Secretary, Department of Health v Peptide Clinics Australia Pty Ltd [2019] FCA 1107
ORDERS
NSD 2213 of 2018 | ||
AND: | PEPTIDE CLINICS AUSTRALIA PTY LTD ACN 165 404 286
| |
JAGOT J | |
DATE OF ORDER: |
THE COURT DECLARES THAT:
1. On each day between 6 March 2018 and 20 March 2019, the Respondent advertised therapeutic goods on its website, its Facebook page and its Instagram page in contravention of s 42DLB(1) of the Therapeutic Goods Act 1989 (Cth) (the TG Act), because:
(a) from 6 March 2018 until around 23 November 2018, the advertisements referred to particular substances included in Schedule 4 to the current Poisons Standard (Schedule 4 Substances) on its website;
(b) from 15 March 2018 until at least 16 November 2018; the advertisements referred to particular Schedule 4 Substances on its Facebook and Instagram pages; and
(c) from 6 March 2018 until 20 March 2019, the advertisements used the word "peptides" and by doing so referred to Schedule 4 Substances
being matters proscribed by s 42DLB(7) of the TG Act.
2. On each day between 22 December 2018 and 20 March 2019, the Respondent advertised therapeutic goods in contravention of s 42DLB(1) of the TG Act by offering them for sale to persons over the telephone as part of a process where:
(a) a person engaged in a telephone consultation with a purported or actual medical practitioner who recommended to the consumer that he or she use particular Schedule 4 Substances; and
(b) the same person was then telephoned by a servant, employee or agent of the Respondent who identified particular Schedule 4 Substances, quoted their prices and solicited an order from the consumer,
being matters proscribed by s 42DLB(7) of the TG Act.
3. On each day between 6 March 2018 and 23 November 2018, the Respondent advertised therapeutic goods in contravention of s 42DLB(1) of the TG Act because such advertisements contained representations that referred to serious forms of cardiovascular disease and diseases of joint, bone, collagen, and rheumatic disease, being diseases, conditions, ailments or defects specified in Part 2 of Appendix 6 of the Therapeutic Goods Advertising Code 2015 (Cth) (2015 Advertising Code), which were therefore restricted representations in relation to which neither an approval under s 42DF of the TG Act nor a permission under s 42DK of the TG Act was in force, being a matter proscribed by s 42DLB(4) of the TG Act.
4. On each day between 6 March 2018 and 21 December 2019, the Respondent advertised therapeutic goods in contravention of s 42DLB(1) of the TG Act because such advertisements contained representations that referred to anxiety and depression, which are mental illnesses, being a condition specified in Part 1 of Appendix 6 of 2015 Advertising Code, and which were therefore prohibited representations in relation to which no permission under s 42DK of the TG Act was in force, being a matter proscribed by s 42DLB(2) of the TG Act.
5. On each day between 6 March 2018 and 23 November 2018, the Respondent advertised therapeutic goods in contravention of s 42DMA(1) of the TG Act, in that the advertisements misled, or were likely to have misled, directly or by implication or through emphasis, comparisons, contracts or omissions and so did not comply with s 4(2)(c) of the 2015 Advertising Code.
6. On each day between 6 March 2018 and 20 March 2019, the Respondent advertised therapeutic goods in contravention of s 42DMA(1) of the TG Act in that the advertisements contained claims, statements and implications that the advertised products are safe or alternatively, cannot cause harm, and so did not comply with s 4(2)(i) of the 2015 Advertising Code and s 10(d)(i) of the Therapeutic Goods Advertising Code (No 2) 2018 (Cth) (2018 Advertising Code).
7. On each day between 6 March 2018 and 20 March 2019, the Respondent advertised therapeutic goods in contravention of s 42DMA(1) of the TG Act in that the advertisements expressly or impliedly encouraged the use of therapeutic goods for one or more of the following uses, when such uses would be inappropriate:
(a) Anxiety/Confidence/Mood Regulation
(b) Anti-ageing
(c) Body Building/Building lean muscle mass/Muscle Building
(d) Tanning/Skin Pigmentation
(e) Injury Repair
(f) Heart Health
(g) Fat/Weight Loss
(h) Libido Enhancement
(i) Premature Ejaculation
(j) Female Sexual Dysfunction
(k) Hair Loss
(l) Insomnia Relief/Jet Lag/Sleep Assistance
and so did not comply with s 4(2)(f) of the 2015 Advertising Code and s 10(c) of the 2018 Advertising Code.
8. On each date between 6 March 2018 and 23 November 2018, the Respondent advertised therapeutic goods in contravention of s 42DMA(1) of the TG Act in that the advertisements presented scientific information in an accurate, imbalanced and misleading manner and so did not comply with s 4(4) of the 2015 Advertising Code.
THE COURT ORDERS THAT:
1. Pursuant to s 42Y(2) of the TG Act the respondent pay a pecuniary penalty to the Commonwealth of $10 million in respect of the contraventions.
2. The respondent pay the applicant's costs as agreed or taxed.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
JAGOT J:
Background
1 In this matter the Secretary of the Department of Health seeks declarations and pecuniary penalties against the respondent, which is now in liquidation. The liquidator of the respondent has not taken any role in the proceeding and neither consents to nor opposes the orders sought.
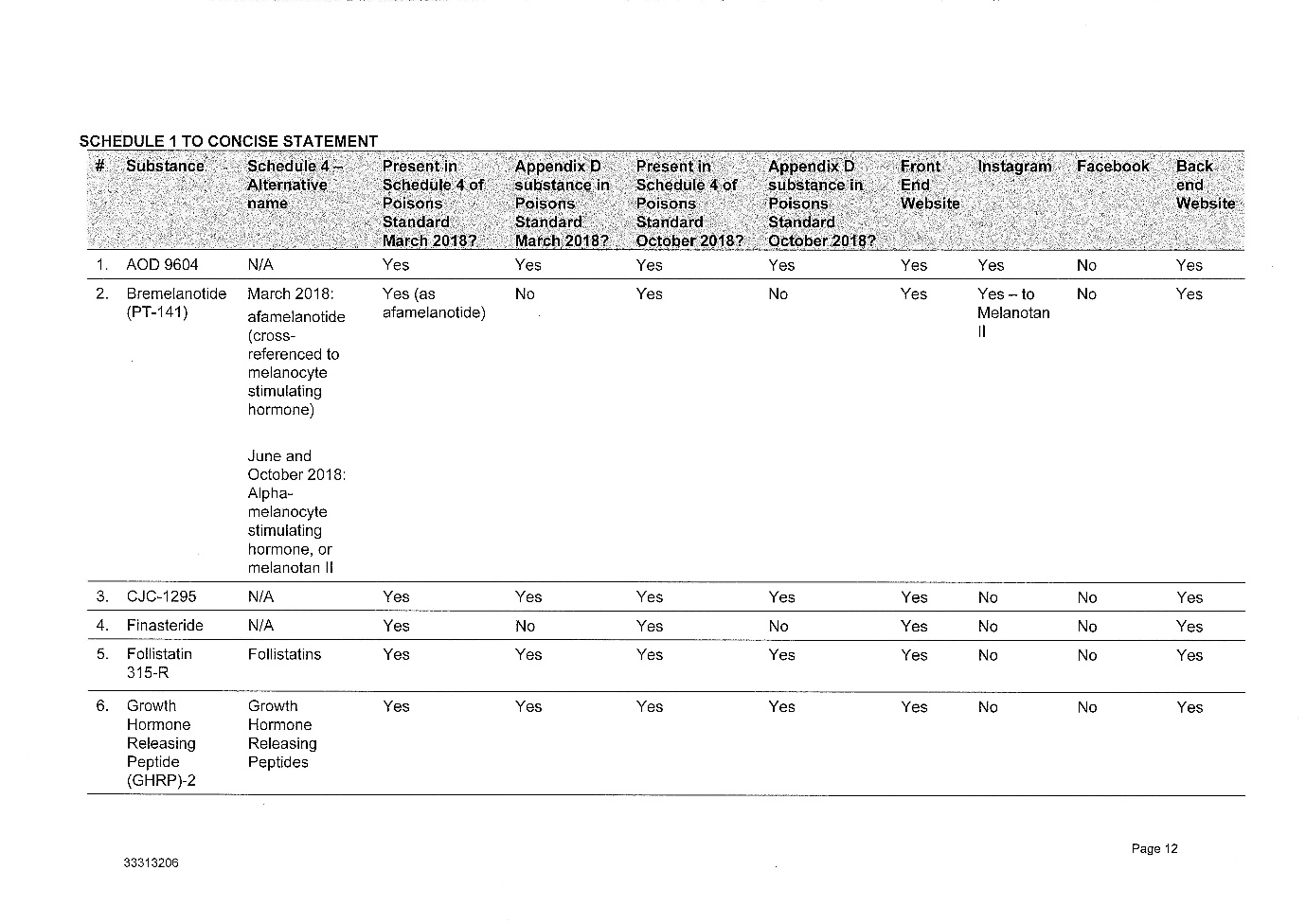
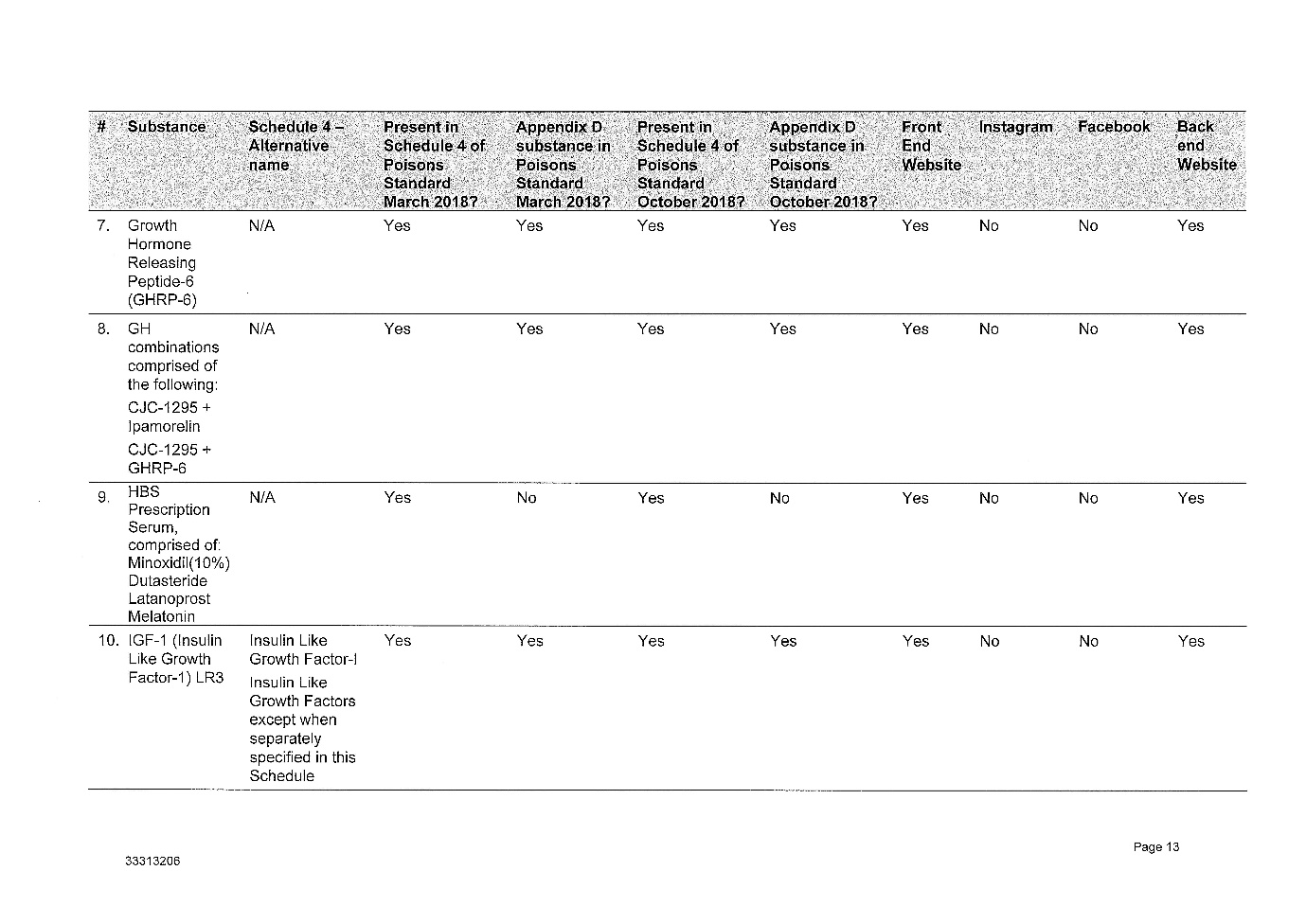
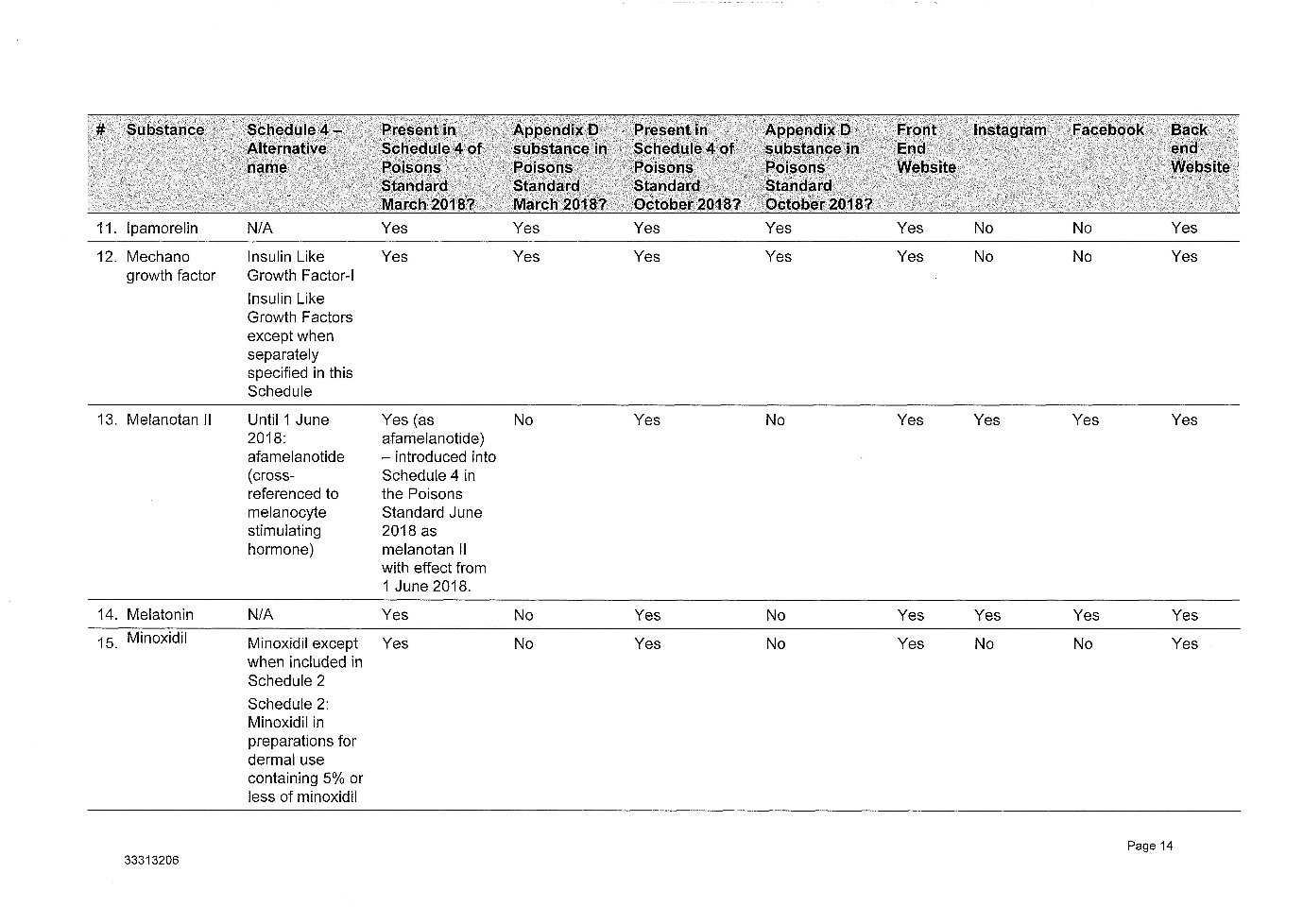
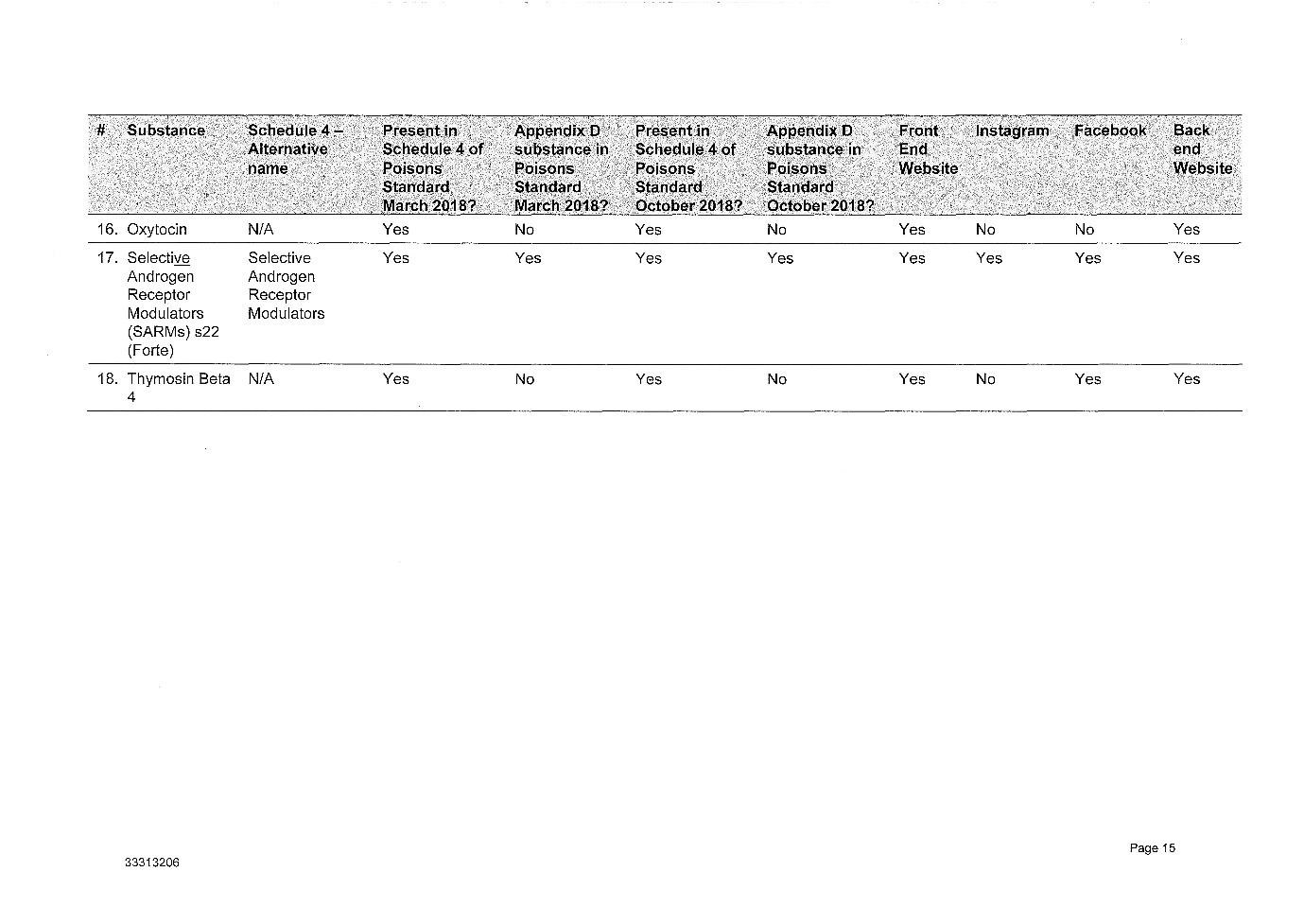
2 The legislative and factual context is complex. Annexed to these reasons for judgment as Annexures 1, 2, 3 and 4 respectively are: (1) the Further Amended Concise Statement filed by the Secretary, (2) the various contraventions of the Therapeutic Goods Act 1989 (Cth) (the TG Act) which the Secretary has adduced evidence to prove, (3) a suggested penalty schedule for the 9 courses of contravening conduct which the Secretary has proved against the respondent, and (4) Annexure B to the Secretary's submissions which describes the regulation of therapeutic goods and Schedule 4 substances.
The legislation
3 The Secretary alleges that Peptide Clinics contravened ss 42DLB and 42DMA of the TG Act which commenced on 6 March 2018, on and from that date until placed into liquidation in March 2019.
4 As the Secretary noted in comprehensive written submissions in support of the case against Peptide Clinics:
According to the second reading speech to the Bill which became this Act [The Hon Mr Hunt MP, Second reading speech to the Therapeutic Goods Amendment (2017 Measures No.1) Bill 2017, House of Representatives, Hansard, 14 September 2017, 10426], the purpose of the amendments included improving compliance and monitoring provisions to strengthen consumer protections, as well as "enhance[ing] enforcement and compliance powers to deter inappropriate or misleading advertising of therapeutic goods".
