Federal Court of Australia
Novartis AG v Pharmacor Pty Limited (No 3) [2024] FCA 1307
ORDERS
First Applicant NOVARTIS PHARMACEUTICALS AUSTRALIA PTY LIMITED Second Applicant | ||
AND: | Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The parties bring in agreed or, if not agreed competing, draft orders giving effect to these reasons by no later than 4.00pm on 20 November 2024.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
YATES J:
1 Novartis AG as patentee, and Novartis Pharmaceuticals Australia Pty Limited (Novartis Australia) as Novartis AG’s exclusive licensee (together, Novartis), sue Pharmacor Pty Limited (Pharmacor) for threatened infringement of claim 1 of Patent No. 2003206738 (the patent) based on Pharmacor’s intended supply in Australia of pharmaceutical products entered on the Australian Register of Therapeutic Goods (ARTG), referred to in these reasons as Valtresto.
2 The application for the patent was filed on 16 January 2003. The patent was granted on 5 April 2007 for a term of 20 years expiring on 16 January 2023. The complete specification filed with the patent application is titled “Pharmaceutical compositions comprising valsartan and NEP inhibitors” (the specification).
3 The specification is directed to pharmaceutical compositions containing certain active pharmaceutical ingredients (APIs); a method of treating or preventing hypertension, heart failure (such as congestive heart failure), or myocardial infarction and its sequelae, by administering a therapeutically effective amount of a combination of certain APIs; and the use of certain APIs in the manufacture of a medicament for treating or preventing one or more of these diseases or conditions. The treatment of hypertension and heart failure is particularly relevant to the issues raised in this case.
4 On 10 June 2016, Novartis AG applied for an extension of the term of the patent under s 70 of the Patents Act 1990 (Cth) (the Patents Act). The application was granted on 5 December 2016. The term was extended to 16 January 2028.
5 The priority date of claim 1 of the patent is 17 January 2002 (the priority date) based on the filing of US Provisional Patent Application No. 60/349,660.
6 Pharmacor denies that it threatens to infringe claim 1. Further, by a notice of cross-claim, it seeks a declaration that claim 1 of the patent is invalid and an order that it be revoked under s 138(3) of the Patents Act.
7 Pharmacor relies on three grounds of revocation, which are pleaded in its further amended statement of cross-claim: (a) the specification of the patent does not comply with s 40(2)(a) of the Patents Act (in its relevant form) in that it does not describe the best method of performing the alleged invention known to Novartis AG at the relevant time; (b) claim 1 of the patent does not comply with s 40(3) of the Patents Act (in its relevant form) in that it is not fairly based on the matter described in the specification; and (c) the invention claimed in claim 1 is not a patentable invention within the meaning of s 18(1)(b)(ii) of the Patents Act (in its relevant form) in that it does not involve an inventive step when compared with the prior art base as it existed before the priority date. Pharmacor has not pursued another pleaded ground of revocation.
8 The relevant form of the Patents Act is the form it took after the Patents Amendment Act 2001 (Cth) and before the Intellectual Property Laws Amendment (Raising the Bar) Act 2012 (Cth) (the RTB Act).
9 Pharmacor also alleges that the term of the patent was wrongly extended because the requirements of ss 70(2)(a) and 70(3)(a) were not satisfied at the time of the application. In its notice of cross-claim, it seeks an order under s 192 of the Patents Act that the Register of Patents (the Register) be rectified by: (a) removing reference to the patent term having been extended to 16 January 2028; and (b) recording that the term of the patent expired on 16 January 2023.
10 If Pharmacor establishes its case on rectification, or its case on revocation on any one of its grounds of invalidity, then Novartis’s case on infringement necessarily fails.
11 For the reasons given below, I have concluded that Novartis has not established that Pharmacor threatens to infringe claim 1 of the patent, having regard to the proper construction of that claim. I have also concluded that the term of the patent was not validly extended. Therefore, Novartis cannot assert the patent rights it claims.
12 It follows from this that Pharmacor’s challenge to the validity of claim 1 does not arise for determination. In any event, its challenge based on the grounds that claim 1 is not fairly based on the matter described in the specification, or because the specification does not describe the best method known to Novartis AG of performing the invention, depends on a construction of claim 1 that I do not accept. Further, even if, contrary to my finding, the patent subsists, Pharmacor has not established that claim 1 is invalid for lack of an inventive step.
13 The cardiovascular system, which includes the heart and blood vessels, is the continuous circulation of blood which returns through the heart twice. Blood is pumped from the right side of the heart to the lungs where it becomes oxygenated. The oxygenated blood then flows to the left side of the heart where it is pumped around the body through blood vessels to deliver oxygenated blood to organs such as the liver and kidneys. Deoxygenated blood is collected by the veins and returned to the right side of the heart to be pumped back to the lungs.
14 The cardiac cycle refers to the steps that occur in a single heartbeat. Around once a second, the cycle commences with an initial “kick” (a rapid increase in pressure as the blood is ejected from the contraction of the left ventricle of the heart into the aorta) occupying about one-tenth of the cycle, followed by a longer period of relaxation as the heart refills with blood, occupying approximately nine-tenths of the cycle. Because of this, blood circulation is pulsatile.
15 In a healthy person, the common measurement of blood pressure is expressed as 120/80—meaning, 120 mmHg (the maximum pressure reached in the aorta during maximal ejection from the left ventricle, which is called the systolic blood pressure) and 80 mmHg (the pressure at which the aortic valve opens, which is called the diastolic blood pressure).
16 The performance of the heart and blood vessels can be assessed by reference to “contractility” (the ability of the heart to contract) and the “ejection fraction” (the percentage of blood pumped out of a ventricle during each cardiac cycle). Assessing contractility involves measuring the rate at which pressure develops in the left ventricle and the speed at which blood is ejected from the left ventricle. The ejection fraction is typically measured from the left ventricle. A normal left ventricle ejection fraction in a healthy adult is around >55% (i.e., in each cardiac cycle more than 55% of the blood in the left ventricle is ejected into the aorta and arteries). The ejection fraction is an indirect measure of contractility.
17 The cardiovascular system is regulated by a number of systems. Two important systems that are particularly relevant to understanding the specification are: (a) the renin-angiotensin-aldosterone system (RAAS); and (b) the natriuretic peptide (NP) system.
18 The RAAS is a neurohormonal system that is responsible for longer term regulation of blood pressure by the release of hormones and blood factors. It is a cascade which is triggered by insufficient blood volume reaching the kidney and results in increased blood pressure.
19 The process begins with cells in the kidneys called juxtaglomerular cells. These cells act as a chemical monitor of matter filtered out of the blood by capillaries in the kidneys. Amongst other things, they detect the rate at which sodium is delivered to the kidneys. If blood pressure is low, less blood will reach the kidneys, and the juxtaglomerular cells will detect less sodium. This activates the RAAS.
20 Once the RAAS is activated, the juxtaglomerular cells cause an enzyme—renin—to be released into the blood. Renin then triggers the production of a hormone—angiotensin I. Angiotensin converting enzyme (ACE), which is diffused throughout the circulatory system, converts angiotensin I into a more active form—angiotensin II.
21 Angiotensin II binds to receptors that cause effects that include: (a) stimulating vasoconstriction; (b) promoting sodium reabsorption (causing sodium retention); (c) inducing the synthesis and release of aldosterone (which promotes sodium and fluid retention); (d) stimulating thirst to encourage fluid intake; and (e) reducing the sensitivity of the baroreceptor reflex—an autonomic nervous system response for maintaining short term stable blood pressure (homeostasis) through triggering a short term increase or decrease in blood pressure by modifying the contractility (power) or rate (speed) of the heart and by changing the vascular tone (the resistance of peripheral arteries).
22 There are two main types of angiotensin II receptors, conveniently referred to as type-1 (AT 1-receptors) and type-2 (AT 2-receptors). Activation of the AT 1-receptors typically causes vasoconstriction and has effects on the heart similar to the baroreceptor reflex to elevate blood pressure. Activation of the AT 2-receptors has almost the opposite effect, reducing blood pressure. However, the effects of activating the AT 2-receptors are less powerful than the effects of activating the AT 1-receptors.
23 The release of aldosterone causes fluid retention, potassium loss, sodium retention, and increases thirst. It does so by blocking the exchange of sodium, potassium and water in the kidneys.
24 As noted, angiotensin II and aldosterone both promote sodium retention. Sodium levels are important because sodium is a substance through which the body controls fluid retention. Increasing sodium concentration in the kidneys means that more water is reabsorbed into the body rather than leaving the body in the form of urine. Fluid content, and hence blood volume, is increased, thereby increasing blood pressure. Decreasing sodium concentration has the opposite effect.
25 In addition to converting angiotensin I to angiotensin II, ACE also interacts with other vasoactive peptides. For example, it inactivates bradykinin, which is a vasodilator that relaxes blood vessels and reduces blood pressure.
26 Heart failure can inappropriately trigger the RAAS into a permanent “on” state leading, ultimately, to damage to the heart and blood vessels.
27 Inhibiting the RAAS is a powerful mechanism for reducing blood pressure.
28 Natriuretic peptides (NPs) promote natriuresis, which is the excretion of sodium via urine.
29 Different parts of the heart produce different NPs: (a) A-type NPs (ANP) (also called atrial natriuretic peptides or atrial natriuretic factor (ANF)) are released by the heart atria when the heart wall muscles are under increased stress by blood volume overload; (b) B-type NPs (BNP) (also called brain natriuretic peptides) are released by the heart ventricles when the heart wall muscles are subject to increased stress by blood volume overload or ventricular hypertrophy (increased muscle mass in the ventricle walls); (c) C-type NPs (CNP) are primarily released by the blood vessel in and around the heart in response to increased blood volume load; and (d) D-type NPs (DNP).
30 At the priority date, CNP and DNP were rarely discussed.
31 ANP and BNP both have effects that act to reduce blood volume. They:
(a) trigger a decrease in sodium reabsorption in the kidneys, causing more sodium to exit the body, and more water to follow;
(b) promote vasodilation, including through the inhibition of vasopressin, primarily on the venous side of the circulatory system, which increases the volume of blood reaching the heart;
(c) promote vasodilation of the blood vessels in the kidney’s filtration system, which increases filtration, causing more sodium (and therefore water) to be excreted as urine;
(d) slightly inhibit the RAAS and sympathetic nervous system; and
(e) reduce aldosterone secretion.
32 Even though ANP and BNP both have these effects, BNP is considered more important because it is released in greater amounts (because of the increased muscle mass of the heart ventricles compared to the heart atria) and has greater effect.
33 ANP and BNP concentration in the blood is a biomarker for the heart and indicates the occurrence and magnitude of heart stressors. Therefore, the measurement of ANP and BNP is a diagnostic tool.
34 NPs are broken down by an enzyme—neutral endopeptidase (NEP). NEP is an important part of the deactivation of NP system. NEP also breaks down bradykinin into inactive fragments.
35 Hypertension (high blood pressure) is largely a disease of the blood vessels. It occurs when the heart is pumping blood under pressure into a vascular system that is restricted. Treatment is directed to stopping the heart creating so much pressure (e.g., by using beta blockers) or reducing the restrictions on the vascular system (e.g., by inhibiting the RAAS to reduce blood volume).
36 Hypertension is more prevalent with age, in part because the blood vessels lose elasticity. During the maximal ejection phase of the cardiac cycle—the “kick”—the blood pressure curve, for a healthy adult, is smoothed out by the elasticity of the aorta and surrounding blood vessels, which expand in response to the spiking blood pressure, increasing the volume of the blood vessels, and thereby reducing the maximum pressure that is reached. As blood vessels become less elastic with age, they do not expand to the same extent. Consequently, blood pressure spikes at a higher level. Approximately two-thirds of the population will develop hypertension over their lifetime.
37 High blood pressure is recognised as a risk factor that is diagnosed as hypertension when it exceeds a “number” at which it is recognised that treatment will provide a meaningful clinical improvement. At the priority date, systolic blood pressure above 140 mmHg (when measured robustly over a 24-hour period) would have been diagnosed as hypertension.
38 Hypertension is largely asymptomatic. However, over time, it can cause significant damage to other organs, including the eyes, brain, kidneys, and heart. For example, persistent high blood pressure can cause blood vessels to burst and bleed, or cause the lining of the blood vessels to thicken and occlude, or make blood plaques worse, which, in turn, can lead to heart attacks, stroke, kidney failure, heart failure, and dementia.
39 Heart failure is a syndrome where the heart ceases to work as expected because of an abnormality in the heart. It is generally a chronic disorder.
40 The diagnosis of heart failure is based on: (a) the presence of one or more classical symptoms (including, shortness of breath, fluid retention, fatigue, and an inability to exercise); (b) the establishment of a causal link between these symptoms and a change in the heart that can be detected through measurement and clinical judgment; and (c) external confirmation that heart failure is occurring, such as by chest X-ray (which may show pulmonary congestion), the measure of NPs, or haemodynamic measurement of the pressure within the heart.
41 There are two types of heart failure, broadly speaking: (a) heart failure with reduced ejection fraction (HFrEF); and (b) heart failure with preserved ejection fraction (HFpEF).
42 As I have noted, a typical left ventricle ejection fraction for a healthy adult is >55%. A patient with a lower ejection fraction is said to have a “reduced” ejection fraction. A patient who has heart failure, but a normal ejection fraction (>55%) is said to have a “preserved” ejection fraction. At the priority date, HFrEF dominated attention and practice, and pharmacotherapies were largely directed to this condition.
43 There are a wide range of causes of heart failure. The most common causes of HFrEF are: (a) ischaemic heart disease (or coronary heart disease), which refers to narrowed arteries (which may be contributed to by hypertension); (b) hypertension; and (c) myocardial infarction (heart attack), which may damage or destroy the heart muscle.
44 Hypertension is significantly more common than heart failure, affecting (as I have said) around two-thirds of the population over their lifetime. Two-thirds of heart failure patients will first have hypertension, which is an underlying risk factor or cause of heart failure, either directly or through ischaemic heart disease. Once a patient with hypertension develops heart failure, in particular HFrEF, their hypertension will remain present. However, in some cases, the patient’s blood pressure will reduce (but still be relatively high) because the heart is not producing as much pressure as it did.
45 All the first line therapies for heart failure reduce blood pressure. For around half the patients with heart failure and hypertension, who are receiving treatment for heart failure, there is no need to separately manage their hypertension. For example, they do not need to take additional pharmacotherapies to reduce their blood pressure.
46 Heart attack is the next most common cause of heart failure. A large heart attack may cause sufficient damage to immediately trigger heart failure. A smaller heart attack may lead to heart failure over time because of deteriorated heart function. For example, the increased stress on the heart wall following a heart attack may result in an enlarged heart through neurohormonal activation. As the heart enlarges, the left ventricle generally becomes more globular and less efficient at pumping than an ordinarily conically-shaped ventricle with smaller muscles.
47 There are other indirect causes of heart failure, as well as contributory risk factors, that need not be detailed for the purposes of these reasons.
48 In Australia, it is common for cardiologists to treat both hypertension and heart failure, although much of the clinical treatment of hypertension is performed by generalists in internal medicine or clinical pharmacology.
49 At the priority date, the development of pharmacotherapies for the treatment of hypertension and heart failure generally involved modifying the various regulatory systems of the cardiovascular system. There were a range of pharmacotherapies and, typically, a patient with hypertension or heart failure would receive more than one type of drug with different modes of action. This was required for heart failure to target different systems to reduce mortality and improve patient outcomes in an additive way. For hypertension, it was possible to use one drug with one mode of action. However, it was common to use multiple drugs with different modes of action to reduce blood pressure without creating unacceptable levels of side effects.
50 In the following paragraphs, I refer to some of the therapies that are of particular relevance to the present case.
51 These drugs inhibit the RAAS by reducing the activity of angiotensin II. They are useful in the treatment of hypertension (they reduce blood pressure by vasodilation) and heart failure.
52 At the priority date, the following ACE inhibitors were available: (a) captopril; (b) enalapril; (c) lisinopril; (d) ramipril; and (e) perindopril. Captopril was one of the first ACE inhibitors with widespread approval and use. It needed to be dosed more frequently and at higher doses than later agents. Enalapril was the next ACE inhibitor to reach widespread approval and use. It was the agent that first demonstrated the benefits of ACE inhibition for heart failure.
53 ACE inhibitors have notable side effects—dry cough and angioedema. Angioedema is a dangerous side effect that involves a dramatic swelling of the tissues of the head and neck. It affects <1% of patients. This, however, is a matter of concern because of the severity of the condition. ACE inhibition leads to a build up of bradykinin, which is associated with both conditions. ACE inhibitors are also contraindicated for patients with renal artery stenosis (blockage of the renal artery).
54 However, when tolerated, ACE inhibitors were considered to be mandatory treatment for chronic heart failure.
55 Angiotensin receptor blockers (ARBs) (also known as angiotensin II antagonists) were the next major development in inhibiting the RAAS. They block the activity of angiotensin II one further step along the RAAS cascade from ACE inhibitors. They do not, therefore, reduce ACE activity as such.
56 ARBs target and predominantly block AT 1-receptors. As I have noted, activation of the AT 1-receptors typically causes vasoconstriction and has effects on the heart similar to the baroreceptor reflex to elevate blood pressure.
57 At the priority date, ACE inhibitors and ARBs were viewed as substitutes, although there was uncertainty as to which was more efficacious in the treatment of heart failure. The ELITE clinical trial—a safety and tolerability study of losartan (an ARB) versus captopril—reported in 1997 that losartan was significantly superior to captopril in reducing mortality. However, ELITE-II—a larger, follow-on trial conducted from 1997—concluded that mortality did not differ significantly between losartan and captopril. The study also confirmed that ARBs had superior tolerability to ACE inhibitors.
58 Notwithstanding the findings of ELITE-II, there continued to be uncertainty as to whether ARBs or ACE inhibitors were superior in the treatment of heart failure. At the priority date, ACE inhibitors remained the first line therapy for heart failure because there had been more studies of their effect in treating heart failure in major clinical trials.
59 ARBs have low rates of side effects (including angioedema) similar to a placebo. As a result, by the priority date, ARBs started to replace ACE inhibitors as first line therapy for hypertension. ARBs also have a wide therapeutic window (the therapeutic window is the range between the concentration of the agent necessary to produce therapeutic effects and the concentration at which there is an unacceptable risk of side effects).
60 At the priority date, a number of ARBs were in use or in development. Their effects were largely thought to be class effects. There were, however, differences in the extent to which each ARB had been proven in large-scale clinical trials.
61 Losartan was the first ARB to receive regulatory approval, globally. As I have noted, it was shown in ELITE-II to be effective in treating heart failure. It was, however, short-acting and generally needed to be administered twice daily.
62 Valsartan is another ARB that has been shown to be effective in treating heart failure. It was the subject of the Val-HeFT clinical trial—a Phase III trial against placebo involving over 5,000 participants, who were also taking the standard heart failure treatments (ACE inhibitors, beta blockers, diuretics and/or digoxin).
63 The results of the Val-HeFT trial were reported in November 2000 at the American Heart Association Meeting and published in Thackray SDR et al, “Clinical trials update: highlights of the scientific sessions of the American Heart Association year 2000: Val HeFT, COPERNICUS, MERIT, CIBIS-II, BEST, AMIOVIRT, V-MAC, BREATHE, HEAT, MIRACL, FLORIDA, VIVA and the first human cardiac skeletal muscle myoblat transfer for heart failure” (2001) 3 European Journal of Heart Failure 117–124 (Thackray). Two relevant conclusions were:
Valsartan significantly reduces the combined end point of mortality and morbidity and improves clinical signs and symptoms in patients with heart failure, when added to prescribed therapy.
and
However, the post hoc observation of an adverse effect on mortality and morbidity in the subgroup receiving valsartan, an ACE inhibitor, and a beta-blocker raises concern about the potential safety of this specific combination.
64 The final results of the Val-HeFT clinical trial were published in December 2001 in Cohn JN and Tognoni G, “A Randomized Trial of The Angiotensin-Receptor Blocker Valsartan in Chronic Heart Failure” (2001) 345(23) The New England Journal of Medicine 167-175 (Cohn).
65 Other ARBs known at the priority date were: (a) candesartan; (b) irbesartan; (c) telmisartan; and (d) eprosartan. Candesartan was the subject of a large-scale clinical trial called the CHARM Program. The CHARM Program was still underway at the priority date. At that time, its results were not expected until 2003.
Aldosterone receptor antagonists
66 Aldosterone receptor antagonists block the effects of aldosterone. As discussed, one function of angiotensin II in the RAAS is to release aldosterone. However, angiotensin II is not the only trigger. Potassium levels can also trigger its release. For this reason, it is useful to inhibit both angiotensin II (by an ACE inhibitor or an ARB) and aldosterone (by an aldosterone receptor antagonist).
67 Spironolactone was the first aldosterone receptor antagonist. It was first used for the treatment of hypertension but was later found to be useful in the treatment of severe heart failure. Its activity is complementary to the activity of ACE inhibitors, ARBs, and beta blockers. Because of this, it was included in various heart failure guidelines, and its use increased significantly. However, its use as an additional drug therapy in mild heart failure was not demonstrated until after the priority date.
68 Spironolactone also reduces blood pressure and was used to treat hypertension (although not commonly prescribed solely for that effect).
69 The main side effects associated with spironolactone were hyperkalaemia (high potassium levels) and gynecomastia (swelling of the breast tissue) in men.
70 Eplerenone was another aldosterone receptor antagonist in development at the priority date.
71 Beta blockers are a broad class of drugs that block beta receptors and inhibit the effects of epinephrine (or adrenaline) and norepinephrine (or noradrenalin). This slows and reduces the power of the heart, thereby reducing blood pressure generated by the heart.
72 Beta receptors, and the mechanisms for blocking them, are diverse and varied. As a result, there can be significant differences in how beta blockers act.
73 Only certain beta blockers are indicated for heart failure, the most commonly used being carvedilol, bisoprolol, metoprolol, and nebivolol. A much wider range can be used for hypertension.
74 Beta blockers have a range of side effects, including depression, sleep impairment, bronchospasm, and erectile dysfunction. Although beta blockers were initially used as primary treatment for hypertension, by the priority date other treatments were being prioritised with fewer side effects.
75 Before the priority date, there was interest in seeking to influence the NP system to treat heart failure and to treat hypertension. As I have noted, NPs are broken down by NEP, which is an important part of the deactivation of the NP system. The action of NPs, which reduces blood pressure, can be prolonged by inhibiting NEPs.
76 The potential role of NEP inhibitors for the treatment of heart failure had been investigated, but none had been approved or were in use. In limited clinical trials, NEP inhibitors had relatively little efficacy.
77 However, before the priority date, there had been development, and widely reported clinical trials, of dual vasopeptidase inhibitors, which are naturally occurring single chemical entities possessing both ACE and NEP inhibitory action—in other words, the complementary action of inhibiting the RAAS and promoting the positive action of NPs. Two such drugs were omapatrilat and sampatrilat.
78 Early clinical trials of omapatrilat were very promising in terms of its efficacy in treating hypertension and heart failure. It was widely reported at leading conferences and in major journals in cardiology. It had also been referred to in at least one teaching text in Australia which reported that it seemed likely that it would prove useful in the treatment of chronic heart failure:
The combination of an NEP antagonist with an angiotensin-converting enzyme (ACE) inhibitor has been shown to result in a more sustained natriuretic effects than has an NEP antagonist alone in experimental animal models of HF, in part because of the inhibition of angiotensin II-mediated effects but also because of the fact that both NEP and ACE degrade bradykinin, a vasodilatory and natriuretic peptide.
79 Other pharmacotherapies (relevant to the consideration of this case) include vasopressin receptor antagonists, endothelin receptor antagonists, and positive inotropic agents.
80 Vasopressin is a vasoconstrictor hormone that also effects the kidneys. At the priority date, vasopressin receptor antagonists were theoretically promising. However, work on them had not resulted in any drug products approved for hypertension or heart failure.
81 Endothelin is also a vasoconstrictor. Endothelin receptor antagonists inhibit the binding of endothelin to receptors in smooth muscle cells. At the priority date, such drugs were also theoretically promising, but work on them had not led to any approved drug products.
82 Positive inotropic agents are drugs which increase the strength of the heartbeat. They are only used for heart failure. They are not used for hypertension because they increase blood pressure. At the priority date, positive inotropic agents were a relatively minor part of the range of pharmacotherapies in use and in development. Their use at the priority date was limited to symptomatic relief where longer term mortality was a less relevant consideration (such as in the case of palliative care or where the patient was in acute heart failure requiring emergency treatment).
83 A prodrug is a compound with little or no pharmacological activity that converts into a pharmacologically active compound by the body’s metabolic processes. Prodrugs are typically used to assist the absorption of a compound that would otherwise be poorly absorbed. If an API has any chemical groups that will affect its absorption when administered, and hence its bioavailability, then a prodrug might be suitable as a strategy for improving its oral bioavailability so that the API can have its intended therapeutic effect in the body.
84 If the API has an appropriate chemical functional group, such as an alcohol group or an acidic group, a non-toxic “prodrug group” can be attached to the compound by an “ester” linkage to form an ester prodrug.
85 Esters are organic compounds formed by a reaction between an alcohol group (OH, which is also referred to as a hydroxy or hydroxyl group) and an acid group (commonly a carboxylic acid group, COOH, which is also referred to as a carboxy or carboxyl group). Esters are a common form of prodrug.
86 More than one API can be combined in a single dosage form. A “fixed dose combination” is a tablet or other dosage form that incorporates two or more APIs in a fixed dosage, or amount. Such a combination, which reduces the number of dosage forms a patient might otherwise be required to take, is a strategy for facilitating patient compliance.
87 Well before the priority date, various fixed dose combination products were available in Australia, including: (a) antihypertensive drugs combined with diuretics in a single tablet; (b) certain antibiotics, including amoxycillin plus clavulanic acid and trimethoprim plus sulphamethoxazole in a single unit dosage form; (c) paracetamol plus doxylamine in a single tablet or capsule; and (d) various analgesic combinations, including paracetamol plus codeine in a single tablet.
88 A complex is a unique single entity consisting of two or more molecules (or charged molecules, i.e., ions) bound together by non-covalent bonds. As a solid, a complex exists as a single, distinct physical material in which all the solid particles have the same chemical composition. This material has a different identity and different properties to the physical materials of its component parts.
89 A solid complex is distinct from a physical mixture of particles. A complex is typically formed by combining its constituent molecules in solution. This process is not equivalent to simply physically mixing the solid components together. It may require specific reaction conditions (e.g., specific temperature, pH, pressure, solvents, catalysts, and, potentially, the use of a complexing agent). The process may be driven by separating the solid complex from solution (e.g., by crystallisation).
90 A complex in a solid state has its own unique set of physical properties, such as solubility, stability, hygroscopicity, melting point (or glass transition for amorphous materials), density, bulk density, powder flowability, adhesion, and physical appearance. Solid complexes also have their own set of analytical properties, such as infrared spectrum, Raman spectrum, solid state NMR spectrum, fluorescence behaviour and, for crystalline materials, X-ray powder diffraction pattern. These properties will be different from those of the separate components of the complex, even when those separate components are physically mixed.
91 Therefore, a solid complex is not the mere sum of its constituent parts. In complexes that involve ions, the ions of opposite charge cannot be physically separated because of the great strength of ionic bonds.
92 A complex can also exist in liquid and semi-solid pharmaceutical dosage forms.
93 A complex can be used in pharmaceutical products to meet a range of product needs, including: (a) taste masking an API; (b) improving the solubility and absorption of an API; (c) retarding the release and absorption of an API; (d) enabling a product to have an adequate storage shelf life; and (e) minimising toxicity.
94 The most important characteristic of complexes in solution, if they do not dissociate completely, is their stability, which can be measured by various, known means.
95 A salt complex can be a so-called “double salt” that comprises one kind of cation and two kinds of anion, or one kind of anion and two kinds of cation, held together by ionic interaction. This does not mean that there are two salts present in the complex. It only means that there are two different ions of one particular charge, and one ion of the opposite charge, reacting to produce a new, single salt—the “double salt”. It is as if the salt-forming reaction has occurred twice to form the one, new salt.
96 Entresto is a film-coated tablet drug product that contains an active ingredient which is a salt complex of the anionic forms of sacubitril and valsartan, sodium cations, and water molecules in the ratio of 1:1:3:2.5, termed TSVH, and excipients. TSVH is a single crystalline complex material that comprises a repeating unit of 18 sodium cations, 6 sacubitril anions, 6 valsartan anions, and 15 water molecules in a coordinated complex arrangement involving ionic bonding, hydrogen bonding and a range of other non-covalent interactions. The overall complex is a single physical material with a unique set of physicochemical properties.
97 The relevant experts agree that TSVH is a single salt. It is also a “double salt” but, as I have already said, this does not mean it is two salts. The anionic sacubitril, anionic valsartan, and sodium cations do not, and cannot, exist independently as distinct materials in the solid state.
98 Following oral administration of the tablet, Entresto disintegrates. The salt complex, TSVH, is released and then dissolved in the gastrointestinal tract fluids. The pharmaceutical composition no longer exists. The salt complex dissociates into solvated anionic sacubitril and anionic valsartan. The anions are then protonated (i.e., they gain a hydrogen atom) into their free acid forms.
99 TSVH was first synthesised in January 2006 following experimental work between March 2005 and January 2006.
100 The relevant experts agree that the specification (discussed below) does not envisage or mention TSVH. However, TSVH is disclosed in an International Patent Application (PCT/US2006/043710) by Novartis AG, published as WO 2007/056546 A1, titled “Pharmaceutical combinations of an angiotensin receptor antagonist and an NEP inhibitor”.
101 Valtresto is a film-coated tablet drug product for the treatment of chronic heart failure in patients with a reduced ejection fraction. It comes in three dosage strengths and comprises an amorphous complex, termed SVT1, and excipients, in which valsartan anions, sacubitril anions, and sodium cations are present in a 1:1:3 ratio.
102 SVT1 is a single chemical entity with a single glass transition. Following oral administration, Valtresto, like Entresto, disintegrates and SVT1 dissociates.
103 The specification commences by referring to ARBs, ACE inhibitors, and NEPs. It refers to ARBs by their alternative name “angiotensin II antagonists” and notes their inhibition of AT 1-receptors and, hence, their use as antihypertensives and in the treatment of congestive heart failure, among other indications.
104 The specification says that inhibitors of the renin angiotensin system “are well known drugs that lower blood pressure and exert beneficial actions in hypertension and in congestive heart failure”. It singles out ACE inhibitors as the most widely studied inhibitors and specifically mentions captopril, enalapril, lisinopril, benazepril, and spirapril.
105 The specification notes that ACE cleaves a variety of substrates, including bradykinin. It says that the prevention of the degradation of bradykinin by ACE inhibitors has been demonstrated, and that:
.. the activity of the ACE inhibitors in some conditions has been reported … to be mediated by elevation of the bradykinin levels rather than the inhibition of Ang II formation. Consequently, it cannot be presumed that the effect of an ACE inhibitors is due solely to prevention of angiotensin formation and subsequent inhibition of the renin angiotensin system.
106 The specification notes that NEP cleaves a variety of peptide substrates including ANP (which it alternately calls ANF), BNP, and bradykinin. It refers to ANPs as “a family of vasodilator, diuretic and antihypertensive peptides which have been the subject of many recent reports in the literature”.
107 After referring to the function of ANPs to maintain salt and water homeostasis, and to regulate blood pressure, the specification says that ANP is rapidly inactivated in the circulation by at least two processes. One process is “enzymatic inactivation via NEP”.
108 The specification says that it has been demonstrated in animal experiments that inhibitors of NEP potentiate the hypotensive, diuretic, natriuretic, and plasma ANP responses. It refers to literature reporting on the potentiation of ANP by specific NEP inhibitors and NEP inhibitors in general.
109 The specification discusses combination therapy:
Prolonged and uncontrolled hypertensive vascular disease ultimately leads to a variety of pathological changes in target organs such as the heart and kidney. Sustained hypertension can lead as well to an increased occurrence of stroke. Therefore, there is a strong need to evaluate the efficacy of antihypertensive therapy, an examination of additional cardiovascular endpoints, beyond those of blood pressure lowering, to get further insight into the benefits of combined treatment.
The nature of hypertensive vascular diseases is multifactorial. Under certain circumstances, drugs with different mechanisms of action have been combined. However, just considering any combination of drugs having different mode (sic) of action does not necessarily lead to combinations with advantageous effects. Accordingly, there is a need for more efficacious combination therapy which has less deleterious side effects.
110 Although this part of the specification addresses a need for new efficacious combination therapy in the context of treating hypertension, later passages speak of the utility of the invention in treating or preventing heart failure, and myocardial infraction and its sequelae.
111 After this introduction, the specification turns to the alleged invention:
In one aspect the present invention relates to pharmaceutical combinations comprising valsartan or pharmaceutically acceptable salts thereof and the neutral endopeptidase (NEP) inhibitor N-(3-carboxy-1-oxopropyl)-(4S)-p-phenylphenylmethyl)-4-amino-2R-methylbutanoic acid ethyl ester or (2R,4S)-5-Biphenyl-4-yl-4-(3-carboxy propionylamino)-2-methyl-pentanoic acid or pharmaceutically effective salts thereof, optionally in the presence of a pharmaceutically acceptable carrier and pharmaceutical compositions comprising them.
112 The pharmaceutical combination is: (a) valsartan (an ARB), which the specification says is disclosed in a European patent (EP 0443983 A) and also in US patent (5,399,578), or its pharmaceutically acceptable salts; and (b) a particular NEP inhibitor as an ethyl ester or as a free acid, or as pharmaceutically acceptable salt of the ethyl ester or the pharmaceutically acceptable salt of the free acid. The ethyl ester is the substance called sacubitril. The free acid is the substance called sacubitrilat. Although not referred to as such in the specification, I will use the name sacubitril to refer to the ethyl ester and the name sacubitrilat to refer to the free acid.
113 The specification discusses, more generally, NEP inhibitors that are useful in combination with valsartan. Page 3 of the specification discloses one such inhibitor:
A NEP inhibitor useful in said combination is a compound of the formula (II)
(II)
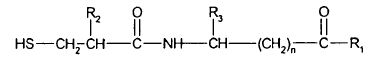
and pharmaceutically acceptable salts thereof wherein:
R2 is alkyl of 1 to 7 carbons, trifluoromethyl, phenyl, substituted phenyl, -(CH2)1 to 4- phenyl, or -(CH2)1 to 4-substituted phenyl;
R3 is hydrogen, alkyl of 1 to 7 carbons, phenyl, substituted phenyl, -(CH2)1 to 4-phenyl, or -(CH2)1 to 4-substituted phenyl;
R1 is hydroxy, alkoxy of 1 to 7 carbons, or NH2;
n is an integer from 1 to 15; and
the term substituted phenyl refers to a substituent selected from lower alkyl of 1 to 4 carbons, lower alkoxy of 1 to 4 carbons, lower alkylthio of 1 to 4 carbons, hydroxy, Cl, Br, or F.
114 Pages 4 to 6 of the specification disclose NEP inhibitors that are said to be “within the scope of the present invention”. The list includes (by chemical name) sacubitril and sacubitrilat. Not all compounds listed on pages 4 to 6, including sacubitril and sacubitrilat, fall within formula (II).
115 Page 6 of the specification reinforces that:
The compounds to be combined can be present as pharmaceutically acceptable salts.
116 On page 6, the specification says that the preferred salts of sacubitril “include the sodium salt disclosed in U.S. Patent No. 5,217,996, the triethanolamine salt and the tris(hydroxymethyl)aminomethane salt”.
117 The specification teaches that the two last-mentioned salts are novel. They are said to be another embodiment of the invention, which includes: (a) their use as NEP inhibitors, especially for preventing and treating conditions and diseases “associated with the inhibition on NEP”; and (b) their combination with valsartan in a pharmaceutical composition, especially for the treatment of conditions and diseases “as disclosed for the combinations of the present invention hereinbefore or hereinafter”. A pharmaceutical composition with either one of these salts is claimed in claim 2.
118 The specification then addresses the effect of combining valsartan with NEP inhibitors:
It has surprisingly been found that, a combination of valsartan and a NEP inhibitor achieves greater therapeutic effect than the administration of valsartan, ACE inhibitors or NEP inhibitors alone and promotes less angioedema than is seen with the administration of a vasopeptidase inhibitor alone. Greater efficacy can also be documented as a prolonged duration of action. The duration of action can be monitored as either the time to return to baseline prior to the next dose or as the area under the curve (AUC) and is expressed as the product of the change in blood pressure in millimeters of mercury (change in mmHg) and the duration of the effect (minutes, hours or days).
Further benefits are that lower doses of the individual drugs to be combined according to the present invention can be used to reduce the dosage, for example, that the dosages need not only often be smaller but are also applied less frequently, or can be used to diminish the incidence of side effects. The combined administration of valsartan or a pharmaceutically acceptable salt thereof and a NEP inhibitor or a pharmaceutically acceptable salt thereof results in a significant response in a greater percentage of treated patients, that is, a greater responder rate results, regardless of the underlying etiology of the condition. This is in accordance with the desires and requirements of the patients to be treated.
It can be shown that combination therapy with valsartan and a NEP inhibitor results in a more effective antihypertensive therapy (whether for malignant, essential, reno-vascular, diabetic, isolated systolic, or other secondary type of hypertension) through improved efficacy as well as a greater responder rate. The combination is also useful in the treatment or prevention of heart failure such as (acute and chronic) congestive heart failure. It can further be shown that a valsartan and NEP inhibitor therapy proves to be beneficial in the treatment and prevention of myocardial infarction and its sequelae.
119 Pages 9 to 12 of the specification summarise the methodology of certain representative studies carried out with the combination of valsartan and sacubitril.
120 At pages 12 – 13, the specification says:
In one aspect … the object of this invention [is] to provide a pharmaceutical combination composition, e.g. for the treatment or prevention of a condition or disease selected from the group consisting of hypertension, heart failure such as (acute and chronic) congestive heart failure, and myocardial infarction and its sequelae, which composition comprises (i) the AT 1-antagonists valsartan or a pharmaceutically acceptable salt thereof and (ii) a NEP inhibitor or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier. A further active ingredient may be a diuretic, especially hydrochlorothiazide.
121 These passages of the specification are followed by the statement:
In this composition, components (i) and (ii) can be obtained and administered together, one after the other or separately in one combined unit dose form or in two separate unit dose forms. The unit dose form may also be a fixed combination.
122 The interpretation of this statement is controversial. I shall return to it when discussing the proper construction of claim 1.
123 Page 14 includes the following statement on which the parties also relied in respect of the proper construction of claim 1:
A therapeutically effective amount of each of the component of the combination of the present invention may be administered simultaneously or sequentially and in any order.
124 Over the following pages, the specification discusses: (a) how pharmaceutical compositions according to the invention can be prepared; (b) typical pharmaceutically acceptable carriers for use in the formulations that are described; (c) combining separate pharmaceutical compositions (a valsartan pharmaceutical composition and a NEP inhibitor pharmaceutical composition) as two separate units in kit form; (d) methods of administering the pharmaceutical compositions; (e) the dosages of valsartan; and (f) the dosages of NEP inhibitors.
125 Pages 16 to 21 of the specification provide example formulations (Examples 1 to 7). Examples 1 to 3 are film-coated tablets. Examples 4 to 7 are capsules. Each formulation contains valsartan but not sacubitril or sacubitrilat.
126 Pages 21A to 21D of the specification report on experiments using two animal models of hypertension (Example 8). The first model used Dahl salt-sensitive rats, where a high salt diet causes suppression of the renin-angiotensin system resulting in hypertension. The second model used SHR rats, which did not require a particular diet to exhibit symptoms of hypertension.
127 It is not necessary to discuss Example 8 other than to note that the substance administered to the rats involved valsartan and a chemical which the relevant experts presumed was sacubitril (it appears that the systematic chemical name used in the title of Example 8 contains a typographical error). The substance was administered as an aqueous solution (in drinking water) by oral gavage. However, the vehicle included polyethylene glycol 400 (a polymeric organic solvent) (PEG 400) and Tween 80 (a surfactant). I will return to the significance of organic solvents when discussing the correct construction of claim 1.
128 After the discussion of Example 8, the specification records three matters.
129 First, the publications and patents mentioned in the specification are incorporated, by reference, in their entirety, as if those documents were set out, in full, in the specification.
130 Secondly, the word “comprise” (and its variations) in the body of the specification and in the claims is to be understood as implying inclusion, not exclusion.
131 Thirdly, references in the specification to any prior publication, or the information derived from any prior publication, or to any matter that is known, are not to be taken as an acknowledgement or admission or suggestion that the prior publication, information, or known matter form part of the common general knowledge.
132 Claim 1, which is central to this case, is:
1. A pharmaceutical composition comprising:
(i) the AT 1-antagonist valsartan or a pharmaceutically acceptable salt thereof;
and
(ii) the NEP inhibitor N-(3-carboxy-1-oxopropyl)-(4S)-p-phenylphenylmethyl)-4-amino-2R-methylbutanoic acid ethyl ester or (2R,4S)-5-Biphenyl-4-yl-4-(3-carboxypropionylamino)-2-methyl-pentanoic acid or pharmaceutically acceptable salts thereof and a pharmaceutically acceptable carrier.
133 The parties accept that the inclusion in integer (ii) of the pharmaceutically acceptable carrier is inapposite, and that integer (ii) should be understood as referring only to the two NEP inhibitors (or the pharmaceutically acceptable salts thereof), with the pharmaceutically acceptable carrier standing apart, as a separate integer of the claim, notionally considered as integer (iii). I will read, and treat, claim 1 accordingly.
134 As I have said, the two NEP inhibitors are, respectively, sacubitril and sacubitrilat (conveniently referred to as sacubitril/at). In integer (ii), sacubitril and sacubitrilat are free acids (meaning that their acidic functional groups are fully protonated). Sacubitril is an ethyl ester and a prodrug of sacubitrilat.
135 Claim 1 is notable for its simplicity. It does not specify the form that the composition should take or its suitability for any particular mode of administration. It does not specify the salts of each component that can be present in the composition (if salts are used), beyond requiring that they be pharmaceutically acceptable. It does not specify the amount (dose) of each component in the composition or the suitability of the composition for any particular dosing regimen. It does not identify the disease or condition for which the pharmaceutical composition is to be used, beyond stating that its identified components are, respectively, an AT 1-antagonist and an NEP inhibitor.
136 Claim 2 claims the pharmaceutical composition of claim 1, where sacubitril is a triethanolamine or tris(hydroxymethyl)aminomethane salt. Claim 3 is also a pharmaceutical composition whose features are dependent on claim 1, and also includes a diuretic.
137 Claim 4 is method of treatment claim, involving the administration of the APIs identified in claim 1 with a pharmaceutically acceptable carrier. Claim 5 is a method of treatment claim, involving the administration of the APIs identified in claim 2 with a pharmaceutically acceptable carrier. Claims 4 and 5 are not limited to the APIs being components of the one pharmaceutical composition.
138 Claims 6 to 8 claim the use of, respectively: (a) valsartan or a pharmaceutically acceptable salt thereof in the manufacture of a medicament to be used in combination with sacubitril/at or their pharmaceutically acceptable salts, to treat or prevent selected conditions or diseases; (b) sacubitril/at or their pharmaceutically acceptable salts in the manufacture of a medicament to be used in combination with valsartan or a pharmaceutically acceptable salt thereof, to treat or prevent selected conditions or diseases; and (c) valsartan or a pharmaceutically acceptable salt thereof and sacubitril/at and their pharmaceutically acceptable salts in the manufacture of a medicament to treat or prevent selected conditions or diseases.
139 Claim 9 claims the use according to any one of claims 6 to 8, wherein sacubitril is in the form of one of the salts identified in claim 2.
140 Claims 6 to 9 are in the form of Swiss type claims (as to which, see Otsuka Pharmaceutical Co Ltd v Generic Health Pty Ltd (No 4) [2015] FCA 634; 113 IPR 191 (Otsuka) at [100] – [121]).
141 I mention these matters because the specification is not directed simply to the invention as defined in claim 1. It contains other teachings directed to supporting the invention as defined by each of the claims.
142 Novartis called expert evidence from:
(a) Professor David Linley Hare OAM. Professor Hare is a Professorial Fellow and cardiologist. He holds Bachelor of Medicine and Bachelor of Surgery degrees and a Diploma in Psychological Medicine from the University of Melbourne. Professor Hare has extensive experience in treating patients with heart failure and hypertension. He has also been involved in cardiovascular research. Professor Hare made two affidavits and provided a joint expert report with Professor Coats (see below).
(b) Professor Jonathan Michael White. Professor White is an organic chemist with over 30 years’ experience in synthetic organic chemistry and physical organic chemistry. Professor White holds a Bachelor of Science (Honours, First Class) degree majoring in chemistry and a PhD in chemistry from the University of Canterbury. He has an extensive understanding of the principles of organic synthesis and the analysis of compounds, including the nature and characteristics of different types of bonds that exist between atoms, ions and molecules. Professor White made three affidavits and provided a joint expert report with Professor Roberts and Professor Steed (see below).
143 Novartis also read affidavits from John Fredrick Howards Collins, a partner in Clayton Utz, the solicitors for Novartis.
144 Pharmacor called expert evidence from:
(a) Professor Andrew Justin Stewart Coats AO. Professor Coats is a cardiologist. Professor Coats holds Bachelor of Medicine and Bachelor of Surgery degrees from the University of Cambridge and a Master of Arts (Physiological Sciences) degree from the University of Oxford. He also holds a Doctorate of Medicine from the University of Oxford and a Doctorate of Science from Imperial College London. He holds specialist accreditations in Australia and the United Kingdom in general internal medicine and cardiology. He has extensive experience in treating patients with heart failure and hypertension. He has been involved in cardiovascular research. Professor Coats made two affidavits and provided a joint expert report with Professor Hare.
(b) Professor Jonathan William Steed. Professor Steed is Professor of Inorganic Chemistry at Durham University. He has over 30 years’ experience in the fields of organic, inorganic and solid-state chemistry. He holds a Bachelor of Science degree majoring in Chemistry (Honours, First Class) and a PhD in Chemistry University College London. His research career has focused on, amongst other things, solid-state chemistry. Professor Steed made two affidavits and provided a joint expert report with Professor Roberts and Professor White.
(c) Professor Michael Stephen Roberts: Professor Roberts is a pharmaceutical scientist with over 50 years’ experience working in the fields of drug formulation, drug absorption, pharmacology, toxicology, and pharmacokinetics. He holds Bachelor of Pharmacy degree from the University of Adelaide. He also holds a Master of Science degree in pharmacy, a PhD in pharmaceutical science, and a DSc degree from the University of Sydney. Professor Roberts made two affidavits and provided a joint expert report with Professor Steed and Professor White.
(d) Dr George Mokdsi: Dr Mokdsi is an intellectual property analyst and searcher, and a director of The Patent Searcher. Dr Mokdsi has a PhD in chemistry from the University of Sydney. He has expertise in the searching of pharmaceutical patents and scientific literature. Dr Mokdsi made one affidavit.
145 Pharmacor also read affidavits from Nina Jacqueline Fitzgerald, a partner in Ashurst Australia, the solicitors for Pharmacor. A redacted version of an affidavit from Ray Cheng, a solicitor at Ashurst Australia, was admitted into evidence as Exhibit I.
146 In closing submissions, the parties advanced criticisms of each other expert witnesses. I do not propose to address those criticisms in specific terms. I am satisfied that all witnesses, whose expertise is unquestioned, gave evidence consistent with their obligations as independent experts. Each provided considerable assistance in resolving the complex issues advanced by the parties.
147 It was a feature of the present case that all the experts were provided with standard instructions in relation to the giving of evidence on the question of inventive step. The background to this happening was that, on 8 June 2023, the Federal Court of Australia IP Practice Area User Group presented a draft of such instructions for discussion at a meeting with Judges in the Court’s Intellectual Property NPA Patents and associated Statutes sub-area. I considered it appropriate that those instructions be used for the assistance of the experts in this case. The instructions are reproduced in the Schedule to these reasons.
148 The “person skilled in the art” is a construct that is used to analyse questions that arise in patent law.
149 It is well-recognised that this construct can be understood as a “team”, particularly if the art is one having a highly developed technology: General Tire & Rubber Co v Firestone Tyre & Rubber Co Ltd [1972] RPC 457 at 485. As such, the construct combines and deploys the knowledge and skills of the members of the “team”. This means that, in real life, the knowledge and skills of some members of the “team” may not be known or shared by others in the “team”. There is, however, but one construct. The person skilled in the art thinks with one mind, speaks with one voice, and draws, when and to the extent necessary, on the disparate knowledge and skills of all the members of the “team”. The person skilled in the art is indivisible: see Otsuka at [127].
150 Depending on the relevant art, the members of the “team” may be highly skilled with research capabilities. In the context of chemical and pharmaceutical patents in particular, it is common to equate the person skilled in the art with a “notional research group” which is confronted with a particular task: Aktiebolaget Hassle v Alphapharm Pty Limited [2002] HCA 59; 212 CLR 411 (AB Hassle) at [53].
151 This does not mean, however, that the person skilled in the art, understood as a highly skilled team or a notional research group, exhibits the capacity for invention. The person skilled in the art, even when understood as a highly skilled team or a notional research group, is taken to have no inventive capacity whatsoever, and is constrained to act only with knowledge that is publicly known, and commonly accepted, by those within the calling of the art in question.
152 The knowledge and skills of the person skilled in the art can be informed by the evidence of expert witnesses. It is important to bear in mind, however, that the person skilled in the art is not a mere avatar of those witnesses: AstraZeneca AB v Apotex Pty Ltd [2015] HCA 30; 257 CLR 356 at [23].
153 The parties accept that, in the present case, the person skilled in the art should be understood as a “team”. However, they disagree on who is in the “team”.
154 They accept that the “team” should include a cardiologist. Pharmacor submits that the cardiologist should have expertise, and a research interest, in the treatment of hypertension and heart failure. Both Professor Coats and Professor Hare are representative of such a cardiologist.
155 Novartis submits that the cardiologist should be one who does not have Professor Coats’s and Professor Hare’s skills and interests, which Novartis describes as “an extreme subset of cardiologists” in Australia. It submits that, at the priority date, cardiologists in Australia were mainly engaged in imaging, interventional or electrophysiological subspecialties. There was no requirement in Australia for a cardiologist to have specialist training in hypertension, and other medical specialists (non-cardiologists) also treated hypertension (e.g., in hypertension clinics), as did general practitioners.
156 Further, Novartis says that, at the priority date, there was no formal training scheme for cardiologists in Australia in relation to heart failure. Novartis accepts that some cardiologists in Australia had an interest in heart failure. It says, however, that, while all heart failure was treated by cardiologists, not all cardiologists who treated heart failure had a special or exclusive interest in that area.
157 The patent specification is directed to those with a real, not a peripheral, interest in its subject matter, which is the development of effective therapy for the treatment of hypertension, the treatment or prevention of heart failure, and the treatment and prevention of myocardial infarction and its sequelae: p 8 of the specification.
158 I am satisfied that the “team” would include a cardiologist who has specialised knowledge and a specialised interest in the development of effective therapy for the treatment of hypertension. The “team” would also include a cardiologist who has specialised knowledge and a specialised interest in the development of effective therapy for the treatment or prevention of heart failure (I need say nothing about the treatment and prevention of myocardial infarction and its sequelae because it does not feature in this case). Given the “pooling” of knowledge within the “team”, it does not matter whether, in real life, there is a cardiologist who has specialised knowledge and a specialised interest in the development of effective treatment for both hypertension and heart failure. That, in my view, is a non-issue. Nor does it matter that, in real life, the pool from which such cardiologists can be drawn is small.
159 I accept that Professor Coats and Professor Hare are representative of the cardiologist on the “team”. For the purpose of defining the attributes of the person skilled in the art, it is their knowledge and skills that are relevant, not those of cardiologists (or other specialists) in the main, or of general practitioners, who just happen to treat hypertension or heart failure.
160 The parties also disagree about whether the “team” also includes a pharmaceutical scientist, a solid-state chemist, an organic chemist, a medicinal chemist, and a formulator.
161 I have little doubt that, as the specification is directed, in part, to pharmaceutical compositions—with claim 1 directed, specifically, to a pharmaceutical composition—a pharmaceutical scientist would be a member of the “team”. I accept that Professor Roberts is representative of such a scientist.
162 I am not persuaded that the specification is addressed, specifically, to a specialist solid-state chemist or specialist organic chemist, and that such persons are part of the “team”. However, I accept that aspects of the knowledge of such persons are relevant to informing the proper construction of claim 1. The evidence of Professor Steed, a solid-state chemist, and Professor White, an organic chemist, was relevant and admissible for that purpose, but not because they were members of the “team”. Their knowledge of chemistry, as relevant to the present case, was shared, substantially, by Professor Roberts, the pharmaceutical scientist.
163 I am not persuaded that a medicinal chemist is part of the “team”. The specification is not concerned with the synthesis of new molecules or variations to known molecules. In any event, no medicinal chemist gave evidence.
164 It is possible that a formulator would be part of the “team” for the purposes of other claims of the patent. However, claim 1 is not an invention for a formulation as such (beyond specifying the need for the pharmaceutical composition to include a pharmaceutically acceptable carrier). To the extent that evidence about formulations arose, it was given by Professor Roberts in his capacity as a pharmaceutical scientist.
The principles of construction
165 Despite the principles of construction applicable to patent specifications and patent claims being settled, the parties nevertheless advanced various statements of these principles in their submissions.
166 Novartis referred to a recent summary of some of the principles in MMD Australia Pty Ltd v Camco Engineering Pty Ltd [2024] FCAFC 38, particularly the following (at [18]):
…
(c) a patent specification should be given a purposive, not a purely literal, construction;
(d) a patent specification is not to be read in the abstract but is to be construed through the eyes of the person skilled in the art in the light of the common general knowledge and the art before the priority date: Jupiters at [67(ii)];
(e) the claims are to be construed in light of the specification as a whole, but the plain and unambiguous meaning of a claim cannot be varied or qualified by reference to the body of the specification: Welch Perrin & Co Pty Ltd v Worrel (1961) 106 CLR 588 at 610 (Dixon CJ, Kitto and Windeyer JJ);
(f) claims in a patent are not to be narrowed by reference to the preferred embodiment: Rehm Pty Ltd v Websters Security Systems (International) Pty Ltd (1988) 11 IPR 289 at 299 (Gummow J); and
(g) the preferred embodiment cannot be used to introduce into the definite words of a claim an additional definition or qualification of the patentee’s invention: Erickson’s Patent (1923) 40 RPC 477 at 491; Globaltech Corporation Pty Ltd v Australian Mud Company Pty Ltd [2019] FCAFC 162; (2019) 145 IPR 39 at [111] (Kenny, Robertson and Moshinsky JJ); Welch Perrin at 612.
167 Novartis also referred the following passage from AstraZeneca AB v Apotex Pty Ltd [2014] FCAFC 99; 226 FCR 324 (Apotex) at [100]:
100 The primary judge set out the principles relevant to the construction of claims of a patent in a manner which was not the subject of criticism by any of the parties to the appeals. Of particular significance was her Honour’s reference to Welch Perrin and Company Proprietary Limited v Worrel and Another (1961) 106 CLR 588 at 610 per Dixon CJ, Kitto and Windeyer JJ and the following passage from the decision of the Full Court of this Court in Kinabalu Investments Pty Ltd v Barron & Rawson Pty Ltd [2008] FCAFC 178 at [44]:
When determining the nature and extent of the monopoly claimed, the specification must be read as a whole. But as a whole it is made up of several parts which have different functions. The claims mark out the legal limits of the monopoly granted. The specification describes how to carry out the process claimed and the best method known to the patentee of doing that. Although the claims are construed in the context of the specification as a whole, it is not legitimate to narrow or expand the boundaries of monopoly as fixed by the words of a claim, by adding to those words glosses drawn from other parts of the specification. If a claim is clear and unambiguous, it is not to be varied, qualified or made obscure by statements found in other parts of the document. It is legitimate, however, to refer to the rest of the specification to explain the background of the claims, to ascertain the meaning of technical terms and resolve ambiguities in the construction of the claims.
168 Novartis emphasised the well-understood passage from Lord Diplock’s speech in Catnic Components v Hill & Smith Ltd [1982] RPC 183 at 243 that:
A patent specification should be given a purposive construction rather than a purely literal one derived from applying to it the kind of meticulous verbal analysis in which lawyers are too often tempted by their training to indulge. The question in each case is: whether persons with practical knowledge and experience of the kind of work in which the invention was intended to be used, would understand that strict compliance with a particular descriptive word or phrase appearing in a claim was intended by the patentee to be an essential requirement of the invention so that any variant would fall outside the monopoly claimed, even though it could have no material effect upon the way the invention worked.
169 Novartis accepted, nevertheless, Lord Hoffmann’s cautionary observation in Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2005] RPC 9 at [34] (accepted in, for example, GlaxoSmithKline Consumer Healthcare Investments (Ireland) (No 2) Ltd v Generic Partners Pty Ltd [2018] FCAFC 71; 264 FCR 474 at [108]):
“Purposive construction” does not mean that one is extending or going beyond the definition of the technical matter for which the patentee seeks protection in the claims. The question is always what the person skilled in the art would have understood the patentee to be using the language of the claim to mean. And for this purpose, the language he has chosen is usually of critical importance. The conventions of word meaning and syntax enable us to express our meanings with great accuracy and subtlety and the skilled man will ordinarily assume that the patentee has chosen his language accordingly. As a number of judges have pointed out, the specification is a unilateral document in words of the patentee’s own choosing. Furthermore, the words will usually have been chosen upon skilled advice. The specification is not a document inter rusticos for which broad allowances must be made. On the other hand, it must be recognised that the patentee is trying to describe something which, at any rate in his opinion, is new; which has not existed before and of which there may be no generally accepted definition. There will be occasions upon which it will be obvious to the skilled man that the patentee must in some respect have departed from conventional use of language or included in his description of the invention some element which he did not mean to be essential. But one would not expect that to happen very often.
170 Pharmacor drew attention to four principles. First, claims are to be construed in the context of the specification as a whole. Secondly, a claim’s function is to define a monopoly with precision with the object of limiting, not extending, the monopoly. Thirdly, the specification is a unilateral document in words of the patentee’s choosing. Fourthly, there is no warrant for adopting a method of construction that gives a patentee what it might wished to have claimed rather than what the words of the claim actually say.
A consequential question of construction
171 The construction of claim 1 is in dispute on one matter only—whether integers (i) and (ii) of the pharmaceutical composition are satisfied by a complex containing anionic valsartan, anionic sacubitril and pharmaceutically acceptable cations (such as sodium cations).
172 The resolution of this question is particularly important because the answer has consequences for several contested issues.
173 Each party advances a “simple” position on this question of construction, but has done so through lengthy submissions, even though they accept that, in this case, the question turns on the meaning of ordinary English words. Those words must, of course, be considered in the context of the specification as a whole as read through the eyes of the person skilled in the art possessing the common general knowledge at the priority date.
174 In this case, the differing positions of the parties was advanced through the competing views of Professor White on the one hand, and Professor Roberts and Professor Steed on the other, as to how the specification should be understood.
175 Novartis contends that claim 1 is to a pharmaceutical composition comprising:
(a) valsartan, or a pharmaceutically acceptable salt thereof;
(b) sacubitril or sacubitrilat, or pharmaceutically acceptable salts thereof; and
(c) a pharmaceutically acceptable carrier.
176 Novartis submits:
… Any pharmaceutical composition that comprises integers (a) to (c) above is within the claim, irrespective of whether it includes any other integers, and irrespective of the form of, or non-covalent association between, the sacubitril/at (or salt), valsartan (or salt), and carrier. … [A] “complex” (whether crystalline or amorphous) is one form of combination of integers (a) and (b).
177 Novartis submits that this construction is consistent with what I said when giving an interlocutory judgment in this proceeding: Novartis AG v Pharmacor Pty Limited [2023] FCA 804 (Novartis 1) at [13] – [19], especially at [17].
178 It is appropriate that I deal with that submission immediately. The relevant paragraphs of the reasons in Novartis 1 are:
13 Claim 1 is:
1. A pharmaceutical composition comprising:
(i) the AT 1-antagonist valsartan or a pharmaceutically acceptable salt thereof;
and
(ii) the NEP inhibitor N-(3-carboxy-1-oxopropyl)-(4S)-p-phenylphenylmethyl)-4-amino-2R-methylbutanoic acid ethyl ester or (2R,4S)-5-Biphenyl-4-yl-4-(3-carboxypropionylamino)-2-methyl-pentanoic acid or pharmaceutically acceptable salts thereof and a pharmaceutically acceptable carrier.
14 Plainly, claim 1 claims a composition which includes valsartan (or a pharmaceutically acceptable salt of valsartan) in combination with one of two identified NEP inhibitors (or a pharmaceutically acceptable salt of the chosen NEP inhibitor). The evidence before me is that the first identified NEP inhibitor—the ethyl ester—is known as sacubitril. The second identified NEP inhibitor is known as sacubitrilat.
15 Claim 1 does not specify, in terms, the particular dose of valsartan or of the NEP inhibitor in the composition. Further, it does not specify the molar or weight ratio between the two active ingredients or their ratio relative to the pharmaceutically acceptable carrier or any other component of the composition (it being noted that the complete specification makes clear that the word “comprising” is to be understood to imply inclusion, not exclusion).
16 Claim 1 does not limit, in terms, the salts that can be used in the composition (other than that they be pharmaceutically acceptable salts) or require that the same salt be used (if the two active ingredients are present as salts).
17 Claim 1 does not limit, in terms, the form of the two active ingredients (such as whether they must be in crystalline form, amorphous form or polymorphic form, or be present as hydrates, or have a supramolecular structure) or limit the way in which the two active ingredients are to be combined.
18 Claim 1 does not limit, in terms, the particular form of the pharmaceutical composition itself.
19 It is clear, however, that claim 1 is more limited in scope than the disclosures made in the complete specification by reference to the “invention”. Its limitation is to compositions that include valsartan (or a pharmaceutically acceptable salt thereof), one or other of the two identified NEP inhibitors (or their pharmaceutically acceptable salts), and a pharmaceutically acceptable carrier.
179 It is important to understand that, in Novartis 1, I was considering the interlocutory question whether discovery should be permitted in aid of Pharmacor’s challenge to the validity of the patent on the ground that, contrary to s 40(2)(a) of the Patents Act, the specification does not describe the best method of performing the invention. The proper construction of claim 1 was not directly before me, as it is now with the benefit of the expert evidence that has been adduced.
180 Moreover, the observation that I made at [17] of Novartis 1 was directed only to the structure of claim 1 and the absence of express words of limitation, as exemplified by Novartis in its submissions, in particular an express limitation such as “wherein the valsartan and sacubitril are combined in the form of a complex”. To note, as I did, that claim 1 does not limit, in terms, the form of the two active ingredients to a supramolecular structure does not mean that claim 1, properly construed, includes the two active ingredients synthesised in a supramolecular structure. I made no such finding and nothing that I said in Novartis 1 constrains my consideration of the proper construction of claim 1 now.
181 Novartis submits that the correct approach to construing claim 1 is to look at each integer (within the patent, integers (i) and (ii) (with integer (ii) read as I have indicated) and notional integer (iii)) and simply ask whether each integer is present in the pharmaceutical composition being considered. It contends that this approach is informed by the following considerations.
182 First, Novartis points to the fact that integer (i) refers to valsartan (or a pharmaceutically acceptable salt) as “the AT 1-antagonist” and that integer (ii) refers to sacubitril/at (and their pharmaceutically acceptable salts) as “the NEP inhibitor”. It submits that the significance of these descriptions is to make clear that the claim is concerned with the pharmacological activity of valsartan and sacubitril/at rather than: (a) the physical form in which each is present in the composition; or (b) the physicochemical properties of that form.
183 In other words, Novartis submits that claim 1 is directed to any pharmaceutical composition including a carrier that delivers valsartan and sacubitril/at as the relevant active agents or ingredients. It submits that the primary emphasis of the patent is on combinations of valsartan and sacubitril/at and the usefulness of that composition in the treatment of certain medical conditions.
184 Novartis submits that this approach to construction is consistent with the accepted approach of importing the requirement of efficacy into the notion of a “pharmaceutical composition”: Boehringer Ingelheim Animal Health USA Inc v Intervet International BV [2022] FCAFC 88; 166 IPR 468 at [96]; Sandoz AG v Bayer Intellectual Property GmbH [2023] FCA 1321 at [524].
185 Secondly, Novartis submits that valsartan and sacubitril/at can exist in their free acid forms or, optionally, as pharmaceutically acceptable salts. Relatedly, it draws attention to the specification’s teaching that the pharmaceutical composition of the invention can be prepared “in a known manner”, including as a solution: see, for example, pp 14 – 15 of the specification. It argues that if the pharmaceutical composition of claim 1 is in the form of a solution, then, in that solution, the pharmaceutically acceptable salts of the active ingredients will be present as anions with balancing cations, which are not ionically bound to each other. That being so, integers (i) and (ii) must comprehend anionic valsartan and sacubitril/at and, therefore, a complex.
186 Thirdly, Novartis submits that the use in claim 1 of the non-exhaustive term “comprising” means that other integers can be present, such as: (a) “wherein the valsartan and sacubitril are combined as a physical mixture”; (b) “wherein the valsartan and sacubitril are combined in the form of a complex”; (c) “wherein the valsartan and sacubitril are amorphous”; (d) “wherein the valsartan and sacubitril are crystalline”; (e) “wherein the valsartan and sacubitril are in the form of a particular polymorph”; (f) “wherein the salt of valsartan is a double salt”; or (g) “wherein the salt of sacubitril is a double salt”.
187 Fourthly, Novartis submits that there is nothing in claim 1 that requires valsartan or sacubitril/at to be in any particular form other than that, if it is in the form of a salt, it be a pharmaceutically acceptable salt. As Novartis puts it:
… All that is required for valsartan to be valsartan is that it not be covalently bonded to other atoms or molecules so as to become a new molecule that is different to valsartan. The same is true for each of sacubitril and sacubitrilat. In this regard, it is important to note that the experts had no difficulty in identifying each of valsartan and sacubitril/at as a molecule, either in its free acid or anionic form (or agnostic as to that distinction), as distinct from other molecules, in the evidence about Valtresto and Entresto.
188 Fifthly, and relatedly, Novartis submits that there is nothing in claim 1 which says that integer (i) cannot also be satisfied by something that satisfies integer (ii). For example, so far as the “pharmaceutically acceptable salt” aspect of integers (i) and (ii) is concerned, there is nothing in claim 1 that says that each integer cannot be embodied by cations that balance the charge of both anions. Should the sodium salt of each active ingredient be in contemplation, there is nothing in the claim that says that the sodium cations cannot be shared between the two ingredients. The pharmacological effects of the active ingredients do not depend on their particular physical form in the composition.
189 By way of summary, Novartis submits:
… Any pharmaceutical composition that comprises integers (i) to (iii) is within the claim, irrespective of whether it includes any other integers, and irrespective of the form of, or non-covalent association between the sacubitril/at (or salt), valsartan (or salt), and carrier. There is no limitation in the claim that excludes a pharmaceutical composition in which there are non-covalent bonds between the valsartan and sacubitril, in salt form or otherwise.
Claim 1 does not say, or require, that integers (i) to (iii) must each be discrete physical substances each with a unique set of physicochemical properties. Whether a pharmaceutical composition comprises each of integers (i) to (iii) is not answered by looking at how the composition was prepared, or by counting distinct ingredients or components used to make the composition, much less by counting salts.
190 Turning particularly to complexes, Novartis submits that a supramolecular complex of valsartan, sacubitril, and sodium, satisfies integers (i) and (ii). It submits that when this complex is included in a pharmaceutical composition with a carrier, the requirements of claim 1 are satisfied. It says:
The entire complex can accurately be described as a salt of valsartan, albeit in the form of a double salt with sacubitiril anions, and can accurately be described as a salt of sacubitril, albeit in the form of a double salt with valsartan anions. … Unlike a reaction that causes a covalent bond to form between two previously separate molecules, non-covalent bonds within a complex do not cause valsartan or sacubitril to cease to be molecules of valsartan or sacubitril.
191 Novartis submits that claim 1 should not be read down to exclude a complex. Further, recourse should not be had to the specification to find words of limitation that are not present in the claim itself.
192 Novartis nevertheless calls in aid the specification’s reference on p 13 to the teaching that “components (i) and (ii) can be obtained and administered together”, including in a “fixed combination”. Novartis submits that these words mean that the combination can include a complex and that the word “together” means “together in any way”. Analysis
193 I do not accept Novartis’s construction of claim 1.
194 Claim 1 is a product claim—a pharmaceutical composition. It is not a method claim—such as a method of using an identified API, or identified APIs, in the manufacture of a medicament (as to which see claims 6 to 9).
195 The composition is defined by identified components, having their identified pharmacological activities, in the state in which those components exist in the composition itself—meaning, in relation to the APIs:
(valsartan or a pharmaceutically acceptable salt of valsartan) + ([sacubitril or a pharmaceutically acceptable salt of sacubitril] or [sacubitrilat or a pharmaceutically acceptable salt of sacubitrilat]) .
196 Although, speaking generally, this composition can be seen as a combination, it is only a combination in the sense that it comprises more than one component. Claim 1 does not use the word “combination” (cf. claim 4), and does not claim a combination in the sense in which that term is sometimes used in patent law, particularly in the context of mechanical inventions, to describe a working interrelationship of known parts that produces something that is new and different from the separate working of those parts. The invention of claim 1 is not defined in terms that implies that integers (i) and (ii) are combined to produce a new chemical entity.
197 Importantly, the composition is not defined by: (a) simply the intended pharmacological activity of each of its components; (b) the form of any substance from which each of its components is derived; or (c) the state of its components after the composition has been administered.
198 So understood, there is no ambiguity in the way in which the pharmaceutical composition is claimed. Claim 1 does not claim, through integers (i) and (ii), a complex formed from valsartan and sacubitril/at or their pharmaceutically acceptable salts. Indeed, to read claim 1 as if it did would be to ignore its clear terms.
199 As explained, a complex is a unique, single entity with its own properties. A complex formed from valsartan and sacubitril/at or their pharmaceutically acceptable salts is not: (a) valsartan or a salt of valsartan; (b) sacubitril or a salt of sacubitril; or (c) sacubitrilat or a salt of sacubitrilat. In short, it is none of the things referred to in claim 1.
200 To support its construction of claim 1, Novartis points to various uses of the word “salt” by Pharmacor’s experts Professor Roberts and Professor Steed, particularly in their affidavit evidence when discussing Entresto and Valtresto, to contend that the words “pharmaceutically acceptable salts thereof”, as used in claim 1, can be read as referring to “salts” of valsartan and sacubitril/at in a salt complex involving valsartan anions and sacubitril/at anions, ionically bonded to, and counterbalanced by, sodium cations to achieve overall electroneutrality.
201 There is, in some parts of Professor Roberts’s and Professor Steed’s affidavits, use of the word “salt” in a somewhat loose sense which lends aid to Novartis’s contention. However, Professor Roberts’s and Professor Steed’s affidavits should not be read with an eye focused on linguistic analysis. They should be read with an eye that discerns their substance.
202 Overall, I am satisfied that, in the passages on which Novartis relies, each was using “salt” to refer to a constituent in a complexing process to explain why particular anions were present in a resultant complex. Professor Roberts and Professor Steed, both in their affidavit evidence, and in their oral evidence, particularly in cross-examination, remained steadfast in their respective, independent opinions that TSVH and SVT1 are single entities and do not comprise distinct salts of valsartan and sacubitril/at.
203 The construction of claim 1 which I accept is supported by the passages on pp 13 and 14 of the specification to which I have drawn attention: see [121] and [123] above.
204 The passage on p 13 talks of components (i) and (ii) as separate components which can be administered “together” or “separately”. The word “together” cannot be used to broaden the construction of claim 1 beyond its ordinary language to include a complex formed from those components. This would be an impermissible use of the specification to place a gloss upon the words of the claim itself. In any event, the passage on p 13 makes clear the intended meaning of “together”. That meaning is “one after the other”, thereby supporting the separate identities of components (i) and (ii) as chemical entities.
205 The word “separately” in this passage also supports the construction of claim 1 which I accept. The relevant phrase is “separately in one combined unit dose form”, meaning that while components (i) and (ii) are in a combined unit dose, they are nevertheless, in that dose form, separate from one another. The passage makes clear that this dose form may be a fixed combination, meaning that that component (i) and component (ii) are present in a fixed amounts.
206 The passage on p 13 also envisages administration of component (i) and component (ii) in “two separate unit dose forms”. Once again, this supports the separateness, in administration, of component (i) and component (ii) and hence the separate chemical identities of the components. When read in the context of the whole passage, this part appears to contemplate the possibility that the separate unit dose forms may not be administered “together” (i.e., in the sense of one after the other), but at different times. Once again, the separate unit dose forms can be fixed dose forms.
207 The passage on p 14 is consistent with all these possibilities. A “therapeutically effective amount” of “each” component of the combination may be administered “simultaneously or sequentially and in any order”.
208 The relevant experts agree that the administration described in the passages on pp 13 and 14 specifies the separate dosing of components (i) and (ii).
209 In my view, the passages on pp 13 and 14 cannot be read, sensibly, to refer to the administration of a complex, which is a single chemical entity. What is more, the relevant experts agree that the pharmaceutical composition of claim 1 includes two APIs or their pharmaceutically acceptable salts. The disagreement between Professor Roberts and Professor Steed on the one hand, and Professor White on the other, is that Professor White argues that, in claim 1:
… there is no restriction that I can see regarding how the two components Valsartan and Sacubitril or their pharmaceutically acceptable salts may or may not be combined in order to prepare the claimed pharmaceutical composition.
210 The difficulty with this explanation, and Professor White’s particular reading of claim 1, is that it is directed to combining components (i) and (ii) to prepare a pharmaceutical composition. It is not directed to the state of components (i) and (ii) when in the pharmaceutical composition. Put another way, Professor White’s explanation conflates the preparation of the pharmaceutical composition with the pharmaceutical composition as prepared. Only the latter is claimed in claim 1. Further, when examining and describing a complex, such as TSVH, Professor White’s approach was to view the components that were combined to make the complex and to look to the roles that each component played in forming the complex.
211 In relation to TSVH, Professor White also said:
…The presence of Valsartan and Sacubitril as their sodium salts in the complex is demonstrated when the solid complex is dissolved in water resulting in a solution containing these both (sic) components whose identities can be demonstrated from solution NMR spectroscopy.
212 This, however, refers to the dissociation of the complex in solution. It is not referring to the state of TSVH in the tablet form of Entresto. As I have said, claim 1 does not address what happens to the components of the composition once it has been administered.
213 As I have noted, Novartis relies on the specification’s teaching that the pharmaceutical composition of the invention can be prepared as a solution. The relevant passage on p 14 is:
The pharmaceutical compositions according to the invention can be prepared in a manner known per se and are those suitable for enteral, such as oral or rectal, and parenteral administration to mammals (warm-blooded animals), including man, comprising a therapeutically effective amount of the pharmacologically active compound, alone or in combination with one or more pharmaceutically acceptable carriers, especially suitable for enteral or parenteral application. Typical oral formulations include tablets, capsules, syrups, elixirs and suspensions. Typical injectable formulations include solutions and suspensions.
214 There are a number of things to be said of this passage.
215 First, the passage says that pharmaceutical compositions according to the invention “can” be prepared in the identified known ways, not that each composition of the described invention must be capable of being prepared in each of those known ways. Novartis gives more work for this passage to perform than its true meaning permits.
216 Secondly, it is not incumbent on a patentee to claim as an invention everything that it describes in the body of the specification as its invention or as an aspect of its invention. The patentee may elect to claim, as an invention, something that is narrower in scope than its description in the body of the specification. A patentee might do so, for example, out of concern that, by claiming too broadly, it might expose its patent to attack—for example, on the ground that what it has claimed as an invention is not novel or on the ground that what it has claimed as an invention lacks utility.
217 Thirdly, and relatedly, whilst a claim must be construed in the context of the specification as a whole, the description in the specification cannot drive the construction of the claims as the patentee has chosen to express them. This is the trite proposition that it is not legitimate to narrow or expand the boundaries of the monopoly, as fixed by the words of a claim, by adding glosses drawn from other parts of the specification. There is nothing in claim 1 that requires the pharmaceutical composition, as there claimed, to be in the form of a solution or to be a pharmaceutical composition that is suitable for administration in a solution.
218 As I have noted, to support its contention that integers (i) and (ii) of the pharmaceutical composition of claim 1 are satisfied by a complex containing anionic valsartan, anionic sacubitril and pharmaceutically acceptable cations (such as sodium cations), Novartis argues that if the pharmaceutical composition of claim 1 is in the form of a solution, then, in that solution, the pharmaceutically acceptable salts of the active ingredients will be present as anions with balancing cations, which are not ionically bound to each other. This is an overstatement.
219 Whether the pharmaceutically acceptable salts of valsartan and sacubitril/at dissociate in solution depends on the solvent. I have previously drawn attention to the reference in Example 8 to the vehicle (the pharmaceutically acceptable carrier) including the polymeric organic solvent PEG 400 and the surfactant Tween 80.
220 The relevant experts agreed that, in PEG 400, valsartan and sacubitril remain as free acids with no significant ionisation of the free acids to anionic valsartan or anionic sacubitril. They also agreed that an example of a pharmaceutical composition that falls within claim 1 is a solution that contains, as the first component, valsartan and, as the second component, sacubitril, with a pharmaceutically acceptable carrier, PEG 400, as the third component.
221 Therefore, even though there is nothing in claim 1 that requires the pharmaceutical composition, as there claimed, to be in the form of a solution or to be suitable for administration in a solution, a pharmaceutical composition in accordance with claim 1, as I have construed it, can be prepared in the form of a solution. It simply depends on the choice of an appropriate solvent.
222 However, to be clear, the pharmaceutical composition of claim 1 cannot be in the form of a solution in which the pharmaceutically acceptable salts of valsartan and sacubitril/at have dissociated into anionic valsartan and anionic sacubitril/at. This is because, in that solution, the salts no longer exist as such. The anions of valsartan and sacubitril/at are not bound to cations.
223 To construe claim 1 as I have is not to “exclude” a complex. It is simply to recognise that, by claiming the pharmaceutical composition in the way it has, Novartis has claimed a composition in which the component of integer (i) and the component of integer (ii) are each present as separate components. One cannot “exclude” that which has not been claimed.
224 Finally, it should be noted that, apart from claims 2 and 3 which are dependent on claim 1, the remaining claims of the patent are not dependent on claim 1. Neither the method of treatment claims nor the Swiss type claims require the involvement of a pharmaceutical composition according to claim 1.
225 As I have recorded, SVT1 is an amorphous complex of valsartan anions, sacubitril anions, and sodium cations, present in a ratio of 1:1:3. This is the form in which SVT1 is present in Valtresto after it has been prepared as a medicament, and before it is administered. The relevant experts agreed that it is most likely that SVT1 is a single salt.
226 Pharmacor submits that, as claim 1 does not cover a complex, such as SVT1, its intended exploitation of Valtresto cannot be a threatened infringement of claim 1. Valtresto comprises one salt—the complex SVT1—not the two separate salts required by components (i) and (ii) of claim 1.
227 Novartis disputes this.
228 First, Novartis relies on a construction of claim 1 that I do not accept.
229 Secondly, it contends that, by para 9(c) of its defence, Pharmacor has made an admission. In para 9(c), Pharmacor pleads:
… the active ingredient in the Pharmacor Products comprises an amorphous mixture of sacubitril, valsartan and sodium in a single unit dosage entity associated by non-covalent bonds …
230 Novartis contends that this is an admission that Valtresto contains sacubitril (or a salt) and valsartan (or a salt)—meaning, two salts. Novartis submits that this is consistent with para 53 of the Joint Expert Report on the topic of complexes and that Pharmacor has not sought to withdraw, and should not be permitted to withdraw, this submission. Novartis submits:
… despite the debate about salts in relation to the construction of claim 1, the only issue on the pleadings for the purpose of infringement appears to be whether the claim is limited to valsartan having to be physically separate from sacubitril (and not on whether there are one or two salts present). Novartis has conducted its case accordingly.
231 Novartis points to a presentation which Professor Steed prepared for the purpose of giving evidence in overseas proceedings involving Entresto: see Ex M. In that presentation, Professor Steed referred to a complex as:
… a material combining two different active ingredients that are non-covalently bonded.
232 When cross-examined on this presentation, Professor Steed said that he had used the expression “active ingredients” loosely and that, by these words, he was intending to mean “active agents”, to refer to the different pharmacological effects of valsartan and sacubitril. Professor Steed said that he did not “mean to imply that [Entresto was] made of two different physical substances, it’s one physical substance.”
233 Thirdly, Novartis contends that the documentary evidence—which includes the product information (PI) for Valtresto, Pharmacor’s documents, and correspondence with the Australian Therapeutic Goods Administration (TGA)—makes clear that Valtresto is a pharmaceutical composition that contains “two active ingredients in combination”, sacubitril and valsartan. It submits that whether a complex can be described as a single entity or a distinct substance is irrelevant because the only question is whether STV1 comprises sacubitril, valsartan, and a carrier, as claimed in claim 1. Novartis submits:
… The correct resolution of that question is that Valtresto (like Entresto) comprises a complex (which might be termed a double salt) of the active ingredients sacubitril and valsartan.
234 Novartis’s submissions contain references to part of the evidence that are covered by orders made under s 37AF(1) of the Federal Court of Australia Act 1976 (Cth). It is not necessary for me to refer, in terms, to that evidence in order to make my reasons comprehensible on this topic. It is sufficient for me to note that the evidence refers to Valtresto containing two salts or two drug substances. The PI for Valtresto refers to sacubitril and valsartan as the therapeutically active ingredients.
235 Novartis submits that the documentary evidence is inconsistent with Valtresto not comprising sacubitril (or a salt thereof) and valsartan (or a salt thereof).
236 Notwithstanding these submissions, Novartis accepts, as it must, that the resolution of the question of infringement follows from the construction of claim 1.
237 Given the construction of claim 1 that I have found, Novartis’s case on infringement fails at the outset, regardless of the validity of claim 1 or whether the term of the patent was validly extended. I do not find its submissions on the question of infringement to be persuasive. Indeed, I regard some of its submissions to be playing with words in a way that ignores the substance of the expert evidence, particularly in the Joint Expert Report on complexes.
238 First, it is not at all clear that Pharmacor has made any admission, let alone one that assists Novartis. Pharmacor contends that it has not made an admission, simply an allegation. This contention is, itself, unpersuasive. The true point is that para 9(c) of Pharmacor’s defence speaks of one active ingredient—an amorphous mixture of sacubitril, valsartan and sodium as a single unit dosage entity associated by non-covalent bonds. It may be that Pharmacor’s use of “mixture” is not apposite. But one should not ignore the substance of what is advanced in para 9(c), which is that, in Valtresto, there is one active ingredient. The active ingredient is sacubitril, valsartan and sodium as a single entity associated by non-covalent bonds—in other words, a complex—not two active ingredients that are separate salts of sacubitril and valsartan.
239 If, contrary to my understanding, para 9(c) of the defence is to be taken as “admitting” that Valtresto contains two separate salts of sacubitril and valsartan, then I would not accept that admission. It is contrary to the evidence.
240 What is more, I am unpersuaded by Novartis’s plea as to the way in which it says it has conducted its case. The nature of complexes; whether claim 1 of the patent covers a complex; and whether STV1 is a complex or comprises two separate salts (being a salt of sacubitril and a salt of valsartan), are issues that have been at the front and centre of this case in the filed evidence and throughout the hearing. They are issues that have been addressed, painstakingly, in the evidence that the parties have adduced.
241 As to this, I do not accept Novartis’s contention that the Joint Expert Report on complexes is consistent with Valtresto containing two salts. To be clear, the paragraph in the Joint Expert Report on which Novartis relies is this:
53. Valtresto is a film coated tablet drug product for the treatment of chronic heart failure in patients with a reduced ejection fraction. It comes in three dosage strengths and comprises an amorphous complex combination of two APIs Sacubitril and Valsartan pharmaceutically acceptable salts), termed SVT1 and excipients. SVT1 is an amorphous complex involving Valsartan anions, Sacubitril anions and sodium cations in a 1:1:3 ratio. It is a single entity which the Valtresto PI states has an IUPAC chemical name of sodium 4-[[(2S,4R)-5-ethoxy-4-methyl-5-oxo-1-(4-phenylphenyl)pentan-2-yl]amino]-4-oxobutanoate and disodium (2S)-3-methyl-2-[pentanoyl[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoate, a molecular formula of C44H55N6O8Na3, a relative molecular mass of 912.96 mol/g, and is depicted by a structural diagram with square brackets containing the three of anions of Sacubitril and Valsartan and three sodium ions in balance, as well as a dot between the Sacubitril and Valsartan, indicating that they are present as a complex. The CAS number 936623-90-4 describes a hydrate and so is not relevant to SVT1. The complex nature of the material is consistent with the DSC data which shows a single glass transition.
242 When this paragraph refers to SVT1 as “an amorphous complex combination of two APIs Sacubitril and Valsartan pharmaceutically acceptable salts”, it is referring to the two APIs being combined into a single entity—a complex. In other words, it is referring to two inputs in the complexing process. As the quoted paragraph makes clear, that entity has a single chemical name, a single relative molecular mass, a structural diagram showing the three anions of sacubitril and valsartan in balance with three sodium ions, and a single glass transition. The next paragraph in the Joint Expert Report makes clear that this single entity is not a mere physical mixture of a crystalline salt of sacubitril and a crystalline salt of valsartan, which would be expected to show two peaks corresponding to the melting point of the two separate materials.
243 The evidence shows that the terms “active agents” and “active ingredients” have not always been used with appropriate precision (when required). But this does not mean that the substance of the evidence should not be grasped and that one should retreat to infelicities of language as the means by which the question of infringement should be determined.
244 I accept, for example, Professor Steed’s evidence that the reference in his presentation (see [231] above) to “two different active ingredients” was “loose” language. However, to be fair to Professor Steed, the context in which he made that reference was to Entresto as a complex, and the combining of ingredients to make a material that is non-covalently bonded. I do not understand him to have been saying, in this presentation, that Entresto, as a pharmaceutical product, contains two separate salts of valsartan and sacubitril. Indeed, his presentation refers to, and depicts, anionic valsartan and anionic sacubitril bonded, non-covalently, with sodium cations, as a single entity.
245 I do not accept that the regulatory documents concerning Valtresto have the weight that Novartis places on them. True it is, they refer to Valtresto as having two salts, or two drug substances, or as being “valsartan and sacubitril”. But these references cannot carry the day. The perspectives from which those statements were made is not clear. Were they made from the perspective of looking at the substances used to make to the product (as opposed to the state of those substances in the product)? Were they made from the perspective of looking to the role or activity of substances after the product has been administered to the human patient for its intended therapeutic effects?
246 Apart from these imponderables, the statements on which Novartis relies cannot have precedence in fact-finding over the evidence which identifies, with precision, the nature of STV1 in Valtresto as a finished product before its administration to a human patient.
247 This brings me back to the different views of Professor White on the one hand, and Professor Roberts and Professor Steed on the other, as to the meaning of claim 1. Those different views resonate in the respective views of Professor White, Professor Roberts, and Professor Steed on whether STV1 comprises two salts.
248 All relevant experts agreed that claim 1 requires two APIs or their pharmaceutically acceptable salts. All relevant experts joined in agreeing on what Valtresto is, and what STV1 is, as quoted above. However, Professor White said:
Valtresto comprises an amorphous complex which forms due to the unique properties of the sodium salt of Sacubitril and the sodium salt of Valsartan when in combination. Hence, I prefer to consider the complex as being a combination of the sodium salts of the two APIs Sacubitril and Valsartan. The presence of dianionic Valsartan and anionic Sacubitril which is present in this material as their sodium salts can demonstrate if the complex is dissolved in water which results in a solution containing these both components which can be demonstrated from solution NMR spectroscopy.
249 I am not persuaded by this point of view. It is based on the same reasoning I did not accept in respect of the construction of claim 1. The above passage conflates the preparation of STV1 and the state of STV1 in Valtresto as a finished product. It also addresses the irrelevant question of what happens to STV1 after Valtresto is administered to a human patient.
250 STV1 is not: (a) valsartan or a pharmaceutically acceptable salt of valsartan; (b) sacubitril or a pharmaceutically acceptable salt of sacubitril; or (c) sacubitrilat or a pharmaceutically acceptable salt of sacubitrilat. Nor is STV1 (valsartan or a pharmaceutically acceptable salt of valsartan) + ([sacubitril or a pharmaceutically acceptable salt of sacubitril] or [sacubitrilat or a pharmaceutically acceptable salt of sacubitrilat]).
251 Novartis’s case on infringement is not established.
252 Section 70 of the Patents Act relevantly provides:
70 Applications for extension of patent
(1) The patentee of a standard patent may apply to the Commissioner for an extension of the term of the patent if the requirements set out in subsections (2), (3) and (4) are satisfied.
(2) Either or both of the following conditions must be satisfied:
(a) one or more pharmaceutical substances per se must in substance be disclosed in the complete specification of the patent and in substance fall within the scope of the claim or claims of that specification;
(b) one or more pharmaceutical substances when produced by a process that involves the use of recombinant DNA technology, must in substance be disclosed in the complete specification of the patent and in substance fall within the scope of the claim or claims of that specification.
(3) Both of the following conditions must be satisfied in relation to at least one of those pharmaceutical substances:
(a) goods containing, or consisting of, the substance must be included in the Australian Register of Therapeutic Goods;
(b) the period beginning on the date of the patent and ending on the first regulatory approval date for the substance must be at least 5 years.
Note: Section 65 sets out the date of a patent.
(4) The term of the patent must not have been previously extended under this Part.
…
253 The Dictionary in the Patents Act defines a “pharmaceutical substance” as follows:
“pharmaceutical substance” means a substance (including a mixture or compound of substances) for therapeutic use whose application (or one of whose applications) involves:
(a) a chemical interaction, or physico-chemical interaction, with a human physiological system; or
(b) action on an infectious agent, or on a toxin or other poison, in a human body;
but does not include a substance that is solely for use in in vitro diagnosis or in vitro testing.
254 In Commissioner of Patents v Ono Pharmaceutical Co. Ltd [2022] FCAFC 39; 291 FCR 1 (Ono), the Full Court noted (at [115] – [116]) the policy objectives of the extension of term regime in Pt 3 of Ch 6 of the Patents Act:
115 Whilst it may be accepted that the object of the extension of term regime is to compensate a patentee of a pharmaceutical substance for time lost in obtaining regulatory approval before it can exploit its invention, it does not follow that ss 70, 71, and 77 should be construed so as to achieve what might be described as a commercial outcome for a patentee. As the High Court noted in Alphapharm, and as his Honour appeared to accept, the extension of term regime seeks to balance a range of competing interests, not just the interests of the patentee. It can be taken that the legislature saw the correct balance as being achieved by the very words it chose to implement that regime.
116 It is, of course, the fundamental duty of a court, when undertaking statutory construction, to give meaning to the legislative command according to the terms in which it has been expressed: Northern Territory of Australia v Collins [2008] HCA 49; 235 CLR 619 at [16]; Alcan (NT) Alumina Pty Ltd v Commissioner of Territory Revenue (Northern Territory) [2009] HCA 41; 239 CLR 27 at [47]; Alphapharm at [42]. Hence, the importance of recognising, as the cases stress, that the task of statutory construction is, essentially, a text-based activity. Here, the balance which the legislature has chosen to strike for the competing interests is clear from the language it has chosen. That balance should not be altered by recourse to a process of reasoning that prefers “a liberal rather than a literal construction”.
255 The Full Court then addressed the requirements of s 70 which, it said, are expressed by reference to three inquiries:
118 The first inquiry is specified in s 70(2). One or more pharmaceutical substances per se, or one or more pharmaceutical substances produced through a process that involves the use of recombinant DNA technology, must, in substance, be disclosed in the complete specification of the patent and, in substance, fall within the scope of the claim or claims of the specification.
119 To emphasise the obvious, s 70(2) recognises that one or more pharmaceutical substances might be involved in the inquiry. Whether one or more substances (and, if more than one, how many substances) are involved, is a matter of objective determination by reference to the complete specification and the claim or claims of the patent in question. This inquiry is not directed to goods that contain, or consist of, the substance or substances. It is directed to the disclosure and claiming of a substance or substances as such.
120 The second inquiry is specified in s 70(3). It takes the one or more pharmaceutical substances identified in the s 70(2) inquiry and asks whether at least one of those substances satisfies each condition of ss 70(3)(a) and (b). Although ss 70(3)(a) and (b) are directed, in terms, to “the substance”, the chapeau to s 70(3) recognises, once again, that one or more substances might be involved in this inquiry. By referring to “at least one of those pharmaceutical substances”, the chapeau establishes a minimum.
121 The first condition (s 70(3)(a)) is that goods containing, or consisting of, the substance must be included in the ARTG. Once again, this is a matter of objective determination. It concerns the state of the ARTG. As such, it looks to all relevant goods. The inquiry is not restricted to the goods of a particular person. The condition does not ask by whom, or on whose behalf, the inclusion was sought or obtained.
122 The second condition (s 70(3)(b)) is that the period beginning on the date of the patent (see s 65 of the Patents Act) and ending on the “first regulatory approval date” for the substance must be, at least, 5 years. This, too, is a matter of objective determination. The definition in s 70(5) of the “first regulatory approval date” sets out the machinery for identifying that date with specificity. The definition recognises that there might be multiple entries in the ARTG for goods that contain, or consist of, a pharmaceutical substance that satisfy the conditions of s 70(2) and s 70(3)(a).
123 If there is one pharmaceutical substance that satisfies the conditions of s 70(2) and s 70(3)(a), the “first regulatory approval date” is the date of commencement of the first inclusion in the ARTG of goods that contain, or consist of, that substance. However, in a given case, there might be multiple pharmaceutical substances that satisfy s 70(2) and s 70(3)(a) and, therefore, potentially, multiple entries in the ARTG in respect of each of those substances. Amongst all these possible entries, s 71(2)(b) takes the “first regulatory approval date” of “any of the pharmaceutical substances”, meaning the first regulatory approval date taken from the set of entries in the ARTG of the pharmaceutical substances that satisfy the conditions of ss 70(2) and (3).
124 The pharmaceutical substances that satisfy both conditions of s 70(3) can be conceptualised as a subset of the pharmaceutical substances that satisfy the conditions of s 70(2), recognising, of course, the possibility that, in a given case, only one substance might satisfy either or both of the conditions of s 70(2) and no substance might satisfy both conditions of s 70(3). In practice, there will be permutations. Section 70(3) calls for the consideration of all substances, and all goods that contain, or consist of, those substances in the particular case at hand.
125 The third inquiry is whether the term of the patent has been previously extended under Ch 6 Pt 3: see s 70(4). This inquiry is uncomplicated. But it, too, is a matter of objective determination.
126 The balance of the extension of term provisions operate on the state of affairs determined, objectively, by reference to the requirements or “conditions” of s 70.
256 The requirement in s 70(2)(a) of “in substance” disclosure has been treated as akin to the test of fair basis—whether there has been a “real and reasonably clear disclosure” of the substance: Pfizer Inc v Commissioner of Patents [2005] FCA 137; 141 FCR 413 (Pfizer) at [75].
257 The requirement in s 70(3) takes one or more of the pharmaceutical substances identified in the s 70(2) inquiry and asks whether at least one of those substances satisfies each condition of ss 70(3)(a) and (b).
258 As regards s 70(3)(a), the inquiry is the factual one of whether the nominated goods, included in the ARTG, contain or consist of the disclosed and claimed “pharmaceutical substance per se”—in other words, is the “pharmaceutical substance per se” an “ingredient” of the nominated goods? The inquiry does not look to the therapeutic effect of the pharmaceutical substance: H Lundbeck A/S v Alphapharm Pty Ltd [2009] FCAFC 70; 177 FCR 151 (Lundbeck) at [239]. Nor is the factual inquiry bounded by what the ARTG, itself, discloses about the goods.
259 Section 70(3)(a) looks, firstly, to whether goods contain or consist of the pharmaceutical substance(s) identified in the s 70(2) inquiry. If the answer is “yes”, the next question is whether those goods are included in the ARTG. The answer to this question is a simple “yes” or “no”. No further interrogation of the ARTG is necessary.
The application to extend the term
260 On 10 June 2016, Novartis AG applied to extend the term of the patent (the extension application). The extension application referred to the “pharmaceutical substance per se” as “a combination of sacubitril and valsartan”.
261 The extension application said that the “pharmaceutical substance per se” was, in substance, disclosed in the specification because:
a pharmaceutical composition comprising the AT-1 antagonist valsartan or a pharmaceutically acceptable salt thereof and the NEP inhibitor N-(3-carboxy-1- oxopropyl)-(4S)-p-phenylphenylmethyl)-4-amino-2R-methylbutanoic acid ethyl ester or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier is disclosed in the patent specification, for example at page 2A. See also attached annex.
262 The extension application was accompanied by a number of documents. Although not marked as such, these documents appear to be the “attached annex” referred to in the application. The reference to “page 2A” of the specification, appears to be the passage quoted at [111] above.
263 The extension application said that the “pharmaceutical substance per se” fell within the scope of the claims because:
a pharmaceutical composition comprising valsartan and sacubitril and a pharmaceutically acceptable carrier is claimed in the patent, for example in claim 1. See also attached annex.
264 The extension application said that the goods containing, or consisting of, the pharmaceutical substance included in the ARTG are:
ENTRESTO sacubitril/valsartan (combined as a sodium salt hydrate complex).
265 The extension application identified the first regulatory approval date as 20 January 2016.
266 As I have recorded, the term of the patent was extended on 5 December 2016 on the basis of this application.
Was the extension of term validly granted?
267 Pharmacor challenges the validity of the extension of term on the basis that one or both of ss 70(2)(a) and (3)(a) of the Patents Act were not satisfied. It contends that, if its construction of claim 1 is accepted, the extension application failed to satisfy s 70(3) of the Patents Act. If, alternatively, this construction is not accepted, the extension application failed to satisfy s 70(2)(a).
268 As to the twin requirements of s 70(2)(a), Pharmacor submits that the “pharmaceutical substance per se” is (a) valsartan or a pharmaceutically acceptable salt thereof; (b) sacubitril or a pharmaceutically acceptable salt thereof; and (c) a pharmaceutically acceptable carrier. This is the pharmaceutical composition disclosed in the specification and claimed in claim 1. If this be accepted, the extension application satisfied s 70(2)(a).
269 If, however, claim 1 is construed so that it covers a complex, Pharmacor submits that the extension application did not satisfy s 70(2)(a) because there was no “in substance” disclosure (i.e., no real and reasonably clear disclosure) of the pharmaceutical substance TSVH. As I have said, the relevant experts agreed that the specification does not envisage or mention TSVH.
270 As to s 70(3)(a), Pharmacor submits that, if its construction of claim 1 is accepted, Entresto does not contain or consist of: (a) valsartan or a pharmaceutically acceptable salt thereof; (b) sacubitril or a pharmaceutically acceptable salt thereof; and (c) a pharmaceutically acceptable carrier. This is because TSVH is a complex, which is one salt, not two salts. Therefore, Entresto does not contain (a) or (b) and, for this reason, s 70(3)(a) was not satisfied.
Novartis’s submissions
271 Novartis submits, firstly, that Pharmacor’s challenge should be dismissed because: (a) claim 1 is to a “combination” of sacubitril (including as a salt) and valsartan (including as a salt) with a carrier; and (b) the actives of Entresto, as recorded in the ARTG and in the PI for Entresto, are sacubitril and valsartan (which also contains a carrier).
272 As to (a), Novartis submits that “a combination of sacubitril and valsartan per se” is disclosed in the specification and, in substance, falls within the scope of claim 1.
273 It submits that this “combination” is a “pharmaceutical substance”, as defined for the purposes of s 70, because it is a “mixture or compound” of two pharmacologically active agents (i.e., an AT 1-antagonist and an NEP inhibitor). Novartis submits that the particular form of the therapeutically active compounds (i.e., sacubitril and valsartan) does not change the identity of the “combination” as a pharmaceutical substance. Novartis submits, therefore, that the absence of non-covalent bonds between the two agents, and the indication that they have discrete pharmacological effects, means that this “mixture or compound of substances” is not a single “substance” for the purposes of the Act.
274 As to (b), Novartis submits that Entresto also contains or consists of a “combination” of two therapeutically active “ingredients”. It submits that the presence of non-covalent bonds in the complex does not alter that fact, or cause valsartan and sacubitril to “lose their identities as distinct molecules and distinct active agents”.
275 Secondly, Novartis submits that the ARTG and Entresto’s PI “conclusively determine the s 70(3) question” in its favour. Novartis relies on the ARTG certificates for Entresto which refer to it as “sacubitril/valsartan (combined as a sodium salt hydrate complex)”. The PI for Entresto says that the “name of the medicine” is “sacubitril and valsartan” and that Entresto tablets contain sacubitril and valsartan “where both drug substances are combined as a sodium hydrate salt complex”. The PI describes Entresto’s mechanism of action as simultaneously inhibiting neprilysin via sacubitrilat as the active metabolite of sacubitril, and by blocking the AT 1-receptor via valsartan.
276 The PI also refers to Entresto’s active ingredient as:
A salt complex of the anionic forms of sacubitril and valsartan, sodium cations and water molecules in the molar ratio of 1:1:3:2.5 respectively
277 I observe that this is a single active pharmaceutical ingredient. The PI also gives Entresto a single chemical name, with a single molecular formula, and a single relative molecular mass. The PI does, however, depict the chemical structure of the complex with the sodium salt of sacubitril and the disodium salt of valsartan.
278 The PI gives a “CAS number”—936623-90-4 for the active ingredients. This is a reference to an entry in the database called the Chemical Abstracts Service. Novartis relies on the entry because, according to it, the entry shows valsartan and sacubitril “compounded” with two other components.
279 The CAS entry was introduced through Professor White’s first affidavit. The entry includes the abbreviation “compd”. So far as I can see, Professor White did not explain this abbreviation. In cross-examination, Professor Roberts expressed his understanding that, ordinarily, “compd” is an abbreviation of “compound”. However, he did not understand the use of that abbreviation in the CAS entry because TSVH is not an organic compound. It is a complex.
280 I should make clear that nothing in Professor White’s evidence indicates that he considered TSVH to be an organic compound. He agreed that TSVH is a single crystalline complex material comprising a repeating unit of 18 sodium cations, 6 sacubitril anions, 6 valsartan anion and 15 water molecules in a coordinated complex arrangement involving ionic bonding, hydrogen bonding and a range of other non-covalent properties.
281 Novartis also relies on the Australian Public Assessment Report (AusPAR) for Entresto published by the TGA in September 2016. The report refers to Entresto as a “sacubitril/valsartan salt complex” and as a “new combination product”. It also refers to Entresto as a “combination product of the two active ingredients” also known as “LCZ696” (this appears to be a reference to the TSVH complex).
282 Novartis refers to these matters to advance a submission that the Secretary of the Department of Health (the Secretary) registered Entresto on the basis that: (a) TSVH is a combination of sacubitril and valsartan; (b) that sacubitril and valsartan exert distinct pharmacological effects; and (c) that Entresto should be registered as a fixed dose combination with separately specified dose strengths for sacubitril and valsartan.
283 Novartis submits that the views of the expert witnesses as to what Entresto “contains or consists of” are not relevant to the determination of the s 70(3) inquiry, except to the extent that they are consistent with, or explain, the Secretary’s views. It contends that “the identity and nature” of the goods referred to in s 70(3) “cannot be impeached or varied by what expert witnesses say about TSVH as described”.
284 Novartis submits that even if Entresto were found to contain a single active ingredient, it nevertheless “contains or consists of” a combination of sacubitril and valsartan in anionic form and anionic sacubitril and valsartan are “identifiable” in TSVH’s crystal structure.
285 Thirdly, Novartis submits that its extension application has operated in “an entirely orthodox manner” to partially ameliorate the detriment it has suffered in taking “over a decade to obtain regulatory approval” for its invention.
286 So far as relevant to the present case, the definition of a “pharmaceutical substance” means a substance for therapeutic use whose application (or one of whose applications) involves a chemical interaction, or physico-chemical interaction, with a human physiological system. The definition makes clear that the “pharmaceutical substance” can be a mixture or compound of such substances. Section 70(2) itself recognises the possibility that a patent specification can, in substance, disclose and claim more than one pharmaceutical substance.
287 The parties addressed the s 70(2) inquiry with reference to claim 1. I have found that the pharmaceutical composition claimed in claim 1 is defined by identified components, having their identified pharmacological activities, in the state in which those components exist in the composition itself—meaning, in relation to the APIs, (valsartan or a pharmaceutically acceptable salt of valsartan) + ([sacubitril or a pharmaceutically acceptable salt of sacubitril] or [sacubitrilat or a pharmaceutically acceptable salt of sacubitrilat]).
288 In my view, the better analysis of the specification, when considered for the purposes of the s 70(2) inquiry, is that two “pharmaceutical substances per se” are, in substance, disclosed and fall within the scope of claim 1—first, the AT 1-antagonist (identified as valsartan or a pharmaceutically acceptable salt of valsartan); second, the NEP inhibitor (identified as either sacubitril or a pharmaceutically acceptable salt of sacubitril, or sacubitrilat or a pharmaceutically acceptable salt of sacubitrilat). These are specific pharmaceutical substances whose application involves, in each case, a chemical interaction or physico-chemical interaction with a human physiological system. However, it does not matter for the purpose of the s 70(2) inquiry in this case if these substances are treated as one pharmaceutical substance (e.g., as a mixture of substances) which acts simultaneously as an AT 1-antagonist and an NEP inhibitor.
289 The critical step, here, is the s 70(3) inquiry. The extension application was based on Entresto and, despite the language in which Novartis AG chose to couch that application, the relevant pharmaceutical substance in Entresto is TSVH—a single crystalline complex that is one salt with a unique set of physiochemical properties.
290 Novartis AG’s description in the extension application of the pharmaceutical substance as “a combination of sacubitril and valsartan” is notable for its obscurity. Entresto does not contain or consist of a “combination” of sacubitril and valsartan unless that description is taken to mean that sacubitril and valsartan, in some form, are inputs in a complexation process that results in the production of a different entity, TSVH, which is then used in Entresto.
291 TSVH is not: (a) valsartan or a pharmaceutically acceptable salt of valsartan; (b) sacubitril or a pharmaceutically acceptable salt of sacubitril; or (c) sacubitrilat or a pharmaceutically acceptable salt of sacubitrilat. Nor is TSVH (valsartan or a pharmaceutically acceptable salt of valsartan) + ([sacubitril or a pharmaceutically acceptable salt of sacubitril] or [sacubitrilat or a pharmaceutically acceptable salt of sacubitrilat]).
292 Novartis’s submission that the form of the pharmaceutical substance or substances does not matter diverts attention from the fundamental problem with its extension application. The pharmaceutical substance or substances that are identified for the purposes of s 70(2) must be the pharmaceutical substance or substances that are considered for the purposes of s 70(3). In the present case, there is a disconnect in the extension application between the requirement of s 70(2)(a) and the requirement of s 70(3)(a), which is obscured by describing the pharmaceutical substance as “a combination of sacubitril and valsartan”.
293 The pharmaceutical substance in Entresto is TSVH. TSVH is not disclosed or even envisaged in the specification. All the relevant experts agree on that. Further, TSVH does not fall within the scope of claim 1. Different pharmaceutical substances are disclosed and claimed in the specification, albeit that they are intended to have the same therapeutic effect as TSVH. But s 70(3)(a) is not satisfied by merely pointing to a different pharmaceutical substance in the registered goods that has the same intended therapeutic effect as the pharmaceutical substance or substances that satisfy s 70(2)(a).
294 I do not accept Novartis’s submission that the ARTG and Entresto’s PI conclusively determine the s 70(3) question or that the expert evidence on this question is irrelevant. Section 70(3)(a) raises two questions: (a) do goods contain or consist of the pharmaceutical substance or pharmaceutical substances that answer the s 70(2) inquiry? (b) are those goods included in the ARTG? Each is a question of fact. The second question, but not the first question, is bounded by the state of the ARTG.
295 In any event, the ARTG and Entresto’s PI make clear that the pharmaceutical substance, for the purposes of the s 70(3) inquiry, is TSVH. That fact is not gainsaid by descriptions of TSVH in the ARTG or Entresto’s PI. Nor is it gainsaid by anything in the AusPAR for Entresto. On the evidence before me, the CAS entry for Entresto appears to use the abbreviation “compd” inappropriately. As I have noted, no one has suggested that TSVH is anything but a complex.
296 The descriptions in the ARTG and Entresto’s PI as to Entresto’s composition have, no doubt, been made for their own purposes. Those purposes are not directed to the requirements of ss 70(2) and 70(3) of the Patents Act. Similarly, the basis on which, or the terms in which, the Secretary chose to register Entresto does not address, still less answer, whether the requirements of ss 70(2) and 70(3) have been met.
297 Finally, the policy consideration that Novartis has raised—the amelioration of the detriment it has suffered by the time taken to obtain regulatory approval of Entresto—is answered by the Full Court’s observation in Ono at [115] – [116] that, although the extension of term provisions in Pt 3 of Ch 6 of the Patents Act take account of a patentee’s interest in obtaining an “effective patent life”, those provisions balance a range of competing interests. It can be taken that the legislature saw the correct balance as being achieved by the very words it chose to implement that regime. The Court’s duty is to give effect to the meaning of the legislative command according to the terms in which it has been expressed.
298 The extension of term application did not meet the requirement of s 70(3)(a). It follows that the extension of term was not validly granted.
299 This conclusion means that Pharmacor’s challenge to the validity of claim 1 falls away. I will, however address Pharmacor’s challenge based on lack of inventive step. The remaining grounds of alleged invalidity not only fall away, they are based on a construction of claim 1 that I do not accept.
300 Sections 7(2) and (3) of the Patents Act, in the form in which they apply in this proceeding, provide:
Inventive step
(2) For the purposes of this Act, an invention is to be taken to involve an inventive step when compared with the prior art base unless the invention would have been obvious to a person skilled in the relevant art in the light of the common general knowledge as it existed in the patent area before the priority date of the relevant claim, whether that knowledge is considered separately or together with the information mentioned in subsection (3).
(3) The information for the purposes of subsection (2) is:
(a) any single piece of prior art information; or
(b) a combination of any 2 or more pieces of prior art information;
being information that the skilled person mentioned in subsection (2) could, before the priority date of the relevant claim, be reasonably expected to have ascertained, understood, regarded as relevant and, in the case of information mentioned in paragraph (b), combined as mentioned in that paragraph.
301 The onus of establishing the lack of an inventive step rests on the party asserting invalidity. The effect of s 7(2) is that the invention, as claimed, is deemed to involve an inventive step unless the contrary is established in accordance with the section: Apotex at [200].
302 It is important to emphasise the words “as claimed” because it is to that precise invention that the inquiry is directed. As I have noted, claim 1 is notable for its simplicity. It does not specify the form that the composition should take or its suitability for any particular mode of administration. It does not specify the salts of each component that can be present in the composition (if salts are used), beyond requiring that they be pharmaceutically acceptable. It does not specify the amount (dose) of each component in the composition or the suitability of the composition for any particular dosing regimen. It does not identify the disease or condition for which the pharmaceutical composition is to be used, beyond stating that its identified components are, respectively, an AT 1-antagonist and an NEP inhibitor. It is simply a pharmaceutical composition which contains the identified AT 1-antagonist and one of the identified NEP inhibitors, with a carrier; nothing more.
303 It is also important to remember that this ground of alleged invalidity is logically distinct from the ground of lack of novelty: see s 7(1) of the Patents Act. In explaining this distinction, Windeyer J said in Sunbeam Corporation v Morphy-Richards (Aust) Pty Ltd (1961) 180 CLR 98 at 111:
When want of novelty was asserted, the thing or process claimed as an invention was assumed to be an invention that is the product of the inventive faculties; but it was said that it was not now at the date of the patent, having been earlier invented and disclosed to the public. When want of subject matter, or lack of inventiveness, was asserted the thing or process claimed as an invention was assumed to be a new thing or process not previously disclosed to the public; but it was said that it was not really an invention and thus not a proper subject matter for the grant of a patent.
304 I mention this only because Novartis’s defence to Pharmacor’s challenge on the ground of lack of inventive step proceeds, in part, on the proposition that, in relation to the treatment of hypertension, and in relation to the treatment of heart failure, there was no approved “combination” product in Australia, before Entresto, that “included a new agent not previously having been through clinical trials in humans”: To the extent that Novartis seeks to support the claimed invention by considerations of novelty, that support should be disregarded.
305 Pharmacor’s challenge to the invention claimed in claim 1 on the ground of lack of inventive step relies on the common general knowledge and prior art information, being the Ksander paper and the Ksander patent (the Ksander documents) referred to below. Satisfaction of the requirement of s 7(3) is, therefore, critical to its challenge. The question whether the requirements of s 7(3) have been met with respect to the Ksander documents provoked significant debate.
306 The word “ascertained” in s 7(3) means no more than “discovered” or “found out”: Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (No 2) [2007] HCA 21; 235 CLR 173 (Lockwood No 2) at [132]. However, s 7(3) requires that there be a reasonable expectation that the prior art information will be “ascertained”. A reasonable expectation requires more than a possibility: Federal Commissioner of Taxation v Peabody [1994] HCA 43; 181 CLR 359 at 386; Aspirating IP Limited v Vision Systems Limited [2010] FCA 1061; 88 IPR 52 at [457]. The question of reasonable expectation is judged with regard to the characteristics of the person skilled in the art and the “problem” which the patentee claims to have solved with the claimed invention: Sequenom Inc v Ariosa Diagnostics Inc [2019] FCA 1011 143 IPR 24 at [599]; Pharmacia LLC v Juno Pharmaceuticals Pty Ltd [2022] FCA 92; 165 IPR 200 (Juno) at [268]; see also Otsuka at [407] – [408].
307 In establishing whether the person skilled in the art could be reasonably expected to have ascertained the prior art information, evidence of a hypothetical search is admissible. However, such evidence is not always necessary. For example, the notoriety of a particular journal that would have been consulted by the person skilled in the art may well suffice to establish the reasonable expectation that the person skilled in the art would have ascertained the prior information: Otsuka at [414] – [424]. Indeed, as Burley J observed in Hanwha Solutions Corporation v REC Solar Pte Ltd [2023] FCA 1017 at [367], unnecessary evidence of a hypothetical search can sometimes result in unnecessary disputation and cost.
308 If evidence of a hypothetical search is relied on, it does not matter that the person skilled in the art could, would, or might have, conducted other searches in addition to the search that ascertained the prior art information in question, or found other prior art information: Sandoz AG v Bayer Intellectual Property GmbH [2024] FCAFC 135 (Sandoz) at [51]; Otsuka at [417]. Also, for the purposes of s 7(3) it is not necessary for evidence to be adduced that the person skilled in the art would prefer, prioritise, or select the ascertained information over other information which the person could be reasonably expected to have discovered or found: Sandoz at [55]; Otsuka at [415] – [417]. Indeed, such evidence is beside the point. The only question arising under s 7(3) is whether the person skilled in the art could be reasonably expected to have ascertained and understood the prior art information and regarded it as relevant to the task at hand.
309 As to the latter requirement, the High Court in Lockwood No 2 (at [152] – [153]) made clear that “relevance” under s 7(3) is not an abstract inquiry:
152 Given the history, context, purpose and specific words of limitation in s 7(3), all of which were addressed by this Court in Firebelt, the phrase “relevant to work in the relevant art” should not be construed as meaning relevant to any work in the relevant art, including work irrelevant to the particular problem or long-felt want or need, in respect of which the invention constitutes an advance in the art. The phrase can only be construed as being directed to prior disclosures, that is publicly available information (not part of common general knowledge) which a person skilled in the relevant art could be expected to have regarded as relevant to solving a particular problem or meeting a long-felt want or need as the patentee claims to have done. Otherwise the words of limitation in the last 40 words of s 7(3) would have no role to play. Any piece of public information in the relevant art would be included, as is the case with the much broader and quite different formulation in the cognate provisions in the United Kingdom, which do not depend on the standard of a skilled person’s opinion of the relevance of the information.
153 The question of what a person skilled in the relevant art would regard as relevant, when faced with the same problem as the patentee, is to be determined on the evidence. The starting point is the subject matter of the invention to be considered together with evidence in respect of prior art, common general knowledge, the way in which the invention is an advance in the art, and any related matters. It should be mentioned that the starting point is not necessarily the inventive step as claimed, or even agreed between parties, because the evidence, particularly in respect of a combination of integers, may support a different inventive step.
(Footnotes omitted.)
310 These passages make clear that, in determining relevance for the purposes of s 7(3), there is a “starting point”. The “starting point” is the subject matter of the invention. Would it be reasonably expected that the person skilled in the art would have regarded the ascertained information as relevant to that subject matter?
311 Although the specification, here, does not posit any “problem” that the patentee faced, it does refer to the fact that, in the treatment of hypertension, drugs with different mechanisms of action have been combined. It speaks of a need for advantageous and more efficacious combinations for the treatment of hypertension. It also speaks of the invention addressing the treatment or prevention of heart failure, and the treatment and prevention of myocardial infarction and its sequelae. The invention can be seen, therefore, as addressing a need for efficacious combination therapy for these conditions or diseases. What is more, the subject matter of the invention (as relevantly claimed) concerns a composition that contains an identified AT 1-antagonist and identified NEP inhibitors. It is in this context that the question of relevance, for the purposes of s 7(3), falls to be addressed.
312 Pharmacor advances its challenge through the reformulated “Cripps question” (see Olin Mathieson Chemical Corporation v Biorex Laboratories Ltd [1970] RPC 157 at 187 – 188), which it preferably expresses in these terms:
Would the notional research group seeking to [come] up with a pharmacotherapy to treat each of HTN and HF, in all the circumstances (which include the CGK and the s 7(3) prior art), have been directly led as a matter of course to try the claimed invention in the expectation that it might well produce a useful or better alternative to other pharmacotherapies?
313 It is to be noted that, in evidence, Pharmacor pursued this question through a more expanded formulation that was directed not simply to a useful or better alternative to “other pharmacotherapies” at the priority date, but to other pharmacotherapies that were “in use, or in development” at that date.
314 Novartis criticises Pharmacor’s formulation because of the inclusion of the words “in development”. It contends that these words introduce uncertainty. It asks: how can the person skilled in the art have a reasonable expectation that a putative pharmacotherapy would be an improvement on a pharmacotherapy in development when the outcome of that development is not known? It also asks: what does “in development” mean? Novartis also raises difficulties about what the common general knowledge with respect to that development might be.
315 In my view, there is a more fundamental problem with the expression of the reformulated “Cripps question” that Pharmacor pursued in submissions. The reformulated “Cripps question” is a tool to assist in analysing whether an invention, as claimed, would have been obvious to the person skilled in the art at the priority date. It is not a question addressing, or directed to attaining, the novelty of a claimed invention. The words “in use, or in development” are an accretion which imports a requirement, or at least suggests the need, for novelty when considering the question. This, however, is irrelevant to the obviousness inquiry. The question, here, is not how the person skilled in the art would have achieved a “new” pharmacotherapy at the priority date (i.e., one not in use or in development at the priority date). It is whether the claimed pharmacotherapy was, at the priority date, obvious. The addition of words “in use, or in development” is, therefore, likely to skew the inquiry of the reformulated “Cripps question” away from prior art information that might well inform the answer to the question. For this reason, I do not propose to consider the reformulated “Cripps question” with these additional words in mind.
316 Novartis also submits that Pharmacor’s expression of the question should be directed (expanded) to pharmacotherapy for treating heart failure alone, in addition to pharmacotherapy for treating heart failure and hypertension. This expansion does not appear to be of any consequence for the case that Pharmacor advances. If the question, as expressed above, is answered in Pharmacor’s favour, that will be sufficient to make good its case on lack of inventive step. In any event, Pharmacor relies on alternative expressions of the reformulated “Cripps question” directed to the notional research group seeking to come up with a treatment for hypertension or a treatment for heart failure. As will become apparent, Pharmacor’s evidence addresses, equally, each variation of the question.
317 The reformulated “Cripps question” is not a necessary test of whether an invention, as claimed, lacks an inventive step. In Generic Health Pty Ltd v Bayer Pharma Aktiengesellschaft [2014] FCAFC 73; 222 FCR 336 at [71], the Full Court referred to it as a test that will be of assistance in some cases.
318 To the extent that the reformulated “Cripps question” does provide assistance, sight should not be lost of the fact that the test under s 7(2) of the Patents Act poses a more fundamental question. Is the invention, as claimed, obvious? Caution should be exercised in proceeding to answer the statutory question through the instrumentality of the reformulated “Cripps question” lest important considerations involved in answering the statutory question are masked.
319 To explain, the reformulated “Cripps question” proceeds on a problem/solution paradigm. It should not be assumed, however, that all inventions are based on that paradigm. It has long been understood that, while the quality of invention might lie in the results of long experiments and profound research to overcome a problem, it might equally lie in, for example, a sudden and lucky thought or in an accidental discovery: Crane v Price (1842) 4 Man & G 580 at 605.
320 In AB Hassle at [39], the plurality quoted, with approval, the following remarks of Graham J in Dow Corning Corporation’s Application [1969] RPC 544 at 560:
An inventor may well arrive at his invention by a flash of genius which causes him no difficulty or concentrated thought at all, but the invention may still be a most brilliant one which would never have occurred to the notional skilled man in the art at all or only after prolonged investigation and the concentrated exercise of his, perhaps lesser, inventive faculty. In such a case, though it is in a sense obvious to the inventor, nevertheless the invention is undoubtedly worthy of patent protection.
321 Further, an invention need not address a felt want, let alone a long-felt want. In Wellcome Foundation Ltd v VR Laboratories (Aust) Pty Ltd (1981) 148 CLR 262, Aickin J observed (at 287) that:
… not all inventions are to be classified as fulfilling a long-felt want. Those which reveal an “unfelt want” are as likely, or sometimes more likely, to involve an inventive step. In such a case experiments and research in perfecting the novel product or process would not be in the same category for it would throw no light on the quality of what was claimed by the patentee to be the inventive step. Such classification of inventions does not comprise a true dichotomy and some patents may have in part each of those qualities.
322 As the plurality said in AB Hassle at [38], an invention that is “an advance of contemporary expectations” and reveals an unfelt want, may well involve an inventive step.
323 In short, there may be no “problem” to address by a “solution”, in which case the application of the reformulated “Cripps question” might not adequately address the statutory question whether, in a given case, the claimed invention lacks an inventive step in the sense that it is obvious to the person skilled in the art.
324 In the present case, I do not feel bound to consider the question whether the invention claimed in claim 1 of the patent is obvious by reference only to the reformulated “Cripps question” as expressed in its various forms by Pharmacor, simply because Pharmacor has chosen to advance its challenge to the validity of claim 1 in that way.
325 Finally, the expectation of success expressed in the reformulated “Cripps question” is not a prediction of success. In Mylan Health Pty Ltd v Sun Pharma ANZ Pty Ltd [2020] FCAFC 116; 279 FRC 354 at [502], the Full Court said:
The reformulated Cripps question does not require certainty of outcome. It requires that the skilled addressee be directly led as a matter of course to try the claimed invention in the expectation that a particular research path “might well produce” a useful result (Hassle v Alphapharm at [53]). It does not require the skilled addressee to know that the steps will produce a useful result.
326 However, and importantly, it is not sufficient that the person skilled in the art would consider that it is “obvious to try” or “worth trying” that which the inventor has claimed is an invention: AB Hassle at [66] – [76]. Any research endeavour that is not undertaken with complete blindness will always be undertaken with some semblance of a chance of success: Application of Tomlinson (1966) 363 F 2d 928 at 931, cited with approval in AB Hassle at [73]. Obviousness to try would be an insufficiently discriminating and, therefore, imperfect standard by which to assess “obviousness” for the purposes of patent law.
327 Pharmacor pursued the reformulated “Cripps question” through an exercise involving the skills of a cardiologist, a pharmaceutical scientist, and a skilled searcher. Pharmacor relied on Professor Coats as representing the cardiologist and Professor Roberts as representing the pharmaceutical scientist. As I will explain, Dr Mokdsi carried out certain searches which were used by Professor Roberts in giving evidence in respect of his task.
328 Professor Coats was set three tasks.
329 First, he was asked to assume that, at the priority date, he was a member of a team seeking to come up with a pharmacotherapy for the treatment of hypertension that would be an improvement on, or a useful alternative to, the existing pharmacotherapies that were “in use, or in development”, at that date, for the treatment of hypertension. He was asked what recommendations, if any, would he make to “the team”?
330 In carrying out this task, Professor Coats did not contemplate the strategy of devising a new molecule (including by varying an existing molecule) on currently known mechanistic pathways. He considered that there was a range of other options that did not involve the difficulty and uncertainty of that path. Similarly, he did not contemplate the strategy of devising a new molecule (including by varying an existing molecule) on unknown mechanistic pathways. In his view, in the context of hypertension and heart failure, there were already a range of known and promising mechanistic pathways. There was no need to engage in a research project to discover new molecules which act on unknown pathways. Professor Coats also considered that devising a new molecule would not be the deployment of the common general knowledge in any event.
331 Professor Coats said that, by the priority date, the effective treatment of hypertension was rarely achieved by a pharmacotherapy that was limited to a single mode of action. He said that pharmacotherapy using multiple drugs with different modes of action was standard practice for hypertension patients whose blood pressure could not be adequately reduced, without unacceptable side effects, by a single drug with a single mode of action. Professor Coats said that, in his experience at the priority date, this was the case for most patients.
332 Professor Coats identified options involving the combination of different classes of agents that could be considered if treating hypertension alone. He said, however, that, in prioritising these combinations, he would have considered it advantageous that the combination be also useful for the treatment of heart failure.
333 Professor Coats explained that, given the significant causal connection between hypertension and heart failure (see [44] above), it is necessary, when prescribing pharmacotherapy for hypertension, to consider what would happen if the patient were to develop heart failure. This would be particularly so for patients with an increased risk of heart failure (such as older patients, or those with diabetes or coronary disease). Professor Coats observed that, at the priority date, all first line therapies for hypertension treated heart failure as well, and a pharmacotherapy that had demonstrated benefits for heart failure would immediately provide those benefits should the patient develop heart failure.
334 Professor Coats said:
While there were a number of clinically plausible treatment pathways that could be explored to treat hypertension, I would have prioritised one that could additionally save lives though the improvement of mortality in heart failure, rather than just lower blood pressure in hypertension.
…
335 Professor Coats said that, at the priority date, the standard practice for the treatment of patients with heart failure was also to use multiple drugs with different modes of action: see [49] above.
336 Professor Coats said that, for pharmacotherapy to treat hypertension and heart failure, he would have considered a combination of: (a) an agent out of the main three recommended classes of pharmacotherapy for the treatment of heart failure; and (b) an agent out of the more promising classes of new agents.
337 Professor Coats identified the three recommended classes of pharmacotherapy for the treatment of heart failure as: (i) an angiotensin II inhibitor (i.e., an ACE inhibitor or an ARB); (ii) a beta blocker indicated for heart failure; or (iii) an aldosterone receptor antagonist. He identified the more promising classes of new agents as: (i) a vasopressin receptor antagonist, (ii) an endothelin receptor antagonist, or (iii) an NEP inhibitor.
338 Professor Coats explained that, to achieve a “new” pharmacotherapy, which was an improvement on existing pharmacotherapies, or at least a useful alternative, it is necessary to identify an additional compelling benefit. Given, on his reasoning, that the pharmacotherapy would have involved the use of an existing class of drugs, Professor Coats reasoned, further, that the additional compelling benefit would have to be provided by, or be the result of, the additional agent—hence, his focus on the most promising new agents.
339 Professor Coats said that the combination of such an agent with an existing drug would enable patients on the existing drug to convert to the new pharmacotherapy. He also said that he would have confidence that the new combination was likely to be successful, at least to the extent of the already established therapy. If the additional administration were of the promising new agent alone—not in combination with an existing agent in the main recommended class—it would need to be tested and proven to be safe and efficacious against all agents in the main recommended class.
340 Turning to the class of new agents, Professor Coats said that he would have first considered an NEP inhibitor. This was principally for three reasons.
341 First, NEP inhibitors are known to reduce the breakdown of NPs, which have beneficial effects for hypertension and heart failure. Secondly, at the priority date, the development projects for vasopressin receptor antagonists and endothelin receptor antagonists had failed to result in any successful product for hypertension or heart failure, including because of lack of sufficient efficacy. Thirdly, Professor Coats said that the development of omapatrilat also provided valuable information that would have facilitated a new pharmacotherapy with an NEP inhibitor. As I have noted ([78]), the publicly reported data for omapatrilat at the priority date had demonstrated that the combination of an ACE inhibitor with an NEP inhibitor produced promising clinical benefits for hypertension and heart failure, albeit with the risk of side effects (e.g., angioedema).
342 Professor Coats said that, given the example provided by omapatrilat, he would have replaced the ACE inhibitor “action” with an ARB, because ACE inhibitors were known to have higher risks of side effects than ARBs, most notably angioedema. Therefore, replacing an ACE inhibitor with an ARB would have ameliorated the risk of angioedema, and resulted in less side effects.
343 Professor Coats said that he would have been confident that the combination of an ARB and NEP inhibitor would have been effective because ARBs were known substitutes for ACE inhibitors in heart failure. He said that this conclusion is supported by: (a) an understanding of how each agent works (namely, in reducing the action of angiotensin II: see [55] above); and (b) the proven use of ARBs as single agents in heart failure (and as substitutes for ACE inhibitors) through clinical trials, such as ELITE-II.
344 Professor Coats reasoned that, whilst it is likely any of the ARBs in use at the priority date would have been safe and effective, his preference would have been for agents which had been shown to be suitable for heart failure in major clinical trials. The data from such trials demonstrates efficacy and reduces the risk of development.
345 At the priority date, losartan was shown to be efficacious in the treatment of heart failure by ELITE-II: see [61] above. Valsartan had also been shown to be efficacious by Val-HeFT: see [62] above. Professor Coats expressed his preference for valsartan because losartan was not able to be dosed once daily. Although less relevant for the treatment of heart failure, this was a downside for the treatment of hypertension (for reasons of patient compliance).
346 Professor Coats’s next choice would have been candesartan, based on the progress of the CHARM Program. Professor Coats said that, although the clinical trials on candesartan had not been reported at the priority date, the fact that it was progressing through major Phase III clinical trials would have provided him with a degree of comfort.
347 Professor Coats said that, while he would not have ruled out selecting eprosartan, irbesartan, or telmisartan, none of these agents had been the subject of major clinical trials in heart failure. For this reason, they would not have been preferred agents.
348 By way of summary, Professor Coats said that his order of preference for the ARB to be used as part of the new pharmacotherapy would have been: first, valsartan; secondly, candesartan or losartan; thirdly, eprosartan, irbesartan, or telmisartan.
349 With respect to the selection of an NEP inhibitor, Professor Coats said that he would have asked the “team” to conduct searches to provide a list of NEP inhibitors that could be used or modified:
… I expect the other members of the team, particularly the clinical pharmacologist, would have greater expertise than me in selecting a complementary ARB and NEP inhibitor combination and I would have sought assistance in this respect. I was not familiar with any NEP inhibitors which had been the subject of major Phase III clinical trials and expect that the NEP inhibitor selected would not have been the subject of such trials.
350 Professor Coats expressed his expectation that the pharmacotherapy that included the ARB and the NEP inhibitor, as devised by the team, would be an effective treatment for hypertension and heart failure. He gave two reasons.
351 First, ARBs were proven effective agents for the treatment of heart failure and hypertension. NEP inhibitors were promising given how the NP system works. Inhibiting the RAAS and the promoting the action of NPs were complementary strategies.
352 Secondly, the reported clinical trials of omapatrilat demonstrated that the combination of ACE inhibition and NEP inhibition was effective. As ARBs and ACE inhibitors were substitutable for the treatment of hypertension and heart failure, it could be expected that the combination of an ARB with and NEP inhibitor would also be efficacious, with lower side effects than omapatrilat. Further, because the team would be using the ARB and NEP inhibitor as separate agents (unlike omapatrilat which was a naturally occurring combination), they would have the flexibility to optimise the two agents.
353 Professor Coats referred to three other matters that he would have recommended as desirable for the pharmacotherapy from a clinical perspective. These matters concern patient compliance. First, the pharmacotherapy should allow for once daily dosing. Secondly, the pharmacotherapy should be administered as a solid oral dosage form (such as a tablet or capsule). Thirdly, the ARB and the NEP inhibitor should be in a single dosage form. Professor Coats noted that, at the priority date, there were examples of pharmacotherapies for the treatment of hypertension and heart failure that were available as single dosage forms with two different modes of action.
354 I should make clear at this point that I do not regard these matters as relevant to the question of whether the invention claimed in claim 1 lacks an inventive step. The claimed pharmaceutical substance is not one limited to a particular dosage form or dosing regimen or indeed by any therapeutic indication beyond the identified activity of components (i) and (ii).
355 After completing the first task, and making the recommendations and giving the reasons I have summarised above, Professor Coats was asked to make the further assumption that, at the priority date, he was a member of a team seeking to come up with a pharmacotherapy for the treatment of heart failure that would be an improvement on, or a useful alternative to, the existing pharmacotherapies that were in use, or in development, at that date, for the treatment of hypertension. Once again, he was asked what recommendations, if any, would he make to the “team”?
356 Professor Coats said that his response to the first task (just discussed) was applicable to the second task, the main difference being that the combinations he originally addressed in the first task would exclude agents that do not treat heart failure and include some agents that do not treat hypertension. Therefore, the initial list of possible agents to combine would be: (a) one agent out of the three recommended classes of pharmacotherapy for the treatment of heart failure (see [337] above); and (b) another, promising agent selected from vasopressin receptor antagonists, endothelin receptor antagonists, NEP inhibitors, and (in light of the heart failure only indication) the positive inotropic agents. He said, however, that the benefit of the new pharmacotherapy treating both heart failure and hypertension remained.
357 Lastly, Professor Coats was asked to assume that, at the priority date, he was a member of a team seeking to come up with a pharmacotherapy for the treatment of hypertension and heart failure that would be an improvement on, or a useful alternative to, the existing pharmacotherapies that were in use, or in development, at that date, for the treatment of those conditions. Once again, he was asked what recommendations, if any, would he make to the “team”?
358 Professor Coats said that his response to the first task was also applicable to this last (third) task except that, instead of de-prioritising agents that only treated hypertension, or only treated heart failure, he would simply exclude them based on the scope of this task (the need to treat both hypertension and heart failure).
359 It will be appreciated that Professor Coats’s third task is the task reflected in Pharmacor’s preferred expression of the “Cripps question”.
360 It is to be recalled that Professor Coats would have asked the “team” to conduct searches to provide a list of NEP inhibitors that could be used or modified for the combination therapy he would have recommended. Professor Roberts was asked to assume that, at the priority date, he was part of that team. He was asked to assume that the team was seeking to come up with a pharmacotherapy for the treatment of hypertension and heart failure which was an improvement on, or useful alternative to, the existing pharmacotherapies that were in use, or in development, for the treatment of those conditions.
361 Professor Roberts was also asked to assume that the team included a cardiologist who had proposed that the pharmacotherapy comprise an ARB and an NEP inhibitor, with the ARB being (in order of preference): firstly, valsartan; secondly, candesartan or losartan; thirdly, eprosartan, irbesartan, or telmisartan.
362 Professor Roberts was also told that, from a clinical perspective, the cardiologist had indicated that it was desirable that the ARB and NEP inhibitor be administered orally, once daily, and preferably as a single combination product. The cardiologist had requested the “team” to identify a suitable NEP inhibitor for the product. Professor Roberts was asked to assume that he had been set this task.
363 However, before being informed of these matters, Professor Roberts had been asked to explain the matters that, before the priority date, he would take into account as a pharmaceutical scientist when considering the suitability of one or more APIs or classes of APIs for inclusion in a pharmaceutical product.
364 By way of broad summary only, Professor Roberts’s evidence was that, if given the name of a particular API to consider, he would, as a matter of course, obtain the chemical structure of the API from one of the key reference sources he had identified (which included the Merck Index and the Chemical Abstracts Service (CAS) Registry) and then conduct a search for the API or drug class using the search tools he described (which included the MEDLINE database and the Chemical Abstracts database).
365 Professor Roberts said that he would then consider: (a) the physicochemical properties of the API or drug class in the context of the intended clinical indication or use; and (b) the biopharmaceutics as defined by the API in the context of the physiology of the absorption site. Biopharmaceutics is the release of the API from its dosage form at the absorption site in the body and the resulting absorption of the API.
366 Professor Roberts said that, in considering the suitability of one or more APIs or classes of APIs for inclusion in a pharmaceutical product, he would also have had regard to the dosage form (such as a tablet or capsule for oral administration; a liquid for injection, an aerosol for inhalation; a cream or patch for topical or transdermal administration; an eye drop for ocular administration; or a suppository for rectal administration).
367 Professor Roberts said that tablets and capsules were particularly advantageous dosage forms. At the priority date, they were, by far, the most commonly used and preferred dosage forms. Amongst other things, they: (a) facilitated accurate dosing of the drug; (b) were stable and easy to handle; (c) were acceptable to most patients; (d) provided a generally reliable and generally safe method of drug administration; and (e) were relatively inexpensive to manufacture.
368 In this context, Professor Roberts also discussed the impact of dosage forms on patient compliance, particularly in the case of chronic illnesses where the patient requires ongoing drug treatment. Professor Roberts said that, where a dosage form cannot be conveniently administered by patients or their carers, or where the drug or its dosage form are unacceptable to patients, patients will be less likely to comply with the dosage regimen for that drug:
118. Two factors known to influence patient compliance and adherence are the frequency with which tablets or other dosage forms are prescribed to be taken, and the total number of dosage forms per day. It was well known by pharmaceutical scientists before the Relevant Date that the more tablets and/or times that a patient needed to take such tablets each day, the greater the risk that a patient would not take their medication as prescribed by their medical practitioner. I published articles on these matters both before and after the Relevant Date, an example of which is annexed to this affidavit … “How to optimise use of medications in the elderly” (Peter I. Pillans, Michael S. Roberts, Medicine Today, 2001, 2(4), 60-64). As we stated on page 63, “[a]n inverse relationship is to be expected between compliance and the number of medications”, meaning that patient compliance typically decreases as the number of medications increases. Although the article focused on the use of medications in the elderly, the above statement applies across the patient population.
369 Professor Roberts explained that there were various ways in which dosage form design can be used to improve patient compliance and adherence. Extended or controlled release dosage forms, which release the API over a prolonged period, can be used to reduce the frequency of administration of the drug over a given period, such as a day. Fixed dose combinations (that incorporate two or more APIs at a fixed dosage or amount) can be used to reduce the total number of dosage forms the patient is required to take over a given period of time.
370 Professor Roberts said that, well before the priority date, fixed dose combination products were available in Australia. These included the products referred to in [87]. Professor Roberts said that he was first involved in designing pharmaceutical products containing more than one API in the 1970s.
371 Having been set his task, Professor Roberts said that he would have agreed with the cardiologist that it was desirable that the ARB and NEP inhibitor be administered orally, once daily and, if possible, in a single combination product.
372 Professor Roberts also said that, before the priority date, he was familiar with ARBs being used for the treatment of hypertension, including from various texts and reference books, and also from discussions at conferences. He said that he would have relied on the cardiologist’s preferences as to which ARB was used. As valsartan was the cardiologist’s preferred ARB, he would have proceeded on the basis that valsartan was the lead candidate for the ARB to be used in the pharmacotherapy. However, Professor Roberts said that this would have been subject to him being able to identify an NEP inhibitor that would be pharmacokinetically and physiochemically compatible with valsartan.
373 As to the task itself, Professor Roberts said that, if the chemical structure of valsartan had not been provided to him, he would have obtained a copy of its chemical structure (from, for example, the Merck Index), in accordance with his usual practice. Professor Roberts was provided an extract from the Merck Index in relation to valsartan. He said that, based on this information, he would have deduced valsartan’s physicochemical properties and its resultant biopharmaceutics. In his earlier evidence, Professor Roberts explained how this could be done.
374 Professor Roberts said that he would have conducted a thorough search for information that may have assisted him to identify and select a suitable NEP inhibitor for use in the pharmacotherapy, using his usual search strategy (which he described). In this case, he said that he would have used the search term “neutral endopeptidase inhibitors”. He said that his initial set of searches would have been: (a) in the MEDLINE database for journal articles, using the search term “neutral endopeptidase inhibitors”; and (b) in the Chemical Abstracts database for journal articles, patents, and other scientific literature, using the broad keyword search phrase “neutral endopeptidase inhibitors”.
375 As it turns out, Professor Roberts did not, in fact, carry out searches for the purpose of giving his evidence. Pharmacor’s solicitors provided him with a document containing search results, which Professor Roberts was asked to assume contained the results he would have obtained if he had carried out the search he described of the MEDLINE database (the MEDLINE results document). I discuss the manner in which the search was actually conducted in the next section of these reasons: see [404] – [409] below.
376 Professor Roberts said that, consistently with his approach to reviewing the results of searches, he reviewed the title and abstract information contained in the MEDLINE results document to identify potentially relevant records for which he would have obtained a full copy. He gave this information a ranking of P.1 to P.5 (“P.1” being for those results that appeared most relevant and most likely to contain information of use for the task). By the letters “NA”, Professor Roberts indicated the results for which he would not obtain the full text of the record.
377 Pharmacor’s solicitors also provided Professor Roberts with a document containing search results, which he was asked to assume contained the results he would have obtained if he had carried out the search he described of the Chemical Abstracts database, restricted to a search only of patents (the Chemical Abstracts results document). I also discuss the manner in which the search of this database was actually conducted in the next section of these reasons.
378 Once again, consistently with his approach to reviewing the results of searches, Professor Roberts reviewed the title and abstract information in the Chemical Abstracts results document to identify potentially relevant records for which he would have obtained a full copy. He considered that some of the results appeared likely to contain information of use for the task, and ranked the information as I have noted above, save that he divided the ranking “P.4”:
(a) “P.4A” to designate those results that were in a foreign language (of which Professor Roberts had no comprehension) which recorded data that he considered to be of use for the task); and
(b) “P.4B” to designate those results that were in a foreign language (of which Professor Roberts had no comprehension) which contained no recorded data that he considered to be of use for the task.
379 After he provided his annotations in respect of the MEDLINE results document and the Chemical Abstracts results document, Pharmacor’s solicitors provided Professor Roberts with two documents: (a) a paper titled “Dicarboxylic Acid Dipeptide Neutral Endopeptidase Inhibitors” (Gary M. Ksander et al., Journal of Medicinal Chemistry, 1995, 38, 1689-1700) (the Ksander paper); and (b) US Patent 5,217,996 titled “Biaryl substituted 4-amino-butyric acid amides” (the Ksander patent). In his review of the MEDLINE results document, Professor Roberts had ranked the Ksander paper as “P.1”. In his review of the Chemical Abstracts results document, Professor Roberts had also ranked the Ksander patent as “P.1”.
380 Professor Roberts reviewed and discussed what he would have understood from the Ksander paper, and described what he would have done in relation to the task he was set, armed with the Ksander paper and the information and knowledge that he considered to be generally accepted in the field of pharmaceutical science in Australia at the priority date.
381 The Ksander paper describes three series of NEP inhibitors (referred to as class 8 compounds, class 10 compounds, and class 21 compounds) on tests in three animal species. The most prominent NEP inhibitor compounds are referred to as compound 19a and compound 21a. Compound 19a is identified as the prodrug of compound 21a.
382 The Ksander paper refers to compound 21a as exhibiting “potent NEP inhibitory activity … in vitro and in vivo”. On Professor Roberts’s understanding of the paper, compound 21a is the most potent compound that is discussed. Professor Roberts said that, as a result, he would have selected compound 21a as the active moiety.
383 Professor Roberts said that he would have considered whether valsartan and compound 21a were pharmacokinetically similar (in terms of having comparable half-lives) after they reach systemic circulation. He would also have considered whether there was a potential for major drug-drug interaction.
384 To consider these questions Professor Roberts characterised compound 21a’s physicochemical properties based on its chemical structure, as he had for valsartan. Having done this, he said that, at the priority date: (a) he would have concluded that valsartan and compound 21a had a number of similar pharmacokinetic properties that would likely lead to them having similar half-lives; and (b) he would not have expected any major drug-drug interactions between valsartan and compound 21a that would substantially impact on each compound’s half-life.
385 Professor Roberts’s next step was to compare the suitability of compound 19a versus 21a as the NEP inhibitor in the pharmacotherapy. For both compound 19a and compound 21a, the Ksander paper teaches that compound 21a is the activity moiety that is distributed, metabolised and excreted from the body (as compound 19a is converted in vivo to compound 21a).
386 However, Professor Roberts said that he would have selected compound 19a as the NEP inhibitor to be used for the purposes of the pharmacotherapy. This is because a tablet would have been his preferred dosage form (for the reasons given above) and compound 19a (as the ethyl ester prodrug of compound 21a) could be administered orally (as shown in the Ksander paper). Professor Roberts said that compound 21a, a dicarboxylic acid, would be poorly absorbed and have low bioavailability. This is because, generally, dicarboxylic acids do not readily permeate the epithelial lining of the gastrointestinal tract into the bloodstream (in the animal studies reported in the Ksander paper, compound 21a was administered intravenously). The Ksander paper showed a rapid increase in ANP (or ANF) plasma concentrations on administration of compound 19a orally (and also intraduodenally), indicating that there was a rapid conversion of compound 19a to compound 21a in vivo.
387 Having reached that decision, Professor Roberts considered compound 19a’s physicochemical properties and resulting biopharmaceutics and said that, at the priority date, he would have deduced (for the reasons he gave) that valsartan and compound 19a are physiochemically comparable and likely to behave in a relatively similar manner in the gastrointestinal tract in vivo, yielding similar rates of absorption.
388 Finally, Professor Roberts said that, before the priority date, he would have been able to prepare a single tablet or capsule containing suitable amounts of valsartan and compound 19a (or the sodium salt thereof), with pharmaceutical excipients, that could be optimised to be dosed daily.
389 Professor Roberts said that, based on the Ksander paper, he would have recommended to the “team” that it pursue a pharmacotherapy with compound 19a as a preferred NEP inhibitor in conjunction with the cardiologist’s preferred ARB, valsartan.
390 As he did with the Ksander paper, Professor Roberts reviewed and discussed what he would have understood from the Ksander patent, and described what he would have done in relation to the task he was set, armed with the Ksander patent and the information and knowledge that he considered to be generally accepted in the field of pharmaceutical science in Australia at the priority date.
391 Professor Roberts said that the Ksander patent covers the same subject matter as the Ksander paper. It describes the invention as relating to certain biaryl substituted 4-amino-butyric acid derivatives of a given, general formula referred to as formula I. It identifies embodiments of the invention designated by formulas Ia, Ib, Ic, Id, Ie, and If, preferably in the enantiomeric form depicted in formula I’. Certain compounds of formula Id are said to be preferred. Certain compounds of formula Ic and Ie are said to be particularly preferred.
392 The patent teaches that:
The novel compounds of the invention are pharmacologically potent neutral endopeptidase enzyme inhibitors which inhibit e.g. the degradation of atrial natriuretic factors (ANF) in mammals. They thus potentiate the diuretic and natriuretic effect of exogenous or endogenous ANF in mammals.
The compounds of the invention are thus particularly useful in mammals as diuretic, natriuretic (saluretic) and antihypertensive agents for the treatment of e.g. hypertension, congestive heart failure and edema.
393 Professor Roberts said that the most prominent compound discussed in the Ksander patent is N-(3-carboxy-1-oxopropyl)-(4S)-(p-phenylphenylmethyl)-4-amino-(2R)-methylbutanoic acid ethyl ester which, for ease of reference, Professor Roberts called Compound A.
394 The Ksander patent describes in vitro and in vivo testing in respect of Compound A. The results of in vivo testing using Compound A showed significant increases in plasma ANF levels and significant reduction in blood pressure in the DOCA-salt hypertensive rat model. The Ksander patent also describes compositions containing the NEP inhibitor compounds.
395 The Ksander patent includes eight examples. Example 1 describes the synthesis of the sodium salt of Compound A. Example 2 describes the synthesis, from Compound A, of the free dicarboxylic acid which, for ease of reference, Professor Roberts called Compound B. Example 8 describes the preparation of 1,000 capsules, each containing 50 mg of the sodium salt of Compound A, together with lactose, modified starch and magnesium stearate as excipients.
396 Professor Roberts said that, at the priority date, he would have understood that Compound A is a prodrug of Compound B, based on descriptions in the Ksander patent. Further, he said that he would have understood that Compound B was the active moiety of Compound A and substantially responsible for the therapeutic results in respect of the oral administration of Compound A. Professor Roberts said:
I would have expected that Compound A likely permeates the GIT membrane and is then converted to Compound B by a de-esterification reaction in the intestinal wall and in the liver as part of the first pass metabolism. I would have expected that the de-esterification of Compound A would result in a fairly substantial and rapid conversion to Compound B.
397 Professor Roberts said that, based on the Ksander patent, he would have selected Compound B as the NEP inhibitor for his task, given: (a) Compound A’s reported effects, in vivo, of increasing plasma ANF levels and reducing blood pressure in the animal model (which Professor Roberts attributed to the active moiety, Compound B); and (b) his understanding from the patent that Compound B was the most effective compound in inhibiting NEP activity, and therefore the most effective compound in potentiating ANF levels and reducing blood pressure.
398 Professor Roberts said that he would have characterised Compound B’s physiochemical properties based on its chemical structure and determined, from those properties, that valsartan and Compound B were pharmacokinetically similar in terms of having comparable half-lives after they reach the systemic circulation. In this regard, Professor Roberts said that he would have anticipated that valsartan and Compound B would each have a moderate to long half-life. He also said that he would not have expected there to be any major drug-drug interactions between valsartan and Compound B that would substantially impact on each compound’s half-life.
399 Professor Roberts said that he would have selected Compound A as the NEP inhibitor prodrug to be used for his task, for the same reasons he would have selected compound 19a over compound 21a disclosed in the Ksander paper: see [386] above. In addition, Example 8 in the Ksander patent describes the formulation of the sodium salt of Compound A as a capsule for oral administration.
400 Having reached that decision, Professor Roberts considered Compound A’s physicochemical properties and resulting biopharmaceutics and said that, at the priority date, he would have deduced (for the reasons he gave) that valsartan and Compound A are physiochemically comparable and were likely to behave in a relatively similar manner in the gastrointestinal tract in vivo, yielding similar rates of absorption. Professor Roberts said that to reduce the already low chance of chemical reaction between valsartan and Compound A, the sodium salt of Compound A could be used.
401 Finally, Professor Roberts said that, before the priority date, he would have been able to prepare a single tablet or capsule containing suitable amounts of valsartan and Compound A (or the sodium salt thereof), with pharmaceutical excipients, that could be optimised to be dosed daily.
402 Professor Roberts said that, based on the Ksander patent, he would have recommended to the “team” that it pursue a pharmacotherapy with Compound A as a preferred NEP inhibitor in conjunction with the cardiologist’s preferred ARB, valsartan.
The Ksander documents combined
403 As I have noted, Professor Roberts considered that the Ksander paper and the Ksander patent cover the same subject matter. He said that he would have combined the information in the two documents for the purpose of his task. He would have understood that: (a) compound 19a in the Ksander paper to be the same as Compound A in the Ksander patent; and (b) compound 21a in the Ksander paper to be the same as Compound B in the Ksander patent.
404 As I have noted, Professor Roberts did not carry out his own searches for the purpose of giving his evidence. This task was entrusted by Pharmacor’s solicitors to Dr Mokdsi who, as I have said, has expertise in searching pharmaceutical patents and scientific literature.
405 Pharmacor’s solicitors asked Dr Mokdsi to conduct a search to identify any scientific literature that would have been identified by a search of the MEDLINE database on 17 January 2002 using the search phrase “neutral endopeptidase inhibitors” and to provide a copy of the full record of each search result displayed in response to that search.
406 Dr Mokdsi carried out that search using the PubMed interface and obtained details for 50 records.
407 Pharmacor’s solicitors also asked Dr Mokdsi to conduct a search to identify what would have been identified by a search of the Chemical Abstracts database on 17 January 2002 using the search phrase “neutral endopeptidase inhibitors” restricted to patents, and to provide a copy of the full record of each search result displayed in response to that search.
408 The Chemical Abstracts database maintained by CAS is called Chemical Abstract PLUS (CAPLUS). Dr Mokdsi carried out a search of CAPLUS using the Scientific & Technical Information Network platform. He obtained details for 51 patent records.
409 Whether the requirements of s 7(3) have been met in respect of the Ksander documents is an important threshold question for the success of Pharmacor’s challenge to claim 1 on the ground of lack of inventive step. Novartis does not dispute that the person skilled in the art would have understood the Ksander documents. It does, however, dispute that the person skilled in the art could be reasonably expected to have “ascertained” the Ksander documents and regarded them as “relevant”, within the meaning of s 7(3).
410 Novartis contends that Pharmacor’s structured approach in the evidence to addressing its expression of the reformulated “Cripps question” is flawed. It submits that Pharmacor’s evidence “demonstrates a highly artificial and tightly controlled exercise in restricting information flow between its key witnesses”—meaning Professor Coats and Professor Roberts. Novartis’s criticism is particularly directed to whether Pharmacor has established that the Ksander documents would have been “ascertained” within the meaning of s 7(3) of the Patents Act. It submits that these documents were only reached after having been “funnelled through a series of exclusionary instructions or choices”—a reference to, primarily, Professor Coats’s evidence.
411 As a consequence, Novartis submits that Pharmacor’s evidence is not capable of supporting a finding that the Ksander documents qualify as s 7(3) documents. It accepts that the evidence demonstrates that these documents could have been returned by a search of relevant databases. It contends, however, that the evidence does not establish that it would have been “reasonable to expect” that the person skilled in the art would have ascertained them.
412 Novartis contends, firstly, that Professor Coats undertook his task very narrowly and did not explore other possibilities, such as considering pharmacotherapies under development, or devising new molecules or modifying existing molecules in conjunction with a medicinal chemist. It submits that Professor Coats was “forced” into the combination he proposed, which included an NEP inhibitor in circumstances where, at the priority date, there were no specific NEP inhibitors in Australia that were available to treat hypertension or heart failure.
413 Secondly, Novartis contends that Professor Coats’s hypothetical request that the “team” conduct searches to provide a list of NEP inhibitors that could be used or modified was not the task that Professor Roberts undertook, rather, the task he undertook was to identify a suitable NEP inhibitor for the product.
414 Thirdly, Novartis contends that only “selective parts” of Professor Coats’s reasoning for his choices were passed on to Professor Roberts. For example, Novartis is critical of the fact that, although Professor Coats was personally aware of Phase II clinical trials of the NEP inhibitor candoxatril in the early 1990s, that information was not passed to Professor Roberts. Novartis also contends that it was “crucial” that Professor Roberts be provided with Professor Coats’s reasoning for choosing an ARB in preference to an ACE inhibitor (which was to avoid the higher risks of side effects with an ACE inhibitor, most notably angioedema). Novartis contends, further, that Professor Coats was not aware of any class effect for NEP inhibitors. For example, different NEP inhibitors may have different effects on different substrates; some might give greater bradykinin release than others. It would be “dangerous to put all NEP inhibitors under one hat”.
415 Fourthly, Novartis challenges the search that was carried out by Dr Mokdsi using, on instructions, the single search term “neutral endopeptidase inhibitors”. Novartis contends that had other terms, namely “bradykinin”, “angioedema”, or “cough” been used as a filter in addition to “neutral endopeptidase inhibitors”, then the Ksander documents would not have been returned because neither of those documents use those additional search terms. Novartis submits that, the Court cannot know what results might have been returned had those additional search terms been used in the present case.
416 Fifthly, Novartis submits that Dr Mokdsi performed only a subset of searches that Professor Roberts would have carried out. As I have recorded, Professor Roberts’s evidence was that he would have carried out initial searches: (a) in the MEDLINE database for journal articles, using the search term “neutral endopeptidase inhibitors”; and (b) in the Chemical Abstracts database for journal articles, patents, and other scientific literature, using the broad keyword search phrase “neutral endopeptidase inhibitors”. However, Dr Mokdsi did not conduct searches of the Chemical Abstracts database for literature other than patent documents.
417 Sixthly, Novartis submits that the searches that Dr Mokdsi undertook were not “reasonable” because the task he was given did not permit him to exercise his discretion as a searcher to use synonyms, truncators, and Boolean operators so as to ensure that his search was not artificially limited.
418 For example, Dr Mokdsi accepted in cross-examination that a search specifically for “neutral endopeptidase inhibitors” would not return a document in which only the term “neutral endopeptidase inhibitor” was used. Novartis said that the Ksander paper does not use the term “neutral endopeptidase inhibitor”. Curiously, its argument appears to be that if Dr Mokdsi had searched using the term “neutral endopeptidase inhibitor” (which he did not do) then his search would not have returned the Ksander paper.
419 Novartis submits that, had Dr Mokdsi been permitted to use his usual discretion in searching—such as by using synonyms, truncators, and Boolean operators—it could be expected that more search results would have been obtained.
420 Seventhly, Novartis submits that, because Dr Mokdsi was constrained by his instructions in carrying out his search (i.e., by searching certain databases using the singular search term “neutral endopeptidase inhibitors”) his search was unreasonably deficient and incapable of establishing the “landscape” of NEP inhibitors. In this regard, Novartis places reliance on Professor Roberts’s evidence that his usual practice is to conduct an iterative search and review process, not a linear one.
421 Eighthly, Novartis relies on the fact that, after having indicated a number of documents in the MEDLINE results document and the Chemical Abstracts results document that he would consider as containing information relevant to his task (see [376] - [379] above), Professor Roberts was only given the Ksander documents. Novartis submits that this could only have been done because Pharmacor wanted Professor Roberts to read and select the Ksander documents as relevant to his task because they are, presumably, the only two pre-priority date documents that disclose sacubitril/at. Novartis also submits that, as a result, the Court does not know how the other documents selected by Professor Roberts from the MEDLINE results document and the Chemical Abstracts document would have informed Professor Roberts’s understanding of the “landscape” of NEP inhibitors, apart from what was stated in the results documents themselves.
422 Overall, Novartis submits that the search and review task that was undertaken, which led to the production of the Ksander documents to Professor Roberts was not thorough. Novartis submits:
… Pharmacor made a series of unexplained forensic choices to constrain or vary what Professor Roberts was told, and to constrain what Professor Roberts and Dr Mokdsi were permitted to do. The evidence … is sufficient to conclude that Pharmacor has not satisfied its onus. In any event, there is also objective evidence that the search was not thorough, and was artificially narrowed. …
423 The evidence establishes that the person skilled in the art could be reasonably expected to have ascertained the Ksander documents by a reasonable search. Novartis’s submissions to the contrary are either irrelevant to the question of ascertainment or are of no consequence.
424 The fact Professor Roberts only recommended compound 19a as a preferred NEP inhibitor (based on the Ksander paper) or Compound A as a preferred NEP inhibitor (based on the Ksander patent), and did not provide a “list” of NEP inhibitors as Professor Coats had requested from the “team”, is a trifling matter. More fundamentally, it is irrelevant to the question whether, before the priority date, the person skilled in the art could be reasonably expected to have ascertained the Ksander documents.
425 Similarly, the fact that Professor Coats’s reasoning for his choices were not communicated to Professor Roberts is irrelevant. Professor Roberts carried out the role of the “team” in seeking an NEP inhibitor that would be complementary to an ARB, in particular the preferred ARB valsartan. Given that the person skilled in the art is a construct, understood in this case as a notional “team”, it is irrelevant to the question of ascertainment to know why the “team” (through Professor Coats) arrived at the selections it had already made. As I have remarked, the person skilled in the art, understood as a “team”, is an indivisible construct that draws, when and to the extent necessary, on the disparate capacities of the members of the “team”. It thinks with one mind and speaks with one voice.
426 In any event, Professor Roberts, who was the only witness representing the skills of a pharmaceutical scientist, did not profess to have any difficulty in carrying out his “team” task on the information he was, in fact, given.
427 Novartis’s submissions challenging the search carried out by Dr Mokdsi are also irrelevant, and difficult to reconcile. On the one hand, Novartis suggests that the search term “neutral endopeptidase inhibitors” should have been filtered (i.e., limited) by additional search terms such as “bradykinin”, “angioedema”, or “cough” (thereby narrowing the search). It argues that the Court will not know what results may have been returned if those filters had been used. On the other hand, it argues that the search should have been widened. In this connection it says that use of the search term “neutral endopeptidase inhibitors” prevented Dr Mokdsi from exercising his searching discretion (such as by not permitting the use of synonyms, truncators, and Boolean operators) which artificially limited the results of the search that should have been undertaken.
428 Professor Roberts considered that “neutral endopeptidase inhibitors” was an appropriate search term for the task he was given. I accept his evidence, particularly in the absence of contrary evidence from someone possessing Professor Roberts’s skills as a pharmaceutical scientist. There is no apparent reason why the search term “neutral endopeptidase inhibitors” should have been filtered by additional search terms, such as those that Novartis suggests. Novartis is correct in saying that the Court will not know the results that may have been returned if additional filtering terms had been used with “neutral endopeptidase inhibitors”, but that inquiry is irrelevant to the question whether the person skilled in the art could be reasonably expected to have ascertained the Ksander documents, by use of the search term “neutral endopeptidase inhibitors”.
429 It is also irrelevant that Dr Mokdsi’s search was more limited than Professor Roberts’s usual practice in searching the Chemical Abstracts database. Dr Mokdsi’s search of the MEDLINE database and the more limited search of Chemical Abstracts database (i.e., not searching for literature other than patent documents) using the search term “neutral endopeptidase inhibitors”, still returned results for the Ksander documents.
430 As to inhibiting Dr Mokdsi’s searching discretion, Novartis argues that the search term “neutral endopeptidase inhibitors” would not have picked up documents which only used the expression “neutral endopeptidase inhibitor”. Hence, had the search term “neutral endopeptidase inhibitor” been used instead of “neutral endopeptidase inhibitors”, the Ksander paper would not have been ascertained. However, Novartis also wants to argue that had Dr Mokdsi been allowed to exercise his searching discretion, he would have used techniques that would have widened the search that he was asked to undertake, which would have returned more results.
431 Once again, Professor Roberts considered that “neutral endopeptidase inhibitors” was an appropriate search term. It is idle to speculate on the results that might have been returned if “neutral endopeptidase inhibitor” was used as the search term. But, even if it be correct that the search term “neutral endopeptidase inhibitors” will not pick up a reference to “neutral endopeptidase inhibitor” (this outcome was only done as a thought experiment), and even if Dr Mokdsi had been permitted to use his searching discretion, I assume that, in the exercise of that discretion, and in accordance with Novartis’s submission, Dr Mokdsi would have used a truncator to broaden the search results to include results for “neutral endopeptidase inhibitor” and “neutral endopeptidase inhibitors”. In other words, the deployment of Dr Mokdsi’s searching discretion would still have returned results that included the Ksander paper.
432 As to Novartis’s submission that Dr Mokdsi’s “linear” search (as opposed to Professor Roberts’s iterative search and review process) would not have established the “landscape” of NEP inhibitors, the relevant question is whether, by using the search term “neutral endopeptidase inhibitors”—on the evidence, an appropriate search term—the Ksander documents could have been ascertained. The evidence answers that question affirmatively. The Ksander documents were ascertained. It is not to the point that an iterative search and review process, which still included the search term “neutral endopeptidase inhibitors”, would have thrown up a wider pool of search results that included the Ksander documents. The Ksander documents would still have been ascertained.
433 Finally, it is irrelevant to the question of ascertainment that, following Dr Mokdsi’s search, Professor Roberts was only given the Ksander documents.
434 Novartis submits that even if the ascertainment requirement of s 7(3) is satisfied, when the Ksander documents are “placed in the context of the appropriate task and the CGK, they are not reasonably to be regarded as documents directed to solving the same or similar problem to that solved by the Patent, as at 2002”.
435 Novartis submits neither the Ksander paper, nor the Ksander patent, discloses the use of sacubitril/at (or any other NEP inhibitor) in combination with another agent. Moreover, neither document contains data on the administration of sacubitril/at to humans. Novartis submits that, whatever the position may have been at the time the Ksander documents were published, “the art (as a matter of CGK) had moved on by 2002”.
436 In this regard, Novartis argues that the Ksander paper teaches that, as at the date of its publication (1995), clinical trials had shown that NEP inhibitors, at best, had moderate pharmacological activity. The Ksander paper advanced the hypothesis that these poor clinical outcomes may have arisen from inferior pharmacokinetics or potency.
437 As at 2002, Kaplan NM, ‘Systemic Hypertension: Therapy” in Braunwald E, Zipes DP and Libby P (eds), Heart Disease: A Textbook of Cardiovascular Medicine (6th ed, W.B. Saunders Company, Philadelphia, 2001) (Braunwald), (a leading textbook on cardiovascular medicine, which Novartis accepts was commonly used for the education of trainee and younger cardiologists, but less commonly by cardiologists later in their professional lives), reported that, in limited clinical trials in patients with heart failure, NEP inhibitors had relatively little efficacy.
438 Novartis says that, apart from ecadotril which, in 1998, had been administered safely to humans and had been well-tolerated, the only active interest in NEP inhibition at the priority date was in relation to omapatrilat which, as I have said, was a vasopeptidase inhibitor possessing both ACE and NEP inhibitory action. Braunwald said:
The combination of an NEP antagonist with an angiotensin-converting enzyme (ACE) inhibitor has been shown to result in more sustained natriuretic effects than an NEP antagonist alone in experimental animal models of HF, in part because of the inhibition of angiotensin II-mediated effects but also because of the fact that both NEP and ACE degrade bradykinin, a vasodilatory and natriuretic peptide.
439 Braunwald also reported that it seemed likely that omapatrilat would prove useful in the treatment of heart failure.
440 Novartis refers to other publications that variously report that: (a) the combination of ACE and NEP inhibitors may be especially useful in hypertension and heart failure; (b) studies with NEP inhibitors alone or in combination with ACE inhibitors had shown “mixed results”, with the largest study (which was the use of ecadotril in heart failure) showing no obvious benefit.
441 Novartis submits that Professor Coats’s recommendation to combine an ARB with an NEP inhibitors was an inventive step, not a routine step. I will consider that submission later. For present purposes, Novartis submits that the person skilled in the art would not have considered either the Ksander paper or the Ksander patent as relevant because of the information which I have summarised above.
442 In support, Novartis submits that the person skilled in the art would not “reasonably” regard a “drug discovery/early drug development paper” (i.e., the Ksander paper) to be relevant to the task. The NEP inhibitor would have to be one that, at the priority date, had already been trialled in humans and “further down the development pathway”.
443 Novartis submits that Professor Roberts’s views on this question are not probative of the question of relevance because he was given the Ksander documents in the context of identifying an NEP inhibitor to combine with valsartan, which was not the task set by Pharmacor’s expression of the reformulated “Cripps question”. Further, neither the Ksander paper nor the Ksander patent teach or suggest the combination of an NEP inhibitor with an ARB.
444 I am not persuaded by Novartis’s submissions on this question, whatever merit they might have for other aspects of its defence to Pharmacor’s challenge.
445 As is made clear in Lockwood (No 2), the starting point for determining relevance under s 7(3) of the Patents Act is the subject matter of the invention. Here, the subject matter of the invention is the development of advantageous and more efficacious combination theory for the treatment of hypertension, the treatment or prevention of heart failure, and the treatment and prevention of myocardial infarction and its sequelae, involving a composition that contains an identified AT 1-antagonist and identified NEP inhibitors. Plainly, documents that concern the therapeutic efficacy of NEP inhibitors are relevant to that subject matter.
446 In any event, Professor Roberts’s evidence is clear on this question and is not disturbed by any of the matters that Novartis has put forward by way of advocacy. Importantly, there is no evidence from any person having Professor Roberts’s skills as a pharmaceutical scientist that contradicts or qualifies his views as to the relevance of the Ksander documents.
The pathway taken for the hypothetical tasks
447 Pharmacor advances its case on obviousness by reference to its expressions of the reformulated “Cripps question” supported by the evidence it has adduced in respect of the three hypothetical tasks. The three hypothetical tasks serve as a tool for answering the reformulated “Cripps question” which, itself, is a tool for answering the statutory question of obviousness. Whether Pharmacor’s expressions of the reformulated “Cripps question” are answered affirmatively depends, therefore, on whether I accept that the steps, decisions, and choices shown by that evidence are the steps, decisions, and choices that the person skilled in the art would likely have taken at the priority date in undertaking those tasks.
448 It is appropriate, therefore, to commence by identifying the critical stages in Professor Coats’s reasoning, when undertaking his hypothetical tasks, that led him to decide on a combination drug containing valsartan and a complementary NEP inhibitor, which later came to be identified by Professor Roberts as sacubitril, through the s 7(3) inquiry.
449 Stage I was Professor Coats’s decision to address the task by devising a drug that provided multiple modes of action. Stage II was his decision that the therapeutic agents should treat both hypertension and heart failure. Stage III was his decision that a selection should be made of one therapeutic agent from the main three recommended classes of pharmacotherapy for the treatment of heart failure and one therapeutic agent from “the more promising classes of new agents”. Stage IV was Professor Coats’s decision to select an NEP inhibitor from “the more promising classes of new agents”. Stage V was his decision that the NEP inhibitor should be combined with an ARB. Stage VI was his decision that the ARB should be valsartan. Stage VII was his decision to defer to the “team” (the pharmaceutical scientist) to provide a list of suitable, complementary NEP inhibitors.
450 Pharmacor’s submissions advance, in particular, Stages II, V, VI, and VII, and Professor Coats’s explanations and reasons for his decisions at these stages, as representing the approach of the uninventive person skilled in the art.
451 For the purposes of analysis, Stages I and II can be considered together. In analysing those stages, it is important to understand that Professor Coats’s first hypothetical task was to come up with a pharmacotherapy for the treatment of hypertension, not a pharmacotherapy for the treatment of heart failure (which came to be his second hypothetical task) or for the treatment of hypertension and heart failure (which came to be his third hypothetical task). It is also important to understand that the second and third hypothetical tasks were put to Professor Coats only after he was asked to consider the first hypothetical task which, itself, was put to Professor Coats only after he was asked to address, and did address extensively, hypertension and heart failure, and the approach to treatment of these conditions before the priority date.
452 It is, perhaps, little wonder, therefore, that, after being asked to address hypertension and heart failure, and the approach to the treatment of these conditions at the priority date, Professor Coats decided to pursue a strategy that involved the treatment of both hypertension and heart failure in a combination product, even though his first task was to consider an improved or useful alternative pharmacotherapy for the treatment of hypertension.
453 In making this observation, I make no criticism of Professor Coats whatsoever. He was an impressive witness whose evidence, and considerable knowledge and expertise, were of great assistance to the Court. However, the sequencing of his evidence in this way placed him at the threshold of a critical pathway that can be seen to have influenced each and every decision and choice he made in undertaking his first hypothetical task. Professor Coats then applied his decision-making in the first hypothetical task to the second and third hypothetical tasks, with only minor modifications.
454 I draw attention to this because the evidence does not establish that, at the priority date, there was a want or need for a pharmacotherapy that treated both hypertension and heart failure in a combination product, let alone a common general knowledge appreciation of such a want or need. Notionally taken at the priority date, Professor Coats’s perception, in the absence of an established need or want, that such a product might be advantageous, was the beginning of invention—the beginning of “an advance of contemporary expectations”: AB Hassle at [38].
455 Stages III and IV of Professor Coats’s decision-making can also be considered together. Stage III was the selection of pharmacotherapy that treated both hypertension and heart failure. As such, it was a step down the pathway on which Professor Coats had been placed. In making that selection, Professor Coats directed himself to one agent from existing classes of pharmacotherapy for treating heart failure and selecting another agent from the “promising classes of new agents”. Step IV was the decision to select an NEP inhibitor from the “promising classes of new agents” in preference to other agents in that class. It was a further step down the pathway on which Professor Coats had been placed. Notably, this step was taken by Professor Coats before his selection of an agent from the existing classes of pharmacotherapy for treating heart failure.
456 Professor Coats’s motivation for selecting an agent from the “more promising classes of new agents” was his perception that it was necessary for his task to demonstrate “an additional compelling benefit” which, in his view, could only be provided by, or be the result of, these new and more promising agents.
457 This reasoning is significant. It marks a departure by Professor Coats from the position of the uninventive person skilled in the art who would not be tasked with striving to find a pharmacotherapy that demonstrates “an additional compelling benefit”. It is apparent, however, that this personal motivation drove Professor Coats to consider agents from “the more promising classes of new agents”. The uninventive person skilled in the art would not be equally or similarly driven.
458 Having reached the point where he needed to consider agents from “the more promising classes of new agents”, Professor Coats selected NEP inhibitors. In doing so, he rejected vasopressin receptor antagonists and endothelin receptor antagonists because, even though, at the priority date, they were the subject of more progressed developed projects and major development programs, those projects and programs had not resulted in a pharmaceutical product for hypertension and heart failure.
459 Having rejected vasopressin receptor antagonists and endothelin receptor antagonists, Professor Coats’s selected NEP inhibitors. His reason for that selection was that:
… natriuretic peptides have a number of beneficial effects for hypertension and heart failure. Accordingly, based on the theoretical understanding of the cardiovascular system at the [priority date], inhibiting NEP and therefore promoting the action of natriuretic peptides was a promising avenue for treatment.
460 As I have noted, Braunwald had reported that, in clinical trials in patients with heart failure, NEP inhibitors had relatively little efficacy. It was only in combination with an ACE inhibitor (namely, in the vasopeptidase inhibitor omapatrilat) that Braunwald had reported that an NEP inhibitor might prove useful in the treatment of heart failure.
461 An article published in 2001, Corti R et al, “Vasopeptidase Inhibitors: A New Therapeutic Concept in Cardiovascular Disease?” (2001) 104(5) Circulation 1856 – 1862 (Corti), reported that the combination of ACE and NEP inhibition may be especially useful in the treatment of hypertension and heart failure by reason of the synergistic effect of the two agents. There is a dispute about whether Corti is part of the common general knowledge. It is not necessary for me to dwell on that question because I do not think that Corti advances Pharmacor’s case in any event. Relevantly, Corti’s teaching is substantially consistent with the teaching in Braunwald that, at the priority date, the possible utility of NEP inhibitors in the treatment of heart failure was only in combination with an ACE inhibitor.
462 In light of that teaching, Professor Coats’s selection of NEP inhibitors because they were a promising avenue for the treatment of heart failure—based simply on the theoretical understanding of the cardiovascular system—is curious. In any event, Professor Coats’s reasoning gives colour to his description “a promising avenue of treatment”.
463 Stage V of Professor Coats’s reasoning—to combine an ARB with the NEP inhibitor—is significant in three respects. First, it proceeds directly from Professor Coats’s selection of NEP inhibitors as a promising avenue of treatment, and not otherwise. Secondly, Professor Coats’s decision was influenced by the promising example provided by omapatrilat. Thirdly, notwithstanding the common general knowledge that the possible utility of NEP inhibitors in the treatment of heart failure was in combination with an agent that was an ACE inhibitor (of which omapatrilat can be taken as an example), Professor Coats decided to recommend a therapy that removed the ACE inhibitor from that combination. He decided to pursue a different, unknown, and therefore untried, combination—namely, an NEP inhibitor and an ARB—in circumstances where (as explained below) ACE inhibitors were considered to be mandatory treatment in heart failure, and ARBs were recommended only when patients were ACE inhibitor-intolerant.
464 Professor Coats’s reasoning was that, by replacing the ACE inhibitor with an ARB, he expected to ameliorate the risk of angioedema associated with ACE inhibition. I note, however, that, regardless of that motivation (which was the subject of detailed criticism in Novartis’s submissions), there was nothing in the common general knowledge at the priority date that suggested NEP inhibition in combination with an ARB was a promising avenue for the treatment of heart failure. Professor Coats’s decision to replace an ACE inhibitor with an ARB, in the only combination in which NEP inhibition in the treatment of heart failure showed promise, was, in fact, a significant departure from the common general knowledge. This placed Professor Coats’s development task(s) in uncharted territory.
465 To elucidate the significance of Professor Coats’s decision, it is appropriate that I say something more about ARBs at the priority date.
466 The first ARB to become available in Australia was losartan. It was approved by the TGA in about 1996 for the treatment of hypertension, not heart failure. It was only available for a short period of time (less than one year) before it was withdrawn. The next ARB was irbesartan, which was approved in about 1998. It was followed by candesartan, which was approved in about 1999. Irbesartan and candesartan were also approved for hypertension, not for heart failure.
467 I have already referred to the fact that the ELITE clinical trial (a safety and tolerability study) investigated losartan and captopril (an ACE inhibitor) in heart failure. In 1997, it reported on results that suggested that losartan was significantly superior to captopril in reducing mortality. However, a larger follow-on trial, ELITE II, conducted from 1997, concluded that mortality did not differ significantly between losartan and captopril.
468 The National Heart Foundation of Australia Guidelines for management of patients with chronic heart failure in Australia published in May 2001 (the NHF Guidelines) which, the parties agree, was common general knowledge at the priority date, recommended, as first line agents for the treatment of heart failure, ACE inhibitors; diuretics; beta-blockers; and spironolactone. The NHF Guidelines also recommended ARBs as first-line agents, with this qualification:
Angiotensin II receptor antagonists may be used as an alternative to ACE inhibitors for patients who are truly ACE-intolerant because of kinin-mediated adverse effects (eg, cough).
469 In relation to this qualification, the NHF Guidelines cited Thackray (see [63] above). Thackray said that the purpose of Val-HeFT was to determine whether the addition of an ARB (valsartan) to an ACE inhibitor would confer additional benefits on mortality and morbidity in patients with heart failure and left ventricular systolic dysfunction. The NHF Guidelines, themselves, reported:
It is possible (but not yet confirmed) that combination therapy with ACE inhibitors and AII antagonists may maximise the benefits of renin-angiotensin system blockade.
470 In Val-HeFT, 5,010 patients were randomly assigned to receive either valsartan (2,511 patients) or placebo (2,499 patients). All patients were receiving background treatment for heart failure: 93% were being treated with ACE inhibitors; 35% were receiving beta-blockers; 5% were being treated with spironolactone.
471 Thackray reported:
Overall, Val-HeFT suggests that there may be an advantage to adding valsartan to an ACE inhibitor in patients with heart failure. Data on all-cause hospitalisation is required before a proper evaluation of the true clinical benefit and cost-effectiveness of this strategy can be judged. The next task will be to decide whether it is an increased dose of ACE inhibitors [3], spironolactone [4] or valsartan that should be added to standard therapy with ACE inhibitors and beta-blockers. Some patients will need all of these strategies, but tailoring treatment to the individual patient’s needs is an important target for future research. Knowing when a patient is receiving too much therapy and which treatment to withdraw is of key importance.
472 In 2001, Braunwald said that an ACE inhibitor was considered to be mandatory treatment in chronic heart failure or asymptomatic left ventricular systolic dysfunction. It said that the only consistent adverse effect noted with ACE inhibitors was a low-level increase in cough. It said that, in such patients, an ARB could be substituted. It also noted that, while clinical trial data on ARBs was emerging, the results were “limited and mixed”:
At this juncture, there is no reason to believe that ARBs will prove superior to ACE inhibitors in patients with HF, with the exception of the side effect of cough … Moreover, based on bradykinin enhancement by ACE inhibitors, it is possible that ARBs will, in fact, be inferior to ACE inhibitors.
473 Braunwald also said:
The future of ARB treatment in chronic HF would appear to be in combination with ACE inhibitors (and potentially spironolactone) to provide more complete inhibition of the RAAS. This view is supported by the observation that the combination of an ACE inhibitor and an ARB produces additive hemodynamic effects. Recently reported clinical trial data also support this concept. … Other studies investigating the combination of an ACE inhibitor and an ARB that have clinical primary endpoints (such as … [Val-HeFT]) are under way, and they will yield a definitive answer regarding ARBs in combination with ACE inhibitors.
For the time being, recommendation for the use of ARBs in chronic HF are limited to patients who are ACE inhibitor intolerant, particularly those with an ACE inhibitor-induced cough.
474 Writing in the International Journal of Cardiology in 2001, (Coats AJS, “Angiotensin Receptor Blockers – finally the evidence is coming in: IDNT and RENAAL” (2001) 79 International Journal of Cardiology 99-102), Professor Coats noted that there were two major options to build on the success of ACE inhibitors—vasopeptidase inhibitors (with omapatrilat showing “tremendous potential in both hypertension and heart failure”) and ARBs.
475 As to vasopeptidase inhibitors, he said:
What made this class look so interesting? It was partly the fact that they inhibit both the ACE enzyme and the neutral endopeptidase that inhibits the break down of the natriuretic peptides and many other interesting vasoactive peptide systems. This accounted for its greater antihypertensive efficacy but also raised the possibility of other disparate effects over and above those seen with ACE inhibitors.
476 As to ARBs, Professor Coats said:
They are more specific and yet certainly retain at least some of the ACE inhibitors effects; similar haemodynamic effects, similar if not identical blood pressure reduction and fewer side effects, especially avoiding the occasionally trouble-some cough associated with ACE inhibitors. With many of the largest pharmaceutical companies backing the ARBs and an enormous clinical trial programme, why has this class not taken over? The answer is that definitive outcome data, by which we mean important clinical end-points from large well designed randomised clinical trials, have been slow in coming.
477 This last-mentioned comment was made before the final results of Val-HeFT were published in Cohn (see [64] above).
478 Cohn reported that, in respect of the two groups (valsartan or placebo), mortality was similar. However, the incidence of the combined end point—mortality and morbidity (defined as the incidence of cardiac arrest with resuscitation, hospitalisation for heart failure, the receipt of intravenous inotropic or vasodilator therapy for at least four hours)—was 13.2% lower with valsartan than with placebo.
479 Cohn also reported:
… Among all 366 patients who were not receiving an ACE inhibitor, whether or not a beta-blocker had been prescribed, there was a significantly lower risk of the combined end point in the valsartan group than in the placebo group (relative risk, 0.56; 95 percent confidence interval, 0.39 to 0.81), as well as a lower risk of death (relative risk, 0.67; 95 percent confidence interval, 0.42 to 1.06).
480 There was a significant dispute between Professor Coats and Professor Hare as to the statistical reliability of this observation. I understand the observation of “significantly lower risk” in the above quotation to be referring to a statistically lower risk having regard to the parameters assigned. I accept that Cohn reported this finding because it was an important clinical finding on the efficacy of valsartan in the treatment of heart failure in respect of the subgroup of participants who: (a) were receiving valsartan, (b) not receiving an ACE inhibitor; and (c) may have been receiving a beta-blocker. It supports the common general knowledge, already made clear in the NHF Guidelines at the priority date (based on Thackray), that an ARB (in this case, valsartan) can be used as a substitute for an ACE inhibitor in the treatment of heart failure.
481 However, Cohn also reported that:
… Within the 30 percent of the population that was being treated with both an ACE inhibitor and a beta-blocker at base line, there was a significant adverse effect of valsartan on mortality and a nearly significant adverse effect of morbidity. Clarification of whether this finding represents a true interaction or it attributable to chance must await the outcome of ongoing trials evaluating the combination of an angiotensin-receptor blocker with an ACE inhibitor and a beta-blocker. Since only 5 percent of the patients in the trial were receiving spironolactone, an aldosterone-receptor blocker, we cannot assess the efficacy or safety of valsartan when given in combination with spironolactone.
482 Although published only shortly before the priority date, I accept that the overall results of Val-HeFT were common general knowledge, particularly in light of the earlier disclosures in Thackray and the general interest in Val-HeFT. I accept that, at the priority date, it was common general knowledge that: (a) valsartan significantly reduces the combined end point of mortality and morbidity and improves clinical signs and symptoms in patients with heart failure, when added to prescribed therapy; (b) valsartan combined with an ACE inhibitor may enhance blockade of the RAAS; (c) valsartan administered with or without a beta-blocker may also be effective in significantly lowering the risk of the combined end point; but (d) there was concern about the potential safety of administering valsartan with an ACE inhibitor and a beta-blocker.
483 I observe that, while Val-HeFT showed the potential efficacy of valsartan in the treatment of heart failure, including with other therapies in use for heart failure, it said nothing about the efficacy or utility of valsartan and an NEP inhibitor in a combination product, including whether such a product might be a useful or better alternative to other pharmacotherapies for the treatment of hypertension, or heart failure, or hypertension and heart failure.
484 Returning to Stages I to V of Professor Coats’s decision-making, I am unable to accept that the steps, decisions, and choices made by Professor Coats, although well-reasoned, are indicative of the steps, decisions, and choices of the uninventive person skilled in the art at the priority date, or that it is likely that the person skilled in the art would have been directly led as a matter of course to take those steps or to make those decisions and choices. The course that Professor Coats took by Stages I to V in respect of each of his hypothetical tasks, leading to a decision to use an ARB/NEP inhibitor combination without an ACE inhibitor, is akin to the “voyage of discovery” described by Buckley LJ in Beecham Group Limited’s (Amoxycillin) Application [1980] RPC 261 at 296 in respect of amoxycillin (a penicillin):
To reach the discovery of the particular characteristics of Amoxycillin and its suitability for treating humans the research worker would have had to embark on a voyage of discovery. It is possible now to see that his voyage would have been short and perhaps uneventfully straightforward, but where each of his two, and possibly more, vessels would make landfall and what those places would be like would not have been obvious to him at the outset. The voyage might have been clearly worth trying but not as a means of reaching a specific hoped-for destination.
485 This is not least because:
(a) The uninventive person skilled in the art would not have been placed at the threshold of a particular pathway, at least in relation to the first hypothetical task and the second hypothetical task.
(b) There was no common general knowledge want or need for a pharmacotherapy that treated both hypertension and heart failure in a combination product although, no doubt, such a product, if available, might be a useful addition to available pharmacotherapy.
(c) Still less was there a want or need for a pharmacotherapy that demonstrated “an additional compelling benefit”.
(d) The uninventive person skilled in the art would not have been striving for a pharmacotherapy that demonstrated “an additional compelling benefit”.
(e) For this reason, it is not likely that the uninventive person skilled in the art would have been driven to consider “the more promising classes of new agents”, or selected NEP inhibitors from such a class.
(f) Even if the uninventive person skilled in the art would have considered NEP inhibitors, that consideration would have been in combination with ACE inhibitors. In clinical trials in patients with heart failure, NEP inhibitors alone had demonstrated relatively little efficacy.
(g) It is unlikely that the uninventive person skilled in the art would have forsaken the combination of NEP inhibitors and ACE inhibitors, which was a promising line of pharmacotherapy, to explore the unchartered territory of a new combination of NEP inhibitors and ARBs for which there was no antecedent.
486 Moreover, in relation to ACE inhibitors and ARBs in the treatment of heart failure, the common general knowledge at the priority date was that:
(a) ACE inhibitors were considered mandatory treatment in heart failure.
(b) Although ARBs could be used in substitution for ACE inhibitors in treating heart failure, this was only when patients were ACE inhibitor-intolerant, specifically because of a low level increase in cough (not, I note, because of the risk of angioedema).
(c) It was unknown whether ARBs would be more effective, less effective, or equally effective than ACE inhibitors in treating heart failure.
(d) However, ARBs could be used with ACE inhibitors in treating heart failure. Braunwald described this as “the future of ARB treatment in chronic HF” to provide “more complete inhibition of the RAAS”. A similar observation was made in the NHF Guidelines.
(e) ARBs could be used with beta-blockers in treating heart failure. There was, however, a potential safety concern in using ARBs (valsartan) in combination with an ACE inhibitor and a beta-blocker.
487 Even if at the priority date the uninventive person skilled in the art happened to have ended up where Professor Coats found himself at the end of Stage V, this could only have been because he or she had reason(s) to think (not necessarily Professor Coats’s reasons to think) that it was worthwhile trying an NEP inhibitor/ARB combination without an ACE inhibitor. However, given the common general knowledge at the priority date, the uninventive person skilled in the art could only have been motivated by the speculative belief that such an NEP inhibitor/ARB combination was worth trying to see if it would provide useful therapy for the treatment of hypertension, or heart failure, or hypertension and heart failure (whichever hypothetical task is in contemplation). I do not accept that the uninventive person skilled in the art would have had a reasonable basis to expect that that particular combination might well provide useful therapy in that regard.
488 These findings and conclusions are fatal to Pharmacor’s challenge to the validity of claim 1 of the patent on the ground of lack of inventive step, even before one reaches the remaining steps of selecting valsartan as the ARB and, ultimately, sacubitril as the NEP inhibitor.
489 As to the selection of valsartan, I have already noted that there is nothing in the common general knowledge with respect to valsartan (including through the knowledge imparted by Thackray and Cohn) that suggests that its use in a combination product with an NEP inhibitor might provide a useful or better alternative to other pharmacotherapies for the treatment of hypertension, or heart failure, or hypertension and heart failure.
490 Further, I do not accept that, from the perspective of the person skilled in the art, the possibility of success of such an ARB/NEP inhibitor combination, even with valsartan as the ARB, would be advanced to an expectation of success at the priority date (in the sense that such a combination might well produce a useful or better alternative to other pharmacotherapies for the treatment of hypertension, or heart failure, or hypertension and heart failure) by information in the Ksander paper and/or the Ksander patent. This is so notwithstanding Professor Roberts’s analysis, based on the disclosures in those documents, that sacubitril would be a preferred NEP inhibitor for use with valsartan. An ARB/NEP inhibitor combination would have remained uncharted territory.
491 For these reasons, Pharmacor’s challenge to the invention claimed in claim 1 on the ground that it lacks an inventive step, fails.
492 Before departing from this topic, I should express my concern about the utility of posing for the consideration of an expert witness a task or tasks that seek the “solution” to a “problem” when the “solution” to the “problem” must already have been known to the witness.
493 Entresto was entered on the ARTG on 20 January 2016 as a sacubitril/valsartan product—combined as a sodium salt hydrate complex—in the form of a tablet for oral administration to adult patients for the treatment of chronic heart failure with reduced ejection fraction. Entresto must have been well-known to Professor Coats when he was asked to undertake his hypothetical tasks.
494 Once again, I make no criticism of Professor Coats who, as I have said, was an impressive witness. However, no matter how conscientiously Professor Coats undertook the tasks he was assigned—and I do not doubt for one moment that he did undertake those tasks conscientiously—Entresto, as a product for treating heart failure, could not have been eliminated from his mind. Hindsight is insidious. An expert, with a background knowledge of Entresto, should not have been put in the position of having to address the hypothetical tasks that Professor Coats was asked to address. The inquiry under s 7(2) is not whether the claimed invention can be explained as a rational choice of integers, but whether, at the priority date, it was obvious.
495 Pharmacor’s challenge to the validity of claim 1 on the basis that the invention, as claimed, is not fairly based on the matter described in the specification (see s 40(3) of the Patents Act as it applies to this proceeding), depends on a finding that integers (i) and (ii) of the pharmaceutical composition are satisfied by a complex containing anionic valsartan, anionic sacubitril and pharmaceutically acceptable cations. I have found to the contrary. Therefore, this challenge to the validity of claim 1 cannot succeed. I do not intend to deal with it on the basis of an alternative construction of claim 1 which I do not accept.
Alleged omission of best method
496 Pharmacor’s challenge to the validity of claim 1 on the basis that the complete specification does not describe the best method known to Novartis AG of performing the invention in claim 1 also depends on a finding that integers (i) and (ii) of the pharmaceutical composition are satisfied by a complex containing anionic valsartan, anionic sacubitril and pharmaceutically acceptable cations. As I have found to the contrary, this challenge to validity cannot succeed.
497 In any event, this challenge depends on s 40(2)(a) of the Patents Act (in the form in which it applies in this proceeding) being construed to require the obligation to describe the best method as operating not only at the date of filing the complete specification (here, 16 January 2003) but also at other, subsequent dates—here, 28 November 2006 (when Novartis AG last amended the complete specification before the patent was granted) and 5 April 2007 (when the patent was granted).
498 Pharmacor’s challenge raises an important question of law which is entirely academic in light of the findings I have made. It is not appropriate that I answer that question in those circumstances.
499 Pharmacor has succeeded in its defence of Novartis’s claim that it has threatened to infringe claim 1 of the patent. Pharmacor has also succeeded in establishing that the term of the patent should not have been extended. It is appropriate that the Register be rectified. Pharmacor’s challenge to the validity of claim 1 has failed.
500 It will be necessary for orders to be made reflecting these outcomes. By 4.00 pm on 20 November 2024, the parties are to bring in agreed or, if not agreed competing, draft orders.
501 In the absence of the parties agreeing on an appropriate costs order, my provisional view is that Novartis should pay Pharmacor’s costs of the issues on which Pharmacor succeeded and that Pharmacor should pay Novartis’s costs of the issues on which Pharmacor did not succeed (including those issues which did not fall for determination because of the construction of claim 1 that I accepted).
502 If necessary, I will hear the parties, on short notice, in respect of any question arising as to the orders that should be made.
I certify that the preceding five hundred and two (502) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Yates. |
Associate:







