FEDERAL COURT OF AUSTRALIA
Biogen International GmbH v Pharmacor Pty Ltd [2021] FCA 1591
ORDERS
First Applicant BIOGEN AUSTRALIA PTY LTD Second Applicant | ||
AND: | Respondent | |
AND BETWEEN: | Cross-Claimant | |
AND: | BIOGEN INTERNATIONAL GMBH (and another named in the Schedule) First Cross-Respondent | |
DATE OF ORDER: | 16 December 2021 |
THE COURT ORDERS THAT:
Upon the respondent undertaking to keep accounts:
1. The applicant’s interlocutory application be dismissed.
2. The time for filing an application for leave to appeal from these orders be extended to 31 January 2022.
3. The applicants pay the respondent’s costs of this application.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ROFE J:
1 This was an application for an interlocutory injunction in proceedings for patent infringement under the Patents Act 1990 (Cth) (the Act) and misleading and deceptive conduct in contravention of the Australian Consumer Law (being Schedule 2 to the Competition and Consumer Act 2010 (Cth) (ACL).
1. INTRODUCTION
1.1 The parties and the broad issues
2 The applicants, Biogen International GmbH (Biogen Switzerland) and Biogen Australia Pty Ltd (Biogen Australia), commenced proceedings seeking final relief pursuant to the Act and the ACL to restrain the respondent Pharmacor Pty Ltd (Pharmacor) from launching its dimethyl fumerate (DMF) products (Pharmacor Products).
3 Biogen Switzerland is the Patentee of Australian patent number 752733 entitled “Utilisation of dialkyl fumarates” (the Patent). Following an extension of term granted by the patent office on 20 October 2014, the Patent expires on 29 October 2024. The patent claims a priority date of 19 November 1998.
4 Biogen Australia and Biogen Switzerland are members of the same group of companies of which Biogen Inc is the parent company. Biogen Australia is not an exclusive licensee of the Patent. Unless otherwise stated the applicants are collectively referred to below as Biogen.
5 Biogen sells dimethyl fumarate formulations in Australia under the name Tecfidera, for the treatment of multiple sclerosis.
6 On 14 July 2021, Pharmacor obtained a listing for the Pharmacor Products on the Australian Register of Therapeutic Goods (ARTG). Pharmacor is seeking to be listed on the Schedule of Pharmaceutical Benefits (PBS Schedule) pursuant to the Pharmaceutical Benefits Scheme (PBS) from 1 December 2021. Biogen seeks urgent interlocutory orders to restrain Pharmacor from marketing and offering the Pharmacor Products for sale.
7 When considering an application for an interlocutory injunction, the court must address two main enquiries, namely whether the applicant for relief has established a prima facie case in the sense explained in Beecham Group Limited v Bristol Laboratories Pty Ltd (1968) 118 CLR 618 at 622-3 (Beecham), and whether the balance of convenience and justice favours the grant of an injunction or the refusal of that relief.
8 Pharmacor contests the application. For the purposes of the interlocutory hearing, Pharmacor admits that the Pharmacor Products fall within all the asserted claims except claim 17. It asserts that the extension of the Patent term was wrongly granted and that the claims asserted against it are invalid for want of novelty over one prior art document.
9 The hearing of the application for injunctive relief was brought on urgently as Biogen apprehended that the Pharmacor Products might be listed on the PBS on the next listing date, 1 December 2021. Subsequent to the hearing, the Court was advised by the parties that the PBS had advised Pharmacor that the Pharmacor Products would not be listed on the PBS before 1 February 2022.
1.2 Summary of conclusion
10 For the reasons set out below I have concluded that the balance of convenience and justice does not lie in favour of the grant of the interim relief sought.
1.3 The witnesses
11 Biogen relies on evidence from five witnesses:
(a) Kylie Michele Bromley is the managing director of Biogen Australia and has a PhD in pharmacology. She has worked in the pharmaceutical industry in Australia for over 20 years. Dr Bromley gives evidence as to the Patent, the market for MS treatment in Australia, Tecfidera, the Pharmacor Products, the background to the dispute and the effect on Biogen if the Pharmacor Products are launched. Biogen relies on two affidavits from Dr Bromley.
(b) Peter James Davey is a health economist with extensive knowledge of, and experience in, the pharmaceutical sector, including with respect to reimbursement and pharmaceutical pricing issues. Mr Davey has worked in the pharmaceutical sector since 1991 and has extensive experience in preparing economic modelling in that sector. Mr Davey gives opinion evidence as to what he considers would be involved in assessing the loss to Biogen if an interlocutory injunction was not granted and it is ultimately determined that the Patent is valid and infringed. Biogen relies on three affidavits from Mr Davey.
(c) Owain Rhys Stone is a Partner at KordaMentha. He is a forensic accountant with significant experience in the pharmaceutical and life sciences sector including in the quantification of economic loss and damage in the context of pharmaceutical injunction disputes. Mr Stone describes the financial analysis he would employ to assess Biogen’s loss if Pharmacor is not restrained and the court finds the Patent valid and infringed. Biogen relies on two affidavits from Mr Stone.
(d) William Neil Charman is a Sir John Monash distinguished Professor in the Faculty of Pharmacy and Pharmaceutical Sciences at Monash University where he was Dean from 2007 to 2019. Professor Charman has expertise in the field of oral drug delivery and the design and assessment of pharmaceutical formulations for oral administration to humans. He gives evidence as to the prior art relied on by Pharmacor and responds to the evidence of Pharmacor’s expert. Biogen relies on one affidavit from Professor Charman.
(e) Rebekah Frances Gay is a partner at Herbert Smith Freehills, Biogen’s solicitors. Ms Gay’s affidavit annexes search results, a decision of the Australian patent office finding claims 13 to 17 of the Patent novel over a prior art application on re-examination, and correspondence and documents relating to United States proceedings concerning Biogen’s related US patent in a dispute with Pharmacor’s ultimate parent company. Biogen relies on one affidavit from Ms Gay.
12 Pharmacor relies on the evidence of three witnesses:
(a) Ashish Mallela is Pharmacor’s CEO. Mr Mallela gives evidence as to the Pharmacor business, the generic pharmaceutical market in Australia, the planned launch of the Pharmacor products in Australia, the irreparable harm that Pharmacor will suffer if it is restrained, and the difficulties in quantifying Pharmacor’s loss. Pharmacor relies on two affidavits of Mr Mallela.
(b) Anthony Samuel is a forensic accountant and the Managing Director at Sapere Research Group Limited. Mr Samuel gives evidence of the exercise required to estimate the value of a loss suffered by a supplier of a generic pharmaceutical product when that supplier is restrained from offering its product, the exercise required to estimate Biogen’s loss if Pharmacor was not restrained, and responds to Mr Stone’s evidence. Pharmacor relies on three reports of Mr Samuel.
(c) Desmond Berry Williams is a senior researcher in Clinical and Health Sciences with expertise in pharmaceutical formulation. Dr Williams gives evidence as to what is disclosed in the two prior art documents and compares the disclosure in those documents with the claims of the Patent. Pharmacor relies on one affidavit from Mr Williams.
13 Due to the exigencies of the COVID-19 pandemic, the hearing took place via Microsoft Teams. Given the confidential nature of most of the evidence addressing the balance of convenience considerations, the hearing took place in two sessions: an open session relating to all issues other than the balance of convenience, and a confidential session attended by only the parties’ instructors and counsel at which the balance of convenience arguments were made. No witnesses were required for cross examination.
14 Much of the evidence was not the subject of substantial disagreement aside from that relating to the complexity of the task of assessing damage for either party. It is not the role of the court in an application such as this to make findings of fact. I do not purport to do so here.
1.4 Multiple sclerosis
15 Multiple sclerosis (MS) is an inflammatory autoimmune disorder affecting the central nervous system that causes a loss of function to the nervous system and loss of communications between the brain and the body. It is the most common cause of neurological disability in young adults, and affects approximately 2.8 million people around the world.
16 MS is a chronic and complex disease, which has a very significant impact on the quality of life of patients.
17 MS is a relatively rare disease. Approximately 25,000 Australians suffer from MS, with the number of patients actively seeking treatment for MS in Australia steadily rising at a rate of about 4% per year. This increase in the patient pool accounts for population growth, disease growth, and patients who have previously opted out of treatment, but are choosing to opt back in as new treatment options become available.
18 There is no known cure for MS. The primary purpose of MS treatment is to reduce the frequency of relapses in symptoms and the progression of the disease.
19 There is also no uniform treatment plan for MS patients in Australia. Generally, a treatment regime is developed for individual patients by a medical practitioner, possibly in consultation with a specialist team which might include a patient’s GP, a neurologist, an MS specialist nurse, and a number of allied health practitioners (such as an occupational therapist or physiotherapist). In determining what treatment to prescribe, physicians will consider a number of factors, including how active the MS is based on a clinical or MRI assessment. For example, if the MS is more active, a patient will likely be prescribed an infusion therapy, while for milder forms, patients will typically take oral treatments. The pregnancy prospects of the patient are also a relevant consideration, given that 70% of patients are female and aged between 25 to 30 years when initially diagnosed. While none of the MS treatments have been approved for use during pregnancy, Tecfidera, for example, is suitable for patients who may fall pregnant.
20 Across a lifetime, a patient will typically use two or more MS treatments. This can be due to:
(a) Reduced efficacy with some drugs after a period of time, which causes patients to experience a decrease in the time between relapses;
(b) The patient having difficulty adhering to a particular treatment regime (particularly if the medication is administered by self-injection or infusion); or
(c) Changes in the safety profile for some treatments; for example, some infusion treatments can increase the chance of a patient developing progressive multifocal leukoencephalopathy (a disease of the white matter of the brain).
1.5 Overview of the MS market
21 Broadly, the available treatments for MS in Australia fall into three types of products:
(a) Injectables: these have been available in Australia for about 20 years and require patients to regularly self-administer sub-cutaneous or intra-muscular injections. Biogen developed one of the first injectables, being Avonex (interferon beta-1a or IFN β-1a). These were breakthrough treatments in the early 2000s. Given the advances in treatments since their introduction, injectables are now the least commonly prescribed form of treatment for MS accounting for only 15% of the market, as they are considered the least effective form of treatment compared with oral medications or infusions.
(b) Oral medications: these are now the largest group of treatments and include Tecfidera. Oral medications are the primary treatment option for mild forms of MS. The first of this group of treatments was introduced onto the MS market around 2011, some 10 years after the introduction of injectables, and again represented a very significant advancement in the treatment of MS. They are still widely used by the MS population today. Oral treatments account for approximately 47% of the MS treatment market in Australia.
(c) Infusions: These are treatments comprising monoclonal antibodies that are administered by infusion. These types of products are becoming more widely used as a result of their high efficacy. Biogen Australia markets Tysabri, which is administered by an intravenous infusion by a health practitioner every four weeks, which takes approximately one hour. Other infusion therapies require administration once or twice a year. These account for approximately 38% of the MS treatment market in Australia.
22 The following table, taken from Dr Bromley’s evidence, summarises the MS treatments available in Australia as at October 2021:
Product | Active Ingredient | Approved | Supplier | Mode of administration |
Injectables | ||||
REBIF | IFN β-1a | 2000 | Merck | SC injection – 3 per week |
AVONEX | IFN β-1a | 2001 | Biogen | IM injection – once a week |
BETAFERON | IFN β-1b | 2002 | Bayer | SC injection – every other day |
COPAXONE | Glatiramer acetate | 2003 | Teva | SC injection – 3 per week |
PLEGRIDY | Pegylated IFN β-1a | 2014 | Biogen | SC injection – once a fortnight |
Oral Medications | ||||
GILENYA | Fingolimod | 2011 | Novartis | Oral – once daily |
AUBAGIO | Teriflunomide | 2012 | Sanofi | Oral – once daily |
APO Teriflunomide | Teriflunomide | 2019 | Apotex | Oral – once daily |
Teriflagio | Teriflunomide | 2019 | Arrow Pharma | Oral – once daily |
Teriflunomide Dr Reddys | Teriflunomide | 2019 | Dr Reddys Labs | Oral – once daily |
Teriflunomide GH | Teriflunomide | 2019 | Generic Health | Oral – once daily |
Pharmacor Teriflunomide | Teriflunomide | 2019 | Pharmacor Ltd | Oral – once daily |
Teriflunomide Sandoz | Teriflunomide | 2019 | Sandoz | Oral – once daily |
Terimide | Teriflunomide | 2021 | Alphapharm | Oral – once daily |
Tecfidera | Dimethyl fumarate | 2013 | Biogen | Oral – twice daily |
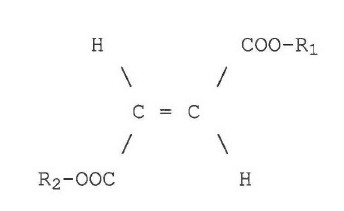
FAMPYRA | Fampridine | 2011 | Biogen | Oral – twice daily |
MAVENCLAD | Cladribine | 2019 | Merck | Oral – yearly regime |
ZEPOSIA | Ozanimod | 2021 | BMS | Oral – once daily |
MAYZENT | Siponimod | 2020 | Novartis | Oral – once daily |
Infusions | ||||
TYSABRI | Natalizumab | 2006 | Biogen | Infusion - monthly |
LEMTRADA | Alemtuzumab | 2013 | Sanofi | Infusion – once a year |
OCREVUS | Ocrelizumab | 2017 | Roche | Infusion – every 6 months |
23 Dr Bromley provided further information about some of the treatments listed in the table which is summarised below.
24 The generic teriflunomide products listed in the table are those that have obtained PBS listing and are on the market. There are additional brands listed on the ARTG, but which are not PBS listed. Dr Bromley’s evidence was that she expected the other generics to also enter the market in the near future. As far as Dr Bromley is aware, Sanofi is no longer providing any significant marketing support for its product, Aubagio, with the result that the overall market share for teriflunomide products has decreased. Sanofi has announced that because of the genericised market it will no longer be marketing Aubagio in Australia beyond October 2021.
25 In March 2021, Novartis obtained approval from the Therapeutic Goods Administration (TGA) for its ofatumumab product, Kesimpta, a sub-cutaneous once a month injectable. Kesimpta was approved for PBS listing on 30 September 2021, with effect from 1 October 2021. Dr Bromley expects the launch of Kesimpta to have an impact on oral MS therapies including Tecfidera, as Novartis is an established participant in the MS market with good relationships with neurologists.
26 The Novartis product Gilenya is the only product containing fingolimod currently available. There are 36 generic products on the ARTG which list fingolimod as the active ingredient.
27 All three experts, Mr Davey, Mr Stone and Mr Samuel described the MS market as a “dynamic market”. I discuss the experts’ views on the MS and DMF markets later.
28 Despite the experts’ comments as to the dynamic nature of the MS market, and the frequency of new products entering the market during the period that Tecfidera has been available in Australia, I observe that [redacted].
