FEDERAL COURT OF AUSTRALIA
Native Extracts Pty Ltd v Plant Extracts Pty Ltd (No 2) [2024] FCA 106
Table of Corrections | |
In paragraph 27, in the first sentence, the words “(defined as Confidential Information)” have been added after the word “misused” | |
26 February 2024 | In paragraph 77, in the first sentence, the words “or the Trust” have been added after the words “Native Extracts” |
26 February 2024 | In paragraph 186, in the first sentence, the word “However,” has been removed and replaced with “That is because” |
26 February 2024 | In paragraph 198, in the second sentence, the word “per” has been removed and the words “Mitchelmore JJA and Griffiths AJA agreeing” have been replaced with “whom Mitchelmore JA and Griffiths AJA agreed” |
26 February 2024 | In paragraph 242, in the first sentence, the words “is then” have been replaced with “must then be” |
26 February 2024 | In paragraph 277, in the second sentence, the words “2.5 times more than” is replaced with “3.5 times” |
ORDERS
DATE OF ORDER: | 23 february 2024 |
THE COURT ORDERS THAT:
1. The claim against the Fifth Respondent be dismissed;
2. Pursuant to section 115(4) of the Copyright Act 1968 (Cth), the First Respondent pay to the First Applicant the sum of $157,198 as additional damages;
3. The parties are to confer on the form of the remaining orders to be made to give effect to these reasons, including on the question of costs;
4. By 4.00 pm on 28 February 2024:
(a) if the parties can agree on the form of orders to be made, including, if agreement can be reached, on the question of costs, they are to provide to the Associate to Downes J draft orders to be made in chambers; or
(b) if the parties cannot agree on the form of orders to be made:
(i) each party is to provide to the Associate to Downes J their proposed draft orders marked up so as to show the areas of disagreement, including on the question of costs, along with any submissions limited to five pages, and also file and serve any affidavit relevant to the question of costs;
(ii) the proceeding be listed for case management hearing on 1 March 2024 at 10.15 am AEST to resolve the form of orders to be made.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
[1] | |
[25] | |
[33] | |
[33] | |
[36] | |
[39] | |
[39] | |
[65] | |
[66] | |
[73] | |
[77] | |
[78] | |
[82] | |
[82] | |
[90] | |
[95] | |
[101] | |
[102] | |
[107] | |
[108] | |
[108] | |
[119] | |
[119] | |
5.2.2 Knowing assistance of breach of equitable and fiduciary duties | [125] |
[132] | |
[132] | |
[180] | |
[194] | |
[197] | |
[197] | |
[202] | |
[202] | |
[204] | |
[208] | |
[220] | |
[226] | |
[230] | |
[232] | |
[232] | |
[239] | |
[243] | |
[262] | |
[263] | |
[277] | |
[288] | |
[295] | |
[296] | |
[298] | |
[307] | |
[309] | |
[311] | |
[315] | |
7.3.3 Conduct after infringement/being informed of infringement: s115(4)(b)(ib) | [321] |
7.3.4 Whether infringement involved conversion: s115(4)(b)(ii) | [327] |
[329] | |
[331] | |
[334] | |
[335] |
DOWNES J:
1 Ms Lisa Carroll and Mr Ross Macdougald, the second respondent, met in September 2011. They commenced living together in January 2012. They have six children between them. One of these children is Mr Jordan Macdougald (his son, not hers) who is the fifth respondent and was approximately 17 years old in January 2012. Other children include Mr Zac Carroll and Mr Cianan Joel (or CJ) Carroll (her sons, not his).
2 Mr Ross Macdougald was and remains a director of a company called FPI Oceania Pty Ltd, which is the fourth respondent. As at 2012, FPI was a wholesale distributor of essential oils, vegetable oils and ingredients used in products sold in the cosmetic and pharmaceutical industry. At this time, FPI sold a small number of botanical plant extracts supplied by Hughes & Co, which extracts had been manufactured by third parties. Mr Stephen Hughes was then a 51% shareholder and Mr Ross Macdougald was a 49% shareholder.
3 As a result of their personal relationship, Ms Carroll commenced to work for FPI on a part-time basis (two days a week) in a sales and marketing capacity. Initially, her role included reception duties, and she was also involved in various marketing activities, being an area in which she deposed to having some expertise.
4 During 2012, Ms Carroll and Mr Ross Macdougald had discussions about starting a new business which would manufacture and sell botanical plant extracts.
5 According to Ms Carroll’s unchallenged evidence, botanical plant extracts are derived directly from plants through a variety of extraction methods and “have been around for centuries”. She also deposed that extracts are used extensively in Australia and internationally, including (in 2012) in the beauty sector.
6 Before the first applicant, Native Extracts Pty Ltd, was incorporated, Mr Ross Macdougald told Ms Carroll about a type of extraction machine which he said he had learned about from his Italian oil supplier, Mr Andrea Parodi. More detail about this conversation appears below. Ms Carroll and Mr Ross Macdougald then travelled to Italy and received hands-on training on how to perform cold liquid extractions using the machine on dry eucalyptus leaves.
7 A 2 litre extraction machine was purchased by FPI and installed at its warehouse by Mr Zac Carroll, who was then a university student. In evidence is a manual for the machine, which relevantly states that the machine uses “an innovative solid-liquid extraction technique” and is designed to (inter alia) extract from officinal plants for the pharmaceutical, homeopathic, botanical and cosmetic sectors.
8 On 12 November 2012, Native Extracts was incorporated with Mr Ross Macdougald as the sole director. Native Extracts operated out of FPI’s warehouse, although the two companies had separate businesses. Ms Carroll became a director of Native Extracts on 22 April 2014.
9 Native Extracts was initially engaged in research and development, and it later commenced manufacturing and selling botanical plant extracts for use in products such as food, beverages, cosmetics and pharmaceuticals, using the 2 litre extraction machine referred to above as well as a 40 litre extraction machine acquired in late 2012. Native Extracts launched its first extracts for sale on 20 March 2013, and by her first affidavit, Ms Carroll explained the lengths to which she went at this time to inform customers about the cellular extraction methods being used by Native Extracts as a “clear point of differentiation” from its competitors.
10 On 3 July 2014, the Native Extracts Holding Trust came into existence, with Mr Ross Macdougald and Ms Carroll as its trustees. Certain transactions were entered into between Native Extracts and the Trust. The current trustee of the Trust is the second applicant.
11 Mr Jordan Macdougald was hired by his father in around October 2014 to work as a warehouse assistant for FPI. He gave evidence that he used one of the extraction machines on a limited number of occasions when Mr CJ Carroll was not present in the warehouse, but this was challenged by Mr CJ Carroll. In any event, Mr Jordan Macdougald’s involvement in the Native Extracts’ business was limited, even on his own evidence.
12 The third respondent, Phytoverse Pty Ltd, was incorporated by Mr Ross Macdougald on 15 December 2014, and it also operated a business out of the same warehouse.
13 Ms Carroll and Mr Ross Macdougald ended their personal relationship in about December 2015, and Mr Ross Macdougald agreed to leave the business of Native Extracts.
14 On 31 March 2016, Mr Ross Macdougald, Phytoverse and FPI entered a Deed of Settlement with (inter alia) Native Extracts and the Trust through its then trustees. Mr Jordan Macdougald witnessed his father’s signature on the deed, but he did not read it.
15 Relevantly, the Deed of Settlement contained a restraint of trade clause restraining Mr Ross Macdougald and any entities under his control from competing with the extract business conducted by Native Extracts, undertaking any work in the botanical extracts industry and contacting (inter alia) any current customers and growers of Native Extracts for a period of 12 months (the restraint period).
16 The Deed also contained a confidentiality clause restraining Mr Ross Macdougald, Phytoverse and FPI and “all employees, agents and contractors of Ross, Phytoverse and FPI” from disclosing to any third party “any knowledge, records or understanding of any information, processes, trade secrets, client records, or any other intellectual property used by” (inter alia) Native Extracts in the conduct of its business.
17 The first respondent, Plant Extracts Pty Ltd was incorporated on 8 July 2016. Mr Jordan Macdougald was appointed as one of its directors, along with a solicitor.
18 Like Native Extracts, Plant Extracts is in the business of manufacturing and selling botanical plant extracts, and it commenced operating that business shortly after its incorporation and within the restraint period. For that purpose, it used two extraction machines which had been acquired by Phytoverse in May 2016 and which were of the same kind as those used by Native Extracts. Both Mr Ross Macdougald and Mr Jordan Macdougald were involved in its business.
19 During the restraint period, Phytoverse provided a start-up working capital loan to Plant Extracts which totalled $58,518.04 by the end of that period.
20 Less than a month after the restraint period elapsed, the ownership and control of Plant Extracts was transferred to Mr Ross Macdougald.
21 The applicants commenced these proceedings in July 2020. At the trial held in October 2023, the respondents (other than Mr Jordan Macdougald) did not call any lay witnesses and amended their defence to admit many of the allegations made against them. After closing submissions on 12 October 2023, the respondents (other than Mr Jordan Macdougald) agreed to a form of order which was made on 19 October 2023: see Native Extracts Pty Ltd v Plant Extracts Pty Ltd [2023] FCA 1265 (Native Extracts (No 1)).
22 Despite those orders, the relief to be granted in respect of certain admitted breaches remains to be determined, and certain allegations concerning liability remain in dispute. All issues concerning Mr Jordan Macdougald remain live.
23 For convenience, the respondents (other than Mr Jordan Macdougald and Biologi Pty Ltd, the sixth respondent) will be referred to as the RM respondents.
24 The trial was held in open court.
25 Each of the claims raised in these proceedings (including those which have been admitted) were summarised by the applicants in their closing submissions. I have adopted that summary as follows:
(1) The applicants claim that Mr Ross Macdougald, Phytoverse and FPI breached the restraint of trade clause in the Deed of Settlement by assisting Plant Extracts to compete with Native Extracts, to undertake work in the extracts industry, to supply extracts and to contact Native Extracts’ growers and customers during the restraint period. They further allege that Plant Extracts was under the control of Mr Ross Macdougald during the restraint period and that, as a result, Native Extracts suffered loss and damage (the restraint of trade claims).
(2) The applicants claim that Mr Ross Macdougald and Phytoverse breached the confidentiality clause in the Deed of Settlement by disclosing information to Plant Extracts that was confidential to Native Extracts or the Trust. They submit that, by reason of those breaches, Mr Ross Macdougald also breached his statutory and equitable duties of confidence which arose by reason of his position as a director of Native Extracts and a trustee of the Trust, and that Plant Extracts, Phytoverse and Mr Jordan Macdougald breached their statutory and equitable duties by aiding and abetting, or by being knowingly concerned in, a party to, or knowingly involved in Mr Ross Macdougald’s breaches. They allege that, as a result, Native Extracts or the Trust suffered loss or damage and that Plant Extracts made unlawful profits (the breach of confidence claims).
(3) The applicants claim that Plant Extracts infringed Native Extracts’ copyright in scientific documents which Native Extracts commissioned from Southern Cross University. The applicants allege that Plant Extracts substantially reproduced and modified these scientific documents before sending them to customers and that, as a result, Native Extracts has suffered loss or damage and Plant Extracts has made unlawful profits. The applicants submit that the circumstances warrant an award of additional damages pursuant to s 115(4) of the Copyright Act 1968 (Cth) (the copyright infringements).
(4) The applicants claim that Plant Extracts and Biologi engaged in misleading or deceptive conduct contrary to ss 18 and 29 of the Australian Consumer Law, being Sch 2 to the Competition and Consumer Act 2010 (Cth) (ACL), and they seek declarations, injunctions and orders for corrective advertising. Damages are also sought by Native Extracts (the ACL contraventions).
26 By way of the Second Further Amended Defence (Defence) to the Second Further Amended Statement of Claim (SOC) tendered during closing submissions, the RM respondents and Biologi admit the bulk of the material facts concerning the restraint of trade claims, the copyright infringements and the ACL contraventions.
27 As for the breach of confidence claims, the applicants plead in the SOC that there were four categories of “confidential information” which had been (or were at risk of being) misused (defined as Confidential Information). Those categories are:
(1) the brand, model number and technical features of the extraction machine used by Native Extracts to manufacture its botanical plant extracts “in circumstances where that machine was not ordinarily used for the purpose of manufacturing botanical plant extracts” (which I will describe in these reasons as the pleaded information concerning the extraction machine);
(2) information concerning the methods, formulas and raw materials used by Native Extracts to manufacture its botanical plant extracts – namely Native Extracts’ Extract Log / Formula Book;
(3) information concerning the growers used by Native Extracts to manufacture its botanical plant extracts – namely Native Extracts’ Grower’s Matrix; and
(4) information concerning the customers and prospective customers to whom Native Extracts sold its botanical plant extracts – namely Native Extracts’ List of Suppliers and Customers.
28 By their Defence, the RM respondents and Biologi admit that:
(1) the Extract Log / Formula Book, Grower’s Matrix and the List of Suppliers and Customers are confidential information;
(2) Mr Ross Macdougald breached his contractual, statutory, fiduciary and equitable duties of confidence to Native Extracts and the Trust;
(3) Plant Extracts and Phytoverse were involved in Mr Ross Macdougald’s breach of his statutory duty of confidence to Native Extracts and assisted, with knowledge, in a dishonest and fraudulent design involving Mr Ross Macdougald’s breach of his fiduciary and equitable duties of confidence towards Native Extracts;
(4) Native Extracts has suffered loss and damage by reason of these matters, including the loss of sales of botanical plant extracts that Native Extracts would otherwise have made;
(5) Plant Extracts received profits which were unlawful.
29 At trial, Mr Jordan Macdougald did not challenge any of the matters referred to in the previous paragraph.
30 Because of the manner in which the respondents ran their respective cases, it is not necessary to decide whether Native Extracts or the Trust is the proper applicant for the purposes of the breach of confidence claims (noting that Native Extracts and the Trust advanced cases in the alternative). The respondents either admitted, or did not challenge at trial, the entitlement of Native Extracts to bring the breach of confidence claims, and so I will proceed on that basis.
31 However, all respondents dispute that the pleaded information concerning the extraction machine is confidential information, with the consequence that they deny the alleged breaches and contraventions, and alleged accessorial liability, insofar as those claims relate to the use of that information.
32 Other than the orders made on 19 October 2023, the balance of the relief sought by the applicants is opposed by the respondents. That relief as finally sought by the applicants was contained in a draft order which was handed up at the conclusion of the trial. Other than costs, no relief appears to be sought against Biologi in those draft orders.
33 The applicants relied upon the evidence of Ms Carroll and Mr CJ Carroll. Both prepared affidavit evidence and were cross-examined by the respondents.
34 Mr Jordan Macdougald filed two affidavits and was cross-examined by the applicants.
35 The RM respondents and Biologi did not lead any lay evidence at trial.
36 The applicants adduced the following expert evidence:
(1) the scientific report of Professor Melissa Fitzgerald dated 13 December 2021;
(2) the affidavit of Professor Fitzgerald affirmed 15 June 2022;
(3) the affidavit of Ms Rita Sellars affirmed 27 June 2022;
(4) the forensic accounting report of Mr David Stephens dated 24 June 2022; and
(5) the supplementary report of Mr Stephens dated 7 June 2023.
37 The RM respondents relied on a forensic accounting report prepared by Mr Elia Lytras dated 10 May 2023, which responded to the evidence contained in Mr Stephens’ first report.
38 On 14 September 2023, the forensic accounting experts, Mr Stephens and Mr Lytras, produced a joint expert report pursuant to Orders made by the Court (JER).
39 The applicants’ pleaded case is that the Confidential Information owned by Native Extracts includes the pleaded information concerning the extraction machine. The key facts relating to the extraction machine will now be addressed in more detail than appears above.
40 During 2012, Mr Ross Macdougald told Ms Carroll that “he had found out about a machine in Italy manufactured by Atlas Filtri, from his Italian oil supplier, Mr Parodi. Mr Parodi knew the engineer and patentee... The machine [was] designed to extract soluble substances in a liquid solid material from a product and suspend it in liquid. The process seemed to be established in Italy in the beverage sector, used to produce limoncello”. There is no suggestion that Mr Parodi supplied this information on a confidential basis, or that he has not informed others about this machine (either before or after 2012).
41 On 23 June 2012, Ms Carroll and Mr Ross Macdougald travelled to Italy, and on about 28 June 2012, they received training on how to operate the machine. Ms Carroll deposed that they received hands-on training on how to perform cold liquid extractions using the machine on dry eucalyptus leaves. There is no suggestion that the training was conducted on a confidential basis.
42 Neither Ms Carroll nor Mr Ross Macdougald had experience performing plant extraction processes prior to travelling to Italy, and after doing so, they “reached out to various sources for information, including [Mr] Hughes” in order to learn about botanical extracts. While information about certain extraction solvents and solvent ratios had been provided in their training, Mr Hughes later provided detailed suggestions on extraction mediums and preservatives to consider as well as general advice on the extraction process.
43 As to the extraction machine itself, Ms Carroll deposed that:
The [machine] is built and supplied by a manufacturer's representative in Italy, Atlas Filtri.
The solid (botanical plant matter) is placed in a filter bag, in a suitable container (the extractor) and is covered with extraction liquid. The patent indicates that this can be performed at a variety of temperatures including zero degrees Celsius.
Another way to describe the process utilised by the … machine would be a system using cold liquids within a closed system.
44 It is apparent from her evidence that Ms Carroll has read the patent of which the extraction machine appears to be an embodiment, and that it discloses information concerning extraction methods involving plants; however, the patent itself was not in evidence. Of course, a patent contains information which is in the public domain.
45 Ms Carroll deposed that, whilst undertaking the training in Italy in 2012, the pair was told by an unidentified person that the extraction machine was the first machine of its kind in Australia and had not been used to make botanical extracts. It must be observed that the conversation deposed to occurred around a decade prior to when Ms Carroll’s evidence was ultimately taken. Given the passage of time and the lack of detail concerning the source of the information, I find it difficult to accept this aspect of Ms Carroll’s evidence and accordingly, I place little weight on it.
46 Sometime after visiting the manufacturer, Ms Carroll and Mr Ross Macdougald determined to purchase a 2 litre model of the extraction machine. Ms Carroll deposed that the purchase was made through FPI for €6,000.
47 Ms Carroll deposed that Mr Hughes co-ordinated the purchase and delivery of the machine from the United Kingdom. As FPI funded the purchase and as he had this direct involvement, it may be inferred that Mr Hughes was aware of the brand, model and intended use of the extraction machine. However, there is no evidence or even a suggestion that Mr Hughes was asked or required to keep any information about the extraction machine confidential.
48 Ms Carroll deposed that, when the extraction machine arrived in Australia, Mr Zac Carroll installed and commissioned it at the premises of FPI at some time before 2 August 2012. There is no evidence that Mr Zac Carroll was asked or required to keep any information about the extraction machine confidential.
