FEDERAL COURT OF AUSTRALIA
Cytec Industries Inc. v Nalco Company [2021] FCA 970
ORDERS
Appellant | ||
AND: | Respondent | |
AND BETWEEN: | Cross-Appellant | |
AND: | Cross-Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The parties confer and supply to the chambers of Justice Burley, within 28 days, short minutes of orders giving effect to these reasons.
2. Insofar as the parties are unable to agree to the terms of the short minutes referred to in order 1, including the appropriate order as to costs, the areas of disagreement should be set out in mark-up.
3. The proceedings be listed for case management at 9.30am on 13 October 2021.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
BURLEY J:
[1] | |
[12] | |
[12] | |
[17] | |
[23] | |
[24] | |
[25] | |
[35] | |
[44] | |
[46] | |
[46] | |
[74] | |
[78] | |
[84] | |
4.5 Some agreed propositions regarding the disclosure of the specification | [86] |
[90] | |
[90] | |
[92] | |
[92] | |
[99] | |
5.3 Do the claims include any limitations as to the synthesis method used to form the reaction product mixture? | [116] |
[119] | |
[119] | |
[122] | |
[129] | |
[140] | |
[150] | |
[150] | |
[152] | |
[158] | |
[164] | |
[202] | |
[202] | |
[204] | |
[226] |
1 These proceedings are an appeal from a decision of a delegate of the Commissioner of Patents by the opponent, and a cross-appeal by the patent applicant, to the grant of patent application number 2012220990 entitled “Reducing aluminosilicate scale in the Bayer process”: Cytec Industries Inc. v Nalco Company [2019] APO 2 (delegate’s decision). The priority date of the patent application is 25 February 2011.
2 Since about 1888, a method developed by Karl Bayer known as the Bayer process has been used to extract alumina from bauxite in order to make aluminium metal. It has been developed over time, but even today it is the dominant method in use around the world. In summary, the process involves crushed and milled bauxite being added to a caustic Bayer liquor at a high temperature. This enables the alumina to be dissolved into the Bayer liquor and unwanted minerals to be left in a residual slurry, known as red mud. The mixture of red mud and Bayer liquor travels through a system of tanks and pipes until the red mud is removed leaving a pregnant liquor. The pregnant liquor is then cooled to allow alumina-containing crystals to precipitate as solids to be removed from it. This process removes about half of the valuable alumina from the Bayer liquor. The spent liquor is then returned to the system to digest more bauxite.
3 One problem with the operation of the Bayer process is that bauxite contains silica which can accumulate and form scale on surfaces within the equipment which eventually has to be removed. The patent application concerns a method of reducing scale.
4 On 7 February 2012, Nalco Company filed its patent application. It was examined and advertised as accepted by the Commissioner of Patents on 21 May 2015 and Cytec Industries Inc. filed a notice of opposition on 21 August 2015 pursuant to s 59 of the Patents Act 1990 (Cth), contending that the patent application should not proceed to grant on the basis that the claims were not novel, that they lacked an inventive step and that the application did not comply with the requirements of s 40(2) and s 40(3) of the Patents Act. On 8 January 2019 a delegate of the Commissioner of Patents found that the description in the specification did not provide a clear enough and complete enough disclosure of the invention and that the claims were not supported. The delegate also found that several of the claims lacked clarity and novelty. Other grounds advanced, including lack of inventive step, were rejected.
5 On 8 January 2019, Cytec filed a notice of appeal pursuant to s 60(4) of the Patents Act from those parts of the decision of the delegate upon which it did not succeed. On 25 February 2019, Nalco filed a cross-appeal which was later amended.
6 On 4 November 2019, Nalco obtained leave of the Court pursuant to s 105(1A) of the Patents Act to amend the claims of the application. In these reasons I refer to the amended form of the complete specification as the patent application.
7 The amendments sought to overcome those aspects of the delegate’s decision that were determined adversely to Nalco. Cytec then amended its statement of grounds and particulars to address the patent application’s amended form. Those grounds are:
(1) That the invention claimed is not supported by the matter disclosed in the specification within s 40(3) of the Patents Act;
(2) That the complete specification does not disclose the invention in a manner which is clear enough and complete enough for it to be performed by a person skilled in the relevant art within s 40(2)(a) of the Patents Act;
(3) That the complete specification does not disclose the best method known to Nalco within the requirements of s 40(2)(aa) of the Patents Act;
(4) That the invention claimed in claims 1, 2, 5, 8, 9 and 12-16 is not novel in light of PCT/US2010/049555 (WO 2011/037873), another patent owned by Nalco, entitled “Reducing aluminosilicate scale in the Bayer process” (WO 873); and
(5) That the invention claimed is not for an “invention” or a “manner of manufacture” within s 18(1)(a) of the Patents Act.
8 In its opening submissions, Cytec abandoned reliance on ground (5).
9 Although styled as an appeal, the present proceeding is in the original jurisdiction of this Court and involves a hearing de novo on the grounds and evidence before the Court. As the opponent to grant, Cytec bears the onus in relation to each ground of opposition raised. Section 60(3A) of the Patents Act, which was introduced with the passage of the Intellectual Property Laws Amendment (Raising the Bar) Act 2012 (Cth) (RTB Act), provides that the Commissioner may refuse an application if satisfied, on the balance of probabilities, that a ground of opposition exists. That is the standard by which the present proceeding is to be judged.
10 The Patents Act that applies to the present dispute is in the form as amended by the introduction of the RTB Act, with the exception that the form of the Patents Act and Patents Regulations 1991 (Cth) applying in relation to the novelty grace period issue arising in ground (4) pre-dates the RTB Act.
11 For the reasons set out in more detail below, I have concluded that the opposition to the grant of all of the claims of the application should succeed on the basis of the lack of support (s 40(3)) and lack of sufficient disclosure (s 40(2)(a)) grounds.
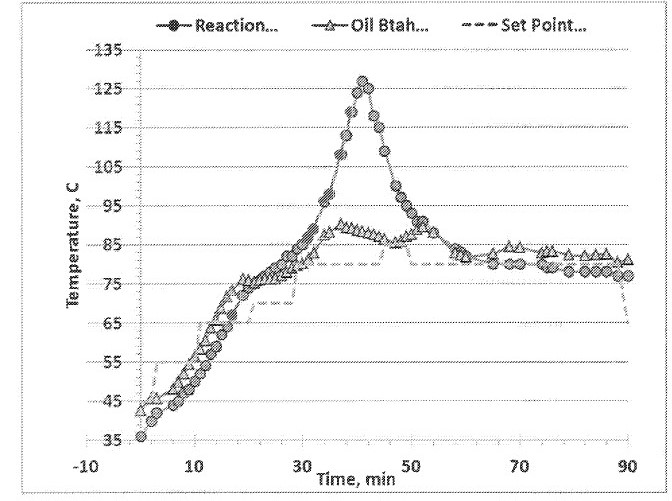
12 Gregory Philip Power has since 2004 been a director and principal consultant of Arriba Consulting Pty Ltd, a company through which he provides consulting services in the bauxite-alumina industry including in relation to Bayer process management, technology, laboratory analysis and research. Since 2004 he has also been an honorary visiting scientist at the Commonwealth Scientific and Industrial Research Organisation where he is a research advisor in mineral chemistry, specialising in Bayer process chemistry and bauxite residue.
13 Dr Power obtained a PhD in chemistry from the University of Western Australia in 1975. He then worked as a senior research scientist for the National Institute of Metallurgy in Randburg, South Africa, before returning to Australia and completing a postdoctoral fellowship at Murdoch University and a research fellowship at the School of Chemistry of the University of Western Australia. In the 1980s, he worked as a laboratory director and then as chief chemist and research manager at Worsley Alumina Pty Ltd. There he commissioned and directed a laboratory, developing a research programme focussed on organics removal and oxalate behaviour, process chemical additives, lime chemistry and analytical methods. In the 1990s and early 2000s, he held roles first at Alcoa of Australia as a chief chemist, chemical and environmental services manager and technology services manager, and then at Alcoa World Alumina Australia as a sustainable technology manager and as technical manager for air quality.
14 Dr Power affirmed one affidavit in these proceedings. He gives evidence about: the Bayer process and how its various parameters can be adjusted depending on the type of bauxite being refined; the problem of scale formation in the Bayer process and approaches to its management as at February 2011; the team of people who would be tasked with finding a useful or better solution to the scaling problem, and their likely approach; the disclosure of WO 873 and how this would inform his approach to managing or controlling scale as at February 2011; and, the disclosure of the patent application and how this compares to that in the WO 873 patent.
15 Christopher John Easton has since 2005 been a Distinguished Professor at the Research School of Chemistry of the Australian National University. Since being awarded a PhD by the University of Adelaide in 1981, he has held teaching and research positions in the chemistry departments of several universities. He has authored or co-authored over 300 publications in the field of organic chemistry and has presented numerous conference papers, the majority of which have been directed to the chemical structure and function of molecules and their synthesis. He has also been involved in a number of projects, including in collaboration with industry partners, involving synthesis, analysis and purification of organic compounds, some of which have been related to the field of industrial chemistry.
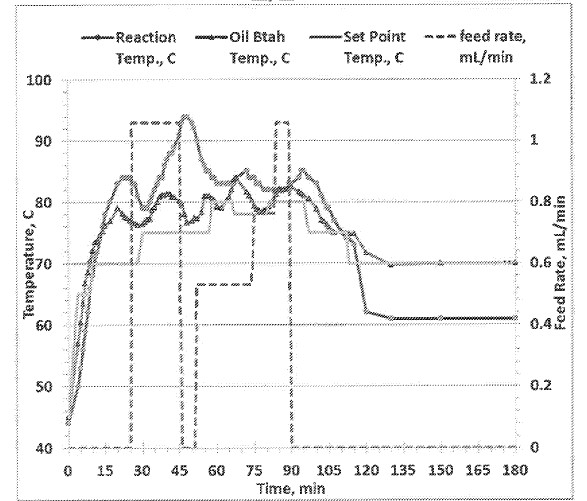
16 Professor Easton affirmed two affidavits in these proceedings. In his first he gives evidence about the Bayer process and the problems caused by aluminosilicate scale formation, and the disclosure of WO 873 and the patent application. Annexed to his first affidavit are copies of declarations made by Professor Easton for the purposes of the opposition proceedings before the delegate. In his second affidavit, he provides further commentary on the patent application.
17 Denis R Audet has since 2015 been a director and principal consultant of Audet Process Audit, a company through which he provides consulting services, principally focussed on Bayer process optimisation, troubleshooting, research and audit, to the alumina industry. He was awarded a Doctorate in Chemical Engineering in 1986 from the Université de Technologie de Compiègne, France.
18 In 1986, Dr Audet commenced working for Alcan International Ltd where he remained, in a range of different roles, until 2015. He was initially involved in developing, and later training others in the use of, simulation software to predict the effect that varying Bayer process parameters would have upon the size and purity of alumina precipitates. He later held roles in the crystallisation and ceramics groups at Alcan where, in addition to working on improving impurity control in the Bayer process and developing alumina products such as toothpaste, paints and fire retardants, he continued to work on making improvements to the simulation software and providing technical assistance to various alumina refineries around the world. He later moved to Australia where, in addition to working on optimising the process by which alumina hydrate is precipitated in the Bayer process, he also developed expertise on the parts of the Bayer process concerned with the way in which the bauxite is introduced into the process. His work has also been concerned with understanding the effect that impurity precipitation, such as silica and oxalate precipitation, has upon alumina hydrate precipitation. Dr Audet is also the co-inventor of two patents relating to the precipitation of alumina in the Bayer process and has published various papers on the topic.
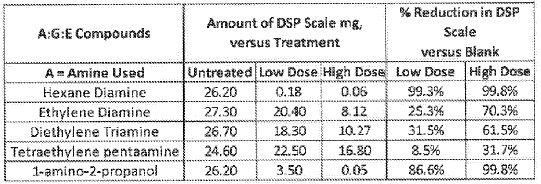
19 Dr Audet affirmed one affidavit in these proceedings. He gives evidence about: the Bayer process and the problems and difficulties associated with it, including scale formation and management; the appropriate team of people to develop a solution to the scaling problem in the Bayer process; the disclosure of the WO 873 patent; the disclosure of the patent application; and whether he could perform the method in the patent application based on the disclosure of the specification. Dr Audet also responded to the affidavit evidence of Dr Power and Professor Easton.
20 John Gerard Bellwood has since 2019 been a part-time consultant to BASF Mining Solutions, principally focussing on supporting projects relating to chemical additive synthesis and application to mining and mineral industries on an industrial scale. He was awarded a Bachelor of Science (Chemistry) in 1982 from the University of Durham, United Kingdom.
21 In 1982, Mr Bellwood commenced working for Allied Colloids Limited where he spent the majority of his time in the laboratory conducting testing on new polymers and chemical reagents for applications related to the oil industry. In this role he worked with chemists who were responsible for developing the compounds he tested and became familiar with their techniques of production and synthesis. He also supported sales personnel by conducting testing to demonstrate how the chemical products might perform in specific customer applications. In 1994 he relocated to Australia to work for Allied Colloids Australia Pty Ltd (later acquired by Ciba then BASF), where he worked on projects including designing and commissioning a polymer production facility, and leading the technical and process support team for a facility manufacturing flocculants for applications such as mineral processing and extraction. He also led a team that developed a new range of flocculants designed to treat waste material in the Bayer process.
22 Mr Bellwood affirmed one affidavit in these proceedings. In it he gives evidence about: the disclosure of the patent application; the disclosure of the WO 873 patent and how that compares to the patent application; and his response to the affidavits of Professor Easton.
2.3 Joint expert report and concurrent evidence
23 Each of the witnesses joined in the preparation of a joint expert report. They also gave oral evidence in a concurrent session, during which they were cross-examined. I found that each expert gave frank and helpful evidence and was careful to refer to matters within their expertise. In this regard, Dr Power has expertise in process chemistry, particularly in the context of the Bayer process. Each of Dr Audet and Mr Bellwood also have experience in the Bayer process. Although Dr Audet has a good understanding of chemistry, he does not have experience working as a synthetic or organic chemist and frankly conceded that his understanding of some aspects under discussion were not as deep as that of Professor Easton, whose strength lies in his extensive experience in synthetic organic chemistry. Professor Easton, however, has only a general familiarity with the Bayer process, and so is less well-placed to give evidence about that process than the Bayer process experts (Dr Power, Dr Audet and Mr Bellwood), whose knowledge in that field was considerably more extensive. I have taken these matters into account in considering the evidence of each of the witnesses.
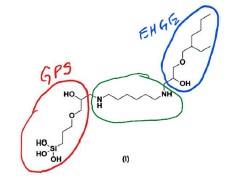
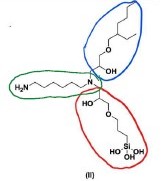
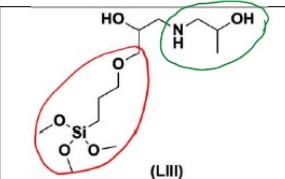
24 The parties co-operated to produce a primer that sets out aspects of the common general knowledge as it stood at the priority date of the patent application. What follows is taken from that document.
25 The Bayer process uses bauxite as the starting material. Bauxite is a rock containing many minerals, including aluminium hydroxides (gibbsite and boehmite) and aluminosilicate clays which are insoluble in water but soluble in strong caustic soda. The other main constituent of bauxite is ferrous (iron) oxide, which gives the bauxite its red appearance. The iron and most other minerals contained in the bauxite are not readily soluble in caustic soda. Silica and/or organic compounds are the main impurities present in bauxite. The amounts and levels of these impurities can depend on where the bauxite was mined. The organic impurities typically come from plant roots or soil that has decayed. The amount of organic impurity depends on how the soil is removed during the mining of bauxite, the geology of the area and climate. For example, bauxite from Western Australia has higher amounts of organic impurities compared to bauxite from the Northern Territory or North Queensland.
26 To dissolve aluminium hydroxide in the form of gibbsite and/or boehmite, crushed and milled bauxite is added to a solution of caustic soda (sodium hydroxide), which has an alkaline pH at high temperature. This basic solution is known as the Bayer liquor, which continuously flows within a closed circuit comprising various machinery that facilitate different stages and conditions for the Bayer process. By taking bauxite, crushing it and heating it to over 100°C (and more typically at least 140-145°C where the bauxite is predominantly gibbsite or at least 250°C where the bauxite is predominantly boehmite) in caustic soda in a pressurised vessel, alumina is extracted into solution (referred to as liquor) and the rest of the minerals are left in a residual sludge/slurry (red mud). The mixture then flows through a series of decanters (operating at about 105°C under atmospheric pressure) in the presence of flocculants to settle and remove the red mud from the liquor, and the liquor is filtered (using, for example, a sand filter) to remove any residual mud particles, leaving a pregnant liquor.