29 The MS market in Australia was described in the evidence as a small, niche market. It is much smaller than the markets in recent damages cases for pharmaceutical products such as venlafaxine (an anti-depressant) in Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (2018) 136 IPR 8, clopidogrel (a platelet aggregation inhibitor) in Commonwealth of Australia v Sanofi (formerly Sanofi-Aventis) (No 5) (2020) 151 IPR 237, or the oral contraceptive pill in Generic Health Pty Ltd & Anor v Bayer Pharma Aktiengesellschaft & Anor (2018) 137 IPR 1.
1.6 Biogen and Biogen’s MS products
30 Biogen is a specialist biotechnology business founded in 1978 which conducts research specifically directed to neurological and neurodegenerative diseases. The core areas on which Biogen’s research has focussed are MS, Alzheimer’s disease and dementia, neuromuscular disorders (including spinal muscular atrophy) and movement disorders (including Parkinson’s disease).
31 Biogen Switzerland owns all Biogen’s non-US intellectual property rights. It is also responsible for the manufacture and distribution of drug products to a number of countries including Australia, and contributes to the funding and management of Biogen’s research activities.
32 Biogen Australia operates Biogen’s business in Australia. It is responsible for the sales, marketing and regulatory approval of Biogen’s products in Australia, relations with regulators, price negotiations with the Australian Government, providing support for clinicians and patients, supporting ongoing Australian research in relevant areas and sponsoring Australian based clinical trials.
33 Biogen Australia’s product portfolio consists of only six products, all of which bar one are dedicated to relapsing MS treatments (Spinraza is indicated for treatment of spinal muscular atrophy).
Product | Active Ingredient | Date listed on ARTG |
Avonex | Interferon beta 1-A | July 2001 |
Tysabri | Natalizumab | November 2008 |
Fampyra | Fampridine | May 2011 |
Tecfidera | DMF | July 2013 |
Plegridy | Peginterferon beta 1-A | November 2014 |
Spinraza | Nusinersen | November 2017 |
34 Biogen has applied for TGA approval for:
(a) Vumerity (diroximel fumarate), a treatment for relapsing MS; and
(b) Aduhelm (aducanumab), a treatment for Alzheimer’s disease.
35 The likely launch of Vumerity and its potential disruption of the DMF market is discussed later in the balance of convenience considerations.
36 The active ingredient of Tecfidera is dimethyl fumarate (DMF). It is an oral medication formulated as enteric-coated microtablets contained within a hard gelatin capsule. Tecfidera is approved in Australia for the treatment of patients with relapsing MS to reduce the frequency of relapses and to delay the progression of disability.
37 Tecfidera was first listed on the ARTG in July 2013, 14 years after the complete specification of the Patent was filed. This was the first regulatory approval date for DMF in Australia. The Patent is the only patent that has had a term extension granted based on DMF (Tecfidera).
38 Biogen Australia is the sponsor on the ARTG of the following two dosage forms of Tecfidera:
(a) 120mg modified release capsule blister packs (ARTG ID 197118); and
(b) 240mg modified release capsule blister packs (ARTG ID 197119).
39 Tecfidera was first listed on the PBS on 1 December 2013. Tecfidera is a prescription only medicine, which means that patients can only obtain it from retail pharmacists after obtaining a prescription from their medical practitioner. The starting dose of Tecfidera is 120mg twice a day administered orally. The maintenance dose, which is started after seven days, is 240mg taken twice a day orally.
40 Tecfidera is a significant part of Biogen Australia’s MS portfolio.
1.7 Pharmacor and Pharmacor’s products
41 Pharmacor’s primary business in Australia is the marketing and supply of generic prescription and over the counter medicines to retail pharmacies and public and private hospitals under its own brand. Pharmacor currently markets and sells 202 discrete products under its own brand.
42 Pharmacor supplies its own products to approximately 3,500 of the more than 5,000 pharmacies in Australia. Part of Pharmacor’s marketing and sales strategy involves offering discounts directly to pharmacists where possible.
43 Pharmacor supplies its products to retail pharmacies in the following ways:
(a) Offering its products for sale to all major pharmaceutical wholesalers (including the three largest, Sigma Healthcare Ltd, Australian Pharmaceutical Industries Ltd and Symbion Pty Ltd) who then supply Pharmacor’s products to retail pharmacies; and
(b) Marketing and selling some products directly to pharmacies and large pharmacy groups such as Chemist Warehouse and Priceline Pharmacy.
44 Mr Davey describes Pharmacor as a mid-sized generic supplier with at least 55 unique molecules listed on the PBS.
45 Alkem is the ultimate parent company of Pharmacor. Alkem was established in India in 1973 and listed on the Indian National Stock Exchange in 2015. Alkem currently operates in over 50 countries and has developed over 800 branded generic products.
46 On 14 July 2021, Pharmacor obtained registration on the ARTG for the following six DMF delayed release capsule blister packs:
(a) FURATEC: for 120mg and 240mg;
(b) AKM: for 120mg and 240mg; and
(c) PHARMACOR: for 120mg and 240mg.
47 On 20 August 2021, Pharmacor applied for listing on the PBS for the two PHARMACOR DMF products (being those products referred to as the Pharmacor Products). Pharmacor admits that if the Pharmacor Products are listed on the PBS, they will be a-flagged as substitutable at the pharmacy level for the Tecfidera products.
48 Since August 2021, Pharmacor has been preparing for the launch and commencement of the supply of Pharmacor Products from 1 December 2021. As noted above, the Pharmacor Products will not be listed until at least 1 February 2022.
1.8 Other ARTG registrations of DMF
49 Accelagen Pty Ltd (Accelagen) is another company with ARTG registration for generic versions of DMF products. Accelagen has four DMF ARTG registrations.
50 On 9 June 2021, Biogen commenced proceedings in this court against Accelagen and MSN Laboratories Private Ltd, India (MSN).
51 On 19 July 2021, the Court made orders by consent enjoining Accelagen and MSN from, amongst other things, seeking listing of the Accelagen DMF products on the PBS whilst the Patent remains in force. Accelagen is not restrained from further transferring its ARTG registrations to third parties. Pursuant to the orders made by Beach J, Accelagen must give Biogen notice, within five days of any transfer of the sponsorship of any of its ARTG registrations to a third party.
52 As a result, at the time of the hearing, Pharmacor was the only generic pharmaceutical company likely to be able to enter the DMF market in Australia on 1 December 2021. Subsequent to the hearing the Court was advised that the earliest date that Pharmacor will be able to enter the market if not restrained is 1 February 2022.
1.9 The issues
53 The dispute concerns the question of the strength of the patent infringement case that Biogen advances and whether the balance of convenience and justice warrants the grant of the interlocutory relief sought.
54 In the amended statement of claim Biogen asserts that the Pharmacor products are pharmaceutical preparations within the scope of at least claims 13, 14, 16, 17, 81, 86, 95, 96, 97 101, 102 and 103 of the Patent.
55 Pharmacor admits that the Pharmacor products fall within the scope of claims 13, 14, 16 (as dependent on 13 or 14), 95, 96, 97, 101(as dependent on claims 95, 96 or 97), 102 (as dependent on any of 95, 96, 97 and 101) and 103 (as dependent on any of claims 95, 96, 97, 101 or 102) of the Patent.
56 Pharmacor denies that the Pharmacor products fall within claims 81 or 86 (both of which are omnibus claims) of the Patent.
57 In light of Pharmacor’s admissions, Biogen does not ask the Court to find a prima facie case of infringement on claims 81 and 86.
58 Pharmacor denies that the Pharmacor products fall within claim 17 which claims a product containing an amount of active ingredient corresponding to 10mg to 300mg of fumaric acid.
59 In the context of the strength the prima facie case the remaining issue on the infringement case is whether claim 17 is infringed.
60 Pharmacor submits that the strength of the infringement case is qualified by its foreshadowed cross-claim for revocation of the asserted claims for invalidity, and the assertion that the extension of term of the Patent was invalidly granted. For the purposes of the interlocutory application, Pharmacor confined its invalidity case at the hearing to a novelty attack to the asserted claims, on the basis of one prior art document: Australian patent application no. 21393/99 (the 593 Application).
61 Biogen asserts that it has a strong prima facie case on its ACL claim on the basis of the strength of its infringement case.
1.10 The Patent
62 The Patent is entitled “Utilisation of dialkylfumarates”. The complete specification was filed on 29 October 1999 and the claims have a priority date of 19 November 1998.
63 The relevant form of the Act is the form the Act took prior to the changes introduced by the Patents Amendment Act 2001 (Cth).
64 The Patent commences:
The present invention relates to the use of dialkyl fumarates for preparing pharmaceutical preparations for use in transplantation medicine or the therapy of autoimmune diseases and pharmaceutical preparations in the form of micro-tablets or micro-pellets containing dialkyl fumarates.
On the one hand, therefore, it relates especially to the use of dialkyl fumarates for preparing pharmaceutical preparations for the treatment, reduction or suppression of rejection reactions of the transplant by the recipient, i.e. host-versus-graft reactions, or rejection of the recipient by the transplant, i.e. graft-versus-host reactions. On the other hand, it relates to the use of dialkyl fumarates for preparing pharmaceutical preparations for treating autoimmune diseases such as polyarthritis, multiple sclerosis, juvenile-onset diabetes, Hashimoto's thyroiditis, Grave's disease, systemic Lupus erythematodes (SLE), Sjogren's syndrome, pernicious anaemia and chronic active (= lupoid) hepatitis.
Both graft rejection and autoimmune diseases are based on medically undesirable reactions or dysregulation of the immune system. Cytokins such as interleukins or tumour necrose factor α (TNF-α) are substantial mediators influencing the immune system. In general, both are treated by the administration of immunosuppressive agents such as cyclosporine.
65 At page 4, the Patent sets out two objects of the invention:
The danger in using immunosuppressive agents lies in weakening the body's defence against infectious diseases and the increased risk of malignant diseases. Therefore, it is the object of the invention to provide a pharmaceutical preparation to be employed in transplantation medicine which may be used to treat, especially to suppress, weaken and/or alleviate host-versus-graft reactions and graft-versus-host reactions, but does not have the above disadvantage.
It is another object of the invention to provide a pharmaceutical preparation which may be employed for treating autoimmune diseases, particularly polyarthritis, multiple sclerosis, juvenile-onset diabetes, Hashimoto's thyroiditis, Grave's disease, systemic Lupus erythematodes (SLE), Sjogren's syndrome, pernicious anaemia and chronic active (= lupoid) hepatitis, without the disadvantages of immuno suppression.
66 There follows at page 5, line 8 and following, description of 13 aspects of the invention. Each aspect of the invention described includes a therapeutic use directed towards transplantation medicine or therapy for autoimmune diseases. For example:
According to a first aspect, the present invention consists in the use of one or more dialkyl fumarates for preparing a pharmaceutical preparation for treating host-versus-graft reactions or graft-versus-host reactions in organ and/or cell transplantation.
67 A third aspect of the invention corresponds to the invention as claimed in claim 13. See for example:
(a) at page 5b, line 29 to page 5c, line 2:
There is disclosed herein certain dialkyl fumarates for preparing pharmaceutical preparations for use in transplantation medicine and for the therapy of autoimmune diseases and pharmaceutical preparations in the form of micro-tablets and micro-pellets containing these dialkyl fumarates. The individual subject matters of the invention are characterised in detail in the claims. The preparations according to the invention do not contain any free fumaric acids per se.
(b) at page 6:
Surprisingly, it has now been found that dialkyl fumarates are advantageous for preparing pharmaceutical compositions for use in transplantation medicine and for the therapy of autoimmune diseases. This is because compositions containing such dialkyl fumarates surprisingly permit a positive modulation of the immune system in host-versus-graft reactions, graft-versus-host reactions and other autoimmune diseases.
European Patent Application 0 188 749 already describes fumaric acid derivatives and pharmaceutical compositions containing the same for the treatment of psoriasis. Pharmaceutical compositions for the treatment of psoriasis containing a mixture of fumaric acid and other fumaric acid derivatives are known from DE-A- 25 30 372. The content of free fumaric acid is obligatory for these medicaments.
(c) at page 7 to the top of page 8:
Specifically, the object of the invention is achieved by the use of one or more dialkyl fumarates of the formula
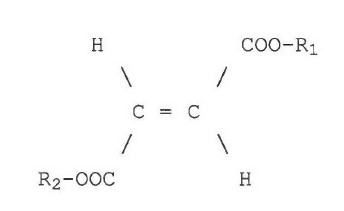
wherein R1 and R2, which may be the same or different, independently represent a linear, branched or cyclic, saturated or unsaturated C1-20 alkyl radical which may be optionally substituted with halogen (Cl, F, I, Br), hydroxy, C1-4 alkoxy, nitro or cyano for preparing a pharmaceutical preparation for use in transplantation medicine or for the therapy of autoimmune diseases.
The C1-20 alkyl radicals, preferably C1-8 alkyl radicals, most preferably C1-5 alkyl radicals are, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, t-butyl, pentyl, cyclopentyl, octyl, vinyl, allyl, 2-hydroxy ethyl, 2 or 3-hydroxy propyl, 2-methoxy ethyl, methoxy methyl or 2- or 3-methoxy propyl. Preferably at least one of the radicals R1 or R2 is C1-5 alkyl, especially methyl or ethyl. More preferably, R1 or R2 are the same or different C1-5 alkyl radicals such as methyl, ethyl, n-propyl or t-butyl, methyl and ethyl being especially preferred.
Most preferably, R1 and R2 are identical and are methyl or ethyl. Especially preferred are the dimethyl fumarate, methyl ethyl fumarate and diethyl fumarate.
(d) at page 8, line 5 and following:
The dialkyl fumarates to be used according to the invention are prepared by processes known in the art (see, for example, EP 0 312 697).
Preferably, the active ingredients are used for preparing oral preparations in the form of tablets, pellets or granulates, optionally in capsules or sachets. Preparations in the form of micro-tablets or pellets, optionally filled in capsules or sachets are preferred and are also a subject matter of the invention. The oral preparations may be provided with an enteric coating. Capsules may be soft or hard gelatine capsules.
The dialkyl fumarates used according to the invention may be used alone or as a mixture of several compounds, optionally in combination with the customary carriers and excipients. …
(e) at page 9, last paragraph:
According to the invention, a therapy with dialkyl fumarates may also be carried out in combination with one or more preparations of the triple drug therapy customarily used in organ transplantations or with cyclosporine A alone. For this purpose, the preparations administered may contain a combination of the active ingredients in the known dosages or amounts, respectively. Likewise, the combination therapy may consist of the parallel administration of separate preparations by the same or different routes.
(f) at page 10, in the middle of the page:
By administration of the dialkyl fumarates in the form of micro-tablets, which is preferred, gastrointestinal irritations and side-effects, which are reduced already when conventional tablets are administered but is still observed, may be further reduced vis- à-vis fumaric acid derivatives and salts.