49 Ms Carroll annexed a manual for the extraction machine, which relevantly states that the machine uses “an innovative solid-liquid extraction technique” and is designed to:
• prepare fluid extracts, mother tinctures, glycerine preparations, oleolites
• extract from officinal plants for the pharmaceutical, homeopathic, botanical and cosmetic sectors
• extract from vegetable substances in the food, diet, zootechnical sectors
• produce fruit and herb spirit
• rehydrate dried vegetables quickly.
(Emphasis added.)
50 It then details the potential uses for the machine as follows:
Particularly suitable for research and analysis laboratories, industries, chemistry, universities, it can treat both dry and fresh materials such as: flowers, leaves, buds, fruit, roots, bark, stems, seeds, officinal plants, herbs and any other vegetable material.
51 After the extraction machine arrived, Ms Carroll and Mr Ross Macdougald started performing research and development at FPI’s warehouse using the machine.
52 Ms Carroll gave this evidence:
I did everything I could to keep the existence and identity of the [machine] confidential, including asking Steve Fawcett, Liam Hampson, my son Cianan Joel Carroll (CJ) and others in the warehouse not to talk about the machine.
53 This evidence is unsatisfactory in many respects. It is not explained by Ms Carroll when she spoke to those people and asked them “not to talk about the machine” (and for how long the machine had been in place when she did this), what position she held at the relevant time, who those “others in the warehouse” were, whether that request was made to others who commenced work at the warehouse at a later date, the substance of what she said to these people on each occasion and how they responded. Without this information, including as to the detail of the words that were used, it cannot be concluded that the circumstances were such as to impose an obligation of confidentiality on those people who were spoken to by Ms Carroll. For example, if the conversations occurred shortly after the extraction machines arrived, it is difficult to elevate a request by the part-time sales and marketing manager employed by FPI to a circumstance which imposes an obligation of confidentiality, especially as Ms Carroll does not appear to have been in any position of authority over the people in the warehouse and Native Extracts did not then exist.
54 Further, it is unclear what Ms Carroll means by doing everything she could, in circumstances where, according to her own evidence, it was not until an unidentified date in 2013, prior to a visit by an outside party to the warehouse, that Ms Carroll took steps to cover up the names of the machines so that the brand and model number could not be seen by those in the warehouse. As to this, Ms Carroll explained:
… I wanted to keep the brand, model number and technical specifications of the machine confidential, so that our competitors would not know that the machine was capable of manufacturing Extracts. At first, this was not a problem, because only Ross, Steve Fawcett, Liam Hampson and I were around the machine. However, in 2013, Chris Oliver from Blackmores visited Native Extracts’ warehouse, and Ross wanted to show Chris the machines. In order to keep our technology confidential, I arranged for all descriptive marks and plates on the 2L and 40L [extraction machines] to be covered up. … Those coverings still remain on the machines at the date of this affidavit.
55 Ms Carroll also gave evidence that a copy of the machine’s operating manual was kept “in or on CJ’s desk” in the warehouse. The copy of the manual in evidence contains multiple references to the machine’s brand, model number and technical specifications, including on the front cover. There is no evidence that there was any attempt to prevent the manual being seen by other employees or by visitors to the warehouse who might have walked past “CJ’s desk”. Indeed, Ms Carroll’s evidence suggests that the manual was seen in 2016 by at least one other employee, a graphic designer. There is no evidence that that employee was instructed to keep confidential any information obtained from the manual.
56 Ms Carroll also gave evidence that it was common for Native Extracts to ask potential partners to sign non-disclosure or confidentiality agreements. While the agreements in evidence define information broadly enough to cover plant and equipment, it does not alleviate my concerns as to the lack of confidentiality of the pleaded information concerning the extraction machine.
57 On 12 November 2012, Native Extracts was incorporated, which was approximately three months after the 2 litre extraction machine was installed at the warehouse. A 40 litre extraction machine was purchased at the end of 2012 and installed at the warehouse.
58 Mr Jordan Macdougald worked in the warehouse for FPI from October 2014, and he saw the extraction machines (although I accept that the machines would have had their brand and model number covered by this time). He gave evidence that he was trained on how to operate the machines, and he also gave evidence as to how to use them. Regardless of whether he used, or was trained on, the machines at this time, there is no evidence or even a suggestion by the applicants that he was instructed by anyone to keep any information about the machines confidential.
59 Ms Carroll and Mr Ross Macdougald had separated by the end of 2015, and they (along with other parties) entered the Deed of Settlement in March 2016.
60 Plant Extracts was incorporated on 8 July 2016. The evidence suggests that in May or June 2016, Phytoverse purchased a 2 litre and 40 litre model of the extraction machine, apparently on Plant Extracts’ behalf.
61 Mr Jordan Macdougald’s evidence was that, during August 2016, the extraction machines were delivered to the warehouse. He deposed that, once the extraction machines arrived, he and Mr Liam Hampson tested the machines and began making extracts. This evidence is consistent with the documentary evidence which shows that Plant Extracts began selling extracts in August 2016.
62 Ms Carroll also deposed that Ms Carla Prendin at Atlas Filtri has confirmed that, other than the machines sold to Native Extracts, Atlas Filtri has only supplied three other extraction machines in Australia, including two that were supplied in 2016. This means that at least one extraction machine has been acquired in Australia by a purchaser other than Native Extracts and Plant Extracts.
63 Ms Carroll also deposed that, to the best of her knowledge, prior to Native Extracts’ purchase of two machines, there were no machines like the extraction machine in Australia. However, the evidence did not establish that if other extraction machines had been purchased and shipped to Australia prior to 2012, Ms Carroll would have been aware of it. This evidence is therefore of no real assistance to the applicants.
64 During her oral evidence and while under cross-examination, Ms Carroll disclosed the brand name of the extraction machine in question.
4.2 Whether breach of equitable duty of confidentiality
65 The applicants accept that, to establish the alleged breaches of the equitable duty of confidence, they must establish that the pleaded information concerning the extraction machine is confidential information as that term is used in equity.
66 An equitable duty of confidence lies in the notion of an obligation of conscience arising from the circumstances in or through which the information was communicated or obtained: Moorgate Tobacco Co Ltd v Phillip Morris (No 2) (1984) 156 CLR 414 at page 438 (Deane J, with whom Gibbs CJ, Mason, Wilson and Dawson JJ agreed); see also Del Casale v Artedomus (Aust) Pty Ltd (2007) 73 IPR 326; [2007] NSWCA 172 at [101] (Campbell JA, McColl JA agreeing).
67 There are four elements that need to be satisfied to establish a claim for breach of confidence: Optus Networks Pty Ltd v Telstra Corporation Ltd (2010) 265 ALR 281; [2010] FCAFC 21 at [39] (Finn, Sundberg and Jacobson JJ); see generally IPC Global Pty Ltd v Pavetest Pty Ltd (2017) 122 IPR 445; [2017] FCA 82 at [189]–[196] (Moshinsky J).
68 First, the information in question must be identified with specificity.
69 Second, the information must have the necessary quality of confidence. This is a question of fact having regard to a range of factors. Such factors include the extent to which the information is known outside the business; the extent of measures taken to guard the secrecy of the information; the value of the information to the applicant and its competitors; the amount of effort or money expended by the applicant in developing the information; the ease or difficulty with which the information could be properly acquired or duplicated by others; and whether the usages and practices in the industry support the claim of confidentiality: see generally Del Casale at [40].
70 Whether the identified information has the necessary quality of confidence has sometimes been described as requiring the information to be relatively secret: see RLA Polymers Pty Ltd v Nexus Adhesives Pty Ltd (2011) 280 ALR 125; [2011] FCA 423 at [42] (Ryan J) adopting Finn J’s observations in Australian Medic-Care Co Ltd v Hamilton Pharmaceuticals Pty Ltd (2009) 261 ALR 501; [2009] FCA 1220 at [632]–[634]. It has also been said that, for information to have a necessary quality of confidence, it must not be something which is public property and public knowledge: Saltman Engineering Co Ltd v Campbell Engineering Co Ltd [1963] 65 RPC 203; [1963] 3 All ER 413 at page 415 (Lord Greene MR).
71 Third, the information must have been received by the respondent in circumstances importing an obligation of confidence. As Campbell JA said in Del Casale at [133] (with whom McColl JA agreed), the question is whether the information was imparted in circumstances where a reasonable person must have realised, on reasonable grounds, that he or she was not free to deal with the information as his or her own or must have realised that he or she could deal with the information only within certain limitations: see also IPC Global at [193].
72 Fourth, there must be an actual or threatened misuse of the information without the applicant’s consent.
73 The first and fourth elements are met in this case and require no further elaboration.
74 By the SOC, the applicants allege that the pleaded information concerning the extraction machine was of a confidential nature, that it was not in the public domain, and that it was the property of Native Extracts or, alternatively, the Trust.
75 However, for the following reasons and as to the second element, the pleaded information concerning the extraction machine did not constitute information which had the necessary quality of confidence and, contrary to the applicants’ pleaded case, it was in the public domain. Further, the pleaded information was not confidential to, and did not constitute trade secrets of, Native Extracts or the Trust such that either of them can claim any entitlement in equity to its protection. That is because, having regard to the facts above:
(1) information about the existence and brand of the extraction machine was the subject of unrestricted disclosures to Mr Ross Macdougald, Ms Carroll, Mr Hughes and Mr Zac Carroll, all prior to the incorporation of Native Extracts and the establishment of the Trust;
(2) information about the brand and technical features of the extraction machine was the subject of an unrestricted disclosure in Italy to Mr Ross Macdougald and Ms Carroll prior to the incorporation of Native Extracts and the establishment of the Trust, including the machine’s ability to perform extractions on eucalyptus leaves;
(3) information about the brand, model number and technical features of the extraction machine are contained in the manual, which was able to be viewed by any entrant to the warehouse when it was on “CJ’s desk”. In addition, there is no basis in the evidence to infer that a version of the manual is anything other than publicly available, or at least that one has been made available on an unrestricted basis to purchasers of the extraction machines. The manual identified that the machine is designed to (inter alia) “extract from officinal plants for the pharmaceutical, homeopathic, botanical and cosmetic sectors” which belies the notion that the machine is “not ordinarily used for the purpose of manufacturing botanical plant extracts”;
(4) information about the technical features of the extraction machine appears to be referred to in its associated patent, which is a public document which Ms Carroll was able to access and review, without any apparent restriction;
(5) having regard to the observations made above in relation to the relevant facts, the evidence was insufficient to demonstrate that information about the brand, model number and technical features of the extraction machine was kept confidential prior to and upon its arrival in Australia, and subsequently in the warehouse, let alone that it was confidential information owned by Native Extracts or the Trust. This is especially as the extraction machine arrived in the warehouse some months prior to the incorporation of Native Extracts;
(6) the evidence was insufficient to establish that all others in the botanical extracts industry (whether overseas or in Australia) were not aware of the extraction machine’s existence including its technical features and ability to manufacture botanical plant extracts, and that not one of them has used the same type of extraction machine for that purpose. This is especially as, even if no-one in the extracts industry was performing cellular extractions prior to Native Extracts doing so (and publicising that fact) in 2013, it does not follow that a competitor did not decide to copy Native Extracts in the interim and was not undertaking this form of extraction process in 2017 or later. This is particularly as Mr Ross Macdougald and Ms Carroll discovered the extraction machine through a contact, learned about its features from public sources and devised extraction processes using the machine. Neither of them appear to have any scientific qualifications, and there is no suggestion that a competitor in the same industry as Native Extracts could not also devise appropriate methods to use the extraction machine to manufacture plant extracts. Nor is there any reason that a competitor could not have discovered the existence of this specific brand and model of extraction machine and its ability to be used for that purpose.
76 As to the third element and having regard to the relevant facts, the evidence does not support a conclusion that the pleaded information concerning the extraction machine was received by Mr Ross Macdougald in circumstances importing an obligation of confidence to Native Extracts or the Trust. Mr Ross Macdougald was told about the extraction machine by a third party before Native Extracts and the Trust existed. There is nothing to suggest that he was prevented from sharing this information with anyone. He was then given further information about the extraction machine in Italy, and was trained to use it, and there was no restriction imposed on him concerning the information which he received or the content of his training (all occurring, again, before Native Extracts and the Trust came into existence). FPI purchased an extraction machine, it was installed at the warehouse and remained in situ for months before Native Extracts was incorporated. Ms Carroll did not depose to discussing with Mr Ross Macdougald that the pleaded information concerning the extraction machine be kept confidential by him.
77 For these reasons, the applicants have failed to establish that Mr Ross Macdougald owed an equitable duty of confidence to Native Extracts or the Trust in relation to the pleaded information concerning the extraction machine. It follows that Mr Ross Macdougald has not breached his equitable duty of confidence in this respect. The applicants also pleaded that Plant Extracts, FPI, Phytoverse and Mr Jordan Macdougald were bound by the same equitable duty of confidence in connection with the extraction machines; however, those claims were not pressed in closing submissions. Had they been pressed, they would have also failed on the basis that (at the least) the second element was not established by the evidence for the reasons given above.
4.3 Whether breach of fiduciary duty
78 The applicants allege that fiduciary duties were owed by Mr Ross Macdougald to each of Native Extracts and the beneficiaries of the Trust. In relation to the pleaded information concerning the extraction machine, this allegation was made on the basis that:
(1) he had obtained the information as a director, and therefore owed a duty not to disclose or use the information for profit without the fully informed consent of Native Extracts;
(2) as trustee of the Trust, he owed the beneficiaries of that trust a duty not to disclose or use any of the information he obtained while trustee for profit without the fully informed consent of the beneficiaries;
(3) the pleaded information concerning the extraction machine was Confidential Information (as defined) to which Mr Ross Macdougald had access to and possession of while he was a director of Native Extracts and trustee of the Trust.
79 However, having regard to the chronology of events established by the evidence (including the timing of the incorporation of Native Extracts and the establishment of the Trust), the applicants did not establish that Mr Ross Macdougald had obtained the pleaded information concerning the extraction machine in his capacity as director or trustee.
80 Further, and for the reasons already given, the pleaded information concerning the extraction machine was not “of a confidential nature and not in the public domain” as alleged by the applicants, with the consequence that it was not information which Mr Ross Macdougald knew or ought to have known was confidential to Native Extracts or trade secrets of Native Extracts and nor was it confidential information owned by Native Extracts such that it was capable of being assigned to the Trust as part of its intellectual property.
81 For these reasons, Mr Ross Macdougald did not breach his fiduciary duties to either Native Extracts or the beneficiaries of the Trust as alleged.
4.4 Whether breach of confidentiality clause
4.4.1 Key provisions of the Deed of Settlement
82 The recitals to the Deed of Settlement include the following statements:
A. Ross and Lisa were in a de facto relationship for the purposes of the Family Law Act 1975 (Cth).
B. During the course of the de facto relationship, Ross and Lisa incorporated the company known as Native Extracts of which:
a. Ross and Lisa are directors; and
b. Ross and Lisa are joint holders of sixty (60) shares in Native Extracts on trust for the Native Extracts Holding Trust.
C. The de facto relationship between Ross and Lisa ended during 2015.
D. Native Extracts sells extracts and native seed oil to various customers.
E. The Native Extracts Holding Trust owned all the intellectual property pertaining to botanical extraction processes including all formulas, research documentation and Southern Cross University analysis.
F. Phytoverse, a company controlled and owned by Ross, sells vegetable oils and other oils to customers.
G. Ross and Lisa are in a dispute about the future direction of Native Extracts and about its management generally.
H. On the terms set out in this Deed, the parties have agreed to a compromise of the dispute in relation to Native Extracts and all of their financial relationship.
83 Clause 3, which concerns the terms of settlement, provides:
3.1 Subject to Clause 3.3 and 3.4 of this Deed, within 2 business days of the date of this Deed:
3.1.1 Ross agrees to relinquish all rights he has (whether beneficially or otherwise) in Lisa’s Entities (including his role as a director, secretary[,] shareholder, appointer, trustee and/or beneficiary) and will transfer all of his right, title and interest in Lisa’s Entities and will sign all necessary transfers (or other documents as may be necessary) to convey the shares and control of Lisa’s Entities to Lisa. Ross further agrees that any descendant, spouse or other lineal or associated person of Ross shall be removed from any entitlement as beneficiary or otherwise that they may have in any of Lisa’s Entities.
…
3.3 In consideration for the transfer and control of Lisa’s Entities, Lisa and Native Extracts jointly and severally agree to pay, and are liable for, the following amounts to the following entities:
3.3.1 to Ross, the RM Loan within 2 business days of the date of this Deed;
3.3.2 to Phytoverse:
3.3.2.1 the Service Charge within 2 business days of the date of this Deed.
3.3.2.2 the Phytoverse Invoices within 2 business days of the date of this Deed;
3.3.2.3 the Phytoverse Management Fee to be paid as follows:
3.3.2.3.1 $60,500.00 (inclusive of GST) within 2 business days of the date of this Deed; and
3.3.2.3.2 $60,500.00 (inclusive of GST) within three (3) months from the date access is granted to the Premises.
84 “Lisa’s Entities” is defined in cl 1 of the Deed of Settlement as follows:
Lisa’s Entities: means:
• Native Extracts Pty Limited
• Onior Pty Ltd;
• Native Extracts Holding Trust;
• Australian Pharmacopoiea Pty Ltd;
• Wild Harvested Organics Pty Ltd; and
• Native Seed Oils Pty Ltd,
which, for the avoidance of doubt includes all plant & equipment, intellectual property and any other assets owned by each and all of the entities.
85 Clause 6 contains the restraint of trade, and provides:
6.1 Subject to clause 6.2, Ross, Phytoverse and FPI and any other entity under his control or that Ross may have an interest in as employee, shareholder or some other beneficial interest hereby agree that subject to payment of the sums set out in paragraph 3.3 of this Deed, that for a period of 12 months commencing on the date of this Deed that Ross, Phytoverse, FPI and any other entity that Ross is in control of be restrained from:
6.1.1 competing with Native Extracts’ current extract business;
6.1.2 undertaking any work in the botanical extract industry;
6.1.3 supplying water soluble botanical extracts;
6.1.4 providing Australian seed oils that will compete with Native Extracts native seed oil range;
6.1.5 undertaking work in the Australian native powder extracts industry; and
6.1.6 contacting any current Native Extracts Customers, Growers, Distributors, Partners or Investors.
6.2 Ross, Phytoverse, FPI or any entity controlled by Ross will be at liberty to trade, supply or sell Oils or other raw material (other than botanical extracts) to any customer (including new customers) and supply Oils or other raw material (other than botanical extracts) to customers that it has customarily supplied such Oils (or raw material) to even if the said customer is also a Native Extracts customer.
