FEDERAL COURT OF AUSTRALIA
Allergan Australia Pty Ltd v Self Care IP Holdings Pty Ltd [2020] FCA 1530
ORDERS
DATE OF ORDER: | 22 October 2020 |
THE COURT ORDERS THAT:
1. Within 14 days of these orders or such further period as may be allowed, the parties are to confer and bring in:
(a) orders that dispose of the proceeding and, so far as relevant, deal with its further conduct, in accordance with the reasons published today;
(b) orders that deal with the costs of the proceeding thus far;
(c) proposed redactions of the reasons published today with reference to the suppression orders in the proceeding; and
(d) failing agreement on the above, competing orders, redactions (if necessary) and short submissions in support of their respective positions.
2. Until further order, the text of the reasons for judgment published today is to be published and disclosed only to the external lawyers (solicitors and counsel), including foreign lawyers, of the parties who may then disclose the substance of the reasons to their respective clients (the parties) without disclosing any facts or information in the reasons which may be the subject of suppression orders in respect of the evidence in the proceeding from which the facts or information is drawn.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ORDERS
NSD 1802 of 2017 | ||
BETWEEN: | ALLERGAN INC Appellant | |
AND: | SELF CARE IP HOLDINGS PTY LTD (ACN 134 308 151) Respondent | |
order made by: | STEWART J |
DATE OF ORDER: | 22 October 2020 |
THE COURT ORDERS THAT:
2. The appellant is to pay the respondent’s costs of the appeal as agreed or taxed.
3. In terms of r 36.03(b) of the Federal Court Rules 2011, the time for the filing of a notice of appeal from these orders shall be 28 days after final orders are entered in proceeding NSD15/2017 as contemplated by order 1(a) of the orders in that proceeding made on 22 October 2020.
[Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.]
STEWART J:
[1] | |
[1] | |
[5] | |
[25] | |
[36] | |
[38] | |
[52] | |
[54] | |
[54] | |
[62] | |
[76] | |
[86] | |
[96] | |
[100] | |
[107] | |
[117] | |
[154] | |
Infringement of BOTOX trade mark under ss 120(1) and 120(2) of TM Act | [154] |
[155] | |
[159] | |
[164] | |
[173] | |
[178] | |
[183] | |
(3) The “goods in respect of which the trade mark is registered” or “goods of the same description” – principles | [185] |
[190] | |
[190] | |
[200] | |
[214] | |
(4) Conclusion: trade mark infringement claim with regard to PROTOX | [227] |
[228] | |
[228] | |
[256] | |
[259] | |
(4) Conclusion: trade mark infringement claim with regard to BOTOX phrases | [262] |
[263] | |
[266] | |
(1) The law | [266] |
(2) Consideration | [274] |
[293] | |
(1) Introduction | [293] |
(2) The law | [296] |
(3) Submissions | [316] |
(4) Consideration | [324] |
[340] | |
[340] | |
[344] | |
[354] | |
Section 44 – deceptively similar in respect of similar goods | [355] |
[359] | |
[388] | |
[405] | |
[413] | |
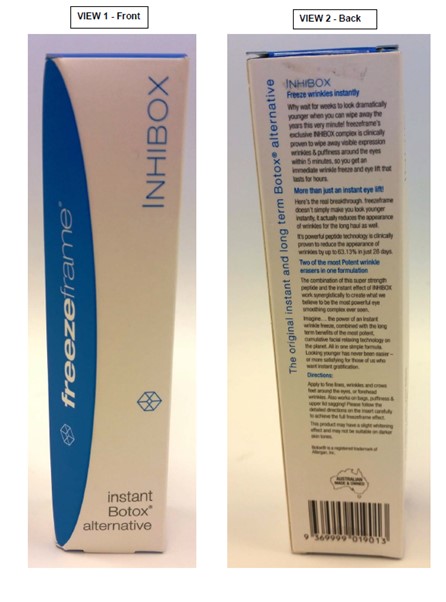
[414] | |
[416] | |
[416] | |
[435] | |
[445] | |
[454] | |
[454] | |
[461] | |
[481] | |
[481] | |
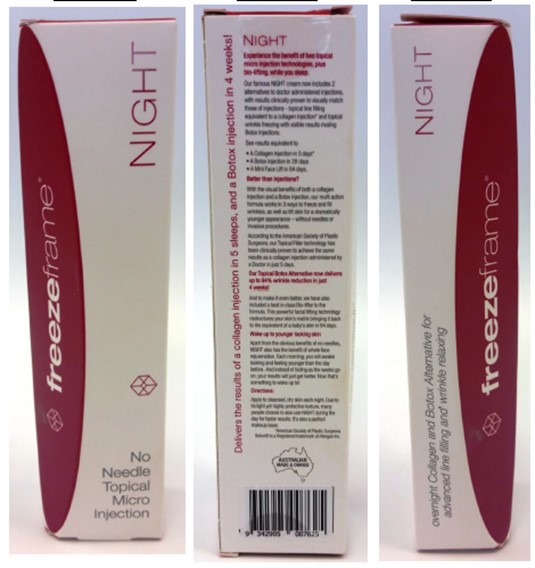
[494] | |
[510] | |
[510] | |
[513] | |
[523] | |
[529] | |
[539] | |
[573] | |
[577] | |
[581] | |
[588] | |
[610] | |
[618] |
1 The eternal human interest in reducing the appearance of ageing underlies the disputes in these cases. The opposing parties have both respectively developed and promoted products with very different modes of action to attract customers who have this Sisyphean interest.
2 This litigation has spawned sprawling testimony and documents before the Court. Standing out amongst the vast number of exhibits are two short segments from the well-known television series and film, Sex and the City. In each, the character Samantha Jones tries to persuade Carrie Bradshaw to submit to treatment with Botox to lessen the appearance of wrinkles on her face and thereby to maintain her apparent youth despite her chronological age. The extracts were tendered as proof of the ubiquitous reputation of Botox.
3 The applicants – who have rights to BOTOX trade marks – contend that the respondents have sought to sell their products by unlawfully leveraging off the reputation of Botox. That includes by way of trade mark infringement, misrepresentations in contravention of the Australian Consumer Law (ACL) and passing off. The second applicant also appeals against a decision of a delegate of the Registrar of Trade Marks to allow one of the respondents’ trade marks – which includes the word PROTOX – to proceed to registration. The respondents, by way of cross-claim, contend that certain of the applicants’ trade marks should be removed from the register or restricted to a smaller class of goods.
4 It is useful to make a note on nomenclature at the outset. Where in these reasons reference is made to a word as a trade mark, e.g., BOTOX and PROTOX, the word is in capital letters. However, when a word is used as the name of a product, it is used as a proper noun and hence has only the first letter capitalised, e.g., Botox and Protox. That, at least, is the approach that has been sought to be followed.
The applicants, their product and their trade marks
5 Allergan Inc is the manufacturer of Botox and the owner of trade marks including for the word BOTOX. BOTOX is an invented portmanteau word, derived from the active ingredient in Botox, namely botulinum toxin, type A. It was first registered as a word trade mark in the United States of America in 1990.
6 Allergan Australia Pty Ltd is the Australian subsidiary of Allergan Inc. It is the sponsor of the Botox products on the Australian Register of Therapeutic Goods and (the applicants contend) is the authorised user of the BOTOX marks in Australia.
7 I shall refer to Allergan Inc and Allergan Australia together simply as Allergan save where it is necessary to distinguish between them.
8 The Botox product is a particular botulinum toxin type A purified neurotoxin complex, supplied as a powder in vials of 50 ml/units, 100 ml/units and 200 ml/units. In use, the product is reconstituted with sterile saline for injection, usually intramuscularly. Botox is used for numerous therapeutic indications (including for the treatment of overactive bladder, cervical dystonia and hyperhidrosis) as well as the following cosmetic indications: temporary improvement in the appearance of upper facial rhytides – glabellar lines (frown lines), lateral canthal lines (crow’s feet) and forehead lines in adults. The present case is concerned with the cosmetic indications.
9 When Botox is injected into the muscle it is expected that most of the Botox will remain close to the site of injection. The core neurotoxin is surrounded by accessory proteins which protect it as it enters the body. The Botox core then dissociates from its accessory protein and enters the nerve cell. Botox blocks the release of acetylcholine, which is a chemical released by the nerve cells to send signals to other cells. When the release of acetylcholine is stopped, muscle contraction cannot occur.
10 Once the Botox is injected, it inhibits the nerve and within roughly 50 hours (or about 2-3 days) the Botox is removed from the body by passing urine. Over time the nerve heals itself and starts releasing acetylcholine again. This gives movement back to the muscle.
11 The effects of Botox may take a few days to manifest. It has effect by relaxing the muscles at the site of injection. Full results may take up to 10-14 days to be seen. The effects will usually last up to four months, then dramatically decrease. Treatment may be repeated every six months.
12 Botox does not moisturise or tighten the skin. People who have been treated with Botox may feel that their skin looks smoother, shiny and moisturised after the treatment due to the fact that the underlying muscle has been relaxed and cannot move. There is, however, no clinical data to show that Botox has these effects on the skin. Important for present purposes is that Botox has a therapeutic effect, i.e., it has a physiological or pharmacological action.
13 In Australia, Botox has been supplied for certain therapeutic indications under the BOTOX marks since November 1993 when approval by the Therapeutic Goods Administration (TGA) was granted. In 2002, Botox received approval for cosmetic indications and it has been available for sale in Australia for that purpose since about April 2002.
14 In Australia, Botox must be administered by a registered medical practitioner (or registered nurse under the direction of a registered medical practitioner) or other authorised health professional under the relevant state or territory legislation. Allergan supplies Botox to approved medical practitioners and other health professionals that hold an account with Allergan Australia. Botox is not advertised or supplied directly to retail customers as the advertising of therapeutic goods, and poisons, is prohibited: s 42DL, Therapeutic Goods Act 1989 (Cth) (TG Act).
15 The evidence is that one 100 ml vial of Botox is sold for approximately $500 (excl. GST). As the product is reconstituted in saline, one vial can apparently be used for more than one treatment. A single treatment, which is to say five or six injections in a single site (e.g., frown lines), including the health professional’s charges, is in the range of $200-$720.
16 In Australia, Allergan Inc is the registered proprietor of the following pending or registered Australian trade marks (which I will refer to as the BOTOX marks):
(1) Trade Mark No. 551279, for the word BOTOX, registered (court action pending) in class 5 and having a priority date of 28 February 1991;
(2) Trade Mark No. 860785 for the composite mark  , registered in class 5 and having a priority date of 14 December 2000 (the 785 mark);
, registered in class 5 and having a priority date of 14 December 2000 (the 785 mark);
(3) Trade Mark No. 860786, for the word BOTOX, registered (court action pending) in class 5 and having a priority date of 14 December 2000 (the 786 mark);
(4) Trade Mark No. 1008655 (International Registration 826203) for the word BOTOX, registered (court action pending) in class 3 and having a priority date of 8 March 2004 (the 655 mark); and
(5) Trade Mark No. 1578426 for the word BOTOX, registered (court action pending) in classes 1, 3, 5, 9, 10, 16, 35, 41, 42, 44, and 45 and having a priority date of 4 September 2013 (the 426 defensive mark).
17 Each of the class 5 registrations is in respect of pharmaceuticals. Relevant to the claim for trade mark infringement under s 120(2) of the TM Act is that the 785 and 786 marks include the following goods: “Pharmaceutical preparations for the treatment of … wrinkles …”.
18 The class 3 registration in respect of the 655 mark is for “cosmetics, face creams and lotions; skin creams and lotions.”
19 The class 3 registration in respect of the 426 defensive mark is for the following:
After sun lotions and preparations; after-shave lotions and creams; anti-ageing creams; anti-wrinkle cream; anti-cellulite preparations; antiperspirant deodorants and preparations; aromatherapy preparations; artificial tanning preparations; astringents for cosmetic purposes; barrier creams and preparations for the skin; bath salts, crystals, oils, lotions and preparations (not medicated); bath soap; beauty care preparations; beauty preparations; beauty care products; beauty products; beauty creams; bleaching preparations (decolourants) for cosmetic purposes; bleaches in the form of creams; bleaching preparations for the hair; body care preparations (non-medicated); body lotions (other than for medical purposes); body moisturisers; body scrubs; breath fresheners; bubble bath preparations; cleaners (preparations) for cleaning surgical instruments; cleaning oils for cosmetic purposes; cleaning preparations; cleaning preparations containing sterilising substances; cleaning preparations for medical and laboratory instruments; cleaning preparations for personal and sanitary use; cleaning preparations for the hands and the skin; cleansers for the face; cleansing facial masks and lotions; cleansing products for removing make-up; collagen preparations for cosmetic application; colouring preparations for cosmetic purposes; conditioners for use on the hair; cosmetic acne creams; cosmetic articles for personal use; cosmetic articles and preparations; cosmetic kits; cosmetic skin care products; cosmetics; deodorant preparations for personal use; deodorants for the body; depilatories; depilatory preparations and products; depilatory wax; dermatological cosmetic preparations; dermatological preparations (other than medicated); detergents having anti-bacterial and disinfecting properties (other than for medical use or for use in manufacturing processes); essential oils; essential oils for cosmetic purposes; exfoliants for the care of the skin; eye make-up; eye wrinkle lotions; facial care products (cosmetic); fragrances; hair care products; hair conditioner; hair colouring preparations; hair cosmetics; hair cream and lotions; hair gel and mousse; hair permanent treatments; hair perming products; hair preparations; hair products; hand cream; impregnated cloths for cosmetic use; impregnated pads for removing make-up; lipsticks; lip gloss; lip pencils and liners; make-up; make-up preparations and products; make-up removing preparations; massage oils and creams (not medicated); moisturisers (cosmetics); mouth washes (not for medical purposes); nail care preparations; nail preparations (cosmetics); non-medicated toiletries; perfumes; perfumery preparations and articles; preparations for care of the teeth; products for cleaning; sanitary preparations (other than for medical purposes); serum (cosmetic preparations); shampoos; shaving soap, creams and gels; skin care preparations and creams (cosmetic); skincare cosmetics; soaps; soap products; sun screen preparations (cosmetics); suntan preparations (cosmetics); tanning preparations (cosmetics); toilet preparations; toiletries; toothpaste
20 The relevance of the details of the class 3 registration, as opposed to all the other classes of registration of the 426 defensive mark, is that the respondents’ challenge to the registration by way of the cross-claim is only to class 3.
21 As dealt with further at [117]-[153] below, sales of Botox have been considerable and BOTOX has achieved significant success as a trade mark and brand, both internationally and in Australia. As at the date of commencement of the proceedings, Botox had a significant and valuable reputation amongst consumers in Australia, including as a highly effective injectable product in reducing the appearance of wrinkles.
22 Allergan maintains a distinction in its business model between the use of Botox for medicinal therapeutic indications and for cosmetic indications. In respect of the cosmetic indications for the Botox product, Allergan uses the website www.botoxcosmetic.com which provides information about the product when used for cosmetic purposes. A different website, www.botox.com, provides information about all uses of the Botox product. That website displays a separate tab to redirect browsers seeking information on its cosmetic uses to the Botox cosmetic website.
23 Consistent with the distinction between the two principal different uses of Botox, Allergan Australia has different sales teams, marketing strategies and marketing material for the Botox product where it is sold for medicinal therapeutic indications and for cosmetic indications. Sales and marketing teams for therapeutic indications target medicinal requirements and treatment of disease as opposed to sales and marketing for cosmetics which focus on the desire for the temporary improvement of appearance. Allergan Australia keeps its business units, including its lists of account holders and records of sales, for the two principal different uses of Botox separate.
24 Other botulinum toxin type A products with therapeutic and cosmetic indications similar to Botox are available in Australia (and overseas), including a product sold under the trade mark DYSPORT manufactured by Ipsen Biopharm Ltd and another under the trade mark XEOMIN manufactured by Merz Pharma GmbH & Co. KGaA.
The respondents, their products and their trade marks
25 Self Care IP Holdings Pty Ltd and Self Care Corporation Pty Ltd are companies incorporated in Australia. When it is not relevant to distinguish between them, I shall refer to them together simply as Self Care. Ms Sonia Amoroso, the third respondent, is a citizen and resident of Australia. Since 24 November 2008, Ms Amoroso has been the sole director and secretary of Self Care IP. Since 14 July 2008, Ms Amoroso has been the sole director and secretary of Self Care Corp.
26 Ms Amoroso was Self Care’s principal witness. She deposed to several affidavits which were read as her evidence in chief, and she was cross-examined for approximately two days. She was a careful witness, by which I mean she was cautious not to be caught out or to give matters away in cross-examination; she required that the questions put to her be clear and understandable by her. I did not get the impression that she was trying particularly to assist the Court, but I also did not get the impression that she was dishonest in her answers. I accept her evidence as honest and, for the most part, as accurately reflecting the facts as they occurred. Where I have reservations as to the reliability of her evidence, I will address those directly. I otherwise accept her evidence.
27 Ms Amoroso founded the Self Care business in about 2008. Self Care supplies cosmetic products, including, in particular, topical anti-wrinkle skincare products under the trade mark FREEZEFRAME.
28 Ms Amoroso came up with the FREEZEFRAME brand and decided that it would be used as an umbrella brand and that various products would form part of the brand. With the Freezeframe products, Ms Amoroso was interested in developing products that could be used as an alternative to botulinum toxin injections.
29 Self Care’s first flagship product was called Freezeframe with Inhibox which was launched in July 2009. The product enjoyed significant retail success.
30 Over subsequent years, Self Care became an award-winning and successful Australian business by reference to the FREEZEFRAME brand. Over time, more than 30 products were developed and sold under that brand, including five that are the subject of these proceedings. Each is dealt with in detail below (at [62]-[106]).
31 Since at least October 2014, Self Care Corp has been the registered owner of the domain name www.freeze-frame.com.au (Freezeframe website), which it uses to promote its Freezeframe range of products.
32 Since at least July 2015, Self Care Corp has been the registered owner of the domain name www.self-care.net.au (Self Care website). Since at least around August 2017, the Self Care website has automatically redirected to the Freezeframe website.
33 In October 2014, Self Care IP lodged an application to the Australian Trade Marks Office to register the trade mark FREEZEFRAME PROTOX. The goods and services in respect of which the FREEZEFRAME PROTOX trade mark is applied for are “anti-ageing serum, anti-wrinkle serum” in class 3.
34 Allergan opposed the registration of the mark, but that opposition was dismissed by a decision of a delegate of the Registrar of Trade Marks on 19 September 2017. That decision is the subject of the appeal in proceeding NSD1802/2017 referred to below (at [340]-[413]).
35 Self Care has a number of other trade marks that are either registered or pending registration, each of which has the word FREEZEFRAME followed by a particular product name. These trade marks thus, relevantly, include FREEZEFRAME NIGHT and FREEZEFRAME BOOST.
36 There are two proceedings that were heard together. There were orders that the evidence in one proceeding is evidence in the other.
37 The parties helpfully agreed a detailed statement of facts which constitutes a substratum of common ground facts which has to be supplemented by factual findings. In that respect, four factual witnesses were cross-examined and two expert witnesses gave evidence concurrently during the course of which they were also cross-examined. There was evidence from several other witnesses, by way of affidavit, who were not required for cross-examination.
38 In proceeding NSD15/2017, Allergan Australia is the first applicant and Allergan Inc is the second applicant. Self Care IP is the first respondent, Self Care Corp is the second respondent, and Ms Amoroso is the third respondent.
39 Allergan claims that Self Care:
(1) has infringed the BOTOX registered trade marks by use of the word BOTOX in various forms (including, e.g., “instant Botox alternative”, “overnight Botox alternative” and “long term Botox alternative”) on the Freezeframe products and packaging, as well as in promotion and advertising contrary to the Trade Marks Act 1995 (Cth) (TM Act). It also claims that use of the trade mark PROTOX is deceptively similar to and infringes the BOTOX mark;
(2) has contravened the ACL by making representations of affiliation with the applicants or their Botox product (the affiliation representations) and representations concerning the efficacy of the respondents’ products (the efficacy representations);
(3) has engaged in passing off; and
(4) has infringed various sections of the TG Act.
40 Allergan contends that Ms Amoroso authorised the infringements of the BOTOX marks, procured or entered into a common design as a joint tortfeasor with Self Care to infringe the BOTOX marks and to engage in passing off, and aided, abetted, counselled, or procured Self Care’s conduct in contravention of the ACL. Allergan relies on Ms Amoroso’s close involvement in the creation, promotion, sale and offer for sale of Self Care’s products.
41 In respect of the trade mark infringement, ACL and passing off claims, Allergan seeks declarations, injunctions, equitable compensation and damages, including exemplary damages, or an account of profits. In respect of the trade mark infringement claim, Allergan also seeks delivery up of the relevant stock of products, packaging and promotional and advertising material. In respect of the TG Act claim, Allergan seeks injunctions.
42 In December 2017, it was ordered that the quantification of the loss and damage suffered by Allergan be heard separately from and after all questions of liability. The questions of liability were identified as including:
(1) Self Care’s liability, if any, for particular heads of loss or damage, the burden falling on Allergan to prove at least one instance of the loss or damage claimed; and
(2) Self Care’s liability, if any, for additional damages.
43 In summary, Self Care says the following with respect to the claims.
44 With regard to the trade mark infringement claims, it says that there has been no infringement of Allergan’s BOTOX trade mark because Self Care’s use of that word on its products is not use “as a trade mark” within the meaning of s 120 of the TM Act. It says that the function of the word BOTOX on the packaging or in the advertising of Self Care’s creams is to say what the product is not – it is not Botox, but an alternative to Botox; it is not an expensive injectable you receive from your medical practitioner, but a modestly priced self-administered cream. It says that Self Care’s branding shows the origin of the goods to be Self Care. It says that the function of the use of the word BOTOX is to compare and contrast its goods to the goods of another trader, which contrast is magnified by the use of the ® symbol with the word BOTOX. Self Care also says that PROTOX is not used “as a trade mark” and is not deceptively similar to BOTOX because the mark is used only in combination with the word FREEZEFRAME.
45 With regard to the ACL claims alleging affiliation and passing off, Self Care says that there is no doubt that consumers associate the word BOTOX with an injectable product used to reduce the appearance of wrinkles. It says that Self Care’s creams/serums are presented as products that reduce the appearance of wrinkles, but explicitly as a “Botox alternative”. It says that the word “alternative” is powerful enough in itself to tell all reasonable consumers that the products are not Botox and are distinct from Botox and the makers of Botox.
46 Self Care says that that distinction from Botox and the makers of Botox is reinforced by other aspects of the context, including Self Care’s own branding on its creams and in its advertising (in which FREEZEFRAME is always prominent), the character of the goods as creams rather than a formulation to be injected, the method of administration being by the consumer themselves rather than by a medical practitioner, and a substantial difference in price. They say that a consumer could not reasonably think that Self Care’s products were affiliated with the makers of Botox, or that the products were in fact Allergan’s goods.
47 With regard to the ACL claims alleging misleading representations about the efficacy of Self Care’s creams, Self Care says that there is a problematic lack of clarity in Allergan’s case in this respect. It says that the claims should be rejected either because the alleged representations are not conveyed by the product packaging or the advertising, or because such representations as were conveyed are not misleading and there were reasonable grounds to make them. In particular, it relies on a number of in vivo studies which, it says, demonstrate that Self Care’s creams reduce the appearance of wrinkles, which is the central message about efficacy.
48 With regard to the claims seeking injunctions against the alleged commission of offences under the TG Act, Self Care says that Allergan has no standing to prosecute criminal offence proceedings or civil penalty proceedings under the TG Act, and that it has not done so. It says that Allergan’s invocation of the exceptional jurisdiction of a civil court to restrain by injunction the commission of an offence should be rejected, including because Allergan has no standing to seek such an injunction and, in any event, there is no contravention of the TG Act because Self Care is not advertising therapeutic goods.
49 Self Care IP and Self Care Corp, as cross-claimants, allege that Allergan has sought to extend its trade mark rights beyond the field of actual use of the trade mark in relation to injectable anti-wrinkle products, to a different and broader field of skin-care creams by way of the class 3 goods specified for the 655 mark and the 426 defensive mark (see [17]-[19] above for the relevant specified goods). It is claimed that those specifications are not valid. It is accordingly sought by Self Care that the 655 mark in relation to those goods be removed and for the 426 defensive mark to be cancelled.
50 With regard to the claims against Ms Amoroso, she says that her actions relevant to the proceeding are squarely within her roles as director and officer of Self Care. She says that they involve, in particular, making and participating in decisions concerning the marketing of Self Care’s products and the claims made about them in marketing materials. Ms Amoroso accepts that her role involves having ultimate responsibility over the operations of Self Care, including over what goes out to consumers and the public, although she is not involved in every decision.
51 Ms Amoroso says that the activities of Self Care of which Allergan complains are squarely activities within the ordinary course of Self Care’s business, which is the supply to the Australian market of topical cosmetics. She says that this is not a case in which she caused Self Care to divert from the usual course of its business.
52 In proceeding NSD1802/2017, Allergan Inc, as appellant, appeals from a decision of a delegate of the Registrar of Trade Marks to allow Self Care IP’s Trade Mark Application No. 1653383 for the words FREEZEFRAME PROTOX to proceed to registration in respect of goods in class 3 (being anti-aging serum and anti-wrinkle serum). Allergan Inc raises ss 42(b), 44, 58, 59, 60 and 62A of the TM Act as grounds of opposition.
53 In summary, Self Care says that each of the numerous grounds on which Allergan opposes the registration of FREEZEFRAME PROTOX ought to be rejected, including because its trade mark is not misleadingly similar to the word BOTOX.
SELF CARE’S MARKET, PRODUCTS AND PACKAGING
54 Ms Amoroso gave evidence that in her experience people who are concerned to reduce the appearance of wrinkles fall into two separate markets, those who are prepared to undergo procedures such as injections which may be painful, invasive and involve the risk of potentially serious side-effects, and those who are interested in the use of anti-wrinkle creams and lotions. Obviously, there are people in the first category who will also use products of interest to the second category.
55 Ms Amoroso also accepted in cross-examination that the market can be split in three ways, namely those who use injectables such as Botox, those who are undecided about whether they will use injectables and those who will not use injectables.
56 In Ms Amoroso’s experience, the term Botox (or botox) is most frequently used to refer to any brand of botulinum toxin injection and not necessarily the particular product traded by Allergan under the BOTOX name. Thus, people refer to “having Botox” even when they are having a different brand of botulinum toxin injection. BOTOX is to a significant degree like marks such as HOOVER, XEROX or BAND-AID which have gained use to describe a whole category of product rather than specifically a particular brand within the category. Ms Amoroso was not challenged on this evidence, and I accept it.
57 The Freezeframe range of products has been sold to retail outlets such as department stores Myer and David Jones and retail chemists such as Priceline, Terry White Chemists, My Chemist and Chemist Warehouse, and to some online stores such as Adore Beauty and AMCAL Pharmacy Online.
58 Self Care’s FREEZEFRAME brand has many competitors in the skincare market. The competitors specialise in cosmetic creams and lotions. They do not manufacture or distribute anti-wrinkle injections. There is no brand which has a skincare product such as skin creams or lotions as well as botulinum toxin injections.
59 Ms Amoroso exhibited photographs of typical in-store displays for the sale of Freezeframe products. These show the whole range of Freezeframe products being displayed together where the common branding is obvious. The same is true of the sale of the products on the Freezeframe website – although each product has its own name, some more obviously incorporating the name FREEZEFRAME than others, all appear clearly and unmistakably within the Freezeframe range. Similarly, products available for sale on the Priceline website are all clearly indicated as being Freezeframe products.
60 Ms Amoroso accepted that some clinics that provide medical treatments also provide cosmetic moisturising products. But, she said that FREEZEFRAME branded products are not sold at such clinics. She was not challenged on that evidence, and I accept it.
61 The Freezeframe range of products has been extremely successful – both in terms of annual revenue and in product units sold. It has been particularly successful at Priceline, which is one of the biggest beauty retailers in Australia.
62 Since the second half of 2014, Self Care has caused to be manufactured and sold in Australia a skincare product which I will refer to as the Protox product. It is sold in a 20 ml tube which is packaged in an elongated box. The packaging includes the word PROTOX prominently on its own, as well as the word FREEZEFRAME® prominently on its own. It also includes the phrases “prolong the look of Botox®” and “clinically proven to prolong the effect of Botox®”.
63 The packaging relevantly looks like this:


64 The tube itself has the words PROTOX and FREEZEFRAME® in close proximity and the phrase “prolongs the skin smoothing effect of Botox® injections”.
65 Inside the Protox product packaging is a product information sheet which contains information about several of Self Care’s products, including the Protox product. Generally, the information sheet prominently displays the word FREEZEFRAME and the phrase “injection free alternatives”.
66 At various times since October 2014, Self Care has sold the Protox product in Australia to various retail outlets and selected pharmacies, as well as through the Freezeframe website. The recommended retail price of the Protox product is around $69.00, including GST.
67 Since April 2015, advertisements that included the Protox product appeared in publications such as the Body+Soul supplement, Woman’s Day and Marie Claire. Also, an article or editorial discussing “cosmeceuticals” with accompanying mini-features of select products in the form of panel advertisements was published in Body+Soul supplements that were included in a number of newspapers in the period between November 2014 and October 2016. The mini-features, or advertisements, on Protox included the following statements:
(1) “Freezeframe Protox ($69, 1800 662 686), can make Botox last longer, and reduce wrinkles even if you haven’t had injections”
(2) “Get smoother skin without Botox”
(3) “IMAGINE A SERUM THAT MAKES BOTOX LAST LONGER
Introducing freezeframe Protox, the anti-aging serum so powerful, it not only gives you a dramatic skin smoothing without the use of Botox, it can actually make the visual effects of Botox last up to three times longer. See smoother, fresher, younger-looking skin in as little as two-to-four weeks. Call 1800 662 686 or visit freeze-frame.com
*Botox is a registered trademark of Allergan, Inc.”
(4) “MAKE BOTOX LAST Imagine a serum that makes Botox® last longer”
(5) “Better without Botox Freezeframe Protox is clinically proven to make Botox look better and last up to three times longer, but it’s the dramatic results this power serum can produce without Botox that has everyone talking. On its own, Freezeframe Protox has a strong Botox-like effect and is proven to reduce deep wrinkles and smooth skin in just 15 days”.
68 From about October 2014, the Protox product together with the statements below have been included on the Freezeframe website at various times, for various periods of time:
(1) “At Last! A serum that can prolong the look of Botox®”
(2) “THE MOST POWERFUL BOTOX® ALTERNATIVE EVER DISCOVERED”
(3) “Introducing freezeframe PROTOX, the anti-aging serum so powerful, it not only gives you dramatic skin smoothing without the use of Botox, it can actually make the visual effect of Botox injections last up to 3 times longer!”
(4) “The Accidental Botox Alternative”
(5) “which could produce a Botox-like visual effect when applied topically to the skin”
(6) “for the first time ever cosmetic ingredients were tested to see what effects they had on skin that has been administered Botox injections”
(7) “freezeframe PROTOX’s peptide complex was proven to make the visual skin smoothing effect achieved from Botox injections last up to 3 times longer than they would on their own!”
(8) “it actually made the visual effect, that lovely smooth skin look achieved with Botox, look even more dramatic than injections alone”
(9) “In fact, skin looked better 6 months after injections than at 2 months, when Botox injections are still at their peak. Botox injections alone would have worn off by then”
(10) “Look better without Botox, or make Botox look better for longer – the power is now in your hands!”
(11) “the ultimate proof: freezeframe PROTOX is so powerful, it can even make Botox look better”
(12) “freezeframe PROTOX intensified the skin smoothing look achieved by Botox, and had prolonged it even after 6 months.”
69 Ms Amoroso came up with the word PROTOX. She intended it to be a play on words, where PRO refers to prolonging and TOX refers to botulinum toxin. She intended the product to be part of the Freezeframe range of products and to be referred to as FREEZEFRAME PROTOX. She expressly disputed that the play on words was intended to reference Allergan’s product Botox, as opposed to the generic understanding of “botox”, namely botulinum toxin.
70 Ms Amoroso said that she decided to use a capital B for Botox in the packaging and advertising to acknowledge the trade mark owner, Allergan, and to avoid the issues that she had had with Allergan during her time at Cat Media Pty Ltd (Cat Media). She was the founder of Cat Media and its managing director from March 1997 until December 2006 when she sold her interest in it.
71 Ms Amoroso denied that she had chosen names like BITOX (when she was at Cat Media), PROTOX and INHIBOX because of the closeness to the BOTOX mark or to play on the name BOTOX. She said that in choosing those names she was interested in communicating an alternative to botulinum toxin, but not that her product had any connection with Botox. She said that the PROTOX name was intended to convey that the product prolongs the effect of botulinum toxin.
72 It might be thought that that is rather undermined by the use of the ® symbol and the recordal that BOTOX is the registered trade mark of Allergan on the product packaging and in some of the advertising. However, in view of the general understanding in the market that “botox” refers to anti-wrinkle injections that are not necessarily Allergan’s product, and Ms Amoroso’s prior experience of Allergan complaining of Cat Media having appropriated its BOTOX mark, she could have had the intention to refer to botulinum toxin injections generically and to wish to take the precaution to specifically acknowledge that BOTOX is Allergan’s trade mark.
73 In email correspondence with people making a video commercial in 2009 when Self Care’s first product, Freezeframe with Inhibox, was launched Ms Amoroso said that “box” or “ox” in the name was a play on “botox” and that she thought that the target market would get the inference. She says that by “botox” she meant botulinum toxin, not the mark BOTOX, which, she says, is shown by the fact that she did not use capital letters. I do not accept that evidence. It strikes me as a convenient reconstruction. Given the fame of BOTOX, the intention was obviously to leverage off that fame by the reference. That is the inference that the target market would “get”.
74 As recorded above, the Protox product was initially advertised and promoted under the lead message that it could be used to prolong the effect of Botox. That was found to be unsuccessful because, on Ms Amoroso’s understanding and assessment, Self Care’s customers do not as a rule use Botox and were accordingly not interested in a product for prolonging its effects. Therefore, the lead messaging was switched to Protox being an alternative to Botox.
75 Where the term “Botox alternative” is used in a statement, Ms Amoroso’s understanding and intention in relation to the meaning of that term was that the product is something that the customer would use themselves instead of using Botox. That is, it is a product which does not involve injections of botulinum toxin.
76 Since around July 2009, Self Care has promoted, sold or offered for sale, in Australia a skincare product which I will refer to as the Inhibox product. It is sold in 10 ml units in a hard plastic syringe-like applicator.
77 Until about February 2017, the applicator was packaged in a box containing a clear plastic window through which one can see the syringe-like applicator. The packaging included the words FREEZEFRAME and WITH INHIBOX appearing separately as well as together. It also included the phrases “instant Botox® alternative”, “Clinically proven to erase wrinkle appearance in 5 minutes” and “The world’s first Instant and Long Term Botox® Alternative”.
78 Since about September 2016 to date, the applicator has also been packaged in a different box without a window. The box prominently displays the words FREEZEFRAME and INHIBOX. It also includes the phrases “instant Botox® alternative”, “clinically proven to erase eye wrinkles & puffiness in 5 minutes” and “The original instant and long-term Botox® alternative”.
79 The current packaging relevantly looks like this:
80 Included in the old and the new packaging is a product information sheet which appears to be the same in each case and the same as the product information sheet for the Protox product.
81 Self Care has sold and offered for sale the Inhibox product in Australia to various retail outlets and pharmacies. The recommended retail price is about $89.00 including GST.
82 From at least October 2012 to date, the Inhibox product has been promoted and sold from the Freezeframe website.
83 From about October 2012, the Inhibox product together with the statements below have been included on the Freezeframe website at various times, for various periods of time:
(1) “CLINICALLY PROVEN
INSTANT BOTOX® ALTERNATIVE
FREEZES WRINKLES INSTANTLY
REDUCES WRINKLES LONG TERM”
(2) “Freezeframe with INHIBOX is the original Instant Botox® Alternative, clinically proven to erase wrinkle appearance under the eyes and on the forehead in just 5 minutes, whilst delivering up to 63.23% reduction invisible [sic] wrinkles in just 28 days”
(3) “a topical alternative to Botox® injections”
(4) “The original Instant Botox® Alternative, freezeframe with INHIBOX has been a number 1 seller all over the world”.
84 The original Freezeframe with Inhibox packaging (Exhibit A) includes the phrase “Stop Time – freezeframe”. Ms Amoroso did not agree that the intention of that phrase was to depict the idea of stopping the passage of time or stopping the ageing process. She said that FREEZEFRAME as a word was intended to give people the impression of being able to capture a point in time. That is because it is an instant product and is not a treatment as such; it does not have long-lasting effects. Ms Amoroso denied that the intention with Freezeframe was to evoke Botox in the sense of “freezing wrinkles”.
85 Ms Amoroso accepted that the syringe-like applicator for the Inhibox product had the intended implication that it is an alternative to a syringe: “a playful, novelty way of not associating with Botox but associating with an alternative to anti-wrinkle injections in a very playful and non-invasive way”.
86 From about February 2017 to date, Self Care has sold or offered for sale a skincare product in Australia which I will refer to as the Night (tube) product. This is to distinguish it from the Night (tub) product which is dealt with next. The Night (tube) product is sold in a 30 ml tube which is packaged in an elongated box. The packaging includes the word NIGHT prominently on its own, as well as the word FREEZEFRAME® prominently on its own.
87 The packaging also includes the phrases “No Needle Topical Micro Injection” and “overnight Collagen and Botox Alternative for advanced line filling and wrinkle relaxing” on the front and side of the box respectively.
88 On the back of the box, the following statements are included:
(1) “Delivers the results of a collagen injection in 5 sleeps, and a Botox injection in 4 weeks!”
(2) “Our famous NIGHT cream now includes 2 alternatives to doctor administered injections, with results clinically proven to visually match those of injections – topical line filling equivalent to a collagen injection* and topical wrinkle freezing with visible results rivalling Botox injections…
*American Society of Plastic Surgeons
Botox® is a Registered trademark of Allergan Inc.”
(3) “See results equivalent to ... A Botox Injection in 28 days”.
(4) “Better than injections? With the visual benefits of both a collagen injection and a Botox injection, our multi action formula works in 3 ways to freeze and fill wrinkles, as well as lift skin for a dramatically younger appearance – without needles or invasive procedures.”
(5) “Our Topical Botox Alternative now delivers up to 94% wrinkle reduction in just 4 weeks!”
89 The packaging relevantly looks like this:
90 The tube itself has the words NIGHT and FREEZEFRAME® in close proximity and the phrase “Discover the overnight Botox® Alternative which relaxes wrinkles and rejuvenates skin whilst you sleep!”
91 Inside the Night (tube) product packaging is a product information sheet which includes the following statements:
(1) “Overnight Collagen and Botox Alternative for advanced line filling and wrinkle relaxing.”
(2) “Our famous NIGHT cream has been reinvented to include not 1, but 2 alternatives to doctor administered injections, with results clinically proven to visually match those of injections – topical line filling equivalent to a collagen injection and topical wrinkle freezing with visible results rivalling Botox injections ... American Society of Plastic Surgeons Botox® is a Registered trademark of Allergan Inc.”
(3) “See results equivalent to... A Botox Injection in 28 days”.
(4) “Better than injections? With the visual benefits of both a collagen injection and a Botox injection, our multi action formula works in 3 ways to freeze and fill wrinkles, as well as lift skin for a dramatically younger appearance – without invasive procedures”
(5) “Our Topical Botox Alternative now delivers up to 94% wrinkle reduction in just 4 weeks!”
(6) “Delivers the results of a collagen injection in 5 sleeps, and a Botox injection in 4 weeks!”
92 Since about February 2017 to date, Self Care sold or offered the Night (tube) product for sale at pharmacy retail outlets and on the Freezeframe website. The recommended retail price of the Night (tube) product is around $79.00, including GST.
93 From about February 2017, the Night (tube) product together with the statements below have been included on the Freezeframe website at various times, for various periods of time:
(1) On the website banner:
(a) “The same visible results of a COLLAGEN INJECTION 15 NIGHTS And a BOTOX® INJECTION 4 WEEKS”
(b) “Collagen Injection 15 DAYS
Botox Injection 28 DAYS
Mini Face Lift 84 DAYS
2 x TOPICAL MICRO INJECTORS”
(2) On the website:
(a) “Imagine ... the same visible results of a collagen injection in just 15 sleeps*, and a Botox® injection in 4 weeks*! ...
*American society of plastic surgeons confirms results ...
*Results may vary. Botox® is a registered trade mark of Allergan Inc. freezeframe NIGHT has no association with any anti-wrinkle injection brand”
(b) “New freezeframe NIGHT contains 2 Topical Micro Injection technologies, both clinically proven to visually match those of injections – topical line filling equivalent to a collagen injection and topical wrinkle freezing with visible results rivaling Botox Injections”
(c) “What’s better than a topical alternative to wrinkle fillers? One formula that includes both an alternative to fillers and alternative to Botox Injections!”
(d) “The same visible results of a COLLAGEN INJECTION 15 NIGHTS And a BOTOX® INJECTION 4 WEEKS
Reduces wrinkle appearance by as much as 94% in 28 days!”
94 Advertisements for the Night (tube) product featured in print advertising in 2017 which included the following statements:
(1) “Imagine... the same visible results of a collagen injection in just 15 sleeps*, and a Botox® injection in 4 weeks*! …
*American Society of Plastic Surgeons – collagen injections deliver a 15% wrinkle reduction
*Botox is a Registered trademark of Allergan Inc.
*NIGHT actives can reduce the appearance of wrinkles by up to 96% as compared to 84% in one Botox study. Data on file. Refers only to the visible effects on skin surface”
(2) “2 x TOPICAL MICRO INJECTORS Collagen Injection 15 DAYS Botox Injection 28 DAYS Mini Facelift 84 DAYS”
(3) “New freezeframe NIGHT contains 2 Topical Micro Injection technologies, both clinically proven to visually match those of injections – topical line filling equivalent to a collagen injection and topical wrinkle freezing with visible results rivaling Botox Injections. But it’s the dramatic face lifting effects that have astounded customers and experts alike, with skin structure brought back to baby’s skin in less than 3 months.”
(4) “What’s better than a topical alternative to wrinkle fillers? One formula that includes both an alternative to fillers and alternative to Botox Injections!”
95 In respect of the Night (tube) product, Ms Amoroso denied that her intention was to convey that the product would do as well as Botox; her intention was to convey that the product achieves a very high level of wrinkle smoothing which is comparable to Botox.
96 In or around 2009, Self Care developed a skincare product which I will refer to as the Night (tub) product. From about October 2012 to about March 2017, Self Care sold or offered for sale the Night (tub) product in a 30 ml glass jar/tub packaged in a cube-shaped box.
97 Inside the Night (tub) product packaging was a product information sheet.
98 Self Care sold the Night (tub) product in Australia to certain retail outlets and pharmacies. The recommended retail price was around $79.00, including GST.
99 Self Care promoted, sold or offered for sale the Night (tub) product in Australia on the Freezeframe website where the following statements were made at various times and for various periods of time:
(1) “Overnight Botox® ALTERNATIVE!”
(2) “LONG TERM BOTOX® ALTERNATIVE”
(3) “This luxurious cream is clinically proven to relax facial expressions and reduce the appearance of wrinkles by up to 63.23% in just 28 days, providing a long term alternative to Botox® injections”.
100 From about March 2017 to date, Self Care has sold or offered for sale a skincare product in Australia which I will refer to as the Boost product. The product is sold in a 12 ml tube with a combined pump/rollerball applicator which is packaged in an elongated box.
101 The Boost product packaging prominently displays the words FREEZEFRAME and BOOST SERUM and contains the statement “Botox® alternative peptides provide targeted results on even the deepest wrinkles.”
102 The packaging relevantly looks like this:

103 The product information inside the Boost product packaging contains the statement “Botox® alternative peptides also add to the anti aging cocktail for dramatic, targeted results on even the deepest wrinkles”.
104 Self Care has promoted, sold or offered for sale the Boost product in Australia on the Freezeframe website and sold it in Australia to the retail outlet Priceline. The recommended retail price is around $59.00, including GST.
105 From March 2017, the Boost product was included on the Freezeframe website with the following statement at various times, for various periods of time: “Botox® alternative peptides also add to the anti aging cocktail for dramatic, targeted results on even the deepest wrinkles”.
106 Since at least October 2012, the Boost Product was included on the Freezeframe website with the following statement at various times, for various periods of time: “LONG TERM BOTOX® ALTERNATIVE”.
SELF CARE’S IMPUGNED STATEMENTS AND ITS UNDERTAKINGS
107 On 18 February 2019, after the filing of Allergan’s further amended statement of claim, and with reference to that pleading, Self Care made a number of undertakings to the Court. The undertakings were expressly made without admission, in order to narrow the issues in dispute.
108 The undertakings were that Self Care will not make certain of the statements of which Allergan has complained. Self Care nevertheless stated that it intends to, and considers that it is entitled to, continue to make a number of statements, including various statements to the effect that the Self Care products are “Botox alternatives”.
109 As indicated, Allergan complains of statements by the respondents in four ways, namely as trade mark infringements, as affiliation representations, as efficacy representations and as contraventions of the TG Act.
110 The tables below, in respect of each product, reflect the statements that Allergan complains of, the ground for the complaint in each case (trade mark infringement, affiliation representation, efficacy representation and TG Act), and whether Self Care has undertaken not to make the statements anymore. These tables are prepared from Schs A, B and C to Allergan’s closing submissions.
No. | Statement | TM inf. Y/N | Aff. rep Y/N | Eff. rep Y/N | TG Act Y/N | U/taking Y/N |
1 | Use of the words “PROTOX” and “FREEZEFRAME PROTOX” | Y | Y | N | N | N |
2 | “Clinically proven to prolong the effect of Botox®” | Y | Y | Y | N | Y |
3 | “PROTOX prolongs the skin smoothing effect of Botox® injections” | Y | Y | Y | N | Y |
4 | “At Last! A serum that can prolong the look of Botox®” | Y | Y | Y | N | Y |
5 | “The most powerful BOTOX® alternative ever discovered” | Y | Y | Y | N | Y |
6 | “The accidental Botox Alternative” | Y | Y | Y | N | Y |
7 | “…Freezeframe Protox ($69, 1800 662 686), can make Botox last longer, and reduce wrinkles even if you haven’t had injections” | Y | Y | Y | N | Y |
8 | “IMAGINE A SERUM THAT MAKES BOTOX LAST LONGER” | Y | Y | Y | N | Y |
No. | Statement | TM inf. Y/N | Aff. rep Y/N | Eff. rep Y/N | TG Act Y/N | U/taking Y/N |
9 | “MAKE BOTOX LAST Imagine a serum that makes Botox® last longer” | Y | Y | Y | N | Y |
10 | “Better without Botox Freezeframe Protox is clinically proven to make Botox look better and last up to three times longer, …” | Y | Y | Y | N | Y |
11 | “Introducing freezeframe PROTOX, the anti-aging serum so powerful, it not only gives you dramatic skin smoothing without the use of Botox, it can actually make the visual effect of Botox injections last up to 3 times longer!” | N | N | Y | N | N |
12 | “…which could produce a Botox-like visual effect when applied topically to the skin” | N | N | Y | N | N |
13 | “freezeframe PROTOX’s peptide complex was proven to make the visual skin smoothing effect achieved from Botox injections last up to 3 times longer than they would on their own!” | N | N | Y | N | N |
14 | “…it actually made the visual effect, that lovely smooth skin look achieved with Botox, look even more dramatic than injections alone” | N | N | Y | N | N |
15 | “In fact, skin looked better 6 months after injections than at 2 months, when Botox injections are still at their peak. Botox injections alone would have worn off by then” | N | N | Y | N | N |
16 | “Look better without Botox, or make Botox look better for longer – the power is now in your hands!” | N | N | Y | N | N |
17 | “the ultimate proof: freezeframe PROTOX is so powerful, it can even make Botox look better” | N | N | Y | N | N |
18 | “freezeframe PROTOX intensified the skin smoothing look achieved by Botox, and had prolonged it even after 6 months” | N | N | Y | N | N |
19 | “Introducing freezeframe Protox, the anti-aging serum so powerful, it not only gives you a dramatic skin smoothing without the use of Botox, it can actually make the visual effects of Botox last up to three times longer. See smoother, fresher, younger-looking skin in as little as two-to-four weeks. Call 1800 662 686 or visit freeze-frame.com *Botox is a registered trademark of Allergan, Inc.” | N | N | Y | N | N |
112 It should be noted that the Protox product statements in the table above that are shaded and numbered 7-10 and 19 appeared in the Body+Soul supplement mini-features referred to at [67] above. That is recorded in the parties’ statement of agreed facts. In Sch A to its closing submissions, Allergan gave various references to the court book as evidence of the statements having been made by Self Care. Some of the references are to pages of the Freezeframe website but the shaded impugned statements do not appear there. The other references are to the Body+Soul mini-features.
113 Ms Amoroso’s evidence, which was not challenged, was that the content of the mini-features in which the statements appeared were controlled by the publishers of Body + Soul and that Self Care had no control over the content. On that basis, I am not able to find that Self Care made the statements, or adopted them or that the statements are otherwise attributable to Self Care. For that reason, those statements are left out of account in what follows.
114 Inhibox product:
No. | Statement | TM inf. Y/N | Aff. rep Y/N | Eff. rep Y/N | TG Act Y/N | U/taking Y/N |
1 | “Instant BOTOX® alternative” | Y | Y | Y | Y | N |
2 | “The World’s first Instant and Long Term Botox® Alternative” | Y | Y | Y | Y | Y re “Long term Botox alternative” only |
3 | “The original instant and long term Botox® alternative” | Y | Y | Y | Y | Y re “Long term Botox alternative” only |
4 | “Clinically Proven, Instant Botox® Alternative” | Y | Y | Y | Y plus “freezes wrinkles instantly” | N |
5 | “Freezeframe with INHIBOX is the original Instant Botox® Alternative” | Y | Y | Y | Y | N |
6 | “…a topical alternative to Botox® injections…” | Y | Y | Y | Y | N |
7 | “The original Instant Botox® Alternative” | Y | Y | Y | Y plus “has been a number 1 seller all over the world” | N |
115 Night products (tube and tub):
No. | Statement | TM inf. Y/N | Aff. rep Y/N | Eff. rep Y/N | TG Act Y/N | U/taking Y/N |
1 | “Discover the overnight BOTOX® Alternative which relaxes wrinkles and rejuvenates skin while you sleep!” | Y | Y | Y | Y | N |
2 | “Overnight Collagen and Botox Alternative for advanced line filling and wrinkle relaxing” | Y | Y | Y | Y | N |
3 | “Delivers the results of a collagen injection in 5 sleeps, and a Botox injection in 4 weeks” | Y | Y | Y | N | Y |
4 | “Our Topical Botox Alternative now delivers up to 94% wrinkle reduction in just 4 weeks” | Y | Y | Y | Y | N |
5 | “Overnight Botox® alternative” “Long term BOTOX® Alternative” | Y | Y | Y | N | Y (tub) N (tube) |
6 | “Botox Injection in 4 weeks Botox Injection in 28 days BOTOX® INJECTION 4 WEEKS Botox Injection 28 DAYS” | Y | Y | N | N | Y |
No. | Statement | TM inf. Y/N | Aff. rep Y/N | Eff. rep Y/N | TG Act Y/N | U/taking Y/N |
1 | “…Botox® alternative peptides provide targeted results on even the deepest wrinkles” | Y | Y | Y | Y | N |
2 | “Botox® alternative peptides also add to the anti aging cocktail for dramatic, targeted results on even the deepest wrinkles” | Y | Y | Y | Y | N |
3 | “Long term BOTOX® alternative” | Y | Y | Y | Y | N |
4 | “Wrinkle relaxing eye-roller for a long term BOTOX® alternative” | Y | Y | Y | Y | N |
117 The reputation of the BOTOX word mark is relevant to a number of the claims that fall to be decided. It is thus convenient to consider the reputation of BOTOX as a separate issue. That is undertaken in this section.
118 Ms Dawn Judith De Cruz, the Associate Director, Legal & Compliance – Australia & New Zealand of Allergan Australia gave evidence. Ms De Cruz testified that in Australia there is coverage of the Botox product by journalists and that Allergan’s overseas marketing material is reproduced or mentioned by third parties in publications circulating in Australia. She annexed to her affidavit internet Google search results for the word BOTOX and publications that were linked to the search results. The search was conducted on 20 November 2017.
119 Most of the search results, even in their summary forms as appearing on Google’s search engine results page, refer to Botox as being an “anti-wrinkle injection”. Also, the knowledge panel (right-hand panel) identifies Botox as botulinum toxin.
120 The media articles identified by Ms De Cruz discuss Botox injections, including their cost and the pain associated with their administration.
121 As recorded above, Ms De Cruz explained that for the purposes of selling, marketing and promoting Botox, Allergan makes a distinction in its business model between the use of Botox for the purpose of medicinal therapeutic indications and its use for cosmetic indications.
122 Ms De Cruz explained that Allergan engaged the company Edelman Australia to provide public relations and media communications related services to Allergan. Edelman regularly monitors Australian media coverage for and on behalf of Allergan in relation to the mark BOTOX. Edelman provided a media audit report on the media coverage of Botox in Australia for the period June 2016 to July 2017. The report shows that there were 1,054 mentions of Botox in the media surveyed in that period, which covered both positive and negative sentiment for Botox.
123 Edelman concluded that most often BOTOX is used as a category term rather than as a brand which, Edelman said, would be used for familiarity with their audience over “anti-wrinkle treatment” or “anti wrinkle injections”. However, given that Edelman’s methodology for reaching that conclusion was not revealed, and it obviously involved some level of judgement and hence opinion, I am not able to give much weight to that conclusion.
124 In the “anti-wrinkle” category of this survey, Edelman found that in every month in the survey BOTOX had overwhelmingly more mentions than any of the other 13 brands surveyed.
125 Ms De Cruz exhibited to her affidavit more than 200 articles from the media that mentioned Botox in the period 2003 to 2017. I reviewed the articles. Almost all of the articles refer to Botox being administered by way of injection and many of them refer to Botox being a toxin. Frequently Botox was referred to in the context of cosmetic surgeries. None of the articles suggests that Botox can be topically applied.
126 Ms De Cruz also exhibited reports of surveys conducted for Allergan by AC Nielsen in relation to brand awareness. Twice in 2004 and then once in each of the following years through to and including 2008, AC Nielsen conducted surveys of more than 400 female respondents over the age of 25 in Australian capital cities. Each survey showed that more than 80%, and in later years more than 90%, of respondents were aware of Botox and more than 20% were aware of botulinum toxin therapy. I do not think that the relatively low awareness of botulinum toxin therapy is indicative of a low awareness that Botox is an injectable product that is only administered by healthcare professionals. All it indicates is that most of the respondents who were aware of Botox were not aware that Botox is constituted by botulinum toxin.
127 Ms De Cruz testified that the survey reports also show that the mark BOTOX had the highest brand awareness for “wrinkle removal procedures” in Australian women aged 25-54. That can be accepted, it being significant that it is “procedures” rather than treatments for which Botox is known.
128 Ms De Cruz also cited mainstream media and television programs that frequently referred to Botox. One of those, as I have already mentioned, is the film Sex and the City. That film was released in Australia in June 2008 and box office sales for the period June to September 2008 grossed more than US$25 million. I take that to indicate that a large number of people watched the film.
129 An episode of the television series Sex and the City, in season five, included a scene mentioning Botox. Season five apparently premiered to nearly eight million viewers, as reported in August 2017, although the evidence does not show when season five aired in Australia.
130 I have reviewed the scenes in the movie and in the episode in season five that mentioned Botox. There is nothing in those scenes to suggest that Botox is topically applied, and in one scene “having Botox” is associated with pain implying widespread knowledge that Botox is an injectable.
131 Ms De Cruz also referred to several other popular television programs that mentioned Botox. One of those is an episode called “Skin Deep” on Catalyst, a television program that aired on ABC in September 2012 to approximately 800,000 viewers in Australia. The program discussed, amongst other things, the use of Botox as an injectable that is required to be administered by a healthcare professional. The program also made a distinction between Botox and its active ingredient botulinum toxin, however referred throughout to “botox” as short hand for anti-wrinkle injections. Other programs include Real Housewives of Sydney and The Bachelorette. The submission is that Botox occupies a place in Australian popular culture. That can be accepted.
132 As one would expect, Allergan regularly publishes press releases to the media, although the evidence does not reveal the extent to which the press releases are picked up by the media and reported on. Allergan also has websites, both internationally and in Australia, where Botox is promoted. The Australian website for Allergan reflects that the active ingredient in Botox is botulinum toxin and that Botox is available only by prescription.
133 Ms De Cruz also gave evidence as to Allergan’s global revenue from sales of the Botox product. That rose from a little under US$1 billion in 2006 to US$2.7 billion in 2016. As at 30 September 2017, Allergan’s revenue for sales of the Botox product for the calendar year 2017 was US$2.3 billion.
134 With regard to the sales of Botox in Australia for cosmetic uses, the evidence is that annual sales in 2016 were about 44% higher than they were in 2009. The annual sales for cosmetic uses have, since 2014, increased year on year by more than 9%. By October 2017, the growth rate for the calendar year 2017 was already reflected at 10.7%.
135 With regard to the sale of Botox for cosmetic uses in Australia, Ms De Cruz referred to a confidential exhibit showing such sales to be worth very substantial amounts each year for the period 2003 to 2017. The strong growth and high net value of sales are indicative of a strong brand awareness.
136 Ms De Cruz gave evidence, the details of which are the subject of a suppression order due to their commercial confidentiality, of the amount spent by Allergan in Australia on promoting the Botox product for cosmetic uses. For present purposes, it is sufficient to observe that the annual spend is, by any measure, very substantial. It can be taken that that spend contributes to widespread brand awareness.
137 Mr Franky Prochilo, Allergan Australia’s Associate Director of Marketing, Facial Aesthetics – Australia & New Zealand, gave evidence. Mr Prochilo testified that Botox has been on the market for almost 30 years, and in Australia for cosmetic indications for more than 15 years.
138 Mr Prochilo said that Allergan provides clinical literature to healthcare professionals, and he refers them to information published by Allergan to help them understand the Botox product and to enable them to provide information to consumers about the product and the procedure for its administration. That information, as would be expected, includes that Botox is a regulated toxin administered by way of injection.
139 An information brochure published by Allergan for healthcare professionals to provide to their patients distinguishes between three types of treatment for facial aging, namely skin treatments that correct texture and pigmentation issues, treatments that can enhance and replace facial volume, and Botox treatments that can relax wrinkle-causing facial muscles. Mr Prochilo accepted in cross-examination, with reference to that information, that Allergan was not putting itself forward as providing any skincare products.
140 A similar brochure also directed to the patients of healthcare professionals identifies different skin treatments amongst which is Botox, which is described as a prescription medicine that should be administered only by trained medical professionals.
141 Mr Prochilo also referred to information that is widely available on the internet which includes that Botox is a regulated toxin administered by way of injection and that Botox therapy is only available on prescription from a doctor.
142 Mr Prochilo gave evidence with regard to the promotional and advertising material produced by Allergan and distributed in Australia. That includes information booklets on how Botox is used for cosmetic purposes, flip charts and consumer brochures containing information for doctors and patients regarding the cosmetic use of Botox, notepads featuring the BOTOX trade marks, anatomical charts of facial muscles featuring the BOTOX trade marks and other selling aids, and sticky notes featuring the BOTOX trade marks. As expected, the consumer information booklets, website material and other promotional materials explain that Botox is a neurotoxin that is administered by way of injection by medical practitioners on a prescription basis only.
143 Mr Prochilo explained that Allergan Australia also produces marketing materials which advertise that Allergan has available injectable muscle relaxants or anti-wrinkle injections, or which provide information about the cosmetic indications and the types of products available for treatment indications. This advertising material refers to the products generally using words such as “injectable muscle relaxants” or “anti-wrinkle injections” without reference to the BOTOX trade mark due to TG Act regulatory requirements. That type of advertising is designed to promote non-brand product category awareness.
144 Allergan’s information and marketing material that it publishes about Botox includes descriptions as to what Botox achieves in its cosmetic applications. This includes descriptions such as “improvement in the severity of [frown] lines”, “improvement in the severity of [crow’s feet] lines”, “relaxes wrinkle-causing muscles, creating an improved appearance”, “improvement after BOTOX® treatment for glabellar lines”, “improvement in crow’s feet”, “enhance and rejuvenate your facial appearance with natural looking results” and “create a rejuvenated appearance”. Thus, as would be expected in respect of the cosmetic applications for Botox, Allergan emphasises the wrinkle-reducing enhancement in the appearance of patients after treatment.
145 The information available on the www.botoxcosmetic.com website that is maintained by Allergan is consistent with that in the information brochures referred to by Mr Prochilo. The home page of the website opens with a warning that Botox may cause serious side effects that can be life-threatening and directs that medical help should be sought right away if any side-effects are experienced at any time “after injection of BOTOX”. The message that Botox is administered by injection is front and centre.
146 In submissions, Allergan relies on the judgment of Stone J in Allergan Inc v Di Giacomo [2011] FCA 1540; 199 FCR 126 in support of the proposition that at least by 2008 Allergan Inc had acquired a significant reputation in the BOTOX marks in respect of a general cosmetic product or procedure for the treatment of facial lines, rather than one specifically involving the use of botulinum toxin. There are, however, a number of reasons why that judgment is not good authority, or evidence, for that proposition.
147 First, and foremost, under s 91 of the Evidence Act 1995 (Cth) a finding of fact in an Australian proceeding is not admissible to prove the existence of a fact that was in issue in that proceeding. The judgment is therefore not admissible to prove the nature of the reputation of the BOTOX marks.
148 Second, that proceeding was undefended with the result that the evidence was unchallenged and no evidence in opposition was adduced.
149 On the basis of the evidence, Allergan submits that the reputation of Allergan in the BOTOX marks in Australia is in connection with a general cosmetic product or procedure for the treatment of facial lines, rather than one specifically involving the use of botulinum toxin.
150 Against that, Self Care submits that the reputation of the mark BOTOX is confined to an injectable anti-wrinkle product. This is because the word BOTOX has only been used in relation to an injectable anti-wrinkle product which must be prescribed by a medical practitioner; it is a focused reputation. Self Care submits that the reputation of BOTOX does not extend to topical cosmetic products rather than an injectable product.
151 There can be no doubt on the evidence, which is accepted by Self Care, that BOTOX has a strong and widespread market reputation. BOTOX is widely known of, frequently referenced in popular culture, and the product by that name has strong sales and substantial advertising spend.
152 As to the nature of that reputation, in my view the evidence, the relevant aspects of which I have sought to identify above, overwhelmingly supports the conclusion that the reputation of Botox is as an injectable anti-wrinkle product administered by healthcare professionals. There is no reputation in the mark BOTOX that extends to topically applied cosmetics or general cosmetic products or treatments. The reputation whilst widespread and strong, is in its nature specific.
153 As already identified, I accept that the general understanding of the use of the word BOTOX is not necessarily as a reference to Allergan’s product which is branded BOTOX, but is rather understood in a generic sense as referring to an anti-wrinkle injection which could be Allergan’s product or one of the other botulinum toxin products on the market.
Infringement of BOTOX trade mark under ss 120(1) and 120(2) of TM Act
154 Allergan provided the following summary of the issues for determination in this part of their claim:
(1) PROTOX as a word mark:
Allergan submits that the use by Self Care of the word PROTOX is an infringement because it is used as a trade mark and it is deceptively similar to BOTOX. Self Care has undertaken to permanently refrain from making what is defined as the “Freezeframe PROTOX statements”, none of which involves the use of PROTOX independently of FREEZEFRAME. Ms Amoroso also gave evidence that Self Care does not use PROTOX independently. Nevertheless, Allergan submits that an infringement of their trade marks by past and possible future uses of PROTOX is still in issue.
(2) Uses of BOTOX in composite phrases – Inhibox:
This refers to the use of phrases such as “Instant Botox® alternative” and “The World’s first Instant and Long Term Botox® Alternative” in relation to the Inhibox product. Self Care has undertaken not to use the phrase “Long Term Botox® alternative” but does not consent to declarations and pecuniary relief for past uses, and expressly does not give an undertaking not to use the statement “Botox Alternative”. It also does not undertake not to use “Instant Botox® Alternative”. Thus, Allergan says that infringement and threatened infringement by uses of the phrases in the manner used by Self Care is in issue, but that infringement by “Botox Alternative” simpliciter is not in issue, and that there are no such uses put in evidence by either party.
(3) Uses of BOTOX in composite phrases – Night (tube):
This refers to the use of phrases such as “overnight Collagen and Botox Alternative”, “Discover the overnight BOTOX® Alternative”, “Delivers the results of a collagen injection in 5 weeks and a Botox injection in 4 weeks!”, and similar phrases. Self Care has undertaken not to use the “Delivers the results of a … Botox injection in 4 weeks” phrases, but expressly states that it does not undertake not to make the statement “provides an alternative to a Botox injection”. Self Care does not undertake not to use the “overnight Botox Alternative” phrases.
(4) Uses of BOTOX in composite phrases – Night (tub):
This refers to the use of phrases such as “Overnight Botox® Alternative” and “Long term BOTOX® alternative”. Self Care has undertaken not to use these phrases but it does not consent to declarations and pecuniary relief for past uses. It also makes it explicit that it does not undertake not to make the statement “Botox Alternative” or “alternative to Botox injections”.
(5) Uses of BOTOX in composite phrases – Boost:
This refers to the use of phrases such as “Long term BOTOX® alternative” and “Botox® alternative peptides”. Self Care has not undertaken not to use these phrases. Infringement and threatened infringement by the use of these phrases is thus in issue.
(6) Use of BOTOX in composite phrases – Protox:
This refers to the use of the phrase “prolong the look of BOTOX®”. Self Care has undertaken not to use this phrase, but it does not consent to declarations and pecuniary relief. Infringement by past use of the phrase is thus in issue. It also makes express that it does not undertake not to make the statement “Botox alternative”.
155 It is convenient to commence with ss 120(1) and 120(2) of the TM Act which provide:
120 When is a registered trade mark infringed?
(1) A person infringes a registered trade mark if the person uses as a trade mark a sign that is substantially identical with, or deceptively similar to, the trade mark in relation to goods or services in respect of which the trade mark is registered.
(2) A person infringes a registered trade mark if the person uses as a trade mark a sign that is substantially identical with, or deceptively similar to, the trade mark in relation to:
(a) goods of the same description as that of goods (registered goods) in respect of which the trade mark is registered; or
(b) services that are closely related to registered goods; or
(c) services of the same description as that of services (registered services) in respect of which the trade mark is registered; or
(d) goods that are closely related to registered services.
However, the person is not taken to have infringed the trade mark if the person establishes that using the sign as the person did is not likely to deceive or cause confusion.
156 By s 17, “a trade mark is a sign used, or intended to be used, to distinguish goods or services dealt with or provided in the course of trade by a person from goods or services so dealt with or provided by any other person.” A sign includes any word or combination of words: s 6.
157 There are three main elements to be established for infringement under s 120(1) or (2), namely: (1) the impugned sign must be used “as a trade mark”; (2) it must be substantially identical with or deceptively similar to the registered trade mark; and (3) it must be used in relation to the registered goods (s 120(1)) or goods of the same description as the registered goods (s 120(2)(a)).
158 In this case, all three elements are in issue in relation to different parts of the claim.
(1) Use as a trade mark – principles
159 Use of a mark “as a trade mark” is use of the mark as a “badge of origin” in the sense that it indicates a connection in the course of trade between goods and the person who applies the mark to the goods. That is the concept embodied in the definition of “trade mark” in s 17 – a sign used to distinguish goods dealt with in the course of trade by a person from goods so dealt with by someone else. See E & J Gallo Winery v Lion Nathan Australia Pty Ltd [2010] HCA 15; 241 CLR 144 at [43] per French CJ, Gummow, Crennan and Bell JJ, and [87] per Heydon J, approving the statement in Coca-Cola Company v All-Fect Distributors Ltd [1999] FCA 1721; 96 FCR 107 at [19] per Black CJ, Sundberg and Finkelstein JJ.
160 In Coca-Cola (at [20]) it was concluded that the authorities provide no support for the view that in determining whether a sign is used as a trade mark one asks whether the sign indicates a connection between the alleged infringer’s goods and those of the registered owner. The question is whether the sign used indicates origin of goods in the user of the sign; whether there is a connection in the course of trade between the goods and the user of the sign.
161 To determine whether the use of a mark constitutes use as a trade mark, the “purpose and nature” of the impugned use must be understood: Shell Company of Australia Ltd v Esso Standard Oil (Australia) Ltd [1963] HCA 66; 109 CLR 407 at 426 per Kitto J (Dixon CJ, Taylor and Owen JJ agreeing). The purpose and nature of the use must be assessed by reference to the context, including the way in which the words have been displayed: PepsiCo Australia Pty Ltd v Kettle Chip Co Pty Ltd [1996] FCA 48; 135 ALR 192 at 211-212 per Sackville J (Lockhart and Sheppard JJ agreeing).
162 It is unhelpful to assess the objectives or intention that was sought to be achieved by the use of the mark because whether or not it was used as a trade mark is to be determined by considering the words as they present themselves to the persons who are to read them and form a view about what they are meant to connote: PepsiCo at 218 per Sackville J; Anheuser Busch v Budejovický Budvar Národní Podnik [2002] FCA 390; 56 IPR 182 at [186] per Allsop J. The task is to examine the way the words are used in their context, including the totality of the packaging, to assess their nature and purpose in order to see whether they are used to distinguish the goods from goods of others: Anheuser Busch at [185] per Allsop J, citing Shell Company at 425 per Kitto J and Johnson & Johnson Australia Pty Ltd v Sterling Pharmaceuticals Pty Ltd [1991] FCA 402; 30 FCR 326 at 347 per Gummow J.
163 The decision of the House of Lords in Irving’s Yeast-Vite Ltd v Horsenail (1934) 51 RPC 110 is particularly pertinent in the present context. Irving complained that Horsenail infringed its YEAST-VITE trade mark by using the phrase “Yeast Tablets, a substitute for Yeast-Vite” in relation to the product. It was held that the impugned use was to distinguish the products from each other: “It is not a use of the word as a trade mark, that is, to indicate the origin of the goods in the Respondent by virtue of manufacture, selection, certification, dealing with or offering for sale” (at 115 per Lord Tomlin).
(2) Deceptive similarity – principles
164 The approach to determining whether two marks are substantially identical and whether they are deceptively similar is different.
165 In considering whether marks are substantially identical they should be compared side by side, their similarities and differences noted and the importance of these assessed having regard to the essential features of the registered mark and the total impression of resemblance or dissimilarity that emerges from the comparison; whether there is substantial identity is a question of fact. See Shell Company at 414 per Windeyer J.
166 As will be seen, Allergan does not rely on substantial identity; it confines its case to deceptive similarity.
167 On the question of deceptive similarity, the marks are not to be looked at side by side. The comparison is between, on the one hand, the impression based on recollection of the applicants’ mark that persons of ordinary intelligence and memory would have; and, on the other hand, the impressions that such persons would get from the respondents’ mark or sign; marks are remembered by general impressions or by some significant detail rather than by any photographic recollection of the whole. See Shell Company at 415 per Windeyer J.
168 In Australian Woollen Mills Ltd v F S Walton & Co Ltd [1937] HCA 51; 58 CLR 641, an infringement case, Dixon and McTiernan JJ (at 658-659) explained the exercise as follows:
But, in the end, it becomes a question of fact for the court to decide whether in fact there is such a reasonable probability of deception or confusion that the use of the new mark and title should be restrained.
In deciding this question, the marks ought not, of course, to be compared side by side. An attempt should be made to estimate the effect or impression produced on the mind of the potential customers by the mark or device for which the protection of an injunction is sought. The impression or recollection which is carried away and retained is necessarily the basis of any mistaken belief that the challenged mark or device is the same. The effect of spoken description must be considered. If a mark is in fact or from its nature likely to be the source of some name or verbal description by which buyers will express their desire to have the goods, then similarities both of sound and of meaning may play an important part. The usual manner in which ordinary people behave must be the test of what confusion or deception may be expected. Potential buyers of goods are not to be credited with any high perception or habitual caution. On the other hand, exceptional carelessness or stupidity may be disregarded. The course of business and the way in which the particular class of goods are sold gives, it may be said, the setting, and the habits and observation of men considered in the mass affords the standard. Evidence of actual cases of deception, if forthcoming, is of great weight.
…
It depends on a combination of visual impression and judicial estimation of the effect likely to be produced in the course of the ordinary conduct of affairs.
169 It is sufficient if persons who only know one of the marks and have perhaps an imperfect recollection of it are likely to be deceived: Cooper Engineering Co Pty Ltd v Sigmund Pumps Ltd [1952] HCA 15; 86 CLR 536 at 538 per Dixon, Williams and Kitto JJ. A mere possibility of confusion is not enough; there must be a real, tangible danger of its occurring: Southern Cross Refrigerating Co v Toowoomba Foundry Pty Ltd [1954] HCA 82; 91 CLR 592 at 595 per Kitto J (adopted on appeal at 608 per Dixon CJ, McTiernan, Webb, Fullagar and Taylor JJ).
170 By s 10 of the TM Act, “a trade mark is taken to be deceptively similar to another trade mark if it so nearly resembles that other trade mark that it is likely to deceive or cause confusion.”
171 In the present case, important issues arise as to whether the following can be taken into account in assessing whether the PROTOX mark is deceptively similar to the BOTOX mark: (1) the nature and reputation of the BOTOX mark, and (2) the manner of use of the PROTOX mark. The issues arise in part from the statement by Kitto J in Southern Cross (at 595) that:
In considering the probability of deception, all the surrounding circumstances have to be taken into consideration. (This includes the circumstances in which the marks will be used, the circumstances in which the goods will be bought and sold, and the character of the probable purchasers of the goods: Jafferjee v Scarlett [(1937) 57 CLR 115 at 120].)
172 However, Southern Cross and Jafferjee v Scarlett [1937] HCA 36; 57 CLR 115 concerned deceptive similarity in the context of opposition to registration rather than in the context of infringement. It is necessary to consider infringement separately.
173 As Gummow J explained in Wingate Pty Ltd v Levi Strauss & Co [1994] FCA 163; 49 FCR 89 at 128, where the comparison of the marks is necessary to determine whether that of the defendant is deceptively similar to the registered mark of the plaintiff, who sues for infringement, the primary comparison must be between any normal use of the registered mark on the one hand and the mark as used by the defendant on the other. The reason for the difference is that in order to give effect to the plaintiff’s monopoly arising from the registration of its mark, protection must be given even if its mark has not been used or, if it has, also in respect of designated goods and services in respect of which it has not been used. Thus it is any normal use of the plaintiff’s mark comprised within the registration that must be considered. On the other hand, it is the infringement by the defendant which is constituted by the actual use of that mark that must be considered. See MID Sydney Pty Ltd v Australian Tourism Co Ltd [1998] FCA 1616; 90 FCR 236 at 245 per Burchett, Sackville and Lehane JJ.
174 Nevertheless, there are circumstances in which matters relevant to the plaintiff’s mark that are broader than the mark itself might be taken into consideration. Section 219 of the TM Act provides that “evidence is admissible of the usage of the trade concerned and of any relevant trade mark, trade name or get-up legitimately used by other persons.” In the context of s 66 of the previous Act, which was in the same terms, in Mond Staffordshire Refining Co Ltd v Harlem [1929] HCA 6; 41 CLR 475 it was concluded that the registered trade mark MONSOL was not infringed by the use of MULSOL on the basis of evidence of a number of marks used for pharmaceutical products, such as those with which that case was concerned, which ended with –SOL or –OL: at 477-478 per Knox CJ, Gavan Duffy and Starke JJ. See Wingate at 127.
175 In MID Sydney (at 245) it was recognised that there are cases, of which that was one, in which it is appropriate to take into account the use, or proposed use, by the alleged infringer and also the character of the registered mark itself. The judgment of Gummow J in New South Wales Dairy Corporation v Murray Goulburn Co-op Co Ltd (No 1) [1989] FCA 124; 14 IPR 26 was cited where it was said (at 67) that in determining the issue of deceptive similarity in the context of infringement it is permissible to have regard to the actual use by the alleged infringer. In MID Sydney (at 246) that included two elements of the character of the mark CHIFLEY, namely that it is familiar not only as the name of a former Prime Minister but also, as the evidence showed, from its use in a number of geographic and other contexts.
176 In Anheuser-Busch Allsop J considered whether the use of the mark BUDEJOVICKÝ BUDVAR infringed the marks BUDWEISER and BUD. That turned in part on whether the former was deceptively similar to the latter. His Honour held (at [153]) that the setting or surrounding circumstances were relevant for the comparison and that they included, relevantly, how the product would be sold and promoted. Relevant also was where and how the allegedly infringing mark was used on the labels on beer bottles: at [181].
177 The Full Court in Melbourne Chinese Press Pty Ltd v Australian Chinese Newspapers Pty Ltd [2004] FCAFC 201; 63 IPR 38, an infringement case, confirmed that the following must be considered: the look and the sound of the words, the goods to which they are to be applied, the nature and kind of customer who would be likely to buy those goods, and “all the surrounding circumstances” (at [16] per Wilcox, Kiefel and Bennett JJ, citing Re Application by Pianotist Co Ltd (1906) 23 RPC 774 at 777 per Parker J). I understand this to be the approach that I am bound to follow.
178 In Registrar of Trade Marks v Woolworths Ltd [1999] FCA 1020; 93 FCR 365, a majority of the Full Court (French and Tamberlin JJ, Branson J dissenting) held that the trial judge’s reference to the familiarity of the name WOOLWORTHS in Australia was appropriate when considering opposition to the registration of the name WOOLWORTHS METRO on the ground that it would be confusing as being deceptively similar to the mark METRO. It was reasoned that “where an element of a trade mark has a degree of notoriety or familiarity of which judicial notice can be taken, … it would be artificial to separate out the physical features of the mark from the viewer’s perception of them”: at [61].
179 In Coca-Cola, the appellant’s registered mark that was said to be infringed was a contour drawing of the glass bottle in which the soft drink Coca-Cola was traditionally sold. The respondent was a confectionery wholesaler which imported and distributed a cola-flavoured confectionery which was shaped like the contour bottle and which had the word COLA inscribed on it. One of the factors listed by the Full Court (at [42] per Black CJ, Sundberg and Finkelstein JJ) as leading to the conclusion that the features of the confectionery were likely to cause confusion in consumers, i.e., to cause them to wonder whether it might be the case that the confectionery came from the same source as Coca-Cola, was that the contour bottle is extremely well-known.
180 In CA Henschke & Co v Rosemount Estates Pty Ltd [2000] FCA 1539; 52 IPR 42, an infringement case, the Full Court directly addressed the relevance of the reputation of the plaintiff’s mark in considering whether the defendant’s allegedly infringing mark is deceptively similar to the former mark. Ryan, Branson and Lehane JJ reasoned (at [46]) that what is to be considered is the significance of a particular word or phrase among traders and consumers in a particular market. If among those persons particular words have come to mean exclusively the goods of a proprietor of a registered mark, the registered mark being or including those words or as a “fancy word” composed of them and, when pronounced, sounding virtually the same, then use by another trader of those words in relation to goods in the class for which the mark is registered will infringe because, necessarily in the circumstances, use of the words by that trader is likely to cause confusion.
181 It was reasoned by the Full Court in CA Henschke (at [50]) that despite reliance on the contour bottle being extremely well-known, the Full Court in Coca-Cola is not to be taken to have decided that reputation evidence of the kind which is undoubtedly relevant in a passing off action is generally relevant to a question of deceptive similarity. It was held (at [52]) that Woolworths suggests, and other authorities support, that in assessing the nature of a consumer’s imperfect recollection of a mark, the fact that the mark, or perhaps an important element of it, is notoriously so ubiquitous and of such long-standing that consumers generally must be taken to be familiar with it and with its use in relation to particular goods or services, is a relevant consideration.
182 The approach to the question of reputation in CA Henschke was followed by the Full Court in Australian Meat Group Pty Ltd v JBS Australia Pty Ltd [2018] FCAFC 207; 268 FCR 624. It was held (at [41] per Allsop CJ, Besanko and Yates JJ) that the limited proposition which the Court accepted Woolworths stood for was not that reputation is relevant generally to deceptive similarity. That proposition was rejected. It was the proposition that deceptive similarity from imperfect recollection might be countered by showing the well-known nature of the registered mark and the lessened likelihood of imperfect recollection. I understand this to be the approach that I am bound to follow.
183 The notion of whether the impugned mark is likely to deceive or cause confusion comes into s 120(2) at two places. The first is in the chapeau, within the concept of “deceptively similar” as defined, and the second is in the chaussette (the closing words of the subsection as referred to by Perram J in SMA Solar Technology AG v Beyond Building Systems Pty Ltd (No 5) [2012] FCA 1483 at [79]), viz., “However, the person is not taken to have infringed the trade mark if the person establishes that using the sign as the person did is not likely to deceive or cause confusion.”
184 The words “as the person did” in the chaussette to s 120(2) direct attention to the way in which, as a matter of fact, the alleged infringer has used the allegedly infringing trade mark. These concluding words pose a different question to that raised by the chapeau of the subsection which, in the context of deceptive similarity, directs attention only to a comparison of the marks in the way discussed above. However, any conclusion about deceptive similarity would usually inform the consideration of whether the actual use was likely to deceive or cause confusion. In a sense, an affirmative answer to the question of whether the alleged infringing mark is deceptively similar would be the starting point. If it was, then it would, in many instances, render it more likely (though not inevitable) that the actual use of the allegedly infringing mark was likely to deceive or cause confusion. Also relevant would be the matters considered in determining whether the alleged infringer’s goods are of the same description of the goods in respect of which the registered mark is registered. See E & J Gallo Winery v Lion Nathan Australia Pty Ltd [2009] FCAFC 27; 175 FCR 386 at [76].
(3) The “goods in respect of which the trade mark is registered” or “goods of the same description” – principles
185 In respect of s 120(1), the inquiry is whether the goods in respect of which the allegedly infringing mark is used are the same as the goods in respect of which the allegedly infringed mark is registered. That involves considering the allegedly infringing goods, and asking whether they are covered by the goods listed in the registration of the allegedly infringed mark. See MID Sydney at 241; Bing! Software Pty Ltd v Bing Technologies Pty Ltd (No 1) [2008] FCA 1760; 79 IPR 454 at [47] per Collier J.
186 In considering whether the impugned mark is used on goods “of the same description” as the goods in respect of which the mark that is said to be infringed is registered (i.e., s 120(2)(a)), the inquiry is broader. The allegedly infringing goods may not be listed in the registration of the allegedly infringed mark, but they may nevertheless be goods of the same description. There may be many matters to be considered apart from the inherent character of the goods. These can be classified as the nature of the goods, the uses of the goods, and the trade channels through which they are bought and sold. No single consideration is conclusive in itself, and the classifications in the schedules for the registration of trade marks are not a decisive criterion as to whether or not two sets of goods are “of the same description”. See Southern Cross at 606 per Dixon CJ, McTiernan, Webb, Fullagar and Taylor JJ.
187 The English Court of Appeal in J Lyons & Co Ltd’s Application [1959] RPC 120 at 128 per Lord Evershed MR adopted the same tripartite classification of considerations (i.e., “the nature and composition of the goods, to their respective uses and functions, and to the trade channels through which respectively they are marketed or sold”). The Court also held that the phrase “goods of the same description” ought not to be given too restrictive a construction – “not, at all events, so as to be limited to goods substantially analogous in kind, or commonly used as mere substitutes or alternatives the one for the other”: at 128. That was adopted by the Full Court of this Court in MID Sydney at 244. See also Polo Textile Industries Pty Ltd v Domestic Textile Corp Pty Ltd [1993] FCA 265; 42 FCR 227 at 240 per Burchett J. Although MID Sydney dealt with services rather than goods, its reasoning applies equally to goods: E & J Gallo at [70].
188 The expression “goods of the same description” is generally to be understood in such a sense that, if two different items are held not to fall within the expression, their sale under the same mark by different companies is not likely to lead to confusion or deception: Polo Textile at 240, adopted in E & J Gallo at [71] per Moore, Edmonds and Gilmour JJ, not disturbed on appeal in E & J Gallo Winery v Lion Nathan Australia Pty Ltd [2010] HCA 15; 241 CLR 144.
189 Product substitutability is one of several factors which may be taken into account, but it is by no means determinative of itself: SMA Solar Technology at [75].
190 Self Care submits that it almost always uses PROTOX in combination with FREEZEFRAME – that FREEZEFRAME is the umbrella brand and PROTOX denotes a product within the range under the umbrella. It submits that it is PROTOX in combination with FREEZEFRAME that does the work of indicating the origin of the product known as Protox – PROTOX does not work alone in this respect.
191 I do not accept that PROTOX is almost always used in combination with FREEZEFRAME. I find that PROTOX is usually used in combination with FREEZEFRAME, but even when that is so the spatial relationship between those words is not consistent.
192 For example, the lid of the elongated box in which the product is packaged has the word PROTOX on its own with no other writing at all, whereas the face and side have the word PROTOX but between them there is a cutaway “surfboard” with the word FREEZEFRAME. The other side panel of the box has FREEZEFRAME without PROTOX, and the back of the box has PROTOX without FREEZFRAME. Much of this is evident from the images at [63] above.
193 The package insert for the Protox product is a composite insert for a number of Freezeframe products. It links the products to the brand FREEZEFRAME, but each product is given its own independent name separately from FREEZEFRAME. Thus, PROTOX appears as the prominent heading and thus name for the Protox product, not FREEZEFRAME PROTOX.
194 Many examples of the promotion and advertising of Protox were tendered in evidence. For example, many ‘advertorial’-type advertisements for Protox appearing in the health and beauty supplement Body + Soul in the period November 2014 to October 2016 were tendered. These depict the product tube with the word PROTOX prominently displayed and “freezeframe” below it in smaller, markedly less prominent writing. Adjacent to the depiction of the product tube is a paragraph of copy that refers to the product as “freezeframe Protox”.
195 Other advertisements in Body + Soul in October and December 2014 were tendered which reflect the Protox product being referred to as PROTOX on its own and also in the combination “PROTOX freezeframe”. An advertisement in Marie Claire in May 2015 refers to the product as “freezeframe PROTOX” and “PROTOX by freezeframe”.
196 Several downloads of pages from the Freezeframe website in the period October 2014 to November 2017 were tendered. Although being on the Freezeframe website, and in that sense linked to FREEZEFRAME, the pages depict the PROTOX name and mark being used independently of FREEZEFRAME, including in banner headings. Within the time period referred to, the more recent examples tend to show more consistent use of PROTOX in immediate proximity to FREEZEFRAME in describing the product as FREEZEFRAME PROTOX. That change appears to have taken place in about July 2015.
197 Examples of advertisements for Protox in the Herald Sun newspaper on 14 June 2015 and in various weekend newspapers on 9 August 2015 refer to the product as FREEZEFRAME PROTOX.
198 In my view it is quite clear that PROTOX is used as a trade mark. It badges the goods to which it applies; it gives those goods a name; it is indicative of trade origin by linking the goods to Self Care which applied the mark. The fact that FREEZEFRAME is the umbrella brand and that PROTOX denotes a product within the Freezeframe range does not detract from the use of PROTOX as a trade mark in respect of the Protox product.
199 The real issue here is not whether the word PROTOX is used as a trade mark – it clearly is, albeit not generally on its own or in isolation. The real issue is whether PROTOX is deceptively similar to BOTOX and, if it is, whether PROTOX as used (i.e., including the way in which it is used with FREEZEFRAME) is not likely to deceive or cause confusion.
200 Allergan relies on the following features in support of the conclusion that it contends for that PROTOX is deceptively similar to each of the BOTOX marks.
201 First, Allergan submits that the dominant feature of PROTOX is -OTOX, both visually and aurally. It submits that in referring to PROTOX, consumers are likely to recall this feature first and that PROTOX is likely to be understood as containing an essential feature of the BOTOX marks.
202 Secondly, Allergan submits that the sign PROTOX has a high degree of similarity as a cue that consumers would ordinarily associate with the Botox product. The cue that is common to both signs is the two syllables “oh” and “tox” (-OTOX). Allergan submits that upon seeing or hearing these, the consumer assumes that the products are, or are in some way, associated with BOTOX. It submits that the use of the PR- is unlikely to dispel a consumer’s recollection of the brand BOTOX. It says that this is particularly the case for consumers who have heard about Botox products, but who have not, or who have barely, seen them and their packaging.
203 Thirdly, Allergan submits that the evidence supports a finding that in adopting the name PROTOX, Self Care intended to trade off the reputation of the brand BOTOX. If so, it submits that the use of the mark PROTOX should be presumed to be fit for the intended purpose and thus likely to deceive in reliance on Australian Woollen Mills at 657 – that is that proof of the respondent’s intention to appropriate part of the trade mark or reputation of a rival may be sufficient in a borderline case to establish deceptive similarity.
204 In my view, the way in which the relevant words are most commonly and easily spoken breaks up the syllables as follows, BO·TOX and PRO·TOX. The first syllable of each word is similar, having the ‘oh’ sound. The words obviously have the second syllable in common, namely TOX. That might readily be understood as a reference to toxin or toxicology, but not necessarily so. It is nevertheless a familiar and common syllable.
205 The words are distinguished by the first syllable in each case, namely BO and PRO. BO has no ready meaning or reference on its own, although well-informed consumers might understand that BO is derived from botulinum. Botulinum is not a common word and knowledge of it would not be widespread.
206 In contrast, PRO is readily understood as meaning “in favour of”, favouring or advantageous, although it can also be a colloquialism for professional: Macquarie Online Dictionary. Ms Amoroso apparently intended to make a portmanteau reference to “prolonging” and “botulinum toxin”. Other than in the specific context of that word used in association with PROTOX, such as in the phrase “prolong the use of Botox with PROTOX”, I do not consider that it would readily be understood in that way.
207 Nevertheless, PRO is a familiar and recognisable element and it has meaning, whereas BO is unfamiliar and does not have a readily recognisable meaning. Also, because the common element, TOX, is readily familiar, I consider that attention is more readily directed to the prefix syllables in each case, i.e., PRO and BO. In any event, the point is that although the words are very similar in look and in sound, they are less so in any idea or meaning conveyed by them.
208 I do not accept Ms Amoroso’s evidence that the TOX part of the word refers to botulinum toxin rather than Botox, or more specifically the word BOTOX. Having regard to Ms Amoroso’s statement that it is a play on words and her reference in contemporaneous correspondence to people understanding the reference, in my view it was clearly intended to be an allusion to Botox (see [71]-[73] above). However, given my acceptance above that the general understanding of the word in the public domain is as an anti-wrinkle injection (see [153]), I do not think that this use demonstrates an intention to appropriate the trade or reputation of the brand BOTOX, but rather as a play on BOTOX and to differentiate from it. The intention was not, in my assessment, to say to the public “this is BOTOX”, but to say the opposite – “this is not BOTOX”.
209 An important factor, in my assessment, is the ubiquitous reputation of BOTOX. As I have found, the word is very widely known, and to such a degree that it has become in ordinary usage a common noun, not only a proper noun. Thus, and within the authority of CA Henschke and Australian Meat referred to above (at [180]-[182]), and as in Mars Australia Pty Ltd v Sweet Rewards Pty Ltd [2009] FCA 606; 81 IPR 354 at [97] per Perram J, the fame of the mark is such as to impact on a consumer’s imperfect recollection of the mark. First, there is not likely to be an imperfect recollection of the mark and, second, even if there is, such a consumer is not on seeing or hearing PROTOX likely to mistake it for BOTOX; they are more likely to be reminded of BOTOX.
210 That conclusion is reinforced by consideration of relevant surrounding circumstances. These include that the PROTOX mark is almost always used in proximity to the FREEZEFRAME mark which identifies a range of products that include marks having no similarity at all with the BOTOX mark.
211 The result is that although the marks are undoubtedly very similar in look and sound, they are sufficiently distinctive that, in my view, persons of ordinary intelligence and memory are not likely to confuse them. The PROTOX mark does not so nearly resemble the BOTOX mark that it is likely to deceive or cause confusion. Put differently, in my view, a person with even an imperfect recollection of the BOTOX mark is not likely to be deceived by the PROTOX mark, or even the general impression left by that mark; there is no real, tangible danger of that occurring. There is also no evidence of actual confusion, which offers some support to that conclusion.
212 In relation to the chaussette to s 120(2) of the TM Act, the PROTOX mark is used in relation to quite different goods, usually in circumstances where it is distinguished from the BOTOX mark because both marks are mentioned and the goods are distinguished by one being described as an alternative to the other, and the PROTOX mark is usually used in combination with or in proximity to the FREEZEFRAME mark. In those circumstances, the use of PROTOX by Self Care in the manner in which it does is not likely to deceive or cause confusion within the meaning of s 120(2). Allergan’s claim of trade mark infringement under s 120(2) in respect of Self Care’s use of PROTOX must accordingly fail.
213 Self Care submits that the mark that is really used is FREEZEFRAME PROTOX which, because of the combination of PROTOX with FREEZEFRAME, is clearly not deceptively similar to BOTOX. Given my conclusion with regard to PROTOX on its own not being deceptively similar it is not necessary for me to make any finding on this submission. However, in case it should be relevant, the evidence does not support a finding that PROTOX is always used in sufficient proximity to FREEZEFRAME such that the relevant mark is FREEZEFRAME PROTOX. I have identified that evidence above (at [191]-[197]).
(3) The registered goods or goods of the same description
214 Allergan’s primary contention is that each of Self Care’s products, including Protox, is a good in class 3 in respect of which Allergan’s class 3 marks are registered, i.e., the 655 mark and the 426 defensive mark. Self Care accepts that, save that it contends by its cross-claim that Allergan’s class 3 registrations should be removed from the register under s 92(4)(b) of the TM Act, in respect of the 655 mark, or cancelled in respect of the goods specified in its registration pursuant to s 88(2)(a) read with s 187(d) of the TM Act, in respect of the 426 defensive mark. Thus, if the cross-claim succeeds then the claim for trade mark infringement under s 120(1), i.e., in relation to goods in respect of which the trade mark is registered, must fail.
215 As will be seen, on the cross-claim I have concluded that the BOTOX 655 mark should be removed on account of non-use but that the BOTOX 426 defensive mark should not be cancelled because it has not been shown or established by Self Care that the use of the BOTOX mark in relation to the goods in class 3, in respect of which that mark is registered, would not be likely to indicate that there is a connection between those goods and the registered owner of the BOTOX mark.
216 The BOTOX 426 defensive mark includes goods in class 3 such as “anti-ageing creams; anti-wrinkle cream; beauty care preparations; beauty preparations; beauty care products; … eye wrinkle lotions; … skin care preparations and creams (cosmetic); skincare cosmetics; …”. Ms Amoroso described the Protox product as “an anti-ageing and anti-wrinkle balm serum”. There can be little doubt that that product is the same product as some of the products in respect of which the BOTOX 426 defensive mark is registered. Thus, although the s 120(1) infringement claim fails at the requirement of deceptive similarity, the requirement of “the same goods” is met.
217 It necessarily follows that the requirement in s 120(2)(a) of “goods of the same description” is also met in respect of the BOTOX 426 defensive mark class 3 goods.
218 Allergan also submits that Protox is a good of the same description as some of the goods in class 5 in relation to which the BOTOX marks are registered. This would only be relevant if I am wrong in my conclusions that the BOTOX and PROTOX marks are not deceptively similar and that the 426 defensive mark class 3 registration should not be cancelled.
219 Allergan relies on the following factors in submitting that Protox is a good of the same description as Botox – by which I take the submission to be that Protox is a good of the same description as the goods in respect of which the BOTOX 785 and 786 marks are registered in class 5:
(1) Self Care has promoted Protox with reference to the product Botox including as “Instant Botox® Alternative”, “Overnight Botox® Alternative” and “Long Term Botox® Alternative”.
(2) Although Botox is administered as an injection by a registered medical practitioner, the evidence shows that skincare goods are sold at the same point of sale where Botox is administered.
(3) The respective products are sold to the same class of customers, namely those customers who are seeking to improve the appearance of facial wrinkles.
220 It will be recalled that the relevant goods in class 5 are “Pharmaceutical preparations for the treatment of … wrinkles.” Botox is clearly such a good.
221 In my view, Protox is not a good of the same description as the BOTOX class 5 registered goods within the meaning of s 120(2)(a). There are a number of reasons for that.
222 First, with regard to the products themselves, Botox is a therapeutic product that is supplied only to healthcare professionals and can be administered only by healthcare professionals. In stark contrast, Protox is a topical cream that is rightly classified as a cosmetic that is sold directly to retail customers and applied by them. To put the matter differently, Botox is a highly toxic poison that is distributed, supplied and administered in a highly regulated manner, whereas Protox is a widely available and easily purchased and applied harmless cosmetic.
223 Second, with regard to the demand side of the market, the evidence is that there are some skincare clinics that sell skincare products and that also offer injectable products such as Botox. The skincare products include skin cleansers, peels, serums, toners, masks, exfoliants, moisturisers, creams and sun creams. However, the fact of these common outlets is not significant considering that what is sold is, on the one hand, a product such as Protox (although not actually Protox) and, on the other, a service being the administration of intramuscular injections. There is little to no risk that they will each be seen as having a common trade origin because they are available at the same place.
224 Third, with reference to the supply side of the market, pharmaceutical manufacture and supply is a quite different business from topical cosmetic product manufacture and supply. There is no evidence of any entity which engages in both. Neither Allergan nor Self Care does both.
225 Fourth, Allergan’s reliance on Self Care describing Protox as an “alternative” to Botox adopts an incorrect meaning of alternative. Protox is an alternative to Botox not in the sense of being a substitute for it, but in the sense of being quite different from it. Allergan’s focus is too much on the common purpose or outcome of reducing the appearance of wrinkles whilst overlooking the very different nature, mode of action and underlying effect of the different products. There is nothing in the marketing and presentation of Protox which suggests that it is a substitute for Botox (in the sense that Pepsi is a substitute for Coca-Cola). They are different in every way, including price, application, associated pain or bodily invasion and length of action – the effect of Botox is measured in months and that of Protox in hours. The only similarity between them is that they are both directed at reducing the appearance of wrinkles.
226 In the result, Protox is not a good of the same description, within the meaning of s 120(2), as a pharmaceutical preparation for the treatment of wrinkles.
(4) Conclusion: trade mark infringement claim with regard to PROTOX
227 For those reasons, Allergan’s claim against Self Care for trade mark infringement for use of its word mark PROTOX must fail.
(1) Use as a trade mark
228 The first question is whether the way in which Self Care uses the word BOTOX in its various composite phrases constitutes use of that word as a trade mark. The various uses that are complained of are identified in columns headed “TM inf. Y/N” in the tables at [111]-[116] above.
229 Those uses can conveniently be categorised as follows:
(1) Phrases including the word “alternative” in relation to Botox, as in “the accidental Botox Alternative”: in respect of the Protox product that is the phrases numbered 5 and 6, the Inhibox product phrases numbered 1-7, the Night products phrases numbered 1, 2, 4 and 5 and the Boost product phrases numbered 1-4.
(2) Phrases referring to increasing the time for which Botox is effective, sometimes including the word “prolongs” or “prolong” in relation to Botox, as in “clinically proven to prolong the effect of Botox®”: in respect of the Protox product that includes the phrases in the relevant schedule numbered 2, 3, 4, 7, 9, 10, 11, 13, 16 and 18.
(3) The phrases “a collagen injection in 5 sleeps, and a Botox injection in 4 weeks”, “Botox Injection in 4 weeks”, “Botox Injection in 28 days”, “BOTOX® INJECTION 4 WEEKS” and “Botox Injection 28 DAYS”: Night products 3, 6.
230 An evaluation of the manner in which Self Care uses the word BOTOX in the phrases that are complained of, including the purpose and nature of the use and the context of the use including the totality of the packaging, leads to the conclusion that those phrases are not themselves used as trade marks within the principles outlined at [159]-[163] above. There are a number of considerations that lead me to this conclusion.
231 In my view, none of the phrases is itself used as a badge of origin. Most of the phrases are narrative or descriptive phrases that include within them badges of origin such as PROTOX, FREEZEFRAME and BOTOX. For the most part, the inclusion of such clearly identifiable badges of origin within the narrative or descriptive phrases counts decisively against the phrases themselves being, or being used as, trade marks.
232 For example, the phrase “clinically proven to prolong the effect of Botox®” describes the product to which it relates and makes a claim about its efficacy, but it does not itself serve to say anything about its origin or about trade connection. Neither does it badge or name the product.
233 Essentially the phrases that are complained of amount to ‘ad-speak’ to spruik the relevant product, most often by either contrasting it to Botox or by saying that it can be used to improve the results of Botox such as by prolonging its effectiveness. They are not used as trade marks.
234 The only phrases that cause some hesitation with regard to whether or not they can themselves amount to trade marks or be used as trade marks are those without verbs such as “the accidental Botox Alternative”, “Instant BOTOX® alternative”, “Overnight Botox® alternative” and “Long term BOTOX® Alternative”. These phrases have the quality of being sufficiently short and catchy such as to possibly amount to labels for products and as such serve to distinguish them.
235 Each is descriptive of the product to which it is attached as an alternative to the well-known Botox product which is identified by name, but that quality of being descriptive does not necessarily mean that it is not used as a trade mark. A sign can both describe a product and serve as a badge of origin: Johnson & Johnson at 339 per Lockhart J and 347 per Gummow J.
236 Allergan submits that the use of capital letters for the first letter of several words in some of the phrases, or even in the spatial arrangement of the words such as one above the other, is indicative of the phrase being used as a label – essentially as a proper noun. I do not find that to be compelling. Capitalisation and spatial arrangement of the words are weak indicators and in any event are used inconsistently.
237 With regard to the phrase “Instant Botox® Alternative”, Allergan relies in particular on the following:
(1) In 2009, Self Care conducted a week-long promotion of its Inhibox product in a large Myer retail department store which included erecting large banners with the phrase “Instant Botox® Alternative” prominently displayed. The banners were estimated by Ms Amoroso to be about two metres tall and the phrase took up about a third of the banner. In some of the banners the phrase was the only writing, whereas in other banners – referred to as tower toppers – there was a subsidiary phrase “freeze wrinkles instantly & long term”. The display is also apparent in a promotional segment from the television program, A Current Affair. From the segment it can be seen that the FREEZEFRAME branding is also prominent.
(2) In August 2016, the Freezeframe website sales catalogue page for the same product labelled it as “Instant Botox® Alternative”.
(3) In August 2018, advertising of the Inhibox product on the Freezeframe website again prominently labelled it as “Instant Botox® Alternative”.
238 There are also other examples of the use of the “Instant Botox® Alternative” phrase in relation to the Inhibox product. As indicated above, it is recorded on the box of the product. There was also evidence of it in other advertising and promotion on the Freezeframe website. There was also evidence of the use of the phrase in relation to the product on the Priceline retail pharmacy’s website at the time of the hearing in this case indicating its continued use.
239 Allergan asks that I consider the different types of uses of the phrases in question separately, i.e., the in-store promotion, on the packaging, on the Freezeframe website, on the Priceline website, and so on. However, in my view none of the uses amounts to use as a trade mark. With the exception of the in-store promotion, the relevant context is a plethora of different messages about the relevant product. It includes the umbrella brand, FREEZEFRAME, and the name of the relevant product to which it speaks which is itself clearly a badge of origin. There are invariably many other phrases that are also used to describe the product or to otherwise say something about it. The context is confusing – capitalisation is inconsistent, punctuation is inconsistent, messaging is inconsistent. In that confusion, the particular short phrases do not stand out as badges of origin.
240 The in-store promotion is perhaps a little different. Although the evidence is scanty, and it may be that other messaging was also present, in my assessment the particularly prominent – outstanding even – use of the catchy phrase “Instant Botox® Alternative” could amount to a badging of the product. It could be understood as saying not only what the product is, but also distinguishing it from other goods on the market and suggesting a connection to Self Care.
241 Against that, Self Care refers to a number of other traders of products that are similar to its products that use similar short comparative phrases with reference to Botox. For example, Dermallure, Celleral, Dermal Meds, Auralei and truvisage all use the phrase “BETTER THAN BOTOX”. Argireline uses the phrase “Botox in the bottle” and “The liquid Botox Alternative”. Heaven Skincare uses “The natural alternative to Botox” in respect of its product Bee Venom Mask. Skin Deva uses “BOTOX ALTERNATIVE”. Natox uses “New Organic Botox Alternative”, “Brand New Natox Anti Ageing Cream Botox Alternative” and “a New Botox Alternative”. Cosima uses “Natural Botox® Alternative”. Xara and StriVectin use “Better than Botox”. Lapureté uses “NATURE’S BOTOX® ALTERNATIVE”.
242 Ms Amoroso’s evidence was that the products referred to in the previous paragraph are “currently” on the market. That was said in April 2018. There was no evidence of how widespread the marketing of those products is or what “market” she was referring to. Many of the products appear to be marketed in other countries and may not be marketed in Australia. Nevertheless, her evidence on this point was not challenged and I take from it that there are at least a number of other products in the Australian market in respect of which traders use phrases of the nature recorded in the previous paragraph which are similar to the kinds of phrases used by Self Care about which Allergan complains in this proceeding.
243 From this, Self Care submits that it has never put itself forward as the only “Botox alternative” on the market or, to adapt the words of Kitto J in Shell Company at 425, it has never attempted to suggest to consumers that where they see those words they are thereby to recognise the goods of Self Care.
244 Whilst that can be accepted, I am not sure that it takes the matter all that far. In any event, all that I am considering at this stage is the use of “Instant Botox® Alternative” at the week-long in-store promotion in 2014. Whether or not Self Care put itself forward as the only Botox alternative, or whether or not consumers on seeing the marketing material at the promotion would have been conscious of there being other products that also promote themselves as alternatives to Botox, does not really answer the question whether the relevant phrase was used as a trade mark in that promotion.
245 Whilst the answer is by no means clear cut, and I am conscious of the conclusion that I have reached (which is set out further below) that the phrase is in any event not deceptively similar to any of the BOTOX marks, I do not consider that use of the phrase in a single in-store promotion for one week amounts to sufficient use or identification to constitute use as a trade mark.
246 That deals with the composite phrases that include BOTOX and whether the phrases are used as trade marks. It remains to consider whether Self Care uses the word BOTOX itself as a trade mark in its use of that word in the phrases that are complained of. There are a number of considerations which lead to the conclusion that Self Care does not use BOTOX as a trade mark.
247 First, Self Care uses BOTOX in a manner which distinguishes Botox from Self Care’s own products. For example, many of the relevant phrases use the word “alternative”, such as “the most powerful BOTOX® alternative”. That is a meaning that conveys the products to be different which suggests also that they have different origins.
248 Similarly, phrases such as those indicating that Self Care’s relevant product “prolongs” the effect of Botox, or makes Botox “last longer”, or reduces wrinkles even without injections, or which makes Botox look better or can produce a Botox-like visual effect, or produce better results than Botox, all serve to distinguish between Self Care’s products and Botox.
249 The Yeast-Vite case, discussed above at [163] above, is a good illustration of why Self Care’s use of the BOTOX mark does not constitute use of it as a trade mark. There, the one product was described as a “substitute” of the other. Describing one product as an “alternative” to another is not materially different. It serves to emphasise that the one is not the other. That was not use as a trade mark.
250 Thus, BOTOX is not used by Self Care as its own badge of origin, i.e., indicating that the product identified by that name originates from Self Care. Indeed, the opposite is true.
251 Second, the use of the ® (registered trade mark) sign adjacent to BOTOX, where that is done, acknowledges that BOTOX is a badge of origin for the well-known product of that name. It does not serve to indicate that it is Self Care’s product. In many instances, it is expressly recorded on the relevant packaging or in the relevant marketing material that BOTOX is a registered trade mark of Allergan Inc. That serves to make it particularly clear that Botox is not Self Care’s product, i.e., BOTOX is a brand of origin and the origin is Allergan, not Self Care.
252 Third, Self Care’s products are branded and marketed under the umbrella brand FREEZEFRAME. As already indicated in relation to the PROTOX mark, the use of the umbrella brand and the product name is not always consistent – the name of the umbrella brand and the product name are not always used in the same spatial relationship. Nevertheless, as a general proposition each product within the range is also branded with the umbrella brand, FREEZEFRAME. In that regard the evidence includes the following:
(1) Ms Amoroso in cross-examination insisted that the name of each product includes the umbrella brand. For example, she said that the product is FREEZEFRAME INHIBOX and not the product INHIBOX within the Freezeframe range.
(2) Although that is reflected in Self Care’s registered trade marks, the way in which the words are presented on the packaging and in relevant advertising material has the result that FREEZEFRAME and the independent product name have independent uses. For example, the Freezeframe website tends to list the products under their individual product names independently of the word FREEZEFRAME, although the range of products is clearly identified as being the Freezeframe range.
253 The result is that one is left with the impression that each product has two prominent brands with which it is associated, the umbrella brand and the product name – the latter sometimes reflected as “freezeframe [PRODUCT]” and other times only as the product name. The descriptive phrases that are also used in relation to the products, such as those that are complained of, have less prominence and serve less to identify the products and do not as a consequence tend to badge or brand the products.
254 Fourth, Self Care’s use of the BOTOX mark does not indicate a connection in the course of trade between Self Care and BOTOX. It does the opposite. By describing Self Care’s products as alternatives or enhancers, comparing and contrasting, attaching the ® symbol and citing Allergan as the owner of that registered trade mark – as identified above – the point is made that there is no connection in the course of trade between BOTOX and Self Care.
255 Thus, in my view Allergan’s trade mark infringement complaint with regard to the various phrases that use the word BOTOX fails at the first hurdle; neither the phrases nor the word BOTOX are used by Self Care in these contexts as trade marks. The single possible exception is the use of “Instant Botox® Alternative” in the in-store promotion in Myer for one week in 2014, but even in respect of that use I have concluded that the phrase was not used as a trade mark.
(2) Deceptive similarity
256 Fairly obviously, the phrases are not in any way similar to the BOTOX marks. For all the reasons already given with regard to why those phrases are not used as trade marks, they cannot be mistaken for any of the BOTOX marks – they serve principally to compare, contrast and distinguish the product to which they are affixed from the product traded under the BOTOX marks that is named Botox. There simply is no deceptive similarity.
257 That conclusion includes the use of the phrase “Instant Botox® Alternative” in respect of its use in the in-store promotion in Myer discussed above. There is nothing about the phrase that might cause it to be confused with any of the BOTOX marks, aside from the fact that it includes the word BOTOX within it. However, the use of “alternative” serves the function of demonstrating that it is not the same as, or linked to, but is quite different from, the BOTOX marks and thus their origin. Indeed, the point of the promotion, and its central message, was to distinguish the product in question from Botox.
258 Of course, the use of the word BOTOX is quite different. It is identical to most of the BOTOX marks, and substantially identical to the BOTOX mark that is a composite mark. Allergan thus satisfies the “similarity” requirement in respect of Self Care’s use of this word, although, as I have concluded, it is not used by Self Care as a trade mark.
(3) The registered goods or goods of the same description
259 For much the same reasons as already concluded with regard to the Protox product, each of Self Care’s other relevant products is the same as goods in respect of which Botox is registered under the 426 defensive mark.
260 In that regard, Ms Amoroso described the products as follows. The Inhibox product is an anti-wrinkle product that contains a tensing agent that forms a film over the skin which, as it dries, shrinks and pulls the skin outwards smoothing and lifting the skin. The Night (tub) product was developed to reduce the appearance of wrinkles and is a wrinkle-relaxing cream or a skin-relaxing cream. The Night (tube) product provides a skin-smoothing action by a combination of intense moisturisation and relaxing the surface of the skin, boosting collagen, lifting and re-densification of the skin. The Boost product is a multifunction serum that increases oxygen uptake in the skin, lifting and smoothing the skin in the process.
261 However, they are not goods of the same description as Botox or the other goods in its class 5 registration. In particular, the following distinctions make that clear: cosmetics versus pharmaceuticals, very different trade channels, very different systems of application and very different prices.
(4) Conclusion: trade mark infringement claim with regard to BOTOX phrases
262 It follows that in my view Allergan’s trade mark infringement claim with regard to Self Care’s use of the various phrases including the word BOTOX, and the use of the word BOTOX itself, must fail.
CROSS-CLAIM: REMOVAL OR CANCELLATION OF TRADE MARKS
263 Self Care’s cross-claim seeks:
(1) Removal (including cessation of protection) from the register of the word mark BOTOX in class 3 of the 655 mark for non-use pursuant to s 92(4)(b) of the TM Act and reg 17A.48D of the Trade Mark Regulations 1995 (Cth) in respect of all goods covered by the registration, i.e., “cosmetics, face creams and lotions; skin creams and lotions”; and
(2) Cancellation of the BOTOX 426 defensive mark pursuant to ss 88(2)(a) and 187(d) of the TM Act in respect of all the class 3 goods, and such other goods as are relied on by Allergan in their claims of trade mark infringement.
264 It is apparent from paras [30] and [33C] of Allergan’s second further amended statement of claim that its reliance on the 426 defensive mark in its s 120(1) infringement claim against Self Care is limited to the goods in class 3. In a footnote (fn 47) to its closing submissions at trial, Allergan states that it relies on all the goods in respect of which the 426 defensive mark is registered. The result is that Self Care’s challenge to the registration of the 426 defensive mark is limited to the goods in class 3.
265 It is convenient to deal with each part of the cross-claim in turn.
266 Section 92 of the TM Act relevantly provides as follows:
92 Application for removal of trade mark from Register etc.
(1) Subject to subsection (3), a person may apply to the Registrar to have a trade mark that is or may be registered removed from the Register.
(2) The application:
(a) must be in accordance with the regulations; and
(b) may be made in respect of any or all of the goods and/or services in respect of which the trade mark may be, or is, registered.
…
(4) An application under subsection (1) … (non-use application) may be made on either or both of the following grounds, and on no other grounds:
…
(b) that the trade mark has remained registered for a continuous period of 3 years ending one month before the day on which the non-use application is filed, and, at no time during that period, the person who was then the registered owner:
(i) used the trade mark in Australia; or
(ii) used the trade mark in good faith in Australia;
in relation to the goods and/or services to which the application relates.
Note 1: For file and month see section 6.
Note 2: If non-use of a trade mark has been established in a particular place or export market, then instead of the trade mark being removed from the Register, conditions or limitations may be imposed under section 102 on the registration of the trade mark so that its registration does not extend to that place or export market.
Note 3: For when the registration of a trade mark is taken to have effect, see sections 72 and 239A.
267 In terms of s 100(1)(c) of the TM Act, in any proceeding relating to an opposed application (for removal for non-use), it is for the opponent to rebut any allegation made under s 92(4)(b) that the trade mark has not, at any time before the period of one month ending on the day on which the opposed application was filed, been used, or been used in good faith, by its registered owner in relation to the relevant goods. Relevantly, in terms of s 100(3)(a), the opponent is taken to have rebutted the allegation that the trade mark has not, at any time during the period referred to, been used, or been used in good faith, by its registered owner in relation to the relevant goods if the opponent has established that the trade mark was used in good faith by the registered owner in relation to those goods.
268 In Lodestar Anstalt v Campari America LLC [2016] FCAFC 92; 244 FCR 557 at [35] per Besanko J (with whose reasons Allsop CJ, Greenwood and Nicholas JJ agreed) and at [119] per Nicholas J (with whose reasons Allsop CJ agreed) it was held with reference to s 100(1)(c) that in an application under s 92(4)(b) the registered owner bears the onus of proof. Thus, Allergan bears the onus of proving use, in good faith, of the 655 mark in relation to “cosmetics, face creams and lotions; skin creams and lotions.”
269 Allergan contends, in the alternative, that if it is concluded that there was non-use of the 655 mark in relation to cosmetics, there should in any event not be removal of that mark in relation to any of the goods in respect of which it is registered as an exercise of the court’s discretion provided for in s 101(3) of the TM Act. In that regard, s 101 relevantly provides as follows:
101 Determination of opposed application—general
…
(2) Subject to subsection (3) and to section 102, if, at the end of the proceedings relating to an opposed application, the court is satisfied that the grounds on which the application was made have been established, the court may order the Registrar to remove the trade mark from the Register in respect of any or all of the goods and/or services to which the application relates.
(3) If satisfied that it is reasonable to do so, the Registrar or the court may decide that the trade mark should not be removed from the Register even if the grounds on which the application was made have been established.
(4) Without limiting the matters the Registrar may take into account in deciding under subsection (3) not to remove a trade mark from the Register, the Registrar may take into account whether the trade mark has been used by its registered owner in respect of:
(a) similar goods or closely related services; or
(b) similar services or closely related goods;
to those to which the application relates.
270 Section 102 of the TM Act, referred to in s 101(2), is not presently relevant.
271 The court may exercise its discretion under s 101(3) to narrow the scope of the registration where it is established that a ground exists in relation to only some of the goods to which the application relates. The subsection assumes that the court may be left in circumstances where some of the grounds have been established in relation to some of the goods but not all and it empowers the court in the exercise of its discretion to remove the trade mark from the register in respect of any or all of the goods to which the application relates. See Hills Industries Ltd v Bitek Pty Ltd [2011] FCA 94; 214 FCR 396 at [296] and [305] per Lander J.
272 The discretion under s 101(3) is said to be broad and without internal limitation: Pioneer Computers Australia Pty Ltd v Pioneer KK [2009] FCA 135; 176 FCR 300 at [167] and [172] per Bennett J. Amongst other things, account may be taken of use of the trade mark on closely related goods: Pioneer at [173]. There is no requirement to establish exceptional circumstances before the discretion can be exercised: Kowa Company Ltd v NV Organon [2005] FCA 1282; 223 ALR 27 at [98] per Lander J; E & J Gallo Winery v Lion Nathan Australia Pty Ltd [2008] FCA 934; 77 IPR 69 at [198] per Flick J; Pioneer at [168].
273 In construing the specification of goods in a trade mark registration, the class number is relevant. The internationally agreed Nice Classification System, although designed for administrative purposes, sets out a useful taxonomy which is a crucial part of the context within which the individual descriptors in the specification of goods are chosen. See Reliance Water Controls Ltd v Altecnic Ltd [2001] EWCA Civ 1928 at [42] per Mummery LJ (Sedly and Kennedy LJJ agreeing).
274 The cross-claim was filed on 1 December 2017. The relevant non-use period is therefore 2 November 2014 to 1 November 2017 – that is because the one-month period before the day on which the non-use application is filed includes the day on which it was filed: Acts Interpretation Act 1901 (Cth), ss 2G and 36(1) item 4. Section 6A of the TM Act does not appear to have application in the case, such as this, of a period ending before the identified event rather than after the identified event. Be that as it may, in this case nothing turns on a day or two at either end of the period.
275 The evidence was, and Allergan accepted, that the 655 mark had only been used in relation to the product Botox. There was no suggestion that that product might be classified or described as “face creams and lotions; skin creams and lotions”. Thus there was no use in that category. In the circumstances, Allergan focused its opposition on the category “cosmetics”, contending that Botox is a cosmetic within the meaning of that word as used in the trade mark registration.
276 The Macquarie Online Dictionary defines “cosmetics” when used as a noun as “a preparation for beautifying the complexion, skin, etc.” It also offers the definitions “serving to beautify; imparting or improving beauty, especially of the complexion”, “designed to effect a superficial alteration while keeping the basis unchanged” and “intended for show; superficial.”
277 Botox is not comfortably regarded as covered by these definitions when read together as I consider that they should be. As recorded at the outset, Botox affects the nerves in the muscle underlying the skin which in turn then affects the muscle. It is thus not “designed to effect a superficial alteration while keeping the basis unchanged”.
278 It was put to Self Care’s expert, Mr Williams, by counsel for Allergan that the term cosmetics is used for products that are applied topically to the skin and not to products that are intended to modify or otherwise inhibit physiological processes, to which he agreed. Mr Williams’s qualifications and experience are dealt with below (at [516]-[518]). Whilst I would not regard his view of the meaning of cosmetic as definitive, I nevertheless give it some weight. That is in particular because of his long experience in the industry of formulating cosmetic products. It is also not insignificant that, through counsel, Allergan adopted that meaning.
279 Further, the relevant class headings for class 3 in the Nice Classification (11th ed., 2019) are “non-medicated cosmetics and toiletry preparations; non-medicated dentifrices; perfumery, essential oils.” In the Nice Classification (8th ed., 2002) applicable at the time of the registration of the 655 mark, i.e., 2004, the applicable class headings for class 3 were not materially different. They were “soaps; perfumery, essential oils, cosmetics, hair lotions; dentifrices.” This suggests that “cosmetics” within class 3 accords with the meanings given above to that term, which is to say substances that are superficially applied with a view to a particular cosmetic outcome and not pharmaceutical or therapeutic substances that are injected into the muscle causing physiological change and which can only be administered by registered medical professionals.
280 Allergan’s first three BOTOX mark registrations, between 1991 and 2000, were all only in class 5. The current class heading for class 5 includes “Pharmaceuticals, medical and veterinary preparations; sanitary preparations for medical purposes; dietetic food and substances adapted for medical or veterinary use, food for babies.” Within that class the goods and services in respect of which the marks were registered included “pharmaceuticals for the therapeutic treatment of neurological disorders, muscle dystonias, smooth muscle disorders, autonomic nerve disorders, headaches, wrinkles, … .” Class 5 is obviously the most appropriate class for the BOTOX marks. That also serves to indicate that “cosmetics” in class 3 may not be a proper descriptor of Botox.
281 Whilst it might be said that Botox is aimed at achieving a particular cosmetic outcome, it cannot in my view ordinarily be described as a cosmetic. By its nature – a Sch 4 poison and a registered medicine on the Australian Register of Therapeutic Goods that causes physiological change and requires to be administered by a registered medical practitioner or health professional – it is a pharmaceutical and a toxin. It is not a cosmetic.
282 Allergan submits that although Botox is an injectable product, not a cream topically applied to the skin, it is used for cosmetic applications; it is sold and administered in doctors’ surgeries and beauty clinics where skin cosmetics are sold; and it has both cosmetic and therapeutic effect. In that regard, Allergan points to the distinction in its own business between the sale of Botox for therapeutic and for cosmetic purposes – it has separate websites, sales teams, marketing strategies and accounting.
283 I am not satisfied that the considerations raised by Allergan establish Botox as a cosmetic. As I have said, relevantly Botox is aimed at a cosmetic or aesthetic outcome, but it remains a pharmaceutical. To call it a cosmetic is too far a stretch.
284 In those circumstances, I conclude that Allergan has not used the 655 mark in respect of any of the goods covered by the registration and, subject to the possible exercise of the Court’s discretion to the contrary, all of them therefore fall to be removed.
285 Insofar as the discretion is concerned, Allergan submits that on the basis of there apparently being a market for “cosmeceuticals”, I should exercise the discretion under s 101(3) of the TM Act not to remove the registration of the 655 mark.
286 Allergan’s expert witness, Dr Graeme Haley, explained that he understood a cosmeceutical to be a cosmetic product which has or claims to have a therapeutic use. However, in the context of Australian regulation there is no recognised category of goods called “cosmeceuticals”. Ms Amoroso said that cosmeceuticals are applied topically as creams, lotions, balms and serums etc., and are often understood amongst consumers to be anti-ageing products. She explained that during the 1990s she saw the idea of cosmeceuticals as a market opportunity and started researching products and ingredients.
287 A brochure produced by Allergan for healthcare professionals to supply to customers describes cosmeceuticals as topical skincare products that contain ingredients that help in the treatment of ageing and photodamaged skin. These, as skin products, are distinguished from products that have an effect on the contours and volume of the face, as another category, and products such as Botox that act on the underlying muscles, as yet another category. Allergan’s witnesses Mr Prochilo and Ms Cain accepted those definitions.
288 The word cosmeceutical is another portmanteau obviously derived from the words cosmetic and pharmaceutical. Clearly enough the meaning it conveys is a cosmetic that has or claims to have curative properties (OED) or a cosmetic product which has or claims to have pharmaceutical benefits (Macquarie Online Dictionary). However, on the evidence in this case I am not satisfied that there is any identifiable market of cosmeceuticals as opposed to cosmetics. The word cosmeceutical appears to be used to give the impression that particular cosmetic products have some additional curative or pharmaceutical benefit, but that would appear to be more in the realm of advertising and marketing then reality. A simple moisturising lotion has a cosmetic effect and also a moisturising effect. That does not make the lotion any less a cosmetic or any more a pharmaceutical.
289 In any event, in the absence of any evidence of an identifiable market of cosmeceuticals as such, I cannot accept Allergan’s submission.
290 Allergan refers in particular to s 101(4)(a) and submits that it has used the 655 mark in respect of “similar goods or closely related services”. It submits that Botox and the service of providing cosmetic procedures that use Botox are addressed to the same market of consumers of cosmetics and often in the same place as cosmetics are offered for sale. That overlap, it submits, should influence the Court to exercise the discretion not to remove the trade mark.
291 There is no evidence that Allergan has used the 655 mark, or indeed any other BOTOX mark, in respect of “closely related services”. The service of administering Botox injections is offered by others, not by Allergan. The question is whether Botox is a closely related good such as to justify the exercise of the discretion. I am not persuaded that it is. That is for the same reasons that distinguish Botox as a pharmaceutical and not as a cosmetic.
292 I am therefore not persuaded that there is any justification for the exercise of the discretion. The class 3 registration of the 655 mark should accordingly be removed for non-use.
Cancellation of the 426 defensive mark
293 The 426 defensive mark was registered by Allergan as a defensive mark within the meaning of s 185 of the TM Act. It claims a priority date of 4 September 2013.
294 The cross-claim seeks cancellation of the 426 defensive mark in respect of: (1) each and all of the goods referred to in the specification in respect of class 3, and (2) such other goods and services referred to in the specification as are relied upon by Allergan in asserting infringement of the 426 defensive mark by Self Care. The ground relied on is that the use of the word mark BOTOX in relation to those goods or services is not likely to be taken to indicate that there is a connection between those goods or services and the registered owner.
295 I shall refer to the goods in respect of which the 426 defensive mark is registered (listed at [19] above) as the class 3 goods.
296 Registration as a defensive mark is intended to give better protection to marks that have been used to such an extent for certain goods or services that their unauthorised use for quite different goods or services would be likely to mislead the public: Davidson and Horak, Shanahan’s Australian Law of Trade Marks & Passing Off (Thomson Reuters, 6th ed., 2016) at [60.05]. Thus, the issue with regard to whether the registration of a defensive mark is maintainable does not concern whether the mark has been, or is intended to be, used in the class in respect of which it is challenged. Rather, it is whether, relevantly, it is likely that the use of the mark by someone else in relation to that class of goods or services will be taken to indicate that there is a connection between those goods or services and the registered owner of the defensive mark.
297 Pursuant to s 88(1) of the TM Act, the court may, on the application of an aggrieved person, order that the register be rectified by cancelling the registration of a trade mark. Such an application may be made, inter alia, on any of the grounds on which the registration of the trade mark could have been opposed: s 88(2)(a) of the TM Act.
298 Self Care, as a trade rival of Allergan’s, is an “aggrieved person”: see Health World Ltd v Shin-Sun Australia Pty Ltd [2010] HCA 13; 240 CLR 590 at [43]-[45] per French CJ, Gummow, Heydon and Bell JJ.
299 The relevant date for determining the parties’ rights under s 88(2)(a) is the priority date: Tivo Inc v Vivo International Corporation Pty Ltd [2012] FCA 252 at [445] per Dodds-Streeton J.
300 Section 185 of the TM Act relevantly provides:
185 Defensive trade marks
(1) If, because of the extent to which a registered trade mark has been used in relation to all or any of the goods or services in respect of which it is registered, it is likely that its use in relation to other goods or services will be taken to indicate that there is a connection between those other goods or services and the registered owner of the trade mark, the trade mark may, on the application of the registered owner, be registered as a defensive trade mark in respect of any or all of those other goods or services.
301 Section 187 of the TM Act relevantly provides:
187 Additional grounds for rejecting application for registration or opposing registration
In addition to any other ground on which:
(a) an application for the registration of a trade mark as a defensive trade mark may be rejected; or
(b) the registration of a trade mark as a defensive trade mark may be opposed;
the application must be rejected or the registration may be opposed:
(c) …; or
(d) in the case of a registered trade mark—if it is not likely that the use of the trade mark in relation to the goods or services in respect of which its registration as a defensive trade mark is sought will be taken to indicate that there is a connection between those goods or services and the registered owner.
Note: Division 2 of Part 4 sets out the main grounds for rejecting an application but section 41 does not apply to defensive trade marks (see section 186). Division 2 of Part 5 sets out the main grounds for opposing registration.
302 In respect of a defensive registration, it is more difficult to gain registration if the word is not invented: Re Applications by Mobile Oil Corp (1995) 32 IPR 535 at 537 and 543. It may thus be of some assistance to Allergan in resisting cancellation of its defensive mark that BOTOX is an invented word.
303 Apart from some passing references, there is apparently no previous judicial consideration of ss 185(1) and 187(d). For that reason, some background is of assistance.
304 Section 185(1) of the TM Act is relevantly identical to s 93(1) of the Trade Marks Act 1955 (Cth) which in turn was modelled, albeit with important differences, on s 27(1) of the Trade Marks Act 1938 (UK). The latter provision was in the following terms:
Where a trade mark consisting of an invented word or invented words has become so well known as respects any goods in respect of which it is registered and in relation to which it has been used that the use thereof in relation to other goods would be likely to be taken as indicating a connexion in the course of trade between those goods and a person entitled to use the trade mark in relation to the first-mentioned goods, then, notwithstanding that the proprietor registered in respect of the first-mentioned goods does not use or propose to use the trade mark in relation to those other goods and notwithstanding anything in the last foregoing section, the trade mark may, on the application in the prescribed manner of the proprietor registered in respect of the first-mentioned goods, be registered in his name in respect of those other goods as a defensive trade mark and, while so registered, shall not be liable to be taken off the register in respect of those goods under the last foregoing section.
305 There are at least two important differences between that provision and s 185(1) of the TM Act. First, s 185(1) requires that the mark be “used” to such an “extent” as opposed to it being “so well-known”. By focusing attention on the extent of the mark’s use as opposed to its fame serves to avoid the inference that defensive registration is available only to “famous” marks: Shanahan’s at [60.515]. Second, s 185(1) requires connection between the goods and the registered proprietor of the mark, as opposed to “connexion in the course of trade”. That would appear to be a less stringent requirement for registration of the defensive mark.
306 Section 27(1) of the Trade Marks Act 1938 (UK) first arose for judicial consideration in Re Ferodo Ltd [1945] Ch 334; 62 RPC 111. FERODO, an invented word, had been applied continuously and on an increasing scale for nearly 40 years to brake and clutch blocks and linings and similar articles for use on machines of various kinds, including motor cars, but also to a few more domestic articles, namely stair and step treads and covers. The applicant sought its defensive registration in respect of all the goods comprised in class 5 (Pharmaceutical substances) and class 34 (Tobacco … smokers’ articles and matches).
307 Evershed J held that the phrase “as respects any goods etc”, which has its equivalent in ss 185(1) and 187(d) of the TM Act the phrase “in relation to the goods”, requires that the knowledge of the mark should be knowledge of it in its application to goods of a specific kind; the conclusion to be reached to justify the defensive registration “must be the inference, in the light of all the known facts, including the general knowledge of the word in its application to the specific goods mentioned, that persons seeing the mark attached to the new class of goods would assume that they originated from the proprietor of the mark or a registered user”: at 337-338.
308 His Lordship held at 338 that in that case it was:
necessary to show that the word [FERODO had] become so well-known in its application to goods of a specific kind, that is, brake and clutch linings and the like …, that its use in relation to ‘other goods’ in classes 5 and 34 – for example, when applied to a bottle of aspirin tablets or a packet of cigarettes – would be likely to lead persons buying or observing those ‘other goods’ – for example, the aspirin tablets or the cigarettes – to suppose that they too originated from the same persons who made and marketed Ferodo brake and clutch blocks and linings, namely, the applicants.
… [Thus] the nature of the goods ‘in respect of which’ the trade mark ‘is registered … and used’ is an important factor, for generally speaking the more special in character those goods are, and the more limited their market, the less likely will be the inference required by the sub-section to be drawn in relation to the goods of a very special kind.
309 It was suggested in argument in that case that some trade marks, with BOVRIL and KODAK being given as examples, are so well-known in one particular field that the use by any person other than the proprietor in respect of any goods whatsoever would prima facie suggest an association of such goods with the proprietors of the marks. His Lordship was not satisfied with the soundness of that proposition, reasoning at 338-339 as follows:
Words do not become known as words without any significance or without appreciation of the things for which they indicate, and so far as trade marks are concerned it may well be that the better known the words become the more closely are they associated in the public mind with the class of articles to which they are in the course of trade applied. … if, for example, Bovril is well known to the great majority of the population of this island, and if it is so known as indicating a particular kind of foodstuff, then, on that very ground, the application of the word to, say, a typewriting machine, might well be held not to be in the least degree likely to lead to the indication referred to in the sub-section.
310 In the particular case, his Lordship held that “the somewhat narrow and special field of manufacture to which the mark [FERODO] has been applied and become known increases the difficulty of establishing, as a matter of fact, that the adoption of the word ‘in relation to’ goods of a widely different character would be likely, according to the standard of common sense, to lead to an inference of common origin”: at 339.
311 Although, as I have indicated, the section under which that case was decided had two important differences to the relevant Australian provisions, the reasoning of his Lordship in relation to the importance of the connection between the mark in question and the particular goods in respect of which it is used and registered is equally apposite in the present context.
312 Although the registered defensive mark does not have to be famous, and neither s 185(1) nor s 187 uses the word reputation, it is obvious that the mark must have some reputation in relation to all or some of the goods and services in respect of which it is non-defensively registered. That is because without that reputation the (unauthorised) use of the mark in relation to other goods or services would not be likely to suggest a connection with the owner of the registered mark.
313 The question arises as to the onus of proof. In Health World (at [23]-[26]) it was explained that if an application is made to have a mark registered there are two opportunities for registration to be prevented and, if a mark has been registered, there is a further opportunity to have the register adjusted. The first opportunity is when the application is lodged. The second opportunity referred to is if the application for registration is accepted, a party who wishes to oppose registration can do so pursuant to s 52 of the TM Act. The third opportunity is that a person who is “aggrieved” by the registration of a mark can have recourse under s 88 or s 92 to have a mark amended, cancelled or removed.
314 In respect of the second opportunity, opposition, it was held in Food Channel Network Pty Ltd v Television Food Network GP [2010] FCAFC 58; 185 FCR 9 (at [14]-[40] per Keane CJ, Stone and Jagot JJ) that the onus of proof lies on the opposing party to prove the ground of opposition relied on. That reasoning applies equally to an application for amendment or cancellation. Thus, the party applying for cancellation of a defensive mark under s 88(2)(a) on one of the grounds on which the registration of the trade mark as a defensive mark could have been opposed under the TM Act bears the onus of establishing that ground of opposition. That must be so because otherwise such a party would be in a better position applying to cancel a mark than it would have been in had it opposed the registration of the mark. That would be illogical.
315 Thus, in the present case, it is Self Care that bears the onus of establishing that it is not likely that the use of the trade mark BOTOX in relation to the goods in respect of which it is registered as a defensive mark will be taken to indicate that there is a connection between those goods or services and the registered owner.
316 Self Care submits that use of the word BOTOX in relation to any of the class 3 goods is not likely to be taken to indicate a connection between those goods and Allergan. It makes the following submissions in that regard.
317 First, it submits that the various class 3 topical cosmetic products do not ordinarily share a common trade source with injectable products such as Botox. It submits that Allergan’s evidence does not demonstrate a single example of a brand of injectable product that is also a brand of topical cosmetic product. It also points to evidence that Allergan’s marketing materials make it clear that Allergan is not in the business of providing skincare products and that consumers should seek advice elsewhere if they wish to know what products they should use.
318 Second, Self Care submits that the manner in which, and the persons to whom, the goods are supplied (which is to say the trade channels) are different in kind. It submits that Self Care’s products are sold to a consumer market in retail stores whereas injectable products such as Botox are sold only to medical practitioners in highly regulated circumstances and at high cost. Self Care submits that these differences in the sources and trade channels of the respective product types reflect the underlying fact that the two forms of business are very different and involve different know-how and expertise.
319 Third, Self Care submits that it is relevant that amongst ordinary reasonable consumers the word BOTOX is frequently used in a general sense to refer to a category of product, being anti-wrinkle injections, rather than a brand. It submits that the existence of that general meaning of the word makes it less likely that a consumer would draw from it a connection of trade association between topical cosmetic products and injectable products.
320 Allergan submits that the class 3 goods are related in the sense that they include products for cosmetic use such as anti-ageing creams, cosmetics, serums, and so on. Implicit in this submission is the contention that Botox is a similar type of product. Allergan also points to an apparent paradox in Self Care’s case, namely that by using phrases such as “instant Botox alternative” it seeks to associate its products, which are class 3 products, with Botox, but at the same time contends that the products are so different that if they had the same name they would not be thought to be connected in the course of trade.
321 Allergan also relies on BOTOX being an invented word and Self Care’s use of “Botox®” which, it submits, is not a use referring to botulinum toxin injections but rather draws a connection between Self Care’s products and the BOTOX mark.
322 Allergan relies on the extensive reputation of BOTOX and submits that Self Care’s attack on the 426 defensive mark falls well short of establishing what is required by s 187(d) of the TM Act.
323 Allergan submits that it is not a particularly far stretch to go from a trade mark used in respect of a product which is for use in “a cosmetic minimally invasive procedure” (being Botox) to use in respect of cosmetic products themselves (being the class 3 goods). It submits that whilst the products are in one sense quite different, they are nevertheless aimed at the same cosmetic outcome and have the same or substantially overlapping markets.
324 As indicated, the registration of the 426 defensive mark is in relation to a long list of goods in class 3. The Nice Classification (10th ed., 2011) applied as at the priority date. The class heading for class 3 was “Bleaching preparations and other substances for laundry use; cleaning, polishing, scouring and abrasive preparations; soaps; perfumery, essential oils, cosmetics, hair lotions; dentifrices.” With reference to the list of goods in the registration, it is apparent that the subheadings “soaps; perfumery, essential oils, cosmetics, hair lotions” are the ones within which Self Care’s class 3 goods would be classified.
325 The question is thus whether it is likely that the use of the trade mark BOTOX in relation to any of the class 3 goods would be taken to indicate that there is a connection between those goods and Allergan, being the registered owner of the BOTOX trade mark.
326 The evidence of the ubiquitous reputation of BOTOX is overwhelming. That reputation is not contested. Indeed, Self Care’s own attempts at differentiating its products from Botox as evidenced by the various phrases about which Allergan complains is itself indicative of the widespread reputation of BOTOX – if BOTOX did not have that reputation no purpose would be served by comparing and contrasting Self Care’s products with Botox.
327 However, as in Ferodo, the reputation of BOTOX is in relation to a very particular type of product. As discussed above (at [222]) in relation to why Protox is not a good of the same description as Botox, Botox is a pharmaceutical that is supplied only to medical practitioners and healthcare professionals and can be administered only by them. It is a highly toxic poison that is distributed, supplied and administered in a highly regulated environment, whereas the goods listed in the 426 defensive mark registration are essentially unregulated topical creams, lotions and cleansers.
328 That said, the reputation of BOTOX is in the same broadly defined or conceptualised area of beauty or appearance treatments as some of the class 3 goods. The types of treatment are very different, as I have explained, but, as Allergan puts it, they are in “the same universe of discourse”. If the reputation of BOTOX was as use as a mark for engine oil, for example, Self Care would not use the word BOTOX on its packaging and in its advertising and product promotions.
329 As dealt with above (at [223]) in relation to Protox and the goods in respect of which BOTOX is registered not being goods of the same description, the trade channels for Botox are quite different from most of the goods listed in class 3. The class 3 goods are essentially cosmetics that are sold as retail products directly to the public. None is a pharmaceutical or a therapeutic good. Also, the trade sources of cosmetics (and cosmeceuticals) are separate from the producers of pharmaceutical treatments for skin ageing and wrinkling such as Botox (see [224] above).
330 However, the class 3 goods and pharmaceutical treatments for skin ageing and wrinkling such as Botox share a substantially common market. Moreover, there is ample evidence of complementary use of certain skin care products and pharmaceuticals. Indeed, as will be dealt with in more detail with regard to the affiliation and efficacy representations, it is part of Self Care’s case that its products complement the use of Botox.
331 Further in that regard, brochures produced by Allergan for healthcare professionals to supply to their customers explain that three treatment types provide results to restore and enhance youthful features, especially when they are used in combination. The three different types are skin treatments that correct texture and pigmentation issues, treatments that can enhance and replace facial volume and treatments that can relax wrinkle-causing facial muscles. The first type uses cosmetics covering the class 3 goods and the third type is goods such as Botox.
332 The brochures explain that combining the benefits of the three treatments can enhance and rejuvenate facial appearance with natural-looking results. Consumers are urged to consult their clinician about treatment plans.
333 I do not consider that the submissions made by Self Care in relation to different trade sources and trade channels, albeit based on facts I have found established, change matters particularly. The mere fact that injectable products such as Botox do not ordinarily share a common trade source with topical cosmetic products, and that Allergan has not up until now itself traded topical cosmetic products, is insufficient to displace a connection that is otherwise likely to be drawn between topical cosmetic products and the owner of the BOTOX mark if that mark was used in relation to such products. Insofar as trade channels are concerned, the fact that typically such channels are different is equally insufficient to displace the connection.
334 Insofar as Self Care’s third submission is concerned, I accept that amongst ordinary reasonable consumers the word BOTOX is frequently used in a general sense to refer to a category of product, being anti-wrinkle injections, rather than a brand. There is evidence to support this as referred to by Self Care. However, BOTOX is nevertheless a powerful brand with a widespread reputation. If it was applied to a topical cosmetic product, i.e., a product that is not an anti-wrinkle injection, I consider that it is likely that ordinary reasonable consumers would draw a connection between the product and the owner of the trade mark BOTOX; it is not likely that ordinary reasonable consumers would consider that the topical cosmetic product is in fact an anti-wrinkle injection; nor is it likely that ordinary reasonable consumers would conclude that the product had a different trade source because it was not an injectable.
335 The point is, simply, that even though the word BOTOX might frequently be used in a general sense to refer to a particular category of product, if it is applied to a product that is not in that category then it will not be being used or understood in that general sense; it will be understood to be a brand for the product.
336 Turning to the list of goods in the registration, in my view several of them are products of a type not so dissimilar from Botox that it is not likely that the use of the mark BOTOX in relation to them would not be taken to indicate that there is a connection between them and Allergan. The products that I have in mind in the list include “anti-ageing creams; anti-wrinkle cream; … collagen preparations for cosmetic application; … dermatological cosmetic preparations; … eye wrinkle lotions; … skin care preparations and creams (cosmetic); skincare cosmetics; …”. In my assessment, if the mark BOTOX was to be used in relation to any such products it is likely that that would be taken to indicate a connection between those goods and the registered owner of the mark.
337 There are other goods in the list in respect of which one would be less confident that it was likely that such a connection would be made. For example “cleaners (preparations) for cleaning surgical instruments; … cleaning preparations for medical and laboratory instruments; … detergents having anti-bacterial and disinfecting properties (other than for medical use or for use in manufacturing processes); … products for cleaning; …”. Those products are all very different from Botox and are apparently unrelated to cosmetic or beauty treatments and anti-ageing or anti-wrinkle solutions.
338 In respect of those products and the remaining products in the list, which lie somewhere in between, I am not able to conclude that it is not likely that the relevant connection will be drawn. As Self Care bears the onus on this point, as discussed above, the case for the cancellation of the registration of the defensive mark in respect of these products is not made out.
339 In those circumstances, it has not been established by Self Care that it is not likely that the use of the trade mark BOTOX in relation to the goods or services in respect of which it is registered in class 3 will be taken to indicate that there is a connection between those goods or services and Allergan. The cross-claim challenging the registration of the 426 defensive mark must accordingly be dismissed.
Introduction
340 By reasons dated 19 September 2017, a delegate of the Registrar of Trade Marks allowed Self Care IP’s application for the registration of the mark FREEZEFRAME PROTOX in class 3 in respect of the goods “anti-ageing serum, anti-wrinkle serum” with a priority date of 20 October 2014: Allergan Inc v Self Care IP Holdings Pty Ltd [2017] ATMO 102. Allergan Inc had opposed the registration.
341 As indicated above, in proceeding NSD1802/2017 Allergan Inc appeals from the decision of the delegate under s 56 of the TM Act. The appeal hearing is a hearing de novo: Jafferjee v Scarlett at 119 per Latham CJ; Woolworths at [32] per French J (Tamberlin J agreeing).
342 Allergan Inc raises the following grounds of opposition to the registration of the FREEZEFRAME PROTOX mark in the appeal:
(1) Section 60: Allergan Inc contends that its mark BOTOX had, before the priority date, acquired a reputation in Australia and that because of that reputation the use of the mark FREEZEFRAME PROTOX would be likely to deceive or cause confusion.
(2) Section 42(b): Allergan Inc contends that the use of the FREEZEFRAME PROTOX mark would be contrary to law on the ground that it would be misleading or deceptive, or is likely to mislead or deceive, in breach of ss 18 and 29 of the ACL, including by falsely suggesting that Self Care IP’s goods are those of Allergan’s, or that Self Care IP or its goods are sponsored, approved, affiliated or licensed by Allergan.
(3) Section 44: Allergan Inc contends that the FREEZEFRAME PROTOX mark is deceptively similar to the BOTOX marks and that the marks cover “similar goods”.
(4) Section 58: Allergan Inc contends that Self Care Corp, and not Self Care IP, is the owner of the FREEZEFRAME PROTOX mark for the purposes of s 58 of the TM Act.
(5) Section 59: Allergan Inc contends that Self Care IP did not, as at the priority date, intend to use the FREEZEFRAME PROTOX mark. The contention is that the intention with respect to the use of the mark was to use FREEZEFRAME and PROTOX separately rather than the composite mark FREEZEFRAME PROTOX.
(6) Section 62A: Allergan Inc contends that the application for the registration of the FREEZEFRAME PROTOX mark was made in bad faith.
343 In respect of the ss 60 and 44 grounds, Allergan Inc relies on the following marks:
(1) the 655 mark for Botox in class 3 in respect of the goods “cosmetics, face creams and lotions; skin creams and lotions”;
(2) the 426 defensive mark for Botox in class 3 in respect of the goods “… anti-ageing creams; anti-wrinkle cream; … preparations for the skin; … beauty care preparations; beauty preparations; beauty care products; beauty products; beauty creams; … cosmetic articles for personal use; cosmetic articles and preparations; … cosmetic skin care products; cosmetics; … serum (cosmetic preparations); … skin care preparations and creams (cosmetic); skincare cosmetics”; and
(3) the 426 defensive mark for Botox in class 5 in respect of the goods “… body care preparations (medicated); chemical preparations and products for medical, veterinary and pharmaceutical purposes … dermatological creams and preparations (medicated) … medicated creams and lotions; medicated facial care preparations; medicated skin care preparations … moisturizers and moisturising preparations (pharmaceutical) … pharmaceutical products and creams … preparations for the skin (medicated) …”.
Section 60 – likely to deceive or cause confusion
344 Section 60 of the TM Act is in the following terms:
60 Trade mark similar to trade mark that has acquired a reputation in Australia
The registration of a trade mark in respect of particular goods or services may be opposed on the ground that:
(a) another trade mark had, before the priority date for the registration of the first‑mentioned trade mark in respect of those goods or services, acquired a reputation in Australia; and
(b) because of the reputation of that other trade mark, the use of the first‑mentioned trade mark would be likely to deceive or cause confusion.
Note: For priority date see section 12.
345 There is no issue in the case that the BOTOX mark had acquired a reputation in Australia before the priority date for the registration of the FREEZEFRAME PROTOX mark, i.e., 20 October 2014. The issue, then, is whether because of the reputation of the BOTOX mark, the use of the FREEZEFRAME PROTOX mark would be likely to deceive or cause confusion. That will turn, at least in part, on the nature of the reputation that the BOTOX mark had acquired.
346 I have already concluded that the reputation of the BOTOX mark is in relation to an injectable anti-wrinkle product administered by healthcare professionals. There is no reputation in the mark BOTOX that extends to topically applied cosmetics or general cosmetic products or treatments. Although that conclusion was reached, in particular, in relation to evidence up to and including late 2017, there is no suggestion in the evidence that the reputation was materially different as at October 2014.
347 Allergan Inc relies on essentially the same submissions in support of the contention that as a result of the reputation of the BOTOX mark the FREEZEFRAME PROTOX mark is likely to deceive or cause confusion as it did in support of the contention that the mark PROTOX is deceptively similar to each of the BOTOX trade marks.
348 For the same reasons as dealt with in relation to why the mark PROTOX is not deceptively similar to the mark BOTOX, in my view the mark FREEZEFRAME PROTOX is not likely to deceive or cause confusion. That is all the more so when, as here, the word FREEZEFRAME is added so as to precede the word PROTOX. The marks are barely similar at all, and certainly not deceptively similar.
349 Allergan Inc submits that the fact that one product is a pharmaceutical and the other is a cosmetic is not determinative. It bases that submission on a finding that it contends for, namely that the pervasive reputation of BOTOX is such that a consumer would draw an association between topical creams, such as the Protox products, and injectables, such as Botox. I have already concluded that that is not the nature of the reputation of BOTOX.
350 Allergan refers to Pfizer v Karam [2006] FCA 1663; 219 FCR 585 (Viagra) at [47] where Gyles J found that there was a risk of consumer confusion between the respective marks for a pharmaceutical (VIAGRA) and non-pharmaceutical herbal or alternative products (HERBAGRA). However, there are important distinguishing features of that case.
351 In Viagra it was concluded (at [31]) that the trade mark VIAGRA is associated with a substance taken orally for enhancement of penile erection for the improvement of sexual performance as well as curing of penile dysfunction, and not that it was only available on prescription. That meant that there was a close similarity between the goods, which together with the similarity between the marks led to the conclusion (at [38]) that a substantial number of members of the public would identify herbal medicines used to aid health, vitality and sexuality marketed under the name HERBAGRA to be a herbal version of VIAGRA. On that basis, deception or confusion was established.
352 In the present case, the reputation of BOTOX does not overlap with the goods for which the FREEZEFRAME PROTOX mark is sought to be registered, and the FREEZEFRAME PROTOX mark is very different from the BOTOX marks.
353 In the circumstances, the ground of opposition based on s 60 of the TM Act has not been established.
Section 42(b) – contrary to law as misleading and deceptive
354 This ground of opposition essentially duplicates the ACL case based on the affiliation representations. For the reasons given in relation to that case, the ground of opposition has not been established.
Section 44 – deceptively similar in respect of similar goods
355 Allergan Inc contends that the FREEZEFRAME PROTOX mark is deceptively similar to Allergan’s prior registered BOTOX mark and covers “similar goods”.
356 Allergan Inc submits that the addition of the word FREEZEFRAME is insufficient to distinguish or differentiate the FREEZEFRAME PROTOX mark. It submits that PROTOX is the distinctive and essential element of the mark for the purposes of assessing substantial identity and deceptive similarity with the BOTOX marks. On that basis it submits that the FREEZEFRAME PROTOX mark is deceptively similar to the BOTOX marks.
357 I have already concluded that PROTOX as a word mark separately from FREEZEFRAME is not deceptively similar to BOTOX. The composite mark FREEZEFRAME PROTOX raises even less possibility of deceptive similarity. That is because in undertaking the comparison, the trade marks are to be considered as a whole: Food Channel at [92]. The FREEZEFRAME component of the combined mark is highly distinctive and serves to clearly distinguish it from the BOTOX mark.
358 With regard to whether the marks are “in respect of similar goods”, once again this involves consideration of a range of factors including the nature and characteristics of the goods, the uses of the goods and the trade channels. For the reasons given previously (at [216]-[217]) with regard to Protox being a good of the same description as goods in respect of which BOTOX is registered, the goods in respect of which the FREEZEFRAME PROTOX registration is sought are similar to the goods in respect of which BOTOX is registered in class 3.
Section 58 – ownership of the opposed mark
359 Section 58 of the TM Act provides that the registration of a trade mark may be opposed on the ground that the applicant is not the owner of the trade mark. In s 6, “applicant” in relation to an application is defined to mean the person in whose name the application is for the time being proceeding.
360 Section 27(1) of the TM Act provides as follows:
(1) A person may apply for the registration of a trade mark in respect of goods and/or services if:
(a) the person claims to be the owner of the trade mark; and
(b) one of the following applies:
(i) the person is using or intends to use the trade mark in relation to the goods and/or services;
(ii) the person has authorised or intends to authorise another person to use the trade mark in relation to the goods and/or services;
(iii) the person intends to assign the trade mark to a body corporate that is about to be constituted with a view to the use by the body corporate of the trade mark in relation to the goods and/or services.
Note: For use see section 7.
361 It will be recalled that Self Care IP made the application, and was hence the applicant, for the registration of the FREEZEFRAME PROTOX mark. Allergan Inc submits that Self Care Corp, and not Self Care IP, is the owner of the mark.
362 As will be seen, it is Self Care’s case that Self Care IP claims to have been the owner of the FREEZEFRAME PROTOX mark at the time of the application for registration and to have authorised or intended to authorise Self Care Corp to use the mark.
363 Ownership of a trade mark is established either by authorship and prior use, or by the combination of authorship, the filing of the application and an intention to use or an intention to authorise use: Pham Global Pty Ltd v Insight Clinical Imaging Pty Ltd [2017] FCAFC 83; 251 FCR 379 at [18]-[19] and [25] per Greenwood, Jagot and Beach JJ. The relevant time is at the time of the application, i.e., 20 October 2014.
364 On the question of authorisation of use, s 8(1) of the TM Act provides that a person is an authorised user of a trade mark if the person uses the trade mark in relation to goods or services under the control of the owner of the trade mark. The nature of “control” referred to is control that is exercised in fact, rather than merely legal control exercised through the provisions of a contract: Lodestar at [97].
365 The parties made extensive references to the evidence in support of their competing contentions on the question of ownership of the opposed mark. In the circumstances, identifying the evidence in some detail cannot be avoided.
366 It will be recalled that both the Self Care companies have Ms Amoroso as the sole director and secretary. Both companies were incorporated and registered in 2008. The shares in both the companies are held on trust by a company of which Ms Amoroso is the sole director, secretary and shareholder, and has been since 15 May 2007. Ms Amoroso had control of decision-making in both companies in relation to product development, branding, marketing and trade marks. These are matters that Allergan not only did not seek to dispute, but sought to confirm in cross-examination of Ms Amoroso.
367 Ms Amoroso’s evidence was that Self Care IP was originally incorporated under the name The Brand Bank Pty Ltd but changed its name in April 2016 to Self Care IP. Ms Amoroso’s purpose in incorporating the company was for it to own some of the intellectual property, including trade marks, used by the Self Care companies and to license that intellectual property to Self Care Corp.
368 Ms Amoroso originally chose the name The Brand Bank because Self Care Corp was having problems with competitors copying its products, including its product names. She had hoped that by not using the words “Self Care” in the company name, competitors would not be given advance notice of the trade marks which were intended to be used by Self Care in the future but that were not yet in the marketplace. After it appeared that competitors had cottoned on to The Brand Bank being a Self Care company, Ms Amoroso decided to change the name to Self Care IP so that it was clear that the company was a Self Care company. The letters “IP” in the company name are plainly an initialism of “intellectual property”. That, and the previous name The Brand Bank, corroborate Ms Amoroso’s stated intention that that company would own Self Care’s trade marks.
369 Ms Amoroso said that in her role as director of Self Care IP she decided whether ownership of a particular trade mark used by the companies would be held by Self Care IP or by Self Care Corp. She said that in circumstances where the mark was owned by Self Care IP it would be licensed to Self Care Corp to use. This arrangement was formalised on 17 August 2013 when Self Care Corp and Self Care IP (then still known as The Brand Bank) entered into a license agreement.
370 The license agreement records that the licensor, i.e., Self Care IP, owns, amongst other things, certain trade marks and that the licensee, i.e., Self Care Corp, is engaged in the business of the manufacture, sale, distribution and provision of various goods. By its operative clause, Self Care IP granted to Self Care Corp “the non-exclusive, non-transferable right and license to use and make use of”, amongst other things, the trade marks. The license agreement is in the nature of an “umbrella” agreement under which, or with reference to which, existing and future trade marks could be licensed.
371 Ms Amoroso’s evidence was that Self Care Corp developed the Protox product that became branded as FREEZEFRAME PROTOX. She said that she came up with the name Protox and that she intended the product to be part of the Freezeframe range of products and to be referred to as FREEZEFRAME PROTOX. At that time, she made the decision that Self Care IP (then still known as The Brand Bank) would own the mark and license its use to Self Care Corp under the terms of the license agreement for the reasons set out above.
372 Even if by failing to record the identity of the particular mark in question the license of its use under the licence agreement failed, as identified above, authorised use is determined by reference to factual control. On the evidence of Ms Amoroso, Self Care Corp used the opposed mark in relation to the Protox product under the control of Self Care IP. That is what she said that she decided, in her capacities controlling both companies, and she was not challenged on that. The license agreement, even if ineffective, serves, as a matter of fact, to confirm that it was Ms Amoroso’s actual intention that Self Care IP would own trade marks and license their use to Self Care Corp.
373 A document referred to as “The Launch Commandments” confirms the intention of the business to register trade marks in the name of Self Care IP in 2014. That document set out Self Care’s instructions about launching a new product. Although the document tendered in evidence is dated 2016, it was put to Ms Amoroso in cross-examination, and she accepted, that the document, or an earlier version of it, was current when the Protox product was launched. The document has as one of its “Commandments” that trade marks for new products must be registered in the name of Self Care IP.
374 It was thus that, on 26 June 2014, Self Care IP lodged an application to the Australian Trade Marks Office to register the mark PROTOX and, on 20 October 2014, it lodged an application to register the mark FREEZEFRAME PROTOX.
375 The results of Australian Trade Mark Searches with IP Australia, which were tendered by the parties, show that there were at least seven trade mark applications lodged in the name of Self Care IP as trade mark owner in the period 2010 to 2015 with FREEZEFRAME in the name of the mark sought to be registered. One of those is the FREEZEFRAME PROTOX mark presently at issue. In contrast, in 2009, i.e., before the period referred to in relation to Self Care IP, applications for the registration of the word mark FREEZE FRAME and one fancy mark for the Inhibox product were lodged in the name of Self Care Corp. All other applications for the registration of marks incorporating the word FREEZEFRAME in the name of Self Care Corp were lodged in 2016, i.e., after the period referred to in relation to Self Care IP, except the one in 2013. The apparent temporal pattern with respect to the companies in whose names the applications were lodged, and the one exception to the pattern in 2013, was not explored in evidence. However, there is nothing inconsistent between that pattern and the evidence of Ms Amoroso with regard to ownership of the respective marks and, where relevant, their use being licensed by Self Care IP to Self Care Corp.
376 Allergan Inc submits that the idea for the word PROTOX came to Ms Amoroso in her capacity as director of Self Care Corp, and not in her personal capacity. It was accepted by Self Care that it was not in her personal capacity, but there is no evidence to support the contention that the name came to her in her capacity as director of Self Care Corp rather than as director of Self Care IP. The fact that it was Self Care Corp that was developing the product says nothing about who would own the trade mark. But in any event, it does not matter. Ms Amoroso was the controlling mind of both companies and had it within her capacity to decide which company would own the intellectual property, and she decided that in the case of the mark FREEZEFRAME PROTOX it would be Self Care IP. There is nothing in the evidence to gainsay that, and Ms Amoroso was not challenged on it in cross-examination.
377 Allergan Inc submits that Self Care IP admitted that it did not create or author the mark FREEZEFRAME PROTOX. In support of that submission it referred to a notice to admit and a response to the notice to admit. The notice to admit, under r 22.01 of the Federal Court Rules 2011 (Cth), was directed to Self Care IP. Amongst other things, it called for the admission that Self Care IP did not create or author the mark FREEZEFRAME and that it was aware that another person or entity had used the mark FREEZEFRAME in respect of the Protox product prior to the priority date. Self Care IP made those admissions although it disputed many other facts that it was asked to admit.
378 It is apparent both from what was asked to be admitted and what was then admitted, that Self Care IP did not admit that it did not author the mark FREEZEFRAME PROTOX. It was not asked about the authorship of that combined mark, or of the use of that mark in respect of the Protox product. It was asked to and made admissions about the mark FREEZEFRAME and not the mark FREEZEFRAME PROTOX. There is therefore no justification or support for the submission that Self Care IP admitted that it did not author the mark FREEZEFRAME PROTOX which is the mark that is the subject of opposition and the ownership of which is disputed with reference to s 58.
379 Allergan Inc also seeks to rely on the fact that the respondents in proceeding NSD15/2017, i.e., the Self Care companies, denied in their defence that Self Care IP advertised, promoted, exhibited, offered for sale, distributed and/or sold in Australia the Protox product under or by reference to the mark PROTOX, or the mark FREEZEFRAME together with the mark PROTOX, or authorised that conduct. Allergan Inc submits that this is consistent with the mark FREEZEFRAME and the mark PROTOX being owned and used by Self Care Corp at the time of the launch of the Protox product on or about 12 October 2014 and at the time of filing the application on 20 October 2014.
380 Paragraph [20] of Allergan’s second further amended statement of claim pleaded the following:
From a date unknown to the Applicants but since at least 7 October 2014, Self Care IP and Self Care Corporation have each manufactured or caused to be manufactured, advertised, promoted, exhibited, offered for sale, distributed and/or sold in Australia a skincare product under the brand name “Protox”, (further or in the alternative, under the brand name “freezeframe” together with the brand name PROTOX) (Protox Product), and/or have applied such market/s in Australia and offered the Protox Product for export, or authorise the above conduct with respect to the Protox Product.
381 Paragraph [20] of Self Care’s third further amended defence pleaded to that paragraph as follows (so far as is relevant):
In answer to paragraph 20 of the second further amended statement of claim, the Respondents:
(a) admit that, from about 7 August 2014 to December 2014, the Second Respondent (Self Care Corporation) caused to be manufactured a skincare product to be sold under the brand name freezeframe PROTOX (freezeframe Protox Product);
(b) admit that at various times and for various periods of time from about 19 October 2014 to 4 March 2019, Self Care Corporation advertised, promoted, exhibited, offered for sale, distributed and/or sold in Australia the freezeframe Protox Product;
(c) admit that from about 19 October 2014 to 4 March 2019 Self Care Corporation applied the freezeframe PROTOX mark to the freezeframe Protox Product and offered for export from Australia on its website at the domain name www.freeze-frame.com.au (freezeframe Website);
…
(f) otherwise deny the matters pleaded in paragraph 20.
382 The pleading by Self Care that it was Self Care Corp that used the FREEZEFRAME PROTOX mark in relation to the Protox product is consistent with Self Care’s case that the FREEZEFRAME PROTOX mark was owned by Self Care IP and licensed for use to Self Care Corp. It is thus apparently the denial in para [20(f)] of the defence that Allergan Inc relies on in submitting that Self Care IP denied that it authorised the use of the FREEZEFRAME PROTOX mark by Self Care Corp.
383 However, that denial does not have that meaning or effect for at least the following reasons. First, when in a defence there is a denial of a fact that is pleaded in a statement of claim, the denial is not proof of the opposite of the pleaded fact. No facts are asserted by a denial. The effect is that the fact asserted in the statement of claim will have to be proved by the party that asserts the fact in the event that that party wishes to rely on it.
384 Another way of looking at that is to recognise that it lies ill in the mouth of Allergan to say that Self Care IP did not authorise the use of the opposed mark on the ground that Self Care IP denied the contention made by Allergan that Self Care IP did authorise the use of that mark. Allergan cannot simultaneously assert one thing and rely on the opposite as having been proved by the denial of what it asserted.
385 Second, para [20] of the statement of claim contains rolled up allegations in a variety of possible combinations such that pleading to it specifically would have been very difficult. In the circumstances, the general denial in para [20(f)] of the defence cannot be understood as a specific denial of a fact not specifically pleaded but capable of being understood to be one of the rolled up allegations in para [20] of the statement of claim. The asserted fact that Allergan Inc relies on as having been denied is that Self Care IP authorised the use of the FREEZEFRAME PROTOX mark, but that fact is not specifically, clearly or separately articulated in para [20] of the statement of claim.
386 Third, and perhaps most tellingly, Allergan’s pleading does not refer to the FREEZEFRAME PROTOX mark. Apparently in a conscientious effort to avoid any suggestion that it accepted that there was such a mark, its pleading refers to the FREEZEFRAME and PROTOX marks separately and them being used together but never refers to the composite FREEZEFRAME PROTOX mark. In those circumstances, the denial of authorisation that is caught up in the general denial in para [20(f)] of the defence cannot be read as a denial that Self Care IP authorised the use of the FREEZEFRAME PROTOX mark.
387 In the circumstances, Allergan Inc’s opposition in reliance on s 58 fails.
388 Section 59 of the TM Act provides that the registration of a trade mark may be opposed on the ground that the applicant does not intend: (a) to use, or authorise the use of, the trade mark in Australia, or (b) to assign the trade mark to a body corporate for use by the body corporate in Australia, in relation to the goods specified in the application. The lack of intention to use the opposed mark must be established at the filing date: Food Channel at [74].
389 Allergan Inc contends that Self Care IP did not, at the priority date, intend to use the opposed mark.
390 The application by Self Care IP for the registration of the mark gives rise to a presumption that the requisite intention to use the mark existed at that time: Aston v Harlee Manufacturing Co [1960] HCA 47; 103 CLR 391 at 401 per Fullagar J and Suyen Corporation v Americana International Ltd [2010] FCA 638; 187 FCR 169 at [193]-[197] per Dodds-Streeton J.
391 Ms Amoroso’s affidavit evidence is that when she came up with the name PROTOX she intended the Protox product to be part of the Freezeframe range of products and to be referred to as FREEZEFRAME PROTOX. She says that at that time she made the decision that The Brand Bank would own the FREEZEFRAME PROTOX mark. If that evidence is accepted then clearly Self Care IP had the requisite intention at the time that the application was made as the application was made because the mark was intended to be used.
392 Although Allergan contends that at the priority date Self Care Corp, and not Self Care IP, had the relevant intention with regard to the use of trade marks in association with the Protox product (although it says that it was the separate marks FREEZEFRAME and PROTOX that were intended to be used), the question of who had the relevant intention has already been dealt with in relation to the s 58 ground of opposition. That is because the intention of ownership of the mark by Self Care IP and its licensed use by Self Care Corp has been decided under that ground, and that incorporates the intention to use the mark.
393 Thus, the remaining question to be dealt with under the s 59 ground of opposition is which mark was intended to be used, and in particular whether there was as at the date of the application for registration of the opposed mark the intention to use that mark.
394 Allergan Inc relies on a portion of the cross-examination of Ms Amoroso in support of the submission that as at the priority date Self Care Corp (not Self Care IP) sought to use the two separate marks FREEZEFRAME and PROTOX and that it did not intend to use the composite mark FREEZEFRAME PROTOX. This is the relevant extract of the cross-examination with the questions numbered for reference purposes:
1. MS GODDARD: Yes. On 19 October 2014. Now, what I want to suggest to you, Ms Amoroso, is that at the date of the launch and for a lengthy period subsequent to the launch, the mark that was in fact used and – was Protox, not Freezeframe Protox? --- It was Protox within the context of Freezeframe-branded elements.
….
2. MS GODDARD: So what I’m suggesting to you is that at the time of the launch of the product, Self Care intended to use the name Protox? --- All within the context …
3. MS GODDARD: Not Freezeframe Protox?--- All within the context of Freezeframe.
4. MS GODDARD: I’m not talking about the context of an umbrella brand. I’m asking you a different thing. You can – of course, Lancome – there’s always the presence of the umbrella brand, but …? --- Yes.
5. MS GODDARD: … you intended to call the product Protox? --- Well, we wouldn’t customarily within our advertising write a product name with Freezeframe every single time it’s mentioned in an advertisement.
6. MS GODDARD: No? --- It’s enough to have it being a Freezeframe-branded advertisement. So, as per our brand guidelines, it would have appeared like that.
7. MS GODDARD: And plainly that’s why you applied for the trademark, isn’t it, because you intended to use the mark Protox in the first – the earlier mark? --- As the product name.
8. MS GODDARD: Yes? --- Yes.
395 The date of 19 October 2014 referred to in question 1 is the date on which Ms Amoroso said that the Protox product was launched. Thus, the answers to questions 1 to 6 establish that as at 19 October 2014, being the day before the date that the application for registration of the opposed mark was lodged, Ms Amoroso had the intention that the Protox product would be known as PROTOX within the Freezeframe range of products. The implication of the answer to question 5 is that sometimes the product name, PROTOX, would appear with FREEZEFRAME but not always.
396 Questions 7 and 8 ask why Ms Amoroso applied for the mark PROTOX and suggest that it was because that was the intended product name. However, the application for registration of the mark PROTOX alone was lodged on 26 June 2014, i.e., four months earlier. Ms Amoroso’s intentions may have changed in the intervening period, which was not explored in cross-examination. Those questions therefore do not assist with regard to her intention on 20 October 2014.
397 Allergan Inc relies on the fact that the application for registration of the mark PROTOX was lodged on 26 June 2014 and that it was only after an adverse report was issued in respect of that application on 27 August 2014 that the application for registration of the mark FREEZEFRAME PROTOX was lodged, but even then the mark actually used on launch of the product was PROTOX.
398 There was evidence that the Protox product was advertised on the Freezeframe website as early as 12 October 2014, i.e., a week earlier than the date that Ms Amoroso said that it was launched. At that time the product was referred to as Protox but on the image of the tube itself there was the word PROTOX with the word FREEZEFRAME immediately beneath it, i.e., more like PROTOX FREEZEFRAME than FREEZEFRAME PROTOX. However, the packaging, as reflected in the first image at [63] above had the word FREEZEFRAME on the “surfboard” and the word PROTOX on the face of the packaging leaving the impression of the word FREEZEFRAME appearing immediately above PROTOX, i.e., FREEZEFRAME PROTOX.
399 Further, Allergan Inc relies on evidence that Self Care’s website referred to the product as FREEZEFRAME PROTOX only after a complaint on behalf of Allergan that PROTOX is deceptively similar to BOTOX and Self Care Corp’s response that it would thereafter ensure that the word PROTOX does not appear otherwise than with the word FREEZEFRAME. Allergan submits that this shows that as at the date of the application for registration of FREEZEFRAME PROTOX there was no intention to use that mark.
400 I do not accept that Allergan Inc has established that Self Care IP did not have the intention on lodging the application for registration of the opposed mark to use it. There are a number of reasons for that.
401 First, in Suyen (at [212]) Dodds-Streeton J observed that because the intention concerns the applicant’s state of mind it is difficult for an opponent to discharge the onus it bears under s 59. Where opposition has succeeded, the intention has been held to be contra-indicated where the applicant company was not operating and failed to provide evidence, had no capacity to trade in the relevant goods or services or was subject to a relevant contractual constraint. In other cases, opposition succeeded because the evidence established the existence of only illegitimate purposes, including the use of registration defensively, speculatively, to gain competitive advantage or to sell the trade mark. None of those circumstances pertains in the present case.
402 Second, the fact that Self Care IP sought first to register PROTOX alone and then sought to register the composite FREEZEFRAME PROTOX mark indicates a positive intention in the mind of Self Care IP to use the mark FREEZEFRAME PROTOX at that time. Ms Amoroso’s evidence that she did not intend to use the product name with FREEZEFRAME “every time”, as I have said, carries with it the implication that she intended that the product name would be used with FREEZEFRAME sometimes which is sufficient intention. That intention was not gainsaid by other evidence.
403 Third, the fact that the Freezeframe website was changed after the complaint correspondence on behalf of Allergan about the PROTOX mark, although indicative of the reason for that change, does not establish an absence of intention at the earlier time. In the period from the launch of the product until the change of the website the words FREEZEFRAME and PROTOX were used in association with each other, both in respect of the name of the product range being FREEZEFRAME and in the tube and on the packaging as I have mentioned, but also as explained by Ms Amoroso in the advertising. That association supports the inference that the intention on registering the FREEZEFRAME PROTOX mark was to use it in the future, and not that it was registered for defensive reasons, speculatively or for illegitimate purposes. Indeed, no suggested purpose of the registration other than the intention to use the mark was put to Ms Amoroso in cross-examination, and there is no evidence to support any other intention.
404 In the circumstances, Allergan Inc’s s 59 opposition must fail.
Section 62A – application made in bad faith
405 Section 62A of the TM Act provides that the registration of a trade mark may be opposed on the ground that “the application was made in bad faith”.
406 In Fry Consulting Pty Ltd v Sports Warehouse Inc (No 2) [2012] FCA 81; 201 FCR 565 at [143]-[166], Dodds-Streeton J undertook a detailed analysis of s 62A and its requirements. In DC Comics v Cheqout Pty Ltd [2013] FCA 478; 212 FCR 194 at [62]-[68], Bennett J sought to distil a number of principles from Dodds-Streeton J’s analysis. I gratefully adopt that distillation, and identify the following as being relevant principles:
Bad faith is a serious allegation and the more serious the allegation, the more cogent the evidence required to support it.
Bad faith does not require dishonesty.
Bad faith is a combined test that involves subjective and objective elements. The subjective element refers to the knowledge of the relevant person at the time of making the application. The objective element requires the decision-maker to decide whether, in the light of that knowledge, the relevant person’s behaviour fell short of acceptable commercial standards.
The question is whether the conduct fell short of the standards of acceptable commercial behaviour observed by reasonable and experienced persons in the particular area. It is whether the knowledge of the applicant was such that the decision to apply for registration would be regarded as in bad faith by persons adopting proper standards.
Mere negligence, incompetence or a lack of prudence to reasonable and experienced standards would not, in themselves, suffice, as the concept of bad faith imports conduct which, irrespective of the form it takes, is of an unscrupulous, underhand or unconscientious character.
407 The analysis of bad faith in Fry Consulting was cited with approval by Perry J in Bauer Consumer Media Ltd v Evergreen Television Pty Ltd [2017] FCA 507; 349 ALR 679 at [341].
408 Allergan Inc’s notice of appeal puts the case that the FREEZEFRAME PROTOX mark was registered in bad faith on the basis that reasonable and experienced people in the field would view that registration as falling short of acceptable commercial behaviour by reason of the following:
(1) Self Care IP was aware of Allergan’s reputation in the BOTOX marks, and intentionally chose the similar opposed mark and the mark PROTOX so as to cause confusion with the distinctive BOTOX marks, thereby falsely suggesting that Self Care IP or its goods were sponsored, approved or affiliated with Allergan and its BOTOX goods;
(2) the Freezeframe website shows frequent and prominent uses of Allergan’s BOTOX marks in the advertising by Self Care IP and/or Self Care Corp in a manner which strengthens the false suggestion of sponsorship, approval or affiliation, and increases the likelihood of confusion between the marks and respective products;
(3) Self Care IP and/or Self Care Corp have used Allergan’s signs and indicia (including BOTOX) and the mark PROTOX (on its own) in the course of trade to strengthen the connection in the minds of consumers between Allergan’s BOTOX marks and the opposed mark in order to leverage or springboard off the existing reputation of Allergan and to obtain a commercial advantage;
(4) Self Care IP and/or Self Care Corp have used Allergan’s signs and indicia (including BOTOX) in the course of trade to falsely represent to consumers a variety of messages including that the Protox product is complementary to the Botox products or a product sold under the BOTOX marks, the Protox product has a therapeutic use that Allergan has verified as correct or that independent clinical trials have proven, that use of the Protox product will give results to the same standard or quality as the Botox products, that use of the Protox product will achieve the same performance characteristics and/or benefits as the Botox products, and so on; and
(5) Self Care IP’s director, Ms Amoroso, was a director of Cat Media in previous proceedings in which she gave undertakings not to use various terms suggestive of BOTOX, not to make representations that the Relaxaderm product (that was in issue in that case) is equally efficacious to Botox and not to use the phrase “better than Botox”.
409 The essential characteristic of this part of Allergan Inc’s case, as will immediately be apparent, is that it offers no more than the case based on the affiliation and efficacy representations and the trade mark infringement case with respect to the use of the word PROTOX as a play on the word BOTOX. That is because the bad faith case asserts that the conduct of Self Care IP in registering the mark FREEZEFRAME PROTOX falls short of the standards of commercial behaviour observed by reasonable and experienced persons in the particular area. In this context, those standards include the standards as set by the ACL and the rules with regard to trade mark infringement.
410 In other words, if Allergan’s case under the ACL based on the affiliation and efficacy representations is good, then bad faith in relation to the registration of the FREEZEFRAME PROTOX mark would likely be established. On the other hand if that case is not good, then it is unlikely that bad faith in the registration of that mark would be found.
411 Similarly, if Allergan’s case of trade mark infringement on the basis that its use of the word PROTOX is use as a trade mark and that it is deceptively similar to the BOTOX mark is good, then it might be that the bad faith case in relation to the registration of the FREEZEFRAME PROTOX mark would be good. Conversely, however, if PROTOX is not deceptively similar to BOTOX then the basis for the bad faith case cannot be established.
412 It will be recalled that I have already concluded that the mark PROTOX is not deceptively similar to BOTOX. Also, it will be seen that my conclusion is that Allergan’s ACL case based on the affiliation and efficacy representations and its passing off case must fail save for one exception. That exception does not relate to the FREEZEFRAME PROTOX mark. In those circumstances, and in the absence of any independent ground of bad faith being established, Allergan Inc’s opposition to the registration of the FREEZEFRAME PROTOX mark based on s 62A must also fail.
Conclusion on the Trade Mark Office appeal
413 For the above reasons, Allergan Inc’s appeal against the decision of the delegate of the Registrar of Trade Marks falls to be dismissed with costs.
AUSTRALIAN CONSUMER LAW AND PASSING OFF
414 It will be recalled that Allergan’s case in reliance on the ACL and the tort of passing off is divided between the case on the affiliation representations (ACL and passing off) and the case on the efficacy representations (ACL only). Each is quite different, and therefore calls to be considered separately. However, before turning to that separate consideration, it is worthwhile to deal with some common aspects.
415 The ACL cases rely on the following provisions of the ACL:
4 Misleading representations with respect to future matters
(1) If:
(a) a person makes a representation with respect to any future matter (including the doing of, or the refusing to do, any act); and
(b) the person does not have reasonable grounds for making the representation;
the representation is taken, for the purposes of this Schedule, to be misleading.
(2) For the purposes of applying subsection (1) in relation to a proceeding concerning a representation made with respect to a future matter by:
(a) a party to the proceeding; or
(b) any other person;
the party or other person is taken not to have had reasonable grounds for making the representation, unless evidence is adduced to the contrary.
(3) To avoid doubt, subsection (2) does not:
(a) have the effect that, merely because such evidence to the contrary is adduced, the person who made the representation is taken to have had reasonable grounds for making the representation; or
(b) have the effect of placing on any person an onus of proving that the person who made the representation had reasonable grounds for making the representation.
(4) Subsection (1) does not limit by implication the meaning of a reference in this Schedule to:
(a) a misleading representation; or
(b) a representation that is misleading in a material particular; or
(c) conduct that is misleading or is likely or liable to mislead;
and, in particular, does not imply that a representation that a person makes with respect to any future matter is not misleading merely because the person has reasonable grounds for making the representation.
…
18 Misleading or deceptive conduct
(1) A person must not, in trade or commerce, engage in conduct that is misleading or deceptive or is likely to mislead or deceive.
(2) Nothing in Part 3-1 (which is about unfair practices) limits by implication subsection (1).
…
29 False or misleading representations about goods or services
(1) A person must not, in trade or commerce, in connection with the supply or possible supply of goods or services or in connection with the promotion by any means of the supply or use of goods or services:
(a) make a false or misleading representation that goods are of a particular standard, quality, value, grade, composition, style or model or have had a particular history or particular previous use; or
…
(g) make a false or misleading representation that goods or services have sponsorship, approval, performance characteristics, accessories, uses or benefits; or
(h) make a false or misleading representation that the person making the representation has a sponsorship, approval or affiliation; or
Framework principles: ACL claims
416 It is useful to identify some framework principles that govern the exercise faced by the court in determining whether conduct is misleading or deceptive within the meaning of s 18 of the ACL or false and misleading within the meaning of s 29. Save for an issue to which I will return concerning the role of the “dominant message” as discussed in Australian Competition and Consumer Commission v TPG Internet Pty Ltd [2013] HCA 54; 250 CLR 640 (TPG HCA), there was no real contest about the principles. The contest was, to a degree, about the facts and, mostly, about the application of the principles to the facts.
417 Section 18 of the ACL prohibits conduct that is misleading or deceptive or likely to mislead or deceive, whereas s 29 prohibits the making of a false or misleading representation. Conduct that contravenes s 18 may involve, but need not involve, the making of a false or misleading representation: Australian Competition and Consumer Commission v TPG Internet Pty Ltd [2020] FCAFC 130; 381 ALR 507 (TPG FCAFC) at [20] per Wigney, O’Byran and Jackson JJ; Campbell v Backoffice Investments Pty Ltd [2009] HCA 25; 238 CLR 304 at [102] per Gummow, Hayne, Heydon and Kiefel JJ.
418 Although s 18 takes a different form to s 29, the prohibitions are similar in nature. Section 18 uses the phrase “misleading or deceptive” whereas s 29 uses the phrase “false or misleading”, but it has been held that there is no material difference between the two expressions: Australian Competition and Consumer Commission v Coles Supermarkets Australia Pty Ltd [2014] FCA 634; 317 ALR 73 at [40] per Allsop CJ; TPG FCAFC at [21].
419 For the inquiry under s 18, it is necessary to identify the impugned conduct and then to consider whether that conduct, considered as a whole and in context, is misleading or deceptive or likely to mislead or deceive: Coles at [38], citing Google Inc v Australian Competition and Consumer Commission [2013] HCA 1; 249 CLR 435 at [89], [102] and [118] and Campomar Sociedad Limitada v Nike International [2000] HCA 12; 202 CLR 45 at [100]–[101]. In the present case, the impugned conduct is readily identified. The inquiry then shifts to whether that conduct, considered as a whole and in context, is misleading or deceptive or likely to mislead or deceive.
420 Conduct is misleading or deceptive or likely to mislead or deceive if it has the tendency to lead into error, i.e., that there is a sufficient causal link between the conduct and the likely error on the part of persons exposed to the conduct: Coles at [39], citing TPG HCA at [39]. Conduct causing confusion and wonderment is not necessarily coextensive with misleading or deceptive conduct: Google at [8]; Campomar at [102]. Conduct is likely to mislead or deceive if there is a real and not remote chance or possibility that a person is likely to be misled or deceived: Global Sportsman Pty Ltd v Mirror Newspapers Pty Ltd [1984] FCA 167; 2 FCR 82 at 87 per Bowen CJ, Lockhart and Fitzgerald JJ; Butcher v Lachlan Elder Realty Pty Ltd [2004] HCA 60; 218 CLR 592 at [112] per McHugh J; TPG FCAFC at [22(a)].
421 Although Allergan submits that the relevant test is that a not insignificant proportion of persons within the relevant class would be likely to be misled, it is doubtful that that is correct: TPG FCAFC at [23].
422 It is not necessary to prove an intention to mislead or deceive: Yorke v Lucas [1985] HCA 65; 158 CLR 661 at 666 per Mason ACJ, Wilson, Deane and Dawson JJ; Google at [9]. However, where there is an intention to deceive, the court may more readily infer that the intention has been or in all probability will be effective: Campomar at [33].
423 It is necessary to view the conduct as a whole and in its proper context. This will or may include consideration of the type of market, the manner in which the goods are sold, and the habits and characteristics of purchasers in such a market: Coles at [41] citing TPG HCA at [52]; Parkdale Custom Built Furniture Pty Ltd v Puxu Pty Ltd [1982] HCA 44; 149 CLR 191 at 199 per Gibbs CJ. The context will also include relevant disclaimers or explanations: Coles at [41], citing Butcher at [49]. In the latter case, the disclaimer was in small print but in a short document and was considered to be “there to be read”.
424 Where the impugned conduct is directed to the public generally (or some relevant section of the public), the court must consider the likely characteristics of the persons who comprise the relevant class of persons to whom the conduct is directed and consider the likely effect of the conduct on ordinary or reasonable members of the class, disregarding reactions that might be regarded as extreme or fanciful: Campomar at [101]-[105]; Kraft Foods Group Brands LLC v Bega Cheese Ltd [2020] FCAFC 65; 377 ALR 387 at [236] per Foster, Moshinsky and O’Bryan JJ. In assessing the effect of conduct on a class of persons such as consumers who may range from the gullible to the astute, the court must consider whether the “ordinary” or “reasonable” members of that class would be misled or deceived: Coles at [43], citing Campomar at [105]. See also Google at [7]; Kraft at [236].
425 The presence or absence of evidence that someone was actually misled or deceived is relevant to an evaluation of all the circumstances relating to the impugned conduct: Coles at [45]. However, it is unnecessary to prove that the conduct in question actually deceived or misled anyone: Google at [6].
426 Returning now to the question of the “dominant message”, with reference to TPG HCA (at [49]-[52]) Allergan submits that when a court is concerned to ascertain the mental impression created by a number of representations conveyed by one communication, it is wrong to attempt to analyse the separate effect of each representation, however it may attribute significance to the “dominant message”.
427 Self Care submits that Allergan’s submission that significance should be attributed to the “dominant message” to consumers should not be accepted for two reasons. First, Self Care submits that TPG HCA did not state a principle of consumer law that marketing or advertising claims must be analysed by a search for the “dominant message” in each case. The initial question is whether the relevant communication to consumers, viewed as a whole, has a tendency to lead a consumer into error (with reference to TPG HCA at [49]). Because of the way that the trader packages or advertises, it may be appropriate to attribute significance to the “dominant message”, but that is a consequence of the facts of the case, not as a principle of law.
428 Second, Self Care submits that on the facts of the case the contents of the packaging of and advertisements for Self Care’s products have little in common with the dominant message of the transient advertisements in TPG HCA. Those are factual matters to which I will return.
429 Insofar as the matter of principle is concerned, TPG HCA concerned a single multi-media advertising campaign with advertisements that had a particular message prominently displayed and then a qualifying message much less prominently displayed: TPG HCA at [1]-[2]. The complaint was that the advertisements were misleading and deceptive by reason of the disparity between the prominent headline message that contained an offer and the less prominent terms qualifying that offer: at [3]. The primary judge found that there was a “dominant message” which would leave a false impression with consumers because the qualifying message was not given sufficient prominence to counter the effect of the dominant message: at [20]-[21].
430 The Full Court of the Federal Court differed from the primary judge in relation to his Honour’s view that the “dominant message” of the advertisements was of critical importance in determining whether they were to be characterised as misleading. The Full Court reasoned that that approach was in conflict with comments in Puxu at 199 that where the conduct complained of consists of words it would not be right to select some words only and to ignore others and that the provision in question was not imposed for the benefit of persons who failed to take reasonable care of their own interests: at [35] and [38].
431 The High Court held that the Full Court erred in holding that the primary judge was wrong to regard the “dominant message” of the advertisements as of crucial importance and that the statements in Puxu which the Full Court applied were not decisive in the circumstances of the case: at [45]. The High Court emphasised the context of the comments in Puxu, namely the sale of items of furniture costing substantial sums of money identical in appearance to those sold by the complainant where potential purchasers were able to inspect the furniture on display and would, acting reasonably, pay attention to the label, brand or mark of the suite that they were minded to buy and, as a result, would not be misled by similarities in the getup of the rival products: at [46].
432 The facts in TPG HCA were said by the High Court to be in stark contrast to those in Puxu in a number of respects including the very different nature of the target audiences and the circumstances of the messaging. In Puxu, the target audience consisted of potential purchasers focused on the subject matter of their purchase in the calm of the showroom to which they had come with a substantial purchase in mind, whereas in TPG HCA the advertisements were an unbidden intrusion on the consciousness of the target audience; the intrusion would not always be welcome, and the very function of the advertisements was to arrest the attention of the target audience: at [47]. The audience of TPG’s advertisements cannot have been expected to pay close attention to the advertisements and might only absorb the “general thrust”: at [47].
433 The upshot is that there is no requirement that the “dominant message” or “general thrust” must be identified. The decision of the High Court was that in the particular context of the case before his Honour, the primary judge did not err in identifying that the advertising campaign in that case had a dominant message which would serve to mislead consumers. In each case, there are factual findings to be made from which the conclusion as to consumers being deceived or misled, or not, can be drawn. In some cases, particularly, but not only, in advertising cases which by their nature involve fleeting regard to uninvited intrusions into the consciousness of the consumer, identification of the “dominant message” will be a valuable analytical tool. In other cases, for example such as where the representation is contained in packaging which is found to be likely to be read and understood as a whole, the “dominant message” analytical tool will be inapposite.
434 In short, whether the “dominant message” analysis is apt will depend on the facts. See Australian Competition and Consumer Commission v Jetstar Airways Pty Ltd [2015] FCA 1263 at [33] per Foster J; Telstra Corporation Ltd v Singtel Optus Pty Ltd [2018] VSCA 347 at [45] per Ferguson CJ, Whelan and McLeish JJA; Australian Competition and Consumer Commission v Birubi Art Pty Ltd [2018] FCA 1595 at [117] per Perry J; Unilever Australia Ltd v Beiersdorf Australia Ltd [2018] FCA 2076 at [27] per Wigney J; TPG FCAFC at [40].
With regard to efficacy in particular
435 It will be seen that in its case based on the efficacy representations, Allergan relies on the representations being representations as to future matters as dealt with by s 4 of the ACL, and it relies on certain principles with regard to comparative advertising and comparative statements. It is therefore necessary to identify the relevant framework principles on these matters.
436 There was no disagreement between the parties as to the application of s 4 of the ACL. Section 4(1) provides that a representation with respect to a future matter is taken to be misleading unless the person who made the representation had reasonable grounds for doing so. It was accepted that by s 4(2), Self Care is taken not to have had reasonable grounds for future representations unless it adduces evidence to the contrary. However, by s 4(3) Allergan retains the overall onus and must show that the evidence put forward by Self Care does not provide a reasonable basis for the representations. See Australian Competition and Consumer Commission v ACM Group Ltd (No 2) [2018] FCA 1115 at [173] per Griffiths J; Australian Competition and Consumer Commission v Woolworths Ltd [2019] FCA 1039 at [95], [96] and [113] per Mortimer J; AFT Pharmaceuticals (Au) Pty Ltd v Reckitt Benckiser (Australia) Pty Ltd [2020] FCAFC 45; 143 ACSR 522 at [8] per Murphy, Wigney and Burley JJ.
437 As will be seen, it is accepted by Self Care that the efficacy representations contended for by Allergan, if established, are representations as to “future matters”. That acceptance is in accordance with the authorities inasmuch as the representations say that the products presently possess characteristics and benefits which will be displayed in the future after the relevant purchase is made and the product is applied. See: Australian Competition and Consumer Commission v Giraffe World Australia Pty Ltd (No 2) [1999] FCA 1161; 95 FCR 302 at [124] per Lindgren J; Australian Competition and Consumer Commission v Purple Harmony Plates Pty Ltd [2001] FCA 1062 at [18] per Goldberg J.
438 Self Care is required to show facts or circumstances, existing at the time of the representations, on which it relied and which are objectively reasonable and support the representations made: Doppstadt Australia Pty Ltd v Lovick & Son Developments Pty Ltd [2014] NSWCA 158 at [189] per Gleeson JA (Ward and Emmett JJA agreeing), applying Sykes v Reserve Bank of Australia [1998] FCA 1405; 88 FCR 511 at 513 per Heerey J.
439 It is insufficient that the person making the representation had a genuinely held view that the representation was not misleading or deceptive: Cummings v Lewis [1993] FCA 190; 41 FCR 559 at 565 per Sheppard and Neaves JJ.
440 Where claims are made of a scientific nature, proof that there is no scientific foundation for those claims may sufficiently establish that the claims are misleading: Reckitt Benckiser (Australia) Pty Ltd v GlaxoSmithKline Australia Pty Ltd [2018] FCAFC 138 at [41] per McKerracher, Yates and Gleeson JJ; AFT Pharmaceuticals at [11]-[13].
441 One of the issues that arises is in what circumstances comparative advertising is misleading or deceptive. In that regard, the characterisation of advertising as comparative does not of itself have legal significance, or create any kind of presumption in favour of a party alleging misleading and deceptive conduct. There is no basis in the ACL for regarding comparative advertising as an inherently disreputable form of commercial conduct, to be viewed with suspicion by the courts. On the contrary, to the extent that comparative advertising provides consumers with accurate facts about competing products, it assists in the making of better informed consumer choices and thereby results in more effective competition. See Telstra Corporation Ltd v Optus Communications Pty Ltd [1996] FCA 1035; 36 IPR 515 at 524 per Merkel J, citing Trade Practices Commission v Telstra Corp Ltd [1993] FCA 567; ATPR 41-256 at 41,454 per Hill J; Gillette Australia Pty Ltd v Energiser Australia Pty Ltd [2002] FCAFC 223; 193 ALR 629 at [20] per Heerey J, [43] per Lindgren J, and [91] per Merkel J.
442 In Gillette, the Court rejected the submission that if the two products that are the subject of comparative advertising are not alike, then, unless the differentiating elements are sufficiently identified, the comparison will be unfair because it will mislead. Provided the factual assertions are not untrue, or misleading half-truths, an advertiser can lawfully compare a particular aspect of its product or service favourably with the same aspect of a competitor’s product or service. See Gillette at [21]-[22] per Heerey J, [43] per Lindgren J, and [93] per Merkel J.
443 A comparative, as distinct from a unilateral, promotion of a product necessarily indicates that the advertisement is not mere advertising puff, but involves representations of fact which are either true or false: Gillette at [44] per Lindgren J; Novartis Pharmaceuticals Australia Pty Ltd v Bayer Australia Ltd [2015] FCA 35; 322 ALR 621 at [177] per Robertson J; Reckitt Benckiser (Australia) Pty Ltd v AFT Pharmaceuticals (AU) Pty Ltd [2018] FCA 1552 at [26] per Gleeson J, upheld in AFT Pharmaceuticals but this point not specifically mentioned.
444 Allergan refers to authority to the effect that particular care needs to be taken by an advertiser when it not only seeks to boost its own product but also seeks to compare its competitor’s products unfavourably: Telstra at 524, citing Stuart Alexander & Co (Interstate) Pty Ltd v Blenders Pty Ltd [1981] FCA 169; 37 ALR 161 at 163 per Lockhart J. However, since even on Allergan’s case the impugned statements are not said to give rise to representations that cast Allergan’s products in an unfavourable light, it is not clear that that principle is applicable. As will be seen, Allergan does not complain that Self Care has made false or misleading statements about Botox.
Framework principles: passing off
445 Although there is an overlap between causes of action arising under Pt 2-1 (i.e., ss 18-19) of the ACL and the common law tort of passing off, the causes of action have distinct origins and the purposes and interests that both bodies of law primarily protect are contrasting. Passing off protects a right of property in business or goodwill whereas Pt 2-1 is concerned with consumer protection. Part 2-1 is not restricted by common law principles relating to passing off and provides wider protection than passing off. See Cadbury Schweppes Pty Ltd v Darrell Lea Chocolate Shops Pty Ltd [2007] FCAFC 70; 159 FCR 397 at [98] per Black CJ, Emmett and Middleton JJ.
446 Passing off protects against injury to the goodwill built up by the activities of the plaintiff: Campomar at [108]. Passing off is not a remedy for the invasion of a right of property in a mark, name or get-up which has been improperly used: AMI Australia Holdings Pty Ltd v Bade Medical Institute (Australia) Pty Ltd (No 2) [2009] FCA 1437; 262 ALR 458 at [81] per Flick J.
447 It has been recognised that there are three core concepts to the tort of passing off, namely: (1) reputation, (2) misrepresentation, and (3) damage. However, it has also been recognised that “the law of passing off contains sufficient nooks and crannies to make it difficult to formulate any satisfactory definition in short form”. See ConAgra Inc v McCain Foods (Aust) Pty Ltd [1992] FCA 176; 33 FCR 302 at 356 per Gummow J.
448 It was observed in Reckitt & Colman Products Ltd v Borden Inc [1990] UKHL 12; [1990] 1 WLR 491 (Reckitt & Colman HL) (at 499) by Lord Oliver of Aylmerton that passing off is not a branch of the law in which reference to other cases is of any real assistance except analogically. The law of passing off can be summarised in one short general proposition – “no person may pass off his goods as those of another”. More specifically, it may be expressed in terms of the elements which the plaintiff in such an action has to prove in order to succeed.
449 First, they must establish goodwill or a reputation attached to the goods or services which they supply in the mind of the purchasing public by association with the identifying “get-up” under which their particular goods or services are offered to the public, such that the get-up is recognised by the public as distinctive specifically of the plaintiff’s goods or services: Reckitt & Colman HL at 499.
450 What is required in respect of this element is proof of a substantial number of persons who are aware of the plaintiff’s product and who are thus potential customers. Such persons represent, in a real sense, a commercial advantage available to be turned to account for the plaintiff’s benefit. See Knott Investments Pty Ltd v Winnebago Industries Inc [2013] FCAFC 59; 211 FCR 449 at [14] per Allsop CJ, citing ConAgra at 346 (Lockhart J), 372 (Gummow J) and 377 (French J).
451 Secondly, they must demonstrate a misrepresentation by the defendant to the public (whether or not intentional) leading or likely to lead the public to believe that goods or services offered by them are the goods or services of the plaintiff. Whether the public is aware of the plaintiff’s identity as the manufacturer or supplier of the goods or services is immaterial, as long as they are identified with a particular source which is in fact the plaintiff. See Reckitt & Colman HL at 499.
452 However, the misrepresentation need not be that there is a common manufacturer or supplier of the goods or services. Pertinent to the present case, is that misrepresentations as to various other “connections” between the plaintiff and the defendant (or its goods) in the form of approval or endorsement by the plaintiff have also been held to be actionable: Shanahan’s at [95.1900]; Moorgate Tobacco Co Ltd v Philip Morris Ltd (No 2) [1984] HCA 73; 156 CLR 414 at 445 per Deane J (Gibbs CJ, Mason, Wilson and Dawson JJ agreeing); Campomar at [109]-[110]; Ricegrowers Ltd v Real Foods Pty Ltd [2008] FCA 639; 77 IPR 32 at [57] per Rares J; Sheldon and Hammond Pty Ltd v Metrokane Inc [2004] FCA 19; 135 FCR 34 at [164] per Conti J; Pacific Dunlop Ltd v Hogan [1989] FCA 250; 23 FCR 553 at 582 per Beaumont J and 583-584 per Burchett J.
453 Thirdly, the plaintiff must demonstrate that they suffer damage by reason of the erroneous belief engendered by the defendant’s misrepresentation that the source of the defendant’s goods or services is the same as the source of those offered by the plaintiff (or that the defendant’s good are endorsed by the plaintiff or the goods are otherwise associated): Reckitt & Colman HL at 499.
Introduction
454 The tables at [111]-[116] above identify each statement made by Self Care about which Allergan complains as amounting to an affiliation representation, and in respect of which Self Care product the statement is made.
455 Allergan pleads that the statements that it complains of in relation to all of the products make the following false representations about the product:
(1) the product is, or is related to, the Botox product or a product sold under the BOTOX trade marks;
(2) the product is a topical cream or serum containing the Botox products;
(3) the product has the licence, sponsorship or approval of Allergan;
(4) the product is affiliated with the business of Allergan; and
(5) Self Care has an approval from or connection or association with Allergan.
456 In respect of the Protox product, Allergan also says that the statements that are complained of make the following false representations:
(1) the Protox product has a therapeutic use Allergan has verified as correct;
(2) Allergan has verified as correct that the physiological processes of the Botox products can be influenced, modified or extended by the use of the Protox product; and
(3) Self Care is legally permitted to advertise the Protox product and/or the Botox products, including under the TG Act.
457 Save for stating that it represented that the Protox product can prolong the visual effects of anti-wrinkle injections, including Botox, Self Care denies that the statements that are complained of make the representations identified by Allergan. I do not understand Self Care to dispute that the identified representations are false – the question is whether by the complained of statements the representations were made. Similarly, I do not understand Self Care to dispute that the statements that are complained of were made in trade or commerce in Australia, and hence that the representations, if made, were similarly made in trade or commerce in Australia.
458 Allergan pleads the conduct of Self Care in making the complained of statements, which in turn made the representations which were false, in the course of trade or commerce in Australia, constitutes misleading or deceptive conduct or conduct which is likely to mislead or deceive in contravention of s 18 of the ACL. Alternatively, it pleads that Self Care represented that each product has a sponsorship and/or approval it does not have, in contravention of s 29(1)(g), or that Self Care has a sponsorship, approval and/or affiliation that it does not have, in contravention of s 29(1)(h) of the ACL.
459 Allergan also pleads that the affiliation representations make false representations of affiliation with Allergan or the Botox products to Australian consumers which cause, or will reasonably foreseeably cause, damage to Allergan including by appropriation or diminution of the reputation and goodwill of Allergan in and to the BOTOX brand, the Botox products and the BOTOX marks. By reason of those matters, it is pleaded that Self Care has engaged in the tort of passing off.
460 Allergan also pleads that Self Care was engaged in deliberately and knowingly making or authorising the making of the affiliation representations with the intention of misleading or deceiving consumers into believing that the products are affiliated.
461 I am not satisfied that the ACL and passing off cases based on the affiliation representations have been established.
462 The principal reason for that conclusion is that Self Care, for the most part, makes it clear that its products are not Allergan’s products; they are advertised and presented as an “alternative” to Botox. Whether or not the word BOTOX as used in that context is understood to be Allergan’s product by that name, or understood more generally as a generic reference to botulinum toxin anti-wrinkle injections, does not matter. The point is that Self Care’s products are an alternative to that other product.
463 Moreover, that other product is also known to be very different, i.e., it is an injectable administered only by healthcare professionals and is significantly more expensive.
464 The description “alternative” carries with it the inevitable and clear message that Self Care’s product is not Botox. “Alternative” is a powerful word and it does a lot of work in this context.
465 Where BOTOX is used in such a way that it is clearly a reference to Allergan’s trade mark, such as when it has the registered trade mark ® symbol next to it and when it is stated that BOTOX is the registered trade mark of Allergan Inc, that does not convey sponsorship, approval or association. It is a factual statement that is entirely accurate. Allergan’s case in this respect in effect asks me to conclude that consumers will misunderstand such statements as conveying sponsorship, approval or association even though in fact they do not. There is no evidence that any consumer has in fact had such a misunderstanding. Indeed, Ms De Cruz stated that Allergan Australia has received no consumer complaint about the Protox product. There is simply no evidential basis on which I can reach a conclusion that the simple factual statements, in the context in which they are made which includes on product packaging and in advertisements for Self Care’s products, that BOTOX is the registered trade mark of Allergan Inc are understood by consumers to convey some association, sponsorship or approval.
466 As I have concluded, the reputation of Botox is as an injectable anti-wrinkle treatment available only from healthcare professionals. It is not a reputation which spreads more broadly into topical creams and serums such as Self Care’s products. In those circumstances, the reputation of Botox is not likely to give rise to consumer confusion between it and Self Care’s products.
467 Allergan relies on what it identifies as Ms Amoroso’s intention with regard to the affiliation representations, which intention it submits can be ascribed to Self Care. There is no difficulty with regard to Ms Amoroso’s intention being ascribed to Self Care. The difficulty is in Allergan’s submission that Ms Amoroso had an intention to mislead or deceive, or to pass off Self Care’s products as Allergan’s, or to be associated with Allergan’s products.
468 Ms Amoroso’s intention, as accepted by her, was to allude to and reference injectable anti-wrinkle products, including Allergan’s product. However, the purpose behind that intention was in order to present Self Care’s products as alternatives to injectable anti-wrinkle products. Rather than to suggest any association between the respective products, the intention was to differentiate them. The intention was also, obviously, that Self Care’s products should compete with anti-wrinkle injectables, including the Botox product, but there can be no complaint about that. Products are launched and marketed every day with the intention that they compete with other products, and the mere mentioning of other products even by name does not on its own amount to any misleading or deceptive message or to a false message of affiliation, sponsorship or approval.
469 Allergan’s submission that Self Care intended to mislead or deceive from which I should infer that that intention has been or is likely to be effective, must accordingly fail.
470 Allergan refers to Sydneywide Distributors Pty Ltd v Red Bull Australia Pty Ltd [2002] FCAFC 157; 234 FCR 549 at [117] per Weinberg and Dowsett JJ (Branson J agreeing) where the following was said with regard to intention:
[W]here a trader, having knowledge of a particular market, borrows aspects of a competitor’s get-up, it is a reasonable inference that he or she believes that there will be a market benefit in so doing. Often, the obvious benefit will be the attraction of custom which would otherwise have gone to the competitor. It is an available inference from those propositions that the trader, with knowledge of the market, considered that such borrowing was “fitted for the purpose and therefore likely to deceive or confuse”. Of course, the trader may explain his or conduct in such a way as to undermine the availability of that inference. Obviously, this reasoning will only apply where there are similarities in get-up which suggest borrowing.
471 However, Self Care has not, in my assessment, borrowed any of Allergan’s get-up. Reference to Botox in product packaging and advertisements, and acknowledging that BOTOX is a registered trade mark of Allergan, is not to adopt Allergan’s get-up.
472 The fact that Self Care’s products, being cosmetic creams and lotions, are so different from Allergan’s Botox, being an injectable, and the very different trade channels through which they are available, also serves to detract from any affiliation, sponsorship or approval representation arising from the complained of statements. Indeed, those differences – in types of product and trade channels – serve to accentuate the point that is sought to be made by Self Care, namely that its products are alternatives to Botox; they are not the same as or similar to or related to Botox.
473 In respect of trade channels, Allergan submits that there is a considerable cross-over in trade channels on the basis that clinics that offer cosmetic injections almost always sell skincare products. That much can be accepted, although the evidence is that no such clinics also offer Self Care’s products. Allergan, however, submits that department stores “like Myer (where [Self Care’s] products have been sold) offer a range of cosmetic skin care products, and have from time to time, offered cosmetic injections”. The evidence Allergan relies upon is the following:
• Articles from the websites of The Australian, anz.businesschief.com and news.com.au on 29 June 2011 quoting from a press release from the CEO of Myer saying that Botox treatments would shortly be available from an outside operator at three Myer stores in Australia.
• An article from The Daily Telegraph on 30 June 2011 quoting a Myer spokeswoman as having said the previous day that the idea of making Botox treatment available in some Myer stores was still being investigated but that research had shown “it is something our customers want”.
474 Even leaving aside the inadmissible hearsay nature of the statements reported in the news articles, that evidence falls hopelessly short of establishing that Botox treatments were ever actually offered in Myer stores. And, if even they were, there is no evidence of for how long they were offered or how widespread the offering was. Ms Amoroso’s evidence that there have been no clinics where injectable anti-wrinkle treatments were offered where Self Care’s products were also sold was not challenged.
475 Allergan also refers to the novelty syringe packaging of the Inhibox product which is an obvious visual reference to injectable products such as Botox. The reference, I think, is clear. The syringe-shaped pump-action applicator is, as Ms Amoroso said, “a play on injections”. Although Ms Amoroso said that it “is not a play on Botox”, I do not accept that. The syringe taken together with the “instant Botox alternative” and similar “alternative” messaging, clearly references or invokes anti-wrinkle injections generically, and also possibly Allergan’s particular product. But once again, the point and effect is to distinguish Self Care’s products from the injectable products. There is nothing in Self Care’s packaging to suggest that its products are injectable – the image of the syringe-shaped applicator catches the eye and makes the association with injectables specifically in order to then differentiate and present Self Care’s product as an entirely different type of product that does not require to be injected.
476 Allergan submits that the “dominant message” conveyed by the affiliation representations is that Self Care’s products are affiliated or associated with, or otherwise sponsored by, the maker of the Botox product. I do not accept that submission, even if a “dominant message” analysis was appropriate in the circumstances. In my view, the dominant message is that Self Care’s products are “alternatives” to injectables such as Botox. That message does not convey any representation of association, affiliation or sponsorship.
477 Allergan refers to Peter Bodum A/S v DKSH Australia Pty Ltd [2011] FCAFC 98; 280 ALR 639 at [244]-[245] per Greenwood J (Tracey J agreeing, Buchanan J dissenting) and submits that the presence of the FREEZEFRAME brand is not sufficient to remove the likelihood of confusion where the consumer erroneously draws an affiliation between the two sources. There are at least two reasons why that submission must be rejected. First, in the case of Self Care the FREEZEFRAME branding is clear, obvious and ubiquitous; it is clearly portrayed to consumers through packaging and advertising that all of the products in question are part of a Freezeframe range of products. In contrast, in the Bodum case the branding of the accused’s product, Euro Line, was not prominent or distinctive and was unknown and unsupported by advertising: at [235]-[238].
478 Second, there is no similarity in appearance between Self Care’s products and Allergan’s products. In contrast, in the Bodum case, for all practical purposes the overall appearance of the competing products was the same: at [227]. So, in the present case not only is there clear differentiating branding, but there is also no basis on which the products would appear to be affiliated.
479 I also reject the submission that Self Care’s use of the phrase “Prolong the look of Botox®”, and similar phrases, suggests affiliation by representing that Protox is an accessory for Botox. That representation was not pleaded. Further, it will be the common experience of consumers that one trader’s product can be used to enhance another trader’s product without there being any suggestion of affiliation. The myriad accessories for Apple devices – covers, charges, headphones, etc. – spring to mind as obvious examples.
480 In the circumstances, Allergan’s case based on the affiliation representations, both in reliance on the ACL and on the tort of passing off, must fail.
Introduction
481 The tables at [111]-[116] above identify each statement that Allergan refers to as giving rise to the efficacy representations made by Self Care in relation to the latter’s different products.
482 In relation to each of the statements, Allergan pleads that they make the following representations, in trade and commerce, to Australian consumers:
(1) use of the product (as a cream) will give results to the same standard or quality as the Botox products (as an injection); and
(2) use of the product will achieve the same performance characteristics, uses and/or benefits as the Botox product.
483 It will be observed that the identified representations that are said to flow from the statements that are complained of, in the context in which the statements are made, essentially track the wording of s 29(1)(a) of the ACL, i.e., a false or misleading representation that goods are of a particular standard, quality, value, grade, composition, style or model or have had a particular history or particular previous use.
484 In addition, in relation to the Protox product statements Allergan pleads that they also make the following representation:
use of the Protox product will work complementarily or synergistically with, or can be used as part of a treatment with, the Botox products to enhance and/or prolong the benefits of the Botox products.
485 It will be observed that in this case the representation is said to be caught by s 29(1)(g) of the ACL, i.e., a false or misleading representation that goods have sponsorship, approval, performance characteristics, accessories, uses or benefits.
486 Allergan pleads that each of the efficacy representations constitutes a representation as to a future matter for the purposes of s 4 of the ACL and that Self Care did not have reasonable grounds for making the representations. In the alternative, to the extent that any of the Protox product efficacy representations do not constitute representations as to a future matter, Allergan pleads that the representations are false.
487 In its defence, Self Care denies that the efficacy representations arise from the statements, admits that the efficacy representations if made constitute representations as to future matters for the purposes of s 4 of the ACL and that they were made in the course of trade and commerce, and otherwise denies the allegations. The defence also avers that Self Care ceased selling or offering for sale the Protox product on or around 4 March 2019.
488 It is to be noted that Allergan initially complained of a number of other representations made by Self Care about its products. These included quantitative representations such as that the Inhibox product is “clinically proven to smooth away over 80% of wrinkles in 5 minutes and to reduce the appearance of wrinkles long term by up to 63.13% in just 28 days”. However, following undertakings made by Self Care, those complaints fell away leaving only the statements which Allergan says directly compare Self Care’s products with the Botox product. Allergan submits that since there is no scientific study that compares the efficacy of the products against each other, there is no reasonable basis for the comparative representations. In that regard, there are authorities dealing with comparative representations where there is no study that compares the products directly against each other head-to-head. The absence of such studies does not mean that there cannot be a reasonable basis for the representations, but it can make it more difficult to establish evidence of an adequate scientific foundation for the representations having regard to the available body of scientific evidence. See AFT Pharmaceuticals at [11], [12], [16], [104], [105] and [110].
489 Much of Self Care’s evidence with regard to the efficacy representations, and the expert evidence on both sides of the case, was directed at the quantitative statements which then, as I have said, fell away. As a consequence, the evidence does not conveniently address the comparative representations that are pleaded other than to identify that there is no head-to-head comparative study.
490 It is also to be noted that Allergan does not assert that Self Care’s products are not effective to reduce the appearance of wrinkles.
491 Allergan puts its case on the basis that the efficacy statements complained of make a wrong comparison with Botox – that is, they describe the attributes of Self Care’s products by reference to Allergan’s Botox product. Put differently, Allergan says that the statements represent that Self Care’s products will “to the same or a similar extent” reduce the appearance of wrinkles as Botox will. That is, use of Self Care’s products will achieve a similar look as a person who has had Botox or such use will achieve the same performance characteristics, uses and/or benefits as the Botox products.
492 Specifically with regard to the Protox product, Allergan says that some of the complained of statements constitute representations that the product will work complementarily or synergistically with or can be used as part of a treatment with the Botox products. This arises from the statement that Protox will “prolong” or “increase” the effect of Botox. The statements in this category are those numbered 2-4, 7-11 and 13-19 in the table at [111] above. The remaining statements which are identified as giving rise to the efficacy representations are, on Allergan’s case, of a comparative nature.
493 The starting point is to identify whether the pleaded representations were made. That exercise includes identifying the statements that are complained of which are said to give rise to the pleaded representations and to construe them in the whole of their context and thereby identify whether, in that context, they make the pleaded representations. This includes consideration of the target consumer or class of consumers and the likely effect on the ordinary or reasonable members of the relevant class of consumer, i.e., disregarding extreme or fanciful reactions: Campomar at [105].
494 The first category of statements are those that are said to give rise to comparative efficacy representations that include the word “alternative”, such as:
“The most powerful Botox® alternative ever discovered” (Protox product statement 5)
“Instant Botox® alternative” (Inhibox product statement 1)
“Overnight Botox® alternative” and “Long term Botox® Alternative” (Night products statement 5)
“Botox® alternative peptides also add to the antiaging cocktail for dramatic, targeted results on even the deepest wrinkles” (Boost product statement 2).
495 Also in this category are Protox statement 6, Inhibox statements 2-7, Night statements 2 and 5, and Boost statements 1 and 3-4.
496 It is to be recalled that I have concluded above that the Protox product statements 7-10 and 19 were not made by Self Care. They therefore fall out of account. The other statements that are complained of were all made by Self Care. That is not in dispute.
497 Importantly, the context in which the statements were made includes the following material elements:
(1) The reputation of Botox is as an injectable anti-wrinkle treatment that can only be administered by healthcare professionals.
(2) The Self Care products are all topical creams, serums and lotions that are typically self-applied.
(3) The cost of a single Botox treatment is substantially more than the cost of an item of any of the Self Care products, and such an item will offer daily treatments for, perhaps, several weeks or even longer depending on individual application habits and techniques.
(4) There is nowhere where the Self Care products and Botox treatments are both offered.
498 Insofar as identifying the class of consumers for Self Care’s products and hence the people who are likely to read the impugned statements is concerned, a few points are worth noting. First, it is people who are interested in treatments for the reduction of the appearance of wrinkles who are liable to have their attention drawn to Self Care’s impugned statements. They would likely be aware of Botox, as the survey evidence shows, and they may be people who have used Botox, or who might consider using Botox, or who would not be interested in using Botox. Secondly, they are for the most part likely to be people, men or women, who know something about anti-ageing and/or anti-wrinkle treatments. That is because if they have an interest in the subject matter, which is the reason why their attention might be drawn to Self Care’s statements about its products, they are likely to have previously looked at materials or to have engaged in conversations on the subject matter.
499 The result is that the ordinary and reasonable consumer on reading the impugned statements in their context is likely to know that Botox is an injectable anti-wrinkle treatment that is available to be administered only by healthcare professionals, that in contrast Self Care’s products are topically self-applied creams, serums and lotions, and Botox is likely to be more expensive than Self Care’s products because it is required to be professionally administered. Also, although probably not being conscious of the fact, such consumers will not have seen or experienced Botox and Self Care’s products being available in the same place.
500 In that context, in my assessment the impugned statements that describe the relevant product as an “alternative” to Botox are likely to be understood by ordinary and reasonable members of the relevant class of consumer as representing that the Self Care product will reduce the appearance of wrinkles to a similar extent as Botox does. The statements convey, in context, that the product will be effective in reducing the appearance of wrinkles. The statements do not say anything expressly about the extent of that effectiveness, particularly with regard to how long any such reduction in the appearance of wrinkles will last. The statements also say nothing about the mechanism of the effect on the reduction in the appearance of wrinkles.
501 The statements that the product is an “alternative” to Botox are likely to be understood as distinguishing the product as not having the qualities of Botox that the consumer might regard to be disadvantageous, such as it being an injectable that can only be administered by healthcare professionals and that it is relatively expensive. They are also likely to be understood as saying that the product will achieve a similar outcome. For the product to be an “alternative” it must, at least, achieve something similar. If it does not have a similar result, then it is not an alternative.
502 I am not persuaded that the statements convey that the effect of the product in question will be the same as the effect of Botox. That is not ordinarily how “alternative” would be understood, and given the ordinary and reasonable consumer’s knowledge of the significant differences between the products, the statements would not be understood as saying that the products are the same or that they have the same effect. The statements also do not imply that the products have the same mechanism or mode of action.
503 The ordinary and reasonable consumer will appreciate that there are many variables to take into account in choosing one product over another. Relevantly, these will include the trouble, pain and expense of purchase and administration or application, how long the effects of the product last, and how significant the effects are. Thus, to say that one is an alternative to the other will not, in my assessment, be understood to say that the one is the same as the other, but neither is it to say that there is no similarity between the products. For the products to be alternatives they must have some similarity with reference to their intended purpose. That is a reduction in the appearance of wrinkles.
504 Simply, the statements carry with them the implication, and hence the representation, that Self Care’s products to which they relate achieve a similar aesthetic outcome with regard to the reduction in the appearance of wrinkles to the outcome that Botox achieves.
505 The second category of statements is comparative statements that expressly make representations as to the effect of the product in question and Botox being the same or similar. These include:
“… which could produce a Botox-like visual effect when applied topically to the skin” (Protox statement 12)
“Delivers the results of a collagen injection in 5 sleeps, and a Botox injection in 4 weeks” (Night statement 3).
506 The promise of a “Botox-like visual effect” might be understood to be a more specific comparative promise than to say that the product is an alternative to Botox. Clearly the promise operates only at the level of visual effect and does not say anything about mechanism of action or longevity, but it comes close to saying that that visual effect will be the same. Still, on balance, given that the word “like” conveys the same meaning as similar and, taken in context, it will be understood that the products are very different, I consider that the representation that is made is that the product will produce a similar visual effect on the appearance in the reduction of wrinkles.
507 The statement that the product “delivers the results of … a Botox injection in 4 weeks”, however, is more exact. It is exact with regard to result, i.e., “the result” is the same result not a similar result, and with regard to time, i.e., it will be achieved in four weeks. That is a representation of exact comparison. However, it is with regard to “results”, not mechanism or mode of action. The results are the results in the reduction in the appearance of wrinkles. Those are the results that are represented to be the same.
508 The third category of statements is of those which make a representation that the product in question works with Botox in some way, such as to “prolong” its effects, to make Botox “look better for longer” or to make the visual effect of Botox “look even more dramatic”. These statements are the Protox statements 2-4, 11 and 13-18. These statements also unavoidably make representations as to the efficacy of the product in question, being that it is effective to “prolong the look of Botox” or to improve the look of Botox.
509 Thus, all the statements that are said to give rise to efficacy representations make representations with regard to the product in question having particular “performance characteristics, … uses or benefits” within the meaning of s 29(1)(g) of the ACL.
Formulations, active ingredients and studies
510 In respect of each of the products in respect of which the efficacy representations were made, Ms Amoroso in her evidence referred to the active ingredients in the products and a number of scientific studies which, she said, she relied on in making claims that the products are effective in reducing the appearance of wrinkles or in prolonging the effect of Botox. Ms Amoroso’s evidence that she relied on the studies was not challenged. I accept it. The central issue for consideration is whether the studies adequately substantiate the claimed effects of Self Care’s products.
511 The formulations for the products, which were tendered and accepted in evidence, are understandably a closely guarded trade secret. For that reason the parties agreed to give the active ingredients letter references. There are ten active ingredients. Thus, reference was made to Active A, Active B, etc., through to Active J.
512 Since the scientific studies naturally record the active ingredients that are their subject, identification of the studies would reveal the active ingredients. There are 23 such studies. They were numbered as Study 1, Study 2, etc., through to Study 24, save that there is no Study 16 or 18 and there are two Study 23s. I do not understand why the numbering was done in that inconsistent manner, but it has no effect on what follows.
513 Allergan relies on the expert evidence of Dr Graeme Haley. Dr Haley holds a Bachelor of Science (Honours) (majoring in polymer science) and a Doctor of Philosophy from the University of New South Wales, conferred in 1968 and 1972 respectively.
514 Dr Haley is an industrial chemist with 37 years’ industrial experience in formulating and manufacturing a wide variety of cosmetic products in Australia and overseas. For the last 15 years he has been a director of a consultancy that provides advice to industry in relation to, inter alia, the manufacturing, marketing and labelling of cosmetic products, making applications for a number of cosmetic and industrial chemicals companies for National Industrial Chemicals Notification and Assessment Scheme notifications and for TGA listings, and advising industry in relation to cosmetic and therapeutic claims and the preparation of safety data sheets.
515 Dr Haley has not had any laboratory experience in formulating skincare products during the last 15 years as it has not been part of his role to make formulating recommendations. He accepted that in some cases his reliance on definitions contained in the INCI Dictionary (International Nomenclature of Cosmetic Ingredients) was possibly due to a lack of direct knowledge or experience of an ingredient’s purpose or effect in cosmetic formulations.
516 Self Care relies on the expert evidence of Mr Richard Williams. Mr Williams holds a Bachelor of Science majoring in pure and applied chemistry at the University of New South Wales in 1980.
517 Since 2012, Mr Williams has been the Business Director and Principal Scientist of a consultancy business that assists and advises its clients to develop various products in the cosmetic, cosmeceutical, therapeutic, nutritional and veterinarian industries. Its business includes making small batches of products for clients so that they can conduct product evaluation studies and clinical studies. Mr Williams is principally engaged in product development from concept to market. This involves taking a market concept (e.g., a cream that will achieve a particular outcome), determining the active ingredients that would support a marketing claim, building a product around the active ingredients and then developing the manufacturing process for that product.
518 In Mr Williams’s prior roles over the preceding 40 years, he was responsible for creating new product formulations and improving existing products. As a result he is familiar with the chemical components of, and ingredients used within, cosmetic products, including within skincare products. He was acknowledged by Dr Haley as being intimately involved in the laboratory experience of formulating skincare products and has “a very expert knowledge of it”.
519 The expert witnesses produced reports by way of affidavits that were read and they produced a joint report. They were also examined, or cross-examined, concurrently following an agenda of issues pre-agreed between counsel.
520 Allergan also relies in this part of the case, to a limited extent, on the evidence of Ms Seona Leanne Cain as an expert. Ms Cain is the Regional Manager, Clinical Specialist employed by Allergan Australia in Sydney. Ms Cain holds a Bachelor of Science (Honours) degree majoring in anatomy from the University of Glasgow in 2000. Her evidence was principally about Botox about which there was little if any controversy.
521 There is only one respect in which Ms Cain’s evidence is relied on by Allergan in relation to Self Care’s products, which is her opinion that it is not possible to make a comparison between a topically applied skincare product and Botox, which is injected into the muscle, given the different modes of action and the number of variables. That obviously depends on the nature of the comparison. This issue is dealt with further below.
522 Where I refer to “the expert witnesses” below, I intend to refer to Dr Haley and Mr Williams and not to Ms Cain. That is not out of any disrespect to her, but only as a shorthand reference to the two witnesses who gave evidence on the same subject matter, produced a joint report and gave evidence concurrently.
The structure of skin and wrinkles
523 There was no disagreement between the experts on the relevant structure of human skin. It has two main layers, the epidermis and dermis. The epidermis is the superficial protective layer at the surface of skin. It varies in thickness in different areas of the body from 0.007 mm to 0.12 mm and is composed of stratified squamous epithelium. The stratified squamous epithelium consists of squamous (flattened) epithelial cells arranged in layers. The epidermis consists of the following five layers, from outermost layer (closest to the surface of the skin) to innermost layer (furthest from the surface of the skin): stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum and stratum basale.
524 The dermis is a thick layer of rough, viscoelastic tissue below the epidermis that contains blood vessels, nerve endings, hair follicles and sweat glands.
525 The skin has a number of protective purposes including keeping out water, UV light, chemicals, drugs, environmental insults and infections. It also keeps water in. The skin also offers mechanical support, neurosensory reception, pain and touch, temperature sensations and endocrinology, and it plays a role in aesthetic appeal and beauty.
526 Ms Cain explained that there are two types of wrinkles which can form in skin, static and dynamic. Dynamic wrinkles are caused by the action of the muscle and all ages can form a dynamic wrinkle, even babies. Static wrinkles are caused by the repetitive action of the muscle on the skin which creates a line or wrinkle that can be seen at rest. As is well known, static wrinkles are more commonly seen in mature adults.
527 Botox impacts the dynamic lines and wrinkles as it stops the movement of the muscle. Static lines can sometimes be improved but it is not guaranteed. Variances in static lines after Botox treatment depends on the skin quality and how deep the line was to begin with. A layperson may also experience that their skin looks smoother and shinier after Botox treatments.
528 The point is that both as a matter of objective fact and as a powerful element of Botox’s reputation, as dealt with in detail above, Botox cosmetic treatments reduce the appearance of wrinkles in the skin. This is notwithstanding that Botox operates, therapeutically, at the level of the underlying muscle. The ordinary and reasonable consumer will understand Botox to effect an improvement in the appearance of wrinkles in the treated areas. However, the improvement is not generally quantified and the ordinary and reasonable consumer will not have a readily available reference point or metric for the quality or extent of the improvement.
Self Care’s identified support for the claims
529 Self Care identified the following ingredients and related studies, and in some cases other evidence as well, as supporting the relevant claims.
530 In support of the proposition that the Protox product reduces the appearance of wrinkles:
(1) Study 8 (in respect of Active F) is an in vivo study showing that Active F, a peptide in solution, has a long-term effect on the reduction in the appearance of wrinkles. Active F is contained in Active E. The active peptide was reported to decrease wrinkle depth by an average of 16.9% and 27.0% after 15 and 30 days respectively, reduce the average roughness (Ra) of skin in volunteers by 20.4% after 28 days, and to ameliorate wrinkle appearance by decreasing wrinkle volume by 20.6% and wrinkle length by 15.9% of after seven days. (Ra = the average of all heights and depths to the reference plane.)
(2) Studies 9 and 10 (in respect of Active G) are in vivo studies showing that Active G has a long-term effect on the reduction in the appearance of wrinkles, and that there is an additive wrinkle reduction effect when Active G is used in combination with Active F. Active G, as with Active F, is contained in Active E.
(3) Studies 6 and 7 (in respect of Active E) are in vivo studies showing that Active E reduces the appearance of wrinkles in the long-term. As indicated, Active E contains Actives F and G. These studies are the subject of detailed discussion at [573]-[576] below.
531 In support of the proposition that after a Botox injection the Protox product prolongs the reduction of the appearance of wrinkles:
Studies 6 and 7 (in respect of Active E) report on an in vivo study showing that Active E prolongs the skin smoothing effect of botulinum toxin type A injections in the periorbital region and frontal region after six months. As indicated, these studies are the subject of further discussion below.
532 In support of the proposition that the Inhibox product reduces the appearance of wrinkles:
(1) Study 5, a short term study using the Inhibox product itself, concluded that the product reduces the appearance of wrinkles in five minutes (i.e., an instant effect). Further measurements after eight hours confirmed that the effect is long lasting. This study is discussed in more detail in the next paragraph.
(2) A video of raw footage of Ms Amoroso applying the product to a woman’s face shows the efficacy of the product. Ms Amoroso’s evidence was that the video was created in about 2014 for educational and advertising purposes. It can be seen that as she applies the product on the woman’s frown lines and crow’s feet and around the eyes a transparent film is formed on the skin which tightens during the drying process and pulls the skin taut. It appears to have the visual effect of smoothing out wrinkles and lifting the area.
(3) In respect of a particular ingredient (identified as item 4 in the formulation), Mr Williams said that it creates the tightening effect on the surface of the skin seen in Study 5. The experts agree that it has an anti-wrinkle effect on skin surfaces – Dr Hayley categorises it as a wrinkle filler and Mr Williams says that it forms a “smooth wrinkle filling form when dry”.
(4) Studies 3 and 4 (in respect of Active H) show an instant tightening effect that reduces the appearance of wrinkles within 10 minutes and lasts for 3-4 hours. The experts agreed that the ingredient in Active H reduces wrinkles by forming a film and tightening the skin when dry.
(5) Studies 1 and 2 (in respect of Active A), are in vivo studies which show that Active A reduces the appearance of wrinkles in 28 days. These studies are discussed in more detail at [549]-[568] below.
533 Study 5 was conducted on the Inhibox product itself in April 2010 by an independent clinical research and bioengineering institute in Italy. The study was conducted with 25 healthy female volunteers between the ages of 48 and 60, with a mean age of 57. It was a controlled, short term study of treated versus untreated side (half face method), i.e., the volunteers applied the test product once only on either the right or the left side of the face according to a predetermined randomisation list at the level of the forehead and the area around the eyes. The study was performed over one day. After a pre-treatment evaluation, there were five post treatment evaluation times, namely at each of 5 minutes, 30 minutes, 2 hours, 5 hours and 8 hours after product application. On each occasion there was a clinical and an instrumental evaluation performed bilaterally at the level of the forehead and the area around the eyes. The evaluations showed the excellent “lifting” activity of the product. A single application indicated a quick reduction of deep and fine wrinkles and a minimisation of under-eye circle and puffiness. The lifted look of the face skin was noticeable until five hours after product application. The experts agreed that the study is reliable.
534 In support of the proposition that the Night (tube) reduces the appearance of wrinkles:
(1) Study 11 (in respect of Active B) is an in vivo study that found that Active B provides a long-term skin smoothing and moisturising effect and increases skin suppleness. Twenty-six female subjects applied a cream containing Active B and a placebo cream twice daily for two months. The study concluded that use of the test product enabled a marked and significant improvement, relative to the placebo, across five parameters. The improvement, already of interest after one month, increased significantly after two months.
(2) Studies 1 and 2 (in respect of Active A) are in vivo studies showing that Active A reduces the appearance of wrinkles in 28 days. As indicated, these are discussed in further detail below.
(3) Study 12 (in respect of Active C) is an in vivo study that found that Active C rejuvenates the skin matrix resulting in the skin appearing smooth. Mr Williams expressed the opinion that Active C has a “tightening” effect on the skin’s surface caused by three identified ingredients in Active C working together. Dr Haley accepted that the improvement shown in the clinical study can be attributed to Active C.
(4) Study 13 (in respect of Active D) includes an in vivo study of Active D which ran for 60 days using 20 volunteers. There were multiple evaluative tests performed using different methodologies at different days of the study using the same volunteers. One of those tests showed a reduction in wrinkle depth and volume in 15 days. The study also showed in tests that Active D increases collagen density, anisotropy index, and skin firmness and elasticity in 15 days.
(5) A further ingredient in Night (tube), which is identified in the tendered formulation as item 26, was identified by the experts as an active ingredient that is a “skin moisturiser”. Mr Williams also said that it provides a reservoir of key ingredients so it assists with efficacy of the actives identified above.
535 In support of the proposition that the Night (tub) reduces the appearance of wrinkles:
(1) Studies 1 and 2 (in respect of Active A) which, as indicated, show that Active A reduces the appearance of wrinkles in 28 days. The “maximum reduction value found for 10% [Active A] Solution was -63.13%”. These studies are discussed in more detail below.
(2) A further ingredient in Night (tub), which is identified in the tendered formulation as item 23, is the same ingredient as item 26 in the Night (tube) formulation dealt with above.
536 Support for the proposition that the Boost serum reduces the appearance of wrinkles:
(1) Mr William’s evidence was that peptides work with other active ingredients in this product to reduce wrinkles as follows: Active I (peptide) plumps the skin, Active A (peptide) softens and relaxes the skin’s surface, [Active J] (not a peptide) stimulates skin cell growth and allows the skin to expand.
(2) Studies 14 and 15 (in respect of Active I) show that Active I increases tissue volume and clumps skin which results in skin smoothing and reduction in the appearance of wrinkles. Study 14, which Study 15 references, is described in more detail in the following paragraph.
(3) Studies 1 and 2 (in respect of Active A) show that Active A (being a peptide) reduces the appearance of wrinkles and 28 days. As indicated, these studies are discussed in more detail below.
(4) The Boost study (Study 16) shows that the product has a significant effect on skin firmness, wrinkledness, dark circles and eye puffiness after 28 days. This was an in vivo study using the Boost product on 30 women volunteers between 35-55 years who were characterised by under-eye bags, dark circles and clinical signs of skin wrinkledness (crow’s feet area). The experts agreed that the study is reliable.
(5) A particular ingredient was identified by Mr Williams as an active. He explained that this ingredient is a skin moisturiser that forms a film reservoir which sits on the surface of the skin and moisturises the skin. He also explained that this ingredient is “known for being one of the best moisturising ingredients for cosmetic skin care products”. Dr Haley also acknowledged that the ingredient “absorbs water to increase water in the top layer of the skin”.
537 Study 14 reports on two in vivo studies, the first to evaluate the effect of Active I on facial volume and the second on breast volume. In the first, a group of 22 female volunteers between 50-60 years old was selected. The subjects applied a cream containing 2% Active I twice a day for 14 days. The volume of the cheeks was analysed by fringe projection at the beginning and at day 14. The treated areas showed an average 11.9% volume growth, and 79% of subjects had a volume growth after 14 days.
538 In the second, a panel of 22 woman between 25-40 years was selected. Volunteers were required to have an 80-90 European bra cup size and a stable weight. They were asked to apply, twice a day, the placebo cream on a breast and the cream containing 2% Active I on the other breast for 56 days. Measurements of breast volume were taken at the beginning and at days 14, 28 and 56 using the Fast Optical In Vivo Topometry Technique (FOITS), which allows reconstructing of the surface and volume of the breast based on the principle of optical interferometry. The relative volume of the area and 3D images were obtained. The differences versus initial time were measured as well as the evolution of the breast volume of the volunteers versus initial time normalised with respect to placebo results. The results at the end of the study showed that Active I generated a clear positive gain tendency in breast volume, while the placebo did not. Moreover, the volume increase in the area where the cream containing Active I was applied was 30 times higher than the placebo at day 56.
539 On the face of it, the studies and other evidence relied on by Self Care amount to a substantial foundation for a representation that the respective products significantly reduce the appearance of wrinkles. Mr Williams gave evidence in respect of each of the products and confirmed that in his view, and on the basis of the formulations of the products and the studies relied on, the products would be expected to significantly reduce the appearance of wrinkles. Dr Haley, in his reports, raised certain queries and criticisms of the studies and in some respects he had a different view of the likely impact of some of the ingredients of some of the products. The disagreements between Mr Williams and Dr Haley were reduced during the process of concurrent examination. Moreover, only some of the issues raised by Dr Haley in his reports were taken up by Allergan in its submissions, written and oral, in support of its case that Self Care’s efficacy representations are misleading or deceptive.
540 As a practical matter, I only intend dealing with the arguments made by Allergan as to why the studies and other evidence relied on by Self Care do not amount to reasonable grounds for the representations. In the light of Allergan apparently not relying on other points, queries and criticisms raised by Dr Haley, I do not intend dealing with those matters if they have not been raised or relied on by Allergan.
541 Allergan makes essentially five arguments against a conclusion that the above studies, and other evidence, amount to “reasonable grounds” for the representations.
542 Allergan’s principal argument is that none of the studies makes a head-to-head comparison of the visible results of the use of Botox with the use of Self Care’s products. It submits that it is not to the point whether or by how much Self Care’s products achieve an improvement in the appearance of expression lines generally. Allergan also submits that it is not possible to make a comparison between a skincare product that is topically applied, such as Self Care’s products, with Botox, which is injected into the muscle, given the differing modes of action and the number of variables.
543 Allergan relies, in particular, on Reckitt Benckiser (Australia) in which Gleeson J (at [307]-[308]) reasoned that because none of the studies that were relied on in that case was directed to a comparison of the two products, Nuromol and Maxigesic, they did not provide an adequate foundation for the representation that one tablet of Nuromol provides equivalent or superior pain relief to two tablets of Maxigesic. Her Honour’s judgment was upheld on appeal, in AFT Pharmaceuticals, where it was reasoned that one real difficulty for AFT in establishing that the primary judge erred in evaluating the scientific evidence was that there was no scientific study which directly related to the efficacy of Maxigesic or Nuromol at their respective recommended daily doses – “None of the scientific studies provided a direct, head-to-head comparison of the efficacy of analgesic products with the same levels of paracetamol/ibuprofen as Maxigesic and Nuromol at maximum daily doses”: at [104].
544 There are a few matters to be observed in considering the application of the reasoning in that case to the present case. First, the representation at issue was a measurable quantitative comparative representation, i.e., that the one tablet was at least twice as good as the other. The representations in the present case, with one exception, are quite different, namely that the efficacy of the relevant products produces similar results in the appearance of the reduction of wrinkles. That representation is not exact or quantifiable in its comparative statement.
545 Second, Gleeson J’s conclusion relied on by Allergan that because there was no head-to-head study of the two products the comparative statement could not be justified was not stated as a principle to be applied across all cases involving comparative claims, and it was not adopted in such terms by the Full Court in the appeal. Inherent in the reasoning of the Full Court is the possibility that the comparative representation could have been justified by scientific studies that were not head-to-head studies of the respective products at the relevant doses. The conclusion was that where some studies favoured the contentions of one side of the case and other studies favoured the contentions of the other side of the case (at [105]), the absence of an applicable head-to-head study created a “real difficulty” in establishing a foundation for the representation: at [104]. The Full Court did not reason that in the absence of a head-to-head study it would be impossible to establish a proper foundation. In this case there is no evidence of opposing studies giving weight to contentions of Allergan that Self Care’s products do not have the effects as represented.
546 Given those differences, I do not accept the submission that the absence of head-to-head comparative studies between Self Care’s products and the Botox product is on its own a basis for finding that Self Care’s efficacy representations are without reasonable grounds.
547 The exception that I have referred to is the representation that the Night (tube) product “delivers the results of … a Botox injection in four weeks”. That representation, as I have said, is exact both as to the comparable results and the time period. As will be seen, the absence of a head-to-head comparison in that case is significant.
548 In view of my conclusion that the representations that are made by the impugned statements say nothing about the mechanism or mode of action of Self Care’s products, Allergan’s submission, and Ms Cain’s opinion, that those products cannot be compared with Botox because they have different mechanisms or modes of action must be rejected. The statements and hence the representations concern the apparent outcomes of the use of the products, i.e., how the skin appears after having used the products. It says nothing about what is actually happening to the skin or how any effects are achieved. The question is whether the claim that the products achieve a similar reduction in the appearance of wrinkles is well-founded.
549 Allergan’s second argument is that some of the studies make highly speculative claims about the efficacy of the products, particularly with reference to the mechanism of action. For example, Studies 1 and 2, in respect of Active A, speculate that the mechanism of action includes effects at the level of the dermis, whereas with reference to another study, referred to as the Kraeling article, Active A would not penetrate to the level of the dermis. The expert witnesses agreed that the peptides would not penetrate to the dermis. The expert witnesses were also in agreement that the mechanism of action of the active peptides in vivo is as a scientific matter not known or explained.
550 However, as I have identified, the question is not about the mechanism of action of the ingredients, but rather the aesthetic outcome. Studies 1 and 2 reported positively on the effect that Active A has on reducing the appearance of wrinkles. They did so by the use of an accepted method of measuring wrinkles known as confocal profilometry which involves taking silicone rubber impression mouldings of a face and then analysing them using a confocal microscope which produces data which is then processed using a digital imaging program on a computer. Study 1, being a supplier’s product brochure on Active A, reports on the results of a number of studies of which Study 2 is one.
551 Allergan’s third argument is that it is not possible to attribute the positive visual effects achieved by the active ingredients of Self Care’s products to the identified “actives” or peptides in those products. It submits that that is because, in relation to the peptides:
(1) only a very small amount of the peptide is used ([redacted until 6 June 2021]% of the product total in most cases);
(2) only a very small amount may get into the viable epidermis (of the order of 0.01% of the peptide applied);
(3) when used in a concentration of [redacted until 6 June 2021]%, only 0.0000005% of the product total or 0.5 ppm would penetrate to the viable epidermis;
(4) the peptides do not penetrate to the dermis, where the muscles or the nerves relevantly operate; and
(5) there are so many other skin conditioning agents in the products (except Boost which is [redacted until 6 June 2021]% water), that they would swamp any effect that reduces the appearance of wrinkles.
552 There are essentially two components to this argument that the peptides in the product do not produce a significant visual effect. The one is that they are in too low a concentration to be effective, and the other is that any effect that is seen is because of moisturising ingredients rather than the peptides.
553 In reliance on the Kraeling article, Dr Haley initially did not attribute any efficacy to the peptide. The Kraeling study showed that the peptide acetyl hexapeptide-8, which was used by both experts as a suitable proxy for all of the peptides under consideration, penetrated to the viable epidermis of human skin in vitro to the extent of 0.01%. From this, Dr Haley concluded that the peptide would not have any significant efficacy in reducing the appearance of wrinkles in vivo.
554 However, Study 2, demonstrates a significant visual effect. Mr Williams accepted that that was a cosmetic effect and not therapeutic because of the level at which it operates, but he was not challenged on there being a positive visual effect on the appearance of wrinkles.
555 The aim of Study 2 was to evaluate the macro-roughness of human skin replicas in silicone to determine the wrinkle reduction efficacy of Active A compared to a placebo cream. The macro-roughness of the silicone replica, which corresponded to the eye contour area, was obtained from 17 volunteers for the test cream and from 10 volunteers for the placebo cream. The treatment lasted four weeks and samples obtained before treatment started and after 28 days were taken. Skin roughness was measured by confocal profilometry across multiple parameters. The study concluded that the test product containing Active A reduced wrinkles by about 34% on average, which is significant compared to the placebo cream which reduced wrinkles by about 3% on average. The reduction in the depth of the wrinkles was about 63% in some instances.
556 Dr Haley challenged these results on the basis that the results for the placebo group (i.e., little or no improvement in the appearance of reduction of wrinkles) were not consistent with his expectations of a product having the ingredients shown in the formulation of the placebo, which included certain moisturising ingredients, in particular glycerin at 2.4%. He said that it is quite feasible to him that the performance of the placebo cream formulation with an additional 0.005% of the peptide coming from Active A (i.e., the test product) could be totally coming from the placebo base formulation.
557 However, Dr Haley accepted that he had done no tests on the performance of the placebo cream, i.e., he was speaking merely from expectation, and he was unable to explain why the test cream and the placebo cream produced markedly different results which he accepted were statistically significant. He accepted that the use of the placebo was designed to exclude the possibility that the results of the active product are attributable to the moisturisers in which it was carried.
558 On the question of the concentration of the active ingredient in the product, Dr Haley accepted that in the absence of testing one cannot make an assumption that any particular ingredient has no effect at any particular concentration, even at very low levels such as 0.1%. Different ingredients will have effect at different concentrations, which is known as the dose response correlation. Mr Williams explained that it is well-known in the cosmetic and therapeutic industries that “the dose makes the activity”, by which I understood him to mean that some substances (such as botulinum toxin as an example) have effect at extremely low dosages and others have effect only at very high dosages, and everything in between. It depends on the dose response correlation for the particular ingredient.
559 Mr Williams explained that it can be misleading to look at the possible effect of an active ingredient in percentage terms as that neglects to take account of the activity of the actual molecule which may be very active, as in the case of peptides. Thus the frequent application of a product with a very low concentration of the peptide may nevertheless deliver a sufficient dosage of the peptide over time to have an active effect.
560 Ultimately Dr Haley accepted, as I do, that Study 2 demonstrates that the peptides are responsible for the improved performance of the test product over the placebo.
561 Allergan’s arguments that the observed positive results cannot be attributed to the active ingredient, which I have rejected above, are applicable to Studies 2, 6, 7 and 11.
562 Allergan’s fourth argument, with reference to the ICH Harmonized Tripartite Guideline for the Structure and Content of Clinical Study Reports (1995), is that ignoring patients who dropped out of Study 2 and drawing conclusions based only on patients who completed a study can be misleading and can introduce bias. It submits that there is a danger in dropping patients with available data from analysis because of poor compliance and eligibility or any other reasons. It submits that an analysis using all available data should be carried out for all studies intended to establish efficacy.
563 The ICH is the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. The ICH Guideline includes the following paragraphs relied on by Allergan:
11.4.2.2 Handling of Dropouts or Missing Data
There are several factors that may affect dropout rates. These include the duration of the study, the nature of the disease, the efficacy and toxicity of the drug under study, and other factors that are not therapy related. Ignoring the patients who dropped out of the study and drawing conclusions based only on patients who completed the study can be misleading. A large number of dropouts, however, even if included in an analysis, may introduce bias, particularly if there are more early dropouts in one treatment group or the reasons for dropping out are treatment or outcome related. …
11.4.2.6 Use of an “Efficacy Subset” of Patients
Particular attention should be devoted to the effects of dropping patients with available data from analyses because of poor compliance, missed visits, ineligibility, or any other reason. As noted above, an analysis using all available data should be carried out for all studies intended to establish efficacy, even if it is not the analysis proposed as the primary analysis by the applicant. In general, it is advantageous to demonstrate robustness of the principal trial conclusions with respect to alternative choices of patient populations for analysis. Any substantial differences resulting from the choice of patient population for analysis should be the subject of explicit discussion.
564 Study 2 records that “about 20 volunteers were chosen for each product” (i.e., for the test cream and the placebo cream), but some of them did not complete the study satisfactorily so they were not included in the analysis of the results obtained. I infer that three volunteers dropped out from the test group and 10 dropped out from the control group. I note that Dr Haley drew the same conclusion.
565 Dr Haley said that it was a matter of concern that 10 of the placebo test subjects had dropped out and that no reasons were recorded. However, he accepted that the study was statistically significant and he failed to explain why the absence of the information about why 10 participants had dropped out would make the study unreliable. Ultimately, he accepted in cross-examination that that matter alone would not constitute a tipping point such as to cause an otherwise reliable study to be unreliable.
566 Insofar as the ICH Guideline is concerned, it describes its objective as being to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ICH regions. Such a clinical study report would be of an individual study of any therapeutic, prophylactic or diagnostic agent conducted in patients. By its own terms, and it was accepted by Dr Haley, the ICH Guideline did not apply to unregulated cosmetics. Further, in its introduction it states that depending on the nature and importance of the study, a less detailed report might be appropriate. Dr Haley was unable to explain why in the case of Study 2, to which the ICH Guideline did not apply, it was inappropriate to not include the reasons why participants dropped out. Since the data measurements were at the commencement of the trial and at its end, clearly there was no relevant data to record in respect of any participant who dropped out before the twenty-eighth day.
567 Mr Williams was cross-examined with reference to the ICH Guideline. He explained that it is not generally applied to cosmetic products. He offered the plausible explanation that 10 out of the original 20 participants in the placebo group may have dropped out because they were not noticing any reduction in the appearance of wrinkles. If that is the reason that they dropped out, that does not detract from the reliability of the study. He said that in his view the difference between the placebo and the test product is sufficient based on the standard deviation in the remaining test subjects to still be reliable.
568 In the circumstances, I do not accept the criticism of Study 2 based on the number of drop-outs and the absence of any recorded reason for them dropping out. I accept that the study offers a reasonable foundation to the claim that Active A produces a significant reduction in the appearance of wrinkles over a 28-day period of application.
569 Allergan’s fifth argument was made by Dr Haley. It was not put squarely in written or oral submissions by Allergan but I deal with it here as an argument advanced by Allergan for the sake of completeness and because there may have been hints at it in submissions. It is what Self Care in its submissions characterised as an “extrapolation issue”. The argument is that one cannot necessarily apply the results of studies of an active ingredient to the performance of a product containing it. It arises because most of the studies that Self Care relies on are studies of the efficacy of the active ingredients in product formulations that may not be exactly the same as Self Care’s formulations.
570 Mr Williams explained that in Australia cosmetic manufacturers tend to use the results of studies on ingredients when formulating their products. The extrapolation is justified where the concentration of the active ingredient in the product is at the same level (or greater) as in the test and if there is nothing in the product which will impair the result that is shown for the active ingredient it is accepted. Although that is not the approach with respect to therapeutics, presumably because of critical safety issues in relation to therapeutic substances which is what justifies their strong regulation, it is the case with respect to cosmetics because of the prohibitive cost relative to the size of the Australian market to conduct studies on each product itself.
571 Dr Haley accepted that the concentrations of the active ingredients in each of the Self Care products, save for the Boost product prior to a correction in its formulation in August 2018, was the same as in the respective studies relied on by Self Care. He also did not identify any component in any of the products that would impair the performance of the peptides in the product formulations.
572 In the result, I accept that the results of each of Studies 3, 4, 6-10 and 13, to which this extrapolation issue applies, can be extrapolated to the respective products.
Prolonging the effect of Botox specifically
573 Studies 6 and 7 investigated the use of peptides to prolong the visible effects of Botox. Each is a report of the same trial the objective of which was to evaluate the anti-wrinkle effect of the Active E peptide after a botulinum toxin type A treatment by injection. A panel of 22 females (51 years on average) received 50 µl (i.e., 0.05 ml) of the toxin in the periorbital (crow’s feet) and frontal region. After the injection, the participants applied either the test formulation with 10% Active E or a placebo formulation (control treatment) twice a day for six months.
574 Skin silicone replicas were obtained before the injection and at different times during the treatments. The skin relief was evaluated by confocal profilometry, quantifying the reduction of the average surface roughness against the baseline. Macroscopic photographs were also taken at baseline and after two, four and six months.
575 In the frontal region, the active topical treatment apparently highly intensified the anti-wrinkle effect of the botulinum toxin type A injection at all times, prolonging its benefit on the skin even after six months. The frontal region skin roughness graph shows that by the six-month period the regions under the control treatment had increased skin roughness from the baseline measurement by 13%. In comparison, the regions under the active treatment had remained at a decrease of 23%. In that way the active can be said to have prolonged the effect (reduction in skin roughness) of the Botox injection past the point when it would be expected that no effect would be present. In the periorbital region, the active treatment potentiated the smoothing effect on wrinkles induced by the injection, improving the results over six months.
576 In his written evidence, Dr Haley said that the clinical test tries to compare the performance of a therapeutic product injected once with a cosmetic product applied topically twice a day for six months which is not a valid comparison. That, of course, misunderstood the study. Dr Haley accepted this in cross-examination. Dr Haley also accepted that the experiment was apt to produce results that allow a comparison between the anti-wrinkle effects of the two different treatments, the only difference between them being the presence of Active E in the test group. He also accepted that the results at six months show that the effect of the botulinum toxin type A injection when administered alone had worn off. In contrast, the results for the combination of the botulinum toxin type A injection and the test product showed strong ongoing efficacy at six months. The results of the study thus apparently demonstrate that Active E prolongs the visible effects of Botox.
Conclusions on reasonable grounds
577 In the result, I find that the representations as to a Self Care product being an alternative to Botox (i.e., the first category of statements identified at [494]-[504] above) are justified as having been made on reasonable grounds. Self Care offered a lot of evidence in support of the proposition that its relevant products produce a noticeable and significant reduction in the appearance of wrinkles. Allergan failed to discharge the onus on it to prove that Self Care did not have reasonable grounds for making the representations. Since, in my evaluation, the achievement of a reduction in the appearance of wrinkles is a similar effect to the use of Botox, the representations were not misleading. That is to say, as concluded above, the ordinary and reasonable consumer would not understand Botox to have a readily quantifiable effect on the appearance of wrinkles, and no evidence has been adduced to actually quantify that effect. Thus, the noticeable and not insignificant effect of Self Care’s products in reducing the appearance of wrinkles is sufficiently similar to the effect of Botox in reducing the appearance of wrinkles that the representation that one is an alternative for the other is not misleading.
578 In the second category of representations (identified at [505]-[507] above), the statement that Protox “could produce a Botox-like visual effect” is also not misleading for the same reasons. The evidence establishes reasonable grounds for the representation that Protox can produce a noticeable and significant visual effect in the appearance of the reduction of wrinkles similar to that produced by Botox (although not quantifiably so).
579 However, also in the second category is the statement that the Night (tube) product “delivers the results of a Botox injection in 4 weeks” which, as I have said, constitutes a representation that the product will deliver the same results as Botox in four weeks. The time period of Study 2 which tested the Active in the Night (tube) product corresponds to the time period of four weeks referred to in the statement. That study does not, however, provide reasonable grounds for the representation that the product produces the same results as Botox. There is no head-to-head study to compare the results of the two products, and although Study 2 quantifies the reduction in wrinkles over four weeks of use of a formulation containing Active A, there is no study which in a comparable way quantifies the efficacy of Botox over four weeks. Self Care has not sought to justify the comparative representation and it is not justified by the evidence. The statement is therefore misleading within the meaning of s 29(1)(g) of the ACL.
580 The third category of statements (identified at [508] above) is those that represent that the product in question prolongs or improves the look of Botox. The studies provide an adequate foundation, and thus reasonable grounds, for the statements with regard to prolonging and improving the look of Botox. Once again, the representations are with regard to appearance. Thus the fact that the underlying physiological effect of Botox is not prolonged by Protox is not to the point. The studies show, albeit in an unexplained way, that the appearance in the reduction of wrinkles that is achieved by Botox is prolonged and enhanced by the use of Protox.
581 As identified (at [42] above), Allergan is required in this part of the case to establish at least one instance of loss or damage under each head of damage that it claims. The quantification of those losses is separated out for later determination.
582 In view of the fact that none of Allergan’s claims, whether as to trade mark infringement, misleading and deceptive conduct under the ACL (with one exception) and passing off has succeeded, the question of loss and damage falls away. The usual practice of a trial court is to decide all the issues, even those that strictly speaking do not arise because of prior findings, to guard against the possibility of the prior findings being held to be wrong and so as to enable a court on appeal to then dispose of all the issues and not to have to refer the matter back to the trial court. That practice is, however, not a rigid requirement. Where there is good reason not to take that course, it is appropriate not to do so but the reasons for not doing so should be explained. See, for example, Chief Commissioner of State Revenue v Adams Bidco Pty Ltd [2019] NSWCA 34 at [3] per Leeming JA (White JA and Emmett AJA agreeing).
583 In my view there is good reason not to separately identify whether an instance of loss or damage has been proved under each head of damage. First, that exercise would involve addressing it in respect of each trade mark infringement that is asserted by Allergan, each asserted basis for passing off, and in respect of each affiliation representation and each efficacy representation. That is a demanding exercise in circumstances where none of it or at least parts of it may prove to be entirely unnecessary.
584 Second, Allergan has indicated, on the assumption of success on liability, that it is likely to seek an account of profits in relation to its asserted trade mark infringements. Moreover, in the event that it was found that no instance of loss or damage had been proved, it would almost certainly seek an account of profits. The result is that even if I was to now conclude that no instance of loss or damage was proved on each of the separate bases on which the claims are put, and that was upheld on appeal, if my findings on liability were overturned there would still have to be a further hearing. Therefore, not dealing now with loss and damage will not in any foreseeable circumstances avoid the need for a remittal and a further hearing in the event that my findings on liability are found to be wrong.
585 Third, in the event that there is an appeal on some limited basis and the parties considered that it would be conducive to the whole case being disposed of by the court on appeal in the event that the appeal is otherwise successful, they could ask me to decide some limited question of loss and damage at this stage. If persuaded, I could then decide that question in time for it also to be dealt with in the appeal.
586 In the circumstances, I have decided not to make any further decision with respect to loss and damage at this stage.
587 The exception is the misleading statement with regard to the results of Night (tube) after four weeks being the same as Botox. The ACL claim in relation to that statement succeeds. I accept the submission that it can be inferred that someone somewhere is likely to have not used Botox, or to have delayed using Botox, because of that statement. I am accordingly satisfied that this claim can properly proceed to the quantification of damages, if Allergan elects to follow that course.
588 Allergan pleads that since 24 November 2008, Ms Amoroso has been the sole director and secretary of Self Care IP and the person responsible for the day-to-day decision-making and management of Self Care IP. Further, Ms Amoroso directed and procured the business dealings and affairs of Self Care IP. That was all admitted by Self Care and Ms Amoroso.
589 Allergan also pleads that since 14 July 2008, Ms Amoroso has been the sole director and secretary of Self Care Corp and the person responsible for the day-to-day decision-making and management of Self Care Corp. Further, Ms Amoroso directed and procured the business dealings and affairs of Self Care Corp. Save that Ms Amoroso was the sole director and secretary as pleaded, the other matters in respect of Self Care Corp were not admitted on the basis that they are unclear.
590 On the strength of the allegations pleaded in the two preceding paragraphs, Allergan pleads three separate bases for the personal liability of Ms Amoroso, namely:
(1) Ms Amoroso infringed the BOTOX marks by having authorised the infringing acts of each of Self Care IP and Self Care Corp within the meaning of s 20(1)(b) of the TM Act;
(2) Ms Amoroso directed, procured or entered into a common design with Self Care IP and Self Care Corp in their acts of infringement of the BOTOX marks pursuant to s 120(1) and/or s 120(2) of the TM Act; and
(3) Ms Amoroso:
(a) is a person who aided, abetted, counselled or procured the conduct of Self Care IP and Self Care Corp in their contravention of ss 18, 29(1)(a), 29(1)(g) and 29(1)(h) of the ACL, within the meaning of s 75B of the Competition and Consumer Act 2010 (Cth);
(b) directed or procured or entered into a common design with Self Care IP and Self Care Corp in engaging in the tort of passing off; and
(c) is a joint tortfeasor with Self Care IP and Self Care Corp in engaging in the tort of passing off.
591 Needless to say, the matters pleaded by Allergan in the preceding paragraph were all denied by Self Care and Ms Amoroso.
592 Before going any further, it can be observed that the pleaded basis for Ms Amoroso’s personal liability for any contravention of the ACL by the Self Care companies is unsustainable. That is because it is pleaded to rest on s 75B of the Competition and Consumer Act 2010 (Cth), but that provision does not apply to Pt XI Div 2 of the Act and hence to the ACL. It may be that the pleader had in mind s 236 of the ACL read with para (a) of the definition of “involved” in s 2, i.e., a person who “has aided, abetted, counselled or procured the contravention”. Allergan does not plead or rely on the wording in para (c) of the definition, i.e., a person who “has been in any way, directly or indirectly, knowingly concerned in, or party to, the contravention”.
593 In any event, Allergan does not make any submissions with regard to Ms Amoroso having been knowingly concerned in the contraventions or having aided or abetted the companies in them. The submissions are put on a narrower basis.
594 It is submitted on behalf of Allergan that Ms Amoroso was so closely involved with the development, the branding, the signing-off and the way in which the products went to market, and that she exercised ultimate decision-making control over the companies, that she is jointly liable with the companies for their infringing conduct. It is submitted that Ms Amoroso had a close personal involvement in the infringing acts of the company to the extent that she went beyond simply acting in her capacity as director, and made the conduct her own.
595 Allergan thus relies on a test of “close personal involvement” in the infringing conduct by the director of the company as expressed by Davies J in Insight Radiology Pty Ltd v Insight Clinical Imaging Pty Ltd [2016] FCA 1406; 122 IPR 232 at [153]. That phraseology was in turn drawn from that of Besanko J in Keller v LED Technologies Pty Ltd [2010] FCAFC 55; 185 FCR 449 at [291].
596 Also in Keller, Emmett J (at [83] and [84]) said that to be liable as a joint tortfeasor with the company the director must have done something more than acting as a director and been involved in invading the applicant’s rights. Jessup J said (at [404]) that the director must be effectively standing apart from the company and directing or procuring it as a separate entity and that there must be a sense in which the director is using the company as the instrument of his or her wrong, and (at [405]) that the director must have made the tort his or her own.
597 More recently, the Full Court has spoken with one voice in JR Consulting & Drafting Pty Ltd v Cummings [2016] FCAFC 20; 329 ALR 625 per Bennett, Greenwood and Besanko JJ after considering a number of authorities including Keller. The Court stated (at [347]) that unless the director is standing apart from the company there will not be concurrence in the act but merely a coincidence of roles in which the only true actor is the company itself, and that “the director must be shown to have directed or procured the tort and the conduct must, clearly enough, go beyond causing the company to take a commercial or business course of action or directing the company’s decision-making where both steps are the good faith and reasonable expression of the discharge of the duties and obligations of the director, as a director” (emphasis in the original): at [350].
598 Ms Amoroso’s role in relation to the impugned conduct of the companies can relevantly be summarised as follows.
599 Ms Amoroso is the sole director and secretary of both the Self Care companies. She has been the sole director of the companies since 2008. The shares of both companies are held on trust by Juventas Group Pty Ltd, a company in respect of which Ms Amoroso has been the sole director, secretary and shareholder since 15 May 2007, as trustee for the Artemis Trust. The Artemis Trust is a family trust which exists for the benefit of various of Ms Amoroso’s family members, including herself.
600 Ms Amoroso incorporated Self Care IP. In her role as director of that company she decided whether ownership of a trade mark used by Self Care Corp would be owned by Self Care IP.
601 Ms Amoroso has been the CEO of Self Care Corp since mid-2013. That company employs around 17 to 19 people at any given time. As part of her role as CEO of Self Care Corp and director of both companies, Ms Amoroso is ultimately responsible for the operations of Self Care. Despite not being involved in every decision and wishing to empower her staff, Ms Amoroso still has ultimate control over what goes out to consumers and the public. That includes ultimate control over the creative direction of any advertising campaign, but not necessarily how it is implemented on a day-to-day basis.
602 Ms Amoroso reviewed and had final sign-off on Self Care’s brand guidelines. All new packaging and advertising is printed and placed on her desk with a sign-off sheet. That enables her to immediately rectify any inconsistency or inadequacy with the packaging or advertising, including the way that the relevant trade marks appear.
603 At the time that the Inhibox product was developed, Ms Amoroso was aware from her own observation that there were a number of products in the market promoting themselves as “Botox alternatives”. Her idea was to develop a similar product that also had an instant effect. Ms Amoroso was part of the team that ultimately selected the statements made by Self Care about this product in respect of which Allergan complains. It was Ms Amoroso who decided to refer to botox as Botox (i.e., with a capital ‘B’) and to acknowledge Allergan as the trade mark owner on the packaging and marketing of the product.
604 Ms Amoroso came up with the brand PROTOX. She made the decision that Self Care IP would own the trade mark. She decided to first register PROTOX on its own rather than FREEZEFRAME PROTOX. A decision was made by the marketing team at Self Care, including Ms Amoroso, to switch the messaging from Protox prolonging Botox to Protox being an alternative to Botox. Ms Amoroso was part of the team that selected the promotional statements that would be made by Self Care in respect of the Protox, Night (tube) and Boost products about which Allergan complains.
605 Only about six people were working in the business at the time that the Night (tub) statements were selected. All those people, including Ms Amoroso, were involved in selecting the relevant statements. Each member of the team reviewed summaries of the clinical studies supporting the product to ensure that the statements were accurate.
606 Ms Amoroso’s evidence in chief put her firmly at the centre of the companies, in ultimate control of their decision-making and in charge of their creative direction. She did not seek to shy away from any of that. However, none of it puts her in a position such as to have gone beyond her role as a director or CEO. In everything she was clearly acting in the interests of the companies and not directly in her own interests; the activities of Self Care of which Allergan complains are squarely activities within the ordinary course of Self Care’s business. There was no indication that anything Ms Amoroso did for the companies was also conduct done on her own behalf.
607 Allergan relies on Ms Amoroso’s previous involvement with Cat Media, and the knowledge and experience she gained there, to submit that the conduct that it complains of with regard to Self Care shows bad faith or consciousness of wrongfulness by Ms Amoroso. I understand Allergan to submit that on that basis she went beyond or outside her role with the companies and made the conduct her own conduct. However, I do not consider that that is established. That perhaps flows inevitably from my findings that, save in one relatively minor respect, the causes of action must fail. Depending on what basis, in the hypothetical alternative now under consideration, it might be found that one or more of the other causes of action succeeded, it might be found that the assertions of bad faith or consciousness of wrongfulness are upheld. But on my factual findings, they must be rejected.
608 In the circumstances, I do not find any basis upon which Ms Amoroso would be personally liable on the non-ACL causes of action against the Self Care companies.
609 With regard to the ACL claims, I have already identified Allergan’s pleading difficulty. It also makes no submissions in support of any finding of accessorial liability based on the relevant (or any) wording of the ACL or with reference to any authority. On the factual findings I have made above, it would face some difficulty in establishing that Ms Amoroso knew that the impugned statements made representations that were misleading or deceptive, or false. In the circumstances, I also do not find any basis upon which Ms Amoroso would be personally liable on the ACL causes of action against the Self Care companies.
610 In this claim, Allergan seeks injunctions against Self Care and Ms Amoroso restraining them from making the statements that are identified in the “TG Act” column in the tables at [111]-[116] above. The asserted basis for the injunctions is that the making of the statements constitute contraventions of the offence provisions in s 42DL(2) or (3) read with s 42DL(10) and the civil penalty provisions in s 42DLB(1) and (7) of the TG Act.
611 The claim is pleaded on the basis that the impugned statements are claims of a therapeutic use, as that term is defined in the TG Act, namely that the physiological processes in a person who has used the relevant product may be inhibited, influenced, intensified, modified or extended by means of the same mode of action as the Botox product.
612 Relevantly, the definition of “therapeutic goods” in the TG Act includes goods “that are represented in any way to be, or that are, whether because of the way in which the goods are presented or for any other reason, likely to be taken to be for therapeutic use”: s 3. The definition of “therapeutic use” includes use in or in connection with “influencing, inhibiting or modifying a physiological process in persons”: s 3.
613 Thus, for a good to be a therapeutic good under the TG Act it need not actually have a therapeutic use or effect; it need only be represented to have that effect. Following the evidence of the expert witnesses in this case, and in particular with reference to the Kraeling article, it is uncontroversial that Self Care’s products have no therapeutic use or effect. Allergan accordingly rests its case on Self Care representing, by the impugned statements in context, that the products have a therapeutic use or effect. In particular, it is pleaded that the statements represent that the products have a therapeutic use or effect “by means of the same mode of action as the Botox product”.
614 In the context of the ACL claims I have found that the statements that are relied on in relation to the TG Act claims do not make any representation with regard to the mode of action of the products and that they do not represent that Self Care’s products have the same mechanism as Botox. I have found that the statements will be understood as saying something about the effect that the products will have with regard to the reduction in the appearance of wrinkles, but not about the mechanism to achieve that effect. See [494]-[509] above.
615 In that regard, it is to be noted that Allergan does not identify Night statement 3 as being one of the statements in respect of which it seeks an injunction. That is presumably because Self Care has given an undertaking not to make that statement. It will be recalled that that is the statement that the product “delivers the results of a Botox injection in four weeks”. In respect of that statement, the case might more readily be made that it makes the representation that the product will have the same mode of action as the Botox product, but I have concluded that that statement also speaks only to observable results and not mode of action. In any event, in view of no injunction being sought in relation to that statement that question need not be considered further.
616 In the circumstances, the TG Act claims fall at the first hurdle. Allergan has simply failed to prove that the statements make the representations that are pleaded and which are in turn said to constitute contraventions of the TG Act. The claims should therefore be dismissed.
617 It should, however, be noted that there are also other possible obstacles to the TG Act claims succeeding even if I am wrong in my conclusion that the statements do not make the pleaded representations with regard to the therapeutic effect of Self Care’s products. The most significant other obstacle is whether Allergan has standing to seek injunctive relief in reliance on contraventions of the TG Act.
618 In the result, for the reasons set out above, I have found as follows in the NSD15/2017 proceeding:
619 Allergan’s claims of trade mark infringement under s 120 of the TM Act against Self Care for its word mark PROTOX, the use of various phrases including the word BOTOX and the use of the word BOTOX itself are not established and should be dismissed.
620 Allergan’s claims that Self Care made representations which were contrary to the ACL of affiliation or that Self Care engaged in passing off are not established and should be dismissed.
621 Allergan’s claims that Self Care made efficacy representations contrary to the ACL are established for only one of the 35 statements complained of, and the rest should be dismissed. The successful claim is with regard to the statement that the Night (tube) product produces the results of Botox in four weeks.
622 Allergan’s claims that Self Care infringed the TG Act are not established and should be dismissed.
623 Self Care’s cross-claim for removal (including cessation of protection) from the register of the word mark BOTOX in class 3 of the 655 mark for non-use is successful and an appropriate order should be made. Its claim for cancellation of the BOTOX 426 defensive mark in respect of all the class 3 goods is not established and should be dismissed.
624 In the NSD1802/2017 proceeding I have found as follows:
625 Allergan Inc’s appeal against the decision of the delegate of the Registrar of Trade Marks to allow Self Care IP’s application for the registration of the mark FREEZEFRAME PROTOX in class 3 should be dismissed.
626 I will require the parties to bring in short minutes of order to dispose of the proceedings in accordance with my reasons, including with regard to costs. Mindful also that much of the evidence is the subject of suppression orders, and conscious that despite my efforts to avoid including suppressed information in my reasons, I may have included such information inadvertently, I will also restrict these reasons to the external lawyers of the parties and give them the opportunity to bring to my attention any redactions that should be made. Only after that will the reasons be published to the parties themselves and made public.
627 The Court will retain the physical exhibits pending the possibility of an appeal.
I certify that the preceding six hundred and twenty-seven (627) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justice Stewart. |
Associate:
NSD 15 of 2017 | |
SELF CARE CORPORATION PTY LTD (ACN 132 213 113) | |
ALLERGAN AUSTRALIA PTY LTD (ACN 000 612 831) |