It is presumed that, upon administration of conventional tablets, the ingredients of the tablet are released in the intestine in a concentration which is too high, causing local irritation of the intestinal mucous membrane. This local irritation results in a short-term release of very high TNF-α concentrations which may be responsible for the gastrointestinal side effects. In case of application of enteric-coated micro-tablets in capsules, on the other hand, very low local concentrations of the active ingredients in the intestinal epithelial cells are achieved. The micro-tablets are incrementally released by the stomach and passed into the small intestine by peristaltic movements so that the distribution of the active ingredients is improved.
68 The Patent has four examples, commencing at page 11, which are directed towards the preparation of:
(1) enteric-coated micro-tablets in capsules containing 120mg of DMF which corresponds to 96mg of fumaric acid;
(2) enteric-coated micro-tablets in capsules containing 120mg of DMF which corresponds to 96mg of fumaric acid
(3) micro-pellets in capsules containing 50mg of DMF which corresponds to 40mg of fumaric acid; and
(4) enteric-coated capsules containing 110mg of DMF which corresponds to 88mg of fumaric acid.
1.11 The claims
69 The Patent has 211 claims. Putting the omnibus claims to one side, each claim (or the claim from which it depends) contains a reference in one form or another to use in transplantation medicine and/or for the therapy of autoimmune diseases, or specific examples of such diseases.
70 The first of the asserted claims, claim 13, claims a pharmaceutical preparation in the form of micro-tablets or micro-pellets consisting of one or more dialkyl fumarates of the formula:

wherein R1 and R2 which may be the same or different, independently represent a linear, branched or cyclic, saturated or unsaturated C1-20 alkyl radical which may be optionally substituted with halogen, hydroxy, C1-4 alkoxy, nitro or cyano excipients, and optionally suitable carriers and excipients for use in transplantation medicine or for the therapy of autoimmune diseases or psoriasis or psoriatic arthritis, neurodermatitis or enteritis regionalis Crohn.
71 Claim 14 claims a pharmaceutical preparation according to claim 13 wherein the autoimmune disease is polyarthritis, multiple sclerosis, juvenile-onset diabetes, Hashimoto’s thyroiditis, Graves’ disease, systemic Lupus erythematodes (SLE), Sjogren’s syndrome, pernicious anaemia or chronic active (lupoid) hepatitis.
72 Claim 15 claims a preparation according to claim 13 or 14 wherein the halogen is Cl, F, I or Br.
73 Claim 16 claims a preparation according to any one of claims 13 to 15 comprising DMF, diethyl fumarate or methyl ethyl fumarate.
74 Claim 17 claims a preparation according to any one of claims 13 to 16 comprising an amount of the active ingredient corresponding to 10mg to 300 mg of fumaric acid.
75 Claim 95 claims a pharmaceutical preparation in the form of micro-tablets or micro-pellets consisting of dimethyl fumarate which has the formula:
and optionally suitable carriers and excipients; for use in therapy of multiple sclerosis.
1.12 Patent term extension
76 On 15 November 2013, Biogen requested that the Commissioner of Patents extend the term of the Patent pursuant to s 70 of the Act (the Request).
77 In the Request, Biogen asserted that the application for an extension of term of the Patent was based on goods containing or consisting of a pharmaceutical substance, being enteric coated micro-tablets of dimethyl fumarate (Tecfidera), currently included on the ARTG.
78 The substance, as it occurs in the goods registered on the ARTG, was said to be in substance disclosed in the complete specification of the Patent as a pharmaceutical preparation in the form of micro-tablets or micro-pellets consisting of a dialkyl fumarate of the formula:

wherein R1 and R2 are CH3 (that is DMF). Biogen refers to this substance as the Propounded Pharmaceutical Substance in its written submissions.
79 In the Request, Biogen also stated that the Propounded Pharmaceutical Substance fell within the scope of at least claims 13 to 17 of the Patent.
80 On 20 October 2014, the Commissioner of Patents granted the extension of term of the Patent. Following the grant of the extension, the Commissioner made an entry in the Register of Patents that the term of the Patent was extended to 29 October 2024.
81 Biogen notes that without the extension, Biogen’s sales of Tecfidera in Australia would only have had patent protection for just over six years. Without the extension, the Patent would have expired on 29 October 2019.
2. RELEVANT LAW
82 The principles concerning the grant of interim injunctive relief are not controversial. When considering an application for an interlocutory injunction the Court must address two main enquiries, namely, whether the applicant for relief has established a prima facie case in the sense explained by the High Court in Beecham, and whether the balance of convenience and justice favours the grant of an injunction or the refusal of that relief.
83 The relevant principles are set out by the Full Court in Samsung Electronics Co. Limited v Apple Inc [2011] FCAFC 156 (Samsung) at [60]-[67]:
At [19] in O’Neill, Gleeson CJ and Crennan J said:
As Doyle CJ said in the last-mentioned case, in all applications for an interlocutory injunction, a court will ask whether the plaintiff has shown that there is a serious question to be tried as to the plaintiff’s entitlement to relief, has shown that the plaintiff is likely to suffer injury for which damages will not be an adequate remedy, and has shown that the balance of convenience favours the granting of an injunction. These are the organising principles, to be applied having regard to the nature and circumstances of the case, under which issues of justice and convenience are addressed. We agree with the explanation of these organising principles in the reasons of Gummow and Hayne JJ.
The requirement that, in order to obtain an interlocutory injunction, the plaintiff must demonstrate that, if no injunction is granted, he or she will suffer irreparable injury for which damages will not be adequate compensation (the second requirement specified by Mason ACJ in Castlemaine Tooheys at CLR 153; ALR 557) was not mentioned in Beecham. Nor was it referred to by Gummow and Hayne JJ in O’Neill. None the less, Gleeson CJ and Crennan J included that requirement in their articulation of the relevant “organising principles” (at [19] in O’Neill). They also agreed with the explanation of those principles given by Gummow and Hayne JJ at [65]–[72] in the same case. One way of reconciling the views of Gleeson CJ and Crennan J with those of Gummow and Hayne JJ on this point is to treat “irreparable harm” as one of the matters which would ordinarily need to be addressed in the court’s consideration of the balance of convenience and justice rather than as a distinct and antecedent consideration. This has been the approach taken by some judges: for example Ashley J in AB Hassle v Pharmacia (Aust) Pty Ltd (1995) 33 IPR 63 at 76–77; Gordon J in Marley New Zealand Ltd v Icon Plastics Pty Ltd [2007] FCA 851 at [3]; Kenny J in Medrad Inc v Alpine Pty Ltd (2009) 82 IPR 101; [2009] FCA 949 at [38]; and Yates J in Instyle Contract Textiles Pty Ltd v Good Environmental Choice Services Pty Ltd (No 2) [2010] FCA 38 at [55]–[64].
The assessment of harm to the plaintiff, if there is no injunction, and the assessment of prejudice or harm to the defendant, if an injunction is granted, is at the heart of the basket of discretionary considerations which must be addressed and weighed as part of the court’s consideration of the balance of convenience and justice. The question of whether damages will be an adequate remedy for the alleged infringement of the plaintiff’s rights will always need to be considered when the court has an application for interlocutory injunctive relief before it. It may or may not be determinative in any given case. That question involves an assessment by the court as to whether the plaintiff would, in all material respects, be in as good a position if he were confined to his damages remedy, as he would be in if an injunction were granted: see the discussion of this aspect in I C F Spry, The Principles of Equitable Remedies, 8th ed, Lawbook Co, New South Wales, 2010, pp 383–9; pp 397–9; and pp 457–62.
The interaction between the court’s assessment of the likely harm to the plaintiff, if no injunction is granted, and its assessment of the adequacy of damages as a remedy, will always be an important factor in the court’s determination of where the balance of convenience and justice lies. To elevate these matters into a separate and antecedent inquiry as part of a requirement in every case that the plaintiff establish “irreparable injury” is, in our judgment, to adopt too rigid an approach. These matters are best left to be considered as part of the court’s assessment of the balance of convenience and justice even though they will inevitably fall to be considered in most cases and will almost always be important considerations to be taken into account.
Gleeson CJ also observed in Lenah Game Meats (at [18]), that, where there is little or no room for argument about the legal basis of the applicant’s claimed private right, the court will be more easily persuaded at an interlocutory stage that a prima facie case has been established. The court will then move on to consider discretionary considerations, including the balance of convenience and justice. But, as his Honour also observed at [18]:
The extent to which it is necessary, or appropriate, to examine the legal merits of a plaintiff’s claim for final relief, in determining whether to grant an interlocutory injunction, will depend upon the circumstances of the case. There is no inflexible rule.
The resolution of the question of where the balance of convenience and justice lies requires the court to exercise a discretion.
In exercising that discretion, the court is required to assess and compare the prejudice and hardship likely to be suffered by the defendant, third persons and the public generally if an injunction is granted, with that which is likely to be suffered by the plaintiff if no injunction is granted. In determining this question, the court must make an assessment of the likelihood that the final relief (if granted) will adequately compensate the plaintiff for the continuing breaches which will have occurred between the date of the interlocutory hearing and the date when final relief might be expected to be granted.
As Sundberg J observed in Sigma Pharmaceuticals (Aust) Pty Ltd v Wyeth (2009) 81 IPR 339; [2009] FCA 595 at [15] (Sigma Pharmaceuticals), when considering whether to grant an interlocutory injunction, the issue of whether the plaintiff has made out a prima facie case and whether the balance of convenience and justice favours the grant of an injunction are related inquiries. The question of whether there is a serious question or a prima facie case should not be considered in isolation from the balance of convenience. The apparent strength of the parties’ substantive cases will often be an important consideration to be weighed in the balance: Tidy Tea Ltd v Unilever Australia Ltd (1995) 32 IPR 405 (Tidy Tea) at [416] per Burchett J; Aktiebolaget Hassle v Biochemie Australia Pty Ltd (2003) 57 IPR 1; [2003] FCA 96 at [31] per Sackville J; Hexal Australia Pty Ltd v Roche Therapeutics Inc (2005) 6 IPR 325; [2005] FCA 1218 at [18] per Stone J; and Castlemaine Tooheys at CLR 54; ALR 558 per Mason ACJ.
84 The Full Court at [67] in Samsung recognised that the issue of whether an applicant has made out a prima facie case and whether the balance of convenience favours the grant of an interim injunction are related enquiries. Pharmacor submits that is of particular relevance in this case where Pharmacor, whilst accepting that the Pharmacor products fall prima facie within the scope of the claims, contends that it has a sufficiently strong case as to the expiry of the Patent and the invalidity of the asserted claims so as to weigh materially in Pharmacor’s favour on the question of balance of convenience.
85 Pharmacor asserts that its case on the wrongful extension of the Patent is so strong that it qualifies the conclusion that Biogen has established a prima facie case on infringement. As Jessup J in Interpharma Pty Ltd v Commissioner of Patents [2008] FCA 1498 (Interpharma) explained at [17]:
That is to say, as a matter of analysis, unless the case for invalidity is sufficiently strong (at the provisional level) to qualify the conclusion that, overall, the applicant has a serious question, or a probability of success, the court should move to consider the adequacy of damages, the balance of convenience and other discretionary matters. It is the applicant’s title to interlocutory relief which is under consideration, and the bottom-line question, as it were, is whether the applicant has a serious question, or a probability of success, not whether the respondent does in relation to some point of defence raised or foreshadowed.
3. ARGUABLE CASE
3.1 Introduction
86 As discussed above, the only claim which Pharmacor denies that the Pharmacor Products fall within is claim 17. Pharmacor contends that the infringement case is qualified by the wrongful extension of term case and the novelty attack.
3.2 Within the scope of the claims
87 Pharmacor admits that the Pharmacor products fall within the scope of all the asserted claims except for claim 17. Claim 17 claims a preparation according to any one of claims 13 to 16 comprising an amount of the active ingredient corresponding to 10mg to 300mg of fumaric acid.
88 Dr Williams’ evidence is that 12.5mg to 375mg of dimethyl fumarate correspond to 10mg to 300mg of fumaric acid. The Pharmacor products contain either 120mg or 240 mg of DMF. Professor Charman’s evidence is that 120mg or 240mg of DMF is the molar equivalent of 96.7mg or 193.4mg of fumaric acid respectively, which is within the range claimed in claim 17. On the evidence of both Professor Charman and Dr Williams the Pharmacor products appear to fall within the scope of claim 17.
89 Pharmacor did not appear to dispute in oral submissions that the Pharmacor products fall within claim 17 as no submissions were addressed to that point.
90 Biogen has a very strong prima facie case on infringement assuming that the asserted claims are extant and valid.
3.3 Patent term extension
91 Section 70 of the Act provides that a patentee of a standard patent may apply for an extension of the term of their patent if the requirements set out in subsections (2), (3) and (4) are satisfied. The relevant subsection for the purposes of this interlocutory application is (2)(a) which provides:
one more pharmaceutical substances per se must in substance be disclosed in the complete specification of the patent and in substance fall within the scope of the claim or claims of that specification.
92 The phrase “pharmaceutical substance” is defined in Schedule 1 to the Act as follows:
pharmaceutical substance means a substance (including a mixture or compound of substances) for therapeutic use whose application (or one of whose applications) involves:
a) a chemical interaction, or physico-chemical interaction, with a human physiological system; or
b) action on an infectious agent, or on a toxin or other poison, in a human body;
but does not include a substance that is solely for use in in vitro diagnosis or in vitro testing.
93 The phrase “therapeutic use” is also defined in Schedule 1 to the Act:
therapeutic use means use for the purpose of:
(a) preventing, diagnosing, curing or alleviating a disease, ailment, defect or injury in persons; or
(b) influencing, inhibiting or modifying a physiological process in persons; or
(c) testing the susceptibility of persons to a disease or ailment.
94 The Explanatory Memorandum to the Intellectual Property Laws Amendment Bill 1998 (Cth), which inserted the current s 70 into the Act, contained the following explanation:
The extension of term provisions will be available for patents that include claims to pharmaceutical substances per se (provided that the other criteria are met) [i.e. a product claim]. These claims to pharmaceutical substances per se would usually be restricted to new and inventive substances. Patents that claim pharmaceutical substances when produced by a particular process (product by process claims) will not be eligible unless that process involves the use of recombinant DNA technology. Claims which limit the use of a known substance to a particular environment, for example claims to pharmaceutical substances when used in a new and inventive method of treatment, are not considered to be claims to pharmaceutical substances per se.
(Emphasis added.)
95 In Boehringer Ingelheim International v Commissioner of Patents [2000] FCA 1918 (Boehringer), Heerey J traced the history and antecedents of the extension of term provisions. That case involved claims directed to a container comprising an aerosol or spray composition for nasal administration or the use of such a composition when used in the container. Heerey J concluded that the Act draws a distinction between a pharmaceutical substance that is the subject of a patent claim and a pharmaceutical substance that forms part of a method or process claim.
96 Heerey J was assisted by the Patent Manual. Inter alia, the Patent Manual said at the relevant time:
25.2.2 Pharmaceutical Substance per se
Except for substances produced by a process involving the use of recombinant DNA technology, an extension of term is only available in respect of a pharmaceutical substance per se being within the scope of a claim of the patent.
The explanatory memorandum to the Intellectual Property Laws Bill of 1997 noted that, except for substances produced by a process involving the use of recombinant DNA technology, claims to pharmaceutical substances per se would usually be restricted to new and inventive substances. The memorandum also mentioned a number of specific instances where an extension would not be available:
‘Patents that claim pharmaceutical substances when produced by a particular process (product by process claims) will not be eligible unless that process involves the use of recombinant DNA technology. Claims which limit the use of a known substance to a particular environment, for example claims to pharmaceutical substances when used in a new and inventive method of treatment, are not considered to be claims to pharmaceutical substances per se.’
This distinction is specifically evident as between the reference in the Act to ‘pharmaceutical substances per se’, and to ‘pharmaceutical substances when produced by a process that involves the use of recombinant DNA technology’. The use of the word ‘per se’ requires the claim to the substance to be unqualified by process, temporal, or environmental, components.
Thus, in order that the term of a patent be extended, the patent must contain one or more claims in the form:
a) A substance of formula ****
b) Substance X mixed with substance Y
Examples of claims that are not directed to substances per se are:
a) Substance X when used …
b) Substance X for use …
c) Substance X when produced by method Y
d) An antiseptic comprising substance X [unless the label ‘antiseptic’ was clearly non-limiting on the scope of the claim.]
e) A method of preparing substance X
f) A substance of formula …, where component Y is produced by …
g) [A specified quantum] of substance X
h) ‘Swiss’ – style claims referring to substance X
i) Use of substance X in the treatment of Y
(Emphasis added.)
97 On appeal from Heerey J in Boehringer, the Full Court in Boehringer Ingelheim International GmbH v Commissioner of Patents (No. 2) (2001) 52 IPR 529, considered the validity of a patent extension based on an ARTG registration for ATROVENT NASAL (ipratropium bromide) said to fall within a claim to compositions for the treatment of a runny nose.
98 The patentee before the Full Court conceded that a container was fundamental to each of the claims, in the sense that absent the container as described in the claim, there would be no infringement. On the patentee’s argument, it was enough that the complete specification disclose one or more pharmaceutical substances, whether as the sole element in an invention or in combination with other elements.
99 In a passage which was approved and adopted by the Full Court and set out in full at [17], the trial judge, Heerey J, stated at [14]–[15]:
[14] Broadly speaking, a claim in relation to substance can be made in three ways:
(i) a new and inventive product alone;
(ii) an old or known product prepared by new and inventive process;
(iii) an old or known product used in a new and inventive mode of treatment.
[15] What is clear in s 70 is that only the first type of claim to a pharmaceutical product is to be subject to extension rights…[T]he policy to be deduced in the light of the legislative history is that Parliament has decided that what is intended to be fostered is primary research and development in inventive substances, not the way they are made or the way they are used, with the sole (and important) exception of recombinant DNA techniques, this being an area particularly worthy of assistance for research and development.
(Emphasis added.)
100 The Full Court held at [38] that the patentee’s argument “effectively reads out of s 70(2)(a) the words “per se””, noting that if that were the legislative intention, the paragraph could have read: “one or more pharmaceutical substances must in substance be disclosed”. There would have been no need for “per se”.
101 The Full Court in Prejay Holdings Ltd v Commissioner of Patents (2003) 57 IPR 424 (Prejay) considered the validity of an extension of term based on “Premia”, said to be a pharmaceutical substance falling within the scope of a claim (claim 14), being:
a method of hormonally treating menopausal or post-menopausal disorders in a woman, comprising administering to said woman continuously and uninterruptedly both progestogen and oestrogen in daily dosage units of progestogen equivalent to laevo-norgestrel dosages of from about 0.025 mg to 0.05 mg and of estrogen equivalent to estradiol dosages of about 0.5 mg to 1.0 mg.
102 The patentee in Prejay sought to distinguish their invention from that in Boehringer on the basis that claim 14 did not have a physical component.
103 The Full Court upheld the trial judge’s decision. In three passages of his reasons at first instance which were quoted by the Full Court, Heerey J said:
[14] In both Boehringer and the present case the claims included integers other than the pharmaceutical substance itself. The fact that in Boehringer the other integer was a particular physical device and in the present case the other integer is a particular method of use is to my mind a distinction without a difference.
….
[16] The fact remains that claim 14 is a method claim and, mere use of the substance otherwise than in accordance with the specified method would not infringe. The reasoning in Boehringer was applicable and correctly applied by the delegate.
….
[26] Where there is a patent for a method, such as “Substance X when used…”, it would usually follow that there is more than one reasonable use of X. If that were not so, one would expect the patent to be only for Substance X per se.
104 Justice Allsop (as his Honour then was) agreed with the decision of the majority, Wilcox and Cooper JJ, and made some additional comments which are apposite to the present case at [39]–[41]:
The argument of the appellant turns on the meaning of the definition of “pharmaceutical substance”. It requires the definition to be sufficiently wide (because of the presence of the phrase per se in s 70(2)(a) of the Act) to incorporate both the substance and a method integer, as long as the additional method integer is concerned with therapeutic use.
I do not see the definition so widely. The definition refers to a substance, which must have a purpose or use — therapeutic use, and whose application involves the other matters identified in the definition. The definition is of a particular kind of substance, but it is of a substance, and only a substance.
So understood, when one turns to s 70(2)(a) of the Act, the substance (that is the combination of the hormones in the amounts) per se must fall within the scope of the claim. If, as is conceded, use of the substance outside the particular administration regime in the claims is outside the scope of the claim, then it cannot be said that the substance per se falls within the scope of the claim.
105 At [42] Allsop J noted that his construction of s 70(2)(a) at [41] accorded with what appeared to him to be “the burden of the secondary materials, exemplified by the following part of the explanatory memorandum”:
The extension of term provisions will be available for patents that include claims to pharmaceutical substances per se provided the other criteria are met. These claims to pharmaceutical substances per se, would usually be restricted to new and inventive substances. Patents that claim pharmaceutical substances when produced by a product by process claims, will not be eligible unless the process involves the use of recombinant DNA technology. Claims which limit the use of a known substance to a particular environment, for example claims to pharmaceutical substances when used in a new and inventive method of treatment, are not considered to be claims to the pharmaceutical substance per se.
(Emphasis added.)
106 The current version of the Patent Manual still lists claims to “substance X for use…” as not being directed to a substance per se. The current version provides the following examples of claims that are not directed to substances per se:
substance X when used .... ;
substance X when produced by method Y;
a method of preparing substance X;
a substance of formula ...., where component Y is produced by .... ;
'Swiss' style claims referring to substance X;
use of substance X in the treatment of Y;
substance X for use .... ;
(a specified quantum) of substance X;
an antiseptic comprising substance X.
(Emphasis added.)
107 Biogen’s extension of term application was based on the Propounded Pharmaceutical Substance which it said fell within the scope of at least claims 13 to 17.
108 Pharmacor illustrates its case that the term was wrongly extended by reference to claim 13 of the Patent. Pharmacor contends that claim 13 is not a claim to a pharmaceutical substance per se for at least three reasons which can be summarised as follows:
(a) The claim is not for a new and inventive substance;
(b) The claim is a purpose limited product claim in that it claims an actual achievement of a therapeutic act being a functional technical feature of the claim. It is therefore, not a product claim for a pharmaceutical substance per se; and
(c) Claim 13 requires that the pharmaceutical formulation be in the form of micro-pellets or micro-tablets. Pharmacor submits that this brings additional features in the claim rendering the claim not to a pharmaceutical substance per se.
109 The Patent makes clear that dialkyl fumarates per se are not new and inventive. The specification makes reference to patents for processes for preparing dialkyl fumarates. Pharmacor draws attention to EP 0 312 697 which describes and claims the use of certain fumaric acid monoalkyl ester salts together with a dialkyl fumarate (such as dimethyl fumarate) for the production of a pharmaceutical preparation for the treatment of psoriatic arthritis, neurodermatitis and regional enteritis Crohns.
110 At the provisional level, Pharmacor’s arguments at (a) and (c) are weak. As I discuss below, the novelty attack is not made out at a provisional level in relation to the micro-pellet or micro-tablet formulations of DMF for the therapy of auto-immune diseases.
111 Pharmacor submits that claim 13 is a claim of the kind “substance X for use in the treatment of disease Y”. In Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd (2013) 304 ALR 1, Crennan and Keifel JJ (as the Chief Justice then was) identify such claims as “purpose” claims, as described by Hoffmann LJ in Merrell Dow Pharmaceuticals In & Ors v HN Norton & Co Ltd (1995) 33 IPR 1.
112 Pharmacor submits that the fact that “for use” in claim 13 limits the use of the claimed pharmaceutical preparation for actual achievement of claimed therapeutic effect is not only apparent from a plain reading of the claim but also when it is read in the context of the specification as a whole including the other claims.
113 Claim 14, for example, claims a pharmaceutical preparation according to claim 13 wherein the autoimmune disease is polyarthritis, multiple sclerosis, juvenile-onset diabetes, Hashimoto’s thyroiditis, Grave’s disease, systemic lupus erythematodes (SLE), Sjogren’s syndrome pernicious anaemia or chronic active (lupoid) hepatitis. If “for use” was non-limiting, Pharmacor submits that claims such as claim 14 to specific diseases or conditions would be redundant because they would not be narrower than the claim from which they are dependent.
114 Pharmacor submits that the limiting effect of the “for use” in the claims has been employed in an attempt to confer novelty and inventiveness over dialkyl fumarates per se. Consistent with this the dependent claims also claim different or specific diseases within the broader class claimed in the independent claims.
115 Pharmacor referred to the discussion of purpose-limited claims by the learned editors of Terrell on the Law of Patents (2021) at 14-109 to 14-110:
14-109
Purpose-limited claims arise in several contexts. First, in respect of claims which are for the use of old products for a new use— sometimes referred to as MOBIL-type claims following the decision of the EPO in MOBIL/Friction reducing additive. (G02/88) EPO Enlarged Board of Appeal decision (G02/88) [1990] E.P.O.R. 73. Secondly, in respect of Swiss-type claims where the claim is for the use of a product in the manufacture of a medicament for a particular therapeutic use. Thirdly, in respect of claims in EPC 2000 format (the successor to Swiss-type claims) where the claim is to a product for a particular therapeutic use (such as “substance X for use in the treatment of Y”).
14-110
Each of these claim types is alike in that their novelty resides not in the product per se but in the new use or purpose for that product. The recognition that an old product can have a new and inventive application is the technical contribution of these claims and leads to the conclusion that it would exceed that technical contribution if the word for in such claims was read in the sense of merely suitable for. After all, the old substance was always “suitable” for the new use, it is just that no-one had recognised the benefits of the new use. For this reason, in determining infringement, regard has to be had to the purpose or intent with which the infringer is putting the old product onto the market.
(Emphasis added)
116 Pharmacor submits that the claims should be construed in the same manner as EPC 2000 claims in the UK and EU. Pharmacor referred to the Full Court in Mylan Health Pty Ltd & Anor v Sun Pharma ANZ Pty Ltd & Anor [2020] FCAFC 116 (Mylan) at [111] which noted that the EU Board of Appeal holds that actual achievement of the therapeutic effect is a functional technical feature of the claim, as opposed to a mere statement of purpose or intention.
117 Biogen contends that the words “for use” when followed by “in transplantation medicine or for the therapy of autoimmune diseases or psoriasis, psoriatic arthritis, neurodermatitis or enteritis regionalis Crohn” do not change the character of the claim. It remains a claim to a pharmaceutical product per se, which is a product claim; not a method of treatment claim. The concluding words identify that the substance is useful for the indicated purposes, but do not serve to limit the product itself.
118 Biogen submits that claims to products “for use” in particular context are conventionally construed as product per se claims. Biogen places reliance on the construction of “for use” in the context of mechanical product claims to submit that claims to products “for use” in particular contexts are conventionally construed as product per se claims: Garford Pty Ltd v Dywidag Systems International Pty Ltd & Anor (2015) 110 IPR 30 at [122]–[124].
119 Biogen asserts that claim 13 is not limited by purpose and should be construed as a claim to the product, or pharmaceutical substance per se. Biogen points to the first paragraph of the Patent and two paragraphs on bottom half of page 10 as providing a basis for an unlimited claim to DMF formulated as micro-tablets or micro-pellets.
120 According to Biogen, the patent makes it abundantly clear when it is confining the statement monopoly to the treatment of various disorders, and the asserted claims are not in this category. A comparison of claims 13 and 40 is said to demonstrate the point. The uses described in claim 13 are for “transplantation medicine or for the therapy of autoimmune diseases or psoriasis, psoriatic arthritis, neurodermatitis or enteritis regionalis Crohn”. Claim 40 is to a preparation according to, amongst others, claim 13 “when used” for the very same uses identified a claim 13.
121 Biogen further submits the claims 14, 16 and 17 merely refine the substance of claim 13 by the identification of suitable uses (claim 14), particular dialkyl fumarates (including DMF) (claim 16), and the quantity of active ingredient (claim 17), respectively. Each too, on Biogen’s analysis, is a claim to a pharmaceutical substance per se, unlimited by therapeutic use.
122 Biogen submitted that Pharmacor’s references to UK and EU construction of Swiss claims and EPC 2000 claims were inappropriate, as the separate jurisprudence that has evolved in the UK and EU in relation to the reason for the existence, and the construction of Swiss claims, and the later EPC 2000 claims, is not part of Australian law.
123 Biogen asserted that the use of DMF micro-tablets for another therapy for which they might also be useful, such as to treat infertility or high blood pressure, would fall within claims 13 and 14, as the micro-tablets would also be suitable for the uses given in claims 13 and 14.
124 I have extracted relevant sections from the Patent above. A review of the specification as a whole does not support Biogen’s contention that claim 13 (and those other claims dependent upon it) is not limited to the therapeutic purposes referred to in the claim.
125 As can be seen from the extracts above, the emphasis of the disclosure in the Patent is on the use of dialkyl fumarates for pharmaceutical preparations for use in transplantation medicine or therapy of auto-immune diseases. Other than the two paragraphs on page 10 referred to by Biogen, each discussion of the invention, the objectives and aspects are tied to use in transplantation medicine or the therapy of autoimmune diseases.
126 Dialkyl fumarates were known at the priority date of the Patent. At page 8, the Patent states that the dialkyl fumarates to be used according to the invention are prepared by processes known in the art. EP 0 312 697 is given as an example.
127 The Patent has 211 claims. Putting aside the two omnibus claims, each of the remaining 209 claims makes express reference to use in transplantation medicine or for therapy of autoimmune diseases. There is not one claim to a pharmaceutical preparation of dialkyl fumarate micro-tablets or micro-pellets alone.
128 The Full Court in Commissioner of Patents v Abbvie Biotechnology Ltd (2017) 125 IPR 398 considered whether claims in the form of Swiss type claims were claims to a pharmaceutical substance for the purposes of s 70(2)(b). Besanko, Yates and Beach JJ held at [60] and [61] that the pharmaceutical substance, adalimumab, even though produced via recombinant DNA technology, did not in substance fall within the scope of the claim in suit. Method of treatment claims or Swiss type claims cannot support an extension of patent term under s 70.
129 Swiss claims in an Australian patent were the subject of consideration by the Full Court in Mylan. The claim in suit was claim 5, which was dependent on claim 1 which claimed:
Use of fenofibrate or a derivative thereof for the manufacture of a medicament for the prevention and/or treatment of retinopathy, in particular diabetic retinopathy.
130 At [194] the Full Court briefly explained the derivation of Swiss claims, being the need to accommodate and satisfy the particular requirements for patentability under the European Patent Convention. Those requirements are not and never have been part of the Australian legal landscape. Nevertheless, as the Court noted, patentees have sought Swiss type claims in their Australian patents.
131 At [195] the Court said it was important to bear in mind that Swiss type claims are method or process claims and to be distinguished from method of treatment claims.
132 The Court at [196] stated that Swiss type claims are purpose limited claims in the sense that the medicament resulting from the method or process is characterised by the therapeutic purpose for which it is manufactured as specified in the claim.
133 The specification of a therapeutic purpose imposes an important limitation on the scope of the Swiss type claim. At [197] the Court noted that in theory it is this limitation which supports the novelty, and hence the patentability of, the invention. The Court continued:
It is appropriate, therefore, to consider this purpose as one that confines the use of the method or process to the achievement of one end and one end only – a medicament for the specified therapeutic purpose; not a medicament for any other therapeutic purpose. Put another way, a Swiss type claim does not claim the invention in terms of a medicament that is useful for, or can be used for, the specified therapeutic purpose and other therapeutic purposes. In order to support its patent ability and preserve its validity, the invention as claimed through a Swiss type claim, is necessarily more limited in scope.
(Emphasis added.)
134 At [198] the Court stated that the characterisation of the medicament by specification of the therapeutic purpose is, therefore, an essential feature of the invention as claimed.
135 The characterisation of the medicament of the Swiss type claim in suit in Mylan by specification of the therapeutic purpose “for the prevention and/or treatment of …” is not dissimilar to the characterisation of the pharmaceutical preparation in claim 13, as “for the therapy of …”.
136 In the context of infringement of Swiss type claims, the Full Court in Mylan at [225] observed that the “mere suitability” of a medicament for a claimed purpose cannot be determinative of the question of infringement. The fact that the patent has been granted on the basis of a second or later therapeutic use necessarily means that there are potentially multiple uses to which the medicament can be put. Use of the medicament for another suitable use (other than the use specified in the claim) would not infringe.
137 Consistent with the disclosure of the invention in the Patent and the reasoning of the Full Court in Mylan, the specified therapeutic purpose in claim 13 is, at least on a provisional view, an essential integer of the claim.
138 On that basis, use of the dialkyl fumarate micro-tablets for a therapy outside the particular therapeutic use in the claims is outside the scope of the claim, and it cannot be said that the substance per se falls within the scope of the claim: see Prejay at [42].
139 My provisional view is that there is a sufficiently strong prospect that the extension of the Patent may have been wrongly granted. That alone is likely to be sufficient to conclude that no injunction should be granted. However, the question of whether or not Biogen has demonstrated a sufficient likelihood of success to justify the preservation of the status quo requires consideration of the basket of discretionary factors which I discuss below: Australian Broadcasting Corporation v O’Neill (2006) 227 CLR 57 at [65].
3.4 Novelty
140 Pharmacor contends that the asserted claims lack novelty in light of Australian Patent Application No. 21593/99 (593 Application)
141 The 593 Application was filed before, but published after, the priority date of the Patent. Pharmacor relies on s 7(1)(c) of the Act, which refers to paragraph (b)(ii) of the definition of “prior art base” in Schedule 1 of the Act (often referred to as whole contents publications).
142 Section 18(1)(b)(i) of the Act requires that the invention be novel when compared with the prior art base as it stood before the priority date. This is to be assessed in accordance with s 7(1) of the Act, which requires a comparison with information made publicly available in documents or through the doing of acts.
143 The principles in respect of novelty are well-established. The basic test remains the “the reverse infringement test” as stated by Aickin J in Myers Taylor Pty Ltd v Vicarr Industries Ltd (1977) 137 CLR 228 at 235. That is, the claim may be anticipated by a document that discloses something within a claim: it does not need to disclose claim across its breadth.
144 A claim will lack novelty if a direction, recommendation or suggestion in a prior publication discloses either expressly or implicitly to a skilled addressee what is claimed or the inevitable result of following a direction, recommendation or suggestion in the prior publication is to arrive at the claimed invention: Bristol Myers Squibb v FH Faulding & Co Ltd (2000) 97 FCR 524 at [67].
145 It is a question of the disclosure to the skilled reader. A disclosure may be explicit or in certain circumstances implicit. This may occur where the prior art information is a publication which does not specify integer but the skilled reader would understand that integer to be present. If the prior art does not expressly specify each and every essential integer of the claimed invention, the evidence must clearly establish that to the skilled reader each and every essential integer is included: Meat & Livestock Australia v Cargill, Inc (2018) 129 IPR 278 per Beach J at [518].
146 The 593 Application is entitled “Utilisation of alkyl hydrogen fumarates for treating psoriasis, psoriatic arthritis, neurodermatitis and regional enteritis”.
147 Dr Williams understands the 593 Application to describe pharmaceutical compositions containing alkyl hydrogen fumarate alone or in combination with dialkyl fumarate in the form of micro-tablets or micro-pellets for treating psoriasis, psoriatic arthritis, neurodermatitis and enteritis regionalis Crohn.
148 The first paragraph on page 3 states:
The compositions in the form of micro-tablets or micro-pellets permit the administration of the free acid instead of its salt without the occurrence of the known side-effects, especially the formation of ulcers. This is probably due to the fact that microtablets or micro pellets permit a uniform distribution in the stomach, thus avoiding irritating local concentrations of the monoalkyl hydrogen fumarate in the form of the free acid.
149 At paragraphs 2 to 4 on page 3, the 593 Application describes a suitable amount of active ingredient for the compositions. In particular, the following amounts and examples:
(a) 20mg to 300mg of the free acid of the article hydrogen fumarate is particularly suitable for oral administration, the total weight of the active ingredients being 100mg to 300mg;
(b) for the start of therapy or the cessation of therapy: 100mg to 120mg of active ingredients, for example, 30mg to 35mg of dimethyl fumarate (a dialkyl fumarate) and 70mg to 90mg of methyl hydrogen fumarate (a monoalkyl fumarate) is advantageous; and
(c) therapeutic dosage after the initial phase: 190mg to 210mg of dimethyl fumarate and 90mg of monoethyl fumarate.
150 Professor Charman notes that this passage is not the only discussion of dosages in the 593 Application. Potential dosage ranges are also discussed in claims 4 and 5, and claim 5 in particular makes it clear that the part portion (by weight) of dialkyl fumarate, which is an optional inclusion, is relative to the part proportion (by weight) of the alkyl hydrogen fumarate. In Professor Charman’s opinion this is significant, as it emphasises that the 593 Application is focused on pharmaceutical compositions that include an alkyl hydrogen fumarate.
151 The Patent was the subject of re-examination proceedings based on the 593 Application in the patent office. The Commissioner of Patents found the Patent claims 13, 14, 16 and 17 to be novel over the 593 Application. The delegate concluded that the preparation of claim 13 consists of one or more dialkyl fumarates and optionally carriers or excipients, and does not contain other components, apart from non-therapeutically effective amounts of impurities or breakdown products of the dialkyl fumarates, which have formed during manufacture or shelf-life of the preparation.
152 Pharmacor relied upon claim 3 of the 593 Application which claims a mixture of methyl hydrogen fumarate and dimethyl fumarate:
The use of methyl hydrogen fumarate in admixture with dimethyl fumarate and optionally customary pharmaceutical excipients and carriers for preparing a pharmaceutical composition in the form of micro-tablets or micro-pellets for treating psoriasis, psoriatic arthritis, neurodermatitis and enteritis regionalis Crohn;
Wherein the composition contains 30.0mg to 35.0mg of dimethyl fumarate and 70 to 90 mg of methyl hydrogen fumarate.
153 The novelty argument for the purposes of the interlocutory injunction application comes down to a question of construction: whether the asserted claims include within their scope active ingredients other than dialkyl fumarates.
154 Pharmacor refers to two references in the Patent specification to support a construction of claim 13 that includes active ingredients in addition to dialkyl fumarates. The first at page 8:
The dialkyl fumarates used according to the invention may be used alone or as a mixture of several compounds, optionally in combination with the customary carriers and excipients.
And the second, the reference to the use of dialkyl fumarates in the context of triple drug therapy at the bottom of page 9 of the Patent (set out above).
155 Biogen contends that claim 3 of the 593 Application does not anticipate the asserted claims of the Patent as those claims do not contemplate a mixture of active ingredients. Biogen submits that the language of claim 13 (and claim 95) use the language “consisting of”, not for example, “comprising” or “including”, and that as a matter of construction the claims do not permit active ingredients other than dialkyl fumarates.
156 Biogen submits that the entire focus of the Patent, including the examples, is on formulations consisting only of dialkyl fumarates as the active ingredient, and when the use of additional therapeutic agents is contemplated, it is expressly mentioned. When read in the context of the specification as a whole, claim 13 does not permit the inclusion of active ingredients other than dialkyl fumarates.
157 Biogen also contrasted the form of the asserted claims with later claims which expressly contemplate a mixture of active ingredients. For example, claim 105 refers to “the pharmaceutical preparation according to any of claims 93 to 104, in combination with a second agent for use in the therapy of multiple sclerosis”.
158 Although acknowledging that construction is a matter for the Court, Biogen also relied on the evidence of Professor Charman’s understanding of claim 13. Professor Charman disagreed that claims 13 and 95 included active ingredients other than dialkyl fumarates (in the case of claim 13) and DMF (in the case of claim 95). He gave the following reasons to support his understanding:
(a) the language used in the claim (“consisting”);
(b) both claims 13 and 95 expressly specify “optional” inclusions and substitutions. Neither of them specify an active ingredient other than a dialkyl fumarate of the relevant formula (and optionally substituted in accordance with the claims) may be included;
(c) the entire focus of the Patent is preparations that comprise one or more dialkyl fumarates as their active ingredient;
(d) the reference to a combination therapy at page 9 of the Patent is to a combination therapy in which therapy with dialkyl fumarates is combined with one or more components of triple drug therapy. It does not contemplate the possibility of a single preparation containing dialkyl fumarates and one or more components of the triple drug therapy; and
(e) other claims of the Patent expressly provide for combination therapies with a second agent. In particular claims 105, 119, 134, 148, 163, 199 and 211 specify a pharmaceutical preparation of those claims which consists of DMF as an active ingredient, in combination with a second agent.
159 At the provisional level, I agree with Biogen’s submission that claim 13 does not include active ingredients other than dialkyl fumarates for the reasons given by Professor Charman. Pharmacor’s submission that claim 13 should be construed to also include other active ingredients should be rejected. Pharmacor impermissibly seeks to “expand the boundaries” of claim 13 by adding glosses drawn from other parts of the specification: Décor Corporation Pty Ltd & Anor v Dart Industries (1988) 13 IPR 385 at 400.
160 For the purpose of the interlocutory hearing, the 593 Application does not anticipate the asserted claims.
3.5 ACL case
161 Biogen Australia is not an exclusive licensee of the Patent. Its claim for relief is based on the allegations of contravention of s 18 of the ACL. Biogen alleges that by marketing and supplying the Pharmacor products in Australia before the expiry of the Patent, Pharmacor is representing to pharmacists that they are entitled to dispense the Pharmacor products for the same indication, or same purposes, as Tecfidera without infringing the Patent. Biogen referred to the proposed packaging of the Pharmacor products in a confidential annexure to Mr Mallela’s affidavit to support its case as to the representations to be made by Pharmacor in marketing and supplying the Pharmacor products.
162 Biogen submits that the strength of the ACL claim is equal to the strength of the prima facie case for relief for infringement of the Patent.
163 Pharmacor submits that the present case is distinguishable from H Lundbeck A/S v Sandoz Pty Ltd (2018) 137 IPR 408 (Lundbeck), and the cases reviewed by Jagot J at [538]-[544] as each of those cases involved misleading and deceptive conduct on the basis of a “failure to warn” customers of the risk of infringement. It submits that the case against Pharmacor is pleaded not as a failure to warn, but rather that Pharmacor will make positive representations that the pharmacist is entitled to dispense the Pharmacor products for the same indication or purposes as Tecfidera without infringing the Patent.
164 The representation alleged by Biogen in this case is of the same kind set out in Lundbeck in the second dot point at [536]: that Sandoz impliedly represented to customers and potential customers that the customers or potential customers could use the Respondent’s Escitalopram Products without infringing AU 144.
165 The failure to warn customers of infringement as discussed in the cases reviewed by Justice Jagot in Lundbeck is implied from the conduct of the alleged infringer in promoting the goods for sale without regard to the patent monopoly. The positive offer of the products constitutes the “failure to warn”. The alleged infringer’s failure to warn its customer of the liability they are inducing them to incur gives rise to an implied representation that the customer can freely deal with the goods. The Full Court said in Ramset Fasteners (Aust) Pty Ltd v Advanced Building Systems Pty Ltd (1999) 44 IPR 481 at [67]:
Whether or not Ramset’s conduct should be analysed as conveying an implicit misrepresentation, it acted misleadingly when it promoted and sold face-lift tilt-up equipment in the way that it did, without informing its customers of the liability it was inducing them to incur. This was conduct calculated to cause a mistaken impression about a significant consequence of the transaction proposed to those customers.
166 As recognised by Bennett and Yates JJ in Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd & Ors (No 2) (2012) 96 IPR 185 at [91], the misrepresentation issue in claims based on patent infringement depends on the existence of patent infringement. As such, for the provisional purposes of the application, it is not necessary to consider the ACL claim separately to the threatened patent infringement case. The ACL case stands or falls with the patent infringement case.
3.6 Conclusions in relation to strength of prima facie case
167 Whilst Biogen has established a clear prima facie case that the Pharmacor products fall within each of the asserted claims, this does not lead to the conclusion that it has established a prima facie case of infringement of those claims. Just as it is axiomatic that an invalid patent cannot be infringed, it is also axiomatic that the claims of an expired patent cannot be infringed. For the reasons set out above, I consider on the current state of the submissions, that Pharmacor’s case that the extension of term of the Patent was wrongly granted is sufficiently strong, at a provisional level, to qualify the conclusion that Biogen has a prima facie case on infringement. As the Full Court in Sanofi-Aventis Deutschland GmbH v Alphapharm Pty Ltd (2019) 139 IPR 409 (Sanofi) stated at [14]:
The approach in Interpharma and Janssen is clear. A case for invalidity which is merely arguable, of itself does not undermine the existence of a prima facie case of infringement which has otherwise been found to exist. However, a sufficiently strong case of invalidity may well qualify the conclusion that there is a prima facie case of infringement. … What is relevant is the strength of the case that the claim or claims said to be infringed are invalid.