6.3 In consideration of the transfer of Ross’s interest in Lisa’s Entities, Native Extracts or any entity controlled by Lisa hereby undertake for a period of 12 months from the date of this Deed to not trade Oils with Natural Beauty Care Pty Ltd or otherwise compete against Phytoverse for the Natural Beauty Care Pty Ltd business in relation to Oil sales.
86 Clause 11, entitled “Confidentiality”, first addresses the confidentiality of the Deed of Settlement within cll 11.1–11.2 as follows:
11.1 Subject to Clause 11.2, the contents of this Deed and all books, documents and information made available to any party for the purposes of entering into this Deed or in the course of the performance of this Deed shall be kept confidential and shall not be disclosed to any other person without the written consent of the other parties, which consent is not to be unreasonably withheld.
11.2 Clause 11.1 shall not apply to any disclosure:-
l l.2.1 required by law;
11.2.2 required by any applicable stock exchange listing rules;
11.2.3 to solicitors, barristers, accountants or other professional advisers under a duty of confidentiality;
11.2.4 by a party to its bankers or other financial institutions, to the extent required for the purpose of raising funds or maintaining compliance with credit arrangements, if such banker of [sic] financial institution first gives a binding covenant to the other parties to maintain confidentiality, in form and substance satisfactory to the other parties;
l l.2.5 required by this Deed or necessary for or incidental to the performance of the obligations and duties contained in this Deed; and
11.2.6 of information in the public domain otherwise than due to a breach of Clause 11.1.
87 Clause 11.3 contains the confidentiality clause which is relied upon by the applicants. It states:
11.3 Ross, Phytoverse, FPI and all employees, agents and contractors of Ross, Phytoverse and FPI shall keep confidential and not disclose to any third party any knowledge, records or understanding of any information, processes, trade secrets, client records, or any other intellectual property used by Lisa’s Entities in the conduct of their businesses and Ross, Phytoverse and FPI will use their best endeavours to ensure compliance with this clause by any applicable person.
88 The “knowledge, records or understanding of any information, processes, trade secrets, client records, or any other intellectual property” which form the subject of the confidentiality clause are not defined in the Deed of Settlement.
89 There is no dispute that cl 11.3 of the Deed of Settlement imposes an obligation of confidence on Mr Ross Macdougald, Phytoverse and FPI. The dispute concerns whether that obligation extends to the pleaded information concerning the extraction machine.
4.4.2 Principles of construction
90 A convenient summary of the general principles of construction is to be found in Mount Bruce Mining Pty Limited v Wright Prospecting Pty Limited (2015) 256 CLR 104; [2015] HCA 37 where French CJ, Nettle and Gordon JJ said at [46]–[51]:
The rights and liabilities of parties under a provision of a contract are determined objectively, by reference to its text, context (the entire text of the contract as well as any contract, document or statutory provision referred to in the text of the contract) and purpose.
In determining the meaning of the terms of a commercial contract, it is necessary to ask what a reasonable businessperson would have understood those terms to mean. That inquiry will require consideration of the language used by the parties in the contract, the circumstances addressed by the contract and the commercial purpose or objects to be secured by the contract.
Ordinarily, this process of construction is possible by reference to the contract alone. Indeed, if an expression in a contract is unambiguous or susceptible of only one meaning, evidence of surrounding circumstances (events, circumstances and things external to the contract) cannot be adduced to contradict its plain meaning.
However, sometimes, recourse to events, circumstances and things external to the contract is necessary. It may be necessary in identifying the commercial purpose or objects of the contract where that task is facilitated by an understanding “of the genesis of the transaction, the background, the context [and] the market in which the parties are operating”. It may be necessary in determining the proper construction where there is a constructional choice…
Each of the events, circumstances and things external to the contract to which recourse may be had is objective. What may be referred to are events, circumstances and things external to the contract which are known to the parties or which assist in identifying the purpose or object of the transaction, which may include its history, background and context and the market in which the parties were operating. What is inadmissible is evidence of the parties’ statements and actions reflecting their actual intentions and expectations.
Other principles are relevant in the construction of commercial contracts. Unless a contrary intention is indicated in the contract, a court is entitled to approach the task of giving a commercial contract an interpretation on the assumption “that the parties ... intended to produce a commercial result”. Put another way, a commercial contract should be construed so as to avoid it “making commercial nonsense or working commercial inconvenience”.
(Footnotes omitted.)
91 In LCA Marrickville Pty Ltd v Swiss Re International SE (2022) 290 FCR 435; [2022] FCAFC 17, Derrington and Colvin JJ (with whom Moshinsky J agreed) observed at [57]–[58]:
It is often identified as “trite law” that the duty of a court when construing a document is to discover its meaning by considering it “as a whole”: Australian Broadcasting Commission v Australasian Performing Rights Association Ltd (1973) 129 CLR 99 at 109 per Gibbs J. The rationale is, as Gibbs J observed, that “the meaning of any one part of it may be revealed by other parts” and, as a corollary, “the words of every clause must if possible be construed so as to render them all harmonious one with another”. In Wilkie v Gordian Runoff Ltd (2005) 221 CLR 522 (Wilkie v Gordian Runoff), a majority of the High Court observed that in construing a policy of insurance, as with other instruments, “preference is given to a construction supplying a congruent operation to the various components of the whole”: at [16] citing Project Blue Sky Inc v Australian Broadcasting Authority (1998) 194 CLR 355 at [69]–[71]. Necessarily, the identification of that construction can only be achieved by ascertaining how a contract or policy might operate as affected by each of the competing interpretations. This “iterative process”, involving “checking each of the rival meanings against the other provisions of the document and investigating its commercial consequences”, “enables a court to assess whether either party’s preferred legal meaning gives rise to a result that is more or less internally consistent and avoids commercial absurdity”: HP Mercantile Pty Ltd v Hartnett [2016] NSWCA 342 at [134] quoting Re Sigma Finance Corp [2010] BCC 40 at [12].
In the interpretive process, the concept of reading a document as a whole involves more than merely acquiring an awareness of the surrounding and related provisions. It requires a substantive intellectual process of evaluating the degree of operative coherence and consistency between a proffered construction and the instrument’s other terms. ...
(Emphasis original.)
92 A contractual obligation may be imposed which requires a person to keep information confidential which extends beyond the subject matter which would otherwise be protected by an equitable duty of confidence: see Zomojo Pty Ltd v Hurd (No 2) (2012) 299 ALR 621; [2012] FCA 1458 at [179(1)] (Gordon J) and the cases cited therein. However, absent an express indication to the contrary, a contractual confidentiality provision will generally be construed as limited only to information which is confidential in character: Isaac v Dargan Financial Pty Ltd (2018) 98 NSWLR 343; [2018] NSWCA 163 at [137] (Gleeson JA, with whom Bathurst CJ and Beazley P agreed). In Maggbury Pty Limited v Hafele Australia Pty Limited (2001) 210 CLR 181; [2001] HCA 70, Gleeson CJ, Gummow and Hayne JJ said at [45]:
Ordinarily, the obligations relating to the use and disclosure of the information would be construed as limited to subject matter which retained the quality of confidentiality at the time of breach or threatened breach of those obligations. An expression of a contrary intent should [be] explicit…
93 Finally, as Lord Diplock said in Antaios Compania Naviera SA v Salen Rederierna AB [1985] AC 191 at 201:
… if detailed semantic and syntactical analysis of words in a commercial contract is going to lead to a conclusion that flouts business commonsense, it must be made to yield to business commonsense.
94 This passage was approved by the majority of the High Court in Maggbury at [43].
95 The applicants assert that the obligation under cl 11.3 attaches to any knowledge, information (and so on) used by Native Extracts or the Trust, such that establishing a breach of cl 11.3 is not dependent on the relevant information being properly characterised as confidential (i.e. the clause can cover subject matter which would not generally be considered as confidential in equity). The necessary consequence of this submission is that Mr Ross Macdougald (for example) would be prevented from ever utilising the extraction machines in the business of Plant Extracts.
96 However, for the following reasons, a reasonable businessperson would immediately reject such a construction as flawed or as a commercial nonsense.
97 First, adopting the applicants’ literal approach has the consequence that cl 11.3 would capture the disclosure of any knowledge or understanding of any information used by Lisa’s Entities in the conduct of their businesses, not just information which is confidential. So, for example, if Native Extracts used a commercially available accounting software program in its business, Mr Ross Macdougald would be prevented by cl 11.3 from disclosing his knowledge of the name of that software program or his understanding of how that program operates to any third party such as Plant Extracts. That would be so even if he had known of the existence of that program before Native Extracts was incorporated and the program had been and continues to be available for purchase by anyone. Such an extreme result cannot have been intended.
98 Second, the Deed of Settlement contains the restraint of trade clause. Construing the Deed as a whole and objectively, a reasonable person would consider that the intention of the parties was that Mr Ross Macdougald would be able to work in the botanical extracts industry and compete with Native Extracts after the expiration of the restraint period. In doing so, it must have been contemplated that he would be able to use commercially available products, such as the extraction machine, and publicly known methods for using that machine, if he resumed work in that industry after the restraint period ended. That is the result which it is assumed that the parties intended as otherwise it would lead to a commercial absurdity.
99 Third, the obligation imposed by cl 11.3 should be construed as limited to subject matter which retained the necessary quality of confidence at the time of breach or threatened breach. In this case and for the reasons given above, the pleaded information concerning the extraction machine has not ever had, let alone retained, the necessary quality of confidence.
100 There is a weak argument that the inclusion of the exceptions in cl 11.2 which apply to cl 11.1, but not cl 11.3, indicate that the parties turned their minds to excluding information in the public domain from the application of cl 11.1 (which is also a confidentiality clause) but not cl 11.3. It might then be said that the failure to have similar exceptions to the operation of cl 11.3 supports the broader construction of that clause. However, such an argument cannot be accepted when one considers the type of information which would be captured by the language used in cl 11.3, and the commercially untenable consequences which follow from such a construction. Further, the failure to include express exceptions to the operation of cl 11.3 is not an explicit expression of a contrary intent of the kind referred to in Maggbury at [45]. Rather, it bears the hallmarks of oversight.
101 For these reasons, the pleaded information concerning the extraction machine does not fall within cl 11.3 of the Deed of Settlement with the consequence that there has been no breach by Mr Ross Macdougald of that clause.
4.5 Whether breach of statutory duty of confidentiality
102 Finally, the applicants contend that, by his alleged disclosure to Plant Extracts of the pleaded information concerning the extraction machine, Mr Ross Macdougald breached his duty owed to Native Extracts pursuant to s 183(1) of the Corporations Act 2001 (Cth) by reason of his former position as a director of that company.
103 There is no dispute that Mr Ross Macdougald owes a continuing duty to Native Extracts under s 183(1). That section relevantly provides:
A person who obtains information because they are, or have been, a director or other officer or employee of a corporation must not improperly use the information to:
(a) gain an advantage for themselves or someone else; or
(b) cause detriment to the corporation.
Note 1: This duty continues after the person stops being an officer or employee of the corporation.
104 The applicants submit that the disclosure of the details of the extraction machine was a breach of s 183(1) as knowledge of the exact model of extraction machine to be purchased, and its usage in the botanical extracts industry, was obtained by Mr Ross Macdougald because of his position as a director of Native Extracts.
105 However, having regard to the chronology of events established by the evidence (including the timing of the incorporation of Native Extracts), the evidence did not establish that Mr Ross Macdougald had obtained the pleaded information concerning the extraction machine in his capacity as director.
106 For these reasons, Mr Ross Macdougald did not breach his duty under s 183(1) of the Corporations Act as alleged.
107 For these reasons, the breach of confidence claims, insofar as they relate to “the brand, model number and technical features of the machine used by [Native Extracts] to manufacture its botanical plant extracts in circumstances where that machine was not ordinarily used for the purpose of manufacturing botanical plant extracts”, must fail.
5. ACCESSORIAL LIABILITY OF MR JORDAN MACDOUGALD
108 The following is a summary of the key allegations in the case brought against Mr Jordan Macdougald as they appear in the SOC.
109 Shortly after the exit of Mr Ross Macdougald from Native Extracts, Mr Jordan Macdougald incorporated Plant Extracts and commenced the following conduct (which I will call the pleaded conduct):
(1) manufacturing and selling botanical plant extracts in competition with Native Extracts;
(2) undertaking research and development in the botanical plant extract industry;
(3) supplying water soluble botanical extracts;
(4) contacting Native Extracts’ growers to source raw materials to manufacture botanical plant extracts; and
(5) contacting Native Extracts’ customers to sell its botanical plant extracts.
110 From its incorporation and during the restraint period, Plant Extracts engaged Mr Ross Macdougald to assist in carrying out the pleaded conduct.
111 Further or alternatively to the above, prior to the incorporation of Plant Extracts, there was an understanding reached between Mr Ross Macdougald, Mr Jordan Macdougald and Mr Ashley Moore (a solicitor) that:
(1) during the restraint period, Mr Ross Macdougald would assist Plant Extracts and Mr Jordan Macdougald in carrying out the pleaded conduct;
(2) Mr Jordan Macdougald and Mr Moore would act in accordance with the wishes and instructions of Mr Ross Macdougald in carrying out the pleaded conduct; and
(3) after the restraint period ended, Mr Jordan Macdougald and Mr Moore would resign as directors of Plant Extracts and its holding company HGWIT Pty Ltd, transfer their shares in HGWIT Pty Ltd to Mr Ross Macdougald, and Mr Ross Macdougald would become the sole director of Plant Extracts and legal owner of all share capital issued in HGWIT Pty Ltd.
112 From its incorporation, Plant Extracts, Mr Jordan Macdougald and Mr Moore gave effect to that understanding. By reason of this, Mr Ross Macdougald acted as a de facto director and shadow director of Plant Extracts, and Plant Extracts was therefore under his control.
113 By reason of the above matters, Mr Ross Macdougald disclosed the Confidential Information to (inter alia) Plant Extracts and Mr Jordan Macdougald or alternatively there was disclosure of the Confidential Information to Mr Jordan Macdougald by Phytoverse and FPI.
114 By no later than August 2016, Plant Extracts had commenced using the Confidential Information in its business to (inter alia) manufacture, supply and sell botanical plant extracts.
115 The Confidential Information (as defined) in the SOC is, apart from the pleaded information concerning the extraction machine:
(1) the Extract Log / Formula Book;
(2) Grower’s Matrix;
(3) List of Suppliers and Customers.
116 In [50] of the SOC, it is pleaded that this occurred in circumstances where Mr Jordan Macdougald knew, or ought to have known, that:
(1) Mr Ross Macdougald had been a director of Native Extracts and a trustee of the Trust;
(2) Mr Ross Macdougald had recently executed the Deed of Settlement;
(3) the Confidential Information (as defined) was information confidential to, and trade secrets of, Native Extracts or the Trust;
(4) Mr Ross Macdougald obtained the Confidential Information while he was a director of Native Extracts and a trustee of the Trust; and
(5) Mr Ross Macdougald was not permitted to use information he obtained whilst he was a director of Native Extracts and a trustee of the Trust for his own benefit or for the benefit of Plant Extracts, Phytoverse, FPI or Mr Jordan Macdougald.
117 Based on these facts and by the SOC, the applicants allege that Mr Jordan Macdougald:
(1) has, for the purposes of ss 79 and 183(2) of the Corporations Act, aided or abetted Mr Ross Macdougald, or been directly or indirectly knowingly concerned in, or a party to, Mr Ross Macdougald’s breach of his Statutory Duty of Confidence;
(2) was bound by an Equitable Duty of Confidence not to use or disclose the Confidential Information without the consent of Native Extracts or the Trust. However, as already noted, this claim was not pressed during closing submissions;
(3) assisted, with knowledge, Mr Ross Macdougald in a dishonest and fraudulent design, involving the breach of his Equitable Duty of Confidence, his Fiduciary Duty (Confidence) and his Fiduciary Duty (Trustee).
118 It should be observed at the outset that, to the extent that these allegations are premised upon the contention that the pleaded information concerning the extraction machine is Confidential Information, those allegations fail for the reasons given above.
5.2.1 Involvement in a breach of the Corporations Act
119 Section 183(2) of the Corporations Act provides that a person who is “involved” in a contravention of s 183 will also contravene s 183.
120 The term “involved” is defined in s 79 of the Corporations Act, which provides that:
A person is involved in a contravention if, and only if, the person:
(a) has aided, abetted, counselled or procured the contravention; or
(b) has induced, whether by threats or promises or otherwise, the contravention; or
(c) has been in any way, by act or omission, directly or indirectly, knowingly concerned in, or party to, the contravention; or
(d) has conspired with others to effect the contravention.
121 Mr Jordan Macdougald cannot be found to have been “involved” in any of the breaches of the Corporations Act unless he intentionally participated in them: Re PFS Wholesale Mortgage Corporation Pty Ltd; Australian Securities and Investments Commission v PFS Business Development Group Pty Ltd (2006) 57 ACSR 553; [2006] VSC 192 at [390] (Hargrave J) citing Yorke v Lucas (1985) 158 CLR 661 (Mason ACJ, Wilson, Deane and Dawson JJ). That is, the applicants must establish that Mr Jordan Macdougald had actual knowledge at the time of the contraventions of each of the essential matters that go to make up the contraventions, although it was not necessary for him to know that the matters amounted to a contravention.
122 Although constructive knowledge is not sufficient, knowledge of matters giving rise to suspicion and the wilful failure to make appropriate inquiry when confronted with the obvious make it possible to infer knowledge of the relevant essential matters: see Re PFS Wholesale at [390]; Australian Securities and Investments Commission v ActiveSuper Pty Ltd (in liq) (2015) 235 FCR 181; [2015] FCA 342 at [400] (White J).
123 However, it is not sufficient for the purposes of s 79 that a person acquires knowledge of the essential matters which go to make up the contravention after it has occurred and, at that time, fails to take appropriate action even if the effect of that action is to conceal, ratify or knowingly derive benefit from the contravention: see Australian Securities and Investments Commission v Australian Investors Forum Pty Ltd (No 2) (2005) 53 ACSR 305; [2005] NSWSC 267 at [114]–[118] (Palmer J) approved in Digital Cinema Network Pty Ltd v Omnilab Media Pty Ltd (No 2) [2011] FCA 509 at [171] (Gordon J) (upheld on appeal in Omnilab Media Pty Ltd v Digital Cinema Network Pty Ltd (2011) 86 ACSR 674; [2011] FCAFC 166 (Jacobson, Rares and Besanko JJ)).
124 The elements of the contraventions of s 183 which the applicants must demonstrate that Mr Jordan Macdougald had knowledge of are that Mr Ross Macdougald:
(1) was, at the relevant time, a director of Native Extracts; and
(2) acquired the Confidential Information; and
(3) acquired the Confidential Information by virtue of his position as director; and
(4) made improper use of that information; and
(5) made that improper use in order to directly or indirectly gain an advantage; and
(6) gained that advantage either for himself or for some other person or persons; or
(7) alternatively, made that improper use to cause detriment to Native Extracts.
See Smart EV Solutions Pty Ltd v Guy [2023] FCA 1580 at [69] (Derrington J) citing Huang v Wang [2015] NSWSC 510 at [41] (Black J).
5.2.2 Knowing assistance of breach of equitable and fiduciary duties
125 The principles concerning liability for knowing assistance (otherwise referred to as the second limb of Barnes v Addy (1874) LR 9 Ch App 244) are well-established. In broad terms, the applicants must establish that:
(1) Mr Jordan Macdougald possessed the requisite degree of knowledge;
(2) Mr Ross Macdougald’s breaches of equitable and fiduciary duty were dishonest and fraudulent; and
(3) Mr Jordan Macdougald actually assisted Mr Ross Macdougald in the dishonest and fraudulent breaches. With this, it must be shown that he had the intention of furthering that dishonest and fraudulent breach – merely permitting or allowing the breach to occur may be insufficient.
See Digital Cinema Network at [172]–[177] (Gordon J).
126 As the High Court clarified in Farah Constructions Pty Limited v Say-Dee Pty Limited (2007) 230 CLR 89; [2007] HCA 22 at [171]–[178], the following four categories of knowledge are sufficient to establish liability for knowing assistance:
(1) actual knowledge;
(2) wilfully shutting one’s eyes to the obvious;
(3) wilfully and recklessly failing to make such inquiries as an honest and reasonable person would make; and
(4) knowledge of circumstances that would indicate the facts to an honest and reasonable person.
127 The applicants submit that the final three categories apply in the present case. In these reasons, these three categories will be described collectively as the requisite degree of knowledge.
128 The allegation that Mr Jordan Macdougald assisted his father in his dishonest and fraudulent breach ought to be assessed having regard to the principles in Briginshaw v Briginshaw (1938) 60 CLR 336.
129 As will be seen, aspects of the case advanced by the applicants in their closing submissions were not pleaded or opened. They ought to have been if it was to be contended by the applicants that there were facts and circumstances from which it ought to be inferred that Mr Jordan Macdougald had actual or the requisite degree of knowledge required to impose liability on him: see r 16.43 Federal Court Rules 2011 (Cth). This is especially as Mr Jordan Macdougald represented himself at the trial.
130 In Say-Dee at [170], the High Court referred to an allegation that a person was liable as a knowing participant in a dishonest and fraudulent design as, “an allegation the seriousness of which means that it ought to have been pleaded and particularised, and the assessment required by Briginshaw v Briginshaw …kept in mind”.
131 In Briginshaw, Dixon J (as his Honour then was) said at pages 361–362:
Fortunately, however, at common law no third standard of persuasion was definitely developed. Except upon criminal issues to be proved by the prosecution, it is enough that the affirmative of an allegation is made out to the reasonable satisfaction of the tribunal. But reasonable satisfaction is not a state of mind that is attained or established independently of the nature and consequence of the fact or facts to be proved. The seriousness of an allegation made, the inherent unlikelihood of an occurrence of a given description, or the gravity of the consequences flowing from a particular finding are considerations which must affect the answer to the question whether the issue has been proved to the reasonable satisfaction of the tribunal. In such matters “reasonable satisfaction” should not be produced by inexact proofs, indefinite testimony, or indirect inferences.
5.3.1 Overarching submissions by applicants
132 The applicants submit that the following facts support an inference that “[Mr] Jordan Macdougald [was] involved in Ross Macdougald’s breach of his Statutory Duty of Confidence and knowingly assisted in a dishonest and fraudulent design involving Ross Macdougald’s breach of his Fiduciary Duty (Confidence), his Fiduciary Duty (Trustee) and/or his Equitable Duty of Confidence” (which are defined terms in the SOC) (the relevant inference).
133 The extraction machines: the first fact relied upon in the applicants’ submissions in support of the relevant inference is that Plant Extracts purchased and uses the exact same 2 litre and 40 litre extraction machines used by Native Extracts.
134 However, for the reasons already given, the pleaded information concerning the extraction machines is not Confidential Information within the meaning of the SOC and the mere fact of the purchase and use by Plant Extracts of the same machines in its business did not constitute a breach of any duty by Mr Ross Macdougald.
135 In any event, there was no evidence that Mr Jordan Macdougald had actual or the requisite degree of knowledge that information about the brand, model number and technical features of the extraction machines constituted information which was not in the public domain (as the applicants allege in the SOC) or that it was confidential information owned by Native Extracts or the Trust. Nor did the applicants establish that Mr Jordan Macdougald had actual or the requisite degree of knowledge that the extraction machines were not ordinarily used for the purpose of manufacturing botanical plant extracts, and that this information was confidential information.
136 To the contrary, under cross-examination, Mr Jordan Macdougald gave this evidence:
Now, we then come to paragraph 44 of your affidavit, where you’re referring to some extraction machines arriving. Now, you know, don’t you, that these machines were ordered and paid for by Phytoverse?---The – the two machines outlined in the – my affidavit?
Yes?---I didn’t at the time. I – I didn’t know at the time who actually paid for them.
But you know that now?---I do know that now. Yes.
[sic] and, again, that was something that – the choosing of those machines wasn’t something that you were involved in?---Well, it’s pretty logical to get the same machines that I had working knowledge of.
Pretty logical. So did you discuss that with your father?---No.
So the machines just turned up?---Essentially, yes.
And were you surprised that they were exactly the same as the ones that you had seen at Native Extracts and ---?---No.
---you say you has [sic] used before?---I – I – I – I wasn’t surprised. No.
You knew, didn’t you, that the reason that they were exactly the same was because the information about what machine to be using and what machine to be ordering was something that Ross had – your father had from his time at Native Extracts?---Well, I just knew you could just go buy the machines. There wasn’t a barrier to entry. It wasn’t – they were, like, an off-the-shelf kind of thing. If you had the contact, then you could buy them.
And you say you know – you knew all about that at the time?---Yes.
But it wasn’t you having any involvement in choosing these machines?---No. But I – yes, I did know that – know that at the time.
But you also knew too, didn’t you, that these were machines that others hadn’t been using for botanical extracts---?---I didn’t know ---
--- other than Native Extracts?---I didn’t know if anyone else was using them or not.
(Emphasis added.)
137 It was not put to Mr Jordan Macdougald that any aspect of this evidence was false. Nor was any evidence adduced by the applicants to contradict this evidence insofar as it relates to Mr Jordan Macdougald’s knowledge. Nor was any submission made by the applicants that this evidence given by Mr Jordan Macdougald should not be accepted and why that is so.
138 The applicants also submit that:
Given the measures taken by Native Extracts to protect the secrecy of the extraction machines, knowledge of the exact model of extraction machine to be purchased could only have been obtained by Ross Macdougald as a result of his position as a director of Native Extracts. There can be no doubt that an honest and reasonable person in Jordan Macdougald’s position would have known that to be the case.
139 However, the use of a commercially available extraction machine by Plant Extracts in a business engaged in manufacturing plant extracts is unremarkable. No suggestion was made by the applicants that Mr Jordan Macdougald knew about the measures taken by Native Extracts to protect the secrecy of the extraction machines, and there was no evidence to support a proposition that an honest and reasonable person in his position would have such knowledge, or why that was so. How then would Mr Jordan Macdougald or an honest and reasonable person in his position know that information as to the exact model of extraction machine to be purchased could only have been obtained by Mr Ross Macdougald as a result of his position as a director of Native Extracts? Other than submitting that there be “no doubt”, the applicants’ submissions do not answer this question.
140 This aspect of the applicants’ submissions is therefore rejected.
141 Absence of research and development: the second fact relied upon in the applicants’ closing submissions in support of the relevant inference is that Plant Extracts “created” its extracts and obtained customers and suppliers without any true initial research and development phase.
142 They submit that Plant Extracts immediately started making extracts after obtaining the extraction machines, selling those extracts to Native Extracts’ customers and purchasing raw materials from Native Extracts’ suppliers. A series of further facts are then listed in [120] of their closing submissions as follows:
(a) Jordan Macdougald pleads in [15(c)(ii)] of his Defence that no research or development in the botanical extract industry was undertaken and gives evidence that immediately after receiving and testing the extraction machines he and Mr Hampson started making extracts;
(b) the evidence set out [above] shows that Ross Macdougald, through Plant Extracts and under the guise of Jordan Macdougald, immediately engaged in a concerted effort to poach Native Extracts’ customers;
(c) as early as 19 July 2016, Ross Macdougald, through Plant Extracts and under the guise of Jordan Macdougald, was offering potential customers “full technical data” for Plant Extracts’ extracts. This is 11 days after Plant Extracts was incorporated. As discussed in Part F of these submissions, in fact, Plant Extracts “Datapacks” included SCU Certificates that belong to, and had been taken from, Native Extracts;
(d) as early as August 2016, Plant Extracts sold extracts that had already been developed by Native Extracts to pre-existing Native Extracts customers;
(e) in Plant Extracts’ first year of operation (FY17):
(i) at least 82 per cent of the sales made by Plant Extracts were sales of extracts Native Extracts had already developed prior to 1 April 2016. If the sales of blends are removed from Plant Extracts’ sales data, 97.8 per cent of Plant Extracts’ sales were sales of extracts that had already been developed by Native Extracts;
(ii) 99.6 per cent of the sales made by Plant Extracts were sales to Native Extracts’ customers prior to 1 April 2016. In other words, of the $86,180 Plant Extracts made in sales in FY17, only $345 of those sales were to customers who were not previously Native Extracts customers. Further, of the $345, $150 of those sales were to a related party (Biologi), leaving only $195 of sales to unique Plant Extracts customers;
(iii) Plant Extracts purchased raw material from Native Extracts’ suppliers.
(Footnotes omitted, emphasis original.)
143 Such a case was not pleaded or opened in these terms for the purposes of seeking the relevant inference in relation to Mr Jordan Macdougald. Rather, some (but not all) of these facts were opened for the purposes of seeking a finding that there had been disclosure of Confidential Information.
144 Further, contrary to their closing submissions, the applicants plead in the SOC (as part of the facts relied upon against Mr Jordan Macdougald) that:
[15] On 8 July 2016, [Mr Jordan Macdougald] incorporated [Plant Extracts] and commenced…undertaking research and development in the botanical plant extract industry…
…
[46] On or about 8 July 2016, [Plant Extracts] commenced using the Confidential Information to … undertake research and development in the botanical plant extract industry…
145 This means that Mr Jordan Macdougald was confronted with closing submissions which contradicted the case which had been pleaded against him.
146 Having regard to the serious allegations which are made and as a matter of procedural fairness, these matters provide a sufficient reason to reject the applicants’ submission concerning this second fact, and I do so.
147 However, for the following reasons, this aspect of the applicants’ case as advanced in the closing submissions lacks merit, and is rejected, in any event. The evidence did not demonstrate that Mr Jordan Macdougald had actual or the requisite degree of knowledge of the matters in the series of facts relied upon by the applicants in [120] of their closing submissions (and the applicants did not identify the evidence to show that he did).
148 As to facts (a) and (c), Mr Jordan Macdougald gave evidence that he was not concerned that Plant Extracts was offering what counsel for the applicants described as “all these extracts and full technical data only 11 days after the company was registered”, and that he believed that this was something that the company had capacity to do. No suggestion was made to him that there were particular facts that should have alerted him to anything or put him on notice of anything, and nor was it suggested to him that he did not have the state of mind which he said that he did. Nor was any submission made by the applicants that his evidence should not be accepted and why.
149 This evidence was given in the context of other evidence given by Mr Jordan Macdougald which was to the effect that, having regard to the way he approached it, the manufacture of the extracts using the extraction machines was not a difficult process. He explained that:
Once the machines were working in the warehouse, I began making extracts and implemented a QA system/batching for the manufacturing of the extracts. I made up the labels for the bottled extracts and Dad provided the graphic design for the logo. I based the system off the Phytoverse labels. For each extract batch produced, I took samples and made up certificates of analysis (CofA), which are one page documents with the extract name, production date, expiry, botanical names, specific gravity and refractive index results, and the colour of the extract.
150 No challenge was made to this evidence on the basis that Mr Jordan Macdougald must have been privy to, or provided with, the content of the Extract Log / Formula Book (being part of the Confidential Information in the case pleaded against him) to manufacture the extracts, or that he was not able to prepare the certificates of analysis in the way that he said that he did without being provided with information by his father.
151 Further, the unchallenged evidence of Mr Jordan Macdougald was that the certificates of analysis prepared by him were not the modified versions of the certificates issued by the Southern Cross University. The suggestion by fact (c) that Plant Extracts had, from as early as 19 July 2016, sent Datapacks to potential customers which included these certificates is therefore not supported by the evidence cited by the applicants.
152 Fact (b) relates to the conduct of Mr Ross Macdougald. In any event, Mr Jordan Macdougald gave evidence that he was told by his father, and believed, that the former customers of Native Extracts who were now buying from Plant Extracts “did not want to work with Lisa” and they were not being serviced by Native Extracts.
153 Mr Jordan Macdougald maintained this position when shown evidence of interactions with customers of Native Extracts during cross-examination as follows:
And you knew, didn’t you, that this company was a customer of Native Extracts at the time?---I believe this was one of the companies that didn’t want to work with Native Extracts.
And you were – believed that because you were told that by your father?---I do, yes. I did.
…
Right. So I was asking you about this Natural Beauty one. Did you know that this was a company – a customer of Native Extracts as well?---No. Again, I – I say that – like, I – all these customers I just assumed they were customers that didn’t want to work with Lisa any more.
And were they – was this a customer that was in the list on your system?---I believe that we sent them oils in Phytoverse, yes.
But you also think that they were – you knew at the time they were a customer that had been a customer of Native Extracts?---I didn’t know that, no.
But you assumed that that was one that was with Native Extracts but no longer wanted to work with them?---Yes.
…
And I’m taking you to … some emails that you exchanged in this period with Premika Chand at Probiotic?---Yes.
And you were familiar with them as a customer that had been purchasing from Native Extracts?---I did. I did know that they were a customer of Native Extracts. But, again, I assumed that they didn’t want to work with them any longer.
Did you talk to your father about that?---My father told me that the liquorice extract that Native Extracts had sent to them had been rejected because it had micro – a micro – microbiomes in it that were found or something like that, and so they sought out a new supplier.
And that’s what he told you at this time – or about this time?---Around this time. Yes.
(Emphasis added.)
154 No suggestion was made to Mr Jordan Macdougald during cross-examination that his evidence was false, that the conversations with his father were not held, that he did not believe what his father told him or, indeed, that Mr Jordan Macdougald had any reason based on any particular fact as to why he should not have believed his father or not formed the view that these customers did not want to purchase from Native Extracts. Nor was any submission made by the applicants that his evidence should not be accepted on these issues, and why.
155 In circumstances where Mr Ross Macdougald was leaving the business of Native Extracts, it is plausible, if not likely, that some customers of that business would want to follow him to any new extracts business which he established. In those circumstances, an honest and reasonable person in Mr Jordan Macdougald’s position would not have any basis to suspect, and nor would it have been obvious, that Mr Ross Macdougald was poaching customers through the misuse of information contained in the List of Suppliers and Customers.
156 It follows that it was not established that Mr Jordan Macdougald had actual or the requisite degree of knowledge that his father “engaged in a concerted effort to poach Native Extracts’ customers”, being the customers in the List of Suppliers and Customers which forms part of the Confidential Information in the case as pleaded against Mr Jordan Macdougald.
157 As to facts (d) and (e)(i), it was common ground that Mr Jordan Macdougald had little or no involvement in the business of Native Extracts. How then did the evidence establish that Mr Jordan Macdougald had actual or the requisite degree of knowledge of the overlap between the extracts offered by Native Extracts prior to 1 April 2016 and the extracts offered by Plant Extracts in FY17, for example? This was not explained by the applicants, and yet they press this fact as a matter which supports the relevant inference. Further, even on Ms Carroll’s evidence, the list of extracts which Native Extracts offered for sale was not kept secret but was a matter of public knowledge. Regarded on its own, there is nothing unlawful about a business deciding to offer the same extracts as one of its competitors, and such a fact would not have indicated to Mr Jordan Macdougald or an honest and reasonable person in his position that Mr Ross Macdougald was misusing information in the Extract Log / Formula Book which forms part of the pleaded Confidential Information.
158 Similarly, as to fact (e)(ii) and in circumstances where Mr Jordan Macdougald had little or no involvement in the business of Native Extracts, there was nothing to indicate his awareness of the extent of the overlap between customers of Native Extracts and Plant Extracts, beyond that explained in his evidence referred to above in relation to fact (b). Further, there was no suggestion that he knew or ought to have realised that his father was misusing information contained in the List of Customers and Suppliers which forms part of the pleaded Confidential Information.
159 As to fact (e)(iii), there is insufficient evidence that Mr Jordan Macdougald knew or had the requisite degree of knowledge that the suppliers being used by Plant Extracts were the same as those used by Native Extracts and that the information about these suppliers was obtained from the List of Suppliers and Customers. This is especially as Mr Jordan Macdougald had limited involvement in the business conducted by Native Extracts. Further, the evidence demonstrated that he had little involvement in contacting suppliers to Plant Extracts other than sending emails at the request of, and often drafted by, his father, with orders otherwise being placed by an employee of Phytoverse or by Mr Hampson. When asked in cross-examination about whether he questioned how his father knew which suppliers to contact, he gave this evidence:
Did you ask [your father] about that? Did you talk to him and say, “How do you know about these people”?---No, I – I never thought about it. It just was, “Send an email off”. I sent it off, and then I would – you know, I would just keep doing my other duties at work.
160 Rapid growth of business compared to Native Extracts: the third fact relied upon in the applicants’ closing submissions in support of the relevant inference is that Plant Extracts grew its sales and business at a substantially faster rate than Native Extracts. A series of facts are then set out in [121] of the closing submissions and cross-referenced to expert evidence to make good that proposition, as follows:
In particular:
(a) Plant Extracts’ sales in its first year of operation were more than double the level of sales of Native Extracts in its first year of operation;
(b) Plant Extracts’ sales in its third year of operation were materially consistent with the level of sales of Native Extracts in its seventh year of operation;
(c) in the first four years of Plant Extracts’ operation (FY17 to FY20):
(i) 78 per cent of Plant Extracts’ sales were of extracts made by Native Extracts prior to 1 April 2016;
(ii) 70 per cent of Plant Extracts sales were to customers that were customers of Native Extracts prior to 1 April 2016;
(iii) 54 per cent of Plant Extracts’ sales were of extracts made by Native Extracts prior to 1 April 2016 to customers that were customers of Native Extracts prior to 1 April 2016;
(iv) only 3 per cent of Plant Extracts sales were to customers that were not customers of Native Extracts prior to 1 April 2016 of extracts that had not been developed by Native Extracts prior to 1 April 2016.
(Footnotes omitted, emphasis original.)
161 Such a case was not pleaded or opened in these terms for the purposes of seeking the relevant inference in relation to Mr Jordan Macdougald. Rather, these facts were opened for the purposes of seeking a finding that there had been disclosure of Confidential Information.
162 Having regard to the serious allegations which are made and as a matter of procedural fairness, this alone is a sufficient reason to reject the applicants’ submission concerning this third fact, and I do so.
163 In any event, there was no evidence that Mr Jordan Macdougald had actual or the requisite degree of knowledge of the facts identified in [121] of the applicants’ closing submissions. This is especially in circumstances where Mr Jordan Macdougald was not even working in the warehouse during the first year of operation of Native Extracts’ business and there was no evidence that he had or would have had access to any of the sales figures of Native Extracts.
164 To the extent that the applicants again rely upon the overlap in customers and extracts, this has already been addressed in relation to the facts asserted in [120] of the applicants’ closing submissions, and applying the same reasoning, this submission fails.
165 This aspect of the applicants’ submissions is therefore rejected.
166 Documents taken by Mr Ross Macdougald: the fourth fact relied upon in the applicants’ closing submissions in support of the relevant inference is that Mr Ross Macdougald took, and Plant Extracts used, other documents belonging to Native Extracts (although, other than certificates issued by the Southern Cross University, the submissions are opaque about what these other documents were).
167 Such a case was not pleaded or opened in these terms. Having regard to the serious allegations which are made and as a matter of procedural fairness, this alone is a sufficient reason to reject the applicants’ submission concerning this fourth fact, and I do so.
168 In any event, there was no evidence that Mr Jordan Macdougald had actual or the requisite degree of knowledge that his father had taken documents from Native Extracts, whatever those documents were.
169 This aspect of the applicants’ submissions is therefore rejected.
170 Business of Plant Extracts is a copy: the fifth fact relied upon in the applicants’ closing submissions in support of the relevant inference is that Plant Extracts’ business appears to otherwise be a wholesale copy of Native Extracts’ business. As part of their closing submissions at [123], the applicants refer to the following:
(a) the name “Plant Extracts” bears striking resemblance to “Native Extracts”;
(b) Plant Extracts’ Product Guide, which shows Plant Extracts’ range of botanical extracts, includes many of the same extracts manufactured and sold by Native Extracts. Plant Extracts also uses unique descriptions of extracts invented by Native Extracts such as “Crown of Gold”, “Snowflower” and “Native Orange Pearl”;
(c) Plant Extracts offers extractions in a 1:1 concentrate and a 1:10 dilution which are the exact same concentrations that were developed by Native Extracts prior to 1 April 2016.
(Footnotes omitted.)
171 Such a case was not pleaded or opened in these terms. Having regard to the serious allegations which are made and as a matter of procedural fairness, this alone is a sufficient reason to reject the applicants’ submission concerning this fifth fact, and I do so.
172 In any event, I would not have accepted that this fifth fact gave rise to the relevant inference.
173 As both businesses are engaged in manufacturing extracts, there is nothing about that same word being used in the name of both businesses which would have alerted Mr Jordan Macdougald or an honest and reasonable person in his position to the prospect that his father was misusing Confidential Information belonging to Native Extracts or the Trust. Indeed, Mr Jordan Macdougald’s oral evidence was that the devising of the company name was a “group effort”, which I understood to mean that he was involved in choosing the name along with his father.
174 As to Native Extracts’ published list of extracts and its sale of extractions in a 1:1 concentrate and a 1:10 dilution prior to 1 April 2016, such matters were disclosed to customers and are therefore in the public domain. Mr Jordan Macdougald or an honest and reasonable person in his position would not perceive that if a competitor (such as Plant Extracts) sold extracts with the same names or concentrations as Native Extracts, there has been or is likely to have been a misuse of Confidential Information by Mr Ross Macdougald, which information is owned by Native Extracts or the Trust. More than this is required.
175 This aspect of the applicants’ submissions is therefore rejected.
176 Respondents’ lack of evidence: the sixth fact relied upon in the applicants’ closing submissions in support of the relevant inference is the lack of evidence from any witness who would be expected to be in a position to contradict the inferences readily available on the documentary record (such as, for example, Mr Ross Macdougald, Mr Hampson or Mr Moore). However, this submission cannot apply to Mr Jordan Macdougald, who gave evidence.
177 As to this, I considered Mr Jordan Macdougald to be a genuine witness who attempted to give his honest recollection of the events in question. Although Mr Jordan Macdougald gave evidence under cross-examination that he was unaware of many aspects of the business of Plant Extracts, I did not form the impression that he was being evasive or obstructive in his evidence. Rather, his answers portrayed a heavy reliance on his father’s advice and that of his father’s solicitor. With respect and based on my impressions of him as a witness, Mr Jordan Macdougald did not appear to be commercially astute.
178 As already noted above, no submission was made by the applicants that Mr Jordan Macdougald’s evidence should not be accepted.
179 For these reasons, I accept the evidence of Mr Jordan Macdougald which is referred to above.
5.3.2 Additional matters relied upon by applicants
180 At [126]–[131] of their closing submissions, the applicants make further submissions which are directed at Mr Jordan Macdougald specifically. These submissions are not aligned with the pleaded case, particularly the allegations in [50] of the SOC, and to some extent overlap with the issues addressed above.
181 For example, it is pleaded in [50] of the SOC that Mr Jordan Macdougald “knew, or ought to have known” the Confidential Information was information confidential to, and trade secrets of, relevantly, Native Extracts and that his father had obtained that information while he was a director of Native Extracts.
182 As already observed, the Confidential Information (as defined) in the SOC includes:
(1) the Extract Log / Formula Book. However, there is no evidence that Mr Jordan Macdougald was even aware of the existence of this book let alone knew that it was information confidential to Native Extracts or that his father had taken it or a copy of it after he left Native Extracts;
(2) Grower’s Matrix. However, there is no evidence that Mr Jordan Macdougald was even aware of the existence of this matrix let alone knew that it was information confidential to Native Extracts or that his father had taken it or a copy of it after he left Native Extracts;
(3) List of Suppliers and Customers. However, there is no evidence that Mr Jordan Macdougald was even aware of the existence of this list let alone knew that it was information confidential to Native Extracts or that his father had taken it or a copy of it after he left Native Extracts.
183 Instead of staying with their pleaded case, the applicants seek findings that Mr Ross Macdougald told Mr Jordan Macdougald that he had “a list of customers that wanted to buy extracts” and that these conversations could have occurred prior to the date of the Deed of Settlement. The applicants further submit that “there can be no doubt, given the timing of these conversations, that an honest and reasonable person, with knowledge of these facts, would have understood that the list of customers could only have been obtained by Ross Macdougald as a result of his position as a director of Native Extracts”.
184 Such a case was not pleaded or opened in these terms. Having regard to the serious allegations which are made and as a matter of procedural fairness, this alone is a sufficient reason to reject the applicants’ submission, and I do so.
185 In any event, such a case is without merit.
186 That is because I infer from his oral evidence (which evidence I accept) that, so far as Mr Jordan Macdougald believed and based on what his father told him, these were customers who no longer wished to purchase extracts from Native Extracts. Mr Jordan Macdougald also gave unchallenged evidence that there was no physical list of these customers. As far as Mr Jordan Macdougald knew, and I consider that an honest and reasonable person would believe in these circumstances, the sharing of information by Mr Ross Macdougald to the effect that there were customers who no longer wanted to purchase extracts from Native Extracts would not give rise to the requisite degree of knowledge that Mr Ross Macdougald was misusing information contained in the List of Suppliers and Customers which forms part of the pleaded Confidential Information.
187 The applicants also submit that Mr Jordan Macdougald gave evidence that he was aware that many of the customers and suppliers his father asked him to write to were customers and suppliers of Native Extracts and “assumed” that the information being supplied to him by his father for this purpose was information his father had obtained as a result of his position as a director of Native Extracts. Their closing submissions extract this passage of evidence:
And you would understand, and understood at the time, that that was information that your father had sent to you because he knew about this, and knew how to make the extracts, and knew what would be needed to make these extracts from his time at Native Extracts? --- I can only assume so, yes.
Did you ask him about that? Did you talk to him and say, “How do you know about these people”? --- No, I – I never thought about it. It just was, “Send an email off”. I sent it off, and then I would – you know, I would just keep doing my other duties at work.
…
Yes. But you – a lot of these suppliers, information was provided to you and – or orders were placed on your behalf on arrangement made by your father; correct? --- Yes.
And the information about who to go to for particular product, raw material needed for your business, that’s information your father had? ---Yes.
And, as you understand it, that’s information he had from his previous experience as a director of Native Extracts? ---I would assume so, yes.
(Emphasis added.)
188 The applicants then submit:
…the circumstances were such that a honest and reasonable person would have realised that the information provided to him, by his father, relating to customers and suppliers was information his father had obtained whilst he was a director of Native Extracts. Jordan Macdougald’s evidence is that he assumed this was the case. The case law is clear that he cannot hide behind his failure to inquire further. His failure to make appropriate inquiries when confronted with the obvious is sufficient to infer actual knowledge.
(Emphasis added.)
189 The flaw in this submission is that when Mr Jordan Macdougald answered that “I can only assume so” and “I would assume so” in the passage above, he appeared to be speaking in the present tense – that is, he appeared to be making the relevant assumption at the time of giving evidence and not saying that he had made the assumption at the time of the events that he was being asked about. At best for the applicants, it is unclear from this passage in the transcript whether he was referring to an assumption he had made in the past or was making in the present. For these reasons, I do not accept that this evidence establishes that Mr Jordan Macdougald made assumptions at the time of the events in question.
190 The applicants submit that Mr Jordan Macdougald “also gave evidence that he was aware that many of the customers and suppliers his father asked him to write to were customers and suppliers of Native Extracts”. However, Mr Jordan Macdougald did not give evidence in these terms. Further, the connection between the customers and suppliers which were referred to by Mr Jordan Macdougald in his evidence and the pleaded List of Suppliers and Customers was not established.
191 By oral closing submissions, the applicants also submit that inquiries ought to have been made by Mr Jordan Macdougald of his father along these lines: “Where is this information coming from? How is it that we have all of these customers? How is it that we can offer all these extracts?” This followed a submission by senior counsel for the applicants that:
…the detail of the particular interactions with customers and suppliers we have set out, but the substance of it is that from very early on he has been told to send emails to specific persons at specific email addresses, offering specific information about a whole range of extracts that they can supply and also specific details to – about raw materials that are to be acquired from certain named suppliers. Now, in a situation where you’ve got a company – standing back from it, they’re supposed to be starting up their own business of making and selling botanical extracts, and suddenly his father comes to him – who he knows is a director of the company, who he knows has entered into a legally binding document – he doesn’t – he says he didn’t read it, but he witnessed it.
192 These submissions diverge from the pleaded and opened case, and even from the closing written submissions. Having regard to the serious allegations which are made and as a matter of procedural fairness, this alone is a sufficient reason to reject these submissions, and I do so. In particular, the emails which Mr Jordan Macdougald sent to suppliers at his father’s request and which identified specific ingredients able to be obtained from those suppliers was not a fact relied upon by the applicants to assert that, having regard to the content of these emails, an honest and reasonable person would have realised that the information about the suppliers was information his father had obtained whilst he was a director of Native Extracts or that his “failure to make appropriate inquiries when confronted with the obvious is sufficient to infer actual knowledge”.
193 Further, for the reasons already given, to the extent that Mr Jordan Macdougald made inquiries of his father, there was nothing to suggest that an honest and reasonable person in his position ought not to have accepted his father’s explanations at the relevant time.
194 The applicants failed to address all of the material facts which are pleaded in the case brought against Mr Jordan Macdougald, identify the evidence which establishes those pleaded facts and justify why that evidence should be preferred to the evidence of Mr Jordan Macdougald, in the event of any conflict. In particular, the applicants failed to establish that Mr Jordan Macdougald had actual or the requisite degree of knowledge of the facts pleaded in [50] of the SOC or that, in the knowing assistance case, Mr Jordan Macdougald actually assisted Mr Ross Macdougald in the dishonest and fraudulent breaches of his equitable and fiduciary duties including that he intended to further those breaches.
195 Further, to a significant extent, the applicants sought to diverge from their pleaded and opened case during closing submissions. For the reasons explained above, that case is rejected as a matter of procedural fairness to Mr Jordan Macdougald.
196 For these reasons, the applicants’ case against Mr Jordan Macdougald must fail, and it should be dismissed.
6. RELIEF FOR BREACHES OF CONFIDENCE
6.1 Alternative orders sought by applicants
197 By the draft order which was provided at the conclusion of the trial, the relief sought against Plant Extracts, Mr Ross Macdougald, Phytoverse and Mr Jordan Macdougald included the following:
2. Pursuant to s 1317H(l) of the Corporations Act 2001 (Cth) (Corporations Act), or in equity, the First Respondent pay to the First Applicant the sum of $3,105,682, on account of profits made by the First Respondent as a result of being involved in the Second Respondent’s contravention of s 183 of the Corporations Act and for assisting, with knowledge, in a dishonest and fraudulent design, involving the Second Respondent’s breach of his Fiduciary Duty (Confidence) and his Equitable Duty of Confidence.
3. Alternatively, the Second Respondent, Third Respondent and Fifth Respondent pay to the First Applicant the sum of $6,203,499, for loss of profits:
(a) in respect of the Second Respondent, as damages for breach of his Contractual Duty of Confidence, compensation pursuant to s 1317H(1) of the Corporations Act for breach of his Statutory Duty of Confidence, or in equitable compensation for breach of his Fiduciary Duty (Confidence) and his Equitable Obligation of Confidence;
(b) in respect of the Third Respondent, as damages for breach of its Contractual Duty of Confidence, compensation pursuant to s 1317H(l) of the Corporations Act for being involved in the Second Respondent’s contravention of s 183 of the Corporations Act, or equitable compensation for assisting, with knowledge, in a dishonest and fraudulent design involving the Second Respondent’s breach of his Fiduciary Duty (Confidence) and his Equitable Duty of Confidence;
(c) in respect of the Fifth Respondent, as compensation pursuant to s 1317H(l) of the Corporations Act for being involved in the Second Respondent’s contravention of s 183 of the Corporations Act, or equitable compensation for assisting, with knowledge, in a dishonest and fraudulent design involving the Second Respondent’s breach of his Fiduciary Duty (Confidence) and his Equitable Duty of Confidence.
(Emphasis original.)
198 The applicants intend to make an election between proposed order 2 and proposed order 3 following the publication of these reasons, and prior to entry of judgment. That it could make such a split election between remedies against different respondents in this way was not opposed by the RM respondents, and is correct as a matter of law, at least insofar as those orders reflect the available relief in equity: see generally Xiao v BCEG International (Australia) Pty Ltd (2023) 111 NSWLR 132; [2023] NSWCA 48 at [68]–[71] (Gleeson JA with whom Mitchelmore JA and Griffiths AJA agreed); see also Personal Representatives of Tang Man Sit v Capacious Investments Ltd [1996] AC 514 at pages 521–522.
199 As the case brought against Mr Jordan Macdougald was not established by the applicants, and the claim against him will be dismissed, no monetary order will be made against him. The reasons which follow will therefore only address the position of Plant Extracts, Mr Ross Macdougald and Phytoverse in relation to the monetary relief sought against them by the proposed orders above.
200 By declarations 6–8 contained in the Order dated 19 October 2023, the RM respondents accepted that:
(1) Mr Ross Macdougald had breached his contractual duty of confidence to Native Extracts and the beneficiaries of the Trust, as well as other duties of confidence defined in that Order as “Duties of Confidence”.
(2) Plant Extracts and Phytoverse were involved in Mr Ross Macdougald’s breach of his statutory duty of confidence to Native Extracts and assisted, with knowledge, in a dishonest and fraudulent design involving Mr Ross Macdougald’s breach of his “Fiduciary Duty (Confidence) and Equitable Obligation of Confidence” to Native Extracts (as defined in that Order).
201 Having regard to the terms of these orders and the closing submissions of the parties, the only issue which remains in relation to Plant Extracts, Mr Ross Macdougald and Phytoverse is the quantum of the monetary relief to be awarded to Native Extracts. As submitted by the RM respondents:
[In] circumstances where the contractual and statutory breaches contended for by the Applicants are made out, some aspects relating to quantum remain in dispute. Specifically, the “price premium differential”, the potential exclusion of certain sales achieved by the First Respondent (as identified by Mr Lytras), the treatment of Research and Development (R&D) costs and the quantum of the award for the “springboard” effect enjoyed by the First Respondent by virtue of its breaches.
(Emphasis original.)
6.2.1 Summary of the position of the parties
202 The applicants, in reliance on Mr Stephens’ evidence, submit that Plant Extracts should disgorge all realised profits made by it from FY17 to FY21 in the amount of $3,105,682. They submit that the true value of the benefit obtained by Plant Extracts is the full capital value of its business and all realised profits to date, and submit that the amount of $3,105,682 is a conservative measure of the actual benefit or gain obtained by Plant Extracts.
203 Plant Extracts accepts that the period from FY17 to FY21 is the correct period, and it accepts that it should be ordered to account for profits derived by it during that period. However, in reliance on Mr Lytras’ evidence, it seeks to limit its liability by excluding certain sales from consideration, and it also submits that any award should be discounted by 50%.
204 The High Court decision of Ancient Order of Foresters in Victoria Friendly Society Ltd v Lifeplan Australia Friendly Society Ltd (2018) 265 CLR 1; [2018] HCA 43 concerned a company, described in the judgment as Foresters, which knowingly took advantage of Messrs Woff and Corby’s dishonest and fraudulent design, which involved breaches of fiduciary duty, in order to enhance its business by appropriating the business connections of its competitors, described as Lifeplan and FPM. It succeeded in doing so.
205 The majority (Kiefel CJ, Keane and Edelman JJ) stated the following at [6]–[9]:
In Consul Development Pty Ltd v DPC Estates Pty Ltd, in a passage accepted as authoritative by both sides in the present case, Gibbs J said that:
“a person who knowingly participates in a breach of fiduciary duty is liable to account to the person to whom the duty was owed for any benefit he has received as a result of such participation.”
So described, the liability to account and to disgorge benefits encompasses “any benefit” received by the knowing participant in a breach of fiduciary duty “as a result of” that participation. The benefit of a business connection is such a benefit. Foresters’ submission fails to come to grips at all with the fact that the benefit that Foresters stood to gain, and in fact acquired, from its participation in the various acts of disloyalty by Woff and Corby was not sporadic deposits from retail customers; it was the business connections of Lifeplan and FPM.
In addition, Foresters’ submission, by framing the issue as involving an enquiry as to whether there was a causal connection between each of the particular acts of Foresters, whereby it participated in the strategy formulated by Woff and Corby, and particular deposits associated with its new business, diverts attention away from the significance of the circumstance that Foresters’ acts of participation in the disloyalty of Woff and Corby were not only informed by, but were also an integral part of, the strategy for despoiling the business of Lifeplan and FPM.
Whether a benefit can be said to be obtained “as a result of” knowing participation in a breach of fiduciary duty by another contrary to the principles of equity is a question of causation or contribution that depends on “a precise examination of the particular facts” of the case, rather than upon attempts to refine the expression “as a result of”, as if that phrase has some determinate operation of its own that may be discerned and applied independently of the equitable principle of which it is part. The equitable disgorgement principle with which we are concerned is a “prophylactic rather than a restitutionary principle”. It is sufficient to show that the profit would not have been made but for dishonest wrongdoing. Further, whatever may be the position for wrongdoing that is not marked by dishonesty, a defendant cannot avoid liability to disgorge profits dishonestly made by showing that those profits might have been made honestly. This is not an approach to causation that is unique to dishonesty in equity. A defendant who is liable to compensate for deceit cannot avoid that liability by showing that the loss would have been suffered even without the deceit; and it is sufficient that the deceit was an inducement to engage in the conduct that occasioned the loss even if there were other inducements. And in taking an account of profits for dishonest infringement of intellectual property rights, courts do not reduce the profit by reference to opportunity cost, that is, the revenue that would have been received by a lawful alternative. As Lord Radcliffe said in the context of disgorgement of profits for a breach of fiduciary duty involving non-disclosure, “it is neither here nor there to speculate whether, if he had done his duty, he would not have been left in possession of the same amount of profit”. For these reasons, the deterrent effect of an order for disgorgement of profits should not be diminished by acceding to Foresters’ attempt to confine the scope of the causal enquiry implicit in the expression “as a result of”.
(Citations omitted.)
206 As to issues of quantification of the benefit to be disgorged, the majority stated at [13]–[16]:
…While it is true that equity will not require an errant fiduciary or a participant in a breach of fiduciary duty to account for an advantage which the breach of fiduciary duty has not caused or to which it has not sufficiently contributed, where causation is sufficiently established the onus is upon the errant fiduciary or participant to show that he or she should not account for the full value of the advantage. That onus is not discharged by mere conjecture or supposition giving the benefit of the doubt to a proven wrongdoer. The requirement of proof conforms with the obligation of a party charged with a breach of fiduciary duty to show why the full value of an advantage obtained in a situation of conflict of duty should not be disgorged.
There are two ways in which the wrongdoer might discharge that onus and reduce the extent of the liability to disgorge profits. The first way, which can involve notorious difficulties in attribution of costs, is by proving his or her entitlement to an allowance for costs incurred, and labour and skill employed…
The second way, which was the focus of this appeal, is by demonstrating that the benefit or advantage is beyond the scope of the liability for which the wrongdoer should account for profits. A wrongdoer might prove that some profit or benefit is beyond the scope of liability for which he or she should account if the profit or benefit has no reasonable connection with the wrongdoing…
No precise test has been prescribed for determining when it will be inequitable to account for a benefit on the basis that it has no reasonable connection with wrongdoing. Nor is there any need for such a test. All of the circumstances must be considered, including the nature of the conduct…
(Citations omitted.)
207 I turn then to the issues in dispute between the parties and their experts.
6.2.3 Total sales by Plant Extracts during FY17 to FY21
208 Mr Stephens and Mr Lytras agree that Plant Extracts derived the sum of $3,998,796 from the sale of extracts between FY17 to FY21 (PE Total Sales). From that starting point, Mr Stephens then seeks to isolate the profit component of that sum. Conversely, Mr Lytras makes deductions to the PE Total Sales figure before he subsequently attempts to isolate the profit component from that residual sum.
209 Table 11 of the JER shows the effect of Mr Lytras’ deductions to the PE Total Sales. That table is replicated here:
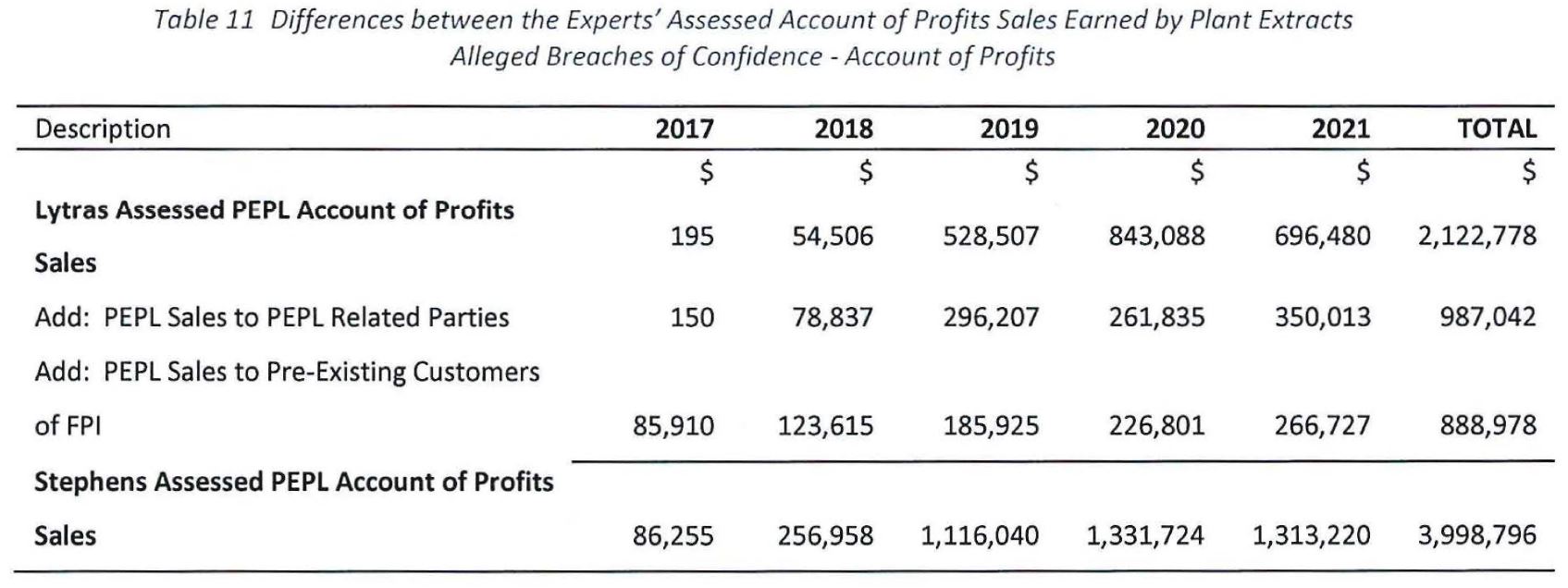
210 Table 11 shows that from the $3,998,796 in PE Total Sales, Mr Lytras deducts $888,978 on account of “PEPL Sales to Pre-Existing Customers of FPI” and $987,042 on account of “PEPL Sales to PEPL Related Parties”. This leaves Mr Lytras with an adjusted total sales figure of $2,122,778.
211 As to the first deduction, Mr Lytras discusses “PEPL Sales to Pre-Existing Customers of FPI” at [6.0]–[6.2] of his report. There Mr Lytras notes that he has relied on a list of alleged pre-existing customers provided on instructions from Stokes Lawyers:
Assumptions and Assessment Premises
The assumptions and premises I have relied upon in conducting my investigations and forming my opinions are categorised into ‘Instructed Assumptions and Premises’ (provided by Stokes Lawyers) and ‘Own Assumptions and Premises’ (made by myself) and are detailed in the sub-sections below.
Instructed Assumptions and Premises
The clients possessing a date against their name in the column ‘FPI Oceania sales prior to extracts’ in the client listing prepared by Ms Morrissey (attached as Annexure 8) are regarded as pre-existing customers of the Respondents collectively (Respondents’ Pre-Existing Customers) and should be excluded from any account of profits and loss of profits calculations for NEPL (as detailed in my supplementary letter of instruction dated 28 November 2022).
(Emphasis original.)
212 The letter of instruction referred to by Mr Lytras above provides the following instructions:
In your report, please consider the following aspects:
1. Please find attached a spreadsheet prepared by our client’s CFO Janel Morrissey. When considering the profits and damages that the applicant is claiming, we request that you discount (from the account of profits calculations) any sales made to the pre-existing customers noted on the spreadsheet.
213 Annexure 8 of Mr Lytras’ report contains the spreadsheet referred to in the letter of instruction. Mr Lytras sets out a summarised version of the spreadsheet at page 20 of his report. Mr Lytras confirmed in cross-examination that, other than the above instructions, he had no further information to support the deduction.
214 Other than an unsuccessful attempt to prove up aspects of the spreadsheet through Ms Carroll, no evidence was adduced by the RM respondents about the spreadsheet such as: the person who compiled it, the information on which it was based such as, for example, business records (which would also require the tender of that underlying information) and how the spreadsheet should be interpreted.
215 As the facts on which his expert opinion is based have not been established, no weight can be attached to Mr Lytras’ opinion that the amount of $888,978 on account of “PEPL Sales to Pre-Existing Customers of FPI” should be deducted, and it follows that this deduction will not be made.
216 As to the second deduction, the term “PEPL Sales to PEPL Related Parties” refers to sales made by Plant Extracts to Biologi and Phytoverse – two entities related to Mr Ross Macdougald and Plant Extracts. In his report, Mr Lytras opines that related parties, like pre-existing customers, “would reasonably be excluded from the Confidential Information definition and represent legitimate PEPL sales and profits provided the sales did not involve the improper use of NEPL’s extract formulae and manufacturing know-how.” Mr Lytras then deducts sales made by Plant Extracts to these entities.
217 However, the assumption on which Mr Lytras’ opinion is based, being that the sales did not involve the improper use of Native Extracts’ extract formulae and manufacturing know-how, was not established by the RM respondents.
218 As the assumption on which his expert opinion is based was not established by the evidence, no weight can be attached to Mr Lytras’ opinion that the amount of $987,042 on account of “PEPL Sales to PEPL Related Parties” should be deducted, and it follows that this deduction will not be made.
219 For these reasons, the correct figure for the PE Total Sales for any account of profits analysis is the sum of $3,998,796.
6.2.4 Research and development costs
220 The experts agreed and parties accept that certain deductions should be made from the PE Total Sales figure as part of any account of profits analysis. The only disputed deduction in terms of quantum related to the research and development costs (R&D costs).
221 Mr Stephens’ position is that R&D costs should be expensed over time, rather than in the year they were incurred, because they have a recurring future benefit that is realised over time. In the JER at [72]–[74], it was stated that:
…Mr Stephens has identified Plant Extracts’ R&D, as extracted from its company tax returns, is as set out in the below table.
Table 14 Plant Extracts R&D Summary
Period
2017
2018
2019
2020
2021
Total
$
$
$
$
$
$
R&D
51,867
226,681
245,515
402,376
521,131
1,447,570
Mr Stephens notes that Plant Extracts’ R&D documents were requested but not provided and on that basis Mr Stephens cannot comment on the extent and nature of R&D undertaken by Plant Extracts. Mr Stephens notes that Plant Extracts’ average R&D cost per extract of $6,491 is materially consistent with Native Extracts average R&D cost of $7,256 (i.e. Plant Extracts and Native Extracts reported similar levels of R&D per extract).
Mr Stephens states that it is his understanding that R&D costs at the relevant times (i.e. 2017 to 2021) were subject to R&D tax incentive schemes (i.e. refundable tax offsets) at a rate of 43.5% of eligible R&D costs. Mr Stephens notes the scheme effectively results in ‘additional tax benefits’ over and above the ordinary deductibility of R&D expenses which effectively reduces the ‘out of pocket’ cost of R&D activities. Mr Stephens notes that any R&D tax incentive benefits have not been accounted for in the assessment of loss.
222 Mr Lytras takes a different approach to the treatment of R&D costs. He takes Plant Extracts’ financial statements and deducts R&D costs in the year they were incurred. Mr Lytras does not particularise any reason for this approach other than that it is consistent with Plant Extracts’ financial statements, and that there is not “sufficient information” concerning the R&D costs incurred by Plant Extracts “to provide him with a sufficient level of comfort to depart” from the approach taken in those financial statements.
223 Mr Lytras also raises concerns that the R&D expense figures calculated by Mr Stephens may include amounts which are operational in nature or which have already been added back to the profit amount. He also expressed concern as to the accuracy of the assumptions and estimations relied on by Mr Stephens in his approach.
224 Both experts agreed that it was unclear on the information before them whether operational expenses had been incorrectly included in the R&D expense figures, and while Mr Stephens accepted that there was a “double count” of some items, he considered that it would result in “no material adjustment”. Further, the concerns of Mr Lytras ultimately stem from the failure of Plant Extracts to provide relevant documents as noted above.
225 In these circumstances, I am not persuaded by Plant Extracts, which bears the onus, that it would be inequitable for it to disgorge the amount of profits in the amount claimed by Native Extracts because it has deducted R&D costs in the year that they were incurred (being the basis upon which Mr Lytras justified his approach). This is particularly the case having regard to the following matters:
(1) Plant Extracts did not provide the documentation requested by Mr Stephens in relation to the R&D expenses;
(2) the calculation by Mr Stephens does not take into account the likely benefit to Plant Extracts of R&D tax incentive schemes;
(3) as Plant Extracts has an ongoing business, the R&D expenses incurred will have a recurring future benefit.
6.2.5 Whether discount should be applied
226 The RM respondents submit that:
While it is accepted that a springboard advantage across this period was enjoyed by the First Respondent, the quantum of that claim is disputed. Specifically, Mr Stephens accepts that the extent to which the enhanced performance of the First Respondent is attributable to the alleged infringements as opposed to other factors (including management capability and market and/or economic factors) is not a matter of accounting expertise. When cross-examined on the issue, Mr Stephens agreed that there were various factors, such as demand in the market, the general desirability of botanical extracts and other economic factors that would bear upon this analysis. Mr Stephens’ contention was that his own analysis would be of assistance to the Court, but did not put it higher than that and accepted such factors as highlighted by Mr Lytras at Paragraphs 14.5 and 14.6 of Lytras 1 were also relevant to the issue. There was no evidence led by the Applicants about these extrinsic factors that may have impacted the springboard effect and the Court could not safely allow for the whole. …
[To] allow the adjustments contended for by Mr Stephens in relation to the springboard effect in full, would not be reasonably likely to be reflective of the actual advantage enjoyed by the First Respondent by virtue of the springboard effect.
Accepting that the springboard effect would have existed to some extent across this period (2017 to 2020), the RM respondents submit that the springboard advantage contended for by Mr Stephens ought be allowed over a four year period but discounted appropriately to reflect the extent to which these other factors would have borne upon the First Respondent’s growth across this period. The RM Respondents [sic] that an appropriate discount rate would be 50%, but appreciate this is a matter within the discretion of the Court, given the limited evidence available to it.
227 In his report, Mr Lytras was critical of the analysis undertaken by Mr Stephens in terms of the claimed springboard advantage including because:
…it compares non‐aligned years, gives no consideration to different market conditions and characteristics (for example, competitive, economic, demand, strategic) prevailing across the various years and gives no consideration to the various capabilities of the owners or the impact that the breakdown of the relationship between Ross and Lisa might have had at the time prior to, and after, the execution of the [Deed of Settlement];
The values in the ‘Sales Comparison’ analysis for [Native Extracts] have been discounted in that sales of oil products have been removed. This masks any effect that increased time and effort [Native Extracts] may have put into this area at the expense of extracts might have occurred;
There is no consideration that [Plant Extracts’] strong early growth after the expiry of the Restraint period might have come from Ross’ extensive experience and background intellectual property in the industry; and
An overlap of customers and products is to be reasonably expected as [Native Extracts] and [Plant Extracts] are direct competitors and located in the same region as each other.
228 However, the onus is on Plant Extracts to demonstrate that it would be inequitable for it to disgorge its profits in the amount claimed by Native Extracts. If there were particular facts which it wished to rely upon and which established that a component of its profits had no reasonable connection to its unlawful conduct, then Plant Extracts was required to adduce evidence to establish those facts – but it failed to do so. It is not sufficient to discharge that onus for it to call an expert to identify a list of possible facts of this kind.
229 For these reasons, no discount will be applied.
230 The applicants submit that, if I accept Mr Stephens’ analysis, it should award Native Extracts, or the Trust, the amount of $3,105,682 on account of profits. Other than proposing a discount, no submission was made to the contrary by Plant Extracts on the premise that Mr Stephens’ analysis is accepted.
231 As I have accepted Mr Stephens’ analysis in all respects, for the reasons explained above, and having regard to the terms of orders 7 and 8 of the Order dated 19 October 2023, it would be appropriate to order that Plant Extracts pay Native Extracts the amount of $3,105,682 on account of profits in the terms sought by the applicants in their draft order, should an election be made for such an order.
6.3.1 Summary of the position of the parties
232 It was common ground that the four year period between FY17 and FY21 was an appropriate period for the purposes of assessing Native Extracts’ loss. Further, the RM respondents did not dispute that damage was suffered by Native Extracts (rather than the Trust) by reason of the unlawful conduct and that Native Extracts was entitled to an award of damages.
233 Importantly and as already observed, it is admitted by the RM respondents in the Defence that Native Extracts suffered loss and damage which was, in effect, a loss of opportunity to make sales of extracts that it would otherwise have made but for the unlawful conduct of Mr Ross Macdougald and Phytoverse.
234 The issue between Native Extracts, on the one hand, and Mr Ross Macdougald and Phytoverse, on the other, was the value of that lost opportunity.
235 As to this, the general position of the applicants is that, but for the admitted unlawful conduct of Mr Ross Macdougald and Phytoverse, all pre-existing customers of Native Extracts who purchased extracts from Plant Extracts would have purchased the same extracts from Native Extracts, in the same quantities and at the higher price that Native Extracts was selling them.
236 By contrast, the general position of Mr Ross Macdougald and Phytoverse is that, but for their unlawful conduct, all pre-existing customers of Native Extracts who purchased extracts from Plant Extracts would have purchased the same extracts from Native Extracts, in the same quantities but at the same price as charged by Plant Extracts.
237 The parties agree upon certain discounts which should be applied, but (in reliance upon their respective experts) disagree about other discounts. The areas of dispute are explored below.
238 I observe that the cases advanced by the parties through their experts regarding the loss of opportunity to make sales are, in some respects, “all or nothing”. In particular, they proceeded on the basis that all sales made by Plant Extracts would have been made by Native Extracts, less particular customers whose total sales were subtracted by way of discount. No submissions were made that any identified customers of Plant Extracts might have purchased from Native Extracts but at a lower volume due to the higher price. For this reason, I will not broaden the case beyond that advanced by the parties and postulate as to the hypothetical number of sales to each of these customers, other than by applying a discount to the total number of sales to reflect the uncertainty of estimating sales generally, discussed below. Instead, I will adopt the approach taken by the parties and include or exclude each respective customer’s sales in toto.
239 It was observed by Beach J in Henley Arch Pty Ltd v Lucky Homes Pty Ltd (2016) 120 IPR 317; [2016] FCA 1217 at [213] that:
…where one is utilising the lost profits method based upon the loss of a chance or opportunity, there are two questions to consider. The first question is whether there has been such a lost opportunity. This is determined on the balance of probabilities. The second question is what is the value of that lost opportunity. That is to be decided on the possibilities or probabilities of the case… But some estimation or even educated guesswork under either question may be required and is justifiable.
(Citation omitted.)
240 The first question has been answered in favour of the applicants by reason of the admission in the Defence.
241 As to the second question and when determining the value of a lost opportunity, a hypothetical counterfactual in which the opportunity was not lost must be considered. One must form an estimate of the probability that the hypothetical counterfactual would have occurred: see Malec v JC Hutton Pty Ltd (1990) 169 CLR 638 at page 639 (Brennan and Dawson JJ), at page 643 (Deane, Gaudron and McHugh JJ); Sellars v Adelaide Petroleum NL (1994) 179 CLR 332 at page 349 (Mason CJ, Dawson, Toohey and Gaudron JJ). Any estimation must be done “with as much precision as the subject matter reasonably [permits]”: see Placer (Granny Smith) Pty Ltd v Thiess Contractors Pty Ltd (2003) 77 ALJR 768; [2003] HCA 10 at [37] (Hayne J).
242 A discount must then be applied to account for other factors and to reflect the uncertainty inherent in the estimation: see Generic Health Pty Ltd v Bayer Pharma Aktiengesellschaft (2018) 267 FCR 428; [2018] FCAFC 183 at [186] (Allsop CJ, Yates and Beach JJ).
6.3.3 The competing contentions
243 The unchallenged and uncontradicted evidence of Ms Carroll was that Native Extracts had the capacity to, and would have, supplied the extracts in the same quantities to the customers who instead purchased extracts from Plant Extracts during the relevant period. I accept that evidence.
244 To determine Native Extracts’ loss of profit, Mr Stephens, the applicants’ expert, identifies each sale made by Plant Extracts during the relevant period. Mr Stephens then applies, by reference to each actual sale by Plant Extracts, the price at which Native Extracts would have sold that same extract to determine the total sales amount which Native Extracts would have made had Native Extracts made those sales. That price was a higher price than was charged by Plant Extracts, which the parties described as the price premium differential. The effect of the price premium differential is to increase the assessed lost revenue by approximately 247% or more than $9.7 million. This is equivalent to multiplying Plant Extracts’ actual sales revenue by 3.47. It should be noted that that percentage as calculated by Mr Stephens is reflective of the average price premium across the total relevant period, but that the actual price premium calculated by Mr Stephens varied year to year and product to product.
245 On this assumption, Mr Stephens’ calculations results in a total figure of $13,758,043 (NE Total Lost Sales). Mr Stephens then makes various deductions to the NE Total Lost Sales figure to account for the expenses that would have been incurred to isolate the “but for” profit component to arrive at an inferred profit margin of 65.2%. He also determined that the lost profit was $8,970,188 in the JER.
246 However, the applicants submit that a 30% discount should then be applied to the NE Total Lost Sales figure. That discount was said to be on the basis that only 70% of all sales made by Plant Extracts from FY17 to FY20 were to pre-existing Native Extracts customers. The applicants submit that, by applying a 30% discount to the NE Total Lost Sales figure, this removes all sales which Plant Extracts made to common customers (after 1 April 2016), related parties (including Biologi) and customers unique to Plant Extracts. The 30% discount was not challenged by the RM respondents. A 30% deduction to the NE Total Sales figure brings the total figure down to $9,630,630. With the application of another unchallenged deduction associated with the sale of extracts to Biocosmetic, the NE Total Sales figure is further reduced to $9,514,569, which the applicants describe as the Adjusted NE Total Lost Sales.
247 The applicants then apply the inferred profit margin of 65.2% arrived at by Mr Stephens to the Adjusted NE Total Lost Sales figure to arrive at a lost profits figure of $6,203,499. This figure is pressed on the basis that Mr Stephens’ treatment of R&D costs is accepted. This issue is addressed below.
248 The applicants also submit that, if Mr Lytras’ treatment of R&D costs is accepted, then the lost profits figure should be $5,328,159, which is calculated by applying Mr Lytras’ inferred profit margin of 56% (as appears in the JER) to the Adjusted NE Total Lost Sales figure.
249 To those figures, the applicants then propose a further discount of up to 20% as a “high-water mark” to make allowance for uncertainties or contingencies, relying on the discount applied in Henley Arch at [221].
250 By contrast, Mr Lytras starts with the total sales made by Plant Extracts during the relevant period (being $3,998,796), ignores the sales price premium, and then makes certain deductions. That is, the RM respondents’ position is to assume that, in effect, Native Extracts would have made the same sales of the same quantities of the relevant extracts but at the price charged by Plant Extracts.
251 Mr Lytras then proposes that further amounts be deducted from this total. It is convenient to address those discounts now.
252 Mr Lytras identifies four categories of customers who, he says, Native Extracts were unlikely to have sold extracts to in the “but for” counterfactual.
253 The first category is “PEPL Sales to Pre-Existing Customers of FPI”. As discussed above, there is no principled basis for Mr Lytras to make this deduction because it is based upon an instruction that these customers used to be FPI customers (but no evidence was adduced to support that instruction). For this reason, no weight is attached to this opinion and the deduction will not be made.
254 The second category is “PEPL Sales to PEPL Related Parties”. The applicants accept that there is merit in Mr Lytras’ exclusion of this category of sales. However, it is already dealt with by applying the 30% discount referred to above.
255 The third category is “PEPL Sales to CR Formulations”. In his report, Mr Lytras gives evidence that sales made by Plant Extracts to CR Formulations should be excluded because “based on their sales conduct (see Schedule 3 of Mr Stephens’ first report) [they] likely would always have taken their custom to PEPL”.
256 Schedule 3 of Mr Stephens’ first report shows that Native Extracts made sales to CR Formulations in each year from FY15 to FY18 in the total amount of $19,615 and Plant Extracts made sales from FY18 to FY21 in the total amount of $854,140. In these circumstances, I accept the opinion of Mr Lytras that the sales to CR Formulations should be excluded from the calculation because the buying behaviour of CR Formulations does not support the assumption which the applicants press, being that CR Formulations would have acquired the same quantities of extracts from Native Extracts during FY18 to FY21 at the higher price charged by Native Extracts. I regard this to be very unlikely.
257 The fourth category is “PEPL Sales to Customers with Little to No History of Sales with NEPL”. In his report, Mr Lytras deducts sales made by Plant Extracts to “Tismor Health & Wellness” and “Biocosmetic” on the basis that Schedule 3 of Mr Stephens’ first report shows that they had “little to no history of sales with” Native Extracts.
258 Biocosmetic has been addressed above, and the applicants accept that it should be excluded from the calculations.
259 As for Tismor Health & Wellness, Schedule 3 of Mr Stephens’ first report demonstrates that Native Extracts first sold extracts to Tismor Health & Wellness in FY17 and continued to sell to them every financial year up to and including FY21, in the total amount of $116,049. Plant Extracts sold extracts to Tismor Health & Wellness each financial year from FY18 to FY21 in the total amount of $771,025. In these circumstances, I accept the opinion of Mr Lytras that the sales to Tismor Health & Wellness should be excluded from the calculation because the buying behaviour of Tismor Health & Wellness does not support the assumption which the applicants press, being that Tismor Health & Wellness would have acquired the same quantities from Native Extracts (instead of Plant Extracts) during FY18 to FY21 at the higher price charged by Native Extracts. I regard this to be very unlikely.
260 As appears in the JER, Mr Lytras applies an inferred profit margin of 56% to his total sales figure of $148,621 to arrive at a lost profits figure of $83,224.
261 The RM respondents then propose a further discount of 50% on the basis that there would have been significant other market factors at play which would have affected the probability of the customers purchasing the extracts from Native Extracts.
262 In the JER, the experts identified that the primary differences between them concerned:
(1) the treatment of R&D costs; and
(2) the differing lost sales assumptions based upon predominantly underlying factual and legal differences which are matters outside of their expertise.
263 The experts adopted different approaches to the treatment of R&D costs, which in turn impacts upon the inferred profit margin calculated by them.
264 Mr Stephens replaced Native Extracts’ recorded R&D costs with an adjusted figure in determining Native Extracts’ cash profit before tax and cash profit before tax margin for application in his loss calculations.
265 Mr Lytras deducted R&D costs (as recorded and treated in Native Extracts’ financial statements) in determining Native Extracts’ cash profit before tax and cash profit before tax margin for application in his loss calculations.
266 For the following reasons, I prefer and will adopt the approach taken by Mr Lytras to that of Mr Stephens.
267 First, Mr Stephens assumed that further R&D costs would not have been incurred by Native Extracts in respect of the lost sales that were in respect of products already developed and sold by Native Extracts. This assumption played a major role in the calculations performed by Mr Stephens. However, that assumption was not verified or established by any lay evidence adduced by Native Extracts. While it has a superficial attraction, not enough is known about the R&D process actually undertaken by Native Extracts to accept, as a blanket proposition, that it would not have incurred any further R&D costs in respect of existing products, whether for the purpose of improving them or perhaps correcting an issue which had been discovered with the formulation of an extract (for example). In this regard, Mr CJ Carroll gave evidence that he had personal involvement in conducting “test extractions” on the extraction machines including for the purpose of “[increasing] the quality and consistency of the extracts”, which indicates that he was involved in performing R&D on existing extracts for the purpose of improving them.
268 Second, in his calculations, Mr Stephens assumed that R&D costs are written off over a period of 10 years. This was said to be on the basis that the R&D costs are expected to have future ongoing benefits/sales. However, as submitted by the RM respondents, such an approach is not reflective of what Native Extracts did during the relevant period, being to write off the R&D costs in the year that they were incurred (being the approach taken by Mr Lytras).
269 In relation to this, Mr Lytras reviewed Native Extracts’ disclosed financial statements (for 2013, 2016 to 2020), income tax returns (for 2014 and 2015) and MYOB management accounts (for 2021) and states in the JER that:
53. …none of these nine sets of financial statements contain or deduct an expenditure item (or items) named, or with a name similar to, ‘Research & Development’ expenditure in determining Native Extracts’ reported net profit before tax for the subject year. Nor is there any capitalisation and subsequent amortisation of a research and development asset.
270 That it treated its R&D costs in this way is the best indicator of what Native Extracts would have done during the relevant period even had it also sold the additional volume of extracts as posited in the applicants’ counterfactual scenario. It is not to the point that Ms Carroll says, as she does in her second affidavit, that she was not made aware that R&D costs could have been spread over many years as an accounting method. That is because, even then, she does not go on to say that, had she been made aware, she would have caused Native Extracts to adopt this alternative accounting method. For these reasons, the higher likelihood is that Native Extracts would have treated its R&D costs in its accounts in the same way irrespective of the volume of sales.
271 Third, after his review of Native Extracts’ disclosed financial statements, Mr Lytras also states in the JER that:
(d) MS Excel files recording R&D activity for 2018 and 2019 provide further detail in relation to amounts claimed as R&D expenditure for tax purposes. Some items appear directly related to R&D:
(i) ‘Consultants R&D’;
(ii) ‘Legal Fees’ (which already would have been added back in the profit calculation in dealing with Legal Expenses);
(iii) ‘R&D Grant Consultant’ (costs associated with obtaining the R&D tax benefit rather than any R&D activity);
(iv) ‘Laboratory Testing R&D’ (predominantly with Southern Cross University as the supplier);
whilst others appear more of an operational and / or allocation of an expense which would arguably have been incurred in any event:
(i) ‘Raw Material’;
(ii) ‘Equipment Hire - Cold Storage’;
(iii) ‘Water Expenses’;
(iv) ‘Store Supplies’;
(v) ‘Manufacturing Expense’;
(vi) ‘Travel and Accommodation’ (to attend CANN10 Cannabis conference);
(vii) ‘Registrations and Subscriptions’;
(viii) ‘Plant and Equipment - At Cost’ (which would already have been added back in the profit calculation in dealing with ‘Depreciation Expenses’);
(ix) 50% of Lisa Carroll’s ‘Wages’;
(x) 30%-40% of Vanessa Minnikin’s ‘Wages’;
(xi) 50% of CJ Carroll’s ‘Wages’;
(xii) 80%-90% of Avishai Karny’s ‘Wages’; and
(xii) 15% of Katherine Bugden’s ‘Wages’.
No MS Excel files recording this level of R&D activity appear to have been disclosed for the 2013 to 2017 or 2020 and 2021 years; and
(e) Generally, the records supporting R&D are incomplete and the adjustments made by Mr Stephen [sic] appear heavily reliant upon his estimates as to likely expenditure.
272 I will describe the items in the list commencing with “Raw Material” and concluding with “15% of Katherine Bugden’s ‘Wages’” as queried operational expenses.
273 During the concurrent evidence session, Mr Lytras explained that, as an accountant, he would want further information about the queried operational expenses and the justification for them being included in the R&D expenses. Mr Stephens explained that he was given the documents relating to the overall R&D costs but was not instructed about specific cost elements included in that amount. No evidence was adduced by the applicants to justify the inclusion of the queried operational expenses in the R&D costs, when this could have been done, and so I accept the concerns of Mr Lytras about the inclusion of these apparently operational costs in the R&D costs.
274 A related issue is that the R&D costs include a level of add-back double count in respect of ‘legal fees’ and ‘plant and equipment’ as they are already added back in dealing with Legal Expenses and Depreciation (which were amounts agreed between the experts).
275 While Mr Lytras raised other concerns with the approach taken by the applicants, and by Mr Stephens, to the calculation of R&D costs, the above is sufficient to dispose of this issue.
276 This has the consequence, as accepted by the applicants, that the inferred profit margin to be applied is that determined by Mr Lytras, namely 56%.
The differing lost sales assumptions
277 The underlying premise of the claimed loss by Native Extracts is an assumption which it presses upon me to adopt, namely that, but for the admitted unlawful conduct of Mr Ross Macdougald and Phytoverse, all pre-existing customers of Native Extracts who purchased extracts from Plant Extracts in the four year period would have purchased the same extracts from Native Extracts, in the same quantities and at the higher price that Native Extracts was selling them. That higher price was nearly 3.5 times the price that Plant Extracts would have charged. However, Mr Stephens did not express an opinion as to the probability of the customers paying the higher price, observing in the JER that:
…the reasonableness of the Price Premium Differential would include an assessment of a broad range of factors including demand elasticity (i.e. price sensitivity), market size and volume; domestic and international competition, market trends, product substitutes/alternatives and customer intentions which are outside the expertise of an accountant.
278 There are several matters which tell against the assumption which Native Extracts presses in this regard and which reduce the probability of its occurrence.
279 First, the evidence of Mr Jordan Macdougald was that his father informed him to the effect that there were customers of Native Extracts who no longer wished to purchase extracts from that company. That evidence was admitted without restriction, and I accept that this was said to Mr Jordan Macdougald by Mr Ross Macdougald. There is a real possibility that at least some customers who had dealt with Mr Ross Macdougald when he was at Native Extracts would have sought to follow him to his new business, or at least been less enthusiastic about purchasing extracts from Native Extracts after he left it. Further, that at least some customers would not have purchased from Native Extracts regardless of the unlawful conduct is supported by the parties’ agreed position in relation to Biocosmetic.
280 Second, there were other manufacturers of botanical plant extracts in the Australian market in 2012 (including one which Ms Carroll described as a major competitor), and there was no evidence to suggest that these competitors were not still operating during the relevant period.
281 While Ms Carroll explained her understanding of the differences between the extraction methods used by Native Extracts and others in the industry in 2012, she also explained the numerous steps taken to promote the extraction methods used by Native Extracts, and the promotion of its extracts from Australian native species. Such promotion was not secret, but a form of publicity. There is therefore a reasonable prospect that a competitor learned of the extraction methods being touted by Native Extracts and sought to adopt them. Notably, even on Ms Carroll’s evidence, Native Extracts was not the only company selling Australian native botanical extracts in 2012 – it was just selling the largest commercial range of them.
282 Although Mr Stephens gave evidence during the concurrent evidence session about his understanding of the market and whether there were substitute products available from other suppliers, this was based on his interpretation of information which had been briefed to him. In any event, according to him, he was not provided with information about the international market (for example), market trends or substitute products, and so his evidence concerning other suppliers or the availability of substitute products is of little assistance, and no weight is given to it.
283 Importantly, there is no evidence that others in the industry had not adopted the same or similar extraction methods as Native Extracts, and were not selling extracts which consumers would regard as substitutable for the extracts sold by Native Extracts during the relevant period. It follows that it cannot be assumed that all customers of Native Extracts would have needed to acquire extracts from Native Extracts if they could not purchase them from Plant Extracts.
284 Third, Ms Carroll explains that price had been a barrier to Native Extracts obtaining customers when it first commenced operations because of the higher prices that it was charging. This indicates that purchasing decisions by customers in this market are affected by price. This aligns with common experience that the decision by a consumer to purchase any product either at all or in a particular quantity will be affected by (inter alia) its price, and that the higher the price, the less likely it is that the consumer will make the same purchase.
285 For these reasons and contrary to the applicants’ submissions, I am not prepared to assume that Native Extracts would have made the same volume of sales to the same customers at the higher price being charged by it during the relevant period.
286 However, the approach taken by Mr Lytras (and advanced by the RM respondents) also cannot be accepted. That is because there is no basis to conclude that there was even a realistic possibility that Native Extracts would have reduced its prices to those which were charged by Plant Extracts; rather, it is more likely that Native Extracts would have continued its existing course of conduct in terms of pricing, but with the result that a lower volume of sales would have been made having regard to the above matters.
287 For these reasons, I am prepared to accept that there would have been some additional sales made by Native Extracts to these customers at the higher price. The application of a discount is required to reflect the uncertainty inherent in the estimation of the quantum of these additional sales, which occurs below.
288 As I have found, CR Formulations and Tismor Health & Wellness are not likely to have acquired the same volume of extracts during the relevant period from Native Extracts. As such, sales attributable to them must be deducted from the lost sales figure. The price premium was applied to the sales to these customers and is included in the Adjusted NE Total Lost Sales figure. However, the price premium was calculated by Mr Stephens at Table 12 of his first report by reference to the sales of types of extract product (such as concentrated, diluted etc.) and by comparing the average prices charged by Plant Extracts and Native Extracts for those products, not on a per customer basis. Mr Lytras does not assess price premium on a per customer basis either. This means it is not possible to make deductions of the sales to CR Formulations and Tismor Health & Wellness using actual figures calculated by the experts by reference to those customers.
289 In light of this, and in an attempt to more accurately deduct the sales to these customers from the Adjusted NE Total Lost Sales figure, I have calculated the average price premium factor across all sales for each year by reference to Schedule 2 of the JER. This was done by dividing the total sales figures inclusive of the price premium for each year (labelled as Sales (Stephens loss of profits)) by Plant Extracts’ actual total sales figures for each year (labelled as Sales (Stephens account of profits)).
290 These price premium factors (without rounding) are then multiplied by the actual sales by Plant Extracts in each year to each of CR Formulations and Tismor Health & Wellness. Relevant figures in these calculations are set out in the table below:
291 This results in lost sales inclusive of the price premium for each customer, totalling $3,052,380 for CR Formulations and $2,683,668 for Tismor Health & Wellness. This results in a total deduction from the Adjusted NE Total Lost Sales figure of $5,736,048 to give a final lost sales figure of $3,778,521.
292 The result of the calculations above can be compared to the result if the actual sales by Plant Extracts to CR Formulations and Tismor Health & Wellness are multiplied by 3.47 to represent the 247% increase to the sales figures caused by the price premium referenced at [244]. Multiplying the total sales by Plant Extracts to those customers by 3.47 results in $5,639,333, which is proximate to the result of $5,736,048 above. I prefer the latter figure as it more accurately reflects the variations in price premium from year to year.
293 Applying the inferred profit margin of 56% to $3,778,521 gives a total of $2,115,972.
294 As set out above, a discount must be applied to account for lack of certainty, even if that discount is only a very modest one: see Generic Health at [186]. The fact that the value of sales to specific customers has been discounted already does not mean that a further discount ought not be applied to the remaining lost sales figure, which remains hypothetical. The applicants appeared to accept that this was so. Taking into account the uncertainties and vagaries involved in assessing the hypothetical counterfactual, I consider that a discount of 10% should be applied to the figure of $2,115,972. Applying this discount results in a figure of $1,904,375, which is equivalent to the hypothetical profits lost by Native Extracts during the relevant period.
295 For these reasons and having regard to the terms of orders 7 and 8 of the Order dated 19 October 2023, it would be appropriate to order that Mr Ross Macdougald and Phytoverse pay Native Extracts the amount of $1,904,375 as damages in the terms sought by the applicants in its draft order, should an election be made for such an order.
7. ADDITIONAL DAMAGES FOR COPYRIGHT INFRINGEMENT
296 The final issue to be determined is the quantum of additional damages to be ordered in respect of the admitted copyright infringement pursuant to s 115(4) of the Copyright Act.
297 Native Extracts submits that an award of at least $157,198 for additional damages is appropriate because it represents the amount of profit that the experts agree that Plant Extracts made from sales of extracts relating to the infringing copyright material. Plant Extracts accepts that Native Extracts is entitled to additional damages but disputes the amount that is claimed.
298 The key facts concerning the copyright infringements are set out below and were either admitted or not challenged by Plant Extracts at trial.
299 As part of the business of Native Extracts, it provided technical documents to customers and potential customers for each of its botanical plant extracts. Those technical documents included parts of Southern Cross University “Certificates of Analysis” (SCU Certificates) which consist of an independent analysis of the composition of each of its botanical plant extracts.
300 From about 2015, Southern Cross University sent SCU Certificates for nine different types of botanical plant extracts to Native Extracts (referred to as the Original SCU Certificates). Copyright in these certificates was assigned to Native Extracts.
301 By at least 2018, Plant Extracts began to send existing and potential customers what it described as Datapacks relating to each of the botanical plant extracts which it produced (which correspond to the nine types of extracts referred to above). Those Datapacks included certificates purporting to be from the Southern Cross University.
302 Each of those certificates reproduced in a material form the whole, or a substantial part, of the Original SCU Certificate relating to the relevant extract. Each certificate also modified the Original SCU Certificate by removing the date, customer details, customer reference number, laboratory reference number and job number. Plant Extracts did not have any licence or authority to reproduce the Original SCU Certificates.
303 On 16 June 2020, Ms Carroll caused a letter of demand to be sent to Mr Ross Macdougald and the entities controlled by him, advising him that they were (among other things) infringing the copyright of Native Extracts by modifying and sending the Original SCU Certificates to existing and potential customers of Plant Extracts.
304 By the Defence, Plant Extracts admitted that it had infringed Native Extracts’ copyright in each of the Original SCU Certificates. It also admitted that, since on or about 8 July 2016, it has continued, and threatens to continue, to engage in these acts of copyright infringement in the course of promoting its business. It was also admitted by Mr Ross Macdougald that he had authorised, and continues to authorise, the conduct by Plant Extracts.
305 As a consequence, I made the following declarations and orders in Native Extracts (No 1):
THE COURT DECLARES BY CONSENT BETWEEN THE APPLICANTS AND THE RESPONDENTS (OTHER THAN THE FIFTH RESPONDENT) THAT:
…
2. The First Applicant is the owner of the copyright subsisting in the Original SCU Certificates, and the First Respondent has infringed that copyright by reproducing in a material form the whole or a substantial part of those works.
…
THE COURT ORDERS BY CONSENT BETWEEN THE APPLICANTS AND THE RESPONDENTS (OTHER THAN THE FIFTH RESPONDENT) THAT:
…
15. Pursuant to section 115(2) of the Copyright Act 1968 (Cth) (Copyright Act), the First Respondent, whether by itself, its directors, officers, employees, agents or otherwise, is permanently restrained from:
(a) infringing the First Applicant’s copyright in the Original SCU Certificates; and
(b) in particular, reproducing in a material form the whole or a substantial part of the Original SCU Certificates.
…
18. Pursuant to section 115(2) of the Copyright Act, the Second Respondent is permanently restrained from authorising the First Respondent or any other person to do acts that infringe the copyright of the First Applicant in the Original SCU Certificates, in particular by reproducing in a material form the whole or a substantial part of those works.
…
27. Pursuant to section 115(2) of the Copyright Act, the First Respondent pay to the First Applicant the sum of $168,053 as compensatory damages.
(Emphasis original.)
306 The amount of $168,053 which was ordered to be paid as compensatory damages represents the profits lost by Native Extracts as a result of the copyright infringements (as agreed by the experts in the JER).
307 Section 115(4) of the Copyright Act provides that the Court may make an order for additional damages if:
(a) an infringement of copyright is established; and
(b) the court is satisfied that it is proper to do so, having regard to:
(i) the flagrancy of the infringement; and
(ia) the need to deter similar infringements of copyright; and
(ib) the conduct of the defendant after the act constituting the infringement or, if relevant, after the defendant was informed that the defendant had allegedly infringed the plaintiff's copyright; and
(ii) whether the infringement involved the conversion of a work or other subject-matter from hardcopy or analog form into a digital or other electronic machine-readable form; and
(iii) any benefit shown to have accrued to the defendant by reason of the infringement; and
(iv) all other relevant matters.
308 The parties were not in dispute about the principles relating to the assessment of additional damages. Those principles were helpfully collected and summarised by Thawley J in QAD Inc v Shepparton Partners Collective Operations Pty Ltd (2021) 159 IPR 285; [2021] FCA 615 (affirmed on appeal in Shepparton Partners Collective Operations Pty Ltd v QAD Inc [2021] FCAFC 206 (Greenwood, Jagot and Rofe JJ)) at [145]:
(1) The award is made under s 115(4)(b) of the Copyright Act and it is the terms of that provision which are of paramount importance. The Court must have regard to the matters in subparas (i) to (iv) of s 115(4)(b) in being satisfied that it is proper to award additional damages. Those matters, to which the Court must have regard, are not in the nature of pre-conditions which must be satisfied in order to make the award: Futuretronics.com.au Pty Ltd v Graphix Labels Pty Ltd (No 2) (2008) 76 IPR 763; [2008] FCA 746 at [17] (Futuretronics).
(2) Subparagraph (iv) of s 115(4)(b) requires the Court to take into account all relevant matters. The discretion is, accordingly, broad and its exercise will be closely tied to the particular facts of the case. No doubt other cases might provide some guidance in various ways, but the focus must be on the particular facts before the Court.
(3) The Court has a broad general discretion to award additional damages not fettered in any arithmetical or mechanical way: LED Builders Pty Ltd v Eagle Homes Pty Ltd (1999) 44 IPR 24; [1999] FCA 584 at [91].
(4) Additional damages need not be proportionate to any award of compensatory damages: Futuretronics at [17]. Damages may be awarded even if the copyright owner is only entitled to nominal compensatory damages: Dynamic Supplies Pty Ltd v Tonnex International Pty Ltd (No 3) (2014) 312 ALR 705; 107 IPR 548; [2014] FCA 909 (Tonnex) at [52].
(5) It is, nevertheless, permissible to calculate additional damages as an “uplift” in a fixed percentage on top of the licence fee that the infringer would have paid — see, for example: Halal Certification Authority Pty Ltd v Scadilone Pty Ltd (t/as Sofra Pizza Pide & Kebab House) (2014) 107 IPR 23; [2014] FCA 614 at [111]–[113] (Halal Certification).
(6) Additional damages may be assessed having regard to principles derived from awards of aggravated and exemplary damages at common law: Autodesk Inc v Yee (1996) 68 FCR 391 at 394; 139 ALR 735 at 739; 35 IPR 415 at 419 (Yee). However, additional damages are not “exceptional”— rather, an element of penalty is an accepted factor: Tonnex at [43]. When an award of additional damages includes a punitive component, the individual circumstances of the infringer must be taken into account in the award: Tonnex at [53].
(7) The blatancy of the infringing conduct may be taken into account — see: Amalgamated Mining Services Pty Ltd v Warman International Ltd (1992) 111 ALR 269 at 286–7; 24 IPR 461 at 479, in which Wilcox J said that “[i]f copyright protection is to mean anything, the courts must take a severe view in cases [that are] blatant”.
309 Native Extracts made submissions regarding each of the relevant matters under s 115(4)(b). In response, Plant Extracts submits that the amount sought should be “discounted” to account for its conduct at trial (that is, its admissions at trial), as well as the fact that quite significant orders for damages would be made against it in relation to other matters. Plant Extracts did not challenge any of the facts relied upon by Native Extracts in relation to its additional damages claim.
310 I consider the parties’ submissions in terms of s 115(4)(b)(i)–(v) below.
7.3.1 Flagrancy: s115(4)(b)(i)
311 The meaning of “the flagrancy of the infringement” has been described in various ways, including as “deliberate and calculated copyright infringement”, “calculated disregard of the plaintiff’s rights, or cynical pursuit of benefit”, conduct which could be described as glaring, scandalous or blatant, and as extending to conduct exhibiting a consciousness of wrongdoing, whether or not it exhibits a consciousness of copyright infringement: see QAD at [147]; see also Futuretronics.com.au Pty Ltd v Graphix Labels Pty Ltd (No 2) (2008) 76 IPR 763; [2008] FCA 746 at [19] (Besanko J); Dynamic Supplies Pty Ltd v Tonnex International Pty Ltd (No 3) (2014) 107 IPR 548; [2014] FCA 909 at [47] (Yates J).
312 Native Extracts submits that Plant Extracts’ infringement was flagrant in that it was deliberate and calculated. That submission must be accepted. It is self-evident that Plant Extracts (by Mr Ross Macdougald) took the Original SCU Certificates belonging to Native Extracts, and digitally modified them so that they would pass as its own. By sending them to its customers, it falsely represented that it had engaged Southern Cross University to conduct independent testing of its extracts and that its extracts contained certain properties as reflected in those certificates.
313 Plant Extracts failed to adduce any evidence to explain this conduct, despite it being open for it to do so. It could have (for example) adduced evidence that Mr Ross Macdougald held a belief that he had a right to reproduce or modify the Original SCU Certificates. For this reason, I infer that this evidence would not have assisted its case. Indeed, given that Plant Extracts was able to cast light on the submission by Native Extracts regarding flagrancy, but has refrained from doing so, I am able to draw the inference that the infringement was deliberate and calculated with greater confidence: see Kuhl v Zurich Financial Services Australia Ltd (2011) 243 CLR 361; [2011] HCA 11 at [63] (Heydon, Crennan and Bell JJ).
314 For these reasons, I am satisfied that the infringing conduct was deliberate and further, done with a view to deceive existing and potential customers.
7.3.2 Deterrence: s115(4)(b)(ia)
315 The concept of deterrence in the context of this provision encompasses both specific and general deterrence: see QAD at [152]. In this case, both specific and general deterrence is necessary.
316 Although Plant Extracts eventually admitted to the copyright infringements and agreed to permanent injunctions, its conduct prior to this demonstrate a disregard for the rights of Native Extracts. In particular, it was only at trial that it admitted that, since on or about 8 July 2016, it has continued the pleaded infringing conduct.
317 Until trial, Plant Extracts has marketed its botanical plant extracts on the false premise that they were scientifically verified, and, as the joint experts agreed, it reaped considerable profits from that conduct. If additional damages were not awarded, Plant Extracts would be required to compensate Native Extracts for the losses it caused but retain the profits which it obtained from its wrongdoing; that, in my view, would not be a sufficient deterrent.
318 In considering specific deterrence, an infringer’s financial standing and resources may be a relevant consideration: see QAD at [154]; Dynamic Supplies at [116]; Henley Arch at [250]; Nesor Nominees Pty Ltd v Big Boys BBQ Pty Ltd (2019) 146 IPR 1; [2019] FCA 1208 at [47] (Anderson J). However, the only evidence before the Court in this regard is the experts’ account of profits analysis from FY17 to FY21. On either expert’s view, Plant Extracts operated a very profitable business and there is no evidence to suggest that Plant Extracts does not have capacity to pay the additional damages award sought.
319 There is also a need for general deterrence. As Ms Carroll deposed in her first affidavit, botanical plant extracts can be used for a variety of purposes including in food, beverages, cosmetics and pharmaceuticals. The modification of the Original SCU Certificates had the effect of giving false information to customers about the composition of products which could be ingested or used on the skin. Those customers were likely to have been misled by the modified Original SCU Certificates with the consequence that at least some of them were induced to buy extracts from Plant Extracts. The prejudice to third parties supports the award of additional damages as sought by Native Extracts as it is necessary to deter other businesses in the industry from engaging in this kind of conduct.
320 These matters all weigh in favour of making the award for additional damages sought by Native Extracts.
7.3.3 Conduct after infringement/being informed of infringement: s115(4)(b)(ib)
321 Native Extracts relies upon the following matters in relation to s 115(4)(b)(ib):
(a) the lack of evidence before the Court that a response was given to Ms Carroll’s letter of demand, or that Plant Extracts ceased to send the modified SCU certificates to customers after receipt of the letter of demand; and
(b) Plant Extracts’ conduct in the litigation.
322 As to the first matter and as already noted, Plant Extracts admitted in October 2023 that it had continued to engage in the conduct after July 2016. There was no suggestion that this conduct ceased upon the letter of demand being received or these proceedings being commenced.
323 As to the second matter, Plant Extracts submits that its conduct in the proceedings weighs against an award of additional damages in the sum sought by the applicants.
324 In some respects, Plant Extract’s conduct of the proceedings was not inappropriate. Certain facts were admitted early and, whilst others were admitted very late, they were still appropriately admitted. Plant Extracts also submitted to a permanent injunction restraining it from infringing Native Extracts’ copyright in the Original SCU Certificates.
325 However, these proceedings have been on foot since 2020. Native Extracts was required to file its evidence and prepare for trial, and it was only at the last minute that Plant Extracts admitted its unlawful conduct, which supports the award of additional damages sought by Native Extracts: see generally Facton Ltd and Others v Rifai Fashions Pty Ltd (2012) 199 FCR 569; [2012] FCAFC 9 at [44] (Lander and Gordon JJ) and [114] (Gilmour J); QAD at [158]–[159]; Henley Arch at [249].
326 Plant Extracts’ delay in admitting that its conduct was unlawful coupled with its belated formal admission that it had continued to engage in the infringing conduct, including after receipt of the letter of demand and after these proceedings were commenced in 2020, support the making of the award for additional damages sought by Native Extracts.
7.3.4 Whether infringement involved conversion: s115(4)(b)(ii)
327 This factor relates to conversion of a work or other subject-matter from hardcopy or analog form into a digital or other electronic machine-readable form.
328 It is unnecessary to consider this factor as it does not arise on the facts of this case.
7.3.5 Benefits accrued: s115(4)(b)(iii)
329 Native Extracts submits that the following benefits should be taken into account:
(a) Plant Extracts obtained a significant pecuniary benefit as a result of selling extracts which were the subject of the modified Original SCU certificates. As to this, the experts agreed that Plant Extracts earned $157,198 in profits as a result of the infringing conduct;
(b) by providing the modified Original SCU certificates, Plant Extracts was able to secure business relationships with customers that it might not otherwise have been able to obtain.
330 Both of these benefits are relevant and weigh in favour of making an award of additional damages. Section 115(4)(b)(iii) is concerned with any “benefit” and is not limited to a pecuniary benefit or a benefit quantifiable at a precise monetary value: Henley Arch at [245].
7.3.6 Other relevant matters: s115(4)(b)(iv)
331 As mentioned, Plant Extracts submits that the amount of additional damages should be discounted on account of the fact that there will be orders for “quite significant damages in relation to other matters”.
332 If this is intended to be a reference to the compensatory damages which have been awarded for copyright infringement, then I accept that this is a relevant factor and I take it into account: see generally QAD at [167].
333 If this is not so intended, then I do not consider this to be a relevant factor within the meaning of s 115(4)(b)(iv). The quantum of additional damages for copyright infringement should not be determined by reference to, or affected by, the quantum of damages awarded for other, unrelated causes of action.
334 Having regard to the above matters and as infringement of copyright has been established by Native Extracts, I am satisfied that it is proper to award additional damages in its favour against Plant Extracts, and that the appropriate amount of such an award is $157,198.
335 For these reasons, the following orders will be made:
(a) The claim against Mr Jordan Macdougald be dismissed;
(b) Plant Extracts pay Native Extracts the sum of $157,198 as additional damages pursuant to s 115(4) of the Copyright Act;
(c) The parties are to confer on the form of the remaining orders to be made to give effect to these reasons, including on the question of costs.
336 The orders to be made will also provide to the effect that, if the parties are able to agree upon a form of orders, then these orders will be made in chambers. If the parties cannot agree, then directions will be made to enable any dispute to be resolved.
I certify that the preceding three hundred and thirty-six (336) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Downes. |
Associate:
SCHEDULE OF PARTIES
QUD 215 of 2020 | |
FPI OCEANIA PTY LTD ACN 089 947 482 | |
Fifth Respondent: | JORDAN RADLEY ROSS MACDOUGALD |
Sixth Respondent: | BIOLOGI PTY LTD ACN 618 697 297 |