27 The Bayer liquor from the decanters then flows to crystallisers which are large tanks (typically around 4,500m3 in volume and usually about 35m in height) to crystallise and precipitate the alumina hydrate. The crystallisers are placed in series (typically 15-30 tanks) usually along a gradient such that the liquor cascades from one crystalliser to the next by gravity flow. Each crystalliser has an agitator to ensure mixing. The liquor and/or slurry flowing to the crystallisers is cooled to a temperature of about 80°C, which initiates the alumina trihydrate (or alumina hydrate) precipitation process by mixing the liquor with fine alumina hydrate “seeds” which are added to the first crystalliser in the series. Alumina-containing solids are then removed from the liquor to be processed and then eventually smelted into aluminium metal.
28 The spent liquor is returned to digest more bauxite. After the liquor leaves the final crystalliser, the liquor remains rich in alumina, however, it is typically half the original alumina concentration present during digestion. A key element of the Bayer process is this recirculation of the liquor.
29 Below is a diagram showing the process flow described in the above paragraphs.
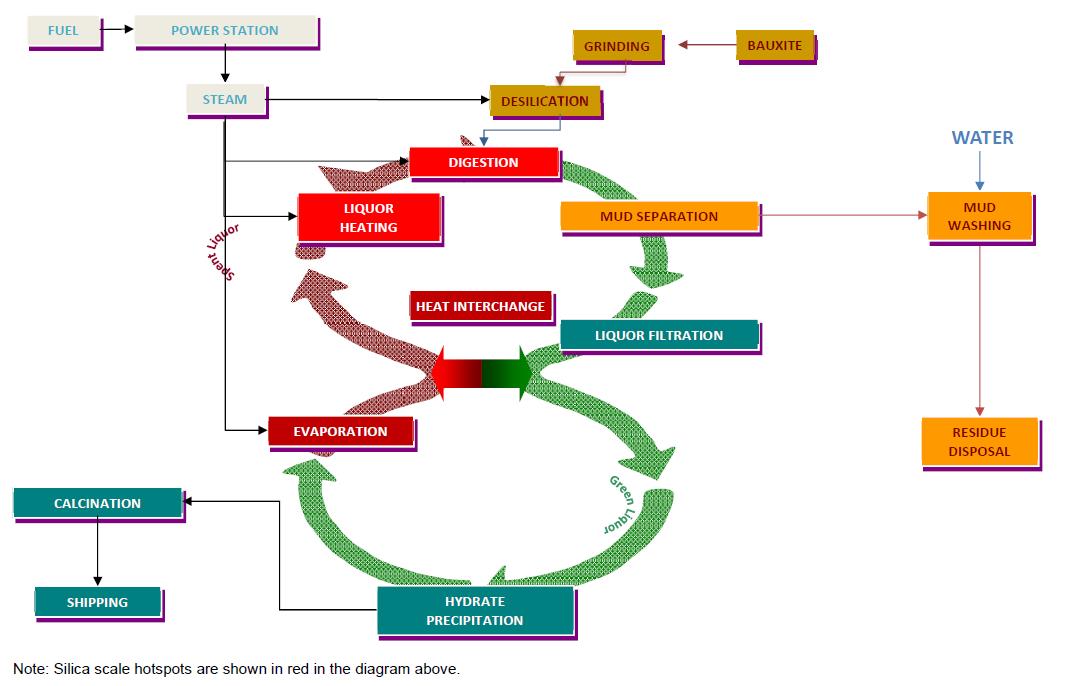
30 The core step of the Bayer process is the removal of the alumina hydrate by precipitation (cooling and seeding). This is done by all refineries and the chemistry of this part of the process is almost always the same. However, the first portion of the Bayer process (up to and including digestion, which is the process of extracting alumina from bauxite by high pressure leaching (digestion) in caustic soda) can occur differently depending on the type of bauxite, as the different types require different pre-processes to extract alumina. For example, lateritic bauxite must be ground and preferably desilicated prior to digestion, as described below. Other bauxites may need to be fired in a kiln, usually with lime, to free up the alumina prior to digestion.
31 After the crushed and milled bauxite has been added to caustic soda, a slurry solution (which is rich in alumina as well as undissolved bauxite) remains at digestion temperature for a time sufficient to dissolve the alumina, which can typically be at least 30 minutes to several hours. The temperature of the slurry is then rapidly reduced in sequential stages, such as by suddenly significantly reducing the pressure and allowing energy to escape. This generates steam which is used to heat up the input Bayer liquor (earlier within the circuit) for digestion via heat exchangers, which are a series of tubes (with heat-conductive surfaces) through which the Bayer liquor runs during the digestion phase. This stage is most prone to silica scale formation on the heat exchangers as silica tends to form in the parts of the Bayer process circuit which are hotter.
32 In Australia, all refineries applying the Bayer process use the process in broadly the same way, subject to variations in bauxite and know-how being used. One key difference is the digestion temperature used as between refineries, which can either be a high temperature (220-270°C where the bauxite contains significant amounts of boehmite) or low temperature (145-175°C where only the gibbsite is intended to be recovered). The reason for the difference is the type of bauxite being processed, or the relative proportions of different forms of alumina-bearing ore. In lateritic bauxite which is found in Australia, there are two key types of aluminium containing ores – gibbsite (aluminium hydroxide – alumina trihydrate) which is soluble in caustic soda at low temperatures (common in Western Australia), and boehmite (aluminium oxy-hydroxide – alumina monohydrate) which requires high temperatures (common in Queensland). Bauxite will generally contain both gibbsite and boehmite and it is their relative proportion that dictates the digestion temperature that is used. In particular, only if there is a significant proportion of boehmite would the higher temperatures for digestion be justified to extract alumina from the boehmite in addition to the gibbsite.
33 After digestion, it is common for refineries to use a range of chemical additives while applying the Bayer process. The same types of chemical additives are used by refineries in the same ways, but the quantities of additives and the specific additives selected could vary between refineries. The selection and variation of additives is a standard and routine part of the process for refinery operators. Chemical additives were and are generally developed and supplied by chemical companies such as Cytec and Nalco.
34 The chemical additives known and commonly used in the Bayer process before February 2011 included settling agents, flocculants, clarifying agents, agglomeration agents, filtration aids and anti-scalants. For example:
(1) A slurry containing fine mud (red mud) is produced after digestion of bauxite, and the mud needs to be separated out. From the 1950s-60s, settlants were developed, where the slurry was placed into a tank to let the mud settle, but fine particles do not settle very well. To improve settling, a settling agent (usually a polymer which will survive in caustic soda and having some charged groups which are oppositely charged to what is holding the mud in suspension) could be added, to attach to the mud particles and cause them to coagulate and settle.
(2) Flocculants neutralise the charge of the mud particles, allowing them to stick together. This is a simpler and more cost effective method of removing mud particles than filtering.
(3) Having flocculated the mud, a clarifying agent was added to eliminate the final remaining particles of fine mud.
(4) Agglomeration agents (often called crystal growth modifiers) were added to help the process of precipitating alumina hydrate.
(5) Filtration aids were used in the process of washing and filtering precipitated alumina hydrate.
(6) Anti-scalants were used to inhibit or reduce the formation of aluminosilicate scale on plant equipment.
3.2 Scale formation and management
35 Scale management is a key issue for the industry because scale of various types impacts on all parts of the process, including operating costs, costs of equipment and health and safety issues. Aluminosilicate scale (DSP), unlike most chemicals, precipitates more readily at higher temperatures. All bauxite contains some clay (aluminosilicate) which dissolves quickly in the Bayer process and results in silica in solution. When this solution is heated for digestion, the silica precipitates as DSP, which grows as a hard glass-like film on the hot equipment. DSP can therefore form obstructions and adversely affect the efficiency of the plant's equipment. In the Bayer process diagram above, the silica scale hotspots are highlighted in red.
36 DSP/scale is a solid growth on the surface of a tank, pump or pipe. The way scale builds up in the Bayer process is similar to scale build up in a kettle. If hard water is boiled in a kettle with an internal heating coil, calcium scale will build up and affect the heat exchange. Consequently, the kettle needs to be descaled with acid (for example, citric acid).
37 The high temperature heat exchangers included within the digesters and evaporators, are key problem areas for scale formation. Scale can grow quickly (with several microns of scale within a few days of operation) and will continue to grow over time. The hard glassy scale is a good heat insulator, meaning the efficiency of the heating tubes starts to reduce as scale builds up, and this reduction will happen proportionately. Scale forms not only in the heat exchangers included within the digesters and evaporators, but they are the main problem area because scale adheres to a hot surface. Scale will also form in other pipes, pumps, and the digestion vessels themselves (especially in places where there may be a join or liquor flow into a vessel where it flashes, resulting in sudden concentration around an entry point). Scaling can happen throughout the processing plant, anywhere there is liquor in contact with a hot surface.
38 After some weeks or months of operation, the scale needs to be cleaned off. This usually involves taking the heat exchanger offline, draining it down and putting it in acid (usually dilute sulfuric acid combined with a corrosion inhibitor). Once the scale is removed, the acid will attack the metal, so the process has to be managed carefully. In general, it is a messy and expensive process. For example, if not enough corrosion inhibitor is in the acid, the tubes will have to be replaced. Taking heat exchangers offline is also a big operational issue, because it means refineries must have spare heat exchangers, which increases capital costs.
39 In addition to acid cleaning, two other ways of managing the problem of aluminosilicate scale build-up known before February 2011 were:
(1) reducing the amount of silica in solution going into digestion through a pre-desilication process; and
(2) additives to the liquor which prevented the scale forming.
40 The patent application concerns additives.
41 The pre-desilication process occurs between milling and digestion, and has been used since approximately the late 1960s or early 1970s for almost all lateritic bauxite processing worldwide. This involves the milled slurry being heated to just under 103°C and being held for approximately 12 hours. The clay in that time will dissolve, releasing silica into solution, which will re-precipitate as aluminosilicate, resulting in less silica in the solution. This process can remove up to approximately 90% of the silica from solution, but not all of it. Therefore, the scale problem remained downstream in the Bayer process and still needed to be addressed. This led to the development of the anti-scalant chemical additives identified in [39(2)], which would be added into the liquor.
42 One chemical solution to the problem of aluminosilicate scale was developed by Cytec. This was an oxysilane-based anti-scalant additive that would keep the silica in solution and prevent scale formation. By February 2011, the product was marketed by Cytec under the brand name Max HT. Max HT is typically added during the evaporation process of the Bayer process. It is intended to remain in the Bayer liquor at the same concentration, albeit with some loss when red mud is removed from the decanters. Max HT has minimal effect on other aspects of the Bayer process, other than reducing or preventing silica scale formation. It stabilises (i.e. keeps dissolved) digested silica from the bauxite by preventing or reducing crystallisation on the heat exchanger. The silica can then be removed by precipitation on red mud in digestion or pre-desilication.
43 In summary, the following matters in respect of scale formation and management were known and regarded as generally well-known and accepted in the field as at February 2011:
(1) build-up of aluminosilicate scale (DSP) in Bayer process equipment was a significant problem;
(2) mechanical cleaning (by drilling out heater tubes or jack-hammering larger vessels) and acid cleaning (by dissolving the scale in sulphuric acid containing a corrosion inhibitor) of the equipment to remove scale meant that equipment had to be taken offline, resulting in added costs and loss of productivity, as well as occupational health and safety issues;
(3) pre-desilication resulted in lower amounts of silica in solution, but did not eliminate the problem entirely;
(4) chemical additives had been developed which were designed to hold silica in solution and prevent the precipitation of DSP, but were not necessarily effective in all Bayer liquors at reasonable (sufficiently low) concentrations; and
(5) those working in the field continued to investigate improved methods of managing and controlling DSP scale formation.
3.3 Developing solutions to the scaling problem
44 In developing a scale inhibitor, while a product would initially be developed on a laboratory scale, it would need to be tested on an industrial scale in a refinery. Particularly when viscosity and miscibility are relevant factors, how the material mixes in the equipment available at a plant will be of great interest, and consideration would be given to how to introduce the product into the liquor stream. Factors to consider would include how the product is pumped (whether it is actively pumped or poured out of a pipe or whether to have a sparging mechanism), how rapidly it is introduced and whether it will settle at the bottom of a mixing tank or float to the top.
45 If the material is very viscous, it will not mix very well with the Bayer solution (which is aqueous, highly alkaline and has a high salt content). Viscous material is also more difficult to pump through pipes and dosing equipment. The degree to which this is a problem depends on the viscosity of the material and how much needs to be added to the liquor. One way to reduce viscosity, as done with flocculants, is to dissolve the material in a carrier to make it more easily dispersed.
4.1 The complete specification
46 The complete specification of the patent application commences by identifying that the field of the invention relates to compositions of matter and methods of using them to treat scale in various industrial process streams, and in particular certain silane based small molecules that have been found to be particularly effective in treating aluminosilicate scale in a Bayer process stream (page 1 lines 4 – 7).
47 The background of the invention notes that the Bayer process is used to manufacture alumina from Bauxite ore. The patent application then says (page 1 lines 15 – 22):
The process uses caustic solution to extract soluble alumina values from the bauxite. After dissolution of the alumina values from the bauxite and removal of insoluble waste material from the process stream the soluble alumina is precipitated as solid alumina trihydrate. The remaining caustic solution, known as “liquor” and/or “spent liquor” is then recycled back to earlier stages in the process and is used to treat fresh bauxite. It thus forms a fluid circuit. For the purposes of this application, this description defines the term “liquor”. The recycling of liquor within the fluid circuit however has its own complexities.
48 The specification then explains that bauxite often contains silica in various forms and amounts, some of which do not dissolve in the Bayer circuit and others, such as clays, which are reactive and dissolve when added to the liquor, thus increasing the silicon concentration in it. The specification provides (page 2 lines 3 – 8):
As liquor flows repeatedly through the circuit of the Bayer process, the concentration of silica in the liquor further increases, eventually to a point where it reacts with aluminium and soda to form insoluble aluminosilicate particles. Aluminosilicate solid is observed in at least two forms, sodalite and cancrinite. These and other forms of aluminosilicate are commonly referred to, and for the purposes of this application define, the terms “desilication product” or “DSP.
49 The specification says that because DSP has an inverse solubility, where precipitation increases at higher temperatures, and because it can precipitate as fine scales of hard insoluble crystalline solids, its accumulation in Bayer process equipment is a problem. As DSP accumulates in pipes, vessels, heat transfer equipment and other process equipment it forms flow bottlenecks and obstructions that can adversely affect liquor throughput. It also reduces the efficiency of heat exchangers. These effects are typically managed through a descaling regime, which involves process equipment being taken off line and the scale being physically or chemically treated, but this leads to regular periods of down-time for critical equipment, and the use of hazardous concentrated acids (page 2 lines 11 – 23). The specification describes that another known approach is deliberately to precipitate DSP as free crystals rather than as scale to reduce the concentration of silica in solution. However, total elimination of silica is impractical, and changing process conditions can lead to changes in the solubility of DSP, resulting in consequent precipitation as scale (page 2 line 24 – page 3 line 7).
50 The specification then refers to the prior art Max HT product at page 3 lines 8 – 20:
Previous attempts at controlling and/or reducing DSP scale in the Bayer process have included adding polymer materials containing three alkyloxy groups bonded to one silicon atom as described in [five patents] and published article Max HTTM Sodalite Scale Inhibitor: Plant Experience and Impact on the Process, by Donald Spitzer et. al., Pages 57-62 Light Metals 2008, (2008) all of whose contents are incorporated by reference in their entirety.
Manufacturing and use of these trialkoxysilane-grafted polymers however can involve unwanted degrees of viscosity, making handling and dispersion of the polymer through the Bayer process liquor problematic. Other previous attempts to address foulant buildup are described in [two patents] both of which are incorporated by reference in their entirety.
51 The reference to adding polymer materials in this passage serves to distinguish the prior art solution to the “small molecule” solution to the scaling problem proposed in the patent application.