5 Therapeutic goods are defined in s 3 of the TG Act as goods that are represented to be or for any reason are likely to be taken to be for, relevantly, therapeutic use. Section 3 also identifies "advertise" in relation to therapeutic goods as including the making of any statement, pictorial representation or design that is intended, whether directly or indirectly, to promote the use or supply of the goods.
6 Section 42DLB, the general civil penalty provision relating to advertisements, provides that:
(1) A person contravenes this subsection if:
(a) the person:
(i) advertises, by any means, therapeutic goods; or
(ii) causes the advertising, by any means, of therapeutic goods; and
(b) subsection (2), (3), (4), (5), (6), (7), (8) or (9) applies to the advertisement.
Maximum civil penalty:
(a) for an individual—5,000 penalty units; and
(b) for a body corporate—50,000 penalty units.
Contravening provisions
(1) This subsection applies to the advertisement if it contains a prohibited representation (whether in express terms or by necessary implication) about the goods and either of the following applies:
(a) no permission under section 42DK is in force in relation to the prohibited representation;
(b) a permission under section 42DK is in force in relation to the prohibited representation but the use of the prohibited representation is not in accordance with the permission or a condition of the permission.
…
(4) This subsection applies to the advertisement if it contains a restricted representation (whether in express terms or by necessary implication) and either of the following applies:
(a) neither an approval under section 42DF nor a permission under section 42DK is in force in relation to the restricted representation;
(b) an approval under section 42DF or a permission under section 42DK is in force in relation to the restricted representation but the use of the restricted representation is not in accordance with the approval or permission or a condition of the approval or permission.
…
(7) This subsection applies to the advertisement if it refers to substances, or goods containing substances, included in Schedule 3, 4 or 8 to the current Poisons Standard but not in Appendix H of the current Poisons Standard, other than a reference authorised or required by a government or government authority (not including a foreign government or foreign government authority).
7 As the submissions for the Secretary explained:
25. For the purposes of Part 5.1, "prohibited representation" means "representations in column 2 of an item in Part 1 of Schedule 2 [to the Therapeutic Goods Regulations 1990 (Cth) ("TG Regulations")] about therapeutic goods in column 3 of that item": TG Act, s 42DJ; TG Regulations, s 6B. The relevant portions of Part 1 of Schedule 2 are as follows:
Part 1—Prohibited representations
Column 1 Item No. | Column 2 Representation | Column 3 Therapeutic goods |
1 | a representation that is a prohibited representation under Part 1 of Appendix 6 to the Therapeutic Goods Advertising Code | all therapeutic goods |
26. Part 1 of Appendix 6 to the Therapeutic Goods Advertising Code 2015 (2015 Advertising Code) is relevantly as follows:
Part 1 – Prohibited Representations
A prohibited representation is defined as:
(i) any representation regarding abortifacient action; or
(ii) any representation regarding the treatment, cure or prevention of the following diseases:
• Neoplastic
• Sexually Transmitted Diseases (STD)
• HIV AIDS and/or HCV; or
• Mental illness;
except for the following representations which are to become restricted representations:
(i) prevention of skin cancer through the use of sunscreens; or
(ii) devices used in contraception or in the prevention of transmission of disease between persons.
