FEDERAL COURT OF AUSTRALIA
McNickle v Huntsman Chemical Company Australia Pty Ltd (Initial Trial) [2024] FCA 807
ORDERS
Applicant | ||
AND: | HUNTSMAN CHEMICAL COMPANY AUSTRALIA PTY LTD (ACN 004 146 338) First Respondent MONSANTO AUSTRALIA PTY LTD (ACN 006 725 560) Second Respondent MONSANTO COMPANY Third Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. Pursuant to s 33ZF of the Federal Court of Australia Act 1976 (Cth) (FCA Act) (and notwithstanding that no application has been made by the representative party in accordance with s 33K(1) of the FCA Act) leave be granted for the group membership to be amended nunc pro tunc so that it is defined as it is in the last version of the statement of claim filed prior to the fixing of the date for opt out (being the second further amended statement of claim filed on 19 October 2020).
2. Pursuant to ss 33ZF and 37P(2) of the FCA Act, the following question, common to the claim of the applicant and all group members (Central Common Question) be answered separately and before any other question or issue in the proceeding:
Throughout the period between July 1976 and 19 October 2020 (relevant period), did or could use of and/or exposure to the herbicide product or products, which contained glyphosate and were branded as “Roundup”, or which contained glyphosate and were otherwise branded with the name “Monsanto” (Roundup Products) increase an individual’s risk of developing non-Hodgkin lymphoma (NHL); and/or cause an individual to develop NHL?
3. The Central Common Question be answered as follows:
It is not proven in this proceeding on the balance of probabilities (in accordance with s 140(1) of the Evidence Act 1995 (Cth)), that throughout the relevant period, use of and/or exposure to Roundup Products increased an individual’s risk of developing NHL; and/or caused an individual to develop NHL.
4. The proceeding be dismissed upon finalisation of any issues relating to costs.
5. Any further evidence as to costs (as contemplated in the reasons (at [1202])) and any submissions as to costs be filed by 12 noon on 30 July 2024.
6. For the purposes of s 33ZB of the FCA Act, Orders 1 to 3 above affect (and hence bind) all current group members in the class action (being those named in the second further amended statement of claim who did not opt out).
7. The proceeding be adjourned for the parties to be heard on the appropriate costs orders in conformity with these reasons at 10:15am on 31 July 2024.
AND THE COURT NOTES THAT:
8. The leave granted after the date of opt out had passed to amend the statement of claim so as to file the third and fourth statement of claim did not include leave being granted on application made by the representative party to alter the description of the group pursuant to s 33K(1) of the FCA Act.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
[1] | |
[11] | |
B.1 Relevant Procedural Background and the Role of the Assessor | [11] |
[25] | |
[36] | |
[49] | |
[66] | |
[73] | |
[75] | |
[82] | |
[85] | |
[90] | |
[93] | |
[93] | |
[93] | |
[103] | |
[109] | |
[109] | |
[117] | |
[118] | |
[120] | |
[123] | |
[124] | |
[126] | |
[126] | |
[131] | |
[131] | |
[142] | |
[155] | |
[162] | |
[169] | |
[173] | |
[173] | |
[188] | |
[199] | |
[212] | |
[228] | |
[231] | |
[238] | |
[256] | |
[270] | |
[278] | |
[289] | |
[294] | |
[296] | |
[299] | |
[302] | |
[306] | |
IV Agricultural Health Study – De Roos (2005) and Andreotti (2018) | [309] |
[309] | |
[317] | |
[326] | |
[330] | |
[348] | |
[358] | |
[362] | |
[370] | |
[371] | |
[371] | |
[375] | |
[387] | |
[399] | |
[400] | |
[410] | |
[410] | |
[410] | |
[414] | |
[429] | |
[429] | |
[433] | |
[433] | |
[446] | |
[463] | |
[468] | |
[470] | |
[475] | |
[477] | |
[503] | |
[508] | |
[517] | |
[519] | |
[521] | |
[540] | |
[550] | |
[565] | |
[573] | |
[576] | |
2. Liver hepatocellular adenomas and carcinomas in male rats | [595] |
[605] | |
[608] | |
[615] | |
[623] | |
[634] | |
G THE THIRD STREAM: MECHANISTIC EVIDENCE (CONCLAVES A, B AND G) | [641] |
[641] | |
[641] | |
[643] | |
[643] | |
[656] | |
[673] | |
[684] | |
[684] | |
[687] | |
[687] | |
[705] | |
[723] | |
[750] | |
[760] | |
[766] | |
[770] | |
[771] | |
[778] | |
[779] | |
[789] | |
[796] | |
[801] | |
[808] | |
[813] | |
[819] | |
[826] | |
[832] | |
[834] | |
[835] | |
[847] | |
[856] | |
[863] | |
[871] | |
[874] | |
[874] | |
[880] | |
[890] | |
[901] | |
[903] | |
[918] | |
[918] | |
[918] | |
[921] | |
[924] | |
[935] | |
[936] | |
[950] | |
[953] | |
[960] | |
[965] | |
[966] | |
[976] | |
[979] | |
[987] | |
[987] | |
[989] | |
[1021] | |
[1024] | |
[1025] | |
[1029] | |
[1038] | |
[1042] | |
[1048] | |
[1048] | |
[1048] | |
[1053] | |
[1053] | |
[1056] | |
[1058] | |
[1059] | |
[1062] | |
[1067] | |
[1067] | |
[1072] | |
[1077] | |
[1088] | |
[1092] | |
[1095] | |
[1095] | |
[1101] | |
[1110] | |
[1120] | |
[1127] | |
[1128] | |
[1133] | |
[1147] | |
[1147] | |
[1153] | |
[1163] | |
[1167] | |
[1177] | |
[1177] | |
[1182] | |
[1197] | |
[1199] | |
[1202] | |
[1203] |
LEE J:
1 Mr Kelvin McNickle lived what might be thought by a young lad to be an Australian idyll. Born in September 1982, he grew up on a rural property on the beautiful Mid North Coast of New South Wales. His father operated a vegetation management business, and, during school holidays, Mr McNickle assisted his dad clearing vegetation.
2 But this was no passing stage. After completing school, Mr McNickle began working full-time for his father. No doubt it was hard work. Almost every working day, Mr McNickle and his father sprayed a weedkiller called “Roundup” on weeds and other unwanted growth using a knapsack sprayer or a drenching unit. As might be expected, drips or leaks of the Roundup soaked through his clothes and onto his skin. Mist or spray would go all over the place and get into his eyes and onto his face and fumes were inhaled.
3 Some years later, after marrying, Mr and Mrs McNickle left Coffs Harbour for Darwin. Mr McNickle is an apparently devout man. The Apostle Paul said: “let him do hard work, doing with his hands what is good work” (Ephesians 4:28). Apparently motivated by the perceived moral value of good and hard manual work, Mr McNickle volunteered to maintain the lawns and gardens of the Kingdom Hall of Jehovah’s Witnesses. His evidence is that in doing so he used Roundup.
4 In May 2018, Mr McNickle was diagnosed with a type of cancer known as non-Hodgkin lymphoma (NHL). Just over a year later, he was informed by a haematologist that he was in ongoing remission. Unfortunately, about six weeks prior to the commencement of the initial trial, his NHL recurred. As a result, Mr McNickle was unable to attend the hearing.
5 I have introduced Mr McNickle, who is the lead applicant in this class action. But this judgment is not, at least directly, about him. This is not the occasion to deal with questions of individual causation.
6 Rather, when this initial trial was fixed, it was contemplated I would determine a set of common questions relating to Mr McNickle’s contention that during a period from July 1976 until an end date defined at the time I made the orders (Relevant Period), glyphosate, glyphosate-based formulations (GBFs), and Roundup Herbicide and Roundup Biactive (Roundup Products) (which have glyphosate as a component), were carcinogenic to humans. The respondents (collectively, Monsanto) reject this contention completely.
7 The common questions were identified in orders made by consent following a case management hearing in April 2023. I set out the terms of those questions in Section J below and why I do not propose to answer them in the form initially proposed.
8 As it happened, issues were refined during the initial trial. In the end, for reasons that will be explained, the determinative question is whether Mr McNickle has discharged his legal onus of proving on the evidence adduced that the use of and/or exposure to Roundup Products can increase an individual’s risk of developing NHL or cause an individual to develop NHL. I will call this, unimaginatively, the central issue.
9 There is no need for me to explain the issues in this proceeding any further. This is a case which has been mired in interlocutory skirmishes and, save for matters which I will mention shortly, I do not propose to rehearse that background here. There have been several judgments of the Court which identify the central issue, the utility of determining it initially, and set out what might be described as the superstructure of the proceeding: see McNickle v Huntsman Chemical Company Australia Pty Ltd (Common Questions) [2023] FCA 662; McNickle v Huntsman Chemical Company Australia Pty Ltd (Additional Expert Conclave) [2022] FCA 1596; McNickle v Huntsman Chemical Company Australia Pty Ltd (Hearing Vacation) [2022] FCA 133; McNickle v Huntsman Chemical Company Australia Pty Ltd (Assessors) [2021] FCA 780; (2021) 285 FCR 244; McNickle v Huntsman Chemical Company Australia Pty Ltd (Expert Evidence) [2021] FCA 370.
10 I will return to the common questions and the utility of answering them below, but at the outset, it is worth saying something about the proper approach to fact finding and the legal method.
B PROPER APPROACH TO FACT FINDING AND THE LEGAL METHOD
B.1 Relevant Procedural Background and the Role of the Assessor
11 Enough has been said to indicate that this case presented significant challenges in fact finding. In the light of the central issue, it is an exercise in considerable understatement to describe the expert opinion evidence and underlying scientific material as dense, complex, and voluminous.
12 As I recorded in McNickle v Huntsman Chemical Company Australia Pty Ltd (Expert Evidence) (at [4]), when this matter first came before me for case management, I indicated my preliminary view was that the Court should adopt the process of appointing referees in a number of scientific disciplines to inquire into, and to report upon, questions concerning the characteristics of the Roundup Products and their alleged carcinogenic effects.
13 The parties vigorously opposed this proposed course.
14 An array of arguments were marshalled and then deployed to oppose referees, including that to proceed along these lines would be constitutionally invalid (an argument wholly devoid of merit and decisively rejected in CPB Contractors Pty Limited v Celsus Pty Limited (formerly known as SA Health Partnership Nominees Pty Ltd) (No 2) [2018] FCA 2112; (2018) 268 FCR 590).
15 With that said, there was one contention made by Mr McNickle that persuaded me (reluctantly) to adopt the course of allowing expert evidence to be deployed in a more traditional way. Mr McNickle’s argument was that prevailing scientific opinion concerning the alleged carcinogenic effects of Roundup had been skewed or somehow manipulated by Monsanto. I concluded that the risk of referees assessing material said to be infected by this allegation of scientific manipulation created a prospect of complicating the inquiry process. Further, the allegation was one of some seriousness and is of a character that meant it should be resolved in open court.
16 Shortly after rejecting the process of references, and faced with what I knew was going to be a raft of highly complex opinion evidence, I noted in McNickle v Huntsman Chemical Company Australia Pty Ltd (Assessors) (at 246 [7]) that almost 100 years ago, Dean Roscoe Pound in his famous work The Spirit of the Common Law (Marshall Jones Company, 1921) (at 214–215) suggested that steps be taken to equip courts with research staffs comparable to those employed by administrative agencies in order to provide judges with specialised investigations, information, and advice. Yet, despite what some have described as centuries of legal history dotted with proof to the contrary, the traditional common law confidence that courts can handle any dispute without assistance has long persisted: see Beuscher, J H “The Use of Experts by the Courts” (1941) 54(7) Harvard Law Review 1105.
17 I also observed that in saying this, there have been attempts by courts to provide, in different ways, expert aid to determination. There are three obvious examples: (1) special juries; (2) referees (properly seen as a version of a special jury); and (3) assessors.
18 Having determined that there was to be no form of special jury, I went on to explain (at 247–253 [11]–[38]) the long history of appointment of assessors tracing back to the sixteenth century; the basis of the Court’s power to order an assessor; and the scope of the role of an assessor. In this last regard, I referred to the judgment of J Forrest J in Matthews v SPI Electricity Pty Ltd (No 32) [2013] VSC 630 and relevantly noted (at 252 [37]) that the role of any assessor would be to assist the judge, but any decision would be that of the tribunal of fact, being the judge alone. Conscious of the need to avoid any procedural unfairness and define the role of the assessor precisely, I explained, with the consent of the parties, that if an assessor was appointed:
(1) the assessor would sit with me during the concurrent evidence sessions, and could question the experts (or counsel) but such questioning would be limited to clarification of the evidence: that is, where the evidence given is perceived by the assessor or me to be ambiguous, unclear or incomplete;
(2) I would consult with the assessor if I found a point of evidence unclear;
(3) I would consult with the assessor in chambers on matters raised by the experts in their oral evidence and in their individual and joint reports, including advice as to any questions the assessor thinks should be asked;
(4) I would seek the guidance of the assessor on scientific and technical matters upon which I lack the requisite knowledge to understand without qualified assistance;
(5) if the assessor raised a theory or opinion that had not previously been identified by the parties, I would disclose this fact and discuss it with counsel; and
(6) the assessor would, from time to time, provide me with advice on matters over which there is dispute between the experts but that such advice is not binding and the determination of a particular issue rests with me.
19 After receiving suggestions from the parties, on 16 August 2021, I made an order pursuant to s 23 and/or s 33ZF of the Federal Court of Australia Act 1976 (Cth) (FCA Act) that Professor Sir John Stewart Savill BA, MBChB, PhD, FRCP, FRCPE, FRCSEd(Hon), FRCPCH(Hon), FASN, FRSE, FMedSci, FAHMS, FRS be appointed as Assessor. In dealing with the Assessor, care has been taken not to depart from the six points identified above and, more generally, to ensure the findings I will make are mine and mine alone, notwithstanding the assistance received from the Assessor in understanding the complex evidence adduced in this case. For completeness, in my dealings with the Assessor, I record he did not raise a theory or opinion privately that had not previously been identified by the parties through their retained experts (see [18(5)] above).
20 Further, and again with the express consent of the parties, when I had completed an initial draft of these reasons without the assistance of the Assessor, I provided a copy of the draft reasons to the Assessor in order to ensure, as it was explained to the parties, that I had not committed any scientific “solecisms” caused by any fundamental misapprehension of the scientific evidence. I only adopted this course after obtaining confirmation by both parties that they regarded any communication between my chambers and the Assessor as being equivalent to an “in chambers” communication and hence wholly confidential, and that they disavowed seeking a copy of any communication of any type between my chambers and the Assessor, including on any appeal.
21 I should record that these reasons would have been significantly impoverished, and the delivery of judgment delayed, without the assistance of Sir John. It would have been much more difficult to identify and then understand the nuances of the opinion evidence without an Assessor, particularly one like Sir John with his happy facility for explaining complex scientific concepts in a way that can be comprehended by an interested layman. On behalf of myself and the Court, I express my appreciation to Sir John for his great assistance.
22 But this was not all the assistance I received.
23 As is well-known, Australia’s version of concurrent evidence typically involves two interrelated processes. First, there is a pre-trial joint expert conferral or conclave phase during which the parties’ experts meet to clarify the areas of agreement and/or disagreement to produce a joint report. The second part of the process is the giving of concurrent evidence.
24 In the present case, the first of these processes became particularly important and I appointed an experienced facilitator and independent barrister, Mr Edward Cowpe, who maintained the integrity of the conclave process and generally ensured the conclave process proceeded effectively and the various joint reports emerging from any conclave were expressed clearly, admissibly, and in a form that would best assist the Court. I am also grateful for his assistance.
B.2 Expert Assistance Generally
25 One of the reasons I was reluctant to abandon a reference and proceed in the time-honoured way in this case is because of my concerns in navigating potentially polarised expert views. In McNickle v Huntsman Chemical Company Australia Pty Ltd (Expert Evidence), I observed (at [1]) that controversy as to how opinion evidence is often deployed by litigants is not new, and some best placed to judge have expressed their criticism with some force. As Lord Woolf MR observed in the report, Access to Justice, Interim Report to the Lord Chancellor on the Civil Justice System in England and Wales (HMSO, London, 1995) (at 183):
Expert witnesses used to be genuinely independent experts. Men of outstanding eminence in their field. Today they are, in practice, hired guns. There is a new breed of litigation hangers-on, whose main expertise is to craft reports which will conceal anything that might be to the disadvantage of their clients.
26 This might be thought as putting the point too highly in this country, but reflecting similar concerns, in Australia there has been extensive law reform discussion concerning the role, deficiencies and remedial responses to perceived difficulties with expert evidence. Most relevantly for present purposes, in the Victorian Law Reform Commission Civil Justice Review (2008) (at 484), reference was made by the Victorian Law Reform Commission (VLRC) to the notion of “adversarial bias”. That conception falls into three varieties:
(1) deliberate partisanship – an expert who deliberately tailors evidence to support the retaining client;
(2) unconscious partisanship – the expert does not intentionally mislead the Court, but is influenced by the situation to give evidence in a way that supports the retaining client; and
(3) selection bias – litigants choose as their expert witnesses persons whose views are known to support their case.
27 Given the evident polarisation of scientific views and the possibility of unconscious partisanship and selection bias, from the moment it became apparent there was to be no special jury, I explained I considered it was likely I would be best assisted if the parties could seek out and retain experts in various disciplines who might be described as “cleanskins”: that is, experts who were not wedded to any particular concluded view and who could approach the questions posed informed by the Expert Evidence Practice Note (GPN-EXPT) (Expert Evidence Practice Note) (see also Harmonised Expert Witness Practice Note (Expert Witness Code of Conduct)) and without feeling the need to justify earlier expressed concluded views.
28 My suggestion was met with a mixed response. Indeed, the implicit (and sometimes explicit) suggestion made in Mr McNickle’s submissions was that my suggestions or concerns in this regard were wrongheaded. I will deal with each of the experts below in detail, but it is worthwhile making four related and general points concerning the criticism that entreating the parties to engage experts who did not hold fixed views was somehow heterodox.
29 First, I accept we are dealing with abstruse areas of specialised knowledge. The relatively limited pool of qualified persons to give opinion evidence and the polarisation of scientific view as to the central issue (and matters adjectival to the central issue) would have perhaps caused some difficulty in identifying appropriate experts; a fortiori experts who had not already formed views.
30 Secondly, notwithstanding the first point, I did not come down in the last shower: like any judge who has been an experienced barrister, I understand the forensic attractions that lead to what the VLRC described as selection bias. I hope I am not being unfair in remarking that my distinct impression throughout the whole of the interlocutory stages of this case was that Mr McNickle’s legal team, on one level understandably, resisted any independent referee process and eschewed engaging “cleanskins” in some areas of specialised knowledge because they felt more comfortable in the traditional approach of fixing upon experts they considered would likely reflect their case theory.
31 Thirdly, in the end, the role of the advocates acting for Mr McNickle was to persuade. As a general proposition, it stands to reason that the more objective, dispassionate, and disinterested an expert is, the more the opinions of the expert are likely to be perceived by the tribunal of fact as being: (a) unaffected by unconscious bias; and (b) persuasive. Of course, I stress this does not mean one assesses the admissibility and then reliability of an expert opinion by reference to some a priori view that it would be better to get a “cleanskin”. Disputed questions of fact must be decided according to the evidence that the parties adduce, not according to some speculation about what other evidence might possibly have been led: Australian Securities and Investments Commission v Hellicar [2012] HCA 17; (2012) 247 CLR 345 (at 412–413 [165]–[167] per French CJ, Gummow, Hayne, Crennan, Kiefel and Bell JJ).
32 Fourthly, Mr McNickle in his submissions repeatedly made the point that the mere fact that an expert witness has a pre-existing opinion on a key issue is an infirm basis for rejecting the expert’s opinion evidence “or placing less weight on it”. He cited Born Brands Pty Ltd v Nine Network Australia Pty Ltd [2013] NSWSC 1646 (and on appeal Born Brands Pty Ltd v Nine Network Australia Pty Ltd [2014] NSWCA 369; (2014) 88 NSWLR 421), to assert a pre-existing opinion does not mean an expert is not open-minded or biased but can rather indicate an expert has come to a considered opinion after years of relevant experience. In other words, preconceived principles can inform opinion evidence, if the principles are scientifically based.
33 Of course, Born Brands was dealing with admissibility and not weight: two different concepts. But I accept the underlying point made by Mr McNickle has real merit. The mere fact that the opinion an expert has expressed in this case as to whether, for example, glyphosate is carcinogenic, is the same as the opinion previously expressed on the same issue in other cases against Monsanto in the United States, is neither a complete nor satisfactory basis for the Court to reduce the weight to be afforded to the expert’s opinion evidence; let alone reject its admission. But depending upon the witness and the characteristics of the evidence, it can be part of the constellation of matters that may be relevant to assessing the persuasiveness of the evidence (depending upon how the evidence of the witness is challenged and whether procedural fairness has been provided to the witness to respond to whether the witness is approaching matters with an open mind or is fixed upon defending a pre-existing opinion).
34 In the end, each witness and the opinions expressed by the witness must be judged on their overall substantive merits, not by reference to the preconceived notion that it might have been thought more compelling to adduce evidence from an expert who first formed opinions through the prism of adherence to the Expert Witness Code of Conduct and approached the task without having formed pre-existing views. Recognising the merit of Mr McNickle’s submissions, I have been careful to approach consideration of the expert evidence in this way.
35 As it happened, there was extensive cross-examination by each party alleging partisanship by some experts. Having foreshadowed this as a potential issue early on, I facilitated the experts engaging usefully with each other under the supervision of an independent barrister and settled the questions to be asked of them following extensive argument. I will deal below with the competing contentions of unconscious bias and partisanship; but I will do so as is appropriate: not by reference to sweeping generalisations; but rather informed by my impressions of the relevant expert’s overall demeanour and credit and, most importantly, the perceived underlying scientific merit of the evidence given by the expert.
B.3 Fact Finding Generally in this Case
36 No lay witnesses were called. As noted above, the spectre of an attack on Monsanto for manipulating the science (decisive in my reasoning to not appoint referees) proved a damp squib, and although it was raised by one expert witness called by Mr McNickle directly, minimal evidence was adduced at this hearing directed to this allegation.
37 Leaving aside agreed facts, all the evidence was either expert or documentary. In respect of the latter, what might be described as a tsunami of scientific articles, studies and other materials was placed before me. I deal below with how, by the consent of the parties, I have approached the admissibility and weight to be given to the representations contained in that material.
38 But it must be emphasised at the outset that the Court’s task in determining the central issue is not an exercise of choice between the evidence of the respective parties’ expert witnesses.
39 It is trite to observe that in accordance with s 140(1) of the Evidence Act 1995 (Cth) (EA), the relevant standard of proof for the Court to apply is on the balance of probabilities, and that the applicant bears the onus of establishing that the evidence satisfies that test.
40 I will return to differences between the approach of both science and the law to causation enquiry below, but as I explained in some detail in Transport Workers’ Union of Australia v Qantas Airways Limited [2021] FCA 873; (2021) 308 IR 244 (at 324–325 [284]–[288]), when the law requires proof of any fact, the tribunal of fact must feel actual persuasion as to its occurrence or existence before it can be found. A party bearing the onus will not succeed unless the whole of the evidence establishes a reasonable satisfaction on the preponderance of probabilities such as to sustain the relevant issue: Axon v Axon (1937) 59 CLR 395 (at 403 per Dixon J). In this way, the facts proved must form a reasonable basis for a definite conclusion affirmatively drawn of the truth of which the tribunal of fact may reasonably be satisfied: Jones v Dunkel (1959) 101 CLR 298 (at 305).
41 As I have also noted, despite the criticisms referred to in Transport Workers’ Union of Australia v Qantas (at 325 [286]), this approach is well entrenched and unquestionably represents the current state of the law. This was reinforced by Kiefel CJ, Gageler and Jagot JJ in GLJ v The Trustees of the Roman Catholic Church for the Diocese of Lismore [2023] HCA 32; (2023) 97 ALJR 857, where their Honours observed (at 875 [60]):
[60] … “[t]o satisfy an onus of proof on the balance of probabilities is not simply a matter of asking whether the evidence supporting that conclusion has greater weight than any opposing evidence ... It is perfectly possible for there to be a scrap of evidence that favours one contention, and no countervailing evidence, but for the judge to not regard the scrap of evidence as enough to persuade him or her that the contention is correct.” The evidence must “give rise to a reasonable and definite inference” to enable a factual finding to be made; mere conjecture based on "conflicting inferences of equal degrees of probability" is insufficient. As Dixon CJ said in Jones v Dunkel, the law:
does not authorise a court to choose between guesses, where the possibilities are not unlimited, on the ground that one guess seems more likely than another or the others. The facts proved must form a reasonable basis for a definite conclusion affirmatively drawn of the truth of which the tribunal of fact may reasonably be satisfied.
(Footnotes omitted)
42 Before passing from these self-directions, it is useful to make two further points.
43 First, in final submissions, Monsanto asserted that the seriousness of Mr McNickle’s allegation, being an allegation that Roundup Products cause NHL, must be considered in deciding whether Mr McNickle has discharged the onus of proof (referring to s 140(2) EA and Briginshaw v Briginshaw (1938) 60 CLR 336 (at 361–362 per Dixon J)).
44 With respect, at best, this is a distraction. Section 140(2) needs to be faithfully applied, but in considering “the gravity of the matters alleged” in s 140(2)(c), the primary focus is upon the nature of the factual allegations in the case (although the consequences of any finding is also a relevant matter). This makes sense when one considers the focus on the gravity of the finding is linked to the notion that the Court takes into account the inherent unlikelihood of alleged conduct, and common law principles concerning weighing evidence: see Qantas Airways Limited v Gama [2008] FCAFC 69; (2008) 167 FCR 537 (at 576 [137]–[138] per Branson J). There is no suggestion of knowing wrongdoing or of other intuitively unlikely conduct; here I am dealing with a question of general legal causation based upon the scientific evidence adduced and Monsanto’s submission is less than helpful when the rationale of Briginshaw is understood.
45 Secondly, on the one hand, Mr McNickle asserts the evidence of general factual causation is pellucid; and on the other hand, Monsanto denies its existence completely. Often these submissions elided the concepts of legal and scientific causation, but it suffices to note for present purposes that the principal case theories adopted are polar opposites. But, as Besanko J observed in Roberts-Smith v Fairfax Media Publications Pty Limited (No 41) [2023] FCA 555 (at [117]–[118]), there is no doubt that a court is not bound to accept the case of one or other of the parties.
46 In this regard, his Honour referred to the “great clarity” of the speech of Lord Brandon of Oakbrook in Rhesa Shipping Co SA v Edmunds (The Popi M) [1985] 1 WLR 948, where his Lordship said the following (at 955):
My Lords, the late Sir Arthur Conan Doyle in his book The Sign of Four, describes his hero, Mr Sherlock Holmes, as saying to the latter's friend, Dr. Watson: “How often have I said to you that, when you have eliminated the impossible, whatever remains, however improbable, must be the truth?” …
In my view there are three reasons why it is inappropriate to apply the dictum of Mr Sherlock Holmes, to which I have just referred, to the process of fact-finding which a judge of first instance has to perform at the conclusion of a case of the kind here concerned.
The first reason is one which I have already sought to emphasise as being of great importance, namely, that the judge is not bound always to make a finding one way or the other with regard to the facts averred by the parties. He has open to him the third alternative of saying that the party on whom the burden of proof lies in relation to any averment made by him has failed to discharge that burden. No judge likes to decide cases on burden of proof if he can legitimately avoid having to do so. There are cases, however, in which, owing to the unsatisfactory state of the evidence or otherwise, deciding on the burden of proof is the only just course for him to take….
47 This reflects the legal reality of how the onus of proof works and the logical reality that a failure to prove a state of affairs does not necessarily mean the state of affairs does not exist. Hence, an acquittal by a jury of a person accused of a criminal charge does not amount to a declaration by the jury of the accused’s innocence (although this state of affairs is taken at law to be the case by reason of the operation of a different presumption of the criminal law).
48 I come back to this important notion in Section J when I deal with the appropriateness of answering the common questions as initially framed.
B.4 The “Weight of Evidence” and the Scientific Studies
49 With metronomic regularity, Mr McNickle asserted that in determining whether glyphosate and/or GBFs can cause or increase the risk of NHL, “it is appropriate to take a weight of evidence approach” to the following three streams of scientific evidence, examined in detail below, being: (1) epidemiological studies of the effects of glyphosate and/or GBFs on humans; (2) long-term studies on experimental animals relating to whether glyphosate and/or GBFs can cause cancer in animals; and (3) mechanistic evidence, that is, evidence relating to whether there is a biologically plausible mechanism by which glyphosate and/or GBFs can induce cancer in humans.
50 The concept of the “weight of evidence” is a common term in published scientific literature, most often seen in the context of risk assessment. The concept is referred to in various conclave reports: Conclave A Joint Report (JRA) (at [19], [125]); Conclave B Joint Report (JRB) (at [72]–[78]); Conclave G Joint Report (JRG) (at [35], [39], [49]–[50], [102]); and Conclave I Joint Report (JRI) (at [13]). But this does not mean the concept is entirely clear; nor does its mere recitation explain how it is called in aid by Mr McNickle.
51 Dr Douglas Weed, an epidemiologist (who was the former Chief, Office of Preventive Oncology, and the Director of the Cancer Prevention Fellowship Program[me] at the National Cancer Institute (NCI), being an agency of the Executive Government of the United States) has published a systematic review of the scientific literature to characterise the “weight of evidence” concept. As I noted during final oral submissions, it is used apparently in three senses:
(1) metaphorical, where weight of evidence refers to a collection of studies or to an unspecified methodological approach;
(2) methodological, where weight of evidence points to established interpretative methodologies or where weight of evidence means that “all” rather than some subset of the evidence is examined, or rarely, where it points to methods using quantitative weights for evidence; and
(3) theoretical, where weight of evidence serves as a label for a conceptual framework. Dr Weed identifies several problems associated with the frequent lack of definition of the term and a lack of consensus about its meaning: see Weed, D L “Weight of Evidence: A Review of Concept and Methods” (2005) 25(6) Risk Analysis 1545 (at 1545–57).
52 Following clarification made during the course of oral submissions, it became apparent that the parties employed the term in the second, methodological sense in that the principled approach requires attention to be given to “all”, rather than some subset, of the scientific evidence.
53 Further, it became apparent during oral submissions that the assessment is suggested to take place on two different levels.
54 First, as is evident from the various conclave reports within each scientific stream, it is common ground that an expert cannot cherry-pick from the peer-reviewed studies those that support the expert’s opinion, but rather carry out a comprehensive assessment of the weight of evidence of regulatory and peer-reviewed studies within that stream of scientific evidence.
55 Secondly, Mr McNickle submits, and Monsanto expressly accepts, that the Court needs to carry out a weight of evidence analysis to “weigh up the three streams of evidence”.
56 I understand the way the concept is used within each scientific stream and its utility, but to the extent the concept is used in this second way, it needs to be approached with some caution and its discrete analytical utility is elusive. As I will explain, scientific causation and the legal question of general causation are different notions and it is trite that my job as a fact finder is to determine the central issue, being a factual issue, according to law, that is, by having regard to all the evidence admitted (and only the evidence admitted).
57 Subject to one matter to which I will come, evidence was admitted because the parties, through their non-objection to its adduction, accepted it was relevant because, if it was accepted, it could rationally affect (directly or indirectly) the assessment of the probability of the existence of a fact in issue at the initial trial: see ss 55 and 56 EA. As explained above, in assessing whether I have reached the level of reasonable satisfaction on the preponderance of probabilities such as to sustain Mr McNickle’s case on the central issue, statute and authority demand I have regard, and only have regard, to the whole of the evidence admitted. My reasoning, inferential and otherwise, can only be based on the evidence admitted and must take account of the entirety of the evidence in reaching conclusions.
58 There is one qualification to what I have said about admissibility. I referred earlier to the scientific articles and studies placed before the Court. There is a lot of law about how a court is to deal with representations of opinion made in such material. As Heydon J explained in Dasreef Pty Limited v Hawchar [2011] HCA 21; (2011) 243 CLR 588 (at 615 [69], 631–632 [110]), there has long existed a qualification to the proof of assumption rule (under a common law exception to the hearsay rule), that experts may give evidence of hearsay matters which go to demonstrate their expertise – what is said in the writings of others in the relevant area of expertise they have read as a basis for their opinion, or what has been said to them in discussions they have had with colleagues and taken into account.
59 In short, experts are generally entitled to rely upon publications and material produced by others in the area in which they have expertise as a basis for their opinions and may give evidence of fact which is based on them. As is explained in Wigmore on Evidence (Little, Brown and Company, 3rd ed) Vol 2 (at 784–785):
The data of every science are enormous in scope and variety. No one professional man can know from personal observation more than a minute fraction of the data which he must every day treat as working truths. Hence a reliance on the reported data of fellow-scientists, learned by perusing their reports in books and journals. The law must and does accept this kind of knowledge from scientific men. On the one hand, a mere layman, who comes to court and alleges a fact which he has learned only by reading a medical or a mathematical book, cannot be heard. But, on the other hand, to reject a professional physician or mathematician because the fact or some facts to which he testifies are known to him only upon the authority of others would be to ignore the accepted methods of professional work and to insist on finical and impossible standards. Yet it is not easy to express in usable form that element of professional competency which distinguishes the latter case from the former. In general, the considerations which define the latter are (a) a professional experience, giving the witness a knowledge of the trustworthy authorities and the proper source of information, (b) an extent of personal observation on the general subject, enabling him to estimate the general plausibility, or probability of soundness, of the views expressed, and (c) the impossibility of obtaining information on the particular technical detail except through reported data in part or entirely.
60 In Karpik v Carnival plc (The Ruby Princess) (Evidential Ruling) [2022] FCA 1318, Stewart J addressed this topic and, after referring to the above extract, noted (at [5]) that “[i]t can thus be seen that expert reliance on such learned publications is not excluded by the rule against hearsay (s 59) or the opinion rule (s 76)”. His Honour was dealing with a case, like here, where the expert reports were replete with references to scientific articles. Having noted the need for caution in making an order under s 136 EA limiting the use of such materials, such a limitation order was not made generally but was made in relation to an article which was: (1) not referred to in any of the expert reports; (2) only referred to in cross-examination; and (3) “deal[t] with an area of expertise not possessed by any of the many expert witnesses in the case” and therefore “there is no other evidence against which to compare, or weigh, the opinions and conclusions expressed in the article”, leaving the Court “in an invidious position with regard to trying to assess the weight to give to those opinions and conclusions” (at [24]).
61 His Honour explained that “[f]or that reason, taken together with the absence of any opportunity for the respondents the test or challenge those opinions and conclusions, I am satisfied that to not limit the use to which the article can be put under s 136 would be unfairly prejudicial to the respondents” (at [24]).
62 To their great credit, the parties to this case (well advised by experienced solicitors and junior counsel), took a sensible and constructive approach to all issues as to admissibility. One aspect of their agreement related to the reception and use of the scientific articles. Taking a slightly different approach to Ruby Princess, I had proposed to the parties a limitation that would restrict reception of material to those articles and studies in the experts’ relevant area of expertise that were read and were said to form a basis for otherwise admissible opinion evidence or were used in cross-examination, accompanied by a general limitation under s 136 EA that the evidence was to be used for the purposes of providing a basis for, or understanding, the expert opinions adduced by the witnesses called.
63 The parties did not embrace this approach. On 19 October 2023, I was informed by the parties that a “joint position” had been reached to the following effect:
All reports or decisions of regulatory authorities and authoritative scientific bodies, as well as scientific articles and reviews and other learned scientific publications, which have been referred to or relied upon by the expert witnesses who participated in the joint conclave should be admitted into evidence, with the parties being at liberty to make submissions in the course of closing submissions as to the weight that the court should place on any such document admitted into evidence.
64 As I noted when informed of this agreement, to the extent it operates to expand the admissibility and use of these scientific materials beyond the principled application of the Pt 3.3 EA exception to the Pt 3.2 hearsay rule, it was open to the parties to dispense, by consent, with the application of Pts 3.2 and 3.3 in relation to particular evidence (see s 190(1)(c) EA).
65 Accordingly, I made orders in accordance with this agreement and will proceed to deal with this aspect of the evidence in accordance with the express agreement of the parties.
B.5 The Scientific and Legal Method and Causation
66 Before passing from fact finding and causation, it is important to make a further point that was somewhat obscured during submissions.
67 In ACQ Pty Ltd v Cook [2009] HCA 28; (2009) 237 CLR 656 (at 661 [14]), French CJ, Gummow, Heydon, Crennan and Bell JJ observed, albeit in a different context, that causation is one of the most difficult fields of debate in the law and one about which “abstract discussion is seldom valuable”. Perhaps recognising this, abstract discussion about causation was largely absent from the parties’ submissions. But one must not gloss over important differences between the scientific and legal method in relation to causal connexions between facts.
68 These differences have been explored in many articles including, by way of example, Korn, H L “Law, Fact, and Science in the Courts” (1966) 66 Columbia Law Review 1080; Brennan, T A “Causal Chains and Statistical Links: The Role of Scientific Uncertainty in Hazardous-Substance Litigation” (1988) 73 Cornell L. Rev. 469; and Geistfeld, M “Scientific Uncertainty and Causation in Tort Law” (2001) 54 Vanderbilt Law Review 1011. It is beyond the scope of this judgment to canvass such a large topic in anything other than the most superficial way, and this judgment should not be unnecessarily diverted into epistemological or philosophical questions, but the nature of differences must be understood, at least in broad terms, so the scientific evidence is properly understood and its relationship to the legal issue of general causation is not distorted.
69 Justice Jonathan Beach has written extra-curially on this topic in a way that repays close attention. Drawing upon his earlier work (“Scientific Evidence: A Need for Caution in Decision-Making” (2010) 42(1) Australian Journal of Forensic Sciences 49 and “Indeterminacy: The Uncertainty Principle of Negligence” (2005) 13 Torts Law Journal 129), in a recent article “Causation: The Interface Between the Scientific and Legal Methods” (2022) 49(1) University of Western Australia Law Review 113, Justice Beach explained (at 113) how the objectives of legal and scientific inquiry differ as science “searches for increased knowledge, with truth as its ideal; its coverage [being] more comprehensive and not time sensitive. But the law’s “objective is to resolve conflicts; its coverage is limited and time sensitive”. In this way, science describes explains and predicts while the law “is concerned with regulating human affairs in accordance with values and objectives”.
70 It is simplistic to say there is one “scientific method” because it depends upon the nature of the scientific task, but speaking generally, these different objectives mean there are critical contextual differences between science and the law concerning methodologies and in the expression of conclusions of their differently focused enquires. Moreover, when it comes to causation, the role of the law might be said to involve an optimistic search for truth but, as my self-directions make clear, the aim is not to discover objective truth, but rather whether the relevant factual issue (in this case of general factual causation) has been proved to the requisite standard.
71 As Justice Beach, with respect, cogently explains in his recent article (at 117–8), while recognising the differences between the legal method and the scientific method:
… when lawyers analyse scientific evidence, the epistemic values that need to be applied correlate with values underpinning the scientific method. A lens must be used which provides a sophisticated picture of the content and reliability of the scientific evidence. And not to analyse scientific evidence through the appropriate lens can lead to outcomes ‘determined by intuitive perceptions of the weight of authority rather than by reasoning from evidence’.
Of course, the language, premises and analytical styles between the scientific method and the legal method have their differences. But when the latter is required to evaluate the former, significant correspondence of epistemic values arises. Lawyers must ‘retrace and evaluate the technical analysts’ logic, from empirical data to subjective judgment’. And complex issues of science require reasoning among purely technical facts analysed through complex models, statistical inference and mathematical instruments that have no comparator in legal reasoning.
Now there are different modes for judicial analysis of scientific evidence. The judge might hear expert evidence in the usual way or through the mechanism of concurrent evidence sessions, which is my preferred option in patent litigation. The judge might appoint his own expert. The judge might obtain assistance from sitting with an assessor who may assist with any explanation of the scientific evidence. The judge might receive a report on the scientific question from a special referee, although this last option is remote from any direct judicial analysis of the scientific question. But whatever the procedural mode, this does not change the epistemic values and methods needed to assess scientific evidence. Moreover, judges are not obliged to accept the ipse dixit of the expert. They are obliged to test propositions, to assess one expert’s opinion against another, and to synthesise a position taking into account all expert, lay and documentary evidence.
72 This is what I have attempted to do. To rely acontextually upon general statements made by scientists as to causation, or to assume that scientific expressions as to possibility or probability necessarily translate to the legal task of considering probability through the lens of general factual causation at law is simplistic. Put in more simple terms, the factual issue before me is, at bottom, a common law jury question and, as was explained by Dixon CJ, McTiernan, Kitto, Taylor and Windeyer JJ in Ramsay v Watson (1961) 108 CLR 642 (at 645):
That some medical witnesses should go into the witness box and say only that in his opinion something is more probable than not does not conclude the case. A qualified medical practitioner may, as an expert, express his opinion as to the nature and cause, or probable cause, of an ailment. But it is for the jury to weigh and determine the probability. In so doing they may be assisted by the medical evidence. But they are not simply to transfer their task to the witnesses. They must ask themselves ‘are we on the whole of the evidence satisfied on the balance of probabilities of the fact?’
73 To limit the scope of the evidence, I required the legal representatives for both parties to attempt to agree upon all relevant non-contentious facts prior to preparing evidence that would be the subject of dispute.
74 Although for reasons that are unnecessary to canvass, this process was somewhat delayed, a statement of agreed facts was prepared, and a final version was provided to the Court. That finalised version of the statement of agreed facts document was admitted into evidence (Agreed Facts or AF) with each fact being an “agreed fact” within the meaning of s 191 EA. The factual findings are made upon an admixture of the Agreed Facts, material drawn from contemporaneous documents, as well as inferences drawn from any communications and documents. Most facts agreed are irrelevant to the scope of the initial trial. I set out some below for the purpose of recording matters which are of relevance (at times, marginal at best relevance) to the present trial.
75 Between around 1974 and 1987, the first respondent, Huntsman Chemical Company Australia Pty Limited (previously called Monsanto Australia Limited, which I will describe as Monsanto Australia (Old)), was a wholly-owned subsidiary of the fourth respondent (Monsanto Company (Old)), and together with associated and related entities conducted an agricultural chemicals business in Australia.
76 In about March 1988, the agricultural chemicals business of Monsanto Australia (Old) was transferred to the second respondent, Monsanto Australia Pty Ltd, (Monsanto Australia (New)) and was thereafter conducted by Monsanto Australia (New) and associated and related entities. Monsanto Australia (Old) ceased to conduct that business.
77 Pursuant to a Share Sale Agreement dated 21 March 1988, all of the assets and liabilities of Monsanto Australia (Old) (which were primarily used prior to 31 March 1988 in the agricultural chemicals business conducted by it) were transferred to an entity which became known as Monsanto Australia Pty Ltd (that is, Monsanto Australia (New)).
78 Until September 2000, Monsanto Australia (New) was a wholly-owned subsidiary of Monsanto Company (Old). From September 2000 until 2018, it was a wholly-owned subsidiary of the third respondent (Monsanto Company (New)). In 2018 Monsanto Australia (New) became a wholly-owned subsidiary of Bayer Aktiengesellschaft (Bayer AG).
79 Monsanto Company (Old) and Monsanto Company (New) are entities incorporated in Delaware in the United States. Until approximately September 2000, Monsanto Company (Old) conducted, among other things, an agricultural chemicals business. Following a corporate restructure, that business including as to Roundup Products, was conducted by Monsanto Company (New) after approximately 1 September 2000.
80 In or around early 2000, Monsanto Company (Old) merged with a publicly owned pharmaceuticals company. Monsanto Company (Old) was the surviving corporation from the merger.
81 Monsanto Company (New) was created on 9 February 2000 as a wholly-owned subsidiary of Monsanto Company (Old). Effective on 1 September 2000, Monsanto Company (New) received the operations, assets and liabilities of the agricultural business previously conducted by Monsanto Company (Old), including in respect of the Roundup Products. Upon completion of the merger Monsanto Company (Old) changed its name to Pharmacia Corporation.
82 The International Union of Pure and Applied Chemistry name for glyphosate is N- (phosphonomethyl) glycine.
83 Glyphosate was first synthesised by a chemist employed by Monsanto Company (Old) in 1970 and patented by Monsanto Company (Old) in 1974 for use as an herbicide.
84 The manufacture of Roundup Products involves the conversion of intermediate products to glyphosate acid (also known as glyphosate technical) which, in turn, is further converted to glyphosate salts for use in the formulation of Roundup Products.
C.3 Roundup Herbicide and Roundup Biactive in Australia
85 Roundup Herbicide is a herbicide product which has been available for sale in Australia since 1976. In the time periods set out below, the entity identified manufactured Roundup Herbicide and/or Roundup Biactive:
(1) Roundup Herbicide
(a) 1979 to 1988 – Monsanto Australia (Old);
(b) 1988 to 2000 – Monsanto Australia (New) and Monsanto Company (Old);
(c) 2000 to 2019 – Monsanto Australia (New) and Monsanto Company (New)
(2) Roundup Biactive
(a) 1996 and 2000 – Monsanto Australia (New) and Monsanto Company (Old);
(b) 2000 to 4 February 2002 – Monsanto Australia (New) and Monsanto Company (New);
(c) 2 February 2002 to 2010 – Monsanto Australia (New);
(d) 2010 to 2019 – Monsanto Australia (New) and Monsanto Company (New).
86 The Roundup Herbicide and Roundup Biactive used in Australia during the Relevant Period was manufactured in accordance with specifications (including as to the formulations) and quality assurances manual set by Monsanto Company (Old) for the period 1976 to 2000, and by Monsanto Company (New) for the period 2000 to 2022.
87 The Roundup Herbicide and Roundup Biactive used in Australia during the Relevant Period was packaged (including labelling) in accordance with specifications and quality assurances manuals set by Monsanto Company (Old) for the period 1976 to 2000, and by the Monsanto Company (New) for the period 2000 to 2020.
88 During the claim period:
(1) Monsanto Company (Old) for the period 1976 to 2000 in respect of Roundup Herbicide and for the period 1996 to 2000 in respect of Roundup Biactive;
(2) Monsanto Company (New) for the period 2000 to 2019;
(3) Monsanto Australia (Old) for the period 1976 to 1988 in respect of Roundup Herbicide; and
(4) Monsanto Australia (New) for the period 1988 to 2020 in respect of Roundup Herbicide and for the period 1996 to 2020 in respect of Roundup Biactive,
directly or through other parties and agencies promoted and marketed Roundup Herbicide and Roundup Biactive in Australia and engaged in activities related to marketing including conducting promotions, educational publications and programmes, product demonstration programmes and print advertisements and in accordance with product use and safety information provided by Monsanto Company (Old) for the period 1976 to 2000 and by Monsanto Company (New) for the period 2000 to 2020.
89 Information concerning the safe use of Roundup Herbicide and Roundup Biactive was communicated by Monsanto to consumers in Australia during the Relevant Period by two predominant means:
(1) the product label including the “Directions for Use” booklet, both affixed to the container of the products; and
(2) the safety data sheets.
D STRUCTURE OF CONSIDERATION OF THE SCIENTIFIC EVIDENCE
90 As noted above (at [49]), the scientific evidence relevant to determining the central issue was divided into three streams, broadly as follows:
(1) epidemiological studies of the effects of glyphosate and/or GBFs on humans (Conclaves C, D and E) (epidemiology stream);
(2) long-term studies on experimental animals relating to whether glyphosate and/or GBFs can cause cancer in animals (Conclaves H, I and J) (animal studies stream); and
(3) mechanistic evidence, that is evidence relating to whether there is a biologically plausible mechanism by which glyphosate and/or GBFs can induce cancer in humans (Conclaves A, B, G) (mechanistic evidence stream).
91 Consistently with the need to have regard to the whole of the evidence, the parties accepted that it is necessary to make factual findings in relation to the evidence adduced in each conclave. It is then appropriate to consider each conclave in the context of the relevant scientific streams and then have regard to all the evidence from each stream. For reasons that will become clear, I will deal separately with Conclave F, which concerned absorption and exposure and the discrete question of dose.
92 Accordingly, I will address each stream separately (although there is necessarily some overlap). In doing so, I will first explain the nature of the stream of scientific evidence; secondly, make general credit findings with respect to each the relevant experts; thirdly, summarise the evidence given in each conclave relevant to each stream; and fourthly, summarise my conclusions in relation to each stream of evidence building upon and supplementing the findings that have been made during the process of setting out the conclave evidence. I will then draw all the threads together in reaching my conclusions.
E THE FIRST STREAM: EPIDEMIOLOGY (CONCLAVES C, D AND E)
93 In 1854, Dr John Snow witnessed an outbreak of cholera in London. Determined to discover its source, he plotted the location of cholera deaths on a dot-map. The dots congregated a short distance from Broad Street (now Broadwick Street) in Soho. There, the local council had installed a water pump. Suspecting that the pump was the source of the outbreak, Dr Snow persuaded the council to remove the handle. The outbreak subsided.
94 But that was not the end of the story: had the handle been contaminated? Was it the water itself? Or was the outbreak due to something else? Dr Snow thought it was the water. He continued to collect data on cholera deaths in the city and identified three water companies that supplied water to districts with the highest mortality rates. It was not clear within each district, however, which company supplied water to households where a cholera death occurred. By collecting data from individual households, Dr Snow inferred that two of the water companies were responsible for transmitting cholera poison and, by 1857, legislation had been enacted to mandate the filtering of all water in London (see Harris, S A “Epidemiology: Theory, Study Design and Planning for Education” (2000) 20 Journal of Continuing Education in the Health Profession 133).
95 Albeit in nascent form, Dr Snow was engaged in epidemiology. For his efforts, he enjoys the not inconsiderable distinction of having a pub named after him.
96 Expressed in broad terms, epidemiology is the study of distribution and determinants of disease in humans. As Spigelman CJ explained in Seltsam Pty Ltd v McGuiness [2000] NSWCA 29; (2000) 49 NSWLR 262 (at 271–272 [59]–[62]), epidemiology is based on the assumption that a disease is not distributed among populations at random: subgroups may be identified which are at increased risk of contracting particular diseases. Epidemiological evidence identifies associations between specific forms of exposure and the risk of disease in groups of individuals.
97 Epidemiologists make judgments about whether a statistical association represents a cause-effect relationship. However, those judgments focus on what is called in the epidemiological literature (as well as the law) general causation, that is, whether the factor is capable of causing the disease. Epidemiologists are not concerned with specific causation, that is, whether the factor caused the disease in an individual case.
98 For the purposes of determining whether exposure to a particular substance is the legal cause of a particular disease, epidemiology only provides evidence of possibility, which (for reasons I have explained), when expressed in opinion form and in epidemiological research, is admissible and part of the whole of the evidence to which the court must have regard: Seltsam v McGuiness (at 274–275 [78]–[79] per Spigelman CJ).
99 At bottom, this is a trial directed to the issue of general factual causation. Obviously enough, given its nature, epidemiological evidence is of real importance. Indeed, as it turns out, successfully demonstrating that the epidemiological evidence adduced in this proceeding supports the conclusion that glyphosate and/or GBFs can increase the risk of and/or cause NHL in humans is a hurdle in accepting Mr McNickle’s case.
100 But it remains one hurdle. As senior counsel for Mr McNickle, Mr Clements KC, submitted in his opening (T10.32–37):
Despite there being very broad agreement between the experts retained by each side, as to the appropriateness of the weight of evidence approach, the respondents, in their written opening, have submitted that the epidemiological evidence should be given primacy by the court. We contend that approach should be rejected. We contend that it’s important for the court to closely consider and weigh up the evidence in each of the three separate streams of scientific evidence.
101 Put another way, the importance of the epidemiological stream does not mean that my conclusion as to whether I have reached a level of reasonable satisfaction on the preponderance of probabilities to sustain Mr McNickle’s case on the central issue is the product of giving primacy to one aspect of the evidence and reducing others to some form of secondary role or, more specifically, placing unwavering and disproportionate focus upon the epidemiological evidence in a vacuum. It is not. Indeed, focussing too intently on one part of the evidence at the expense of proper consideration of another part and then assessing the accumulated whole, would not only be a legal but a logical error.
102 Of course, some parts of the evidence may emerge as being more persuasive or important than other aspects, but any conclusion on the central issue must be made with regard to the overall weight of all of the evidence.
103 It is largely uncontroversial among epidemiologists as to the principles or postulates which should be applied to assess evidence of a statistical correlation or association between the exposure in question and health outcome of interest: see Seltsam v McGuiness (at 285 [139] per Spigelman CJ).
104 The first step in assessing the epidemiological evidence and whether it indicates an etiologic relationship (that is, a causal relationship) between exposure to glyphosate and/or GBFs and development of NHL in humans is to identify the relevant peer-reviewed literature. The second step is to identify strengths and limitations of each publication, which, in turn, allows an identification of publications that are most relevant to the specific topic of concern, and an evaluation of the weight that should be placed on such publications (Conclave C Joint Report (JRC) (at [63])).
105 In evaluating the relevant literature, epidemiologists may attempt to distinguish between causal and non-causal explanations (at JRC [64]–[65]). While there is no agreed set of criteria to assess causation that exists in the field, lists of criteria have become popular. The most well-known of these among epidemiologists is one devised by Sir Austin Bradford Hill, then Professor Emeritus of Medical Statistics at the University of London, in his Presidential address to the Section of Occupational Medicine: “The Environment and Disease: Association or Causation” (1965) 58 Proc R Soc Medicine 295.
106 The following summary of the “Bradford Hill criteria” is extracted from French CJ’s judgment in Amaca v Booth Pty Ltd [2011] HCA 53; (2011) 246 CLR 36 (at 54–55 [44]):
[44] … The [Bradford Hill] criteria were expressed as the aspects of an association between two variables that should be considered before inferring that the most likely interpretation of the association is causation. In summary, they are:
strength of association – eg, reflected in the ratio of the death rates between groups exposed to a suspected agent and those not so exposed;
consistency in the observed association – eg, has it been repeatedly observed by different persons in different places, circumstances and times;
the specificity of the association – if the association is limited to specific workers and particular sites and types of disease and there is no association between the work and other modes of dying, that is a strong argument in favour of causation;
temporality – the temporal relationship of the variables;
biological gradient – whether the association reveals a biological gradient or dose-response curve;
plausibility – whether the expected causation is biologically plausible – a consideration which depends upon the biological knowledge of the day;
coherence – the cause and effect interpretation of the data should not seriously conflict with the generally known facts of the natural history and biology of the disease;
experiment – whether experimental or semi-experimental evidence supports a causation hypothesis;
analogy – eg, given the effects of thalidomide and rubella it is easier to accept slighter but similar evidence with another drug or another viral disease in pregnancy.
107 These factors are not intended as a necessary condition for a cause-and-effect relationship, nor a checklist or set of hard and fast rules to be applied in evaluating causation among epidemiologists. Rather, the Bradford Hill criteria constitutes a guide or set of commonsense propositions which may be taken into account in determining whether or not the Court should infer, on the balance of probabilities, that a particular exposure caused injury (see Amaca v Booth Pty Ltd (at 54–55 [44]–[45] per French CJ); Seltsam v McGuiness (at 285 [139]–[141] per Spigelman CJ); (at JRC [66]–[71])). As was noted in a discussion of the application of the Bradford Hill criteria in the Restatement Third, Torts: Liability for Physical and Emotional Harm §28, Comment c(3) (at 406–407):
Whether an inference of causation based on an association is appropriate is a matter of informed judgment, not scientific methodology, as is a judgment whether a study that finds no association is exonerative or inconclusive. No algorithm exists for applying the Hill guidelines to determine whether an association truly reflects a causal relationship or is spurious. Because the inferential process involves assessing multiple unranked factors, some of which may be more or less appropriate with regard to a specific causal assessment, judgment is required.
108 Before going further, and in an attempt to make what follows less unreadable, it is worth identifying some key terminology and demystifying some jargon frequently deployed in the epidemiological literature, which is also relevant to the two other streams of evidence.
Relative risk, odds ratios and confidence
109 As Dr Ian Freckelton AO KC explained in Expert Evidence: Law, Practice, Procedure and Advocacy (Thomson Reuters, 6th ed, 2021) (at 1199), epidemiologists identify the strength of an association between a particular form of exposure and the risk of contracting a particular disease by a measure described as “relative risk” (RR). The RR describes the rate of a disease of interest occurring in persons who have been exposed to a particular environmental factor divided by the corresponding rate in persons without a history of that exposure. For example, a RR of 2.0 indicates a two-fold risk in exposed people compared to non-exposed people.
110 Another term frequently used in the literature is “odds ratio” (OR). The OR represents the odds that an outcome will occur given a particular form of exposure, compared to the odds of the outcome occurring without that exposure. Although the OR can indicate the strength of an association between exposure and outcome, it is not the same as RR. For example, when there is no association between exposure and outcome, the OR and RR are identical (1.0). However, when there is an association between an exposure and an outcome, the OR can exaggerate the estimate of their relationship.
111 ORs can be statistically significant or not. As Morling J explained in Re Australian Federation of Consumer Organisations Inc v Tobacco Institute of Australia Ltd (1991) 27 FCR 149 (at 163–164), statistical significance is an expression of the relative confidence that a particular result (say, an OR of 2.0), is a true effect and not due to chance.
112 Statistical significance is frequently expressed in the scientific literature in terms of a “confidence level” (CL) which, conventionally, is the 95% CL. That is, if a result (or a range of results) is statistically significant at the 95% CL, the probability that result is due to chance is approximately 1 in 20. An example is usefully provided by Morling J (at 163):
[A]n investigation of the association between exposure (to an agent) and outcome (disease), having opted for a confidence level of 95%, may produce a point estimate of 2.5, with upper and lower confidence limits of 3.5 and 1.5. This means that based on the raw data, the best estimate is that those exposed have a 2 times greater chance of experiencing the disease than those not exposed. Further, one can be 95% sure that the true relative risk is somewhere between 1.5 and 3.5.
113 The range between the upper and lower confidence limits is known as the “confidence interval” (CI). If the CI is wide, then the point estimate is less reliable than if it is narrow. Ordinarily, wide CIs indicate a small number of observations.
114 It is also worth noting the relationship between CIs and statistical significance.
115 The relative risk of disease normally associated with pure chance is 1.0: that is, no increased risk, no decreased risk. Using the 95% CL, if the value 1.0 lies between the reported upper and lower CLs, then the probability that the result is due to chance has not been satisfactorily excluded at the 95% CL. Whenever the confidence interval straddles 1.0, then the result is not statistically significant. To borrow Morling J’s example (at 164), assuming a 95% CL, Study A reports a point estimate of 2.5 with a CI of 1.5 to 3.5. Study B reports a point estimate of 2.5 with a CI of 0.8 to 4.0. The results in Study A are statistically significant, whereas the results in Study B are not. Importantly, a CI which includes the value of 1.0 is frequently referred to in the literature as including the “null hypothesis” (that is, no association between the particular form of exposure and risk of contracting the disease).
116 Decisions in the United States have demanded a relative OR of greater than 2.0 to establish causation on the balance of probabilities: see Daubert v Merrell Dow Pharmaceuticals Inc 43 F 3d 1311 (9th Cir, 1995); Re Joint Eastern and Southern District Asbestos Litigation 758 F Supp 199 (1991) (at 202–203). This is not the law in Australia. As Spigelman CJ explained in Seltsam v McGuiness (at 285 [137]), the test of actual persuasion does not require epidemiological studies to reach the level of a relative risk of 2.0, notwithstanding that the closer the ratio approaches 2.0, the “greater the significance that can be attached to the studies”. The “strands in the cable” must be capable of bearing the weight of the ultimate inference: see also Re Australian Federation of Consumer Organisations (at 211 per Morling J).
117 It is also worth briefly explaining some key differences between the types of studies found in the epidemiological literature.
118 In a cohort study, a group of individuals is identified and then divided into those who have been exposed to the event in question (say, exposure to glyphosate and/or GBFs) and those who have not. The group is then followed for a number of years, and individuals who contract the disease are identified in order to determine if there is any difference in incidence between the groups (at JRC [16]).
119 Associations in cohort studies are typically expressed in terms of RR.
120 In contrast, case-control studies identify individuals with the disease in question (the cases) from the outset, which are then matched to a group of individuals who do not have the disease (the controls). Data about exposure to a risk factor (or several risk factors) are then collected retrospectively, typically by interview, abstraction from records, or questionnaires.
121 Controls can be individually matched to cases or matched on a group basis in which distributions of age, sex, and other covariates are similar. Matching is conducted to reduce confounding by causes of the disease of interest other than those being studied that are correlated with the exposures under investigation (at JRC [15]).
122 Associations in case-control studies are typically expressed as ORs.
123 Pooled analysis is a method which provides for the combining of results of multiple studies. It is often used when the results of individual studies are too small or not definitive enough to draw any firm conclusion as to causation. In contrast to meta-analyses, pooled analyses can only be conducted if the included studies used the same study design and statistical models, and if their respective populations were homogeneous. If done properly, a pooled study can provide more statistically precise estimates of risks associated with the exposures of concern and allow for the evaluation of associations among subpopulations or subgroups or subtypes of disease (at JRC [17]).
124 It is useful here to set out some other relevant terminology, some of which will be relevant to the other streams. What follows is largely drawn from definitions provided in Section D of the JRC:
(1) Environmental exposures – chemical, biological, and physical agents that occur in the air, water, or soil that can be hazardous to health. Chemical agents of particular concern globally are air pollution (for example, particulate matter), water pollution (for example, toxic metals), and food contaminants (for example, E coli bacteria).
(2) Meta-analysis – a meta-analysis statistically summarises the risk estimates from multiple studies and can provide an overall estimate of risk. When properly conducted, a meta-analysis can combine evidence from individual studies and can provide more stable summary estimates of effect. Typically, systematic reviews of the literature and meta-analysis of the results are conducted to obtain summary risk estimates that can help to inform causal inference.
(3) Confounding – confounders are variables that are associated with the health outcome of interest and are correlated with exposures being studied as risk factors. Confounders may be demographic variables (for example, age, sex, ethnicity) or other exposures that are risk factors for the health outcomes of interest. For example, findings from studies of a particular pesticide and a particular health outcome may be confounded if the exposures of interest are correlated with other exposures that are etiologically related to the outcome of interest. Results that are confounded can lead to erroneous results and conclusions.
(4) Exposure assessment – the method of exposure assessment involves estimating study participants’ exposures to environmental or lifestyle factors. The assessment can range from binary precision (“yes” or “no”); to high / medium / low (ordinal); to quantitative levels (continuous) (for example, micrograms of the chemical exposure per milligram of soil).
(5) Etiologic association – a causal relationship between the exposure and the health outcome being studied.
(6) P-value – the probability that a finding was due to chance. A p-value of less than 0.05 is often regarded as an indicator of a “real” (non-chance) finding. The underlying assumption is that 5% of findings are likely to be due merely to chance. For example, if p=0.01, then there is no more than a 1% possibility that the finding was merely a chance observation.
(7) Dose-response trend – the pattern of relative risk estimates in relation to increasing exposure levels. An upward (“positive”) consistent trend is indicative of an etiologic relationship between exposure and disease risk.
(8) Recall bias – a systematic error that occurs when study participants do not remember previous events or experiences accurately.
(9) Selection bias – a distortion in a measure of association due to a sample selection that does not accurately reflect the target population.
125 I turn now to the relevant experts in the epidemiology stream.
126 The epidemiology stream spanned Conclaves C, D and E and involved the following expert witnesses:
(1) Conclave C – Epidemiology (epidemiology concerning glyphosate)
Professor Checkoway and Associate Professor Harris
(2) Conclave D – Epidemiology and biostatistics (the correct allocation of person years in the cohort studies which have been conducted into glyphosate)
Professor Gordon and Associate Professor Harris
(3) Conclave E – Biostatistics (statistical analysis of epidemiology studies)
Professor Gordon and Dr Crump
127 As noted earlier, in addition to the giving of concurrent evidence in open court and the preparation of joint reports in respect of each conclave, several individual reports were prepared by each of the experts and relied upon by the parties for the purposes of the epidemiology stream, as set out in the table below:
Report | Date | Report Topic |
Expert Report of Professor Ian Gordon (First Gordon Report) | 29 September 2021 | Statistical methodologies used, and the consequent reliability of the conclusions drawn, in Andreotti (2018), De Roos (2005) and Crump (2020). |
Expert Report of Dr Shelly Harris (First Harris Report) | 28 January 2022 | Whether human epidemiological evidence supports an association between exposures to GBFs and increased risk of NHL. |
Expert Report of Dr Kenny Crump (First Crump Report) | 15 July 2022 | The extent to which the body of epidemiologic science provide reliable and cogent evidence as to the effect, if any, that exposure to glyphosate-based herbicide has on a person’s risk of developing NHL. |
Expert Epidemiological Report of Harvey Checkoway (First Checkoway Report) | 1 August 2022 | Methods or processes used in epidemiology to evaluate whether a substance or chemical is carcinogenic to humans and whether glyphosate and/or GBFs, such as Roundup, are carcinogenic. |
Expert Report Professor Ian Gordon addressing questions of the Court (Second Gordon Report) | 16 August 2023 | Whether the error described and summarised in the First Gordon Report (at [173]) was made in De Roos (2005) and Andreotti (2018) and reliability of those studies. |
Expert Epidemiological Report of Harvey Checkoway (Second Checkoway Report) | 7 September 2023 | Review of the Hardell (2023) report which addressed potential etiologic relations of GBFs and risk of NHL. |
Supplemental Expert Report of Shelly Harris (Supplementary Harris Report) | 9 September 2023 | Response to Second Gordon Report in relation to cumulative exposure categories. |
Supplemental Expert Report of Shelly Harris (Second Supplementary Harris Report) | 10 September 2023 | Review of Chang and Andreotti (2023) and response to Smith dated 10 September 2023. |
Expert Report Professor Ian Gordon addressing questions of the Court (Third Gordon Report) | 15 September 2023 | Whether the error described and summarised in the First Gordon Report (at [173]) was made in De Roos (2005) and Andreotti (2018) and reliability of those studies. |
Sensitivity Analysis for Agricultural Health Study, Andreotti (2018) (AP Harris Sensitivity Report) | 20 September 2023 | Sensitivity analysis conducted following Professor Gordon’s suggestion in the Third Gordon Report (at [124]) in relation to cumulative exposure in Andreotti (2018). |
Response to AP Harris Sensitivity Report (Fourth Gordon Report) | 24 September 2023 | Professor Gordon’s opinion regarding Professor Villeneuve and AP Harris’ conclusions in the AP Harris Sensitivity Report. |
128 In the following section, I will introduce each of the experts who participated in the epidemiology stream, before turning to making some general observations as to credit. In doing so, I am conscious that the parties have made submissions as to credit which loom large in respect of some witnesses and not others. I have had regard to all these submissions, but it would add further to an already lengthy and oppressive judgment to set them out in full, except where I consider them to be of especial importance. As will be seen, in this section I make repeated references to various aspects of the evidence and various studies. This evidence and the studies referred to are dealt with comprehensively later in my judgment.
129 With that said, for reasons that will already be obvious, a significant amount of time was expended by both sides in mounting attacks upon witnesses (not limited to the epidemiology stream) whose evidence, it is said, was marred by partisanship. As I explained in Russell v Australian Broadcasting Corporation (No 3) [2023] FCA 1223 (at [438]), many experienced judges have expressed the caution that any criticisms of a witness, which go beyond the legitimate necessities of the occasion, should be avoided. Indeed, unnecessary credit findings should be avoided partly because of a body of research casting doubt on the ability of judges to make accurate credibility findings based on demeanour: see Fox v Percy [2003] HCA 22; (2003) 214 CLR 118 (at 129 [31] per Gleeson CJ, Gummow and Kirby JJ).
130 Despite an adverse credit finding with respect to one witness, issues going to credit are not the centrepiece of this case. As the High Court explained in Fox v Percy (at 129 [31]), the Court’s task is to reason its conclusions, as far as possible, on the basis of contemporary materials, objectively established facts and the apparent logic of events (in this initial trial, the scientific materials). This is not to say that my assessment of the scientific evidence in the three streams is not partly informed by impressions I have gained as to the relevant expert’s overall demeanour and credit. It is. But there is an extent to which such impressions can distract the Court from the principal task of assessing the underlying scientific merit of the evidence adduced in this proceeding.
131 Professor Harvey Checkoway is an applied epidemiologist specialising in occupational and environmental risk factors for multiple different malignant diseases and neurological disorders, especially Parkinson’s disease. Professor Checkoway was engaged by Mr McNickle.
132 Professor Checkoway holds a bachelor’s degree in psychology from Boston University (1971), an MPH in epidemiology from Yale University (1975), and a PhD in epidemiology from the University of North Carolina (1979). Since 2013, he has been a Professor at the University of California, San Diego Departments of Family Medicine and Public Health. Professor Checkoway previously held faculty positions at the University of North Carolina in the Department of Epidemiology and the University of Washington in the Departments of Environmental and Occupational Health Sciences and Epidemiology.
133 Much of the contest concerning Professor Checkoway’s evidence went to the basis for his conclusion in the JRC (at [132]) that there is “generally supportive evidence that environmental GBF exposures are etiologically related to NHL” (emphasis added). It is important to note from the outset that Professor Checkoway, in reaching that conclusion, stated that he relied predominantly on four individual case-control studies, namely Hardell and Eriksson (1999); McDuffie (2001); Eriksson (2008); and Meloni (2021). I will return to these studies below.
134 Mr McNickle submits that Professor Checkoway gave evidence in a careful and considered manner and that the Court should find that he was a reliable witness, for the following reasons.
135 First, Professor Checkoway responded directly to questions posed to him and made appropriate concessions without hesitation. He did not seek to dismiss, nor set aside, epidemiological studies which did not support his conclusion that there is generally supportive evidence that GBF exposures are etiologically related to the risk of humans developing NHL.
136 Secondly, and relatedly, Professor Checkoway gave evidence that it is important for the Court to have regard to all of the relevant studies, and that he did not consider the studies which did not indicate an etiologic relation as inferior to those which did, but that those studies were inconsistent with other studies (namely, the four individual studies) on which he relied and found to be “especially meaningful” (T866.11–13; T866.36–39).
137 Subject to matters to which I will return momentarily, my impression of Professor Checkoway is that he generally did his best to give candid answers when challenged on critical aspects of his evidence. Having said that, it is worth mentioning two examples which were reflective of a tendency on the part of Professor Checkoway, from time to time, to not explain persuasively the reasons why he placed less weight upon studies which did not support his ultimate conclusion that there is an etiologic association between exposure to glyphosate and/or GBFs and NHL.
138 First, and most significantly, Professor Checkoway omitted reference to the Agricultural Health Study (AHS) (to which I will return) in the JRC (at [128]–[132]) when setting out his conclusions as to whether there exists evidence for an etiologic association between glyphosate exposure and development of NHL in humans. Notwithstanding that Professor Checkoway addressed the AHS and its strengths and weaknesses (at JRC [102]–[104]), for reasons that will become all too apparent, its omission when offering his ultimate conclusion as to the central issue is, at best, puzzling. This is highlighted by Professor Checkoway ultimately accepting that the AHS is an “excellent study” and “[i]n terms of study design and methodology, I think [the AHS is] perhaps the best study we have” (T959.14–15; T959.44–45).
139 Secondly, in the JRC (at [95]), Professor Checkoway sets out in Table 1 a summary of findings from publications that address potential etiologic relationships between glyphosate and/or GBFs and NHL. It does not, however, include the case-control study of Cantor (1992) (to which I will return). At some risk of oversimplification, that study suggests that there is no significant risk elevation of NHL for exposure to glyphosate. When asked about why he chose not to include Cantor (1992) in Table 1, Professor Checkoway explained that it was an accidental omission and that it should have been included in the table (T1031.44–1032.3). It is regrettable the omission of the Cantor study in Table 1 was the result of an inadvertent slip given that it is included in Table 2 of the JRC (at [113]), albeit as part of a list of limitations.
140 Thirdly, and relatedly, Professor Checkoway did not resile from his position in the JRC that greater weight ought to be placed upon the individual studies, as opposed to the pooled studies, for the conclusion that there is an etiologic link between glyphosate and/or GBF exposure and development of NHL in humans. Despite this, when asked about the weight that should be attributed to Cantor (1992), compared to De Roos (2003) (a pooled study, of which the Cantor study contributes 26 of the 36 exposed cases and 49 of the 61 controls), Professor Checkoway’s evidence was that greater weight should be placed on De Roos (2003) (T1032.19–36).
141 These examples may not be particularly significant in isolation and do not detract from my overall impression that Professor Checkoway was a generally impressive witness who sometimes made concessions. It is necessary, however, to approach Professor Checkoway’s evidence with increased scrutiny in the light of his tendency to place weight upon studies which support his conclusion and downplay weaknesses which detract from that conclusion.
142 Associate Professor Shelly Harris (AP Harris) is an epidemiologist and an associate professor in the Division of Epidemiology and the Division of Occupational and Environmental Health in the Dalla Lana School of Public Health, University of Toronto. AP Harris was engaged by Monsanto.
143 AP Harris holds a PhD in epidemiology in the Department of Preventive Medicine and Biostatistics (now Public Health Sciences), Faculty of Medicine, University of Toronto. AP Harris is a scientist in the Occupational Cancer Research Centre and previously was appointed as a scientist in the Division of Prevention and Cancer Control at Cancer Care Ontario.
144 Relevantly for present purposes, AP Harris’ ultimate conclusion (at JRC [233]–[236]) is that based upon her critical evaluation of all the epidemiological studies, despite some case-control studies indicating some statistically significant elevations in ORs for NHL, the evidence does not support a causal connexion between exposures to glyphosate and/or GBFs and increased risk of NHL overall (or its subtypes).
145 Mr McNickle submits that the Court should reject AP Harris’ evidence and conclusion that the scientific literature does not indicate a causal connexion between exposure to glyphosate and/or GBFs and development of NHL because AP Harris was not a wholly credible witness and, at least at times, did not approach her role as an expert witness impartially and strayed into advocacy. In the light of that tendency, Mr McNickle submits that the Court should prefer the evidence of Professor Checkoway and Professor Gordon.
146 Four points are made.
147 First, in the First Harris Report, the JRC, and the Conclave D Joint Report (JRD), it is said that AP Harris expressed opinions and conclusions that are inconsistent with opinions that she had expressed previously in peer-reviewed, published papers and in presentations to the wider scientific community, and that accordingly, the opinions expressed by AP Harris in this proceeding are more favourable to Monsanto than conveyed previously to the scientific community.
148 Secondly, AP Harris sought to defend and endorse the AHS with a degree of impartiality that was inappropriate for an expert witness. Three points are developed in relation to this submission, namely: (1) AP Harris produced five reports (totalling, remarkably, 335 pages) in respect of the NCI data in De Roos (2005) (despite the orders of the Court providing for one report); (2) AP Harris prepared a “sensitivity analysis” (that is, the AP Harris Sensitivity Report) on her own initiative in respect of Professor Gordon’s conclusions in the Third Gordon Report because it remained a live issue in the proceeding; and (3) AP Harris told the Court that the conclusions in the AHS papers “must be relied upon” for the evaluation of the association between glyphosate use and the risk of NHL as their study design offers many advantages and strengths over other studies. By contrast, it is said that AP Harris referred to aspects of Chang and Andreotti (2023), which was prepared by many of the same authors and used the same AHS data but reported an association between glyphosate exposure and oxidative stress, as “misleading”.
149 Thirdly, Mr McNickle submits that AP Harris strayed into ad hominem attacks upon Professor Gordon’s professional expertise and character, including an assertion that Professor Gordon’s opinion was “purposively misleading or demonstrates a lack of knowledge of the fundamentals of analysing data from epidemiological cohort studies” (Supplementary Harris Report (at [19])).
150 Fourthly, it is said that in contrast to Professor Checkoway and Professor Gordon, AP Harris often gave unresponsive answers to questions put to her in cross-examination and that she had to be asked several times to answer the question asked.
151 On balance, I consider these criticisms to be overstated. I do not think that AP Harris was intent on avoiding concessions that she considered adverse to the case of Monsanto, and, to the extent that she strayed into any perceived advocacy, I consider that that was a product of a genuine attempt to get at the truth of the scientific evidence, as opposed to an attempt to bolster the case of Monsanto or her own conclusions in her primary report or joint reports.
152 One example will suffice. When giving oral evidence in Conclave D, AP Harris was asked about the cross-sectional study Blair (2011) which, in summary, assessed the impact of exposure misclassification on estimates of relative risks in the AHS papers, using the range of correlation coefficients observed between measured post-application urinary levels of 2,4-D and chlorpyrifos (two herbicides) and exposure estimates. Blair (2011) did not include data on exposure to glyphosate and, therefore, was of general relevance only to an understanding of the AHS papers. However, when questioned about the correlation coefficient in the study, AP Harris opined that glyphosate may “behave similarly” to 2,4-D or other herbicides (T799.4). The exchange continued (T799.19):
MS SZYDZIK: Just a couple of questions arising out of that. Associate Professor Harris, you just said that you would expect glyphosate might behaviour similarly to 2,4-D, are you just able to explain why you think that’s the case?
ASSOC PROF HARRIS: It’s a possibility. I think it’s because it’s not a highly volatile compound and often they’re spraying and using similar types of application methods. So they might be spraying it off a tractor or something like that. But they’re not going to be putting it in a backpack and spraying it by hand. So there’s some similarities in the way these compounds are applied and also their physical characteristics, as I understand.
153 Having had the benefit of seeing this evidence unfold, I have difficulties in accepting the submission that AP Harris was intent on supporting the case of Monsanto at the expense of her own impartiality. There can be little doubt that if AP Harris was so minded, she would have been reluctant to proffer a view that glyphosate may behave similarly to other herbicides, such as 2,4-D. As noted earlier, this was indicative of what I perceived to be a desire on the part of AP Harris to be faithful to her oath.
154 While I accept far too much material was produced contrary to the directions of the Court, I suspect that had more to do with over-enthusiasm (including of lawyers) rather than any lack of objectivity on the part of AP Harris.
155 Professor Ian Gordon is an applied statistician specialising in biostatistics and epidemiology, including methodological issues in randomised controlled trials, cohort studies, case-control studies, meta-analysis, and survival analysis. Professor Gordon was engaged by Mr McNickle.
156 Professor Gordon holds a bachelor’s degree in statistics from the University of Melbourne, a MSc (by thesis) from La Trobe University concerning conditioning in statistical inference, and a PhD from the University of Melbourne on sample size determination for discrete data. He is currently a Professor of Statistics at the University of Melbourne and the director of the Statistical Consulting Centre.
157 It will suffice to note for present purposes that much of the controversy over Professor Gordon’s evidence concerned his scrutiny of the methodology used in the AHS papers which, in summary, were that first, Andreotti (2018) did not obtain exposure data up to the point in time that it obtained data about cancer incidence; and secondly, about the way in which Andreotti (2018) utilised multiple imputations to address missing data. As it turns out, these criticisms were largely unfounded or, if they applied, had a negligible effect on the ultimate conclusions expressed by the authors of the AHS papers. For reference, I deal with this topic in the context of addressing the AHS in Section E.3 below.
158 Mr McNickle submits that notwithstanding this, the Court should find that Professor Gordon was a credible and reliable witness who gave evidence in a careful and considered manner. It is said that his willingness to change and revise his opinions in the light of new information and data indicates that he kept an open mind throughout the trial, which is demonstrative of his impartiality as an expert.
159 It was difficult not to gain the impression that Professor Gordon, at times, appeared inflexibly wedded to his criticisms of the AHS papers. Several examples may be cited, and I do not intend to set them all out here.
160 With that said, I do not accept that this was the product of a deliberate attempt on Professor Gordon’s part to obfuscate the scientific literature to bolster his own conclusions or the case of Mr McNickle. When challenged on his criticisms of the methodologies adopted in the AHS papers, Professor Gordon, albeit belatedly, made appropriate concessions and accepted when the evidence plainly tended against his conclusions (see, for example, at JRD [66], [230]). At one point, during cross-examination, Professor Gordon even went so far as to concede that he could not comment upon the issue of latency between exposure and clinical manifestation of NHL because he lacked expertise in cancer processes (T675.24–31).
161 Overall, my impression is that Professor Gordon approached the task of giving evidence with integrity, and that if his evidence was less than satisfactory in respects, it was not for want of candour.
162 Dr Kenny Crump is a professional consultant and biostatistician. He was engaged by Monsanto.
163 Dr Crump holds a bachelor’s degree in electrical engineering from Louisiana Tech University, a bachelor’s degree in mathematics from the University of Denver, and a PhD in mathematics with an emphasis in probability theory from Montana State University. In 1980, Dr Crump started a consulting company focussed on providing advice and conducting research on problems related to health risk assessment, and worked for several consulting companies in which he performed quantitative risk assessments, including for exposure to ethylene oxide and inorganic arsenic.
164 Little can be gained by way of an assessment of Dr Crump’s credibility in giving oral evidence because it was obvious during the Conclave E concurrent evidence session that Dr Crump had trouble hearing and comprehending questions put to him in cross-examination. I do not propose to speculate as to why this was the case, but I raised that it may have been partly due to a combination of jetlag and fatigue.
165 The consequence was that senior counsel for Mr McNickle, Ms Szydzik SC, was constrained in her ability to effectively cross-examine Dr Crump on matters squarely falling within his area of expertise and the subject matter of his primary report and published article, Crump (2020). Monsanto, on the other hand, contends that this concern is overstated, and further submitted (in my view, surprisingly) that Dr Crump had little difficulty engaging with what he was asked by Ms Szydzik. I do not accept that submission. The transcript is an incomplete guide to the difficulties as I perceived them unfold while closely observing what was occurring in Court, but Dr Crump’s significant hesitations and evident vexation caused me to ask Dr Crump to leave the witness box and then intervene as follows (T1201.18–23):
HIS HONOUR: … I just raise this issue because I’m just wondering how utile this process is. I’m not convinced that Dr Crump is really understanding [Ms Szydzik’s] questions. Whether he’s tired or jetlagged, or whatever, but he’s obviously having difficulty hearing and I don’t want there to be an unfairness associated with that. Now, I don’t know what the position is. There has been no – I mean, I raise it because I’m concerned about his ability to comprehend what’s being put to him.
166 I did not make this comment without carefully reflecting upon whether it was necessary to intervene. Sometimes, these impressions can only be gauged if one observes what is happening in real time and I thought what was happening could occasion a real unfairness to Dr Crump.
167 I will return to Dr Crump’s evidence below in relation to Conclave E, but I hasten to note that I must bear in mind that there was a limited ability for Mr McNickle to test Dr Crump’s evidence, which must be borne in mind steadily when evaluating his evidence and assessing the ultimate weight the Court ought to place upon it.
168 With these general observations in mind, I come to my findings as to the contested aspects of the epidemiological and biostatistical evidence.
E.3 The Epidemiological and Biostatistical Evidence
169 Mr McNickle contends that the body of epidemiological and biostatistical literature provides generally supportive evidence that exposure to glyphosate and/or GBFs increase an individual’s risk of developing NHL, and therefore that glyphosate and/or GBFs are carcinogenic to humans.
170 As noted earlier (at [133]), Mr McNickle relies upon Professor Checkoway’s conclusion in the JRC (at [132]) that there is “generally supportive evidence that environmental GBF exposures are etiologically related to NHL” (emphasis added). Subject to a matter to which I will return, Professor Checkoway primarily relied upon four individual case-control studies which, in his opinion, indicate an “etiologic contribution” of exposure to GBFs to NHL; namely Hardell and Eriksson (1999); McDuffie (2001); Eriksson (2008); and Meloni (2021) (primary case-control studies). Professor Checkoway also relied upon the pooled studies of Hardell (2002); De Roos (2003); Pahwa (2019); Lee (2004); and De Roos (2022) (pooled case-control studies) in formulating his conclusion in the JRC. It will also be necessary to deal with some other articles referred to in the course of the concurrent evidence session.
171 In dealing with the various studies relied upon by the parties in the epidemiological stream, it is convenient first to set out, at a high level, the key conclusions of each study; secondly, summarise the findings of each of the experts in respect of each study; and thirdly, make findings as to the weight to be attributed or assistance derived from the various studies and expert opinion evidence.
172 In doing so, I will adopt the following structure:
Section I will detail my findings as to the primary case-control studies;
Section II will set out my findings in relation to the pooled case-control studies;
Section III will address some other studies referred to in Conclave C;
Section IV will set out my findings as to the AHS in the context of Conclave D;
Section V will detail my findings as to recall and selection bias in Conclave E; and
Section VI will set out my conclusion as to the epidemiology stream.
I Primary case-control studies
173 This study was a population-based study in northern and central Sweden encompassing 442 cases and approximately twice as many controls. Exposure data was obtained by questionnaires, which were supplemented by telephone interviews. In total, 404 cases and 741 controls answered the questionnaire.
174 The authors found there was increased risk for NHL for subjects exposed to herbicides, specifically an OR of 2.3 (95% CI: 0.4–13) for ever/never exposure to GBFs (that is, subjects who were ever exposed to GBFs compared to those who were never exposed).
175 Professor Checkoway considered that despite the wide CI, the OR “in terms of its direction” was nonetheless suggestive of an etiologic relation between exposure to GBFs and development of NHL in humans (T917.46). In his analysis, CIs are only a measure of statistical “precision” and are not a “necessary criterion” for assessing causation (at JRC [128]).
176 Further, in cross-examination, Professor Checkoway said that it could be inferred from the fact that the multivariate analysis produced much higher ORs than the univariate analysis that glyphosate exposure was not confounded (that is, a variable whose presence affects the variables being studied so that the results do not reflect the actual relationship) by other pesticides (T914.22–25) (although he acknowledged that it was unclear what variables were controlled for in the multivariate analysis (T915.20)). While Professor Checkoway placed less weight on the multivariate analysis, he nonetheless considered that the data presented was valid and he did not disregard that it showed an elevated OR (T916.3–4; T916.21–22).
177 Professor Checkoway and AP Harris agreed that the study had a design issue in that the selected reference group had no exposure to any pesticides (T918.10–22). However, a crude OR can readily be calculated using as the baseline reference group all cases and controls that were unexposed to glyphosate (but still exposed to other pesticides), by taking the total number of cases (404) and controls (741) in the study and subtracting from that the cases and controls exposed to glyphosate (four and three, respectively), which results in an unadjusted or crude OR of 2.46. In Mr McNickle’s submission, the calculation of the unadjusted OR using the baseline reference group of all cases and controls that were unexposed to glyphosate (but still exposed to other pesticides) shows that the OR is higher using the revised baseline group.
178 Professor Checkoway acknowledged that recall bias was a “possibility” as in any case-control study. But it was not possible for him to say how much of an effect it may have had, or whether it would inflate or deflate the estimate (T918.32–919.3). AP Harris gave evidence that there was evidence of recall bias “potentially” because of the elevations of risk across multiple pesticide categories, and Professor Checkoway agreed with the proposition that “this might lead you to think” there has been recall bias (T919.21–35). Ultimately, Mr McNickle submits that the evidence of the two experts went no higher than recall bias having a possible or potential impact on the results and no evidence was given that this was likely or probable.
179 Proxy respondents (that is, someone who assists the intended respondent in the study or responds on their behalf) were used for around 43% of deceased subjects in this study, with each deceased case being matched to two deceased controls: see Hardell and Eriksson (1999) (at 1354) (T920.1–34). Professor Checkoway agreed with Monsanto’s proposition that it was likely that a proxy would provide less accurate exposure information than the exposed person but clarified that this applied to both cases and controls which results in non-differential misclassification, and which typically leads to an underestimate of relative risk (T920.36–46). AP Harris gave evidence that a lack of adjustment for proxy respondents might bias the reported OR estimates (at JRC [172]), but did not give evidence as to how, in this study, the results may be biased. AP Harris agreed that for the most part, proxy respondents cannot provide as much detail regarding pesticide use as the subjects themselves, which would tend to bias estimates of relative risks downward (T994.32–995.1).
180 I consider that Hardell and Eriksson (1999) is of limited assistance, for the following reasons.
181 First, and most importantly, Professor Checkoway conceded that the result he nominated in Table 1 as the “major finding” from this study (that is, OR 2.3, 95% CI: 0.4–13) was “extremely wide” (T910.25). As explained earlier, because the CI straddles 1.0, it does not exclude the null hypothesis. Professor Checkoway conceded that the effect of the CI he identified as the “major finding” in the study was that it did not exclude the possibility of no association between exposure to GBFs and development of NHL in humans.
182 Secondly, Professor Checkoway accepted that the sample size which produced the result that he identified as the “major finding” was too small to derive any real assistance from the study in respect of any association of glyphosate with NHL (T912.26). With only four cases and three controls, the statistical power of the study is small, and the ORs are elevated non-significantly. Professor Checkoway also agreed with AP Harris’ observation that (T915.2–4):
ASSOC PROF HARRIS: [t]he numbers of cases [and] controls could be even smaller than four and three because of missing data in the other variables. So when they actually do the control for the analysis, the numbers might be quite small …
183 Thirdly, Professor Checkoway agreed with AP Harris’ observation that in respect of the multivariate analysis (which had the wide CI of 0.6–5.4) the authors did not identify what was adjusted for in that analysis (T915.20). AP Harris explained the significance of not knowing what was adjusted for (T916.8–12) as follows:
ASSOC PROF HARRIS: So we don’t know what they adjusted for and then we really have no way [to evaluate] that because they haven’t actually presented any basic demographic information or comparison between the cases and controls in the manuscript itself. So you have no idea really whether the controls are representative of the population from which the cases were done.
184 Professor Checkoway agreed with AP Harris’ explanation (T916.14) and noted that he did not place any significant weight on the multivariate analysis.
185 Fourthly, Professor Checkoway conceded that despite his assertion (at JRC [97]) that “[a]nother strength was the comprehensive questionnaire that was the source of the exposure assessment”, he has never seen the questionnaire (T908.22). Professor Checkoway agreed that he could not tell whether the questionnaire was more or less extensive than the questionnaire used in the AHS (T909.1).
186 Fifthly, Professor Checkoway accepted that the study said nothing about dose-response, as it was in his view “impossible” because of the small number of cases and controls (T917.26–32).
187 For these reasons, I do not consider Hardell and Eriksson (1999) is of significant assistance.
188 This study was a population-based study among men resident in six Canadian provinces to test the pesticide-exposure hypothesis related to four rare tumours. The report is based solely on cases diagnosed with NHL. Data from postal questionnaires based on responses from 517 NHL cases and 1506 control subjects were analysed. For the highest glyphosate exposure category (greater than two days per year), the authors reported an OR of 2.12 (95% CI: 1.20–3.73).
189 AP Harris described the McDuffie (2001) results as a “statistically significant increase” (at JRC [152]) and “certainly elevated” (T1053.27). Professor Checkoway’s evidence was that the study showed an increasing and significant dose-response trend across the three exposure categories, which was suggestive of a dose-response relationship between GBF exposure and development of NHL (T924.23–37; T925.31–38; T926.31; see also JRC (at [129])). In his view, the study had various strengths including an appropriate study design, the large sample size, detailed NHL diagnostic methods that included pathological confirmation, and data analysis methods, especially “conditional logistic regression” which is a well-established, robust approach to estimate ORs controlled for confounding variables (at JRC [99]).
190 Mr McNickle submits that McDuffie (2001) provides support for an etiologic connexion between exposure to GBFs and NHL and that the OR estimates in the McDuffie (2001) study are not unreliable due to confounding, selection bias, recall bias or use of proxy respondents, for the following reasons.
191 First, as to confounding, the authors do not state expressly whether they adjusted for other pesticides. Professor Checkoway and AP Harris agreed that if the authors had not adjusted for other pesticides the results for glyphosate may potentially be confounded by other pesticides (T928.36–930.29).
192 Secondly, as to selection bias, AP Harris considered that the poor response rates for both cases and controls “could lead to selection bias” (at JRC [154]). Professor Checkoway agreed there was some difference between the response rates of the cases and controls, but noted the difference was not large and did not agree with Monsanto’s proposition that it was “significant” (T931.22–24). He did not consider over-reporting was an issue (T931.33). AP Harris gave evidence, and with which Professor Checkoway agreed, that there is some difference in case and control participation rates in all case-control studies, and that because participation rates were “not great” in both the case and control groups, “you just always need to be concerned about the potential bias” (T931.40–44).
193 Thirdly, as to recall bias, the authors acknowledge only the “potential for recall bias” which is inherent in any case-control study (at 1161). Neither AP Harris nor Professor Checkoway gave evidence to the effect that recall bias did impact, or is likely to have impacted, the OR estimates in the paper. Moreover, the authors conducted a validation study of the questionnaire used to collect exposure information and found “excellent concordance” between the questionnaire answers and pesticide purchase records (at 1156), suggesting the reported information was accurate and recall bias was not a significant issue.
194 Fourthly, as to the use of proxy respondents, it is said there is no evidence that the use of proxy respondents had any substantive impact on the OR reported. AP Harris’ assertion that McDuffie (2001) did not adjust for proxy respondents in the multivariate analyses which “would likely bias results”, it is said, should be rejected. As acknowledged by AP Harris (at JRC [152]), the authors did not report any results for glyphosate for the multivariate analysis that she criticises. Further, as observed by AP Harris and her co-authors in Pahwa (2019), deceased cases and controls were ineligible for inclusion in the McDuffie paper, meaning there is reduced potential for bias being introduced using proxy respondents.
195 I consider that McDuffie (2001) does not provide supportive evidence of an etiologic connexion between GBFs and development of NHL of any real significance.
196 First, as with Hardell and Eriksson (1999), Professor Checkoway agreed that for the ever/never results reported for glyphosate in Table 2 of McDuffie (2001), both the unadjusted and adjusted results did not exclude the null hypothesis, as the lower bound of each confidence interval was less than 1.0 (T923.6).
197 Secondly, Professor Checkoway agreed that the study does not indicate that the results in Table 8 (which contained the result of OR 2.12, 95% CI: 1.2–3.73, and which he identified in Table 1 of the JRC as the “major finding” from this study) were adjusted for exposure to pesticides other than glyphosate (T929.1–29). Although it is not entirely clear from the report itself, Professor Checkoway observed that the authors “didn’t report it so I would assume that they didn’t do it” (T929.36–37). The corollary is that the results in respect of glyphosate may have been confounded for exposure to other pesticides (T929.25–29; T930.5–13).
198 Thirdly, AP Harris observed (T933.6–8) that the authors had reduced the number of surrogate (or proxy) respondents by excluding deceased persons from the definition of eligible subjects for the study. As a result, the analyses were not adjusted for proxy respondents, which AP Harris identified as a flaw of the study. Professor Checkoway agreed (T933.13).
199 This study was a population-based study of NHL risk factors in Sweden. Subjects aged between 18 and 74 years were included. In total, 910 cases and 1016 controls participated. The authors concluded that the study indicated an association between exposure to phenoxyacetic acids and NHL and “the association with glyphosate was considerably strengthened” (at 1657).
200 The authors reported an OR of 2.02 (95% CI: 1.10–3.71) for ever exposed to glyphosate, which increased to 2.36 (95% CI: 1.04–5.37) for the high dose glyphosate exposure category (greater than ten days a year exposure). The authors also reported a multivariate analysis which gave an OR of 1.51 (95% CI: 0.77–2.94) for glyphosate.
201 Mr McNickle submits that this study had several strengths as agreed by Professor Checkoway and AP Harris. Each referred to the study subjects and pathological confirmation of clinical diagnoses as strengths of the study, among other strengths (at JRC [106], [182]).
202 It is contended by Mr McNickle that there is no basis for the Court to dismiss the results of this study as unreliable, or overestimated, due to confounding, selection bias and or recall bias issues, for the following reasons.
203 First, as to confounding, Professor Checkoway and AP Harris agreed that the presence of other pesticides “could be a confounder”, but neither agreed with the proposition put by senior counsel for Monsanto that the other pesticides “is a confounder” (T939.1–8). Further, Professor Checkoway gave evidence to the effect that the lack of adjusting for other pesticides diminished somewhat the “non-trivial odds ratio” of 1.51 for glyphosate exposure, but it did not mean that the recorded association went to the null value (T939.42–44).
204 Secondly, as to selection bias, AP Harris and Professor Checkoway agreed the use of a control group with no exposure to pesticides was not an optimal approach (T936.22–937.7). Professor Checkoway gave evidence, with which AP Harris agreed, that he would not describe the results as “incorrect”, but the control group meant that the “results may be somewhat overestimated” (T937.1–7). As with Hardell (1999) addressed above, the unadjusted OR can be calculated using the baseline reference group of all cases and controls that were unexposed to glyphosate (but still exposed to other pesticides) (that baseline group can be calculated as follows: for cases, 910–29=881; for controls, 1016–18=998). This would result in an OR of 1.82 (calculated by taking the odds of exposure for cases (29/881=0.03292) and dividing it by the odds of exposure for controls (18/998=0.01804)). This calculation of the unadjusted OR shows that using the baseline reference group of all cases and controls that were unexposed to glyphosate (though still exposed to other pesticides) reduces the OR marginally (from 2.02 to 1.82, noting that the OR in the paper is adjusted for age, sex and year of diagnosis or enrolment).
205 Thirdly, as to recall bias, Professor Checkoway observed that like the other studies, exposure assessment is based on questionnaire responses (that is, self-reporting). However, his opinion was that this is less of an issue in this study because of the “very thorough” questionnaire and use of trained interviewers to collect supplementary exposure information (at JRC [107]). Professor Checkoway was not cross-examined on his opinion in relation to recall bias and this paper. Further, as noted elsewhere by Professor Checkoway, inaccurate exposure estimation resulting from self-reporting typically leads to “non-differential misclassification”, the usual consequence of which is underestimation of risk association.
206 Notwithstanding these points, I do not consider that Eriksson (2008) is of much assistance, for the following reasons.
207 First, Professor Checkoway gave evidence that none of the three glyphosate results in Table II were adjusted for exposure to other pesticides (T935.16–17).
208 Secondly, and relatedly, Professor Checkoway agreed that the result he extracted in Table 1 of the JRC was a univariate result and, further, that the results of the multivariate analysis in Table VII in Eriksson (2008) were lower than the univariate results (T935.35–37). This is significant because the authors of the study expressly noted (at 1660) that exposure to multiple pesticides was common among the study participants:
Multivariate analysis
Since mixed exposure to several pesticides was more a rule than an exception, and all single agents were [analysed] without adjusting for other exposure, a multivariate analysis was made to elucidate the relative importance of different pesticides. Criteria for agents to be included in this analysis are defined in Statistical Methods above. As seen in Table VII increased ORs were found but in general lower than in the univariate analysis.
209 Significantly, the multivariate result in Table VII from the study for glyphosate (that is, an OR of 1.51, 95% CI: 0.77–2.94) was not only lower than the univariate result, but the lower bound of the CI was less than 1.0. Professor Checkoway conceded that as a result, the multivariate result for exposure to glyphosate in this study did not exclude the null hypothesis (T939.34).
210 Thirdly, Professor Checkoway agreed with AP Harris’ view (T936.29–32) that by dint of the study’s design, the unexposed group was comprised of subjects who were unexposed to all pesticides, meaning that the comparison between exposed and unexposed subjects for glyphosate was invalid (see, for example, T936.12–27). As Professor Checkoway explained (T937.15–27) (with which AP Harris agreed (T937.31)):
PROF CHECKOWAY: [O]ne of the reasons it’s not an optimal comparison group is that people not exposed to any of the, in this case, pesticides, maybe have different characteristics, maybe different socioeconomic status ... and so forth, and so it’s – it’s not the optimal comparison ... You consider all the data you have for both groups, in my view, and then do an adjustment for the multiple variables.
211 Relatedly, AP Harris noted that another effect of the study design was that the unexposed group excluded exposures to various confounders that may have been associated with excluded pesticides (T937.31–46). Professor Checkoway agreed that the consequence of the design of the study that compared an unexposed group comprised of people unexposed to all pesticides with an exposed group of people exposed to glyphosate and possibly other pesticides is that the presence of the other pesticides could be a confounder if one were trying to elucidate the effects of glyphosate (T939.1–4)
212 This study was conducted in six Italian centres between 2011 and 2017 and involved 867 incident lymphoma cases and 774 controls. The authors reported an OR of 1.4 (95% CI: 0.62–2.94) for ever/never exposure to glyphosate. For the medium-high cumulative exposure category, the authors reported an OR of 1.5 (95% CI: 0.49–4.81).
213 Professor Checkoway considered that Meloni (2021) provides evidence of an etiologic association between GBF exposure and NHL (T869.1–24; T884.16–17; T885.28–30) and shows a dose-response trend, with a low exposure OR of 1.2 and a medium to high exposure OR of 1.5 (T871.32–872.27; T885.27–30). In his view, a strength of this study was the “high calibre” exposure assessment methodology (at JRC [113]; T869.38–40; T903.37). Similarly, AP Harris gave evidence that the study’s exposure assessment methodology was “state of the art” and said that “it should result in more valid and reliable estimates of pesticide exposure for all subjects” (at JRC [188], [191]) (T1105.28–1106.11).
214 Both Professor Checkoway (at JRC [113]) and AP Harris (at JRC [191]) opined that a limitation of the study was the small number of participants exposed to glyphosate, which they agreed meant there would be less precision in the estimates (T884.28–36; T893.5–7). Nonetheless, according to Professor Checkoway, the trend was in the direction of an etiologic relation (T885.7–30).
215 Mr McNickle submits that there is no basis for the Court to place limited weight on this study because of any suggestion by Monsanto that the authors had low confidence in certain participants’ exposures and or that the control group was not appropriate, for the following reasons.
216 First, Professor Checkoway did not agree with Monsanto’s proposition that it would be “unsafe” to draw conclusions from the low confidence exposure category; his evidence was that he would not disregard the information, even though it weakened the conclusion somewhat (T892.36–42). Similarly, AP Harris did not suggest that the low confidence exposure category should be discounted: her evidence was that while she would focus on the high confidence exposure category, she would also consider the other results and how they differ (T895.11–14).
217 Secondly, Professor Checkoway did not agree with Monsanto’s suggestion that a “weakness” of the study was that the control group of sick people in hospital was not a fair representation of unexposed people in the population. Although Professor Checkoway and AP Harris agreed that the control group was not a fair representative sample of the general population without NHL, they did not consider this issue was related to exposure per se (T902.6–21). Mr McNickle contends that no evidence was given as to how, if at all, the nature of the control group may or may not impact the study results and, accordingly, there is no basis for the Court to dismiss this study because of the nature of the control group.
218 Again, I do not find these submissions particularly persuasive and I consider that Meloni (2021) is of limited assistance, for the following reasons.
219 First, Professor Checkoway conceded that the opinion he outlined in the JRC was not supported by the authors of the study, including noting as follows: “When looking at our cases fitting the definition of NHL, we did not find an association with exposure to glyphosate” (at 5). Senior counsel for Monsanto put to Professor Checkoway the following passages from the study:
(1) “Our findings provide limited support to the IARC decision to classify glyphosate as a probable human carcinogen (Group 2A), with specific lymphoma subtypes as the target” (T879.40–880.4); and
(2) “When looking at our cases fitting the definition of NHL, we did not find an association with exposure to glyphosate” (T880.16–24).
220 Professor Checkoway agreed that the conclusions of the authors of Meloni (2021) did not support his conclusion that the study provided evidence that environmental exposure to GBFs are etiologically related to NHL risk as follows (T882.34–38):
MR FINCH: And you would accept that the authors’ own view doesn’t support your view?
PROFESSOR CHECKOWAY: That – it’s not – that’s correct, it doesn’t – it doesn’t agree with my interpretation.
221 Secondly, and relatedly, in attempting to explain how he came to conclude that Meloni (2021) supported the conclusion he reached about exposure to GBFs being related to NHL risk despite the conclusions of the study authors, Professor Checkoway explained that he “did not rely on the discussion of section – of almost any of the manuscripts” (T881.42–43). Absent from the JRC is any explanation of this divergence and, despite contending that his interpretation of the findings in Meloni (2021) was “more accurate” than the study authors’ own interpretations (T882.22), Professor Checkoway did not provide an explanation (at least in a way I could understand) of the basis for this belief, nor did Mr McNickle attempt to elicit any detailed explanation from him in re-examination.
222 Thirdly, as is common with the individual case-control studies, Professor Checkoway had noted (at JRC [115]) that one “limitation of this study is the small numbers of participants ever exposed (21 cases, 15 controls) and small numbers exposed to medium/high level cumulative glyphosate exposure (seven cases, six controls).” It was put to him in cross-examination that “the authors say their study suffered from low statistical power to detect associations and from a higher probability of chance findings due to small numbers which are major interpretive limitations” (T883.29–32). Professor Checkoway agreed (T883.34).
223 Fourthly, Professor Checkoway accepted that only a small fraction of the study’s participants had ever been exposed to GBFs (T891.1–8). He agreed that of the 36 study participants who were recorded as having been ever exposed to GBFs, the study’s authors had low confidence that 21 of them were ever actually exposed to GBFs (T891.47).
224 Fifthly, despite having included an ever/never result from Meloni (2021) as one of the two “major findings” he extracted and included in Table 1 of the JRC, Professor Checkoway nevertheless gave oral evidence that he does not rely on ever/never results for his conclusions (T897.33–41):
MR FINCH: … Professor Checkoway, am I over-simplifying your approaching to this problem by saying that although you don’t discount the import of the confidence interval, you are focusing more on the trend or the dose response which is evident from the figures; is that an undue simplification?
PROFESSOR CHECKOWAY: No. That’s – I would agree with your interpretation. One thing I should mention, I think I’ve said it before, is that the ever versus never comparison is a very crude metric and I wouldn’t – I don’t rely on conclusions based on ever never.
225 Sixthly, for the two results from Meloni (2021) that Professor Checkoway extracted as “major findings” in Table 1 of the JRC, he agreed that because the lower bound of the CI for each one was less than 1.0, the result did not exclude the null hypothesis (T896.46). Professor Checkoway indicated that his interpretation of these results was that “it’s an elevated estimate of the relative risk and it’s statistically imprecise” (T897.6–7).
226 Seventhly, Professor Checkoway accepted that because the controls in Meloni (2021) were hospital patients, that control group was unlikely to reflect the population from which the people exposed to GBFs might typically be drawn and further agreed that this was a weakness of the study (T901.45–902.17).
227 Accordingly, I have not derived significant assistance from Meloni (2021).
II Pooled case-control studies
228 As noted earlier, pooled studies combine data from individual case-control studies and reanalyse the data. As noted above, Professor Checkoway and AP Harris agreed that if conducted properly, pooling data can increase the study power to detect more statistically precise associations (at JRC [17]) and that pooled studies should be considered in the overall weight of evidence analysis (at JRC [83], [89], [136]; T952.32–35; T862.13–19).
229 Mr McNickle submits that the Court should find that five of the six pooled case-control studies provide evidence of an etiologic association between GBFs and NHL: Hardell (2002), De Roos (2003), Lee (2004), Pahwa (2019) and Hardell (2023). The sixth pooled study is De Roos (2022).
230 I will deal with each in turn.
231 This study analysed data from Hardell and Eriksson (1999) and Nordstrom (1998), which compares 515 NHL cases and 1141 controls. The authors reported a univariate OR of 3.04 (95% CI: 1.08–8.52) for ever/never glyphosate exposure. When adjustment was made for other pesticide exposure, the OR reduced to 1.85 (95% CI: 0.55–6.20).
232 Professor Checkoway opined (at JRC [117]) that the study had various strengths, including a relatively large dataset from high-calibre case-control studies and thorough statistical analysis methods (see also Table 2 (at 27)). His opinion was not the subject of challenge in cross-examination and, in his view, the main limitation of the study is that the exposure assessment was based on questionnaire responses (self-reporting) (at JRC [117]). The usual consequence of this limitation, it is said, is underestimation of etiologic relations (at JRC [61(d)(iv)]).
233 As with Hardell (1999) and Eriksson (2008) addressed above, the unadjusted OR can be calculated using the baseline reference group of all cases and controls that were unexposed to glyphosate (but still exposed to other pesticides) (that baseline group can be calculated as follows: for cases, 515–8=507; for controls, 1141–8=1133). This results in an OR of 2.23 (calculated by taking the odds of exposure for cases (8/507=0.0158) and dividing it by the odds of exposure for controls (8/1133=0.0071)).
234 Mr McNickle submits this recalculation of the OR shows that with the baseline reference group of all cases and controls that were unexposed to glyphosate (but still exposed to other pesticides) the OR remains significantly elevated (reduced from 3.04 to 2.23, noting that the OR in the paper was adjusted for study, study area and vital status whereas the recalculated OR was unadjusted).
235 I do not consider that Hardell (2002) provides persuasive evidence of an etiologic association between GBFs and NHL, for two reasons.
236 First, only eight subjects and eight controls reported glyphosate exposure (see Table I), with an unadjusted univariate OR of 3.04 (95% CI: 1.08–8.52) which, when corrected for exposure to other pesticides by multivariate analysis (Table VII), was not significantly elevated and included the null hypothesis (OR 1.85, 95% CI: 0.55–6.2).
237 Secondly, Professor Checkoway reports (at JRC [117]) that the main limitation of the study is use of a questionnaire (as is common for all other studies) but does not highlight the negligible gain in exposed cases over the parent study.
238 This study pooled data from three case-control studies conducted in the United States, namely Hoar (1986); Zahm (1990); and Cantor (1992). Prior to De Roos (2003), the glyphosate data that had been collected in two of those case-control studies (Hoar (1986) and Zahm (1990)) had not been reported on or analysed.
239 The authors reported an increased OR of 2.1 (95% CI: 1.1–4.0) for ever exposed to glyphosate, or glyphosate exposure, using logistic regression and an OR of 1.6 (95% CI: 0.9–2.8) using hierarchical regression (that is, a methodology in which predictors are added in blocks to improve statistical validity).
240 Professor Checkoway (at JRC [119]) noted that the study has various strengths, including the selection of datasets from similar populations with multiple pesticide exposures assessed, large sample size, and rigorous data analysis methodology and there were no serious limitations to the methodology. AP Harris acknowledged (at JRC [148]) that there was benefit in the large sample size (that is, statistical power) and adjustment for other pesticides.
241 AP Harris, however, identified various “methodological issues” with the study, namely: (1) the lack of controls for proxy respondents; (2) exclusion of data due to missing and unknown responses; and (3) no observed consistency between the De Roos (2003) results and authors of the previous analyses.
242 As to proxy respondents, AP Harris noted (at JRC [150]) that the lack of control for proxy respondents meant that OR estimates “are very likely biased upwards, as demonstrated in Waddell et al. (2001)”. That study used the same three pooled case-control studies as De Roos (2003) to evaluate the associations between organophosphate insecticides and the risk of NHL.
243 Mr McNickle submits that the Court should reject AP Harris’ evidence that proxy respondents “very likely” biased the results in De Roos (2003) upwards, for the following reasons.
244 First, AP Harris conceded in cross-examination that she could not be certain that because there was an observed trend for organophosphates in Waddell (2001), that the same trend would be observed for glyphosate (T993.19–24). As AP Harris noted under cross-examination, whether proxy respondents under-report or over-report exposures will “depend on the chemical” in question and how well-known that chemical is at the time of exposure information collection (T990.24–26; T993.13–41). It is said that that evidence suggests, contrary to AP Harris’ evidence in the JRC, Waddell (2001) (which was about organophosphates) cannot “demonstrate” that the OR estimates in De Roos (2003) for glyphosate are “very likely” biased upwards.
245 Secondly, Baris (1998), which was a study which used the same three pooled case-control studies as De Roos (2003) and Waddell (2001) to evaluate the associations between chemical exposure and the risk of NHL, did not demonstrate any upward biased OR trend for proxy respondents. That study evaluated associations between DDT and NHL and showed a downward bias for ORs for proxy respondents compared to direct respondents (T992.44–993.9). As noted above, whether proxy respondents under-report or over-report exposures (and thus whether there is an upward or downward biased OR trend) will depend on the chemical in question and how well known that chemical is at the time of exposure information collection (T990.24–26; T993.13–32). Glyphosate was not a well-known chemical at the time of the US studies which were pooled in De Roos (2003) (T993.32–41).
246 Thirdly, Lee (2004) pooled data from two of the same US case-control studies and evaluated glyphosate exposure and NHL. As was conceded by AP Harris in cross-examination, it reported “the same pattern of results” for direct and proxy respondents (T996.28–997.9). That is, for glyphosate, Lee (2004) shows that the data from two of the US studies did not show any difference between direct and proxy respondents.
247 As to the exclusion of data due to missing and unknown responses, Mr McNickle submits that this can be put to one side. AP Harris agreed in cross-examination that it was appropriate for the authors to exclude the data for the purpose of the analysis (T1003.35–38). Further, AP Harris did not explain in her reports how the exclusion of data might have biased the OR estimates in De Roos (2003), or in her oral evidence (T1004.4–10).
248 As to the alleged lack of “some consistency” between the De Roos (2003) results and other analyses, Mr McNickle contends AP Harris’ point is that the ORs in De Roos (2003) should be discounted in part or whole by reason of this purported inconsistency. It is said that this contention should be rejected. AP Harris stated (at JRC [147]) that the estimates in De Roos (2003) were elevated in contrast to those reported in Lee (2004), and that the differences could not be explained by the addition of the Hoar (1986) data. However, in cross-examination, AP Harris conceded that the ORs and CIs for glyphosate in De Roos (2003) and Lee (2004) were within the same range (T1037.1–1038.43).
249 AP Harris also accepted in cross-examination that the absence of specific reporting of glyphosate in Hoar (1986) and Zahm (1990) could be because the authors were not interested in reporting on it at that time (T1046.23–31; T1047.45–1048.4). Mr McNickle contends that, properly analysed, the three US studies, and Lee (2004), do not provide support for any proposition that the ORs in De Roos (2003) are artificially elevated or unreliable. To the contrary, Mr McNickle contends that the consistency in results across De Roos (2003) and Lee (2004) should give the Court comfort that these pooled analyses individually provide supportive evidence of an association between exposure to glyphosate and the development of NHL.
250 These submissions are well put and have a superficial attraction, but they tend to distract from some key weaknesses of the study.
251 First, De Roos (2003) pooled various studies to provide 650 NHL cases exposed to at least one of 47 pesticides. When adjusted for use of other pesticides, analysis of 36 cases and 61 controls reporting exposure to glyphosate yielded statistically significantly elevated ORs of 2.1 (95% CI: 1.1–4.0) (Table 3). However, using hierarchical regression analysis, the elevation was not significant and included the null hypothesis (OR 1.6, 95% CI: 0.9–2.8). Professor Checkoway did not identify this as a limitation of the study (at JRC [119]).
252 Secondly, and relatedly, AP Harris, when asked why the apparent addition of only 10 cases and 12 controls to those reported in Cantor (1992) had shifted the OR from 1.1 to 2.1, she explained (T1032.46):
ASSOC PROF HARRIS: What I believe has happened in De Roos is that to do the [hierarchical] regression analysis they have to exclude all observations that have missing data for any of the 47 or 57, I can’t remember which, any of those pesticides that they’ve analysed in the higher model, so they excluded those observations to do that calculation which ended with an odds ratio of 1.6, but they also used the same dataset to calculate the odds ratios using logistic regression and that’s how they ended up with the odds ratio estimate of 2.1, I believe it was. So that’s on what I would call a restricted dataset that excluded quite a few observations for the pesticides, plus some other variables, so it differed from the Cantor dataset, primarily by restricting the data to a small – smaller group.
253 AP Harris was confident that the results were reported accurately, but that in contrast to larger pooled studies (such as the North American Pooled Project (NAPP), to which I will return), different results were observed in relation to a fuller dataset (T1033.13). Accordingly, conventional logistic analysis was conducted in De Roos (2003) on a dataset restricted in ways which the publication itself does not make pellucid.
254 Thirdly, I do not consider the issue of whether or not the failure to exclude proxy respondents “very likely” biased the results of the study upwards is particularly significant. Even if I were persuaded that AP Harris’ contention that the ORs were overestimated due to the failure to exclude proxy respondents was wrong, it would not detract from the key limitations of the study to which I have already referred. In any event, the failure to exclude proxy respondents has been consistently identified in relation to other studies as a limitation simpliciter (see above (at [179], [194], [198], [244])), regardless of whether the omission tended to increase or decrease OR estimates. The same may be said of Mr McNickle’s contention with regard to the exclusion of data due to missing and unknown responses (T1004.4–6), and the unequal contribution of cases and controls being not conducted during the same timeframe (see T1004.29–1006.11).
255 For these reasons, I consider De Roos (2003) is of limited assistance.
256 Pahwa (2019) was a pooled study authored by AP Harris and others. It combined data from four case-control studies, namely McDuffie (2001); Cantor (1992); Hoar (1986); and Zahm (1990) to evaluate associations between pesticide use and the risk of haematological cancers, including NHL and its subtypes. Pahwa (2019) was conducted as part of the NAPP (to which I have already referred (at [253])). The NAPP pooled the US and Canadian case-control studies in order to evaluate associations between pesticides and haematological cancers, including NHL and its subtypes. AP Harris was the lead principal investigator of the NAPP, which was supported by the NCI (at JRC [158]).
257 The authors reported that ever/never exposed subjects resulted in an OR of 1.43 (95% CI: 1.11–1.83) for NHL overall and an OR of 2.42 (95% CI: 1.48–3.96) for use of more than two days per year for NHL overall, which reduced to OR 1.73 (95% CI: 1.02–2.94) after adjustment for other pesticides. The authors also reported an OR of 1.53 (95% CI: 0.94–2.49), for the highest stratum of lifetime days of glyphosate use (greater than seven days), which was reduced to OR 1.06 (95% CI: 0.62–1.81) after controlling for other pesticides.
258 Mr McNickle submits that Pahwa (2019) provides suggestive evidence of an association between GBF exposure and NHL, as reflected in Professor Checkoway’s opinion (at JRC [125]). In doing so, Mr McNickle draws attention to some differences between AP Harris’ opinions and conclusions in relation to Pahwa (2019) (as set out in her reports in this proceeding) and other material which reported on the NAPP results for glyphosate and NHL and other studies emerging from the NAPP co-authored by AP Harris.
259 It is contended that those differences include important omissions from her reports in this proceeding of matters that were directly addressed in the published material, and which downplay the extent to which Pahwa (2019), and the NAPP data on which that paper was based, provides supportive evidence of an association between GBFs and NHL.
260 Mr McNickle submits that the Court should find that AP Harris’ “true opinion” about glyphosate and NHL based upon the NAPP data is that which was stated in Pahwa (2019) and other published material. That is, the NAPP data provides “some limited evidence of an association between glyphosate use and NHL” and evidence that “glyphosate may be associated with an increased risk of NHL”.
261 Mr McNickle’s submissions may be summarised as follows.
262 First, AP Harris gave evidence that published scientific papers and presentations at epidemiological conferences are two of the three ways in which results of scientific research are disseminated to the scientific community (T1064.27–1065.22), and that it is important for the results and conclusions expressed in such publications and presentations to be accurately expressed (T1065.26–30). The conclusion in Pahwa (2019) that the NAPP data provides “some limited evidence of an association between glyphosate use and NHL” is the scientific finding that AP Harris and her co-authors contributed to the literature before her engagement as an expert witness for Monsanto. Mr McNickle also refers to two presentations in June and August 2015 in which AP Harris and her co-authors reported a similar conclusion to that in Pahwa (2019) that “glyphosate use may be associated with increased risk of NHL”.
263 Secondly, AP Harris’ presentation of the results of Pahwa (2019) in the JRC, it is said, is not a balanced or accurate account of the paper’s findings. AP Harris used language (at JRC [163]; T1080.31–1081.24) which “downplay[ed] the association that was observed for SLL [a subtype of NHL]”; omitted reference to unadjusted OR results and the discussion of those results from Pahwa (2019) (at JRC [164]; T1083.10–1084.5; T1086.29–43); omitted reference to text in Pahwa (2019) that set out positive evidence of an association between glyphosate and NHL (T1084.6–1085.18); and noted that the paper had “no statistically significant findings for duration of use for lifetime days for use of NHL overall or any subtype”, when Pahwa (2019) had concluded that there were statistically significant findings for SLL (T1087.9–45). Mr McNickle submits that these omissions and differences are significant and deliberate, which cannot be explained away by AP Harris having conducted a cursory summary of Pahwa (2019).
264 Thirdly, AP Harris sought to explain differences between the presentation of the results of Pahwa (2019) in her reports and the paper itself by noting, among other things, that there was disagreement between the authors of Pahwa (2019) (T1081.15–1081.32); that she needed to review the exact wording of the JRC to ascertain any inconsistencies with the study (T1082.24–1083.4); and that she had omitted parts of the evidence relating to frequency on the basis that she did not consider the omitted results to be important (T1086.4). Mr McNickle submits that these explanations are implausible and ought not to be accepted by the Court.
265 Fourthly, it is said that AP Harris conceded that based on the results of Pahwa (2019), it is “quite possible” that glyphosate may be associated with increased risk of NHL (T1078.41–44) and agreed with the conclusions set out in Pahwa (2019) (T1082.11–15). She also noted that Pahwa (2019) was unlikely to be affected by recall bias (T1098.15–19) and that she had included possible recall bias as a limitation despite, among other things, not citing recall bias as a concern in relation to (1) the JRC or in any discussion of the pooled individual studies in the JRC (T1091.18–39); (2) in Pahwa (2019) itself (T1092.32–1093.29); (3) the NAPP papers (T1096.34–1097.14); and (4) in other papers such as Cantor (1992) and Lee (2004) (T1093.31–1094.4). Mr McNickle submits that AP Harris identifying recall bias as a concern in relation to Pahwa (2019) in this proceeding is an example of her “straying into the role of an advocate for Monsanto”.
266 Subject to matters to which I will return, these were issues that did cause me some concern. For example, it was a significant omission, in my view, for AP Harris not to include unadjusted OR results in the JRC (at [164]) and portions of text from Pahwa (2019) which suggest a causal connexion between glyphosate and/or GBF exposure and NHL.
267 Before returning to this matter, it is necessary to focus on the paper itself.
268 The authors’ conclusion in Pahwa (2019) is that there is “some limited evidence of an association between glyphosate use and NHL, but that consistent patterns of association across different metrics and sub-types were not observed” (at 600). Specifically, although there was a statistically significant association found for handling glyphosate more than two days per year (OR 1.73, 95% CI: 1.02–2.94) (albeit with an insignificant p-trend of 0.2), this association was “attenuated and became no longer statistically significant when adjusted for reported use of 2,4-D, dicamba, and malathion” (at 606). Notwithstanding the most consistent evidence was found for the SLL subtype of NHL, where “positive patterns were observed for duration, frequency, and lifetime days of glyphosate use”, the authors found that there was “no pattern of increasing risk of NHL overall with increasing years of use of glyphosate” (emphasis added) (at 606).
269 In the end, subject to the qualifications in Pawha (2019) itself, it does provide some limited evidence of an association between glyphosate and NHL as AP Harris had opined prior to her engagement. With respect, her apparent hesitancy in seeking to distance herself from the logic of her earlier conclusions is relevant to the assessment of her entire evidence, notwithstanding that as I have found, I have regarded her as a generally impressive witness.
270 Lee (2004) combined data from two US case-control studies used in De Roos (2003), Cantor (1992) and Zahm (1990). The authors investigated whether asthma modifies the risk of NHL associated with pesticide exposure, including glyphosate. The study reported an OR of 1.4 (95% CI: 0.98–2.1) for non-asthmatics and an OR of 1.2 (95% CI: 0.4–3.3) for asthmatics.
271 AP Harris opined that the overall average OR as between the two groups would be approximately 1.38, and that the lower bound of the 95% CI would likely be above 1.0 (T1038.24–35). AP Harris also agreed that the upper bound of the 95% CI would be 2.1, consistently with De Roos (2003).
272 Mr McNickle contends that Lee (2004) provides supportive evidence of causal connexion between GBFs and NHL, drawing upon a larger group of cases and controls than De Roos (2003) (36 cases, 61 controls); the non-asthmatic group comprising 53 cases and 91 controls, and the asthmatic group 6 cases and 12 controls. It is said the study demonstrates consistency across studies indicating a causal relationship between GBF exposure and NHL such that using the data from two of the same studies as De Roos (2003) and conducting a different type of analysis, the study produced similar, and relatively precise, ORs and CIs.
273 I consider Lee (2004) is of limited assistance, for the following reasons.
274 First, although Lee (2004) drew upon a larger group of cases and controls exposed to glyphosate than De Roos (2003), in this instance this does not necessarily equate to greater statistical power. To illustrate, set out below is Table II of Lee (2004) (at 300):
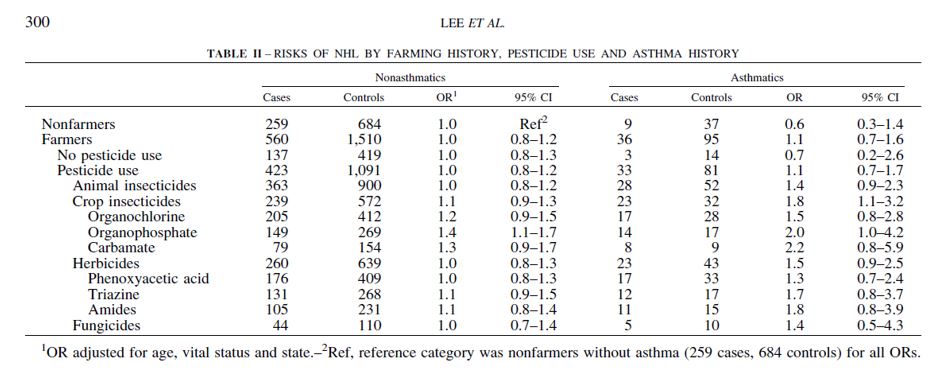
275 Among non-asthmatics, Lee (2004) drew upon data from 423 cases and 1091 controls reporting exposure to pesticides generally. Among asthmatics, there were 33 cases and 81 controls. In De Roos (2003), the total number of cases exposed to pesticides was 650, with 1193 controls.
276 Notwithstanding that De Roos (2003), being the larger study overall, yielded a smaller group of subjects exposed to glyphosate (36 cases, 61 controls) compared to Lee (2004) (53 cases, 91 controls), it would be misleading to attribute greater weight to Lee (2004). This is because the studies use different pre-exclusion criteria. In Table III of Lee (2004), for example, the total number of non-asthmatic cases reporting exposure to a particular pesticide is 1665, which is approximately four times greater than the total number of non-asthmatic cases exposed to any pesticide (423 in Table II). In other words, in Lee (2004), one non-asthmatic case of NHL was on average exposed to about four pesticides. In De Roos (2003), the same issue is apparent, but it is approximately 2.3 times greater than the total number of exposed cases. The effect of this is that while Lee (2004) does draw upon a larger group exposed to glyphosate than De Roos (2003), it is likely to have less statistical precision because of the failure in Lee (2004) to correct for multiple pesticide exposures.
277 Secondly, it was far from AP Harris’ firm conclusion that if the OR was average as between the two groups in the study (asthmatics and non-asthmatics), the 95% CI would likely be above 1.0. It was apparent AP Harris was giving an “eyeball” estimate of the combined ORs and CIs without having undertaken a formal analysis of the combined primary data used in Lee (2004). The corollary is that absent some formal demonstration, the non-asthmatic case-control comparison in Lee (2004) of OR 1.4 (95% CI: 0.98–2.1) does not exclude the null hypothesis.
278 Hardell (2023) pooled data from three Scandinavian studies, namely Hardell and Eriksson (1999), Eriksson (2008) and Nördstrom (1998). The results were based on 1425 cases of NHL (including the subtype hairy cell leukemia (HCL)) with 2157 controls. Professor Checkoway did not refer to this pooled study in the JRC but it is necessary to say something about it, given it is the subject of the Second Checkoway Report.
279 The authors reported an OR of 2.2 (95% CI: 1.3–3.8) for exposure to glyphosate in the univariate analysis, which reduced to OR 1.51 (95% CI: 0.77–2.94) in the multivariate analysis. For the group with greater than 13 days cumulative exposure, a higher OR of 2.4 (95% CI: 1.2–5.0) was reported. In the three latency groups, highest risk was obtained for greater than 20 years with an OR of 2.6 (95% CI: 1.3–5.1). The authors concluded that the pooled analysis yielded “an increased risk for NHL including the subtype HCL for exposure to pesticides”, and that “of special concern” is the increased risk associated with common herbicides including glyphosate.
280 Mr McNickle contends that Hardell (2023) supports an etiologic relationship between glyphosate and/or GBFs and NHL. The study was the subject of the Second Checkoway Report, in which Professor Checkoway concluded (at [5]):
The results generated by the pooled analysis of data from these studies add support to an etiologic relation between GBF’s and NHL risk insofar as the individual studies were all appropriately designed. Strengths of the pooled analysis are enlarged sample size and a homogeneous approach to data analysis.
281 I consider that Hardell (2023) provides limited evidence of an etiologic link between GBF exposure and NHL, for the following reasons.
282 First, notwithstanding reporting significantly elevated “ever” results for glyphosate (OR 2.2, 95% CI: 1.3–3.8) (37 cases, 26 controls), exposure to glyphosate at less than or equal to 13 days was non-significant (OR 2.0, 95% CI: 0.96–4.3) with the authors observing no clear dose-response effect.
283 Secondly, Professor Checkoway qualified his comments in the Second Checkoway Report by noting (at [6]) that a limitation of the study is the relatively small number of study participants who reported GBF exposure (possibly due to the number of participants who were enrolled in the late 1990s and early 2000s before glyphosate was a suspected health hazard), limiting statistical power. Professor Checkoway cites Nördstrom (1999) as a notable example of a study that did not include findings for a dose-response analysis, which would have been based on only four cases and five controls exposed to glyphosate.
284 Thirdly, the authors indicate (at 999) that in the calculations of risk estimated for different pesticides, “exposure to other types were not included for cases and controls”. As a result, the comparison group of cases and controls included subjects with no exposure to any type of pesticides. Professor Checkoway noted that this was “not an optimal approach” (T942.5–13). AP Harris agreed, adding that the “poor definition of a reference group” carried over into the pooled analysis (T942.5–13).
285 Fourthly, and relatedly, counsel for Monsanto put to Professor Checkoway that this group definition had been present in Hardell and Eriksson (1999), Eriksson (2008) and Nördstrom (1998) and, as a result, Hardell (2023) has no greater ability to isolate the effect of glyphosate compared to the individual studies (T943.1–2). Professor Checkoway agreed, noting that he placed “more weight” on the individual studies than the pooled analysis (T943.4–6).
286 Fifthly, Professor Checkoway agreed with AP Harris that the consistently elevated ORs in Table 1 for all pesticides could indicate recall bias. In this regard, AP Harris and Professor Checkoway noted during the concurrent session that (T945.1–17):
ASSOC PROF HARRIS: I think it was probably two things. I think that it could potentially be recall bias when we see [OR] elevations across the board but it could also be, in part, due to the definition of the reference group. So it could be two things influencing those results is my take on that.
PROFESSOR CHECKOWAY: Yes. I would agree with that. I think, as I said, you know, not exposed to any of these, it’s a different – I want to say species but it’s a different type of group of persons.
287 Sixthly, and connected to this last point, Professor Checkoway opined that the results in relation to glyphosate in the study may have been confounded. As AP Harris observed, in Table 1 of Hardell (2023), there are “a lot of elevated significant associations for many compounds” and suggested that some of the herbicides and phenoxy acids are “associated with glyphosate use as well and correlated” (T943.26–27; T943.39–40). As a result, as AP Harris noted, “a lot of those people are going to have multiple exposures and then again they’re still comparing them to that reference group that has none” (T943.44–944.2). Professor Checkoway agreed and explained that if one is comparing “exposed versus non-exposed to anything, the chances are that you will find elevated odds ratios for the exposures” (T944.35–41).
288 For these reasons, I consider that Hardell (2023) provides limited assistance to the applicant’s case theory.
289 Professor Checkoway did not refer to this study in the JRC, but it is necessary to say something about it. De Roos (2022) pooled ten studies of multiple herbicides from North America, Europe, and Australia to compare 9229 NHL cases with 9626 controls. Table 3 of the study indicates no association between glyphosate use and risk of NHL and its subtypes. For ever/never exposed to glyphosate, the study reported an OR of 1.03 (95% CI: 0.83–1.29); for exposure of eight years or less, an OR of 1.11 (95% CI: 0.87–1.43); for greater than eight years to 15.5 years, an OR of 0.96 (95% CI: 0.65–1.42); and for greater than 15.5 years, an OR of 0.90 (95% CI: 0.59–1.37) (p-trend non-significant (0.54)).
290 AP Harris considered De Roos (2022) to be the “most statistically powerful yet” and that it supports the “null findings of the North American NAPP study [Pahwa (2019)]”. Professor Checkoway did not address the findings in De Roos (2022) and accepted that he had failed to respond to AP Harris’ analysis of the study in the JRC (T973.39–974.8).
291 I consider that De Roos (2022) is of some assistance to Monsanto’s case theory.
292 As the authors note, a strength of De Roos (2022) is the large, pooled sample from which the authors characterised a broad spectrum of occupational herbicide use, in both farming and non-farming occupations (at 804). The authors of the study (and AP Harris (at JRC [231])) noted that the findings of the study are consistent with other large studies (including the AHS), concluding (at 804) that the study demonstrated:
little evidence of an association between glyphosate use and all NHL, and meta-analysis indicated substantial heterogeneity of effect among the studies. Our findings for all NHL agree with recent, large studies, including an updated analysis of the AHS cohort that reported only small, non-significant increases in NHL risk with higher intensity-weighted lifetime days of use, lagged by 20 years (55 cases, OR=1.12, 95% CI: 0.83 to 1.51 for the highest vs lowest quartile) and the AGRICOH meta-analysis of three cohorts (including the AHS) that found no association between ever-use and all NHL (OR=0.95, 95% CI: 0.77 to 1.18).
(Emphasis added, footnotes omitted)
293 AP Harris accepted that variation in relation to, inter alia, exposure assessment, control selection and the matching of controls for cases across the underlying studies may constitute exposure misclassification (T1110.39–1111.19). The authors also recognised the possibility of recall bias, especially among those underlying studies with open-ended elicitation of exposures in any job. With that said, as AP Harris observed, I do not consider that these weaknesses detract significantly from the relative statistical power of the study.
294 As noted earlier, Professor Checkoway’s Table 1 (at JRC [95]) references eight publications, four of which are the primary case-control studies which I have addressed above in Section I. The remaining four studies relied upon by Professor Checkoway are Orsi (2009); Hohenadel (2011); De Roos (2005); and Andreotti (2018). It is necessary to say something about Orsi (2009) and Hohenadel (2011). I will also address the study omitted by Professor Checkoway from Table 1 of the JRC, namely Cantor (1992), and the pooled cohort study of Leon (2019).
295 I will come to De Roos (2005) and Andreotti (2018) when dealing with the AHS.
296 Orsi (2009) was a hospital-based case-control study of pesticide exposures and NHL conducted in France. The study included 436 participants with 244 incident cases of NHL. Pesticide exposures were inferred from questionnaires. The study reported RR for glyphosate exposure of 1.0 (95% CI: 0.5–2.2) based upon 12 cases and 24 controls who were deemed to have been exposed to glyphosate.
297 I consider that Orsi (2009) is of limited assistance to Mr McNickle’s case theory.
298 Notwithstanding that some strengths of the study include a suitable study design and a detailed questionnaire, it shares a fallback common to the individual case-control studies in that a small number of cases and controls were exposed to GBFs (at JRC [109]). Further, notwithstanding that experts reviewed the study questionnaire (including an agronomist and an industrial hygienist), they allocated a chemical to study participants based on crop type, rather than the chemical being identified by the participant. As AP Harris noted in the joint session, the likely effect of this was to introduce exposure misclassification into the study (T1112.34–43):
MS SZYDZIK: And so it is not necessarily the case that somebody who was exposed or not exposed to a chemical was correctly identified as such by reason of this allocation method; you would agree with that?
ASSOC PROF HARRIS: Yes. We don’t – we can’t – we don’t know.
MS SZYDZIK: And so that’s going to introduce exposure misclassifications into this case control study?
ASSOC PROF HARRIS: Likely, yes.
299 Hohenadel (2011) was a population-based case-control study which was conducted in six Canadian provinces between 1984 and 1991. The study included 513 cases of NHL and 1506 controls and is notable for examining the combination of malathion (an agricultural pesticide) with various other pesticides, including glyphosate. Pesticide exposures were determined from questionnaires. After adjustment for age, province and proxy questionnaire responses, glyphosate alone (19 cases, 78 controls) yielded OR 0.92 (95% CI: 0.54–1.55) and OR 2.10 (95% CI: 1.31–3.37) for the combination of glyphosate and malathion. This suggested that glyphosate alone was not a strong risk factor for NHL, but that a synergistic effect with malathion exposure was strong (at JRC [110]).
300 As Professor Checkoway notes, some of the strengths of the publication include an appropriate study design, a relatively large sample size and assessment of associations of multiple pesticides (at JRC [111]). With that said, a limitation was reliance on non-quantitative exposure assessment based on self-reported lifetime use. As the authors note (at 2327):
A major limitation of our analysis is that our proxy measures for pesticide exposure were based on self-reported lifetime use. It is not clear whether use of combinations of pesticides were from actual tank mixtures, combinations used during the same growing season, or use in different years over a lifetime. These are quite different exposure scenarios and, even if the pesticides were carcinogenic, we might expect quite different biologic effects from these different exposure patterns.
301 For these reasons, I have not derived significant assistance from Hohenadel (2011).
302 Cantor (1992) was a population-based case-control study of 622 men with a new diagnosis of NHL with 1245 controls between 1981 and 1983. Trained staff conducted in-person interviews with subjects (or their proxy respondent) who were selected from the Iowa State Health Registry (1981–1983) and Minnesota hospital and pathology laboratory records (1980–1982). The interview included personal, demographic, family history, smoking as well as residential and occupational histories. For any subject who worked on a farm for at least six months (since age 18), a detailed farming and pesticide use history was collected. Pesticide lists were developed and included 23 insecticides for animal use, 34 crop insecticides, 38 herbicides, and 16 fungicides. Information on ever/never use, first and last year of use, application method, and if the subject had personally applied, mixed or handled the pesticide, was collected for each pesticide (at JRC [141]).
303 The study reported ORs for farming and different crops farmed and for various pesticide groups. No single herbicide group was significantly associated with NHL overall (Table 3), although it is unclear how glyphosate was classified. When evaluating specific herbicides (Table 6), 26 cases reported having ever used glyphosate with 49 controls, resulting in an OR estimate of 1.1 (95% CI: 0.7–1.9). These ORs were adjusted for vital status, age, state of residence, cigarette smoking status, family history of lymphopoietic cancer, high-risk occupations, and high-risk exposures (at JRC [142]).
304 As AP Harris summarised in the JRC (at [143]), the authors found notable and mostly consistent elevations in NHL risk were observed for other pesticides including carbaryl, chlordane, DDT, diazinon, lindane, malathion and nicotine. Of the positive and significant associations observed with the insecticides, the authors reported that the NHL risk was greater for farmers who used these pesticides prior to 1965, which was 15–18 years prior to NHL diagnosis in the study (case recruitment started in 1981).
305 I consider that Cantor (1992) is of neutral assistance. Some of the notable strengths of the study include its relatively large sample size and interview process through which detailed information was collected in relation to exposure use and history. A notable limitation of the study, however, as AP Harris notes (at JRC [144]) is that given glyphosate was not registered for use until 1975 in the United States, no study participants reported use of glyphosate prior to 1965 based on the stratified analysis presented. The corollary is that study participants could have a maximum of 6–7 years of exposure. The authors acknowledged that there was a need for a longer latency period to evaluate risks associated with glyphosate (at 2452; JRC [144]).
306 This study pooled data from the AHS (Alavanja (1996)) and cohort studies in Norway (Kreistensen (1996)) and France (Leveque-Morlais (2015)). The authors considered 2,430 cases of NHL in 316,270 farm workers. For 1,131 cases of NHL (including subtypes), the adjusted glyphosate “hazard ratio” was RR 0.95 (95% CI: 0.77–1.18).
307 Although Leon (2019) pooled data from a review of a large number of pesticides, neither the Norwegian nor the French study specifically addressed the relationship between GBF exposure and NHL, which was inferred from a crop exposure matrix (CEM) which indicates the use of specific pesticides for specific crops (at JRC [121]). As Professor Checkoway noted in the JRC (at [122]), the CEM introduces the potential for inaccuracy because first, registration and data for approval for pesticides may have been incomplete and may be erroneous; and secondly, information on specific pesticide active ingredients and registration and recommendation for use on specific crops may not be as accurate as reports by agricultural workers who applied pesticides (given that documentation that the pesticide was approved and sold does not necessarily mean that it was applied by individual agricultural workers). These limitations may have introduced exposure misclassification, which, as Professor Checkoway notes, is typically greater for less common exposures (such as GBFs) in the relevant time period.
308 For these reasons, I do not consider Leon (2019) is of significant assistance in demonstrating an etiologic relationship between glyphosate and/or GBF exposure and NHL.
IV Agricultural Health Study – De Roos (2005) and Andreotti (2018)
309 It is not an overstatement to describe the AHS as a highly significant study. Much time was devoted to it in oral and written submissions and, as will become evident, the AHS constituted a study that Monsanto pointed to as being the single most important aspect of the evidence contrary to Mr McNickle’s case. Despite this, at least initially, its reception into evidence was far from certain and the road to its adduction was a long and windy one.
310 As I noted in McNickle v Huntsman Chemical Company Australia Pty Ltd (Additional Expert Conclave) (at [1]), in December 2022, an interlocutory application was filed by Mr McNickle whereby permission was sought to communicate with Mr Cowpe, the independent barrister, who convened the expert conclaves (see above (at [24])) for the purpose of requesting that he facilitate the provision of a data transfer agreement, and the data underlying two epidemiology studies based on the AHS papers. As I noted (at [7]–[11]):
[7] What emerged following Conclave D was that the main disagreement between the experts in analysing both De Roos and Andreotti relates to what is described as the “the correct allocation of person years and consequent calculation of rate ratios.” It is Professor Gordon’s opinion that the allocation of person years to levels of cumulative exposure to glyphosate was incorrectly carried out, and led to incorrect calculations of rate ratios. Dr Harris’ opinion, however, was that the allocation of person years was correctly carried out, and led to accurate calculations of rate ratios.
[8] Hence, the parties are at issue as to what Professor Gordon describes as a “serious analytic error”. The consequence of this error is said to be that it could result in a “downward bias in the risk of NHL in the highest category of glyphosate exposure”. It is said by Dr Harris that Professor Gordon has provided “no direct evidence of this error”, and that this belief could only be substantiated “if the underlying AHS data were obtained and reanalysed”.
[9] No doubt I am missing something, but it seems to me it is a tad curious that the applicant, Mr McNickle, is the party seeking to obtain the AHS data. It is the respondents who seek to deploy opinions contained in articles from both Dr Roos and Andreotti in their case. If there is some prima facie, rational basis for suggesting that this material might have a serious analytical error, which can only be ascertained upon the checking of the underlying data, the failure to produce the data by the party seeking to adduce and rely upon the evidence, might have been relied upon to ground an application by the other party for rejection, or discretionary exclusion, or limitation of the material or, alternatively, support submissions going to the weight of the representations contained in the articles. But as it happens, both parties have reached a consensus that it is appropriate that the AHS data be obtained and reanalysed
…
[10] So far, so good. One would have thought this would be a relatively straightforward process of obtaining the data, providing it to the experts and obtaining their further opinions on it. But like so many matters in this case, it is not quite as simple as that. The AHS data is maintained by the National Cancer Institute (NCI), a United States government body that is part of the Department of Health and Human Services, being part of the executive government of the United States of America. For well over a year, efforts have been made by the solicitors for the applicant to obtain a copy of this data.
[11] Remarkably, since August 2021, around 60 communications have been exchanged concerning the appropriate facilitation of the data to experts. I had indicated at a case management hearing in September 2022 that I was content for the parties to continue to make efforts to obtain material to be provided to the facilitator in the same way as other documents in order for the experts, if they thought the material relevant, to express their opinions as to the questions posed to them. What has become apparent, however, following communications by the solicitors for both parties and representatives of the NCI, is that the position of the NCI is as follows (as explained in the Affidavit of Mr Lee Taylor sworn 7 November 2022 (at [18])):
(a) The NCI explained that the raw data are not contained in simple spreadsheets that are legible or reviewable by non-specialists. Rather, the data – which comprise the records of over 50,000 participants in the AHS – are raw text which can only be analysed within statistical software platforms. The NCI maintains the data in a software suite known as SAS (Statistical Analysis System) which, I understand, was developed in part by the National Institute of Health, of which the NCI is a part. Dr Beane-Freeman explained that they are able to export the data in a form that can be used in comparable analytic platforms (such as MATLAB).
(b) Because the data are in the form described above, there would be no utility to providing the AHS Data in its raw form to the parties’ lawyers or indeed to the Court. The data cannot be interpreted or “interrogated” by non-specialists without appropriate statistical qualifications, training, and experience, as well as the necessary statistical analysis software. To the extent, therefore, that the parties’ lawyers need to challenge any expert’s analysis of the AHS Data, they will likely need to rely on their own expert(s) (who will have the data).
(c) The NCI’s policy and practice of providing AHS Data to expert researchers is critical to their overarching mission as a public health body. The AHS Data comprise personal medical information of rural farmers enrolled in the AHS, and if the NCI is unable to guarantee that its subjects’ privacy is fully protected, the integrity of the AHS (and similar projects) would be fundamentally at risk. That imperative is served by providing data only to experts in relevant fields who are properly trained in managing and using health data, and who are experienced in deploying that data in a manner consistent with the privacy requirements (such as in publications using the data), and whose institutions typically use such data and therefore have appropriate systems in place to manage and protect the data.
(d) The NCI is aware that the AHS Data are sought in connection with this proceeding and that they would be used by expert witnesses.
(e) The Proposed DTA does not restrict the experts or the parties from using the experts’ analyses of the data (as distinct from the raw data themselves) in open court, nor would it preclude those analyses being incorporated into the Court’s analysis.
(f) Under the Proposed DTA, the lawyers and/or the Court can request the experts to generate data outputs from the raw AHS Data. There is no restriction on how many or what type of outputs can be generated by the experts, other than that those outputs cannot be based on fewer than five individual study participants (of over 50,000). This is to ensure that study participants’ privacy is protected. A data output is generated by aggregating the raw data of individual AHS participants to allow this data to be analysed and studied under different circumstances. Data outputs would reflect the results of particular analytic queries or calculations performed on the raw data; for example, the number of AHS participants were exposed to glyphosate in a particular year, might be extracted and then rendered in a form that would be intelligible to laypersons, such as a graph or chart, a table, or a textual description. Similarly, more complex outputs might be generated such as a re-calculation of participants’ intensity-weighted cumulative lifetime days exposure (which is a focus of Professor Gordon’s analysis), which would also be rendered in a form intelligible to the parties’ legal representatives and the Court.
(g) These outputs will enable the data to be digested and analysed so that the parties’ lawyers and the Court can properly understand what the data show. These outputs from the raw data can be used by the parties’ lawyers or the Court to test the experts’ opinions, methodology and analyses. The outputs from the raw AHS Data can be referred to in open court.
(h) The lawyers and the Court would then be able to ask the experts questions regarding their analyses, opinions and methodology, including based upon any outputs generated by the experts from the raw AHS Data.
311 As I then explained (at [19]–[25]), I facilitated an arrangement agreed between the parties for material to be provided by the NCI to the two experts in Conclave D.
312 Without access being obtained to the underlying data so that the conclusions of the AHS could be tested, it would have at least been open to Mr McNickle to move for the discretionary exclusion of opinions based on the AHS, as the refusal of the NCI to produce those materials would have occasioned prejudice to Mr McNickle in seeking to challenge the conclusions drawn from the AHS. But this was not the forensic course adopted by Mr McNickle and a pragmatic solution was reached in order to allow this important material to be placed before the Court.
313 Returning to the AHS itself, the study is a prospective cohort study conducted in Iowa and North Carolina. It includes 57,311 private and commercial licensed pesticide applicators, who were recruited between 1993 and 1997. Among private and commercial applicators, 75.5% (or approximately 43,000 people) reported ever using glyphosate. Exposures to glyphosate and other pesticides were assessed from responses to self-administered questionnaires completed by study participants.
314 Two studies (that is, the AHS papers) are follow-up reports on the AHS, namely:
(1) De Roos A J, Blair A, Rusiecki JA, et al. (2005) “Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study” 113 Environ Health Perspect 49 (De Roos (2005)); and
(2) Andreotti G, Koutros S, Hofmann J N, et al. (2018) “Glyphosate use and cancer incidence in the Agricultural Health Study” 110 J Natl Cancer Inst 509 (Andreotti (2018)).
315 For this reason, and partly because of the way in which Mr McNickle developed his arguments with respect to its findings, I will deal with it in a slightly modified sequence.
316 First, I will outline, at a high level, the conclusions of the AHS papers. Secondly, I will deal with Mr McNickle’s contentions made in the context of Conclave D with respect to the reliability of the AHS papers. Thirdly, I will set out what I consider to be the key strengths of the AHS and make findings as to the weight to be attributed to it in the light of the above.
317 Findings from the AHS reported by De Roos (2005) and Andreotti (2018) do not indicate an association between glyphosate exposure and the risk of development of NHL in humans.
318 De Roos (2005) represents the first follow-up study of the AHS. Among 54,315 subjects included in age-adjusted analyses, 41,035 (75.5%) reported having ever personally mixed or applied products containing glyphosate and 13,280 (24.5%) did not. As the authors note (at 51), the cohort, both exposed and never exposed, was composed primarily of male, middle-aged, private applicators with a relatively low smoking prevalence. In both the exposed and never-exposed groups, more than half of the subjects reported that they had never smoked.
319 The authors of De Roos (2005) found that glyphosate exposure (grouped as ever/never) was not significantly associated with cancer overall (age-adjusted RR: 1.0, 95% CI: 0.9–1.1; fully-adjusted RR: 1.0, 95% CI: 0.9–1.2) or with most of the cancer subtypes identified (at JRC [202]). Table 2 below extracted from De Roos (2005) (at 51) sets out these results:
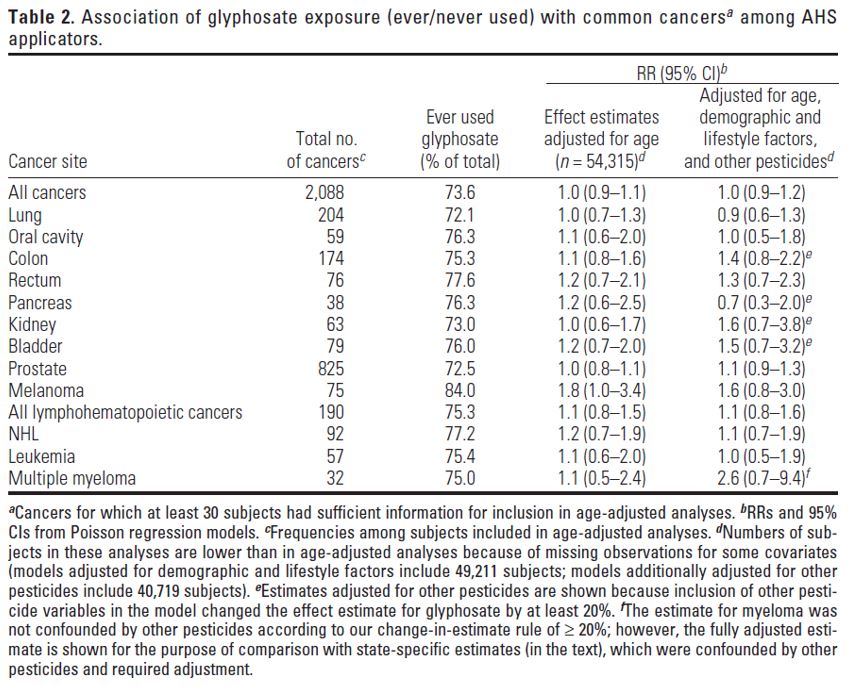
320 As can be seen, the age-adjusted and fully-adjusted RRs for NHL were 1.2 (95% CI: 0.7–1.9) and 1.1 (95% CI: 0.7–1.9) based on 92 NHL cases and a prevalence of exposure of 77.2% indicating no risk of NHL associated with glyphosate use (at JRC [202]; De Roos (2005) (at 51)). Further, the evaluation of additional glyphosate exposure metrics using the lowest tertile as a reference group found no positive associations between glyphosate use and NHL. For cumulative exposure days (divided into tertiles), RR estimates for the second and third exposure tertiles were RR: 0.7 (95% CI: 0.4–1.4) and RR: 0.9 (95% CI: 0.5–1.6) respectively as compared to the reference group. For the third exposure metric, intensity-weighted exposure days, the RR estimates decreased to RR: 0.6 (95% CI: 0.3–1.1) and RR: 0.8 (95% CI: 0.5–1.4) indicating a non-significant protective effect (at JRC [203]; De Roos (2005) (at 52)).
321 Andreotti (2018) represents the second follow-up of the AHS cohort. It included 57,310 participants who enrolled between 1993 and 1997, with telephone interviews being conducted approximately five years after enrolment (1999–2005). Andreotti (2018) extended the cancer incidence follow-up to 2012 in North Carolina and 2013 in Iowa and, in relation to the latter, extended the median time follow-up of 6.7 years in De Roos (2005) by 11 to 12 years (at JRC [207]–[208]; Andreotti (2018) (at 509)).
322 Of the initial 57,310 private and commercial applicators, those who reported a history of cancer at enrolment, those who lived out of state, and those who did not report any glyphosate information (that is, missing data) at enrolment, were excluded from the analysis. This resulted in an analytic sample of 54,251 individuals. Based on this sample, 44,932 subjects (82.8%) used glyphosate, including 5,779 incident cancer cases (79.3%) (at JRC [213]; Andreotti (2018) (at 509)).
323 Andreotti (2018) concluded that glyphosate was not statistically significantly associated with total cancer (RR: 0.99, 95% CI: 0.91–1.08) or cancer at any site in the unlagged analysis. Glyphosate use was not associated with lymphohematopoietic malignancies (RR: 1.0, 95% CI: 0.74–1.34) and it was not associated with NHL or any NHL subtypes (see Table 3 of Andreotti (2018) (at 514–515); at JRC [214]). In the top exposure quartile, the RR was 0.87 for NHL (95% CI: 0.64–1.20) and the RR for multiple myeloma (MM) was 0.87 (95% CI: 0.45–1.69). The authors reported that the association for NHL was unchanged when MM was excluded from the NHL category (RR: 0.85, 95% CI: 0.62–1.18) (at JRC [214]; Andreotti (2018) (at 514–515)). Based on these results, subject to some observations in relation to positive findings in previous case-control studies, the authors concluded (at 515):
In our study, we observed no associations between glyphosate use and NHL overall or any of its subtypes. This lack of association was consistent for both exposure metrics, unlagged and lagged analyses, after further adjustment for pesticides linked to NHL in previous AHS analyses, and when we excluded multiple myeloma from the NHL grouping. The lack of association between glyphosate and NHL is also consistent with the previous AHS analysis.
(Footnotes omitted)
324 As noted earlier, the AHS was the subject of detailed discussion over the course of the Conclave C and D joint sessions. Professor Checkoway opined that the AHS is an “excellent study” and “[i]n terms of study design and methodology, I think it is perhaps the best study we have” (T959.14–15; T959.44–45). AP Harris also concluded that “the AHS analysis conducted and presented by [Andreotti (2018)] represents the most comprehensive analysis of the relationship between glyphosate and NHL” (at JRC [220]).
325 Before coming to the reasons for those conclusions and my findings as to the AHS, it is necessary first to address Mr McNickle’s contentions.
326 One could not help but reach the conclusion that having recognised the threat posed by the AHS to Mr McNickle’s epidemiological case, every possible attempt was made to undermine its veracity, which started with the sort of arguments referred to in McNickle v Huntsman Chemical Company Australia Pty Ltd (Additional Expert Conclave) (at [7]–[8]), being Professor Gordon’s opinion that the study contained a “serious analytical error” in that the allocation of person years to levels of cumulative exposure to glyphosate was incorrectly carried out, and led to incorrect calculations of rate ratios. This attack, for reasons I will explain, ultimately ran into the sand on detailed analysis.
327 The apogee of Mr McNickle’s attack upon the AHS later emerged in the context of Conclave D, relying largely upon Professor Gordon’s identification of three alleged methodological flaws with the AHS papers, which I address below.
328 Having failed to make out its “serious analytical error”, Mr McNickle’s revised position is that the AHS papers should be considered by the Court in weighing all the epidemiological evidence. With that said, he contends that the Court should not accept Monsanto’s submissions that: first, the AHS papers are more reliable than the case-control studies; secondly, the fact that the AHS papers did not show a positive association between glyphosate and NHL means that there is no such association; or thirdly, the AHS papers suggest that the many case-control studies indicating a positive association can or should be discounted or dismissed. Mr McNickle’s submissions may be summarised as follows:
(1) the AHS papers are affected by exposure misclassification, the effect of which is bias that results towards the null hypothesis (exposure misclassification proposition);
(2) there is a fundamental problem with the multiple imputation procedure used in Andreotti (2018) such that the results of the paper are not reliable; (multiple imputation proposition); and
(3) the absence of a positive association in the AHS does not disprove the fact of positive associations evident in the case-control studies (no positive association proposition).
329 I will deal with each in turn.
330 Mr McNickle submits that the Court should find the results in the AHS papers are affected by exposure misclassification. This is said to be for three reasons: first, the way in which exposure information was obtained in the AHS (that is, self-reporting); secondly, exposure misclassification would bias RR estimates towards the null hypothesis; and thirdly, exposure was not calculated for any period after the follow-up questionnaire (that is, cumulative exposure). It is necessary to elaborate on each below.
331 First, as noted above, the AHS relied on self-reporting to obtain pesticide exposure information. This can lead to exposure misclassification and, in turn, the underestimation of an association between the exposure and outcome of interest. It is said that the way in which exposure information was sought in the initial enrolment questionnaire likely led to exposure misclassification in the AHS. As explained by Professor Gordon (at JRD [106]–[107]), the questionnaire’s use of cumulative exposure variables means that two persons with different exposure histories will be regarded as having the same cumulative exposure in the analysis conducted in the AHS papers. Professor Gordon and AP Harris indicated that the effect of exposure misclassification would likely bias estimates in the direction of an apparent lack of association (at JRD [108]; T806.24–807.24). It is also said that exposure misclassification is likely in the AHS papers because exposure information was collected by self-administered questionnaire, and not by trained interviewers. Professor Checkoway described this aspect of the AHS as a “noteworthy limitation” which “typically leads to non-differential misclassification that often results in underestimated association” (at JRC [104]). AP Harris gave evidence that interviews as opposed to self-report has advantages, one of which is that participants can ask clarifying questions about the information being sought (T800.35–47).
332 Secondly, Mr McNickle relies upon Professor Gordon’s view in the Second Gordon Report (at [47]–[49]) that the impact of non-differential exposure measurement error will always bias relative risks towards the null. The cross-sectional study Blair (2011) mentioned earlier (at [152]) is cited in support, which assessed the impact of exposure misclassification on estimates of relative risks in the AHS.
333 Blair (2011) compared 83 agricultural workers participating in the AHS between urinary levels of pesticides before and after application and the exposure intensity scores derived from subjecting the AHS questionnaire answers given at the time of application to analysis by the AHS exposure algorithm, which assigns varying weight to factors including mixing, application method, use of protective equipment, and so on. AP Harris and Professor Gordon agreed that the following conclusions reached by the authors (at 6) were reflected by the study data about the AHS (Figure 1) (T782.19–44):
First, the accuracy of reporting of pesticide use by farmers is comparable to that for many other factors commonly assessed by questionnaire for epidemiologic studies. Second, except in situations where exposure estimation is quite accurate (i.e., correlations of 0.70 or greater with true exposure) and true relative risks are 3.0 or more, pesticide misclassification may diminish risk estimates to such an extent that no association is obvious, which indicates false negative findings might be common.
334 Thirdly, exposure in the AHS was not calculated for any period after the time of the follow up questionnaire, such that it is possible that cumulative exposure did increase after that point for many individuals (Third Gordon Report (at [75], [121]); T672.32–673.19). Professor Gordon suggested that the consequence was that for some cases of NHL, there will be lower cumulative exposure “leading to bias in the estimates of the rate ratios” (Third Gordon Report (at [122])). Professor Gordon opined that this problem was not addressed by the sensitivity analyses conducted by Andreotti (2018) (T813.20–814.3), nor by AP Harris in the AP Harris Sensitivity Report (T1147.1–1148.9).
335 I do not find these submissions persuasive.
336 First, in relation to self-reporting, Mr McNickle’s submission does not relevantly turn on the fact that the AHS utilised self-reporting in the initial questionnaire. Rather, as I apprehend it, the concern is largely directed towards the contemporaneous categories of exposure that were available for selection, specifically the fact that people with differing levels of actual exposure would have been regarded as having the same cumulative exposure for the purposes of the study. This issue, however, as AP Harris recognised, is not unique to the AHS: it arises with any epidemiological study that collects exposure data in a group manner, regardless of whether or not self-reporting is utilised. As AP Harris noted in the JRD (at [151]), this manner of categorisation “can introduce minimal measurement error” but that it is “often how epidemiological studies collect data”.
337 Secondly, with respect to Blair (2011) and the effect of exposure misclassification biasing relative risks towards the null, Professor Gordon claims in the Third Gordon Report (at [50]) that the risk estimates in Andreotti (2018) are subject to non-differential exposure measurement error which causes this risk to be closer to the null value (RR: 1.0) than it ought to be. However, as AP Harris explains in her second supplementary report (at [39]), what this means is that the true risk is further away from the null value. As the RR shows a protective association of RR: 0.80 (95% CI: 0.60–1.04), Professor Gordon’s reasoning implies that the “true” protective effect of glyphosate exposure is greater than this and that the true RR would be less than 0.80. In other words, Professor Gordon’s logic suggests that had non-differential exposure not existed, the inverse exposure NHL associations reported by Andreotti (2018) would be more protective (T722.46–723.6).
338 Thirdly, as noted earlier, in response to Professor Gordon’s concerns in relation to the cumulative exposure issue in Andreotti (2018), AP Harris (with the assistance of Professor Villeneuve) prepared a sensitivity analysis (AP Harris Sensitivity Report) which, Monsanto contends, directly addresses the form of criticism that Professor Gordon makes in the Third Gordon Report (at [80]). Professor Gordon suggested that a sensitivity analysis could project cumulative exposure between the first and second AHS surveys, up to a presumed retirement age, or (if known) actual cessation of exposure (Third Gordon Report (at [124])).
339 Professor Gordon prepared a report in response to AP Harris’ report on 24 September 2023 (Fourth Gordon Report) in which he raised three concerns with respect to the AP Harris Sensitivity Report. First, the report was limited to those participants who provided data at the second survey and did not project cumulative exposure for 37% of participants who did not do so (Fourth Gordon Report (at [11], [19]); T1148.1–4). Secondly, the report used one imputation only and did not deal with the missing data according to principles of multiple imputation (to which I will return) (Fourth Gordon Report (at [25])). Thirdly, the report used only one metric, namely cumulative lifetime days of exposure, as opposed to utilising intensity-weighted cumulative lifetime days (Fourth Gordon Report (at [26])).
340 These concerns, however, are largely beside the point, for two reasons. First, Professor Gordon’s suggested solution as implemented by AP Harris in her sensitivity report resulted in a rate ratio lower than those published in Andreotti (2018). Secondly, the resulting point estimates in Professor Gordon’s response to AP Harris’ sensitivity analysis (Fourth Gordon Report) are still consistent with Andreotti (2018), which revealed no relationship between glyphosate and/or GBF exposure and the risk of NHL. As Professor Gordon concluded (Fourth Gordon Report (at [29])):
Using the dataset provided by Associate Professor Harris, the estimated RR* for this category (23 NHL cases) is 0.97 (95% CI: 0.60 to 1.55). Using the same dataset, but restricted to those with projected exposure only, the estimated RR* for this category (18 NHL cases) is 1.12 (95% CI: 0.65 to 1.95).
(Emphasis added)
341 Before leaving this topic, for completeness I should deal with a further matter raised by Mr McNickle concerning the issue of cumulative exposure in the AHS papers.
342 Some time was devoted in oral and written submissions to Professor Gordon’s belief that the authors of the AHS papers had committed what is known as a “Duck error”. This is not some logical flaw based on anthropomorphic imagery, but rather derives from the study Duck (1975). It refers to an error in allocating person-years of study participants all to the category of cumulative exposure they were in at the end of the study (see First Gordon Report (at [137]– [145])).
343 Professor Gordon conceded that he was wrong to have believed that exposure in the AHS papers was measured until the end of the study participation (at JRD [66], [230]), and that there was in fact a conceptual difference between a Duck error and the kind of alleged error explained above (at [330]) in relation to accounting for participants’ exposure after enrolment (T688.1–6). It is unnecessary to make any observations about whether or not Professor Gordon (in Monsanto’s submission) had “reimagined” or “revised” the nature of this error following the JRD.
344 It is necessary, however, to say something about why the difference between the two concepts matters. A Duck error is a misallocation of a measured or recorded exposure, which is different to the problem of how epidemiologists deal with measuring continuing exposure following enrolment. As AP Harris explained during the joint session in relation to Professor Gordon’s conclusion (at JRD [231]) (T665.5):
ASSOC PROF HARRIS: So what he’s talking about here is a different concept. So he’s talking about measurement error that is occurring after the collection of data. Whereas the Duck error is actually – it’s almost like a calculation error given the data that you’ve already collected. So there are two separate things. Although they sound similar, and they’re easy to confuse, they’re two separate issues.
345 The difference is important because there is a need to allow for a latency period between the exposure of interest and the development of cancer. It is necessary to ensure that enough time elapses between exposures measured and the development of cancer because, obviously enough, cancer does not develop from recent exposures. As AP Harris explained, measuring exposure up and until cancer follow-up can lead to error because it can eliminate any allowance for latency (T677.12–17):
ASSOC PROF HARRIS: … if the latent period is longer than six or seven years, which it is likely for most cancers, maybe 15 years or more, you’re basically introducing error into it because you’re collecting additional exposure data to the pesticide, or whatever it is. That’s not actually relevant for that cancer outcome because it’s not exposures up to the day of disease that cause cancer. It’s usually exposures that occurred, you know, five, 10, 15 years ago.
346 It is for this reason that a design feature of the AHS papers was to measure and include exposure until the respective questionnaires, but to then defer ascertaining cancer outcomes for a period of time (T674.40–44). Although Professor Gordon conceded that he was “not an [expert] on the cancer process” (T675.29), he accepted that allowing for a latency period between the exposure of interest and cancer development is an intended feature of epidemiological studies (T675.33–676.21). It follows that to continue to allocate person-years of cumulative exposure until the end of the follow-up period would eliminate the latency period and introduce error into the analysis.
347 For these reasons, I do not consider that the AHS is affected by exposure misclassification to a degree which significantly detracts from its key strengths identified above. As AP Harris observed, exposure misclassification is a limitation inherent to all epidemiological studies, particularly studies involving many participants over the course of several years. It is not, therefore, a question of whether or not the AHS is affected by exposure misclassification: there are good reasons to suggest that a study as significant as the AHS is affected by it (see Blair (2011)). The question is whether the relevant study is affected by it to a degree which would render its findings unreliable. In the light of the supplementary reports prepared by AP Harris and Professor Gordon, and the resulting negligible effect on the RR estimates in Andreotti (2018), in my view the AHS is not such a case and, accordingly, reject the emphasis sought to be placed on the exposure misclassification proposition.
348 As noted earlier, approximately 37% of participants in the AHS did not complete the follow-up questionnaire (Andreotti (2018) (at 510)). To deal with this problem, the authors of Andreotti (2018) employed a procedure known as multiple imputation (MI). MI is a technique which attempts to address the common issue of missing data by creating several different imputed datasets and combining results from each of them to obtain reliable estimates.
349 Professor Gordon and AP Harris agreed that the MI dataset used by the authors of Andreotti (2018) was not a complete dataset. Mr McNickle submits that as a result, the conclusions of Andreotti (2018) are unreliable. This submission was developed as follows.
350 First, Professor Gordon gave evidence that MI datasets are always complete datasets, citing his own experience and two texts on MI (Rubin (1987) and Schafer (1997)). Professor Gordon added that he had never before encountered a MI dataset with missing data (Third Gordon Report (at [86]–[90]); T708.14–19).
351 Secondly, AP Harris conceded in cross-examination that missing data in a MI dataset was “not common” (T836.10–21). It is said that AP Harris sought to justify the missing data in the MI dataset on the basis that it had been assessed and validated by the authors of Heltshe (2012) (Supplementary Harris Report (at [65])), but that nowhere in that paper do the authors refer to, or approve of, the use of a non-standard MI technique using a dataset with missing data (T837.35–838.28).
352 Thirdly, due to his concerns about the MI dataset employed in Andreotti (2018), Professor Gordon contacted the NCI. Without objection, evidence was adduced that the NCI advised him that as part of the reason for the missing data, a miscode had been identified and that they intended to publish an “errata” (by which I presume they mean “erratum”). It is said that in the light of the fact that the NCI response does not explain what the erratum will say, how the miscode arose, or whether the NCI has conducted an evaluation of the entire dataset to determine whether any other coding errors exist, the Court should be concerned about the reliability of the findings of Andreotti (2018).
353 Some time was spent at the hearing on the spectre of missing data and miscoded entries, and I do not propose to rehearse that discussion here or wade further into a debate as to whether the use of incomplete MI datasets is an unorthodox approach or not. This seems to me to be missing the forest for the trees. The fact is that the authors of Andreotti (2018) conducted two sensitivity analyses with complete datasets to account for the imputed exposure data and gauge the validity of the results of the study. I set out the relevant passage in Andreotti (2018) below (at 512):
To evaluate the impact of using imputed exposure data for participants who did not complete the follow-up questionnaire, we limited the analysis to 34,698 participants who completed both questionnaires, reducing the total number of cancer cases to 4,699. Glyphosate use was not associated with NHL (n=306 total cases; RR (Quartile 4) = 0.90, 95% CI = 0.63 to 1.27, P-trend = 0.54), and there was a non-statistically significantly elevated risk for AML (n = 35 exposed cases; RR (Tertile 3) = 2.64, 95% CI = 0.78 to 6.86, P-trend = 0.18; data not shown).
(Emphasis added)
354 In other words, Andreotti (2018) restricted the analysis to the complete dataset (that is, participants who completed both the enrolment and follow-up questionnaires, including over 34,000 registered pesticide applicators, approximately 4,600 cases of cancer and 306 cases of NHL), which excluded the MI exposure and found that the results of the sensitivity analysis remained consistent with the results of the primary analysis: that is, exposure to glyphosate and/or GBFs was not associated with NHL. As Professor Gordon noted (T720.5–13):
MR FINCH: Professor Gordon, isn’t this an answer to one of your points, when you do this sensitivity analysis, you are using a complete dataset?
PROFESSOR GORDON: Yes. You’re avoiding the use of any missing data.
MR FINCH: When they do that we can read for ourselves what they came up with respect to the risk ratio?
PROFESSOR GORDON: Yes.
355 Further, during the joint session, the Assessor asked Professor Gordon about the sensitivity analyses conducted in Andreotti (2018) (T813.8–18):
ASSESSOR: … the conclusions that are drawn from that subset [Andreotti (2018) sensitivity analysis (at 512)] would not be affected by multiple imputation problems. That’s my question. Is that correct?
PROFESSOR GORDON: Yes.
ASSESSOR: Yes.
PROFESSOR GORDON: They could be affected by missing data. They’re certainly affected by missing data. But not by multiple imputation problems, correct, because there’s no multiple imputation used there. Yes.
356 What this means is that regardless of the method adopted by the authors of Andreotti (2018) as to the issue of MI, it is clear from the sensitivity analysis and Professor Gordon’s evidence that it did not make any significant difference to the risk ratio results.
357 Accordingly, I do not accept the emphasis sought to be placed on the multiple imputation proposition.
358 Mr McNickle submits that the fact that one epidemiology study among many does not show evidence of a positive association between glyphosate and/or GBF exposure and NHL is not conclusive evidence that there is no such association. It is said that such an approach would be inconsistent with the weight of evidence approach propounded by Professor Checkoway and AP Harris (at JRC [25]).
359 Mr McNickle cites a paper authored by AP Harris and others as an example, namely Koutros S, Harris S, Spinelli J et al. (2019) “Non-Hodgkin Lymphoma Risk and Organophosphate and Carbamate Insecticide Use in the North American Pooled Project” 127 Environ Int. 199 (Koutros (2019)). There, the authors identified a positive association between exposure to malathion and NHL, with a statistically significant OR of 1.63 (unadjusted) and 1.43 (adjusted). In contrast, the AHS paper on malathion (Alavanja (2014)) showed an inverse association between malathion and NHL (that is, OR less than 1.0). Mr McNickle notes that nowhere in Koutros (2019) is it suggested that the results of that study should be discounted or assigned limited importance by reason of the results of Alavanja (2014). It is said that this is demonstrative of the correct approach to be taken with regard to the findings of the AHS in this proceeding.
