FEDERAL COURT OF AUSTRALIA
Commonwealth of Australia v Sanofi (formerly Sanofi-Aventis) (No 5) [2020] FCA 543
Table of Corrections | |
Para [523] amended to add to the end of the third sentence the following words: “to have given the Famvir and Sandostatin precedent” |
ORDERS
Applicant | ||
AND: | SANOFI (FORMERLY SANOFI-AVENTIS) First Respondent SANOFI-AVENTIS US LLC Second Respondent BRISTOL-MYERS SQUIBB INVESTCO LLC Third Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The application for compensation filed on 1 April 2013 be dismissed.
2. The applicant and the respondents exchange written submissions (limited to five pages in length) in relation to the question of costs of the application by 4.00pm, 8 May 2020.
3. The applicant and the respondents exchange written submissions in reply (limited to four pages in length) by 4.00pm, 19 May 2020.
4. The contents of the paragraphs in the Reasons for Judgment specified in the Schedule to these orders (“the specified paragraphs”) not be further published or disclosed except in accordance with inter partes arrangements previously agreed with Apotex Pty Ltd or prior orders of this Court in relation to the disclosure of any document referred to in any of the specified paragraphs.
5. Subject to any further order or direction, order 4 shall operate until 4.00pm, 12 May 2020 after which time neither order 4 nor any other non-publication or confidentiality order made in this proceeding shall prevent any person from publishing or disclosing the contents of any of the specified paragraphs.
6. For the avoidance of doubt, nothing in these orders, or any other non-publication or confidentiality order made in this proceeding, shall prevent any person from publishing or disclosing any part of the reasons for judgment not contained in the specified paragraphs.
7. Any application to vary order 4 or 5 shall be made by way of interlocutory application which (together with any affidavit upon which the party seeking such an order seeks to rely) must be filed and served by 4.00pm on 7 May 2020.
8. Any interlocutory application filed pursuant to order 7 be fixed for hearing at 9.30am on 12 May 2020 before Nicholas J.
SCHEDULE
Paragraphs [227], [234], [296], [311], [550], [551]
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
[1] | |
[36] | |
[36] | |
[45] | |
[54] | |
[56] | |
[58] | |
[59] | |
Setting of the AP2P and AEMP and price reductions under the PBS | [61] |
12.5% statutory price reduction on first listing of a generic | [64] |
[66] | |
[68] | |
[71] | |
[77] | |
[78] | |
[80] | |
[80] | |
[82] | |
[89] | |
[100] | |
[109] | |
[121] | |
[131] | |
[133] | |
[133] | |
[139] | |
[148] | |
[148] | |
[158] | |
[164] | |
[174] | |
[177] | |
[177] | |
[190] | |
[197] | |
[197] | |
[201] | |
Would Apotex would have applied to list its clopidogrel products from 1 April 2008? | [219] |
Would the Minister or the Delegate have listed Apotex’s clopidogrel products on 1 April 2008? | [353] |
[422] | |
Did the Commonwealth’s loss flow directly from the grant of the interlocutory injunction? | [433] |
[452] | |
[482] | |
[490] | |
[499] | |
[530] | |
[560] | |
[568] | |
[571] | |
[587] | |
[596] | |
[629] | |
[645] | |
[653] |
[661] | |
The Commonwealth’s loss in the interrupted supply counterfactual | [664] |
The Commonwealth loss in the continuous supply counterfactual | [666] |
[695] | |
[698] |
NICHOLAS J:
1 Clopidogrel is a medication which inhibits blood platelet aggregation (ie. the formation of blood clots). It is a drug that, in Australia, can only be prescribed by medical practitioners. It is usually prescribed to patients who have suffered a heart attack or stroke, are at risk of suffering a heart attack or stroke, or who suffer from some other condition that is treated by prescription of a blood platelet aggregation inhibitor. It is a “blockbuster” drug that generated worldwide annual sales equivalent to more than US$1 billion revenue for the Sanofi group of companies (“the Sanofi Group”) and the Bristol-Myers Squibb group of companies (“the BMS Group”).
2 Clopidogrel, in the form of hydrogen sulfate, has been supplied in Australia in tablet form by Sanofi Australia Pty Ltd (“Sanofi Australia”) under the brand name Plavix and also by Bristol-Myers Squibb Australia Pty Ltd (“BMS Australia”) under the brand name Iscover. Both products were registered on the Australian Register of Therapeutic Goods (“ARTG”) on 2 December 1998 and listed on the Pharmaceutical Benefits Scheme (“PBS”) on 1 November 1999.
3 There is a global co-marketing arrangement in place between the Sanofi Group and the BMS Group in relation to the marketing and sale of clopidogrel around the world. In Australia, these arrangements provided for co-marketing of clopidogrel by Sanofi Australia under the Plavix brand and by BMS Australia under the Iscover brand.
4 An essential step that must be taken before a drug can be marketed and supplied in Australia is to obtain approval from the Secretary of the Department of Health (“Department”) or a Delegate in the Therapeutic Goods Administration (“TGA”) for registration on the ARTG.
5 Clopidogrel products sponsored by Apotex Pty Ltd (“Apotex Australia”) were registered on the ARTG on 21 August 2007. The effect of these registrations was to allow Apotex Australia to supply such products under the brand names “Genrx Clopidogrel”, “Chemmart Clopidogrel” and “Terry White Chemists Clopidogrel” in blister packs, and “Apo-Clopidogrel” in blister packs and bottles.
6 After a sponsor secures ARTG registration, an application can be made to list the registered brand on the PBS. The PBS listing of a brand of pharmaceutical item usually provides a substantial benefit to the supplier or sponsor, essentially because the Commonwealth subsidises, often heavily, the cost of supplying PBS listed medicines to patients. PBS listing was an essential component of Apotex Australia’s plan to launch its generic clopidogrel products in Australia.
7 On 16 August 2007 Apotex Australia, which was until 20 September 2007 called GenRx Pty Ltd, commenced a proceeding in this Court against Sanofi-Aventis (“Sanofi”) (“the patent proceeding”) in which it sought an order revoking Australian Patent No 597784 for clopidogrel (“the Patent”) registered in the name of Sanofi. On 17 September 2017 Sanofi filed a cross-claim against Apotex Australia in the patent proceeding seeking injunctive and other relief for threatened infringement of the Patent.
8 The Patent was for the dextro-rotatory enantiomer of methyl alpha-5 (4,5,6,7-tetrahydro (3,2-c) thieno pyridyl) (2-chlorophenyl)-acetate, (commonly known by the non-proprietary name “clopidogrel”), a process for its preparation, and pharmaceutical compositions containing it.
9 Some drugs can be produced in different salt forms which may have advantages over their parent molecules. In particular, a drug salt may be more stable or easier to administer than its non-salt parent molecule, even though they are therapeutically equivalent. Claim 1 of the Patent encompassed clopidogrel in all of its pharmaceutically acceptable salts. Each of claims 2 to 5 covered particular forms of salt. The active pharmaceutical ingredient in Plavix and Iscover and Apotex Australia’s generic clopidogrel products is clopidogrel hydrogen sulfate which is the compound specified in claim 3.
10 In early September 2007, Apotex Australia applied for PBS listing of its clopidogrel products from 1 December 2007. It was soon after informed that its application had been lodged too late for its clopidogrel products to be listed on 1 December 2007. This led Apotex Australia to withdraw the application. The next available date for a listing of a first generic brand of clopidogrel, which would result in a change to the price of the existing listed brands of the drug (ie. the Plavix and Iscover brands), was 1 April 2008. An application for listing on that date had to be made by 1 December 2007.
11 On 25 September 2007 Sanofi obtained from Gyles J an interlocutory injunction (“the interlocutory injunction”) against Apotex Australia restraining it from infringing the Patent (including) by importation or sale of pharmaceutical products which had clopidogrel as their active ingredient. The interlocutory injunction was expressed to operate until the determination of the proceeding or further order. Sanofi gave the usual undertaking as to damages to the Court in support of the interlocutory injunction.
12 Apotex Australia also gave to the Court an undertaking (“the first Apotex undertaking”) that it would not apply to list its clopidogrel products on the PBS until the determination of the proceeding or further order. Sanofi did not give any undertaking as to damages in return for the first Apotex undertaking.
13 On 20 February 2008 Spirit Pharmaceuticals Pty Ltd (“Spirit”) also commenced a proceeding (“the Spirit proceeding”) against Sanofi, Sanofi-Aventis US LLC (“Sanofi US”), and Bristol-Myers Squibb Investco LLC (collectively “Sanofi BMS” or “the Sanofi BMS companies”) seeking revocation of the Patent. On 7 March 2008 Gyles J ordered that the two proceedings be heard together.
14 The trial of the patent proceeding before Gyles J commenced on 28 April 2008. Reasons for judgment were published by his Honour on 12 August 2008. Final orders were made by his Honour on 19 August 2008 revoking various claims of the Patent. Importantly, however, Gyles J found that claims 2 to 5 were valid including, in particular, claim 3 which was directed to clopidogrel as hydrogen sulfate. His Honour granted Sanofi a final injunction on 19 August 2008 (“the final injunction”) restraining Apotex Australia from infringing that claim. His Honour also noted in the relevant minute of order that Apotex Australia was released from the first Apotex undertaking. There was an appeal filed by Apotex Australia and a cross-appeal filed by the Sanofi BMS companies against his Honour’s orders. An appeal and a cross-appeal were also filed against Gyles J’s orders in the Spirit proceeding.
15 On 15 September 2008 various interlocutory undertakings were given to a Judge of the Court (Moore J) to operate until further order or the determination of the appeal. The appeal was heard by the Full Court of the Federal Court (Bennett, Emmett and Middleton JJ) in February 2009. On 29 September 2009 the Full Court delivered its reasons for judgment in the appeal. On 13 October 2009 the Full Court made orders allowing Apotex Australia’s appeal and setting aside the final injunction. The Full Court also ordered that the Patent be revoked.
16 Further undertakings were provided to the Full Court by the Sanofi BMS companies and Apotex Australia pending the determination of an application made by Sanofi for special leave to appeal to the High Court. That application was heard and refused on 12 March 2010. The first generic clopidogreneric clopidogrel product to be listed on the PBS was the clopidogrel Sandoz brand of clopidogrel products by Sandoz Pty Ltd (“Sandoz”) on 1 April 2010. Apotex Australia’s clopidogrel products were not listed on the PBS until 1 May 2010.
17 On 4 May 2010 Apotex Australia and two related entities, Apotex Inc (“Apotex Canada”) and Apotex Research Private Limited (“ARPL”) filed a notice of motion in the patent proceeding seeking orders against the Sanofi BMS companies for the payment of compensation pursuant to the various undertakings given to the Court in support of the interlocutory injunction and other undertakings that Apotex Australia subsequently gave to the Court which I will refer to in greater detail later in these reasons.
18 The compensation claims made by Apotex Australia, Apotex Canada and ARPL were discontinued by those companies by a notice of discontinuance filed on 7 November 2014 pursuant to a settlement deed entered into between those companies and Sanofi BMS companies on 4 November 2014 (“the Settlement Deed”).
19 In this proceeding, which was commenced by interlocutory application filed in the patent proceeding on 11 April 2013 (ie. more than three years after the High Court refused special leave), the Commonwealth, as applicant, seeks orders requiring the Sanofi BMS companies to pay to it compensation for the loss said to have been suffered by the Commonwealth as a result of Apotex Australia having been prevented from supplying its generic clopidogrel products in Australia and obtaining a PBS listing of such products on 1 April 2008.
20 The Commonwealth contends that the interlocutory injunction granted by Gyles J had the effect of delaying significant reductions in the payments it was obliged to make to pharmacists under the PBS for the supply of Plavix and Iscover and a number of related combination products between 2008 and 2014.
21 The cost to the Commonwealth of subsidising the supply of clopidogrel under the PBS in the financial year ended 30 June 2008 was approximately $171 million, making clopidogrel the third most heavily subsidised prescribed drug available under the PBS during that financial year. The Commonwealth contends that the listing of Apotex Australia’s clopidogrel brands on the PBS on 1 April 2008 would have triggered an automatic and immediate statutory reduction of 12.5% to the existing listed prices for Plavix and Iscover with the ex-manufacturer price for Plavix and Iscover reduced from $64.94 a pack to $56.82. In addition, the Commonwealth contends that there would have been a further 2% statutory price reduction on 1 August 2009, and further price reductions from 1 April 2010 arising from the operation of the system of mandatory price disclosure that was introduced into the National Health Act (1953) (Cth) (“NHA”) with effect from 1 August 2007.
22 The total amount of compensation claimed by the Commonwealth is approximately $325 million excluding interest and costs. Of this, approximately $51 million relates to mandatory price reductions that would have automatically taken effect on 1 April 2008 had Apotex Australia obtained and maintained a PBS listing of its clopidogrel products with effect from that date. The balance of the claim relates to further price reductions that the Commonwealth says would have occurred when the first of the relevant price disclosure price reductions would have first taken effect from 1 April 2010.
23 The loss claimed by the Commonwealth consists of the difference between:
the amount paid by the Commonwealth in respect of the supply of clopidogrel products under the PBS and the Repatriation Pharmaceutical Benefits Scheme (“RPBS”) since 1 April 2008; and
the amount that would have been paid by the Commonwealth in respect of the supply of those products under the PBS and the RPBS since 1 April 2008 if Apotex’s clopidogrel products had been listed on the PBS from that date.
24 There are three different components that make up the difference claimed by the Commonwealth (exclusive of interest):
a total of $50,718,312 in respect of mandatory statutory price reductions that would have occurred on 1 April 2008 and 1 August 2009;
a total of $215,922,465 in respect of price disclosure price reductions that would have occurred between 1 April 2010 and 31 December 2014;
a total of $58,307,301 in respect of payments made by the Commonwealth in respect of clopidogrel with aspirin combination products in the period 1 December 2009 to 31 March 2016.
25 Much of the evidence in the proceeding is concerned with the issue of whether Apotex Australia’s clopidogrel products would have been listed on the PBS on 1 April 2008 but for the interlocutory injunction granted by Gyles J.
26 There are two specific questions that arise in relation to that issue. The first is whether the evidence establishes that Apotex Australia would have applied for a PBS listing of its clopidogrel products by 1 December 2007 so that they would be listed on the PBS from 1 April 2008. The second question is whether the Minister, or a Delegate, would have accepted such an application and listed those products on the PBS with effect from 1 April 2008.
27 It is common ground that both questions are to be decided on the balance of probabilities. It is also common ground that if either of these questions is answered in the negative, then the Commonwealth’s application for compensation must fail.
28 Another issue concerns the first Apotex undertaking whereby Apotex Australia undertook not to apply for a PBS listing of its clopidogrel products until the determination of the patent proceeding. As previously mentioned, this undertaking was not supported by any cross-undertaking as to damages. For reasons that will be further explained, Sanofi BMS contends that the existence of the first Apotex undertaking is fatal to the Commonwealth’s case because it was the first Apotex undertaking rather than the interlocutory injunction that prevented Apotex Australia from applying to list its clopidogrel products on the PBS.
29 Another issue concerns the final orders made by Gyles J on 19 August 2008. There is a contest between the parties as to the significance to be attributed to those orders in the counterfactual analysis. The Commonwealth contends that the appropriate counterfactual analysis (which the parties referred to as the “continuous supply counterfactual”) requires that it be assumed that:
there was no interlocutory injunction or final injunction preventing Apotex Australia from supplying its clopidogrel products;
Apotex Australia’s clopidogrel products had been listed on the PBS since 1 April 2008 and had been on the market from or shortly before that date.
30 There is an issue between the parties as to whether that is the appropriate counterfactual analysis and, even if it is, whether Apotex Australia would have continued supplying its clopidogrel products once the outcome of the trial before Gyles J became known.
31 There is also an issue as to whether, in what Sanofi BMS contended was the appropriate counterfactual analysis (which the parties referred to as the “interrupted supply counterfactual”) the Commonwealth would have responded to any cessation of supply by not only delisting the Apotex clopidogrel products from the PBS, but also by restoring the ex-manufacturer price for Plavix and Iscover to the level that it was before the 12.5% statutory price reduction took effect on 1 April 2008, which is the date on which the Commonwealth says Apotex Australia’s clopidogrel products would have been listed but for the interlocutory injunction.
32 There is a further issue as to what effect the listing of Apotex Australia’s clopidogrel products on the PBS on 1 April 2008 may have had on price negotiations between the Commonwealth and Sanofi Australia and BMS Australia in relation to the clopidogrel with aspirin combination products sold by Sanofi Australia under the CoPlavix brand and by BMS Australia under the DuoCover brand.
33 Other issues that arise in the proceeding include the questions whether the Commonwealth was a person adversely affected by the operation of the interlocutory injunction, whether the Commonwealth, as a body politic, has suffered any compensable loss or damage, and whether any such loss or damage was a direct and reasonably foreseeable consequence of the interlocutory injunction.
34 There are a number of other matters that were relied upon by Sanofi BMS in support of their contention that the Commonwealth’s claim to compensation should be rejected (either in whole or substantial part) on discretionary grounds. The matters relied upon included the Commonwealth’s delay in bringing its application, whether considered either alone or in combination with what Sanofi BMS contended would have involved, in any counterfactual scenario in which Apotex’s clopidogrel products were launched by Apotex Australia in March or April 2008, an infringement by Apotex Australia of Sanofi Australia’s copyright in product information documentation, and an infringement of Sanofi’s Canadian Patent No 1336777 (the “Canadian Patent”) by Apotex Canada.
35 Other issues in the proceeding are relevant to the quantum of the Commonwealth’s damages claim. The principal question to be decided in relation to quantum is what market share Apotex Australia was likely to have captured had it continuously supplied its clopidogrel products from 1 April 2008.
THE PHARMACEUTICAL BENEFITS SCHEME
Listing of a medicine on the PBS
36 The PBS is a scheme for subsidising medicines. It is not necessary for a medicine sold in Australia to be listed on the PBS. Medicines that are not listed on the PBS are not subsidised and are known as either “private prescriptions” or “over-the-counter medicines”. Clopidogrel products have been subsidised on the PBS since 1 November 1999.
37 The PBS legislation is found in Pt VII of the NHA. Relevantly, s 85(1) provides: “Benefits shall be provided by the Commonwealth, in accordance with this Part, in respect of pharmaceutical benefits”. Section 85(2) provides that the drugs and medicinal preparations in relation to which Part VII applies are “declared by the Minister, by legislative instrument” pursuant to one of the limbs of that subsection.
38 Section 85(2AA) empowers the Minister by legislative instrument to revoke or vary a declaration under s 85(2) in relation to a drug or medicinal preparation. However, before doing so the Minister must obtain the advice in writing of the Pharmaceutical Benefits Advisory Committee (“PBAC”) in relation to the proposed revocation or variation: s 85(2AB). The PBAC is established by s 100A of the NHA. Its functions include making recommendations to the Minister from time to time as to the drugs and medicinal preparations which it considers should be made available as pharmaceutical benefits under Part VII: s 101(3A). PBAC is required to give consideration to the effectiveness and cost of therapy when making such recommendations: s 101(3A).
39 The relevant legislative instruments are referred to as “Listing Instruments” and are made or amended monthly. Listing Instruments do not record the price paid by the Commonwealth to dispensers, but dispensed prices under the PBS are included in a document published by the Department of Health called the Schedule of Pharmaceutical Benefits (“PBS Schedule”). The PBS Schedule is updated monthly to incorporate revisions made in or to the Listing Instruments.
40 Any new therapeutic good (including a generic brand of a drug) must be registered on the ARTG before it can be generally marketed in Australia. However, there are no requirements in the NHA concerning the information that has to be provided in support of an application to list a generic brand of a listed drug on the PBS. At the relevant time, an application for listing a generic brand was not required to be considered by the PBAC. Such applications were referred to the Pharmaceutical Evaluation Branch (“PEB”) of the Department for consideration.
41 For any applications to list generics which would result in a change to the price subsidised by the Commonwealth for a drug, there were, at the relevant time, three application deadlines and three listing dates (1 April, 1 August and 1 December) each year. Applicants seeking to list a generic brand were required to furnish to PEB a letter of application (for which there was no specified form); a completed “Application to list a Drug or Medicinal Preparation as a Pharmaceutical Benefit” form; a completed “Request for Price Alteration” form; a copy of the letter from the TGA approving the entry of the product in the ARTG; a copy of the current Certificate of Medicine Registration or Certificate of Listing for the product issued by the TGA; a copy of the current product information approved by the TGA, if applicable; a copy of the primary pack label or final artwork; and written assurance that stock of the product will be available on the proposed date of listing in the PBS Schedule.
42 Applicants usually also provided a completed form entitled “Notification of Responsible Person under National Health Act 1953” which, from 1 April 2008, confirmed that the applicant was the responsible person under s 84AF seeking a determination from the Minister. They also had to request the TGA to provide to the PEB a statement indicating that it is appropriate for an equivalence indicator to be shown in the PBS Schedule and against which other brands (ie. a statement of bioequivalence of the generic brand with the existing listed brands).
43 Applicants were also instructed to contact the Director, Pharmaceutical Pricing and Estimates Section, of the PEB in relation to the pricing of the new brand, and to check the “reference pricing groups” document to determine whether the 12.5% price reduction policy may affect the drug for which the application is being made.
44 The Commonwealth submitted that, in practice, applicants seeking PBS listing for a generic brand of a pharmaceutical item already listed on the PBS were approved as a matter of course subject to them complying with the administrative requirements referred to above. It further submitted that if PEB requested a Delegate to list a generic brand by executing the relevant Legislative Instrument, the Delegate invariably did so. The Commonwealth also submitted that from 1 August 2007, the question of whether the generic brand seeking listing might be the subject of a patent (or the subject of any patent infringement or revocation proceedings) was not relevant and not considered by the Delegate. It also submitted that assurances of supply were taken at face value. I will return to these submissions later in these reasons.
45 Important changes were made to the NHA by the National Health Amendment (Pharmaceutical Benefits Scheme) Act 2007 (Cth) (“the 2007 Amendment Act”). The 2007 Amendment Act received royal assent on 28 June 2007 and these changes (“the 2007 Amendments”) took effect from 1 August 2007. Relevantly, the changes made provision for the creation of the F1 and F2 formularies and various mandatory price reductions that would occur (inter alia) when a pharmaceutical item moved from F1 to F2. From 1 August 2007 to 30 November 2010 F2 was further divided into Part A of F2 and Part T of F2: see s 84AD. On 1 December 2010 Part A and Part T were merged resulting in a singular F2 formulary.
46 According to the Second Reading Speech for the National Health Amendment (Pharmaceutical Benefits Scheme) Bill 2007 (Cth) in the House of Representatives (“the Second Reading Speech”):
One of the main changes proposed in this bill is the separation of PBS medicines into two formularies – F1 and F2 – each with different pricing arrangements. Medicines where there is only a single brand listed will be referred to as F1. It will contain both on-patent and off-patent medicines that are not substitutable with other brands or medicines. With this formulary, there will be no mandatory price reductions, and existing price linkages will be retained within this group. This means that single-brand medicines will retain their higher prices until they become subject to competition, providing companies with a greater certainty about the prices of these medicines. This allows the Australian government to ensure these medicines remain subsidised by the PBS, hence continuing their availability at affordable prices. Medicines where there are brands listed and groups of medicines that are interchangeable between patients will be referred to as F2.
47 Since 1 August 2007, the listing of a new brand of an existing listed pharmaceutical item has various price consequences. The listing of the new brand of a pharmaceutical item (eg. a generic version of the listed pharmaceutical item) triggers an automatic and immediate 12.5% price reduction: ss 99ACH. While there are various exceptions to this, it is common ground that none of them are relevant for the purpose of the present proceeding. Prior to 1 August 2007, the listing of a new brand of pharmaceutical item did not automatically trigger a 12.5% price reduction; whether or not such a price reduction would be imposed was a matter left to the discretion of the Minister or Delegate.
48 It appears that a 12.5% reduction was never imposed prior to 1 August 2007 where the generic supplier chose to launch its new brand “at risk” (ie. in circumstances where the pharmaceutical item was within the scope of an unexpired patent owned by the innovator of the pharmaceutical item). However, the 12.5% discount was imposed if the patent had expired or the generic brand was to be supplied by or with the licence of the owner of the patent. Hence, the 12.5% discount applied to what were referred to in the industry as “autogenerics” or “pseudogenerics” which were generic products marketed by or with the licence of the innovator. From 1 August 2007, the 12.5% price reduction was imposed automatically when the first generic brand was listed on the PBS irrespective of whether or not it was launched at risk.
49 From 1 August 2007, the NHA imposed supply obligations with respect to newly listed brands: see Part VII Div 3C which include those provisions that relate to what is usually referred to as the “guarantee of supply”. According to the Second Reading Speech the guarantee of supply would:
… guarantee the supply of all new brands listed on the PBS from 1 August 2007 and the already listed medicines for which price reductions are offered to all patients who require these medications. This will significantly help patients on these medications, as the guarantee of supply period will be 24 months, or until another new brand is listed, or a further price reduction is offered for another already listed medicine. Any company which believes that it will be unable to supply, or has failed to supply, will be required to notify the minister. A criminal penalty will apply for failure to notify the minister. There is also a $33,000 fine for a corporation.
Companies which fail to supply or which are unable to supply may have that brand or other brands on the PBS delisted or they may be refused the listing of new brands. These tough measures introduced with respect to noncompliance with the new price disclosure and supply guarantee measures are another advantage for patients, as they aim to ensure that companies adhere to their commitments. Patients have a right to know that the prices that they are paying for their medications are truly reflective of manufacturing costs. They also have a right to have a continual, guaranteed supply of medications available to them. Patients do not need the added stress of costs often incurred by having to change their medications because they are too expensive or unavailable. These reforms aim to further ensure patients’ quality of life by increasing the accountability and compliance of companies which supply the medication.
50 Section 99AEB provided, subject to some specific exceptions, that the responsible person for a newly listed brand (“the guaranteed brand”) “must supply” that medicine for 24 months beginning on the day that the brand is listed on the PBS: see ss 99AEC-99AED. If the responsible person failed to supply, or was unable to supply the brand (as defined by ss 99AEE and 99AEF respectively), or forms the belief that they will fail to supply or be unable to supply, then they must notify the Minister as soon as possible: s 99AEG. Failure to notify the Minister in accordance with the requirements of ss 99AEG is a criminal offence.
51 Importantly, s 99AEB provided that the period of the guarantee of supply (ie. “the guaranteed period”) might end sooner than it otherwise would in a number of situations including upon the listing of another generic brand of the same pharmaceutical item. Hence, if the innovator of the pharmaceutical item obtained a listing of an autogeneric product in response to the prior listing of a generic brand then the guarantee of supply in respect of that generic brand would no longer apply to it. It follows that the relevant guaranteed period might last for considerably less than 24 months.
52 If during the guaranteed period the responsible person failed to supply, or was unable to supply the guaranteed brand on one or more occasions, it was open to the Minister to cancel the PBS listing for that brand or any other listed brand of the responsible person. The Minister could also refuse to list any other brands of the responsible person on the PBS. Those and other actions that could be taken by the Minister are fully described in s 99AEH(2). Section 99AEH(3) refers to various matters that the Minister may take into account when considering what action to take pursuant to s 99AEH(2) which include:
the number of times the responsible person failed to supply, or was unable to supply the guaranteed brand;
the period in which those failures or inabilities occurred;
the duration of those failures or inabilities;
the reasons for those failures or inabilities; and
whether those reasons are, in the Minister’s opinion, reasonable.
53 In the event that the Minister exercises the power under s 99AEH revoking or varying a determination under s 85(6) to issue a Listing Instrument in relation to a brand of a pharmaceutical item then there are various actions that he or she may also take to ameliorate the effects that were triggered by the listing of that brand. These include making a determination under s 99AEI to increase the price of a brand of a pharmaceutical item and, under s 99AEJ, restoring a pharmaceutical item to F1 where the requirements of that section are satisfied.
54 The Act provides for the listing of pharmaceutical items that contain more than one drug. Under s 84 a “combination item” is a pharmaceutical item that has a drug that contains at least two other drugs, at least one of which is a PBS listed drug. The medicine containing the drug clopidogrel with aspirin is a combination item.
55 Since 1 August 2007, combination items where there is only one listed brand have not been allocated to either F1 or F2: see s 85AB(5). The Department maintains a (non-statutory) list of combination items known as the Single Brand Combination Drug list (“SBCD list”).
Co-marketed pharmaceutical items
56 Whether two or more brands of a pharmaceutical item are co-marketed brands for the purpose of the Act is determined by reference to s 84AE. Co-marketing involves two responsible persons, usually the innovator and one of its licensees, co-marketing a pharmaceutical item for which there is no generic competition under different brands.
57 As at 1 August 2007 co-marketed brands of a pharmaceutical item, including combination items, were treated as if they were one brand of the pharmaceutical or combination item: s 84AE(1).
58 As previously mentioned, applicants for a PBS listing of a new brand of a pharmaceutical item are required to provide what is referred to as an “assurance of supply”. The assurance of supply (unlike the guarantee of supply) is a purely administrative requirement imposed by the Department; there is nothing in the NHA that requires that it be given as a condition of listing. The form of the assurance of supply required from an applicant prior to listing on the PBS has evolved over time. From June 2005 to December 2006, the PBAC Guidelines required applicants to provide to the Department written assurance that stock of the product would be available on the proposed listing date. Since December 2006, the Department has required applicants to provide a written assurance that sufficient stock of their brand of the relevant pharmaceutical item would be available on the proposed date of listing to meet anticipated demand.
Payment by the Commonwealth for listed products
59 The amount a patient pays for pharmaceutical products subsidised under the PBS is usually set by the NHA. The amount paid by patients (referred to as the “Patient co-payment”) for clopidogrel has always been capped. The general co-payment as at 1 April 2008 was $31.30 and the concessional co-payment as at that date was $5.00. The amount of the co-payments is adjusted each year. By 1 January 2016 the general co-payment was $38.30 and the concessional co-payment was $6.20.
60 The remainder of the cost of a PBS-subsidised product is paid by the Commonwealth directly to the dispenser. Section 99 provides that a pharmacist or approved medical practitioner who has supplied a pharmaceutical benefit is “entitled to be paid by the Commonwealth … an amount equal to the Commonwealth price of the pharmaceutical benefit as at the time of the supply” (or, if certain conditions are satisfied, an amount based on the Commonwealth price), where the “Commonwealth price” is as defined in s 84(1).
Setting of the AP2P and AEMP and price reductions under the PBS
61 Section 85AD empowers the Minister and the responsible person for a listed brand of a pharmaceutical item to agree the appropriate maximum price for sales of the brand of the pharmaceutical item by wholesalers or manufacturers to approved pharmacists. At all material times, price agreements existed between the Minister and Sanofi Australia (and its predecessors) and BMS Australia (and its predecessors) in respect of the clopidogrel products Plavix and Iscover respectively. The price agreed, until 30 September 2012, was known as the AP2P (ie. the approved price to pharmacists) and since that date, the AEMP (ie. the approved ex-manufacturer price).
62 The AP2Ps and AEMPs set in price agreements can be, and were, reduced by virtue of statutory provisions that did not require new agreements to be executed. These provisions are found in Div 3A and Div 3B of Part VII of the NHA.
63 There are three kinds of price reductions to the AP2P/AEMP under the NHA that are relevant: statutory price reductions, negotiated price reductions (as also known as “administrative price reductions”) and statutory price disclosure price reductions (“PDP reductions” or “PDPR”).
12.5% statutory price reduction on first listing of a generic
64 Pursuant to s 99ACB, upon the listing of the first generic brand of an existing listed drug, the AP2P of the existing brand of the drug would be reduced by 12.5% (with the price of the first generic also set at the same level).
65 As at 31 March 2008, the AP2P for a pack of clopidogrel was $69.82; the effect of a 12.5% reduction on 1 April 2008 would have been to reduce it to $61.09. A reduction in the AP2P would not have affected the dispensing fee for clopidogrel (which was a fixed dollar amount) but would have reduced the amount paid under the dispenser mark-up, as this was a percentage of the AP2P.
Other statutory price reductions
66 In 2008, once a mandatory 12.5% statutory price reduction took effect, further 2% price reductions automatically followed on nominated days. As at 1 April 2008, these were 1 August 2008, 1 August 2009 and 1 August 2010: s 99ACI. The third 2% price reduction would only occur if there was no prior price disclosure price reduction resulting from the listing of the first generic brand. Amendments made to the NHA in 2010 provided that these 2% reductions also occur on 1 February 2011 in certain circumstances: (see s 99ACF, s 99ACIA and Schedule 2 of the National Health Amendment (Pharmaceutical Benefits Scheme) Act 2010 (Cth)).
67 From 2008 if a combination item was listed on the PBS and recorded on the SBCD list, and a listed component drug of the combination item became subject to a statutory price reduction, including under s 99ACB, then pursuant to s 99ACC the existing price for the combination item ceased to have effect the day before the statutory price reduction took effect and the Minister could then agree a new price for the brand(s) of combination item.
Negotiated (administrative) price reductions
68 In addition to changes to AP2Ps and AEMPs dictated by statute, it is also possible for the Minister and the responsible person to agree a price change for a particular listed brand. This occurred twice in respect of clopidogrel.
69 The first occasion was on 1 February 2009, when the listing conditions for clopidogrel were expanded to cover a new therapeutic use. The Pharmaceutical Benefits Pricing Authority (“PBPA”) determined that a reduction in the price of clopidogrel would be a reasonable offset for the increased sales that would result from the extended listing conditions, and sent letters to Sanofi Australia and BMS Australia suggesting price reductions. Each of Sanofi Australia and BMS Australia agreed to a 3% reduction in price.
70 The second occasion was on 1 December 2011, when Alphapharm Pty Limited (“Alphapharm”) negotiated a 20% price reduction with the Minister. The Minister invited the responsible persons of other listed brands to agree to similar reductions or risk having their brands delisted. Sanofi Australia and BMS Australia agreed a corresponding price reduction.
Price disclosure price reductions
71 Between 1 August 2007 and 30 November 2010, a new generic brand that was listed on the PBS was subject to mandatory price disclosure requirements under s 99AD: see s 99ADD. Regulations specified information that had to be supplied to the Department, which used the information to calculate what is known as the Weighted Average Disclosed Price (“WADP”) for a pharmaceutical item (or group of pharmaceutical items with the same listed drug and same manner of administration): see s 99ADB(4)-(6) and reg 37F of the National Health (Pharmaceutical Benefits) Regulations 1960 (Cth).
72 WADP was calculated according to a formula which took into account the volume of sales of the particular brands the subject of price disclosure and the extent to which the responsible persons of those brands offered discounts and other incentives which resulted in the price actually paid by pharmacists being less than the AEMP plus the applicable wholesale mark-up.
73 The responsible person for existing listed brands could elect to comply with the price disclosure requirements for those brands: s 99ADE. Once made, the election could not be revoked by the responsible person but the Minister could (at the responsible person’s request) determine to revoke the election (with prospective effect) if the listing of the generic brand that activated the PDPR regime was varied or revoked: s 99AEL.
74 On 23 March 2010 BMS Australia elected to participate in the PDPR regime for Iscover before the listing of the first generic clopidogrel product took effect on 1 April 2010. Sanofi Australia elected to participate for Plavix on 28 April 2010.
75 If the WADP as calculated and recorded in a determination by the Minister was more than 10% below the current AEMP, then the AEMP was reduced to the WADP. The AP2P was also reduced in accordance with a formula set out in Reg 37C. The effect of the PDPR regime was, therefore, to impose mandatory price reductions aimed at ensuring that the subsidy paid by the Commonwealth under the PBS for a particular drug more closely reflected the price actually paid for that drug by pharmacists. The earliest date on which a PDP reduction can take effect is two years after the PDP reduction regime is activated by the listing of a generic product.
76 If Apotex Australia’s clopidogrel products had been listed on 1 April 2008, all listed clopidogrel products would have moved from F1 to F2 on that date. In fact this did not occur until 1 April 2010 when Sandoz’s clopidogrel products were first listed on the PBS. PDP reductions consequent upon that listing did not occur until 1 April 2012, that being the earliest date that such price reductions could have taken effect. There was a 32.62% reduction in the AEMP (from $58.07 to $39.13) that took effect on 1 April 2012. Subsequent reductions took place on 1 August 2013, 1 April 2014 and 1 October 2014, at which point the AEMP reduced to $10.17.
The Repatriation Pharmaceutical Benefits Scheme
77 The RPBS is a subsidy scheme administered by the Department of Veterans’ Affairs for eligible veterans, war widows/widowers and their dependants. The pricing and reimbursement arrangements for the supply of pharmaceutical benefits under the PBS are automatically translated across to the RPBS. However, under the RPBS, the patient generally never pays more than the concessional rate of the patient co-payment.
ARTG REGISTRATIONS FOR GENERIC CLOPIDOGREL
78 Records produced by the TGA that are in evidence show that both Sandoz and Spirit applied for ARTG registration of generic clopidogrel products about twelve months after Apotex Australia commenced the patent proceeding. Applications were made by other generic suppliers in 2009 and 2010. As the following table shows, Sandoz’s clopidogrel products were the first to be registered after Apotex on 20 November 2009:
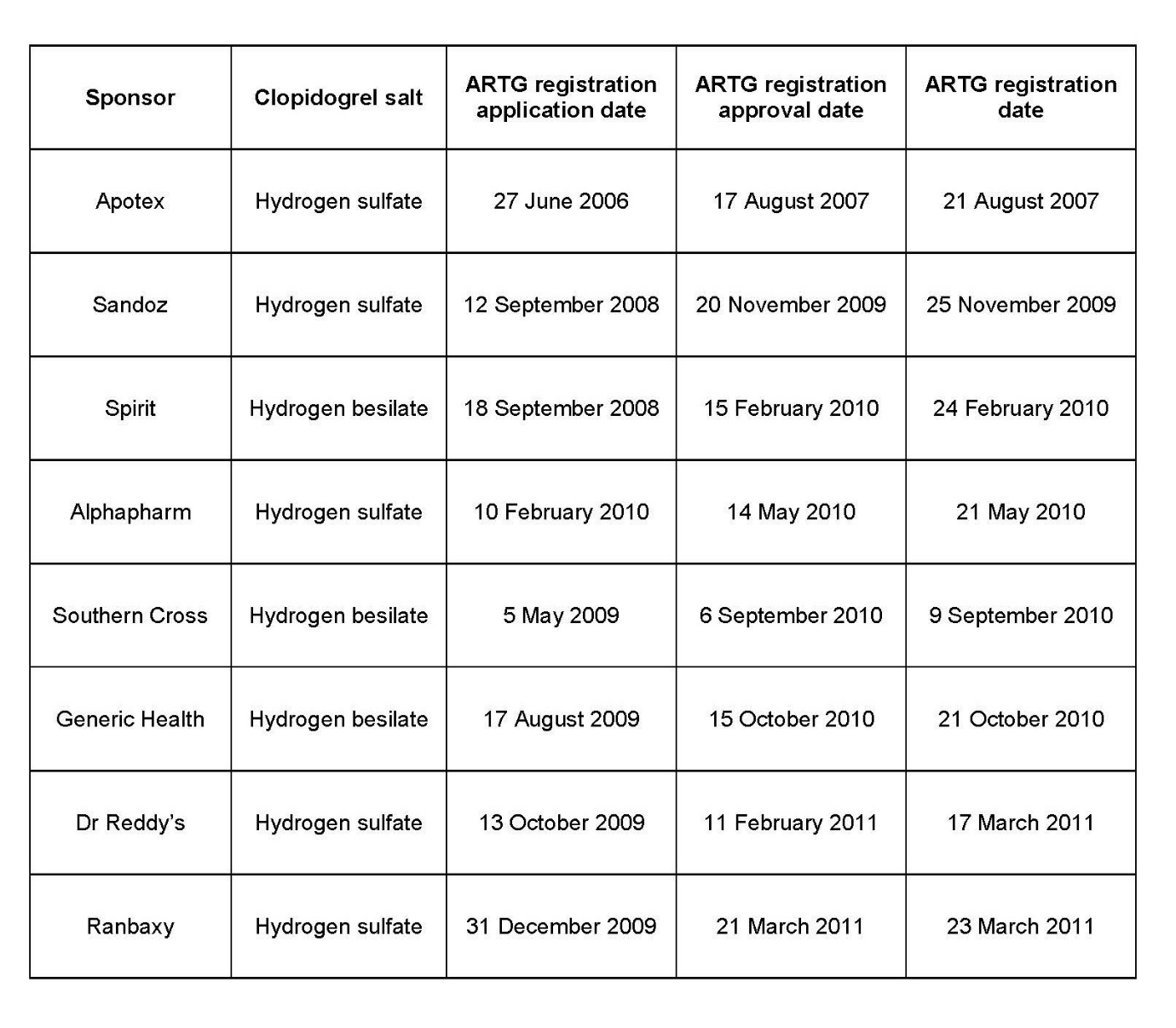
79 The evidence does not show why any of these other generic suppliers did not apply for ARTG registration any sooner than they did. In particular, it is not possible to say on the evidence whether any of them was deterred by the existence of the Patent and the prospect of being sued by Sanofi were they to have entered the market for clopidogrel in Australia or whether there is some other explanation for them not applying for ARTG registration sooner than they did. What is clear, however, is that, based on the ARTG application dates, no generic supplier apart from Apotex Australia could have obtained a PBS listing of a generic clopidogrel product on 1 April 2008.
THE PRINCIPAL PROCEEDING BETWEEN APOTEX AND SANOFI
Commencement of the patent proceeding
80 Apotex Australia commenced the patent proceeding by an originating application filed on 16 August 2007 seeking orders revoking the Patent. On 17 September 2007 Sanofi filed a defence and cross-claim.
81 By its cross-claim, Sanofi sought two separate injunctions, on a final basis, and also on an interim basis pending the determination of the proceedings. The first injunction sought by Sanofi (proposed order 1 in the cross-claim) was an injunction which, in broad terms, restrained manufacture and supply. The second injunction sought by Sanofi (proposed order 2 in the cross-claim) was an injunction restraining Apotex Australia from “taking any steps to obtain listing of any of the [Apotex Australia] Clopidogrel products under the pharmaceutical benefits scheme maintained by the Commonwealth under the National Health Act 1953 (Cth) [ie, the PBS]”.
82 The interlocutory hearing before Gyles J took place on 18 September 2007. The evidence includes the transcript of the hearing before his Honour and of a brief hearing on 25 September 2007 when his Honour made the interlocutory orders.
83 In its written submissions dated 18 September 2007 in support of its claim for interlocutory relief, Sanofi stated that it offered the usual undertaking as to damages in respect of both of its proposed interlocutory injunctions including that which would have prevented Apotex Australia from taking steps to list its clopidogrel products on the PBS.
84 During the course of the hearing before Gyles J on 18 September 2007, his Honour indicated that he did not see any proper basis on which the Court could prevent Apotex Australia from taking steps to list its clopidogrel products on the PBS. Senior Counsel for Apotex Australia, Mr DK Catterns QC, stated that his client’s position was that, if the Court was minded to restrain supply, “the question of needing to restrain us from doing anything with the PBS or restraining the PBS doesn’t arise”. That statement prompted the following exchange between his Honour and Mr Catterns QC:
MR CATTERNS: We would be prepared, if your Honour is against us and grants an injunction against us from selling, we will not apply to the PBS during the period of that restraint with liberty to apply.
HIS HONOUR: Yes. All right.
MR CATTERNS: Because, in short your Honour, it’s pointless for us, the question of needing to restrain us from doing anything with the PBS or restraining the PBS doesn’t arise. If your Honour were against us…
HIS HONOUR: Why would that be, why would that be, because - - -
MR CATTERNS: It’s of no value to us, your Honour. We don’t want to get listed, to be candid, we don’t want to be listed on the PBS if we can’t sell. It would damage our friends by bringing their price down twelve and a half per cent.
HIS HONOUR: Yes, that’s a risky thing anyway.
MR CATTERNS: Yes, it will only damage our friends, it’s of no benefit to us, and we will make enemies in the industry.
HIS HONOUR: Yes. All right. I’m not inquiring really why, I just want to know what the situation is.
MR CATTERNS: So, your Honour, we would be happy to – if your Honour, we would be unhappy, but if your Honour makes an injunction against us selling, we would agree on an appropriate undertaking that would fix that up.
HIS HONOUR: Yes. All right. Thank you.
85 This exchange is relied upon by the Commonwealth to show that, were it not for Gyles J’s decision to grant an interlocutory injunction, Apotex Australia would never have proffered the first Apotex undertaking not to take steps to list its clopidogrel products on the PBS.
86 On 21 September 2007, Gyles J published reasons for concluding that an interlocutory injunction should be granted provided that the undertaking as to damages was adequately secured. His Honour said in his reasons at [15]:
I am not satisfied the damages would be an adequate remedy if Sanofi succeeds in the suit, notwithstanding the offer on the part of GenRx to adequately secure a very substantial amount to cover any reasonable likely assessment of damages and offering other undertakings to ameliorate the effect of commencing to trade. It is put on behalf of Sanofi that the assessment of damages would be difficult, if not impossible, for reasons spelled out in detail with some force. No doubt the assessment of damages would be a formidable exercise but I am not persuaded that it would be as complex as suggested. It should not be all that difficult to assess the effect on the incumbent of a new entrant who would have to keep clear records of what took place. I do not see that task as being any more complex in principle than working out the damages which Sanofi would have to pay GenRx under the undertaking for damages for keeping it out of the market if revocation succeeds. However, I am much influenced by the effects of disturbing the status quo, particularly as it relates to the operation of the PBS. A new entrant in this field would have an effect which may be both unpredictable and irreversible. There is also likely to be interference with the trade patterns of Sanofi with its customers, both wholesale and retail, that may not be detectable or measurable in money terms. Sanofi has built up a considerable trade since 2003, and the establishment to go with it, as GenRx looked on. Temporary disturbance of that status quo is not justified …
87 His Honour’s observations in this paragraph were largely based on evidence from witnesses who made affidavits for Sanofi including, in particular, Mr Lindsay and Mr Dick which explained, amongst other things, the potential price effects if Apotex Australia were to obtain a PBS listing of its clopidogrel products and the losses Sanofi would suffer if the injunctive relief sought by it was not granted. Those losses would arise, according to their evidence, from a statutory reduction in the price to pharmacists for clopidogrel, the erosion of Sanofi’s clopidogrel sales to pharmacists and the erosion of Sanofi’s sales of other drugs to pharmacists. Mr Lindsay’s and Mr Dick’s evidence was also relied upon by Sanofi to show that the statutory price reductions may not be reversed (even if Sanofi succeeded at the trial) and that Sanofi’s potential losses were unpredictable and unquantifiable.
88 As the Commonwealth’s submissions in the present case correctly pointed out, all of that evidence concerning potential loss was predicated on the assumption that, if the interlocutory relief sought by Sanofi was not granted, Apotex Australia would obtain a PBS listing for its products.
Gyles J’s orders of 25 September 2007
89 On 25 September 2007 his Honour published his revised reasons for judgment which he had delivered orally the previous week. His Honour’s reasons for judgment show that he was satisfied that there was a substantial issue to be tried as to the validity of the Patent, but that he considered that the balance of convenience weighed in favour of granting an interlocutory injunction. It is apparent from the 25 September 2007 transcript that the parties had reached agreement with respect to the interlocutory relief that should be granted in light of his Honour’s reasons for judgment delivered orally the previous week. This included agreement in relation to the form of undertakings that were to be provided to the Court by both Apotex Australia and Sanofi.
90 The minute of the orders made by Gyles J on 25 September 2007 includes a notation of undertakings given to the Court on that date by Sanofi and Apotex Australia, an interlocutory injunction restraining Apotex Australia from infringing the Patent, and an order requiring Sanofi to provide security. Other orders made by his Honour provided for security, pleadings, discovery, the filing and service of affidavit evidence, and a final hearing before his Honour fixed to commence on 28 April 2008 for a period of two to three weeks.
91 It was common ground before me that by reason of s 72 of the Constitution, Gyles J’s appointment as a Judge of the Court expired on 22 August 2008. This is a matter that would most likely have been known to the parties’ legal representatives who would have understood that since the trial was to be before Gyles J, his Honour would be required to deliver judgment on or before that date.
92 Relevantly, the minute of orders made on 25 September 2007 state:
UPON the Respondent/Cross-Claimant undertaking to the Court to:
(a) submit to such order (if any) as the Court may consider to be just for the payment of compensation, to be assessed by the Court or as it may direct, to any person whether or not a party, adversely affected by the operation of Order 1 set out below or any continuation (with or without variation); and
(b) pay the compensation referred to in (a) to the person or persons there referred to.
THE COURT:
1. ORDERS that, pending the determination of the proceedings or further order, the Applicant/Cross-Respondent whether by itself, its directors, officers, servants, agents or otherwise, be restrained from infringing Australian Letters Patent No 597784 (the Patent) and, in particular, from engaging in the following acts within the patent area (as that term is defined in Patents Act 1990 (Cth)), without the license or authority of the Respondent/Cross-Claimant:
(a) making, selling or otherwise disposing of the products known as GenRx Clopidogrel, Apo-Clopidogrel, Chemmart Clopidogrel and Terry White Chemists Clopidogrel or any other pharmaceutical composition the active ingredient of which is clopidogrel bisulfate, a compound claimed in claims 1, 3, 10 and 11 of the Patent (collectively, the GenRx Clopidogrel Products);
(b) offering to make, sell or otherwise dispose of the GenRx Clopidogrel Products;
(c) using or importing the GenRx Clopidogrel Products;
(d) keeping the GenRx Clopidogrel Products for the purpose of doing any of the acts described in sub-paragraphs (a) to (c) above;
(e) authorising other people to engage in any of the acts described in sub-paragraphs (a) to (d) above.
2. NOTES that the Applicant/Cross-Respondent undertakes to the Court that, pending the determination of the proceedings or further order, it will not, whether by itself, its directors, officers, servants or agents or otherwise, take any steps to obtain listing of any of the GenRx Clopidogrel products under the pharmaceutical benefits scheme maintained by the Commonwealth under the National Health Act 1953 (Cth).
93 There are six important points to make about this order and various undertakings.
94 First, the interlocutory injunction referred to in numbered paragraph 1 of the orders operated “pending the determination of the proceedings or further order”. In the events that happened, the interlocutory injunction operated in accordance with its terms until final orders were made by Gyles J on 19 August 2008 at which time it ceased to operate.
95 Secondly, paragraphs (a)-(e) of the interlocutory injunction did not include, among the specified activities in respect of which Apotex Australia was expressly restrained, taking steps to list on the PBS. Nevertheless, the description of the particular acts in the interlocutory injunction is preceded by the general words “… be restrained from infringing …” the Patent and it is clear that the scope of the restraint could extend beyond the infringing activities specified in subparas (a)-(e) to the order.
96 Thirdly, although the interlocutory injunction restrained Apotex Australia from infringing the Patent, it would not have been an infringement of the Patent for Apotex Australia to apply to list its clopidogrel products on the PBS. This proposition derives from the Full Court’s decision in Warner-Lambert Company LLC v Apotex Pty Ltd (2017) 249 FCR 17 (“Warner-Lambert”) in which it was held that a person who makes an application to list a pharmaceutical product on the PBS does not thereby “exploit” a patented invention. It was Apotex Australia’s contention at the interlocutory hearings on 18 and 25 September 2007, and at the hearing on 19 August 2008 at which the form of the final orders was debated, that taking steps to obtain a PBS listing of a pharmaceutical product was incapable of constituting an act of patent infringement. But until the Full Court’s decision in Warner-Lambert was published, the correctness of that proposition was open to debate.
97 Fourthly, the undertaking given to the Court by Apotex Australia referred to in numbered paragraph 2 of the orders specifically prevented Apotex Australia from taking any steps to list its clopidogrel products on the PBS until the determination of the proceeding or further order. Importantly, however, that undertaking was not supported by any undertaking as to damages by Sanofi. The minute of order shows that Sanofi’s undertaking as to damages required it to submit to an order for compensation in favour of any person adversely affected by the operation of the interlocutory injunction but not the undertaking given to the Court by Apotex Australia. This is a critical point to which I will return.
98 Fifthly, the undertaking as damages given to Gyles J permitted the Court to make an order for compensation that “it may consider just … to any person whether or not a party, adversely affected by the operation of [the relevant order] …”. This language reflected the form of what by that time had become the usual undertaking as to damages that an applicant is required to give in this Court in return for an interlocutory injunction.
99 Sixthly, the usual undertaking as to damages given to Gyles J was given by Sanofi but not by Sanofi US or BMS. Neither of those entities was a party to the patent proceeding at that time and neither of them was bound by the undertaking as to damages given to his Honour by Sanofi.
Gyles J’s orders of 19 August 2008
100 At the time of granting the interlocutory injunction, Gyles J fixed the matter for a final hearing commencing 28 April 2008. The trial before Gyles J commenced on that date. Apotex Australia did not contest infringement. The principal issue in dispute was the validity of the Patent.
101 Reasons for judgment were published by Gyles J on 12 August 2008. His Honour upheld the validity of claims 2-5 of the Patent, but found the other claims to be invalid. His Honour held (inter alia) that claim 3 (which covered clopidogrel bisulfate, which was the active ingredient in Apotex Australia’s clopidogrel products) was valid.
102 On 14 August 2008, the proceeding was again before Gyles J for argument regarding the form of final orders. At the hearing on that date, Sanofi sought a final injunction restraining supply of Apotex Australia’s clopidogrel products, in terms similar to the interlocutory injunction, and also a final injunction restraining Apotex Australia from taking any steps to obtaining a PBS listing of those products. The latter form of injunction was sought by Sanofi on the basis that a listing would require a guarantee of supply which would constitute offering to sell and, therefore, an exploitation of the invention.
103 Apotex Australia resisted the making of a final injunction restraining the taking of steps to list on the PBS, and sought a release from the first Apotex undertaking, on the basis that taking such steps would not constitute exploitation. Gyles J refused to grant the injunction sought by Sanofi in respect of PBS listing and granted Apotex Australia the release it sought from the first Apotex undertaking. The transcript of the hearing before his Honour on 14 August 2008 clearly suggests that his Honour doubted that by applying for a PBS listing of its clopidogrel products, Apotex Australia would infringe the Patent.
104 Apotex Australia also sought from Gyles J a stay of the final injunction pending the determination of its appeal or, alternatively, an undertaking from Sanofi in lieu of such a stay allowing the final injunction to operate on condition that, if so directed, Sanofi would pay compensation to Apotex Australia for any damage suffered by it in the event its appeal was successful with the final injunction being discharged: see Minnesota Mining & Manufacturing Company v Johnson & Johnson Limited [1976] FSR 139 at 145 per Buckley LJ. It is apparent that Gyles J (quite understandably) regarded this as an exceedingly ambitious application given that Apotex Australia had been restrained by the interlocutory injunction since 25 September 2007. His Honour indicated that Apotex Australia’s application for a stay of the permanent injunction should be made to the Full Court.
105 On 19 August 2008 Gyles J made orders declaring that claims 2, 3, 4 and 5 of the Patent were valid and that Apotex Australia’s clopidogrel bisulfate products infringed claim 3. The final injunction granted by his Honour was in these terms:
The Applicant/Cross-Respondent whether by itself, its directors, officers, servants, agents or otherwise, be restrained from infringing claim 3 of the Patent and, in particular, from engaging in the following acts within the patent area (as that term is defined in Patents Act 1990 (Cth)), without the license or authority of the Respondent/First Cross-Claimant:
(a) making, selling or otherwise disposing of the products known as GenRx Clopidogrel, Apo-Clopidogrel, Chemmart Clopidogrel and Terry White Chemists Clopidogrel, each being pharmaceutical compositions the active ingredient of which is clopidogrel bisulfate, or any other pharmaceutical composition the active ingredient of which is clopidogrel bisulfate, a compound claimed in claim 3 of the Patent (collectively, the Apotex Clopidogrel Products);
(b) offering to make, sell or otherwise dispose of the Apotex Clopidogrel Products;
(c) using or importing the Apotex Clopidogrel Products;
(d) keeping the Apotex Clopidogrel Products for the purpose of doing any of the acts described in sub-paragraphs (a) to (c) above;
(e) authorising other people to engage in any of the acts described in subparagraphs (a) to (d) above.
106 Thus, the terms of the final injunction granted on 19 August 2008 (“the final injunction”) was in similar terms to the interlocutory injunction except that it was no longer expressed to operate until further order or the determination of the proceeding and was also expressed in terms which only restrained infringement of claim 3 of the Patent.
107 His Honour also ordered that claims 1, 6 to 9, 10 and 11 be revoked. That order was stayed until the final determination of any appeal subject to Sanofi providing a number of undertakings including “… not to threaten any person other than the Applicant in these proceedings or the Applicant, Spirit Pharmaceuticals Pty Ltd, in Federal Court Proceeding No. NSD 214 of 2008 with infringement of [those] claims ...”.
108 Gyles J also recorded, by a note included in the minute of orders, that the undertaking given by Apotex Australia to the Court on 25 September 2007 (ie. the first Apotex undertaking) ceased to have effect.
The undertakings given to Moore J on 15 September 2008
109 On 19 August 2008 Apotex Australia filed an appeal from Gyles J’s judgment and orders against (inter alia) the final injunction granted by his Honour based on claim 3. For reasons not clear from the evidence, Sanofi US and BMS were joined as second and third respondents to Apotex Australia’s appeal. On 8 September 2008 the Sanofi BMS companies filed a cross-appeal.
110 On 15 September 2008 Moore J made the following order by consent:
1. Upon the parties giving the undertakings noted in paragraphs 2 and 3 below, the security in the sum of A$40 million provided by the First Respondent pursuant to paragraph 3 of the orders made by Gyles J on 25 September 2007 in Federal Court of Australia Proceeding No NSD 1639 of 2007 remain in place pending the final determination of this appeal, as security for such compensation, if any, as the Court may consider should be paid to the Appellant should it be found to have been adversely affected by any of:
(a) paragraph 1 of the orders made by Gyles J on 25 September 2007 in Federal Court of Australia Proceeding No NSD 1639 of 2007;
(b) paragraph 2 of the orders made by Gyles J on 19 August 2008 in Federal Court of Australia Proceeding No NSD 1639 of 2007; and
(c) the undertakings of the Appellant noted in paragraph 3 below.
111 His Honour also noted the following undertakings that were given to the Court by Apotex Australia (the appellant) and the Sanofi BMS companies (the respondents):
2. The Respondents undertake to the Court to:
(a) submit to such order (if any) as the Court may consider to be just for the payment of compensation, to be assessed by the Court or as it may direct, to any person whether or not a party, adversely affected by the operation of any of paragraph 1 of the orders made by Gyles J on 25 September 2007 in Federal Court of Australia Proceeding No NSD 1639 of 2007, paragraph 2 of the orders made by Gyles J on 19 August 2008 in Federal Court of Australia Proceeding No NSD 1639 of 2007 and the undertakings of the Appellant noted in paragraph 3 below, or any continuation of any of them (with or without variation); and
(b) pay the compensation referred to in (a) to the person or persons there referred to.
3. The Appellant undertakes to the Court that, pending the final determination of this appeal or further order:
(a) it will not, whether by itself, its directors, officers, servants or agents or otherwise, take any steps to obtain listing of any of the Apotex Clopidogrel Products (as defined in paragraph 2(a) of the orders made by Gyles J on 19 August 2008 in Federal Court of Australia Proceeding No NSD 1639 of 2007) under the pharmaceutical benefits scheme maintained by the Commonwealth under the National Health Act 1953 (Cth);
(b) it will not, whether by itself, its directors, officers, servants or agents or otherwise:
(i) infringe any of claims 1, 6, 7, 8, 9, 10 and 11 of Australian Letters Patent No 597784;
(ii) take any steps to obtain listing of clopidogrel or any salt of clopidogrel under the pharmaceutical benefits scheme maintained by the Commonwealth under the National Health Act 1953 (Cth).
112 The only order made by Moore J is that recorded in paragraph 1 of the minute of orders which, in substance, preserved the security previously given by Sanofi in support of its undertakings as to damages given to Gyles J on 25 September 2007 and 19 August 2008 and the various additional undertakings provided by the Sanofi BMS companies to Moore J as recorded in paragraph 2 of the minute of order.
113 It will be necessary to say more about the effect of these undertakings later in these reasons but it is worth noting a few key points at this stage.
114 The form of orders and undertakings of 15 September 2008 were in a form agreed between Apotex Australia and the Sanofi BMS companies. The agreed undertakings prevented Apotex Australia, until the determination of the appeal or further order, from supplying clopidogrel or any salt of clopidogrel including those within the scope of the claims held invalid by Gyles J. This is likely to have been important to Sanofi because the final injunction granted by Gyles J and his Honour’s other orders left Apotex Australia free to make and supply a clopidogrel product using a different clopidogrel salt as the active ingredient.
115 The agreed undertakings also prevented Apotex Australia from taking any steps to have any clopidogrel product listed on the PBS until the determination of the appeal or further order.
116 The agreed undertakings required each of the Sanofi BMS companies to pay compensation, if so directed by the Court, to any party adversely affected by the interlocutory injunction granted by Gyles J on 25 September 2007, the final injunction granted by his Honour on 19 August 2008, and the various undertakings given to Moore J by Apotex Australia referred to in paragraph 3. These included the undertaking not to take steps to have any clopidogrel product listed on the PBS.
117 An important point to note in relation to the agreed orders and undertakings of 15 September 2008 is that they did not include any undertaking as to damages in respect of the first Apotex undertaking given by Apotex Australia to Gyles J on 25 September 2007. Although paragraph 3 of the agreed orders and undertakings is an undertaking not to take any steps to obtain a PBS listing of any of Apotex Australia’s clopidogrel products, clopidogrel or any salt of clopidogrel, this undertaking was essentially forward looking in the sense that it could only apply to activities engaged in from the time the undertaking took effect, which was when it was proffered to and accepted by Moore J.
118 Although it must now be accepted that taking steps to apply for a PBS listing of a pharmaceutical product the supply of which infringes a patent does not constitute an act of infringement, it does not follow that the Court lacked power to accept undertakings from Apotex Australia forbidding it from taking such steps. The Court has power to receive undertakings from parties in settlement of a dispute where they are reasonably related to the subject matter of the proceedings before the Court: Thomson Publications (Australia) Pty Ltd v Trade Practices Commission (1979) 40 FLR 257 at 280 per Deane and Fisher JJ. The same is also true of the undertakings as to damages given by the Sanofi BMS companies in relation to the various undertakings referred to in paragraph 3 of the minute of order of 15 September 2008.
119 It was submitted by Sanofi BMS that the undertaking given by BMS should be construed as permitting the making of an order for the payment of compensation in respect of any loss or damage suffered by a person adversely affected by the interlocutory injunction from the date the undertaking was given but not any loss or damage suffered before then. In support of that submission it was suggested that the undertaking (at least in so far as it applied to BMS) could not be construed as applying to any loss or damage suffered prior to the date it was given in the absence of clear language to that effect.
120 The language of the undertaking is in my opinion clear. It is not open to the narrow construction for which Sanofi BMS now contend. Nor do I see any reason why the clear language of the undertaking should be read down. I should also note that there was no suggestion in either the submissions or the evidence that the undertaking was intended by any of the Sanofi BMS companies to have some limited operation that depended on whether a claimant’s alleged loss or damage was suffered before or after the date the undertaking was given.
The Full Court’s orders of 13 October 2009
121 Apotex Australia’s appeal against the judgment of Gyles J was successful. On 13 October 2009 the Full Court (Emmett, Bennett and Middleton JJ) held that all of the claims of the Patent were invalid. The Full Court ordered (inter alia) that the final injunction granted by Gyles J be vacated and that the Patent be revoked. These orders were stayed to allow the Sanofi BMS companies to seek special leave to appeal to the High Court.
122 The minute of orders made by the Full Court (which was expressed to be by consent) includes paragraphs 1-7 as follows:
1. ORDERS that, subject to order 4 below, orders 1, 2, 3 and 11 of the orders made by his Honour Justice Gyles on 19 August 2008 be vacated.
2. ORDERS that, subject to order 4 below, Australian Patent Number 597784 (the Patent) be revoked.
3. ORDERS that order 4 of the orders made by his Honour Justice Gyles on 19 August 2008 be vacated.
4. ORDERS that, upon the Respondents / Cross-Appellants giving the undertakings noted in paragraph 7 of these orders and agreeing to provide the security referred to in paragraph 6 of these orders, Orders 1 and 2 be stayed:
a. initially for a period of 28 days; and
b. if an application for special leave to appeal to the High Court of Australia is lodged by the Respondents / Cross-Appellants, until the determination of the special leave application by the High Court of Australia; and
c. if special leave is granted and a Notice of Appeal is filed by the Respondents / Cross-Appellants, until the determination of that appeal by the High Court of Australia,
provided that if the Respondents / Cross-Appellants fail to provide the security referred to in paragraph 6 of these orders by the date specified in paragraph 6, the stay in respect of Orders 1 and 2 will immediately lapse.
5. ORDERS that the security referred to in order 1 made by Moore J on 15 September 2008 remain in place until further order.
6. ORDERS that the Respondents / Cross-Appellants provide by 3 November 2009 to the Appellant / Cross-Respondent additional security in the sum of A$X, either by way of a cash deposit into a joint account or by way of bank guarantee or irrevocable standby letter of credit or any combination of these, pending the determination of the amount of compensation, if any, that the Court may consider should be paid to the Appellant / Cross-Respondent should it be found to have been adversely affected by order 4 of these orders, paragraph 1 of the orders made by Gyles J on 25 September 2007 in Federal Court of Australia Proceeding No NSD 1639 of 2007, paragraph 2 of the orders made by Gyles J on 19 August 2008 in Federal Court of Australia Proceeding No NSD 1639 of 2007, or the undertakings of the Appellant noted in paragraph 3 of the orders made by Moore J on 15 September 2008.
7. NOTES that the Respondents / Cross-Appellants undertake to the Court to:
a. submit to such order (if any) as the Court may consider to be just for the payment of compensation, to be assessed by the Court or as it may direct, to any person whether or not a party, adversely affected by the operation of any of order 4 of these orders, paragraph 1 of the orders made by Gyles J on 25 September 2007 in Federal Court of Australia Proceeding No NSD 1639 of 2007, paragraph 2 of the orders made by Gyles J on 19 August 2008 in Federal Court of Australia Proceeding No NSD 1639 of 2007, the undertakings of the Appellant noted in paragraph 3 of the orders made by Moore J on 15 September 2008; and
b. pay the compensation referred to in (a) to the person or persons there referred to.
123 The effect of the Full Court’s order 1 was to vacate the declarations made by his Honour and also the final injunction restraining infringement of claim 3. The Full Court’s order 4 provided for a stay of order 1 in aid of an application for special leave to appeal subject to the Sanofi BMS companies providing the undertakings noted in paragraph 7 and the additional security referred to in paragraph 6 of the orders.
124 It is common ground that the conditions imposed in relation to security were complied with and, as a consequence, the stay granted by the Full Court took effect. The effect of the stay was that the final injunction granted by Gyles J restraining infringement of claim 3 remained in force until the determination of the Sanofi BMS companies’ special leave application.
125 Paragraphs 6 and 7 of the Full Court’s orders referred to the interlocutory injunction granted by Gyles J on 25 September 2007 and the final injunction granted by his Honour on 19 August 2008. However, as previously mentioned, the former order ceased to have effect when his Honour granted the final injunction on 19 August 2008.
126 In accordance with the Full Court’s orders the discharge of the final injunction granted by Gyles J (as provided for in paragraph 1) was stayed (as provided for in paragraph 4) pending the determination of any application for special leave to appeal made by the Sanofi BMS companies.
127 The orders and undertakings of 13 October 2009 were made by consent. Like those of 15 September 2008, they reflected the terms of a compromise made between Apotex Australia and the Sanofi BMS companies.
128 There are two further points to make in relation to the Full Court’s orders of 13 October 2009.
129 The first is that the undertakings as to damages were again given by the Sanofi BMS companies in relation to the interlocutory injunction and the final injunction granted by Gyles J, but not the first Apotex undertaking given to Gyles J by Apotex Australia on 25 September 2007. It follows that the first Apotex undertaking has never been supported by way of any cross-undertaking as to damages given to the Court by Sanofi or the other Sanofi BMS companies at any stage of the proceeding before Gyles J or the subsequent appellate proceedings. From this it may be inferred that at no stage did any of the Sanofi BMS companies agree to submit to an order for the payment of compensation in respect of loss arising out of the first Apotex undertaking. By contrast, they did agree to do so in respect of the undertaking to the same general effect that was provided by Apotex Australia to Moore J on 15 September 2008.
130 The second point is that the effect of the stay granted by the Full Court in paragraph 4 of its orders ensured that the undertakings given to Moore J on 15 September 2008, including Apotex Australia’s undertaking not to take steps to obtain a listing of any clopidogrel product on the PBS, remained in place until the determination of the Sanofi BMS companies’ application for special leave.
131 On 12 March 2010 the Sanofi BMS companies’ application for special leave to appeal was refused by the High Court. In accordance with the terms of the Full Court’s order, the stay of its orders ceased to operate as soon as the High Court pronounced its order. From that moment on Apotex Australia was free to sell its clopidogrel products and to take steps to have them listed on the PBS.
132 Apotex Australia commenced taking orders for its clopidogrel products on 12 March 2010 and received its first shipment from ARPL on 23 March 2010. Apotex Australia’s clopidogrel products were listed on the PBS on 1 May 2010 which was, as previously mentioned, one month after Sandoz became the first generic supplier to obtain a PBS listing of generic clopidogrel.
The Apotex companies’ discontinued claims
133 On 4 May 2010 Apotex Australia, Apotex Inc and ARPL (“the Apotex companies”) filed a notice of motion applying for orders requiring the payment of compensation under the various undertakings given to the Court by the Sanofi BMS companies. The amount of compensation eventually claimed by the Apotex companies was slightly in excess of $138,000,000.
134 The Apotex companies’ application for compensation was settled by the Apotex companies and Sanofi BMS companies in accordance with the Settlement Deed dated 4 November 2014. In accordance with the terms of the Settlement Deed, the Apotex companies filed a notice of discontinuance of their claims on 7 November 2014.
135 The Settlement Deed was the subject of a previous judgment by me declaring that clause 6 was partly unenforceable: see Commonwealth of Australia v Sanofi (formerly Sanofi-Aventis) [2017] FCA 382. Clause 6 of the Settlement Deed provided:
6. ASSISTANCE TO OTHERS
Otherwise than by compulsion of law, the [the Apotex companies] agree not to voluntarily assist in any way or encourage:
(a) the Commonwealth in relation to the Commonwealth Compensation Claim by way of waiving any claim for legal professional privilege that any or all of the Applicants may have, or releasing any third person from any obligation of confidence in respect of information relevant to the Commonwealth Compensation Claim or the Apotex Compensation Claim, or by the provision of documents;
(b) any third person in a claim against any of the Respondent Parties in connection with the Undertakings as to Damages by way of waiving any claim for legal professional privilege that any or all of the Applicants may have, or releasing any third person from any obligation of confidence in respect of information relevant to the Apotex Compensation Claim, or by the provision of documents.
136 I found that clause 6 was contrary to the public interest because of its strong propensity to prevent or hinder the Commonwealth’s legal representatives’ efforts to interview various witnesses who had been employed by the Apotex companies. It was on that basis that I granted a declaration that clause 6 was unenforceable in so far as it prevented the Apotex companies from releasing any witness or prospective witness from any obligation of confidence in respect of the information relevant to the Commonwealth’s claim for compensation.
137 I was not satisfied that clause 6 was unenforceable to the extent that it prevented the Apotex companies from waiving legal professional privilege or providing documents to the Commonwealth. As I said at [83]:
[83] I am not persuaded that cl 6 is otherwise contrary to the public interest in so far as it relates to the Commonwealth’s claim. There was no attempt by the Commonwealth in its submissions to explain why cl 6 was contrary to public policy in so far as it prevents Apotex from waiving legal professional privilege or providing documents. It may be accepted that in these two respects cl 6 may cause the Commonwealth some difficulties if it is assumed that Apotex would agree to waive privilege or provide documents were it not for cl 6. But that does not provide a sufficient basis for holding that cl 6 is contrary to public policy in so far as it prevents Apotex from taking either of those steps. Litigation is usually conducted on the assumption that one party will not assist an opposing party by voluntarily waiving legal professional privilege or voluntarily providing documents unless the first party is of the view that it is in its interests to take either of those steps. The difficulties facing the Commonwealth are not out of the ordinary.
138 I went on to state that it was a matter for each of the relevant witnesses (ie. those who had previously made an affidavit or expert report filed by the Apotex companies) to decide whether he or she wished to participate in any interview with the legal representatives acting for the Commonwealth. It was not suggested by the Commonwealth in its final submissions in the present application that clause 6 (or any other relevant provision of the Settlement Deed) prevented it from speaking with or calling any witness or potential witness. In particular, it was not suggested that clause 6 prevented the Commonwealth from calling Dr Sherman as a witness.
139 The Commonwealth filed its interlocutory application seeking compensation from Sanofi BMS on 11 April 2013. The interlocutory application filed by the Commonwealth seeks orders for the payment of compensation with interest pursuant to the undertakings given by Sanofi to Gyles J on 25 September 2007, the undertakings given by the Sanofi BMS companies to Moore J on 15 September 2008, and the undertakings given by the Sanofi BMS companies on 13 October 2009.
140 The Commonwealth’s claim is pleaded in its Amended Points of Claim (“CAPOC”). The CAPOC makes reference to the “Restraints” which are defined to comprise:
(a) the interlocutory injunction granted by Gyles J on 25 September 2007;
(b) the final injunction granted by Gyles J on 19 August 2008;
(c) the second Apotex undertaking given by Apotex Australia to Moore J on 15 September 2008; and
(d) the stay granted by the Full Court on 13 October 2009 of its order also made on that date vacating the final injunction.
141 There is also reference in paragraph 11 of the CAPOC to the first Apotex undertaking given to Gyles J on 25 September 2007 (but which is not one of the “Restraints” as defined) whereby Apotex Australia undertook not to take any steps to have its clopidogrel products listed on the PBS pending the determination of the proceeding or further order. Paragraphs 8 and 11 of the CAPOC alleges:
8. On 18 September 2007, Apotex informed the Court, in effect, that, if the Court were to grant the injunction sought by Sanofi-Aventis, Apotex would undertake not to take steps to list certain products (set out in subparagraph 9(a) below) under the Pharmaceutical Benefits Scheme (PBS) established under the National Health Act 1953 (Cth) (NHA). The Court was informed by Apotex that it adopted this position because listing of those products under the PBS would have obliged Apotex to supply them (which it could not do if the injunction sought by Sanofi-Aventis were to be granted).
…
11. On 25 September 2007, as a direct consequence of the grant of the Interlocutory Injunction and as previously foreshadowed to the Court (see paragraph 8 above), Apotex gave an undertaking to the Court that, pending the determination of the proceedings or further order, it would not, whether by itself, its directors, officers, servants or agents or otherwise, take any steps to obtain listing of any of the Apotex Clopidogrel Products under the PBS (First Apotex Undertaking).
142 Paragraphs 62-65 of the CAPOC allege as follows:
Effect of the Restraints
62. By reason of the matters referred to in paragraphs 6 to 25 above, Apotex was restrained from making, selling or otherwise disposing of, offering to make or sell, using or importing, the Apotex Clopidogrel Products from 25 September 2007 to 12 March 2010 (Injunction Period).
63. By reason of the matters referred to in paragraphs 6 to 25 and 62 above, Apotex was prevented from obtaining the listing of any of the Apotex Clopidogrel Products on the PBS with a listing date prior to 1 May 2010.
64. If not for the Restraints during the Injunction Period:
(a) Apotex would have sought PBS listing for the Apotex Clopidogrel Products on or by 1 December 2007; and
(b) Apotex would have obtained PBS listing for the Apotex Clopidogrel Products on 1 Apri12008.
65. Further to paragraph 64 above, if not for the Restraints during the Injunction Period:
(a) from no later than 1 April 2008, the Apotex Registered Clopidogrel Products (or some of them) would have competed with PLAVIX and ISCOVER; and
(b) for the purposes of the NHA (Part VII, Division 3B), the difference between the Weighted Average Disclosed Ex-Manufacturer Price and the approved ex-manufacturer price (calculated in accordance with NHA ss 99ADB and 99ADH) in respect of each brand of clopidogrel would have been at least 10% on one or more of:
(i) a prescribed day in 2010; and/or
(ii) a prescribed day in 2011; and/or
(iii) a prescribed day in 2012.
(strikethrough/underlining omitted)
143 Paragraph 64 specifies what the Commonwealth alleges Apotex Australia would have done if not for the existence of the Restraints (ie. apply for a PBS listing of the Apotex clopidogrel product by 1 December 2007 and obtain such a listing on 1 April 2008). It is not alleged that, if not for the Restraints, Apotex Australia would have applied for a listing on any subsequent listing date.
144 Paragraph 66 pleads what the Commonwealth alleges were the adverse effects suffered by it as a result of the existence of the Restraints as follows:
Adverse effects suffered by the Commonwealth
66. By reason of the effect of the matters referred to in paragraphs 62 to 65 above and in the context of the matters set out in paragraphs 32 to 57 above:
(a) clopidogrel did not move to Part A of F2 on 1 April 2008;
(b) a statutory reduction of 12.5% to the Approved Price to Pharmacists (referred to in sub-paragraph 45(c) above):
(i) did not take effect in respect of clopidogrel on 1 April 2008; and
(ii) was deferred until 1 April 2010;
(c) a further statutory reduction of 2% to the Approved Price to Pharmacists (referred to in sub-paragraph 45(d) above) did not take effect in respect of clopidogrel on one or more of:
(i) 1 August 2009; and
(ii) 1 August 2010;
(d) the statutory price disclosure regime referred to in paragraphs 45(e) to 46 above did not take effect with respect to clopidogrel on 1 April 2008 such that the Commonwealth did not receive the benefit of a price reduction on one or more of the following:
(i) a prescribed day in 2010; and/or
(ii) a prescribed day in 2011; and/or
(iii) a prescribed day in 2012.
It is the common ground that 1 April and 1 August were “prescribed days” for each of the 2010, 2011 and 2012 years.
145 Paragraphs 67, 68 and 69 then plead matters relied upon by the Commonwealth in support of its claim based on the clopidogrel with aspirin combination products marketed in Australia by Sanofi Australia under the CoPlavix brand and by BMS Australia under the DuoCover brand since about 1 December 2009 when they were listed on the SBCD List.
67. By reason of the effect of the matters referred to in paragraphs 62 to 65 above and in the context of the matters set out at paragraphs 32 to 61 above:
(a) the Approved Price to Pharmacists applicable to COPLAVIX and DUOCOVER from the date of listing on the PBS did not reduce to reflect:
(i) a statutory reduction of 12.5% to the Approved Price to Pharmacists for clopidogrel having taken effect on 1 April 2008 (referred to in subparagraph 66(b) above); and/or
(ii) a statutory reduction of 2% to the Approved Price to Pharmacists for clopidogrel having taken effect on 1 August 2009 (referred to in subparagraph 66(c) above);
(b) the NHA s 99ACC (referred to in paragraph 53 above) did not apply to COPLAVIX and DUOCOVER during the period 1 December 2009 to 31 March 2010 (inclusive) at all;
(c) the NHA s 99ACC (referred to in paragraph 53 above) did not apply to COPLAVIX and DUOCOVER from 1 April 2010 to 30 September 2011 (inclusive) to the same effect as would have been the case but for the Restraints; and
(d) the Commonwealth did not receive the benefits of one or more new price agreement(s) commencing in respect of COPLAVIX and DUOCOVER:
(i) on one or more of NHA s 99ADH reduction days that would have applied to clopidogrel but for the Restraints (or any of them) (see sub-paragraphs 47(c) and 66(d) above); and/or
(ii) on 1 August 2010 (see sub-paragraphs 45(d) and 66(c) above).
68. In the alternative to paragraph 67 above, if, but for the Restraints (or any of them) COPLAVIX and DUOCOVER would have been listed on F2 on 1 December 2009, then, by reason of the matters referred to in paragraphs 62 to 65 and in the context of the matters set out at paragraphs 32 to 61 above:
(a) the Approved Price to Pharmacists applicable to COPLAVIX and DUOCOVER from the date of listing on the PBS did not reduce to reflect:
(i) a statutory reduction of 12.5% to the Approved Price to Pharmacists for clopidogrel having taken effect on 1 April 2008 (referred to in sub paragraph 66(b) above); and/or
(ii) a statutory reduction of 2% to the Approved Price to Pharmacists for clopidogrel having taken effect on 1 August 2009 (referred to in sub paragraph 66(c) above);
(b) the Commonwealth did not receive the benefits, in respect of payments for the supply of COPLAVIX and DUOCOVER, of a price reduction on 1 August 2010 (referred to in sub-paragraphs 45(d) and 66(c) above).
69. By reason of the matters referred to in paragraphs 66 to 68 above:
(a) the Commonwealth has suffered, and will continue to suffer, loss, being amounts paid (or payable) under the PBS and RPBS which would not have been paid (or would not be payable) if the listing and supply of the Apotex Registered Clopidogrel Products under the PBS had not been subject to the Restraints; and
(b) the amount of the Commonwealth’s loss is the difference between:
(i) the amount actually paid or payable by the Commonwealth in respect of the supply of clopidogrel products under the PBS or RPBS since 1 April 2008; and
(ii) the amount that would have been paid or payable in respect of the supply of clopidogrel products under the PBS or RPBS since 1 April 2008 if the listing and supply of the Apotex Registered Clopidogrel Products under the PBS had not been subject to the Restraints.
146 The Commonwealth’s alleged loss in respect of the combination products is the difference between the price it agreed to pay in May 2009 and the price the Commonwealth alleges it would have agreed to pay in the counterfactual world. In the real world, the combination products were the subject of a PBAC recommendation that had the effect that the price of clopiodgrel with aspirin was to be no higher than the price of clopidogrel, subject to adjusting for different pack sizes.
147 It is common ground that 1 April 2010 was the first possible date on which listed clopidogrel products could have been the subject of a price disclosure price reduction if a generic clopidogrel product had been listed on 1 April 2008. However, the Commonwealth’s case is that if a generic clopidogrel product had been listed on 1 April 2008, then by around 31 July 2009 it would have known whether a price disclosure price reduction would occur on 1 April 2010 and, in those circumstances, it would have sought to postpone any agreement in relation to the price for the combination products until it could negotiate a price that took account of the forthcoming price reduction, the amount of which would have been ascertainable in or about July 2009.
148 A number of the witnesses called by the Commonwealth were employed by Apotex Australia, Apotex Canada or ARPL. Many of the affidavits made by those witnesses had been prepared and filed before those companies discontinued their damages claims. The most important of these witnesses (“the Apotex witnesses”) was Mr Roger Millichamp who was, at all relevant times, the managing director of Apotex Australia.
149 Mr Millichamp was the subject of a detailed cross-examination which involved a substantial challenge to his credit, the most significant aspect of which related to the Commonwealth’s failure to call evidence from his superiors within the Apotex group of companies and what was put to him as a deliberate effort on his part to conceal the identity of the actual decision-maker who would have determined if Apotex Australia would apply for a 1 April 2008 PBS listing of its clopidogrel products if no interlocutory injunction had been granted.
150 Another of the Apotex witnesses called by the Commonwealth was Mr Gordon Fahner who was, at all relevant times, the Senior Vice-President of Business Operations and Finance at Apotex Canada. Mr Fahner gave evidence concerning the financial capacity of Apotex Canada and related entities to provide security in relation to any award of damages that might be made in favour of Sanofi against Apotex Australia in the counterfactual scenario in which no interlocutory injunction was granted on terms that required Apotex Australia to provide such security.
151 Another of the Apotex witnesses who was called by the Commonwealth was Mr Rajesh Goel, the Corporate Finance Controller of ARPL, who gave evidence concerning manufacturing capabilities at its facility in Bangalore, India, and the ability of ARPL to supply Apotex Australia with clopidogrel products in the event that, in any relevant counterfactual analysis, it were to be assumed that Apotex Canada would have been unable to do so due to the existence of Sanofi’s Canadian Patent. His evidence was of no real consequence in light of the submissions ultimately advanced by Sanofi BMS in relation to the Canadian Patent.
152 Leaving aside Mr Millichamp, the most significant witness called by the Commonwealth was Ms Felicity McNeill. She is a senior Commonwealth public servant who is experienced in exercising various statutory powers under the NHA. Her evidence was relied upon by the Commonwealth in support of its counterfactual analysis both in relation to the continuous supply counterfactual and the interrupted supply counterfactual and, in particular, whether the Apotex clopidogrel products would have been listed on the PBS on 1 April 2008, and whether the 12.5% statutory price reduction triggered by any such listing would have been reversed once Gyles J made his final orders. She also gave evidence, as did Mr Phillip Spann, in relation to the question of delay and the Commonwealth’s involvement in earlier patent proceedings involving a PBS listed medicine.
153 Another witness called by the Commonwealth was Ms Philippa Horner who is also a senior Commonwealth public servant and Principal Legal and Policy Adviser to the Health Products Regulation Group of the Department. Her evidence was primarily directed to the copyright issue. Although Ms Horner’s evidence provided some useful background in relation to the use of innovators’ copyright material in the product information documents prepared by generic suppliers of bioequivalent or biosimilar products, her evidence was uncontentious and of no real consequence in light of the submissions ultimately advanced by Sanofi BMS in relation to the copyright issue. This is also true of evidence given for the Commonwealth by Dr Mariana Coghlan, Mr David Pearson and Mr Andrew Korbel.
154 There was evidence given by a number of witnesses called by the Commonwealth directed to establishing the quantum of its alleged loss including payments made by the Commonwealth in respect of clopidogrel and clopidogrel with aspirin under the PBS and the RPBS up to 31 December 2016. The quantum of the payments made by the Commonwealth was ultimately not a matter of dispute and it is unnecessary to refer to the detail of the evidence given on that topic.
155 The Commonwealth relied on the evidence of Mr John Montgomery, a former CEO of Alphapharm and an Adjunct Professor of Pharmacy at the University of Technology, Sydney. Mr Montgomery is an expert in the market for generic brands of pharmaceuticals in Australia and he gave evidence concerning the estimated market share that Apotex Australia would have achieved in the Australian clopidogrel market if the Apotex clopidogrel products had been listed on the PBS on 1 April 2008. He also gave evidence in relation to the likely discounts that would have been applied in the Australian clopidogrel market in both the continuous supply counterfactual and the interrupted supply counterfactual.
156 The Commonwealth also relied on the evidence of Mr Andrew Ross, a partner at Korda Mentha, who has expertise in valuation and accounting. He gave evidence in respect of the quantification of the Commonwealth’s loss.
157 I will comment on some aspects of the evidence of Mr Millichamp, Ms McNeill and Mr Montgomery when I turn to consider it in more detail. But as to all other witnesses who were cross-examined (whether called by the Commonwealth or Sanofi BMS) I should record that I did not see any reason to think that any of them was doing anything other than giving their evidence honestly, and to the best of their recollection and belief.
158 Of the 35 witnesses who gave evidence for Sanofi BMS, 24 of them gave evidence that solely related to the subsistence and ownership of copyright in product information documents relating to Sanofi’s clopidogrel products. None of these witnesses was cross-examined. The Commonwealth ultimately accepted that copyright subsisted in these documents and that it was at all relevant times owned by Sanofi Australia.
159 Two important witnesses called by Sanofi BMS were Mr Jeremy Moulding, the former General Manager of Sanofi Australia, and Mr Paul Lindsay, the former Director of Public Affairs of Sanofi Australia. Mr Lindsay made an affidavit that was read at the interlocutory hearing before Gyles J in August 2007 which was again read at the hearing of the present application. More recent affidavits made by him were also relied upon by Sanofi BMS. That evidence, and evidence given by Mr Allan Dick, Mr Robert Wilson and Mr Paul Sim, was directed to showing how, for the purpose of the relevant counterfactual analysis, Sanofi Australia was likely to have responded to entry by Apotex Australia into the clopidogrel market in 2008 as part of a “generic defence strategy” involving the launch of an autogeneric product, and price discounting of Sanofi’s existing clopidogrel products.
160 The evidence of Mr Sim, a former Pricing Manager of Sanofi Australia, was primarily concerned with the clopidogrel with aspirin products and what recommendations he would have made to Mr Moulding (and others) in order to mitigate the effects of any subsequent price disclosure reductions with respect to clopidogrel that might flow through to Sanofi’s clopidogrel with aspirin combination products in the counterfactual scenario in which the Apotex clopidogrel products were listed on the PBS on 1 April 2008.
161 Sanofi BMS also called evidence from Dr Kim Sweeny, Principal Research Fellow at the Victoria Institute of Strategic Economic Studies, Victoria University, who has expertise in the PBS and the market for generic pharmaceuticals in Australia. He gave evidence concerning the market share achieved by generic manufacturers for drugs other than clopidogrel for which a first generic equivalent was listed on the PBS between 1 August 2007 and 31 December 2010 and what he considered to be useful “comparator molecules”.
162 Evidence was also given by Mr Michael Hobbs concerning generic market share and factors affecting generic substitution and generic market share. Mr Hobbs, who provides consultancy services in the pharmaceutical industry, has many years of experience working for both innovators and generic suppliers of PBS listed pharmaceuticals.
163 Sanofi BMS also relied on evidence given by Mr Terrence Potter. Mr Potter is an accounting expert who responded to the evidence given by Mr Ross. Mr Potter and Mr Ross were able to agree upon an extensive set of damages calculations that could be applied across a range of different counterfactual scenarios.
164 Sanofi BMS made a number of submissions in relation to the failure of the Commonwealth to call evidence from relevant decision-makers including, in particular, Dr Bernard (Barry) Sherman and Ms Diana MacDonell (who I shall for convenience refer to as the “missing witnesses”) and whose evidence was said to be of direct relevance to the counterfactual analysis including as to what Apotex Australia and the Commonwealth would have done if no interlocutory injunction had been granted restraining Apotex from supplying its clopidogrel products.
165 I will refer to the specific submissions that were made when considering the hypothetical events in which the missing witnesses were likely to have been involved had those events occurred. However, it is first necessary to refer to some of the relevant authorities.
166 The principle of law with which I am concerned is commonly known as the rule in Jones v Dunkel (1959) 101 CLR 298. The principle was considered in Payne v Parker [1976] 1 NSWLR 191 (Hutley, Glass and Mahoney JJA) in an appeal against the jury verdict for the defendant in a medical negligence case. The judgment of Glass JA (who was in dissent) is widely recognised as correctly stating the relevant legal principles: see, for example, Manly Council v Byrne & Anor [2004] NSWCA 123 at [53] per Campbell JA (with whom Beazley JA and Pearlman AJA agreed).
167 After referring to Lord Mansfield CJ’s well-known dictum in Blatch v Archer (1774) 1 Cowp 63 at p 65; 98 ER 969 at p 970 that “… all evidence is to be weighed according to the proof which it was in the power of one side to have produced, and in the power of the other to have contradicted” and various other authorities, Glass JA said at 200-201:
From these various sources it is possible, I believe, to deduce the following propositions:
(1) The rule is a principle of the law of evidence whereby a particular form of reasoning is authorized.
(2) The reasoning which is permissible involves the treatment of a failure to adduce evidence as a reason for increasing the weight of the proofs of the opposite party or reducing the weight of the proofs of the party in default: … The principle may be invoked for a deficiency in the evidence either of a party bearing the legal onus of proving an issue, or of a party bearing the evidentiary burden only: … If the failure is of the latter kind, the direct evidence of the party with the onus of proof can be more readily accepted, and inferences in his favour may be more confidently drawn: Jones v. Dunkel. If the failure is of the former kind, a consonant formulation would be that the direct evidence of the party carrying the onus may be more readily rejected, and the inferences for which he contends may be treated with greater reserve. The default “brings a great slur on his cause”: …
(3) The failure to call a particular witness is merely one instance of evidentiary deficiency which brings the principle into operation. Other instances are the failure to adduce any evidence at all: … the failure to produce a particular document, and the failure to prove a particular fact: [Wigmore on Evidence, 3rd ed. (1940) Vol II, par. 285].
168 His Honour referred at points (4)-(5) to the operation of the principle in the context of trial by judge and jury. His Honour then turned to consider in more detail the conditions attaching to the operation of the principle as follows at 201-202:
…
(6) Whether the principle can or should be applied depends upon whether the conditions for its operation exist. These conditions are three in number: (a) the missing witness would be expected to be called by one party rather than the other, (b) his evidence would elucidate a particular matter, (c) his absence is unexplained.
(7) The first condition is also described as existing where it would be natural for one party to produce the witness: Wigmore, par. 286, or the witness would be expected to be available to one party rather than the other: … or where the circumstances excuse one party from calling the witness, but require the other party to call him: … or where he might be regarded as in the camp of one party, so as to make it unrealistic for the other party to call him: … or where the witness' knowledge may be regarded as the knowledge of one party rather than the other: … or where his absence should be regarded as adverse to the case of one party rather than the other: … It has been observed that the higher the missing witness stands in the confidence of one party, the more reason there will be for thinking that his knowledge is available to that party rather than to his adversary: … If the witness is equally available to both parties, for example, a police officer, the condition, generally speaking, stands unsatisfied. There is, however, some judicial opinion that this is not necessarily so: … Evidence capable of satisfying this condition has been held to exist in relation to a party's foreman: … his safety officer: … his accountant: … his treating doctor: …
(8) According to Wigmore, par. 285, the second condition is fulfilled where the party or his opponent claims that the facts would thereby be elucidated. Under other formulations, the condition is made out when the witness is presumably able to put a true complexion on the facts: Jones v. Dunkel, might have proved the contrary: … would have a close knowledge of the facts: … or where it appears that he had knowledge: … I would think it insufficient to meet the requirements of principle that one party merely claims that the missing witness has knowledge, or that, upon the evidence, he may have knowledge. Unless, upon the evidence, the tribunal of fact is entitled to conclude that he probably would have knowledge, there would seem to be no basis for any adverse deduction from the failure to call him.
(9) The third condition is satisfied if no explanation is offered for the absence of the witness, or the tribunal thinks that the explanation given is unsatisfactory. The explanation tendered may be that the witness is ill, overseas, dead or refuses to waive his privilege: Wigmore, par. 286.
(some citations and footnotes omitted)
169 Sanofi BMS submitted that the Commonwealth’s failure to call the missing witnesses gives rise to a number of deficiencies in the evidence which, in the absence of any evidence from them, allows the Court to more readily reject the evidence that the Commonwealth relies on to make good the evidentiary gap including, in particular, the evidence of Ms McNeill. That submission is correct in so far as it goes. The real question is whether the conditions attracting the operation of the Jones v Dunkel principle are satisfied.
170 The three conditions that must be fulfilled before the rule can operate concern, first, the relationship of the party to the missing witness and (inter alia) whether the missing witness would be expected to be available to one party rather than the other; second, whether it appears that the missing witness is likely to have knowledge of the relevant facts; and, third, whether any satisfactory explanation has been given by the relevant party in respect of its failure to call the missing witness.
171 In the present case the evidence that Sanofi BMS says could have been given by the missing witnesses primarily relates to their state of mind and what each of them would have done in the relevant counterfactual scenario.
172 If the relevant conditions are satisfied, it will be open to the Court to infer that any evidence the missing witnesses may have given would not have assisted the Commonwealth’s case: see, for example, Air Express Ltd v Ansett Transport Industries (Operations) Pty Ltd (1981) 146 CLR 249 (“Air Express”) per Gibbs J at 314 and Brandi v Mingot (1976) 12 ALR 551 at 559-560 per Gibbs ACJ, Stephen, Mason and Aickin JJ. It may also be open to the Court to draw an inference unfavourable to the Commonwealth’s case that the Court might not have been willing to draw if the relevant conditions had not been satisfied. As Heydon J said in ASIC v Hellicar (2012) 247 CLR 345 at 432 [232]:
… two consequences can flow from the unexplained failure of a party to call a witness whom that party would be expected to call. One is that the trier of fact may infer that the evidence of the absent witness would not assist the case of that party. The other is that the trier of fact may draw an inference unfavourable to that party with greater confidence.
His Honour went to say at 443-444 [256]:
… the greater the failure of a party bearing the onus of proof to call available witnesses with valuable evidence to give, the harder it is to satisfy that onus. … The cases illustrate nothing more than Dawson J’s observation: ‘When a party’s case is deficient, the ordinary consequence is that it does not succeed’ [Whitehorn v The Queen (1983) 152 CLR 657 at 682].
173 In the present case the Commonwealth submitted that none of the three conditions referred to by Glass JA in Payne v Parker was satisfied. I will consider the correctness of that submission when considering each of the counterfactual scenarios to which the missing witnesses’ evidence was said by Sanofi BMS to be relevant.
174 I have previously referred to clause 6 of the Settlement Deed which prevented Apotex Australia or Apotex Canada from waiving legal professional privilege in documents relating to the Commonwealth’s claim for compensation at least in so far as doing so may have assisted or encouraged the Commonwealth in relation to its claim. The Commonwealth’s challenge to the validity of that aspect of clause 6 was unsuccessful. In the result, neither Apotex Australia nor Apotex Canada was able to waive legal professional privilege in relation to any document relevant to the Commonwealth’s claim without risk of breaching clause 6 of the Settlement Deed.
175 There were many claims of legal professional privilege made by Apotex Australia and Apotex Canada particularly in relation to documents relevant to the question of whether Apotex Australia would have applied for a PBS listing of its clopidogrel products with effect from 1 April 2008 but for the interlocutory injunction. Some of the documents which I refer to in my analysis of the evidence on that issue include portions that have been redacted on the ground of legal professional privilege. Some of these redactions have been made to highly significant communications that are of direct relevance to the question of what Apotex Australia would have done had there been no interlocutory injunction.
176 Of course, no inference is to be drawn on account of legal professional privilege having been claimed. Nevertheless, it is important to note that the claims for legal professional privilege that have been made create a situation in which a number of written communications that are highly relevant to a central issue in the proceeding have been produced in incomplete form. In respect of some of these communications I cannot be satisfied that the relevant document, in its redacted form, conveys an accurate or sufficiently complete picture of the author’s state of mind or how the document was likely to be understood by a recipient in its unredacted form. This necessarily affects what weight may be given to various documents and what Mr Millichamp had to say about some of them in his evidence.
177 In Air Express a company (the claimant) which was added as defendant in an action for breach of contract brought by the plaintiff in the High Court applied for damages pursuant to an undertaking as to damages. The undertaking had been given by the plaintiff on the grant of an ex parte interim injunction to restrain the original defendants to the action, the Commonwealth and the Secretary of the Department of Transport, from issuing permission to the claimant to import freighter aircraft into Australia. The undertaking as to damages was extended in favour of the claimant when it was added as a defendant and the interim injunction was continued until the suit was disposed of or further order.
178 Aickin J, who heard the application for damages, was not satisfied that the Secretary would have issued the permission even if the interlocutory injunction restraining him from doing so had not been continued, and rejected the application on that basis.
179 Aickin J said at 266-267:
In a proceeding of an equitable nature it is generally proper to adopt a view which is just and equitable, or fair and reasonable, in all the circumstances rather than to apply a rigid rule. However the view that the damages should be those which flow directly from the injunction and which could have been foreseen when the injunction was granted, is one which will be just and equitable in the circumstances of most cases and certainly in the present case. No doubt the view as expressed in the two decisions of the Court of Appeal does not constitute a rigid rule and circumstances may sometimes require a different approach. However it will in my opinion be seldom that it will be just or equitable that the unsuccessful plaintiff should bear the burden of damages which were not foreseeable from circumstances known to him at the time.
His Honour continued at 268:
It is important in all cases, and particularly in the present case, to bear in mind the distinction adverted to in many of the cases (e.g. per Myers C.J. in Newman Bros. Ltd. v. Allum; S.O.S. Motors Ltd. (In liq.) [No. 2] [[1935] N.Z.L.R. Suppl., at p.18]) between damages flowing from the injunction and damages flowing from the litigation itself. There may not in every case be any difference between the two but, where there is a difference, it is essential that the damage flowing from the litigation should not be confused with the damage flowing from the interlocutory injunction. This is necessarily required by the form of the undertaking itself.
180 His Honour found that it was probable that the Minister would not have issued the written permission while the litigation was continuing and that it was not the continuation of the injunction which brought about such damage as the claimant may have suffered, but the litigation itself.
181 On appeal, Barwick CJ expressed his agreement with Aickin J’s reasons.
182 Gibbs J said at 311-312:
The object of requiring a plaintiff who seeks an interlocutory injunction to enter into an undertaking of this kind is to attempt to ensure that a defendant will receive compensation for any loss which he suffers by reason of the grant of the injunction if it appears in the event that the plaintiff was not entitled to obtain it. The insistence upon the giving of an undertaking is a very important, if not an essential, means of preventing injustice from being done by the court when it makes an order at an interlocutory stage, before the rights of the parties have been finally determined. The court has a discretion not to enforce such an undertaking, but unless the defendant has been guilty of conduct that would render it inequitable to enforce the undertaking it would seem just, speaking generally, that a plaintiff who has failed on the merits should recompense the defendant for the damage that he has suffered as the result of the making of the interlocutory order. However, it is perfectly clear, and it appears from the words of the undertaking themselves, that the only damages to which a defendant is entitled are those which he has sustained by reason of the grant of the injunction. The generally accepted view is that the damages must be confined to loss which is the natural consequence of the injunction under the circumstances of which the party obtaining the injunction has notice: see Smith v. Day [(1882) 21 Ch.D. 421, at p. 430.] and the cases cited in Kerr on Injunctions, 6th ed. (1927), p. 667, and Halsbury, 4th ed., vol. 24, par. 1077. However, in the present case the question is not whether loss caused by an injunction was a natural consequence of making it, but whether any loss which the appellant suffered was caused by the making of the injunction.
…
In a number of authorities the court has distinguished between loss which was caused by the injunction and loss which arose from the litigation: see Bingley v. Marshall [(1863) 9 L.T.N.S. 144, at p. 145]; Gault v. Murray [(1892) 21 OR 458, at p. 462]; Douglass v. Bullen [(1913) 12 DLR 652, at p. 655] and Newman Bros. Ltd. v. Allum, S.O.S. Motors Ltd. (In liq.) [No. 2] [[1935] N.Z.L.R. Suppl. 17, at p. 18]. There is no reason to doubt that it is correct in principle to draw such a distinction if the facts warrant it. If the pendency of the litigation, rather than the making of the order, was the cause of the plaintiff's loss, the terms of the undertaking have no application, since the plaintiff has not sustained loss by reason of the order. Moreover, except in certain cases analogous to malicious prosecution, a defendant is not entitled to recover damages for loss resulting from legal proceedings brought against him — the only liability of the unsuccessful plaintiff is to pay costs. The court should no doubt scrutinize with care an assertion by a plaintiff that loss which has been suffered by a defendant has resulted from the litigation rather than from the making of the interlocutory order, since a plaintiff should not be allowed to evade payment of the price which he has agreed to pay for the grant of the injunction. In the end however the question becomes one of fact: did the making of the order cause the loss? The onus of proof must, in accordance with general principles, lie on the defendant who asserts that he sustained damage by reason of the order.
It was submitted on behalf of the appellant that it is enough that the making of the order should have been a cause of the damage, so that if both the making of the order and the continuance of the litigation are concurrent causes the undertaking will be applicable. However, in almost every case in which an injunction is granted the injunction will play some part in causing the party bound by it to act in accordance with its terms. To order a plaintiff to pay damages where it appears that the party bound by the injunction would have acted as he did even if the injunction had not been granted, would be to give the undertaking an effect obviously not intended. The party seeking to enforce the undertaking must show that the making of the order was a cause without which the damage would not have been suffered. It was further submitted that the onus lies on the plaintiff, against whom the undertaking is sought to be enforced, to disentangle any damage arising from the litigation from that which was caused by the making of the order. However, the onus of proof does not shift in this way; the defendant, who seeks to enforce the undertaking, must prove that the damage he has sustained was caused by the making of the order.
183 Stephen J said at 319-320:
But a plaintiff who sues for an injunction and obtains interlocutory relief, giving an undertaking to the court as the price of that relief, commits no wrongful act, no breach of contract or of duty when, at the trial, he fails to obtain any perpetual injunction. If, as a result of the grant of interlocutory relief, the defendant has been harmed there will, however, have been injustice and, an undertaking having been given, the court will thereby have been armed with jurisdiction, otherwise lacking, to right that injustice and compensate the defendant for the harm done to him.
From this it can be seen that it will only be if damage is suffered because of the grant of the injunction, and would not have been suffered but for it, that the court should compensate a defendant who claims damages under the undertaking. Its grant must be shown to be the causa sine qua non of the damage complained of before the defendant can be entitled to be compensated for what turns out to be the erroneous grant by the court of the injunction against it.
184 Mason J, who dissented, did not agree that it should be inferred that the applicant’s loss was caused by the litigation rather than the injunction. However, his Honour said at 324-325:
The object of the undertaking is to protect a party, normally the defendant, in respect of such damage as he may sustain by reason of the grant of the interim injunction in the event that it emerges that the plaintiff is not entitled to relief. It is no part of the purpose of the undertaking to protect the defendant against loss or damage which he would have sustained otherwise, as for example, detriment which flows from the commencement of the litigation itself. That is loss or damage which the defendant must bear himself, as he does when no interim injunction is sought or granted. Consequently, it is for the party seeking to enforce the undertaking to show that the damage he has sustained would not have been sustained but for the injunction.
185 It is apparent from these passages from the reasons of Gibbs, Stephen and Mason JJ that their Honours were each of the view that the interim injunction in relation to which the claim on the undertaking as to damages was made had to be a cause of the relevant damage. Gibbs J said that the making of the order must be a cause without which the damage would not have been suffered. Stephen J said that the grant of the order must be shown to be the causa sine qua non of the damage. Mason J spoke to the need for the claimant to show that his damage would not have been suffered but for the injunction. This test, which is sometimes referred to as the sine qua non test or, more often, the “but for” test, is concerned with the question whether a defendant’s conduct (or in this case the interlocutory injunction) is a cause in fact of the plaintiff’s (or in this case the Commonwealth’s) damage.
186 Even when the “but for” test is satisfied, it remains necessary to determine whether the cause in fact is also the cause in law. This is because not all causes which satisfy the sine qua non test will entail legal responsibility. It is in this context that expressions such as “direct cause”, “proximate cause”, “effective cause” and “supervening cause” come into play: see McGregor H, McGregor on Damages (19th ed, Sweet & Maxwell, 2014) at 114-115 and see also March v E & MH Stramare Pty Ltd (1991) 171 CLR 506 (“March”) at 515-517 per Mason CJ, at 522-523 per Deane J.
187 There is one other matter arising out of the High Court’s decision in Air Express that I must address. Senior Counsel for Sanofi, Mr Sheahan QC, submitted that, for the purpose of determining the cause of the Commonwealth’s loss, it was necessary to consider whether the loss claimed would have been suffered by it had no interlocutory injunction been sought or granted. In particular, Mr Sheahan QC submitted that it was necessary for the Commonwealth to show what loss would have been suffered by the Commonwealth had Sanofi not made any application for an interlocutory injunction. In support of this submission Mr Sheahan QC drew attention to what was said by Mason J in Air Express in the penultimate sentence of the passage at 324-325 which I have previously set out.
188 The point now under consideration assumes significance in Sanofi’s submissions because, while there was much evidence directed to the question of how Apotex Australia would have acted if the interlocutory injunction had not been granted, the evidence did not establish how Apotex Australia would have acted in the event that Sanofi had not made any application for an interlocutory injunction.
189 I do not accept Mr Sheahan QC’s submission as to the effect of what Mason J said on this point in Air Express. It does not seem to me that his Honour was suggesting that the question of recoverability on the undertaking had to be assessed by reference to a hypothetical scenario (or counterfactual) in which the interlocutory injunction that had in fact been granted was never sought. I think it is clear from the final sentence in the relevant passage that his Honour considered that the “but for” analysis was to be undertaken by reference to the interim injunction granted in that case and not the application for the interlocutory injunction. On that basis, I would not regard the absence of any evidence from the Commonwealth as to how Apotex Australia would have acted in the event that Sanofi had not applied for an interlocutory injunction as fatal to the Commonwealth’s case.
190 Certain of Aickin J’s observations in Air Express at 266-267 were approved by French CJ, Gummow, Hayne, Heydon and Kiefel JJ in European Bank Limited v Robb Evans of Robb Evans & Associates (2010) 240 CLR 432 (“European Bank”). Their Honours said at 439 [15]-[18]:
[15] The undertaking as to damages and its origins in equity practice of the nineteenth century, if not earlier, were explained by Aickin J in Air Express [(1981) 146 CLR 249 at 260-261] and by Gleeson CJ, Gummow, Kirby, Hayne and Crennan JJ in Mansfield v Director of Public Prosecutions (WA) [(2006) 226 CLR 486 at 497-499 [30]-[34]]. The authorities discussed in Mansfield included Russell v Farley [(1881) 105 US 433 at 438], where Bradley J had explained the requirement of the undertaking as a response to the anxiety entertained by the court that otherwise its interlocutory order might lead to damage for which there could be no redress except by an order for costs.
[16] In Air Express, Mason J said that there was little to be gained from an examination of the authorities dealing with causation of damage in contract, tort and other situations; the Court was better advised to look to the purpose which the undertaking as to damages is to serve and to identify the causal connection or standard of causal connection which is most appropriate to that purpose [(1981) 146 CLR 249 at 324].
[17] A party seeking an equitable remedy is required to “do equity” and this is the origin of the requirement that the party giving an undertaking as to damages submit to such order for payment of compensation as the court may consider to be just. Given its origin and application to varied circumstances in particular cases, the process of assessment of compensation cannot be constrained by a rigid formulation.
[18] These considerations, bearing upon the interests of justice in the particular circumstances of the litigation, support the following statement by Aickin J in Air Express [(1981) 146 CLR 249 at 266-267. See also R v Medicines Control Agency; Ex parte Smith & Nephew Pharmaceuticals Ltd [1999] RPC 705 at 714], made with respect to interlocutory injunctions, but applicable to the interlocutory order made by the Court of Appeal against European Bank. His Honour said:
In a proceeding of an equitable nature it is generally proper to adopt a view which is just and equitable, or fair and reasonable, in all the circumstances rather than to apply a rigid rule. However the view that the damages should be those which flow directly from the injunction and which could have been foreseen when the injunction was granted, is one which will be just and equitable in the circumstances of most cases and certainly in the present case.
The phrase “could have been foreseen” should be noted.
191 Their Honours went on to consider the findings made by the primary judge in that case, (Gzell J) which they said were of great importance and not disturbed by the New South Wales Court of Appeal. One of these was that, but for the interlocutory order made in that case, the appellant would have converted the relevant funds from United States dollars to Euros. The High Court held that this was an advantage the appellant would have enjoyed were it not for the existence of the interlocutory order for which it was entitled to recover compensation. Their Honours said at 442-443 [28]-[29]:
[28] The appropriate outcome in this case was to be reached by the following path.
[29] On the inquiry before Gzell J the first question was “What is the loss that is now alleged?”, the second “Did that loss flow directly from the order of 18 May 2004?” and the third “Could the loss sustained have been foreseen at the time of that order?” The inquiry presented by the third question is an inquiry as to whether a loss of the kind actually sustained could have been foreseen. Contrary to the submission by the respondent, Mr Evans, the inquiry is not as to whether the actual loss suffered was foreseen at the time the undertaking was given. In the present case there was a finding by the primary judge, as indicated above, that the loss directly flowed from the order and his Honour further found that the loss could have been foreseen. That result should not have been disturbed.
(emphasis original)
192 The focus of the High Court’s attention in European Bank was on the foreseeability of the appellant’s loss at the time the relevant undertaking as to damages was given. Although their Honours expressly approved of the statement by Aickin J in Air Express to which I have referred, nothing was said by the High Court in European Bank to suggest that the “but for” test which Gibbs, Stephen and Mason JJ each required the claimant to meet was not generally applicable. Nevertheless, it is also true that the High Court in European Bank cautioned that the process of assessment of compensation should not be constrained by a rigid formulation. In my opinion this caution would extend to the rigid application of the “but for” test of causation.
193 The “but for” test as it is applied to the assessment of causation in the field of negligence resulting in personal injury was the subject of detailed consideration by the High Court in March. As Mason CJ said at 516:
The “but for” test gives rise to a well-known difficulty in cases where there are two or more acts or events which would each be sufficient to bring about the plaintiff's injury. The application of the test “gives the result, contrary to common sense, that neither is a cause”: Winfield and Jolowicz on Tort, 13th ed. (1989), p. 134. In truth, the application of the test proves to be either inadequate or troublesome in various situations in which there are multiple acts or events leading to the plaintiff's injury: see, e.g., Chapman v. Hearse; Baker v. Willoughby [[1970) AC 467]; McGhee v. National Coal Board; M’Kew (to which I shall shortly refer in some detail). The cases demonstrate the lesson of experience, namely, that the test, applied as an exclusive criterion of causation, yields unacceptable results and that the results which it yields must be tempered by the making of value judgments and the infusion of policy considerations.
194 Deane J said at 522:
For the purposes of the law of negligence, the question of causation arises in the context of the attribution of fault or responsibility whether an identified negligent act or omission of the defendant was so connected with the plaintiff's loss or injury that, as a matter of ordinary common sense and experience, it should be regarded as a cause of it (cf. Barnes v. Hay [(1988) 12 NSWLR 337, at p 339]). The “but for” (or “causa sine qua non”) test may well be a useful aid in determining whether something is properly to be seen as an effective cause of something else in that sense. In particular, the test will commonly exclude causation for the purposes of the law of negligence if the answer to the question it poses is that the accident which caused the injuries would have occurred in the same way and with the same consequences in any event: see, e.g., Duyvelshaff v. Cathcart & Ritchie Ltd. [(1973) 47 ALJR 410, at pp 414-417,419; 1 ALR 125, at pp 134-135, 138, 142-143]. There are however, in my view, convincing reasons precluding its adoption as a comprehensive definitive test of causation in the law of negligence.
Gaudron J agreed with the reasons of both Mason CJ and Deane J.
195 McHugh J spoke at 532 of the need to apply the “but for” test in “a practical commonsense way”.
196 Applying the reasoning in Air Express and European Bank to the present case, the following questions must be addressed in relation to the Commonwealth’s claims:
Would the relevant loss have been sustained but for the grant of interlocutory injunction?
Did such loss flow directly from the interlocutory injunction?
Could loss of the kind sustained have been foreseen at the time the interlocutory injunction was granted?
197 At all material times, the Apotex group of companies has been a very substantial manufacturer and distributor of generic medicines worldwide. The principal operating company in the group is Apotex Canada. It was founded in 1974 and became the largest Canadian owned pharmaceutical company.
198 The Apotex group operated in Australia through Apotex Australia which was known as GenRx until 20 September 2007, and is now known as Apotex Pty Ltd. Mr Roger Millichamp was appointed Managing Director of Apotex Australia on 1 March 2006.
199 Dr Bernard Sherman was co-founder of Apotex Canada, its CEO and Chairman, and the ultimate controller of the Apotex group, including Apotex Canada, Apotex International Inc (“Apotex International”), Apotex Corporation (“Apotex US”) and Apotex Australia.
200 Mr Millichamp reported to Mr Michael Weingarten from about March 2006 to about June 2007 and thereafter to Mr Andrew Kay. Until his departure in about June 2007 Mr Weingarten was the Vice President of Sales at Apotex Canada. Mr Kay, to whom Mr Weingarten reported, was the President of Apotex International. Mr Kay remained in that position until late 2008 or early 2009 around which time he left the Apotex group. Both Mr Weingarten and Mr Kay were based at the head office in Canada (“Apotex HQ”) and they reported ultimately to Dr Sherman.
Proceedings in Canada and the USA
201 On 10 March 2003, Apotex Canada commenced the process of seeking to obtain a Notice of Compliance (“NOC”) from the Canadian Minister of Health for a generic version of clopidogrel bisulfate tablets. On 28 April 2003, Sanofi and related entities initiated a proceeding in the Federal Court of Canada seeking an order prohibiting the Canadian Minister of Health from issuing a NOC to Apotex Canada in respect of its clopidogrel bisulfate product until expiry of Sanofi’s Canadian Patent.
202 On 21 March 2005, the Federal Court of Canada granted an order of prohibition which prevented issuance of such a NOC to Apotex Canada. Apotex Canada appealed to the Federal Court of Appeal. On 22 December 2006, the Federal Court of Appeal dismissed Apotex Canada’s appeal from the judgment of the Federal Court of Canada preventing issuance of a NOC.
203 On 5 July 2007, Apotex Canada was granted leave to appeal against the judgment of the Federal Court of Appeal to the Supreme Court of Canada. However, on 6 November 2008 the Supreme Court of Canada dismissed that appeal.
204 On 8 June 2009, Sanofi and related entities commenced an action for patent infringement against Apotex Canada and various of its related entities. The relief claimed included declaratory, injunctive and pecuniary relief in respect of what was alleged to be infringements of Sanofi’s Canadian Patent by the Apotex entities that were involved in manufacturing clopidogrel products in Canada for export to other countries. On 6 December 2011 the Federal Court of Canada held that each of the claims of the Canadian Patent were invalid and, consequently, a patent infringement proceeding brought by the Sanofi entities against the Apotex entities was dismissed.
205 However, on 24 July 2013 that judgment was set aside by the Federal Court of Appeal which held that the patent was valid and infringed. The Apotex entities then sought from the Supreme Court of Canada special leave to appeal which was granted on 30 January 2014. Their appeal to the Supreme Court of Canada was discontinued on 3 November 2014 pursuant to a Settlement Deed dated 4 November 2014 entered in between the Sanofi and Apotex entities.
206 On 21 November 2001, Apotex Canada, through its US affiliate Apotex US, filed an application with the United States Food and Drug Administration (“FDA”) seeking approval to sell generic clopidogrel bisulfate tablets in the United States.
207 On 21 March 2002, Sanofi and BMS (“the Sanofi BMS entities”) filed a claim in the United States District Court for the Southern District of New York against Apotex Canada and Apotex US (“the Apotex entities”) for infringement of Sanofi’s US Patent No 4847265 (“US Patent”) for clopidogrel. The Apotex entities filed a counterclaim alleging that the US Patent was invalid and unenforceable.
208 On 20 January 2006, the FDA gave final approval to the Apotex entities’ application to sell clopidogrel bisulfate tablets in the United States.
209 On 17 March 2006, the Apotex entities and the Sanofi BMS entities reached an agreement to settle their litigation. That agreement was subject to approval by the Federal Trade Commission and a consortium of US state attorneys-general. In early May 2006, the state attorneys-general informed the parties that they would not approve the settlement agreement.
210 On 26 May 2006, the parties reached an amended settlement agreement. On 28 July 2006, the parties were informed by the state attorneys-general that the states objected to and would not approve the amended settlement agreement.
211 The amended settlement agreement permitted any party to declare that there had been “regulatory denial” if the required regulatory review had not been completed by 31 July 2006. It also provided for litigation between the parties to resume in the event of such regulatory denial.
212 On 31 July 2006, the Apotex entities declared that there had been regulatory denial. The Sanofi BMS entities then sought a temporary restraining order to prevent the Apotex entities from launching their generic clopidogrel bisulfate product in the United States. On 4 August 2006, the District Court denied relief.
213 On 8 August 2006, the Apotex entities launched their clopidogrel bisulfate product in the United States. On 15 August 2006, the Sanofi BMS entities filed a motion seeking a preliminary injunction restraining the Apotex entities from infringing the US Patent and an order recalling all of the products that had been manufactured and distributed since the 8 August 2006 product launch by the Apotex entities.
214 On 31 August 2006, the District Court granted the Sanofi BMS entities a preliminary injunction preventing further supply by the Apotex entities of their clopidogrel products, but refused to order a product recall. The Apotex entities sought a stay of the preliminary injunction, which was denied on 21 September 2006 by the District Court. An appeal brought by them against the decision to grant the preliminary injunction was dismissed on 8 December 2006.
215 On 19 June 2007, the District Court held that Sanofi’s US Patent was not invalid or unenforceable and granted a final injunction restraining Apotex Canada from further infringement of the US Patent. Apotex Canada filed an appeal to the United States Court of Appeals for the Federal Circuit, which affirmed the District Court’s decision on 12 December 2008.
216 On 24 July 2009, the Apotex entities filed a petition for a writ of certiorari in the Supreme Court of the United States which was denied on 2 November 2009.
217 On 19 October 2010, the United States District Court awarded Sanofi US$442,209,362. Those damages were calculated under clause 14(ii) of the amended settlement agreement which established a limit on the damages that Sanofi could recover from the Apotex entities for infringement of Sanofi’s US patent. The damages awarded represented 50% of the Apotex group’s net sales. Thus, the Apotex group retained US$442,209,362 of net sales, and had to pay the same amount to the Sanofi and BMS entities. This represented the realisation of the worst-case scenario that the Apotex group faced at the time it elected to launch in the USA. It also reflected a highly favourable outcome for Apotex which, were it to have launched clopidogrel in the USA in the absence of the amended settlement agreement, could have resulted in an award of damages greatly in excess of that amount.
218 Contrary to a suggestion made in the Commonwealth’s opening submissions, I do not think the US launch was genuinely “at risk” in the sense those words were used in the evidence and submissions. Apotex’s exposure depended not on how much profit Sanofi lost, but how much profit Apotex made. Apotex knew what its worst case would be in the US (as a percentage of its profit) should the validity of the US Patent be upheld.
Would Apotex would have applied to list its clopidogrel products from 1 April 2008?
219 In early 2006, Apotex Australia began exploring the possibility of launching a generic clopidogrel product in the Australian market. At that time there were no generic clopidogrel products registered on the ARTG or listed on the PBS in Australia.
220 Beginning in March 2006 when he took up his position as Managing Director, Mr Millichamp required his senior management team to prepare regular launch forecasts and plans for all key Apotex Australia generic product launches (including for clopidogrel) generally covering a three-year period following launch. These included calculations of possible market share, discounts to be offered by Apotex Australia, and forecasts as to the amount of stock needed.
221 Mr Millichamp sent an email on 2 May 2006 to Karen McTavish (Apotex Australia’s Supply Chain Manager) in which he stated with reference to clopidogrel “[c]learly we are keen to get to market as soon as we can with this molecule”. The email is heavily redacted. Appended to Mr Millichamp’s email was an email from Mr Michael Ascnzo, whose position is shown as Analyst, Product Selection, Apotex International. The whole of Mr Ascnzo’s email is also redacted.
222 On 16 May 2006 Marnie Peterson, Assistant Marketing Manager with Apotex Australia provided to Mr Millichamp what she described as an updated forecast model in respect of clopidogrel.
223 On 19 June 2006, Mr Weingarten sent to Dr Sherman forecasts of net margins for the first three years supplying clopidogrel in Australia assuming a “launch date” in October 2007. Mr Weingarten said:
The information contained above outlines a very conservative scenario, with [Apotex Australia] taking 20% generic segment market share. The reality is, assuming that we have a solid patent strategy, that we should be the only generic on the market on approval in October 2007. I would like your advice on how to proceed, in order to ensure that we are able to launch, on approval, in Australia.
224 On the same day, Dr Sherman responded to Mr Weingarten’s email as follows:
By then [October 2007] the litigation should be over in both US and Canada. If we win, we will launch at risk in Australia on approval. If we lose in US and Canada, we will not launch.
225 At that point in time, therefore, it appears to have been Dr Sherman’s intention to launch at risk once TGA approval was obtained (expected to be obtained by October 2007) but only if Apotex succeeded in the litigation that was pending in the United States and Canada.
226 On 27 June 2006, Apotex Australia (then still known as GenRx) submitted a dossier for its generic clopidogrel products to the TGA for acceptance as of 30 June 2006. That was the earliest possible date following the expiry of the five year “data exclusivity” period, during which Sanofi Australia (as submitter of the original data supporting the quality, safety and efficacy of the originator product for registration on the ARTG) could have objected to the TGA relying upon that data in order to evaluate the generic products.
227 On 1 January 2007, Apotex Canada and Apotex Australia entered into a Transfer Pricing & Indemnity Agreement in respect of clopidogrel (“the TPI Agreement”). It recited that “Apotex will supply the Product” – defined as “the generic pharmaceutical product Clopidogrel” – “to GenRx to market and sell the Product in the [Australia]” and provided that Apotex Canada would defend, indemnify and hold Apotex Australia harmless against, inter alia, any claims, damages, liabilities or obligations which Apotex Australia “may suffer, incur or become liable for as a result of or in connection with any claim asserted against [Apotex Australia] to the extent such claim is based upon a contention that the manufacture of the Product by [Apotex Canada] for distribution and sale by [Apotex Australia] in [Australia] infringes the patent or other intellectual property rights of [Sanofi and/or its affiliates] or any other third party in [Australia]”. The TPI Agreement also gave Apotex Canada “the sole right to determine if the parties will or will not commence legal proceedings to challenge any third party patent which may affect the sale of the Product in [Australia]”.
228 On 15 February 2007, Mr Millichamp sent an email to Mr Weingarten (who was at the time Mr Millichamp’s immediate superior) stating:
As you know we expect TGA approval for clopidogrel in July / August of this year. Our current understanding is that we should wait to see if we launch depending on the outcome (successful or not) of Canadian and US litigation.
[redacted]
[redacted] The potential upside to the Australian business is huge if we can launch.
[redacted]
229 On 20 February 2007, Dr Sherman sent an email to Mr Weingarten and Mr Ivor Hughes. Mr Hughes was a lawyer who appears to have worked closely with Apotex Canada’s senior management (including Dr Sherman) providing advice on intellectual property and litigation strategy in respect of Apotex’s operations in Canada, the USA and Australia. Dr Sherman’s email which he copied to Mr Millichamp, stated:
Plan is as follows:
[redacted]
2. In May or June, we will file suit in Australia to invalidate the patent. [redacted]
3. We will then advise Sanofi that we will launch unless they move for and obtain an injunction, in which case they will have to give an undertaking for our damages.
4. If they do not give an undertaking for our damages and do not get an injunction, we will launch.
230 Although the first step in the plan has been redacted for privilege, the remaining three steps were to commence proceedings against Sanofi to revoke the Patent at or about the time Apotex Australia obtained ARTG registration for its clopidogrel products (then thought to be May or June 2007), to notify Sanofi that Apotex Australia intended to launch those products on the Australian market unless restrained by an interlocutory injunction, and to launch at risk if not restrained.
231 There was some cross-examination of Mr Millichamp that focused on the language used in point 4 to describe the conditions that would trigger the launch at risk. However, in my view the message conveyed was clear. According to this email, Dr Sherman was, at this stage, intending to have Apotex Australia launch at risk in the event that Sanofi did not obtain any interlocutory injunction. The email also reflects a clear awareness on Dr Sherman’s part of the requirement for Sanofi to give an undertaking as to damages in return for any interlocutory injunction that it may obtain.
232 By 20 February 2007 the plan for clopidogrel in Australia no longer depended on the outcome of the litigation in Canada or the United States. By that date, the Federal Court of Appeal in Canada had already dismissed Apotex Canada’s appeal from the decision preventing the issuance of a NOC, and the US Court of Appeal had dismissed the Apotex entities’ appeal against the preliminary injunction granted by the US District Court on 31 August 2006.
233 In an email sent by Mr Kay to Mr Steven Lydeamore concerning clopidogrel on 13 April 2007, Mr Kay stated that “Australia is first up and Barry’s instruction is to attempt to launch at risk and then invalidate”. It is not clear whether the “launch at risk” referred to in this email was one that would involve a PBS listing for a generic clopidogrel product. In any event, it is apparent that Mr Kay understood at that time that Dr Sherman was proposing to “attempt to launch at risk” in Australia before a final hearing at which the validity of the Patent would be determined.
234 On 22 June 2007, after the US District Court gave its decision of 19 June 2007 upholding the validity of Sanofi’s US Patent, Mr Millichamp sent an email to Mr Kay. Up until that point, Mr Millichamp had reported to Mr Weingarten, but Mr Millichamp commenced to report to Mr Kay directly after Mr Weingarten suffered a heart attack. Mr Millichamp’s email referred to a recent discussion he had with Mr Kay with regard to clopidogrel and it explained Apotex Australia’s recommendation that it launch a range of clopidogrel products in Australia. Mr Millichamp’s email included the following:
Our recommendation is that we should launch for the following reasons:
1) Commercial rationale – A launch of a product like Clopidogrel personifies everything that Apotex stands for as a company.
• Apotex/ GenRx has positioned itself as an organisation that is prepared to involve itself in litigation and fight to bring high quality and cost effective medicines to the market as soon as possible.
• We have been first to market with Perindopril, Perindopril / Idapamide, Carvedilol and Meloxicam – We want to build on our credibility and success to date by, in this case, launching a “blockbuster” molecule.
• We strongly believe that if we handle the communication appropriately (which we will) a launch of Clopidogrel will only serve to build our reputation, enhance our credibility and most importantly gain us significant incremental business through the acquisition of new customers. As recently as yesterday we were asked by a major customer (API) for whom we are tendering to win their business, if we will continue to bring products to market whilst challenging patents as they see that as a major reason to trade with us and support our business.
• The financial upside from the launch of the molecule itself is considerable notwithstanding [sic] the additional sale of products in the rest of our portfolio that we will gain as our customer base expands.
• Scenario 1 – no Authorised Generic Launch – 35% Market share – discount 50% (after 12.5% price drop) – Year 1 net margin at Management price = 20,077,236 AUD
• Scenario 2 – Authorised Generic launch – 17% Market share – discount 60% (after 12.5% price drop) – Year 1 net margin at Management price = 9,751,800 AUD
…
235 The email included some projected sales figures for each of the two scenarios discussed.
236 It is important to note that Mr Millichamp’s email was heavily redacted for privilege. However, what the disclosed portions of Mr Millichamp’s email do not disclose is any consideration of the financial impact on Apotex Australia or Apotex Canada were the validity of one or more of the relevant claims to have been upheld. I will return to this issue shortly when considering email correspondence exchanged between Mr Millichamp and Mr Kay in late July 2008 as to how Apotex Australia should respond in the event that Gyles J found in favour of Apotex Australia and refused Sanofi final injunctive relief.
237 On 22 June 2007, Mr Kay sent an email to Mr Millichamp asking him for some additional information including “a timetable scenario of events going forward, registration date, launch, PBS listing, visibility to Sanofi etc”. Mr Millichamp sent an email to Mr Kay on 25 June 2007 providing the following additional information:
Our expected timetable of events going forward is as follows:
* Registration (ARTG) approval date - Expected towards the end of July, latest early August (Please note however that the TGA have been surprising us and approving our dossiers earlier than expected. Gabapentin 600mg and 800mg tablets are likely to be approved 3 months ahead of our expected timing).
* We propose launching to the trade, by taking orders, on the day that we appear on the ARTG i.e. have regulatory approval.
* We propose notifying Sanofi of our planned launch by applying to revoke their patent either on the same day that we launch or a few days earlier. The idea is to notify Sanofi as soon as we know for sure that we can launch, i.e. when we know that TGA approval will be granted but not give Sanofi time to try and block our initial launch efforts.
* We will submit our application for PBS listing once we are sure that no injunction will be imposed by the courts (PBS listing is granted roughly three months after initial application).We will, of course, have to get to court before we know this outcome. If we apply for PBS listing as soon as we launch we will trigger the 12.5% price drop and Sanofi will argue that pricing has been dropped and therefore their damages cannot be readily measured, will cause irreparable harm and the decision cannot be reversed etc.
When we get to court we will need to let the judge know that we plan to PBS list. In court the idea, in relation to the 12.5% drop and further launch activities, is that we will argue that damages are an adequate remedy, give undertakings that we will record all sales, and provide a bank guarantee in order to pay costs and damages in the event that we are not successful at final trial. We will have to give a bank guarantee that is effective in Australia, but supported by Apotex, as GenRx have no assets or profits at this stage in this jurisdiction to prove that we can pay out in the event of a Sanofi success.
* It is difficult to forecast the amount that we would have to put up as a bank guarantee but we estimate that it could be in the range of 50 -70 Million AUD (for one year’s damages) depending on what Sanofi argues in court.
* We would not propose continuing with launch activities i.e. takings orders and supplying stock if we do not gain PBS listing. Our sales will be very low, virtually zero without PBS reimbursement. In this case we would continue with our patent revocation case but not apply for PBS listing and so not trigger the 12.5% price reduction. We will of course be notifying our customers when we launch that this is a high risk launch and subject to legal action so that they are not misled if we are not able to deliver stock for a considerable time.
Andrew, this is what we are planning but if there is a different approach that is proposed from your side we will be happy to take that advice and recommendation.
238 There are a number of observations to make in relation to this email from Mr Millichamp to Mr Kay.
239 First, the email is consistent with other evidence which indicates that Apotex Australia had no real interest in launching and marketing its clopidogrel products unless it could obtain a PBS listing for them. I am satisfied there would have been no significant market for the Apotex Australia clopidogrel products in Australia unless they could be supplied to pharmacists at prices that were subsidised under the PBS and that there would have been no commercial benefit to Apotex Australia if it were to market its clopidogrel products in Australia in the absence of a PBS listing.
240 Secondly, in his email Mr Millichamp foreshadowed the need for Apotex Canada to facilitate the provision of a bank guarantee to secure any costs and damages that might ultimately be payable to Sanofi in the event Apotex Australia was not successful at trial. Mr Millichamp said that the amount of the bank guarantee could be in the range of A$50 to A$70 million dollars in respect of “one year’s damages”. Beyond that indication, there is nothing in Mr Millichamp’s email to show that he had undertaken any analysis of Apotex’s financial exposure if it were to launch its clopidogrel products, obtain a PBS listing for them, but later see its challenge to the validity of the Patent fail.
241 On the same day Mr Millichamp sent his email, Mr Kay sent an email to Dr Sherman which included the following:
Would you please re-confirm or otherwise our approach in Australia.
We expect TGA approval for our application July / August this year.
Upon grant we will move towards launch and initiate revocation proceeds of the enantiomer patent.
[redacted]
We shall pursue a PBS re-imbursement listing, this will trigger a 12.5% price cut for the product in the market if we are not injuncted and able to sell.
[redacted]
The cost of pursuing revocation are likely to be up to $2m. [redacted]
[redacted]
Utilising the HBr salt under development will require a new dossier and filing and a delay of some 18 months.
Do you want us to continue to launch and to invest in the revocation proceedings?
242 Mr Kay’s email to Dr Sherman of 25 June 2007 is also heavily redacted for privilege. It was followed by other email communications on that date, over which privilege was claimed by the Apotex parties, involving Mr Hughes, Mr Peter Chalk (Apotex Australia’s lawyer), Mr Millichamp, Mr Baxter, and Dr Jeremy Desai. Mr Baxter was the next most senior officer within the Apotex group after Dr Sherman.
243 On 27 June 2007, Mr Kay sent a further email to Dr Sherman (copied to, amongst others, Mr Baxter, Mr Millichamp and Dr Desai) asking him to “advise” when he had “decided whether to pursue revocation of the enantiomer patent, and if you wish us to move to launch at risk”. This is a significant communication because it reflects an understanding on the part of Mr Kay that it was necessary, or at least desirable, to obtain confirmation from Dr Sherman as to his intention with respect to Australia and whether or not Apotex Australia should seek to revoke the Patent and “launch at risk”.
244 Dr Sherman responded to Mr Kay’s email on 27 June 2007 at 4.44am at what appears to be Australian time, but privilege has been claimed over the whole of its contents. The email was addressed to (amongst others) Mr Kay and Mr Hughes. It was also copied to Mr Millichamp, Ms Keast (Apotex Australia’s Regulatory Affairs Manager), Mr Baxter and Dr Desai.
245 Dr Sherman sent a supplementary response to the same people a little under 30 minutes later at 5.12am. Apotex Australia has also claimed privilege over the whole of that communication so it is not possible to know what Dr Sherman said in that email in answer to Mr Kay’s emails to Dr Sherman of 25 and 27 June 2007. Later that morning Mr Millichamp sent an email to some of his colleagues in Apotex Australia with the subject “Clopidogrel in Australia” appending copies of the email correspondence of 22, 25 and 27 June 2007 and saying nothing more than “FYI – game on!!!”
246 The evidence also includes an email sent from Mr Millichamp to Mr Haas on 27 June 2007 at 1.59pm. Again, it has been heavily redacted for privilege but it includes the following:
[redacted]
[redacted] If we are successful in avoiding an injunction we will plan to launch subject to Barry’s further advice / approval.
If anything changes I’ll let you know.
247 Appended to Mr Millichamp’s email was a copy of Dr Sherman’s email of 27 June 2007 sent at 5.12am. It is therefore clear that Mr Millichamp had already received that email at the time of sending his email to Mr Haas.
248 Mr Millichamp’s email to Mr Haas suggests that at the time it was sent it was Mr Millichamp’s understanding that even if Apotex Australia was successful in avoiding an interlocutory injunction, the launch of a generic clopidogrel was subject to Dr Sherman’s further advice or approval. That is not consistent with the understanding that Mr Millichamp claimed to have held at this time based on Dr Sherman’s email of 20 February 2007. On the contrary, it reflects an understanding on Mr Millichamp’s part that even if the application for the interlocutory injunction was successfully resisted by Apotex Australia, Mr Millichamp would still need to consult with Dr Sherman (either directly or more likely through Mr Kay or Mr Baxter) for the purpose of obtaining Dr Sherman’s authority to proceed with a product launch.
249 Mr Millichamp was cross-examined by Mr Sheahan QC on the email to Mr Haas as follows:
MR SHEAHAN: Now, what you were indicating to Mr Hass, I suggest, was that the final decision to launch, even if you were successful in avoiding an injunction, would be a decision made by Dr Sherman at that time; correct? ––– And I think the thinking was that Dr Sherman gave the initial instructions. Andrew Kay had then clarified things with Dr Sherman. Again, we need to show him respect. If there was anything we needed to go to him with, we would. But, if there was no change on the instructions he had given, we would just move ahead.
So do you say to his Honour that what – the only possibility you’re addressing here is that after having informed Canada of your success on the interlocutory injunction, then, out of the blue, as it were, they might indicate to you that they had had a change of heart? ––– Very unlikely.
…
MR SHEAHAN: You were saying that it’s very unlikely that out of the blue Canada would – having been told of your success – just send you a message saying, “We’ve changed our mind.” You agree? ––– I agree with that.
…
And because that scenario was unlikely, what I want to suggest to you is that that is not the scenario you were addressing in this email by saying:
We plan to launch subject to Barry’s further advice/approval
You were addressing another possibility, weren’t you? ––– It’s – in – in retrospect I – or in – in hindsight or in – I can’t actually remember what scenario I was discussing here. It’s to Steven Hass [sic]. I guess I’m just covering all angles and just saying look, subject to Barry’s further advice/approval – it’s a – it’s almost a throwaway line. But you know, there could be a scenario, I guess, where Barry could change his mind. I mean, I ..... think he would. But – it would be very unlikely.
What you were addressing here, I suggest, was your intention to confirm the instructions that you had after a successful interlocutory proceeding, to make sure that Canada still wanted to proceed at risk in Australia? ––– It’s an email to the – I already had the instructions on what to do and had already explained to my boss – this is another email to somebody in production, and I’ve just put that comment in there. I never at any stage would – I think it would be very, very unlikely indeed that Barry would ever change his mind. And it’s only to Steven Hass [sic], who actually is a personal friend of mine. And I put that – put that line in there – I didn’t think that I would have to go through another round of approval with – with – with Barry.
250 I found this evidence most unconvincing. In my view it was largely non-responsive and evasive. Mr Millichamp was not asked whether he believed that Dr Sherman was unlikely to change his mind, but he was at pains to emphasise how unlikely it was that Dr Sherman would do so (“very, very unlikely indeed”). The suggestion that Mr Haas was his personal friend, or that he was only “Stephen Haas” or “somebody in production” does not explain why the launch was said by Mr Millichamp to be subject to Dr Sherman’s “further advice / approval”.
251 I do not accept the explanation given by Mr Millichamp concerning his email to Mr Haas. In particular I do not accept that the relevant statement was a “throw away” line or that Mr Millichamp was merely seeking to “cover all angles”. Nor do I accept that it was unlikely that Dr Sherman would change his mind. On Mr Millichamp’s own evidence, Dr Sherman had already changed his mind in that the revocation/launch strategy for Australia was originally predicated on a successful outcome of the litigation in the US and Canada. In my view, the terms of Mr Millichamp’s email are more likely than not to be correct in so far as they suggest that Dr Sherman had not finally committed to a launch at risk in Australia and that it would be necessary for Mr Millichamp to obtain final approval to launch at risk in the event that no interlocutory injunction was granted.
252 On 8 August 2007, Mr Haas sent an email to Mr Millichamp, Dr Sherman and Mr Kay (amongst others) concerning clopidogrel in Australia which sought information in relation to manufacturing requirements in the event that Apotex Australia required stock of clopidogrel if it were to obtain a PBS listing of its products on 1 December 2007. Mr Kay responded by email sent the same day to (among others) Mr Haas, Mr Millichamp and Dr Sherman. The email is significant because, in my view, it indicates that Mr Kay was not expecting that Apotex Australia would be successful in its efforts to resist an application for an interlocutory injunction. Mr Kay said:
The current plan is to put Sanofi on notice of our intention to launch and so invite them to seek an interlocutory against us launching. We are assuming that this will be successful and are thus not planning to launch at this stage. I’m not sure why this information has not reached you.
253 Mr Kay would have appreciated that it would be necessary for Sanofi to give an undertaking as to damages in the event that it obtained an interlocutory injunction.
254 Several minutes after Mr Kay sent his email, Dr Sherman replied to it stating: “We should put Sanofi on notice ASAP. Who is taking care of it?” Dr Sherman’s email does not express any view as to the likelihood of Apotex Australia successfully resisting the application for an interlocutory injunction, but does not express disagreement with what Mr Kay said in his email. Mr Kay responded to Dr Sherman’s email the following day but the whole of his response has been redacted for privilege.
255 On the basis of the emails exchanged between Mr Kay and Dr Sherman on 8 August 2007, and in the absence of evidence to the contrary, I infer that neither Dr Sherman nor Mr Kay were expecting that Apotex Australia would be successful in resisting an application for an interlocutory injunction by Sanofi.
256 On 14 August 2007, Mr Millichamp signed a certificate under s 26B(1) of the Therapeutic Goods Act 1989 (Cth) (“the TG Act”) on behalf of Apotex Australia, by which Apotex Australia certified that “acting in good faith, it believes on reasonable grounds that it is not marketing, and does not propose to market, clopidogrel hydrogen sulphate in a manner or circumstance that would infringe a valid claim of a patent that has been granted in relation to the therapeutic good”. That certificate was provided to the TGA on 15 August 2007.
257 On 15 August 2007, Apotex requested the TGA to provide a statement regarding bioequivalence in respect of its clopidogrel products which it would later need to provide to the PBAC in support of its application for a PBS listing of those products.
258 On 16 August 2007, Apotex Australia commenced the patent proceeding seeking an order revoking the Patent.
259 On 17 August 2007, Apotex Australia received letters from the TGA confirming approval and registration of Apotex Australia’s clopidogrel products on the ARTG effective from 21 August 2007. On the same day Mr Millichamp wrote to Sanofi Australia notifying it that Apotex Australia had obtained an ARTG registration for its clopidogrel products, that it had applied to the Federal Court of Australia for an order revoking the Patent, and that it was preparing to launch its clopidogrel products into the Australian market in the near future.
260 On 17 August 2007, Mr Millichamp also provided a letter to the sales team at Apotex Australia suggesting that it could be circulated by them to customers which is in fact what subsequently occurred. The letter stated:
GenRx Pty Ltd obtained registration of clopidogrel 75mg (as hydrogen sulfate) tablets under the provisions of the TGA on 17th August 2007. We intend to launch this product into the Australian market in the near future, initially using the brands GenRx Clopidogrel, Chemmart Clopidogrel and Terry White Chemists Clopidogrel. However, before launching these products we need to take the actions outlined below:
° GenRx has applied to the Federal Court of Australia for an order to revoke Sanofi-Aventis's patent covering clopidogrel and its pharmaceutically acceptable salts (Australian Patent No. 597784) and notified Sanofi-Aventis of our intention to begin marketing and selling clopidogrel 75mg (as hydrogen sulfate) tablets in Australia.
° Sanofi-Aventis may respond to our application for revocation of its patent and signalled intention to launch by applying for an interim injunction to prevent us from launching our product into the Australian market. Of course, we would vigorously defend any such application. However, if they do this, we will not seek PBS listing for clopidogrel or take orders from pharmacists until the outcome of that application has been determined. This interim injunction application process would normally be decided in the next one or two months.
° Assuming Sanofi-Aventis do apply for an interim injunction, then the decision whether to launch these products will be delayed until the outcome of that application has been determined (one or two months). We will continue to keep you informed of the status of the proceedings.
Regardless of the outcome of Sanofi-Aventis application for an interim injunction (should they make one), we will continue with our challenge to the validity of the clopidogrel patent before the Federal Court of Australia. We anticipate that the revocation proceedings for Australian Patent No. 597784 would be likely to proceed to trial within approximately 12-18 months.
261 Apotex Australia submitted an application to list its clopidogrel products on the PBS with effect from 1 December 2007. The application was dated 1 September 2007 but was not received by PBAC until 4 September 2007.
262 Ms Keast said in the letter enclosing the application form and related documentation:
As discussed last week, at this time GenRx Pty Ltd provides an assurance that supplies of this product will be available as per listing in the Schedule of Pharmaceutical Benefits from the date of listing on 1 December 2007, dependent on possible litigation. We will confirm supply by or before 12th October 2007 as discussed.
We would like to confirm that if we wish to defer or withdraw this application that we can do so without triggering the 12.5% price decrease providing we notify you in writing by or before the 12th October 2007. This application is lodged on the basis that this understanding is correct. If our understanding is incorrect, please inform us at your earliest convenience as this may affect whether we proceed with the application at this time.
263 Ms Keast’s letter indicates that the PBAC would permit an application for a PBS listing to be withdrawn after it had been submitted. In the case of Apotex Australia’s application for the 1 December 2007 PBS listing, this would have had to be done by 12 October 2007 (ie. approximately six weeks prior to the PBS listing date).
264 The application was withdrawn by Apotex Australia on 4 September 2007 after it was advised by PBAC that it had been received after the 1 September 2007 deadline for lodgement of applications for PBS listings on 1 December 2007. Having missed that deadline, it was not possible for Apotex Australia to obtain a PBS listing of its clopidogrel products before 1 April 2008. The deadline for filing an application for a listing on that date was 1 December 2007. I infer that it would have been open to Apotex Australia to withdraw an application filed on that date by 14 February 2008 or thereabouts.
265 On 6 September 2007, Apotex Australia provided Sanofi with an undertaking not to have its clopidogrel product listed on the PBS until the outcome of Sanofi’s application for interlocutory relief had been determined provided that any such application was commenced and determined by 11 October 2007.
266 The patent proceeding was listed for the first directions hearing before Gyles J on 13 September 2007. At that directions hearing, counsel for Apotex Australia proffered an undertaking to provide security in the sum of A$50 million for any damages and costs that might ultimately be awarded in favour of Sanofi. Gyles J raised the possibility of fixing the proceeding for a final hearing to take place within the next six months. A trial in six months was much sooner than Mr Millichamp had until this time anticipated. He had previously understood that the trial was likely to occur in somewhere between 12 and 18 months’ time.
267 Later that day Mr Millichamp reported to Mr Kay that “as it stands today, we give undertakings (attached) turn up in court next Tuesday and we will know where we stand by the end of next week. Our plan is to launch to customers (take orders) immediately, assuming that we are free to sell, and then supply product in early 2008 for an April PBS listing”. He also said “We are comfortable with the undertakings as we have essentially given them before. The key difference is of course the amount of the Bank guarantee. As you will know we have foreshadowed a sum of this magnitude in previous correspondence”. Mr Kay replied that “The undertakings look routine so do not present a problem” and “I had presaged the likely bank guarantee with Barry and Craig, and from Gord’s [ie. Mr Fahner’s] response it does not look an issue”.
268 Mr Kay questioned whether anyone else had a TGA approval or PBS listing and stated that the only concern was “a pre-emptive competitor launch … given we are 7 months from launch”. Mr Millichamp replied that no-one else had a product on the ARTG and, “[a]s a result no company, apart from us, can get a PBS listing yet”. He also stated that “[o]ur strategy will be to launch ASAP, assuming no injunction, and ensure we sell in stock and lock in customers to optimize our revenues and block any potential competition for as long as we can”.
269 On 13 September 2007, Mr Paddy Smith (Chief Financial Officer of Apotex Australia) sent an email to Mr Fahner, copying Mr Kay, Mr Adams and Mr Millichamp regarding the bank guarantee. He explained:
As the market for Clopidogrel is considerably larger than that for Carvedilol, and the introduction of the GenRx product will result in a 12.5% price reduction for the product sold by Sanofi, then the amount of damages is potentially much larger. The amount is AUD50 million. Senior Management of Apotex are aware of and have previously approved this amount (see emails below and attached).
…
Should the Court find in our favour, this will be BIG news in Australia! We would wish to start taking orders for the product quite rapidly, so we would be keen to finalise the bank guarantee in less than 28 days (we cannot take orders until we comply with all the requirements of the undertaking).
The process would be (I suggest) similar to last time: a L/C from Toronto Dominion to NAB (our bank in Australia); on the back of which the NAB would issue a bank guarantee to Sanofi. (We have to involve an Australian bank as the Court will not accept a guarantee from anything other than an Australian based bank).
The purpose of this email is to set this process in motion.
270 Mr Fahner responded that same day to Mr Smith’s email stating, “we will start the ball rolling to ensure the availability for an L/C in case it happens”. The same day, Dr Sherman sent an email to people in Apotex Canada including Dr Desai, Mr Caccamo and Mr Haas, stating “[w]e may be launching in Australia soon” and asking whether “the inventory of tablets that we have on hand for US [is] saleable in Australia. i.e. can we repackage for Australian launch?” Mr Haas informed Dr Sherman that he was looking into this and that Apotex Australia had been “unable to get listed in the PBS book for December”. He also told Dr Sherman that “[t]he formulation submitted to Australia is identical to that of the USA”, and that approved shelf life was two years. Dr Sherman responded, asking “When were the tablets made? What is expiry dating? I expect that the tablets are in fact good for years. What is required to extend expiry dating for Australia to 3 years?” Mr Haas provided a response that appears to suggest that the US product would have had an expiry date of 29 August 2008 which (I infer) would not be sufficient for retail sale in Australia after 1 April 2008. On 17 September 2007, Ms Keast advised Rekha Panchal, who was part of the regulatory affairs team of Apotex Canada, of the stability data that would be required to support a three year expiry date.
271 On 14 September 2007, Mr Smith sent an email to Mr Corkery of National Australia Bank (“NAB”) referring to a meeting that Mr Smith had with NAB that afternoon to discuss the arrangements relating to the bank guarantee. Also on 14 September 2007, Ms Peterson emailed Mr Haas attaching the artwork for clopidogrel packaging.
272 On 17 September 2007, Sanofi filed its defence and cross-claim against Apotex Australia alleging infringement of the Patent. The cross-claim included the claim for interlocutory injunctive relief. On 18 September 2007, Gyles J heard the application for the interlocutory injunction. At the hearing, Apotex offered security of A$50 million, to be increased as appropriate “as the proceeding progresses”. Apotex also offered to maintain comprehensive accounting records of all clopidogrel products sold. During the course of the hearing, Mr Catterns QC was explicit and direct in stating that Apotex would apply for listing as at 1 April 2008 if there was no interlocutory injunction.
273 Following the hearing, Mr Millichamp reported to Dr Desai that Apotex Australia would learn the outcome of the interlocutory hearing in a few days and that:
…
Even if we get an injunction imposed I am very confident that we will win at final trail [sic]. In that case we will obviously seek damages (a large amount).
We are of course hoping that we get some great news on Friday that we are free to sell. We certainly did everything we possibly could do to prove that damages would be an adequate remedy.
Fingers crossed!!!!
274 On 19 September 2007, Mr Smith sent Mr Fahner an email informing him that Apotex Australia would know within about 24 hours whether it would need to provide a bank guarantee in the amount of A$50 million. Mr Smith said:
We will have 28 days to put this into place (if required), but as previously advised, we would like to complete this asap, as we cannot begin to take orders until we have the guarantee in place. And as this would be a major event in the Australian generic market, to be able to get maximum positive impact of the ruling (if in our favour), it is important that we begin taking orders as soon as possible.
275 On 21 September 2007, Gyles J delivered oral reasons as to why an interlocutory injunction restraining infringement of the Patent should be granted. His Honour published his revised written reasons on 25 September 2007. I have previously set out the terms of the interlocutory injunction granted by his Honour, the undertaking as to damages given by Sanofi in relation to that interlocutory injunction, and the undertaking given to the Court by Apotex Australia that was not supported by an undertaking as to damages from Sanofi.
276 In cross-examination Mr Millichamp’s evidence was that he had received from Dr Sherman by the email of 20 February 2007 instructions to get Apotex’s clopidogrel products on the market as soon as practical and proceed to launch at risk. He also said that he understood that he needed to take whatever action was appropriate to launch the product at risk. In particular, Mr Millichamp said:
I had instructions to try and launch at risk at the interlocutory stage in 2007. So that’s why we had the interlocutory fight [sic], because we were trying to get on the market as soon as we practically could and launch at risk. And at that period in time, I had instructions to do that, and I was implementing the plan of action to do that.
277 Mr Millichamp’s evidence was that it was not necessary for him to seek any further instructions from Dr Sherman before launching at risk, and that the instructions contained in the 20 February 2007 email were all that he required by way of authority or approval from Dr Sherman for Apotex Australia to launch its clopidogrel products at risk in Australia.
278 There were two related developments in September 2007 which could have led Dr Sherman to conclude that it might be preferable not to launch at risk even if Gyles J was to have refused the interlocutory injunction.
279 The first of these occurred in early September when Apotex Australia became aware that its application to list its clopidogrel products on the PBS on 1 December 2007 had been filed too late for that to occur, and that the earliest possible date for a PBS listing was 1 April 2008. Mr Millichamp advised Mr Kay of this fact in an email sent on 4 September 2007. The email, which was not copied to Dr Sherman, states:
We have had a conversation with the Pricing and PBS listing Department of Health and Ageing.
The new guidelines on the 12.5% price drop and timings for PBS listing have resulted in us being unable to get listed in the PBS book for December.
In the event that we are successful in defending against an application for interlocutory relief from Sanofi the earliest time that we can get PBS reimbursement is now April 2008. In this case we will require stock in Australia towards the end of February / Start March 2008 assuming that we are able to launch.
We please request that the supply team remain on standby to supply us if we are successful in the initial stages of our litigation. The timing has just moved back three months later than originally communicated.
280 This information appears to have been conveyed to Dr Sherman by email from Mr Haas on 14 September 2007.
281 The second development occurred on 21 September 2007 when Gyles J published his reasons for refusing the interlocutory injunction. At the directions hearing on 13 September 2007 his Honour had said that he thought that there should be an early final hearing of the proceeding, but it was only after his Honour indicated he would grant the interlocutory injunction that he confirmed when the final hearing would take place. On 21 September 2007 his Honour informed the parties that the proceeding would be fixed for final hearing before himself commencing on 28 April 2008 (ie. in approximately six months’ time).
282 Since the application for the relevant PBS listing did not need to be filed before 1 December 2007 to obtain a listing on 1 April 2008, there was no reason why Apotex Australia had to make any final decision in relation to PBS listing before 1 December 2007. Even then, it still would have been possible for Apotex Australia to withdraw any such application at any time up to 14 February 2008 or thereabouts.
283 It was put to Mr Millichamp in cross-examination that there was no particular urgency about making a final decision to launch at risk. He gave the following evidence:
MR SHEAHAN: Having regard to that time gap, there was plenty of opportunity for a further decision to be made about whether or not to launch at risk? ––– A decision had already been made to launch at risk. That’s why we were having an interlocutory fight [sic].
…
To put it slightly differently, on the assumption that Dr Sherman had given you clear instructions, there was plenty of time for him to change his mind in that period, if he saw a reason to do so; correct? ––– Well, I don’t see why he would change his mind, bearing in mind he said if an injunction wasn’t granted we would launch.
HIS HONOUR: That’s not what you’ve been asked? ––– But was the – I guess the question was, was there time for him to change his mind. Is that the question? After the – after an interlocutory hearing, whichever way it would be, was there time for Dr Sherman to make – to change his mind. Is that – is that the question?
MR SHEAHAN: Yes? ––– He could change his mind.
Mr Millichamp, you’re the businessman, not me, but I had – would you agree that it is common sense with decisions that are, potentially, critical to the future of a business, that you would make them at the latest possible point in time, so that you could make them with the benefit of the most up-to-date information? ––– That’s a very good question, by the way. I’m just thinking how to – how I would answer that properly, or appropriately. Key strategic decisions, as to whether or not to launch a product, whether to litigate, whether to invest huge amounts of capital expenditure, etcetera, etcetera, are made relatively earlier on, if I can characterise it in that way. And as litigation moves along, or as capital projects move along, or as other business decisions, IT implementations move along, etcetera, there are variations to the decision, or to – to – variations to the activities and the actions that take place after the key strategic decision is made. I think that’s probably the best way I can characterise it. So make a key decision and then tactical changes will be made, as part of the process, as – as matters change.
HIS HONOUR: Could – I mean, one matter that would have borne on your thinking, perhaps – and I’m asking – is at what time there was going to be a final hearing of your case? ––– Your Honour, absolutely. We – we were trying to get a – a final hearing as early as possible.
And what if the judge had said, “I can give you a final hearing in December”, for example? ––– After the interlocutory in September?
Yes? ––– We would be delighted with that, because we wanted to have a hearing as practically possible [sic].
But would that – my question is, would that not bear on the question of whether or not you would launch? ––– Sorry - - -
At risk. At risk? ––– Is that – that’s assuming, your Honour, that we – no - - -
There was no injunction? ––– Well, we – we would – we would, almost certainly, still absolutely launch – launch at risk. It’s actually, probably, better for us, because if there’s going to be – if decisions were finally going to be against us, we would know earlier rather than later what the outcome was.
…
So another consideration might be the amount of security that the judge ordered that you provide in the event that there was no injunction granted? ––– That – yes. The amount of – that would be a consideration. And, as your Honour will have seen, we – we proffered $50 million. That is a consideration.
The amount, though? ––– Yes. The amount is a consideration. I mean, we – we – we thought 50 million would be sufficient as an initial sum. And I can’t remember the terms, but with liberty to increase that as time goes by.
All right? ––– But it is part of the decision-making process. Yes.
284 Mr Millichamp’s evidence based on the hypothetical proposition that there could be a final hearing in December 2007 to the effect that “… we would almost certainly … launch at risk” seems to me to assume that this would be Dr Sherman’s instruction to him in such circumstances. It does not explain why Dr Sherman would have wished to launch at risk before the final hearing in the event that it was scheduled to occur in the very near future, and in the same month as the next available PBS listing date. The risks of doing so were substantial if Apotex Australia were to launch at risk in such circumstances given the possibility that, in the event that it failed to persuade the trial judge that the Patent was invalid, it could find itself having to cease any further sales following the grant of a final injunction shortly after obtaining a PBS listing that triggered a price reduction that might not be reversed or, at least, might not be reversed for some significant period of time.
285 It is important to recall that it was the PBS listing that was likely to give rise to what would have been perceived by Apotex Australia at the time to be a substantial exposure for damages which Sanofi may later seek to recover from Apotex Australia if it was successful at the final hearing.
286 In the absence of evidence from Dr Sherman, I am not persuaded that he would have authorised a launch at risk in circumstances where an interlocutory injunction had been refused, but a final hearing was fixed to commence on 28 April 2008. The advice previously communicated by Mr Millichamp in his letter of 17 August 2007 was that a trial was to take place within approximately 12 to 18 months. In the absence of evidence to the contrary I infer that Apotex Canada was also acting on this understanding until no earlier than 13 September 2007.
287 The evidence is, in my view, consistent with Dr Sherman having deferred any final decision as to whether to launch at risk until after the outcome of the interlocutory application was known. The fact that he may not have expected Apotex Australia to be successful in its opposition to the interlocutory injunction suggests that he did not consider it necessary to make any final decision until such time as there was a decision in Apotex Australia’s favour.
288 In its written communications with pharmacists, Apotex Australia had not committed itself to launching in the event no interlocutory injunction was granted. But by publicly resisting the interlocutory application Apotex Australia would be signalling to the market its intention to aggressively contest the validity of the Patent. There was therefore some advantage to be gained in resisting the interlocutory application even if Dr Sherman was later to decide, based on an up to date risk/reward analysis, that it would be undesirable for Apotex Australia to launch at risk.
289 It is not possible to know how Dr Sherman would have reacted to news that the proceeding had been fixed for hearing on 28 April 2008 in the hypothetical situation in which no interlocutory injunction had been granted. He may well have wanted to know how long the final hearing would take and how long it may take for Gyles J to deliver his judgment. Had Dr Sherman asked, he would most likely have been told that his Honour would cease to hold his judicial office from 22 August 2008 (unless he retired sooner) and that he would need to deliver judgment before then.
290 I do not think there is any doubt that Mr Millichamp was eager to launch at risk. He believed that if Apotex Australia could get its clopidogrel product on the market in early 2008 followed by a PBS listing on 1 April 2008 then it would lead to a substantial increase in sales. However, his evidence shows that he was heavily focused on the positive consequences of getting to market quickly, but much less focused on the negative consequences of doing so, and the potential financial consequences should the challenge to the validity of the Patent fail.
291 The evidence provides no information as to the legal advice that informed any assessment of the strength of the case for invalidity or the extent of Apotex Australia’s exposure in the event that the challenge to the validity of the Patent failed. Statements in Mr Millichamp’s oral evidence to the effect that “we always believed that all of the claims of the patent were invalid” are not persuasive in circumstances where any legal advice upon which such a belief was based is not in evidence particularly in circumstances where the validity of the US Patent had already been upheld by the US District Court in a decision that was later affirmed on appeal.
292 The risk/reward calculations that were later performed in July 2008 are revealing because they show what Apotex Australia perceived its financial exposure to Sanofi could be if it successfully resisted Sanofi’s application for an interlocutory injunction and then proceeded to launch its clopidogrel products in Australia with the benefit of PBS listing from 1 April 2008.
293 In late July 2008, several weeks before the final judgment was handed down by Gyles J, Mr Millichamp sought instructions from Mr Kay as to how Apotex Australia should respond to a favourable decision from Gyles J holding the Patent invalid. Mr Millichamp sent an email to Mr Kay seeking instructions from “Apotex HQ” on how to proceed in that event:
If we are successful we need to make a decision on whether or not to launch at risk of the Full Court reversing the judgment on appeal. It is inevitable that Sanofi will try and appeal the judgment if they lose.
Our preferred position here is of course to launch, i.e. start to take orders immediately on the day judgment is handed down, if we win our case.
Our potential exposure to damages will be in the range of 166 M AUD if Sanofi were to prevail on the Full Court appeal. Please see the attached spreadsheet which indicates the summary calculations we have used to estimate the damage we may have to pay if we lost on appeal.
As we will only have a maximum of one to two days notice before we know the date of the judgment we need to be clear of Apotex HQ position now so that we can plan a potential launch strategy and secure a major commercial advantage if we win.
Could you please let us know if Apotex HQ is comfortable for us to launch at risk of Full Court reversal on Sanofi appeal knowing the potential risk of damages should Sanofi prevail.
294 The spreadsheet attached to Mr Millichamp’s email contained the assumptions and calculations which produced the A$166 million damages exposure. The elements of the “downside” analysis included:
the effect of the 12.5% statutory price reduction that would result from the listing of a first generic clopidogrel product (calculated to the end of the patent term in February 2013);
a period of lost sales to Sanofi over 18 months (the time it was assumed the appeal process would take);
a market share assumption for Apotex Australia of 17.5%; and
a profit margin assumption for Sanofi of 90%.
295 The spreadsheet also included an “upside” analysis of the profits that Apotex Australia forecast it would make if it were in the market selling clopidogrel for a three year period.
296 The calculations showed that Apotex Australia’s A$166 million exposure to Sanofi would be more than fivefold greater than the profits that Apotex Australia was expecting to make on the sale of its clopidogrel products if it were to have launched them immediately following a successful outcome before Gyles J.
297 Mr Millichamp’s email indicates that it was Apotex Australia’s preferred position to launch if it was to win the case. However, as the evidence makes clear, whether or not to launch in those circumstances was not his decision to make. Mr Smith sent an email to colleagues on 4 August 2008 which was copied to Mr Millichamp in which he said:
If the Court finds in our favour, then it is likely we will launch but it is not an automatic decision. In this situation, the innovator (Sanofi) would inevitably appeal - and that may require Apotex to give guarantees against possible damages should we lose the appeal. The numbers are very large and this will not be a decision made in Australia!
298 It is clear that the general understanding within Apotex Australia was that Sanofi would appeal against any judgment given in favour of Apotex Australia and that any decision to launch Apotex’s clopidogrel products in Australia prior to the determination of the appeal would have to be made by Apotex Canada.
299 Mr Kay’s initial reaction to Mr Millichamp’s request for instructions appears in Mr Kay’s 28 July 2008 email to Mr Baxter. Even on the assumption that Gyles J would find in Apotex Australia’s favour, Mr Kay’s view was that “we need to see the judgement first” and that “if we chose to launch the potential exposure if Sanofi win at appeal exceeds $150m”.
300 Mr Baxter responded to Mr Kay’s email later that day by asking:
How are damages assessed in Australia? Is it our profits, or their losses? Would there be any punitive damages?
Mr Baxter would have appreciated that Sanofi’s losses would prove to be far greater than Apotex’s profits if damages ever had to be assessed in Australia.
301 Mr Baxter’s response was sent by Mr Kay to Mr Millichamp with a request for an answer to Mr Baxter’s questions. Almost the whole of Mr Millichamp’s response to Mr Kay’s request is redacted for privilege. However, it can be inferred that Mr Millichamp obtained legal advice which he then conveyed to Mr Kay. While it is not appropriate to speculate about the content of the legal advice that was provided, it would have been readily apparent to any competent legal advisor at this time that Apotex Australia would have a substantial exposure to Sanofi for compensatory (but not punitive) damages for patent infringement calculated by reference to Sanofi’s loss of profits. As I have explained, this is different from the position that existed in the US when the Apotex entities launched their clopidogrel products on the US market on 8 August 2006 and the terms of the amended settlement agreement applied.
302 On 6 August 2008 Mr Kay sent an email to Mr Millichamp in which he said:
I wonder if the best outcome would be that we win at first instance, [Sanofi] appeal and the injunction remains in place pending appeal. That way, we don’t expose ourselves to potentially ruinous damages, but would collect damages off [Sanofi] for the further period of being off the market in the event the SA appeal fails.
303 That email was sent at a time when Mr Kay had received both Mr Millichamp’s risk/reward calculations and Mr Millichamp’s answers to Mr Baxter’s questions. It is not clear to what extent he had by this point discussed the matter with Mr Baxter although it is clear that at that time Mr Baxter was yet to respond to Mr Millichamp’s response to Mr Baxter’s questions.
304 On 10 August 2008 Mr Millichamp sent an email to Mr Baxter and Mr Kay advising that the judgment was to be handed down on 12 August 2008. A part of the email has been redacted for privilege. However, Mr Millichamp clearly understood that there was a possibility that Apotex Australia may be successful. This gave rise to what he referred to as the “Million’s [sic] of Dollar” question:
The “Million’s of Dollar” question of course is how big is the risk of us losing on appeal? If we are successful tomorrow we will try and reach a view on this although it is likely to be very difficult indeed to predict an outcome with any degree of surety.
305 It is apparent that what Mr Millichamp proposed was that Apotex Australia would take time to consider any judgment given in its favour with a view to forming a view as to the likely outcome of any appeal by Sanofi. Mr Millichamp recognised that it would be difficult to come to any firm view as to the likely outcome of any such appeal.
306 Mr Kay sent another email to Mr Millichamp on 12 August 2008 which he copied to Mr Baxter. As previously mentioned, Gyles J published his reasons for decision on 12 August 2008. Mr Kay’s email to Mr Millichamp and Mr Baxter was sent on the day (but before) judgment was delivered. Mr Kay said:
As discussed last week my view is that in the event of our success and should [Sanofi] decide to appeal we should in some way allow the injunction to continue, and seek damages should any appeal fail to go [Sanofi’s] way.
307 There are two short points to make about Mr Kay’s email.
308 First, it is apparent that what may have been a quite tentative view held by Mr Kay on 6 August 2008 had developed into a firm view by 12 August 2008 that Apotex Australia should not launch until Sanofi’s appeal had been decided.
309 Secondly, Mr Kay’s remarks suggest that he saw a benefit in obtaining an undertaking as to damages against which a claim might later be made despite his view that the risk that Apotex Australia may have to pay very substantial damages weighed against launching clopidogrel in Australia while any appeal by Sanofi remained unresolved. This appears to me to reveal an intention to use the undertaking as to damages as a means of having Sanofi underwrite the decision not to launch.
310 A similar risk/reward analysis to that undertaken in July 2008 was undertaken by Apotex Australia following the Full Court’s decision at the end of September 2009 finding in favour of Apotex Australia.
311 Adopting some different assumptions (a 40% Apotex Australia market share, a period of lost sales to Sanofi exceeding four years, and updated market size and pricing information), the figures showed a potential exposure to Sanofi of more than $650 million. In that analysis the Sanofi damages figure was more than sevenfold greater than the Apotex Australia profit figure.
312 The Commonwealth submitted that Apotex Australia had in June 2007 estimated that one year worth of potential damages to Sanofi would be in the range of A$50 million to A$70 million. These figures appear in Mr Millichamp’s email to Mr Kay of 25 June 2007 in which Mr Millichamp provided an estimate of the amount of the bank guarantee that may need to be established.
313 The bank guarantee estimate was expressed to relate to damages in respect of a period of one year and not Apotex Australia’s total exposure in respect of any loss that Sanofi may have suffered beyond that period. The risk/reward analysis prepared in July 2008 specified a figure of just over $52 million as a loss that would be suffered in the first year following launch of Apotex Australia’s clopidogrel products based on an initial 12.5% price reduction and a 17.5% loss of market share. That figure, which is at the lower end of the June 2007 estimate, did not take into account price reductions triggered in subsequent years by the PBS listing of the Apotex Australia clopidogrel products or any profits that would be lost by Sanofi in the second or subsequent years.
314 Sanofi’s loss was likely to have exceeded the $52 million estimate by a substantial margin if Apotex Australia was to have obtained PBS listing on 1 April 2008 and it was not finally established until 2009 or 2010 (following any appeals) that the Patent was valid. Much of that loss would have been referable to the 12.5% statutory price reduction. Needless to say, whether or not Apotex would be legally liable for that loss was at that time (and is perhaps still) open to debate, but it is apparent that Apotex Australia was proceeding on the basis that it could be liable for the financial impact on Sanofi of the 12.5% price reduction should it be held that the Patent was valid and infringed.
315 In cross-examination Mr Millichamp was questioned as to what he had told Apotex Canada about Apotex Australia’s potential exposure to Sanofi for damages in the event that it launched at risk and was ultimately unsuccessful in the patent proceeding. In particular, when asked whether he had informed Apotex Canada that the potential exposure could be between $100 million to $200 million, he said he could not recall whether he mentioned those figures but said that “… certainly they were aware there was a huge exposure, or a very substantial exposure”.
316 During the course of his cross-examination Mr Millichamp said that “… the Apotex position is that we always try and push the boundaries, we always try and get on the market at the earliest time possible for our commercial advantage and we always believed that all of the claims of the patent were invalid …”. The views he expressed in July 2008 predicated on the assumption that Apotex Australia would succeed before Gyles J at trial appear to be inconsistent with that claim. This point was taken up with Mr Millichamp in cross-examination as follows:
MR SHEAHAN: Mr Millichamp, you said to his Honour that Apotex always tries to get on the market as soon as possible – correct – that’s what you just said? ––– Yes.
Right. You didn’t do that after the decision of the Full Court, did you?--- ..... the Full Court being?
The Full Court of the Federal Court in the proceedings - - -? ––– The year - - -
- - - concerning - - -? ––– Sorry. The year - - -
- - - 2009 – the end of 2009? ––– No, we didn’t do it on then – then, but – so, I should rephrase that. Can I rephrase the point to - - -
HIS HONOUR: Yes. Go on? ––– Your Honour, can I rephrase the point? Your Honour, when we look at the patent – and I apologise for, perhaps, misleading you and not being clear in my response to you. When we look at the patent landscape and the opportunity to launch a product at risk and challenge a patent in Australia, clearly we’ve done the work up-front and looked at our probabilities of success, based on either non-infringement or invalidity and our negotiating position with the other party. And on that basis, we will make the decision to challenge, to try and get on the market as soon as we practically can. And your Honour may be aware that in 2006, we got in the market with perindopril erbumine at risk pending initial trial. We also got on the market with Carvedilol pending a trial. And our intention was to get on the market as early as possible with clopidogrel. Now, we ended up having an interlocutory injunction, as was just covered, and being kept off the market, but for the injunction, we would have tried to launch, just like we did with Carvedilol, just like we did with perindopril, and, in the past, we also tried with alendronate, but was the subject of an injunction as well. So when I mentioned to your Honour we always try and launch on the market as soon as practically possible, I was referring to the early stages of the litigation. Now, over a period of time, depending on the interlocutory injunction, the decision that’s handed down then, the first instance judgment and the reasons that are handed down then and any appeal and any subsequent – or special leave application to the High Court, the scenario and the risk profile will change over time, but at the outset, when we decide to launch at risk or challenge a patent the idea is to get on the market as early as practicably possible.
317 I did not find this evidence persuasive. The evidence does not suggest that the business case for Apotex Australia launching its clopidogrel products in August or September 2008 followed by PBS listing from 1 December 2008 would have been significantly less attractive to Apotex Australia than a launch at risk in February or March 2008 followed by a PBS listing from 1 April 2008. In his email to Mr Kay, Mr Millichamp had referred to the opportunity to get the Apotex clopidogrel product to market following a successful outcome before Gyles J as “a major commercial advantage if we win” and indicated that a product launch immediately after judgment in Apotex’s favour was Apotex Australia’s preferred position.
318 One matter of concern is Mr Millichamp’s statement that “… in 2006, we got in the market with perindopril erbumine at risk pending initial trial”. It is clear that Mr Millichamp was seeking to compare Apotex’s launch of that compound, at risk, and the proposed launch of clopidogrel at risk. However, there are two basic differences between perindopril erbumine and clopidogrel in this context; first, the compound patent for perindopril erbumine had already expired by the time Apotex launched its own product in November 2006; second, the relevant pharmaceutical item had already been the subject of a 12.5% administrative price reduction on 1 August 2006, three months prior to the listing of Apotex’s perindopril erbumine product on 1 December 2006.
319 It is true that there was litigation in 2006 brought against Apotex Australia that related to perindopril erbumine. However, this litigation was based on an allegation that Apotex Australia had engaged in misleading and deceptive conduct (not patent infringement) and was dismissed by Heerey J with no order as to costs on 30 November 2006: see Servier Laboratories (Australia) Pty Ltd v GenRx Pty Ltd [2006] FCA 1763. There was a further proceeding commenced in February 2007 in which it was alleged by Les Laboratories Servier (“Servier”) that Apotex Australia had infringed Australian Patent No 606992 by manufacturing and supplying Apotex’s perindopril erbumine products. What became of that proceeding is not apparent from the evidence. There was another proceeding brought by Apotex Australia against Servier related to two different patents. What connection they had with perindopril erbumine is also not apparent from the evidence. In any event, what is clear is that by the time Apotex Australia’s perindopril erbumine products were listed on the PBS, the price of the innovator’s products had already been reduced by 12.5%.
320 So far as Mr Millichamp’s reference to carvedilol is concerned, the listing of Apotex’s generic products on the PBS did not result in a 12.5% price reduction of the innovator’s products. An application for an interlocutory injunction brought by the innovator against Apotex Australia was refused. The Judge hearing the application (Emmett J) was satisfied that the launch of the Apotex product was “less than likely” to result in a 12.5% price reduction: see Roche Therapeutics Inc v GenRx Pty Ltd (2007) 71 IPR 546 at [100]. There is no evidence to suggest that the listing of the Apotex products resulted in any such price reduction.
321 The Commonwealth submitted that it was not appropriate to use the July 2008 and September 2009 analyses for the purposes of drawing inferences as to whether or not Apotex Australia would have listed on the PBS from 1 April 2008 but for the interlocutory injunction. In essence, it submitted that the circumstances existing at those later dates were different from those that it faced in August 2007.
322 I accept that the July 2008 and September 2009 risk/reward analyses addressed different circumstances from those that existed in August 2007. Nevertheless, those analyses and Apotex Canada’s ultimate response to them tends to contradict the assertion made in Mr Millichamp’s oral evidence that Apotex tries to get on the market as soon as possible.
323 An important matter when assessing the veracity of Mr Millichamp’s evidence on this topic is that the discussion that occurred in July 2008 concerning risk/reward were predicated on the assumption that Apotex Australia would be successful at the trial. Mr Kay’s view that it might be better if the injunction remained in place (presumably through the operation of a stay of the trial judge’s final orders) reflects a decidedly timid approach and one that is not consistent with the bullish corporate philosophy espoused by Mr Millichamp in his evidence.
324 Another important matter when assessing the veracity of Mr Millichamp’s evidence on this topic is that the profit forecasts forwarded to Mr Kay by Mr Millichamp in his email of 22 June 2007 incorporated two scenarios that essentially mirrored the first two of the three scenarios included in the July 2008 analysis. The first of these scenarios assumed that there would be no authorised generic, that there would be a 12.5% price drop on PBS listing, and that Apotex Australia would take a 35% market share. The second assumed that there would be one authorised generic, a 12.5% price drop on PBS listing and that Apotex would take a 17% market share. The additional (third) scenario included in the July 2008 analysis assumed two authorised generics, a 12.5% price drop and an 8.5% market share to Apotex Australia. The forecast profit in the additional scenario was slightly over half of that for the second scenario, but was still substantial. However, in all three scenarios the potential exposure arising out of a claim for damages by Sanofi greatly exceeded the forecast profit.
325 According to his affidavit evidence, Mr Millichamp considered that it was highly unlikely that either Sanofi or BMS would have sought to launch an autogeneric clopidogrel product until after the patent proceeding had been finally determined and any rights of appeal by Sanofi against an unsuccessful outcome had been exhausted. Admittedly, that was merely Mr Millichamp’s perception of how Sanofi and BMS would have responded to the launch by Apotex Australia of a generic clopidogrel product prior to the final determination of the proceeding before Gyles J and any appeal against findings of invalidity. In any event, that appears to have been his view at all the relevant times and was later put forward in his affidavit evidence as his expert view based on his experience in the industry. As he said in his affidavit with reference to the period 2008 and 2009:
In my experience, it is very unlikely that [S]anofi and BMS Australia would have taken any steps during that time, that is, while the litigation remained on foot, to jeopardise the Australian market for PLAVIX and ISCOVER, for example, by discounting prices to any significant degree, which would have resulted in a larger reduction in the AP2P as a result of price disclosure (assuming sanofi voluntarily chose to disclose its prices) or by launching other competing generic products which would have triggered the 12.5% price drop in the AP2P for clopidogrel (if they had been PBS listed before Apotex's products) and also been subject to mandatory price disclosure. These actions would have had long term impacts on the market, in the sense that they would have either triggered the 12.5% drop in the AP2P, or at least potentially affected it via the price disclosure calculations, and these price changes could not have been reversed even if [S]anofi had ultimately been successful in removing the Listed Apotex Clopidogrel Products from the market and withdrawn its own generic product from the market. In addition, launching a competing generic product is something that in my opinion, based on my experience, an originator will only do after careful consideration because their new generic brand will devalue the market for the originator brand, not just through its potential impact on the AP2P, as I have described above, but because the originator needs to discount their own generic product to differentiate it from the originator brand. This causes a shift in sales away from the originator brand and therefore a loss of value and market share to the originator brand.
326 It is not necessary for present purposes to determine whether Sanofi and BMS would have responded to the launch of generic clopidogrel products by Apotex Australia in the way that Mr Millichamp expected. What is significant is that the second and third scenarios described in the 2007 and 2008 analyses were considered to be unlikely because it was expected that Apotex Australia would not face any competition from autogeneric clopidogrel products for so long as the patent proceeding (including any appeal) remained on foot. I do not think that there is any doubt that Mr Millichamp would have understood this to allow Apotex Australia a significant period of time during which to capture market share in the absence of any competition from an autogeneric product.
327 Mr Millichamp made an affidavit on 14 August 2008 by which time he would have read Mr Kay’s email of 12 August 2008 and Gyles J’s reasons for judgment of 12 August 2008. This affidavit, at least on its face, was prepared for the purpose of providing evidence in support of an application by Apotex Australia for a stay of the final injunction restraining Apotex Australia from infringing claim 3 of the Patent which Gyles J had by this time already held to be valid. I say on its face because I do not consider this was the outcome that Apotex Australia was actually seeking or working towards at the time Mr Millichamp made the affidavit. The affidavit was most likely prepared for the purpose of extracting additional undertakings from Sanofi that would provide Apotex Australia with the opportunity to make a claim for compensation in relation to losses that it could later claim it had suffered by reason of the final injunction that Gyles J subsequently granted in the event that it was set aside on appeal.
328 While Mr Millichamp’s affidavit includes a statement suggesting that the commercial advantage available to Apotex Australia may have already been eroded significantly, it clearly conveys the impression (without stating in so many words) that Apotex Australia desired to launch its clopidogrel as soon as possible and that it wanted the final injunction stayed pending the determination of an appeal to enable it to do so. And yet, evidence to which I have previously referred shows that Mr Millichamp’s immediate superior, Mr Kay, would have strongly preferred to see the permanent injunction continue until such time as any appeal from Gyles J’s judgment had been determined. Mr Kay’s view no doubt reflected his assessment of Apotex Australia’s financial exposure in the event that there was a launch of the Apotex clopidogrel products before any appeal from Gyles J’s judgment had been determined.
329 There is nothing in Mr Kay’s communications with Mr Millichamp to suggest that Mr Kay believed that the value of the commercial opportunity available to Apotex had diminished significantly since September 2007 or that there had been some fundamental shift in any risk/reward calculus which would have justified Apotex Australia launching at risk in early 2008 (with a PBS listing on 1 April) but not late 2008 (with a PBS listing on 1 December).
330 In his affidavit of 14 August 2008 Mr Millichamp explains what the consequences for Apotex Australia would be in the event that a final injunction was not stayed. He referred to the possibility of other generic clopidogrel products entering the market with different salts of clopidogrel not covered by claim 3 with the result that Apotex Australia would lose what Mr Millichamp referred to as its first mover market advantage. However, as at that date, no other generic supplier (including Spirit) had obtained ARTG registration for any clopidogrel product. The deadline for applying for a PBS listing on 1 December 2008 was 1 September 2008. In the circumstances, it is difficult to see how Apotex Australia’s opportunity to secure the first mover advantage was significantly diminished and I do not think it was. It should also be recalled that according to Mr Millichamp’s July 2008 email to Mr Kay, Apotex Australia’s preferred position was to launch.
331 Mr Sheahan QC submitted that Mr Millichamp was an unreliable witness who had deliberately sought to mislead the Court. Central to this submission were various affidavits made by Mr Millichamp which were said by Mr Sheahan QC to have conveyed the impression that the decision to launch at risk in Australia was one for Mr Millichamp to make. As Mr Sheahan QC correctly observed, it was only in Mr Millichamp’s affidavit of 7 July 2017 (which was the seventh affidavit made by him in the proceeding) that he made any reference to Dr Sherman. It was submitted that the failure to make any mention of Dr Sherman in any of the earlier affidavits made by Mr Millichamp was part of a deliberate attempt by him to conceal the identity of the principal decision-maker who would ultimately decide whether Apotex Australia would launch its clopidogrel products at risk.
332 In considering this submission it is important to examine some of Mr Millichamp’s early affidavits in context. The first two were made prior to the interlocutory hearing before Gyles J at a time when Sanofi and its witnesses were positively asserting that Apotex Australia would launch its clopidogrel products at risk in the event that no interlocutory injunction was granted. I would not read anything into Mr Millichamp’s failure to make any mention of Dr Sherman in his first two affidavits in those circumstances.
333 Mr Millichamp’s third affidavit was originally made in support of Apotex Australia’s opposition to Spirit’s application to have its proceeding heard with Apotex Australia’s proceeding. It did not provide an occasion for Mr Millichamp to make any reference to Dr Sherman’s role as the principal decision-maker within the Apotex group of companies.
334 The fourth affidavit was that made on 14 August 2008 explaining why there should be a stay of any permanent injunction granted by Gyles J. Once again, I do not see that affidavit as one in which Mr Millichamp should reasonably have been expected to say anything about Dr Sherman’s decision-making role within the Apotex group. Although there are criticisms that might be made in relation to that affidavit, not mentioning Dr Sherman’s role in the Apotex group is not one of them.
335 However, the position in relation to Mr Millichamp’s fifth affidavit is somewhat different. It was an affidavit made on 9 April 2011 that was specifically prepared in support of the claims made on the various undertakings as to damages, not only by Apotex Australia, but also Apotex Canada and ARPL. Nothing is said in that affidavit about Dr Sherman’s decision-making role in the Apotex group. In this affidavit, Mr Millichamp stated that it was his intention in August, and the early part of September 2007, that Apotex would obtain PBS listing of Apotex’s clopidogrel products with effect from the earliest possible date and that he would have begun marketing and selling those products as soon as Apotex Australia was sure that PBS listing would be obtained. He also stated that if the interlocutory injunction had not been granted, and Apotex Australia had been able to launch its products in preparation for PBS listing on 1 April 2008, he would have instructed his sales team to begin marketing and pre-selling those products from on or around 1 January 2008.
336 Mr Millichamp’s sixth affidavit was an affidavit made on 30 April 2013 which, for the most part, replied to affidavits filed by Sanofi’s witnesses. This affidavit did not refer to Dr Sherman’s decision-making role in the Apotex group although, given its general subject matter, I do not think there was any reason why it should have done so.
337 The seventh affidavit filed by Mr Millichamp was made on 7 July 2017. It is the first affidavit in which Mr Millichamp provides any information as to Dr Sherman and his decision-making role and the fact that there could be no launch at risk in Australia without his approval.
338 The submission that Mr Millichamp deliberately sought to mislead the Court in relation to Dr Sherman’s decision-maker role is not a submission I would accept unless it was clearly supported by the evidence. I do not think it is. However, there were aspects of Mr Millichamp’s oral evidence (to which I have previously referred) that I regard as unsatisfactory. He also seemed inclined to volunteer evidence (which I give little weight) as to Dr Sherman’s state of mind in what appeared to me to be a conscious effort to make up for the lack of any direct evidence from Dr Sherman himself. That said, although I did not regard Mr Millichamp as an entirely satisfactory witness, I do not accept the submission that he deliberately sought to mislead the Court.
339 Mr Millichamp’s cross-examination confirmed, as he had acknowledged in his affidavit of 7 July 2017, that Dr Sherman was the key decision-maker who would have had to give his approval before there could be any launch at risk of Apotex’s clopidogrel products in Australia. In cross-examination Mr Millichamp asserted that Dr Sherman had given such approval in the email of 20 February 2007 and that it contained an instruction from Dr Sherman to try to launch at risk in 2007. Mr Millichamp went on to suggest that the same email provided him with all the authority he needed to cause Apotex Australia to launch at risk and that this constituted the authority he would have relied upon had Gyles J refused to grant the interlocutory injunction.
340 I do not accept that the 20 February 2007 email was an instruction on which Mr Millichamp would have considered himself entitled to act in late 2007 without reverting, either directly or indirectly, to Dr Sherman for the purpose of obtaining confirmation of that instruction and the necessary final approval from him authorising a launch at risk. The suggestion that Mr Millichamp did not need to obtain any further approval from Dr Sherman before launching at risk is, in my view, inconsistent with the email correspondence between Mr Kay and Dr Sherman and Mr Millichamp and Mr Haas.
341 Nor do I consider that the email sent by Dr Sherman on 20 February 2007 is persuasive evidence as to what Dr Sherman’s thinking may have been eight or nine months later. The 20 February 2007 email was sent prior to any decision by the US District Court in relation to the US Patent. Some of the developments that subsequently occurred in 2007 were expected; the ARTG registration of Apotex’s clopidogrel products was one of these. However, the coincidence of events that would have led to both a PBS listing on 1 April 2008 (in the counterfactual scenario in which no interlocutory injunction was granted) followed by the commencement of the trial of the patent proceeding (as actually occurred) later that month was a result of developments that could not have been anticipated until about the time the interlocutory application was determined.
342 As previously mentioned, the Commonwealth called evidence from a number of employees, or former employees, of Apotex Canada and ARPL. Mr Fahner, the Senior Vice-President of Business Operations and Finance at Apotex Canada, is a senior employee of Apotex Canada, but not someone who would have had any direct involvement in deciding whether or not to launch the Apotex clopidogrel products at risk in Australia. What is significant for present purposes is that he made a number of detailed affidavits that were read by the Commonwealth (including one affidavit made some months after the Apotex companies discontinued their own claims for compensation) and also travelled from Canada to Australia to make himself available for cross-examination. Mr Goel and, of course, Mr Millichamp, were witnesses who worked for one of the Apotex companies, who made affidavits in support of the Commonwealth’s case. Mr Goel travelled from India to Australia for cross-examination.
343 Sanofi BMS submitted that the Commonwealth’s failure to call Dr Sherman should lead the Court to infer that his evidence would not have assisted the Commonwealth’s case. This brings me to the question whether, in respect of Dr Sherman, the three conditions referred to by Glass JA in Payne v Parker are satisfied.
344 In this case I would have expected Dr Sherman to have been available to the Commonwealth rather than to Sanofi BMS. Contrary to a submission made by the Commonwealth, I think it would be quite unrealistic to have expected Sanofi BMS to have called Dr Sherman in its defence of the Commonwealth’s claim. Apotex Canada and Sanofi BMS were at relevant times trade rivals engaged in significant litigation in at least three jurisdictions. The US litigation culminated in a judgment in favour of Sanofi BMS against members of the Apotex group (including Apotex Canada) for in excess of US$442 million. In Australia, the Apotex companies made claims against Sanofi BMS in excess of A$138 million under the same undertakings as to damages upon which the Commonwealth’s case is based. And in Canada, Apotex Canada was found to have engaged in infringement of Sanofi’s Canadian Patent on what was likely to have been a significant scale by manufacturing and exporting clopidogrel products for the US market. In my view it would be unreasonable and contrary to common sense to have expected Sanofi BMS to call Dr Sherman.
345 The fact that Apotex Canada made Mr Fahner available to make an affidavit for the Commonwealth and to travel to Australia for cross-examination raises the obvious question of why the Commonwealth did not seek to call evidence from Dr Sherman on an issue of critical importance to its case. In circumstances where Mr Fahner, a senior executive at Apotex Canada, was called by the Commonwealth, it seems to me it would be reasonable to expect it to have also called Dr Sherman to give direct evidence of his state of mind at relevant times by affidavit and, if required, orally either in person or perhaps, by video link if he was unwilling or unable to travel to Australia.
346 There was no evidence called by the Commonwealth that would provide any explanation as to why it did not call Dr Sherman or what attempts, if any, it made to call him. I am mindful that Dr Sherman was, at the time of the trial, based in Canada. However, as I have mentioned, this did not prevent the Commonwealth calling Mr Fahner or other Apotex group executives resident overseas to give evidence in support of its case.
347 I conclude that Dr Sherman was a witness who I would have expected to have been available to the Commonwealth and who would have had a close knowledge of relevant facts. In circumstances where the Commonwealth’s decision not to call Dr Sherman was wholly unexplained, I infer that the Commonwealth chose not to call him because it considered that his evidence would not have assisted its case.
348 I am not prepared to infer, based on the 20 February 2007 email, or any of the subsequent correspondence in evidence which was said to justify the drawing of such an inference, that Dr Sherman was likely to have instructed Mr Millichamp to procure the listing of Apotex’s clopidogrel products with effect from 1 April 2008.
349 In my opinion, the Commonwealth’s case suffers from an evidentiary deficiency which cannot be made good by drawing inferences from correspondence written by Dr Sherman in the lead up to the hearing of the interlocutory application. In particular, I do not think it can be inferred that if Dr Sherman had known that the trial of the patent proceeding would commence in the same month that Apotex Australia obtained a PBS listing of its clopidogrel products (triggering a 12.5% statutory price reduction), that he would have, in those circumstances, authorised Apotex Australia to obtain such a listing before judgment was delivered or, at least, until the trial had concluded (by which time he and his colleagues and his legal advisers may have had a clearer view of the strength of Sanofi’s case).
350 I should add that I do not regard Mr Fahner’s evidence as to the capacity of Apotex Australia to provide security as a condition of avoiding an interlocutory injunction as relevant to any issue in the case. The critical question for present purposes is whether Apotex Australia would have applied for PBS listing in the absence of the interlocutory injunction. The question whether it would have been required to provide security as the price of avoiding an interlocutory injunction does not arise in the counterfactual analysis which presupposes that there was no entitlement to the interlocutory injunction that was granted. It would be incorrect, as a matter of principle, to instead presuppose that there was no interlocutory injunction if, and only if, Apotex Australia was able to provide security.
351 In the result, I am not persuaded that Apotex Australia would have sought and obtained a PBS listing of its clopidogrel products from 1 April 2008 even if the interlocutory injunction had not been granted. It follows that the Commonwealth’s claim for compensation must be dismissed.
352 Given that conclusion, it is unnecessary for me to decide any of the other issues that were the subject of evidence and submissions in the case. However, given the extent of the evidence and submissions, and the possibility that the case will go further, I have also made findings in relation to other key issues. The first of these is whether, if Apotex had applied for a PBS listing of its clopidogrel products from 1 April 2008, the Minister or Delegate would have decided to list those products on the PBS from that date.
Would the Minister or the Delegate have listed Apotex’s clopidogrel products on 1 April 2008?
353 By the time they made their final submissions, Sanofi BMS did not contend that Apotex Australia would not have complied with the formal requirements of an application for listing from 1 April 2008. I find that, had Apotex Australia decided to proceed to apply for and obtain a PBS listing from that date, it would have prepared and lodged an application that complied with all relevant administrative requirements. A more difficult question is whether the Minister or her Delegate would have approved any such application.
354 Sanofi BMS contended that the evidence does not establish that the Minister or her Delegate would have accepted Apotex Australia’s application for listing if it is assumed that a listing with effect from 1 April 2008 would have occurred in the same month that the infringement proceeding brought by Sanofi against Apotex Australia was to be heard by Gyles J and that the Minister or her Delegate would have been aware of that fact. In fact, Sanofi BMS went so far as to contend that for the Commonwealth to have listed Apotex’s clopidogrel products in those circumstances would have involved an unreasonable exercise of the relevant statutory discretion.
355 The Commonwealth did not accept that Sanofi BMS’ counterfactual analysis was accurate. I will return to consider the arguments raised in response to it shortly. However, on the assumption that I was to find that it would be appropriate to assume for the purpose of the counterfactual analysis that the listing application would have been made in circumstances where the Minister or her Delegate would have known that the infringement proceeding was to be heard by Gyles J in April 2008, the Commonwealth contended that Apotex Australia’s products would still have been listed from 1 April 2008. The principal evidence relied upon in support of that contention was given by Mr Lindsay, Mr Moulding and Ms McNeill.
Sanofi’s counterfactual analysis
356 There are a number of points to make in relation to the counterfactual analysis relied upon by Sanofi in support of its contention that the Minister or her Delegate would not have listed Apotex Australia’s clopidogrel products on the PBS from 1 April 2008.
357 Sanofi’s counterfactual analysis involved two possible scenarios.
358 The first scenario was one in which Sanofi, on becoming aware of Apotex Australia’s application to list from 1 April 2008, would have lobbied the Minister with a view to persuading her to refuse Apotex Australia’s application. As I understood the submission, Sanofi contends that it would have pointed to the fact that there was a trial of the patent proceedings imminent with a final judgment to follow some four or five months thereafter, and that this would have justified the Minister in rejecting Apotex Australia’s application apparently on the basis that Sanofi’s products would otherwise be the subject of an immediate 12.5% price reduction upon listing of the Apotex Australia products, and that there was some doubt as to whether or not Apotex Australia could comply with either its assurance of supply or the guarantee of supply.
359 For reasons explained in greater detail below, I am not persuaded that Sanofi would have lobbied the Minister in the manner suggested. I am satisfied that Sanofi was of the view that unless it could obtain an interlocutory injunction that had the effect of preventing Apotex Australia from applying for a PBS listing, then Apotex Australia would proceed to apply for such a listing and that it was, to borrow an expression used by Mr Brereton SC in his cross-examination of Sanofi’s witnesses, “all but inevitable” that such an application would have been approved.
360 There was some evidence given by Sanofi’s witnesses to the effect that they would have sought to lobby the Minister. I did not find that evidence persuasive. To my mind it was the product of hindsight and does not reflect what was likely to be the thinking of Sanofi’s senior management at the time. Importantly, there is no contemporaneous evidence to suggest that Sanofi planned to lobby the Minister in the event that it failed to obtain the interlocutory relief it sought. The first time it was suggested that it would have done so was in affidavits made by Sanofi’s witnesses more than seven years later.
361 Although it was not put to Sanofi’s witnesses that lobbying for a rejection of Apotex’s application was futile, I am satisfied based on the oral evidence given by Mr Lindsay and Mr Moulding (discussed below) that they were unlikely to have sought to lobby the decision-maker essentially on the basis that it was their belief at the time that unless Apotex Australia was restrained from lodging its application for a 1 April 2008 listing, it was almost certain that the application would be approved by the relevant decision-maker.
362 The second scenario postulated by Sanofi assumed that Apotex would have, when making its application for listing from 1 April 2008, disclosed to the Minister or the Delegate the existence of the patent proceeding, the date of the trial, and the fact that there was likely to be a final judgment not later than the end of August 2008. According to Sanofi, the substance of the disclosure by Apotex Australia would have been to the following general effect:
We believe we will be able to supply on 1 April for two years but whether in fact we will be able to do so will depend upon the outcome of a patent trial to be heard in April and likely to be decided not later than the end of August. There is also the possibility that between now and then Sanofi will apply for an interlocutory injunction, if it hadn’t already.
363 In developing its submissions in relation this counterfactual analysis, Sanofi returned to the submission (which I have previously rejected) that it must be assumed, when determining what was likely to have happened if Apotex Australia had applied for a PBS listing from 1 April 2008, that no interlocutory injunction had been applied for by Sanofi. The significance of this point for present purposes is that Sanofi’s counterfactual analysis assumes that (whether informed by Sanofi or Apotex Australia) the Minister or her Delegate would have approached the exercise of the discretion under s 85(6) on the understanding that Sanofi had not made any application for an interlocutory injunction, and that Sanofi may yet do so.
364 I do not accept that this is the appropriate counterfactual analysis. An essential point that must be included in the counterfactual analysis is that Sanofi had made an application for interlocutory relief that had been refused. The possibility that Sanofi would not have sought interlocutory relief is in my opinion so remote as to be fanciful. Not only did Sanofi make such an application, but I am satisfied that it would have understood that not applying for the interlocutory relief at the earliest opportunity after it first became aware of Apotex’s intentions was not a serious option if it was to have any chance of preventing Apotex Australia from obtaining a PBS listing for its clopidogrel products which would have a significant impact on Sanofi Australia’s profits.
365 It was further submitted by Sanofi that for the purposes of the relevant counterfactual analysis it should be assumed that Apotex would only have made a conditional listing application with a qualified assurance of supply in the same form that it made on 1 September 2007. The justification for assuming that Apotex would have made another conditional application seems to be that, in the counterfactual scenario postulated by Sanofi, the possibility that Sanofi might make an application for an interlocutory injunction restraining Apotex from applying to list from 1 April 2008 would still exist (because on Sanofi’s analysis no such application should be taken to have been made) and that the application to list from 1 April 2008 should therefore be assumed to be attended by the same uncertainty as that which had been lodged by Apotex on 1 September 2007.
366 I do not think that is the correct approach. One does not assume for the purpose of the counterfactual analysis that there is no interlocutory injunction, but that there might well be one at any minute. On the other hand, and contrary to a submission made by the Commonwealth, no particular assumptions should be made as to the reasons why no interlocutory relief was obtained. It would not be appropriate to assume, for example, that interlocutory relief was withheld based on the strength of the invalidity case.
367 What is in my opinion critical is that, in the appropriate counterfactual analysis, it is to be assumed that the opportunity for Sanofi to obtain an interlocutory injunction restraining Apotex Australia from either supplying its clopidogrel products, or applying to list them on the PBS from 1 April 2008, was foreclosed.
368 At the very least this means that, for the purpose of the relevant counterfactual analysis, it may be assumed that it would have been open to Apotex Australia to inform the relevant decision-maker (if it considered it necessary or desirable to do so) that there was no serious prospect of Sanofi obtaining any interlocutory relief that would prevent Apotex Australia proceeding with an application to list its products on the PBS from 1 April 2008. But on no view should it be assumed that Apotex Australia would have had to inform the relevant decision-maker that it faced the prospect of an interlocutory hearing that might at any minute culminate in an interlocutory injunction preventing it from supplying its clopidogrel products.
369 There is a logical inconsistency in Sanofi’s counterfactual analysis which assumes that the relevant decision-maker would know that Gyles J had fixed the matter before himself for a trial in April 2008 and that, having regard to his imminent retirement, there would need to be a final judgment by August 2008, but not that an interlocutory injunction had previously been refused. Any counterfactual analysis that assumes that Sanofi might at any time obtain an interlocutory injunction preventing Apotex Australia from supplying its clopidogrel products involves a hypothetical scenario that would never have eventuated in the real world.
370 At the time of the interlocutory hearing before Gyles J, Mr Paul Lindsay was employed by Sanofi Australia as its Director of Public Affairs. He held that position from July 2006 until July 2010. By September 2007 he had considerable experience working in the pharmaceutical industry and maintaining a thorough knowledge of the operation of the PBS. He made an affidavit in September 2007 that was relied on by Sanofi at the interlocutory hearing in which he described the PBS system and the effect of the 2007 Amendments in some detail.
371 In his affidavit Mr Lindsay described the price changes that would be triggered in the event that Apotex Australia’s clopidogrel products were listed on the PBS from 1 April 2008 including the various mandatory price reductions that would result from such a listing and the price disclosure reductions that would be due to take effect from 1 April 2010.
372 Mr Lindsay’s affidavit does not suggest that he gave any consideration to the possibility that the Minister might decline to list Apotex Australia’s clopidogrel products from 1 April 2008 if no interlocutory injunction was granted. In fact the interlocutory hearing before Gyles J appears to have been conducted on the clear understanding common to both parties that in the event that Apotex was not prevented from making application to list its clopidogrel products from 1 April 2008 then that application would be approved.
373 During his cross-examination Mr Lindsay agreed that, at the time he made the affidavit, he believed that if Sanofi did not obtain an interlocutory injunction to restrain Apotex from selling its clopidogrel products, it was “all but inevitable” that Apotex would apply to list on the PBS, and that it was also “all but inevitable” that Apotex would obtain a PBS listing of its clopidogrel products from 1 April 2008.
374 In an affidavit made by Mr Lindsay in 2016, he stated that if Apotex had not been restrained in September 2007 from listing its products on the PBS, he would have recommended to the General Manager of Sanofi Australia (Mr Moulding) that the company engage with the Minister to request that she exercise her discretion not to list Apotex’s clopidogrel products on the PBS on the basis that:
the listing of Apotex’s products on the PBS would result in those products infringing Sanofi’s patent; and
the validity or otherwise of Sanofi’s patent had not yet been determined by the courts.
375 Mr Lindsay said in the same affidavit that this request would most likely have taken the form of a letter to the Department prepared by him (or someone who reported to him) and signed by him or the General Manager.
376 There are several points to note about Mr Lindsay’s written evidence on this topic. The first is that, as previously mentioned, it is not a matter raised in any of his prior written evidence. Secondly, and more importantly, he does not explain why in his view the mere existence of a patent proceeding would be a matter that might result in the Minister declining to accept an unconditional application for the listing of Apotex’s clopidogrel products from 1 April 2008. Thirdly, Mr Lindsay’s 2016 affidavit says nothing about the finer details of the Sanofi BMS’ counterfactual analysis as developed in their written and oral submissions with regard to either the April trial date or the anticipated judgment date.
377 Mr Lindsay was asked some questions in his cross-examination concerning the possibility of Sanofi lobbying the Government with a view to preventing Apotex obtaining a PBS listing of its products. The relevant exchange was as follows:
Mr Brereton: … It’s right, isn’t it, that at the time you swore your affidavit, the first affidavit, that you thought that the prospect of Sanofi-Aventis lobbying the government on this matter would have been a pointless exercise? ––– I’m not sure I would agree. It’s never a pointless exercise to speak to the Minister.
Mr Brereton: But I’m right in saying, aren’t I, that it was – if you thought about it, you would expect that lobbying the government was unlikely in the extreme to prevent Apotex from achieving listing? ––– I think it’s true that the chances were low. […]
HIS HONOUR: Why, in your view, would the chances be low? ––– Because it had changed from administrative implementation to legislative implementation.
HIS HONOUR: And when we talk about the chances, you’re talking about the chance of persuading the Minister not to list the Apotex products on the PBS? ––– Yes.
MR BRERETON: You thought the chances of persuading the Minister not to list were low? ––– Yes.
378 I regard Mr Lindsay’s oral evidence as significant because he appears to have been the person within Sanofi who was best placed by reason of his prior knowledge and experience to form a view as to the likelihood of Apotex Australia obtaining a PBS listing of its clopidogrel products in the event that an interlocutory injunction was not granted and what response Sanofi might reasonably expect to receive to a submission to the Minister that she not list Apotex Australia’s clopidogrel products on the PBS until final resolution of the outstanding patent proceeding.
379 Sanofi BMS submitted that the oral evidence given by Mr Lindsay on these matters should be disregarded, or at least, heavily discounted. There were two criticisms of Mr Lindsay’s oral evidence that were made in support of that submission. The first focused on Mr Lindsay’s limited experience of the Department’s practice following the introduction of the 2007 Amendments. The second focused on what was said to be the generality of the relevant parts of his oral evidence.
380 I do not accept either of these criticisms of Mr Lindsay’s oral evidence. Mr Lindsay was the expert within Sanofi Australia who was advising the company’s General Manager in relation to Apotex Australia’s proposed PBS listing. I do not see any reason to question the reliability of his understanding of the PBS system or the Department’s practices in the period from July 2006 until July 2010. I am satisfied that he is likely to have had a practical understanding of the dynamics of the situation in which Sanofi found itself at the time of the interlocutory hearing before Gyles J and the various options that were open to Sanofi in resisting Apotex Australia’s effort to break into the clopidogrel market.
381 As to the generality of Mr Lindsay’s evidence, Sanofi’s submission seems to assume that, because Mr Lindsay merely agreed with some general propositions in cross-examination without further elaboration, that the weight that may be given to his evidence is diminished as a result of him not having exposed his reasoning in any greater detail. I do not accept that submission.
382 The evidence Mr Lindsay gave is in my view consistent with the totality of the evidence as to the likelihood of Sanofi convincing the relevant decision-maker not to approve an application by Apotex Australia to list its clopidogrel products on the PBS from 1 April 2008 and is also consistent with my own view of the practical difficulties that such a course of action would have created for the relevant decision-maker. I identify these difficulties later in these reasons. I am also mindful that it was open to Sanofi to re-examine Mr Lindsay with a view to having him elaborate (for the purposes of demonstrating why his evidence should not carry weight) but that no questions of that kind were asked.
383 I regard Mr Lindsay’s evidence as persuasive; in my view it provides me with a reliable insight into what the thinking of Sanofi’s senior management in Australia was at the time in relation to the likely response to an application by Apotex Australia for the listing of its clopidogrel products including the likely outcome of any attempt by Sanofi to persuade the Minister that Apotex Australia’s products should not be listed from 1 April 2008 on account of the existence of the patent proceeding but in circumstances where no relevant interlocutory relief was in place.
384 Mr Moulding, who was at all relevant times the General Manager of Sanofi Australia, was also of the understanding in September 2007, that if Gyles J declined to grant Sanofi an interlocutory injunction, then the listing of Apotex Australia’s clopidogrel products on 1 April 2008 was all but inevitable. It is apparent from his evidence that this view was based on advice he received from what he referred to as his “specialist teams” led by Mr Lindsay and which no doubt included others with relevant legal and regulatory experience.
385 Mr Moulding also gave evidence that, in the event interlocutory relief had been refused, Sanofi would have contacted the Minister notifying her that:
listing Apotex Australia’s clopidogrel products on the PBS would involve an infringement of Sanofi’s patent; and
there was ongoing litigation between Apotex Australia and Sanofi regarding the validity of the Patent which had not yet been determined.
386 Mr Moulding said that he would have requested the Minister to refrain from listing Apotex’s clopidogrel products on the PBS in circumstances where the Patent was still valid (by which I took him to mean, neither expired nor revoked).
387 Much of what I have said in relation to Mr Lindsay’s evidence on this topic applies to Mr Moulding’s written evidence. It seems to me that it would have been apparent to both Mr Lindsay and Mr Moulding (who clearly enough was acting on Mr Lindsay’s advice) in late 2007 and early 2008 that the prospects of achieving a favourable outcome by lobbying the Minister were remote if the Minister was also made aware that Sanofi’s application for interlocutory relief had been refused or, at the very least, that Sanofi had elected not to make any such application.
388 In the circumstances, I am not persuaded that Sanofi, if confronted by a rejection of its claim for interlocutory relief, would have embarked on an alternative strategy that involved lobbying the Minister. In any event, for reasons that I will later explain, even if Sanofi had written to the Minister seeking to dissuade her from listing Apotex Australia’s clopidogrel products, I am satisfied that it is most unlikely that Sanofi would have been successful in that endeavour.
389 Ms Felicity McNeill was the Assistant Secretary of the PEB between 4 January 2010 and 27 September 2010. On 27 September 2010 she became First Assistant Secretary of the Pharmaceutical Benefits Division (“PBD”).
390 PEB is a branch of PBD. It provides listing, pricing and policy advice to the Minister on issues relating to the PBS, and to that end, manages and supports a number of advisory bodies, including the PBAC and its sub-committees, and formerly the PBPA. It is the part of the Department which was at all relevant times responsible for reviewing and processing applications for listing of generic brands.
391 Ms McNeill’s affidavit evidence was admitted on two bases.
392 First, her affidavit evidence was admitted as evidence of relevant policies and practices of PBD and what was likely to have occurred in the counterfactual scenario in which Apotex Australia applied for PBS listing with effect from 1 April 2008. According to the Commonwealth, Ms McNeill’s evidence, when read with certain documents published by the PBD in 2007 and 2009, demonstrates that there was no material change in relevant policies and practices of the PBD between August 2007 and January 2010.
393 Secondly, her affidavit evidence was also admitted as evidence of the opinions of a person having specialised knowledge in relation to the PBD’s policies and practices in the period from August 2007 to January 2010. This specialised knowledge was, in so far as it related to matters pertaining to any 1 April 2008 listing, knowledge said to have been acquired by her after she became Assistant Secretary of PEB in January 2010.
394 I found Ms McNeill to be an honest and diligent witness who displayed a thorough knowledge of the relevant statutory scheme. However, the fact that Ms McNeill’s affidavit evidence was admitted into evidence does not necessarily mean that it is persuasive or that it should carry significant weight in relation to matters that depend on an assessment of how other persons might have exercised a statutory discretion under the NHA.
395 The cross-examination of Ms McNeill suggested that she had not given prior consideration (at least not until after she made the last of her four affidavits) to the particular counterfactual relied upon by Sanofi BMS. During the course of her cross-examination she accepted that the counterfactual raised with her in cross-examination involved novel circumstances that neither she nor, to the best of her knowledge, anyone else in the PBD had actually encountered or perhaps even contemplated until raised in this proceeding.
396 Ms McNeill was not a relevant decision-maker in 2007, 2008 or 2009 and the question whether or not Apotex Australia’s clopidogrel products would have been listed on 1 April 2008 was one that would have been decided by another Delegate, if not the Minister personally. Neither the Minister, nor any other person who was likely to have been involved in the decision-making process at that time, was called to give evidence.
397 Ms McNeill displayed a detailed knowledge of the relevant administrative requirements and statutory provisions including s 85 of the NHA which provides, in subsection (6) that “the Minister may, by legislative instrument, determine a brand of a pharmaceutical item”. Section 85(6) is the source of the Minister’s power to list a generic supplier’s brand of a pharmaceutical item.
398 In practice, it appears that the Delegate who exercised the discretion under s 85(6) of the NHA could either be the Assistant Secretary who heads PEB or the First Assistant Secretary who heads PBD.
399 Ms McNeill agreed that the Delegate who would have exercised the discretion whether or not to list Apotex Australia’s clopidogrel products on the PBS from 1 April 2008 would have been Ms Diana MacDonell who left the Department in 2011 and is now working as a consultant in the pharmaceutical industry. I will say more in relation to Ms MacDonell later when dealing with Sanofi BMS’ submission that an inference should be drawn against the Commonwealth based on its failure to call her.
400 Ms McNeill’s first affidavit of 28 November 2014 details the process undertaken by the Department after receipt of an application for listing of a first generic brand of a listed drug. It is a process which Ms McNeill says was routine and which did not change in any material respect from August 2007. The Commonwealth submitted that Ms McNeill’s evidence was persuasive and should be given considerable weight because, as head of PEB and then head of PBD, she was well placed to become aware of the relevant policies and practices of the PBD and to express views about how they were likely to be administered.
401 Ms McNeill’s evidence highlighted the importance of two published documents which gave policy guidance to the exercise of the discretion to list in 2007 and 2009. The first of these was the document entitled “Guidelines to Applicants” which set out what information “must be provided to allow an additional brand to be added to the [PBS Schedule]” (the “Guidelines”). The Guidelines provide a statement of what an application for a PBS listing of an additional brand must include. The Guidelines require (inter alia) certain information from the TGA including proof of registration of the brand on the ARTG and a statement of “brand equivalence”. The Guidelines also require “written assurance that stock of the product will be available on the proposed date of listing”. This requirement, which is an administrative rather than statutory requirement, was referred to by Ms McNeill as the assurance of supply.
402 The second document is the Department’s “Listing Unit Requirements”. That document also sets out the documents an applicant for listing is required to provide and the relevant timelines. Other versions of the Listing Unit Requirements published during the period 1 October 2008 to 1 February 2010 are also in evidence. Ms McNeill’s evidence (which I accept) is that there was no material change to the Listing Unit Requirements between late 2007 and 2015.
403 Ms McNeill’s evidence was that if the information required under the Guidelines and the Listing Unit Requirements was provided with an application, and provided that the listing price was agreed to by the generic applicant, then the generic brand would be listed as a matter of course. She said that if Delegates were formally requested to list a generic (by signing the relevant listing instrument), they did so, and that she was not aware of any instances where a Delegate did not adopt a request by the PEB to list a generic brand.
404 Ms McNeill also gave evidence to the following effect:
(a) she was not aware of any Delegate proposing to list a generic brand having conducted, or requested, any searches or inquiries as to whether the application (or the subsequent listing of the generic brand) would infringe a patent;
(b) she never conducted or requested such searches or inquiries, or received a request from another Delegate to do so;
(c) she has never considered patent matters to be relevant to an application for the listing of a generic brand;
(d) she is not aware of any evidence of a Delegate taking into account patent matters when making a determination to list a first generic brand on the PBS between 1 August 2007 and 1 April 2010;
(e) since she joined the Department in January 2010, PEB has not questioned assurances of supply given by any new generic brand prior to listing; she maintained that the assurance is taken at face value;
(f) based on her knowledge and experience, the same approach in respect of the assurance of supply was taken during the period 2007 to 2009;
(g) if an applicant did not provide a written assurance of supply, or if an applicant withdrew the assurance, PEB would not request the Delegate to list the applicant’s generic brand;
(h) conversely, if an assurance of supply was provided and not withdrawn before listing, it was taken at face value, and patent issues were not inquired into or considered.
405 In her first affidavit, Ms McNeill stated:
I have never considered patent matters to be relevant to an application for the listing of a generic brand … I proceed on the basis that matters of patent law, if any, are the concern of the relevant patentees and the applicant seeking to list its generic brand. There may be a patent or there may not; and if there is a patent it might be the subject of a licence or it may not; and the patent may be valid or it may not. The question of the existence of a patent, or a patent dispute, may be relevant to the applicant seeking to list its generic brand in determining whether to give an assurance of supply … but that is a matter for the applicant.
406 Ms McNeill was cross-examined in relation to certain statements appearing in the Explanatory Memorandum and Second Reading Speech relating to the 2007 Amendments which introduced the guarantee of supply requirements (see Part VII Div 3C of the NHA). It was suggested to her in cross-examination that those statements indicated a concern on the part of the legislature that the generic suppliers who obtained PBS listings of their products should be able to supply their listed products on a long term basis so as to avoid interruptions to supply that might be disruptive and harmful to the interests of innovators and consumers. She said that one of the objects of the relevant provisions was to seek to avoid the harmful effects caused by an interruption to supply after listing, but that there were other objects relevant to the question of whether a generic product should be listed on the PBS. However, it is apparent from her evidence as a whole that she also considered the desirability of promoting competition in the market for pharmaceutical items listed on the PBS by (inter alia) the listing of generic brands was an important object of the relevant provisions.
407 The Second Reading Speech to the National Health Amendment (Pharmaceutical Benefits Scheme) Bill 2007 (Cth) suggests at the time the 2007 Amendment Act was enacted that the Government was concerned about the high cost of many medicines and the need to ensure that it paid reasonable prices for medicines without increasing the costs to patients and taxpayers. As the Minister stated:
One of the main changes proposed in this bill is the separation of PBS medicines into two formularies – F1 and F2 – each with different pricing arrangements. Medicines where there is only a single brand listed will be referred to as F1. It will contain both on-patent and off-patent medicines that are not substitutable with other brands or medicines. With this formulary, there will be no mandatory price reductions, and existing price linkages will be retained within this group. This means that single-brand medicines will retain their higher prices until they become subject to competition, providing companies with a greater certainty about the prices of these medicines. This allows the Australian government to ensure these medicines remain subsidised by the PBS, hence continuing their availability at affordable prices. Medicines where there are brands listed and groups of medicines that are interchangeable between patients will be referred to as F2.
408 With regard to the guarantee of supply, the Minister also said:
This will guarantee the supply of all new brands listed on the PBS from 1 August 2007 and the already listed medicines for which price reductions are offered to all patients who require these medications. This will significantly help patients on these medications, as the guarantee of supply period will be 24 months, or until another new brand is listed, or a further price reduction is offered for another already listed medicine. Any company which believes that it will be unable to supply, or has failed to supply, will be required to notify the minister. A criminal penalty will apply for failure to notify the minister. There is also a $33,000 fine for a corporation.
Companies which fail to supply or which are unable to supply may have that brand or other brands on the PBS delisted or they may be refused the listing of new brands. These tough measures introduced with respect to noncompliance with the new price disclosure and supply guarantee measures are another advantage for patients, as they aim to ensure that companies adhere to their commitments. Patients have a right to know that the prices that they are paying for their medications are truly reflective of manufacturing costs. They also have a right to have a continual, guaranteed supply of medications available to them. Patients do not need the added stress of costs often incurred by having to change their medications because they are too expensive or unavailable. These reforms aim to further ensure patients’ quality of life by increasing the accountability and compliance of companies which supply the medication.
409 It is apparent from the elaborate attention that the guarantee of supply receives in the 2007 Amendments (including by the introduction of the relevant criminal penalty provision) that it was regarded as an important measure aimed at ensuring the availability of pharmaceutical items under the PBS.
410 The various statutory provisions relating to the guarantee of supply (which I have previously set out) not only impose a guarantee of supply in respect of the guaranteed period but also include detailed provisions relating to disclosures that must be made to the Minister by the relevant supplier in the event that it has failed to supply or has reasonable grounds to believe that it will be unable to supply in the future.
411 What is most significant about the guarantee of supply provisions for present purposes is that they impose obligations on the supplier that only take effect once the supplier’s products have been listed on the PBS.
412 Importantly, none of the 2007 Amendments require the supplier to make any relevant disclosure either prior to, or at the time of lodging the application for PBS listing, in relation to the supplier’s ability to comply with the guarantee of supply. In particular, none of the relevant provisions require that the supplier satisfy the Minister or the Delegate, or that the Minister or the Delegate be satisfied, that the supplier will comply, or that the supplier has reasonable grounds to believe that it will be able to comply, with its supply obligations throughout the guaranteed period or at all. Nor do the relevant provisions include any express requirement that the Minister or her Delegate give consideration to these matters prior to listing the supplier’s products on the PBS.
413 Further, none of the 2007 Amendments require the supplier to make any disclosure of the existence of a patent proceeding the outcome of which may at some time in the future affect the supplier’s ability to comply with the guarantee of supply. Nor do the relevant provisions include any express requirement that the Minister or her Delegate enquire into the existence of any such proceeding or its potential impact on the supplier’s ability to comply with the guarantee of supply.
414 The discretion under s 85(6) is conferred in terms that are unconfined. There is nothing in s 85 or any other provision of the NHA that is to be understood as requiring the Minister or the Delegate to have regard to the existence or possible outcome of any patent proceeding relevant to the ability of the applicant for listing to comply with the guarantee of supply. While it was contended by Sanofi BMS that this was in fact a matter that the decision-maker was bound to take into account (ie. that it was a mandatory consideration) I do not think that is correct. There is nothing to be found in the subject matter, scope and purpose of the NHA that would support that proposition: Minister for Aboriginal Affairs v Peko-Wallsend Ltd (1986) 162 CLR 24 at 39-40 per Mason J.
415 I fully accept that the existence of a patent proceeding relevant to the applicant’s capacity to supply at the time of listing or during the guaranteed period is a matter that the decision-maker may take into account. What weight should be given to it is a matter for the person exercising the discretion under s 85(6).
416 Sanofi BMS placed considerable reliance in their submissions on the hypothetical nature of Ms McNeill’s evidence and the failure of the Commonwealth to call any person who would have been actually involved in a consideration of any application by Apotex Australia to have its clopidogrel products listed on a PBS from 1 April 2008.
417 I have had regard to the failure of the Commonwealth to call either the Minister or any Delegate (including Ms MacDonell) who was likely to have been involved in any actual decision to list. So far as the failure to call the Minister is concerned, I do not think any inference unfavourable to the Commonwealth should be drawn. It seems to me unlikely that the Minister would have been involved in the making of that decision which is more likely than not to have been left to the Delegate. Even if Sanofi had lobbied the Minister, Sanofi would have been unlikely to have influenced the decision in circumstances where Sanofi, as patentee, had either not applied for, or had been refused, an interlocutory injunction restraining Apotex Australia from supplying its clopidogrel products.
418 What I find most persuasive on this issue is not Ms McNeill’s evidence, but the evidence given by Mr Lindsay and Mr Moulding who were refreshingly frank in their implicit acceptance that any application by Apotex Australia for a listing of its clopidogrel products would most likely have been approved subject to Apotex providing an assurance of supply and complying with all other necessary formalities.
419 I do not think it likely that the Delegate would have refused the application on the basis that a trial of the patent proceedings would shortly take place or that a judgment might be expected to be given some time between May 2008 and August 2008. In my view the Delegate is likely to have been most influenced by two matters: first, the willingness of Apotex Australia to provide an assurance of supply and, second, the absence of any interlocutory injunction restraining any such supply. I think it unlikely that a Delegate would have questioned the ability of Apotex Australia to either comply with its assurance of supply or comply with its obligations under the guarantee of supply. So far as the latter was concerned, I consider it most likely that the Delegate would have proceeded on the basis that, in the event that there was some failure on the part of Apotex to supply during the guaranteed period, then it would be open to the Minister in that situation to exercise one or more of the powers available under the relevant provisions of the NHA including the power to delist the Apotex Australia clopidogrel products and the power to reverse the 12.5% statutory price reduction.
420 One of the difficulties that would confront the Minister or Delegate who was made aware of the trial dates and the estimated judgment date would be understanding who was likely to prevail at the trial or on appeal. The decision-maker would not obtain much assistance from the protagonists each of whom would almost certainly proclaim the strength of its position. Nor could the decision-maker be expected to independently investigate the strength of each party’s case given the complexity of the factual and legal questions that usually arise in proceedings brought to enforce or revoke a pharmaceutical patent. All this leads me to conclude that the decision-maker would be unlikely to question Apotex Australia’s capacity to supply its clopidogrel products.
421 I find that an application made by Apotex Australia to list its clopidogrel products on the PBS from 1 April 2008 would most likely have been approved by the Minister or Delegate.
THE SIGNIFICANCE OF THE FIRST APOTEX UNDERTAKING
422 As previously explained, the interlocutory injunction did not in terms (whether expressly or impliedly) prevent Apotex Australia from taking steps to have its clopidogrel products listed on the PBS. It was the first Apotex undertaking that prevented Apotex Australia from taking such steps. Sanofi submitted that the loss claimed by the Commonwealth as a result of Apotex Australia having been prevented from taking steps to have its clopidogrel products listed on the PBS was not a loss that would have been suffered but for the interlocutory injunction because it was the operation of the first Apotex undertaking rather than the interlocutory injunction that prevented Apotex Australia from taking such steps.
423 The Commonwealth contended that Sanofi’s reliance on the “but for” test was excessively technical, that it reflected an overly rigid approach, and that it did not reflect the reality of a situation in which the first Apotex undertaking would never have been given but for the decision of Gyles J to grant the interlocutory injunction.
424 The giving of the first Apotex undertaking was not a necessary or natural consequence of the making of the interlocutory injunction. The undertaking was volunteered by Apotex Australia in spite of its stated position (later held to be correct) that taking steps to obtain a PBS listing of its clopidogrel products would not constitute an act of patent infringement.
425 In its submissions the Commonwealth referred to the course of the argument before Gyles J and the expressed desire on the part of Apotex Australia to avoid what it referred to as a “jurisdictional argument” about the legal significance of an application for a PBS listing and whether the making of such an application could constitute an act of infringement.
426 While a consideration of those matters may explain why Apotex Australia agreed to provide the first Apotex undertaking, it does not explain the absence of an undertaking as to damages with respect to that restraint. There is no evidence to explain why no undertaking as to damages was given in relation to the first Apotex undertaking. But there is also no evidence to suggest that this departure from the usual practice to give an undertaking as to damages in respect of an interlocutory injunction or undertaking to the Court was anything other than a matter to which the parties to the patent proceeding knowingly and willingly agreed.
427 The Commonwealth accepted that it needed to establish that the interlocutory injunction had the effect of preventing Apotex Australia from obtaining a PBS listing of its clopidogrel products. It advanced a number of arguments as to why the interlocutory injunction should be found to have had that effect. It submitted that the interlocutory injunction prevented Apotex Australia from obtaining PBS listing of its clopidogrel products because it prevented Apotex Australia from giving an assurance of supply and from complying with the statutory guarantee of supply. On this basis, the first Apotex undertaking was said by the Commonwealth to make explicit what was in any event a practical consequence of the restraint imposed by the interlocutory injunction.
428 Because the interlocutory injunction prevented Apotex Australia from supplying its clopidogrel products, it would have made it impossible for Apotex Australia to give an assurance of supply or to comply with its supply obligations during the guaranteed period for so long as it remained in force. I therefore accept that the interlocutory injunction had the practical effect of preventing Apotex Australia from applying for a PBS listing of its clopidogrel products from 1 April 2008 assuming it was otherwise willing and able to do so.
429 The Commonwealth also submitted that there was a clear and direct relationship between the interlocutory injunction and the first Apotex undertaking. It submitted that the undertaking was ancillary to the interlocutory injunction in that, had there been no interlocutory injunction, the first Apotex undertaking would never have been given. I accept that submission. If Gyles J had decided not to grant an interlocutory injunction, then the first Apotex undertaking would never have been given.
430 In their submissions Sanofi BMS contended that the but for test could not be satisfied in this case because, if there had been no interlocutory injunction, Apotex Australia would still have been prevented by the operation of the first Apotex undertaking from applying for PBS listing. However, in my view that is not the appropriate way to approach this issue. Rather, it is necessary to ask whether, if there had been no interlocutory injunction, Apotex would have been free to apply for PBS listing.
431 If one asks whether the interlocutory injunction prevented Apotex Australia from applying for a PBS listing of its clopidogrel products then the answer to that question would be yes for the reasons previously given. The correctness of that proposition can also be tested by asking whether, if the first Apotex undertaking had not been given, the interlocutory injunction would have prevented Apotex from applying for PBS listing of its clopidogrel products. The answer to that question is also yes for the reasons previously given. The fact that there was a further and more direct restraint imposed by the first Apotex undertaking does not mean that the interlocutory injunction did not also prevent Apotex Australia from making such an application.
432 I accept that if the interlocutory injunction had not been granted, then the first Apotex undertaking would also not have been given. I find that but for the interlocutory injunction, there would have been no restraint in place that would have prevented Apotex Australia from taking steps to apply for a PBS listing of its clopidogrel products were it otherwise willing and able to do so.
Did the Commonwealth’s loss flow directly from the grant of the interlocutory injunction?
433 The question whether the connection between the interlocutory injunction and the alleged loss is sufficiently “direct” is distinct from the question whether the alleged loss was reasonably foreseeable at the time the interlocutory injunction was granted.
434 What does the concept of “directness” require in this context?
435 Aickin J referred to a number of relevant authorities in Air Express. The first of these was Smith v Day (1882) 21 Ch D 421 which was a case in which the plaintiff had obtained an interlocutory injunction restraining the erection of a new building. The interlocutory injunction was subsequently discharged, and the defendant made a claim on the plaintiff’s undertaking as to damages. The only damage alleged by the defendant was that he had agreed to lease part of the property within the new building to a tenant, and was prevented from doing so by the interlocutory injunction preventing his building work.
436 Much of what is said in Smith v Day is obiter because the defendant failed to establish that he had entered into a binding agreement for lease with the prospective tenant. Nevertheless, each member of the Court considered what the position would have been if the defendant had established the existence of such an agreement and that he was in breach of it by reason of the existence of the interlocutory injunction. Brett LJ said at 428:
Here an alleged agreement for a lease is relied on. In the first place I do not think the existence of such agreement proved. If it did exist, the next question is, whether the injunction so interfered with the erection of the buildings as to entitle the tenant to throw up the agreement. I am not satisfied that it did. But assume that it did, and that the agreement was broken in consequence of the injunction, still I agree with the Vice-Chancellor in thinking that the breach is not by reason of the injunction, but is a consequence too remote to be regarded. If anyone obtains an injunction preventing another from proceeding with a building, he must be taken to have notice of everything in the building contract, and all liabilities which the person stopped incurs to his contractor by reason of the stoppage, are a natural and immediate consequence of the injunction. But the fact that the injunction prevents the carrying out of an entirely independent agreement as to the property is too remote.
437 Cotton LJ said at 430:
I think that the damages must be confined to loss which is the natural consequence of the injunction under the circumstances of which the party obtaining the injunction has notice …
438 Jessel MR expressed the view at 426-427 that, even if the underlying facts were proven, the damage claimed would not be recoverable on the undertaking as to damages on the basis that it was too remote.
439 Another case referred to by Aickin J was Schlesinger v Bedford (1893) 9 TLR 370. In that case Lindley LJ (with whom Lopes and AL Smith LJJ agreed) said at 370-371:
The real nature of an undertaking of this kind and the extent to which damages ought to be awarded thereunder were carefully explained by the late Master of the Rolls in the well-known case of Smith v. Day. That case was instructive for this reason, that it showed that all the remote consequences of obtaining an injunction which was afterwards dissolved, were not to be taken into account in assessing the damages to be paid to the defendant under the plaintiff's undertaking. It would be unduly straining such undertaking to include in it damages which did not naturally flow from the injunction. In Smith v. Day it was held that the damage was too remote. The defendant there claimed that he had lost a good tenant by reason of the injunction, but it turned out that there had not at the date of the injunction been any agreement for a lease, although negotiations had been entered into with a view to a lease. That case was followed by Ex parte Hall: In re Wood, where a receiver obtained an injunction restraining a man from the selling of certain goods, and damage resulted from the receiver restraining him from removing the goods. The Court held that the man against whom the injunction was obtained was not entitled to recover any damage except such as resulted naturally from his being restrained from selling and that the damage was too remote. So here the plaintiffs ought not to be exposed to damages which were not fairly consequential upon the injunction, and which they could not have foreseen when the injunction was granted.
(citations omitted)
440 Other cases referred to by Aickin J in Air Express at 264-265 speak of the need for the damage to have “necessarily and naturally flowed” from the interlocutory injunction for it to be recoverable. It is therefore necessary when applying this test to have regard to the precise terms of the interlocutory injunction in order to assess whether the damage allegedly suffered as a result of the restraint imposed by it flows naturally from it.
441 Sanofi submitted that the interlocutory injunction did not directly affect any legal right, obligation or interest of the Commonwealth and that its alleged loss was therefore merely an “indirect or consequential” effect of the operation of the interlocutory injunction upon the legal rights and obligations of Apotex Australia.
442 The Commonwealth submitted that its legal obligations were directly affected by the interlocutory injunction. It submitted that, if Apotex Australia’s clopidogrel products had been listed from 1 April 2008, the statutory price reduction would have automatically been triggered with the consequence that the amount of the payments that the Commonwealth was legally obliged to make would also have been reduced.
443 In my view, the interlocutory injunction did not directly affect the legal rights, obligations or interests of the Commonwealth. It did not prevent the Commonwealth from receiving and accepting an application for PBS listing by Apotex Australia of any one or more of its clopidogrel products or the clopidogrel products of any other generic supplier. At most it prevented Apotex Australia from entering the market for clopidogrel in Australia which in turn had the practical effect of denying the Commonwealth the financial benefits it would have obtained were it to have received and accepted an application by Apotex Australia to list its clopidogrel products on the PBS from 1 April 2008.
444 The Commonwealth also submitted that it was not necessary for it to show that its legal rights, obligations or interests were directly affected by the interlocutory injunction. It submitted that the question to be addressed was whether the loss suffered by it was the direct consequence of the legal or practical operation of the interlocutory injunction.
445 Even if it is accepted, as I have found, that the first Apotex undertaking would never have been given if the interlocutory injunction had not been granted, it does not follow that the Commonwealth’s loss flowed directly from the interlocutory injunction. The terms of the interlocutory injunction did not prevent Apotex Australia from applying for a PBS listing of its clopidogrel products or from taking any other steps to obtain such a listing. Doing so would not have involved a breach of the interlocutory injunction. The Commonwealth’s loss was a natural and direct consequence of Apotex Australia not being able to apply to list its clopidogrel products on the PBS with effect from 1 April 2008, which was the precise conduct to which the first Apotex undertaking was directed, but not something the interlocutory injunction expressly or implicitly prohibited. This strongly suggests, in my view, that the loss alleged by the Commonwealth in this case was an indirect consequence of the interlocutory injunction.
446 It is also necessary to take account of the objective intention of the parties as reflected in the form of orders and undertakings agreed between Sanofi and Apotex Australia once Gyles J informed them that he would grant an interlocutory injunction. The form of the interlocutory injunction (which did not by its terms prevent listing), the form of the first Apotex undertaking (which by it terms did prevent listing), and the fact that the former but not the latter was supported by Sanofi’s undertaking as to damages, provides strong contextual support for the view that the undertaking as to damages should not be interpreted as extending to loss suffered by the Commonwealth (or any other person) as a result of Apotex Australia being prevented from applying to have its clopidogrel products listed on the PBS.
447 The Commonwealth placed reliance on some observations I made in Otsuka Pharmaceutical Co Ltd v Generic Health Pty Ltd [2015] FCA 848 (“Otsuka”). It was a case in which I granted the Commonwealth leave to intervene for the limited purpose of being heard on the appellant’s application for a stay of final orders that (inter alia) revoked various claims of the patent in suit. The Commonwealth opposed the grant of a stay and also sought, in the alternative, an order requiring that the appellant provide security in relation to its undertaking as to damages which the Commonwealth submitted should be modified so as to expressly permit it to recover compensation for any financial loss it suffered in the event the appellant’s appeal was unsuccessful. The financial loss against which the Commonwealth sought to be protected included the loss that would arise if one or more generic products (including those that the respondent was proposing to supply) could not be listed on the PBS prior to the determination of the appeal.
448 I said at [45]:
The fact that the person seeking security is not a party to the relevant proceeding, or bound by the relevant restraint, may well suggest that it is not likely to be adversely affected by the order, at least not in any direct or foreseeable way. However, this could not be true of the Commonwealth in the circumstances of the present case. The loss it will suffer if a stay is granted and the appeal is later dismissed seems to me to be both direct and foreseeable.
In its submissions the Commonwealth focused attention on the last sentence of this passage.
449 Otsuka was an interlocutory decision given without the benefit of full argument. I would not feel constrained by what I said in that case if I was persuaded that it was incorrect. But it is important to note the facts in Otsuka that distinguish it from this case.
450 First, the interlocutory relief that was granted by me was not an interlocutory injunction granted before trial but a stay of final orders including an order revoking the relevant claims of the patent in suit and discharging an interlocutory injunction granted before the trial. Secondly, and more importantly, the relevant interlocutory relief granted by me included an order requiring the respondent to withdraw any application it had made to list its product on the PBS. This relief was granted upon the patentee giving an undertaking as to damages. It was therefore not a case in which the relevant restraint was not supported by any undertaking as to damages.
451 In my view, any financial loss suffered by the Commonwealth as a result of Apotex Australia’s clopidogrel products not having been listed on the PBS from 1 April 2008 was not a loss that flowed directly from the existence of the interlocutory injunction but was the direct and immediate consequence of the first Apotex undertaking. This was an undertaking given by Apotex Australia to the Court that was not supported by any cross-undertaking as to damages. The Commonwealth’s loss is therefore not recoverable pursuant to the undertaking as to damages given in support of the interlocutory injunction.
Could loss of the kind sustained have been foreseen at the time the interlocutory injunction was granted?
452 The next question that arises is whether the loss suffered by the Commonwealth as a result of Apotex Australia being prevented from listing its clopidogrel products on the PBS from 1 April 2008 was loss of the kind that could have been foreseen at the time the interlocutory injunction was granted.
453 Sanofi accepts that it knew at the time it gave the first undertaking as to damages that the listing of Apotex clopidogrel products on the PBS from 1 April 2008 would trigger an immediate 12.5% reduction in the price paid by the Commonwealth for clopidogrel and that it would also activate a price disclosure regime that might lead to further price reductions in the future. These matters would have been obvious to Sanofi and were in fact the focus of much of the affidavit evidence it relied upon before Gyles J at the interlocutory hearing on 18 September 2007 and in the submissions made to his Honour at that time.
454 Nevertheless, Sanofi contends that the loss suffered by the Commonwealth was not reasonably foreseeable at the time the interlocutory injunction was granted because:
(a) the interlocutory injunction did not restrain Apotex Australia from applying for a PBS listing of its clopidogrel products;
(b) no other generic supplier was restrained by the interlocutory injunction or any other relevant restraint. As a result, it was possible that clopidogrel products of another generic supplier might be listed on the PBS from 1 April 2008;
(c) the automatic triggering of the 12.5% price reduction was a result of amendments made to the NHA which only took effect from 1 August 2007;
(d) the Commonwealth had never, until it did so in this proceeding, applied for compensation pursuant to the usual undertaking as to damages in a proceeding brought by a patentee to restrain infringement of a pharmaceutical patent;
(e) Sanofi had notified the Commonwealth on 29 August 2007 that it intended to commence a proceeding for patent infringement against Apotex Australia and, in spite of having been given such notice, the Commonwealth did not notify Sanofi that it considered itself adversely affected by the interlocutory injunction; and
(f) the claim that the Commonwealth was “adversely affected” by making higher payments under PBS, was not to be foreseen because no legal right or liability of the Commonwealth was affected by the interlocutory injunction.
455 I will deal with each of Sanofi’s contentions in turn.
456 The fact that the interlocutory injunction did not itself prevent Apotex Australia from taking steps to list its clopidogrel products on the PBS does not mean that the loss alleged to have been suffered by the Commonwealth as a result of the grant of the interlocutory injunction was not reasonably foreseeable. It was reasonably foreseeable at the time Sanofi applied for the interlocutory injunction that, if Apotex Australia was prevented from supplying its clopidogrel products, then it would not make any application to list those products on the PBS for so long as the interlocutory injunction remained in force. Although I found that this was an indirect consequence of the grant of the interlocutory injunction, it was nevertheless a consequence that was reasonably foreseeable at the time the interlocutory injunction was granted.
457 The fact that there was a possibility that another generic supplier might apply to list its own clopidogrel products from 1 April 2008 does not mean that any loss suffered by the Commonwealth if Apotex was prevented from supplying its clopidogrel products could not be foreseen. Merely because any loss that might be suffered by the Commonwealth as a result of Apotex Australia not listing its clopidogrel products on the PBS from 1 April 2008 might have been avoided if another generic supplier was willing and able to obtain such a listing does not mean that the Commonwealth’s alleged loss was not reasonably foreseeable. All this shows is that the Commonwealth’s alleged loss would not have been seen as certain to occur.
458 The fact that the automatic price reduction that would have been triggered by the PBS listing of Apotex Australia’s clopidogrel products was a result of changes made to the NHA which took effect in August 2007 is quite irrelevant. It is clear from the evidence before Gyles J that Sanofi well understood the effect of the relevant changes.
459 Nor is the fact that the Commonwealth had not sought compensation pursuant to the usual undertaking as to damages in any prior proceeding relevant to the question whether the alleged loss sustained by the Commonwealth was a reasonably foreseeable consequence of the interlocutory injunction that was granted. The same is also true of any failure on the part of the Commonwealth to notify Sanofi that it may consider itself adversely affected by the interlocutory injunction. While these matters may have encouraged Sanofi to believe that the Commonwealth was unlikely to make a claim on Sanofi’s undertaking as to damages (though there is no evidence to that effect) it does not bear on the question of whether the loss alleged by the Commonwealth was a reasonably foreseeable consequence of the interlocutory injunction.
460 The last of Sanofi’s contentions on this topic confuses the question of directness with foreseeability. The fact that the interlocutory injunction did not directly affect the Commonwealth’s legal rights, obligations or interests, does not make any loss arising out of an indirect effect of the interlocutory injunction not reasonably foreseeable. Of course, the more indirect the effect of the interlocutory injunction, the more difficult it may be to establish that any resulting loss was foreseeable. But I do not think this is true in the present case. Subject to what follows, it is my view that the loss of the kind alleged by the Commonwealth was a reasonably foreseeable consequence of the operation of the interlocutory injunction.
461 One component of the Commonwealth’s alleged loss that requires separate consideration is that which arises out of the operation of the price disclosure price reduction regime.
462 It is common ground that if Apotex Australia’s clopidogrel products had been listed on the PBS on 1 April 2008, the price disclosure provisions would have been activated which would in turn have given rise to the possibility of a price disclosure reduction for all listed clopidogrel products on 1 April 2010.
463 Although Sanofi BMS submitted that the possibility that such a reduction would in fact eventuate was not reasonably foreseeable at the time the interlocutory injunction was granted, it is clear from the evidence (including evidence relied upon by Sanofi before Gyles J at the interlocutory hearing) that Sanofi was well aware prior to the grant of the interlocutory injunction that there was a serious possibility, if not a likelihood, that a price disclosure price reduction would occur on 1 April 2010 unless Apotex Australia was restrained from obtaining a PBS listing of its products on 1 April 2008. It is apparent from Mr Moulding’s evidence that he understood at the time the interlocutory injunction was granted that if Apotex Australia’s clopidogrel products were listed on the PBS on 1 April 2008, then this would activate the price disclosure regime and could lead to further price decreases in future years that went beyond the mandatory price reductions triggered by such a listing. It is also apparent from Mr Lindsay’s evidence that activation of the price disclosure regime could also lead to price reductions which could “… result in a large reduction in the approved ex-manufacture price for drugs containing clopidogrel”.
464 Another component of the Commonwealth’s alleged loss that requires separate consideration is that which relates to the combination products containing clopidogrel with aspirin (Sanofi’s CoPlavix and BMS’ DuoCover).
465 Both of these combination products were listed on the PBS on 1 December 2009 at a price that had been agreed between the Commonwealth and Sanofi on or about 13 May 2009 and which reflected the price of clopidogrel at that time. The agreed price for the combination products was based upon a PBAC recommendation which stipulated that the price of the combination products should be no higher than the price of clopidogrel (ie. that there should be no addition to the price in respect of the aspirin component).
466 There were price agreements in place with respect to both combination products for the period 1 December 2009 to 30 September 2012. Each of these agreements was based upon the clopidogrel price prevailing as at 1 December 2009.
467 The Commonwealth’s alleged loss includes an amount referable to the difference between what it actually agreed to pay in respect of the combination products and what it contends it would have agreed to pay in the counterfactual world. It is not disputed that the first possible date on which listed clopidogrel products could have been the subject of a price disclosure price reduction if a generic clopidogrel product had been listed on 1 April 2008 was 1 April 2010.
468 The combination products were not registered on the ARTG until September 2009 and were not listed on the PBS until 1 December 2009 (ie. more than two years after the interlocutory injunction was granted).
469 The Commonwealth sought to attribute this component of its alleged loss to the existence of the interlocutory injunction on the basis of a counterfactual analysis that involved a number of steps.
470 First, if a generic brand of clopidogrel had been listed on the PBS on 1 April 2008, then by about 31 July 2009, it would have been apparent to the Department and the responsible persons for all listed brands of clopidogrel whether or not clopidogrel would be the subject of a price disclosure reduction on 1 April 2010. This is because such a price disclosure reduction would have been calculated, determined and notified by about 31 July 2009.
471 Secondly, if Sanofi and BMS had sought to list their combination products on the PBS from 1 December 2009 in circumstances where clopidogrel was expected to be the subject of a price disclosure price reduction on 1 April 2010, the Department would have sought to negotiate an agreed price for the combination products that took account of the scheduled price reduction for clopidogrel that was due to take effect on 1 April 2010.
472 None of the Sanofi witnesses was cross-examined with a view to showing that at the time the interlocutory injunction was granted Sanofi knew or could reasonably have foreseen that there was a real possibility that the interlocutory injunction may have had some adverse financial impact on the Commonwealth arising out of the sale of any combination products that were not then listed on the PBS or the subject of any application for such a listing.
473 The Commonwealth’s counterfactual analysis in relation to the pricing of the combination products finds support in the evidence of Ms McNeill who explained that the Commonwealth would have only agreed a price for the combination products after 31 July 2009 that took account of the price reduction of clopidogrel that was expected to take effect on 1 April 2010. Her evidence on this topic was logical and persuasive.
474 Even if the exact amount of the price reduction expected to take effect on 1 April 2010 would not have been known to the Department or Sanofi at the time they entered into a pricing agreement, there would have been nothing to stop the Department from agreeing a price that was either the same (if not less) than the price for clopidogrel as at 1 April 2010 in circumstances where it was known that a price disclosure price reduction would occur on that date. Such an agreement would have taken into account both the anticipated price disclosure price reduction and the PBAC recommendation.
475 It seems to me unlikely that it would have been possible for Sanofi to negotiate a price for the combination products that was higher than the price that was to be paid for clopidogrel given the terms of the PBAC recommendation. No witness suggested that Sanofi would have attempted to negotiate a price greater than that recommended by PBAC.
476 Sanofi BMS submitted that the counterfactual analysis relied upon by the Commonwealth should not be accepted because it was based on matters that were “unknown and unknowable” at the time the interlocutory injunction was granted and depended on what changes (if any) the Commonwealth might make to the rules governing price disclosure price reductions.
477 The possibility that the rules governing price disclosure price reductions might change does not answer the Commonwealth’s point that a reasonable person in Sanofi’s position at the time the interlocutory injunction was granted could have anticipated that it may well have had the effect of delaying a price disclosure price reduction at some time in the future. The possibility of any such price reduction did not depend on there being any change to the existing rules.
478 I also consider that it was reasonably foreseeable at the time of the grant of the interlocutory injunction preventing the listing of Apotex Australia’s clopidogrel products on 1 April 2008 and activation of the price disclosure price reduction regime could well affect price negotiations in respect of any combination products of which clopidogrel was a key component in the months leading up to any price disclosure price reduction such as was likely to occur on 1 April 2010.
479 Although it would not have been possible to know with any measure of certainty what impact the listing of Apotex products on 1 April 2008 may have had on such negotiations, a reasonable person in Sanofi’s position would have understood that it may well influence future price negotiations relating to clopidogrel products (including new combination products) by such that the Commonwealth would pay less for those products than it otherwise would have done.
480 I find that loss of the kind said to flow from the interlocutory injunction due to its effect on the pricing of the combination products was reasonably foreseeable at the time the interlocutory injunction was granted.
481 As to what would have actually happened in the event that Apotex Australia’s clopidogrel products had been listed on the PBS on 1 April 2008 and at all material times thereafter (ie. in the continuous supply counterfactual), I think it is likely the Minister, acting on the advice of her departmental officers, would have declined to enter into any price agreement in respect of the combination products until such time as the size of the expected 1 April 2010 price disclosure price reduction was known. At the very least I would have expected any price agreement entered into before 1 April 2010 to have included some mechanism that took any such price reduction into account and passed on the benefit of it to the Commonwealth.
THE SIGNIFICANCE OF THE FINAL ORDERS
482 Some important questions arise concerning the final orders made by Gyles J on 19 August 2008 in various counterfactual scenarios in which there was (inter alia) no interlocutory injunction granted, the first Apotex undertaking was never given, and the Minister or her Delegate had received and approved an application made by Apotex Australia for PBS listing of its clopidogrel products with effect from 1 April 2008.
483 In their submissions the Commonwealth and Sanofi BMS referred to two principal counterfactual scenarios in their analysis of the effect of Gyles J’s judgment of 19 August 2008.
484 The first counterfactual scenario was referred to as the “interrupted supply counterfactual” which comprises a hypothetical scenario in which there was no interlocutory injunction, and in which Apotex Australia, having obtained PBS listing of its clopidogrel products from 1 April 2008, supplied those products continuously from in or about March or April 2008 through until 19 August 2008 or shortly thereafter, at which time they were withdrawn from sale pending the determination of the appeal from Gyles J’s final orders.
485 The second counterfactual scenario was referred to in submissions as the “continuous supply counterfactual” which comprises a hypothetical scenario in which there was neither an interlocutory injunction nor a final injunction and in which Apotex Australia, having obtained PBS listing of its clopidogrel products from 1 April 2008, continued to supply those products from in or about March or April 2008 through to 12 March 2010 when the High Court dismissed Sanofi BMS’ application for special leave to appeal.
486 Sanofi BMS submitted that the interrupted supply counterfactual is the appropriate scenario against which to assess the Commonwealth’s alleged loss, the effect of Gyles J’s final orders, and the significance that is to be attributed to what may have been an unwillingness on the part of Apotex HQ to permit Apotex Australia to commence supplying its clopidogrel products on or shortly after 19 August 2008 prior to resolution of the litigation.
487 The Commonwealth submitted that the continuous supply counterfactual is the appropriate scenario against which to assess the Commonwealth’s alleged loss because it gives effect to the separate undertakings as to damages given in relation to both the interlocutory injunction and the final injunction. The continuous supply counterfactual requires a consideration of various matters including how both Apotex Australia and the Commonwealth would have responded to Gyles J’s final judgment in circumstances where there was no final injunction restraining Apotex from continuing to supply products that had been on the market since March or April 2008.
488 I have previously set out the terms of the declarations and orders made by Gyles J on 19 August 2008 following the trial of the proceeding. They included a declaration as to the validity of (inter alia) claim 3 of the Patent, a declaration that Apotex Australia’s clopidogrel products infringed claim 3, and a final injunction restraining Apotex Australia from infringing claim 3.
489 As previously stated, the interlocutory injunction ceased to operate upon the making of the final orders. From the moment those orders were made on 19 August 2008, it was not the interlocutory injunction, but the final injunction, that prevented Apotex Australia from supplying or offering to supply its clopidogrel products. And unless there was a stay granted in respect of the final injunction until the determination of any appeal by Apotex Australia against Gyles J’s final orders, it would not have been possible for Apotex Australia to continue to meet its supply obligations under the guarantee of supply.
The Interrupted Supply Counterfactual
490 On the assumption that Apotex had obtained a PBS listing for its products on 1 April 2008 and had been supplying its products from March or April 2008 up to the date Gyles J made his final orders, three questions arise. First, would Apotex Australia have applied for a stay of the final injunction to enable it to continue to supply and to maintain its PBS listing until the determination of any appeal? Secondly, would such a stay have been granted and, if so, on what terms? Thirdly, would Apotex Australia have been willing and able to comply with any terms that may have been imposed by the Court as a condition of granting a stay including, in particular, any requirement that it provide security by way of bank guarantee?
491 Mr Millichamp dealt with this counterfactual scenario in his 2017 affidavit. In that affidavit he says:
Assuming that Apotex Australia undertook a March 2008 Launch, I have been asked what Apotex Australia would have done on 12 August 2008, if Gyles J delivered judgment in favour of Sanofi, and on 19 August 2008 when the Final Orders were made. In those circumstances, I would have:
(a) advised the Department on 12 August 2008 of the judgment, and told them that there was a possibility that Apotex Australia may not be able to meet its supply obligations;
(b) taken legal advice and, if there was a reasonable basis for doing so, sought a stay of the Final Orders pending an appeal by Apotex Australia (as I say at paragraph 70 above, Apotex Australia did in fact appeal certain aspects of Gyles J's judgment and the Final Orders);
(c) if necessary, proffered an undertaking as to damages and a bank guarantee to support the undertaking; and
(d) if that stay were refused by Gyles J on 19 August 2008 or some other date, I would have immediately advised the Department and moved to de-list the Listed Apotex Clopidogrel Products from the PBS Schedule.
492 Mr Millichamp’s evidence is qualified in so far as it indicates that the steps that he says he would have taken depended on the legal advice he would have obtained. Legal advice in relation to the implications of seeking and obtaining a stay would most likely require a consideration of the strength of the appeal and the likelihood of Gyles J’s final orders being set aside. Consideration would also need to be given to the extent to which Apotex Australia would be exposing itself to an award of damages in respect of loss suffered by Sanofi during the period from the grant of the stay until the determination of the appeal. Given Apotex Australia’s financial circumstances, it was highly likely that Apotex Australia would be required to provide a bank guarantee to secure any potential damages award as a condition of it being granted a stay of the final injunction.
493 These are all matters upon which Apotex HQ would need to be consulted and about which senior management of the Apotex group would make relevant decisions.
494 There is a fundamental difficulty with Mr Millichamp’s evidence in that it says nothing at all about the need to consult with Apotex HQ in relation to any application for a stay. The question whether Apotex Australia would have sought a stay of the final injunction and whether it would have been willing to comply with the terms upon which any such stay might be granted is a matter about which Mr Millichamp would have had to take instructions from Dr Sherman, Mr Baxter or Mr Kay. The ultimate decision would most likely have had to be made by Dr Sherman acting on advice and information provided by Mr Baxter and Mr Kay. It is likely that Dr Sherman would also have consulted Mr Hughes.
495 To the extent that the appropriate counterfactual analysis depends upon Apotex having applied for and obtained a stay of the final injunction, Sanofi BMS submitted that the evidence does not establish that Apotex Australia would have applied for any such stay, or at least not with a view to continuing to supply clopidogrel products pending the determination of Apotex Australia’s appeal. I accept that submission. It cannot be inferred in the absence of evidence from the relevant decision-maker at Apotex HQ that Apotex Australia would have sought to apply for a stay so that it could continue to supply.
496 Sanofi BMS also submitted that even if Apotex Australia had applied for a stay, it is unlikely that it would have been granted. I think that is right.
497 The starting point is that a “successful party is presumed to be entitled to the benefits of the judgment obtained”: Advanced Building Systems v Ramset Fasteners (Aust) Pty Ltd (1997) 145 ALR 121 at 122. The judgment after a hearing on the merits is not to be regarded as provisional in character pending the determination of an appeal: Red Bull Australia Pty Ltd v Sydneywide Distributors Pty Ltd [2001] FCA 1750 at [6] per Hely J. The applicant for a stay has the burden of persuading the Court that it should be granted.
498 Apotex Australia’s clopidogrel products would have been on the market for a relatively short period of time. The whole purpose of having an early final hearing was to ensure that Sanofi’s application for final relief could be promptly determined. It seems to me unlikely that, in circumstances where Apotex Australia had failed at trial, it would have been permitted to remain in a market that it had first entered, with eyes open, shortly before the trial commenced and less than six months before final relief was granted.
Reversal of the 12.5% Price Reduction
499 According to Mr Millichamp’s evidence, he would have immediately advised the Department and moved to de-list Apotex Australia’s clopidogrel products if a stay had been refused by Gyles J on 19 August 2008 or some other date. His evidence does not indicate what he would have done in the event that he was not provided with instructions to obtain a stay. However, I infer that he would have taken the same steps if he had been instructed not to apply for a stay of the final injunction.
500 The Commonwealth submitted that, even if Apotex Australia ceased supplying its clopidogrel products on or about 19 August 2008 while it awaited the determination of its appeal, the relevant decision-maker in the Department would not have reversed the 12.5% statutory price reduction that took effect on 1 April 2008.
501 The Commonwealth accepted that in the interrupted supply counterfactual the relevant decision-maker would have been faced with a set of novel circumstances. The only evidentiary support for the Commonwealth’s contention that the 12.5% price reduction would not have been reversed in the interrupted supply counterfactual is in the evidence of Ms McNeill.
502 In her written evidence Ms McNeill expressed her opinion as to what she would have done in the event that Apotex Australia had advised that it could no longer supply its clopidogrel products. She said in that situation she would have delisted Apotex Australia’s clopidogrel products once Apotex Australia advised that it could no longer supply them.
503 That Apotex Australia’s clopidogrel products would have been delisted from the PBS in the interrupted supply counterfactual is not in dispute. The question is whether, notwithstanding that the products would have been delisted, the relevant decision-maker would have declined to reverse the 12.5% price reduction.
504 In her evidence Ms McNeill considered whether, in circumstances where Apotex’s clopidogrel products had been delisted, she would have decided to exercise powers under s 99AEI (to reverse the 12.5% price discount) and s 99AEJ (to return Plavix and Iscover to the F1 formulary). Relevantly, Ms McNeill said in her written evidence:
17. Following the delisting of a guaranteed brand, and assuming that PLAVIX and ISCOVER were still co-marketed, the delegate would also have had to consider whether to exercise the powers under sections 99AEI (to increase the approved price to pharmacists of PLAVIX and ISCOVER) and 99AEJ (to move clopidogrel back to F1 and associated powers).
18. … If I had been required to consider whether to exercise the powers under sections 99AEI and 99AEJ, I would have considered a range of issues, including the financial impact on the Commonwealth of exercising those powers, weighed up against the effect on Sanofi of not exercising those powers. I would have considered as key factors:
(a) whether it was possible for Apotex to appeal the Federal Court decision and/or its injunction orders and, if so, the time for lodging an appeal, and the likely timeframe for the result of the appeal;
(b) if I was to determine under section 99AEI to reverse the statutory price reduction or otherwise increase the agreed price for the PLAVIX and ISCOVER brands of clopidogrel after the delisting of any guaranteed brands, whether the Commonwealth would have the right to claim back from Sanofi or some other person the additional amounts spent by the Commonwealth as a result of that price increase if:
(i) the appeal was successful; and
(ii) Apotex’s generic brands of clopidogrel were re-listed following the appeal; and
(iii) the price was then reduced to the level before the delisting of Apotex’s generic brands of clopidogrel;
(c) whether I could defer a decision to exercise the section 99AEI and 99AEJ powers until the period in which the right to appeal had expired and, if appeals were lodged, await the outcome of the appeals; and
(d) if the statutory price reduction was not reversed and the agreed price was not increased while an appeal was being determined, whether Sanofi would be entitled to claim compensation from Apotex (or even from the Commonwealth) for that period if the appeal was not successful.
19. I would have sought input about those matters from other persons within the Department as necessary, including the General Counsel. I would also have raised it with one or more of the Deputy Secretary of the Department to whom I reported, the Secretary or the Minister the issue of the possible exercise of the powers under sections 99AEI and 99AEJ. I expect that Sanofi would also have raised the same matters with one or more of them.
20. If I was informed that Apotex was going to appeal the grant of a final injunction, and I was advised that:
(a) because the section 99AEI and 99AEJ powers are discretionary, I could decline to exercise the powers until the outcome of any appeal was known (the appeal period); and
(b) if I exercised any of the powers under section 99AEI and an appeal was successful, it was unlikely the Commonwealth could recover from any person the additional amounts it would have spent due to the increased PBS price of PLAVIX and ISCOVER during the appeal period; and
(c) if I did not exercise any of the powers under section 99AEI and an appeal was unsuccessful, it was more likely than not that Sanofi could claim compensation or damages (from someone other than the Commonwealth) in respect of the continuing reduced PBS price of PLAVIX and ISCOVER during the appeal period,
then taking into account the considerations referred to at paragraph 18 above, it is likely I would have declined to exercise any of the section 99AEI and 99AEJ powers while the appeal process was on foot.
505 This evidence of Ms McNeill was admitted over Sanofi BMS’ objection. At the time it was admitted I indicated that I considered that it was admissible as evidence of how a reasonable decision-maker may have acted in the interrupted supply counterfactual. I also indicated that the arguments made by Sanofi BMS in support of its objection were matters that went to the question of what weight would be given to Ms McNeill’s evidence rather than its admissibility. It will have been apparent from what I said at the time that it did not follow from my ruling as to the admissibility of Ms McNeill’s evidence that it would ultimately carry any weight.
506 There are a number of features of the relevant parts of Ms McNeill’s evidence to which I draw attention.
507 First, it is important to recognise that in the hypothetical situation addressed by Ms McNeill’s evidence, the Apotex clopidogrel products were or would be delisted due to Apotex Australia’s inability to supply. I think that is an appropriate assumption to make when considering the interrupted supply counterfactual.
508 Secondly, Ms McNeill did not occupy any relevant position in the Department until January 2010. Ms Diana MacDonell was the Acting Assistant Secretary of PEB in August 2008. Any decision whether or not to reverse the 12.5% statutory price reduction would most likely have been made by her in her capacity as a Delegate of the Minister or, if not by Ms MacDonell, then by the Minister herself.
509 Thirdly, Ms McNeill’s evidence is based upon the assumptions referred to in paragraph 18(a)-(d) and paragraph 20(a)-(c) of her affidavit. So far as the assumptions referred to in paragraph 20(a)-(c) are concerned, these refer to three pieces of advice which she said, if received by her, and if taken into account together with the considerations referred to in paragraph 18 of her affidavit, would likely have led her to decline to exercise any of the relevant powers while the appeal process was still on foot. In her cross-examination, Ms McNeill acknowledged that she was unable to say what she would have been likely to have done in the hypothetical situation addressed in her evidence if she did not obtain each of the three pieces of advice referred to in paragraph 20(a)-(c).
510 The third piece of advice referred to in Ms McNeill’s evidence was advice to the effect that if the statutory price reduction was not reversed, and the agreed price was not increased before the appeal was determined, it was more likely than not that Sanofi could later claim compensation or damages in respect of the impact of the 12.5% price reduction during the period that would be taken up by the appeal process. Of course, whether any such claim would be available would necessarily depend on whether Apotex Australia succeeded in its appeal against Gyles J’s final orders. However, for the purposes of the counterfactual analysis, the question is whether a lawyer would have advised the relevant decision-maker in August or September 2008 that it was more likely than not that Sanofi could recover such compensation or damages from Apotex Australia in the event that any such appeal was unsuccessful.
511 In my view it is likely that any advice of the kind referred to by Ms McNeill would have been highly qualified and would have stopped well short of advising that Sanofi was likely to recover compensation or damages in respect of the relevant loss from Apotex Australia or anyone else. A lawyer providing such advice would have had to grapple with the question of whether, by applying for a PBS listing of its clopidogrel products, Apotex Australia had infringed the Patent. That question had not, as at August 2008, been the subject of judicial determination.
512 The fact that the Full Court later decided (in Warner-Lambert) that taking steps to apply for a PBS listing or obtaining a PBS listing does not constitute an act of infringement does not mean that a lawyer advising the relevant decision-maker in 2008 would have given advice to that effect. It is now clear that any advice given by a lawyer contrary to the Full Court decision would have been wrong. I think it appropriate to proceed on the basis that a lawyer advising the decision-maker would have provided correct advice or, at the very least, would have made clear that there were reasonable arguments both ways and that it was not possible to say with any confidence that Sanofi would be likely to recover its losses from Apotex Australia on the basis that, by obtaining a PBS listing for its clopidogrel products, Apotex Australia had infringed the Patent. But the question remains whether he or she would have also advised that Sanofi’s loss of profits would have been recoverable on some different basis.
513 It is significant that the requirement to give an assurance of supply would have required Apotex Australia to either import or manufacture substantial quantities of infringing product so that it was available in sufficient quantities on the listing date. There does not seem to me to be any obvious reason why the loss suffered by Sanofi as a result of a statutory price reduction might not be recoverable as a reasonably foreseeable loss that was caused by those particular acts of infringement. However, the most I would have expected a lawyer advising the relevant decision-maker to say is that there was a reasonably strong argument that Sanofi should be permitted to recover profits from Apotex Australia lost by Sanofi as a consequence of the 12.5% price reduction. Advice to that effect falls well short of what Ms McNeill’s evidence suggests that she would have required.
514 A further difficulty with Ms McNeill’s evidence is that it does not make any allowance for the advice or opinions of other persons in the Department who she says she would have consulted before making a decision including the Deputy Secretary, Secretary or Minister. It seems likely, particularly in circumstances where it must be assumed that Sanofi would have made representations to one or more of those persons, that their views would have been important, if not determinative, when deciding whether to reverse the 12.5% statutory price reduction. There is no evidence to indicate how any of them would have responded to such representations.
515 The evidence shows that the Novartis sponsored Famvir and Sandostatin products were the subject of 12.5% price reductions that took effect on 1 August 2006 following the listing on the PBS of bioequivalent products sponsored by a generic supplier. The 12.5% price reduction was not a mandatory price reduction because the 2007 Amendments had not taken effect.
516 On 9 August 2006 the originator, Novartis, wrote to the Minister (the Hon. Tony Abbott) complaining that, as at that date, stock of the generic products was not available for supply, and requesting the Minister reverse the 12.5% price reduction for each unit of the originator’s products sold after 1 August 2006. PBPA minutes of 16 August 2006 show that the Minister promptly agreed to this request.
517 The patent covering Novartis’ products had already expired by 1 August 2006 leaving Novartis without any conceivable right to restrain supply of the generic products. And yet it may be inferred that the Minister considered that it would be unfair not to reverse the 12.5% price reduction in circumstances where the generic suppliers had been unable to supply their products.
518 Ms McNeill accepted in cross-examination that fairness toward the originator who had suffered a price reduction was a relevant consideration that might inform a decision whether or not to reverse a 12.5% price reduction if the generic supplier was unable to supply. Oddly, this is not a consideration that Ms McNeill refers to in her affidavit when explaining how and why she would have acted in the counterfactual scenario in which Apotex Australia was unable to supply its clopidogrel products for so long as Gyles J’s final injunction remained in force.
519 Sanofi submitted that a Jones v Dunkel inference should be drawn against the Commonwealth in respect of its failure to call Ms MacDonell. The Commonwealth submitted that no such inference could be drawn essentially because Ms MacDonell left the Commonwealth’s employment in 2011 and she was now working as a consultant to the pharmaceutical industry.
520 The Commonwealth first gave notice to Sanofi BMS that it intended to make a claim against the respondents on 27 February 2012, by which date Ms MacDonell had already left the Commonwealth’s employment. However, the fact that she was no longer employed by the Commonwealth then, or at any time thereafter, does not prevent the Court from drawing a Jones v Dunkel inference.
521 Ms MacDonell was a senior public servant who occupied a position of considerable trust and confidence and who exercised important statutory functions in her capacity as a Delegate. There is no evidence to explain why she was not called by the Commonwealth.
522 Ms MacDonell was the Delegate most likely to have been involved in exercising relevant powers and discretions under the NHA in relation to the Apotex clopidogrel products. The fact that she may now be working as a consultant in the pharmaceutical industry is not a matter that is likely to have prevented the Commonwealth from calling her. She is a person who I would have expected the Commonwealth to call. The inference I draw is that Ms MacDonell’s evidence would not have assisted the Commonwealth’s case.
523 The Minister’s reversal of the price reduction to which the Famvir and Sandostatin products were subject in 2006 provided Sanofi with a strong precedent upon which to draw in any negotiations in which it sought to secure a reversal of any 12.5% statutory price reduction in respect of its clopidogrel products based on Apotex Australia’s failure to supply. It is true that in the case of the Famvir and Sandostatin products, the price reduction was triggered by the listing of the generic products and the exercise of a discretionary power to reduce the price of those products. In my view, however, that does not diminish the weight that one might expect a decision-maker seeking to exercise the relevant power in a logical and consistent manner to have given the Famvir and Sandostatin precedent. If anything, the case for reversal in the interrupted supply counterfactual would be far stronger because, as the Minister would be bound to acknowledge, the statutory price reduction had occurred consequent upon the listing of generic products which had since been held to be infringing.
524 The Commonwealth submitted that the Court may conclude that there was a possibility that the 12.5% price reduction would have been reversed and, for that reason, when assessing the Commonwealth’s loss, the Court may think it appropriate to reduce the Commonwealth’s damages to take into account the possibility of the reversal occurring. In support of these submissions the Commonwealth relied on the principles discussed in Malec v JC Hutton Pty Ltd (1990) 169 CLR 638, Commonwealth of Australia v Amann Aviation Pty Ltd (1991) 174 CLR 64 and Sellars v Adelaide Petroleum NL (1994) 179 CLR 332.
525 In Malec v JC Hutton Pty Ltd (1990) 169 CLR 638 Deane, Gaudron and McHugh JJ said at 643:
If the law is to take account of future or hypothetical events in assessing damages, it can only do so in terms of the degree of probability of those events occurring ... But unless the chance is so low as to be regarded as speculative - say less than 1 per cent - or so high as to be practically certain - say over 99 per cent - the court will take that chance into account in assessing the damages. Where proof is necessarily unattainable, it would be unfair to treat as certain a prediction which has a 51 per cent probability of occurring, but to ignore altogether a prediction which has a 49 per cent probability of occurring. Thus, the court assesses the degree of probability that an event would have occurred, or might occur, and adjusts its award of damages to reflect the degree of probability.
526 That passage was cited with approval by Mason CJ, Dawson, Toohey and Gaudron JJ in Sellars v Adelaide Petroleum NL (1994) 179 CLR 332 at 350. Their Honours said at 349:
[W]here there has been an actual loss of some sort, the common law does not permit difficulties of estimating the loss in money to defeat an award of damages. The damages will then be ascertained by reference to the degree of probabilities, or possibilities, inherent in the plaintiffs succeeding had the plaintiff been given the chance which the contract promised.
This approach is not confined to contracts relating to games of chance, sporting contests or other competitions. Fink v. Fink concerned a contract to provide an opportunity for a reconciliation, breach of which was held to entitle the wife to damages. And there can be no doubt that a contract to provide a commercial advantage or opportunity, if breached, enables the innocent party to bring an action for damages for the loss of that advantage or opportunity. So, in The Commonwealth v Amann Aviation Pty Ltd, Mason CJ. and Dawson J. [at p 92], Brennan J. [at pp 102-104] and Deane J. [pp 118-119) concluded that a lost commercial advantage or opportunity was a compensable loss, even though there was a less than 50 per cent likelihood that the commercial advantage would be realized. Damages for breach of contract were assessed by reference to the probabilities or possibilities of what would have happened.
527 Their Honours also said at 350:
In Malec v J C Hutton Pty Ltd ([(1990) 169 CLR 638], this Court drew a distinction between, on the one hand, proof of historical facts what has happened - and, on the other hand, proof of future possibilities and past hypothetical situations. The civil standard of proof applies to the first category but not to the second, particularly when it is necessary to determine future possibilities and past hypothetical situations for the purpose of assessing damages [pp 639-640, per Brennan and Dawson JJ; pp 642-643, per Deane, Gaudron and McHugh JJ].
…
Neither in logic nor in the nature of things is there any reason for confining the approach taken in Malec concerning the proof of future possibilities and past hypothetical situations to the assessment of damages for personal injuries. The reasons which commended the adoption of that approach in assessments of that kind apply with equal force to the assessment of damages for loss of a commercial opportunity, as the judgments in Amann acknowledge.
528 In the present case I think it more likely than not that the 12.5% statutory price reduction would have been reversed in the interrupted supply counterfactual. I acknowledge that the evidence in support of that conclusion is sparse. But this is due at least in part to the absence of any evidence from any relevant decision-maker. I regard the hypothetical evidence of Ms McNeill as problematic for reasons previously stated and also because it is in my view likely to have been influenced by her knowledge that Gyles J’s final orders were later set aside. I give far greater weight to the evidence of the Novartis experience in 2006.
529 Were it necessary to express my conclusion in percentage terms, I would estimate the chances of the Minister or the Delegate not reversing the 12.5% statutory price reduction to be substantially less than 50%, and in the region of 10%.
The Continuous Supply Counterfactual
530 It was not contended by the Commonwealth that in circumstances where Apotex Australia had been restrained by the interlocutory injunction through to 19 August 2008, that Apotex Australia would have sought to commence supply of its clopidogrel products from that date forward were it free to do so either because the proceeding against it was dismissed or, alternatively, a stay of the final injunction was granted. Nor did the Commonwealth contend that Apotex Australia would have applied to list its clopidogrel products on or after 19 August 2008 were it free to do so in the period through to the determination of any appeal.
531 The Commonwealth’s position is no doubt explained by Mr Kay’s correspondence of 12 August 2008 which indicates that he was against launching such products in circumstances where the correctness of Gyles J’s conclusion on validity (whichever way it went) would most likely be challenged on appeal. Mr Kay thought it was too risky to launch before any appeal by Sanofi was determined even if Apotex Australia were to persuade Gyles J that all relevant claims of the Patent were invalid. The commercial strategy Mr Kay favoured in that scenario was to postpone any launch until Sanofi had exhausted its rights of appeal and, in the meantime, attempt by negotiation to secure a further undertaking as to damages from Sanofi on which Apotex Australia might later make a claim.
532 In its written submissions the Commonwealth submitted:
370. …[W]ithout the interlocutory injunction Apotex would have secured listing on 1 April 2008 and launched its clopidogrel products. The litigation itself would not have deterred Apotex. Once this is accepted, there is no reason to suppose that, in this counterfactual world, the products would not have remained listed through at least until August 2008.
371. It is in this counterfactual world that one must consider the effect of the operation of the final injunction and the second Apotex undertaking. If Apotex was listed on 1 April 2008, the final injunction would have had the effect of compelling Apotex to apply for delisting. The final injunction would have placed Apotex in breach of the guarantee of supply obligations and it would have been compelled to notify the Minister and delisting would have followed. The question is whether Apotex, having obtained listing on 1 April 2008, would have maintained that listing after 18 August 2008, on the hypothesis that there was no final injunction.
372. What is being tested is whether the Commonwealth is adversely affected by the operation of the interlocutory injunction and the final injunction. The way to do that is to consider what the world would have looked like if, to use the phrase of Gibbs J in Air Express, “for some reason” there was no interlocutory injunction or final injunction, but the litigation was otherwise on foot. That is, would Apotex have been deterred from obtaining and maintaining the clopidogrel listing even had there been no interlocutory or final injunction? This is different from the question of whether Apotex would have applied for PBS listing for the first time in late 2008 had it failed at the interlocutory stage but succeeded at trial.
(original emphasis)
533 On the Commonwealth’s analysis, Mr Kay’s view is irrelevant because he was addressing a hypothetical situation in which Apotex won at trial after having previously been prevented from supplying by the interlocutory injunction. The Commonwealth says that this is not the appropriate counterfactual because it must be assumed that there never was either an interlocutory injunction or a final injunction.
534 This leads the Commonwealth to ask what would have occurred in the event that Apotex Australia had not been restrained at any stage (whether by an interlocutory or final injunction) from supplying its clopidogrel products. For my part, assuming for the moment that the Commonwealth’s submission as to the appropriate counterfactual is correct, I would frame the relevant question in these terms: Has the Commonwealth established that, on the assumption that Apotex Australia was supplying its clopidogrel products in the period from March 2008 to August 2008, it would have continued doing so in the absence of a final injunction restraining it from infringing claim 3?
535 Before addressing that question it is necessary to refer to another submission made by the Commonwealth. It submitted that for the purpose of the counterfactual analysis it could not be assumed that there would have been a trial in April 2008 or a judgment delivered in August 2008. According to the Commonwealth, the fact that a trial had occurred before Gyles J in April 2008 and a judgment was delivered by his Honour in August 2008 are matters that are to be disregarded in the counterfactual analysis.
536 I do not think there is any substance to this submission. It reflects an approach that is fundamentally at odds with the discussion of the relevant principles in Air Express which emphasises the need to disentangle the consequences of the injunction from the consequences of the litigation. In the present case, the relevant findings and declarations were consequences of the litigation.
537 The Commonwealth’s submission is also at odds with the normal course of counterfactual analysis which requires that the counterfactual world be treated as being the same as the real world minus the event the subject of the counterfactual inquiry. The Court asks how things would have turned out if the relevant event was removed from the equation. The Court does not assume away other real world facts such as (in this case) the identity of the trial judge, the timing of the trial or final judgment, or the findings made.
538 The Commonwealth also submitted that the outcome of the appeal process demonstrated that Sanofi was never entitled to the declaratory relief granted because the Patent was invalid. It is true, as the Commonwealth emphasised, that Gyles J’s judgment was later set aside by the Full Court. However, it was not suggested by the Commonwealth that his Honour’s judgment was obviously wrong or that a litigant in the position of Apotex Australia could make any decision regarding continuing supply on the basis that the judgment would be set aside. The appeal raised issues which, although ultimately decided in Apotex Australia’s favour, could never have been fairly characterised as issues on which Apotex Australia was sure or even likely to prevail.
539 Sanofi BMS submitted that the Commonwealth’s submission elevated the effect of the second undertaking as to damages given to Moore J to an undertaking not to rely upon the fact that Gyles J made findings adverse to Apotex Australia or declarations reflecting such findings. I do not think that is how the Commonwealth puts its submission but, in any case, I agree that there is no basis for treating the undertaking given to Moore J as having that effect.
540 It follows that even if one accepts that it is necessary to disregard both the existence of the interlocutory injunction and the final injunction in the counterfactual analysis, it is still necessary to have regard to the fact that Gyles J made findings and declarations to the effect that claim 3 was valid and infringed.
541 The Commonwealth’s written submissions continue:
374. If Gyles J had not granted a final injunction, Apotex would not have taken steps to have its Apotex products delisted. That is true even assuming that all other things were the same: including the judgment of 12 August 2008 and the declarations as to validity.
375. The fact that Apotex would already have entered the market at risk and launched in April 2008 provides very strong evidence that it would have stayed on the market in this counterfactual world. A finding that Apotex would have launched at risk on 1 April 2008 carries with it a very strong implication that it was serious about selling clopidogrel – and serious about selling despite the risk of an adverse litigation result.
376. The real world demonstrated that Apotex was intent to prosecute an appeal against the decision of Gyles J with vigour. Dr Sherman approved an appeal within 4.5 hours of receiving Mr Millichamp’s report. The appeal was filed on the same day that final orders were made. We do not know what legal advice Apotex was given about its prospects on appeal. We do know that the decision of Gyles J was erroneous. There is reason to suppose that Apotex considered that it had substantially good prospects of overturning the judgment.
377. Having launched at risk in April 2008, Apotex would already be exposed to the potential of paying damages to Sanofi if Apotex was ultimately unsuccessful. The issue for Apotex would have been to consider what additional damages it would have to pay in the event that its appeal was unsuccessful. Apotex may have taken the view that it may already be exposed to the full consequences of the 12.5% price reduction and that this would not change by delisting.
(footnotes omitted)
542 The fact that Dr Sherman gave instructions to lodge an appeal within 4.5 hours of receiving news that Apotex Australia had lost at trial is not evidence that he would have been willing to permit Apotex Australia to continue supplying its clopidogrel product in the face of Gyles J’s judgment. It cannot be inferred that Dr Sherman, Mr Baxter and Mr Kay would have been content for Apotex Australia to continue to supply once Gyles J had found claim 3 to be valid and infringed. They may well have preferred to adopt the more cautious approach of ceasing to supply what Gyles J had found to be infringing products until after the outcome of the appeal was known.
543 The Commonwealth asserted in its written submissions that the fact that, in the counterfactual world, Apotex Australia had already entered the market at risk in early 2008 provides very strong evidence that it would have stayed on the market in the absence of a final injunction restraining it from doing so. But even if Apotex Australia was to have launched at risk in early 2008, it does not follow that it would have continued to supply its clopidogrel products once there had been a trial and findings made that claim 3 was valid and infringed.
544 Another circumstance that may well have changed was the risk tolerance of senior management at Apotex HQ. Even if they had been willing to authorise a launch at risk in early 2008, the risk involved in continuing to supply after having lost at trial may well have been perceived by them as excessive.
545 It is clear that Mr Kay had significant concerns about Apotex Australia’s potential exposure to a claim for damages in the event there was a product launch prior to the determination of any appeal. So great was his concern, he concluded in the days leading up to delivery of judgment that there should be no product launch even if Gyles J was to find in favour of Apotex Australia. Of course, it is to be remembered, as the Commonwealth correctly emphasised, that those views were expressed against a background in which Apotex Australia was already the subject of the interlocutory injunction and had not yet entered the market. But Mr Kay’s view on the topic still reflects a highly risk averse view and suggests that Apotex Canada was, at least by August 2008, far more cautious in its approach to risk than the Commonwealth’s submissions might suggest.
546 This brings me to the question of how Dr Sherman, other members of senior management at Apotex HQ, and the Canadian lawyer, Mr Hughes, would have reacted to a final judgment which, although the subject of a right of appeal, had found that Apotex’s clopidogrel products infringed a valid claim of the Patent.
547 It is a matter of mere conjecture as to whether Dr Sherman or, for that matter, Mr Baxter or Mr Kay, would have considered it in either Apotex Australia’s or Apotex Canada’s interests to continue supplying their clopidogrel products in Australia while awaiting a decision in an appeal against a judgment of the trial judge finding that such products infringed a valid claim of the Patent. In my view it is not open to infer that Dr Sherman, or anyone else at Apotex HQ, would have instructed Mr Millichamp to continue to supply in the face of that finding.
548 It is also a matter of mere conjecture to assert, as the Commonwealth does, that Apotex HQ may have taken the view that Apotex Australia was already exposed to the full consequences of the 12.5% price reduction. Implicit in that view is an assumption that the Minister or Delegate would refuse to reverse the 12.5% price reduction in the event that Apotex Australia decided to cease suppling its clopidogrel products until the determination of its appeal from Gyles J’s judgment. I do not think there would have been any reasonable basis to assume that there would be no price reversal in such circumstances.
549 Even if the 12.5% price reduction was not reversed once the Minister was made aware of Gyles J’s judgment, Apotex Australia’s financial exposure would have been significantly reduced if it were to have ceased supplying its clopidogrel products until the determination of its appeal. This is because if Apotex Australia ceased supply, every further sale that would otherwise have gone to it was likely to have gone to Sanofi’s licensees in Australia (ie. Sanofi Australia and BMS Australia) and without them having to engage in the additional price discounting made necessary by Apotex Australia’s entry into the clopidogrel market.
550 The figures provided by Mr Millichamp to Mr Kay on 28 July 2008 show that Apotex Australia’s financial exposure to Sanofi could be expected to increase substantially if Apotex Australia continued to supply its product after 19 August 2008 until the determination of its appeal, especially if the 12.5% price reduction that took effect on 1 April 2008 was not reversed. Mr Millichamp’s figures suggest that damages for a 12 month period might range somewhere between about $40.0 million and about $50.0 million. For the purpose of estimating Apotex Australia’s net exposure, it is necessary to allow for the profits that would have been made by Apotex Australia during that period. Mr Millichamp estimated that these profits could range from anywhere between about $5.0 million to about $23.0 million depending on if, when, and how many, autogenerics were launched. It therefore seems likely that Apotex Australia’s exposure, in the event that it continued to supply after 19 August 2008, was likely to result in a substantial award of further damages in the event that Gyles J’s finding that claim 3 was valid was upheld on appeal.
551 On the assumption that no autogenerics were launched, the net detriment to Apotex Australia based on Mr Millichamp’s calculations would have been in the vicinity of between about $30.0 million to $40.0 million over an 18 month period in which an appeal might be heard and determined. The net detriment would have been considerably larger than this if any autogenerics had been launched.
552 The Commonwealth submitted:
380. Against the potential exposure to Sanofi for additional damages by remaining on the market, and the likelihood of that exposure being realised, Apotex would have to weigh up the logistical and financial hardship that it would face in removing its clopidogrel products from the market, knowing that it (at least) might win on appeal and be allowed to re-enter the market. Its reputation may be tarnished. If it voluntarily delisted (there being no injunction), Apotex would certainly suffer logistical and financial hardship in recovering stock and failing to realise profits. It would not be able to recover those losses even if it won the appeal. Against those certain losses, it would weigh up the risk of having to pay additional damages to Sanofi if it lost the case on appeal. The kind of company that made the decision to launch at risk in the first place would undoubtedly have held firm and remained on the market if it could.
553 I do not accept that Apotex’s reputation would be tarnished if it were to have withdrawn its products from sale in circumstances where they had been found to infringe. With regard to any logistical and financial hardship, there was little if any evidence to suggest that there would be any significant hardship that would compare to the financial risk involved in continuing to supply in the face of a finding by the trial judge that claim 3 was valid and infringed. Evidence from Mr Montgomery indicated that the logistical difficulties of withdrawing a product after launch would have been considerable, but his evidence does not say anything about the countervailing financial risk of continuing to supply in the face of such a finding.
554 As the Commonwealth submitted, it would be necessary for Apotex Australia to weigh up the risk of having to pay additional damages to Sanofi if Apotex Australia lost its case on appeal against the profits it would make if it were to continue supply and win its appeal. These are matters that the decision-makers at Apotex HQ would have had to consider in light of any legal advice that was obtained as to the prospects of an appeal, the time that any such appeal would take to be heard and determined, and the likelihood that Sanofi Australia and BMS Australia would launch one or more autogenerics, assuming, in this counterfactual scenario, that they had not already done so.
555 The most useful insight that the evidence provides as to how Apotex HQ would have responded in August 2008 to a judgment declaring that the products Apotex Australia had been supplying since March or April 2008 were infringing emerges from Mr Kay’s email correspondence (discussed above) which, although addressing a different situation, reflects what in my view was a highly risk averse attitude to the litigation.
556 In the absence of evidence from any relevant decision-maker at Apotex HQ as to what instructions would have been given to Mr Millichamp in the Commonwealth’s counterfactual scenario in which there was no interlocutory injunction and no final injunction, I am not persuaded that Apotex Australia would have continued to supply its clopidogrel products after 19 August 2008 while its appeal was pending.
557 The Commonwealth also submitted that in the counterfactual scenario in which Apotex Australia had obtained a PBS listing on 1 April 2008, and there was no final injunction, Apotex Australia would not have taken steps to have its products de-listed. I do not accept that submission.
558 Whether or not Apotex would have applied to de-list its clopidogrel products if there was no final injunction would necessarily depend on whether Dr Sherman, or any other relevant decision-maker at Apotex HQ, would have been willing to allow Apotex Australia to continue supplying products which Gyles J had found infringed a valid claim of the Patent.
559 If the decision had been made not to continue to supply in those circumstances, then Apotex Australia would almost certainly have applied to have its clopidogrel products de-listed for the simple reason that, given such a decision, it could not guarantee supply prior to the determination of its appeal against Gyles J’s final judgment.
560 A more fundamental difficulty with the Commonwealth’s continuous supply counterfactual that Sanofi BMS points to arises out of the way in which the Commonwealth has put its damages case. In essence, the Commonwealth contends that it has suffered loss as a consequence of Apotex Australia being prevented from listing its clopidogrel products on the PBS on, and no later than, 1 April 2008. Every element of the loss which the Commonwealth is alleged to have suffered is said by it to flow from Apotex Australia’s failure to obtain listing of its clopidogrel products on that date.
561 If the Commonwealth’s loss resulted from the inability of Apotex Australia to obtain a PBS listing on 1 April 2008, then this is not a loss that could ever be said to have been caused by the existence of the final injunction. Similarly, the existence of the interlocutory injunction cannot account for the failure of Apotex Australia to obtain a PBS listing of its clopidogrel products at any time after 19 August 2008.
562 In the absence of the final injunction, it would have been open to Apotex Australia to apply for and obtain a listing on 1 December 2008 which would have immediately triggered the first statutory price reduction and also activated the price disclosure regime. However, the evidence does not establish that, but for the existence of the final injunction, Apotex Australia would have obtained a PBS listing on the first available date after Gyles J delivered final judgment (ie. 1 December 2008). It follows that the evidence cannot satisfy the but for test in respect of any loss suffered by the Commonwealth arising out of a failure by Apotex Australia to list on 1 December 2008.
563 This may explain why the Commonwealth’s claim was confined to one based on Apotex Australia’s failure to obtain a listing on 1 April 2008 and not any later date. However, even if it is assumed that the interlocutory injunction may have somehow prevented Apotex Australia from obtaining a listing of its products after the final judgment, there is a question whether any of the loss alleged by the Commonwealth that would not have been suffered if Apotex Australia had listed on 1 December 2008 was a direct or a reasonably foreseeable consequence of the operation of the interlocutory injunction.
564 As to directness, it was the final injunction that restrained Apotex Australia from supplying its products after 19 August 2008. To the extent that the operation of the interlocutory injunction may have made any contribution to that result, this was not something that naturally flowed from the interlocutory injunction. Any loss suffered by the Commonwealth as a consequence of Apotex Australia being unable to supply after 19 August 2008 could only be an indirect effect of the interlocutory injunction.
565 The Commonwealth contends (at least implicitly) that it was reasonably foreseeable at the time Gyles J granted interlocutory relief on 25 September 2007 that the interlocutory injunction might somehow have prevented Apotex Australia from obtaining a PBS listing until at least 1 April 2010. In my opinion that contention is not tenable. It was known at the time the interlocutory injunction was granted that there would be a trial of the proceeding before Gyles J commencing in late April 2008 and that his Honour, who was due to retire in August 2008, would need to deliver judgment before vacating his position as a Judge of this Court.
566 I do not consider that it could have been reasonably contemplated or foreseen at the time the interlocutory injunction was granted that it might prevent Apotex Australia from listing on any other listing date subsequent to the date of final judgment. It was no doubt for this reason that no submission was put to Gyles J at the interlocutory hearing on 18 September 2007 that the interlocutory injunction could or might have any such effect.
567 Given that the next available listing date after August 2008 was 1 December 2008, it would have been reasonably foreseeable that the interlocutory injunction might have had the practical effect of preventing Apotex Australia from obtaining a PBS listing until then and that the 12.5% statutory price reduction triggered by such a listing might therefore be delayed from 1 April 2008 until 1 December 2008 (ie. a delay of eight months). But I do not accept that it was reasonably foreseeable at the time Gyles J granted the interlocutory injunction that the interlocutory injunction could have any legal or practical operation that could delay PBS listing until after 1 December 2008. Similarly, it was not reasonably foreseeable that the interlocutory injunction might delay activation of the price disclosure price reduction regime until after 1 December 2008.
RELEVANCE OF OTHER GENERIC SUPPLIERS
568 The Sanofi BMS companies submitted that the Commonwealth was required to show that the fact that no generic supplier was listed on the PBS before 1 April 2010 was a direct result of the interlocutory injunction rather than the existence of the patent proceeding. They submitted that the evidence says nothing about whether any generic supplier apart from Apotex Australia could have taken steps to obtain ARTG registration earlier than it did but instead chose not to do so because of the existence of the Patent and the possibility that it may also be sued for patent infringement.
569 I do not accept that submission. Whether or not other generic suppliers were deterred from taking steps to enter the clopidogrel market sooner than they did due to the existence of the Patent or the possibility that they may be sued for infringement is irrelevant to the question whether the Commonwealth suffered loss as a direct result of Apotex Australia having been prevented from listing its clopidogrel products on the PBS on 1 April 2008.
570 Even if it is assumed that other generic suppliers were deterred by the existence of the Patent or the possibility of infringement proceedings being commenced against them, it does not follow that the Commonwealth has not suffered loss as a result of Apotex Australia not being listed before them. The fact that other generic suppliers may have had their own reasons for not obtaining ARTG approval or PBS listing earlier than they did merely demonstrates that there may have been a number of different causes of the Commonwealth’s alleged loss. It is not necessary for the Commonwealth to establish that the existence of the interlocutory injunction was the sole cause.
IS THE LOSS CLAIMED BY THE COMMONWEALTH COMPENSABLE?
571 The Sanofi BMS companies contended that, even if all of the other legal requirements necessary to permit the Commonwealth to recover were satisfied, the loss claimed by it was not recoverable either because the Commonwealth was not a person “adversely affected” by any relevant order or undertaking, or because it had not suffered any compensable loss. In particular, Sanofi BMS contended:
The claimed adverse effect of the interlocutory injunction and the final injunction – namely, the consequential impact on the level of costs incurred in the operation of a legislative scheme for social welfare – is not a compensable loss;
The alleged loss was caused by the operation of the Commonwealth’s own laws, and in particular the operation of the PBS provisions introduced into the NHA in August 2007 which resulted in a mandatory 12.5% reduction on the listing of a second brand, irrespective of whether or not there was a patent in respect of the relevant molecule;
The Commonwealth cannot be “adversely” affected by the operation of either the interlocutory injunction or the final injunction in any relevant sense for compensatory purposes, as it must be in the interests of the Commonwealth for orders made by a superior court of the Commonwealth, in aid of rights asserted under the laws of the Commonwealth, to operate, and be complied with, according to their terms.
572 In their written submissions Sanofi BMS characterised the PBS as a Commonwealth funded “social welfare scheme”. That is not an inapt description of the PBS. Taking that as their starting point, Sanofi BMS submitted:
450. The Commonwealth’s funding of that social welfare scheme, which operates according to rules which the Commonwealth itself can and does amend from time to time, is not in any relevant sense a “loss” or “damage”. The funding of PBS expenditure is a matter of government policy, and the policy and rules by which price reductions are determined can be, and are, changed from time to time by the Commonwealth itself …
451. The Commonwealth is committed to, and plainly has an interest in, funding the PBS, having regard to public health considerations. Because it is so committed, the Commonwealth will adjust taxes and spending in other areas to ensure that commitment to the PBS is always met.
452. As such, there is no basis on which the Court could conclude that the Commonwealth itself suffered some financial detriment in the period from April 2008 by reason that expenditure in respect of one particular drug pursuant to one particular social welfare scheme was higher than might have otherwise been the case in that period. For the Commonwealth, any such additional expenditure may have been neutral if offset by other savings. By way of example, if any decrease of, say, $50m in the amount actually spent on clopidogrel under the PBS in 2008-2010 would have led the Department to spend an additional $50m on some other programme which it did not pursue because that money was not available within its budget in that period, then the position is neutral so far as the Commonwealth is concerned – that is, it is in entirely the same financial position (a balanced budget) whether or not a generic brand of clopidogrel had been listed in the period 2008-2010.
573 Sanofi BMS went on to say that the Commonwealth does not suffer any compensable loss when, in accordance with its policy objectives, it fully funds the PBS, and has control of all decisions to list medicines on the PBS, the prices at which they are listed, and the regulatory regime by which those prices are subsequently adjusted.
574 Although it is not necessary to determine the correctness of these contentions, I will briefly explain why I do not accept any of them.
575 The vast bulk of the Commonwealth’s revenue consists of payments made by taxpayers to the Consolidated Revenue Fund (“CRF”) which is used to fund a multitude of Commonwealth activities including the operations of the PBS and the subsidisation of PBS listed medicines. In the present case it may be inferred that if the Commonwealth paid more for clopidogrel than it would have done but for the interlocutory injunction, then the CRF is depleted by a corresponding amount.
576 If the Commonwealth had made adjustments to its activities due to the price of clopidogrel remaining at the 1 April 2008 level, then it does not follow that the Commonwealth has not suffered a loss. The point is best illustrated by the following example. Let it be assumed that the Commonwealth paid more than it would have had to pay when purchasing medicine for use in hospitals due to the operation of the interlocutory injunction. The Commonwealth might recoup that difference from taxpayers or patients or, alternatively, by eliminating or reducing other expenditure incurred by it in running those hospitals. Either way, I do not think it would be correct to say that the Commonwealth has not suffered a loss due to the operation of the interlocutory injunction. The fact that the Commonwealth may make adjustments to taxes, or rates of taxes, in order to increase its receipts, or that it may curtail other expenditure, does not mean that the Commonwealth cannot have suffered a loss reflecting the difference between what it would have paid but for the injunction and what it actually paid.
577 In British Westinghouse Electric and Manufacturing Company, Limited v Underground Electric Railways Company of London, Limited [1912] AC 673 Viscount Haldane LC referred to various cases in which a subsequent transaction provided a plaintiff who suffered loss as the result of a legal wrong with a compensating benefit. His Lordship said at 690-691:
The subsequent transaction, if to be taken into account, must be one arising out of the consequences of the breach and in the ordinary course of business. This distinguishes such cases from a quite different class illustrated by Bradburn v Great Western Ry. Co. [L.R. 10 Ex. 1], where it was held that, in an action for injuries caused by the defendants' negligence, a sum received by the plaintiff on a policy for insurance against accident could not be taken into account in reduction of damages. The reason of the decision was that it was not the accident, but a contract wholly independent of the relation between the plaintiff and the defendant, which gave the plaintiff his advantage. Again, it has been held that, in an action for delay in discharging a ship of the plaintiffs' whereby they lost their passengers whom they had contracted to carry, the damages ought not to be reduced by reason of the same persons taking passage in another vessel belonging to the plaintiffs: Jebsen v East and West India Dock Co. [L.R. 10 C.P. 300], a case in which what was relied on as mitigation did not arise out of the transactions the subject-matter of the contract.
578 The Commonwealth’s circumstances are not relevantly different from those of a natural person, who as a result of a legal wrong, is required to spend more money than he or she otherwise would, and who then makes corresponding adjustments, either by consuming or spending less, or by relying on the generosity of a friend, or the proceeds of an insurance policy, to make good the shortfall. The benefit derived from the making of such adjustments or from payments by a friend or insurer need not be brought to account when assessing the loss if they are properly characterised as collateral. The benefit will be properly characterised as collateral if it arises out of a wholly independent transaction.
579 I do not think the possibilities referred to by Sanofi BMS in their submissions relating to offsetting expenditure or balanced budgets has any bearing on the Commonwealth’s entitlement to recover under any of the relevant undertakings as to damages because it has not been shown that the Commonwealth obtained any compensating advantage or benefit due to the existence of either the interlocutory injunction or the final injunction. Nor has it been alleged by Sanofi BMS that the Commonwealth failed to take steps to secure any such advantage or benefit in breach of any duty to mitigate its loss.
580 Of course, as Sanofi BMS emphasised, this is not a case in which the Commonwealth is the victim of a legal wrong. The Sanofi BMS companies did not commit any legal wrong. However, in my view, this makes no difference. The principles to which I have referred still apply for the purpose of assessing the Commonwealth’s loss.
581 One of the other points made by Sanofi BMS is that the Commonwealth “… is [the] author of its own loss”. There seem to be two arguments relied upon in support of that assertion: first, that the Commonwealth was responsible for the 2007 Amendments to the NHA enacted by the Parliament, including, in particular, the amendment to s 99ACB of the NHA that imposed a 12.5% price reduction on first generic listing irrespective of whether the relevant drug was on patent. The second, although only faintly mentioned in the submissions, was that the Commonwealth was responsible for its own loss because it allowed an invalid patent to proceed to grant. I do not think there is any substance to these arguments.
582 The 2007 Amendments, including the amendment to s 99ACB of the NHA, came into effect on 1 August 2007. Those amendments formed part of the relevant legal matrix in which Sanofi applied for and obtained its interlocutory injunction. One purpose in seeking the interlocutory injunction was to prevent Apotex Australia from taking steps that might trigger the mandatory price reduction that would automatically occur in the event that the Apotex clopidogrel products were listed on the PBS.
583 The Sanofi BMS companies’ principal argument, as I understand it, is that because the Commonwealth was the architect of the PBS, including those features of the statutory scheme introduced by the 2007 Amendments, the Commonwealth was the cause of any loss it may have suffered as a result of the operation of the scheme. This argument, it seems to me, is devoid of common sense. It is a little like saying that the loss suffered by the proprietors of a business shut down by an interlocutory injunction that was later set aside were the authors of their own loss because they elected to engage in the trading activities that gave rise to the interlocutory injunction, or taking the argument to its logical extreme, because they chose to go into business in the first place. The Sanofi BMS companies’ other argument based on the grant of an invalid patent is equally unattractive. Both arguments involve a form of “but for” reasoning that the law is astute to eschew: see Faulkner v Keffalinos (1970) 45 ALJR 80 at 86 per Windeyer J, in the passage quoted with approval by Mason CJ (with whom Toohey and Gaudron JJ agreed) in March at 516.
584 The last of the points made by Sanofi BMS in support of the contention that the loss alleged by the Commonwealth was not recoverable was that the Commonwealth could not be a person adversely affected by the operation of the orders of this Court. The essence of the argument appears in the following paragraphs of the Sanofi BMS companies’ written submissions:
476. … [A]t all times from 25 September 2007 when the Interlocutory Injunction was granted until 12 March 2010 when the Final Injunction was set aside, it was in the Commonwealth’s interests for there to be no supply by Apotex of any of its clopidogrel products (which would have amounted to a contempt of a court of the Commonwealth), even if an effect of those injunctions being obeyed was that the Commonwealth itself might suffer some increased expense.
477. In that sense, the Commonwealth cannot in any relevant sense be characterised as having been “adversely” affected by the operation of the injunctions in accordance with their terms, despite any consequential financial impact upon the costs of a particular Commonwealth scheme, because the Commonwealth at all times had a vital interest in ensuring that those injunctions were given full force and did operate in accordance with their terms.
585 In support of these submissions Sanofi BMS referred to the authorities that support the well-established principle that a judgment of a superior court of record is binding on the parties to the proceeding in which it is given and must be obeyed unless and until it is properly set aside: Eastman v The Queen (2008) 166 FCR 579 (Spender, Gray and Logan JJ) at 588 [22] and Isaacs v Robertson [1985] 1 AC 97 (PC) at 101-102. The argument was that the Commonwealth has a special interest in ensuring that orders made by this Court, as a superior court of record, are obeyed, a point that was said to have added force in the case of a final order made in exercise of the judicial power of the Commonwealth that gives rise to a final and binding determination of the parties’ rights: Huddart, Parker & Co Pty Ltd v Moorehead (1909) 8 CLR 330 at 357.
586 The answer to these submissions is that the Commonwealth has not, by making its claims for compensation, disobeyed any of the orders made by Gyles J, encouraged any other person to do so, or engaged in any conduct capable of diminishing the authority or standing of the Court. There is no doubt that the Commonwealth has an interest in ensuring the orders of this Court are observed. But that does not provide any basis for holding that the Commonwealth is not entitled to recover on an undertaking as to damages when an order in relation to which the undertaking is given is later set aside. There is no logical connection between the Commonwealth’s interest in ensuring that orders of superior courts of record are observed and the entitlement of the Commonwealth to recover pursuant to such an undertaking if the terms of the undertaking and the applicable legal principles otherwise allow.
587 Sanofi BMS contended that the Commonwealth’s application for compensation should be refused, if not in whole, at least in substantial part, on discretionary grounds. Given my previous findings, there is no occasion for me to exercise any discretion. However, I think it desirable that I refer to the arguments that were advanced in relation to the question of discretion and that I make findings relevant to it.
588 The High Court noted in European Bank at 438-9 [14] that the undertaking as to damages “is not a contract between parties or some other cause of action upon which one party can sue the other”. Similarly, in Barratt Manchester Ltd v Bolton Metropolitan Borough Council [1998] 1 All ER 1 (“Barratt”) Millett LJ (with whom Kennedy LJ and Sir Brian Neill agreed) pointed out that a party seeking to enforce the undertaking as to damages has no cause of action but has a right to apply for compensation under the undertaking which the Court, in its discretion, may award.
589 In Barratt the question was whether an inquiry into the damages that should be awarded under a cross-undertaking as to damages should be dismissed on account of a failure by the plaintiff (who was the applicant for compensation) to comply with orders for the service of documents. The Court of Appeal held that the inquiry should be allowed to proceed notwithstanding the plaintiff’s delay, which was found to have occurred in the context of a complex and difficult case, and which was not shown to have caused the defendant (the Attorney-General) any prejudice. In the course of his judgment Millett LJ referred with approval to the judgment of Sir Michael Kerr in C T Bowring & Co (Insurance) Ltd v Corsi Partners Ltd [1994] 2 Lloyd’s Rep 567 at 582. Millett LJ said at 7:
An inquiry as to damages under a cross-undertaking, however, possesses a number of special features. The cross-undertaking in question is given to the court, not to the party opposite, and may be enforced or discharged by the court in its discretion. The party seeking to enforce the undertaking has no cause of action. Although entitled to apply to enforce the cross-undertaking, he has no legal right to its enforcement or to damages: see Cheltenham and Gloucester Building Society v Ricketts (1993) 4 All ER 276, (1993) 1 WLR 1547. Any loss which he may have sustained is occasioned, not by a legal wrong, but in consequence of an order of the court. Since there is no cause of action there is no period of limitation either; but the cross-undertaking cannot be enforced without the leave of the court, which may be withheld if not applied for promptly: see Smith v Day (1882) 21 Ch D 421 and Exp Hall, re Wood (1883) 23 Ch D 644. As those cases show, the court does not inquire whether the other party has been prejudiced by the delay. The only question is whether the applicant has behaved with reasonable despatch.
If sufficient ground be shown, the court can not only strike out the inquiry but discharge the cross-undertaking, so that there can be no question of starting enforcement proceedings again. In C T Bowring & Co (Insurance) Ltd v Corsi Partners Ltd [1994] 2 Lloyd’s Rep 567 at 582 Sir Michael Kerr said that in relation to a cross-undertaking:
... the Court acts or declines to act in its own right, not merely as an umpire in an adversarial process between the parties, though obviously having full regard to the position of the parties and the interests of justice. In deciding how to deal with such an undertaking the Court exercises a broad equitable jurisdiction ...
590 His Lordship continued at 10:
In my judgment this provides the solution to the problem posed in this appeal. In conducting an inquiry of the present kind and ascertaining the amount of the loss suffered by the plaintiff which is covered by the defendant's cross-undertaking, the court is not engaged in determining the legal rights of the parties. The discharge of the defendant's cross-undertaking does not deprive the plaintiff of his legal right to damages, for he has none. It may expose him to irrecoverable loss in consequence of an earlier order of the court, and this may seem to be unfair; but any appearance of unfairness is dispelled by the reflection that the plaintiff has been afforded an opportunity to recover his loss and has failed to take it by proceeding with reasonable diligence.
In my judgment, the same principles ought to apply to the discharge of the cross-undertaking for failure to prosecute the inquiry as apply to the grant or refusal of the inquiry in the first place. The enforcement of the cross-undertaking should be regarded as being conditional on the inquiry being applied for promptly and prosecuted with reasonable diligence. This would allow for a desirable degree of flexibility. Just as the court may decline to enforce the cross-undertaking if the plaintiff does not apply to enforce it with reasonable promptitude, so it ought to be willing to discharge it where the plaintiff does not conduct the enforcement proceedings with reasonable diligence.
This is not to say that the presence or absence of prejudice to the defendant is irrelevant. Its presence will always be highly material. Where the delay has occasioned significant prejudice, it will almost always be right to dismiss the inquiry and discharge the cross-undertaking. But the greater the delay, the less the need to establish prejudice; and the court should not hesitate to discharge the cross-undertaking and dismiss the inquiry where there has been excessive and prolonged delay, even though it cannot be shown to have occasioned any prejudice to the other party.
591 In Air Express Aickin J said at 266:
In a proceeding of an equitable nature it is generally proper to adopt a view which is just and equitable, or fair and reasonable, in all the circumstances rather than to apply a rigid rule.
That statement of principle was approved by the High Court in European Bank at 439 [18].
592 The Commonwealth submitted that an undertaking as to damages will be enforced save in exceptional or special circumstances. In support of that submission it relied on the judgment of Gibbs J in Air Express. His Honour said at 311-312:
The court has a discretion not to enforce such an undertaking, but unless the defendant has been guilty of conduct that would render it inequitable to enforce the undertaking it would seem just, speaking generally, that a plaintiff who has failed on the merits should recompense the defendant for the damage he has suffered as the result of the making of the interlocutory order.
593 It is apparent from what Gibbs J and Aickin J said in Air Express that in every case the Court must determine what is “just and equitable” or “fair and reasonable” having regard to the facts of the case.
594 The discretion to award compensation is exercised judicially and in accordance with settled principles. There will therefore be many cases in which a party who has suffered loss as a direct and foreseeable result of an interlocutory injunction granted against it can properly expect that there will be an order for the payment of compensation made in its favour. Nevertheless, the remedy is in the discretion of the Court which may take into account any matter that it considers relevant to the question of whether it is fair and reasonable in the circumstances of the case to grant or withhold relief. In my opinion there is no rule that requires a party resisting an application for compensation on discretionary grounds to establish the existence of exceptional or special circumstances.
595 Sanofi BMS submitted that the Commonwealth’s claim for compensation should be refused or reduced having regard to the following four matters:
(a) the Commonwealth’s delay in notifying the respondents that it would or might make a claim pursuant to the undertakings as to damages;
(b) the fact that any application for listing by Apotex, and any subsequent supply, would have involved an infringement of Sanofi’s copyright in the product information relating to Plavix (“the Plavix PI”) and in the consumer medicine information relating to Plavix (“the Plavix CMI”);
(c) the fact that any products which Apotex supplied in the period of the Restraints (that is, from 25 September 2007 until 12 March 2010) would have been manufactured in Canada, in breach of Sanofi’s Canadian Patent; and
(d) any award of compensation would be contrary to the public interest or the interests of justice, including that it would be unjust to order the respondents to pay compensation in respect of loss attributable to a final order of the court in their favour after a trial on the merits.
Each of these matters was relied on individually and collectively.
596 It has long been established that an application to enforce an undertaking must be brought within a reasonable time, and that an otherwise meritorious claim for compensation instituted pursuant to an undertaking may be refused due to delay: see Air Express at 261 per Aickin J.
597 The Commonwealth first became aware of the existence of the patent proceeding in August 2007. On 16 August 2007, the Commissioner of Patents was given various documents in accordance with O 58, r 14(1)(c) of the Federal Court Rules 1979 (Cth). These included a letter from Apotex Australia’s lawyers to the Commissioner of Patents serving, in accordance with the rule, copies of the application, the statement of claim, and the particulars of invalidity filed by Apotex Australia in the patent proceeding.
598 On 29 August 2007 Sanofi gave a certificate under s 26C(2) of the TG Act to the Secretary of the Department referring to infringement proceedings that Sanofi proposed to commence against Apotex Australia. The statutory scheme that provides for the giving of such certificates was considered by the Full Court in Commonwealth v Sanofi (2015) 237 FCR 483 at 496-499 [49]-[58].
599 On 17 September 2007, the solicitor for Sanofi, Mr Phillip Kerr of Allens Arthur Robinson (“Allens”), wrote to the Commissioner enclosing a copy of Sanofi’s defence and cross-claim. The cross-claim included claims for final and interlocutory injunctive relief restraining Apotex Australia from (inter alia) supplying the Apotex Australia clopidogrel products and from taking any steps to obtain a listing of any of those products under the PBS.
600 Also included in Mr Kerr’s letter was a copy of the notice of motion filed by Sanofi which contained the same claim for interlocutory relief and, in addition, set out the terms of the usual undertaking as to damages which was expressed to apply to both of the interlocutory injunctions sought. It would have been apparent to the Commissioner from the notice of motion that Sanofi was intending to give the usual undertaking as to damages in respect of any order restraining supply or the taking of steps to list by Apotex Australia.
601 On 3 September 2008 Mr Kerr wrote to the Commissioner advising her of the orders made by Gyles J and enclosing a copy of the minute of his Honour’s orders. It would have been apparent to the Commissioner or any person acting on her behalf that Gyles J had ordered that various claims of the Patent be revoked but that these did not include claim 3 which was held to be valid and infringed by Apotex Australia and that a final injunction had been granted restraining the infringement of that claim.
602 The Commissioner was not notified of the undertakings given to Moore J on 15 September 2008. Nor is there any evidence to suggest that the Commissioner was aware of the existence of those undertakings or that she could have had any reason to assume that the Sanofi BMS companies (or any of them) had given any further undertakings as to damages to the Court in respect of either the interlocutory injunction or the final injunction. For all the Commissioner knew, the interlocutory injunction had been overtaken by the final injunction that prevented Apotex Australia from supplying its clopidogrel products for the duration of the term of the Patent.
603 The Commissioner was not notified of the orders of the Full Court until 29 April 2010 when Apotex’s lawyers forwarded a copy of the Full Court’s minute of order to her under cover of a letter that drew attention to the fact that the Full Court had ordered the Patent be revoked and that the application for special leave to appeal against the Full Court’s judgment had been refused more than six weeks earlier. That letter was not sent to the Commissioner until some weeks after the High Court had refused the Sanofi BMS companies’ application for special leave to appeal.
604 Before turning to some evidence given by Ms McNeill in relation to the issue of delay, it is important to recognise the limitations in the information that was provided to the Commonwealth in the form of those communications addressed to the Commissioner of Patents.
605 It appears that the Commissioner was not given any notice that any interlocutory injunction had been granted or that any cross-undertaking as to damages had been given. The Commissioner was notified of the final orders made by Gyles J on 3 September 2008 but was not provided with any further information in relation to the patent proceeding, including the outcome of the appeal to the Full Court or the application for special leave, until 29 April 2010.
606 There is evidence that suggests that officers of the Department understood in 2005 that the Commonwealth could be adversely affected by interlocutory orders made in patent proceedings and that it might be entitled to make a claim on a cross-undertaking as to damages given in relation to such an order based on its impact on the pricing of drugs listed on the PBS including, in particular, any 12.5% price reduction that might be expected to be imposed if no order was made.
607 On 23 September 2005, Mr Phillip Spann, as Deputy Commissioner of Patents, determined that the Register of Patents should be amended to bring forward the date of expiry of patents held by Pfizer for various drugs listed on the PBS. Pfizer appealed to the Federal Court in a proceeding (“the Pfizer proceeding”) to which the Commissioner of Patents was the respondent and in which Pfizer also sought a stay of Mr Spann’s decision.
608 Mr Spann gave instructions to the Australian Government Solicitor (“AGS”), who was acting for the Commissioner of Patents, to notify both Spirit (a generic supplier seeking to enter the market) and the Department of Pfizer’s stay application.
609 By letter dated 18 October 2005, the AGS notified Pfizer’s lawyers that there were interested third parties whom the Commissioner considered should be notified of the stay application. The AGS’s letter identified the Department as one of the interested third parties, stating that the Department had an interest because the proceeding related to a drug that was listed on the PBS.
610 The AGS’s letter of 18 October 2005 was copied to the Secretary of the Department, Ms Halton, and the First Assistant Secretary of the Medical and Pharmaceutical Services Division (which was the previous name of PBD), Ms Huxtable. Both Ms Halton (as Secretary) and Ms Huxtable (as Deputy Secretary) were still at the Department as at 3 March 2010. However, in January 2008, Ms Huxtable moved to a different role in the Department leading a Health Policy Taskforce after which she no longer had any responsibility for the PBS.
611 The Commonwealth (acting though the Department) sought to intervene in the Pfizer proceeding for the purpose of submitting that Pfizer should be required to give the usual undertaking in support of any stay so that the Commonwealth would have some protection if the stay resulted in increased PBS expenditure. The Commonwealth filed an affidavit in support of the application that was affirmed by Ms Corbett, who was at that time Assistant Secretary of the Pharmaceutical Benefits Branch (the previous name of the PEB). The Commonwealth also filed written submissions in support of its intervention which were directed to persuading the Court that Pfizer should be required to give the usual undertaking as to damages as the price of obtaining a stay.
612 Ms Corbett’s affidavit explained that the sooner that the generic competitor (in that case Spirit) was able to list and sell, the sooner a 12.5% price reduction could be applied to the drug Norvasc and four other PBS listed drugs.
613 Judgment on Pfizer’s stay application was given by Bennett J on 11 September 2006. The stay was granted and later continued upon Pfizer giving an undertaking as to damages while Pfizer pursued what was ultimately an unsuccessful appeal against Bennett J’s decision upholding the Commissioner’s decision in respect of each of the patents.
614 It was put to Ms McNeill in cross-examination that Ms Corbett was still in the Department through until March 2010. Her recollection was that Ms Corbett had retired well before then, but when she was shown a document (that does not appear to have been tendered by any party) she accepted that Ms Corbett “… was still an officer of the department in the 2009/2010 year”.
615 Contrary to a submission made by Sanofi BMS, Ms McNeill’s evidence does not establish that Ms Corbett was still working in the Department until at least 10 March 2010. The evidence does not permit me to say when it was that Ms Corbett left the Department or, in particular, whether she was still there when the Sanofi BMS companies’ application for special leave was refused.
616 As previously mentioned, Ms McNeill joined the Department as Assistant Secretary of PEB on 4 January 2010. She gave evidence that the organisational structures in place in the Department were such that any decision by the Commonwealth to pursue a claim for compensation was a decision that would have been the responsibility of the First Assistant Secretary of PBD to recommend and take forward. That is the position Ms McNeill held on an acting basis from 27 September 2010 and on a permanent basis from 5 January 2012.
617 Mr Learmonth, who was the Deputy Secretary of the Department, received an email communication from the TGA on 12 March 2010 forwarding details of a press announcement issued by Apotex Australia which included a statement indicating that it would seek compensation from Sanofi for loss suffered as a result of having been kept out of the clopidogrel market. He forwarded a copy of the email to Ms McNeill on the same day with a one word message conveying his relief at the outcome. While he no doubt appreciated the importance of the decision so far as PBS spending on clopidogrel was concerned, there is nothing in the email, or any other evidence, that would suggest that he, or any other person then reporting to either Ms McNeill or himself, understood that the Commonwealth may also have a right to claim compensation from Sanofi as a result of undertakings as to damages given in the patent proceeding.
618 Ms McNeill gave evidence that she first became aware of the possibility that the Commonwealth may be able to recover compensation from Sanofi as the result of a meeting of the Generic Medicines Working Group that she attended on 22 March 2011 (“the GMWG meeting”). Mr David Learmonth was also present at the GMWG meeting. It appears from Ms McNeill’s evidence that he too was unaware until the GMWG meeting that the Commonwealth may have a claim against Sanofi for compensation arising out of orders made against Apotex Australia in the patent proceeding.
619 Shortly after the GMWG meeting Ms McNeill sought legal advice from the General Counsel of the Department. That advice was provided to her in July 2011. Advice was sought from Corrs Chambers Westgarth (“Corrs”). It was not until 27 February 2012 that Corrs wrote to Allens informing them that the Commonwealth intended to seek compensation pursuant to the undertakings as to damages provided by Sanofi.
620 Ms McNeill’s evidence in relation to these matters was not challenged and I accept it. In particular, I accept that neither she nor Mr Learmonth was aware before March 2011 that the Commonwealth may be able to recover compensation from Sanofi under any of the relevant cross-undertakings as to damages given in relation to orders made against Apotex Australia. I think it more likely than not that other persons working within the PBD, even if aware of the patent proceeding between Apotex Australia and the Sanofi BMS companies, were also unaware of the Commonwealth’s right to make a claim for compensation under any of the undertakings as to damages.
621 If at any time between the date of the interlocutory injunction granted by Gyles J and the order of the High Court refusing special leave to appeal, the Department had sought advice in relation to whether or not the Commonwealth could recover on the undertaking as to damages given by Sanofi in support of the interlocutory injunction, the advice would have been that this necessarily depended on the outcome of the trial and any appeal. Under the terms of the various interlocutory orders made by the Full Court that were in place up until the High Court made its order, the final injunction granted by Gyles J remained in force. It was quite simply not open to the Commonwealth to make any claim until the High Court refused Sanofi BMS special leave to appeal.
622 The Sanofi BMS companies seek to address this by submitting that the Commonwealth was guilty of delay by not giving notice at any time prior to the determination of the application for special leave that the Commonwealth may seek to make a claim for compensation in the event that the final judgment of Gyles J was later set aside. That submission would have been slightly more attractive if there was any evidence (which there is not) to suggest that the Sanofi BMS companies believed, whether based on the Commonwealth’s silence during the period from September 2007 to March 2010 or at all, that no claim for compensation would be made by the Commonwealth. What is said by Sanofi BMS to be delay up until March 2010 was not, in my view, delay at all and is certainly not delay which would support the refusal of relief.
623 So far as delay after 12 March 2010 is concerned, nothing appears to have been done by the Commonwealth with a view to investigating the possibility of a claim until after the GMWG meeting. There is no explanation for that delay other than that Ms McNeill, and other officers within the Department, did not appreciate that the Commonwealth may have a right to claim compensation from Sanofi or BMS until the GMWG meeting took place.
624 Between 22 March 2011, when the GMWG meeting took place, and 27 February 2012, when Corrs wrote to Allens, the Commonwealth took steps to investigate the possibility of making a claim against Sanofi. The Commonwealth appears to have spent about 12 months investigating the possibility of such a claim before arriving at the point at which it felt able to instruct Corrs to write to Allens asserting, in very general terms, the Commonwealth’s entitlement to compensation.
625 It was suggested by Sanofi BMS in their submissions that the process of obtaining any necessary legal advice and making a claim should not have taken anywhere near 12 months. I do not accept that submission. The potential claim was novel and complex. And, even though the total period of delay following notification of the refusal of special leave on 12 March 2010 is substantial, it is explained, in my view, by the fact that the relevant officers within the Department had no appreciation until legal advice was obtained that the Commonwealth may have a right to claim compensation from Sanofi. It is apparent that any knowledge that may have been gained by Ms Corbett and others within the Department as a result of the Pfizer litigation did not trigger any such appreciation. Whether or not it should have is another question, although it is not one that was taken up with Ms McNeill in her cross-examination.
626 Sanofi BMS placed considerable emphasis in their submissions on the fact that there had never been a claim made by the Commonwealth on an undertaking as to damages given in relation to an interlocutory injunction preventing a generic supplier from listing its products on the PBS before now. Be that as it is, there is nothing in the evidence to indicate that this led Sanofi BMS to believe that there would be no claim made by the Commonwealth in this case. The possibility that such a claim might be made was implicitly acknowledged by Mr Bannon SC when appearing for Sanofi before Gyles J on 18 September 2007. After noting that the Commonwealth had not applied to be made a party to the proceeding, Mr Bannon SC said “… but our undertaking as to damages, as your Honour would know, is not limited to the parties”.
627 Although the delay that occurred after 12 March 2010 was significant, it has in my view been explained. I accept the explanation is not entirely satisfactory in circumstances where the Commonwealth had such a significant financial interest in generic competition in the clopidogrel market. On the other hand, in assessing whether or not it is just and equitable, or fair and reasonable, to refuse the Commonwealth relief based upon the existence of such delay, I have had regard to the fact that Sanofi BMS do not assert that they have suffered any prejudice as a result of any delay by the Commonwealth in notifying them of its claim.
628 In all the circumstances, I am not persuaded that any delay by the Commonwealth in asserting its claim against Sanofi BMS justifies the refusal of the relief sought.
629 If Apotex Australia wished to list its clopidogrel products on the PBS on 1 April 2008 it was required, under the Policy and Procedures Manual of the PBPA, to provide to the PBAC (inter alia) a copy of the current approved product information document (“PI”) for those products. The PI that would have been used by Apotex Australia to meet this requirement (“the Apotex PI”) was the same as that which was current as at 1 December 2007 and which had been approved by the TGA in August 2007 when Apotex obtained the ARTG registration of its clopidogrel products. The Apotex PI was provided to the Department by Apotex Australia when it made its conditional application for PBS listing in early September 2007.
630 The Commonwealth accepted that copyright subsisted in the Plavix PI and that it was at all relevant times owned by Sanofi Australia.
631 The Apotex PI reproduced almost the entire text of the Plavix PI. The Commonwealth did not dispute, in the course of its final submissions, that by reproducing the Plavix PI in the Apotex PI, Apotex Australia had infringed copyright in the Plavix PI.
632 The Therapeutic Goods Legislation Amendment (Copyright) Act 2011 (Cth), which introduced s 44BA into the Copyright Act 1968 (Cth), received royal assent on 27 May 2011. It is common ground that this amendment eliminated any scope for Sanofi Australia to make any claim against Apotex Australia in relation to any infringement of copyright in the Plavix PI and the Plavix CMI occurring after that date.
633 I find that if Apotex Australia had applied for a PBS listing of its clopidogrel products from 1 April 2008, it would have infringed Sanofi’s copyright in the Plavix PI by reproducing in the Apotex PI a substantial part of the Plavix PI when applying for such listing.
634 Regulation 9A(2) of the Therapeutic Goods Regulations 1990 (Cth) at all relevant times required that any supply of Apotex’s clopidogrel products be accompanied by a CMI document. If Apotex had supplied any of its clopidogrel products in any of the relevant counterfactual scenarios, it would have been necessary for Apotex Australia to comply with this regulation. The CMI for a product is typically much shorter and less complex than the PMI.
635 It was accepted by the Commonwealth that copyright subsisted in the Plavix CMI and that it was at all relevant times owned by Sanofi Australia. The form of the CMI that Apotex Australia in fact supplied with its clopidogrel products from March 2010 included large parts that were identical to, or substantially similar to, corresponding parts of the Plavix CMI.
636 I find that if Apotex Australia supplied its clopidogrel products at any time between 2008 and 2010, it would have most likely infringed Sanofi Australia’s copyright in the Plavix CMI due to the need to comply with reg 9A(2).
637 All that being so, the next question is what significance, if any, these copyright findings have to the Commonwealth’s claim for compensation and whether, as Sanofi BMS contend, they should lead the Court to refuse to grant the Commonwealth relief.
638 Sanofi BMS made clear that they did not contend that, in any relevant counterfactual scenario, Sanofi, or Sanofi Australia, would have taken any action against Apotex Australia for infringement of copyright in either the Plavix PI or the Plavix CMI. In particular, it was not suggested that either of them would have written a letter of demand or made any other complaint, much less commenced legal proceedings, in respect of any such infringement.
639 The Commonwealth contends that, in circumstances where it is accepted that neither Sanofi nor Sanofi Australia would have taken any action against Apotex Australia in relation to any infringement of copyright in either the Plavix PI or the Plavix CMI, Sanofi BMS should not be permitted to rely on copyright infringement in any counterfactual scenario as an answer to the Commonwealth’s claim.
640 The Sanofi BMS companies’ submissions focused on what was said to be the ability of Sanofi Australia to assert a copyright claim in 2007 and 2008. While they do not contend that copyright is a matter that they would have raised in seeking to prevent Apotex Australia from listing and supplying its clopidogrel products, it was submitted that it does not follow that any infringement by Apotex Australia of copyright in the Plavix PI or the Plavix CMI could not be taken into account by the Court when exercising its discretion to withhold relief.
641 There was considerable evidence given as to how difficult and time consuming a task it would be for Apotex Australia to rewrite the Apotex PI so that it did not infringe Sanofi Australia’s copyright. Mr Millichamp’s evidence on this issue, which I accept, is that this would take about three weeks, but that it could take some months of interaction with the TGA before the rewritten PI was accepted. His evidence that it would take about two to three weeks to rewrite a PI is consistent with Sanofi’s own internal documents which are to the same general effect.
642 In their written submissions the Sanofi BMS companies contended:
… [I]f Sanofi had asserted in September 2007 that Apotex had, in the preparation of the Apotex PI, infringed its copyright in the Plavix PI, it would have taken at least 6-7 months in order for the Apotex PI to be rewritten, submitted to the TGA for approval, and approved by the TGA (that is, until around April 2008). Until that occurred, Apotex would not have been able to submit the rewritten PI, as approved by the TGA, to the Department with an application for listing. Accordingly, Apotex would not have been able to apply, by 1 December 2007, for a 1 April 2008 listing. Also, there must be real doubt whether Apotex would have been in a position (if it chose to do so) to apply, by 1 April 2008, for a 1 August 2008 listing.
643 The difficulty with this submission is that it assumes that copyright would have been asserted by Sanofi in any relevant counterfactual scenario and that Sanofi would also have been able to prevent Apotex Australia from using its existing PI by obtaining what would have had to be (given the relevant timeline) an interlocutory injunction. It cannot be assumed that Apotex Australia would not have defended any proceeding brought against it for copyright infringement or that Sanofi would have been granted an interlocutory injunction without demonstrating that damages would not be an adequate remedy. There is good reason to believe that a judge would have refused to grant an interlocutory injunction if the evidence showed (as was the fact) that the Apotex PI could be rewritten in two or three weeks.
644 I am therefore not persuaded that copyright in the Plavix PI or the Plavix CMI provided Sanofi with any opportunity to delay any PBS listing of the Apotex clopidogrel products. Nor do I think that it would be just and equitable to reduce any amount that would otherwise be awarded to the Commonwealth on the basis of a postulated infringement of a legal right which the Sanofi BMS companies seem to accept would never have been asserted against Apotex Australia in any relevant counterfactual scenario.
645 As previously mentioned, Sanofi commenced an action against Apotex Canada for infringement of the Canadian Patent in June 2009. A judgment of the Federal Court of Canada holding the Canadian Patent invalid was set aside by the Federal Court of Appeal in July 2013. An appeal against that decision, brought pursuant to special leave to appeal granted by the Supreme Court of Canada in January 2014, was discontinued following execution of the Canadian Settlement Agreement between Sanofi and Apotex Canada on 13 November 2014.
646 At no stage was there any injunctive relief in place in Canada that prevented Apotex Canada from manufacturing and exporting to Australia the Apotex clopidogrel products. Had Apotex Australia launched its clopidogrel products in Australia in early 2008, it would have sourced its supply of those products from Canada where they would have been made by Apotex Canada. In the present case, Sanofi BMS contend that any clopidogrel product manufactured in Canada for supply to Apotex Australia would have constituted an infringement of the Canadian Patent and that this provides another reason why the Commonwealth should be refused the relief it seeks in this case.
647 The judgment of the Federal Court of Appeal is in evidence but not for the purposes of proving any fact in issue in the Canadian proceedings or any facts found in the judgment. The critical issues in the proceeding in the Federal Court of Appeal included whether the Canadian Patent was invalid for either lack of utility or because the invention described was obvious. The trial judge had found the Canadian Patent invalid on both grounds and the correctness of key findings relevant to those grounds were in issue in the appeal.
648 In this case Sanofi BMS submitted that there was no issue before the Federal Court of Appeal that the Canadian Patent was infringed. That is correct in the sense that Apotex Canada accepted the clopidogrel products it manufactured fell within the scope of one or more of the claims of the Canadian Patent. But there certainly was an issue as to whether or not those claims were valid. Needless to say, even if Apotex Canada’s products fell within the scope of any asserted claim, no relief could be obtained in respect of any alleged infringement of that claim if the claim was held to be invalid and revoked.
649 The Commonwealth submitted that the judgment of the Federal Court of Appeal in Canada does not establish that any of the asserted claims of the Canadian Patent was valid. It argued, correctly in my view, that given the limited basis on which the judgment of the Federal Court of Appeal was admitted into evidence, it cannot be relied on to prove the validity of the Canadian Patent.
650 It was not suggested by Sanofi BMS that the Commonwealth was precluded as a matter of law from disputing the validity of the Canadian Patent. The Commonwealth was not a party to the Canadian proceedings or to the Settlement Agreement that led to the discontinuation by Apotex Canada of its appeal to the Supreme Court of Canada. Nor is there any presumption of validity upon which Sanofi BMS can rely in this proceeding.
651 In my view the Commonwealth’s submission should be accepted. It has not been established that, in any relevant counterfactual scenario, the manufacture of the Apotex clopidogrel products in Canada by Apotex Canada would have infringed a valid claim of the Canadian Patent. The whole basis of the Sanofi BMS argument on this topic depends on an assumption that has not been proven.
652 In my view it would not be just or equitable to reduce any compensation otherwise payable to the Commonwealth based on an unproven assertion that the Canadian Patent was valid.
653 There is a public interest in ensuring that an applicant who obtains an interlocutory injunction on condition that it gives a cross-undertaking as to damages is held to the undertaking in the event that it is later decided that the applicant is not entitled to a final injunction. However, the right of any person adversely affected by the interlocutory injunction to recover compensation is governed by the terms of the relevant undertaking and the relevant legal principles previously discussed, which are informed by the interests of justice and, in particular, whether it is just and equitable that a person who has obtained an interlocutory injunction but fails at trial to obtain final relief should be required to pay compensation to a person adversely affected.
654 I do not think there is any additional public interest consideration that arises in this case that should prevent the Commonwealth from recovering compensation under the undertaking as to damages given by Sanofi in return for the interlocutory injunction.
655 Sanofi BMS’ submission in relation to the public interest focused on the circumstances in which the further undertaking as to damages was given to Moore J. While there is precedent for the grant of an undertaking as to damages in relation to a final injunction (usually as a condition imposed in return for not ordering a stay of such an injunction pending appeal) there are particular features of the undertaking as to damages and the circumstances in which it was given that were said to stand out.
656 In their submissions Sanofi BMS drew attention to the fact that the undertaking as to damages was given some weeks after the final injunction was granted. I do not regard this as unusual. In those cases where such an undertaking has been given as a condition imposed in return for refusing an application for a stay, the undertaking will often be given sometime after the final injunction has been granted, especially if (as in this case) the primary judge takes the view that the question of whether his or her final orders should be stayed is a matter that should be dealt with in the appellate jurisdiction.
657 Another feature of the undertaking as to damages given by Sanofi BMS in relation to the final injunction which was said to be unusual is that it extended not just to the parties to the proceeding in which it was given but also to any other person adversely affected by the final injunction. It was submitted by Sanofi BMS that the undertaking as to damages was most likely framed in those terms so as to permit other companies within the Apotex group to make claims for compensation in the event that Gyles J’s judgment was set aside. That may be so, but it is also likely that the terms of the undertaking given merely reflect the view that there was no reason why an undertaking in any terms narrower than those of the “usual undertaking” should be given.
658 In any event, the evidence as to any prior negotiations relating to the undertaking as to damages (and the other agreed undertakings given to Moore J) is scant and it is not possible to say which of those two explanations is likely to be correct. But I do agree with the submission made by Sanofi BMS that the fact that the Commonwealth found itself in a position where it could make a claim under an undertaking as to damages given in relation to a final injunction was purely fortuitous. The Commonwealth did not even learn of its existence until some 18 months after the undertaking was given and Ms McNeill and her colleagues seem to have been taken by complete surprise when later told that the Commonwealth may be able to make a claim for compensation.
659 On the other hand, the undertaking as to damages was given to Moore J by Sanofi BMS in the usual form and in terms that are very clear. There is nothing in the evidence that would enable me to infer that Sanofi BMS did not understand that the Commonwealth may be entitled to recover compensation from them pursuant to that undertaking in the event that Gyles J’s judgment was later set aside.
660 I am not persuaded there is any additional public interest consideration that would, even if the Commonwealth were otherwise entitled to recover compensation, justify the refusal of such relief.
661 Although it is unnecessary to do so, I propose to make findings on various issues relating to the quantum of the Commonwealth’s alleged loss.
662 The accounting experts have agreed the calculation methodologies for the computation of both loss and interest. The differences between them concern the appropriate assumptions they should make.
663 All dollar figures quoted in this section of the judgment are exclusive of interest.
The Commonwealth’s loss in the interrupted supply counterfactual
664 The accounting experts agree that if it is found that:
Apotex Australia would have ceased supplying its clopidogrel products from 19 August 2008 until 1 April 2010; and
the 12.5% statutory price reduction triggered on 1 April 2008 would have been reversed;
then the loss suffered by the Commonwealth is $11,038,663. They also agree that if there was no reversal then the Commonwealth’s loss is $55,724,324 which includes $1,995,963 in respect of the fixed statutory price reductions as they would have applied to clopidogrel with aspirin. There would have been no price disclosure price reductions in the interrupted supply counterfactual.
665 As previously mentioned, the Commonwealth submitted that so much of its alleged loss that is attributable to any decision not to reverse the 12.5% statutory price reduction (ie. $44,685,661) could be adjusted to reflect the possibility that no such reversal would have occurred. I have previously found that there was about a 10% chance that the 12.5% price reduction would not be reversed which would have justified an award of compensation in that scenario of $15,507,229.
The Commonwealth loss in the continuous supply counterfactual
666 The Commonwealth’s claim based on the continuous supply counterfactual has three components; the first is that based on the fixed statutory price reductions that would have occurred on 1 April 2008 and 1 August 2009, the second relates to price disclosure price reductions that the Commonwealth alleges would have occurred from 1 April 2010 through to 31 December 2014, and the third relates to the alleged impact that the price disclosure price reduction regime would have had on price negotiations in respect of clopidogrel with aspirin in 2009 in the lead up to any price disclosure price reduction on 1 April 2010.
667 The quantum of the Commonwealth’s claimed loss in respect of the fixed statutory price reductions that would have occurred on 1 April 2008 and 1 August 2009 has been agreed between the accounting experts at $50,718,312. The agreed figure does not include any amount in respect of price disclosure price reductions, the first of which would not have occurred until 1 April 2010. Nor does the agreed figure include any amount in respect of the Commonwealth’s claim arising out of price negotiations for clopidogrel with aspirin.
668 If I had found that the Commonwealth was entitled to recover any compensation in respect of price disclosure price reductions, then it is necessary to determine what market shares would have been obtained by Sanofi BMS and generic suppliers throughout the relevant period and what level of discounts would have been offered by them from time to time. This is because the market shares and price of each of the clopidogrel products in the continuous supply counterfactual are used in calculating the WADP that would have been determined periodically in the counterfactual world. The WADP would then have been used to determine whether a price disclosure price reduction was to occur on any of the prescribed dates.
669 Sanofi BMS invited me to apply the discount levels which Mr Montgomery had applied in his expert report. The Commonwealth did not contend for any different approach. The discount levels used by Mr Montgomery have been used in calculating price disclosure price reductions that would have occurred in the continuous supply counterfactual.
670 There was a substantial disagreement between Mr Montgomery and Mr Hobbs as to the market share for clopidogrel that would have been obtained by Apotex Australia in the continuous supply counterfactual.
671 The Commonwealth relies on the evidence of Mr Montgomery who estimated that the market share that would have been achieved by Apotex Australia in the continuous supply counterfactual in the period 1 April 2008 to 31 March 2010 would have been higher (on average 5%) than the aggregate of the market shares of all suppliers of generic clopidogrel products that were achieved in the real world in the period 1 April 2010 to 31 March 2012. Mr Hobbs, on the other hand, estimated that the market share that would have been achieved by Apotex Australia during the first two years after launch was likely to be in the order of 5% to 8% below the total market share that was achieved by all generic suppliers in the real world during that two year period.
672 The loss claimed by the Commonwealth based on Mr Montgomery’s market share estimates in respect of clopidogrel (but excluding any amount in respect of clopidogrel with aspirin) is agreed by the accounting experts to be $266,640,777 which includes the amount of $50,718,312 previously referred to in respect of the fixed statutory price reductions on 1 April 2008 and 1 August 2009. If the Commonwealth’s case based on the price negotiations for clopidogrel with aspirin were to have been accepted, then the Commonwealth would be entitled to a further $58,307,301 in respect of clopidogrel with aspirin giving a total amount in respect of the continuous supply counterfactual of $324,948,078.
673 Adopting Mr Hobbs’ market share estimates, the agreed figure in respect of clopidogrel (but excluding any amount in respect of clopidogrel with aspirin) is $233,560,796. To that it would be necessary to add an additional amount of $1,414,103 in respect of the fixed statutory price reductions as they would have applied to clopidogrel with aspirin. No additional amount has been included in the accounting experts’ agreed figures in respect of the effect of any price disclosure price reduction on the negotiations relating to clopidogrel with aspirin. Were it necessary for me to have done so I would have directed the accounting experts to calculate a precise figure in respect of that component of the Commonwealth’s alleged loss.
674 It is important to note that Mr Montgomery’s market share estimates were based on a “bottom up” analysis of the market for clopidogrel in 2008 and 2009 and did not involve any analysis of sales made in the real world. It was Mr Hobbs who adopted what was referred to in submissions as the “backdated actual market share” approach which took actual sales figures for the period 1 April 2010 to 31 March 2012 as its starting point. For reasons that I will now explain I prefer the approach taken by Mr Hobbs.
675 It would be surprising if Apotex Australia would have achieved a greater market share in the continuous supply counterfactual than it and all other generic suppliers achieved in the real world a few years later. Some of those generic suppliers which Apotex Australia found itself competing with in the clopidogrel market in 2010 were large and well-known businesses (eg. Sandoz, Sigma, Alphapharm and Generic Health) all of which would have been striving to acquire market share. Mr Montgomery conceded that the “rational, normal expectation” is that a single generic supplier would perform less well than a group of generic suppliers competing for business because there would be less pressure to maximise discounts and offer the best terms. However, he maintained that this expectation did not apply in the circumstances of the continuous supply counterfactual.
676 Before considering Mr Montgomery’s reasoning in more detail, it is necessary to say something about the likelihood of an autogeneric product being listed on the PBS in the continuous supply counterfactual. As previously explained, an autogeneric is a generic product marketed by or with the licence of the innovator. In the pharmaceutical industry, an autogeneric is not usually thought of as a true generic. An innovator will usually launch an autogeneric as part of a broader strategy to defend its market share for a particular drug in circumstances where the innovator has or will soon lose its exclusivity in respect of that drug.
677 Mr Montgomery was of the view that Sanofi was likely to have listed its autogeneric Winthrop brand clopidogrel products on 1 November 2008 if Apotex Australia had listed its clopidogrel products on 1 April 2008. This evidence may be contrasted with that given by Mr Millichamp who said that it was “very unlikely” that Sanofi would have launched an autogeneric clopidogrel product before the patent proceedings had been finally determined and any rights of appeal had been exhausted. Mr Millichamp’s evidence on that issue was against the weight of the evidence given by Sanofi witnesses and Mr Montgomery. In fairness to the Commonwealth, it did not place any reliance on Mr Millichamp’s evidence on this particular point and it agreed that if Apotex Australia listed its clopidogrel products on the PBS on 1 April 2008 and supplied them continuously thereafter, then the autogeneric product would have been listed on the PBS on 1 November 2008.
678 However, in its closing submissions the Commonwealth sought to rely on a number of other statements made by Mr Millichamp in his written evidence (which were said to be unchallenged) in support of its contention that Apotex Australia would have achieved a greater market share in the continuous supply counterfactual than was later achieved in the real world by all generic suppliers combined. I do not regard Mr Millichamp’s evidence on this topic as reliable and I give it no weight.
679 Mr Montgomery’s estimate of the market shares that would have been achieved by generic suppliers in the continuous supply counterfactual was based on a consideration of the following three factors:
clopidogrel offered a large profit opportunity for pharmacists since it combined a high volume of scripts and a highly subsidised price;
Apotex Australia would have enjoyed an extended period of exclusivity (or clean air) of approximately 23 months duration;
the August 2007 PBS reforms would have motivated pharmacists to more enthusiastically substitute generic products to offset the impact of those reforms on their profit margins.
I will deal with each of these factors in turn.
680 The “profit opportunity” for pharmacists referred to by Mr Montgomery had three components: the volume of scripts a pharmacist can dispense; the government subsidised price of the product against which a percentage discount is offered by the generic supplier; and the level of the discount offered by the generic supplier. However, Mr Montgomery accepted in cross-examination that the profit opportunity available to pharmacists was greater in 2010 than it was in 2008. That was because each of the three components of the profit opportunity was equally or more advantageous to pharmacists in 2010.
681 Mr Montgomery reasoned that the high volume of clopidogrel sales (2,111,423 PBS scripts dispensed in the 12 months to 30 June 2007) was a factor that would have driven generic substitution in 2008. However, the evidence shows that clopidogrel PBS script volume grew significantly from 2,309,279 in the 12 months to 30 June 2008 to 2,676,941 in the 12 months to 30 June 2010. Mr Montgomery accepted that in terms of volume, the opportunity for pharmacists was greater in 2010 than in 2008.
682 Mr Montgomery also reasoned that the relatively high price of clopidogrel was a factor that would have driven generic substitution in 2008. However, he accepted that the AP2P for clopidogrel was not materially different between 2008 and 2010.
683 Mr Montgomery pointed to the aggressive discounting by Apotex Australia as a factor that would also have driven generic substitution in 2008. However, the evidence shows that the deals offered to pharmacists in the real world in 2010 were more favourable than they would have been in the counterfactual world in 2008. In particular:
the discounts that Apotex Australia would have offered in 2008 in the counterfactual were 10% smaller than those which Sandoz and Apotex Australia in fact offered in the real world (Apotex Australia would have offered a 60% discount in 2008 whereas Sandoz offered a 70% discount in 2010 that Apotex Australia matched);
the purchasing terms that Apotex Australia would have offered in 2008 were less attractive than those it offered in the real world (Apotex’s 60% discount in 2008 would have required the purchase of 13 months of stock, the offer in the real world in 2010 was 70% with a minimum buy of six months of stock, or 65% with no minimum buy requirement).
684 Mr Montgomery accepted that Apotex Australia’s 65% discount with no minimum buy requirement was a “better deal” than what would have been offered in 2008.
685 Mr Montgomery suggested that in the counterfactual world Apotex Australia would have had a period of 23 months of “clear air” in which to maximise generic substitution in circumstances where it would not have been “distracted by other generics with competing messages and competitive pricing”.
686 If Apotex Australia had launched its clopidogrel products in early 2008, it would have found itself in direct competition with Sanofi, which would have adopted an aggressive defence strategy in an attempt to minimise the growth of the generic market for clopidogrel. The evidence shows Sanofi would have offered a 30% discount on Plavix with a six month buy requirement soon after Apotex Australia began offering deals on its products in January 2008. Mr Montgomery accepted that an offer of that kind in 2008 would have been unprecedented, and would have offered a very large profit opportunity for pharmacists.
687 If the Apotex clopidogrel products had been listed on the PBS on 1 April 2008, Sanofi’s autogeneric Clopidogrel Winthrop product would have been listed on the PBS on 1 November 2008. Sanofi would have commenced to market this product aggressively from 5 August 2008 when it was registered on the ARTG. The evidence shows that it would most likely have been offered at a discount of 75% which Apotex Australia would have then had to match.
688 Mr Montgomery also accepted that Clopidogrel Winthrop had an advantage over the Apotex clopidogrel products in that at least some pharmacists would have preferred to purchase an authorised generic product as opposed to a true generic.
689 In any event, it seems to me that what Mr Montgomery refers to as “clean air” is an environment free from aggressive competitive activity engaged in by multiple generic suppliers all of who would be seeking to increase their market share for clopidogrel products. In my opinion, competitive activity of that kind would be likely to lead to increased generic substitution resulting in the generic suppliers enjoying a larger aggregate market share of the clopidogrel market than would be achieved by one generic supplier.
690 Mr Montgomery emphasised that at the time of a hypothetical launch by Apotex Australia in early 2008, the 2007 reforms to the PBS would have come into effect. He considered that these changes impacted pharmacy profit margins and that “[p]harmacists were … keenly interested in addressing profit declines from PBS reforms, and the need to drive generic substitution was seen by many pharmacists as the most effective way to address those profit declines”. However, as Mr Montgomery conceded in cross-examination:
pharmacists were as focused on making profits from the sale of discounted generic products in 2008 as they were in 2010;
by 2010 pharmacists were more effective at switching patients to generic products as generic brands were more widely accepted by patients; and
the rate of generic substitution increased between 2008 and 2010.
691 I am not persuaded that Apotex Australia would have achieved a higher level of generic substitution in the continuous supply counterfactual than was achieved by multiple generics suppliers in the real world.
692 The Commonwealth submitted that if Mr Montgomery’s market share estimates were not accepted, then its loss should be assessed using the backdated actual market share approach on the basis that Apotex Australia would have sold as much generic clopidogrel between 1 April 2008 and 31 March 2010 as was sold by all generic suppliers in the two year period commencing from 1 April 2010. Sanofi, on the other hand, submitted that it was unlikely that Apotex Australia would have had that level of success and that some discount should be applied to the real world figures to reflect this. Sanofi BMS submitted that an appropriate discount factor was 10%.
693 The Commonwealth submitted there was an insufficient evidentiary basis to support a finding that Apotex Australia’s market share in its first two years selling clopidogrel would have been any lower than the aggregate market share that was achieved by all generic suppliers in the real world. It was on that basis that the Commonwealth submitted that there should be no discount applied to the figures obtained using Mr Hobbs’ backdated market share approach. I do not accept that submission because I think it is unlikely that Apotex Australia would have achieved the same level of market penetration in the continuous supply counterfactual that was later achieved by all generic suppliers in the real world. It seems to me that some discount should be applied. I accept that arriving at an appropriate discount involves an element of speculation and guess work.
694 The discount that I would have adopted in this case is 5% on the basis that it represents the midpoint between the parties’ competing positions on this particular issue which, it seems to me, is most likely to approximate what would have occurred in the continuous supply counterfactual. In that regard, I note that while the evidence supports the application of some discount to the figures derived using Mr Hobbs’ backdated market share approach, Sanofi’s reliance on a 10% discount figure finds no direct support in Mr Hobbs’ evidence. The 5% discount would need to be applied to the figures previously quoted in [673] based on Mr Hobbs’ market share estimates to arrive at the final figure.
695 There was a dispute between the parties as to whether or not the Court had any power to order the payment of interest in this case. That dispute raised the question of whether the present proceeding is “… for the recovery of any money … in respect of a cause of action …” within the meaning of those words as used in s 51A of the Federal Court of Australia Act 1976 (Cth). It was acknowledged by Sanofi BMS that at least two judges of this Court have awarded interest under s 51A in a proceeding to recover compensation under an undertaking as to damages: Specsavers Pty Ltd v The Optical Superstore Pty Ltd (No 3) (2012) 290 ALR 263 (Katzmann J), Principal Strategic Options Pty Ltd v Coshott [2003] FCA 736 (Branson J). However, it was submitted by Sanofi BMS, correctly in my view, that in neither of those cases was the question now raised addressed. In particular, neither of those cases considered the implications of the reasoning in Elsinora Global Ltd v Deputy Commissioner of Taxation (2006) 155 FCR 413 for a claim for interest under s 51A in a proceeding of this kind: see the judgment of Young J (with whom Gyles J and Stone J agreed) at 422-423 [44]-[48].
696 The point raised by Sanofi BMS in relation to interest was not articulated until Sanofi BMS delivered their closing submissions. It was not foreshadowed in their written or oral openings or in a statement of issues to which they agreed. In my view, the point is one that Sanofi BMS was required to expressly plead: see r 16.08(b) of the Federal Court Rules 2011 (Cth). I have no doubt that the point took the Commonwealth by surprise which is understandable given that nothing was said about it until closing submissions.
697 The point raised by Sanofi BMS is a pure question of law which is not necessary for me to decide given the other conclusions I have reached. But if I was to have concluded that the Commonwealth was entitled to recover on any one or more of the relevant undertakings, and that s 51A did not permit the Court to award interest, I would have included in the award an additional sum for simple interest calculated from 1 April 2008 on the basis that it was just and equitable to do so: see Love v Thwaites (No 4) [2012] VSC 521 at [91] per Dixon J and, on appeal, Love v Thwaites [2014] VSCA 56 at [51].
698 The Commonwealth’s application for compensation filed on 11 April 2013 will be dismissed. The parties will be given an opportunity to file written submissions on the question of costs.
I certify that the preceding six hundred and ninety-eight (698) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Nicholas. |