27. For the purposes of Part 5.1, "restricted representation" means "a representation in an advertisement about therapeutic goods that refers to a form of a disease, condition, ailment or defect identified in a part of the 2015 Advertising Code as a serious form of a disease, condition, ailment or defect": TG Act, ss 42B and 42DD. The relevant portion of the 2015 Advertising Code is relevantly located in Appendix 6 as follows:
"Part 2 – Restricted Representations
An advertisement for therapeutic goods may refer, expressly or by implication, to a disease, condition, ailment or defect specified in Table 1, provided that prior approval for such a reference is obtained from the Secretary of the Department of Health.
Approval may be given by a delegate of the Secretary in the TGA, taking into consideration any recommendation from the TGACC and appropriate expert committee or committees.
Table 1. Diseases, conditions, ailments and defects for which the advertising of serious forms is restricted
…
• Diseases of joint, bone, collagen, and rheumatic disease
…
• Endocrine diseases and conditions including diabetes and prostatic cancer
…
• Mental disturbances
…
Serious in the context of this table will mean forms of those diseases, conditions, ailments or defects which are:
• Generally accepted not to be appropriate to be diagnosed and/or treated without consulting a suitably qualified healthcare professional, and/or
• Generally accepted to be beyond the ability of the average consumer to evaluate accurately and to treat safely without regular supervision by a qualified healthcare professional."
28. Section 5 of the 2015 Advertising Code is as follows:
"Prohibitions
(1) An advertisement for therapeutic goods must not contain, expressly or by implication, a representation specified in Part 1 of Appendix 6.
(2) An advertisement for therapeutic goods must not refer, expressly or by implication, to serious forms of diseases, conditions, ailments or defects specified in Part 2 of Appendix 6, unless prior approval is given under the Act."
8 Section 42DMA, the civil penalty provision for non-compliance with the Advertising Code, provides that:
(1) A person contravenes this section if:
(a) the person:
(i) advertises, by any means, therapeutic goods; or
(ii) causes the advertising, by any means, of therapeutic goods; and
(b) the advertisement does not comply with the Therapeutic Goods Advertising Code.
Maximum civil penalty:
(a) for an individual—5,000 penalty units; and
(b) for a body corporate—50,000 penalty units.
9 As the submissions for the Secretary explained:
30. Advertisements for therapeutic goods directed to consumers must comply with the 2015 Advertising Code: 2015 Advertising Code, s 3(1)(a). "The conformity of an advertisement with this Code should be assessed in terms of its probable impact upon the reasonable person to whom the advertisement is directed": 2015 Advertising Code, s 3(2).
31. Section 4 of the 2015 Advertising Code prescribes what an advertisement for therapeutic goods must and must not contain:
"(1) An advertisement for therapeutic goods must:
(a) comply with the statute and common law of the Commonwealth, States and Territories; and
(b) contain correct and balanced statements only and claims which the sponsor has already verified.
(2) An advertisement for therapeutic goods must not:
(a) be likely to arouse unwarranted and unrealistic expectations of product effectiveness;
(b) be likely to lead to consumers self-diagnosing or inappropriately treating potentially serious diseases;
(c) mislead, or be likely to mislead, directly or by implication or through emphasis, comparisons, contrasts or omissions;
(d) abuse the trust or exploit the lack of knowledge of consumers or contain language which could bring about fear or distress;
(e) contain any matter which is likely to lead persons to believe:
(i) that they are suffering from a serious ailment; or
(ii) that harmful consequences may result from the therapeutic good not being used.
Sunscreen preparations are exempted from (ii) if the claims made in the advertisement are consistent with current public health messages.
(f) encourage, or be likely to encourage, inappropriate or excessive use;
(g) contain any claim, statement or implication that it is infallible, unfailing, magical, miraculous, or that it is a certain, guaranteed or sure cure;
(h) contain any claim, statement or implication that it is effective in all cases of a condition;
(i) contain any claim, statement or implication that the goods are safe or that their use cannot cause harm or that they have no side-effects; or
…
(4) Scientific Information
Any scientific information in an advertisement should be presented in a manner that is accurate, balanced and not misleading. Scientific terminology must be appropriate, clearly communicated and able to be readily understood by the audience to whom it is directed. Publication of research results must identify the researcher and financial sponsor of the research.
(5) Comparative Advertising
Comparative advertisements must be balanced and must not be misleading or likely to be misleading, either about the therapeutic goods advertised or the therapeutic goods, or classes of therapeutic goods, with which it is compared. Points of comparison should be factual and reflect the body of scientific evidence. Comparisons should not imply that the therapeutic goods, or classes of therapeutic goods, with which comparison is made, are harmful or ineffectual."
32. The Therapeutic Goods Advertising Code (No 2) 2018 ("2018 Advertising Code") commenced on 1 January 2019. Section 10 of the 2018 Advertising Code provides:
Advertising for therapeutic goods must:
(a) support the safe and proper use of therapeutic goods by:
(i) presenting the goods in accordance with directions or instructions for use; and
(ii) not exaggerating product efficacy or performance; and
(b) not be likely to lead to people delaying necessary medical attention or delaying the use of, or failing to use, treatment prescribed by a medical practitioner; and
(c) not encourage inappropriate or excessive use of the therapeutic goods; and
(d) not contain any claim, statement, implication or representation that:
(i) the therapeutic goods are safe or that their use cannot cause harm, that they have no side-effects; or
(ii) the therapeutic goods are effective in all cases of a condition or that the outcome from their use is a guaranteed or sure cure; or
(iii) the therapeutic goods are infallible, unfailing, magical or miraculous; or
(iv) harmful consequences may result from the therapeutic goods not being used — unless the claim, statement, implication or representation is permitted under section 42DK of the Act or approved under section 42DF of the Act.
10 Section 42AA of the TG Act creates an exception to the application of Pt 5-1 and is in these terms:
(1) This Part does not apply to advertisements directed exclusively to:
(a) medical practitioners, psychologists, dentists, pharmacists, optometrists, chiropractors, physiotherapists, nurses, midwives, dental hygienists, dental prosthetists, dental therapists or osteopaths; or
(b) persons who are:
(i) engaged in the business of wholesaling therapeutic goods; or
(ii) purchasing officers in hospitals; or
(c) herbalists, homoeopathic practitioners, naturopaths, nutritionists, practitioners of traditional Chinese medicine or podiatrists registered under a law of a State or Territory; or
(d) a class of persons specified under subsection (1A). …
(2) This Part does not apply to advertisements directed exclusively to persons who are members of an Australian branch (however described) of one of the bodies prescribed for the purposes of this subsection
…
(4) This Part does not apply to advice or information given directly to a patient by a person referred to in paragraph (1)(a) or (c) or subsection (2) in the course of treatment of that patient.
11 By s 42Y, the Court may order a pecuniary penalty in respect of a contravention of a "civil penalty provision" (as defined in s 42YA) of the TG Act.
12 The contravening conduct constituted advertising appearing over considerable periods of time on digital platforms. As a result, the Secretary contended, and I accept that, a contravention occurred on each day the advertising appeared on the digital platform. Appendix 2 discloses the number of days over which the various advertisements in contravention of the legislation appeared on the digital platforms.
Expert evidence
13 The advertisements all related to peptides. These are chains of amino acids which form proteins and can be used to stimulate growth hormones in the body. As explained in the Secretary's submissions, the evidence from Professor David Handelsman, a Professor of Medicine and Reproductive Endocrinology and Andrology, establishes that:
(1) With the exception of using Minoxidil for hair loss, there is no medical justification for using the peptides and peptide treatments advertised by Peptide Clinics to treat the so-called "conditions" that were listed on its website.
(2) The risks of taking such substances for the conditions is "completely unacceptable".
(3) "…doctors should simply not prescribe such non-approved substances for frivolous non-medical complaints, such as tanning", which is one of the "conditions" listed on the Peptide Clinics' website for which peptides were available.
(4) The peptides advertised and retailed by Peptide Clinics are Schedule 4 Substances, meaning they can only be supplied on prescription.
(5) The substances listed in Schedule 1 to the Amended Concise Statement are in the Poisons Standard as at March 2018, October 2018 and January 2019.
14 Professor Handelsman also said:
A complete medical assessment involves taking a full and careful medical history, conducting a proper physical examination and discussing the risks and benefits of any proposed treatment with full disclosure (including providing written information) of what is known about that drug, how widely it has been used and its safety profile. The goal is to form a thorough picture of the patient's medical status, relevant medical history and potential risks for any new proposed treatment, especially for a product (not simply the active ingredient) that has no soundly established safety and efficacy profile.
The medical history should be based on open-ended questions with the doctor following-up answers to the standard questions to determine the deeper significance of responses. It is simply completely inadequate to have a check-list of standard questions. While a minority of doctors do use such a check list of questions, these are a prelude to clarifying any responses (including important and relevant negative responses) with further open-ended questions to define the response in greater depth.
A complete physical examination is an indispensable component of the medical assessment. A written Information Sheet should name the drug, its source (or sponsor), the agency taking responsibility for the drug administration, the medical indication for the administration, the likely expected effects and side-effects as well as known risks, warning about teratogenic risk (for potentially fertile women) and the extent of clinical experience in using that product. Use of such substances without fully informed consent would be a major dereliction of professional responsibility by any doctor.
None of these criteria are satisfied by the inadequate online Medical Questionnaire as the sole medical assessment of the patient.
15 Professor Rebecca Mason, who specialises in endocrine physiology, examined the Peptide Clinics website and explained why, overall, "the scientific information is not balanced, is substantially misleading and in some important instances contradicts key public health messages. The information on the website could mislead even someone with a scientific background".
16 Associate Professor Hespe, a GP Clinician and Head of General Practice and GR Research at the University of Notre Dame, Sydney, described the business model of Peptide Clinics as a patient driven medication transaction with minimal medical oversight and considerable risk of provision of medication to customers with contraindications. The Associate Professor said:
The medical information gathered in the medical form is only one part of the medical diagnostic and management process. The next step is for the medical practitioner to interrogate the patient about the answers and ensure a fully informed history has been gained.
There does not appear to be any undertaking by the website doctor to proceed any further into the diagnostic or management process. This therefore appears to be a transactional relationship between the website and the customer for purchase of goods rather than a doctor/patient relationship.
I am particularly concerned that the customer may believe that they are being provided with a medically "safe" prescription of medication that is in fact unsuitable or unsafe for that person under this regime.
17 Further:
[T]here is no information provided on an individualised basis to the customer. They are merely provided with a range of medications to purchase with no discussion about what are the risks and/or benefits of the treatment for this person.
18 In respect of the altered business model of Peptide Clinics involving a telephone call from a medical practitioner, the Secretary's submissions observed the following:
98. Associate Professor Hespe gives evidence that at no stage during the telephone conversation recorded at paragraph 14 of the Tan Affidavit or the telephone conversation recorded at paragraph 45 of the Second McIntosh Affidavit, does either TGA officer become the Doctor's "patient" because in both cases the Doctor "makes no attempt to conduct a medical history interview, fails to establish a diagnosis and does not talk about any need to have physical examination findings to confirm a diagnosis". Associate Professor Hespe opines that "this does not fulfil the description of someone who is taking on the role and responsibility of a medical practitioner for the patient. He is conducting the role of informing a customer about their particular options regarding peptides to purchase, on the basis of the completed online survey", being the "medical questionnaire" Associate Professor Hespe has previously stated is entirely unfit for purpose. Associate Professor Hespe also gives evidence that at no stage does the Doctor and the TG officer enter into a Doctor-Patient relationship. She observes that:
"He fails to ensure that the product is medically correct for either of the TGA officers and also fails to offer any detailed medical advice regarding potential adverse effects or outcomes at this time. He states all follow up will be via the clinic and will not be via any further contact with himself. He also specifically distances himself from the "business" of the peptide sales. In my opinion the management plan fails to meet the requirements of a medically supervised treatment as there is no diagnosis of a medical condition, there is no 'management plan' for the condition and there is no option given for medical review after commencement of the peptides."
99. Associate Professor Hespe concludes that the conversations "never meet the minimum requirements of a medical consultation and more importantly the Doctor distances himself from any ongoing care or responsibility for the prescribed treatment." They are "really just a sales pitch about what products are available to meet the requests of both clients with no attempt to establish a doctor/patient relationship or any sense of ongoing medical care or responsibility."
The advertisements
19 The Secretary explained in the submissions:
44. Peptide Clinics advertised therapeutic goods on its Website, Facebook and Instagram, in a way which the Secretary alleges fell within s 42DLB(7) of the TG Act such that Peptide Clinics contravened s 42DLB(1) of the TG Act. However, its business model changed over time as did the nature of what it advertised and it is necessary to trace through the changes to show the various ways in which the conduct fell within s 42DLB(7). It is convenient to group the contraventions into the following periods:
(a) From at least 6 March 2018 (when the relevant legislative provisions commenced) until around 23 November 2018, Peptide Clinics advertised Schedule 4 Substances on the Front End of its Website;
(b) From 15 March 2018 until at least 16 November 2018, Peptide Clinics advertised Schedule 4 substances by name on its Facebook and Instagram pages ("Social Media Channels");
(c) From at least 6 March 2018 until 20 March 2019 (the date the Website became inoperative) in the case of the Front End of the Website and Social Media Channels, Peptide Clinics advertised Schedule 4 Substances (but no longer specifically identified them by their specific names);
(d) From at least 6 March 2018 until 21 December 2018, Peptide Clinics advertised Schedule 4 substances by name on the Back End of the Website. Consumers gained access to the Back End by completing an online medical questionnaire which was allegedly reviewed by medical practitioner who then granted the consumer access to the Back End (generally, within a very short period of time and without even speaking with that consumer);
(e) From 22 December 2018 (when Peptide Clinics provided an undertaking) until 20 March 2019, Peptide Clinics no longer advertised Schedule 4 Substances by name on the Back End. Rather, after a consumer submitted a medical questionnaire, the consumer would be contacted by a medical practitioner who advised the consumer what Schedule 4 Substances they should purchase and thereafter a representative of Peptides Clinics would telephone the consumer, explain the costs of the goods and take their order.
20 From the evidence and submissions I am satisfied that the Secretary has established contraventions as alleged:
(1) "In the period 6 March 2018 to 23 November 2018, Peptide Clinics advertised Schedule 4 Substances by name of the Front End of the Website. Since these advertisements contained references to substances, or goods containing substances, included in Schedule 4 to the current Poisons Standard, s 42DLB(7) of the TG Act applied. As a result, Peptide Clinics contravened s 42DLB(1) of the TG Act."
(2) "From 15 March 2018 until at least 16 November 2018, Peptide Clinics advertised Schedule 4 Substances by name on its Facebook and Instagram pages. On its Facebook Page, Peptide Clinics advertised Melanotan II from 4 June 2018; Melanotan II from 25 May 2018; SARMs s22 from 16 May 2018; Melanotan II from 10 May 2018; AOD-9604 from 8 May 2018; and Thymosin Beta 4 from 15 March 2018. On its Instagram Page, Peptide Clinics advertised Melanotan II from 25 May 2018; SARMs s22 from 16 May 2018; Melanotan II from 10 May 2018; and AOD-9604 from 8 May 2018...This conduct also fell within s 42DLB(7) of the TG Act such that s 42DLB(1) was contravened."
(3) "From at least 6 March 2018 until 20 March 2019 (the date that the Website became inactive) in the case of the Front End of the Website, and from around 8 November 2018 in the case of the Social Media Channels until 20 March 2019, Peptide Clinics advertised Schedule 4 Substances (but no longer specifically identified them by their specific names). The conduct nevertheless fell within s 42DLB(7) because the advertisements still referred to substances or goods that contained substances in Schedule 4."
(4) "During the period 6 March 2018 to 23 November 2018, the Website described a 'five-step' process for purchasing Peptides and Peptide Treatments from Peptide Clinics." The steps involved a questionnaire which Professor Handelsman described as "grossly inadequate" for the purpose of providing a medical practitioner with sufficient information to enable him or her to prescribe the Schedule 4 Substances safely. The questionnaire, if approved by an unknown medical practitioner, and email confirmations, enabled the consumer to access the back end of the website which advertised various peptides for purchase.
(5) "On about 13 December 2018, Peptide Clinics provided an undertaking in substantially similar form to the interlocutory injunction that had been sought by the Secretary, to have effect from 5pm on 21 December 2018. After this time, Peptide Clinics changed its business model and dispensed with the Back End of the Website. In its place, Peptide Clinics introduced a telephone ordering system." This involved the same questionnaire and a consultation with a medical practitioner over the telephone about the products which involved Schedule 4 substances.
(6) "From 6 March 2018 to around 23 November 2018, Peptide Clinics contravened s 42DLB(1) of the TG Act by advertising therapeutic goods by means of advertisements containing representations that referred to serious forms of cardiovascular disease and diseases of joint and bone, being diseases, conditions, ailments or defects specified in Part 2 of Appendix 6 of the TG Advertising Code, which were therefore 'restricted representations' for the purposes of s 42DD of the TG Act in relation to which neither an approval under s 42DF nor a permission under s 42DK was in force such that 42DLB(4) applies."
(7) "From 6 March 2018 to 23 November 2018, Peptide Clinics contravened s 42DLB(1) of the TG Act by advertising therapeutic goods by means of advertisements containing representations that refer to anxiety and depression, which are mental illnesses, being a condition specified in Part 1 of Appendix 6 of the 2015 Advertising Code, and which were therefore 'prohibited representations' in relation to which no permission under s 42DK was in force such that 42DLB(2) applies."
(8) "From 6 March 2018 to 23 November 2018, the Respondent contravened s 42DMA(1) of the TG Act by advertising therapeutic goods, such advertisements being non-compliant with s 4(2)(c) of the 2015 Advertising Code in that the advertisements misled, or were likely to have misled, directly or by implication or through emphasis, comparisons, contrasts or omissions."
(9) "From 6 March 2018 to 20 March 2019, the Respondent contravened s 42DMA(1) of the TG Act by advertising therapeutic goods, such advertisements being non-compliant with s 4(2)(i) of the 2015 Advertising Code and s 10(d)(i) of the 2018 Advertising Code in that the advertisements contained claims, statements and implications that the advertised products are safe or alternatively, cannot cause harm, when in fact they are not safe and can cause harm."
(10) "From 6 March 2018 to 20 March 2019, Peptide Clinics contravened s 42DMA(1) of the TG Act by advertising therapeutic goods, such advertisements being non-compliant with s 4(2)(f) of the 2015 Advertising Code and s 10(c) of the 2018 Advertising Code in that the advertisements expressly or impliedly encouraged the use of substances, or goods containing substances, included in Schedule 4 to the current Poisons Standard for one or more of the following uses, when such uses would be inappropriate:
Anxiety/Confidence/Mood Regulation
Anti-ageing
Body Building/ Building Lean Muscle Mass/Muscle Building
Tanning/Skin Pigmentation
Injury Repair
Heart Health
Fat/Weight Loss
Libido Enhancement
Premature Ejaculation
Female Sexual Dysfunction
Hair Loss
Insomnia Relief/Jet Lag/Sleep assistance."
(11) "From 6 March 2018 to 23 November 2018, the Respondent contravened s 42DMA(1) of the TG Act by advertising therapeutic goods, such advertisements being non-compliant with s 4(4) of the 2015 Advertising Code in that the advertisements presented scientific information in an inaccurate, imbalanced and misleading manner."
21 The exception in s 42AA(4) of the TG Act is inapplicable. It will be recalled that this section requires that there be advice of information given directly to a patient by, relevantly, a medical practitioner in the course of treatment of that patient. In summary, as set out in the submissions for the Secretary:
(1) Section 42AA(4) of the TG Act did "not apply to any of the advertisements that appeared of the Front End of the Website, Facebook or Instagram because they were publicly accessible. There was no medical practitioner involved at all".
(2) Section 42AA(4) did not apply to the Back End of the Website for these reasons:
(a) there was no direct contact between the customer and the medical practitioner;
(b) "the persons to whom the information was given are not Peptide Clinics' or the medical practitioners' 'patients', in fact or at law, for the purposes of s 42AA(4)" because the "relationship lacks the elemental features of a doctor/patient relationship by reason of the facts that: (1) the customer does not know the identity of the medical practitioner; (2) the medical practitioner has not satisfied the minimum requirements for conducting a patient consultation and a clinical examination of the customer or the patient; and (3) the medical practitioner has not treated the customer, including because the medical practitioner has not advised the customer of the risks associated with treatment involving the use of the Advertised Products"; and
(c) any advice or information given cannot be said to have been in the course of treatment of the patient because the medical practitioners involved, according to the evidence including expert evidence, "(1) do not take a fully informed medical history from the customers; (2) do not provide the customers with all current treatment options available for managing their condition; (3) do not inform the customers of the risks and benefits of each treatment option; (4) do not follow-up the management process of the customer's condition; and, (5) generally fall well short of the minimum standards expected of a doctor when treating a 'patient' in respect of a medical condition".
(3) Section 42AA(4) did not apply to the altered business model involving medical practitioners telephoning the customers for the same reasons.
Penalty
22 Section 42Y of the TG Act empowers the Court to impose a pecuniary penalty on a person for contravention of a civil penalty provision. By s 42Y(3):
In determining the pecuniary penalty, the Court must have regard to all relevant matters, including:
(a) the nature and extent of the contravention; and
(b) the nature and extent of any loss or damage suffered as a result of the contravention; and
(c) the circumstances in which the contravention took place; and
(d) whether the person has previously been found by the Court in proceedings under this Act to have engaged in any similar conduct.
23 By s 42YA:
A subsection of this Act (or a section of this Act that is not divided into subsections) is a civil penalty provision if the words "civil penalty" and one or more amounts in penalty units are set out at the foot of the subsection (or section).
24 It follows that ss 42DLB and 42DMA are civil penalty provisions.
25 The maximum penalty for each contravention of either provision is $10,500,000. This is because the sections provide for a maximum penalty of 50,000 penalty units for a corporation, and s 4AA of the Crimes Act 1914 (Cth) relevantly provides that in a law of the Commonwealth or a Territory Ordinance, unless the contrary intention appears, "penalty unit" means the amount of $210.
26 Pecuniary penalties are payable to the Commonwealth: s 42YD(a).
27 I accept the submissions for the Secretary as follows.
28 The objects of the TG Act in s 4 are relevant. They include providing for "the establishment and maintenance of a national system of controls relating to the quality, safety, efficacy and timely availability of therapeutic goods that are used in Australia" and providing "a framework for the States and Territories to adopt a uniform approach to control the availability and accessibility, and ensure the safe handling, of poisons in Australia".
29 The object of the imposition of a civil penalty is deterrence, specific and general, so that the penalty imposed cannot be regarded as a mere cost of doing business: Australian Building and Construction Commissioner v Construction, Forestry, Mining and Energy Union [2017] FCAFC 113 (ABCC v CFMEU) at [98], Australian Competition and Consumer Commission v Woolworths Limited [2016] FCA 44 at [125], Singtel Optus Pty Ltd v Australian Competition and Consumer Commission [2012] FCAFC 20 at [62]-[63].
30 This said, the penalty should not be oppressive in the sense that it should be no higher than necessary to achieve the object of deterrence: Australian Competition and Consumer Commission v Leahy Petroleum Pty Ltd (No 2) [2005] FCA 254; (2005) 215 ALR 281 at [9].
31 The maximum penalty, $10,500,000 for each contravention, must be considered because it shows the penalty for the worst possible case and enables comparison with the case at hand: Markarian v The Queen [2005] HCA 25; (2005) 228 CLR 357 at [31].
32 The exercise, however, is not a mathematical one starting with the maximum penalty and adjusting by reference to mitigating and aggravating circumstances: ABCC v CFMEU at [166].
33 Regard needs to be had to the "course of conduct" principle which ensures that an offender is not punished multiple times for the same criminality: Construction, Forestry, Mining and Energy Union v Cahill [2010] FCAFC 39; (2010) 269 ALR 1 at [39]. Accordingly, as described in the submissions for the Secretary:
136. Thus, rather than imposing separate penalties for each contravention, the Court may seek to group them by reference to the principle of "single course of conduct" or "one transaction" if there is sufficient interrelationship between the legal and/or factual elements of those contraventions. This principle guards against the risk that the respondent is "doubly punished" in respect of the relevant acts or omissions that make up multiple contraventions. In effect, consideration is given to whether the contraventions arise out of the same course of conduct or the one transaction, in order to determine whether it is appropriate that a "concurrent" or single penalty should be imposed for the contraventions.
34 Regard needs to be had to the principle of totality. By this principle the total penalty imposed for multiple contraventions must not exceed that which is just and appropriate in the circumstances of the case: ABCC v CMFEU at [117].
35 The fact that Peptide Clinics is in liquidation and has ceased trading is relevant. For one thing, the object of specific deterrence is now moot. In Australian Competition and Consumer Commission v Australian Institute of Professional Education Pty Ltd (in liq) [2017] FCA 521 at [26] Bromwich J observed that:
(1) the purpose of a civil penalty, and thus of such proceedings, is primarily if not wholly protective in promoting the public interest in compliance, by putting a price on contravention that is sufficiently high to deter repetition by the contravener [not a factor in this case] and by others tempted to contravene: Commonwealth v Director, Fair Work Building Industry Inspectorate [2015] HCA 46; (2015) 326 ALR 476 (the CFMEU civil penalty case) at 490 [55], quoting Trade Practices Commission v CSR Ltd (1991) ATPR 41–076 at 52,152;
(2) capacity to pay any penalties imposed was not a proper or relevant consideration: Australian Competition and Consumer Commission v Leahy (No. 2) [2005] FCA 254; (2005) 215 ALR 281 at 285 [11];
(3) even if a company is in liquidation, it may still be appropriate to order that it pay penalties as a measure of the Court's disapproval of the contraventions and as a measure of the seriousness in which they are regarded, including for the purposes of general deterrence: Australian Competition and Consumer Commission v SIP Australia Pty Limited [2003] FCA 336; (2003) ATPR 41-937 at 47,077-8 [59] – it was not suggested that this principle was diminished in a material way by such penalties not ultimately being recoverable by reason of the respondent being in liquidation;
(4) the ACCC [or in this case, the Secretary] as the body enforcing the civil penalty provisions in question has a real interest in seeking declaratory relief to vindicate a public right that … has been breached: Australian Competition and Consumer Commission v Goldy Motors Pty Ltd [2000] FCA 1885; (2001) ATPR 41-801 at 42,630 [30]; Australian Competition and Consumer Commission v Pacific Dunlop Limited [2001] FCA 740; (2001) ATPR 41-823 at 43,098-9 [63]-[69] – a point that may be seen to apply equally in respect of the other relief sought; and
(5) there is a significant public interest in declarations of contravening conduct and imposition of penalties being on the public record in aid of deterrence, which is not defeated by the fact that the company is in liquidation and unable to pay the penalties: Secretary, Department of Health and Ageing v Prime Nature Prize Pty Ltd (in liq) [2010] FCA 597 at [22]-[23].
36 As to the nature and extent of the contraventions:
(1) As the Secretary submitted, the contraventions involved numerous serious breaches of Pt 5-1 of the TG Act, which regulates the advertising of "therapeutic goods" and was introduced to promote the protection of public health.
(2) The contraventions formed part of a deliberate marketing strategy to promote the supply and use of the peptides sold by the business.
(3) The contraventions occurred over a 12 month period commencing when the operative provisions came into force and not ceasing until Peptide Clinics was placed into liquidation.
(4) In the Secretary's words, the modified business practices in response to the TGA's concerns was to involve doctors to give the business "the veneer of medical legitimacy" and compliance with the TG Act when, in substance, there was none. I accept that the conduct was "egregious because it gave consumers the false impression that the information contained in the advertisements was provided by a medical practitioner acting in their best interests to treat their individual needs".
(5) The contraventions had a wide reach with the company advertising that it had 50,000 satisfied patients.
(6) The conduct exposes a deliberate decision to pursue profits at the expense of public health and safety, and continued despite the TGA repeatedly raising concerns about the conduct.
37 As to the nature of any specific loss or damage caused by the contraventions, none can be proved. Nevertheless, based on the expert evidence, there was potential for harm to human health. As the Secretary submitted the expert evidence is clear – "it was dangerous for Peptide Clinics to promote the Schedule 4 Substances for the non-therapeutic purpose for which they were advertised, especially without proper supervision by a qualified healthcare professional". Further:
The products sold by Peptide Clinics were expensive. The evidence shows that products were being sold for amounts ranging from $99.95, to $209.95, $900 and $1,399.85. It can be inferred that for products that were at best, ineffective (and likely dangerous), consumers would have suffered significant financial loss in addition to risking their health and safety.
38 There is also evidence of online queries after treatment including:
(a) concerns about rashes and irritation after injecting the relevant substances; this query was not answered,
(b) issues raised concerning knee pain, the response to which did not directly address the question asked,
(c) heart palpitations to which the response was to "reduce the dose",
(d) gynecomastia, in response to which the Peptide Clinics representative prescribed a further medication without diagnosing the patient.
39 As to the circumstances in which the conduct took place, this has been described above, namely, the business model involved a deliberate strategy to pursue profit at the risk of public health and safety. I accept the Secretary's submission that "Peptide Clinics has deliberately and recklessly pursued its own financial self-interest at the expense of its legal obligations and the interests of public health".
40 Peptide Clinics has not previously been found by the Court to have engaged in any similar conduct.
41 I accept the submissions for the Secretary that:
165. The TGA gave Peptide Clinics every opportunity to address its concerns and correct its conduct; Peptide Clinics failed to do so. At all times between 23 July 2018 and 23 April 2019 Peptide Clinics was legally represented by Mills Oakley. There was substantial correspondence between the TGA and Peptide Clinics' solicitors regarding the concerns that the TGA had with the Website. However, Peptide Clinics refused to make the necessary changes to its Website to bring its Website into conformity with its obligations under the TG Act.
166. Peptide Clinics was obstructionist. It refused to provide the TGA with information when requested. For instance, it refused to identify to the TGA the names and registration numbers of the medical practitioners who were said to grant access to the Back End of the Website. It was later revealed that certain doctors that Peptide Clinics used to write prescriptions for its products had previously had limitations placed on their medical licences which prevented them from providing substances like peptides.
167. This Court would find that Peptide Clinics had no intention of complying with its obligations under the TG Act unless it was forced to do so.
168. Peptide Clinics did not co-operate with the Secretary in the course of this proceeding. This proceeding was commenced in November 2018. Peptide Clinics was not placed into liquidation until March 2019. During that time, it never filed a concise statement in response. While it did provide an undertaking, Peptides Clinics thereafter altered its business model in a way that still flouted the relevant provisions of the TG Act. Moreover, it never filed any admissions regarding its wrongdoing. Peptide Clinics intended to contest this proceeding until it was placed into liquidation.
169. It has shown no contrition for its conduct. It appears that there was a complete lack of compliance culture at the company. Peptide Clinics failed to act promptly and appropriately when the contraventions of the TG Act were brought to its attention.
42 As the business model of Peptide Clinics depended on contravening the statutory provisions, it should be inferred that all revenues derived from the contraventions. On the evidence, in the period from 1 July 2018 to 22 March 2019, Peptide Clinics received $2,150,740 in income and made a gross profit in the amount of $889,961. As at 22 March 2019, it had retained earnings of $856,478.
43 As to the number of contraventions, I accept the approach reflected in Appendix 2 to the effect that the different advertisements contravened the applicable provisions of the TG Act from day to day.
44 As to the course of conduct principle, I accept the approach in Appendix 3 that the conduct can sensibly be described as involving nine separate courses of conduct. I accept also that before applying the totality principle the range of penalties suggested by the Secretary for each contravening conduct is an appropriate range having regard to the maximum penalty, the circumstances considered above and the need for deterrence. With the object of deterrence in mind, I consider that penalties at the higher end of each range are called for as follows:
(1) Course of conduct 1 - $2.5 million
(2) Course of conduct 2 - $2 million
(3) Course of conduct 3 - $3 million
(4) Course of conduct 4 - $2 million
(5) Course of conduct 5 - $2.5 million
(6) Course of conduct 6 - $2 million
(7) Course of conduct 7 - $2.5 million
(8) Course of conduct 8 - $2 million
(9) Course of conduct 9 - $1.5 million
Total $20 million
45 Having regard to the totality principle I consider that a total penalty in the mid-range proposed by the Secretary should be imposed, in the sum of $10 million. As the Secretary submitted, although "it is no longer necessary to deter Peptide Clinics from re-contravening, a penalty of this size would secure the objective of general deterrence by making it clear message that companies will not be able to profit from their wrongdoing".
46 I also accept that the making of declarations is appropriate in the public interest given the seriousness of the contraventions and the associated risks to public health. I accept that "the declarations are desirable and appropriate because they would record the Court's disapproval of the conduct, vindicate the concerns of consumers, assist the Secretary to carry out the duties conferred on her by the TG Act, assist in clarifying the law, and make clear to other would-be contraveners that such conduct is unlawful".
I certify that the preceding forty-six (46) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Jagot. |
Associate:
ANNEXURE 1











ANNEXURE 2
Non-compliance with s 4(2)(c) of the 2015 Advertising Code | ||||
Webpage | FACS ref | Evidence/CB reference | Date range | Days |
Homepage - www.peptideclinics.com.au | 19.1 | Annexure RSM-3, CB Tab 7.3, Pages 281-284 | 6 Mar – 23 Nov 2018 | 262 days |
Treatments - www.peptideclinics.com.au/treatments | 19.2 | Annexure RSM-3, CB Tab 7.3, Pages 285-288 | 6 Mar – 23 Nov 2018 | 262 days |
Increasing melanin production -www.peptideclinics.com.au/how-to-increase-melanin-production; | 19.3 | Annexure RSM-3, CB Tab 7.3, Pages 289-293 | 6 Mar – 23 Nov 2018 | 262 days |
UV radiation and Sun Exposure- www.peptideclinics.com.au/uv-radiation-sun-exposure | 19.4 | Annexure RSM-3, CB Tab 7.3, Pages 294-299 | 6 Mar – 23 Nov 2018 | 262 days |
Tanning options -www.peptideclinics.com.au/tanning-options | 19.5 | Annexure RSM-3, CB Tab 7.3, Pages 300-302 | 6 Mar – 23 Nov 2018 | 262 days |
MSH -www.peptideclinics.com.au/treatments/sun-safety-australia/msh-melanocyte-stimulating-hormone | 19.6. | Annexure RSM-3, CB Tab 7.3, Pages 303-305 | 6 Mar – 23 Nov 2018 | 262 days |
Safe enhanced tanning -www.peptideclinics.com.au/store/treatments/safe-enhanced-tanning.html | 19.7 | Annexure RSM-3, CB Tab 7.3, Page 306 | 6 Mar – 23 Nov 2018 | 262 days |
Vitamin D -www.peptideclinics.com.au/safe-enhanced-tanning-and-vitamin-d | 19.8 | Annexure RSM-3, CB Tab 7.3, Pages 308-311 | 6 Mar – 23 Nov 2018 | 262 days |
Sun Safety - www.peptideclinics.com.au/treatments/sun-safety-australia | 19.1 | Annexure RSM-3, CB Tab 7.3, Pages 312-314 | 6 Mar – 23 Nov 2018 | 262 days |
| Total: | 2,358 | ||
Non-compliance with s 4(2)(f) of the 2015 Advertising Code / s 10(c) of the 2018 Advertising Code | ||||
Treatments -www.peptideclinics.com.au/treatments | 20.1 | Annexure RSM-3, CB Tab 7.3, Pages 285-288 | 6 Mar – 23 Nov 2018 | 262 days |
Back end Treatments Tab | 20.2 | Exhibit EPM-1, Tab 22 | 6 Mar – 21 Dec 2018 | 290 days |
Homepage - www.peptideclinics.com.au | 20.3 | Annexure RSM-3, CB Tab 7.3, Pages 281-284 | 6 Mar – 21 Dec 2018 | 290 days |
Medical questionnaire page - https://www.peptideclinics.com.au/store/vip/medical/index/ | 20.3/20.4 | Annexure CMH-4, CB Tab 8.4, Pages 436-439 | 6 Mar 2018 – 20 Mar 2019 | 379 days |
| Total: | 1,221 | ||
Non-compliance with s 4(2)(i) of the 2015 Advertising Code / s 10(i) of the 2018 Advertising Code | ||||
Sun Safety - www.peptideclinics.com.au/treatments/sun-safety-australia | 21 | Annexure RSM-3, CB Tab 7.3, Pages 312-314 | 6 Mar – 23 Nov 2018 | 262 days |
Homepage - www.peptideclinics.com.au | 22.2 and 22A.1 | Annexure RSM-3, CB Tab 7.3, Pages 281-284 | 6 Mar 2018 – 20 Mar 2019 | 379 days |
Medical Questionnaire -https://www.peptideclinics.com.au/store/vip/medical/index/ | 22.1 and 22A.2 | Annexure CMH-4, CB Tab 8.4, Pages 436-439 | 6 Mar 2018 – 20 Mar 2019 | 379 days |
Peptides Australia - https://www.peptideclinics.com.au/peptides-australia/ | 22.3 and 22A.3 | Annexure AJGT-1, CB Tab 16.1, Pages 755-757 | 6 Mar 2018 – 20 Mar 2019 | 379 days |
Back End Treatment and education tabs | 22.4 | Exhibit EPM-1, Tabs 22, 43-50 | 6 Mar – 21 Dec 2018 | 290 days |
| Total: | 1,689 | ||
Non-compliance with s 4(4) of the 2015 Advertising Code | ||||
Sun Safety Australia -www.peptideclinics.com.au/treatments/sun-safety-australia/ | 23 | Annexure RSM-3, CB Tab 7.3, Pages 312-314 | 6 Mar – 23 Nov 2018 | 262 days |
| Total: | 262 | ||
Total contraventions of s 42DMA: 5,530 | ||||
ANNEXURE 3
Course of conduct | FACS ref | Duration of conduct | No. of contraventions | Comments | Penalty | Declaration | ||
1. | Advertisement of specific (named) Sch.4 Substances on Front End of Website, contrary to ss.42DLB(1) and (7) of TG Act | [8] | 6 March 2018 - 23 November 2018 | 262 days x 18 products – 4, 716 | Blatant disregard of law | $1.5m to $3m | 1(a) | |
2. | Advertisement of Sch.4 Substances generally (eg "peptides") on Front End of Website, contrary to ss.42DLB(1) and (7) of TG Act | [11A] | 6 March 2018 – 20 March 2019 | 379 days | Blatant disregard for law | $1m to $2.5m | 1(c) | |
3. | Advertisement of Sch.4 Substances on Back End of Website, contrary to ss.42DLB(1) and (7) of TG Act | [10] | 6 March 2018 – 21 December 2018 | 290 days x 18 products = 5,220 | Tried to "game" the law Conduct created risk to public health and safety as it was by this device that the peptides were retailed | $2m to $4m | 1(a) | |
4. | Advertisements of Sch.4 Substances via telephone calls, contrary to ss.42DLB(1) and (7) of TG Act | [10A] | 22 December 2018 to 20 March 2019 | 88 days | Tried to "game" the law even after proceedings had been commenced | $1 to $2.5m | 2 | |
5. | Advertisements of specific (named) Sch.4 Substances on Social Media Channels, contrary to ss.42DLB(1) and (7) of TG Act | [9] | AOD 9604 | 8 May – 16 Nov 2018 | 192 days x 2 (FB+IG) = 384 | Blatant disregard of law Feeder to the Website | $1.5m to $3m | 1(b) |
10 May – 16 November (IG only) | 190 days | |||||||
Melanotan II | 10 May – 16 Nov 2018 | 190 days x 2 = 380 | ||||||
25 May – 16 Nov 2018 | 175 days x 2 = 350 | |||||||
4 June– 16 Nov 2018 (FB only) | 165 days | |||||||
SARMs s22 (Forte) | 16 May – 16 Nov 2018 | 184 days x 2=368 | ||||||
Thymosin Beta 4 (Facebook only) | 15 Mar – 16 Nov 2018 | 246 days | ||||||
Total | 2,083 | |||||||
6. | Advertisements of Sch.4 Substances generally (eg "peptides") on the Social Media Channels, contrary to ss.42DLB(1) and (7) of TG Act | [9A]-[9B] | 6 Mar 2018 – 20 Mar 2019 | 379 days x 2 for Instagram and Facebook = 758 | $1m to $2.5m | 1(c) | ||
7. | Advertisements containing prohibited representations, contrary to ss.42DLB(1) and (2) of TG Act | [16] | 6 Mar – 21 Dec 2018 | 290 days | Very dangerous because gave false assurances to consumers | $1.5m to $3m | 4 | |
8. | Advertisements containing restricted representations, contrary to ss.42DLB(1) and (4) of TG Act | [12] | 6 Mar – 23 Nov 2018 | 262 days x 3 (for each page) = 786 | $1.25 to $2.5m | 3 | ||
9. | Advertisements that were not compliant with the Advertising Code, contrary to s.42DMA of TG Act | [19]-[23] | 6 March 2018 – 20 March 2019 | 5,530 (see separate schedule of contraventions) | $1m to $2m | 5-8 | ||
Total contraventions | 19,850 | Total statutory maximum: over $200 billion | ||||||
TOTAL | $11.75m to $25m | |||||||
APPLY TOTALITY AND CONSIDER DETERRENCE | $8m to $12m | |||||||
ANNEXURE 4










