FEDERAL COURT OF AUSTRALIA
Quaker Chemical (Australasia) Pty Ltd v Fuchs Lubricants (Australasia) Pty Ltd (No 2) [2020] FCA 306
ORDERS
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. Within 14 days of the date of these orders the parties file an agreed form of orders, including as to costs. Failing agreement, the parties file and serve competing forms of orders within 21 days of the date of these orders.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ROBERTSON J:
Introduction
1 These proceedings concern two Patents, the Standard Patent, Australian Patent No. 2012304245, entitled Method for Detecting Fluid Injection in a Patient, with a priority date of 2 September 2011, and the Innovation Patent, Australian Patent No. 2013100458 which in part is in similar terms. The Innovation Patent is a divisional of the Standard Patent claiming the same priority date, and was certified on 25 July 2013.
2 The Patents relate generally to a method for detecting high pressure fluid injection (HPFI or HPI) injuries. These injuries can occur where fluid is kept under high pressure in machinery, such as hydraulic machinery used in mines. For example, longwall systems are used to extract coal resources from underground. They utilise many very large hydraulic roof supports which operate under extremely high pressures, often 5000 psi and above, and require very large amounts of hydraulic fluid given their size, length of hosing and pressure requirements.
3 Fluid may escape from the machinery, for example, through a small crack in a tube carrying the fluid. When it escapes, it can emerge under high velocity, as a fine stream of liquid, which is capable of injecting itself into the body of a person. Injuries of that type can be difficult to detect, but can lead to severe health consequences.
4 As explained under the heading “Preferred Embodiment of the Invention”, Figure 1 in each of the Patents shows a view of a fluid injection according to the invention:
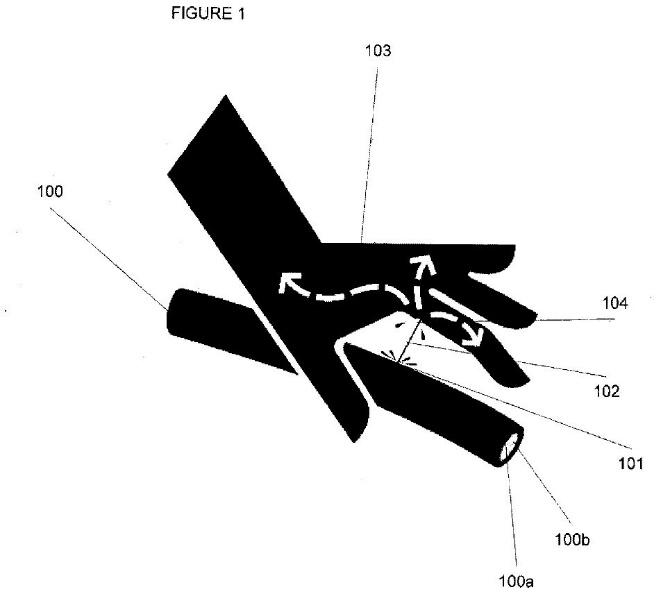
…
Tubing 100 has pressurised fluid such as hydraulic fluid 100a flowing through it. The hydraulic fluid 100a has fluorescence 100b added to it. Tubing 100 includes a leak 101 that has resulted in a high pressure leak 102. In this instance a patient’s hand 103 is in the way of the stream and the hand 103 receives a fluid injection through injection point 104.
5 The Patents are directed to a method for detecting fluid injection in a patient, having certain specified steps. In broad outline, the method involves the inclusion in the machine fluid, such as the hydraulic fluid, of an additive, which is a dye that fluoresces. That dye will fluoresce in the presence of ultraviolet light, and the method involves the use of that property to detect fluid injection in a patient.
6 The parties appeared to be at one in saying that the nature of the invention was easily conveyed in a single sentence. “It is simply a method where fluorescent dye is added to the fluid in a hydraulic system and, if it is suspected that fluorescently-dyed fluid has been accidentally injected into a person, then a UV/blue light is shone on the site of the suspected injection, with fluorescence in or under the skin said to assist in indicating where the injection has occurred.”
7 The inventor recorded in the Patents is Mr Wayne Thompson. The original applicants for the Patents were two companies, Thommo’s Training and Assessment Systems Pty Ltd (TTAAS) and T&T Global Solutions Pty Ltd (T&T), of which Mr Thompson was a director.
8 Quaker Chemical (Australasia) Pty Ltd (Quaker) acquired the Patents from those companies in late 2014.
9 Fuchs Lubricants (Australasia) Pty Ltd (Fuchs), the respondent/cross-claimant, was a supplier of fluid products capable of being used in the claimed method. In particular, Fuchs supplied hydraulic fluid.
10 Two categories of hydraulic fluid supplied by Fuchs were water-based fluids, used in longwall machinery, and mineral oils, used in other hydraulic machinery.
11 Fuchs supplied water-based longwall fluids and concentrates under the “Solcenic” brand and mineral oil products under the “Renolin” brand.
12 TTAAS, and later Quaker, offered two “Oil-Glo” fluorescent dye additive products: Oil-Glo WB (water-based) (also called WD 802 or Oil-Glo 802) for use in longwall fluids, and Oil-Glo OB (oil-based) (also called Oil-Glo 33) in mineral oil systems. These two products were later renamed FluidSafe WB and FluidSafe OB. Quaker had earlier offered a water-based fluorescent dye additive product called “Quinto Glow”.
13 The essence of the case brought by Quaker was that Fuchs had infringed the Patents by supplying, or offering to supply, to certain of its customers, Solcenic and Renolin containing fluorescent dye, in circumstances where the dye or product was not acquired from the patentee. That is, the infringement case was concerned with the supply of Solcenic or Renolin products with a different dye included in particular cases that was not Oil-Glo or FluidSafe. The contention was that Fuchs had infringed the Patents by supplying products in circumstances where the use of those products by the persons to whom they were supplied would infringe the claimed method.
14 Quaker did not complain that the supply by Fuchs of Solcenic or Renolin products which included, or was later mixed by the customer with, Oil-Glo or FluidSafe sourced from the patentee was an infringement. It was accepted that the Oil-Glo or FluidSafe dye was supplied by TTAAS or Quaker on the basis that it could be used for that purpose, and that there was therefore an implied licence, or an authorisation, for that use. As I have said, Quaker contended that subsequently Fuchs sought to, and did, include in its products fluorescent dye additives not sourced from the patentee. It was that conduct that gave rise to the infringement claim. In particular, Quaker claimed, it was the supply by Fuchs to three customers, in specific instances, that involved an infringement by supply. Those customers were: first, from 2016 onwards, BHP Billiton Mitsubishi Alliance (BMA), which was the operator of a mine site at Broadmeadow; second, from 2015 onwards, Glencore, which was the operator of various mine sites, relevantly including Oaky North, Bulga, Ulan No. 3 and Ulan West; and third, from 2015 onwards, Yancoal, the operator of, relevantly, Moolarben and Ashton mine sites.
15 In terms of the legal principles, there were three ways in which the case for Quaker was put. The first relied on s 117 of the Patents Act 1990 (Cth). The second relied on the concept of authorisation under s 13 of the Act: infringement by authorising the act of another. The third relied on the law of joint tortfeasance in a similar way.
16 Fuchs cross-claimed against Quaker, and alleged that both Patents were invalid and liable to be revoked on the grounds of lack of clarity, insufficiency and failure to disclose best method, lack of utility, lack of novelty and secret use. It also alleged that the Standard Patent and Innovation Patent were invalid and liable to be revoked on the ground of lack of fair basis and lack of support, respectively.
17 2 September 2011 was the date of filing of the provisional specification. One significant date, besides the priority date of 2 September 2011, was 2 September 2010, being the date 12 months before the earliest priority date of the claims of the Standard Patent and the Innovation Patent. 2 September 2010 is relevant in considering the publication or use of the invention for the purpose of reasonable trial or experiment.
18 2 February 2012 was the date of filing of the complete specification for the Standard Patent. 2 February 2011 is therefore the date which is 12 months before the filing date of the complete specification for the Standard Patent. That date is relevant because there is an additional grace period provided under the Act and the Patents Regulations 1991 (Cth). In short, information made publicly available by publication or use of the invention by or with the consent of the inventor after that date is to be disregarded.
19 10 April 2013 was the date of filing of the complete specification for the innovation patent.
20 On 21 April 2017, Jagot J ordered that the issue of the quantum of any pecuniary relief be determined separately from, and subsequent to, the determination of all other issues in the proceeding. That leaves for determination the question of liability for additional damages, not the quantification of them, as part of the first hearing.
The witnesses
21 Quaker relied on affidavits of:
(a) Wayne Thompson affirmed on 22 May 2018 and 5 September 2018; Mr Thompson was cross-examined;
(b) Harold Kraus, General Manager and Business Director of Quaker, sworn on 22 May 2018 and 31 August 2018; Mr Kraus was cross-examined;
(c) Peter Gray, consulting engineer, who was called as an independent expert, affirmed on 20 December 2018; Dr Gray produced a joint report with Mr Russell Smith dated 4 February 2019 and gave oral evidence.
22 Fuchs relied on affidavits of:
(a) Craig Roberts, account manager, affirmed on 27 April 2018; Mr Roberts was cross-examined;
(b) Christopher Bate affirmed on 3 May 2018 and 7 March 2019; Mr Bate was cross-examined;
(c) Stuart Knight, Deputy Managing Director of Fuchs, affirmed on 4 May 2018, 17 August 2018 and 2 November 2018; Mr Knight was cross-examined;
(d) Katrina Crooks, solicitor, affirmed on 10 May 2018; Ms Crooks was not required for cross-examination;
(e) Russell Smith, longwall hydraulics consulting engineer, who was called as an independent expert, affirmed on 5 October 2018; as noted above Mr Smith produced a joint report dated 4 February 2019. He gave oral evidence.
The statutory provisions
23 Section 117 stated:
117 Infringement by supply of products
(1) If the use of a product by a person would infringe a patent, the supply of that product by one person to another is an infringement of the patent by the supplier unless the supplier is the patentee or licensee of the patent.
(2) A reference in subsection (1) to the use of a product by a person is a reference to:
(a) …; or
(b) if the product is not a staple commercial product—any use of the product, if the supplier had reason to believe that the person would put it to that use; or
(c) in any case—the use of the product in accordance with any instructions for the use of the product, or any inducement to use the product, given to the person by the supplier or contained in an advertisement published by or with the authority of the supplier.
24 The term “supply” was defined in Sch 1, the Dictionary, as follows:
supply includes:
(a) supply by way of sale, exchange, lease, hire or hire-purchase; and
(b) offer to supply (including supply by way of sale, exchange, lease, hire or hire-purchase).
25 Section 13 provided:
13 Exclusive rights given by patent
(1) Subject to this Act, a patent gives the patentee the exclusive rights, during the term of the patent, to exploit the invention and to authorise another person to exploit the invention.
(2) The exclusive rights are personal property and are capable of assignment and of devolution by law.
(3) A patent has effect throughout the patent area.
26 Section 18 provided:
Patentable inventions for the purposes of a standard patent
(1) Subject to subsection (2), an invention is a patentable invention for the purposes of a standard patent if the invention, so far as claimed in any claim:
(a) is a manner of manufacture within the meaning of section 6 of the Statute of Monopolies; and
(b) when compared with the prior art base as it existed before the priority date of that claim:
(i) is novel; and
(ii) involves an inventive step; and
(c) is useful; and
(d) was not secretly used in the patent area before the priority date of that claim by, or on behalf of, or with the authority of, the patentee or nominated person or the patentee’s or nominated person’s predecessor in title to the invention.
Patentable inventions for the purposes of an innovation patent
(1A) Subject to subsections (2) and (3), an invention is a patentable invention for the purposes of an innovation patent if the invention, so far as claimed in any claim:
(a) is a manner of manufacture within the meaning of section 6 of the Statute of Monopolies; and
(b) when compared with the prior art base as it existed before the priority date of that claim:
(i) is novel; and
(ii) involves an innovative step; and
(c) is useful; and
(d) was not secretly used in the patent area before the priority date of that claim by, or on behalf of, or with the authority of, the patentee or nominated person or the patentee’s or nominated person’s predecessor in title to the invention.
(2) Human beings, and the biological processes for their generation, are not patentable inventions.
Certain inventions not patentable inventions for the purposes of an innovation patent
(3) For the purposes of an innovation patent, plants and animals, and the biological processes for the generation of plants and animals, are not patentable inventions.
(4) Subsection (3) does not apply if the invention is a microbiological process or a product of such a process.
[Note: see also sections 7 and 9.]
27 Section 7 provided:
7 Novelty and inventive step
Novelty
(1) For the purposes of this Act, an invention is to be taken to be novel when compared with the prior art base unless it is not novel in the light of any one of the following kinds of information, each of which must be considered separately:
(a) prior art information (other than that mentioned in paragraph (c)) made publicly available in a single document or through doing a single act;
(b) prior art information (other than that mentioned in paragraph (c)) made publicly available in 2 or more related documents, or through doing 2 or more related acts, if the relationship between the documents or acts is such that a person skilled in the relevant art would treat them as a single source of that information;
(c) prior art information contained in a single specification of the kind mentioned in subparagraph (b)(ii) of the definition of prior art base in Schedule 1.
Inventive step
(2) For the purposes of this Act, an invention is to be taken to involve an inventive step when compared with the prior art base unless the invention would have been obvious to a person skilled in the relevant art in the light of the common general knowledge as it existed in the patent area before the priority date of the relevant claim, whether that knowledge is considered separately or together with the information mentioned in subsection (3).
(3) The information for the purposes of subsection (2) is:
(a) any single piece of prior art information; or
(b) a combination of any 2 or more pieces of prior art information;
being information that the skilled person mentioned in subsection (2) could, before the priority date of the relevant claim, be reasonably expected to have ascertained, understood, regarded as relevant and, in the case of information mentioned in paragraph (b), combined as mentioned in that paragraph.
Innovative step
(4) For the purposes of this Act, an invention is to be taken to involve an innovative step when compared with the prior art base unless the invention would, to a person skilled in the relevant art, in the light of the common general knowledge as it existed in the patent area before the priority date of the relevant claim, only vary from the kinds of information set out in subsection (5) in ways that make no substantial contribution to the working of the invention.
(5) For the purposes of subsection (4), the information is of the following kinds:
(a) prior art information made publicly available in a single document or through doing a single act;
(b) prior art information made publicly available in 2 or more related documents, or through doing 2 or more related acts, if the relationship between the documents or acts is such that a person skilled in the relevant art would treat them as a single source of that information.
(6) For the purposes of subsection (4), each kind of information set out in subsection (5) must be considered separately.
28 Section 9 provided:
For the purposes of this Act, the following acts are not to be taken to be secret use of an invention in the patent area:
(a) any use of the invention by or on behalf of, or with the authority of, the patentee or nominated person, or his or her predecessor in title to the invention, for the purpose of reasonable trial or experiment only;
(b) any use of the invention by or on behalf of, or with the authority of, the patentee or nominated person, or his or her predecessor in title to the invention, being use occurring solely in the course of a confidential disclosure of the invention by or on behalf of, or with the authority of, the patentee, nominated person, or predecessor in title;
(c) any other use of the invention by or on behalf of, or with the authority of, the patentee or nominated person, or his or her predecessor in title to the invention, for any purpose other than the purpose of trade or commerce;
(d) any use of the invention by or on behalf of the Commonwealth, a State, or a Territory where the patentee or nominated person, or his or her predecessor in title to the invention, has disclosed the invention, so far as claimed, to the Commonwealth, State or Territory.
29 The operation of ss 7 and 9 is affected by s 24, extracted at [37] below.
30 The new s 7A provided:
(1) For the purposes of this Act, an invention is taken not to be useful unless a specific, substantial and credible use for the invention (so far as claimed) is disclosed in the complete specification.
(2) The disclosure in the complete specification must be sufficient for that specific, substantial and credible use to be appreciated by a person skilled in the relevant art.
(3) Subsection (1) does not otherwise affect the meaning of the word useful in this Act.
31 The parties agreed that certain parts of the post-Raising the Bar version of the Act applied to the Innovation Patent, including the requirements of the new s 7A, in addition to s 18(1A)(c) of the Act.
32 The previous s 40 provided:
(1) A provisional specification must describe the invention.
(2) A complete specification must:
(a) describe the invention fully, including the best method known to the applicant of performing the invention; and
(b) where it relates to an application for a standard patent—end with a claim or claims defining the invention; and
(c) where it relates to an application for an innovation patent—end with at least one and no more than 5 claims defining the invention.
(3) The claim or claims must be clear and succinct and fairly based on the matter described in the specification.
(4) The claim or claims must relate to one invention only.
33 The new s 40 provided:
Requirements relating to provisional specifications
(1) A provisional specification must disclose the invention in a manner which is clear enough and complete enough for the invention to be performed by a person skilled in the relevant art.
Requirements relating to complete specifications
(2) A complete specification must:
(a) disclose the invention in a manner which is clear enough and complete enough for the invention to be performed by a person skilled in the relevant art; and
(aa) disclose the best method known to the applicant of performing the invention; and
(b) where it relates to an application for a standard patent—end with a claim or claims defining the invention; and
(c) where it relates to an application for an innovation patent—end with at least one and no more than 5 claims defining the invention.
(3) The claim or claims must be clear and succinct and supported by matter disclosed in the specification.
(3A) The claim or claims must not rely on references to descriptions or drawings unless absolutely necessary to define the invention.
(4) The claim or claims must relate to one invention only.
34 The parties agreed that the post-Raising the Bar versions of ss 40(2)(a) and (3) applied to the Innovation Patent.
35 Section 138 provided:
138 Revocation of patents in other circumstances
(1) Subject to subsection (1A), the Minister or any other person may apply to a prescribed court for an order revoking a patent.
(1A) A person cannot apply for an order in respect of an innovation patent unless the patent has been certified.
(2) At the hearing of the application, the respondent is entitled to begin and give evidence in support of the patent and, if the applicant gives evidence disputing the validity of the patent, the respondent is entitled to reply.
(3) After hearing the application, the court may, by order, revoke the patent, either wholly or so far as it relates to a claim, on one or more of the following grounds, but on no other ground:
(a) that the patentee is not entitled to the patent;
(b) that the invention is not a patentable invention;
(d) that the patent was obtained by fraud, false suggestion or misrepresentation;
(e) that an amendment of the patent request or the complete specification was made or obtained by fraud, false suggestion or misrepresentation;
(f) that the specification does not comply with subsection 40(2) or (3).
36 The pre-Raising the Bar versions of the Act and Patents Regulations apply to the disclosures relied upon, which occurred before 15 April 2013: See Intellectual Property Laws Amendment (Raising the Bar) Act 2012 (Cth) s 3 and Sch 6 items 32, 33 and 133(4) (concerning the application of amendments to s 24) and the Intellectual Property Legislation Amendment (Raising the Bar) Regulation 2013 (No 1) (Cth) reg 4 and Sch 6 item 7 (substituting new regs 2.2 and 2.3), and reg 23.36(4) item 3 of the Patents Regulations (concerning the application of those amendments).
37 Section 24 provided:
24 Validity not affected by certain publication or use
(1) For the purpose of deciding whether an invention is novel or involves an inventive step or an innovative step, the person making the decision must disregard:
(a) any information made publicly available, through any publication or use of the invention in the prescribed circumstances, by or with the consent of the nominated person or patentee, or the predecessor in title of the nominated person or patentee; and
(b) any information made publicly available without the consent of the nominated person or patentee, through any publication or use of the invention by another person who derived the information from the nominated person or patentee or from the predecessor in title of the nominated person or patentee;
but only if a patent application for the invention is made within the prescribed period.
(2) …
38 Turning to the Patents Regulations, regs 2.2 and 2.3 relevantly provided:
2.2 Publication or use: prescribed circumstances
(1) …
(1A) For paragraph 24 (1) (a) of the Act, the circumstance that there was a publication or use of the invention within 12 months before the filing date of the complete application, is a prescribed circumstance.
(2) For paragraph 24 (1) (a) of the Act the following are also prescribed circumstances:
(a) …;
(b) …;
(c) … ; or
(d) the working in public of the invention within the period of 12 months before the priority date of a claim for the invention:
(i) for the purposes of reasonable trial; and
(ii) if, because of the nature of the invention, it is reasonably necessary for the working to be in public.
(3) …
(4) …
2.3 Publication or use: prescribed periods
(1A) For information of the kind referred to in paragraph 24 (1) (a) of the Act, if the applicant relies on the circumstance in subregulation 2.2 (1A), the prescribed period is the period of 12 months after the information was first made publicly available.
(1) For information of the kind referred to in paragraph 24 (1) (a) of the Act, if the applicant relies on a circumstance in subregulation 2.2 (2), the prescribed period is:
(a) in the case of a circumstance mentioned in paragraph 2.2 (2) (a) or (b):
(i) if the application claims priority from a basic application made within 6 months of the date of the first showing or use of the invention at a recognised exhibition — 12 months from the making of the basic application; and
(ii) in any other case — 6 months after the first showing or use of the invention at the exhibition; and
(b) in the case of the circumstance mentioned in paragraph 2.2 (2) (c):
(i) if the application claims priority from a basic application made within 6 months of the date of the first reading or publication referred to in that paragraph — 12 months from the making of the basic application; and
(ii) in any other case — 6 months after the first reading or publication; and
(c) in the case of the circumstance mentioned in paragraph 2.2 (2) (d) — 12 months from the start of the first public working of the invention referred to in that paragraph.
(2) For the purposes of subsection 24 (1) of the Act, in the case of information of the kind referred to in paragraph 24 (1) (b) of the Act, the prescribed period is 12 months from the day when the information referred to in that paragraph became publicly available.
(3) Subregulation (4) applies:
(a) if an application for a patent is a divisional application:
(i) under section 79B of the Act for an invention disclosed in the specification filed with a previous application for a standard patent (the original application); or
(ii) under section 79C of the Act for an invention disclosed in the specification filed in respect of an application for an innovation patent (the original application); and
(b) only to information disclosed in the divisional application that was disclosed in the original application.
(4) For determining the prescribed period for subsection 24 (1) of the Act, the filing date of the divisional application is taken to be the filing date of the original application.
39 The following grace periods were said by Quaker to be relevant:
(a) Pursuant to s 24(1)(a) of the Act and regs 2.2(1A) and 2.3(1A), any publication or use of the invention after 2 February 2011 by or with the consent of the patentee cannot be taken into account for the purposes of novelty (General Grace Period).
(b) Pursuant to s 24(1)(a) of the Act and regs 2.2(2)(d) and 2.3(c), any publication or use of the invention after 2 September 2010 by or with the consent of the patentee in the context of the working in public of the invention for the purposes of reasonable trial, where, having regard to the nature of the invention it was reasonably necessary for the working to be in public, cannot be taken into account for the purposes of novelty (Reasonable Trials Grace Period).
(c) Pursuant to s 24(1)(b) of the Act and reg 2.3(2), any publication or use of the invention after 2 September 2010 without the consent of the patentee by a person who derived the information from the patentee, cannot be taken into account for the purposes of novelty (Unauthorised Disclosures Grace Period).
The names of the products
40 The names and variations of the water-based longwall hydraulic fluid products were:
Fuchs Solcenic 3002 (concentrate, undyed)
Fuchs Solcenic 3002 (concentrate, dyed)
Fuchs Solcenic 2020 (concentrate, undyed)
Fuchs Solcenic 2020 D (concentrate, dyed)
Fuchs Solcenic 2020 4% Emulsion (pre-mixed emulsion, 96% water, undyed)
Fuchs Solcenic 2020 D 4% Emulsion (pre-mixed emulsion, 96% water, dyed)
Fuchs Solcenic GM20 (concentrate, undyed)
Fuchs Solcenic GM20 D (concentrate, dyed)
Fuchs Solcenic GM20 LD (concentrate, dyed)
Fuchs Solcenic GM20 3% (pre-mixed emulsion, 97% water, undyed)
Fuchs Solcenic GM20 D 3% Premix Emulsion (pre-mixed emulsion, 97% water, dyed)
Fuchs Solcenic GM20 D Plus 3% Premix Emulsion (pre-mixed emulsion, 97% water, dyed)
Fuchs Solcenic GM20 D Plus 3% Premix Emulsion (pre-mixed emulsion, 96% water, dyed)
Fuchs Solcenic 2B (concentrate, dyed)
Fuchs Solcenic 7 (concentrate, dyed)
Fuchs Solcenic 3C (concentrate, dyed)
Quaker Quintolubric 814
Quaker Quintolubric 818-02
41 The names of the mineral oil products were:
Fuchs Renolin B 68 [Renolin B 68 Plus when different base oil used] (undyed)
Fuchs Renolin B 68 [Renolin B 68 Plus when different base oil used] (MET) (dyed)
Fuchs Renolin B 68 [Renolin B 68 Plus when different base oil used] D (NG) (dyed)
Fuchs Renolin B 68 [Renolin B 68 Plus when different base oil used] D (dyed)
Fuchs Renolin B PLUS 68 LD (dyed)
42 The names of the fluorescent dye products were:
Quaker [previously TTAAS] Oil-Glo 33, also known as Oil-Glo OB, rebranded FluidSafe OB in early 2012 (oil-based fluorescent dye additive)
Quaker [previously TTAAS] WD 802, also known as Oil-Glo WB or Oil-Glo 802, rebranded FluidSafe WB in early 2012 (water-based fluorescent dye additive)
Quaker Quinto Glow (water-based fluorescent dye additive)
Fuchs [sourced from third party] Fluoresceine LT (water-based fluorescent dye additive)
Fuchs [sourced from third party] Uniqua W400X (water-based fluorescent dye additive)
Fuchs [sourced from third party] Uniglow F-4 HF (oil-based fluorescent dye additive)
The Patents
43 Under the heading “Field of the Invention” in the Standard Patent, the description states:
The present invention relates to the detection of fluid in the human body and in particular to the detection of hydraulic and fuel fluid within the human body. The invention has been developed primarily for use in detecting the presence of hydraulic and diesel fuel in the human body as a result (sic) accidental fluid injection and will be described hereinafter with reference to this application. However, it will be appreciated that the invention is not limited to this particular field of use.
44 Under the heading “Background of the Invention” is stated:
…
Hydraulic and diesel fuel systems machinery such as those in mining and other industrial areas, operate at very high pressures, often 207 bar (3000 psi) and above. Loose connections or defective hoses can cause a fine, high velocity stream of fluid. Testing has shown that even in systems pressurised to as little as 7 bar (100 psi), this fluid stream can penetrate human skin.
The injury sustained in a high pressure injection incident is usually worse than it will first appear. The injury is relatively rare and it may be that some medical practitioners or hospital services will not be alert to the severity of an injury of this type.
An accidental fluid injection beneath the skin may initially only produce a slight stinging sensation. The danger is that a victim will tend to ignore this, thinking that it will get better with time. This is not often the case and the fluid can cause serious tissue damage. Fluid injected directly into a blood vessel can spread rapidly through a victim’s circulatory system leaving the human body with little ability to purge these types of fluid.
A fluid injection injury can become very serious or even fatal if not dealt with promptly and properly. Typically the only treatment available is to surgically remove the fluid within a few hours of injection. The longer the delay in getting professional medical aid, the further the tissue damage can spread. If left untreated, the injury could result in disfigurement, amputation of the affected part or death of the victim.
Accidental fluid injections can be difficult to confirm. That is, victims may be covered by fluid externally however there may be uncertainty on whether any fluid has penetrated the victim’s skin. As discussed above any delay in treating a victim can cause sever (sic) harm and as such it would be advantageous if confirmation of fluid injection can be confirmed.
45 Under the heading “Summary of the Invention” it is said as to the first aspect:
It is an object of the present invention to overcome or ameliorate at least one of the disadvantages of the prior art, or to provide a useful alternative.
According to a first aspect of the invention there is provided a method for detecting fluid injection in a patient, the method including the steps of:
providing a fluid storage tank;
providing fluid for use in machinery and adding said fluid to the fluid storage tank; and
providing a fluorescent dye and adding the fluorescent dye to the fluid such that the fluid fluoresces in the presence of ultraviolet light.
Preferably the method includes the step of providing a fluorescent light to detect the presence of the fluid.
Preferably the light is a high intensity blue light.
Preferably the method includes the step of a possible fluid injection occurring in a patient.
Preferably the method includes the step of providing a dark room and examining the patient with the blue light in the dark room to determine whether possible fluid injection has occurred.
Preferably the method includes the step of washing the patient.
Preferably the method includes the step of washing the point of possible fluid injection on the patient.
Preferably the method includes the step of examining the patient with the blue light in the dark room to determine whether possible fluid injection has occurred after the step of washing the patient.
Preferably the method includes the step of determining whether or not fluid injection has occurred.
…
46 Under the heading “Preferred Embodiment of the Invention” it is said:
The preferred embodiment of the invention provides a method for detecting fluid injection in a patient following a possible fluid injection. The method including (sic) the step of providing a fluid storage tank in the form of equipment having a diesel or hydraulic tank. The appropriate fluid such as diesel or hydraulic fluid is added to the fluid storage tank. A fluorescent dye is then provided and adding (sic) to the fluid such that the fluid fluoresces in the presence of ultraviolet light. It would be understood that the fluorescent dye can be added to any suitable fluid and the scope of the invention is not limited to diesel and hydraulic fluid.
The preferred embodiment of the invention also provides a fluid reservoir, the fluid reservoir adapted to store hydraulic fluid and fluorescent dye such that the hydraulic fluid and the fluorescent dye mix in the fluid reservoir. The fluid reservoir is connected to and is in fluid communication with at least one hydraulic actuator and/or motor such that the flow of hydraulic fluid and the fluorescent dye such (sic) drives the actuator and/ore (sic) motor. As would be understood, the hydraulic system can be used to drive any number of motors, pumps and the like and many different types of machinery used in mining an (sic) in other applications. The fluid reservoir is connected to the (sic) at least one hydraulic actuator and/or motor by at least one of the following: a hydraulic tube; a hydraulic pipe; a hydraulic hose or the like such that a leak in the hydraulic tube, hydraulic pipe or hydraulic hose results in both hydraulic fluid and fluorescent dye leaking therefrom.
As would be understood, a broad spectrum of light could be used in embodiments of the invention and the invention is not limited to light of any particular wavelength or colour.
In the preferred embodiment the fluorescent dye used is sold under the brand name OILOil Glo (sic) and is manufactured by T&T Global Solutions Pty Ltd. As would be understood any other suitable fluorescent dye can be used and chosen according to the particular application and specifications required.
Following a suspected fluid injection, the method includes the step of providing a fluorescent light to detect the presence of the fluid on a patient. In the preferred embodiment the light is a high intensity blue light although it would be understood that any suitable ultra violet light could be used. That is, following a suspected fluid injection of a person as a result of a high pressure stream of fluid from hydraulic machinery, if the person has been injected, hydraulic fluid mixed with the fluorescent dye would be injected into the patient. As a result of this injection with both hydraulic fluid and the dye, the injection point along with the injected tissue can be found using the ultra violet light which causes the dye to fluoresce.
Once a suspected fluid injection occurs, the method includes the step of providing a dark room and examining the patient with the blue light in the dark room to determine whether possible fluid injection has occurred. After the initial examination, the method includes the step of washing the patient and particularly the point of possible fluid injection preferably with water and detergent.
After the step of washing the patient and the point of possible fluid injection, the method includes the step of re-examining the patient with the blue light in the dark room to determine whether possible fluid injection has occurred.
By following the steps of the preferred embodiment, it is possible to determine whether or not fluid injection has occurred in the patient.
…
47 The claims in the Standard Patent, claims 1 to 22, were as follows, claims 1 and 9 being the independent claims:
THE CLAIMS DEFINING THE INVENTION ARE AS FOLLOWS:
1. A method for detecting fluid injection in a patient, the method including the steps of:
providing a fluid storage tank;
providing fluid for use in machinery and adding said fluid to the fluid storage tank;
providing a fluorescent dye and adding the fluorescent dye to the fluid such that the fluid fluoresces in the presence of ultraviolet light; and
a possible fluid injection occurring in a patient.
2. A method according to claim 1 including the step of providing an ultraviolet light to detect the presence of the fluid.
3. A method according to claim 2 including the step of providing a dark room and examining the patient with the ultraviolet light in the dark room to determine whether possible fluid injection has occurred.
4. A method according to claim 3 including the step of washing the patient.
5. A method according to claim 4 including the step of washing the point of possible fluid injection on the patient.
6. A method according to claim 5 including the step of examining the patient with the ultraviolet light in the dark room to determine whether possible fluid injection has occurred after the step of washing the patient.
7. A method according to claim 6 including the step of determining whether or not fluid injection has occurred.
8. A method substantially as herein described with reference to the accompanying drawings.
9. A method of detecting an accidental hydraulic or fuel fluid injection in a patient having a possible fluid injection, the fluid having been added to a storage tank provided for use in machinery and a fluorescent dye having been added to the fluid such that the fluid fluoresces in the presence of UV light, the method including the steps of:
examining the patient by exposing the patient to UV light; and
determining the presence of fluorescence,
wherein fluorescence indicates a hydraulic fluid injection occurring in the patient.
10. A method according to claim 9, wherein the hydraulic fluid is water or oil or a combination of both water and oil.
11. A method according to claim 10, wherein the hydraulic fluid is a combination of water and oil.
12. A method according to claim 11, wherein the fuel is diesel or petrol.
13. A method according to any one of claims 9 to 12, wherein the step of examining the subject by exposing the patient to UV light occurs in a dark room.
14. A method according to any one of claims 9 to 13, wherein the method includes the step of washing the patient.
15. A method according to claim 14, wherein the step of washing the patient is at a site of possible fluid injection.
16. A method according to claim 15, wherein after washing the site of possible fluid injection, the method includes re-examining the subject by exposure to the UV light.
17. A method according to any one of claims 9 to 16, wherein the hydraulic fluid is used in machinery at a pressure of about 100psi to about 3000psi.
18. A method according to any one of claims 9 to 16, wherein the hydraulic fluid is used in machinery at a pressure of about 150psi to about 2000psi.
19. A method according to any one of claims 9 to 16, wherein the hydraulic fluid is used in machinery at a pressure of about 500psi to about 1500psi.
20. A method according to any one of claims 9 to 19, wherein the fluorescent dye is present in the fluid in at least about 0.0 1% (v/v).
21. A method according to any one of claims 9 to 20, wherein the fluorescent dye is present in the fluid in at least about 0.2% (v/v).
22. A method according to any one of claims 9 to 21, wherein a colouring dye is also added to the hydraulic fluid such that the hydraulic fluid changes colour.
48 By its cross-claim, as amended, Fuchs sought an order revoking claims 1 to 22 of the Standard Patent pursuant to s 138.
49 The claims in the Innovation Patent, claims 1 to 3, were:
THE CLAIMS DEFINING THE INVENTION ARE AS FOLLOWS:
1. A method for detecting fluid injection in a patient, the method including the steps of:
providing a fluid storage tank;
providing fluid for use in machinery and adding said fluid to the fluid storage tank;
providing a fluorescent dye and adding the fluorescent dye to the fluid such that the fluid fluoresces in the presence of ultraviolet light;
a possible fluid injection occurring in the patient; and
providing a high intensity blue ultraviolet light to detect the presence of the fluid.
2. A method according to claim 1 wherein the fluorescent dye has colour characteristics such that when the dye is mixed with an industrial fluid the fluid takes on the colour characteristics of the dye.
3. A method according to claim 2 wherein the industrial fluid is one or more of the following: hydraulic fluid; petrol; or diesel.
50 By its cross-claim, as amended, Fuchs sought pursuant to s 138 an order revoking claims 1 to 3 of the Innovation Patent.
Chronology of events
51 In mid-2009, Mr Thompson/TTAAS was contracted by Peabody Energy Australia to develop mechanical and hydraulic-based training for the mechanical trade staff at Peabody’s Metropolitan mine (Mechanical Training). Mr Thompson designed and manufactured for TTAAS a fluid injection simulator as part of the practical component of his Mechanical Training. This Mechanical Training was provided from around the end of 2009 or beginning of 2010 until around late 2010.
52 On 6 May 2010, a fitter working on a longwall was struck in the hand by high pressure fluid at Metropolitan.
53 In mid-2010, an HPFI task force was established by Peabody. Between 6 May 2010 and 30 July 2010 the taskforce visited Metropolitan.
54 Also in 2010, Mr Thompson and TTAAS were engaged to prepare and provide fluid injection awareness training at Metropolitan (HPFI Training).
55 On 3 September 2010, an electrician at Metropolitan was struck on the arm by pressurised fluid.
56 In mid to late September 2010 or in October 2010 Mr Thompson conceived of the claimed method.
57 In October or November 2010, Mr Thompson disclosed the use of Oil-Glo fluorescent dye in hydraulic fluids to facilitate detection of HPFI using a UV/blue light to Mr Andy Withers of Peabody and demonstrated detecting fluid injection with fluorescent dye using a simulator in the car park at Metropolitan. In November 2010, Mr Thompson conducted tests using pigskin and the simulator and took photographs and videos of these tests.
58 During November 2010, there was disclosure, via in person conversation, telephone and/or emails, by Mr Ken Risk of Peabody to Mr Craig Roberts of Fuchs, of the method of adding a chemical dye to the longwall fluid at Metropolitan so as to enable detection of fluid under a person’s skin in the case of a suspected HPFI.
59 In early December 2010, Mr Thompson conducted compatibility tests of Oil-Glo 33 on a Bobcat machine. On 16 December 2010, TTAAS submitted a risk assessment report for Oil-Glo 33 to be tested in Metropolitan’s machinery.
60 On 21 March 2011, there was a suspected fluid injection recurrence at Metropolitan. The incident report stated, under recommendations: “Implement the oil glow to high potential equipment when trial complete. (Trial currently on 6 shooter, Eimco and Shuttle Car)”.
61 On 23 March 2011, Metropolitan completed its risk assessment for, the introduction of “Oil Glo 802” into its longwall emulsion mix. Metropolitan completed the risk assessment tasks for Oil-Glo and responsibilities around protocols and training in April-May 2011.
62 On 31 March 2011, TTAAS provided to Metropolitan a tax invoice for the supply of “six 20 litre drums of solcenic – Premix each drum with 6.125 litres of Oil Glo 802”.
63 On 16 April 2011, Dr Sean Nicklin from Sydney Hospital emailed Mr Thompson suggesting that “some product testing of the fluid in deeper tissues” could be done using pigs trotters.
64 Early in May 2011, TTAAS provided HPFI training at Peabody’s North Goonyella mine.
65 Also in May 2011, Oil-Glo (WD802 or Oil-Glo 802) was added to Metropolitan longwall fluid. On 26 May 2011, a sample of Solcenic fluid containing Oil-Glo was taken by Fuchs from the longwall at Metropolitan for testing.
66 Between May and June 2011 various supplies were made by TTAAS of Oil-Glo and related products to North Goonyella and Metropolitan.
67 In July 2011, Oil-Glo 802 (WD 802) was added to North Goonyella longwall fluid.
68 Between 24 and 27 July 2011, the NSW Minerals Council Occupational Health & Safety Conference was attended by Mr Bate. Mr Peter Doyle of Peabody presented, referring to a strategy for detection and disclosing HPFI detection using fluorescent dye in hydraulic fluid and UV/blue light.
69 On 26 July 2011, Felix Radu of Quaker emailed Mr Thompson, referring to a discussion on the same date and requesting Mr Thompson’s availability “to discuss Oil Glow supply for Quaker longwall fluids”.
70 On 18 August 2011, Mr Thompson met with Dr Sean Nicklin.
71 On 26 October 2011, there was a suspected HPFI incident at North Goonyella. The first aid report recorded under “Initial Treatment” that the patient was “Examined with Blue light for fluid presence and or penetration” and “nil evidence found”, and under “Signs and Symptoms” there was “evidence of ‘Oil Glo’ on initial or subsequent examination with ‘Blue light’”.
72 On 23 December 2011, Mr Thompson was informed that an HPFI injury had occurred at North Goonyella with fluid that contained the fluorescent additive.
73 On 22 February 2012, an email from Mr Wayne Pearce of Fuchs recorded that Mr Thompson “would like to meet with Fuchs as site dosing is not his preferred option”, and had informed Mr Pearce that “Fuchs is the only oil company he has provided samples to and although other companies have requested samples at this stage he would prefer to work with us”.
74 On 13 March 2012, Mr Pearce emailed Fuchs global personnel outlining some “major concerns” relating to Fuchs’ business arising from the “Oil-Glo situation”.
75 On 26 April 2012, Mr Michaelson of Fuchs emailed Mr John Snape and Mr Peter Crossland of Xstrata (now Glencore) to enquire about Xstrata’s position with regard to the use of Oil-Glo for HPI mitigation.
76 Fuchs admitted that since 2012, it had supplied Solcenic longwall fluid products and Renolin hydraulic mineral oil products in Australia which contained fluorescent fluid additives. It contended that since August 2012 it had on occasion supplied hydraulic fluids mixed with FluidSafe dyes purchased from the patentee (TTAAS until the end of 2014 and Quaker thereafter) to customers in Australia in response to requests from customers for HPFI detection.
77 On 6 September 2012, Tahmoor Colliery (Xstrata) informed Fuchs that FluidSafe would be added to its hydraulic oil and requested information regarding estimated time and costs of the supply.
78 On 25 March 2013, Mr Knight sent an internal email to Fuchs stating that “We have been instructed by North Goonyella, Metropolitan and Wambo Mines (Peabody) to introduce a product called Fluidsafe into their Solcenic and Hydraulic oils” and that “[w]e recently had a request from Yancoal to put the oil based product in their hydraulic oil.”
79 On 28 March 2013, Mr Michaelson emailed Mr Knight and Wayne Hoiles of Fuchs in relation to requests received from Peabody, Donaldson, Tahmoor (Xstrata) and Ravensworth (Xstrata) for FluidSafe. Mr Michaelson expressed concern about liability if the dye did not work in its intended role in identifying the HPI.
80 On 6 August 2013, NRE No 1 (Gujarat NRE) requested that Fuchs work with Mr Thompson to “ensure that GM20 concentrate is provided with a suitable level of fluid dye to ensure that our emulsion always has the correct amount of dye”.
81 On 23 August 2013, Mr Michaelson noted an inquiry from Glencore concerning the potential use of dye added to its longwall fluids for leak detection and instructed Mr Pearce to contact Paul Littley in the UK to jointly provide information on a suitable dye and “the method of enhanced detection” and noting “I do not know if we can purchase the dye from Spectroline as Wayne Thompson may be the Australian agent but there must be products available elsewhere”. Mr Pearce emailed Mr Littley stating: “Glencore (Xstrata) have requested a Fluorescein dye in the Solcenic we provide to assist in leak detection. As it is only for leak detection (not HP detection) we can use any fluorescein dye, they do not want to use the FluidSafe product as it is too expensive. We worked out the concentration of dye in the Quaker product at the recent Chemist meeting so I am thinking we go with the same dose rate for this.”
82 On 28 August 2013, Mr Pearce emailed Mr Esdaile of Glencore regarding “[l]eak detection in longwall fluid”, stating “I phoned you yesterday and left a message regarding the plan to add a leak detection dye to our Solcenic products supplied to Xstrata. I would like to discuss this matter further with you either over the phone or if preferred I could come and meet you in person.”
83 On 30 August 2013, Mr Pearce emailed Mr Esdaile of Glencore regarding “Leak Detection – Longwall Fluids” stating, in part, “To summarise our conversation earlier this week”:
- Fuchs is able to supply longwall Fluids to Glencore sites with a fluorescent dye in them for the use of leak detection only (not HP injection detection).
Mr Esdaile responded stating “Thanks Wayne. Appreciate the follow up. I will be in touch.”
84 On 6 September 2013, Mr Graham Mackenzie of Oaky No 1 (Glencore) requested information from Fuchs in relation to dye additives for both HPFI injection detection and leak detection.
85 Mr Pearce responded:
There is a lot of information on this topic so I will attempt to provide you with the nuts and bolts.
HPI Detection: The dye currently being marketed for use in cases of high pressure injection (HPI) is branded as Fluidsafe WB, this product is supplied by a company called n AAS which is owned and managed by Wayne Thompson. Wayne has recently been granted a patent for the use of his product for HPI detection and it has been used for several years in coal mines in QLD and NSW with some success. The required concentration is quite high for a dye of this type and the product is rather expensive thus implementation of the dye has a significant financial cost.
Fuchs has tested it’s (sic) range of Solcenic products with the Fluidsafe WB dye at the recommended treat rate and have deemed that the dye has no impact on the key performance characteristics of the longwall fluid (seal compatibility, biostability, corrosion protection etc.). I have attached some information from TTAAS on the dye in question.
Leak detection: There are many dyes on the market that can be added to oil based and water based products to assist in leak detection using a blue light. Dyes used for leak detection are significantly lower in cost and require a much lower treat rate than the dye discussed above for HPI detection. Fuchs is able to add dyes to its range of products with full confidence that the performance of its product will not be affected.
It must be understood however that dyes added to products for leak detection shall not be used for HPI detection.
As mentioned on the phone I have had a similar request for information from Andrew Esdaile for XCN and provided him with information as requested.
86 On 24 October 2013, Mr Roberts sent an email to Mr Michaelson:
Justin Ryan indicated that he would like Fuchs to introduce a dye into Solcenic 2020 for their next longwall block in 2014. He mentioned we have had this discussion with Andrew (I’m assuming Andrew Esdale – Group Engineer) on this matter and raised concerns with the FluidSafe patent.
Fluidsafe is only used at a low concentration for leak detection and NOT for identifying fluid injection.
87 On the same date, Mr Pearce sent an email to Mr Roberts:
Please confirm whether they want dye for leak detection only or injection detection?
I have informed Andrew that we can add a dye for leak detection at no extra cost to the customer. The dye used in this scenario is not Fluidsafe.
If they want injection detection we will need to look at extra costs associated with this.
88 On 25 October 2013, Mr Thompson provided a copy of the certified Innovation Patent to Fuchs and stated “my legal advice with respect to leak detection and prior knowledge is captured in the certification.”
89 Also on 25 October 2013, Broadmeadow (BMA) asked Fuchs to provide a dye equivalent to Quinto Glow for HPFI detection. Mr Michaelson contacted Mr Thompson for him to send details of FluidSafe to Broadmeadow.
90 On 26 October 2013, Mr Michaelson responded to Mr Roberts’ email of 24 October 2013 stating “Gents we may have a legal problem with Wayne Thompson on this leak detection as he says his patent covers leak detection. So let’s take a breather and discuss before moving on Ulan.”
91 On 29 October 2013, Mr Michaelson stated in an email that “Presently there is a patent covering the use of dyes for leak detection but particularly HPI injuries.”
92 On 21 November 2013, Mr Jeff Daveson of Oaky No 1 (Glencore) emailed Mr Garry Cooper of Fuchs asking whether he had “heard of or have info/feedback/benefits on any mines using phosphorescent dye in LW emulsion”.
93 On 22 November 2013, representatives of Quaker Chemical Corporation (Quaker US) filed a Notice of Opposition to the grant of the Standard Patent.
94 Also on 22 November 2013, Mr Cooper replied to Mr Daveson in substantially the same terms as Mr Pearce’s email of 6 September 2013 to Mr Mackenzie of Oaky No 1.
95 On 20 January 2014, Mr Michaelson sent an email to Mr Knight stating that he had been informed by Mr Thompson that “he has been approached by Quaker Australia and US to discuss the purchase of his Patent and rights to the use of Fluid Safe for use in Longwall fluid in both regions.” Mr Michaelson further stated in his email:
The following mines use Fluid Safe WB in Australia Russel Vale NRE North Goonyella Metropolitan Austar Coal Mine Wambo Coal Ulan #3
Broadmeadow mine is pursing (sic) the Fluid Safe approach although this has waned in the recent past.
Xstrata at a high level has stopped Tahmoor adopting Fluid Safe but it has been introduced to Ulan #3 on the basis of leak detection
96 On 21 January 2014, Mr Cooper sent a response to Mr Daveson’s email of 21 November 2013 to Mr Kimber of Glencore in substantially the same terms as Mr Pearce’s email of 6 September 2013 to Mr Mackenzie of Oaky No 1.
97 On 23 January 2014, Mr Elliot of Fuchs sent an internal email to Mr Lingg and others stating:
We have used standard green fluorescein dye for a number of years in association with longwall fluid leak detection. In the USA, we received MSHA approve to add this dye to our Solcenic products in 1991.
Fluidsafe is an Australian company that sells a similar green florescent dye for detection of high pressure injection incidents with people.
Fluidsafe provides detection equipment (black light technology) and has conducted medical research that shows their dye to be detectable after injection to the body. Fluidsafe has applied for a patient (sic) to protect this technology, and they appear to be protected in the Australian market. Because this process is considered medical, it is not patentable in the UK and possibly other European markets.
Peabody Coal has driven the use of Fuildsafe (sic) dye in the emulsions being used in all of their longwall mines.
Fuchs Australia has used the Fluidsafe dye in all Solcenic sales to Peabody over the last couple of years.
In the USA, we are supplying Peabody with a Spectroline Blue UV dye for use with our Solcenic GM-20.
In their recent tender, Peabody requested this dye be used in their Twentymile mine (USA).
We have begun an emulsion high pressure injection study with Dr. Pittermann (Germany).
This testing so far has involved two different dyes; standard green florescent dye and the blue UV dye being used at Peabody’s Twentymile mine.
This test involves the injection of dyed emulsion into a live cow udder.
The preliminary results indicate that the blue dye is more difficult to detect due to fatty tissue also showing up as bluish.
The standard green is more easily detectable.
Our position on dye, is to find a comparable product to Fluidsafe and do the research in order to show similar performance.
It would appear to us that the market will lean towards the use of dyed longwall fluids if they can be marketed as a safety advantage.
The level of dye required to treat fluids for pressure injection is considered higher that (sic) what would be necessary for leak detection, on the order of six times as much. This amount of green dye makes the fluids look very intense in the working fluid. There have been incidents where the green dyed emulsion has been pumped to an outside holding pond which has created problems for some customers. Quaker had to remove their dye from some mines due to environmental issues being raised.
98 On 24 January 2014, Mr Knight emailed senior global Fuchs personnel in relation to Quaker’s proposed purchase of the FluidSafe business, stating:
The relationship between Quaker and Wayne Thompson has not been good as Quaker produced their own product ‘Quintoglo’ which Wayne believed breached patent.
Fuchs Australia have used Fluidsafe in Solcenic and hydraulic oil at Peabody sites. The Fluidsafe was purchased by Peabody and delivered to Fuchs where we blended it into Peabody product. This business was lost in a tender situation to Quaker and BP Castrol in September 2013.
Further to Georg’s comments
*This is a threat to our business however uptake has been slow with some organisations reticent to move to the use of dye in longwall fluid. There is pressure from Unions and the Mines Inspectorate to move in this direction given the product has been proven to be successful in identifying High Pressure Injection Injuries (HPI) on several occasions and also identifying where a HPI has not taken place
…
If a customer wants a dyed product for HPI detection we will play with a straight bat and inform them that the product is patented in Australia and we must buy the patented product. We will be open book on the dye.
99 On 2 April 2014, a Ulan No. 3 (Glencore) document titled “Toolbox Talk” stated:
To assist to reducing cost to the operation Fuch (sic) our oil supplier are introducing Fluorescenine LT dye into the hydraulic oil and solcenic fluid. This dye will assist in locating leaks.
…
• Similar to the dye used previously on the longwall (lower concentration), this dye CAN NOT be used for detecting fluid injections.
…
• There will be a change in the colour of the hydraulic fluid, from an orange/yellow to a crisp yellow
…
• When exposed to a blue light the oil will glow, helping to locate the leak.
100 On 21 May 2014, Mr Justin Ryan of Ulan No. 3 (Glencore) requested information on products from Fuchs that could be added to hydraulic oil to identify leaks. Mr Ryan stated in his email:
Andrew Esdaile when looking into fluid safe said that Fuchs have products that can be used for leak detection, we still have some fluid safe for the longwall (will be interested in products when this runs out), but I am looking at products for hydraulic oil. Does Fuchs have products that can be added to hydraulic oil to identify leaks?
101 On 22 May 2014, Mr Roberts forwarded the email from Mr Ryan of 21 May 2014 internally, stating: “This is back on the radar again and we were advised yesterday of concerns with HP injections for both hydraulic oil and Solcenic”. Mr Pearce responded stating:
Attached is my correspondence with Andrew Esdaile regarding fluorescent dye in Solcenic for leak detection only.
As for the hydraulic oil, the Fluidsafe when used at the correct level adds about [redacted] and we already have this product setup and supply several sites frequently. We can however source other dyes at lower concentrations to significantly reduce this cost however this would create further complexity in our range.
(Emphasis in original.)
102 On 27 May 2014, Mr Roberts sent Mr Michaelson a draft response to Mr Ryan for approval posing two options for hydraulic oil:
1. Hydraulic oil containing Fluid Safe OB which will incur an extra cost of [redacted] per litre
2. Hydraulic oil with dye under investigation
103 On the same day, Mr Michaelson forwarded Mr Roberts’ draft email to Mr Knight stating:
We are at a cross roads with dye in hydraulic and longwall fluids.
Ulan is asking can we supply a dye for leak detection in the longwall and mineral oil applications.
Ulan are presently using fluid safe on their longwall but want to explore other alternatives.
Initially this fellow introduced fluid safe based on leak detection not HPI.
Craig’s concern is that by not proceeding that this could provide an avenue for Quaker who has not been approached or approached Ulan.
This is a quandary given we have stayed close to Fluid Safe and Wayne Thompson and if we move in this direction that relationship will be irreparably damaged. Thompson believes he has patent coverage of leak detection.
The decision needs to be taken by a director.
Do we proceed and supply dyed longwall fluid and hydraulic oil using a standard dye which has no additional product cost impact or defer to the expensive fluid safe option.
104 Again on the same day, Mr Knight responded stating:
Where we are selling for HPI detection we need to us (sic) FluidSafe. For leak detection only Wayne’s patent cannot hold as other products have been around for many years. Therefore lower cost alternatives can be used for leak detection.
105 On 11 June 2014, Mr Roberts responded to Mr Ryan of Glencore’s email stating:
Fuchs are (sic) currently reviewing and conducting R&D along with QC testing with dyes for both mineral hydraulic oils and longwall fluids.
…
It should be clear at all stages that Fuchs makes no undertakings in relation to the use and monitoring of such products by Ulan Coal Mines and specifically and explicitly rules out any correlation of the use of such products for High pressure injection injuries detection.
106 Also on 11 June 2014, Mr Burns of Oaky Creek (Glencore) requested information from Fuchs on compatibility of FluidSafe with Solcenic 2020. Mr Michaelson responded to Mr Burns’ email stating that Fuchs had “previously supplied Solcenic 2020 with Fluid Safe WB blended into the final delivered product.”
107 On 13 June 2014, lawyers acting for TTAAS and T&T wrote to Whitehaven Coal Ltd, referring to the applications for the Standard Patent and Innovation Patent, and stating:
We hereby advise that the use of any product by you incorporating a fluorescent dye for detecting fluid injection when that product and system has not been supplied by our clients may render you liable to a claim for damages by our clients for the breach of our clients (sic) intellectual property rights.
108 On the same day, lawyers acting for TTAAS and T&T wrote to Anglo Coal Aus Ltd, stating:
Our client has information to indicate that your company amongst others may have been approached by Quaker Chemical seeking to sell you products which they claim incorporate a fluorescent dye which would allow you to detect high pressure fluid injection. It is our clients’ view that the method proposed by Quaker Chemical for detecting such injuries is in breach of our clients’ intellectual property rights as established under its patents.
109 On 16 July 2014, Mr Ryan of Glencore responded to Mr Roberts’ email stating:
As per our discussions Ulan would like to progress as soon as practical adding the leak detection dye (not for fluid injection) to our hydraulic oil and within 2 months for our solcenic.
Could you please provide the technical specs … and procedure for detection etc, so we can assess the level of risk control we will need to implement when we make this change.
110 On 18 July 2014, Mr Pearce sent an email to Glencore titled “Proposed Colour Change to Fuchs Hydraulic oil” which stated:
A couple of months ago we were approached by Ulan underground mine regarding the potential use of dyes in our hydraulic products to assist in leak detection.
The scope of this enquiry encompassed standard mineral oil based hydraulic oil (Renolin B PLUS range) and longwall Hydraulic fluid type HFA (Solcenic 2020).
In response to this request Fuchs proposes to make a modification to the dye that is used in our Renolin B PLUS range. This change will result in a slight change in the colour of the product from an orange/yellow to a crisp yellow as seen in figure 1 below.

Figure 1 Proposed colour vs Existing colour
The total dye concentration in the product is unchanged as this is a simple partial substitution of dye.
The additive pack and base stock in the product have not been altered and as such there is no impact on product performance.
Under the right light and conditions the product will fluoresce enough for leak detection.
Due to the fact that this improvement is cost neutral Fuchs is proposing to make this change across the entire Renolin B PLUS range.
…
I must stipulate that these proposed changes described above are in direct response to the enquiry raised by Ulan Underground for leak detection only.
Under no circumstances does the type of, or concentration of dye in these products make them suitable for HPI detection.
Fuchs is offering to supply products with fluorescing capabilities at no extra cost to Glencore, equipment used to detect the fluid and associated methods cannot be supplied by Fuchs at this stage.
111 On 21 July 2014, David Young of Glencore forward Mr Pearce’s email of 18 July 2014 to Mr Thompson, asking “Is this FluidSafe they are referring too (sic)?”
112 On 28 July 2014, Mr Pearce emailed Mr Thompson requesting that he “provide details as to whether or not the changes proposed by Fuchs is (sic) in breach of your patent.” Mr Thompson responded that “TTAAS’s and T&T’s lawyers had already been instructed (by me) to write to Fuchs setting out our position in relation to its conduct with respect to use of fluorescent additives.”
113 Prior to mid-2014, TTAAS and Fuchs had a working relationship and shared sensitive commercial information.
114 On 30 July 2014, TTAAS and T&T, through Silberstein & Associates, issued a letter of demand to Fuchs alleging patent infringement by supply.
115 Also on 30 July 2014, in response to the letter of demand, Mr Pearce made the following observations in internal Fuchs correspondence:
I have made comment against many of the points. Basically he has made many assumptions that are unfounded. He is trying to link it all to HPI injection which is not the intended use of our product and we have made this clear.
…
Just by scratching the surface it is clear to see that the use of fluorescent dyes for leak detection with a UV light is in the public domain and evidence is readily available. This opens the question why he is not hitting the dye suppliers in Australia?
The easier option is to roll over to TTAAS, this would prevent us from fulfilling the customer’s needs and potentially give opportunity to a competitor. We know that Quaker use a fluorescent dye in their LW fluids and as this was used prior to patent they would not be under the same restrictions that we are. This would give them a point of difference in the market.
Glencore do not support TTAAS at the higher level in the business and it does not look great for Fuchs if we do not stand our ground on an issue where we appear to be in the right. Andrew Esdaile and Garry Horner have both made comments about challenging Wayne Thompson due to the precarious nature of the patent and they are aware that Quaker have done just that. The Quaker challenge is much more contentious being that they introduced a dye for HPI injection.
I am no lawyer but all the conversations I have had internally at Fuchs and abroad (Martyn, Paul etc.) on this matter [redacted]. We have worked in good faith with Wayne Thompson and have supported the implementation of his product into the market, his product however has a purpose and the patent is titled as such “The Method for detecting Fluid Injection in a Patient”. His claims at present are all based on assumptions which I do not believe should prevent us from providing the best products and solutions to our customers possible.
116 On 8 August 2014 Fuchs, through Collison & Co, responded to letter of demand.
117 On 9 October 2014, in response to a request from Mr Russell of Fuchs in relation to an update on FluidSafe, Mr Pearce stated:
Regarding the patent; we continue to recommend and use Fluidsafe WB and Fluidsafe OB where the customer requires a dye specifically for the purpose of high pressure injection detection (HPID).
We have not and have no plans to challenge TTAAS on the validity of his patent in regards to HPID.
We did however challenge the patent validity in regards to fluorescein dyes used for leak detection and are quite comfortable that dyes added to fluids for this application are not covered by the scope of the patent.
…
… my concern with adding another dye is that without concrete test data we cannot be sure that the dye will remain adequately detectible for 24 hours in the human body. Remember that TTAAS claimed that special additives were added to stabilise the dye in the human body, we do not have any evidence to prove or disprove this claim and as we are dealing with a medical process I would be reluctant to assume that the Fluidsafe product is simply straight Fluorescein.
118 On 20 or 22 October 2014, Fuchs met with Broadmeadow (BMA) to discuss fluorescent dye for use in HPFI detection.
119 On 21 November 2014, Mr Pearce sent an internal Fuchs email which stated:
As the concept of changing the dye across the whole Renolin B PLUS hydraulic range seemed to come up against a significant amount of hurdles the decision has now been made to create a stand-alone product, ingeniously named Renolin B 68 LD.
As this will now be a Ulan No.3 specific product we do not need to keep the colour in line with the existing product and as such can remove the previous dye completely and increase the fluorescent dye up to the point where the product is cost neutral.
Please update the R&D brief for this project with the new target outcome, we aim to supply site at the beginning of 2015.
120 Also on 21 November 2014, Austar (Yancoal) provided Fuchs with FluidSafe WB to mix into their emulsion.
121 On 8 December 2014, Appin (BMA) requested information from Fuchs for fluorescent additive.
122 On 12 December 2014, TTAAS and T&T sold its FluidSafe business to Quaker, assigning to Quaker the patents in suit and any right to sue for prior infringement.
123 On 24 February 2015, a Ulan No. 3 document entitled “Change Management” for a change summarised as the “Introduction of dye to Fuchs Solcenic and hydraulic for leak detection” was approved by Mr Gavin Foster and Mr Ryan. The purpose of the change was said to be “A dye is being added to the solcenic and hydraulic fluid by the supply (sic) Fuch (sic), to aid in the dection (sic) of leaks.”
124 On 26 February 2015, Mr Ryan emailed Mr Roberts stating: “The change management is completed as per Grants (sic) Email, it was done both for solcenic and hydraulic oil. One of the action (sic) is to develop a procedure for using the product to detect a leak. What tools you need etc. Can you please provide details so I can put together?”
125 On 3 March 2015, Mr Roberts of Fuchs forwarded to Mr Ryan an email from Mr Pearce, with the subject line “[l]eak detection equipment” and body text as follows:
On the attached image you have the lamp cover for use underground, the torch for surface use and some yellow tinted safety glasses (any make will do).
The torch and glasses can be supplied in a kit with chargers for the torch. The lamp covers where purchased from Wayne Thompson. I have a couple that I can spare Ulan if required.
To detect leaks essentially shine the blue light in the direction of the equipment whilst wearing the glasses and look for the illuminated fluid.
126 On 24 March 2015, Mr Roberts’ weekly report noted in relation to Ulan West (Glencore): “Leak detection dye for Solcenic GM20 for next longwall block”.
127 On 13 May 2015, Mr Pearce emailed Mr Ben Withers and Mr Besley of Glencore stating, in part: “Fluorescein dye offered for leak detection only is a sodium salt of fluorescein. There is no specific data from the supplier however fluorescein dye is classified as biodegradable which indicates that it will rapidly biodegrade in the environment. The quantity used in the final emulsion supplied to site is <0.01% m/m (SDS attached).”
128 On 18 May 2015, Narrabri (Whitehaven Coal) requested pricing on Solcenic with HPFI dye. In an internal email to Robert Fryer of Fuchs about this request Mr Pearce stated: “Quaker supply a HPI dye for Narrabri, this gives them a significant cost advantage as we need to by (sic) the dye from them. I will confirm price of dye and get back to you.”
129 On 21 May 2015, Mr Roberts’ weekly report noted in relation to Ulan No. 3: “SWP for leak detection”.
130 In May 2015, a Fuchs document titled “Business Activity Report” addressed to Mr Pearce from Mr Roberts stated in relation to Ulan No. 3: “6. Leak detection procedure required ASAP.”
131 On 3 June 2015, Newlands (Glencore) requested information from Fuchs on “the UV dye that can be put into emulsion to assist with fluid injection detection.” Mr Pearce responded on 5 June 2015 stating that Fuchs “can supply the product with the dye that claims to assist with HPI” for an additional cost. Mr Pearce also said that “There is (sic) some question marks over this technology including, stability of the dye, maintaining adequate concentration of dye and training in the medical industry” and also that “Fuchs is able to supply a dye for leak detection free of charge in your Solcenic 2 B-W however we do not recommend the use of dye for HPI detection, our advice is always to seek professional medical attention for suspected HPI.”
132 Also on 5 June 2015, Whitehaven Coal requested pricing from Fuchs on longwall fluids with and without dye. Mr Pearce responded with pricing and stating “The dye matter is more complex. We can supply a dye for leak detection only free of charge however the cost of the dye required for HPI injection is extremely expensive and will add an extra [redacted] to the price.” (Emphasis in original.) Mr Pearce also referred to “some question marks over this HPI detection technology” in similar terms as in his other 5 June 2015 email.
133 On 16 June 2015, Ulan No. 3’s leak detection procedure was prepared.
134 On 24 June 2015, Mr Roberts’ weekly report noted in relation to Ulan No. 3: “leak detection products”.
135 On 6 July 2015, Mr Pearce sent an email to Mr Darren Stephens of Ashton (Yancoal) stating “With increased focus on cost savings and minimising leaks across the face we have incorporated a very small amount of fluorescent dye into our GM20 product.”
136 In August 2015, Fuchs commenced supplying Solcenic GM20 D to Ulan No. 3 (Glencore), to Bulga (Glencore) and to Ashton (Yancoal). The supply to Bulga (Glencore) was later described in December 2016 “Updated Sales Data” as having been initiated by “Leak detection promotion”.
137 On 4 August 2015, Kevin Meyer of Broadmeadow (BMA) requested information from Fuchs on “glow additive”. Mr Pearce replied that Fuchs could “provide a glow product for leak detection” and asked “Are you only wishing to use it for leak detection?” and further stated “FYI, I am on site next Thursday week for my routine visit.” Mr Meyer replied “Not so much leak detection but more in regard to high pressure fluid injection detection.” Mr Pearce responded stating:
To achieve the target concentration of 0.015% HPI dye in the emulsion at 2% operating concentration the dye must be supplied in the concentrate at 0.75%.
The dye itself is very expensive and at 0.75% will increase the cost of your longwall fluid from [redacted].
There are however some question marks over this technology including, stability of the dye, maintaining adequate concentration of dye and training in the medical industry.
Fuchs is able to supply a dye for leak detection free of charge in your Solcenic GM20 however we do not recommend the use of dye for HPI detection, our advice is always to seek professional medical attention for suspected HPI
(Emphasis in original.)
138 On 16 September 2015, Ben Withers of Bulga (Glencore) made an enquiry of Fuchs regarding Injection Awareness Training and asked whether “Fuchs have any training available to roll out to our workforce. We are looking for something along the lines of the actual simulated pig injections as offered by Wayne Thompson.” Mr Pearce forwarded the email internally, stating:
We have had a request for info on HPI awareness. Are you able to have the work we have done in Germany sent over, it may need to be sent on a USB stick due to the size.
This customer is a Glencore site and it is imperative that we provide adequate information and training to ensure Quaker do not get a foot in.
After some further correspondence Mr Littley responded:
I will collect info but just advise caution that if we are to fight Quaker we have to poo poo (sic) the idea and the dye is not an indicator of tissue damage and only is an aid to seeing the initial point of injection.
…
Are you already dying the product for Glencore using the Quaker dye.
Mr Pearce responded “I won’t go into too much detail but will ensure the theory behind the Quaker product is discredited as I have already done to this site in the past.” In response to a further email from Mr Littley asking, in part: “Are you already dying the product for them using the Quaker Dye?”, Mr Pearce stated: “We are no longer dying any products with the HPI dye. We do have a little fluorescein in the product for leak detection. … My advice as we agreed will be to seek medical treatment for suspected HPI in all cases.”
139 On 17 September 2015, Mr Littley replied to Mr Pearce stating, in part: “Please take great care with this topic. Suggest you get advice from Martyn and Stew since if info gets back to Quaker on what we are doing it will tip them off and also probably kick off the war with Quaker ahead of us challenging the validity of the Patents!”
140 In October 2015, Fuchs commenced the supply of Renolin B 68 Plus LD to Ulan No. 3 (Glencore).
141 Two presentations dated 7 October 2015, titled “Fuchs / Glencore: Ulan # 3” and “Fuchs / Glencore: Ulan West Review Meeting”, state “RENOLIN B PLUS 68 LD – contains a specially designed fluorescent dye to assist with detecting hydraulic fluid leaks in equipment (clear bright pink)” and, under the heading “SOLCENIC GM20 D – leak detection” state:
• Ensure fluid has been dyed using a fluorescing dye.
• Inspect equipment after dye has been circulated through system focusing on areas at risk of leaks such as hose fittings, fluid will exhibit a green/yellow colour.
• The dye can be detected much more readily by utilising yellow tinted safety glasses and shining a UV light/torch on the area to be checked for leaks. Any UV light/torch must be intrinsically safe for use in underground environment.
142 On 3 December 2015, Mr Krysko of Lake Coal made an enquiry to Fuchs regarding fluid injection dye. Mr Pearce responded on 4 December 2015 stating: “We can supply the product with the injection dye however we would need to re-quote as this adds extra cost to the product. All our products are compatible with the dye as we worked closely with TTAAS who introduced this dye in the early stages.”
143 Also on 3 December 2015, Fuchs requested re-examination of the Standard Patent and Innovation Patent.
144 On 9 December 2015, Broadmeadow (BMA) requested information from Fuchs regarding Solcenic GM20 and use of dye for “safety reasons”.
145 In the period 16-23 December 2015, there was internal Fuchs correspondence exchanged regarding how to deal with a request from Broadmeadow for dyed Solcenic for the purpose of HPFI detection. That correspondence included an email from Mr Pearce of 23 December 2015 which attached a draft presentation and stated:
Think we are getting close. This might actually be enough for me to start serious discussion with site and hopefully avoid them even consulting Quaker for pricing.
I do think we will need a more committal comment on HPI to satisfy the site HS&E team (actually state that the dye we use will stain the skin and muscle tissue and can assist in HPI detection).
That said I would like to convince site where possible verbally rather than in writing.
My draft presentation is attached, I invite and expect feedback on whether you believe I have gone too far or not far enough.
Keep in mind that I aim to convince site that as a lubricant specialists company we have the expertise on dyes and the capability to test and investigate their use. This presentation provides unquestionable evidence that our dye is the same and at the same concentration to the Fluidsafe dye at the centre of the initial patent, a dye that we worked closely with as a show of good faith to Wayne Thompson.
The draft presentation stated, in part:
• Products containing Fluorescein based dyes will on contact with skin or human tissue, result in staining.
• It is therefore impossible to separate the use of dyes from the well established detection of fluid leakage to that which is being reported as diagnostic methods for medical use such as aiding in the identification of potential High Pressure Injection of fluids.
146 On 24 December 2015, Mr Knight replied to Mr Pearce’s email of 23 December 2015 stating: “Excellent presentation Wayne. Please do not leave a copy with the mine until we have patent determination though.”
147 Also on 24 December 2015, Mr Pearce confirmed by email that Fuchs could supply to Broadmeadow a dyed product with minimal impact to production cost and requested availabilities for a presentation to be made the following year.
148 On 20 January 2016, Oaky Creek (Glencore) requested information from Fuchs regarding “fluoresce tell-tale additives used for the identification of fluid injection”.
149 On 21 January 2016, Mr Pearce delivered a presentation at Broadmeadow on the use of fluorescent dyes.
150 On 29 January 2016, Mr Pearce offered to present to Mr Chamberlain of Oaky Creek (Glencore) on Fuchs’ 2% emulsion product and fluorescent additives for identification of fluid injection. Mr Pearce stated: “I think on these two topics it would be best for me to present and give you an opportunity to ask any questions that you may have in person.”
151 On 4 February 2016, IP Australia issued Re-examination Reports. Fuchs’ re-examination requests were unsuccessful.
152 On 23 February 2016, Mr Brennan Long of Broadmeadow (BMA) emailed Mr James of Fuchs, stating: “Can you please help us out on the contract side of things to implement the purchase of GM20 Emulsion with fluorescein dye include? (sic)”.
153 On 2 March 2016, Mr Pearce presented information on Solcenic GM20 and fluorescein dye to Oaky Creek North (Glencore).
154 On 11 March 2016, Moolarben (Yancoal) requested information from Fuchs regarding FluidSafe for hydraulic oil and the supply price for a fluid injection test kit.
155 In March 2016, Fuchs commenced supply of Renolin B 68 D containing FluidSafe OB to Moolarben (Yancoal).
156 On 21 April 2016 Ethan Searson of South 32 emailed Mr Pearce and asked “Can you please confirm, if we wanted to go down the path of adding the fluid injection and leak detection dye, this would be free of charge?” Mr Pearce responded “Dye for the purpose of leak detection is free of charge only.”
157 In May 2016, Fuchs commenced to supply to Solcenic GM20 D to Ulan West (Glencore).
158 On 18 May 2016, Austar (Yancoal) requested Fuchs to supply Solcenic GM20 with FluidSafe.
159 On 8 June 2016, Mr Pearce emailed Mr Littley and others stating in relation to Broadmeadow (BMA): “there [is] a real risk that we could find ourselves in court soon for the supply of a high concentration of dye in a product for Broadmeadow mine. The customer under their own guidance plan to use the dye for the patented purpose and have approached Wayne Thompson (Quaker) for training which will alert them to the fact that we are supplying a dyed product.” (Mr Knight admitted in cross-examination that he shared Mr Pearce’s concern about the risk of litigation.)
160 Also on 8 June 2016, Mr Roberts’ weekly report noted in relation to Ulan West (Glencore): “Leak detection kit requested by Clinton. [Action Required:] Purchase leak detection kits”.
161 On 9 June 2016, Mr Pearce stated in an email to Mr Littley that he had “just had another site request Fluidsafe dye”. The source of the request was not stated.
162 On 14 June 2016, Mr Roberts’ weekly report noted: “Leak detection details for Ulan West”.
163 On 21 June 2016, Quaker, through Silberstein & Associates, issued a letter of demand to Fuchs alleging patent infringement by supply.
164 In response to Quaker’s letter of demand dated 21 June 2016, on 22 June 2016, Mr Pearce made the following observations in internal Fuchs correspondence: “We have used the patented dye in every case where HPI dye has been requested and a standard dye at much lower concentrations where leak detection was required.” Mr Pearce also set out a table of supplies which he described in the email as “Sales data … to the best of my memory.”
165 In July 2016, Fuchs commenced the supply to Broadmeadow (BMA) of Solcenic GM20 LD.
166 On 1 July 2016 Fuchs, through Collison & Co, replied to Quaker’s letter of demand stating that “Fuchs completely denies any wrongdoing” and reiterating that “in the past, and at no time since 2012, have they ever included as part of their point of sale promotional material, labelling, technical advice and so forth, any suggestion or inducement to customers of their product that an intended use of our client’s products could be used (sic) as part of a method incorporated into your client’s claimed invention involving ‘a possible fluid injection occurring into the patient’” (original emphasis). (In cross-examination Mr Knight accepted that this statement was incorrect insofar as it related to Broadmeadow.) A further paragraph of the 1 July 2016 letter referred to “information or documentation which suggests [Fuchs] is selling product for which there is referencing that it could be used in an application associated with your client’s inventions” and stated that “our client confirms that there is no such information or documentation available as its products are not intended to be used for the purpose associated with your client’s claimed inventions.” (Again, in cross-examination Mr Knight accepted that this statement was incorrect: there was information and documentation available of that kind in relation to Broadmeadow. Mr Knight gave evidence that he was not involved in giving instructions to Collinson & Co for the purpose of them acting on behalf of Fuchs on this issue, or for that statement to be included in the letter.)
167 On 7 July 2016, Fuchs’ Contract review Meeting Minutes for Ulan No. 3 stated: “Further information required on … UV Leak Detection Kit” and included the action item “Email details and quotation for UV Leak Detection” which was stated be “Completed 11/7/16” by Craig Roberts.
168 By an originating application dated 26 July 2016, the present proceedings were commenced.
169 On 8 August 2016, in an internal email chain titled “UV Dye we discussed last week” Mr Alexander Franke of Fuchs received a query regarding “UV Dye” from Rolleston Coal (Glencore). On 23 August 2016, Rolleston Coal requested information from Fuchs regarding “UV dye in hydraulic oils”. Mr Franke responded on 23 August 2016 stating “A dye for the hydraulic is available. It’s not so easy to get it as there is only one company selling it as they have a patent on this. We are checking availability and cost.”
170 On 25 August 2016, Mr Roberts’ weekly report noted “leak detection details for Scott Cook from Ulan West”.
171 On 22 September 2016, Bulga (Glencore) requested information from Fuchs about dye for HPFI detection. Fuchs responded by providing the PDS for Renolin B 68D, described in the email as “a specifically designed hydraulic oil with a fluorescent dye”.
172 On 12 January 2017, Mr Hoiles, Managing Director of Fuchs, sent a copy of a document named “Updated Sales Data Dec 31 2016”, which contains a schedule recording data in columns including labelled “Product”, “Customer”, “Comments” (which records details of supplies) and “Initiated by” (which is one of “HPI request”, “Leak detection request” or “Leak detection promotion”), which Fuchs contended shows the reason for the supply of dye in each case and expressly distinguished between leak detection and HPFI products.
173 On 20 September 2017, Fuchs received a request from Moolarben (Yancoal) for fluorescent dye in its longwall for HPFI detection and Mr Pearce provided pricing information to Moolarben (Yancoal) for “the patented dye that has the reported use of aiding in HPI incidents”.
174 On 19 June 2018, Appin West (South 32) enquired about an additive to Solcenic GM 20 and hydraulic oil which would be detectable for potential fluid injection cases.
175 On 4 July 2018, Mr Pearce emailed Mr Roberts stating: “It would assist us in closing the Narrabri deal if we could get something in writing from someon (sic) at Ulan 3 or West stating that they are happy with GM20 and the dye allows them to effectively identify any leaks across the face. Would this be possible and please avoid any HPI mention?” Mr Roberts responded to Mr Pearce’s email stating: “Grant Patrick is a big supporter of this and he utilized a (sic) IR gun to identify leaks on the face.”
176 On 10 July 2018, Mr Kirkhope of Ulan West (Glencore) responded to Mr Pearce’s request (which was forwarded to him by Mr Robinson of Fuchs). Mr Kirkhope stated: “I have no involvement in the leak detection properties. We don’t do that here and I have no interest in doing it. Generally you can either hear or see a leak and it’s way too labour intensive to bother UV leak detection.”
177 On 20 July 2018, Mr Knight sent an email to Mr Rushton confirming that Fuchs had “won Narrabri Coal’s longwall from Quaker. Product contains low concentration dye for leak detection. Supply commences in 3 weeks.”
178 Also on 20 July 2018, Fuchs provided pricing information for “FluidSafe WB (as supplied by Quaker)” to South 32.
179 On 23 August 2018, Whitehaven (mine unknown) requested information from Fuchs “for dye inclusion in the GM20 for leak detection”. Mr Smith of Whitehaven also stated: “Note: there is consensus that the use of dye for fluid injection management is not required.”
180 On 7 September 2018, Ravensworth (Glencore) requested information from Fuchs about traceable dye detection for HPFI. Fuchs responded by stating its belief that prevention is better than detection and directing its enquiry to Mr Horner of Glencore.
181 On 17 October 2018, Oaky North (Glencore) requested pricing from Fuchs of adding dye to Solcenic GM20, stating: “Primary purpose is in the event of a suspected fluid injection”. Mr Pearce responded by stating “Fuchs will only supply its longwall fluids containing fluorescent dye suitable for HPI detection by adding FluidSafe dye, which needs to be sourced from Quaker” and that “Quaker, not Fuchs, represents that longwall fluids containing FluidSafe are suitable for HPI detection.”
182 On 18 October 2018, Axel Cutts, Undergraduate Mechanical Engineer at Oaky North (Glencore) sought further information on “Quaker dye”.
183 Mr Pearce responded to Mr Cutts on 29 October 2018 stating it was “quite a sensitive topic” and directing Mr Cutts to Mr Horner of Glencore.
184 On 16 January 2019, (Yancoal) Moolarben indicated that it would like to change to the leak detection dye product as soon as possible.
Particulars of invalidity
185 Fuchs’ particulars of invalidity were as follows, as per its Fifth Further Amended Particulars of Invalidity, and excluding particulars no longer relied upon:
FIRST PATENT [Standard Patent]
Lack of clarity
1. Claims 1 to 22 of the First Patent are invalid and liable to be revoked pursuant to s 138(3)(f) of the Act on the ground that those claims are not clear and succinct, as required by s 40(3) of the Act.
PARTICULARS
…
(b) As to claim 1, there is no requirement for any ultraviolet light (which is introduced as an essential feature in claims 2 and 9), such that it is not clear in the context of the specification as a whole how fluid injection is to be detected.
…
(i) There is no requirement in any of claims 1 to 19 or 22 for fluorescent dye to be present at a particular concentration or within a particular concentration range in the fluid, such that it is not clear in the context of the specification as a whole what concentration of fluorescent dye must be present in the fluid for a possible fluid injection to be detected.
Insufficiency and failure to disclose best method
2. Further or in the alternative to paragraph 1 above, claims 1 to 22 of the First Patent are invalid and liable to be revoked pursuant to s 138(3)(f) of the Act on the ground that those claims do not describe the invention fully, including the best method known to the Applicant/Cross-Respondent or its predecessors in title, of performing the alleged invention, as required by s 40(2)(a) of the Act.
PARTICULARS
…
(c) The specification does not disclose any or the particular concentration of fluorescent dye which should be present in the fluid, other than to “0.01% (v/v)” and “0.2% (v/v)” as preferments.
Lack of utility
3. Further or in the alternative to paragraphs 1 to 2 above, claims 1 to 22 of the First Patent are invalid and liable to be revoked pursuant to s 138(3)(b) of the Act on the ground that the alleged invention as claimed is not useful, as required by s 18(1)(c) of the Act.
PARTICULARS
(a) The alleged invention, so far as claimed in claims 1 to 22, fails to meet the promise of the specification across the full scope of the claims.
(b) The specification indicates that the patient should be examined with an ultraviolet light in a dark room after washing the point of possible fluid injection. However, claims 1, 2, 3, 4, 5, 9, 10, 11, 12, 13, 14 and 15 do not require any such sequential washing and examination steps or any such conditions for examination. The method defined in the claims will not work over the full scope of claims 1, 2, 3, 4, 5, 9, 10, 11, 12, 13, 14 and 15 which encompass the examination of patients prior to, or without any, washing of the skin, since fluorescence cannot be used to determine whether a possible fluid injection beneath the skin has occurred where the fluid carrying the Fluorescent Fluid Additives remains on the surface of the skin or in conditions in which fluorescence cannot be observed or detected.
…
(d) The specification does not indicate that fluorescent dye must be present at a particular concentration in the fluid. The method as claimed will not work over the full scope of claims 1 to 19 and 22 which encompass fluorescent dyes present in the fluid at concentrations which are too low to be detected under a patient’s skin and therefore could not be used to determine whether a fluid injection has occurred.
…
Lack of fair basis
4. Further or in the alternative to paragraphs 1 to 3 above, claims 1 to 22 of the First Patent are invalid and liable to be revoked pursuant to s 138(3)(f) of the Act on the ground that those claims are not fairly based on the matter described in the specification, as required by s 40(3) of the Act.
PARTICULARS
(a) Claim 1 travels beyond the matter disclosed in the specification because it is not limited to detection of fluid using ultraviolet light, and there is no real and reasonably clear disclosure in the body of the specification of the invention in that form.
…
(h) Claims 1 to 19 and 22 each travel beyond the matter disclosed in the specification because those claims are not limited to fluorescent dye to be present at a particular concentration or within a particular concentration range in the fluid, such as about 0.01% (v/v) or 0.2% (v/v), and there is no real and reasonably clear disclosure in the body of the specification of the invention in that form.
…
Lack of Novelty
6. Further or in the alternative to paragraphs 1 to 5 above, claims 1 to 22 of the First Patent are invalid and liable to be revoked pursuant to s 138(3)(b) of the Act on the ground that the alleged invention the subject of each claim is not a patentable invention within the meaning of s 18(1)(b)(i) of the Act because, when compared with the prior art base as it existed before the priority date of each claim, the alleged invention is not novel.
PARTICULARS
(a) The Respondent/Cross-Claimant relies on information made publicly available in each of the following documents:
…
(viii) Email from Ken Risk to Craig Roberts dated 18 November 2010 with the subject “UV oil additive”.
(b) The Respondent/Cross-Claimant relies on information made publicly available in each of the following acts:
…
(vi) the disclosure of the invention between September and November 2010 at Peabody’s Metropolitan mine by Wayne Thompson to Andy Withers, and by Wayne Thompson to Andy Withers and the Metropolitan mine manager, on the occasions referred to in paragraphs 40 and 47 of the second affidavit of Wayne Thompson dated 5 September 2018;
…
(ix) the disclosure on or about 9 November 2010, via in-person oral conversation, by Ken Risk to Craig Roberts of the possibility of adding a chemical dye to the longwall fluid at Peabody’s Metropolitan mine so as to enable detection of fluid under a person’s skin in the case of a suspected or possible fluid injection;
(x) the disclosure on or about 17-18 November 2010, via oral in-person conversations, telephone oral conversations and/or emails, by Ken Risk to Craig Roberts of the possibility of adding a “UV oil additive” called “Oil-Glo” to the longwall fluid at Peabody’s Metropolitan mine so as to enable “verification of oil injections” under persons’ skin;
…
(xv) any combination of one or more of the acts described in sub-paragraphs 6(b)(ix), and 6(b)(x), above, considered as a single source of information.
…
Secret use
7A. Further or in the alternative to paragraphs 1 to 7 above, claims 1 to 22 of the First Patent are liable to be revoked pursuant to s 138(3)(b) of the Act on the ground that, before the priority date, the alleged invention the subject of each claim was secretly used in the patent area within the meaning of s 18(1)(d) of the Act.
Particulars
(a) Before 2 September 2011, the product known as “Oil Glo” (Oil Glo) was offered, promoted and sold commercially by the Applicant/Cross-Respondent’s predecessors in title, T&T Global Solutions Pty Ltd (T&T) and/or TTAAS Thommo’s Training and Assessment Systems Pty Ltd (TTAAS), as an “oil injection indicator” to Peabody Energy Australia Pty Ltd and/or Peabody Australia Mining Pty Ltd (collectively, Peabody).
(b) T&T and/or TTAAS disclosed to Peabody and to the Respondent/Cross-Claimant use of Oil Glo in the method comprising the alleged invention disclosed in the First Patent. The disclosures were made, at least, by Mr Wayne Thompson (for and on behalf of T&T and/or TTAAS) in or around mid-2010 to early 2011 to representatives of Peabody and the Respondent/Cross-Claimant, as described in sub-paragraphs 6(b)(vi) to 6(b)(x) above.
(c) In around May to June 2011, Peabody issued purchase orders to TTAAS and/or T&T for the purchase of Oil Glo for delivery to its mine sites at Metropolitan and North Goonyella.
(d) In around May to July 2011, TTAAS and/or T&T delivered Oil Glo purchased by Peabody to its mine sites at Metropolitan and North Goonyella.
(e) TTAAS’s and/or T&T’s offers, promotions and entering into agreements and arrangements to supply Oil Glo were offered and promoted, including for use in accordance with the methods claimed in the First Patent, were secret and:
(i) were not for the purpose of reasonable trial or experiment only;
(ii) did not occur solely in the course of a confidential disclosure of the invention by or on behalf of, or with the authority of, the patentee, nominated person, or predecessor in title;
(iii) were not for any purpose other than the purpose of trade or commerce; and
(iv) did not involve disclosure of the invention to the Commonwealth or any State or Territory.
(ea) Between September 2010 and March 2011, Peabody added Oil Glo to the mineral hydraulic oil used in at least one rubber-tyred vehicle operating in the outbye of Peabody’s Metropolitan mine. One purpose of adding Oil Glo was to enable the detection of the mineral hydraulic oil under a person’s skin by shining a UV/blue light on the skin surface at the point of suspected or possible injection.
(eb) Oil Glo was added to the Solcenic 3002 longwall fluid was used in the longwall at Peabody’s Metropolitan mine from at least early May 2011. One purpose of adding Oil Glo was to enable the detection of the mineral hydraulic oil under a person’s skin by shining a UV/blue light on the skin surface at the point of suspected or possible injection. This is reflected in updates to treatment protocols for high-pressure injection injuries, communications to the workforce and the fact that almost immediately from the time Oil Glo was introduced to the longwall at Metropolitan a UV light was shone on the skin of workers presenting with suspected high-pressure injection injuries. The addition of Oil Glo followed risk assessments performed and completed by or for Peabody in late 2010 and early 2011 at its Metropolitan mine.
(ec) Oil Glo was added to the Solcenic 2020 longwall fluid used in the longwall at Peabody’s North Goonyella mine from at least 30 June 2011. One purpose of adding Oil Glo was to enable the detection of the mineral hydraulic oil under a person’s skin by shining a UV/blue light on the skin surface at the point of suspected or possible injection. This is reflected in updates to treatment protocols for high-pressure injection injuries and communications to the workforce. The addition of Oil Glo followed risk assessments performed and completed by or for Peabody in late 2010 and early 2011 at its Metropolitan mine, which were communicated to the North Goonyella mine.
(ed) In or around November 2010, Peabody made inquiries with the Respondent/Cross-Applicant regarding the possibility of Fuchs adding a chemical dye to longwall fluid supplied to Peabody’s Metropolitan mine so as to enable detection of fluid under a person’s skin in the case of a suspected or possible fluid injection.
(f) Further particulars are matters for evidence.
186 It is unnecessary to set out the particulars of invalidity for the Innovation Patent. The particulars were the same as for the Standard Patent except: the invalidity was pleaded in respect of claims 1 to 3 of the Innovation Patent; particular 1(b) was not used in relation to the Innovation Patent; s 40(2)(aa) and its language were relied on under the heading “Insufficiency and failure to disclose best method; s 7A was referred to under the heading “Lack of utility”; the particulars under the heading “Lack of fair basis” in relation of the Standard Patent appeared under the heading “Lack of support” in relation to the Innovation Patent, and do not include particular 4(a); the particulars under the heading “Lack of novelty” referred to s 18(1A)(b)(i) instead of s 18(1)(b)(i); and the particulars under the heading “Secret use” referred to s 18(1A)(d) rather than to s 18(1)(d).
The parties’ submissions
Validity
187 Fuchs submitted the Patents were invalid at the priority date, 2 September 2011, as articulated in its Fifth Further Amended Particulars of Invalidity.
Novelty
1. Overview
188 Fuchs submitted that both Patents were invalid at the earliest priority date because during 2010, before the priority date and outside the scope and operation of any grace period exception, Mr Thompson disclosed the method to personnel, Mr Andy Withers, Mr Ken Risk and Mr Chris Bate, at Peabody both orally and by way of demonstration. Those personnel plainly understood the method of the invention, and one of them (Mr Risk) freely and unambiguously disclosed it to Fuchs (Mr Roberts) both orally and by email in November 2010. Mr Thompson was copied on that email. When the relevant provisional patent application was filed on 2 September 2011, and the complete patent application for the Standard Patent was filed on 2 February 2012, they were simply too late, Fuchs submitted.
189 Further, Fuchs submitted (in the alternative to its novelty case) that Quaker’s Patents were also invalid because Mr Thompson secretly used the invention before the priority date by commercially exploiting it through his sales of Oil-Glo fluorescent additives to Peabody, and by authorising Peabody to use that Oil-Glo in the method the subject of his “invention”.
190 Fuchs submitted Quaker essentially treated the concept and requirements for “reasonable trial” of an invention as being the same for both the novelty “grace period provisions” and the exclusion from the definition of “secret use”. However, each applied to different types of conduct and imposed different requirements. Quaker could not benefit from either the novelty “grace period” or the exclusion from “secret use”. At a threshold level, Mr Thompson’s disclosures by conversations and demonstrations were not “working of the invention in public”. Nor did any tests which were performed relate to the invention claimed in the Patents. Nor were any of the disclosures or demonstrations connected with any of the tests that were performed in any event. To the extent the simple method required any testing or verification of efficacy once the idea had been conceived, such testing had been completed prior to the time when Metropolitan (in May 2011) and North Goonyella mine (in June 2011) introduced the Oil-Glo additive to their production longwall mining systems on a commercial basis and scale. In addition, immediately thereafter, and well before 2 September 2011, workers at those mines who had presented with suspected HPFI injuries had a UV/blue light shone on their affected areas in each mine’s first aid room.
191 Fuchs’ submissions on secret use are treated separately below.
192 Quaker submitted that to the extent that any disclosures did occur, they fell within the scope of the relevant “grace periods” for novelty under the Act and did not amount to secret use.
193 The grace periods in [39] above were relevant: the General Grace Period (publication or use after 2 February 2011); the Reasonable Trials Grace Period (publication or use after 2 September 2010—the working in public of the invention for the purposes of reasonable trial where it was reasonably necessary for the working to be in public) and the Unauthorised Disclosures Grace Period (publication or use after 2 September 2010 by a person who derives the information from the patentee).
194 As the General Grace Period applied to all uses and disclosures of the invention by or with the consent of the patentee, Quaker’s submissions, and Fuchs’ novelty case, focused on the period prior to 2 February 2011. Further, following Mr Bate’s disavowal of his original evidence, it was now clear that there was no evidence of any disclosure or use prior to 2 September 2010.
2. General principles
2.1 Novelty-destroying disclosures
195 Fuchs submitted that s 7(1) of the Act provided the circumstances in which an invention will lack novelty at the priority date. Those circumstances comprised prior public documentary disclosures and public acts. Information said to anticipate must be of a kind that would disclose to a person skilled in the relevant art all of the essential features or integers of the invention: RD Werner & Co Inc v Bailey Aluminium Products Pty Ltd (1989) 25 FCR 565 at 593-4. The information must “enable” the person skilled in the art at once to perceive, to understand, and practically to apply the discovery, without the need to carry out further experiments.
2.2 Novelty grace periods
196 Fuchs submitted that Quaker bore the onus of establishing its entitlement to rely on these grace period provisions. The onus was on Fuchs to establish that the relevant disclosures were novelty destroying. Once that had been established, the claims of the patent will be anticipated unless Quaker could establish that, pursuant to s 24 and reg 2.2, those disclosures should be disregarded.
197 Fuchs submitted that the onus of proving that the reasonable trial grace period applied to an otherwise novelty destroying disclosure was on Quaker. The grace periods provided a positive defence based on circumstances known only to the patentee or its predecessor in title.
198 Fuchs submitted that the regulation did not make provision for general disclosures for the purposes of a trial. Cases concerning public trial were concerned with the disclosure effected by the working of the invention. The distinction drawn in regulation 2.2(2)(a) and (b) between publication and use was informative: where associated publications were meant to be covered, express provision was made.
199 Fuchs submitted that no assistance (for Quaker) was provided by cases concerning secret use. First, the relevant statutory expression was different (“secretly used”). Second, the provisions concerning secret use were a separate ground of invalidity (on a separate policy basis, being that a person should not be able to extend their monopoly by secret use) that did not require considerations of novelty and whether there was prior publication. Third, related disclosures were not public and therefore not anticipations. Fourth, the cases did not suggest that mere disclosure (whatever that might mean in this context) amounted to “use”.
200 With respect to purported disclosures after 2 September 2010 and before 2 February 2011 which were relied upon by Fuchs, Quaker submitted the onus was on Fuchs to prove that the disclosures occurred, their form and content, when they occurred, and whether a relevant grace period applied. The Court had to consider, with respect to any particular alleged disclosure relied upon by Fuchs, whether or not the disclosure contained “clear and unmistakable directions” to the person skilled in the art (PSA) to do what the patentee claimed in the Patents (AstraZeneca AB and Another v Apotex Pty Ltd and Others [2014] FCAFC 99; 226 FCR 324 at [293]) and would enable them to apply that invention using only common general knowledge and routine trial and experimentation: Olin Corporation v Super Cartridge Co Pty Ltd (1977) 180 CLR 236 at 260-1.
201 It was common ground that the Patents had the same grace periods.
202 As to the Reasonable Trials Grace Period, Quaker submitted that Fuchs bore the onus of proving that any disclosure or use was not for the purposes of a reasonable trial.
203 Qhaker submitted that the grace periods in respect of novelty operated in a similar manner. The Court had applied the principles developed in the context of reasonable trial under s 9 of the Act, to the context of reasonable trial under s 24(1) of the Act: Australian Mud Company Pty Ltd v Coretell Pty Ltd (No 4) [2015] FCA 1372 at [659] per McKerracher J.
204 Quaker submitted that trials can constitute “‘seeing how it goes’ when the invention is employed in the field”: Grove Hill at [231]; Bradken Resources Pty Ltd v Lynx Engineering Consultants Pty Ltd [2012] FCA 944; 210 FCR 21. Reasonable trials may be for the purposes of assessing or evaluating the performance of the invention, and could extend to determining whether a product or process needed to be improved, how to perfect a product or process, or the suitability of a product or process for commercial use: Australian Mud Company Pty Ltd v Coretell Pty Ltd (No 4) at [659]-[660]. The fact that a trial occured in a commercial context did not preclude it from being a reasonable trial: Grove Hill at [229]; Bradken at [351]-[365].
205 That the grace periods were beneficial or remedial provisions was demonstrated by their intended effect, Quaker submitted, being to protect a patentee from what would be harsh outcomes in certain circumstances.
206 Quaker submitted reg 2.3(1)(c) envisaged that the working in public could occur over a period of many months. It was absurd to suggest that any ancillary information arising in respect of that working of the invention which was disclosed to the public would invalidate the patent.
207 Quaker submitted it was implausible that nothing less than a trial involving an actual or suspected HPFI injury in a living person (dead people or animal tissue not answering the description of a “patient”) would constitute a reasonable trial.
3. Contentions
208 Fuchs submitted information disclosing the invention was made publicly available before 2 February 2011. The claimed invention was a simple method, easily conveyed in a single sentence. Once apprised of this simple concept, the relevant skilled addressee would have immediately understood all of the steps required to perform the method.
209 Fuchs submitted that prior to 2 February 2011, the invention was disclosed by Mr Thompson to personnel at Metropolitan, and by those personnel to personnel of Fuchs, in the following discrete disclosures:
a. two disclosures in October/November 2010 to Andy Withers at Peabody’s Metropolitan mine;
b. disclosure in November 2010 by Ken Risk (Peabody) to Craig Roberts (Fuchs).
210 Fuchs submitted that there was no evidence from Mr Thompson that those to whom he disclosed the invention were subject to obligations of confidence. Nor was there any evidence that Mr Thompson did not consent to further disclosures, including by Peabody to Fuchs, or that he sought to impose confidentiality obligations on any person to whom he disclosed or demonstrated his method. Prima facie, the disclosures made at Metropolitan were novelty defeating, Fuchs submitted.
3.1 Disclosures by Mr Wayne Thompson – particular 6(b)(vi)
211 First disclosure: Fuchs submitted that Mr Thompson disclosed the method the subject of the Patents in a conversation with Mr Andy Withers, the manager of mechanical engineering at Metropolitan in October 2010. Mr Thompson admitted disclosing what he described as “the Method” to Mr Withers in this conversation.
212 Fuchs submitted that the conversation took place shortly after Mr Thompson had “a sudden idea one night” to use the fluorescent Oil-Glo dye to enable detection of HPFI injuries. The conversation was in the context of the possibility of adding dye to the hydraulic fluid in machinery at Metropolitan.
213 Fuchs submitted that in cross-examination Mr Thompson agreed that in his conversation with Mr Withers in October 2010 he disclosed to Mr Withers that the best way in which to determine whether a person had been injected with high pressure fluid (which used fluorescent dye) was to shine an ultraviolet light on them. The conversation was in public. The freedom which representatives of Metropolitan considered they had in dealing with the information was readily apparent from their conduct in relation to Fuchs.
214 Second disclosure: Fuchs submitted that there was a second disclosure by Mr Thompson when he demonstrated his invention to Mr Withers and the Metropolitan mine manager in October or November 2010. Mr Withers had earlier received a demonstration of Mr Thompson’s simulator (prior to the addition of any Oil-Glo). Mr Thompson subsequently used that simulator (with Oil-Glo) to demonstrate his invention to Mr Withers and the Metropolitan mine manager in October or November 2010. Specifically, he used his simulator (being a simulator with a hydraulic system) with the fluorescent dye in it to demonstrate the penetration of pig skin by the fluorescent dye. The demonstration utilised a pig skin specimen as part of the simulation of detection of an HPFI injury. Pig skin was used as an analogue for human skin.
215 Fuchs submitted that Mr Thompson’s simulator: (a) was utilised to demonstrate HPFI injuries, (b) utilised a hydraulic system similar to hydraulic systems that were used in larger pieces of machinery, (c) had a hydraulic system that was an oil-based system and utilised a hydraulic pump, (d) had a fluid storage tank of around 40 litres, (e) had Oil-Glo added to it, (f) was able to simulate HPFI injuries across a range of pressures via an industrial gauge, (g) had a range of pressures able to be demonstrated from 150 psi to 4,800 psi, and (h) was able to simulate an HPFI injury even at the lowest possible setting of the industrial gauge. There was little doubt that Mr Withers would have understood that the simulator had a hydraulic system and had these features: that was the whole point of the simulator.
216 Fuchs submitted that after conducting a simulated high pressure injection of hydraulic fluid containing Oil-Glo into the pig skin specimen, Mr Thompson took the specimen out of the simulator and showed it to Mr Withers and the mine manager so that they could observe the injection. Although Mr Thompson did not on that occasion use a UV light for the purpose of aiding detection of the fluorescent dye, given that Mr Thompson agreed in oral evidence that in his conversation with Mr Withers in October 2010 he explained the use of the UV light to aid detection, the Court ought infer that Mr Thompson made the same obvious point to Mr Withers and the mine manager during the course of the demonstration. In any event, it had been disclosed previously, Fuchs submitted.
217 Fuchs submitted that what was disclosed by Mr Thompson to people at Metropolitan was also evidenced by what was disclosed contemporaneously to Fuchs. Mr Roberts gave evidence that Mr Risk of Metropolitan said the following to him in November 2010:
We are looking at introducing a dye to our longwall fluid to enable it to be detected under a worker’s skin so we can tell if there has been an injection.
…
It’s UV dye so it will glow when you shine a blue light on it. The dye will glow from under the skin. You need to wash the skin first to make sure what you see glowing is from under the skin.
218 Fuchs submitted that it was clear that Mr Risk, who reported to Mr Withers, was passing on information that Metropolitan had learned from Mr Thompson.
219 Fuchs submitted that the information disclosed, in the relevant sense, the integers of the claims of the Patents, and made detailed submissions to this effect. It was not necessary that the prior art information disclosed every integer in a claim, provided that the skilled addressee would add the missing integer as a matter of course and without the need for inventive ingenuity or undue experimentation.
220 Quaker submitted that, having regard to its evolving nature, Mr Bate’s evidence was only of assistance to the Court regarding when events occurred if it could be tied to objective material.
221 Quaker provided a summary of the findings it submitted the Court should make. Quaker submitted any disclosures of the method the subject of the Patents were part of reasonable trials which necessarily occurred in public, and were undertaken either by Mr Thompson himself, or at Metropolitan by employees there, by Mr Thompson’s initiation and with his oversight.
3.2 Disclosures by Mr Ken Risk – particulars 6(a)(viii) and 6(b)(ix)
222 Fuchs submitted that, on or about 9 November 2010 and on or about 17-18 November 2010, Mr Ken Risk (Peabody) disclosed to Mr Craig Roberts (Fuchs) the patented method during the course of oral in-person conversations, telephone conversations and emails. Mr Roberts said that Mr Risk informed him of the method of adding a “UV oil additive” called “Oil-Glo” to the longwall fluid at Metropolitan so as to enable “verification of oil injections” under persons’ skin. Based on these communications, Mr Roberts said he learnt about Mr Thompson’s patented method, and of Peabody’s intention to introduce fluorescent dye into the longwall mining operations at Metropolitan.
223 Fuchs submitted it was clear that the disclosure was for the purpose of ascertaining whether Fuchs considered it was possible to add Oil-Glo to Solcenic 3002 for the purpose of Mr Thompson’s method. Before Oil-Glo was introduced into Metropolitan’s longwall operations in early May 2011, no trials were ever done using Solcenic 3002 to which Oil-Glo had been added. All that was required was to conduct a compatibility test (ie. that the dye was compatible with Solcenic 3002 – this did not occur though because it had already been conducted successfully with Oil-Glo in mineral hydraulic fluid), followed by the completion of a risk assessment by Metropolitan (which concerned ensuring procedures were in place for the safe keeping and utilisation of the dye).
224 Again, Quaker relied primarily on grace periods in respect of this disclosure.
3.3 Novelty grace periods
225 As to the grace period provisions, Fuchs submitted that any disclosures of the invention made before 2 September 2010 would be novelty defeating. Further, any disclosures of the invention before 2 February 2011 which were not for reasonable, and necessarily public, trial, and which were made with Mr Thompson’s consent, would be novelty defeating.
226 Fuchs submitted, in relation to s 24(1)(a) and “prescribed circumstances”, that there were three requirements that must be satisfied. Fuchs submitted none was satisfied in the present case.
227 As to the first element, Fuchs submitted a “working in public of the invention” required a demonstration or other working of the invention. In the case of a method, it meant performing the method. The disclosures in the present case did not amount to this. Even Mr Thompson’s demonstration did not involve this, because there was no “possible fluid injection” (claim 1) or fluid injection and examining of a patient (claim 9).
228 Fuchs submitted that the method of the Patents as set out in the claims of the Patents was quite unusual in that it required a fluid injection, or at least a possible fluid injection, in a human: i.e. an injury. Thus claim 1 of the Standard Patent required “a possible fluid injection occurring in a patient”. Claim 9, the other independent claim of the Standard Patent, required “examining the patient”, “determining the presence of fluorescence” “wherein fluorescence indicates a hydraulic fluid injection occurring in the patient”. Likewise, claim 1 of the Innovation Patent (the only independent claim) required “a possible fluid injection occurring in the patient”. Quaker had not identified any “working in public of the invention” involving any of these integers in the relevant grace period.
229 Fuchs submitted that the second element required that the “working in public of the invention” be for the purposes of reasonable trial. This was a difficult element to address, it submitted, because there was no identified “working in public of the invention” for the reasons it advanced as to the first element, and the relevant anticipation was an oral disclosure in any event. Quaker had not identified with precision what it relied upon in this regard, or specifically what activity amounted to a working in public of the specific integers of the claims of the Patents.
230 Fuchs submitted that it appeared Quaker wished to rely upon “trials” being conducted at Metropolitan as trials of the invention. However, Fuchs submitted, they were not the relevant disclosures. Nor did they involve the working in public of the invention, because there was no fluid injury, or “possible fluid injury”.
231 Fuchs submitted that even if these difficulties were able to be overcome, the relevant trial had to be a reasonable trial of the invention. In this regard, in assessing what might be a working of the invention for the purpose of reasonable trial, it was necessary to keep in mind the nature of the invention in question, the tasks for which it was designed, and the conditions under which it was to be used.
232 Fuchs submitted that, to the extent to which there were any tests, these were tests of a particular dye (of a particular brand, being Oil-Glo) to confirm compatibility with the relevant hydraulic fluid, rather than tests of the invention. In this regard, “confirm” was the correct word. For mineral oil hydraulic fluid, Mr Thompson selected a dye that was specified as suitable for such use, and the use of the dye in his simulator confirmed what he expected to happen, namely that the dye would be compatible. He subsequently tested it in a Bobcat, which had nothing to do with any disclosures to Metropolitan, and once he finished that test he understood that his invention was likely to work in hydraulic systems in mobile machinery (i.e. oil-based systems).
233 Fuchs submitted that likewise, in relation to the introduction of dye into the fluid of the longwall, the use of dyes in longwall fluid was well-established. Quaker had been selling a fluorescent dyed product (Quintolubric) for longwalls since 1985. Mr Thompson selected something which he expected to work. Indeed, he was so confident that it would work that he placed it into the longwall fluid in a working mine even though a failure of the hydraulic fluid could have catastrophic consequences for workers working on the longwall.
234 Fuchs submitted that, as Mr Kraus confirmed, if a person was to take a licence of the Patents and use a new dye in carrying out the method of the Patents, it would be necessary to go through a process of obtaining administrative approval from a mine before incorporating that dye into mining machinery (such as the longwall). That would include a risk assessment, a change request, and so on. That would not involve testing the Patents, but rather testing the particular way in which the licensee chose to implement the Patents. Testing a particular application of an invention (method) was not trialling the invention itself.
235 Fuchs submitted that in any event, the reality was that the inclusion of Oil-Glo into the longwall at Metropolitan was not a reasonable trial at all. There was no doubt that it would work. Rather, it was a commercial implementation by the patentee of the invention, for which profits were derived. It could properly be described as a roll out of the invention.
236 Therefore, Fuchs submitted, the alleged “reasonable trial” was not a trial at all. To the extent it was a trial, it was not a trial of the invention, but rather a particular dye. The second element was not satisfied.
237 Fuchs submitted that the third element was also not satisfied. It required that because of the nature of the invention it was reasonably necessary for the working to be in public. That imported a test of necessity, Fuchs submitted. It submitted that this would be expected to be a difficult criterion to satisfy; and so it proved here.
238 First, Fuchs submitted, it was not necessary to test the product in a mine. Mr Thompson tested the Oil-Glo 33 in a Bobcat on a private farm in October 2010. Mr Thompson conducted this testing himself. As noted above, following the testing in the Bobcat, Mr Thompson confirmed that the Oil-Glo 33 was compatible with machinery including mining machinery. There was no suggestion that this testing was done in public or that it was necessary for this testing to be done in public.
239 Fuchs submitted that by the time the Oil-Glo came to be added to the mine, there was no doubt that it would operate in that mine. There was no doubt as to compatibility and no doubt about the production of fumes: this was evident from the preparedness of Metropolitan to introduce fluorescent fluid dye into longwall operations in May 2011. By that time, the mine had completed the risk assessment dated 23 March 2011.
240 Second, Fuchs submitted, there was no reason why that test had to be in public. Mine sites are private property. Confidentiality arrangements can be established. Workers can be under obligations not to disclose to others. No such approach was adopted here, but that was not because it was not possible. There were secret use cases involving mining. There was no reason for concluding that the disclosures and the subsequent use had to be open and unrestricted. The relevant legal or equitable obligation of confidence could have been imposed.
241 Fuchs submitted that the same position applied to the disclosure by Mr Risk. Although Quaker submitted that this disclosure was also made without the consent of Mr Thompson, so as to fall within s 24(1)(b), Fuchs submitted there was no obligation of confidentiality imposed on Mr Withers or the mine manager, and there was nothing preventing them from discussing the matter with Mr Risk.
242 Plainly, Mr Thompson had a willingness to discuss his method, and ran the risk that Peabody personnel might pass on details of his method to third parties such as Mr Roberts at Fuchs.
243 Therefore, Fuchs submitted, it was not the case that the disclosure was without consent.
244 Quaker submitted that the initial disclosure of the idea of the invention by Mr Thompson to Mr Withers in September or October 2010, and the subsequent demonstration of the injection of mineral oil with Oil-Glo in it into pig skin by Mr Thompson to Mr Withers and the Metropolitan mine manager in October 2010, fell within the Reasonable Trials Grace Period.
245 Quaker submitted that prior to the disclosure to Mr Withers by Mr Thompson, the invention was still entirely conceptual. While Mr Thompson had at least demonstrated to himself that Oil-Glo could be added to his simulator to make the mineral oil easier to see, that told him little about whether the claimed method would work in practice. The fluorescence was not the focus of his testing to date.
246 Quaker submitted that Mr Thompson determined that it was necessary to undertake a number of trials of the proposed invention to determine whether it would work, and determined that it was necessary to trial it in the field, including in respect of the kind of hydraulic equipment and locations at which the method might be used. While the invention was of broad application, clearly an important application for it, and one Mr Thompson was familiar with, would be at mine sites. Quaker submitted there was no basis to suggest that it was reasonable to undertake field trials of the product claims but not method claims, referring to Coretell and Australian Mud Company.
247 Quaker submitted that this was particularly so because Mr Thompson lacked the facilities or resources to trial the invention in the field, which was a relevant matter referred to in Bradken.
248 Quaker submitted that Mr Thompson could hardly get permission to carry out trials at the mine site without telling Mr Withers about what he proposed to do. Therefore, Mr Thompson told Mr Withers about his idea, and they discussed what steps would need to be taken to implement it. Such disclosure was directed towards, and related to, the carrying out of a trial in public of the invention at Metropolitan.
249 Quaker submitted that the subsequent demonstration before Mr Withers and the mine manager was of the same character. Further, it was necessary for the demonstration to be in public in order to receive the feedback of others who had to be persuaded of the idea, namely Mr Withers and the mine manager.
250 Quaker submitted, in respect of the alleged novelty-destroying disclosures by Mr Risk, that Mr Roberts’ email of 9 November 2010 to Mr Pearce was prompted by Mr Risk’s discussion of the proposal to include a dye into Metropolitan’s hydraulic systems that same day. Mr Risk would have learned of the idea from Mr Withers and ultimately from Mr Thompson, and it was unsurprising that Mr Risk then reached out to Fuchs in early November 2010.
251 Quaker submitted that, therefore, the 9 November 2010 emails were excluded from consideration with respect to novelty for at least two reasons.
(a) They were a disclosure within the scope of the Reasonable Trials Grace Period, given that they were was prompted by Mr Risk’s discussions about the need for Fuchs to confirm that Oil-Glo could be added to their Solcenic products, which would require testing to be undertaken by Fuchs before Fuchs would “sign off” on the addition of the dye.
(b) Alternatively, they were a disclosure made: (i) without the consent of Mr Thompson and TTAAS, as Mr Thompson disclosed the claimed method to Mr Withers for the purpose of himself undertaking trials of the method; (ii) based on information ultimately derived from Mr Thompson; and (iii) after 2 September 2010, and thus fell within the Unauthorised Disclosures Grace Period.
252 Quaker submitted that the 18 November 2010 and later emails and oral communications between 17 and 18 November relied upon by Fuchs were clearly excluded from an assessment of novelty as part of the reasonable trials being undertaken at Metropolitan for the same reasons.
253 As to Fuchs’ submission regarding obligations of confidence, Quaker submitted that the Reasonable Trials Grace Period was only necessary where information had been made publicly available (s 24(1) of the Act). Therefore, it could not be that the failure to restrict disclosure affected whether the disclosure was part of a reasonable trial.
Secret use
254 Fuchs’ case in relation to secret use was put as an alternative to the case on public disclosure – that is, if the disclosures were not public but were confidential. If that was so, Fuchs submitted, then the use in the mine would be confidential by parity of reasoning. If the offer or promotion and/or sale before 2 September 2011 of Oil-Glo by Quaker’s predecessors in title was not a novelty defeating anticipation of the invention the subject of the Patents, Fuchs contended that TTAAS and/or T&T engaged in and authorised acts constituting prior secret use in their dealings with Peabody (including by authorising Peabody’s use of the method) between about mid-2010 and June 2011 and subsequently through to 2 September 2011 (the priority date). The grace period exemption applicable for novelty purposes did not apply in the context of prior secret use under the relevant form of the Act for considering the validity of the Patents: s 9(e) of the Act, which extended the grace period to secret use, was added by the Intellectual Property Amendments (Raising the Bar) Act 2012. See also Intellectual Property Amendments (Raising the Bar) Bill 2011 Explanatory Memorandum, Items 28 and 29 at 117. Any secret use before the priority date (2 September 2011) would invalidate the Patents, Fuchs submitted.
255 Fuchs submitted that the rationale behind the prohibition on issue of a patent following secret use, s 18(1)(d), was that the inventor should not, by using the invention for a period of time prior to disclosing it in a patent application, derive greater advantage from it than was contemplated by the Act: Grove Hill at 313. The inquiry as to secret use essentially involved two questions: (a) had there been a “use” and (b) if so, was it “secret”? As to the first question, the word “use” was found in the definition of “exploit” in the Act. It had been said that “use” had a narrower meaning than the term “exploit”. In Azuko Pty Ltd v Old Digger Pty Ltd [2001] FCA 1079; 52 IPR 75, Gyles J described at [186] the question of “use” as involving a practical test: “Has what occurred amounted to a de facto extension of the patent term?” He then stated that the answer to this question will usually depend on whether the patentee reaped a commercial benefit from what was done before the priority date.
256 As to the second question, Fuchs submitted, the use must be “secret”. Relevant to the question of “secret” use was the extent to which the patentee’s operations (including its testing facilities) were accessible to the public, and likewise accessible to people who were free to disclose to others. Overall, it was ultimately a question of fact in each case as to whether conduct by a patentee constituted prior secret use.
257 Fuchs submitted that in the period between mid-2010 and June 2011, Peabody conducted an investigation into HPFI at its mine sites in Australia, and methods suitable for the detection of HPFI injuries. During that same period, Mr Thompson disclosed the invention the subject of the Patents to Peabody, and that method was put into use by Peabody, with the authorisation of TTAAS and/or T&T, at its Metropolitan and North Goonyella mines.
258 Fuchs submitted that Peabody’s use of the method did not involve any form of “testing” to determine whether the invention worked. In about May or June 2011, Peabody issued purchase orders to TTAAS and/or T&T for the purchase of Oil-Glo fluorescent dye for delivery to its mine sites at Metropolitan and North Goonyella. Following the placing of those orders, TTAAS and/or T&T delivered Oil-Glo products to those Peabody mine sites in around May to July 2011. This constituted a commercial purchase of the Oil-Glo for use in Peabody’s commercial operations at each of those mines, specifically for the purpose of implementing the since-patented method. This conduct by Peabody was authorised by Mr Thompson. Both TTAAS/T&T and Peabody obtained a commercial benefit from the use made of the method.
259 Quaker submitted that secret use and public use were mutually exclusive: Grove Hill at [212]; Coretell at [221]. For a use to be “secret”, there must be some “deliberate concealment as a necessary element of the process”: Bradken at [345]. Further, a use of the invention for the purpose of reasonable trial or experiment only did not amount to secret use: s 9(a) of the Act.
260 In relation to Fuchs’ reliance on Azuko, Quaker submitted that the question posed by the Full Court regarding whether the patentee brought about a de facto extension of the patent term was asked in respect of conduct which was, relevantly, secret. The concept of a de facto extension of the patent term was not a substitute for the statutory test.
261 Further, Quaker submitted, various uses of the invention on which Fuchs relied were for the purpose of reasonable trial or experiment only. Given the integrated nature of the longwall, there would be no way to trial new fluid in it other than in situ and under operation.
262 Even if that was incorrect, Quaker submitted, Fuchs’ submissions as to secret use ignored uncontested evidence that as of April 2011 at the latest, the method claimed in the invention was public. Quaker referred to an email from Mr Thompson to Peter Doyle of 19 April 2011.
263 Further, Fuchs had provided no evidence that any person supplied with Oil-Glo by Mr Thompson exploited the claimed method prior to 2 September 2011, when the provisional specification was filed. The first document recording the use of Oil-Glo to determine whether a worker had suffered an HPFI at Metropolitan, or indeed any mine site, was dated 26 October 2011, after the filing of the provisional application.
Utility (s 18(1)(c)) and Insufficiency, Clarity and Fair Basis (s 40)
4. Overview
264 Fuchs submitted that both Patents possessed deficiencies which rendered some or all of the claims of those Patents invalid on the grounds of lack of utility, insufficiency, lack of clarity and lack of fair basis.
265 Quaker submitted that these grounds of challenge to the Patents suffered from a range of difficulties. They were in part not pleaded and were based on a mischaracterisation of the claims and/or contrary to evidence or authority.
5. General principles
266 At the level of principle, Quaker submitted that claim construction was a matter for the Court. The claims should be read in a purposive manner, with a generous measure of common sense. Quaker submitted that most, if not all, of the expert evidence regarding claim construction did not assist as the experts were not drawing upon any relevant expertise in construing the claims.
5.1 Utility
267 As to utility, Fuchs submitted ss 18(1)(c) and 18(1A)(c) provided that an invention was a patentable invention if the invention, so far as claimed in any claim, was useful. An invention must achieve the result promised by the patentee in the specification, and the result promised must be useful. Such requirements applied to everything within the scope of the relevant claim.
268 Generally, Fuchs submitted, it may be of assistance to consider utility by reference to the following questions: What had the patentee promised for the invention as described in the relevant claim? Was the promise useful? Had that promise been met? Fuchs referred to Meat & Livestock Australia Ltd v Cargill, Inc [2018] FCA 51; 354 ALR 95 at [821].
269 Fuchs provided the following additional references. A claim may lack utility if an additional integer or integers needs to be added: Rescare Ltd v Anaesthetic Supplies Pty Ltd (1992) 25 IPR 119 at 143. As there stated, a distinction was to be drawn between cases where the invention claimed was not useful unless an additional integer or integers be added (such claims being invalid) and those cases where qualifications and expedients necessary to make work the article which had been claimed can be, and are left to the skilled reader to supply for himself. Esco Corporation v Ronneby Road Pty Ltd [2018] FCAFC 46; 131 IPR 1 emphasised two matters: first, the question of what are the promises of the invention is a matter of construction of the specification as a whole and, secondly, it is necessary to consider the question of inutility on a claim by claim basis. In Inverness Medical Switzerland GmbH v MDS Diagnostics Pty Ltd [2010] FCA 108; 85 IPR 525 at [117] Bennett J observed that all within the scope of a claim must be useful if the claim is not to fail for want of utility, so that a claim was bad if it covered something that would not produce the desired result, even if a skilled person would know which means to avoid it. A claim will fail for inutility if within its scope there is subject matter claimed which will not achieve the desired result: Norton and Gregory Ltd v Jacobs (1937) 54 RPC 271 at 275 and 276. Norton was discussed and applied in Austal Ships Pty Ltd v Stena Rederi AB [2005] FCA 805; 66 IPR 420 at [227] and [230].
270 Fuchs further submitted that, even if the claims of the Innovation Patent complied with s 18(1A)(c) read alone, they failed to comply with the additional requirement imposed by s 7A.
271 Turning to Quaker’s submissions, Quaker accepted that certain parts of the post-Raising the Bar version of the Act applied to the Innovation Patent, and therefore the Innovation Patent must meet the requirements of the new s 7A of the Act, in addition to s 18(1A)(c).
272 Quaker submitted that the relevant Explanatory Memorandum stated that s 7A was intended to align Australian law with that in the United States, and referred to the meaning of the constituent terms used in s 7A by reference to United States authority.
273 Quaker submitted that claims limited by result were “necessarily useful since it is claimed in terms of the result said to be achieved”: Interlego AG v Toltoys Pty Ltd (1973) 130 CLR 461 at 472. By reference to SNF (Australia) Pty Ltd v Ciba Specialty Chemicals Water Treatments Ltd [2011] FCA 452; 92 IPR 46, Quaker submitted that: the usefulness of an invention was a question of fact; the onus of proof rested solely upon Fuchs as the party challenging validity; and the patent specification did not need to positively establish the utility of the invention.
274 Quaker made submissions about the insufficiency of speculative or theoretical evidence to establish inutility. Quaker submitted that a claim may have utility even if a promised advantage cannot be achieved in all cases or with the same degree of success, referring to Rescare Ltd v Anaesthetic Supplies Pty Ltd (1992) 25 IPR 119 at 142-3. Close attention should be paid to any “promises” said to be conveyed, and unless the specification clearly asserted that the invention would achieve a particular outcome across the breadth of the claims, inutility will not be demonstrated by showing that in some cases that outcome would not be achieved, referring to Austal Ships at [254]-[257]; Washex Machinery Corporation v Roy Burton & Co Pty Ltd (1974) 49 ALJR 12 at 19 per Stephen J; and Rescare Ltd v Anaesthetic Supplies Pty Ltd (1992) 25 IPR 119 at 142 per Gummow J. Section 18(1)(c) did not require an invention to be useful, or even practicable, in a commercial sense: Advanced Building Systems Pty Ltd v Ramset Fasteners (Aust) Pty Ltd [1998] HCA 19; 194 CLR 171 at [24].
275 Quaker submitted that there were limits on the principle that everything within the scope of a claim must be useful. If qualifications and expedients necessary to make the invention work were left to the reader to supply, that did not equate with inutility: Blanco White T A, Patents for Inventions (4th ed, Stevens, 1974), § 4-408, cited in AB Hassle v Alphapharm Pty Ltd [1999] FCA 628; 44 IPR 593 at [227] per Lehane J; WM Wrigley Jr Co v Cadbury Schweppes Pty Ltd (2005) 66 IPR 298 at [138]; Coopers Animal Health Australia Ltd v Western Stock Distributors Ltd (1986) 6 IPR 545 at 572.
5.2 Sufficiency
276 As to sufficiency, Fuchs submitted that s 40(2)(a) of the Act (at the relevant time) required that the specification of a complete application must “describe the invention fully, including the best method known to the applicant of performing the invention”. The requirements of sufficiency and best method were separate requirements. The test for sufficiency was that stated by the High Court in Kimberly-Clark Aus Pty Ltd v Arico Trading International Pty Ltd [2001] HCA 8; 207 CLR 1 at [25]. The specification must disclose sufficient information to enable the PSA to perform the invention: Samuel Taylor Pty Ltd v SA Brush Co Ltd (1950) 83 CLR 617 at 623-4 per Latham CJ.
277 Warner-Lambert Co LLC v Apotex Pty Ltd [2018] FCAFC 26; 129 IPR 205 had the potential to mislead if not understood in the context in which the issue arose in that case, including in relation to a separate process that might be required before a clinical application of the compound in question is made. Fuchs relied on that judgment at [123]-[130].
278 Fuchs submitted that the pre-RTB form of s 40(2)(a) required that a complete specification “describe the invention fully”. This had, for many years, had two overlapping but distinct aspects. A specification “describes the invention fully” if “[(a)] it makes the nature of the invention plain to persons having reasonably competent knowledge of the subject and [(b)] also makes it plain, to persons having reasonable skill, how to perform the invention”: Patent Gesellschaft AG v Saudi Livestock Transport and Trading Company (1997) 37 IPR 523 at 530. The “invention” for the purpose of s 40(2) was “the embodiment which is described, and around which the claims are drawn”: Kimberly-Clark at [21].
279 Fuchs further submitted that, even if the claims of the Innovation Patent complied with the unamended “pre-RTB” form of s 40(2)(a), they failed to comply with the applicable and more onerous requirements of the amended form of s 40(2)(a).
280 Quaker submitted the best method of performing an invention need not be claimed in the claims, it merely needed to be referred to at some point in the specification: PhotoCure ASA v Queen’s University at Kingston [2005] FCA 344; 64 IPR 314 at [116].
281 Quaker submitted s 40(2)(a), as applicable to the Standard Patent, provided that a complete specification must describe the invention fully. On the Kimberly-Clark test, the patent specification need only enable the performance of a single embodiment within each claim.
282 Quaker submitted the specification was to be read in the light of the common general knowledge and the art before the priority date: Kimberly-Clark at [24]. The hypothetical addressee should be taken to know the basic principles of patentability.
283 Quaker submitted that insufficiency will not be made out if the work required to implement the invention was routine in character, having regard to the nature of the field, even if the steps involved were difficult, expensive and time-consuming: Warner-Lambert Co LLC v Apotex Pty Ltd [2018] FCAFC 26; 129 IPR 205 at [99]-[136].
284 Quaker submitted the new form of ss 40(2)(a) and 40(3) raised difficult questions. Encompass Corporation Pty Ltd v Infotrack Pty Ltd [2018] FCA 421; 130 IPR 387 made clear that it was simplistic merely to assert that the new ss 40(2)(a) and 40(3) “strengthen” or makes “more onerous” the previous requirements. Quaker submitted that the Court should reject Fuchs’ challenge to the Innovation Patent based on the new requirements given Fuchs advanced no submissions by reference to the requirements of the new form of the provisions. In any event, the evidence did not establish that the specification did not disclose the invention in a clear enough and complete enough manner for the invention to be performed by a skilled person, or did not support the claims, or did not provide an “enabling disclosure” in the relevant sense.
5.3 Clarity
285 As to lack of clarity, Fuchs submitted s 40(3) (at the relevant time) required that the claims “must be clear and succinct”, referring to Meat & Livestock at [932]-[937] per Beach J.
286 Fuchs submitted that a claim which contained an irresolvable ambiguity or uncertainty, and as a result was fairly open to two or more meanings, was liable to be revoked on the ground of lack of clarity: Freeman v TJ and FL Pohlner Pty Ltd (1994) 30 IPR 377 at 381-2.
287 Quaker submitted that a lack of precise definition in claims was acceptable so long as they provided a workable standard suitable to the intended use: Minnesota Mining & Manufacturing Co v Beiersdorf (Aust) Ltd (1980) 144 CLR 253 at 274. Further, “any purely verbal or grammatical question that can be resolved according to ordinary rules for the construction of written documents, does not, once it has been resolved, leave uncertain the ambit of the monopoly claimed”: Welch Perrin & Co Pty Ltd v Worrel (1961) 106 CLR 588 at 610.
288 Quaker’s submissions as to the new form of s 40(3) are addressed at [284] above.
5.4 Fair basis
289 As to fair basis, Fuchs submitted that s 40(3) (at the relevant time) required that the claims must be fairly based on the matter described in the specification. The test of fair basis was set out by the High Court in Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (No 1) [2004] HCA 58; 217 CLR 274 (Lockwood (No 1)) at [69].
290 Fuchs submitted that the existence in the body of the specification of a consistory statement that was worded identically to a claim did not necessarily mean that the claim was fairly based on that specification: Lockwood (No 1) at [87]. In the present case, it was therefore necessary to consider the specification as a whole, not just some coincidence of language shorn of context.
291 Fuchs further submitted that, even if the claims of the Innovation Patent complied with the unamended “pre-RTB” form of s 40(3), they failed to comply with the applicable and more onerous requirements of the amended form of s 40(3).
292 Quaker relied on the statement of Barwick CJ in Olin Corporation v Super Cartridge Co Pty Ltd (1977) 180 CLR 236 at 240, approved in Lockwood (No 1) at [57]. This emphasised that the essential question was one of breadth.
293 Quaker submitted that the claims need not be restricted to precise embodiments described in the specification: Sartas No 1 Pty Ltd v Koukourou & Partners Pty Ltd (1994) 30 IPR 479 at 497 per Gummow J, upheld in Leonardis v Sartas No 1 Pty Ltd (1996) 67 FCR 126 at 143. A “consistory clause” in the specification describing the invention in similar terms to a claim may provide fair basis for that claim, provided the balance of the specification was not inconsistent with that description: eg, Lockwood (No 1) at [38], [72], [91]-[93], [99]-[101].
294 Quaker’s submissions as to the new form of s 40(3) are addressed at [284] above.
6. Contentions
6.1 Detection step
295 Fuchs submitted that claim 1 of the Standard Patent claimed a method of detecting fluid injection in a patient. However, none of the steps provided in the method included a step of detecting the presence of the fluid. The step of detecting the presence of the fluid by providing an ultraviolet light was added in claim 2 of the Standard Patent.
296 Fuchs submitted that if there was to be a detection step then it had to be read in as a matter of construction of the claim language. It was not present simply because the method was said to be a “method for detecting fluid injection”: that was the result. Dr Gray did not identify the words that were said to import the step. The requirement that the fluid “fluoresces in the presence of ultraviolet light” was not a step of detection, but a statement that the fluid will fluoresce in the presence of UV light. The claim did not say where, or in what circumstances, the fluid will fluoresce. By contrast, claim 2 required the detection of the presence of fluid. Mr Smith regarded claim 1 as deficient by reason that it omitted any reference to a detection step. Fuchs submitted Mr Smith’s construction better reflected the language of the claim.
297 Fuchs submitted that the lack of a detection step meant that claim 1 of the Standard Patent was invalid for lack of utility, clarity and fair basis. It lacked utility because it claimed a method for detecting fluid injection in a patient but failed to provide a detection step. It lacked clarity because, in the absence of a detection step, it was not clear in the context of the specification as a whole how fluid injection was to be detected. It was not fairly based because the only detection step disclosed in the specification for the Standard Patent was detection of fluid using ultraviolet light: to the extent claim 1 was construed by the Court to include some other form of detection, claim 1 was not fairly based on the matters described in the specification because there was no real and reasonably clear disclosure in the body of the specification of any other detection step other than through use of an ultraviolet light.
298 Quaker submitted that Mr Smith’s evidence was based on a reading of claim 1 that was contrary to common sense, which became apparent in his cross-examination. The PSA, employing the method of claim 1, would as a matter of course include a step of detecting whether or not a fluid injection occurred. By the term “including”, claims 1-6 did not exclude steps not to referred to. Claims 1-6 were limited by result and anything which did not constitute a method for detecting a fluid injection was not captured by them.
299 Quaker submitted that claim 7 did not “introduce” a step of determining whether a fluid injection had occurred, but was a more specific version of the invention claimed in claim 1. It did not suggest that previous claims, including claim 1, did not require that step.
6.2 Promise of the invention
300 Fuchs submitted that the promise of the invention, as identified in the specifications, was to provide a method to detect and confirm whether a fluid injection had occurred in a patient. Both experts agreed that the method claimed in the Patents could not be used completely to rule out whether a fluid injection had occurred. In the context of an invention that was concerned to provide enhanced workplace safety, any chance that the method might produce a false negative (which could result in severe harm to a worker who did not receive treatment as a result of the false negative) defeated the purpose of the invention, Fuchs submitted. The method would not constitute a diagnostic tool. The method would be unreliable.
301 Further, Fuchs submitted, the evidence showed that implementation of the invention provided no practical difference in the outcome for a person with a suspected fluid injection injury: she/he will ultimately be sent for medical treatment. Both experts agreed that even if the method claimed in the Patents was followed, it would be prudent to send the patient for medical treatment. That step, they said, ought to occur even if there was an absence of fluorescence at the injection site at that time. Mr Smith’s evidence was that protocols used on industrial sites and mine sites recommended immediate specialist medical assessment and/or treatment be sought in the event of a suspected fluid injection injury. Mr Smith’s evidence was confirmed by two fluid injection protocols in evidence.
302 Quaker submitted the specification disclosed that by following certain steps it was possible to determine that a fluid injection had not occurred. Given those steps, which included examining a patient, washing the patient, and then examining the patient again after washing, one could determine that a fluid injection had not occurred because of the absence of any fluorescent dye.
303 Quaker submitted that Dr Gray considered it difficult to imagine circumstances where hydraulic fluid containing a fluorescent dye would not leave some trace of fluorescence if it had penetrated the skin. Mr Smith’s evidence was based on propositions he had retreated from as to whether fluorescent fluid under the skin could be washed away or would fluoresce.
304 Quaker submitted that Fuchs’ submission that anyone suspected to have suffered an HPFI would go to hospital anyway relied on a procedure constituting only one embodiment of the invention. Further, fluid injection protocols would vary from location to location. Mr Smith could not possibly speak to all locations in all relevant industries, nor did he. Even in the mining context, there was evidence that not all protocols required those suffering potential HPFI injection to be taken to hospital. Metropolitan did not invariably send workers to hospital. Glencore’s draft procedure was drafted before it acquired dyed products from Fuchs and was used by mine sites which, on Fuchs’ case, did not use the claimed method.
6.3 Washing requirement
305 Fuchs submitted that claims 1-5 and 9-15 of the Standard Patent, and claims 1-3 of the Innovation Patent, did not require any step of washing or examination after washing. Fluorescence could not be used to determine whether a possible fluid injection beneath the skin had occurred where the fluid carrying the fluorescent dye remained on the surface of the skin. That was because there was no way of distinguishing between dye detected which was on the surface of the skin and dye detected beneath the skin. Dr Gray accepted that to “detect” whether there was a fluid injection you would need a separate step of working out whether the fluid had penetrated beneath the skin of the patient which involved additional steps such as washing.
306 Fuchs submitted that the method defined in the claims would not work over the full scope of those claims, as they encompassed the examination of patients prior to, or without any, washing of the skin, and accordingly, claims 1-5 and 9-15 of the Standard Patent, and all of the claims of the Innovation Patent lacked utility.
307 Quaker submitted that Fuchs’ submission was based on the flawed premise that claims which did not refer to washing excluded washing. Further, there was no evidence that a worker would inevitably be splashed with fluid which needed to be washed off, or that the invention would have no utility in determining a fluid injection had not occurred where there was no fluid or fluorescence present on the patient at all.
6.4 Dye concentration issues
308 Fuchs submitted that claims 1-19 and 22 of the Standard Patent, and claims 1-3 of the Innovation Patent, did not specify any particular concentration at which the fluorescent dye was to be added to the fluid to enable a fluid injection injury to be detected. Dye concentration levels were only introduced in claims 20 and 21 of the Standard Patent. The dye concentration levels provided in claims 20 and 21 were based on tests of Oil-Glo in particular hydraulic fluids.
309 Fuchs submitted that Mr Smith’s evidence was that adding any amount (however small) of fluorescent dye to a fluid, for fluorescence in the presence of ultraviolet light (as required by the claims), was not sufficient to enable detection of fluid injection injuries. Fluorescent dye needed to be added at a concentration such that the fluorescence was visible from beneath the skin. Dr Gray accepted that it would be necessary to establish what concentration of fluorescent dye was necessary to be able to see the fluid fluoresce under the skin. His evidence was that this would involve more than some visual testing in a laboratory to achieve a similar level of fluorescence to the Oil-Glo in the hydraulic fluid at the relevant concentration given in the Patents. Additional testing would need to be done to any new product to make sure that it will fluoresce under a range of different conditions. He accepted this would involve some level of research work, which would be more than trivial but, as he was not a medical professional or a physicist, he said he could not comment on how much work would need to be done.
310 Fuchs submitted that Dr Gray said that the concentration levels of Oil-Glo disclosed by the examples in the Patents were unclear in two respects. First, it was unclear to what the Oil-Glo was being added. Second, it was unclear as to what was being referred to as 100% Solcenic at page 8 of the Standard Patent and the Innovation Patent. Fuchs submitted the same issues arose in relation to the hydraulic oil example given at page 7 in each Patent. The concentration levels disclosed were not shown to provide detectable fluorescence under the skin. Figures 8a, 8b, 9a and 9b only showed fluorescence in a beaker.
311 In relation to claims 20 and 21, which referred to particular minimum concentrations, the difficulty with these claims was that different dyes have different properties. Dr Gray said that stating a particular concentration may be “misleading”, because it depended upon what was being used as the dye, and therefore the concentrations in claims 20 and 21 would give him “a starting point only” which would require further research to apply.
312 It followed, Fuchs submitted, that claims 1-19 and 22 of the Standard Patent and claims 1-3 of the Innovation Patent lacked utility, clarity, sufficiency and fair basis. As to utility, the claims encompassed the use of fluids in the method claims which had concentrations of fluorescent dye too low to be detected under a patient’s skin, and therefore could not be used to determine whether a fluid injection has occurred. As to clarity, it was not clear in the context of the specification as a whole what concentration of fluorescent dye must be present in the fluid for a possible fluid injection to be detected. As to sufficiency, the claims did not state a workable concentration level required for the dye without the need for the PSA to undertake an extensive research program. As to fair basis, the claims were not limited to a particular concentration, or to a particular concentration range, in the fluid, and there was no real and reasonably clear disclosure in the body of the specification of an invention in that form.
313 Fuchs submitted that claims 20 and 21 of the Standard Patent, which each stated a concentration level, also lacked sufficiency. This was because different fluorescent dyes have different properties meaning the skilled addressee would understand that concentrations based on Oil-Glo, like those set out in claims 20 and 21, could not simply be applied to a different fluorescent dye and would merely be starting point for further research. Such research would also need to take into account further variables such as the properties of the hydraulic or other industrial fluid to which the fluorescent dye was to be added and the nature and intensity of the UV or blue light that was to be used in detecting fluorescence. Even using Oil-Glo, the concentration levels disclosed in the specifications of the Patents would not enable the invention to be performed without extensive further research because of the deficiencies in the disclosures in the specification regarding the testing done with Oil-Glo.
314 Quaker submitted that the short answer on this issue was that there was no evidence that the PSA would be unable, based on the specification and routine trial and experimentation, to determine the necessary dye concentration in hydraulic fluid to work the invention. The PSA could include a large amount of dye to make sure that it would readily be visible, or take the concentration of Oil-Glo in mineral oil as specified in the patent and use an alternative dye at varying concentrations until it matched in intensity. Mr Gray said he would defer to others such as medical experts as to the extent of experimentation required, and there was no evidence from those PSAs. Finally, the appropriate concentration of dye would vary depending on the dye and hydraulic fluid, so was not a matter which the Patents needed to specify with certainty.
315 With respect to fair basis and best method, Quaker submitted it was enough that the Patents specified a concentration with respect to Oil-Glo in mineral oil, being one embodiment of the invention.
Infringement
316 Quaker submitted that Fuchs infringed the Patents by supplying certain of Fuchs’ customers with longwall fluid and mineral oil products containing fluorescent dye, where those products, and the dye, had not been acquired from the patentee. In summary, Quaker alleged that Fuchs: (a) had reason to believe that the products it sold would be used by its customers in the method claimed in the Patents, and/or (b) induced, authorised or procured its customers to use those products in the method claimed in the Patents.
General principles
317 Quaker submitted that s 117(2)(b) of the Act applied where the supplier of a product (not being a staple commercial product) had reason to believe that the person supplied would put the product to an infringing use. Section 117(2)(c) applied where the supplier provided instructions or inducements for a product to be put to such a use. In either case, by reason of s 117(1), the act of supply was deemed to be an act of infringement.
318 Quaker submitted that the infringing use of a product referred to in s 117(1) included the use of a product as part of a patented method. Given the use of the word “would”, it was not necessary to prove that any of Fuchs’ customers had, in fact, carried out the claimed method. For this purpose, “supply” of a product included an offer to supply. The test of “reason to believe” in s 117(2)(b) was objective.
319 The parties agreed that infringement by authorisation or joint tortfeasance required there to be a primary infringement.
320 Quaker submitted that the concept of authorisation was of the same scope as that used in copyright law. It would be relevant to consider whether the alleged authoriser sanctioned, approved or countenanced infringement, whether they had the power to prevent it but did not do so, and whether they purported to license the conduct of the infringer.
321 In respect of joint tortfeasance, Quaker submitted, the principles were summarised by French J in Collins v Northern Territory [2007] FCAFC 152; 161 FCR 549 at [24]-[30]. Infringement by joint tortfeasance would also occur where parties acted in concert in a manner which brought about an infringement, Quaker submitted.
322 Fuchs submitted that s 117(2)(b) required “a reasonable belief of the likelihood that a person put a product to that [infringing] use”. The likelihood was not “may”, but “would”. The test required all relevant circumstances to be considered: Generic Health Pty Ltd v Otsuka Pharmaceutical Co Ltd [2013] FCAFC 17; 100 IPR 240 at [106].
Contentions
7. Preliminary and general contentions
323 By way of overview, Quaker submitted that from August 2012, Fuchs incorporated fluorescent dye additives, initially sourced from the patentee, into its Solcenic and Renolin products for the purpose of enabling the products to be used HPFI detection. However, from 2014 Fuchs offered to supply, or did supply, hydraulic fluid products mixed with fluorescent dye additives not sourced from TTAAS or Quaker to BMA (at Broadmeadow), Glencore (at Ulan No 3, Ulan West, Bulga and Oaky North), and Yancoal (at Moolarben and Ashton). Those supplies were in circumstances where Fuchs had reason to believe its customers would use, or Fuchs induced its customers to use, the products in a method claimed in the Patents.
324 Quaker submitted that it was highly relevant that Fuchs had earlier included Oil-Glo and FluidSafe in its products for the purpose of HPFI detection. Additionally, Quaker submitted the documents, and the evidence of Mr Knight, disclosed the lengths to which Fuchs went to disguise its infringing conduct. This included characterising its marketed uses for the products as being the detection of leaks in machinery, as opposed to HPFI detection. A number of these documents were only recently provided to Quaker, because they were the subject of an unsuccessful claim by Fuchs for legal professional privilege.
325 Quaker submitted that Fuchs’ assertion that it had no reason to believe its products (other than as supplied to Broadmeadow) would be used in the claimed method by reason of the low concentration of fluorescent dye in them was contrary to the view of Fuchs at the time as expressed in internal correspondence at the highest levels within the business.
326 Quaker contended that, prior to 2012, Mr Thompson, Mr Bernie Michaelson (Manager – Engineering Services) and Mr Wayne Pearce (Product Manager – Mining) of Fuchs had been in regular contact in respect of TTAAS’ Oil-Glo fluorescent dye, which it was promoting for mixing in hydraulic fluids to assist in the detection of HPFI injections by the claimed method. In February 2012, Mr Thompson sought to have Fuchs premix Oil-Glo into longwall and mineral oil products that Fuchs was supplying to certain customers, and expressed an interest in working with Fuchs exclusively. To that end, in early 2012, Fuchs acquired samples of Oil-Glo OB (oil based) from TTAAS in order to test the compatibility of it with Fuchs’ products (Fuchs having previously received Oil-Glo WB, water based, to test).
327 Quaker submitted that it was apparent from a Fuchs email dated 13 March 2012, sent from Mr Pearce to global representatives of Fuchs, that by that time Fuchs considered Oil-Glo to be a strategic threat to its business. The email noted that the dye was effective in detecting HPFI, and there was significant and growing demand for it at Australian mines. Mr Pearce was concerned both because of the increase in cost of products Fuchs would be supplying to its customers, and because Quaker at that time was supplying a product containing dye, which could potentially be used to detect HPFI, without purchasing it from TTAAS. Later internal Fuchs emails expressed similar concerns.
328 Quaker submitted that, over the course of the period from 2012 until 2018, Fuchs received, from a variety of mine sites owned by a variety of customers, dozens of requests for information regarding its ability to supply hydraulic products including a fluorescent dye for the purpose of detecting HPFI. Those requests demonstrated that Fuchs was well aware of the widespread knowledge and demand within the mining industry for use of fluorescent dyes in hydraulic fluid in accordance with the claimed method. A table of alleged HPFI requests was annexed to Quaker’s submissions, and is set out at [421] below with Fuchs’ responses.
329 Prior to mid-2014, Quaker submitted, Mr Thompson and TTAAS had a good working relationship with Fuchs. As well as supplying Oil-Glo and FluidSafe to Fuchs, Mr Thompson and Fuchs employees shared sensitive commercial information and TTAAS offered Fuchs discounts on Oil-Glo and FluidSafe products. Mr Thompson was in regular contact with Mr Pearce, shared information with Fuchs about TTAAS litigation strategy against Quaker, and sought to supply Oil-Glo and FluidSafe exclusively through Fuchs. However, the relationship deteriorated arising from Fuchs’ offer to add fluorescent dye not purchased from TTAAS to Renolin products it was supplying to Ulan, Quaker submitted.
330 Quaker submitted that, following a fall-out leading to TTAAS issuing a letter of demand to Fuchs in mid-2014, and in particular following the sale of the Patents to Quaker on 12 December 2014, Fuchs decided to adopt a very different tone towards FluidSafe, and the method claimed in the Patents. In correspondence with customers from 2014 or 2015, Fuchs sought to pooh pooh and discredit FluidSafe. In cross-examination, Quaker submitted, Mr Roberts repeatedly offered up what was evidently a rote speech about the technology not having been proven to work, which was not responsive to the questions he was being asked. Fuchs’ attempts to discredit in the industry a technology designed to protect workers, a technology it had accepted worked, reflected poorly on its and its employees’ credit and credibility, and were consistent with its attempts to hide its infringement of the Patents, Quaker submitted.
331 Quaker noted the absence of any evidence from Mr Pearce, Mr Michaelson or Mr Wayne Hoiles in these proceedings.
332 Quaker submitted that Mr Pearce was a central, if not the central, figure within Fuchs regarding FluidSafe and the method underlying the Patents. He also led the development of Fuchs’ alternative fluorescent dyes from 2014 onwards, and was integral as a senior customer contact for both Glencore and BMA at Broadmeadow and Yancoal. He presented to Broadmeadow regarding what became Solcenic GM20 LD in January 2016, and to Oaky North the next month in what Quaker alleged was an offer of supply infringing s 117 of the Act. Mr Pearce had been at Fuchs throughout the relevant period, and remained at Fuchs as at the hearing in a senior role.
333 Quaker submitted that Mr Michaelson was a central customer contact, and was in control of the underground coal mining arm of Fuchs’ business at least until 2014.
334 Quaker submitted that Mr Hoiles was the Managing Director of Fuchs throughout the entirety of the relevant period, was responsible for the decision to proceed with the supply of Solcenic with dye to Broadmeadow for the purpose of HPFI detection, and was responsible for giving instructions in respect of: (a) Fuchs’ patent attorneys’ response to Quaker in July 2016; (b) Fuchs’ defence in these legal proceedings; and (c) Fuchs’ decision to assert privilege in respect of certain documents the subject of the privilege dispute between Quaker and Fuchs.
335 Quaker submitted that Fuchs had provided no explanation as to why any of Mr Pearce, Mr Michaelson and Mr Hoiles had not been called to give evidence in the proceedings. Although Quaker accepted that Mr Michaelson was no longer an employee of Fuchs, this did not mean he could not have been called by Fuchs. Further, and in any event, the evidence was that Mr Pearce and Mr Hoiles remained at Fuchs.
336 Given the above matters, Quaker submitted, the Court could readily draw an inference that the evidence of Mr Pearce, Mr Michaelson and Mr Hoiles would not have assisted Fuchs, particularly in respect of key events for which there was no direct evidence before the Court which would be particular to those witnesses’ knowledge. These events include Mr Pearce’s and Mr Michaelson’s oral conversations with key Glencore personnel such as Andrew Esdaile, and Mr Pearce’s conversations when presenting regarding fluorescent dye to the Broadmeadow and Oaky North mine site in January and February 2016. Indeed, where there was doubt or ambiguity in respect of a matter based on the evidence in respect of which Mr Pearce, Mr Michaelson or Mr Hoiles could have given evidence, any such doubt or ambiguity should be resolved against Fuchs, that is, it should not receive the benefit of the doubt.
337 Quaker submitted that the fact that Fuchs did not bear the onus of proof with respect to infringement was immaterial; it was material that it was within Fuchs’ power to rebut inferences to be drawn from evidence (or absences of evidence).
338 Fuchs made the following general submissions in relation to infringement.
339 Fuchs submitted the addition of fluorescent dye to hydraulic fluid may be useful for other purposes not the subject of the Patents, including to enable workers to detect leaks of fluid that could otherwise be difficult to see, particularly underground.
340 Fuchs submitted that indirect infringement of any broad claim of either Patent did not mean there was also indirect infringement of any narrower claim. Quaker must establish that the requirements of s 117 (or the authorisation case or the common design case) were met with regard to all of the features of a claim. Quaker had failed to articulate how each of the dependent claims of its Patents might be indirectly infringed, or indeed how each of those claims might be valid if the Court were to find that broader claims were invalid.
341 In respect of the aspects of Quaker’s case based on s 117(2)(c), Fuchs submitted that it was only ever reactive to requests for any dye. This included the case of Broadmeadow. The fact that the customer was specifying the need for HPFI detection made it clear that the customer was not being instructed or induced. On the contrary, Fuchs merely responded to a request for a fluid suitable for HPFI detection and provided sufficient information to BMA to convey to it that the product could be used for that purpose if it wished. The comparison of Fuchs’ product with FluidSafe was merely to convey such information and could not be construed as an instruction. As to the significant “undercutting” of the cost of supplying Solcenic GM20 with FluidSafe, if this was any form of inducement, it was an inducement for BMA to purchase the product, not to use the product in accordance with the patented method. Nor, Fuchs submitted, could some inference be drawn that Mr Pearce provided an instruction or inducement. It was clear from the documents that Mr Pearce’s concern, expressed more than once, was that the customer wanted the product for a particular purpose and he thought that the response being provided was too non-committal as to whether it was suitable for that purpose. This was the subject matter of any discussion, Fuchs submitted.
342 Furthermore, Fuchs submitted, since Quaker contended the express terms of any instructions did not reflect Fuchs’ true intentions or beliefs as to likely use of the Fuchs products by its customers, the s 117(2)(c) case conflicted with the s 117(2)(b) case. Fuchs’ express instructions to customers (as to suitability for leak detection only) could not support a s 117(2)(c) case. Quaker could only advance its case that Fuchs engaged in “disguising” conduct via s 117(2)(b).
343 In respect of the aspects of Quaker’s case concerning s 117(2)(b), Fuchs made the following general submissions.
344 Fuchs submitted it had never supplied fluorescent fluid additives per se, ie, not mixed into other fluid products. Fuchs accepted that products containing fluorescent fluid additives may be capable of being used in a method of detecting fluid injection in a person caused by injection of fluid from machinery.
345 Since August 2012, Fuchs submitted it had on occasion supplied products mixed with FluidSafe, which was with the implied licence of the patentee and so could not constitute infringing conduct. Since around August 2015, Fuchs had also on occasion supplied hydraulic fluid products containing third party fluorescein dyes. Prior to any such supply, Fuchs confirmed that the purpose of the supply was for leak detection only and was not for fluid injection detection. (The position at Broadmeadow was addressed separately.)
346 Fuchs submitted that Quaker’s contention that numerous Fuchs documents and communications were fake and designed to create a false impression was extraordinary, not supported by any evidence, and untenable.
347 Further, Fuchs submitted, Quaker had adduced no evidence of any acts of primary infringement by any Fuchs customer.
348 Fuchs submitted that the concentrations of dye required for HPFI detection and leak detection were very different. The concentration of fluorescent dye in fluid provided by Fuchs for leak detection was 22.5 times less than the equivalent amount of FluidSafe directed by Quaker to create fluids suitable for use in HPFI detection: the product suitable for leak detection was not suitable for HPFI detection, Fuchs submitted.
349 In mid-2013, Glencore sought information as to whether dye could be added to longwall fluids for leak detection. Fuchs’ position was summarised in an email from Mr Pearce of Fuchs to Mr Mackenzie of Glencore of 6 September 2013. It was clear that Fuchs was offering the customer a choice: to have solution suitable for HPFI detection with the FluidSafe dye sourced from TTAAS at higher cost, or to have a much more diluted dye suitable for leak detection only, at lower cost. Fuchs’ internal documents likewise recorded the distinction.
350 Fuchs submitted that claims 1 and 9 of the Standard Patent, and claim 1 of the Innovation Patent, required that there be a “possible” fluid injection injury for the purposes of the claimed invention. That is, the claims required that there be a prospect that fluid injection injuries will occur: that prospect must be realistic. While Mr Knight believed that, at Broadmeadow, the high concentration fluorescent dye supplied was for HPFI detection, the evidence did not establish (whether for Broadmeadow or any other mine) that the patented method would in fact be performed. That was important because if there was no real or likely prospect that the patented method would be performed, that had a bearing on the question of whether Fuchs had “reason to believe”. The evidence was that HPFI injuries were especially rare.
351 Fuchs submitted that, despite the vigour of Quaker’s assault on the credibility of Mr Knight and Mr Roberts, each of them responded to questions in a considered and helpful manner. Their oral evidence was candid, and each made concessions when that was warranted and appropriate. Their evidence ought to be accepted as reliable and honest, Fuchs submitted.
352 As to the non-Broadmeadow mines, Fuchs submitted, first, that Fuchs was supplying leak detection products, which had different characteristics from HPFI products. Second, Fuchs acted at all times consistently with the conservative approach taken by Australian mines to the introduction and use of products at mine sites. Third, Quaker’s approach ignored the development of the approach adopted by Fuchs in its business over time. Fourth, in cross-examination it was not suggested to Mr Knight that the sales data record he was taken to, which distinguished between the leak detection and HPFI products, was other than what it appeared to be on its face. Fifth, Quaker’s case that Fuchs deliberately falsified documents and concealed the true position was never put to Mr Knight in cross-examination. Sixth, Quaker focused on statements made in a particular circumstances of BMA’s Broadmeadow mine, which were exceptional. The mass of internal email communications relating to Broadmeadow was not repeated with respect to any other Fuchs customer, nor was this put to Mr Knight.
353 Fuchs submitted that Jones v Dunkel inferences should not be drawn against Fuchs by reason of its failure to call Mr Pearce, Mr Hoiles and Mr Michaelson, because there was no gap in the evidence and no basis for drawing them in the other evidence. The failure was not unexplained: Quaker suggested for the first time at the trial that Fuchs’ documents were false or self-serving.
8. “Staple commercial product” issue
354 Fuchs submitted that s 117(2)(b) would not apply if fluorescent fluid additives and/or products containing fluorescent additives were staple commercial products. It submitted that Quaker bore the onus of establishing that the Fuchs products, and fluorescent fluid additives, were not staple commercial products. The phrase “staple commercial product” should not be given a narrow meaning. A staple commercial product was a product supplied commercially for various uses: Northern Territory v Collins [2008] HCA 49; 235 CLR 619 at [41] and [50] per Hayne J and [145] per Crennan J, Heydon J agreeing. Another relevant criterion may be whether the product was traded for use in various ways. Fluorescent dyes such as the fluorescein added by Fuchs to its longwall and mineral hydraulic oil products were quintessentially staple commercial products. Fluorescein had a wide array of uses, including in microscopy, in dye lasers, in forensics and serology to detect blood stains, and as a tracer for fluids (such as in leak detection). To the extent that Fuchs supplied fluorescein mixed into its longwall fluids or mineral hydraulic oils, those fluids merely acted in this context as carriers for the fluorescein and the supply remained a supply of fluorescein. Additionally, products containing fluorescent dyes such as fluorescein were themselves capable of various different uses. In the present case, the Fuchs products were traded for two purposes – leak detection and product identification. Therefore, Fuchs’ supplies of fluorescein, and products containing fluorescein, were supplies of staple commercial products, Fuchs submitted.
355 Fuchs submitted that it missed the point to say that, to the extent that the correct product was the combined product, the products in question were specialised. The dyed products had a number of different commercial uses and functions. The dye in hydraulic fluid assisted it to be: identified; not accidentally consumed; used to detect leaks; and, for higher concentration products, used for HPFI detection.
356 Quaker submitted that Mr Knight specifically noted that Fuchs had never supplied fluorescent fluid additives per se to any of its customers. Thus the product the subject of the analysis was not “fluorescein”, nor a generic fluorescent dye and a generic hydraulic fluid that happen to be supplied together. The hydraulic products supplied by Fuchs were highly specialised products provided with respect to the particular needs of the customers and the particular needs of their machinery. The phrase “staple commercial product” was intended to refer to basic products or raw materials which were commonly available and had a multitude of uses: Astrazeneca AB v Apotex Pty Ltd [2014] FCAFC 99; 226 FCR 324 at [428]-[431].
9. Dye concentration issue
357 Fuchs contended that it had no reason to believe that any supply of Renolin or Solcenic products with third party dyes to mines sites (except Broadmeadow) would be used as part of a method to detect HPFI, given the low concentration of fluorescent dye added to those products.
358 Fuchs’ submissions in this respect were that the concentration of fluorescent dye (other than FluidSafe) in the Fuchs products (other than supplied to Broadmeadow) was approximately 22.5 times lower than the equivalent amount of FluidSafe directed by Quaker to create fluids suitable for use in HPFI detection. It relied on Mr Knight’s evidence.
359 The concentration of fluorescent dye in Fuchs’ Solcenic and Renolin products sold to the Glencore and Yancoal mines was the minimum amount Fuchs considered sufficient to facilitate leak detection. The treat rate of 8g dye per 100L of longwall fluid was adopted based on the Fuchs group’s leak detection experience in Poland. The treat rate of 1g of dye per 100L of mineral hydraulic oil was selected as testing showed it was sufficiently fluorescent for leak detection.
360 The only Fuchs product supplied at a higher concentration was the Solcenic GM20 LD supplied to Broadmeadow. The treat rate for FluidSafe WB directed by Quaker was 750mL per 100L of Solcenic GM20 concentrate. Testing done prior to the supply to Broadmeadow established equivalence of Uniqua W400X at a concentration of 180g/100L.
361 Fuchs submitted that Mr Knight attested to Fuchs’ concerns as to possible harm that might be suffered by workers if the method claimed in the patents did not work as promised, and to use FluidSafe dyes at the treat rates directed by TTAAS and later Quaker. The evidence did not support an inference that Fuchs knowingly supplied fluid having 22.5 times less fluorescence than directed by Quaker expecting it to be used for HPFI purposes, and that Fuchs’ customers conspired with it in such conduct and then used that fluid for HPFI detection. Quaker led no evidence that leak detection products could be used for HPFI purposes, and indeed had distinguished in its own product lines between suitability for leak detection and HPFI.
362 Quaker submitted that this was a convenient exercise in hindsight and motivated reasoning, and should be rejected. Crucially, Quaker submitted, Fuchs never did any testing that would allow it, or any reasonable person in its position, to conclude that the products being supplied to Glencore and other sites could not be used for the detection of HPFI. Excluding the supply to Broadmeadow, the extent of Fuchs’ testing of dyed products was, at best, superficial. It largely consisted of working out whether the proposed dyes glowed or not.
363 This generalised testing would have not allowed any reasonable person in the position of Fuchs to reach any conclusion that such dye could not be used as part of the claimed method. Mr Knight accepted that Fuchs had never undertaken any testing to determine the minimum concentration of dye which could be used as part of the claimed method.
364 Further, Quaker submitted, the dyes used by Fuchs were different to FluidSafe. The mere fact TTAAS and Quaker recommended different concentrations of FluidSafe for use in detection of leaks versus detection of HPFI, gave no guidance as to the appropriate dose rates (or the differences between those dose rates) for leak detection versus HPFI detection in respect of Fuchs’ different dyes. This exposed as misleading the comparison of concentrations of dyes used by Fuchs in its opening submissions, such as its reference to a “22.5 times” difference in concentration. Mr Smith was of the view that it was entirely possible in some circumstances that dye at a concentration appropriate for leak detection could be used as part of the claimed method.
365 Finally, Quaker submitted, whatever the actual position be, as a matter of fact Fuchs did not think that the use of fluorescent dyes for leak detection could be separated from HPFI detection whatever the concentration. It followed from this that the concentration of dye in the product supplied could not have had any material bearing on whether or not Fuchs had reason to believe that the product would be put to an infringing use. The issue of concentration was a recent invention and did not affect Fuchs’ reason to believe at the relevant time.
10. Supply to BMA – Broadmeadow mine site
366 As an example of infringement, Quaker submitted, Broadmeadow was instructive for two important reasons. First, it was an occasion on which Fuchs let its guard down and exposed its tactic of using leak detection as a “front” to disguise infringing conduct. Second, and crucially, it exposed that, as a matter of fact, Fuchs thought that there was and is no way to separate the use of fluorescent dye for leak detection from use in HPFI detection. That is, the defence now put forward by Fuchs to the effect that (a) it had no reason to believe hydraulic fluid containing dye supplied for leak detection with lower concentrations of dye would be put to use of HPFI detection, and (b) “there were two quite different products sold in the marketplace with two quite different characteristics”, was wrong, and was contrary to Fuchs’ actual belief at the relevant times. Therefore, it was appropriate to consider Broadmeadow before turning to other instances of infringement.
367 Quaker referred to the concessions made in cross-examination by Mr Knight, and to emails revealing a view at the very highest levels in Fuchs that, as Mr Rushton and Mr Littley themselves said, there was no separating the use of fluorescent dye for leak detection from detection of HPFI. None of these statements were made by reference to a particular concentration, let alone a high concentration. Nor could they be, given that they were expressly stating that dye used for leak detection (and thus presumably supplied at a concentration suitable for that purpose) could be used for HPFI detection. Their view was that the type of dye was relevant, namely fluorescein, not its concentration. While Mr Knight sought to explain away these statements in his evidence by inserting an assumption as to concentration, if the authors at the time had thought that concentration was relevant, they would have said so. Mr Knight did not seek to gainsay what the authors of the relevant statements said at the time.
368 Quaker also noted that, had it not challenged Fuchs’ privilege claim, many of these communications would have remained hidden from Quaker and the Court. On their face, these documents clearly could not be privileged.
369 Quaker submitted that the internal exchanges exposed Fuchs’ awareness that it was seeking to supply Solcenic with fluorescent dye in circumstances in which the use of that product by the customer would infringe the Patents. They also exposed Fuchs’ attempts to cover up that infringement, and to provide a “front” of plausible deniability on the basis that it was seeking to supply a product for leak detection, not HPFI detection.
370 In summary, Quaker submitted, the conduct of Fuchs in offering to supply and then supplying Solcenic GM20 LD to BMA satisfied s 117(2)(b) of the Act. Fuchs had reason to believe that the product would be used as per the claimed method; indeed, it knew it was intended to be.
371 Quaker also submitted that Fuchs actively induced or instructed BMA to use the Solcenic GM20 LD product as part of the claimed method within the meaning of s 117(2)(c), by means of: expressly comparing its product to FluidSafe when it knew Broadmeadow wanted to use a dye to aid in HPFI detection; providing such instruction or inducement at a meeting in January 2016, noting Mr Pearce had not given evidence about what was discussed at that meeting and had contemporaneously stated Fuchs would like to “convince site verbally rather than in writing”; and significantly undercutting the cost of supplying Solcenic GM20 using FluidSafe.
372 Quaker submitted, for similar reasons, that Fuchs also authorised and was a joint tortfeasor in the use of the product by BMA for detection of HPFI at Broadmeadow. It submitted the Court should infer that direct infringements occurred, in circumstances where: Broadmeadow was seeking the dye for use of the method claimed in the Patents; there was evidence that while HPFI injuries were rare, incidents in which the escape of fluids injured people were far more common, and the patented method would assist in distinguishing the two; and there was evidence that where FluidSafe was used, the patented method was used regularly to determine whether HPFI had occurred. For example, following the roll-out of Oil-Glo at Metropolitan there was a “steady stream” of workers seeking to use a UV light to determine whether they had suffered an HPFI.
373 Fuchs submitted that it adopted a different approach to BMA’s Broadmeadow mines, when compared with the Glencore and Yancoal mines, as appeared from the documents.
374 There were, nevertheless, a number of issues in relation to Broadmeadow. The first was that the products in question were staple commercial products, and s 117(1)(b) therefore did not apply. The second was whether Fuchs reasonably expected those Fuchs products would be used in the infringing method, which required not only that fluorescent dye be added to the fluid of operating machinery but also that a suspected HPFI injury occur and that a UV/blue light be used to detect the presence of fluorescence. Such incidents were not common and instances of actual HPFI injuries were especially rare.
375 Despite issuing subpoenas to numerous Fuchs customers, including BMA, Glencore and Yancoal, targeting this exact information, Quaker had not identified a single instance at any mine supplied with Fuchs products where the patented method was used even to inspect a suspected HPFI injury, let alone where an HPFI injury was confirmed. Indeed, given the treatment outcome for a person suffering a suspected HPFI injury would be the same irrespective of whether the patented method of fluorescent detection was used, Fuchs had no reason to believe the dyed Fuchs products would in practice be used in accordance with Quaker’s patented method.
376 In cross-examination, Mr Knight acknowledged that the provision of Fuchs’ fluorescent fluid products to Broadmeadow might run the risk of patent infringement. In a series of internal communications Fuchs’ staff debated how that customer’s request might be met in a manner that could minimise any suggestion that Fuchs had engaged in infringing conduct.
377 Fuchs submitted that Mr Pearce gave a presentation to Broadmeadow in a bid to secure the mine’s business. Before giving his presentation, a draft was circulated internally for comment. Amendments were made to the draft presentation. Mr Knight agreed that the purpose of the presentation was to persuade Broadmeadow that Fuchs could supply a product suitable for HPFI purposes.
378 With respect to the issue of “reason to believe”, Mr Knight acknowledged in cross-examination that at the time of the supply of the high concentration product to Broadmeadow for use in HPFI detection he expected Broadmeadow would put it to that use. However, it was not put to Mr Knight in cross-examination whether he had any reason to believe that the Broadmeadow mine (or any other Fuchs customer in Australia) might perform, with a Fuchs product, the method contained in any of the claims of the Standard Patent or the Innovation Patent.
379 Fuchs submitted that this was significant. Claims 1 and 9 of the Standard Patent and claim 1 of the Innovation Patent required a fluid injection injury (or “possible” fluid injection injury). If claim 1 was invalid because of a failure to contain a detection step, then claim 2 would require the provision of ultraviolet light to detect the present of the fluid. There was no basis for Fuchs to have any particular belief in relation to this step.
380 Likewise, Fuchs submitted, it was not put to Mr Knight whether he expected the Broadmeadow mine might engage in washing a worker having a suspected HPFI injury as part of any HPFI detection method used at the mine (noting the requirement for washing in claims 4 and 14 and their dependent claims). As a related point, there was no evidence that Broadmeadow (or any other mine site) possessed the equipment required to perform the method the subject of each of the claims in suit. By way of further example, Mr Knight was not asked in cross-examination whether he was aware whether the Broadmeadow mine (or any other mine site) might have a medical facility which included a darkened room and a UV light for the purpose of performing the patented HPFI detection method. Nor was he asked whether he was aware whether any personnel at the mine had been trained in performing the patented HPFI detection method.
381 Fuchs submitted that Quaker bore the onus of establishing infringement under ss 117(2)(b) or (c).
382 Fuchs submitted that Quaker’s infringement case relied solely on inferential reasoning which was dependent on Mr Knight’s candid acknowledgments in cross-examination that the Broadmeadow mine asked Fuchs for a dyed product to be used for HPFI detection, that there was a risk of HPFI injuries in longwall mining operations, and that Fuchs supplied a product for HPFI purposes. It also depended on making assumptions about the terms of any HPFI protocol used at the Broadmeadow since the mine’s protocol was not in evidence. Fuchs submitted this did not establish a proper basis from which the Court ought to infer that the particular combination of features in any one or more of the method claims in suit was likely to be performed at the Broadmeadow mine.
383 By way of example, Fuchs referred to the following:
(a) Quaker noted that claim 9 of the Standard Patent and claim 1 of the Innovation Patent required ultraviolet lights, and said that while Fuchs did not supply those lights, it knew that Broadmeadow had them because they were asking for product for HPI detection and “given that Fuchs employees visited the mine site regularly”. This was pure speculation. Quaker then submitted that “as Mr Pearce was not called to give evidence”, Quaker could not ask questions of him, but “that absence of evidence should not assist Fuchs”. This attempted to reverse the onus, and should not be accepted.
(b) Likewise, Quaker submitted, in relation to the requirement for a “potential fluid injection”, that Mr Knight “eventually accepted the obvious, which is that Fuchs believed that if such a suspected injection did occur, Broadmeadow would have used the claimed method”. That was not sufficient. The question was not whether, if it occurred, the method would be used. The potential fluid injection itself formed part of the method – it was a specified integer.
11. Supply to Glencore – Various mine sites (Ulan No 3, Ulan West and Bulga); Offer to supply to Glencore – Oaky North
384 Quaker submitted that Fuchs acknowledged that it had supplied its Solcenic and Renolin products containing a fluorescent dye to Glencore’s Ulan No 3, Ulan West mine and Bulga mines, said to be for the purpose of “leak detection”. Quaker submitted that, despite such public statements, Fuchs had reason to believe that those mine sites would, if a suspected fluid injection occurred, use the products supplied by Fuchs in an infringing manner.
385 Quaker submitted that the following matters demonstrated that Fuchs had reason to believe that the supply of Renolin and Solcenic products containing fluorescent dye made to the Ulan No 3, Ulan West and Bulga mines would be used at those mine sites in the claimed method:
(a) Mr Michaelson sent an email to Mr Pearce on 28 August 2013 noting that he had received an enquiry from Glencore regarding potential use of dye for leak detection, but then: (i) noted that Mr Pearce should contact Mr Littley to provide information on the recommended use of a suitable dye and the method of “enhanced detection”; and (ii) said “I do not know if we can purchase the dye from Spectroline as Wayne Thompson may be the Australian agent but there must be products available elsewhere”. There was no evidence as to the precise nature of that enquiry – there was no documentary evidence of it, suggesting it was an oral conversation. Mr Michaelson has not been called to give evidence.
(b) That day, Mr Pearce wrote to Mr Andrew Esdaile of Glencore regarding “the plan to add a leak detection dye”, but stated he wished to discuss the matter over the phone or in person. This conversation was then purportedly summarised in an email on 30 August 2013 to the following effect: “Fuchs is able to supply longwall Fluids to Glencore sites with a fluorescent dye in them for the use of leak detection only (not HP injection detection).” The Court should infer this summary was self-serving, and that what was discussed verbally included the purpose of HPFI detection. Again, the absence of direct evidence of the content of the oral conversation because Mr Pearce had not been called was not a matter in Fuchs’ favour.
(c) In response to an email from Mr Graham McKenzie of Glencore at the Oaky North mine site on 6 September 2013 for information “on dye additives for emulsion/solsenic in regards to fluid injection”, Mr Pearce appeared to have called Mr McKenzie, the substance of that call not being in evidence, creating a lacuna in the evidence which allowed the Court to infer that its contents would not have assisted Fuchs. Mr Pearce then sent a follow up email with prepared text that was used by Fuchs in respect of a number of other queries stating:
HPI Detection: The dye currently being marketed for use in cases of high pressure injection (HPI) is branded as Fluidsafe WB…Wayne has recently been granted a patent for the use of his product for HPI detection and it has been used for several years in coal mines in QLD and NSW with some success. The required concentration is quite high for a dye of this type…thus implementation of the dye has a significant financial cost ...
Leak detection: There are many dyes on the market that can be added to oil based and water based products to assist in leak detection using a blue light. Dyes used for leak detection are significantly lower in cost and require a much lower treat rate than the dyes discussed above for HPI detection … It must be understood however that dyes added to products for leak detection shall not be used for HPI detection.
After this, Mr Pearce said “I have had a similar request for information from Andrew Esdaile for [Xstrata Coal NSW] and provided him with information as requested”. The reference to “shall not” rather than “could not” was telling. As with Broadmeadow, it was clear from Fuchs’ own internal emails that its own view was that it was not possible to separate the two uses.
(d) On 21 May 2014, Mr Justin Ryan at the Ulan No 3 mine site sent an email to Mr Roberts of Fuchs asking whether Fuchs had products that could be added to hydraulic oil to identify leaks. Mr Roberts forwarded that email to Mr Michaelson stating: “This is back on the radar and we were advised yesterday of concerns for HP injections for both hydraulic oil and Solcenic” (Quaker’s emphasis). Mr Roberts gave evidence that he understood from Mr Ryan’s reference to FluidSafe that he was seeking to use the product supplied for HPFI detection, hence his reference to “HP injections” in his email to Mr Michaelson. That is, he understood Mr Ryan’s request to relate to HPFI injections.
(e) This exchange led to discussions within Fuchs about the supply of fluorescent dyes purportedly for leak detection, with a decision being made by Mr Knight that where Fuchs was selling for HPFI detection, FluidSafe would need to be used, but where it was for leak detection, Fuchs could provide its own dyes. This statement was made in a self-serving manner, and did not reflect Fuchs’ true intention. Indeed, the statement was admittedly ignored by Fuchs itself with respect to the supply of Solcenic GM20 LD to Broadmeadow.
(f) On 27 May 2014, Mr Roberts prepared a draft response to Mr Ryan which he sent to Mr Michaelson for approval. In that response he posed two options to Mr Ryan with respect to mineral oil fluids: “1. Hydraulic oil containing Fluidsafe OB which will incur an extra cost of [*] per litre 2. Hydraulic oil with dye under investigation.” Given the form of this comparison, Mr Roberts’ email was apt to convey to Mr Ryan that the two options would be equivalent. Further, noting that Mr Roberts understood FluidSafe to be used for HPFI detection, the statement suggested that option 2 would also function for that purpose. This exposed Mr Roberts’ protestations that he instructed Mr Pearce, his superior, that the product under investigation should be only suitable for leak detection, as implausible.
(g) On 11 June 2014, Mr Roberts prepared a further draft reply to Mr Ryan which was sent to Mr Michaelson for approval. That response confirmed that Fuchs was undertaking investigations but it believed it would be able to provide products meeting Mr Ryan’s “requirements”. Evidently the ambiguity of this response was deemed too risky for Fuchs, as Mr Roberts’ response was amended. Again, Mr Michaelson had not been called to explain the changes he made to Mr Robert’s draft email.
(h) On 11 June 2014, Mr Roberts responded to Mr Ryan (who Mr Roberts understood to be requesting a product for use in HPFI detection), stating that Fuchs was undertaking R&D with respect to dyes for longwall fluid and mineral oils and that pending further testing they would be able to “meet your requirements as requested.” In what was clearly a self-serving statement, the email concluded “Fuchs makes no undertaking in relation to the use and monitoring of such products … and explicitly rules out any correlation of the use of such products for High pressure Injection injuries detection”.
(i) On 16 July 2014, Mr Ryan replied to Mr Roberts stating “Ulan would like to progress as soon as practical adding the leak detection dye (not for fluid injection) to our hydraulic oil and within 2 months for our solcenic … Could you please provide the technical specs … and procedure for detection etc, so we can assess the level of risk control we will need to implement when we make this change” (Quaker’s emphasis).
(j) On the same day, Mr Peter Faulkner of Fuchs sent an email to Mr Fryer expressing concern at the idea of including a fluorescent dye in all hydraulic products for all Glencore mines as was proposed, arising from Ulan’s request. A few days later Mr Fryer said: “I think the request was driven from Bernie’s Underground business unit (possibly to detect high pressure fluid injection related injury)”. Mr Fryer was a senior representative of Fuchs, and his unguarded view of the purpose of the supply was instructive to demonstrate Fuchs’ understanding of its purpose.
(k) On 18 July 2014, Mr Pearce sent an email to Mr Esdaile and Mr Garry Horner of Glencore, stating that Ulan had requested a change of dye for leak detection, and that Fuchs was proposing to change dyes used in Renolin mineral oils supplied to all Glencore sites to add in a fluorescent dye. The email said that there would be no additional cost for the supply, and that the concentration of dye was not suitable for HPFI detection and that “equipment used to detect the fluid and associated methods cannot be supplied by Fuchs at this stage.” No explanation had been given as to why such equipment and methods could not be supplied, if the supply was genuinely for leak detection. The Court should find that Fuchs was attempting to provide itself with a paper trail so that it could deny any reason to believe the product would be used as part of the claimed method at a later point in time, as it sought to do at Broadmeadow. Further, given that Fuchs had not carried out testing to determine the minimum concentration of dye which could be used as part of the claimed method it had no basis to suggest that the concentration of dye was not suitable for HPFI detection.
(l) On 19 September 2014, Mr Esdaile asked Mr Pearce how “the legal wrangle over the dye in the hydraulic oil [was] going”. Mr Pearce said he had sought an update and was awaiting a response. No further response had been produced by Fuchs. Again, the absence of any evidence from Mr Pearce of any further reply is not a matter in Fuchs’ favour.
(m) In February 2015, Mr Roberts met with representatives of the Ulan West mine site and a request was made for the inclusion of a fluorescent dye in their Solcenic GM20. Mr Roberts implausibly suggested that he specifically recalled that Clinton Maynard of Ulan West had specifically asked for a dye for leak detection purposes and specifically said it was not HPFI detection purposes. If that was indeed what Mr Maynard said, it was at least consistent with the script from which other Glencore employees operated. However, there was no such suggestion on the documentation at the time, which referred simply to “Solcenic GM20 dye request…”
(n) Fuchs began supplying Solcenic with fluorescent fluid additives to the Bulga mine site in August 2015. There was no documentary evidence in respect of the request for that supply and Mr Pearce had not given evidence in respect of requests for that supply from the Bulga mine site. Again, this was not a matter that assisted Fuchs. Indeed, it appeared that the supply was not made arising from a request from the customer, but was initiated by Fuchs and was “promoted” for leak detection. This was an important matter, as Fuchs could not claim in respect of this supply to have had any view as to what the customer intended to use the product for based on the customer’s request.
(o) Further, in a month after supply commenced, in September 2015, Mr Ben Withers of the Bulga mine site asked Mr Pearce whether Fuchs had “any training available to roll out to our workforce? We are looking for something along the lines of the actual simulated pig injections as offered by Wayne Thompson.” While this was HPFI awareness training, it was significant that it was being requested very shortly after fluorescent dye was added to the Solcenic at the Bulga mine site. That this was a sensitive topic was confirmed by the fact that Mr Littley said to Mr Pearce:
Please take great care with this topic. Suggest you get advice from Martyn and Stew since if info gets back to Quaker on what we are doing it will tip them off and also probably kick off the war with Quaker ahead of us challenging the validity of the Patents!
The reference to tipping off Quaker suggested a concern that the training was being implemented at that mine site specifically because of the roll out of Solcenic with dye. Indeed, in the following year in the context of Broadmeadow, Mr Pearce sent an email to Mr Fryer on 25 May 2016 stating “Got word that Broadmeadow approached Wayne Thompson for HPI training hence Quaker will soon know our intention to supply a dye to site.”
386 Quaker submitted that it appeared that versions of Solcenic and Renolin products containing fluorescent dye were supplied to Ulan No 3 from 2015, and were then rolled out to other Glencore sites at Ulan West and Bulga. In particular, Mr Knight gave evidence that: Renolin B 68 Plus LD mineral oil containing fluorescent fluid additives other than FluidSafe had been supplied to Ulan No 3 since October 2015; and Solcenic longwall emulsion containing fluorescent fluid additives other than FluidSafe had been supplied to Ulan No 3 and Bulga since August 2015, and at Ulan West since May 2016.
387 Quaker noted that Fuchs submitted it had no reason to believe that those Glencore mine sites wished to use the products supplied by Fuchs as part of the method claimed in the Patents, based on the correspondence in the period from 2014 to 2015 and outlined above. However, Quaker submitted, the question was an objective one, and was also a dynamic one. It was not to be decided only based on the circumstances of the initial supply. The supply had been ongoing over many years, and Fuchs had an intimate relationship with its customers and their mine sites, with its employees regularly visiting mine sites and meeting with representatives, reviewing stock, and analysing the quality of the products it supplies.
388 Quaker submitted that subsequent correspondence either reinforced Fuchs’ understanding of the intended use in HPFI, or led Fuchs to have reason to believe that supply of Renolin B 68 Plus LD and Solcenic products including fluorescent dye to Glencore in more recent years would be used as part of the method claimed in the Patents. In an email from Mr Pearce to Mr Roberts on 4 July 2018, he said: “It would assist us in closing the Narrabri deal if we could get something in writing from someone at Ulan 3 or West [about leak detection]…Would this be possible and please avoid any HPI mention?” (Quaker’s emphasis.) If Fuchs had believed the dye was only to be used for leak detection, there would be no reason to include such warning. Again, Mr Pearce had not been called to give evidence as to why he would include such a statement in this email.
389 The responses to this request were even more telling, Quaker submitted. Mr Roberts replied to Mr Pearce stating that Mr Patrick of Glencore “utilized a IR gun to identify leaks on the face.” Mr Roberts explained that the term “IR” referred to infra-red, and said that this technology did not require any fluorescent dye in the hydraulic fluid. That is, Mr Roberts must have understood, and Mr Pearce must have understood, that if the mine site was utilising an IR gun to detect leaks, it was not using the fluorescent dye to do so, so it did not need the fluorescent dye for leak detection. If it did not need the dye for leak detection, the only other use of the dye was for detection of HPFI. Even more emphatically, Mr Wayne Kirkhope of Ulan West replied to Mr Pearce’s request for information stating that: “I have no involvement in the leak detection properties. We don’t do that here and I have no interest in doing it. Generally you can either hear or see a leak and it’s way too labour intensive to bother UV leak detection.” Whatever was the case before, Fuchs could not have continued to have had no reason to believe following this email that Ulan West was using the fluorescent dye supplied as part of the claimed method on the basis the mine was using the dye for leak detection, as Mr Kirkhope had expressly disavowed any use of the dye for leak detection.
390 Quaker submitted that Glencore, like Fuchs, appears to have adopted a self-serving “position” on this issue. On 29 October 2018, Mr Axel Cutts at Oaky North emailed Mr Pearce about a dye supplied by Quaker for its GM20 longwall fluid. Mr Pearce replied: “Sorry for the delay in response, this is quite a sensitive topic. Glencore is familiar with this dye so can I suggest that you touch base with Garry Horner for more info on corporate position.” To similar effect was an email from Mr Fryer to Mr Brett Simpson of Ravensworth on 7 September 2018: “The high pressure injection debate is the subject of a global court case at present. We argue prevention is better than detection. If you contact Garry Horner he will give you Glencore’s position on the matter which we fully agree with.” Mr Simpson replied “Agree with in body detection dye being a mitigating factor only after the event. Definitely the prevention aspect is our focus.”
391 Quaker noted that Fuchs submitted that based on this email Glencore’s approach was to focus on prevention of HPFI, implying this was somehow inconsistent with it also seeking to use the claimed method if such prevention was not effective, and implying that Glencore did not think the invention worked. However, Mr Simpson’s email suggests nothing of that sort. Of course prevention of HPFI would be the focus of any mine site, but that did not mean that “mitigation” was not also relevant and important, and Mr Simpson did not suggest it was not relevant or important to Glencore in this correspondence. Ultimately, Fuchs had no evidence that Glencore’s focus on prevention meant it did not want to use the claimed method. It had not adduced any evidence from any representative of Glencore regarding its belief. Despite a question in re-examination clearly intended to invite such evidence from Mr Roberts, he did not give it.
392 Further, Quaker noted, Fuchs had submitted that Glencore’s own internal documents confirmed that the dyed products supplied by Fuchs to Glencore were only used for leak detection. Again, there was no evidence that was so. Fuchs relied on four documents: (a) a procedure with respect to leak detection; (b) a change management report; (c) a document entitled “Toolbox Talk”; and (d) and a draft procedure regarding fluid injections.
393 Quaker submitted that the change management document and the Toolbox Talk document were drafted by Mr Roberts and provided to Glencore. They were not “independent” internal documents created by Glencore. Fuchs was concerned to conceal its awareness of the prospect that Glencore would seek to use the products as part of the claimed method. Its creation of documents recording provision of products for leak detection was not inconsistent with that concern but consistent with it. Further, the Toolbox Talk document was expressly stated to be a draft document. There was no evidence it was ever actually published or promulgated to the workforce at any Glencore mine site. Also, the change management and Toolbox Talk documents related to Ulan No 3 only; there were no equivalent documents from Ulan West or the Bulga mine sites.
394 Second, Quaker submitted, the protocol for treating fluid injections was in draft form, and besides which, as noted by Mr Knight, it was last modified on 2 December 2014. This was well before any dyed Renolin or Solcenic products were supplied to any of the Ulan No 3, Ulan West or Bulga mine sites in 2015 and 2016. A document showing a procedure without reference to use of the claimed method prior to the supply of a product which could be used as part of the claimed method did not indicate anything.
395 Third, Quaker submitted, with respect to the leak detection procedure, it was unsurprising that a document referring to a leak detection procedure would not refer to use of the claimed method. Quaker did not submit that at least some of the mine sites to which the dyed product were supplied were interested in using the products for leak detection. Quaker merely submitted that that fact was not inconsistent with them also wishing to use the products as part of the claimed method.
396 In relation to Oaky Creek, Quaker submitted there was an offer of supply in circumstances which would infringe under either of s 117(2)(b) or (c).
397 On 20 January 2016, Daryl Chamberlain of the Oaky Creek mine site sent an email to Mr Pearce asking “What experience do you have with fluoresce (sic) tell-tale oil additives used for the identification of fluid injection.” Mr Pearce then forwarded this email to Mr Knight and Mr Fryer, stating: “I will let you know how my presentation goes tomorrow at Broadmeadow and we can discuss tactic (sic) from there depending on how it is received.” On 29 January 2016 (shortly after having presented to Broadmeadow on the subject), Mr Pearce replied to Mr Chamberlain: “I am on site at this stage on the 24th of February … I think on these two topics it would be best for me to present and give you an opportunity to ask any questions you may have in person.” In a monthly business activity report provided to Mr Fryer for February 2016, Mr Pearce reported with respect to Oaky Creek North that “1. Longwall move commencing in March, expected to take 4 weeks … 2. Presented info on Solcenic GM20 and Fluorescein dye.” No presentation had been produced.
398 Quaker submitted that Fuchs’ submission that there was no evidence of what was discussed at this meeting, apart from what was in the documents, was not in Fuchs’ favour. Mr Pearce had not been called to give evidence, thus the Court could more readily draw an inference based on the evidence before the Court that Mr Pearce offered to supply to Oaky Creek the Solcenic product including dye for the purposes of HPFI detection.
399 While it did not appear that any fluids containing fluorescent dye were subsequently supplied to Oaky Creek after the requests, Quaker submitted it should be inferred that Fuchs offered to supply that product in circumstances where it had reason to believe that Oaky Creek would put that product to an infringing use. This gave rise to further conduct within at least s 117(2)(b), independently of what the Court decided in respect of the supply of other products to Glencore.
400 Fuchs submitted that presentations given to Ulan No 3 and Ulan West dated 7 October 2015, described Renolin B Plus 68 LD and Solcenic GM20D as containing “a specially designed fluorescent dye to assist with detecting hydraulic fluid leaks in equipment”. There was no evidence of any direction by Glencore to its employees to use the patented method.
401 In cross-examination, Mr Roberts explained that his reference to “HP Injections” in his internal email of 22 May 2014 reflected rumours he had heard in the industry, and not any conversation with Mr Ryan. Mr Roberts was steadfast that his “draft” email reply to Mr Ryan’s email of 21 May 2014 related to the supply of a product suitable for leak detection only. The reply ultimately sent expressly stated that Fuchs products were not suitable for HPFI detection. Mr Ryan’s email of 16 July 2014 stated the product was not intended for HPFI purposes.
402 In relation to Ulan West, Mr Roberts confirmed that Mr Maynard (an engineering manager the equivalent of Mr Ryan) had only ever sought Fuchs products for leak detection purposes. It was not suggested to him that his evidence, expressed clearly and confidently, was false. In cross-examination, Mr Roberts said that he believed Mr Peace, in stating “please avoid any HPI mention”, was referring to Fuchs’ position that it did not endorse HPFI detection. Mr Roberts could not be shaken from his views on the inutility of HPFI detection. Further, Mr Roberts said that, at the time he sent his email of 4 July 2018, Ulan West was using the IR gun and also Solcenic GM20 to detect leaks. Finally, Fuchs submitted it was plain from the last sentence of Mr Kirkhope’s email of 10 July 2018 that Ulan West was using Solcenic GM20 for leak detection without using a UV light.
403 In relation to Bulga, Fuchs submitted that it was of no moment that Mr Withers requested HPFI awareness training a month after Fuchs’ initial supply of fluid containing leak detection dye. HPFI awareness training was separate from training to teach the method of HPFI detection. As to Mr Littley’s email to Mr Pearce. in context it was apparent Mr Littley was concerned with Fuchs’ bovine udder testing.
404 In relation to the alleged offer to Oaky Creek, Fuchs submitted that Quaker did not lead any evidence of any offer actually made. Quaker’s contention, that the Court should infer an offer was made by Mr Pearce, yet at the same time acknowledge that Fuchs never supplied any product to Oaky Creek, was spurious.
12. Supply to Yancoal – Ashton and Moolarben mines
405 Quaker submitted that for many years prior to 2016, Fuchs supplied Yancoal mines with Solcenic and Renolin products mixed with FluidSafe, which it knew to be used for the purpose of detecting HPFI, given correspondence exchanged with those mines in 2012-2014. This intended use was confirmed by an email in May 2016 from Mr Warren Ekert (Regional Engineering Manager) to Ms Julie Hill (Procurement Manager), both of Yancoal, referring to FluidSafe.
406 Quaker complained of two instances of infringing supply.
407 The first was with respect to the Ashton mine site. Quaker submitted that Fuchs admitted that it had supplied Solcenic GM20 3% Premix Emulsion since August 2015 including fluorescent dye to that mine site. This supply of dyed Solcenic appeared to have been initiated by Fuchs, and in particular by Mr Pearce. Mr Knight was unaware of the documentation regarding this supply, so his evidence as to the circumstances of the supply should be given no weight.
408 Quaker submitted that, apart from Mr Pearce’s email, there was otherwise no documentation regarding the supply to Ashton. That gap in evidence had been created by Mr Pearce not being called to give evidence, so the Court should infer his evidence would not have assisted Fuchs. Given that Fuchs was aware that other Yancoal mine sites such as Abel and Austar were using Solcenic and Renolin products dyed with FluidSafe for the purposes of HPFI detection, Fuchs would have had reason to believe that another Yancoal mine site would have had a similar intention, particularly where the supply was not initiated by the mine based on a particular request, but by Fuchs. Fuchs’ submissions as to the issue of concentration should not be accepted, as they provided no basis for it to have had any view to the contrary.
409 The second instance Quaker complained of was the supply or offer to supply Solcenic GM20 with fluorescent dye to the Moolarben mine. Quaker submitted that Fuchs was aware that Moolarben was concerned about HPFI and believed that the use of dyed fluid to detect HPFI was a viable method. On 11 March 2016, Mr Mark Fogarty of Moolarben emailed Mr Roberts to request dyed Renolin products which he wished to use for detection of HPFI. Mr Fogarty also sought a kit for detection of HPFI. Despite Mr Roberts’ assertion that a kit for the detection of HPFI was different from one for leak detection, which was absurd, he was forced to accept that they were the same. It followed that Fuchs supplied to Mr Fogarty a kit with a UV light and glasses that could be used to detect HPFI when used with dyed hydraulic fluid and the claimed method.
410 Quaker submitted that Fuchs had supplied Renolin B 68 D to Moolarben since March 2016, containing FluidSafe for the purposes of HPFI detection. No complaint was made with respect to that supply. However, the Court should find that Fuchs had commenced, or was intending to commence, supplying Solcenic dyed with fluorescent dye, given that at a monthly meeting with representatives of the site in January 2019, they requested “leak detection product” “ASAP”.
411 Quaker submitted that, relevantly, Exhibit C had Mr Robinson of Fuchs meeting with three “attendees” of Yancoal at Moolarben and noting in the meeting minutes:
5. Brief discussion with Christian & James about changing to the Fuchs Leak Detection Die. James would like Moolarben to change to the Lak (sic) Detection Product ASAP.
412 Quaker submitted that while Mr Knight gave evidence of Fuchs’ supply as at the date of his affidavit on 2 November 2018, he had not made further enquiries before giving evidence. Thus in respect of the supply to Moolarben as of March 2019, his evidence was unreliable and incomplete.
413 Quaker submitted that if that supply, or offer to supply, occurred, it would infringe s 117(2)(b) of the Act. While Exhibit C referred to “leak detection product”, Fuchs was aware that Moolarben was concerned about HPFI and was currently supplying dyed Renolin to be used as part of the claimed method. It was implausible that a mine site using dyed mineral oil as part of the claimed method would not want a dyed Solcenic product which could be used as part of the claimed method in its longwall, as workers near the longwall were at particular risk of HPFI. As Mr Pearce said in an email dated 16 December 2015 in the context of the supply to Broadmeadow, “this dye issue is preventing us from having a major crack at any of Quakers (sic) business as no site wants to take an apparent step back on safety.” Quaker repeated its submissions with respect to concentration, and noted that regardless there was no evidence as to the concentration of dye in such Solcenic product.
414 Fuchs submitted it had only ever supplied fluorescently-dyed fluid for leak detection to one Yancoal mine, Ashton, on an ongoing basis. As Ashton was never supplied with fluorescently-dyed Renolin of any kind, Mr Pearce’s email to Ms Hill of 11 August 2016 was merely an offer to remove the FluidSafe dye, not to replace it with an alternative dye.
415 In relation Ashton, Fuchs submitted that in July 2015 it offered, in the context of “minimising leaks across the face”, to supply Yancoal with a replacement product called Solcenic GM20 containing “a very small amount of fluorescent dye”. In its records Fuchs identified this supply as for leak detection. As to Quaker’s reliance on Fuchs being aware other Yancoal mines were using FluidSafe for HPFI detection: each mine had its own procedures and preferences.
416 In relation Moolarben, Mr Knight gave evidence that when Moolarben ran out of undyed Solcenic emulsion in December 2017 and urgently required more, it temporarily accepted the premixed dyed Solcenic fluid being supplied to Ashton (Yancoal) and Bulga (Glencore). Mr Knight said in cross-examination he was not aware of any intention to supply Moolarben in the future with a Solcenic product including a fluorescent dye. All that could safely be drawn from the “Site Meeting Minutes” document dated January 2019 was that Moolarben may have had a recent intention to acquire product for leak detection purposes. Fuchs submitted that there was an obvious reason why a mine might not want to acquire Solcenic with a dye for HPFI detection: it was expensive. There was no basis for thinking that Moolarben would use a low concentration leak detection product for HPFI detection.
417 Finally, Fuchs submitted that Mr Roberts’ evidence that the quote he provided to Mr Fogarty on 15 March 2016 was for a leak detection kit for leak detection purposes, although he agreed it was “possible” it could be used for HPFI detection, was steadfast and inherently plausible.
13. Quaker’s authorisation and joint tortfeasorship cases
418 Both parties accepted that in order to succeed in relation to its joint tortfeasorship and authorisation cases, Quaker had to establish that there were acts of primary infringement.
419 Quaker’s submissions in respect of authorisation and joint tortfeasorship in respect of BMA (Broadmeadow) and Glencore are recorded above in respect of each mine.
420 Fuchs submitted that neither the authorisation case, in all its guises, nor the joint tortfeasorship case, could succeed because no acts of primary infringement at a third-party mine had been established. If the Court did not agree, Fuchs accepted that liability for authorisation followed any finding as to indirect infringement under ss 117(2)(b) or (c).
14. Quaker’s table of requests to Fuchs for HPFI detection dye, and Fuchs’ response
421 In response to Quaker’s table of requests made to Fuchs for dye for HPFI detection, Fuchs provided the following, with its comments in the right hand column:
Requests made to Fuchs for dye for HPFI – Fuchs’ Response
The Respondent does not accept that each of these documents contains a request for the supply of dye for HPFI. Rather, each occasion needs to be considered in turn.
No. | Date/Time | Customer | Mine Site | Outcome of Request [Fuchs] |
1. | 06/09/2012 9:19am | Xstrata (Glencore) | Tahmoor | No supply of dyed fluids |
2. | 08/01/2013 9:56am | Donaldson Coal (Yancoal) | Abel Mine | RENOLIN B 68 D supplied (from May 2013 to Jun 2016) Dye was FluidSafe |
3. | 19/20/2013 9:17am | Yancoal | Austar | RENOLIN B 68 D supplied (from November 2013) SOLCENIC 2020 D 4% EMULSION supplied (from Dec 2014 to Jun 2015) SOLCENIC GM20 D PLUS 3% PREMIX EMULSION supplied (from May 2016) All dyes were FluidSafe |
4. | 25/03/2013 6:40am | Fuchs Internal re Peabody & Yancoal | Peabody and Yancoal mines supplied with FluidSafe prior to 2015 – only discrete supplies of other dyes at low concentrations to Yancoal’s Ashton (from Aug 2015) and Moolarben (Dec 2017 to Feb 2018) mines | |
5. | 27/03/2013 09:57am | Xstrata (Glencore) | Ravensworth | No supply of dyed fluids |
6. | 28/03/2013 11:30am | Fuchs internal re Peabody; Yancoal; Xstrata | North Goonyella; Metropolitan; Tahmoor; Ravensworth; Blakefield; Wallsend | None of these mines were ever supplied with fluorescent dye other than FluidSafe (if they were supplied with any dyed fluids) |
7. | 20/06/2013 10:14pm | BHP Billiton | Illawarra Coal | No supply of dyed fluids |
8. | 10/07/2013 3:45pm | Yancoal | Unknown | Yancoal mines supplied with FluidSafe prior to 2015 – only discrete supplies of other dyes at low concentrations to Yancoal’s Ashton (from Aug 2015) and Moolarben (Dec 2017 to Feb 2018) mines |
9. | 06/08/2013 10:55am | Gujarat NRE Coking Coal Limited | NRE No 1 | No supply of dyed fluids |
10. | 06/09/2013 8:24am | Glencore | Oaky No. 1 | No supply of dyed fluids |
11. | 25/10/2013 9:03am | BHP Billiton | Broadmeadow | Supplied SOLCENIC GM20 LD supplied (from Jul 2016) High concentration generic dye supplied from Jul 2016 |
12. | 26/10/2013 4.00 pm | Whitehaven Coal | Narrabri mine | SOLCENIC GM20 D supplied (from Aug 2013, after Whitehaven disavowed HPFI detection – see FTB tab 221) Low concentration generic dye |
13. | 21/11/2013 4:28pm | Xstrata / Glencore | Oaky No. 1 | No supply of dyed fluids |
14. | 11/06/2014 12:41pm | Xstrata / Glencore | Oaky Creek | No supply of dyed fluids |
15. | 20/10/2015 11:44am | BHP Billiton | Broadmeadow | See 11 above. |
16. | 21/11/2014 3:05am | Yancoal | Austar Paxton | See 3 above. |
17. | 08/12/2014 9:38am | BHP Billiton | Appin Mine | No supply of dyed fluids |
18. | 19/03/2015 | Vale | Carborough Downs | RENOLIN B 68 D supplied once thereafter (Dec 2017) Dye was FluidSafe |
19. | 18/05/201 11:40am | Whitehaven Coal | Narrabri Mine | See 12 above. |
20. | 03/06/2015 1:29pm | Xstrata / Glencore | Newlands Northern Underground | No supply of dyed fluids |
21. | 05/06/2015 3:31pm | Whitehaven Coal | Narrabri Mine | See 12 above. |
22. | 04/08/2015 2:25pm | BHP Billiton | Broadmeadow | See 11 above. |
23. | 16/09/2015 12:19pm | Glencore / Xstrata | Bulga | SOLCENIC GM20 – 3% PREMIX EMULSION (Fluoresceine LT) supplied from Aug 2015 to Mar 2016) SOLCENIC GM20 – 3% PREMIX EMULSION (Uniqua W 400X) supplied (from Apr 2016) Low concentration generic dye |
24. | 03/12/2015 9:50pm | Lake Coal / Yancoal | Not mine specific | No supply of dyed fluids |
25. | 09/12/2015 12:48pm | BHP Billiton | Broadmeadow | See 11 above. |
26. | 16/12/2015 11:04pm | BHP Billiton | Broadmeadow | See 11 above. |
27. | 21/12/2015 3:54pm | BHP Billiton | Broadmeadow | See 11 above. |
28. | 23/12/2015 10:34pm | BHP Billiton | Broadmeadow | See 11 above. |
29. | 24/12/2015 10:00am | BHP Billiton | Broadmeadow | See 11 above. |
30. | 20/01/2016 5:52pm | Xstrata / Glencore | Oaky Creek | No supply of dyed fluids |
31. | 09/02/2016 5:43pm | BHP Billiton | Broadmeadow | See 11 above. |
32. | 11/03/2016 2:54pm | Moolarben Coal (Yancoal) | Moolarben | SOLCENIC GM20 – 3% PREMIX EMULSION (Uniqua W400X) supplied (from Dec 2017 to Feb 2018) Low concentration generic dye RENOLIN B 68 D supplied (from Mar 2016) Dye was Fluidsafe |
33. | 21/04/2016 10:20am | South 32 | Not mine specific | No supply of dyed fluids |
34. | 18/05/2016 7:28am | Yancoal | Austar Paxton | See 3 above. |
35. | 09/06/2016 4:44pm | Unknown | Unknown | No supply of dyed fluids |
36. | 08/08/2016 2:31pm | Glencore | Rolleston Coal | No supply of dyed fluids |
37. | 23/08/2016 3:04pm | Glencore | Rolleston Coal | No supply of dyed fluids |
38. | 22/10/2016 7:17am | Glencore | Bulga | See 23 above. |
39. | 20/09/2017 1:07pm | Yancoal | Moolarben | See 32 above. |
40. | 27/08/2018 12:56pm | Glencore / Xstrata | Clermont | No supply of dyed fluids. |
41. | 07/09/2018 7:51am | Glencore / Xstrata | Ravensworth | No supply of dyed fluids |
42. | 17/10/2018 7:51am | Glencore / Xstrata | Oaky North | No supply of dyed fluids |
43. | 18/10/2018 9:38am | Glencore / Xstrata | Oaky North | No supply of dyed fluids. |
44. | 19/06/2018 1:40pm | South 32 | Appin West | No supply of dyed fluids. |
15. Relief
15.1 Injunction
422 Both parties sought an opportunity to be heard in relation to the appropriate form of any relief in the event of a finding of infringement.
15.2 Additional damages
423 Quaker sought a finding that Fuchs was liable to pay additional damages to Quaker in an amount to be assessed. This was a clear case in which additional damages should be awarded arising from the infringing conduct, Quaker submitted.
424 Quaker submitted that:
(a) In respect of the supply to Broadmeadow:
(i) Fuchs was aware in advance that its supply of Solcenic GM20 LD to Broadmeadow would be infringing, but it nonetheless went ahead with that supply;
(ii) Fuchs attempted to disguise its infringement with respect to Broadmeadow by seeking to concoct a paper trail to suggest that Broadmeadow merely wanted to use the product for leak detection, and attempted to hide or avoid documenting any communications which would suggest to the contrary;
(iii) Fuchs expressed a view internally following the supply that such supply would be infringing;
(iv) Fuchs expressly denied to Quaker it had any reason to believe that the supply to Broadmeadow was infringing in correspondence by its patent attorneys, despite that being false and contrary to its own internally expressed view; and
(v) Fuchs had continued to deny in these proceedings, right up until the end of the hearing, that it had any reason to believe such supply would be infringing, despite that being contrary to its own internally expressed view;
(vi) Because of Fuchs’ infringement, it was able to maintain its contract to supply Solcenic to Broadmeadow. Without it, it was almost certain that Broadmeadow would have turned to Quaker to supply it with longwall fluid, and potentially other fluids (given the convenience of one supplier of such products, as demonstrated by Fuchs’ pitch to Yancoal).
(b) In respect of the supply to Glencore mines:
(i) If the Court accepted Quaker’s submissions that Fuchs (and Glencore) used requests for leak detection as a “front”, Fuchs engaged in extensive attempts to conceal its infringing conduct, which also provided as basis to conclude that Fuchs knew that its supply was infringing.
(ii) Fuchs had made assertions in these proceedings about the relevance of concentration of dye, which were contrary to its own internally and contemporaneously expressed views.
(iii) Again, because of Fuchs’ infringement, it was able to maintain its contracts to supply Solcenic and Renolin to Glencore mine sites. Without it, it was possible that those mine sites would have turned to Quaker for their supply of those and other hydraulic fluids.
(c) Further, in respect of both Broadmeadow and Glencore mines, Fuchs sought to claim privilege over numerous documents disclosing its internal views on a number of critical issues:
(i) The documents on their face were clearly not privileged. Indeed, after Rares J had made some initial rulings in respect of a number of documents, Fuchs did not assert before his Honour that various other documents were privileged at all.
(ii) Fuchs did assert before Rares J that some other documents were privileged, leading to his Honour making rulings in respect of those documents. A review of those documents and Rares J’s comments in the transcript made it clear that the original claims of privilege made by Fuchs were not properly based.
(iii) The views expressed in these documents, many of which were significant, would have remained hidden to Quaker and to the Court had Quaker not taken steps to challenge Fuchs’ privilege claims.
(iv) As discussed, Mr Hoiles, the Managing Director of Fuchs, was responsible for giving instructions in respect of Fuchs’ privilege claim. He has not been called by Fuchs to give evidence in these proceedings.
Quaker submitted that the above conduct readily came within s 122(1A)(1)(a), (c) and (d) of the Act. The case was one in which an award of additional damages was appropriate having regard to the authorities.
425 Quaker anticipated that Fuchs would attempt to assert that because Quaker sought to challenge the grant of the Standard Patent, this provided some explanation or excuse in respect of Fuchs’ infringing conduct. However, there was no evidence whatsoever that any of the relevant personnel within Fuchs had this issue in their mind when engaging in any of the conduct referred to. Rather, the evidence was very much to the contrary. Mr Knight effectively admitted that he and Fuchs thought what they were doing was infringing and sought to cover it up.
426 Fuchs contended that its submissions could only sensibly address Broadmeadow, because the infringement case in relation to the other sites was completely unmeritorious.
427 The case in relation to Broadmeadow was a secondary infringement case. Such a case could not put the infringer in a worse position than an ordinary case of patent infringement. Fuchs has always maintained the genuine view that the Patents were invalid, and the contrary was not suggested to Mr Knight in cross-examination. By the time of its dealings with Broadmeadow in August 2015 to July 2016, Fuchs had, on 4 December 2015, requested re-examinations of both Patents and, although these were dismissed, Fuchs maintained that the Patents were invalid in these proceedings by its cross-claim. Fuchs also relied on an email from Mr Pearce to Mr Knight dated 30 July 2014 as demonstrating Fuchs’ awareness of Quaker’s challenge to the Patents’ validity, which had been supported by evidence of Mr Peter Doyle regarding prior disclosure of the invention at Metropolitan.
428 Fuchs submitted that its conduct at Broadmeadow did not involve copying the FluidSafe dye. Rather it sourced its own dye which it sought to match with the absorbance of FluidSafe.
429 Fuchs submitted that its decision to “run the risk” did not warrant additional damages in circumstances where Fuchs doubted whether the patented method worked, proceeded cautiously in dealing with customer requests for HPFI products, and considered it had strong grounds upon which to regard the Patents as invalid.
430 Fuchs submitted there was no basis for an inference that Fuchs deliberately claimed privilege in documents that it knew were not privileged. The privilege claim failed for lack of proper evidence of the purpose of the communications, which did not address whether privilege claims were properly made in the first place nor the company’s state of mind as to their making.
The evidence of the lay witnesses
Mr Wayne Thompson
431 In 2005, Mr Thompson established TTAAS to provide training and assessment and subsequently transferred his Registered Training Organisation (RTO) registration to it.
432 In or around 2008 to 2009, while he was working for Waratah Engineering, Mr Thompson was also engaged by the NSW Department of Industry & Investment to assist in the preparation and development of the Mining Design Guidelines 41 titled Fluid Power Safety Systems at Mines (MDG 41).
433 When an HPFI incident would occur, the mine site’s engineering managers would receive a safety alert or an incident report generally issued by the NSW Department of Primary Industries, which would contain recommendations with references to the MDG 41, other guidelines, and Australian standards. This would then usually be relayed on to the workforce through site safety talks to ensure that they were aware of the issues so that the incident would not happen again.
434 By 2009, Mr Thompson had left his employment with Waratah Engineering. He resolved to pursue his passion of improving safety in the mining industry through education, training and innovation.
435 Since around 2005 until around December 2014, Mr Thompson provided training to numerous underground and open cut coal and gold operations, particularly in New South Wales, Queensland and New Zealand.
436 Mr Thompson said he was aware from his experience and qualifications that one serious safety concern associated with fluid power systems, particularly with hydraulic systems, was HPFIs. HPFI injuries were uncommon but could sometimes be fatal or have serious health consequences, with all injured persons requiring surgery and many requiring amputations. These injuries also frequently led to mental health issues, including posttraumatic stress disorders.
437 He gave evidence that HPFI injuries were difficult to detect as the injured person may only have felt a small sting. The difficulty was magnified where the fluid under high pressure that caused the injection originated from a hairline fracture in a pipe or hose, or adjacent to a loose fitting. HPFIs were thus often overlooked. However, their seriousness had to be responded to by surgical intervention. Surgery, the only viable treatment option, could cause disfigurement and amputation. It was for those reasons at least that HPFI injuries had been a major concern in the mining industry for some time. In or around 2002, the NSW Department of Mineral Resources (as it was then called) released a Fluid Injection Protocol which outlined a procedure to follow, and also attached documents containing information about HPFI. This “protocol” was to go with the patient and to the doctor or hospital.
438 In or around mid-2009, Mr Thompson was engaged as a contractor by Peabody to develop a site-specific competency based training package for Helensburg Coal Pty Ltd, known as Metropolitan Colliery. During the delivery of these training packages to select groups of Metropolitan employees, he was requested to conduct the training more broadly for the Metropolitan workforce, particularly in relation to the safety issues around hydraulics and high-pressure injuries including HPFI.
439 From around July 2009, as part of the practical component of the mechanical training, TTAAS (Mr Thompson) started to design and develop a fluid injection simulator. Mr Thompson also thought that the simulator should include a mini hydraulic system in which he could easily and transparently (for the sake of people undertaking the training) regulate the pressure of the machine fluid used in the system. It seemed to him that a mineral oil-based hydraulic system would be the most sensible to adopt for the simulator because it was very difficult and expensive to purchase water-based hydraulic pumps adapted to be used in a small system such as that which he had proposed for the simulator.
440 The simulator was created to demonstrate safely what would happen if fluid escaped under pressure and penetrated human tissue. The simulator contained other component parts (not specific to an HPFI demonstration) including a hydraulic ram, a hydraulic pump, and other items specific to MDG 41.
441 Initially, Mr Thompson used model hands made of ballistic gelatine to show that it did not require extremely high pressures for fluids to penetrate the human tissue. He was aware from his training and experience that HPFI injuries could occur with as little as 6.9 Bar (100 psi) of pressure, approximately twice the typical tap water pressure in a home.
442 The simulator was completed in or around September 2009, at which time Mr Thompson demonstrated it for the first time to Mr Withers, the manager of mechanical engineering at Metropolitan, and Mr Nathan Thompson, who was a Peabody employee.
443 The simulator was placed in the training area at Metropolitan colliery and was not used until some months later when the training began. Later in 2010 at the earliest, specific HPFI training was provided included demonstrations using the simulator.
444 At the beginning of 2010, TTAAS started to provide mechanical training to Metropolitan. For the purposes of the mechanical training, only the ballistic gelatine hand was used in the demonstration. Once a gelatine hand had been injected, it could not be re-used. Accordingly, Mr Thompson had to make several gelatine hands for each training session, typically around 6-8 per training session depending on the intended number of attendees. The mechanical training did not involve any demonstrations using pig skin or pig trotters. TTAAS provided mechanical training to Metropolitan from early 2010 to around late 2010.
445 Towards the end of 2010, whilst TTAAS was still providing training for Metropolitan, Mr Withers asked Mr Thompson whether he would be willing to assist Peabody’s Fluid Injection Task Force (Peabody Task Force) and informed him that Peter Doyle, the General Manager of Corporate & Operations Support in Peabody, would contact him. Mr Thompson was aware of the Peabody Task Force and that it had been formed earlier in 2010 following a number of suspected and actual incidents of HPFIs within the Peabody mine sites. The cost implications of even suspected HPFIs were substantial. The Peabody Task Force was created to investigate those incidents, identify the risks and provide recommendations for risk management.
446 Mr Thompson’s role in assisting the Peabody Task Force was to provide information and develop materials in regard to HPFI injuries to be presented to the workforce. He was also contacted regularly by various members of the Task Force for information associated with HPFI such as training material developed by TTAAS and, at one stage, for material for the NSW Mineral Council Occupational Health and Safety Conference of July 2011.
447 During the course of providing the mechanical training in 2010, from time to time Mr Thompson received feedback from some trainees in relation to the fluid injection demonstration that they could not easily see the penetration of the machine fluid into the gelatine hand. Often only a small hole in the gelatine hand could be observed. To that end, Mr Thompson would often pass around the gelatine hand so that those undertaking the training could see the hole(s) in the hand.
448 Mr Withers requested Mr Thompson to develop an HPFI specific training program. Mr Thompson thought it important to resolve the visibility issue of the fluid injection simulator to improve the practical demonstration. After some deliberation, in around mid-August 2010 he looked into a dye for adding to the oil-based hydraulic fluid he was using in the simulator so that the demonstration would provide greater visibility. As part of this deliberation, Mr Thompson undertook a Google search for dyes which he could use in the simulator, specifically for dyes that could be introduced into oil-based hydraulic systems. Those searches identified the business of Spectronics. Mr Thompson then undertook a further search for Australian distributors of Spectronics’ dyes, and he found out that Russell Fraser Sales Pty Ltd was an Australian distributor of them.
449 On 3 September 2010 Mr Thompson purchased a sample only bottle of Oil-Glo 33 from Russell Fraser Sales to test its compatibility in the fluid injection simulator. The volume of a sample only bottle was 473 ml. In evidence was a copy of the invoice dated 3 September 2010 from Russell Fraser Sales for the purchase of the sample only bottle of Oil-Glo 33 and a leak detection kit, including a blue LED flashlight.
450 Spectroline Oil-Glo 33 was a fluorescent dye specific for use in systems that used oil-based fluids, including transport machines such as loaders, dozers and man transports. Oil-Glo 33 was not compatible with longwall systems where water-based fluids were used.
451 Mr Thompson undertook testing of the Oil-Glo 33 dye in the simulator to work out whether it was compatible with the oil-based mechanical system within it. Oil-Glo 33 was found to be compatible and was then implemented into the fluid injection simulator soon after that for use in the training. At that stage, the fluorescent dye was used solely to improve the visibility of the fluid injection in the gelatine hand.
452 Mr Thompson deposed that during the delivery of the training using Oil-Glo 33 in the simulator, he had a sudden idea one night of using this fluorescent dye to identify actual instances of HPFI rather than just using it as a visual aid in the gelatine hand of the simulator. He wondered whether it may be possible for the dye also to be visible under human skin which would allow detection of HPFI injuries if the dye was first mixed into the hydraulic fluid (the Method). He did not remember the precise date when he had the idea of the Method. However, he knew it was some time after he had introduced Oil-Glo 33 into the simulator, so it was likely to have been some time in mid to late September 2010, or possibly in October 2010.
453 Mr Thompson thought that the addition of the dye into hydraulic systems at a mine site had the potential to improve mine safety and the significant risks associated with HPFI. However, in order to be able to determine whether the Method would work, he considered that he would need to conduct a number of trials and experiments.
454 He was aware from his experience in the mining industry that mines would not agree to add materials to their hydraulic systems without satisfactory results from extensive testing, risk assessments and the attainment of relevant approvals to ensure that such introduction would not impact upon the machines or worker safety. To that end, he was aware that he would have to discuss the Method with a mine site in order to be able to have suitable trials and experiments undertaken. He considered that he had good relationships with people at Metropolitan, particularly Mr Withers, and so it made sense to him that he explore with Mr Withers whether Peabody might consider having the Method tested at Metropolitan or at any of its other mine sites. Further, Mr Thompson was aware that the machines at Metropolitan were older and out of the original equipment manufacturer (OEM) warranties which meant that the risks associated with the commercial implications were not as severe in comparison to new machines that were still within the OEM warranty period. He knew that Peabody would also be more willing to consider trialling the Method at Metropolitan for this reason.
455 Mr Thompson was also not certain whether the Oil-Glo dye would effectively show an HPFI injection under the skin. The gelatine hand which he had been using with the dye in the simulator was translucent, and quite different in appearance, texture and composition to a person’s skin. He considered that he would need to undertake testing to determine whether the Method would work by reference to a much better analogue for human flesh and tissue.
456 In or around October 2010, Mr Thompson spoke to Mr Withers about running some trials and experiments to test the Method at Metropolitan. This was the first occasion on which he had told anyone at Peabody about the Method. After Mr Thompson had explained the proposed Method to him, they had a conversation to the following effect:
Mr Withers: That sounds interesting. What do you need to do in order to understand whether that would work?
[Mr Thompson]: I’m not sure at this stage but first, I would need to make sure that it’s compatible with the machines before we do anything. I can’t put anything in there unless it’s compatible with all the machines.
Mr Withers: Yes, before anything, make sure its (sic) compatible with the machines and is not going to be detrimental to the machines. Come back to me when you have something.
457 Mr Thompson was also aware that the Oil-Glo 33 product, which was designed for an oil-based hydraulic system, could not be safely introduced into a predominantly water-based hydraulic system, such as would be found in a longwall mine like Metropolitan, and where HPFI injuries appeared to be more prevalent. Accordingly, Mr Thompson considered that even if he could establish that the Oil-Glo 33 product could be introduced into an oil-based hydraulic system, he would still need to identify an alternative dye suitable for introduction into a water-based hydraulic system before he would have any real sense of whether the Method would work.
458 Mr Thompson considered that successful introduction of the Method into the mines in a way that would benefit workers would necessarily require dyes useful for introduction into both oil-based and water-based hydraulic systems because both such systems were in operation in most mines, notably most underground mines. It was already clear to him at this point that determining whether the Method would work in practice would take significant effort, planning, testing and trialling. Those things would also likely cost a lot of money that TTAAS/T&T did not necessarily have. He was concerned about whether he had (or could get) what he anticipated it would take to determine whether the Method would work. He considered that there was likely to be a long road ahead with challenges that he might not be able to overcome, including, for example, providing for himself and for his family, especially if he was going to launch into the project full swing as he believed was necessary.
459 At this stage, Mr Thompson deposed, he was still unaware of whether the Oil-Glo 33 dye would effectively highlight the injection of fluid into human skin. Further, while he had discussed the Method with Mr Withers (as set out above), he considered that he had to do more to “sell” the idea to Mr Withers to convince him to agree to have Mr Thompson working on the necessary testing and trials at Metropolitan to determine whether the Method was feasible. To this end, Mr Thompson prepared a demonstration using the fluid injection simulator, illustrating an injection of high pressure fluid containing Oil-Glo 33 into pig skin. He decided to use pig skin as research he had done on the internet led him to believe that pig skin was similar to human skin. This testing proved to be difficult and time consuming to set up as it was difficult to get the pig skin to hold firmly in place. If not glued on properly, the impact of the high pressure would cause the piece of pig skin to fly off from the unit holding it, rather than being pierced by it. Due to these difficulties, Mr Thompson did not use the pig skin in the HPFI training or other training. Outside of trials and experiments he did on his own, he only used pig skin as a “once off” to present the concept to Mr Withers.
460 The demonstration was conducted in the carpark of Metropolitan and Mr Withers and the Metropolitan mine manager at the time were present. Mr Thompson did not recall the exact date on which the demonstration occurred, but said it must have occurred after the construction of the pig skin specimen but before he had undertaken any further testing of the pig skin. Therefore, he believed it occurred in October or November 2010. He did not recall anyone else viewing the demonstration.
461 After the pig skin was injected with the hydraulic fluid containing the fluorescent dye, Mr Thompson took the pig skin specimen out of the simulator and allowed Mr Withers and the mine manager to observe the injection. During this presentation, Mr Thompson did not shine a blue light over the pig skin to show the injection, as the demonstration was in an open air carpark and lighting was good. The fluorescent dye could be seen at the injection site with an unaided eye.
462 In or around mid-November 2010, shortly after the presentation in the carpark to Mr Withers and the mine manager, Mr Thompson conducted further tests in his own time using the pig skin and took photographs and videos of the tests. He injected the fluid at different distances and at different pressures to see under what conditions a fluid injection would occur.
463 The risks associated with introducing new substances into a hydraulic system are mitigated by conducting suitable trials and experiments before that substance is introduced, Mr Thompson deposed.
464 In early December 2010, Mr Thompson conducted compatibility tests of Oil-Glo 33, using the balance of the sample bottle of Oil-Glo 33 purchased from Russell Fraser Sales on 3 September 2010, on an aboveground Bobcat. A Bobcat is typically a single driver operated machine capable of excavating via a hydraulic powered mechanism. Bobcats are used in mine sites. However, Bobcats suitable for underground work typically tend to be constructed slightly differently to aboveground Bobcats.
465 The testing of the Bobcat was conducted by Mr Thompson alone on a farm, not in an underground environment. The Bobcat was not particularly needed or being used and so the risks associated with any damage that the introduction of Oil-Glo 33 would cause were not material. Although Mr Thompson knew the testing would ultimately need to be conducted on underground machines, one first step he considered that he needed to undertake was to perform compatibility tests on an effectively dispensable piece of equipment that operated above ground, as the risk of disrupting production of an underground mine with so much uncertainty could not be justified. It was also much easier for him to perform the tests on an above ground machine in the first instance.
466 Mr Thompson still had the balance of the sample bottle of Oil-Glo 33 that he had used in the fluid injection simulator. As Russell Fraser Sales had sold the product to him on the basis that it ought to be compatible with mining machines, and as the Bobcat was effectively dispensable, he decided to introduce a very generous amount of Oil-Glo 33 into the Bobcat’s hydraulic system with a view to really putting the machine through its paces with Oil-Glo 33 on board. In fact, he used essentially all of the remaining Oil-Glo 33 in the container.
467 After introducing the Oil-Glo 33, Mr Thompson then started up the Bobcat and operated it through all of its functions to their respective relief pressures at normal operating temperature. Relief pressure is the maximum pressure at which each individual function can operate. The inclusion of the Oil-Glo 33 product into the Bobcat did not adversely impact the functionality of the machine. Mr Thompson regarded the compatibility testing of Oil-Glo 33 on the Bobcat to have been a success.
468 Mr Thompson was also aware that, as part of the trialling of the introduction of Oil-Glo 33 into those systems, it would be necessary for mine workers to be able to clearly observe the Oil-Glo 33 in the system while underground, as testing would have to occur in machinery which operated underground. He had previously purchased an Optimax leak detection kit which included a blue light to cause the Oil-Glo 33 to fluoresce. The standard torches that came with the Optimax leak detection kits were not approved to be used underground. Equipment which is to go underground has to be carefully checked, so as to avoid the risk of sparks which could ignite flammable gasses which can build up in mine sites underground. As Mr Thompson did not want to waste time trying to have the Optimax torches approved, he opted instead to start with a blue lens cap which could be clipped onto a torch already approved to go underground. In evidence was a recipient created tax invoice dated 14 December 2010 for the supply to TTAAS of 50 “off Cap lamp blue lense (sic) covers”.
469 Mr Thompson conducted a risk assessment on or around 16 December 2010 for the Oil-Glo 33 product, and its proposed introduction into machines with oil-based hydraulic systems.
470 It was only after the provision of the risk assessment that Mr Withers agreed to the conduct of trials of Oil-Glo 33 in the hydraulic systems of a roof bolting machine (called a Multibolter) and an Eimco 4 at Metropolitan to carry out forensic and engineering compatibility testing. As Mr Thompson did not have a sufficient quantity of Oil-Glo 33 available (having used most of it in the simulator and Bobcat), he needed to buy some more so he could supply it to Peabody. However, TTAAS also needed some money because it and Mr Thompson did not have sufficient funds to acquire the minimum purchase order quantity. A sample only container would not contain a sufficient quantity of the product to conduct the compatibility testing on both the Multibolter and Eimco. The amount of time he was spending on the project was also starting to cause him the financial concern that he had earlier anticipated. On 18 January 2011 Mr Thompson raised an invoice, which was in evidence, from TTAAS to Metropolitan. He raised the invoice so that TTAAS was able to pass on the costs to Metropolitan for the acquisition of more Oil-Glo 33, as well as other matters.
471 Due to the level of uncertainty in respect of the Oil-Glo 33 product, Mr Withers and Mr Thompson spent some time ensuring that the additive would work in the machines. There were no issues with the Multibolter or the Eimco after adding the Oil-Glo 33 product. He did not recall Mr Withers ever sending him anything or discussing any issues as a result of adding Oil-Glo 33 to those machines.
472 Mr Thompson knew that Peabody was likely to agree to introducing a fluorescent dye into the longwall system at Metropolitan as HPFI injuries were prone to occur in the longwall. To test whether the Method would work in a longwall system, another fluorescent fluid additive equivalent dye to Oil-Glo 33 but which was compatible with a water-based system was required. Mr Thompson therefore looked into the dyes manufactured by Spectronics Corporation and noticed that the Material Safety Data Sheet (MSDS) for Oil-Glo 33 was very similar to the MSDS for WD 802. On that basis, he recommended Peabody conduct a risk assessment on the introduction of WD 802 into the longwall fluid at Metropolitan.
473 In or around the end of March 2011, Peabody raised a purchase order for, and TTAAS supplied Metropolitan colliery with, a pallet load of WD 802 for trialling in Metropolitan’s longwall system. The mine workers then premixed the WD 802 with Solcenic, a Fuchs product, which was the longwall fluid used by Peabody at the time. The premixing was done in accordance with Mr Thompson’s instructions, as was the addition of the premixed fluid into the longwall system.
474 In or around April 2011, Mr Thompson prepared MSDSs for Oil-Glo 33 and for WD 802. The “Product Use” in both MSDSs was recorded as “Leak Detection” as trials were still ongoing at this stage and it was not confirmed whether Oil-Glo 33 or WD 802 would be suitable for use in HPFI detection. He considered the MSDSs for Oil-Glo 33 and WD 802 which were prepared and distributed by the manufacturer, Spectronics, in the US. He believed Spectronics to have undertaken thorough testing and analysis of the additives that would be reflected in its MSDSs.
475 In or around May 2011, WD 802 was introduced into the Metropolitan longwall system for monitoring and testing. TTAAS continued to supply Metropolitan with fluorescent fluid additives until 2014 when TTAAS/T&T sold the business and all underlying intellectual property to Quaker.
476 Mr Thompson deposed that he was aware from his workings with Peabody that hydraulic fluids used in the machinery at its Metropolitan, North Goonyella and Wambo mines were supplied by Fuchs. He was aware that Fuchs was its supplier of hydraulic fluids until around 2013.
477 Mr Thompson deposed that, prior to the commencement of compatibility testing of Oil-Glo 33 with Metropolitan’s machinery in 2011, he was reluctant to disclose or discuss the Method with Fuchs as it was still in its early stages, and he did not do so. However, once testing on the machinery was underway, it became necessary to communicate with Fuchs in relation to testing the compatibility of Oil-Glo 33 and WD 802 with its hydraulic fluids, particularly because Peabody had difficulties with dosing on site.
478 By mid-2011, Mr Thompson deposed, once the fluorescent fluid additives were included into the longwall system at Metropolitan, he discussed the Method with mining companies and other companies including Joy and Pirtex. He received numerous enquiries on the Method, and numerous discussions were had whilst he provided HPFI training after mid-2011. He deposed that he did not discuss the Method during HPFI training prior to then.
479 Between April and June 2011, Mr Thompson had several exchanges with Mr Doyle in relation to the NSWMC OH&S Conference and other matters relating to the Peabody Task Force. On 20 April and on 13 and 16 June 2011, he sent Mr Doyle various information including a presentation he prepared in relation to HPFI injuries.
480 On 23 June 2011, Mr Thompson received an email from Mr Doyle attaching a copy of the presentation for the NSWMC OH&S Conference for his suggestions and comments. In the email, he requested that Mr Thompson delete the presentation from his system after reviewing it. Shortly after receiving the email, Mr Thompson reviewed the presentation and saw that Mr Doyle had incorporated information that Mr Thompson had forwarded to him. He sent Mr Doyle a reply confirming the presentation looked “great”.
481 By July 2011, following the NSWMC OH&S Conference, Mr Thompson considered that the Method was widely known as a result of Peabody’s Peter Doyle’s presentation about the Peabody Task Force and his invention.
482 Mr Thompson deposed that he was aware that Fuchs conducted compatibility testing of WD 802 with its longwall fluids in around mid to the end of 2011 as Fuchs occasionally requested information from him to assist it at that time. He received a copy of the laboratory test reports Fuchs produced for the compatibility of WD 802, which Fuchs referred to as “Oil Glo” or “Glo Dye”, with the following longwall fluids:
(a) Solcenic 2B-W;
(b) Solcenic 2020; and
(c) Solcenic GM20.
483 The addition of WD 802 to the Solcenic products appeared to have no significant effects on the chemistry, stability or corrosion inhibiting the capacity of those products.
484 Mr Thompson was also aware that Fuchs conducted compatibility testing of Oil-Glo 33 with its hydraulic oil called Renolin B Plus 68 in or around March 2012, as he provided Fuchs with a copy of the MSDS for Oil-Glo 33 upon its request.
485 As the Method involved injection of the fluorescent dye into the human body, Mr Thompson considered that he needed to consult medical professionals, and did so in late February and between March and August 2011.
486 Mr Thompson deposed that whilst HPFI injuries were a serious safety risk, those injuries were relatively rare. However, in order to be certain that the Method actually worked, a person needed to have been injected with fluid containing a fluorescent fluid additive. As it was unethical to conduct human testing, there were benefits for trials to be conducted in more than one mine site. Following HPFI training delivered at Peabody’s North Goonyella mine, WD 802 was delivered to North Goonyella at the end of June 2011. In around July 2011, Mr Thompson deposed, trialling of WD 802 in the longwall system at North Goonyella commenced.
487 Mr Thompson deposed to an event on 23 December 2011 when a contractor fitter at North Goonyella was accidentally injected with hydraulic fluid in his hand/wrist and the on-site first aid staff and paramedics used the fluid injection detection kit to confirm that an HPFI injury had occurred. Mr Thompson believed that the incident was the first time that a person received an HPFI injury with machine fluid which contained a fluorescent fluid additive. It was only after this incident that Mr Thompson was certain that the Method worked. Following this incident, he pushed for the implementation of fluorescent fluid additives in hydraulic systems and the Method more widely.
488 As with Metropolitan, Mr Thompson continued to supply North Goonyella with fluorescent fluid additives until 2014. He also supplied Wambo with fluorescent fluid additives until 2014.
489 In early 2012 Mr Thompson/TTAAS rebranded the Oil-Glo 33 and WD 802 to FluidSafe-OB for oil-based systems and FluidSafe-WB for water-based and emulsion systems, and FluidSafe-FB for fuel-based systems, respectively.
490 Mr Thompson deposed that he was the sole inventor of the invention described and claimed in the following patent applications and patents:
(a) Australian provisional patent application no. 2011903536 titled “Method for detecting fluid injection in a patient” which was filed on 2 September 2011 (‘536);
(b) Patent Cooperation Treaty (PCT) patent application no. PCT/AU2012/000094 titled “Method for detecting fluid injection in a patient” which was filed on 2 February 2012 claiming priority to ‘536 (‘094);
(c) The Standard Patent, which entered the Australian national phase from ‘094 having as its effective date of patent the same filing date and having the same priority claim as ‘094; and
(d) The Innovation Patent, which was filed as a divisional patent application from the Standard Patent keeping the same effective date of patent and priority claim.
491 As to the claims of the Patents, Mr Thompson deposed that when a volume of machine fluid containing the fluorescent dye escaped from a machine, that escaped machine fluid was detectable by shining an ultraviolet light adjacent to the area where the escaped machine fluid was expected to have ended up.
492 His invention involved adding fluorescent fluid additives to machine fluid so that, in the event of a suspected HPFI injury, an assessment could be made as to whether that suspicion was correct by shining an ultraviolet light adjacent the suspected site of injection. If that assessment confirmed an HPFI injury, then the ultraviolet light could be used by medical personnel/surgeons to determine the extent to which the machine fluid that penetrated the skin had tracked into the body. Until his invention, and still at the date of his first affidavit, in circumstances where a fluorescent fluid additive had not been added to machine fluid, surgical methods of extracting machine fluid from the human body following an HPFI injury involved incising a surgical wound adjacent to the suspected point of injection and extending the surgical wound until the surgeon believed that machine fluid had not tracked any further in the human body beyond the furthest edge of the surgical wound.
493 Mr Thompson said the technology had tended to be named by reference to the name of the fluorescent fluid additives he used from time to time. Initially, he referred to the technology as Oil-Glo, named after the fluorescent fluid additive Oil-Glo 33. This was an additive that he determined to be suitable for oil machine fluids. Around the same time, he also identified an additive for water based machine fluids which was called WD 802. However, Oil-Glo was the preferred acronym used for the technology, whether introduced into water based - or oil based - machine fluids. Later, in early 2012, he came up with the name FluidSafe and thereafter the technology was referred to as FluidSafe.
494 The ‘536 application was filed in the name of T&T. The ‘094 PCT application was filed in the names of T&T and TTAAS. Consequently, the Patents were filed in the names of T&T and TTAAS.
495 Mr Thompson was at all material times a director of TTAAS, and he was a director of T&T prior to its deregistration in 2016.
496 The Standard Patent was laid open to public inspection on 7 March 2013 and the Innovation Patent was laid open to public inspection on 20 June 2013 and certified 25 July 2013.
497 TTAAS and T&T were, until 12 December 2014, the registered owners of the patents.
498 Acceptance of the Standard Patent application was published on 22 August 2013, and Quaker US lodged a notice of opposition to grant of the patent on 22 November 2013. What transpired over the next 12 months or so between Quaker, on the one hand, and T&T and TTAAS, on the other hand, was a series of contentious exchanges, followed by a prolonged negotiation which resulted in Quaker acquiring the FluidSafe business in December 2014. When that occurred, Quaker US withdrew their opposition and the Standard Patent proceeded to grant.
499 On 12 December 2014, TTAAS and T&T assigned all right, title and interest (including the right to sue for past infringement) in and to the Patents to Quaker.
500 Between December 2014 and 13 January 2017, Mr Thompson was employed by Quaker. Since around 13 January 2017 he had been engaged by Quaker as a paid consultant, and retained his Quaker email address to fulfil his ongoing role as a consultant.
501 Mr Thompson accepted in cross-examination that at the time that he devised the invention the subject of those patents, he did not have any particular familiarity with patent law.
502 Mr Thompson did not accept that in 2010, as part of his training, he disclosed to workers, including by demonstrating using his simulator, that fluorescent dye could be used to detect fluid injuries and people. He also did not accept that he suggested to those workers that they could use a UV light to detect such injuries or that they could use UV light in a darkened room to detect such injuries.
503 Mr Thompson was asked if, during this process of testing the dye in pieces of mine machinery and then the introduction of the dye into the longwall, he had wanted it all to be a secret, he could have entered into some form of confidentiality agreement with Metropolitan. He answered that he was not aware that he could have and he was not thinking about that at the time. The communications that he had with the mine were between himself and that specific mine, the same as every other mine that he had done training for. He accepted that if he had wanted to keep it secret he could have asked them to enter into some confidentiality arrangement.
504 Mr Thompson said that from his perspective the relevant dye was being trialled until it was confirmed to be working in December 2011 at the North Goonyella mine.
505 Mr Thompson accepted that in or around October 2010 he disclosed to Mr Withers that he had the idea of adding fluorescent dye to hydraulic fluid in a machine in order to identify whether a worker had been injected with hydraulic fluid. He said he did not disclose to Mr Withers that the fluid might fluoresce in the presence of UV light. Mr Thompson said that using fluorescent dye to detect whether a person had actually received fluid into the body was an idea at that time, a concept, and not something which he knew because there was not a person injected and he could not confirm the idea. Mr Thompson accepted that one of the things he was trying to get across to Mr Withers was that he had had a good idea which might allow injuries beneath the skin of a person to be able to be detected by fluorescent dye. He discussed with Mr Withers that he had an idea that this would assist workers who might be injected from equipment, including equipment on the longwall. This included all equipment at the mine that had hydraulic fluid in it. Mr Thompson understood that in order for this to be useful some sort of test or some sort of process was needed and people would need to be educated about that process. He discussed those things with Mr Withers. Mr Withers was quite clear that before any piece of equipment had any dye in it, it needed to go through a process, including a risk assessment.
506 Mr Thompson agreed that when he met with Mr Withers and first disclosed his method he, Mr Thompson, well understood that to have the best chance of detection and to use the fluorescent dye in the best way, he would want to shine an ultraviolet light on someone. That would give the clearest indication of whether or not there had been injected. It was likely he discussed this with Mr Withers as well.
507 I accept Mr Thompson’s evidence. As to the initial dispute between Mr Thompson on the one hand and Mr Bate on the other as to the date of certain conversations between them, Mr Bate revised his evidence having studied Mr Thompson’s affidavit. Rather than some events occurring in June or July to August 2010, Mr Bate, having reconsidered the sequence of events as they occurred, placed those things later, some towards the end of 2010. I consider Mr Bate’s evidence more fully below.
Mr Harold Kraus
508 Mr Kraus, General Manager and Business Director of Quaker, the other lay witness called by Quaker, deposed in his two affidavits as follows.
509 Quaker’s main business focus was directed towards the supply of longwall fluids, and Quaker held about 40% market share in Australia for the supply of longwall fluids. Quaker’s main competitor in the Australian market for longwall fluids was Fuchs, with about 60% of the market share in Australia for the supply of longwall fluids.
510 In 1985, Quaker entered the longwall fluid marketplace through launching what it called “Generation 2” products, now marketed as Quintolubric 814. The longwall fluids which were previously in the market were a macro emulsion containing a relatively high concentration of mineral oil, and had a milky appearance. It called these longwall fluids “Generation 1” products.
511 Quintolubric 814 was a HFA (high water content fire resistant) semi-synthetic biodegradable micro emulsion containing a relatively low concentration of mineral oil. Having less mineral oil content meant that the product was easier to filter and had less environmental impact as a result of accidental spills and mine water discharge.
512 In 2010, Quaker released longwall fluids from a Generation 3 product range to the Australian marketplace, marketed as Quintolubric 818-02 (818-02). 818-02 was a full solution synthetic HFA hydraulic which Quaker believed provided superior characteristics compared to those of Generation 1 and 2 products. When the Quintolubric range of longwall fluids was first introduced in Australia in 1985, the workers in the mine sites were not very receptive as those fluids were semi translucent and had a watery appearance. Visually, the workers in the mining industry were used to the milky appearance of longwall fluids and were not comfortable with an almost clear/clear fluid as it could not be readily seen. Further, a fluid which had characteristics that made it easily visually identifiable was preferred, especially in the process of preparation of the longwall fluid if the product was supplied as a concentrate to be mixed at the mine sites. There was also a potential risk that people could drink the fluid mistaking it for water.
513 Quaker subsequently decided to add a green fluorescent dye to its longwall fluids so that they were visually identifiable. The presence of a dye also allowed Quaker to market and sell the product as being able to be used for leak detection.
514 In mid-April 2012, Quaker and Quaker US received an email from TTAAS’s patent attorneys bringing to their attention the ‘094 PCT application that had been filed in the names of T&T and TTAAS on 2 February 2012.
515 Quaker investigated T&T’s and TTAAS’s patent position around that time and concluded that, because there had been no application for a patent made in Australia, there was not yet a serious cause for concern.
516 Quaker had already been marketing longwall fluids that had a greenish colour from the dye that it was adding. Quaker saw merit in offering to customers a new longwall fluid that contained a fluorescent fluid additive that would: (a) improve leak detection over its existing products; and (b) have the added benefit of allowing HPFI injuries to be detected (as was the subject of the ‘094 PCT application). It was Quaker’s view that offering a product of this kind would enhance the safety of mine sites in Australia.
517 Accordingly, Quaker went about developing in-house its own fluorescent fluid additive that could be premixed with its longwall fluid products.
518 By early 2013, Quaker had already started marketing Quinto Glow, Quaker’s fluorescent fluid additive which, on request, it would premix with its Quintolubric longwall fluid products. Although Quaker’s promotional material talked about delivering Quinto Glow as an additive, this never occurred.
519 Around 27 March 2013, Quaker and Quaker US each received a letter from Benetatos White, a law firm acting for T&T and TTAAS. The letter alleged that Quaker had been supplying products to its customers in a manner that infringed T&T’s and TTAAS’s patent rights.
520 Quaker also heard from a number of Quaker’s customers around that time that Benatatos White had written to them alleging that they were also infringing T&T’s and TTAAS’s patent rights by using products supplied to them by Quaker.
521 Between April 2013 and December 2014, Quaker had a number of heated exchanges with T&T and TTAAS and during that period Quaker US opposed the granting of the Standard Patent, which Mr Kraus understood was a national Australian patent filing based on the ‘094 PCT application. Ultimately, the issues between the companies were resolved by Quaker buying the FluidSafe business from T&T and TTAAS.
522 With a view to ensuring the smooth transition of the FluidSafe business into Quaker’s offerings, Quaker negotiated a contract of employment with Mr Thompson. Mr Thompson remained employed full time until mid-January 2017, following which time he had stayed on as a paid consultant to assist Quaker with various service activities.
523 Quaker also withdrew the opposition to the Standard Patent, which was subsequently granted.
524 At no time since Quaker had been the owner of the Patents had it ever granted any licence to a third party to exploit the invention, or otherwise disposed of the rights, claimed in either patent.
525 On 4 December 2015, Collison & Co filed requests for re-examination of the Patents. Knowing that Collison & Co had represented Fuchs in its earlier contentious exchanges with T&T and TTMS, Quaker surmised that the re-examination requests had been filed on behalf of Fuchs.
526 On 4 February 2016, IP Australia issued two re-examination reports - one in respect of each of the Patents. Both Patents were upheld in their entirety, despite the extensive prior art cited and submissions made by Collison & Co.
527 By around 10 February 2016, Fuchs had placed orders for FluidSafe OB (being the oil based, rather than WB, or water based, dye). Fuchs had not placed any orders for FluidSafe WB for more than 18 months before that time.
528 Based on Mr Kraus’ instructions, Silberstein & Associates sent a letter to Fuchs with a copy to Collison & Co on 21 June 2016. The letter alleged that Fuchs had been supplying fluorescent fluid additives or machine fluids containing them to mines since at least 7 March 2013, and that such supply would infringe the Patents. It then made a number of demands, failing compliance with which it confirmed that Quaker would commence proceedings for patent infringement. Collison & Co sent a reply to that letter on 1 July 2016.
529 Mr Kraus then instructed Silberstein & Associates to prepare draft documents to commence proceedings and send them to Collison & Co with a cover letter offering Fuchs a final opportunity to respond. That letter was sent to Collison & Co on 22 July 2016.
530 Having not received a reply to Silberstein & Associates’ letter dated 22 July 2016, on 26 July 2016, in accordance with Mr Kraus’ instructions, Silberstein & Associates commenced the present proceedings in this Court.
531 In Mr Kraus’ second affidavit he deposed, form his experience, to mine sites being generally unwilling to adopt new technology until sufficient data had been provided that supported the adoption of that technology. The kinds of data that were typically demanded by a mine site before it would be willing to consider adopting and using chemical substances included:
(a) an MSDS;
(b) an approval from the OEM of the equipment in which the substance would be used, stating that the new technology may be safely used within their machinery design;
(c) evidence of prior field tests and experience; and
(d) an internal risk assessment by the mine site.
532 Mr Kraus deposed that not all new technologies were particularly well suited to OEM approval testing. Proponents of those new technologies needed to look to alternative mechanisms for justifying to mine sites the introduction of those new technologies. An example of such technologies was a hydraulic fluid additive, because additives of that nature had attributes that would not necessarily fit within the parameters typically tested through established OEM approval regimes.
533 Absent OEM approval, in Mr Kraus’ experience, mine sites pressed to embrace new technology would typically seek the results of field tests conducted in relation to that new technology. As the industry was relatively conservative, many mine sites would seek results of field tests conducted within other well-established markets, but preferably from within the same market in which they operated. Consequently, Mr Kraus deposed, an alternative to OEM approval was demonstrable feasibility obtained through field testing. In his experience, although a successful field test would not garner OEM approval, in some cases it had been a more expeditious and/or more appropriate method for supporting the introduction of a new technology into mine sites.
534 Specific examples of situations and circumstances in which a field test may be more appropriate than traditional OEM approval regimes included novel hydraulic fluids, and technology akin to Mr Thompson’s invention which involved the introduction of an additive to hydraulic fluid. Given the limited nature of OEM approval testing regimes, there were no relevant acceptance criteria which the additive could be tested against.
535 Mr Kraus deposed that although field tests, by their nature, were intended to provide tangible evidence of the relative ability to implement new technology, they carried a high risk. A field test presented a risk to the productivity of the mine, the machines directly and indirectly involved in the testing, mine site employees, and the environment. Because of the risks involved, a significant amount of planning, preparation, trials, and experiments were typically required to be undertaken before a mine site was willing to agree to take on those risks by conducting field trials with that new technology.
536 Mr Kraus deposed that one of the penultimate steps prior to implementation of an otherwise favourable prospective new technology into a mine site was an internal risk assessment. The internal risk assessment was generally conducted by either an internal team comprised of employees of the mine, or independent external risk assessment professionals under contract to the mine, or possibly a combination of the two.
537 Mr Kraus deposed that a mine site would generally only begin an internal risk assessment once it had been provided with an appropriate MSDS as well as any other specific materials. In some cases, the other specific materials may include OEM approvals, results of field tests, preliminary safety reports, and risk assessments conducted by the proponent of the new technology.
538 Mr Kraus deposed that, in his experience, an internal risk assessment carried out by a mine site would vary between mines. To his knowledge it was standard practice for a majority of mine sites to carry out the following, all of which was documented and compiled into a completed internal risk assessment report:
(a) A review of current safety protocols and procedures that could be impacted by implementation of the new technology. This could include statistical analysis of incidents that had occurred despite current safety measures.
(b) A technical assessment by OH&S specialists, either internal or external, who were familiar with the mine site, and perhaps the more intricate workings of the machinery which could be affected by implementation of the new technology.
(c) Compilation of a list of foreseeable hazards associated with implementation of the new technology based on the data and resources presented to the mine site in respect of that new technology (such as MSDSs, OEM approvals (if any) and technical reports). This list would also include a ranking of the likelihood of a hazard occurring, the consequences and effects of any hazards eventuating, and options/suggestions for mitigating that risk.
(d) Internal safety assessment and technical implementation meetings could be held between mine site managers, onsite engineers, OH&S managers, and other personnel as appropriate, to discuss implementation measures (such as change management processes).
539 Once the completed internal risk assessment report had been generated, the report would be delivered and reviewed by general and senior managers of the mine sites, who then made an informed decision as to whether or not the new technology would be adopted.
540 Following a successful internal risk assessment as conducted by the mine site, and should the mine still wish to adopt and/or implement the new technology, a document known as a change management process (CMP) document would be, or would have been, created/finalised. The CMP document set out the steps necessary to introduce a novel technology including:
(a) a description of the new technology, including its function;
(b) the timeline for any progressive stages of staggered or stepped implementation;
(c) how and when the replaced, older technology would be dealt with, or removed, if applicable; and
(d) methods and timetables for review of the new technology as it was introduced and used onsite.
541 In cross-examination Mr Kraus accepted that after Quaker added a fluorescent dye coloured green to the Quintolubric fluid, its leak detection properties were enhanced: one of the features that Quaker would then refer to in marketing the product was that it had these leak detection properties and that product, including the fluorescent dye, was used in a number of mines for many years.
542 When FluidSafe commenced to be used in mines in Australia, Quaker decided to respond with a competitive product called Quinto Glow, being a fluorescent dye that could be mixed by Quaker with its Quintolubric longwall fluid. The resulting mixture was significantly more fluorescent than the Quintolubric product alone: the intensity was enhanced significantly to be at least as strong as the FluidSafe.
543 Quinto Glow as such was not intended to be marketed as a standalone product but as an additional option to Quaker’s current range in particular. Customers could either buy the existing Quintolubric product, or they could have the Quintolubric product mixed with Quinto Glow, which Quaker would supply as one mixed product. Quaker would then combine them at the point of manufacture and deliver to the mine site. The usual way of delivering this to mine sites was to deliver the mixed product as a concentrate to which the mine site would then add water, and that much diluted product would then be used in the longwall system. Quaker’s mixed product of Quintolubric with Quinto Glow was sold as suitable for HPFI detection.
544 Quaker developed the Quinto Glow dye, which had the same core component as the existing dye in Quintolubric, in-house and tested it in-house. Once Quaker had completed the colour testing and the dermal and ocular sensitivity testing, it commenced to market Quinto Glow by offering it to its existing customers as an optional addition. Each mine would then generally conduct a risk assessment and have change management procedures for the new product.
545 Mr Kraus accepted that if a third party to whom Quaker had licenced the Patents wished to sell a new product to provide HPFI detection capability: they would need to supply an MSDS for the new product; the mine would have to conduct a risk assessment for the new product; the mine would have to change management procedures for the new product; if OEM approval were desired, the third party would need to satisfy the OEMs that the product would work with their equipment, including by conducting testing and analysis. Mr Kraus added there would be a lot of other elements as well, including that there would need to be retention samples and a clear monitoring regime in place, particularly until the third party was satisfied that the product would perform as advertised in the market for a suitable period of time.
546 I accept Mr Kraus’ evidence.
Mr Craig Roberts
547 Turning to the evidence of the witnesses called by Fuchs, Mr Roberts was employed by Fuchs in the role of Account Manager for underground coal mines in southern and western New South Wales, including in particular the Illawarra region.
548 He deposed that between 2005 and 2013, Fuchs supplied Metropolitan with fluids for use in its longwall operations. The composition of longwall fluids over decades was essentially water (around 96-98% by volume), with natural or synthetic oil additives (around 2-4% by volume) to provide various functions. The water base was advantageous because it was much cheaper than pure oil, was far more resistant to fire, was much less hazardous (to people handling or exposed to it) and was more environmentally-friendly as leaked fluid did not harm the environment.
549 Mr Roberts deposed that shearers, and various other machinery used at a longwall mine site, used much smaller volumes of hydraulic fluid and had less vulnerability to leaks because there were fewer hoses, valves and fittings. These machines used mineral oils as their hydraulic fluids.
550 During 2005-2013, the longwall fluid Fuchs supplied to Metropolitan was a fluid product called Solcenic 3002, which was a synthetically produced mixture that provided lubricating, antibacterial, anti-corrosion and other beneficial features to the longwall fluid, but did not contain any mineral oil. Solcenic 3002 would be mixed with water to form an emulsion that was predominantly water: 98 parts water to 2 parts Solcenic 3002 concentrate. The concentrate was mixed underground with water, at the 98:2 ratio, by a mixing unit.
551 Mr Roberts’ primary contact at Metropolitan was Mr Ken Risk, Peabody’s longwall engineer. He also received instructions from time to time from the senior site engineer, Mr Andy Withers, who was Mr Risk’s boss. Mr Roberts also spoke and dealt with various other personnel at Metropolitan from time to time, as necessary and appropriate.
552 Mr Roberts deposed that in late 2010 (and for at least 4 years beforehand), the safety risks of escaped pressurised fluids were a major concern for him and he was aware that it was also a concern for many of Fuchs’ customers, including Peabody, BHP Billiton and NRE Gujarat.
553 Mr Roberts gave evidence of a conversation he had with Mr Risk during a routine site visit to Metropolitan on 9 November 2010. Mr Risk referred to having “brought in a guy to run a training program for all of our staff to raise awareness about the risks of fluid injections.” During that same conversation Mr Risk said words to the effect: “We are looking at introducing a dye to our longwall fluid to enable it to be detected under a worker’s skin so we can tell if there has been an injection. Can you help us with adding that chemical to the Solcenic concentrate?” Mr Roberts said that he was not told, or understood, that any of the content of his discussions with Mr Risk was in any way confidential.
554 In an email sent on 9 November 2010, Mr Roberts raised with the relevant Fuchs personnel the possibility of adding dye to Solcenic to enable it to be detected under human skin without surgery. He said that Mr Risk’s suggestion of adding dye was the reason he, Mr Roberts, sent the email: that was where he got the idea from.
555 Mr Roberts became aware that the dye Mr Risk was considering adding to the longwall fluid was “ultraviolet” but he could not remember whether that was mentioned in the conversation on 9 November 2010 or during conversations on 17 November or 18 November 2010. Mr Roberts’ understanding was that for the ultraviolet dye within the longwall fluid to become visible or detectable under the skin, an ultraviolet or blue light needed to be shone on it so that it would glow or fluoresce.
556 On 18 November 2010, Mr Risk sent Mr Roberts an email on the topic of the ultraviolet additive he wanted to add to the longwall emulsion at Metropolitan. Mr Risk provided to Mr Roberts in that email the contact details of Mr Wayne Thompson, “who has demonstrated the Oil-Glo product which I am keen to look at to put in our longwall emulsion.” Mr Risk asked Mr Roberts to contact Mr Thompson for the details of the product to see whether that was possible.
557 Mr Roberts understood from Mr Risk’s 18 November 2010 email, in light of the earlier conversations, that Oil-Glo was an ultraviolet additive that could be added to hydraulic fluids, such as hydraulic mineral oil and Solcenic longwall fluids before they were put into hydraulic machinery. Mr Roberts understood that Oil-Glo was an additive, in that it was a liquid that could be poured and mixed into the hydraulic fluid at appropriate ratios to achieve the suitable concentrations. He also understood that it would be easier to add the Oil-Glo to Solcenic 3002 concentrate, before that concentrate was mixed with water, simply because the volumes of the concentrate were so much lower and it was much easier to pour in small amounts to the tank on the surface than to tamper with the existing machine underground that mixed the concentrate with the water.
558 At that point, Mr Roberts’ understanding included that: Peabody’s purpose in adding the Oil-Glo dye to its longwall fluid, if it proceeded with doing so, was to enable the fluid to be detected underneath a person’s skin, without the need for exploratory surgery; the Oil-Glo dye Peabody was considering adding to the longwall fluid was UV, such that it would glow/fluoresce and that would make it visible and detectable under the skin; Peabody contemplated that the presence of the Oil-Glo dye under the skin would be detected (or not) by shining a UV/blue light on the area where the HPFI injury was suspected to have occurred; it would be easier to detect the presence of Oil-Glo under the skin if the UV/blue light was shone in a darkened room; and properly washing the surface of a patient’s skin before shining the UV/blue light would mean any fluorescence detected could be more easily confirmed as being from under the skin rather than on its surface.
559 Mr Roberts said that the first step for Fuchs in considering whether Oil-Glo could or should be added to Solcenic 3002 or any other Solcenic longwall fluid was to obtain a sample of Oil-Glo and the MSDS, so that compatibility tests could be undertaken. Mr Thompson declined to provide Fuchs with any sample or MSDS for some time. Neither the MSDS nor an Oil-Glo sample was provided to Fuchs by Mr Thompson until sometime in early to mid-2011.
560 Mr Roberts became aware by reason of a conversation on 5 May 2011 with an employee of Peabody that Metropolitan had begun adding Oil-Glo to the Solcenic 3002 longwall emulsion in early May 2011. At about that time he was shown the first aid room which had been set up to enable testing the fluid injections using a UV/blue light. There was a cap lamp, which fitted onto a miner’s helmet, with a blue light cover that was put over the end to create a UV/blue light.
561 On one visit to Metropolitan in May or June 2011, Mr Risk explained to Mr Roberts that they had been mixing the Oil-Glo into the Solcenic 3002 concentrate manually but it was messy and not really accurate enough, and that their preference would be for Fuchs to mix the Oil-Glo into Solcenic at the factory level to achieve the exact concentrations of dye required and reduce the mess created on site.
562 Mr Roberts deposed that the first time he observed, during routine sampling, that the colour of the Solcenic 3002 longwall emulsion had changed to fluorescent yellow was in late May 2011. The change was from clear and translucent yellow colours, for mixed emulsions of Solcenic 3002, to fluorescent yellow, a marked change attributable to the Oil-Glo dye.
563 Mr Roberts’ understanding from discussions with Mr Risk and Mr Withers during his visits to Metropolitan was the Peabody was satisfied with the performance of Oil-Glo in the Metropolitan longwall. Mr Risk told him on 26 May 2011 that adding Oil-Glo would be one of the requirements when Metropolitan next went to tender for longwall fluids. This was confirmed in a further conversation with Mr Risk on 1 July 2011. At that time Fuchs still did not assist Peabody with mixing Oil-Glo into the longwall emulsion.
564 In cross-examination, Mr Roberts accepted that the different Solcenic products were specially formulated products having regard to matters such as the requirements of the particular OEM or particular standards that were introduced, and they were special purpose products in that they were formulated having regard to a particular purpose. He also agreed that the different Renolin products and the different grades within Renolin B were each specially formulated products intended to have a particular balance of properties for a particular use.
565 Mr Roberts agreed that he was not able to recall when during the period December 2010 through to April 2011 Mr Risk said words to the effect: “We are pushing ahead with the introduction of Oil-Glo to our longwall fluid.”
566 Mr Roberts was cross-examined on a series of emails dated 11 to 15 March 2016 but denied that he understood that, by the email from Mr Fogarty of Yancoal, Mr Fogarty was requesting from him dyed fluid and a test kit that would be capable of being used in the detection of HPFI injuries.
567 He did not accept that Renolin B 68 D was a product that had been supplied by Fuchs as being suitable for use in the detection of HPFI injuries, as well as leak detection. (In re-examination, Mr Roberts confirmed it was his understanding that Renolin B 68 D had FluidSafe dye in it.)
568 The following question was put and answer given:
Mr Roberts, at the time of this email, that is, July 2018, there was a concerted effort within Fuchs, to your knowledge, to ensure that no mention is made in internal Fuchs correspondence of Fuchs’ products, including dye other than FluidSafe, being suitable for use in the detection of HPI injuries. Do you agree with that?---Yes, but we’re defined that we would not support the - the definition of HPI for fluids, and the … only exclusion was the FluidSafe that was provided by Wayne Thompson at the time. And, again, all the way through we’ve never said it’s for HPI. As I keep saying to you over again, the idea and the science did not stack up.
569 Mr Roberts did not agree that efforts were made within Fuchs to avoid mentioning the suitability of its dyed products other than those with FluidSafe dye for use in detecting HPFI injuries so as to enable Fuchs to say down the track, if it needed to, that it had never supported that use of its products.
570 In re-examination, Mr Roberts said that in his opinion, the large majority of his customer base was not of the opinion that injection detection was an effective method and was of the opinion that the method of prevention was a much better option than detection.
571 I deal with Quaker’s challenge to Mr Roberts’ evidence bellow when considering the internal Fuchs documents and client correspondence upon which it was based.
Mr Christopher Bate
572 Mr Bate was another witness called by Fuchs. His first affidavit was affirmed on 3 May 2018. He was employed, from 2005-2014, by Peabody in roles relating to occupational health and safety at Metropolitan. At the time he affirmed his first affidavit he was employed at Sydney Water. At the time he gave his oral evidence he was employed at Metropolitan by a subcontracting company to the mine called Sada Coal, where he was employed on the surface coal handling preparation operation at the mine. It was the same mine site as when he was employed by Peabody.
573 He deposed that the hydraulic fluid used in the Metropolitan longwall during 2009-2012 was called Solcenic and was made up of water and various other components. Other hydraulic machinery used at Metropolitan during that period, such as rubber-tyred vehicles, used hydraulic mineral oils that were not diluted with water.
574 He said that in mid-2010 there were particular concerns at Metropolitan about HPFI injuries, following several incidents at the site involving escapes of high pressure from the longwall and other equipment. He was particularly concerned about HPFI injuries at this time. Mr Withers, the most senior mechanical engineer at the Metropolitan site, was a major driver of initiatives to address the risk of HPFI injuries.
575 A few weeks before he first met or contacted Mr Thompson (that is, in the period around May-June 2010), Mr Bate was instructed by Mr Withers to retain Mr Thompson’s services to provide fluid injection risk awareness training to all staff (over 400 people) working at the Metropolitan site in roles where they might be exposed to hydraulic or other high-pressure machinery.
576 A few weeks later (in the period June to July 2010), Mr Withers formally introduced Mr Bate to Mr Thompson when he was on site at Metropolitan.
577 Mr Bate originally deposed that he met and spoke with Mr Thompson in the fortnight or so before his training sessions began (that is, between June and August 2010) to review his proposed training content and format. This included a one-on-one meeting on site at Metropolitan where, Mr Bate said, Mr Thompson ran him through his proposed PowerPoint presentation and showed him his assessment tools for training participants. Of the presentation he first reviewed with Mr Thompson, for the purposes of confirming it was appropriate for use in his training Mr Bate recalled: that it included photographs showing samples of flesh that had been injected at various pressures; that the fluid was visible on and in the flesh because it was fluorescently dyed; and that in the photos the fluid was glowing against a dark background, which Mr Bate understood was because a fluorescent light was shone on the sample in a darkened room. Mr Bate recalled that Mr Thompson said words to the effect of:
That’s pig’s flesh that has been injected by hydraulic oil. The hydraulic oil contains a glowing dye so you can see it under the skin when you shine a blue light on it. That fluorescent dye is called “Oil Glo”. It can be added to longwall fluids and other hydraulic oils before they are pumped in, without affecting the machinery, and then you can use the blue light to see of someone’s been injected.
578 Mr Bate said that as a consequence of Mr Thompson having run him through his training materials during that one-on-one meeting, his understanding was that:
(a) Mr Thompson had a fluorescent dye, called “Oil Glo”, that could be added to hydraulic fluids (such as hydraulic mineral oil) in their storage drums or tanks, before they were put into hydraulic machinery;
(b) Oil-Glo fluorescent dye would fluoresce when a blue light was shone on it, and was even more visible when the blue light was shone in a darkened room;
(c) when hydraulic fluids containing the Oil-Glo fluorescent dye were injected under the skin, the fluorescent dye would still fluoresce when a blue light was shone on the skin; and
(d) in this way, adding the Oil-Glo fluorescent dye to hydraulic fluids before they were used in a hydraulic machine or system would make it possible to detect the presence of that fluid under the skin of a person, if that person had been injected, by shining a blue light on the skin.
579 Mr Bate deposed that he also appreciated at that time from what Mr Thompson had told him that washing the patient’s skin to remove the dyed hydraulic fluid from the surface would better enable the dyed hydraulic fluid to be seen fluorescing from under the skin, if there had been an injection—otherwise it would have been difficult to distinguish between the dyed hydraulic fluid fluorescing above and below the skin.
580 Mr Bate originally deposed that in the fortnight or so before Mr Thompson began conducting his training, but after his one-on-one meeting with him (that is, during June to August 2010), Mr Thompson attended a monthly meeting of the OHS committee at Metropolitan’s site, at Mr Bate’s invitation. The OHS committee comprised about 12 persons including Mr Bate and Mr Withers, senior management of the mine and union representatives. Mr Bate deposed Mr Thompson’s presentation to the OHS committee during that meeting included the same presentation as Mr Bate had reviewed. Mr Bate said that Mr Thompson said words to the following effect:
Here is an injection detection kit that includes all you need to inspect a person who might have been injected with pressurised fluid containing the Oil Glo fluorescent dye. It includes a blue fitting that can be attached to a miner’s lamp and safety glasses. Shining the light on the skin where the injection is suspected to have occurred will show whether or not there has been an injection, as any fluid injected under the skin will glow. It is important to wash the skin before you shine the light so the only glowing fluid is that under the skin. The dye will glow more clearly if the room is dark.
581 Mr Bate said that Mr Thompson explained to the OHS committee that as part of his training presentation he would show a DVD video entitled “Lethal Strike”, a dramatised scenario where a worker receives a seemingly innocuous prick on his finger when checking hoses on a truck, which he thought was from a wire but was in fact an HPFI injection. Mr Bate deposed that Mr Thompson played this video for the OHS committee.
582 Mr Bate said that he recalled members of the OHS committee standing around Mr Thompson’s trailer in the surface car park at Metropolitan and that Mr Thompson said words to the effect:
I am going to demonstrate the impact of pressurised fluid on the hand at relatively low pressures and at higher pressures. I am using pig flesh because pig skin is very similar to human skin in terms of its structure and hardness. The oil I’m spraying contains the Oil Glo fluorescent dye I showed you in my presentation inside, so it will be detectable under the pig’s skin where there has been an injection but the pressure is not high enough to blow a hole through. This means we can shine a UV light to pick up the presence of oil under the skin, once it has been cleaned, in situations where the spray has been at lower pressures and you may not otherwise know or believe there is a problem at all.
Mr Bate said Mr Thompson added words to the effect that “[t]here are versions of the Oil Glo dye which can be added to mineral oil and to Solcenic longwall fluids before they are put into the machinery.”
583 Mr Bate recalled Mr Thompson giving a practical demonstration involving turning off the lights in the first aid room and shining the blue light on a piece of flesh he had sprayed with the dyed mineral oil in his trailer, and fluid that had been injected under the pig’s skin began to glow an iridescent blue colour.
584 Mr Bate said that he understood, as a result of Mr Thompson having taken him and other members of the OHS committee through his training materials and having given practical demonstrations of injections and the use of blue light to observe Oil-Glo under the pig’s skin, that:
(a) Oil-Glo fluorescent dye could be added to hydraulic mineral oil or Solcenic longwall fluid before they were put into hydraulic machinery or the longwall system;
(b) Oil-Glo mixed into hydraulic mineral oil or longwall fluid would fluoresce when a blue light was shone on it;
(c) when hydraulic mineral oil or longwall fluid containing Oil-Glo was injected under the skin of a pig (which he understood to be very similar to that of a person), the Oil-Glo would still fluoresce when a blue light was shone on the skin;
(d) it was easier to see the Oil-Glo under the skin if the room was darkened;
(e) it was easier to discern that the Oil-Glo was glowing from underneath the skin if the surface of the skin had been washed to remove any dyed fluids;
(f) in this way, adding Oil-Glo to hydraulic mineral oil or longwall fluid before either was used in a hydraulic machine or system would make it possible to detect the presence of that oil/fluid under the skin of a person, if that person had been injected, by shining a blue light on the skin.
585 Mr Bate deposed that each training session run by Mr Thompson was attended by about 25 to 30 workers from Metropolitan, and there were also over 100 additional people, such as people working at the adjacent coal washery, who Mr Bate regularly invited to Peabody training because they were involved in the processing of coal extracted from Metropolitan and exposed to many similar safety risks, including HPFI risks from high-pressure water jets and hoses they used to wash coal and hydraulic machinery used the lift and move. The training, Mr Bate deposed, was also attended by underground contractors who were not permanent Peabody employees but who were at the time working at Metropolitan and attended some of the sessions. No participants were ever required to sign any confidentiality undertaking, agreement or deed in relation to the content of that training nor were any of them told by Mr Bate or, to the best of his knowledge, Mr Thompson or anyone else that any part of the training session or content was in any way confidential.
586 Mr Bate deposed that Mr Thompson’s training format included the PowerPoint presentation, “Lethal Strike” video, assessment and practical demonstrations which had been shown to the OHS committee, with some modifications necessitated by practical issues.
587 Mr Bate deposed that none of: his one-on-one session with Mr Thompson, his presentation and demonstration to the OHS committee, or any of the training sessions Mr Thompson conducted Metropolitan, were stated by him or by Mr Bate or by any other person to be confidential. Mr Bate did not consider any of the content to be confidential. His understanding was that he and all others who attended Mr Thompson’s training sessions were entirely free to discuss any and all of the content of his training sessions and practical demonstrations. At one point, Mr Bate invited a friend from another mine (BHP Billiton’s Appin West mine) to come down to have a look at the training. It was in this spirit that he invited the staff from the coal washery facility adjacent to Metropolitan, which was owned by an entirely separate company, to come and attend some of Mr Thompson’s training sessions free of charge. Mr Thompson did not object to the washery staff attending. Mr Bate was impressed that Metropolitan was going to be an industry leader in adopting the new safety initiative and was eager to promote that fact to the industry.
588 Mr Bate deposed that it was important to test the Oil-Glo in a working machine to ensure it did not affect the machine’s operation or performance. He recalled the test conducted under Mr Withers’ direction involving the addition of Oil-Glo to the hydraulic mineral oil of an Eimco rubber-tyred vehicle used in the underground part of the mine. He recalled that this testing of Oil-Glo in this machine began within about three months after Mr Thompson’s training finished, therefore sometime between September and December 2010. The testing ran for at least 4-6 weeks, Mr Bate deposed.
589 Following that successful trial, in the period November 2010 to March 2011 Mr Bate deposed that he was involved in aspects of the processes for working towards introducing Oil-Glo in the longwall fluid at Metropolitan.
590 Mr Bate also deposed to attending a meeting of Peabody’s Fluid Injection Task Force either during or shortly after Mr Thompson’s training program, therefore, in around June to September 2010. He recalled that Metropolitan’s representative on the Task Force was a fitter who had suffered an actual HPFI injury in May 2010. Mr Bate recalled Mr Withers providing the Task Force with descriptions of the Oil-Glo fluorescent dye additive and the practical demonstrations of pressurised injections that Mr Thompson gave using the chamber on the back of his trailer.
591 Mr Bate deposed that in around July 2011 he attended the New South Wales Minerals Council Safety Conference in the Hunter Valley. One of the presenters at the conference was Mr Peter Doyle from Peabody’s Corporate team, who had led the Task Force. Mr Bate recalled that part of Mr Doyle’s presentation included slides relating to the use of Oil-Glo as an additive to hydraulic fluids to enable detection under the skin using a UV light, to identify instances of HPFI. This was at a time when Oil-Glo had been introduced to the Solcenic longwall emulsion fluid at Metropolitan and they had already begun to see workers presenting with suspected HPFI injuries and having the UV light shone on them.
592 A second affidavit by Mr Bate was affirmed on 7 March 2019. In it he did no more than provide, first, further detail of a conversation he recalled having during the period of November 2010 to March 2011 with Mr Ron Brown, the long-standing Vice President of the workers’ union. Mr Bate deposed to having said to Mr Brown, in answer to a question of when Oil-Glo was going to be added to the longwall fluid, “we’re still going through the process of testing it in machinery, working out dosing and working out the safety procedures - but hopefully it will be very soon.… We just need to finish going through all the processes.”
593 Mr Bate deposed that the inclusion of Oil-Glo in the longwall fluid was not a test, but rather was the implementation of the Oil-Glo product at Metropolitan once the testing and trialling had been concluded. By that time, Mr Bate said, Mr Thompson had demonstrated that the Oil-Glo dye could enable injected fluid to be detected using a UV/blue light, the mechanical compatibility testing in the Eimco rubber-tyred vehicle, the dosage testing and the risk assessment had been completed, and the Oil-Glo additive had been approved for addition to the fluid of the operating longwall.
594 When Mr Bate gave his oral evidence on 15 March 2019, he made a significant correction or change to his written evidence. He accepted as “probably true” that Mr Thompson displayed the dye in pig flesh in or around October 2010. Mr Thompson had given a demonstration to Mr Withers and to Mr Nathan Thompson and after a short period of time, which could have been up to two weeks, Mr Wayne Thompson showed Mr Bate by demonstration that the dye could be seen in a piece of pig flesh. Mr Bate thought this could have been “the answer we were looking for for detecting hydraulic injection and not cutting people open, you know, needlessly and causing all that trauma.” To him the big driver was preventing unnecessary surgery.
595 In effect, rather than the events to which he had referred being in the period June and August 2010, Mr Bate’s evidence now was that the presentation, including the photos of pig flesh that had been injected and the blue light shining on it so that the dye underneath the skin could be seen, was “in the back end of 2010”.
596 In cross-examination, Mr Bate accepted that the corrections he had made in his oral evidence resulted in numerous passages in his first affidavit being incorrect.
597 Mr Bate accepted that, contrary to [22] of his first affidavit, he was not at a meeting between June and August 2010 when Mr Thompson provided him with a proposed PowerPoint presentation including photographs showing samples of pig flesh that had been injected by fluid with dye in it that glowed fluorescently under UV light. Mr Bate also accepted that Mr Thompson did not make the statement recorded in Mr Bate’s first affidavit as to the pig flesh being injected by hydraulic oil and the hydraulic or containing a fluorescent dye.
598 Mr Bate now accepted that when he first met Mr Thompson he did not see from him a presentation that included the pig skin and the fluorescent dye and the fluorescence of the dye. Mr Bate did not accept that he first saw the presentation including the pigskin and the dye and the fluorescent dye well into 2011 or towards the middle of 2011.
599 Mr Bate accepted that the matters that he said he had become aware of, listed at subparagraphs (a) to (d) of [578] above were not matters of which he was aware at all at that time.
600 Mr Bate accepted that the timing of his evidence, that at that time he appreciated from what Mr Thompson told in that washing the patient skin to remove the dyed hydraulic fluid from the surface would better enable the dyed hydraulic fluid to be seen fluorescing from under the skin if there had been an injection, was incorrect.
601 Mr Bate agreed that contrary to [24] of his first affidavit, in particular [24(a)] and part of [24(d)], the presentation Mr Thompson made to the OHS committee did not include pictures of the pig’s flesh injected with hydraulic oil and fluorescent dye and Mr Thompson did not discuss those matters with the committee at that time. Mr Bate accepted that Mr Thompson did not make any statement about the pig flesh and the Oil-Glo fluorescent dye in the UV light at the OHS committee meeting in July to August 2010 and neither did he say that there were versions of the Oil-Glo dye which could be added to mineral oil and to Solcenic longwall fluids before they were put into the machinery. Mr Bate also agreed that, contrary to his first affidavit at [24(d)], Mr Thompson did not then take members of the OHS committee to the first aid room and use the blue light to show the fluorescence of the dyed fluid on the pig flesh.
602 Mr Bate accepted that what he had said at [25(a)-(f)] of his first affidavit (see [584] above) was incorrect in so far as it said that he, Mr Bate, was aware of those matters as a result of the OHS committee meeting in July to August 2010.
603 Mr Bate accepted that what he had said at [27(b)] of his first affidavit was incorrect and that during his training in the period June to late August 2010 Mr Thompson did not hold up examples of the blue light and safety goggles from his injection detection kit and explain that they were how you could detect the presence of the dyed oil/fluid under the skin.
604 Mr Bate accepted that what he had said at [27(c)] of his first affidavit was incorrect and that Mr Thompson did not, at his training in a practical demonstration, allow workers to fire a shot of fluid at pig flesh during the period June to August 2010. Neither did Mr Thompson shine a blue light on any of the flesh he “shot” during these demonstrations to workers during his training sessions because Mr Thompson was not making any demonstration using flesh or a blue light at his training in the period July to August 2010. Similarly, Mr Bate accepted that Mr Thompson did not provide an explanation in giving his PowerPoint presentation in the period June to August 2010 that a blue light could be shone on skin to detect the presence of any dyed oil/fluid under the skin due to an injection.
605 Mr Bate accepted that Mr Thompson’s training sessions that Mr Bate attended in the period June to August 2010 did not disclose to him the matters at [25(a)-(f)] of his first affidavit (see [584] above).
606 Mr Bate agreed that, contrary to [72] of his first affidavit, a document annexed to his affidavit and referred to at [71] being a presentation entitled “Fluid Injection Testing - Demonstrating the force required to penetrate human tissue”, containing pictures of pig flesh injected with oil containing the fluorescent dye was not “very similar or possibly identical to the one [he] was first shown by Wayne Thompson in [their] one-on-one meeting…, that was shown to the OHS committee… and that was presented to participants in Wayne Thompson’s training program” in the period June to August 2010. Similarly, Mr Bate accepted that [72(d)] and [72(e)] of his first affidavit were incorrect in that what was recorded in the pictures did not reflect what happened in the period June to August 2010.
607 Mr Bate stated that the conversation he deposed to at [39] of his first affidavit that he had had with Mr Withers “in the two to three months immediately following the completion of Wayne Thompson’s training sessions (therefore, in around September to December 2010)” was possibly towards the end of 2010.
608 Mr Bate accepted, in relation to [42] of his first affidavit, that Mr Thompson did not show the blue light test kit during his training course in the period June to August 2010.
609 Mr Bate accepted, in relation to [43] of his first affidavit, that the testing of Oil-Glo in the outbye rubber-tyred machine at Metropolitan could have occurred later than in the period between September and December 2010 and it could have commenced early in 2011. He agreed that those tests were ongoing as at 21 March 2011.
610 Mr Bate accepted, in relation to [44] of his first affidavit, that the statement that he attributed to Mr Withers could have been made in March, or perhaps April, 2011.
611 Mr Bate accepted, in relation to [45] of his first affidavit, that the processes for working towards introducing Oil-Glo in the longwall fluid at Metropolitan could have happened in and around March, or perhaps April, 2011.
612 Mr Bate accepted that it would be likely that the steps involved in the dosing of the dye into the Solcenic, including the purchase and use of a pump to transfer dye from a 20 litre drum into the thousand litre pod of Solcenic and the subsequent steps involving decanting, occurred around the period March into early April 2011 based on a Control Implementation Action Plan.
613 Mr Bate believed that the dosing for the longwall itself was around the time March 2011 into early April 2011.
614 Mr Bate accepted that if [57] and [58] of his first affidavit were to be read as referring to the meeting of the Peabody task force that occurred at Metropolitan in or around June 2010, Mr Withers did not provide the task force with descriptions of Oil-Glo and the practical demonstrations involving Oil-Glo or the range of matters that Mr Bate had set out at [58] at that meeting.
615 Mr Bate accepted that if, as stated at [66] of his first affidavit, there were instances of workers presenting with suspected or possible HPFI injuries (and having a UV/blue light shone on them) prior to 2 September 2011, one would expect to be able to find a first aid form filled out in relation to those instances.
616 In light of these significant changes and concessions, I accept Quaker’s submission that I should have regard to Mr Bate’s evidence as to the timing of events only if it can be tied to objective material. Ultimately, I did not consider it necessary to have regard to Mr Bate’s evidence in deciding any of the contested issues of fact.
Mr Stewart Knight
617 Mr Knight was Deputy Managing Director, employed by Fuchs. He affirmed three affidavits.
618 In his first affidavit, dated 4 May 2018, Mr Knight deposed that he was responsible for all mining activities, human resource and purchasing. He deposed that he had extensive knowledge and experience of underground coal mining, particularly in relation to the variety of machinery used and the hydraulic, lubricating and other fluids used to run and maintain it. He deposed that the underground coal mining community in Australia was relatively small and tight-knit. He understood that his knowledge on topics relating to hydraulic fluids was substantial, given his experience, but by no means unusual or exceptional.
619 Mr Knight outlined some aspects of the historical development of the longwall and other hydraulic fluids used in underground coal mining, from at least the late 1980s in Australia. He deposed that Fuchs manufactured and marketed mineral hydraulic oils under the Renolin brand name. The most common hydraulic fluids were made primarily of mineral oil, but there were many variants including water-based products, such as longwall fluids. The Fuchs brand name for longwall fluids was Solcenic.
620 In use, Mr Knight deposed, longwall fluid concentrates were typically mixed with water (95-98 percent). He deposed that longwall fluid concentrate must mix with the water to be used, provide lubricity to seals and moving parts, be compatible with sealed materials, be rapidly biodegradable, prevent corrosion, be stable in a wide range of waters, the filterable and must have relevant equipment manufacture and government approvals.
621 Mr Knight deposed that in Australia, currently and since the late 1980s, there were two main suppliers of longwall fluids: Fuchs and Quaker. The larger market share has always been held by Fuchs, with Quaker holding the second largest market share.
622 Mr Knight provided some historical examples of dyes, including fluorescent dyes, which have been used in longwall fluids, including Fuchs’ Solcenic products and Quaker’s Quintolubric products, in Australia since the early 1980s. Mr Knight deposed that throughout his 33 years’ experience, dyes had been commonly used in hydraulic fluids for the purpose of identification and/or leak detection. He deposed that many of the longwall fluid concentrates sold by Fuchs in Australia over the past 33 years he had been working in the industry have produced, when mixed with water, a translucent “milky” emulsion that appeared to have a white colour. This milky fluid was readily detectable to the naked eye when it leaked from hydraulic longwall machinery underground in a coal mine.
623 Mr Knight deposed that Solcenic products in different regions had been died, including with fluorescent dye, during the time he had worked in the industry. He gave an example in the United States in the early 1990s when a Fuchs related company sought and obtained regulatory approval to add fluorescent dye to Solcenic 2B longwall fluid to enable the detection of leaks using a blue light.
624 Mr Knight deposed that, in Australia, Quaker’s Quintolubric longwall fluids, since they were first introduced to the market in the late 1980s, have contained a fluorescent green dye. Many Quintolubric products were synthetic and therefore transparent and almost colourless, and the green florescent dye therefore enabled the fluids to be readily identifiable, distinguishable and also detectable, including in underground coal mines using blue (ultraviolet) lights. The fluorescent green dye also provided additional leak detection capabilities to Quintolubric fluids, which had been touted by Quaker in its marketing materials. Mr Knight provided some examples.
625 In his second affidavit, dated 17 August 2018, Mr Knight deposed that since before 2 September 2011 he had been familiar with the addition to longwall fluids and mineral hydraulic oils of dyes that fluoresce in the presence of light and the supply of longwall fluids and mineral hydraulic oils containing such fluorescent fluid additives in Australia. He deposed to his understanding that Fluorescein was the active component of many fluorescent fluid additives that had been used by Fuchs and its related companies since at least the early 1990s in longwall fluids, mineral hydraulic oils and other hydraulic fluids, lubricants and industrial fluids.
626 Mr Knight deposed that since at least 1998, Fuchs had added fluorescein based fluorescent fluid additives to a variety of its products supplied in Australia. He gave examples based on his search of Fuchs’ business records. He deposed that fluorescein had also been used as the active fluorescing component of fluorescent fluid additives used in longwall fluids for several decades, since at least 1992. He provided examples.
627 Mr Knight deposed that since August 2012, Fuchs had supplied some of its customers with longwall fluid concentrate and emulsion products containing the FluidSafe WB fluorescent fluid additive promoted and supplied to the Australian underground coal mining industry by Quaker and previously by TTAAS.
628 Specifically, in so doing, Mr Knight deposed (taking account of corrections made by his third affidavit) that in accordance with the directions published by TTAAS and later by Quaker:
(a) Fuchs had added 750 ml of FluidSafe WB liquid dye per 100 litres of Solcenic 3002 concentrate and therefore 0.015% FluidSafe WB (volume/volume) after the concentrate was mixed with water at 2% to create the longwall fluid emulsion supplied to Peabody’s Metropolitan underground coal mine between August 2012 and April 2014;
(b) Fuchs had added 750 ml of FluidSafe WB liquid dye per 100 litres of Solcenic 2020 concentrate to create Solcenic 2020 D longwall fluid concentrate and therefore 0.015% FluidSafe WB (volume/volume) after the concentrate was mixed with water at 2% to create the longwall fluid emulsion supplied to Peabody’s North Goonyella underground coal mine between September 2012 and September 2013;
(c) Fuchs had added 750 ml of FluidSafe WB liquid dye per 100 litres of Solcenic 2020 concentrate before mixing that died concentrate at 4% with water to create a FluidSafe WB concentration in the final emulsion of 0.03% (volume/volume) in the Solcenic 2020 D 4% Emulsion longwall fluid products supplied to Yancoal’s Austar underground coal mine between December 2014 and June 2015; and
(d) Fuchs had added 750ml of FluidSafe WB liquid dye per 100 litres of Solcenic GM20 concentrate before mixing that died concentrate at 3% with water to create a Fluidsafe WB concentration in the final emulsion of 0.0225% (volume/volume) in Solcenic GM20 D Plus 3% Premix longwall fluid products supplied to Yancoal’s Austar underground coal mine since May 2016.
629 Mr Knight deposed that since August 2012, Fuchs had supplied some of its customers with mineral hydraulic oil products containing the FluidSafe OB fluorescent fuel additive promoted and supplied to the Australian underground coal mining industry by Quaker and previously by TTAAS. Specifically, Mr Knight deposed, in accordance with the directions published by TTAAS and later by Quaker, Fuchs had added 60 ml of FluidSafe OB liquid dye to 100 litres of Renolin B 68 to create a FluidSafe OB concentration of 0.06% by volume in:
(a) the Renolin B 68 D (MET) mineral hydraulic oil products supplied to Peabody’s Metropolitan underground coal mine pursuant to orders between September 2012 and December 2013;
(b) the Renolin B 68 D (NG) mineral hydraulic oil products supplied to Peabody’s North Goonyella underground coal mine pursuant to orders between September 2012 and November 2013;
(c) the Renolin B 68 D mineral hydraulic oil products supplied to Yancoal’s Austar underground mine pursuant to orders since November 2013;
(d) the Renolin B 68 D mineral hydraulic oil products supplied to Donaldson Coal’s Abel underground coal mine between May 2013 and June 2016;
(e) the Renolin B 68 D mineral hydraulic oil products supplied to Yancoal’s Moolarben underground coal mine pursuant to orders since March 2016.
In his oral evidence, Mr Knight made clear that Renolin B 68 is a product that contains no dyed material. Renolin B 68 D is the product that contains FluidSafe.
630 Mr Knight annexed to his second affidavit a bundle of copies of historical and current Product Information, MSDS and application ratio for the FluidSafe WB and FluidSafe OB products promoted and supplied in Australia by TTAAS and by Quaker.
631 Under the heading “FLA’s supply of Fluorescent Fluid Additives to Glencore mines for leak detection” Mr Knight set out in his affidavit details of Fuchs’ supply of longwall fluid products, and mineral hydraulic products, containing fluorescent fluid additives to Glencore mines since August 2015 for the purpose of facilitating leak detection. He deposed that between August 2015 and March 2016, Fuchs added a fluorescein-based water-soluble fluorescent fluid additive (in the form of a liquid created from powder known as Fluoresceine LT) to some of its longwall fluids. He provided details of the treat rate, that is, how much Fluoresceine LT powder Fuchs added per 100 litres of Solcenic longwall fluid concentrate or emulsion to create the products supplied to Glencore’s Ulan and Bulga mines.
632 Since around March 2016, Mr Knight deposed, Fuchs had been unable to source further Fluoresceine LT powder. As a replacement, since around April 2016 Fuchs had added another fluorescein-based water-soluble fluorescent fuel additive to its longwall fluids, that additive being Uniqua W400X. Mr Knight set out details of the treat rate using that liquid dye to achieve similar levels of fluorescence to Fluoresceine LT.
633 Mr Knight deposed that Fuchs had also supplied Glencore’s Ulan mine with another fluorescent fluid additive since October 2015. That product was a fluorescein-based oil-soluble fluorescent fluid additive, called Uniglow F-4 HF, which Fuchs added to its Renolin B Plus 68 mineral hydraulic oil to create the Renolin B Plus 68 LD product supplied to Ulan since October 2015. He provided details of the treat rate.
634 It was Mr Knight’s understanding, he deposed, that the Solcenic GM20 D containing Fluoresceine LT and Uniqua W400X, and Renolin B Plus 68 LD containing Uniglow F-4 HF, had only ever been made by Fuchs for Glencore for the purpose of facilitating the detection of leaks of those products when used in the longwall and other hydraulic equipment at Glencore’s mine sites.
635 His understanding, based on the reports he received from Fuchs’ sales representatives and product managers, and directions he gave to Fuchs’ sales representatives and product managers with respect to Fuchs’ supplies of Solcenic GM20 D and Renolin B Plus 68 LD to Glencore sites, was that: the concentration of fluorescent dye in the final product used at the Ulan and Bulga mine sites was the minimum and most cost-effective amount that Fuchs considered was sufficient to facilitate leak detection; the fluorescent characteristics of these Solcenic GM20 D and Renolin B Plus 68 LD products were only suitable and would only ever be used by Glencore at the Ulan and Bulga mine sites to facilitate detection of leaks using a UV/blue light; Glencore’s purpose and intentions with respect to requesting that fluorescent dye be added to longwall fluids and/or mineral hydraulic oils was confined to facilitating leak detection at the Ulan and Bulga mine sites; and Fuchs’ purpose in adding fluorescent dye to the Solcenic GM20 D and Renolin B Plus 68 LD products were only ever to facilitate Glencore’s detection of leaks of that fluid at the Ulan and Bulga sites.
636 Mr Knight annexed to his affidavit a bundle of copies of emails and attached documents by way of illustration. He set out in his affidavit what he stated the emails and their attachments demonstrated with respect to the statements made by Fuchs and by Glencore with respect to the purpose for which fluorescent dyes were added by Fuchs to longwall fluids and mineral oils supplied to Glencore, and the expected use of those products.
637 In his third affidavit, dated 2 November 2018, Mr Knight explained that the Peabody provided to Fuchs the FluidSafe WB added to products supplied to its Metropolitan and North Goonyella mines. Peabody sent the FluidSafe WB and FluidSafe OB to Fuchs’ production plant at Wickham, in Newcastle, New South Wales. At that Wickham production plant, Fuchs added FluidSafe WB to Solcenic 3002 concentrate supplied to Metropolitan and to Solcenic 2020 concentrate (to create Solcenic 2020 D concentrate) supplied to North Goonyella.
638 Mr Knight deposed that Fuchs purchased from TTAAS:
(a) the FluidSafe OB added to Renolin B 68 to create the Renolin B 68 D (MET) mineral hydraulic oil supplied to Metropolitan and Renolin B 68 D (NG) supplied to North Goonyella;
(b) the FluidSafe WB added: to the Solcenic 2020 concentrate (to create Solcenic 2020 D 4% Emulsion) supplied to Austar for orders dating from 16 December 2014 to 18 June 2015; and to the Solcenic GM20 concentrate (to create Solcenic GM20 D Plus 3% PREMIX) supplied to Austar for orders dating from 24 May 2016;
(c) the FluidSafe OB added to the Renolin B 68 (to create Renolin B 68 D) mineral hydraulic oil supplied to Austar and Moolarben;
(d) the FluidSafe OB added to the Renolin B 68 (to create Renolin B 68 D) mineral hydraulic oil supplied to Abel. Fuchs had never supplied longwall fluid (undyed or dyed) to the Abel mine as it did not have a longwall before it was placed into care and maintenance in June 2016.
639 Mr Knight deposed that from 1 January 2010 to August 2015, the only Solcenic longwall fluid products Fuchs supplied to any customers in Australia containing any fluorescent fluid additives were the Solcenic 3002, Solcenic 2020 D, Solcenic 2020 D 4% and Solcenic GM20 D Plus 3% Premix products containing FluidSafe WB to which he had already referred. Prior to August 2015, Fuchs did not supply any customer in Australia with any Solcenic longwall fluid products containing Fluoresceine LT, Uniqua W400X or any fluorescent fluid additive other than FluidSafe WB.
640 From 1 January 2010 to 8 October 2015, Mr Knight deposed, the only Renolin mineral hydraulic oil products Fuchs supplied to any customers in Australia containing any fluorescent fluid additives were the Renolin B 68 D products containing FluidSafe OB to which he had already referred. Before October 2015, Fuchs did not supply any customer in Australia with any Renolin mineral hydraulic oil products containing Uniglow F-4 HF or any fluorescent fluid additive other than FluidSafe OB.
641 At no time since 1 January 2010, Mr Knight deposed, had Fuchs supplied any fluorescent fluid additives, FluidSafe WB, FluidSafe OB, Fluoresceine LT, Uniqua W400X, Uniglow F-4 HF or otherwise, as standalone products.
642 Mr Knight referred to the supplies of products containing fluorescent fluid additives described in his second affidavit and deposed that the only other customers in Australia to which Fuchs had supplied any Solcenic longwall fluid products containing fluorescent fluid additives since August 2015 (and, indeed, since 1 January 2010) were:
(a) Solcenic GM20 D, containing 8g of Uniqua W400X per 100 litres of Solcenic GM20 concentrate (orders dated from 2 May 2016) to the Ulan West underground coal mine operated by Glencore;
(b) Solcenic GM20 3% Premix Emulsion, containing 8g of Fluoresceine LT per 100 litres of Solcenic GM20 concentrate (orders dated from August 2015 to March 2016), and later 8g of Uniqua W400X per 100 litres of Solcenic GM20 concentrate (orders dating from April 2016), in each case before that concentrate was mixed with water at 3% (at Fuchs’ Wickham site, in Newcastle), to the Ashton underground coal mine operated by Yancoal;
(c) Solcenic GM20 LD, containing 180g of Uniqua W400X per 100 litres of Solcenic GM20 concentrate (orders dated from 14 July 2016), to the Broadmeadow underground coal mine operated by BMA;
(d) Solcenic GM20 3% Premix Emulsion, containing 8g of Uniqua W400X per 100 litres of Solcenic GM20 concentrate (orders dated from December 2017 to February 2018), before that concentrate was mixed with water at 3% (at Fuchs’ Wickham site, in Newcastle), to the Moolarben underground coal mine then operated by Yancoal; and
(e) Solcenic GM20 D, containing 8g of Uniqua W400X per 100 litres of Solcenic GM20 concentrate (orders dated from 29 August 2018), to the Narrabri underground coal mine operated by Whitehaven Coal.
643 In his third affidavit Mr Knight referred to the explanation in his second affidavit that the Solcenic GM20 D longwall fluid products containing Fluoresceine LT and Uniqua W400X, and the Renolin B 68 Plus LD mineral hydraulic oil products containing Uniglow F-4 HF, had only ever been supplied by Fuchs to Glencore’s Ulan (Solcenic GM20 D and Renolin B 68 Plus LD) and Bulga (Solcenic GM20 3% Premix Emulsion only) mines, on his understanding, for the purpose of facilitating leak detection.
644 Mr Knight deposed that the concentration of fluorescent fluid additives in the final Solcenic GM20 D (Fluoresceine LT or Uniqua W400X dye) products supplied to Glencore’s Ulan and Bulga (Solcenic GM20 3% Premix Emulsion only) mine sites to his understanding was the minimum and a cost-effective amount that Fuchs considered was sufficient to facilitate leak detection. He deposed that Fuchs adopted the treat rate of 8g Fluoresceine LT per 100 litres of Solcenic concentrate on advice provided to members of Fuchs’ technical team in Newcastle by Fuchs’ Global Head of Water-Based Chemistry, Mr Paul Littley.
645 In his oral evidence, Mr Knight explained this reference to the minimum and a cost-effective amount as follows:
… we were making sure that we had a product that was capable of leak detection and also was the most cost-effective way that we could do it, and we had sourced material and adopted a treat rate that had been taken up by Fuchs Poland, and we believed that to be the best low-cost and cost-effective leak detection treat rate. … We were trying to keep the cost of the material as neutral as possible … compared to selling our product without the dye in it.
646 Similarly, Mr Knight deposed, the same minimum and cost-effective concentration of fluorescent fluid additives (8g per 100 litres of Solcenic concentrate) was added to the Solcenic GM20 D longwall fluid products supplied to Glencore’s Ulan West mine and Whitehaven Coal’s Narrabri mine, and to the Solcenic GM20 3% Premix Emulsion longwall fluid products supplied to Yancoal’s Ashton and Moolarben mines.
647 Mr Knight explained Fuchs’ selection of the treat rates for Fluoresceine LT and Uniqua W400X in Solcenic longwall fluid products.
648 Mr Knight deposed to an absence of further testing by Fuchs of these products. For example, he deposed that Fuchs had never undertaken any further or separate testing of its longwall fluid products containing 8g Fluoresceine LT or Uniqua W400X per 100L (Solcenic GM20 D and Solcenic GM20 3% Premix Emulsion) to evaluate their suitability or effectiveness in facilitating leak detection for any customers. Rather, once it had been selected, the relevant treat rate continued to be adopted in preparing all Solcenic GM20 D and Solcenic GM20 3% Premix Emulsion products supplied by Fuchs to customers.
649 In cross-examination, Mr Knight agreed that Yancoal’s Ashton mine prior to August 2015 used Solcenic 2020 with no fluorescent dye included and accepted there was a change from Solcenic 2020 at 4% to Solcenic GM20 at 3% with dye in it.
650 Mr Knight agreed that a particular table of supplies of Solcenic and Renolin products, which included entries referable to 2015, indicated that although in some cases Fuchs supplied products, including a fluorescent dye, in response to a request by the customer for a product that could be used for leak detection, in other cases the product was supplied not in response to a request by the customer, but rather as a proposal by Fuchs of a product that could be used for leak detection.
651 Mr Knight agreed that in some instances Fuchs supplied a product in response to a request by customer for a high concentrate product that could be used in HPFI detection. He also accepted that some customers requested a high concentration product for use in HPFI detection but in at least some cases a customer simply requested a product for use in HPFI detection without reference to any particular concentration.
652 It was Mr Knight’s understanding that Fuchs had been supplying Yancoal’s Moolarben mine with Renolin products containing the FluidSafe fluorescent dye for HPFI detection. His understanding was that as at the present day there was no dye in the Moolarben long wall. He said Fuchs had no intention to supply the Moolarben mine with Solcenic product including a fluorescent dye in the near future.
653 Mr Knight was taken to a statement he made in an email dated 27 May 2014: “Where we are selling for HPI detection, we need to us (sic) Fluidsafe.” Mr Knight agreed that Fuchs was selling FluidSafe for HPFI detection with Solcenic and that customers had requested that product for use in the detection of HPFI injuries. That was why Fuchs sourced from TTAAS at that point in time. Mr Knight said that Fuchs did not support the HPFI-dye methodology and was only providing the dye in an HPFI scenario where it was requested by the customer and that the only dye that Fuchs were supplying at that time was FluidSafe.
654 The following exchange occurred:
You would agree with this proposition, would you not, Mr Knight? Fuchs, in cases where a customer requested it, was selling its Solcenic product with Fluid Safe in it for HPI detection?---We were selling the Solcenic product with the Fluid Safe in it at the recommended concentration, because we could manufacture that concentration and ensure that was what was specified by the Fluid Safe supplier, and we sold that to the customer.
And you sold that product to the customer for HPI detection, did you not?---Yes, we did.
655 Mr Knight agreed that at the time in 2014 his view was that if Fuchs did not use FluidSafe in a product sold for HPFI detection there was a risk of infringement of the TTAAS or Quaker patent. Also, FluidSafe was the only material that Fuchs was aware of that was available for HPFI at that point in time. There was data in relation to the use of FluidSafe for HPFI detection.
656 Mr Knight agreed that subsequently Fuchs did supply to Broadmeadow for HPFI detection its Solcenic product including a dye that was not FluidSafe. Fuchs matched the colour of the FluidSafe dye at recommended concentration with an alternative dye and that is what Fuchs sold to Broadmeadow, which had specifically requested HPFI dye. Mr Knight accepted that the purpose of the supply of the product to Broadmeadow was HPFI detection. Mr Knight agreed that he had reason to believe at the time of the supply the product to Broadmeadow for use in HPFI detection that Broadmeadow would use it for that purpose.
657 Mr Knight accepted that Fuchs had not conducted any testing of any product that it had supplied for use in leak detection to ascertain whether or not that product would also be capable of being used by Fuchs’ customers for the purpose of HPFI detection.
658 Mr Knight confirmed that for North Goonyella and Metropolitan, those mines bought the FluidSafe dye and Fuchs put that product into the Solcenic at their request. Those mines were dealing direct with Mr Thompson. Mr Knight accepted that Fuchs were supplying the hydraulic oil products, including the FluidSafe dye, on the understanding that it was to be used by the customer for HPFI detection. On the other hand, Wambo and NRE Gujarat were themselves mixing on the site FluidSafe dye with Solcenic to be used in their longwall systems.
659 Mr Knight agreed that it was his view as at 26 March 2013 that the supply of a product including fluorescent dye for use in HPFI detection without a licence from the owner of the patent, at that time TTAAS, would be in violation of the patent and that remained his view subsequently when Fuchs supplied product including a fluorescent dye to Broadmeadow mine for use in HPFI detection.
660 Mr Knight agreed that at the time he wrote an email on 24 January 2014 his view was that the product had been proved to be successful in identifying HPFI injuries on several occasions and also identifying where an HPFI had not taken place, his view being on the basis of the documentation that Fuchs saw from Wambo, North Goonyella and Metropolitan.
661 Mr Knight did not accept by reference to an email of 18 December 2015, copied to him, that Mr Littley was indicating there his belief that a Fuchs product containing a dye for leak detection purposes could also be capable of being used for HPFI detection. Mr Knight said it had been clear that a dye for leak detection was a lot lower concentration than a dye for HPFI detection.
662 Mr Knight said that a dye at a low concentration would be very difficult to pick up in an HPFI scenario but accepted that that understanding was not based on any testing.
663 Mr Knight was taken to an email from Mr Pearce dated 21 December 2015 which referred in particular to Broadmeadow and agreed that at the time of that email he understood Mr Pearce to be referring to the use of a dye other than FluidSafe in a product to be supplied by Fuchs to Broadmeadow for use in HPFI detection. He also agreed that Mr Pearce, at least, considered that it would be necessary to convey to the customer in some way that Fuchs’ view was that the product could assist in HPFI detection. By Mr Pearce’s reference to the statement being worded carefully, Mr Knight understood that to convey a concern on Mr Pearce’s part that if Fuchs were too explicit in its statement that the product could assist in HPFI detection that may be able to be used against it down the track if there was any issue of infringement with the Patents. Mr Knight agreed that he had formed the view at that time, December 2015, that Fuchs should proceed to consider providing such a product to Broadmeadow, preparing a statement of that kind and taking the risk that there may be some claim of infringement of the patent.
664 Mr Knight agreed that Mr Littley’s response to his email suggested that notwithstanding the customer had requested the use of the product for HPFI detection, Fuchs should ensure that the written request from the customer specified leak detection.
665 Mr Knight did not agree that Mr Rushton in an email dated 21 December 2015 was indicating to him that a product containing a fluorescein dye for leak detection was also one that would be capable of being used in HPFI detection.
666 Mr Knight was taken to the following in a Fuchs draft statement from that period:
It is impossible, to separate the use of dyes from the well-established detection of fluid leakage to that which is being reported as diagnostic methods for medical use such as aiding in the identification of high-pressure injection of fluids.
Again, Mr Knight’s evidence that the statement was not saying that the use of dye for leak detection could not be separated from the use of dye for HPFI detection but that it was saying that the dye that was used to leak detection was the same dye that was used for HPFI detection. He agreed that the passage did not make any statement about concentration.
667 Mr Knight said he was not comfortable that the product that was proposed to be supplied to Broadmeadow, to which this statement would relate, would be capable of being used in the detection of HPFI. He was satisfied that Fuchs had made a product with high concentration and match the colour of the FluidSafe product which, purportedly, was good for HPFI and he believed that the product Fuchs proposed to supply would be capable of being used by the customer, Broadmeadow, in HPFI detection. Fuchs’ intention, he said, was to sell the product to the customer to cover HPFI that the customer wanted. He wanted to convey to the customer that the product could be used for that purpose.
668 Mr Knight’s understanding was that the draft statement that had been prepared was “suitably vague” in that it did not explicitly say that this product was suitable for use in HPFI detection.
669 Mr Knight said that although the draft statement did not express anything about concentration, it was implicit, in the knowledge of Mr Rushton and Mr Littley, that the dye going into Broadmeadow was for HPFI and was at high concentration.
670 Mr Knight understood Mr Littley to expressing the view that Broadmeadow should provide a statement to Fuchs to say that Broadmeadow wanted a fluid with dye to enable leak detection so that Fuchs would not be in breach of patent by supplying a high concentration product for HPFI injection.
671 Mr Knight understood that Mr Pearce was proposing that he would attend the site at Broadmeadow and attempt to convince the customer verbally rather than in writing, to the extent that he needed to do so, that the product could assist in HPFI detection. Mr Pearce gave a presentation at Broadmeadow and sought to convince Broadmeadow that Fuchs had a product that satisfied their request for HPFI detection.
672 Mr Knight agreed that the dye in the product that was proposed to be supplied would stain the skin and muscle tissue and could assist in HPFI detection; the dye was designed to assist in HPFI detection.
673 In relation to a later version of the presentation, Mr Knight said that Mr Pearce later presented to Broadmeadow at the site about the proposed supply of the product for HPFI detection and Mr Pearce did use a presentation of that kind. Mr Knight was not present at the presentation and could not indicate whether the presentation Mr Pearce used was the one to which he was being taken in cross-examination or a different one.
674 As to the form of the presentation seen by Mr Knight on or about 24 December 2015, Mr Knight asked Mr Pearce not to leave a copy of it at Broadmeadow because of the concern on Mr Knight’s part that that sort of document would provide evidence that Fuchs understood and believed that the product it proposed to supply at Broadmeadow was capable of and suitable for use in HPFI detection. Fuchs did not want Mr Pearce to leave a copy because Fuchs believed that it would be in breach of patent and did not want the draft presentation to be available down the track to show that Fuchs believed that the product would be used in HPFI detection.
675 Mr Knight agreed that at the time of an 8 June 2016 email he shared a concern that there was a real risk that Fuchs could find itself in court soon for the supply of a high concentration dye in a product for Broadmeadow. He believed at that time that Broadmeadow planned to use for the patented purpose the product supplied by Fuchs.
676 Mr Knight was taken to a letter dated 1 July 2016 by Fuchs’ firm of patent attorneys and was asked whether he agreed, in light of Mr Pearce’s presentation to Broadmeadow, that it was incorrect to state that Fuchs had never included as part of its point of sale promotional material, labelling, technical advice and so forth any suggestion to customers of their product that an intended use of the product was in HPFI protection. Mr Knight agreed that it was incorrect in so far as it related to Broadmeadow.
677 Mr Knight agreed that in light of Broadmeadow it was wrong to say that no information or documentation suggesting that Fuchs was selling a product for which there was referencing that it could be used in HPFI detection was available at the time of that letter from Fuchs’ patent attorneys, and it was false to suggest that in relation to Broadmeadow.
678 As with Mr Roberts, I deal with Quaker’s challenge to Mr Knight’s evidence when considering the documents on which that challenge was based.
Ms Katrina Crooks
679 I note that Fuchs initially relied on an affidavit of Ms Katrina Crooks, legal practitioner, affirmed 10 May 2018, who annexed to her affidavit copies of a number of brochures. These were admitted as limited to establishing that the statements were made and subject to relevance. The relevance was said to be to Fuchs’ misleading and deceptive conduct case which was ultimately not pressed. It follows that this affidavit is not relevant and I reject it.
The evidence of the expert witnesses
680 Each side called an expert. There was a general objection on each side. The general objection was to the expertise, the field of expertise and how that field, if any, related to the evidence of each of the experts. My ruling is that each of the experts was sufficiently qualified for their evidence to be admissible in relation to the technology available and as to general circumstances prevailing at mines. Neither witness was qualified to give medical evidence.
Mr Russell Smith
681 Mr Smith, called by Fuchs, was the founder and managing director of Quantise Consulting Engineers Pty Ltd and previously employed in various engineering roles by an engineering consultancy firm. His roles in those two firms had focused primarily on the provision of engineering services in relation to hydraulic machinery and equipment used in underground and open-cut (surface) mining. Mr Smith deposed that he had 39 years’ experience as an engineer working in different parts of the mining industry of Australia, including 28 years’ experience in roles relating to the operation, maintenance and relocation of longwall equipment, particularly its hydraulic components and systems.
682 Mr Smith deposed that he was one of seven inventors named in Australian Patent No. 2013315063 for an invention entitled “Cutter head for mining machine”.
683 Mr Smith described safety issues, protocols and tools used in longwall operations during the period up to and immediately before 2 September 2011.
684 He deposed that, given the escape of fluids at very high hydraulic pressures was the major risk associated with visiting and working on a longwall, he was prior to 2 September 2011 aware of the potential HPFI injuries that could be caused by such escaping fluids. He referred to MDG 41, published in December 2010, which included in its Appendix D a table of common types of HPFI injuries and near misses relating to escapes of hydraulic fluid. The table focused on describing the nature, cause and effects and recommended actions relating to HPFI injuries and near misses from the perspective of dealing with the mechanical machinery and hoses and following regimented incident management and risk assessment protocols. Appendix E was a safety, health and environment procedure entitled “Fluid Injection Protocol”. That protocol’s procedure for treating persons with apparent HPFI injuries essentially involved calling an ambulance immediately, keeping the patient rested and immobilised and not giving any fluids or food. It included additional information explaining the severity of HPFI injuries, even if they appeared innocuous, and that all suspected incidents should be treated as medical emergencies with specialist medical assistance sought immediately.
685 Mr Smith was asked to comment upon each claim of the Patents in light of the descriptions in the specification and the common general knowledge of an engineer experienced in working with hydraulic longwall machinery and equipment as at 2 September 2011. He said that the invention described in the specification was a simple method of adding fluorescent dye to the fluid in a hydraulic system and, if it was suspected that fluorescently-dyed fluid had been injected into a person, shining a UV light on the site of suspected injection, with fluorescence in or under the skin indicating an injection had occurred. His overall understanding from reading the specification was that the technical advances achieved by that method were the inclusion of a fluorescent dye in hydraulic fluid, with the dye remaining in the hydraulic fluid during the normal operation of the machinery at hydraulic pressures, and the use of a UV light to examine sites of potential fluid injection for the presence of fluorescence that indicated injection had occurred.
686 In Mr Smith’s view, the invention, to facilitate detection of an injury rather than detection of a leak, was very similar to long-established leak detection methods. Mr Smith accepted that he did not expressly in his affidavit characterise the invention, as claimed, as a version of a long-established fluorescent dye leak detection method repurposed for the detection of fluid injection injuries.
687 Mr Smith deposed that, other than claims 20 and 21 of the Standard Patent, the minimum concentrations of dye required to meet all the fluorescence requirements were not features of the other claims but he nonetheless understood there would be a minimum concentration of the fluorescent dye required to achieve its functional requirements, which concentration would likely be different depending on the particular fluorescent dye being used.
688 Mr Smith’s opinion was that, to his understanding, the Method did not necessarily enable the absence of HPFI injuries to be confirmed, as nowhere in the specification was the ability to confirm non-injection described. Absence of fluorescence would not, in his view, be sufficient definitively to determine that no HPFI injury had occurred: if no fluorescence was detected, it still would be possible fluid injection had occurred. All persons potentially suffering HPFI injuries would still be treated according to standard protocol, whether or not the method detected fluorescently-dyed fluid in or under the skin. A person who may have suffered an HPFI injury was, following the standard HPFI treatment protocol, sent to hospital for medical attention. That would not necessarily occur any sooner as a result of the method being performed than if the method had not been performed. Mr Smith’s opinion was that the medical attention, and any invasive surgery, were likely to commence just as quickly after the incident whether or not the method was used, meaning any injected fluid was likely to have been within the body for the same amount of time.
689 Mr Smith deposed that claim 1 of the Standard Patent required fluorescent dye to have been added to the fluid of a hydraulic system and a possible fluid injection to have occurred, but did not actually require any UV light to be shone on the patient or any determination of whether fluid injection had occurred. This, he said, was an incomplete description of the method, as he understood from the specification, as shining a UV light on the patient and detecting fluorescence and thereby determining an injection had occurred were essential steps in the method. Leaving aside the description in the specification, Mr Smith said this was not a “method of detecting fluid injection in a patient” as it lacked the actual detection step involved in shining the UV light. In his view the method defined by claim one of the Standard Patent could not be performed unless a possible fluid injection occurred in a patient. The difficulty he had with claim 1 of the Standard Patent was that there was no step at all following a possible fluid injection occurring in a patient, let alone any step that might actually detect a fluid injection, such as shining a UV light.
690 In relation to claim 1 of the Innovation Patent, Mr Smith repeated his comments regarding claims 1 and 2 of the Standard Patent.
Dr Peter Gray
691 Dr Gray, called by Quaker, was the Managing Director of Ground Support Services Pty Ltd, an engineering consulting company. He had more than 42 years’ experience in the civil and mining engineering industries and a PhD in civil and mining engineering. From 1973 to 1985 Dr Gray was employed by BHP Collieries as a Geotechnical Engineer in its Rock Mechanics Group. He was also personally experienced in developing technologies for the mining, civil and steel industries, and he listed patents and patent applications on which he was named as an inventor.
692 Dr Gray gave his understanding of what was required in order to supply a new technology to a coal mine: the testing required would depend on the product and/or components of the product (for example, chemical, electrical, et cetera) proposed to be introduced. Such supply would need to demonstrate that the technology would be beneficial to the mine in some way.
693 He formed the view that the Patents in suit were directed to a method for the early detection of an accidental fluid injection injury in a human. The invention, he said, provided a solution to the problem of rapidly detecting fluid injection into human skin which may be otherwise difficult to see with the naked eye, particularly in a mining setting (both above and below ground), though not only in that environment.
694 Dr Gray deposed that despite his 42 years of experience in the mining industry, he had never heard of nor learnt of fluorescent dyes being used to detect HPFI injuries prior to reading the Patents. He was only generally aware of the use of fluorescent dyes for groundwater tracing by hydrologists.
695 Dr Gray agreed with Mr Smith that the method specified in the Patents was simple, but in his opinion it was a clever method and its simplicity was of great benefit.
696 Dr Gray distinguished between a method for detecting fluid injection in a patient, that is, detecting the presence or absence of fluid injection, from diagnosis of a fluid injection which had a high threshold. In his opinion, the word “detecting” as it appeared in the claims did not mean diagnosing, and he understood the word “detecting” to merely require the identification of something that may or may not be there. While the method may not diagnose every suspected fluid injection, Dr Gray believed it was highly likely that the invention would at least detect the absence of a fluid injection. He set out the circumstances in which the method may not identify a suspected fluid injection injury. He estimated that the risk of a fluorescent dye which had come into contact with a person’s skin not leaving a trace, especially in circumstances where that fluid had penetrated the person’s skin, was extremely low.
697 Dr Gray disagreed with Mr Smith’s statement that the medical attention and any invasive surgery were likely to commence just as quickly after the incident whether or not the method was used. Dr Gray’s opinion was that the method would help to distinguish between other physical injuries and fluid injection injuries and, if a fluid injection had occurred and fluorescence were detected under the skin, that it may also assist hospital staff more accurately to assess the extent of fluid injection under the skin. The method simply provided a mechanism to detect whether a fluid injection injury had occurred.
698 Dr Gray disagreed with Mr Smith’s views of claims 1 and 2 of the Standard Patent.
Joint report and concurrent oral evidence
699 The experts filed a joint report dated 14 February 2019. The matters on which they disagreed were far more extensive than the matters on which they agreed.
700 The joint report addressed: (1) each expert’s understanding of the invention claimed in the Patents; (2) whether claim 1 was incomplete as not reciting a step of providing or using any light; (3) the concentration of the “fluorescent dye” in the “fluid for use in machinery” required to perform the claimed methods in the Patents; (4) whether injected fluid could be detected by performing the claimed methods in the Patents; and (5) whether washing the patient as claimed in claims 4, 5, 14 and 15 of the Standard Patent could have the effect of washing away the fluorescent fluid under the skin.
701 The experts agreed on the following principal points: the method could be used to detect fluid injection injuries occurring in a broad range of industries and pressurised fluid systems; fluid injection injuries might be shallow just under the skin or may extend deeper into limbs or the body; many different fluorescent dye types might be used, and different concentrations may be required for different dyes used in different hydraulic fluids and for different applications; both the dye concentration and the intensity of the ultraviolet light must be sufficient for fluorescence beneath the skin to be visually detectable; shallow injection injuries should be detectable using the method; where fluid has penetrated deep into the body, limbs or tissue, the full extent of the injury might be undetectable; some dyed fluid could be washed from the injection point depending on the shape and dimensions of the entry wound; it was very unlikely that dyed fluid would be washed from beneath the skin.
702 The experts disagreed as follows. As to topic (2) above, Mr Smith’s opinion was that claim 1 of the Standard Patent was an incomplete method for detecting fluid injection in a patient because it lacked any step of exposing the suspected injury to ultraviolet light in order to detect it, whereas Dr Gray’s opinion was that claim 1 was complete because ultraviolet light was present. As to topic (4) above, Mr Smith’s opinion was that the method was unable to detect the absence of fluid injection injuries, whereas Dr Gray’s opinion was that the method was highly likely to be able to detect the absence of fluid injection injuries.
703 These experts also gave concurrent oral evidence.
704 Relevant, in my opinion, to topic (1) above, the following exchange occurred in cross-examination:
MR DIMITRIADIS: Indeed, a method would not be a method for detecting fluid injection in a patient if one didn’t have that step; correct.
MR SMITH: That’s my position.
MR DIMITRIADIS: It’s perfectly clear to you, applying your common sense reading this claim, that you would need to include in the method some detection step in order to (sic) it to meet the claim; correct?
MR SMITH: Correct.
705 Relevant, in my opinion, to topic (3) above, Mr Smith gave evidence that: “You can buy the fluorescent dyes from a number of vendors. They … provide them so that they fluoresce in whatever fluid it is you’re using them in. … They will tell you how much to put in in order to do that.”
706 Dr Gray agreed in cross-examination that determining the presence of fluorescence by itself may be at the step of seeing it on the skin, and you may need to go further to see whether it is under the skin.
707 Mr Smith accepted in cross-examination that he was not able to say categorically that a product supplied for use in leak detection, including a particular concentration of dye, would not be capable of being used in the method of the patents.
708 The following further exchange occurred in the cross-examination of Mr Smith:
MR DIMITRIADIS: Your view is, having read the patents, that shallow injection injuries should be detectable using the method of the invention.
MR SMITH: Yes, I think they should be.
MR DIMITRIADIS: And your view is that where the fluid has penetrated deep into the body, limbs or tissue, the full extent of the injury might not be detectable; correct?
MR SMITH: That’s – that’s right, certainly the full extent.
MR DIMITRIADIS: But even in that circumstance where there is a deep penetration of fluid into the body, limbs or tissue, it may well be the case that a shallow part of that injury could be detectable; correct?
MR SMITH: It will depend on the nature of the wound. If it’s a very fine stream that’s gone in, I have no idea.
MR DIMITRIADIS: Would you defer to a medical professional on that topic?
MR SMITH: Well, I would defer to a medical professional on any injection risk injury.
709 On the issue of concentration, the following exchanges occurred:
MR MOORE: … you’re agreeing with me, I think, that in order to work out what would fluoresce, how much … you would need to fluoresce beneath the skin for it to be workable, you would need some information.
DR GRAY: You would need information on that before you actually did it … in that particular application, which might be different to another application.
…
MR DIMITRIADIS: In short, it would be possible to, on that assumption, in the laboratory, combine the proposed dye with the hydraulic fluid and do some visual tests alongside the Oil-Glo in the hydraulic fluid at the relevant concentration given in the patent to see the point at which one achieved a similar level of fluorescence; correct?
MR SMITH: There should be some comparison test possible.
MR DIMITRIADIS: And that would give you some comfort that whatever the concentration of the proposed dye is in the hydraulic fluid would be appropriate for use in the method; correct?
MR SMITH: Yes.
710 In my opinion, both experts gave their evidence in a forthright manner, although I regard Dr Gray’s evidence as more cogent. For example, I was not persuaded by Mr Smith’s reasons for why he did not include in his affidavit, but emphasised in the joint report and in his oral evidence, his proposition as to the close similarity between the use of dye in fluids for leak detection and the use of dye in fluids for HPFI detection. I also consider that Dr Gray gave more direct answers to the questions he was asked by counsel in cross-examination. Mr Smith, in my opinion, on a number of occasions did not initially answer the question he was asked.
Consideration
Construction of the Patents and their alleged deficiencies
711 In Davies v Lazer Safe Pty Ltd [2019] FCAFC 65 at [42]-[45], the Full Court said that although the claims are to be construed in the context of the specification as a whole, it is not legitimate to narrow or expand the boundaries of monopoly as fixed by the words of a claim by adding to those words glosses drawn from other parts of the specification. It is legitimate to refer to the rest of the specification to explain the background to the claims, to ascertain the meaning of technical terms and resolve ambiguities in the construction of the claims.
712 It is an error to construe the claims with an eye to the alleged infringement: Welcome Real-Time SA v Catuity Inc [2001] FCA 445; 113 FCR 110 at [21].
713 The Full Court in Fresenius Medical Care Australia Pty Limited v Gambro Pty Limited [2005] FCAFC 220; 224 ALR 168 at [94] emphasised the importance of the actual language used in the claim to define the invention, as follows:
The whole of the specification must be read in order to construe the claim. So much is not in dispute. However, that does not mean that the words of the claim are to be ignored. Nor can it be ignored that the patentee has chosen not to include in a claim matters, integers or aspects of the invention that have been referred to in the body of the specification. This accords with the well-accepted principle enunciated in Welch Perrin [& Co Pty Ltd v Worrel (1961) 106 CLR 588] at 610 that glosses drawn from the body of the specification cannot be used to narrow or expand the boundaries of the monopoly as fixed by the words of a claim.
714 In the Standard Patent, under the heading “Field of the Invention” the Patent states that the invention relates to the detection of fluid in the human body and in particular to the detection of hydraulic and fuel fluid within the human body. It states that the invention has been developed primarily for use in detecting the presence of hydraulic and diesel fuel in the human body as a result of accidental fluid injection, but the invention was not limited to this particular field of use.
715 Under the heading, “Background of the Invention”, the Patent states that an accidental fluid injection beneath the skin can cause serious tissue damage. A fluid injection injury can become very serious or even fatal if not dealt with promptly and properly. The longer the delay in getting professional medical aid, the further the tissue damage can spread. If left untreated, the injury could result in disfigurement, amputation of the affected part or death of the victim. Importantly, the Patent states:
Accidental fluid injections can be difficult to confirm. That is, victims may be covered by fluid externally however there may be uncertainty on whether any fluid has penetrated the victim’s skin. As discussed above any delay in treating a victim can cause sever[e] harm and as such it would be advantageous if confirmation of fluid injection can be confirmed.
716 The “Summary of the Invention” section is set out over several pages of the specification. It states that “According to a first aspect of the invention there is provided a method for detecting fluid injection in a patient, the method including three steps where the first word is “providing” and a further eight steps where the first word is “preferably”.
717 According to a ninth aspect of the invention, there is a method for detecting fluid injection in a patient, the method including the steps of: providing a fluid storage tank; providing fluid for use in machinery and adding said fluid to the fluid storage tank; providing a fluorescent dye and adding the fluorescent dye to the fluid such that the fluid fluoresces in the presence of ultraviolet light; and a possible fluid injection occurring in a patient.
718 The specification includes 14 figures, although it refers as well to “Figures 12 – 25” as showing a sample training course according to the invention, some of which are then described.
719 The specification provides, under the heading “Preferred Embodiment of the Invention”, that the preferred embodiment of the invention provides a method for detecting fluid injection in a patient following a possible fluid injection. It then refers to the method including the step of providing a fluid storage tank (equipment having a diesel or hydraulic tank); and a fluorescent dye being provided once the appropriate fluid is added to the fluid storage tank such that the fluid fluoresces in the presence of ultraviolet light. The following passage appears central:
Following a suspected fluid injection, the method includes the step of providing a fluorescent light to detect the presence of the fluid on a patient.… [F]ollowing a suspected fluid injection of a person as a result of a high pressure stream of fluid from hydraulic machinery, if the person has been injected, hydraulic fluid mixed with the fluorescent dye would be injected into the patient. As a result of this injection with both hydraulic fluid and the dye, the injection point along with the injected tissue can be found using ultraviolet light which causes the dye to fluoresce.
Once a suspected fluid injection occurs, the method includes the step of providing a dark room and examining the patient with the blue light in the dark room to determine whether possible fluid injection has occurred. After the initial examination, the method includes the step of washing the patient and particularly the point of possible fluid injection preferably with water and detergent.
After the step of washing the patient and the point of possible fluid injection, the method includes the step of re-examining the patient with the blue light in the dark room to determine whether possible fluid injection has occurred.
By following the steps of the preferred embodiment, it is possible to determine whether or not fluid injection has occurred in the patient.
720 I then turn to the claims. Claim 1 and claim 9 are independent claims.
721 For ease of reference I set out claim 1 again:
1. A method for detecting fluid injection in a patient, the method including the steps of:
providing a fluid storage tank;
providing fluid for use in machinery and adding said fluid to the fluid storage tank;
providing a fluorescent dye and adding the fluorescent dye to the fluid such that the fluid fluoresces in the presence of ultraviolet light; and
a possible fluid injection occurring in a patient.
722 The first contention against validity was that there was no detection step. Fuchs submitted that as a matter of ordinary language the reader could not discern in claim 1 of the Standard Patent any step of detection. The requirement that the fluid fluoresce in the presence of ultraviolet light was not itself a step of detection, Fuchs submitted, but was a statement of properties. The fluid would fluoresce in the presence of ultraviolet light without there being any step of detecting anything. This claim did not say when or in what circumstances the fluid will fluoresce. Put another way, if claim 1 was not invalid, Quaker could contend that the conduct infringed the patent without there being an actual detection of any injection, Fuchs submitted. A detection step was not, in fact, added until claim 2, which was a method according to claim 1 including the step providing an ultraviolet light to detect the presence of the fluid.
723 In my opinion, the contention on behalf of Fuchs reduces to the proposition that there is no separate detection step set out in claim 1. So viewed, it has an inherent unreality about it.
724 It is plain that the claim refers to a method for detecting a fluid injection injury and the list of steps that are set out are inclusive, not exhaustive. I accept Quaker’s submission that any person skilled in the art employing that method would understand that it would be necessary to include some step directed to the detection of the fluid injection injury, which is what the claim is directed at. To suggest that claim 1 be tested by excluding any detection step is not a common sense way of assessing the utility or clarity of that claim or whether it is fairly based.
725 I am also of the view that the contrast with claim 2, relied on by Fuchs, is not well made since the point of claim 2 is not to add a detection step but to add the step of providing an ultraviolet light. In claim 1, the ultraviolet light may be present but not provided.
726 The second problem, Fuchs submitted, was that the claimed method was not able to confirm whether or not a fluid injection had occurred whereas the promise of the invention was to provide a method to detect and confirm whether a fluid injection had occurred in a patient. Any chance that the method might produce a false negative defeated the purpose of the invention because the diagnostic tool would be entirely unreliable, Fuchs submitted. Following the instructions of the method would provide no practical difference because, whether or not fluorescence was detected, the worker would ultimately be sent for medical treatment.
727 In my opinion, that the method could not be used completely to rule out whether a fluid injection had occurred is not an objection establishing inutility, except in a theoretical sense. The weight of the evidence is that those involved at the mine sites considered the method to be useful in practice. I do not regard the evidence of either of the experts, Dr Gray or Mr Smith, as establishing the contrary. If the method were capable of showing, as I find it is, that a high pressure injection has occurred in a “patient” then that is sufficient. If the method were capable of showing, as I find it is, that a fluid injection was unlikely to have occurred then that also is sufficient. Relatedly, I would not regard the promise of the invention as being as high as to provide a method for confirming whether or not an HPFI injury has occurred with absolute certainty. The specification does not demand such a conclusion.
728 I do not consider the reference to “the sites (sic) fluid injection protocol” in the preferred embodiment of the invention as a difficulty even where the mine protocols about which there was evidence tended to provide that if there is any suspected fluid injection the patient is to be taken to medical care in any event.
729 Quaker objected to Fuchs pressing its contentions that the Patents were invalid because they lacked a detection step, or could not confirm whether a fluid injection had occurred, or because a person with a suspected fluid injection would inevitably be taken to hospital, on the basis that these contentions were insufficiently particularised. Fuchs relied on general grounds alleging inutility in its Fifth Further Amended Particulars of Invalidity. I allow Fuchs to press the contentions. Although they were not raised with the same level of particularity as the remainder of Fuchs’ grounds of invalidity, they do fall within the general pleaded grounds, and Quaker did not suggest it was unable to deal with them. However, as I have said, the contentions fail on their merits.
730 The third problem, Fuchs submitted, was the washing requirement. Fuchs submitted that a number of the claims did not require any step of washing or examination after washing but to detect whether there was a fluid injection it was necessary to have a separate step of working out whether the fluid had penetrated beneath the skin of the patient. The step of washing and then examining the patient did not come in until claim 6.
731 In my opinion, claim 1 does not include a step of washing but washing is not an essential step in every case, for example where the “patient” was not covered in fluid on the surface of their skin but considered that they might have been injected. Because it is not always an essential step it was appropriately the subject of a dependent claim, claim 3 and claim 4, as submitted by Quaker.
732 The fourth problem, Fuchs submitted, related to dye concentration. Fuchs submitted that apart from claims 20 and 21 dye concentration was not dealt with at all in the Standard Patent. There was no specification of any particular concentration at which the fluorescent dye was to be added to the fluid to enable a fluid injection injury to be detected. And it could not be the case, Fuchs submitted, that any amount, however small, of the fluorescent dye would enable detection of a fluid detection injury. Fuchs also submitted that even claims 20 and 21 had this problem as the concentrations there specified for Oil-Glo could not simply be applied to a different dye, and would require further research.
733 In my opinion, as submitted by Quaker, it was sufficient to meet the test for fair basis and disclosure of best method for the patent specification to enable the performance of one embodiment of the invention within the scope of each claim and one could apply the invention by simply using the Oil-Glo dye, referred to in the specification, and employ it at a sufficient concentration by choosing the particular concentrations described in the specification for that purpose. To do so would be straightforward. I do not accept Fuchs’ submission that the claims are invalid because it is unclear to what the Oil-Glo is being added - it is plain that it is a fluid as referred to in claim 1 - or what was being referred to as “100% Solcenic” in the body of the specification for the Standard Patent and Innovation Patent. An embodiment of the invention can be performed by adopting the relevant concentration of Oil-Glo in any chosen hydraulic fluid, whether or not of the Solcenic brand.
734 Separately, I also accept the submission by Quaker that the specification is to be construed from the perspective of the skilled person, taking into account common general knowledge and Fuchs has not established that the employment of any other embodiment of the claimed invention would involve too much work on the test in Kimberly-Clark Australia Pty Ltd v Arico Trading International Pty Ltd [2001] HCA 8; 207 CLR 1 at [25]:
The question is, will the disclosure enable the addressee of the specification to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty?
See also Apotex Pty Ltd v Warner-Lambert Company LLC (No 2) [2016] FCA 1238; 122 IPR 17 at [267] and, on appeal, Warner-Lambert Co LLC v Apotex Pty Ltd [2018] FCAFC 26; 355 ALR 44 at [99]-[136].
735 I have referred above to claim 9 as an independent claim. It was in the following terms:
9. A method of detecting an accidental hydraulic or fuel fluid injection in a patient having a possible fluid injection, the fluid having been added to a storage tank provided for use in machinery and a fluorescent dye having been added to the fluid such that the fluid fluoresces in the presence of UV light, the method including the steps of:
examining the patient by exposing the patient to UV light; and
determining the presence of fluorescence,
wherein fluorescence indicates a hydraulic fluid injection occurring in the patient.
736 What I have said in relation to the four issues or problems relied on by Fuchs applies also to claim 9.
737 For these reasons I consider that the problems or difficulties to which Fuchs pointed are not made out in relation to the Standard Patent.
738 In terms of the Innovation Patent, Fuchs did not repeat its claim that it lacked an explicit detection step.
739 The second issue or problem claimed by Fuchs, concerning the claimed method not being able to confirm whether or not a fluid injection had occurred, also applies to the Innovation Patent and I repeat my reasons for rejecting that issue.
740 Next, there is no reference to washing in the claims in the Innovation Patent but I reach a similar conclusion for similar reasons as in relation to the Standard Patent.
741 Again, there is no reference in the claims in the Innovation Patent to dye concentration but I repeat my reasons in relation to that issue or problem as for the Standard Patent.
742 For these reasons I consider that the problems or difficulties to which Fuchs pointed are not made out in relation to the Innovation Patent.
743 On the questions of construction, I did not derive any assistance from the expert evidence.
Alleged novelty-destroying disclosures
744 I turn next to the issue of prior publication as it impacts on validity.
745 It may also be recalled that the relevant regulations are referable to s 24 of the Act, which provided as follows:
24 Validity not affected by certain publication or use
(1) For the purpose of deciding whether an invention is novel or involves an inventive step or an innovative step, the person making the decision must disregard:
(a) any information made publicly available, through any publication or use of the invention in the prescribed circumstances, by or with the consent of the nominated person or patentee, or the predecessor in title of the nominated person or patentee; and
(b) any information made publicly available without the consent of the nominated person or patentee, through any publication or use of the invention by another person who derived the information from the nominated person or patentee or from the predecessor in title of the nominated person or patentee;
but only if a patent application for the invention is made within the prescribed period.
746 The first prescribed circumstance relied on by Quaker was pursuant to s 24(1)(a) of the Act and regs 2.2(1A) and 2.3(1A), by which any publication or use of the invention after 2 February 2011 by or with the consent of the patentee cannot be taken into account for the purposes of novelty. By reg 2.2(1A), for s 24(1)(a) of the Act, the circumstance that there was a publication or use of the invention within 12 months before the filing date of the complete application, is a prescribed circumstance. This was referred to in submissions as the general grace period.
747 A second prescribed circumstance for the purposes of s 24(1)(a) is the working in public of the invention within the period of 12 months before the priority date of a claim for the invention, ie 2 September 2010, for the purposes of reasonable trial where, because of the nature of the invention, it is reasonably necessary for the working to be in public: reg 2.2(2)(d). The prescribed period is 12 months from the start of the first public working of the invention referred to in reg 2.2(2)(d). This was referred to in submissions as the reasonable trials grace period.
748 A third prescribed circumstance is that, pursuant to s 24(1)(b) of the Act and reg 2.3(2), any publication or use of the invention after 2 September 2010 without the consent of the patentee by a person who derived the information from the patentee, cannot be taken into account for the purposes of novelty. By reg 2.3(2), for the purposes of s 24(1) of the Act, in the case of information of the kind referred to in s 24(1)(b) of the Act, the prescribed period is 12 months from the day when the information referred to in s 24(1)(b) became publicly available. This was referred to in submissions as the unauthorised disclosures grace period.
749 Speaking generally, I accept the submissions on behalf of Quaker that whether the doing of something is for the purposes of a reasonable trial is a matter of fact and degree, given the nature of the invention. Trials may constitute “‘seeing how it goes’ when the invention is employed in the field”: Grove Hill Pty Ltd v Great Western Corporation Pty Ltd [2002] FCAFC 183; 55 IPR 257 at [231]. I also accept that by virtue of the language of s 24(1) the provision relates to any information made publicly available through any publication of the invention in the prescribed circumstances. Thus it is not merely the working of the invention itself, or the actual working of the whole of the invention in public, to which the prescribed circumstances are directed.
750 I take into account, while noting they were made in relation to the expression “for the purpose of reasonable trial or experiment only” in s 9 dealing with acts that were not to be taken to be secret use of an invention, the statements of principle by the Full Court in Coretell Pty Ltd v Australian Mud Company Pty Ltd [2017] FCAFC 54; 250 FCR 155 at [226] and [232]-[233] per Burley J, with whom Jagot and Nicholas JJ agreed, as follows:
226 Fourthly, the operation of s 9(a) also calls for consideration of what use is reasonable trial and experimentation. In Grove Hill, Dowsett J found (at [232]) that this will be determined based on the nature of the equipment in question, the tasks for which it was designed and conditions under which it is to be used. Again, it is a question of fact.
…
232 The observations of the High Court [in Longworth v Emerton (1951) 83 CLR 539] in the emphasised passage above do not support the proposition advanced by the appellants that use for trial or experiment must in all cases be in order to bring an invention into a form suitable for applying for a patent. The facts must enable a finding that the trial or experiment is for the development or validation of the invention disclosed and described. But is not a requirement of s 9(a) that the trial or experiment be for the purpose of conceptualising or drafting the specification. Tests conducted to prove or refine the concept will suffice, so long as the purpose is reasonable trial or experiment.
233 Accordingly, I do not accept that it is a necessary requirement of s 9(a) that the trial or experiment be directed towards the drafting of the patent application (or provisional application).
751 I accept the evidence of Mr Kraus as to the necessity for testing. I note that the evidence of Mr Roberts and Mr Bate was to the same effect, that is, that testing or trialling of the hydraulic fluids with the fluorescent dye added was necessary.
752 I accept Mr Thompson’s evidence that in mid to late September or in October 2010, during the delivery of the training using Oil-Glo 33 in the simulator, Mr Thompson had a sudden idea one night of using this fluorescent dye to identify actual instances of HPFI in people rather than merely using it as a visual aid in the gelatine hand of the simulator. He wondered whether it may be possible for the dye also to be visible under human skin which would allow detection of HPFI injuries if the dye was first mixed into the hydraulic fluid.
753 I further accept that testing and trials was necessary in respect of at least two matters: first, whether the fluorescent dye in hydraulic fluid in human tissue could identify HPFI in people and, second, whether the fluorescent dye could be added into the hydraulic fluids of hydraulic systems without damaging them by way of corrosion, ignition, blockage of filters and unplanned movements of the machines. I find that it was reasonably necessary for trials to take place in equipment actually used in mines where harm to humans from HPFI could occur.
754 I find that the first time Mr Thompson told anyone at Peabody about the Method involved in his idea was in late September or in October 2010. At that stage Mr Thompson did not know whether his idea would work effectively to highlight the injection of fluid into human skin.
755 I find that it was in October 2010 that Mr Thompson first undertook trials using samples of pig flesh as a substitute for human tissue. Later in October or in early November 2010 Mr Thompson showed this to Mr Withers and the Metropolitan mine manager at the Metropolitan car park. Mr Thompson then conducted, by himself, further tests using the pig skin and took photographs and videos of the tests, injecting the fluid at different distances and at different pressures to see under what conditions a fluid injection would occur. It was also necessary to consult medical practitioners and this was done between March and August 2011.
756 The testing for compatibility began in early December 2010, but not in an underground environment. First, there were trials of Oil-Glo 33 in the hydraulic systems of a Multibolter and an Eimco. I find that on or about 16 December 2010 Mr Thompson conducted a risk assessment for the Oil-Glo 33 product and its proposed introduction into machines with oil-based hydraulic systems. Such a risk assessment was a necessary precursor to any trials being undertaken.
757 Thereafter, but in 2011, to test whether the Method would work in a longwall system Mr Thompson first needed to acquire a dye compatible with water-based hydraulic systems. The required WD 802 was introduced into the Metropolitan longwall system for monitoring and testing in or around May 2011. By mid-2011, Mr Thompson discussed the Method more generally, “with mining companies and other companies including Joy and Pirtex.”
758 I find that Mr Thompson did not discuss the Method during HPFI training prior to mid-2011. I reject Mr Bate’s evidence to the contrary. I therefore reject Mr Bate’s evidence that disclosure was made to Mr Bate’s friend from another mine (BHP Billiton’s Appin West mine) who came to have a look at the training or to the staff Mr Bate invited from the coal washery facility adjacent to Metropolitan, which was owned by an entirely separate company, to come and attend some of Mr Thompson’s training sessions free of charge.
759 I find that the disclosures of the method the subject of the Patents were part of reasonable trials which necessarily occurred in public, and were undertaken either by Mr Thompson or by employees at Metropolitan under Mr Thompson’s supervision.
760 I find that the original disclosure of the idea of the invention by Mr Thompson to Mr Withers and the subsequent demonstration by Mr Thompson to Mr Withers and the Metropolitan mine manager in October 2010 fall within the period of 12 months before the priority date of a claim for the invention, ie after 2 September 2010, as the working in public of the invention for the purposes of reasonable trial, and where, because of the nature of the invention, it was reasonably necessary for the working to be in public: reg 2.2(2)(d).
761 I find that these disclosures were for the purpose of and part of the working in public of the invention as they were a necessary precursor for the reasonable trial. Mr Thompson could not carry out trials at Metropolitan without Mr Withers’ assistance and permission and it was therefore necessary for Mr Thompson to tell Mr Withers what he proposed. As I have said, the disclosures were for the purpose of then directed towards the working in public of the invention for the purposes of reasonable trial.
762 I find that, because of the nature of the invention, it was reasonably necessary for the working to be in public. This is because an important application of the invention was in working machinery at a working mine site where the invention would be used in public. This was a trial in “real world conditions” and a reasonable form of trial in relation to the nature of this invention. It follows that I reject the submission by Fuchs that in the circumstances reasonable trial did not extend beyond Mr Thompson’s testing of Oil-Glo in his simulator and in a Bobcat. I reject the related submission by Fuchs that the trial at Metropolitan was merely a test of compatibility of a fluorescent dye additive with the hydraulic fluid used at Metropolitan, or merely a testing of a particular application of the invention, and what may be implicit in it, which is that there is a mutual exclusivity between such testing and a trial of the invention.
763 Further, I accept that Mr Thompson was in straitened financial circumstances at the time and certainly did not have his own large-scale machinery which he could use for the purposes of a non-public trial. I find it was necessary that the workers in the mine were informed of the fact of the hydraulic fluid being dyed and the purpose of it. I do not regard it as a reasonable alternative that the workers and other staff at the mine could each have entered into a form of confidentiality agreement. This was a matter of occupational health and safety in the Australian mining industry and I accept the evidence that there was and is a strong culture of information sharing in that respect.
764 Third party fluid manufacturers, such as Fuchs, also needed to be consulted about the addition by way of trial of fluorescent dye to their products. The 9 November 2010 emails were thus correspondence arising from and in relation to the reasonable trial of the invention at Metropolitan. Mr Pearce confirmed how critical the testing of FluidSafe with Fuchs’ products was in a later email dated 9 June 2016.
765 I also find that the disclosure by the 9 November 2010 emails and conversation between Mr Risk and Mr Roberts of Fuchs was with the consent of Mr Thompson. I find that Mr Risk would have been told something of the method the subject of the Patents by Mr Withers to whom it had been communicated by Mr Thompson. It may be recalled that Mr Roberts gave evidence of a conversation he had with Mr Risk during a routine site visit to Metropolitan on 9 November 2010. Mr Risk said words to the effect: “We are looking at introducing a dye to our longwall fluid to enable it to be detected under a worker’s skin so we can tell if there has been an injection. Can you help us with adding that chemical to the Solcenic concentrate?” Mr Roberts said that he was not told, or understood, that any of the content of his discussions with Mr Risk was in any way confidential. To the contrary, I would infer that Mr Thompson would have been aware, when he disclosed the invention to Mr Withers, that Mr Withers would need to consult his superiors and that Peabody would have to consult third party fluid manufacturers before trialling the addition of Oil-Glo to the longwall at Metropolitan, and that he impliedly consented to that course. I would therefore regard the disclosures for that purpose by Mr Withers to Mr Risk and by Mr Risk to Mr Thompson as being with the consent of Mr Thompson. I would not regard this position as inconsistent with Mr Thompson’s subsequent email communication on 30 November 2019 indicating he was “cautious” about “releasing any details to a third party”: as the balance of that email demonstrates he was “more than happy” to have a meeting with Mr Roberts and to “give [him] the intent”.
766 Even if I am wrong, however, and Mr Thompson did not consent to those disclosures, then Quaker succeeds on its alternative argument, which was that these disclosures were of information made publicly available without the consent of the patentee by Metropolitan, who derived the information from the patentee: see s 24(1)(b) and reg 2.3(2), referred to as the unauthorised disclosures grace period. As I have found, the information communicated by Mr Withers to Mr Risk, and by Mr Risk to Mr Roberts, was derived originally from information communicated to Mr Withers by Mr Thompson. On either view, the disclosures are to be disregarded for the purpose of novelty.
767 For these reasons, in my opinion, regs 2.2(2)(d) and 2.3(c) applied since the publication or use of the invention after 2 September 2010 was by or with the consent of the patentee (s 24(1)(a)) in the working in public of the invention for the purposes of reasonable trial, where, having regard to the nature of the invention it was reasonably necessary for the working to be in public. Accordingly, the disclosures are to be disregarded for the purposes of novelty.
768 I find that Oil-Glo was not added to the Solcenic fluid for use in Metropolitan’s longwall until May 2011 and North Goonyella’s longwall until June 2011, both dates being after 2 February 2011. I also find that that publication or use of the invention was by or with the consent of the patentee. Thus the general grace period referable to regs 2.2(1A) and 2.3(1A) applies, in my opinion.
769 In so far as Fuchs maintained that there was secret use of the invention, I find that the supply of Oil-Glo in 2011 to Peabody either for or after trialling the method was not secret. By no later than April 2011, the method claimed in the invention was public, as evidenced by the email from Mr Thompson to Mr Peter Doyle of 19 April 2011. Further, Mr Thompson gave evidence which I accept that he discussed the method with other mining companies and received numerous enquiries about the Method. Fuchs’ case on secret use was expressly put as an alternative to its case on public disclosure which was advanced only if the disclosures and use of the invention at Broadmeadow were not public.
770 The Innovation Patent, as a divisional patent, has the same grace periods as the Standard Patent: regs 2.4(3), (4). Therefore, the Patents have the same grace periods.
Infringement
Infringement by supply - s 117(2)(b)
771 The s 117(2)(b) case centred on, first, BMA, the operator of a mine site at Broadmeadow; secondly Glencore, which was the operator of various mine sites; and third Yancoal, the operator of various other mine sites.
772 In each case, the first issue is whether the product supplied to the operator was a staple commercial product. If it was not, the second issue is whether Fuchs would have had reason to believe that the actual method in the valid claims of the patent would be used by the operator at the relevant mine site. Rather than some general question of whether the operator was interested in HPFI detection, the question is whether Fuchs had reason to believe the operator would actually use the method of the patent.
1. “Staple commercial product”
773 Section 117(2)(b) only operates in respect of the supply of a product which is not a “staple commercial product”. The immediate context of that phrase is as follows:
117 Infringement by supply of products
(1) If the use of a product by a person would infringe a patent, the supply of that product by one person to another is an infringement of the patent by the supplier unless the supplier is the patentee or licensee of the patent.
(2) A reference in subsection (1) to the use of a product by a person is a reference to:
(a) …; or
(b) if the product is not a staple commercial product-any use of the product, if the supplier had reason to believe that the person would put it to that use; or
(c) in any case-the use of the product in accordance with any instructions for the use of the product, or any inducement to use the product, given to the person by the supplier or contained in an advertisement published by or with the authority of the supplier.
774 In my opinion, the product referred to in s 117(1) is in this case a product supplied by Fuchs, the use of which by a person, in the senses described by s 117(2), would infringe the patent.
775 Plainly, and leaving aside for the moment the terms of s 117(2)(b), an infringing use would include the use of an oil-based or water-based hydraulic fluid product, to which fluorescent dye had been added, in the patented method.
776 In my opinion, that analysis identifies what the relevant product is when asking the question whether or not it is a staple commercial product purposes of s 117(2)(b). I find it is the product as supplied which falls for consideration rather than the components of that product as supplied. In that respect, I find that Fuchs supplied special-purpose hydraulic fluid products designed for particular uses in hydraulic machinery, which products included fluorescein. As Fuchs itself submitted, it had never supplied fluorescent fluid additives per se, that is, not mixed into one of its fluid products. I reject Fuchs’ submission that the hydraulic fluids acted merely as “carriers” for the fluorescein, and that there remained a separate supply of the fluorescein contained in them: that is an artificial approach to characterising the supply. The hydraulic fluid products were not staple commercial products within s 117(2)(b).
777 I note and accept the evidence of Mr Roberts that the different Solcenic products he had referred to were specially formulated products having regard to matters such as the requirements of the particular OEM or particular standards that were introduced and they were special purpose products in that they were formulated having regard to a particular purpose. He also agreed that the different Renolin products and the different grades within Renolin B were each specially formulated products that was intended to have a particular balance of properties for a particular use.
778 Speaking generally, the phrase “staple commercial product” refers to a product, such as a product in the nature of raw materials, which is commonly available and has a multitude of different uses. See Sanofi-Aventis Australia Pty Ltd v Apotex Pty Ltd (No 3) [2011] FCA 846; 196 FCR 1 at [267]-[271] (not challenged on appeal: see Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd (No 2) [2012] FCAFC 102; 204 FCR 494 at [147]) and AstraZeneca AB v Apotex Pty Ltd [2014] FCAFC 99; 226 FCR 324 at [428]-[431]. Contrary to the submission on behalf of Fuchs, I do not regard the plurality as meaning to qualify at [431] what the High Court had said in Northern Territory v Collins [2008] HCA 49; 235 CLR 619.
779 In any event, applying Fuchs’ approach, that one is engaged in an inquiry as to whether the product is used for multiple uses rather than an inquiry as to whether it is a staple within some ordinary meaning of that term, to the product as I have described it, my conclusion is the same: the product is a special-purpose product for a specific use and is therefore not a staple commercial product within the meaning of the provision.
2. “Reason to believe”
780 Said by Fuchs to be relevant to this issue in respect of each mine site is the question of product concentration. In broad terms Fuchs contended that there was a clear difference between low concentration products, supplied for leak detection, and high concentration products promoted as suitable for HPFI detection. But the context is Fuchs’ reason to believe that the products would be used in the patented method. The most direct way of addressing that issue is to examine whether the operator of the mine sites in question said that HPFI detection was the purpose for which it wanted to buy the product. Quaker submitted that the product for use in leak detection was in effect substitutable and the same product could be used for HPFI. As part of its defence, Fuchs submitted that a product supplied for the purpose of leak detection had a different and substantially higher concentration of the dye in the fluid, it being the case that fluorescent dye had for many years been added and used for leak detection.
781 Quaker provided a list or table in the proceedings of what it described as requests made to Fuchs for dye for HPFI. There were 44 such instances in Quaker’s document. Nine of the 44 were referable to BMA’s Broadmeadow mine site where, as I explain in more detail below, I find that Fuchs did have reason to believe that the product, not using the FluidSafe dye, would be used for HPFI.
782 Leaving aside Broadmeadow, I do not accept Quaker’s submission that where Fuchs supplied product containing fluorescent dye for leak detection it had reason to believe that the operator of the mine would use the product for HPFI detection. In my opinion, while aware of what occurred in relation to Broadmeadow, each supply is to be looked at on its own terms. In other words, hindsight should be put to one side and I would not accept that what occurred in relation to Broadmeadow showed that Fuchs had a propensity to make such supplies.
783 Many of the instances in Quaker’s document are not requests made to Fuchs for dye for HPFI but are no more than enquiries in circumstances where, as I find, the possibility of using fluorescent dye for HPFI detection was a matter of great interest at the time to those concerned with mine safety. Even where there were requests, in many instances such as the Xstrata (Glencore) mine site at Ravensworth it was not established that there was any supply by Fuchs of dyed fluids. In other instances although dyed fluids were supplied, the dyes were FluidSafe and it appeared to be accepted by Quaker that the supply by the patent holder of FluidSafe carried with it an implied licence to use the method in the Patents. I consider the correspondence relating to the specific mines relevant to these proceedings in more detail below.
784 I find that although FluidSafe could be used for both leak detection and HPFI detection, in the latter case the dye was used in a higher concentration than for leak detection alone. I also accept the evidence of Mr Knight that that was his understanding.
785 Quaker’s “HYDRAULIC FLUIDS: FLUIDSAFE - OB HYDRAULIC FLUID ADDITIVE” Application Sheet stated, under the heading “Concentration Control”, for the FluidSafe OB hydraulic fluid additive for “Fluid injection detection” the concentration of 600ml per 1,000 litres, and for “Leak detection” 300ml per 1,000 litres, that is, half the HPFI concentration. For the water-based product, Quaker’s Application Sheet “HYDRAULIC FLUIDS: FLUIDSAFE - WB HYDRAULIC FLUID ADDITIVE” stated, under the heading “Concentration Control”, for the FluidSafe WB hydraulic fluid additive for “Fluid injection detection” the concentration of 7.5 litres per 1,000 litres concentrated longwall fluid, and for “Leak detection” 3 litres per 1,000 litres concentrated longwall fluid. The evidence of Mr Knight in his second and third affidavits, which I accept, was consistent with and confirmed that these concentrations were in fact used in the supplies made by Fuchs where FluidSafe was used.
786 I find that Quaker’s marketing materials distinguished between different uses based on concentrations.
787 Where a third party dye was used by Fuchs in respect of the relevant mines apart from Broadmeadow, Mr Knight’s evidence, which I accept, was that 8g of dye was added per 100L of Solcenic concentrate, 0.24g per 100L of Solcenic emulsion (containing 97% water), or 1g per 100L of Renolin mineral hydraulic oil. In the case of Broadmeadow, Fuchs added 180g of dye per 100L of Solcenic concentrate. In that case, Mr Knight’s evidence was to the effect that the treat rate had been selected to match the colour, light absorbance, light transmittance, and saturation in Solcenic of FluidSafe at Quaker’s recommended treat rate for HPFI detection. At least as regards Solcenic, I find that there is a clear difference between the treat rate adopted for third party dyes at Broadmeadow, developed as suitable for HPFI detection, and those at other relevant mines. The evidence does not go so far as to establish that the non-Broadmeadow Solcenic products could not be used for HPFI detection, but because of the stark difference in treat rates it cannot simply be assumed that they could. The question of whether Fuchs had reason to believe they would be put to that use thus falls to be determined by reference to what I have already described as the most direct evidence on that topic, being its communications with customers around the time of the supply.
788 I regard the references by Fuchs to the additional expense of FluidSafe for HPFI detection as containing two elements referable to an increased cost to Fuchs: one element is the greater concentration of the fluorescent dye for HPFI detection and the other is an element of monopoly pricing in the supplies of FluidSafe by the holder of the Patents, carrying with them an implied licence to use that product in the method of the Patents.
789 I note the references in the documents of which Mr Pearce was the author to the purpose of the supplies but where there is obscurity or ambiguity I give less weight to what is stated in those documents as Mr Pearce was not called to give evidence.
790 Further, I do not accept Quaker’s contention that, leaving aside Broadmeadow, references to the supply of dyed product for leak detection should be taken as code for the supply of dyed product for HPFI detection. I have considered the list provided by Quaker of references to leak detection in the documents in the tender bundle but I do not draw an inference from those references that all or any of them constituted or contained a covert reference to HPFI detection.
791 I find that Fuchs was conscious of the possibility of infringing the Patents by its supplies and, Broadmeadow apart, took steps to communicate to the operators of the relevant mine sites that the product in question was not to be used for HPFI detection. I do not infer from these references that Fuchs proceeded on the basis that the product having a concentration of fluorescent dye suitable for leak detection could be used for HPFI detection.
792 As a further general point, Quaker in its submissions drew attention to Fuchs’ unsuccessful claim for legal professional privilege, that claim having been dismissed by Rares J on 13 February 2019. Quaker submitted that the privilege claim was not properly based.
793 Quaker’s contention was put primarily as going to the question of additional damages and s 122 but it was also put that on a fair reading of the documents as they sat in the tender bundle it was clear that they were not properly the subject of a claim for privilege and Quaker pointed to this as further conduct on the part of Fuchs in endeavouring to ensure that evidence of its knowledge of the use of the product for HPFI did not come to light. It was put that objectively the documents were not properly the subject of a claim for privilege and there was no basis for them to be withheld.
794 Fuchs submitted that the Court could not reach the conclusion that Fuchs had deliberately claimed privilege in documents that it knew were not privileged on the basis of the documents themselves. That did not address the question as to whether the privilege claims were properly made in the first place, Fuchs submitted.
795 Quaker responded that what emerged from the course of events was that Fuchs took every step it could to try to ensure that the documents in question did not come to light and one could see objectively that its conduct in relation to the privilege claim had that character.
796 To the extent that this issue was pressed by Quaker otherwise than in relation to additional damages, I do not accept it. In my opinion the contention as developed was put at too abstract a level to be of assistance in relation to the matters of fact the Court has to decide, which could be the only relevance of the contention. As will appear below, I have taken into account in relation to Broadmeadow Fuchs’ attempts to obscure the character of what it was supplying to BMA at that site and in evaluating what occurred at the other mine sites I have taken into account Fuchs’ attempts in relation to Broadmeadow.
797 I return later to the question of additional damages, including the relevance of Fuchs’ unsuccessful claim of legal professional privilege.
2.1 BMA (Broadmeadow mine)
798 It may be recalled that the product complained of by Quaker in relation to BMA’s Broadmeadow mine was Solcenic GM20 LD supplied to that mine site since July 2016.
799 I note in this respect the evidence of Mr Knight and Fuchs’ acceptance in its submissions that for Broadmeadow Fuchs had developed a high concentration product to match FluidSafe in response to the customer insisting on a product for use in HPFI detection.
800 Fuchs accepted that its submissions as to the low concentration of fluorescent dye added to the Fuchs products did not apply in relation to Broadmeadow. It accepted that its Solcenic GM20 LD supplied to Broadmeadow was supplied at a higher concentration.
801 I find that if a suspected HPFI injury did occur, Fuchs believed that BMA would have used the claimed method at the Broadmeadow site. It may be recalled that Mr Knight accepted in cross-examination that he had reason to believe, at the time of the supply of the Fuchs product to Broadmeadow for use in HPFI detection, that Broadmeadow would use it for that purpose. He also accepted that he believed that the product Fuchs proposed to supply to Broadmeadow would be capable of being used by Broadmeadow in HPFI detection.
802 In terms of claim 1 of the Standard Patent, I find that at the time of supply Fuchs had reason to believe, by reason of its products being requested and supplied as suitable for “HPFI detection”, that BMA would use the Solcenic GM20 LD at Broadmeadow in a method for detecting fluid injection in a patient, including the steps of: providing a fluid storage tank; providing fluid, for use in machinery, and adding it to the tank; providing fluorescent dye and adding it to the fluid (this step having been performed by Fuchs) such that the fluid fluoresces in the presence of ultraviolet light; and a possible fluid injection occurring in a patient.
803 In terms of claim 2 of the Standard Patent, I find that Fuchs had reason to believe that BMA would, in addition to the steps of claim 1, provide an ultraviolet light to detect the presence of the fluid. I do not accept the submission on behalf of Fuchs that it was pure speculation that Broadmeadow had an ultraviolet light to use in HPFI detection. BMA specifically requested the supply of the Fuchs product in relation to HPFI detection. I infer that Mr Pearce presented a slideshow at Broadmeadow which stated, albeit not expressly directed to HPFI detection, that fluorescein was “[p]articularly effective in areas with low light where the use of a blue light will result in the fluid fluorescing (glowing)”, which appeared in both the draft of a presentation Mr Pearce was to present to Broadmeadow, and a similar presentation later provided to a different client. These circumstances provided sufficient reason for Fuchs to believe: that BMA knew in respect of its Broadmeadow mine site how the method of using dyed fluids to detect HPFI injuries worked; that it was known by BMA that using an ultraviolet light could improve the efficacy of that method; that BMA had or would obtain such a light, and that BMA would use that light to detect the presence of fluid. For the same reasons, I find that Fuchs had reason to believe that its products would be used in methods as claimed in claim 9 of the Standard Patent and in claim 1 of the Innovation Patent.
804 In terms of claims 10 and 11 of the Standard Patent, I find that the Solcenic GM20 LD fluid product supplied to BMA at Broadmeadow was an emulsion, and therefore a combination, of water and oil. Accordingly, Fuchs had reason to believe that methods according to those claims would be performed.
805 I also find, in terms of claims 14-16 of the Standard Patent, that Fuchs similarly had reason to believe that staff at Broadmeadow would, if fluid remained on the surface of the subject’s skin following the steps involved at [802]-[803], wash the subject, at the site of possible fluid injection, and then re-examine them by exposure to the UV light. Again, this follows from Fuchs’ awareness that those at BMA requesting its products for HPFI detection knew generally how that method worked, and the obviousness of a washing step should it prove necessary in a particular case.
806 However, I am not persuaded that Fuchs had reason to believe that BMA would use its products at Broadmeadow in methods as claimed in claims 3-7 and 13 of the Standard Patent. There is no evidence that there was, or that Fuchs had reason to believe there was, a location at Broadmeadow which could be used as a dark room and in which suspected fluid injection injuries could be examined.
807 No argument was directed towards the meaning of the dependent claim 2 of the Innovation Patent, which claims a “method according to claim 1 wherein the fluorescent dye has colour characteristics such that when the dye is mixed with an industrial fluid the fluid takes on the colour characteristics of the dye”.
808 Plainly, the dye as described in claim 2 of the Innovation Patent is one that is not only fluorescent, as covered by claim 1, but which also changes the colour of the fluid. I find Uniqua W400X, which was added to the Solcenic GM20 supplied to Broadmeadow, had that property: product information described it as having a colour shade of “fluorescent yellow dye”. Further, photographs in a Laboratory Test Report, dated 28 January 2016 and provided to BHP in relation to a supply of Solcenic GM20 with a lower concentration of fluorescein, depict a clear difference in colour between fluids labelled “GM20-2% Standard” and “GM20-2% Standard + Fluorescein”. Although that test would have been conducted using Fluoresceine LT, which Fuchs used from August 2015 to March 216, Mr Knight’s evidence was that samples of Solcenic dyed with Fluoresceine LT and Uniqua W400X had a “near equivalence” of colour. Accordingly, I find that Fuchs had reason to believe that its product, being a hydraulic fluid, would be used in a method according to claims 2 and 3.
809 I do not accept the submission on behalf of Fuchs that because HPFI injuries were especially rare there was no real or likely prospect that the patented method would be performed and that therefore Fuchs did not have reason to believe that BMA would put the product to an infringing use. I construe s 117(2)(b) as applying where an alleged infringer has reason to believe that, if the occasion (here, a suspected fluid injection) for a product to be put to a particular use arose, the product would be put to that use. I see no reason, advancing a discernible purpose of the provision, to construe s 117(2)(b) as going further and requiring the alleged infringer also to have reason to believe that the occasion would in fact arise. But even if s 117(2)(b) were to be so construed, the evidence did not establish that events giving rise to a “possible fluid injection”, to adopt the language of the claims, were so rare as to negate any reason to believe on the part of Fuchs that its products would be put to the very use they were requested for, that is, to determine whether a suspected HPFI injury had occurred, and in a way that would infringe the claims of the Patents as explained above.
810 I therefore find that Fuchs had reason to believe that its product would be used in methods as claimed in claims 1, 2, 9-12 and 14-16 of the Standard Patent, and claims 1-3 of the Innovation Patent: Fuchs intended its product so to be used. I find that the conduct of Fuchs in offering to supply and then supplying Solcenic GM20 LD to BMA for its Broadmeadow mine site satisfied s 117(2)(b) of the Act.
2.2 Glencore (Ulan No 3, Ulan West, Bulga and Oaky North mines)
811 The products complained of by Quaker in relation to Glencore’s Ulan No 3 mine site were Solcenic GM20 D supplied since August 2015 and Renolin B 68 [Plus] LD supplied there since October 2015. For Ulan West, the product was Solcenic GM20 D supplied since May 2016. For Bulga, the product was Solcenic GM20 [Plus] 3% Premix Emulsion, supplied since August 2015. For Oaky North it was said by Quaker to be a Solcenic product containing fluorescent dye offered by Fuchs to that Glencore mine site in February 2016.
812 Quaker accepted that supplies to the Ulan No 3, Ulan West and Bulga mines were said to be for the purpose of “leak detection” but submitted that the Court should find that, despite such public statements, Fuchs had reason to believe that those mine sites would, if a suspected fluid injection occurred, use the products supplied by Fuchs in an infringing manner.
813 I do not accept that submission and I do not accept Quaker’s reading of the correspondence set out at [81]-[85], [100]-[102], [105], [109]-[110] and [138]-[139] above. I take into account that neither Mr Pearce nor Mr Michaelson was called by Fuchs to give evidence but I do not for that reason more readily draw the inferences for which Quaker contends as in my opinion there is no sufficient basis in the evidence for me to draw those inferences.
814 In relation to Ulan West, I do not accept Quaker’s submission that Fuchs had reason to believe that that site was not using the product for leak detection. In my opinion the reference to an infra-red gun to identify leaks in Mr Roberts’ email of 4 July 2018, set out at [175] above, and Mr Kirkhope’s email of 10 July 2018 set out at [176] above, constitute too slender a basis to find that “Fuchs could not have continued to have had no reason to believe following this email that Ulan West was using the fluorescent dye… for leak detection”, which was Quaker’s submission. I accept Mr Roberts’ evidence that Mr Maynard had at all times only ever sought Fuchs products for leak detection and had not asked Fuchs for products suitable for HPFI detection. I also accept Mr Roberts’ evidence that at the time he sent his email of 4 July 2018 Ulan West was using the infra-red gun and also Solcenic GM 20 to detect leaks.
815 In my opinion it is unlikely that Glencore communicated with its supplier Fuchs by stating that it proposed to use a product supplied for one purpose, leak detection, but that in truth it intended to use the product for another purpose, HPFI. There was no plain evidence to this effect and the clear statements are to the effect that the supply was the purpose of leak detection. I decline to draw the inferences for which Quaker contended. It is to be recalled that HPFI related to the safety of employees.
816 I do not construe the correspondence on which Quaker relies as having a sinister aspect, given the existence of the Patents: the use of a fluorescent dye for HPFI was a sensitive topic. Indeed in an email sent on 25 October 2013 Mr Thompson asked Mr Michaelson of Fuchs to note “that my legal advice with respect to leak detection and prior knowledge is captured in the certification” (emphasis added). A copy of the Patents was part of that correspondence.
817 I accept the evidence of Mr Roberts and Mr Knight in respect of the supplies to these Glencore mines.
818 In relation to Mr Roberts, I accept in particular his rejection of the proposition put to him that Renolin B 68 D was a product that had been supplied by Fuchs as being suitable for use in the detection of HPFI injuries, as well as leak detection. I do not accept Quaker’s submission that the evidence of Mr Roberts that he instructed Mr Pearce that the product under investigation should be only suitable for leak detection was implausible in light of Mr Roberts’ draft response to Mr Ryan of Glencore of 27 May 2014, set out at [102] above: I would not regard the lack of express acknowledgement in that email that the product under investigation was being developed for leak detection as sufficient to doubt Mr Robert’s evidence to that effect.
819 I accept the evidence of Mr Roberts where he did not agree that efforts were made within Fuchs to avoid mentioning the suitability of its dyed products other than those with FluidSafe dye for use in detecting HPFI injuries so as to enable Fuchs later to say, if it needed to, that it had never supported that use of its products.
820 I accept Mr Roberts’ evidence as to his internal email 22 May 2014, extracted at [101] above, and the reference there to “concerns with HP injections”: Mr Roberts explained that these words did not reflect any conversation he had had with Glencore. I also accept Mr Roberts’ evidence as to the email he ultimately sent to Mr Ryan in response to Mr Ryan’s email of 21 May 2014.
821 In relation to Mr Knight, I accept in particular his evidence that Fuchs did not support the HPFI dye methodology, that Fuchs was only providing the dye for HPFI where it was requested by the customer and that the only HPFI dye that Fuchs were supplying at that time, that is other than at Broadmeadow, was FluidSafe. I also accept Mr Knight’s evidence that he did not agree, by reference to an email of 18 December 2015, copied to him, that a Fuchs products containing a dye for leak detection purposes could also be capable of being used for HPFI detection. Mr Knight considered it clear that a dye for leak detection had a much lower concentration than a dye for HPFI detection.
822 Contrary to Quaker’s submission, I find that Fuchs did not infringe under s 117(2)(b) of the Act in respect of the supply of Renolin B 68 Plus LD mineral oil and Solcenic longwall emulsion to Glencore’s Ulan No 3, Ulan West and Bulga mine sites.
823 I do not accept Quaker’s additional submission, concerning Oaky Creek, that Fuchs offered to supply product containing fluorescent dye in circumstances where it had reason to believe that Oaky Creek would put that product to an infringing use. I note in this respect that Fuchs did not at any point supply any product to Oaky Creek and I am not persuaded that any offer was in fact made by Fuchs to supply product containing fluorescent dye to Oaky Creek for HPFI.
2.3 Yancoal (Ashton and Moolarben mines)
824 The product complained of by Quaker in relation to Yancoal’s Ashton mine site was Solcenic GM20 3% Premix Emulsion supplied to Yancoal’s Ashton mine site since August 2015. In relation to Yancoal’s Moolarben mine site, the complaint was in relation to a Solcenic product containing fluorescent dye offered to, or supplied to, Yancoal from January 2019.
825 In relation to the Ashton mine site, the documentation centred on an email sent by Mr Pearce to Mr Darren Stephens of Yancoal on 6 July 2015, which stated: “With increased focus on cost savings and minimising leaks across the face we have incorporated a very small amount of fluorescent dye into our GM20 product. This will not impact product performance but will produce a slight yellow colour to make the product a little more visible…”. I see no reason to interpret this email as meaning other than what it says.
826 Mr Knight gave evidence about this supply and, contrary to Quaker’s submissions, I am not persuaded that his evidence should be given no weight in this respect. His understanding was that the concentration of fluorescent fluid additives in the product supplied was the minimum and a cost-effective amount that Fuchs considered was sufficient to facilitate leak detection. I also do not accept the submission on behalf of Quaker that because Fuchs was aware that other Yancoal mine sites were using Solcenic and Renolin products dyed with FluidSafe for the purposes of HPFI detection, Fuchs would have had reason to believe that Yancoal would have had a similar intention in relation to the Ashton mine site. In my opinion, each mine had its own procedures and preferences.
827 Contrary to Quaker’s submission, if pressed, I see nothing in Mr Pearce’s email to Ms Julie Hill of Yancoal dated 11 August 2016 founding a contrary conclusion. That email referred to “the hydraulic oil without the dye as supplied to Ashton” not to Solcenic.
828 I accept the submission on behalf of Fuchs that Ashton was only supplied with Solcenic fluid dyed with an alternative fluorescent dye for the purpose of leak detection.
829 Contrary to Quaker’s submission, I find that Fuchs did not infringe under s 117(2)(b) in respect of its supply of Solcenic GM20 3% Premix Emulsion to Ashton since August 2015.
830 In relation to Yancoal’s Moolarben mine site, it may be recalled that no complaint was made with respect to the supply of Renolin B 68 D to Moolarben since March 2016, containing FluidSafe for the purposes of HPFI detection, but Quaker submitted that in and since January 2019 Fuchs had commenced, or was intending to commence, supplying Solcenic dyed with fluorescent dye, given that the minutes of a monthly meeting with representatives of the site in January 2019 record, in the apparent context of a discussion about Solcenic, that a Mr James White of Yancoal “would like Moolarben to change to the Lak (sic) Detection Product ASAP”.
831 Those minutes were admitted into evidence as Exhibit C. They are dated 16 January 2019 and consist of a one-page “Site Meeting Minutes” in relation to “Moolarben (Underground)”. The site attendees from Yancoal were Mr Christian Paredes, Mr White and Mr Errol Flack, and the Fuchs attendee was Mr Andrew Robinson. The document does not refer to FluidSafe or to HPFI. Item 5 of the topics discussed is set out at [411] above.
832 I do not draw the inference from this entry and the surrounding circumstances that Fuchs had commenced or was intending to commence supplying to the Moolarben mine site Solcenic dyed with fluorescent dye for the purpose of HPFI. I do not accept the submission by Quaker in relation to Moolarben that it was implausible that a mine site using dyed mineral oil as part of the claimed method would not want a dyed Solcenic product which could be used as part of the claimed method in its longwall, as workers near the longwall were at particular risk of HPFI. Rather, I find that the reference to leak detection in Exhibit C accurately reflected Moolarben’s interest and, further, gave rise to no reason to believe on the part of Fuchs that the product, if supplied, would be used for HPFI.
833 I do not accept Quaker’s criticism of Mr Knight’s evidence in this respect, which was that it was unreliable and incomplete. That contention assumes that, contrary to the terms of Exhibit C, the meeting attendees referred to HPFI. It is true that Mr Knight did not in his affidavit refer to the site meeting minutes which became Exhibit C, but he was taken to that document and gave evidence that he was not aware of any request or suggestion by Moolarben that it wished to change the use Fuchs Solcenic product, including a fluorescent dye. In my view there is nothing in Exhibit C from which I would infer that Mr Knight’s evidence was unreliable or incomplete, or that the meeting attendees actually discussed HPFI.
834 Contrary to Quaker’s submission, I find that Fuchs did not infringe under s 117(2)(b) in respect of its supply or intended supply of Solcenic dyed with fluorescent dye to Moolarben since January 2019.
Infringement by supply - s 117(2)(c)
835 Quaker also relied on s 117(2)(c). It was put that that provision was applicable in the case of Broadmeadow, although the issue remained in play in relation to the other mine sites also. There were three propositions that were advanced. The first was that Fuchs, in its presentation and discussions with Broadmeadow, compared the qualities of its product to FluidSafe, which was a product that was used for HPFI detection. And it knew in those circumstances that Broadmeadow wanted to use a dye to aid HPFI detection. Quaker submitted that an inference should be drawn that Mr Pearce convinced the customer, verbally, at his site meeting in January 2016, and the instructions or inducements to that effect were given by him at that time. Third, Quaker submitted, Fuchs in adopting this approach was concerned to undercut the cost of what it would supply by using a dye other than FluidSafe. And that, in conjunction with the other matters was also an inducement to BMA at Broadmeadow to use the Fuchs product, that is, Solcenic GM 20 LD because it was an offer of a substantially cheaper product, indeed, a product at a fraction of the price of a product including FluidSafe.
836 In my opinion, this aspect of Quaker’s case succeeds in relation to Broadmeadow. In so concluding I apply the (obiter) observations of the plurality in AstraZeneca AB v Apotex Pty Ltd at [425]-[427] where, at [427], their Honours held “the Watson product information document provided a clear inducement to consumers of its 20 mg dosage product to engage in tablet splitting.”. Although I am not persuaded that the use of the product was in accordance with any instructions given by Fuchs for the use of the product, I find that there was an inducement to use the product in the patented method, given to BMA by Fuchs. Although I am not prepared to infer that Mr Pearce gave instructions for the use of the product at his site meeting at Broadmeadow in January 2016, I do infer that the cheaper pricing of the product supplied by Fuchs compared to FluidSafe, in circumstances where BMA wanted a product for HPFI detection, constituted an inducement to use the product in the method claimed by the patent even though it was BMA which was pressing for Fuchs to supply the product for HPFI detection. Contrary to Fuchs’ submissions, I would not regard the inducement as being limited to the purchase of the product, having regard in particular to the comparison drawn with FluidSafe and Broadmeadow’s expressed intentions. This reasoning and conclusion apply only in the case of the Broadmeadow mine.
837 In relation to the other mines in respect of which Quaker advanced a case based on s 117(2)(c), Glencore’s Ulan No 3, Ulan West, Bulga and Oaky North mines, that case fails for the same reason as the one based on s 117(2)(b). As I have said, I do not accept Quaker’s reading of the relevant correspondence. That correspondence does not evidence any instructions or inducements provided by Fuchs to use the products it supplied, or offered to supply, in any method as claimed in the Patents. I take into account the failure to call Mr Pearce, but there is an insufficient basis in the evidence to infer that any such instructions or inducements were provided.
Authorisation and joint tortfeasance
838 I next consider Quaker’s authorisation and joint tortfeasance case. Quaker submitted that, for similar reasons, Fuchs also authorised and was a joint tortfeasor in the use of the product by BMA for detection of HPFI at Broadmeadow. On the evidence, Fuchs knew that Broadmeadow intended to use the Solcenic GM20 LD as part of the claimed method. It told Broadmeadow its product could be used for that purpose. It could have refused to supply Solcenic for that purpose without FluidSafe, but chose not to do so for commercial reasons, and instead facilitated Broadmeadow’s proposed infringement of the Patents by specifically formulating for Broadmeadow a chemically identical product to FluidSafe and supplying it to Broadmeadow.
839 Quaker accepted that, in order to make out authorisation and joint tortfeasorship, there had to be direct infringement. It submitted the Court should infer that such direct infringements occurred in the case of Broadmeadow, in circumstances where: (a) Broadmeadow was seeking the dye for the purposes of use of the method claimed in the Patents, (b) there was evidence that while HPFI injuries were rare, incidents in which the escape of fluids injured people were far more common, and the patented method would assist in distinguishing the two; and (c) there was evidence that where FluidSafe was used, the patented method was used regularly to determine whether HPFI had occurred. For example, following the roll out of Oil-Glo at Metropolitan there was a “steady stream” of workers seeking to use a UV light to determine whether they had suffered a HFPI.
840 Accordingly, Quaker submitted that the Court should find that Fuchs infringed the Patents by the supply and offer of supply of its Solcenic GM20 LD product to Broadmeadow.
841 Fuchs submitted that Quaker did not provide any particular instances of the acts alleged to constitute Fuchs authorising or threatening to authorise any conduct which would infringe either Patent. The same was said of the second authorisation case based on the allegation of aiding, abetting, counselling or procuring conduct which would infringe either Patent.
842 Fuchs denied the mere supply of fluorescent fluid additives in Australia constituted authorisation of conduct by third parties which might infringe either Patent. Moreover, Quaker had failed to prove any primary acts of infringement by customers of Fuchs. In the absence of a primary act of infringement, there could be no authorisation or common design.
843 Fuchs submitted that the authorisation case, in all its guises, could not succeed in the event that no acts of primary infringement had been established, which they had not. That was sufficient to dispose of the authorisation case based on s 13(1) of the Act.
844 To the extent Quaker was permitted to rely on a case of common design (ie, joint tortfeasorship), the same applied, Fuchs submitted. Infringing conduct of a third party mine must be established. No evidence had been led in that respect. Fuchs’ submitted that the supplies in this proceeding were to be distinguished from supplies of pharmaceutical products in pharmaceutical patent cases in which an inference may be drawn that a product was prescribed for a certain purpose and would be used for that purpose. Quaker had subpoenaed each of Fuchs’ customers seeking to prove that the patented method had been used and had failed to adduce any such evidence.
845 In my opinion Quaker’s authorisation and joint tortfeasor case fails for lack of sufficient evidence of direct infringement. I do not infer such direct infringements in the case of Broadmeadow, nor in the cases, if put, of the other mines in respect of which Quaker alleged indirect infringement. In my opinion, the inferences relied on by Quaker are speculative such that I am not satisfied that direct infringement is made out on the balance of probabilities.
846 In particular, I would not infer that the step of “a possible fluid injection occurring in a patient”, in terms of claim 1 of the Standard Patent and the Innovation Patent, or the circumstance of “a patient having a possible fluid injection”, in terms of claim 9 of the Standard Patent, occurred at Broadmeadow or any of the relevant Glencore mines. Contrary to Quaker’s submission, I do not regard as a sufficient basis for such an inference Mr Bate’s evidence that, following the introduction of Oil-Glo to the longwall at Metropolitan, “there was a steady flow of workers presenting to the first aid room who had been hit or splashed with dyed Solcenic longwall emulsion fluid and who wished to have the UV light shone on them to confirm no injection had occurred”. This is a different question to that posed by s 117(2)(b), being whether Fuchs had reason to believe, at the time of supply, that its products would be put to an infringing use.
Additional damages
847 Section 122(1A) of the Act provides:
(1A) A court may include an additional amount in an assessment of damages for an infringement of a patent, if the court considers it appropriate to do so having regard to:
(a) the flagrancy of the infringement; and
(b) the need to deter similar infringements of patents; and
(c) the conduct of the party that infringed the patent that occurred:
(i) after the act constituting the infringement; or
(ii) after that party was informed that it had allegedly infringed the patent; and
(d) any benefit shown to have accrued to that party because of the infringement; and
(e) all other relevant matters.
…
848 It is clear from Oxworks Trading Pty Ltd v Gram Engineering Pty Ltd [2019] FCAFC 240 at [66] and following that where an alleged infringer had a reasonably arguable defence to the allegation of infringement based on its construction of the claims of the patent, a strong factor telling against the award of additional damages is that the decision of the alleged infringer to continue to pursue its own commercial interests in the face of allegation of infringement could objectively be considered to be reasonably defensible.
849 I have summarised the competing submissions of the parties on this point at [423]-[430] above.
850 In light of the findings I have made, the claim for additional damages could be maintainable only in relation to the supply to Broadmeadow. I have rejected Quaker’s submissions that generally Fuchs and Glencore used requests for Fuchs’ products to be supplied for leak detection as a front.
851 I consider that Fuchs’ supply to the Broadmeadow site was reasonably defensible, objectively considered. Fuchs had a reasonably arguable defence to the allegation of infringement based on its construction of the claims of the patent. I also take into account, but in that context, Mr Knight’s evidence that it was his view as at 26 March 2013 that the supply of a product including fluorescent dye for use in HPFI detection without a licence from the owner of the patent, at that time TTAAS, would be in violation of the patent and that remained his view subsequently when Fuchs supplied product including a fluorescent dye to Broadmeadow mine for use in HPFI detection. Further, Mr Knight agreed that Mr Littley’s response to his email suggested that notwithstanding the customer had requested the use of the product for HPFI detection, Fuchs should ensure that the written request from the customer specified leak detection. Thus, I find that Fuchs in that context attempted to disguise its potential infringement with respect to Broadmeadow so as to make it less likely to be discovered in any subsequent litigation involved in the Patents. I take into account that Fuchs did not directly copy the FluidSafe dye but sourced its own dye which it tried to match to the FluidSafe dye. I take into account Fuchs’ unsuccessful claim for legal professional privilege in respect of a substantial number of documents but I do not draw the inference that Fuchs claimed privilege in documents that it knew were not privileged.
852 I also take into account that the letter dated 1 July 2016 by Fuchs’ firm of patent attorneys was incorrect to state that Fuchs had never included as part of its point of sale promotional material, labelling or technical advice any suggestion to customers of their product that an intended use of the product was in HPFI protection. This was incorrect in relation to Broadmeadow.
853 Nevertheless, I find that it is not appropriate to award additional damages. Although Fuchs made the supplies to Broadmeadow having regard to its commercial position vis-à-vis Quaker and to guard against losing market share in relation to other products as well, I do not consider its conduct as warranting additional damages.
854 In respect of both Broadmeadow and Glencore mines, Quaker submitted that Fuchs sought to claim privilege over numerous documents disclosing its internal views on a number of critical issues when the documents on their face were clearly not privileged. A review of the documents relied on by Quaker in this respect shows that while some the claims may well have been unsustainable, and such claims should not have been made, there is no pattern to the documents giving them a uniform or special significance to the case such as to ground a liability to additional damages.
855 I also note that the quantification of any additional damages is not part of the separate question now being heard and determined by the Court.
Conclusion and orders
856 The parties asked for an opportunity to consider the Court’s reasons before the making of any orders, including costs orders. I agree with that common position and will make directions for the parties to attempt to agree on a form of orders within 14 days of the date of this judgment or, failing agreement, a further seven days within which the parties should file and serve their competing form of orders.
I certify that the preceding eight hundred and fifty six (856) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Robertson. |
Associate:
Dated: 17 March 2020