52 Under the heading “Summary of the Invention” the first aspect of the invention is said to provide a method for the reduction of aluminosilicate containing scale in a Bayer process comprising the steps of (page 4 line 19 – page 4b line 3):
adding to the Bayer process stream an aluminosilicate scale inhibiting amount of a composition comprising at least one small molecule, the at least one small molecule comprising at least three components, one being an R1 component, one being an R2 component and one being an R3 component, the components within the small molecule arranged according to the general formula:
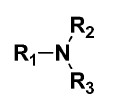
wherein the small molecule may be at least one of: carbonates, bicarbonates, carbamates, ureas, amides and salts thereof and:
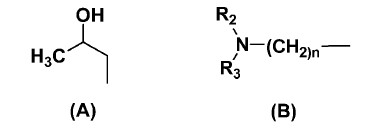
R1 is selected from the group consisting of: H, alkyl, amine, structure (A) and structure (B);
each R2 is G, and each R3 is independently selected from the group consisting of H, alkyl, amine, G and E,
wherein each G is one item independently selected from the group consisting of: 3-glycidoxypropyltrimethoxysilane, 3-glycidoxypropyltrialkoxysilane, 3-glycidoxypropylalkyldialkoxysilane, 3-glycidoxypropyldialkylmonoalkoxysilane, 3-isocyanatopropyltrialkoxysilane, 3-isocyanatopropylalkyldialkoxysilane, 3-isocyanatopropyldialkylmonoalkoxysilane, 3-chloropropyltrialkoxysilane, 3-chloropropylalkyldialkoxysilane, and 3-chloropropyldialkylmonoalkoxysilane and wherein G is optionally hydrolyzed;
each E is independently selected from the group consisting of ethylhexyl glycidyl ether, C3-C22 glycidyl ether, C3-C22 isocyanate, C3-C22 chloride, C3-C22 bromide, C3-C22 iodide, C3-C22 sulfate ester, C3-C22 phenolglycidyl ether, and any combination thereof, and
n is an integer from 2 to 6.
53 This was a consistory clause for the unamended version of claim 1. This aspect relates to silane-based small molecules added to the Bayer process stream.
54 The silane based small molecules of this embodiment are defined by the Markush diagram wherein each of R1, R2 and R3 are “selected from the group consisting of” different chemical compounds. R1 is selected from a very broadly described group which includes amines and structures (A) and (B). Structure (A) relevantly includes 1-amino-2-propanol (AP), and structure B relevantly includes hexane diamine (H) and ethyl diamine (ED). R2 is G, which relevantly includes 3-glycidoxypropyltrimethoxysilane (GPS) and R3 relevantly includes 2-ethylhexyl glycidyl ether (E). These inclusions are relevant to the claims under consideration.
55 The specification describes that a second aspect of the invention involves a method for the reduction of aluminosilicate containing scale in a Bayer process involving mixing with the stream an aluminosilicate scale inhibiting amount of a composition comprising at least one small molecule comprising three components, the first being a multiamine having from 2 to 5 amine groups, the second being selected from silanes similar to G, as defined in the first aspect, and the third being similar to E, as defined in the first aspect. It is not necessary for present purposes to describe the third aspect.
56 Two further embodiments are then identified at page 4d. One is a method for reducing siliceous scale in a Bayer process comprising the step of adding to a Bayer liquor an aluminosilicate scale inhibiting amount of reaction product between an amine-containing molecule and an amine-reactive molecule containing at least one amine-reactive group per molecule and at least one –Si(OR)n group per molecule, where n = 1, 2 or 3, and R = H, C1-C12 Alkyl, Aryl, Na, K, Li or NH4 or a mixture of such reaction products (page 4d lines 12 – 18). The expert evidence indicates that this covers a large range of unidentified reaction products which can reduce siliceous scale using two reagents, namely (1) an amine-containing molecule and (2) an amine reactive molecule containing at least one amine-reactive group per molecule and at least one –Si(OR)n group per molecule.
57 The other embodiment described on page 4d involves adding to a Bayer liquor “an efficacious amount of reaction product” between (1) an amine-containing “small molecule”; (2) an amine-reactive “small molecule” containing at least one amine reactive group per molecule and at least one –Si(OR)n group per molecule, where n = 1, 2 or 3 and R = H, C1-C12 Alkyl, Aryl, Na, K, Li or NH4 or a mixture of such reaction products; and (3) a non-polymeric amine reactive hydrophobic hydrocarbon. The expert evidence indicates that this embodiment is similar to the previous embodiment, however, the molecules produced are defined as “small molecules” and use three reactants.
58 A next embodiment is described to be a method of reducing DSP in a Bayer process comprising the step of adding to the Bayer process stream an aluminosilicate scale inhibiting amount of a mixture of the products previously defined (page 4e lines 2 – 4).
59 The detailed description of the invention commences by defining “polymer” to mean a chemical compound comprising essentially repeating structural units each containing two or more atoms and “small molecule” to mean a chemical compound comprising essentially non-repeating structural units. It states that the terms “small molecule” and “polymer” are mutually exclusive (page 4e lines 15 – 24).
60 After defining other terms, the specification describes the Bayer process in general terms (page 6 lines 10 – 20) before stating, at page 6 lines 21 – 26:
In this invention, it was discovered that dosing of various types of silane-based products can reduce the amount of DSP scale formed.
In at least one embodiment of the invention, an effective concentration of a silane-based small molecule product is added to some point or stage in the liquor circuit of the Bayer process, which minimizes or prevents the accumulation of DSP on vessels or equipment along the liquor circuit.
(Emphasis added)
61 The specification repeats (page 7 line 8 – page 8 line 14) the general embodiment identified as the “first aspect of the invention” (see [52] above). It then says (page 9 lines 1 – 2):
In at least one embodiment said small molecule is selected from the group consisting of: (I), (II), (III), (IV), (V), (VI), (VII), (VIII), and (IX):
then depicting nine drawings of individual structures I-IX, each of which is a compound formed from the reaction of H, GPS and E.
62 The specification continues by setting out nine further sets of embodiments by reference to the said small molecule being selected from groups of numbered small molecules, each of which is drawn. In total 65 small molecules are depicted.
63 The patent application then points out that the invention resides in the use of small molecules as opposed to the higher molecular weight polymers of the prior art. It says at page 33 lines 5 – 17:
The effectiveness of these small molecules was unexpected as the prior art teaches that only high molecular weight polymers are effective. Polymer effectiveness was presumed to depend on their hydrophobic nature and their size. This was confirmed by the fact that cross-linked polymers are even more effective than single chain polymers. As a result it was assumed that small molecules only serve as building blocks for these polymers and are not effective in their own right. (WO 2008/045677 [0030]). Furthermore, the scientific literature states “small molecules containing”...“[an] Si-O3 grouping are not effective in preventing sodalite scaling”...because...“[t]he bulky group”...“is essential [in] keeping the molecule from being incorporated into the growing sodalite.” Max THTM Sodalite Scale Inhibitor: Plant Experience and Impact on the Process, by Donald Spitzer et. al., Page 57, Light Metals 2008, (2008). However it has recently been discovered that in fact, as further explained in the provided examples, small molecules such as those described herein are actually effective at reducing DSP scale.
64 The patent application then sets out three advantages to using small molecule based inhibitors as opposed to the polymeric inhibitors (page 33 lines 20 – 26). First, the smaller molecular weight means that there is a larger number of active, inhibiting moieties available around the DSP seed crystal sites at the DSP formation stage. Secondly, the lower molecular weight allows for an increased rate of diffusion of the inhibitor, which favours fast attachment of the inhibitor molecules onto DSP seed crystals. Thirdly, the lower molecular weight avoids high product viscosity and so makes handling and injection into the Bayer process stream more convenient and effective.
65 The specification then provides two examples. Example 1 is entitled “Example of a Synthesis Reaction A, E and G” and provides (page 34 line 7 – page 35 line 20):
In a typical synthesis reaction the three constituents: A (e.g. hexane diamine), G (e.g. 3-glycidoxypropyltrimethoxysilane) and E (e.g. ethyl hexyl glycidl ether) are added to a suitable reaction vessel at a temperature between 23-40°C and allowed to mix. The reaction vessel is then warmed to 65-70°C during which time the reaction begins and a large exotherm is generated. The reaction becomes self-sustaining and depending on the scale of the reaction, can reach temperatures as high as 125 to 180°C. (see FIG. 1).
66 Figure 1, depicted at the end of the specification, is earlier described as “a graph illustrating a batch reaction profile of the invention” and is as follows:
67 The specification continues (page 34 lines 13 – 24):
Typically the reaction is complete after 1 to 2 hours and then the mixture is allowed to cool down. As an aspect of this invention this un-hydrolyzed product mixture can be isolated as a liquid or gel or a solid in a suitable manner. Alternatively, the reaction product mixture can be hydrolyzed, via a number of methods, to prepare a solution of the hydrolyzed product mixture in water. The hydrolysis of the alkoxysilane groups in the component G results in the formation of the corresponding alcohol (e.g. methanol, ethanol etc,. depending on the akloxysilane used in the synthesis).
It is common to those skilled in the art to conduct the ring opening of an epoxide with a reactive amine in a batch mode (where the components are mixed together), heated to an initiation temperature above room temperature (e.g. 50-65°C) with the reaction temperatures allowed to reach as high as 125 to 180°C. This can cause internal cross-linking and side reactions to occur - which is often desired in the resin manufacturing processes.
68 The reference to resin manufacturing processes is to polymers which, as noted above at [59], the specification contrasts with the small molecules of the invention. The specification continues (page 34 line 25 – page 35 line 6):
However, at least one embodiment involves the use of a continuous or semi-batch synthesis method which provides several advantages over the batch process commonly used. This involves adding only a portion of the G and E constituents either together or sequentially or individually in a form of a slow feed to initiate the primary epoxide ring opening reaction, followed by the slow continuous feeding of the two constituents G and E (either together or separately and at the same time or sequentially). This method allows for a much better control over the overall reaction, the reaction temperature and provides a better overall yield of the active compounds in the product also avoiding the undesired side reactions. (see FIG. 2).
(Emphasis added)
69 Figure 2 is described earlier in the specification as “a graph illustrating a semi-batch reaction profile of the invention” and is as follows:
70 The specification continues (page 35 lines 7 – 16):
In at least one embodiment the synthesis reaction utilizes constituent G = 3- glycidoxypropyltrimethoxysilane. Prolonged exposure at high temperatures above 120°C can result in internal coupling reactions and multiple substitutions with the reactive amine groups such as hexane diamine or ethylene diamine. The resulting un-hydrolyzed reaction products will turn to a gel over shorter time period accompanied by an increase in the reaction product viscosity. Use of a semi-batch process or continuous or separate or slow sequential or individual or combined feed of the E and G epoxides into the reaction mixture allows better control of the reaction temperature thereby reducing the amount of methanol that is generated and isolated during the reaction. Furthermore the reaction mixture has a lower viscosity and accounts for fewer undesired side reactions (see Table l).

71 Example 2 is entitled “Examples of the relative DSP scale inhibition of various A:G:E small molecules formed during the synthesis reaction disclosed above”. The example begins (page 36 lines 1 – 9):
The scale inhibition performance of the small molecule is typically performed as follows:
l) A small amount of sodium silicate (0.25 - 1.5 g/L as SiO2) is added to a Bayer refinery spent liquor at room temperature to raise the silica concentration in the liquor.
2) Portions of this liquor sample are dosed with varying amounts of the new scale inhibitor compound or mixture.
3) Dosed and untreated (or Blank) liquor samples are subjected to elevated temperatures between 96 to 105°C for 4 to 6 hours.
4) Samples are then cooled and the amount of DSP scale formed in each of the dosed liquors samples are measured and compared to that formed in the untreated or blank samples.
72 Table 2 then shows the relative DSP scale inhibition for several A + G + E synthesized mixtures “using the synthesis reaction disclosed earlier”. The five synthesized mixtures are the result of the reaction of five different amines with glycidoxypropyltrimethoxysilane (the G component) and 2-ethylhexyl glycidyl ether (the E component):
73 The specification states, at page 37 line 6, that the term “comprising” means “including, but not limited to”.
74 Claim 1 provides (with integer numbers added):
(1) A method for the reduction of aluminosilicate containing scale in a Bayer process comprising the step of:
(2) adding to the Bayer process stream an aluminosilicate scale inhibiting amount of a composition comprising at least one small molecule
(3) selected from the group consisting of compounds (I) through (XIII), (XV) through (XXX), (XXXII) through (XLVII), (LIII) through (LVIII) and (LX)
(4) within a product mixture formed from the reaction of a) hexane diamine, ethylene diamine or 1-amino-2-propanol; b) 3-glycidoxypropyltrimethoxysilane; and c) 2-ethylhexyl glycidyl ether:
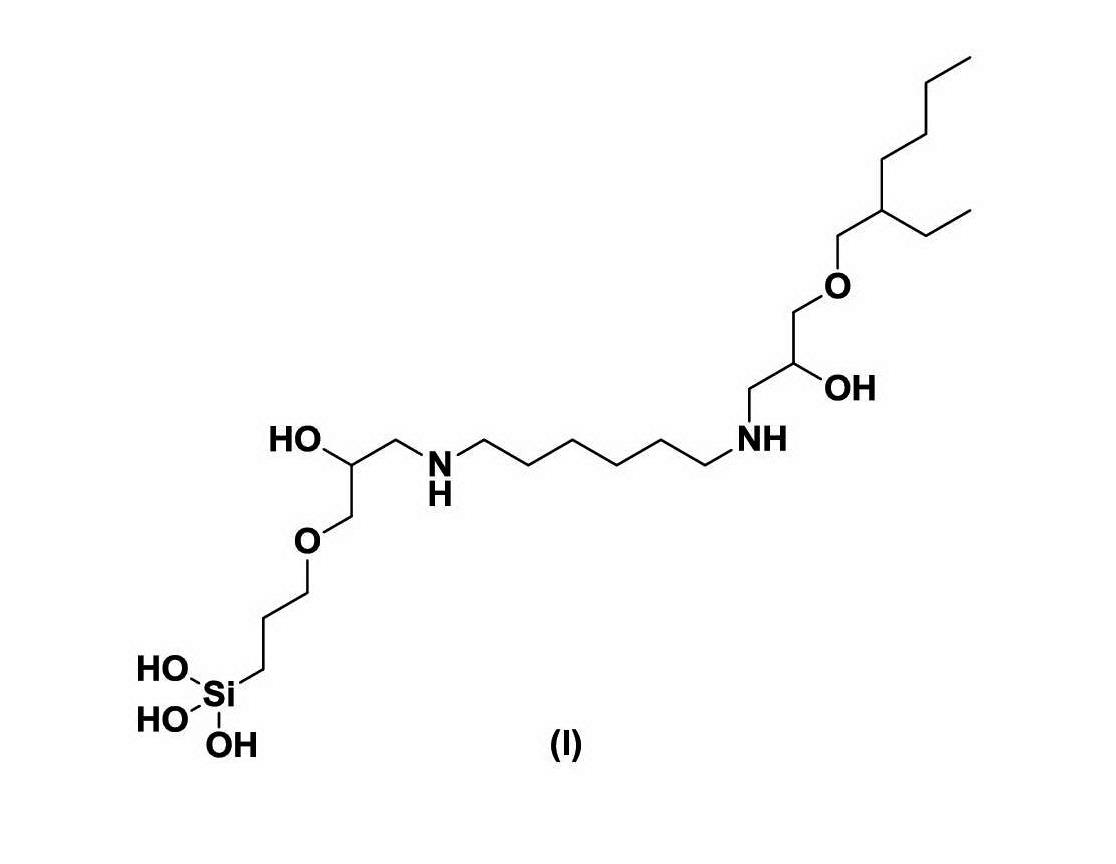
[drawn thereafter are the 46 further compounds referred to in integer (3)]
75 It will be seen that integer (4) identifies three constituent reactants from which the product mixture is to be formed:
(1) Hexane diamine (H), ethylene diamine (ED) or 1-amino-2-propanol (AP) being amines (abbreviated conveniently to A (or amine) which can mean any one of H, ED or AP);
(2) 3-glycidoxypropyltrimethoxysilane (GPS) being an amine-reactive compound that has a silane (Si(OR)3) group; and
(3) 2-ethylhexyl glycidyl ether (E) being an amine-reactive hydrophobic hydrocarbon.
76 Claims 2 – 16 are as follows:
2. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (I) through (XIII), (XV) through (XXX) and (XXXII) through (XLII).
3. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (LIII) through (LVIII).
4. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (LIII), (LIV) and (LV).
5. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (I) through (IX).
6. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (X) through (XIII) and (XV) through (XIX).
7. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (XX) through (XXII).
8. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (XXIII) through (XXIX).
9. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (XXX) and (XXXII).
10. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (XXXIII) though (XLII).
11. A method according to claim 1, wherein the small molecule is selected from the group consisting of compounds (LVI) through (LVIII) and (LX).
12. A method according to claim 1, wherein the small molecule is present in a solution in an amount ranging from about 0.01 to about 100 wt%.
13. A method according to claim 1, wherein the composition further comprises an item selected from the list consisting of: amines, activators, antifoaming agents, co-absorbents, corrosion inhibitors, coloring agents, and any combination thereof.
14. A method according to claim 1, wherein the composition comprises a solvent, the solvent is selected from the group consisting of: water, alcohols, polyols, other industrial solvents, organic solvents, and any combination thereof.
15. A method according to claim 1, wherein the composition is isolated from a synthesis reaction in the form of a solid, precipitate, gel, salt and/or crystalline material in pHs ranging from 0 to 14.
16. A method according to claim 1, wherein the composition is hydrolyzed prior to being added to the Bayer process.
4.3 The chemistry of the claims
77 At this point it is convenient to refer again to the primer, which sets out some of the background organic chemistry relevant to an understanding of the claims.
78 The drawn small molecule structures in claim 1 consist of 47 of the 65 structures depicted in the body of the specification. The primer records that they can be placed conceptually into five groups, examples of which are set out below, where the amine (or A) component is circled in green, the GPS component is circled in red and the ether (or E) component, if present, is circled in blue. Those five groups are:
(1) hexane diamine (i.e. the A component), with 3-glycidoxypropyltrimethoxysilane (GPS) and 2-ethylhexylglycidyl ether (E), (noting that some molecules do not include an E component, and the trimethoxysilane (Si(OCH3)3 groups on the molecules have been hydrolysed to Si(OH)3);
|
|
(2) ethylene diamine (i.e. the A component), with GPS and E, (noting that some molecules do not include an E component, and the trimethoxysilane (Si(OCH3)3 groups on the molecules have been hydrolysed to Si(OH)3);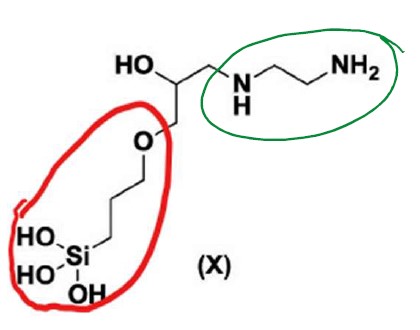
(3) ethylene diamine (i.e. the A component), with GPS and E, (noting that some molecules do not include an E component, and the trimethoxysilane (Si(OCH3)3) groups on the molecules have not been hydrolysed);
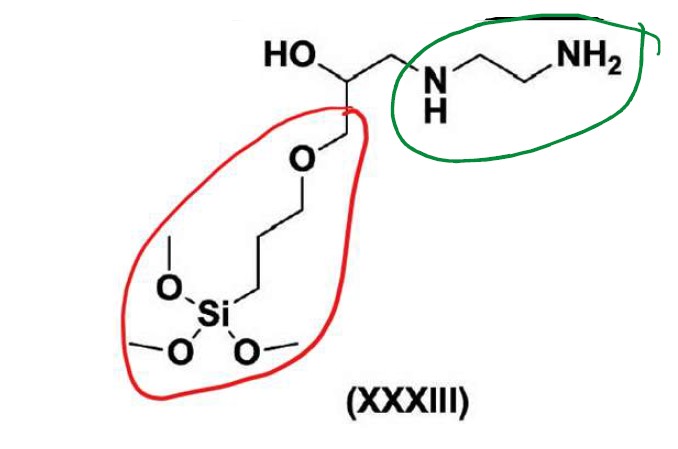
(4) 1-amino-2-propanol (i.e. the A component), with GPS and E, (noting that some molecules do not include an E component, and the trimethoxysilane (Si(OCH3)3 groups on the molecules have been hydrolysed to Si(OH)3); and
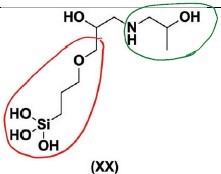
(5) 1-amino-2-propanol (i.e. the A component), with GPS and E, (noting that some molecules do not include an E component, and the trimethoxysilane (Si(OCH3)3) groups on the molecules have not been hydrolysed).
79 The small molecules identified are amine-based (each must have an A component) which must also have at least one silane group (from the GPS component) and may optionally also have one or more non-silane hydrophobic group(s) (the E component). This is apparent from the fact that the structures identified in integer 3 of claim 1 include a number of structures with no E group at all, and some where E is present.
80 Each of the small molecules in the claims of the patent application are relatively simple molecules. The structures are formed from the A, GPS and E reactants by a reaction between the epoxide ring at the end of GPS or E (both have the same single epoxide ring at one end) and an amine group of the A reactant. The amine nitrogen (N) attaches to a carbon atom adjacent the oxygen (O) of the epoxide ring, causing the epoxide ring to open. A hydrogen (H) from the amine is transferred to the oxygen which was originally part of the epoxide ring to form a hydroxyl (OH) group. All of the claimed structures are formed by way of this reaction, with different permutations of GPS and E attaching to the relevant amine.
81 This epoxide-amine reaction is exothermic, due to the epoxide ring opening during the reaction, which releases energy in the form of heat, and the resulting lower energy state when the carbon atom in the epoxide ring has bound to the amine nitrogen (N), substituting for one of the hydrogens (H) previously bound to the amine nitrogen (N).
82 Each primary amine group (NH2) on hexanediamine, ethylene diamine and 1-amino-2-propanol, before reacting, has two hydrogen (H) groups and is therefore able to react with two epoxide reactants. The compounds shown in claim 1 show different combinations and degrees of epoxide-substitution on the amine. Hexanediamine and ethylene diamine are both diamines, having two primary amine groups (one at each end of their carbon chain) and can react with four epoxide groups. 1-Amino-2-propanol is a monoamine with only one primary amine group (at the end of the carbon chain) and can react with two epoxide groups.
83 When reference is made to compounds as being hydrolysed or not hydrolysed, this refers to the GPS component of the molecules, which in some of the structures appears as a trimethoxysilane (Si(OCH3)3) and in others appears hydrolysed as a trihydroxysilane (Si(OH)3).
4.4 The person skilled in the art
84 The subject matter of the patent application is chemistry, particularly organic chemistry as it relates to the synthesis of reaction product mixtures containing the small molecules claimed and the chemistry of their application to the inhibition of silica scale formation in Bayer liquor. The disclosure of the specification focusses on the use of synthesised small molecule product mixtures in the Bayer process to reduce the formation of scale.
85 There is no dispute that each of the experts called to give evidence fall within the class of persons with a practical interest in the subject matter of the invention.
4.5 Some agreed propositions regarding the disclosure of the specification
86 The experts in their joint report agreed that the product mixtures described and claimed in the patent application are formed from the reaction of one of the specified amines (H, ED or AP) and the amine-reactive silane containing molecule (GPS) and the amine-reactive hydrophobic hydrocarbon (E). In their joint report they say:
17. [The application] provides structural drawings which are conceptual representations of the silane-containing products that would be formed through amine-epoxide ring opening reactions and expected to be present in the resulting product mixtures. Why these silane compounds were chosen to be illustrated instead of others that would inevitably be present in the complex reaction product mixtures, or their proportions or individual effectiveness, is not disclosed.
18. Claim 1 states that a product mixture must contain at least one of the small molecules listed, and that the product mixture is added to the Bayer stream in an inhibiting amount. Claims 2-11 are dependent on claim 1 limiting [sic] to specific subsets of the possible structures. The scale-inhibiting action of a product mixture will inevitably be attributable to the aggregate influences of all of the silane compounds present in the complex mixture, rather than any specific individual compound which may be present in the mixture in only a small amount.
19. Dependent Claims 12-15 relate to how the product may be formulated.
20. Dependent Claim 16 allows for the product to be hydrolysed prior to being added to the Bayer process.
87 In their oral evidence there was little difference between the experts as to the complexity of the product mixtures. When one of the three different forms of amine is reacted with GPS and E, the consequent reaction mixture will contain an extremely large variety of small molecules and will include all of the small molecules listed in claim 1, subject only to being limited by those molecules containing the same form of amine backbone that was added to the mixture. Professor Easton explained why so many different small molecules would be formed, despite the input being simply three reactants:
PROF EASTON: Because each reaction has at least four possibilities. Each time the amine reacts with an epoxide, and each time the substitutions are sequential, so the second substitution, it’s at least four more; the third one, it’s at least four more. So if you look at the compound like compound 1 or 2, I could probably expect to produce a reaction mixture that would have 10 per cent – something like that – of those molecules in there with a whole lot of other molecules from subsequent reactions, but once I get up to a compound that’s tetrasubstituted like compound 8 or 9, because of the number of possible reactions, there’s literally thousands of possibilities...
88 They agree that there was no known way to avoid the production of a complex product mixture including within it all of the other compounds specified within the claim.
89 The experts also agree that the examples in the patent application disclose methods for making the product mixtures identified in the laboratory, and that beyond general guidance, not enough detail is provided in the specification to enable the examples to be replicated. They add that the focus of the synthesis method disclosed is a semi-batch process in which reactants are added gradually. They agree that the patent application states that the semi-batch process allows for much better control of the overall reaction and the reaction temperature and provides a better overall yield of the active compounds in the products, also avoiding undesired side reactions. They agree that whilst the patent application discloses an empirical laboratory method for testing the scale-inhibiting effectiveness of the product mixtures “[n]ot enough detail is provided to enable the examples to be replicated”.
90 It is appropriate to address two issues of claim construction before turning to the grounds of invalidity advanced by Cytec. They may be summarised as follows:
(1) whether the claims encompass an embodiment wherein the composition added to the Bayer process stream comprises only one type of small molecule selected from the group of compounds identified in claim 1 (the single small molecule argument); and
(2) whether the claims include any limitations as to the synthesis method to be employed.
91 The principles of claim construction are not in dispute and are conveniently set out in the decision of the Full Court in Jupiters Ltd v Neurizon Pty Ltd [2005] FCAFC 90; 222 ALR 155 (Hill, Finn and Gyles JJ):
[67] There is no real dispute between the parties as to the principles of construction to be applied in this matter although there is some difference in emphasis. It suffices for present purposes to refer to the following:
(i) the proper construction of a specification is a matter of law: Décor Corp Pty Ltd v Dart Industries Inc (1988) 13 IPR 385 at 400;
(ii) a patent specification should be given a purposive, not a purely literal, construction: Flexible Steel Lacing Company v Beltreco Ltd (2000) 49 IPR 331 at [81]; and it is not to be read in the abstract but is to be construed in the light of the common general knowledge and the art before the priority date: Kimberley-Clark Australia Pty Ltd v Arico Trading International Pty Ltd (2001) 207 CLR 1 at [24];
(iii) the words used in a specification are to be given the meaning which the normal person skilled in the art would attach to them, having regard to his or her own general knowledge and to what is disclosed in the body of the specification: Décor Corp Pty Ltd at 391;
(iv) while the claims are to be construed in the context of the specification as a whole, it is not legitimate to narrow or expand the boundaries of monopoly as fixed by the words of a claim by adding to those words glosses drawn from other parts of the specification, although terms in the claim which are unclear may be defined by reference to the body of the specification: Kimberley-Clark v Arico at [15]; Welch Perrin & Co Pty Ltd v Worrel (1961) 106 CLR 588 at 610; Interlego AG v Toltoys Pty Ltd (1973) 130 CLR 461 at 478; the body of a specification cannot be used to change a clear claim for one subject matter into a claim for another and different subject matter: Electric & Musical Industries Ltd v Lissen Ltd [1938] 56 RPC 23 at 39;
(v) experts can give evidence on the meaning which those skilled in the art would give to technical or scientific terms and phrases and on unusual or special meanings to be given by skilled addressees to words which might otherwise bear their ordinary meaning: Sartas No 1 Pty Ltd v Koukourou & Partners Pty Ltd (1994) 30 IPR 479 at 485-486; the Court is to place itself in the position of some person acquainted with the surrounding circumstances as to the state of the art and manufacture at the time (Kimberley-Clark v Arico at [24]); and
(vi) it is for the Court, not for any witness however expert, to construe the specification; Sartas No 1 Pty Ltd, at 485–486.
5.2 The single small molecule argument
92 Cytec submits that claim 1 encompasses:
(a) embodiments where the listed small molecules are contained “within” a complex reaction product mixture; in other words the “composition” that is added to the Bayer process is the complex reaction product mixture;
(b) embodiments where the reaction product mixture composition contains a single type of small molecule, being one of those small molecules identified in integer (3); and
(c) embodiments where the listed small molecules have been “selected from...within a product mixture” by isolating them from the complex product mixture within which they were formed.
93 Cytec draws support for (b) and (c) from the language of claim 1 and also from claim 12 which claims a method according to claim 1 wherein the small molecule is present in a solution in an amount ranging from about 0.01 to about 100% by weight. Read in the context of its dependency on claim 1, Cytec submits that claim 12 further demonstrates that within claim 1 is a composition where there is no product mixture, but rather consists of 100% of one of the selected small molecule structures.
94 Nalco accepts that claim 1 includes within its scope the result of the reaction of A, GPS and E within (a), being a product mixture which will include at least one, and most likely all, of the claimed small molecules (depending on which of the amine reactants is used) in various proportions. It submits that the experts agree that in practice the reaction of those three reactants will almost inevitably form each of the claimed small molecules, corresponding to the selection within A (whether H, ED or AP), in various proportions within the product mixture. It submits that Mr Bellwood and Professor Easton understood that the formation of any of the claimed small molecules will occur randomly. As at the priority date, three of the experts thought it was possible but statistically unlikely that only one of the claimed molecules and not other of the claimed molecules would be within a product mixture formed by the reaction of A, GPS and E.
95 As I note further below, Nalco also accepts that a product mixture within the claim may theoretically include a single type of small molecule, being one of those identified in integer (3), within the construction (b). However, it submits that this would be a random happenstance that the claim does not “require”.
96 Nalco, contests that the claim includes any embodiment wherein a single type of small molecule is isolated from the product mixture within construction (c).
97 Nalco submits that the dependent claims do not detract from its construction. It submits that the reference to “the small molecule” in claims 2 to 12 refer to the “at least one small molecule” within the product mixture formed from the reaction of A + GPS + E and that this term should be given the same meaning as in claim 1. With respect to claim 12, Nalco submits that it concerns the dilution of the product mixture and therefore the small molecules within it, and does not claim a product mixture having only one of the claimed small molecules and not others.
98 Nalco submits that by defining the scope of the claims by reference to particular small molecules that will inevitably form from the reaction of A + GPS + E, it is plain that the product mixture must be the result of “the reaction” disclosed in the patent application. This, it submits, demonstrates that the selection of the small molecules in claim 1 is not arbitrary. Nalco also submits that the product mixture formed from the reaction of A + GPS + E need not contain only the small molecules identified in the claims, and that this is supported by the expert evidence that other compounds formed from the reaction will inevitably be present in the product mixture.
99 The scientific evidence concerning claim 1 is not substantially in dispute. The compounds illustrated in claim 1 would conceptually be derived as a product of the three identified reactants (depending on the selection of the amine), but the patent application does not describe how the specific small molecules could be separated from that product mixture. The patent application also does not provide any explanation as to why the specifically drawn compounds, as opposed to other molecules which would also inevitably be contained in the product mixture made from the reactants, have been specifically identified as effective in reducing aluminosilicate scale when added to the Bayer process. Example 1 in the patent application describes general methods of producing the synthesis reaction of A + GPS + E and recites a preference for a semi-batch method. Example 2 identifies various different amines used and indicates that hexane diamine is the most preferred of the amines to use in the synthesis reaction.
100 The compounds illustrated in claim 1 are all derived from the same silicon-containing moiety (GPS) and hydrophobe (E), with the only variation being the amine component, which may be in the form of H, ED or AP.
101 The 47 specifically illustrated compounds in the claim are likely to be present as the product of the reaction identified in integer (4) and would be within a very complex mixture, which would include small molecules and polymers. The person skilled in the art reading the specification would attribute the aluminosilicate scale inhibiting effect to the combination of all of the silane compounds in the mixture formed from A + GPS + E, rather than any specific individual small molecule which would be present in the mixture in small amounts.
102 The evidence indicates that it is not possible to say, based on the disclosure of the specification, whether any particular compound in the mixture is responsible for all, most, or only some of the effect. In his affidavit, Mr Bellwood provided a slight qualification to this general statement by making the point that the listed compounds in the patent application did not include compounds containing only A and E reactants (i.e. compounds without the silane group GPS) because such compounds would not be active in DSP scale inhibition or reduction.
103 The reason for the complexity of the mixture was discussed in the evidence and concerns the number of potential primary and later reactions within the mixture as the explanation given by Professor Easton set out in [87] above indicates.
104 Against this immediate background and the common general knowledge, I turn to consider the language of claim.
105 For the reasons that follow, in my view, the language of claim 1 encompasses a composition that is made up of a single type of small molecule selected from the group identified within the product mixture formed from the reaction of A + GPS + E (within the construction propounded by Cytec in (b) above) as well as a composition that includes a complex mixture of many of the small molecules depicted in the claim as well as many other compounds (within (a) above). It is not apparent that the claim encompasses an embodiment that involves isolating a small molecule from the reaction product mixture within the construction propounded by Cytec in (c).
106 The claim refers to adding to the Bayer process stream an “aluminosilicate scale inhibiting amount of a composition comprising at least one small molecule selected from the group” consisting of 47 identified compounds. The word “comprising” is defined to mean “including, but not limited to” and the words “at least one small molecule” includes the selection of one molecule only. It is apparent from the breadth of the meaning of “including” that the entirety of the composition may be one type of small molecule or more than one such type. It is difficult to read the claim in a different way. The type of small molecule may be one or more selected from the group of nominated compounds that are “within a product mixture” that is “formed from the reaction of a) hexane diamine, ethylene diamine or 1-amino-2-propanol; b) 3-glycidoxypropyltrimethoxysilane; and c) 2-ethylhexyl glycidyl ether”.
107 I reject the submission that the words “selected from” refer to the selection of the three alternative amines, H, ED or AP. That is not a natural or common sense way to understand the words “selected from”; the verb “selected” applies to the compounds identified in the claim, not the amines identified in integer (4). Although the choice of either H, ED or AP as the amine “backbone” will influence which of the small molecule compounds identified in integer (3) may appear within the reaction product mixture, that choice does not account for the language of “comprising at least one small molecule”.
108 The result is that, properly construed, the claim includes within its scope both: (a) a complex reaction mixture, formed from the reaction of A + GPS + E, that includes within it many of the identified small molecules as well as many more compounds; and (b) a reaction mixture, formed from the reaction of A + GPS + E, that is made up of a single type of small molecule identified in the claim. The language of “comprising at least one small molecule” indicates that the claim covers the spectrum between these two possibilities.
109 Indeed, as I have noted, Nalco accepts that the scope of the claim includes a composition comprising a single type of the same small molecule. As Nalco put it in closing submissions:
MR COOKE: ...it’s impossible, your Honour, to be able to say, “I’m going to get this particular compound”. You can’t do it. And there’s no evidence that you could, and we don’t submit that you can. And with the…“at least one”, your Honour, right, what that’s dealing with is the fact that when there’s a random reaction, right – because you don’t – because all you’ve got control of is the reaction itself, and not the molecules which are formed from that reaction. But you know, as a matter of chemistry, the probabilities you’re going to get them, and we know, as a matter of chemistry, that you’re more likely – you’re most likely to get a proportion –differing proportions of each of those small molecules – three of the experts, as at the priority date, although they said it’s statistically unlikely that you would only get one of the claimed small molecules and not other of the claimed small molecules in the reaction within the product mixture, it was still a possibility....But the point is…just consider the situation where it didn’t have the words “at least one of the molecules” in there...Because it’s a random event, how could you be actually 100 per cent sure?...You can’t because you can’t control it....
HIS HONOUR: But the – I don’t think there was any disagreement that the - the probability of actually getting one was infinitesimally small, even if you set to one side Dr Power.
Mr COOKE: Yes
HIS HONOUR: So you’re saying the drafting was done for the purpose against that infinitesimally small prospect.
MR COOKE: Well – well, it – yes.
110 The reference to the small prospect that one would obtain only one type of small molecule in the composition arises from the expert evidence. As Mr Bellwood put it, the probability of a single small molecule emerging within the reaction mixture would be like trying to win the lottery in every country in the world with the same six numbers on the same weekend. Dr Power considered that the prospect of this occurring was an impossibility. Even so, it is apparent from the foregoing that Nalco does not dispute that claim 1 encompasses a single type of molecule forming the composition to be added to the Bayer process stream, but it submits that this is necessary for the patentee to have claim coverage against the infinitesimally unlikely event to which it refers.
111 In my view the Nalco concession is properly made. However, claim 1 is not to be understood as claiming only the infinitesimally small happenstance to which the evidence refers. The claim is not limited in scope as to how the single type of molecule becomes the result of the reaction product mixture. Claim 1 simply encompasses a circumstance where 100% of the reaction mixture formed from the reaction of A + GPS + E is composed of a single type of one of the identified small molecules. Put another way, the claim includes within its scope any circumstance where a single type of the small molecules identified in integer (3) emerges as the outcome of the product mixture, regardless of the conditions in which the reaction is conducted.
112 It is true that the claim does not “require” an outcome of a single type of molecule as the reaction product, but the question of claim construction does not concern what the claim “requires” but rather the breadth of the claim. In my view this construction provides content to the language of integer (3), whereby the composition comprising at least one small molecule involves a “selection from the group” of compounds. Otherwise, that wording would be surplusage.
113 Furthermore, this construction sits comfortably with claim 12 which is for a method according to claim 1 “wherein the small molecule is present in a solution in an amount ranging from about 0.01 to about 100 wt%”. In my view, the reference to the small molecule must be a reference to the “at least one small molecule” in claim 1. In circumstances where the “at least one small molecule” of claim 1 is just one small molecule, claim 12 provides for the composition to be a solution that contains 100% of that type of small molecule.
114 This construction applies to all of the dependent claims 2 – 16.
115 I am not satisfied, however, that claim 1 includes within its scope a circumstance where the listed small molecules have been “selected from...within a product mixture” by isolating them from the complex product mixture within which they were formed, within construction (c) propounded by Cytec. That construction involves selecting at least one small molecule from the product mixture formed from the reaction of A + GPS + E, which distorts the language of the claim by transposing the selection of (in this instance) one compound as the type of small molecule that is to be the result of the product mixture of integer (4), to the selection of one small molecule from the resulting product mixture.
5.3 Do the claims include any limitations as to the synthesis method used to form the reaction product mixture?
116 Nalco makes the following submission:
...the product mixture is the result of “the reaction” of A + G + E, understood in the context of the specification as a whole (i.e. in a manner that controls the temperature to avoid prolonged exposure to high temperatures above 120°C, so as to maximise the yield of the small molecules).
117 Cytec submits that the claims are not limited to any mode of performing the synthesis reaction, and contain no limitation which requires the yield of the specific, drawn molecules identified in the claims to be maximised or even efficacious.
118 To the extent that Nalco submits that claim 1 is to be read as including within it a limitation as to the way in which the synthesis reaction is brought into effect, in my view, that submission must be rejected. No language in the claims identifies that the product mixture formed from the reaction must be undertaken under any particular conditions. The specification teaches in example 1 that a product mixture may be produced using a batch method or (preferably) a semi-batch method but, as Mr Bellwood says in his evidence, the specification explains that a product mixture containing at least one of the small molecules will be produced using a simple batch reaction. Accordingly, as Cytec submits, even the gloss that Nalco seeks to import does not arise from the specification. In any event, it is not permissible to read the claims down by reference to glosses drawn from the specification.
119 Section 40(3) requires that the claim or claims be clear and succinct and supported by matter disclosed in the specification.
120 In Merck Sharp & Dohme Corporation v Wyeth LLC (No 3) [2020] FCA 1477; 155 IPR 1, I said:
[545] ...As said in the Explanatory Memorandum, in exchange for the exclusive rights given to the patentee, the patentee must share with the public the information necessary to make and use the invention. This is the essential exchange between inventors and the public which has long been a feature of patent law in Australia: see Lockwood No 1 at [57].
[546] In CSR Building Products Ltd v United States Gypsum Company [2015] APO 72, Dr S D Barker adopted the summary provided by Aldous J in Schering Biotech at 252 – 253, which has been often followed in the United Kingdom (emphasis added):
…to decide whether the claims are supported by the description it is necessary to ascertain what is the invention which is specified in the claims and then compare that with the invention which has been described in the specification. Thereafter the court’s task is to decide whether the invention in the claims is supported by the description. I do not believe that the mere mention in the specification of features appearing in the claim will necessarily be a sufficient support. The word “support” means more than that and requires the description to be the base which can fairly entitle the patentee to a monopoly of the width claimed.
[547] That approach encapsulates broadly the claim support obligation under s 40(3). To it may be added the requirement that the technical contribution to the art must be ascertained. Where it is a product, it is that which must be supported in the sense that the technical contribution to the art disclosed by the specification must justify the breath of the monopoly claimed.
121 The parties accept that this is the appropriate way to approach this requirement.
122 Cytec contends that the technical contribution to the art may be described as the synthesis of a reaction product mixture by reacting an amine, an amine-reactive silane compound and an amine reactive hydrophobe to form a mixture of silane-based small molecules for reducing DSP scale in a Bayer process. It contends that the claims lack support for the following reasons.
123 First, as a matter of claim construction, all claims include within their scope embodiments where a single molecule constitutes the whole small molecule component of the composition, whether because the reaction mixture contains a single type of molecule only or because a single type of small molecules is isolated from the reaction mixture. This is the subject of the construction debate that is addressed in section 5.2 above. Cytec submits that there is no supporting disclosure of the synthesis of any such composition or any supporting disclosure of isolating a single small molecule from the complex reaction product mixtures. Furthermore, even if the claims were construed as including embodiments with two or more individual small molecules, Cytec submits that they would lack support, because the technical contribution of the invention relates only to the complex reaction product mixture, scale inhibition being achieved by the aggregate effect of the large number of molecules that are present within it.
124 Secondly, Cytec submits the purported “selection” of the small molecule(s) within the claims from other small molecules that will inevitably be present in the reaction mixture lacks support. Each of the reactions of A (including as it does, H, ED or AP) + GPS + E will always contain an extremely large variety and number of other small molecules. Cytec notes that it is agreed between the experts that the scale inhibiting action of a product mixture will inevitably be attributable to the aggregate influences of all of the silane compounds present in the complex mixture, rather than any specific individual compound which may be present in the mixture in only a small amount. Cytec also notes the agreement of the experts that the patent application does not disclose why the saline compounds that were drawn and disclosed in the patent application were chosen instead of others that would inevitably be present in the complex reaction product mixtures.
125 Cytec submits that from these matters it is apparent that there is no disclosure in the specification teaching the skilled person why any small molecule in the complex mixture is selected over any other. In the absence of an explanation directing the person skilled in the art to select a specific “at least one small molecule” in preference to the very large number of other molecules that will inevitably be present, the scope of the claims is not supported by the disclosure and is simply an arbitrary selection. Put differently, Cytec submits that the claimed selection is meaningless where scale inhibition is caused by the aggregate and the claims do not require any individual selected molecule to be present in any particular amount or concentration. On this basis, Cytec submits that no technical contribution is provided to support the “selection” aspects of the claims.
126 In answer to the first argument, Nalco submits that the technical contribution of the patent application is the discovery that the small molecule based inhibitors are effective in reducing DSP scale in Bayer refineries and that the patent application describes the reaction conditions for making and maximising the yield of those small molecule based inhibitors. It contests the construction advanced by Cytec as set out in section 5.2 above.
127 In answer to the second argument, Nalco submits, first, that the claims do not require the product mixture formed from A + GPS + E only to contain the small molecules identified in claim 1. Secondly, that in practice, each of the small molecules identified will inevitably form in varying proportions in the product mixture, depending on the particular amine selected. Accordingly, the selection is in no sense arbitrary. Thirdly, the claims do not require the scale-inhibiting action of the product mixture to be attributable to any specific small molecule or only the small molecules identified in the claim. Fourthly, the specification discloses that the silane based small molecules of the general structure defined by the Markush diagram (set out above at [52]) are effective in reducing DSP scale in Bayer refineries, and that there are at least three advantages to using small molecule based inhibitors such as those claimed over polymeric inhibitors with repeating units of silane and hydrophobe. Nalco notes that the specification also discloses the reaction conditions for making those small molecule based inhibitors, which minimises undesired side reactions to maximise the yield of those small molecules in a product mixture. The technical contribution of the patent application is therefore the discovery that these small molecule based inhibitors are effective in reducing DSP scale in Bayer refineries, and it describes the reaction conditions for making and maximising the yield of those small molecule based inhibitors.
128 Nalco submits that it follows that the claims are supported by the description; the monopoly claimed does not include a method that goes beyond the technical contribution to the art provided by the specification. The test for lack of support does not involve an assessment of whether the claimed invention is “arbitrary” and the specification need not explain why any particular limitation has been adopted in the claims. What s 40(3) requires is that the claims do not extend beyond the technical contribution disclosed.
129 I have in section 5.1 concluded that claim 1 includes within its scope not only a complex reaction mixture but also a composition where the product mixture formed from the reaction of integer (4) consists entirely of one type of small molecule.
130 There is no dispute that the specification does not disclose how such a composition may be made. Indeed, the expert evidence was clear that the person skilled in the art would not know how to achieve such a composition and the specification does not teach how this would be done.
131 Whilst the evidence does disclose that there is an infinitesimally small prospect that the product of the reaction of A + GPS + E in claim 1 may yield a single type of small molecule within the reaction product mixture, that would arise only by random happenstance, and not by virtue of any disclosure in the specification. I reject that as an adequate teaching for the purpose of the support requirement within s 40(3). Indeed, Nalco did not seek to defend the claim on the basis that the technical contribution to the art was a means of producing such a composition, or that the random happenstance identified would provide adequate support. Rather, it submitted that the technical contribution was the more general discovery that small molecule based inhibitors are effective in reducing DSP scale in Bayer refineries and that the patent application broadly describes the reaction conditions for making and maximising the yield of those small molecule based inhibitors. I agree with that characterisation. The body of the specification does not identify or describe the beneficial effects of particular small molecules. Nor does it identify or describe how to produce a reaction mixture that consists of only one or other of those small molecules. Indeed, the advantages of the small molecules are identified in the specification at page 33 as being: first, that the small molecular weight means that there is a larger number of active, inhibiting moieties available around the DSP seed crystal sites at the DSP formation stage; secondly, that the lower molecular weight allows for an increased rate of diffusion of the inhibitor, which favours fast attachment of the inhibitor molecules onto DSP seed crystals; and, thirdly, the lower molecular weight avoids high product viscosity and so makes handling and injection into the Bayer process stream more convenient and effective. Each of these advantages is linked in terms to the molecular weight of small molecules generally. Understood in this way, the technical contribution is directed to small molecules generally, not to any specific small molecules or how to produce a reaction mixture confined to any one small molecule.
132 In my view the absence of any technical contribution sufficient to encompass the full scope of the monopoly claimed in claim 1 renders claim 1, and all of the claims dependent on it, lacking in support. Nalco has claimed something despite not disclosing how it is to be done. It has not shared with the public the information necessary to make and use of the invention as claimed. In so doing, the necessary consideration for the grant has not been provided, and Nalco has claimed the benefit of an invention that it has not made.
133 Accordingly, I find that the opposition to the grant of the all of the claims succeeds on this basis.
134 I now briefly consider, against the prospect that I am wrong in my view as to the construction of the claims, whether Cytec’s second support argument should succeed. This argument is to be understood in the context of a construction whereby claim 1 is limited to including within its scope only a complex reaction product including many different small molecules.
135 In essence, the contention advanced by Cytec is that the specification does not explain why it is that the particular small molecules that are identified within claim 1 have been singled out. Accordingly, Cytec contends that the identified molecules amount to little more than an arbitrary parameter.
136 However, the question of support falls to be determined by consideration of what is claimed and comparing that with the invention described in the specification, to determine whether the description in the specification, in the form of the technical contribution to the art, fairly entitles the patentee to a monopoly of the width claimed: Schering Biotech Corp’s Application [1993] RPC 249 at 253 (Aldous J).
137 The technical contribution to the art in the present context includes that small molecules produced by the reaction of A + GPS + E overcome or ameliorate at least one of the disadvantages of the prior art or at least provide a useful alternative. The prior art is identified on page 3 of the specification by reference to previous attempts to control or reduce DSP scale by adding polymer materials containing three alkyloxy groups bonded to one silicon atom. The advantages of the small molecules are identified, as I have noted above, in the specification at page 33 as being concerned with small molecules generally.
138 Claim 1 identifies small molecules that will form within the product mixture formed from the reaction of A + GPS + E. The specification provides guidance as to how that product mixture can be synthesised to produce small molecules. Whilst the expert evidence indicates that additional reaction products are likely to arise from the synthesis reaction, there is no dispute that the product mixture formed from the reaction will produce a complex mixture including small molecules and that this is explained and taught in the body of the specification. In that sense it is properly enabled.
139 It is true that in this sense (and upon this construction of the claims), the identification of the specific small molecules depicted is redundant, in that the disclosure of the specification does not explain why those compounds are singled out. However, that aspect of the claim and the disclosure of the specification is addressed in response to Cytec’s first support argument. Insofar as the claim includes a reaction product mixture containing only particular small molecules identified in the claim, that aspect of the claim is not supported (as I have found in answer to Cytec’s first support argument). However, the broader embodiment encompassed within the claim, where the reaction product is a complex mixture containing many of the listed small molecules and many more compounds, does not suffer from lack of support.
7. LACK OF CLEAR AND COMPLETE DESCRIPTION
140 Section 40(2)(a) of the Patents Act requires that the complete specification disclose the invention in a manner which is clear enough and complete enough for the invention to be performed by a person skilled in the relevant art.
141 Cytec contends in its statement of grounds and particulars that for substantially the same reasons that the claims lack support within s 40(3), the complete specification fails to comply with s 40(2)(a).
142 In Merck in section 16, particularly at [511] – [514] and [523] – [526], I addressed the introduction into the Patents Act of the current form of s 40(2)(a). I said the following in relation to the manner in which this requirement has been applied in the United Kingdom under the name “classical sufficiency”:
[525] In Novartis AG v Johns & Johnson Medical Ltd [2010] EWCA Civ 1039; [2011] E.C.C. 10, Jacob LJ said at [74]:
The heart of the test is: “Can the skilled person readily perform the invention over the whole area claimed without undue burden and without needing inventive skill?”
[526] In Terrell on the Law of Patents (19th ed, Sweet & Maxwell, London, 2020), at page 404 the learned editors (Sir Colin Birss et al) propose as a convenient summary of the elements of this aspect of classical insufficiency the following passage provided by Kitchin J (as his Lordship then was) in Eli Lily v Human Genome Sciences [2008] 7 WLUK 978; RPC 29 at [239]:
The specification must disclose the invention clearly and completely enough for it to be performed by a person skilled in the art. The key elements of this requirement which bear on the present case are these:
(i) the first step is to identify the invention and that is to be done by reading and construing the claims;
(ii) in the case of a product claim that means making or otherwise obtaining the product;
(iii) in the case of a process claim, it means working the process;
(iv) sufficiency of the disclosure must be assessed on the basis of the specification as a whole including the description and the claims;
(v) the disclosure is aimed at the skilled person who may use his common general knowledge to supplement the information contained in the specification;
(vi) the specification must be sufficient to allow the invention to be performed over the whole scope of the claim;
(vii) the specification must be sufficient to allow the invention to be so performed without undue burden.
143 In CSR Building Products Ltd v United States Gypsum Company [2015] APO 72, Dr S D Barker considered at [95] that it involved the following three steps:
(1) Construe the claims to determine the scope of the invention as claimed;
(2) Construe the description to determine what it discloses to the person skilled in the art; and
(3) Decide whether the specification provides an enabling disclosure of all the things that fall within the scope of the claims.
144 In the present case, the delegate (at [54]) added to these the further questions to be considered, citing Evolva SA [2017] APO 57 at [45]:
(1) Is it plausible that the invention can be worked across the full scope of the invention?
(2) Can the invention be performed across the full scope of the claims without undue experimentation?
145 It will be observed that the steps proposed in Terrell on the Law of Patents (19th ed, Sweet & Maxwell, London, 2020) (set out above at [142]) do not differ materially from those set out in the decisions of the Commissioner. The emphasis in Evolva that the question involves consideration of whether the invention can be performed across the full scope of the claims without undue experimentation represents a significant shift from the previous law, as set out by the High Court in Kimberly-Clark Australia Pty Ltd v Arico Trading International Pty Ltd [2001] HCA 8; 207 CLR 1 at [25], and represents the established position in the United Kingdom.
146 The language of s 40(2)(a) now indicates that this is the correct approach in Australia, a position that is amplified by the secondary materials. In his second reading speech introducing the Intellectual Property Laws Amendment (Raising the Bar) Bill 2011 (Cth) (RTB Bill), the Hon. Mark Dreyfus QC MP observed that the Bill sought to raise patent standards to address concerns that Australia’s standards are lower than elsewhere, which discourages export of technology developed in Australia, and inhibits the growth of Australian business. He said:
A theme in this Bill is one of recalibrating and raising Australian standards, to align them more closely with those of other major trading partners around the world. Some patent standards around the world have developed to the point where there is an aligned approach internationally. In such cases...the Bill raises Australian patenting standards to that common approach.
The Bill raises patent standards in three important areas.
First, it raises standards with respect to the information provided in patent applications and specifications. Disclosure is a cornerstone of the patent system. Patents are an exchange between inventors and the public. In exchange for a 20-year monopoly on commercialisation, the innovator must tell the public how their idea works. This disclosure allows for potential follow-on innovators to build on initial work to produce even better innovations.
The Bill raises standards to require that the patent right is consistent with the information provided in the patent specification. There must be enough information disclosed for the public to make and use the invention. Also, a specific, substantial and credible use for the invention must be also disclosed and the scope of the claims for patent protection must not extend beyond what has been disclosed.
Importantly, the amended provisions mirror similar provisions in the United Kingdom and Europe. It is intended that Australian courts will have regard to developments in the law in the courts of those other jurisdictions when interpreting the new provisions and will develop Australian law in a consistent fashion…
(Emphasis added)
147 The Explanatory Memorandum to the RTB Bill also makes plain that the intention of the changes was to bring the law into conformity with the laws of Australia’s major trading partners, with particular reference to the United Kingdom and Europe: see Commonwealth Scientific and Industrial Research Organisation v BASF Plant Science GmbH [2020] FCA 328; 151 IPR 181 at [188]-[190] (Beach J). For both, the equivalent requirement is that the disclosure obligation, expressed in equivalent terms, applies across the breadth of the claim: see Novartis AG v Johns & Johnson Medical Ltd [2010] EWCA Civ 1039; [2011] E.C.C. 10 at [74] (Jacob LJ); Patents Act 1977 (UK) s 14(3); Art 83 of the Convention on the Grant of European Patents, opened for signature 5 October 1973, 1065 UNTS 199 (entered into force 7 October 1977).
148 In Merck I referred additionally to the following two cases at [523] – [524]:
[523] ...[classical sufficiency] requires an assessment by the court of the steps which it would be necessary for the skilled reader or team to take in following the teaching of the specification and in order to arrive within the claim: Zipher Ltd v Markem Systems Ltd [2009] EWHC 1379; FSR 1 at [362] – [363] (Floyd J).
[524] In Mentor Corp v Hollister Inc (No 2) [1992] 7 WLUK 465; [1993] RPC 7 at 14, the Court of Appeal (per Lloyd LJ, with whom Stuart-Smith and Scott LJJ agreed) endorsed a passage from Aldous J at first instance (Mentor Corp v Hollister Inc [1991] 3 WLUK 167; FSR 557 at 562) who said:
The section requires the skilled man to be able to perform the invention, but does not lay down the limits as to the time and energy that the skilled man must spend seeking to perform the invention before it is insufficient. Clearly there must be a limit. The subsection, by using the words “clearly enough and completely enough”, contemplates that patent specifications need not set out every detail necessary for performance, but can leave the skilled man to use his skill to perform the invention. In so doing he must seek success. He should not be required to carry out any prolonged research, enquiry or experiment. He may need to carry out the ordinary methods of trial and error, which involve no inventive step and generally are necessary in applying the particular discovery to produce a practical result. In each case, it is a question of fact, depending on the nature of the invention, as to whether the steps needed to perform the invention are ordinary steps of trial and error which a skilled man would realise would be necessary and normal to produce a practical result.
149 In the present case, my resolution of the construction of claim 1 in section 5.2 is also determinative of the ground of opposition under s 40(2)(a). For the reasons set out in section 6, it is apparent that the disclosure of the specification, including the claims, is not sufficient to enable the invention claimed to be performed without undue experimentation insofar as those claims include a product mixture made up of a single type of small molecule. Accordingly, for substantially the same reasons why Cytec succeeds in its first argument based on lack of support, it succeeds in its first argument based on lack of sufficient disclosure. Cytec failed, however, in relation to its second lack of support argument. It advanced no additional argument to suggest that it should, in that event, succeed in relation to this contention based on lack of sufficiency. For the reasons set out in my consideration of that argument above, Cytec’s second argument also fails under this ground.
150 Section 40(2)(aa) of the Patents Act provides that a complete specification must disclose the best method known to the applicant of performing the invention. The requirement is one of long standing, and was not relevantly amended with the introduction of the RTB Act.
151 In its statement of grounds and particulars, Cytec contends that the invention defined in the specification, including the claims, does not comply with s 40(2)(aa). It gives particulars of two alternative ways in which it is alleged that the specification fails to comply. First, the patent application states that a continuous or semi-batch synthesis method provides advantages over a batch process, such as better control over the overall reaction, better overall yield of the active compounds in the reaction product mixture, lower viscosity and fewer undesired side reactions. However, the best method of performing the invention is not disclosed because the specification does not disclose the specific semi-batch method, including details that would have a significant impact on the reaction product produced such as: (1) the order of addition of the reactants; (2) the concentration of reactants used; (3) whether a solvent is used; (4) the feed rate of reactants being added to the mixture; (5) the reaction temperature; and (6) the reaction time. Secondly, the patent application discloses that Nalco was aware that a specific “synthesis reaction” produced the reaction product mixtures that achieved the DSP scale inhibition reported in Table 2 of the specification. The details of that synthesis reaction formed part of the best method known to Nalco, but the specification does not disclose the reaction including details of whether a semi-batch synthesis method was used, details of the matters identified in (1) – (6) above, the dosage of the reaction product added to the Bayer liquor, or the length of application of the reaction product to the Bayer liquor. For both allegations Cytec contends that the absent information would have a significant impact on the reaction product produced when the person skilled in the art performs the invention.
152 The best method requirement provides a safeguard against a patent applicant holding back information in its possession with a view to getting the benefit of a patent monopoly without conferring on the public the full consideration for the grant of that monopoly: Pfizer Overseas Pharmaceuticals & Ors v Eli Lilly & Co [2005] FCAFC 224; 225 ALR 416 at [374] (French and Lindgren JJ) and [408] (Crennan J agreeing); see also Les Laboratoires Servier v Apotex Pty Ltd [2016] FCAFC 27; 247 FCR 61 at [64] (Bennett, Besanko and Beach JJ).
153 The starting point of the factual enquiry is to ascertain “the invention”. This is the embodiment which is described and around which the claims are drawn (cf the invention so far as claimed in any claim): see Kimberly-Clark at [21] (Gleeson CJ, McHugh, Gummow, Hayne and Callinan JJ); Sandvik Intellectual Property AB v Quarry Mining & Construction Equipment Pty Ltd [2017] FCAFC 138; 126 IPR 427 at [94] (Greenwood, Rares and Moshinsky JJ).
154 In GlaxoSmithKline Consumer Healthcare Investments (Ireland) (No 2) Limited v Generic Partners Pty Limited [2018] FCAFC 71; 264 FCR 474 (Middleton, Nicholas and Burley JJ), the Full Court said:
[185] The best method requirement has a long history in patent law which predates the introduction in 1932 of s 25(2)(j) into the Patents and Designs Act (1907) (UK) which made it a distinct statutory ground of revocation. As Fletcher Moulton LJ explained in Vidal Dyes Syndicate Ltd v Levenstein Ld. [1912] 29 RPC 245 at 269:
It is settled law that a patentee must act towards the public uberrimȃ fide, and must give the best information in his power as to how to carry out the invention. He is therefore bound to tell the public all the steps that can advantageously be taken in carrying out the invention.
[186] The patent applicant is required to disclose information not already known to the skilled addressee by way of the common general knowledge that is required to perform the invention in the best manner known to the patent applicant as at the date the complete specification is filed.
[187] Whether there has been a failure to make the required disclosure is essentially a question of fact. Every case will depend on its own facts including the nature of the invention, and the significance of what is and what is not disclosed. And like many other questions that arise in relation to the interpretation of a patent specification and the scope of its disclosure, the question whether there has been sufficient disclosure of the best method should be addressed in a practical and common sense manner. It is also necessary to have regard to the public policy justification that supports the best method requirement.
155 The patent applicant is not entitled to withhold information that is necessary to enable the skilled addressee to perform the invention in accordance with the best method merely because the skilled addressee could ascertain such information by routine experiment. There is a great deal of experimentation which, although routine and not requiring the application of any ingenuity, may be time consuming and expensive. For the patent applicant to withhold information which it knows is necessary to perform the invention in accordance with the best method merely because the information could be obtained by routine experimentation is inconsistent with what Fletcher Moulton LJ described as settled law; that the patentee is required to give the best information in its power as to how to carry out the invention: see GlaxoSmithKline at [191]. Whether or not it will be open to the patent applicant to not disclose relevant information on the basis that it is available to the skilled addressee by routine experimentation will depend on the importance of the information in question, the practicality of disclosing it, and the extent of the burden imposed on the skilled addressee who is left to rely upon routine experimentation: GlaxoSmithKline at [192].
156 In Servier the Court was concerned with whether or not the patentee had satisfied the obligation to disclose the best method by generally disclosing that the arginine salt was prepared according to “a classical method of salification of organic chemistry”, but failing to disclose the specific salification method that was used to make the claimed arginine salt.
157 The Full Court in GlaxoSmithKline repeated (at [189]) the following paragraphs in Servier, which provide a useful explanation of the correct approach:
[134] Perindopril arginine is generally a more stable product than perindopril erbumine and the claim is not to a specific form of the arginine salt. If Servier knew of a method that provides a form of the salt with the characteristics exemplified in the Patent, which characteristics provided the stated advantages of the invention over the prior art, it was incumbent on it to provide that method. This would relieve the skilled worker from making the choices within those necessarily made or available in a classical salification. The disclosure of the method known to Servier would not only have relieved the skilled addressee of confronting blind alleys and pitfalls which may not be uncommon in a general sense but also, and importantly, would tell the skilled addressee the methodology to achieve the form that obtains the result which constitutes the invention, that is increased stability and storage length. While claim 1 does not refer to any particular form of perindopril arginine, crystalline or otherwise, if Servier had a method that produced a product that was at least sufficiently crystalline or in a sufficiently good form so that it could be used in the API for the tablets used in the stability study described in the specification, that is precisely what should have been disclosed.
[135] Accepting that there was no lack of sufficiency, the mere fact that a complete specification described a method which conveyed sufficient information to a skilled addressee to enable him or her to work the invention does not necessarily satisfy the Patentee’s additional obligation to describe the best method. The patentee has an obligation to include aspects of the method of manufacture that are material to the advantages it is claimed the invention brings.
[136] In the present circumstances, the inventor, Mr Damien, had not characterised the products of the two methods that he utilised but he did know that those methods created the arginine salt in a useable form and, as a person skilled in the art, he knew that there were many alternatives available from which to choose. As the skilled worker, he knew that the method of classical salification was sensitive to choices such as the choice of solvent. He knew that some were likely not to be as good as others. That is consistent with the expert evidence, although it was not specifically put to Mr Damien.
[137] The method of making the perindopril arginine affects the form of that compound and that of the properties of the compound itself, including its stability and usability of formulation. Its making can also involve unnecessary choices and difficulties.
158 Cytec submits that the heart of the invention disclosed in the patent application is the synthesis of a reaction product mixture that can be used to inhibit DSP scale in the Bayer process stream. Whilst claim 1 adopts the form of a method claim, in reality it is this synthesis that leads to a product mixture efficacious to inhibit DSP scale. The specification does not address the work required in plant testing or scale up for manufacturing, because that is not the invention.
159 Cytec submits that the best performing product mixture known to the inventors and Nalco at the time of the filing of the patent application was the H + GPS + E mixture (HGE product), identified in row 1 of Table 2, which was produced using a semi-batch synthesis method. Cytec submits that Nalco failed to disclose that method because it failed to disclose a number of specific details about the manner in which the best product mixture was produced. It submits that the information omitted from the patent application is important and defines the “personality” of the best candidate product mixture that the skilled addressee could take forward. It submits that as a consequence there is no certainty that the person skilled in the art can obtain a reaction product mixture as good as the best product mixture in the patent application achieved by Nalco. It submits that the missing parameters include information that Nalco’s own experts considered formed part of the best method. Cytec contends that the missing information could easily have been provided in the specification, as it would have been information that the inventors had to hand in performing the experiments reported in the patent application, a point demonstrated by the fact that WO 873, another Nalco patent, included within it information such as the molar ratios of reactants and dosages.
160 Nalco disputes Cytec’s characterisation of the invention. It submits that invention disclosed is the use of the product mixture containing the identified silane based small molecules on an industrial scale to treat aluminosilicate containing scale in a Bayer process. The invention does not include making the product mixture on a laboratory scale, and the parameters used in laboratory scale methods are not transferrable to an industrial scale. It submits that it has given the best method of performing the invention by disclosing the selection of hexane diamine as the amine (in Table 2) and the use of the semi-batch method so as to control the reaction temperature and avoid prolonged temperatures over 120°C, thereby increasing the yield of those small molecules.
161 Nalco submits that by focussing on the examples in the patent application Cytec misconstrues the invention and the purpose of the examples, which is simply to illustrate, by way of laboratory experiments, three aspects of the method: first, that the semi-batch method is preferred; secondly, that using different amines will give rise to different efficacies of DSP inhibition; and thirdly, to illustrate that hexane diamine is the most effective of those amines tested.
162 Nalco submits that the particular matters that Cytec contends were not disclosed in the patent application are ancillary matters relevant to laboratory scale testing of the examples and do not form part of the invention. It submits that the experiments cannot be understood to contain information about the best method on an industrial scale, save for establishing that a semi-batch method is the preferred method of controlling the temperature to maximise the small molecule yield, that using different amines with different sized carbon backbones will give rise to different efficacies and that hexane diamine is the most effective of those amines tested. It further submits that: (a) the application of a semi-batch method in an industrial context is routine and a matter of optimisation depending on the equipment in the plant and the manufacturer; and (b) the selection of the molar ratios of A, GPS and E reactants is also a matter of optimisation for each refinery, and that there is no evidence to suggest that Nalco had done that at the time of the filing of the complete specification. Nalco then addresses each of the particular matters said by Cytec to be absent from the patent application, and contends that no absence of best method is established.
163 I note that Cytec’s case as presented in its closing submissions does not conform precisely with the case as advanced in its statement of grounds and particulars, which identifies two alternative arguments in relation to the best method ground. However, Nalco made no complaint about this apparent change and expressed no difficulty in meeting the case as advanced in closing submissions. It is that argument that I consider below.
164 The advance in the art disclosed in the specification is the discovery that certain silane based small molecules have been found to be effective in treating aluminosilicate scale in a Bayer process stream (page 1 lines 4 – 7). The background to the invention describes the problems associated with DSP scale in the Bayer process and highlights the prior art solution that was based on the addition of polymer materials containing three alkyloxy groups bonded to one silicon atom (page 3 lines 8 – 10). That solution had problems with unwanted degrees of viscosity, which made handling and dispersion (miscibility) of the polymer through the Bayer liquor problematic (page 3 lines 16 – 18). The specification notes that it is an object to address or ameliorate these problems, which is said to have been done in several aspects of the invention.
165 Each aspect of the invention then described is prefaced with a reference to it being a method for the reduction of aluminosilicate containing scale in a Bayer process. There is no dispute that this process is conducted at an industrial, refinery level. The focus of each aspect of the invention is on the synthesis of the at least one small molecule comprising at least three components, described most generally by reference to the Markush diagram:

where R1, R2 and R3 are selected from an extremely broad group of constituents and are bonded to a nitrogen atom. Later aspects and embodiments narrow the selection.
166 The detailed description states that it was “discovered that dosing of various types of silane-based products can reduce the amount of DSP scale formed” (page 6 lines 21 – 22). The embodiments described in the detailed description again focus on the reaction product to be produced. To reinforce the point, the specification says that although some of the small molecules have been identified in the prior art in relation to other unrelated applications, “their effectiveness in reducing Bayer Process scale was wholly unexpected” because the prior art taught that only high weight polymers are effective (page 32 line 10 – page 33 line 17). The specification then sets out the three advantages said to be conferred by using small molecule based inhibitors as opposed to polymeric inhibitors (page 33 lines 18 – 26).
167 It is in this context that the specification sets out the two examples which are said to be presented “for the purposes of illustration and are not intended to limit the scope of the invention” (page 34 lines 3 – 5). I have set out much of the content of the examples in section 4.1 above.
168 Example 1 identifies a semi-batch method as being preferred for performing the synthesis of A, G and E when compared with a batch method (both of which were known to those skilled in the art).
169 The primer records that the general concept of “batch”, “batch-on-batch” and “semi-batch” methods is well known among chemists. In summary:
(1) A “batch” method is a method where all reactants are reacted together with one another at the same time.
(2) A “batch-on-batch” method is a method where all reactants are reacted together with one another, but the reaction is split into multiple “batch” reactions (e.g. 10% of the total amount of the reactants is reacted in a first “batch” reaction; then the next 10% of the total amount of the reactants is reacted in a second “batch” reaction, and so on). A “batch-on-batch” method may be a series of smaller scale batch processes, to reduce the scale of each reaction (e.g. for safety reasons in an industrial manufacturing process, or to manage the amount of heat produced by a reaction that is exothermic), with all of the reaction products ultimately combined.
(3) A “semi-batch method” is a method where all reactants eventually end up in the same reactor, but the order of addition (including the rate, timing, amounts and proportions of the different reactants present) is varied. The purpose of the variations in a “semi-batch method” is to control the reaction conditions during the course of the reaction, particularly where the reaction is exothermic.
170 The semi-batch method is said in the patent application to allow for a much better control over the overall reaction by adding only a portion of the G and E in a form of a slow feed to initiate the primary epoxide ring opening reaction followed by the slow continuous feeding of G and E (page 35 lines 1 – 4). The evidence indicates that the person skilled in the art would understand that A is added first before the feed of G and E is commenced. The semi-batch method is said to provide a better overall yield of the active compounds in the product, also avoiding undesired side reactions (page 35 lines 4 – 6). It is also said to reduce the amount of methanol that is generated and isolated during the reaction and to produce a reaction mixture with a lower viscosity (page 35 lines 12 – 20). Dr Power observes that Table 1 records that the reaction temperature of the semi-batch method (as opposed to the batch and batch-on-batch methods) was more controlled, with very little methane involved, which suggests that the methoxysilane groups remained mostly intact. The end product also did not show the same degree of coupling-related viscosity of the batches produced using the batch and batch-on-batch methods.
171 Example 2 is said to provide examples of the relative DSP scale inhibition of various A:G:E small molecules formed during the synthesis reaction “disclosed above”. Although Professor Easton in his written evidence was unsure which of the three methods described in Example 1 was used, by the hearing Cytec agreed that it was the semi-batch method. The example is of a laboratory test that involved adding a small amount of sodium silicate to a Bayer liquor to increase the super saturation of silica in solution, dosing the liquor with the scale inhibitor being tested, raising the temperature to 105°C (just below the boiling point of a Bayer liquor) and heating it for a few hours, and then cooling the samples and measuring the amount of DSP scale formed. The results illustrate that of the five amines tested (in conjunction with GPS and E) as the scale inhibitor, hexane diamine performed the best at high and low doses, the next best was 1-amino-2-propanol and the third best was ethylene diamine. These are the three amines claimed in claim 1.
172 Having regard to the disclosure of the specification and the claims, the embodiment which is described and around which the claims are drawn is the use of a product mixture containing small silane-based small molecules synthesised from the reaction of components A (either H, ED or AP), GPS and E, which mixture is to be added to Bayer process stream in an aluminosilicate scale inhibiting amount. The method is directed to the application of the synthesised product mixture in a Bayer process stream which is on an industrial scale. The invention described and claimed resides in the discovery that the use of small molecules of the type described and claimed can be useful in the inhibition of scale in the Bayer process. It is not an invention for a method of industrial (or indeed laboratory) methods of synthesis.
173 For the following reasons I am not persuaded that further provision of specific information going to the manner in which the semi-batch process outlined in example 1, and more particularly the details of the synthesis reaction used to achieve the HGE product in the laboratory in example 2, was necessary in order for Nalco to satisfy the best method requirement of s 40(2)(aa) of the Patents Act.
174 First, at a general level, it must be noted that examples 1 and 2 concern laboratory tests performed by Nalco. There is no suggestion that, by the time of the filing of the complete specification, Nalco had produced any industrial-scale batches of product mixtures that were suitable for use in accordance with the method of the claims. This provides an important distinction to the circumstances in Servier, where the evidence indicated that the inventors had developed a method of making something that did fall within the claims. The focus of the debate in the present case is one step removed from the invention claimed in the patent application, and concerns the extent to which the outcome of laboratory testing might or would be useful to the person skilled in the art if they wished to make something that could be applied within the method of the claims.
175 Secondly, examples 1 and 2 taught that the inventors had learned that the use of a semi-batch method of production was preferable, that using different amines would give rise to different efficacies of DSP inhibition and that the HGE product was the most effective of the mixtures that the inventors had tested. The semi-batch method is a standard process known in the field that, the specification notes, allows for better control of the overall reaction and the reaction temperature and provides a better overall yield of the active compounds in the products, also avoiding undesired side reactions that would result from using a batch process. In such a process, the temperature control may be achieved by adjusting the feed rate of the reactants as well as the cooling mechanisms used, which will vary from plant to plant depending on the available equipment. The specification provides information that controlling the temperature of the reaction is important in order to achieve the optimal yield of small molecules. Professor Easton considered that it also provides a number of temperature parameters for the reaction, being an initial temperature of 65-70°C to initiate the reaction and an optimal temperature range of between 82-93°C. It also teaches that a prolonged temperature above 120°C is to be avoided in order to avoid unwanted side reactions.
176 I accept that once that information has been provided, it would be a matter of routine for the skilled process engineer to design an industrial synthesis process according to the equipment available. Viewed in this light, Figure 2 in the patent application provides an indication of the preferred general approach that should be taken to synthesising the reaction product mixture. This, of course, is not a complete answer to an allegation of a failure to disclose the best method (see Servier at [135]) but it goes some way towards that outcome, depending on the materiality of facts held by the inventor not disclosed in the specification.
177 Thirdly, typically, a scale inhibiting amount of a composition of the type claimed in the patent application (and also the prior art Max HT product) would be dosed continuously into the Bayer stream at a rate of between 10-50 kg/hr, amounting to hundreds of tonnes each year per refinery. That is a radically different amount of the composition to that produced in a laboratory scale synthesis.
178 The evidence demonstrates that laboratory-scale methods of synthesis can only provide a rough starting point for the development of a method of industrial scale production of synthesised reaction mixtures in quantities necessary for industrial application. I am not persuaded that the provision of more granular information detailing the way in which the HGE product was made in the laboratory, including the various parameters of the semi-batch synthesis method used, would provide information of sufficient use to the person skilled in the art seeking to perform the invention. This is because the skilled addressee would, in any event, have to develop an industrial-scale production method of synthesis suitable for synthesising the small molecule product mixture for industrial application. As I have noted, there is no suggestion that Nalco had performed any industrial scale production of the reaction product mixture described.
179 Mr Bellwood gave evidence that to work the synthesis reaction on an industrial scale he would put the amine into the reactor, bring it up to the initiation temperature and then, very quickly, feed in an amount of the epoxide (that is, GPS and E) which he would have established was enough to “kick off” the reaction. He would then, once the temperate in the exotherm started to kick off, commence a continuous feed of GPS and E to maintain that reaction. At the same time he would also apply coolant to the vessel in order to extract the heat, the aim being to balance the heat generated by the exotherm with the heat extraction system of the reactor, to maintain as close to a constant temperature as possible. He would be “looking constantly to balance the rate of reaction through the feed rate of the G and E” and then continue that to completion when all of the reactants have been added.
180 Dr Power did not disagree with that approach. He said in response:
DR POWER: I don’t disagree; it’s one way of doing it, absolutely, but there’s any number of other ways, and the chemical engineering of it would be a whole thing in itself. You might want to use a series of continuous stir tank reactors if you were wanting to produce a lot of this stuff in a commodity way. It would be unlikely that if you were wanting to produce large quantities of it that you would use batch reactors. It probably is continuous to tank reactors, and then that would – and I also would say that, having all the A there at the start is one assumption about how the chemistry would work better. As I pointed out before, there’s other ways of doing that as well, so there’s any number of ways of doing this, and then the chemical engineering of it is a whole – another set of developments. So the patent doesn’t give you any guidance at all on any of that. This is just a way of producing a sample in the lab. That’s all it is.
(Emphasis added)
181 Dr Audet gave evidence to similar effect.
182 These factors persuasively illustrate that the parameters of the semi-batch synthesis reaction used to produce the best performing reaction product mixture in examples 1 and 2 of the patent application could not in any event simply be scaled-up for industrial scale production. The skilled industrial chemist must design the industrial scale reaction having regard to the plant equipment used, including its size and cooling capacity, which will affect the feed rate of the reactants necessary to maintain the temperatures that will provide an optimal yield. This is a balancing exercise. I accept that laboratory scale reactions such as those in example 1 can do little more than provide general guidance as to the preferred approach to be adopted on an industrial scale. In line with this, example 1 guides the skilled addressee to the optimal reaction conditions known to the applicant based on its laboratory testing. I am not satisfied that the supply of more specific details of the process used by the applicant in the examples would be of practical assistance to the person skilled in the art.
183 Fourthly, there are relevant differences between individual Bayer liquors such that the results provided in the examples are unlikely to have a material bearing on a final process of synthesis of the reaction product for use on an industrial scale. In this regard the following background matters may be noted:
(1) All Bayer liquors are aqueous solutions of sodium aluminate which inevitably contain varying amounts of inorganic impurities including sodium carbonate, sodium sulphate, sodium chloride, sodium silicate, and a complex mixture of organic impurities.
(2) There are significant variations between individual refineries in all of these parameters, particularly organic impurities.
(3) The amounts of suspended solids in Bayer liquors varies between Bayer liquors, including the levels of suspended solids in the form of red mud.
(4) The types of aluminosilicate scale differ between Bayer liquors and include sodalite, cancrinite and vishnevite.
(5) The tendency and type of aluminate scale to form at a particular refinery will depend on a wide variety of factors including the type of bauxite, which will in turn determine the process conditions.
(6) Variables such as these mean that some Bayer refineries perform digestion at a lower temperature range than others: where the bauxite is predominantly gibbsite, the temperature is at least 140-145°C, and where it is boehmite, the temperature is at least 250°C.
184 Mr Bellwood gave evidence that the composition of the Bayer liquor is something that has a significant bearing on the fine detail of the product mixture which is most efficient at a particular refinery. It is his experience that the composition of the reagent, relative to its chemical family, will need to be optimised in relation to the varying properties and composition of the Bayer liquor. He anticipated that in applying the method of the patent application, the molar ratios (i.e. the relative proportions) of reactants would need to be adjusted to optimise the composition for specific refineries. In this regard, whilst the overall chemistry approach is likely to be the same for all refineries, specific parameters in the synthesis, including molar ratios, are routinely and necessarily adjusted as part of the process to optimise for product performance. Dr Audet gave evidence to similar effect.
185 Dr Power agreed that varying the molar ratios of the reactants may cause the product mixture to be more or less effective at inhibiting DSP scale depending on the temperature of the Bayer liquor before it goes into the heat exchangers in a particular Bayer refinery and also the pH of the Bayer liquor.
186 Dr Power considered that the similarities between Bayer liquors, including high ionic strength, strong alkalinity, and high levels of alumina and silica, to be more important than their differences when it comes to the design of scale inhibitors. However, his evidence in cross-examination in relation to the Max HT product (and its subsequent iterations Max HT 500 and 550) served to demonstrate that: (1) the performance of Max HT varied across different Bayer liquors such that it was necessary to adjust its dosage from refinery to refinery to achieve scale inhibition efficacy; (2) Max HT was developed over time to become more tolerant to the temperature and pH of Bayer liquors, as well as to perform better in the presence of suspended solids, factors which vary between refineries; and (3) in principle, one option open to an additive manufacturer who wished to improve the efficacy of an additive would be to vary the molar ratio of the reactants to optimise it for a particular Bayer liquor. In my view, this evidence tends to support the position advanced by Nalco that the synthesis of the reaction product mixture will be informed by the particular characteristics of the Bayer liquor in which it is intended to be used. Certainly it serves to demonstrate that testing on one Bayer liquor in a laboratory could not be regarded as particularly determinative of the final form of a synthesised product mixture useful in any given industrial operation.
187 Furthermore, the product mixture produced from the reaction envisaged in claim 1 has the potential to include a greater or lesser amount of hydrophobic constituents. There is some uncertainty as to how those hydrophobes would interact with suspended organic solids in a particular Bayer liquor, either by adsorbing to those solids or instead interacting, as intended, with the silane groups of the aluminosilicate seed crystals. Depending on that interaction, the scale inhibiting aspect of the reaction mixture would be more or less effective. Dr Power accepted that it was a matter of speculation as to how that would be revealed in terms of efficacy. Professor Easton accepted that to determine the effect of a greater number of hydrophobes on a molecule against its tendency to adsorb to solids would require testing with the Bayer liquor in question. Such speculation serves to demonstrate the need for testing of any product mixture in the Bayer liquor in which it is intended to be used.
188 Fifthly, based on evidence given by Professor Easton, Cytec contended in closing submissions that the examples ought to have included, in relation to the semi-batch synthesis method used to produce the HGE product, the following details:
(1) the order and sequence of the addition of the reactants;
(2) the rate of addition (feed rate) and mixing of each of the reactants being added to the reaction;
(3) the amount and relative ratio of the reactants;
(4) the temperature controls used for the reaction; and
(5) the reaction time.
189 In dissecting the examples in this way, Cytec tends to distract attention from the correct approach. As noted, the invention disclosed and claimed is not to a method of industrial or laboratory synthesis of the identified small molecules but to the novel application of the reaction product mixture on an industrial scale in a Bayer process stream.
190 Sixthly, and further to the fifth point, the evidence adduced by Cytec does not serve to demonstrate that there is a relevant correlation between, on the one hand, laboratory test results of the type it alleges are omitted from the patent application and, on the other, industrial scale process development. Indeed, Professor Easton was not asked in his evidence in chief to address the utility of the information in the examples for a subsequent industrial application, which might have been more material to the present question. His evidence focussed upon whether he would be able to replicate the best results set out in the examples. It was upon this premise that the five omitted items of information now relied upon by Cytec are based. However, as I have noted, I am not satisfied that the laboratory experiments exemplified in the examples of the patent application would provide a useful proxy for industrial manufacture in any event.
191 Finally, I turn specifically to the five points made by Cytec set out above at [188].
192 The first of these (order and sequence of addition of reactants) is, as noted earlier, addressed in example 1. The evidence of the Bayer process experts was that in light of the disclosure in the specification, they would understand it to teach to add the amine to the reaction vessel first, then add a small amount of GPS + E to kick off the reaction, and then feed in GPS + E together to maintain the reaction. Although the language used in the example is not completely clear, I accept that this is how the person skilled in the art would understand the teaching.
193 In relation to the second (feed rate and mixing of reactants), the patent application discloses that using the semi-batch process is recommended as it enables the feed rate of the reactants to be controlled, which controls the exothermic reaction. For reasons I have explained in more detail above, the specific feed rate and mixing used for the HGE product is not material to the disclosure when it is applied on an industrial scale.
194 In relation to the third (the amount and relative ratio of the reactants), each of the Bayer process experts agreed that if seeking to implement the invention in an industrial setting they would commence with a 1:1:1 molar ratio of the reactants and test the reaction product mixture for efficacy. They would do this even if example 2 had provided specific or different molar ratios for the HGE product. I have noted above that Dr Audet and Mr Bellwood explained that they would do this because the molar ratios should be optimised for the particular Bayer liquor used in each refinery. I accept this evidence.
195 In relation to the fourth (the temperature controls used for the reaction), the patent application discloses: (a) that the epoxide ring opening reaction between the A reactant and the G and E reactants is initiated at a temperature of approximately 65-70°C; (b) prolonged exposure at high temperatures above 120°C is to be avoided so as to maximise the yield of the small molecules; (c) the temperature of the reaction must be controlled to avoid undesired side reactions and to provide a reaction mixture with lower viscosity (as exemplified by Table 1); (d) the semi-batch synthesis method is recommended as it enables the feed rate of the reactants to be controlled, limiting the rate of the exothermic reaction, and ensuring that the temperature is controlled within the optimal range of approximately 82-93°C (as exemplified by Figure 2).
196 In relation to the fifth (reaction time), the Bayer process experts agreed that the time it will take for the reaction to complete will differ depending on the amount of the reactants used, the temperature at which the reaction is performed, the production facility used, and the feed rates.
197 Having regard to the matters addressed above, in my view, Cytec has not demonstrated that the patent applicant has failed to disclose the best method known to it of performing the invention within s 40(2)(aa).
198 I should note that in cross-examination, Cytec suggested to Dr Audet that if he were to have been provided with a “recipe book” containing all of the details used to synthesise the HGE product as at the priority date, and he were asked by a commodity chemical company to develop the best possible Bayer process antiscalant product using the patent application, he would “take it without hesitation”, and indeed pay for it if he was short on time. Dr Audet accepted these propositions, qualifying his acceptance by pointing out that he would buy the recipe book if he wanted a “guaranteed solution”, because without the recipe book, he could not be so guaranteed.
199 Understandably, Cytec placed reliance on this evidence in its closing submissions. However, the difficulty with this exchange is that it appears to be inconsistent with Dr Audet’s more detailed and reasoned evidence that he would need to optimise the synthesis parameters of the reaction product mixture for industrial sized manufacture, as well as the individual Bayer liquor in each refinery in which the reaction product mixture is to be used. I do not think, by this evidence, Dr Audet intended to recant from his earlier views, and it was not suggested to him that he did. I take this evidence to indicate that such a recipe book could assist the skilled addressee in developing a reaction product mixture, on a laboratory scale, for the particular Bayer liquor used in example 2. However, this is not the invention claimed in the patent.
200 In a similar vein, Mr Bellwood accepted that whilst that is not an approach that he would take, having the recipe book would provide him with a “starting point” for the optimisation process that he would undertake in any event. Mr Bellwood accepted that having the recipe book would mean that he could make a reaction product mixture that had the scale reduction qualities of the HGE product. He also accepted that he could perform tests on other Bayer liquors to confirm the efficacy of the mixture in the liquor of those refineries. If satisfactory, he could take the product forward to plant testing and scaling up. By these answers Mr Bellwood accepted that having more information of this type could be useful. However, again, I am not satisfied that by this short passage of answers that Mr Bellwood should be understood to have retreated from the more reasoned explanations to which I have referred above about the distinction between the outcome of laboratory tests and the method ultimately likely to be used for industrial scale synthesis. Most scientists offered more information would accept it, but that fact does not of itself mean that there has been a failure to supply a best method.
201 Accordingly, the challenge advanced on the basis of s 40(2)(aa) of the Patents Act fails.
202 Cytec contends, as an alternative to its case based on the lack of clear and complete disclosure within s 40(2)(a) of the Patents Act, that WO 873 anticipates the claims of the patent application. It accepts that in order to succeed in this regard it must establish that:
(1) the “grace period” provision in s 24(1)(a) of the Patents Act does not apply;
(2) WO 873 anticipates the claims of the patent application; and
(3) WO 873 meets the requirements of the definition of “whole of contents” novelty, as set out in the definition of “prior art base”.
203 Cytec accepts that if it succeeds in its contention that the claims of the patent application lack sufficient description within s 40(2)(a) because of the presence of the words “at least one small molecule” in claim 1, then it cannot succeed in establishing the whole of contents requirements necessary under s 7(1)(c) of the Patents Act (because the notional claims proposed by Cytec also used the words “at least one small molecule”), with the consequence that its novelty argument must fail. That eventuality has arisen. Furthermore, for the reasons set out below, I conclude that the grace period provision does apply, with the consequence that the lack of novelty argument must fail on that basis also. As a consequence, I have not considered the disclosure of WO 873 or the “whole of contents” requirement (identified in (2) and (3) above) which Cytec would also have had to establish to succeed in its novelty challenge.
9.2 Does the “grace period” apply?
204 There is no dispute about the relevant facts underlying this argument:
(1) WO 873 claims a priority date of 25 September 2009;
(2) WO 873 was filed on 21 September 2010;
(3) WO 873 was published on 31 March 2011;
(4) The patent application claims a priority date of 25 February 2011; and
(5) The patent application was filed on 7 February 2012.
205 By s 18(1)(b)(i) of the Patents Act, an invention so far as claimed in any claim will be taken to be novel when compared with the prior art base as it existed before the priority date of that claim unless it is not novel in the light of any one of three kinds of information specified in s 7(1), each of which must be considered separately, being:
(a) prior art information (other than that mentioned in paragraph (c)) made publicly available in a single document or through doing a single act;
(b) prior art information (other than that mentioned in paragraph (c)) made publicly available in 2 or more related documents, or through doing 2 or more related acts, if the relationship between the documents or acts is such that a person skilled in the relevant art would treat them as a single source of that information;
(c) prior art information contained in a single specification of the kind mentioned in subparagraph (b)(ii) of the definition of prior art base in Schedule 1.
206 Sub-paragraph (b)(ii) of the dictionary definition in the Patents Act provides a definition of “prior art base” that is sometimes referred to as “whole of contents” novelty:
prior art base means:
...
(b) in relation to deciding whether an invention is or is not novel:
...
(ii) information contained in a published specification filed in respect of a complete application where:
(A) if the information is, or were to be, the subject of a claim of the specification, the claim has, or would have, a priority date earlier than that of the claim under consideration; and
(B) the specification was published on or after the priority date of the claim under consideration; and
(C) the information was contained in the specification on its filing date.
207 Cytec contends that WO 873 is a prior art document to which s 7(1)(c) applies on the basis that it has been published, that it contains information filed in respect of a complete specification that would have a priority date earlier than the claims of the patent application (within (A)), that the WO 873 specification was published on 31 March 2011 which was after the priority date of the application being 25 February 2011 (within (B)) and the information relied upon was in the specification of WO 873 on its filing date, being 21 September 2010 (within (C)).
208 There is no dispute that the relevant form of s 24 and regs 2.2(1A) and 2.3(1A) is as they stood prior to the amendments made in the RTB Act. This is because the relevant “information” (being the publication of WO 873) was made available before 15 April 2013: see RTB Act s 3 and schedule 6, items 32, 33 and 133(4); Intellectual Property Legislation Amendment (Raising the Bar) Regulation 2013 (No 1) (Cth) reg 4 and schedule 6 item 7; Regulations reg 23.36(4) item 3.
209 Section 24(1)(a) provided:
24 Validity not affected by certain publication or use
(1) For the purpose of deciding whether an invention is novel or involves an inventive step or an innovative step, the person making the decision must disregard:
(a) any information made publicly available, through any publication or use of the invention in the prescribed circumstances, by or with the consent of the nominated person or patentee, or the predecessor in title of the nominated person or patentee…
…
but only if a patent application for the invention is made within the prescribed period.
210 Regulation 2.2 was entitled “Publication or use: prescribed circumstances” and reg 2.2(1A) provided:
For paragraph 24 (1) (a) of the Act, the circumstance that there was a publication or use of the invention within 12 months before the filing date of the complete application, is a prescribed circumstance.
211 Regulation 2.3 was entitled “Publication or use: prescribed periods” and reg 2.3(1A) provided:
For information of the kind referred to in paragraph 24 (1) (a) of the Act, if the applicant relies on the circumstance in sub-regulation 2.2 (1A), the prescribed period is the period of 12 months after the information was first made publicly available.
212 Nalco submits that a straightforward application of s 24(1)(a) requires that WO 873 be disregarded from consideration as a prior art document because it is “information made publicly available, through....publication...in the prescribed circumstances, by or with the consent of the...patentee”. Nalco submits that WO 873 satisfies the “prescribed circumstances” because it is “a publication...within 12 months before the filing date of the complete application” in accordance with regs 2.2(1A) and 2.3(1A).
213 Cytec submits that the grace period afforded by s 24(1)(a) and the Regulations has no application because the words made publicly available in that section serve to limit its operation only to the types of prior art information identified in s 7(1)(a) and (b), but not s 7(1)(c). Cytec submits that ss 7(1) and 24(1)(a) must be read together, given that the latter operates to limit the scope of the former. The term “publicly available” is well understood to mean information being accessible to the public, yet documents available by reference to the whole of contents provision in s 7(1)(c) are not made “publicly available” or even accessible to the public, and s 24(1)(a) must be construed accordingly.
214 Cytec submits that its construction would result in a rule of general application for whole of contents publications which would reflect their special status as unpublished patent filings of the same patentee. It submits that the purpose of the grace period under s 24(1)(a) is generally intended to protect inventors who inadvertently disclose their invention before seeking protection and to encourage the sharing of research results between inventors, thereby allowing researchers and academics to publish and discuss results in journals and peer reviewed literature, without putting at risk any patentable subject matter that is disclosed. Furthermore, the legislature has separately provided in s 79B for inventors to be able to make multiple successive divisional applications in relation to an invention that is disclosed in an earlier filing. By providing that divisional applications are entitled to the same priority date as the parent application (reg 3.13D) the risk of self-anticipation is avoided by this mechanism, and abuse is also avoided by confining the later patent to the same term (ss 65, 67 and reg 6.3). Cytec submits that if s 24(1)(a) is construed as applying to documents within s 7(1)(c), this would make a simple filing strategy available to patentees who might, instead of filing a divisional application under s 79B, file a provisional application for a second, later in time, patent on the same date that the patentee files the complete specification for the first application. In so doing, the grace period would apply to the earlier filed documents and the second application will enjoy a patent term that is not tied to that of the first application, thereby benefitting from a de facto extension of term.
215 Cytec also contends that the Patents Act 1952 (Cth) provided no exemption for inventors who engaged in prior claiming, and the whole of contents provisions were introduced as an extension of that concept. Had the legislature intended to depart from the position that self-disclosure exemptions did not apply to the patentee’s own earlier patent filings then, Cytec submits, one might have expected that clear language in the legislative scheme would have made that clear. Further, Cytec submit that had this been the intention of Parliament, an express statement to that effect might have been made in the extrinsic materials.
216 Finally, Cytec submits that there is a difficulty construing s 24(1)(a) by reference to anything in the Regulations, or by reference to the decision of the Full Court in Mont Adventure Equipment Pty Ltd v Phoenix Leisure Group Pty Ltd [2009] FCAFC 84; 176 FCR 575 as the Court there was considering the proper construction of the Regulations and, in any event, s 24(1)(a) was enacted over a decade before the Regulations upon which reliance is now placed and it is difficult to see a logical means by which the latter can assist in the construction of the former.
217 Despite the care with which Cytec’s submissions were put, I am unable to accept them.
218 Section 24(1)(a) states, in plain terms, that for the purposes of deciding whether an invention is (relevantly) novel, the person making the decision must disregard “any information made publicly available, through any publication or use...” of the invention. That is to be understood by reference to the accepted meaning of “publicly available” and “publication”. The coincidence of the words “publicly available” in ss 7(1)(a) and (b) does not warrant a reading down of the meaning of those words in s 24(1)(a) as being applicable only to subsections 7(1)(a) and (b), and not to information that has been made publicly available as part of a disclosure that falls within s 7(1)(c). There is no doubt that information may be publicly available within the definition of prior art base identified by reference to s 7(1)(c).
219 So understood, s 24(1)(a) provides an exception to the prior art information that may be considered when assessing novelty for each of the kinds of prior art documents identified in s 7(1), regardless of whether they fall within subsections (a), (b) or (c). I am unable to glean from the language of s 24(1)(a), or the context in which it appears in the Patents Act, any means by which it may be said that Parliament intended that whole of contents documents be precluded from benefitting from the grace period provision.
220 By allowing for the Regulations to identify the prescribed circumstances and the prescribed period, it is apparent that Parliament intended for those circumstances and that period to be varied from time to time, all the while requiring that the information be of a type that has been made publicly available. In this context, it may be noted that reg 2.2(1A) provides that the relevant circumstance is the publication of the invention within 12 months of the filing date of the complete application. By tethering the prescribed circumstance to filing date, rather than the priority date, it is apparent that the legislature intended information of the type in s 7(1)(c) to be within the ambit of s 24(1)(a). That is because none of the prior art information referred to in ss 7(1)(a) or (b) is published after the priority date and before the filing date of the complete application. As Nalco submits, had the legislature intended only to exclude information of the kinds referred to in s 7(1)(a) and (b), then it could have used the priority date and not the filing date in the Regulations. Indeed, had Parliament intended to confine the operation of s 24(1)(a) to the kinds of information identified in ss 7(1)(a) and (b), then it could have done so in terms.
221 Whilst it may be accepted that one does not construe the language of s 24 by reference to the Regulations, it is apparent that the drafting of the Regulations has been done with the operative section in mind.
222 I do not accept that in so construing s 24(1)(a) the consequence is to thwart the aim to promote free discussion of inventions. Indeed, the construction that I have preferred no less promotes such free discussion than the construction propounded by Cytec.
223 Nor do I consider that the concern raised by Cytec – that patentees will, in effect, gain an additional year of the patent term by adopting the strategy to which Cytec refers in its submissions – is determinative. That submission somewhat oversimplifies the position. For instance, by filing the patent application a year later and not availing itself of divisional status under s 79B, the patent application loses the benefit of the earlier priority date, with the consequent adjustment of the common general knowledge and exposure to other prior art information.
224 Accordingly, in my view, the grace period provision applies to WO 873. There is no dispute that the result is that WO 873 is not information that may be taken into account in assessing novelty.
225 Accordingly, Cytec fails in its novelty challenge to the patent application.
226 For the reasons set out in more detail above, I have found that the grounds of opposition to the grant of all of the claims of the patent application based on lack of support within s 40(3) and lack of clear enough and complete enough disclosure within s 40(2)(a) succeed, but that the grounds based on lack of novelty within s 7(1) and failure to disclose the best method within s 40(2)(aa) fail. My preliminary view is that Cytec should have 70% of its costs of the proceedings, which allows for its success in the appeal, but also for its failure in respect of some of the grounds of opposition.
227 The parties should confer and supply to my chambers, within 28 days, short minutes of orders giving effect to these reasons. Insofar as they are unable to agree to the terms of the orders (including as to costs), the areas of disagreement should be set out in mark-up. The proceedings will be listed for case management at 9.30am on 13 October 2021 to determine what further steps, if any, are required.
I certify that the preceding two hundred and twenty-seven (227) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Burley. |
Associate: