FEDERAL COURT OF AUSTRALIA
Hood v Bush Pharmacy Pty Ltd [2020] FCA 1686
Table of Corrections | |
Para [283] fourth sentence delete the words “the bottles supplied by Ms Levinson and Heritage Oils with” | |
Para [308] second sentence delete the word “not” | |
Para [344] second sentence replace the word “application” with the word “applicable” | |
Para [388] fourth sentence replace the words “cannot assume or infer” with the words “cannot be assumed or inferred that” | |
ORDERS
Applicant | ||
AND: | BUSH PHARMACY PTY LTD (ACN 149 740 741) Respondent | |
AND BETWEEN: | BUSH PHARMACY PTY LTD (ACN 149 740 741) Cross-Claimant | |
AND: | Cross-Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The applicant file and serve a proposed minute of order within 7 days.
2. The proceeding be listed for any argument in relation to the form of the orders (including in relation to costs) at a date to be fixed.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ORDERS
NSD 1271 of 2017 | ||
| ||
BETWEEN: | JOHN JAMES DAVID HOOD Applicant | |
AND: | DOWN UNDER ENTERPRISES INTERNATIONAL PTY LIMITED (ACN 127 755 971) Respondent | |
AND BETWEEN: | DOWN UNDER ENTERPRISES INTERNATIONAL PTY LIMITED (ACN 127 755 971) Cross-Claimant | |
AND: | JOHN JAMES DAVID HOOD Cross-Respondent | |
JUDGE: | NICHOLAS J |
DATE OF ORDER: | 23 November 2020 |
THE COURT ORDERS THAT:
1. The applicant file and serve a proposed minute of order within 7 days.
2. The proceeding be listed for any argument in relation to the form of the orders (including in relation to costs) at a date to be fixed.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ORDERS
NSD 1272 of 2017 | ||
| ||
BETWEEN: | JOHN JAMES DAVID HOOD Applicant | |
AND: | NEW DIRECTIONS AUSTRALIA PTY. LIMITED (ACN 052 973 743) Respondent | |
AND BETWEEN: | NEW DIRECTIONS AUSTRALIA PTY. LIMITED (ACN 052 973 743) Cross-Claimant | |
AND: | JOHN JAMES DAVID HOOD Cross-Respondent | |
JUDGE: | NICHOLAS J |
DATE OF ORDER: | 23 november 2020 |
THE COURT ORDERS THAT:
1. The applicant file and serve a proposed minute of order within 7 days.
2. The proceeding be listed for any argument in relation to the form of the orders (including in relation to costs) at a date to be fixed.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ORDERS
NSD 2175 of 2017 | ||
| ||
BETWEEN: | JOHN JAMES DAVID HOOD Applicant | |
AND: | NATIVE OILS AUSTRALIA PTY LTD (ACN 154 612 487) Respondent | |
AND BETWEEN: | NATIVE OILS AUSTRALIA PTY LTD (ACN 154 612 487) Cross-Claimant | |
AND: | JOHN JAMES DAVID HOOD Cross-Respondent | |
JUDGE: | NICHOLAS J |
DATE OF ORDER: | 23 november 2020 |
THE COURT ORDERS THAT:
1. The applicant file and serve a proposed minute of order within 7 days.
2. The proceeding be listed for any argument in relation to the form of the orders (including in relation to costs) at a date to be fixed.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ORDERS
NSD 2176 of 2017 | ||
| ||
BETWEEN: | JOHN JAMES DAVID HOOD Applicant | |
AND: | HERITAGE OILS PTY LTD (612 556 626) First Respondent JULIA GAY LEVINSON Second Respondent | |
AND BETWEEN: | HERITAGE OILS PTY LTD (612 556 626) Cross-Claimant | |
AND: | JOHN JAMES DAVID HOOD Cross-Respondent | |
JUDGE: | NICHOLAS J |
DATE OF ORDER: | 23 november 2020 |
THE COURT ORDERS THAT:
1. The applicant file and serve a proposed minute of order within 7 days.
2. The proceeding be listed for any argument in relation to the form of the orders (including in relation to costs) at a date to be fixed.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
[1] | |
[9] | |
[9] | |
[15] | |
Down Under Enterprises International Pty Limited (NSD 1271 of 2017) | [17] |
[18] | |
[19] | |
Heritage Oils Pty Ltd / Julia Gay Levinson (NSD 2176 of 2017) | [20] |
[22] | |
[24] | |
[36] | |
[36] | |
[36] | |
[41] | |
[45] | |
[48] | |
[50] | |
[51] | |
[51] | |
[55] | |
[58] | |
[59] | |
[59] | |
[62] | |
[74] | |
[82] | |
[82] | |
[84] | |
[87] | |
[114] | |
[119] | |
[154] | |
Is Kunzea ambigua essential oil a staple commercial product? | [174] |
[190] | |
[207] | |
[224] | |
[255] | |
[270] | |
[281] | |
[302] | |
[312] | |
[322] | |
[332] | |
[338] | |
[347] | |
[360] | |
[367] | |
[374] | |
[384] | |
[391] |
NICHOLAS J:
1 Before me are five proceedings for patent infringement, trade mark infringement and contravention of s 18 and s 29 of the Australian Consumer Law (“ACL”). The trade mark case against all respondents was abandoned by the applicant during the course of the hearing.
2 The patent in suit (Standard Patent No 721156) expired on 20 October 2017 almost a year before the hearing of the proceedings commenced. It is common ground that the priority date of the claims is 23 October 1996 (“the Priority Date”) based on a provisional application filed on that date.
3 In its broadest form, the invention, as defined in claim 1, is “[a]n essential oil derived from shrubs of the genus Kunzea”. The claims include various other product claims (claims 2-4) together with various method of treatment claims (claims 5-13) that use such an oil.
4 The validity of the claims of the patent on which the respondents are sued is challenged by the respondents who seek orders revoking all such claims. The applicant now accepts that the product claims 1-4 (inclusive) are invalid.
5 The respondents say that none of the method of treatment claims are valid and, if they are, then they have not been infringed. The infringement case is based solely on s 117 of the Patents Act 1990 (Cth) (“the Act”).
6 The applicant claims pecuniary relief for infringements of the patent that are alleged to have occurred during the six year period prior to the commencement of each proceeding and up to the expiry date. He also claims additional damages under s 122(1A) of the Act.
7 The trial of the proceedings was confined to questions of liability and any entitlement on the part of the applicant to additional damages (but not the quantum thereof) with all questions concerning the quantification of any pecuniary relief being deferred for consideration at a later date.
8 Also before me is an application by the applicant to amend the claims of the patent.
9 Mr Hood, the applicant, is the named inventor and the patentee. He made a number of affidavits and was cross-examined. He describes himself as a farmer and an inventor.
10 Mr Hood purchased a farm in 1986 which was called the “DuCane Estate”. The farm is approximately 400 hectares in size and is situated in North East Tasmania, around 34 kilometres east of Bridport. In 1990 Mr Hood went to Western Australia to work in a gold refinery. He returned to Tasmania in December 1990. He said that it was at this time that he first paid attention to the native shrubs growing on his property including Kunzea ambigua.
11 Mr Hood did not know the name Kunzea ambigua until sometime in 1993 or 1994 when he asked a botanist at the Queen Victoria Museum in Launceston to identify a sample of the shrub from his farm. The botanist, Ms Mary Cameron, identified the shrub as Kunzea ambigua. She also identified a number of other shrubs, Melaleuca squarrosa (lemon scented tea tree), Melaleuca ericifolia (swamp paperbark, or lavender tea tree), and Leptospermum lanigerum (woolly tea tree), which were also growing on Mr Hood’s property at the time.
12 Mr Hood learnt about Kunzea ambigua from various botanical books which he borrowed from friends in the Launceston area and from conversations with them, including Mr Peter Duckworth, a retired forester. From the reading and the conversations, Mr Hood came to understand that it was the German botanist, Gustav Kunze, that discovered the genus Kunzea and it was given its botanical name after him in 1851 or thereabouts. From this same research, Mr Hood came to understand that it was George Claridge Druce who identified Kunzea ambigua in 1917.
13 In March 1995 Mr Hood sent samples of Kunzea ambigua, Melaleuca squarrosa and Melaleuca ericifolia to Professor Bob Menary at the University of Tasmania (“UTAS”). According to Mr Hood, he asked Professor Menary to see if any of the shrubs he had provided contained oils that might have a commercial use. He later received a telephone call from one of Professor Menary’s assistants who told Mr Hood that they “… had identified 23.3% 1,8-cineole in the Kunzea ambigua shrub, young regrowth and 15.3% 1,8-cineole in mature regrowth (being shrubs of 2-3 feet in height)”. The assistant also said that “the highest percentage of all the shrubs that [Mr Hood] provided to the University of Tasmania was 39.9% 1,8-cineole was from Melaleuca squarrosa and next was Melaleuca ericifolia with 39%”.
14 It was not until 23 October 1996 that Mr Hood filed his provisional specification. The complete specification was filed on 20 October 1997 and was first open to public inspection on 15 May 1998. The obvious errors in the claims to which I refer later in these reasons all appear in the complete specification as filed on 20 October 1997.
Bush Pharmacy Pty Ltd (NSD 1267 of 2017)
15 Bush Pharmacy Pty Ltd (“Bush Pharmacy”) is a company that operates a farm on Flinders Island, Tasmania, where it harvests wild growing flora which it distils into essential oils. The essential oils that Bush Pharmacy has extracted and sold include those derived from Kunzea ambigua.
16 Bush Pharmacy sells Kunzea ambigua essential oil to commercial customers in Australia and overseas and has done so since in or about April 2016. It does not supply Kunzea ambigua to consumers. Mr Stephen Backhaus, a director of Bush Pharmacy, made two affidavits and was cross-examined.
Down Under Enterprises International Pty Limited (NSD 1271 of 2017)
17 Down Under Enterprises International Pty Ltd (“Down Under”) was established by Dee-Ann Seccombe Prather and her husband, Phillip Prather, in or about September 2007. Between June 2016 and January 2017 Down Under sold to various customers located outside Australia 1kg and 2kg packets containing Kunzea ambigua essential oil. Ms Prather, who is the sole director of Down Under, made two affidavits and was cross-examined.
New Directions Australia Pty Limited (NSD 1272 of 2017)
18 New Directions Australia Pty Ltd (“New Directions”) is a wholesaler of natural skin products and natural raw materials including essential oils. New Directions has sold Kunzea ambigua essential oils since about 2004. The majority of New Directions’ customers are commercial customers. Mr Domenic Ardino is a director of New Directions. He made two affidavits and was cross-examined.
Native Oils Australia Pty Ltd (NSD 2175 of 2017)
19 Native Oils Australia Pty Ltd (“Native Oils”) was incorporated in 2011, but did not commence trading until 2013. Mr David Johnson, the Managing Director of Native Oils, made two affidavits, and was cross-examined. He gave evidence concerning sales of Kunzea ambigua essential oil made by Native Oils the first of which occurred in or about April 2014.
Heritage Oils Pty Ltd / Julia Gay Levinson (NSD 2176 of 2017)
20 Heritage Oils Pty Ltd (“Heritage Oils”) sold bottles of Kunzea ambigua essential oils and various other products that contained Kunzea ambigua essential oil, in the period 27 December 2012 to 18 May 2017. In that period, approximately 36.7 kgs of Kunzea ambigua was sold by Heritage Oils to customers in Australia. Ms Julia Gay Levinson, who is also a respondent in the proceeding against Heritage Oils, is a director of that company. She previously carried on business as a sole trader under the name Heritage Oils until the company took over her business. The claim against her relates to trading activities carried on by her prior to that time. Ms Levinson made two affidavits and was also cross-examined. Another director of Heritage Oils, Mr David Brocklehurst, also gave evidence for that company. He made one affidavit and was cross-examined.
21 At the hearing Mr Hood was represented by Mr M Green SC, who appeared with Ms B Oliak, Dr G O’Shea and Mr B Cameron. Mr A Fox, with Mr S Hallahan, appeared for Bush Pharmacy, Down Under and New Directions. Mr S Reuben and Ms J Whitaker appeared for Native Oils, Heritage Oils and Ms Levinson.
The Therapeutic Goods Act LISTING
22 Mr Hood caused “DUCANE KUNZEA OIL Kunzea ambigua” in 1mL/ml liquid multipurpose bottles to be listed on the Australian Register of Therapeutic Goods (“ARTG”) maintained pursuant to s 9A of the Therapeutic Goods Act 1989 (Cth) (“the TG Act”). This listing, numbered 72143, and effective from 3 July 2002, is for the following standard indications:
Relief of the symptoms of influenza/flu.
Temporary relief of the pain of arthritis. (or) Temporary relief of arthritic pain.
Helps relieve nervous tension, stress and mild anxiety.
Relief of muscular aches and pains.
Temporary relief of the pain of rheumatism. [or] Temporary relief of rheumatic pain.
23 According to the public summary of the registration, the active ingredient of the medicine is Kunzea ambigua 1mL/mL. The dosage form is shown as “Liquid-multipurpose” and the route of administration is shown as “Inhalation”.
24 The patent is entitled “Essential oil and methods of use”.
25 The specification acknowledges that essential oils have been used for medicinal purposes for many hundreds of years. It also acknowledges that essential oils have been extracted in a number of different ways, most often by steam distillation.
26 The specification states that the object of the invention is to provide “a new essential oil which has beneficial properties”. The invention “in its broader sense” is said to include an essential oil derived from plants of the genus “Kunzea”. According to the specification:
More specifically, it relates to the essential oil derived from the shrub Kunzea ambigua. The essential oil is adapted for treatment of ailments of the human body, and is applied topically to relieve pain, minimize bruising and to assist in healing, and may be used either pure or in a carrier.
…
The shrub from which the oil is obtained, a member of the Myrtaceae family, genus Kunzea, species ambigua.
27 The specification does not suggest that Mr Hood was the first to “discover” Kunzea ambigua. As is apparent from the passage just quoted, at the time the specification (and the provisional specification) was prepared Kunzea ambigua was a known plant species that had already been classified as a member of a known family (the Myrtaceae family) and a known genus (the Kunzea genus). This is also borne out by Mr Hood’s dealings with Ms Cameron, the botanist, who identified for Mr Hood the sample of the plant obtained by him from his farm. It is also borne out by other evidence to which I will refer later in these reasons.
28 The specification describes the area in Tasmania in which Kunzea ambigua is found and the manner in which it may be harvested. This is followed by a brief description of a known process that may be used to extract the essential oil from the plant.
29 The specification also includes a discussion of the chemical composition of the oil obtained from Kunzea ambigua based on gas chromatograph analysis with reference to examples. The specification states:
The attached examples show the results of gas chromatograph analysis of the oil made from the shrub, and it will be seen that its principal components in each example are mono- and sesqui-terpenes.
Considering Example 1, the largest components are α pinene, 50.52 per cent, 1-8-cineole, 14.08 per cent, and sesquiterpene alcohols, globulol, 6.81 per cent, and viridiflorol, 5.96 per cent.
In Example 2, again the largest components are α pinene, 41.5 per cent, 1-8-cineole, 12.1 per cent and in this case, there are identified 3 sesquiterpene alcohols which together are 25.5 per cent.
Whilst other analyses of the oils have varied in absolute percentage, the relative percentages of the various components are very similar, with a preponderance of the above components.
30 A comparison of Example 1 and Example 2 (set out below) shows that there are some significant differences in the composition of the different samples. However, what both Examples have in common is that their largest components are alpha-pinene and 1-8-cineole which are present in quantities of about 40-50% and 12-14% respectively. The results of the gas chromatograph analysis as set out in the specification are as follows:
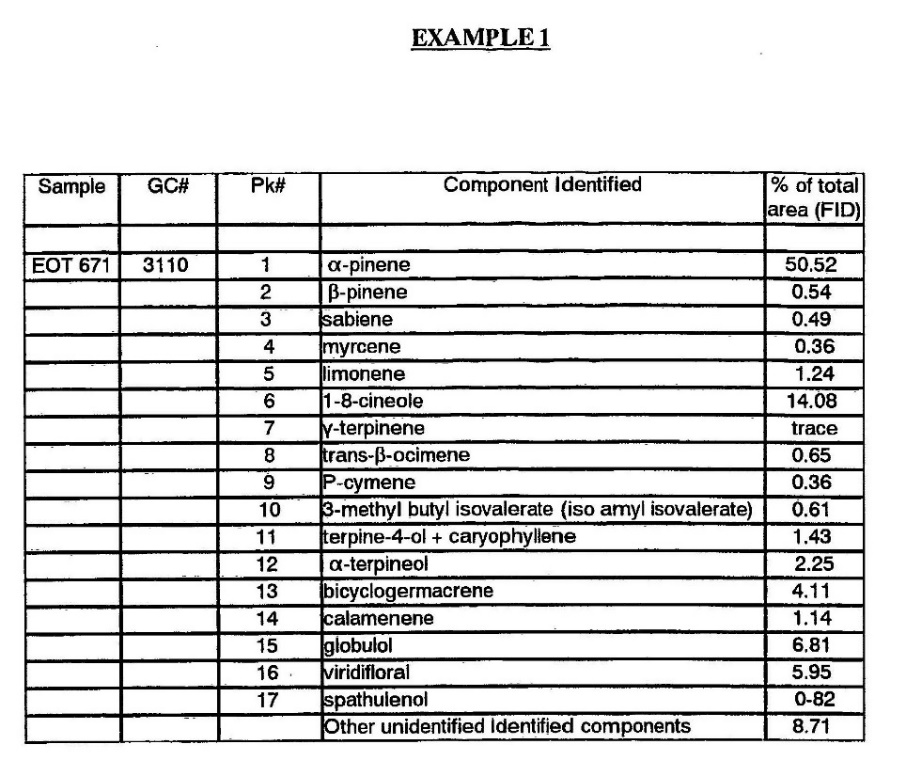
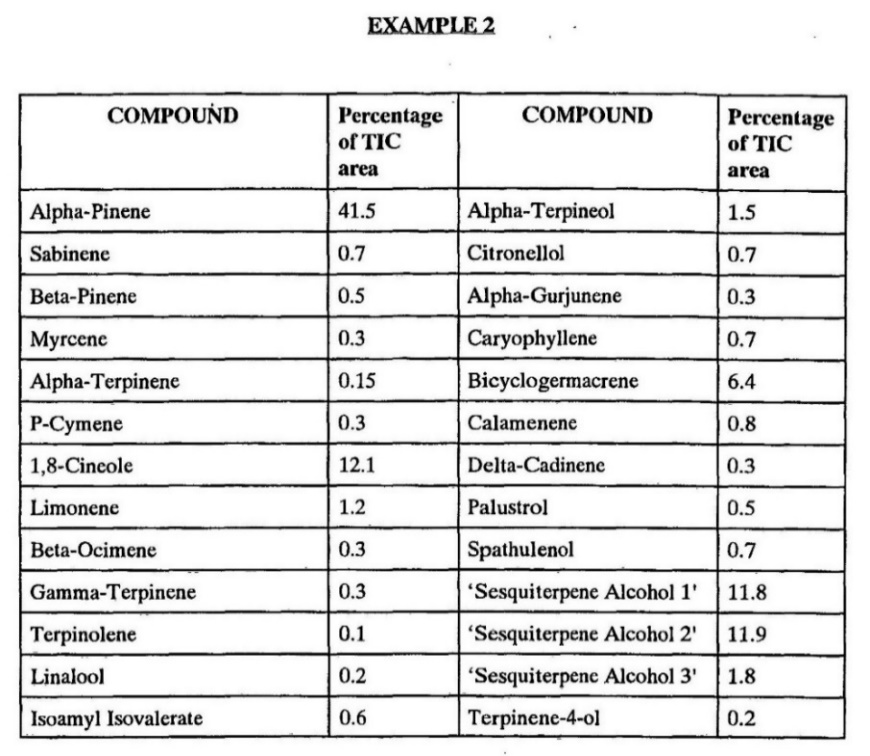
31 The specification also includes a discussion of the “trials” that Mr Hood conducted. Mr Hood does not purport to have conducted any scientific tests (for example, any form of randomised trial) and the evidence referred to in the specification is essentially anecdotal. According to the specification:
I have had trials done with the oil in a number of therapeutic applications, and although full trials has not been completed, I have found, qualitatively, that the oil has the ability to reduce pain caused by muscle and tendon strain and impact trauma, and it has also been found to reduce pain from gout, headache and bites, particularly insect and spider bites.
It has also had positive results in the treatment of rashes, skin irritations and acne.
It also seems to provide a positive result in relieving sinus congestion and as a result of this, aids in the return of the senses of smell and taste.
It also appears to have an ability to prevent the progress of cold sores, although to date the results in treating genital herpes have not been so positive.
One area which has provided a surprising reaction is in the treatment of bruising.
If applied to the area after the trauma and before bruising has started or is only incipient, I have found that it is almost completely ameliorated.
The oil appears to have no strongly adverse reaction with the skin, although as with all essential oils, people with sensitive skin should be careful before they apply it at full strength, but it can readily be applied, diluted in a carrier oil, preferably a pure vegetable oil, a lotion which can be an aqueous suspension, or an ointment, which can often be a petroleum emulsifying ointment base.
Where the Kunzea ambigua oil is applied, either pure or in a carrier oil or a lotion, the oil seems to pass through the skin relatively quickly, so no doubt it has a molecular size to enable this to occur, although to date I have not studied the size of the molecule.
…
Apart from its pharmaceutical benefits, the oil can be used in other ways.
32 Pausing there, it can be seen that the specification discloses that the oil may be applied topically either in undiluted form or using a carrier, preferably a pure vegetable oil, a lotion, or an ointment. While the specification clearly contemplates that the oil may be applied in these ways, it is also clear that the function of the carrier is to facilitate the topical application of the Kunzea ambigua oil in diluted form. In my view the specification is not in this passage contemplating the use of the Kunzea oil as part of a blend of different essential oils (except in so far as any other oil acts as a carrier). Nor do I understand this passage to be suggesting the use of Kunzea oil, or Kunzea ambigua oil, in blended products that include other active ingredients, the function of which is to cleanse or hydrate the body.
33 The specification also states that the oil may be used as “an oil disseminator”, “a very good rust inhibitor”, and a timber polish. The specification then continues as follows:
Whilst I have described the oil itself, its method of production and various uses, it is to be understood that these may be developed as the use of the oil continues.
Whilst in the above embodiment, I have described the use of the oil derived from the shrub Kunzea ambigua, it is believed that the oils derived from other plants of the genus Kunzea may have similar properties.
34 There are 18 claims. Claims 1-4 and claims 16-17 are product claims. Claims 5-13 are method of treatment claims. There is also a claim for the use of the essential oil as an insect repellent (claim 14) and as a rust inhibitor (claim 15). Claims 16 and 18 are omnibus claims and claim 17 is a claim based on the Examples.
35 The claims of the patent are as follows:
1. An essential oil derived from shrubs of the genus Kunzea.
2. The essential oil as claimed in claim 1 which oil is derived from the shrub Kunzea ambigua.
3. The essential oil of claim 1 in which the oil is obtained from the distillation of the green matter of the shrub.
4. The essential oil of claim 2 wherein the distillation is by steam distillation.
5. A method of treatment in which the essential oil as claimed in any one of claims 1 to 4 is applied topically as a treatment to relieve pain, minimize bruising, or to assist in healing.
6. A method of treatment as claimed in claim 4 wherein the pain relieved is pain from muscle and tendon strain, impact trauma, gout, headaches and insect and other bites.
7. A method of treatment as claimed in claim 4 wherein the use of the essential oil relieves sinus congestion.
8. A method of treatment as claimed in claim 4 wherein the essential oil limits the progress of cold sores, dries up dermatitis and aids in the healing of contusions.
9. A method of treatment as claimed in claim 4 wherein the essential oil assists in treatment of rashes, skin irritations and acne.
10. A method as claimed in any of claims 4 to 7 wherein the essential oil is applied in a carrier.
11. A method as claimed in claim 8 wherein the carrier is a vegetable oil.
12. A method as claimed in claim 8 wherein the carrier is a lotion.
13. A method as claimed in claim 8 wherein the carrier is an ointment.
14. The essential oil of any one of claims 1 to 3 wherein the oil is used as an insect repellent.
15. The essential oil of any one of claims 1 to 3 wherein the oil is used as a rust inhibitor.
16. An essential oil substantially as hereinbefore described.
17. An essential oil having an analysis substantially as described in relation to the Examples.
18. The use of the essential oil as claimed in any one of claims 1 to 4 and 15, substantially as hereinbefore described.
The Applicant’s expert witnesses
36 Professor Robert Menary OAM is Emeritus Professor in the Tasmanian Institute of Agriculture at UTAS. He has undertaken research and teaching in the field of horticultural science over a long career commencing in 1967 when he was first employed as a Senior Lecturer at UTAS. He has published extensively and supervised a large number of PhD students. He also supervised the Master’s thesis (discussed later in these reasons) of Valerie Dragar (now Dr Dragar) as part of her work toward the degree of Master of Agricultural Science.
37 Professor Menary gave evidence of his dealings with Mr Hood in the mid-1990’s which broadly corroborated Mr Hood’s evidence. According to Professor Menary, he provided the sample Kunzea ambigua oil from Mr Hood to Dr Davies who performed a gas chromatographic analysis, the results of which were then provided to Mr Hood. The evidence indicates that this type of analysis was routine and was typically employed at the Priority Date to ascertain the composition of oil extracted from plant matter.
38 Professor Menary said that he did not have any particular knowledge or experience in relation to therapeutic uses or qualities of essential oils and, on that basis, did not comment on any aspects of the patent (including claims 5 to 13) which related to the therapeutic uses for Kunzea ambigua oil. His knowledge and experience focused on the flavours and fragrances of essential oils rather than their therapeutic uses.
39 One matter upon which Professor Menary gave evidence was that relating to the ascertainability of two documents that were relied upon by the respondents and which were said to contain information made relevant pursuant to s 7(3) of the Act. The respondents abandoned reliance upon those documents as s 7(3) information in closing submissions.
40 Professor Menary provided a written report which includes a response to Dr Carson’s affidavit evidence. However, I did not find this response of much assistance due to its lack of specificity. In particular, it failed to engage with Dr Carson’s evidence as to what was commonly and generally known by people working in the field of essential oils as at the Priority Date. I believe this most likely reflects the fact that Professor Menary’s knowledge and experience was, as I have said, focused on fragrances and flavours of essential oils.
41 Dr Narkowicz is an academic in the School of Medicine at UTAS. He teaches and performs research, primarily in the area of pharmaceutical product evaluation. He obtained a Bachelor of Science (Hons) in 1985 majoring in chemistry. His Honours thesis was on Tasmanian marine natural products, including terpendoids from soft corals. In 2003 he was awarded a Doctor of Philosophy which focused on the isolation and characterisation of marine natural products with anti-parasitic activity.
42 He has since led investigations at the School of Pharmacy at UTAS on Tasmanian natural products with potential pharmaceutical applications. These included two formulations containing Kunzea oil that were developed by a Tasmanian pharmacist from Launceston for treating psoriasis and greasy heel in horses.
43 With respect to Kunzea oil, Dr Narkowicz has led investigations into the constituents of Kunzea oil and variations in Kunzea oil constituents. In particular, he has researched the antibacterial and antifungal activity of Kunzea oil and different fractions of Kunzea oil. He has conducted clinical trials of formulations containing Kunzea oil for psoriasis in humans, greasy heel in horses and onychomycosis (toenail infection) in humans. He was part of a team that successfully obtained a RIRDC grant for the onychomycosis study. Mr Narkowicz has also lead projects investigating other essential oils.
44 Since 2009, Dr Narkowicz has been the Director and CEO of Xderma Pty Ltd. Xdera was created to commercialise Greasy Heel KO, a pharmaceutical product containing Kunzea oil for use on horses. This product was released into the market in 2010 under a minor use permit. Full registration for the product was sought but ultimately refused. The company is currently pursuing the commercialisation of other topical skin care products. He is also associated with a company that has obtained a TGA listing for a product that incorporates Kunzea ambigua oil into a product which “may assist with the management of toenail fungal infections such as onychomycosis”.
45 Dr Southwell is a plant research chemist. He is an Adjunct Professor of Plant Science at Southern Cross University. He was awarded a Bachelor of Science (Hons) from the University of Sydney in 1967. He subsequently completed a Masters of Science in 1972 and was then awarded a Doctor of Philosophy in 1982 from the University of Manchester. He then returned to Australia and took up a position as Head of the Essential Oil Research Unit at the Wollongbar Agricultural Institute and progressed to Senior Research Scientist in 1984 and Principal Research Scientist in 1991. As a plant research chemist, he conducted research into new, unusual and commercial secondary metabolites present in flora. Given the commercial importance of tea tree and eucalyptus, he has performed a significant amount of work using these oils.
46 Since 1995, Dr Southwell has been a Committee Member of Standards Australia’s CH/21 Essential Oil Committee. From around the same time, he has been a delegate to the International Standards Organisation’s TC54 Essential Oil Committee. In both committees, Dr Southwell has participated in the development of new standards and voted on changes to existing standards. The standards developed by the TC54 Committee are used by oil traders to establish acceptable baselines for the quality and authenticity of various traded essential oils. Through this work, Dr Southwell is familiar with the specifications of significant commercial essential oils.
47 In 2005 Dr Southwell set up his own consulting company called Phytoquest which advises on the chemistry of volatile constituents including essential oils and insect and plant chemistry ecology.
48 Mr Archer was an executive with the Australian Therapeutic Goods Administration. He has over 17 years’ experience at a senior level. He began as Head of the OTC Medicines Section and then took up a variety of other positions including Secretary of the Medicines Evaluation Committee and Secretary of the Therapeutics Goods Committee. He is now the Director of Archer Emergy & Associates which is an independent consulting group providing advice and assistance to companies that import, manufacture or sell medicines, medical devices, cosmetics and foods in Australia and New Zealand. Mr Archer was not cross-examined.
49 Large parts of the expert report made by Mr Archer which was tendered by the applicant were rejected on the basis that they constituted expressions of opinion about the meaning and effect of various statutory provisions or were irrelevant to any issue in the case.
50 As I have mentioned, Mr Hood gave evidence and was cross-examined. His solicitor, Mr Stephen Sharpe, also gave evidence, but was not cross-examined. Mr Sharpe’s evidence was mostly directed to proof of documents relating to the prosecution history of the patent and included the file obtained from the patent attorney firm A Tatlock & Associates (which acted for Mr Hood in connection with his patent application), various search results, trap purchases, webpages and letters of demand, some of which are documents I will make reference to later in these reasons.
The Respondents’ expert witnesses
51 Dr Carson is a Research Associate in the School of Biomedical Sciences at the University of Western Australia. She is a microbiologist. She completed a Bachelor of Science (Honours) at the University of Western Australia in 1989 and which was conferred in 1990. After completing that degree, Dr Carson commenced working towards a PhD focused on antimicrobial agents derived from plants which she completed in 2001. The title of her PhD thesis was “Antimicrobial activity of Melaleuca alternifolia Oil”.
52 Melaleuca alternifolia is one of a number of plants which have commonly been referred to as tea tree. The essential oil derived from Melaleuca alternifolia is commonly referred to as tea tree oil.
53 In 1994, Dr Carson commenced working as a member of a research group funded by what was then known as the Rural Industries Research and Development Corporation in relation to the study of the antimicrobial activity of tea tree oil. Her research work during the period from 1994 to 2014 was primarily in relation to tea tree oil. Dr Carson completed her PhD research (which she completed on a part-time basis) in 2001. In 1993, Dr Carson published a review paper co-written with Professor Thomas Riley entitled “Antimicrobial activity of the essential oil of Melaleuca alternifolia” in Letters in Applied Microbiology, 1993.
54 Dr Carson was an impressive witness. I consider she is well qualified by reason of her training and experience to give evidence as to the content of the common general knowledge as at the Priority Date.
55 Dr Clark is an ethnobotanist and anthropologist. In 1982 he completed a Bachelor of Science at the University of Adelaide majoring in botany and zoology. As part of that degree, he studied the biochemistry and taxonomy of plants. From 1990 to 1995 he undertook doctoral research in social anthropology and human geography at the University of Adelaide and was awarded a Doctor of Philosophy in 1995.
56 From 1982 to 2011 he was employed at the South Australian Museum. He held various positions at the South Australian Museum, including Registrar and then Senior Collection Manager of the Anthropology Division. This role involved organising and managing approximately 30000 artefacts and conducting field work relevant to the collection. In 1992 he became a Curator of Anthropology and from 1994 he was the Senior Curator of Aboriginal Collections. In 1998 he became Principal Curator of the Australian Aboriginal Cultures Gallery Project. In 2000 he became Head of Science at the Museum, and shortly after, Head of Anthropology/Manager of Sciences.
57 Since 2011 he has been self-employed as a consultant and an author. Some of his publications relate to Aboriginal uses of plants as medicines and foods. He has also been consulted from time to time by chemists who are interested in plants with potential therapeutic uses.
The Respondents’ lay witnesses
58 As previously noted, Mr Backhaus, Ms Prather, Mr Ardino, Mr Johnson, Ms Levinson and Mr Brocklehurst gave evidence. The only other lay witness for the respondents was Ms Debra Wilson, a senior librarian at UTAS. She gave evidence as to the public availability of the Dragar Thesis and the Morrison article both of which are discussed later in these reasons. Ms Wilson was not required for cross-examination.
59 The skilled addressee is a notional person skilled in the relevant art who may have an interest in using the products or methods of the invention, making the products of the invention, or making products used to carry out the methods of the invention either alone or in collaboration with others having such interest: see Aristocrat Technologies Australia Pty Ltd v Konami Australia Pty Ltd (2015) 114 IPR 28 at [26], Apotex Pty Ltd v Warner-Lambert Company LLC (No 2) (2016) 122 IPR 17 at [27]. The notional skilled addressee may, in an appropriate case, consist of a team of persons having different fields of expertise: General Tire & Rubber Co Ltd v Firestone Tyre & Rubber Co Ltd [1972] RPC 457 at 485, Root Quality Pty Ltd v Root Control Technologies Pty Ltd (2000) 49 IPR 225 at [71]. The notional skilled addressee may be, depending on the field of the invention, highly qualified, but lacks the capacity for invention. For this reason the notional skilled addressee is sometimes referred to as the hypothetical “non-inventive worker in the field”: Wellcome Foundation Ltd v VR Laboratories (Aust) Pty Ltd (1981) 148 CLR 262 (“Wellcome”) at 270. In the present case I will refer to this notional person as the person skilled in the art.
60 In the present case it was common ground that Mr Hood, even though he was the inventor, was not a person skilled in the art.
61 Counsel for Mr Hood submitted that the person skilled in the art in this case consisted of a team of persons possessing a number of different skills including a botanist, a plant or organic chemist, pharmacologists and, “… persons involved in regulatory compliance and marketing research”. I think this description of the notional team is too broad. I accept that the notional team may include a plant or organic chemist, a microbiologist and a horticultural scientist, all with training and experience in the field of the use of essential oils. It may also include pharmacologists, pharmacists and dermatologists but it will not include persons involved in the regulatory or marketing fields. Of those that gave relevant expert evidence, it seems to me that Dr Carson (a microbiologist), Dr Southwell (a plant chemist) and, to a lesser extent, Professor Menary (a horticultural scientist) are most likely to reflect the combined knowledge and experience of the person skilled in the art as at the Priority Date. I say to a lesser extent in the case of Professor Menary because, as he clearly acknowledged in his evidence, his considerable experience in the field of essential oils was focused on the flavour and fragrance of essential oils rather than any therapeutic use.
62 There was no dispute between the parties as to the relevant principles of construction. These were summarised by the Full Court (Hill, Finn and Gyles JJ) in Jupiters Ltd v Neurizon Pty Ltd (2005) 222 ALR 155 at [67]. I do not think it necessary to set out their Honours’ often quoted summary.
63 It is alleged by the respondents that each of claims 6-9 lack clarity because they refer to a method of treatment as claimed in claim 4 in circumstances where claim 4 does not claim a method of treatment. There is no doubt that the reference in these claims to claim 4 is an obvious drafting error and that they should refer instead to claim 5. Of course, the error would be readily corrected by an amendment substituting the reference to claim 4 with a reference to claim 5 in each of claims 6-9.
64 In closing submissions the applicant initially submitted that there was no error in claim 7 and that it was indeed intended to refer to claim 4. The difficulty with that submission is that, while claim 7 refers to a method of treatment as claimed in claim 4, there is no method of treatment referred to in claim 4. In my view claim 7, if read in that way, makes no sense at all and is liable to be revoked on the ground that it lacks clarity.
65 If claim 7 is read with claim 5, then the method of treatment referred to in claim 7 must be one in which the essential oil is applied topically to relieve sinus congestion. Other methods of treatment, such as those involving the inhalation of emitted vapours from the essential oil in order to relief sinus congestion, are not methods of treatment within the scope of claim 7.
66 Ultimately, the applicant accepted that the reference to claim 4 in claim 7 (and all other claims affected by the same error) should be understood as referring to claim 5 and that, if those claims were not open to that interpretation as a matter of construction, then these are obvious errors which should be corrected by way of a series of simple amendments. I will say more on this issue when considering the amendment application.
67 In circumstances where claims 6-9 refer to claim 4 rather than claim 5, each of those claims is invalid for lack of clarity. An error of this kind appearing in a claim cannot be disregarded or corrected on the basis that the skilled addressee would understand that an error had been made in the drafting of the claim: see Glaxosmithkline Consumer Healthcare Investments (Ireland) (No 2) Ltd and Another v Generic Partners Pty Ltd (2018) 264 FCR 474 at [117]-[121], [126]-[130].
68 Claims 11, 12 and 13 contain another obvious error in that they refer to claim 8 rather than claim 10. Those claims are also invalid for lack of clarity. Once again, the lack of clarity arising out of the obvious error is easily corrected by an amendment substituting the reference to claim 8 in claims 11, 12 and 13 with a reference to claim 10.
69 There are other issues that arise in relation to claims 10, 11, 12 and 13.
70 Claim 10 refers to a method of treatment “wherein the essential oil is applied in a carrier”. Claims 11, 12 and 13 require that the carrier be “a vegetable oil”, “a lotion”, and “an ointment” respectively.
71 There was no expert evidence led by any of the parties aimed at elucidating the meaning of the words “applied in a carrier” in claim 10.
72 I have previously referred to statements appearing in the specification concerning the use of the essential oil in a carrier. The relevant paragraphs of the specification state:
The oil appears to have no strongly adverse reaction with the skin, although as with all essential oils, people with sensitive skin should be careful before they apply it at full strength, but it can readily be applied, diluted in a carrier oil, preferably a pure vegetable oil, a lotion which can be an aqueous suspension, or an ointment, which can often be a petroleum emulsifying ointment base.
Where the Kunzea ambigua oil is applied, either pure or in a carrier oil or a lotion, the oil seems to pass through the skin relatively quickly, so no doubt it has a molecular size to enable this to occur, although to date I have not studied the size of the molecule.
73 There are a couple of matters to note about these paragraphs in the specification which provide the basis for claims 10, 11, 12 and 13. The use of a carrier is discussed in the context of the desirability of directly applying the oil to the skin in a diluted form rather than at full strength. It is clear from what is said that the function of the carrier is to enable the oil to be topically applied at less than full strength. Importantly, there is no suggestion in the body of the specification that the carrier, whether it is a vegetable oil, a lotion, an ointment, or something else, may consist of a formulation including other ingredients that cannot be sensibly understood to act as a carrier for the oil. A good example of such an ingredient would be a colouring agent the function of which is to colour the formulation rather than to carry it.
Proposed amendments to the patent
74 It is useful at this point to identify the amendments that the applicant originally proposed to the patent. The proposed amendments, at least in the form they were propounded until the applicant’s closing submissions, are set out in the second amended originating application filed in Proceeding NSD 1267 of 2017. Orders to the same effect were also sought in each of the other proceedings brought by the applicant. The Commissioner of Patents was given notice of the application to amend the patent in accordance with Rule 34.41 of the Federal Court Rules 2011 (Cth).
75 If the amendments in the form first proposed by the applicant were made, claims 3-13 would read as follows:
3. The essential oil of claim 1 2 in which the oil is obtained from the distillation of the green matter of the shrub.
4. The essential oil of claim 2 3 wherein the distillation is by steam distillation.
5. A method of treatment in which the essential oil as claimed in any one of claims 1 to 4 is applied topically as a treatment to relieve pain, minimize bruising, or to assist in healing.
6. A method of treatment as claimed in claim 4 5 wherein the pain relieved is pain from muscle and tendon strain, impact trauma, gout, headaches and insect and other bites.
7. A method of treatment in which the use of the essential oil as claimed in claim 4 wherein the use of essential oil relieves sinus congestion.
8. A method of treatment in which the use of the essential oil as claimed in claim 4 wherein the essential oil limits the progress of cold sores, dries up dermatitis and aids in the healing of contusions.
9. A method of treatment in which the use of the essential oil as claimed in claim 4 wherein the essential oil assists in the treatment of rashes, skin irritations and acne.
10. A method as claimed in any of claims 4 to 7 5 to 9 and 19 to 26 wherein the essential oil is applied in a carrier.
11. A method as claimed in claim 8 10 wherein the carrier is a vegetable oil.
12. A method as claimed in claim 8 10 wherein the carrier is a lotion.
13. A method as claimed in claim 8 10 wherein the carrier is an ointment.
76 The applicant also sought to make amendments to the complete specification to include the following additional claims:
19. A method of treatment in which the essential oil as claimed in anyone of claims 1 to 4 is applied topically as a treatment to minimize bruising.
20. A method of treatment which the essential oil as claimed in anyone of claims 1 to 4 is applied topically as a treatment to assist in healing.
21. A method of treatment as claimed in claim 5 wherein the pain relieved is pain from impact trauma.
22. A method of treatment as claimed in claim 5 wherein the pain relieved is pain from gout.
23. A method of treatment as claimed in claim 5 wherein the pain relieved is pain from headaches.
24. A method of treatment as claimed in claim 5 wherein the pain relieved is pain from insect and other bites.
25. A method of treatment as claimed in claim 8 wherein the essential oil dries up dermatitis.
26. A method of treatment as claimed in claim 8 wherein the essential oil aids in the healing of contusions.
27. A method of treatment as claimed in claim 9 wherein the essential oil assists in the treatment of skin irritations.
28. A method of treatment as claimed in claim 9 wherein the essential oil assists in the treatment of acne.
77 The applicant stated in its opening submissions in relation to the proposed amendments:
18. Section 102 of the Patents Act applies in relation to the application to amend in the form it existed prior to the amendments effected by the Intellectual Property Laws Amendment (Raising the Bar) Act 2012 (Cth). The basis for the Proposed Amendments is set out in Mr Hood’s Statement of Grounds for Application to Amend Patent (the Grounds), and include that: (a) proposed amendments are to correct obvious mistakes in claim numbering in dependent claims (and as such s.102(3) provides so that s.102 is not operative); and (b) in respect of all proposed amendments, the proposed claims as amended are supported by the matter disclosed in the specification and in substance fall within the scope of the claims prior to amendment, and so are allowable under Patents Act: s.102.
(footnotes omitted)
78 I do not propose to say too much at this stage about the amendments originally proposed by the applicant to claims 7 to 13 except to note that, contrary to the submission made by the applicant, those amendments, if made, would have broadened the scope of the claims so that they encompassed methods of treatment that did not involve the topical application of the oil. In my view the submission that the proposed amendments to those claims were intended to correct an obvious error was only half true. While it is true that there is an obvious error in those claims, the applicant sought to correct it not by merely substituting a reference to claim 5 in place of claim 4, but by making other changes to the language of the claims that would have enlarged their scope.
79 In closing submissions the applicant did not press its original application to amend claims 3-13. But it did propose amendments to correct what were said to be obvious errors:
the reference in claim 3 to claim 1 which the applicant says should refer to claim 2;
the reference in claim 4 to claim 2 which the applicant says should refer to claim 3;
the reference in each of claims 6-9 to claim 4 which the applicant says should be a reference to claim 5;
the reference in claim 10 to claim 4-7 which the applicant says should be a reference to claims 5-9; and
the reference in each of claims 11-13 to claim 8 which the applicant says should be a reference to claim 10.
80 The following amendments were also proposed to claims 5, 14, 15 and 18 as consequential to any finding that claims 1-4 are invalid:
5. A method of treatment in which the essential oil as claimed in any one of claims 1 to 4an essential oil derived from the shrub Kunzea ambigua, obtained by steam distillation of the green matter of the shrub, is applied topically as a treatment to relieve pain, minimize bruising, or to assist in healing.
14. The essential oil of any one of claims 1 to 3An essential oil derived from the shrub Kunzea ambigua, obtained by steam distillation of the green matter of the shrub, wherein the oil is used as an insect repellent.
15. The essential oil of any one of claims 1 to 3An essential oil derived from the shrub Kunzea ambigua, obtained by steam distillation of the green matter of the shrub, wherein the oil is used as a rust inhibitor.
18. The use of the essential oil as claimed in any one of claims 1 to 4An essential oil derived from the shrub Kunzea ambigua, obtained by steam distillation of the green matter of the shrub, and substantially as hereinbefore described.
81 I note that the proposed amendment to claims 14-18 are problematical because they claim matter that is within the scope of the existing claim 4 which the applicant has accepted is invalid. I will say more about the proposed amendments later in these reasons.
82 The submissions made by Mr Fox on the issue of validity of the relevant claims were adopted by Mr Reuben on behalf of the respondents for whom he appeared.
83 The main focus of the validity attack was directed to the contention that the relevant claims were not to a manner of manufacture and did not involve an inventive step. It is important to recognise, at the outset, that there was no allegation that any of the claims was to an invention that was not useful. In particular, none of the respondents contended the claimed methods of using essential oil derived from plants of the Kunzea genus were not useful methods of treating the conditions referred to in the claims or that the invention, as claimed, did not fulfil any promise conveyed by the specification. It follows that the invalidity case is to be determined on the basis that the methods of treatment referred to in the relevant claims are useful in the treatment of those different conditions.
84 The respondents allege that claim 8 is invalid for lack of fair basis. They submitted there was no disclosure in the body of the specification of any method of treatment that “dries up dermatitis” and “aids in the healing of contusions”. A similar submission was made in relation to claim 9 which is to “[a] method of treatment as claimed in claim 4 [sic] wherein the essential oil assists in the treatment of rashes, skin irritations and acne”.
85 The test to be applied for the purpose of ascertaining whether a claim is fairly based on the matter described in the specification as required by s 40(3) of the Act (as it stood at the time the specification became open for public inspection) requires that the specification contain “a real and reasonably clear disclosure” of what is claimed: Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (2004) 217 CLR 274 at [68]-[69]. I agree with the respondents that there is no real or reasonably clear disclosure of the method of treatment claimed in claim 8. Claim 8 is therefore invalid for lack of fair basis.
86 The same is not true of claim 9. The specification includes a statement that “[i]t has also had positive results in the treatment of rashes, skin irritations and acne”. In my opinion there is a real and reasonably clear disclosure of the use of the essential oil derived from Kunzea ambigua as a treatment for those three conditions. I do not think claim 9 is invalid for lack of fair basis.
87 For an invention to be patentable, it must meet the relevant requirements of the Act including s 18(1)(a) which requires that the invention, as claimed, be a manner of manufacture within the meaning of s 6 of the Statute of Monopolies 1624 21 Jac 1 c 3. Schedule 1 to the Act defines “invention” as “any manner of new manufacture the subject of letters patent and grant of privilege within section 6 of the Statute of Monopolies, and includes an alleged invention”. This element of patentability was considered in National Research Development Corporation v Commissioner of Patents (1959) 102 CLR 252 (“NRDC”) and, more recently, D'Arcy v Myriad Genetics Inc (2015) 258 CLR 334 (“Myriad”). According to NRDC, as explained in Myriad, for an invention to constitute a manner of manufacture in the relevant sense, it must first be “for a product made, or a process producing an outcome as a result of human action” and, secondly, it must have “economic utility”: see Myriad at [28] per French CJ, Kiefel, Bell and Keane JJ. However, while these two characteristics are essential to the existence of a patentable invention, they will not always provide a sufficient criteria against which to determine whether an invention is a manner of manufacture. As their Honours also observed at [28]:
… When the invention falls within the existing concept of manner of manufacture, as it has been developed through cases, they will also ordinarily be sufficient. When a new class of claim involves a significant new application or extension of the concept of “manner of manufacture”, other factors including factors connected directly or indirectly to the purpose of the Act may assume importance …
Their Honours went on to identify at [28] a number of additional factors that may need to be considered when a new class of claim is in issue.
88 In their submissions the respondents did not suggest that the claims in suit in this case involve any new class of claim and did not make any submissions directed to the additional factors identified in Myriad at [28]. Rather, the focus of the respondents’ submissions was on the principle considered by the High Court in Commissioner of Patents v Microcell Limited (1959) 102 CLR 232 (“Microcell”) and the finding that what was claimed in that case was not patentable because (at 251):
We have in truth nothing but a claim for the use of a known material in the manufacture of known articles for the purpose of which its known properties make that material suitable. A claim for nothing more than that cannot be subject matter for a patent, and the position cannot be affected either by the fact that nobody thought of doing the thing before, or by the fact that, when somebody did think of doing it, it was found to be a good thing to do.
89 In that case the patent specification described an invention consisting of a self-propelled rocket projector comprising a tube of synthetic resinous material reinforced with mineral fibres. It was apparent on the face of the specification that the invention was said to reside in the use of such material in the construction of the self-repelled rocket projector. In the course of the High Court’s judgment in Microcell, reference was made to the “old and well-established principle that the mere discovery of a new use of a particular known product is not what is meant by invention … [and] that where by the alleged invention no new product is obtained, no new method of manufacture suggested nor an old one improved, the discovery cannot be protected by a grant of Letters’ Patent …”. Their Honours said at 250:
Here the specification does not on its face disclose more than a new use of a particular known product. To use Lord Buckmaster’s words, no new product is obtained, and there is no new method of manufacture suggested or an old one improved. Tubular self-propelled-rocket projectors were at the relevant time well-known articles of manufacture. Synthetic resinous plastics reinforced with mineral fibres, and in particular polyester plastics reinforced with glass or asbestos fibres, were well-known materials. These things are to be gathered from the specification itself, which contains no suggestion of novelty in relation to the article to be manufactured or the material to be used. It further appears from matter published in Australia as early as 1946 that the reinforced plastic materials referred to in the specification had been used in the manufacture of a wide variety of articles. The properties of those materials were known generally, and in particular it was well known that they possessed that combination of great strength and lightness wherein, according to the specification itself, lies their virtue for the purpose in hand. The matter published in 1946 refers to their “extraordinary strength in relation to weight” – they are “stronger for their weight than steel” – and to their high tensile strength – another quality which the specification regards as a virtue for the purpose in hand. It was well known too that they possessed high impact strength and high resistance to heat. In these circumstances we do not think it can be said, merely because it does not seem previously to have occurred to anyone to make a rocket projector out of reinforced plastic, that any inventive idea is disclosed by the specification.
90 Gageler and Nettle JJ referred to Microcell in Myriad. Their Honours said, in a passage that was relied on by the respondents in this case, at [129]-[131]:
[129] Admittedly, it has occasionally been doubted that there is any longer a threshold requirement of inventiveness as opposed to the specific requirements of inventive step and novelty for which s 18(1)(b) provides. It has also been suggested that it would be desirable to collapse the subject matter requirement into the specific inquiries of inventive step and novelty. The Advisory Council on Intellectual Property concluded that it would make sense for “questions of newness to be dealt with under the specific provisions for novelty and inventive step, rather than under the general umbrella of manner of new manufacture”.
[130] But for present purposes, the law on the point appears to be tolerably clear. In Commissioner of Patents v Microcell Ltd, the Full Court held that the subject matter of a claim as disclosed in the specification must possess a quality of inventiveness or, in other words, the use of ingenuity that adds to the sum of human knowledge. In NV Philips Gloeilampenfabrieken v Mirabella International Pty Ltd, the majority recognised that the quality of inventiveness must appear on the face of the specification. In Advanced Building Systems Pty Ltd v Ramset Fasteners (Aust) Pty Ltd (1998) 194 CLR 171 (Ramset), the majority held that whether claimed subject matter is an invention for the purposes of s 100(1)(d) of the Patents Act 1952 (Cth) is distinct from inquiries as to inventive step, obviousness and novelty under s 100(1)(e) and (g), and that the court below had erred in considering “inventive merit” in light of prior art for the purposes of s 100(1)(d). The majority distinguished Philips on the basis that it was decided under the Patents Act 1990 (Cth). But, at a later point in the judgment, the majority also acknowledged that, where the subject matter of a claim as disclosed in the specification is plainly not an invention, the claim should be dismissed.
[131] Notwithstanding that Microcell did not establish a discrete “threshold” test, each of those decisions is consistent with the requirement, essential to the concept of a “manner of manufacture”, that the subject matter of a claim have about it a quality of inventiveness which distinguishes it from a mere discovery or observation of a law of nature. That requirement is separate and distinct from the other requirements of inventive step and novelty. As Brennan, Deane and Toohey JJ stated in Philips, an alleged invention will:
“remain unsatisfied if it is apparent on the face of the relevant specification that the subject matter of the claim is, by reason of absence of the necessary quality of inventiveness, not a manner of new manufacture for the purposes of the Statute of Monopolies. That does not mean that the threshold requirement of ‘an alleged invention’ corresponds with or renders otiose the more specific requirements of novelty and inventive step (when compared with the prior art base) contained in s 18(1)(b). It simply means that, if it is apparent on the face of the specification that the quality of inventiveness necessary for there to be a proper subject of letters patent under the Statute of Monopolies is absent, one need go no further.”
(some footnotes omitted)
91 The particulars given by the respondents in support of their manner of manufacture case were as follows:
(a) The alleged invention, as claimed in each of claims 1 to 4 of the Patent, is not the proper subject matter for the grant of a patent, in that it is not an “artificially created state of affairs”:
(i) The essential oil claimed in each of claims 1 to 4 of the patent is naturally occurring.
(ii) The essential oil claimed in each of claims 1 to 4 of the patent is not “made”.
(b) Further, the alleged invention, as claimed in each of claims 1 to 4 of the Patent, is not the proper subject matter for the grant of a patent, in that what is claimed is “the use of a known material in the manufacture of known articles for the purpose which its known properties make that material suitable”: Commissioner of Patents v Microcell Ltd (1959) 102 CLR 232. That is, at the priority date of the Patent the processes of distillation and steam distillation were known processes by which essential oils could be, or were, derived from native Australian shrubs.
(c) As to claims 5 to 9, the alleged invention as claimed in each claim is not the proper subject matter for the grant of a patent as the use of the essential oil in the method of treatment (in each claim) claims a known use for a known material. It was known at the earliest priority date of the Patent that essential oils obtained from native Australian shrubs referred to in paragraphs [29] to [36] of the affidavit of Dr Philip Allan Clarke sworn 20 June 2018 had, amongst other potential uses and applications, medicinal and therapeutic applications, including those mentioned in the said claims. No patentable subject matter resides in an alleged invention which seeks to claim a monopoly over attributes or capabilities inherent in and to, and/or attributes or capabilities known to be inherent in and to, essential oils.
(d) Further or in the alternative to (a) and (c), no manner of manufacture resides in a purported invention to a mere discovery (if there be one) that an essential oil derived from an Australian native shrub, such as the kunzea ambigua, may be a product and/or used in a method of treatment involving medicinal or therapeutic applications such as those mentioned in the claims. Such a discovery (if there be one) is not an invention capable of being the proper subject matter of a standard patent and is not a manner of manufacture.
92 As I have mentioned, the applicant accepts that claims 1-4 are invalid on the basis that they lack novelty. In those circumstances it is unnecessary to determine whether those claims are also invalid on the ground that they are not to a manner of manufacture. However, there are a number of observations that I will make in relation to that issue.
93 The respondents submitted that Mr Hood was not the first to discover an essential oil derived from Kunzea ambigua because the authors of the Morrison Paper published in 1922 and the Dragar Thesis published in 1984 had already extracted essential oil from Kunzea ambigua in connection with their investigations of the species many years before Mr Hood first produced essential oil from Kunzea ambigua growing on his property.
94 The difficulty with that submission is that the specification itself does indicate that the essential oil derived from Kunzea ambigua was a known substance that had been previously extracted from the plant. On the contrary, the specification states that it is the object of the invention described to provide a new essential oil. There is in my view nothing in the specification to suggest to the person skilled in the art that the essential oil of Kunzea ambigua had been extracted from the plant before the Priority Date. It is not apparent on the face of the specification that the quality of inventiveness necessary for there to be a proper subject of letters patent is absent. The present case is therefore outside the ratio of the decision of the majority in NV Philips Gloeilampenfabrieken v Mirabella International Pty Ltd (1995) 183 CLR 655 (“Philips”) at 663-664 per Brennan, Deane and Toohey JJ.
95 However, in Philips the majority (Brennan, Deane and Toohey JJ) went on to express the opinion that the same conclusion would follow even if the lack of inventiveness was not apparent on the face of the specification. Their Honours said at 666-667:
Strictly speaking, it is unnecessary to answer the question whether a process which could not be a proper subject matter for a patent according to traditional principle, for the reason that it is merely a new use of a known product, can nonetheless be a “manner of manufacture within the meaning of section 6 of the Statute of Monopolies” for the purposes of s 18(1)(a). However, in view of the fact that the argument in this Court and the judgments in the Full Court were primarily directed to that question, it is appropriate that we indicate that we consider that the above construction of s 18(1)’s threshold requirement of “an invention” goes a long way towards answering it since it would border upon the irrational if a process which was in fact but a new use of an old substance could be a “patentable invention” under s 18 if, but only if, that fact were not disclosed by the specification. In the context of that construction of s 18(1)’s threshold requirement of an “invention”, the preferable conclusion is that the phrase “manner of manufacture within the meaning of section 6 of the Statute of Monopolies” in s 18(1)(a) should be understood as referring to a process which is a proper subject matter of letters patent according to traditional principle.
96 In Bristol-Myers Squibb Co v F H Faulding & Co Ltd (2000) 97 FCR 524 (“Faulding”) the Full Court treated the majority decision in Philips as authority for the proposition that if, on the basis of what was known, as revealed on the face of the specification, the invention was obvious or did not involve an inventive step, the threshold requirement of inventiveness imposed by s 18(1) of the Act would not be met. Black CJ and Lehane J said at [30]:
The majority of the High Court in Philips explicitly say that their observations about a case where want of the threshold requirement of inventiveness is not apparent on the face of the specification are not necessary to their decision. And, in discussing the commencement point (what is “known”) of the inquiry about inventiveness, their Honours refer only to the Microcell principle. In our view, in the light of the authorities to which we have referred, Philips stands for the proposition (as a matter of construction of the 1990 Act) that if, on the basis of what was known, as revealed on the face of the specification, the invention claimed was obvious or did not involve an inventive step – that is, would be obvious to the hypothetical non-inventive and unimaginative skilled worker in the field […] – then the threshold requirement of inventiveness is not met. Some elaboration, however, is required in relation to what the specification reveals as “known”. If a patent application, lodged in Australia, refers to information derived from a number of prior publications referred to in the specification or, generally, to matters which are known, in our view the Court – or the Commissioner – would ordinarily proceed upon the basis that the knowledge thus described is, in the language of s 7(2) of the 1990 Act, part of “the common general knowledge as it existed in the patent area”. In other words, what is disclosed in such terms may be taken as an admission to that effect. In substance, we think, that is what happened, both in Microcell and in Philips. If, however, the body of prior knowledge disclosed by the specification is insufficient to deprive what is claimed of the quality of inventiveness, then the only additional knowledge or information which will be taken into account is knowledge or information of a kind described in s 7(2) of the 1990 Act. That again, in our view, is consistent with the approach taken in Microcell. It is also, with respect, the only approach which does not, in practical terms, render s 18(1)(b)(ii) otiose. Of course, once that additional knowledge is taken into account, one is applying s 18(1)(b)(ii), not the opening words of s 18(1) – unless, perhaps, one might apply either, there being, in this respect, no difference between them.
The decision in Faulding is authority for the proposition that it is not enough to establish that an invention is not a manner of manufacture by showing that it lacks inventiveness unless the lack of inventiveness is apparent on the face of the specification. This is the approach followed in a number of subsequent Full Court decisions including Novozymes A/S v Danisco A/S (2013) 99 IPR 417 per Jessup J and AstraZeneca AB v Apotex Pty Ltd (2014) 226 FCR 324 (“AstraZeneca”) at [379]-[387] per Besanko, Foster, Nicholas and Yates JJ.
97 In Myriad, Gageler and Nettle JJ said at [126]-[128]:
[126] For a claimed invention to qualify as a manner of manufacture it must be something more than a mere discovery. The essence of invention inheres in its artificiality or distance from nature; and thus, whether a product amounts to an invention depends on the extent to which the product “individualise[s]” nature. As Professors Sherman and Bently wrote:
“What then was required in order to move from the realm of discovery to that of invention? The simple answer was that it was necessary to show that abstract principles had been reduced to practice, that Nature had been individualised or activated … While philosophical or abstract principles could not on their own be patented, their embodiment in a material or practical form could. In these circumstances it was clear that in law it was the artificial or created nature of the final product, its distance from Nature, which ensured that an object became an invention rather than a mere discovery.”
[127] The question then is whether the subject matter of the claim is sufficiently artificial, or in other words different from nature, to be regarded as patentable.
[128] Relevantly, the artificiality of a product may be perceived in a number of factors, including the labour required to create it and the physical differences between it and the raw natural material from which it is derived. Regardless, however, of the amount of labour involved or the differences between the product and the raw natural material from which it is derived, it is necessary that the inventive concept be seen to make a contribution to the essential difference between the product and nature.
(footnotes omitted)
98 In the present case each of claims 1-4 is to an essential oil derived from plant matter of the kind identified in the claims. These claims are to the essential oil so derived in whatever form the extracted oil takes. This is significant because, according to the evidence, the chemical composition of the plant will vary depending on growing conditions and this will in turn affect the composition of the essential oil derived from the plant. While the chemical composition of the oil extracted from different plants will vary, this is due to the way in which the plant responds to the conditions in which it is grown and the stage at which it is harvested. The composition of the essential oil will mirror what is found in the plant. Moreover, the usual method by which the essential oil is extracted from the plant (ie. steam distillation) is one of great antiquity. It was common ground that essential oil has been extracted from plants using this process for many hundreds (perhaps thousands) of years. In those circumstances, a question arises as to whether the essential oil the subject of each of claims 1-4 is (adopting the language used by Gageler and Nettle JJ in Myriad at [127]) sufficiently artificial or different from nature to be regarded as patentable.
99 Of course, as the applicant now concedes, an essential oil of the kind referred to in each of claims 1-4 had been extracted and described in the documents that form part of the prior art base before the Priority Date with the consequence that each of those claims is invalid for lack of novelty. It is therefore unnecessary for me to express any final conclusion with respect to the issue of manner of manufacture as it relates to claims 1-4. However, it seems to me that if the question posed by Gageler and Nettle JJ in Myriad at [127] is the right question to ask, it is strongly arguable that the difference between the raw oil extracted from the Kunzea ambigua shrub is insufficiently artificial or different from nature to qualify as patentable subject matter.
100 I now turn to the challenge to the validity of claims 5-9 each of which is to a method of treatment. It is clear that each of claims 6-9 mistakenly refer to claim 4 when they should refer to claim 5. For the purposes of addressing the validity issues I will proceed as if the reference in claims 6-9 to claim 4 was a reference to claim 5.
101 The respondents accepted that a method of treatment may be patentable subject matter. The correctness of that proposition was confirmed by the High Court in Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd (2013) 253 CLR 284 (“Apotex”). In that case the High Court rejected the submission that the subject matter of the claim in suit was “essentially non-economic” and therefore not to a manner of manufacture: see Apotex at [277] per Crennan and Kiefel JJ. Their Honours said at [283]:
[283] … a method claim in respect of a hitherto unknown therapeutic use of a (known) substance or compound satisfies the general principle laid down in the NRDC Case. Such a method belongs to a useful art, effects an artificially created improvement in something, and can have economic utility. The economic utility of novel products and novel methods and processes in the pharmaceutical industry is underscored by s 119A of the 1990 Act and by their strict regulation in the Therapeutic Goods Act 1989 (Cth) (the TGA).
102 It is apparent from the particulars of invalidity which I have previously set out that claims 5-9 of the patent are said by the respondents not to be to a manner of manufacture not because they lack economic utility but because they are for the “known use of a known material”. The respondents rely on the principle discussed in Microcell to which I have previously made reference.
103 Dr Clark gave some evidence on a range of different plants in Australia from which essential oils may be derived. According to this evidence, there are roughly 20 to 50 different plant families in Australia from which essential oils may be derived. However, a large proportion of oils (approximately 70%) come from a small number of families (approximately 12 families). The main families of essential oil producing plants are the following:
(a) Myrtaceae;
(b) Rutaceae;
(c) Lamiaceae;
(d) Fabaceae;
(e) Poaceae;
(f) Santalaceae;
(g) Solanaceae.
104 Dr Clark also gave evidence that the genera of plants from Australia which were commonly and generally known by October 1996 for producing oils with therapeutic uses included:
(a) Eucalyptus/Corymbia – Myrtaceae family;
(b) Melaleuca (“tea tree”) – Myrtaceae family;
(c) Leptospermum (“tea tree”) – Myrtaceae family;
(d) Kunzea (“tea tree”) – Myrtaceae family;
(e) Backhousia – Myrtaceae family;
(f) Santalum (“sandalwood”) – Santalaceae family;
(g) Boronia – Rutaceae family;
(h) Prostranthera (“mint bush”) – Lamiceae family;
(i) Cymbopogon (“Lemongrass”) – Poaceae family;
(j) Callitris (“Australian pine”) – Cupressaceae family.
105 One of the most well-known Australian essential oils in October 1996 was tea tree oil. There are also tea tree oils from New Zealand (and other places), which were commonly and generally known in Australia in October 1996.
106 Whilst I accept Dr Clark’s evidence in relation to the different types of essential oils and their uses, I do not think it establishes that the essential oil derived from Kunzea ambigua had been used for therapeutic purposes before the Priority Date. It is true that Dr Clark’s evidence shows that Australian Aboriginal people have used native plants for therapeutic purposes for thousands of years, including to treat infections, skin problems, colds and nasal conditions. However, Dr Clark did not suggest that any of those treatments involved extracting the essential oil of the plants nor did he suggest that Kunzea ambigua had been used by them for therapeutic purposes.
107 In submissions it was accepted by Mr Fox, correctly in my view, that the evidence did not establish that the essential oil derived from Kunzea ambigua had been used for therapeutic purposes before the Priority Date. Even if it is correct to say that such an essential oil was a known material, it has not been shown that it had known properties that made it suitable for use in the treatment of any of the ailments referred to in the claims. It is true that a person skilled in the art, if he or she was to ascertain the chemical composition of oil derived from Kunzea ambigua, may infer that there were some chemical components in the oil that may be useful as a treatment for one or more of the ailments referred to in the method of treatment claims. But the evidence given by Dr Carson and some of the other witnesses called by the respondents to that effect was more in the nature of a conjecture, it being recognised that the essential oil would need to be tested to determine whether it was suitable for such a purpose.
108 In my view the evidence does not support a finding that any of the method of treatment claims was for a known use of a known material. In any event, as I have previously observed, there is nothing on the face of the specification to suggest that the essential oil derived from Kunzea ambigua was not new or that the methods of treatment that used such an oil lacked the quality of inventiveness necessary to support the grant of a valid patent.
109 The respondents submitted that what Mr Hood claims to have discovered is that Kunzea ambigua is suitable for a number of uses including as a treatment for various ailments. They then submitted that the methods of treatment defined by the claims, and which Mr Hood is said to have invented, were based on anecdotal evidence obtained from family and friends to whom Mr Hood supplied his essential oil before the Priority Date. It was submitted that any discovery made about the use of Kunzea ambigua oil as a method of treatment was therefore made not by Mr Hood but by his family and friends.
110 These submissions involve an assertion that the methods of treatment defined by the relevant claims were either discovered by Mr Hood’s family and friends to whom he supplied his essential oil or that those methods of treatment were first performed by Mr Hood’s family and friends rather than Mr Hood himself. At their core these submissions involve an unparticularised contention that the use of the relevant methods of treatment was not novel because they were first used by Mr Hood’s family and friends. I do not think that the involvement of Mr Hood’s family and friends (as, in his words, “guinea pigs” who he asked to test his essential oil) can advance the respondents’ manner of manufacture case. No case based on lack of novelty arising out of such use is pleaded. Nor is it pleaded that any one or more of Mr Hood’s family or friends were inventors of the relevant methods of treatment and that the patent was obtained on the basis of a representation to some contrary effect.
111 The respondents also submitted that Mr Hood, the alleged inventor, was not a person skilled in the art and did not possess any scientific or technical qualification or experience which might provide a technical foundation for comprehending his assertion of having made any relevant discovery. It was submitted that nothing was discovered by Mr Hood in relation to the potential therapeutic uses of the essential oil which could “add to the sum of human knowledge”. Reference was made in support of this submission to what was said by Gageler and Nettle JJ in Myriad at [165] where their Honours cited with approval the following well-known statement of Buckley J in Reynolds v Herbert Smith & Co Ltd (1902) 20 RPC 123 at 126:
Invention … adds to human knowledge, but not merely by disclosing something [not previously known]. Invention necessarily involves also the suggestion of an act to be done, and it must be an act which results in a new product, or a new result, or a new process, or a new combination for producing an old product or an old result.
112 As the patent specification makes clear, the method said to have been discovered by Mr Hood is one that takes advantage of the therapeutic properties of the essential oil referred to in the claims and the usefulness of those oils when topically applied as a treatment for various ailments. If this use of the essential oil provides an effective treatment for the different ailments referred to in the claims, then it is difficult to see why it is not correct, as a matter of principle, to describe Mr Hood as the inventor of a new method of treatment in circumstances where it is not alleged that any of the methods of treatment were used before the Priority Date except by Mr Hood and his family and friends.
113 The suggestion that Mr Hood was not a person skilled in the art and that he was not in any real position to know why the methods of treatment discovered by him were effective, wrongly assumes that it is only a person skilled in the art who is capable of conceiving of a patentable invention. As Aickin J reminded us in Wellcome at 286 “a valid patent may be obtained for something stumbled upon by accident [or] remembered from a dream […] if it otherwise satisfies the requirements of the legislation”. There is nothing in the Act that requires that the inventor be a person skilled in the relevant art or that the inventor must have the same level of understanding of the invention as a person skilled in the art.
114 Section 18(1)(b)(ii) of the Act provides that an invention is a patentable invention for the purposes of a standard patent if the invention, so far as claimed in any claim, involves an inventive step when compared with the prior art base as it existed before the Priority Date of that claim. At the relevant time s 7(2) and s 7(3) of the Act provided:
(2) For the purposes of this Act, an invention is to be taken to involve an inventive step when compared with the prior art base unless the invention would have been obvious to a person skilled in the relevant art in the light of the common general knowledge as it existed in the patent area before the priority date of the relevant claim, whether that knowledge is considered separately or together with either of the kinds of information mentioned in subsection (3), each of which must be considered separately.
(3) For the purposes of subsection (2), the kinds of information are:
(a) prior art information made publicly available in a single document or through doing a single act; and
(b) prior art information made publicly available in 2 or more related documents, or through doing 2 or more related acts, if the relationship between the documents or acts is such that a person skilled in the relevant art in the patent area would treat them as a single source of that information;
being information that the skilled person mentioned in subsection (2) could, before the priority date of the relevant claim, be reasonably expected to have ascertained, understood and regarded as relevant to work in the relevant art in the patent area.
115 The expression “prior art information” is relevantly defined to mean information that is part of the prior art base for the purposes of s 7(3) in relation to deciding whether an invention does or does not involve an inventive step. At the relevant time the expression “prior art base” was defined in para (a) of the definition to mean information that is made publicly available whether in or out of the patent area. The term “patent area” is relevantly defined in Sch 1 to mean Australia.
116 Section 7(2) of the Act uses the word “obvious” in the course of describing what must be established before an invention can be held not to involve an inventive step. Something may be “obvious” in light of the common general knowledge, or the common general knowledge coupled with relevant s 7(3) information, if it is “plain or open to the eye or mind, something which is perfectly evident to the person thinking on the subject” (Olin Mathieson Chemical Corp v Biorex Laboratories Ltd [1970] RPC 157 at 188) or something which “would at once occur to anyone acquainted with the subject and desirous of accomplishing the end” (Vickers, Sons & Co Ltd v Siddell (1890) 15 App Cas 496 at 502).
117 An invention may also be obvious in light of the common general knowledge if the person skilled in the art faced with the same problem as the inventor would have taken as a matter of routine whatever steps might have led from the prior art to the invention, whether they be the steps of the inventor or not (Wellcome at 286 per Aickin J) or (using the language of the “modified Cripps question”) if the person skilled in the art would be directly led as a matter of course to take such steps in the expectation that doing so might well produce a useful or better alternative to the prior art (Aktiebolaget Hassle v Alphapharm Pty Ltd (2002) 212 CLR 411 at [50]-[53]). However, a claimed invention is not obvious merely because the person skilled in the art would consider that it was “worthwhile to try”.
118 Much of the evidence adduced by the respondents in support of their contention that each of the relevant claims was invalid for lack of inventive step took as it starting point the Dragar Thesis. In their opening submissions the respondents relied on the Dragar Thesis as a document containing s 7(3) information that the person skilled in the art could, before the Priority Date, be reasonably expected to have ascertained, understood and regarded as relevant. Ultimately, the respondents abandoned their reliance upon the Dragar Thesis. The evidence does not support a conclusion that the Dragar Thesis constituted common general knowledge or information made relevant pursuant to s 7(3) of the Act. It follows that the question whether the claimed invention is invalid for lack of inventive step is to be determined solely by reference to the common general knowledge as it existed at the Priority Date.
119 The notional skilled addressee will possess the common general knowledge in the field of the invention. This will include the background knowledge and experience available to all those persons engaged in the field and will include publications to which they would refer as a matter of course: see Minnesota Mining and Manufacturing Company v Beiersdorf (Australia) Ltd (1980) 144 CLR 253 at 292 per Aickin J, ICI Chemicals & Polymers Ltd v Lubrizol Corporation Inc (1999) 45 IPR 577 at [112] per Emmett J.
120 Dr Carson gave extensive evidence as to what was commonly and generally known as at the Priority Date. I find that the information referred to in paras [121]-[142] below was part of the common general knowledge that would have been known to the notional person skilled in the art at the Priority Date.
121 Melaleuca alternifolia is one of a number of plants commonly referred to as tea tree, which have been used medicinally. Other species of plants in the Melaleuca, Leptospermum and Kunzea genera have been referred to as tea tree and used medicinally. For example, Manuka (Leptospermum scoparium) and Kanuka (Kunzea ericodes) are two types of tea tree from which essential oils are derived. Those essential oils have been promoted and sold for claimed medicinal uses.
122 Tea tree has been used for medicinal purposes since Australia was colonised in 1788. There are reports of Captain Cook making tea from leaves of a tea tree plant. It is from this type of early use that the name tea tree is derived. However, the taxonomy of Australian native plants had not been developed at the time, and what was referred to as tea tree was in fact various species of plants which are now classified in the Melaleuca, Leptospermum and Kunzea genera. Those species were all referred to as tea tree because of their similar appearance and scent.
123 Although different species of plants were being used for the same purposes, those plants were all generally found to be suitable for those purposes. Related species of plants (e.g. species within the same genus or the same family) may share similar properties (e.g. similar appearance and similar scent), and, generally, the more closely related two plants, the greater the likelihood that there will be similarities between their physical and chemical properties. Those physical and chemical properties include the composition of oil derived from the plants (e.g. both having high concentrations of the same types of chemical compounds).
124 There is a very long history of Australian Aboriginal people using native plants for medicinal purposes. There are reports of Australian Aboriginal people using tea tree by crushing the leaves to make poultices, and reports of use of species closely related to Melaleuca alternifolia for the treatment of headaches, aches and pains, colds, skin conditions and as an insect repellent. The methods of use included crushing leaves and small branches to inhale the vapour, and soaking bruised leaves in water and then drinking it or pouring it over the body. Crushed leaves of tea trees were inhaled to treat coughs and colds or were sprinkled on wounds, after which a poultice was applied. In addition, tea tree leaves were soaked to make an infusion to treat sore throats or skin ailments.
125 Although there are different plants which have commonly been referred to as tea trees and which are still referred to as being varieties of tea tree, the term tea tree oil is generally only used to refer to oil from one or two species of the Melaleuca genus.
126 Two of the most well-known tea tree oil products sold in Australia from about the 1920s containing Melaleuca alternifolia were oil in water emulsions called “Melasol” and “Ti-trol”.
127 Beginning around the late 1980s, there was a resurgence of interest in natural products, including tea tree oil. Commercial products containing tea tree oil which were available at that time included oil, gel, cream, hand and body lotion, shampoo, conditioner, foot balm, soap, linament, pessaries, toothpaste, insect repellent, veterinary shampoo and commercial air conditioner germicides.
128 By October 1996, tea tree oil was used for a wide variety of treatments including cuts and bruises, the cleansing of wounds, colds, arthritis, coughs, congestion, dermatitis, minor burns and insect bites. However, in the early 1990s there was little scientific literature substantiating or explaining tea tree oil’s effectiveness in treating such conditions.
129 Essential oils can be obtained from various parts of a plant or tree, such as leaves, bark or wood. The most oil rich parts of a plant differ depending on the particular plant.
130 Essential oils can be distilled from plants using different distillation methods. One common method of distillation of essential oils is steam distillation. Steam distillation has been used for hundreds of years.
131 Tea tree oil has for many years been produced by steam distillation of the leaves and terminal branchlets of Melaleuca alternifolia. A branchlet is a small branch on a plant which is generally young and green (as opposed to older branches which may have become brown and dry). A terminal branchlet is the small branch that holds the terminal leaves. Terminal branchlets are generally young and green since they occur at the growing tips of the branches.
132 Essential oils are complex chemical mixtures comprising many different compounds in varying concentrations. As the technology for analysing essential oils has developed and improved, it has become possible to identify more compounds in essential oils. For example, by 1937 the principal constituents identified in tea tree oil were simply identified as: “d-α-pinene, α- and γ- terpinene, cymene (cineol 8%), A 1-terpinenol-4, sesquiterpenes, etc”. In 1989, Brophy et al published a paper which identified 97 different compounds present in tea tree oil. Later analysis identified over 114 different compounds present in tea tree oil.
133 The most common way of analysing the composition of essential oils is using gas chromatography mass spectrometry (GC-MS). This method of analysis was well-known in October 1996 and is the process by which tea tree oil was analysed in the Brophy paper.
134 The Brophy paper was a significant development in understanding the composition of tea tree oil, and was cited by many other researchers who were writing about tea tree oil and other essential oils. That paper and the information described in it were commonly and generally known in Australia before October 1996.
135 The following are the primary compounds present in tea tree oil and their respective concentrations (as identified by Brophy et al):
136 The precise concentrations of compounds present in an essential oil can vary widely between samples. This is because the composition of the oil from one plant may be different from the composition of the oil from another plant of the same species. The differentiation of different strains of the same species based upon higher concentrations of particular chemicals in their essential oils is referred to as differentiation based upon “chemotype”.
137 By the late 1980s, it was considered that terpinen-4-ol was the active component in tea tree oil acting either alone or synergistically with one or more minor components. However, the contribution of individual components to the antimicrobial activity of tea tree oil was unknown.
138 In 1995, Professor Riley and Dr Carson published an article in which they reported findings of tests in relation to the antimicrobial activity of individual chemical constituents of tea tree oil. One of the reasons for undertaking this research was because, although it was generally recognised that tea tree oil had some antimicrobial activity, and although it was thought that terpinen-4-ol was primarily responsible for that activity, that latter proposition had not been established scientifically and the extent to which the other constituents of tea tree oil contributed to the antimicrobial activity of tea tree oil (if at all) was not known.
139 Professor Riley and Dr Carson tested the following 8 chemical constituents of tea tree oil individually for antimicrobial activity against 12 different microorganisms, 11 of those being bacteria and 1 being fungal in nature:
(a) 1,8 cineole;
(b) terpinen-4-ol;
(c) p-cymene;
(d) linalool;
(e) α-terpinene;
(f) γ-terpinene;
(g) α-terpineol; and
(h) terpinolene.
140 Professor Riley and Dr Carson found that four of the oil compounds tested demonstrated notable antifungal activity, and six of the oil compounds tested showed consistent antibacterial activity. They published their findings in a 1995 article entitled “Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia”. The title of the article refers to “antimicrobial activity” because a substance (e.g. an essential oil, or a chemical compound) that has both antibacterial activity and antifungal activity may be described as having “antimicrobial” activity (ie. “antimicrobial” covers both antibacterial and antifungal).
141 Prior to 1995, reports had attributed the antimicrobial activity of tea tree oil almost entirely to terpinen-4-ol. However, Professor Riley’s and Dr Carson’s research demonstrated that both a-terpineol and linalool have antibacterial and antifungal activity equivalent to that of terpinen-4-ol. Professor Riley’s and Dr Carson’s study also demonstrated that 1,8-cineole has somewhat less but still a significant level of antimicrobial activity when compared with terpinen-4-ol, a-terpineol and linalool.
142 Dr Carson’s evidence was that if a chemical analysis of an essential oil shows the presence in significant concentrations of compounds known to have particular types of activity (e.g. antimicrobial activity or anti-inflammatory activity), then it can be expected that the essential oil will demonstrate that type of activity. Generally, if a particular compound is present in an essential oil at concentrations greater than around 15-20%, then Dr Carson would expect the essential oil to demonstrate activity consistent with the type(s) of activity that compound is known to demonstrate. Further, if a particular compound is present in an essential oil at concentrations greater than around 15-20%, then Dr Carson would expect that essential oil to demonstrate activity consistent with the type(s) of activity demonstrated by other essential oils that contain that compound at similar or greater concentrations.
143 On the basis of Dr Carson’s and Dr Southwell’s evidence, the respondents submitted that it would have been “very plain” to a person skilled in the art that an essential oil derived from Kunzea ambigua would be likely to be suitable for use in the treatment of a range of conditions described in the method of treatment claims.
144 According to the respondents’ submissions, the question whether the method of treatment claims were obvious should be assessed by reference to Dr Carson’s evidence to the effect that, if someone brought her a sample of Kunzea ambigua before the Priority Date to determine whether it would yield a useful essential oil, she would have first ascertained its biological heritage and then arranged for tests to be done including GC-MS tests to determine its chemical profile. Armed with details of the plant’s chemical profile, Dr Carson would have immediately observed the high quantity (around 63%) of alpha-pinene and 1,8-cineole (known monoterpenes with antimicrobial activity) and concluded that the essential oil could reasonably be expected to be useful in the treatment of the ailments disclosed in the claims. The respondents submitted that Dr Southwell’s evidence was to the same general effect except in relation to the use of the essential oil as a treatment for gout.
145 The difficulty with the respondents’ submissions in relation to inventive step is that they take as their starting point a hypothetical situation in which the person skilled in the art was provided with a sample of Kunzea ambigua for evaluation in much the same way that Mr Hood provided to Professor Menary the sample of Kunzea ambigua found by Mr Hood on his property. Approached in that way, Mr Hood’s decision to send Professor Menary a sample of Kunzea ambigua for the purpose of testing it to determine whether it might yield a useful essential oil is excluded from the inventive step analysis even though the idea of undertaking such testing was the first step along the road that led to the claimed invention.
146 There is nothing in the evidence that would indicate that, as at the Priority Date, a person skilled in the art would have had any reason to test Kunzea ambigua for the purpose of determining whether it might yield an essential oil derived from the plant that could be useful as a method of treatment.
147 The evidence showed that essential oil extracted from a number of plant species have been used to treatment various conditions. It was well-known at the Priority Date that tea tree oil products produced from Melaleuca alternifolia could be used for therapeutic purposes and as a treatment for cuts and bruises, congestion, insect bites and various other ailments. But that does not provide a sufficient basis for concluding that a person skilled in the art would be sufficiently motivated to analyse the essential oil derived from Kunzea ambigua in the expectation that it might well provide a useful treatment for such ailments.
148 The respondents’ evidence on the question of inventive step shows that as at the Priority Date there were a number of researchers who were investigating essential oils extracted from native plants with a view to supplying a growing market for such products. However, there is no evidence to suggest that it occurred to any person other than Mr Hood to investigate the possibility of using the essential oil derived from Kunzea ambigua as a treatment for any one or more of the various ailments described in the claims.
149 The idea that it would have been obvious to a person skilled in the art, at the Priority Date, to do what Mr Hood did relies on an ex post facto analysis that assumes that the person skilled in the art would have been asked to analyse a sample of Kunzea ambigua for the purpose of ascertaining whether it might be a promising candidate for evaluation in clinical trials aimed at determining whether it would provide an effective method of treatment of one or more such ailments.
150 There is a gap in this ex post facto analysis in that it does not adequately explain how Kunzea ambigua would have presented itself as a candidate to begin with. To say that it was a natural choice because it has a chemical profile which would be likely to have antimicrobial activity does not explain why the person skilled in the art would have been drawn to consider Kunzea ambigua as a potential therapeutic agent in the first place.
151 In my view the evidence does not show that any of the methods for treatment claims lacks an inventive step.
152 A similar issue arose in AstraZeneca in that part of the plurality’s judgment that discussed the “starting point issue”. As the plurality (Besanko, Foster, Nicholas and Yates JJ) observed at [201]-[203]:
[201] Except to the extent that any two or more documents or acts may be treated as a single source of information pursuant to s 7(3)(b), the combining of individual documents or acts that constitute s 7(3) information is prohibited. There may be many documents and acts that qualify as s 7(3) information in any particular case. However, unless s 7(3)(b) is engaged, the question arising under s 7(2) must be addressed by reference to the common general knowledge considered separately from, or together with, what will necessarily be a single piece of prior art information.
[202] Accordingly, whether a claim of a patent is invalid for lack of inventive step is to be determined by comparing the invention, so far as claimed, against the common general knowledge and any s 7(3) information. The question is then whether the invention would have been obvious to the hypothetical person skilled in the art in light of that knowledge considered separately from, or together with, the s 7(3) information. So understood, it is apparent that the relevant provisions of the Act do not expressly or impliedly contemplate that the body of knowledge and information against which the question whether or not an invention, so far as claimed, involves an inventive step is to be determined may be enlarged by reference to the inventor’s (or patent applicant’s) description in the complete specification of the invention including, in particular, any problem that the invention is explicitly or implicitly directed at solving.
[203] If the problem addressed by a patent specification is itself common general knowledge, or if knowledge of the problem is s 7(3) information, then such knowledge or information will be attributed to the hypothetical person skilled in the art for the purpose of assessing obviousness. But if the problem cannot be attributed to the hypothetical person skilled in the art in either of these ways then it is not permissible to attribute a knowledge of the problem on the basis of the inventor’s “starting point” such as might be gleaned from a reading of the complete specification as a whole. There are a number of reasons why this should be so.
153 The plurality went on to identify a number of reasons why an inventive step analysis should not commence from the same starting point from which the inventor commenced to develop his or her invention if that starting point was not common general knowledge or s 7(3) information.
154 The allegation of patent infringement based on the method of treatment claims depends on s 117 of the Act.
117 Infringement by supply of products
(1) If the use of a product by a person would infringe a patent, the supply of that product by one person to another is an infringement of the patent by the supplier unless the supplier is the patentee or licensee of the patent.
(2) A reference in subsection (1) to the use of a product by a person is a reference to:
(a) if the product is capable of only one reasonable use, having regard to its nature or design—that use; or
(b) if the product is not a staple commercial product—any use of the product, if the supplier had reason to believe that the person would put it to that use; or
(c) in any case—the use of the product in accordance with any instructions for the use of the product, or any inducement to use the product, given to the person by the supplier or contained in an advertisement published by or with the authority of the supplier.
155 Schedule 1 to the Act includes the following definition of “supply”:
supply includes:
(a) supply by way of sale, exchange, lease, hire or hire-purchase; and
(b) offer to supply (including supply by way of sale, exchange, lease, hire or hire- purchase).
156 The question whether “the use of a product would infringe a patent” raised in s 117(1) turns on whether such use would infringe the exclusive rights given to the patentee. These rights are defined in s 13. Section 13(1) provides that “[s]ubject to this Act, a patent gives the patentee the exclusive rights, during the term of the patent, to exploit the invention and to authorise another person to exploit the invention”. Section 13(3) provides that a patent has effect throughout the patent area.
157 Schedule 1 to the Act includes the following definition of the word “exploit”:
exploit, in relation to an invention, includes:
(a) where the invention is a product—make, hire, sell or otherwise dispose of the product, offer to make, sell, hire or otherwise dispose of it, use or import it, or keep it for the purpose of doing any of those things; or
(b) where the invention is a method or process—use the method or process or do any act mentioned in paragraph (a) in respect of a product resulting from such use.
158 Although the applicant’s pleaded case relies on s 117(1)(a), (b) and (c), the applicant no longer places any reliance on subpara (a).
159 Section 117 of the Act was considered by the High Court in Northern Territory of Australia v Collins (2008) 235 CLR 619 (“Collins”).
160 One question that arose in Collins was whether standing cypress pine trees (of the Callitris Intratropica species) were a staple commercial product within the meaning of s 117(2)(b) of the Act. The question was answered by the High Court in the affirmative.
161 Before referring in detail to the judgments in Collins it is desirable to refer to the judgment of French J (as his Honour then was) who dissented in the Full Court of the Federal Court of Australia on the question of whether the trees were a staple commercial product: Collins v Northern Territory (2007) 161 FCR 549 (French, Branson and Sundberg JJ). His Honour held at [99] that millable timber in the form of standing trees is not a product manufactured to a particular use.
162 One matter raised by his Honour’s judgment concerns the proper characterisation of the relevant product for the purposes of determining whether or not it is a staple commercial product. French J said at [97]-[98]:
[97] … There is a preliminary question about the classification of what was supplied for the purpose of determining whether it was, at the time of supply, a staple commercial product. That translates, in the present case, to the question whether the relevant class is unmilled timber or unmilled timber of the species Callitris Intratropica. If the product class were rightly described as unmilled timber there would be no debate. The Court could take judicial notice of the fact that unmilled timber is a staple commercial product.
[98] A different example may illustrate the point. No one would doubt that nails, bolts or screws are staple commercial products. These were examples offered in Pavel v Sony Corporation [1993] FSR 177. It may be that the bulk of nails, bolts and screws which are sold fall within a particular range of sizes. There may be some of unusual size or dimension or composition for which there is very little demand comparatively speaking. They are still part of the class of staple commercial products comprised by nails, bolts or screws as the case may be. Of course a nail, bolt or screw built to specification for an infringing use, like the top structure for convertible automobiles which was considered in Aro Manufacturing Company v Convertible Top Replacement Company Inc 377 US 476 (1964) would not be a staple article or commodity of commerce. In any event, it would be caught by s 117(2)(a) and the question of its character as a staple commercial product would be irrelevant.
163 In the High Court, Gummow ACJ and Kirby J referred with approval to French J’s reasoning. At [27] their Honours referred to French J’s reasoning at 582-583 with which their Honours expressly agreed. In particular, their Honour’s quoted with approval French J’s statement that the relevant product class to which Callitris Intratropica timber belonged for the purpose of s 117(2)(b), was “millable timber”.
164 A similar issue arises in the present case. Is the relevant product an essential oil derived from native shrubs or should it be defined more specifically as an oil derived only from shrubs of the genus Kunzea or, alternatively, shrubs of the Kunzea ambigua species?
165 None of the other judgments in the High Court address the question raised by French J. Other members of the Court referred to “the timber in question” without finding it necessary to distinguish millable timber and millable timber of the relevant species. All other members of the Court found that the timber in question was a staple commercial product within the meaning of s 117(2)(b). Both Hayne J and Crennan J each gave detailed reasons concerning the scope and operation of s 117 and the role played by the exclusion from the operation of s 117(2)(b) of “staple commercial products” in the context of the statutory scheme and the concept of “indirect” or “contributory” infringement both at common law and under the patent legislation enacted in the United States of America and United Kingdom.
166 Hayne J said at [34]-[38]:
[34] Section 117(1) is engaged where there is “the supply of [a] product by one person to another”. At the relevant time, the dictionary in Sch 1 to the Act provided that “supply” includes “supply by way of sale, exchange, lease, hire or hire-purchase”. The dictionary did not (and does not) contain any definition of “product” but the dictionary’s treatment of the word “exploit” distinguishes between “where the invention is a product” and “where the invention is a method or process”. Read as a whole, s 117 can be seen to proceed on the footing that the word “product” has its ordinary meaning and is not confined to a patented product or a product that is itself the result of applying a patented method or process.
[35] Although s 117(1) is engaged only where there is “the supply of [a] product by one person to another”, s 117 is directed to an identified sub-set of such transactions. That sub-set is identified first by the introductory words of s 117(1) – “[i]f the use of a product [the product that is supplied] by a person would infringe a patent” – and second by the amplification in s 117(2) of what is meant by “the use of a product by a person”. At the risk of undue abbreviation the amplification provided by s 117(2) can be described as inviting attention, in the particular cases identified in each paragraph of the sub-section, to “only use” (s 117(2)(a)), “known use” (s 117(2)(b)) and “instructed use” (s 117(2)(c)). In many, perhaps most, cases a convenient point at which to begin consideration of an issue about the application of s 117(1) will be to examine what is said to be the use of the product that is alleged to engage the provision. It is that use which must be identified as the use which would infringe the patent because the hinge about which s 117 turns is its introductory words: “[i]f the use of a product by a person would infringe a patent.”
[36] When the question is approached in that way, it will be observed that to ask whether supply of an input for a patented method or process (or resulting product) is capable of attracting s 117(1) may direct attention away from the relevant statutory questions. Those questions are: is there a supply of a product; what is the use of the product (as use is elucidated in s 117(2)); and does that use infringe the patent?
[37] The answer that is to be given to the last of the three questions just identified will, of course, turn upon whether the use in question contravenes the patentee’s exclusive rights under s 13 of the Act, during the term of the patent, to exploit the invention and to authorise another person to exploit the invention. And that requires close attention to what is meant by “exploit”. In particular, it requires close attention to what is said in the dictionary in Sch 1 to the Act about “exploit”, namely that:
“exploit, in relation to an invention, includes:
(a) where the invention is a product – make, hire, sell or otherwise dispose of the product, offer to make, sell, hire or otherwise dispose of it, use or import it, or keep it for the purpose of doing any of those things; or
(b) where the invention is a method or process – use the method or process or do any act mentioned in paragraph (a) in respect of a product resulting from such use.”
[38] The question which the appellant submitted arose in this matter – is the supply of an input for a patented method or process (or resulting product) capable of attracting the operation of s 117(1) of the Act? – was framed as it was in the expectation that it could and should be answered in the negative. When the questions presented by s 117 are identified in the manner set out earlier in these reasons it is apparent that a variety of quite different cases may arise for consideration under s 117. In particular, the different kinds of use that are identified in s 117(2) may present radically different issues about the relationship between the relevant use and the patentee’s exclusive rights to exploit the relevant invention. That is reason enough not to attempt some singular answer to a general question about when s 117 may be engaged.
167 Moving to the expression “staple commercial product” Hayne J said at [41]-[43]:
[41] I agree with Crennan J that a staple commercial product is one that is supplied commercially for various uses. While I agree with her Honour that this does not mandate an inquiry into whether there is “an established wholesale or retail market”, I greatly doubt that a product could be described as a “staple commercial product” if there were not some market for its sale for various uses. The doubt lies in the fact that the product must be a commercial product and that, to be a “staple commercial product”, it must be an article of commerce that not only can be used in a variety of ways but also is traded for use in various ways. But no question of that kind arises here. As Crennan J points out, leaving aside any supply to Australian Cypress Oil Co Pty Ltd (ACOC), timber of the kind at issue in this case was supplied to various licensees for a variety of uses. Those transactions were not so few or infrequent as to deny the existence of a market for the supply of that kind of timber for a variety of different uses.
[42] The phrase “staple commercial product” must be read as a whole and it must take its meaning from the context in which it sits. In particular, it is to be recalled that s 117 creates a liability in a supplier of a product where the act of supply would otherwise not infringe a patentee’s rights. Section 117 imposes liability on the supplier if use of the product supplied by the person to whom it is supplied would infringe.
[43] In this setting “staple commercial product” should not be given a narrow meaning. To do so would expand the classes of supply which are reached by s 117, thus expanding the rights of the patentee where, by hypothesis, the act of supply is not otherwise an infringement of the patentee’s monopoly. Further, the meaning given to “staple commercial product” must recognise that the central focus of s 117 falls upon the use of a product. The construction of the section must be approached with these two matters at the forefront of consideration.
(footnotes omitted)
168 His Honour referred to the drafting history of s 117, and the 1984 Report of the Industrial Property Advisory Committee. His Honour then returned to s 117(2)(b) and the expression “staple commercial product”. His Honour said at [48]-[50]:
[48] To read “staple commercial product” as identifying a product that is supplied commercially for various uses does not reflect the notion of principal or chief importance sometimes conveyed by the adjective “staple”. But as Crennan J concludes, “staple”, used adjectivally in the compound expression “staple commercial product”, should not be read as directing attention to the economic significance of the product concerned. Rather, it should be read as inviting attention to the variety of uses to which the product both can be, and is in fact, put. It is that variety of uses which, when the product is supplied commercially, makes the product a staple commercial product.
[49] As the reasons of Crennan J show, this construction of the provision is not inconsistent with the desire, expressed in the government’s published response to the report of the Industrial Property Advisory Committee, to harmonise Australian patent law with the laws of Australia’s major trading partners. But, as those reasons also show, the laws of the United States of America and the United Kingdom relating to indirect infringement are each expressed in terms that differ in important respects from s 117. There is, therefore, only limited assistance to be gained from considering the expressed desire for harmony with major trading partners.
[50] It may be thought that to read “staple commercial product” as identifying a product that is supplied commercially for various uses leaves little effective work to be done by s 117(2)(b). In particular it can be observed that cases of “only one reasonable use” are dealt with in s 117(2)(a), and yet many cases in which a product has various uses will not fall within s 117(2)(b). The resolution of this apparent tension between the two provisions lies in the recognition that s 117(2)(a) is concerned with a product capable of only one reasonable use, whereas “staple commercial product” takes its operation from what is seen to occur in the market-place. The two paragraphs pose radically different questions. The question posed in s 117(2)(a) is: For what can the product be used? By contrast, the question posed in s 117(2)(b) is: To what uses is the product in fact put? If it is in fact supplied commercially for various uses, it is a staple commercial product and the supplier of such a product is not to be held liable as an infringer because the person to whom the product is supplied uses it in a way that infringes, even if the supplier has reason to believe that it may be used in that way. Reading the provision on this basis would bring within the reach of s 117(2)(b) the supply, for example, of a product previously traded for only one use where the supplier has reason to believe that it will be used for a new and infringing use. It would leave beyond the reach of s 117 the supply of a product that has previously been traded for various uses unless the supply falls within s 117(2)(c) – where the supplier instructs or induces a particular use which infringes, or advertises the product for that use.
(footnote omitted)
169 Crennan J considered the meaning of the expression “staple commercial product” at [138]-[145]. Her Honour said at [142]-[145]:
[142] The precise scope of the expression “staple commercial product” is not clear. One ordinary adjectival use of “staple”, applied to raw materials, conveys the meaning that the material is capable of being used as a constituent element in a number of other products. That focuses on the inherent qualities of the product. Another equally well-known ordinary adjectival use of “staple” conveys the meaning that a product has a foremost place among products, for example, in a particular location. That focuses on the distribution of a product rather than upon its inherent capacity to be a constituent in a number of other products and overlaps with the idea that the product be a “commercial” product.
[143] It has been suggested by at least one writer in respect of European rights that a “staple commercial product” has two main qualities: first, it must be “[a] basic product commonly used for various purposes”, and secondly, it must be “[g]enerally available on the market”.
[144] Raw materials such as wool or timber undoubtedly have the first quality. As to the second quality, it is necessary to recognise that s 117(2)(b) operates to limit liability for contributory infringement. Policy arguments in favour of imposing liability for contributory infringement are much weaker with a product that has significant non-infringing uses. The legislative intention evinced in the statutory language, and apparent also from the relevant secondary materials, is to except from liability, the supply of products with significant non-infringing uses, or as it has been put in relation to the American provisions, products with “lawful as well as unlawful uses”. A preference for such a construction has also been essayed in respect of s 60(3) of the Patents Act 1977 (UK) by a writer who states “the intention is to stop material particularly adapted to the use of an invention being made available to a putative infringer, but that material which has and, importantly, had, a general purpose of more than de minimis utility, falls within the [UK] exception.”
[145] The phrase “staple commercial product” means a product supplied commercially for various uses. This does not mandate an inquiry into whether there is “an established wholesale or retail market” or into whether the product is “generally available” even though evidence of such matters may well be sufficient to show that a product is a “staple commercial product”. The relevant inquiry is into whether the supply of the product is commercial and whether the product has various uses. Leaving aside the supply to ACOC, the timber here was supplied on commercial terms to various licensees for a variety of non-infringing uses. Accordingly, the Northern Territory is protected by the limitation in s 117(2)(b).
(footnotes omitted)
170 Heydon J agreed with Crennan J’s reasons with respect to the operation of s 117(2)(b) of the Act.
171 In AstraZeneca the plurality (with whom Jessup J agreed) noted that the considerations relevant to the question whether a product is a staple commercial product include how widely the product is used and for what range of purposes. In that case the Court was concerned with the question whether a pharmaceutical compound known as rosuvastatin was a staple commercial product within the meaning of s 117(2)(b). The trial judge had rejected such an argument on the basis that:
Despite the fact that I accept that rosuvastatin has a number of medical uses, not just the treatment of hypercholesterolemia, I cannot accept that it should be characterised as a “staple commercial product”. The difficulty I have arises from the word “staple”, which does indicate something more than merely a “commercial product”. The reasoning in Collins does not lead me to the view that the fact that a product can be used in one or even a number of non-infringing ways is itself sufficient to make the product a staple commercial product. While “staple” is not concerned with the economic significance of uses, it is concerned with the variety of uses. The variety of uses in this case is confined by the nature of the product to a limited class, being the treatment of diseases of a particular kind or class (albeit different diseases) in humans. Rosuvastatin, despite its usefulness for a variety of disease conditions, is not able to be compared to timber (as in Collins) or, for example, types of pharmaceutical products which might be useful for many human conditions. It is for these reasons I conclude that the rosuvastatin products proposed to be supplied by the generic parties are not staple commercial products.
172 After referring to the trial judge’s reasons for declining to hold that rosuvastatin was a staple commercial product, the Full Court, after referring to that part of the trial judge’s reasons which I have just quoted, said at [429]-[431]:
[429] In argument before us, the generic parties placed considerable reliance on the judgment of Crennan J in Collins at [145]. Her Honour said that the phrase “staple commercial product” means “a product supplied commercially for various uses”. Hayne J (at [41]) and Heydon J (at [57]) agreed. However, Crennan J’s statement must be read in its proper context including the factual setting in which it came to be made. Collins involved the supply of a species of timber which Crennan J acknowledged at [143]-[144] constituted “[a] basic product commonly used for various purposes”.
[430] Whether a product meets that description is a question of fact. Considerations relevant to the question whether a product is a staple commercial product include how widely the product is used and for what range of purposes. In a passage in Thorley S, Miller R, Burkill G, Birss C and Campbell D, Terrell on the Law of Patents (16th ed, Thomson Sweet & Maxwell, 2006) at [8-37] referred to in a footnote to Crennan J’s judgment at [144] the learned authors, referring to the use of the expression “staple commercial product” in s 60(3) of the Patents Act 1977 (UK), state:
The use of the word “staple” is presumably a reference to raw materials or other basic products commonly available and with a multitude of possible applications, and the purpose of the subsection is to protect the supplier of such products even if he has knowledge that they are to be put to an infringing purpose. The scope of the words is far from clear and the dividing line between protecting the supplier of raw materials on the one hand and giving a fair monopoly to the patentee must be a question of fact in each case.
The authors of the IPAC report referred (at paragraph 14.2) to the undesirability of preventing a person from selling “a staple commodity with a wide variety of possible uses” suggesting that they also considered that a staple commodity was one that had a wide range of uses.
[431] We are not satisfied that rosuvastatin is a staple commercial product. The fact that it may be used for both infringing and non-infringing purposes is not conclusive. There are many products capable of being used for both infringing and non-infringing purposes that cannot be characterised as either raw materials or basic products commonly used for a variety of purposes. The uses to which rosuvastatin may be put appear to us to be limited to the prevention or treatment of cardiovascular disease and its associated risk factors (e.g., high cholesterol). This is apparent from the evidence of Dr Wilson, a cardiologist called by AstraZeneca, who said that he does not prescribe rosuvastatin (or any other statin) for any indication other than cardiovascular disease or its associated risk factors. It is true that Dr Hay, a general practitioner called by AstraZeneca, gave evidence that he also prescribes rosuvastatin for the treatment of conditions such as cerebrovascular disease, chronic renal disease and diabetes. However, as we read his evidence, rosuvastatin is prescribed by Dr Hay in order to prevent or treat cardiovascular disease in situations where there is increased risk of it occurring due to the existence of these conditions.
173 It is apparent that the Full Court, like the trial judge in that case, focused on the range of uses to which rosuvastatin could be put which appeared to be limited to the prevention or treatment of a particular disease (ie. cardiovascular disease and associated conditions such as high cholesterol) contributing to its progression. In the present case the therapeutic uses to which the relevant oil may be put are very broad indeed and extend to the treatment of a wide range of conditions that include bruising, sinus congestion, cold sores, dermatitis, rashes and acne. The oil may also provide relief from the symptoms of influenza, relief from the pain of arthritis and relief of nervous tension, stress and anxiety.
Is Kunzea ambigua essential oil a staple commercial product?
174 The applicant submitted that Kunzea ambigua essential oil is not a staple commercial product. In support of that submission he relied primarily on the evidence of Dr Narkowicz.
175 Dr Narkowicz’s evidence was that Kunzea ambigua oil is essentially a niche product and was “not a commodity or raw material”. He referred to the fact that there were few manufacturers or suppliers of the oil, that it was not readily available in supermarkets, pharmacies or from other retailers, and that for the most part it could only be obtained from specialist suppliers of essential oils. His evidence was that the most common commercial use of Kunzea ambigua is for therapeutic use (including aromatherapy) as a means to improve, alleviate or cure a disease or ailment.
176 Dr Narkowicz also said that the oil is expensive to produce because Kunzea ambigua is not a high yielding crop and this makes it more costly to produce than lavender, tea tree, eucalyptus and fennel oils which derive from high yield crops. He said that the high cost of producing Kunzea ambigua oil meant that it would not be economic to use for other purposes.
177 Dr Narkowicz accepted that Kunzea ambigua oil is supplied not only in its pure (or raw) form but was also incorporated into soaps, lotions and topical creams. He said that this was most often to take advantage of the therapeutic properties of the oil.
178 Dr Narkowicz said that other oils like tea tree oil and eucalyptus oil are sometimes used for household and industrial applications because of their antimicrobial properties. He said that while Kunzea ambigua oil could theoretically be used for those purposes, this is not commercially practical because of its high cost.
179 I accept that Kunzea ambigua oil is commonly used for its perceived therapeutic properties. But I do not accept Dr Narkowicz’s evidence that the oil is not properly considered a commodity or a raw material. The evidence shows that it was and it is supplied in its raw state for use as an ingredient used to manufacture various consumer products. Dr Narkowicz did not suggest this was an uncommon or de minimis use.
180 Dr Narkowicz undertook some research for the purpose of preparing his expert report that involved searching the internet for products containing Kunzea ambigua oil. The products that he found contained either Kunzea ambigua oil by itself, or in combination with other oils, or in a lotion, soap or the like. He said that many of these products were advertised as having therapeutic properties. Although he did not mention products advertised for use in aromatherapy, it is clear from other evidence that Kunzea ambigua oil is also used for that purpose and in a variety of personal care products used for hair and skin care.
181 Dr Narkowicz also said that some people are sensitive to tea tree oil and cannot use it. Kunzea ambigua oil may offer an alternative to tea tree oil.
182 The respondents contend that Kunzea ambigua oil is a staple commercial product. They submit that the oil is a raw material which the evidence shows is used in the manufacture of a range of products including hair and skin care products and massage oils for which there is an established market.
183 I accept the respondents’ submission.
184 My reasons for concluding that Kunzea ambigua essential oil is a staple commercial product are as follows.
185 First, the oil is a raw material that is supplied commercially for use in either its raw form or as an ingredient in a range of hair and skin care products. It is a basic product with a wide variety of possible uses. It belongs to a range of natural oils (including tea tree and eucalyptus oils) derived from native plants that are widely used to promote healing and well-being and which may assist healing when used either alone or when blended with other ingredients.
186 Secondly, the oil may be used for a range of therapeutic purposes due to its anti-inflammatory and antimicrobial properties. Although the method of treatment claims are extremely broad, they do not extend to many of the uses for which the oil is supplied. For example, none of the relevant claims cover the inhalation of vapours produced from the oil (ie. aromatherapy) or the use of the oil in a blend of essential oils. Use of the oil in these ways would not infringe any of the method of treatment claims of the patent. In this regard, I am satisfied that the oil can be, and is in fact, used for a range of different non-infringing purposes.
187 Thirdly, the oil has been registered on the ARTG since 2002 for a wide range of indications. Not all of these indications are within the scope of the claims. For example, the oil is registered for use in the relief of nervous tension, stress and mild anxiety. None of the claims cover the use of the oil for that purpose. Even the wide language of claim 5 (“to assist in healing”) would not extend to the use of the oil for the relief of nervous tension, stress or mild anxiety. Ironically, the applicant’s listing on the ARTG shows the route of administration as “Inhalation”.
188 Fourthly, the crucial question is whether the relevant product is supplied commercially for various uses. As Crennan J observed in Collins at [145], the relevant inquiry is into whether the supply of the product is commercial and whether the product has various uses. The fact that the oil is not widely available and is supplied by a comparatively small number of wholesalers and retailers, or that it is expensive when compared to some other essential oils, is beside the point. In my view the evidence establishes that Kunzea ambigua oil is commercially supplied and used for various purposes.
189 I have previously referred to the issue of product classification raised by French J in Collins. In the present case, even if the relevant class of products is narrowly defined as consisting of essential oils derived from shrubs of the Kunzea genus or, alternatively, the Kunzea ambigua species, then products within this class are still, in my view, to be treated as staple commercial products. That said, I am inclined to think that the proper classification of the product for the purpose of s 117(2)(b) is not so limited but encompasses all essential oils derived from native shrubs. It seems to me that these are all members of a homogenous class of products comprising essential oils derived from various native plant species many of which have well-known antimicrobial and anti-inflammatory properties. While some essential oils may be more effective in the treatment of various conditions than others (just as certain species of timber are likely to be more suitable for building than others), they are nevertheless within the same class of products. On that basis each member of that class (which would include kunzea, tea tree, eucalyptus, lavender and sandalwood oils) can be regarded as a staple commercial product within the meaning of s 117(2)(b) of the Act.
190 The respondents also contend that s 117 does not apply to what I shall refer to as export sales by which I mean sales of product by the respondents to customers situated outside the patent area.
191 Under s 117 the act of infringement is the act of supplying, or offering to supply, a product in the circumstances specified in the section. Neither s 117(1) nor (2) expressly requires that the act of supply, or offering to supply, occur in the patent area. The question is whether a limitation to that effect should be implied.
192 There is a presumption that general words used in a statute are interpreted to apply within territorial limits: see Jumbunna Coal Mine, NL v Victorian Coal Miners’ Association (1908) 6 CLR 309 at 363 per O’Connor J, Barcelo v Electrolytic Zinc Co of Australasia Ltd (1932) 48 CLR 391 at 423-433 per Dixon J. The presumption applies to the general words used in s 117. Accordingly, the act of supply, or the act of offering to supply, upon which liability under s 117 hinges, must be an act that occurs within the territorial limits which, for present purposes, corresponds to what is defined in the Act as the “patent area”.
193 There is another issue that arises in relation to export sales. Can s 117 apply in circumstances where a person supplies a product to another person for use outside the patent area?
194 In the case of a claim for a method of treatment the person who infringes such a claim will usually be a person who performs the method (e.g. a medical practitioner or patient). In the absence of s 117, a person who merely supplies the product used to perform the method of treatment (e.g. a pharmacist) does not infringe the claim because that person does not perform the method.
195 However, liability under s 117 may arise even though the person alleged to have infringed by supplying the product has not supplied it to a person who actually uses the product. Further, liability under s 117 may arise even if the supplied product is never used to perform the relevant method at all. This is because the use referred to in s 117(1) is a notional use postulated to occur in the circumstances specified in s 117(2).
196 Section 117(1) applies if the use of the product by a user would infringe a patent. Section 117(2) gives meaning to the expression “use of a product” in s 117(1). But the use of the product, ascertained by reference to s 117(2), must still be a use that would infringe the patent.
197 There are two ways to interpret the opening words of s 117(1).
198 The first is to treat the words “infringe a patent” as if they refer to nothing more than using the product in a way that is within the scope of a claim. On that interpretation the use postulated in s 117(1) is a use that is postulated to occur in the patent area. It would be an infringement for a person to supply a product in the patent area to another person where the product is capable of only one reasonable use, and that use is within the scope of a relevant claim, even if the product is later used outside the patent area and, therefore, in circumstances where such use could not give rise to liability for patent infringement.
199 An alternative interpretation of s 117(1) involves giving the words “would infringe a patent” full effect and asking whether use of the product would constitute an act of infringement. On that interpretation, if the product is to be used outside the patent area, then the supplier would not be liable under s 117.
200 Section 117(2)(a) focuses on the nature or design of the product. In circumstances where subpara (a) applies, the state of mind of the supplier is irrelevant. Liability will arise under s 117(1) when read with s 117(2)(a) simply because the supplier has supplied a product within the patent area which is capable of only one reasonable use. The fact that the product was supplied for use outside the patent area would not spare the supplier from liability if the product was, by reason of its design, only capable of infringing use.
201 The position under s 117(2)(b) and (c) is different.
202 Subparagraph (b) of s 117(2) refers to “the person”. This is a reference to the same person referred to in the opening words of s 117(1). It is this person’s postulated use of the product that is to be considered when determining whether s 117(2)(b) applies. In circumstances where s 117(2)(b) applies, the use of the product must be a use to which the supplier had reason to believe the person would put the product.
203 But even if the supplier has reason to believe that the product will be put to a use within the scope of a claim of the patent, it does not necessarily follow that such use would infringe the patent. An obvious situation in which the use of a product by the user would not infringe the patent is where the use takes place outside the patent area.
204 It seems to me that it would not be in keeping with the purpose or policy underlying s 117 to apply s 117(2)(b) in situations where the supplier has no reason to believe that the product will be used in the patent area. Use of a product outside the patent area is not a use that would infringe a patent granted under the Act. That is not to say that for s 117(2)(b) to apply, it is necessary for the supplier to have reason to believe that the person would infringe. That would depend on a number of considerations including an awareness of the existence of the patent and a correct understanding of the scope of the relevant claims. But it seems to me that in requiring that the supplier have reason to believe that the person would put the product to a use that would infringe a patent, the words “the use of a product” in s 117(1) are necessarily referring to a use of the product in the patent area. Hence, s 117(2)(b) should be understood as referring to a use of the product in the patent area.
205 Subparagraph (c) of s 117(2) raises different considerations. Like subpara (a), it is not concerned with the supplier’s state of mind.
206 The way in which subpara (c) is expressed is slightly awkward but I think the intention is clear enough. It is directed at instructions or inducements for use either given to the user of the product or contained in an advertisement published by or with the authority of the supplier of the product which must occur (by implication) in the patent area. Liability can arise under s 117(1) when read with s 117(2)(c) even if the supplier has no reason to believe that the person who had been given the relevant instruction or inducement will follow that instruction or act in accordance with that inducement.
207 The applicant’s infringement case against Bush Pharmacy relies on sales of Kunzea ambigua oil by Bush Pharmacy in the period February 2016 to June 2017. Some of those sales were made to persons in Australia while others were made to persons outside Australia.
208 Bush Pharmacy accepts that it supplied product to customers located in Australia in the period February 2016 to June 2017. Although I am not presently concerned with any question of quantification of damages, or the precise number of such sales made, it is nevertheless necessary for the applicant to show that one or more of the sales made by Bush Pharmacy during this period constituted a supply of product to which s 117 of the Act applies.
209 On the assumption that Kunzea ambigua oil is not a staple commercial product (contrary to my previous finding), the applicant submitted that Bush Pharmacy would be liable under s 117(2)(b) on the basis that it had reason to believe that the product sold would be put to a use that infringed claims 5, 6 and 7. He also submitted that Bush Pharmacy had given or published an inducement to use the product in a manner that would infringe claims 5, 6 and 7.
210 The applicant relied on the contents of the website for Bush Pharmacy as it stood at 17 November 2016 which included the following entry on a webpage entitled “Our Products”:

211 The applicant also relied on the terms of the TG Act listing (“TGA listing”) which I have previously set out and the various indications covered by that listing on the basis that it is referred to in the first paragraph on the webpage.
212 I do not think the reference to the TGA listing assists the applicant. A reader of the advertisement may understand from that reference that Kunzea ambigua oil has been approved for use in the treatment of one or more medical conditions. But the advertisement does not say what those indications are. Since the content of the TGA listing is not disclosed, the reference to the TGA listing does not in itself suggest that the essential oil is suitable for the treatment of any particular indication. Nor does it indicate how the oil is to be used.
213 Most of the webpage for Kunzea ambigua consists of a description of the plant used to produce the oil and the manner in which it is distilled and supplied. Relevantly, it also states that the essential oil can be applied without the need for dilution under the guidance of a medical professional and that it is clean and suitable for medical applications and aromatherapy.
214 The applicant accepts that there is no evidence of any specific instructions given by Bush Pharmacy as to the use of the relevant product. Hence, this is not a case concerned with any product instructions of the kind referred to in s 117(2)(c). The infringement case based on subpara (c) turns on whether the evidence establishes that Bush Pharmacy has given a relevant inducement to a person who may use the product or whether it has published any advertisement or authorised the publication of any advertisement containing any such inducement.
215 The word “inducement” as used in s 117(2)(c) refers to something that is directed to persuading or leading a person to do something which would result in that person using the relevant product in a manner that infringes the patent. Statements that expressly or impliedly encourage or promote the use of the relevant product in a manner that infringes the patent may amount to an inducement for the purposes of s 117(2)(c). In determining whether the relevant material contains an inducement it will often be necessary to look not only to the actual words used but also to the impression they are likely to convey in their broader context, remembering that there are many different ways in which an advertiser may by words or conduct persuade another to adopt a particular course of action using something less than clear or unambiguous language.
216 Mr Backhaus’ evidence was that Bush Pharmacy is a wholesaler of essential oils which it supplies in bulk quantities to either traders in essential oil (he referred to them as “oil traders”) or other persons who repackage the oils for retail sale.
217 The Bush Pharmacy website does not appear to be targeted at users of the essential oil. In those circumstances I am not persuaded that what appears on the website could constitute an inducement given to consumers to use the product in a manner that would infringe the patent. But that is not the end of the matter.
218 For an advertisement to constitute an inducement of the kind referred to in s 117(2)(c), it must be published by or with the authority of the supplier. However, the language of subpara (c) does not support the existence of any additional requirement that the advertisement be published to any person who uses the product. Hence, it would be no answer for a wholesale supplier of medicine who promoted the infringing use of its product in a trade magazine read only by pharmacists to say that s 117(2)(c) was not engaged because the advertisement was not targeted at, or ever seen by, consumers. In my opinion it would be sufficient to find that the advertisement was directed at persuading or leading persons to engage in an infringing use of the medicine.
219 A difficulty for the respondents is the wide scope of claim 5. Claim 5 is infringed by a person who uses the relevant oil as a method of treatment by applying it topically to (inter alia) “assist in healing”. Does the webpage seek to persuade or lead persons to use the oil in a method of treatment within the scope of claim 5? Given the width of that claim I think the answer is yes. The webpage states that the oil “may be applied without the need for dilution under guidance of a medical professional” and that “it is suitable for medical applications”. In my opinion those statements are directed at persuading or leading persons to use the oil in a method of treatment within claim 5.
220 The respondents relied on the decision of Yates J in Otsuka Pharmaceutical Co Ltd v Generic Health Pty Ltd (2012) 291 ALR 763. In that case his Honour was concerned with an application brought by the patentee for an interlocutory injunction restraining the infringement of a patent for a method of treating a patient using a particular pharmaceutical compound (aripiprazole) in the treatment of schizophrenia. I do not propose to set out the relevant claims, which are referred to by his Honour at [60]-[64] and which include claim 7. His Honour found that there was a prima facie case of infringement established based on s 117(1) when read with s 117(2)(b). With regard to the patentee’s case based on s 117(2)(c) his Honour said at [101]-[104]:
[101] Section 117(2)(c) proceeds on the basis of an instruction or an inducement to use a product in a way that would infringe the patent. In this connection the applicants rely upon the Product Information registered with respect to the GH products. I was not taken to any particular part of that product information. However, I observe that, relevantly, it simply recites the indication for which the GH products are registered. The product information also discloses that aripiprazole can be switched for other antipsychotic drugs and describes how that might be done.
[102] Two things should be noted. First, the product information does not, in terms, refer to the treatment of cognitive impairment of the kind specifically referred to in claim 7. Second, although the product information refers to switching, it does not refer to switching because of the patient’s failure to respond to other antipsychotic drugs, let alone those specified in claim 7.
[103] It follows that the applicants’ case in this regard must lie, substantially, in some implied disclosure or inducement to use the GH products in accordance with the specific integers of claim 7. There is, however, no evidence that would enable me to conclude, with any reasonable level of confidence, that prescribing doctors would read into the product information an instruction that the GH products are to be used or can be used in the method of treatment specifically claimed in claim 7. Certainly the product information does not give that instruction or inducement expressly. In this connection, it is not sufficient that prescribing doctors will or might use the GH products for a method of treatment as so claimed. Section 117(2)(c) requires there to be an instruction or inducement.
[104] I am not satisfied, therefore, that there is a prima facie case of threatened infringement of the 772 patent arising under s 117(1) of the Act based on the application of s 117(2)(c). Once again, the evidence at trial might support a different conclusion.
221 A review of the relevant claim in that case indicates that it was for a method of treating patients suffering from a cognitive disorder caused by a number of different conditions and which fails to respond to the use of a number of different antipsychotic drugs. The relevant claim was very narrowly expressed. It is against that background that his Honour concluded that there was no prima facie case of inducement to use the relevant product in accordance with the specific integers of the claim.
222 Here, as I have said, claim 5 is exceedingly broad. A reasonable person reading the entry for Kunzea ambigua oil on Bush Pharmacy’s webpage would be led to understand that the oil can be applied topically to assist in healing. Of course the website does not indicate what particular conditions the oil can be used to treat. In many cases involving method of treatment claims, that would be fatal to a case based on s 117(2)(c). However, the broad scope of claim 5 puts this case in a different category. The reader is told that the oil is suitable for medical applications and that it can be applied without the need for dilution under the guidance of a medical professional. He or she will therefore be led to understand that the oil is suitable for topical application for medical purposes. In my view the reader would also infer from what appears on the webpage that the oil can be used to assist in healing. I find that the statements appearing on the webpage are likely to lead a reasonable person who read them to use Kunzea ambigua oil in that manner and for that purpose.
223 I find that Bush Pharmacy has infringed claim 5 by supplying Kunzea ambigua oil to persons in Australia in circumstances referred to in s 117(1) when read with s 117(2)(c). I am not satisfied that claims 6 or 7 have been infringed.
224 The applicant’s case against Down Under relies on s 117(1) when read with s 117(2)(b) and (c). His pleaded case is that Down Under has sold, offered to sell, supplied and offered to supply Kunzea ambigua oil. In circumstances where I found that Kunzea ambigua oil is a staple commercial product, the case based on s 117(2)(b) must fail.
225 With regard to s 117(2)(c), the applicant relied on the contents Down Under’s website (“the Down Under website”).
226 The pleaded case as against Down Under is found in the second amended statement of claim filed on 8 March 2018. At para 10 of that document, the applicant alleges:
For a period of time beginning on a dale unknown to the Applicant to the present (the “Patent Infringement Period”), but within the six year limitations period (the “Limitations Period”), the Respondent has sold, offered to sell, supplied and offered to supply products comprising an essential oil derived from shrubs of Kunzea ambigua (the “Infringing Oil Products”).
Particulars
(a) Web pages at http://www.downunderenterprises.com/Kunzea dated 8 August 2014 showing “Australian Kunzea Oil” offered for sale in 1 kg amounts;
(b) Web pages at http://www.downunderenterprises.com/100-Pure-Australian-Kunzea-Oil-Kunzea-ambigua-1-kg dated 11 November 2015 showing “Australian Kunzea Oil” offered for sale in 1 kg amounts; and
(c) Further particulars to be provided after discovery.
227 The relevant webpages upon which the applicant relies are in fact dated 16 November 2016. No webpages dated 8 August 2014 or 11 November 2015 are in evidence. Nor were any further particulars provided contrary to what was foreshadowed in the particulars to paragraph 10.
228 In para 10 of its defence, Down Under answers the allegation that it has offered to supply and supplied relevant products as follows:
(a) says that the website referred to in the particulars to paragraph 10 of the amended statement of claim (the Down Under Enterprises (USA) Website) is operated by a company named Down Under Enterprises, Inc (Down Under Enterprises (USA)), with which Down Under Enterprises does business;
(b) further says that Down Under Enterprises (USA) has supplied essential oil derived from shrubs of Kunzea ambigua (Kunzea Essential Oil) to persons outside of Australia;
(c) further says that any Kunzea Essential Oil which Down Under Enterprises (USA) supplied to persons outside of Australia between about February 2007 and about November 2015 originated from the Applicant (or persons associated with the Applicant) at “Ducane Estate”;
(d) admits that it has supplied Kunzea Essential Oil to Down Under Enterprises (USA), including Kunzea Essential Oil that originated from the Applicant (or persons associated with the Applicant) at “Ducane Estate”, for Down Under Enterprises (USA) to sell to persons outside of Australia; and
(e) otherwise denies the paragraph.
Particulars
The Kunzea Essential Oil obtained by Down Under Enterprises or Down Under Enterprises (USA) which originated from the Applicant (or persons associated with the Applicant) at “Ducane Estate”, and which was supplied by Down Under Enterprises (USA) to persons outside of Australia, is identified as follows:
(i) June 2006 - 1 kg of “Ducane Kunzea Oil” (Batch 010) with Certificate of Analysis dated 5 June 2006 purchased from Chris Condon;
(ii) February 2007 - 5kg of “Ducane Kunzea Oil” (Batch 010) with Certificate of Analysis dated 5 June 2006 purchased from “Ducane Estate”;
(iii) February 2015 - 300g of “Ducane Kunzea Oil” (Batch no. DUC010 DU_0115) purchased from “Ducane Estate”.
229 According to Ms Prather, who is the sole director of Down Under and the President of Down Under Enterprises, Inc (“Down Under USA”), the Down Under website is maintained by Down Under USA which she says has owned the domain name at which the website is located “… since the founding of the US company in 2001”. She explained in her affidavit evidence how the US company came to be established. She said that it was formed in 2001 while she and her husband were living in the USA. She also said that Down Under USA has been wholly owned by her husband (who is a citizen of the USA) since about mid-2007.
230 Down Under was established in September 2007 when Ms Prather and her husband moved from the USA to live in Australia.
231 Down Under USA first purchased Kunzea ambigua oil from a supplier located in Los Angeles in June 2006 which was produced by the applicant. It acquired a further quantity of Kunzea ambigua oil in August 2006 which was also produced by the applicant. A further 5kg of the oil was purchased in February 2007 directly from the applicant. According to Ms Prather, approximately 3.5kg of the oil acquired directly from the applicant had been sold by the end of 2014.
232 In early 2015 Ms Prather approached Mr Hood seeking to purchase some more oil. For reasons that are unclear, Mr Hood refused to supply Ms Prather. Perhaps he understood that she might be intent on reselling the oil to retailers who he preferred to supply directly. In any event, whatever Mr Hood’s reason for not wanting to supply Ms Prather, it was necessary for her to look to other suppliers from whom to purchase Kunzea ambigua oil. Her evidence on these matters was not challenged and I accept it.
233 In or about May or June 2016, Down Under made two separate 1kg purchases of the oil from The Paperbark Co Pty Ltd (“TPBC”). TPBC was a supplier of various essential oils, including Kunzea ambigua oil. Documents produced by Ms Prather relating to these transactions clearly show that TPBC represented to Ms Prather that the oil supplied by TPBC to Down Under was produced by the applicant. This is consistent with Ms Prather’s understanding and expectation at the time since she was unaware of any other manufacturer of Kunzea ambigua oil.
234 The applicant, in his written evidence, referred to what he contended were various discrepancies in the batch numbers and certificates and analysis provided by TPBC to Ms Prather. But it appears that TPBC used its own batch numbers and produced its own certificates of analysis in respect of products that it supplied. That does not mean that the product it supplied was not produced by the applicant.
235 In his oral evidence, the applicant accepted that TPBC was a supplier of the applicant’s product in the Australian marketplace. In fact Mr Hood said in his evidence that Mr Day, who I infer is the proprietor of TPBC, was “a long term, good, regular customer”.
236 I accept Ms Prather’s evidence that the Kunzea ambigua oil acquired by Down Under between 2006 and 2016 was oil that was produced by the applicant.
237 Ms Prather’s first affidavit included details of sales of Kunzea ambigua oil made by Down Under in the period June 2016 to August 2017. Some of these sales (to the value of approximately $6000) were made by Down Under to customers located in Hong Kong, China, Japan, Taiwan, Korea, the Philippines and Singapore. During the same period Down Under USA made two sales, the first in February 2017 and the second in August 2017 (with a combined value of approximately US$1,000) to customers in the USA.
238 The sales information provided by Ms Prather also includes details of free samples that were sent to Asia and the USA to develop a Kunzea oil market in those territories. Her evidence also shows that as at May 2017 there was some small quantities of stock on hand in Australia and in the USA.
239 In her second affidavit Ms Prather stated that all sales of Kunzea ambigua oil made between June 2006 and October 2017 by either Down Under or Down Under USA were to commercial customers outside of Australia. To the extent that evidence was challenged in Ms Prather’s cross-examination, that challenge was extremely faint. The closest that counsel came to suggesting to Ms Prather that her evidence was untrue was during a brief exchange concerning the Down Under website and the facility that appears to have been available on that site to make online purchases of essential oil. Ms Prather was asked about an entry on the website for Kunzea ambigua essential oil offered for sale in 1kg bottles. The cross-examination went as follows:
MR GREEN: Do you see, there’s a photo of a bottle …? ––– Yes.
But that’s why – that’s from your website? ––– Yes.
And that’s where – do you see how it says “Buy” on the right-hand side? ––– Yes, yes.
Is that a way in which commercial customers could buy on – from the website? ––– Yes.
And so if they wished to buy a one-kilo, 100 per cent pure Australian Kunzea oil – Kunzea ambigua – they could click, and they could then obtain a price? ––– At that point, yes.
All right. And was that in place from around 2012 as well, when you revamp [sic] the website? ––– I believe so, yes.
And you fulfilled orders through the website? ––– Rarely, but it’s possible.
Did you get orders from all over the world, or only from Australia? ––– We’re global. We sell mostly into the United States. I don’t ever remember selling an oil online to anyone in Australia.
If I was in Australia, I could click on this website, and … from 2012, and certainly in 2016 – I could purchase from this website? ––– I think in 2016 we had taken down this capability. I would have to check the date, because we took the ability to do online purchasing off the website.
And the reason for that is why? ––– Because the prices – we found that a lot of competitors were going onto our website and just looking at our prices.
And undercutting you? ––– I don’t know whether they were or not, but they were looking at our prices.
240 Ms Prather’s evidence suggested that the facility allowing online purchases (“the online purchase facility”) and related price information were removed from the website in 2016. The printed extract from the Down Under website that is in evidence is dated 16 November 2016 which would suggest that, if the online purchase facility was removed in 2016, then this must have happened very late in the year. That in itself is not inconsistent with Ms Prather’s recollection that the online purchase facility was taken down in 2016. I note in her oral evidence she said that she would have to check whether that was so. But that evidence was never followed up, and was not the subject of any further elaboration in cross-examination or otherwise.
241 There is a statement in the applicant’s written evidence in which he asserts that Down Under had been selling Kunzea ambigua oil products on its website “since at least 16 November 2016”. He annexed a copy of the relevant extracts from the website as they appeared on 16 November 2016 to his affidavit. There was no additional evidence adduced by the applicant which would lead me to believe that Ms Prather’s recollection was inaccurate.
242 In 2017 Ms Prather commenced sourcing Kunzea ambigua oil from Bush Pharmacy. It is common ground that was not oil produced by Mr Hood but had been produced by Bush Pharmacy presumably using crop grown at its Flinders Island plantation. However, Ms Prather’s evidence, which I accept, was that the Kunzea oil she supplied from 2006 to 2016 was product produced by Mr Hood and that she did not purchase Kunzea ambigua oil from Bush Pharmacy until 2017.
243 It is for the applicant to prove that Down Under supplied or offered to supply Kunzea ambigua oil in the patent area that was not produced by the applicant. Having regard to the evidence to which I have referred, I am not persuaded that any Kunzea ambigua oil supplied or offered for supply by Down Under prior to late 2016 was oil produced by anyone other than the applicant.
244 For the applicant to succeed in its infringement case against Down Under it must establish, on the balance of probabilities, that some of the product it acquired from Bush Pharmacy in 2017 was supplied or offered for supply by Down Under in the patent area.
245 I note that subpara (d) to para 10 of Down Under’s defence contains an admission that Down Under has supplied Kunzea essential oil to Down Under USA to sell to persons outside Australia. However, there is no evidence to indicate whether the supply of Kunzea essential oil referred to in subpara (d) occurred within the patent area. As I have explained, Down Under USA is a United States corporation with a place of business in the USA.
246 In closing submissions the applicant sought to tender a number of invoices that I was told would show that the oil was supplied in Australia “free on board”. The tender was objected to and not pressed when it was drawn to the applicant’s attention that the invoices related to transactions that occurred after the patent had expired. In my view it is not open to me to find that any supply of product by Down Under to Down Under USA took place within the patent area. Nor does the evidence establish that Down Under supplied any product to any other person in the patent area. Ultimately, counsel for the applicant did not seek to develop any submission to the contrary.
247 I accept that if the Down Under website had, at any time after Down Under commenced supplying product it acquired from Bush Pharmacy, included the online purchase facility, then this may amount to offering such product for sale. But there is little evidence of the form the website took after 16 November 2016 and Ms Prather’s evidence, so far as it goes, suggests that by the time Down Under commenced to supply product sourced from Bush Pharmacy, the online purchase facility had been removed.
248 The nature of an “offer to sell” was considered by me in Apotex Pty Ltd v Warner-Lambert Co LLC (No 3) (2016) 123 IPR 30. As I said at [29]-[32]:
[29] In contract law, the exposure of goods for sale in a shop has usually been treated as no more than an indication of a shopkeeper’s willingness to treat rather than an offer to sell although this will not always be the case: Pharmaceutical Society of Great Britain v Boots Cash Chemists (Southern Ltd) [1952] 2 QB 795 at 801; [1952] 2 All ER 456, Reardon v Morley Food Pty Ltd (1980) 33 ALR 417. In the latter case, Smithers J said at 423:
… The display of goods in the shop window or shelf, complete with price markings is usually interpreted as an invitation to treat. Whether it is such an offer depends on the intention of the trader to be gathered from all the circumstances.
An offer may be described as an expression of willingness to contract, made with the intention, actual or apparent, that it shall become binding on the person making it as soon as it is accepted by the person to whom it is addressed. It may be made to an individual, to a specified group of persons or to the world at large. It may be made expressly by words or by conduct …
[30] However, the word “offer” in the context of the statutory definition of exploit should not be given a narrow meaning based upon contract law. In Gerber, Jacob J said at 411:
Does advertisement or any negotiation without a firm offer, amount to an “offer to dispose of”[?] Miss Heilbron suggests not, relying on authorities in English law distinguishing between an “offer” and an “invitation to treat”. An offer, in contract, is an indication of terms of an [sic] contract by which the offeror will consider himself bound if the terms are accepted. Anything short of that, in pre-contractual negotiations or an advertisement, will not do. Most advertisements do not constitute that kind of an offer (contrast the classic case of Carlill v Carbolic Smoke Ball Co [1893] 1 QB 256; [1891] All ER Rep 127) …
Mr Floyd suggests that “offer to dispose of” should not be construed so restrictively, that the matter should be looked at as one of commercial substance. “Was the product being made available to the market?” was his way of looking at it.
I have not hesitation in rejecting Miss Heilbron’s legalistic argument. Section 60 is not intended to reflect the English law of contract …
[31] I respectfully agree with his Lordship’s approach. I do not think that there is any doubt that what may be characterised for the purposes of contract law as a mere invitation to treat may constitute an “offer to sell or otherwise dispose of” a product within the statutory definition of exploit. In my opinion a display of product for sale in a shop may be within the statutory definition whether or not the display is an offer or an invitation to treat in a contractual sense.
[32] The question whether there is an “offer to sell or otherwise dispose of” a product within the statutory definition is to be determined as a matter of substance by asking whether the respondent is expressly or impliedly offering to sell or dispose of the relevant product. In deciding that question, it is necessary to have regard to all the relevant circumstances in which the alleged offer is made. These may include the subjective intention of the party who is alleged to have made the offer.
249 The circumstances of that case were different from those with which I am now concerned. For one thing, the statutory language being construed (“offer to sell or otherwise dispose of”) in that case appear in the definition of “exploit” in Sch 1 of the Act and not the definition of “supply”. Nevertheless, what is said in relation to those words also applies to the words “offer to supply” that are included in the definition of “supply” provided it is also recognised that the offer to supply a product must be an offer to supply the product in the patent area.
250 In the present case there are a number of considerations that lead me to think that there is no offer to supply by means of the Down Under website if, as Ms Prather’s evidence suggested, the online purchase facility and related pricing information were removed in late 2016.
251 The few pages of the website that are in evidence (which relate only to Kunzea ambigua oil) mainly consist of product information of a technical nature. Ms Prather’s evidence to the effect that the website was directed at wholesalers (even though it could be accessed by any member of the public) was not challenged and appears to me to be correct.
252 The removal of the online purchase facility and pricing information is significant and suggests that the website, even if it may have been visited by potential customers who may have been interested in purchasing essential oils supplied by Down Under or Down Under USA, was not one that conveyed any willingness to supply goods in the patent area. The evidence is unclear as to how Ms Prather would have responded had a visitor to the website contacted Down Under or Down Under USA and asked to be supplied with the Kunzea ambigua oil in Australia. There is no evidence of any person in the patent area having responded to the website as if it was advertising product for supply in Australia. In fact there is no evidence of any person in Australia (with the possible exception of Mr Hood in November 2016) having visited the Down Under website.
253 In the result, I am not persuaded that in 2017 or any material time thereafter, Down Under offered to supply Kunzea ambigua essential oil in the patent area.
254 It follows that the case brought against Down Under based on s 117 of the Act fails.
255 It is not disputed that at all relevant times after 17 November 2016 New Directions supplied, and offered to supply, Kunzea ambigua essential oil in the patent area.
256 The infringement case brought against New Directions was based on s 117(1) when read with s 117(2)(b) and s 117(2)(c). Given my finding that Kunzea ambigua oil is a staple commercial product, the case based on s 117(2)(b) must fail.
257 So far as the applicant’s case based on s 117(2)(c) is concerned, the evidence relied upon by the applicant consisted of various webpages found at the website newdirections.com.au (“the New Directions website”). That is a website at which New Directions sells its Kunzea ambigua essential oil (and other essential oils) to customers in containers ranging in size from 100ml to 20kg.
258 It was submitted on behalf of the applicant that the New Directions website contains instructions or an inducement to use the product in a manner that would infringe.
259 The New Directions website includes a description of Kunzea ambigua oil and its uses. On various pages the following statement appears:
Common uses: Kunzea has traditionally been used for relieving muscular aches and pain. It has also been used to relieve symptoms of cold and flu when used in steam inhalations.
260 The first question is whether the New Directions website provides “instructions for use” to use the product in a manner that would infringe the patent.
261 According to the Oxford English Dictionary, “instructions” are “directions about how something should be done or operated; detailed information given as to the use, assembly, etc., of a product”.
262 It is necessary to distinguish between the two uses referred to on the New Directions website.
263 The second of the uses referred to on the New Directions website is “to relieve symptoms of cold and flu when used in steam inhalations”. For reasons previously explained, none of the claims covers the use of “steam inhalations” and on that basis that statement cannot amount to an instruction or inducement to use the oil in a manner that would infringe the patent.
264 The first of the uses referred to (ie. for relieving muscular aches and pains) describes a use for Kunzea ambigua essential oil but says nothing about how the product is to be used. The webpage does not indicate whether or not the product should be applied topically. While the webpage specifies a use, it does not provide any instructions for use.
265 This brings me to the question of whether the contents of relevant webpages contain any inducement to use the product in a manner that would infringe the patent.
266 Mr Fox submitted on behalf of New Directions that this statement does not constitute an inducement to use Kunzea ambigua oil in a manner that would infringe the patent. He placed particular reliance on the fact that the common uses described do not instruct or encourage the topical application of the oil.
267 In order to infringe claim 5 (the broadest of the relevant claims) the oil must be topically applied. A person familiar with the use of essential oils may well understand that this is how the oil should be used, but in my view the relevant webpage does not seek to encourage or persuade a user to use the oil in any particular way. Although it is true that a reader may infer that the oil is to be topically applied, he or she may just as readily infer that the oil is to be diluted in a bath or administered by inhalation. The website says nothing about how the oil is to be used as a treatment for muscular aches and pains.
268 In my view the relevant webpage does not contain any statement that is properly characterised as an instruction or inducement to use Kunzea ambigua oil in a manner that would infringe the patent.
269 I find that the case against New Directions based on s 117 of the Act fails.
270 The case against Native Oils based on s 117(1) relies only on s 117(2)(b). Given my finding that Kunzea ambigua oil is a staple commercial product that case must fail.
271 I should for completeness make some further findings in relation to the application of s 117(2)(b) in the case of Native Oils. The applicant contended, as it had to if it was to succeed in a case based on s 117(2)(b), that Native Oils had reason to believe that the Kunzea ambigua oil supplied by it would be put to a use that would infringe the patent.
272 In its defence, Native Oils did not admit that it had reason to believe that persons to whom it supplied Kunzea ambigua essential oil would put it to any of the uses claimed in the patent.
273 The applicant’s approach to this aspect of the case was to ask Mr Johnson, in the course of a lengthy cross-examination, whether he believed that Kunzea ambigua oil would assist in the treatment of various ailments. Mr Johnson denied that he had reason to believe that many of the uses to which he was referred in cross-examination were familiar to him. For example, he was asked whether he had “… heard that it could be used for minimising bruising”. He denied that he had heard that. He also said he had never heard of the oil being used to treat pain from headaches or insect and other bites.
274 The applicant submitted that Native Oils had “reason to believe” that persons would use the products for at least the uses claimed in claims 5, 6 and 9 because Mr Johnson:
was aware that it was quite possible that some of his customers would resell the product into a therapeutic market;
believed that the product would assist with the treatment of tired muscles and joints;
had reason to believe that the product would be used to treat dry or itchy skin; and
believed that the product could possibly be used to reduce muscle pain.
275 For the purpose of putting Mr Johnson’s cross-examination in its proper context, it is necessary to refer to some of the documentary evidence. The applicant tendered several webpages from the Native Oils website. These pages were dated 17 November 2016. Although Mr Johnson was asked some questions in cross-examination concerning those documents, he was also asked some questions concerning what was said to be pages from a new website created in May 2018. The timing is significant because that is approximately six months after the patent expired. Neither the applicant nor the respondents tendered any of the pages from the new website by reference to which the cross-examination I am about to refer to proceeded and those pages are therefore not in evidence.
276 During the course of the cross-examination the following exchange occurred with reference to the new website:
MS OLIAK: Okay. So on the website you have stated that it can help with tired muscles and joints. So that was one thing that you believe that this oil would be useful for; correct? ––– Yes.
Okay. And then you say it can also be used on dry or itchy skin. So that’s another use that you had reason to believe the oil would be used for; correct? ––– Yes.
And you would – would you agree that dry or itchy skin is a skin irritation? ––– Yes, I would.
Yes? And for tired – tired muscles and joints, would you also agree that Kunzea – you would have reason to believe that Kunzea ambigua would be used for relieving muscle pain, for example? ––– Possibly, but several oils could be, I assume.
And had you also, back when you first started selling the Kunzea oil, were you also aware of any uses, for example, of the oil to relieve sinus congestion? ––– I hadn’t heard of that, no.
277 Mr Johnson agreed that Kunzea ambigua oil “can help with tired muscles and joints” and that this was something that he believed the oil would be useful for. That, in my view, falls short of a concession that either Mr Johnson, or his company, had reason to believe that Kunzea ambigua oil supplied by Native Oils during the term of the patent was likely to be used for that purpose.
278 It was put to Mr Johnson that the oil could be used on dry or itchy skin and that this was another use that he would have reason to believe the oil would be used for. Mr Johnson agreed. It was also put by counsel for the applicant to Mr Johnson that “dry or itchy skin” is a “skin irritation” in the language of claim 9. He agreed.
279 As I have mentioned, Native Oils’ defence does not deny the allegation of “reason to believe”. The relevant allegation is traversed by a mere non-admission. More significantly, however, is Mr Johnson’s evidence-in-chief (which was given by affidavit supplemented by some brief oral evidence) which does not address the question of whether Mr Johnson or his company had reason to believe that in the period up to the expiry of the patent in which Native Oils was admittedly supplying Kunzea ambigua oil that such oil would be used in an infringing manner.
280 In light of the evidence given by Mr Johnson in relation to the use of Kunzea ambigua oil as a treatment for dry or itchy skin, coupled with the absence of any pleaded denial or any countervailing evidence-in-chief from Mr Johnson on the point, I am satisfied that, at all material times when Native Oils was supplying Kunzea ambigua oil, it had reason to believe that the oil would be used to treat dry or itchy skin in a manner that would result in an infringement of claim 9.
281 Heritage Oils is a company that was incorporated on 23 May 2016. At all material times since its incorporation, Ms Levinson, and her partner, Mr Brocklehurst have been its only directors. It appears from the evidence that the company took over the business that had been previously conducted by Ms Levinson sometime after the company was incorporated under the business names “Heritage Oils” and “Heritage Oils Tasmania”. It is alleged Ms Levinson supplied Kunzea ambigua essential oil and related products under those business names prior to 23 May 2016 and that the company has done so since then. According to Ms Levinson’s evidence, which I accept, she first commenced selling such products on about 27 December 2012. However, she did not become aware of the existence of the patent until she received a letter of demand from the applicant’s solicitors in or about December 2016.
282 The applicant tendered various samples of Heritage Oils’ products and product labelling. One such exhibit (Exhibit 8) was a 10ml bottle of Kunzea oil, the label of which read as follows:
Kunzea Oil
Kunzea ambigua
Use with a vaporiser as it can relieve respiratory
complaints and a wonderful relaxing bath oil
10ml Batch: 0021112 Expiry Date: August 2016
Heritage Oils Tasmania
16A Dane Street, East Victoria Park, WA 6101
+61 (0) 427 416 681
www.heritageoilstasmania.com.au
283 At the time the bottle was tendered I raised with Mr Green SC the relevance of the label and the requirement in the relevant claims that the oil be applied topically. The label refers not to topical application but to use of the oil with a vaporiser and as a bath oil. Although I was informed that counsel would address this in final submissions, he did not return to it and never sought to explain how what appeared on the bottle could amount to an instruction or inducement to use the oil in an infringing manner. In my view labelling in the form of that shown in Exhibit 8 cannot amount to an instruction or inducement to use the oil in an infringing manner.
284 There were other labels tendered by the applicant (Exhibit 9) which reflected labelling used by Heritage Oils as at September 2018. These labels fall into two categories. The first relates to labels for bottles containing “100% pure” Kunzea ambigua oil. The only indication of use appearing on those labels is the word “aromatherapy”. There is nothing on those labels that could amount to an instruction or inducement to use the oil in an infringing manner.
285 There are other labels included in Exhibit 9 for a product called “Smitten” which is a blend of Calendula, Arnica, Sandalwood Nut oil, Almond oil and Kunzea oil. For reasons previously explained, a blended oil does not fall within any of the relevant claims.
286 What I have said in relation to Smitten applies equally to another Heritage Oil product called “Joint and Muscle Massage Oil” which is also a blend of oils that includes Rosehip oil, Sandalwood Nut and Kunzea ambigua oil. Ms Levinson’s unchallenged evidence was that it was the Sandalwood Nut oil in the Joint and Muscle Massage Oil that was the main ingredient.
287 The use of Joint and Muscle Massage Oil in a relevant method of treatment could only be within claims 10-13 if all of the other oils included in the blend are regarded as a carrier of the Kunzea ambigua oil. There was no evidence which would support that characterisation of the role of the other oils. It may well be that the Joint and Muscle Massage Oil includes a carrier but it is not established that it is the function of all the other oils in the blend (including the main ingredient) to act as a carrier of Kunzea ambigua oil. It is more likely than not, in my view, that at least some of the other oils have been included in the blend on account of their aromatic or therapeutic properties. In my view they do not constitute, either individually or collectively, a carrier within the meaning of claims 10-13.
288 Other products supplied by Ms Levinson and Heritage Oils upon which the applicant relies includes various shampoos, conditioners and soaps which included amongst their ingredients Kunzea ambigua essential oil. In my view none of these products is within any of the claims relied upon by the applicant. In particular, the evidence does not establish in respect of any of those products that the other ingredients constitute, either individually or collectively, “a carrier” within the meaning of claims 10-13.
289 It is apparent from the description of the ingredients of the shampoo that appears on the Heritage Oils website that they include purified water, Lauryl Glucoside, Cocoamodopropyl, Betaine derived from Coconut oil, Sodium Laurel (sic), Sarconsonate, Sodium Lauroamphoacetate, Coco Glucoside, Glyceryl Oleayte, Sodium Hydroxymethylglycinate and Citric acid. Not surprisingly, at least some of these ingredients are likely to have been included for their cleaning and emulsifying properties (e.g. Sodium Lauryl sulfate). I am not satisfied on the evidence, which, if any, of these ingredients could properly be regarded as a carrier in the overall formulation.
290 It is also apparent from the description of the ingredients of the conditioner that appears on the Heritage Oils website that they include Sandalwood Nut oil, purified water, Cetyl Stearyl alcohol derived from vegetable oil, Cetrimonium chloride derived from vegetable oil, Citric acid and Sodium hydoxymethylglycinate water. Again, it seems likely that at least some of these ingredients are included in the formulation not to act as a carrier of the Kunzea ambigua oil, but for their softening or hydrating properties. Again, I am not satisfied on the evidence which, if any, of these ingredients could properly be regarded as a carrier in the overall formulation.
291 This brings me to the “pure Kunzea oil soap”. The relevant entry on the Heritage Oils website for the product is as follows:
Pure Kunzea Oil Soap
Heritage Oils handmade Kunzea Essential Oil soap is a rich, foaming soap which can be used daily over the face and body by the whole family. Made with the purest Kunzea Oil, this soap leaves your skin invigorated, refreshed and fully cleansed.
Try Kunzea soap if you have any skin disorders and rashes as the oil helps rehydrate the skin and clear blemishes.
A perfect compliment for your body after a gentle massage with Joint & Muscle Massage Oil.
Free from Palm Oil and added chemical fragrances and colours.
Ingredients
Kunzea Essential Oil, Kunzea ambigua, Coconut Oil, saponfied [sic] olive oil
292 There was no evidence to indicate what the role of the coconut oil or saponified olive oil was in the product formulation for Kunzea ambigua soap. In particular, there was no evidence directed to establishing that the function of either the coconut oil or the saponified olive oil was to act as a carrier of the Kunzea ambigua oil. While that may well be their function, that is not an inference I am willing to draw in the absence of any evidence to that effect. It was for the applicant to prove that the use of Heritage Oils’ Kunzea ambigua oil soap for a relevant method of treatment would infringe claims 10-13. In my view the evidence does not support such a finding.
293 It follows that any case against Ms Levinson and Heritage Oils under s 117 must be confined to the supply of bottles of pure Kunzea ambigua oil. The question then is whether any of those bottles were supplied by Ms Levinson or Heritage Oils in the circumstances referred to in either s 117(2)(b) or (c). For reasons previously explained, s 117(2)(b) does not apply. So the infringement case against Ms Levinson and Heritage Oils based on the supply of bottles of pure oil turns on the application of s 117(2)(c).
294 I do not accept that the label on the bottles of Kunzea ambigua oil supplied by Ms Levinson or Heritage Oils contains any relevant instructions or inducement. However, the applicant also relies on statements appearing on webpages at the Heritage Oils Tasmania website and the Heritage Oil website.
295 The evidence includes various reproductions of webpages from the Heritage Oils Tasmania website (www.heritageoilstasmania.com.au) as it stood at 11 June 2014. The website at that time included the following entry (“the 2014 entry”) in respect of small bottles of pure Kunzea oil that were being offered for sale on the website:
Kunzea Oil - Pure
Our Kunzea Oil is processed from the Tasmanian Kunzea - Kunzea ambigua - grown and processed sustainably [sic] on farms on the pristine slopes of north-eastern Tasmania and Flinders Island, Australia. The tree is sometimes referred to as ‘White Cloud’ due to its profusion of white flowers during flowering season.
Kunzea Oil is a 100% pure essential oil with known properties to aid in the relief of muscular and joint aches and pains. A few drops of Kunzea Oil has been proposed to assist in the relief of sinus which comes with coughs and colds and serves as a more gentle inhalant for children than some euacalyptus [sic] oils.
Kunzea oil has been used to help relieve nervous tension and is known to reduce anxiety of which there have been many anecdotal reports. Kunzea Oil has a soft, mildly eucalyptus note and when combined with Tasmanian Lavender Oil can produce a beautiful calming aroma. We have blended these oils in our Smitten which has been used for this purpose - see our testimonials. Kunzea Oil is known to assist in relieving the itchiness from bites and stings which will avoid further infection. It is implied that Kunzea Oil can assist in the reduction of skin rashes and blemishes, applied with a chosen carrier oil like Rosehip oil to help rehydration [sic]. Kunzea Oil is used for Aromatherapy and when combined with Sweet Almond oil, makes a wonderful addition to a stress free massage. Try a few drops of Kunzea Oil in a vaporiser or in the bath for its calming effect and cleansing aroma. It will make your bath a rewarding experience.
There are no known side effects of the use of Kunzea Oil, it is a safe and non-toxic oil for general use, pregnancy included. However, as with all essential oils, it is advised to try a few drops on the skin before application, although it is known to be well tolerated on the skin in an undiluted state. Safe for family use.
Store your Kunzea ambigua oil out of direct light and keep out of the reach of children.
Highlights of Kunzea ambigua Oil
Known to be effective in the reduction of joint aches and pains and the reduction of redness in skin blemishes and rashes. Can be used as an inhalant and a relaxant.
296 The 2014 entry suggests a range of possible uses for Heritage Oils’ pure Kunzea ambigua oil. In the second paragraph it refers to the use of the product as a gentle inhalant for “the relief of sinus which comes from coughs and colds”. In that context it does not expressly indicate how the oil is to be applied but a little further down, in the third paragraph, it does refer to the use of a vaporiser. It seems to me that the 2014 entry stops short of encouraging or promoting the use of the topical application of the oil for the relief of sinus congestion.
297 The 2014 entry also states that Kunzea ambigua oil is known to be effective in the reduction of joint aches and pains. There is no express indication given as to how the oil should be applied when used to treat that condition. However, there is also a statement to the effect that Kunzea ambigua oil is known to be well tolerated on the skin in an undiluted state. In the circumstances, I think the 2014 entry does encourage and promote the use of the oil as a treatment for the relief of joint aches and pains by, inferentially, topical application of the oil to the skin. Although there is no direct statement in the 2014 entry that when the oil is used for the relief of muscular and joint pain, that it should be applied topically, I think there is a clear inference conveyed when the entry is read as a whole, that this is at least one way in which the oil may be used. In my opinion the 2014 entry constitutes an advertisement that contains an inducement to use the pure oil in a method of treatment within the scope of claim 5.
298 The evidence includes various printouts of webpages downloaded from the Heritage Oils website (www.heritageoils.com.au) on 20 July 2016. These webpages include the following entry for Kunzea essential oil (“the 2016 entry”):
Kunzea essential oil can be identified as one of nature’s most effective multi-use oils. Much research has been done on this versatile oil.
• As an anti-inflammatory. It’s known properties are said to aid in the relief of muscular and joint aches and pains.
• A few drops of Kunzea Oil is a mild decongestant to assist in the relief of sinus and serves as a more gentle inhalant for children than some eucalyptus oils.
• It can help relieve nervous tension and is known to reduce anxiety of which there are various anecdotal reports.
• It is implied that Kunzea Oil has been used to assist in healing skin disorders and reduce skin rashes like eczema and psoriasis.
• Kunza [sic] essential oil is used in Aromatherapy and when combined with a good carrier oil makes a wonderful addition to a stress free massage. Try our Joint and Muscle Massage Oil.
• Try a few drops of Kunzea Oil in a vaporiser or in the bath for its calming effect and cleansing aroma. It will make your bath a rewarding experience.
• Can be used on pets to ward off ticks and fleas - see story from a user in the News report.
• Blends well with other oils: Lavender, WA Sandalwood, Rosalina, Lemon
We use Kunzea Oil in our Bodycare products - pure handmade soap blended with coconut oil to help reduce irritation and skin disorders, shampoo and conditioner to aid scalp conditions and blended with Sweet Almond oil, in our aromatherapy massage oils to help reduce arthritis and muscular pain.
We have blended Kunzea essential oil with Lavender Oil and WA Sandalwood Nut Oil to create our product Smitten (blended essential oils) to assist in relieving irritation and inflammation from bites and stings. With children this can reduce further infection by reducing the urge to scratch - (see Testimonials)
There are no known side effects with the use of Kunzea Essential Oil, it is a safe and nontoxic oil for general use. However, as with all essential oils, it is advised to try a few drops on the skin before application. Safe for family use.
Store your Kunzea oil out of direct light in a cool and dry place and keep out of the reach of children.
(original emphasis)
299 The 2016 entry also suggests a range of possible uses for Heritage Oils’ pure Kunzea oil. Not all of these uses would be within the relevant claims. Use of the oil with a vaporiser would not amount to an infringement regardless of what condition the oil was being used to treat. Similarly, for reasons previously explained, use of the oil in a blend with another oil (except where the other oil functions as a carrier) would not constitute an infringement.
300 The 2016 entry states that the oil is known to aid the relief of muscular and joint aches and pains. A little further on, there is a statement suggesting that the oil be tested on the skin before (by implication) topical use.
301 In my view the 2016 entry encourages and promotes the use of the oil as a treatment for the relief of joint aches and pains by topical application of the oil to the skin. I therefore find that the 2016 entry is an advertisement that contains an inducement to use the pure oil in a method of treatment within the scope of claim 5.
302 Section 122(1A) of the Act provides:
(1A) A court may include an additional amount in an assessment of damages for an infringement of a patent, if the court considers it appropriate to do so having regard to:
(a) the flagrancy of the infringement; and
(b) the need to deter similar infringements of patents; and
(c) the conduct of the party that infringed the patent that occurred:
(i) after the act constituting the infringement; or
(ii) after that party was informed that it had allegedly infringed the patent; and
(d) any benefit shown to have accrued to that party because of the infringement; and
(e) all other relevant matters.
303 In each of the proceedings, the applicant contends that he is entitled to additional damages under s 122(1A) of the Act based on the matters pleaded in para 20C of the second further amended statement of claim filed in the proceeding against Bush Pharmacy in which it is alleged:
20C. The Applicant is also entitled to and claims additional damages under section 122(1A) of the Patents Act based on the following:
(a) the Respondent did the infringing acts flagrantly (sub-section 122(1A)(a)):
(i) the Respondent was aware of the Applicant, the Applicant’s business and that the Applicant was the inventor and registered owner of the ‘156 Patent;
(ii) the Respondent knew or had reason to believe that it did not have the licence or authority of the Applicant to do the infringing acts;
(iii) the Respondent knew or had reason to believe that doing the infringing acts would constitute an infringement of the ‘156 Patent;
(iv) the Respondent never sought the permission or authority of the Applicant to perform the infringing acts (or any other acts) in relation to the ‘156 Patent; and/or
(v) the Respondent believed that the Applicant did not have the financial and/or other resources necessary to enforce the ‘156 Patent and took a calculated risk in that regard;
(b) there exists a need to deter persons and entities from deliberately, unfairly and improperly infringing patents owned by others particularly where a calculated risk has been taken by those persons in relation to the potential enforcement of the patents against them (sub-section 122(1A)(b));
(c) after the Respondent was informed by the Applicant of the patent infringement referred to herein, the Respondent wrongfully asserted that it had a defence to patent infringement knowing that no valid defence existed and continued to perform the infringing acts (sub-section 122(1A)(c)); and/or
(d) the Respondent benefited from its patent infringement because it was able to offer a relatively uncommon essential oil product to its customers and obtain a financial benefit from any revenue derived from any sales of such products (sub-section 122(1A)(d)).
The same matters are relied upon against each of the respondents.
304 The case for additional damages is predicated on a finding that the respondents not only knew of the existence of the patent, but understood that they did not have any valid defence to a claim for its infringement.
305 The evidence relied upon by the applicant includes letters of demand sent to the various respondents on 2 December 2016. In all relevant respects, the letters of demand are in identical terms.
306 The letters of demand refer to the patent, the date that the complete specification first became open for inspection, and sets out claims 1-3. The letters of demand asserted that claims 1-3 of the patent had been infringed by each respondent.
307 There are several observations to make in relation to the claim for additional damages.
308 First, as previously noted, the applicant accepts that claims 1-3 are invalid. To the extent the issue of the validity of those claims was explored in the cross-examination of the respondents’ witnesses, there was a consistent theme to the effect that none of the respondents believed that an essential oil could be patented. Given the applicant accepts that claims 1-3 were invalid for lack of novelty, it is not necessary for me to reach any conclusion in relation to the manner of manufacture point but it is fair to say that the argument raised by the respondents to the effect that an essential oil extracted from a native shrub is not patentable subject matter is reasonably (perhaps even strongly) arguable. In any event, the claims upon which the applicant based his letters of demand are admitted to be invalid. Non-compliance with the letters of demand in those circumstances could hardly be evidence of flagrancy or provide any other basis for awarding the applicant additional damages.
309 Secondly, the letters of demand did not make any reference to any of the methods claims or to s 117 of the Act. Once proceedings were commenced, the applicant pleaded a case based on s 117(2)(a), (b) and (c). It was only at the hearing that the applicant abandoned reliance upon s 117(2)(a). Further, I have found that s 117(2)(b) cannot apply because the essential oil derived from the Kunzea ambigua shrub is a staple commercial product. Even if that conclusion were found to be incorrect, I think it clear that the respondents’ argument that s 117(2)(b) did not apply was a perfectly reasonable one.
310 Thirdly, while the applicant has had some success against the respondents based on s 117(2)(c), the arguments raised in answer to the claim based on that provision involved what in my view amounted to a bona fide and reasonable testing of the applicant’s infringement case. It was never put to the respondents’ witnesses (including Ms Levinson) that they believed that their company was infringing, or was likely to be infringing, any one or more of the method of treatment claims.
311 Nor do I accept that the evidence shows that any of the respondents engaged in any deliberate, unfair or improper conduct involving what is referred to in the applicant’s pleading as “a calculated risk” or that any of them have “wrongfully asserted” a defence to patent infringement “knowing that no valid defence existed”. In the circumstances, I think the whole of the pleaded claim for additional damages falls away.
The Patent amendment application
312 I have previously set out details of the amendments to the patent which the applicant now seeks. These are not the amendments that were notified to the Commissioner of Patents or advertised in the Australian Official Journal of Patents. As I have explained, the notified amendments were not pressed by the applicant.
313 The proposed amendments, in their revised form, correct what are in my view obvious errors in claims 6, 7, 8, 9 and 10, each of which should have referred to claim 5 rather than claim 4, and in claims 11, 12 and 13, each of which should have referred to claim 10 rather than claim 8.
314 The proposed amendments were resisted by the respondents primarily on the basis of the applicant’s delay in making the amendment application. There are a number of reasons why I do not think delay provides a proper basis to refuse orders correcting what in my view are obvious mistakes.
315 First, the respondents who were found to have committed acts of patent infringement were found liable on the basis of claim 5. This is a claim in which the obvious error does not appear and does not require amendment.
316 Secondly, the proposed amendments to claims 6-10 and 11-13 do no more than correct obvious errors that, in my view, were unlikely to have, and did not, mislead any of the respondents.
317 The applicant also proposes amendments to claim 5, 14, 15 and 18 that are said by him to be purely consequential upon my finding that claims 1, 2, 3 and 4 are invalid.
318 Although I propose to allow the proposed amendment to claim 5, I will not allow the proposed amendments to claims 14, 15 or 18.
319 Proposed claims 14 and 15 are to “an essential oil … wherein the oil is used” either as an “insect repellent” (claim 14) or a “rust inhibitor” (claim 15). There are difficulties associated with claims expressed in this form because they blur the distinction between product and method claims which informs the definition of “exploit” in Sch 1 of the Act by reference to which the patentee’s exclusive rights are defined in s 13 of the Act.
320 In substance each of proposed claims 14 and 15 is to an essential oil derived from the shrub Kunzea ambigua and obtained by steam distillation of the green matter of the shrub. The use of the word “for” in the claim merely requires that the oil be suitable for the stated purposes. A claim in that form would be invalid for lack of novelty. Further, proposed claim 14 would not be fairly based on (or supported by) matter disclosed in the specification which makes no reference to the use of the relevant oil as an insect repellent.
321 Proposed claim 18 is to an essential oil derived from the Kunzea ambigua shrub “obtained by steam distillation of the green matter of the shrub and substantially as herein before described”. A claim in that form would also be invalid for lack of novelty.
322 As I have mentioned, the applicant abandoned his trade mark infringement case against each respondent towards the end of the hearing. But having regard to other issues that arise in the proceedings, including allegations that the respondents have contravened provisions of the ACL, and questions of costs that are also likely to arise, it is necessary for me to say something about the trade mark issues even though they are no longer directly in play.
323 At all relevant times the applicant was the registered owner of Australian Trade Mark No 744122 (“the Trade Mark”) comprising the words “DUCANE KUNZEA” which has been registered since 17 September 1997 for the following goods:
Essential oil and products containing essential oils including lotions, aromatics, incense, creams, cosmetics, perfumes, scents, shampoos, toiletries, skin care, anti-oxidant preparations
324 As will be apparent from some of the evidence to which I have already referred, the use made of the word “Kunzea” by the respondents on product labels and on webpages included the use of names or descriptions such as:
“Kunzea”
“Kunzea ambigua”
“Kunzea essential oil”
“Kunzea oil”
“Pure Australian Kunzea oil”
“Pure Australian Kunzea oil (Kunzea ambigua)”
“Australian Kunzea essential oil”
“Kunzea conditioner”
“Kunzea medicating shampoo”
“Pure Kunzea oil soap”
325 The applicant’s case, as pleaded and opened, was that the use of these names and descriptions was infringing use under s 120(1) of the Trade Marks Act 1995 (Cth) (“TM Act”). Infringement was denied on the basis that the name Kunzea had not been used as a trade mark, and was neither substantially identical or deceptively similar to “DUNCANE KUNZEA”. The respondents also relied on the defence under s 122(1)(b) of the TM Act.
326 Of course not every use of the word “KUNZEA” disclosed in the evidence could possibly amount to trade mark use. More often than not, the words “Kunzea” and “Kunzea ambigua” are being used by the respondents to describe the essential oil or the Kunzea ambigua plant from which the essential oil has been derived.
327 According to the applicant’s written opening submissions, the word “Kunzea” is substantially identical to “Ducane Kunzea”. He contended that, applying a side by side comparison of “DUNCANE KUNZEA” and “KUNZEA”, the word “DUNCANE” made a relatively unimportant contribution to the overall impression conveyed in a comparison of the two marks. That argument is, and always was, untenable and it was rightly abandoned by Mr Green SC, not before time, in his oral opening submissions.
328 Even when assessing “deceptive similarity” it is necessary to have regard to the whole of the registered trade mark and not simply some part of it. In the present case the applicant’s case focused entirely on the word “KUNZEA” which is the botanical name used to identify the genus of certain plants from which the essential oil supplied by the respondents is derived. The applicant’s trade mark case focused entirely on the least distinctive element of the mark.
329 The applicant also pleaded a claim for additional damages in respect of such infringements under s 126(2) of the TM Act. For example, as against Bush Pharmacy, the applicant alleged, in its second amended statement of claim at para 28A:
28A. The Applicant is also entitled to and claims additional damages under section 126(2) of the TM Act based on the following:
(a) the Respondent did the infringing acts flagrantly (sub section 126(2)(a));
(i) the Respondent was aware of the Applicant, the Applicant's business and that the Applicant was the registered owner of the Trade Mark;
(ii) the Respondent knew or had reason to believe that it did not have the licence or authority of the Applicant to do the infringing acts;
(iii) the Respondent knew or had reason to believe that doing the infringing acts would constitute an infringement of the Trade Mark;
(iv) the Respondent never sought the permission or authority of the Applicant to perform the infringing acts (or any other acts) in relation to the Trade Mark; and/or
(v) the Respondent believed that the Applicant did not have the financial and/or other resources necessary to enforce the Trade Mark and took a calculated risk in that regard;
(b) there exists a need to deter persons and entities from deliberately, unfairly and improperly infringing trade marks owned by others particularly where a calculated risk has been taken by those persons in relation to the potential enforcement of the trade marks against them (sub-section 122(2)(b));
(c) after the Respondent was informed by the Applicant of the infringement of the Trade Mark referred to herein, the Respondent wrongfully asserted that it had a defence to trade mark infringement knowing that no valid defence existed and continued to perform the infringing acts (sub-section 122(2)(c)); and/or
(d) the Respondent has benefited and continues to financially benefit from its trade mark infringement because it derives revenue from the sales of its Infringing Goods (sub-section 122(2)(d)).
330 It is appropriate to draw particular attention to the allegations made in para 28A(a)(iii) and (c) which are, and always were, completely unsupportable.
331 The applicant is not to be criticised for abandoning the trade mark case. But, in due course, when considering questions of costs, it may be necessary to consider whether the claims for trade mark infringement should ever have been made in the first place.
332 The applicant seeks declaratory, injunctive and pecuniary relief in relation to what was said in closing submissions were misleading and deceptive representations made by Bush Pharmacy, Down Under, New Directions, Heritage Oils and Ms Levinson, each of which is alleged to have thereby engaged in conduct in contravention of s 18 and s 29 of the ACL. No such claim is made against Native Oils.
333 The applicant’s pleaded case against the relevant respondents is problematical in that nowhere in his pleadings does the applicant plead any of the representations that he sought to rely on in closing submissions. It is also complicated by the abandonment of his trade mark case or any related allegation of passing off.
334 It was submitted for the applicant in his closing submissions that:
60. Mr Hood originally asserted two interrelated species of conduct contrary to the ACL: the first concerning passing off of the Respondents’ products as those of Mr Hood; and the second that each Respondent had referred to the TGA Listing in relation to Kunzea ambigua, either directly, or by referring to the five Standard Indications listed in respect of Mr Hood’s product: SASOC [31]-[36]; (CB:1:7). Only the ACL contraventions arising from the second class of conduct is maintained by Mr Hood.
335 It was also submitted for the applicant in his closing submissions that:
69. The pleaded cases. There was some suggestion during the hearing that the Applicant’s pleaded case in each proceeding did not raise the misrepresentations by each Respondent concerning the TGA Listing. The Applicant submits that the issue was clearly pleaded and no surprise could be said to arise. In summary, the pleading in each proceeding pleads: the TGA Listing in relation to five Standard Indications; that each Respondent did not itself obtain registration under the TGA; that Bush Pharmacy and Down Under referred to Mr Hood’s TGA Listing, while Down Under, New Directions and Heritage Oils / Ms Levinson referred to a number of the Standard Indications for the TGA Listing, in selling and offering to sell the Infringing Goods; that each Respondent made representations in relation to the TGA registration status of the Infringing Goods (as that term is defined in the pleadings), the qualities or properties of the Infringing Goods, the Infringing Goods being sponsored or approved by the Applicant, and that each Respondent having a sponsorship, approval or affiliation with Mr Hood, Ducane Kunzea or Ducane Kunzea Oil. The pleading necessarily entails the representations further summarised below (at paragraphs 72, 76, 82, and 86).
(footnotes omitted)
336 Contrary to what appears in the paragraph above, I do not accept that the ACL case was “clearly pleaded”. However, I do accept that in relation to what I see as the central issue in the ACL case none of the respondents could have been taken by surprise. I refer here to the allegation that at least some of the respondents have represented that their Kunzea ambigua oil products are listed on the ARTG (ie. “listed goods”) or registered on the ARTG (ie. “registered goods”) when in fact they are not.
337 The last line of para 69 identifies a number of paragraphs in the applicant’s closing submissions which are said to identify the representations upon which the applicant relies in support of his ACL case. I will refer to those paragraphs in more detail when dealing with the specific matters relied upon by the applicant in support of his ACL case against each of the relevant respondents.
Therapeutic Goods Act 1989 (Cth)
338 It is necessary to explain some aspects of the regulatory scheme governing the supply and marketing of therapeutic goods in Australia.
339 Section 3 of the TG Act defines “therapeutic goods”. Relevantly, these include goods:
(a) that are represented in any way to be, or that are, whether because of the way in which the goods are presented or for any other reason, likely to be taken to be:
(i) for therapeutic use; or
(ii) for use as an ingredient or component in the manufacture of therapeutic goods; or
(iii) for use as a container or part of a container for goods of the kind referred to in subparagraph (i) or (ii); or
…
340 Section 3 of the TG Act relevantly defines “therapeutic use” to mean:
use in or in connection with:
(a) preventing, diagnosing, curing or alleviating a disease, ailment, defect or injury in persons; or
(b) influencing, inhibiting or modifying a physiological process in persons; or
…
341 Section 3 of the TG Act defines “medicine” to mean:
therapeutic goods (other than biologicals) that are represented to achieve, or are likely to achieve, their principal intended action by pharmacological, chemical, immunological or metabolic means in or on the body of a human; and
…
342 As previously mentioned in the context of the applicant’s listing, the ARTG is a register maintained under s 9A of the TG Act. The ARTG is divided into a number of parts including a part for goods known as “registered goods” and a part for goods known as “listed goods”. These goods may or may not be medicines within the terms of the relevant definition. The applicant’s goods are listed in the ARTG as a medicine, ie. they are goods that meet both the definition of therapeutic goods and the definition of medicine that appear in s 3 of the TG Act.
343 Section 23 of the TG Act allows for the making of applications for registration or listing of therapeutic goods. Broadly speaking, applications for listing of eligible medicines are entered in the ARTG based on assurances provided by the applicant relating to their quality, safety and efficacy. The requirements in respect of such assurances are found in s 26A of the TG Act.
344 The relevant application form includes a declaration certifying that the medicine complies with the requirements specified in s 26A(2) of the TG Act. Among other things the applicant must certify that the medicine is eligible for listing (s 26A(2)(a)), that the medicine is safe for the purposes for which it is to be used (s 26A(2)(b)), and that the medicine complies with all prescribed quality and safety criteria that are applicable to the medicine (s 26A(2)(f)). Listed goods can be called in for evaluation by the Secretary under s 31 of the TG Act.
345 Section 3 of the TG Act defines the word “sponsor” in relation to the therapeutic goods as follows:
(a) a person who exports, or arranges the exportation of, the goods from Australia; or
(b) a person who imports, or arranges the importation of, the goods into Australia; or
(c) a person who, in Australia, manufactures the goods, or arranges for another person to manufacture the goods, for supply (whether in Australia or elsewhere);
but does not include a person who:
(d) exports, imports or manufactures the goods; or
(e) arranges the exportation, importation or manufacture of the goods;
on behalf of another person who, at the time of the exportation, importation, manufacture or arrangements, is a resident of, or is carrying on business in, Australia.
346 Broadly speaking, a person may be liable under the TG Act:
(a) for supplying therapeutic goods that are not registered, listed or exempt goods unless that person establishes that he or she was not the sponsor at the time of the supply (s 19B(4) and s 19D(1));
(b) for supplying therapeutic goods for which he or she is not a sponsor if the goods are not registered, listed or exempt goods, where the person supplied is not the ultimate consumer of the goods (s 21);
(c) for representing that therapeutic goods that are not included in the ARTG are so included (s 21B(3); and
(d) for publishing or broadcasting an advertisement about therapeutic goods that are not entered in the ARTG (s 42DL(g)).
347 The second amended originating application seeks a declaration that Bush Pharmacy has, in trade or commerce, engaged in conduct that was misleading or deceptive or likely to mislead or deceive in contravention of s 18, and that it has made false or misleading representations in contravention of s 29. An injunction is also sought under s 232 of the ACL preventing the respondent from engaging in further activities which contravene the ACL.
348 The form of the declaratory and injunctive relief sought against Bush Pharmacy is problematical in so far as it does not identify any relevant conduct. In particular, it does not identify any misrepresentation.
349 The ACL case pleaded against Bush Pharmacy appears in paras 29-39 of the second further amended statement of claim. Relevantly, paras 29-37 allege:
29. The Respondent has engaged in trade or commerce in selling and offering to sell the Infringing Goods.
30. The Applicant repeats paragraphs 21 to 24 herein regarding the use of the Infringing Trade Mark by the Respondent in selling and offering to sell the Infringing Goods.
31. The Applicant, through DuCane, has received the approval of the Therapeutic Good Administration (“TGA”) for DUNCANE KUNZEA OIL (the “Listing”).
Particulars
The number tor the Listing is 72143.
32. From 2 December 1999 until 13 July 2017, the Listing was the only entry on the TGA register which used the name KUNZEA or KUNZEA OIL in the name of the product.
33 There are five Standard Indications for the Listing.
Particulars
The Standard Indications in the Public Summary for the Registration are:
(a) Relief of the symptoms of influenza/flu. [Warnings S and COLD required];
(b) Temporary relief of the paint of arthritis. (or) Temporary relief of arthritic pain. [Warning S required];
(c) Helps relieve nervous tension, stress and mild anxiety. [Warning S required];
(d) Relief of muscular aches and pains. [Waring S required]; and
(e) Temporary relief of the pain of rheumatism. [or] Temporary relief of rheumatic pain. [Warning S required].
34. The Respondent has not obtained TGA approval for the Infringing Goods.
35. The Respondent has referred specifically to the Registration in selling and offering to sell the Infringing Goods.
Particulars
The web pages on which the Infringing Goods are sold state “Listed under Therapeutic Goods Australia”.
36. The conduct of the Respondent in using the word KUNZEA and referring to the Registration in selling and offering to sell the Infringing Goods has misled or has had the potential to mislead persons as to:
(a) the source of the Infringing Goods;
(b) the TGA registration status of the Infringing Goods;
(c) the qualities or properties of the Infringing Goods;
(d) the Infringing Goods being sponsored or approved by the Applicant; and
(e) the Respondent having a sponsorship, approval or affiliation with the Applicant, DUNCANE KUNZEA or DUNCANE KUNZEA OIL.
37. In the premises, the Respondent has, in selling and offering to sell the Infringing Goods:
(a) engaged in conduct that was misleading or deceptive or likely to mislead or deceive in contravention of section 18 of Schedule 2 to the Competition and Consumer Act 2010 (Cth) (the “ACL”); and/or
(b) made false or misleading representations in contravention of section 29 (subsections (1)(a), (1)(g) and/or (1)(h)) of the ACL.
350 As already noted, none of these paragraphs identify any representation said to have been conveyed and said to be misleading or deceptive. However, in para 72 of the applicant’s closing submissions, the two representations ultimately relied upon by the applicant were expressed in these terms:
Bush Pharmacy’s website therefore represents that: Bush Pharmacy’s Kunzea ambigua oil products were “listed under Therapeutic Goods Australia” and Bush Pharmacy’s Kunzea ambigua oil products were able to be used for the ARTG indications for which Mr Hood’s Kunzea ambigua oil products are listed.
351 In my view, the relevant page of the Bush Pharmacy website does convey the first of the representations relied on, ie. that the Kunzea ambigua essential oil products supplied by Bush Pharmacy are listed goods that are included in the ARTG. It is admitted that Bush Pharmacy’s products were not so listed. Accordingly, the representation conveyed by the relevant website entry was false and, it follows, misleading or deceptive and likely to mislead or deceive.
352 The fact that the first representation that I have found was conveyed is not pleaded does not in my view provide a sufficient basis for rejecting the ACL case against Bush Pharmacy as it is now put based upon that representation. In my view it has been tolerably clear throughout the hearing that there was an issue as to whether or not Bush Pharmacy was entitled to represent that its Kunzea ambigua products were listed goods and that it was contended by the applicant that this representation was conveyed and that it was misleading and deceptive.
353 The second of the representations identified in para 72 of the applicant’s closing submissions is more problematic. The applicant says that the relevant webpage conveyed a representation that Bush Pharmacy’s Kunzea ambigua oil products were able to be used for the indications referred to in the listing for which the applicant’s Kunzea ambigua oil products are listed. He alleges that representation is false.
354 For the second representation to be misleading or deceptive, or likely to mislead or deceive, it would be necessary for the applicant to establish that the Kunzea ambigua oil products supplied by Bush Pharmacy could not be used to treat the conditions referred to in the listing. However, the applicant has not pleaded or sought to prove that the Bush Pharmacy Kunzea ambigua oil products cannot be used in the treatment of any of the relevant conditions. Accordingly, I reject the applicant’s ACL case in so far as it depends on the second of the relevant representations.
355 If it is suggested by the applicant that the second representation was misleading or deceptive or likely to mislead or deceive because purchasers of Kunzea ambigua oil supplied by Bush Pharmacy could not lawfully use it for the treatment of any of the conditions referred to in the listing for the applicant’s goods, then there are two difficulties with that way of putting the applicant’s misrepresentation case.
356 First, I was not referred to any provision in the TG Act which would make it unlawful for a person who purchased Kunzea ambigua essential oil from Bush Pharmacy to use it to treat any of the conditions referred to in the applicant’s TGA listing. Although the TG Act, broadly speaking, prevents a person from supplying or marketing therapeutic goods unless they are registered goods or listed goods, there does not appear to be any provision which would prevent a consumer who had acquired such goods using them as he or she saw fit.
357 Secondly, it has never been pleaded by the applicant that a person acquiring Kunzea ambigua essential oil from Bush Pharmacy was under any legal restriction preventing him or her from using those products as he or she saw fit. The vice in Bush Pharmacy’s conduct lies in it having represented that its goods were listed goods when they were in fact not listed goods. It is on that basis that the applicant’s ACL claim is entitled to succeed.
358 I am satisfied that there should be a declaration and an injunction based on the misleading and deceptive representation that I have found to have been made by Bush Pharmacy in contravention of s 18 of the ACL.
359 As to the applicant’s case based on s 29 of the ACL, I am satisfied that the representation which I found to be misleading and deceptive also constitutes a false representation that Bush Pharmacy’s goods are listed goods. In my view, that representation is caught by s 29(1)(a) of the ACL in that it constitutes a representation that Bush Pharmacy’s goods are of a particular standard or quality (ie. that they have met the standards required for listing under s 26 of the TG Act and related provisions) when they have not. I am therefore satisfied that Bush Pharmacy has contravened s 29(1)(a) of the ACL.
Down Under
360 The ACL case pleaded against Down Under appears in paras 29-39 of the second amended statement of claim. The pleading is essentially identical to that filed in the proceeding against Bush Pharmacy.
361 In the particulars to para 35 there is a reference to “the webpages on which the Infringing Goods sold refer to the Registration”.
362 In para 76 of the applicant’s closing submissions it is alleged that:
76. The Down Under Website therefore represents that:
(a) Down Under’s Kunzea ambigua oil products are “registered with the Australian Therapeutic Goods Administration”;
(b) Down Under’s Kunzea ambigua oil products are listed on the ARTG with ARTG listing number “AUSTL 72143”, being the number of the TGA Listing;
(c) Down Under’s Kunzea ambigua oil products were able to be used for the ARTG indications for which Mr Hood’s Kunzea ambigua oil products are listed.
363 The Down Under website includes the express statement “Kunzea oil is registered with the Australian Therapeutic Goods Administration (AUSTL 72143)”. That statement clearly indicates that the relevant goods offered for sale by Down Under are registered goods or (at least) listed goods included in the ARTG.
364 I am satisfied that the first and the second of the representations referred to in para 76 was conveyed by the relevant entry appearing on the Down Under website. I am also satisfied that each of those representations was false.
365 What I have said in relation to the second of the representations relied upon by the applicant against Bush Pharmacy also applies to the third of the representations now alleged in para 76 of the applicant’s closing submissions to have been made by Down Under. Accordingly, for the reasons previously stated, the applicant’s case based on that representation must fail.
366 I am satisfied that there should be a declaration and an injunction based on the misleading and deceptive representations that I have found to have been made by Down Under in contravention of s 18 and s 29(1)(a) of the ACL.
New Directions
367 The ACL case pleaded against New Directions appears in paras 29-39 of the second amended statement of claim. Subject to one important difference, the pleading is essentially identical to that filed in the proceedings against Bush Pharmacy and Down Under.
368 The ACL case against New Directions is different from that alleged against Bush Pharmacy and Down Under in that it is not alleged that New Directions has made any representation to the effect that New Directions’ Kunzea ambigua oil products are listed goods. Rather, what is alleged in para 35 is as follows:
35. The Respondent has referred to two of the Standard Indications in selling and offering to sell the Infringing Goods.
Particulars
The web pages on which the Infringing Goods are sold state:
“Common uses: Kunzea has traditionally been used for relieving muscular aches and pain. It has also been known to relieve symptoms of cold and flu when used in steam inhalations.”
369 I have previously set out the relevant extract from the webpage on which the “common uses” statement appears.
370 In his written closing submissions at para 82, the applicant identifies a single representation that is said to have been made by New Directions:
82. The New Directions Website therefore represents that their [sic] Kunzea ambigua oil products are able to be used for two of the ARTG indications for which Mr Hood’s Kunzea ambigua oil products are listed (“Relief of the symptoms of influenza/flu” and “Relief of muscular aches and pains”).
371 That representation is relevantly the same as the second of those relied upon against Bush Pharmacy and the third of those relied upon against Down Under.
372 The applicant has not alleged, nor proven, that the Kunzea ambigua oil supplied by New Directions cannot be used for the relief of symptoms of cold and flu or for the relief of muscular aches and pains. On that basis the claim brought against New Directions for alleged contravention of the ACL must fail.
373 It may well be that New Directions has contravened relevant provisions of the TG Act by supplying or marketing therapeutic goods in Australia that are not registered or listed in the ARTG. And it may well be that some consumers who purchased Kunzea ambigua oil from New Directions did so under the misapprehension that such goods were being offered for sale lawfully because they had been registered or listed in the ARTG. However, no case based upon such conduct was pleaded or particularised. Nor was any such case addressed in the applicant’s closing submissions.
Heritage Oils/Ms Levinson
374 The ACL case pleaded against Heritage Oils and Ms Levinson appears in paras 34-44 of the amended statement of claim. As pleaded, the ACL case brought against them is essentially the same as that alleged against New Directions.
375 In paragraph 40 of the statement of claim it is alleged that both Heritage Oils and Ms Levinson have “referred to four of the Standard Indications in selling, offering to sell, supplying and offering to supply the Infringing Goods”. In the particulars to that paragraph reference is then made to paras 13(c), 13(d), 13(g) and 13(h) of the amended statement of claim, all of which refer to various webpages on the Heritage Oils Tasmania website and the Heritage Oils website to which I have previously referred.
376 At paras 85 and 86 of the applicant’s closing submissions it was submitted:
85. The exemplar conduct to which the Court may have reference in relation to Heritage Oils and Ms Levinson are representations on the Heritage Oils Website and the Heritage Oils Website [sic] which include the following relevant statements:
“Kunzea Oil is a 100% pure essential oil with known properties to aid in the relief of muscular and joint aches and pains. A few drops of Kunzea Oil has been proposed to assist in the relief of sinus which comes with coughs and colds and serves as a more gentle inhalant for children than some eucalyptus oils” … “Kunzea oil has been used to help relieve nervous tension and is known to reduce anxiety of which there have been many anecdotal reports” … and “Known to be effective in the reduction of joint aches and pains and the reduction of redness in skin blemishes and rashes. Can be used as an inhalant and a relaxant”…
86. The Heritage Oils Website therefore represents that Heritage Oils’ Kunzea ambigua oil products are able to be used [sic] a number of the ARTG indications for which Mr Hood’s Kunzea ambigua oil products are listed.
377 As is apparent from para 86, the sole representation relied upon in support of the ACL case against both Heritage Oils and Ms Levinson is that Heritage Oils’ Kunzea ambigua oil products are able to be used to relieve a number of conditions referred to in the applicant’s TGA listing.
378 The applicant sought to establish that this representation was false by reference to evidence given by Ms Levinson and Mr Brocklehurst. The applicant submitted at paras 88-89 of his written submissions:
88. During cross-examination, Ms Levinson gave evidence that she was responsible for Heritage Oils’ website that Heritage Oils sold bottles of Kunzea ambigua oil via its website and that statements concerning the use of Kunzea ambigua essential oil for the relief of muscular and joint aches and pains, sinus and to help relieve nervous tension and reduce anxiety included on Heritage Oils’ website are “appropriate”.
89. Relevantly, during cross-examination Mr Brocklehurst of Heritage Oils gave evidence that he did not “believe that there is any scientific evidence to support” certain claims made on Heritage Oils’ website in relation to therapeutic effects of Kunzea ambigua essential oil, and that he “disagreed with [his] partner”, Ms Levinson, on this matter.
(citations omitted)
379 The evidence referred to by the applicant was directed to the question of whether Ms Levinson and Heritage Oils had “reason to believe” for the purpose of s 117(2)(b) of the Act.
380 The relevant part of the cross-examination referred to by the applicant in his closing submissions really begins when Ms Levinson is taken to a paragraph on the Heritage Oils’ website. The cross-examination was as follows:
MR GREEN: Yes. And then you – and then – and I take it you wrote these words, the next paragraph:
Kunzea oil’s known properties are to aid in the relief of muscular and joint aches and pains. A few drops of Kunzea oil is a mild decongestant to assist in the relief of sinus and serves as a more gentle inhalant for children than some eucalyptus oils.
Do you see that? ––– Yes.
Continuing:
Kunzea oil has been used to help relieve nervous tension and is known to reduce anxiety, of which there are various anecdotal reports.
? ––– Yes.
Those anecdotal reports were enough to satisfy you, were they not, that you could include this on your website? ––– Like everybody else. So that – all this – this information has been copied from a lot of websites and yes, I agree that it is appropriate.
381 Counsel never put to Ms Levinson that she had no reasonable basis for the statements made on the website or that anything she said concerning the “Kunzea oil’s known properties” was incorrect or inappropriate.
382 The cross-examination of Mr Brocklehurst also involved the cross-examiner putting to him that Kunzea ambigua oil could provide relief from pain, muscle and tendon strain, that it limited the progress of cold sores, assisted in the treatment of rashes, and helped to minimise bruising. Mr Brocklehurst said that he did not agree with Ms Levinson in relation to some of these matters but that the difference of opinion arose out of Mr Brocklehurst not placing much weight on “anecdotal evidence” of the kind relied upon by Ms Levinson. But Mr Brocklehurst’s evidence cannot establish that Kunzea ambigua oil cannot be used to treat the various conditions referred to in his cross-examination especially when that proposition was never put to Ms Levinson in her cross-examination.
383 The ACL case against Heritage Oils and Ms Levinson must fail for the same reason that the ACL case against the other respondents based upon a representation to a like effect fails. It has not been pleaded nor proven that the Kunzea oil supplied by Heritage Oils cannot be used for any of the indications referred to on the Heritage Oils website including, in particular, those referred to in the passage extracted in para 85 of the applicant’s closing submissions.
Damages under the Australian Consumer Law
384 The applicant has claimed compensation in each of the proceedings for which it made an ACL claim pursuant to s 237(1) and s 243(e) of the ACL for any loss and damage suffered by, or likely to be suffered by, the applicant.
385 Section 237 of the ACL permits the Court to make an order for the payment of compensation in favour of a person who has suffered, or is likely to suffer, loss or damage because of the conduct of another person in contravention of s 18 or s 29. Section 243 of the ACL permits the Court to make various orders, including, an order directing the respondent to pay the injured person the amount of “the loss or damage”.
386 While questions of quantification of damages have been deferred, it is still necessary for the applicant to show that he has suffered, or is likely to suffer, loss or damage because of the contravening conduct that I have found to have occurred. This is a pre-condition to the award of any compensation under s 237(1) or s 243(e) of the ACL.
387 In his closing submissions the applicant submitted:
94. The Court should also proceed to undertake an enquiry in relation to the damage suffered or likely to be suffered by Mr Hood for the purposes of making compensation orders, because the evidence establishes that: therapeutic uses are important uses to which Kunzea oil is sought to be put by those acquiring the product (T159.38-39); each of the ACL Respondents have made material sales of Kunzea ambigua essential oil [Annexure SB-1 (CB:2:48.01); Annexure DSP-1 (CB:2:50.01); Annexure DA-1 (CB:2:52.01); Levinson 1 [4] (CB:2:56)]; there are limited suppliers of Kunzea ambigua essential oil (Mr Hood, Bush Pharmacy and Mr Wagner) (T190.5-15); and Mr Hood has unutilised potential to produce additional Kunzea ambigua essential oil [Hood 1 [38]-[39], [88] (CB:2:44)].
388 The statement that therapeutic uses are important uses to which Kunzea ambigua oil is sought to be put by those acquiring the product is not supported by the evidence referred to by the applicant. The evidence referred to in para 94 of his closing submissions is evidence given by Mr Backhaus during cross-examination. Mr Backhaus did not accept that therapeutic uses for Kunzea ambigua oil are important uses nor was that proposition put to him. In any event, it cannot be assumed or inferred that purchasers would not have acquired Kunzea ambigua oil from any of the respondents were it not for them representing that the oil supplied was listed on the ARTG. The evidence shows that New Directions made sales of Kunzea ambigua oil without making any such representation.
389 The applicant has not established that he suffered any loss, or is likely to suffer any loss, as a result of the misrepresentations found to have been made. In particular, there was no evidence to suggest that any person to whom Bush Pharmacy or Down Under supplied Kunzea ambigua oil was likely to have been induced to do so by reason of the relevant misrepresentations.
390 It was for the applicant to show that he has or is likely to suffer some loss or damage as a result of the relevant misrepresentations. In my view the applicant has not met the conditions for an award of compensation under either s 237(1) or s 243(e) of the ACL.
391 Claims 1, 2, 3, 4 and 8 of the patent are invalid.
392 The applicant accepted that there should be declarations of non-validity made in relation to claims 1-4. I do not know whether the applicant has in mind to bring proceedings in respect of infringements that occurred during the term of the patent against other suppliers of Kunzea ambigua oil but it is theoretically open to him to do so. In the circumstances, I think the appropriate order to make in relation to claims 1-4 is that they be revoked. There should also be an order revoking claim 8.
393 The applicant has established that Bush Pharmacy, Heritage Oils and Ms Levinson have infringed claim 5 of the patent by supplying Kunzea ambigua essential oil for use in accordance with the requirements of that claim and an inducement to use the oil in that manner contained in advertisements published by or with their authority.
394 There will be declaratory relief granted reflecting those findings. There should also be an enquiry as to the quantum of the pecuniary relief to which the applicant is entitled for patent infringement.
395 The patent infringement case brought against the other respondents (Down Under, New Directions and Native Oils) fails.
396 In relation to the ACL claims, the applicant is entitled to declaratory and injunctive relief in an appropriate form in respect of Bush Pharmacy’s and Down Under’s contraventions of s 18 and s 29(1)(a) of the ACL. The declaratory and injunctive relief should identify the particular representations which those respondents were found to have made.
397 So far as the proposed amendments to the patent are concerned, I propose to allow the application to amend to correct the obvious errors (as previously identified) appearing in the method of treatment claims and to take account of the revocation of claims 1-4 and 8 on terms that the applicant submit to a revocation order in respect of the other product claims that are dependent on any one or more of claims 1-4 (ie. claims 14-18). In my view, if the claims are to be amended at all to take account of the revocation of claims 1-4, then the other product claims that are dependent on claims 1-4 should also be revoked. The result of the amendment application, assuming the applicant submits to an order for the revocation of claims 14-18, will be that the claims of the patent, as amended, will be confined to eight method of treatment claims.
398 I will hear from the parties in relation to the form of the declarations, injunctions, the s 105(1) order and all necessary procedural orders dealing with the quantification of pecuniary relief for patent infringement. I will also hear from the parties in relation to costs.
399 The applicant must file and serve a proposed minute of order within 7 days. The proceedings will be listed for any argument in relation to the form of the orders (including in relation to costs) at a date to be fixed.
I certify that the preceding three hundred and ninety-nine (399) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Nicholas. |