360 To the extent that this submission is said to go beyond the fact that one ought not give disproportionate weight to one epidemiology study over the entirety of the epidemiological evidence, I confess that I have some difficulty understanding the burden of this submission.
361 I have already said enough to indicate (including above in Section B) what the proper approach to the scientific evidence demands in a case such as the present. Put shortly, it necessitates viewing the central issue with reference to the whole of the evidence. It is inevitable that in this process, some parts of the evidence will emerge as more significant than other parts, and, accordingly, may involve assigning greater weight to those parts, for a variety of reasons. But this does not mean that such evidence is conclusive to sustain a finding on the central issue: it must always be viewed in the light of the whole. It is for this reason, as Monsanto submits, that the significance of the Alavanja (2014) results and the appropriateness of AP Harris’ conclusions about malathion must be considered in the context of a weight of evidence analysis of all available literature on the risks of malathion exposure, rather than treated in isolation.
362 Having dealt with Mr McNickle’s contentions with respect to the AHS, I set out below my findings and what I consider to be the key strengths of the AHS and why it is appropriate that significant weight should be placed upon it.
363 First, in this context, size matters. The AHS involved more than 57,000 participants; over three-quarters of whom were ever exposed to glyphosate. As a result, the AHS offers a level of statistical power which vastly exceeds that of the individual case-control studies addressed above, which, on average, ranged between a few hundred to a few thousand participants. Professor Checkoway agreed that the large number of participants in the AHS “dwarfed any of the case [control] studies” (T951.7–10) and that as a result, offered greater statistical precision (T949.5–8; T952.9–10). AP Harris also identified the number of participants in the AHS as a key strength of the study (at JRC [204]). It was no doubt for this reason that numerous attempts were made by Mr McNickle, starting with Professor Gordon’s conjecture that it contained a “serious analytic error” (see McNickle v Huntsman Chemical Company Australia Pty Ltd (Additional Expert Conclave) (at [8])) to undermine the cogency of the study.
364 Secondly, the sample used in the AHS is highly relevant in assessing an etiologic relationship between exposure to glyphosate and/or GBFs and cancer, including NHL and its subtypes. It consisted of private (mostly farmers) and commercial applicators of pesticides who are regularly exposed to GBFs and other exposures thought to be linked to cancer. As AP Harris noted (at JRC [204]):
The AHS is a large prospective cohort study with many applicators occupationally exposed to levels much higher and with greater variation than those that occur in population-based/community studies. The collection of exposure information occurred prior to the cancer outcomes (i.e. no issues with recall bias) and included additional details on pesticide use and possible exposures that are often not collected in retrospective studies.
(Emphasis added)
365 AP Harris reiterated this point during the joint session when highlighting the prevalence of exposure among participants, noting that “whether it’s a case control or a cohort study and – but here we’ve got a much higher prevalence of exposure. So we’re going to have more power to detect those associations in the AHS given its sufficient follow-up” (T951.42–952.1). Professor Checkoway agreed with this proposition (T952.1).
366 Thirdly, the participants in the AHS have been followed for a significant period of time. Enrolment in the study occurred between 1993 and 1997. As noted earlier, De Roos (2005) was the first follow-up study which considered cancers up and until December 2001, which was a median follow-up period of 6.7 years. The second follow-up, Andreotti (2018), obtained further exposure information from follow-up questionnaires completed between 1999 and 2005 and considered cancers identified until 2012 (for North Carolina) and 2013 (for Iowa). As AP Harris noted in relation to the findings of Andreotti (2018), the additional years of follow-up increased the statistical power of the study and allowed for a study of NHL overall including several of its subtypes, adding that the five-, 10-, 15- and 20-year lagged analyses did not significantly affect results for NHL (at JRC [217]).
367 Fourthly, coupled with the large number of participants involved in the AHS, the questionnaires were designed in a way to procure detailed and granular responses from subjects concerning, among other things, the types of pesticides used, the duration and frequency of exposure, the way the pesticide was applied, and personal health information. The AHS questionnaire was the only questionnaire available for inspection by the expert witnesses in this proceeding and, when asked about its level of detail, despite initial criticisms with respect to the utilisation of self-reporting, Professor Checkoway agreed that the enrolment questionnaire for the AHS “dug down to an appropriately granular level of exposure to particular pesticides” (T955.44–956.1).
368 Fifthly, and relatedly, the reliability of exposure histories given by licensed pesticide applications (as in the AHS) has been verified in other specific studies. The authors of Andreotti (2018) note this as a strength of the study, stating (at 7) that “this AHS analysis includes only licensed pesticide applicators who have been shown to reliably report their pesticide use (28, 29)”. The two studies referenced by the authors are:
(1) Blair A, Tarone R, Sandler D, et al. (2002) “Reliability of Reporting on Lifestyle and Agricultural Factors by a Sample of Participants in the Agricultural Health Study from Iowa” 13 Epidemiology 94; and
(2) Hoppin J A, Yucel F, Dosemeci M, et al. (2002) “Accuracy of Self-reported Pesticide Use Duration Information from Licensed Pesticide Applicators in the Agricultural Health Study” 12(5) J Expo Anal Environ Epidemiol. 313.
369 Professor Checkoway agreed that the authors of the AHS papers had regard to studies which specifically addressed the reliability of the type of study participants who comprised its participants (T962.21; T963.33), noting that there had not been “any studies equivalent to those set out in [Blair (2002)] and other validating the accuracy of the recall of the cohorts of people used in any of those case controlled studies” (T963.40).
370 For these reasons, it is difficult to come to any conclusion other than that the AHS represents the largest, most comprehensive and reliable analysis to date concerning whether there exists an etiologic relationship between exposure to glyphosate and/or GBFs and NHL.
V Conclave E – Recall and selection bias
Introduction
371 Conclave E addressed the discrete question of whether the methodologies used, and conclusions drawn in Crump, K “The Potential Effects of Recall Bias and Selection Bias on the Epidemiological Evidence for the Carcinogenicity of Glyphosate” (2020) 40(4) Risk Analysis 696 (Crump (2020)) are reliable.
372 That study concludes that four case-control studies relied upon by Mr McNickle, namely McDuffie (2001); Hardell (2002); Eriksson (2008); and Orsi (2009) (collectively, Crump (2020) case-control studies) are “contaminated by statistical bias, likely stemming in the main from recall bias” (Conclave E Joint Report (JRE) (at [125])). In addition to recall bias being potentially responsible for increased ORs in the case-control studies cited, Crump (2020) suggests that Hardell (2002) and Eriksson (2008) used a non-standard method of computing ORs liable to cause a form of selection bias, which exacerbates the effect of any recall bias.
373 Dr Crump’s extra-curial reasoning in his article (which was admitted without objection by Mr McNickle, notwithstanding the difficulty in testing his evidence given in Court) is that systemic bias is present in these studies because of the high proportion of ORs elevated above 1.0 (Table II, Crump (2020) (at 700)). It is contended that the proportion of ORs he calculated as being above one is consistent with two possible hypotheses: first, all the pesticide groups considered are capable of causing NHL; and secondly, all of the OR calculations are biased. Given that it is very unlikely that every category of pesticide examined is capable of causing NHL, Dr Crump concludes that results from at least four of the five case-control studies (that is, the Crump (2020) case-control studies, excluding De Roos (2003)) are biased due to a form of recall bias, which is possibly exacerbated in Hardell (2002) and Eriksson (2008) by selection bias (at JRE [106]–[109]).
374 Before turning to my findings, it is necessary to address Mr McNickle’s contentions with respect to Crump (2020) (that is, out-of-court representations made by Dr Crump).
Mr McNickle’s contentions
375 Mr McNickle contends that there are several flaws in the analysis conducted in Crump (2020).
376 First, it is said that Dr Crump’s description of the two possible hypotheses (at JRE [107]) overstates the results which his hypotheses purport to explain. It is convenient here to set out Table II of Crump (2020) (at 700) (footnotes omitted):
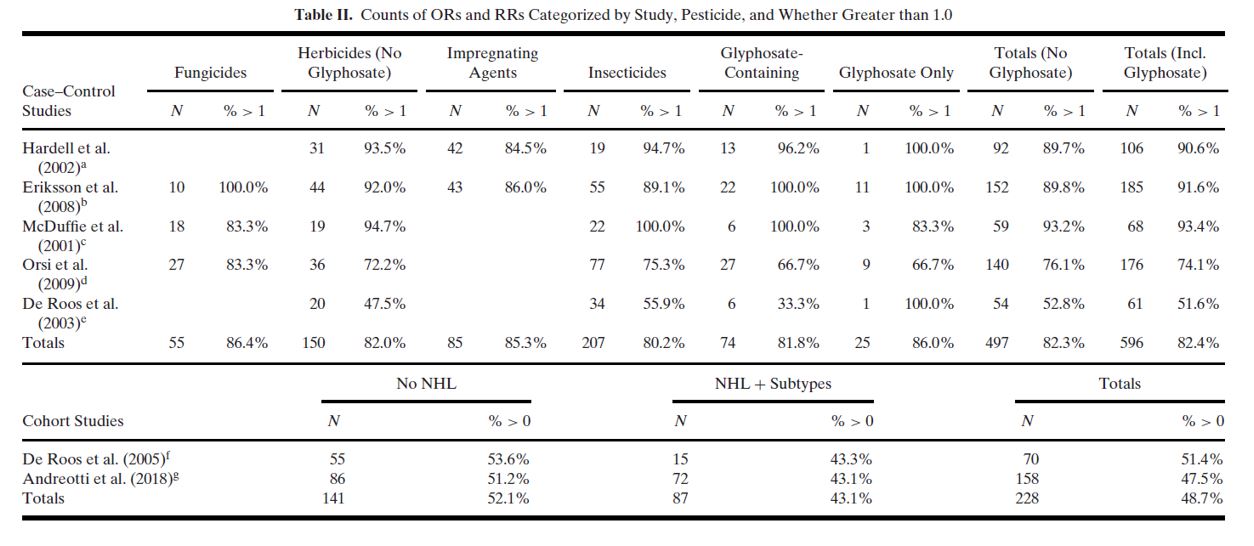
377 Dr Crump refers to “all” of the pesticide groups causing NHL and “all” of the OR calculations being biased in his articulation of the “two possible hypotheses” (at JRE [107]). However, as can be seen above, on Dr Crump’s calculations, it is not the case that all ORs are above 1.0. For example, the proportion of ORs above 1.0 are as low as 33.3% in De Roos (2003), 66.7% in Orsi (2009) and 83.3% in McDuffie (2001). It is contended that by virtue of those numbers alone, it cannot be said that the ORs are consistent only with two possible hypotheses, being that all the pesticide groups considered are causing NHL or that all the ORs are biased.
378 Secondly, it is contended that Dr Crump’s methodology for calculating the proportion of ORs above 1.0 in Table II involved, in effect, double-counting (at JRE [131]–[134]; First Gordon Report (at [336]–[344]); T1168.10–16, T1169.46–47, T1242.4–18, T1246.5–1247.4). This is said to be for two reasons.
379 The first reason is that the same exposed cases and controls were included in the calculation of more than one OR used by Dr Crump (T1238.44–1239.3). As a result, the ORs used by Dr Crump were not statistically independent, which has the effect of inflating the proportion of ORs above 1.0 (T1240.26–34).
380 The second reason is that the same individuals were likely exposed to more than one pesticide. Dr Crump conceded that that would mean the number of ORs above 1.0 could be the result of several pesticides showing an association across the population: (T1241.40–45). For example, an OR for Pesticide A might be above 1.0 even if Pesticide A is not associated with NHL, because some of those exposed to Pesticide A were also exposed to Pesticides B and C, where Pesticides B and C do cause NHL. This is another form of double counting, because Pesticides B and C may cause the ORs to appear elevated for Pesticide A as well as for Pesticides B and C, even though Pesticide A itself is not causing the OR to be elevated.
381 It is said that this lack of statistical independence or double counting means that the proportion of ORs above one is consistent with more than the two hypotheses Dr Crump identified (at JRE [107]). Two other hypotheses that the pattern of ORs are consistent with are: (1) the same exposed cases and controls are included in multiple of the ORs, and thus if there is an association for that exposure, one would expect that the ORs based upon those same exposed cases and controls would all be elevated; and (2) exposure to multiple pesticides by the same cases and controls could also explain the pattern of elevated ORs in the studies.
382 Thirdly, Professor Gordon and Dr Crump agreed that although Dr Crump’s simulation of the effect of recall bias looks at the effect on the OR assuming there is an absence of an association (that is, where the true OR equals 1.0), where the true OR has some different value (that is, greater than 1.0), then recall bias can cause a decrease in the OR, depending on the accuracy of exposure recall (sensitivity) and the accuracy of non-exposure recall (specificity) (at JRE [170]–[171]; T1233.18–1234.1). This downward effect on the OR is demonstrated by the sensitivity analysis undertaken by Professor Gordon for De Roos (2003) (see First Gordon Report (at [268])). Professor Gordon notes that in many of the combinations that would reflect recall bias (that is, where there is differential recall between cases and controls), the OR is underestimated and not overestimated and, as a result, contradicts Dr Crump’s simulated results (First Gordon Report (at [290]–[301]) and Figures 6, 7).
383 Fourthly, the literature cited by Dr Crump in support of his opinion that recall bias is a well-known problem does not support the conclusion that recall bias will necessarily be present or will necessarily result in ORs being inflated (at JRE [147], [149], [150]; T1194.44–1201.4).
384 Fifthly, it is said that the conclusions drawn by Dr Crump are contradicted by what the data in the papers show about the presence (or absence) of recall bias, as reported by the authors of the papers themselves or of other papers that analysed the same data. This is apparent from a review of McDuffie (2001) (at 1161); De Roos (2003) (at 8); Pahwa (2019) (at 607), Cantor (1992) (at 2543–2545), Lee (2004) (at 301); Hardell (2002) (at 1046) and Eriksson (2008) (at 1658, 1660) and other papers.
385 In relation to each of McDuffie (2001) and De Roos (2003), based upon what was said in those papers about whether recall bias was present (and other papers), Dr Crump said that “[t]here was not evidence for case recall bias” in those studies (T1218.40–1219.1; see also T1207.23–34; T1209.1–34; T1213.5–38; T1215.12–16). In relation to Hardell (2002), Dr Crump conceded that if recall bias was present, then it would be expected that farmers as an occupation would show an elevated trend (T1220.1–10). As a result, the absence of an association being observed for farmers points against recall bias being the cause for the elevated ORs (as was identified by the authors of the paper). Dr Crump did not disagree that that conclusion followed from the concession he made, instead stating that he “would have to think about it more seriously” and “[s]pend more time thinking about it” (T1220.16–17).
386 In relation to Eriksson (2008), Dr Crump conceded that there was some information available from the paper itself that there was no recall bias (T1226.1–4). In relation to Eriksson (2008), the paper showed elevated ORs for some, but not all, lymphoma types. Dr Crump accepted earlier in his evidence that where different diseases are studied within the one study and an association is identified for one but not another disease, that would suggest that recall bias was unlikely (T1200.38–46). It is said the Court should not conclude that this constitutes evidence that recall bias is “likely” and that Dr Crump’s concessions in cross-examination undermine the reliability of Crump (2020).
Consideration
387 Before going further, it is important to keep two things separate. The first are the out-of-court representations contained in Crump (2020) and the second are the in-court representations of Dr Crump in his opinions adduced in chief, including through the JRE. As noted above, the out-of-court opinions were admitted without objection by Mr McNickle and following the difficulties encountered during his cross-examination, no suggestion was made that those out-of-court representations be rejected or the subject of some limitation – indeed, the submissions about the diminished weight being given to his evidence were focussed on his in-court representations. In particular, there was no attempt by Mr McNickle to develop an argument that I ought to approach the out-of-court representations made by Dr Crump any differently to any other scientific article that was placed in evidence without objection.
388 Despite all this, in assessing the evidence, it is necessary to keep in mind that the Conclave E concurrent evidence session was hampered by Dr Crump’s difficulty hearing and comprehending questions, to which I have already made reference above (at [162]–[168]). In any event, it is difficult to come to any other conclusion that Crump (2020) is of limited assistance.
389 The chief reason for this is that broadly speaking, I have already found the Crump (2020) case-control studies suffer from a series of limitations to which I have already referred above in Sections I, II and III. Nothing about Crump (2020) changes this conclusion, notwithstanding that recall and selection bias was a factor in my assessment as to the case-control studies. Although I consider this issue to be somewhat of a distraction, for completeness, however, I will make the following observations.
390 First, with respect to the preponderance of ORs being above 1.0 in Table II, that table must be read in conjunction with Figure 1 in the study, which illustrates that the mean and median OR of the Crump (2020) case-control studies (that is, with the exception of De Roos (2003)) are well-over 1.0. As Dr Crump notes (at 699):
Fig. 1 shows plots of the tabulated ORs or RRs by study, with the pesticide groupings upon which the ORs are based (including ORs derived for both individual pesticides and groups of pesticides), classified as to fungicides, herbicides not containing glyphosate, impregnating agents, insecticides, and pesticide groupings that include glyphosate. The individual pesticides and groupings for each study are listed in the footnote to Table II. The tabulated RRs from the two cohort studies are similarly classified into cancer groupings not containing NHL and groupings containing NHL. Since the logarithms of ORs are plotted, we are interested in the proportion of log-transformed ORs that are greater than 0.0 (equal to the proportion of untransformed ORs greater than 1.0). … These figures show that ORs in McDuffie et al. (2001), Hardell et al. (2002), and Eriksson et al. (2008) are all practically greater than 1.0. Also, there is an excess of ORs greater than 1.0 in Orsi et al. (2009). These excesses of ORs greater than 1.0 occur in all categories of pesticides considered in these studies.
(Emphasis added)
391 Professor Gordon accepted in cross-examination that the preponderance of ORs for NHL greater than 1.0 for practically all categories of pesticides suggested that recall bias may be responsible for the observed associations in the Crump (2020) case-control studies, the effect of which will tend to make glyphosate appear carcinogenic even if it is not (T1160.7–12); at JRE [158]). As Dr Crump explains, “[t]his bias would be expected to be present in exposure of all pesticides because cases and controls would respond differently when questioned about their past exposure” (at JRE [130]).
392 Secondly, with respect to double-counting, it was suggested to Dr Crump that he counted ORs for pesticide groups (for example, phenoxy herbicide) and also ORs from the individual chemicals within that group (for example, 2,4-D and mecoprop) such that double-counting would artificially generate a higher proportion of elevated ORs. It is clear from the Crump (2020) supplementary files that in relation to McDuffie (2001), the calculation of the generic pesticide ORs (phenoxy herbicide) was based on a different number of exposed participants than the ORs for individual chemicals within the relevant pesticide group. That is, they are different exposures with different ORs. The table below extracted by Monsanto helpfully illustrates that the participants exposed to phenoxy herbicide (generic) were different to those exposed to the specific phenoxy herbicides, necessitating separate OR calculations:
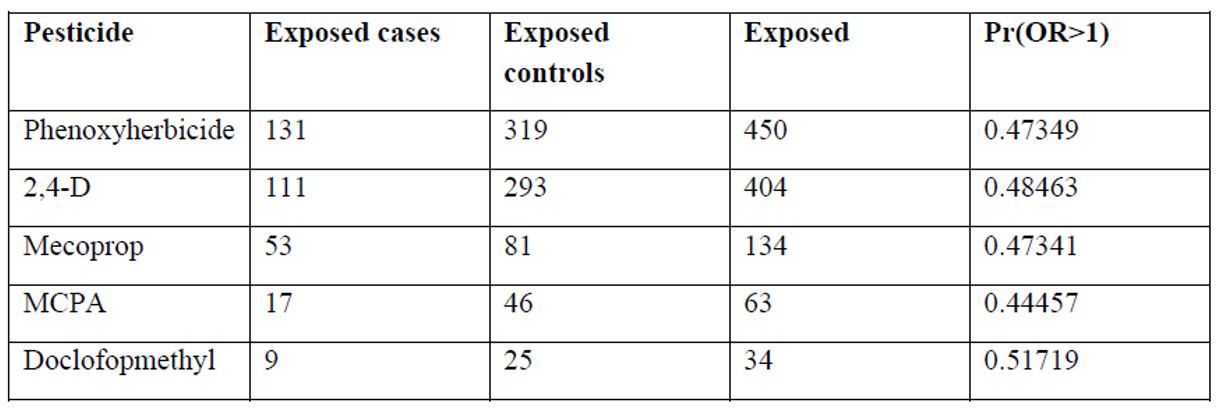
393 The following exchange occurred between the Assessor and Professor Gordon (T1243.22–32):
ASSESSOR: So I’m still intrigued why they reported phenoxy herbicide generic as well as the individual phenoxy herbicide. Aren’t they all different exposures with different odds ratios?
PROFESSOR GORDON: Well, potentially, yes, that’s right, Sir John, they are. But sometimes people might consider, you know, a grouping of exposures, I guess, if they think they can be meaningfully grouped in some way.
ASSESSOR: But they’re different exposures.
PROFESSOR GORDON: Yes, they are.
394 With respect to the second form of double-counting alleged, being that the same individuals were likely exposed to more than one pesticide, Professor Gordon accepted that even if the premise were true that a confounding agent (that is, Pesticides B and C, see above (at [380])) is capable of causing NHL, there is no evidence that the relevant agent is glyphosate (T1168.22–1169.10). As Dr Crump noted in the JRE (at [129]):
If there is only one such carcinogenic pesticide, the presence of that pesticide would need to be consistently highly correlated with all the other pesticide categories to see the consistent pattern of ORs > 1 in all categories of pesticides examined. Although I acknowledge that exposure to different pesticides may be correlated, I know of no evidence for such high correlations, and Professor Gordon has not presented such evidence.
395 This is not to say that some form of double counting in Crump (2020) did not, in fact, occur. As Professor Gordon recognised, there is duplication present, for example, in rows 14 and 15 in relation to dinitroanilines and trifluralin (T1246.43–1247.4) which pertain to the same study participants. Mr McNickle, however, did not present any modelling of the effect of removing such duplications which, as Dr Crump notes, would not alter the excess of ORs above 1.0 (and would be, in any event, not possible in this study) (T1247.9–1247.26).
396 Thirdly, although Professor Gordon and Dr Crump agreed that it is theoretically possible that in circumstances where the OR is greater than 1.0, then recall bias can decrease the OR (rather than increase it) (at JRE [170]–[171]; T1233.18–1234.1), this proposition was not tested with respect to Dr Crump’s simulated results in the JRE. Accordingly, it is difficult to come to any conclusion as to whether Dr Crump’s simulation can be contradicted on this basis.
397 Fourthly, as to the literature and data cited in Crump (2020) and the contention that the case-control studies referred to by Dr Crump tended against the presence of recall bias in those studies, three points should be made: (1) the apparent differences between the degree of elevation in ORs for one disease (but not others) in any given study is not compelling evidence against the presence of recall bias. In Eriksson (2008), for example, the other disease with a non-elevated OR was an outlying subset of NHL (that is, unspecified B-cell lymphoma, which exhibits unusually low ORs); (2) recall bias in any given study is not excluded by claims from authors that pilot studies or samples indicated a correlation between reported exposures and objective measures of exposure (such as the purchase of pesticides); and (3) recall bias does not arise solely from publicity about glyphosate, therefore it is not excluded by pre-publicity dates of undertaking questionnaires.
398 Fifthly, without making any firm conclusion as to Crump (2020) and recall and selection bias for reasons I have explained, Professor Gordon accepted (at JRE [183]) that the exclusion of the unexposed cases and controls in the two studies was “strange and non-standard” and that he was “not convinced” that Eriksson (2008) and Hardell (2002) excluded other exposures among the unexposed to glyphosate group. In cross-examination, however, Professor Gordon accepted that this method had been used to derive the ORs in Eriksson (2008) (T1184.16) and Hardell (2002) (T1184.5), and accepted that to the extent there are ORs that are infected by selection bias “[they are] the odds ratios that Dr Crump included in table 2 [of Crump [2020])” (T1185.18–21).
Conclusion
399 For these reasons, I have derived only limited assistance from Crump (2020).
400 Overall, having regard to the weight I have attributed to the epidemiological evidence adduced, I am not satisfied that the epidemiological evidence supports an etiological association between glyphosate and/or GBFs and NHL.
401 Broadly summarised, that conclusion follows for three reasons.
402 First, I have derived limited assistance from the case-control studies addressed in Sections I, II and III above. As I have explained, the case-control studies share common limitations which, when viewed collectively, significantly diminish the reliability of those publications in demonstrating an etiologic connexion between glyphosate and/or GBF exposure and NHL. It is worth highlighting two, namely:
(1) the failure to correct for exposure to other pesticides (that is, confounding); and
(2) the small number of study participants who reported exposure to glyphosate.
403 As to the first limitation, confounding is a frequently recurring issue in the case-control studies. To take four examples, in Eriksson (2008), Professor Checkoway conceded that none of the results for glyphosate in Table II were adjusted for exposure to other pesticides, with the authors noting (at 1660) that exposure to multiple pesticides was “common among the study participants” (see above (at [208])). In Hardell (2023), the authors indicated (at 999) that in calculating the risk estimate for different pesticides, “exposure to other types were not included for cases and controls”, thereby including subjects with no exposure to any type of pesticides (see above (at [284])). In Orsi (2009), the authors allocated an agent to study participants based upon crop type, thereby introducing potential exposure misclassification (see above (at [298])). In Lee (2004), one non-asthmatic case of NHL was on average exposed to about four pesticides. The corollary is that in a large proportion of the case-control studies, there was a failure or inability to address confounding factors or to isolate appropriately the purported carcinogenic effect of glyphosate as an agent.
404 As to the second limitation, most of the case-control papers involved a relatively small number of cases and controls who were exposed to glyphosate. In Meloni (2021), for example, Professor Checkoway and AP Harris agreed that a limitation of the study was the small number of participants ever exposed to glyphosate (21 cases and 15 controls). The authors acknowledged this as a “major interpretive limitation” of the study (see above (at [222])), and further noted that it was not clear in any event whether the 21 cases had in fact ever been exposed to glyphosate. In Hardell (2023), a similar number of cases (37) and controls (26) had ever been exposed to glyphosate, which was noted by Professor Checkoway as a weakness of the study (see above (at [283])). In Hardell and Eriksson (1999), Professor Checkoway accepted that the sample size for glyphosate (four cases and three controls) was too small to derive any reliable conclusion from the study as to an etiological association between glyphosate and/or GBFs and NHL. As noted earlier, he agreed with AP Harris’ observation that the number of cases and controls in that study could have been even smaller than four and three respectively due to missing data in other variables (see above (at [182])). As a result, due to the relatively small sample sizes involved, the case-control studies generally suffer from low statistical precision and an inability to extrapolate the results for the population at risk.
405 Secondly, putting to one side the issue of confounding and small sample sizes, to the extent that the case-control studies can be relied upon for the purposes of examining whether there is a causal connexion between glyphosate and/or GBF exposure and NHL, it is clear that the OR results in many of the studies include the null hypothesis (that is, do not exclude the possibility of no causal relationship between exposure to glyphosate and/or GBFs and NHL). To list five examples (emphasis added):
(1) Hardell and Eriksson (1999) (OR 2.3, 95% CI: 0.4–13);
(2) McDuffie (2001) (OR 1.26, 95% CI: 0.87–1.80) (unadjusted);
(3) Eriksson (2008) (OR 1.51, 95% CI: 0.77–2.94);
(4) De Roos (2003) (OR 1.6, 95% CI: 0.9–2.8) (adjusted for hierarchical regression); and
(5) Meloni (2021) (OR 1.4, 95% CI: 0.62–2.94).
406 It is worth reiterating in respect of these results that a wide confidence interval is indicative of a small number of observations, and hence a less reliable point estimate (see particularly Hardell and Eriksson (1999) and Meloni (2021)).
407 Thirdly, for reasons I have explained and in contrast to the bulk of the case-control studies, the AHS represents the most comprehensive and reliable analysis to date of whether there is a causal relationship between exposure to glyphosate and/or GBFs and NHL.
408 At the risk of repetition, it is well to emphasise two points: (1) in the light of the sheer number of study participants involved, the AHS offers a level of statistical precision which vastly exceeds any comparable study on the etiologic relationship between glyphosate and NHL. Professor Checkoway and AP Harris agreed that this represented a “key strength” of the AHS in contrast to the case-control studies, which frequently ranged from a few hundred to a few thousand participants; and (2) in contrast to population-based and community studies, the sample in the AHS consists of applicators who were occupationally exposed to GBFs and other agents thought to be carcinogenic. As noted earlier, Professor Checkoway and AP Harris agreed that as a result of the high prevalence of exposure, the AHS offers a far greater facility to detect potential associations between GBFs and various cancers.
409 Having dealt with epidemiology, it is now necessary to turn to the rats and mice.
F THE SECOND STREAM: ANIMAL STUDIES (CONCLAVES H, I AND J)
410 The animal studies stream concerns long-term studies conducted on experimental animals. It is comprised of studies which address whether a substance or chemical is capable of inducing cancer development in laboratory animals, and if so, whether this information provides insight into carcinogenic potential or risk for humans (Conclave H Joint Report (JRH) (at [16])).
411 Animal studies can provide two insights into the carcinogenic potential or risk for humans of a substance: first, whether there is a potential carcinogenic hazard; and secondly, whether there is a dose-response relationship. As stated by the US Environmental Protection Agency (USEPA) “Guidelines for Carcinogen Risk Assessment” (March 2005) (USEPA Guidelines) (at JRH [17]):
The objective of long-term carcinogenesis bioassays is to determine the potential carcinogenic hazard and dose-response relationships of the test agent. Carcinogenicity rodent studies are designed to examine the production of tumours as well as preneoplastic lesions and other indications of chronic toxicity that may provide evidence of treatment-related effects and insights into the way the test agent produces tumours.
412 Mr McNickle contends that the evidence adduced in the animal studies stream establishes that more likely than not, glyphosate causes cancer in rats and mice, and that the results of the animal bioassays provide important evidence as to glyphosate’s carcinogenicity in humans.
413 Before turning to that evidence, it is necessary to make some further introductory observations with respect to the methodology used in animal studies the subject of this stream.
414 Animal studies involve experiments on mammals. They are typically performed on rats and mice over a period of 18 to 24 months under a set of hygienic, procedural, and humane conditions known as “good laboratory practices” (GLP). Consistently with the first law of toxicology that “the dose makes the poison”, GLP is achieved by procuring, randomising, dosing and caring for the animals to ensure that the only difference between the animal groups is the amount of chemical they receive (First Bayard Report (at [1])).
415 A typical experiment will involve an animal bioassay set (a term to which I will return shortly) of at least 50 animals per sex, per dose group in three treatment groups, and in a concurrent control group (at JRH [20]). One of the groups receives none of the chemical (that is, the concurrent control group), while the treatment groups receive different amounts of the chemical up to a maximum dose, known as the maximum tolerated dose (MTD). Testing at the MTD is not done because humans are expected to be exposed at such high doses, rather it is done to determine if the tested chemical has carcinogenic potential, and to limit the numbers of animals in the study. If a chemical is found to cause cancer at high doses, it is almost always considered to cause less (but not zero) cancer at lower doses (at JRI [44]).
416 When the animals die or are slain, they are autopsied for findings of chronic toxicity and cancer in most organs or systems. Reports are prepared and the results are analysed by toxicologists, and by statisticians who perform a series of statistical tests to determine whether or not the cancers in the treatment groups differ from those in the control group (First Bayard Report (at [1])).
417 Cancer biologists gain insights into cancer biology by using animal model systems. The term “animal model” usually refers to studying an animal and its susceptibility to the agent administered to develop a disease which is the same as or like a disease in humans. Some animal models are specifically developed by modifying the animal host to increase susceptibility. In other models, the animal is administered the disease for the purpose of further experimentation, such as slowing the progress of the disease by using a drug (at JRI [18]).
418 Animal models are evaluated according to whether experimental findings can be translatable to humans (at JRI [16]). With respect to murine models (that is, models involving rats and mice) used in cancer research, the vast majority are developed for the purposes of (at JRI [19]):
(1) studying an animal and its susceptibility to the agent administered to develop a disease which is the same as or like a disease in humans;
(2) studying the biological processes that occur during cancer development. In such cases, the mice may start the experiment without cancer or already have the cancer at the start of the experiment; and
(3) establishing a cancer in a small animal and assessing the response to a given therapy (that is, a new drug). Such animal model systems can be considered “contrived or artificial”, in that the mouse biology is somehow manipulated to “host” a cancer.
419 Such studies frequently involve use of “bioassays”. Bioassays are a quantitative assessment of the potency of a chemical or biological substance by observing and measuring its effects on cells, tissues, living organisms, or humans (at JRI [21]), and include experiments conducted on living animals (in vivo) and on living cells or organ tissue (in vitro).
420 Animal bioassays are an important tool in evaluating the carcinogenic potential of most chemicals. Bioassays on murine subjects are typically conducted for registration purposes for commercial products, including pesticides, if and as required by regulatory agencies or bodies and by law. If a registration requirement under law requires the conduct of long-term carcinogenic studies, then it is incumbent upon the registrants of a chemical that is being developed with the intention of it becoming commercially available to conduct such studies and submit them to the relevant regulatory authorities (at JRH [14]). For pesticide registrations globally, there exist standardised test guidelines for various and multiple toxicological effects and outcomes, but specific to evaluation of carcinogenic potential, there are existing test guidelines (TG) for both the Organisation for Economic Co-operation and Development (OECD) and USEPA regulatory authorities. The TGs specify the framework and specific scientific details and parameters by which long-term cancer studies are conducted on laboratory animals and also provide guidance on test result interpretation relative to carcinogenic potential of a chemical or substance.
421 The following represent some of the key study design or data interpretation guidance from the OECD when conducting chronic or carcinogenicity studies in laboratory animals (at JRH [19]; see further Guidance Notes for Analysis and Evaluation of Chronic Toxicity and Carcinogenicity Studies (2002) OECD (OECD Guidance Notes)):
(a) OECD Test Guideline 451 (i.e., for carcinogenicity evaluation) requires at least three dose levels, as well as controls for determination of the dose-response relationship.
(b) The largest administered dose should not compromise the biological interpretability of the observed responses.
(c) In a carcinogenicity study, this dose should not significantly affect the survival rate except through tumour production or cause a body weight decrement greater than 10-12% of concurrent control values.
(d) Overt toxicity or inappropriate toxicokinetics as a result of excessive dosing may result in tumorigenesis that is secondary to toxicity rather than directly attributable to the agent.
(e) The clearest indication of a positive carcinogenic response is obtained when the incidence of tumours rises above concurrent and historical control levels in both sexes and is higher at higher doses. Further significant observations in treated animals include an increase in rare types of tumour, metastases, reduced latency, and the presence of tumours at multiple sites.
(f) Although statistical comparisons are of treated animals and concurrent controls, additional insights into the significance of tumours can be obtained from examination of historical control data.
422 The USEPA’s key study design requirements and data interpretation guidance for animal studies are as follows (at JRH [20]):
(a) Current standardised carcinogenicity studies in rodents test at least 50 animals per sex per dose group in each of three treatment groups and in a concurrent control group, usually for 18 to 24 months.
(b) The high dose (i.e., maximum tolerated dose or MTD) in long-term studies is generally selected to provide the maximum ability to detect treatment-related carcinogenic effects while not compromising the outcome of the study through excessive toxicity or inducing inappropriate toxicokinetics (e.g., overwhelming absorption or detoxification mechanisms).
(c) The purpose of two or more lower doses is to provide some information on the shape of the dose-response curve.
(d) Trend tests and pairwise comparison tests are the recommended tests for determining whether chance, rather than a treatment-related effect, is a plausible explanation for an apparent increase in tumour incidence. A statistically significant response may or may not be biologically significant and vice versa.
(e) A trend test such as the Cochran-Armitage test (Snedecor and Cochran, 1967) asks whether the results in all dose groups together increase as dose increases. A pairwise comparison test such as the Fisher exact test (Fisher, 1950) asks whether an incidence in one dose group is increased over that of the control group. By convention, for both tests a statistically significant comparison is one for which p is less than 0.05 that the increased incidence is due to chance. Significance in either kind of test is sufficient to reject the hypothesis that chance accounts for the result. A statistically significant response may or may not be biologically significant and vice versa.
(f) The standard for determining statistical significance of tumour incidence comes from a comparison of tumours in dosed animals with those in concurrent control animals. Additional insights about both statistical and biological significance can come from an examination of historical control data. Historical control data can add to the analysis, particularly by enabling identification of uncommon tumour types or high spontaneous incidence of a tumour in a given animal strain.
423 The USEPA also provides guidelines on the issue of multiple comparisons, to which I will return below (at [481]) and which assumes some importance in the context of Dr Bayard’s evidence.
424 While the OECD and USEPA guidance above contain similar recommendations relative to study design or data interpretation, this reflects the desire by regulatory authorities for global harmonisation of toxicology study design and data interpretation. Relative to evaluation of results from long-term cancer bioassays in laboratory animals include the following (USEPA Guidelines (at 2–20) (at JRH [21])):
Assessment of evidence of carcinogenicity from long-term animal studies.
In general, observation of tumors under different circumstances lends support to the significance of the findings for animal carcinogenicity. Significance is generally increased by the observation of more of the factors listed below. For a factor such as malignancy, the severity of the observed pathology can also affect the significance. The following observations add significance to the tumor findings:
• uncommon tumor types;
• tumors at multiple sites;
• tumors by more than one route of administration;
• tumors in multiple species, strains, or both sexes;
• progression of lesions from preneoplastic to benign to malignant;
• reduced latency of neoplastic lesions;
• metastases;
• unusual magnitude of tumor response;
• proportion of malignant tumors; and
• dose-related increases.
In these cancer guidelines, tumors observed in animals are generally assumed to indicate that an agent may produce tumors in humans. Mode of action may help inform this assumption on a chemical-specific basis. Moreover, the absence of tumors in well-conducted, long-term animal studies in at least two species provides reasonable assurance that an agent may not be a carcinogenic concern for humans.
425 Tumours at multiple sites, for instance, show that the chemical can be absorbed, distributed widely throughout the body, and have adverse effects on multiple systems. Tumours occurring in multiple species, strains and both sexes add to the confidence that the chemical’s effects are not constrained to just one site or laboratory strain. These studies include a broad spectrum of cancers, including NHL. Furthermore, in the case of NHL in humans, malignant lymphomas in mice have been used as laboratory models for NHL based on similarities of lymphocyte development.
426 Further to the USEPA and OECD guidelines, the International Agency for Research on Cancer (IARC) has adopted a system for classifying chemicals for their carcinogenic potential to humans, namely (at JRI [24]–[25]):
(1) Group 1 (Carcinogenic to humans);
(2) Group 2A (Probably carcinogenic to humans);
(3) Group 2B (Possibly carcinogenic to humans); and
(4) Group 3 (Not classifiable as to its carcinogenicity to humans).
427 Classification of a chemical depends upon an evaluation of the three streams of evidence the subject of this proceeding, not only animal studies. With that said, the IARC notes that for some agents, carcinogenicity in experimental animals was demonstrated before epidemiological studies identified carcinogenicity in human beings. While this observation does not mean that all agents which can cause cancer in experimental animals can cause cancer in humans, it is biologically plausible that agents for which there is sufficient evidence of carcinogenicity in experimental animals present a carcinogenic hazard to humans. Importantly, although such agents are considered to pose a potential carcinogenic hazard, that inference does not imply tumour concordance across species (at JRI [27]).
428 In terms of the evaluation of human carcinogenic potential, bodies (such as the USEPA) utilise carcinogen classification like that used by the IARC. In arriving at a rating, the Bradford Hill criteria (see above (at [105]–[106])) are often used. The USEPA Guidelines, for example, uses the Bradford Hill criteria for evaluation of human carcinogenic potential, with “evidence from experimental animals” relating to “consistency”, “strength”, “temporal, “biological gradient”, “biological plausibility” and “coherence” criteria.
429 The animal studies stream spanned Conclaves H, I and J and involved the following expert witnesses:
(1) Conclave H – Animal studies A (toxicological analysis of results of animal studies)
Dr Bayard and Dr Juberg
(2) Conclave I – Animal studies B (relevance of animal studies and bioassays to humans)
Dr Bayard and Professor Prince
(3) Conclave J – Animal studies C (statistical analysis of results of animal studies)
Dr Bayard and Dr Crump
430 I should pause to note that Monsanto, for reasons I will explain, made a forensic decision not to call Dr Crump to give evidence in Conclave J. Accordingly, I have not had regard to Dr Crump’s contribution to the JRJ or his individual report in informing my conclusions below (except to the extent that it is appropriate to make something of Monsanto’s failure to call him in this part of its case).
431 As with the other streams, individual reports were prepared by each of the experts, as set out in the table below:
Report | Date | Report Topic |
Expert Report of Dr Bayard (First Bayard Report) | 4 April 2022 | The carcinogenicity of glyphosate in laboratory rodent lifetime studies. |
Supplementary Report of Professor Prince (Second Prince Report) | 16 July 2022 | Response to the First Bayard Report and basis for presumption that “growth control mechanisms at the cellular level are homologous between species (US EPA, 2005)”. |
Supplementary Expert Report of Dr Juberg (Second Juberg Report) | 14 July 2022 | Response to the First Bayard Report (at [141]–[151]) which addresses, among other things, whether it is more likely than not that glyphosate causes cancer in animals and should be considered a human carcinogen. |
432 In the following section, I will introduce each of the experts who participated in the animal studies stream before turning to making some general observations as to credit.
433 Dr Steven Bayard is a retired statistician and risk assessor with over 40 years of experience in health and quantitative risk assessment of toxic chemical hazards. Mr McNickle had initially engaged Dr Christopher Portier to give evidence in the animal studies stream, however, Dr Bayard was engaged by the applicant following Dr Portier’s decision to no longer take part in this proceeding (a matter to which I will return below).
434 Dr Bayard holds a bachelor’s degree in mathematics from Tufts University (1965) and a PhD in biostatistics from Johns Hopkins University (1971). He also holds graduate credits in toxicology from the Massachusetts Institute of Technology (1979) (a non-degree programme). Dr Bayard’s experience includes working with the CPSC, USEPA and OSHA where, among other things, he authored cancer risk assessments for various chemicals. Dr Bayard also has experience teaching graduate courses in statistics, biostatistics, epidemiology and demography, and in various project management and consulting roles.
435 Dr Bayard’s conclusion in the animal studies stream is that based upon his analysis and evaluation of 13 lifetime glyphosate cancer bioassays, glyphosate is carcinogenic in mammals (at JRH [24]; JRI [54]). Monsanto contends that the Court should reject this proposition principally because Dr Bayard was not an impartial witness.
436 This is said to be for three interrelated reasons, namely that Dr Bayard:
(1) recited the work of Dr Portier with little or no independence;
(2) approached the task of giving evidence as an advocate, rather than an impartial expert; and
(3) came to this proceeding with a fixed view from his involvement in glyphosate litigation in the United States.
437 As to the first reason, Monsanto contends that critical aspects of Dr Bayard’s evidence involved a replication of Dr Portier’s analysis. It will suffice to set out a few examples given by Monsanto. When Dr Bayard was asked about the unavailability of data underlying one of the studies on which he relied (Takahashi (1999)), Dr Bayard said that he had “relied on the JMPR and Dr Portier” (T1644.25–30), before clarifying that he had “relied mostly – more on the JMPR than I did on Dr Portier” (T1645.1–2). Further, when Dr Bayard was asked whether he looked at Dr Portier’s data when he made corrections to his report in the week leading up to the concurrent evidence session, Dr Bayard said that he did look at the data beforehand and must have drawn some of his data from the analyses conducted by Dr Portier (T1666.39–43). Monsanto also submits that Dr Bayard had replicated the method Dr Portier had used for adjusting for multiple comparisons, which apparently had not been featured in the scientific literature and had never been used other than by Dr Portier (T1772.12–13; T1774.46; T1773.8; T1774.17–28).
438 As to the second reason, Monsanto submits that aspects of Dr Bayard’s oral evidence indicated that he approached his task as an advocate for Mr McNickle, rather than as an independent expert witness. It is said that Dr Bayard, among other things:
(1) resisted the proposition that he is not a qualified toxicologist and that he was not an expert on the mode of action of glyphosate on the basis that he had read Wang (2019) (T1613.41–1614.3; T1629.23–1630.9; T1629.32, 44; T1630.8);
(2) failed to disclose to the Court that the original study pathologist in Takahashi (1999) revised the incidence of tumours upon re-examination (T1647.15–20; T1649.26–33);
(3) conceded that he did not give any reasons as to why the pathologist’s re-examination results should not be preferred over the old slides (T1650.6–10), noting that “I probably should have said that there was a re-examination” (T1647.26–27); and
(4) approached his evidence with a view to “defend[ing] the report [he] wrote in court” (T1778.22).
439 Monsanto further submits that Dr Bayard’s approach was obfuscatory advocacy, highlighting that he was repeatedly entreated by the Court and by counsel to attend to the question asked (see, for example, T1614.11–21; T1615.25; T1623.24–25; T1627.43; T1644.45; T1646.11; T1677.18; T1681.27–31; T1691.38; T1697.13; T1699.17; T1713.6–11; T1727.38–39; T1733.30; T1747.15; T1763.17; T1767.43; T1664.10–16; T1728.30; T1755.31–32; T1756.5–6; T1774.15).
440 As to the third reason, Monsanto highlights that Dr Bayard had been engaged as a consultant to a US law firm, Lundy Lundy Soileau & South, in glyphosate litigation prior to replacing Dr Portier in this proceeding (T1619.28–36). When asked as to whether he had seen Dr Portier’s evidence when giving evidence previously in glyphosate litigation in the United States (namely, Kane v Monsanto (No. 1622-CC10172) in the Circuit Court of the City of St Louis, State of Missouri (Kane v Monsanto)), Dr Bayard recalled reviewing deposition material of Dr Portier in forming his opinion (T1622.25–46) and that he had reviewed analyses that Dr Portier had prepared in another case that Dr Portier had participated in prior to Dr Bayard preparing his report in Kane v Monsanto (T1625.45–1626.6). Further, when it was put to Dr Bayard that when he gave evidence in those proceedings in 2018 that he had a “fixed view” that glyphosate was an animal carcinogen, he responded that “I probably forgot about it soon after that but yes” and that “in 2018 I thought that way” (T1617.35–45).
441 Although I think these submissions are, in part, put too highly, Dr Bayard was not a responsive witness and did at times stray into advocacy, rather than presenting as a disinterested expert in the Anglo-Australian tradition. Having said that, by his own lights, I suspect that Dr Bayard was attempting to assist the Court and it is worthwhile making the following points.
442 First, as I have noted earlier (at [32]–[33]), the fact that an expert witness has expressed an opinion previously in analogous litigation as to whether, for example, glyphosate is carcinogenic is not a satisfactory reason simpliciter to reduce the weight to be afforded to the expert’s present opinion. There is no dispute that Dr Bayard had formed an opinion that glyphosate was an animal carcinogen in April 2018 (T1618.1–2). Although this does not necessarily mean Dr Bayard approached his evidence in this proceeding as an advocate or with a fixed view, sometimes he did fall into this trap. But having said that, I do not think, for example, that Dr Bayard deliberately attempted to obfuscate the findings of Takahashi (1999) by failing to explain that the original study pathologist in that study had conducted a re-examination. Nor do I consider that Dr Bayard intentionally gave a less than complete picture of the evidence by concluding that Lankas (1981) provided a reliable foundation for the proposition that glyphosate causes Thyroid C-cell adenomas and carcinomas in Sprague-Dawley (SD) rats (T1739.15–1740.34; at JRH [70]–[76]).
443 Secondly, it is neither necessary nor helpful to wade into the question of the extent to which Dr Bayard saw or relied upon materials prepared by Dr Portier in advance of the initial trial. For the reasons I have explained above, this is an exercise liable to distract from the task of assessing the underlying scientific merit of the relevant expert’s opinion. To some extent, Dr Bayard relied upon Dr Portier’s work, but I do not think that it amounted to a “faithful replication”. Dr Bayard’s evidence, which I accept, is that Portier (2020) used similar method to his own but that Dr Portier “did not look at tumours where [Dr Bayard] analysed them by type” (T1773.8–1774.17). In any event, insofar as Dr Bayard did rely upon Portier (2020), he was entitled to do so for the reasons I have explained above (at [62]) with reference to Karpik v Carnival.
444 Overall, although I accept Monsanto’s submission that Dr Bayard was, at times, less than responsive and frequently engaged in speeches, in my view this was reflective of a natural tendency, common among the expert witnesses, to come to the defence of one’s own work, rather than any conscious attempt to obscure the evidence.
445 Before moving on, it is important to distinguish this finding as to credit from Monsanto’s submission that Dr Bayard’s evidence should be afforded less weight because of: (1) his alleged flawed statistical approach with respect to the animal bioassays; and (2) his alleged limited expertise to conduct a critique of multi-disciplinary animal bioassay studies. It is convenient to deal with these submissions when I come to the animal studies evidence in Section F.3 below.
446 Dr Daland Juberg is an independent consultant with Juberg Toxicology Consulting LLC. Dr Juberg earned a PhD in toxicology and MS in Environmental Health Sciences (water quality), from the University of Michigan, along with a bachelor’s degree in biology from Wittenberg University. Dr Juberg was engaged by Monsanto.
447 Dr Juberg was formerly the Global Leader of Human Health Science Policy at Corteva agriscience, and previous roles included Global Leader, North American Leader, and Senior Toxicologist of the Human Health Assessment group within Dow AgroSciences (Dow). Dr Juberg was also a Principal with the International Centre for Toxicology and Medicine (Washington, DC), Senior Toxicologist for Eastman Kodak Company (NY), and Environmental Scientist with Environmental Control Technology Corporation (MI).
448 Dr Juberg’s conclusion in the animal studies stream is that the long-term cancer bioassays in rats and mice do not demonstrate that glyphosate is carcinogenic in mammals (at JRH [103]). Mr McNickle submits that Dr Juberg did not bring an independent mind to giving evidence in the initial trial, primarily because Dr Juberg deferred to the decisions of several regulatory bodies in support of his opinions without actively engaging with that material.
449 This submission was developed as follows.
450 First, it is said that Dr Juberg relied upon decisions reached by the OECD regarding the carcinogenicity of glyphosate, despite the OECD never having conducted an evaluation of glyphosate. When Dr Juberg purported to set out the conclusions of the OECD, what he most often did, Mr McNickle submits, was set out the submission made by the “Glyphosate Taskforce” (GTF) in support of its application for renewal of glyphosate’s registration, represented by Monsanto (T1892.5–1894.12). The corollary is that Dr Juberg’s evidence, at least in part, consisted of repeating what was submitted by the GTF, as represented by Monsanto, instead of any independent analysis or conclusions reached by a regulator. Relatedly, Mr McNickle submitted that notwithstanding Dr Juberg’s opinion regarding the USEPA’s compliance with their guidelines (at JRH [150]), he was nonchalant about the vacation of the 2020 Interim Decision by the 9th Circuit Court on the bases that the USEPA had failed to comply with its own guidelines (T1867.40–1868.36).
451 Secondly, it is said that Dr Juberg took a slapdash approach to his evidence by failing to respond to Dr Bayard’s opinions in the JRH. In short, when purporting to respond to Dr Bayard’s opinions about whether glyphosate is carcinogenic to mammals (Question 3(a)), he in fact addressed the wrong question (Question 4) which related to the specific issue of timing. It is said that Dr Juberg missed the substantial body of Dr Bayard’s analysis of the bioassays concerning carcinogenicity to mammals (in response to Question 3(a)). Further, it is contended that Dr Juberg repeated aspects of his evidence from the Second Juberg Report in the JRH in response to Dr Bayard’s answers to Questions 3(b) and (c) (at JRH [128]–[135]).
452 Thirdly, and relatedly, to the extent that Dr Juberg provided opinions based upon his review of the animal bioassays, in contrast to Dr Bayard’s analysis, Dr Juberg did not undertake any statistical analysis himself (T1781.3–14). He relied on the statistical analysis and conclusions reached in the study reports, and therefore relied on the decisions and judgments made by the study authors in undertaking their analysis and in reaching those conclusions (T1781.7–9; T1793.32–1795.8). Those decisions and judgments include information about what statistical test was used, what threshold was used for statistical significance, and what data was used in the statistical analysis (such as whether males and females were analysed separately or were combined) (T1793.35–1794.25). Mr McNickle contends that as a result, Dr Juberg’s opinions on the animal bioassays must be seen through the prism of an expert whose opinions are premised on the recitation of another expert’s analysis.
453 Before turning to my findings, it is worth pausing to say something about Dr Juberg’s evidence in the animal studies stream.
454 Naturally enough, Mr McNickle’s submissions as to the animal bioassays are replete with criticisms of Dr Juberg’s evidence which, broadly speaking, reiterate the points made above, and can otherwise be grouped into two categories:
(1) that Dr Juberg made criticisms of Dr Bayard’s findings in circumstances where he did not perform the statistical analyses himself (at AS [398], [404], [428], [431], [436], [442], [444], [449], [452]); and
(2) that Dr Juberg did not review or have regard to particular reports or matters relied upon by Dr Bayard (at AS [412], [421], [423]).
455 I have had regard to all these submissions in evaluating the animal bioassays evidence and in informing my conclusion as to Dr Juberg’s credit. But it is important at this juncture to reinforce a point I made earlier in these reasons (at [32]–[33]).
456 Putting to one side Dr Juberg’s credit, even if I was satisfied that there was some force in Mr McNickle’s criticisms, it would not perforce follow that Dr Bayard’s evidence would, as a consequence, necessarily become more persuasive. As will become clear when turning to the animal studies evidence, I have formed the view that there are not insignificant limitations associated with Dr Bayard’s findings. For that reason, this makes it strictly unnecessary to address Mr McNickle’s criticisms above.
457 To the extent that it matters, however, and for the purpose of informing my conclusion as to Dr Juberg’s credit, I do not find Mr McNickle’s submissions persuasive, for the following reasons.
458 First, as to Dr Juberg’s references to findings purportedly made by the OECD, those initial references in the JRH were corrected in a letter to Mr McNickle’s solicitors (Maurice Blackburn) dated 30 September 2023, which was tendered in the proceeding. As was acknowledged in cross-examination (T1894.39–1895.6):
MS SZYDZIK: And so I think this is now probably clear from the corrections table that has been provided to us. But in a number of instances – actually almost entirely, but there are some exceptions, where you rely upon the 2015 RAR, what you are including in your table is a statement that was made by the GTF Glyphosate Taskforce?
DR JUBERG: Yes. We tried – again, we tried to amend and provide more clarity as to what my summary was, certainly what the notifier’s conclusions are, and then if there was an RMS comment. I would agree with you.
MS SZYDZIK: Yes. But to the extent that you are – you are identifying matters that have been included in that unitalicised text, that is almost verbatim what, according to this document, the RAR, what the Glyphosate Taskforce itself put into its submission?
DR JUBERG: Yes. I think that’s reflected in this revised table there.
459 Further, with respect to the US 9th Circuit Court decision, it is not unusual that Dr Juberg, as a toxicologist, was not particularly focussed on those findings. The USEPA has made it clear that although its Interim Registration Review Decision for Glyphosate has been withdrawn, this does not mean that the decision (or its underlying findings) are incorrect as a matter of science. As noted in the USEPA Memorandum “Withdrawal of the Glyphosate Interim Registration Review Decision” (September 2022) (at 5):
Although the glyphosate ID is now vacated in part and the remainder withdrawn, that does not automatically mean that EPA’s underlying scientific findings regarding glyphosate, including its finding that glyphosate is not likely to be carcinogenic to humans, are either incorrect or cannot be used as support for a future decision following reconsideration in accordance with the court’s decision.
460 Secondly, I do not consider that Dr Juberg failed in any fundamental respect to respond to Dr Bayard’s opinions in the JRH. I accept Monsanto’s submission that Dr Juberg’s evidence on the animal studies placed Dr Bayard’s evidence in its biological context, rather than engaging with Dr Bayard’s statistical analysis at large. As Dr Juberg explained in the Second Juberg Report (at 19):
In his expert report, Dr. Bayard focuses on statistical analyses, at the exclusion of multiple other biological factors which must be considered in an evaluation of carcinogenic potential for a compound, and then relies on the Wang et al (2019) study as the basis for his conclusions on the carcinogenicity of glyphosate, both in animals and in humans.
461 Thirdly, in my view Dr Juberg did not jeopardise his independence by relying upon the statistical analysis of other experts in the study reports. As he explained during cross-examination “[I went] to the pathology in all eight bioassays and look for what I referred to this morning as a star or an asterisk indicating that that would be statistically significant” (T1781.7–9). In any event, insofar as Dr Juberg did rely upon such analyses, like Dr Bayard, provided his reasoning was transparent, he was entitled to do so for the reasons I have explained earlier with reference to Karpik v Carnival (see above (at [62]))
462 Overall, my impression is that Dr Juberg did his best to bring an independent mind to the initial trial. It will, however, be necessary to say something further with regards to Dr Juberg’s evidence in the third stream.
463 Professor Miles Prince AM is a professional haematologist and holds a bachelor’s degree in medicine and a bachelor’s degree in surgery from Monash University. Professor Prince is a full Professor of Medicine at both the University of Melbourne and Monash University. He was engaged by Monsanto.
464 Previously, Professor Prince was head of haematology at the Peter MacCallum Cancer Centre in Melbourne. In 2016, he was appointed the Professor/Director of Molecular Oncology and Cancer Immunology at Epworth Healthcare; a joint appointment between Epworth Healthcare and the University of Melbourne overseeing the clinical practice, research and teaching conducted at the Molecular Oncology and Cancer Immunology Department at Epworth Healthcare. In the same year, Professor Prince founded Precision Haematology. There, he is the lead clinician with two other clinical haematologists, a specialist haematology nurse, a haematology and oncology dietitian and an oncology psychologist.
465 As noted above, Professor Prince participated in the JRI and concurrent evidence session, in which he concluded, in summary, that the field of animal studies are not robust enough to determine if the use of or exposure to glyphosate and/or GBFs can cause NHL.
466 Mr McNickle made extensive submissions as to why the Court should have significant concerns about Professor Prince’s independence. These submissions, however, are largely made in the context of Professor Prince’s evidence in the JRA and JRB concurrent sessions and, for that reason, I do not intend to address them here. Subject to some matters to which I will return, it suffices to note for the purposes of my approach to Professor Prince’s evidence in the animal studies stream and the JRI that I consider these concerns to be somewhat overstated and, broadly speaking, I have formed the view that Professor Prince was an impressive witness.
467 I will return to the topic of Professor Prince’s credit in Section G.2 below.
Dr Crump
468 I have already introduced Dr Crump as a witness above in the context of the epidemiology stream (at [162]) and explained then why little can be gained by way of an assessment Dr Crump’s credibility. Less can be gained from the concurrent evidence session in Conclave J because, as I have explained, that session did not proceed as a result of Monsanto’s decision not to call Dr Crump or rely upon evidence in respect of the questions asked in Conclave J, nor the parts of Dr Crump’s report relevant to the conclave.
469 As a result of Monsanto’s decision not to call Dr Crump in Conclave J, Mr McNickle submits that the Court should draw a Jones v Dunkel inference. Mr McNickle contends that two consequences flow from that decision: first, that the Court should draw an inference that Dr Crump’s evidence on multiple comparisons would not have assisted Monsanto; and secondly, that the Court should more readily accept Dr Bayard’s evidence. I will address this submission below in the context of Mr McNickle’s submissions as to the issue of multiple comparisons.
F.3 The Animal Studies Evidence
470 Mr McNickle contends that the evidence establishes that glyphosate more likely than not causes cancer in mice and in rats, and that the results of the animal bioassays provide important evidence as to glyphosate’s carcinogenicity to humans. This contention is founded upon Dr Bayard’s evidence and conclusion (at JRH [24]) that:
… based on my analysis and evaluation of 13 lifetime glyphosate cancer bioassays in both sexes of rats and mice, I conclude that glyphosate is carcinogenic in mammals. Since glyphosate is the active and predominant ingredient in GBF’s, I conclude that GBF’s are also carcinogenic in mammals. This reasoning follows the guidance of the USEPA (EPA, 1986, 2000) on chemical mixtures.
471 In evaluating the animal studies evidence, it is necessary to address the 13 bioassays (animal bioassays) relied upon by Dr Bayard, which consist of seven studies of glyphosate oral administration in rats, and six in mice. The animal bioassays are set out in the table below:
No. | Study | Species |
1 | Lankas G P “A Lifetime Study of Glyphosate in Rats (1981) Report No. 77-2062 (Bio/Dynamics, Inc.) (Lankas (1981)) | Rat |
2 | Knezevich A L and Hogan G K (1983) “A Chronic Feeding Study of Glyphosate in Mice” Report No. 77-2061 (Bio/Dynamics, Inc.) (Knezevich and Hogan (1983))
| Mouse |
3 | Stout L D and Ruecker P A (1990) “Chronic Study of Glyphosate Administered in Feed to Albino Rats” MRID No. 41643801 (Stout and Ruecker (1990))
| Rat |
4 | Atkinson C, Strutt A, Henderson W et al. (1993a) “104-week Chronic Feeding/Oncogenicity Study in Rats with 52-week Interim Kill” MRID No. 49631701 (Atkinson (1993a)) | Rat |
5 | Atkinson C, Martin T, Hudson P, and Robb D (1993b) “Glyphosate: 104-week Dietary Carcinogenicity Study in Mice” Inversek Research International (Tranent, EH33 2NE, Scotland) (Atkinson (1993b)) | Mouse |
6 | Suresh T P, “Combined Chronic Toxicity and Carcinogenicity Study with Glyphosate Technical in Wistar Rats” (1996) Toxicological Department Rallis Research Centre (Rallis India Limited, Syngenta) (Suresh (1996)) | Rat |
7 | Sugimoto K, “18-month Oral Oncogenicity Study in Mice, Vol. 1 and 2” (1997) Institute of Environmental Toxicology 2-772 (Suzuki-cho, Kodaria-shi, Tokyo, 187, Japan) (Sugimoto (1997)) | Mouse |
8 | Enemoto K “24-month Oral Chronic Toxicity and Oncogenicity Study in Rats, Vol. 1” Institute of Environmental Toxicology (Kodaria-shi, Tokyo, Japan) (Enemoto (1997)) | Rat |
9 | Takahashi M “Oral Feeding Carcinogenicity Study in Mice with AK-01” (1999) Nippon Experimental Medical Research Institute Co. Ltd (Agatsuma, Gunma, Japan) (Takahashi (1999)) | Mouse |
10 | Kumar D P S “Carcinogenicity Study with Glyphosate Technical in Swiss Albino Mice” (2001) Toxicology Department Rallis Research Centre (Rallis India Limited) (Kumar (2001)) | Mouse |
11 | Brammer A “Glyphosate Acid: Two Year Dietary Toxicity and Oncogenicity Study in Rats” (2001) Central Toxicology Laboratory (Alderley Park, Macclesfield, Cheshire, UK, Syngenta) (Brammer (2001)) | Rat |
12 | Wood E, Dunster J, Watson P and Brooks P (2009) “Glyphosate Technical: Dietary Combined Chronic Toxicity/Carcinogenicity Study in the Rat” Harlan Laboratories Limited (Shardlow, Derbyshire DE72 2GD, UK) (Wood (2009a)) | Rat |
13 | Wood E, Dunster J, Watson P and Brooks P “Glyphosate Technical: Dietary Carcinogenicity Study in the Mouse” (2009) Harlan Laboratories Limited (Shardlow, Derbyshire DE72 2GD, UK) (Wood (2009b)) | Mouse |
472 In addition to the animal bioassays, Dr Bayard also relied upon a mechanistic study of MM in mice, namely Wang (2019).
473 As noted earlier, the thrust of Monsanto’s submissions in the animal studies stream consisted of an attack upon Dr Bayard’s alleged flawed statistical approach and limited expertise to conduct a multi-disciplinary critique of the animal bioassays. It is convenient to address those submissions first, before turning to the animal bioassays; Dr Bayard’s reliance on Wang (2019); and the relevance of the animal studies evidence to humans in Conclave I.
474 Accordingly, this section will adopt the following structure:
Section I will address Dr Bayard’s alleged flawed statistical approach;
Section II will address Dr Bayard’s alleged limited expertise in conducting multi-disciplinary animal bioassay studies;
Section III will set out my findings as to the animal bioassays;
Section IV will address Dr Bayard’s reliance on Wang (2019);
Section V will address the relevance of animal studies to humans in Conclave I;
Section VI will set out my conclusion as to the animal studies stream.
I Dr Bayard’s alleged flawed statistical approach
475 Monsanto contends that Dr Bayard’s approach to the animal bioassays was flawed in two fundamental respects: first, that he failed to account for multiple comparisons; and secondly, that he engaged in a pooling exercise which undermined the integrity of the bioassay process.
476 I will deal with each in turn.
477 The multiple comparisons problem arises when a set of statistical inferences are considered simultaneously. The more inferences that are made, the more likely it is that erroneous inferences are drawn.
478 A good illustration of the multiple comparisons problem comes from calculating the family-wise error rate (FWER). The FWER is the probability of drawing at least one false-positive in a family of hypotheses tests. It can be calculated using the following formula:
1 – (1 – p-value) number of tests
479 As I have explained above (at [124]), the p-value represents the probability that a finding was due to chance and is conventionally set at p=0.05 (that is, an assumption that there is a 5% chance of rejecting the null hypothesis when it is in fact true). If a single outcome is tested at a p-value of 0.05, then the assumption that there is a 5% chance of drawing a false-positive inference is true. However, where multiple outcomes are tested, the more likely it is that a false-positive result is drawn. This can be illustrated by increasing the number of tests in the FWER formula:
Test | Formula | FWER |
1 | 1 – (1 – 0.05)1 | 5% |
2 | 1 – (1 – 0.05)2 | 10% |
3 | 1 – (1 – 0.05)3 | 14% |
4 | 1 – (1 – 0.05)4 | 19% |
5 | 1 – (1 – 0.05)5 | 23% |
480 As seen above, when conducting five tests at a p-value of 0.05, there is a 23% chance of drawing a false-positive result. For 100 tests conducted at p=0.05, the probability of drawing at least one false-positive is approximately 99.4%. In order to correct for the inflation of false-positives, methods such as the Bonferroni or the Benjamini-Hochberg method are often used to adjust the p-value to account for the false-positive error rate (T1766.24–27; T1768.1–12).
481 As would already be obvious, the issue of multiple comparisons is well-known in the field of animal studies given the necessity to evaluate large animal bioassay datasets. As the USEPA Guidelines state (at 2–20):
Considerations of multiple comparisons should also be taken into account. Haseman (1983) analyzed typical animal bioassays that tested both sexes of two species and concluded that, because of multiple comparisons, a single tumor increase for a species-sex-site combination that is statistically significant at the 1% level for common tumors or 5% for rare tumors corresponds to a 7–8% significance level for the study as a whole. Therefore, animal bioassays presenting only one significant result that falls short of the 1% level for a common tumor should be treated with caution.
482 Mr McNickle submits that Dr Bayard, consistently with the USEPA Guidelines, took into account the problem of multiple comparisons in concluding that glyphosate more likely than not causes cancer in rats and mice, and that the results of the animal bioassays provide important evidence as to glyphosate’s carcinogenicity to humans (T1633.32).
483 These submissions may be summarised as follows.
484 First, as Dr Bayard explained, multiple comparisons should be considered by evaluating consistency, reproducibility and other weight of evidence factors. Dr Bayard did so by treating a statistically significant result as a “signal” to look for consistencies and inconsistencies for that tumour response in other studies (at JRJ [125]; JRH [32]; First Bayard Report (at [46]–[47]); T1643.33–38). For any one tumour site in a study, Dr Bayard conducted his statistical tests and, depending on the outcome, the response was either statistically significant or not. Dr Bayard explained his method in the First Bayard Report (at [47]) as follows:
For each study the probability is 1 in 20 that a P<0.05 could happen by chance. Repeating the same process 5 times, once for each study, would result in one P-value <0.05 about 20% of the time, even if there is no true response. Thus, concluding a positive carcinogenic response for a chemical solely on one positive cancer response study out of five would result in declaring too many false positives. However, the probability of 2 chance positive (false positive) responses in the same tumor site from those 5 studies is only slightly more than 2%. So, if I see 2 positive response studies, and three negative studies, in my professional judgement I would initially call that a positive tumor response for that species/strain/sex. I also need to consider the magnitude of the P-value. A highly statistically significant P-value, say P<0.01, has more weight than one that is just statistically significant at P<0.05.
485 Dr Bayard’s evidence was that this was method was a statistical method (T1772.37).
486 Secondly, it is said that there is nothing in the USEPA Guidelines which mandates how multiple comparisons are to be accounted for, nor do the guidelines require any statistical adjustment to the p-value. Indeed, the method for accounting for multiple comparisons described in the guidelines (that “animal bioassays presenting only one significant result that falls short of the 1% level for a common [tumour] should be treated with caution”) does not involve any adjustment to p-value results.
487 Thirdly, and relatedly, Mr McNickle submits that the aide-memoire (and the documents summarised therein) relied upon by Monsanto (MFI-46A, which excludes reference to documents not tendered which had been previously included in MFI-46 (T2248.37–2249.24)) in support of the proposition that statistical adjustment for multiple comparisons is a well-known issue in the conduct and evaluation of animal cancer bioassay reports should be disregarded by the Court. In short, it is said that first, the documents referred to in MFI-46A do not support that proposition; and secondly, that those documents provide no evidentiary foundation which contradicts Dr Bayard’s methodology or its appropriateness.
488 Fourthly, it is said that Monsanto mischaracterised Dr Bayard’s evidence about the ways in which multiple comparisons can be taken into account. Contrary to Monsanto’s submissions, Dr Bayard did not identify two statistical methods for considering multiple comparisons (the Bonferroni method and the Benjamini-Hochberg correction method) but noted that there are various ways to address the problem of multiple comparisons (T1720.27–41; 1772.31–33).
489 Fifthly, as I noted earlier (at [469]), in the absence of calling Dr Crump to give evidence in Conclave J, Mr McNickle submits that the Court should draw a Jones v Dunkel inference that Dr Crump’s evidence concerning multiple comparisons would not have assisted Monsanto’s case, and that the Court should more readily accept Dr Bayard’s evidence that he did account for the problem of multiple comparisons.
490 When the matter returned for hearing in late January 2024, it became evident that the real point of departure between the parties as to this issue was whether it was necessary for Dr Bayard to have made a statistical adjustment for multiple comparisons, rather than having to account for the issue (in the words of the USEPA Guidelines) (see above (at [481]); T2147.41–2149.43). As Ms Szydzik explained (T2149.32–40):
MS SZYDZIK: So, in our submission, the caution that the US EPA guidelines that you need to approach certain results with in order to account for multiple comparisons when analysing the animal bioassays is exactly what Dr Bayard did, and he did that by looking for consistency, reproducibility and other weight of evidence factors, and that was his evidence. His evidence also was that his approach was the common approach in risk assessment. Now, as I’ve already said, the guidelines don’t say that a statistical adjustment is required, Dr Bayard did not say that a statistical adjustment was required, and the respondents have led no evidence to say that statistical adjustment was required. …
491 While I recognise there is some force in the proposition that the necessity to account for the problem of multiple comparisons is a broader concept than the necessity to make a statistical adjustment or correction, when one has regard to the USEPA Guidelines and the evidence as a whole, a laser-like focus on use of the word “account” distracts from the underlying rationale for the existence of recognised methods which address the issue of multiple comparisons.
492 As I explained above (at [478]–[480]), the FWER can be used to illustrate the inflation of false-positives when conducting multiple hypotheses tests. Plainly enough, the inflation of the false-positive error rate is a statistical problem, and properly accounting for that problem necessitates a corrective method which admits of a statistical answer. As the authors of Haseman (1983) (the report referred to in the USEPA Guidelines when discussing the necessity to account for multiple comparisons) note (at 336):
It should be emphasized that no rigid statistical decision rule is currently being employed by the NTP in the evaluation of tumor incidence data. Moreover, it is not the purpose of this paper to derive and advocate the use of such a procedure. Nevertheless, to obtain meaningful estimates of false-positive rates, one should base calculations on a statistical procedure whose “statistical positives” correlate highly with tumor increases that are regarded as “biological positives.”
(Emphasis added)
493 There are several recognised statistical procedures in the literature for correcting the false-positive rate; two of which Dr Bayard recognised (T1768.1; T1772.23–33) (and to which I have already referred (at [480], [488])), being the Bonferroni method and the Benjamini-Hochberg method. The latter was used in the USEPA’s “Revised Glyphosate Issue Paper: Evaluation of Carcinogenic Potential” (December, 2017) (USEPA Issue Paper), where it was noted (at 71):
… the 2005 EPA Guidelines for Carcinogen Risk Assessment state that “considerations of multiple comparisons should also be taken into account”. Multiple comparison methods control the familywise error rate, such that the probability of Type I error (incorrect rejection of the null hypothesis or “false positive”) for the pairwise comparisons in the family does not exceed the alpha [p-value] level. In the current evaluation, the Benjamini-Hochberg correction method was used to adjust for multiple comparisons (Benjamini and Hochberg, 1995).
(Emphasis added)
494 It follows, having regard to the USEPA Guidelines and its broader context, that in order to account properly for multiple comparisons, it is necessary to undertake a statistical procedure which is capable of correcting outcomes for false positives. Dr Bayard, in my view, did not undertake such a procedure and, accordingly, I am not persuaded that Dr Bayard appropriately accounted for the issue of multiple comparisons. Two observations should be made in this regard.
495 First, Dr Bayard’s method, in summary, involved excluding tumours from his analysis that did not have at least one statistically significant value (T1772.31–1773.2). Despite his claim that a similar method was employed in Portier (2020) (T1773.4–26), it did not involve the application of an established or recognised procedure for correcting outcomes for multiple comparisons (such as the Bonferroni or Benjamini-Hochberg method). During cross-examination, I asked whether this approach was reflected anywhere else in the scientific literature (T1773.4–26):
HIS HONOUR: Other than Portier, which you’ve mentioned now on a number of occasions, I’m asking for anyone else other than that – other than that gentleman?
DR BAYARD: I probably do. But it’s just kind of common type of analyses that you’re doing. That people who do risk assessment the way I do, they look by tumour types and they figure out - - -
HIS HONOUR: I’m not asking you to explain it. I’m not asking you to explain the method.
DR BAYARD: Sorry.
HIS HONOUR: You’ve been asked now several times and I would be very grateful if you would assist me with a direct responsive answer: is it referred to anywhere in the literature?
DR BAYARD: I haven’t seen this type exactly done in any way.
496 Secondly, whether the method undertaken by Dr Bayard can be classified as a “statistical method”, for the reasons above, I do not think much assistance can be derived from it. The process of excluding tumours from the analysis which do not have at least one statistically significant result does not admit of a statistical outcome which can provide information as to: (1) whether that outcome is a false positive; and (2) the probability of obtaining a false positive result. Put another way, Dr Bayard’s method does not meaningfully assist in estimating false positive rates because it does not involve any adjustment to the p-value which accounts for the problem of multiple comparisons.
497 This conclusion has caused me to view Dr Bayard’s calculations in the animal studies stream with real caution. With that said, in the light of my overall conclusion as to this stream (and for reasons I will come to when assessing the animal studies evidence itself), my overall conclusion would not change even if I was wrong to have found that Dr Bayard did not account properly for the problem of multiple comparisons.
498 Before leaving this topic, however, it is necessary to say something about Jones v Dunkel.
499 During the initial trial, there was some back and forth about the precise reason Monsanto elected not to call Dr Crump to give evidence in Conclave J. From my vantage point, I assumed the reason was obvious and related to the evident personal difficulties he had in giving evidence, but Monsanto was at pains to disabuse me of what they contended was my misapprehension. Indeed, on the final day of the trial, senior counsel for Monsanto, Mr Craig KC, having disavowed any other reason, identified the reason for not calling Dr Crump as follows (T2218.24; referring to T1947.12–21):
MR CRAIG: … Having regard to the evidence adduced in conclave H, regarding the tumours and studies relied upon by Dr Bayard, and Dr Bayard’s evidence on pooling and multiple comparisons, I don’t need to and I’m not going to call Professor Crump in conclave J.
500 I found Monsanto’s approach passing strange. Foundational to the consideration as to whether an adverse inference should be drawn is whether there is a reasonable excuse for the failure to call a witness whose evidence would be expected to be adduced by a party. Given the difficulties I had seen unfold before me, a reasonable excuse was obvious. However, in the light of the fact that Dr Crump’s earlier difficulties in giving evidence at the trial were expressly disavowed as the reason why he was not called, it seems to me that I must put what I assumed was the reasonable reason to one side.
501 It was open for Monsanto to take a robust view that they did not call Dr Crump in Conclave J because they considered that Dr Bayard concessions adverse to Mr McNickle’s case. But this seems to me simplistic: we are dealing with a complex scientific inquiry where there is a degree of prima facie cogency in arguments being put by competing experts. To decide not to call an expert who has been subject of a joint conclave, and has put before the Court apparently reasoned opinions, is no small thing. I think, in all the circumstances, it would be appropriate to conclude the forensic decision by Monsanto was made because a considered view was taken it was in the interests of Monsanto that he not enter the witness box again on the basis that his evidence would not assist its case. An adverse inference should be drawn.
502 I hasten to add, however, that the drawing of a Jones v Dunkel inference cannot be used to fill gaps in the evidence. In all the circumstances, it is of limited utility to Mr McNickle’s case in circumstances where Dr Crump’s evidence on multiple comparisons could not have made up for deficiencies in Dr Bayard’s approach to the animal bioassays.
503 Dr Bayard’s analysis in the JRH included results which pooled data from the animal bioassays (see, for example, JRH [52]–[53]; Table 4; First Bayard Report (at [102])). As I noted in the first stream (at [122]–[123]), pooled studies can increase statistical precision, provided the studies included in the pool have the same or similar study design. As explained in Gart J, Chu K, Tarone R “Statistical Issues in Interpretation of Chronic Bioassay Tests for Carcinogenicity” (1979) 62(4) National Cancer Institute 957 (at 963):
Usually, the control incidences are limited to the laboratory where the compound is tested. If a formal significance test is to be made involving such a “pooled laboratory” control group, it is usually required that the pathologic examination for the control groups and the treated groups be done by, or at least be reviewed in all instances by, the same pathologist. Other aspects of the protocol should be the same or similar for all the control groups to be pooled. These aspects include the length of time on study, the supplier, and the mean birth dates for the animals. Statistical tests of homogeneity among the individual control groups that are candidates for pooling should be done for both the survival curves and the [tumour] incidences.
504 I have not derived significant assistance from Dr Bayard’s pooled analyses, for the following reasons.
505 Dr Bayard accepted that there were a variety of differences between the animal bioassays including that they: (1) operated for different durations; (2) operated across different laboratories; (3) involved different generations of CD-1 mice; (4) had different reviewing pathologists; and (4) involved such different doses that a mouse in the high-dose group in one study could in fact be a mouse in the mid-dose or low-dose group in another study (T1722.45–1723.41). For these reasons, Dr Bayard conceded that he did not place weight on his pooled analyses in arriving at his conclusion that glyphosate more likely than not causes cancer in rats and mice. As Dr Bayard said in cross-examination (T1700.10–45):
DR BAYARD: Yes. But I don’t put – I talked about it in my – in my report that I don’t put much weight into pooling because not much has been written on it and because of what it does to the control groups.
MR CRAIG: All right. Let’s talk about pooling then. When you were preparing your report, you didn’t make any evaluation in your report for whether or not the mice in the various studies were fed the same or a similar diet?
DR BAYARD: No, I did not factor diet in.
MR CRAIG: And you did not do that for the rat studies either?
DR BAYARD: No. I basically assumed that the controls were fed the same diet and so the comparison should be against treated versus controlled.
MR CRAIG: But when you’re pooling you’re comparing animals from two different studies, aren’t you?
DR BAYARD: You’re absolutely right. That’s why I put no weight on it.
MR CRAIG: His Honour should put no weight on pooled studies, should he?
DR BAYARD: You can try and see what happens. But I agree that – the problem is that you’re really messing up your control groups because they – they just don’t have much to compare against. You’re pooling controls groups and that’s – that – that really contaminates the study.
506 The following was later put to Dr Bayard in cross-examination (T1724.1–10):
MR CRAIG: Yes. Thank you. Now – and you accept that pooling studies across laboratories, generations, different pathologists and different operating time periods is a highly unusual procedure, don’t you?
DR BAYARD: You mean for statistical purposes, the way I did it?
MR CRAIG: Yes.
DR BAYARD: Yes. I – again I didn’t put much weight on it, I just did the analysis so I put it in.
507 For these reasons, I do not consider much can be gained from Dr Bayard’s pooled analyses in informing my ultimate conclusion in the animal stream.
II Dr Bayard’s alleged limited expertise
508 Monsanto submits that Dr Bayard lacked expertise to conduct a critique of the multi-disciplinary animal bioassays in two respects. The first is that Dr Bayard, who is not a pathologist, was not qualified to challenge the conclusions of the pathologists who conducted the animal bioassays. The second is that Dr Bayard could not give reliable evidence as to how a particular dose exposure to glyphosate in animals translates to human exposures because he is not a toxicologist.
509 As it emerged when the matter returned in late January, the high watermark of this submission (and where Dr Bayard’s alleged lack of expertise in pathology and toxicology converged) concerned the distinction between whether Dr Bayard, in forming his opinion as to the animal bioassays, was engaged in an exercise of merely interpreting the results of the animal bioassays, or whether (as Monsanto contends) that exercise involved an assessment of the biological significance (that is, whether the outcome has an effect on the health or survival of a living organism) of a statistically significant result (T2200.13–30). This is important because as Dr Bayard accepted, a “major role” of the pathologist and toxicologist in undertaking an animal cancer bioassay is to assess the biological significance of the findings in the study (a proposition with which Dr Juberg agreed) (T1765.31–46).
510 To the extent that it matters, my impression of Dr Bayard’s evidence is that he did occasionally stray outside of his area of expertise and gave firm scientific opinions on topics in which he lacked the requisite qualifications, including the biological significance of statistically significant results in the animal bioassays. This is not to be unduly critical of Dr Bayard: as I said earlier (at [444]), I do not consider that he made any deliberate attempt to obscure the evidence. Most scientists, after all, are not lawyers, and do not approach the task of giving evidence (particularly oral evidence) conscious at all times that their words will be poured over by teams of solicitors and barristers.
511 With that said, it will suffice here to set out one example of where I consider Dr Bayard gave evidence beyond his field of expertise. I will return to other instances when I come to the animal bioassays and Dr Bayard’s evidence given in relation to Wang (2019) in Section IV below.
512 Dr Bayard in the JRH (at [57]; Table 5) relied upon figures from Knezevich and Hogan (1983) which had been calculated following a re-examination by a pathology working group (PWG) (T1714.41–1716.4). The PWG reached the opposite conclusion that Dr Bayard had as to causation and whether the tumours the subject of the study were caused by glyphosate, noting (at 24–25):
This PWG firmly believes and unanimously concurs with the original pathologist and reviewing pathologist that the incidences of renal tubular-cell neoplasms in this study are not compound related.
The following points were taken into consideration in reaching this decision:
a) Renal tubular-cell tumors are spontaneous lesions for which there is a paucity of historical control data for this mouse stock. However, clustering can occur and the incidence in this study is comparable to the available historical control range from several laboratories (Appendix B). Since there were 3 treated groups and only 1 control group, there is a greater possibility of more variation from mean control incidences in the treated mice.
b) None of the treatment groups differed from the controls by the Fisher exact test at the 0.05 level of significance. Over all groups there was not evidence of a significant linear trend at the 0.05 level by a one-tailed CochranArmitage Test.
c) Multiple renal tumors were not found in any animal.
d) Compound related nephrotoxic lesions, including preneoplastic changes, were not present in this study. In addition, renal toxicity was not noted in the 3-month subchronic toxicity study reported in December 1979.
(Underlining in original)
513 During cross-examination, however, Dr Bayard first sought to challenge the PWG’s conclusion that renal tubular cell tumours are spontaneous lesions (T1717.26–39):
HIS HONOUR: No. So you disagree with the statement of fact that renal tubular cell tumours are spontaneous lesions?
DR BAYARD: From what I’ve seen of data I do disagree with that. They can also be compound related.
HIS HONOUR: I just wanted to understand your evidence. Thank you.
DR BAYARD: Thank you.
MR CRAIG: You’re not an expert of whether renal cell tumours are spontaneous lesions, are you, Dr Bayard.
DR BAYARD: I am not an expert on that, that’s correct.
514 Dr Bayard then went on to give the following evidence (T1718.1–9):
MR CRAIG: And so these views of the reviewing pathologists are important qualifications to any conclusion that these tumours were caused by glyphosate, aren’t they?
DR BAYARD: There’s certainly opinions.
MR CRAIG: Yes. Held by those who have expertise that you do not have?
DR BAYARD: That’s correct
515 Dr Juberg also gave evidence that focus should be placed on the conclusions of the PWG (T1800.1–7).
516 In the light of this evidence, I do not think that Dr Bayard was in a position to substitute his opinion with the findings of the PWG and the reviewing pathologist in relying upon the figures in Knezevich and Hogan (1983) and, accordingly, I do not think much assistance can be derived from it. As Mr Craig submitted, an assessment of the biological significance of the tumours the subject of the animal bioassays cannot be divorced from the expertise that is brought to bear by those pathologists who have actually inspected and evaluated the tumours. Consistently with my approach to the expert evidence as a whole, a proper assessment of the totality of the evidence necessitates engaging with the statistical and pathological conclusions, and the overall coherence of the animal bioassays, to which I will now turn.
517 It is well to note from the outset that in the light of my findings above, I have approached the task of evaluating aspects of Dr Bayard’s evidence in relation to the animal bioassays through a somewhat different prism. I use the term aspects advisedly because those specific findings do not constitute a broader finding that the Court, when having regard to the whole of the evidence, should approach the relevant task cautious of placing any weight upon Dr Bayard’s conclusions in the animal studies stream as if his credit has been seriously impugned (notwithstanding that in certain respects, it is necessary to be cautious about aspects of his evidence).
518 Where relevant, and in order to aid comprehension, I have explained in the following section how those specific findings bear upon my conclusions with respect to each of the specific tumours Dr Bayard contends were caused by exposure to glyphosate. Further, I should note that several of Dr Bayard’s calculations in Conclave H were the subject of corrections, which were set out in a schedule provided to the Court and tendered (which document I will refer to as the Bayard Corrections).
519 Dr Bayard gave evidence that glyphosate induces four types of tumours in mice, namely: (1) malignant lymphomas; (2) hemangiomas and hemangiocarcinomas; (3) kidney tumours; and (4) lung adenocarcinomas.
520 I will address each in turn.
521 Mr McNickle submits that based on Dr Bayard’s findings, the Court ought be satisfied that glyphosate causes malignant lymphomas in two strains of mice and in both sexes (First Bayard Report (at [90]; JRH, Table 2); at JRH [37]–[43]). Alternatively, it is contended that glyphosate causes malignant lymphoma in both sexes of CD-1 mice (at JRJ [37]–[38]). I have reproduced Table 2 below:
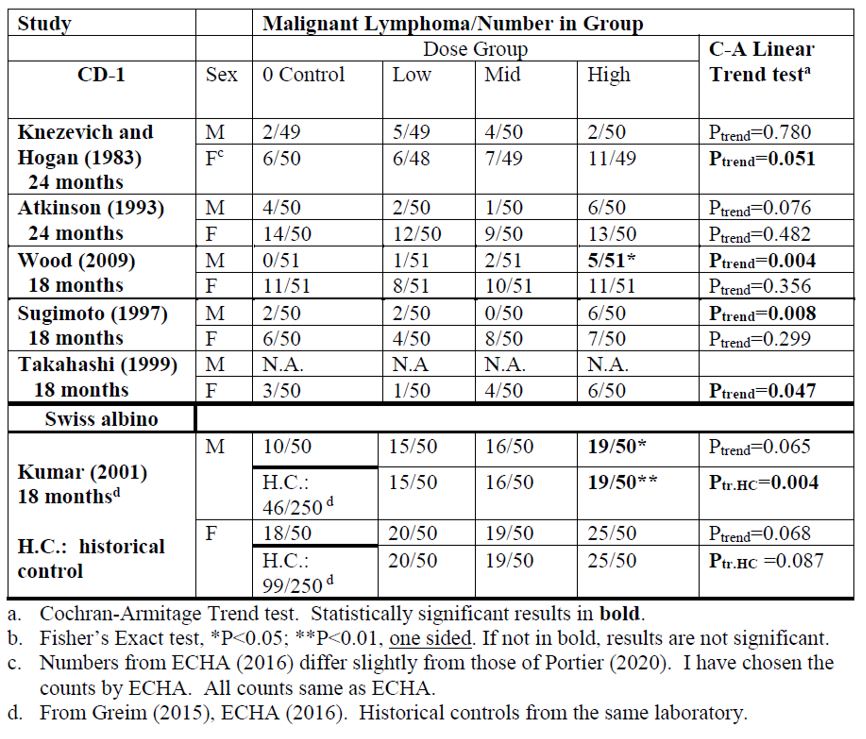
522 As can be seen, Dr Bayard relied on six studies in support of these contentions, namely Knezevich and Hogan (1983); Atkinson (1993); Wood (2009b); Sugimoto (1997) and Takahashi (1999) (as reported in the Joint Meeting on Pesticide Residue (2016) (JMPR)); and Kumar (2001).
523 Mr McNickle’s submissions may be summarised as follows.
524 First, Dr Bayard found “highly statistically significant” linear trends in male CD-1 mice using the Cochran-Armitage (C-A) test (Ptrend=0.004 in Wood (2009b); Ptrend=0.018 in Sugimoto (1997)) (see Bayard Corrections), whereas Atkinson (1993) indicated “near statistical significance” (Ptrend=0.076) (at JRH [37]). Dr Bayard gave evidence that a highly significant p-value of less than 0.01 bears more weight than one that is only statistically significant (First Bayard Report (at [23], [24], [47])). He concluded (at JRH [37]):
… the probability those two highly statistically significant studies [Wood (2009b) and Sugimoto (1997)] (i.e. those two studies being false positives at the P<0.01 level), out of four studies being false positives is less than 1 in 1,000. With such a small probability of two false positives out of four studies, I conclude that glyphosate causes malignant lymphomas in CD-1 male mice.
525 Secondly, in relation to female CD-1 mice, Dr Bayard relied on Knezevich and Hogan (1983) and Takahashi (1999) (Ptrend=0.047) in support of his view that glyphosate likely causes malignant lymphoma in female CD-1 mice. His evidence was that female CD-1 mice were statistically significant for trend (Ptrend=0.051) (at JRH [38]) (later corrected to Ptrend=0.058 in the Bayard Corrections) and noted that the chance of having two or more false positives out of the (total) five studies was “2 in 100”.
526 Thirdly, Dr Bayard contends that Kumar (2001) also strongly supports the cancer findings in relation to CD-1 mice. Kumar (2001) was a study involving Swiss Albino mice, and although it was unique in this regard, Dr Bayard noted that both male and female trend responses were “near statistically significant” (Ptrend=0.068 for males and Ptrend=0.065 for females) and noted the high dose male response (P=0.024). Mr McNickle submits that Dr Bayard only relied on Kumar (2001) to the extent that it supported his conclusions already expressed in relation to CD-1 mice, rather than evidence on a stand-alone basis that could support his contention that glyphosate caused malignant lymphomas in mice.
527 Based on this evidence, Dr Bayard concluded (at JRH [42]) that glyphosate causes malignant lymphomas in CD-1 mice (male and female). He noted that together, “four of the five CD-1 mouse studies showed significant or highly significant trends for either males or females, and the fifth study showed a linear trend that was near statistical significance” (at JRH [42]). He noted further (at JRH [43]):
With all six mouse studies showing increased malignant lymphoma trends, five were statistically or highly statistically significant in at least one sex, I conclude that it is far more likely than not that glyphosate causes malignant lymphomas in two strains of mice and in both sexes.
528 I do not consider that much assistance can be derived from Dr Bayard’s evidence in support of a conclusion that glyphosate causes malignant lymphomas in mice, for the following reasons.
529 First, based on the statement by the authors of Greim (2015) (at 201), malignant lymphoma is the “most common spontaneously occurring tumour category in the mouse”. Indeed, as Dr Bayard accepted, malignant lymphoma is the most common category of tumour in the CD-1 mouse (T1655.23–26) (and noted that it was even more prevalent in Swiss mice (T1653.37–38). In cross-examination, Dr Bayard was shown Giknis (2005), which was an 18-month study of spontaneous neoplastic lesions in CD-1 mice (T1656.25–28) and identified the naturally occurring incidences of particular tumours in Charles River Laboratory CD-1 mice (T1656.30–37). Dr Bayard accepted that in some of the studies reviewed by Giknis (2005), there were very high numbers of naturally occurring malignant lymphomas. As he noted then (T1658.30–44):
MR CRAIG: Thank you. And so in some of – in some of these studies there were as many as 20 malignant lymphomas in a particular study occurring naturally?
DR BAYARD: It was more than that. I mean you had one outlier study had 35 out of 70 animals had malignant lymphomas. I think those were the females, I’m almost positive, and I’ve got that in my tables.
MR CRAIG: Thank you.
DR BAYARD: But that was such an outlier that it could have skewed anything.
MR CRAIG: But the point is that there are studies where the incidence of malignant lymphoma within a cohort of animals, 50 animals, is very, very high, isn’t it?
DR BAYARD: Yes. And there’s some very, very low too.
530 Dr Bayard also accepted that because of how common lymphoma are as “spontaneously” occurring tumours in CD-1 mice, their association with glyphosate needs to be evaluated by reference to the full range of available studies (T1659.1–10).
531 Secondly, the majority of Dr Bayard’s findings in relation to CD-1 mice were not statistically significant. In the JRH and First Bayard Report, Dr Bayard had identified a predetermined test for statistical significance (being P=0.050) (T1663.15–44):
MR CRAIG: So of the five CD-1 mice bioassays, focusing on females, four out of the five studies did not meet your predetermined test for statistical significance, did they?
DR BAYARD: You’re talking only males now?
MR CRAIG: No. Females.
DR BAYARD: Females. One – well, again, you and I would differ from .051 to .058.
MR CRAIG: Well, .058. Dr Bayard, is more than .05?
DR BAYARD: It’s a whopper.
MR CRAIG: Well, Dr Bayard, let’s not laugh about it. You identified a predetermined test for statistical significance and that was .05, wasn’t it?
DR BAYARD: Yes. But I’m not hard and fast on it.
MR CRAIG: Okay. Now, I’m asking questions - - -
DR BAYARD: Go ahead.
MR CRAIG: - - - by reference to the predetermined test for statistical significance and I’m putting to you what should be a fairly clear proposition that four out of the five studies for female CD-1 mice did not meet your predetermined test for statistical significance, did they?
DR BAYARD: They weren’t less than .05, yes.
532 Using Dr Bayard’s predetermined criteria, there were no statistically significant findings for male CD-1 mice in most of the studies considered (Knezevich and Hogan (1983); Atkinson (1993); and Takahashi (1999)). Moreover, for female CD-1 mice, as highlighted in the transcript above, four out of the five studies did not satisfy Dr Bayard’s predetermined test for statistical significance, the exception being Takahashi (1999) (Ptrend=0.047).
533 In relation to Takahashi (1999), that study included data extracted from the JMPR and there was some divergence between the parties as to first, the extent to which its findings could be given weight, given that the experts did not have access to the underlying bioassay report, nor the histopathological data (T1644.22–26); and secondly, whether a reference to “lymphoma” in the JMPR was a reference to “malignant lymphoma” (T1665.3–10). Be that as it may, what did emerge from the evidence was that the top dose received by female mice was the highest of the six studies considered by Dr Bayard (8690 mg / kg / day) (at 153), which Dr Bayard and Dr Juberg accepted as indicating signs of toxicity (T1652.38; T1653.18–24). Dr Bayard’s result was also derived without having corrected for multiple comparisons (see above (at [494]–[497])), whereas the JMPR did not yield a statistically significant result for female CD-1 mice after having corrected for it. For these reasons, and having regard to the whole of the evidence, I do not consider Takahashi (1999) significantly assists in demonstrating a casual connexion between glyphosate and the development of malignant lymphomas in female CD-1 mice.
534 Thirdly, Dr Bayard’s findings in relation to Kumar (2001) are of limited assistance. In cross-examination, Dr Bayard identified the existence of endoparasites in the animals the subject of the study (T1679.44–46). Although he could not give an answer as to whether the existence of endoparasites in a cohort of mice affected the reliability of a study into the oncogenic effects of a substance on mice (T1680.4–13), Dr Juberg identified the presence of endoparasites as a “nontreatment related confounder” (T1680.15–20). I asked Dr Juberg about this issue during his cross-examination (T1857.29–42):
HIS HONOUR: Well, I might be able to short-cut this by asking a couple of questions. You say at line 17:
It sounds to me like a non-treatment related confounder.
That should be. I would be interested to understand – you explain that a little bit, and if there is a confounder present, does that, in your opinion, render Kumar’s data on malignant lymphoma unreliable in assessing possible causation by glyphosate?
DR JUBERG: Yes, I would agree, your Honour. I would stand by my statement here that if we take it at face value that there were endoparasites perhaps involved that were in the animals themselves, that to me could certainly compromise the health status and the ability of any animal to detoxify or handle a chemical exposure. So to me that would be a confounder that would have to be looked at.
535 Further, the type of mouse under consideration was the MF-1 strain of mouse, which was a cross-breed of the CFW mouse (T1682.41–1683.1). As Taddesse-Heath (2000) identified in their study (cited in Greim (2015)), CFW mice under study develop a high incidence of lymphoma in addition to murine leukaemia virus infection (T1684.24–28). The authors concluded (at 6836) that there was a “clear association” of virus expression with lymphoma and leukemia development and that use of CFW mice will be problematic in long-term studies.
536 I accept that the fact that there was no evidence of health deterioration due to suspected viral infection in the Kumar (2001) study itself is of little assistance because one would not necessarily expect oncogenic viruses to be evident by health deterioration (T1863.16–37). As Dr Juberg noted (T1863.44–46) “animals can harbour retroviruses and other types of viruses and they – sometimes they just exist within the animal species itself without exerting or displaying signs of health deterioration”. In any event, as noted above, Dr Bayard only relied on Kumar (2001) in calculating p-values (unadjusted for multiple comparisons) to the extent that it supported his conclusions already expressed in relation to CD-1 mice and, accordingly, I consider it is of limited utility in demonstrating that glyphosate causes malignant lymphomas in mice.
537 Fourthly, I do not consider that significant assistance can be derived from Dr Bayard’s findings in relation to Sugimoto (1997), for the following reasons. Dr Bayard accepted in cross-examination that the high dose level (4348 mg / kg / day) generated “evident signs of toxicity” (T1688.10–17), such as “loose stool, retarded growth, reduced food consumption and food efficiency, cecum distension and increased absolute and relative cecum weight …” (see Greim (2015), Table 17). Although there was some debate as to evidence of “excessive toxicity” as defined by the USEPA Guidelines (with Dr Juberg agreeing that there were signs against it (T1843.39–43)). Finally, the dose rates in Knezevich and Hogan (1983) were similar to Sugimoto (1997) and demonstrated no correlation between the administration of glyphosate and development of malignant lymphoma.
538 Fifthly, the evidence established issues concerning the reliability of the Wood (2009b) study of CD-1 male mice, and I think it is of limited assistance, for the following reasons:
(1) Dr Bayard’s statistical result for the Wood (2009b) mouse (Ptrend=0.004) was affected by the fact that there were zero malignant lymphomas recorded in the control group (0/51), which Dr Bayard agreed one would normally expect to see in control male mice (T1668.43–1669.4; T1677.8–12) (the consequence being that if the control represented historical control values, the statistical significance would fall) (T1677.43–1678.1). As the OECD Guidance Notes state (at 62), “caution should be exercised in interpreting results that are barely statistically significant, or in which incidence rates in concurrent controls are unusually low relative to historical controls (US EPA, 1996)”;
(2) relatedly, the authors of Wood (2009b) concluded (at 38):
For male mice there was a higher incidence of malignant lymphoma for high dose group animals but this was almost certainly due to the absence of malignant lymphomas among control males. A low incidence of malignant lymphomas would normally be expected for control male mice since malignant lymphoma is a background neoplastic condition in both sexes in the CD-1 mouse. Findings from a concurrent control group of male mice suggested an incidence of 6/50 or 12% for malignant lymphoma in male mice of this strain. This illustrates the variability in incidence of malignant lymphoma between control groups even when concurrently housed at the same facility (Brooks et al 2007). In any event the incidence of the condition among high dose male mice is not sufficiently great to suggest a relationship to treatment.
(Emphasis added)
(3) Dr Bayard’s calculations did not account for multiple comparisons, whereas the USEPA did correct for multiple comparisons and found no statistically significant outcome (USEPA Issue Paper (at 72, 88)).
539 For these reasons, Dr Bayard’s evidence in support of the contention that there is a casual connexion between glyphosate and the development of malignant lymphomas in male and female CD-1 mice is of limited assistance.
2. Hemangiomas and hemangiosarcomas
540 Dr Bayard gave evidence that glyphosate induces the development of hemangiomas and hemangiosarcomas, which are tumours of blood vessels. Hemangiomas are benign, whereas hemangiosarcomas are malignant (T1689). It is convenient here to reproduce Table 4 of the JRH which sets out Dr Bayard’s findings (an additional row was inserted by Dr Bayard in the Bayard Corrections in relation to Knezevich and Hogan (1983) where for hemangiosarcomas, female mice counts were 1/49, 0/49, 3/50, 2/50, resulting in Ptrend=0.074; and for the “Pool 24-months” row, the tumour type should read “Hemangioma + Hemangiosarcoma” and the tumour count was corrected by Dr Bayard to 1, 0, 3, 2 (Ptrend=0.051)):
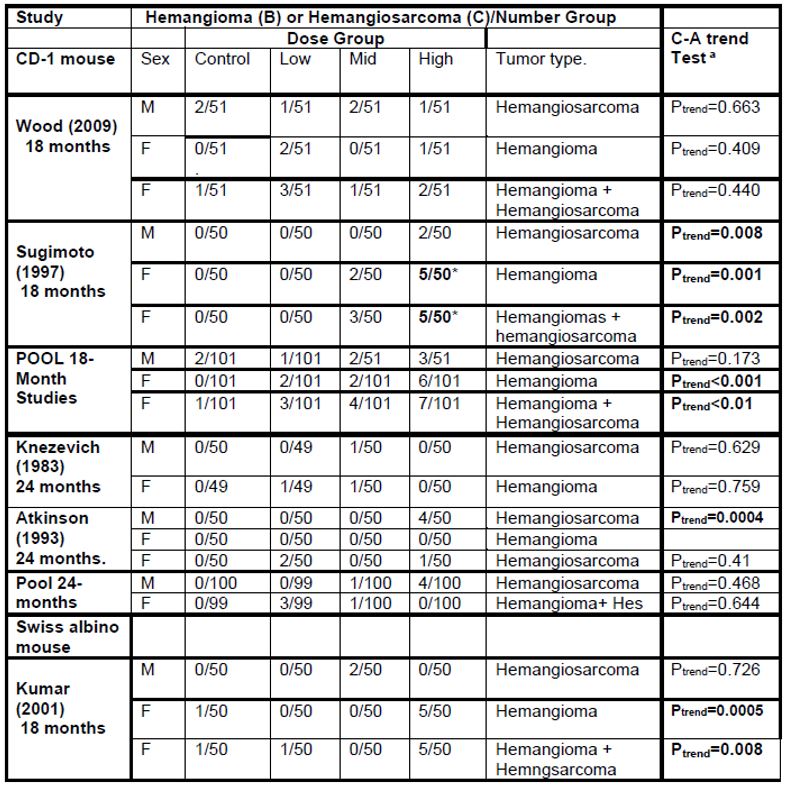
541 Mr McNickle relies on Dr Bayard’s conclusion that Sugimoto (1997) and Atkinson (1993) provide “strong evidence” that glyphosate causes hemangiosarcomas in male mice (at JRH [51], Table 4). In relation to the former, Dr Bayard gave evidence that when one study shows statistically significant increases in the same tumour type in both sexes, that is considered strong evidence that the agent causes cancer in animals (at JRH [51]). He also gave evidence that Kumar (2001) had a “very highly statistically significant” result (Ptrend=0.0005) showing increases in hemangiomas in female mice (at JRH [51]). In relation to males, his evidence is that only two hemangiosarcomas were seen, both in the mid dose group, but because he could not find any historical data on hemangiosarcomas for the Swiss Albino mouse, he could not comment any further on whether the two mid-dose tumours were noteworthy. Based on these findings, Dr Bayard concluded that there is “convincing evidence to me that glyphosate causes hemangiomas and hemangiosarcomas in mice” (at JRH [53]).
542 I consider that Dr Bayard’s evidence in relation to hemangiomas and hemangiosarcomas is of limited assistance, for the following reasons.
543 First, none of the studies relied upon by Dr Bayard indicated statistical significance with respect to hemangiomas in male mice in any study (at JRH [51], Table 4; T1699.1–20). For hemangiosarcomas, according to Dr Bayard’s predetermined test, the majority of studies were not significant for male mice (T1689.32–1690.6). The two studies that did meet Dr Bayard’s predetermined test were Sugimoto (1997) (Ptrend=0.008) and Atkinson (1993) (Ptrend=0.004).
544 In relation to the former, two points may be made: (1) it was noted by the authors of Sugimoto (1997) that they did not report statistically significant results for hemangiosarcomas because they corrected for multiple comparisons, whereas Dr Bayard did not (T1690.8–31; see also USEPA Issue Paper (at 72, 88)); and (2) in circumstances where other studies conducted at higher dose levels than Sugimoto (1997) (namely, Knezevich and Hogan (1983)) and lower (Wood (2009b)) revealed no statistically significant occurrences of hemangiosarcoma, it is not evident that the study provides a reliable foundation for glyphosate causing the induction of hemangiosarcomas in male mice. This is especially so where the actual incidence of hemangiosarcomas in Sugimoto (1997) was 0, 0, 0 and 2 – in other words, it is not clear how the presence of two hemangiosarcomas in the high dose group in Sugimoto (1997) constitutes reliable evidence that glyphosate causes hemangiosarcomas in mice in the light of Knezevich and Hogan (1983) and Wood (2009b).
545 In relation to Atkinson (1993), as seen above, the incidence of hemangiosarcomas in male mice was 0/50, 0/50, 0/50 and 4/50. Similarly to Sugimoto (1997), it is not clear that this result represents a reliable foundation for a conclusion that glyphosate causes hemangiosarcomas in male mice in circumstances where: (1) the doses administered in Atkinson (1993) were similar to those administered in the “negative” Wood (2009b) study; (2) the number of tumours in Wood (2009b) (6/200) exceeds that found in Atkinson (1993); (3) the outcome in Atkinson (1993) is within the historical control data range of between 1.67 to 12% incidence of hemangiosarcomas in male CD-1 mice (see Giknis (2005), Table 3); and (4) there is no evidence of significant hemangiosarcomas developing in female mice in Atkinson (1993).
546 Secondly, in relation to female mice, Dr Bayard calculated for:
(1) Sugimoto (1997), a p-trend of 0.001 for hemangiomas, and a p-trend of 0.002 for hemangiomas plus hemangiosarcomas; and
(2) Kumar (2001), a p-trend of 0.0005 for hemangiomas, and a p-trend of 0.008 for hemangiomas plus hemangiosarcomas.
547 In cross-examination, however, Dr Bayard conceded that there was no independent suggestion that hemangiosarcomas in female mice were statistically significant (T1698.19–32):
MR CRAIG: … There’s no study that you’ve looked at in the context of table 4 which suggests there is a statistically significant correlation between glyphosate and hemangiosarcomas as opposed to hemangiosarcomas and hemangiomas combined; that’s correct, isn’t it?
DR BAYARD: In females.
MR CRAIG: In females. That’s correct, isn’t it?
DR BAYARD: I will just restate it: I’m not going to – not going to divert, digress, but your question, I – is that, for hemangiosarcomas alone, not counting hemangiomas in females, there is no independent suggestion that they are statistically significant. I agree with that.
(Emphasis added)
548 Further, it is noteworthy that three out of the four CD-1 mice studies demonstrated no statistical significance for females according to Dr Bayard’s predetermined test. The only cancer bioassay which met the predetermined test for significance of hemangiomas in female CD-1 mice was Sugimoto (1997), which, even if one were to disregard the evident signs of toxicity (as noted above (at [537])), when compared to the high dose level in Knezevich and Hogan (1983) and the low dose level in Wood (2009b) (which studies revealed no statistically significant occurrences of hemangioma in female mice) indicates no reliable foundation for the contention that glyphosate causes hemangioma in female CD-1 mice.
549 For these reasons, I consider Dr Bayard’s evidence of limited assistance in demonstrating a causal connexion between glyphosate and the development of hemangiomas and hemangiosarcomas in mice.
550 Dr Bayard stated that there is “strong evidence” glyphosate causes kidney adenomas and carcinomas in male CD-1 mice and that glyphosate causes kidney adenomas in Swiss Albino male mice (at JRH [62]). In reaching that conclusion, Dr Bayard relies upon the data reproduced from the JRH below (Table 5):
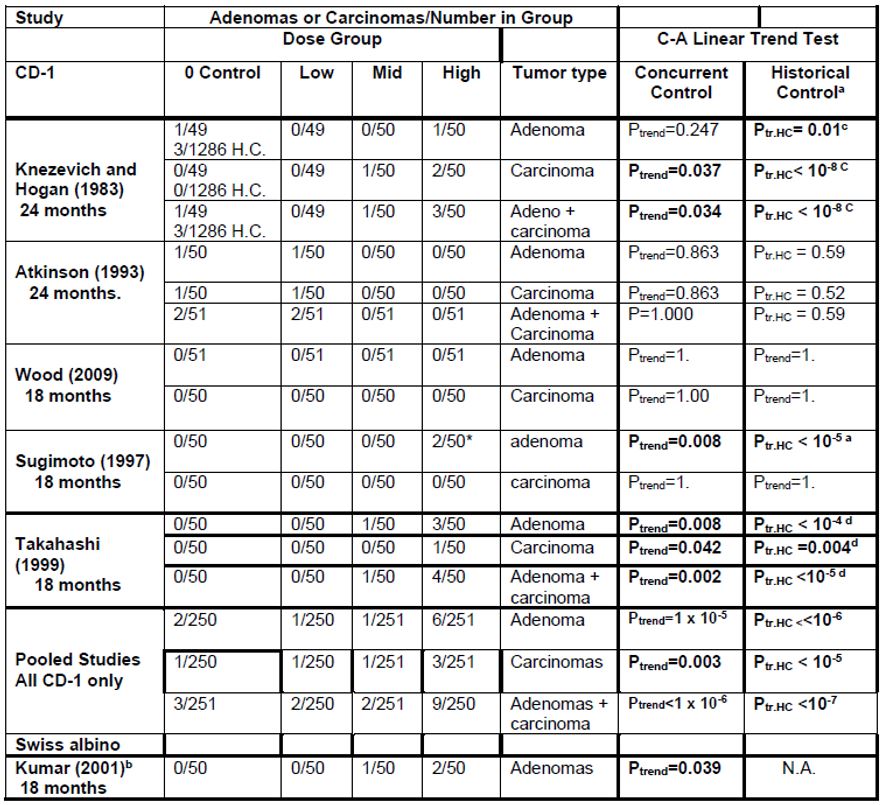
551 Mr McNickle contends that the Court should accept Dr Bayard’s evidence that glyphosate more likely than not causes kidney tumours in male mice for the following reasons.
552 First, spontaneous kidney tumours, both adenomas and carcinomas, are rare in male and female CD-1 mice. As Dr Bayard explained in the JRH (at [55]):
The incidences seen in Tables 3A and 3B, which are extracted from Giknis and Clifford (2005), and compared with Giknis and Clifford (2010), show just how rare these tumours are in control animals. In their 2005 document the authors recorded only 7 kidney adenomas and 4 kidney carcinomas among 2939 male control mice (0.2% and 0.1%, respectively) from a total of 59 cancer bioassay studies initiated between the years 1987-2000. For studies in female mice during these same years, the numbers of tumour-bearing animals were even smaller; there was only 1 adenoma and 1 carcinoma reported among 3227 control female mice (0.03% for each tumour type, or 3 tumour bearing animals per 10,000 animals).
553 It is said that consistently with Dr Juberg’s evidence and the USEPA Guidelines, the rarity of tumours is a matter of biological significance to be considered in the evaluation of the animal bioassays and, in turn, in assessing whether a tumour response is treatment related or due to chance (T1795.44–1796.6). Dr Bayard concluded that with so few observed spontaneous kidney tumours in control CD-1 mice, any reported increase among the groups of mice treated with glyphosate would be highly notable (at JRH [56]).
554 Secondly, and relatedly, of the five CD-1 mice studies, three showed statistically significant linear-dose-responses for adenomas and/or for adenomas/carcinomas combined (Knezevich and Hogan (1983); Sugimoto (1997); and Takahashi (1999)) and two for carcinomas alone (Knezevich and Hogan (1983) and Takahashi (1999)). It is contended that Kumar (2001) supports these results (JRH, Table 5).
555 In my view, Dr Bayard’s evidence is of limited assistance in favour of a finding that glyphosate more likely than not causes kidney tumours in male mice, for the following reasons.
556 As a preliminary matter, it is significant that Dr Bayard limited his finding to the incidence of kidney tumours in male mice only. There was no incidence of kidney tumours in female mice across the mouse studies, and hence no cross-gender significance (T1712.25–32). According to the USEPA Guidelines (see above (at [424])), the presence of tumours in both sexes is a criterion in assessing significance. As Dr Bayard noted in his individual report (at [102]):
… Third, the analysis illustrates that these rare tumors should be viewed and analyzed [sic] differently from common tumors. This was recognized [sic] very early on in the U.S. National Cancer Institute’s bioassay testing program[me] by the Head of the Biometry Branch, who wrote:
“In an organ that has a spontaneous tumor rate of less than 1 %, the observance of small, dose-related tumor increases in both male and female animals of the same species may be considered as strong evidence of carcinogenicity by a pathologist or a toxicologist, even if all the significance tests have P-values exceeding 0.05.” Gart et al. (1976).
(Emphasis added)
557 This was raised with Dr Bayard in cross-examination (T1726.22–1727.13):
MR CRAIG: Okay. In the quote on which you rely, the author identifies that:
Small dose related tumour increases in both male and female animals may be considered as strong evidence of carcinogenicity of a pathologist.
Do you see that?
DR BAYARD: Yes.
MR CRAIG: Okay. Now pausing there, you’ve already accepted that there is no incidence of kidney tumours in female mice, haven’t you?
DR BAYARD: In the – in the studies – in the five CD-1 mouse studies that you’re talking about.
MR CRAIG: Yes.
…
MR CRAIG: So let’s be very clear about this: there is no dose related tumour increase in female mice with respect to kidney tumours, is there, Dr Bayard?
DR BAYARD: I think that’s – I mean, not only is that true, I think I didn’t see any tumours in the females – in the - -
MR CRAIG: Thank you. And so that quote is inapplicable to kidney tumours in mice, isn’t it, with respect to glyphosate because there is no dose related tumour increase in respect of female mice?
DR BAYARD: I wouldn’t – I wouldn’t eliminate that quote. I can see limitations on it based on that quote, limitations on my analysis based on that quote, but I – it doesn’t say that if you – it doesn’t give the negative of that or the obverse.
MR CRAIG: That quote does not say, “It is appropriate to pool cancer bioassays with respect to a single sex across 26 different years, multiple laboratories, and different pathologists”, does it?
DR BAYARD: Not in so many words.
558 In the light of that evidence, Dr Bayard’s conclusion that glyphosate more likely than not causes kidney tumours in mice was limited to findings in respect of male mice (T1712.34–37) which, for the following reasons, I find are of limited assistance.
559 First, in relation to kidney adenomas, Dr Bayard relied upon Takahashi (1999) and Sugimoto (1997). In relation to the former, Dr Bayard identified a statistically significant p-trend of 0.008 (JRH, Table 5). In cross-examination, however, it was put to Dr Bayard that the tumour count in the study was adjusted upon re-examination by a pathologist, which meant that result was no longer statistically significant (T1713.44–1714.1):
MR CRAIG: Okay. And in respect of those studies, if one was to use the tumour count identified by the pathologist upon re-examination in Takahashi, the Takahashi figures would no longer be significant, would they?
DR BAYARD: That’s correct on the re-examination of the new slides.
560 Dr Bayard accepted that he had “ignored the re-examination 13 years later of new slides” and accepted that it was necessary to have included reference to the subsequent examination in his report (T1649.19–34). He said further (T1650.1–10):
MR CRAIG: And you didn’t draw to the court’s attention the final sentence of that – the final two sentences of that paragraph, did you?
DR BAYARD: No. I made my choice of which set of slides were important.
MR CRAIG: And you didn’t set out any reasoning as to why the re-examination results should not be preferred to those of the – under the original study?
DR BAYARD: Why the new slides should not have been referred instead of the original slides, I did not say that. I did not give reasonings.
561 In relation to Sugimoto (1997), I have already noted above (at [522]) the high dose level in that study and Dr Bayard’s agreement that there were evident signs of toxicity. In circumstances where studies conducted at dose levels higher than Sugimoto (1997) (Knezevich and Hogan (1983)) and lower than Sugimoto (1997) (Wood (2009b)) did not establish any statistically significant incidences of kidney adenomas, I do not consider any real assistance can be derived from Sugimoto (1997) in support of a conclusion that glyphosate induces kidney adenomas in male mice. This is particularly so where: (1) there was no progression from adenomas to carcinomas in the study (nor in Wood (2009b) or Kumar (2001)); and (2) when corrected for multiple comparisons, the authors of Sugimoto (1997) identified no statistically significant incidences of kidney adenomas (T1714.3–14).
562 Secondly, with respect to kidney carcinomas in male mice: (1) the evidence indicates no association between glyphosate and kidney carcinomas on Dr Bayard’s predetermined test for statistical significance in Atkinson (1993), Wood (2009b) or Sugimoto (1997) (see Table 5 above (at [550])); (2) Dr Bayard relied upon the incidence of a sole kidney carcinoma in Takahashi (1999) (0/50, 0/50, 0/50, 1/50) in circumstances where the study authors noted (at 155, as reported in the JMPR) that: (a) the sole carcinoma was within historical control levels; (b) “tubular epithelial cell hypertrophy was localized” and that “[t]hese findings indicate no association between the tubular epithelial cell hypertrophy and the development of renal tumours”; and (c) the results in Atkinson (1993) (1/50, 1/50, 0/50, 0/50) suggest that incidences of kidney carcinomas occur without a dose-response relationship; (3) as noted above in Section II, Dr Bayard reached the opposite conclusion on causation in contrast to the PWG and reviewing pathologist in relation to the findings in Knezevich and Hogan (1983) in circumstances where he lacked the requisite pathological expertise.
563 Thirdly, in relation to Kumar (2001), to the extent that it matters given Dr Bayard only relied upon the study peripherally with respect his contention that glyphosate induces kidney tumours in mice (see above (at [539])), I consider it is of limited assistance in circumstances where: (1) the study was affected by the existence of a endoparasites in the test animals which amounted (in Dr Juberg’s view) to a “nontreatment related confounder” (see above (at [534])); and (2) in any event, the study used the MF-1 Swiss Albino strain of mouse, in respect of which there an absence of historical control data in relation to adenomas.
564 For these reasons, I am not satisfied the evidence establishes that glyphosate induces kidney adenomas and carcinomas in CD-1 and Swiss Albino mice.
565 Dr Bayard’s evidence in relation to lung adenocarcinomas was brief.
566 Dr Bayard noted (at JRH [63]) that Wood (2009b) indicated a statistically significant trend (Ptrend=0.024) for lung adenocarcinomas in which a “highly significantly increased dose-response trend” was found in malignant lymphomas, and that this result is supported by Sugimoto (1997) which found a non-statistically increased trend (Ptrend=0.135) in lung adenocarcinomas along with a “highly statistically significant” (Ptrend =0.008) increased trend in malignant lymphomas. Dr Bayard concluded that evidence of the same two cancers in two like studies of the same species, strain, and sex from two different laboratories is strong evidence of a causal connexion between glyphosate and the induction of cancer in mice.
567 Mr McNickle submitted that although Dr Bayard’s evidence was short, the presence of lung adenocarcinomas added to the weight of evidence in favour of a finding that glyphosate induces cancer in mice. The fact that lung adenocarcinomas were not found in female mice does not lessen the statistical significance given that lung adenocarcinomas were found with malignant lymphomas in male mice across two studies. Further, Dr Bayard, it is said, carefully explained in cross-examination why he did not consider that such tumours fell within historical controls, where he noted (T1734.11–21):
MR CRAIG: What I want to suggest to you is that you made no evaluation in your report for whether or not the occurrence of the lung adenocarcinomas in Wood fell within historical controls?
DR BAYARD: I saw no reason to because it’s not an uncommon tumour.
MR CRAIG: Thank you.
DR BAYARD: Historical controls are – when you listen to – when you read the guidelines, they talk about the – because of the type of study, they talk about the effectiveness or the use in rare tumours.
568 I do not think that much assistance can be derived from Dr Bayard’s evidence with respect to the occurrence of lung adenocarcinomas in mice.
569 First, as Dr Bayard explained, lung adenocarcinomas are not rare in mice (T1733.1–4; T1734.15). As is evident from the exchange above, Dr Bayard found it unnecessary to assess whether the tumour type fell within historical controls due to its prevalence. Indeed, in Giknis (2005) (at 8, Table 3), the range of naturally occurring lung adenocarcinomas in male CD-1 mice fell between 1.43 and 26% (T1733.22–47).
570 Secondly, Dr Bayard gave evidence that the only cross-gender study which gave rise to a statistically significant result (according to his predetermined test) was Wood (2009b) and agreed there was no statistically significant finding for lung adenocarcinomas in male or female mice in Knezevich and Hogan (1983); Atkinson (1993); Sugimoto (1997); Takahashi (1999); Kumar (2001); or Wood (2009b) (female mice) (T1732.19–30):
MR CRAIG: And the only study across male and female mice, which gave rise to a significant unadjusted result according to your predetermined hypothesis, was Wood?
DR BAYARD: That’s correct.
MR CRAIG: And so there was no statistically significant finding for male or female mice, with respect to lung adenocarcinomas across Knezevich and Hogan, Atkinson, Sugimoto, Takahashi, Kumar or Wood female mice?
DR BAYARD: Yes. I explain all that. The only other study that found a near significant trend was Sugimoto.
571 In the light of the above, Dr Bayard gave the following evidence (T1735.22–36):
MR CRAIG: … I want to suggest to you that there’s an unreliable – your report provides an unreliable foundation for concluding that glyphosate causes lung adenocarcinomas and I put to you that the reason for that is there’s no statistically significant result in females and of all the studies you look at with respect to males there’s only a statistically significant result in the Wood study; do you accept that?
DR BAYARD: I do. With the condition – the reason I put this in was because it shows it – for strength of evidence is shows that multiple tumours are appearing in the same study, that’s all.
MR CRAIG: And what that means is one cannot reliably conclude that lung adenocarcinomas are caused by glyphosate, can they?
DR BAYARD: I would not go that far. I wouldn’t – I would agree with you, but I wouldn’t go that far as to say that was causal.
572 For these reasons, I am not persuaded Dr Bayard’s evidence provides a firm foundation for a conclusion that glyphosate induces lung adenocarcinomas, or that it adds significantly to the weight of evidence in favour of such a conclusion.
573 Dr Bayard gave evidence that it is more likely than not glyphosate causes cancer in rats (at JRH [87]). This conclusion, however, was qualified by his opinion (at JRH [69]) that the evidence of carcinogenicity in rats is “not as strong, or, at least, not as consistent, as it is in mice”.
574 With that said, Dr Bayard contended that glyphosate induces three categories of tumours in rats, namely: (1) thyroid C-cell carcinomas and adenomas; (2) liver hepatocellular adenomas and carcinomas (in male rats); and (3) kidney adenomas.
575 I will address each in turn.
1. Thyroid C-cell carcinomas and adenomas
576 Dr Bayard gave evidence that it is more likely than not that glyphosate causes thyroid C-cell carcinomas and adenomas in both sexes of SD rats (at JRH [76]). In reaching this conclusion, Dr Bayard relied upon, inter alia, two rat bioassays which indicate statistical significance, namely Lankas (1981) (for carcinomas) and Stout and Ruecker (1990) (for carcinomas and adenomas) (at JRH [70]–[72]). I have reproduced Dr Bayard’s findings in Tables 6A (carcinomas) and 6B (adenomas and carcinomas) from the JRH below (noting Table 6B was the subject of the Bayard Corrections as follows: (1) in relation to Stout and Ruecker (1990), for the dose groups named “Low and “L-med”, the total animals should be 48 in both; and (2) in relation to Atkinson (1993), in the H-med dose group, there is one additional thyroid C-cell adenoma (3/21) (revised Ptrend=0.193)):
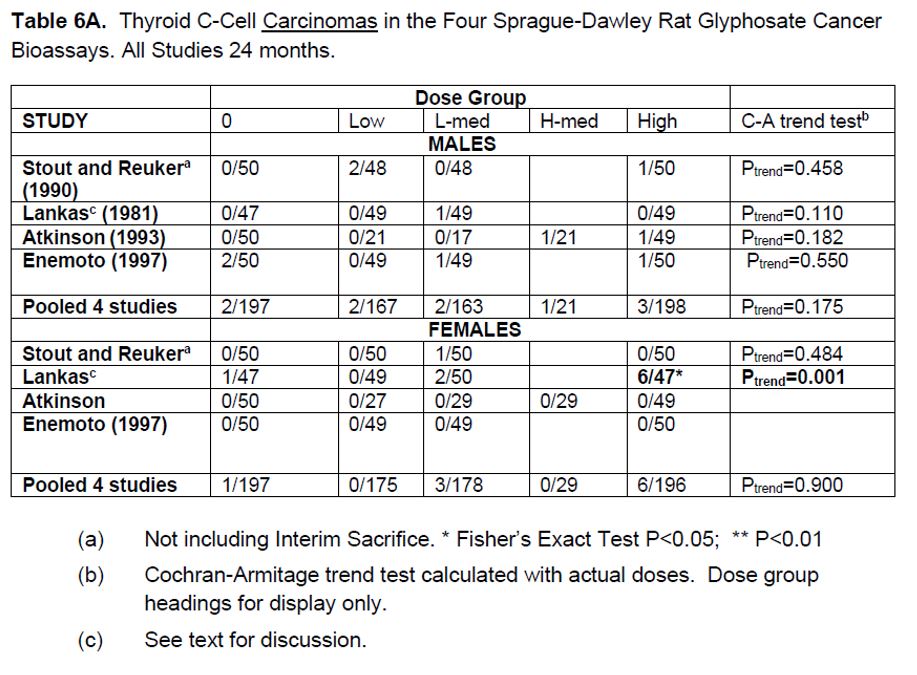
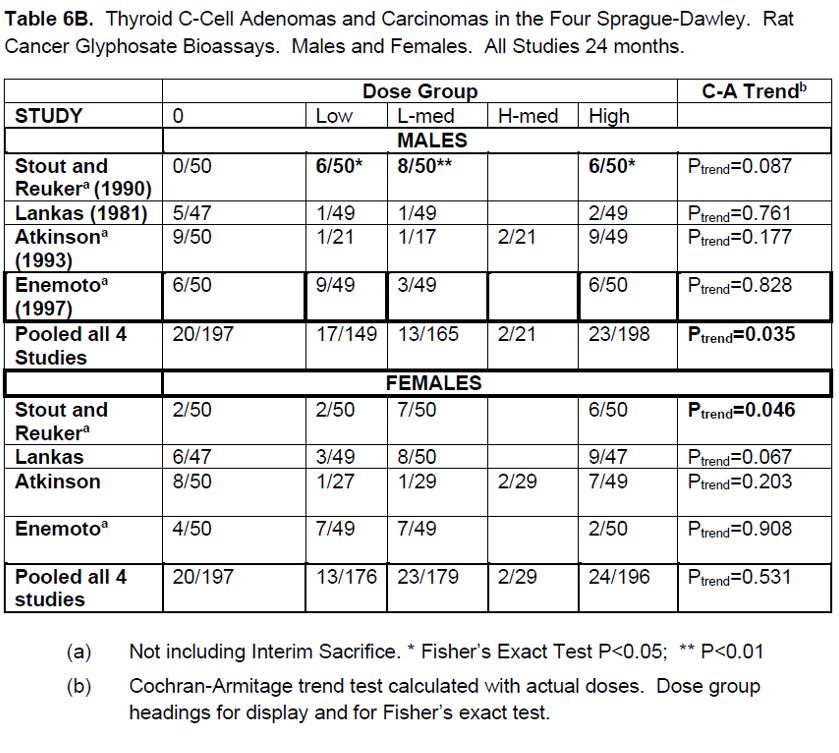
577 First, Dr Bayard noted (at JRH [70]–[71]) that for carcinomas in female rats, Lankas (1981) indicated a “highly significant” linear trend (Ptrend=0.001) and a statistically significant pairwise comparison of high dose versus control, but that: (a) the other three studies (Stout and Ruecker (1990); Atkinson (1993); and Enemoto (1997)) did not; (b) the pooled C-cell carcinomas for the four studies do not show a statistically significant p-trend (Ptrend=0.900); and (c) the high dose in the Lankas (1981) female SD rats is relatively low (34 mg / kg / day) (lower than the low dose in two of the other SD rat studies).
578 Secondly, when combining results for adenomas and carcinomas, Dr Bayard noted that Stout and Ruecker (1990) indicate both a significant trend for female rats (Ptrend=0.046) and all three statistically significant pairwise results for male rats, and a marginally significant p-trend (Ptrend=0.087). Accordingly, he concluded that statistically significant findings in combined thyroid C-cell adenomas and carcinomas in both sexes in the same study is “sufficient evidence for carcinogenicity in animals” by any guidelines. Dr Bayard also noted the “highly statistically significant” pooled result of the four glyphosate male studies (Ptrend=0.035) (at JRH [71]).
579 Mr McNickle submits that the Court should accept Dr Bayard’s evidence supporting his conclusion that it is more likely than not that glyphosate causes thyroid C-cell carcinomas and adenomas in both sexes of SD rats. It is said that in reaching his overall conclusion, Dr Bayard considered historical control data which indicated that although thyroid C-cell adenomas are common in male and female SD rats, thyroid C-cell carcinomas are rare in SD rats (at JRH [73]–[74]). Dr Bayard also considered further, and more contemporaneous, historical control data in relation to Stout and Ruecker (1990) which supported his level of confidence in the conclusion of glyphosate causing thyroid C-cell cancers.
580 In my view, Dr Bayard’s evidence is of limited assistance in demonstrating a causal relationship between glyphosate and thyroid C-cell carcinomas and adenomas, for the following reasons.
581 First, in relation to thyroid C-cell carcinomas in male rats, there was no statistically significant finding from any of the studies relied upon by Dr Bayard in Table 6A (T1736.39–46). In cross-examination, Dr Bayard agreed that this presented a problem for his thesis as to carcinogenicity (T1752.1–6):
MR CRAIG: And I’m putting to you that that tends to suggest that glyphosate does not cause or provides no firm foundation for a conclusion that glyphosate causes thyroid C-cell carcinomas in male rats?
DR BAYARD: I think I agree that – that there is an argument, if you – if you consider only carcinomas.
582 In relation to thyroid C-cell adenomas in male rats, it may be observed that:
(1) the majority of studies in Table 6B did not indicate a statistically significant result for thyroid C-cell adenomas;
(2) when adenomas were considered alone, Dr Bayard gave evidence that the results in relation to male rats were within the historical control levels which he had identified and relied upon from the Isobe (2016) study (T1749.41–1750.1):
MR CRAIG: Yes. And what I want to suggest to you is that the adenoma figures in male rats were within historical controls identified in the Isobe 2016.
DR BAYARD: And what figure did I give you for that?
MR CRAIG: 11 per cent.
DR BAYARD: For adenomas, it sounds about right.
(3) although Stout and Ruecker (1990) did indicate a statistically significant pairwise result for combined thyroid C-cell adenomas and carcinomas in male rats, Dr Bayard accepted that the zero incidence of adenomas and carcinomas was unusual (given one would ordinarily expect a higher naturally occurring incidence of adenomas and carcinomas in SD rats) (T1750.32–36).
583 Taking into account these matters, it was put to Dr Bayard in cross-examination that there was not a reliable foundation for concluding that glyphosate causes thyroid C-cell carcinomas and adenomas in male rats, in respect of which he accepted that the evidence was “not very strong” (T1753.24–44):
MR CRAIG: … Dr Bayard, one certainly can’t draw any firm conclusion that glyphosate causes thyroid C-cell carcinomas and adenomas in male rats?
DR BAYARD: Well, that’s – the data don’t say that but – because of the Stout and Ruecker calculations for adenomas and carcinomas, but I – I do suggest to you that the evidence isn’t as strong as – in rats as it is in mice and that’s true for at least thyroid – thyroid C-cell adenomas and carcinomas too.
MR CRAIG: It would be quite unsafe for his Honour to proceed on the basis that the data presented is actually persuasive as to the existence of thyroid C-cell adenomas and carcinomas being caused by glyphosate in male rats?
DR BAYARD: I – if you break it down piece by piece by piece, one can say one out of four studies showed statistical significance in male rats but you haven’t included the females.
MR CRAIG: Looking at males alone, his Honour cannot be actually persuaded that glyphosate causes thyroid C-cell carcinomas and adenomas, can he?
DR BAYARD: Together in total there’s evidence there but it’s not very strong.
584 Secondly, turning to thyroid C-cell carcinomas in female rats, the only study relied upon by Dr Bayard which met his predetermined test for statistical significance was Lankas (1981) (Ptrend=0.001) (T1736.33–37).
585 Some time was spent on the reason why Dr Bayard had included Lankas (1981) in his analyses in circumstances where other studies with the same or similar disqualifying features (such as low doses and a poor Klimisch score (as reported in Greim (2015)) had been deliberately excluded (a Klimisch score, derived from Klimisch (1997), is a method of evaluating the reliability of study data, particularly for regulatory purposes) (see Brusick (2016) (at 58)) (T1737.39–47). Dr Bayard explained the reason for including it as follows (First Bayard Report (at [77])):
I included the Lankas (1981) Sprague-Dawley rat study, despite its lower Klimsch score in Greim (based on low doses used), because there were carcinogenic effects observed. In my opinion, the study was of acceptable quality and conducted properly as judged by Greim (2015), and the data are available. To omit it on the basis that the highest dose wasn’t high enough to determine carcinogenic potential is contradicted by the response. Omitting it would be to lose potentially valuable information.
586 For present purposes, whether it was appropriate for Dr Bayard to include Lankas (1981) for the reasons identified above is largely beside the point. To the extent that it matters, Dr Juberg acknowledged that it was not appropriate to discard tumour results in their entirety even in the event of a compromised study, but rather they should be considered together with other study results and other lines of evidence (T1845.18–30).
587 What is plain, however, is that Dr Bayard in cross-examination identified several limitations in Lankas (1981), namely: (1) that it was a “really unusual study” due to the low dose versus carcinogenic potential (T1738.25–41) (indeed, Dr Bayard speculated whether the authors had “miscounted the dose” (T1740.27–28)); (2) relatedly, that the study is an “outlier result” and had to be “put in a lower class” having regard to other studies on the incidence of thyroid C-cell carcinomas in SD rats (T1740.15–34); (3) that the study ran for 26 months (instead of 24 months), which caused Dr Bayard to opine “… you wonder what happened in those extra two months, whether age caught up with them, I don’t know” (T1759.1–7).
588 A further limitation associated with the low dose in Lankas (1981) was identified by the USEPA as follows (USEPA Issue Paper (at 72)):
There were no treatment-related effects on survival at any dose level. The highest dose tested of approximately 31 mg/kg/day was not considered a maximum tolerable dose to assess the carcinogenic potential of glyphosate. Consequently, a second study (Stout and Ruecker, 1990) was conducted at higher doses …
589 Related to the final point made by the USEPA is that on Dr Bayard’s analysis, there was no correlation between glyphosate and thyroid C-cell carcinomas in female rats in Stout and Ruecker (1990). As Dr Bayard explained in cross-examination (T1741.1–19):
MR CRAIG: … what is also correct is that in the study that followed at the higher doses there was no statistically significant result for thyroid C-cell carcinomas in Sprague-Dawley rats?
DR BAYARD: That’s correct. You’re talking about table 7?
MR CRAIG: No, I’m looking at table 6A. We’re talking about thyroid C-cell carcinomas.
DR BAYARD: You’re just talking about carcinomas now, not the adenomas?
MR CRAIG: Correct. Thyroid C-cell carcinomas.
DR BAYARD: I’m sorry, I just want to go back. Just let’s say, no significant increase at all – in Stout and Ruecker you’re saying, right?
MR CRAIG: Yes.
DR BAYARD: I agree with you.
590 In relation to thyroid C-cell carcinomas in female rats, Dr Bayard gave evidence that the incidence of thyroid C-cell adenomas in female SD rats was far more common than the incidence of thyroid C-cell carcinomas (T1742.28–33):
MR CRAIG: Thank you. So you accept, I take it, Dr Bayard, that the incidence of thyroid C-cell adenomas in female Sprague-Dawley rats is far more common than the incidence of thyroid C-cell carcinomas?
DR BAYARD: It certainly was at that time and I think that was true historically too.
591 In Dinse (2016), the authors identified a historical control level for thyroid C-cell adenomas of 25% in female SD rats (at 5, 15). Notably, the occurrence of thyroid C-cell adenomas across the studies relied upon by Dr Bayard was within that figure (as well as the historical control levels for female SD rats of 5.9% relied upon in the First Bayard Report (at [113])).
592 Further, apart from Stout and Ruecker (1990), none of Dr Bayard’s combined thyroid C-cell adenoma and carcinoma results for female rats in Table 6B indicate statistical significance. In relation to Stout and Ruecker (1990), Dr Bayard gave evidence that the concurrent controls for males and females were “unusually low” (2/50) (T1756.40–45). In addition, there was no evidence in that study of thyroid C-cell adenomas progressing to carcinomas (see Table 6A).
593 When these matters were put to Dr Bayard, he concluded that there is “some evidence” that glyphosate induces thyroid C-cell adenomas and carcinomas in female rats, as follows (T1756.10–1757.41):
MR CRAIG: On the basis of the evidence that we’ve gone through in this proceeding, Dr Bayard, his Honour cannot be actually persuaded that glyphosate causes thyroid C-cell carcinomas and thyroid C-cell adenomas in female rats, can he?
DR BAYARD: I would say there is some evidence and so it’s – I don’t know if he’s persuaded or not. If you take the evidence from - - -
HIS HONOUR: All right. So you say there’s some evidence. Would you say in your professional opinion it is more likely than not?
DR BAYARD: Based on – more likely than not that it causes cancer in female rats?
HIS HONOUR: Yes.
DR BAYARD: I do. But only because it matches up with the other part of the Ruecker study with the males, adenomas and carcinomas also, and it’s so unusual to get both males and females in the same study having increases in adenomas and carcinomas together.
…
DR BAYARD: But if you break it down and you say let’s look at this one or just look at this one then I don’t think it’s persuasive. But I think if you take a look at all of the evidence you say there is some evidence there.
MR CRAIG: Okay. And you rely on, in answering his Honour’s question, you hone in on and rely solely on the Stout and Ruecker study, don’t you?
DR BAYARD: Without the Stout and Ruecker study I would not make that claim.
MR CRAIG: And you accept that, in the Stout and Ruecker study in table 6B, that the concurrent controls for males and females are unusually low?
DR BAYARD: Yes. They seem to be. But that was the study. I mean, that’s – if you look at the – if you look at guidelines, the guidelines say use the concurrent controls.
MR CRAIG: And that, importantly, you identify and should identify for his Honour as casting doubt on relying solely on Stout and Ruecker?
DR BAYARD: I – I – when you say “doubt”, it probably depends where you’re sitting. It is some evidence. It’s not complete evidence. And one has to make the decision.
…
MR CRAIG: Okay. I’m going to put it to you one last time, Dr Bayard. There is simply insufficient evidence for his Honour to be actually persuaded that glyphosate causes thyroid C-cell adenomas and carcinomas in rats, isn’t there?
DR BAYARD: Well, that’s his decision. But I feel that there was some evidence that there was a combination of adenomas and carcinomas in the good study in rats because it was in both sexes.
594 It is worth making two points as to the above extract. The first is a particular one, namely that I do not think Dr Bayard’s evidence is of significant assistance in demonstrating that glyphosate induces thyroid C-cell carcinomas and adenomas in male and female rats. The second is a more general one. This extract is a good and relatively representative example of a tendency by the experts called by both Mr McNickle and Monsanto to slip between the notion of identifying some evidence for a proposition to making more definitive statements of opinion in support or against a particular proposition. I will return to this issue when stepping back and considering the whole of the evidence and, based on that evidence, reaching a conclusion as to whether Mr McNickle has discharged his onus on the central issue.
2. Liver hepatocellular adenomas and carcinomas in male rats
595 Dr Bayard gave evidence that it is more likely than not that glyphosate causes hepatocellular adenomas in SD and Wistar male rats (at JRH [80]). As Mr McNickle submits, this conclusion should be tempered by Dr Bayard’s evidence that he did not consider glyphosate causes cancer in rats by reference to liver hepatocellular information simpliciter – rather (as with his evidence with respect to lung adenocarcinomas in mice (see above (at [565]–[572])), Dr Bayard considered that in conjunction with his conclusions in relation to the incidence of other tumours, it added to the weight of evidence in support of his overall conclusion that glyphosate is carcinogenic to rats (at JRH [87]). As Dr Bayard said in cross-examination (T1758.34–37):
MR CRAIG: You don’t conclude that glyphosate causes cancer in Sprague-Dawley and Wistar rats in the liver, do you?
DR BAYARD: That’s correct.
596 And later (T1761.23–26):
MR CRAIG: Thank you. Now, Dr Bayard, what I want to suggest to you is that his Honour could not conclude that glyphosate causes cancer in rats by reference to the liver hepatocellular data, could he?
DR BAYARD: Could not?
MR CRAIG: Conclude that glyphosate causes cancer in rats by reference to the liver hepatocellular information?
DR BAYARD: I didn’t.
MR CRAIG: Thank you. And nor should his Honour.
DR BAYARD: Well, I try not tell him what to do.
597 With that aside, Dr Bayard noted two “highly statistically significant” trend results, one for each strain, in Stout and Ruecker (1990) and Brammer (2001) (at JRH [78]), in addition to a significant trend in Lankas (1981) for liver carcinomas (at JRH [78]). He also noted that pooling the studies resulted in a “highly statistically significant” trend for liver adenomas in the Wistar rat, and a near statistically significant trend for adenomas in the SD rat (at JRH [78]). I have extracted Dr Bayard’s results below from Table 7 of the JRH (noting that in the Bayard Corrections: (1) in relation to Atkinson (1993), for both adenomas and carcinomas, the low-medium dose group total animals should read 49; and (2) in relation to Brammer (2001), in the low dose group, the total animals should read 51 (p-trend unchanged for both)):
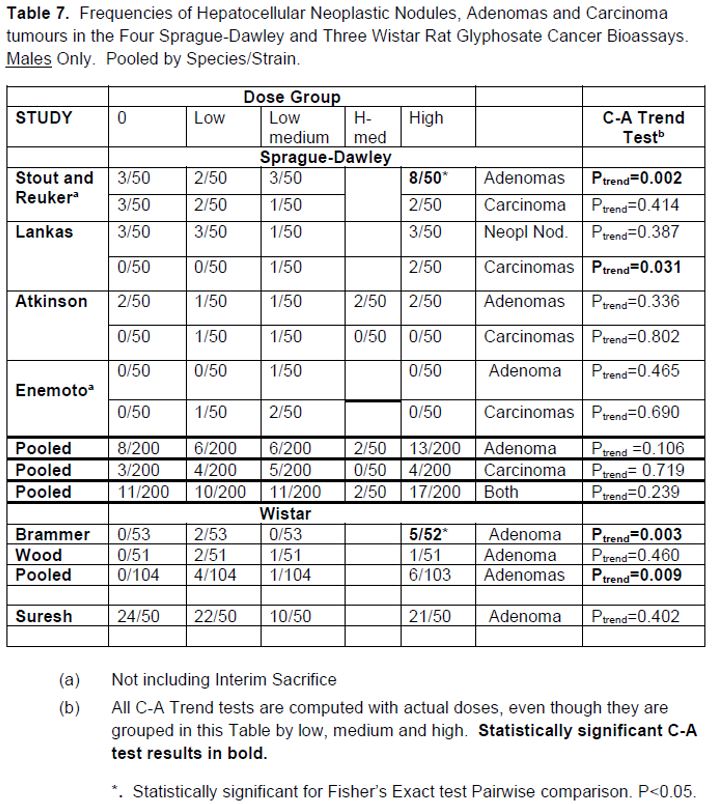
598 Putting to one side Dr Bayard’s concession that the evidence concerning liver hepatocellular adenomas and carcinomas cannot on its own stand as evidence in favour of a conclusion that glyphosate is carcinogenic in rats, I am not persuaded that his evidence significantly assists in support of his thesis as to carcinogenicity, for the following reasons.
599 First, the only statistically significant result for adenomas in male SD rats was in Stout and Ruecker (1990) (Ptrend=0.002). As Dr Bayard noted in cross-examination (T1758.39–42; T1759.34–37):
MR CRAIG: … the only statistically significant result for male rats was in Stout and Ruecker for adenomas; that’s correct, isn’t it?
DR BAYARD: Yes. But there was one for carcinomas in Lankas.
…
MR CRAIG: Thank you.
DR BAYARD: - - - other than the Stout and Ruecker study, I see no increase in the males for adenomas alone.
600 Secondly, as would already be evident, none of the studies relied upon by Dr Bayard in Table 7 generated results which were significant across male and female SD rats (T1759.39–11) (see above (at [543], [557])).
601 Thirdly, as Dr Bayard noted (at JRH [78]), there was “little evidence” of progression from liver hepatocellular adenomas to carcinomas (T1642.12). As Dr Juberg noted in cross-examination, while benign (that is, adenomas) and malignant (that is, carcinomas) tumours should be taken into account in any analysis of carcinogenicity, in any evaluation of oncogenicity (that is, the capability to induce tumour formation), one is “[primarily] interested in malignancy versus a benign tumour that doesn’t progress to a malignant state” (T1798.11–25) (see also USEPA Guidelines (at 2–20) (at JRH [21]) (see above (at [424])).
602 Fourthly, in relation to Wistar rats the subject of Brammer (2001), it can be observed that: (1) the study generated a statistically significant result which was inconsistent with Wood (2009a) (see Table 7 above (at [597])); (2) the pathology evaluation of Brammer (2001) indicated that there were “no treatment related non-neoplastic lesions in any organs of either sex at any dose level” (USEPA Issue Paper (at 80)); and (3) relatedly, as the USEPA noted, there was improved survival in the high dose group (USEPA Issue Paper (at 80)):
The improved survival in the high dose group may help explain a higher incidence of an age related background tumour like liver adenomas and this corresponds with the lack of associated lesions observed in the study.
603 Although Dr Bayard considered that statement to be speculative, Dr Juberg agreed that improved survival likely contributed to a modestly higher incidence of age-related background tumours (T1761.15–21).
604 For these reasons, Dr Bayard’s evidence concerning liver hepatocellular adenomas and carcinomas in male rats is of limited assistance in support of the conclusion that glyphosate is carcinogenic to rats.
605 Dr Bayard’s evidence in relation to this tumour was brief. He contended that it is more likely than not that glyphosate causes kidney adenomas in the SD rat; a conclusion based on Enemoto (1997) which found an increase (Ptrend=0.0004) based on four tumours (at JRH [83]).
606 I do not think significant assistance can be derived from this evidence.
607 As Dr Bayard noted, aside from Enemoto (1997), there was “no other supporting evidence in the other three S-D rat glyphosate studies and no evidence of increased kidney carcinomas” (emphasis added), nor any supporting evidence in the Wistar rat studies (at JRH [83]). Further, the result in Enemoto (1997) was not replicated in females, nor any other study for female SD rats. Notwithstanding Dr Bayard’s evidence that kidney adenomas in the SD rat are comparatively rare (at JRH [83]), I consider Enemoto (1997) to be of limited assistance in demonstrating that it is more likely than not glyphosate induces kidney adenomas.
608 Dr Bayard gave further evidence in relation to other tumours in rats, namely mammary gland tumours, testis interstitial cell tumours and pancreas islet cell adenomas (at JRH [81], [84]–[86]). Although Dr Bayard was not cross-examined on this evidence, Mr McNickle submits that his evidence in relation to other tumours contributes to his thesis on carcinogenicity in rats (at JRH [87]).
609 I do not consider much assistance can be derived from this evidence, largely due to the limitations identified by Dr Bayard in the JRH.
610 First, in relation to mammary gland tumours in rats, as Dr Bayard noted (at JRH [81]), only one out of the seven Wistar rat studies (Wood (2009a)) resulted in a statistically significant trend for adenomas (Ptrend=0.008). The trend for adenocarcinomas alone was also statistically significant (Ptrend=0.031). Notwithstanding that, Dr Bayard explained that only Suresh (1996) reported a supportive result (a mid-dose significant increase in adenomas in the SD rat), concluding that glyphosate “may cause mammary gland cancer in the Wistar rat, but that the data lack enough consistency to make a more definitive statement” (at JRH [81]).
611 Secondly, with respect to testis interstitial cell tumours, Dr Bayard gave evidence that Lankas (1981) reported a highly significant trend (Ptrend=0.009) in the SD rat (at JRH [84]). However, as Dr Bayard noted: (1) there was no evidence of cancers and no increase seen in the other three SD rat studies, which were conducted at much higher doses; and (2) testicular interstitial cell tumours are not rare in the SD rat (averaging approximately 6.5%). Despite the response in Lankas (1981) was double that result, Dr Bayard concluded “it is not more than likely than not that glyphosate causes testis interstitial cell tumours in rats” (at JRH [84]).
612 Thirdly, in relation to pancreas islet cell adenomas, Dr Bayard noted that Stout and Ruecker (1990) and Lankas (1981) in male SD rats found statistically significant increases in control versus some dose-group comparisons, but that there was less evidence for trends and that the only carcinoma in Stout and Ruecker (1990) was in the control group (at JRH [85]). Dr Bayard noted further (at JRH [85]) that:
[t]he positive adenoma result was only slightly supported by a third study (Atkinson, 1993), which found only adenoma type, and only in the high-dose group. The fourth study, Enemoto (1997) reported 8 pancreatic cell tumours, but four of these were in the control group, 1 carcinoma in the mid-dose group, with no evidence of dose-response. Pancreatic Islet Cell adenomas are fairly common in the Sprague-Dawley rat, averaging 7.5%.
613 Based on this evidence, Dr Bayard concluded that there was “some evidence” that glyphosate causes pancreatic islet cell adenomas in the SD rat, but that the lack of a dose-response trend in any of the four studies, and lack of supportive evidence in the Wistar rat, prevented a more definitive conclusion.
614 For these reasons, I consider that Dr Bayard’s evidence in relation to the development of mammary gland tumours, testis interstitial cell tumours and pancreas islet cell adenomas in rats of limited assistance.
615 This is a study which assumes some importance in the mechanistic evidence stream, and, for that reason, I do not propose to deal with it at any length here. In any event, for present purposes, I can be brief with respect to Dr Bayard’s reliance upon it in this stream.
616 Wang (2019) was a 72-week mechanistic study of a Vk*MYC mouse model for the purposes of assessing the development of MM in humans. Dr Bayard gave evidence that it claimed to provide direct evidence of in vivo mode of action (or mechanism of action) that glyphosate causes increased MM, a cancer that affects B-cells (and a subtype of NHL) (at JRH [45]; JRI [61]), and concluded that the Vk*MYC mouse is a reasonable model for determining potential carcinogenic effects for glyphosate in humans (at JRI [66]). Mr McNickle submits that Dr Bayard’s evidence on Wang (2019) supports the evidence given by Professor Smith and Dr Flecknoe-Brown in the third stream and their contention that glyphosate can cause NHL by causing an upregulation of activation-induced cytidine deaminase (AID).
617 It is not an overstatement to say that the evidence adduced in the third stream revealed some not insignificant methodological problems with Wang (2019). It is unnecessary for present purposes to wade into those problems, to which I will return, because we are here focussed on Dr Bayard’s reliance on the study and the extent to which it provides supportive evidence of his thesis as to carcinogenicity. I do not think that it does, for the following reasons.
618 First, as I noted earlier in Section II, Dr Bayard is not a toxicologist. In cross-examination, Dr Bayard accepted that in assessing the reliability or otherwise of the results of Wang (2019), it is a matter for toxicologists as to whether the evidence indicates that glyphosate provides a mechanism of action by which it can cause cancer (T1776.20–1778.1). As the following exchange records (T1630.5–22):
MR CRAIG: … are you an expert on the mode of action of glyphosate and I suggest to you that you’re not.
DR BAYARD: I know what I learned from the Wang study but I – that is the extent of my knowledge on the mechanism of glyphosate.
MR CRAIG: I’m going to put it to you one more time.
HIS HONOUR: As I understand your evidence – and please correct me if I’m wrong – you accept that, prior to reading the Wang study, that you weren’t an expert?
DR BAYARD: I did not know anything about that mechanism.
HIS HONOUR: And any expertise that you gained you gained from your analysis of the Wang material?
DR BAYARD: On the mechanism. That’s correct.
619 Dr Bayard later gave evidence that he had no expertise in NHL (T1761.38–41):
MR CRAIG: Okay. Dr Bayard, you don’t claim any expertise in non-Hodgkin lymphoma, do you?
DR BAYARD: I do not.
620 Secondly, Wang (2019) was not a compliant animal carcinogenicity study. Dr Bayard accepted that the study did not comply with the USEPA or OECD guidelines for the conduct of a carcinogenicity study (T1604.1–4). To take four examples highlighted by Dr Juberg: (1) the number of animals per dose group was 10, which is well below the 50 animals per sex per dose group required by the regulatory authorities (see above (at [416])) (at JRH [133(f)]); (2) a singular dose was used instead of the required three, which precludes any ability to assess dose-response relationships for any of the reported effects (particularly for the noted increase in MM) (at JRH [133(b)]); (3) no historical control data on any tumour type or finding was reported by the authors of the study (at JRH [133(g)]); and (4) the use of 1000 mg/L from is approximately 50,000,000 times greater than the Median US surface water concentration of 0.02 ug/L as reported in the study, which “raises serious doubt as to the relevance of this administered dose for humans” (at JRH [133(b)]).
621 I accept Mr McNickle’s submission, so far as it goes, that Wang (2019) was not required to comply with the regulatory guidelines because it did not purport to be a long-term animal carcinogenicity or toxicity study (with which Dr Juberg agreed (T1565.37–44)). It does not follow axiomatically, however, that the deficiencies associated with Wang (2019) borne out by the evidence merely “fall away” as a result. The examples I have set out above, among others (to which I will return in the third stream), disclose some significant issues in relying upon it as evidence that there exists a mode or mechanism of action by which glyphosate can cause MM in Vk*MYC mice, which evidence can be extrapolated to the development of NHL in humans.
622 For these reasons, Dr Bayard’s evidence with respect to Wang (2019) is of limited assistance.
V Conclave I and the relevance of animal studies to humans
623 As noted above, Conclave I (Animal Studies B) involved Dr Bayard and Professor Prince and concerns the relevance of the animal studies and bioassays to humans. It should be noted from the outset, however, that there is an unavoidable degree of overlap between this conclave and the other streams. This is because, as I have explained earlier (at [420]), although the animal bioassays provide important evidence when assessing the potential carcinogenicity of a chemical in humans, classification of a chemical into one of IARC’s categories depends upon an evaluation of evidence from all of the streams in order to assess carcinogenicity overall (at JRI [24], [28], [35], [37]).
624 In the final version of the JRI in evidence (which was the subject of agreed rulings), Dr Bayard gave evidence that if an agent is shown to cause cancer in non-human mammals, it is capable of causing cancer in humans (at JRI [51]). A related question is whether site concordance of carcinogenic potential between animals and humans is pertinent, and to what degree (at JRI [51]). Using the example of malignant lymphoma, Dr Bayard points to site concordance between animals and humans to strengthen the confidence that glyphosate caused lymphomas in animals will also occur in humans (at JRI [86]). As Mr McNickle submits, however, this is not a matter considered in isolation, and there are a range of matters relied on by Dr Bayard in reaching his opinion including Wang (2019) (at JRI [55], [61]).
625 I consider Dr Bayard’s evidence to be of limited assistance in demonstrating the translatability of animal bioassays and evidence to humans, for the following reasons.
626 First, it is uncontroversial that the conduct of long-term animal carcinogenicity studies is a feature of the process of seeking registration for the relevant chemical. The utility of such studies differs once a chemical becomes registered and is available for commercial use. That is because prior to that time, the absence of human exposure precludes the ability to conduct relevant epidemiological studies, which attempt to ascertain whether there is in fact a relationship between exposure and cancer in humans. After that time however, it becomes possible to conduct such studies, and the need to rely on long-term animal carcinogenicity studies to predict cancer in humans decreases.
627 As the Agreed Facts record (see Section C above), glyphosate was first registered for use in the mid-1970s and has been available for commercial use for decades. In the time since its initial registration, there have been a multitude of epidemiological studies conducted. Accordingly, there is far less need to rely on the results of long-term animal carcinogenicity studies to predict what effect exposure to glyphosate and/or GBFs will have in humans than prior to its initial registration.
628 Secondly, while I do not consider that Dr Bayard deliberately obfuscated the evidence, in my view Dr Bayard overstated the scientific basis for the presumption that chemicals which can cause cancer in animals can do so in humans. As Dr Bayard noted (at JRI [31]):
Agencies that regulate use of these chemicals presume that if they cause cancer in animals, they can also cause cancer in humans. The scientific basis for this presumption is that growth control mechanisms at the cellular level are homologous between species (U.S. EPA, 2005). See comment below by Professor Prince in paragraph 46. Site concordance between species is not a requirement.
(Emphasis in original)
629 In contrast to the above passage cited by Dr Bayard: (1) the USEPA Guidelines (at [2.2.2.1.5]) is not concerned with the presumption that “growth control mechanisms at the [level of the cell] are homologous [among mammals]” simpliciter, but in the context of a much narrower issue, namely site concordance; and (2) relatedly, that sentence is prefaced by the USEPA with the words “there is evidence”, as opposed to amounting to a scientific presumption.
630 Thirdly, Professor Prince gave evidence (at JRI [46]) as to why Dr Bayard’s statement that “growth control mechanisms at the cellular level are homologous between species” (at JRI [31]) is “incorrect and outdated” in the light of various differences between species, which can be summarised as follows: (1) it does not consider the differences in the immune system structure and responses across species; (2) it ignores that genes and gene expression differ across species; (3) it does not consider tumour-predisposing or tumour-protective gene mutations, which vary within and across species; and (4) it disregards variations of epigenetic changes that occur across species.
631 Professor Prince’s evidence was not to the effect that animal studies cannot provide any meaningful evidence that could be extrapolated to humans, and I accept Mr McNickle’s submission that he gave evidence to the effect that: (1) mice share a great deal of genetic similarities with humans (T305.42–47); (2) the laboratory mouse is a highly effective model for lymphoid malignancies due to its small size and significant physiological and molecular similarities between humans and mice (T308.7–13); (3) animal modelling helps add to the weight of evidence (T308.36); (4) animal models are useful (T309.9–10); and (5) animal models, including the mouse model, has greatly impacted the study of lymphoid cancer, biology and its treatment (T309.12–17). As Dr Juberg noted (T1465.29):
DR JUBERG: We – ethically, we can’t do cancer studies in humans, but we do the next best thing. We do them in two species – mice, rats – and so that’s why we follow them up with required 18-month studies in mice, which is the lifetime of a mouse, two years in rats to mimic the lifetime of a human, which is 70 years. So that’s – that’s – you know, it’s a – you’ve got different biological layers of organisation. You’ve got mutagenic gene tox, we talked about chronic toxicity, but then we ultimately go to oncogenicity studies.
632 Notwithstanding the utility of such models generally, it is important to recognise, however, there exist a number of criteria by which an animal model would need to be evaluated in order to extrapolate any findings validly from such a model to humans. As Professor Prince notes (at JRI [56]–[57]):
[56] I believe that animal studies do have the theoretical potential to provide reliable data to predict behaviour of GBFs in humans. In other words, if the study methodology is appropriate, it may theoretically be able to demonstrate positive associations. Thus, to reliably predict the effect of GBFs on humans, the animal model must demonstrate high translatability to the human.
[57] In my opinion, currently there is no animal model that has demonstrated any meaningful translatability.
(Italics in original)
633 Fourthly, as I have noted above in Section IV, Dr Bayard gave evidence that Wang (2019) claimed to provide direct evidence of in vivo mode of action (or mechanism of action) that glyphosate causes MM (at JRH [45]; JRI [61]), concluding that the Vk*MYC mouse is a reasonable model for determining potential carcinogenic effects for glyphosate in humans (at JRI [66]). I do not propose to rehearse the limitations I have already found with respect to that study in demonstrating the translatability of the findings in relation to the Vk*MYC model to humans (to which I will return in the third stream), save to reiterate that in the light of those limitations, I do not consider Dr Bayard’s evidence to be at all compelling in demonstrating the translatability of the animal studies evidence to humans.
634 Having regard to the whole of the evidence before me in the animal studies stream, I am not persuaded that the weight of the evidence in this stream supports the conclusion that glyphosate more likely than not causes cancer in mice and in rats, nor that the results of the animal bioassays provide important evidence as to glyphosate’s carcinogenicity to humans.
635 In broad terms, that conclusion follows for three reasons.
636 The first is that Dr Bayard’s evidence from the outset was marred by an approach to the animal bioassays which: (1) did not meaningfully assist in estimating false-positive rates because it did not involve any adjustment to the p-value which accounted for the problem of multiple comparisons; (2) included pooled analyses in circumstances where there existed a variety of substantive differences between the animal bioassays (such as different durations and dose-levels); and (3) involved Dr Bayard giving scientific opinions on fields outside of his expertise.
637 As I noted above, these matters caused me to view Dr Bayard’s evidence as to the animal bioassays in a different light and noting, where necessary, how those findings affected the relative assistance I derived from Dr Bayard’s findings in relation to a particular study. To take two examples: (1) Dr Bayard reached a diametrically opposite conclusion on causation in contrast to the PWG and reviewing pathologist in relation to the findings in Knezevich and Hogan (1983); and (2) considered that Wang (2019) provided direct evidence of in vivo mode of action (or mechanism of action) that glyphosate causes increased MM – both in circumstances where he is not a qualified toxicologist.
638 Secondly, as would already be evident, I have derived limited assistance from each of the animal bioassays (and Wang (2019)) relied upon by Dr Bayard in the light of several limitations which, when viewed collectively, can be grouped into several common categories. It is well to say something about four of them:
(1) In relation to each tumour type in rats and mice, most of the studies did not indicate a statistically significant result according to Dr Bayard’s predetermined criteria for significance. To take two examples: (a) for malignant lymphomas, it will be recalled that there were no statistically significant findings for male CD-1 mice in the majority of studies (Knezevich and Hogan (1983); Atkinson (1993); and Takahashi (1999)), and for female CD-1 mice, four out of five studies did not satisfy Dr Bayard’s predetermined test for statistical significance (the exception being Takahashi (1999)); and (b) for liver hepatocellular adenomas and carcinomas, the only statistically significant result for adenomas in male SD rats was in Stout and Ruecker (1990). Mr McNickle submits that this lack of consistency ought not diminish the overall weight to be placed on the animal bioassays on the basis that the absence of a statistically significant result in one study does not entail that a highly significant result in a second study should be discounted (echoing the submission made in the first stream in relation to Koutros (2019) and Alavanja (2014)) (see above (at [358]–[361])). To repeat, for the purposes of emphasis, my conclusion as to the animal studies (which in due course will affect my ultimate conclusion) rests upon an analysis of the whole of the evidence in this stream. This does not involve weighing the relative strength of the animal studies in isolation but mandates, among other things, an assessment of the consistency of the evidence viewed as a whole. As Dr Bayard explained (at JRH [32]):
If a tumour increase is seen in one study of, for example, male CD-1 mice, then, theoretically it should be seen in every study. These animal studies are designed such that the only difference among the four groups in each study is the amount of glyphosate they receive. By comparing individual tumour types within the same species/strain and sex, the multiple studies should allow a good estimate of consistency. However, there are several differences among the studies that limit these direct comparisons. Chief among these are (1) different sources for stocks of animals, (2) genetic drift of the animals’ DNA over time, (3) differences in diets among labs, (4) different study dosing levels among labs, (5) different laboratories, and (6) different pathologists’ diagnoses. All these contribute to different results. Nevertheless, consistency is the backbone of science and the evaluation of it is primary in my analysis.
(Emphasis added)
(2) Relatedly, a number of the animal bioassays reported inconsistent results when assessing relative dose levels. This was seen most clearly in Lankas (1981) and Stout and Ruecker (1990) where no correlation between glyphosate and thyroid C-cell carcinomas in female rats was found in relation to the latter study in circumstances where it was conducted at higher doses; and in relation to Sugimoto (1997) in circumstances where other studies conducted at higher dose levels (namely, Knezevich and Hogan (1983)) and lower (Wood (2009b)) revealed no statistically significant occurrences of hemangiosarcoma in mice.
(3) A number of the tumours identified by Dr Bayard are not rare in rats and mice. The evidence established, for example, that malignant lymphoma is the “most common spontaneously occurring tumour category in the mouse” and, as Dr Bayard accepted, the most common category of tumour in CD-1 mice (T1655.23–26). Further, with respect to testis interstitial cell tumours, Dr Bayard gave evidence that such tumours are not uncommon in the SD rat (averaging approximately 6.5%).
(4) Several of the animal bioassays lacked cross-gender significance. For kidney tumours in mice, for example, there were no incidences in female mice across all of the mouse studies. Further, for kidney adenomas in rats, the result in Enemoto (1997) was not replicated in females, nor any other study for female SD rats. Mr McNickle submits that this is not a reason to reduce the weight of such studies because the absence of a statistically significant result in one sex does not justify discounting statistically significant results in the other sex, given that the USEPA Guidelines specify that tumours in both sexes “adds to the significance of tumour findings” (see JRH [21]). When read in context, however, I do not consider that is a fair reading of the USEPA Guidelines. Although it was not expressly referred to by Monsanto, the USEPA noted in the Issues Paper (at 73) when referring to the relevant passage of the guidelines:
Other observations can strengthen or lessen the significance of tumor findings in carcinogenicity studies. Such factors include: uncommon tumor types; tumors at multiple sites; tumors in multiple species, strains, or both sexes; progression of lesions from preneoplastic to benign to malignant; reduced latency of neoplastic lesions (i.e., time to tumor); presence of metastases; unusual magnitude of tumor response; and proportion of malignant tumors (EPA, 2005).
(Emphasis and underlining added)
639 Thirdly, in relation to Dr Bayard’s reliance on Wang (2019) and his evidence given in Conclave I, I do not consider Dr Bayard’s evidence to be of assistance in demonstrating the translatability of the animal studies evidence to humans, primarily for two reasons: (1) notwithstanding the limitations associated with Wang (2019) that were borne out by the evidence, Dr Bayard lacked the requisite qualifications to conclude that that study provided evidence that glyphosate provides a mode or mechanism of action by which it can cause MM and NHL; and (2) despite the utility of animal models generally and the theoretical potential of animal studies to provide reliable data to predict the behaviour of diseases in humans (such as GBFs), as Professor Prince noted, the extrapolation of findings the subject of such models to humans is a different question which necessitates “high translatability” and the application of a number of criteria, including, inter alia, the animal species, dose exposure, and similarities in biological response (at JRI [132]).
640 For the foregoing reasons, on the material before me, I am not satisfied the animal studies evidence supports a conclusion that glyphosate more likely than not causes cancer in mice and in rats, nor that the results of the animal bioassays provide important evidence as to glyphosate’s carcinogenicity to humans.
G THE THIRD STREAM: MECHANISTIC EVIDENCE (CONCLAVES A, B AND G)
641 The third stream concerns mechanistic evidence. Broadly speaking, it involves two questions: first, whether the mechanistic studies support a finding that glyphosate and/or GBFs have any of the ten key characteristics of carcinogenicity; and secondly, whether the mechanistic studies support a finding that there is a biologically plausible mechanism by which glyphosate and/or GBFs cause NHL in humans.
642 Before turning to the mechanistic evidence, it is necessary to say something about the methodologies adopted in this stream.
643 Conclave A concerned with NHL causation and the field of cancer biology, which may be defined as follows (at JRA [17]):
Cancer occurs when cells develop changes that allow them to grow uncontrollably. Our cancer biology researchers are working to understand what causes these changes, how this leads to cancer, and what factors determine the success of cancer treatments. This is leading to better ways to diagnose and treat cancer. Our cancer researchers are discovering:
• The changes that make a normal cell become cancerous.
• The genes and proteins required for the growth and progression of cancer.
• New ways to treat cancer that target molecules essential for the cancer cell’s growth and survival.
• How to select the best treatment for a person with cancer.
644 Cancer is defined by the National Institute of Health (NIH) as “[a] term for diseases in which abnormal cells divide without control and can invade nearby tissues. Cancer cells can also spread to other parts of the body through the blood and lymph systems” and is divided into several subtypes (some of which we have already encountered above), including (at JRA [27]–[28]):
(1) carcinomas (which begin in the skin or in tissues that line or cover internal organs);
(2) sarcomas (which begin in bone, cartilage, fat, muscle, blood vessels, or other connective or supportive tissue);
(3) leukaemia (which forms in blood-forming tissue, such as the bone marrow, and causes too many abnormal blood cells to be made);
(4) lymphoma (Hodgkin type and NHL) and MM (which are cancers that begin in the cells of the immune system); and
(5) central nervous system cancers (which are begin in the tissues of the brain and spinal cord).
645 Lymphoma is a general term given to blood cancers that develop in the lymphatic system, which is a critical part of the immune system that includes the spleen, thymus, tonsils, adenoids and bone marrow. Lymphatic tissue is also found in the skin, gut and stomach, and brain. The lymphatic system generates specialist white blood cells (lymphocytes) to fight disease and infection (at JRA [55]). The NIH defines lymphoma and its subtypes as follows (at JRA [60]):
Cancer that begins in cells of the immune system. There are two basic categories of lymphomas. One kind is Hodgkin lymphoma [(also known as Hodgkin’s disease)], which is marked by the presence of a type of cell called the Reed-Sternberg cell. The other category is non-Hodgkin lymphomas, which includes a large, diverse group of cancers of immune system cells. Non-Hodgkin lymphomas can be further divided into cancers that have an indolent (slow-growing) course and those that have an aggressive (fast-growing) course. These subtypes behave and respond to treatment differently. Both Hodgkin and non-Hodgkin lymphomas can occur in children and adults, and prognosis and treatment depend on the stage and the type of cancer.
646 Around 85% of lymphomas are diagnosed as NHL. The remainder are Hodgkin lymphoma (at JRA [61]).
647 In lymphoma, the lymphocyte is altered so that it grows and multiplies uncontrollably and can form a tumour, usually in the lymph nodes (at JRA [42]). Lymphocytes include a number of different cell subtypes, including (at JRA [51]):
(1) B-cells (also known as B lymphocytes) which are critical to the making of antibodies that, among other things, target micro-organisms such as bacteria and viruses; and
(2) T-cells (also known as T lymphocytes) and NK cells that kill viruses, parasites and cancer cells, and produce cytokines (chemicals involved in messaging in growth of white blood cells and the immune response) which can recruit other cells to make antibodies that target micro-organisms.
648 B-cells and T-cells have receptors which are formulated within the cell to recognise foreign proteins (antigens). These are called B-cell receptors (BCR) and T-cell receptors (TCR), respectively. To make these receptors unique to the thousands of different antigens that a human can be exposed to, these cells undergo a very specific engineering process in the lymph nodes which involves multiple complex gene mutations aimed at giving these lymphocytes antigen-specificity. This is called somatic hypermutation (SHM). These mutational processes go wrong in NHL development: a phenomenon not observed in other cancers (at JRA [56]).
649 B-cells (or B lymphocytes) are also critical in the process of antibody production. Antibodies are a part of the immune system’s response to “foreign” invaders like viruses. Antibodies recognise, target and bind to specific proteins (referred to as antigens) expressed on the surface of bacteria, viruses and some cancer cells, and cause the cell’s destruction (at JRA [57]).
650 Lymph nodes are found at various points around the lymphatic system and are filled with lymphocytes, being B-cells and T-cells, which act as filtering stations for the lymph fluid. Lymphocytes help protect the body against infection by seeking out and destroying bacteria, viruses, parasites, and fungus. An example is when the body experiences a sore throat: the lymph nodes under the jawbone become swollen and tender because the B-cells and T-cells in the lymph node become activated and multiply in response to the identified virus or bacteria causing the infection (at JRA [58]).
651 NHL arises when developing B-cells or T-cells undergo a malignant change and multiply in an uncontrolled way and without order. These cells also become long-lived. The increasing numbers of abnormal lymphocytes (called lymphoma cells) accumulate and form the collections of cancer cells. These cancer cells initially become a single tumour in a lymph node and usually, over time, spread to other parts of the body (at JRA [59]).
652 NHL, however, is not a single entity: its different subtypes are broadly divided into the following two main groups: (1) B-cell NHL (arising from developing B-cells); and (2) T-cell NHL (arising from developing T-cells or NK cells) (at JRA [62]). There are more than 80 different sub-types of lymphoma currently recognised by a classification system developed by the World Health Organisation (WHO). Five of these subtypes belong to a group of diseases called Hodgkin lymphoma. All other subtypes are commonly grouped together and are called NHL (at JRA [66]).
653 In evaluating the evidence on GBFs and NHL causation, the experts employ three principles (at JRA [32]):
(1) over-arching biological plausibility based on what is known of the pathogenesis (that is, the development of a disease and the chain of events leading to it);
(2) the current biological experimental evidence (in vivo and in vitro experimental data) that has been proposed that GBFs can cause NHL in humans; and
(3) one’s interpretation of the clinical relevance of the epidemiological evidence that exposure to GBFs is associated with the development of NHL.
654 As would already be evident, this approach mirrors the weight of evidence approach taken by IARC and most authoritative bodies that perform carcinogenicity evaluation (at JRA [32]). In the context of Conclave A, however, Dr Flecknoe-Brown and Professor Prince are particularly concerned with using the field of cancer biology to: (1) examine the scientific evidence of the biological plausibility based on what is known of the pathogenesis of NHL; and (2) evaluate the current biological experimental evidence (in vivo and in vitro experimental data) that contends that exposure to GBFs can cause NHL in humans (at JRA [33]).
655 Notwithstanding that both Dr Flecknoe-Brown and Professor Prince have some understanding and familiarity with the interpretation of epidemiological studies, they are not experts in the field and defer to the interpretation of such studies the subject of the epidemiological stream (as discussed in Section E.3 above).
656 Replete throughout the transcript are references to the “key characteristics” (KCs). This was not a reference to those members of the inner bar who appeared in the initial trial and who are His Majesty’s counsel, but rather a reference to the ten key characteristics of carcinogenicity. The KCs, to which I will return shortly, aid in our understanding of carcinogenesis (that is, how cancers grow). Carcinogenesis is a multi-step process by which normal cells are transformed into cancer cells by acquiring various properties that allow them to form tumours (at JRB [40]).
657 The “Hallmarks of Cancer” is a concept developed by Hanahan and Weinberg (2000) and is central to understanding carcinogenesis, which comprises eight biological capabilities acquired during the multistep development of human tumours (hallmarks). The hallmarks constitute an organising principle for rationalising the complexities of cancer and include (as of 2011) (at JRB [41]–[42]):
(1) sustaining proliferative signalling,
(2) evading growth suppressors,
(3) resisting cell death,
(4) enabling replicative immortality,
(5) inducing angiogenesis,
(6) activating invasion and metastasis;
(7) reprogramming of energy metabolism; and
(8) evading immune destruction.
658 Underlying the hallmarks are the following enabling characteristics: (1) genome instability, which generates the genetic diversity that expedites their acquisition; (2) inflammation, which fosters multiple hallmark functions; and (3) creation of a “tumour microenvironment” (at JRB [44]). Although the hallmarks provide a framework as to what is required for a cancer to develop, it is not comprehensive in that the importance of each of the hallmarks will vary across different cancer types (at JRA [46]).
659 The KCs, on the other hand, were developed by IARC in 2015 to support cancer hazard identification and reflect the known properties of established carcinogens (at JRB [47]), as follows:
(1) Is electrophilic or can be metabolically activated to an electrophile (KC1);
(2) Is genotoxic (KC2);
(3) Alters DNA repair or causes genomic instability (KC3);
(4) Induces epigenetic alterations (KC4);
(5) Induces oxidative stress (KC5);
(6) Induces chronic inflammation (KC6);
(7) Is immunosuppressive (KC7);
(8) Modulates receptor-mediated effects (KC8);
(9) Causes immortalization (KC9);
(10) Alters cell proliferation, cell death, or nutrient supply (KC10).
660 Whereas the hallmarks are the properties of cancer cells, the KCs are the properties of human carcinogens that induce cancer. Thus, while the hallmarks describe what biology exists in a cancer, the KCs describe the actions of carcinogens that can cause those hallmarks to become acquired. The hallmarks and the KCS are, however, interrelated in that the hallmarks set boundaries for the KCs in biological terms. Indeed, some of the KCs produce effects analogous to the hallmarks (for example, carcinogens that induce genome instability (KC3) can produce this hallmark in both normal and cancer cells, and those that induce immunosuppression produce an effect analogous to cancer cells evading immune destruction by suppressing immune surveillance mechanisms). However, there often is not a one-to-one relationship and it is generally not possible to identify individual relationships between specific KCs of carcinogens and a single hallmark of cancer (at JRB [54]–[55]).
661 In relation to KC1, many chemicals that induce cancer and are carcinogenic to humans are either electrophilic or are converted in the body to electrophiles. Electrophilic substances can bind to DNA directly and cause DNA damage or can bind to the active sites of proteins thereby blocking key biochemical reactions, such as DNA repair. This typically means they are genotoxic (DNA damaging) and/or able to inhibit proteins which maintain the structure and integrity of DNA. Hence, many known human carcinogens possess KC1 and KC2 (and less frequently, KC3). Possession of these KCs indicates that the chemical is very likely to produce mutations in DNA (an inherited change in the sequence of DNA) that may lead to cancer. These mutations may take the form of a single change in the DNA sequence or a change at the chromosome level causing a large change. If these changes occur at the locations of genes controlling cell growth and division then cancer may be initiated or promoted (at JRB [48]).
662 In contrast to these irreversible mutations, epigenetic alterations (KC4) are reversible mechanisms that cause altered gene expression. Epigenetic modifications include methylation of the DNA, modifications of histone proteins which coat the DNA, and differential expression of non-coding RNAs (such as microRNAs) which control how genes are expressed. Many carcinogens induce these epigenetic alterations and therefore possess KC4 (at JRB [49]).
663 For reasons that will assume some importance later, many human carcinogens, including radiation and asbestos, induce a phenomenon known as “oxidative stress”. This can lead to damage to DNA and other cell components and the ability to induce it is KC5 (at JRB [50]).
664 The remaining five KCs do not involve DNA. Rather they are properties which increase the likelihood that cells containing mutations, however generated, be it by spontaneous mutation, oxidative stress, viruses, or other chemicals, will divide, proliferate and escape the immune system. Chemicals which possess KC6 will induce chronic inflammation that promotes the growth of small, undetected tumours into larger, more aggressive ones and those which possess KC7 will suppress the immune system and allow tumour cells to escape surveillance and spread. Chemicals which possess KC8 will alter hormone levels and promote the growth of hormone sensitive cancers (at JRB [51]).
665 In relation to KC9, normal cells have a finite lifespan controlled by sections of DNA at the end of chromosomes called telomeres. However, cancer cells can become immortal and do not die unless killed by an outside agent, such as a chemotherapy drug. Chemicals and other agents, such as viruses, may promote this immortalisation process and therefore possess KC9 (at JRB [52]).
666 Finally, with respect to KC10, cancer cells usually divide faster than normal cells; do not undergo programmed cell death; and often have an altered metabolic state that allows them to survive. Chemicals which enhance these processes possess KC10 and will promote the proliferation of cancers cells and tumours in general.
667 The KCs are not a mandatory component in determining the carcinogenicity of a substance but a set of guiding principles which, together with sufficient evidence of cancer induction in humans (usually because of epidemiological studies) can assist in determining whether the substance is capable of causing cancer (at JRB [58]). If, however, the evidence in humans is limited (that is, less than sufficient to determine carcinogenicity) or non-existent (for example, because of a lack of epidemiological studies), then an evaluation of the mechanistic evidence for carcinogenicity becomes important (at JRB [59]).
668 One way to assess this mechanistic evidence is to use the KCs. Since this approach allows the reviewer to identify and assess all the available information in a systematic manner, it has become the method of choice for IARC and other institutions (such as the USEPA’s IRIS program[me] and California EPA Proposition 65 committee and is endorsed by the US National Academies of Sciences, Engineering and Medicine) (at JRB [60]).
669 When the epidemiological evidence for carcinogenicity in humans is limited or insufficient, then the evaluation of the mechanistic evidence using the 10 KCs becomes critical, as does the evaluation of evidence from long-term studies in experimental animals. If, for example, there is sufficient evidence of cancer induction in two species of experimental animals, or in multiple studies in one species, combined with strong mechanistic evidence – based on the KCs in exposed humans – then IARC would conclude based on its preamble (a document which describes how it reaches its conclusions and guides all working groups) that the substance was “carcinogenic to humans”) (at JRB [60]). Specifically, the relevant sections for IARC evaluation are (at JRB [61]):
(1) exposure data;
(2) studies of cancer in humans which summarises all of the pertinent epidemiological studies and identifies tumour sites for which there is sufficient, limited, or inadequate evidence of carcinogenicity in humans;
(3) studies of cancer in experimental animals;
(4) mechanistic and other relevant data;
(5) summary; and
(6) evaluation and rationale.
670 As Professors Smith and Prince explain, in another scenario, if the evidence for cancer in humans was limited from the available epidemiological studies (that is, there is a positive association, but bias, chance and confounding cannot be ruled out) and the evidence in experimental animals was either insufficient or inconclusive, then the mechanistic evidence based on the 10 KCs can play an important role. If such evidence based on the KCs is considered strong, then the substance would be considered “probably carcinogenic to humans”. In other words, the available scientific evidence indicates that such a substance is reasonably anticipated to be a human carcinogen (at JRB [62]). Conversely, the mechanistic evidence can also play a role in downgrading the evaluation of a substance. This would occur if there were little or no evidence for one or more KCs, or if the mechanism of cancer induction in animals is not thought to operate in humans (at JRB [63]).
671 Viewed in isolation, the 10 KCs are not definitive and/or accurate for demonstrating that a substance is carcinogenic. If there is inadequate evidence of cancer in humans and less than sufficient evidence in experimental animals, then even if there is strong evidence for multiple KCs in humans, human cells, or other experimental systems, it can only be concluded that the substance in question is “possibly carcinogenic to humans”. This indicates that further study is required before a definitive and/or accurate determination can be made. However, in evaluating the evidence of mechanisms alone, the use of the KCs is a valuable tool (at JRB [64]).
672 Lastly, Professors Smith and Prince emphasise that the interpretation or evaluation of the quality of the scientific data examining the KCs is based on expert judgment. Indeed, as all such evaluations are, different individual or groups of scientists may reach different conclusions regarding the strength of the evidence for each of the 10 KCs. Further, the KCs are not intended to be static, but are likely to evolve over time as knowledge of the biological understanding of cancer and the mechanisms of carcinogenesis improve, and new methodologies of testing the KCs appear (at JRB [65]–[66]).
673 In determining whether there is a biologically plausible mechanism by which glyphosate and/or GBFs can cause NHL in humans, mechanistic toxicology plays an important role.
674 As Professor Smith and Dr Juberg note (at JRG [26]), the field mechanistic toxicology aims to understand how a chemical works to exert its effects or cause toxicity at the molecular level. It is essentially the identification and understanding of how a substance works in a living system related to the subsequent expression of effects or toxicity. A toxicological mechanism of action relates to a sufficient understanding of the molecular basis to assist in establishing causality between exposure to a substance or chemical and a specified outcome or effect.
675 Toxicological mechanisms can be identified for numerous toxicological outcomes including carcinogenicity, neurotoxicity, and developmental toxicity. Scientists often design and conduct mechanistic studies which aim to provide insight into possible toxicological mechanisms and these studies can be used in conjunction with other sources of information (for example, laboratory animal studies, human or epidemiological data) to make decisions about the probability of a chemical causing harm to humans (at JRG [27]). Other mechanistic toxicology studies can be designed and conducted to determine if one or more components of a toxicological mechanism can be demonstrated and may increase our insight and confidence in the proposed toxicological mechanism for a chemical or substance (at JRG [29]).
676 While one goal of conducting mechanistic toxicology studies is to probe and further understand toxicological mechanism between exposure and effect (for example, in the present case related to carcinogenicity), this is not always possible and hence a less refined, but nevertheless important priority is to propose a mode of action for tumour induction by a chemical or substance. Mode of action outlines the thought processes involved in making use of mechanistic data in risk assessment in a structured way. In this context, a supported mode of action would have evidence provided by robust mechanistic data to establish a biologically plausible explanation for a particular toxicological outcome (at JRG [30]).
677 The USEPA Guidelines places importance on mode of action for carcinogens, as follows (at JRG [31]):
The use of mode of action in the assessment of potential carcinogens is a main focus of these cancer guidelines. Elucidation of a mode of action for a particular cancer response in animals or humans is a data-rich determination. Significant information should be developed to ensure that a scientifically justifiable mode of action underlies the process leading to cancer at a given site.
(Emphasis added)
678 Importantly, mechanistic studies can provide evidence for each of KCs. In turn, this evidence can be categorised to determine if it is sufficiently strong to conclude that the chemical or substance possesses that KC (at JRG [32]). As noted above, it is recognised in the field of mechanistic toxicology that the KCs in isolation are not an appropriate and sufficient framework for evaluating and/or determining whether a substance, such as glyphosate and/or GBFs, causes cancers, including NHL. However, in combination with epidemiological studies of exposed humans and long-term cancer studies in experimental animals, they form an important component of the weight of evidence approach (at JRG [48]).
679 Further, all relevant mechanistic toxicology studies, data, and other scientific information which inform carcinogenic potential may need to be considered along with other evidence before rendering a conclusion on the potential for glyphosate and/or GBFs to be capable of causing cancer in humans. Ordinarily, the process involves using the data from the mechanistic studies in combination with data from epidemiological studies of exposed humans and long-term cancer studies in experimental animals. Together they form the components of the weight of evidence evaluation to determine if exposure to glyphosate and/or GBFs is carcinogenic, including as a cause of NHL (that is, an evaluation of the three streams) (at JRG [33]–[35]).
680 Mechanistic studies may include laboratory studies in experimental animals, micro-organisms like bacteria and yeast, cell cultures including cultures of human cells, sub-cellular fractions and purified enzymes and studies using biological markers (biomarkers) of injury in humans. For example, blood can be drawn from persons occupationally exposed to a chemical or substance of interest and the number and types of cells in the blood measured and compared with those levels in a matched control set of unexposed people to see if there are differences. Similar experiments can be performed in experimental animals by exposing them to different doses of the same chemical and measuring the cell make-up of their blood (at JRG [41]).
681 To determine if the chemical damages DNA, multiple different types of tests can be performed, including measures of DNA damage in the white blood cells of exposed humans. Alternatively, simple test systems can be used, such as Salmonella bacteria in a petri dish. One such test is called the Ames test, which allows one to screen for mutations in the DNA of bacteria exposed to a chemical of interest (at JRG [42]).
682 Mechanistic toxicology studies have been consistently used to provide evidence regarding the ability of a chemical or substance to cause cancer, especially regarding the relevance to humans of findings in experimental animals and to screen for the ability of the chemical to cause genotoxicity and mutagenicity. The goal is to gain insight into how a chemical may produce toxic effects. In addition to established types of mechanistic studies, another approach towards understanding toxicological mechanism is to conduct hypothesis-based testing, whereby one is able to develop an a priori hypothesis related to how a chemical may cause a certain effect; properly design an approach to test that hypothesis; and then conduct the study to ascertain whether the hypothesised outcome was realised (at JRG [43]–[44]).
683 The results of these mechanistic studies are then used in conjunction with other sources of information to make decisions about the probability of a chemical’s propensity for causing harm to humans, including causing cancer or another toxicological outcome. The weight of evidence approach discussed above is the methodology that it is typically used by both authoritative and regulatory bodies when assessing the relationship between exposure to a chemical or substance and subsequent toxicological outcomes (at JRG [45]).
684 The mechanistic evidence stream spanned Conclaves A, B and G and involved the following expert witnesses:
(1) Conclave A – Cancer biology (causation of NHL)
Dr Flecknoe-Brown and Professor Prince
(2) Conclave B – Cancer biology (hallmarks of cancer and key characteristics of human carcinogens)
Professor Smith and Professor Prince
(3) Conclave G – Mechanistic toxicology
Professor Smith and Dr Juberg
685 Several individual reports were prepared by each of the experts for the purposes of this stream, as set out in the table below. For the purposes of Conclave A, Dr Flecknoe-Brown did not prepare an individual report:
Report | Date | Report Topic |
Expert Report of Professor Smith (First Smith Report) | 11 August 2021 | Whether mechanistic studies of glyphosate provide the necessary biological plausibility and mechanistic basis for the conclusion that use or exposure to glyphosate and/or GBFs such as Roundup Biactive and Roundup Herbicide can cause NHL. |
Expert Report of Professor Prince (First Prince Report) | 1 February 2022 | The relationship, if any, between glyphosate and/or GBFs and NHL and Mr McNickle’s prognosis. |
Expert Report of Dr Juberg (First Juberg Report) | 2 February 2022 | Evaluation of the mutagenic and genotoxic profile for glyphosate, GBFs, surfactants, and aminomethyl phosphonic acid and to form a scientifically-based opinion on whether these materials possess mutagenic or genotoxic potential and whether they represent potential genotoxic carcinogens. |
Supplementary Expert Report of Dr Juberg (Second Juberg Report) | 16 July 2022 | Response to the First Bayard Report (at [141]–[151]) which addresses, among other things, whether it is more likely than not that glyphosate causes cancer in animals and should be considered a human carcinogen. |
Supplementary Report of Professor Smith (Second Smith Report) | 7 September 2023 | The significance of the Chang (2023), Alvarez-Moya (2023) and Rana (2023) studies and whether those studies support the conclusion that use or exposure to glyphosate and/or GBFs such as Roundup Biactive and Roundup Herbicide can cause NHL. |
Further Supplementary Report of Dr Juberg (Third Juberg Report) | 10 September 2023 | Review and commentary on the Second Smith Reports noting any specific agreement or disagreement with statements in that report. |
686 In the following section, to the extent I have not already done so in the preceding streams, I will introduce each of the experts who participated in this stream and make some general observations as to their credit.
687 Dr Stephen Flecknoe-Brown is a medical practitioner in the field of haematology and an Associate Professor of Medicine at the University of New South Wales. Dr Flecknoe-Brown was engaged by Mr McNickle.
688 Dr Flecknoe-Brown commenced practice as a haematologist in 1982. Dr Flecknoe-Brown studied at Haileybury College and Luther College and the Melbourne Monash University Faculty of Medicine before commencing residency at the Royal Prince Alfred Hospital. Between 1980–1982, Dr Flecknoe-Brown was admitted as a fellow in the Royal Australasian College of Physicians (RACP); the Royal College of Pathologists of Australasia; the University of California (as a hematology-oncology fellow); and as a research fellow at the Ludwig Institute for Cancer Research Sydney. He holds a number of honours and appointments, including, among other things: a life fellowship in the Royal Society of Medicine and the RACP and a fellowship in the Royal College of Physicians in Edinburgh; and an appointment on the Governance Committee of the RACP and as Secretary of the Regional Medical Specialists’ Association. He is also the editor of Common Sense Pathology. Dr Flecknoe-Brown ceased practising as a haematologist in 2020 and currently practises in general medical work.
689 In summary, Dr Flecknoe-Brown’s conclusion in the JRA is that it is more likely than not that glyphosate and/or GBFs cause NHL in humans (at JRA [176]). Monsanto contends that the Court should place little to no weight on Dr Flecknoe-Brown’s conclusion and opinions, for four reasons: (1) he lacks independence from Mr McNickle (or his solicitors); (2) he lacks the requisite expertise in areas in which he purported to give expert opinion evidence; (3) he failed to disclose limitations in various studies he relied upon; and (4) his treatment of the epidemiological evidence was contrary to the approach of the expert epidemiologists who gave evidence for both parties and were therefore better qualified to give that evidence.
690 Monsanto developed these submissions as follows.
691 First, in relation to his alleged lack of independence, Dr Flecknoe-Brown was initially engaged by Maurice Blackburn as a consulting expert (T118.6–7). It is said that he understood that: (1) the firm was acting for plaintiffs in a class action against Monsanto concerning an allegation that exposure to Roundup caused NHL; (2) understood that Maurice Blackburn was seeking compensation for people in that category (T116.37–38); (3) had already provided advice to another law firm in a class action on this question (T117.3–16); (4) had carried out research to support that advice (T117.18); (5) stated that he “admire[d] Maurice Blackburn and … its mission” (T117.38–42); and (6) understood that at least part of that “mission” was “to achieve the compensation which was going to be sought in the action” (T117.44–45).
692 It is also said that over a number of years, Dr Flecknoe-Brown carried out a variety of tasks which are ordinarily carried out by a consulting expert, as opposed to an independent expert, including, for example, drafting and reviewing pleadings (T121.8–11; T118.13–17; T137.5; T121.24–33) and discussing the forensic debate that would ensue arising out the concept of carcinogenicity (T120.23–24), including the implications of the narrowness or width of what “carcinogenicity” meant (T120.37–39). Further, it is contended that Dr Flecknoe-Brown understood that his role included, among other things, providing advice to Maurice Blackburn concerning evidence that might emerge from Monsanto’s experts (T123.30–32) and attended to multiple research tasks given by Maurice Blackburn (T125.4–9; T127.8–15), which are matters that are the kind of tasks ordinarily carried by consulting experts.
693 Secondly, it is contended that Dr Flecknoe-Brown repeatedly omitted from his opinions in the JRA limitations and qualifications that were expressly identified by the authors of the studies on which he relied. For example, Dr Flecknoe-Brown relied upon Donato (2020) in support of the proposition that the epidemiology evidence indicates an association between glyphosate and/or GBFs and NHL in humans in circumstances where the authors of Donato (2020) stated that “we found no consistent indication of an association between exposure to glyphosate and risk of NHL or MM” (at 9) (T162.36–44). In cross-examination, it is said Dr Flecknoe-Brown conceded that this observation applied to the highest exposure categories to glyphosate (T163.12–15) and amounted to the Donato (2020) study indicating that its findings were of no significance (T163.36–38). However, neither Dr Weisenburger (a prior expert who ultimately did not participate in the proceeding) nor Dr Flecknoe-Brown disclosed that limitation (T164.1–5). To take another example, Dr Flecknoe-Brown relied on Paz-y-Mino (2007) in support of his opinion, which he considered was a “fair summary” and “wouldn’t modify the wording terribly much” (T206.31–35), but then went on, it is said, to make a series of concessions in relation to it, including, among other things, that: (1) the study does not disclose whether any of the participants developed any sort of cancer at all (T210.19–23); (2) blood sampling from exposed individuals varied from two weeks to two months after exposure, thereby allowing time for confounders to affect the study (T210.25–211.29); and (3) it is unknown whether participants were exposed to other pesticides in the two months after the first group was tested (T211.33–45). I will return to other examples below when addressing the mechanistic evidence.
694 Relatedly, it is submitted that Dr Flecknoe-Brown recited the work of Dr Weisenburger with little to no independence. Dr Flecknoe-Brown generally accepted that the stated basis for his contribution to the JRA as to his belief concerning the carcinogenicity of glyphosate and/or GBFs was the IARC Monograph 2017 (IARC Monograph 2017) and Weisenburger (2021) (T158.44–159.7; T15922–26; T159.34–38; T160.16–34; T169.1–30; T170.18–26; T191.24–40; T203.31–46; T204.5–11; T205.34–37). Dr Flecknoe-Brown gave evidence that the paper “seemed a good guide so that I didn’t miss anything” (T195.27–28) and accepted that he assumed Dr Weisenburger’s paper was an exercise of objective and impartial analysis (T195.30–38). It is said the significance of this is that, given Dr Weisenburger’s history and involvement in glyphosate litigation in the United States (which he acknowledged in his disclosure statement for the article (at 8), Dr Flecknoe-Brown cannot be expected to bring an objective mind to this proceeding.
695 Thirdly, it is contended that Dr Flecknoe-Brown lacked expertise in a number specialised fields relevant to his opinion, namely that: (1) after admission to the College of Physicians, Dr Flecknoe-Brown has undertaken no higher research study (T112.32–33); (2) none of the research he has undertaken has concerned the causes of NHL (T113.10; T114.40–43; T115.3–5); (3) none of his research activities have involved examining the relationship between glyphosate, GBFs and NHL (T114.42–43); (4) he has never published a paper on the causes of NHL, or the relationship between glyphosate and cancer (T114.29–33); (5) research has not comprised a large proportion of his professional work (T114.45–47); (6) his publication record only contains four publications that relate to NHL; (7) he does not consider himself to be an expert in epidemiology (T115.21–23); (8) he has not trained in toxicology and would not describe himself as a toxicologist, but says he has a working knowledge of toxicology concepts (T115.25–30); and (9) he has never conducted a micronucleus assay (T218.23–26).
696 As to the approach Dr Flecknoe-Brown took in making his contribution to the JRA, it is said he conceded first in giving the overview of epidemiology (at JRA [178]–[179]), he had only reviewed the individual epidemiology studies “in the generalist perspective” because he said, “I don’t think I’m qualified to interrogate them in detail” (T191.15–16); and secondly, when expressing his opinion concerning the USEPA’s treatment of the animal bioassays (at JRA [184]): (a) he was not qualified to express a view about the efficacy or methodological security of any animal studies as an expert (T200.25–28); and (b) he had not reviewed the same data that the USEPA had reviewed (T200.30–32).
697 Fourthly, it is contended that Dr Flecknoe-Brown’s approach to the epidemiology evidence was misguided. It is said that his treatment of the epidemiology evidence concerning glyphosate and/or GBFs (at JRA [178]–[179]) was contrary to the approach taken by AP Harris and Professor Checkoway in that it was solely reliant on meta-analyses and conceded that he would accept their views about the utility of meta-analyses (T193.4).
698 Although I did not find all aspects of Dr Flecknoe-Brown’s evidence persuasive, in my view, I think he did his best to provide assistance to the Court.
699 As Monsanto notes, prior to Dr Flecknoe-Brown’s engagement, I had expressed some preliminary concerns that in the light of his previous consulting work, Dr Flecknoe-Brown may not bring an independent mind to the task of giving expert evidence in this proceeding (see, for example, T40.18–19; T40.31–33; T40.42–46; T41.15–20 (Case Management Hearing, 3 August 2022)). Notwithstanding those preliminary views, as I noted earlier (at [32]–[33]), the mere fact that an expert has expressed an opinion in this case which aligns with a previously held opinion on the same issue is not a complete nor satisfactory basis for the Court to reduce the weight to be afforded to the expert’s opinion evidence, let alone reject its admission. It would be an error to allow a rigorous attempt to uncover alleged motivations of an expert as directing attention away from an assessment of the overall substantive merits of the witness’ evidence.
700 Although the circumstances of his retention and the fact that Dr Flecknoe-Brown had been involved in making minor amendments to the pleadings are less than ideal, in the end I think that Dr Flecknoe-Brown tried to live up to his obligations under the Expert Witness Code of Conduct as best he could. Moreover, I do not accept Monsanto’s submission that his failure to highlight various limitations identified by the authors of studies he relied upon (such as Donato (2020) or Paz-y-Mino (2007)) as conduct deliberately aimed at obfuscating the scientific evidence. The principal reason for this, having had the benefit of seeing this evidence unfold, is that notwithstanding that he occasionally demonstrated a degree of reluctance, Dr Flecknoe-Brown made appropriate concessions, particularly when certain matters were outside of his area of expertise. It will suffice to note two examples.
701 The first is that in relation to Bolognesi (2009), a study relied upon by Dr Flecknoe-Brown, the authors noted that “[t]he smaller number of subjects recruited in this study and small amount of information about the exposure precluded any conclusions. Many pesticides are used in conventional agriculture in Colombia and many pesticides are used in the production of coca … however, there is not sufficient information to correlate the frequency of MN to the pesticide exposure” (at 544). Although Dr Flecknoe-Brown omitted that qualification in the JRA, he conceded, inter alia, that: (1) he would not place his expertise above that of the authors themselves (T220.29–35); (2) he went further than the authors themselves were prepared to go (T232.35–38); and (3) he was not familiar with the other agricultural chemicals used in South America (T233.27–28). Dr Flecknoe-Brown later gave evidence that he should have referred to these qualifications in the JRA (T241.1–14):
MR FINCH: Well, it wasn’t really the best you could do, was it? You left out important qualifications.
PROFESSOR FLECKNOE-BROWN: That’s correct.
MR FINCH: And you substituted your opinion for those of the authors.
PROFESSOR FLECKNOE-BROWN: My independent assessment, yes.
MR FINCH: But that’s not the best you can do to give a fair assessment of the data, is it?
PROFESSOR FLECKNOE-BROWN: If – yes, I agree that strengths and weaknesses are best included in reports and qualifications should be acknowledged.
702 The second is that in relation to the overview given of the epidemiology studies (at JRA [178]–[179]), Dr Flecknoe-Brown conceded that he only gave an overview in a “generalist perspective” and “[was not] qualified to interrogate them in detail” (T191.15–22). Later, when the opinion of AP Harris and Professor Checkoway was put to Dr Flecknoe-Brown concerning the utility of meta-analyses and the consistency of the epidemiology studies, Dr Flecknoe-Brown deferred to their assessment, noting (T193.1–4):
MR FINCH: … Now, putting it bluntly, you would accept the views of the expert epidemiologists, wouldn’t you, about that?
PROFESSOR FLECKNOE-BROWN: They’re the experts, yes.
703 For these reasons, I do not consider that Dr Flecknoe-Brown came to this trial with a fixed view, intent on avoiding concessions adverse to Mr McNickle’s case theory. Overall, my impression is that he is a knowledgeable and highly experienced clinician who conscientiously sought to assist the Court. That is not to diminish the reality, however, that a combination of his lack of expertise in certain areas and omission of various limitations in the studies he relied upon in the JRA has rendered aspects of his evidence unsatisfactory.
704 I will return to these issues when addressing the mechanistic evidence below.
Professor Prince
705 I have already introduced Professor Prince in Section II above and noted that Mr McNickle made extensive submissions as to why the Court should have significant concerns about his evidence. In summary, Professor Prince gave evidence in support of his conclusion that he was not satisfied that exposure to GBFs increases the risk of NHL in humans or can cause an individual to develop NHL (at JRA [267]). Professor Prince also expressed the opinion that he does not believe that there is a biologically plausible mechanism by which GBFs could cause NHL (at JRA [207]; JRB [118]).
706 Mr McNickle’s submissions may be summarised as follows.
707 First, Professor Prince gave untrue evidence in relation to the joint reports he had reviewed for the purposes of the initial trial. On 6 September 2023, Professor Prince gave evidence that the only joint report that he had been given, other than those of which he was a joint author, was the JRG, whereas the true position, it is said, was that two weeks earlier, on or around 23 August 2023, he had been given a folder which included two other joint reports of which he was not a co-author, being the JRC and JRH, both of which he had read to differing extents (T309.26–45). Mr McNickle contends that that evidence casts a pall of unreliability over Professor Prince’s evidence and raises doubts as to his independence.
708 Secondly, approximately three weeks before the initial, Monsanto’s solicitors (Freehills) provided Professor Prince with a table which set out experimental concerns that Dr Juberg had expressed in the First Juberg Report and in the JRG in relation to Paz-y-Mino (2007) and Bolognesi (2009) (Juberg Table). Professor Prince also attended a meeting with Freehills in August 2023 at which time there was a discussion about those studies (T528.35–43). Mr McNickle contends that the provision of the Juberg Table to Professor Prince, less than one month before the start of the trial, at the very least, created a significant risk that his opinions about Paz-y-Mino (2007) and Bolognesi (2009) would be inappropriately influenced by having been informed of what another one of the Monsanto’s experts had said about those studies in another conclave.
709 It is said that this risk materialised when Professor Prince gave evidence and made the same (or very similar) criticisms of those studies, despite Professor Prince not expressing such criticisms in his written reports. To take two examples: (1) Professor Prince made the same criticism of Paz-y-Mino (2007) that Dr Juberg had made relating to sample collection and processing having occurred at different times, as acknowledged by Professor Prince (T210.38–211.16; T533.31–37); and (2) Professor Prince made the same criticism of Paz-y-Mino (2007) that Dr Juberg had made relating to individuals who were the subject of the study possibly having been exposed to pesticides other than glyphosate, as acknowledged by Professor Prince (T540.34–39).
710 Thirdly, when asked whether he had been given any other tables drawing his attention to various aspects of evidence given in other conclaves, Professor Prince stated: “the only other table that I’ve ever been given was the one … about the discrepancies of the quotations, et cetera, between what I had written and what needed to be … clarified” (T541.33–36). It is said that evidence was incorrect. Professor Prince was shown another document prepared by Freehills entitled “Studies to Show Prof Prince from Applicant’s Opening Submissions”, followed by the exchange (T571.17–23):
MR CLEMENTS: Thank you, your Honour. Professor, having now seen that document, does that refresh your memory that, in fact, you had received more than two tables?
PROFESSOR PRINCE: Yes, it is. It’s formally a table. Yes. I misrepresented because I didn’t think of this as a table.
711 Fourthly, it is said Professor Prince gave inconsistent evidence about whether he had looked for EZH2 in Table S3 of Lohr (2012). In cross-examination, Professor Prince gave evidence that he was not able to find EZH2 in that table (T409.35–38), which was consistent with a note he had provided to Freehills during the initial trial (T187.29–47). However, Professor Prince later gave evidence that he was able to find EZH2 (T413.42–47). Professor Prince gave evidence (T415.13–36):
HIS HONOUR: Sorry, can I just ask a question. I just want to understand Professor Prince’s evidence because it may be – I will have a look at the transcript. But I had thought you earlier said that, when you looked at the table, you couldn’t find any reference to EZH2 and that led to your chain of reasoning. Now, that was part of your chain of reasoning. But I think in response to a question – the last question you were asked you said you did look at the table and did find EZH2 or I’m just wondering – did I misunderstand your evidence?
PROFESSOR PRINCE: So I wasn’t able to fully explain but it’s about the numbers of genes. So - - -
HIS HONOUR: No. No. No. I’m just asking you, when you looked at the table - - -
PROFESSOR PRINCE: Yes.
HIS HONOUR: - - - for the purposes of preparing, did you find EZH2 or did you not find it?
PROFESSOR PRINCE: In that table, yes, I did.
712 Fifthly, it is contended that Professor Prince made a substantial change to the JRI on the eve of the initial trial when prompted by Monsanto’s solicitors. In a series of email exchanges between him and Freehills on 1 September 2023, Professor Prince agreed to amend the statement (at JRI [78]) that “… it is recognised that increased expression of AID always leads to more mutations” to “it is recognised that increased expression of AID does not always lead to more mutations” (emphasis added). In cross-examination, Professor Prince gave evidence (T373.3–34):
MR CLEMENTS: … but there has been no relevant change in the science between the time you signed off on the joint report in December 2022 and the time of this email exchange last Friday, has there?
PROFESSOR PRINCE: No.
MR CLEMENTS: The only thing that has changed is that – and this isn’t your fault – the only thing that has changed is that the respondent’s solicitors have done an exercise in trying to establish if there’s any inconsistencies between your first report and your second to try to assist their defence in this case; that’s true, isn’t it?
PROFESSOR PRINCE: Yes. Because both statements are true. It will be – it can always and it can not always, depending on the situation – and depending on the situation it’s working in under, whether it’s working in under an abnormal scenario, so for the layman as terms, an increased in AID is considered as just an increase in AID but when we’re thinking about its capacity to – to be translated and then produce functioning AID, they’re two different scenarios and that’s all that I was trying – that we discussed in the two different situations. In the Conclave we were talking about the normal situation of AID.
MR CLEMENTS: Well, you didn’t qualify it like that in paragraph 78 of the Conclave, did you?
PROFESSOR PRINCE: No, I didn’t. No.
713 Mr McNickle submits that Professor Prince’s evidence “both statements are true …” and his readiness to accept the amendment to the JRI should diminish his reliability as an expert witness.
714 Sixthly, it is contended that Professor Prince gave argumentative and inconsistent evidence concerning whether smoking increases the risk of follicular lymphoma (FL) (a type of NHL). Professor Prince was cross-examined in relation to a paper he co-authored with Odutola et al in 2022, which paper concluded “the totality of these epidemiological findings implicates tobacco smoke as carcinogenic for FL” (at 18) (T318-321). It is said that Professor Prince refused to admit in cross-examination that persons with a history of smoking tobacco had an increased risk of FL, noting that “I just don’t know if smoking causes – is associated with follicular lymphoma” (T318.27–31; T319.33–43), but later conceded that when he signed off on Odutola (2022) as co-author, he was satisfied that the risk of FL increases with both smoking and pesticide exposure (T327.5–9). Mr McNickle submits that Professor Prince’s refusal, a short time earlier in cross-examination, to accept smoking increases the risk of FL reflected poorly on his reliability and credibility as a witness.
715 It is fair to describe Professor Prince as someone uninhibited by any discernible self-doubt. His overweening confidence in the correctness of his opinions was manifest in his presentation in the witness box. He was adamant that he was right in asserting that glyphosate does not cause NHL.
716 Although this assertive approach was somewhat disconcerting, it is perhaps unsurprising for two reasons.
717 The first is that in his role as Professor/Director of Molecular Oncology and Cancer Immunology, Professor Prince is often responsible for difficult life and death decisions in the treatment of cancer patients. It is not to stretch unduly the bounds of s 144 EA to observe that is a role and clinical culture which necessitates eschewing indecisiveness and equivocation. The second is that as Monsanto’s “star” expert witness in the proceeding, having participated in three of the ten expert conclaves, and in the light of his qualifications and extensive clinical experience and research on NHL, Professor Prince considered himself one of the most authoritative voices on NHL causation in Australia. I mention these matters not because I have placed more or less weight on Professor Prince’s opinions as a consequence, but because it goes some way in explaining some of his answers given in cross-examination and general demeanour in the witness box.
718 With that aside, subject to a matter to which I will return shortly, I have some difficulty accepting Mr McNickle’s submissions as to Professor Prince’s credit.
719 The bulk of Mr McNickle’s submissions above can be broadly categorised as allegations seeking to impugn Professor Prince’s credit on the basis he knowingly misrepresented that he had (or had not) been provided with certain material, or that he had (or had not) read certain material in advance of giving evidence (see above (at [707]–[713])).
720 I do not regard these submissions as helpful. Apart from distracting from the substantive merits of the evidence, there may be several explanations for Professor Prince: (1) giving evidence as to the joint reports that he had (or had not) read prior to the trial; (2) reviewing material prepared by another expert witness prior to the trial; (3) giving evidence as to whether what he had read constituted a “table” or not; (4) giving evidence as to whether EZH2 was found in Table S3 of Lohr (2012); and (5) giving evidence as to opinions previously expressed in journal articles he co-authored – chief among them being mere inadvertence. This is not to exclude the possibility that Professor Prince did give incorrect evidence as to one or more of these matters, but I am not satisfied on the evidence that they are explicable for a reason other than a lapse of memory or inadvertence. In any event, insofar as Professor Prince had regard to the analyses of other experts in the proceeding, he was entitled to do so for the reasons I have explained earlier with reference to Karpik v Carnival (see above (at [62])).
721 The only remaining matter which gave me some pause was Professor Prince’s amendment to the JRI (at [78]) at the eleventh hour. Although not directly relevant to this stream, I consider it was more than a mere oversight for Professor Prince to have approved the initial wording of the JRI and not to have qualified his subsequent amendment to the JRI with the explanation adduced from him in cross-examination (see above (at [712])).
722 With that said, and notwithstanding my observations as to Professor Prince’s general demeanour in the witness box, my overall impression is that Professor Prince approached the task of giving evidence with integrity and attempted to assist the Court. As will be seen, when pressed on his evidence, Professor Prince made some (albeit grudging) concessions, and his tendency to engage in long-winded answers was largely a product, in my view, of a genuine attempt to explain his strongly held opinions in the JRA, JRB and JRG and the scientific evidence, rather than stray into the role of an advocate for Monsanto.
723 Professor Martyn Smith is a Professor of Toxicology and holds the Kenneth and Marjorie Kaiser Chair of Cancer Epidemiology in the School of Public Health, University of California at Berkeley. Professor Smith was engaged by Mr McNickle.
724 Professor Smith holds a Bachelor of Science in Biology from Queen Elizabeth College, University of London (1977) and a PhD in Biochemistry from the Medical College of St. Bartholomew’s Hospital, London, England (1980). His PhD research involved studies of oxidative drug metabolism using quantitative cytochemical and biochemical methods. Professor Smith performed post-doctoral research on mechanisms of toxicity in the Department of Toxicology at the Karolinska Institute, Stockholm, Sweden (1980–1981) under the supervision of Professor Sten Orrenius, before returning to the University of London in 1981 to teach the first combined Bachelor of Science degree course in toxicology and pharmacology offered in the United Kingdom. As of the date of his first report, Professor Smith teaches toxicology classes in “Health Risk Assessment and Regulation” and “Riskscapes and Environmental Justice” to approximately 20–30 graduate students each year and supervises the research of undergraduate and graduate students and post-doctoral fellows and participates on thesis committees.
725 Professor Smith has been a full professor since 1992 and has been employed at Berkeley since 1982. Previously, Professor Smith held the titles of Assistant Professor (1982–1987) and Associate Professor (1987–1992). Between 1993–2003 and 2007–2010, Professor Smith served in the rotating positions of head and deputy-head of the Division of Environmental Health Sciences. From 2003–2005, he served as Chair of the Faculty in the School of Public Health and, from 2012–2014, served as Director of the Berkeley Institute of the Environment. Professor Smith is also Director of the Superfund Research Centre at Berkeley (a position held continuously since 1987). Professor Smith holds several awards, including as a Fellow of the American Association for the Advancement of Science and as a member of the Collegium Ramazzini, an independent international academy comprised of 180 internationally renowned experts in the fields of occupational and environmental health. Professor Smith is also a member of the editorial boards of the journals Cancer Epidemiology, Biomarkers and Prevention, Exposome and Cell Biochemistry and Function and has previously served on the board of several other peer-reviewed journals.
726 Professor Smith’s conclusion in the JRB and JRG, in summary, is that there exist biologically plausible mechanisms by which use of or exposure to glyphosate and/or GBFs can lead to the development of NHL (at JRB [169]), and that glyphosate and/or GBFs are genotoxic, mutagenic, and genotoxic carcinogens (at JRG [93]).
727 Monsanto devoted some time developing submissions as to why the Court should find that Professor Smith was not an independent witness. In short, it contends that in the light of his professional background, Professor Smith is a “hired gun” for plaintiff law firms pursuing litigation concerning the alleged carcinogenic effects of glyphosate.
728 Regrettably, it is necessary to descend into some detail concerning this allegation.
729 First, Monsanto submits that Professor Smith has extensive experience working in plaintiff-litigation. It is said Professor Smith has been involved in litigation as an expert for 40 years (T1276.26–28); has given depositions in hundreds of items of litigation in the United States (T1276.33–34); testified in court between 15–20 times (T1276.30–31); and has been retained by over 100 law firms in the United States (T1276.40–41). All those items of litigation have involved a substance of some kind or medical product (T1276.36–38). Since 1998, he has only given evidence for plaintiffs (T1276.43–45). Professor Smith first received money for glyphosate litigation in February or March 2016 from the US law firm, Andrus Wagstaff (T1277.1–3). He first acted as a consultant to Andrus Wagstaff before his role was changed into a “testifying” witness (T1284.12–16). Professor Smith has given evidence by deposition or at trial in four different glyphosate proceedings in the United States over a total of eight days between September 2018 and May 2023 (T1277.5–10) and has spent at least 300 hours liaising with lawyers based in the United States on glyphosate litigation (T1278.10–18; T1317.15–17).
730 Secondly, it is contended that Professor Smith is motivated by a desire to defend IARC and his colleagues, being a member of IARC’s Scientific Advisory Board between 2010–2014 (T1278.26–27). He was also an editor of the IARC publication known as Molecular Epidemiology Principles and Practice in April 2012 (T1278.29–31); was a speaker and presenter on KCs at an IARC conference in June 2016 (T1278.33–37); was a speaker and presenter at IARC in September 2018 (T1282.14–24); was a co-author of several articles, papers or presentations that appear in IARC publications (T1282.26–27); and has served on IARC Working Groups (T1282.29). Against this background, it is said Professor Smith conceded that a reason underpinning his decision to become a testifying witness in glyphosate litigation was that he was “annoyed” by what he regarded to be an attack on IARC and its scientists so that he felt he should step up and act as a testifying witness (T1284.18–25).
731 Thirdly, Monsanto contends that consistently with that motivation, Professor Smith has propagated a number of allegations about Monsanto. It is said that during the time that Professor Smith was engaged as a testifying expert for plaintiffs in glyphosate litigation in the United States, he used his Twitter account to re-tweet serious allegations against Monsanto. For example, on 18 January 2019, he re-tweeted an allegation that Monsanto had engaged in improper practices in briefing journalists (T1284.37–1285.31) and, on 17 January 2019, he re-tweeted a further message disparaging Monsanto because he “wanted to support IARC and the IARC Working Group” (T1286.23–25). On 27 January 2019, Professor Smith re-tweeted a message by Dr Chris Wild disparaging of Monsanto (T1286.30–1287.12), and by doing so sought to demonstrate his support for Dr Wild and IARC (T1287.14–15). On 3 June 2019, he re-tweeted another aspersion about Monsanto (T1287.20–1288.29), allegedly because “I just wanted to make people aware of the article” (T1288.41–42).
732 Fourthly, it is said that Professor Smith prioritises defending IARC over scientific candour. Professor Smith accepted that, in the pursuit of scientific accuracy, publishers should have processes in place to address conflicts of interest (T1279.7–8). When asked whether an agency dedicated towards scientific candour should disclose studies that the agency is aware of that do not support a particular conclusion, Professor Smith at first gave a non-responsive answer (T1279.22–24), then said “it depends on the agency” (T1279.26–28), then conceded “generally, I agree with you agencies should do that” (T1279.28), and then, it is said, conceded more definitively “[y]es. Generally yes” (T1279.34–36). When asked whether it was desirable in the pursuit of scientific knowledge that draft reports and revisions of draft reports by agencies be made available for public comment to obtain the benefit of comments from experts outside an organisation, Professor Smith said, “[m]aybe in some circumstances, yes” (T1280.36–39). Monsanto submits that contrary to these concessions, Professor Smith was a signatory to a letter submitting that IARC funding should not be made contingent on adopting a review process in which drafts and revisions were publicly available online (T1281.19–22), a proposal to introduce a process to address conflicts of interest at IARC (T1281.34–40), and providing summaries of studies that did not support the agency’s conclusions (T1282.4–12).
733 Fifthly, Monsanto contends that Professor Smith is a supporter (and benefits from) toxic tort suits in the United States. Professor Smith was a co-founding member of an organisation in the known as the Council for Education and Research on Toxics (CERT), together with others including Mr Raphael Metzger, who brings toxic tort lawsuits (T1292.32–43). The activities of that organisation included the filing of toxic tort lawsuits (T1292.45–47). Professor Smith has been allegedly hired as an expert in proceedings commenced by CERT (T1293.7–9), including proceedings regarding chemicals created when making potato fries (T1293.7–9) and proceedings against Starbucks (T1293.11–12). CERT has given more than $150,000 to advance research topics of Professor Smith’s interest (T1293.14–21).
734 Sixthly, it is submitted that Professor Smith withheld from the Court important limitations on studies upon which he relied. Professor Smith accepted under cross-examination that “in the event and to the extent that [a] study was demonstrated [to have] limitations, then the conclusions must also be modified to that extent” (T459.3–7; T500.11–16); that “it was important to draw to the Court’s attention not only the findings that might establish whether or not glyphosate and [GBFs] were carcinogenic but what the limitations were on that hypothesis” (T1314.10–13); that “studies done with unreliable methods or design deficiencies must be given less weight” (T1327.17–21); that an important consideration when assessing the quality of studies is whether the study accounts for important confounding and modifying variables (T1328.23–36); and that a good quality mechanistic toxicology study is one that is designed to account for confounding variables (T1329.8–11). However, it is said the evidence demonstrates that Professor Smith omitted from his evidence fundamental limitations and qualifications that were often expressed in the direct terms by the authors of the studies on which he relied. Monsanto refers to a number of examples in its submissions to which I will return in the context of addressing the mechanistic evidence below.
735 Seventhly, it is said Professor Smith has approached this proceeding with a fixed view from his involvement in glyphosate litigation in the United States. Professor Smith accepted that, in the process of giving evidence by way of deposition in 2018 in glyphosate proceedings, he had reached the view that glyphosate and/or GBFs were carcinogenic (T1316.10–11). He expressed that view in 2018 (T1316.17–18) and maintained that view at the time he was retained by Maurice Blackburn to give evidence in this proceeding (T1316.20–21). He confirmed that he had a firm view about the carcinogenic nature of glyphosate at the time he was retained as an expert in this proceeding by Maurice Blackburn (T1316.27–31). Monsanto submits that although he asserted that he always held an open mind, Professor Smith’s approach to giving evidence suggests otherwise, and questions put to him in re-examination did not elicit any explanation that would allow the Court to understand what, if anything, would have changed his mind as to the carcinogenicity of glyphosate.
736 Based on the conduct described above, Monsanto submits that the Court should find that Professor Smith was determined to act as an advocate for Mr McNickle. In the alternative, Monsanto contends that Dr Juberg’s opinion should be preferred to Professor Smith’s in the third stream. Those submissions may be summarised as follows.
737 First, it is said Professor Smith failed to carry out any thorough review of the mechanistic studies despite propounding the KCs typology on the basis that it enables a systematic review of the studies to be undertaken (First Smith Report (at [74])). Professor Smith accepted in cross-examination “that the question of whether or not glyphosate and glyphosate-based formulations cause cancer needs to be assessed by reference to the full set of reliable and relevant studies” (T1321.14–18). He also accepted that “mechanistic toxicology studies on a single substance may be numerous and diverse” (T1325.4–7) and “often report on a multitude of end points and toxicity pathways” (T1325.9–12).
738 On several occasions, however, Professor Smith conceded that he had identified peer-reviewed studies that purported to show a positive association between glyphosate and five of the KCs without attempting to examine basic features of those studies that are well-accepted to define the reliability of the study. To take one example, Professor Smith placed significant weight on the three human biomonitoring studies concerning genotoxicity even though each of those studies was carried out on populations who lived among illicit cocaine crops (T1294.38–39), and even though Professor Smith had not looked at the issue of whether cocaine is genotoxic (T1336.46–1337.1). Professor Smith also admitted that Paz-y-Mino (2011) did not control for drug use (T1339.9–25).
739 Secondly, and relatedly, it is said that Professor Smith included in his analysis studies which he ought to have rejected as bad science. Professor Smith, for example, identified Geier (2023) for the first time in this proceeding when giving oral evidence in Conclave B (T512.5–14, T1298.46–1299.8). Professor Smith described the paper in terms (T512.9–14):
PROFESSOR SMITH: … there is a recent paper correlating the levels of glyphosate in the urine as measured in the American and Hayne study, which is an American study of large numbers of people throughout the US, and they’ve correlated lower oestrogen levels being present in people with high levels of glyphosate in their urine.
740 Professor Smith accepted in oral evidence that he relied on that study despite knowing that Dr Geier had been suspended as a licensed medical practitioner (T1299.32–33); that Dr Geier falsely claimed to be a board-certified geneticist and a board-certified epidemiologist (T1299.41–42); that he “generally was aware of the controversies surrounding Dr Geier” (T1299.44–45), and that “Dr Geier was a controversial physician” (T1302.25). Monsanto submits that Professor Smith did not seek to bring any of those matters to the attention of the Court when identifying Geier (2023) as scientific evidence on which the Court should make findings in respect of KC8 (T1300.1–5; T1302.35).
741 Thirdly, Monsanto submits that Professor Smith drew conclusions beyond what was available from the evidence and in such a way consistently to support Mr McNickle’s case. In his first report (at [138]), Professor Smith cited a number of studies for the proposition that “multiple laboratories [had found] that glyphosate and/or GBFs, including Roundup, alter the make-up of the gut microbiome in several species and cause extensive inflammation”. However, it is contended that those studies provided no basis for a conclusion that there is any evidence (let alone evidence from “multiple laboratories”) that glyphosate and/or GBFs cause “systemic chronic inflammation”.
742 Fourthly, Monsanto repeats its submission above (at [734]) that Professor Smith failed to disclose a range of limitations associated with Paz-y-Mino (2007); Bolognesi (2009); and Paz-y-Mino (2011). It is said that his failure to identify in his written reports the limitations of the studies on which he relied particularly detracts from his credibility and is especially strong evidence of his willingness to act as an advocate for Mr McNickle, given that Professor Smith had reason to know that his responsibilities to the Court as an expert required him to identify those limitations. Professor Smith stated in cross-examination that he “guess[ed]” (T1313.31–32) that he was aware of the criticism that had been made by the US District Court of his evidence in Perry v Novartis 564 F Supp 2d 452 (2008) (at 466 per Dalzell DCJ) when the Court found:
It therefore appears that Dr. Smith’s analysis of the i3 report focused not on the findings that were most relevant to the hypothesis he sought to test but on the findings that were most helpful to his paying client. While this approach is, sadly, not uncommon, it is incompatible with the reliable application of the scientific method.
743 Fifthly, it is said that Professor Smith refused to make reasonable concessions in cross-examination. For example, after Professor Smith had given evidence that chronic inflammation “needs to be prolonged” (T1307.44) and, is in that respect, different from “sub-chronic inflammation” (that is, inflammation of a shorter duration) (T1307.46–1308.1), Professor Smith refused to concede that Hamdaoui and Naifar (2008) (being a study that Professor Smith cited as evidence that GBFs cause chronic inflammation) was not a chronic inflammation study. Monsanto submits that Professor Smith avoided making that concession on four occasions (T1308.17–31). To take another example, when asked whether the nutritional deficiencies of children in Paz-y-Mino (2011) was a potential confounder that needed to be considered, Professor Smith gave evidence that was “hypothetically” a confounding factor (T1336.12–44). It is said that Professor Smith sought to deflect attention from discussion about the extent of the malnutrition in the children of the families by contending that the nutritional status related only to children and the studies concerned adults, and ultimately gave evidence that the nutritional problems were “potentially” a confounding factor (T1337.35–45).
744 For essentially the reasons that Monsanto identifies, I have reservations about the way in which Professor Smith approached the task of giving evidence in this proceeding. Without any intended disrespect, he seemed to me to be representative of a certain type of expert who has committed himself to one side of a controversy. Although this criticism was directed at a number of experts on both sides in this case, it was most effectively made in relation to Professor Smith (and another expert to whom I will return below). In saying this, I have reminded myself that the focal point of the relevant task is not the preconceived opinions of the relevant expert, but the overall substantive merits of their evidence given in the witness box and in their expert reports.
745 In this regard, it is worth making the following observations.
746 First, as noted above (at [735]), Professor Smith gave evidence that by the time he was retained by Maurice Blackburn in this proceeding, it would be fair to say that he held a “firm view” about the alleged carcinogenic nature of glyphosate (T1316.27–31). He also gave evidence that since 2018, he has remained open to be persuaded otherwise as to whether glyphosate and/or GBFs are carcinogenic or can cause NHL (T1437.1–3). I have some misgivings about the cogency of that evidence after observing him in the witness box, even though he has kept abreast of recent studies concerning the potential carcinogenic effects of exposure to glyphosate and/or GBFs.
747 Secondly, albeit reluctantly, Professor Smith made concessions, including: (1) in relation to Paz-y-Mino (2007), Bolognesi (2009), and Paz-y-Mino (2011), noting the studies had “some limitations” (at [83]); (2) in relation to Bolognesi (2009) specifically, that its findings were “somewhat conflicting” (First Smith Report (at [90])); (3) that there were a number of limitations in Lucia (2022) (T493.44–496.11); and (4) significantly, for reasons that will become clear, that the KCs are not conclusive of carcinogenicity. As Professor Smith said in cross-examination (T445.29–35):
MR FINCH: Now, I will use the expression ticking the boxes. Ticking the box of five of the 10 KCs may suggest a possibility that a substance is carcinogenic but is not conclusive, of course?
PROFESSOR SMITH: Yes. If you only had that information, and you had no epidemiological information and no toxicological data in experimental animals, you could only say that the chemical was possibly carcinogenic in humans.
748 A further example of Professor Smith making a concession in an area where there are conflicting findings (only some of which support his view as to the carcinogenicity of glyphosate) is provided by an exchange he had with the Assessor (T603.45–604.20).
ASSESSOR: This is a question first for Professor Smith and it’s about that figure 2 in Wozniak et al 2020. You said, perfectly correctly, I think, that reduction in p53 expression might ultimately lead to tumour formulation. The question I have for you is whether you think that reduction that’s seen at 100 [micromolar] is significant? It’s statistically significant but is halving the level of gene expression likely to have a big consequence for the functions of p53? And I ask that because, of course, the knockout mouse doesn’t have a phenotype if it’s a heterozygote, it only has a phenotype if it’s a homozygotes. So the level of p53 would have to be pretty low to lead to a tumour was my thinking. What do you think about that level shown in that bottom right hand panel?
PROFESSOR SMITH: I think it’s – I think your scepticism is reasonable because – but I think it’s not the only mechanism by which you can get changes in p53. So, for example, through the mutagenic actions of glyphosate on things like 8-hydroxyguanosine and other things, you could get mutations in one copy of p53, and that mutated copy, you could then get – in the regular copy you could then get ..... of 50 per cent change and you could significantly change the amount of p53 present. So, on its own, with such a high concentration, I’m not going to put my hat on. That’s really tremendously important. For the purposes of key characteristics though if you want to say does glyphosate induce epigenetic alterations? Then these papers significantly contribute to that statement. But I – I understand your scepticism that that alone wouldn’t be enough.
ASSESSOR: Right.
PROFESSOR SMITH: You would need other things.
749 Thirdly, Professor Smith’s involvement in CERT of which he was a board member (T1294.22–24); his previous involvement in toxic tort lawsuits in the US commenced by CERT; or (to the extent they were tendered) his views published on Twitter or other media reinforced my impression above (at [744]) that Professor Smith had placed himself on one side of this scientific controversy. Although I do not think Professor Smith acted as an advocate or surrogate for the views of CERT, IARC or any other organisation in such a way as to jeopardise his independence in this proceeding, these matters did cause me to give increased scrutiny to his evidence.
Dr Juberg
750 I have already made findings about Dr Juberg’s credit above (at [446]–[462]). It is necessary, however, to say something further about his evidence in the context of this stream.
751 Mr McNickle submits there is an important difference between the expertise of Dr Juberg and Professor Smith which is particularly relevant to the mechanistic evidence. Dr Juberg accepted that he has no specialised knowledge on the topic of NHL in humans (T1443.15–16), whereas Professor Smith has authored or co-authored several articles that specifically relate to NHL (First Smith Report (at [12])). It is said that Dr Juberg adhered to the view that glyphosate and/or GBFs performed for regulatory purposes are more reliable and should carry more weight than studies on glyphosate and/or GBFs published in peer-reviewed, publicly available journals, which Dr Juberg allegedly discounted (at JRG [61], [113]; T1502.29–45).
752 It is said further that Dr Juberg’s preference for the regulatory studies is of limited utility in this proceeding because the central issue is whether the use of or exposure to Roundup Products (which contain glyphosate) can increase the risk of NHL in humans, whereas most regulatory studies are on bacteria, mice, rats and other animals. Few of them, it is said, are in human lymphocytes and none are in vivo studies on humans. Regulatory studies on bacteria will show whether glyphosate is mutagenic to bacteria but, as Dr Juberg accepted, the results of those bacterial studies do not reveal whether glyphosate is genotoxic or mutagenic to humans (T1480.26–34).
753 Mr McNickle also submits that Dr Juberg’s approach runs counter to IARC’s recommendation that studies in exposed humans or in human cells should be emphasised in the assessment of whether a substance is carcinogenic to humans (IARC Monographs on the Identification of Carcinogenic Hazards to Humans Preamble (2019) (at 27)). Professor Smith gave evidence in (at JRG [92]) that he considers the regulatory animal studies and bacterial mutagenicity tests on glyphosate and/or GBFs to be “of little value, compared to studies in actual humans and human cells” (see also T1508.21–26). Professor Prince and Dr Flecknoe-Brown expressed a similar opinion (T246.1–17).
754 It is perhaps unsurprising that in the light of his professional background, Dr Juberg displayed a preference for the regulatory studies. There may be advantages to such an approach. Regulatory studies are conducted according to internationally recognised standardised guidelines (such as the OECD Test Guidelines for Mutagenicity, Genotoxicity, and Carcinogenicity (at JRG [56]); and the USEPA Test Guidelines for Mutagenicity, Genotoxicity, and Carcinogenicity (at JRG [57])). As Dr Juberg explained (at JRG [111]), he placed higher confidence on such studies because the development of standardised and harmonised test guidelines, coupled with GLP when conducting toxicological testing, “results in an objective and high-quality testing regime aimed at critically evaluating the toxicological profile of pesticides”. The use of guidelines ensures that there is “general harmonisation across the globe when data are generated for various toxicological effects” and that there is “a standard for how, and by which, to conduct toxicity testing regardless of who is generating the data for regulatory purposes” (at JRG [53]).
755 In contrast, according to Dr Juberg, peer-reviewed studies are not limited in their design and conduct by any a priori set of standardised guidelines. Dr Juberg explained that “[a]cademic studies or other publicly available literature studies typically do not adhere to either test guideline study design or GLP, but often use other approaches to serve their research needs and goals” (at JRG [53]; see also T1330.7–44; T1331.3–7). The authors of the peer-reviewed studies are free to design their studies in a way that best serves whatever “research needs and goals” they have (at JRG [53]). Notwithstanding this freedom may facilitate exploratory science, it is said that a potential drawback is that it leaves open the possibility that publication bias may infect the reliability of the studies’ design, conduct, and results. As Professor Smith explained in cross-examination (T1332.1–15):
MR CRAIG: And publication bias effects peer reviewed studies because peer reviewed studies are reviewed for the purposes of publication, aren’t they?
PROFESSOR SMITH: They are.
MR CRAIG: But it doesn’t affect regulated studies because those studies are generally not submitted for publication, are they?
PROFESSOR SMITH: No. It’s unfortunate but they are not.
MR CRAIG: So an example of publication bias might include a study author providing an unpublished manuscript which includes an important caveat but the publishing editor removing that important caveat?
PROFESSOR SMITH: Perhaps. Yes.
756 With that said, the danger with this approach, it seems to me, is to assume that those dealing with the regulators will play with a straight bat. For obvious reasons, this may be an unsafe assumption in the circumstances of this case, and, in this regard, it is necessary to say something about the evidence adduced concerning Professor James Parry.
757 Professor Parry was the Professor of Genetics and Chairman of the Centre for Molecular Genetics and Toxicology at the University of Wales. In or around early 1999, Monsanto engaged Professor Parry to conduct a review of four published genotoxicity studies which identified that glyphosate and/or GBFs may be genotoxic, being: Rank (1993); Bolognesi (1997); Lioi (1998); and Peluso (1998). Professor Parry prepared a report on those four studies (Parry (1999)) which he provided to Monsanto. Professor Parry concluded (at 7):
The overall data provided by the four publications provide evidence to support a model that Glyphosate is capable of producing genotoxicity both in vivo and in vitro by a mechanism based upon the production of oxidative damage.
758 Professor Parry also recommended that further work be undertaken to clarify the potential genotoxic activity of glyphosate. Parry (2019), however, was not provided to regulatory authorities, including the APVMA, by Monsanto. In cross-examination, I raised this issue with Dr Juberg (T1472.36–1473.4):
HIS HONOUR: … Dr Juberg, if you go to page 7 of the document in front of you, the second paragraph, “The overall data,” down to the word “damage” and then you’ve already pointed out the second sentence there, “If confirmed.” That is, there’s a representation made and then – on the basis of the work that has been done by Professor Parry. Thinking back to your time at Dow and your responsibility for dealing with regulators, if you at Dow in respect of a matter in which you were engaging with regulators had from a respected scientist that the overall data provided by publications, irrespective of whether you had reason to think the publications were in the hands of the EPA, provide evidence to support a model the product is capable of producing genotoxicity in both in vivo and in vitro by a mechanism based on the production of oxidative damage, would you regard it as – would you think, given your knowledge of dealing with regulators, it would be important to convey that information to the regulator?
DR JUBERG: Yes.
759 Although it was not the subject of detailed evidence, this is a matter of some concern and is redolent of the kind of danger I have referred to above in assuming that agrochemical companies will provide all relevant studies to agrochemical regulators. Having said that, it must be balanced against the fact that: (1) Parry (1999) did not involve a weight of evidence approach; (2) each of the studies the subject of Parry (1999) appear to have been considered by the relevant regulators; and (3) Professor Smith and Dr Juberg gave evidence concerning three of the four studies the subject of Parry (1999) (Rank (1993); Bolognesi (1997); Lioi (1998)) (Professor Smith did not rely upon Peluso (1998) (T1586.39–1587.5)). Although it caused me some disquiet and would certainly give me concerns about accepting anything from Monsanto at face value, in the light of these matters, and in the absence of any cross-examination of Professor Parry, I consider the evidence concerning Parry (1999) is of limited assistance in resolving the central issue.
760 Mr McNickle contends that the mechanistic evidence strongly supports the conclusion that glyphosate and/or GBFs (and thus Roundup Products) are human carcinogens; increase the risk of NHL in humans; and can cause NHL in humans. This is for two reasons.
761 First, it is said glyphosate exhibits five KCs of carcinogenicity, namely:
KC2 (genotoxicity);
KC4 (induces epigenetic alterations);
KC5 (induces oxidative stress);
KC6 (induces chronic inflammation); and
KC8 (modulates receptor-mediated effects).
762 Secondly, and relatedly, Mr McNickle submits that there are five identifiable biologically plausible mechanisms by which use of or exposure to glyphosate and/or GBFs (and thus Roundup Products) can cause NHL in humans, as follows:
(1) glyphosate and/or GBFs are genotoxic to human lymphocytes and capable of causing double-strand breaks of DNA in human lymphocytes, and thus could cause the kind of genetic mutations which can lead to the development of NHL in humans (first hypothesised mechanism);
(2) glyphosate and/or GBFs cause an upregulation of the AID enzyme in humans that increases the amount of SHM, class switch recombination (CSR) and V(D)J recombination taking place in B-cells and therefore increases the number of mutations occurring within the B-cells including the kind of mutations that cause NHL (second hypothesised mechanism);
(3) glyphosate and/or GBFs cause oxidative stress in humans which leads to damage to DNA, including double-strand breaks of DNA, and cause haematological cancers such as NHL (third hypothesised mechanism);
(4) glyphosate and/or GBFs induce epigenetic alterations in human lymphocytes, including alterations of DNA methylation and hence the expression of key cancer genes, including NHL (fourth hypothesised mechanism); and
(5) glyphosate and/or GBFs are preferentially distributed to the bone marrow, where it remains longer than in other tissues. Given that lymphocytes develop from lymphoid stem cells, which develop in the bone marrow, it is said that they may there be exposed to the alleged genotoxic and mutagenic effects of glyphosate and/or GBFs (fifth hypothesised mechanism).
763 It should be reiterated here that although a substance “ticks the box” of one or more of the KCs, it does not necessarily follow that there is a corresponding biologically plausible mechanism by which exposure to that substance can cause cancer. As Professor Smith explained in cross-examination (T1324.17–21):
MR CRAIG: … in terms of your KC framework, the 10 KCs do not themselves determine whether a substance has a mechanism of action for causing cancer?
PROFESSOR SMITH: No. You have to use the information from that to form one.
764 It is convenient therefore to deal with the former question separately from what might be described as the latter “significance” question when I address whether there is any biological plausible mechanism by which use of or exposure to glyphosate and/or GBFs can cause NHL.
765 Accordingly, I will adopt the following structure:
Section I will detail my findings as to KC2 (genotoxicity);
Section II will set out my findings in relation to KC4 (induces epigenetic alterations);
Section III will address some other studies referred to KC5 (induces oxidative stress);
Section IV will set out my findings as to KC6 (induces chronic inflammation);
Section V will detail my findings as to KC8 (modulates receptor-mediated effects);
Section VI will set out my findings as to the hypothesised biologically plausible mechanisms of action; and
Section VII will set out my conclusion as to the mechanistic evidence stream.
766 As noted earlier (at [661]), KC2 (genotoxicity) is the ability of a chemical substance to cause DNA damage. This damage may or may not produce a mutation, which is a permanent and heritable change in the structure of the genome. If it does, it may lead to cancer (at JRG [13]).
767 Genotoxicity can include a wide range of effects on the genome, including, among other things, DNA adducts, strand breaks, sister chromatid exchanges, and chromosome aberrations. These types of genotoxic effects may lead to mutations where only a single base is changed in the DNA or to much larger ones where a whole chromosome is lost, or parts of chromosomes are deleted entirely (at JRG [14]). A mutagen is a genotoxic agent that produces mutations. All mutagens are genotoxic, but not all genotoxicants are mutagenic (that is, leading to a permanent and heritable change in the DNA or genetic material) (at JRG [15]).
768 Professor Smith concluded that based upon the studies pertaining to glyphosate and/or GBFs genotoxicity, there is “strong evidence” that they are genotoxic, mutagenic and can cause NHL (at JRB [160]; JRG [84]). Dr Flecknoe-Brown similarly concluded that glyphosate and/or GBFs are genotoxic and mutagenic to humans (at JRA [185]–[189], [205], [283], [326]).
769 For reasons that will become clear, KC2 is near the top of the shopping list for Mr McNickle’s case in the third stream. Indeed, the question of whether glyphosate and/or GBFs exhibit KC2 was the subject of prolix submissions and extensive evidence adduced in the initial trial. It is necessary to delve into some detail in the following sections of these reasons which, for convenience, will adopt the following structure:
(1) the in vitro studies, which will address: (a) the regulatory studies on the genotoxicity of glyphosate and/or GBFs sponsored by Monsanto or other corporations (see First Smith Report (at [104]–[120])); (b) the in vitro studies, namely: Santovito (2018); Wozniak (2018); Kwiatkowska (2017); Nagy (2021); Mladinic (2009); Alvarez-Moya (2023); and Roma (2023) (in vitro studies); and (c) Dr Flecknoe Brown’s evidence given in Conclave A;
(2) the in vivo studies, which will include consideration of four studies (Paz-y-Mino (2007); Bolognesi (2009); Paz-y-Mino (2011); and Chang and Andreotti (2023)) (in vivo studies); and
(3) the in vivo significance of in vitro studies, which will address the question of the “real world” significance of concentrations at which glyphosate and/or GBFs may, in artificial in vitro conditions, cause damage to DNA.
770 It will be recalled that in vitro studies are studies conducted on living cells or organ tissue outside of their normal biological context (often referred to colloquially as “test-tube experiments”). It is convenient first to address the regulatory studies, before turning to the in vitro studies and Dr Flecknoe-Brown’s evidence given in Conclave A.
771 It is not an understatement to describe the regulatory studies as voluminous. It is partly for this reason that Dr Juberg’s approach (at JRD, Annexure D (at [1])) involved reviewing:
… a sufficiently large and representative sample of different types of regulatory mutagenicity and genotoxicity studies from various laboratories and multiple registrants (where available) … conducted over several decades, so as to be able to form a weight of the evidence opinion on the results generated based on regulatory test guidelines and conducted under GLP.
772 Professor Smith referred to a sample of regulatory studies (at JRG [76]; First Smith Report (at [104]–[120])) and, importantly, both experts agreed that the regulatory studies were either largely or uniformly negative in demonstrating that glyphosate and/or GBFs are genotoxic.
773 Dr Juberg identified (at JRG, Annexure D, Table 1 (entitled “Representative Regulatory Mutagenicity and Genotoxicity Studies for Glyphosate Technical”)) the relevant particulars of 18 studies, each of which was negative. Similarly, in Table 2 in Annexure D (entitled “Representative Monsanto Regulatory Mutagenicity and Genotoxicity Studies for GBFs”), Dr Juberg identified the relevant particulars of 60 studies (including studies done on human lymphocytes) which were also negative in terms of demonstrating that glyphosate and/or GBFs are genotoxic.
774 Professor Smith concluded from his review of regulatory studies sponsored by Monsanto and other corporations that “they are largely negative” (First Smith Report (at [104])) and later, in cross-examination, accepted that they were “uniformly negative for genotoxicity” (T1358.25–28). Professor Smith noted, however, that those negative results “often have flaws which make them unreliable or partly unreliable” and “conflict with positive findings in better designed studies in the peer-reviewed literature” (First Smith Report (at [120])).
775 Those alleged flaws may be summarised as follows (pinpoint references are to the First Smith Report): (1) for bacterial studies using the Ames test, 12 out of 24 studies had “severe shortcomings” according to the authors of Knasmueller (2021), which included (at 11), among other things: (a) the number of strains was not adequate (in seven trials); (b) inadequate positive controls were used in one report; and (c) inadequate negative controls are reported in two studies (at [105]–[107]); (2) relatedly, the Ames test, according to Professor Smith, is a poor predictor of human carcinogenicity (at [107]); (3) the studies did not score sufficient cell counts (sometimes less than that recommended by the OECD guidelines) and three studies presented no historical controls (at [108]–[110]; [115]–[117]); (4) the use of high doses of relatively pure glyphosate and only one or two donors (at [111]–[112]); (5) differences in the nature of the donor (at [114]); and (6) the majority of studies using the bone marrow micronucleus assay were not found to be in agreement with the OECD guidelines and or were inconclusive (at [118]).
776 Two difficulties arise in this context. The first is that in contrast to Dr Juberg (see above (at [771])), there is little explanation by Professor Smith as to the process he used to search for the regulatory studies that he selected for inclusion in his report, notwithstanding his evidence that it is “necessary for a toxicologist to conduct a comprehensive search of the mechanistic toxicology literature before reaching conclusions about the KCs or mechanisms of action” (T1326.35–39). The second difficulty is that to the extent they were addressed (even if only generally in relation to the reliability or otherwise of the regulatory studies versus the peer-reviewed literature (see above (at [754]–[759])), the purported limitations referred to above were not the subject of any detailed cross-examination.
777 I have, of course, had regard to the evidence and purported limitations associated with the regulatory studies relied upon by Professor Smith, but it is partly for these reasons that I can be brief in relation the regulatory studies. In the light of Dr Juberg and Professor Smith’s agreement that the regulatory studies are either largely or uniformly negative in demonstrating that glyphosate and/or GBFs exhibit KC2 (see above (at [773]–[774])), it is difficult to come to any conclusion other than they are of limited assistance to Mr McNickle’s case on genotoxicity. I hasten to add that this conclusion does not constitute a view one way or the other as to the debate which emerged between the parties concerning the general reliability of the regulatory studies versus the peer-reviewed literature (notwithstanding my concerns about those dealing with regulators “playing with a straight bat” (see above (at [754]–[759])) but is one reached upon an assessment of the overall substantive merits of Professor Smith and Dr Juberg’s evidence given in the witness box, the joint reports, and individual expert reports.
2. The in vitro studies
778 The in vitro studies, however, paint a different picture. Bar Roma (2023), all of the studies were conducted on human lymphocytes, and it is convenient to address each in turn before saying something about Dr Flecknoe-Brown’s evidence given in Conclave A.
779 This study conducted an analysis of the clastogenic and/or aneugenic effects of glyphosate by chromosomal aberrations and micronuclei assays. Peripheral venous blood was collected from six healthy subjects (two males and four females), non-smoking, not alcoholics, not under drug therapy, and with no recent history of exposure to mutagens. Human lymphocytes were exposed to five glyphosate concentrations (0.500, 0.100, 0.050, 0.025, and 0.0125 μg/mL, where 0.500 μg/mL represents the established acceptable daily intake value) and the other concentrations were tested in order to establish the genotoxicity threshold for the compound (at 34693–4). The authors concluded (at 34693):
We observed that chromosomal aberration (CA) and micronuclei (MNi) frequencies significantly increased at all tested concentrations, with exception of 0.0125 μg/mL. Vice versa, no effect has been observed on the frequencies of nuclear buds and nucleoplasmic bridges, with the only exception of 0.500 μg/mL of glyphosate that was found to increase in a significant manner the frequency of nucleoplasmic bridges.
780 As Professor Smith explained (First Smith Report (at [91])), the authors found that the cytokinesis-block proliferation index and the mitotic index were not significantly reduced, indicating that glyphosate did not produce significant cytotoxicity (that is, damage or death to the cell, rather than genotoxicity which involves disruption to the DNA) or effects on the proliferation of the cells at the tested concentrations. Glyphosate therefore produced significant chromosome damage (a form of genotoxicity) in the human lymphocytes in the absence of cytotoxic effects.
781 It is worth noting from the outset that Santovito (2018) is one (if not the only) study that Monsanto concedes provides some evidence that glyphosate and/or GBFs are genotoxic. Notwithstanding some minor limitations, I accept that the evidence established that the study does provide reliable evidence that glyphosate is genotoxic, for the following reasons.
782 First, the study demonstrated that glyphosate was genotoxic by two pathways, namely: (1) where exposure to a substance causes increased chromosomal aberrations in lymphocytes; or (2) where exposure to a substance causes increased frequency of micronuclei (MN) in lymphocytes, either or both circumstances is an indication that the substance is genotoxic to lymphocytes (T554.32–43). As noted above, the authors observed that chromosomal aberrations and micronuclei frequencies significantly increased at all tested concentrations except for the lowest concentration. Professor Prince in cross-examination accepted that this result demonstrated genotoxicity (T555.18–27):
MR CLEMENTS: Professor Smith opines in his report that those results showed, in that study, glyphosate produced significant chromosome damage which is a form of genotoxicity in human lymphocytes; he’s right about that, isn’t he?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: So the Santovito study showed that glyphosate was genotoxic to human lymphocytes in two different ways, didn’t it?
PROFESSOR PRINCE: Yes.
783 Professor Prince was then taken to the kind of chromosomal aberrations that the authors observed were caused by glyphosate (at 34695), where it was noted:
Glyphosate was found to induce the following structural CAs: gaps, chromatid and chromosome breaks, dicentric chromosomes, rings, tri-tetraradials, and acentric fragments. This last, together to chromatid breaks, represent the most frequent observed aberrations (Table 1). Because of the conflicting opinions about the possibility to consider gaps as indicators of genomic damage, we decided to exclude gaps from statistical analysis.
784 Professor Prince agreed that this presented evidence of double-strand breaks in the DNA (T557.8–20):
MR CLEMENTS: I suggest it would be wrong to say that the Santovito study was unable to demonstrate any double strand DNA breaks, wouldn’t it?
PROFESSOR PRINCE: The Santovito study, correct. Yes.
MR CLEMENTS: It showed double strand breaks, didn’t it?
PROFESSOR PRINCE: Yes.
785 Secondly, despite concerns that: (1) the “purity of the test material and presence of possible impurities were not noted which creates doubt as to the reliability of the study results”; and (2) that Santovito (2018) was not conducted according to OECD Guideline 487 (T1519.6–14), Dr Juberg accepted in cross-examination that Santovito (2018) presented evidence that glyphosate is genotoxic (T1542.28–35).
786 Thirdly, Professor Smith gave evidence (at JRG [87]) that it was notable the study used six healthy donors (two males and four females) and scored 200 cells per donor such that 1200 cells were scored for each dose rather than only 100 or 200 in the studies sponsored by Monsanto “making the study much more powerful than those performed under OECD guidelines”. As Professor Smith noted in response to a question from the Assessor (T1501.43–5):
PROFESSOR SMITH: … And in this particular study [Santovito (2018)], they didn’t just use one donor; they used six. And this is a combination of the pooled data from six people, and they’ve scored 200 cells in each person. So they’ve scored 1200 cells, which is much more than normal. And so this is why this study, I believe, is a very important one because it has a very large number of cells scored, it has a large number of individuals studied and this means that – and it also is using a chromosome aberration test, which is more sensitive than the – than the comet assay and less variable because it’s scored under a microscope blind. And you see a stronger dose response with this. So I would – I would rely more on this than I would on the comet assay. Variability with the comet does not surprise me too much.
787 Fourthly, subject to matter to which I will return later in this section, as Professor Smith explained (at JRG [88]), the study used much lower doses of glyphosate than the studies sponsored by Monsanto (ranging from 0.0125 to 0.5 ug/ml) and, subject to matter to which I will return, were likely to be more relevant to levels of human exposure. A total of five doses was used rather than three and glyphosate was added to the cultures at 24 hours (the same as the industry studies) but was not washed off and remained in the cultures exposing the cells until they were harvested 28 hours later.
788 Overall, for these reasons, and notwithstanding the authors noted that: (1) there are limitations associated with an in vitro study due to the reduced sample size (at 34698); and (2) the increased cytogenetic damage at glyphosate concentrations equal and lower than the established acceptable daily intake (ADI) value “requires further investigations” in order to establish the effective genotoxicity threshold of glyphosate (at 34699) (a matter to which I will return), I am satisfied that Santovito (2018) presents reliable evidence that glyphosate is genotoxic.
789 This study assessed the effect of glyphosate, its formulation (Roundup 360 PLUS) and its main metabolite (aminomethylphosphonic acid, AMPA) in the concentration range from 1–1000 μM on DNA damage in human peripheral blood mononuclear cells (PBMC).
790 As Professor Smith explained (First Smith Report (at [92])), the compounds studied and formulation induced DNA single and double strand-breaks and caused purine and pyrimidine oxidation. Roundup 360 PLUS caused damage to DNA at 5 μM, while glyphosate and AMPA induced DNA lesions only at higher concentrations of 250 μM and 500 μM, respectively. The authors concluded that the observed changes were not likely associated with the direct interaction of herbicide components studied with DNA, but most probably occurred through effects related to the induction of oxidative stress (to which I will return below).
791 In my view, Wozniak (2018) presents some evidence that glyphosate is genotoxic, for two reasons.
792 First, in cross-examination, Professor Prince agreed that the effect of glyphosate and Roundup 360 Plus on PBMCs (the predominant cell type in which are lymphocytes) showed that those substances induced DNA damage in the PBMCs, including single and double strand-breaks (T558.16–25):
MR CLEMENTS: And the results of the test by Wozniak was that both glyphosate and the Roundup formulation and AMPA induced DNA damage in the PBM cells including single strand breaks and double strand breaks; correct?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: And Wozniak et al also observed that the Roundup formulation caused greater DNA damage than glyphosate on its own; is that correct?
PROFESSOR PRINCE: Yes.
793 Professor Prince went on to give evidence (T559.1–10):
MR CLEMENTS: Yes. All right. Thank you. So one thing was crystal clear from the study [Wozniak (2018)], the effect of exposing human PBM cells to glyphosate or the Roundup formulation was damage to DNA including single and double strand breaks?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: And so this adds to the body of evidence, I suggest to you, that glyphosate and GBFs are genotoxic to human lymphocytes; do you agree with that.
PROFESSOR PRINCE: Yes.
794 Secondly, notwithstanding his concern (at JRG, Annexure E), that there were “no effects reported at lower exposure concentrations … which indicates that these findings have a practical threshold and have little relevance for humans if one extrapolates the experimental concentrations to expected human exposures. The laboratory proficiency for study conduct and availability of historical control data were not noted, which represent potential experimental variables that influence the interpretation and reliability of the study results”, Dr Juberg accepted in cross-examination that Wozniak (2018) provided some evidence that glyphosate and Roundup are genotoxic (albeit in concentrations higher than real world applications) (T1550.15–19):
MR CLEMENTS: Do you accept that the Wozniak study shows that glyphosate and Roundup are genotoxic in concentrations higher than you would expect humans to encounter in real-world use of GBFs?
DR JUBERG: Yes.
795 Accordingly, I consider that Wozniak (2018) provides some evidence that glyphosate is genotoxic. I will return to this study in the context of addressing KC5 (induces oxidative stress).
796 This study assessed DNA damage (determination of single and double strand-breaks by the comet assay) and DNA methylation (global DNA methylation and methylation of p16 (CDKN2A) and p53 (TP53) promoter regions) in human PBMCs exposed to glyphosate. PBMCs were incubated with the compound studied at concentrations ranging from 0.1–10 millimolar (mM) for 24 hours.
797 The authors concluded that glyphosate induced DNA lesions, which were effectively repaired. However, PBMCs were unable to repair completely DNA damage induced by glyphosate. The study, in summary, indicated that glyphosate (at high concentrations from 0.5–10 mM) may induce DNA damage in leucocytes such as PBMCs and cause DNA methylation in human cells (at 93).
798 I accept that Kwiatkowska (2017) provides some evidence that glyphosate is genotoxic.
799 Notwithstanding that there was some disagreement as to whether the study indicated evidence of double-strand breaks, Professor Prince ultimately agreed with Professor Smith’s conclusion and gave evidence that the study showed that glyphosate was genotoxic to human lymphocytes (T578.4–37):
MR CLEMENTS: Perhaps I will break it up: do you agree that the 2017 Kwiatkowska study shows that glyphosate causes DNA damage to human lymphocytes in the form of single strand breaks?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: But you disagree that it shows double strand breaks, do you?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: And on what basis do you say that?
PROFESSOR PRINCE: Because I don’t think the assay that they’ve done, that they’ve performed and described in the methods section, has the capacity to differentiate between single and double strand breaks. There may be double strand breaks in there but they can’t demonstrate them clearly.
MR CLEMENTS: So you say it’s an issue with the sensitivity of the particular assay used?
PROFESSOR PRINCE: Correct.
MR CLEMENTS: And what do you say about that, Professor Smith?
PROFESSOR SMITH: I say that they used the classical comet assay which measures both single and double strand breaks. I agree with Professor Prince that the assay can’t separate them but there’s no reason to suggest that there’s only single and no double strand breaks present.
MR CLEMENTS: Thank you. Just back to you for a minute, Professor Prince, do you agree with this: the study – this is another study that shows that glyphosate is genotoxic to human lymphocytes?
PROFESSOR PRINCE: Correct.
800 For this reason, I consider that Kwiatkowska (2017) provides some evidence that glyphosate is genotoxic.
801 This study compared the cytogenetic effect of the active ingredient glyphosate and three marketed GBHs (Roundup Mega, Fozat 480, and Glyfos) by investigating cytotoxicity with fluorescent co-labelling and WST-1 cell viability assay as well as genotoxicity with cytokinesis block micronucleus assay in isolated human mononuclear white blood cells.
802 The authors concluded that glyphosate had no notable cytotoxic activity over the tested concentration range (0–10,000 micromolar (µM)), whereas all the selected GBHs induced significant cell death from 1,000 µM regardless of metabolic activation. MN formation induced by glyphosate and its formulations at sub-cytotoxic concentrations (0–100 µM) exhibited a diverse pattern. Glyphosate caused statistically significant increase of MN frequency at the highest concentration (100 µM) after 20 hours of exposure.
803 Further, Roundup Mega exerted “a significant genotoxic effect” at 100 µM both after four- and 20-hour exposures showing a stronger and more rapid effect than glyphosate alone (at 1). Moreover, Glyfos and Fozat 480 also resulted in a statistically significant increase of MN frequency from the lower concentration of 10 µM after 4-h and 20-h treatment, respectively. The authors also concluded that the differences observed between the active principle (glyphosate) and formulations (at 1):
… confirm the previous concept that the presence of co-formulants in the formulations or the interaction of them with the active ingredient is responsible for the increased toxicity of herbicide products, and draw attention to the fact that GBFs are still currently in use, the toxicity of which rivals that of POEA-containing formulations (e.g., Glyfos) already banned in Europe. Hence, it is advisable to subject them to further comprehensive toxicological screening to assess the true health risks of exposed individuals, and to reconsider their free availability to any users.
804 I consider that limited assistance can be derived from Nagy (2021) in support of a conclusion that glyphosate is genotoxic.
805 In cross-examination, Professor Prince agreed with Professor Smith’s conclusion that the results of the study indicated that glyphosate and/or GBFs are genotoxic to human cells (T580.9–12; T584.8–14). Professor Smith, however: (1) could not opine what chemicals were used in Glyfos and Fozat 480 and did not research their composition (T1381.46–1382.1); and (2) had not seen data that suggested that the 10 µM dose for four hours or 20 hours mimics a does realistically possible in human applicators (T1382.3–7). Further, Nagy (2018) only observed damage above 100 µM glyphosate and observed no effects at 0.1, 1 or 10 µM (which result is inconsistent with Santovito (2018)).
806 Dr Juberg also gave evidence on Nagy (2021) and noted, among other things, the presence of unknown contaminants in the use of Glyfos and Fozat 480 (at JRG, Annexure E):
The formulations used in [Nagy (2021)] were reportedly obtained from applicators which does not follow good laboratory practice and is not a typical experimental practice. The presence of possible contaminants is unknown and thus attribution of effects to glyphosate or GBHs is not certain. This study used a different positive control than recommended by the standard test guideline and laboratory proficiency and historical control data were not noted, all of which create doubt as to the reliability of the study results.
807 For these reasons, I do not think much assistance can be derived from Nagy (2021).
808 Mladinic (2009) studied the genotoxic and oxidative potential of glyphosate on human lymphocytes. Testing was conducted with and without metabolic activation. Genotoxicity was evaluated by the alkaline comet assay and analysis of MN and other nuclear instabilities applying centromere probes (at 800).
809 Following glyphosate exposure, the comet assay showed significantly increased tail length (20.39 μm) and intensity (2.19%) for 580 μg/ml, and increased tail intensity (1.88%) at 92.8 μg/ml, compared to control values of 18.15 μm for tail length and 1.14% for tail intensity. With S9, tail length was significantly increased for all concentrations tested: 3.5, 92.8, and 580 μg/ml (at 800).
810 I do not think much assistance can be derived from Mladinic (2009).
811 The authors conclusion in Mladinic (2009) was that “no clear dose-dependent effect was observed, [indicating] that glyphosate in concentrations relevant to human exposure do not pose significant health risk”. Professor Smith disagreed with this conclusion (First Smith Report (at [96])), noting that the data “clearly show a genotoxic effect for all concentrations of glyphosate tested using the comet assay, including the very low dose of 3.5 microgr/ml”, but did not include that qualification in his reports. As Dr Juberg noted (at JRG, Annexure E):
It is questionable whether the in vitro concentrations used [in Mladinic (2009)], coupled with lack of a clear dose-response, as noted by the authors, are relevant to expected human exposures.
812 With respect to this latter point, I should pause here to note that during the concurrent evidence session, Mladinic (2009) was the subject of an important exchange between Professor Smith and the Assessor concerning the apparent variation between the assays in that study and how the threshold concentration for detecting the genotoxicity (or otherwise) of glyphosate can vary significantly by dint of the use of different assays. This is an important issue and one to which I will return below when addressing the real world significance of the in vitro studies.
813 This was a study of the effect of various concentrations of glyphosate and three commercial GBFs on human lymphocytes. Human PMBCs obtained from 40 donors were exposed to glyphosate at 0.1, 1, 10 and 50 mM as well as to equivalent concentrations of glyphosate as commercial formulations.
814 Genetic damage was assessed in this study using the comet assay and was significantly increased at all concentrations of glyphosate and with two formulations. These formulations showed genotoxicity that was concentration-dependent but in a higher proportion compared to pure glyphosate. Professor Smith relied on this study in the Second Smith Report (at [10]) to conclude that glyphosate and/or GBFs are genotoxic.
815 I accept that Alvarez-Moya (2023) provides some evidence that glyphosate and/or GBFs are genotoxic, for the following reasons.
816 First, Professor Smith highlighted in his second report (at [10]) the fact that 40 separate human donors were used for this study as a typical study of human blood cells, whereas under the OECD guidelines, one to two donors are recommended. Professor Smith gave evidence that the use of a much larger number of donors will “clearly provide a better understanding of how a human population will respond to a chemical exposure and the use of 40 donors by Alvarez-Moya and Reynoso-Silva makes it the largest study to date of glyphosate’s effects on human lymphocytes from differing donors” (Second Smith Report (at [10])).
817 Secondly, Dr Juberg accepted that the data obtained in the study showed the genotoxicity of glyphosate and the GBFs tested in the study. In cross-examination, Dr Juberg was taken to the conclusion of the authors (at 4) that “our data clearly showed the genotoxicity of [glyphosate] and GBH …”. Dr Juberg noted the qualification by the authors in the previous paragraph that “although there is evidence of the genotoxic activity of [glyphosate], this is controversial, and it is necessary to increase the degree of certainty of its genotoxicity” but gave evidence (T1559.22–43):
MR CLEMENTS:
Our data clearly showed the genotoxicity of G – glyphosate – and GBH – glyphosate-based herbicide.
MR CLEMENTS: I suggest to you that conclusion is correct, the data generated by this study did clearly show the genotoxicity of glyphosate and GBH. Do you agree with that?
DR JUBERG: Well, I would just counter that with maybe the first sentence of the discussion:
Although there is evidence of the genotoxic activity of [glyphosate], this is controversial and it is necessary to increase the certainty of its genotoxicity.
So within this study, I would agree with you. But I would refer to that upper paragraph as well in – in all fairness, bring that into play.
818 Accordingly, I consider that Alvarez-Moya (2023) provides some evidence that glyphosate and/or GBFs are genotoxic.
819 In this study, HEK293 cells were exposed to various concentrations of Roundup Control Max. The authors found that in the three highest glyphosate concentrations (70, 700, and 3,500 μg/L) increased levels of DNA damage compared to the control at the three exposure times tested and that cells initially exposed to 3,500 μg/L of glyphosate for 24 hours were unable to repair the breaks in DNA strands after four hours of incubation in culture medium (at 1). The authors concluded (at 1):
The present study demonstrated for the first time that Roundup® Control Max may induce genetic damage and cause alterations in the DNA repair system in human embryonic kidney cells even at concentrations found in blood and breast milk of people exposed through residues of the herbicide in food, which values have been poorly assessed or not studied yet according to the existent literature.
820 I consider that Roma (2023) is of limited assistance in demonstrating that glyphosate and/or GBFs are genotoxic, for the following reasons.
821 First, as noted above, Roma (2023) was conducted on immortalised kidney cells (HEK293), as opposed to human lymphocytes; the corollary being that it is of little relevance to the development of NHL in humans. Dr Juberg gave evidence that to his knowledge, the cell line used in Roma (2023) was not referred to in either the OECD or USEPA Guidelines (T1944).
822 Secondly, as Dr Juberg explained (T1942.14–1943.19): (1) the study incorrectly referred to the US OSHA permissible exposure limit (where there is no such exposure limit for glyphosate); and (2) the highest concentration of glyphosate used in the study is 175,000 times the median water concentration for glyphosate. Relatedly, the authors’ claim that Roundup Control Max was deployed at “environmentally relevant concentrations” is questionable. The relevant source cited by the authors (Aris and Leblanc (2011)) reported that none of the 30 pregnant women and only two of the 39 non-pregnant women had detectable glyphosate in their blood (with the mean of the two outliers being 73.6 micrograms per litre (0.43 µM)), whereas the authors of Roma (2023) suggest that this is an “environmentally relevant concentration” routinely found in “people exposed mainly through the consumption of food containing pesticide residues” (at 6).
823 Thirdly, Dr Juberg identified that there were findings in Roma (2023) which indicated there was a threshold below which DNA damage was not seen because effects were seen in the top three dose levels and not the bottom two (T1943.14–16), and that DNA repair was reported with reversibility at both 70 and 700 micrograms per litre after 24 hour exposure within four hours (T1943.16–19).
824 Fourthly, the precise formulation of Roundup Control Max (“79.2% w/w of glyphosate monoammonium salt, plus adjuvants”) is not provided in the study; the corollary being that even in the event Roma (2023) provided reliable evidence as to the presence of genotoxicity, those results could not be isolated to glyphosate.
825 For these reasons, I do not consider that Roma (2023) is of significant assistance in demonstrating that glyphosate and/or GBFs are genotoxic.
Dr Flecknoe-Brown (Conclave A)
826 It is convenient here to address Dr Flecknoe-Brown’s evidence in relation to in vitro studies conducted on humans as to whether glyphosate and/or GBFs exhibit KC2. The basis for his conclusion is summarised (at JRA [185], [188]–[189]) as follows:
185 The IARC 2017 monograph concluded that there was sufficient evidence that glyphosate and GBFs were genotoxic in various systems to support its conclusion that glyphosate is probably carcinogenic to humans.
…
188 The IARC 2017 monograph analysed 16 genotoxicity studies using standard methods on cultured human cells. All but two showed significant or highly significant evidence of genotoxicity in cells exposed to glyphosate or GBFs. Genotoxicity was shown in four of five studies of cultured non-human mammalian cells exposed to glyphosate or GBFs.
189 Weisenburger [(2021)] presents results of another nine in vitro studies using human and non-human cells published since the IARC 2017 monograph. These show evidence of DNA strand breaks, DNA aberrations, micronuclei formation and epigenetic effects in cells exposed to glyphosate or its metabolite, aminomethylphosphonic acid.
827 As mentioned earlier (at [694]), the IARC Monograph 2017 analysed a number of genotoxicity studies which broadly overlap with the studies referred to in Professor Smith’s evidence (see, for example, Table 4.1 “Genetic and related effects of glyphosate in exposed humans” (at 367)). Dr Flecknoe-Brown gave evidence that he believed he did review the 16 genotoxicity studies the subject of the IARC Monograph 2017 individually (T241.33–34), but then gave evidence (T241.36–242.4):
MR FINCH: But you say nothing in your report about the limitations and qualifications expressed in any of those reports, do you?
PROFESSOR FLECKNOE-BROWN: That’s probably, right. Yes. I haven’t got it in front of me.
MR FINCH: And we can’t conclude from reading your report what the qualifications or limitations were in those studies, can we?
PROFESSOR FLECKNOE-BROWN: I, again, looked at them carefully and drew my conclusions from them.
MR FINCH: Well, you said you did that in the Colombian and Ecuadorian studies too but you left out the qualifications of those, didn’t you?
PROFESSOR FLECKNOE-BROWN: Yes.
828 Although I have recorded above (at [698]–[704]) the reasons Dr Flecknoe-Brown did his best to assist the Court, aspects of his evidence were unsatisfactory. As was seen in relation to Bolognesi (2009) (to which I will return), Dr Flecknoe-Brown conceded that he had omitted several limitations which rendered the findings of that study in demonstrating that glyphosate and/or GBFs are genotoxic of little assistance (see above (at [701])). Similarly, in relation to Weisenburger (2021), which study he relied upon (at JRA [189]) in support of his conclusion that glyphosate and/or GBFs are genotoxic, Dr Flecknoe-Brown gave evidence as follows (T242.28–46):
MR FINCH: Thank you. Now, at paragraph 189 of your joint report paragraphs, you summarise the article from Dr Weisenburger of 2021. You see there he presents results of another nine in vitro studies, do you see that?
PROFESSOR FLECKNOE-BROWN: Yes. Yes.
MR FINCH: And did you look at each of those nine studies?
PROFESSOR FLECKNOE-BROWN: I believe so. I - - -
MR FINCH: You don’t extract from them anything - - -
PROFESSOR FLECKNOE-BROWN: I haven’t – if I haven’t commented on them I can’t say that I’ve looked at them beyond a read.
MR FINCH: You haven’t extracted from any of those studies any of the limitations or qualifications in those studies either, have you?
PROFESSOR FLECKNOE-BROWN: No.
829 Dr Flecknoe-Brown was then taken to extracts of Weisenburger (2021) and gave evidence as follows (T246.39–7; T247.32–43):
MR FINCH: So that Dr Weisenburger, whose report you summarised, places, as he says, greater weight on those two studies, but you would agree with me, Dr Flecknoe-Brown, wouldn’t you, that Dr Weisenburger nowhere qualifies his article by mentioning the reservations that we’ve already gone through in those two studies; that’s true, isn’t it?
PROFESSOR FLECKNOE-BROWN: Without a detailed re-examination for that flaw I couldn’t - - -
MR FINCH: For the moment, you will accept me subject to me getting it wrong?
PROFESSOR FLECKNOE-BROWN: Yes.
MR FINCH: Is that right?
PROFESSOR FLECKNOE-BROWN: Yes.
…
MR FINCH: So out of all the studies he refers to, including the 16 referred to in paragraph 188 and the nine referred to in paragraph 189, the only two that we can positively identify are the two that we’ve already dealt with, the Ecuadorian and the Colombian studies?
PROFESSOR FLECKNOE-BROWN: Yes.
MR FINCH: And he leaves out any qualification, subject to your reservation about not having read it, about those?
PROFESSOR FLECKNOE-BROWN: Yes. Although later in the paper I’m sure he does come to those other stuff.
830 In short, with respect to his conclusion as to genotoxicity, I accept Monsanto’s submission that Dr Flecknoe-Brown largely recited and, as a result, did not adequately interrogate the studies underlying the findings of the IARC Monograph 2017 and Weisenburger (2021) which resulted in the omission of a number of salient limitations in the JRA.
831 Accordingly, I consider that Dr Flecknoe-Brown’s evidence is of limited assistance in assessing whether glyphosate and/or GBFs exhibit KC2.
832 In contrast to the regulatory studies (which Dr Juberg and Professor Smith agreed were largely or uniformly negative) (see above (at [773]–[774])), the in vitro studies provided satisfactory evidence that glyphosate and/or GBFs are capable of generating a genotoxic signal in laboratory conditions. Save for Roma (2023) and Dr Flecknoe-Brown’s evidence given in Conclave A, the experts broadly agreed that the in vitro studies demonstrate genotoxicity by, among other things, increased frequency of chromosomal aberrations, including single and double-strand DNA breaks, and increased frequency of MN in lymphocytes (see particularly Santovito (2018) and Wozniak (2018)).
833 I will return to this evidence below when giving my conclusion as to KC2.
834 The in vivo studies analyse the effects of accidental or incidental exposure to glyphosate and/or GBFs in humans. It is necessary to address four studies which, for reasons I will explain below, provide limited assistance as to whether glyphosate and/or GBFs exhibit KC2.
835 This study analysed the consequences of aerial spraying with glyphosate added to a surfactant solution in northern Ecuador. Since January 2001, this region has been subjected to aerial spraying by the Colombian Government with Roundup-Ultra, an herbicide formulation containing glyphosate, polyethoxylated tallowamine surfactant and the adjuvant Cosmoflux 411F (Cosmoflux) (which I will say something about later). The main purpose of spraying glyphosate in this formulation is to eradicate illicit crops grown in this area, and several research projects have been carried out to investigate the consequences of the use of this formulation in Ecuador (MRE, Ecuador, 2003; Acción Ecológica, 2003) (at 456).
836 A total of 24 exposed and 21 unexposed control individuals were investigated in the study using the comet assay (that is, a standard technique for analysing DNA damage or repair, biomonitoring, and genotoxicity testing) which showed a higher degree of DNA damage in the exposed group compared to the control group. The authors concluded that these results suggest that in the formulation used during aerial spraying, glyphosate had a genotoxic effect on the exposed individuals (at 456).
837 Professor Smith gave evidence that taken together with the other in vivo studies, Paz-y-Mino (2007) suggests that glyphosate and/or GBFs pose a genotoxic and mutagenic risk in exposed humans (at JRG [83]). Professor Prince in cross-examination agreed that the study indicated that GBFs pose a genotoxic risk to humans (T550.20–25).
838 Notwithstanding that the study does indicate a statistically significant lengthening of the DNA tail in comet assays of blood from glyphosate-exposed subjects versus controls and some indication of an association between “baseline frequency of binucleated cells with micronuclei” (BNMN) and exposure to pesticides in general (at 994), the evidence established that Paz-y-Mino (2007) suffers from limitations which render it of limited assistance in support of the conclusion that glyphosate and/or GBFs are genotoxic, for the following reasons.
839 First, and perhaps most importantly, the control group of 21 individuals (who were located 80 kilometres away from the exposure area) were not matched to the exposed group and no blood samples or health documentation were taken prior to the spraying. The consequence is that the study results did not account for any pre-existing level of exposure in the control group. As the authors noted (at 458):
The unexposed (U) control group consisted of 21 unrelated healthy individuals living 80 km away from the spraying area. They were similar to the exposed group regarding their demographic characteristics and occupation but were not matched controls. Blood samples were collected and processed as for the exposed group, but not concomitantly.
(Emphasis added)
840 Dr Flecknoe-Brown and Professor Prince gave evidence that this presented as a limitation of the study (T212.1–213.8):
MR FINCH: And you would accept, also in this study, that no blood examples were taken before the spraying, were they?
PROFESSOR FLECKNOE-BROWN: No. Again, the controls here were people who were living in unexposed areas.
MR FINCH: Yes. But no blood samples were taken for the cases.
PROFESSOR FLECKNOE-BROWN: Nothing I saw, no.
MR FINCH: So it doesn’t account at all for what their pre-existing level of exposure might have been?
PROFESSOR FLECKNOE-BROWN: Again, I guess I rely – I rely on the fact that the other pesticides that – the other various agricultural chemicals that can cause problems have not been used in this – in this group of people.
MR FINCH: We don’t know?
PROFESSOR FLECKNOE-BROWN: No, we don’t know. But they’re hard to come by and - - -
MR FINCH: Yes. But the mere fact that the case group was not sampled before the period means that we don’t know what level of toxic chemicals was in their bloodstream at all?
PROFESSOR FLECKNOE-BROWN: No, we don’t. But we can say that the control group didn’t have them.
MR FINCH: Now, Professor Prince, what do you think of that limitation on the study?
PROFESSOR PRINCE: I am totally reliant on what they – what’s written in the paper. I don’t know how these people travel, whether 80 kilometres is close or far in terms of distance, what other – what other things that they’ve been exposed of. I suppose whether they do batches of – I mean, farming involves, you know, putting different things on different – on different crops at different times and it’s often time-related. So I would have thought a period of two months, a lot can happen.
…
MR FINCH: There’s two doorways through which confounders may enter, the two months after the testing and non-testing of the case group before the study; isn’t that right?
PROFESSOR PRINCE: Absolutely. I mean, I think this is a study which is done in a relatively poor country where there’s probably a small number of people doing it and to getting around to all of the people took them time and that was the practical solution but does not make for great science.
841 Professor Smith later gave the following evidence as to this limitation (T465.25–46):
MR FINCH: And so we don’t know what, if anything, people were exposed to in that time?
PROFESSOR SMITH: No.
MR FINCH: No blood samples were taken prior to the first exposure?
PROFESSOR SMITH: Not in this study, no.
MR FINCH: So there was no baseline results?
PROFESSOR SMITH: No. That’s very common though in all the of the studies I’ve done on benzene. We’ve never had a baseline.
MR FINCH: This was an area where spraying was pretty common to try to eradicate illegal crops?
PROFESSOR SMITH: I have no real information on that.
MR FINCH: They actually say so.
PROFESSOR SMITH: They say so in the paper? Okay. I agree then.
842 Secondly, the authors noted that the application rate of the formulated product greatly exceeded the maximum recommended rate (at 458):
The aerial spraying on the border between Ecuador and Colombia used 44% of Roundup-Ultra (see above) but the recommended application rate of this formulation in the USA is 1.6% to 7.7% up to a maximum concentration of 29% (MREE, 2003) and according to Acción Ecológica (2003) the application rate of the formulated product must not exceed 0.95 L ha-1. In the area of our study the application rate was 23.4 L ha-1 (10.3 L ha-1 with respect to glyphosate) and therefore more than 20 times the maximum recommended application rate for the formulated product, which may explain our comet assay results (Table 1) (Acción Ecológica, 2003, Nivia, 2001).
(Emphasis added)
843 Professor Smith acknowledged in cross-examination that he had not disclosed this limitation in his report (T1296.35–41).
844 Thirdly, as the authors of Bolognesi (2009) highlighted (at 987), the number of subjects in Paz-y-Mino (2007) (21 control and 24 exposed) was “small and there were 23 females and only 1 male in the exposed group, making interpretation of the results difficult”. Professor Smith gave evidence that this was a description of the study which indicated the necessity for further analysis, but accepted it was a limitation which he did not disclose in his report (T1297.45–7).
845 Fourthly, as identified in Paz-y-Mino (2011) (at 49), the authors noted that the population had nutritional problems as a result of the spraying. In cross-examination, Professor Smith accepted that this could constitute a further confounding factor (T1337.18–1338.4).
846 Accordingly, in the light of these limitations, I consider that Paz-y-Mino (2007) provides limited assistance as to whether glyphosate and/or GBFs are genotoxic.
847 This study was carried out in five regions of Colombia, with different potential exposure to glyphosate as reported by Sanin (2009).
848 A total of 274 individuals were included in the study: women of reproductive age and their spouses were interviewed to obtain data on current health status, history, lifestyle, including past and current occupational exposure to pesticides, and factors including those known to be associated with increased frequency of MN. In regions where glyphosate was being sprayed, blood samples were taken prior to spraying (indicative of baseline exposure), five days after spraying, and four months after spraying.
849 The authors’ overall conclusion was that although there was some indication of an association between glyphosate exposure and genotoxicity, the data suggested that genotoxic damage associated with glyphosate spraying for control of illicit crops is “small and appears to be transient” and that the evidence indicates that the genotoxic risk potentially associated with exposure to glyphosate in the areas where the herbicide was applied for coca and poppy eradication is low (at 994–995). Although Professor Prince gave evidence that the study demonstrated genotoxic risk to humans (T553.10–15), and Dr Flecknoe-Brown noted that Bolognesi (2007) “is a high quality study demonstrating in vivo genotoxicity” (T232.31–33), the evidence established some not insignificant limitations associated with the study, as follows.
850 First, the highest frequency of BNMN was in a place where there was no aerial eradication spraying of GBFs. As Professor Smith noted in cross-examination (T1305.36–1306.3):
MR CRAIG: And what you did not disclose to the court, I suggest to you, is the conclusion that was then expressed at page 990 that the highest frequency of BMNM was in Boyaca where no aerial eradication spraying of glyphosate was carried out and Valle del Cauca where aerial spraying was for maturation of sugar cane. You did not disclose that to the court, did you?---I can’t reproduce every single thing in the article.
MR CRAIG: No. But the point that’s being made by the study authors there, Professor Smith, is that the highest frequency of BNMN was in the place where there was no aerial eradication spraying and the place where aerial spraying was of Roundup 747 and not the overall formulation?---Yes. But Boyaca has the highest rate because there’s multiple pesticides being sprayed in that particular region.
MR CRAIG: You did not draw the court’s attention to the fact that the highest frequency of BNMN was in the place where there was no aerial eradication spraying and in Valle del Cauca, did you?---I did not.
851 Secondly, the authors noted (at 992) that the data suggest “greater exposure to genotoxic agents in these populations is independent of the exposure to glyphosate products”. Professor Smith gave evidence that he did not include that limitation in his reports.
852 Thirdly, the authors highlighted a number of limitations with the study itself; the most significant of which was that it was not possible to infer causality from the study results (at 995):
Given the situation, the best approach possible, a prospective cohort, was used but the need to use better procedures to estimate the exposure is acknowledged. Based on the applicable Bradford-Hill guidelines (Hill, 1965), it is not possible to assign causality to the increases in frequency of BNMN observed in our study. There was a smaller frequency of BNMN and MOMN in the region of no pesticide use compared with the regions where pesticides (including glyphosate) were used, which is consistent with other reports in the literature. Although temporality was satisfied in the increase in frequency of BNMN after spraying, this response did not show strength as it was not consistently correlated with the rate of application. Recovery was also inconsistent with decreases in frequency of BNMN in the areas of eradication spraying but not in the area where lower rates were applied on sugar cane.
(Emphasis added)
853 Professor Smith and Dr Flecknoe-Brown agreed with the authors’ conclusion (T479.33–37; T239.14–18). The authors went on to note (at 995):
Further studies are needed to better characterize the potential genotoxic risk associated with the application of glyphosate for sugar cane maturation. The smaller number of subjects recruited in this study and small amount of information about the exposure precluded any conclusions.
(Emphasis added)
854 Professor Smith ultimately gave evidence that in the light of these limitations, Bolognesi (2009), at its highest, could only present evidence of “potential genotoxic risk” (T478.13–31):
MR FINCH: You will see the authors acknowledge that: Further studies are needed to better characterise the potential genotoxic risk. And you would agree with the application of that word, “Potential to the genotoxic risk”, given the limitations of the study?
PROFESSOR SMITH: Yes.
MR FINCH: And they say – and this is something you’ve already referred to: The smaller the number of subjects recruited in this study and small amount of information about the exposure precluded any conclusions. You would agree with their assessment of the data?
PROFESSOR SMITH: I think what their study shows is there is – despite its limitations, that there is an increased incidence of micronuclei in the exposed population but you cannot make any firm conclusions about it for the reasons we’ve discussed.
855 For these reasons, I consider Bolognesi (2009) is of limited assistance in demonstrating that glyphosate and/or GBFs exhibit KC2.
856 Paz-y-Mino (2011) was a study conducted two years after aerial spraying of pesticide (a glyphosate mixture) on the northern Ecuador border of 144 individuals. A total of 521 medical diagnoses and 182 peripheral blood samples were obtained during the study. The authors found that in 84.7% of families, an individual fell ill during the spraying, and the symptoms were respiratory, digestive, and ophthalmological problems, cephalea, and skin conditions, whereas a little after the spraying, the latter became the most important problem.
857 After analysing the metaphases and karyotyping 92 individuals who belonged to the different communities of the province of Sucumbios located in Ecuador’s northeastern border, the authors observed that all the analysed women obtained a normal karyotype (46, XX) (that is, a normal appearance of a complete set of chromosomes). The authors also observed that 33% of the 92 individuals with normal karyotype had a low percentage of chromosomal fragility (5%), whereas 67% of the individuals did not present this feature. All the studied population came within the normal parameters considered for studies of chromosomal fragility (see Table 3) (at 48).
858 I do not consider that Paz-y-Mino (2011) provides significant assistance as to whether glyphosate and/or GBFs are genotoxic, for the following reasons.
859 First, as to the health conditions caused by the spraying, Professor Smith gave evidence that they are suggestive of “toxicity due to the extremely high levels of the formulation used” (T1335.35–42). Professor Smith qualified that statement, noting “but not to the blood forming system or the blood, not to the lymphocytes” (T1335.41–42), but did not identify why the health conditions identified by the authors could not be a confounding factor.
860 Secondly, as noted earlier (at [845]), the nutritional status of the people studied in Paz-y-Mino (2011) was another confounding factor. Professor Smith accepted that this could constitute a further confounding factor and that he did not identify it as acknowledged by the authors (T1337.18–1338.4). As the authors note (at 49):
This information clearly indicates that during the aerial spraying, the population had nutritional problems due to the broad spectrum herbicides that caused harm in the agricultural products essential for the population feeding, whereas the analyses obtained 2 years after the last aerial spraying confirmed improvement in the general nutritional status of the population.
861 Thirdly, the authors concluded (at 50):
Several research studies related to glyphosate exposure have been conducted in Colombia by Bolognesi et al. (8), Sanin et al. (21), and Solomon et al. (22), which state that the studied populations have low genotoxic risk associated with glyphosate. Regarding our study, we obtained results showing no chromosomal alterations in the analyzed individuals. Nevertheless, the aerial spraying had a socially and psychologically negative impact on the Ecuadorian communities. Carrying out studies in the short and long term is very important for taking control of population health and for monitoring possible disease development in the coming future.
(Emphasis added)
862 Accordingly, I consider that Paz-y-Mino (2011) provides limited assistance as to whether glyphosate and/or GBFs are genotoxic.
863 This is a study which assumed some importance in relation to KC5 but given Professor Smith’s reliance upon it for the purposes of KC2, it is necessary to say something about it here.
864 Chang and Andreotti (2023) used a group of 268 male farmers exposed to glyphosate in the AHS to investigate associations between glyphosate exposure and urinary oxidative stress biomarkers, including the chemical 8-OHdG which is a biomarker of oxidative stress and is also known to cause DNA strand breaks. The results indicated that: (1) there was a significant positive association between urinary glyphosate concentrations and oxidative stress markers, including levels of 8-OHdG; and (2) there was also an association between use of glyphosate the previous day and significantly increased 8-OHdG in the urine.
865 Professor Smith initially gave evidence that Chang and Andreotti (2023) provided “conclusive” evidence that glyphosate and/or GBFs were genotoxic (T479.27), and noted in his second report (at [7]) concerning the finding that urinary glyphosate concentrations were positively associated with increased levels of 8-OHdG and MDA:
This finding by Chang, Andreotti and co-investigators in the AHS shows that glyphosate exposure induces oxidative stress in humans that results in damage to the DNA in the form of the DNA adduct 8-OHdG. “This adduct has been widely used as a biomarker of oxidative DNA damage, and determination of 8-OHdG levels may be useful in defining a chemical’s mode of action” (Brusick, Aardema et al. 2016). Demonstration of the formation of 8-OHdG in glyphosate-exposed humans in the USA by Chang and co-authors is therefore clear evidence of a mutagenic and genotoxic mode of action for glyphosate and provides the strongest evidence to date that glyphosate induces oxidative stress (key characteristic 5) and is genotoxic (key characteristic 2) in humans.
(Emphasis added)
866 Later, during an exchange with the Assessor, Professor Smith conceded that the authors in fact drew no conclusion about the genotoxicity of glyphosate which, despite its length, is useful to set out in full (T619.3–621.47):
ASSESSOR: … So firstly, Professor Smith, while we’re looking at your supplementary report, you have this phrase that glyphosate induced oxidative stress key characteristic 5 and is genotoxic, key characteristic 2, and the first question I’ve got for you was that is that what Chang et al actually conclude? ... did they actually conclude that this was evidence of genotoxicity in the humans they studied?
PROFESSOR SMITH: They only go so far as to say it shows oxidative stress.
ASSESSOR: Fine. So what makes you confident that this show that is there’s genotoxicity in humans?
PROFESSOR SMITH: Because – because genotoxicity is damage to the DNA and they’re showing that there is an adduct which we know is mutagenic is damaged on the DNA.
ASSESSOR: Can I again, can I ask where is the DNA that’s damaged when something is genotoxic? Are you referring to DNA that’s in the nucleus of a cell that would potentially mutate, or are you referring to DNA anywhere in the body?
PROFESSOR SMITH: Well, in this particular case you don’t actually know where the DNA damage is occurring.
ASSESSOR: Right.
…
ASSESSOR: The point I was driving at was that it’s not proof positive of damage to DNA in nuclei of living cells?
PROFESSOR SMITH: It’s not. I agree with you. It’s possible that there’s a thing but it’s not fully conclusive, I agree.
ASSESSOR: And your additional point is, of course, it doesn’t tell you which cell linings in the living human have potentially been infected either?
PROFESSOR SMITH: Right. I just think it really tells you that in people exposed to glyphosate you get oxidative stress.
ASSESSOR: Do you think it’s still fair to say to say that this is the strongest evidence yet that there’s genotoxicity or would you want to perhaps change the emphasis a bit?
PROFESSOR SMITH: Well, I think actually the strongest evidence for genotoxicity is some of the papers we’ve already talked about today and yesterday.
ASSESSOR: Right.
PROFESSOR SMITH: And this sort of adds to it. This provides you a mechanism by which that genotoxicity could have occurred. So I think, from a mechanistic viewpoint, this is the strongest evidence you could get. But I agree that we have got other things like micronucleus formation in people that is better. So, yes, I would agree that I should say strongest evidence to date for oxidative stress and helps contribute to our understanding of the mechanism of genotoxicity.
ASSESSOR: Right. So contribute to our understanding?
PROFESSOR SMITH: Yes.
867 Further, although not directly relevant to the question of genotoxicity, a matter which did give me some pause as to the reliability of Chang and Andreotti (2023) was AP Harris’ evidence concerning factors other than glyphosate exposures contributing to higher levels of stress biomarkers in the farming and non-farming controls (Second Supplementary Harris Report (at [16])). It is convenient here to set out Supplementary Table 2 and 3 of Chang and Andreotti (2023):
868 As AP Harris explained (Second Supplementary Harris Report (at [15])), the relationship between urinary concentration and stress biomarkers can be evaluated using Supplementary Table 3, which indicates that unadjusted glyphosate concentrations were moderately related to concentrations of 8-OHdG. Examinations of the correlations between exposure groups, which removes some of the bias, due to differences in dilution of urine samples between groups, indicates that associations were the strongest in the non-farming controls (r ranged between 0.53–0.64, authors did not present p-values). However, as AP Harris notes (Second Supplementary Harris Report (at [15])) when the authors adjusted for creatinine:
… these associations disappeared (or reversed) for the recently and lifetime exposed farmers. In contrast, the only positive associations between adjusted urinary glyphosate and the stress biomarkers occurred in the farming and non-farming controls. Specifically, in nonfarmers a weak association was observed for 8-OHdG (r=0.32) and MDA (r=0.21) and in farming controls, with MDA (r=0.22). Note that in the non-farming group, the adjusted glyphosate quantified in the urine samples (0.37 µg/g) was on average (GM comparison) less than ½ of the recently exposed group (0.79 µg/g). Similarly, the farming controls had lower exposures (0.42 µg/g) than the glyphosate exposed groups.
869 AP Harris gave evidence that the effect of this is that factors other than glyphosate exposure contributed to higher levels of stress biomarkers in both the farming and non-farming controls. Further, the relationship between glyphosate concentration (adjusted and not adjusted for creatinine) and 8-OHdG is modified by exposure group, meaning that the findings should be reported separately for different groups (Second Supplementary Harris Report (at [16]); see also T1390–1392).
870 In the light of this evidence, Professor Smith’s initial contention that Chang and Andreotti (2023) provided anything approaching “conclusive” evidence that glyphosate and/or GBFs are genotoxic cannot be sustained.
871 Overall, the in vivo studies did demonstrate some evidence of glyphosate and/or GBFs exhibiting genotoxicity. Professors Smith and Prince agreed that Paz-y-Mino (2007) demonstrated genotoxicity by (among other things) a statistically significant lengthening of the DNA tail in comet assays, and Professor Prince and Dr Flecknoe-Brown agreed that Bolognesi (2009) demonstrated that glyphosate presents a genotoxic risk to humans.
872 With that said, the evidence established some not insignificant limitations with the in vivo studies as a whole which rendered their findings of limited assistance, including, inter alia: (1) the failure to match controls and account for pre-existing levels of exposure; (2) the high levels of concentration used; (3) the small number of subjects and controls utilised in the studies; and (4) the presence of nutritional problems as a potential confounding factor. It is worth noting here that although Monsanto contended that the use of Cosmoflux in the Paz-y-Mino studies and Bolognesi (2009) presented as a potential confounder, which was the subject of some evidence given by Professor Smith and Dr Flecknoe-Brown, I think this was largely a distraction in the light of Dr Juberg’s evidence (which I accept) that in his experience in dealing with various herbicides and pesticides, he was unaware of any adjuvants that could be described as genotoxic (T1585.34–38).
873 Accordingly, in contrast to the in vitro studies, I do not consider that the in vivo studies present reliable evidence that glyphosate and/or GBFs exhibit KC2.
The real world significance of the in vitro studies
874 Before going further, it is worth saying something about why Mr McNickle considered it necessary to wage a battle over the real world significance of the in vitro studies.
875 It has been demonstrated that the in vitro studies presented reliable evidence that glyphosate, in artificial laboratory conditions, can cause damage to DNA, and therefore exhibit KC2. In contrast, the in vivo studies overall did not provide reliable evidence that glyphosate possesses KC2. This presents a problem for Mr McNickle’s case on genotoxicity: if exposure to glyphosate can cause DNA damage in laboratory conditions, one would expect that outcome to be replicated when humans are exposed to the aerial spraying of glyphosate in the real world (that is, in vivo conditions).
876 It is for this reason that Mr McNickle spent some time in the initial trial attempting to demonstrate the in vivo significance of the in vitro studies or, put another way, that the in vitro evidence which demonstrates that glyphosate is genotoxic matters in the real world.
877 Much of this debate centred upon cytotoxicity, which is a term we have encountered in relation to the in vitro studies. Cytotoxicity is cell damage or death, and a potential by-product of cytotoxicity is genotoxicity. This is important because Mr McNickle submits that the in vitro studies which used low concentrations of glyphosate and observed a genotoxic signal (for example, Santovito (2018)) are in the range of concentrations that can be observed in healthy humans with no history of exposure to glyphosate, and thus provide evidence that glyphosate is genotoxic in vivo. Monsanto, on the other hand, contends this proposition is not reconcilable with the fact that most of the positive results for genotoxicity obtained from the in vitro studies can be explained by the induction of cytotoxicity caused by doses of glyphosate which no human would have any prospect of being exposed to in the real world.
878 The following table records (in descending order, except for Roma (2023)) the lowest concentrations of glyphosate at which a statistically significant genotoxic signal was found in the in vitro studies:
Report | Concentration in micromolar (µM) |
Kwiatkowska (2017) | 500 |
Wozniak (2018) | 250 |
Nagy (2021) | 100 |
Alvarez-Moya (2023) | 100 |
Mladinic (2009) (tail intensity with S9) | 2.9 |
Santovito (2018) | 0.145 |
879 Before going further, it is convenient to address two studies together which received some attention from the parties in relation to this topic.
2. Zouaoui (2013) and Kwiatkowska (2020)
880 Monsanto relied upon Zouaoui (2013), which was a study of humans who (as bizarre as it may sound) ingested glyphosate in suicidal attempts and whose blood plasma concentrations were then measured. Case 9 was a woman who ingested a glass of Glyper, glyphosate, in a suicidal attempt, and Case 12 was man who voluntarily drank two formulations, Verdys, glyphosate, and Decis, deltamethrin (T1377.44–1378.6). Serum and urine samples were taken 24 hours after the ingestion (T1571.5–18) and, following the ingestion of glyphosate, the blood concentration of these cases was 0.678 mg/L for Case 12 and 150 mg/L for Case 9 (e20–21).
881 Although it was not tendered, the parties expressly agreed (T2067.28–35) that the calculations in an aide memoire provided to Professor Smith (MFI-34) which converted 0.7 mg/L (having rounded up the 0.678 mg/L obtained from Case 12 to 4.1 µM) were correct and not bona fide in dispute. I have set those calculations out below:
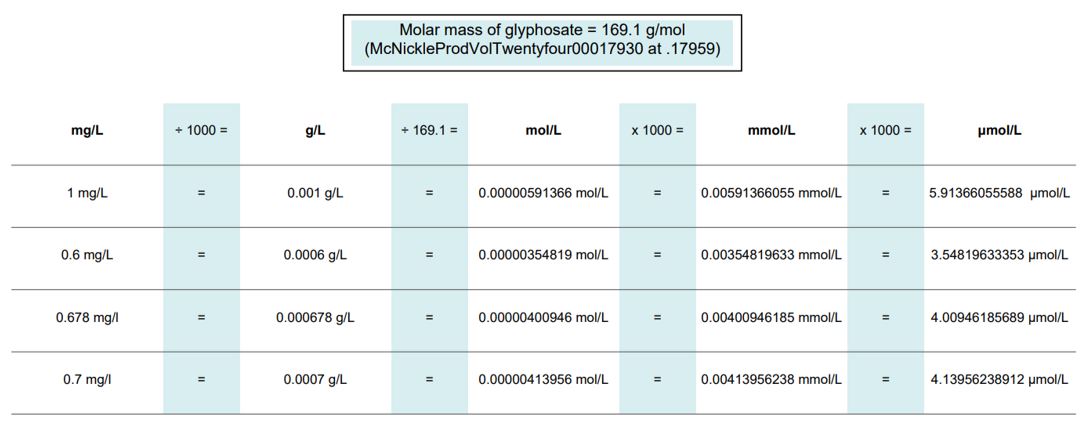
882 Kwiatkowska (2020) relied upon the findings in Zouaoui (2013) to identify the lower range of the concentrations “that [correspond] to the concentration detected in humans after acute poisoning with glyphosate formulation” (at 2–3). The authors identified that lower range as 4.1 mM, as compared with 4.1 µM that Zouaoui (2013) identified as the lower range of the toxic concentrations. In cross-examination, Professor Smith accepted that the authors of Kwiatkowska (2020) erred, as follows (T1380.21–42):
MR CRAIG: And so if the assumption is correct that the calculations on the aide-mémoire that I have provided you are right, the authors of Kwiatkowska are mistaken in recording the concentration range of being – starting at 4.1 millimolar, are they not?
PROFESSOR SMITH: They are.
MR CRAIG: Thank you. I pause there just to inquire as to whether Sir John has any questions arising from that.
ASSESSOR: That was one of the clarifications I was thinking of seeking.
MR CRAIG: Certainly.
ASSESSOR: So that range should read 4.1 to 44.2 micromolar, and it has been misprinted as “millimolar”.
PROFESSOR SMITH: That seems to be correct.
ASSESSOR: You’re agreed on that.
PROFESSOR SMITH: Yes.
883 Mr McNickle submits that Monsanto’s reliance upon Zouaoui (2013) is misplaced, for the following reasons.
884 First, it is said that Monsanto misstated what was measured or reported by Zouaoui (2013). That study did not measure or report what doses of glyphosate were “cytotoxic to human blood”, or “toxic to humans” or “genotoxic to humans”. Rather, the study measured the glyphosate levels found in blood at varying time intervals after ingestion of glyphosate, irrespective of whether the relevant individual was suffering from, or showing, any signs of intoxication at the time that the blood sample was taken. The words cytotoxic and genotoxic are not mentioned in Zouaoui (2013).
885 Secondly, and relatedly, the glyphosate blood concentration figures of 0.678 mg/L for Case 12, and 3.7 mg/L for Case 1, in Table 1 of Zouaoui (2013) do not provide reliable information in relation to what blood concentration of glyphosate corresponds with intoxication or poisoning caused by ingestion of glyphosate, for the following reasons:
(1) For Cases 1 and 12, the serum collection time was 24 hours after ingestion of the GBF. During the 24 hours between glyphosate ingestion and sample collection, the level of glyphosate in the body is falling (T1570.20–35). Hence, the difference between serum time collection post glyphosate poisoning for different individuals would have a significant impact on the level of glyphosate found in the serum (T1571.44–1572.7). There is no suggestion in the study that by the time serum samples were taken from Cases 1 and 12, 24 hours after glyphosate ingestion, they had any signs of symptoms of intoxication.
(2) The dose ingested in Zouaoui (2013) is estimated, in non-specific terms, by reference to estimates of how much is in a mouthful and a glass and so on, which Dr Juberg agreed is likely to lead to unreliable information (T1572.6–24).
(3) Case 12, in respect of whom the lowest concentration figure of 0.678g/L was recorded, was stated to have ingested two poisons (Verdys containing glyphosate and a Decis solution containing deltamethrin) (at 2). Dr Juberg agreed that a large asterisk needs to be put next to Case 12 because of the potential confounding factor introduced by the deltamethrin (T1572.26–32) and stated that the deltamethrin could have affected the measure of glyphosate because Case 12’s body was trying to metabolise two different chemistries simultaneously (T1578.40–1579.17).
886 For these reasons, Mr McNickle contends that there is no reliable basis for concluding that a glyphosate blood concentration level of 0.678 mg/L (4.1 µM) corresponds with intoxication arising from the ingestion of glyphosate. Relatedly, Mr McNickle submits that the variability between the concentrations which produced positive results for genotoxicity in the in vitro studies does not detract from his case on genotoxicity, for the following reasons.
887 First, both haematologists, Professor Prince and Dr Flecknoe-Brown, and two of the three toxicologists in the case, Professor Smith and Dr Sawyer, concluded that glyphosate and/or GBFs are genotoxic to humans. Professor Prince accepted that Kwiatkowska (2017) and Santovito (2018) showed that glyphosate is genotoxic to humans (T555.18–27; T578.33–37). Professor Smith also expressly relied on Kwiatkowska (2017), Mladinic (2009) and Santovito (2018) for his conclusion that glyphosate is genotoxic to human lymphocytes (First Smith Report (at [91]–[96]); at JRG [84]).
888 Secondly, it is said that there is no safe level of exposure to, or safe dose of, a genotoxic carcinogen. Therefore, it could not be argued persuasively that the large variability between the concentrations of glyphosate that produced positive results for genotoxicity in various assays in the Kwiatkowska (2017), Mladinic (2009) and Santovito (2018) studies could mean that there are some concentrations of glyphosate and/or GBFs that do not pose a genotoxic risk to humans.
889 Thirdly, Mr McNickle cites three further studies in support of his contentions as to genotoxicity: (1) Suárez-Larios (2017), which examined lymphocytes taken from healthy young men exposed to glyphosate and found “a statistically significant increase in double DNA strand breaks” (0.4 µM); (2) Alvarez-Moya (2014), which indicated positive results for genotoxicity at 0.7 µM (see IARC Monograph 2017 (at 379)); and (3) Roma (2023) (addressed earlier (at [819]–[825])) which demonstrated the capacity of “Roundup Control Max” to cause genetic damage in human embryonic kidney cells at a concentration of 70 μg/L.
890 I do not regard Mr McNickle’s submissions as persuasive, for the following reasons.
891 First, as is evident from the table set out above (at [878]), the large variability between the concentrations which produced positive results for genotoxicity in the in vitro studies is, in my view, of some significance.
892 This necessitates some explanation.
893 Professor Smith gave evidence that in Mladinic (2009), several threshold concentrations for detecting a genotoxic signal were identified (T1499.6–44):
ASSESSOR: … Now, this is quite a complicated table [Mladinic (2009), Table II], and the point I wanted to make was that even within this one study, various assays of genotoxicity under various conditions produce very different threshold concentrations for an effect for glyphosate. So for example, on the left-hand column, using tail length in the comet assay without S9 added, the – would you agree, Professor Smith, that the only concentration that shows evidence of genotoxicity is 580 micrograms per mil?
PROFESSOR SMITH: Yes.
ASSESSOR: Which I calculate to be 3.43 millimolar.
PROFESSOR SMITH: Okay.
ASSESSOR: Then if you move across to the second column, which is the intensity of the table, still without S9, would you agree that the lowest concentration with an effect is 3.5 micrograms per mil?
PROFESSOR SMITH: Yes.
ASSESSOR: And I’ve calculated that at 20.7 micromolar.
PROFESSOR SMITH: Okay.
ASSESSOR: And then as we go over and the S9 is added into the third column, the tail length, would you agree that the lowest concentration with a positive difference is the same, 3.5 micrograms per mil?
PROFESSOR SMITH: I would.
894 Professor Smith went on to agree with the Assessor and give evidence (T1500.1–15):
ASSESSOR: So within – would you agree within this one figure – this one table, we’ve got almost a thousandfold range in the glyphosate concentration that produces a – the lowest glyphosate concentration that results in a positive outcome in the test?
PROFESSOR SMITH: Tail intensity is clearly more sensitive than tail length.
ASSESSOR: Yes.
PROFESSOR SMITH: And so I don’t think the tail length is so different. And also – but there is – I fully agree with you, there is - - -
ASSESSOR: Yes.
PROFESSOR SMITH: There does appear to be somewhat of a dose response. That’s all I would say.
895 As the exchange above established, in Table II of Mladinic (2009), the comet assay tail length in glyphosate-treated lymphocytes had a lowest threshold at 580 micrograms per ml (3430 µM) but, when adding the “metabolic activation system” to S9 lymphocyte culture, this figure was brought down by two orders of magnitude to 3.5 micrograms per ml (20.7 µM). This concentration was the lowest threshold for detection of a glyphosate-associated increase in comet tail intensity, which by including S9 in the lymphocyte culture, was reduced a further order of magnitude to 0.5 micrograms per ml (2.9 µM). The corollary is that in one study, the choice of assay type and cell culture conditions resulted in a thousand-fold variation in the lowest glyphosate concentration that generated a genotoxic response.
896 Another example comes from Santovito (2018) where a further ten-fold reduction in the lowest concentration of glyphosate associated with a genotoxic signal was observed by artificially activating and driving proliferation of lymphocytes by culture for 72 hours with the stimulus PHA (phytohaemaglutinin). Table 1 of Santovito (2018) indicates a statistically significant increase in chromosomal aberrations with the lowest glyphosate concentration threshold of 0.025 micrograms per ml (0.145 µM).
897 Seen in this light, the large variability between the concentrations which produced positive results for genotoxicity in the in vitro studies is significant because Mladinic (2009) and Santovito (2018) suggest that toxicologists can generate in vitro conditions and use various assays to detect a genotoxic hazard at ever-lower concentrations of glyphosate. The fact that genotoxicity was observed from the use of low concentrations of glyphosate in Suárez-Larios (2017) and Alvarez-Moya (2014) is further evidence of this notion.
898 Secondly, in relation to Zouaoui (2013), three of the in vitro studies relied upon by Professor Smith cited that study as a benchmark of the cytotoxic dose of glyphosate to human blood (Santovito (2018) (at 34698); Wozniak (2018) (at 511); and Kwiatkowska (2017) (at 94)). The authors of Wozniak (2018), for instance, noted (at 511):
Moreover, some cases of acute poisoning with glyphosate have been reported (Roberts et al., 2010). Zouaoui et al. (2013) showed that in the case of glyphosate intoxication, its content in blood was in the range from 0.6 to 150 mg/L (3.54 ± 887.21 μM).
899 In any event, the focus on Zouaoui (2013) was largely a distraction. It is plain as a pikestaff that even if 4.1 µM cannot be fastened upon as the lowest threshold level of intoxication, four of the in vitro studies relied upon by Professor Smith were conducted at glyphosate concentrations which exceeded the lowest intoxication threshold identified in evidence by Professor Smith (5 µM) by a multiple of between (at least) 20 to 100 (see above (at [878])).
900 Thirdly, Kwiatkowska (2020) cited Aris and Leblanc (2011) in support of the proposition that a glyphosate concentration of 0.01 mM is “quite similar to the concentration determined in blood of humans who were not directly exposed to this herbicide [0.435 ± 0.167 μM]” (at 2). As explained above in relation to Roma (2023) (at [822]), however, Aris and Leblanc (2011) reported that none of the 30 pregnant women and only two of the 39 non-pregnant women had detectable glyphosate in their blood (with the mean of the two outliers being 73.6 micrograms per litre (0.43 µM)). It is therefore doubtful whether these two outliers could be said to be representative of the whole population studied and indicates that the authors of Kwiatkowska (2020) presumed that blood concentrations of glyphosate are higher in healthy individuals than they are in reality.
901 As noted above, it was necessary for Mr McNickle to wage this battle largely due to the evident problems associated with the in vivo studies in demonstrating that glyphosate is genotoxic in the real world. I am not persuaded, however, that the in vitro studies can ameliorate these problems, broadly for two reasons: first, Santovito (2018) and Mladinic (2009) demonstrated that it is possible to detect a genotoxic signal at ever-lower concentrations of glyphosate by changing in vitro conditions and using different assays; and secondly, that a majority of the in vitro studies were conducted at concentrations which exceeded the lowest threshold for induction of cytotoxicity identified in evidence by Professor Smith (5 µM).
902 It will be necessary to return to this topic below when addressing in the first hypothesised mechanism in Section VI.
903 Before setting out my conclusion as to whether glyphosate exhibits KC2, it is useful to give an overview of my findings above as to the evidence given in relation to the regulatory studies; in vitro studies; in vivo studies; and other studies.
904 First, with respect to the regulatory studies relied on by Mr McNickle, Dr Juberg and Professor Smith agreed that the regulatory studies were either largely or uniformly negative in terms of demonstrating that glyphosate and/or GBFs are genotoxic. In contrast, however, notwithstanding the presence of some minor limitations, and despite Roma (2023) and Dr Flecknoe-Brown’s evidence being of limited assistance, the experts were largely in agreement that the in vitro studies provided reliable evidence that glyphosate and/or GBFs are genotoxic.
905 In short: (1) Professors Prince and Smith gave evidence that the results of Santovito (2018) demonstrated genotoxicity by: (a) a number of increased chromosomal aberrations, including double-strand DNA breaks; and (b) increased frequency of MN in lymphocytes; (2) Professor Smith, Dr Juberg and Professor Prince agreed that Wozniak (2018) indicated that glyphosate induced DNA damage in PMBCs, including single and double-strand breaks; (3) Professors Smith and Prince accepted that Kwiatkowska (2017) demonstrated DNA damage to lymphocyte in the form of single-strand DNA breaks; (4) Professors Smith and Prince agreed that the results of Nagy (2021) showed that glyphosate and/or GBFs are genotoxic; and (5) Dr Juberg (subject to the authors’ qualification) and Professor Smith agreed that Alvarez-Moya (2023) provided evidence of genotoxicity.
906 Secondly, however, the in vivo studies presented a different picture. Although the experts were largely ad idem in relation to two of the in vivo studies demonstrating that glyphosate and/or GBFs pose a genotoxic risk (Paz-y-Mino (2007) and Bolognesi (2009)), the evidence established that the in vivo studies suffered from several limitations which rendered their findings of limited assistance, which may be broadly categorised as follows: (1) failure to match controls and account for pre-existing levels of exposure (Paz-y-Mino (2007); Bolognesi (2009)); (2) aerial spraying exceeding the maximum application rate for the formulated product (Paz-y-Mino (2007); Paz-y-Mino (2011)); (3) the small number of subjects and controls used (Paz-y-Mino (2007)); and (4) the presence of nutritional problems as a confounding factor (Paz-y-Mino (2007) (Paz-y-Mino (2011)). It was also seen in relation to Chang and Andreotti (2023) that contrary to Professor Smith’s evidence, the authors only went so far as to conclude that the study demonstrated the presence of oxidative stress, rather than evidence of genotoxicity, and that the authors’ reliance on Aris and Leblanc (2011) rendered its findings less reliable in relation to any conclusion as to KC2.
907 Subject to a matter to which I will return momentarily, I am satisfied, having regard to the whole of the mechanistic evidence before me, that glyphosate and/or GBFs are genotoxic and therefore exhibit KC2. Notwithstanding the limitations to which I have referred, it is difficult to come to any other conclusion, particularly given the weight placed upon the in vitro studies by the experts and Professors Smith and Prince agreeing they demonstrate that glyphosate and/or GBFs satisfy the requirements of KC2.
908 The rub, however, is one I have introduced earlier in relation to the in vivo significance of the in vitro studies: that is, the weight of the evidence may demonstrate that glyphosate and/or GBFs may exhibit genotoxic effects on human blood cells, but whether this constitutes evidence that those substances are capable of inducing genotoxicity, let alone cancer or NHL, in the real world is a different question. I will return to this issue in the context of the first hypothesised mechanism in Section VI below.
909 Before leaving this section, however, it is necessary to say something briefly in relation to mutagenicity.
910 Professor Smith concluded in his reports that there is strong evidence that glyphosate and/or GBFs are genotoxic and mutagenic (at JRB [160]; JRG [84]). Dr Flecknoe-Brown similarly concluded (at JRA [198]–[205], [270]) that GBFs have a “mutagenic effect” or contain “mutagenic substances”. It will be recalled that a mutagen is a genotoxic agent that produces mutations and while all mutagens are genotoxic, not all genotoxicants are mutagenic (that is, leading to a permanent and heritable change in the DNA or genetic material) (see above (at [766]–[767])).
911 Notwithstanding my conclusion above as to genotoxicity, I am not persuaded that the evidence establishes that glyphosate and/or GBFs exhibit mutagenicity, for the following reasons.
912 First, Professors Smith and Prince agreed that mutagenicity involves the induction of permanent mutations in the DNA (T316.34–36; T462.41) and that genotoxic damage must cause permanent cell mutation in order to lead to cancer (T462.32–36). As Professor Prince explained in cross-examination (T584.27–585.15):
MR CLEMENTS: I suggest to you it is right because double strand breaks in lymphocytes results in the kind of chromosomal rearrangement commonly seen in human NHL.
PROFESSOR PRINCE: There’s no evidence for that. There’s no evidence that the chromosomal breaks that we see – that glyphosate is, one, that they persist; and, secondly, if they do persist, are they the chromosomal breaks that we see in lymphoma. If I had that evidence, I would be able to answer you a different way.
…
MR CLEMENTS: So you disagree with me?
PROFESSOR PRINCE: Totally. Are you looking for proof of the actual mutation caused by double strand breaks, caused by glyphosate?
PROFESSOR PRINCE: Yes. We’re all looking for that, yes.
MR CLEMENTS: But you say this, do you – you say, “I accept that exposure to glyphosate will resulted in double strand breaks in lymphocytes, but I can’t [prove] that those double strand breaks actually happen in chromosomes which can then trigger NHL”; is that - - -
PROFESSOR PRINCE: Absolutely correct.
913 Secondly, notwithstanding evidence of genotoxicity in the studies relied upon by Professor Smith, none of the studies demonstrated that glyphosate and/or GBFs caused DNA damage that was permanent or lead to mutations which cause NHL in humans.
914 Thirdly, I do not regard Dr Flecknoe-Brown’s evidence in the JRA (at [198]–[205], [270]) concerning the mutagenicity of glyphosate and/or GBFs as persuasive, for the reasons identified by Professor Prince above (at [905]). Further, Dr Flecknoe-Brown does not state the basis for his assumption in those paragraphs that glyphosate and/or GBFs are mutagenic substances.
915 Accordingly, although I am satisfied, subject to the qualification above, that glyphosate and/or GBFs exhibit KC2, I do not consider that the evidence establishes that glyphosate and/or GBFs are mutagenic substances.
II KC4 (induces epigenetic alterations)
916 It will be recalled that epigenetic alterations (KC4) are reversible mechanisms that cause altered gene expression. Epigenetic modifications include methylation of the DNA, modifications of histone proteins which coat the DNA, and differential expression of non-coding RNAs (such as microRNAs) which control how genes are expressed. Many carcinogens induce these epigenetic alterations and therefore possess KC4 (see above (at [662])).
917 Professor Smith concluded that there is strong evidence that glyphosate and/or GBFs induce epigenetic alterations, including in human lymphocytes, and therefore possess KC4 (at JRB [112], [157]; First Smith Report (at [129])). Professor Smith maintained this opinion in oral evidence, adding that “epigenetic changes are a common event in the development of non-Hodgkin lymphoma” (T487.33–34). In support of his opinion, Professor Smith primarily relied on Rossetti (2021) and, to a lesser extent, Lucia (2022).
918 This study is a “mini-review” which summarises the current evidence concerning alleged glyphosate, GBH, and amino methylphosphonic acid (AMPA)-induced epigenetic modifications in humans and rodents and proposes them as potential mechanisms through which these compounds could alter body functions (at 2).
919 The authors suggest that studies indicate that in human lymphocytes and blood mononuclear cells, glyphosate (and at much lower concentrations, its metabolite AMPA) alter the DNA methylation status and hence the expression of key cancer genes p16, p53, and p21. These genes encode proteins with important functions in the DNA-damage repair pathways that are among the most frequently compromised pathways in pathological conditions such as tumour growth.
920 Further, the authors note that GBFs have also been shown to induce aberrant DNA methylation and histone acetylation/methylation of the estrogen receptor alpha gene in rats. This receptor is the main protein which signals the effects of estrogens to the nucleus of cells and plays an important role in many biological processes in the body, including lymphoma development (First Smith Report (at [129])). The authors concluded (at 6):
Recent findings have shown that the exposure of glyphosate, GBH, or AMPA could affect epigenetic mechanisms. These include the decrease of global DNA methylation, alterations in the methylation pattern of specific regions, including ER and tumor suppressor genes, histone modifications, and differential expression of non-coding RNAs involved in, for example, Wnt and Notch pathways. These epigenetic markers have been involved in several physiological and pathological processes that were also reported after glyphosate, GBH, or AMPA exposure in animal models. In this sense, several lines of evidence indicate that the exposure to these compounds could alter the epigenome, disrupting the mRNA expression and protein levels of key genes involved in normal functions and thus, producing negative consequences (Figure 1). These epigenetic alterations could be heritable and could have a manifestation in health impacts and disease after the exposure has ended. Overall, more studies are needed to identify epigenetic targets, to define how they are dysregulated in human disease and their functional role, and to determine the critical windows of vulnerability by herbicide exposures.
921 Lucia (2022) was an epigenome-wide association study to identify DNA methylation loci associated with urinary glyphosate and its metabolite AMPA levels. Secondary goals were to determine the association of epigenetic age acceleration with glyphosate and AMPA and develop blood DNA methylation indices to predict urinary glyphosate and AMPA levels (at 047001-1).
922 White blood cell DNA methylation was measured from 392 women who participated in the study. Glyphosate and AMPA were measured in two urine samples per participant using liquid chromatography–tandem mass spectrometry. Methylation differences at the probe and regional level associated with glyphosate and AMPA levels were assessed using a resampling-based approach (at 047001-1).
923 The authors identified 24 CpG sites (a region of DNA) whose methylation level was associated with urinary glyphosate concentration and two associated with AMPA. Four regions were associated with glyphosate levels, along with an association between ESR1 promoter hypomethylation and AMPA (at 47001-1). The authors concluded (at 047001-12):
This study identified differential DNA methylation associated with the herbicide glyphosate and its metabolite AMPA and developed a methylation index that accurately predicted urinary glyphosate concentration and tertile in an internal validation sample. Glyphosate- and AMPA-associated methylation occurred near genes associated with cancer (SF3B2,109,110,111 MSH4,113 and TRIM31117,118,119) and endocrine disruption (ESR1132), and AMPA was associated with greater epigenetic age acceleration. These results suggest that exposure to these common chemicals affects the epigenome, informing the hypothesis that glyphosate and/or AMPA exposure might elevate the risk for disease, including cancer. Further studies are warranted to replicate our results, determine the functional impact of glyphosate- and AMPA-associated differential DNA methylation, and explore whether DNA methylation could serve as a biomarker of long-term glyphosate exposure.
924 Before going further, it is worthwhile to say something about the “significance” question as it relates to KC4.
925 As noted above (at [916]), epigenetic alterations can include methylation of the DNA, modifications of histone proteins which coat the DNA, and differential expression of non-coding RNAs (such as microRNAs). That is, to satisfy KC4 simpliciter, it is sufficient that the evidence demonstrates that the substance in question causes (for instance) methylation of the DNA. The next step involves asking whether the evidence demonstrates that the epigenetic alteration points to a change in gene expression which leads to cancer – in other words, does the epigenetic alteration matter?
926 As Professors Smith and Prince noted during the concurrent evidence session (T494.2–19):
PROFESSOR SMITH: … so for the purposes of saying key characteristic 4, independent of whether there’s functional changes, gene expression or whatever, it shows you that in humans you see epigenetic alterations which is really what KC4 is all about.
MR FINCH: Yes. So do you accept that, Professor Prince, that you see KC4?
PROFESSOR PRINCE: Yes. … KC4 – and I didn’t write the KC – is the recognition of epigenetic changes - - -
MR FINCH: Yes.
PROFESSOR PRINCE: - - - I would say two things: one would have to be, well, what is a significant epigenetic change and an insignificant epigenetic change?
MR FINCH: Sure.
PROFESSOR PRINCE: So I think that’s what we’re arguing over.
927 Professors Smith and Prince went on to give evidence (T495.10–25):
PROFESSOR SMITH … I mean, the idea for KC4 is: does it induce epigenetic alterations? And this study adds to – adds to the findings from the intact human blood cells in culture where we see methylation changes also that glyphosate, both in human beings and in human cells, induces epigenetic alteration which is what KC4 is.
MR FINCH: Yes.
PROFESSOR SMITH: What the consequences of those are and whether they’re truly significant alterations is an open question. But for the purposes of checking the box KC4, [methylation of the DNA] would do it.
MR FINCH: Now, without going back over things you’ve already said, Professor Prince, do you agree with that?
PROFESSOR PRINCE: Yes, I agree with that.
928 This is important because although the studies suggest that glyphosate and/or GBFs cause epigenetic changes (such as methylation of the DNA), there is less evidence as to whether those changes are “significant” or linked to the development of cancer. As noted earlier, we are here concerned with the first question, namely whether glyphosate and/or GBFs “tick the box” of inducing epigenetic alterations: the significance question will be addressed when dealing with the fourth hypothesised mechanism of action below in Section VI.
929 With that aside, the evidence established that Rossetti (2021) and Lucia (2022) suffered from several limitations which largely went to the significance question. I will address those limitations later, but, for the following reasons, I am satisfied that glyphosate can induce epigenetic alterations and therefore exhibits KC4.
930 Professor Prince agreed with Professor Smith’s conclusion that exposure to GBFs causes epigenetic changes in B-cells (T403.37–41; at JRA [208]). In cross-examination, Professor Prince was taken to Rossetti (2021) and its summary of the findings of Kwiatkowska (2017) (which we have seen earlier in relation to KC2). He gave the following evidence:
MR CLEMENTS: So in terms of the Rossetti paper … this is at page 3 and it’s the last paragraph on the right-hand column … so you will see there it says:
The effect of glyphosate on DNA methylation was first reported by Kwiatkowska et al in vitro. These authors showed that high concentrations of glyphosate induced DNA lesions in peripheral blood mononuclear cells, decreased global 5mC percentage and increased methylation of p53 promoter.
I suggest to you that’s a fair summary of the findings of Kwiatkowska in relation to the epigenetic changes induced by glyphosate. Bearing in mind it’s – the summary is silent, I’m just wondering about that expression?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: So you agree with that?
PROFESSOR PRINCE: I agree.
931 Professor Prince then gave the following evidence in relation to two studies authored by Wozniak et al cited in Rossetti (2021) (at (34) and (60)) (T597.47–598.5):
MR CLEMENTS: … Do you agree that – and you tell me if you need to look at the abstract or read the whole paper but do you agree that the two Wozniak 2020 studies reported similar results to Kwiatkowska in relation to the effect of glyphosate on DNA methylation?
PROFESSOR PRINCE: Correct.
…
932 In relation to Wozniak (2021), Professor Prince gave evidence in the JRI (at [129]) (see also T601.7–45):
Woźniak et al. found that glyphosate changes the expression of DNMT1, DMNT3A, and histone deacetylase (HDAC) 3 in PBMCs. These enzymes are involved in the regulation of chromatin architecture and, thus, could affect methylation patterns and histone modification, leading to changes in gene expression but they did not actually study this.
(Citations omitted)
933 Professor Prince then gave the following evidence in relation to the studies referred to above in Rossetti (2021):
MR CLEMENTS: Just trying to wrap this up, Professor Prince, I suggest the study I’ve asked you about; namely, Kwiatkowska 2021, and the three Wozniak ones in 2020 and 2021, are consistent in that they’ve all showed that when human cells are exposed to glyphosate in vitro that causes global hypomethylation of DNA in the cells; that is, a reduction of methylation of the DNA in the cells; do you agree with that?
PROFESSOR PRINCE: Correct.
MR CLEMENTS: And, similarly, they have consistently showed that there was hypermethylation; that is, increased methylation, in the TP53 tumour suppressor gene?
PROFESSOR PRINCE: Correct.
MR CLEMENTS: And, similarly, they’ve consistently showed that there was hypomethylation; that is, reduced methylation, in the p21 promoter gene?
PROFESSOR PRINCE: Correct.
MR CLEMENTS: And so I suggest to you it’s fair to say that the studies that have examined the effect of glyphosate on methylation of human cells in vitro are consistent; do you agree with that?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: And it’s fair to say, I suggest, that those studies have consistently showed that exposure to glyphosate results in aberrant DNA methylation; correct?
PROFESSOR PRINCE: I agree.
934 In short, Professors Smith and Prince agreed that the Wozniak (2020) studies, Wozniak (2021), and Kwiatkowska (2017) the subject of Rossetti (2021) demonstrated evidence of epigenetic alterations resulting from glyphosate and/or GBFs in the form of: (1) global hypomethylation; (2) hypermethylation in the TP53 tumour suppressor gene; (3) hypomethylation in the p21 promoter gene; and (4) aberrant DNA methylation.
935 For these reasons, I am satisfied that glyphosate and/or GBFs induce epigenetic alterations. I will return to this topic in relation to the “significance” question when addressing the fourth hypothesised mechanism of action in Section VI below.
III KC5 (induces oxidative stress)
936 KC5 (oxidative stress) occurs when cells produce an excess of reactive oxygen species (ROS). As noted earlier (at [663]), many human carcinogens can induce oxidative stress which can lead to damage to DNA and other cell components.
937 Professor Smith’s opinion is that there is “strong evidence” that glyphosate and/or GBFs induce oxidative stress (First Smith Report (at [130]–[134]); at JRB [112]).
938 Professor Smith notes that there are more than ten in vitro studies that have reported oxidative stress from glyphosate and/or GBFs resulting in increased ROS formation; decreased antioxidant activity; decreased oxidative detoxification; increased oxidative DNA damage; and increased lipid peroxidation. It is said that when cells are exposed to glyphosate and/or GBFs, increased ROS formation causes oxidative stress and the re-regulation of cell signalling associated with apoptosis or necrosis. Reduced antioxidant activity also reduces the defensive ability for inflammation response in cells (First Smith Report (at [132])). Professor Smith notes further that numerous animal studies have shown that glyphosate induces oxidative stress. Biomarkers of oxidative stress have been reported in various tissues in rats and mice, including liver, skin, kidney, brain, and plasma (First Smith Report (at [133])).
939 Notwithstanding that I harbour some concerns as to whether Professor Prince has sufficient expertise in toxicology and inflammation to give a comprehensive opinion as to whether glyphosate exhibits KC5, I am satisfied on the evidence before me that glyphosate and/or GBFs can induce oxidative stress, for the following reasons.
940 First, in cross-examination, Professor Prince agreed that exposure to glyphosate and/or GBFs “tick the box” of KC5 but that oxidative stress induced by glyphosate falls short of acting as a biologically plausible mechanism to induce lymphoma (T500.38–43):
MR FINCH: … is it too broad a summary to say that your view is you may see oxidative stress being induced by glyphosate exposure but you don’t see it moving all the way to an expression in the human body or is that too rough a summary?
PROFESSOR PRINCE: No. That’s a perfect summary.
…
941 Professor Prince went on to note (T501.19–25):
PROFESSOR PRINCE: … I think that it’s a question of what we were talking about before in terms of – I agree that there’s enough data to put [glyphosate] into tick[ing] the box of oxidative stress as a KC but it’s a question of whether it is relevant or not relevant to lymphoma. The second issue is that there’s just a huge amount in the data as to what causes these measurements of oxidative stress and the non-specific nature of we’re getting oxidative stress all the time through a variety of things and how they relate to is what complicates the area.
942 Secondly, Professor Smith relied on Chang and Andreotti (2023) (which I have addressed above in the context of KC2 (at [863]–[870])). That study indicated that: (1) there was a significant positive association between urinary glyphosate concentrations and oxidative stress markers (including levels of 8-OHdG; and (2) there was an association between use of glyphosate the previous day and significantly increased 8-OHdG in the urine, notwithstanding that Professor Smith conceded that in theory, the 8-OHdG detected could have arisen by oxidation of cell-free DNA living outside of living cells (T619.35–41). Despite a close scrutiny of the supplementary data establishing that it was unsafe for the authors to conclude that glyphosate induced oxidation of DNA in vivo (a topic dealt with earlier in relation to KC2 (at [867]–[870])), the authors of concluded that:
Our findings contribute to the weight of evidence supporting an association between glyphosate exposure and oxidative stress in humans and may inform evaluations of the carcinogenic potential of this herbicide.
943 Thirdly, notwithstanding Dr Juberg expressed some ambivalence as to what conclusions could be drawn from Chang and Andreotti (2023) (T1583.4–9; T1584.5–9; Third Juberg Report (at [6])) and some limitations highlighted by Monsanto (which, broadly speaking, go to the question of whether oxidative stress induced by glyphosate is a biologically plausible mechanism for cancer, to which will return in Section VI), Professors Smith and Prince both agreed that the study provided reliable evidence that exposure to glyphosate induces oxidative stress in humans.
944 As noted earlier (at [865]), Professor Smith concluded in the Second Smith Report (at [7]–[9]) that the findings by the authors of Chang and Andreotti (2023) indicate that glyphosate induces oxidative stress in humans which results in damage to the DNA, and that constitutes “the strongest evidence to date that glyphosate induces oxidative stress [KC5] and is genotoxic [KC2] in humans”. Professor Prince agreed with that conclusion, save for Professor Smith’s statement with regards to KC2 (see above (at [865])). In cross-examination, Professor Prince gave evidence as follows (T615.8–616.1):
MR CLEMENTS: … The results of the Chang Andreotti study showed that there was a positive association between urinary glyphosate concentrations and levels of 8-OHdG; that’s correct?
PROFESSOR PRINCE: Yes. I understand, in certain – there was certain correlations, yes.
…
MR CLEMENTS: … there was also an association between use of glyphosate the previous day and significantly increased 8-OHdG in the urine; is that correct?
PROFESSOR PRINCE: Correct.
MR CLEMENTS: And in their conclusion on page 1 the authors state:
Our findings contribute to the weight of evidence supporting an association between glyphosate exposure and oxidative stress in humans.
I suggest to you that’s a fair conclusion from the results of the study; do you agree?
PROFESSOR PRINCE: I agree.
945 Fourthly, Professors Prince and Smith agreed that Sidthilaw (2022), a study introduced by Mr McNickle during the concurrent evidence session concerning maize farmers in Thailand who were exposed to glyphosate, demonstrated that the spraying of glyphosate induced oxidative stress. The authors’ conclusion (at 5) was put to Professors Smith and Prince, who gave the following evidence (T623.21–624.16):
MR CLEMENTS: And the results are set out on page 5 of the study… :
The comparison of urinary glyphosate levels, oxidative stress, inflammation and lung function before and after applying glyphosate, showed that there was a statistically significant increase in urinary glyphosate levels, oxidative stress and serum MDA, while serum GHS levels showed a statistically significant decrease. There was a statistically significant increase in inflammation and CRP. However, lung function decreased statistically significantly.
CRP stands for C-reactive protein; is that correct?
PROFESSOR PRINCE: Correct.
MR CLEMENTS: And that’s a biomarker of inflammation; is that correct?
PROFESSOR PRINCE: Once it exceeds a threshold, it’s not a continuous variable.
MR CLEMENTS: Right. Okay. Is CPR secreted by the body as a result of inflammatory processes?
PROFESSOR PRINCE: Yes. CRP – it’s secreted by certain sorts of cells.
MR CLEMENTS: Yes. Thank you. MDA, which is referred to in that Results paragraph, stands for malondialdehyde?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: Is that a biomarker of oxidative stress.
PROFESSOR PRINCE: Yes, it is.
MR CLEMENTS: The higher the MDA level the more oxidative stress the cell has been subjected to; is that right?
PROFESSOR PRINCE: I can’t answer that. I’m not actually sure.
MR CLEMENTS: Professor Smith, do you have anything to say about that? Is it correct to say the higher the MDA level the more oxidative stress a cell has been subjected to?
PROFESSOR SMITH: Yes.
946 Professor Prince was then asked about the biomarker “glutathione” (GHS) and its relationship to oxidative stress (T624.18–38):
MR CLEMENTS: GHS stands for glutathione, which, I suggest, is another biomarker of oxidative stress. Professor Prince, do you agree with that?
PROFESSOR PRINCE: It’s an uncommon one but I believe it has been used, yes.
MR CLEMENTS: GHS plays a role in protecting cells from damage from oxidative stress; is that correct?
PROFESSOR PRINCE: My understanding, yes.
MR CLEMENTS: And when a cell is subjected to oxidative stress GHS gets used up in a process of breaking down – sorry, I will go back a step: when a cell is subjected to oxidative stress by glyphosate, GHS gets used up in a process of breaking down glyphosate; is that correct?
PROFESSOR PRINCE: Correct.
MR CLEMENTS: And, therefore, a reduction in the levels of GHS in cells is an indication that the cells have been subjected to oxidative stress?
PROFESSOR PRINCE: Yes, that’s correct.
947 Professor Prince then gave evidence, based on those results (T624.40–625.4):
MR CLEMENTS: At the bottom of the left-hand column of page 5, the authors state as follows – there’s a paragraph that starts with the words, “In the case of.” So:
In the case of serum oxidative stress and inflammation, our results indicate that serum MDA and CRP levels increased statistically significantly after the application of the glyphosate but that GHS decreased.
So I suggest to you what that study showed is that spraying glyphosate induced oxidative stress in the bodies of the farmers who had sprayed the glyphosate.
PROFESSOR PRINCE: Yes.
948 Fifthly, it will be recalled that Professor Prince agreed in cross-examination that Wozniak (2018) demonstrated that glyphosate induced single and double-strand DNA breaks (thus exhibiting KC2) (see above (at [793])). He also gave evidence as follows (T558.30–44):
MR CLEMENTS: The Wozniak study also observed that glyphosate and the Roundup formulation generated reactive oxygen species in the PBM cells; correct?
PROFESSOR PRINCE: Yes.
MR CLEMENTS: And they concluded that the observed DNA damage was not associated with direct interaction between glyphosate and Roundup with the DNA molecule.
PROFESSOR PRINCE: Yes.
MR CLEMENTS: But likely occurred as a result of the effects of the reactive oxygen species generated as a result of exposure to glyphosate and Roundup.
PROFESSOR PRINCE: That was their interpretation of “likely” and I think it’s reasonable.
949 In the light of this evidence, I am satisfied that glyphosate and/or GBFs induce oxidative stress. It will be necessary to return to this topic in the context of addressing the third hypothesised mechanism of action below in Section VI: that is, whether oxidative stress of the type observed in the studies is implicated in causing NHL in humans.
IV KC6 (induces chronic inflammation)
950 KC6 (chronic inflammation) concerns inflammation caused by the body’s response to potentially harmful irritants or pathogens. The function of inflammation is to eliminate the cause of cell injury and initiate tissue repair. It has been shown that chronic inflammation can increase the risk of some cancers, including, for example, colorectal cancer and mesothelioma (T622.12–27; T1307.44).
951 Professor Smith’s opinion is that there is “strong evidence” that glyphosate and/or GBFs cause chronic inflammation (First Smith Report (at [135]–[143])). In reaching that conclusion, Professor Smith relied upon the following studies:
(1) Pandey (2019);
(2) Hamdaoui (2018);
(3) Kumar (2014);
(4) Yamamoto (2013), Kruger (2013), Shehata (2013), Aitbali (2018), Davoren (2018), Kittle (2018), Lozano, Defarge (2018), Mao (2018), Motta (2018), Nielsen (2018), Blot (2019) and Tang (2020) (gut microbiome studies); and
(5) Mesnage (2021)
(collectively, chronic inflammation studies).
952 It is convenient first to summarise Professor Smith’s findings with respect to the chronic inflammation studies and other studies relied upon by Mr McNickle in relation to KC6.
Relevant studies
953 First, in relation to Pandey (2019) and Kumar (2014), the former study exposed rats to various doses of Roundup (0, 5, 10, 25, 50, 100 and 250 mg/kg bodyweight glyphosate) orally, every day for 14 days. On day 15, liver and adipose tissues from dosed rats were analysed for inflammation markers. CRP in liver, cytokines IL-1β, TNF-α, IL-6, and inflammatory response markers, and prostaglandin-endoperoxide synthase were upregulated in liver and adipose of rats exposed to higher (100 and 250 mg/kg bw/d) doses of Roundup. Professor Smith notes that these data show that short-term GBF exposure induces inflammation in the liver and adipose tissue. In relation to the latter, Kumar (2014) indicated that inhalation of glyphosate-rich air samples and glyphosate induced airway inflammation in mice (First Smith Report (at [136])).
954 Secondly, Professor Smith notes that chronic inflammatory outcomes from longer term exposures have also been reported. It is said that Hamdaoui (2018) indicated that histological examination of liver tissue from rats sub-chronically exposed to GBFs revealed the presence of Kupffer cells (a form of macrophage associated with inflammation), hepatocyte vacuolisation, inflammatory reaction, and necrosis (though the latter occurred in the absence of fibrosis or tissue remodelling) (First Smith Report (at [137])).
955 Thirdly, it is said that another way in which glyphosate and/or GBFs may induce systemic chronic inflammation has been discovered that may be especially important for the induction of lymphoma (Yamamoto (2013)). According to Professor Smith, with reference to the gut microbiome studies, this mechanism relates to the finding from multiple laboratories that glyphosate and/or GBFs, including Roundup, alter the make-up of the gut microbiome in several species and cause extensive inflammation (First Smith Report (at [138])). An altered microbiome can cause both local and systemic chronic inflammation and, as a result, produce systemic oxidative stress and genotoxicity (Yamamoto (2013)). This, in turn, can promote lymphoma-genesis (the growth and development of lymphoma) (First Smith Report (at [140])). As Professor Smith notes (First Smith Report (at [141])):
As stated above multiple studies show that GBFs and glyphosate can alter the gut microbiome. In 2013, it was reported that glyphosate altered the gut microbiomes of cattle and poultry (Kruger, Shehata et al. 2013, Shehata, Schrodl et al. 2013), leading Samsel and Seneff to speculate that glyphosate’s effects on the gut microbiome may be responsible for a plethora of diseases, including nonceliac gluten sensitivity and autism spectrum disorders (Samsel and Seneff 2013). These proposed associations remain controversial, but it is clear that glyphosate and GBFs can alter the gut microbiome.
956 During the concurrent evidence session, Professor Smith also relied upon Lehman (2023), which, it is said, demonstrated changes in gut microbiota and upregulation of pro-inflammatory cytokine IL-17A in mice exposed to low doses of glyphosate (T506.39–46; T634.35–635.29).
957 Fourthly, and relatedly, Mesnage (2021) used a combination of “shotgun-metagenomics” and metabolomics to investigate the gut microbiome of rats exposed to increasing doses of glyphosate and the GBF, MON 52276. The authors demonstrated that glyphosate treatment resulted in “higher levels of intermediates of the shikimate pathway in the ceca, suggesting inhibition of EPSPS in the cecum microbiome”. Professor Smith notes that this mechanism also leads to increases in shikimic acid in soil micro-organisms following application of GBFs. Serum metabolomics suggested that MON 52276 had a greater impact than glyphosate on the serum metabolome, indicating additional systemic toxic effects. Hence, the purported safety of glyphosate and/or GBFs, based on the fact that mammals including humans lack the shikimate pathway, therefore ignores the presence of this pathway in micro-organisms which play a central role in the well-being of many species from honey-bees to humans. The toxic effect of glyphosate on these beneficial micro-organisms in our gut and elsewhere could have “profound consequences including producing chronic inflammation and contributing to lymphomagenesis” (First Smith Report (at [142])).
958 Mr McNickle also relies upon Sidthilaw (2022) as evidence for the proposition that exposure to glyphosate and/or GBFs induce chronic inflammation. In that study, the authors concluded (at 5):
The comparison of urinary glyphosate levels, oxidative stress, inflammation, and lung function before and after applying glyphosate showed that there was a statistically significant increase in urinary glyphosate levels, oxidative stress and serum MDA (p < 0.001), while serum GHS levels showed a statistically significant (p < 0.001) decrease. There was a statistically significant increase in inflammation and CRP (p < 0.001), however lung function decreased statistically significantly (p < 0.001) (Fig. 1).
(Emphasis added)
959 In cross-examination, Professor Prince gave evidence (see above (at [945]–[946])) that CRP is a biomarker of inflammation as it is secreted by certain cells of the body due to inflammatory processes (T623.32–43). Professor Smith also gave evidence that Sidthilaw (2022) adds to the evidence that exposure to GBFs induces chronic inflammation (T625.26–30).
960 I do not consider the evidence supports the conclusion that glyphosate and/or GBFs exhibit KC6, for the following reasons.
961 First, Professor Smith gave evidence that “chronic inflammation associated with the development of cancer is a prolonged response to persistent infections or irritants that inflict cell death and tissue injury” (T1307.44) and is therefore different from: (1) “sub-chronic inflammation” (that is, inflammation of a shorter duration) (T1307.46–1308.1); and (2) acute or temporary inflammation in that “you would require repeated exposure to the irritant or the infectious agent in order to generate that chronic inflammation” (T1307.40–43). This is significant because Professor Smith accepted that Pandey (2019), Hamadoui (2008) and Kumar (2014) upon which he relied in his first report are not studies in chronic inflammation but studied sub-chronic inflammation (T1308.3–1309.10):
MR CRAIG: Now, when one comes to your evidence on chronic inflammation you deal with chronic inflammation at paragraph 136 of the primary report that you filed in this proceeding – if I can ask that that be brought up, please – and at paragraph 136 you rely on Pandey and Kumar as two of the studies relied upon for the purposes of demonstrating chronic inflammation. That’s correct, isn’t it?---Well, no, I mean, if you look at the next paragraph we’re talking on Pandey that it’s really about more acute or sub-chronic inflammation.
MR CRAIG: Okay. So it’s not a study in respect of chronic inflammation, is it?---It’s a study showing that there is acute or sub-chronic inflammation from exposure but not - - -
MR CRAIG: Answer my question: it’s not a study demonstrating chronic inflammation, is it?---It’s not long enough. It’s only 14 days. It couldn’t do.
MR CRAIG: Thank you. Now, at paragraph 137 you refer to a study entitled Hamdaoui, Naifar et al?---Yes.
MR CRAIG: And, again, that’s not a chronic inflammation study, is it?---Again, it’s a sub-chronic longer-term exposure.
MR CRAIG: Thank you. So, again, it’s not a study that demonstrates chronic inflammation, is it?---It demonstrates inflammation that’s for sure.
MR CRAIG: Answer my question, Professor Smith. It’s not a study that demonstrates chronic inflammation, is it?---Well, they’re sub-chronically exposed. So it’s a sub-chronic inflammation.
MR CRAIG: Thank you. So it’s not a prolonged exposure. It’s a exposure of a shorter duration?---It’s not a lifetime or lengthy exposure but it is a longer-term exposure.
…
MR CRAIG: Thank you. Now, if we go back to paragraph 136, you will see reference to a study Kumar and Khodoun?---Yes.
MR CRAIG: That’s not a study demonstrating chronic inflammation, is it?---I don’t recall the study exactly how long it was – there were exposed to.
…
MR CRAIG: [Kumar (2014)] ran for a shorter period of time than the Pandey study which you referred to earlier as not being a study demonstrating chronic inflammation?---True.
MR CRAIG: And so it is not a study demonstrating or evidencing chronic inflammation, is it?---No, it’s sub-chronic.
962 Professor Smith then gave evidence (T1309.12–18):
MR CRAIG: Thank you. Now, I want to suggest to you that nowhere in your primary report do you draw his Honour’s attention to the fact that each of Pandey, Hamdaoui and Kumar are sub-chronic rather – are not studies of chronic inflammation, are they?---They’re studies of inflammation. That’s all.
MR CRAIG: Thank you. But you rely on them to ask his Honour to conclude, or as demonstrative of the existence of KC6, being chronic inflammation, don’t you?---I do.
963 Secondly, in relation to the gut microbiome studies, Professor Smith conceded in cross-examination that those studies do not measure inflammation; nor demonstrate the existence of extensive inflammation, and mainly concern “glyphosate-induced dysbiosis of the microbiome”. Indeed, Kruger (2013), Aitbali (2018), Mao (2018) and Blot (2019) do not mention the word “inflammation” (T510.32–35). As Professor Smith said in cross-examination (T1310.17–37):
MR CRAIG: And I want to also suggest to you that those studies, upon proper consideration, do not demonstrate the existence of extensive inflammation?---I would agree with you that that was an overinterpretation in the report. I agree.
MR CRAIG: Thank you. And is sitting here today the first time it has occurred to you that it was a overinterpretation in your primary report?---Yes. That’s the first time I’ve done a direct comparison.
MR CRAIG: And so before coming to court today to give evidence, and after publishing a supplementary report on Rana itself, it has not occurred to you that you have overinterpreted those studies in opining that they cause excessive inflammation – sorry, extensive inflammation?---I think the more accurate statement is the one in Rana.
MR CRAIG: Now, is it your evidence to his Honour that those studies demonstrate the existence of actual inflammation?---They’re mainly about the glyphosate-induced dysbiosis of the microbiome.
MR CRAIG: Yes. And I want to suggest to you that those studies do not demonstrate the existence of actual inflammation, do they?---I don’t think they measure inflammation in those studies, no.
HIS HONOUR: Does that mean the answer is no the question? I will just have counsel repeat the question.
MR CRAIG: Yes.
MR CRAIG: I want to put to you, Professor Smith – and we can do this the short way or do this the long way – the studies that are referred to in paragraph 138 of your report do not identify the existence of actual inflammation, do they?---No, they look for dysbiosis of the microbiome.
964 Thirdly, in relation to Sidthilaw (2022), it is doubtful whether that study can assist with respect to chronic inflammation. Although the study demonstrates some correlation between glyphosate exposure and a particular biomarker CRP (which is described in that study as a “marker for inflammation”), the study only took samples from farmers for the 24 hours prior and 24 hours after exposure to glyphosate. Accordingly, it did not show any result in a period over which a “chronic” response could be measured and there is no “prolonged” or “repeated” exposure which is required for chronic inflammation according to Professor Smith’s evidence (see above (at [961]–[962])). Indeed, the authors concluded that “further studies to assess the long-term effects of glyphosate are warranted” (at 9).
965 For these reasons, I am not satisfied that the evidence supports the conclusion that glyphosate and/or GBFs induce chronic inflammation.
V KC8 (modulates receptor-mediated effects)
966 KC8 refers to effects that may be caused by chemical carcinogens which act directly on specific cell receptors or modulate synthesis, transport, distribution, biotransformation, and clearance of the natural ligand, such as a hormone. As Professor Smith explained (T510.43–7):
MR FINCH: … We move lastly in the terms of KCs I think to KC8, that is modulates receptor mediated effects; what does that mean?
PROFESSOR SMITH: Well, the hormones in your body which control much of your life, things like testosterone, oestrogen and other things, they act through receptors and so a chemical may modulate receptor effects by changing levels of hormones but it may also change it by changing the receptor itself, here is the expression, or the amount of protein on the cell surface or the type of receptor that is expressed. And so modulates, receptor, mediated effects, was meant to be a catch-all phrase for hormone changes and changes in the receptors that interact with hormones.
967 In his characteristically assertive way, Professor Smith opined that there exists “strong evidence” that glyphosate and/or GBFs impact the synthesis of estrogen in human cells and experimental animals and, in this manner, modulate receptor-mediated effects (First Smith Report (at [146]–[148])). In summary, Professor Smith cites several studies in his first report in support of the following propositions: (1) that glyphosate and/or GBFs interfere with the synthesis of estrogen, lowering circulating levels and potentially promoting the development of NHL (at [146]); (2) glyphosate and/or GBFs may act by disrupting the hypothalamic-pituitary axis by reducing adrenocorticotrophic hormone levels, protein kinase A activity, and steroidogenic acute regulatory protein mRNA, including phosphorylated StAR, a protein that carries out a key regulatory step in steroidogenesis (at [147]).
968 During the concurrent evidence session, however, it became clear that the evidence was lacking in several key respects.
969 First, Professor Smith conceded that in his written reports, none of the studies cited in support of his opinion that glyphosate and/or GBFs module receptor-mediated effects were conducted on humans (T1419.31–39):
MR CRAIG: Thank you. In your written expert reports in this proceeding, you did not cite any studies done in humans to conclude that there is strong evidence that glyphosate modulates receptor mediated effects; is that correct?
PROFESSOR SMITH: In my report?
MR CRAIG: In your written reports.
PROFESSOR SMITH: Correct.
970 Secondly, the evidence established that the IARC Working Group 112 (2015) did not share Professor Smith’s opinion that the evidence for glyphosate for KC8 was strong and instead said it was weak (T511.47). Professor Smith gave evidence that the study which changed his view as to KC8 since that time was Geier (2023) (T512.5–14) (recalling the controversies surrounding Dr Geier set out above (at [739]–[740])), but that he did not “particularly” rely upon that study in advancing his opinions before the Court (T1413.19–21).
971 Thirdly, the USEPA Memorandum “Weight of Evidence Conclusions on the Tier 1 Screening Assays for the List 1 Chemicals” (2015) (USEPA 2015 Memorandum) subjected 52 chemicals to a weight of evidence evaluation. The USEPA concluded that the evidence for glyphosate exhibiting KC8 was weak and that it was not necessary to undertake a mammalian or wildlife EDSP Tier 2 testing. It concluded (at 34):
Based on weight of evidence considerations, mammalian or wildlife EDSP Tier 2 testing is not recommended for glyphosate since there was no convincing evidence of potential interaction with the estrogen, androgen or thyroid pathways.
972 In his third report, Dr Juberg explained (at [14]–[16]) the reasons why the USEPA’s EDSP programme did not provide evidence that glyphosate modulates receptor-mediated effects:
14. I strongly disagree with the Rana et al (2023) [which study cited Geier (2023)] conclusion “a growing body of evidence indicates that glyphosate and its formulations are endocrine-disrupting chemicals…” Rana et al (2023) looked at but a fraction of the available information for glyphosate related to endocrine disruption, focusing primarily on estrogen synthesis, estrogen receptor activity, and endocrine ligand synthesis. Glyphosate has been thoroughly tested in the USEPA’s EDSP Testing Program[me] (USEPA, 2015) (EPA-HQ-OPP-2009-0361-0047) not only for estrogen-mediated interactions/effects (including aromatase activity and steroidogenesis), but also for androgenmediated effects, and thyroid activity. In reviewing Rana et al (2023), their evaluation of KC8 only included discussion on estrogen and aromatase and did not consider or discuss other important facets of the endocrine system including androgen, thyroid-, or steroidogenesis-related effects. The USEPA’s EDSP Program[me] took more than 10 years to develop and validate and their review for glyphosate (USEPA, 2015) was extensive and exhaustive as noted by their inclusion of the following:
• For evaluation of estrogen-related effects, the analysis incorporated 7 different endocrine assays and 20 different studies (other scientifically relevant information or OSRI)
• For evaluation of androgen-related effects, the analysis incorporated 5 different endocrine assays and 18 OSRI studies.
• For evaluation of thyroid-related effects, the analysis incorporated 3 different endocrine assays and 11 OSRI studies.
• The assays used in the evaluation of glyphosate’s endocrine activity potential included in vitro (i.e., cellular-based), in vivo (i.e., whole animal), and considered both mammalian and ecological species.
• In the USEPA’s evaluation of estrogen, androgen, and thyroid (EAT) effects from glyphosate exposure, there were multiple measurements and parameters including receptor binding, receptor activation, relevant organ weights, histopathology, systemic toxicity, among others.
15. In total, there were multiple lines of evidence that the EPA evaluated for EAT activity including some 15 parameters for EAT which cross multiple layers of biological organization and which in my opinion are superior to the limited review by Rana et al (2023), which only looked at estrogen and aromatase endpoints and which did not include review of any of the EDSP findings for glyphosate which are publicly available.
16. The USEPA review of numerous empirical studies that were conducted on glyphosate according to established and validated test methods for endocrine activity concluded that “Based on weight of evidence considerations, mammalian or wildlife EDSP Tier 2 testing is not recommended for glyphosate since there was no convincing evidence of potential interaction with the estrogen, androgen or thyroid pathways.”
973 Professor Smith gave evidence that he did not place weight on the results of the USEPA 2015 Memorandum (T1417.29–34). The reason given was that (T512.40–44):
PROFESSOR SMITH: … the EPA only look at influence directly onto the oestrogen receptor. So they have a very strict interpretation. And although it appears as though glyphosate in some studies can alter the expression of the oestrogen receptor, I don’t think that’s really a major component, and I think that they have missed the association with oestrogen itself in the body.
974 Professor Smith conceded, however, in forming those opinions, that he did not recall having regard to Table 2 of the USEPA 2015 Memorandum, which was subject of the following evidence (T1419.11–19):
MR CRAIG: Thank you. Let’s go to table 2, then, on the following page. The first row of the table states:
Lines of evidence indicating potential interaction with oestrogenic/anti-oestrogenic pathways for glyphosate.
Do you see that?
PROFESSOR SMITH: Yes.
MR CRAIG: And this table demonstrates that the EPA tested more than just the influence of glyphosate directly onto the oestrogen receptor, doesn’t it?
PROFESSOR SMITH: Well, they’re testing on the oestrogen receptor and its subsequent effects in the pathway.
MR CRAIG: Yes. So the answer to my question is “yes”, isn’t it?
PROFESSOR SMITH: Yes.
975 For these reasons, I am not satisfied that the evidence supports the conclusion that glyphosate and/or GBFs exhibit KC8.
VI Hypothesised biologically plausible mechanisms
976 It will be recalled that Mr McNickle contends that there are five identifiable biologically plausible mechanisms by which use of or exposure to glyphosate and/or GBFs (and thus Roundup Products) can cause NHL in humans (see above (at [762])). It is important here to distinguish evidence which supports the existence of a biologically plausible hypothesis or theory, and evidence which establishes a biologically plausible mechanism. As Professor Prince explained in relation to KC4 (T405.14–18) (to which I will return):
PROFESSOR PRINCE: It’s a biologically plausible theory or hypothesis. It’s not a biologically plausible mechanism because we haven’t proven the mechanism.
MR CLEMENTS: In other words, it’s plausible but you say not yet established by experiments; is that a fair - - -
PROFESSOR PRINCE: Yes.
977 Related to this notion is that the process of identifying whether a chemical substance possesses a biologically plausible mechanism of action does not axiomatically follow from a conclusion that the substance exhibits one or more of the KCs simpliciter. Indeed, it is a “data-rich determination” (see above (at [677])) and, as Sonich-Mullin (2001) noted (at 2):
… a supported mode of action would have evidence provided by robust mechanistic data to establish a biologically plausible explanation. Mechanism of action, in contrast, relates to sufficient understanding of the molecular basis to establish causality; it is at the other end of the continuum from little or no evidence of mode of action to scientific proof of mechanism of action.
(Emphasis added)
978 Bearing these matters in mind, I turn now to addressing each of the five hypothesised mechanisms of action advocated for by Mr McNickle.
979 The first hypothesised mechanism is that glyphosate and/or GBFs are genotoxic to human lymphocytes and capable of causing double-strand breaks of DNA in human lymphocytes, and accordingly, are capable of causing the kind of genetic mutations which can lead to the development of NHL in humans. Although I have found that the weight of evidence supports the conclusion that glyphosate and/or GBFs satisfy KC2, the next step of whether there exists reliable evidence that those substances are capable of inducing genotoxicity, let alone cancer or NHL, in the real world is a different question (see above (at [875]–[877])).
980 For the reasons set out below, I am not persuaded that Mr McNickle has established the first hypothesised mechanism.
981 First, shortly after the exchange between Professor Smith and the Assessor concerning the variability between the concentrations which produced positive results for genotoxicity in Mladinic (2009) referred to above (at [893]–[894]), Professor Smith gave the following evidence in response to a question from the Assessor (T1503.1–14):
ASSESSOR: … And then final question, which we touched on yesterday, was I explained how I’m obsessed by risk rather than hazard and why that’s the case. So does this huge variation in the concentration of glyphosate that has a positive result in these studies in vitro – does that tell us anything about risk in the real world?
PROFESSOR SMITH: … I don’t think so, no. I mean, I think the way that we do – the way that this works typically in the – in the environmental and occupational arena is that you first of all decide, is the chemical a carcinogen or probable carcinogen on the basis of epidemiological studies, studies in animals and all of these sort of cell studies and mechanistic studies, and a weight of the evidence evaluation as we explained. But after that – after you’ve decided it’s a carcinogen – of course, you could buy a bottle of a carcinogen and put it in your garage and never open it. And as long as it’s not volatile, unless somebody goes and drinks it or something, there’s no risk, really, right?
982 Put another way, as I explained earlier (at [875]–[877]), although it is possible to demonstrate that glyphosate and/or GBFs have a genotoxic effect on human blood cells under artificial in vitro cell culture conditions, this evidence says very little about the risk of genotoxicity to humans resulting from exposure to glyphosate and/or GBFs in the real world.
983 This is for two reasons: first, the majority of the in vitro studies (Wozniak (2018); Kwiatkowska (2017); Nagy (2021) and Alvarez-Moya (2023)) (Mladinic (2009) may also be included in this list (see Table 2; T1499.3–15)) used concentrations of glyphosate which exceeded the lowest intoxication threshold identified in evidence by Professor Smith (5 µM) by several orders of magnitude (in other words, at doses that no human would have any prospect of being exposed to in the real world) (see above (at [901])); and secondly, even with respect to the results in studies which did not exceed that threshold (Santovito (2018) and Mladinic (2009)), it was demonstrated that it is possible to detect a genotoxic signal at ever-lower concentrations of glyphosate by changing in vitro conditions and using different assays (see above (at [901])).
984 Secondly, and in any event, none of the studies the subject of the mechanistic evidence suggested that the genotoxic effect observed from glyphosate and/or GBFs is implicated in causing NHL in humans. A fortiori, it will be recalled at least three studies suggested that the effect of glyphosate is “transient” and capable of repair: (1) in relation to Paz-y-Mino (2011), Professor Smith accepted that the effects of exposure are “[t]ransient only in the sense that lymphocytes in your body turn over. So the damaged ones are removed and the regular ones, the new ones, appeared and basically that took away the damage that as observed in the first study” (T472.13); (2) in relation to Bolognesi (2009), the authors concluded that the data suggested that genotoxic damage associated with glyphosate spraying for control of illicit crops is “small and appears to be transient” (at 994–995); and (3), in relation to Roma (2023), Dr Juberg gave evidence that DNA repair was reported with reversibility at both 70 and 700 micrograms per litre after 24 hour exposure within four hours (T1943.16–19).
985 Thirdly, as noted earlier (at [912]), Professors Smith and Prince agreed that mutagenicity involves permanent mutations in the DNA and that genotoxic damage must cause permanent cell mutation to lead to cancer or NHL. None of the mechanistic studies, however, demonstrated that glyphosate and/or GBFs induced DNA damage that was permanent or could lead to mutations which would induce NHL in humans.
986 In short, although I am satisfied on balance that glyphosate and/or GBFs exhibit KC2, this conclusion must be tempered by the reality that genotoxicity has been demonstrated in what might be described as extreme in vitro conditions which, on the evidence before me, is not translatable to a form of carcinogenic risk in humans. Accordingly, for the reasons set out above, I do not consider there is robust mechanistic data to support a conclusion that there exists a biologically plausible mechanism by which glyphosate and/or GBFs are capable of causing the kind of genetic mutations which can lead to the development of NHL in humans.
987 The second hypothesised mechanism is that glyphosate and/or GBFs can cause an upregulation of the AID enzyme in humans that increases the amount of SHM, CSR and V(D)J recombination taking place in B-cells, and therefore increases the number of mutations occurring within the B-cells, including the kind of mutations that cause NHL.
988 This hypothesised mechanism is somewhat unique in that it does not, at least directly, relate to one or more of the KCs. For this reason, we have not encountered many of the scientific concepts relating to the upregulation of AID and, regrettably, it is necessary here, among other things, to wade manfully into the details of Wang (2019).
989 It will be recalled that Wang (2019) was a 72-week mechanistic study of a Vk*MYC mouse model for the purposes of assessing the development of MM in humans (see above (at [616])).
990 Mr McNickle contends that Wang (2019) is significant because it demonstrates, in an in vivo mice study, that an effect of the administration of glyphosate to two different types of mice was increased expression of AID. It is said that in humans, the activity of the AID enzyme plays a critical role in the genesis of NHL because it enables and regulates the processes of SHM and CSR in B-cells, which are both error prone and capable of causing the mutations that lead to NHL.
991 This necessitates some further explanation.
992 As noted in the JRA (at [104]), all cancers have a common problem: they all must have mutations in their genetic material to become cancerous. Many cancers have these mutations caused by external factors that mutate the DNA (for example ultraviolet light in the case of melanoma and cigarette smoke in the case of lung and bladder cancer). This can be described as an external mutagen (a foreign substance causing mutations).
993 The genetic processes of SHM and class-switching are well known mutagenic processes that strongly increase the risk for a B-cell to undergo malignant transformation. This is called spontaneous mutation. The key evidence that spontaneous mutations are crucial events in B-cell NHL-genesis are as follows (at JRA [105]):
(1) During B-cell development, SHM can increase the chance of spontaneous mutations in genes other than the genes involved in immunoglobulin (Ig) production. This off-target activity of SHM can cause point mutations in cancer-promoting genes (proto-oncogenes). SHM plays an important role in NHL-genesis and AID can mutate genes (in addition to Ig genes).
(2) Diffuse large B-cell lymphoma (DLBCL) accumulate AID-dependent somatic mutations in many other genes, including oncogenes such as MYC and PIM1.
(3) CSR also involves DNA breaks, and errors in its regulation can lead to chromosomal switch translocations, which are frequently detected in lymphoid malignancies. AID is the likely candidate to mediate these translocations, as AID is required for spontaneous MYC/IgH translocations in mice.
994 AID activity increases the mutation rate in Ig genes by a factor of approximately one million and contributes to genomic instability (at JRA [332]) and has been implicated in several other cancers, which are not B-cell malignancies, including hepatocellular carcinomas, gastric cancers and colorectal cancer (T627.8–34).
995 Wang (2019) examined the effect of acute and chronic exposure to glyphosate on the expression of AID in both wild type and Vk*MYC mice. The authors concluded (at 7), that “glyphosate upregulated AID in the spleen and bone marrow of both wild-type and Vk*MYC mice”.
996 Dr Flecknoe-Brown also gave evidence (at JRA [190]) that the study:
… showed that those laboratory animals [exposed] to glyphosate developed monoclonal gammopathy of undetermined significance (MGUS). On continued exposure to glyphosate, this progressed to multiple myeloma (MM), which is a blood cancer closely related to NHL. This is also highly relevant to the question of glyphosate causing NHL.
997 In a letter dated 28 August 2023, Maurice Blackburn informed Freehills that during the Conclave A concurrent evidence session, Mr McNickle would seek leave to adduce additional evidence from Dr Flecknoe-Brown in relation to Wang (2019) in substance as follows:
(1) MM in mice is sufficiently similar to NHL in humans to make the Wang (2019) relevant to humans;
(2) Wang (2019) shows that exposure to glyphosate causes a substantial increase in the amount of AID which can lead to NHL. That is because a substantial increase in the amount of AID will result in a substantial increase in the amount of SHM and thus a substantial increase in the amount of mutations that can cause NHL. The B-cells’ governing mechanisms, which in various ways can repair or render harmless potentially dangerous mutations which occur during SHM, are overwhelmed by the substantial surge in SHM and mutations, and thus become much less effective at repairing or rendering harmless those mutations.
998 The parties agreed during the concurrent evidence session that (2) had been led and properly reflected Dr Flecknoe-Brown’s opinion (T167.27–36). In addition to his evidence above, Dr Flecknoe-Brown gave the following evidence:
(1) that he examined Wang (2019) in detail and could not identify any significant methodological flaws (T108.8–13);
(2) there was an increase in AID expression in both the wild type mice and Vk*MYC mice who had glyphosate in their drinking water (T108.17–21; T387.45–388.25);
(3) Wang (2019) is significant because AID is “the key player in somatic hypermutation and class-switch recombination in lymphocytes and, therefore, considered the most important cause of mutations in lymphocyte and lymphoma production” (T108.33–36);
(4) overexpression of AID, particularly in combination with germinal centre hyperplasia, will predictably lead to overwhelming of the DNA damage response mechanism and increase the risk of lymphoma formation (T108.37–39);
(5) Wang (2019) is relevant to NHL in humans because of the key role that AID plays in the processes of SHM and CSR (which are known to lead to NHL in humans) and, for probably the first time, upregulation of AID was demonstrated in response to feeding mice glyphosate (T110.35–40; T278.42–279.3);
(6) the AID upregulation found was dose dependent (in respect of acute glyphosate exposure) (T271.30–272.9; T281.23–33); and
(7) that he reposed confidence in Wang (2019) (T288.39–43).
999 I have already referred to some limitations associated with Wang (2019) in the animal studies stream (at [619]–[621]) and noted then that it is not an overstatement to say the evidence established some not insignificant methodological problems with the study. In my view, those problems, among other things, render the study’s findings of little assistance in support of the second hypothesised mechanism and, accordingly, Wang (2019) is not capable of constituting the data-rich or robust data that proves that glyphosate and/or GBFs cause an upregulation of AID in humans that increases the amount of SHM, CSR and V(D)J recombination taking place in B-cells and increases the number of mutations occurring within the B-cells including the kind of mutations that cause NHL, for the following reasons.
1000 First, Wang (2019) provides little assistance as to the question of whether glyphosate and/or GBFs cause NHL in humans because MM and NHL are different diseases with different cells of origin. In response to Dr Flecknoe-Brown’s evidence (at JRA [190]) “a study on mice conducted by [Wang (2019)] showed that those laboratory animals to glyphosate developed monoclonal gammopathy of undetermined significance (MGUS). On continued exposure to glyphosate, this progressed to [MM], which is a blood cancer closely related to NHL”, Professor Prince gave the following evidence:
PROFESSOR PRINCE: … I think the first statement above it is incorrect in that it – glyphosate developed monoclonal – animals to glyphosate developed monoclonal gammopathy of undetermined significance. They actually developed monoclonal gammopathy not undetermined significance. That’s a human term. The next line:
On continued exposure to glyphosate this progressed to multiple myeloma.
Well, no, I would say, yes, they were exposed to glyphosate by definition as were the control – they were exposed to glyphosate. The animals who were not exposed to glyphosate were in the control group and then the next sentence which is a blood cancer closely related to non-Hodgkin lymphoma. They’re not closely related at all.
MR FINCH: Why not?
PROFESSOR PRINCE: So I think the first thing to say is that, if we look at the big – at the top level of the answer to that is that the WHO classify blood disorders into a series of diseases and they do that for a reason. I won’t go through it but it’s very detailed. But they define it as a different disease. Number two, they have a very different cell of origin.
1001 This latter point is significant, and it is necessary to pause here to say something about Professor Prince’s evidence and the difference between lymphoma-genesis and myeloma-genesis with reference to a diagram from Nogai (2011) (at JRA [289]):
1002 The term “cell of origin” refers to the phase or ontogeny of a B-cell as it goes through its development (from left to right in the diagram above) (T178.9–12) (for example, the cell of origin for FL, and so on, is the pro B-cell). As the cell matures (following antigen contact), it enters the germinal centre of the lymph node where the cell is exposed to several reactions driven by AID (T178.25–31). The events during the germinal centre reaction include AID-mediated SHM and CSR, which are critical events for lymphoma evolution (at JRA [289(c)]; T178.39–46). If, for example, a malignancy occurs at that point in the centrocyte, it can cause FL. Importantly, the centrocytes (that is, the earliest B-cell) can only function within the germinal centre (T179.1–9). In turn, the cells undergo further growth becoming “centroblasts” as they move out of the germinal centre. From that point, the cells can be activated, and the “plasma-blast” is the next cell of origin (which induces what is described as “ABC diffuse large B-cell lymphoma” (ABC DLBCL) (T179.1–9)).
1003 The two major types of large-cell lymphoma are: (1) germinal centre B-cell lymphoma (GCB DLBCL); and (2) ABC DLBCL (T179.11–12). These cells then transform into plasma cells and most of those cells lose their B-cell receptor (T179.13–16). Importantly, given they are no longer in the germinal centre, those cells have very low levels of AID and are no longer undergoing SHM – that is, SHM has occurred much earlier in the developmental ontogeny of the cell (T179.16–19).
1004 This is significant in demonstrating the difference between lymphoma-genesis and myeloma-genesis for two reasons: (1) the plasma-cell (that is, the cell of origin for MM) is no longer undergoing SHM and only produces Ig (lymphocytes are incapable of producing Ig) (T179.29–35), which is relevant because lymphocytes do not have monoclonal clones (T179.30) (cf. Wang (2019); at JRA [190]); and (2) secondary mutations that can occur to develop into lymphoma are different in myeloma because: (a) myeloma is driven by Ig and all forms of myeloma have some form of Ig abnormality (T179.37–40); (b) in contrast to lymphoma, all forms of myeloma have “MYC activation” (T179.40–41); and (c) myeloma develop late line mutations which are uncommon in lymphoma (T179.42–43). In the light of these factors, Professor Prince’s conclusion is that “they are totally different diseases” (T179.42–43).
1005 Dr Flecknoe-Brown agreed that lymphoma and myeloma are “different entities”, “behave quite differently” and the that the mutational signatures of the diseases “look different”, but that the diseases are related insofar as both cells of origin have gone through the germinal centre (T180.1–13). In response, Professor Prince noted in the context of Wang (2019) (T180.17–20):
PROFESSOR PRINCE … I think it’s important, when we’re talking about the mouse model, is that AID, the function of AID, is in the germinal centre and so it can’t function outside of the germinal centre and the – but it’s critical for the development of a lymphocyte, plasma cells don’t require AID.
1006 Secondly, Wang (2019) used the Vk*MYC mouse model which was specifically designed to be susceptible to MM and increase the chance of developing a MYC-related tumour (T173.38–45). In short, the Vk*MYC model produces large amounts of MYC protein which has a substantial effect on the pathways of the genome and which influence the production of AID. As Professor Prince explained in relation to Wang (2019) (T174.10–13):
PROFESSOR PRINCE: … I don’t think the glyphosate is causing the increase in AID. I think what’s causing the increase in AID is the large amounts of MYC. Complicating the whole issue is that the study is flawed in numerous ways which disallow us to draw really interpretations of some of the patterns that we see in the gels.
1007 Consistently with the above, Dr Flecknoe-Brown observed that the authors had reported an upregulation of AID in the untreated Vk*MYC mice and gave the following evidence (T174.35–175.13):
MR FINCH: You also observed in the study though that untreated Vk*MYC mice had increased levels of AID too, didn’t they?
PROFESSOR FLECKNOE-BROWN: Yes. Yes.
MR FINCH: Which lends some weight to Professor Prince’s view?
PROFESSOR FLECKNOE-BROWN: Well, except for the significant difference in the quantity.
MR FINCH: Yes.
PROFESSOR FLECKNOE-BROWN: Almost double.
MR FINCH: Nevertheless, the process was observed that mice – Vk*MYC mice, untreated by glyphosate, were observed to have increased levels of AID?
PROFESSOR FLECKNOE-BROWN: Yes. But they had twice as much with glyphosate.
MR FINCH: Indeed.
PROFESSOR FLECKNOE-BROWN: Yes.
MR FINCH: But does that not lend some support to the mechanisms that Professor Prince is talking about?
PROFESSOR FLECKNOE-BROWN: Yes. Yes.
1008 Thirdly, and relatedly, there were inconsistencies between the results obtained for Vk*MYC and wild type mice in the study. It is convenient here to extract Figure 5 from Wang (2019):
1009 The density of the “bands” in the above diagram are said to indicate a dose-response to glyphosate compared to the controls. As seen above in Figure 5a, the densest band in the AID analysis of spleen for chronic glyphosate exposure is the wild type mouse, whereas according to the authors’ conclusions (and for the reasons explained above (at [1006]–[1007])), it ought to have been in the exposed Vk*MYC mouse which is designed to drive AID expression from an excess of MYC. Despite its length, it is worth setting out here Dr Flecknoe-Brown’s evidence in response to questions from the Assessor broadly on this topic as follows (T277.4–278.40):
ASSESSOR: … Okay. So let’s go to figure A and let’s look at spleen expression of AID in wild-type without glyphosate. What do we see there?
PROFESSOR FLECKNOE-BROWN: It’s there, yes.
ASSESSOR: It’s there.
PROFESSOR FLECKNOE-BROWN: Yes.
ASSESSOR: So these are individual mice, aren’t they, and it says in the legend that hoary old phrase of one represents mouse shown for each observation, one representative mouse per treatment group is shown.
PROFESSOR FLECKNOE-BROWN: Sorry.
ASSESSOR: So my question is with regard to this position about whether AID is expressed in the spleen of wild-type mouse that don’t see glyphosate, which of those two observations is the reliable one?
PROFESSOR FLECKNOE-BROWN: I’m sorry, I’ve re-read the legend.
ASSESSOR: Yes.
PROFESSOR FLECKNOE-BROWN: Figure B is with that dioxin compound that we were not - - -
ASSESSOR: Yes, that’s the positive control, but the wild-type hasn’t seen anything.
PROFESSOR FLECKNOE-BROWN: Yes.
ASSESSOR: And we agreed that the spleen, without glyphosate, showed no expression of AID by the - - -
PROFESSOR FLECKNOE-BROWN: Yes.
ASSESSOR: - - - technique, whereas in the wild-type, spleen in the group of animals that were seeing glyphosate, these were the controls that didn’t, we see that AID is expressed.
PROFESSOR FLECKNOE-BROWN: Yes.
ASSESSOR: So these two mice were maintained under identical conditions.
PROFESSOR FLECKNOE-BROWN: Yes.
ASSESSOR: Which of the two mice is giving the reliable information about AID expression in those circumstances?
PROFESSOR FLECKNOE-BROWN: Well, given that – given that all the wild-type mice survived, it would be better to see the rest of the – of the – of the western blot studies, yes.
ASSESSOR: So all the comparisons in this figure are made comparing one mouse with another. So I’ve just seen a big discrepancy in two mice that should be the same. So how does that affect your confidence about the reliability of comparing bands, one mouse to another?
PROFESSOR FLECKNOE-BROWN: Well, I – I haven’t examined the supplementary material. There may be some information – some other - - -
ASSESSOR: Doesn’t seem to be.
PROFESSOR FLECKNOE-BROWN: Doesn’t seem to be.
ASSESSOR: Yes.
PROFESSOR FLECKNOE-BROWN: All I can say is there is obviously a variability in the amount of AID expressed in the untreated wild-type mice.
ASSESSOR: So I agree with you that western blotting is not a reliable technique for quantitating protein. If you had to do that what techniques might be use check and obviously you would do that in more than one mouse.
PROFESSOR FLECKNOE-BROWN: Yes. I think nephelometry would be the way I’m going.
ASSESSOR: Okay.
PROFESSOR FLECKNOE-BROWN: That’s a reliable method of quantitative - - -
ASSESSOR: The authors didn’t use any of these other reliable methods?
PROFESSOR FLECKNOE-BROWN: Didn’t see it, no.
1010 Fourthly, and relatedly, notwithstanding his evidence that he could not identify any significant methodological flaws in Wang (2019), the following evidence was adduced from Dr Flecknoe-Brown during the concurrent session:
(1) Dr Flecknoe-Brown did not know how much glyphosate each mouse in the study consumed (T258.25–33; T286.12–14);
(2) the study does not record how much water was consumed by each of the four groups of mice (T286.6–10);
(3) the study is silent on whether the mice were housed together or separately (T258.44–45);
(4) for each of the groups presented in Figure 5a, only one mouse out of 10 per group was presented (T269.5–11; T284.43–285.19);
(5) only three of the Vk*MYC mice treated with glyphosate survived to 72 weeks (T273.30–34);
(6) there is no recorded measurement of the actual glyphosate levels in any of the surviving mice (T286.1–4);
(7) the authors do not explain the basis upon which each of the mice chosen for presentation in Figure 5a or 5c were considered to be representative (T285.30–37);
(8) the results of the Western blotting analysis in Figure 5a have been presented without quantitating the data – Dr Flecknoe-Brown agreed that it appears that “[t]heir conclusions are based on eyeballing the gel” (T276.25–27);
(9) significantly, Professor Prince and Dr Flecknoe-Brown agreed that the study did not constitute reliable evidence that glyphosate upregulates AID (T281.1–16):
ASSESSOR: … Dr Flecknoe-Brown first: are you [confident] that this data demonstrates that glyphosate upregulates AID? I’m looking at reliability here. Is this reliable evidence that glyphosate upregulates AID, knowing what you know about western blotting and this particular dataset.
PROFESSOR FLECKNOE-BROWN: Yes. To me it looks more likely than not but I’m not confident.
ASSESSOR: Professor Prince?
PROFESSOR PRINCE: No, I can’t see any evidence that convinces me. You might – I might be pre-empting your second question but it relates to functional AID as well. But I can see these results, the controls, the changes, there’s so many inconsistencies and lacking data that – and, in fact, all I can see is that it is an interesting model that’s producing – that shows that MYC produces a large amount of AID.
(10) for the only two mice in Figure 5a, who were exposed to glyphosate, the authors do not record much glyphosate was absorbed or the AID levels for those mice were not recorded (T288.1–9) – a fortiori, it is not known what size or gender the mice were, which would influence how much water and consequently how much glyphosate each would consume (T288.45–289.18); and
(11) Dr Flecknoe-Brown gave evidence that he did not analyse the epidemiological studies cited by the study in support of its hypothesis that there was a link between exposure to glyphosate and risk of MM (T290.20–41).
1011 Fifthly, Dr Juberg gave evidence in his second report (at [14]–[15]) which, not without some overlap and although it was not required to conform to regulatory guidelines (see above (at [621])), highlights a number of limitations of Wang (2019), as follows: (1) that no information was provided as to test material purity; (2) a single dose was used, instead of three, which precludes the ability to assess dose-response relationship for reported effects; (3) it is not known where the concentration used by stands relative to the MTD (the dose used in Wang (2019) was approximately 15-fold the ADI (at 10)) (see also T73.20–12); (4) daily clinical observations were not noted to have occurred and thus there is no information on the general health of the mice used over the course of the study; (5) as noted above (at [1010]): (a) no information on body weight gain or decrements were reported and thus it is not known whether the glyphosate dose administered was associated with palatability or overt toxicity; (b) information on the survival rate and animals which had to be euthanised for humane reasons among both treated and control animals was inadequately provided; and (c) the inclusion of fewer animals (10 per group) precludes a proper and robust statistical analysis of the study data, and which has a major implication on determination if the presence of tumours or other findings are treatment-related; and (6) no historical control data on any tumour type or finding was reported in the study.
1012 Sixthly, Professor Smith gave evidence that Wang (2019) is a “significant paper on the mechanism of NHL production by glyphosate because Figure 5 in [Wang (2019)] paper shows that both dioxin (positive control) and glyphosate enhance the expression of AID, suggesting that glyphosate causes lymphoma by the same mechanism as dioxin” (First Smith Report (at [123])). In the concurrent evidence session, however, Professor Smith caveated that conclusion by highlighting the authors’ conclusion:
MR FINCH: And in the second sentence there you say:
This is a significant paper on the mechanism of NHL production by glyphosate exposure.
And you refer to figure 5 in that paper.
PROFESSOR SMITH: Yes.
MR FINCH: And that’s where you’ve taken the suggestion that you have in this paragraph from?
PROFESSOR SMITH: Yes.
MR FINCH: And you refer to it again, I suggest, quite deliberately as what it suggests?
PROFESSOR SMITH: Correct.
MR FINCH: Right. And again at a high level of generality you would agree that the terms in which that paper are expressed are just that. It’s a suggestion but not a conclusion.
PROFESSOR SMITH: Well, it’s my opinion that that at a probable mechanism and of importance.
MR FINCH: Yes.
PROFESSOR SMITH: It’s not a firm scientific conclusion because the studies in mice, there isn’t – there aren’t studies in humans of AID and glyphosate.
1013 Professor Smith later gave evidence consistent with Dr Juberg’s observations above (at [1011]) that the dose used in Wang (2019) was excessive compared to any human dose:
MR CRAIG: … Just before the conclusion of [Wang (2019)], the study authors observed that they were cognisant that an individual would unlikely consume such an excessive dose of glyphosate. Do you see that?
PROFESSOR SMITH: Yes, that’s typical in experimental and toxicological studies where you use a high dose to see an effect.
MR CRAIG: Thank you. So my question for you was that the dose used in Wang was an excessive dose of glyphosate, wasn’t it?
PROFESSOR SMITH: Excessive compared to a human dose, yes.
1014 Seventhly, even in the event Wang (2019) constitutes evidence that exposure to glyphosate causes an upregulation of AID, I am not satisfied that Dr Flecknoe-Brown’s hypothesis with regards to the process of mutated EZH2 genes is persuasive, having regard to the whole of the evidence.
1015 Before going further, it is necessary here to provide some further background.
1016 Dr Flecknoe-Brown hypothesises that a “biologically plausible mechanism whereby the mutagenic substances in GBFs can lead to the development of NHL is by mutation of one of the genes involved in the DNA damage response” – that is, a mutation of the EZH2 gene (at JRA [270]). The function of the EZH2 gene without mutation is to control the proliferation of cells in the germinal centre – a mutation causes “excessive proliferation of the germinal centre, with malignant transformation a frequent sequel” and a “mutant EZH2 also induces differentiation blockade in DLBCL cells, allowing them to proliferate in their malignant form” (at JRA [270]). The genetic aberrations which comprise the essential characteristics of double-hit lymphoma are the rearrangements of the MYC and either one of the BCL2 or BCL6 genes. Dr Flecknoe-Brown does not contend that exposure to glyphosate and/or GBFs causes these genetic aberrations directly, but rather that these rearrangements “are a result of the vastly increased amount of somatic hypermutation occurring in these lymphoproliferative tissues” and the “lymphoproliferation is … a direct result of the presence of the Tyr641 mutated EZH2 enzymes” (at JRA [282]).
1017 In cross-examination, however, Dr Flecknoe-Brown conceded that there is no laboratory evidence that he is aware of which demonstrates glyphosate and/or GBFs can induce the mutation of the EZH2 gene which was observed in the Mr McNickle’s genetic sequencing. As Dr Flecknoe-Brown and Professor Prince said (T200.45–201.12):
MR FINCH: … I think you agree, Dr Flecknoe-Brown, that you don’t cite any laboratory evidence where EZH2 mutations have been induced in experiments with glyphosate or GBFs?
PROFESSOR FLECKNOE-BROWN: No, I wasn’t aware of any.
MR FINCH: Sure. And, Professor Prince, are you aware of any such studies?
PROFESSOR PRINCE: No, I’m not.
MR FINCH: Would there be any difficulty in doing such a study from either of you?
PROFESSOR PRINCE: No, there wouldn’t be. I mean, it’s – it would be subject to the different – looking at a different number of lymphoma models in mice and then the actual technique of assessing for EZH2 mutations is very simple.
1018 Further, some time was spent on Table S3 of Lohr (2012) (see above (at [711])) and whether that study constituted reliable evidence in favour of the EZH2 gene being a target for AID-induced mutation. It is unnecessary to rehearse it here, save to note that the evidence established that Table S3 of Lohr (2012) can be interpreted as showing that associations between AID and mutations in potential targets such as MyD88 and EZH2 are not statistically significantly different in frequency (and therefore most likely arose by chance), whereas the more frequent mutations in recognised AID targets such as BCL-2 and PIM1 are statistically significant. As Professor Prince explained in response to questions from the Assessor (T429.41–431.1):
ASSESSOR: And then the other question was, in the discussion of table S3 in the Lohr paper, you wanted to tell us what those numbers about enrichments meant and that kind of got lost in the discussion. So could you just explain your analysis of table S3?
PROFESSOR PRINCE: Thank you. So the issue about making these sorts of analyses is more than just looking at a few numbers. It has got to be statistically proven. So one of the other papers which I presented, which was to make that point, was the Khodabakhshi paper because it talks about the stringent analyses that are required to draw statistical conclusions as to what is the chance of that mutation in that – and that motif being caused by an SHM.
So the conclusions that are – that are drawn in the Lohr paper are very carefully – and I would say – appropriately worded where they say that they have found a number of – a number of areas in genes where the AID target had been – the AID target is being detected. However, it’s a statistical issue because those can be mutated. There are mutations going on in our body all the time and there are amino acids being substituted and they can occur in those motifs. It doesn’t just – AID is not the only thing that will cause changes in those for DNA. Statistically, there’s masses of that motif throughout your genome.
So the second paper, which is the Khodabakhshi paper, spends a whole column in the results in the method section and the results and discussions as to how they statistically show whether that genes are targeted by AID. So it’s no surprise that EZH2 is in the table. In fact, when I went to that table I got some papers which look at known – what are called known classic and non-classic targets for AID, which are extremely well,-known and they’re in that table listed between 2 to 5 genes. Every gene is there. I mean, I can’t say that. I haven’t looked at every gene. But there is a large number of genes in the genome that are in that table. It’s a matter of how many.
So that’s why I was making the point about the PIM1 where you could see the list is so long and BCL-2 which was the point of that table. The issue of the – of the – the top genes that are being named, there’s nothing – that has got nothing to do with the EZH2. That has got nothing to do with AID preference. It’s just newly discovered genes are important in DLBCL. So the – that was what I wanted to say about table 3.
…
ASSESSOR: So the reason that Dr Flecknoe-Brown sees these associations in your mind is that that’s by chance, it’s not a statistically significant association. The link between EZH2 and various motifs that AID recognises has arisen by chance?
PROFESSOR PRINCE: Yes. And it has not been proven.
1019 Eighthly, largely for the reasons identified above (at [695]–[696]), I am not satisfied that Dr Flecknoe-Brown possesses the requisite expertise to conduct a thorough analysis into the second hypothesised mechanism or hypothesise that glyphosate and/or GBFs can induce the mutation of the EZH2 gene in the manner alleged. I have no doubt that Dr Flecknoe-Brown is a highly experienced haematologist and clinician, but without any intended disrespect, I do not consider that his expertise is of real assistance in identifying the extent to which a particular mechanistic toxicology study supports a finding that there is a biologically plausible mechanism by which glyphosate and/or GBFs can cause NHL. As Dr Flecknoe-Brown conceded in cross-examination (T114.40–43):
MR FINCH: None of it [Dr Flecknoe-Brown’s research] is research into the causes of NHL?---That’s correct.
MR FINCH: Or to the relationship between glyphosate or glyphosate-based formulations to cancers?---Correct.
1020 For these reasons, I do not consider that Wang (2019) provides any persuasive evidence as to the existence of the second hypothesised mechanism.
1021 Before concluding, it is necessary to say something briefly about this study.
1022 Mr McNickle relied on Suárez-Larois (2017) in support of his contention that glyphosate causes an upregulation of AID for the reasons set out by Professor Smith in his first report (at [125]), as follows:
The production of DNA strand breaks by AID in CSR engage the non-homologous end-joining repair pathway. In a supporting finding, Suárez-Larios and colleagues exposed whole blood taken from healthy young men to glyphosate and found a statistically significant increase in double DNA strand breaks and phosphorylated Ku80, a critical protein downstream of AID required for the repair of the ends of CSR DNA strand breaks ([Suárez-Larios], Salazar-Martinez et al. 2017). This study indicates that glyphosate may alter DNA repair.
1023 I do not consider that Suárez-Larois (2017) constitutes strong evidence in favour of the existence of the second hypothesised mechanism. In cross-examination, Professor Smith conceded that the authors expressed their conclusion in the following terms (T458.42–459.7):
MR FINCH: … Can I suggest to you a couple of things, that is, in fact, the strength with which they express their conclusions, that it’s a “may”?
PROFESSOR SMITH: Correctly.
MR FINCH: And, secondly, you would agree that, in the event and to the extent that the study was demonstrated, have limitations, then the conclusions must also be modified to that extent?
PROFESSOR SMITH: Correct.
1024 For the foregoing reasons, I do not consider that the state of the evidence is such that it points persuasively to the conclusion that glyphosate and/or GBFs can cause an upregulation of the AID enzyme in humans that increases the amount of SHM, CSR and V(D)J recombination taking place in B-cells, and therefore increases the number of mutations occurring within the B-cells, including the kind of mutations that cause NHL.
1025 The third hypothesised mechanism is that glyphosate and/or GBFs cause oxidative stress in humans which leads to damage to DNA, including double-strand breaks of DNA, and cause haematological cancers such as NHL.
1026 I have already found above (at [949]) that glyphosate and/or GBFs can induce oxidative stress, but I am not satisfied that it is a biologically plausible mechanism which leads to the development of NHL, for the following reasons.
1027 First, Professor Smith gave evidence as follows (T500.1–3):
MR FINCH: Yes. Now, is it fair to say that, up until you saw the Chang 2023 study, you had expressed the view, had you not, that oxidative stress was probably the weakest of the 10 KCs?
PROFESSOR SMITH: Well, I didn’t mean that for glyphosate. That was expressed as in general because as we’ve already talked about, there are several things that will produce oxidative stress that are not necessarily carcinogenic.
1028 Secondly, as noted earlier, although Professor Prince agreed that glyphosate and/or GBFs “tick the box” of KC5, he gave evidence that it falls short of acting as a biologically plausible mechanism because there is little to indicate that the data supports the conclusion that the non-specific nature of oxidative stress can cause NHL (T500.38–43; T501.19–25). As Professor Smith explained shortly after the exchange above (T500.22–36):
PROFESSOR PRINCE: At a high level I think it’s important to recognise that it’s not one of the hallmarks for cancer per se. Oxidative stress. So somehow it has got to get to one of the hallmarks of cancer. And so that’s part of the dilemma that I have particularly in the context of lymphoma. So it’s the question: how does it get there? And of the – because it can affect a variety of pathways, lipids, protein, inflammatory pathway and direct DNA binding, the formation of hydroxy radicals on the surface. And the issue has been is that that removal becomes the measurement, the so-called eight hydroxy deoxyguanosine, and that then becomes a suggestion that that’s demonstrating DNA damage, which it is, but that DNA damage can then go the next step and has implications and an automatic assumption that that’s a genotoxic effect leading to lymphoma. And there’s a number of reviews that have been reported in that – in the report – in the documents. And really none of them demonstrate a clear association with reactive oxygen species causing lymphoma in humans. So I think the – it really steps from how does it fit from the hallmarks of cancer all the way through to lymphoma.
1029 The fourth hypothesised mechanism is that glyphosate and/or GBFs induce epigenetic alterations in human lymphocytes, including alterations of DNA methylation and hence the expression of key cancer genes, including NHL.
1030 As noted earlier, I have found that glyphosate and/or GBFs exhibit KC4, but that there is less evidence as to whether those changes are “significant” or linked to the development of cancer. Accordingly, I am not satisfied that there is persuasive evidence in favour of the fourth hypothesised mechanism, for the following reasons.
1031 First, as noted earlier (at [929]), the authors of the relevant studies highlighted several limitations which lessen their weight in favour of a conclusion that glyphosate and/or GBFs induce epigenetic alterations which could be functionally implicated in the development of NHL. In relation to Rossetti (2021), for example, the authors concluded (at 1) that “[o]verall, more studies are needed to identify epigenetic targets, to define how they are dysregulated in human disease and their functional role, and to determine the critical windows of vulnerability by herbicide exposures” (emphasis added). In relation to Lucia (2022), Professor Smith accepted from the outset that the authors highlighted several limitations (T491.10–11), as follows: (1) the study consisted of post-menopausal women aged between 45 and 66, therefore the results “may not be generalisable to other populations or those residing outside California” (T492.10–24); (2) that a further limitation of the study was “the lack of gene expression data” (T493.5–19) (that is, as Professor Prince explained, it is not clear whether the changes in methylation affect the gene expression or are otherwise inert (T493.26–32); (3) that no inference can be drawn regarding the “temporal relationship between DNA methylation differences and glyphosate exposure” (T494.21–495.2); (4) that although the authors did not observe a relationship between recent herbicide use and glyphosate and AMPA concentrations, “those data were missing for 37 per cent of participants which should be considered another limitation of the study” (T496.1–31); (5) importantly, that the authors’ conclusion was tentative (“that informs the hypothesis that glyphosate and/or AMPA exposure might elevate the risk for disease” (emphasis added)); and (6) that “[f]urther studies are warranted to replicate our results, determine the functional impact of glyphosate- and AMPA-associated differential DNA methylation, and further explore whether DNA methylation could serve as a biomarker of glyphosate exposure” (emphasis added) (T496.43–497.15).
1032 Secondly, Professor Prince gave evidence that any conclusion that glyphosate and/or GBFs are capable of causing epigenetic alterations does not axiomatically lead to the conclusion that glyphosate and/or GBFs cause NHL in humans. As he explained, quoting Professor Smith’s 2020 article (at JRB [144]–[145]):
[144] KC 4- Epigenetic alterations: The inability of the KCs to evaluate epigenetics is clearly stated in [Smith (2020)]:
The difficulty with interpreting the KC ‘induces epigenetic modifications’ is that the relevance of a particular epigenetic change for carcinogenesis by a specific agent may not be clear and causality is hard to establish. More validation is clearly needed to fully understand which epigenetic endpoints are most indicative of carcinogenic risk.
[145] Indeed, this issue is definitely true for NHL – while epigenetic changes are extremely common in NHL, it is generally accepted by cancer biologists that epigenetic changes are not the earliest events in NHL development. Put another way, epigenetic changes (particularly non-mutational epigenetic changes) do not cause the NHL initiation but are rather part of the proliferation and pro-survival process that develops later during the growth of the NHL.
(Emphasis added)
1033 In relation to this latter point, as Professor Prince explained in his first report (at [197]), “[i]n summary, although epigenetic changes are commonly observed in B-cell NHLs, they are generally considered to be late events that contribute to, but are not proven drivers of, the initiation of [NHL-genesis]” (emphasis added). He also noted (at [200]) with respect to studies involving glyphosate and/or GBFs that “epigenetic changes have been observed in some in vitro lymphocyte studies (where lymphocytes have been exposed to glyphosate-based herbicides)” but that “these in vitro changes are inconsistent and non-specific and there is no evidence that they induce lymphoma-genesis. These epigenetic changes are not dissimilar to changes that we observe in aging cells” (emphasis added).
1034 In cross-examination, Professor Prince gave further evidence as follows (T488.5–25):
MR FINCH: And I will come back to that article in a moment. So we will spend a little time on that, not much. Professor Prince, what’s your view about the significance, if any, of epigenetic alteration?
PROFESSOR PRINCE: So my opinion is, having looked at the data pretty carefully, the issue is about the level of evidence of the epigenetic – true epigenetic influence. In other words, what can – what we see – and I agree with the studies – is that we see hypermethylation in some of the promoters and we see hypomethylation across the genome and we know that that is associated with lymphoma. The key, though, is that there’s no studies that have shown a clear association between those and the expression of genes.
So one of the things that we clearly show – have to show in the laboratory is that those genes that are being methylated or hypomethylated actually somehow changes expression. Now, there is one paper, which is a later Wozniak paper, that comments that there is expression changes but, in fact, when you look at that paper it’s not correlated with the methylation status. So something is changing expression but the methylation isn’t. And so for me to be convinced that the glyphosate is having an impact on the pathogenesis of lymphoma, I need to see the hyper or hypomethylation on the specific gene and then I need to see a change in the gene expression and then preferably the protein production.
All of those things are possible. In fact, there’s a lovely paper showing exactly that with the use of antibiotics. So just because you can see these changes it doesn’t, last of all, necessarily mean that it leads to lymphoma.
1035 Professor Prince conceded that the “disruption of the proper functioning of epigenetic processes can cause NHL” (T403.37–46), but qualified that statement by explaining (T405.4–20):
MR CLEMENTS: … I suggest to you that what follows from that is if exposure to glyphosate disrupts the proper functioning of epigenetic processes in B-cells, that is a biologically plausible mechanism by which exposure to glyphosate can cause NHL, isn’t it?
PROFESSOR PRINCE: It’s a biologically plausible theory or hypothesis. It’s not a biologically plausible mechanism because we haven’t proven the mechanism.
MR CLEMENTS: In other words, it’s plausible but you say not yet established by experiments; is that a fair - - -
PROFESSOR PRINCE: Yes.
1036 Professor Smith agreed with Professor Prince’s assessment insofar as the mechanism has not yet been established by the evidence (T608.4–16):
PROFESSOR SMITH: … I agree with Professor Prince we don’t have absolute proof that certain genes are changed at specific time in a specific level but overall the evidence where you see multiple epigenetic changes in genes, that we know are important in the development of NHL, and this predictive hypomethylation that you see, I think, is very strong evidence that aberrant gene methylation and DNA methylation play a role in development of NHL from glyphosate exposure.
1037 As Professor Prince explained earlier in cross-examination (T607.33–45):
MR CLEMENTS: Yes. Hence, I suggest to you, the conclusion that ought be drawn is that the because several studies show that glyphosate induces epigenetic changes in human cells and epigenetic changes in human cells can cause NHL, that supports the proposition that glyphosate is a human carcinogen and can cause NHL, doesn’t it?
PROFESSOR PRINCE: No. As Sir John just pointed out, you can change – you need to see a significant change of gene expression for that hypothesis. And so the significant change can both be firstly demonstrated change and there’s lacking data in the literature about a clear demonstration of that change, both methylation and histone modification, and then that change in expression needs to be clinically significant and translatable to the development of lymphoma. So those steps are missing and that’s why I have to disagree with you.
1038 I can be even briefer in relation to this hypothesised mechanism.
1039 The fifth hypothesised mechanism is that glyphosate and/or GBFs are preferentially distributed to the bone marrow, where it remains longer than in other tissues. Given that lymphocytes develop from lymphoid stem cells, which develop in the bone marrow, it is said that they may there be exposed to the genotoxic and mutagenic effects of glyphosate and/or GBFs.
1040 In support of the fifth hypothesised mechanism, Mr McNickle relies on Dr Sawyer’s opinion that exposure to glyphosate and/or GBFs can cause NHL in humans and that it is preferentially distributed to the bone marrow (Conclave F Joint Report (JRF) (at [36]–[40])). It is said that Dr Sawyer’s opinion is cumulative evidence which adds to the opinion evidence to the same effect given by Dr Flecknoe-Brown, Professors Smith and Checkoway.
1041 For reasons that will become apparent, Dr Sawyer withdrew his evidence on the hypothesised mechanisms, deferring to the “oncologist” (T2036.24–25). Further, Professor Prince and Dr Flecknoe-Brown did not give evidence in support of the fifth hypothesised mechanism and, accordingly, I do not consider the evidence supports the existence of the fifth hypothesised mechanism.
1042 The third stream might be regarded as the strongest aspect of the evidence in favour of Mr McNickle’s case theory (although I use this adjective in a relative sense).
1043 The evidence established that: (1) genotoxic effects on human blood cells can be observed from exposure to glyphosate and/or GBFs, and therefore exhibit KC2 (genotoxicity) (see above (at [903]–[907])); (2) glyphosate and/or GBFs induce epigenetic changes, including, among other things, methylation of the DNA (see above (at [935])); and (3) glyphosate and/or GBFs induce oxidative stress which can damage the DNA (see above (at [949])). With that said, having regard to the mechanistic evidence, the KCs, and the hypothesised mechanisms of action, I am not satisfied that the whole of the mechanistic evidence supports the conclusion that there exists a biologically plausible mechanism by which glyphosate and/or GBFs can cause NHL.
1044 Notwithstanding my findings as to the KCs, as I have explained, the mechanistic evidence stream turned on the “significance” question: that is, a chemical may “tick the box” of one or more of the KCs, but the next step of establishing a biologically plausible mechanism is the relevant question for the purposes of concluding that the mechanistic evidence supports Mr McNickle’s case. This is particularly evident in relation to KC2 where although the weight of the evidence demonstrated that glyphosate and/or GBFs may exhibit genotoxic effects in artificial in vitro environments, the mechanistic evidence did not support the conclusion that that finding is translatable to a genotoxic risk to humans in the real world due to either the use of excessive doses or the potential to detect a genotoxic signal at ever-lower concentrations of glyphosate by modifying in vitro conditions and using different assays (see above (at [901])). Similarly, in relation to KC4, it is evident that there does not exist a data-rich or robust association between epigenetic changes induced by exposure to glyphosate and/or GBFs (such as hypomethylation) and the development of NHL in humans. As Professor Smith noted in his 2020 article “[t]he difficulty with [KC4] is that the relevance of a particular epigenetic change for carcinogenesis by a specific agent may not be clear and causality is hard to establish” (see above (at [1032])).
1045 For those hypothesised mechanisms which did not relate directly to one or more of the KCs, Mr McNickle devoted some time to the second hypothesised mechanism and Wang (2019).
1046 The evidence established, however, that Wang (2019) suffered from several significant methodological problems, which may be summarised as follows: (1) NHL and MM are different diseases with different cells of origin which limits the extent to which any findings can be applied to the pathogenesis of NHL in humans (see above (at [1001]–[1004])); (2) the use of Vk*MYC mice, which are bred to produce large amounts of MYC protein (which, in turn, can influence the production of AID) resulted in inconsistent findings as to the upregulation of AID (see Figure 5 and above (at [999]–[1002])); (3) Dr Flecknoe-Brown conceded that the study suffered from a number of significant methodological flaws, including, to highlight three: (a) that only one “representative” mouse was demonstrated for each experimental condition; (b) the authors did not record the actual glyphosate levels in any of the surviving mice; and (c) Dr Flecknoe-Brown and Professor Prince were not confident that the data presented in the study demonstrated reliable evidence that glyphosate and/or GBFs cause an upregulation of AID; (3) Professor Smith and Dr Juberg agreed that the dose used in the study was “excessive” compared to any dose a human would be exposed to in the real world; and (4) even in the event that Wang (2019) is evidence that glyphosate and/or GBFs cause an upregulation of AID, there is no compelling evidence that there exists a biologically plausible mechanism whereby the mutagenic substances in glyphosate and/or GBFs can lead to the development of NHL by mutation of the EZH2 gene.
1047 For these reasons, having regard to the whole of the mechanistic evidence, I am not satisfied that there exists a biologically plausible mechanism by which the use of or exposure to glyphosate and/or GBFs can cause NHL in humans.
H CONCLAVE F (ABSORPTION AND EXPOSURE) AND DOSE
1048 Conclave F concerned the absorption and exposure of glyphosate and/or GBFs. The two expert participants in this conclave were Dr Sawyer and Dr Jeffrey Driver. Dr Sawyer was engaged by Mr McNickle and Dr Driver was engaged by Monsanto.
1049 The concurrent evidence session involving both experts was due to commence in the sixth week of the initial trial. However, on the evening of the prior Friday, the solicitors for Monsanto sent the following email to my Associate:
Dear Associate
The Respondents wish to provide an update in relation to Conclave F which is scheduled to commence on Monday, 9 October. In light of the common questions and the written and oral evidence provided to date, the Respondents do not consider it necessary and do not intend to call Dr Jeffrey Driver to give evidence at this initial trial.
We have copied the Applicant’s solicitors to this email.
…
1050 As foreshadowed in this email, Dr Driver was not called as a witness and hence Monsanto did not rely on Dr Driver’s report dated 18 March 2022 (Driver Report); and, by consent of the parties, JRF was redacted to remove the portions setting out Dr Driver’s opinions before being adduced into evidence. As a result, the evidence comprising reports prepared by the experts in relation to Conclave F that were ultimately tendered (subject to limitations pursuant to s 136 EA) is the JRF and the Expert Report of Dr Sawyer dated 8 September 2021 (Sawyer Report).
1051 Several consequences are said to flow from Monsanto’s forensic decision not to call Dr Driver, primarily that a Jones v Dunkel inference should be drawn, to which I will return later in this section. More importantly, however, as I foreshadowed earlier, Dr Sawyer’s evidence revealed some not insignificant issues.
1052 Before turning to those issues, it is convenient first to give an overview of the JRF and some key concepts relating to absorption and exposure.
1053 For a compound or chemical to reach a tissue, it usually must be taken into the bloodstream from a route of exposure, which is generally via the oral, dermal and/or inhalation route. For example, following oral exposure, chemicals often enter the body via mucous surfaces like the digestive tract (intestinal absorption), before being taken up by the target cells. Factors such as poor compound solubility, gastric emptying time, intestinal transit time, chemical instability in the stomach, and inability to permeate the intestinal wall can all reduce the extent to which a chemical is absorbed after oral administration (at JRF [21]).
1054 Dermal absorption is influenced by factors such dose density, duration of exposure, molecular size, lipophilicity, water solubility, and skin thickness. Absorption critically determines the compound's bioavailability (that is, the percentage of the chemical that makes it into the body). Routes of administration are an important consideration for pharmacokinetics and dynamics (an area of science which measures movement of chemicals into and throughout the body and their interaction with target or affected tissues, cells, and biochemical processes) (at JRF [15], [21]). Some factors can increase dermal bioavailability such as chemicals, for example, dimethyl sulfoxide that affect barrier function, irritations of the skin, sweating, dermal inflammation and epidermal damage from scratches, cuts, and other injuries (at JRF [22]).
1055 It is said that absorption of Roundup ingredients, glyphosate and polyoxyethylene amine occurs principally through the skin, which is called dermal absorption or “percutaneous absorption”. The skin is the predominant route of exposure for most occupational uses of pesticides, including GBFs. As a result, knowledge of skin penetration or dermal absorption is critical to evaluate absorbed dose of a pesticide in an operator. Airborne absorption via inhalation of glyphosate is more efficient but contributes much less to systemic dose than dermal absorption. (at JRF [23]–[25]).
1056 Compounds are most often carried to its target site via the bloodstream. From there, the compound may distribute into muscle and organs, usually to differing extents. After entry into the systemic circulation, either by intravascular injection or by dermal absorption from any of the various extracellular sites, the chemical is subjected to various distribution processes that tend to lower its concentration in plasma while the concentration at other sites may differ (at JRF [26]).
1057 Distribution is defined as the reversible transfer of a chemical between one compartment (for example, fat, blood, muscle, and bone) to another. Some factors affecting chemical distribution include regional blood flow rates, molecular size, water and lipid solubility and binding to serum proteins or bone minerals, forming a complex (at JRF [27]).
1058 Compounds begin to break down as soon as they enter the body. The majority of small-molecule chemical metabolism is carried out in the liver. As metabolism occurs, the initial (parent) compound is converted to new compounds called metabolites. When metabolites are pharmacologically inert, metabolism deactivates the chemical and usually reduces the toxicity effects on the body (at JRF [28]).
1059 Compounds and their metabolites are removed from the body via excretion, usually through the kidneys or the liver. Unless excretion is complete, accumulation of foreign substances can adversely affect normal metabolism (at JRF [29]).
1060 There are three main sites where systemically circulating chemical excretion occurs. The kidney is an important site, and it is where products are excreted through urine. Hepatic faecal excretion is the process that initiates in the liver and passes through to the gut until the products are finally excreted along with waste products or faeces. Excretion through pulmonary exhalation can occur with certain volatiles (at JRF [30]).
1061 Excretion or elimination of a chemical can be characterized by its half-life (that is, the time it takes for the concentration of that substance to fall to half of its initial value). For human exposure and dose assessment, human data are the most relevant and reliable for estimating half-life. However, given ethical considerations when dealing with humans, such studies have unavoidable limitations resulting in some uncertainties in interpreting the data. Human biomonitoring studies (studies which measure chemical concentration in biological samples such as blood or urine) with multiple collection intervals allow estimates of excretion rate of absorbed dose (at JRF [31]–[32]).
H.2 The Absorption and Exposure Evidence
1062 Mr McNickle contends that Dr Sawyer’s evidence supports the conclusion that glyphosate and/or GBFs are capable of being absorbed through human skin and into circulation in the body, and that exposure to glyphosate and/or GBFs increases the risk of NHL and can cause NHL in humans (at JRF [36], [40]).
1063 These submissions were developed as follows.
1064 First, Mr McNickle relies on Dr Sawyer’s opinions in the JRF that absorption of Roundup products occurs principally through the skin via dermal absorption (and to a lesser extent inhalation), which occurs at an approximately permeability factor of between 2–3% (at JRF [23], [36], [90]). Once it is absorbed, glyphosate is distributed via the vascular and lymphatic system to all tissues in the body (at JRF [92]). Roundup Products can be absorbed by exposed skin and/or by penetrating through clothing which comes into prolonged contact with the skin (at JRF [147]). Further, although aerosol inhalation represents a minor route of exposure (20% or less), it is nonetheless relevant particularly when spraying in a horizontal position and should be afforded weight (at JRF [153]). It is also necessary to consider exposure through irritated or damaged skin in the light of studies that have demonstrated that glyphosate deposition in damaged skin is approximately five times that of healthy skin, and penetration through damaged skin is increased 20-fold (at JRF [154]).
1065 Secondly, it is said that: (1) Professor Prince and Dr Flecknoe-Brown accepted that glyphosate can be absorbed through human skin exposed to Roundup Products (at JRA [182]); (2) that glyphosate absorption through the skin is supported by a number of studies (including Chang and Andreotti (2023) and Sidthilaw (2022), which studies found that glyphosate was present in the urine of individuals exposed to GBFs (see also T623.1–625.5)); (3) insofar as Dr Sawyer’s opinion that dermal absorption of glyphosate occurs at an approximate permeability factor of between 2–3% (at JRF [36]), that opinion is consistent with the “Dermal Penetration Figure” of 3% referred to in Monsanto’s Operator Exposure Assessment for MON2139 (at 2) (at JRF [80]).
1066 Thirdly, as noted earlier (at [1040]), Dr Sawyer contends that exposure to glyphosate and/or GBFs increases the risk of NHL and can cause NHL because glyphosate is cleared more slowly from the bone marrow than other parts of the body (which opinion is relied on by Mr McNickle as cumulative evidence to that given by Dr Flecknoe-Brown, Professors Smith and Checkoway). It is said that Brewster (1991) demonstrates that following initial absorption, the concentration of glyphosate is shown to decrease in the tissues examined over the time frame with the rate of elimination from the plasma far exceeding the elimination rate from the bone marrow (at JRF [38]). Other than the carcass, the last organ or tissue to clear the glyphosate was the bone and bone marrow. Dr Sawyer notes that Brewster (1991) “clearly demonstrated that there was significant retention of glyphosate in bone marrow versus plasma. This is a highly significant finding as bone marrow can play a fundamentally important role in the early development of NHL” (at JRF [38]).
1067 It will be recalled that in the context of the first stream, I recorded (at [130]) that with the notable exception of one witness in this proceeding, issues going to credit are not the centrepiece of this case. That witness is Dr Sawyer and, regrettably, for reasons that will shortly become apparent, issues going to his credit assumed some significance.
1068 Before going further, however, it is well to emphasise two related matters.
1069 The first is that I have reiterated more than once (at [32]–[33]) that the mere fact that an expert witness has a pre-existing opinion on a key issue is an infirm basis for rejecting the expert’s opinion evidence or placing less weight on it. The focus of the Court is not the preconceived opinions of the relevant expert; but the overall substantive merits of their evidence given in the witness box and in their expert reports.
1070 The second matter is that both parties made extensive oral and written submissions as to Dr Sawyer’s credit which, broadly speaking, relate to the allegation that he is a professional “glyphosate-plaintiff” expert. But without wishing to add to an unduly long judgment, I do not propose to set them out in full and to focus on those aspects of issues going to Dr Sawyer’s credit which, in my view, merit particular attention.
1071 In the following subsections, I will set out briefly Dr Sawyer’s background before turning to highlighting several matters informing my conclusion as to his creditworthiness.
1072 Dr Sawyer is a professional toxicologist with a doctorate in toxicology from the Indiana University School of Medicine. Dr Sawyer has an associate degree from the State University of New York Agricultural and Technical College at Morrisville in 1976; a Bachelor of Science degree in biology from the State University of New York in 1978; and a master’s degree in cellular and molecular biology, also from the State University of New York, in 1982. He subsequently earned a doctorate in toxicology from the Indiana University School of Medicine in 1988 (Sawyer Report (at [13])).
1073 During his training at the Indiana University School of Medicine’s Department of Toxicology and Pharmacology, Dr Sawyer studied under the late Dr Robert Forney. Dr Forney was the department chairman, the director of the State Department of Toxicology. Dr Sawyer studied the mechanisms and toxicity of organophosphates and organophosphorus chemicals as specialised research with respect to sarin and carbamates were ongoing within the department. He also received forensic training and worked in the State Department of Toxicology laboratory facility at the medical centre in Indianapolis, Indiana, performing forensic analyses for poisons, pharmaceuticals, drugs of abuse and alcohol (Sawyer Report (at [14])).
1074 Subsequent to his training, Dr Sawyer served full-time in public health as the toxicologist for the Onondaga County Department of Health, Syracuse, New York. His work experience included municipal and civil risk assessment, evaluation of environmental toxic exposures including petroleum products, design and execution of environmental monitoring, health assessment studies, methods to identify and effectively reduce community toxic exposures and assessment of public health matters. Further, during his employment as toxicologist for the health department, he assisted the medical examiner’s office with investigations of accidental and intentional poisonings (Sawyer Report (at [15])).
1075 Dr Sawyer has previously been certified by the State of New York’s Department of Health in forensic toxicology and received certificates of qualification as a clinical laboratory director and as a licensed environmental laboratory director by the States of New York, New Jersey, California and South Carolina. He has also previously been trained and certified under OSHA 29 CFR1910.120 for Hazardous Waste Operations and Emergency Response (Sawyer Report (at [16])).
1076 Dr Sawyer is presently the toxicologist for Toxicology Consultants and Assessment Specialists, LLC, in Sanibel, Florida. He has previously served as a peer-reviewer for the journal The Forensic Examiner and as a member of its editorial board and was previously an adjunct assistant professor at the Upstate Medical Centre in the Department of Medicine at SUNY Health Science Centre. Dr Sawyer is a member of various professional societies including the Sigma Xi, the Scientific Research Society, the American Academy of Forensic Sciences and the International Association of Forensic Toxicologists (Sawyer Report (at [17])).
1077 Dr Sawyer was an unsatisfactory witness. His evidence gave me some pause and it is well to highlight those aspects which caused me concern.
1078 First, Dr Sawyer’s first piece of glyphosate-litigation in which he gave sworn evidence was Johnson v Monsanto (Cal. Superior Ct., San Francisco) (T1961.29–32), where he commenced as a consulting expert on the case in late 2016 or early 2017 (T1961.34–35). Dr Sawyer confirmed that by the time he gave evidence in that litigation in July 2018, he had determined that glyphosate was a human carcinogen (T1962.37–39). Dr Sawyer gave evidence that he has not resiled from that view since then (T1970.1–3, T1970.33–35, T2006.26–34). He accepted that when he was retained by Maurice Blackburn in this proceeding, he held a fixed view that glyphosate was carcinogenic (T2007.42–43) and that he proposed to give evidence in this case consistent with evidence he gave in glyphosate litigation in the United States (T2006.32–34):
MR CRAIG: … And as you’ve said earlier today, you had formed by that stage the opinion that glyphosate was carcinogenic?---Yes.
MR CRAIG: And you had determined your methodology with respect to exposure days based on the epidemiological studies?---Yes.
MR CRAIG: And what you were proposing to do in this case is give evidence consistent with that you had already provided in the Pilliod litigation?---Yes. With respect to general causation, absolutely.
1079 Secondly, Dr Sawyer presented as anything but a disinterested and dispassionate witness. He described Monsanto as a “criminal” and “unethical” organisation (T1998.36–38; T2000.33–39). Dr Sawyer initially asserted that it was only after his expert conclave in this proceeding that he formed the view that Monsanto engaged in “criminal” conduct (T2000.7–8), but later gave evidence that it was since “approximately December 2022” that he held the view that Monsanto has interfered with the publication of various articles, including articles authored by him (T2021.31–32). Dr Sawyer gave evidence (T1998.36–41):
DR SAWYER: … And I also have proof in 13 different emails from Monsanto how they intentionally interfered and stopped two publications, which was criminal in my opinion, and I didn’t want to be in a – I didn’t check with any lawyers. This was a scientific article that I submitted to a peer review journal. It had nothing to do with a law firm. I don’t even know if the law firms knew I was writing the article.
1080 It later emerged that no later than November 2022 that Dr Sawyer had formed the view that: (1) Monsanto may sabotage or interfere with his publication (T2021.26–27; T2026.41–46); (2) he was concerned about that occurring (T2021.23–24); (3) he refused to answer questions on deposition about his proposed publication with Dr Benbrook and Dr Mesnage (T2021.1–24); (4) he held the belief that Monsanto has been guilty of ghost-writing (T2022.1–15); and (5) he had expressed the view that Monsanto had engaged in unethical behaviour “in any way you looked at it” (T2022.17–33).
1081 Dr Sawyer later gave evidence that he had a suspicion about Monsanto’s conduct dating back to the Johnston trial in the United States, which occurred in July 2018. He then gave the following evidence (T2023.3–30):
MR CRAIG: And you allege that just prior to that trial somebody from Monsanto or related to Monsanto stole the trash from outside your house?---I don’t know that I – you know, I made it clear that I have no evidence that Monsanto did that. But mysteriously my trash was stolen the night before it was to be picked up. It was very unusual and well documented by other family members. Cans were ..... but the trash was gone.
As a consequence of that you formed the view, as you expressed on oath at lines 8 and 9, that you believe that the trash had been stolen by somebody related to Monsanto, didn’t you?---I believe it was possible that it was related to Monsanto because it coincided with the full week of my deposition. I think I was deposed four or five days that week and then on Saturday or Sunday the trash was stolen. Two large cans. The containers were left behind.
In your sworn evidence – sorry, in your sworn deposition in the Cody proceeding, you didn’t say you thought it was possible, you thought that somebody from – related to Monsanto had stolen your trash, didn’t you?---Not exactly. If you read the full transcript - - -
HIS HONOUR: I don’t think that’s an accurate reflection of what he said. He believed – he agreed with the proposition he believed that it was somebody related to Monsanto.
DR SAWYER: Right. But I was not certain of that and I made that clear too that I couldn’t say with any certainty. I couldn’t prove it.
MR CRAIG: Thank you. But your belief, as expressed at the deposition by sworn evidence, Dr Sawyer, was that it was somebody related to Monsanto that had stolen your trash?---Yes.
1082 Thirdly, Dr Sawyer gave evidence that he had deliberately destroyed documents that he knew were required to be produced in glyphosate litigation in the United States. Dr Sawyer understood that when he attends a deposition in the United States, he could be compelled to produce documents (T1959.1–2); that he needed to follow that compulsory process (T1959.4); and that such a notice to produce documents is compulsory (T1997.9–10). He gave evidence, however, that he was prepared to not comply with any compulsory process that, in his view, was an “unethical, illegal request” (T1997.37–39).
1083 After receiving such a compulsory notice in glyphosate litigation in the United States in January 2023 (Berns, et al. v. Monsanto (Circuit Ct., St. Louis, MO)) ordering the production of documents relating to a publication that he co-authored with Dr Benbrook and Dr Mesnage, Dr Sawyer destroyed documents that, to his knowledge, came within the scope of the notice (T1997.45–1998.24):
MR CRAIG: Now, after publication of your article with Dr Benbrook, you intentionally destroyed all of the documents relating to the publication of that document, did you not?---Yes, because Monsanto was demanding documents that – I checked with the journal and also the international rules, and to disclose information in a paper that was undergoing peer review, including information that could lead Monsanto to contact the peer reviewer, it is unethical and illegal.
MR CRAIG: And you unilaterally destroyed those documents without checking as to whether that was a legally available course to you, didn’t you?---I did check with the journal and the – there’s an actual international agency that lays out the rules for peer-reviewed literature, and I – I read those guidelines. And - - -
MR CRAIG: You took no step to have that request set aside, did you?---I don’t know what that means.
MR CRAIG: Well, you didn’t communicate with your lawyers, with the lawyers acting for Berns or with Monsanto to say, “I’m prevented from providing you with this information by journal requirements, and I refuse to do so,” did you?---I read the guidelines and contacted the journal personnel and learned that – and I already knew this, but just double-checking that any documents – the rough draft manuscript, etcetera, that’s under peer review is completely confidential. And I also have documents showing that Monsanto intentionally interfered with two different publications in the past. And - - -
MR CRAIG: You destroyed those documents because you didn’t want Monsanto to get them, didn’t you?---That’s correct. And interfere with it getting published, as they did in two other cases, which I have the documents to prove.
1084 Dr Sawyer later gave evidence (T2000.41–43):
MR CRAIG: And so you took it into your own hands to destroy documents rather than allowing Monsanto to examine on them in the course of a deposition process?---You bet. I did. I did the right ethical thing, and I stand behind my actions.
1085 Fourthly, it is apparent that the majority of Dr Sawyer’s work and income is derived from giving expert evidence for plaintiffs in glyphosate-litigation. He gave evidence that:
(1) most of his income has come from litigation consulting over the past 20 years (T1970.44–45);
(2) he has given evidence in approximately 34 American States (T1970.41–42);
(3) has testified as a hired expert in approximately 300 or more depositions (T1971.7–8);
(4) has been deposed as an expert witness in over 200 trials;
(5) has prepared over 100 reports, spanning 49 proceedings in which there has been a deposition or trial, in Roundup litigation (T1968.36–1969.2);
(6) conducts a litigation consulting business through a website “experttoxicologist.com” (T1971.28);
(7) his largest clients have “very much” been plaintiffs’ attorneys in Roundup cases (T1972.27–29);
(8) he had received approximately US$2.5 million in fees in connexion with Roundup litigation in the United States over a period of six years (T1972.31–33);
(9) his rate for Roundup matters is US$785 per hour and full-day rate for giving evidence at a deposition or trial is US$8,200 per day (T1972.38–1973.16); and
(10) at the time he was engaged by Mr McNickle in this proceeding, more than half of his income was derived from Roundup cases (T2008.17–18; T2008.24–28).
1086 Fifthly, Dr Sawyer gave evidence that he approached the task of giving expert evidence in this proceeding in the same manner as he would in the United States. When asked about the litigation process in the United States, his understanding included that he could be first retained either as a consulting expert or a testifying expert (T1957.19–20), and that one can change from being a consulting in a case to a testifying witness (T1958.6–7). This caused me to ask Dr Sawyer, having familiarised himself with the Expert Witness Code of Conduct, whether there is anything he did in relation to approaching his role as an expert in this proceeding differently from the way in which he would have approached giving evidence, and has approached giving evidence, in United States proceedings (T1989.29–37), to which he responded (T1989.47–1990.16):
DR SAWYER: … The only difference I see is actual written questions in black and white that are very succinct and clear. I never in my experience have been greeted with a very specific list of questions. And in the US, I know attorneys are very fearful of writing such because, in the US, I’m always asked to turn over my file. And I turn it over. I turn over everything, and sometimes the – so – so what happens in the US, the questions are usually asked verbally by phone. So I see that as a – as a – an outstanding difference. In terms of item 3.2(b), that your opinions are based wholly or substantially on specialised knowledge arising from your training and/or experience, that’s exactly what I do. However, in the US, I – I don’t recall ever being asked that question in black and white, and I think it’s a very good question.
HIS HONOUR: All right. Well, thank you for that. So I take it from that answer that essentially you proceeded – apart from the fact that you were asked specific questions in writing in this engagement, your approach to it was essentially the same as that you would take in relation to providing expert evidence in an American case. Would that be fair?---Yes.
1087 Sixthly, Dr Sawyer accepted that because of his extensive experience in glyphosate-litigation, he can “cut and paste” sections of his reports and include them in new reports (T1975.1–10; T1986.27–43). He accepted that, subject to any such additions and subtractions, the opinions in the reports remain essentially the same (T1975.9–10) and conceded that, in his report in the present proceeding, he had cut and pasted sections from reports he prepared in other Roundup cases, including work that he had prepared in 2021 and 2020 (T1987.1–26, T1988.24–26), without disclosing that he had replicated or recited those opinions (T1987.28–30). The copied sections came from reports that he had prepared before he received the questions or letter of instruction from Maurice Blackburn in this proceeding (T1987.32–36; T1988.1–18).
1088 Following Dr Sawyer’s cross-examination, senior counsel for Monsanto submitted that in the light of his evidence, the Court cannot proceed on the basis that the JRF is the product of two independent expert witnesses doing their best to assist the Court in arriving at the truth of the scientific evidence consistently with the Expert Witness Code of Conduct (T2044.35–38). Accordingly, Monsanto made an application to exclude Dr Sawyer’s evidence on the basis that it might give rise to a danger of unfair prejudice or be misleading or confusing, and that as a result, the Court should exercise its discretion to exclude it pursuant to s 135 EA.
1089 In McNickle v Huntsman Chemical Company Australia Pty Ltd (Evidentiary Ruling) [2023] FCA 1268, I dealt with Monsanto’s application and noted then (at [14]–[21]):
[14] None of this is a bolt from the blue.
[15] As long as two and a half years ago in McNickle v Huntsman (Expert Evidence), I anticipated that an objection will be made by Monsanto to the evidence of, inter alios, Dr Sawyer, who I described then (at [17]) as a witness:
[w]ho has allegedly given evidence in 28 proceedings apparently dealing with the Expert Issues.
[16] I went on to note (at [18]) that:
Cognisant of potential issues of adversarial bias, particularly in a case such as the present involving allegations of scientific manipulation, on 22 December 2020 (and having abandoned the proposed reference), I indicated to the parties that the Court would be best assisted if the experts chosen by the parties in each area of speciality were non-partisan and were “truly independent about the issues and about their view”: see T9.43–7.
[17] As I indicated to the parties then (at [21]), if there was to be some attack on the independence of, among others, Dr Sawyer, then, consistently with the overarching purpose in Pt VB of the Federal Court of Australia Act 1976 (Cth), it would be utile to ensure that any such objection was resolved sooner rather than later. During that discussion, I had referred to the possibility that it may be appropriate to make an order facilitating an advance ruling pursuant to s 192A of the Evidence Act.
[18] As it happened, Mr McNickle opposed this course. As I explained then (at [32]):
Both in written submissions in advance of the case management hearing and orally, Mr Rush QC, counsel for Mr McNickle, submitted that it would be beyond power for the Court and the parties to embark on a process of presently determining whether any expert evidence which may be given by any expert witness would be excluded pursuant to s 135 of the Evidence Act or on admissibility grounds. Five points were made:
(1) First, the “threshold jurisdictional” question for exercising the discretion in s 192A has not been satisfied. Section 192A may be enlivened where a “question arises” in relation to any of the three matters set out in subparagraphs (a)–(c) of that section. No such question presently arises. This is said to be because:
(a) there is presently no “evidence” proposed to be adduced and thus, subparagraphs (a) and (b) of s 192A cannot apply; and
(b) no question arises about the giving of leave, permission or direction under s 192: see s 192A(1)(c).
(2) Secondly, further to the threshold issue, it would be inappropriate for the Court to exercise the discretion under s 192A at this time. Here, no expert reports have been filed, and as such, the Court would be making its discretionary decision in the absence of information which is critical to that decision. It was said that the Court is in no position to, and ought not to, make an advance ruling where all matters relevant to the issues have not been, and cannot be, ascertained.
(3) Thirdly, difficulties arise in relation to any contemplated rulings since it is not clear what precise rulings would in fact be sought by Monsanto.
(4) Fourthly, while it seems the process presently contemplated is intended to avoid disruption to the trial date, it will have the opposite effect. It was said that Monsanto appears to make a global complaint in respect of all of Mr McNickle’s experts, and as such, five experts would need to give evidence and be cross examined at a voir dire. The processes around production of documents and materials relevant to such a voir dire will take further time, and will inevitably lead to delay in the balance of the timetable to an initial trial.
(5) Fifthly, Order 1 made on 5 March 2021 focuses the inquiry concerning the independence of the experts and whether those experts have expressed certain opinions previously. It was submitted that the questions posed indicated a misunderstanding of what is required by the notions of “independence” and “impartiality” in the expert evidence context. It was further submitted that even if an expert has expressed an opinion previously (even on many occasions), this does not mean that the expert is not independent or impartial within the court processes. It was said that it simply does not follow from the fact that an expert has extensive experience and expertise, and based on that experience and expertise has formed opinions about certain questions of science (which may have been expressed previously, including in previous litigation), that the expert lacks independence or impartiality. Accordingly, it was said that as a consequence, the rulings proposed are based on an incorrect premise.
[19] The fifth point was repeated during oral submissions today: that is, any issues concerning a perceived lack of independence and impartiality cannot go to issues of admissibility but rather only to weight. In this respect, I was referred to the judgment of Wigney J in Rush v Nationwide News (No 5) [2018] FCA 1622 (at [35]–[36]), where his Honour noted:
[35] The relevant principle established by those and other authorities was neatly summarised by Dodds-Streeton J in Ananda Marga in the following terms (at [35]):
In my opinion, relevant authority establishes that while (as reflected by the Federal Court Practice Note and like curial protocols) objectivity and independence are sought of expert witnesses, such qualities are not preconditions of competence, even in the case of expert witnesses. The sanction for failure to fulfil the obligations imposed by relevant authority and curial protocols is not the exclusion of the expert’s evidence, but rather, the significant risk that it will fail to persuade.
[36] In other words, an actual or perceived lack of independence, impartiality or objectivity of an expert witness goes to weight, not admissibility.
[20] In response to that argument, I expressed the following views in McNickle v Huntsman (Expert Evidence) (at [34]–[35]), to which I adhere:
[34] In Rush, Wigney J (at [11]–[43]) dealt with what was described as “the independence ground”, which, unsurprisingly, was an argument advanced by the publisher in that case that the alleged lack of independence of two experts proposed to be called by the applicant in a defamation proceeding ought not be admitted or alternatively excluded. His Honour dealt with an argument that the applicant had not complied with Pt 23 of the FCR because, in the circumstances of the case, the expert was an advocate for the cause of the party who proposed to call him. In doing so, his Honour dealt with a decision of Mortimer J in Guy v Crown Melbourne Limited [2017] FCA 1104, expressing (at [36]) the view that:
… an actual or perceived lack of independence, impartiality or objectivity of an expert witness goes to weight, not admissibility.
[35] I think Mr McNickle puts the point too highly. A broad point was being made by his Honour in Rush, which is no doubt correct in the general run of cases. But I do not think this means there cannot ever be circumstances in which a lack of impartiality or objectivity would go to more than weight and would form a basis by which the proposed expert evidence would not be admitted. One would conceive of some circumstances where proposed expert evidence could fail to survive the balancing exercise required when applying s 135 of the Evidence Act, in that receiving such evidence would be of such limited assistance (by reason of its lack of independence, impartiality or objectivity) that it would be unfairly prejudicial to the other party or would result in an undue waste of time.
[21] As is evident from the above, the position that Mr McNickle took in the early interlocutory stages of this proceeding was clear: the Court can only, and must only, deal with any objections to the expert evidence in the context of the initial trial. I noted then (at [39]) that I had formed no view as to whether or not the objections foreshadowed had any merit, but that I was nonetheless conscious they had been advanced by responsible counsel and solicitors who had formed the view that there is a reasonable basis for saying there was a want of independence on behalf of the relevant experts such that their evidence should be the subject of (at least) discretionary exclusion. I went on to note (at [41]–[42]) that:
[41] I did think initially that it was possible for an advance ruling hearing to be held some time prior to the initial trial if it was with the active cooperation of both parties and there was no objection to this course. However, given the opposition to this course by Mr McNickle, I am not convinced that this is appropriate. Indeed, there is some prospect that adopting this course would lead to a potential interlocutory appeal. If there had have been cooperation by both parties, I would have grasped the nettle and proceeded to have had an advance ruling hearing in advance of the initial trial, even if, at the end of that hearing, I was persuaded that it was inappropriate for a ruling to be provided. Given that course is opposed, then not without some misgivings, it appears that I will have to leave the ruling on admissibility of the expert material (on presently identifiable grounds) to the initial trial (as is said to be necessary by Mr McNickle).
[42] As I stressed above, I have reached no view one way or the other as to whether or not any allegations of a lack of impartiality have any substance. No doubt Mr McNickle will maintain his contention that the evidence given by the nominated experts will be of importance in resolving the issues and carries great weight. All that is yet to be seen. Of course, if at the initial trial the objection which has now been clearly flagged is upheld, I will be required to determine the appropriate remedial response. In determining any remedial measure proposed by Mr McNickle, no doubt Monsanto will submit that its attempt to resolve this issue now pragmatically (which has been objected to) will be a relevant consideration in the event that the objection is ultimately upheld on the basis of material now known to exist or able to be obtained.
1090 I explained then that the consistent position of Mr McNickle (see above (at [32]–[33])) was that the proper approach to assessing the evidence of an expert witness in respect of whom it is alleged that they have not brought an independent mind is to consider the scientific material adduced from any expert and judge it on its merits, irrespective of whether the expert has adhered to a fixed view prior to being engaged to assist the Court or, indeed, irrespective as to whether the expert harbours some justified or unjustified animus towards a party.
1091 Ultimately, I formed the view that it was unnecessary to deal with that submission because the appropriate course was to admit Dr Sawyer’s evidence. As I noted then (at [24]–[27]):
[24] There can be little doubt that an assessment of the weight of Dr Sawyer’s opinion evidence will be the subject of detailed closing submissions. One aspect of that assessment may involve consideration of the forensic decision not to call Dr Driver, another will be the evidence given by Dr Sawyer in cross-examination, including the evidence summarised at Section B above.
[25] All these aspects, and others, will require close consideration. The points made by Monsanto today attacking the cogency of the Dr Sawyer’s evidence in the context of discretionary exclusion apply equally to the weight that should be afforded to that evidence. I do not propose to express any views here about the ultimate reliance that ought to be placed on the opinions expressed by Dr Sawyer or canvass any of the alleged deficiencies in the evidence relied upon by Monsanto. For the purposes of this ruling, it is necessary I form an impressionistic assessment of its probative value, but to descend into further detail without the benefit of final submissions in a matter such as this, involving complex scientific evidence, would be premature and inappropriate.
[26] In exercising the discretion, I have had regard to s 192 of the Evidence Act. Without seeking to list all relevant considerations exhaustively, to exclude the evidence at this late stage of the initial trial would not shorten the length of any hearing. Relatedly, it is relevant that the forensic course taken by Monsanto was not to attack the evidence on the voir dire and then seek to object to the adduction of evidence in chief in the trial (being the more orthodox course), but to allow the evidence in at trial without objection and then seek to discredit and apply to exclude it. Further, weighing significantly in the exercise of discretion is that the topic of the opinion evidence is of some importance given the determinative issues in the proceeding.
[27] In the end it must be demonstrated that the danger of unfair prejudice or relevant confusion substantially outweighs the probative value of the evidence. In the light of the potential probative value of the evidence and the reality that the matters now raised by Monsanto can be dealt with through the prism of an assessment of the weight of the evidence, I do not consider that unfair prejudice or confusion is established to a degree necessary to enliven the discretion to exclude the opinion evidence pursuant to s 135 of the Evidence Act.
1092 I am conscious I am not conducting what amounts to a Royal Commission into various conduct that has occurred between Dr Sawyer and Monsanto and, unaided as I was by any relevant re-examination, I have not been invited to make findings on the cogency of various allegations that Dr Sawyer made during his colourful cross-examination. Irrespective of whether there is any basis for his evident animus, the fact is that it was manifest that Dr Sawyer did hold a real and abiding animus against Monsanto. In circumstances where I repeatedly requested the parties to pay close attention to the law and practice relating to experts as it has developed in Anglo-Australian law, and the desirability of avoiding any actual or apprehended partisanship, it was surprising that someone with the deep-rooted hostility of Dr Sawyer to one of the parties was called by Mr McNickle. Although it is necessary, as I have repeatedly stressed, to reach conclusions on the substantive merits of his opinions, it is unrealistic to consider that assessment can be wholly insulated from the distinct impression I gained from Dr Sawyer’s evidence, and his manner of his giving it, that he presented as a “hired gun” and not as an independent expert in the sense contemplated by the Expert Witness Code of Conduct.
1093 It is plain that: (1) at the time he executed the JRF in December 2022, Dr Sawyer, among other things: (a) maintained a belief that glyphosate is a human carcinogen; (b) believed that Monsanto is a criminal enterprise; (c) had deliberately destroyed documents the subject of a compulsory process in glyphosate-litigation in the United States; (d) believed that Monsanto had engaged in illegal and unethical conduct, including by engaging in ghost-writing and inferring with the publication of various articles; and (e) believed that Monsanto (or its agents) had rifled through his rubbish; and (2) by the time he was engaged by Mr McNickle, intended to give evidence in this proceeding consistent with his view as to general causation.
1094 When he entered into Conclave F, Dr Sawyer was charged with the responsibility of forming a view in accordance with the dictates of the Expert Witness Code of Conduct, which provides, inter alia, that “[a]n expert witness is not an advocate for a party and has a paramount duty, overriding any duty to the party to the proceedings or other person retaining the expert witness, to assist the Court impartially on matters relevant to the area of expertise of the witness” (Expert Witness Practice Note, Annexure A (at [2])) (emphasis added). Regrettably, and perhaps inevitably given his fixed views and his hostility towards one the parties, I consider that Dr Sawyer fell short of these standards and acted as an advocate for those that retained him. Having noted this, I will now turn to the underlying substance of his evidence which, leaving aside questions of partiality, has inherent difficulties.
1095 It is important to recognise from the outset that this part of my reasons has, as its point of departure, the notion that the whole of the evidence has established that glyphosate and/or GBFs are genotoxic and/or mutagenic carcinogens. It concerns the question of dose: principally whether the evidence establishes that there is no safe level of exposure to a genotoxic and/or mutagenic carcinogen. It is a discrete question which is not strictly confined to issues arising out of Conclave F and, given some back-and-forth concerning Mr McNickle’s case on dose (and its accompanying nomenclature), it necessitates some explanation.
1096 Mr McNickle contends that: (1) there is no safe level of exposure to a genotoxic and/or mutagenic carcinogen; and (2) the extent of the increased risk of developing NHL relates to the intensity and duration of exposure to glyphosate and/or GBFs (T2096.16–2097.14; T2220.41–2223.15; AS [5], [15], [523B]). It is worth noting that implicit in (2) (which I will call the alternative dose proposition) is the notion that glyphosate and/or GBFs are carcinogenic at some threshold dose (hence the terms “threshold carcinogen” or “non-threshold carcinogen”).
1097 Early in the initial trial, I was mindful to dispel any misapprehensions about the way in which Mr McNickle had framed his contentions as to dose (T86.35–87.47):
HIS HONOUR: … Mr McNickle contends that any use of or exposure to Roundup herbicide and Roundup Biactive increases an individual risk to developing NHL and could cause an individual to develop NHL. … I might find that I accept that proposition, in which case, I will simply answer yes to question 1. I might find that, well, look, I’m not satisfied that any use or exposure to Roundup Herbicide or Roundup Biactive increases an individual’s risk, there might 40 be a risk at a very, very low level but it’s only really – I’m only satisfied on the evidence and reasonably satisfied the evidence is capable of causing cancer at a greater level – a greater duration or intensity of exposure, in which case, I would answer yes but qualify that answer. That’s the point I made at the last case management hearing where there was certain attempts to reframe the questions.
MR CRAIG: Your Honour, our point is a simple one: if your Honour is going to answer question 1 yes, your Honour must determine the dose at which that is said to occur because, implicitly in our learned friend’s framing of the question, they contend that an answer of yes is possible, even without identifying for your Honour the dose - - -
HIS HONOUR: That’s their primary case and their alternative case. Their alternative case is that there’s a point where the duration of intensity of exposure means that it’s more likely than not that those Roundup products are carcinogenic and will cause an individual to develop NHL.
MR CRAIG: And what’s really important about that, your Honour, is what they are contending or what is implicit in that contention is that there is a systemic dose at which the product is capable of causing cancer. And they don’t adduce any evidence as to that systemic dose.
HIS HONOUR: The evidence hasn’t started yet.
MR CRAIG: Well, your Honour - - -
HIS HONOUR: That might emerge during the process of cross-examination for all I know. But they’re the two – I don’t want there to be any pleading point in this case and I understand that the way they put it is in paragraph 8 [of the applicant’s opening submissions] and I think that’s within the pleadings and we will see where the evidence takes us in relation to what I described as their primary position and the more nuanced position.
MR CRAIG: Yes, your Honour. And I just – at the risk of repeating myself, we accept that it’s not a pleading point. In terms, paragraph 26 - - -
HIS HONOUR: You say it’s a no evidence point.
MR CRAIG: It’s a no evidence point and it’s really important, your Honour, to hone in on the relevance of dose both at the thresholds carcinogen – as any use or exposure and at what your Honour’s calling the alternative case which we really would submit to your Honour isn’t being pressed in any meaningful way because you didn’t hear our learned friend’s open on conclave F at all and they haven’t adduced any evidence as to systemic exposure in a way that would allow your Honour to determine that so-called alternative case. We say there really is in truth one case and one case only, that is it’s an unsafe at any speed case.
HIS HONOUR: No, there isn’t. And I don’t want that just left unanswered on the transcript. That is not the way they’re running their case. The way they’re running their case is paragraph 8 – now, you might say that the evidence doesn’t support that contention but that’s a different point.
1098 The issue was revisited when the matter was last before the Court, when senior counsel for Monsanto made the following submissions (T2221.26–42):
MS O'GORMAN: If I can note firstly, your Honour, that this second proposition from paragraph 8 of the opening submissions [that is, the alternative dose proposition] is scientifically an entirely different proposition on dose from the one that the applicant addresses in closing submissions, namely, that genotoxic substances necessarily cause cancer at any dose. The second proposition - - -
HIS HONOUR: Sorry, you say that’s what?
MS O'GORMAN: It’s an entirely different proposition, and it doesn’t flow from the first proposition. The first proposition is genotoxic substances are carcinogenic at any dose, and I will come to address the Calabrese paper specifically on that.
HIS HONOUR: Yes.
MS O'GORMAN: But the second proposition is that even having established it’s carcinogenic at any dose, the risk then increases proportionately as the exposure increases. So there’s a linear dose response relationship.
1099 Shortly after that exchange, I made an order that the parties each provide the Court with a note as to whether the evidence establishes that for a genotoxic carcinogen and/or mutagenic substance, there is no safe level of exposure, and as to why the approach adopted by any regulatory agency as to linear extrapolation as a default establishes, as a matter of fact, that there is no safe level of exposure. This was done and Mr McNickle identified the following evidence in support of his primary case as to dose:
(1) Professor Smith’s evidence that “if the compound is genotoxic, then the US EPA and most of the regulatory agencies throughout the world draw a linear no threshold line to this, and then they – society sets a limit because there is no safe dose, and that’s generally accepted” (T1555.29–44);
(2) Dr Juberg’s evidence:
(a) in relation to Professor Smith’s evidence at (1), “that that’s how cancer risk is done” (T1556.5);
(b) that he was not aware of the USEPA having ever set a threshold or safety factor in respect of a genotoxic carcinogen (T1556.12–16); and
(c) that there is “great variability between individuals as to how susceptible they are to chromosomal aberrations and or mutations” (T1510.29–43); and
(3) Dr Flecknoe-Brown’s opinion that there is no safe level of exposure to mutagenic substances (at JRA [201]–[202]).
1100 It is convenient to summarise Mr McNickle’s contentions as to his primary case on dose before turning, to the extent necessary, the alternative dose proposition.
1101 Mr McNickle contends that the evidence adduced from Professor Smith, Dr Juberg and Dr Flecknoe-Brown establishes that there is no safe level of exposure to a genotoxic and/or mutagenic carcinogen.
1102 First, Professor Smith gave evidence that in the human population, there is no safe level of exposure to, and no safe dose of, genotoxic carcinogens (T1551.20–1552.6). A short time later (T1555.29–35) Professor Smith stated again, in reference to genotoxic carcinogens, that “there is no safe dose, and that’s generally accepted”. Dr Juberg disagreed and stated that he was “of a belief that thresholds do exist within the human population” (T1552.21). Mr McNickle submits that the Court should prefer Professor Smith’s opinion over Dr Juberg’s opinion on this issue because: (1) Dr Juberg’s opinion on this issue is difficult to reconcile with his evidence in relation to the variability between individuals within the human population as to how susceptible they are to chromosomal aberrations and or mutations; (2) Dr Juberg’s opinion is based on the views of Professor Calabrese which, Mr McNickle submits, for reasons referred to below, should be given no weight; and (3) Dr Juberg’s opinion on this issue is also inconsistent with the evidence of Dr Flecknoe-Brown.
1103 Secondly, Mr McNickle contends that the variability between individuals within the human population as to how susceptible they are to chromosomal aberrations and or mutations weighs in favour of a conclusion that there is no safe level of exposure to genotoxic carcinogens (T1551.20–1552.6 (Professor Smith); T1510.29–43 (Dr Juberg)). As a consequence of the variability between individuals within the human population as to how susceptible they are to mutations, some people might suffer from a mutation more easily than their neighbour, which can be due to genetic variability (T1510.38–43). Therefore, as Dr Juberg accepted, some people might cope with a large exposure to a genotoxic substance, whereas other people might end up with cancer if they just have a very small exposure to a carcinogenic substance (T1511.12–16).
1104 Thirdly, despite Mr McNickle not objecting to the representations made by Professor Calabrese in the articles tendered into evidence (or seeking a s 136 EA limitation in relation to representations contained in them), Dr Juberg relied on the views of Professor Calabrese in support of his opinion that “there are practical thresholds for carcinogens” (T1556.1–5). Mr McNickle submits that the Court should place no weight on the views expressed by Professor Calabrese. He was not called as a witness in the initial trial, and hence the views he expressed in Calabrese (2022) could not be explored or challenged. This is said to be particularly problematical because Professor Calabrese, in effect, acknowledges that he is an outlier in his criticisms of the linear non-threshold model, which he describes as the “sacrosanct dose response model used in cancer risk assessment for over half a century” and of “overwhelming pre-eminence” and which he states has, at its core, “the belief that a single carcinogenic molecule or a single ionization [sic] can initiate the complex process of carcinogenesis”.
1105 Fourthly, Dr Flecknoe-Brown’s evidence strengthens the evidence that there is no safe level of exposure to a mutagenic substance like glyphosate because, as he explained, “a mutation is a one off event” (at JRA [202]). Dr Flecknoe-Brown said (at JRA [201]) that “a mutation occurring due to exposure to a mutagen [such as GBFs] could directly cause the malignant change in the cell”, continuing (at JRA [202]):
Although a mutation is a one-off event, the more times that a person’s DNA is exposed to a mutagen, the more opportunities there are for such a one-off event to occur. In the case of glyphosate and GBFs, the amount of exposure to the mutagenic substance is determined by not only the number of occasions on which the person is exposed (intensity and duration of use), but also if any barriers to exposure to the GBF are used.
1106 It is said that Professor Prince did not contradict Dr Flecknoe-Brown’s opinions that “a mutation is a one off event”, “a mutation occurring due to exposure to a mutagen could directly cause the malignant change in the cell” or “[a]lthough a mutation is a one-off event, the more times that a person’s DNA is exposed to a mutagen, the more opportunities there are for such a one-off event to occur”. Mr McNickle submits that as a result, the Court should accept Dr Flecknoe-Brown’s evidence and his analysis, set out above, that there is no safe level of exposure to a mutagenic substance.
1107 Fifthly, Mr McNickle submits that evidence of the approach taken by the USEPA, or any other regulatory agency, as to linear extrapolation, or the application of safety factors or thresholds, in respect of genotoxic carcinogens, does not prove, one way or the other, whether there is, in fact, no safe level of exposure to genotoxic and or mutagenic carcinogens. Instead, such evidence can only prove, if accepted, that a regulator takes a certain approach in respect of a certain issue. The only evidence adduced in relation to the USEPA’s approach in this regard is said to be that given by Professor Smith and Dr Juberg. Professor Smith gave evidence (T1555.29–44):
PROFESSOR SMITH: So for genotoxic carcinogens you do not apply safety factors… So if the compound is genotoxic, then the US EPA and most of the regulatory agencies throughout the world draw a linear no threshold line to this, and then they – society sets a limit because there is no safe dose, and that’s generally accepted. So if you have no safe dose, how do you actually set a public health limit? …. You do not use safety factors. You do not say there’s a threshold. The US EPA has never, in my understanding, for a genotoxic carcinogen ever set a threshold or safety factors setting a standard for the human population.
1108 Dr Juberg agreed with Professor Smith’s conclusion stating that “I would agree with him [Professor Smith] that that’s how cancer risk is done” (T1556.5). Dr Juberg also gave evidence that he was not aware of the USEPA having ever set a threshold or safety factor for the human population in respect of a genotoxic carcinogen (T1556.12–16).
1109 Sixthly, contrary to Monsanto’s submission, Mr McNickle submits that it is not possible for the USEPA Guidelines to “disprove” that the USEPA has never set a threshold or safety factor for a genotoxic carcinogen. It is said that is not possible because the USEPA Guidelines can prove no more than their content. The USEPA Guidelines themselves cannot prove what the USEPA actually does in practice (as distinct from what the guidelines say). Mr McNickle acknowledges that the guidelines (at 3.3.1) refers to the possibility of using a non-linear approach in particular situations, rather than the default approach of linear extrapolation, but points out that it is clear from the evidence of Professor Smith and Dr Juberg that, in fact, in practice, the USEPA has never set a threshold, or used safety factors, in respect of a genotoxic carcinogen.
1110 To the extent it is necessary to form a concluded view, I am not satisfied that the evidence supports the proposition that genotoxic and/or mutagenic carcinogens are carcinogenic at any dose, for the following reasons.
1111 First, notwithstanding the evidence summarised above (at [1101]), the USEPA does not “draw a linear no threshold line” (T1555.29–44) in respect of all genotoxic and mutagenic substances. The USEPA defaults to the assumption that some substances are carcinogenic at any dose only in circumstances where the “observed data” relevant to the issue is insufficient, only to make precautionary public health decisions (and not to make any reliable scientific finding) and only because the assumption of carcinogenicity at any dose is “a health-protective approach”. Professor Smith accepted that the OECD and USEPA Guidelines are “the internationally accepted guidelines for the conduct of mechanistic toxicological carcinogenicity studies” (T1330.7–15). Dr Juberg also accepted under cross-examination that many regulatory bodies follow the OECD and USEPA Guidelines (T1461.18–24), and Professor Smith and Dr Juberg (at JRG [53]) jointly described the OECD and USEPA Guidelines as “two of the more recognised and cited sets of test guidelines” that “[m]any global regulatory bodies follow”. The USEPA Guidelines (at 3.3) provide:
3.3. EXTRAPOLATION TO LOWER DOSES
The purpose of low-dose extrapolation is to provide as much information as possible about risk in the range of doses below the observed data. The most versatile forms of low-dose extrapolation are dose-response models that characterize risk as a probability over a range of environmental exposure levels. These risk probabilities allow estimates of the risk reduction under different decision options and estimates of the risk remaining after an action is taken and provide the risk information needed for benefit-cost analyses of different decision options. … When the weight of evidence evaluation of all available data are insufficient to establish the mode of action for a tumor site and when scientifically plausible based on the available data, linear extrapolation is used as a default approach, because linear extrapolation generally is considered to be a health-protective approach.
1112 However, the USEPA considers it safe to infer that (as a matter of scientific fact, as compared with a matter of public health assumption) a carcinogen is carcinogenic at any dose only if: (1) “the MOA data” from the dose-response assessment for the carcinogenicity mode of action of a substance suggests that “the likely shape of the dose-response curve at lower doses” is linear; and (2) the observed “extent of inter-individual variation” supports that conclusion. As the USEPA Guidelines state (at 3.3.1):
3.3.1. Choosing an Extrapolation Approach
The approach for extrapolation below the observed data considers the understanding of the agent’s mode of action at each tumor site (see Section 2.4). Mode of action information can suggest the likely shape of the dose-response curve at lower doses. The extent of inter-individual variation is also considered, with greater variation spreading the response over a wider range of doses. Linear extrapolation should be used when there are MOA data to indicate that the dose-response curve is expected to have a linear component below the POD. Agents that are generally considered to be linear in this region include:
• agents that are DNA-reactive and have direct mutagenic activity, or
• agents for which human exposures or body burdens are high and near doses associated with key precursor events in the carcinogenic process, so that background exposures to this and other agents operating through a common mode of action are in the increasing, approximately linear, portion of the dose-response curve
1113 Although that passage indicates that substances that are carcinogenic by a mutagenic mode of action are “generally considered” (emphasis added) to be carcinogenic on a linear dose, the USEPA Guidelines provide that the issue of whether a mutagenic substance is carcinogenic at any dose is not to be assumed. Indeed, the issue depends on an analysis of the evidence derived from a dose-response assessment (at 3.3.1):
When the weight of evidence evaluation of all available data are insufficient to establish the mode of action for a tumor site and when scientifically plausible based on the available data, linear extrapolation is used as a default approach, because linear extrapolation generally is considered to be a health-protective approach. Nonlinear approaches generally should not be used in cases where the mode of action has not been ascertained. Where alternative approaches with significant biological support are available for the same tumor response and no scientific consensus favors a single approach, an assessment may present results based on more than one approach.
A nonlinear approach should be selected when there are sufficient data to ascertain the mode of action and conclude that it is not linear at low doses and the agent does not demonstrate mutagenic or other activity consistent with linearity at low doses. Special attention is important when the data support a nonlinear mode of action but there is also a suggestion of mutagenicity. Depending on the strength of the suggestion of mutagenicity, the assessment may justify a conclusion that mutagenicity is not operative at low doses and focus on a nonlinear approach, or alternatively, the assessment may use both linear and nonlinear approaches.
(Emphasis added)
1114 In the absence of a proper dose-response assessment on the evidence, it follows that the question of whether a genotoxic or mutagenic carcinogen is carcinogenic at any dose or at a threshold dose cannot be answered reliably (T2229.27–40).
1115 Secondly, I do not consider that inter-individual variability assists Mr McNickle’s primary case as to dose. The fact that that there is variability between individuals as to how susceptible they are to chromosomal aberrations and or mutations (T1510.29–43) is – of itself and absent any quantification of the range of variation – irrelevant to the issue of dose. As noted above (at [1114]), the fact of inter-individual variation does not ground the inference that a substance is carcinogenic at any dose. Instead, the data as to the “extent of inter-individual variation” is data that needs to be considered, when conducting a dose-response assessment, in order to determine whether a carcinogen is, as a matter of fact, carcinogenic at any dose or carcinogenic only at a threshold dose. No evidence is before me as to the extent of inter-individual variation by which glyphosate causes cancer in individuals, and that variation cannot, in my view, provide support for Mr McNickle’s contention that glyphosate is carcinogenic at any dose.
1116 Thirdly, largely for the reasons I identified earlier (at [695]–[696]), I do not consider that Dr Flecknoe-Brown’s evidence supports Mr McNickle’s contentions above. Dr Flecknoe-Brown does not cite any scientific inquiry upon which he based his assertions (at JRA [201]–[202]) and, accordingly, it is of limited assistance in determining Mr McNickle’s primary case as to dose.
1117 Fourthly, notwithstanding that Professor Smith characterised Professor Calabrese as “on the fringe of the community and widely considered to be a little bit crazy”, I do not resile from the view that there is no evidence before me corroborating that opinion and that I must assess Calabrese (2022) like any other article in evidence (T2232.22–26). If Mr McNickle wished to maintain a submission that the representations in Calabrese (2022) ought not to be before the Court, it was open for him to take a different course. The fact that Dr Juberg relied upon Calabrese (2022) in support of his evidence and Professor Smith’s criticisms of it were all matters apparent as early as 28 September 2023 – that is, well before the parties reached the joint position on the adduction of scientific articles (among other things) referred to above (at [63]–[65]). Mr McNickle could have contended that Calabrese (2022) should be treated somewhat differently, but submissions as to a lack of weight were not developed by reference to any cogent and detailed criticism of the article. As it happens, Calabrese (2022) suggests why it is not safe to assume that all mutagenic substances are carcinogenic at any dose:
5. Pharmacokinetic and pharmacodynamic factors and carcinogen dose response
Mutagen genetic damage is strongly dependent on and affected by the pharmacokinetic processes of absorption, distribution, metabolism, storage and excretion. These processes involve the interaction of component parts of the biological system with unique physico-chemical properties of the mutagenic molecules, which may include both the parent mutagen and/or possible mutagenic metabolites. The occurrence of a mutagenic event having relevance to a carcinogenic outcome will be affected by the following factors: (1) the number of mutagenic molecules that survive the pharmacokinetics and can reach the DNA of targeted cells, (2) the thermodynamic parameters that affect the activation and covalent binding of the mutagenic molecules to DNA, and (3) the biological capacity and rate of DNA repair in the target cells. While each of these factors alone tends to reduce the likelihood for any single mutagen molecule (the smallest dose possible) producing a mutational event, the combination of all three factors would only further greatly reduce the likelihood, virtually eliminating the possibility. To increase the probability of a mutational outcome, it would require continually increasing the number of mutagens (i.e., dose or level of exposure) until some threshold value is exceeded and the likelihood of evading these barrierlike factors is sufficient to register a mutational event. Essentially, this describes and lends rational support to a threshold dose-response model where some low-dose value must first be exceeded before a mutagenic effect can be observed.
(Emphasis added)
1118 Dr Juberg relied on Calabrese (2022) in support of his contentions as to dose (T1555.1–13; 1578.31–32) and given this line of reasoning was not the subject of challenge by Mr McNickle (and the views therein are not inherently improbable), I accept Monsanto’s submission that it is evidence weighing against Mr McNickle’s primary case on dose.
1119 For these reasons, to the extent it is necessary to form a view, I do not consider that the evidence establishes that genotoxic and/or mutagenic carcinogens are carcinogenic at any dose.
IV Alternative dose proposition
1120 Mr McNickle’s alternative dose proposition relies upon Dr Sawyer’s evidence as to cumulative exposure and exposure days. Dr Sawyer’s methodology as to cumulative exposure and exposure days is summarised in the executive summary to his primary report as follows (at 5):
Cumulative exposure duration is the only valid toxicological dose metric methodology for glyphosate exposures. The measurement method is based on 8- hour time-weighted “exposure-days” for comparison to threshold values in human epidemiological studies of applicators revealing statistically significant elevated NHL [ORs]. This is the only methodology that has been used to determine a measurement of exposure dose in the published in the peer-reviewed human studies. This methodology facilitates direct comparison of Mr. McNickle’s exposure history to that of the epidemiological studies. It is generally accepted that childhood exposures to mutagenic carcinogens results in a 2.8-fold increased risk of malignancies compared to that expected from adult-only exposures.
1121 Dr Sawyer recognised that a causation-based enquiry, whether general or specific, requires an assessment of systemic dose (at JRF [40]–[42]; T1977.16–35).
1122 I am far from satisfied Dr Sawyer’s evidence supports the alternative dose proposition, for the following reasons.
1123 First, Dr Sawyer conceded in cross-examination that: (1) his exposure threshold metric cut-off is not in any way based on results from the animal studies. He sought to justify this on the basis that, under the relevant rules of evidence in the United States, animal extrapolation is disallowed for causation purposes but then conceded that this is only in respect of specific causation (T2029.10–17); (2) his exposure threshold metric cut-off is similarly not based on any results from the mechanistic toxicology studies (T2029.19–21); (3) his exposure threshold metric cut-off is based solely on a selection of the epidemiology studies which demonstrate a positive association between glyphosate and NHL, the majority of which we have encountered in the first stream and in respect of which a number of errors and limitations were found (T2029.23–35); (4) he relies on exposure days as a metric to attempt to estimate the dose of glyphosate to which people were exposed, but that this does not measure systemic dose (T2029.37–42); (5) with the exception of Eriksson (2008), the exposure days in the epidemiology studies that he relied on did not measure the duration of exposure to glyphosate either and accepted that those studies in fact use a minimal exposure and include that in the exposure day calculation (T2032.17–24); (6) there exists a body of evidence which demonstrates that cancer risk at a cumulative dose varies substantially with the duration of exposure (T2031.14–16); (7) despite each individual being counted as having one exposure day, the dose that is relevant for each individual could be very different (T2033.10–12); and (8) that the epidemiological studies which assess the association between GBFs and NHL “do not measure dose” and that the studies use a dose metric frequency of exposure, expressed as “exposure days” (at JRF [134]).
1124 Secondly, Dr Sawyer justified the use of exposure days on the basis that the cumulative metric of “pack years” has some validity as a predictor of lung cancer risk (at JRF [160]):
It should be noted that epidemiological studies have used cumulative exposure dose metrics for many decades in other studies. The best example is that of cigarette smoking and lung cancer measured in units of "pack-years"; that is the (number of packs per day) x (years). For example, a person smoking 1 pack per day for 40 years has a 40 pack-year dose metric. The scientific literature reveals numerous cigarette smoking studies assessed with in units of pack-years. More recently, biomonitoring has employed nicotine and cotinine in units of mg/kg/day in urine or blood; however, biomonitoring is incapable of assessing previous years of smoking.
1125 In cross-examination, however, Dr Sawyer was taken to an article (Peto (2012)) and gave the following evidence (T2030.32–2031.6):
MR CRAIG: … Now, you will see the title of this journal article is That the Effects of Smoking Should Be Measured in Pack Years Misconceptions; do you see that?---Yes.
MR CRAIG: Above the title there is an editorial. Can you read that editorial to yourself starting with the word, “Misconceptions”?---It needs to be blown up a little. Well – no, it is blown up. Okay. Yes, I read that.
MR CRAIG: Thank you. I suggest to you that the use of pack years in the epidemiological literature is one of the misconceptions and ill-founded theories to which the editors refer in this editorial?---According to this editorial. However, pack years is what was originally used to determine cigarette smoking as a cause of cancer and there is a reason that sometimes we have to use a weaker methodology as opposed to a stronger methodology. For example, measuring benzoapyrene in the blood would not provide any information on how many years or at what doses smoking occurred. And the same goes true with glyphosate: measuring somebody that presents for NHL, there are no blood tests that are going to go back and historically document what the blood levels were 10, 20, 30 years earlier while working on the farm. I mean, it’s just – the same holds true for cigarette smoking. Measuring – if a smoker came into the office and I, as a toxicologist, was asked to perform analyses, there are no analyses I could perform that would give me a full history of this person’s smoking.
1126 In short, Dr Sawyer acknowledged that the use of “pack years” was a weaker methodology and accordingly, it is doubtful whether it can be used to enable the epidemiological studies relied upon by Dr Sawyer to provide a reliable exposure assessment.
1127 For these reasons, to the extent it is necessary to form a view, I am not satisfied that the evidence establishes that genotoxic and/or mutagenic carcinogens are carcinogenic at any dose, nor that the evidence establishes a threshold dose at which glyphosate and/or GBFs (assuming they are carcinogenic) cause cancer or NHL.
1128 In the light of my conclusion as to Dr Sawyer’s evidence in Section H.3 above, Mr McNickle no longer has much to gain from a Jones v Dunkel inference flowing in his favour because of Monsanto’s decision not to call Dr Driver to give evidence.
1129 It is necessary, however, to say something about it.
1130 Mr McNickle submits that because of Monsanto’s decision not to call Dr Driver: (1) the Court should draw an inference that Dr Driver’s evidence would not have assisted the Monsanto’s case; and (2) the Court should more readily accept the opinion evidence of Dr Sawyer on topics on which Dr Driver could have opined. Mr McNickle submits that those consequences should flow from the non-calling of Dr Driver because:
(1) Dr Driver is a witness which Monsanto could reasonably have been expected to call, given he provided the Driver Report to Freehills, and then, in December 2022, participated in Conclave F with Dr Sawyer and contributed to the JRF, in which Dr Driver and Dr Sawyer set out their opinions in relation to the questions agreed by the parties; and
(2) if Monsanto contends that some of the opinions expressed by Dr Sawyer are incorrect and should be rejected, Dr Driver should have been called to contradict Dr Sawyer and/or to explain any assertion to be made by Monsanto that Dr Sawyer’s opinions are incorrect.
1131 I accept there is some force in Mr McNickle’s submission that a Jones v Dunkel inference would ordinarily flow in circumstances where Dr Driver was in a position to contradict Dr Sawyer’s evidence, in particular, those matters set out above (at [1064]–[1066]). But I am not persuaded that the matters identified by Dr Sawyer and summarised in those paragraphs are strictly relevant to the contest as to general causation in this initial trial (that is, Question 4).
1132 As senior counsel for Monsanto pointed out, the only portions of Dr Sawyer’s evidence that remained unchallenged were those in respect of which it was unnecessary for Monsanto to adduce evidence. As I noted earlier (at [502]), Jones v Dunkel cannot be used to fill gaps in the evidence or to convert conjecture into inference. Even if I proceed on the basis that Dr Driver’s evidence would not have assisted Monsanto’s case and the Court could more readily accept Dr Sawyer, his evidence was so inherently problematical that I have not reached the requisite level of persuasion as to the cogency of his opinions given in Conclave F and as to dose.
I DRAWING THE THREADS TOGETHER
1133 We now come to the step required to be undertaken by a Court and which reflects the imperative that a judge (unlike a scientist) must decide, one way or another, whether the burden of proof is satisfied by the party positing a general causation hypothesis based upon scientific evidence.
1134 As Mr McNickle correctly submits, it would be an error of reasoning to reach the conclusion that just because each of the three streams of scientific evidence do not establish general causation that this axiomatically provides an answer to the general causation issue.
1135 As I have explained, I am required to direct myself by having regard to all the streams of scientific evidence and any other material admitted in ascertaining whether Mr McNickle’s onus has been discharged.
1136 I recently noted in Lehrmann v Network Ten Pty Limited [2024] FCA 369 (at [98]), assessing whether this burden has been discharged does not involve a simple estimate of probabilities; it requires ascertaining a subjective belief in a state of facts on the part of the tribunal of fact. Mr McNickle, being the party bearing the onus, will not succeed unless the whole of the evidence establishes a reasonable satisfaction on the preponderance of probabilities such as to sustain the relevant general causation issue; put another way, I am required to assess whether I have formed a subjective belief or actual persuasion of the existence of the necessary causal connexion. As Justice Hodgson put it, I am dealing with two questions: “not just what are the probabilities on the limited material which the court has, but also whether that limited material is an appropriate basis on which to reach a reasonable decision”: see Hodgson, D H “The Scales of Justice: Probability and Proof in Legal Fact-finding” (1995) 69 Australian Law Journal 731; Ho v Powell [2001] NSWCA 168; (2001) 51 NSWLR 572 (at 576 [14]–[16] per Hodgson JA).
1137 Bearing these matters in mind, the whole of the evidence in the three streams revealed some not insignificant issues with the evidence presented by Mr McNickle in favour of his contention that use of and/or exposure to glyphosate and/or GBFs can increase an individual’s risk of developing NHL or cause an individual to develop NHL. Indeed, as has been seen, much of that evidence was directed towards establishing plausible or theoretical possibilities and, as Monsanto was wont to emphasise throughout the initial trial, it is not enough to persuade the Court that glyphosate and/or GBFs might cause or increase the risk of NHL – actual persuasion necessitates more: namely, that the Court be reasonably satisfied that glyphosate and/or GBFs are capable of causing NHL in humans.
1138 It is partly for this reason that I earlier described the evidence adduced in the epidemiological stream as causing difficulty in accepting Mr McNickle’s case. Without placing disproportionate focus upon it, it is, after all, the stream of evidence before the Court that examines the actual occurrence of cancer in humans exposed to glyphosate and/or GBFs in the real world. This was recognised as long ago as February 2022 when Mr Clements accepted that one of the “critical areas” of expertise in which expert opinion evidence will be adduced in this case is in the field of epidemiology: McNickle v Huntsman Chemical Company Australia Pty Ltd (Hearing Vacation) (at [13]). In saying this, however, I am conscious that epidemiology may not establish a positive link between glyphosate and/or GBFs and NHL, but this does not entail that such a link does not exist, although it would have to be established by other evidence. In other words, epidemiology which establishes a positive link between agent and effect is neither a necessary nor sufficient condition for establishing causation: see Justice Jonathan Beach “Causation: The Interface Between the Scientific and Legal Methods” (at 142).
1139 But in the end, the evidence adduced in the first stream established (in order of importance) that: (1) the AHS represents the most comprehensive and reliable analysis to date of whether there is a causal relationship between glyphosate and/or GBFs and NHL because, inter alia: (a) in the light of the sheer number of study participants involved, the AHS is of a different character to any comparable study on the etiologic relationship between glyphosate and NHL; and (b) in contrast to population-based and community studies, the sample in the AHS consists of applicators who were occupationally exposed to GBFs and other agents thought to be carcinogenic; (2) the vast majority of the case-control papers: (a) involved a relatively small number of cases and controls who were exposed to glyphosate; and (b) suffered from the failure to correct for exposure to other pesticides (that is, confounding). It is for these reasons that the epidemiological literature does not support the conclusion that exposure to glyphosate and/or GBFs is capable of causing NHL in humans. It is well to emphasise again here that the AHS was not only described by Professor Checkoway as “excellent” (T952.14–15) and “perhaps the best study we have” (T959.44–45), but it is plain that in the light of its size and nature of exposure the study participants experienced, if glyphosate and/or GBFs were causative of NHL, one is faced with the reality that the relevant association was not observed.
1140 As for the animal studies stream, the evidence was insufficient to establish that: (1) glyphosate causes cancer in murine subjects; or (2) even if it does, that causation of cancer in rats and mice entails that it also causes cancer (and NHL) in humans with real world exposures. It will be recalled that: (1) Dr Bayard’s evidence was marred by an approach to the animal bioassays which: (a) did not meaningfully assist in estimating false-positive rates because it did not involve any adjustment to the p-value which accounted for the problem of multiple comparisons; (b) included pooled analyses in circumstances where there were a variety of substantive differences between the animal bioassays (such as different durations and dose-levels); and (c) involved Dr Bayard giving scientific opinions on fields outside of his expertise; (2) the animal bioassays (and Wang (2019)) suffered from several limitations including, among other things: (a) in relation to each tumour type in rats and mice, and perhaps most importantly, the majority of the studies did not indicate a statistically significant result according to Dr Bayard’s predetermined criteria for significance; (b) a number of the animal bioassays reported inconsistent results when assessing relative dose levels; (c) a number of the tumours identified by Dr Bayard are not rare in rats and mice; and (d) several of the animal bioassays lacked cross-gender significance; and (3) Dr Bayard’s reliance on Wang (2019) in the context of the JRI was not of assistance in demonstrating the translatability of the animal studies evidence to humans because: (a) Dr Bayard lacked the requisite qualifications to conclude that that study provided evidence that glyphosate provides a mode or mechanism of action by which it can cause cancer; and (b) the extrapolation of findings the subject of such models to humans is a question which necessitates “high translatability” and the application of a number of criteria, including, inter alia, the animal species, dose exposure, and similarities in biological response.
1141 Lastly, in relation to the mechanistic evidence stream, although the evidence established that glyphosate and/or GBFs exhibit KC2 (genotoxicity); KC4 (induces epigenetic changes); and KC5 (induces oxidative stress), the evidence did not support the “next step” that there exists a corresponding biologically plausible mechanism by which glyphosate and/or GBFs can cause NHL. This was particularly evident in relation to KC2 where genotoxicity was demonstrated in artificial in vitro environments, but it was not evident that that risk was translatable to the risk of humans developing NHL from exposure to glyphosate and/or GBFs in the real world. Further, it was apparent that Mr McNickle’s reliance upon the second hypothesised mechanism and Wang (2019) was misplaced in the light of a slew of limitations which rendered it of limited assistance in demonstrating whether an upregulation of AID contributes to the development of NHL in humans. Even in the event that the mechanistic evidence allowed a more definitive conclusion to be drawn as to any association between glyphosate and/or GBFs and the development of cancer, it is well to emphasise again that if there is inadequate evidence of cancer in humans and insufficient evidence in experimental animals, the mechanistic evidence and KCs can only suggest that the substance in question is “possibly carcinogenic to humans” (see above (at [671])).
1142 I am acutely conscious that during his opening, Mr Clements submitted (T34.24–31):
MR CLEMENTS: I mean, it’s clear from the approach [Monsanto has] taken to the three streams. So, as I said earlier, in the epidemiology conclaves, Professor Checkoway refers to four case controlled epidemiological studies that support the conclusion that exposure to GBFs increases NHL risk. What do the respondents say? All of the four studies have flaws. Two of them are not statistically significant. So it’s the same old approach: ignore the smoke, there’s no fire here. They do the same thing with animal studies. There are numerous studies in experimental animals which support the conclusion that glyphosate is carcinogenic in mammals.
1143 Monsanto’s drumbeat as to the results of the AHS in its submissions was potentially deafening. But consistently with Mr McNickle’s submissions, I have guarded against being distracted from the full sweep of the evidence by mistakenly elevating the epidemiology evidence (and, in particular, the AHS) as some form of necessary condition for establishing causation. More generally, I have sought to ensure I have not been diverted from weighing the whole of the evidence by focussing too intently on complaints about individual studies, which have ranged from the highly significant to the pettifogging. Mr Clements correctly urged me to view the evidence in the round and I have attempted to do so.
1144 In an 1898 article on British frontier policy in India, Churchill wrote:
I shall take refuge in Kinglake’s celebrated remark, that “a scrutiny so minute as to bring a subject under a false angle of vision is a poorer guide to a man’s judgment than the most rapid glance that sees things in their true proportions”.
1145 The difference here is that my consideration of the whole has been no mere rapid glance and I do not think Monsanto’s minute scrutiny of individual studies has meant I have obtained any false angle of vision.
1146 Stepping back and viewing the evidence as a whole, and faithfully giving weight to those aspects I consider most cogent, but without giving disproportionate emphasis to any particular stream, the state of the evidence is not such as to sustain the necessary causal connexion. This is not to say that the evidence suggestive of a causal link did not give me some pause, and I will say something shortly about the appropriate answer to the questions posed in this initial trial and the ongoing scientific controversy more generally. But the weight of the evidence adduced in the three streams made it difficult to come to any conclusion other than the applicant has not established his general factual causation case to the requisite legal standard.
1147 The questions posed for determination prior to the initial trial were in the following terms:
1. Throughout the period July 1976 until 4 July 2022 (the Relevant Period), were:
a. Glyphosate; and/or
b. Glyphosate based formulations (GBFs)–
carcinogenic to humans?
2. Throughout the Relevant Period, were:
a. Roundup Herbicide; and/or
b. Roundup Biactive–
carcinogenic to humans?
3. Throughout the Relevant Period, if Roundup Herbicide and/or Roundup Biactive contacted the skin, did any surfactants present increase absorption in the bloodstream by reason of the factors identified in [the fourth further amended statement of claim at [29]]?
4. Throughout the Relevant Period, did or could use of and/or exposure to:
a. Roundup Herbicide;
b. Roundup Biactive;
c. Glyphosate; and/or
d. GBFs–
i. increase an individual’s risk of developing NHL; and/or
ii. cause an individual to develop NHL?
5. If use of or exposure to Roundup Herbicide or Roundup Biactive can cause NHL in humans, would use in accordance with the Labels, Safety Data Sheets and Safety Directions detailed in paragraphs 52 to 60 of the Statement of Agreed Background Facts … amount to a use or exposure that is capable of causing NHL by reason of that use?
1148 I find myself at the conclusion of this initial trial in a similar position to that which I found myself in Lloyd v Belconnen Lakeview Pty Ltd [2019] FCA 2177; (2019) 377 ALR 234. I noted in that case (at 328–332 [374]–[385]) that in Gill v Ethicon Sàrl (No 3) [2019] FCA 587, I had earlier described s 33ZB of the FCA Act as being the most important provision within Pt IVA and noted (at [4]–[5]):
[4] … This provision provides that a judgment given in a representative proceeding must describe or otherwise identify the group members affected by it and binds all such persons other than any person who has opted-out of the proceeding under s 33J. This provision was described by the Full Court in Femcare Ltd v Bright [2000] FCA 512; (2000) 100 FCR 331 at 338 [25] (Black CJ, Sackville and Emmett JJ) as, in one sense, the “pivotal provision” in Pt IVA.
[5] In Timbercorp Finance Pty Ltd (in liquidation) v Collins [2016] HCA 44; (2016) 259 CLR 212…. French CJ, Kiefel, Keane and Nettle JJ explained at 235–236 [52]–[53] as follows:
Part 4A creates its own kind of statutory estoppel. Section 33ZB requires that a judgment in a group proceeding identify the group members affected by it and, subject to a provision not presently relevant, provides that that judgment “binds all persons who are such group members at the time the judgment is given”. In order to understand that to which the group members are bound, it is necessary to read s 33ZB in the context of Pt 4A as a whole and ss 33C(1) and 33H in particular. By that process it will be seen that group members are bound by the determination of the claims giving rise to the common questions. …
1149 The orders I made defining the issues for trial are commonly known as “Merck orders”.
1150 As I explained in Belconnen Lakeview, that description comes from the decision of the Full Court in Merck Sharp & Dohme (Australia) Pty Ltd v Peterson [2009] FCAFC 26; (2009) 355 ALR 20. Merck was an application for leave to appeal and appeal from the decision of the primary judge, Jessup J, who, consistently with the then prevailing orthodoxy, had proposed that following the conclusion of the initial trial, the Court would hear further argument and make common findings which (following reflection in s 33ZB orders) would be relevant to determining the individual cases of group members. The Full Court (Moore, Sundberg and Tracey JJ), however, adopted a different approach noting (at 22 [5]) that the difficulty was that the pleading raised questions of the liability of the respondent to all group members and not just to the applicant. Their Honours went on to observe (at 22–23 [6]–[7]):
[6] In our opinion it is desirable, if not necessary, to identify precisely what issues will be determined in the “trial” (and those that will not be determined) on the assumption, which the parties did not gainsay, that at the end of the “trial”, orders will be made which reflect the determination made by the trial judge on both questions of fact and law or mixed questions of fact and law. That the “trial” will result in a determination of Mr Peterson’s claim (personal to him), is a given. As Sackville J did in Courtney v Medtel Pty Ltd (2003) 126 FCR 219 (by making orders on 16 August 2002 posing a number of questions which were partly answered on 3 March 2003 [2003] FCA 129), an order can be made identifying Mr Peterson’s claim as a matter (in the sense of subject matter entailing questions of fact and law) to which the trial will be directed.
[7] Also, common issues can be determined. There is plainly a controversy about which issues are common. Merck disputes that the issues pleaded in Mr Peterson’s statement of claim are, in truth, common questions. However, it is tolerably clear that the scheme of Part IVA of the Federal Court Act is that whilst a proceeding continues as a representative proceeding, the Court should, in the ordinary course (at least in relation to proceedings involving a sizable group where liability may depend on each member’s individual circumstances), initially deal with issues that are common to all members of the representative group or a sub-group of that group. So much is apparent from ss 33Q and 33R. Indeed an important procedural step is contemplated by that latter section whereby an individual group member might, by direction, be permitted to appear in the proceeding for the purposes of determining an issue that relates only to the claims of that member. At the very least the Court would need to consider whether such a direction is made before determining an issue which was not a common issue and might be characterised as an issue that relates only to the claims of a particular member.
1151 I further remarked that Merck orders are useful in identifying what issues should, and are to be, determined, but like in so many instances with Pt IVA proceedings, care must be taken to avoid elevating specific decisions as to practice and procedure which may arise in one case as if they were determinative precepts and principles of universal application: see Regent Holdings Pty Ltd v State of Victoria [2012] VSCA 221; (2012) 36 VR 424 (at 429 [19] per Nettle, Redlich and Osborn JJA). In Belconnen Lakeview, I made clear (at 330 [377]) that a Merck order is not set in stone: it is an interlocutory order which may be adapted and changed as circumstances require and that it is not uncommon for deficiencies in the approach taken at an interlocutory stage to be recognised only during or at the conclusion of the initial trial.
1152 There are two issues that arise in relation to the questions identified in the Merck order: first, whether it is necessary or appropriate to answer them as initially framed; and secondly, the form of any answer to any appropriate question given my findings explained above.
1153 The central issue (which, in the end, has turned out determinative) can be seen reflected in the terms of Question 4. This is not an unexpected development and, as I explain below, informed my desire to have an initial trial on the question of general factual causation.
1154 At the close of evidence, I invited submissions as to whether I should necessarily answer any question other than a refined version of Question 4. I accept there may have been utility in answering all questions in the event that Mr McNickle had generally succeeded, but as Monsanto correctly submits, this initial trial is about the central issue and, as Mr McNickle accepts: “if the Court answers ‘no’ to all parts of [Question 4], it would follow that this proceeding would be dismissed” (AS [561]).
1155 The problem with Question 4 is that it is unnecessarily complicated and does not reflect the pleading. This is a case concerning Mr McNickle and other persons who have used or have been exposed, during an identified period, to Roundup Products within Australia. “Roundup Products” are defined in the pleading as the herbicide product or products, which contained glyphosate and were branded as “Roundup”, or which contained glyphosate and were otherwise branded with the name “Monsanto”.
1156 No-one during the trial has sought to draw any relevant distinction whatsoever between the two Roundup Products used by Mr McNickle (Roundup Herbicide or Roundup Biactive) and any other Roundup Product or, more generally (and subject to one qualification irrelevant for present purposes) any material distinction between any Roundup Product and any other GBF.
1157 The use of Roundup Products is a common thread linking all group members. Although the case was run without distinction between the carcinogenic properties of Roundup Products and other GBFs, the question posed and answered by the Court should be grounded in the common facts between group members and not stray into some hypothetical statement about any other GBFs (even if the answer would be the same on the evidence in this case).
1158 Despite written submissions contemplating some possible mixed response to Question 4 by Mr McNickle, this position was subsequently modified, and each party accepts Question 4 can be more simply expressed, and reflect the central issue, by being expressed as follows:
Throughout the period between July 1976 and 19 October 2020 (relevant period) [a period I will explain below], did or could use of and/or exposure to the herbicide product or products, which contained glyphosate and were branded as “Roundup”, or which contained glyphosate and were otherwise branded with the name “Monsanto” (Roundup Products) increase an individual’s risk of developing non-Hodgkin lymphoma (NHL); and/or cause an individual to develop NHL?
1159 Obviously enough, an answer, which amounts to a declaration, should be clear on its terms, and hence the question and answer should be self-contained and not adopt defined terms taken from another document. I will call this question the central common question.
1160 This is not the place to discuss the logical difficulties of a criterion of group membership which includes a causal element, which I have discussed before (see, for example, Perera v GetSwift Limited [2018] FCA 732; (2018) 263 FCR 1 (at 27–28 [79]–[81])). But adopting a realistic approach, when one has regard to the group definition (discussed below), by reason of the causal element introduced by the pleader (the group member must have been diagnosed with NHL by reason of the use of and/or exposure to Roundup Products), an adverse answer to the central common question is determinative of the claims of group members unless general causation is established.
1161 On 26 April 2023, an order was made pursuant to ss 33ZF and 37P(2) of the FCA Act that this proceeding be listed for an initial trial of the common questions specified in an annexure to that order (being those identified above (at [1147])). There is no need to modify that interlocutory order given its terms, but for reasons I have explained, the best approach in the light of the conduct of the trial and the conclusions I have reached, is to just answer the central common question.
1162 Having identified the central common question, it is now appropriate to identify the answer, and then the persons the answer should bind.
1163 The difficulty is that my resolution of the central issue is not captured by a simplistic binary response to the central common question. The answer to a separate question, such as the central common question, operates as a form of declaration of legal rights. As I have already explained, and as reflects the legal approach to proof of a fact, and that just because a moving party fails to persuade a Court to make a declaration in the terms it seeks, does not mean the opposing party is entitled to a declaration in opposite terms.
1164 If, as Monsanto asserts, I simply answer “no”, this would amount to a distortion of my resolution of the central issue according to law. This is because to say Mr McNickle has not proved to my reasonable satisfaction on the balance of probabilities on the state of the evidence adduced at this trial that the use of and/or exposure to Roundup Products increases an individual’s risk of developing NHL or causes an individual to develop NHL, is not the same thing as saying affirmatively that it does not. As I have explained, whatever else is unclear, one thing is plain: the science is all not one way. Even leaving aside evidence adduced on behalf of Mr McNickle:
(1) Professor Prince accepted there is some evidence that exposure to GBFs cause or contribute to the development of NHL (T631.36–40); further, he accepted “there’s aspects of what GBF can do which are known to be causative of lymphoma” (although asserting “there’s no compelling link to me to demonstrate that it causes lymphoma” (T631.46–632.2));
(2) AP Harris accepted it is “quite possible” that glyphosate use may be associated with increased risk of NHL (T1078.41–44); and
(3) Dr Juberg gave evidence (at JRH [154]) that “[b]ased on my review of available and accessible cancer bioassays for glyphosate in rats and mice … I conclude that none of these studies support a clear and compelling basis for carcinogenicity of glyphosate in mammals” (emphasis added).
1165 As Justice Beach remarked in his recent article (see above (at [69])), “at the forensic level in litigation, truth is static”, but the scientific method involves the positing of testable hypotheses based upon empirical observation and any theory formed, by its nature, is generally contestable (at 116, 120). It suggests hubris and defies commonsense to assume future scientific research and analysis could not mean more definitive conclusions may be able to be drawn using the scientific method, including buttressing the notion that Roundup Products are possible carcinogens. One cannot foreclose the possibility that further research may reveal a “compelling link” (a fortiori, a sufficiently persuasive link on the balance of probabilities) demonstrating that the use of and/or exposure to glyphosate and/or GBFs causes NHL. As I have been at pains to emphasise, I am deciding as to my reasonable satisfaction on the balance of probabilities based upon the evidence selected by the parties and which can only represent a snapshot of the state of scientific knowledge as at the time the evidence closed.
1166 Although any s 33ZB order would not operate in rem and would only relevantly bind Mr McNickle and group members specified in the order to the answer to the central common question, it is important the orders of the Court not distort the result of the forensic contest in this trial.
1167 Mr McNickle has brought this case as the representative for a finite group of persons. The parties now say that they are those persons who: (a) have been diagnosed with NHL by reason of the use of and/or exposure to, at any time between July 1976 and 4 July 2022, Roundup Products, within Australia; or (b) are the executors or administrators of, or beneficiaries of or persons with an interest in, the estates of deceased persons who would have been group members had they not died prior to 4 July 2022; or (c) the dependents of persons who would otherwise have been group members where a cause of action had vested in or may be brought by that person.
1168 The italicised terminal date of the now pleaded “Relevant Period” is alleged to be the date of the fourth further amended statement of claim. At the time leave was granted to amend, the parties (and, in particular, Mr McNickle who sought the amendment), did not seek leave to amend the group definition pursuant to s 33K of the FCA Act. For the reasons explained by the Full Court in Ethicon Sàrl v Gill [2018] FCAFC 137; (2018) 264 FCR 394 (Allsop CJ, Murphy and Lee JJ) this course should not have occurred. As was explained by the Full Court (at 401 [29]), Pt IVA puts in place an opt out regime. Irrespective of any positive step taken by a claimant, if at the time of an order which changes the group definition in a proceeding, they fall within the group definition as amended, they would become group members.
1169 The purported inclusion of claimants here (who may have had no knowledge about being purportedly joined to the class) is problematical. Opt out occurred pursuant to orders made on 28 May 2021 and, pursuant to s 33J(1) of the FCA Act, 20 July 2021 was fixed as the date by which group members could opt out of this class action.
1170 I raised this issue when it belatedly became apparent to me. My research suggests no s 33K order was sought by either party; nor was one made when the third further amended statement of claim was filed on 10 February 2022. Hence, the composition of group members at the time of opt out was that as defined in the second further amended statement of claim filed on 19 October 2020.
1171 My initial response, adopted by the parties, was to propose that I make a regularising s 33K order to operate nunc pro tunc from the date of the fourth amended statement of claim. But, upon reflection, I am not satisfied this is an appropriate course.
1172 As was explained in Ethicon Sàrl (at 406–407 [50]–[52]):
[50] By the amendment of a group definition, a new group member affected is not becoming a party, still less is the group member an existing party seeking to bring a new cause of action arising out of similar circumstances. The legal consequence is that the claim of a new group member, which claim gives rise to at least one substantial issue of law or fact with others, has become subject to the operation of the Part, subject to opt out or declassing. When one recognises that the regime expressly contemplates and provides for the individuality of claims within a group proceeding, what is brought into focus is that an order for amendment, which has the consequence of expanding the group definition, is sui generis and that analogies drawn from other contexts are apt to mislead.
[51] It is consistent with the scheme introduced by Part IVA and, in particular, the need for there to be certainty as to the persons who comprise the class at all times, that the Group Definition Amendment should have been ordered to take effect from the date of amendment…. To adopt that course is consistent with the expansion of the class effected by the earlier Amendment Orders which, unfortunately, were not drawn to the attention of the primary judge in the present context. Apart from anything else, this prevents the topsy turvy notion that someone retrospectively becomes a group member on commencement, when the Court has thus far proceeded on the basis that they are not group members. As a matter of principle, such an approach would avoid the vice of potentially resuscitating causes of action by persons who have never sought to agitate them. It would be odd that by becoming a group member through the augmentation of a class, substantive rights were conferred on a claimant that had been either extinguished or barred by operation of statute and could not otherwise be advanced by that claimant.
[52] Before leaving this topic it is worth making a further point. Given the suspension of limitations caused by the operation of s 33ZE, the question of inclusion (or, as occurred here, unusually, exclusion) of group members has potentially important consequences on substantive rights. As does the date when any such order is to take effect. Whatever might be the nature of other amendments to a statement of claim, or to relief claimed in an application, attention must be given by parties to the legal consequences of class composition changes. Irrespective as to when other amendments might take effect, the consequences of amendments to group definition can affect the rights of third parties, being the absent group members or proposed group members. Although it would be inappropriate to lay down inflexible rules, the default position is that a s 33K order (or an equivalent order under s 33ZF) has effect from the time the definition is changed, consistent with the requirements of class certainty. It is a sound practice for applicants, in seeking such orders, to deal separately with amendments concerning class composition to assist in avoiding any confusion.
1173 The failure to heed this warning has led to the current problem. No doubt I should have been more vigilant, but there is a limit as to how much time one can spend double-guessing and checking consent orders provided by experienced class action lawyers.
1174 Whatever the reason or reasons for this problem, the result is there are a group of persons who, subject to leave being granted to augment the class, could have become group members because they first fell within its literal terms after 19 October 2020 but prior to 4 July 2022. I will call these people potential group members.
1175 A belated s 33K order being now made which operates from 4 July 2022 in order to bind potential group members to a s 33ZB order would, of course, operate adversely to the interests of these third parties without them having had any opportunity to be heard. Moreover, I would be substantively affecting their legal rights, notwithstanding they have been deprived of the fundamental right in an opt out regime, being the making of an informed choice as to whether they wish to be part of this class action. There may have been a range of steps I could have taken at an earlier time to direct notices to potential group members if I had known of this problem in a timely way.
1176 I raised this issue with the parties when the matter was last before the Court in January 2024, but I remain unconvinced a retrospective order should be made which operates adversely to non-parties. If a statutory estoppel is to bind strangers, the strangers have to be group members and be dealt with fairly and in accordance with Pt IVA. Any s 33ZB order should be made binding the current group members as defined in the second further amended statement of claim filed on 19 October 2020 other than those that used the opportunity thereafter given to them to opt out.
I The Nature of the Discretion
1177 It continues to be said that the Court’s discretion as to awarding costs is “unfettered” but that is, at best, overly simplistic and, at worst, inaccurate.
1178 As the Full Court (Besanko, Jagot and Lee JJ) explained in LFDB v SM No 2 [2017] FCAFC 207 (at [7]):
… in exercising the discretion to award costs, s 37N(4) of the Act requires the Court to take account of any failure by a party to comply with the overarching purpose of the civil procedure provisions, namely to facilitate the just resolution of disputes according to law …
1179 Moreover, and more generally, any power to award costs must be exercised in a way “that promotes the overarching purpose” (s 37M(3)): Bellamy’s Australia Limited v Basil [2019] FCAFC 147; (2019) 372 ALR 638 (at 643 [24] per Murphy, Gleeson and Lee JJ).
1180 These mandatory statutory considerations inform the discretion, but so do a miscellany of principles emerging from the cases, for example: a costs order should reflect the degree of success attained; a successful party may be ordered to pay some costs in respect of unsuccessful aspects of the case; and that costs are compensatory in nature and not punitive: see, for example, Hockey v Fairfax Media Publications Pty Ltd (No 2) [2015] FCA 750 (at [37] per White J). Further, and importantly, although there is “no absolute rule”, one of the “general propositions” regarding an award of costs is that “the award is discretionary but generally that discretion is exercised in favour of the successful party”: Foots v Southern Cross Mine Management Pty Ltd [2007] HCA 56; (2007) 234 CLR 52 (at 62–63 [25] per Gleeson CJ, Gummow, Hayne and Crennan JJ).
1181 Apart from the obvious and weighty consideration that Monsanto has prevailed at the initial trial, there are three factors which bear upon the discretion as to making an award of costs, which have not been the subject of submissions.
II The Parties’ Conduct and the Overarching Purpose
1182 The first is the relevance of the conduct of this litigation by the parties. To adopt what I said in a defamation case (Kumova v Davison (No 2) [2023] FCA 1 (at [86])), Pt VB of the FCA Act does not contain empty rhetoric. There is a statutory duty requiring practitioners to assist their clients in facilitating the just resolution of disputes according to law and as quickly, inexpensively and efficiently as possible (s 37N(2)). This means that in conducting litigation and identifying issues, including separate issues which may be determinative, the parties, including a respondent, must be engaged in trying to ascertain ways to simplify and expedite the resolution of matters. This obligation is reflected in the Central Practice Note: National Court Framework and Case Management (CPN-1) (at [7.2]).
1183 This obligation at the initial stages of a proceeding to give real thought as to how a case might be most efficiently resolved is particularly important in large class actions, which demand and consume so many public resources. Any notion practitioners can just roll the arm over and conduct litigation and incur costs like they are in a time warp that has transported them back to a time when provisions such as Pt VB did not exist cannot persist.
1184 My preliminary view, subject to hearing further on this issue from the parties, is that despite the efficient and skilled way the initial trial was eventually conducted, it is open to conclude that both parties share responsibility for this case not proceeding to trial much earlier and with vastly less cost.
1185 The first time this matter came before me, as long ago as 1 May 2020, I raised with senior counsel for Monsanto my view that “it seems to me that whether or not the allegation [in] paragraphs 23, 24, 25 and 26 are made out or not made out [the paragraphs of the pleading alleging the carcinogenic properties of Roundup Products] is likely to be of central importance to the claim” (T55), and that it is a scientific matter, that is, the scientific evidence would inform the factual issue, that is, jury question raised on the pleading.
1186 I then requested the parties to think about a way these scientific (and potentially determinative) issues could be resolved by a special jury as efficiently as possible and foreshadowed listing argument on that question later that day. This was opposed, and I then said (T60):
HIS HONOUR: … there are two ways of doing it, … we could debate this at greater length, but I might as well indicate to the parties the various options now so they can go away and think about them and consider it. I’m anxious to try to hear this case and deliver a judgment in it by the latter part of next year, if at all possible. I think it’s important for the court that these sort of cases do not languish in the list for years, as they’ve been allowed to in various other matters, and it’s in the interests – if we’re doing more than paying lip service to the overarching purpose and trying to resolve these cases quickly, we’ve got to approach and manage these cases in a way which allows us to do that. I appreciate the force in what is said, that it would be appropriate to give some detailed consideration as to (1) whether or not a referee ought be appointed and, secondly, if a referee is to be appointed, should there be a series of what’s been described elsewhere as a subject matter referees or, alternatively, a referee who’s hearing from a series of subject matter experts? And the two courses since – I mean, when referees first came about, they originated in the second Judicature Bill in 1873. There were really two types of referees. There were official referees, and one of the reasons why in some court rules you had this legacy of special referees was the distinction between an official referee, who was actually an officer of the High Court, and you had special referees.
…
HIS HONOUR: And so there’s two types of referees, and you could either have a retired judge referee, like a Mr Lindgren or somebody else, who is dealing with the parties adducing – in a less formal and less costly way – expert material that they deploy and inquiring and reporting and preparing a report for the court. Now, that’s one mode of special jury, one mode of reference. The other is to have a series – if it’s a complex matter, to have a series of subject matter referees. Now, ordinarily it would be for that referee, and the usual orders that I make for reference, which I can provide to the parties, would be laws of evidence don’t apply, the legal representatives will have no involvement in relation to that. And the referee will be the one that makes such inquiries of experts and any other person as they see fit, and then provide a report to the client.
So it’s a very flexible arrangement which has a series of different ways that one can do it. And I don’t have any particular view save for the fact that in my experience at least – and I have been involved in both – subject matter referees generally are far cheaper, far more efficient, and actually do serve to engender the sort of cost savings and time savings than a judge hearing all these issues at a trial. The beauty of it also is the fact that it can happen well in advance of the trial. So there would be proceedings on the reference, I would hope, in the early part of next year after the reference process has been completed. Parties then have certainty of, if the report is adopted, fine, if the report is rejected, then there may need to be consideration given as to whether the case can proceed on the fixed date because it may be the parties would need to then seek to adduce some expert evidence.
Or alternatively, as you can see in the rules, there’s the ability for supplementary reports and the like to be prepared if that was thought appropriate. So I just thought I would indicate to you that there are a variety of different ways of doing this. You know, if this is a particular type of issue that simply is too complex and too difficult and inapt for the appointment of a referee, then we will proceed in the orthodox way. But I’ve said enough to make it clear that my view that these are the sort of scientific type issues which may very well be apt for the appointment of a referee.
1187 When the matter next came before me on 12 August 2020, when the spectre of scientific manipulation had been raised and the parties were united in their opposition to any reference process, I observed (T39):
HIS HONOUR: … common ground ha[s] broken out about the folly of the course that I had proposed… I thought it might – there might be – this is relevant for the exercise of discretion either by me or by a full court [as to a reference], you’ve suggested some alternatives, but there may be others. I mean, one of the complications about this case which is similar to the complications about the PFAS case is that – and this presented some challenges in PFAS but was able to be overcome – is that there are aspects of the questions which reflected a significant divide in the scientific community, and that is people were of particular camps.
And … we don’t want this like – a District Court personal injuries case in the 1980’s where there’s plaintiff’s doctors and defendant’s doctors, and one knows exactly what they’re going to say, and with all the unsatisfactory aspects of that. So even if we didn’t go down the reference course, it may be that at least one alternative is the idea of one or two court appointed experts together with perhaps an assessor. Anyway, I raise this because it – I accept the force of the submissions that have been made by the parties, and it’s something which does cause on pause, and you have to think through these things about what’s best for not only the parties and the Court, and the just determination of the issues.
But, I’m just not going to let this case proceed down the path where there is entrenched experts on one side, entrenched experts on the other, and I’m sit here with this tsunami of expert material having to make a determination of which I think, in some respects, courts are ill-equipped to do. Anyway, I say that apropos of nothing other than just to entreat you to start thinking about these issues even if the reference process isn’t to proceed, because I think those sorts of issues need to be thought through very carefully before we were to make decisions about processes for conclaves and the like.
1188 On 22 March 2021, when issues had been raised about the extent of discovery, upon which it became evident an enormous amount of money was being spent, a roundtable conference was held and attended by counsel, solicitors and the eDiscovery and technology mangers for the solicitors for the parties, during which I observed (T101):
HIS HONOUR: … I must say, when I see the limited relevance of these documents in the overall case, I can’t help getting the feeling that a vast amount of money is being spent in relation to this discovery process – like in a large number of other class actions, which is not consistent with the overarching purpose. This case is likely to be determined on – and which has been conceded before in response to my proposal we have a referee – the assessment of the expert evidence about the properties of… these products. It’s not going to be determined – I can see some issues in the case to which the relevance of Monsanto Australia – of the carcinogenic – of the alleged carcinogenic property – that character of these products to be relevant, but – arguably irrelevant, but it’s the tail – I just get the feeling it’s just the tail wagging the dog – that a vast amount of money is being spent…which is not directed to the primary issue about what these – what – whether or not these two products were toxic and carcinogenic.
1189 These entreaties again fell on deaf ears. After coming back to the need to conduct this litigation in accordance with the overarching purpose on several occasions in 2021 and 2022, by 9 December 2022 (and after having vacated an earlier hearing of the initial trial in February 2022) and perhaps revealing my exasperation that the resolution of the case had been made unnecessarily costly and complicated, the following exchange occurred (T20):
HIS HONOUR: The whole case comes down to whether or not this thing is carcinogenic. And I just want to entreat you again to think about ways that we could make this trial simpler.
MS SZYDZIK: We will do so.
HIS HONOUR: Yes. I know I was talked out of having a separate question about whether or not it’s carcinogenic. I can’t quite understand how I was talked out of that, but I was.
MR FINCH: ...
HIS HONOUR: … I decided it was a good idea after hearing submissions, but not I’ve seen the expert reports and I still can’t understand why that’s not a preferable course.
MS SZYDZIK: We maintain that it isn’t, but perhaps we don’t really want to re-enliven that.
HIS HONOUR: Well, either it’s me or somebody else that’s going to have to sit down at a trial in separate next year and decide whether or not this is – you win or you lose. And that’s going to turn on the question of carcinogenicity, it seems to me.
1190 After delivering an ex tempore judgment on an unrelated matter, later that day the following exchange occurred (T30):
HIS HONOUR: … Now, just remind me why – just remind me again – humour me by reminding me again as to why it’s still not sensible to deal with just the issues of carcinogenicity first?
MS SZYDZIK: Because it’s not as straightforward as that. Because it is an issue that is bound up with questions relating to the respondent’s knowledge and, as your Honour has also said, questions relating to the tainting conduct that has been set out in the reply.
HIS HONOUR: So peripheral this tainting business for reasons I’ve explained, I just – even more that I’ve seen when I’ve read the joint expert reports. It just does not seem to me – we have really real misgivings about this. I was talked out of a reference process. Having seen the joint reports, I don’t know why. This spectre of scientific fraud has been – which doesn’t seem to have anything to do with the way in which the experts have expressed their views. It only goes to the question of what the knowledge was of Monsanto at relevant times for the negligence case if you’re going to win on carcinogenicity anyway. And it can’t be determined. It’s fairyland to think that’s going to be determined if it seems to me it’s a safety defect point. So anyway, all I’m saying I will ask you to think about it again. And I will entertain – if anyone wanted to make an application again now that I’ve had the benefit of the expert reports and after I’ve seen the rejoinders, as to some other more efficient way of dealing with this which would not involve a judge of the court having to spend a vast, vast amount of its time on the balance of issues in the case if the view was formed that it’s not carcinogenic. And if a view is formed that it is carcinogenic, then order the parties to a mediation after that finding has been made. Then I will entertain it on 2 February.
But again, look, you may be able to convince me again that it’s inappropriate. It’s just that I’m finding it really hard to reconcile the amount of time that’s allocated to this when there seems to me a fairly confined question which is going to leave the parties, irrespective of which ways it goes, in a much better position. And if we then have to have a subsequent hearing, we have a subsequent hearing. But I know I have expressed these views before and it must be an element of frustration, but I don’t think we should just necessarily continue to going down the same path if the path becomes increasingly rocky and the alternative path seems to lead to the broad sunlit uplands of making life easier for the person deciding the case.
1191 Indeed, as it turned out, it was “as straightforward as that”. Finally, on 2 February 2023, senior counsel for Monsanto said (T3–4):
MR FINCH: On two occasions on 9 December, your Honour entreated the parties to give some thought about ways in which the hearing can be made more simple - - -
HIS HONOUR: I did.
MR FINCH: - - - and shorter, not to put too fine a point on it, with particular reference to the issue of the causation of NHL/cancer/carcinogenicity issue more generally. Can I be disarmingly frank about this. There’s a great deal of attraction in the debate that your Honour has instigated on a number of occasions about focusing on that. There are some difficulties, so far as we can tell for the moment, in having a traditional separate question concerning that, and this is an issue your Honour has heard about before, because our learned friends wish to, amongst other things, put to our expert witnesses that their conclusions might be different had they been aware of certain scientific misconduct as it affects some of the basal data. Scientific misconduct, of course, doesn’t affect the witnesses; it affects some of the data, some of the articles, if it be established.
I have some problems, if I can be forgiven for speaking in the first person, with forcing the applicants not to be able to do that, because they say, “This is something which we want to say which vitiates the respondent’s scientific case about causation.” And, with respect, it seems to us to be dangerous to ask your Honour to force them not to do that either. But there is another way forward which we want to float now…
1192 What was then suggested was an embryonic version of the trial plan eventually put in place. Unfortunately, those acting for Mr McNickle were not able to respond that day and said they required an adjournment, but this trial plan was eventually put in place. Why the parties resisted simplification of the initial trial and why a version of the final trial plan could not have been put in place years before is, on my present understanding of the material, difficult to fathom.
1193 To repeat, my instinctive view, raised at the beginning of the case, is that it was possible to separate out potentially determinative issues. Subject to hearing submissions, it might be thought the reasons given from time to time as to why a simplification or staging of issues did not occur at a much earlier stage did not have any substance. Although it might be said those acting for Mr McNickle steadfastly refused any simplification of the case whenever it was raised (except at the heel of the hunt), Monsanto was also responsible for facilitating and giving effect to the overarching purpose. Notwithstanding my unfeigned respect for all those involved and my gratitude to the solicitors and all counsel for conducting the final hearing with such skill and courtesy, I do not consider this case has been conducted optimally or even efficiently.
1194 As noted above, these large class actions involve the consumption not only of the private resources of the parties, but also public resources. Everyone is entitled to their day in court, but they are not entitled to another litigant’s day in court. The resisting parties also have a separate obligation to identify and then promote the optimal way of resolving the dispute consistent with their separate obligations under Pt VB of the FCA Act.
1195 Over and over in these large class actions, judges of the Court see huge process costs being expended which are ultimately wasted. As I have previously remarked, a respondent is entitled to spend as much money as it wants to defend litigation and, subject to ethical constraints, a solicitor is entitled to work as instructed, which may include doing work which could be later characterised as objectively unnecessary. But this does not mean that unnecessary costs should be recoverable against an unsuccessful litigant. A singular aspect of this case is that despite active case management and judicial intervention aimed towards constraining costs from the very outset, much more money has been spent on this litigation than in truth was necessary for a fair disposition of the issues – particularly relating to issues as to Monsanto’s knowledge.
1196 I wish to receive submissions on how these issues inform any order for costs.
III Public Interest Litigation
1197 The second factor is that in Turner v MyBudget Pty Limited (No 2) [2018] FCA 1509, I dealt with a costs application made in a class action by a successful respondent. After noting the submission that the “usual rule that costs follow the event” is somewhat of an oversimplification, I observed (at [8]–[14]):
[8] Although the fact that [the respondent] has been successful in resisting the claim by [the applicant] is a very powerful discretionary factor militating in favour of an award of costs, it is not necessarily determinative. While the discretion as to costs is a very broad one, it is restrained in the case of representative proceedings by the prohibition on making an award for costs against group members except in defined circumstances: see s 43(1A) of the [FCA Act]. Although this provision is not directly relevant in the case of costs sought against a representative party, it recognises the reality that the questions determined in representative proceedings have a public dimension which transcends ordinary inter partes litigation and the rights of the parties to the litigation inter se.
[9] The case brought by [the applicant] is an exemplar of the type of proceeding that the Australian Law Reform Commission had in mind when, in 1988, it first recommended the introduction of a class action regime in ALRC Report 46. Here, a very large number of persons were affected by a common issue of law (in this case being the proper construction of the Interest Provision). It would have made no sense whatsoever for any individual litigant to have commenced a proceeding seeking clarification of this issue given the very small amount of money at stake for any individual litigant. The benefit of a grouping of claims by a Part IVA proceeding could only be achieved in the event that one person was prepared to come forward and act as the representative applicant. Unless [the applicant] had been prepared to bring the claim… uncertainty would have continued to exist relating to the true effect of the Interest Provision. Although I have reached a view that the proper construction is as [the respondent] contended, the arguments advanced by [the applicant] were far from being unarguable and, if I may say so, were advanced in an efficient and comprehensive manner by Counsel for the applicant, and in a way which was consistent with the overarching purpose.
[10] This is a world away from a commercial class action, where the applicant is part of some form of common enterprise which seeks to use the Court’s processes not only for the vindication of the applicant’s personal claim but also as a means by which a managed investment scheme is seeking to derive a significant financial advantage for the participants in the scheme including a litigation funder.
[11] [The respondent] recognises the fact that a proceeding brought in the “public interest” may be a basis, at least in some circumstances, for not awarding against an unsuccessful applicant: see Ruddock v Vadarlis (No 2) [2001] FCA 1865; (2001) 115 FCR 229. It asserts, however, that there is no general principle that the usual “costs rule” should not apply if the subject matter of litigation is a matter of public importance. It also points to QANTAS Airways Limited v Cameron (No 3) (1996) 68 FCR 387, a case which involved the question as to whether an unsuccessful applicant in a class action should be ordered to pay costs. QANTAS Airways was a case brought to vindicate mixed public and private interests: see at 390. Lindgren and Lehane JJ considered that the litigation served the “public interest” to the extent that it elucidated the duty of care owed by international airlines to passengers in relation to environmental tobacco smoke and that this should be given some weight, but damages were claimed and it was “impossible to view the proceedings as having been brought and pursued purely in the public interest”: see 389-390. In those circumstances, the applicant was ordered to pay some costs.
[12] Similarly, it is submitted, [the applicant] sought monetary relief and hence “his case cannot be viewed as having been pursued purely in the public interest”.
[13] It may be accepted that this was not a “pure” public interest claim, but a striking feature of this case was that any claim of [the applicant] (or any group member) was very modest, yet the collective benefit was potentially large. Although the class action did seek to vindicate [the applicant’s] claim, it had a very significant benefit transcending the parties relative to the stake of the personal financial claim of the applicant. 24,222 others have obtained certainty as to their position. This number includes, it is safe to infer, many persons likely to have some financial vulnerability.
[14] Although I do take into account the important consideration that [the applicant’s] individual case has failed, he (together with his lawyers) has performed a valuable service for the benefit of others. In all the circumstances, while taking fully into account that the costs discretion is generally to be exercised in favour of the successful party, my view is that no order for costs should be made. Although on one level this might be thought to operate unfairly on [the respondent] which has incurred not insignificant costs, it must be recalled that it has now received the benefit of quelling the controversy relating to the operation of the Interest Provision not only in relation to [the applicant], but with regard to all group members. Costs applications are not determined solely by identifying who was responsible for the litigation, but by a broad assessment as to whether the order does occasion an injustice...
1198 Given the significant public interest in this litigation, the resolution of the central issue (at least for present purposes) transcends the interests of the parties. How this informs the discretion as to costs is also something in respect of which I seek assistance.
IV The Position of Mr McNickle
1199 Thirdly, and related to the last point, as I noted in the introduction, Mr McNickle is a man of modest means; is presently unwell; and has a wife to support. However strongly I empathise with the position Mr McNickle finds himself in, I am aware, in ordinary, inter partes litigation, that any discretion exercised in such a way so as to deprive a successful party of their costs must be exercised judicially and “according to rules of reason and justice, not according to private opinion … or even benevolence … or sympathy”: Williams v Lewer [1974] 2 NSWLR 91 (at 95 per Rath J); Oshlack v Richmond River Council (1998) 193 CLR 72 (at 81 per Gaudron and Gummow JJ).
1200 But this is not ordinary litigation, and he has relied on others to bring the claim and, despite being unwell, he has performed a signal service in acting as a representative applicant. Mr McNickle will no doubt be disappointed in the result, but his labours have not been in vain. His individual case has provided a means by which the evidence currently marshalled has been evaluated and assessed thus resolving the legal rights of a great many people. At the end of the day, any ordinary costs rules are subject to the ability of the Court to make further or other orders as required to achieve an overall just result: Lombard Insurance Co (Australia) Ltd v Pastro (1994) 175 LSJS 448; GEC Marconi Systems Pty Ltd v BHP Information Technology Pty Ltd [2003] FCA 688; Furber v Stacey [2005] NSWCA 242.
1201 In this last respect, I am conscious that Monsanto has made submissions as to why the Court “should not be concerned as to the ramifications” of making a costs order against Mr McNickle but, so far as I can see, those submissions go beyond any evidence that was adduced at the hearing and, subject to any “without prejudice” material relevant to costs that may be admissible because of s 131(2)(h) EA, the parties agreed that the entire “universe of material” to which I am to have regard in making any final orders has already been adduced and is in evidence (T2087.40–2088.11).
1202 If a costs order against Mr McNickle is pursued, subject to any application, I will allow for further evidence as to costs (restricted to material admissible because of the exception contained in s 131(2)(h) EA) and submissions to be made to address relevant considerations.
1203 For these reasons, I make the following orders and make the notation set out below:
1. Pursuant to s 33ZF of the Federal Court of Australia Act 1976 (Cth) (FCA Act) (and notwithstanding that no application has been made by the representative party in accordance with s 33K(1) of the FCA Act) leave be granted for the group membership to be amended nunc pro tunc so that it is defined as it is in the last version of the statement of claim filed prior to the fixing of the date for opt out (being the second further amended statement of claim filed on 19 October 2020).
2. Pursuant to ss 33ZF and 37P(2) of the FCA Act, the following question, common to the claim of the applicant and all group members (Central Common Question) be answered separately and before any other question or issue in the proceeding:
Throughout the period between July 1976 and 19 October 2020 (relevant period), did or could use of and/or exposure to the herbicide product or products, which contained glyphosate and were branded as “Roundup”, or which contained glyphosate and were otherwise branded with the name “Monsanto” (Roundup Products) increase an individual’s risk of developing non-Hodgkin lymphoma (NHL); and/or cause an individual to develop NHL?
3. The Central Common Question be answered as follows:
It is not proven in this proceeding on the balance of probabilities (in accordance with s 140(1) of the Evidence Act 1995 (Cth)), that throughout the relevant period, use of and/or exposure to Roundup Products increased an individual’s risk of developing NHL; and/or caused an individual to develop NHL.
4. The proceeding be dismissed upon finalisation of any issues relating to costs.
5. Any further evidence as to costs (as contemplated in the reasons (at [1202])) and any submissions as to costs be filed by 12 noon on 30 July 2024.
6. For the purposes of s 33ZB of the FCA Act, Orders 1 to 3 above affect (and hence bind) all current group members in the class action (being those named in the second further amended statement of claim who did not opt out).
7. The proceeding be adjourned for the parties to be heard on the appropriate costs orders in conformity with these reasons at 10:15am on 31 July 2024.
AND THE COURT NOTES THAT:
8. The leave granted after the date of opt out had passed to amend the statement of claim so as to file the third and fourth statement of claim did not include leave being granted on application made by the representative party to alter the description of the group pursuant to s 33K(1) of the FCA Act.
I certify that the preceding one thousand two-hundred and three (1203) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Lee. |
Associate:
Dated: 25 July 2024




