Federal Court of Australia
Abbott v Zoetis Australia Pty Ltd [2022] FCA 1390
ORDERS
Applicant | ||
AND: | Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The parties confer and on or before 7 December 2022 file draft orders to give effect to the Court’s reasons for judgment delivered today and in default of agreement draft orders marked up to show any disagreements and written submissions limited to 3 pages.
2. The proceeding stand over to 9 December 2022 for the making of final orders.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
RARES J:
1 This is a representative proceeding under Pt IVA of the Federal Court of Australia Act 1976 (Cth). It concerns a vaccine, EquivacHeV Hendra virus vaccine (Equivac HeV), developed by the respondent, Zoetis Australia Pty Limited, which until about March 2013 was called Pfizer Animal Health Pty Limited, in conjunction with the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and others, to provide immunity for horses against the zoonotic (ie: capable of being transmitted from animals to humans) virus of the henipavirus family. That virus has taken the eponymous name Hendra virus (or HeV) from the Brisbane suburb where it first became evident in 1994. On that occasion, a number of racehorses and a veterinarian died and a veterinary nurse suffered long-term effects from HeV.
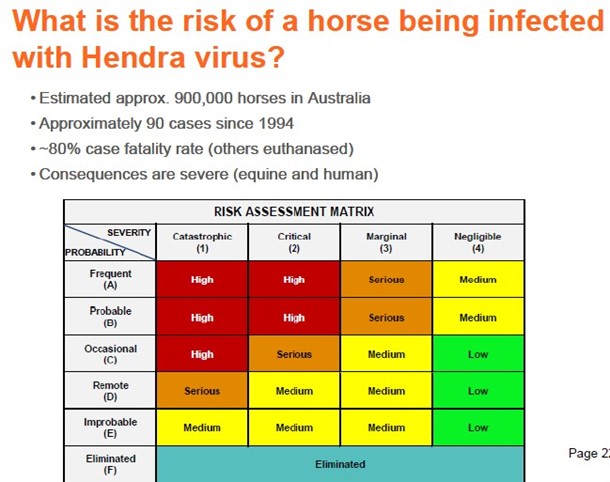
2 There are approximately 900,000 domesticated, and an unknown number of feral, horses in Australia. Equivac HeV has proved particularly effective in providing immunity to vaccinated horses.
3 The applicant representative party, Rachael Abbott, is a stockperson who owned a number of horses, including the mare, Primetime, which was vaccinated with an initial injection and a booster injection of Equivac HeV in, respectively, July and August 2014. In addition, commencing in July 2013, a sample group member, Kelly Hinton, had her horses, including Quinn, a gypsy cob stallion, vaccinated.
4 Ms Abbott claims that Primetime suffered enduring adverse physical conditions as side effects resulting from the administration of Equivac HeV. Ms Hinton claims that Quinn died within five days after receiving his fifth vaccination on 28 January 2016, again, as a result of the vaccination itself.
5 The applicant claims that over the relevant period, between 10 August 2012 and 20 March 2018, Zoetis engaged in conduct that was misleading or deceptive or likely to mislead or deceive in contravention of s 18(1) of the Australian Consumer Law (ACL) in Sch 2 of the Competition and Consumer Act 2010 (Cth), by making three representations, that she pleaded as the geographic spread, the no serious side effects and the all horses representations in a number of different publications. (For brevity and because nothing turns on it in this proceeding, in these reasons I will use the shorthand expression “misleading” to encapsulate the various permutations in s 18(1)).
6 In addition, the applicant claims that during the relevant period, Zoetis guaranteed to consumers, within the meaning of s 54(1) and (2) of the ACL, that Equivac HeV was of acceptable quality. The applicant claims compensation under s 236 of the ACL. The group members are all persons who suffered loss or damage or personal injury in the relevant period because of Zoetis’ alleged conduct in contravention of ss 18(1) or 54(2) of the ACL.
7 The essential issues are whether, first, each of the representations on which the applicant relies was conveyed by the publications identified, secondly, it was, in fact, incorrect or misleading or deceptive and, thirdly, to the extent that it was made with respect to a future matter within the meaning of s 4 of the ACL, Zoetis had reasonable grounds for making it, as it contends.
8 The three representations as pleaded in the second further amended statement of claim and the particulars of publications in which the applicant alleged they were conveyed as pressed at trial (with the shorthand names of the publications by which each was conveyed) are:
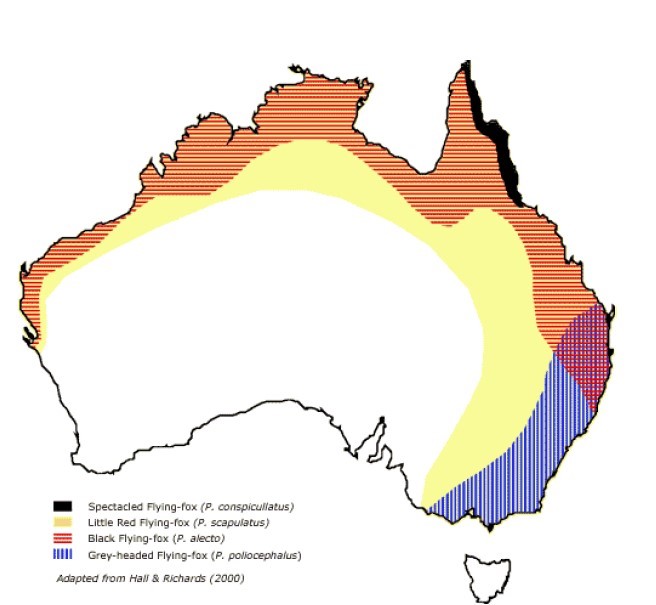
(1) The geographic spread representation
From 2 March 2013 and throughout the relevant period, Zoetis represented to the Class that there was a serious risk of horses contracting Hendra in all areas of Australia in which flying foxes were present.
Particulars
The representation was contained, separately and together, in:
(a) the 2013 fact sheet;
(b) the March 2013 media release;
(c) the mythbusting pamphlet;
(d) the September 2013 seminar;
(e) the Horses 4 Horses website information; and
(f) the every horse owner pamphlet.
(2) The no serious side effects representation
From 10 August 2012 and throughout the relevant period, Zoetis represented to the class that Equivac HeV had no serious side effects.
Particulars
The representation was contained, separately and together, in:
(a) the first and second permit disclosures;
(b) the registration module;
(d) the third permit disclosure;
(c) the mythbusting pamphlet;
…
(f) the Horses 4 Horses website information;
(g) the facts about HeV pamphlet; and
(h) the Equestrian Life letter.
(3) The all horses representation
From 2 March 2013 and throughout the relevant period, Zoetis represented to the class that all horses in Australia should be treated with Equivac HeV.
Particulars
(a) The representation was made expressly in the 2013 fact sheet and March 2013 media release.
(b) Further to (a), the representation was contained in the registration module materials and Equestrian Life letter.
9 I will describe the contents of each of the particularised and pressed publications below when considering whether each representation was conveyed to an ordinary, reasonable member of the class. Ms Abbott pleaded that she relied on each of the representations whereas Ms Hinton pleaded that she relied only on the geographic spread and no serious side effect representations.
10 If the no serious side effects representation is true, the claim under s 54(1) and (2) of the ACL necessarily fails and, likewise, if that representation was untrue, the guarantee will succeed. In addition, it is necessary to consider what, if any, damages Ms Abbott and Ms Hinton have been able to prove.
11 Relevantly, the ACL provided during the relevant period:
4 Misleading representations with respect to future matters
(1) If:
(a) a person makes a representation with respect to any future matter (including the doing of, or the refusing to do, any act); and
(b) the person does not have reasonable grounds for making the representation;
the representation is taken, for the purposes of this Schedule, to be misleading.
(2) For the purposes of applying subsection (1) in relation to a proceeding concerning a representation made with respect to a future matter by:
(a) a party to the proceeding; or
(b) any other person;
the party or other person is taken not to have had reasonable grounds for making the representation, unless evidence is adduced to the contrary.
(3) To avoid doubt, subsection (2) does not:
(a) have the effect that, merely because such evidence to the contrary is adduced, the person who made the representation is taken to have had reasonable grounds for making the representation; or
(b) have the effect of placing on any person an onus of proving that the person who made the representation had reasonable grounds for making the representation.
(4) Subsection (1) does not limit by implication the meaning of a reference in this Schedule to:
(a) a misleading representation; or
(b) a representation that is misleading in a material particular; or
(c) conduct that is misleading or is likely or liable to mislead;
and, in particular, does not imply that a representation that a person makes with respect to any future matter is not misleading merely because the person has reasonable grounds for making the representation.
…
18 Misleading or deceptive conduct
(1) A person must not, in trade or commerce, engage in conduct that is misleading or deceptive or is likely to mislead or deceive.
…
54 Guarantee as to acceptable quality
(1) If:
(a) a person supplies, in trade or commerce, goods to a consumer; and
(b) the supply does not occur by way of sale by auction;
there is a guarantee that the goods are of acceptable quality.
(2) Goods are of acceptable quality if they are as:
(a) fit for all the purposes for which goods of that kind are commonly supplied; and
(b) acceptable in appearance and finish; and
(c) free from defects; and
(d) safe; and
(e) durable;
as a reasonable consumer fully acquainted with the state and condition of the goods (including any hidden defects of the goods), would regard as acceptable having regard to the matters in subsection (3).
(3) The matters for the purposes of subsection (2) are:
(a) the nature of the goods; and
(b) the price of the goods (if relevant); and
(c) any statements made about the goods on any packaging or label on the goods; and
(d) any representation made about the goods by the supplier or manufacturer of the goods; and
(e) any other relevant circumstances relating to the supply of the goods.
12 Zoetis is, together with its associated company, Zoetis Australia Research and Manufacturing Pty Limited, an Australian subsidiary of a global veterinary pharmaceutical business, Zoetis Inc. Previously, Zoetis had formed part of the Pfizer Animal Health business prior to Pfizer divesting itself of its Zoetis businesses.
13 Equivac HeV is an award-winning veterinary medicine. The Australian Pesticides and Veterinary Medicines Authority (also called APVMA) registered Equivac HeV on 4 August 2015 in the Register of Agricultural and Veterinary Commercial Products under s 20(2) in the Schedule to the Agricultural and Veterinary Chemicals Code Act 1994 (Cth) (the Agvet Code). Before this, from the launch of the product on 10 August 2012 until its registration, the Authority had granted Zoetis three minor use permits under s 112 of the Agvet Code that strictly controlled the use and distribution of Equivac HeV to horses.
14 The Authority required Zoetis, as a condition of each of the permits, to label, and include a leaflet in, the container in which it supplied Equivac HeV, that contained certain information referred to as the first, second or third permit disclosures, as to possible side effects of vaccination on which the applicant relies as conveying the no serious side effects representation. Until the Authority registered Equivac HeV, the permits only allowed Zoetis to distribute the vaccine to registered veterinary surgeons (veterinarians or vets), who had to administer it.
15 The applicant alleged that each of the labels and leaflets for permit PER13510, issued for the period 10 August 2012 to 3 August 2014 (the first permit), permit PER14876, issued for the period 4 August 2014 to 4 August 2015 (the second permit), and the superseding permit PER14887, issued for the period 31 March 2015 to 4 August 2015 (the third permit), conveyed the no serious side effects representation, as I explain later.
16 It was common ground that a large number of publications accompanied great public controversy and discussion about the Hendra virus since its discovery in 1994 with the tragic results that I have mentioned.
17 Earlier in this proceeding, Lee J ordered that Professor Michael Ward PhD; DVSc, FACVSc be appointed as an expert referee. Professor Ward was a registered specialist in veterinary epidemiology. The referee had to report about:
the risk of horses contracting HeV in all areas of Australia in which flying foxes were present;
the nature and magnitude of that risk, as well as the state of knowledge of transmission of HeV from flying foxes to horses, between horses and from horses to humans;
the state of knowledge of the geographic distribution and movement of flying fox species;
which species of flying fox was or were capable of transmitting HeV to horses in Australia; and
what factors affected the nature and magnitude of the risk of HeV being passed from flying fox to flying fox, flying foxes to horses, horses to horses and horses to humans.
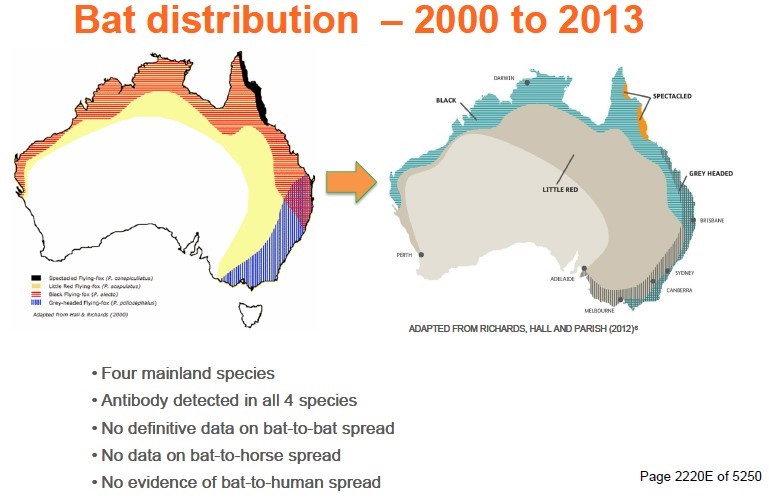
18 Professor Ward made a report, dated 11 September 2020, which became an exhibit. He supplemented the report with answers to further questions which the parties posed through the Court. Professor Ward opined, and I find, that during the relevant period:
(1) there was a risk of horses contracting HeV, whether directly from flying foxes or otherwise, in all areas of Australia in which flying foxes were present;
(2) the magnitude of the risk of horses contracting HeV in all areas of Australia in which flying foxes were present primarily was dependent on the presence of two species, Pteropus (or P.) alecto and Pteropus (or P.) conspicillatus, being the black and spectacled flying fox;
(3) the magnitude of the risk ranged from very low, or about 1 to 2 per 10,000, within the Hendra area (defined as the area where the horses had suffered HeV infection, namely the coastal strip from Far North Queensland, and on the eastern side of the Great Dividing Range in tropical areas of Queensland to the mid North Coast of New South Wales and, in one instance, in Southern Queensland) to negligible or extremely low outside the Hendra area, namely, or about 1 or 2 per 1,000,000;
(4) except for the transmission of HeV from horses to humans during the relevant period, the state of knowledge of the means of transmission of the virus between flying foxes, from flying foxes to horses and between horses was poor;
(5) there was good knowledge of the geographic distribution of flying fox species and fair knowledge of their local and long-distance movements. A scientific consensus developed that the black and spectacled varieties were the flying fox species more likely to be capable of transmitting HeV to horses in Australia; and
(6) a range of factors was proposed that might affect the nature and magnitude of the risk of HeV being passed from flying fox to flying fox and from flying foxes to horses, but there was a lack of scientific consensus. In contrast, there was a consensus that the transmission of the virus from horses to horses and from horses to humans depended on very close contact with an infectious horse.
19 Professor Ward’s view, on which the parties largely proceeded, was that there was a risk throughout those areas of Australia in which flying foxes were present, as depicted on the map below that summarised the distribution of the four species, of both horses and humans contracting HeV, but that the risk outside the Hendra area was extremely low, but still present.
20 During the course of the hearing, Zoetis sought to have Professor Ward comment on provisional and speculative results released by some researchers, in a document entitled “Horses as Sentinels Research”, which suggested that another species of flying fox, other than the black or spectacled ones, carried a slightly different variant of the Hendra virus. Professor Ward said that he was generally aware at the time he wrote his report that research into this topic was progressing, but he was not aware of its content or of its provisional findings and had not taken those into account.
21 The researchers hypothesised that, at the stage their research had reached, the grey-headed flying fox might intermittently cause a variant of HeV to be transmitted or spillover into other species, such as horses and humans. They stated that they had speculated that different strains of HeV could predominate in different species of flying fox. However, at that stage, their work had not been formulated into a paper, far less peer reviewed.
22 In those circumstances, I formed the view that it would not be appropriate to have the referee report on that material, or to allow it to be introduced into evidence, because the applicant would not be able to meet it. It may well be that if and when that research matures into a more concrete form, some of the conclusions that I reach may need reconsideration in another context.
4.2.1 The applicant’s lay witnesses
23 Ms Abbott had been a stockperson for her working life. She had experience in handling horses, cattle, and, as a lay person, veterinary products to treat livestock. She had certification and training in farm safety and feeding cattle. She had also competed, with some success, in stockhorse handling competitions. Part of her working experience had involved her riding her own horses as a pen rider. She explained that this required her to ride through numerous pens of between 150 to 250 cattle to check on their apparent health, the amount of feed and water, and identify any that were in need of attention. She said that, as a pen rider, ultimately she needed to check about 5000 head daily and spent about three minutes in each pen. To do this, she needed a good, healthy horse, called a stockhorse. Ms Abbott obviously cared deeply about her horses, as was apparent when she gave evidence.
24 Ms Hinton has a master’s degree in business administration and, at the time of the trial, was studying for a master of financial planning degree. She had a lifelong interest in horses, having begun riding when she was six years old, and had her own horse at about 15. She bought her first horse as an adult when she was 18 and has owned horses ever since. She and her husband, David Hinton, traded as a horse stud partnership from about August 2008 under the name Dellifay Gypsy Cobs and Performance Horses.
25 Dr Richard L’Estrange graduated as a bachelor of veterinary science from the University of Queensland in 1987. He became a member of the Australian and New Zealand College of Veterinary Scientists in veterinary pharmacology in 2008 and studied vaccinology and pharmacology to pass the examination for that qualification. He worked in private practice in Queensland and the United Kingdom from graduation until 2010 when he began employment with Pfizer. In 2012, he became responsible for Pfizer’s equine products portfolio, including Equivac HeV.
26 Dr Phillip Lehrbach was a technological expert in vaccines and biological products. He obtained degrees of bachelor of science from the University of Sydney in 1974, a doctor of philosophy from the University of Melbourne in 1980, a master of business administration from the University of Technology, Sydney, and a master of medical science drug development from the University of New South Wales in 2005. He worked on post-doctoral research fellowships in the 1980s at the Universities of Edinburgh (in molecular biology) and Geneva (in medical biochemistry). He also lectured at the Universities of Sydney and New South Wales on molecular biology and its influence on the development of animal health vaccines, as well as authoring or co-authoring over 60 peer reviewed articles on topics of molecular biology, animal health vaccines and microbial genetics. He also had roles assisting governmental bodies. He worked in senior positions in companies that eventually became part of the Pfizer group in October 2009. In 2010, he became director of regulatory affairs Asia-Pacific.
27 Dr John Messer, in 2012, was Zoetis’ regulatory affairs manager. Dr Messer was experienced in collecting data and investigating adverse reaction reports for the purpose of Zoetis’ pharmacovigilance of its products. He graduated as a bachelor of veterinary science in 1990 and then practised as a vet in Australia and the United Kingdom for about 12 years, predominantly with farm animals, including horses. As part of his practice, from time to time, he vaccinated horses. In December 2002, he began working with the German multinational, Bayer, as a product support veterinarian and eventually became a technical development manager. His roles at Zoetis required him to deal with consumers, vets and the Authority in respect of, among other matters, pharmacovigilance. He began working for Zoetis in 2011. During 2011 to 2013, as regulatory affairs manager, he worked under Dr David Chudleigh who until late 2013 was Zoetis’ departmental director new product developments, regulatory and scientific affairs and whose role included being in charge of pharmacovigilance, including for Equivac HeV. Dr Messer succeeded to Dr Chudleigh’s position.
28 Pharmacovigilance involves the assessment of reports of clinical signs that an animal presents in a temporal association with the earlier administration of a vaccine or medication and an assessment, based on the known pharmacology and toxicity of the introduced product or the likelihood that it may have caused the clinical sign to present.
29 There were three expert witnesses, Associate Professor Ben Sykes, who was called by the applicant, Dr Charles El-Hage and Professor Josh Slater, who were called by Zoetis. Each prepared individual reports and they all prepared a joint report and gave concurrent evidence.
30 Associate Professor Sykes has numerous tertiary qualifications including degrees as a bachelor of veterinary medicine and surgery and of science from Murdoch University, master’s degrees in science, from the Virginia-Maryland Regional College of Veterinary Medicine, and business administration from the University of Liverpool and a doctor of veterinary pharmacology from the University of Queensland. He had over 20 years’ clinical experience as a veterinarian with particular focus on high performance horses and large animal internal medicine. His research had focussed on gastrointestinal diseases of horses. He had taught at numerous universities around the world and was, at the time of the trial, an associate professor at Massey University in New Zealand. He also holds diplomas from both the European College of Equine Internal Medicine and the American College of Veterinary Internal Medicine, which are the highest internationally recognised veterinary internal medicine qualifications.
31 Dr El-Hage is a bachelor of veterinary science with honours and a doctor of philosophy (in infectious diseases focussed on equine immunology and vaccinations) from the University of Melbourne. He is a member of the Australian and New Zealand College of Veterinary Scientists (Equine Medicine). He had been a lecturer in clinical sciences at the University of Melbourne since 2006 and since 2012 had taught students for the degree of doctor of veterinary science. He practised for 16 years from 1984 as a clinician, mainly with horses, in Australia and overseas. He also carries out research.
32 Professor Slater is a bachelor of veterinary medicine and surgery from the University of Edinburgh, and a doctor of philosophy in equine virology from the University of Cambridge. He also holds a diploma from the European College of Equine Internal Medicine. He was a lecturer, then senior lecturer, in equine medicine at Cambridge and professor of equine clinical studies for 13 years at the Royal Veterinary College of the University of London. Since 2019, he has been a professor of veterinary medicine at the University of Melbourne. At London, he was director of the equine referral hospital responsible for all aspects of the College’s clinical activities, treating animals referred to it by general practitioners, as well as leading its research and teaching programs. He specialised in research into equine infectious diseases, particularly equine influenza and equine strangles in collaboration with other institutes in the United Kingdom, United States of America, and Melbourne. Strangles is a bacterial infection that affects a horse’s upper respiratory system (in its head, neck and throat), causing its lymph glands to swell and compress its respiratory tract, restricting its ability to breathe and, as it were, strangling it. Professor Slater was a research group leader and supervised doctoral and masters students. At London, he led and had overall responsibility for its specialist and medical team. In that role, he performed surgeries and treated horses.
33 In terms of its chemical structure, Equivac HeV is relatively simple. It consists of an antigen, being a genetically engineered, but inert, copy of the G protein on the surface of the living HeV, a biological adjuvant, excipients (that give each dose bulk but are neutral substances), and a preservative.
34 The G protein in the antigen replicates the part of the virus that binds to cells within the horse’s body on infection. On the past and present understanding of the operation of the vaccine, it is necessary for a booster injection to be given 21 days after the first administration and, thereafter, every six months to maintain the horse’s immunity. The purpose of the vaccine is to introduce the G protein in its inert form into the horse’s immune system and generate an immune response, leading to immunity.
35 The second ingredient of Equivac HeV is a biological adjuvant. An adjuvant stimulates the immune response in the animal when the inoculation occurs. The adjuvant used in Equivac HeV is a particular immune stimulating complex (ISCOM) known as the ISCOMatrix, and it, together with the excipients and the preservative, are all also used in the Equivac equine flu vaccine. The only difference between Equivac HeV and Equivac is the antigen in each.
36 As is common in human experiences, from time to time, horses also will develop a side effect, being an injection site reaction, at the area where the body reacts to the introduction of the vaccine by generating an immune response. Naturally, the questions of whether there are other side effects from the administration of any vaccine, what their nature is and what risks they present to the organism, are critical to any person making an informed choice about the wisdom or otherwise of vaccinating.
37 When Zoetis originally submitted Equivac HeV for registration by the Authority, it based its data on the results of a very small clinical trial of only about 30 horses. That was why the Authority required, as a condition of each of the three permits that it issued, that the vaccine be administered only by vets, and that any clinical signs or adverse reactions had to be reported to Zoetis or the Authority by those vets or horse owners.
38 As the evidence made clear, horses suffer a variety of illnesses and other apparent ailments or conditions that occur with regularity, some of which are also manifestations of clinical signs of HeV itself, and others of reactions, or potential reactions, to the administration of one or more vaccines.
5. Was each representation conveyed?
39 At the outset, it is necessary to consider whether each of the representations was conveyed by each particular publication on which the applicant relied. Over the course of the relevant period, knowledge developed about, first, HeV itself and its geographic spread, in terms of published instances of infections of horses or humans and, secondly, the actual or possible side effects of Equivac HeV were appearing as clinical signs or adverse reactions in horses in a temporal, but coincidental, relationship to, or as a result of, the administration of the vaccine. Thus, a publication may have been accurate at one time but rendered inaccurate later by new experiences.
40 Each of the 12 publications was made to a somewhat different audience. Some, such as the three permit disclosures that the Authority required be made on the label or in a leaflet with which the vaccine was distributed for use, were made primarily or probably exclusively to veterinarians. Others, such as Zoetis’ publicity materials, were made to the general public, but more particularly to group members and other persons in the equine industry and those who owned horses or whose business involved horses, such as farms or feedlots, who were, or were likely to be, interested in protecting themselves and or their horses or businesses from any adverse impact or risk that HeV posed or might pose. Thus, Zoetis made the 12 publications to wide but diffuse audiences comprised of members of the public as well as, in some cases, a more limited audience that primarily included veterinarians.
41 Whether or not each of the 12 publications on which the applicant relied conveyed one or more of the three representations is a question of fact that must be addressed in the context of the surrounding circumstances: Campomar Sociedad, Limitada v Nike International Ltd (2000) 202 CLR 45 at 84-85 [100]-[101] per Gleeson CJ, Gaudron, McHugh, Gummow, Kirby, Hayne and Callinan JJ; see too: H Lundbeck A/S v Sandoz Pty Ltd (2022) 399 ALR 184 at 199 [69] per Kiefel CJ, Gageler, Steward and Gleeson JJ.
42 The evaluation of whether a particular publication conveyed to its audience one or more of the three representations as alleged has to be undertaken by considering whether an ordinary reasonable member of the class comprised of that particular audience would have so understood it: Nike 202 CLR at 85 [102]; see too Ethicon Sarl v Gill (2021) 288 FCR 338 at 516-519 [798]-[810] per Jagot, Murphy and Lee JJ. Importantly, s 18(1) of the ACL does not relieve a person to whom a publication is made, that is alleged to convey a representation that is misleading, from taking reasonable care of, or for, his or her or its own interests: Nike 202 CLR at 85 [102]-[103], applying Parkdale Custom Built Furniture Pty Ltd v Puxu Pty Ltd (1982) 149 CLR 191 at 199 per Gibbs CJ and 210-211 per Mason J.
43 Thus, in each case of publication on which the applicant relies, the question is whether the effect of the communication to reasonable members of the class to whom it was made was to convey to them the relevant representation or representations: Nike 202 CLR at 85 [103].
44 I will describe each publication in the chronological sequence in which it was made before turning to whether it conveyed any representation the applicant alleged it did and then its accuracy.
5.1 The first permit disclosure
45 The first permit, which was in force for two years from 3 August 2012, restricted the use of Equivac HeV to vets who had been accredited by completing the Equivac HeV module (which comprised the registration module on which the applicant also relied in respect of conveying the no serious side effects and all horses representations). The first permit required Pfizer (as Zoetis was then named) to supply the Equivac HeV vaccine in a container that complied with the Agricultural and Veterinary Chemicals Code Regulations 1995 (Cth) and was labelled in the manner that the permit specified, including a statement: “READ ENCLOSED LEAFLET”. Pfizer and veterinarians had to maintain records of, among other matters, each vaccinated horse, its owner and any reported adverse reaction, including lack of efficacy, resulting from use of the vaccine. Pfizer had to fully investigate and report all adverse reactions to the Authority within 48 hours of receiving a report of an adverse reaction. The form of the leaflet that was part of the first permit stated:
Side Effects
Transient swelling may develop at the site of vaccination in some horses but should resolve within one week without treatment.
46 The leaflet also described the safety trials that Pfizer had conducted that founded the statements about the then-known side effect. It stated that the safety profile of Equivac HeV had been assessed in completely randomised, negatively controlled safety trials in foals and adult horses. The foals had remained healthy throughout the study and there was no evidence of decreased appetite, altered demeanour or abnormal gait. One animal in the vaccinated control group had a higher short-lived rectal temperature following a double dose that resolved by the following morning. Another demonstrated marked pyrexia (ie: increased temperature) at the same time, which, the leaflet stated, indicated that the event might not have been vaccine related. It said 3.4% of adult horses in the vaccinated group had an injection site reaction following the first dose and 34.5% had one the day after the second dose, but seven days after the second dose, all of those reactions had resolved apart from one small lesion in a particular horse that may not have been vaccine related. The leaflet stated there were no systemic vaccine reactions or other abnormal reactions to the vaccine and no adverse events had been reported in the course of the study.
47 Dr L’Estrange said that Equivac HeV went into use in about November 2012.
48 Shortly after Equivac HeV’s public release, Zoetis (then still called Pfizer), the CSIRO, through its Australian Animal Health Laboratory, the Australian Veterinary Association (AVA), Equine Veterinarians Australia and two bodies based in the United States of America, the Henry M Jackson Foundation for the Advancement of Military Medicine and the Uniformed Services University of the Health Sciences published a document headed “Hendra Virus Fact Sheet” (the 2013 fact sheet).
49 The 2013 fact sheet said that HeV was a deadly disease found exclusively in Australia that was transmitted from flying fox to horse, horse to horse and horse to people, having first been discovered in 1994 in the Brisbane suburb of Hendra. It stated:
The virus occurs naturally in flying fox populations across most Australian states and territories with the potential for the disease to appear wherever there are flying fox colonies.
(emphasis added)
50 The 2013 fact sheet said that since 1994, HeV had claimed the lives of 81 horses with more than 30 of those deaths recorded in 2011 and 2012. It stated that of seven confirmed cases of HeV infection in humans, four persons had died, with the most recent death occurring in August 2009. It stated:
Why is the Hendra virus a concern for Australian horse owners?
While the prevalence is low, the Hendra virus is one of Australia’s most lethal viruses. 75 per cent of horses infected with the virus die as a result of the disease, usually within the first two days of showing signs of illness.
The Hendra virus is just as deadly to the humans that come into close contact with infected horses. 57 per cent of humans diagnosed with the disease have died.
How is the Hendra virus spread?
It is thought that horses contract the Hendra virus by ingesting food or water contaminated with infected flying fox body fluids and excretions. The virus can then be passed onto humans if they come into contact with an infected horse’s nasal discharge, blood, saliva or urine.
(emphasis added)
51 The 2013 fact sheet said that, to date, no evidence supported direct or zoonotic transmission or spillover from flying foxes to humans. It stated that clinical signs of HeV infection in horses included, but were not limited to, acute onset of illness, increased body temperature, shifting of weight between legs, depression, increased respiratory rate, nasal discharge, head tilting or circling, muscle twitching and urinary incontinence. The 2013 fact sheet said that ways to reduce the risk of infection in horses included increasing hygiene and cleaning practices, moving horse feed and water containers that were beneath trees to be under shelter so as to avoid potential contamination by flying fox fluids, cleaning and disinfecting all equipment such as halters, lead ropes and twitches, that had been exposed to horses’ bodily fluids, before use on other animals and quarantining any horse thought to be infected. It continued:
How can horse owners and vets protect themselves and others from infection?
In keeping with the Australian Veterinary Association’s policy briefing on the Hendra virus horse vaccine (Equivac® HeV), it is strongly recommended that all horses in Australia are vaccinated against Hendra virus to protect humans from its potentially fatal outcome.
(emphasis added; footnotes omitted)
5.3 The March 2013 media release
52 On 20 March 2013, Zoetis issued a media release, in which it identified itself as formally having changed its name from Pfizer and confirmed its ongoing commitment to educating the equine industry and making Equivac HeV available to horse owners. It stated that:
Zoetis recognised that HeV continued to be a public health concern, “as it is an unpredictable virus that can cause disease and death in both humans and horses.”
Zoetis was working closely with key equine industry members, government bodies and veterinary wholesalers to promote a Hendra vaccination month in April 2013, ongoing education of vets and horse owners, and:
The CSIRO have recently completed a duration of immunity study and greater flexibility around the administration of the vaccine by vets in the near future is a possibility. Additionally, the latest safety data shows that Equivac HeV has exceptionally low incidence of adverse events, with 0.2% of horses displaying minor events.
Equivac HeV vaccine continues to be crucial to breaking the cycle of Hendra virus transmission from flying foxes to horses and then people.
As such, Zoetis remains committed to making the vaccine available in Australia and to supporting veterinarians in their efforts to control Hendra virus. Zoetis also supports the Australian Veterinarian Association’s position that all horses in Australia should be vaccinated against Hendra to reduce equine and human infection.
(emphasis added)
53 Next, in about March 2013, Zoetis produced a two-page pamphlet entitled “Myth Busting Hendra Vaccine” (the myth busting pamphlet). The front page of the pamphlet had a photo of a young woman feeding her horse, under which, in bold yellow and black, appeared the wording, “Vaccinate before it’s too late”. The format of the myth busting pamphlet was to state a myth and then provide Zoetis’ response. The front side relevantly appeared as below:

54 The myth at the bottom of the front page read:
MYTH: There’s no risk of Hendra virus in my area
Hendra virus has been found in all four mainland species of flying foxes in Australia. These bats are found in all mainland states and territories of the country, making exposure possible. For example, bats have tested positive to the Hendra Virus in Melbourne and more recently in South Australia, indicating exposure is possible.
There are many unknowns about how Hendra virus is contracted and bats can fly hundreds of kilometres in a few days – meaning that an apparent absence of bats on a property does not eliminate risk.
55 In addition, on the reverse page of the pamphlet, among other matters, there appeared:
MYTH: The vaccine isn’t safe
Data from the first 24,500 does of the Hendra vaccine administered to horses resulted in only 58 reports from horse owners and veterinarians, with 53 horses categorised as having had a side-effect. The majority of these reports involved injection site swellings, which is not uncommon with any injection in a horse. The adverse event rate to date is approximately 0.22%, placing it in-line with the expected adverse event rate for most vaccines. None of the side-effects reported were serious, and all resolved.
(emphasis added)
56 After about May 2013, and over 25,000 doses in the first six months of use, Zoetis issued an online module to already accredited vets entitled “NEW Hendra virus e-learning module: An update on Equivac HeV vaccine” (the registration module). The registration module stated that:
the module was designed to bring addressees up to date with recent developments on the duration of immunity, the safety, as well as the administration protocol, of Equivac HeV and an update on HeV itself;

Hendra Virus Update
As of 28th April 2013 there have been two confirmed cases of Hendra virus in 2013.
One horse was infected near Mackay, in January 2013.
One horse was infected in the Tablelands area of North Queensland in February 2013.
A Hendra-positive bat was discovered in Adelaide in January 2013, meaning Hendra positive bats are now known to be in six states or territories around Australia.

because clients heavily relied on a vet’s recommendation, vets should recommend vaccination to their clients by saying, among other things, that:
it was “the safest thing for your family and your horses”; and
“[t]he consequences of a Hendra virus outbreak are so severe, it isn’t worth taking the risk. Vaccination is a low cost for the security it provides”;
the AVA’s recommendation was to vaccinate all horses against HeV to protect them and humans from its potentially fatal outcome.
57 The registration module included multiple choice questions based on the information it contained, including about the adverse reaction rate from the first 25,000 doses.
5.6 The September 2013 seminar
58 In September 2013, Dr L’Estrange approved the 30 slides entitled “Hendra virus and the Hendra virus vaccine” for Zoetis staff to use in the September 2013 seminar, which included:
59 The applicant relied on the statement in the slide above, that the antibody had been detected in all four species of flying fox, as conveying the geographic spread representation.
60 The next slide was interactive and set out a timeline from the discovery of HeV in 1994 to 2013, which had details of occurrences of HeV including each location, the numbers of deaths and infected humans and horses in each occurrence and observations of symptoms. The following slide was as below:
61 Other slides described the research from which Equivac HeV resulted, the protocol for administering it, including reference to the first permit, Zoetis’ Health4Horses webpage, that enabled veterinarians to register and check vaccination of a horse using its microchip number, a summary of the results of clinical studies, details of the numbers of vets accredited to administer the vaccine and numbers of vaccinated horses. Zoetis presented the reader with further slides that recorded “current stakeholder positions”, including statements:
“The AVA believes that all horses should be vaccinated against the Hendra virus” under the heading “Reducing Hendra risk”,
recommending vaccination of horses by then Queensland Minister for Agriculture, Fisheries and Forestry, the Hon John McVeigh MLA, Racing Queensland, and the New South Wales and Queensland Governments’ biosecurity agencies, and
Equestrian New South Wales’ mandate that any horse attending any event in that State had to be vaccinated with Equivac HeV as of 1 January 2014.
5.7 The Health4Horses website information
62 On 10 October 2013, Zoetis posted on its Health4Horses webpage a page headed “Widespread Risk” with a subheading “Hendra Virus: Presumed Transmission Pathway” (the Health4Horses website information). The page reproduced a flying fox distribution map that was similar to those at [19] and [58] above. Above the map, this page stated:
Hendra virus can occur wherever there is overlap of flying foxes and horses. Because of the large distances that flying foxes travel, Hendra virus outbreaks could occur across a large proportion of the country.
(emphasis added)
63 Underneath the map, the page stated: “Bats carrying the Hendra virus are present in six states or territories around Australia”.
5.8 The every horse owner pamphlet
64 In December 2013, Zoetis published a pamphlet headed “Hendra Vaccination: What every Australian horse owner needs to know” (the every horse owner pamphlet). The pamphlet stated, among other matters:

65 After a page entitled “The enormous impact of Hendra virus” that discussed the fatality rate and health consequences for the three humans who survived the virus and the effect of infection on those in the equine industry, the next page appeared as below:

66 The remainder of the every horse owner pamphlet identified the signs of HeV in horses and humans and urged that vaccination was the best protection against it.
5.9 The second permit disclosure
67 The second permit continued the restrictions on the administration and use of Equivac HeV and the requirements to report and investigate fully all adverse reactions that the first permit had imposed. The second permit expanded the content of the leaflet under the side effects heading to the following:
Side Effects
Transient swelling may develop at the site of vaccination in some horses but should resolve within one week without treatment.
In some horses transient post-vaccination reactions including injection site reaction, pain, increase in body temperature, lethargy, inappetance [sic], and muscle stiffness have also been observed. Rarely reported symptoms have included urticaria, mild peripheral oedema and mild transient colic. Symptoms may vary in severity and on some occasions may require veterinary intervention.
Systemic allergic reactions such as anaphylaxis are thought to occur rarely with all vaccines and may require parenteral treatment with adrenaline, corticosteroid and antihistamine as appropriate and should be followed with appropriate supportive therapy.
(emphasis added)
68 Urticaria is hives or small swelling under the skin, and colic is a general term for abdominal pain. Oedema is swelling. I have discussed urticaria, colic and oedema below in dealing with the expert evidence at [240]-[251]. Inappetence is loss of appetite. The leaflet included substantially the same information as to the safety profile of Equivac HeV as in the first permit’s leaflet.
5.10 The facts about HeV pamphlet
69 In about December 2014, Zoetis published as part of its Health4Horses series a two page pamphlet headed: “Facts About: The Hendra vaccine” (the facts about HeV pamphlet). The applicant relies on this pamphlet as conveying the no serious side effects representation.
70 The pamphlet outlined on the left hand side of the front page the research studies and testing of Equivac HeV that provided the basis of the Authority’s grant of the permit for its use. It told the reader that in the field studies, “the only reactions seen were temporary injection site swellings in some horses”.
71 The front page then stated on its right hand side:

72 Across the foot of its front page, the pamphlet stated the following:
“The Hendra vaccine has been subject to the same level of rigorous safety and efficacy testing as other vaccines on the equine market.”
Dr Deborah Middleton: Research Team Leader and Senior Veterinary Pathologist, CSIRO
73 On the reverse page of the pamphlet, Zoetis explained that it had an obligation to report suspected reactions to the Authority and it advised horse owners to report to their vet, Zoetis or the Authority any suspected reaction to the Equivac HeV.
74 The reverse page had quotes attributed to, first, Dr Padraig Kelly, the resident veterinarian of Coolmore stud in the Hunter Valley in New South Wales and, secondly, Megan Jones, an Olympic silver medal winner, who operated a stud farm in the Adelaide Hills. Both recommended vaccinating horses with Equivac HeV. In his quote, Dr Kelly expressly acknowledged that the Hunter Valley “is not located in a high-risk area, [but] we deem it prudent to be proactive in terms of protecting against the risks posed by the disease”. The quote did not refer to any reaction of a horse at Coolmore stud. Ms Jones stated that in the Adelaide Hills, “there is a risk of Hendra virus from fruit bats. In addition, a number of our horses and our clients’ horses travel interstate for competitions, which also poses a risk”. She said that her stud was not prepared to take a risk of contracting HeV and that they had “seen only one minor site reaction, which was temporary and not threatening to the horse’s health”.
75 A summary at the foot of the reverse page of the facts about HeV pamphlet stated:

5.11 The third permit disclosure
76 The third permit again continued the restrictions on the administration and use of Equivac HeV as well as the requirements for Zoetis to report and investigate fully all adverse reactions that the first and second permits had imposed. The description of side effects that had to be stated in the leaflet was slightly, but not materially, different to that required in the second permit. I have highlighted the changes below, namely from “Rarely reported symptoms” to “Additional reported clinical signs”, “Symptoms” to “Clinical signs” and, where the ellipsis is in the last paragraph, the deletion from the side effects stated in the second permit leaflet in [67] above of “are thought to occur rarely with all vaccines and”):
Side Effects
Transient swelling may develop at the site of vaccination in some horses but should resolve within one week without treatment.
In some horses transient post-vaccination reactions including injection site reaction, pain, increase in body temperature, lethargy, inappetence, and muscle stiffness have also been observed. Additional reported clinical signs have included urticaria, sweating, mild peripheral oedema and mild transient colic. Clinical signs may vary in severity and occasionally may require veterinary intervention.
Systemic allergic reactions such as anaphylaxis […] may require parenteral treatment with adrenaline, corticosteroid and antihistamine as appropriate and should be followed with appropriate supportive therapy.
(emphasis added)
5.12 The Equestrian Life letter
77 The January 2016 issue of Equestrian Life Australia Magazine published Dr L’Estrange’s letter to its editor dated 2 November 2015. He appeared to be responding to a single critic’s views that the magazine had published in its November/December 2015 edition. Dr L’Estrange wrote:
Hendra virus is a deadly zoonotic disease that has killed four people and nearly 100 horses.
Its incidence is increasing. In the past five years, there have been more than 40 spillover events in Queensland and New South Wales. In every case, at least one horse dies. I have witnessed the devastating effects this virus has on those involved: the fear of exposure, an agonising 30-day wait to be cleared, and the regret expressed by owners that they didn’t protect themselves, their families, friends and horses with vaccination.
Hendra virus is rare, but its symptoms in horses are non-specific and easily mistaken for common conditions. Some horses appear to have colic, others may simply go off their feed or appear lethargic. There have even been infected horses without any noticeable symptoms. Veterinarians face the difficulty of diagnosis, suiting up and suiting down in full PPE in heat and humidity, unable to leave behind medications and warning owners to stay away from a horse that probably will not have Hendra but where the safety-first approach must be paramount. Communication from Queensland government authorities to veterinarians recently has been to the effect that “if Hendra cannot be ruled out, then it must be ruled in.”
…
In 2014, six horse owners had exposure to Hendra virus considered so extreme they were offered an experimental treatment in a Brisbane hospital. The treatment is administered intravenously, with adrenalin at the ready, in case of anaphylaxis.
This year, workplace health and safety prosecutions were commenced against three veterinarians who were accused of not maintaining a safe workplace. Horses they treated turned out to have Hendra virus.
Veterinarians face a real threat to their health and now a legal threat to their livelihoods when diagnosing and treating unvaccinated horses. Veterinarians, of course, are not the only people with workplace health and safety obligations, which is why sporting bodies, event organisers and others often conclude that vaccination is an obvious, sensible precaution.
The Hendra vaccine became available in late 2012 following ground-breaking work by the CSIRO, Zoetis and international research partners. As part of the original permit, veterinarians were required to report every suspected adverse event, and every dose has been documented and administered by a veterinarian.
Zoetis assesses each report received and classifies it as to the likelihood of the symptoms reported being attributable to vaccine administration. All reports are conveyed to the APVMA which makes an independent assessment.
(emphasis added)
78 Dr L’Estrange continued:
I have personally examined every adverse event report concerning the Hendra vaccine received by Zoetis. The majority are typical of the experiences many people have with human vaccines: temporary soreness or swelling around the injection site, sometimes a temperature, or being off colour for a day or so. Phenylbutazone (bute) is commonly recommended by veterinarians for the treatment of horses experiencing an adverse reaction or as a preventative for horses that have previously experienced a reaction, but is not recommended for horses that have not experienced a reaction.
Australia’s very thorough regulator had the benefit of seeing the adverse report data generated from 350,000 doses administered as part of the registration application. The APVMA was satisfied as to the safety of the vaccine and proceeded to register it. The vaccine is demonstrably safe and effective. It is widely used as a health and safety tool by owners, breeders and the mounted units of state police forces.
(emphasis added)
Everyone involved with horses in Australia is fortunate to have a safe, high quality vaccine to help protect against a deadly virus. The Hendra vaccine deserves wider use to protect horses, veterinarians, owners and their families.
Owners should speak with their veterinarian in order to make a sensible and informed decision as to whether to vaccinate their horses.
(emphasis added)
6. Was the geographic spread representation (there was a serious risk of horses contracting Hendra in all areas of Australia in which flying foxes were present) conveyed?
6.1 The applicant’s submissions
80 The applicant argued that each of the following six publications (the geographic spread publications) read separately and together conveyed the geographic spread representation, namely, the 2013 fact sheet, the March 2013 media release, the myth busting pamphlet, the September 2013 seminar, the Health4Horses website information, and the every horse owner pamphlet.
81 The applicant argued that each of the geographic spread publications misrepresented the nature and degree of the risk of horses contracting HeV. She contended that each of the six publications falsely asserted the magnitude of the risk as being “a serious risk of horses contracting Hendra in all areas of Australia in which flying foxes were present” (emphasis added). She contended that, as Professor Ward’s report made clear, the risk was extremely low outside the Hendra area, indeed, “miniscule”, being 1 or 2 in 1,000,000. She asserted that each of the six publications had overpitched the magnitude of the risk of horses catching HeV both in and out of the Hendra area. She argued that the referee’s finding that, even in the Hendra area, the risk of a horse being infected with HeV was only 1 or 2 in 10,000, and that in 25 years no horse outside the Hendra area had been infected, rendered the geographic spread representation misleading.
82 The applicant contended that in the myth busting pamphlet, Zoetis’ campaign reached its high watermark by impelling owners to “Vaccinate before it’s too late”, based on the fear that a horse could catch the virus anywhere in Australia and so put both the animal and those humans who came in contact with it at risk. The prompt to take immediate action, she claimed, was reinforced by the statement that wherever bats were the serious risk existed that they could carry and spread the virus.
83 The applicant also relied on the unqualified statement in the every horse owner pamphlet as to the areas in which bats can be found. That pamphlet was focused on, and directed to, horse owners.
84 The applicant claimed that each of the six geographic spread publications conveyed misleadingly that, wherever flying foxes were present in Australia, there was a serious risk that a horse could become infected with HeV and that the horse owner should therefore vaccinate their horse to prevent that serious risk maturing. She contended that the six publications conveyed that because all species of flying fox carried HeV, were thought to be the means by which horses became infected and were present throughout Australia, the risk of equine infection was “serious”. She submitted that the miniscule magnitude of the risk, as found by the referee, was never communicated to the public, including group members. She asserted that the group members had been “entitled to know the magnitude of the risk before they wasted their money” on paying for vaccination or treatment of horses that suffered an adverse side effect from it.
85 The applicant argued that because Chinchilla was just on the west side, but not west, of the Great Dividing Range, and was the place furthest to the west where there had been a confirmed HeV infection, it was misleading for Zoetis to represent that there was a serious or significant risk in the vast land mass to the west of the Great Dividing Range. She contended that it was syllogistic for the geographic spread publications to posit that, because HeV occurred naturally in flying foxes and they were present across most States and Territories, therefore there was a serious risk of infection wherever they were without adverting to the fact that no cases had been detected outside the Hendra area.
86 She argued that, for example, Agriculture Victoria had published “An Assessment of the Hendra virus Situation in Victoria” on 12 July 2011 in which it stated that there had been no cases of HeV infection in any horse in Victoria. The applicant asserted that, in approximately June 2013, the Chief Veterinarian of Victoria had asserted to a vet in private practice in Maffra, a dairy district in that State, that “IT WONT [sic] BE HENDRA, we don’t get hendra in Victoria”, as Dr L’Estrange had recorded in an email dated 26 June 2013 to officers in the Queensland Department of Agriculture, Fisheries and Forestry. She argued that the Victorian Chief Veterinarian “knew of the real level of risk” in that State and that Zoetis ignored this information in its publications, such as in the heading “Widespread Risk”, on the Health4Horses website information that it posted on 10 October 2013.
87 The applicant also contended that Zoetis did not have reasonable grounds for making the geographic spread representation to the extent that it was made with respect to any future matter within the meaning of s 4 of the ACL. The applicant argued that contemporaneous scientific literature, of which Zoetis was aware, confirmed that no cases of HeV had occurred outside the Hendra area and that the risk of the virus being transmitted (or there being a spillover) from flying foxes to horses or humans was sporadic and connected to an outbreak in a colony. She cited the abstract of a paper entitled “Hendra Virus Infection Dynamics in Australian Fruit Bats” by H Field and others published in December 2011. The abstract stated that the researchers’ findings showed that flying fox HeV excretions occurred periodically, “rather than continuously, and in geographically disparate flying fox populations in the state of Queensland”. The applicant argued that this, and similar publications which Dr L’Estrange had read before writing or approving the geographic spread publications, had identified HeV infections as only having ever occurred in the Hendra area, so that the generalised expression in those publications in respect of the risk of HeV infection wherever bats were found was misleading. She also contended that the geographic spread publications were misleading because there had been no known case of horse to horse transmission of HeV infection, yet the publications, such as the every horse owner pamphlet, suggested that this was possible (see [65] above). The applicant accepted that the transmission mechanism for HeV to horses was unknown, but she contended that the geographic spread publications’ “elevat[ion of] speculation to a statement of fact is misleading”.
88 The issue is whether the geographic spread publications convey that “there was a serious risk of horses contracting Hendra in all areas of Australia in which flying foxes were present”?
89 Mason J explained in Wyong Shire Council v Shirt (1980) 146 CLR 40 at 47-48, with the agreement of Stephen J and Aickin J, what the perception and evaluation of the nature of a risk involves in ordinary human experience (on which a jury would have to act) as:
The perception of the reasonable man’s response calls for a consideration of the magnitude of the risk and the degree of the probability of its occurrence, along with the expense, difficulty and inconvenience of taking alleviating action and any other conflicting responsibilities which the defendant may have. It is only when these matters are balanced out that the tribunal of fact can confidently assert what is the standard of response to be ascribed to the reasonable man placed in the defendant’s position.
The considerations to which I have referred indicate that a risk of injury which is remote in the sense that it is extremely unlikely to occur may nevertheless constitute a foreseeable risk. A risk which is not far-fetched or fanciful is real and therefore foreseeable. But, as we have seen, the existence of a foreseeable risk of injury does not in itself dispose of the question of breach of duty. The magnitude of the risk and its degree of probability remain to be considered with other relevant factors.
(emphasis added)
90 In my opinion, the 2013 fact sheet explained in a balanced way that the prevalence of the virus was low but that, if a horse became infected with HeV, there were life threatening consequences for the animal and any human who came into contact with it that had to be considered and prevented. It conveyed that the reason for this was not because, necessarily, there was a real possibility of a horse contracting HeV anywhere in Australia where flying foxes were present but because, although there was some possibility of that occurring, the gravity of the consequences were that event to occur would be severe.
91 The ordinary reasonable member of the class or other reader (whom I will describe in these reasons as the ordinary reasonable reader or the reader) would have read the 2013 fact sheet as a whole.
92 The reader would have read in it that since 1994, HeV had claimed the lives of 81 horses, which comprised 75% of the total number of horses infected. The 2013 fact sheet said in terms that “the prevalence is low” but that HeV “is one of Australia’s most lethal viruses”. The thrust of the 2013 fact sheet was that the risk of infection was small, but real, and connected to flying foxes in which HeV occurs naturally. The reader would have understood that HeV infections in horses and humans were very rare but were likely to be deadly when they did occur.
93 The 2013 fact sheet conveyed to the ordinary reasonable reader that the risks of equine HeV infection and potential horse and human transmission would be minimised by vaccinating horses with the new Equivac HeV. The reader would have understood the 2013 fact sheet to say that the magnitude of the risk maturing into an infection is very slight, but not fanciful or far-fetched, and that if there were an infection the consequences would be serious.
94 The ordinary reasonable reader would have understood that prior outbreaks of HeV had been the subject of considerable publicity and public concern but that those outbreaks were rare. The 2013 fact sheet accurately conveyed the consequences of past HeV outbreaks and the fact that while only 81 horses had died since 1994, over 30 of those deaths had occurred in the previous two years (in 2011 and 2012).
95 The ordinary reasonable reader of the 2013 fact sheet would have understood from it that, over the previous 18 years, the consequences of horses and humans contracting HeV were extremely grave and that the risk of that happening, while not large, was real wherever flying foxes could travel. This was the basis for the recommendation of Zoetis (then named Pfizer) and its co-publishers (the CSIRO, the AVA, Equine Veterinarians Australia and the two United States medical bodies) that all horses in Australia be vaccinated to protect humans from HeV’s potentially fatal outcome.
96 I reject the applicant’s argument that, based on the referee’s findings, the magnitude of the risk that a horse (or human) could contract HeV, even in the Hendra area, precluded any suggestion that the risk, in the sense of the magnitude of the risk occurring (as opposed to its consequences if it did), was “weighty” or “grave” or “threatening” (see the definitions of “serious” in Macquarie Dictionary Online: sense 5, Oxford English Dictionary online: senses 3a, b).
97 In my opinion, the 2013 fact sheet did not convey the geographic spread representation. That is because the ordinary reasonable reader would not have understood it to be saying that the magnitude of the risk of horses contracting HeV in all areas of Australia was a “serious risk” in the sense which the applicant asserted. The publication conveyed in a balanced way that there was a real, albeit very low, risk but it was one which merited the precaution of vaccination rather than taking the chance of the potential serious or dire consequences of inaction.
6.2.2 The March 2013 media release
98 The March 2013 media release made the point that HeV continued to remain a health concern and “an unpredictable virus that can cause disease and death in both humans and horses.” Equivac HeV was “crucial to breaking the cycle of Hendra virus transmission from flying foxes to horses and then people”. Zoetis said in it that it supported the recommendation of the AVA that all horses ought be vaccinated (as set out at [52] above).
99 The ordinary reasonable reader would not have understood the March 2013 media release as conveying that there was a “serious risk”, in terms of magnitude of the likelihood of horses catching the disease, in the sense on which the applicant relied.
100 I am of opinion that the applicant’s reading of the 2013 March media release is strained and far-fetched. The reader would have understood it to convey that inoculating horses with Equivac HeV would provide protection against the possibility that, although unpredictable, HeV could infect them and, through that mechanism, humans. The reader would not have read it as conveying that there was a “serious” risk of a horse contracting HeV.
101 It is important to distinguish between what is a real, foreseeable risk of a serious consequence occurring if a horse became infected with HeV, on the one hand, and the magnitude or degree of probability of that risk occurring, on the other. This is the point that Mason J made in Shirt 146 CLR at 47-48 in the passage I have quoted in [89] above. The applicant’s attempt to discern the geographic spread representation as conveyed in the March 2013 media release (and the other geographic spread publications) distorts the distinction. The publication was stating a truism in recounting to the reader that HeV is a virus that can cause disease and death and, as such, is a serious matter of concern for persons employed in the equine industry in Australia.
6.2.3 The myth busting pamphlet
102 The myth busting pamphlet stated in terms on the first page (see [53] above):
There are many unknowns about how Hendra virus is contracted and bats can fly hundreds of kilometres in a few days – meaning that an apparent absence of bats on a property does not eliminate risk.
(emphasis added)
103 The applicant also relied on the injunction to “Vaccinate before it’s too late” as conveying that there was risk of considerable magnitude in all areas in which flying foxes were present.
104 In my opinion, the ordinary reasonable reader of the myth busting pamphlet would have understood it to be conveying that, in terms of the old adage, it is better to be sure than sorry. The pamphlet made clear that there were many unknowns about how HeV is contracted. It stated the fact that bats are found in, and can travel across, large areas of Australia where horses could be present.
105 The reader would also have been aware, particularly since a horse owner is likely to have followed stories about HeV and horses over the course of the previous 18 years, that the virus had caused known infections very rarely but that on those occasions there had been deleterious consequences.
106 In that context, the reader would have understood the pamphlet to be doing no more than asserting, as an advertisement by Zoetis, that, now that a vaccine was available, it was better to vaccinate one’s horse now rather than to regret the realisation of the risk that HeV might appear on the owner’s property and affect his or her horse. The reader would not understand the myth busting pamphlet to be saying that there was a risk of considerable or significant magnitude in all areas of Australia that horses would contract HeV. Rather, it was saying that no one knows whether or not the virus is able to be transmitted in all areas of Australia where there are bats, so that, as a matter of common sense, it would be prudent to use the new vaccine that could eliminate the risk entirely.
6.2.4 The September 2013 seminar
107 The slides, read as a whole, conveyed to the ordinary reasonable reader that “Australian flying fox populations coincide with the areas of greatest risk for Hendra virus” and that flying foxes were a natural host for HeV. The slides informed readers, accurately, that HeV antibodies are present in all four mainland species of flying fox, but that there was no definitive data on how the disease spread from bat to bat; no data as to how it spread from bat to horse and no evidence of it spreading from bats to humans. The seminar slides set out a brief history of the virus from 1994 to a case that had appeared in 2013 in Mackay in the Tablelands area of Queensland. It noted that there had been about 30 occurrences affecting a total of around 80 horses over the 19-year timeframe including two large outbreaks, one in 1994 in which 14 of the 20 horses affected died, and one of the two people infected had died, and the second in 2008 in Redlands in Queensland where four of the five horses infected had died and one of two people infected had died.
108 The slide headed “What is the risk of a horse being infected with Hendra virus?” (see [60] above) stated that there were approximately 900,000 horses in Australia, 90 cases since 1994, with an 80% fatality rate, and other horses had had to be euthanised that had come into contact with the deceased horses.
109 The ordinary reasonable reader of the September 2013 seminar slides would not have understood them to convey that there was a substantial or serious risk that horses could contract HeV in all areas of Australia. Such a construction would be fanciful. The reader would know that, if there had only been about 90 cases in 30 outbreaks, including the two larger ones in Hendra and Redlands, over the preceding period of 19 years, equine HeV infection was an extremely rare event, apparently somewhat random and connected to the presence of flying foxes in the area. The ordinary reasonable person would not have understood that the September 2013 seminar conveyed the geographic spread representation.
6.2.5 The Health4Horses website information
110 The Health4Horses website information had a heading “Widespread Risk” and a subheading “Hendra Virus: Presumed Transmission Pathway” that informed the reader that HeV “can occur wherever there is overlap of flying foxes and horses” and that, because flying foxes have a wide geographic presence, outbreaks of HeV could occur across a large proportion of Australia.
111 Those statements were accurate and did not inflate, to “a serious risk”, the magnitude of the real and foreseeable possibility that horses could contract HeV. Rather, as with the other geographic spread publications, the website information conveyed, in a balanced way, that there was a real, but unpredictable, risk of an outbreak of HeV where flying foxes were present and the reader might wish, as a matter of common sense, to guard against it by vaccinating his or her horses.
6.2.6 The every horse owner pamphlet
112 The every horse owner pamphlet identified that HeV had caused over 90 horse deaths (directly or because of the need to euthanise) and that, of seven people infected, four of them had died.
113 The ordinary reasonable reader of the pamphlet would not have understood it to convey that there was a serious risk throughout Australia of horses contracting HeV. Like the other geographic spread publications, the pamphlet was factually accurate in stating how all species of flying fox carried HeV, outbreaks were rare and had occurred sporadically but randomly in Queensland and more recently in New South Wales and west of the Great Dividing Range. The reader would have understood that, among other things, the pamphlet conveyed that movement of horses could also pose a risk of spreading infection from HeV. It quoted Associate Professor James Gilkerson, the director of the Centre for Equine Infectious Diseases at the University of Melbourne, as saying:
This is an endemic disease. It is not going away … The best way to protect ourselves and the best way to protect our horses is to have the horses vaccinated.
(emphasis added)
114 In my opinion, that was a statement of obvious common sense and captured what the ordinary reasonable reader of the pamphlet would have understood it to be saying, namely, that as a matter of common sense, Equivac HeV could eliminate any risk of the uncommon and random chance that there could be an outbreak of HeV which would put not only the horses but humans who came into contact with them in peril of serious consequences, including death.
115 For the reasons above, I am not satisfied that any of the geographic spread publications, read in isolation or together, conveyed the geographic spread representation.
116 I reject the applicant’s argument that, because the geographic spread publications failed to convey to the reader that between 1994 and 2012, there had been no case of any horse contracting HeV outside the Hendra area, the absence of such a qualification would have led the reader to believe that the magnitude of the risk was serious, being far greater than was the reality.
117 If I am wrong and the omission of some further definition of the risks in and out of the Hendra area, of the nature that the referee’s report specified, in any of the geographic spread publications caused the geographic spread representation to be conveyed, that omission could not amount to conduct that contravened s 18(1) of the ACL. That is because, in making a considered decision whether to vaccinate one or more horses, an owner or a vet or other group member can be expected to do more than act on the vaccine vendor’s self-promotion. An ordinary reasonable member of the class is not a person who would make an impulsive decision to vaccinate his or her horse without reflection or thought.
118 A consumer who looks at furniture that resembles another manufacturer’s product can be expected to look more closely for any distinguishing marks or labels as part of making a considered decision whether to buy. The person cannot say that he or she was misled by a mark where the label identifies the maker: Puxu 149 CLR at 199, 210-211. As Mason J said of such a decision (at 211):
taking into account the importance both financially and aesthetically of the relevant furniture, my conclusion is that a purchaser who considered it important to acquire Post and Rail “Contour” furniture could reasonably be expected to look for and find the label or to take comparable steps, such as inquiring of a salesman, to ensure that furniture from the “Contour” range was being purchased.
(emphasis added)
Similarly, Gibbs CJ said (at 199):
Although it is true, as has often been said, that ordinarily a class of consumers may include the inexperienced as well as the experienced, and the gullible as well as the astute, the section must in my opinion be regarded as contemplating the effect of the conduct on reasonable members of the class. The heavy burdens which the section creates cannot have been intended to be imposed for the benefit of persons who fail to take reasonable care of their own interests. What is reasonable will of course depend on all the circumstances. The persons likely to be affected in the present case, the potential purchasers of a suite of furniture costing about $1,500, would, if acting reasonably, look for a label, brand or mark if they were concerned to buy a suite of particular manufacture.
The conduct of a defendant must be viewed as a whole. It would be wrong to select some words or act, which, alone, would be likely to mislead if those words or acts, when viewed in their context, were not capable of misleading.
(emphasis added)
119 In my opinion, the geographic spread publications conveyed only that there was a real risk of horses contracting HeV in all areas of Australia in which flying foxes were present. As Professor Ward’s report found, that was correct, bearing in mind that the risk was nonetheless real and could be guarded against. The publications did not convey the magnitude of the risk as “serious” or suggest that there was any significant probability of it occurring. Rather, the thrust of the geographic spread publications’ discussions of the risk was to recognise its unforeseeability, the randomness and rarity of its occurrence over the preceding years since 1994 in juxtaposition with the very serious consequences that HeV infection posed for horses and humans if and when it did occur.
120 It is ordinary human experience to recognise that some situations can arise that are quite unlikely to occur yet are foreseeable and likely to have serious consequences if they did. Sharks can, and very rarely do, attack swimmers at Sydney or other beaches, and when they do it is often with fatal consequences. Despite knowing the potentially devastating consequences of a shark attack if it were to occur, most people make a considered decision to swim, relying on what they understand are precautions that the authorities have put in place. Likewise, statements about the seriousness of diseases like smallpox or poliomyelitis and the availability of vaccinations that can immunise persons against catching them, do not convey, without more, that there is a serious risk of contracting the disease without vaccination. Rather, the ordinary reasonable person would understand that vaccination offers protection against a real, even if unlikely or remote, risk of contracting such a disease that would have serious consequences. Those risks can be contrasted with the recent COVID-19 pandemic where the risk of contagion among the population was very high and immunisation offered only some protection against being infected or, if one was, of serious health consequences from it. The geographic spread publications did not convey that the risk of equine or human HeV infection was in, or anywhere in the nature of, the latter class of risk.
121 The September 2013 seminar expressly identified when and where outbreaks had occurred. The 2013 fact sheet and the every horse owner pamphlet told the reader the statistics, citing the small numbers of infections and deaths of humans and horses over the preceding 19 years. The other geographic spread publications did not become misleading by reason of the applicant’s assertion that they needed to contain, but omitted, the qualification that outbreaks had not occurred outside the Hendra area. First, the ordinary reasonable reader would be likely to be aware of any outbreaks of HeV locally to him or her, given their rarity. Secondly, until early August 2015, Equivac HeV could only be administered by an accredited veterinarian. Any horse owner or person considering the vaccination of a horse would need to engage the services of, and could be expected to seek advice from, such a veterinarian as to the need for, or the advisability of, vaccinating the horse. Here, an owner, who, acting reasonably, was concerned as to the proper veterinary treatment of his or her horse, would enquire of the vet about the risk of HeV contagion in the circumstances of that owner, which would include the horse’s location. Thirdly, a horse owner or person with responsibility for making decisions about whether to vaccinate a horse who read any of the geographic spread publications could be expected to take reasonable care of his or her own interests in deciding whether to do so: Puxu 149 CLR at 199, 210-211, Nike 202 CLR 84-85 [100]-[103]. That reasonable deliberation would include the person assessing the need to vaccinate, and the cost and advisability of doing so, based on veterinary advice in the location and circumstances of the owner and the horse.
122 For the reasons I have given, none of the geographic spread publications conveyed the geographic spread representation. The express or implied omission from them of the specific information that no cases of HeV had occurred outside the Hendra area did not cause any of the geographic spread publications to convey that representation.
7. Was the all horses representation (all horses in Australia should be treated with Equivac HeV) conveyed?
123 It is convenient at this point to deal with the all horses representation. That is because the issues relevant to the no side effects representation involve detailed consideration of, among other matters, the conditions exhibited by Ms Abbott’s and Ms Hinton’s horses and the expert evidence.
124 The applicant alleged that the all horses representation was conveyed by each of the 2013 fact sheet, the March 2013 media release, the registration module and the Equestrian Life letter (the all horses publications). She pleaded that the all horses representation contravened s 18(1) of the ACL because the risk of a horse becoming infected was low in the Hendra area and extremely low outside it.
125 Zoetis argued that none of the all horses publications conveyed the all horses representation. It contended that if that representation was conveyed, first, it was a statement of opinion, or secondly, it was not misleading or deceptive or likely to mislead or deceive. Rather, it contended that it had simply passed on statements by the AVA or others about the desirability of vaccinating horses.
126 In her opening submissions, the applicant argued that the all horses representation would contravene s 18(1) of the ACL if the serious risk element of the geographic spread representation were incorrect. She did not devote much argument separately to the all horses representation in closing. She contended that Professor Ward had found that of the 60 occasions of HeV infection since 1994, there was evidence only in two of likely horse to horse transmission, namely in the outbreaks at Hendra in 1994 and at Redlands in 2008. She submitted that, because of Professor Ward’s finding that HeV is not highly contagious, horse to horse transmission is rare and the capacity for an infection to spread is limited. She argued that because the mechanism for transmission is unknown, one possible route was through shared equipment, which could account for the above two outbreaks.
127 I reject Zoetis’ argument that the all horses representation was not conveyed by it in the 2013 fact sheet, the March 2013 media release, and the registration module. Zoetis was the publisher of each of the four all horses publications. It did so with a view, among others, to informing the potentially consuming public and, relevantly, group members, of the virtues of the vaccine that it was marketing. It did not distance itself from the recommendations of others to vaccinate; rather, it was using those recommendations and adopting them for itself as part of the promotion of its product.
128 In Webb v Bloch (1928) 41 CLR 331 at 363-364, Isaacs J discussed the common law rules as to who is a publisher of allegedly defamatory matter. Like the tort of defamation, liability for a contravention of s 18(1) of the ACL by engaging in conduct that conveys a representation that is misleading does not depend on any intention of the person who engaged in the conduct to convey the representation. As Kiefel CJ, Keane and Gleeson JJ held in Fairfax Media Publications Pty Ltd v Voller (2021) 392 ALR 540 at 548 [32] (see also per Gageler and Gordon JJ at 554 [68]), applying the principle that Isaacs J had explained:
a person who has been instrumental in, or contributes to any extent to, the publication of defamatory matter is a publisher. All that is required is a voluntary act of participation in its communication.
129 The 2013 fact sheet stated in terms (at [51]) that “it is strongly recommended that all horses in Australia are vaccinated against Hendra virus to protect humans from its potentially fatal outcome”, which Zoetis told the reader was “in keeping” with the AVA’s policy briefing on Equivac HeV. In so doing, Zoetis was putting that statement forward as its recommendation.
130 Similarly, in the March 2013 media release, Zoetis expressed its support for what it communicated to readers was the AVA’s recommendation (see [52]). That was also a publication by Zoetis of the all horses representation.
131 In the registration module, Zoetis advocated that vets should tell their clients that “[v]accination is the safest thing for your family and your horses” and it was not worth taking the risk of the severe consequences of infection with HeV. Zoetis told the vets who were its readers of the AVA’s strong recommendation to vaccinate “all horses in Australia” (see [52] above). Zoetis conveyed the all horses representation in the registration module, read as a whole through those statements.
132 The ordinary reasonable reader would not have understood that Zoetis was simply passing on, without endorsing, information about the AVA’s views that all horses should be vaccinated: cf Yorke v Lucas (1985) 158 CLR 661 at 666 per Mason ACJ, Wilson, Deane and Dawson JJ; Butcher v Lachlan Elder Realty Pty Ltd (2004) 218 CLR 592 at 605 [38]-[39] per Gleeson CJ, Hayne and Heydon JJ. Here, Zoetis was a veterinary pharmaceutical manufacturer that had brought Equivac HeV to market and it could be assumed to know its properties, likely efficacy and purpose. Zoetis was in the business of marketing its product and, clearly enough, the AVA’s recommendation was a good selling point for it which the reader would have understood in the context of the registration module read as a whole, as a statement that Zoetis adopted.
133 However, I am not satisfied that Zoetis conveyed the all horses representation in the Equestrian Life letter. That is because the reader would understand it to be conveying Zoetis’ or Dr L’Estrange’s response to criticism of Equivac HeV. Dr L’Estrange sought to demonstrate in this letter: first, the safety and efficacy of the vaccine, and secondly, the understandable propensity of horse owners to confuse the non-specific symptoms of HeV with common conditions suffered by horses.
134 The reader would not have understood the letter to be asserting that every horse in Australia should be vaccinated, as opposed to advocating the vaccine’s “wider use to protect horses, veterinarians, owners and their families”, about which Dr L’Estrange said in the concluding sentence (see [79]): “Owners should speak with their veterinarian in order to make a sensible and informed decision as to whether to vaccinate their horses”.
135 Read as a whole, the Equestrian Life letter would not have conveyed to the reader that all horses should be vaccinated. Rather, the reader would have understood from it that the vaccine was safe, had been rigorously tested, had obtained regulatory approval and gave protection to horses and those who came into contact with them from HeV, but an owner should make any decision about whether to vaccinate after obtaining expert advice about his, her or its individual circumstances. No doubt, the reader would perceive that the article was saying that vaccination was a sensible course. But, he or she would not have understood it to be saying that it was the only option, especially since it left open that consultation with a vet may result in “a sensible and informed decision” that, in the owner’s particular circumstances, vaccination might not be necessary. After all, the letter was not directed to urging vaccination but rather was concerned to defend Equivac HeV from an apparent attack by the single critic to whose complaints Dr L’Estrange had referred.
7.2.1 Was the all horses representation misleading?
136 The applicant pleaded that the all horses representation was misleading because:
the risk of a horse becoming infected with HeV in the Hendra area was low and elsewhere, where there were flying foxes, was extremely low;
the risk of a person becoming infected with HeV as a result of interacting with an infected horse was low; and
the side effects of Equivac HeV included serious and potentially debilitating adverse reactions.
137 As I explain below in dealing with the no serious side effects representation, there were no serious or debilitating adverse reactions to, or side effects of, Equivac HeV.
138 The fact that the risks of spillover from bats to horses and horses to humans were low did not render the all horses representation misleading. Dr L’Estrange knew in 2013 that the governmentally mandated Australian Veterinary Emergency Plan (Aust Vet Plan) stipulated that any animal that had been infected with HeV had to be destroyed regardless of whether it had recovered from the disease. Experience by 2012 had shown that the risk from HeV was real, foreseeable and, if it came to pass, potentially fatal for humans and, until about 2016, because of the Aust Vet Plan requirement for euthanising any horse that came into contact with an infected horse, always fatal for horses. As Mason J explained in Shirt 146 CLR at 47-48, in the passage I have cited at [89] above, the evaluation of how to respond to a reasonably foreseeable risk of harm involves elements of judgment in which a reasonable person must balance the degree of probability of the risk eventuating with any possible alleviating action, including the cost, if any, of taking such a step.
139 When imposing a duty of care, a court must identify an objective answer to whether a reasonable person would have acted in a particular way in responding to a foreseeable risk. However, that cannot be the basis on which a court evaluates whether a person in Zoetis’ position has a reasonable basis to express an opinion about the advisability of vaccinating horses to prevent the possibility of fatal consequences to horses and humans.
140 There is a difference between fact and opinion. Opinions about the same subject can be reasonable but diametrically (or to a lesser degree) opposed, as is fundamental in political and intellectual debate. A person on one side of an issue can think the other view is wrong, and vice versa, while all (or at least all reasonable people) would accept that each side has a reasonable basis for its view. Ordinarily, the nature of opinion is that it reflects belief, based on the selection of some facts or theories, which admit of different viewpoints or outcomes and about which there is no single demonstrably correct answer.
141 Here, the all horses representation, as pleaded, namely “all horses in Australia should be treated with Equivac HeV”, would be understood by the reader of the relevant publication in which it was conveyed as the publisher’s (Zoetis’) exhortation or opinion that vaccination ought to occur because it is a good idea or precaution. That was the context in which each of the 2013 fact sheet, the March 2013 media release and the registration module conveyed that representation. And they did so in the context that the ordinary reasonable reader would have appreciated that Zoetis was not only the publisher but also the manufacturer or supplier of Equivac HeV and had an interest in promotion of its commercial success and sale.
142 The 2013 fact sheet informed the reader that the prevalence of HeV was low, only 81 horses had died in the previous 19 years and of the seven humans infected, four had died. It told the reader that HeV occurred naturally in flying fox populations in most Australian States and Territories, so that there was potential for the disease to spread wherever they were found. The 2013 fact sheet culminated with endorsing the AVA’s policy briefing by saying “it is strongly recommended that all horses in Australia are vaccinated against Hendra virus to protect humans from its potentially fatal outcome” (emphasis added).
143 The 2013 media release was self-evidently a promotion of Equivac HeV by Zoetis, under its new name. The opening paragraph emphasised to the reader Zoetis’ intention to educate the industry “on the vaccine’s safety profile and its role in reducing the risk of Hendra virus infection” (emphasis added). The reader would understand from it that Zoetis and the CSIRO had studied the immunity given by, and safety of, Equivac HeV, before he or she read the concluding two paragraphs (set out at [52] above) which included Zoetis endorsing the AVA’s recommendation that all horses in Australia be vaccinated.
144 Again, the ordinary reasonable reader would have understood the 2013 media release as an opinion by Zoetis and as self-endorsement of its product.
145 The ordinary reasonable reader of the registration module necessarily was a professional veterinarian who had or was seeking accreditation to administer Equivac HeV. That reader could not have mistaken, as wholly objective or factual, the puffery and sales pitch nature of some of the material including the following slide:

146 The ordinary reasonable reader of this material, being a veterinarian, would be expected to make his or her own professional appraisal of the necessity or advisability of administering any form of treatment, including Equivac HeV, to a horse (or other animal) before doing so, having regard to all relevant circumstances. Of course, he or she would also be expected to give serious consideration to the AVA’s recommendations as well as the manufacturer’s recommendations as to the circumstances, functionality and use of the vaccine.
7.2.2 Was the all horses representation conveyed as a statement of Zoetis’ opinion?
147 I am satisfied that, in reading each of the 2013 fact sheet, the March 2013 media release and the registration module, being the three publications that conveyed the all horses representation, the reader would have understood it to be an expression of an opinion by Zoetis as to a desirable course of action to guard against the risks of HeV to humans and horses.
148 The ordinary reasonable reader would have understood the nature of the all horses representation as an opinion conveyed in Zoetis’ promotional materials for its own products. He or she would have appreciated that there was a degree of puffery inherent in that expression of opinion. But, the reader would also have understood that the opinion related to dealing with the risk of contracting HeV, however small or remote, that existed in the context where knowledge of the existence of the disease had only emerged in the previous 19 years and not a great deal was known of how it was transmitted. The reader would have understood that the all horses representation conveyed Zoetis’ opinion that use of the Equivac HeV on all horses in Australia would give persons who could come into contact with horses, including vets, certainty that they would not be exposed to any risk of catching HeV.
7.2.3 Did Zoetis have reasonable grounds for making the all horses representation?
149 A statement of opinion is a representation of the maker’s state of mind, and when made by an expert, such as Zoetis was in respect of animal health products, including vaccines, comes with an implication that the maker had reasonable grounds for holding it: RAIA Insurance Brokers Ltd v FAI General Insurance Co Ltd (1993) 41 FCR 164 at 165-166 per Davies J, 175 per Beaumont and Spender JJ.
150 The all horses representation was also a representation with respect to a future matter, namely what should occur to all horses is that, in the future, they should be vaccinated. That attracts the operation of s 4 of the ACL. Zoetis had an evidential burden to adduce some evidence of reasonable grounds for making the representation (which, of course, can be the same as those on which it based the opinion). Once Zoetis adduced some evidence of its reasonable grounds, which it did, as I explain below, the dispositive burden of proof, that on the whole of the evidence, there were no reasonable grounds, fell onto the applicant, as Mansfield, Greenwood and Barker JJ held in North East Equity Pty Ltd v Proud Nominees Pty Ltd (2012) 285 ALR 217 at 224-225 [30]-[34]. As they said of s 51A(1) of the Trade Practices Act 1974 (Cth), which is an analogue of s 4(1) of the ACL (285 ALR at 224 [29]):
the relationship between the burden cast upon the respondents to adduce evidence of reasonable grounds, and the dispositive burden upon the appellant, once the respondents had adduced evidence of reasonable grounds, was important in the present case. When relying upon s 51A(1), taken alone, to establish a contravention of s 52, two integers must be satisfied by an applicant. The first is that the representation relied upon must be a representation with respect to a future matter, and the second is that the representor did not have reasonable grounds for making the representation.
(emphasis added)
151 I am satisfied that Zoetis (through Dr L’Estrange) had reasonable grounds for making the all horses representation in the three publications in which it was conveyed. I am not satisfied that in making the all horses representation Zoetis engaged in conduct that contravened s 18(1). That is because the all horses representation was an expression of an opinion and was based on reasonable grounds.
152 Dr L’Estrange gave the following evidence as to why he supported the view of the AVA:
Because I felt that any horse in Australia could potentially be exposed to Hendra virus either directly from flying foxes or indirectly via an infected horse that might travel. You might remember … the incubation period for Hendra virus is up to 16 days, which is plenty of time for a horse to catch the virus and then move to a different part of the country, perhaps a part of the country that doesn’t have flying foxes even, and then be infectious to either a human or to another horse. And so I felt that it wasn’t just the areas that had flying foxes that were potentially at … risk, and so that led to encompassing all horses in Australia.
(emphasis added)
153 The all horses representation also reflected the opinion or recommendation of the AVA (in each of the 2013 fact sheet, the 2013 media release, and the registration module) and, so far as it was conveyed by the 2013 fact sheet, the opinion held also by other apparently expert bodies, namely the CSIRO, AVA, Equine Veterinarians Australia and the two United States medical research bodies, which joined Pfizer in endorsing it.
154 During the relevant period:
on 16 August 2012, the AVA issued a policy briefing that expressed as its policy the all horses representation, namely “The AVA strongly recommends that all horses in Australia are vaccinated against Hendra virus to protect them and to protect humans from its potentially fatal outcome”;
in May 2013, Workplace Health and Safety Queensland issued a statement, “Hendra virus – information for horse properties and other horse businesses”, that stated “[t]he vaccine is the single most effective way of reducing the risk of Hendra virus infection in horses”. The Queensland Government reaffirmed that this was the position that it promoted in response to a 21 October 2016 report tabled by the Agriculture and Environment Committee of the Queensland Parliament;
on 30 July 2013, the board of Equestrian NSW issued a policy that, effective on 1 January 2014, all horses attending any event in New South Wales had to be vaccinated and strongly encouraged its members “to vaccinate ALL their horses … if they are at risk. Risk factors include: living in a bat habitat area, humans having close contact with their horses and/or competing against horses from other areas or with unknown vaccination status”;
in August 2013, the NSW Department of Primary Industries published the eighth edition of its “Primefact” on HeV. It noted that since 1994 the majority of HeV cases had occurred east of the Great Dividing Range with only one to the west, at Chinchilla in Queensland, in July 2011. It noted that incidents were sporadic but in 2011 there were 18 in northern NSW and south-east Queensland and in 2012 cases had occurred north of Rockhampton. It said that HeV had also infected dogs (which had not shown signs of illness) on properties with infected horses. It said that horses could be infectious before showing signs of illness and that HeV “vaccination is the single most effective step horse owners can take to reduce the chance of infection”. The Department repeated its advice in a biosecurity bulletin issued on 4 September 2015.
155 Moreover, each of the three expert witnesses, Associate Professor Sykes, Dr El-Hage, and Professor Slater, said that the efficacy of Equivac HeV was excellent. In their joint report they opined that, based on its formulation as a subunit protein vaccine with an ISCOM adjuvant, “we would expect it to be a relatively safe vaccine with relatively few side effects” and considered that Equivac HeV to be at the safer end of the spectrum compared with other vaccine technologies.
156 Dr L’Estrange held a similar opinion about the vaccine. He considered that it was common sense to vaccinate all horses with such a vaccine to eliminate the risk of HeV infection as he expressed in the Equestrian Life letter. I accept his evidence generally.
157 I found Dr L’Estrange to be a careful, honest witness having had the opportunity to observe him giving evidence over five days. When he became responsible for Pfizer’s equine portfolio in 2012, which included Equivac HeV, he made it his business to read whatever scientific literature he could that dealt with HeV, including from Dr Deborah Middleton, the Chief Scientist of the CSIRO animal health laboratory in Geelong, Dr Peter Reid, who had been the attending veterinarian for the original 1994 HeV outbreak and who had since published research on HeV, Biosecurity Queensland and from online materials, such as Google Scholar alerts that provided Dr L’Estrange with access to the latest published literature on HeV and a related virus that occurs in Asia, called Nipah virus. Dr L’Estrange communicated with Dr Chris Broda, an American expert, who helped develop Equivac HeV and who was studying Nipah virus. Dr L’Estrange also regularly attended professional seminars, lectures and conferences to keep himself informed throughout the relevant period. He had had experience with other animal vaccines when in private practice.
158 Dr Middleton was one of the co-authors of the 2011 paper “Hendra virus: what do we know” Vol 22 (5-6) NSW Public Health Bulletin 118, which Dr L’Estrange reviewed at the time that he familiarised himself with his new responsibilities in 2012. He understood that paper to state that spillover of HeV could occur from all four species of flying fox within their range that spanned over most States and Territories as depicted in a map in the paper similar to that at [19] above. The abstract of that paper stated:
Hendra virus infection is an emerging infectious disease that is not well understood. Most cases of Hendra virus infection have occurred in Queensland, with one case in a horse in NSW. Hendra virus infection has a high mortality rate in horses and humans and as cases could occur anywhere in Australia it is important to be ready for prompt action should an outbreak occur in NSW. This paper: reviews the current knowledge on Hendra virus infection including methods for preventing the disease; explains the animal health and human health response for an outbreak within NSW; and discusses possible future avenues for post-exposure prophylaxis and prevention by vaccination.
(emphasis added)
159 The applicant argued that other parts of Dr Middleton’s paper described the risk of spillover of HeV infections from each of bats to horses and horses to humans or horses as rare. However that argument does not negate the logic or reasonableness of what each of the 2013 fact sheet, the March 2013 media release and the registration module was conveying were the grounds in support of the reasons it gave for the all horses representation.
160 The paper stated, in its text, that HeV infection “could theoretically occur anywhere in Australia where there are Flying-foxes” and cited as the source of that statement a May 2010 publication by the South Australian Department of Primary Industry and Resources. The paper concluded saying that HeV “is an emerging infectious disease that is still not well understood”.
161 On 21 January 2013, an article appeared in The Land reporting that tests on 100 dead flying foxes found in North Adelaide revealed that they had HeV. Dr L’Estrange said that he reviewed the article and information in it in January 2013. He also understood that there was an incubation period of up to 16 days for HeV to manifest. Dr L’Estrange said that, as a result, “there’s also the possibility of horses becoming infected without anybody realising it and then those horses then travelling to areas of the country where there are no flying foxes and them becoming unwell and then, potentially, infecting people or other horses”. That was a common sense appreciation of a real risk about HeV and its potential for transmission in circumstances where little was known about the mechanisms of transmission of the virus by horses, other than the fact that there have been cases of infection of more than one horse on the same property and humans at a property where there was an infected horse.
162 The referee found that, objectively, the risk of horses contracting HeV outside the Hendra area was extremely low or about 1 to 2 per 1,000,000. HeV is spread by mechanisms not fully understood.
163 Over the course of the relevant period, more cases outside the Hendra area were reported and became known, for example, the infected bats found in Adelaide in January 2013. As some of the all horses publications noted, and as a matter of common knowledge, horses are mobile and can be moved all over Australia, including to Tasmania. The evidence showed that persons such as Ms Abbott needed their horses for their work and, as they needed, took them to various parts of the country. Others, such as racehorses, are transported frequently interstate. And, of course, the original outbreak in Hendra involved a racing stable. Horses are also moved from one part of the country to another for the purposes of shows or sporting competitions, such as equestrian events.
164 The ordinary reasonable reader of the 2013 fact sheet, the March 2013 media release and the registration module would have understood that if, in some way, horses or equipment on which their bodily fluids might be present can travel long distances within Australia, it was reasonably possible for those horses or the equipment to come into contact with other horses or humans in a different location, as a result of which there was a potential for HeV to spread. The reader would have appreciated that, because so little is known about how HeV is transmitted, a recommendation or opinion in terms of the all horses representation was, first, common sense and, secondly, not a statement of fact.
165 I accept Dr L’Estrange’s evidence that he believed in that opinion. I am satisfied he had reasonable grounds for doing so. I do not consider that the ordinary reasonable reader of any of the 2013 fact sheet, the March 2013 media release or the registration module would have understood it to convey more than that Zoetis was making a common sense recommendation, as a statement of its opinion, including where the relevant publications contained others’ opinions, such as that of the AVA (and Zoetis’ adoption of that opinion as its own).
166 The fact that others may have had a different opinion did not preclude Zoetis from having the opinion that it expressed in the three publications in which it expressed the all horses representation. I am satisfied, for the reasons above, that the material in evidence provides ample reasonable grounds on which Zoetis and Dr L’Estrange (on its behalf) acted in making the all horses representation, including to the extent that it may have involved, or been with respect to, any future matter for the purposes of negating the presumption in s 4(1) of the ACL. I am comfortably satisfied that Zoetis and Dr L’Estrange had reasonable grounds for making the all horses representation at the time and in the circumstances that they published the 2013 fact sheet, the March 2013 media release and the registration module. In any event, the applicant failed to discharge her onus of proof under s 4 of the ACL to negate that Zoetis had reasonable grounds with respect to any future matter: North East Equity 285 ALR at 224 [29]-[31].
167 For those reasons, I am not satisfied that the all horses representation was misleading in contravention of s 18(1) of the ACL or that Zoetis or Dr L’Estrange did not have reasonable grounds, including with respect to any future matter within the meaning of s 4(1), when publishing it.
8. Was the no serious side effects representation (Equivac HeV had no serious side effects) conveyed?
168 The applicant particularised, and by the time of final address, relied on eight publications as conveying the no serious side effects representation, namely each of the first, second, and third permit disclosures, the registration module, the myth busting pamphlet, the Health4Horses website information, the facts about HeV pamphlet and the Equestrian Life letter (the no serious side effects publications).
169 There was a substantial dispute between the parties about what the words “serious side effects” meant in the no serious side effects representation. The applicant pleaded that serious side effects were “serious and potentially debilitating adverse reactions” (whether as a result of one side effect or more than one acting in combination). She relied on dictionary definitions of “serious” and “debilitating” to support the sense that she assigned to the word “serious” in the representation, as being the meaning that each of the no serious side effects publications conveyed. She asserted that “serious” meant “giving cause for apprehension”, “debilitating” meant “to make weak or feeble, to weaken, to enfeeble” and “potentially” meant “not actually, but possibly”. The parties joined issue on this claim.
170 During final address, the applicant argued that a serious side effect was anything beyond a minor site reaction or a small rise in temperature. In addition, the applicant said that if a side effect lasted more than transiently, it was ex hypothesi serious, particularly if it lasted for more than a few days.
171 In addressing the factual issue of whether a condition was in fact a side effect of Equivac HeV, the applicant contended that a side effect or clinical sign was serious if, first, a veterinarian had to attend to treat the horse following its vaccination because of the appearance of some condition or symptom, regardless of the nature of the treatment or the length of time in which the horse was affected, or, secondly, if the vaccination was plausibly scientifically related to the side effect or clinical sign. She contended that a finding that the administration of Equivac HeV had caused a side effect or clinical sign could be made if she established a sequence of events from which that conclusion could be inferred as a matter of common sense, as long as medical science did not exclude such a finding. Alternatively, she asserted that a temporal association and the scientific plausibility of the reported clinical sign being a side effect provided a sufficient basis for a finding to be made. She relied on what Mason J said in Tubemakers of Australia Ltd v Fernandez (1976) 50 ALJR 720 at 725.
172 The applicant contended that, since August 2014, the side effects described in the second and third permit disclosure were “serious” and, because they had not been mentioned in any of the earlier publications that conveyed the no serious side effects representation, being the first permit disclosure, the registration module, the myth busting pamphlet, and the Health4Horses website information, those publications were misleading. Further, she argued that until the second permit disclosure, the leaflet had said that there could be a site reaction of transient swelling even though in the preceding period Zoetis had recorded progressively, through Dr L’Estrange’s work, significantly more reactions and clinical signs that came to be included in the second permit disclosure.
173 When driven to elucidate what side effects she relied on to make good the claim that over the course of the relevant period the no serious side effects representation was misleading, the applicant produced in final address a schedule on which she relied. Before producing that schedule, the applicant had pleaded that the side effects associated with Equivac HeV vaccination were set out in a much longer list in Appendix A to the second further amended statement of claim.
174 I reject Zoetis’ argument that the first, second and third permit disclosures were, or would have been understood to be, publications by the Authority and not by Zoetis. First, in order to sell Equivac HeV, Zoetis chose to accept the conditions that the Authority imposed in each permit requiring it to distribute the label and leaflet containing the relevant disclosures of the actual or reported side effects to date. That made Zoetis a publisher of the leaflet about its own product: Voller 392 ALR at 548 [32], 554 [68]. Moreover, Dr L’Estrange and Dr Lehrbach gave evidence that the wording of each of the first, second and third permit disclosures resulted from discussions between them on behalf of Zoetis and the Authority. Secondly, the ordinary reasonable reader, being a vet, would have understood that the manufacturer of a medication or vaccine (here Zoetis) was informing him or her of necessary and relevant information about the product, including whatever the manufacturer had to disclose, such as possible side effects, under the relevant regulatory regime (here, the Agvet Code).
175 Before addressing the factual questions, I think it is important to determine the parties’ dispute about what the ordinary reasonable reader of a no serious side effects publication would have understood it to convey. That will also affect the question of what evidence is relevant to establish whether a condition is proved to have been caused by, and is a side effect of, administration of Equivac HeV.
8.1 The meaning of the no serious side effects representation
176 The Oxford English Dictionary online defines “serious” (in sense 3b) as “of an injury, condition, etc.: significant or worrying; giving cause for anxiety or concern; grave, threatening, or dangerous”. The Macquarie Dictionary online defines “serious” (in sense 6) as “giving cause for apprehension; critical; a serious illness.”
177 However, the use of dictionaries to limit or construe how a publication ought be understood is problematic. That is because the sense in which a word or expression used in the publication should be understood is a matter ultimately based on what an ordinary reasonable reader would have understood it to mean in the context in which the publication occurred. The expression “no serious side effects” is used in some of the no serious side effects publications, so the question is what, in its context, each publication that carried the no serious side effects representation would have conveyed to its ordinary reasonable reader about the sense of the word “serious”.
178 I reject the applicant’s attempt to use dictionary definitions to explain the sense of “serious or potentially debilitating” that she pleaded the word “serious” carried in the no serious side effects representation. The definitions on which she relied did not capture the sense of gravity that the ordinary and natural meaning of a negation of there being “serious side effects” would convey to the ordinary reasonable reader. The reader would understand that there is a distinction of substance between a side effect of a vaccination, such as an injection site reaction persisting, perhaps annoyingly and painfully for a few days, and one that causes a significant change to a person’s (or animal’s) medium to long term health or has an immediate, highly disruptive impact. The applicant’s reliance on meanings such as “to make weak or feeble, to weaken, to enfeeble” would capture the normal human reaction after a vaccination for a day or so while the body’s immune response took its course. An assertion that a vaccination had “no serious side effects” would not be understood by an ordinary reasonable reader as conveying that such an immune response would not occur. Such a reading would distort and unrealistically strain the natural and ordinary meaning of the expression. The applicant’s argument reflected the weakness of the evidence that she led on the nature of clinical signs that could be found to be, in fact, side effects of Equivac HeV, as I will explain later in these reasons.
179 I am of opinion that the reader would have understood the word “serious” in this context in the same way as one refers to a “serious illness”, that is an illness giving rise to considerable apprehension or concern because of its significant consequences for the horse.
180 Here, the reader, whether a vet or a lay person, would have understood the first, second, and third permit disclosures, the registration module, the myth busting pamphlet, and the facts about HeV pamphlet (the non-disclosure publications) to convey that vaccination with Equivac HeV had “no serious side effects” in the sense that those side effects that had occurred, or would occur, were not life threatening, dangerous to the horse’s health or such as to give rise to any substantive veterinary condition affecting its welfare, beyond a transitory or short term impact that would have no lasting effect on its health or wellbeing, other than conferral of immunity from HeV.
181 I reject the applicant’s submission that the non-disclosure publications would have been understood by the ordinary reasonable reader as meaning that there were no side effects that “gave rise to apprehension in horse owners and the possibility, sometimes an actuality, that the vaccine had caused the horses to become weak or feeble” regardless of its duration. I also reject the applicant’s contention a horse owner would hold such an apprehension if he or she considered that it was necessary to seek veterinary intervention after a vaccination. In my opinion, those arguments reflect an unreasonable and strained understanding of ordinary English words. A person’s subjective apprehension that some medical or veterinary condition is “serious” cannot be the basis on which to assess the objective effect of the condition on the human or animal about whom or which it is perceived. The argument endarkened the issue. It ignored the possibility that the vet comes at the anxious owner’s behest and diagnoses that there is nothing wrong with the horse or it has an unconnected disease or injury or concludes that the owner simply misinterpreted a transient and not serious side effect or clinical sign. The judgment of the owner, which might be unreasonable or mistaken, cannot be used as the determining factor of whether, because of its contemporaneous appearance, some reaction, real, perceived or imaginary, was connected to Equivac HeV, regardless of whether there was, in fact, any causal relationship.
182 The ordinary reasonable reader of the non-disclosure publications would have understood the no serious side effects representation as an objective assurance that the horse would not suffer some life threatening, potentially debilitating or dangerous reaction to the vaccine. Transient discomfort, including discomfort that requires the attendance of a vet and or the administration of a temporary pain killer medication, is not a “serious side effect”, even though the horse owner or an observer is worried by the perceived discomfort. Ordinary experience of human vaccinations includes transient feelings of pain and or swelling at the injection site, as well as sometimes feeling weak or symptomatic of the disease for which one has been inoculated. However, the ordinary reasonable reader would not understand that such a transient reaction is appropriately described as a “serious side effect”, the more so if the reader was a vet.
183 In my opinion, the ordinary reasonable reader would not have understood the no serious side effects representation to convey that use of Equivac HeV would entail that there would be no need to call a vet to treat a transient clinical sign or side effect. The reader would have understood a serious side effect to be one involving a life-threatening or potentially debilitating condition or serious illness, or be likely to incapacitate the horse for a substantial period.
184 The mere fact that an owner decides to call a veterinarian to attend a horse cannot be a defining feature of whether a side effect is serious. Different owners will have different views and different financial capacities that will affect their approach as to whether or not to call a vet. Some vets are owners who do not need to call themselves. Different people will have more or less concern about the appearance of a side effect or clinical sign in a horse. For example, if the horse has pyrexia (ie: a raised temperature) that cannot, of itself, be a serious side effect. The administration of phenylbutazone (bute), which is an anti-inflammatory drug, as a frequently used remedy is comparable to the administration in humans of analgesics that one buys at the supermarket and takes for a headache or temperature, enabling the patient to resolve his or her condition to normality within a relatively short time. If a vet needs to attend the horse shortly after the administration of the vaccination, that does not signify that the clinical sign that appeared was in fact a reaction to the vaccination, and cannot be the criterion for whether it was necessarily serious.
185 Zoetis also noted that the no serious side effects representation could be understood as conveying a statement that was an opinion, rather than a statement of fact; however, in my opinion, that argument should be rejected. The statement about the nature of the side effects was unqualified and, among other things, conveyed on the product’s label or leaflet as required by the regulator, being the Authority. Zoetis also sought to defend the no serious side effects representation as a statement with respect to a future matter, being a statement about what was obviously known at the particular times of publication in the relevant period about likely side effects. It contended that it had a reasonable basis for its opinion over that period.
8.2 The no serious side effects publications
186 Because the applicant pleaded that serious side effects were “serious and potentially debilitating adverse reactions”, she had to establish that the ordinary reasonable reader of a no serious side effects publication would have understood it to convey that Equivac HeV had no serious side effects in the sense pleaded of serious or potentially debilitating adverse reactions.
187 For the period from 10 August 2012 to 3 August 2014, the leaflet that the Authority required Zoetis to include in the container in which it distributed Equivac HeV to vets contained the following (see [45] above):
Side Effects
Transient swelling may develop at the site of vaccination in some horses but should resolve within one week without treatment.
188 The reader of the first permit disclosure was an accredited vet who intended to and did administer Equivac HeV to horses in the two years during which the first permit remained in force. The leaflet conveyed that the vaccine had no serious side effects. The description “transient swelling” was the veterinary equivalent of the common transient side effect from vaccination that humans experience of swelling at the injection site as the immune system reacts to the stimulus of the vaccine in developing the body’s response to the relevant disease. Accordingly, I find that the first permit disclosure conveyed the no serious side effects representation.
189 The myth busting pamphlet conveyed the no serious side effects representation in the statements set out at [55] above, which, in substance, stated that the experience of reactions to the first 24,500 vaccinations had resulted in only 53 horses having side effects, the majority of which were injection site reactions. The reader was told that this was only 0.22% and an expected result. The myth busting pamphlet made this conclusion pellucid when stating “[n]one of the side-effects reported were serious, and all resolved”. I find that the myth busting pamphlet conveyed the no serious side effects representation.
190 Zoetis also published the registration module to vets. In the section headed ‘Vaccine Safety Update’ (see [56] above), the ordinary reasonable reader, being an accredited, or to be accredited, vet, was told that there was an adverse event result rate of about 0.22% based on what the module described as a “Classification system”. The vet reader would have understood this to be a reference to a system of pharmacovigilance known as the ABON system. There are four or five classifications in the ABON system (the O1 category was required for the European Union, but not by the Authority, and Zoetis used it for classifying reported reactions to Equivac HeV) namely:
A = probably related to the product
B = possibly related to the product
O = unclassified (and or, as required by the Authority, unclassified / inconclusive)
O1 = inconclusive
N = unlikely to be product related
191 The ABON system requires a vet to use professional judgement to decide whether a report of a side effect or adverse reaction to the administration of a vaccine or other medicinal treatment is, in the classifier’s view, first, reasonably associated temporally with the vaccination’s onset and duration, secondly, for a probable classification (namely that the vaccination probably caused the reaction) the description of the clinical phenomena should be consistent with, or at least plausibly, based on the known pharmacology and toxicology of the medication or product and, thirdly, if there is a sufficient amount of information, there is no other equally plausible explanation.
192 The ordinary reasonable reader of the registration module, being a vet, would have understood that out of the first 25,500 doses of Equivac HeV there had been only 58 instances of an adverse reaction in a horse, of which about half were injection site reactions. That reader would have understood that none of the 58 instances involved a serious or worse outcome. Moreover, that understanding would have been reinforced because the vet would also have read the label and leaflet containing the first permit disclosure that the Authority required to be supplied with the vaccine. There was nothing else in the registration module that could have affected that understanding. Accordingly, I am satisfied that the registration module conveyed the no serious side effects representation.
193 The Health4Horses website information did not make any reference to side effects. The applicant did not explain how the information that dealt with the presumed transmission pathway for HeV to pass from bats to horses, one horse to another, and from one or more horses to humans, conveyed anything about the side effects. Accordingly, the Health4Horses website information did not convey the no serious side effects representation.
194 The second permit disclosure (see [67] above) set out many more details about side effects than the first permit disclosure. Again, this disclosure was made to accredited vets, who formed the class of ordinary reasonable readers of it. The second paragraph of the part headed “Side Effects” commenced by describing “transient post-vaccination reactions”. The vet reader would have understood all the reactions that the second paragraph described, including the rarely reported symptoms, to be minor. The last paragraph referred to the possibility of systemic allergic reactions, including anaphylaxis, “thought to occur rarely with all vaccines”, but did not describe such a reaction as having occurred. The joint report said that “[m]inor anaphylactic (allergic reactions, eg urticaria) are documented in the horse following the administration of a range of different vaccines”.
195 For these reasons, the second permit disclosure conveyed to the reader the no serious side effects representation, given its omission to state that there was any side effect of a serious nature.
196 The facts about HeV pamphlet also conveyed the no serious side effects representation by omitting any reference to what the reader would have understood was a serious side effect while discussing, in an anodyne way, those reactions and side effects that had resulted in a 0.28% reaction rate from over 300,000 doses. The reader was told that some horses had mild injection site reactions about which were “no reason for concern”, the vast majority of those reactions “resolve with little or no treatment”, and no deaths had occurred. The pamphlet stated that “[m]ost reactions are similar to those that people would expect when receiving a simple tetanus vaccine and are much less common”. It emphasised the “rigorous safety and efficacy testing” that Equivac HeV had received at the foot of the first page and the favourable experiences of a vet and former Olympic medallist at two different stud farms.
197 The third permit disclosure was substantially the same as the second. The ordinary reasonable reader, being a vet, would not have understood it to convey a different message based on the minor differences that I noted at [76] above. I am satisfied, for the same reasons as I gave in respect of the second permit disclosure, that the third permit disclosure conveyed the no serious side effects representation.
198 In the Equestrian Life letter, Dr L’Estrange informed readers that every dose of Equivac HeV for the preceding years had been documented and administered by a vet, who had to report every suspected adverse event. The letter told the reader that Zoetis had to assess each such report and classify the likelihood whether the reported symptom was attributable to the vaccination (see [77] above). Dr L’Estrange then informed the reader that he had examined every adverse event report that Zoetis had received about Equivac HeV. He summarised his findings, saying the majority were typical of reactions humans had when vaccinated, namely temporary soreness or swelling of the site of the inoculation, sometimes a temperature or being off colour for a day or two. He said that vets commonly administered bute to treat an adverse reaction. Dr L’Estrange informed the reader that the Authority had seen every adverse reaction report for the 350,000 doses administered as part of the registration process for Equivac HeV, was satisfied as to the safety of the vaccine and had proceeded to register it. He wrote: “The vaccine is demonstrably safe and effective”.
199 I reject the applicant’s argument that, because Dr L’Estrange did not identify in his letter the other reactions of which he was aware, this omission rendered what he wrote misleading and so conveyed the no serious side effects representation. The question is whether an ordinary reasonable reader of the Equestrian Life letter would have understood it to convey that Equivac HeV had no serious side effects. That is a different issue to what else it may or should have conveyed.
200 The ordinary reasonable reader of the Equestrian Life letter would not have understood Dr L’Estrange to be conveying an unqualified statement that Equivac HeV had no serious side effects at all. Rather, he said that the majority of adverse reactions were typical or similar reactions to those that humans experience and that the Authority, as regulator, was satisfied, after examining all the adverse reaction reports for 350,000 doses, that the vaccine was safe and could be registered. The letter concluded with his admonition to owners to speak to their vet “in order to make a sensible and informed decision as to whether to vaccine their horses”.
201 For these reasons, I am not satisfied that the Equestrian Life letter conveyed the no serious side effects representation.
202 Because horses cannot communicate in the same way as human beings, veterinarians must look for clinical signs. Their diagnosis of what condition the horse presents with may be very different to what an owner perceives the horse to be presenting with, even though the owner will often know the horse very well and understand intuitively many of its reactions or behaviours that signify it being other than in a normal condition.
203 It is important to appreciate that a clinical sign or adverse event that a horse exhibits after vaccination will be only recognised as a side effect of that vaccination if the two are causally connected. Causation was a central issue in the trial. And, as all of the experts agreed, it is common for horses, after vaccination, to display one, more or all of the clinical signs of a transient reaction at the injection site with transient increase in temperature, lethargy and inappetence (ie: lack of appetite), which could be interrelated. The experts said that those clinical signs are of conditions that are transitory and cannot be characterised as serious unless another mechanism or disease affecting the horse’s immune system or body connects them.
204 The three experts agreed that all of the clinical signs reported to Zoetis or the Authority (the reported clinical signs) in connection with the use of Equivac HeV reflected conditions that horses experience in the ordinary course of their lives regardless of the administration of a vaccine. Thus, a clinical sign can manifest coincidentally, but unrelatedly, to a vaccination.
8.3.1 The need to prove causation of a side effect
205 Whether a reaction was a serious side effect of vaccination of a horse with Equivac HeV was a matter on which the applicant bore the onus of proof. The degree to which the animal is affected by any immediate, medium or long-term, debilitating or grave clinical sign that appears at or within a reasonably short period after the vaccination will affect the characterisation or degree as to the impact of the side effect.
206 In Fernandez 50 ALJR at 724, Mason J, with whom Barwick CJ and Gibbs J agreed, held that there was medical evidence from which the jury could find that the plaintiff’s injury had been caused by his employer’s negligence. Mason J explained:
To establish on the probabilities that a medical condition or disability from which he suffers is “caused or materially contributed to” by the defendant’s wrongful conduct (Bonnington Castings Ltd. v. Wardlaw, [1956] AC 613, at p. 620, per Lord Reid). Consequently, as the decision in that case demonstrates, the plaintiff will fail if all that he can show is that his disability might have been so caused (see also St. George Club Ltd. v. Hines (1961), 35 A.L.J.R. 106, at p. 107, where it was pointed out that mere proof of default followed by injury does not show that the default caused the injury).
(emphasis added)
207 His Honour added (at 725) that a sequence of events, first, can confirm expert evidence where the sequence tends to support the probability of a causal connection, or, secondly, where the sequence aids in drawing an inference that, according to expert evidence, is open to be drawn.
208 In Amaca Pty Limited v Booth (2011) 246 CLR 36 at 61-63 [69]-[70], Gummow, Hayne and Crennan JJ said:
Even if the issue is one to which other disciplines may not be able to give any conclusive answer, questions of causation, as a step in the ascertainment of rights and the attribution of liability in law, call for sufficient reduction to certainty to satisfy the relevant burden of proof for the attribution of liability (Amaca Pty Ltd v Ellis (2010) 240 CLR 111 at 121-122 [6]). In Tubemakers of Australia Ltd v Fernandez ((1976) 136 CLR 681 (note); 50 ALJR 720 at 724; 10 ALR 303 at 311), Mason J, with the concurrence of Barwick CJ and Gibbs J, referred to a statement by Dixon J as elaborating the general onus which lies upon the plaintiff where the issue of causation lies outside the realm of common knowledge and experience. In Adelaide Stevedoring Co Ltd v Forst ((1940) 64 CLR 538 at 569. This statement may be compared with the passage in Australian Knitting Mills Ltd v Grant (1933) 50 CLR 387 at 426 in which Dixon J declined to act on the evidence of the chemist called by the plaintiff), Dixon J said:
“I think that upon a question of fact of a medical or scientific description a court can only say that the burden of proof has not been discharged where, upon the evidence, it appears that the present state of knowledge does not admit of an affirmative answer and that competent and trustworthy expert opinion regards an affirmative answer as lacking justification, either as a probable inference or as an accepted hypothesis.”
(Emphasis added.)
The “but for” criterion of causation proved to be troublesome in various situations in which multiple acts or events led to the plaintiff’s injury (March v Stramare (E & MH) Pty Ltd (1991) 171 CLR 506 at 516-517), for example, where the development of a particular medical condition was the result of multiple conjunctive causal factors. In such cases what may be unclear is the extent to which one of these conjunctive causal factors contributed to that state of affairs. These situations have been addressed by the proposition stated by Lord Watson in Wakelin v London & South Western Railway Co ((1886) 12 App Cas 41 at 47) that it is sufficient that the plaintiff prove that the negligence of the defendant “caused or materially contributed to the injury” (See March v Stramare (E & MH) Pty Ltd (1991) 171 CLR 506 at 514 per Mason CJ; Athey v Leonati [1996] 3 SCR 458 at 466-468 per Major J; Tse, “Tests for factual causation: Unravelling the mystery of material contribution, contribution to risk, the robust and pragmatic approach and the inference of causation”, Torts Law Journal, vol 16 (2008) 249, at pp 252-256). In that regard, reference may be made to the well-known passage in the speech of Lord Reid in Bonnington Castings Ltd v Wardlaw ([1956] AC 613 at 621). Of that case it was said in the joint reasons in Amaca Pty Ltd v Ellis ((2010) 240 CLR 111 at 136 [67]):
“The issue in Bonnington Castings was whether exposure to silica dust from poorly maintained equipment caused or contributed to the pursuer’s pneumoconiosis, when other (and much larger) quantities of silica dust were produced by other activities at the pursuer’s workplace. Those other activities were conducted without breach of duty. As Lord Reid rightly pointed out ([1956] AC 613 at 621), the question in the case was not what was the most probable source of the pursuer’s disease: dust from one source or the other. The question was whether dust from the poorly maintained equipment was a cause of his disease when the medical evidence was that pneumoconiosis is caused by a gradual accumulation of silica particles inhaled over a period of years.”
(italic emphasis in original; bold emphasis added)
209 Moreover, causation is a question of fact that must be determined as a matter of common sense: Ethicon 288 FCR at 529 [849] per Jagot, Murphy and Lee JJ; Commonwealth of Australia v McLean (1996) 41 NSWLR 389 at 410A per Handley and Beazley JJA. They said at 411B-C:
The issue was causation in fact of an actual event, and not causation of an hypothetical event where the court allows for the probability that the event would or would not have occurred: see Malec v J C Hutton Pty Ltd (1990) 169 CLR 638. Causation in fact of an actual event is an all or nothing issue. The plaintiff was not entitled to recover anything for his throat cancer unless he established, on the balance of probabilities, that it had been caused by the tort.
(emphasis added)
210 The closer the temporal connection between the administration of Equivac HeV and the appearance of the relevant clinical sign or signs may make it more reasonable, depending on the other clinical information available, to reach a view as to whether the clinical sign or signs is or are a side effect or effects.
211 Moreover, the experts agreed that the mere fact that there may be more reports of one or more of the clinical signs that are themselves commonly experienced by horses does not enable one to draw a conclusion of a causal relationship between it or them and the administration of the vaccine. That is because, in the absence of a background disease or condition in the individual horse, one needs to identify a causal mechanism to link the clinical sign to the vaccination and to understand the latter’s role. The coincidence of timing between the appearance of one or more of the commonly occurring clinical signs and vaccination still requires a thorough investigation or at least a full, contemporaneous history, before it will be reasonable to make a clinical veterinary diagnosis of a connection. Thus, a veterinarian can physically examine a horse and make a clinical diagnosis of what he or she assesses about a horse’s condition that may or may not be borne out if further diagnostic tests or pathology results are returned.
212 In contrast, pharmacovigilance reporting and assessment or ABON classification, ordinarily, does not produce the same degree of accuracy of either a clinical, veterinary diagnosis or diagnostic tests. And, as Professor Slater explained, none of the three experts had pharmacovigilance expertise. Rather, each expert used his expertise to analyse from data reported to the Authority and Zoetis using the pharmacovigilance information process, whether there was, in fact, a recognisable, immunological connection or mechanism between the observation of the clinical sign and the vaccination.
213 Associate Professor Sykes explained that he had been asked to opine in his reports about whether it was biologically plausible that a reported clinical sign following vaccination with Equivac HeV was caused by the vaccination.
214 It is also important to bear in mind that in equine vaccinations the injection point is not usually cleaned, swabbed or disinfected (unlike the alcohol or other disinfecting swab used to clean a human vaccination site before injection). Thus, as Dr El-Hage explained, horses have hair all over their skin that holds dirt and debris. He said that a known possible risk of equine vaccination is that the injection process may introduce a bacteria or other source of infection into the horse’s bloodstream from the surrounding material in the horse’s hair or on its skin. In this way, any source of infection from the horse’s skin or hair can manifest as a clinical sign in temporal proximity to the vaccination but will not be a side effect of the vaccine. This reinforces the analytical need to move beyond the mere coincidence in timing between vaccination with Equivac HeV and the appearance or duration of any clinical sign.
215 Senior counsel for the applicant questioned the experts about a list of reported clinical signs observed after vaccination of horses with Equivac HeV as to the possible causal connection between the two.
216 The experts agreed during their concurrent evidence that there was no documented side effect of the administration of any vaccine to a horse causing systemic inflammatory responses or systemic inflammatory response syndrome (SIRS). That agreement was significant because Associate Professor Sykes’ expert reports had postulated that vaccination with Equivac HeV could cause SIRS.
217 Associate Professor Sykes explained that SIRS is a very specific description of a precise process, namely the dysregulated and, to a large degree, over-activation of, the immune response. He said that it was more likely to be connected to the idiosyncratic response of an individual animal than as a general side effect of the administration of a vaccine.
218 Professor Slater explained that SIRS is an “out-of-control immune response”. It is dysregulated, in the sense that it manifests as a set of clinical signs and can occur in all animal species, but it develops principally through an infection in the organism. He explained that the body releases different sets of chemical signals, called cytokines, in response to the different stimuli from, respectively, SIRS, which is one out of control immune response, and an adjuvant in a vaccine. He said that anaphylaxis is also an out of control immune response that has never been documented in horses and is triggered by a different mechanism to SIRS. The different cytokines released in response to the different stimuli can be (and have been) analysed in blood samples. The signals caused by the cytokines released in response to the stimulus drive cell, antibody and immune responses in the organism which may generate an external clinical sign or signs. Such a clinical sign may also be indicium of a different condition than that caused by the original stimulus. Professor Slater said that the end point, in the manifestation of clinical signs, was the same presentation whether the animal suffered an out of control immune response due to the very different stimuli of SIRS and anaphylaxis. That is, outwardly, the animal will exhibit the same clinical signs as a consequence of each of the two very different mechanisms that actually triggered the immune response which generated those signs.
219 Associate Professor Sykes reasoned that it was biologically plausible that a SIRS immune response could be caused by the vaccination in association with an underlying condition and present with the same clinical signs that a different mechanism, such as anaphylaxis, can also produce.
220 But, as Professor Slater said, the various immune responses to SIRS and or any underlying condition are each a different mechanism to the responses produced by a vaccination. Because the ISCOMatrix adjuvant, unlike a chemical adjuvant, only remains in the body for a short period, Professor Slater’s view was that it was not reasonable to conclude that a vaccination with Equivac HeV could trigger an out of control immune response like SIRS.
221 All the experts agreed that there are no documented cases of horses having either SIRS of anaphylaxis as a response, neither of which had ever been recorded after equine vaccination.
222 Associate Professor Sykes accepted that because of the absence of any academic research, observational research or clinical trials, and given that SIRS was a well-known and well-researched immune system reaction, it “would be a big step to take” to infer that any of the clinical signs reported in horses which the experts gave evidence about was caused by the vaccination with Equivac HeV.
223 Both Professor Slater and Dr El-Hage explained why such a causative link was not open on the current state of scientific knowledge about both SIRS and the way in which the adjuvant used in Equivac HeV works to produce an immune response. They said that because the ISCOM or ISCOMatrix adjuvants (used in Equivac and Equivac HeV) are biological substances they do not remain or persist long in the body after administration in a vaccine, unlike chemical adjuvants, that often included aluminium or alum which remained in the body.
224 The experts agreed that the efficacy of Equivac HeV was excellent. The G protein used in it is an artificial, not natural or living, substance. That means that the administration of the vaccine involves no risk of infection with HeV so that, in this respect, it is very safe. As Professor Slater said, the use of the artificial inert G protein greatly reduces the likelihood of unexpected side effects while producing an efficient immune response. The use of an artificial, or non-living, antigen (like the G protein in Equivac HeV) and a biological adjuvant, like the ISCOMatrix, makes it reasonable to expect, as Professor Slater explained, that Equivac HeV will generate effective and long-duration protective immune responses with a low incidence of side effects.
225 A biological adjuvant, such as the ISCOMatrix, produces a more effective, balanced immune response than a chemical adjuvant. That is because biological adjuvants, that have been used in medical vaccines since the 1980s, contribute to antigen stability and interact efficiently with antigen presenting cells.
226 Here, Associate Professor Sykes accepted that his attribution of a “biologically plausible” or possible connection between vaccination and the presentation of a side effect, where neither Professor Slater nor Dr El-Hage supported such an attribution, was based on “potentially” seeing a link.
227 I am not satisfied that Associate Professor Sykes’ evidence of the possibility of a link based on biological plausibility rose to the level that it was probable that a causal connection existed between the vaccination and any clinical sign, other than the transient ones on which all the experts agreed (see [266]-[267]) and, as he acknowledged, such a connection was not an accepted (or documented) hypothesis: cf Adelaide Stevedoring Co Ltd v Forst (1940) 64 CLR 538 at 569 per Dixon J as applied in Fernandez 50 ALJR at 724 and Amaca 246 CLR at 62 [69]. At best, Associate Professor Sykes speculated that there might be a causal connection between the administration of the vaccine and the presentation of a clinical sign if SIRS, or some other stressor, were present, even though there was no evidence at all that such a stressor (including SIRS) was ever present or likely to be. Associate Professor Sykes addressed the connection issue on the basis of whether it was “biologically plausible” to draw the link based on an “indirect mechanism” such as SIRS or anaphylaxis. However, neither of those indirect mechanisms had any support in scientific literature.
228 The existence of a plausible explanation that there may be a link between the vaccination of a horse with Equivac HeV and the presentation of a clinical sign does not necessitate or justify a finding that the vaccine caused or was a cause of the clinical sign: Merck Sharp & Dohme (Australia) Pty Ltd v Peterson (2011) 196 FCR 145 at 182 [143] per Keane CJ, Bennett and Gordon JJ.
8.4 Are any of the reported clinical signs a side effect?
8.4.1 Consideration of expert evidence
229 During their concurrent evidence, senior counsel for the applicant asked each of the experts which of the following reported clinical signs was a side effect of a horse’s vaccination with Equivac HeV. The experts explained that the reported clinical signs about which they gave oral evidence were common conditions that horses experience in the ordinary course of their lives. They said that, while some of those signs were, or may have been, also side effects of vaccination, they were transient, lasting for up to a day or so but sometimes longer, and equivalent to human reactions to vaccination such as soreness, possible swelling around the injection site, an increase in body temperature, lethargy or feeling a bit off. None of these side effects for horses (or humans) had any consequence that could be described as serious or debilitating or of the character of a serious or grave illness.
230 Ataxia is a specific neurological condition in a horse a clinical sign of which can be sore feet, weakness or wobbliness on its feet and it not able to be ridden. However, the manifestation of that clinical sign is not necessarily evidence that the horse is ataxic, as opposed to experiencing a common, but transient, side effect of feeling weak or off colour as an immediate effect of vaccination. Humans experience such side effects for a short time, sometimes a day or more after vaccination.
231 Associate Professor Sykes asserted that it was biologically plausible for Equivac HeV to produce ataxia. He approached his analysis on the basis of his instructions to express an opinion on whether it was biologically plausible that a clinical sign could be a side effect of the vaccination of a horse with Equivac HeV. He opined in his first report that such a biologically plausible causative mechanism was open on his mistaken understanding (or assumption) that the vaccine had a chemical adjuvant. He said:
the best recognised mechanisms by which the Equivac HeV vaccine could cause the listed side effects are overactivation of the innate immune response, or via the induction of hypersensitivity or autoimmunity. These processes can be induced by either the organism itself, (or in this case) its subparts, or to other constituents of the vaccines. In particular, adjuvants are commonly incriminated in the development of adverse side effects in modern vaccines.
(emphasis added)
232 During their concurrent evidence, the experts discussed whether there was a possible mechanism that vaccination with Equivac HeV would introduce into the horse’s immune system that would lead to the conclusion that one or more particular clinical signs was or were a side effect or side effects. As I have found below, some clinical signs were transient side effects of Equivac HeV, including injection site reactions (such as swelling and or pain reaction), pyrexia, lethargy, inappetence and tachycardia ([266]-[267]). I accept Professor Slater’s and Dr El-Hage’s evidence that, as the former said:
to follow Professor Sykes’ comment about the possibility, what I would say is that … these untoward autoimmune-type reactions, as best as we understand it, occur because of persistence of the adjuvant and a persistent immune drive from that persisting adjuvant, which is a strong feature of the chemical adjuvants, but it’s best to understand it is not a feature of the biological adjuvants like ISCOM. They don’t persist for that length of time. So, again, from a mechanistic point…of view, it’s …relatively easy to understand how a chemical adjuvant like aluminium could be associated with these autoimmune disease events, much harder to see a mechanism by which a shorter persistent adjuvant like ISCOMS could do so.
(emphasis added)
233 Associate Professor Sykes accepted that the ISCOMatrix adjuvant in Equivac HeV was far less likely to provide a mechanism for an autoimmune inflammatory syndrome (or ASIA) response in a horse than a chemical adjuvant. He agreed with Professor Slater that, in any event, there was no evidence or basis in literature to suggest that ASIA had occurred in a horse in connection with a biological adjuvant like ISCOM or ISCOMatrix. Yet, he qualified his agreement saying “it remains biologically plausible”. In contrast, as Dr El-Hage pointed out, there was no evidence of such a reaction in the many millions of horses vaccinated for equine flu with Equivac, that had the same adjuvant as Equivac HeV. Associate Professor Sykes’ persistence with his reliance on biological plausibility as providing a basis for clinical signs reported in association with Equivac HeV vaccination included what he said about SIRS (see [216]-[227] above). This was one of the several bases that I explain in these reasons why I found Associate Professor Sykes’ evidence less persuasive than Dr El-Hage’s and Professor Slater’s.
234 In his first report, Associate Professor Sykes had stated that a range of inflammatory neurological diseases had been associated with vaccination including demyelination of nerves associated with motor functions or ataxia.
235 Both Professor Slater and Dr El-Hage disagreed with Associate Professor Sykes’ hypothesis. Professor Slater said that, in humans, demyelination side effects had been associated with, and were believed to be related to, chemical adjuvants in vaccines but not biological ones. Associate Professor Sykes responded that, while he agreed with what Professor Slater had said, “our knowledge base on the newer generation adjuvants is much lower because they just simply haven’t been in use as long as … the old adjuvants”. I do not accept that qualification. As Dr El-Hage said, Equivac (ie: the equine flu vaccine), which other than its antigen has the same ingredients as Equivac HeV, including the same ISCOMatrix adjuvant, had been used in vaccinations of millions of horses annually without any reports of the kinds of reaction humans had to chemical adjuvants. And, Professor Slater gave the evidence quoted in [232] above.
236 However, all three agreed that there are no reported post-vaccination diagnoses of ataxia in horses. As Associate Professor Sykes said:
So at one end of the spectrum you have the potential for weakness, pain and a range of other things, even laminitis, to be interpreted as ataxia, because the horse is stumbling, and we know that pain and those sorts of things will cause that. At the other end we have the specific diagnosis of ataxia, and I agree that post-vaccination demyelination of ataxia is, to my knowledge, not being reported in the horse.
(emphasis added)
237 Professor Slater explained the need for a proper diagnosis of the horse’s condition in order to evaluate whether a clinical sign is or is reasonably able to be connected to vaccination, saying:
A proportion of people after they’ve had a flu vaccine for a short period — a few hours, perhaps, up to a day — do report some flu-like symptoms. This is the immune response to the vaccine. We’re very good at self-reporting, of course. We can say, “I don’t feel so good after that vaccine.” I sincerely doubt we would observe that in a horse. And I think when a horse owner or a horse vet reports that a horse appears to have muscle stiffness, that’s a whole different level of disease process. That is not a transient, “I don’t feel so good.” You know, if I had a flu vaccine and had some muscle aches, you wouldn’t notice that from how I was walking and moving around. Whereas for an owner or a horse vet to notice that a horse has got muscle stiffness and pain, that’s an entirely different disease process that’s happening compared with that transient and expected post vaccine response.
(emphasis added)
238 Both Dr El-Hage and Associate Professor Sykes agreed with Professor Slater that such a “disease process” was different to a response to the vaccine and observed that different breeds of horse have their own idiosyncratic propensities and reactions, such as well-muscled quarter horses with big bottoms that are prone to myositis, muscle soreness or stiffness, which may be exacerbated, for a short time, by various stimuli including vaccination.
239 I am not satisfied, on the basis of the expert evidence, that ataxia is a side effect of Equivac HeV vaccination.
240 Urticaria or swelling is a very common clinical sign in horses. It is possible that, if the condition appeared within hours of vaccination, it was caused by, and was a side effect of, the vaccine depending on the circumstances.
241 Pyrexia is also a very common clinical sign of any vaccination. It can be associated with localised sweating, because it is an indication of the animal’s immune response. Thus it can be a side effect.
242 A lump or lumps that develop at the vaccination site is a local, transient reaction that is associated with vaccination. I will discuss this clinical sign further below when dealing with Ms Abbott’s particular allegation about what occurred with her horse, Primetime (see [312]-[320], [362]-[372] below).
243 None of the experts regarded erratic behaviour as a side effect of vaccination. Similarly, none of the experts considered that recumbency or lying down was a side effect of vaccination. They considered the presentation of clinical signs of erratic behaviour or recumbency would be linked to a separate intermediary process (ie: condition or disease) distinct from the coincidence in timing of the vaccination.
244 Anaphylaxis has never been documented as a side effect of a horse’s vaccination.
245 Lethargy is a non-specific clinical sign of a transient immune response immediately after vaccination of a horse that can last a few hours.
246 Pain reaction is a known transient side effect of any vaccination. Injection with Equivac HeV can produce transient pain at the injection site but any persisting pain is likely to be associated with another condition or disease. A rare complication of any vaccination is an acute site reaction.
247 Laminitis is an inflammation of the hoof laminae between the bone in the hoof wall. It is ubiquitous among horses and can have very serious consequences. Both Professor Slater and Dr El-Hage considered that it was not plausible that vaccination with Equivac HeV could cause laminitis. Associate Professor Sykes believed that it was “biologically plausible” that the vaccine could cause laminitis as “the straw that breaks the camel’s back if we induce a stressor on that horse, and that stressor potentially being just a severe acute injection site reaction” if it were pre-sensitivised. He said that if the horse had no risk or pre-disposing factor, Equivac HeV vaccination was “highly unlikely” to cause laminitis. Associate Professor Sykes said that if the horse had SIRS, vaccination could increase stress on it and trigger laminitis because increased stress increased cortisol which is a trigger for laminitis. However, he accepted that horses could be stressed for many reasons including vigorous activity.
248 I do not accept Associate Professor Sykes’ evidence. First, he had not reviewed any reports of laminitis with which he had been briefed. Secondly, he knew of no research or documentation that laminitis had been causally linked to vaccination with Equivac HeV. Professor Slater had research groups at both London and Melbourne working on laminitis. He said that there had been extensive epidemiological investigations in Europe, North America and Australia and in none of the many published reports had vaccination ever been suggested as a trigger for laminitis. Dr El-Hage agreed saying that there had definitely been no documentation that drew a connection between vaccination and the development of laminitis.
249 Accordingly, I do not accept that laminitis is a side effect of Equivac HeV.
250 The experts agreed that joint stiffness, or synovitis is not a side effect of Equivac HeV.
251 Colic is a very common, non-specific clinical sign in horses. Professor Slater observed that the majority of horses experiencing abdominal pain had a temperature rise because this reflected body-wide responses to what was occurring in the abdomen, especially if it had serious gastrointestinal disease. He said that fever would not cause colic, but if a horse had colic for another reason, it may well have a fever associated with its abdominal issues. Both Dr El-Hage and Associate Professor Sykes agreed.
252 Both Professor Slater and Dr El-Hage could not see any mechanism that would link administration of Equivac HeV to colic. Associate Professor Sykes agreed generally with them but observed:
I do potentially see a link when we talk about anaphylaxis that colic would be potentially a clinical sign associated in that immediate post-vaccination period if we were to have an anaphylactic event.
253 However, after Professor Slater reiterated that anaphylaxis, while theoretically possible, had never been documented as a post-vaccination event for a horse, and was otherwise a rare event for horses, Associate Professor Sykes accepted that it was unlikely that a horse would get colic because, as he said, “it’s unlikely that you would have anaphylaxis in the first place”.
254 In my opinion, this was another example of Associate Professor Sykes seeking to support a suggestion that it was biologically plausible that Equivac HeV could produce a side effect in a horse without any reasonable basis for doing so. He had no basis to suggest that colic, if linked to anaphylaxis, was a possible side effect of vaccination, rather than accepting from the outset that the rarity of equine anaphylaxis, and the absence of any link to vaccination, was such that his postulated link was even less likely.
255 Tachycardia (or increased heart rate) is a possible transient immune response, side effect or response to pain at the injection site of vaccination with Equivac HeV. However, tachycardia is a clinical sign that can be caused by many disease processes and is a feature of pain response and inflammation. As Professor Slater said, with Associate Professor Sykes’ and Dr El-Hage’s agreement, all vaccines create an immune response and, in a proportion of individuals, a measurable body response including a transient increase in feebleness and/or a moderate increase in heart rate. That would occur within a few hours of administration and persist for a few hours.
256 Lameness is not a side effect of Equivac HeV. It is the most common problem that horses develop. The experts all agreed that it was very difficult to draw a connection between vaccination and lameness. That is because lameness occurs with changes in bones, joints and muscles as opposed to immune responses to vaccination.
257 Abortion is a common occurrence in mares due to disease or placental insufficiency.
258 Red bagging occurs in a mare when she is close to giving birth. It involves the placenta detaching, because of disease, and the foal being aborted. Similarly, other trauma can cause abortion, to which mares are particularly susceptible at an early stage, such as when they experience trauma or are transported for several hours.
259 Professor Slater and Dr El-Hage agreed that it was very hard to see any causal link between vaccination and either abortion or red bagging. They said that pre-existing disease or factors other than vaccination would cause (early) abortion or red bagging to occur. However, Associate Professor Sykes asserted that “indirectly … if we get severe disease, we potentially would see that as a consequence, rather than a direct effect of vaccination on the placenta per se”. He said that the connection could include SIRS or anaphylaxis. I do not accept that, again, speculative reasoning of Associate Professor Sykes for the reasons I have given.
260 Hair loss and effect on coat (including depigmentation) are very common background clinical presentations that result from a large variety of conditions that lead to poor hair growth, general ill health and or ill thrift, as Dr El-Hage explained. Neither he nor Professor Slater considered that body-wide hair loss or any other effect on a horse’s coat could be a side effect of vaccination. However, they said that there could be transient change of the horse’s hair or coat in the localised area of the injection.
261 Associate Professor Sykes considered that hair loss, effect on coat and depigmentation were biologically plausible. He said that depigmentation was permanent in the area in which it occurred but not serious, unless the colour of the horse was important, for example if the owner wanted to use it in dressage and, even then, he said “[it’s] certainly not a serious problem”. He added that hair loss and effect on coat were transient and the hair “typically comes back”. As Associate Professor Sykes said, the possibility (which I do not consider realistic based on my acceptance of the evidence of Professor Slater and Dr El-Hage) of the hair loss or effect on coat of vaccination is transient and not a serious problem.
262 Diarrhoea is a common clinical sign in horses. The experts agreed that short-term diarrhoea can be caused if a horse is stressed, including by being vaccinated, but any non-transient diarrhoea would be linked to systemic illness, not vaccination. They all discussed this further in relation to Quinn, as I explain below.
263 A combination of more than one clinical sign can present after vaccination. The experts agreed that post-vaccination side effects of Equivac HeV could include combinations of transient injection site reactions, pyrexia (ie: increase in temperature), inappetence and lethargy. As Associate Professor Sykes said, when clinical signs present as transient, all will come and go at approximately the same time and overall would have a mild effect on the horse.
264 On 8 January 2015, the Authority informed Zoetis that it had made an ABON assessment that it had reclassified Zoetis’ pharmacovigilance assessment to describe vaccination with Equivac HeV as a possible cause of seven horses’ deaths. Zoetis disputed this as I describe below (see [508]).
265 Professor Slater considered that it was impossible to see a causal mechanism linking administration of the vaccine to a horse’s death. As Dr El-Hage observed, one would need to review a comprehensive post-mortem to rule out other causes of disease in order to conclude that, instead, the vaccination had caused the death. Associate Professor Sykes reiterated that anaphylaxis may be a possible cause as well as other indirect pathways, such as he postulated for Ms Hinton’s horse, Quinn, which I consider below. I do not accept Associate Professor Sykes’ evidence because of the absence of any scientific or veterinary experience of a horse experiencing it caused by vaccination.
8.4.2 Conclusion as to expert evidence
266 For the reasons above, the only reported clinical signs that senior counsel put to the expert witnesses in support of the applicant’s case that I find were side effects of, or caused by, vaccination of a horse with Equivac HeV are urticaria, pyrexia, one or more lumps at the vaccination site, lethargy, pain reaction (including injection site reaction), hair loss or effect on coat at the injection site and tachycardia.
267 I am satisfied that, first, the presentation of a horse with any or a combination of those clinical signs after vaccination can be characterised as the horse suffering a side effect of, or caused by, the vaccination, and secondly, the suffering of each of those side effects, whether alone or in combination, is transient and does not cause any serious or debilitating effect on the horse.
268 I am also satisfied that if (which I do not accept) presentation of a horse with hair loss occurs after vaccination with Equivac HeV, the hair loss is localised around the infection site and, other than any change in pigmentation, is transient. As I will explain below, based on the expert evidence, I am not satisfied that any clinical sign that, first, Primetime, or secondly, Quinn, presented after vaccination with Equivac HeV was a side effect of, or caused by, vaccination with Equivac HeV.
8.5 Other issues arising in respect of the no serious side effects representation
269 The applicant advanced the results of pharmacovigilance reports as a basis independent of the expert evidence on which she asserted that the no serious side effects representation could be shown to be misleading. She sought to rely on the aggregation and contemporaneous evaluations of the ABON classifications from the pharmacovigilance data that the Authority and Zoetis accumulated during the relevant period which, she asserted, demonstrated that the reported clinical signs were, in fact, side effects of vaccination that were serious and or debilitating.
270 I should also emphasise that there is no issue that the vaccine overall is very safe. The question is whether, in the small proportion, 0.22% to 0.28%, of those instances of a vet or owner reporting a perception of a clinical sign as related to the administration of the vaccine it is reasonable to infer that more than an insignificant number were serious side effects.
271 In my opinion, the applicant’s approach to characterising a serious side effect based on pharmacovigilance reports and related ABON classifications of clinical signs should be rejected, for the reasons below.
272 I have found that, based on the expert evidence, none of the clinical signs on which the applicant relied as serious or debilitating side effects was, in fact, a side effect of Equivac HeV, except in respect of those that I found were transient side effects (see [266]-[267] above). The applicant’s argument depended on the coincidence of the timing of a horse’s vaccination and the presentation of one or more clinical signs (of a more severe character than that of one or more of its transient side effects that I have accepted or of one or more other reported clinical signs that I have not accepted as side effects). As I have explained, the fact that a horse may suffer one or more or a combination of transient side effects is not enough to support the applicant’s case that the no serious side effects representation contravened s 18 of the ACL, because she contended that, when there are several, “it is axiomatic that the side effects are more severe”.
273 Because I have rejected Associate Professor Sykes’ evidence of a biologically plausible link between vaccination with Equivac HeV and the reported clinical signs on which the applicant relied as serious or debilitating side effects, there is no expert opinion evidence to support her case that those were side effects of the vaccine and, accordingly, it is not necessary to analyse the pharmacovigilance material in any detail. However, I will deal with some of that material to explain why this argument cannot be supported.
274 The applicant argued that, based on what Basten JA, with whom Beazley JA agreed, said in Elbourne v Gibbs [2006] NSWCA 127 at [78] (and see too at [75]-[76]) evidence of a defendant’s or respondent’s failure to provide information, particularly as to potential risks of a procedure, combined with the materialisation of those risks, is sufficient to establish, prima facie, causation in the absence of a more plausible contrary inference. She contended that each of the first, second, and third permits required Zoetis to “fully investigate and report all adverse reactions” to the Authority, but its investigations failed to eliminate the plausibility that the reported clinical signs (other than the non-contentious transient side effects I have found) were not side effects.
275 The applicant relied in her closing written submissions on an asserted “striking similarity” between the reaction of Quinn and a reaction that Derina McLaughlin reported in her horse to Dr L’Estrange in her emails of 1 and 3 September 2014. She wrote:
My beautiful black show mare got deathly ill after her vaccine booster for Hendra. She was ok initially, then went down hill, with dramatic temps of 39 degrees, swaying, listless, not eating. She would stand in her spring paddock with her head down. My vet came at night and denied that it could be the vaccine but said they had no idea what it could be. (hello? Healthy horse has vaccine and has uncontrollable temperatures of 39 degrees and has sore neck?) I have had and shown horses for 30 years. I got another opinion. 15 days since the vaccine, she is now recovering and sparky enough to have a buck in the paddock. She had the vaccine before, so this was a booster only. I found the same description of her symptoms from another person online. I thought I was being responsible as a horse owner, keeping all her vaccines updated. Now I am devastated, as I have had 14 days of misery, and worry.
(emphasis added)
276 To illustrate what I have found above about the problem with treating pharmacovigilance reports as a basis to arrive at a reliable veterinary diagnosis or conclusion, I will discuss the evidence relating to Ms McLaughlin’s horse. The evidence of what Dr L’Estrange did to investigate Ms McLaughlin’s report showed that there was no relevant similarity between the condition of her horse and that of Quinn, other than that each developed fever or pyrexia at an imprecise time 24 or more hours after vaccination. The veterinary information that Dr L’Estrange collected showed that any suggestion of a connection between Equivac HeV and Ms McLaughlin’s horse’s condition was unlikely, and that the pyrexia was of unknown origin (or “PUO”). Neither vet suggested that there was any connection, other than coincidence, between the Equivac HeV vaccination and the subsequent clinical signs that Ms McLaughlin’s horse presented.
277 The apparent lack of substance in Ms McLaughlin’s asserted side effects of Equivac HeV, that Dr L’Estrange’s thorough pharmacovigilance investigation revealed, demonstrated the flawed approach in the applicant’s argument based on pharmacovigilance reports.
278 Dr L’Estrange investigated this complaint, speaking to both Ms McLaughlin and the attending vet. The vet said that, on 19 August 2014, the horse had been vaccinated on opposite sides of its neck with Equivac HeV as well as the combined equine flu and strangles vaccine at the same time as the vet had performed a dental procedure while it was sedated. In addition, the vet said that she had diagnosed a suspected gastric ulcer syndrome and it began a course of omeprazole as treatment. The vet advised Ms McLaughlin to rest the horse and not work it excessively.
279 Ms McLaughlin told Dr L’Estrange that the horse was off colour for the next four of five days, but she rode it on the sixth day after vaccination. She reported that it was listless the next day, had a high temperature, showed difficulty moving its neck and appeared to be swaying. She called the vet on 27 August 2014 reporting a temperature in excess of 39℃. The vet told her to give the horse bute and attended later that day to find that all the horse’s vital signs were within normal limits and it was eating hay. The vet asked Ms McLaughlin to call if the fever returned but received no such call. Ms McLaughlin told Dr L’Estrange that 15 days after vaccination she called a different vet who took blood samples that returned a result for anaemia. Dr L’Estrange considered that anaemia was consistent with the diagnosis of ulcers as the original vet had found. The second vet informed Dr L’Estrange that he had been given a history that the horse had a temperature and was lethargic three days after vaccination and that those clinical signs persisted despite administration of bute and ulcer treatment. The vaccinating vet also reported that she suspected that Ms McLaughlin had worked the horse more than she revealed because it was in training for the imminent Royal Melbourne Show.
280 The applicant also relied in her closing written submissions on her endarkened characterisation of the “progress of the deterioration of [Primetime that] mirrored what had occurred with [Lisa] Busby’s horses” (emphasis added).
281 According to the pharmacovigilance reports in evidence, on 28 August 2013, three of Ms Busby’s horses received a second Equivac HeV vaccination. There were numerous entries in the pharmacovigilance reports and Dr L’Estrange’s contemporaneous handwritten notes about Ms Busby’s assertions as to her horse’s clinical signs, when and how they presented and the attending vets’ reports. Ms Busby also recounted to him that, shortly after her horses received boosters on 26 February 2014, they presented with discomfort.
282 In her closing written submissions, the applicant characterised one pharmacovigilance report about one of Ms Busby’s horses as recording “a very serious reaction” to the vaccine. That reaction was classified in the causality assessment as “possible” (emphasis in original). The submission referred to Ms Busby’s, not a vet’s, description in an email that the horse’s neck had:
“swelled quite badly along the whole neck – not just the site of the injection and he would not allow you to even think about touching him for four days”. This is exactly the same as what happened to Ms Abbott’s horse, Primetime.
(footnote omitted)
283 One issue with that submission is that it did not analyse the evidence that it cited. The pharmacovigilance report by Zoetis dated 3 March 2014 had discussed the vaccination of Ms Busby’s horse Ruff Coconut on 28 August 2013 by Dr Mitchell Edwards. That reported referred to Dr Edwards attending and examining the horse the next day and prescribing bute in response to Ms Busby’s report that he was off colour, had a poor appetite, and was sore all over. Dr Edwards took blood samples that the report noted had found mild elevations in four results of no significance. It noted that, after 10 days on bute, the horse’s lethargy and pain appeared to have improved and that the bute treatment continued for a further five days, when Ms Busby reported that it was comfortable without bute. The report concluded that the “acute reaction, experienced in the 2 weeks post vaccination resolved” and that it was “possible the signs are related to vaccination, but the duration of the signs is slightly off”. It noted that Ms Busby’s subsequent reports in January and February 2014 about the horse having muscle soreness and irritability from October 2013 “are likely to be due to back pain” and were unlikely to be a consequence of Equivac HeV.
284 Dr L’Estrange’s email to which the applicant’s closing submission referred was dated 16 March 2014 and responded to Ms Busby’s email to him of 14 March 2014, in which she set out her observations of two other horses, Mac Me Again and Mr Mysterious, that on 26 February 2014, she had had vaccinated with the six-month booster. She did not vaccinate Ruff Coconut on that occasion. She reported that Mr Mysterious, which had not reacted to the previous vaccinations, “swelled quite badly along the whole neck – not just the site of the injection and he would not allow you to even think about touching him for 4 days [sic]”. Ms Busby wrote that she had left Ruff Coconut to “recover from his previous ordeal” and his behaviour since being spelled “is he almost looks a little spastic … and I am not saying this in a funny way”.
285 Dr Edwards emailed Dr L’Estrange on 17 March 2014 saying that Mr Mysterious’ “neck swelled right up for 2 days and resolved over another 2 days. Not off feed at all, still stiff and reluctant to do anything, crtanky [sic] and ill tempered and cannot be ridden”. He speculated that Ms Busby’s horses may have had a genetic predisposition. Dr Edwards wrote, in respect of Ruff Coconut, that Ms Busby “has said to me that you have come to the conclusion that the vaccine and subsequent reaction has ‘fried his brain’”. Dr L’Estrange responded saying that he had not come to any such conclusion about Ruff Coconut. Rather, he wrote, another vet had diagnosed that the horse had back pain in the croup area and recommended that he be put on bute and spelled indefinitely. Dr L’Estrange said that he was not aware of any such genetic predisposition but he had limited knowledge of the diseases that Dr Edwards mentioned.
286 The various other reports in the pharmacovigilance material dealing with Ms Busby’s horses, which is not necessary to detail here, reinforced the factually intensive nature of any investigation of whether there was a causal relationship between the presentation of a clinical sign and the horse’s vaccination.
287 I reject the applicant’s argument based on the pharmacovigilance reports. First, I have found that there is no basis on the expert evidence that the reported clinical signs on which the applicant relied as serious side effects of Equivac HeV were in fact serious or debilitating or side effects at all. Secondly, s 140(2) of the Evidence Act 1995 (Cth) requires the Court to take into account in determining whether it is satisfied of a fact, on the balance of probabilities, the nature of the cause of action or defence, the subject-matter of the proceeding and the gravity of the matters alleged. Here, the applicant alleged that Zoetis had contravened s 18(1) of the ACL by making the no serious side effects representation in marketing to the public Equivac HeV. That allegation was centred on the factual proposition that the vaccine in fact caused serious side effects. At highest, about 0.28% of all vaccinations resulted in a report of a possible adverse reaction. In Briginshaw v Briginshaw (1938) 60 CLR 336 at 361-362, Dixon J said:
Except upon criminal issues to be proved by the prosecution, it is enough that the affirmative of an allegation is made out to the reasonable satisfaction of the tribunal. But reasonable satisfaction is not a state of mind that is attained or established independently of the nature and consequence of the fact or facts to be proved. The seriousness of an allegation made, the inherent unlikelihood of an occurrence of a given description, or the gravity of the consequences flowing from a particular finding are considerations which must affect the answer to the question whether the issue has been proved to the reasonable satisfaction of the tribunal. In such matters “reasonable satisfaction” should not be produced by inexact proofs, indefinite testimony, or indirect inferences.
(emphasis added)
288 In Murray v Murray (1960) 33 ALJR 521 at 524, Dixon CJ, with whose reasons Taylor J agreed, explained what he meant in Briginshaw 60 CLR at 360-363, saying, relevantly:
What the civil standard of proof requires is that the tribunal of fact, in this case the judge, shall be “satisfied” or “reasonably satisfied”. The two expressions do not mean different things but as in other parts of the law the word “reasonably”, which in origin was concerned with the use of reason, makes its appearance without contributing much in meaning. However, its use as a qualifying adjective seems to relieve lawyers of a fear that too much unyielding logic may be employed. But the point is that the tribunal must be satisfied of the affirmative of the issue. The law goes on to say that he is at liberty to be satisfied upon a balance of probabilities. It does not say that he is to balance probabilities and say which way they incline. If in the end he has no opinion as to what happened, well it is unfortunate but he is not “satisfied” and his speculative reactions to the imaginary behaviour of the metaphorical scales will not enable him to find the issue mechanically. … courts did not impose on the parties, or one may perhaps say claim from the parties, the same strictness or exactness of proof about all questions arising in a civil trial without regard to their triviality or importance, the unlikelihood or the probability of their occurring. In other words the tribunal might reason upon the evidence to a conclusion as a responsible and sensible man would in all the circumstances.
(emphasis added)
289 Thirdly, as Zoetis submitted, the applicant’s attempt to use particular pharmacovigilance reports to make out her case cannot be accepted. None of Ms McLaughlin, Ms Busby, or their horses’ treating vets was called to give evidence, no expert evidence was adduced in relation to any of their horses and the full relevant veterinary records for them are not in evidence. It is inappropriate to use those reports as a basis for making a finding as to whether any of those or other horses in other pharmacovigilance reports in evidence suffered any side effect that vaccination with Equivac HeV caused. I reject the applicant’s submissions based on those pharmacovigilance reports.
290 Here, the best that can be said of the morass of the factual disputes and issues relating to the clinical signs that Ms Busby’s horses reportedly displayed, the connection of those signs to the various vaccinations, the various and sometimes conflicting statements in pharmacovigilance reports, emails, and Dr L’Estrange’s handwritten notes and oral evidence, is that these constitute one or more “inexact proofs, indefinite testimony or indirect inferences”. It is not reasonable to conclude from those that the applicant has established that the vaccine was a “possible” cause of any one or more of the clinical signs, or that the clinical signs were, on the balance of probabilities, actually a cause of one or more side effects of Equivac HeV manifested in the presentation of the sign or signs: Briginshaw 60 CLR at 361-362 and s 140(2) of the Evidence Act.
291 The three experts were briefed with all the material that the parties could gather to opine about Primetime and Quinn, yet found that those were insufficient to allow them to draw a conclusion that the vaccination was causative of any clinical sign reported or documented about either Primetime or Quinn. The pharmacovigilance material does not have any sufficient degree of precision to enable me to make a finding on the balance of probabilities that a reported clinical sign was caused by Equivac HeV, particularly in circumstances where I have rejected Associate Professor Sykes’ evidence of biological plausibility where it conflicts with Professor Slater’s and or Dr El-Hage’s views.
292 Given the uncertainty as to what occurred with the horses referred to in the pharmacovigilance material, it is unsatisfactory for the applicant to assert that Zoetis contravened s 18(1) of the ACL in making the no serious side effects representation (and, to the extent her case relied on that being proved, the all horses representation) in the absence of any direct evidence from persons such as Ms McLaughlin, Ms Busby, the treating vets of their horses, the blood or other pathology reports and expert evidence that analysed that material and could be tested at the trial.
293 While the pharmacovigilance reports and Dr L’Estrange’s notes were admissible as business records under s 69 of the Evidence Act, they have very limited utility when it comes to drawing conclusions of a veterinary or scientific character as to the causation of a side effect, in light of s 140(2) of the Evidence Act and Briginshaw 60 CLR at 361-362. Given my findings that there were no serious side effects, based on the expert evidence about the various reported clinical signs on which the applicant relied, in the abstract, it would be futile to examine piecemeal each pharmacovigilance report on which the applicant relied. That is because the expert evidence that I accepted does not support the conclusion that the applicant advanced and none of the experts was asked to give evidence on any of those pharmacovigilance reports or the reported clinical signs displayed by Ms McLaughlin’s or Ms Busby’s horses.
294 In order to make any accurate or reliable evaluation of whether a clinical sign is a side effect, first, one must have an accurate and sufficiently detailed history, secondly, there must be sufficient contemporaneity and if possible, some veterinary diagnosis, and, thirdly, some expert evidence that supports the conclusion of the clinical sign being a serious side effect (of which there is none that I have accepted). Given that the applicant postulated that Ms McLaughlin’s report of the clinical signs presented by her horse was one of “striking similarity” when the facts negate that description and the absence of reliable expert evidence to support the ascription of “serious side effects”, it is unnecessary to trawl through each of the other pharmacovigilance reports of clinical signs.
295 The expert evidence establishes that the stimulation of a horse’s immune response by the biological ISCOM adjuvant is transient, lasting in the order of 24 hours or so. Thus, as the expert evidence explained, some causative mechanism within the horse’s biology must be identified to explain how the presentation of a particular clinical sign can have been caused by the administration of Equivac HeV. The applicant’s reliance on the pharmacovigilance material does not sufficiently establish that Equivac HeV caused any of the reported clinical signs.
296 Apart from having different inert antigens, the chemical structures of Equivac HeV and Equivac are identical. No serious side effects of the kind on which the applicant relied have been associated with, or found to have been caused by, Equivac in many millions of uses.
297 As Ms Abbott’s evidence demonstrated, an owner’s description of a clinical sign can be inaccurate or exaggerated, often because of understandable concern. It is a common heuristic way to reason that coincidence of the occurrence of two or more facts equates to them being causally, rather than accidentally, connected. She drew such a connection in her mind and its influence affected her in recounting what occurred to Primetime. The applicant sought to use the material in evidence relating to Ms McLaughlin’s, Ms Busby’s and other persons’ reports of a horse’s presentation of clinical signs after vaccination as establishing that, first, the sign was biologically plausibly a side effect caused by Equivac HeV and, secondly, the postulated side effect was “serious” or “serious or debilitating”, based on those reports.
298 I had the benefit of the expert evidence that explained how difficult it is to draw the conclusion that a clinical sign is a side effect of Equivac HeV without the identification of some clear mechanism that reasonably can support a finding of causation. The expert evidence that I accepted did not suggest the existence of such a mechanism for any of the clinical signs on which the applicant relied as a side effect (in the cases I found above) that was other than transitory. I cannot form the opinion, based on the applicants’ reliance on the pharmacovigilance and related evidence, that the other reported clinical signs (including ones that were reported as more than transitory) were in fact caused as a side effect of the administration of Equivac HeV. That is because, absent some scientific veterinary basis, I would be speculating, in the same way that Associate Professor Sykes did, that the presentation of each such clinical sign was not only biologically plausibly caused by the vaccine, but more probably than not it was: Murray 33 ALJR at 524.
299 I am unpersuaded that any of that evidence established that it was more probable than not that the vaccine caused or was a cause of the various reported clinical signs (other than the ones I have accepted as transitory) or that any was or amounted to a serious side effect of it. I have not discounted that it is possible that some horse or horses may have had an idiosyncratic reaction in presenting one or more of those reported clinical signs and that it was or they were a side effect or effects of Equivac HeV. But one swallow does not a summer make. The expert evidence that I have accepted does not support such a conclusion and the likelihood of inaccuracy and incompleteness in the various untested documentary records of other persons’ reports in evidence leaves too many uncertainties to enable me to find with any feeling of persuasion that the vaccine caused, or was a cause of, any serious side effect: Murray 33 ALJR at 524.
9. The claim under s 54(1) of the ACL
300 The applicant pleaded that Zoetis supplied Equivac HeV to consumers, including vets, in trade or commerce during the relevant period and thereby contravened the guarantee of acceptable quality in s 54(1) of the ACL because it had serious side effects which rendered it not free from defects or unsafe within the meaning of s 54(1) and (2)(c) and (d).
301 Under s 3(2)(a) of the ACL, which defined “consumer”, a person was not taken to be a consumer if the person acquired the goods for the purposes of resupply. Thus, if a vet acquired Equivac HeV, as had to occur under each of the first, second, and third permits, Zoetis did not supply the vaccine to a consumer, as defined, but supplied it to vets for resupply. Here, vets supplied Equivac HeV to Ms Abbott and Ms Hinton for Primetime and Quinn. Accordingly, s 54(1) of the ACL did not apply to the supply of Equivac HeV to either of Ms Abbott or Ms Hinton.
302 The applicant pleaded that, unless the contrary were established, the vets were presumed to be consumers in relation to Equivac HeV by force of s 3(10) of the ACL. However, once the purpose of the relevant acquisition by the vets who administered the vaccine to each of Primetime and Quinn was established on the evidence (as mandated in each of the first, second and third permits), namely that the vet had acquired the dose to resupply it, the presumption in s 3(10) of the ACL was displaced. In addition, Ms Hinton’s evidence established that on 28 January 2016, she acquired the vaccine, which by then was registered, from a veterinary clinic.
303 Because this is a representative proceeding under Pt IVA of the Federal Court Act, it is possible that Zoetis did supply a consumer directly with Equivac HeV at some point during the relevant period after the Authority registered it on 4 August 2015. However, based on the applicant’s pleaded case, in paragraphs 46 and 63 of the statement of claim, the only reason why she alleged that Equivac HeV was not of acceptable quality within the meaning of s 54(2) was that there were serious side effects, the existence of which would have established that the no serious side effects representation also contravened s 18(1) of the ACL. Since I have not found that the no serious side effects representation contravened s 18(1) or s 54(2), it follows that the pleaded claim of a contravention of s 54(1) and (2) that may have applied to group members other than Ms Abbott or Ms Hinton must fail.
304 Ms Abbott had suffered a serious injury on 29 October 2003 when she fell off a horse while working for her then employer. She had a right shoulder reconstruction in November 2006. On 24 July 2012, she received an award of weekly workers compensation from the Workers Compensation Commission of New South Wales in respect of injuries to both shoulders and her spine, which it deemed to have occurred on 4 July 2010. The Commission found that her subsequent employment at another feedlot had exacerbated her earlier injury and resulted in a partial diminution in her earning capacity of $207 per week.
305 In 2008, Ms Abbott had received the gift of her mare, Primetime, for her 40th birthday. She said that Primetime had a heritage bloodline. She was, in Ms Abbott’s view, a high performance horse and, in due course, she expected her to be good for breeding. She also had care of another horse, Ervines Jive, which she had selected for purchase on behalf of another owner, and she had bought and sold other stockhorses in the past.
306 Ms Abbott gave evidence that, by March 2014, she regarded working in a feedlot as a stockperson or rider as “too physical for me”.
307 However, in June 2014, Ms Abbott commenced employment with JBS Australia Pty Ltd at its Caroona feedlot, near Quirindi, New South Wales. JBS is part of a group owned by Brazilian interests that is one of the largest beef producers in the world. JBS’ business here included buying and fattening cattle for its veal and export beef markets. Ms Abbott used Primetime for her work at the feedlot together with Jive, and later, other horses. She used two horses per shift, but favoured Primetime.
308 By early July 2014, JBS had implemented a policy that all horses at its facilities should be vaccinated. On 4 July 2014, James Palfreeman, JBS’ Australian manager of feedlots, emailed Richard Nicholls, the Caroona feedlot manager, responding to a query as to whether horses that were not, or not fully, vaccinated could be held in a designated paddock at the feedlot. Mr Palfreeman responded saying:
Unfortunately the simple answer is that this will pose a risk to the site as horses won’t have immunity and the business wants no risk….
Just as an aside Will tells me that when they discussed vaccination with one shift of pen riders this week one of them said that he had been looking to buy a horse from Murwillumbah in the last month for work at Riverina Beef. A horse died about 10 days ago there and 5 people were exposed to Hendra. The risk of this disease is going to be hard to manage without full vaccination.
(emphasis added)
309 On 8 July 2014, Mr Palfreeman sent an email to JBS’ feedlot managers informing them that Equestrian Australia had made a bylaw on HeV vaccination. He noted that a post on its website set out local council areas in Queensland and New South Wales that fall into “the Hendra Risk Zone but [it] also note[s] that in times of severe food shortage bats have been found in unusual places like Melb[ourne]”. He concluded this email saying that “[w]e should never lose sight of the fact that 60% of cases have proven fatal in humans so we are protecting people by doing this” (emphasis added).
310 Mr Palfreeman’s emails, from which I have quoted above, made pellucid that JBS had decided its own policy that it was not prepared to take any risk of HeV infection and that all horses at JBS’s facilities had to be vaccinated.
311 On 10 July 2014, Meg Wippell, the livestock manager at the Caroona feedlot, convened a meeting with stockpersons then employed there. Ms Wippell said that JBS was implementing a Hendra vaccination program and intended that all horses at the feedlot be vaccinated so as to be immune to HeV by 1 August 2014. She handed to each stockperson JBS’ HeV policy consultation document, for receipt of which Ms Abbott signed. At that or a subsequent meeting, Ms Abbott was told that JBS’ policy was that, if a stockperson did not have his or her horses vaccinated against HeV, their employment would be terminated. At a later meeting, Mr Nicholls told the stockpersons that JBS would pay for four of each of their horses to be vaccinated with the first two injections 21 days apart and booster shots 6 and 12 months later.
10.1.2 Primetime’s vaccinations with Equivac HeV
312 Ms Abbott was rostered off on the day that Dr Richard Frith, of Quirindi Veterinary Clinic, attended at the feedlot to vaccinate all the horses there. She went with her horses the next day, 29 July 2014, to Quirindi Clinic where Dr Lisa Goodchild vaccinated and microchipped them. She said in chief that she pointed out to the female vet (Dr Goodchild) “two very small, little bumps on her neck, which, laughably, was [sic] so tiny that only a mother would point it out on a child” and that these were like “insect bites”. She said that she asked the vet about them but the latter did not respond. Ms Abbott then reloaded her horses and returned to her lodgings. Ms Abbott said that Ms Wippell and Dr Goodchild told her that the vaccination was safe and the only side effect could be “minor [injection] site swellings and some increase in temperature, but nothing serious”, but if anything serious occurred a vet would be on call.
313 When cross examined about why in her earlier affidavit she had made no mention of the two bumps or her having indicated them to Dr Goodchild, Ms Abbott asserted that “it was an oversight”. I do not accept that Ms Abbott pointed out the bumps to Dr Goodchild or that they were evident on Primetime at this time. First, she omitted referring to this seemingly important matter when making her affidavit and, secondly, had she pointed them out to Dr Goodchild, it is implausible that Ms Abbott would have tolerated the vet ignoring her raising a concern. As she demonstrated in giving evidence, Ms Abbott had a forceful personality and was not one to be fobbed off, especially in relation to the welfare of horses about which she cared deeply. While it is possible that the lump existed at that time, it was not of any concern to Ms Abbott until it began to enlarge weeks later (see [330] below). Ms Abbott’s criticism of Dr Goodchild as insouciant illustrated her willingness to find fault with others.
314 Ms Abbott had drawn the picture below of Primetime’s head and neck that Associate Professor Sykes included in an addendum to his first report showing the location of the two lumps and the granulation and swelling that she said occurred later. She marked on the picture where the injection site was:
315 Ms Abbott gave oral evidence of a further lump that she claimed developed from where she marked “fluid” on the drawing. She described it:
now, it goes from there past the middle of her mane line,.. and then reduced to the back of her ears where the fluid settled, it continued then to grow. It is massive and it is now both sides of the horse’s neck. It goes from that spot from the bottom of the wither to, if I’m sitting on top of her, to halfway up her neckline – it is large. It looks like a Brahman hump and then it has little bumps all the way to the back of her ears.
(emphasis added)
316 There is no expert or other evidence about a lump of this “Brahman” like size and Ms Abbott did not record its presence in her emails to Dr L’Estrange on 5 October 2014 and 17 March 2015, or her letter to JBS of 9 March 2015. I do not believe that Primetime developed such a “Brahman hump” at any time related to her vaccinations.
317 The morning after the vaccination, Ms Abbott said that she noticed some slight swelling on Primetime’s neck as she was brushing her before work. She said that the mare was sensitive in that area and “not quite herself”, as the vet said might happen. She said that the lump, being the injection site swelling, lasted four or five days.
318 Ms Abbott said that between the initial vaccination and the first booster, on 20 August 2014, one of the two lumps grew rapidly to “something that was an inch in size”, which she pointed out to Dr Frith when he came to the feedlot to administer the booster. Dr Frith said that he did not have the expertise to remove the lump. I find that that was the first occasion on which Ms Abbott had mentioned the lump to a vet. Dr Frith attended with a student vet, Joanne Brumley. He said that they were also going to administer the combined 2 in 1 tetanus and strangles vaccinations and drench the horses, because JBS required this. Ms Abbott told him that her horses had had those vaccinations when they started working at the feedlot, but because they had never reacted to those vaccines she allowed the further vaccinations. She rode Primetime for a short period after her vaccinations and then put her in her trailer and returned to her lodgings.
319 The next day Ms Abbott said that Primetime was “supersensitive all over” and that “I felt like I was poking her with needles”, which had never occurred before. She said that there was injection site swelling on her neck, the hair on her mane had started to thin, her gums were white, and her coat had gone dull. She said that when she began riding, the horse began puffing fast, stressing and sweating. She soon changed horses to ride Jive because Primetime was not coping. Ms Abbott said Primetime remained in that condition, not herself, for some days.
320 When she was next rostered off she took Primetime to Dr Timothy Hill, a vet at Tamworth, to remove the lump, which she said was in the vicinity of the injection site. Dr Hill surgically removed the lump. Ms Abbott asked for it to be tested and blood samples taken.
10.1.3 Ms Abbott’s conversation with Dr L’Estrange
321 On 4 October 2014, after the removal of the lump, Ms Abbott telephoned Dr L’Estrange. He made a contemporaneous file note of their conversation, as was his practice. I prefer Dr L’Estrange’s evidence where it conflicts with Ms Abbott’s in relation to their discussions because, first, he made a contemporaneous note and, as was his practice, is likely to have recorded any matter of significance as to what Ms Abbott stated about the possible effects on, or condition of, her and other horses relating to their vaccinations, secondly, I found him to be a reliable and truthful witness, and thirdly, for reasons that I explain below, I found Ms Abbott to be an unreliable witness.
322 The conversation lasted about one hour. Ms Abbott told Dr L’Estrange that her horses had a relaxed nature. She said that Dr Frith had vaccinated them at precisely the same spot for the Equivac HeV and combined tetanus and strangles vaccines. When she was cross-examined about that assertion, she answered that Ms Brumley had performed the two injections, saying “I didn’t actually look for exactly the same dot, but too close, too close. Had I been aware of the possible problems I would have actually asked to go to the other side” (emphasis added). When pressed that, for all she knew, the injections have been a centimetre apart, she agreed. I formed the view that her account was a reconstruction based not on any recollection but on her belief that the vaccinations had caused the later health problems that Primetime experienced. I do not accept that Ms Brumley used the same injection site for the two vaccinations. Indeed, Ms Abbott admitted, in the words I have emphasised in her answer above, that she had not looked for that site at the time.
323 Ms Abbott also said that after the second vaccination, horses at the feedlot had a generalised sensitivity all over. She said that Primetime had not cycled (ie: come into season) since being vaccinated, even though she had been due to cycle one week after the vaccination. She told Dr L’Estrange that, subsequently, Dr Hill had removed a tumour from the horse’s neck and taken blood and more tumours had appeared. She told him that her horse had white gums (which Dr L’Estrange noted as pale mucous membranes) for 13 days, and from two days after vaccination did not want to be brushed or saddled, became worse for the next 21 days, and was stiff and unsteady on her feet. She told him that some of the horse’s mane had fallen out. She then said to him that the mare “actually looked fabulous and you wouldn’t know [any of what Ms Abbott recounted] if you didn’t know the horse”. She admitted in cross-examination that this description was true and that Primetime did then look fabulous. She complained to Dr L’Estrange that the tetanus and strangles vaccination had been given at the same time as Equivac HeV. She also said that the horse had been wormed (ie: drenched).
324 She told him that other riders’ horses had issues with loss of mane hair and diarrhoea, and that the feed for horses at the feedlot was “dodgy”, which he suggested to her may have accounted for the diarrhoea. Ms Abbott agreed that she had told Dr L’Estrange that she thought that the food for the horses at the feedlot was “dodgy” and that other riders’ horses had had diarrhoea. I do not accept her denial that she also had said to him that their horses had lost mane hair. She agreed that if food was not of good quality that fact needed to be considered as a possible cause of the presentations of conditions that the horses at the feedlot gave, saying “I wanted to know what was wrong, yes”. In my opinion, she decided to ignore the poor food as a cause and instead focussed on the vaccinations with Equivac HeV.
325 Ms Abbott told Dr L’Estrange, at a later meeting with Dr Frith, all the riders indicated what observations they had made of their horses. She said that Dr Frith pressed his professional opinion that those observed conditions were not due to the vaccine but, as Ms Abbott said in cross examination, she did not accept his opinion: “And I still don’t. I was actually more prepared to accept it then than now”. That answer demonstrated that Ms Abbott had convinced herself that Equivac HeV was responsible for the conditions that Primetime and the other horses presented regardless of the facts. Later, she changed her evidence to say that she had used her own, not the feedlot’s food. I do not accept that evidence.
326 Dr L’Estrange told Ms Abbott that he would be happy to speak to the other riders. He had already spoken to one, Chris Hopkins, who had contacted him earlier. Dr L’Estrange told Ms Abbott that the most likely adverse event from a vaccination would be swelling at the injection site.
327 On 5 October 2014, Ms Abbott emailed Dr L’Estrange the pathology report for Primetime’s blood sample that Dr Hill had taken on 22 September 2014. This showed that the horse’s red cell count was normal, not anaemic, as it would have been if the horse had white gums. Dr L’Estrange said that the blood tests indicated that only three results were outside the normal range, including one for urea, indicating muscle damage that could be caused by bringing the horse into the yard to be tested, and none was indicative of any significant illness.
10.1.4 Dr L’Estrange’s enquiries about Ms Abbott’s concerns
328 On 9 October 2014, Dr L’Estrange spoke to Dr Hill, who referred to Ms Abbott and her complaint concerning the alleged effects of vaccination with expletives such as “bullshit”, “nutcase” and “crazy”. Dr Hill told him that none of the issues with her horse was vaccine related and that the mare most probably had a viral infection or one of the seasonal viruses that were common in the area.
329 Dr L’Estrange had a practice of reporting conversations giving feedback or information relating to possible clinical signs or side effects associated with vaccination to Zoetis’ pharmacovigilance section, the senior person in which was Susan Boorer. On 9 October 2014, he emailed Dr Tina Skuodas, who was also a member of Zoetis’ pharmacovigilance section a summary of his conversations with Ms Abbott and Dr Hill.
330 Ms Abbott provided Dr L’Estrange with a copy of the histopathology report for the tumour that Dr Hill had removed on 5 September 2014 from the lower third of Primetime’s neck close to her shoulder (withers). That report noted that the tumour had been “Observed three weeks previously and reported to have noticeably enlarged since”. The diagnosis was that the tumour was “chronic” and “focal”, which Dr L’Estrange explained meant that it had not suddenly appeared, but had been long term and in a small, localised area. The report said that the tumour appeared to have blood cells evidencing an allergic reaction, perhaps from parasites or biting insects. The report stated: “while the cause is not known, a chronic localised hypersensitivity response, possibly to biting insects, is suspected”. Dr L’Estrange opined (and I accept that), based on the report, the tumour was not caused by any vaccine.
331 Ms Abbott said in evidence that in about early 2015, she sold Jive for $16,000. She said that, from September 2014, “it was all decline” with Primetime, the granulation spread on her neck and presented as swelling on both sides. She said that one of the mare’s eyes was dull and disinterested and she was stiff in movement and never recovered.
332 On 17 October 2014, Ms Abbott visited Dr Alan Simson with Primetime. Ms Abbott said that his speciality was as a reproductive vet. He noted that the mare had been vaccinated with Equivac HeV and, in the previous month, Dr Hill had surgically removed lesions from her neck. Dr Simson wrote an undated note on his clinic’s letterhead, after he saw the mare again on 14 January 2015, recording his earlier observations. On 17 October 2014, Ms Abbott told him that she proposed to breed the mare in the next few days and he examined her for that purpose. He observed that the mare presented with scarring and stiffness in her neck and that because of her neck lesions she would be unsuitable to ride. She appeared “a little dull”, so Dr Simson took blood which revealed anaemia and increased bilirubin. Dr Simson suggested a course of iron, vitamins and minerals for Primetime.
333 Ms Abbott asserted in cross examination that on 17 October 2014, Dr Simson did not tell her not to ride Primetime but only had done so after he received the results of the blood pathology. She disputed that he had told her not to ride the horse because of the neck lesions. I do not accept that evidence. Ms Abbott took Dr Simson’s advice and gave Primetime iron, vitamins and minerals which improved her condition. This showed that the condition of the horse, as revealed by blood tests, had deteriorated after Dr Hill had taken blood samples on 22 September 2014 (see [327]), well after the Equivac HeV vaccinations, and that Ms Abbott’s reconstruction of events and observations were unreliable.
334 On 26 October 2014, Dr Skuodas, on behalf of Zoetis, provided a pharmacovigilance report to the Authority in relation to Primetime and Ms Abbott’s reporting of her concerns. The report gave an N (unlikely) ABON classification in respect of Ms Abbott’s report and referred to Dr L’Estrange’s discussion with her and Dr Hill. It expressed the view, based on Dr Hill’s assessment, that none of Ms Abbott’s reported observations of Primetime were vaccine related and those were most likely to be due to seasonal viruses.
335 Ms Abbott mated Primetime in early November 2014, but she did not fall pregnant until her next cycle.
336 On 10 November 2014, the Authority sent Zoetis a report that it had received from Ms Abbott. It recorded that she asserted that Dr Simson would be treating Primetime and that her blood tests had indicated that her “immune system is being compromised”.
337 On 8 December 2014, Ms Abbott injured her neck at work and required medical treatment including x-rays. She took time off work and had pain in her left shoulder and neck. By this time, she was disaffected with JBS as an employer and its compliance with what she regarded as its animal welfare obligations, both in respect of horses and cattle. She said that, in her belief, JBS was “absolutely not” even providing the bare necessities for the horses at the Caroona feedlot. She criticised JBS’ treatment of cattle as “disgusting”, but it was the only large facility near to where her parents lived and she wanted to be near to her father who was then unwell.
338 On 14 January 2015, Ms Abbott took Primetime to Dr Simson again for a pregnancy examination at which he found that she had conceived. He took blood and noted that the results had improved.
339 On 10 February 2015, Ms Abbott emailed Ms Wippell informing her that there was “a huge shadow over the Hendra vaccination reactions”. She implored JBS, hoping that “a higher power will put a stop to this un-necessary use of this ‘un-registered vaccine’ which may also have legal implications”.
10.1.5 JBS’ dismissal of Ms Abbott
340 By about March 2015, when booster shots were due, Ms Abbot had formed the view that it was not in Primetime’s best interests to be vaccinated against HeV again “given that she was unable to perform work duties any more”. She said that she made the decision after “I had done some research and sought professional advice”. She wrote to JBS on 6 March 2015, saying that she would not be revaccinating her horses against HeV because the vaccine was an unregistered product subject to a minor use permit “for high risk locations, which we are not” and she was concerned for their welfare. She noted that JBS had refused to reimburse her expenses or those of other riders after the earlier vaccinations. At this time, Ms Abbott was on leave recovering from shoulder reconstruction surgery to treat an old injury.
341 Also on 6 March 2015, Ms Abbott’s fellow stockpersons, including Mr Hopkins, wrote to JBS that they too would not be revaccinating their horses.
342 On 8 March 2015, Lauren McNally, JBS’ compliance supervisor of the Caroona feedlot, emailed Ms Abbott, following their conversation earlier that day, with a letter, written by Mr Nicholls as manager. The letter referred to Ms Abbott’s recent breach of JBS’ Work Health and Safety Policy and standard terms and conditions of employment because of her deliberate insubordination in failing to follow JBS’ instructions based on her refusal to have her horses revaccinated. The letter informed her that JBS had formed the preliminary view that her employment should be terminated, but offered her the opportunity of responding with reasons to the contrary by 11 March 2015. The letter enclosed a copy of JBS’ policy dated 2 September 2014 that required all horses that worked or resided on a JBS site to be vaccinated against HeV, as “the most effective means of preventing infection and spread of the disease to horses and employees”. The policy acknowledged:
JBS recognises that the risk of infection is lower in areas where environmental conditions are unsuitable and fruit bats are not endemic. JBS sites located in southern inland New South Wales are a lower risk.
343 Later on 9 March 2015, Ms Abbott wrote a long response to JBS. She asserted that Primetime and Jive had been vaccinated with Equivac HeV twice, with the second injection occurring only 18 days after the first. When cross examined about the veracity of that timing, Ms Abbott agreed that this was untrue but prevaricated, first suggesting that the interval was 20 days before accepting that she knew that it was 21 days and then backtracking to a gap of 20 days. She said “I’m surprised I’ve got 18 days there”. She knew that her letter had sought to exaggerate problems about the vaccinations and the impacts she alleged they caused to her horses. In the next paragraph she stated:
The horses scoured, went off their feed, developed dull coats, displayed soreness over their entire body, swelling over Kidneys, joint swelling, weakness, hair loss, swelling on the neck, lack of energy, severe [sweats] and continued to show declining health. On day 9 Chris Hopkins’ horse died and from all reports terrible symptoms prior to being ‘Put To Sleep’.
344 She gave evidence that this paragraph referred to the effects on her horses, Primetime and Jive, as well as on the majority of the other stockpersons’ horses at the Caroona feedlot. She had made no complaint earlier to Dr Frith or Dr L’Estrange about her or other horses having swelling over the kidneys or scouring or soreness over their entire bodies, and I do not accept her oral evidence that she had made such a complaint to Dr L’Estrange. However, by early November 2014 she had complained to the Authority about Primetime suffering “total body pain for 7 weeks after” and was scoured.
345 Apart from Ms Abbott’s assertions as to vaccination being the cause of death for Mr Hopkins’ horse, Subbie, there was no expert or other admissible evidence that identified the cause of that horse’s death. I can make no findings about that unfortunate event.
346 In the letter of 9 March 2015, she accused Dr Frith of failing “to advise the chemistry test results which we later discovered had abnormalities”. She explained, in cross examination, that the “abnormalities” were the three minor blood test results that previously Dr Frith and Dr Hill had told her had no clinical significance. Associate Professor Sykes confirmed, in his expert report, the elevations in the three results for Primetime’s samples taken on 22 September 2014 were clinically insignificant. When cross examined about why she criticised Dr Frith in this letter, she said:
--- Because he’s not being open and honest with the people who actually own these horses and are … concerned about them.
All right? --- He’s withholding information. The second meeting he walked into the room with the bundle of the biochemistry portion [sic] and said, I didn’t bring these last time. Am I bad?
347 That evidence, which I do not accept, is another example of Ms Abbott’s propensity to overdramatise and exaggerate matters that she conceived would advance her case.
348 In the letter, she asserted that Primetime had “been valued in excess of $25,000”. There was no expert evidence of any value for that horse, or for Jive to which she ascribed a value of $16,000 in the letter. Ms Abbott gave oral evidence that in 2012, at the Paradise Lagoons Campdraft, Phillip Kirkby, a breeder of stockhorses and a past president of the Australian Stock Horse Society remarked of Primetime that “She would be worth $25,000 or $30,000”. Ms Abbott said in oral evidence that the Landmark Classic sale in 2015 was the largest stockhorse sale in Australia and that a mare of Primetime’s then age of 11 had a value of $14,000. She asserted that as a result of her vaccinations in 2014, the mare was then worth about $3,000 “because you can’t ride her and her breeding performance is extremely poor”.
349 Zoetis tendered the catalogue and results of the 2015 Landmark Classic sales. This showed an average price of $12,379 for the 480 lots sold. The average price for the 281 mares sold was $14,270 with the highest of those sales being $80,000. Most of the mares appeared to be four or five year olds, much younger than Primetime. The applicant provided no analysis to relate those averages or prices to the worth of Primetime. I limited the use of Ms Abbott’s statements relating to value under s 136 of the Evidence Act as evidence of communications or her assertions only and not of the truth of their content, which needed to be made by a person qualified to give an expert opinion.
350 On 13 March 2015, Mr Nicholls, on behalf of JBS, responded to Ms Abbott’s 9 March 2015 letter. He said that JBS had made its decision to implement its HeV vaccination policy “after lengthy consultation with industry experts including the Principal Veterinary Epidemiologist at Biosecurity Queensland – Department of Agriculture, Fisheries and Forestry”. He wrote that JBS recognised the risk that HeV posed and was committed to providing a safe working environment. He offered Ms Abbott one more opportunity to indicate her acceptance of JBS’ vaccination policy by 17 March 2015.
351 On 17 March 2015, Ms Abbott replied, adhering to her decision not to revaccinate her horses. She said that she “would have preferred to see JBS consult Biosecurity New South Wales” and that State’s Department of Primary Industry.
352 In her letters, Ms Abbott had sought that JBS compensate her for all her expenses, which she also claimed as damages in this proceeding, in dealing with what she asserted were the consequences, among others, of Primetime’s HeV vaccinations. JBS declined.
353 Also on 17 March 2015, Ms Abbott emailed Dr L’Estrange. As she accepted in cross examination, by this time Ms Abbott was trying to establish that the vaccinations had caused Primetime’s problems. She wrote:
Last August (you may recall) our horses experienced quite severe reactions after being given 3 in1 vaccines and also drenched.
My mare Primetime was given the 3 in 1 but no drench. Her reactions so severe that my Vet, Dr Alan Simson phoned me after follow up bloods had been taken from Primetime and instructed me to stop riding her. After very expensive treatments I have managed to regain what seems now to be healthy horse BUT what remains in her neck is a mass of granulated tissue in her neck that irritates her when touched.
I am being stood down from JBS because I will not allow re-vaccination of my horses because I have been advised from my vet and [the Authority] that reactions as a rule become worse. Is this correct?
(emphasis added)
354 Also, on 17 March 2015, the Authority wrote to Ms Abbott informing her that it had given “possible” classifications to the presenting signs of, first, Primetime, for alopecia (localised), lump (local), pain, pale mucuous membranes and stiffness, and secondly, Jive, for injection site reaction, oedema, pain and pyrexia.
355 On 18 March 2015, Dr L’Estrange spoke to Dr Simson about Primetime. He requested copies of the blood test results and any recollection of clinical signs that the horse presented. Dr Simson said that he had not recorded a history. He told Dr L’Estrange that Primetime was pregnant. Dr Simson also told him that he had seen a number of horses with delayed signs after vaccination and Dr L’Estrange asked him to provide him with the details, however Dr Simson did not appear to have done so.
356 On 20 March 2015, Mr Nicholls on behalf of JBS wrote to Ms Abbott terminating her employment on the basis foreshadowed in its letter of 9 March 2015.
357 On 23 March 2015, Dr Simson emailed Dr L’Estrange informing him that when he saw Primetime on 16 October 2014 she had marked engorgement of her neck blood vessels, a divot where Dr Hill removed “a supposed abscess or reaction to the vaccine” and “indication of inflammation and infection of blood vessels on the left side of the neck”. He said that when he re-examined the mare in mid-January 2015, the neck “looked pretty close to normal”. Dr Simson enclosed two blood test results. Given that Dr Simson did not record a history of his examination on 17 October 2014 and his account was different to that in his undated note written after 14 January 2015, I place little weight on his email of 23 March 2015 where its contents differ from the more precise undated note (see [332] and [338] above).
358 Ms Abbott asserted in evidence that when she later tried to use Primetime again as a stockhorse, after breeding a foal from her, she was unstable on her legs and too dangerous to ride for her work. She said that she could not keep the horse in foal and the mare had lost a foal to red bag after 2015. Since then, she said that there have been no significant problems with Primetime.
359 In 2014, Ms Abbott began communicating on a “Say No to the Hendra Vaccination” Facebook page (the “Say No” page). She said that contributors to the “Say No” page had horses that had experienced adverse reactions to Equivac HeV and offered each other support. She said that there were thousands of members. She made a post that stated that one vaccination had been fatal (meaning her assertions about Mr Hopkins’ horse, which she said, in evidence, he had shot when mustering at Merewether 10 days after its vaccination and that no vet had diagnosed its condition or performed an autopsy). Ms Abbott said that she had “strong feelings about this product” and had made her postings to the “Say No” page expressing them. For example, she said that she had made another post alleging that two horses had died because of Equivac HeV and that a replacement horse, that she had obtained, died of colic because the vet would not treat the animal as a result of it not being vaccinated. She gave evidence that, in one post, she had intended “absolutely” to call Dr Frith a goose and to affect his reputation.
360 When pressed in cross examination about how she could have made the definite allegation concerning Mr Hopkins’ horse when she knew that extremely serious things could happen to horses independently of any vaccine, including the example of the horse that had died of colic without being vaccinated, she gave the following evidence:
I myself was not against … the vaccine. That’s why I had allowed it to be given to my horse. I believed what I was told, that it was a safe product. I’m not an anti-vaxxer but I don’t like this product.
You vaccinated your horse because you wanted to keep your job? --- No. Incorrect. Incorrect. I would have much preferred to have kept my horse and gone to a different job.
…
At the time that you made this unqualified statement, that the vaccine caused this fatality, you knew that bloods had been taken of a number of horses at the feedlot where this horse had been vaccinated. You knew that experts had looked at those bloods because there was a concern about whether or not the vaccinations were causing problems with the horses and Dr Frith had come along and assured the stockies that the vaccine wasn’t causing the problems. That’s right, isn’t it? You didn’t accept that? --- I wasn’t convinced.
Dr Hill told you that the bloods taken of your horse didn’t establish any connection- - -? --- No.
- - - between the condition of the horse and the vaccine but you didn’t accept that, did you? --- No.
Dr L’Estrange was also of the same mind, wasn’t he? Having reviewed the pathology he said to you that it wasn’t related to the vaccine? --- Correct.
(emphasis added)
361 The following is an example of her bias or implacable view that coloured how she gave her evidence about her horses and any possible relationship of their presentation with their vaccinations:
And you accept, don’t you that the decisions that were being made by vets as to whether they would expose themselves to a risk of treating horses with the Hendra virus was for their own wellbeing?---Once again, the circumstances around how those vets contracted the virus from the horses is questionable.
…
… it is a fact that’s well-known to you and you accept that … vets treating horses with the virus, which were not being vaccinated obviously, died?---Who? But they’ve changed their practices since then. The vets have learnt a lot out of that.
But you’re critical of vets for not – you were talking about the product being – the vets boycotting the – or some words of that nature?---Boycotting the entire horse industry. They did.
…
And one of the major advantages of vaccinating your horse is that you can be treated by any vet?---And the downside is it could cause them death.
And you’re very happy to tell people on this website that it is causing death?---No. I’m saying it could.
(emphasis added)
10.3 Did Primetime suffer any side effects from Equivac HeV?
362 Associate Professor Sykes and Dr El-Hage gave expert evidence about whether Primetime had suffered any side effects from her vaccination with Equivac HeV. Professor Slater was not asked to opine on this horse.
363 In the joint report, Associate Professor Sykes and Dr El-Hage agreed that there was insufficient veterinary diagnostic information about Primetime to enable an accurate assessment as to whether the vaccination caused any side effect in her.
364 As Associate Professor Sykes noted, because Primetime’s second Equivac HeV vaccination occurred concurrently with the administration of the tetanus and strangles vaccination, no specific causal link to the former could be made in respect of any reported clinical sign. Moreover, there were no contemporaneous blood tests or veterinary examinations of Primetime immediately after either Equivac HeV vaccination. He observed that the three results that were outside normal range in the blood sample that Dr Hill took on 22 September 2014 were clinically insignificant, two were mild and the slight elevation in her albumin was most likely to be due to biological variation rather than disease. He observed that there was a reported temporal association between the administration of Equivac HeV and the reported increase in mass of the lump subsequently removed but he said that it was not possible to attribute Equivac HeV as the cause of that increase.
365 Associate Professor Sykes also believed that the chronic clinical signs that Ms Abbott reported between September 2014 to January 2015 were “biologically plausibly attributable to injection with the Equivac HeV vaccine in a manner similar to reports of the post-vaccination phenomenon ASIA [autoimmune inflammatory syndrome] in people”.
366 However, ASIA is primarily linked to the use of chemical adjuvants. As I noted above (at [231]), at the time he wrote his first report, Associate Professor Sykes mistakenly understood that Equivac HeV had a chemical adjuvant.
367 In his report, Dr El-Hage observed that despite her affidavit description of Primetime as lethargic, having sloppy faeces and reduced appetite in the period following her first vaccination, Ms Abbott had no hesitation in riding her on that and the following days. She then reported an injection site lump that persisted for four days. Dr El-Hage observed that Ms Abbott had reported that, after the second vaccination, Primetime at first was sweating, intolerant of exercise and later touch sensitive, especially over the injection site, irritable, suffered hair loss over her loin, had a dull coat, had developed a cough, had nasal discharge and loose stools. None of these reported clinical signs was investigated by a vet. He noted that Ms Abbott reported that the mare made steady improvements over the following months. He noted the blood samples revealed that her fibrinogen level was 5 g/L, slightly higher than the 4 g/L normal level, which was indicative of mild inflammation and consistent with recent vaccination, especially if there were some swelling and inflammation of the injection site.
368 Dr El-Hage noted Dr Simson’s observation that (as at 17 October 2014) her coat was dull and her demeanour “dull and listless”. Dr El-Hage opined that Dr Simson was a well-regarded equine reproduction practitioner who was satisfied that, at that time, Primetime was sufficiently suitable and robust to be presented in three days’ time to a stallion to become pregnant. As Dr El-Hage said, Dr Simson would have been unlikely to have advised proceeding with that recommendation if the mare was obviously ill and of unsound health.
369 Dr El-Hage noted that Ms Abbott had reported, without specific dates, history, any pathology or testing, that Primetime had slipped (ie: aborted) two premature foals. He said that abortion was a serious matter but the absence of any detail as to what had happened and any veterinary or pathology reports as to any cause of any abortion excluded the capacity to make any link.
370 I accept his evidence that none of the signs, other than the abortions, as he said Ms Abbott had recorded in her affidavit, could be categorised as a serious adverse experience. He opined that the reported clinical signs after vaccination were consistent with transient lethargy and inappetence. I note that Dr El-Hage accepted Ms Abbott’s assertions at face value. She did not repeat all of them in her oral evidence (and her affidavit was not read). I have little confidence in Ms Abbott’s accuracy as an historian given her tendency to view past events through her fixed perspective that the vaccinations were the cause of all her (and other) horses’ clinical signs.
371 Dr El-Hage opined, and Associate Professor Sykes agreed, that severe infectious conditions that are endemic in eastern Australia could have caused clinical signs that Ms Abbott reported in Primetime. In their joint report, they opined that due to the extended period over which Ms Abbott reported clinical signs for Primetime and the lack of detailed veterinary diagnostic information for that period, it was not possible to distinguish mere temporal association (or coincidence) from any causal mechanism linked to the Equivac HeV vaccinations. Moreover, no vet conducted a thorough diagnostic evaluation of the mare, other than Dr Simson in respect of her fitness to breed, and the information in the blood tests (that were not taken close to the times of vaccination) and the pathology reports for the removed lump did not reveal any causal link to the vaccination. Both Dr El-Hage and Associate Professor Sykes agreed that a sampling error or storage artefact compromised the interpretation of Primetime’s blood analysis and other results that Ms Abbott had reported. Thus, Dr El-Hage and Associate Professor Sykes concluded that they did not have sufficient diagnostic information to enable them to make an accurate assessment of what, if any, connection existed between Ms Abbott’s reported clinical signs of Primetime’s condition and her Equivac HeV vaccinations.
372 Accordingly, there is no expert evidence that establishes a causal link between any of the clinical signs that Ms Abbott reported (even had I accepted the accuracy of all of her reports) and Equivac HeV. I am not satisfied that Equivac HeV had any role in any clinical sign or conclusion that Primetime experienced.
373 It follows that Ms Abbott has failed to prove her claim and it must be dismissed.
10.4 The paucity of the evidence on damages
374 During the early part of the hearing, I gave the applicant leave to introduce non-expert evidence of loss and adjourned the oral evidence on that topic of both Ms Abbott and Ms Hinton to later in the trial. That was because the lawyers for the applicant had not addressed the topic of damages in pre-trial evidence or exchanges of information. This emerged as an issue when senior counsel for the applicant, Mr C Barry QC, sought to lead oral evidence in chief from Ms Abbott about Primetime’s value, when she was not qualified to express an opinion as to the value of horses.
375 Mr Barry QC explained that, when Wigney J was case managing the proceeding, his Honour had made observations that questions as to quantification of damages that any group member had sustained would have to be decided after resolution of the common questions. Mr Barry QC said that his side had mistakenly interpreted his Honour’s remarks to mean that there would be a separate hearing on damages for all group members, including Ms Abbott and Ms Hinton, when, as Mr Barry QC later realised, his Honour had not been discussing their claims.
376 I accepted that an error had occurred but was not prepared to adjourn the trial part-heard. I allowed Ms Abbott and Ms Hinton to give some evidence as to damages on the voir dire before each was cross-examined and gave them and their lawyers more time to collect documents and prepare lay evidence to support their claims for damages. I sought to balance their right to bring their claims, despite the genuine error of their lawyers, with Zoetis’ entitlement to know and be able to prepare for the case on damages as part of the trial. Had I allowed the applicant and Ms Hinton to lead expert evidence, Zoetis would be entitled to do the same, which could not have occurred if the trial were to continue.
10.5 Ms Abbott’s damages claim
377 Ms Abbott claimed that, because of Zoetis’ misleading conduct, JBS had terminated her employment. She claimed that she had suffered past economic loss of $69,287.63 for the period 20 March 2015 to 30 June 2018, being loss of future earnings based on her income at JBS prior to her termination, the cause of which she asserted had been one or more of the geographic spread, no side effects and all horses representations. She also claimed treatment expenses for Primetime of $2,165.81, damages for the diminution of her value of between $25,975 and $68,000 based on sale results of other horses at the 2021 National Classic sale (being, apparently, the new name for what in 2015 was called the Landmark Classic sale) about which Ms Abbott had given evidence earlier in the trial (see [348]-[349] above) and the value of the loss of four foals of $86,264 (an average of $21,566 each).
378 Had I found Zoetis contravened s 18(1) of the ACL, nonetheless I would have rejected each of Ms Abbott’s claims under the three heads of damage.
379 Ms Abbott’s past economic loss claim was dubious because she is unlikely to have undertaken any further work as a stockperson for JBS or anyone. At the time of her dismissal, Ms Abbott was 46 years old. On 9 February 2015, Ms Abbott underwent shoulder reconstruction surgery at the expense of one of her previous employers’ insurers, Allianz Australia Workers’ Compensation. She provided JBS with a medical certificate that she would be unfit for any work between 9 February 2015 and 20 March 2015 and advised that she would probably need a total of 10 weeks’ leave to obtain a sufficient level of rehabilitation.
380 After her dismissal, she began working as a farrier. She asserted that, had she not been dismissed, she could have returned to work at JBS on light duties, once her shoulder had resolved sufficiently after surgery. However, had she remained in its employ immediately after 20 March 2015, she was obviously dissatisfied with working for JBS and is likely to have sought other work. Given her antipathy to JBS at that time and the wide range of skills that her work experience had given her, I consider that she would have sought other employment as soon as she could, had she not been dismissed. I do not accept her claim that she would have stayed working for JBS until 30 June 2018. She did not work as a stockperson again. She did not lead evidence of other employers who employed stockpersons but did not require their horses to be vaccinated with Equivac HeV.
381 As Zoetis established in its closing written submissions, Ms Abbott’s tax returns for the period between 1 July 2014 and 30 June 2018 showed that, first, she earned income and received some workers compensation payments over the period after 20 March 2015 and, secondly, she claimed tax deductions for the expenses incurred in earning her assessable income that were not factored into her claim for past economic loss. By the 2017-18 financial year, her taxable income was $42,122 which was nearly twice what it was when she worked for JBS. Had it been necessary to resolve this claim, I would have found a loss in the order of about $15,000 for the period of about 39 months to June 2018, after allowing for vicissitudes.
382 Ms Abbott claimed treatment expenses for blood and hair tests of $181.50 and $1,196.14 that Dr Simson performed on Primetime. She also claimed $216.22 for a consultation with Dr Hill on 21 October 2014, but did not provide any invoice to support its connection to her claim. I am not satisfied that the claim for these expenses has been established. Had she established that Primetime suffered serious side effects from Equivac HeV, proved expenses such as these would have been compensable.
383 Last, Ms Abbott claimed $571.95 in expenses incurred in February 2021 for which she gave no basis to connect these to the vaccinations in mid-2014. I would have rejected this claim.
384 Ms Abbott also claimed for diminution in Primetime’s value and her loss of four foals. Ms Abbott contended that she was entitled to damages for the loss of the value of Primetime because she could no longer use her, so she claimed, as a working horse for her occupation as a stockperson but had to use her to breed. She gave no evidence of working as a stockperson after 2014. As I said, there was no evidence that Primetime suffered what is called red bagging or a form of miscarriage as a result of Equivac HeV. Primetime bred two mares but there is no evidence of their value or sale prices.
385 Apart from Mr Barry QC asking her to verify that she was making this claim and tendering, as part of it, the 2021 National Classic sale results, there was no additional evidence of value beyond Ms Abbott’s evidence about communications discussing horse values (see [348] above) and her referring to the average sale price from 2015 Landmark Classic sales of $12,379 (see [349] above). Because Ms Abbott gave no probative evidence about these matters, I am unable to find any value for Primetime or that the mare lost, or lost the capacity to bear, any foals. As Ms Abbott said, the mare produced two foals but she gave no evidence about their sale or condition. The value claimed for the foals is near to the average sale price of 497 lots of geldings, mares and stallions sold at the 2015 Landmark Classic sale. There was no breakdown of prices for foals. That average price proved nothing about the particular value of Primetime or any of her foals. Without expert evidence as to Primetime’s value before and after the alleged impact (which I have rejected) of Equivac HeV on her and of the value of foals as progeny of such a mare in each year in which that progeny might have been sold had they existed, this claim has no basis and I would have rejected it as unproven.
386 As I pointed out during the course of argument, the sale prices in the 2015 Landmark Classic or 2021 National Classic sales were unhelpful given that there were presumably mares in a similar condition to Primetime, assuming she was sound at the time of the sale, and a similar age. But the applicant’s counsel were not even prepared in her final submissions to perform the exercise of identifying a mare that may have been comparable or her sale price. And, even if they had, that would not address the critical issue of what, if any, diminution in value occurred between the assumed condition of Primetime being equivalent to the average mare of the same age and condition sold at that sale, and her value after the vaccination. Presumably, Primetime still had a value as a horse that was capable of producing, and had produced, two foals.
387 I am not satisfied that Equivac HeV had any role in the presentation of any clinical sign or condition that Primetime experienced on which Ms Abbott relied as a serious side effect of vaccination. It follows that Ms Abbott had failed to prove her claim and it must be dismissed.
388 Ms Hinton based her claims on the geographic spread and no serious side effects representations being misleading and Zoetis having contravened the consumer guarantee that Equivac HeV was of acceptable quality in s 54(1) of the ACL.
389 I considered that Ms Hinton was an honest witness. She was intelligent and thoughtful. She developed an interest in gypsy cob horses. She explained that the breed originated in Ireland where Romani used them to pull wagons. They are sturdy, quiet and with a good temperament, suitable for children. She said that they have coats with a lot of colour in them, describing them as “flashy” and like a “miniature Clydesdale”. They are used and compete in a variety of sports, pony clubs and dressage.
390 In 2008, Ms Hinton was looking at horses and saw a photo of a black and white pinto gypsy cob and, as she described, “I immediately fell in love” and began researching about the breed. She learnt that there were not many in Australia. She and her husband decided to buy a gypsy cob and searched for a suitable stallion at studs in the United Kingdom and United States.
391 Eventually, they decided to buy a two year old pinto coloured stallion, Quinn, from a stud in Oregon for USD15,000. By the time Quinn arrived in January 2009 at the family property at Worongary in the hinterland of the Gold Coast, Queensland, Mr and Mrs Hinton had outlaid a total of about AUD57,000 (including the purchase price). They arranged for a professional trainer to break him in and train him. Ms Hinton competed with him in dressage events including the Queensland State Championships. She showed him at the Ekka on multiple occasions and also entered a competition at the Pan Pacific Masters Games where they won a bronze medal in elementary freestyle dressage. Ms Hinton became president of the Australian Gypsy Horse Society.
392 Ms Hinton worked full-time and she and her husband had a young son. They ran Dellifay as a hobby. They charged $1,500 as Quinn’s service fee and they also obtained straws of his semen which were frozen and stored.
11.1.2 Decision to vaccinate Quinn
393 When Equivac HeV became available, Ms Hinton read about, saw advertisements and discussed it as a topic of interest in the equine industry. She saw it advertised in numerous magazines including Horse Deals, Hoofbeats and Equestrian Life.
394 As emerged more fully in her cross examination, between at least 2012 and 2015, Ms Hinton had made herself very familiar, and kept herself up to date, with a considerable amount of publicly available material about the risks to humans and horses of contracting HeV. This included where those risks were present, in terms of location, and more acute, such as the area in which she and her family lived. She searched and or subscribed to online and printed information from government, equine industry and other sources. And, as she said, by the end of 2012, she understood from the “biosecurity newsletters and stuff” that there had been cases of Hendra virus in Queensland and “it was everywhere when a confirmed case was found”. In doing so, she came across and read many publications that urged that all horses be vaccinated with Equivac HeV because it was the most effective way of preventing the risk of equine and human infection and a prudent occupational health and safety measure. This general information was of similar effect to the geographic spread representation.
395 In 2012, Ms Hinton was attending a meeting about classes for gypsy cobs in 2013 when she learnt that the Ekka’s head vet was considering making HeV vaccination mandatory for horses at the show. That caused Ms Hinton concern because the effect of Equivac HeV on breeding stock, such as Quinn, was unknown. She was not aware of any testing on breeding stock that established that the vaccine would not cause any difficulties.
396 Dr Christine Perry and Dr Michael Higgins were vets at Nerang Equine Vets, the practice that Ms Hinton used predominantly for her horses. She discussed HeV vaccination with Dr Perry and other vets when they attended her property. The vets told Ms Hinton that Coolmore stud had vaccinated about 400 horses, including some of the most valuable in the country, without any problem or adverse reactions. She also attended a seminar at the Nerang Country Hotel that Pfizer or Zoetis had run to inform people about Equivac HeV. She wanted to obtain as much information as she could both for her own knowledge and understanding but also in her capacity as president of the Society.
397 Ms Hinton and her husband knew bats flew over their property at certain times of the year. Because of that and her understanding that bats are attracted to fig trees, they chopped down the fig tree in one of their horse paddocks “so that they [the bats] would not be … near it at the time that it flowers”. She knew that her property was susceptible to the risk of HeV because it was in the Hendra area.
398 Ms Hinton read the myth busting pamphlet before July 2013 and, in particular, its negation of the “myth” that “The vaccine isn’t safe” (see [55] above). She said:
… certainly, when I was coming to make that decision about whether I would vaccinate my horses, as in my statement, I called the vets and when they were out checking and scanning mares, I would ask them, on occasion, what they were seeing and was there any truth to some of the stories that were coming out about more severe reactions…. not that I believed there were serious side effects. There was rumours around was there any serious side effects, of which I remember asking my vets was that the case, and they had said no, they hadn’t seen any, and they thought that a lot of stuff going on was, you know, rubbish out there, so I trusted on my vet’s opinion that it was, that, you know, they hadn’t seen any adverse reactions at that time. So when it came time to vaccinate my horses, I called them and asked for their opinion, again, before booking in the appointment to get them to come and do the vaccination.
(emphasis added)
399 Zoetis sought to argue that Ms Hinton could not be found to have relied on the no serious side effects representation because she trusted her vets who conveyed the same information. However, that submission was unrealistic in seeking to separate out the causative impact of its own active campaign to disabuse participants in the equine industry from the “myths” that it was “busting”: Ethicon 288 FCR at 516-519 [798]-[810]. In Gould v Vaggelas (1984) 157 CLR 215 at 236, Wilson J explained the principles applicable in respect of the causative effect of an alleged fraudulent representation, that are equally appropriate in respect of a misleading representation in trade or commerce, that induces a person to act or refrain from acting (and see too Sellars v Adelaide Petroleum NL (1994) 179 CLR 332 at 348 per Mason CJ, Dawson, Toohey and Gaudron JJ) that:
1. Notwithstanding that a representation is both false and fraudulent, if the representee does not rely upon it he has no case.
2. If a material representation is made which is calculated to induce the representee to enter into a contract and that person in fact enters into the contract there arises a fair inference of fact that he was induced to do so by the representation.
3. The inference may be rebutted, for example, by showing that the representee, before he entered into the contract, either was possessed of actual knowledge of the true facts and knew them to be true or alternatively made it plain that whether he knew the true facts or not he did not rely on the representation.
4. The representation need not be the sole inducement. It is sufficient so long as it plays some part even if only a minor part in contributing to the formation of the contract.
(emphasis added)
400 In early 2013, Ms Hinton began working at a job in Brisbane, and started commuting daily. Before that, she had been working closer to home on the Gold Coast. Her husband fed the horses with their three year old son daily, when she went to Brisbane to work. Ms Hinton said that everyone was very nervous about HeV at the time and wanted to know how to protect themselves and their horses. She also kept up-to-date through her discussions with the vets whom she knew.
401 In July 2013, Ms Hinton became aware of an outbreak of HeV at Mudgeeraba, about five kilometres away from her home. She was very concerned. She understood that Dr Higgins was the treating vet for that case of HeV.
402 Mr Hinton was not as experienced with horses as his wife and she was worried that he may not perceive subtle changes in behaviour that could alert one to a horse being unwell. She said that she spoke to Dr Higgins’ wife, Lucy, who worked in the practice’s office, about what the practice was experiencing and if vets had been called out to attend suspected HeV cases. Mrs Higgins told her that the practice had not seen any other cases. Ms Hinton understood at this time that the only risk to her horses of vaccination was the possibility of infertility because Equivac HeV had not been tested for that. However, she said that at the Zoetis presentation, and possibly in something she had read, she had learnt that, because the make up of the vaccine was like another one, the expectation was that there would not be any adverse consequences for breeding. She remained concerned about the possibility of an adverse effect on Quinn’s fertility because he was a breeding stallion, and was conscious that Equivac HeV had not been fully registered but distributed under the minor use permits.
403 On 11 July 2013, the day after she had spoken to Mrs Higgins and six days after the outbreak near her home, either Dr Perry or Dr Higgins came to her property and they had a further discussion. By then, she already had a lot of information from government departments, the AVA, Equine Australia and biosecurity bulletins, all of which she understood were urging that owners vaccinate their horses because HeV was a serious problem. The problem had become acute for her because of the proximity of the outbreak nearby at Mudgeeraba. She explained her decision to vaccinate all her seven horses, including Quinn:
the fear at that point in time was that, you know, my horse will get Hendra and, therefore, my family will get Hendra. And that’s when I made that risk-based assessment that if it was something that I did that would protect my husband and son, who were feeding the horses in the afternoon for me during the week and I had to just risk the infertility issues, that was a risk that I was … happy to take at that time. I was never assessing it on a risk that there was severe reactions. I didn’t know at the time that there were others that … had horses suffering, or died, or laminitis or, you know, bigger than the normal reactions that we were told about with the lumps on the neck or the high temperatures.
All right. But you would accept that with the information you had, principally being information from your own vet’s recommendations, and the government departments, AVA, Equine Australia, at that point in time once you read about that case up there, you really had no choice, did you? You had to vaccinate in the interests of your own family and your own horses? --- I had made the decision to vaccinate. Whether I had to or not was a decision that I and my husband made …
[Y]ou moved very quickly, didn’t you, to do it? --- Yes, but no one held a gun to my head. It wasn’t a mandated thing that I had to vaccinate. It wasn’t a requirement by anyone to make me vaccinate. It was a decision that I had made in assessing ---
But the simple fact that you knew that you might be putting Quinn’s ability to sire foals at risk shows how serious you thought this risk was; that’s right, isn’t it? When I say the risk, how serious the risk associated with the Hendra virus was? --- Particularly when the publications were coming out about, you know, protect … your family and you will, you know, you will get, your horse will get Hendra, you will get Hendra, and people that catch Hendra die.
And particularly when they were coming from the government and the people who you understood knew what they were doing? --- Well, I hoped they were doing the right thing, just as you hope with all governments.
(emphasis added)
404 Later, at the end of her evidence, I asked Ms Hinton the following:
Well, Ms Hinton, so, you’ve told me that you took this decision because you were concerned about your family’s health and, your son and your husband particularly, having regard to what you had just learned had happened very close by with the detection of the Hendra virus in the area neighbouring you, and I wanted to know, if you had been told, when you asked the vets if there’s any more information about it just before the vaccination, they had said, “Yes, well, there’s a possibility in which the horse might react and die”, would you have still gone ahead? --- I think it’s a hard question, because I obviously made that decision to vaccinate on the understanding that there was, you know, that wasn’t a possibility that that would happen. I think if it was likely that there was the possibility that he would die, I think all of the material that I would have seen at that time would have been vastly different, because they wouldn’t have been promoting, you know, “It’s all good”, and, you know, “It’s safe”, and, you know, “Protect yourself, protect your family”, and there’s the very real possibility that a different decision would have been made on the balance of risk. And I would have, I would have thought, sought more information than what had been provided at the time.
(emphasis added)
405 As Ms Hinton acknowledged in that last answer, obviously with her subsequent knowledge of the profoundly distressing experience that she had of losing her beloved horse, Quinn, five days after his 28 January 2016 vaccination, she would have had to weigh the possibility that Equivac HeV might cause her horse to die in coming to a decision. However, having seen and heard her, I am comfortably satisfied that, even though she may have felt deeply conflicted, she would still have been faced with and taken the same “risk-based assessment that if it was something that I did that would protect my husband and son, who were feeding the horses in the afternoon for me during the week … I had to just risk the infertility issues”.
406 No doubt she feels a responsibility that Quinn might not be dead if she had not decided to vaccinate. As I will explain, on the expert evidence, the vaccination had no role to play in his death. Moreover, had she not had the understanding, that she had in giving her answers quoted above, that Equivac HeV had serious side effects, which the evidence does not support, she would have made the same decision, namely to vaccinate. And that is so even if she had weighed the possibility that there was a serious risk (contrary to my findings) that those side effects, including possible death of a horse, might occur.
407 At the end of the day, Ms Hinton valued the lives of her husband and son above the great love she also had for her horses. The threat of HeV was literally just around the corner for her and her family in July 2013 and she knew that it was a risk of potential death for her husband, son and herself if one of the horses caught HeV, as horses at Mudgeeraba had.
408 It is pellucid that, in Ms Hinton’s case, the geographic spread representation was not misleading. She was within the Hendra area and, in late July 2013, her family and horses were on a property about five kilometres away from an actual outbreak of HeV at Mudgeeraba. The only way to protect her family, herself and their horses from the real and present risk of HeV then was by vaccinating the horses with Equivac HeV. Ms Hinton knew those facts and the need to take precautions against the gravity of the real and proximate risk of HeV appearing on their property. The only common sense cause of her decision to vaccinate the horses was that immediate risk. To the extent that past publications may have conveyed the geographic spread representation to her, it played no role in her decision making process. And, even if it did, it was accurate in her then situation.
409 Ms Hinton did not understand the no serious side effects representation to have been conveyed. Rather, she understood Zoetis to be saying that there were no known serious side effects. But, she believed that there was a real question as to its side effects, at least so far as the potential of Equivac HeV to cause a serious impact on a horse’s reproductive ability. The no serious side effects representation could only have the capacity to be misleading or deceptive if there were, in fact, one or more undisclosed serious or debilitating side effects that, had it or they been disclosed, would have led Ms Hinton into error in deciding, in the circumstances in which she was placed in late July 2013 (and at the time of each subsequent vaccination) whether to proceed with vaccinating her horses.
410 In my opinion, if I were wrong in concluding, as I have, that Equivac HeV had no serious or debilitating side effects, and she had known of such a side effect (including any potential to cause the death of a horse), as a matter of common sense, Ms Hinton would have acted as she did. First, her driving and, in my assessment, overwhelming concern as a caring and loving mother and wife was the protection of her young son and husband from the proximate and real risk of HeV and its potentially lethal consequences. Secondly, she actually took the decision that the horses should be vaccinated despite her deep concern that there was a real risk that Equivac HeV could cause her horses to become infertile. She realised that there were possibly unknown risks of its use as a new and not fully tested product.
411 Invidious as it would be for a person in Ms Hinton’s position, having to choose between the possibility that vaccination of her horses, and in particular Quinn, may have had possible side effects that could cause his death, and the risk that, without the vaccination the horse could contract HeV and zoonotically pass it to her son or husband (or herself), I am satisfied that she would have done as she did. Her reasoning, as she explained in her evidence in chief, bespoke the common sense way in which she would have acted were she informed (contrary to the facts as I have found) that there were one or more serious side effects, namely:
So along the lines of seeing all the articles and advertisements about the risk that was faced with Hendra and the fact that if your horse got Hendra you were highly likely to get it yourselves and die. That was a risk I wasn’t prepared to take for my husband and my son.
(emphasis added)
412 In chief, Ms Hinton said that if she had been told that the vaccine was unsafe and that death was a possibility, and “now that I know and what came out in the Parliamentary inquiry, I would never have vaccinated my horses”. Subsequently to the initial vaccination of her horses in July 2013, she also saw a double page advertisement by Zoetis, that appeared in Horse Deals magazine published in March 2014, which the applicant had not pleaded or particularised as conveying any of the three representations. As she said of it, “I don’t think there was anything new in this page that I hadn’t already seen before”. She agreed that government departments and independent bodies had conveyed some information, about the understanding that flying foxes were the source of HeV, that vaccination was recommended because of the risk HeV posed and that vaccinated horses should receive booster shots every six months.
413 Prior to January 2016, Ms Hinton had vaccinated her horses against various diseases, including HeV, many times without any serious incidents. One mare, Gypsy Elite Pride’s Gem, had a temperature spike after her first and, possibly, second Equivac HeV dose that resolved within 24 hours.
414 In about August or September 2014, Ms Hinton had Gem treated by another veterinary practice, West Vets, for colitis. The vet did tests on her and found that she had salmonella. Gem made a full recovery.
415 Ms Hinton, like Ms Abbott, was a member of the “Say No” page but was unsure whether she joined before or after Quinn’s death. After his death she “went looking for answers” with group members. Ms Hinton said that: “Quinn’s loss was so devastating that we decided to close up our stud”.
11.1.3 Quinn’s vaccination on 28 January 2016 and the events leading to his death
416 Before January 2016, Ms Hinton was aware that Quinn had had multiple vaccinations over his life, including in the United States and as a part of satisfying the Australian requirements to enable him to be imported. She was not aware that Quinn had ever experienced an adverse reaction to any vaccination, including with Equivac HeV. She described him as “a very healthy horse”.
417 Before vaccinating Quinn, she was very interested to know from her vets about their experience with Equivac HeV and had also sought to inform herself about it from her own researches. She knew that Coolmore was a big thoroughbred stud, with a large number of brood mares and stallions, located in the Scone area and that many articles had stated that it was using Equivac HeV to vaccinate its horses. Before January 2016, vets whom she trusted told her of their experiences using Equivac HeV. She understood that the main benefit of the vaccine was that her horses would not get HeV and “to protect myself and my family”. She reiterated:
The deciding factor was the fact that there was a Hendra case close to home, and I couldn’t risk my husband or son contracting Hendra if they, if, you know, exposed themselves to the horse like, we were led to believe that they would get Hendra.
(emphasis added)
418 She said the vaccination gave her peace of mind about protecting her horses, herself and her family. She had purchased a HeV PPE (personal protective equipment) kit in case one of her horses displayed any symptoms. She and her husband had chopped down trees, put up shelters over water feeders and taken the other steps that the Horse Council had recommended to safeguard their property and horses from the risk of catching HeV. She understood that after a vaccination with Equivac HeV, she should monitor her horses for injection site swellings and increases in temperature.
419 As part of giving regular booster vaccinations late in the afternoon of Thursday, 28 January 2016, Dr Rachel Wilson of Gold Coast Equine Clinic came to Ms Hinton’s property and gave Quinn his fifth vaccination.
420 As was her practice, Ms Hinton monitored her horses for any adverse reactions over the next 24 hours. She checked their temperatures in the morning and afternoon and looked at their necks. The horses were fed twice daily.
421 On the morning of Friday, 29 January 2016, Quinn’s temperature was normal (between 37.5℃ and 38.5℃). By that evening he had a temperature, but there was no evidence of when Ms Hinton took it or what it was. Otherwise, he appeared to be normal. Ms Hinton called the Clinic. The Clinic told her to give him 10 ml of bute, which she administered in paste form, and that a heightened temperature can be a normal response to a vaccination, because of the challenge to the immune system, but should resolve within 24 to 48 hours.
422 At 8 am on Saturday, 30 January 2016, Quinn’s temperature had increased to 40.6℃. Ms Hinton rang Dr Tim Hawthorne at the clinic and told him of the temperature. He advised her to give 10 ml of bute. By the afternoon, Quinn’s temperature had come down to 40℃ and he was still eating some of his foods. On Dr Hawthorne’s advice, she administered more bute.
423 On Sunday, 31 January 2016, Quinn’s temperature was up to 41.2℃. She spoke to Dr Hawthorne again and then gave Quinn more bute. Ms Hinton also was hosing him to cool him. By 6.30 pm in the afternoon, his temperature had reduced to 39.8℃, but he was quiet and nibbling at food, not drinking or eating as much as usual.
424 On Monday, 1 February 2016, when she woke up, Ms Hinton looked towards the paddock and saw Quinn uncharacteristically standing at the far end of the paddock covered in mud and very restless, moving from one spot to another, lying down, getting up, and obviously unhappy. Ms Hinton watched his erratic behaviour for about 20 minutes and then went to the paddock. She said that normally he would be at the gate neighing to greet her and waiting to be fed. In the paddock, she listened with her ear to his stomach to check if there were any sounds in his gut, which she said is a typical precaution if one observes a horse in pain. She did not hear any noises. She took his temperature and saw that it had dropped slightly but said that it was still well above the normal range.
425 At this point, Ms Hinton called the Clinic which said that Dr Hawthorne would come out some time later that morning. About 10 minutes later, she led Quinn on a rope to the next paddock so that she could observe him more closely. He then started to lie down at the end of the lead in a lot of pain. She rang the Clinic back and said that a vet had to come urgently because he was rolling around, visibly in pain and showing very typical signs of colic, namely looking at his gut and wanting to lie down. The Clinic said that Dr Hawthorne would come immediately. Ms Hinton, understandably, became very emotional in reliving her traumatic experience in the witness box. She said that Dr Hawthorne arrived near the time when her son would leave for school, which I infer was around 8 am to 8.30 am.
426 I limited the use of the following part of what Ms Hinton said that Dr Hawthorne next told her, under s 136 of the Evidence Act, to being evidence of the statement he made but not evidence of the truth of any of its content, including the opinion or other matters that she said he stated. I did so because the applicant was not proposing to call Dr Hawthorne and evidence of Quinn’s clinical signs and condition at this time was likely to be significant for the experts and in resolving the cause of Quinn’s death. In the event, the applicant did not call Dr Hawthorne or explain his absence.
427 Ms Hinton said that Dr Hawthorne told her that he had some experience with gypsy cobs when he had practiced in the United Kingdom. He said that he felt Quinn’s immune system was being challenged. He said sometimes horses are so healthy and stoic that vaccines can cause their systems to overload. He told her that was why, on occasion, he gave miniature horses (such as gypsy cobs) only half a dose of Equivac HeV.
428 Zoetis did not seek, and I did not make, any limitation under s 136 of the following evidence of the balance of the conversation. Dr Hawthorne confirmed to Ms Hinton that there were no gut noises. He recommended that she take Quinn to the Clinic so that they could give him intravenous fluids, rehydrate him and get his gut moving. He gave Quinn some pain relieving medication before leaving. In his clinical notes, Dr Hawthorne recorded that at Ms Hinton’s property, Quinn was “[i]n a bad way”, had “[p]ale gums” and he had referred him to the practice at the Clinic.
429 As soon as Dr Hawthorne left, Ms Hinton and a friend got Quinn into a horse float and drove him on the 20 minute trip to the Clinic, arriving there at between 10.00 am to 10.30 am. She then went up the road to a veterinary supplies store and bought some Hartmann’s fluid for intravenous administration. She took the fluid back to the Clinic and went home to wait for news. She understood that the Clinic conducted ultrasounds, blood tests and rectal palpitations on Quinn and put him on intravenous fluids. She returned home.
430 During the day, Ms Hinton spoke with Dr Hawthorne for updates while Quinn was at the Clinic. He told her that they were running tests and mentioned the possibility that he may have colitis. She told him that previously Gem had had colitis and had tested positive for salmonella. About two hours later at 5 pm, Dr Hawthorne phoned and told Ms Hinton that the Clinic could not assist further and they had referred Quinn for suspected colitis to the UQ VETS Equine Specialists Hospital at the Gatton campus of the University of Queensland.
431 The Clinic’s clinical notes recorded:
Hendra vaccinated on Thursday, pyrexia noticed on Friday. Temp 41 Saturday morning. Was eating fine over weekend, Monday morning lying down with colic pain, very dehydrated and not eating. Admitted to GCEC for fluids and further workup. Some hard faeces noted at pelvic flexure. Drenched, over next few hours became less comfortable, even following NSAIDS. Referred to UQ as watery diahhorea [sic] and collitis a possibility. Another horse previously had salmonella on same property. Tight band on R side but normal on U/S.
432 Ms Hinton, her husband and son collected Quinn from the Clinic in the float. The Clinic administered more pain relief medication before the two and a half hour drive to Gatton where they arrived at about 8 pm. Dr Joan Norton admitted Quinn and he was taken to the intensive care stables for more tests. In his expert report, Dr El-Hage noted that the University records indicated that the cause of Quinn’s colic was difficult to localise although blood tests indicated likely damage to his intestines.
433 Dr Norton told Ms Hinton that she would call her later that evening when the test results arrived. The family drove home. As they arrived home, Dr Norton rang and said that the preliminary tests showed no infection and Quinn had no reflux, which were good signs. The vet said that she was hopeful that Quinn would recover and that the focus of treatment would be to keep him comfortable and to firm up his watery manure.
434 At about 3.00 am on Tuesday, 2 February 2016, Dr Norton phoned Ms Hinton. The vet reported that things were not going well, Quinn’s heart rate was highly elevated (ie: tachycardia) and the pain relief medication was not helping. Ms Hinton said that the vet said that they were not sure what was going on with Quinn and wished to do exploratory surgery (Dr El-Hage explained that, based on his review of Gatton’s clinical records, which were in evidence that the vet had recommended an exploratory laparotomy to assist diagnosis). Ms Hinton said that she and Dr Norton had a detailed conversation about what they would look for in the surgery and what prognosis or diagnosis she could offer. Ms Hinton said that she would call Dr Norton back after discussing what the vet had told her with her husband, which she then did.
435 The couple decided that the most humane course was to euthanise Quinn and conveyed this to Dr Norton at about 3.30 am. Quinn was put down.
436 The Gatton clinical records recorded that Quinn was started on intravenous fluids (calcium, polymixin B and plasma). He was initially comfortable but progressively experienced more pain, began lying down and rolling with worsened tachycardia (100 bpm) and increased lactate. The worsening of his condition occurred despite the administration of several doses of painkillers and sedatives (detomidine, bute and buscopan).
437 On 5 February 2016, an autopsy on Quinn occurred at Gatton. The post-mortem report found that Quinn had an ulcerated colon, diffuse typhlitis (being, as Dr L’Estrange explained inflammation of the horse’s caecum, which is like a human appendix but a much larger and important part of the large bowel) and colitis (being inflammation of the colon). The post-mortem report concluded:
An invasive bacterial agent such as salmonella or clostridium would fit with these gross pathological findings.
(emphasis added)
438 As Dr L’Estrange noted, this suggested diagnosis was never confirmed because no tests were performed on the tissues or other organic material examined or recovered in the post-mortem of Quinn.
11.2.2 The expert evidence as to Quinn’s cause of death
439 The substantial facts and timeline in the evidence I have summarised in [419]-[438] above was distilled in Exhibit 8. It was put to and used by Associate Professor Sykes, Dr El-Hage and Professor Slater during their concurrent evidence.
440 In the joint report, Professor Slater (who had been asked to opine on Quinn’s case), Associate Professor Sykes and Dr El-Hage agreed that there was a paucity of diagnostic information. They pointed, particularly, to the lack of, first, any detailed veterinary diagnostic information between the time of administration of Equivac HeV to Quinn on 28 January 2016 and Dr Hawthorne’s attendance at the Hintons’ property on the morning of 1 February 2016 and, secondly, any comprehensive and timely post-mortem examination and report. The experts agreed that this lack of data made the task of arriving at what caused Quinn to become sick depend heavily on their own individual experience and subjective interpretation. They also agreed that Quinn’s was a complex case with many factors that may have contributed to the ultimate outcome of colitis. Those factors included the horse’s history of vaccination with Equivac HeV, the administration of bute, his prolonged pyrexia (ie: increased temperature) and the possibility of his having an undiagnosed, underlying or latent salmonella or other disease.
441 The experts agreed that Quinn was suffering colitis at the time of his death. They also agreed that horses can suffer colitis from a range of causes, so that the diagnostic issue was whether the vaccination could have created a causal connection to Quinn contracting or developing the disease. However, the experts disagreed about Associate Professor Sykes’ postulation that “activation of a latent salmonella carrier state into clinical Salmonellosis is the most likely pathophysiological scenario”.
442 The experts noted that there was no evidence that Quinn displayed any clinical signs of a reaction to the vaccination until Ms Hinton observed his raised temperature over 24 hours afterwards. Ms Hinton was a careful, caring owner and was concerned to monitor her horses, including Quinn, for clinical signs of any adverse reactions to the vaccine. In my opinion, given Ms Hinton’s concern for Quinn’s (and her other horses’) welfare, and her care in taking temperatures twice daily, it is safe to infer (as I do) that Quinn had no injection site reaction of any substance, such as swelling or pain, or that caused him any noticeable discomfort. He had had a similar experience to his four previous Equivac HeV vaccinations.
11.2.3 Associate Professor Sykes’ changes of his evidence
443 Associate Professor Sykes changed his evidence over time, including in relation to Quinn. During the concurrent oral evidence, he appeared to be seeking ways to justify the existence of a possible means of connection between the vaccination and appearance of colitis in Quinn, as well as the various clinical signs for which he postulated a “biologically plausible” link, that I have described above, rather than assessing objectively what the data revealed. This caused me to approach his suggestion of a causal link between Equivac HeV and the reported clinical signs with considerable caution and, ultimately, find it unpersuasive.
444 In his first report, Associate Professor Sykes recorded an assumption that the clinical signs that Ms Hinton had reported about Quinn in the first three days after his vaccination were “consistent with a severe, acute injection site reaction with secondary, systemic manifestations of disease” (emphasis added), that those clinical signs were associated with the vaccination and:
In assessing this, it warrants note that although mild, acute injection site reactions are recognised as a common complication of vaccination the severity, persistence, and refractory nature of the pyrexia reported in the case is atypical.
(emphasis added)
445 In his second report made on 28 October 2020, Associate Professor Sykes referred to that evidence, and new information from the clinical notes of the Clinic and Gatton, including the post-mortem report, saying:
Distinction of the relative contribution of the post-vaccination local inflammatory response and the gastrointestinal disease to the presentations at both the Gold Coast Equine Clinic and the UQ Vets – Equine Specialist Hospital is difficult. However, given the chronology of clinical signs and the severity of the changes reported in the “Pathology – final report”, in my opinion the development of the acute, severe gastrointestinal disease was the dominant clinical syndrome at the time.
…
The aetiology of the acute, severe gastrointestinal disease and clinical SIRS was not identified, partly due to a lack of pathogen specific testing. However, in my opinion, acute Salmonellosis is the most likely cause. This is based on the descriptive pathology and histopathology in the University of Queensland’s Laboratory Services’ “Pathology – final report” and the history of Salmonella on Quinn’s home property as per the Gold Coast Equine Clinic's clinical records.
…
Similarly, the additional information makes the development of SIRS secondary to the sustained release of inflammatory mediators from the local site of injection less likely. However, it warrants note that the vaccine injection site was not examined post-mortem. As such, the potential for the localised reaction to be a contributor to the clinical syndrome observed cannot be excluded.
(emphasis added)
446 In answer to a question I asked, Associate Professor Sykes said that in his second report he had assumed that Quinn had had an observed injection site reaction and, based on that, this reaction was the stressor that plausibly could have led to the development of salmonella. Earlier in his evidence, he agreed that the existence of an acute injection site reaction of Quinn’s was “a fundamental part of [his] reasoning in establishing that trigger and plausible connection between the vaccine and the death”.
447 In his third report, made on 29 January 2021, Associate Professor Sykes noted that Professor Slater had pointed out in his report that there was no reported injection site reaction in Quinn, saying:
I acknowledge that no specific reference is made to the injection site in the clinical records and accordingly clarify my characterisation of the initial reaction as an acute reaction to vaccination.
(emphasis added)
448 He gave this evidence about that “clarification”:
HIS HONOUR: When you write your third report and clarify that it’s an acute reaction, you’re talking not about a localised reaction at all. You’re talking about a totally different aetiology, namely, it was a fever; is that right?
ASSOC PROFESSOR SYKES: Correct.
(emphasis added)
449 Associate Professor Sykes explained why he did not review his reasoning process on the basis that he now knew that there was no injection site reaction, saying that:
I believe that I had referred to the fever as part of the acute injection site reaction, but obviously had not. So to me, the absence of localised signs in terms of an injection site reaction, per se, I agree, decreases the likelihood of that association, but I was remiss, but I believe that the development of the fever post-vaccination is still plausibly associated with the vaccine and then, potentially, as a stressor to the development of salmonellosis. But equally, I agree on the counterpoint that the background rate of disease as a possible contributor or as possible alternative explanation is valid.
(emphasis added)
450 When I asked him why he had not adopted the background rate of disease as much more likely to have been the cause after he became aware that there had been no acute injection site reaction that was a significant feature in his earlier reasoning as to the aetiology of Quinn’s death, he gave the following evidence:
Because I think that the fever still provides the central link between those two, albeit not specifically referenced to in either of those statements. As I said, I agree that the absence of the localised response decreases the likelihood of that association, but I think that the development of systemic disease post-vaccine administration still provides a plausible explanation for then the subsequent trigger in relation to salmonella into salmonellosis.
MR GYLES: The systemic disease is pyrexia; is that right?
ASSOC PROFESSOR SYKES: Pyrexia. Yes.
MR GYLES: Right.
ASSOC PROFESSOR SYKES: It should more specifically have been referred to as pyrexia.
(emphasis added)
451 In his third report, after making his “clarification”, he later asserted in answer to Dr El-Hage’s report:
However, I disagree that the failure of either the Gold Coast Equine Vets or UQ Vets – Equine Specialist Hospital to recognise swelling when placing an intravenous catheter precludes the possibility of a localised reaction being present simply because the injection site is unknown and could have been on the contralateral side of the neck.
… the absence of a rise in CK or AST post-vaccination does not definitely rule out an injection site reaction as they are only elevated when significant muscle damage which would not necessarily occur if the inflammatory response were localised to the subcutaneous tissue.
(emphasis added)
452 Associate Professor Sykes said that the notes of the Clinic and Gatton would have been expected to record swelling consistent with an injection site reaction “[i]f it was obvious” before saying “I’m happy to agree that there is no evidence that there was … a local injection site reaction”.
453 Associate Professor Sykes’ was determined to continue postulating, after he became aware of the errors in his assumptions that Quinn had an injection site reaction and that Equivac HeV had a chemical adjuvant (see [231] above), that an injection site reaction was possible in order to support his theory of the vaccine being a realistic cause of Quinn’s contraction of colitis and or salmonella. That determination, despite the lack of any evidence that Quinn had any injection site reaction, as Associate Professor Sykes ultimately accepted, suggested that he was seeking to make the facts fit to his theory, rather than reasoning objectively from the available facts.
454 In the end, Associate Professor Sykes’ references to it being biologically plausible the vaccination with Equivac HeV could provide a causative mechanism for any of the reported clinical signs (other than the transient ones that I have found to be side effects) had no support in scientific literature or from either Professor Slater or Dr El-Hage.
455 I am not persuaded that Associate Professor Sykes’ theory, that any reported clinical sign associated with Equivac HeV (other than those transient ones that I have found earlier in these reasons) is biologically plausibly a side effect of the vaccination, provides a basis to conclude that it is, in fact, a side effect: Ethicon 288 FCR at 529 [849], Amaca 246 CLR at 61-62 [69]-[70], McLean 41 NSWLR at 411B-C, Peterson 196 FCR at 182 [143], Forst 64 CLR at 569, Briginshaw 60 CLR at 361-362, Murray 33 ALJR at 524.
456 The applicant had the onus to prove that the no serious side effects representation was incorrect or otherwise contravened s 18(1) of the ACL. Even if it were biologically plausible (which I do not accept, on the evidence, it is) that some or all of the reported clinical signs was or were to be a side effect or effects, that possibility is far removed from it being the fact that the vaccine causes, or is a cause of, the asserted side effect.
457 No treating veterinarian was called to establish a source of any of the clinical signs in respect of Primetime or Quinn reported to the Authority or Zoetis as part of the pharmacovigilance reports. All that is in evidence consists of Zoetis’ business records produced through reports to it, including Dr L’Estrange’s recordings of what he was told in discussions with horse owners and vets, documents or reports that the Authority produced, and other correspondence.
458 As the experts said in their concurrent evidence, in order to be able to make any kind of authoritative diagnosis of cause and effect between a vaccination and a reported clinical sign, it is necessary to have detail about the circumstances in which the vaccine was administered, the time between then and when the clinical sign first appeared, together with, if possible, pathology or blood sample reports to identify whether there is or is arguably any physiological connection between them beyond coincidence.
459 The apex of the applicant’s approach to causation of her suggested side effects was Associate Professor Sykes’ use of biological plausibility given the absence of any research or diagnostically rigorous analysis of the aetiology of the observed condition. A similar use of coincidence in the timing of vaccination and appearance of a clinical sign occurs in the pharmacovigilance material in evidence which, also, reflects various degrees of biological plausibility in the ABON classifications assigned in each pharmacovigilance report. However well qualified the vet making an ABON classification may be, his or her evaluation depends on the quality of data that is reported.
460 Crucially, pharmacovigilance is a different discipline to clinical (or veterinary) diagnosis of an individual animal’s actual condition and the cause or causes of it, including any actual, as opposed to coincidental, relationship between that condition and the vaccination. No expert in pharmacovigilance gave evidence.
461 Moreover, because many of the conditions that have been reported as clinical signs that appear with, concurrently or shortly after, the administration of Equivac HeV are commonly experienced by horses quite apart from any vaccination, there is no necessary connection between those clinical signs or conditions and Equivac HeV. There was no epidemiological, statistical or other evidence to show that, for example, the rates reported of clinical signs reflecting common conditions experienced by horses, including pyrexia, sweating, heavy breathing, laminitis and the like were out of proportion with what one might expect in the ordinary course if horses were not vaccinated.
462 Again, this called into question the whole hypothesis underpinning the applicant’s case that Zoetis’ collection, that the Authority required, of the pharmacovigilance reports of any clinical sign that might be a side effect or adverse reaction that could be classified as a side effect of the vaccination is not necessarily one in which any causal relationship exists between the administration of the vaccine and the presentation of the clinical sign. An event that satisfies the hypothesis may simply be the appearance of a condition in the horse that it would have developed or was already developing before or without the vaccination. That is why one needs specific information about the condition of the horse before vaccination, the time and development of any subsequent clinical sign said to be a side effect of its administration and a diagnostic analysis based on pathology or veterinary investigation.
463 As Zoetis pointed out, Associate Professor Sykes was only prepared to go so far as to suggest that while it may be biologically plausible that clinical signs are side effects of Equivac HeV, “we have no specific evidence to say that they do. But to some degree, to a large degree, we also have no specific evidence to say that they don’t”.
464 Although Quinn’s post mortem report referred to the possibility that “[a]n invasive bacterial agent such as salmonella or clostridium would fit with these gross pathological findings”, no tests were performed to analyse whether any, and if so, what, bacterial agents were present in his body. Nor was any pathological or physiological analysis done to detect any connection between Equivac HeV and the colitis, pyrexia, or other disease or diseases that led to Quinn’s death.
465 However, over 24 hours after the vaccination, Ms Hinton noticed that Quinn had a fever but she did not recall what his temperature was. That was the first time that Ms Hinton noticed Quinn manifesting any clinical sign or a change in his condition after the vaccination. Thus, for most of the 24 hours or possibly some time further after the vaccination Quinn had presented no clinical signs of any condition.
466 As Professor Slater explained, the stimulating effect of the biological adjuvant on the horse’s immune system is “a short-lived phenomenon” which largely, but not necessarily completely, would have run its course over the initial 24 hours immediately following vaccination. Dr El-Hage agreed. Associate Professor Sykes said that where, as with Quinn, vaccination occurred in the afternoon, “we would expect the majority of acute reactions to occur by the next morning”. Thus, by the time that Ms Hinton first noticed any change in Quinn, being his increased temperature, it was unlikely, but of course still possible, that the adjuvant was continuing to have an effect on his immune system.
467 Hence, as the experts discussed, there was a need to search for a mechanism that linked Equivac HeV to the presentation of a clinical sign, pyrexia, that, in the ordinary understanding of the way in which ISCOMatrix adjuvant could affect the immune system, would not be expected to be a cause of its appearance so long after the vaccination. As Professor Slater explained, if an adverse reaction to Equivac HeV were occurring in Quinn, one would expect to see pain or a chemical signal from an injection site reaction, yet none was observed. And Dr El-Hage opined that the manifestation of a fever in the evening, about 24 hours after vaccination, was not in any way consistent with an adverse reaction to that vaccination.
468 Importantly, as Professor Slater explained, Equivac HeV used common and understood technology in respect of its antigen and adjuvant that could not be classed as untried or “completely new”. He said that there was no reason to expect that Equivac HeV would have a different safety profile from similar vaccines that used the ISCOM or ISCOMatrix biological adjuvant and, because the antigen was genetically engineered but inert, and the structure of Equivac HeV was safer than traditional protein based vaccines because it was a purer, non-living compound. Both Dr El-Hage and Associate Professor Sykes agreed. I found Professor Slater’s evidence compelling.
469 Associate Professor Sykes considered that a stressor, such as the vaccination, could trigger colitis or another disease within the following 48 hours. However, this view was based on his unsupported theory that it would be biologically plausible for Equivac HeV to cause or trigger a range of diseases beyond the transient side effects that I have accepted. The speculative nature of Associate Professor Sykes’ approach appeared when he explained that in Quinn’s case:
…the cornerstone challenge with this case is the paucity of information that we have in this, sort of, black hole of a window. I think we, as per our report, were in agreement that the horse died ultimately of colitis, a fairly peracute colitis, given that it didn’t appear to even develop diarrhoea. It just simply developed severe colitis, and I agree with Dr El-Hage that the administration of the non-steroidals may have been a contributory factor to that deterioration. I think, Dr El-Hage and I agreed that it was most likely salmonella, but again, the paucity of diagnostic testing makes that difficult to be certain.
(emphasis added)
470 On the Clinic’s advice, Ms Hinton began administering bute to him. Professor Slater said that, normally, if a horse experienced a fever (or pyrexia) after a vaccination, a single dose of bute would reduce it and lead it to disappear. As Associate Professor Sykes said, bute is a non-steroidal anti-inflammatory drug for horses, the equivalent of which in humans is ibuprofen.
471 Importantly, all the experts agreed that the fact that bute was administered twice daily to Quinn from early in the evening of 29 January 2016, up to the evening of 31 January 2016 suggested that there was something else than a reaction to the vaccine that caused the fever, particularly in the absence of any injection site reaction.
472 Associate Professor Sykes sought to support the plausibility of a connection between Quinn’s vaccination and the persistence of his fever despite the repeated doses of bute by suggesting that the horse reacted to a behavioural or physiological stress that the vaccination had caused. However, he said that the act of injection was “extremely unlikely to be an adequate stress” to trigger salmonella or salmonellosis. In my opinion, based on the evidence of Professor Slater and Dr El-Hage, the same unlikelihood is true of colitis or any other disease that may have caused the death of Quinn. All the experts agreed that the two trips in the horse float that Quinn made on 1 February 2016, first to the Clinic and then to Gatton, would have created significant stress on him.
473 Later in the concurrent evidence, Associate Professor Sykes identified “the point of dispute” between him and Professor Slater “as whether you need to have the injection site … local reaction as prerequisite requirement for vaccination reaction”. That was a recognition by Associate Professor Sykes that his theory that Quinn’s clinical signs and those reported for other horses could have been biologically plausibly caused by Equivac HeV did not require any injection site reaction, despite that having been previously a fundamental part of his reasoning (see [446] above). I found this reasoning unpersuasive.
474 Each of Professor Slater and Dr El-Hage explained cogently why, on the evidence (or more properly lack of evidence), none of Quinn’s clinical signs suggested the existence of any connection between his vaccination and death. I accept Professor Slater’s evidence that there was no plausible link between Quinn’s vaccination and the development of colitis. The three experts discussed the possibility that the repeated dosages of bute that Quinn received may have delayed the acute presentation of colitis symptoms. Both Dr El-Hage and Associate Professor Sykes agreed that Quinn’s dosages of bute may have damaged his gut sufficiently to contribute to his rapid deterioration on 1 February 2016. However, the lack of any veterinary or pathological evidence of the horse’s condition from the time of vaccination to 1 February 2016 meant that none of the experts could give any definitive opinion about what disease Quinn developed that led to his death.
475 Associate Professor Sykes encapsulated that, even on his reasoning, the highest that the applicant’s case rose was that the paucity of information about Quinn made it difficult to attribute or discount a cause of his death because “we don’t know”. That entails that the applicant has not discharged her burden of proof: Murray 33 ALJR at 524.
476 Moreover, I am satisfied that Quinn’s death was not caused by his vaccination with Equivac HeV. I accept Dr El-Hage’s and Professor Slater’s evidence that because Quinn did not present any clinical sign for about 24 hours, was not sore, and was eating, he did not suffer any adverse reaction from the Equivac HeV injection.
11.3 Ms Hinton’s damages claim
477 Zoetis accepted that, had Equivac HeV caused Quinn’s death, Ms Hinton could recover $4,144.62 in respect of her claim for his veterinary treatment expenses.
478 Ms Hinton also claimed damages for the value of Quinn, $150,000 for the loss of the opportunity to market and promote him and the gypsy cob brand and $114,773.04 in respect of an annual loss of income of $9,564.42 for 12 years from his services as a stallion.
479 As with Ms Abbott, because of the applicant’s lawyers’ misunderstanding of the issues for trial, there was no expert valuation evidence of Quinn or expert accounting evidence in support of Ms Hinton’s claims for loss of opportunity or other loss or damage. The applicant’s lawyers assisted Ms Hinton in presenting her damages claim.
480 Ms Hinton provided detailed financial records of Dellifay in an effort to support her claim, but these revealed that Dellifay was the partnership name of a business that she and her husband operated. Because Ms Hinton was selected as a sample group member and I have found that Zoetis is not liable, the failure to include her husband as a co-sample member with her is of no moment since he would have been a group member and entitled to make the claim jointly with his wife. However, the difficulties with the claim for damages based on loss of opportunity to earn income or profit from Quinn’s activities as a stallion were more profound.
481 Based on the partnership’s tax returns for the seven years between 1 July 2009 and 30 June 2015, Dellifay lost over $400,000, as Zoetis set out in the table below that it included in its closing submissions (the pre-tax loss of Dellifay in each year being in the left hand column and the tax deduction for the partners (Mr and Ms Hinton) in the right):
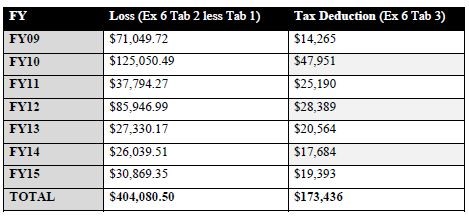
482 Quinn’s service fees comprised the only substantive source of income for Dellifay in the period covered in the table above. That income met only some of the expenses necessarily incurred in keeping him and otherwise operating the business of the partnership. Dellifay had other gypsy cobs included as assets and no doubt incurred some of its expenses in maintaining them. Ms Hinton said that Dellifay owned two purebred mares that she and her husband were hoping to breed and to sell their progeny. She suggested that a foal sold as a gelding might realise $9,000 and a filly between $18,000 and $25,000. During 2013 and 2015, Quinn earned no service fees, but Ms Hinton excluded those fallow years from the figures she used to calculate the expected future earnings of $9,564.42. As Ms Hinton explained, the thoroughbred industry standardises all horses’ birthdays as occurring on 1 August and the gestation period is about 11 months. She said that the preferred breeding time for mares was from late September to early in the next year. She explained that in 2013 there was a severe drought and owners, including she and Mr Hinton, were struggling to feed their existing horses. She said that in 2015, she was heavily pregnant and because they were hand serving mares, she decided not to offer Quinn. That was because she did not want to take any risk of unpredictable behaviour by a mare while she was being serviced. Ms Hinton noted that the stud had been in a start-up phase and that few of the horses that Dellifay sold were purebreds. She anticipated, however, that purebreds would comprise future sales, rendering her estimates of loss conservative.
483 Quinn had in the past experienced some health issues including an eye ulcer. None of Ms Hinton’s calculations allowed for vicissitudes.
484 She accepted that from an economic perspective the stud farm that Dellifay ran up to Quinn’s death had been loss making but she considered that the amounts she and her husband had invested (including meeting the losses) were an investment that would yield profits in future years. The partners chose to run the business at a loss while they were prepared to support it using Quinn as a, indeed the principal, means of earning income and so reducing their losses in the start-up phase. As Ms Hinton said, the losses that the partnership incurred reduced the taxable income of the partners. It was a matter for them how they chose to run their lives including to support a business that, at the time of Quinn’s death, was yet to turn a profit but which they believed would do so in the future.
485 In my opinion, the evidence adduced in support of Ms Hinton’s claim does not establish that, from an economic perspective, Dellifay lost any opportunity to make profits from its business, which seemed to be consistently loss-making. However, Quinn was an income producing asset of a business that, albeit not then profitable, the partners were supporting. Thus, his death caused his owners loss reflected in whatever his market value was as a purebred functioning stallion or, as the claim was formulated, as an asset that the partnership could use to generate income to defray its expenses.
486 In essence, the combination of the claims for loss of opportunity to earn future service fees and to use Quinn to promote the value of the gypsy cob brand may be seen as another way of valuing Quinn as an income producing asset. But, the difficulty with the second of those claims is the absence of any evidence on which to ascribe any value to the brand or its promotion.
487 Importantly, Ms Hinton and her husband closed the stud because of Quinn’s death. On the evidence, whatever income he may have been able to generate from stud fees and brand promotion would not have been likely to contribute to Dellifay making any profits in the foreseeable future, as opposed to assisting it to reduce its ultimate losses, as had occurred in the past. Moreover, it would be necessary to discount any projected future income for contingencies, including further fallow years, and the possibility of Quinn suffering death or an incapacitating injury before his expected longevity.
488 I am not satisfied, on the basis that this claim was propounded, that Ms Hinton or Dellifay suffered any compensable loss of opportunity from Quinn’s death. All that they proved was that he would have reduced the future losses of a loss-making business that has now ceased to operate. That is not compensable because, from an economic perspective, the death saved Dellifay from continuing to make losses. There is no other evidence enabling a valuation of Quinn as an income producing asset.
489 I have concluded that, because Zoetis did not convey the geographic spread representation as alleged, and each of the no serious side effects and all horses representations was accurate, the applicant has not established that any of the three representations contravened s 18(1). However, it is still necessary to decide whether Zoetis had reasonable grounds for making either the no serious side effects or the all horses representation to the extent that it was made with respect to any future matter within the meaning of s 4 of the ACL.
490 Conceptually, it is difficult to understand how a true representation can be misleading or deceptive or likely to mislead or deceive, even if made with respect to a future matter, unless the circumstances are like those that Lord Blackburn described in his speech in Smith v Chadwick (1884) 9 App Cas 187 at 201, namely:
if with intent to lead the plaintiff to act upon it, they put forth a statement which they know may bear two meanings, one of which is false to their knowledge, and thereby the plaintiff putting that meaning on it is misled, I do not think they can escape by saying he ought to have put the other. If they palter with him in a double sense, it may be that they lie like truth; but I think they lie, and it is a fraud. Indeed, as a question of casuistry, I am inclined to think the fraud is aggravated by a shabby attempt to get the benefit of a fraud, without incurring the responsibility. But I do not think there is any case made out against the defendants of that sort.
(emphasis in original)
491 However, as the Full Court held in North East Equity 285 ALR at 225 [34], because s 4(1) deems a representation, including one that is true, to be misleading, unless the representor has reasonable grounds for making it, it is necessary to deal with this issue. This could leave a person who made a true representation (or one which turned out to be true) with respect to a future matter liable if he, she or it cannot point to there being some reasonable ground for it and the applicant suffered loss by relying on it. The representor’s position would be a little like King Lear’s fool who told him (William Shakespeare, King Lear, Act 1, sc 4, cc 182-188):
I marvel what kin thou and thy daughters are.
They’ll have me whipped for speaking true, thou’lt
have me whipped for lying, and sometimes I am
whipped for holding my peace. I had rather be any
kind o’ thing than a Fool. And yet I would not be
thee, nuncle. Thou hast pared thy wit o’ both sides
and left nothing i’ th’ middle.
492 The three representations as conveyed to the ordinary reasonable reader in the publications complained of were accurate and were not misleading or deceptive or likely to mislead or deceive.
493 Since I found that none of the geographic spread publications conveyed the geographic spread representation ([115]), I do not consider it necessary to deal with any issue as to whether Zoetis had reasonable grounds for it.
494 As I explained in rejecting the applicant’s submission that the all horses representation contravened s 18(1) of the ACL, if that representation were understood as an expression of opinion, it conveyed that Zoetis had reasonable grounds for holding the opinion and I have found that was so ([149]-[167]).
495 In her closing written submissions, the applicant accepted that Zoetis had established through the evidence of Dr L’Estrange and Dr Lehrbach that Zoetis had reasonable grounds for the no serious side effects representation based on their understanding of Equivac HeV’s components at the time that Equivac HeV first went to market. Accordingly, the applicant must have accepted there were reasonable grounds for Zoetis to make the no serious side effects representation in the first permit disclosure, when it was published in the leaflet for it on 10 August 2012.
496 Unhelpfully, the applicant never identified with any precision the time by which she asserted Zoetis must have come to lack reasonable grounds for the no serious side effects representation.
497 Relevantly, I found each of the first, second and third permit disclosure ([188], [195], [197]) the registration module ([192]), the myth busting pamphlet ([189]) and the facts about HeV pamphlet conveyed the no serious side effects representation ([196]). It is not necessary to deal, under s 4 of the ACL, with the applicant’s claims about the Health4Horses website materials and the Equestrian Life letter because I found that they did not convey the no serious side effects representation ([193], [201]).
498 The applicant argued that as Zoetis received progressively more and more adverse reaction reports, which Dr L’Estrange and the Authority reviewed and then assigned more ABON “probable” or “possible” classifications, Zoetis should have concluded at some unspecified time that it was no longer reasonable to continue publishing the no serious side effects representation.
499 During the relevant period, Zoetis supplied nearly 600,000 doses of Equivac HeV that vaccinated nearly 360,000 horses as appears in the following table:
Calendar Year | Number of vaccine doses administered in the calendar year per Registry | Number of horses to which vaccine doses were administered in the calendar year per Registry |
From 1 November 2012 (product launch) | 16,018 | 8,657 |
2013 | 155,426 | 76,264 |
2014 | 146,134 | 82,986 |
2015 | 101,787 | 63,838 |
2016 | 76,056 | 55,471 |
2017 | 73,496 | 55,000 |
Up to 20 March 2018 (end of Relevant Period) | 17,873 | 15,896 |
Total | 586,790 | 358,112 |
500 Dr L’Estrange appraised himself of how Equivac HeV was developed including its composition when he became responsible in 2012 in relation to it. He created an Excel spreadsheet to record information relating to adverse reaction pharmacovigilance reports that Zoetis received beginning on 1 November 2012. He caused spreadsheets to be made that recorded that information separately for each financial year until 30 June 2017 to give himself a snapshot of the reported clinical signs that Zoetis classified as probable or possible in its pharmacovigilance reports to the Authority and the numbers, breeds and ages of the horses that had received vaccination in that period. That information is in the table at [499] above. The spreadsheet’s list of clinical signs evolved and increased based on the reports of adverse reactions that Zoetis received and adverse reaction information from vets and horse owners. After the receipt of each report of an adverse reaction, Dr L’Estrange set about investigating the circumstances in order to form a view as to whether there might be a causal connection between the clinical sign and the vaccination.
501 He expected that when Equivac HeV began being distributed in November 2012 it would perform similarly to other equine vaccines. He knew that the initial chemical trials had produced “minimal adverse events”. Thus, he expected, for example, there would be reports of injection site swelling because that was a common consequence of vaccination and that those reports did not need further investigation. This was because an injection site reaction was expected to be a temporary, not a serious, clinical sign and could be relieved by bute if need be. He said this was an immune response that the adjuvant stimulated. Likewise, he considered that a clinical sign of lethargy in a horse after vaccination was similar to the frequent human reaction to vaccination of feeling tired that would resolve within a few days.
502 Throughout the relevant period, Dr L’Estrange did not understand or believe that Equivac HeV caused any serious or debilitating side effects based on his own knowledge and understanding of Equivac HeV, his investigations, and the information in discussions with treating vets, the reports of clinical signs that he received or the spreadsheet’s information.
503 Dr L’Estrange was diligent in investigating and analysing adverse reaction reports, then compiling and from time to time updating, the pharmacovigilance reports that he caused to be sent to the Authority.
504 Dr L’Estrange participated in settling the final wording of the second permit disclosure and believed that its wording was accurate based on his knowledge, experience and the information he had accumulated at that time and his knowledge and experience. He gave evidence, that I accept, that at the times of the respective publications of each of the others of the 12 publications complained of (that the applicant alleged conveyed the three representations), he believed that each was accurate and based on his understanding of scientific knowledge.
505 In any event, I accept Dr L’Estrange’s evidence that throughout the relevant period he believed, based on his knowledge, experience, the records he caused to be kept and his evaluation of all adverse events reported to Zoetis, and the investigations and information he had about them, that Equivac HeV was safe and not causing serious and potentially debilitating side effects.
506 Dr Messer made twice daily checks with members of Zoetis’ pharmacovigilance team at its offices in Rhodes, a suburb of Sydney. In the relevant period, Dr Skuodas was Zoetis’ officer, principally responsible under Dr Messer, for pharmacovigilance in respect of Equivac HeV and they worked together on a daily basis. Dr Messer said that in 2014 he saw a high rate of reported adverse events, but observed that the majority were injection site reactions or their sequelae, such as fever. Dr Messer explained that Zoetis made its own ABON assessments in its pharmacovigilance reports to the Authority, while the Authority performed its ABON assessments independently.
507 In a letter dated 8 January 2015, the Authority informed Zoetis that it had reclassified to “possible” Zoetis’ assessment of “unknown” or “unlikely” for the causation of seven horse deaths. Dr Messer reviewed those reassessments using the Authority’s algorithm and concluded that its reassessments were erroneous.
508 On 6 February 2015, Dr Messer responded to the Authority’s 8 January 2015 letter disputing, with detailed reasons, the Authority’s view, and adhering to the original classifications that Zoetis had made. Dr Messer gave evidence, which I accept, that he believed that the Authority was wrong and what he wrote was correct. He explained that there was a subjective element to the selection of an ABON classification based on an evaluation of three factors, namely, first, a reasonable association in time between vaccination and the reported adverse event, secondly, the plausibility of a causative connection by reason of the pharmacology and toxicology of the product and, thirdly, the absence of any other equally plausible explanation for the adverse event.
509 On 19 March 2015, the Authority responded and agreed to reclassify to “unknown” one of its disputed classifications but maintained its other six reclassifications of “possible” which, it wrote, gave “a reporting incidence of 0.002% for death”.
510 Shortly after, on 31 March 2015, the Authority issued the third permit with its wording of the third permit disclosure that the label should include under the heading “Side Effects” (see [76] above).
511 When Dr Lehrbach began employment at Pfizer, work in respect of Equivac HeV was the subject of a research development project. He was involved in both the vaccine’s development and regulatory compliance as he had been with many others. He dealt with all aspects of Equivac HeV’s research and the steps up to its registration including Zoetis’ application for patents in respect of it. He explained that his understanding (including in November 2012 when Equivac HeV went to market) was this was a subunit or synthesised genetically engineered, not live, vaccine. He understood that it used the G protein as the surface glycoprotein of the virus that Zoetis could make in sizeable quantities. A viral glycoprotein is involved in causing infection in mammalian cells. He also explained that the adjuvant, ISCOMatrix, stimulates the animal’s immune response to the particular (glyco)protein. He was also familiar with the other ingredients of Equivac HeV, namely, the preservative, thiomersal, which had been commonly used, including in Zoetis’ vaccines for horses, for many years before 2012, and the excipients (which make up the volume but are inert). The preservatives and excipients are excreted after vaccination by the horse, as is the adjuvant after it has been metabolised.
512 Dr Lehrbach said that the G protein is genetically engineered from the living HeV in a fermentation process. The process involves taking a single strand of the particular G protein gene (living RNA) of the virus and converting that to a DNA copy of the G protein gene by cloning it into an animal cell to express the inert G protein, which is sterile. He said the inert G protein mimics the G protein on the surface of the Hendra virus. He said that, in 2012, the technology to create the G protein was the subject of a wealth of literature on similar approaches to create vaccines for other diseases.
513 Dr Lehrbach gave evidence, that I accept, that he believed that each of the first, second and third permit disclosures was correct. He said that the wordings were finalised by agreement between the Authority and Zoetis and that he was involved in and approved each of the final versions.
514 He said that the first permit disclosure was an accurate description of the results of the clinical work to that time. He said that:
based on my knowledge of the components of the vaccine, the way it’s made and … the results of clinical testing, … I do not agree that this vaccine would cause serious and debilitating side … reactions.
(emphasis added)
515 Dr Lehrbach said that there was nothing to show that Equivac HeV was unsafe when it went to market. Thereafter, including up to the end of 2018, he kept abreast of the pharmacovigilance reports and regularly spoke with Dr L’Estrange and Dr Messer about the adverse reports that Zoetis was receiving. He said that, had he believed that Equivac HeV had caused or would cause serious and debilitating side effects, he would have informed the Authority. He said that at the time of his evidence he still believed that Equivac HeV did not and would not cause serious and debilitating side effects. He based that belief on the clinical trials, the vaccine’s method of manufacture and his general knowledge and experience of the development of animal vaccines.
516 Dr Lehrbach did not accept that tachycardia, tachypnoea (ie: rapid breathing) (unlike Dr L’Estrange), lethargy, colic or death would be associated with, or were side effects of, Equivac HeV. However, he agreed to include lethargy, muscle stiffness and the other observations of clinical signs that were stated in the second and third permit disclosures under the heading “Side Effects” because they were consistent with the pharmacovigilance reports that Zoetis had received and the Authority’s request, even though those were not reactions that he would associate with the vaccine.
517 I accept the evidence of Dr Lehrbach, Dr Messer, and Dr L’Estrange that they believed that Equivac HeV would not, and as reports of adverse reactions came in to Zoetis over the course of the relevant period, had not caused any serious (or debilitating) side effect. That was because of their expertise as vets and their understanding of the technology involved in the vaccine, the experience that they had had or knew of with the adjuvant, preservative, and excipients it used and the inert character of the antigen.
518 For the reasons I have given, they had reasonable grounds for believing that any future matter with respect to the no serious side effects representation was accurate. More importantly, the applicant did not establish that each of them (as the officers of Zoetis responsible for conveying the no serious side effects representation) did not have reasonable grounds for making it at any time in the relevant period.
519 The experts agreed that the expected safety of Equivac HeV was high. That being so, it is pellucid that Zoetis had reasonable grounds for making the no serious side effects representation and the all horses representation (to the extent that the applicant relied on the existence of serious side effects to support her claim that the all horses representation was misleading or contravened s 18(1) of the ACL).
520 In addition, the no serious side effects representation in each of the first, second, and third permit ceased to be made by Zoetis progressively as the next disclosure replaced its predecessor and remade the representation. Once the Authority registered Equivac HeV the vaccine was no longer distributed or sold under the third permit and there is no evidence that any subsequent supply of Equivac HeV had any information in the leaflet or that Zoetis made the no serious side effects representation.
521 Similarly, the registration module conveyed the no serious side effects representation to the vets who completed it in the period immediately following about May 2013 when it was published. However, the applicant led no evidence of the duration of the period in which Zoetis published the registration module. It would have been pellucid to a vet to whom it was published that the no serious side effects representation had been based on the initial 25,500 doses, as it stated ([56] above). By no later than the publication of the second permit disclosure, any future matter conveyed in the no serious side effects representation in the registration module would no longer have been conveyed to a vet. Likewise, to the extent that the no serious side effects representation was made with respect to a future matter in the myth busting pamphlet and the facts about HeV pamphlet, there is no evidence of their continuing publication after their initial publications and, in particular, after the Authority registered Equivac HeV in August 2015.
522 The applicant did not explain or lead any evidence of how any publication of the no serious side effects representation in any of the non-disclosure publications had any effect after that registration occurred, given that there was no evidence that any of them continued to be published.
523 In any event, I am not satisfied that the applicant has proved that the no serious side effects representation was not true or that Zoetis did not have reasonable grounds for its making with respect to any future matter.
524 In light of my findings above, I am not satisfied that:
(1) any of the geographic spread publications conveyed the geographic spread representation;
(2) the Equestrian Life letter conveyed the all horses representation;
(3) the Horses4Horses website information or the Equestrian Life letter conveyed the no serious side effects representation;
(4) to the extent that it was conveyed, each of the all horses representation or the no serious side effects representation contravened s 18(1) of the ACL or, so far as it was made with respect to any future matter, Zoetis did not have reasonable grounds for making it;
(5) Equivac HeV was not of acceptable quality within the meaning of s 54(2) of the ACL or that Zoetis contravened the consumer guarantee in s 54(1) in supplying it.
525 In my opinion, subject to hearing from the parties, the following answers should be given to the common questions that I have formulated below:
Question 1: During the period between 10 August 2012 and 20 March 2018, did Zoetis engage in conduct that was misleading or deceptive in contravention of s 18(1) of the ACL (or with respect to any future matter, by not having reasonable grounds for making, in contravention of s 4(1) of the ACL) representations in trade or commerce that:
(a) there was a serious risk of horses contracting Hendra virus in all areas of Australia in which flying foxes were present;
(b) Equivac HeV had no serious side effects; or
(c) all horses in Australia should be treated with Equivac HeV.
Answer 1:
(a) Zoetis did not make this representation;
(b) Zoetis made this representation, but, in doing so:
(i) it did not contravene s 18(1) of the ACL; and
(ii) to the extent that the representation was made with respect to any future matter, it did not contravene s 4(1) of the ACL;
(c) Zoetis made this representation, but, in doing so:
(i) it did not contravene s 18(1) of the ACL; and
(ii) to the extent that the representation was made with respect to any future matter, it did not contravene s 4(1) of the ACL.
Question 2: During the period between 10 August 2012 and 20 March 2018, did Zoetis supply, in trade or commerce, Equivac HeV to a consumer when the vaccine was not of acceptable quality for the purposes and within the meaning of the guarantee in s 54 of the ACL that it was of acceptable quality?
Answer 2: No.
526 Because I have rejected the factual cases of Ms Abbott and Ms Hinton that the vaccinations of Primetime and Quinn with Equivac HeV caused either horse to suffer a serious side effect, it follows that the application must be dismissed and that costs should follow the event. I will give the parties an opportunity to address the form of final orders.
I certify that the preceding five hundred and twenty-six (526) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Rares. |
Associate:




