Federal Court of Australia
Elanco Australasia Pty Ltd v Abbey Laboratories Pty Ltd [2024] FCA 640
ORDERS
Appellant | ||
AND: | Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The parties confer and supply to the chambers of Justice Burley by 4pm on 20 June 2024 draft short minutes of order giving effect to these reasons and in so far as is necessary, a proposed timetable for further steps to be taken in the proceedings.
2. Insofar as the parties are unable to agree to the terms of the draft short minutes of order referred to in order 1, the areas of disagreement should be indicated in mark-up.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
[1] | |
[10] | |
[12] | |
[23] | |
[33] | |
[42] | |
[42] | |
[63] | |
[64] | |
[69] | |
[69] | |
[77] | |
[86] | |
[86] | |
[90] | |
[101] | |
[102] | |
[103] | |
[110] | |
[121] | |
6.3.5 The general approach to developing thiacloprid dosages in a pour-on | [122] |
[129] | |
[137] | |
[137] | |
[144] | |
[155] | |
[168] | |
[169] | |
[173] | |
[184] | |
[213] |
BURLEY J:
1 This is an appeal from two decisions of a delegate of the Commissioner of Patents who allowed an opposition to the grant of patent application No 2014280848 entitled “Ectoparasitic treatment method and composition”. The priority date of the patent application is 12 June 2013. The patent application relates to methods of treating biting lice on sheep and goats using the insecticide thiacloprid.
2 The applicant for the patent was initially Bayer Australia Ltd, which subsequently assigned it to Elanco Australasia Pty Ltd, the present appellant. The respondent is Abbey Laboratories Pty Ltd.
3 Abbey filed a notice of opposition under s 59 of the Patents Act 1990 (Cth) on 9 August 2018. It advanced its opposition to grant on the grounds of: lack of clear enough and complete enough disclosure; lack of support; lack of clarity; lack of novelty; lack of inventive step; and secret use. The delegate in her first decision allowed the opposition on the basis that: claims 1 – 10 lacked support pursuant to s 40(3) of the Patents Act; claims 1 and 4 – 11 lacked novelty in the light of the prior publication of a document entitled “Trade Advice Notice on thiacloprid in the Product Piranha Dip for Sheep, APVMA product number 63766”; and, claims 1 – 3, 6 and 7 lacked an inventive step in light of the Trade Advice Notice and the common general knowledge in the art; Abbey Laboratories Pty Ltd v Bayer Australia Ltd [2020] APO 25 at [236].
4 Elanco then amended the patent application. A second opposition hearing was then conducted at which Abbey argued that the amendments did not serve to overcome the ground of lack of inventive step in the light of the common general knowledge and the Trade Advice Notice. In her second decision, the delegate again allowed the opposition and refused the application; Abbey Laboratories Pty Ltd v Elanco Australasia Pty Ltd [2021] APO 30.
5 In its Amended Notice of Appeal, Elanco contends that the delegate erred in concluding in the first decision that claims 1 – 3, 6 and 7 of the original claims lacked an inventive step and that she erred by concluding in the second decision that claims 1 – 10 of the patent application as amended lacked an inventive step.
6 Despite the appeal being brought by Elanco, the effective moving party is Abbey. That is because the present proceeding is in the original jurisdiction of this Court and involves a hearing de novo on the grounds and evidence before the Court. As the opponent to the grant, Abbey bears the onus in relation to each ground of opposition raised. Section 60(3A) of the Patents Act provides that the Commissioner of Patents may refuse an application if satisfied, on the balance of probabilities, that a ground of opposition exists. That is the standard by which the present proceeding is to be judged.
7 Abbey filed a statement of several grounds upon which it contended that the patent application should not proceed to grant. As a result of admirable co-operation between the parties, they agreed that the sole issue now between them is whether or not the amended claims of the patent application involve an inventive step in accordance with s 7(3) of the Patents Act, in the light of the common general knowledge and the content of the Trade Advice Notice. They also agree that this can be determined by examination of claim 1 on the basis that none of the other claims will serve to confer an inventive step if claim 1 does not. The parties provided a statement of agreed common general knowledge as at the priority date.
8 Examination of the patent request and complete specification was requested on 6 December 2016. As a result, all questions of validity are to be determined under the Patents Act as amended by the Intellectual Property Laws Amendment (Raising the Bar) Act 2012 (Cth).
9 For the reasons set out below I have found that the appeal must be dismissed.
10 Abbey relies on the evidence of Chhaya Mahashabde, pharmaceutical formulator and Kim Agnew, a veterinarian. Elanco relies on the evidence of Guiseppe (Joe) Pippia, an analytical chemist who provides consultancy services to the animal and human pharmaceutical industries and Edward Whittem, a veterinary pharmacologist.
11 I consider that each of the experts was qualified to give evidence of assistance in understanding the patent application and consideration of the issues. Where questions or weight arise, I refer to these more specifically below.
12 Ms Mahashabe graduated from the University of Mumbai with a Bachelor of Pharmacy in 1988 and completed a Master of Pharmaceutical Sciences from the same university in 1990. From 1991 to 1997, she was a research and development executive at Lupin Laboratories Ltd, a generic pharmaceutical company. From 1997 until 1999, she was responsible for product development, including the identification of natural active ingredients, establishing safety and efficacy profiles of new ingredients for incorporation into wound and skin care products, at Johnson & Johnson, India. From 1999 until 2002, she was a research scientist at the Regional Technology Centre at Colgate Palmolive, India where she was responsible for conducting formulation development and stability studies in relation to personal care products. In 2002, she immigrated to New Zealand where, from April 2002 until March 2010, she held various roles, including in new formulation development at Nutralife Health & Fitness (now Vitaco Health Limited), a nutraceuticals company. From April 2010 until July 2013, Ms Mahashabde was a formulation chemist at Virbac Australia Pty Ltd, where she was responsible for formulation development and evaluation of various products for sheep, cattle and horses.
13 In July 2013, she was promoted to Head of Pharma Formulation and became responsible for the formulation development of all vet pharma projects at Virbac Australia. In September 2021, she was appointed to her current role as Head of Formulation and Analytical Chemistry in which she is responsible for formulation development of new formulations and reformulation of existing products, and managing a team, including a formulation scientist and analytical chemist.
14 Ms Mahashabde was instructed to provide her evidence in several stages. She was first provided with the agreed common general knowledge and then explained, as at June 2013, how she would keep up with developments in the methods for controlling lice in an animal such as sheep or goats, the products known to her for use in controlling ectoparasites on an animal and the steps she would take to develop a pour-on formulation for the treatment of ectoparasites on a sheep or goat. Included in the agreed common general knowledge that Ms Mahashabde was provided was the agreed dipping calculation to which I refer at [39] below.
15 In her second affidavit, Ms Mahashabde was asked to assume that she had been asked, as at the priority date, to develop a formulation for controlling lice on an animal such as sheep and goats and provided with the Trade Advice Notice. She was then asked to explain the types of formulations containing thiacloprid she would consider developing for controlling lice on such animals, if there were any formulations she would consider taking to the development stage, and whether any different steps would need to be taken to ensure persistent control of the lice (for at least two weeks). The annexures to her second affidavit include a letter of instruction by Bird & Bird, solicitors and a copy of the Trade Advice Notice.
16 In her third affidavit, she was asked to provide her comments on the patent application. In her fourth affidavit, she provided comments on certain matters raised in Mr Pippia’s affidavit.
17 Mr Pippia obtained a Bachelor of Applied Science from the Queensland University of Technology in 1991 and a Master of Science and Technology from the University of New South Wales in 2004. From 1991 to 2000 he was a senior analytical chemist in the Queensland Department of Primary Industries where he was responsible for various analytical techniques, including trace residue analysis of veterinary drugs and pesticides, such as gas chromatography, high performance liquid chromatography and liquid chromatography mass spectrometry.
18 From 2000 until 2004, he was a senior chemist at Virbac Australia, where he worked in a product development team. His role was to provide analytical expertise to formulation development and product registration activities.
19 From 2004 until 2011, he was a technical manager at Troy Laboratories (Australia) Pty Ltd, a manufacturer of animal health products. In that role, he managed product development, product quality, regulatory affairs and quality control laboratories. He and his team developed tablets and topical formulations for the treatment and prevention of ecto- and endo-parasites and various other topical, oral and parenteral liquids and suspensions.
20 Since 2012, Mr Pippia has provided consultancy services to the animal and human pharmaceutical industries as Managing Director of Pia Pharma. This role includes the provision of analytical services, formulation development, product development, good manufacturing practice consulting and regulatory consulting.
21 Mr Pippia was provided sequentially with each of Ms Mahashabde’s first, second and third affidavits and provided his comments on each in turn.
22 Ms Mahashabde and Mr Pippia joined in the preparation of a joint expert report (formulation JER) and gave evidence concurrently, during which they were cross-examined. I found that both presented as witnesses who used their best endeavours to assist the Court.
23 Dr Agnew obtained a Bachelor of Science in Clinical Biochemistry from Massey University in New Zealand in 1980 and a Bachelor of Veterinary Science from the same university in 1987. From 1987 until 1995, he practised as a vet specialising in dairy cattle, pigs and sheep.
24 In 1995, he became the Veterinary Technical Manager for Elanco Animal Health New Zealand where he was responsible for product development and registration of veterinary products. In the late 1990s, he was a member of a team tasked with the development of a pour-on, dip and aerosol formulation containing spinosad (a parasiticide) as its active ingredient, for the control of sheep lice and flies. He recalls that Elanco’s dip formulation was the first spinosad product that was commercially available, followed by its aerosol and pour-on products. He was responsible for conducting clinical studies and preparing regulatory submissions for those products.
25 In 2006, Dr Agnew moved to Australia and became the Research and Regulatory Manager for Elanco Animal Health Australia where he led a team that applied for and managed the registration of spinosad products. In 2009, he became Elanco’s Innovations Business Unit Manager which involved, amongst other things, leading the regulatory team responsible for compliance maintenance of product registrations, conducting studies to support line extensions and new product development and managing product development work.
26 From 2011 until 2013, Dr Agnew was the Emerging Markets Regional Regulatory, Research and Product Development Manager, where he led teams responsible for: compliance maintenance of all new product registrations and submissions; conducting studies to support line extensions and new product development; and working on product development projects. From 2013 until 2015, he was the Associate Director ANZ R&D Hub and was responsible for leading the initiation of a regional research and development hub with a team comprising clinical, regulatory and scientific technical expertise, developing team strategy and leading due diligence assessment of new technologies including novel sources of parasiticide actives.
27 From 2015 until 2020, he was the R&D Leader for Australia and New Zealand at Merial/Boehringer-Ingelheim.
28 Since 2020, Dr Agnew has been a Director of KAP Consulting Pty Ltd, APAC Managing Partner at Paul Dick and Associates and Principal Investigator at PharmAust Monepantel in the treatment of canine lymphoma.
29 Dr Agnew was asked to provide his evidence in several stages. In his first affidavit he was asked to explain his knowledge as at the priority date of products and methods of administration used for controlling ectoparasites on an animal and the qualifications and roles of a team tasked with developing a formulation for administration by pour-on, dip or backlining of an ectoparasiticide for use on a sheep or a goat. He stated that as at the priority date, knowledge of an active with a known method of administration would be useful in developing a composition with the same active but designed for a different form of administration. In his second affidavit, Dr Agnew was provided with a copy of the patent application and also the Trade Advice Notice and asked to provide his comments on the range or amount of active ingredient used in the formulation in the claims and comment on the relationship between that range and the amount of active identified in the Trade Advice Notice. In his third affidavit Dr Agnew responded to the affidavit of Professor Whittem.
30 Professor Whittem has since 2021 been a Professor at James Cook University in Townsville and Dean of the College of Public Health, Medical and Veterinary Sciences. He obtained a Bachelor of Veterinary Science from the University of Melbourne in 1980 and a Doctor of Philosophy in Veterinary Pharmacology from the University of Georgia, in the United States, in 1991. He also holds a Residency Certificate in Veterinary Clinical Toxicology from the University of Georgia and a Fellowship in Therapeutics and Chemotherapy from the Australian and New Zealand College of Veterinary Scientists. Professor Whittem held various academic positions from 1991 until 2000. From 2000 to 2001, he was a Research Program Manager – Discovery at Schering-Plough Animal Health. From 2001 to 2008, he was employed by Jurox Pty Ltd as Head of Research and Development and a member of the Jurox senior executive team. In that role, he supervised the development and registration of various new veterinary products including for sheep and cattle. Between 2008 and 2021, he held various roles at the University of Melbourne in the Faculty of Veterinary and Agricultural Sciences and on the Academic Board. From 2019 to 2022, he was President of the Veterinary Practitioners Registration Board of Victoria.
31 Professor Whittem gave evidence in the course of the opposition proceedings before the delegate and in the present proceedings annexed three declarations that he had provided then. He responded sequentially to the affidavits of Dr Agnew.
32 Dr Agnew and Professor Whittem collaborated in the production of a joint expert report (veterinarian JER) and gave oral evidence concurrently, during which they were each cross-examined. I found that both presented as witnesses who used their best endeavours to assist the Court.
3. AGREED COMMON GENERAL KNOWLEDGE POSITION STATEMENT
33 The following is a summary of the agreed common general knowledge as at 12 June 2013.
34 The most prevalent species of lice to affect sheep in Australia is biting louse, Bovicola ovis. A range of chemical parasiticides were available for the treatment and prevention of sheep lice, such as insect growth regulators, spinosyns and macrocyclic lactones. Neonicotinoids is a class of insecticides that were known.
35 External application methods to administer pharmaceutically effective amounts of compositions to an animal such as sheep and goats included:
(a) dipping, such as by plunge, shower or cage dip;
(b) jetting; and
(c) topical administration, where the medicament is applied to only part of the outside of an animal, such as backlining. Treatment by backlining is recommended to be used within a day (or at most a week) of shearing and involves small volumes of product being applied along the backs of the animal from head to rump.
36 Administration of a lower concentration of active ingredient was generally known to be desirable, including to reduce cost and reduce residue on an animal, provided the active remains efficacious at a lower concentration.
37 In administering chemical parasiticides to an animal, such as a sheep or goat, by dipping or spraying, it is essential that the fleece be thoroughly wetted, regardless of the method being used.
38 When administration is via a dip:
(a) for stripping actives, the concentration of active decreases during use, and the dip liquid must be reinforced (addition of product) and topped up (combined product and water) to keep the dip at an effective level and strength as monitored during the treatment of the animals;
(b) for non-stripping actives, the concentration remaining in the dip liquid stays constant and the dipping liquid is topped up to maintain the desired volume of liquid as monitored during the treatment of the animals.
39 For dipping, the amount of an active ingredient that is applied to a sheep in mg/kg body weight can be calculated knowing the volume (mg/L) administered and the weight of the animal. For example, at 48ppm, assuming between 2L and 6L of formulation is “take-off”, this equates to an application of:
Sheep weight (kg) | Active applied (mg/kg) |
20 | 4.8 – 14.4 |
30 | 3.2 – 9.6 |
40 | 2.4 – 7.2 |
50 | 1.92 – 5.76 |
60 | 1.6 – 4.8 |
I refer to this paragraph below as the agreed dipping calculation.
40 As Ms Mahashabde explained, a sheep retains a volume of dip from a bath when it is treated with a dip product. This can be used to estimate the amount of active with which the sheep is treated by reference to the “take off” from the dip. In the agreed dipping calculation, the assumed take off is between 2L and 6L of formulation for which the application amounts may be determined as set out in the table.
41 Dosage determination studies, dosage confirmation and field trials are conducted to determine the appropriate dosage range in which a compound for treating lice on sheep is efficacious, including for determining the appropriate dosage range to provide persistent control.
42 The patent application is entitled “Ectoparasitic treatment method and composition”.
43 The field of the invention relates to an ectoparasitic treatment method and composition particularly relating to the treatment of biting lice on sheep.
44 The description of the “Background Art” identifies that ectoparasites on animals are of significant concern and that a particular problem for farmers in Australia is parasite infestation on sheep. It identifies that lice and blowflies are the two most significant external parasite problems that affect sheep and that lice are generally species specific. The most prevalent and significant type of sheep lice is the biting louse, Bovicola (formerly Damalinia) ovis.
45 The background refers to the harm caused by sheep lice to the wool industry and to sheep, noting that the control of lice requires effective chemical treatment of an entire flock and subsequent biosecurity measures to prevent re-infestation. It also refers to the impacts on animal welfare and production due to sheep lice and notes that although most lice are observed in the fleece, away from the skin surface, they are quite mobile and are likely to feed on substrates other than just the loose debris present at that location. Lice are noted to be transmitted between sheep via direct contact.
46 The specification continues (page 3 lines 5 – 8):
External administration of ectoparasiticides to sheep is usually most effective when applied immediately after shearing (off-shears). In particular, treatment by backlining either by pour-on or spray on is recommended to be applied within 24 hours of shearing, although within 7 days may be suitable for some products.
(Emphasis added)
47 The amended claims focus entirely on backlining (or “pour-on”), which is distinguished from other methods of administration.
48 The specification identifies the advantages of using smaller quantities of parasiticides (page 3 lines 20 – 27):
The administration of any chemical parasiticide will to some degree result in residue on the animal. If a smaller quantity of parasiticide can be used, the resultant residues may also be lower. For high volume applications such as dipping or jetting, residues in the environment may also be reduced by using lower concentrations of suitable actives. However, the actives must maintain efficacy at lower concentration to be suitable. The use of lower quantities of actives is also desirable to lower cost and/or potentially decrease safety issues. Lower residues are desired in that they may provide shorter withhold times, avoid export limitations, and increase market acceptability.
49 The specification then lists common types of parasiticides, the last of which is the neonicotinoid, imidacloprid. The claims in suit concern the use of a different neonicotinoid, which is thiacloprid. It says (page 3 line 29 – page 4 line 30):
In Australia the common types of externally administered chemical parasiticides suitable for the treatment and/or prevention of sheep lice include insect growth regulators (IGRs), such as triflumuron; spinosyns, such as spinosad; macrocyclic lactones, such as ivermectin; magnesium fluorosilicate; organophosphates; synthetic pyrethroids; and the neonicotinoid imidacloprid.
IGRs do not affect adult lice, resulting in a delay of up to 14 weeks for the developing stages to be killed and for the adult lice to die off. There have also been reports of emerging resistance to IGRs in Australia.
Spinosyns have low toxicity and provide rapid knock-down, but have a short duration of action.
Macrocyclic lactones also provide rapid knock-down, but are required to be administered in relatively large quantities to be effective.
Organophosphates are under increased scrutiny in regard to safety. In Australia the use of diazinon, the most commonly used organophosphate for control of sheep lice, has been discontinued since May 2007.
Synthetic pyrethroids can take 6-8 weeks to kill lice, which may lead to further spreading of lice across a flock. Significant resistance has also occurred with synthetic pyrethroids, being reported since the 1980s. The resistance has progressed to the extent that the use of synthetic pyrethroids in many locations may no longer be effective in the control of sheep lice.
In Australia, imidacloprid is the active ingredient in the parasiticide product Avenge™, sold by Bayer Australia Ltd, for the control of lice on sheep. Avenge™ contains imidacloprid at a concentration of 3.5% w/v, and is applied as a backline treatment. When used for lice control on sheep, Avenge™ provides up to four weeks of persistent activity when applied within 24 hours following shearing, which can prevent reinfestation by lice.
50 The specification identifies an objective of the invention as follows (page 5 lines 2 – 8):
There is a clear need for alternative methods for controlling ectoparasites such as sheep lice. Ideally such methods would use an active with high potency against lice to allow for relatively low quantities and concentrations of actives during use, be suitable for both off-shears (short wool) and long wool treatments, and have high persistency.
It is an object of the present invention to address on or more of the foregoing problems and/or to at least provide the public with a useful choice.
51 The “Disclosure of the Invention” then provides (emphasis added) (page 6 lines 1 – 7):
The invention is based in part on the surprising discovery that thiacloprid has high potency and suitability for the control of lice, when applied externally to an animal, thiacloprid is as shown in the chemical formula:
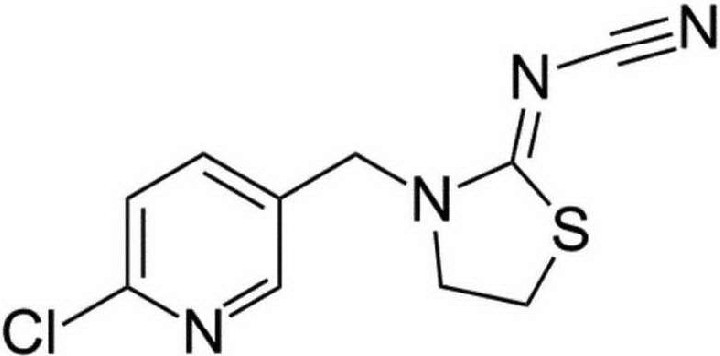
Thiacloprid: [3-[(6-Chloro-3-pyridinyl)methyl]-2-thiazolidinylidene] cyanamide is an insecticide of the neonicotinoid class.
52 The specification then provides a list of 28 items to which the invention is said to relate. The first three of these may be said to comprise the broadest statements of the invention (page 6 lines 10 – 15):
1 A method of controlling lice on an animal, characterised by the step of administering externally a pharmaceutically effective amount of thiacloprid.
2 The method of item 1, characterised by the step of topically administering a composition containing from 0.0001% to 3.5% w/v of thiacloprid.
3 The method of item 1 or 2, wherein the amount of thiacloprid administered to the animal is from 1 to 45 mg/kg by weight of the animal.
53 The invention is then described by 10 consistory paragraphs which match the language of the claims.
54 Under the heading “Modes for Carrying out the Invention” the specification states that the use of the invention will be particularly described in relation to the control of biting lice on sheep. A series of aspects of the invention are set out at pages 9 to 12 before the specification sets out a series of preferred methods of administration. Most of the aspects concern methods that were included within the scope of the claims of the patent application before they were narrowed by the amendments after opposition. Those included treatment by dipping and spraying.
55 The first preferred method is dipping, a “traditional form” of which is said to be plunge dipping, where sheep are mustered through a deep trough containing the treatment liquid and the liquid is allowed to penetrate into the fleece. An alternative identified is “shower dipping” where the sheep are sprayed with the treatment liquid. The second preferred method, “jetting” is then described.
56 More relevantly, the specification describes “backlining”, a further preferred method, as follows (page 13 line 26 – page 14 line 2):
Backlining
The backlining technique, otherwise referred to as pour-on or spray-on, is the application of a relatively small amount of liquid to back of an animal. The liquid may be applied as a spray or a stream or jet of liquid, using manual applicators or power assisted applicators. The volume, concentration viscosity and other properties of the treatment liquid may vary depending on the requirements. Typically a pour-on is administered in two or more bands along either side of the spine of the sheep.
57 The specification then says that the inventors have surprisingly discovered that thiacloprid has unexpectedly high efficacy in the control of lice on sheep. It says that one particular advantage provided by the invention is the long persistency following administration, for example, “when thiacloprid is administered as a plunge-dip and/or pour-on, four weeks of persistency has been measured” (page 14 lines 8 – 9). It continues (page 14 line 11 – page 15 line 2):
There is limited understanding in the prior art of the usefulness of thiacloprid in controlling parasites on animals.
The superior potency of thiacloprid compared to imidacloprid in the control of lice on sheep is a surprising result. The dose of imidacloprid recommended for the product AVENGE™ for the control of lice on sheep is around 49 mg/kg of the body weight of the sheep. By comparison, thiacloprid has been demonstrated as effective when used as a pour-on treatment at a dose as low as 5 mg/kg thiacloprid.
In one embodiment, the present invention provides a method of controlling lice on sheep, characterised by the step of administering externally thiacloprid in an amount of from 5 to 40 mg/kg by weight of the treated animal, wherein the thiacloprid is administered by localised topical application to the back of the animal. In one embodiment, the thiacloprid is administered as a pour-on. In one embodiment, the thiacloprid is administered as one or more bands along either side of the spine of the animal.
In one embodiment of the invention, the high potency of thiacloprid is utilised by the provision of a 1% w/v composition of thiacloprid for use as a backline treatment for lice control on sheep. By comparison, the backline imidacloprid formulation Avenge™, uses the substantially higher concentration of 3.5% w/v.
58 The specification identifies how it is said that the invention achieves the earlier stated objectives (page 15 lines 4 – 8):
This significantly higher potency allows for lower concentrations and/or quantities of thiacloprid to be used compared to similar parasiticides such as imidacloprid. It can be seen that the present invention has a number of advantages over the prior art, including lower residues both on the animal and the environment, lower costs for manufacture, ease of manufacture, and a lower chance of lice developing resistance.
59 Under the heading “Examples”, the specification provides that table 1 is a formulation example of the present invention, noting that the formulation provided may be used, in particular, as a concentrate that is diluted with water for use in dipping. Typically, it says, this would be diluted to around 48 ppm for such use. The detail is then set out (page 15 lines 15 – 30).:
Table 1: Formulation of thiacloprid concentrate
Formulation 1 | ||
Ingredients | Proportion | |
Thiacloprid | 48% | Active |
Polysorbate 80 | 10-15% | Surfactant |
Plasdone K25 | 2.0-5.0% | Dispersing |
Xanthan gum | 0.1- 0.3% | Thickening |
Wacker silica | 0.5- 1.5 | Thickening / dispersing |
Methyl paraben | 0.2 | Preservative |
Propyl paraben | 0.04 | Preservative |
Defoamer RD | 0-0.1 | Anti foam |
Propylene glycol | 10- 20% | Anti-freeze |
Deionised water | Qs to 100% | |
60 Table 2 is an example composition of the invention with particular suitability for use in a backlining treatment, such as a pour-on (page 16 lines 1 – 5):
Table 2: Formulation of thiacloprid pour-on
Formulation 2 | ||
Ingredients | Proportion | |
Thiacloprid | 1.0% | Active |
DMA (Dimethyl acetamide) | 40% | Solvent |
BHT | 0.2% | Antioxidant |
Dipropylene glycol methyl ether | Qs to 100% | Co-solvent |
61 The specification then sets out 11 ‘trial examples’. Examples 1 – 3 concern jetting. Examples 4 – 7 and 9 concern plunge dipping. Example 8 concerns in vitro assessment by means of an adult louse bioassay.