168 In my view, Biogen has not established a prima facie case of infringement of extant claims. The question of whether Biogen has demonstrated a sufficient likelihood of success to justify the preservation of the status quo, requires a consideration of the balance of convenience discretionary factors, which I discuss below.
169 As was said by the Full Court in Sanofi at [8] the strength of the prima facie case is relevant to the balance of convenience, but the weighing process involved in evaluating where the balance of convenience lies does not affect the assessment of the existence or strength of the prima facie case. The Full Court then quoted the Full Court in Samsung at [59]:
The critical integer in the test … is the need for the Court to assess the strength of the probability of ultimate success on the part of the plaintiff. The strength of that probability of will depend upon the nature of the rights asserted and the practical consequences likely to flow from the grant of the injunction which is sought.
170 As I have stated above, I am of the view that Pharmacor has presented a sufficiently strong case that the patent term was wrongly extended to qualify Biogen’s prima facie case on infringement. However, as noted above, this conclusion alone is likely to be sufficient to determine that Biogen should not be granted the relief it seeks. For completeness, I now turn to consider the various discretionary factors that are relevant to the balance of convenience to see whether the status quo should be maintained in Biogen’s favour.
4. BALANCE OF CONVENIENCE
4.1 Overview of the Pharmaceutical Benefit Scheme
171 Dr Bromley provided an overview of the ARTG and PBS, which is extracted below:
1. Before a prescription-only medicine such as Tecfidera can be supplied in Australia, it must be included on the ARTG. For a prescription medicine this process is known as registration of the good.
2. The sponsor is responsible for applying to the TGA to have the therapeutic good included on the ARTG. The sponsor is a person or company that exports, imports, manufactures or arranges for the export, import or manufacture of a therapeutic good, such as a prescription medicine.
3. To obtain an ARTG entry (for a new prescription medicine) or to vary an ARTG entry (for a change to a prescription medicine), a sponsor must submit an application to the TGA (category 1 application for a new medicine, category 3 application/self-assessable request/safety-related request for changes to a prescription medicine).
4. To obtain registration for a new medicine or a change to a prescription medicine, a sponsor must submit a category 1 application to the TGA.
5. The process of applying for registration is similar for both originator and generic prescription medicines but the data required to be submitted to support the efficacy and safety of originator and generic drugs differs considerably.
......
26. Generally, it is approximately 12 to 18 months from the date the application for registration is submitted until the approval is granted by the TGA. Although the Transition document does not distinguish timing targets for originator and generic medicines, the ARGPM document outlines that the TGA’s target for evaluation times of new chemical entities allowed for 50 extra working days, as compared to new generics. This is set out at pages 11 and 12 of the Transition document, and page 31 and 32 of the ARGPM document.
Pharmaceutical Benefits Scheme
27. After obtaining registration on the ARTG, a sponsor can apply for listing on the PBS. The PBS is administered by the Australian Government Department of Health and Ageing under the National Health Act 1953 (Cth) (NH Act) and the National Health (Pharmaceutical Benefits) Regulations 1960 (Cth), and is the mechanism by which the Australian Government provides access to subsidised medicines for patients in Australia. Pharmaceutical manufacturers apply to have their medicine listed on the PBS so that the pharmacies dispensing the medicine are reimbursed, and the cost of the medicine to patients is subsidised by the government for an agreed price.
28. The PBS Schedule is a list of all the medicines that are subsidised by the Australian Government, and is published monthly by the Department of Health. It lists all of the pharmaceutical products included in the PBS and their corresponding “dispensed price for maximum quantity” (DPMQ), being the price at which a PBS-listed medicine may be dispensed at the retail pharmacy level. The maximum quantity is also specified for each product. The DPMQ incorporates the price ex-manufacturer, in addition to all fees, mark-ups and patient contributions. The price ex-manufacturer, commonly referred to as the Approved Ex-Manufacturer Price (AEMP), is the maximum price at which a pharmaceutical manufacturer can supply the product to a wholesaler. When a patient purchases a PBS-listed medicine they are required to pay the specified patient contribution, referred to as the Patient Co-Payment. The Patient Co-Payment price is fixed by legislation, and differs depending on a person’s status as a patient. Patient copayment amounts are adjusted on 1 January each year in line with the Consumer Price Index (CPI). Figures for the last four years are as follows:
Effective Date | General patients | Concession patients |
1 January 2017 | $38.80 | $6.30 |
1 January 2018 | $39.50 | $6.40 |
1 January 2019 | $40.30 | $6.50 |
1 January 2020 | $41.00 | $6.60 |
1 January 2017 | $41.30 | $6.60 |
29. The process for listing a medicine on the PBS is governed by the NH Act and the National Health (Pharmaceuticals and Vaccines – Cost Recovery) Regulations 2009 (Cth).
30. To apply for listing on the PBS the sponsor must lodge an application with the Pharmaceutical Benefits Advisory Committee (PBAC). The PBAC is a statutory expert advisory committee with the primary role of recommending to the Minister of Health which medicines should be subsidised by the Australian Government under the PBS. The PBAC meets three times a year, usually March, July and November. Special meetings of the PBAC can be called outside these periods.
31. There are six different types of submissions for the listing of medicines on the PBS, namely:
(a) Category 1 Submissions;
(b) Category 2 Submissions;
(c) Category 3 Submissions;
(d) Category 4 Submissions;
(e) committee secretariat submissions; and
(f) new brand of existing pharmaceutical item submissions.
32. An application to the PBAC for the listing of a new medicine is done by way of Category 1 or 2 Submissions. Category 1 or 2 Submissions generally relate to requests for the listing of a new medicine or vaccine, a new indication for a currently listed medicine, or to make material changes to a currently listed indication. For all these submissions, an economic model is required. This model is provided to support a claim of cost-effectiveness, cost-utility or cost-minimisation.
33. Category 1 and 2 Submissions to the PBAC consists of five sections:
(a) Section 1 establishes the context for the submissions, including a description of the medicine and its proposed use.
(b) Section 2 presents the best available clinical evidence to support the effectiveness and safety of the proposed medicine for its proposed use.
(c) Section 3 presents an economic evaluation of the consequences of substituting the proposed medicine for the main comparator in the context of the listing requested.
(d) Section 4 provides information regarding the use of the medicine in practice, including the predicted extent of use, and financial analysis for the PBS and the Australian Government health budget.
(e) Section 5 is an optional section which allows the sponsor to present any other additional information relevant to the assessment.
34. All Submissions must be submitted 17 weeks before each scheduled PBAC meeting.
35. Generally, the PBAC considers the application, particularly the cost effectiveness of the product (that is, the cost of treatment compared with the benefit gained by the patient), and makes a recommendation to the Minister of Health as to whether the product should be subsidised.
36. If a positive recommendation has been made by the PBAC to list a medicine, there are several steps that the sponsor must undertake before the medicine will be listed on the PBS by the Minister. These include:
(a) Pricing negotiations: It is necessary for the sponsor and the Minister to agree on the price that the Government will pay for the medicine. Negotiations around the pricing of the medicine are conducted by the Pricing Section of the PBS. After a price has been negotiated, the sponsor is required to submit a Request for AEMP (PB11a) form in order to formalise the agreed pricing arrangement.
(b) Restrictions: any restriction on the circumstances of prescribing that should apply to the new listing must be finalised, including if necessary, through negotiations with the Minister.
(c) Assurance of supply: As a prerequisite to having a medicine listed on the PBS, the Department of Health requires the sponsor to provide a written undertaking that sufficient stock of the product to meet demand will be available to allow for delivery to PBS dispensers (e.g. pharmacies, hospitals or other suppliers) as at the date that the medicine is listed on the PBS.
37. Generally, the PBAC does not consider submissions for new generic brands of existing pharmaceutical products. Instead the submission is considered by the PBS Listings Unit. A sponsor of a generic medicine is required to submit the following documentation to the PBS Listings Unit:
(a) a submission cover letter on company letterhead signed by an authorised representative. The cover letter must include a signed supply assurance undertaking that the sponsor will ensure that there is sufficient stock of the product to meet demand and the stock will be available to allow for delivery to PBS dispensers (e.g. pharmacies, hospitals or other dispensers) in time for the PBS listing day. The letter must also include a copy of, or link to the TGA-approved Product Information;
(b) a TGA bioequivalence statement which is included within the Approval Letter;
(c) a completed and signed Responsible Person form, which includes the details of the authorised representative; and
(d) a ‘Request for approved ex-manufacturer price’ form (Form pb11a).
First listing of a generic medicine on the PBS ‘a’-flagging
38. At the time of determining to list a brand of medicine on the PBS, the Minister for Health may also determine that the brand is to be ‘a’-flagged. A medicine that receives ‘a’-flagging is treated as therapeutically equivalent to one or more brands of pharmaceutical items already listed on the PBS Schedule. A generic product that is ‘a’-flagged against an originator medicine is able to be substituted for the originator brand, at the pharmacy level. In practical terms, this means that, unless a medical practitioner indicates on a script that only the originator brand can be prescribed, and no substitution is allowed (which is done by checking a box on the script that states “Brand substitution not allowed”), an ‘a’-flagged generic can be dispensed by a pharmacist in place of the originator medicine.
39. It is not necessary for a manufacturer to apply to have their product ‘a’-flagged in order for the Minister to make the determination. The Minister may decide to 'a'-flag a generic even if not requested by the manufacturer.
Mandatory Price Reductions
40. The medicines on the PBS are divided into two ‘formularies’ being:
(a) F1 Formulary: This category comprises medicines for which there is only a single brand available, and which do not have any other medicines in the same therapeutic group. That is, generally drugs in the F1 formulary are those for which there is no generic version on the market.
(b) F2 Formulary: This category comprises medicines for which there are multiple brands available that are interchangeable at the pharmacy level, or which are in a therapeutic group with other drugs with multiple brands.
41. On first listing of a generic medicine, the originator medicine (all forms strengths and indications) will automatically move from Formulary F1 to F2 and, as a result, is subject to a mandatory 25% reduction to the AEMP. This is often referred to as “the mandatory price drop”. The new price of the generic medicine after the 25% price reduction becomes the ‘Benchmark Price’ for that product. This Benchmark price also applies to the generic medicine.
42. Once a price drop occurs, the originator manufacturer can elect to supply its product at the Benchmark Price or apply a ‘brand price premium’. A brand price premium is an additional price charged by the originator manufacturer which must be paid by the consumer. Regardless of any brand price premium, the Commonwealth Government will only subsidise the cost of the medicine up to the Benchmark Price.
Price Disclosure
43. The PBS first implemented price disclosure arrangements in 2007 and since then the arrangements have undergone a number of reforms. The purpose of the price disclosure regime is to ensure that the price the Government pays for PBS-listed medicines aligns more closely with the price at which they are actually sold into the market.
44. Under the price disclosure arrangements, suppliers of certain PBS-listed brands of medicines, namely those drugs on the F2 formulary that have not been exempt from price disclosures, are required to provide pricing information to the Government every six months, relating to a single ongoing rolling cycle of 12 months. The data is then used by the Department of Health to determine the price at which the medicines are in fact being supplied in the market. This is known as the weighted average disclosed price (WADP).
45. The WADP is calculated on the basis of one six month batch of data, and any price reduction arising from that calculation occurs every six months on 1 April and 1 October. In 2015, changes were made to the price disclosure regime including the removal of originator brands, or biological medicines, as part of the calculation of the WADP for medicines listed on the F2 formulary for 3 years or more where this results in a lower adjusted price. Therefore, once an originator brand has been listed on the F2 formulary for a period of time, it is removed from the calculation of the WADP for the medicine. This means only the prices offered by the generic manufactures, which are often significantly lower than the originator price, are taken into consideration in the determination of the market value of a product.
46. Once the Department has determined the WADP at which the medicines are actually sold, the WADP of the medicine is compared with the AEMP for the medicine. If the difference between the WADP and the AEMP is 10% or greater, the AEMP for all brands of the medicine will be reduced by the same percentage when the price review takes effect. For example, if the WADP is 30% less than the AEMP, then the AEMP will reduce by 30%. Subject to a few exceptions, recent changes to the NH Act means that this 10% or greater rule is generally now only applied to 7 price disclosures cycles, after which it changes to greater than 30%. If, however, 2 consecutive price disclosure cycles where at least 30% or greater, then the threshold will revert to 10%.
47. This process continues such that, where the price at which the medicines are sold by manufacturers continues to be reduced, medicines on F2 formulation may be subject to a price reduction twice a year.
4.2 Effect of listing the Pharmacor products on the PBS
172 Pharmacor admits that if the Pharmacor products are listed on the PBS they will be ‘a-flagged’ to Tecfidera. This means that the Pharmacor products will be considered therapeutically equivalent to, and substitutable for, Tecfidera.
173 Currently, the Dispensed Price for Maximum Quantity for Tecfidera is not more than $649.96 for 120mg and $1291.80 for 240mg. In both cases, the cost to non-concessional patients is the legislated co-payment amount of $41.30, and the balance is paid by the Australian Government under the PBS.
174 The listing of the Pharmacor products as the first generic DMF to be listed on the PBS will cause Tecfidera to automatically move from Formulary F1 to F2 and to be subject to a mandatory 25% reduction to the AEMP.
175 Whilst Mr Mallela notes that the Department of Health has the discretion to reverse the 25% statutory price reduction if a generic is removed from the market, there is yet to be an occasion where the Department of Health has exercised its discretion to reverse the statutory price reduction to put the price back up to the original price.
176 Following the initial mandatory price drop, Tecfidera would be subject to mandatory price disclosure requirements. Any discounting by Pharmacor beyond the mandatory price drop would affect the weight average disclosed price and result in further mandatory price reductions to the AEMP for Tecfidera.
177 In Dr Bromley’s experience it is usual for the generic companies operating in Australia to fix the price for their products at a price that is lower than the reduced AEMP. The pharmacists are the primary beneficiary of this discounting. The patient continues to pay the legislated co-payment amount and the government pays the pharmacist the DPMQ, with the result that the pharmacist will derive a greater profit from selling the cheaper generic than the originator, if the originator company does not match the discount.
178 Pharmacor notes it is currently the only generic in a position to launch immediately, so the heavy discounting beyond the 25% price drop contemplated by Dr Bromley is unlikely to eventuate, unless and until another generic launches a DMF product. However, the evidence was that Pharmacor does intend to discount further than the 25% mandated drop.
4.3 Summary of the balance of convenience arguments
179 Biogen submits that the launch of the Pharmacor products would:
(a) cause an immediate minimum 25% mandatory statutory reduction of the price of Tecfidera under the PBS;
(b) cause ongoing downward pressure on the price of Tecfidera due to generic discounting and the mandatory price disclosure regime;
(c) result in a significant loss of Tecfidera’s market share;
(d) affect the price of Tecfidera in Taiwan and South Korea, which are tied via international reference pricing to the price of Tecfidera in Australia;
(e) [redacted];
(f) negatively impact Biogen Australia’s operations, including;
(i) compromising its funding of education activities for health professionals;
(ii) compromising the standard of its community education and patient and carer support;
(iii) reducing the amount available for funding clinical trials and local research; and
(g) cause irreparable and unquantifiable harm to Biogen and others.
180 Pharmacor contends that Biogen has not established a prima facie case of infringement in light of the strength of its challenge to the validity of the asserted claims of the Patent and the extension of term of the Patent.
181 Secondly, Pharmacor submits that the harm that it will suffer in the event that an injunction is granted but subsequently discharged after trial will be significant and difficult to quantify. As one aspect of the harm that it will suffer, Pharmacor submits that Biogen will, [redacted]. Pharmacor contends that, if an injunction is granted, Biogen by its own actions will destabilise the status quo and substantially reduce the effect of Pharmacor’s entry on the market by the time the proceedings are determined. Further, Pharmacor contends that it will lose its “first mover” advantage if an interlocutory injunction is granted.
182 Thirdly, Pharmacor submits that Biogen Australia is not the exclusive licensee of the Patent and therefore it has no entitlement to relief for infringement of the Patent. Pharmacor accepts that Biogen Switzerland has established a separate head of loss, but it notes that Biogen Australia’s claim for damages is based solely on the allegations in relation to s 18 of the ACL. Pharmacor submits that there is no evidence in support of Biogen’s ACL claim, and no evidence of Biogen Australia’s potential loss if the Pharmacor products are allowed to launch.
183 Even though Biogen Australia does not have a right to bring infringement proceedings in respect of the Patent, the Court is required to assess and compare the prejudice and hardship likely to be suffered by third parties, which includes Biogen Australia, in the exercise of its discretion to grant or refuse an injunction: see Samsung at [66].
4.4 Consideration of the balance of convenience
184 As the Full Court noted in Samsung at [62], the assessment of the harm to the applicant should no injunction be granted, and the harm to the respondent if an injunction is imposed, is at the heart of the basket of discretionary considerations which must be addressed and weighed as part of the Court’s consideration of the balance of convenience and justice.
185 The question of whether damages will be an adequate remedy for the alleged infringement of the applicant’s rights will always need to be considered when the Court has an application for interlocutory injunctive relief before it. The interaction between the Court’s assessment of the likely harm to the applicant, if no injunction is granted, and its assessment of the adequacy of damages as a remedy, will always be an important factor in the Court’s determination of where the balance of convenience and justice lies: Samsung at [62]-[63].
186 The Full Court in Samsung warned against elevating the assessment of the adequacy of damages as a remedy to a separate and antecedent inquiry, saying “these matters are best left to be considered as part of the Court’s assessment of the balance of convenience and justice, even though they will inevitably fall to be considered in most cases and will almost always be important considerations to be taken into account.
187 The resolution of the question of where the balance of convenience and justice lies requires the Court to exercise a discretion. I start by considering the position of Biogen in the event that an interlocutory injunction is not granted (the Damages Scenario).
4.5 Reduced Tecfidera revenue
188 The evidence indicates that Pharmacor lodged a PBS application on 20 August 2021 and if not restrained, the projected date for Pharmacor Products to be listed on the PBS is now 1 February 2022.
189 Pharmacor admits that the Pharmacor Products will be a-flagged as substitutable with Tecfidera. As a consequence, a pharmacist can supply the Pharmacor Products to a patient with a prescription for Tecfidera unless the “no substitution” box has been checked. Dr Bromley referred to a 2010 article published in the Medical Journal of Australia, which reported that the “no brand substitution” box was ticked in only 1% of computer generated prescriptions, and 0.7% of handwritten prescriptions.
190 The launch of the Pharmacor Products on the PBS will impact the revenue to Biogen Australia from Tecfidera which Biogen submits will have a number of significant flow on consequences for Biogen’s operations.
191 The hit to Biogen Australia’s revenue from the launch of the Pharmacor Products is said to be fourfold:
(a) mandatory 25% price drop;
(b) further discounting by Pharmacor on top of the 25% price drop;
(c) reduced market share; and
(d) reduction of the total DMF market in Australia.
4.5.1 25% reduction in annual revenue
192 As noted above, the first revenue effect from the listing of the Pharmacor Products on the PBS, and their a-flagging with Tecfidera is the immediate mandatory 25% price drop to the AEMP of Tecfidera. Thus, the annual revenue from Tecfidera in Australia will be reduced by 25%.
193 Whilst the Commonwealth has a discretion to reverse the price drop if a generic product is removed from the market, Dr Bromely and Mr Davey have never known a price drop to be reversed. Biogen submits that given the Commonwealth’s interest in reducing the costs of administering the PBS, a reversal is not only unprecedented but inherently unlikely. I agree that it is unlikely that that the Secretary would reinstate the price of Tecfidera if the Pharmacor products were later removed from the market if Pharmacor was ultimately restrained from selling the Pharmacor Products.
4.5.2 Further discounting by generic entrant
194 Mr Mallela’s evidence is that Pharmacor will sell the Pharmacor Products at a modest discount to the 25% reduced AEMP.
195 [Redacted].
196 Tecfidera will be subject to the mandatory price disclosure reporting regime. Any discounting by Pharmacor will result in further reductions to the AEMP for Tecfidera and the Pharmacor products over time.
197 The position of Accelagen and whether it might seek to challenge its current restraint if Pharmacor were to enter the market is unknown.
4.5.3 Loss of Tecfidera market share
198 Dr Bromley gives confidential evidence as to the amount of market share she expects that Biogen Australia would lose in the first year, and within five years of the launch of the Pharmacor products. Mr Mallela’s confidential estimate of Pharmacor’s first year market share is to a lesser amount, but it would still constitute a significant reduction in Biogen Australia’s Tecfidera market. If nothing else, this evidence shows that there is uncertainty in estimating the potential DMF market share that will be captured by the Pharmacor products.
199 In addition to the reduced revenue due to the mandatory 25% price drop, and generic discounting, Biogen Australia’s revenue from Tecfidera will be further reduced by the diminished sales from its smaller share of the DMF market.
200 Dr Bromley expects that if Pharmacor was ultimately restrained Biogen Australia would be unlikely to claw back its original DMF market share. Mr Davey agrees with Dr Bromley’s expectation. He considers that the total DMF market volume would irreversibly change as a consequence of generic launch and Biogen Australia withdrawing at least some support for its current education and support programs. This is discussed further below.
4.5.4 Diminution of total DMF market volume
201 Dr Bromley considers that total sales of DMF (the DMF market volume) will start declining from the date of launch of the Pharmacor products.
202 Biogen Australia’s ability to continue to promote and support Tecfidera through various marketing, support programs, educational activities and research grants will be reduced as a result of the decline in Tecfidera revenue following the launch of the Pharmacor products. Biogen submits that with the reduction of marketing and educational activities focussed on Tecfidera it will no longer be “front of mind” to prescribing clinicians.
203 Dr Bromley also notes that the launch of the Pharmacor products will be at a time when she considers that the MS treatment market in Australia will be “particularly dynamic”, including with the launch of new oral MS treatments such as Mayzent and Zeposia, and the injectable Keysimpta, each of which may eat into the DMF market.
4.5.5 Impact on overseas Tecfidera pricing
204 Biogen submits that the pricing of Tecfidera in Australia also has an effect on the price that is set for Tecfidera in other countries. Dr Bromley’s evidence was that the price set for Tecfidera by the relevant authorities in Taiwan and South Korea takes into account the publicly listed price for Tecfidera in Australia, as well as the price in certain other reference jurisdictions. Dr Bromley did not expect the international prices to be adjusted upwards if the Pharmacor products were removed from the market in Australia.
205 I accept that Tecfidera’s Australian price may feed into the pricing of Tecfidera in other countries. However, there is no evidence as to what other countries are taking into account in the setting of the overseas price, and the weighting of the Australian price relative to the price setting formulae of the other countries. Whilst there may be a decrease in the price of Tecfidera in overseas markets, in particular Taiwan and South Korea, without evidence it is not possible to estimate the likely magnitude of such a decrease.
4.5.6 Vumerity pricing
206 Biogen has a new product, Vumerity, [redacted]. The launch of Vumerity is discussed further below.
4.5.7 MS Alliance program
207 Biogen Australia provides MS patients who have been prescribed a Biogen MS medication with access to a patient support program, known as the MS Alliance. The program is run by experienced MS nurses employed by Biogen Australia and has been recognised with an award for Sustained Excellence at the PRIME awards, an awards programme which recognises excellence in healthcare.
208 Through the MS Alliance program, patients are provided with information and support regarding their treatment, well-being and support services. The support may be provided via an online meeting platform such as Skype, phone, face to face or a password protected website. The MS Alliance program is available to all patients prescribed all Biogen MS products, not just Tecfidera. There is greater use of the MS Alliance program in rural areas where patients are more likely to take oral medications rather than use infusions or injectables.
209 The evidence suggests that Biogen Australia has made considerable investment in the MS Alliance program over the years.
210 Dr Bromley notes that Biogen Australia is a member of the peak body for pharmaceutical companies in Australia, Medicines Australia, which administers the Medicines Australia Code of Conduct. Biogen Australia considers that pursuant to the Code, it cannot continue to provide the MS Alliance support to patients who transfer from Tecfidera to the Pharmacor products. As the switch would take place at the pharmacy level, it may be difficult for Biogen Australia to know which patients are no longer taking Tecfidera.
211 As a result of the reduced revenue from Tecfidera, Biogen Australia may scale back the MS Alliance program, or some aspects of it. This may affect a broader range of patients than just those on Tecfidera. For example, those patients requiring personal instruction on how to self-inject may suffer if the number of available nurses on the MS Alliance program is reduced.
4.5.8 Other impacts on Biogen’s operations from reduced Tecfidera revenue
212 Biogen submits that a reduction in the revenue generated by Tecfidera will have a number of confidential flow on effects to the Biogen Australia operations in addition to those outlined above. Dr Bromley gave confidential evidence as to the nature of these flow on effects.
213 At a general level, Biogen Australia has a sales and marketing team. [Redacted].
214 Biogen Australia organises a number of professional education events for healthcare professionals, and community disease education. It also sponsors a range of third party conferences and scientific meetings.
215 Biogen Australia also supports research into MS conducted by hospitals, healthcare institutions and universities through grants for MS related projects.
216 Dr Bromley gave confidential evidence as to the details, including the amounts of funding provided, of the educational programs, sponsorships and clinical trials and research that would be impacted if Biogen Australia reduced its financial support to these activities due to reduced revenue from Tecfidera. The evidence suggests that Biogen Australia has made considerable investment in the MS education programs, research and clinical trial support over the years.
217 Biogen submits that the harm it will suffer as outlined above, if an injunction is not granted, is irreparable and unquantifiable.
218 I then move to consider the consequences to Pharmacor (and third parties) if an interlocutory injunction is granted to restrain it from launching the Pharmacor Products (the Restraint Scenario).
4.6 Loss of first mover advantage
219 At the time of the hearing, Pharmacor was the only sponsor of generic DMF products in a position to enter the market in Australia on 1 December 2021. Accelagen, the only other generic company with an ARTG listing, is currently enjoined from launching until the expiry of the Patent.
220 Pharmacor contends that if it is restrained from launching the Pharmacor Products on 1 December 2021, it will lose the significant commercial advantage of being the first generic to market: the “first mover advantage”. In the event that Pharmacor is successful at trial and the restraint is lifted, Pharmacor expects that there would be other generic companies also ready to launch a generic DMF product at that time. Pharmacor submits that the loss of the first mover advantage would be irreparable and unquantifiable.
221 Mr Mallela’s evidence is that Pharmacor is able to target the retail pharmacies known to supply Tecfidera. The profit margin to the pharmacist on the 240mg Pharmacor Product is such as to make it an attractive prospect to a pharmacist, even on low monthly sales numbers. It is Mr Mallela’s evidence that the small monthly sales make it less likely that a pharmacist would change to another generic supplier if one became available.
222 Mr Davey rejects the proposition that Pharmacor would obtain a first mover advantage with the Pharmacor products given the large number of pharmacies relative to the small size of the DMF market. The possibility of a first mover advantage in the DMF market is strongly contested as between Mr Mallela and Mr Davey. Both gave further confidential evidence to support their position.
223 On a provisional level it is not possible to exclude the possibility of a first mover advantage. It may be that because of the small size and niche nature of the market, it is not as attractive for the larger generic suppliers as it is to Pharmacor, and for that reason it is possible that Pharmacor may achieve a period where it is the “first mover”.
224 Whilst the loss of a first mover advantage is a matter to be weighed in the balance, it is not a decisive consideration in assessing where the balance of convenience lies. As Yates J said in Otsuka Pharmaceutical Co Ltd v Generic Health Pty Ltd (No 4) (2015) 113 IPR 191 at [178]:
I accept that the evidence discloses a benefit of being the “first mover” in the sense in which the respondent uses that term. It is clear, however, that that advantage is one that derives from what I have found to be a prima facie case of threatened infringement of the 772 patent. Thus it is an advantage that is sought to be derived at the expense of the applicants’ asserted monopoly rights.
In this case, where there is a sufficiently strong case that the extension of the patent term was wrongly granted, so as to qualify the prima facie infringement case, the loss of first mover advantage remains a factor to be considered in assessing where the balance of convenience lies.
4.7 Vumerity
225 As at the date of the hearing of the injunction application, the status quo is that the Pharmacor Products are not on the market.
226 Biogen has applied for TGA approval for Vumerity.
227 Pharmacor submits that there is no status quo to preserve as Biogen’s launch of Vumerity will alter the market as patients will shift from Tecfidera to Vumerity, and thus significantly diminish the market in which the Pharmacor Products can compete.
228 Biogen argued that the relevant status quo is that at the time of the hearing and that the future launch of Vumerity is not a relevant consideration for the balance of convenience.
229 The [redacted] launch of Vumerity [redacted] is a relevant factor to consider in assessing where the balance of convenience and justice lies. In exercising the discretion, the Court is required to assess and compare the prejudice and hardship likely to be suffered by Biogen and Pharmacor in the Damages and Restraint Scenarios. Both exercises are necessarily forward looking, involving estimates as to the likelihood of future occurrences (for example, loss of future market share). Future activities of Biogen that are likely to affect the future DMF market during the Restraint Scenario are relevant to include in the balance of convenience consideration.
230 Dr Bromley’s evidence is that the launch of Vumerity by Biogen Australia is not part of an intentional strategy to switch the Tecfidera market to Vumerity because of generic competition in the DMF market. Vumerity has been in development for several years.
231 Vumerity was launched in the US in around December 2019 and there was confidential material before the Court as to the effect of the launch of Vumerity on the US DMF market.
232 A PBS application may be made concurrently with an application for registration on ARTG. Pharmacor is concerned that Biogen has made such a concurrent application, and if so there may be a PBS listing of Vumerity prior to the hearing and determination of the trial in this matter.
233 A key benefit of Vumerity is that it can be used by those patients who have not tolerated Tecfidera well, or at all, as Vumerity is an improved and better tolerated product for the MS community. Dr Bromley gave evidence that Biogen is launching Vumerity as a separate and distinct oral treatment for MS. Dr Bromley expects that when Vumerity is launched in Australia, the uptake of the product will be from a number of patient groups, including:
(a) newly diagnosed MS patients;
(b) patients currently prescribed Tecfidera (or a DMF generic, if such products are on the market at the time of the launch); and
(c) patients using other non-DMF therapies.
Biogen Australia expects that a significant proportion of patients will continue treatment with Tecfidera. Dr Bromley strongly rejects the proposition that Biogen would use its MS Alliance program to promote products to patients or to switch them to a different treatment.
234 Pharmacor put on evidence to show that in the US, Biogen markets Vumerity as superior to Tecfidera. Studies of the stomach problems experienced by patients taking Tecfidera, compared with those experienced by patients taking Vumerity, show that Vumerity is better tolerated. The Vumerity patients reported fewer days with stomach problems than people taking DMF, and their stomach problems were generally less severe. Patients were less likely to stop taking Vumerity because of stomach problems than DMF.
235 [Redacted]. Consistent with Dr Bromley’s expectation for Australia, there was still a market in the US for DMF after the launch of Vumerity.
236 Dr Bromley does not expect Vumerity to launch in the first quarter of 2022. [Redacted]. The launch of Vumerity prior to the determination of the proceeding would be a significantly complicating factor for the calculation of harm to and quantification of damages to Pharmacor.
237 [Redacted]. On [redacted] Dr Bromley’s evidence as to the likely source of Vumerity patients, at least some patients currently taking Tecfidera will change to Vumerity. In that case, [redacted].
238 If Pharmacor were free to launch now, it would be able to obtain a share of the current DMF market prior to the launch of Vumerity and the reduction of the DMF market volume following the move of patients from DMF products to Vumerity.
239 [Redacted]. However, the size of the likely resultant reduction to the total DMF market following the launch of Vumerity is uncertain.
240 Biogen submitted there was no need for it to undertake not to de-list Tecfidera from the ARTG as it had no intention of doing such an act, and therefore there was no need to for it to be restrained.
4.8 Costs of launch
241 Pharmacor has incurred costs in preparing to launch the Pharmacor Products and submits that if it is enjoined these costs will be wasted.
242 These costs would be easily quantified if it were determined that any injunction was wrongly granted.
243 Biogen also notes that any costs of launch were incurred by Pharmacor with its “eyes wide open” to the potential risk and submits that Pharmacor should have cleared the way before making preparations to launch.
244 The launch costs are not a relevant factor.
4.9 Assessment of loss and damage
245 Both parties submit that they will suffer significant irreparable harm which is incapable of quantification.
246 Mr Davey described the MS market as a complex and dynamic market. There are a large number of treatment options currently available. Most of the treatment options are alternatives in that each can be considered as a potential treatment for a patient with relapsing MS and the choice between them will depend on the individual patient’s circumstances and preferences as well as their clinician’s opinion and preferences. Mr Davey observed that new products, both originator and generic, launch into the market relatively frequently.
247 Mr Davey did not consider the DMF market or the MS treatment market to be mature or predictable markets. He described a mature market as one where there have been no new treatments for a significant period of time, and where there is a steady and stable pattern of change in the market. In a mature market, all patients who are eligible for treatment will have their treatment in place, with only the newly diagnosed patients adding to the market.
248 As a consequence of the immaturity of the DMF and MS markets, Mr Davey considered that the DMF market volume would be very difficult to predict, and that any prediction would be associated with significant uncertainty in a hypothetical analysis.
249 Mr Davey identified the following further complexities involved in assessing loss to Biogen in the Damages Scenario:
Recreation of a hypothetical market from the date of launch of the Pharmacor Products to sometime in the future three to seven years beyond the expiry of the Patent;
Impact of the launch of new, non-substitutable DMF products such as new injectables, infusions or new oral products and the launch of generic versions of other existing alternative treatments;
Clinical practice, including clinician and patient preferences, may affect uptake between oral and other forms of medication;
The extent to which pharmacy purchasing is driven by the cheapest generic product available (to produce maximum profit), their relationship with the generic supplier or other incentives on offer;
Entry of other generic DMF products;
Loss of brand awareness due to reduced marketing expenditure;
Erosion of Biogen’s ability to launch defensive strategies, such as its own generic product prior to expiry of the Patent; and
Entrance of Vumerity to the MS market.
250 Mr Davey considered that the preparation of a hypothetical model of the total DMF market size, both in the period leading up to expiry of the Patent and into the future, would be very complex and difficult. His evidence was that the economic modelling of the counterfactual will be difficult in either case and associated with a significant degree of uncertainty. Mr Davey was unable to say with certainty whether the Restraint or Damages Scenario would require more counterfactual scenarios to be modelled.
251 Mr Stone considered the an assessment of Pharmacor’s loss and damages in the Restraint Scenario would be subject to similar difficulties as the assessment of Biogen’s loss and damage in the Damage Scenario. Each assessment would have its unique complexities. The assessment of loss and damage in both scenarios is complex, time consuming, will require considerable estimation and will therefore be subject to uncertainty.
252 Mr Samuel illustrated the areas of uncertainty involved in assessing the loss to Pharmacor in the Restraint Scenario. This was done by reference to the considerable variations in assumptions given to the experts in the Venlafaxine and Clopidogrel cases in relation to matters such as:
Hypothetical launch dates for and number of other generic DMF products;
Hypothetical generic market share;
Hypothetical net sales price per unit; and
Number of first line pharmacist customers secured by the generic.
253 Mr Samuel noted that each of the matters listed above had a material consequence to the estimate of loss.
254 Mr Samuel also listed the following complexities in assessing Pharmacor’s loss in the Restraint Scenario:
Lost sales volumes and market share are affected by the entry of other generic DMF products, the possible launch of an authorised generic and the pricing of these;
Absence of any actual trading data;
The period of restraint;
Estimating future cash flows; and
Loss of first mover advantage.
255 Mr Samuel agreed that estimating the loss and damage for Pharmacor or Biogen were both complex tasks with their own particular complexities. However, Mr Samuel considered the assessment of Pharmacor’s loss and damage in the Restraint Scenario would be considerably more complex than estimating Biogen’s loss and damages in the Damages Scenario.
256 Biogen objected to Mr Samuel’s qualification to give evidence as to the assessment on the basis that he was a forensic accountant, like Mr Stone, and he had no experience, qualification or specialised knowledge in health economics. Mr Samuel and Mr Stone have been an expert witness in most of the recent damages or the undertaking as to damages cases including Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (2018) 136 IPR 8 and other pharmaceutical related damages such as Novartis Pharmaceuticals Australia Pty Ltd v Bayer Australia Ltd (2015) 111 IPR 1.
257 Whilst I agree that Mr Samuel does not have the qualifications and experience of Mr Davey in health economics, I consider that for the purposes of reaching a provisional view as to the complexity of the assessment task of both Biogen and Pharmacor, his experience enables him to give such evidence, particularly as his evidence is based heavily on his experience in the Venlafaxine and Clopidogrel cases.
258 A number of the complicating factors identified by Biogen and Pharmacor overlap. A prime example being the entry of Vumerity to the MS market.
259 The evidence is the assessment of the loss of either party would be a complex, time consuming exercise, each with their own unique complexities, which would require considerable estimation and be subject to uncertainty. At the provisional level it is not possible to determine that the complexity of estimating the loss of one party is more complex than that of the other.
4.10 Eyes wide open/clearing the way
260 In June 2017, Biogen commenced proceedings in the US against Pharmacor’s parent company Alkem for infringement of three patents, including the US equivalent to the Patent in respect of its threatened launch of a Tecfidera generic product in the US.
261 Pharmacor decided to enter the Australian DMF market after the commencement of the US proceedings and sometime before making its application for PBS listing. The date by which the decision had been made appears on the artwork for packaging designs for the Pharmacor products in a confidential annexure to Mr Mallela’s first affidavit.
262 On 8 July 2021, Pharmacor provided the Secretary to the Department of Health with a certificate pursuant to s 26B(1) of the Therapeutic Goods Act 1989 (Cth) certifying that acting in good faith, it believed on reasonable grounds that it was not marketing, and did not propose to market the Pharmacor Products in a manner or circumstance that would infringe a valid claim of a patent that has been granted in relation to DMF.
263 Biogen submits that at the time Pharmacor provided the certificate pursuant to s 26B(1), Pharmacor must have formed a view as to why it did not infringe the Patent at that time, yet, Pharmacor took no action to “clear the way”.
264 The authorities say that a failure to clear the way is not a significant factor in the balance of convenience, but it is still a factor to be weighed in the balance.
265 Biogen submits that in this case, there was no pressing reason why Pharmacor had to launch prior to clearing the way. Biogen contrasted the subject matter of this case to that in Samsung, a mobile phone which would be obsolete before the next Christmas.
266 It may be a more significant factor in this case where the balance of convenience is evenly balanced. Unlike challenges involving multiple grounds of invalidity, each, such as inventive step, requiring significant expert evidence, Pharmacor’s attack on the extension of term is a discrete issue requiring limited evidence.
267 In response Pharmacor points to the potential patent “thicket” it faces. In correspondence Biogen has raised the prospect of bringing “at least” two other patents into the infringement action. Pharmacor says that there is no requirement to clear the way through a patent thicket before launching.
4.11 Parties’ conduct
268 Both parties sought to make much of the other’s conduct in offering or refusing to agree to an expedited trial in the first quarter of 2022. Both saw merit in the prospect of an expedited trial. Each sought to hold the other’s refusal to agree to an expedited trial as a factor to weigh against them in the balance of convenience.
269 Biogen asserts that Pharmacor proposed, and was prepared to agree to, an expedited trial in February 2022. Biogen contends that Pharmacor was prepared to be restrained until March 2022, and that it has not identified any prejudice that it would suffer if the period of restraint were to be extended from March 2022 to late 2022.
270 Pharmacor contends that it was only prepared to agree to be restrained until the expedited trial if certain undertakings protecting its position were given by Biogen. Biogen was not prepared to provide the undertakings sought. In the absence of the protection of the undertakings, Pharmacor submits that no point can be made as to there being no further prejudice if Pharmacor was to be restrained for a few more months.
271 I was taken to the correspondence between the parties as to the possibility of an expedited hearing.
272 On 25 August 2021, Pharmacor made an offer to Biogen that it would agree to withdraw its application for PBS listing, and to an interlocutory injunction restraining the exploitation of the Pharmacor Products on the following conditions:
(a) Biogen undertake not to assert any other patents against Pharmacor in relation to the exploitation in Australia of the Pharmacor Products;
(b) Biogen provide the usual undertakings as to damages;
(c) Biogen undertake to provide notice of, and take action against, any third party seeking to exploit a DMF product in Australia and not to launch an authorised generic during the period the injunction is in force;
(d) The final hearing be listed in February 2022, and final judgment delivered on or before 24 March 2022; and
(e) Pharmacor be permitted to lodge an application for PBS listing of the Pharmacor Products on or before 1 January 2022, on the basis that the listing would be withdrawn in the event that Biogen obtained final judgment in their favour.
273 On 27 August 2021, Biogen refused Pharmacor’s offer. In particular it noted that it could not agree as to when judgment would be delivered or to allow Pharmacor to make a guarantee of supply in circumstances where Biogen considered the guarantee could not be properly given. In addition, Biogen observed that the Pharmacor Products may infringe two other Biogen patents and it requested a sample of the Pharmacor Products or information as to the percentage by weight of DMF in, and the tensile strength of, the Pharmacor Products.
274 Pharmacor contends that its offer was reasonable, and Biogen acted unreasonably in refusing to accept Pharmacor’s offer of an expedited trial, [redacted].
275 Biogen contends that it was reasonable to refuse the offer as it came with unacceptable conditions and Biogen was seeking information from Pharmacor as to the Pharmacor Products to determine whether to bring two further patents into the infringement action.
276 Pharmacor submits that the undertakings sought by it make clear that it was not prepared to accept the loss of the first mover advantage because Pharmacor would have had advanced notice of the entry of any other new entrants to the market and they would also be restrained (or if not, Pharmacor would have been able to apply to discharge the injunction).
277 In any event, the Court was unable to accommodate the expedited trial contemplated by the parties.
278 I am loathe to attribute any weight to either party on the conduct debate. Parties are to be encouraged to work together to achieve the efficient resolution of disputes and use of Court resources contemplated by s 37M of the Federal Court of Australia Act 1976 (Cth), without the threat that anything they say or do will be used against them in a balance of convenience fight.
279 Neither party adopted a position that was so unreasonable or untenable in the circumstances so as to merit consideration as a relevant factor against them affecting the balance of convenience.
4.12 Third parties — Commonwealth
280 The Commonwealth, as the entity responsible for funding the PBS, is a third party that would be affected by the grant of an interlocutory injunction. As noted by Burley J in Sanofi at [179] the Commonwealth has made claims on the undertaking as to damages given in relation to Venlafaxine, Clopidogrel and Rosuvastatin. The challenges likely to be associated with calculating the Commonwealth’s loss, if claimed, in the event that an injunction is allowed but subsequently found to be wrongly made is a further factor to take into account, but at the low end of the spectrum.
4.13 Security
281 Both parties offered to provide security if required.
4.14 Undertakings
282 Biogen offered to provide an undertaking to Pharmacor to provide notice of, and take action against, any third party seeking to exploit a generic version of Tecfidera in Australia that appears to infringe the Patent, and not to launch an authorised generic during any period in which Pharmacor is restrained from launching the Pharmacor Products.
5. CONCLUSIONS AND DISPOSITION
283 The balance of convenience factors (other than the likely launch of Vumerity) are quite evenly balanced. On the evidence, the calculation of loss in either the Restraint or Damages Scenario is of comparable complexity, such that it is not possible at the provisional level to determine which damages calculation exercise would be more complex.
284 The [redacted] introduction of Vumerity at some point [redacted] is a factor which tends against the granting of the injunction sought. The effect of any launch of a product to be marketed as superior to DMF [redacted] would have a profound effect on the DMF market such that the DMF market would be very different (and likely much smaller) [redacted] to its current state.
285 In my view the balance of convenience does not favour the grant of an injunction to restrain the launch of the Pharmacor Products.
286 To my consideration of the balance of convenience, I also add my conclusion as to the weakness of Biogen’s prima facie case given my provisional view that the extension of term of the Patent was wrongly granted. Taken together, these lead me to conclude that there is insufficient likelihood of success in all the circumstances to justify the grant of the injunction sought. Accordingly the application is dismissed.
I certify that the preceding two hundred and eighty-six (286) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Rofe. |
Associate:
Dated: 23 December 2021
VID 443 of 2021 | |
BIOGEN AUSTRALIA PTY LTD |




