Federal Court of Australia
Calix Limited v Grenof Pty Ltd [2023] FCA 378
ORDERS
Applicant | ||
AND: | GRENOF PTY LTD ACN 166 936 894 First Respondent AQUADEX PTY LTD (ACN 102 261 727) Second Respondent | |
AND BETWEEN: | GRENOF PTY LTD ACN 166 936 894 Cross-Claimant | |
AND: | Cross-Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The amended originating application filed on 2 March 2023 be dismissed.
2. Claim 1 of Australian Patent No 2014339743 be revoked.
THE COURT DECLARES THAT:
3. The letter dated 19 February 2021 sent by Alder IP on behalf of the applicant to the first respondent constituted an unjustified threat.
THE COURT FURTHER ORDERS THAT:
4. The amended statement of cross-claim filed on 8 July 2022 be otherwise dismissed.
5. Subject to order 6 below, the applicant is to pay the respondents’ costs of the proceeding (including the cross-claim).
6. The first respondent is to pay the applicant’s costs of the interlocutory application filed on 2 December 2022.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
NICHOLAS J:
INTRODUCTION
1 This is a proceeding brought by the applicant (“Calix”) against the first respondent (“Grenof”) and the second respondent (“Aquadex”) for infringement of claim 1 of Australian Standard Patent No 2014339743 (“the Patent”) entitled “Process and apparatus for manufacture of hydroxide slurry”. The priority date of claim 1 is 24 October 2013.
2 Grenof has filed a cross-claim seeking revocation of claim 1 on the grounds that the invention, as claimed, lacks novelty, and does not involve an inventive step. Grenof also contends, by way of its cross-claim, that the Patent does not disclose the invention of claim 1 in a manner which is clear enough and complete enough for the invention to be performed by a person skilled in the art as required by s 40(2)(a) of the Patents Act 1990 (Cth) (“the Act”) and that claim 1 is not supported by the matter disclosed in the specification as required by s 40(3) of the Act. Grenof also contends that Calix has made unjustified threats for which it seeks relief pursuant to s 128 of the Act.
3 The respondents are alleged to have infringed Claim 1 of the Patent by manufacturing, supplying, or offering to supply hydroxide slurry. It is admitted that Grenof has manufactured and supplied hydroxide slurry under the name Phodine and Phodine Plus and that Aquadex has supplied hydroxide slurry under the name Phodine. The respondents admit that those products are made using what is relevantly the same process (“the Grenof process”).
4 For the reasons that follow I have concluded that claim 1 of the Patent is not infringed and is, in any event, invalid for lack of inventive step and non-compliance with s 40(3) of the Act.
WitnessES
5 There were three witnesses who provided written and oral evidence. Two independent experts, Professor Charles Sorrell, called by Calix, and Mr Steven Messiter, called by the respondents, gave their oral evidence in a concurrent session. Oral evidence was also given (though not in the concurrent session) by Mr David Redfern who developed the Grenof process while working for Grenof. Mr Redfern now works for Aquadex.
6 Professor Sorrell is a Professor of Ceramic Engineering at the University of New South Wales (“UNSW”). He was appointed to that position in 1997. He holds a PhD awarded in 1987 in the field of Ceramic Engineering, a Master of Science in Ceramic Science, a Bachelor of Science in Ceramic Engineering, and a Bachelor of Science in Chemistry. He gave evidence in relation to the interpretation of the Patent, the common general knowledge, the prior art, and infringement.
7 Professor Sorrell in his Stage 1 Report claimed that he “has practical knowledge of the then-existing process for producing hydroxide slurries, which [he] derived through [his] teaching, researching, and consulting.” In his oral evidence, he said he produced a series of magnesium hydroxide slurries when undertaking his PhD and that a colleague of his “basically bounced ideas off him” at some stage in connection with some consultancy work that the colleague was undertaking for a company engaged in the manufacture of hydroxide slurries. It was on the basis of that involvement that Professor Sorrell would not accept in cross-examination that he had not worked in the field of the production of hydroxide slurries for industrial purposes.
8 The respondents originally objected to the whole of Professor Sorrell’s evidence on the basis that he was not a person skilled in the relevant art. The parties were content for me to admit Professor Sorrell’s evidence subject to that objection which I would rule on in the reasons for judgment. However, in closing submissions, the respondents informed me that they now object only to those parts of Professor Sorrell’s evidence relating to the interpretation of the Patent. For reasons which I will explain, I admit Professor Sorrell’s evidence, including his evidence relating to the interpretation of the Patent, but give it very little weight. It is clear that Professor Sorrell has had very little experience in the manufacture of hydroxide slurries. Further, in my view, for reasons that will be explained, Professor Sorrell is not a reliable witness.
9 Mr Messiter is a Chemical Engineer who has worked in the mining and chemical industries since 1984. Mr Messiter holds a Bachelor of Engineering (Chemical) with First Class Honours awarded in 1984 from UNSW. He has been employed by BHP Engineering as a Process Design Engineer, and by ICI Operations (“ICI”) as a Process Engineering and Technical Superintendent and as a Senior Process Engineer. Between 1995 and 1996 he worked for ICI as an Engineering Manager responsible for (inter alia) managing the design and construction of a magnesium hydroxide slurrying plant. Mr Messiter gave evidence in relation to the interpretation of the Patent, the common general knowledge, the prior art, and infringement. It is clear that Mr Messiter is a skilled addressee and Calix did not suggest that he was not. I regard him as a generally reliable witness. In all areas where there was a material disagreement between Mr Messiter’s evidence and Professor Sorrell’s evidence, I prefer Mr Messiter’s evidence.
10 Mr Redfern is a Chemist and holds a Bachelor of Science (Synthetic Organic Chemistry) with Honours. He gave evidence concerning the Grenof process, including a temperature measurement device (ie. the thermocouple) and a mixing apparatus used in the Grenof process, and other matters relevant to infringement. There were aspects of Mr Redfern’s evidence that I considered unsatisfactory. Some of his written evidence concerning the mixing apparatus used in the Grenof process was inaccurate and misleading. As to the location of the thermocouple in the reaction vessel used in the Grenof process, I prefer the evidence of the measurement made by Professor Sorrell. However, for reasons that will be explained, I prefer Mr Redfern’s evidence to that of Professor Sorrell on the question of whether the maximum temperature of the reaction mixture in the Grenof process reaches boiling point.
The Patent
11 The Patent includes 24 claims. Claims 1-22 are method claims. Claim 23 is an apparatus claim. Claim 24 is a product claim.
12 The technical field of the invention is described in the complete specification (“CS”) as follows at [0001]:
The present invention relates to relates [sic] broadly to a process and apparatus for manufacture of high solids hydroxide slurries from caustic calcined carbonate powders that may be produced from calcination of magnesite, dolomite and limestone and mixtures thereof, whereby the slurries have low resistance to shear thinning to facilitate reconstitution after months of storage with mild agitation.
13 The CS includes the following discussion of the nature and uses of hydroxide slurries at [0002]:
Hydroxide slurries are aqueous suspensions of solid hydrated oxides of primarily magnesium, as Mg(OH)2, calcium Ca(OH)2 and mixtures thereof, in water. They are widely used in many industrial processes. An example of which is the treatment of water to raise the pH and eliminate odours, particularly for sewerage treatment, and to precipitate heavy metals. These slurries are increasingly replacing sodium hydroxide because of their inherent properties.
14 The CS goes on to describe the use of hydroxide slurry in various applications including the use of magnesium hydroxide slurry in sewerage treatment. CS [0005] refers to the production of magnesium hydroxide slurries from either precipitated magnesium hydroxide (Mg(OH)2), or hydrating magnesium oxide (MgO) produced from the calcination of the mineral magnesite.
15 Preferred properties of hydroxide slurries are described at CS [0006]. Although Professor Sorrell (Calix’s expert) suggested that some of these properties related to the raw materials used in the manufacturing process, I think it is reasonably clear that the preferred properties described are those of hydroxide slurries or, in other words, finished products.
16 The CS refers to prior art including various US patents. One of these is US Patent 5906804 (“Aral”) entitled “Magnesium Hydroxide Slurries” which is one of four publications relied on by the respondents in support of its lack of novelty allegation.
17 The CS purports to identify problems to be solved at [0021] – [0023]. It is stated at CS [0022] and [0023]:
[0022] The present invention may aim to provide a process, system, device and apparatus for production of hydroxide slurries from caustic calcined carbonate or hydroxide powders.
[0023] It is an object of the present invention to overcome or ameliorate at least one of the disadvantages of the prior art, or to provide a useful alternative.
18 The CS identifies two aspects of the invention at [0025] – [0026]:
[0025] A first aspect of the present invention may relate to a process for producing a hydroxide slurry from caustic calcined carbonate powder, comprising the following steps: mixing caustic calcined carbonate powder with water in a reactor vessel and forming a reaction mixture; applying a shearing force to the reaction mixture using a mixing apparatus; allowing heat of hydration to raise the temperature of the reaction mixture to near the boiling point, preferably about 95ºC, and allowing steam to evaporate from the reaction mixture as hydration proceeds, to remove excess heat and control reaction temperature to just below or at the boiling point.
[0026] A second aspect of the present invention may relate to a process for producing a hydroxide slurry from caustic calcined hydroxide powder, comprising the following steps: mixing caustic calcined hydroxide powder with water in a reactor vessel and forming a reaction mixture; applying a shearing force to the reaction mixture using a mixing apparatus; allowing heat of hydration to raise the temperature of the reaction mixture to near the boiling point, preferably about 95C [sic], and allowing steam to evaporate from the reaction mixture as hydration proceeds, to remove excess heat and control reaction temperature to just below or at boiling point.
19 I draw attention to the last few words of those paragraphs each of which refers to a reaction temperature “just below or at boiling point”. Those words do not reflect the language of claim 1 which refer to a maximum reaction temperature “near the boiling point”. The CS does not include any consistory statement mirroring the language of claim 1.
20 A preferred process is described as follows at CS [0029]:
The preferred process may additionally comprises [sic] the following steps: metering an input of a viscosity modifier to enable the mixing apparatus to maintain uniform mixing under thin slurry conditions promoted by the viscosity modifier; allowing the reaction to proceed spontaneously, during boiling, until the water has ceased to boil and the temperature dropped to a first set point; and quenching the slurry to drop the temperature to a second set point.
21 The specification also describes a further aspect of the invention which is said to relate to a reaction apparatus. A reaction apparatus is claimed in claim 23. According to CS [0039]:
A third aspect of the present invention may relate to a reaction apparatus for producing a hydroxide slurry from a reaction mixture of at least caustic calcined carbonate powder or caustic calcined hydroxide powder and water, wherein the reaction apparatus comprises: a reaction vessel having a first inlet adapted for receiving caustic calcined carbonate powder and a second inlet adapted for receiving water and a controller that is adapted to electronically control the process within the reaction vessel; shearing apparatus positioned within the reaction vessel for shearing the reaction mixture and wherein the rate of shearing is controlled by the controller; a viscosity sensor positioned within the reaction vessel adapted to supply viscosity information about the reaction mixture to the controller; a temperature sensor positioned within the reaction vessel adapted to supply viscosity information about the reaction mixture to the controller; and a steam outlet for release of steam from the reaction vessel, such that in use the reaction is controlled by the controller so that the heat of hydration may raise the temperature of the reaction mixture, allowing water to boil off from the reaction mixture as hydration proceeds, and removing steam via the steam outlet to remove excess heat and control reaction temperature at boiling point.
22 CS [0041] – [0042] state:
[0041] In one form, the disclosure provides a process of producing hydroxide slurry from caustic calcined carbonate or hydroxide powder, including:
(a) mixing caustic calcined carbonate or hydroxide powder with water in a reactor vessel;
(b) shearing the reaction mixture; and
(c) allowing heat of hydration to raise the temperature of the reaction mixture to a maximum near the boiling point, and allowing water to boil off from the reaction mixture as hydration proceeds, to remove excess heat. The maximum and reaction temperature is bounded by the boiling point of water in the mixture.
[0042] Optional, and preferred, process steps include one or more of:
(d) metering an input of a viscosity modifier to enable the mixing system to maintain uniform mixing under thin slurry conditions promoted by the viscosity modifier;
(e) allowing the reaction to proceed spontaneously, during boiling, until the water has ceased to boil and the temperature dropped to a first set point; and
(f) quenching the slurry to drop the temperature to a second set point.
23 Means by which the reaction may be controlled are referred to in CS [0044] – [0047]:
[0044] Further preferred aspects of control of the reaction include one or more of:
[0045] (i) minimising the heat losses such that the hydration heat liberated spontaneously heats the slurry and accelerates the hydration process such that the water boils, to provide the constant conditions at the boiling temperature and pressure to allow the remainder of the hydration to be controlled in a simple self-regulating manner; and
[0046] (ii) mixing of the water and particles to reduce the formation of bubbles, to break up the formation of aggregates during the slurry production, and to provide mixing so that the hydration reaction at the surfaces of all particles can occur at a fast, uniform rate; and
[0047] (iii) adding a viscosity modifier to maintain a thin slurry during production, with the modifier being added at a rate and to a degree necessary to allow the mixing to take place without a substantial change in the energy consumption of the mixing system.
24 Further reference to the temperature of the reaction mixture appears at CS [0049]:
A further aspect of the present invention may provide for a reaction apparatus for producing hydroxide slurry from caustic calcined carbonate powder or caustic calcined hydroxide powder, including: a reaction vessel having an inlet for the powder and a water inlet; shearing apparatus for shearing the reaction mixture; and a steam outlet for release of steam from the reaction vessel, such that in use the reaction is controlled by allowing heat of hydration to raise the temperature of the reaction mixture, allowing water to boil off from the reaction mixture as hydration proceeds, and removing steam via the steam outlet to remove excess heat and control reaction temperature at boiling point.
25 The description of the invention by reference to drawings and what are said to be examples is found at CS [0058] – [0087] and includes at CS [0061]:
The basis for the process is that the energy released from hydration of the MgO to Mg(OH)2 by liquid H2O is used to heat the products of the reaction and the excess water to 100 C , and the excess heat spontaneously boils a portion of the excess water. In the ideal case of a reactor at ambient pressure, with no heat loss and inputs at 25 C, a 60% slurry can be made in which the heat released spontaneously raises the temperature to 100 C, and the remaining heat from the subsequent reaction spontaneously boils the water. Thus one tonne of 60% slurry (containing 600 kg of Mg(OH)2 and 400 kg of water) is produced at 100 C by boiling off an additional 76 kg of water. This slurry is made by mixing 415 kg of MgO and 661 kg of water at 25 C. From the known thermodynamics of the reactions, the hydration of the MgO by liquid water releases 387 MJ of heat of which 186 MJ is used to heat the materials to 100 C, and 201 MJ is used to heat and evaporate the water. In the design of the reactor, with inputs at 25 C, it follows that about 48% of the hydration reaction is complete before the boiling of the excess water occurs. Real reactors have heat losses, and minerals have impurities, so these quantities provided above are for guidance only. In the prior art, the released heat is removed using heat exchangers, or for very slow reactors the heat is lost by convection or conduction. In this invention, the evaporation of the water is used to remove the heat, and the boiling point of water provides a stable operating condition for rapid processing.
(Errors in original.)
The first sentence of that paragraph is significant in that it refers to excess heat spontaneously boiling a portion of the excess water. The next sentence describes an embodiment in which the slurry is heated to 100ºC (at ambient pressure) “and the remaining heat from the subsequent reaction spontaneously boils the water”.
26 At CS [0062] there is further discussion of the process in which the following statements appear:
The kinetics of hydration plays a very important role in the formation of slurries from DBM and CCM. It is well established that the initial reaction rate (a) scales proportionally to the surface area of the solid particles, and (b) has an activation energy of about 60 kJ/mol This means that the hydration reaction rate at 50 C, 75 C, and 100 C is, respectively, 4.3, 18.1 and 57.6 times faster than that at 25 C. However, it is often observed that the rate of reaction slows down significantly before the reaction is complete, and this is attributed to the low solubility of Mg(OH)2, such that Mg(OH)2 crystallites coats the pore surfaces. This is particularly evident from dead burned magnesia. The solubility of Mg(OH)2 also increases with temperature, so this effect become less important at higher temperatures. For dead-burned materials the very low porosity is such that it is believed that the Mg(OH)2 crystals formed during the reaction are separate from the parent particle. Wet milling of DBM will remove any coating, and expose new surfaces, on the particles. While the grinding process of DBM is essential, the prior art also describes the use of hot water to increase the hydration rate. The milling conditions then determine the time to produce the slurry. In contrast, for very high surface area CCM particles, the specific surface area may exceed 100 m2/gm, and there is little evidence of pore blocking effects. Without being limited to theory, migration of water to such CCM particles is probably not a rate limiting step because of the high porosity of the particles. The most important observation is that the hydration reaction of CCM, in a well stirred thermally insulated reactor, exhibits thermal run-away. For example, using a material with a surface area of 190 m2/gm, the temperature of the well-stirred reactor initially rises spontaneously to 50 C over 30 minutes, and this is followed by a fast process in which the temperature spontaneously rises to 100 C within 10 minutes. The heat released by the initial hydration increases the water temperature, which increases the reaction rate so that heat released further increases the temperature. This is thermal run-away. Importantly, the boiling point of water is reached preferably within thirty minutes, and the temperature stabilizes, such that the remaining reaction can be completed, say, with an additional 120 minutes of processing at a fixed temperature through the release of steam. In this invention, the boiling of the water circumvents the need to control the temperature of the reactor to avert damage or hazards. Furthermore, the signature that the reaction is substantially complete is that boiling ceases and the temperature begins to fall, at a rate determined by reactor heat losses and residual hydration. It would be appreciated by a person skilled in the art that PCCM produced with a high surface area, in the range of 100-200 m2/gm will be preferred as a source of PCCM, compared to PCCM with a surface area of 20-60 m2/gm because the processing time will be shorter, and less susceptible to heat losses that might otherwise result in the slurry not reaching the boiling point of water.
“DBM” refers to “dead burned magnesia” which is generally produced by the calcination of mineral magnesite. “CCM” refers to caustic calcined magnesia. “PCCM” refers to powdered CCM.
27 There is a debate between the experts as to what “thermal run-away” as described in CS [0062] is. Professor Sorrell says that this is an implicit reference to “localised boiling” which occurs at the interface between the aqueous phase of the suspension and the particulate. There was a good deal of discussion in Professor Sorrell’s evidence concerning “localised boiling” and “superheating” which describe physical phenomena that appears to be central to what he says is his understanding of the invention as described and claimed. I will say more about this later in these reasons but I note that it is common ground that the CS specification makes no explicit reference to either “localised boiling” or “superheating”.
28 CS [0077] includes the following statement:
Importantly, there is no adverse effect of boiling water on the slurry characteristics provided that the water content is managed to account for the loss.
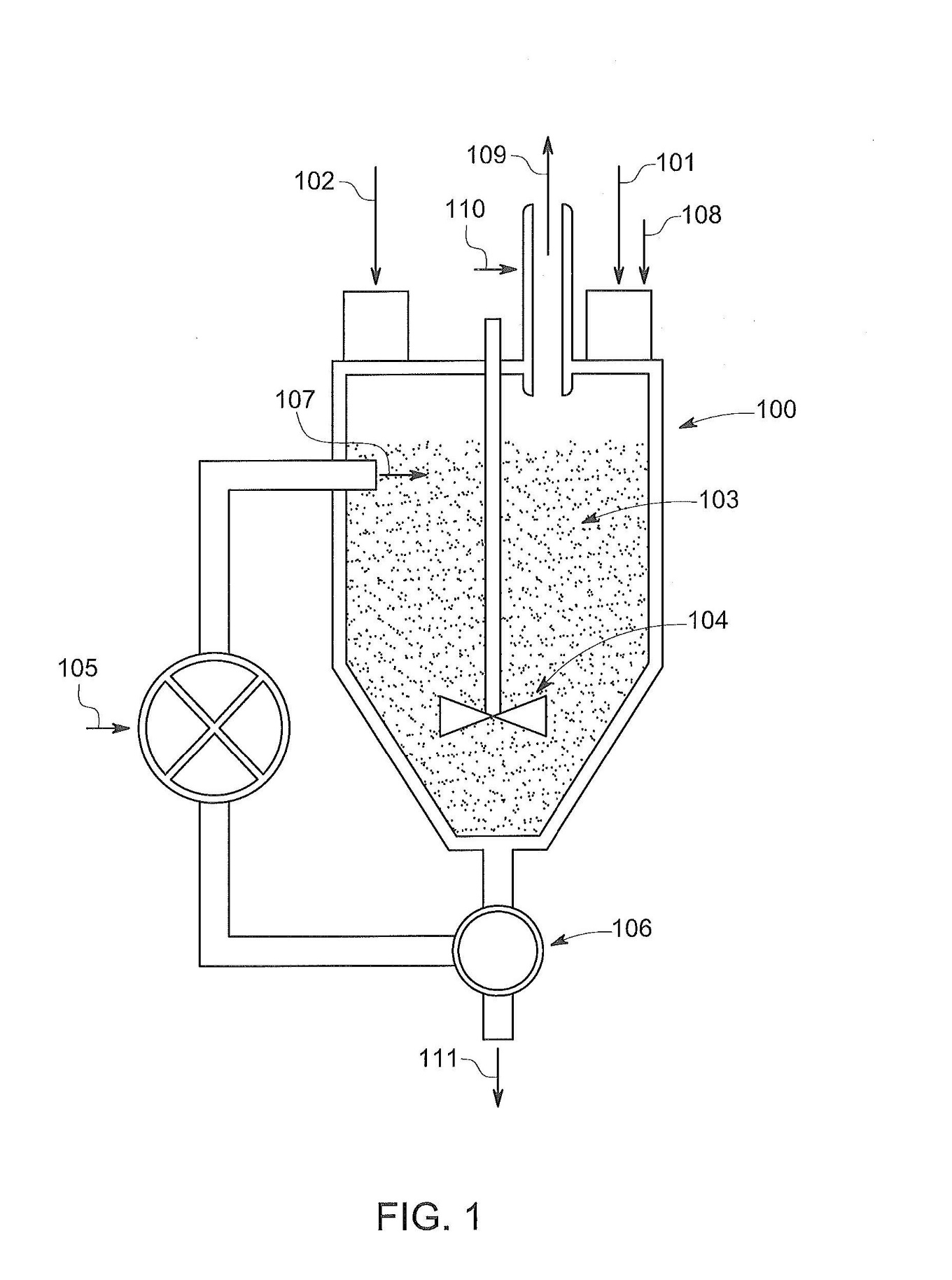
29 CS [0078] describes an embodiment of the invention by reference to Fig 1 which is reproduced as Annexure A to these reasons. The apparatus depicted in Fig 1 includes a reaction vessel (100) in which, as the reaction proceeds, the temperature of the reaction rises due to the exothermic hydration process. As the water approaches the boiling point, slightly below 100ºC, steam (109) is ejected through the stack (110). When the reaction is nearly complete, the boiling ceases, and the temperature of the reaction begins to fall. According to CS [0079]:
Process control is thereby simplified, and costs reduced, by using boiling point as a bound to control the process temperature, and optionally by using a simple quenching mechanism to stop the reaction.
These paragraphs of the CS suggest that the boiling point of water is the upper limit of the temperature of the reaction mixture.
30 In the discussion of preferred embodiments, there are a number of references to the use of a “high shear mixing apparatus” which are directly relevant to the debate between the parties as to the proper construction of the words “applying a shearing force” as used in integer (iii) of claim 1. For example at [0073] and [0075]:
[0073] Notwithstanding the concepts described above, experiments show that the formation of a stable slurry is facilitated by the use of a high shear mixing apparatus which is capable of inducing each of the mechanisms described above. In more general terms, the formation of a stable slurry is rendered more difficult to achieve without the use of such a high shear mixing apparatus …
…
[0075] In summary, the mixing of the solids is preferably accomplished using a high shear mixing apparatus that substantially dissipates concentration gradients, agglomerates, steam bubbles and induces comminution.
31 Although only claim 1 is sued on, for the purpose of providing context it is useful to refer to claim 1 and a number of its dependent claims. Claims 1-4 provide:
1. A process for producing a hydroxide slurry from caustic calcined carbonate powder or caustic calcined hydroxide powder, comprising the following steps:
(a) mixing the powder with water in a reactor vessel and forming a reaction mixture;
(b) applying a shearing force to the reaction mixture using a mixing apparatus;
(c) allowing heat of hydration to raise the temperature of the reaction mixture to near the boiling point, and
(d) allowing steam to evaporate from the reaction mixture as hydration proceeds, to remove excess heat and control the maximum reaction temperature to near the boiling point.
2. The process of claim 1, wherein the process is adapted for the production of a high solid fraction hydroxide slurry from the reaction mixture, wherein the slurry has a relatively low resistance to shear thinning.
3. The process of claim 2, wherein the process additionally comprises the following steps:
(e) metering an input of a viscosity modifier to enable the mixing apparatus to maintain uniform mixing under thin slurry conditions promoted by the viscosity modifier;
(f) allowing the reaction to proceed spontaneously, using evaporation to balance the heat release, until the water temperature has reached a maximum and the temperature dropped to a first set point; and
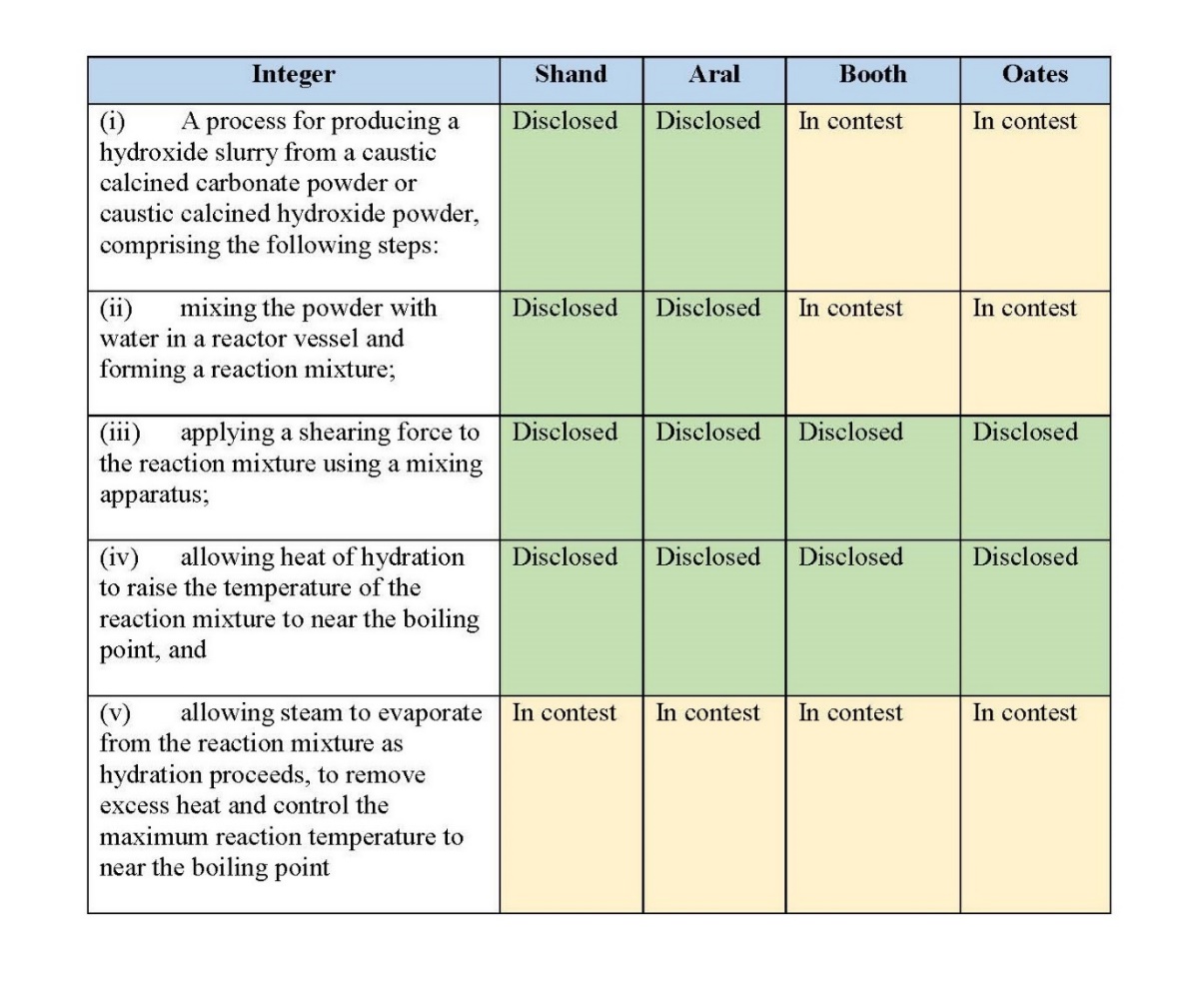
(g) quenching the slurry to drop the temperature to a second set point.
4. The process of claim 3, wherein the maximum temperature is near the boiling point of the reaction mixture.
32 Claim 23, which I previously mentioned, is to a reaction apparatus. It claims:
23. A reaction apparatus for producing a hydroxide slurry from a reaction mixture of at least caustic calcined carbonate or caustic calcined hydroxide powder, and water, wherein the reaction apparatus comprises:
(a) a reaction vessel having a first inlet adapted for receiving caustic calcined carbonate powder and a second inlet adapted for receiving water and a controller that is adapted to electronically control the process within the reaction vessel;
(b) shearing apparatus positioned within the reaction vessel for shearing the reaction mixture and wherein the rate of shearing is controlled by the controller;
(c) a viscosity sensor positioned within the reaction vessel adapted to supply viscosity information about the reaction mixture to the controller;
(d) a temperature sensor positioned within the reaction vessel adapted to supply temperature information about the reaction mixture to the controller; and
(e) a steam outlet for release of steam from the reaction vessel, such that in use the reaction is controlled by the controller so that the heat of hydration raises the temperature of the reaction mixture, allowing water to boil off from the reaction mixture as hydration proceeds, and removing steam via the steam outlet to remove excess heat and control reaction temperature at boiling point.
This claim defines an apparatus that includes a temperature sensor to supply temperature information to a controller which controls the reaction by allowing steam to escape via a steam outlet to remove excess heat and “control reaction temperature at boiling point”.
33 It is fair to say that the CS is a confused and muddled document especially when regard is had to the temperature limitation (“near the boiling point”) referred to in claim 1. The broadest description of the invention as it appears in CS [0025] and [0026] speaks of controlling the reaction temperature “to just below or at the boiling point”. There are many other paragraphs (including those to which I have previously referred) that suggest that the water that makes up the aqueous phase of the reaction mixture is allowed to boil: see, in particular, CS [0029], [0032], [0039], [0041], [0042], [0045], [0049], [0060], [0061], [0062], [0077], [0078] and [0079].
THE INSPECTION
34 Pursuant to an interlocutory application filed by Calix on 23 February 2023, I made orders on 1 March 2023 that the respondents make the equipment used to perform the Grenof process available for inspection by Professor Sorrell so that he could take measurements necessary to respond to the applicant’s evidence, including that of Mr Redfern and Mr Messiter. Professor Sorrell and Mr Messiter were both present at the inspection but Mr Redfern was not. The intention was to allow Professor Sorrell to examine the equipment used in the Grenof process and to observe its operation. That occurred on 8 March 2023.
35 The inspection was audio-visually recorded and parts of the video recording were played at the hearing during the examination of Professor Sorrell and Mr Messiter. Exhibit 2 is a compilation of screenshots from the video recording. I will say more about the inspection later in these reasons.
Common General knowledge
36 The person skilled in the art will possess the common general knowledge in the relevant field. This will include the background knowledge and experience available to all those persons engaged in the relevant field within the patent area including publications to which they would refer as a matter of course: Minnesota Mining and Manufacturing Company v Beiersdorf (Australia) Ltd (1980) 144 CLR 253 at 292 per Aickin J, ICI Chemicals & Polymers Ltd v Lubrizol Corporation Inc (1999) 45 IPR 577 at [112] per Emmett J. The High Court emphasised in Aktiebolaget Hassle v Alphapharm Pty Ltd (2002) 212 CLR 411 at [31] that information cannot be treated as part of the common general knowledge in the absence of evidence of its general acceptance and assimilation by persons skilled in the art.
37 The Patent is concerned with the manufacture of hydroxide slurries including, in particular, magnesium hydroxide slurries and calcium hydroxide slurries.
38 As at the priority date, the process of producing hydroxide slurries from hydroxide powder (MgO or CaO) and water (H2O) was widely known. This was typically done by mixing the oxide powder with water in a reaction vessel to form a reaction mixture known as a slurry. The slurry was in the form of a suspension with an aqueous phase (excess H2O) and a particulate phase (Ca(OH)2 or Mg(OH)2). This process can be described by the chemical reaction (in the case of calcium hydroxide) CaO + H2O Ca(OH)2 + heat or (in the case of magnesium hydroxide) MgO + H2O Mg(OH)2 + heat.
39 The reaction is an exothermic reaction, which means that it produces heat. The heat generated is called “heat of hydration.” The amount of heat generated by the reaction is dependent on the amount of reagent (ie. CaO or MgO) added.
40 As the reaction between the reagent and H2O occurs, the heat of hydration produced will raise the temperature of the reaction mixture. If the reaction mixture is allowed to boil, the release of steam will remove excess heat. Assuming the reaction occurs at normal pressure (ie. one atmosphere) the temperature of the reaction mixture will not exceed the boiling point of the excess water.
41 The reaction mixture can be brought to the boil by the addition of extra reagent or the use of less water. Boiling can be avoided by the use of less reagent or the addition of more water. The reaction mixture can be brought to near boiling point by adjusting the ratio of reagent to water, which (I infer) would be done through a process of calculation and routine “trial and error” testing. I find that these are all matters of basic chemistry that would have been well known to the person skilled in the art at the priority date.
42 Mr Messiter gave evidence that the maximum temperature of a typical process used to produce calcium hydroxide slurries (known as a batch lime slaker) would be kept below the boiling point. He said the process was relatively well understood and involved mixing solid oxide with water in a controlled manner to produce a slurry. In his written evidence, Mr Messiter said:
Process for manufacturing magnesium hydroxide slurries prior to 2013
…
17. Typically, these processes would be designed so that the reaction temperature would be sufficient to ensure a relatively short batch time with the maximum temperature controlled by the feed rate. Generally, in my experience, the maximum temperature of a typical batch lime slaker would be kept below the boiling point to ensure that there were no issues of pressure. This process was conducted in a non-pressurised vessel, and therefore at atmospheric pressure.
18. In respect of calcium oxide (lime) hydration, there were four main types of lime slakers, namely, batch slakers (the most common in my experience), slurry detention slakers, paste slakers and ball mill slakers, which were all readily available from equipment vendors in Australia. Batch slakers were commonly used to provide automated and reliable onsite production of calcium hydroxide slurries for relatively low usage rates. In batch slakers, the ingredients are added together in a single batch to form the hydroxide slurry. Typically, the batch cycle would be automatically controlled, and would include adding lime in a metered quantity to water in an agitated tank. Temperature control would be typically achieved by metering the feed quantity added, and the maximum temperature would be kept at a level that would ensure a short batch time and would generally be below the boiling point. The systems would have usually included dust control in the form of venting of the feeder and the batch tank to a vent scrubber and the system would generally operate at atmospheric pressure …
Mr Messiter also said that the production of magnesium hydroxide slurries was less common than the production of calcium hydroxide slurries but that the process for producing these slurries was in principle very similar.
43 None of this evidence was challenged in cross-examination or contradicted by other evidence. I accept it and find that the common general knowledge included the process of producing hydroxide slurries in the manner described by Mr Messiter.
Professor Sorrell
44 The patent specification is “addressed to those likely to have a practical interest in the subject matter of [the] invention”…“with a practical knowledge and experience of the kind of work in which the invention was intended to be used”: Catnic Components Ltd v Hill & Smith Ltd (1982) RPC 183 at 242-243. The skilled addressee (referred to in s 7 and s 40 of the Act as a person skilled in the relevant art) is a notional person who is deemed to have the common general knowledge of those working in the field to which the invention relates but who does not possess any capacity for invention: Root Quality Pty Ltd v Root Control Technologies Pty Ltd (2000) 177 ALR 231 at [70]-[72] and [93], American Cyanamid Co v Ethicon Ltd [1979] RPC 215 at 245-246.
45 As I have explained, I do not consider Professor Sorrell to be a reliable witness. This has created a difficulty for me in determining whether or not he is in any sense representative of a person skilled in the relevant art. His evidence in relation to his prior experience and interest in the subject matter of the patent specification was not persuasive and, if anything, confirmed that he had little or no real training or experience in the production of hydroxide slurries.
46 In circumstances where I have decided to give Professor Sorrell’s evidence in relation to the interpretation of the Patent little weight, the question whether he is a skilled addressee is somewhat moot. However, I am not persuaded that Professor Sorrell is a person with a practical interest in the subject matter of the Patent or that his prior training or experience qualifies him to give relevant evidence as to the common general knowledge or state of the relevant art at the priority date. That said, I have addressed the substance of Professor Sorrell’s evidence in these reasons against the possibility that my conclusion on that issue is found to be incorrect.
47 Before commenting on aspects of Professor Sorrell’s evidence in relation to infringement, I must explain why I found him to be an unreliable witness. For this purpose, I refer to three key examples of what I regarded as his contradictory and unreliable evidence.
CS [0062]
48 Professor Sorrell gave evidence as to his understanding of CS [0062]. He was asked by Mr Green SC (who appeared for Calix) about the reference to “boiling” that appears in that paragraph and, in particular, the statement “… the signature that the reaction is substantially complete is that boiling ceases and the temperature begins to fall …”. Professor Sorrell said that this reference was “… about boiling of the reaction mixture [and] not about localised boiling”. Professor Sorrell also gave the following evidence:
MR GREEN: Can you explain why you consider that that sentence was not referring to localised boiling?
PROF SORRELL: I read that as a volumetric issue, and it’s true that the boiling that occurs here, below the boiling point of the solution, has to be at the interface, so I understand your nuance here. The boiling at the interface occurs, but I think this is about that process below the actual boiling point, and they’re using boiling as a – in the sense of a bounding temperature.
49 I think it is tolerably clear from Professor Sorrell’s evidence at this point that he was saying that the boiling referred to in CS [0062] was not localised boiling but volumetric boiling. Localised boiling refers to the boiling of water at the interface between the reagent (eg. MgO) and water. Volumetric boiling, at least as understood by Professor Sorrell (Mr Messiter was not familiar with the term), refers to boiling of the reaction mixture as a whole.
50 Later in the oral evidence, Mr Dimitriadis SC (who appeared for the respondents) asked Professor Sorrell further questions concerning CS [0062] and the references to the boiling of water that appear in that paragraph. Professor Sorrell gave the following evidence:
MR DIMITRIADIS: And you would agree with me, would you not, that that passage is clearly describing a system in which the reaction mixture is allowed to boil, and it is that mechanism which has the effect of controlling the temperature of the reaction mixture at the boiling point.
PROF SORRELL: No. I believe you’re making my point, with this paragraph, about localised boiling. Look at the phrase, “This is thermal runaway.” That’s what happens at the interface. It’s really hot. That’s thermal runaway, and that’s localised boiling.
…
HIS HONOUR: But just so I understand this, you say – you interpret claims [sic] – paragraph 62 to be referring not to boiling of the reaction mixture as a whole, is that right - - -
PROF SORRELL: I interpret the - - -
HIS HONOUR: - - - but something else.
PROF SORRELL: I interpret this phrase, thermal runaway, to be a direct reference to localised boiling, and that is the boiling that can be observed, because if the microbubbles rise to the surface, you can mistake it for boiling, but it is steam. So in a sense, it’s boiling, but it’s not volumetric boiling.
HIS HONOUR: But where it refers to, a bit further down:
In this invention, the boiling of the water circumvents the need –
et cetera, you say that’s not talking about boiling of the entire reaction mixture. It’s talking about something else, is it?
PROF SORRELL: In this paragraph - - -
HIS HONOUR: Yes.
PROF SORRELL: - - - I make a – I can see a clear distinction, so I have to conclude that this is about localised boiling, and it’s not volumetric boiling.
HIS HONOUR: It’s not talking about boiling of the entire reaction mixture.
PROF SORRELL: No.
HIS HONOUR: All right - - -
PROF SORRELL: It’s microbubbles, but - - -
HIS HONOUR: - - - meaning it’s not.
PROF SORRELL: But if there are lots of microbubbles, it can give the appearance of completely boiling.
MR DIMITRIADIS: Professor Sorrell, my note is that, in some earlier evidence that you gave when my learned friend, Mr Green, was asking questions, you gave evidence that this passage was talking about volumetric boiling. Do you recall that?
PROF SORRELL: No, I don’t.
MR DIMITRIADIS: If you did give that evidence, was that incorrect?
PROF SORRELL: I don’t remember the details.
MR DIMITRIADIS: You’re saying now that it’s not talking about volumetric boiling. Is that the position?
PROF SORRELL: As I said, the reference to thermal runaway tells me it’s localised boiling.
MR DIMITRIADIS: Now, you’ve referred - - -
HIS HONOUR: But could I just ask why is that? What is it about that sentence that tells you it’s localised boiling.
PROF SORRELL: If the system were limited to 100 degrees, there’s no thermal runaway. It stops. But if it says “thermal runaway”, then it’s above 100 degrees, and if it’s above 100, that’s the particle of magnesia, and that’s what’s causing localised boiling. This is one of the implicit references to localised boiling in the text.
51 Professor Sorrell’s evidence as to CS [0062] was in my opinion contradictory. Having said in terms that CS [0062] was “about boiling of the reaction mixture [and] not about localised boiling” he went on to insist that CS [0062] was about localised boiling.
52 A fair reading of CS [0062] indicates that the temperatures of the reaction mixture can rise to, but may not exceed, 100ºC. According to CS [0062]:
the temperature of the reactor initially rises to 50ºC over 30 minutes;
over the next 10 minutes the temperature rises to 100ºC; and
preferably, the boiling point of water (ie. 100ºC) is reached within 30 minutes.
What is being described here is not just the temperature of the reactor but also its contents and, in particular, the water comprising the aqueous phase. Moreover, the process described is a gradual one, in which the reaction mixtures takes between 30 to 40 minutes to reach the boiling point.
53 The statement in CS [0062] that “[t]he heat released by the initial hydration increases the water temperature which increases the reaction rate so that heat released further increases the temperature” is not referring to temperatures exceeding 100ºC. So much is clear from the reference to “initial hydration”. The heat from the hydration reaction (ie. the heat of hydration) raises the temperature of the reaction mixture to the boiling point of water (ie. 100ºC).
Superheating
54 The video recording of the Grenof process in operation shows what Mr Messiter and Mr Redfern consider to be vigorously boiling. In his oral evidence, Professor Sorrell attributed what he referred to as extreme surface turbulence to “superheating on the surface”. Professor Sorrell gave the following evidence:
MR DIMITRIADIS: … Professor Sorrell, to come back to the question his Honour asked you, which was about determining whether the reaction mixture is near to or at, or above the boiling point, could I suggest this to you? One way to determine whether the temperature of a reaction mixture is at the boiling point is to look at it and to observe whether or not it’s boiling.
PROF SORRELL: That’s provided you can see it.
MR DIMITRIADIS: Well, it’s pretty obvious if a reaction mixture is boiling, isn’t it?
PROF SORRELL: If you can see it, yes, you should be able to determine that, but it’s not necessarily black and white, because there are microbubbles formed, which might be interpreted as boiling, and then we – we foreshadowed that I attribute the extreme turbulence to super-heating, which blocks vision of anything below, and so to say that you can see the boiling is not certain, and to differentiate the microbubbles from large volumetric boiling probably can be done, but it might not be absolutely straightforward.
MR DIMITRIADIS: I would suggest to you that a simple observation, as to whether or not there is vigorous bubbling, due to the formation of bubbles of gas in the liquid phase, is a much safer guide to assessing whether the temperature of the reaction mixture is at the boiling point, than measurements of the kind you referred to, using a thermocouple.
PROF SORRELL: No. I would disagree. I think it’s superheating on the surface.
55 In the Joint Experts’ Report (Exhibit A), Professor Sorrell stated that “the extreme surface turbulence is attributed to superheating”. In his final Stage 6 Report, Professor Sorrell disagreed with Mr Messiter’s opinion that the reaction mixture in the Grenof process is boiling and stated at para 86:
a) The onset of extreme surface turbulence occurs at a measured (display) temperature of 95.3°C (Table 3.2, Point 42). This indicates that the extreme surface turbulence takes place at least 4.7°C below the boiling point of water at atmospheric pressure. I consider that this shows that the extreme surface turbulence is not caused by boiling.
…
e) In short, I conclude that the causative effect is superheating, which is a well known thermodynamic process that occurs when a liquid (or gas) is exposed to temperatures in excess of the boiling point of the liquid but in the absence of boiling. When it occurs, it generates large volumes of superheated gas. A basic introduction to the phenomenon is appended … This text documents that the temperatures causing superheating of water at atmospheric pressure can occur up to 280°C or possibly even 302°C.
56 The “basic introduction” referred to by Professor Sorrell is the textbook, Metastable Liquids – Concepts and Principles by Pablo Debenedetti, which refers to the possibility of heating water to above its normal boiling point (100ºC at one atmosphere). According to the author, “superheated water exists in a state of precarious equilibrium”. The author also states that “[e]xperiments show, however, that a condition is eventually reached in which boiling … can no longer be prevented, and a new phase [ie. gaseous] appears suddenly”. Superheating is therefore a phenomenon in which the temperature of a liquid (usually water) exceeds its boiling point but without boiling. It can be followed by a sudden and violent release of gas.
57 In cross-examination, Mr Dimitriadis SC drew Professor Sorrell’s attention to statements in his earlier Stage 5 Report in which he ruled out superheating occurring in the Grenof process. In that earlier Stage 5 Report he expressed the opinion that the conditions required for superheating of the liquid did not exist. The conclusion in his earlier Stage 5 Report is at odds with the conclusion in his later Stage 6 Report extracted above.
58 Professor Sorrell was cross-examined in some detail in relation to his conclusion that the extreme turbulence at the surface of reaction vessel was caused by superheating and his change of opinion between his Stage 5 and 6 Reports. It was suggested to him, correctly in my view, that the textbook on which he relied indicated that various factors, at least some of which were present in the Grenof process, were not conducive to superheating. Professor Sorrell described the question asked of him (which in my view was a perfectly reasonably one) as a “non-sequitur”. The cross-examination continued as follows:
MR DIMITRIADIS: Can you give your explanation as to how you say superheating is relevant to this process, please, Professor Sorrell?
PROF SORRELL: I can answer it in one sentence. The superheating of slurries is a very well-known phenomenon.
MR DIMITRIADIS: That’s it, is it? That’s your explanation?
PROF SORRELL: That’s all I need to explain. It’s very well known that you can superheat slurries.
59 A little later, in answer to a question from the Court, Professor Sorrell said again that superheating, in the context of slurries, is very well-known. When asked by the Court why there was no reference in his earlier report to the fact that superheating of slurries was a well-known phenomenon, he said:
PROF SORRELL: No. I don’t mean it’s commonly observed. I said it’s common you can make it happen. When you look at the published literature, you can see there are papers on superheating of slurries, but it’s not common in industry. It’s not very well known. We – we do superheating in clays when we fire them. That involves superheated water as well, but generally the ceramic industry wouldn’t even think about it. It – it just is [sic].
(Emphasis added.)
None of Professor Sorrell’s reports refer to any literature relating to the superheating of slurries. His evidence concerning superheating in slurries was in my opinion contradictory and inconsistent.
60 Later in his evidence, Professor Sorrell went on to state that the Grenof system “is not an equilibrium system”. He said it was undergoing mixing and was “dynamic”. However, this observation is at odds with what the textbook on which he relied makes clear, which is that superheating occurs in “a state of precarious equilibrium”. The fact that the system is undergoing mixing and is dynamic, would suggest that it is not in equilibrium and that the extreme turbulence observed in the Grenof process is therefore not attributable to superheating.
61 Professor Sorrell was also asked some questions by Mr Green SC, in what was effectively re-examination concerning the difference between superheating and localised boiling. In answer, Professor Sorrell equated superheating with localised boiling by referring to what he described as “localised superheating.” Professor Sorrell also proposed the “evolutionary” nature of his reports as an explanation for his change of opinion regarding whether the conditions for superheating were present in the Grenof process. He gave the following evidence:
MR GREEN: Can you explain to his Honour the difference between the phenomena of localised boiling and superheating that you’ve been discussing?
PROF SORRELL: I think there – there’s a good chance they’re the same. Because the particle is really hot, and in principle that should cause superheating. However, down in the bulk of the slurry, you have a cooling mechanism and you have pressure suppressing bubble formation. So it might be appropriately viewed as, you know, localised boiling. But it also could be considered localised superheating. I don’t know. I don’t know the temperature at the interface that actually happens and the actual phenomenon.
MR GREEN: And what caused you to introduce the concept of superheating?
PROF SORRELL: All of these reports are evolutionary. I have new thoughts, and I change my mind sometimes.
62 There are various difficulties with this evidence. In his last Stage 6 Report, Professor Sorrell described superheating as a thermodynamic process that occurs when a liquid is heated to temperatures in excess of its boiling point but in the absence of boiling. In other evidence, he made clear that localised boiling occurs at the interface between the reagent and the water and that this occurs throughout the reaction mixture which he regarded as essentially homogeneous in temperature. I generally found Professor Sorrell’s evidence concerning superheating, localised boiling and the temperature of the reaction mixture unconvincing and often inconsistent.
Boiling
63 As mentioned above, the video recording of the inspection of the Grenof process was shown during the examination of Professor Sorrell and Mr Messiter. It is evident from my viewing of the video recording that the maximum temperature recorded by the thermocouple during the Grenof process was 101.5°C (at around the 38 minute mark).
64 Professor Sorrell detailed his measurements from the inspection in his final Stage 6 Report. He recorded that the depth of the reaction vessel was 1.8m and the depth of the thermocouple was 1.78m (being approximately 20mm above the bottom of the vessel). Professor Sorrell also gave evidence that he estimated that the depth of the thermocouple in the slurry was 1.48m.
65 Professor Sorrell’s evidence of the dimensions of the reaction vessel differed from the measurements provided by Mr Redfern. In his oral evidence, Mr Redfern provided that the depth of the reaction vessel was 1.37m and the depth of the thermocouple was 1.07m (being 300mm from the base of the reaction vessel). However, in his affidavit dated 16 February 2023, Mr Redfern stated that the depth of the reaction vessel was approximately 1.3m and the depth of the thermocouple was 1.17m (being 130mm from the base of the reaction vessel). Given the differences in the figures provided by Mr Redfern for the depth of the thermocouple were not explained, I prefer the measurements provided by Professor Sorrell that were taken during the inspection.
66 Professor Sorrell recorded that the highest reaction temperature recorded at the thermocouple during the Grenof process was 101.5°C. It is important to note at this point that Calix’s infringement case against the respondents relies on proving that the maximum reaction temperature reached during the Grenof process was near the boiling point. It is accepted by Calix that this does not include at or above the boiling point (ie. 100°C at one atmosphere).
67 To explain why the maximum reaction temperature recorded at the thermocouple (ie. 101.5°C) was above 100°C, Professor Sorrell gave evidence concerning boiling points of water as a function of depth (ie. at pressure greater than one atmosphere) and concluded that at the depth of the thermocouple in the slurry (1.48m) the boiling point of water was 103.8°C. Accordingly, he concluded that given the temperature at the thermocouple (101.5°C) was less than the boiling point of water at the depth of the thermocouple in the slurry (103.8°C) the mixture was not boiling anywhere in the reaction vessel.
68 Mr Dimitriadis SC cross-examined Professor Sorrell regarding his depth and temperature calculations and his conclusion that the mixture was not boiling anywhere in the reaction vessel. Professor Sorrell gave the following evidence:
MR DIMITRIADIS: Professor Sorrell, the boiling point of the reaction mixture you have calculated as being 103.8 degrees at the location of the thermocouple, correct?
PROF SORRELL: Yes.
MR DIMITRIADIS: And because the boiling point is dependent on the pressure at a lesser depth in the reaction vessel, the boiling point will be closer to 100, correct?
PROF SORRELL: That’s correct.
MR DIMITRIADIS: And at some point in the reaction vessel the boiling point will be 101, won’t it?
PROF SORRELL: That’s correct.
MR DIMITRIADIS: Assuming your calculations are correct. Is that right?
PROF SORRELL: Yes.
MR DIMITRIADIS: And at that point the temperature – if the temperature of the reaction mixture is 101 degrees it will be at its boiling point?
PROF SORRELL: At that pressure it will be at the theoretical boiling point, yes.
MR DIMITRIADIS: Yes. And because of your view that the reaction mixture is well mixed and it’s homogeneous in terms of its temperature, you would conclude from the fact that the temperature is 101 degrees at the thermocouple that it’s also 101 degrees at that higher point in the reactor vessel, correct?
PROF SORRELL: Approximately, yes. Except for the pressure effect and the thermal gradient, which will change it.
MR DIMITRIADIS: Yes. Let’s put aside the thermal gradient which you said wouldn’t be a significant factor earlier today, correct?
PROF SORRELL: Yes.
MR DIMITRIADIS: Doesn’t it follow from all of that that at the point of lesser depth in the reactor vessel where the boiling point of the reaction mixture is 101 degrees, the temperature of the reaction mixture will be at that boiling point?
PROF SORRELL: No, because it’s mixed.
MR DIMITRIADIS: How does it being mixed undermine that proposition? It actually makes the point I’m trying to put to you, doesn’t it?
PROF SORRELL: It homogenises the temperature. So if you were to say that the temperature up here in the reactor is different from down here, the mixture mixing tends to homogenise it and the differentiation is from the pressure and the radiant heat loss and what have you from the surface. So there’s a gradient. So perhaps I’m missing the point of your question.
MR DIMITRIADIS: You’ve accepted that the boiling point of the reaction mixture at some depth will be 101 degrees, correct?
PROF SORRELL: Yes.
MR DIMITRIADIS: And you’ve accepted that the temperature measured by the thermocouple, if it’s 101 degrees, reflects the temperature throughout the reaction mixture.
PROF SORRELL: Yes.
MR DIMITRIADIS: Therefore the temperature of the reaction mixture at that location that I referred to where the boiling point is 101 degrees will be at its boiling point?
PROF SORRELL: Okay, now I understand the confusion. We’re talking about equilibrium versus non-equilibrium. Under equilibrium conditions, you are correct. But this is not an equilibrium system. It’s undergoing mixing. So it’s dynamic. And so it changes. So in theory, at equilibrium conditions you are correct. But under the actual conditions you are incorrect.
MR DIMITRIADIS: This is the first time you’ve introduced the concept of equilibrium conditions as being relevant, isn’t it?
PROF SORRELL: No. I’ve referred to phase equilibrium all throughout the report.
MR DIMITRIADIS: I suggest to you that the evidence you’ve just given is not valid scientifically and that it’s clear to you that this reaction mixture is boiling. Do you accept that?
PROF SORRELL: No. It is scientifically correct.
69 Contrary to Professor Sorrell’s evidence, there was in my opinion no confusion at all. He agreed that the temperature throughout the reaction mixture would be 101ºC (101.5ºC according to the thermocouple) and that the boiling point of water would be 101ºC at some depth below the surface. He stubbornly refused to accept the obvious implication of his own evidence that at least some part of the reaction mixture will have reached boiling point. His evidence that this would not be so in the Grenof process because equilibrium conditions did not exist was in my opinion implausible. He had previously accepted, more than once, that the temperature throughout the reaction mixture was relatively constant. Continuous mixing of the reaction mixture by the mixing apparatus would help maintain the reaction mixture at a relatively constant temperature (ie. around 101.5ºC as measured by the thermocouple). At the upper level of the reaction vessel (to a depth of around 0.5 metre) the reaction mixture is likely to be boiling and producing steam. That the reaction mixture is boiling and giving off steam is consistent with Mr Messiter’s and Mr Redfern’s evidence and my own observation of the Grenof process based on my viewing of the video recording.
Construction issues
“applying a shearing force”
70 The first issue concerns the meaning of “applying a shearing force” as that expression is used in integer (iii) of claim 1. The respondents submit that “applying a shearing force” refers to a high level of shear which involves something more than merely mixing the contents of the reaction mixture using a mixing apparatus. According to the respondents’ expert, Mr Messiter, the reference to “a shearing force” should be understood, when claim 1 is read in the context of the CS as a whole, to refer to a “high level of shear force”. In support of his opinion, Mr Messiter refers to various references in the CS to “a high shear mixing apparatus”.
71 I do not accept the respondents’ interpretation. It imposes on the clear language of the claim a gloss based on the description in the CS of preferred methods of the invention. So much is clear from CS [0075] which states that “the mixing of solids is preferably accomplished using a high shear mixing apparatus” (emphasis added). There is nothing in the CS to indicate that a high level shear apparatus must be used to perform the invention. Claim 1 merely requires use of a mixing apparatus that applies a shearing force, not a mixing apparatus that applies any particular degree of shearing force.
“allowing steam to evaporate from the reaction mixture as hydration proceeds, to remove excess heat and control the maximum reaction temperature to near the boiling point”
72 It is common ground that “to near the boiling point” refers to temperatures near to and below the boiling point and does not encompass temperatures equal to or above the boiling point. The boiling point referred to, is in my opinion, the boiling point of water (ie. 100ºC at one atmosphere) since the aqueous phase of the reaction mixture is wholly or predominantly made of water.
73 As to the meaning of the word “control” in the context of integer (v) of claim 1, it is in my view used in the sense of restricting or restraining. The Concise Oxford English Dictionary (Eleventh Edition, Revised) defines “control” to mean (inter alia) “the restriction of an activity, tendency or phenomenon”. The Oxford English Dictionary Online defines control to mean (inter alia) “[t]he action or fact of holding in check or restraining”. In my opinion, that is the sense in which the word control is used in claim 1. Hence, claim 1 defines a process in which steam is allowed to evaporate to remove excess heat, thereby restricting or restraining the maximum temperature of the reaction mixture to near to, but below, the boiling point.
74 There was also some debate as to the meaning of “the maximum reaction temperature” as those words are used in claim 1. In my opinion, they would be understood by the skilled addressee as referring to the maximum temperature of the reaction mixture at any given depth. If the temperature of the reaction mixture is allowed to equal or exceed the boiling point at any given depth then the invention as claimed is not performed.
Infringement
75 It is accepted by the respondents that if the construction which I have given to the phrase “applying a shearing force” is correct, then the Grenof process applies a shearing force to the reaction mixture as required by claim 1. I find that this feature of claim 1 is present in the Grenof process.
76 I do not accept that in the Grenof process, the reaction mixture or any part of it is superheated. It follows that I do not accept that what appears to be boiling of the reaction mixture as seen in the video recording is attributable to superheating.
77 In his oral evidence, Professor Sorrell gave as an example of superheating the superheating of water in a container in which an extremely hot object is inserted. Professor Sorrell explained that it is the rapid increase in the temperature of water and the temperature differential that causes the water to exceed its boiling point without actually boiling.
78 It appears from the video recording that the temperature of the reaction mixture in the Grenof process increases gradually, and over an extended period of time. This is not consistent with the occurrence of superheating. Professor Sorrell was asked why, if the reaction mixture is superheating, it never reaches boiling point. His answer was as follows:
PROF SORRELL: Yes. In the bulk of the liquid, the localised boiling is cooled by the water and the water exerts a pressure. So when the bubbles try to nucleate, they’re suppressed by the opposing pressure. So if it’s superheating or boiling under water, there’s a control mechanism to keep the bubbles from nucleating and growing, plus there’s a cooling mechanism. But when you’re on the surface, it’s a completely different situation. So very low vapour pressure, nothing to suppress gas formation. There’s an insulating layer of air which has a lower thermal conductivity than water. So there’s ideal conditions for the process to superheat because there’s nothing to suppress the superheating of the surface, but underneath there is.
79 In his last Stage 6 Report, Professor Sorrell stated that the onset of extreme turbulence as shown in the video recording takes place at least 4.7ºC below the boiling point of water at atmospheric pressure. He went on to say that “this shows that the extreme surface turbulence is not caused by boiling”. Picking up on this point, Calix submitted that the extreme surface turbulence is first observed in the video recording when the thermocouple shows 95.3ºC and that this cannot constitute boiling.
80 The difficulty with Professor Sorrell’s evidence and Calix’s submission is that they ignore the delay in the temperature read-out. Professor Sorrell’s evidence makes no allowance for any delay. It was put to Mr Messiter in oral evidence that the extreme surface turbulence seen on the video recording “was way below the boiling point”. Mr Messiter did not accept this. His evidence (which I accept) was that thermocouples may have a one or two minute delay when they are not in a thermowell. In addition to Mr Messiter’s evidence, there is also evidence from Mr Redfern (which I also accept) who said there was a delay between the temperature reached in the reactor vessel and the temperature measured by the thermocouple. He said that it was not surprising to him that the reaction mixture appears to be boiling for two to three minutes before the temperature recorded by the thermocouple exceeded 100ºC.
81 The temperature shown on the thermocouple at 28.22 minutes into the video is 95.3ºC. At 29.00 minutes it has increased to 100ºC (ie. 38 seconds later). Thus, a 38 second delay understates the temperature at the thermocouple sensor by around 5ºC at 28.22 minutes.
82 As I have mentioned, the textbook on which Professor Sorrell relied refers to superheated water as being in a state of precarious equilibrium (or “metastable equilibrium”). The author also states:
Care must be taken to prevent … superheated water from boiling. Vibrations, suspended impurities, or irregularities on the walls of a container can trigger the appearance of a new phase … Experiments show, however, that a condition is eventually reached in which boiling … can no longer be prevented, and a new phase appears suddenly …
83 Professor Sorrell’s evidence does not explain how the Grenof process is managed (according to his theory) to achieve superheating and to avoid boiling. The evidence suggests that the state of precarious equilibrium necessary to produce superheating rather than boiling is difficult to achieve and maintain. The evidence does not indicate what advantage would be gained by superheating the slurry rather than boiling it. That the Grenof process is either designed or managed to achieve superheating, and not boiling, seems to me to be highly unlikely.
84 In any event, even if I was to accept that the extreme turbulence seen in the video recording of the Grenof process is attributable to superheating rather than boiling, given Professor Sorrell’s evidence that superheating involves the liquid reaching or exceeding its boiling point without actually boiling, then it must follow that the maximum temperature of the reaction mixture will necessarily have exceeded its boiling point.
85 Finally, I should mention s 121A of the Act, which was relied on by Calix so as to shift the burden of proof with respect to infringement onto the respondents. Section 121A provides:
121A Burden of proof—infringement of patent for a process
(1) This section applies only to a patent for a process for obtaining a product.
(2) If, in proceedings for infringement of a patent started by the patentee or the exclusive licensee:
(a) the defendant alleges that he or she has used a process different from the patented process to obtain a product (defendant’s product) identical to the product obtained by the patented process; and
(b) the court is satisfied that:
(i) it is very likely that the defendant’s product was made by the patented process; and
(ii) the patentee or exclusive licensee has taken reasonable steps to find out the process actually used by the defendant but has not been able to do so;
then, in the absence of proof to the contrary the onus for which is on the defendant, the defendant’s product is to be taken to have been obtained by the patented process.
(3) In deciding how the defendant is to adduce evidence for the purposes of subsection (2), the court is to take into account the defendant’s legitimate interests in having business and manufacturing secrets protected.
86 The structure of s 121A indicates that it is concerned with the drawing of inferences with respect to a process based on an examination of a product of that process. The section is primarily aimed at circumstances in which the plaintiff has access to the defendant’s product, but not the defendant’s process (eg. because it is used by a third party overseas and is unavailable for inspection by the plaintiff).
87 In the present case, the respondents’ product is the hydroxide slurry made and supplied by the respondents under the names Phodine and Phodine Plus. Section 121A can only apply if the Court is satisfied (inter alia) that it is “very likely” that those products were made by the patented process (ie. the method of claim 1).
88 There was no evidence to show that the making of a hydroxide slurry of the kind made and supplied by the respondents could not be made by raising the temperature of the reaction mixture to its boiling point (as opposed to “near the boiling point”) or that there was anything about the product that made it unlikely that the reaction mixture was allowed to boil during its production. There is nothing about the respondents’ product which satisfies me that it is likely, much less very likely, that it was made by the method of claim 1. In those circumstances, s 121A of the Act cannot apply.
89 I find that in the Grenof process, the reaction mixture reaches boiling point and boils. I also find that the maximum reaction temperature reaches boiling point. The Grenof process is therefore outside the scope of claim 1.
Validity
Novelty
90 Section 18(1)(b)(i) of the Act provides that an invention is a patentable invention, for the purposes of a standard patent, if the invention, so far as claimed in any claim, when compared with the prior art base as it existed before the priority date, is novel. Section 7(1) of the Act provides that an invention is taken to be novel when compared with the prior art base unless it is not novel in light of relevant prior art information (as defined in the Act).
91 The test for whether a patent claim lacks novelty by reason of a prior publication (or is “anticipated” by a prior publication) has been described as follows in General Tire & Rubber Co Ltd v Firestone Tyre & Rubber Co Ltd [1972] RPC 457 at 486:
To anticipate the patentee's claim the prior publication must contain clear and unmistakable directions to do what the patentee claims to have invented … A signpost, however clear, upon the road to the patentee's invention will not suffice. The prior inventor must be clearly shown to have planted his flag at the precise destination before the patentee.
(Citations omitted.)
92 The question is whether the prior publication is sufficient to enable the skilled addressee to perceive, understand and, where appropriate, apply the prior disclosure necessarily to obtain the invention without the necessity of making further experiments and gaining further information before the invention can be made useful: Hill v Evans (1862) 45 ER 1195 at 1199; 1A IPR 1 at 6. Common general knowledge can be used to construe a prior art document, but cannot “…be deployed complementarily to arrive at a disclosure which the document alone, properly construed, does not make”: AstraZeneca AB v Apotex Pty Ltd (2014) 226 FCR 324 at [352] (“AstraZeneca”).
93 The respondents rely on four prior art documents:
(a) United States Patent 5906804, entitled “Magnesium Hydroxide Slurries”, published on 8 February 1996 (“Aral”);
(b) United States Patent 2904401, entitled “Viscosity Control Method and Apparatus for Hydrating Lime”, published on 15 September 1959 (“Booth”);
(c) Chapter 22 of Lime and Limestone: Chemistry and Technology, Production and Uses by J.A.H. Oates, published in 1998 by Wiley-VCH Verlag GmbH (“Oates”); and
(d) Section 9.4 of Chapter 9 of The Chemistry and Technology of Magnesia by Mark A Shand, published in 2006 by Wiley Interscience (“Shand”).
94 Calix accepts that each of the prior art documents discloses at least some of the integers of claim 1. The integers in contest are highlighted in yellow in the following table:
Shand
95 Shand describes a method of producing magnesium hydroxide slurry by reacting magnesium oxide in water in excess of that required for hydration. The hydration reaction is described as exothermic and is “carried out in large, well-agitated tanks that are open to the atmosphere to allow the escape of steam and mitigate any pressure buildup”. Crucially, Shand states that “the MgO and water are mixed together in the reactor and allowed to boil”.
96 Given my previous finding that “near the boiling point” in integer (v) of claim 1 does not include “at the boiling point,” it is clear that integer (v) is not disclosed in Shand. Accordingly, Shand does not anticipate claim 1.
Aral
97 Aral describes another method of producing magnesium hydroxide slurries in which MgO powder is mixed with water. In each of the examples referred to in Aral, the reaction mixture is allowed to boil. It follows that Aral does not anticipate claim 1.
Booth
98 Booth describes a method and apparatus for hydrating lime. It calls for the mixture of water and quicklime (which the skilled addressee would understand to be calcium oxide (CaO)). The specification states that the temperature of the reaction mixture ordinarily rises to about 212ºF (ie. the boiling point of water) and that “… it has been unnecessary to control the temperature in any way”. Accordingly, Booth does not anticipate claim 1.
Oates
99 Oates describes a traditional method of hand slaking using a wooden or metal trough in which water is mixed with quicklime which is then brought to the boil. Here again, it is clear that Oates does not anticipate claim 1 because it requires that the water be brought to the boil.
Inventive Step
100 An invention is a patentable invention if the invention, when compared with the prior art base, involves an inventive step. Sections 7(2) and (3) of the Act identify the nature of the enquiry. They provide:
(2) For the purposes of this Act, an invention is to be taken to involve an inventive step when compared with the prior art base unless the invention would have been obvious to a person skilled in the relevant art in the light of the common general knowledge as it existed (whether in or out of the patent area) before the priority date of the relevant claim, whether that knowledge is considered separately or together with the information mentioned in subsection (3).
(3) The information for the purposes of subsection (2) is:
(a) any single piece of prior art information; or
(b) a combination of any 2 or more pieces of prior art information that the skilled person mentioned in subsection (2) could, before the priority date of the relevant claim, be reasonably expected to have combined.
101 As this is a post-Raising the Bar patent, there is no requirement of “ascertainment” under s 7(3) of the Act.
102 In Wellcome Foundation Ltd v VR Laboratories (Aust) Pty Ltd (1981) 148 CLR 262, Aickin J stated at 286:
The test is whether the hypothetical addressee faced with the same problem would have taken as a matter of routine whatever steps might have led from the prior art to the invention, whether they be the steps of the inventor or not.
See also AstraZeneca AB v Apotex Pty Ltd (2015) 257 CLR 356 at [15] per French CJ; Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (No 2) (2007) 235 CLR 173 (“Doric No 2”) at [53]. “Routine” in this context means steps of a routine character to be tried as a matter of course.
103 A “scintilla of invention” can sustain a valid patent, but there must be “some difficulty overcome, some barrier crossed” or something “beyond the skill of the calling”: Doric No 2 at [52]; RD Werner & Co Inc v Bailey Aluminium Products Pty Ltd (1989) 25 FCR 565 at 574; Allsop Inc v Bintang Ltd (1989) 15 IPR 686 at 701.
104 No reliance was placed by Calix on any secondary indicia of inventiveness (ie. a long felt want or need, commercial success, etc) and no submission was made along those lines: cf. Doric No 2 at [116].
105 In approaching the question of inventive step, I am mindful of the dangers of hindsight: see Garford Pty Ltd v Dywidag Systems International Pty Ltd (2015) 110 IPR 30 at [44] and the authorities referred to by the Full Court in that paragraph.
106 Calix placed considerable reliance on paras 82 and 83 of Professor Sorrell’s Stage 2 Report. Professor Sorrell was asked:
Does the process described in Claim 1 solve any of the problems of processes for producing hydroxide slurries that existed on or before 24 October 2013? If so, explain by reference to the Patent, the common general knowledge and/or any other matter which you deem relevant.
107 Paragraphs 82-83 record Professor Sorrell’s answer to this question which was as follows:
82. I agree with the problems for producing hydroxide slurries that existed on or before 24 October 2013, as described in the Patent, being:
(a) Large particle size and associated low surface area and hence low reactivity. (e.g., dead burned magnesia (DBM) and lime) (Paragraphs [0030] and [0031]). The patent provides a process using caustic calcined carbonate powder or caustic calcined hydroxide powder as starting material, which is a fine (micron-scale) and reactive powder of high surface area.
(b) Suboptimal rheology, manifested by poor suspension stability, insufficient shear thinning, and agglomeration (Paragraphs [0027], [0028], [0035], [0069], [0070], [0071], and [0072]. The Patent provides a process using shearing force for reduction of concentration gradients, deagglomeration, comminution, and prevention of formation of large bubbles, all of which reduce the resistance to shear thinning and the stability of the suspension.
(c) Low solids loading, resulting in low efficiency (Paragraphs [0027], [0028], and [0032]). The Patent provides a process to produce a suspension of high solids loading, which increases throughput capacity and therefore the efficiency.
(d) Retention of passivating layer, which slow down or stops hydration reaction (Paragraphs [0027], [0028], [0029], [0034]). The Patent provides a process using shearing force for progressive removal of the passivating hydroxide surface layer, thereby both enhancing and regulating the rate of hydration.
(e) Localised mass and heat concentration gradients around the particles during hydration, which would slow down or stop hydration reaction (Paragraph [0036]). The Patent provides a process using localised boiling at the particle-liquid interface, thereby producing micro-bubbles, which, in combination with the shearing force, homogenise and distribute both mass and heat by raising them to the surface of the reaction mixture.
(f) Volumetric mass and heat concentration gradients throughout the vessel during hydration slow down or stop hydration reaction (Paragraph [0036]). The Patent provides a process using shearing force for homogenisation and distribution of both mass and heat, thereby accelerating and controlling the hydration reaction rate. The associated release of steam provides the complementary mechanism of cooling to balance the heating from hydration.
83. I consider the process described in Claim 1 solved all of the problems, as discussed above.
108 The first point to make about Professor Sorrell’s response is that it does not answer the question asked. He merely recites various matters that either appear in, or which he gleans from, the CS without reference to the common general knowledge.
109 Professor Sorrell states that the process described in claim 1 “solved all of the problems” discussed. He does not explain how it did so by reference to the common general knowledge. For example, he does not indicate whether caustic calcined powder or caustic calcined hydroxide powder were known and used at the priority date in the manufacture of hydroxide slurries. Furthermore, he does not say whether the mixing apparatus applying a shearing force was known and used in the manufacture of hydroxide slurries at the priority date. In short, his response provides no information at all as to the state of the common general knowledge at the priority date.
110 A hydroxide slurry made using the method defined by claim 1 need not exhibit any particular physical characteristics. Various dependent claims (not sued on) further define the method by reference to what may be desirable properties of a slurry produced using the method of claims 1 and 3. For example, claim 2 (dependent on claim 1) refers to a slurry that is a “high solid fraction hydroxide slurry” and that “has a relatively low resistance to shear thinning”. Similarly, claim 8 (dependent on claims 1-3) refers to a slurry with a specified solids content.
111 Paragraph 82 of Professor Sorrell’s Stage 2 Report focuses on various qualities of a hydroxide slurry that are not reflected in the method defined by claim 1. There is nothing in claim 1 that requires (whether expressly or impliedly) that the slurry produced using that method will include particles having a particular size, surface area, solids content or shear resistance.
112 The use of a mixing apparatus which applies a shearing force to distribute both mass and heat in a reaction mixture comprising water and hydroxide powder was a well-known and well understood technique at the priority date. The person skilled in the art would understand that the hydration reaction rate could be controlled (in the sense of restricted or restrained) by the use of a suitable mixing apparatus operating at a suitable speed and a suitable combination of water and reagent.
113 It would also be understood by the person skilled in the art that the release of gas (ie. water vapour) from the reaction mixture produced by the heat of hydration would restrict or limit the rate of the hydration reaction. The person skilled in the art would also understand that by adjusting the speed of the mixing apparatus, the quantities of water and reagent, and the “feed rates” of those components, the temperature of the hydration reaction could be kept close to (but below) the boiling point. These adjustments would be routine and would not require the exercise of any imagination or invention.
114 For reasons previously explained, I also accept Mr Messiter’s evidence concerning methods that were used to make hydroxide slurries at the priority date which, as I have found, were common general knowledge. His evidence establishes that the method the subject of claim 1 was well-known and actually being performed by persons skilled in the relevant art before the priority date who were, I infer, seeking to maximise reaction temperatures (in order to reduce batch times) while at the same time avoiding boiling (in order to avoid excessive pressure).
115 Further, each of Shand and Aral are documents which are made relevant by s 7(3) of the Act. Each is to be considered individually (ie. not in combination) together with the common general knowledge as at the priority date.
116 As to Shand, it would be obvious to the person skilled in the art equipped with that document and the common general knowledge that it would be possible (through the use of the well-known techniques previously referred to) to restrict the maximum temperature of the reaction mixture used in Shand so that it was near to, but did not equal or exceed, the boiling point. No imagination or invention would be required in order for the person skilled in the art to achieve that result. In my opinion, the invention defined by Claim 1 was obvious in light of Shand and the common general knowledge. I make the same findings in relation to Aral.
117 In my opinion, the invention as defined by claim 1 is invalid for lack of inventive step.
Section 40(2)(a)
118 Section 40(2)(a) of the Act requires that a complete specification “disclose the invention in a manner which is clear enough and complete enough for the invention to be performed by a person skilled in the relevant art.” Whether an invention is disclosed in a manner which is clear enough and complete enough depends on whether, given what is contained in the specification, the skilled person can readily perform the invention without undue burden or the need for further invention.
119 The language of s 40(2)(a) reflects that of s 72(1)(c) of the Patents Act 1977 (UK) (“UK Act”). That section has been considered in numerous cases in the UK including by Aldous J in Mentor Corp v Hollister Inc [1991] FSR 557. His Lordship said at 562:
The section requires the skilled man to be able to perform the invention, but does not lay down the limits as to the time and energy that the skilled man must spend seeking to perform the invention before it is insufficient. Clearly there must be a limit. The subsection, by using the words "clearly enough and completely enough," contemplates that patent specifications need not set out every detail necessary for performance, but can leave the skilled man to use his skill to perform the invention. In so doing he must seek success. He should not be required to carry out any prolonged research, enquiry or experiment. He may need to carry out the ordinary methods of trial and error, which involve no inventive step and generally are necessary in applying the particular discovery to produce a practical result. In each case, it is a question of fact, depending on the nature of the invention, whether the steps needed to perform the invention are ordinary steps of trial and error which a skilled man would realise would be necessary and normal to produce a practical result.
That statement of the law was approved by the Court of Appeal in Mentor Corp v Hollister Inc [1993] RPC 7 at 14.
120 In Novartis AG v Johnson and Johnson Medical [2010] EWCA Civ 1039 Jacob LJ said at [74]:
The heart of the test is: “Can the skilled person readily perform the invention over the whole area claimed without undue burden and without needing inventive skill?”
121 Both of those authorities were referred to and applied by Burley J in Merck Sharp and Dohme Corporation v Wyeth LLC (No 3) (2020) 155 IPR 1 (“Merck”) at [524]-[525]. At [526] his Honour also referred to the judgment of Kitchin J (as his Lordship then was) in Eli Lily v Human Genome Sciences [2008] RPC 29 at [239]. Kitchin J identified seven key elements of the requirement under s 72(1)(c) of the UK Act which included the following:
sufficiency of the disclosure must be assessed on the basis of the specification as a whole including the description and the claims;
the disclosure is aimed at the skilled person who may use his common general knowledge to supplement the information contained in the specification;
the specification must be sufficient to allow the invention to be performed over the whole scope of the claim; and
the specification must be sufficient to allow the invention to be so performed without undue burden.
122 In their submissions, the respondents relied on the evidence of Professor Sorrell including evidence given by him concerning the disclosure of the specification and the large number of parameters that he considered potentially relevant to the manufacture of hydroxide slurries. In circumstances where I have found that Professor Sorrell is not a person skilled in the relevant art, I do not give any significant weight to the respondents’ challenge to the validity of claim 1 based on that evidence. More relevant to this challenge are the findings as to common general knowledge based largely on the evidence of Mr Messiter.
123 The respondents point to the fact that the CS does not include any working examples of the method defined by claim 1. However, it is important to observe here that the invention defined by claim 1 reflects what I found to be a well-known method of producing hydroxide slurries that would most likely require the person skilled in the art to undertake routine experimentation aimed at determining suitable quantities and feed rates for water and reagent necessary to increase the temperature of the reaction mixture close to boiling point. Working examples might assist the person skilled in the art but the fact that there is none provided does not mean that he or she would not be able to perform the method of claim 1 without undue burden.
124 In my view, it follows from my construction of claim 1 and my findings as to the common general knowledge that a person skilled in the art would be able to perform the invention over the whole of the area covered by claim 1 without undue burden and without the need for invention. In the circumstances, I am not persuaded that the CS does not comply with the requirements of s 40(2)(a) of the Act.
Section 40(3)
125 Section 40(3) of the Act requires (inter alia) that the claims be supported by matter disclosed in the specification.
126 The test for support that has been applied in the UK and more recently in Australia, is that adopted by Aldous J in Schering Biotech Corp’s Application [1993] RPC 249. His Lordship said at 252-253:
… to decide whether the claims are supported by the description it is necessary to ascertain what is the invention which is specified in the claims and then compare that with the invention which has been described in the specification. Thereafter the court's task is to decide whether the invention in the claims is supported by the description. I do not believe that the mere mention in the specification of features appearing in the claim will necessarily be a sufficient support. The word "support" means more than that and requires the description to be the base which can fairly entitle the patentee to a monopoly of the width claimed …
127 That test was referred to by Burley J in Merck at [546]-[547] who referred also to the requirement “that the technical contribution to the art disclosed by the specification must justify the breath of the monopoly claimed”.
128 Although discussion of s 40(3) is often focused on the breath of the claim, there may be some claims which lack support not because they are too broad, but because they define an invention that is materially different to what is described in the body of the specification. Hence, a claim that includes a feature not disclosed in the specification, or omits a feature that is disclosed, may lack support because the invention claimed is materially different from the invention disclosed. Whether or not the claim will lack support in such circumstances will depend on the proper characterisation of the invention disclosed in the body of the specification and the invention claimed. See, for example, the invention described in the relevant priority document and the invention claimed in AstraZeneca at [254]-[255] which were characterised by the Full Court of the Federal Court as “fundamentally different” inventions. It is difficult to see how a claim to an invention that is fundamentally different from that which is disclosed in the specification could be “supported by matter disclosed” in accordance with s 40(3) of the Act.
129 I have previously drawn attention to various passages in the CS which indicate that the method of making hydroxide slurry as most broadly described in the CS is a method in which the reaction temperature is restricted to “just below or at the boiling point”: see CS [0025]-[0026] and [0041]. In describing various preferred embodiments of the invention, reference is also made to boiling, heating the reaction mixture to 100ºC, and boiling the excess water: see CS [0061], [0062] and [0078]. The CS states that “[i]mportantly, there is no adverse effect of boiling water on slurry characteristics provided that the water content is managed to account for the loss”: CS [0077]. The CS also discusses the use of the boiling point “as a bound to control the process temperature …” CS [0079]. What is disclosed by the CS is a method in which the maximum temperature of the reaction mixture is permitted to reach boiling point.
130 None of those references, or the description of the invention as a whole, supports a claim to a method in which the maximum temperature of the reaction mixture is restricted to “near the boiling point”. The invention as defined by claim 1 is fundamentally different from the invention disclosed in the body of the specification. In my opinion, claim 1 is not supported by matter disclosed in the CS and is invalid for non-compliance with s 40(3) of the Act.
Unjustified Threats
131 It is not disputed by Calix that it threatened Grenof with infringement proceedings by letter from Alder IP to Grenof dated 19 February 2021. Given my previous findings, it follows that the threat was unjustified. There is no evidence of any other threat having been made. In those circumstances, an injunction is not warranted. There is no evidence to suggest that Grenof suffered any loss by reason of Calix having made the unjustified threat.
Disposition
132 There will be:
an order dismissing the amended originating application;
an order revoking claim 1 of the Patent;
a declaration that the threat made by Calix to Grenof was unjustified;
an order otherwise dismissing the amended statement of cross-claim.
133 With respect to costs, subject to what follows, Calix must pay the respondents’ costs of the proceeding including the cross-claim.
134 I previously reserved the costs of an interlocutory application brought by Calix for discovery of documents. Grenof opposed the order sought by Calix essentially on the basis that the documents were not relevant. On 20 December 2022, I made an order for discovery substantially in the terms sought by Calix. The list of documents filed by Grenof pursuant to that order referred to a very small number of documents. In my view, it was unreasonable for Grenof to resist the application for discovery of those documents. They were clearly relevant and there was no reason why they should not have been disclosed to Calix’s solicitor when first requested. In the circumstances, Grenof should pay Calix’s costs of the interlocutory application.
135 Orders accordingly.
I certify that the preceding one hundred and thirty-five (135) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Nicholas. |
Annexure A