62 Example 10 concerns backlining and is of immediate relevance for present purposes as it is the only example that concerns backlining, which is the subject of the claims. It provides (page 18 lines 9 – 19):
Trial Example 10
A study was performed using 10 g/L thiacloprid pour-on formulation, on four groups of sheep at different dose rates including a negative control group, as summarised in table 3. The results of the study demonstrated that thiacloprid 10 g/L pour-on effectively controlled a natural infestation of sheep lice when applied at a dose level as low as 0.5 ml per kg bodyweight (approximately 200 mg thiacloprid per square metre of sheep body surface area). At each assessment time, including 22 weeks after treatment, there was an absence of live lice on all three groups.
Table 3: Trial of 1% w/v thiacloprid pour-on
Group | Treatment | Volume administered | Nominal Dose level | Number of Sheep | Assessment times |
1 | Thiacloprid 10 g/L Pour-On | 1.5 ml/kg | 600 mg/m2 | 8 | Pre-shearing, 4, 8, 12 and 22 weeks post treatment |
2 | Thiacloprid 10 g/L Pour-On | 1.0 ml/kg | 400 mg/m2 | 7 | |
3 | Thiacloprid 10 g/L Pour-On | 0.5 ml/kg | 200 mg/m2 | 8 | |
4 | Negative controls | Nil | Nil | 7 |
63 The claims are as follows:
1. A method of controlling lice on an animal, characterised by the step of administering by backlining a pharmaceutically effective amount of thiacloprid, wherein the amount of thiacloprid administered to the animal is from 10 to 30 mg/kg by weight of the animal, wherein the thiacloprid is administered in a composition containing 0.5%-2% w/v of thiacloprid, and wherein the animal is a sheep or a goat.
2. The method of claim 1, wherein the composition contains 1% w/v of thiacloprid.
3. The method of claim 1 or 2, wherein the amount of thiacloprid administered to the animal is from 10 to 15 mg/kg by weight of the animal.
4. A method of providing persistent control of lice on an animal, characterised by the step of administering by backlining a pharmaceutically effective amount of thiacloprid, wherein the amount of thiacloprid administered to the animal is from 10 to 30 mg/kg by weight of the animal, wherein the thiacloprid is administered in a composition containing 0.5%-2% w/v of thiacloprid, and wherein the animal is a sheep or a goat.
5. The method of claim 4, wherein persistent control is provided for at least two weeks following administration.
6. Use of thiacloprid in the manufacture of a composition for controlling lice on an animal, wherein the amount of thiacloprid administered to the animal is from 10 to 30 mg/kg by weight of the animal, wherein the composition is administered by backlining to the animal, wherein the composition contains 0.5%-2% w/v of thiacloprid, and wherein the animal is a sheep or a goat.
7. The method of any one of claims 1 to 5, or the use of claim 6, wherein the animal is a sheep.
8. A method of controlling lice on an animal, characterised by the step of topically administering a pharmaceutically effective amount of thiacloprid, wherein the amount of thiacloprid administered to the animal is from 10 to 30 mg/kg by weight of the animal, wherein the thiacloprid is administered in a composition containing 0.5%-2% w/v of thiacloprid, and wherein the animal is a sheep or a goat.
9. A method of providing persistent control of lice on an animal, characterised by the step of topically administering a pharmaceutically effective amount of thiacloprid, wherein the amount of thiacloprid administered to the animal is from 10 to 30 mg/kg by weight of the animal, wherein the thiacloprid is administered in a composition containing 0.5%-2% w/v of thiacloprid, and wherein the animal is a sheep or a goat.
10. Use of thiacloprid in the manufacture of a composition for controlling lice on an animal, wherein the amount of thiacloprid administered to the animal is from 10 to 30 mg/kg by weight of the animal, wherein the composition is administered topically to the animal, wherein the composition contains 0.5%-2% w/v of thiacloprid, and wherein the animal is a sheep or a goat.
4.3 The person skilled in the art
64 There is no dispute that the person skilled in the art in respect of the claimed invention would be a skilled team including: a formulation chemist; an analytical chemist; a clinical veterinarian or animal scientist; and a statistician. I accept that each of these people will have a high degree of skill.
65 The veterinarian experts agreed that their role would be to conduct studies concerning food safety, efficacy and animal safety required for registration in Australia, and that their expertise is useful to have in the team early in the conception of a product. The role of the statistician would be to ensure the correct design of studies and then process the data obtained.
66 Ms Mahashabde gave evidence that the formulation chemist decides the concentration of the formulation for the initial dose determination studies or the proof of concept studies and then has input in how much needs to go onto the animal, in consultation with the other members of the team. Mr Pippia considered that the formulation chemist does not determine the dose and it is the wider team that determines the dose and runs a dose determination study.
67 I accept that the formulator, in consultation with the other members of the team and most relevantly the clinical veterinarian, would propose starting doses for the determination of dose amounts. That accords with the fact that it is the formulator who would need to take into account the means of administration of the active ingredient to the animal in question. After the range is determined, the formulator would proceed to formulate the product.
68 Ultimately, in my view the contribution of the analytical chemist and statistician relates to implementing and checking data based on tests conceived and directed by the clinical veterinarian or animal scientist and the formulation chemist. Accordingly, it is the evidence of the veterinarian and formulation chemists that is of most relevance to the inventive step enquiry; Wellcome Foundation Ltd v VR Laboratories (Aust) Pty Ltd [1981] HCA 12; (1981) 148 CLR 262 at 281.
5. INVENTIVE STEP – THE SUBMISSIONS
5.1 The case advanced by Abbey
69 Abbey advances its lack of inventive step case on the basis of the state of the common general knowledge when considered together with the Trade Advice Notice, a document that is accepted by the parties to contain information that falls within s 7(3)(a) of the Patents Act.
70 Abbey submits that proof of concept studies formed an early part of dose determination and that they were of a routine nature as at the priority date, with the process of dose determination being well-defined and varying little between companies and product types.
71 Abbey submits that a range of known chemical parasiticides, including pour-on formulations, were available for the treatment and prevention of sheep lice before the priority date, that the veterinarian experts agreed that the amount of active ingredient used in each was set out on the product label and that information on those labels formed part of the common general knowledge. The AVENGE product was a known and successful product used in a pour-on and was considered by the formulation experts to be a useful reference product in developing a pour-on composition for thiacloprid. Abbey submits that the contents of the AVENGE product label fall within the common general knowledge and indicate that a 60mL volume of AVENGE applied to a 50kg sheep equates to a dose of imidacloprid of about 42 mg/kg. Imidacloprid and thiacloprid are both in the neonicotinoid class of insecticides. They have the same parent compound (the differences are in the side chains and the addition of one sulphur in thiacloprid) and their chemical structures formed part of the common general knowledge. They would have been considered as a matter of course and were readily available on resources such as PubChem.
72 Abbey contends, and the formulation experts agreed, that three basic propositions as to the development would form part of the common general knowledge. First, in determining the required amount of active for a pour-on product, it is ideal to keep the volume of dose that goes on the animal as constant as possible. Secondly, 60mL is an appropriate volume for a pour-on product. Thirdly, if one uses a fixed volume such as 60mL and a desired mg/kg has been set, then the calculation of the weight to volume concentration necessarily follows from those parameters.
73 Abbey submits that developing a pour-on product when there is an existing dipping product for the same active was known to have taken place for three of the six available dip products in the market. In such development, the administration of a lower concentration of active is generally known to be desirable, including to reduce cost and reduce residue on the animal. In estimating the amount of active to be used for a pour-on formulation when there is an existing dip or spray product, allowance must be made for the different method of application as set out in the agreed dipping calculation.
74 Abbey relies on the content of the Trade Advice Notice as providing the basis for the skilled team to move forward with a process of development. Abbey submits that in view of the Trade Advice Notice and the common general knowledge, arriving at the invention claimed before the priority date would have involved the following:
(a) Selecting the pour-on administration method for thiacloprid;
(b) Identifying a dosage or dosage range based on mg/kg;
(c) Identifying a concentration range in w/v %.
75 Abbey submits that the skilled team would have taken steps very close to those described in Example 10 of the patent application. It submits that one approach to the obviousness enquiry is whether “the hypothetical addressee faced with the same problem would have taken as a matter of routine whatever steps might have led from the prior art to the invention, whether they be the steps of the inventor or not”, citing Wellcome Foundation at 287. It submits in the alternative that the reformulated Cripps question, as set out in Aktiebolaget Hässle v Alphapharm Pty Limited [2002] HCA 59; (2002) 212 CLR 411 (AB Hässle) at [52] – [53], might be considered. Abbey would pose the reformulated Cripps question in the present case as “whether the person skilled in the art would have been directly led as a matter of course to try dosages within the claimed dosage range/concentration in the expectation that this might well produce a better or useful alternative to the existing sheep lice treatments or some other useful result”.
76 Abbey submits that the evidence supports the contention that the person skilled in the art would have had the requisite expectation of success.
77 Elanco disputes the case advanced by Abbey on several levels. It challenges the contention that the common general knowledge includes: thiacloprid and its chemical structure; the solvents used in AVENGE; and product labels used on other products in the market.
78 Elanco submits that the Trade Advice Notice does not teach the use of thiacloprid for use in a composition for a pour-on product nor the amounts to be administered or concentrations in any such product. Although the Trade Advice Notice provides a potential candidate for investigation, it is only the first step in a series of stages in the developmental process. Early stages also include determining the solubility and chemical structure of thiacloprid. Neither Ms Mahashabde nor Dr Agnew considered those matters in their affidavit evidence. Both are critical to the development pathway. Elanco submits that Professor Whittem gave evidence of the importance of chemical structure and the differences between imidacloprid and thiacloprid which meant that the person skilled in the art could not assume from information about imidacloprid that thiacloprid would work in a similar way. This meant that there could be no real expectation of success in the developmental pathway proposed by Abbey.
79 Elanco submits that the claimed dose and concentration ranges are narrow and although the evidence shows that the hypothetical team could have developed a pour-on formulation using thiacloprid, the skilled team could have no expectation of success in setting out to formulate thiacloprid as a pour-on product, citing, amongst other authorities, Apotex Pty Ltd v ICOS Corporation (No 3) [2018] FCA 1204; (2018) 135 IPR 13 at [354] – [358] (Besanko J).
80 Elanco submits that the approach taken by Abbey to the starting dosage of an hypothetical development process involves assumptions as to the amount of take-off in dipping, converting information from the Trade Advice Notice to devise a starting dosage for hypothetical testing of a pour-on formulation and then continuing on an assumption that every step of the theoretical development process identified would be successful. However, the evidence undermined the correctness of that assumption.
81 Elanco submits that Abbey’s case is essentially that, given the disclosure in the Trade Advice Notice of the use of thiacloprid in a plunge dipping formulation, provided that sufficient dose determination studies were undertaken, the skilled team would eventually arrive at a formulation and dose for a pour-on product containing thiacloprid in the concentration and dosage ranges claimed. However, it is apparent that as a “starting point” the dose that is proposed is a dose that would be tested in a preliminary dose determination study.
82 Elanco submits that Ms Mahashabde’s evidence is flawed for several reasons:
(a) First, her process of determining the solubility of thiacloprid in known solvents used in pour-on formulations, which is said to be a crucial step, relies on documents which Elanco contends did not form part of the common general knowledge as at the priority date.
(b) Secondly, her approach to calculating a starting point for a dose of thiacloprid in a pour-on formulation is based on impermissible assumptions. Ms Mahashabde estimates the dose required in a dipping formulation by reference to the amount of thiacloprid administered in the dip disclosed in the Trade Advice Notice. Her calculation involves what Elanco submits is an impermissible assumption as to the amount of take-off (being the amount that remains on a sheep after it is dipped). Ms Mahashabde arrives at an estimate of 4.8mg/kg and then converts that estimated dipping dose into a potential dose for a pour-on formulation in an impermissible manner. Despite acknowledging that it is not technically possible to correlate the dose of a dip formulation to a pour-on formulation, she doubles the dipping dose to arrive at a starting dose for a pour-on, on the basis that pour-on formulations are more concentrated and the safety information in the Trade Advice Notice discloses that residue studies were carried out at the double dose amount.
(c) Thirdly, she calculates the concentration of her hypothetical formulation based on the volume used for the AVENGE product (being 60mL for a 50kg sheep). This again has difficulties, given that AVENGE uses the separate active ingredient imidacloprid and the AVENGE product label was not part of the common general knowledge as at the priority date. Elanco notes that concentration will be heavily dependent on the solubility of thiacloprid, and that Ms Mahashabde’s evidence concerning the ultimate concentration of a formulation of a pour-on product involves assumptions as to the degree of solubility of that active, in comparison to imidacloprid.
83 In relation to the evidence of Dr Agnew, Elanco notes that he made no attempt to calculate the dose of thiacloprid but gives evidence in relation to two alternative pathways to a starting point. The first is to calculate a minimum effective dose for thiacloprid in a pour-on. This is not an approach that Professor Whittem would take (though the experts agreed this was for commercial reasons). Elanco criticises the approach taken by Abbey to this evidence, because: (a) it is based on the proposition that first, a “routine” minimum effective dose study would be conducted; (b) it would yield the same results as Trial Example 10 in the patent application; and (c) by this means, the dosage amounts and concentrations claimed were obvious.
84 The second is to calculate an effective dose by reference to the AVENGE product label, which Elanco submits was not common general knowledge. In any event, Abbey contends that this approach would not be adopted because of the structural differences between imidacloprid and thiacloprid but in any event, the dosing range for AVENGE is around 38 to 79 mg/kg, depending on the weight of the sheep, with the average dose being around 49 mg/kg. That is significantly higher than the range of 10 to 30 mg/kg claimed in the patent application.
85 Elanco submits that none of the hypothetical pathways proposed by Abbey would have the chance of success that the authorities require, emphasising the decision of Besanko J in ICOS Corporation at [338] – [356], and the authorities cited therein.
86 It is necessary first to consider the question of inventive step by reference to the steps that Abbey contends the hypothetical skilled worker – here a team – would have taken from the common general knowledge, with the addition of the Trade Advice Notice, to the invention claimed.
87 The invention as claimed is a method of controlling lice on sheep or goats by administering by backlining a pharmaceutically effective amount of thiacloprid where the amount administered is in a dose of 10 to 30 mg/kg by weight of the animal and the concentration of thiacloprid in the composition is 0.5% to 2.0% w/v.
88 Elanco accepts that the claims are concerned with thiacloprid used in a particular dosage and concentration range. It also accepts that no aspect of the inventive step lies in the mechanics of the formulation. That concession is properly made. The disclosure of the invention identifies no aspect of the formulation of any composition. The disclosure is of an invention of alternative methods for controlling ectoparasites to achieve high potency and high persistency by any means of external administration of thiacloprid. The “surprising discovery” identified in the section of the specification entitled “Disclosure of the Invention” is not of any particular type of formulation but of the finding that thiacloprid has high potency and is suitable for the control of lice when applied externally to an animal. The broadest statements of the invention, quoted at [52] above, concern the step of administering a pharmaceutically effective amount of thiacloprid externally. That invention is then narrowed to the topical administration of a composition containing “0.0001% to 3.5% w/v of thiacloprid”. The examples and preferred embodiments of the invention disclosed in the specification traverse administration by backlining, dip treatment or jetting treatment.
89 The delegate found in the first decision that the problem addressed by the specification is the need for alternative methods for controlling lice using actives of high potency and high persistency that allow for the use of relatively low quantities of actives for short and long wool treatments. In my respectful view, that summary accords with the disclosure of the specification. The claims, however, have been narrowed (after they were found by the delegate to be too broad and lacking an inventive step) such that the characterisation of the invention must be considered in light of their final form. Here, as I have noted, they are for a dose of thiacloprid in a particular mg/kg range with a concentration in a particular range. The invention does not concern formulation, but the identification of dosage amounts for use in the method of backlining, which is referred to in the evidence as a “pour-on formulation”.
90 By s 7(2) of the Patents Act an hypothetical person skilled in the art, notionally possessed with the common general knowledge as it existed before the priority date, must find the invention to be obvious, whether or not the common general knowledge is supplemented by prior art information within s 7(3): AstraZeneca AB v Apotex Pty Ltd [2015] HCA 30; (2015) 257 CLR 356 (AstraZeneca (HC)) at [18] (per French CJ).
91 The law concerning the requirement for an inventive step reflects the balance of policy considerations in patent law of encouraging and rewarding inventors without impeding advances and improvements by skilled, non-inventive persons: Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (No 2) [2007] HCA 21; (2007) 235 CLR 173 (Lockwood No 2) at [48] (Gummow, Hayne, Callinan, Heydon and Crennan JJ). The cases over the years have made a number of statements as to what is required to answer the jury question of whether or not an invention is obvious. It is a question of fact. The question is not what is obvious to a court but depends on analysis of the invention as claimed having regard to the state of the common general knowledge, any information relied upon for the purpose of s 7(3), and the approach taken to it by the person skilled in the art: Lockwood No 2 at [51].
92 As a basic premise, the question is always “is the step taken over the prior art an ‘obvious step’ or an ‘inventive step’?”. This is often an issue borne out by the evidence of the experts: Lockwood No 2 at [52]. Whilst the question remains one for the courts to determine, the courts do so by reference to the available evidence, including that of persons who might be representative of the skilled person in the art: AstraZeneca (HC) at [70] (Kiefel J, as the former Chief Justice then was).
93 Various formulations of the question have been set out in the cases. In R D Werner & Co Inc v Bailey Aluminium Products Pty Ltd [1989] FCA 57; (1989) 25 FCR 565 at 574, Lockhart J said that there must be “some difficulty overcome, some barrier crossed”.
94 A “scintilla of invention” is sufficient to support the validity of a patent: AB Hässle at [48] (per Gleeson CJ, Gaudron, Gummow and Hayne JJ), although as I have noted previously, this proposition may be seen to be somewhat circular, because the requirement of the qualitative assessment remains that the scintilla so identified must be inventive.
95 In Allsop Inc v Bintang Ltd [1989] FCA 428; (1989) 15 IPR 686 at 701 the Full Court (Bowen CJ, Beaumont and Burchett JJ) noted that for the invention to be inventive, it must be “beyond the skill of the calling”.
96 In AstraZeneca (HC), French CJ noted at [15] that relevant content was given to the word “obvious” by Aickin J in Wellcome Foundation at 286, where Aickin J posed the test:
whether the hypothetical addressee faced with the same problem would have taken as a matter of routine whatever steps might have led from the prior art to the invention, whether they be the steps of the inventor or not.
97 At [15], French CJ (with whom Gageler and Keane JJ and Nettle J agreed) explained:
The idea of steps taken “as a matter of routine” did not, as was pointed out in AB Hässle, include “a course of action which was complex and detailed, as well as laborious, with a good deal of trial and error, with dead ends and the retracing of steps”. The question posed in AB Hässle was whether, in relation to a particular patent, putative experiments, leading from the relevant prior art base to the invention as claimed, are part of the inventive step claimed or are “of a routine character” to be tried “as a matter of course”. That way of approaching the matter was said to have an affinity with the question posed by Graham J in Olin Mathieson Chemical Corporation v Biorex Laboratories Ltd. The question, stripped of references specific to the case before Graham J, can be framed as follows:
“Would the notional research group at the relevant date, in all the circumstances, which include a knowledge of all the relevant prior art and of the facts of the nature and success of [the existing compound], directly be led as a matter of course to try [the claimed inventive step] in the expectation that it might well produce a useful alternative to or better drug than [the existing compound]?”
That question does not import, as a criterion of obviousness, that the inventive step claimed would be perceived by the hypothetical addressee as “worth a try” or “obvious to try”. As was said in AB Hässle, the adoption of a criterion of validity expressed in those terms begs the question presented by the statute.
(Citations committed and square brackets in original)
98 The approach proposed by Graham J in Olin Mathieson Chemical Corporation v Biorex Laboratories Ltd [1970] 1 WLUK 202; [1970] RPC 157 to which French CJ refers is often referred to as the “modified Cripps question”. The application of the modified Cripps question was the subject of consideration in Generic Health Pty Ltd v Bayer Pharma Aktiengesellschaft [2014] FCAFC 73; (2014) 222 FCR 336, where the Full Court said at [71] (Besanko, Middleton and Nicholas JJ):
We do not think that the plurality in [AB Hässle] were saying that the reformulated Cripps question was the test to be applied in every case. Rather, it is a formulation of the test which will be of assistance in cases, particularly those of a similar nature to [AB Hässle]. The plurality did not reject as an alternative expression of the test the question whether experiments were of a routine character to be tried as a matter of course (The Wellcome Foundation Limited v VR Laboratories (Aust) Proprietary Limited (1981) 148 CLR 262, at 280-281, 286, per Aickin J). We do not think there is a divide here in terms of whether an expectation of success is relevant between a test which refers to routine steps to be tried as a matter of course and the reformulated Cripps question. It is difficult to think of a case where an expectation that an experiment might well succeed is not implicit in the characterisation of steps as routine and to be tried as a matter of course. On the other hand, we think a test formulated in terms of worthwhile to try was firmly rejected by the High Court in [AB Hässle] (see also [Pfizer Overseas Pharmaceuticals v Eli Lilly and Co (2005) 225 ALR 416], at 476, [287], per French and Lindgren JJ). The fact (if it be the fact) that the position in the United States may have shifted does not affect the binding nature of what the plurality said in [AB Hässle].
(Emphasis added)
99 In Nichia Corporation v Arrow Electronics Australia Pty Ltd [2019] FCAFC 2; (2019) 175 IPR 187, the Full Court (Jagot J, with Besanko and Nicholas JJ agreeing) picked up on the emphasised passage above in concluding that, in finding that there were “a number of unknowns” and that the patentee “did not know” that a combination would produce a satisfactory result within the claim, the primary judge strayed from “the test of steps taken in an expectation that they might well produce the invention or a useful result towards a test of an expectation of knowing that steps will produce a useful result based on predictive capacity” (at [88] – [89]). The relevant test is merely expecting that the steps may well work, rather than knowing that steps will or would or even may well work (at [99]).
100 In relation to having multiple avenues to try, in Nichia the Full Court adopted as orthodox the statement of Laddie J in Brugger v Medic-Aid Ltd [1996] 6 WLUK 122; [1996] RPC 635 at 661:
…if a particular route is an obvious one to take or try, it is not rendered any less obvious from a technical point of view merely because there are a number, and perhaps a large number, of other obvious routes as well. If a number of obvious routes exist it is more or less inevitable that a skilled worker will try some before others. The order in which he chooses to try them may depend on factors such as the ease and speed with which they can be tried, the availability of testing equipment, the costs involved and the commercial interests of his employer. There is no rule of law or logic which says that only the option which is likely to be tried first or second is to be treated as obvious for the purpose of patent legislation.
6.3 Findings of common general knowledge
101 I have in section 3 above identified aspects of the agreed common general knowledge. Set out below are my findings in respect of other aspects of the common general knowledge relied upon by Abbey in support of its obviousness case.
6.3.1 Backlining is a preferred method
102 Backlining with small volumes of product being applied to the back of the sheep was the most preferred form of administration for treatment of ectoparasites on animals at the priority date because it was an easier and more efficient method of administration and was in many ways more convenient than dips.
103 During development, the investigation of a dose or dosage range of an active ingredient sufficient to kill a target parasite was routinely undertaken by conducting dose studies, which were a standard part of product development. This would involve the team selecting several bench scale formulations to take into proof of concept studies, where efficacy and animal safety data could be obtained.
104 Proof of concept studies involve in-vivo studies which are conducted at (sheep) pen scale typically involving multiple groups of 3 – 6 sheep, each with one of the selected formulations.
105 In the veterinarian JER, the experts agree that proof of concept studies are typically conducted on more than one test formulation that may not be the final one for commercialisation. Proof of concept studies use a wider dose range than would be used in the dose determination studies, with an aim that the dose range cover both effective and ineffective doses. There is a dispute between the parties as to the focus of proof of concept studies, and in particular whether they are used to identify an estimated minimum dose rate, which I address in section 6.5.4.1 below.
106 After proof of concept studies, dose determination studies would typically be conducted using more animals (30 – 100 sheep) for one or two of the most preferred test formulations to investigate a tighter dosage band (i.e. narrower range) than assessed in the proof of concept studies.
107 The size, number and design of dose determination studies varied between companies, but in general terms the studies were designed to identify the necessary dose for a formulation of the active ingredient. In the veterinarian JER, the experts agreed that the results of the proof of concept studies and dose determination studies can sometimes be unexpected and accordingly such studies are necessary in new formulation development.
108 The World Association for the Advancement of Veterinary Parasitology publishes guidelines (WAAVP Guidelines) for evaluating the efficacy of ectoparasiticides against, amongst other things, biting lice. The veterinary experts agreed that these were well known in the field and give advice as to how to conduct dose determination studies. They contain advice that for a dose determination study, four groups of animals adequately infested with the target parasite are administered with either a negative control, 0.5, 1 and 2 times the anticipated effective dose. This approach was known to both veterinarian experts without referring to the WAAVP Guidelines and I find it to form part of the common general knowledge. The experts further agreed that generally the proof of concept studies used a larger range of doses than used in the subsequent dose determination studies.
109 The final stage of investigation is the conduct of dose confirmation studies, which involve larger groups of sheep to assess the efficacy of the doses chosen. No party suggested that these are relevant to the question of inventive step.
110 One matter in dispute was whether the information contained in the product labels of commercially available parasiticides formed part of the common general knowledge.
111 Abbey submits, and the veterinarian experts agree, that a range of chemical parasiticides were available for the treatment and prevention of sheep lice before the priority date, as listed in the first affidavit of Dr Agnew and that the amount of active ingredient in each of the commercially available products was set out in the product label. It submits that the labels for the known parasiticides formed part of the common general knowledge as a resource routinely identified and referred to by those in the field.
112 Abbey relies on the following evidence to support its submission, which I accept.
113 First, that the product labels of commercial products as identified by Dr Agnew in his evidence were regarded in the field as standard reference tools which were accessed by veterinarians where necessary. Secondly, labels were generally available for access on the website of the Australian Pesticides and Veterinary Medicines Authority (APMVA). Thirdly, product labels were consulted as a matter of course, and in preference to being retained knowledge. As Professor Whittem said in his oral evidence, he does not teach his students to learn dosages by heart, but that they should “always look at the label”. Mr Pippia gave evidence that he would consult labels as part of his technical resources for product analysis.
114 Against this background, the question arises as to whether or not the information contained in the product labels formed part of the common general knowledge in June 2013. The evidence of Dr Agnew, with which Professor Whittem agreed, is that some 17 listed products used in controlling ectoparasites, and their methods of administration were known to them before the priority date. Included in that list is the AVENGE product which has as its active ingredient imidacloprid. The amount of active used for the various means of administration (which included pour-on, dipping, jetting and dressings) was included on the product label. The label generally also provided a guideline or recommended dosage based on the size of the animal, which for dips provided an initial charge and, depending on whether the active is striping or non-stripping, a replenishment plus topping up or topping up amount.
115 For the reasons that follow, I accept that the product label for AVENGE formed part of the common general knowledge.
116 The notion of common general knowledge involves the use of that which is known or used by those in the relevant trade and which forms the background knowledge and experience which is available to all in the trade; Minnesota Mining and Manufacturing Company v Beiersdorf (Australia) Limited [1980] HCA 9; (1980) 144 CLR 253 at 292 (Aickin J). Information that is merely ascertainable by a routine literature search is not of itself to be regarded as common general knowledge; AB Hässle at [31], [57] (Gleeson CJ, Gaudron, Gummow and Hayne JJ). It would be wrong to consider a document part of the common general knowledge simply because skilled persons could readily locate and assimilate its contents because that would leave open for inclusion contestable portions of journals and articles that would not satisfy the description of common general knowledge; British Acoustic Films Ltd v Nettlefold Productions Ltd [1935] 1 WLUK 2; (1936) 53 RPC 221 at 250 per Luxmoore J:
In my judgment it is not sufficient to prove common general knowledge that a particular disclosure is made in an article, or series of articles, in a scientific journal, no matter how wide the circulation of that journal may be, in the absence of any evidence that the disclosure is accepted generally by those who are engaged in the art to which the disclosure relates. A piece of particular knowledge as disclosed in a scientific paper does not become common general knowledge merely because it is widely read, and still less because it is widely circulated. Such a piece of knowledge only becomes general knowledge when it is generally known and accepted without question by the bulk of those who are engaged in the particular art; in other words, when it becomes part of their common stock of knowledge relating to the art.
(Emphasis added)
117 In an often-cited passage in ICI Chemicals & Polymers Ltd v Lubrizol Corporation Inc [1999] FCA 345; (1999) 45 IPR 577 at [111] – [112], Emmett J said:
The notion of common general knowledge involves the use of that which is known or used by those who are in the relevant field or area. It forms the background knowledge and experience which is available to all in that field in considering the making of new products or making of improvements in old products. It must be treated as being used by an individual as a general body of knowledge …
The common general knowledge is the technical background to the hypothetical skilled worker in the relevant art. It is not limited to material which might be memorized and retained at the front of the skilled worker’s mind but also includes material in the field in which he is working which he knows exists and to which he would refer as a matter of course. It might, for example, include:
• standard texts and handbooks;
• standard English dictionaries;
• technical dictionaries relevant to the field;
• magazines and other publications specific to the field.
118 Jagot J observed in Gilead Sciences Pty Ltd v Idenix Pharmaceuticals LLC [2016] FCA 169; (2016) 117 IPR 252 at [216] (affirmed in Idenix Pharmaceuticals LLC v Gilead Sciences Pty Ltd [2017] FCAFC 196; (2017) 134 IPR (Nicholas, Beach and Burley JJ)):
…Justice Emmett [in ICI Chemicals] is not suggesting anything more than that the common general knowledge might include information in articles (etc) if the skilled addressee knows the article (etc) exists and would refer to it as a matter of course. In other words, what his Honour is allowing for is that the skilled addressee does not have to have instant recall of every matter for it to be common general knowledge. If the skilled addressee knows that certain information exists in an article (etc) and would refer to that document as a matter of course to refresh his or her memory about the details of that which the skilled addressee already knows in broad outline then those details might themselves form part of the common general knowledge. Justice Emmett is not suggesting that merely because the skilled addressee could locate a document and, having located it, could read and assimilate its contents, the document and its contents would form part of the common general knowledge. This would be inconsistent with Minnesota Mining, in particular, that the common general knowledge is the background knowledge and experience which is available to all in the trade. For the possibility which Emmett J recognised in ICI Chemicals to arise there would have to be evidence that the particular document was known and would be referred to as a matter of course by those in the field.
(Emphasis added)
119 In each case, determination of whether identified information falls within the common general knowledge will be a matter of weight and evaluation according to these principles.
120 I am satisfied, on the basis of the evidence that I have summarised above, that those skilled in the art knew of the existence of the AVENGE label, knew of the type of information that was available on it and would refer as a matter of course to that label to provide them with information about the products in question.
6.3.4 Chemical structure of thiacloprid
121 Abbey submits that the chemical structure of thiacloprid formed part of the common general knowledge. There is no doubt that imidacloprid was a known active ingredient (in AVENGE) and that it, as well as thiacloprid, were both part of the neonicotinoid class of insecticides. Abbey contends that the chemical structures of both formed part of the common general knowledge because they were readily accessible on resources such as PubChem. However, I am not satisfied that this level of knowledge is sufficient for Abbey to discharge the burden on it to established that the chemical structures fall within the range of information that the skilled addressee knows to exist and would refer to as a matter of course to refresh their memory of that which they already know in broad outline (to paraphrase Gilead).
6.3.5 The general approach to developing thiacloprid dosages in a pour-on
122 Abbey relies on several aspects concerning the development of a pour-on product as forming part of the common general knowledge which I consider below.
123 It is not in dispute that the administration of a lower concentration of active ingredient is generally desirable, provided that the active ingredient maintains its efficacy at that concentration. As Ms Mahashabde and Mr Pippia agreed, in developing a product, the aim is to use the least amount of active ingredient as possible.
124 The formulation experts agreed, and I accept that in determining the required amount of active for a pour-on product, it is ideal to keep the volume of the treatment that goes on the animal as constant as possible.
125 Abbey contends that when calculating the amount of active for a new composition, the skilled team typically considers a 50kg sheep as a target. As the amount of active ingredient is calculated in terms of mg/kg, the selection of a particular weight is of no particular significance and to some extent arbitrary. The evidence indicates that an assumed 50kg animal is regarded by those in the field to be a convenient approach.
126 Abbey submits that if there is a fixed volume chosen and the desired mg/kg is determined (a matter of significant contest between the parties), the calculation of the weight to volume percentage concentration of the active ingredient follows as a matter of arithmetic. This follows as a matter of logic and was agreed between Mr Pippia and Ms Mahashabde.
127 I have referred in section 3 to the agreed dipping calculation, which I repeat for convenience:
For dipping, the amount of an active ingredient that is applied to a sheep in mg/kg body weight can be calculated knowing the volume (mg/l) administered and the weight of the animal. For example, at 48ppm, assuming between 2L and 6L of formulation is “take-off”, this equates to an application of:
Sheep weight (kg) | Active applied (mg/kg) |
20 | 4.8 – 14.4 |
30 | 3.2 – 9.6 |
40 | 2.4 – 7.2 |
50 | 1.92 – 5.76 |
60 | 1.6 – 4.8 |
128 I accept the evidence of Ms Mahashabde that dipping compositions are more diluted than pour-on treatments, which are more concentrated. As she says in the formulation JER, pour-on products are applied by backlining the animal and the active ingredient in the formulation has to migrate over the body of the animal to reach the lice at an effective concentration, hence they are required to be more concentrated than dips, which cover the entire body of the animal and allow the active ingredient to come in direct contact with the lice. This accords with the evidence of prior art formulations given by Mr Pippia, where he observes that the concentration of the active ingredient for a dip is a fraction of the concentration of the active ingredient in a pour-on.
129 The Trade Advice Notice was published in February 2013. There is no dispute that it forms part of the prior art base for the purposes of s 7(3) of the Patents Act.
130 The Trade Advice Notice is entitled “Trade Advice Notice on thiacloprid in the Product Piranha Dip for Sheep, APVMA product number 63766” and relates to an application by Bayer Australia Ltd (Animal Health) for the registration of a new product called “Piranha Dip for Sheep”, containing an approved active constituent thiacloprid (480 g/L).
131 Under the heading “About this document” it provides:
… the Australian Pesticides and Veterinary Medicines Authority (APVMA) is considering an application to vary the use of an existing registered agricultural or veterinary chemical. It provides a summary of the APVMA’s residue and trade assessment.
132 The “Introduction” provides:
The [APVMA] has before it an application from Bayer Australia Limited (Animal Health), for the registration of a new product, Piranha Dip for Sheep, which contains the approved active constituent thiacloprid (480 g/L). The new product is a plunge dip that is intended for the control of the sheep body louse (Bovicola ovis) on short wool sheep.
To date, there are no products containing thiacloprid that are registered for use on food-producing animal species. However, there are two thiacloprid-based products registered for agricultural uses in Australia.
The existing Australian maximum residue limits (MRLs) for thiacloprid in animal commodities were established to cover the occurrence of residues as a result of animals consuming feedstuffs containing thiacloprid residues. Therefore, the current application does not involve the establishment of Australian MRLs for thiacloprid in edible sheep tissues.
However, the application does require the setting of meat and milk withholding periods (WHPs), establishment of an export slaughter interval (ESI), and approval of the proposed product label.
133 In section 2, entitled “Residues in Livestock”, the Trade Advice Notice includes the following:

134 Under the sub-heading 2.2, “Current Australian MRLs and residues definition”, entries for thiacloprid in the MRL (Maximum Residue Limits) standard are listed. Relevantly, the Trade Advice Notice provides, in relation to “residue definition/marker residue”, that it was concluded that the current residues definition/marker residue of parent thiacloprid “remains appropriate and is consistent with the use of the [Joint FOA/WHO Expert Committee on Food Additives (JECFA)]-approach to assessing residues of veterinary drugs”.
135 In relation to “Application rate (dip strength) and dipping process”, the Trade Advice Notice says:
Most of the residues trials were conducted at elevated rates (1.5 to 2x the label rate), and different dipping processes were used in each trial. The variability of the dipping processes seen in the residues trials is likely to reflect the variability of on-farm practices. Further, there is a broad range of factors that impact on the residues profiles of treated sheep. Therefore, in this instance, it was not considered appropriate to correct the residues data for dip strength (to the 1x rate). Analysis of the residues data, without correction for dose rate, reflects the “worst case” residues scenario.
136 In the conclusion, the Trade Advice Notice says that APVMA had considered whether the use of Piranha Dip for Sheep in accordance with the label instructions could potentially unduly prejudice trade and commerce between Australia and overseas. The risk was considered to be “low when the recommended [Expert Slaughter Interval (ESI)] of 60 days (2 months) is observed…, as residues in all tissues are expected to be below the method [Limit of Quantification (LOQ)]”. It states that the APVMA “is seeking comment from relevant industry groups and stakeholders in relation to the perceived level of risk to Australia’s export trade in sheep meat and live sheep”.
6.5 Consideration of the Evidence
6.5.1 The approach to formulating thiacloprid
137 Ms Mahashabde gave written evidence before she was shown the patent application that the following are the typical steps she would take in the development of a pour-on formulation before the priority date. Mr Pippia generally accepted this evidence as a “condensed summary” of a significant amount of work that would take several years to complete, assuming each step proceeds successfully:
(a) Determine the solubility of thiacloprid in known solvents used in pour-on formulations, (she named several solvents);
(b) Preliminary dose determination studies using a basic formulation in which the active was soluble to determine the dosage or dose range;
(c) Pre-formulation compatibility tests in solvent(s);
(d) Consideration of the need to add stabilisers and any other special excipients like antioxidants to protect the molecule from oxidation, preservatives, viscosity modifiers and dyes;
(e) Stability studies on a few possible prototype formulations;
(f) Proof of concept trials on at least two probable formulations which may contain different concentrations of the active to ascertain the safety and efficacy, and to confirm the dose;
(g) Stability studies on the chosen formulation in the final container system to establish shelf life and storage conditions;
(h) Conducting pivotal safety, efficacy and residue trials on the proposed formulation.
138 The inventive step challenge focusses on steps (a) and (b).
139 In her oral evidence, Ms Mahashabde clarified that the preliminary dose determination studies referred to in [137(b)] involve taking a concentrate solution that is believed to be a good starting point and applying higher and lower doses of that target concentration and then checking at what level good efficacy is obtained. I understand this to equate to the proof of concept studies to which the veterinarian experts refer, and which I have summarised in my findings of common general knowledge in section 6.3.2 above. Ms Mahashabde’s evidence is that she would select the target concentration and the studies would be conducted in consultation with the clinical team. I have referred to the role of the skilled team in the context of the typical interaction between the formulators and clinical experts in section 4.3 above.
140 In her written evidence, Ms Mahashabde says that if there was an existing formulation for a product that contained the same active ingredient or a related active that was a dip or spray product, then she would start her formulation process with regard to that product, noting that she would still follow the steps outlined at [137] above. Mr Pippia agrees that if an active constituent was formulated for dipping sheep or goats, then it would be worth investigating the potential to formulate that active into a suitable pour-on formulation, although he considered that the formulation development process would be essentially starting from the beginning. This approach points squarely to the content of the Trade Advice Notice, which refers in terms to thiacloprid as an active used in dipping.
141 When Ms Mahashabde was given the Trade Advice Notice she was asked in her letter of instruction to explain the types of formulations that she would consider developing for controlling lice on an animal such as sheep and goats for a formulation containing thiacloprid, and whether there are any formulations that she would consider taking forward into development.
142 Ms Mahashabde’s evidence is that she would have sought to develop a pour-on formulation containing thiacloprid, having regard to the dip product disclosed in the Trade Advice Notice and the preference for pour-on formulations as at the priority date. This accords with the evidence of Mr Pippia, who agreed in the formulation JER that a pour-on would be a logical choice for development if there was a pre-existing dip product.
143 Ms Mahashabde would then have followed the steps listed at [137(a) – (h)] above using ingredients usual for pour-on formulations. I have noted above that Mr Pippia broadly agrees with this approach.
6.5.2 Selecting a solvent or solvents
144 In such a formulation, Ms Mahashabde would typically include one solvent (possibly two if necessary to ensure spreadability and stability, reduce cost and improve penetration of the active), an antioxidant, and a scourable dye to enable treated sheep to be distinguished from untreated sheep.
145 As I have noted earlier in these reasons, properly characterised, the invention the subject of the claims is not for the formulation of thiacloprid. Indeed, Elanco accepts that this is so. Rather the invention that is described and claimed concerns the identification of a particular dosage and concentration range for the use of thiacloprid in a pour-on formulation (for the treatment of sheep or goats).
146 Ms Mahashabde would have considered whether there was an existing formulation for an ectoparasiticide product that contained thiacloprid or a related active ingredient. As at June 2013, she knew that imidacloprid was a related active of thiacloprid, being part of the same class of neonicotinoids, and that imidacloprid was the active ingredient in a well-known lousicide AVENGE, which was available in pour-on form. Mr Pippia agreed that AVENGE was a well-known product as at the priority date. I have no doubt that the skilled team would have, in developing any pour-on product using thiacloprid, had regard to the label of the AVENGE product.
147 To the extent that she could not precisely recall the chemical structure of thiacloprid or imidacloprid, Ms Mahashabde gives evidence that she could “easily access this information” through publicly available sources. She gives evidence that she would not have recalled the precise formulation of AVENGE but was aware that this would have been available from the AVENGE Safety Data Sheet, which she could obtain from the internet, a copy of which she annexed to her affidavit.
148 There is no dispute that it was generally known in the field that thiacloprid was a neonicotinoid or that imidacloprid, the active ingredient in AVENGE, was in the same chemical class. I have found above that the product label of AVENGE formed part of the common general knowledge. This identified imidacloprid. However, as I note in section 6.3.4 above, I am not satisfied that the chemical structure of either thiacloprid or imidacloprid formed part of the common general knowledge. This is not necessarily fatal to the case advanced by Abbey. The veterinarian experts agreed that the chemistry of the active ingredient may not be a determining factor during the initial investigations into the development of a new product. However, I set to one side access to the chemical structures in my consideration of the case advanced by Abbey.
149 Based on the evidence of Ms Mahashabde, Abbey submits that the skilled team would have determined the solubility of thiacloprid in a pour-on composition. Elanco disputes that it would have, before June 2013, as a matter of course been able to identify a suitable solvent for thiacloprid in the concentrations identified and contends that the significant differences between a dip and pour-on formulation meant that it could not be known what properties the mixture of solvent and thiacloprid would have in a pour-on formulation. This, it submits, means that the hypothetical task of developing a pour-on formulation fails at an early hurdle.
150 For the following reasons, I am satisfied that the skilled team would readily have identified a solvent or solvents for thiacloprid such that the determination of dosage amounts could proceed.
151 Ms Mahashabde considers that it would be a straightforward task to identify a suitable solvent or solvents for thiacloprid and provides a list of a number of potential solvents with which she was familiar. She excludes from that list n-methyl pyrrolidone, which was one of the solvents used in AVENGE and identified in the AVENGE Safety Data Sheet referred to above. It has been classified by the European Chemicals Agency as a substance of high concern because of its potential to cause human reproductive harm and so she would avoid using it. Nevertheless, she is confident that a solvent or solvents suitable for thiacloprid in a pour-on formulation would be identified, especially given that it is in the same class as imidacloprid, which had been solubilised.
152 I understand that Mr Pippia agrees with this evidence. In his affidavit, he takes issue only with the rejection of n-methyl pyrrolidone, but otherwise does not dispute Mr Mahashabde’s evidence that she would readily determine the solubility of thiacloprid using known solvents. Mr Pippia reviewed the list of 13 potential solvents named by Ms Mahashabde as candidates and agreed that it was a reasonable list to assess. He would have added n-methyl pyrrolidone to the list because, despite being “off limits” in Europe, it is not classified as a substance of concern in Australia. In his oral evidence Mr Pippia does not dispute Ms Mahashabde’s opinion that solubilising thiacloprid would be straightforward, he merely adds his view that each of the potential solvents would have a different solubility of thiacloprid and that some may be less desirable and others would give more options. He considers that one may end up with “five that could sort of produce a reasonable range”. He considers that solubility studies would be required to understand how the molecule behaves.
153 Having regard to these matters, in my view, the evidence does not support the conclusion that the step of finding a solvent for thiacloprid in a pour-on product is a relevant barrier to progress for the skilled team. The fact that there may be several options by which a skilled formulator is likely to find a solvent, or that it takes some time and effort, does not change the analysis. To the extent that there may be a disagreement between Ms Mahashabde and Mr Pippia (one is not apparent to me) then I prefer the view of Ms Mahashabde summarised above.
154 In my view, the evidence establishes that it would have been straightforward for a skilled formulator to place thiacloprid into solution by one of several means. Furthermore, the fact that each of imidacloprid (in AVENGE) and thiacloprid (in PIRANHA) had been placed in solution would give the skilled team a confident expectation of success.
6.5.3 Interpreting the Trade Advice Notice
155 Abbey relies on the content of the Trade Advice Notice to point the way to the solution to the problem posed in the patent application and offer a basis upon which the skilled team would have confidence that a backlining method of treatment within claim 1 would likely be developed before the priority date.
156 Ms Mahashabde gives evidence about the several steps that she would take to arrive at a starting dose for using thiacloprid as the active ingredient in a pour-on formulation. She notes that the Trade Advice Notice provides a dosage regime of 480 g/L which is diluted to 10 ml/L or 48 mg/L in water. From that information, she makes several deductions which warrant analysis.
157 First, she estimates that the weight of the sheep used in the Trade Advice Notice would be around 50kg. This is not controversial.
158 Secondly, she estimates that a 50kg sheep would retain between 3L and 5L of liquid while exiting the dip (“take-off”). The range of 3L to 5L is controversial. Elanco submits that Ms Mahashabde made an impermissible assumption in this regard. A wider range of 2L to 6L was provided in the agreed dipping calculation. In her oral evidence Ms Mahashabde was unable to provide a basis for the narrower range, other than by noting that she had consulted others in the course of preparing her evidence as to the appropriate range. Such an extraneous source of information cannot be relied upon and I set it to one side.
159 In the broader range, the agreed dipping calculation yields the result that for a 50kg sheep, assuming a range of 2L to 6L of take-off and at a concentration of 48 mg/L (48ppm), the dose range of thiacloprid is between 1.92 mg/kg and 5.76 mg/kg.
160 Thirdly, Ms Mahashabde understands that thiacloprid is a very potent molecule in terms of efficacy in the control of lice, particularly in comparison with AVENGE which used the active imidacloprid at a concentration of 35 g/L, being a 60mL dose for a 50kg sheep at 42 mg/kg of imidacloprid.
161 Elanco criticises the use of the agreed dipping calculation by contending that the range of take-off is necessarily imprecise and can be no more than an estimate. In this context it relies on the evidence of Professor Whitten as to inaccuracies inherent in such estimations. He considers that estimates of the dose of active compound based on take-off volume are likely to be overestimates. Dr Agnew, on the other hand, considered that it was common practice, and indeed an industry standard, as at the priority date, to calculate the amount of active applied to an animal in mg/kg for a dipping formulation and that this could be estimated by reference to the take-off volume.
162 In their concurrent evidence, the veterinarian experts agreed that their differences in this regard arose from their different professional backgrounds and experiences.
163 Professor Whittem characterised his industry experience as research and development at a small family-owned company with a relatively constrained budget, meaning the nature and detail of the studies done in preparation of products for registration were more confined. In the veterinarian JER and in concurrent evidence, Professor Whittem explained that he approached the determination of a dose as a calculation from a molecular and clinical pharmacology point of view, where the calculation of a dose rate is a computation aimed at arriving at a specific, accurate and precise dose for an individual animal. He would also consider the regulator’s point of view, taking into account factors affecting the absorption, distribution, metabolism and elimination of a drug. He considers that estimating the amount of active ingredient administered to a sheep by reference to an assumed amount of take-off usually leads to overestimates of the dose delivered.
164 Dr Agnew, on the other hand, worked with large multinational companies; Elanco, Merial and Boehringer Ingelheim, which had fewer budgetary constraints affecting the design and conduct of study programs. He approached the determination of dose in sheep louse studies from a clinician and product developer’s point of view and would be comfortable to arrive at an estimate of the effective dose. Dr Agnew considers that he took a more practical, commercial approach to product development.
165 I accept that the difference of approach reflects their differences in background and experience. I am satisfied that the skilled team would take the more practical and less academic approach that Dr Agnew favoured in terms of providing an appropriate – but not necessarily precise – estimate of the amount of active ingredient in a dip formulation and converting it by reference to the take-off amount into a dose estimate. I accept that the skilled team would proceed on the basis that the agreed dipping calculation reflects a calculation that the skilled team would make on the basis of the disclosure in the Trade Advice Notice.
166 From this calculation, the skilled team would understand that thiacloprid was considerably more potent as a parasiticide than the active ingredient in the AVENGE product (imidacloprid). As the agreed dipping calculation demonstrates, for a 50kg sheep, at a concentration of 48 mg/L (48ppm), the amount of active thiacloprid applied will be between 1.92 mg/kg and 5.76 mg/kg. The amount of imidacloprid required for an equivalent sheep was 42 mg/kg, being some seven times more than thiacloprid.
167 Once it is accepted, as I do, that the skilled team would convert the amount of thiacloprid administered in the dip solution to a range of mg/kg administered, it is readily apparent that the skilled team would immediately appreciate that it was likely that a significantly lower amount of thiacloprid would need to be administered in a pour-on formulation than imidacloprid, and that it was likely to be useful.
6.5.4 Arriving at doses within claim 1
168 Abbey takes two approaches to the question of the dose range set out in claim 1. It first submits that the skilled team would have arrived at dosages within the claimed dose ranges and concentrations irrespective of any initial range of dosages used for the studies by reason of undertaking routine proof of concept studies. Secondly, it contends that in any event, the effect of the evidence is that the skilled team’s initial range of dosages for the studies, estimated based on the amount of thiacloprid disclosed in the Trade Advice Notice, would have included dosages and concentrations within the claim. I address each in turn below.
169 Abbey contends that the skilled team would have determined the solubility of thiacloprid in a pour-on formulation and then undertaken proof of concept studies and dose determination studies to determine the doses. Such studies were routine in nature and followed a standard pathway. It submits that the purpose of proof of concept studies was to determine an appropriate or optimal dose or dosage range. Elanco submits that determination of an optimal or robust dose is not done during proof of concept studies but later at the dose determination stage.
170 The evidence of the veterinarian experts supports the view that proof of concept studies are indeed routine.
171 One area of disagreement between the experts concerned the aim or purpose of the proof of concept studies. Dr Agnew’s experience is that in those studies the skilled team focusses on trying to determine the dose where the product became ineffective (being a minimum dose) because the product to be commercialised will be applied in great numbers of sheep and in a broad range of conditions and so there is a drive to understand the range of dose that would or would not work. Professor Whittem considered that in an industry where there are constrained resources, as long as there is an adequate safety margin between the labelled dose and the lowest dose which causes adverse effects, then there is no need to probe for the lowest effective dose, unless there is a cost of goods issue. His view is that if one hits upon an effective dose and it is safe and commercially viable, in terms of margin for the final product, there is no benefit in spending more money on further research.
172 In my view, this evidence tends to confirm that the process of ascertaining a dose level range is indeed routine to the skilled team and that it would be conducted at the proof of concept stage. Nothing in Professor Whittem’s evidence suggests that there is any doubt that the skilled team could seek to ascertain a minimum effective dose or an effective dose range. Whilst it may be that at a later stage precise pharmacokinetic metrics are ascertained for dose efficacy in order to satisfy regulatory requirements, I am satisfied that at the earliest stage the skilled team is seeking to determine an efficacious dose, with a preference, for the reasons explained by Dr Agnew, towards the lower end of the scale. Professor Whittem questioned whether it would be necessary to seek a minimum, having regard to commercial concerns which perhaps apply to smaller companies in the field but do not apply to larger companies. In this regard, I prefer the evidence of Dr Agnew as to the likely approach of the skilled team being to use the smaller and less expensive proof of concept studies (conducted on small pens of sheep) to seek to ascertain a dose range. To the extent that commercial considerations are relevant to the question of inventive step, I consider that the more practical and less academic approach of Dr Agnew, which is reflected in his work experience, is the more likely approach to be taken by the notional skilled team.
6.5.4.2 Selecting a dosage range
173 Ms Mahashabde was asked to assume that she had been asked as at the priority date to develop a formulation for controlling lice on an animal such as sheep or goats and provided with a copy of the Trade Advice Notice. In response, she reiterated the approach she would take to formulating any product, but this time in the context of the disclosure in the Trade Advice Notice in relation to thiacloprid. After determining the solubility of thiacloprid (to which I have referred in 6.5.2 above) she would have estimated a dose to kill lice on sheep from the information in the Trade Advice Notice in the manner to which I have referred in section 6.5.3. In that section I have concluded that the skilled team would consider that the dose would be between 1.92 mg/kg and 5.76 mg/kg.
174 Ms Mahashabde explains that she would, as a starting point in developing a pour-on containing thiacloprid, double the mg/kg plunge dipping dose that she had estimated from the Trade Advice Notice. The reason for the doubling is because: (a) the concentration of active ingredient in pour-on formulations is generally known to be higher than for dips and sprays; and (b) the safety information in the Trade Advice Notice says that residue studies were carried out at twice the dose rate which provides a basis for her to be confident that such a dose is safe.
175 In cross-examination about the “start dosage”, she maintained her view that she would have started with twice her estimated maximum dipping dose of 4.8 mg/kg (i.e. 2 x 4.8 = 9.6 mg/kg) because she was aware from the residue trials reported in the Trade Advice Notice that the double dipping dose was safe. As I have noted in 6.5.1 above, the studies to test these doses equate to proof of concept studies.
176 Ms Mahashabde would apply a dose volume of 60mL for a 50kg sheep, that being similar to the amount used for the AVENGE product. As I have noted, the choice of 60mL was not controversial. Mr Pippia agreed that it was an acceptable volume to give to a 50kg sheep and he too would have looked to AVENGE as a starting point for a yardstick as to potential dose.
177 Mr Pippia criticises Ms Mahashabde’s approach of doubling the higher dose from the dipping dose range estimated from the Trade Advice Notice as being arbitrary. His evidence is that if he were starting with the dose range given in the agreed dipping calculation, he would try each of the lower amount in the range for a 50kg sheep (being 1.92 mg/kg) and the higher amount (being 5.76 mg/kg) and test that in an “initial dose determination study”, which I take to be equivalent to a proof of concept study as mentioned in section 6.3.2 above, and then test using multiples of that range. As he said:
So we’re talking about the initial dose determination study. Now, I would be guided by the data that I get from that particular range. If I don’t see efficacy, I know that I have to widen.
178 As Mr Pippia said in his affidavit evidence, studies with multiples of the starting dosage would be a “logical step towards efficacy”. He gave oral evidence, when considering Ms Mahashabde’s approach, that he would take a more conservative approach than her and test from the lower limit for a 50 kg sheep of 1.9 mg/kg and then up to three times that amount and the same for the upper limit of 5.8 mg/kg.
179 Mr Pippia’s affidavit evidence was that, rather than considering the Trade Advice Notice, he would have looked to AVENGE as a starting point for a yardstick as to potential dose, which provided a pour-on formulation of an active constituent in the same class of compound. Relying on the AVENGE information, a 60mL volume of AVENGE applied to a 50kg sheep equates to a 42 mg/kg dose. On that basis, he would consider a similar dosage formulation of thiacloprid. He accepts, however, that this would be a guide only as it “could be possible” that the potency of thiacloprid is significantly higher for lice than imidacloprid. He would expect to trial a range of doses.
180 Mr Pippia does not in my view adequately explain why he would set the Trade Advice Notice to one side for the purpose of obtaining information for determining an effective dose amount. He accepts, as I have found, that it is possible to calculate the dose amount of thiacloprid given to a sheep from the Trade Advice Notice, which indicates that a significantly lower amount of thiacloprid is required than imidacloprid in order to control lice.
181 In my view, it is less logical to go to a different active ingredient (being imidacloprid) to work out the starting point when the Trade Advice Notice provided information about thiacloprid. Indeed, as Professor Whittem said, and Dr Agnew agreed, even with structurally similar compounds such as imidacloprid and thiacloprid, it is difficult to make assumptions of similar efficacy, potency or persistence. I consider that the approach of Ms Mahashabde to rely on information provided in the Trade Advice Notice as providing the basis for her approach is more persuasive and the route likely to be taken by the skilled team.
182 Having regard to the evidence of Mr Pippia and Ms Mahashabde, I consider it most likely that the skilled formulator would propose to the skilled team that multiples of the estimated dipping dose be tested in initial proof of concept studies for the development of a pour-on. I consider that if those studies did not yield any efficacious results then they would have broadened the range. In this regard, I accept that in the notional skilled team, formulators and veterinarian experts would consult in relation to doses and select several starting doses to test. For the reasons given above, in my view it is most likely that the starting doses would be at least two and three times the estimated doses of thiacloprid derived from the Trade Advice Notice.
183 On the basis of this approach, the initial testing by the skilled team would be of the following dosage amounts, using a dose volume of 60mL for a 50kg sheep. The formulation experts agreed that 1.92 to 5.76 mg/kg from the agreed dipping calculation translates to 96 to 288 mg of thiacloprid on a 50kg sheep. The double and triple doses of thiacloprid have been highlighted in bold where they fall within claim 1 of the patent application.
Active applied (mg/kg) (by body weight) | Total active (mg) (50kg sheep) | Weight/volume % (60mL dose) |
Initial range (agreed dipping calculation) | ||
1.92 mg/kg | 1.92 x 50 = 96 mg | 96 mg / 60 mL = 1.6 mg / mL = 0.16 % w/v |
5.76 mg/kg | 5.76 x 50 = 288 mg | 288 mg / 60 mL = 4.8 mg / mL = 0.48 % w/v |
Double dose | ||
3.6864 mg/kg | 3.6864 x 50 = 184.32 mg | 184.32 mg / 60 mL = 3.072 mg / mL = 0.31 % w/v |
11.5 mg/kg | 11.5 x 50 = 575 mg | 575 mg / 60 mL = 9.583 mg / mL = 0.96% w/v |
Triple dose | ||
5.76 mg/kg | 5.76 x 50 = 288 mg | 288 mg / 60 mL = 4.8 mg / mL = 0.48 % w/v |
17.28 mg/kg | 17.28 x 50 = 864 mg | 864 mg / 60 mL = 14.4 mg / mL = 1.4% w/v |
184 Claim 1 of the patent application provides:
A method of controlling lice on an animal, characterised by the step of administering by backlining a pharmaceutically effective amount of thiacloprid, wherein the amount of thiacloprid administered to the animal is from 10 to 30 mg/kg by weight of the animal, wherein the thiacloprid is administered in a composition containing 0.5%-2% w/v of thiacloprid, and wherein the animal is a sheep or a goat.
185 Abbey submits that in testing whether or not there is an inventive step on the facts, the Cripps question can be modified to read as follows:
Whether the skilled team would have been directly led as a matter of course to try dosages within the claimed dosage range/concentration in the expectation that this might well produce a better or useful alternative to the existing sheep lice treatments or some other useful result.
186 It submits that this must be answered in the affirmative, but in any event submits that the inventive step question may be answered separately by reference to the test set out in Wellcome Foundation at 287 (set out in section 6.2 above), namely whether the hypothetical addressee, faced with the same problem would have taken as a matter of routine whatever steps might have led from the prior art to the invention, whether they be the steps of the inventor or not. Elanco disputes this, submitting that the skilled team would not have been directly led as a matter of course to try thiacloprid in the ranges claimed.
187 In the course of its argument, Elanco submits that it would not be sufficient for the hypothetical skilled team to arrive at a dosage of thiacloprid in a pour-on formulation to treat lice in sheep that was within the range of dosage and concentration claimed in claim 1 of the patent application. It submitted that the range itself would need to be determined.
188 I do not consider this proposition to be supported by the authorities. In my view, it is sufficient for Abbey to establish that it was obvious to achieve something that falls within the range claimed to invalidate the entirety of the claim for want of inventive step. As the Full Court said in AstraZeneca AB v Apotex Pty Ltd [2014] FCAFC 99; (2014) 226 FCR 324 (Besanko, Foster, Nicholas and Yates JJ) at [195] (Jessup J agreeing at [447]):
In assessing novelty or obviousness, the focus is on the claim as construed in light of the complete specification read as a whole and the common general knowledge as it stood at the priority date of the claim. In particular, in assessing whether or not an invention was obvious and, consequently, lacking an inventive step, the relevant inquiry is whether anything within the claim (not merely embodiments described in the complete specification) would have been obvious to the hypothetical person skilled in the art: Blanco White TA, Patents for Inventions (5th ed, Stevens & Sons, 1983) at [4-201]; see also Grove Hill Pty Ltd v Great Western Corporation Pty Ltd (2002) 55 IPR 257 at [364] per Gyles J. The position in Australia and United Kingdom is no different in this respect. It is the claimed invention that must involve an inventive step: Conor Medsystems Inc v Angiotech Pharmaceuticals Inc [2008] RPC 28 at [17] to [19] per Lord Hoffman.
(Emphasis added)
189 Just as the novelty of the claim would be defeated by a disclosure within the range, so too is the inventive step.
190 In this regard, I note that whilst claim 1 identifies a range, nowhere in the specification is there any suggestion that the inventive step is in the selection of the range. Indeed, as a review of the patent application demonstrates, the broadest statements of the invention involve assertions that the method may be characterised by the step of topically administering a composition containing from 0001% to 3.5% w/v of thiacloprid (see [52] above). Trial Example 10, which is the only example referring to the backlining method, does not purport to show a range. Indeed, the results of the four trials conducted report efficacy in pour-on treatments with dosage levels of 15 mg/kg, 10 mg/kg and 5 mg/kg, all of which are said to have resulted in an absence of live lice on the sheep tested. The dose of 5 mg/kg is outside the dose range of claim 1. Accordingly, whilst claim 1 is for a method of treatment within a range, there is no basis upon which it may be said that the range, rather than a dose within the range, forms part of the inventive step.
191 Abbey submits that the skilled team would, as a matter of routine, have conducted the solubility, proof of concept and dose determination studies leading from the Trade Advice Notice to the claimed invention in the expectation that they might well succeed in finding a dose within the claimed range, citing Merck Sharp & Dohme Corporation v Wyeth LLC [2020] FCA 1477; (2020) 155 IPR 1 (MSD v Wyeth) at [255] and [822] and the cases there cited.
192 Elanco contends that the evidence demonstrates that it was not routine to develop the minimum effective dose of the active ingredient. It disputes that the formulation of a pour-on dosage form of thiacloprid would be straightforward, identifying two difficulties facing the skilled team, first the question of solubility and secondly the question of dosage amount.
193 I have addressed the evidence concerning questions of formulation in sections 6.5.1 and 6.5.2 above. It is not in dispute that the present patent application is not for a formulation, but rather for a dose range for use in a method of treatment. I have concluded in section 6.5.2 that no inventive step lay in finding a suitable solvent for thiacloprid.
194 The question of dosage amount, or as some of Elanco’s submissions suggested, a “starting dosage” was, to some extent, a distraction. This was perhaps generated by the manner in which the evidence of Ms Mahashabde was presented. However, neither Ms Mahashabde nor any other expert stands as a proxy for the notional skilled team. The task of the Court is to assimilate the evidence and determine whether the invention claimed lacks an inventive step.
195 The distinction between characterising steps taken as a matter of routine that would invalidate a claim (Wellcome Foundation at 286; AstraZeneca (HC) at [15]) and steps taken because the skilled team regards them to be “worth a try” but would not invalidate a claim (AB Hässle at [72]; AstraZeneca (HC) at [15]) is not always clear. In Generic Health, the Full Court explained at [71] that one way of distinguishing the two is by reference to the expectation of the skilled team that it may succeed in its endeavour:
… It is difficult to think of a case where an expectation that an experiment might well succeed is not implicit in the characterisation of steps as routine and to be tried as a matter of course. On the other hand, we think a test formulated in terms of worthwhile to try was firmly rejected by the High Court in [AB Hässle] …
196 As I have noted in section 6.2 above, in Nichia the Full Court observed that the relevant test for expectation will be satisfied if the Court finds that the hypothetical skilled team expects that the steps may well work, rather than knowing that steps will or would or even may well work (at [99]).
197 Elanco relies on the analysis by Besanko J in ICOS Corporation to demonstrate that in the present case the evidence does not rise to the level required. In that case, after a thorough examination of the authorities, many of which have been referred to above, his Honour found, in relation to the 946 patent that although the development team would as a matter of course, be directly led to try the compound tadalafil with the required expectation of success, he was not satisfied that the team would, as a matter of course, be directly led to the claimed doses with such an expectation (at [465]). The factual matrix that led his Honour to that conclusion is quite different to the present case. The skilled team here, in possession of the Trade Advice Notice, has been provided with a template for the use of a potent active ingredient that has been shown to be efficacious and safe in a dip formulation. There is no obstacle to its use in proof of concept studies. The only task is for the skilled team to identify dosage amounts to be applied.
198 In this regard, the evidence of the veterinarian experts to which I have referred in section 6.5.4.1 above persuades me that the conduct of proof of concept studies forms a basic tool for the skilled team to ascertain an effective dose to thiacloprid in a pour-on product. I am not persuaded that there was any relevant obstacle to the skilled team in developing such a dose. Indeed, the evidence was that there were before the priority date several pour-on formulations within the common general knowledge used for backlining treatments that were also formulated for use in dips. Armed with the Trade Advice Notice the skilled team had every reason to expect that an efficacious dosage would be achieved.
199 Furthermore, I accept that the skilled team would work to ascertain an efficacious dosage amount of active ingredient that sat in the lower end of the range. I have concluded in section 6.5.4.1 that this was the routine approach.
200 Within the skilled team, the formulator would work in conjunction with other members to determine appropriate dosage amounts. Ms Mahashabde determined that she would take the dose amount from Trade Advice Notice by estimating the amount used in the dip formulation and then applying that estimate to several proof of concept studies. Mr Pippia, if using the Trade Advice Notice to select a starting point, would have taken much the same approach. Both would use those studies to check for efficacy using multiples of the dosage range identified. I do not accept that this approach is arbitrary – that there was nothing scientifically rigorous about the selection of a starting dose, at the early stage, or that it simply involved guesswork.
201 I accept that at the outset the skilled team would not know the dosage amount that would be ultimately selected. But that is precisely why the tests are required. Such tests are, however, in my view in the nature of tests for checking and verifying of the type referred to in Wellcome Foundation at 280-281. The plurality in AB Hässle considered at [51] what Aickin J had in mind by his use of “routine” by referring to an earlier passage of Wellcome Foundation (at 280-281):
Evidence of what he did by way of experiment may be another matter. It might show that the experiments devised for the purpose were part of an inventive step. Alternatively it might show that the experiments were of a routine character which the uninventive worker in the field would try as a matter of course. The latter could be relevant though not decisive in every case. It may be that the perception of the true nature of the problem was the inventive step which, once taken, revealed that straightforward experiments will provide the solution. It will always be necessary to distinguish between experiments leading to an invention and subsequent experiments for checking and testing the product or process the subject of the invention. The latter would not be material to obviousness but might be material to the question of utility.
202 In the present case, no inventiveness lies in the perception of the true nature of the problem. Once armed with the Trade Advice Notice the skilled team immediately perceives the desirability of moving to a backlining method using thiacloprid. The question remaining is whether the process of arriving at a formulation within the claimed range was an inventive step.
203 In my view the hypothetical skilled veterinarian in the team would be robust about the likelihood that an efficacious dose of thiacloprid for use in a pour-on product would be ascertained. Dr Agnew held the view that it would be a straightforward step to determine the dose range of a pour-on product, including where information is available about a dip product using the same active. He explained that in his career he had never experienced a circumstance where an active ingredient that was administered as a dip was not able to be adapted to be used as a pour-on. In cross examination he explained that when he saw the Trade Advice Notice he automatically assumed that it would work for a pour-on formulation. In this regard, he gave an example of his own experience:
MR FOX: Yes, yes. But you don’t – you don’t look at, for example, the trade advice notice and see it referring to thiacloprid being useful for dipping and then automatically assume that it will work for pour-on formulation, do you?
DR AGNEW: Actually, I did. And so the reason – the reason for that is, again, based on my experience from the Spinosad work where we started with a concentrate, a suspension concentrate product. We then moved to a suspension concentrate, a same formulation but much less concentrated for the sheep dip. And because the efficacy of Spinosad against sheep lice was fantastic, then the step of deciding to develop a pour-on was quite straightforward because we had information based on the species; so sheep were the same. We had information that the dip was efficacious against sheep lice. So that was the parasite we were going after. And so the biggest challenges in terms of assessing are that you’ve got a reasonable chance of success with a product. So – so when I – when I see a trade advice notice with a product where the active that I’m thinking of developing as a pour-on and I see it’s used on sheep and against lice, I actually would be very confident that you could backline formulation with that active.
204 Professor Whittem noted that one could not make assumptions about dose when moving from a dip to a pour-on product, but that one would have, from the dip formulation, evidence that efficacy in a pour-on product is possible if one got the distribution right.
205 To the extent that there is variance in their approach, I accept the evidence of Dr Agnew as more persuasive.
206 In relation to dose, I have accepted at [172] above that proof of concept studies would typically aim to determine a range, with the focus being to establish a minimum effective dose. The evidence of Ms Mahashabde and Mr Pippia focussed on the starting doses to be tried in proof of concept studies, although it is apparent from their evidence, and I accept, that the skilled team would broaden the range of the doses used in proof of concept studies as results came in. As Mr Pippia said, he would be “guided by the data”. Although I accept that the process of determining the dosage amounts would be time consuming and expensive, and would possibly take the team a long time to achieve, I am satisfied that their conduct is well within the range of the skills of the skilled team and falls within the range of conduct described in the authorities as “routine steps”. On the basis of their approach, as set out in the evidence considered in section 6.5.4.2 above, I am satisfied that even their starting ranges would include dosage amounts within the range claimed in claim 1.
207 In my view, the present facts are analogous to those in AstraZeneca (HC). In that case, the Watanabe Article did not disclose dosage amounts. French CJ relevantly said at [42] – [44]:
Jessup J concluded that the primary judge had not erred in concluding that a person skilled in the art would have been led directly and as a matter of course to try rosuvastatin at a dosage of 5-10 mg/daily in the expectation that it might well be an efficacious treatment for a patient suffering from hypercholesterolemia. The evidence of Professor O’Brien and of Dr Reece was sufficient to sustain the conclusion that the skilled person, having read the Watanabe Article in the light of the common general knowledge, would have entertained the expectation that rosuvastatin might well be at least as efficacious a treatment as atorvastatin. No error was involved in that reasoning. In the light of the evidence it would be a routine step to test rosuvastatin at the lowest efficacious dose.
Jessup J rejected a submission by AstraZeneca that the primary judge’s conclusion that the skilled person would have proceeded to try rosuvastatin at any dose, let alone a 5 or 10 mg dose, with any reasonable expectation of success was not open on the evidence before her. That rejection was plainly correct. As his Honour said (91): “Whether an invention is obvious is a question to be answered by the Court.” The question posed by this Court in Wellcome Foundation Ltd and AB Hässle does not require that, in order to sustain an obviousness case, a party has to lead evidence which echoes the terms of that question. A similar conclusion was open, as the primary judge found, on Patent 471.
AstraZeneca submitted in this Court that the “claimed invention” is a treatment using a once daily, 5-10 mg dosage of rosuvastatin. The only dosage expert, Dr Reece, confirmed that neither the Watanabe Article nor Patent 471 contained animal or human trial safety data. He had given evidence that such data were essential to determining what dosage should be tested in clinical trials. The person skilled in the art would never have chosen the dose to be tested simply by trying the doses that worked for other statins. That evidence was referred to by Jessup J, who said (92):
“But that evidence also made it quite clear that such trials would conventionally be carried out. They would fall within the concept of working towards the invention with an expectation of success referred to in AB Hässle.”
No error is disclosed in that reasoning.
208 Justice Kiefel said at [93] – [95]:
The final submission was that, armed with the Watanabe article and the common general knowledge, the skilled person would not have been led directly to the invention because the starting dosages are an essential element of the invention and were not revealed by that article or by any other prior art document.
This may be accepted. The dosages were revealed to AstraZeneca by clinical trials on humans, as inevitably they would be if undertaken. The point is that the Watanabe article contained sufficient information, including as to the results of the pre-clinical trials on animals, for Professor O’Brien to consider that clinical trials were warranted. Dr Reece was of the same view.
Professor O’Brien’s evidence about what the right dosages might be, although accurate, was largely speculative and this was not his area of expertise. However, Dr Reece, who has a background in clinical pharmacology and research, gave evidence that dose sizes which would be trialled could be expected to start from 5 mg, 10 mg and 20 mg. I do not understand AstraZeneca to contend that the starting dosages disclosed in the Patent would not be identified by normal clinical trials, which utilise certain standards and procedures. The evidence therefore shows that the skilled person would be led to the invention.
209 Justices Gageler and Keane said at [116]:
It is also wrong to suggest, as AstraZeneca did, that the need for further testing of human subjects was an obstacle to a conclusion that rosuvastatin would have been tried as a matter of course in the expectation of a solution to the problem in the common general knowledge. Section 7(2) does not invite a consideration of the notional addressee’s motivation to carry out any tests that would need to be done. In particular, it does not contemplate consideration of whether the skilled addressee would be sufficiently encouraged by the available information to undertake the expense and inconvenience of further tests necessary to bring the solution to the stage of implementation.
210 Justice Nettle said at [123]:
In fact, however, there is no basis in the objection. Both the primary judge and the Full Court approached the matter in accordance with that requirement. As Jessup J said, although Dr Reece’s evidence was that the Watanabe article contained no safety data the result of either animal or human trials, the evidence also disclosed that trials of that kind would conventionally be carried out. Accordingly, carrying out the trials fell within the concept of working towards an invention with an expectation of success and that was consistent with the conclusion that the invention was obvious.
(Footnote omitted)
211 In my view, that team would be confident, in commencing its proof of concept studies, that it would obtain an efficacious backlining treatment using thiacloprid. It would not know the dosage amounts, but adopting routine testing of the type described and with the approach identified for the selection of a dosage range in 6.5.4.2 in my view the skilled team would have arrived at a dosage amount that falls within the claims. Indeed, although on my analysis it was not necessary for it to do so, the results set out in the table in section 6.5.4.2, where the approach to commencing proof of concept studies would lead to a dose amount within the range, support this conclusion. Such results were not necessary because in my view, on the basis of the promising information in the Trade Advice Notice, the skilled team would, if it did not find an efficacious dose in initial proof of concept studies, vary the range until they did.
212 As a footnote, I observe that there is a degree of arbitrariness about the range claimed in claim 1. Trial Example 10 demonstrates that the administration of 5 mg/kg (0.5 ml/kg) was effective. This is half the minimum amount claimed. No evidence suggests that there was any rational basis for excluding the lower dose from the claim. For present purposes it is not necessary to dwell on the consequences of that exclusion for the validity of the claim. However, in light of the matters to which I have referred, no invention will lie in arbitrarily limiting an effective range.
213 For the reasons set out above, the appeal should be dismissed. Elanco must pay Abbey’s costs of the appeal.
I certify that the preceding two hundred and thirteen (213) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Burley. |
Associate:




