FEDERAL COURT OF AUSTRALIA
Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2018] FCA 1556
ORDERS
DATE OF ORDER: | 19 October 2018 |
THE COURT ORDERS THAT:
1. Until 5.00pm on 26 October 2018, pursuant to s 37AF of the Federal Court of Australia Act 1976 (Cth) and on the ground that it is necessary to prevent prejudice to the proper administration of justice under s 37AG(1)(a), there be no disclosure (by publication or otherwise) of the reasons for judgment delivered on the date of this order in proceedings VID 195 of 2009, NSD 596 of 2009 and NSD 1124 of 2009 (Proceedings) to any person other than to the external solicitors and counsel for the parties in the Proceedings, including the external solicitors and counsel for the Commonwealth of Australia (despite the discontinuance of its interlocutory application).
2. By 4.00pm on 26 October 2018 any party wishing to claim that any part of the reasons for the judgment should be subject to a further confidentiality order is to notify the Associate to Jagot J and the other parties, as well as the Commonwealth, by email of the claim including:
(a) details of the matter claimed to be confidential;
(b) a short statement of the reasons the matter is said to be confidential; and
(c) a statement identifying whether the claimant consents to the confidentiality claim being determined by Jagot J on the basis of the email or seeks an oral hearing.
3. If no notice by email is received in accordance with order 2, the reasons for judgment will be published forthwith.
4. If notice is received in accordance with order 2, the reasons for judgment will be published forthwith with the claimed confidential matter redacted pending determination of the confidentiality claim.
5. The parties are to confer and, by 4.00pm on 2 November 2018, are to propose in a joint email (including agreed and disagreed matters) to the Associate to Jagot J further directions to enable the matter to be finalised.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ORDERS
NSD 596 of 2009 | ||
BETWEEN: | ALPHAPHARM PTY LTD (ACN 002 359 739) Applicant | |
AND: | WYETH First Respondent WYETH AUSTRALIA PTY LTD (ACN 000 296 211) Second Respondent | |
AND BETWEEN: | WYETH (and another named in the Schedule) First Cross-Claimant | |
AND: | ALPHAPHARM PTY LIMITED (ACN 002 359 739) Cross-Respondent | |
IN THE INTERLOCUTORY APPLICATION: | ||
BETWEEN: | ALPHAPHARM PTY LTD (ACN 002 359 739) First Applicant GENERIC HEALTH PTY LTD (ACN 110 617 859) Second Applicant PHARMATHEN S.A. Third Applicant | |
AND: | WYETH First Respondent WYETH AUSTRALIA PTY LTD (ACN 000 296 211) Second Respondent | |
JUDGE: | JAGOT J |
DATE OF ORDER: | 19 OCTOBER 2018 |
THE COURT ORDERS THAT:
1. Until 5.00pm on 26 October 2018, pursuant to s 37AF of the Federal Court of Australia Act 1976 (Cth) and on the ground that it is necessary to prevent prejudice to the proper administration of justice under s 37AG(1)(a), there be no disclosure (by publication or otherwise) of the reasons for judgment delivered on the date of this order in proceedings VID 195 of 2009, NSD 596 of 2009 and NSD 1124 of 2009 (Proceedings) to any person other than to the external solicitors and counsel for the parties in the Proceedings, including the external solicitors and counsel for the Commonwealth of Australia (despite the discontinuance of its interlocutory application).
2. By 4.00pm on 26 October 2018 any party wishing to claim that any part of the reasons for the judgment should be subject to a further confidentiality order is to notify the Associate to Jagot J and the other parties, as well as the Commonwealth, by email of the claim including:
(a) details of the matter claimed to be confidential;
(b) a short statement of the reasons the matter is said to be confidential; and
(c) a statement identifying whether the claimant consents to the confidentiality claim being determined by Jagot J on the basis of the email or seeks an oral hearing.
3. If no notice by email is received in accordance with order 2, the reasons for judgment will be published forthwith.
4. If notice is received in accordance with order 2, the reasons for judgment will be published forthwith with the claimed confidential matter redacted pending determination of the confidentiality claim.
5. The parties are to confer and, by 4.00pm on 2 November 2018, are to propose in a joint email (including agreed and disagreed matters) to the Associate to Jagot J further directions to enable the matter to be finalised.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
ORDERS
NSD 1124 of 2009 | ||
BETWEEN: | GENERIC HEALTH PTY LTD (ACN 110 617 859) First Applicant | |
AND: | WYETH Respondent | |
AND BETWEEN: | WYETH (and another named in the Schedule) First Cross-Claimant | |
AND: | GENERIC HEALTH PTY LTD (ACN 110 617 859) Cross-Respondent | |
IN THE INTERLOCUTORY APPLICATION: | ||
BETWEEN: | GENERIC HEALTH PTY LTD (ACN 110 617 859) First Applicant ALPHAPHARM PTY LTD (ACN 002 359 739) Second Applicant PHARMATHEN S.A. Third Applicant | |
AND: | WYETH First Respondent WYETH AUSTRALIA PTY LTD (ACN 000 296 211) Second Respondent | |
JUDGE: | JAGOT J |
DATE OF ORDER: | 19 OCTOBER 2018 |
THE COURT ORDERS THAT:
1. Until 5.00pm on 26 October 2018, pursuant to s 37AF of the Federal Court of Australia Act 1976 (Cth) and on the ground that it is necessary to prevent prejudice to the proper administration of justice under s 37AG(1)(a), there be no disclosure (by publication or otherwise) of the reasons for judgment delivered on the date of this order in proceedings VID 195 of 2009, NSD 596 of 2009 and NSD 1124 of 2009 (Proceedings) to any person other than to the external solicitors and counsel for the parties in the Proceedings, including the external solicitors and counsel for the Commonwealth of Australia (despite the discontinuance of its interlocutory application).
2. By 4.00pm on 26 October 2018 any party wishing to claim that any part of the reasons for the judgment should be subject to a further confidentiality order is to notify the Associate to Jagot J and the other parties, as well as the Commonwealth, by email of the claim including:
(a) details of the matter claimed to be confidential;
(b) a short statement of the reasons the matter is said to be confidential; and
(c) a statement identifying whether the claimant consents to the confidentiality claim being determined by Jagot J on the basis of the email or seeks an oral hearing.
3. If no notice by email is received in accordance with order 2, the reasons for judgment will be published forthwith.
4. If notice is received in accordance with order 2, the reasons for judgment will be published forthwith with the claimed confidential matter redacted pending determination of the confidentiality claim.
5. The parties are to confer and, by 4.00pm on 2 November 2018, are to propose in a joint email (including agreed and disagreed matters) to the Associate to Jagot J further directions to enable the matter to be finalised.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
TABLE OF CONTENTS:
[1] | |
[4] | |
[29] | |
[31] | |
[32] | |
[48] | |
[67] | |
[72] | |
[77] | |
[82] | |
[87] | |
[104] | |
[104] | |
[105] | |
[111] | |
[115] | |
[116] | |
[117] | |
[119] | |
6 Principles relating to proof of loss by the generics – supply by them to pharmacists | [152] |
7 Principles relating to proof of loss by the manufacturers/suppliers | [206] |
[214] | |
[217] | |
[229] | |
[234] | |
[238] | |
[238] | |
[242] | |
[273] | |
[287] | |
[287] | |
[296] | |
[317] | |
[331] | |
[333] | |
[334] | |
[334] | |
[335] | |
[355] | |
15.4 Events between 6 March and 22 May 2009 (and the evidence of Mr de Alwis and Mr Ellis) | [373] |
[445] | |
[471] | |
[473] | |
[474] | |
[474] | |
[491] | |
[503] | |
[504] | |
[504] | |
[505] | |
[509] | |
[548] | |
[644] | |
[645] | |
[648] | |
[648] | |
[652] | |
[661] | |
[667] | |
[679] | |
[796] | |
[797] | |
[798] | |
[799] | |
[799] | |
[802] | |
[806] | |
[821] | |
[822] | |
[822] | |
[824] | |
[832] | |
[838] | |
[846] | |
[847] | |
[847] | |
[848] | |
[848] | |
[851] | |
[854] | |
[857] | |
[865] | |
[869] | |
[870] | |
[870] | |
[874] | |
[874] | |
[883] | |
[894] | |
[901] | |
[907] | |
[934] | |
[934] | |
[938] | |
[980] | |
[980] | |
[992] | |
[1019] | |
[1020] | |
[1030] | |
[1033] | |
[1038] | |
24 Contemporaneous evidence relevant to generic market shares | [1041] |
[1063] | |
[1063] | |
[1116] | |
[1155] | |
[1155] | |
[1199] | |
[1230] | |
[1230] | |
[1232] | |
[1232] | |
27.2.2 Disputes between generics and manufacturers/suppliers | [1240] |
[1259] | |
[1268] | |
[1276] | |
[1279] | |
[1282] | |
30 Calculations of loss based on the probabilities and possibilities | [1316] |
[1332] | |
[1336] | |
JAGOT J:
The claims
1 These matters concern claims for compensation pursuant to undertakings given as the price of the grant of interlocutory injunctions restraining infringement of a patent ultimately found to be invalid. The patent is referred to as the method patent. It relates to an anti-depressant known as venlafaxine.
2 On 21 December 2011, having found the method patent to be invalid, the Full Court made orders consequential upon reasons for judgment, Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 3) [2011] FCAFC 165 as follows:
NSD 1533 of 2010
8. Any application that has or may be made by any person seeking an order for the payment of compensation pursuant to the undertaking as to damages given by the respondents to the Court on 3 June 2009 be remitted to the primary judge for determination.
NSD 1603 of 2010
8. Any application that has or may be made by any person seeking an order for the payment of compensation pursuant to the undertaking as to damages given by the respondents to the Court on 25 August 2009 be remitted to the primary judge for determination.
NSD 1644 of 2010
8. Any application that has or may be made by any person seeking an order for the payment of compensation pursuant to the undertaking as to damages given by the respondents to the Court on 10 November 2009 be remitted to the primary judge for determination.
3 Six claimants sought compensation pursuant to the undertakings. Three claimants were parties to the original proceedings, Sigma, Alphapharm and Generic Health. Where there is no need to distinguish between them I refer to these parties together as the generics. Three claimants were non-parties. Alembic and Pharmathen were manufacturers and suppliers of generic venlafaxine products to the generics. The Commonwealth was also a claimant but it settled its claim after the hearing. The remaining claimants relied on evidence and submissions of the Commonwealth, so it is necessary to refer to these despite the fact that the Commonwealth is no longer a claimant. Where there is no need to distinguish between the respondents I refer to them together as Wyeth.
Summary of conclusions
4 I have concluded that orders for compensation should be made in favour of each claimant.
5 My principal conclusions are as follows.
6 Each claimant has been adversely affected by the operation of one or more of the interlocutory injunctions. It is just that Wyeth be ordered to pay compensation to each claimant assessed by reference to the adverse effect of the interlocutory injunction or injunctions upon them.
7 The fact that I granted final injunctions on 8 November 2010 does not mean that the interlocutory injunctions were not wrongly granted. I made final orders on the basis of my conclusion that the method patent was valid. The Full Court held that the method patent was invalid. It follows that the interlocutory injunctions were wrongly granted.
8 The primary adverse effect of the interlocutory injunctions is that they prevented the generics from supplying their generic venlafaxine products to pharmacists until 8 November 2010. On 8 November 2010, when I granted final injunctions on the basis that the method patent was valid, the interlocutory injunctions were discharged and thus ceased to operate from that date. Because I granted final injunctions which also prevented supply of the products, the adverse effects of the operation of the interlocutory injunctions ceased on 8 November 2010. No claims on Wyeth’s undertakings beyond that date can be sustained. The claimed loss of opportunity to obtain a stay of my final orders, which was not pleaded, is not compensable as a matter of principle or fact.
9 The claimants claim for lost opportunities. The primary opportunity was to supply venlafaxine products. Because the opportunity to supply depended on a statutory approval which each of the generics held before the interlocutory injunctions were granted, the interlocutory injunctions necessarily deprived the generics of an opportunity of some value. This loss having been proved on the balance of probabilities, the extent of the generics’ losses is to be assessed by reference to the probabilities and possibilities which should be inferred from the evidence.
10 Only possibilities of a less than 1% likelihood may be disregarded. Only possibilities of a greater than 99% likelihood may be treated as certain. Accordingly, it is necessary to assess the possibilities of any generic having sought and obtained PBS listing of its products to enable supply under the PBS and the possibilities of any generic having supplied its products outside of the PBS on the private market if the interlocutory injunctions had not been granted. For the generics, this is the best way of identifying the adverse effect of the interlocutory injunctions upon them.
11 Because the opportunity of the manufacturers/suppliers to supply the products to the generics depended on the generics placing orders (which in turn depended on the generics being able to supply pharmacists), the manufacturers/suppliers had to prove on the balance of probabilities that they would have supplied products if not for the interlocutory injunctions. This having been proved on the balance of probabilities, the losses of the manufacturers/suppliers are also to be assessed by reference to all of the possibilities which should be inferred from the evidence.
12 The kinds of losses claimed were all reasonably foreseeable at the time the interlocutory injunctions were granted. Other than in one respect, the kinds of losses claimed were also the direct and natural consequence of the operation of the interlocutory injunctions. The claims of Generic Health and the derivative claims of Pharmathen for the lost opportunities to supply the products to other generic companies are too remote to be compensable and otherwise should not be permitted on a discretionary basis.
13 By the undertakings Wyeth submitted to such orders as the Court considers just to compensate a person adversely affected by the operation of the interlocutory injunctions. The undertakings do not extend to any adverse effect caused by the existence of the method patent or the litigation including all of the usual exigencies of litigation. Anticipating an interlocutory injunction is one of the exigencies of litigation. The fact that the grant of an interlocutory injunction against one person may lead another to anticipate that they also will be the subject of an interlocutory injunction does not make conduct taken in such anticipation an adverse effect of the interlocutory injunction as granted.
14 The approach which best reflects the terms of the undertakings is to assess what the position would have been if Wyeth had not prosecuted its applications for interlocutory relief on the days it did so and thus had not been granted the interlocutory injunctions. To go further and disregard the mere threat or anticipation of interlocutory relief by the generics would be to expand the scope of the undertaking so as to permit compensation to be granted for the mere existence of the method patent or the litigation which is impermissible. If, however, I am wrong about this, the only material difference from the hypothetical facts as I have found them would be one of timing in relation to the prospects of PBS listing and PBS supply. For Sigma, the relevant date for PBS listing of its products would change from 1 December 2009 to 1 August 2009. For Alphapharm and Generic Health, the relevant date would change from 1 March 2010 to 1 December 2009.
15 Subject to these matters, none of Wyeth’s answers to the claims for compensation should be accepted. In particular, Wyeth’s copyright claims involve an abuse of process and should not be permitted.
16 Discretionary considerations weigh heavily in favour of enforcement of the undertakings.
17 My primary conclusions about the probabilities and possibilities are the result of an evaluative process in which within the range of reasonable inferences from the evidence I have resolved remaining doubts in the claimants’ favour because Wyeth had the benefit and the claimants suffered the harm of the wrongly granted interlocutory injunctions. As a result of this process, and on the basis that at any given time, the probabilities and possibilities for each claimant must equal 100% or 1:
(1) Sigma’s lost opportunity of supply should be valued on the basis of the following hypothesised facts:
(a) from 1 May 2009 until 1 December 2009:
(i) Sigma would have supplied its products on the private market: 100%;
(ii) Sigma would have supplied its products under the PBS: 0%; and
(iii) Sigma would not have supplied its products at all: 0%.
(b) from 1 December 2009 until 8 November 2010:
(i) Sigma would have supplied its products on the private market: 80%;
(ii) Sigma would have supplied its products under the PBS: 20%; and
(iii) Sigma would not have supplied its products at all: 0%.
(2) Alphapharm’s lost opportunity of supply should be valued on the basis of the following hypothesised facts:
(a) from 22 July 2009 until 1 March 2010:
(i) Alphapharm would have supplied its products on the private market: 90%;
(ii) Alphapharm would have supplied its products under the PBS: 0%; and
(iii) Alphapharm would not have supplied its products at all: 10%.
(b) from 1 March 2010 until 8 November 2010 if Alphapharm was supplying its products on the private market (the 90% probability) and assuming the Sigma 20% possibility of PBS listing on 1 December 2009:
(i) Alphapharm would have continued to supply its products on the private market: 10%;
(ii) Alphapharm would have supplied its products under the PBS: 90%; and
(iii) Alphapharm would not have supplied its products at all: 0%.
(c) from 1 March 2010 until 8 November 2010 if Alphapharm was not supplying its products on the private market (the 10% possibility) and assuming the Sigma 20% possibility of PBS listing on 1 December 2009:
(i) Alphapharm would have supplied its products on the private market: 0%;
(ii) Alphapharm would have supplied its products under the PBS: 80%; and
(iii) Alphapharm would have continued to not supply its products at all: 20%.
(d) from 1 March 2010 until 8 November 2010 if Alphapharm was supplying its products on the private market (the 90% probability) and assuming the Sigma 80% probability of not PBS listing on 1 December 2009:
(i) Alphapharm would have supplied its products on the private market: 90%;
(ii) Alphapharm would have supplied its products under the PBS: 0% and
(iii) Alphapharm would not have supplied its products at all: 10%.
(e) from 1 March 2010 until 8 November 2010 if Alphapharm was not supplying its products on the private market (the 10% possibility) and assuming the Sigma 80% probability of not PBS listing on 1 December 2009:
(i) Alphapharm would have supplied its products on the private market: 0%;
(ii) Alphapharm would have supplied its products under the PBS: 0% and
(iii) Alphapharm would not have supplied its products at all: 100%.
(3) Generic Health’s lost opportunity of supply should be valued on the basis of the following hypothesised facts:
(a) from 10 November 2009 until 1 March 2010:
(i) Generic Health would have supplied its products on the private market: 80%;
(ii) Generic Health would have supplied its products under the PBS: 0% and
(iii) Generic Health would not have supplied its products at all: 20%.
(b) from 1 March 2010 until 8 November 2010 if Generic Health was supplying its products on the private market (the 80% probability) and assuming the Sigma 20% possibility of PBS listing on 1 December 2009:
(i) Generic Health would have continued to supply its products on the private market: 10%;
(ii) Generic Health would have supplied its products under the PBS: 90%; and
(iii) Generic Health would not have supplied its products at all: 0%.
(c) from 1 March 2010 until 8 November 2010 if Generic Health was not supplying its products on the private market (the 20% possibility) and assuming the Sigma 20% possibility of PBS listing on 1 December 2009:
(i) Generic Health would have supplied its products on the private market: 0%;
(ii) Generic Health would have supplied its products under the PBS: 80%; and
(iii) Generic Health would have continued to not supply its products at all: 20%.
(d) from 1 March 2010 until 8 November 2010 if Generic Health was supplying its products on the private market (the 80% probability) and assuming the Sigma 80% probability of not PBS listing on 1 December 2009:
(i) Generic Health would have supplied its products on the private market: 80%;
(ii) Generic Health would have supplied its products under the PBS; 0% and
(iii) Generic Health would not have supplied its products at all: 20%.
(e) from 1 March 2010 until 8 November 2010 if Generic Health was not supplying its products on the private market (the 20% possibility) and assuming the Sigma 80% probability of not PBS listing on 1 December 2009:
(i) Generic Health would have supplied its products on the private market: 0%;
(ii) Generic Health would have supplied its products under the PBS: 0% and
(iii) Generic Health would not have supplied its products at all: 100%.
18 Accordingly, I have concluded that the chance of Alphapharm and Generic Health obtaining PBS listing of their products depends on the chance that Sigma would have obtained PBS listing of its products, which I have assessed to be a possibility of 20%.
19 Compensation should be assessed generally as proposed by Mr Samuel in his calculations of 8 July 2018, based on the revised work of Professor Hausman, without any discount for risk, and should include interest at the rates specified in Interest on Judgments Practice Note (GPN- INT).
20 Mr Samuel’s approach needs to be the subject of further calculations by reference to my conclusions about the probabilities and possibilities. I have identified a method for the required calculations based on my conclusions which involves the following:
The probabilities and possibilities may be represented by:
PBS + PM + N = 1
Where:
PBS is the chance of the generic supplying under the PBS
PM is the chance of the generic supplying on the private market
N is the chance the generic would not have supplied its products at all.
Further if:
VPBS means the estimated profits from posited PBS supply.
VPM means the estimated profits from posited private market supply.
VN means the no supply position, which must always equal zero.
VT means the total profits from posited PBS and/or private market supply,
then:
Sigma’s lost profits would be calculated as follows:
Sigma VT for 1/5/09 to 1/12/09 = 1 x VPM + 0 x VPBS + 0 x VN.
Sigma VT for 1/12/09 to 8/11/10 = 0.80 x VPM + 0.2 x VPBS + 0 x VN.
Sigma total VT = Sigma VT for 1/5/09 to 1/12/09 + Sigma VT for 1/12/09 to 8/11/10.
Alphapharm’s lost profits would be calculated as:
Alphapharm VT for 22/7/09 to 1/3/10 = 0.9 x VPM + 0 x VPBS + 0.1 x VN.
Alphapharm VT for 1/3/10 to 8/11/10 = 0.666 x VPM + 0.178 x VPBS + 0.156 x VN.
Generic Health’s lost profits would be calculated as:
Generic Health VT for 10/11/09 to 1/3/10 = 0.8 x VPM + 0 x VPBS + 0.2 x VN.
Generic Health VT for 1/3/10 to 8/11/10 = 0.528 x VPM + 0.176 x VPBS + 0.296 x VN.
21 The calculations for Alphapharm and Generic Health which both involve dependent possibilities (Sigma PBS listing and prior private market supply) are explained in Schedule 5. As this issue of dependent possibilities was not explored in the hearing and my method may be incorrect, the parties will be given an opportunity to be heard in respect of this issue and other outstanding issues as identified.
22 Alphapharm should also be compensated for 90% of the cost of the products it had to destroy due to their limited shelf life when they were ultimately delivered to it. But for the Alphapharm interlocutory injunction Alphapharm would not have suffered this loss.
23 The compensation to the manufacturers/suppliers should be assessed on the same basis having regard to the supply prices from the manufacturers/suppliers to the generics that Mr Samuel proposed and otherwise reflecting the evidence they adduced about their capacity and costs.
24 Alembic should also be compensated for the ingredients it had to destroy as a result of the Sigma interlocutory injunction.
25 I consider that my approach to compensation appropriately reflects the terms and purpose of the undertakings which Wyeth gave as the price for the interlocutory injunctions to which, in the event, it was not entitled.
26 If, however, each lost opportunity of supply must be proved on the balance of probabilities it will be apparent from the conclusions above that each claimant would have succeeded in proving a lost opportunity of supply on the private market and would have failed to prove a lost opportunity of supply under the PBS. In that event, the compensation should be assessed by reference to 100% of the value of private market supply for the relevant periods up to 8 November 2010. As the claims of the manufacturers/suppliers are derivative they cannot do better than the generics.
27 Costs should be determined after all outstanding issues are resolved and in the usual course.
28 It is possible that I have not resolved an issue which needs to be resolved. If that is the case, the parties will be given an opportunity to draw such matters to my attention.
Abbreviations, basic concepts and people
29 Schedule 1 explains abbreviations and basic concepts. It is based on the two statements of agreed facts in evidence. Those statements, excluding annexures, are in Schedules 2 and 3.
30 Schedule 4 identifies the people who are referred to in these reasons for judgment.
31 In this section I record only those facts which set the context for the claims.
ARTG registration
32 The ARTG registrations of the generics’ products obtained before the interlocutory injunctions were granted are fundamental to my conclusions.
33 Details about ARTG registration are set out in the statement of agreed facts in Schedule 2 on which the following section is based.
34 A product containing the active pharmaceutical ingredient venlafaxine hydrochloride must be registered on the ARTG before it can be imported into, manufactured or supplied in, or exported from, Australia.
35 Section 9A(1) of the Therapeutic Goods Act provides that:
The Secretary is to cause to be maintained a register, to be known as the Australian Register of Therapeutic Goods, for the purpose of compiling information in relation to, and providing for evaluation of, therapeutic goods for use in humans.
36 Section 3(1) of the Therapeutic Goods Act contains definitions, including relevantly, of “sponsor” in relation to therapeutic goods.
37 By s 23, a person may make an application to the Secretary for registration or listing of therapeutic goods.
38 By s 25(3), after an evaluation, the Secretary must decide to register or not to register the goods.
39 Division 1 of Pt 3-2 of the Therapeutic Goods Act provides for a range of civil penalties and criminal offences in relation to therapeutic goods.
40 The person or company holding the registration of a pharmaceutical product registered on the ARTG is called the sponsor of the relevant product. A sponsor may offer to supply, and supply, that therapeutic product to pharmacies, pharmaceutical distributors/wholesalers, government authorities and private or public hospitals in Australia when the product has been registered on the ARTG.
41 To register a therapeutic product on the ARTG the sponsor must obtain approval from the TGA.
42 If a pharmaceutical product is approved by the TGA, the brand name and the active ingredient of the product are entered on the ARTG, along with the name of the sponsor, the approved indication(s), the dosage form and the pack sizes for which the product may be supplied.
43 A sponsor wishing to supply a bioequivalent version (generic product) of a pharmaceutical product which is already registered on the ARTG (originator product) may make an application that does not include all the information that was required of the originator to register the originator’s product.
44 A sponsor of a generic product may rely on the safety and efficacy data submitted by the originator in respect of the originator product if the sponsor of the generic product demonstrates that the generic product is “essentially similar” or bioequivalent to the originator product.
45 In order to demonstrate bioequivalence a sponsor of the generic product should normally conduct clinical bioavailability studies comparing the generic product to the originator product. The sponsor of the generic product is required to conduct such a study or studies with the Australian originator product or demonstrate that the comparator used in the studies against which the generic product is compared is identical to the Australian originator product.
46 Once a pharmaceutical product is registered on the ARTG, it can be sold, offered for sale and otherwise supplied in Australia, including to pharmacies.
47 Pharmacists may dispense a generic product registered on the ARTG which is equivalent to the originator product (called a generic equivalent) when a doctor prescribes the originator product by brand name but does not identify on the prescription that substitution is not permitted and the patient consents, prescribes a different generic equivalent by brand name but does not identify on the prescription that substitution is not permitted and the patient consents, prescribes the product by its active pharmaceutical ingredient (API) name, such as venlafaxine, or prescribes the generic equivalent by its brand name.
The PBS scheme
48 It is necessary to understand some aspects of the PBS scheme. The scheme is explained in the statement of agreed facts in Schedule 2 on which the following section is based.
49 The PBS scheme is established in Pt VII of the National Health Act. The existence and management of the PBS by the Commonwealth is fundamental to the health and well-being of all Australians.
50 The PBS Schedule is a list of all of the pharmaceutical products available to be dispensed to patients through the PBS at a Commonwealth-subsidised price. The PBS Schedule is usually updated and published on a monthly basis. A pharmaceutical product can only be listed on the PBS Schedule after the product has been registered on the ARTG.
51 Section 85(1) of the National Health Act provides for the Commonwealth to provide benefits “in respect of pharmaceutical benefits” (defined in s 84(1) to mean, insofar as relevant, a drug the subject of a declaration under s 85(2)). Section 85(2) provides that the drugs and medicinal preparations in relation to which Pt VII applies are those, relevantly, “declared by the Minister, by legislative instrument, to be drugs and medicinal preparations to which this Part applies”. By s 85(3) the Minister may by legislative instrument determine, by reference to strength, type of unit, size of unit or otherwise, the form or forms of a listed drug (listed drug being defined in s 84(1) to mean “a drug or medicinal preparation in relation to which a declaration under subsection 85(2) is in force”). By s 85(6) the Minister may, by legislative instrument, determine a brand of a pharmaceutical item. In practice these declarations and determinations are made by a delegate of the Minister within the relevant branch of the Department of Health.
52 As Efexor-XR had been PBS listed since 1999 (for the 150mg and 75mg strengths) and since 2005 (for the 37.5mg strength), the relevant power for the listing of the generic brands of extended release venlafaxine was s 85(6) of the National Health Act.
53 Division 3C of Pt VII of the National Health Act, inserted in 2007, contains the guarantee of supply provisions. By s 99AEB:
The responsible person for a guaranteed brand of a guaranteed item must supply the guaranteed brand of the guaranteed item during the guaranteed period for the guaranteed brand of the guaranteed item.
54 Section 99AEB applied to the PBS listing of a generic version of Efexor-XR. As a result, by ss 99AED, the responsible person in respect of any such brand “must supply” the brand until the first of 24 months beginning on the day that the brand is listed, the listing of another new brand of the drug, another brand of the drug offering a price reduction is accepted by the Minister, or the delisting of the brand. By s 99AEG, the responsible person must notify the Minister as soon as practicable if the responsible person for a brand subject to a guarantee of supply fails to supply or is unable to supply the brand or forms the belief that they will fail to supply or be unable to supply the brand. By s 99AEH if the responsible person for a guaranteed brand fails to supply, or is unable to supply the brand on one or more occasions, the Minister may cancel the PBS listing for the brand.
55 As the Commonwealth explained in its submissions:
There are no requirements in the NHA [National Health Act] concerning the information that has to be provided in support of an application to list a generic brand of a listed drug on the PBS, although any new therapeutic good (including a generic brand of a drug) must be registered on the ARTG before it can be generally marketed in Australia. At the relevant times, an application for listing a generic brand was not required to be considered by the PBAC [the Pharmaceutical Benefits Advisory Committee established under s 100A], but rather just sent to the Pharmaceutical Evaluation Branch (“PEB”) within the Department.
The administrative requirements of the PEB for applications to list generics that were followed at all material times…have been largely the same since 2007. For any such applications which would result in a change to the price subsidised by the Commonwealth for a drug (i.e. the listing of a first generic brand of a listed drug), there were three application deadlines and three listing dates (1 April, 1 August and 1 December) per year. Once a first generic had been listed, subsequent applications for listing of another generic of that medicine could occur on the first day of any month.
The guidelines provided that applications required: a letter of application (for which there was no specified form); a completed “Application to list a Drug or Medicinal Preparation as a Pharmaceutical Benefit” (“PB11”) form; a completed “Request for Price Alteration” (“PB11a”) form; a copy of the letter from the TGA approving the entry of the product in the ARTG; a copy of the current Certificate of Medicine Registration or Certificate of Listing for the product issued by the TGA; a copy of the current Product Information (“PI”) approved by the TGA, if applicable (but only until 30 August 2009); a copy of the primary pack label or final artwork; and “written assurance” that “sufficient stock of the product to meet anticipated demand will be available at the time of listing on the PBS”.
56 Section 85AD of the National Health Act provided for the Commonwealth and the supplier of a medicine to enter into a supply agreement agreeing the maximum price the supplier would charge a pharmacist. If a price agreement was in force then the maximum price in the agreement was defined by s 98B(3) to be the APP (approved price to pharmacists) of that medicine. By reg 37D of the National Health (Pharmaceutical Benefit) Regulations 1960 (Cth), the maximum price a manufacturer could supply the medicine to a wholesaler was the APP less the amount of the wholesale mark-up. From 1 October 2012 the Approved Ex-Manufacturer Price or AEMP, the maximum wholesale price at which a manufacturer could supply to a wholesaler, replaced the APP. The AEMP was also agreed between the Commonwealth and the supplier of the medicine.
57 Section 99 of the National Health Act provides that a pharmacist who has provided a pharmaceutical benefit “is entitled to be paid by the Commonwealth”:
(a) where the prescription for the supply of the pharmaceutical benefit was an entitlement card prescription, and the supply was not an early supply of a specified pharmaceutical benefit - an amount equal to the Commonwealth price of the pharmaceutical benefit as at the time of the supply; and
(b) in any other case - the amount (if any) by which the Commonwealth price of the pharmaceutical benefit, as at the time of the supply, exceeded the amount (without any allowable discount) that the pharmacist or approved medical practitioner was entitled to charge under subsection 87(2) or (3).
58 The PBS Schedule lists the price at which a PBS-listed medicine may be dispensed at retail pharmacy level (the PBS price, also referred to as the Commonwealth price), which is referred to in the PBS Schedule as the dispensed price for maximum quantity (DPMQ) (the maximum quantity is also specified for each product on the PBS Schedule). At all relevant times until October 2012 the PBS price comprised:
(1) the approved price to pharmacist, which is the maximum price allowed to be charged to the dispensing pharmacist for the medicine under the National Health Act and related legislation (the National Health Legislation) which in turn comprised:
(a) the ex-manufacturer price, which is the maximum price allowed to be charged by the sponsor or manufacturer of the medicine under the National Health Legislation;
(b) the wholesaler mark-up, which is the maximum mark-up that can be charged by a wholesaler under the National Health Legislation;
(2) the pharmacist mark-up, which is the maximum mark-up that can be charged by a pharmacist under the National Health Legislation (giving effect to the Fourth and Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia); and
(3) a dispensing fee charged by pharmacies under the National Health Legislation (giving effect to the Fourth and Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia).
59 When a patient buys a PBS listed medicine they must pay the patient co-payment amount which is different for general patients compared to concessional patients. At 1 August 2009, this was $5.30 for concessional patients and $32.90 for general patients. The PBS scheme also provided for a “safety net” so that if the amount a patient had paid exceeded the safety net threshold in any given year, concessional patients paid $0 and general patients paid the concessional co-payment amount ($5.30) for the remainder of the year. As required by s 99 of the National Health Act, the Commonwealth would pay the pharmacist the difference between the Commonwealth price and the amount the patient had paid. This amount represented the PBS subsidy.
60 Part VII, Division 3A of the National Health Act, sets out price reductions which apply to pharmaceutical products that are already listed on the PBS Schedule, when the first PBS listing of one or more generic versions of that product (first generic equivalents) occurs. For the period between 1 January 2009 and 31 January 2011, a PBS listing of the first generic version of an originator product would have triggered a 12.5% price reduction in the APP of the originator brand provided certain circumstances were met and any exceptions did not apply. In the present case, in respect of generic venlafaxine products, the relevant circumstances were met and the exceptions did not apply. The new APP would also have been applied to any other generic equivalent version subsequently listed unless the APP was otherwise further reduced by the time of their listing.
61 As the Commonwealth explained, there were other potential statutory price reductions once the 12.5% price reduction had occurred. Further 2% price reductions may automatically follow on nominated days. For example, if a generic brand of venlafaxine had been listed on 1 August 2009 or 1 December 2009, a 2% statutory price reduction would have occurred on 1 August 2010 under s 99ACI.
62 There were also price disclosure price reductions. Under s 99ADB and reg 37 information had to be given to the Department which formed the basis for calculation of the Weighted Average Disclosed Price (or WADP) for a pharmaceutical item. The Commonwealth explained:
The WADP was calculated according to a formula which took into account:
(a) the volume of supplies of the particular brands the subject of price disclosure; and
(b) the extent to which the responsible persons of those brands offered discounts and other incentives which resulted in the price actually paid by pharmacists being less than the AEMP and the applicable wholesale mark-up.
If the WADP calculated, and recorded in a determination, by the Minister was more than 10% below the current AEMP, then the AEMP was reduced to the WADP. The AP2P was also reduced in accordance with a formula set out in regulation 37C. The effect of the price disclosure regime was, therefore, to effect price reductions to the cost of drugs resulting in the subsidy paid by the Commonwealth under the PBS more closely reflecting the price actually paid for a drug by a pharmacist.
Until 1 December 2010, the responsible person for existing listed brands could elect to comply with the price disclosure requirements for those brands: section 99ADE. Once made, the election could not be revoked by the responsible person (section 99ADE(5)) but the Minister could (at the responsible person's request) determine to revoke the election (with prospective effect) if the listing of the generic brand that triggered the price disclosure regime was varied or revoked: section 99AEL. From 1 December 2010, price disclosure was mandatory for all brands of all drugs listed on the F2 formulary.
PDPRs took effect according to a cycle that depended on when the first generic brand was listed. For venlafaxine:
(a) if the first generic brand had listed on 1 August 2009, the first PDPR could have occurred on 1 August 2011, and then subsequent PDPRs could have occurred on 1 August 2012 and 1 April 2014; and
(b) if the first generic brand had listed on 1 December 2009, the first PDPR could have occurred on 1 April 2012, and then subsequent PDPRs could have occurred on 1 April 2013 and 1 April 2014.
63 By operation of Commonwealth policy , there have been only three occasions per year on which a product whose listing on the PBS would result in a reduction in the APP of a pharmaceutical product could be listed on the PBS (that is, included on the PBS Schedule), on 1 April, 1 August and 1 December. To obtain listing on these dates, the application to list must be submitted by certain dates. An application to list a first generic equivalent on the PBS on 1 April must be filed by 1 December of the preceding year. An application to list a first generic equivalent on the PBS on 1 August must be filed by 1 May of that year. An application to list a first generic equivalent on the PBS on 1 December must be filed by 1 September of that year. Thereafter, a second or subsequent generic equivalent could be listed on the PBS on the first day of any month, provided that the application for listing is made by the 15th of the month which is three calendar months before the listing date.
64 A pharmacist may dispense products privately, that is, not through the PBS, even where the products are PBS listed. Prescription products which are not listed on the PBS are dispensed privately. If a pharmacist dispenses a product privately the patient bears the full purchase price of the product as no Commonwealth subsidy is paid under the PBS.
65 The PBS Price and the ex-manufacturer price are maximum values, and a sponsor or manufacturer may elect to reduce the actual price it charges the pharmacist, at any time, by offering its products at a discount to the ex-manufacturer price component of the PBS Price.
66 In the case of PBS listed medicines that are purchased by a pharmacist at the listed APP, the profit made by a pharmacist on the sale of those medicines comprises the set pharmacist mark-up and the dispensing fee. However, where a pharmacist is able to purchase a PBS listed medicine at a price below the listed APP, an additional profit is earned which reflects the difference between their cost of goods and the listed APP. The greater the difference between the actual cost of goods and the listed APP, the greater the profit margin for the pharmacist.
Efexor-XR
67 Wyeth was the patentee of Australian Patent No 567524 for the compound, venlafaxine hydrochloride, a selective noradrenaline re-uptake inhibitor used for the treatment of depression and related illnesses. The compound patent reached the end of its term and expired on 6 December 2008.
68 Wyeth was also the patentee of Australian Patent No 2003259586 granted on 11 May 2007 for an “extended release formulation” of venlafaxine hydrochloride, which is referred to as the method patent. The method patent would reach the end of its term and expire in 2017.
69 Wyeth Australia was the sponsor of pharmaceutical products, Efexor-XR in 37.5mg, 75 mg and 150mg formulations. Efexor-XR is an extended release formulation of venlafaxine hydrochloride. The 75 mg and 150mg formulations of Efexor-XR were listed on the PBS in 1999. The 37.5mg formulation of Efexor-XR was listed on the PBS in December 2005. The Efexor-XR products were the only products listed on the PBS containing venlafaxine hydrochloride as the active pharmaceutical ingredient until 1 April 2012.
70 Efexor-XR was the leading anti-depressant brand in Australia in terms of units sold and value of sales. Annual sales of Efexor-XR were in the order of $114 million.
71 On 1 February 2009 Wyeth’s product Pristiq, in which the active pharmaceutical ingredient was desvenlafaxine (a metabolite of venlafaxine), was listed on the PBS. Desvenlafaxine was the subject of Australian Patent No 2002250058 which will reach the end of its term and expire in 2022.
72 Sigma was the sponsor of registrations on the ARTG on 5 March 2009 of Evelexa XR 150 venlafaxine (as hydrochloride) 150mg extended release capsule, Evelexa XR 75 venlafaxine (as hydrochloride) 75mg extended release capsule, and Evelexa XR 37.5 venlafaxine (as hydrochloride) 37.5mg modified release capsule.
73 The Evelexa XR products were registered on the ARTG on the basis of being essentially similar to the corresponding Efexor-XR products and are substitutable (including on the PBS) for those products.
74 Sigma commenced proceeding VID 195 of 2009 on 1 April 2009 against Wyeth and Wyeth Australia challenging the validity of the method patent. On 1 May 2009 Wyeth filed a cross claim in proceeding VID 195 of 2009 alleging infringement and threatened infringement of the method patent including an application for an interlocutory injunction.
75 On 3 June 2009 Sundberg J granted the Sigma interlocutory injunction which was as follows:
Pending the determination of the proceeding or further order the cross-respondent whether by itself, its directors, officers, servants, agents or otherwise be restrained from marketing, taking orders for, selling, supplying, offering to supply or otherwise exploiting in Australia the products listed on the Australian Register of Therapeutic Goods under the name Evelexa-XR or any other product comprising the same generic modified release formulation of venlafaxine hydrochloride, without the licence of or authority of the cross-claimants.
76 The Sigma interlocutory injunction was granted:
Upon the cross-claimants by their Counsel undertaking:
(a) to submit to such order (if any) as the Court may consider to be just for the payment of compensation, to be assessed by the Court or as it may direct, to any person, whether or not a party, adversely affected by any operation of the order below or any continuation (with or without variation) thereof, and
(b) to pay the compensation referred to in (a) to the person there referred to.
Enlafax-XR
77 Alphapharm was the sponsor of registrations on the ARTG on 30 April 2009 of Enlafax-XR venlafaxine 150mg modified release capsule and Enlafax-XR venlafaxine 75mg modified release capsule.
78 The Enlafax-XR products were registered on the ARTG on the basis of being essentially similar to the corresponding Efexor-XR products and are substitutable (including on the PBS) for those products.
79 Alphapharm commenced proceeding NSD 596 of 2009 on 19 June 2009 challenging the validity of the method patent. On 23 July 2009 Wyeth and Wyeth Australia Pty Ltd filed a cross-claim in proceeding NSD 596 of 2009 alleging infringement and threatened infringement of the method patent including an application for an interlocutory injunction.
80 On 25 August 2009 I granted the Alphapharm interlocutory injunction which was as follows:
1 Pending the determination of the proceeding or further order, Alphapharm Pty Limited (whether by itself; its directors, officers, servants, agents or otherwise) be restrained from infringing claims 4 and 27 (insofar as claim 27 is dependent on claim 4) of Australian Patent No 2003259586 (the method patent), including without limitation by, during the term of the method patent and without the licence or authority of Wyeth, importing, marketing, taking orders for, selling, supplying, offering to supply in Australia the Enlafax-XR Products or any other product comprising the same generic modified release formulation of venlafaxine hydrochloride for use by persons for the Registered Indications or otherwise exploiting the invention the subject of the method patent.
2 Alphapharm Pty Limited be restrained from applying to list the Enlafax XR Products on the Schedule of Pharmaceutical Benefits.
81 The Alphapharm interlocutory injunction was granted:
UPON the cross-claimants by their counsel undertaking:
(a) to submit to such order (if any) as the Court may consider to be just for the payment of compensation, to be assessed by the Court or as it may direct, to any person, whether or not a party, adversely affected by any operation of the orders below or any continuation (with or without variation) thereof; and
(b) to pay the compensation referred to in (a) to the person there referred to; and
(c) until further order, not to make any application to de-list Efexor-XR from the Pharmaceuticals Benefits Scheme.
82 Generic Health was the sponsor of registrations on the ARTG on 6 August 2009 of (as now known) Venlafaxine Sandoz XR venlafaxine (as hydrochloride) 150mg modified release capsule, (as now known) Venlafaxine Sandoz XR venlafaxine (as hydrochloride) 75mg modified release capsule, Venlafaxine GENERICHEALTH XR venlafaxine (as hydrochloride) 150mg modified release capsule, and Venlafaxine GENERICHEALTH XR venlafaxine (as hydrochloride) 75mg modified release capsule, as well as (as now known) Apo-Venlafaxine XR venlafaxine (as hydrochloride) 150mg modified release capsule and (as now known) Apo-Venlafaxine XR venlafaxine (as hydrochloride) 75mg modified release capsule, and (as now known) Venlexor XR venlafaxine (as hydrochloride) 150mg modified release capsule (as now known) Venlexor XR venlafaxine (as hydrochloride) 75mg modified release capsule.
83 The Generic Health venlafaxine products were registered on the ARTG on the basis of being essentially similar to the corresponding Efexor-XR products and are substitutable (including on the PBS) for those products.
84 Generic Health commenced proceeding NSD 1124 of 2009 on 6 October 2009 challenging the validity of the method patent. On 9 November 2009 Wyeth and Wyeth Australia Pty Ltd filed a cross-claim in proceeding NSD 1124 of 2009 alleging infringement and threatened infringement of the method patent including an application for an interlocutory injunction.
85 On 10 November 2009 I granted the Generic Health interlocutory injunction which, pending further order, was as follows:
(a) Generic Health Pty Ltd (whether by itself its directors, officers, servants, agents or otherwise), be restrained from infringing claims 4 and 27 (insofar as claim 27 is dependent on claim 4) of the Patent, including without limitation by, during the term of the Patent and without the licence or authority of the respondent, importing, marketing, taking orders for, selling, supplying, offering to supply in Australia any products the subject of ARTG registration numbers 151874, 151875, 151876, 151877,151878, 151880, 151884, 151885 (the GH Products) or any product comprising the same generic modified release formulation of venlafaxine hydrochloride for use by persons for major depression and social anxiety disorder or otherwise exploiting the invention the subject of the Patent;
(b) Generic Health Pty Ltd (whether by itself, its directors, officers, servants, agents or otherwise) be restrained from selling or assigning its rights in the registrations for the GH Products or any product comprising the same generic modified release formulation of venlafaxine hydrochloride the subject of the Patent without notifying the purchaser or assignee of the restraints placed upon Generic Health Pty Ltd by operation of these orders in relation to the GH Products; and
(c) that Generic Health Pty Ltd make it a condition of any purchase or assignment of the rights in the registrations for the GH Products, that the purchaser or assignee provides undertakings, without admission of liability, in the following terms to the respondent:
…
(d) Generic Health Pty Ltd be restrained from applying to list the GH Products on the Schedule of Pharmaceutical Benefits.
86 The Generic Health interlocutory injunction was granted upon:
The respondent’s and Wyeth Australia Pty Ltd’s undertaking, by their counsel:
(a) to submit to such order (if any) as the Court may consider to be just for the payment of compensation, to be assessed by the Court or as it may direct, to any person, whether or not a party, adversely affected by any operation of the orders below or any continuation (with or without variation) thereof; and
(b) to pay the compensation referred to in (a) to the person there referred to; and
(c) until further order, not to make any application to de-list Efexor-XR from the Pharmaceuticals Benefits Scheme.
Other events
87 On 19 August 2009 I listed the proceedings between Sigma, Alphapharm and Wyeth for hearing, on a provisional basis, starting on 8 March 2010. On 28 August 2009 I vacated this order and listed the proceedings for hearing from 12 to 30 April 2010. Although the proceedings involving Generic Health had not been commenced at that time, Generic Health was aware of the hearing dates by no later than September 2009. Subsequently, orders were made by which the proceedings involving Generic Health were also to be heard at the same time as the proceedings involving Sigma and Alphapharm.
88 On 15 October 2009, Pfizer Inc acquired Wyeth and Wyeth Australia Pty Ltd. The sale process had commenced much earlier in or around January 2009. Pursuant to the sale arrangements Pfizer Australia Pty Ltd, a subsidiary of Pfizer Inc, acquired all of Wyeth Australia’s products.
89 Ranbaxy Australia Pty Ltd was the sponsor of registrations on the ARTG on 22 January 2010 of Venlafaxine Ranbaxy venlafaxine (as hydrochloride) 150mg modified release capsules, Venlafaxine Ranbaxy venlafaxine (as hydrochloride) 75mg modified release capsules, and Venlafaxine Ranbaxy venlafaxine (as hydrochloride) 37.5mg modified release capsules.
90 I heard the proceedings between 15 April and 31 May 2010.
91 Spirit Pharmaceuticals Pty Ltd was the sponsor of registrations on the ARTG on 30 July 2010 of Elaxine SR 150 venlafaxine (as hydrochloride) 150mg modified release capsule, Elaxine SR 75 venlafaxine (as hydrochloride) 75mg modified release capsule, and Elaxine SR 37.5 venlafaxine (as hydrochloride) 37.5mg modified release capsule.
92 SC Pharma Pty Ltd was the sponsor of registrations on the ARTG on 30 July 2010 of Stada Venlafaxine SR venlafaxine (as hydrochloride) 150mg modified release capsule, Stada Venlafaxine SR venlafaxine (as hydrochloride) 75mg modified release capsule, Stada Venlafaxine SR venlafaxine (as hydrochloride) 37.5mg modified release capsule, Venlafaxine SR SCP venlafaxine (as hydrochloride) 150mg modified release capsule, Venlafaxine SR SCP venlafaxine (as hydrochloride) 75mg modified release capsule, and Venlafaxine SR SCP venlafaxine (as hydrochloride) 37.5mg modified release capsule.
93 On 23 September 2010 I provided the draft reasons for judgment to the parties to review for the purpose of identifying any confidential information which ought not to be disclosed in the published reasons. These draft reasons were in the same terms as the reasons for judgment published subsequently in which I decided the method patent was valid and that the generic parties should be subject to final injunctions.
94 On 8 November 2010 I made orders consequential on reasons for judgment published on that day, Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2010] FCA 1211; (2010) 88 IPR 459. I dismissed the challenges to the validity of the method patent, made final orders restraining each of Sigma, Alphapharm and Generic Health from infringing the method patent, and discharged the interlocutory injunctions and released Wyeth and Wyeth Australia from each of the undertakings which they had given in order to secure the interlocutory injunctions. I also refused applications by Sigma and Alphapharm to stay the final injunctions pending their foreshadowed appeals: Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 2) [2010] FCA 1212.
95 First Sigma, and then Alphapharm and thereafter Generic Health appealed against the orders I made on 8 November 2010.
96 On 12 November 2010 Jacobson J refused Sigma’s further application to stay the final injunctions against it pending hearing of the appeal which it had filed: Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2010] FCA 1258.
97 Pfizer Australia was the sponsor of registrations on the ARTG on 10 December 2010 of Venlafaxine Wyeth venlafaxine (as hydrochloride) 150mg modified release capsule, Venlafaxine Wyeth venlafaxine (as hydrochloride) 75mg modified release capsule, Venlafaxine Wyeth venlafaxine (as hydrochloride) 37.5mg modified release capsule, Altven venlafaxine (as hydrochloride) 150mg modified release capsule, Altven venlafaxine (as hydrochloride) 75mg modified release capsule, and Altven venlafaxine (as hydrochloride) 37.5mg modified release capsule.
98 On 28 January 2011 Sigma’s ARTG registration for Evelexa XR was transferred to its parent company, Sigma Company Limited. This was done as part of the arrangements for the sale of Sigma to Aspen Asia Pacific Pty Ltd, after which Sigma’s named was changed to Aspen Pharma Pty Ltd. Aspen Asia acquired the shares in Sigma pursuant to a share sale agreement completed on 31 January 2011. Apart from Evelexa XR and one other product, all generic pharmaceutical products owned or in-licensed by Sigma or its subsidiaries (including Arrow Pharmaceuticals Pty Ltd, Chemists’ Own Pty Ltd and Herron Pharmaceuticals Pty Ltd), including the associated intellectual property rights and ARTG registrations were transferred with the shares in Sigma.
99 Generic Health was the sponsor of registrations on the ARTG on 25 March 2011 of Apotex-Venlafaxine XR venlafaxine (as hydrochloride) 75 mg modified release capsule, Apotex-Venlafaxine XR venlafaxine (as hydrochloride) 150 mg modified release capsule, Chemmart Venlafaxine XR venlafaxine (as hydrochloride) 75 mg modified release capsule, Chemmart Venlafaxine XR venlafaxine (as hydrochloride) 150 mg modified release capsule, Terry White Chemists Venlafaxine XR venlafaxine (as hydrochloride) 75 mg modified release capsule, Terry White Chemists Venlafaxine XR venlafaxine (as hydrochloride) 150 mg modified release capsule.
100 On 28 October 2011 the Full Court delivered judgment to the effect that the appeal should be allowed as the method patent was invalid: Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2011] FCAFC 132; (2011) 119 IPR 194. On 11 November 2011 the Full Court made orders allowing the appeal and setting aside the orders I made on 8 November 2010 to the effect that each of Sigma Pharmaceuticals Australia, Alphapharm and Generic Health be restrained from infringing the method patent. On 21 December 2011 the Full Court made further orders including a declaration that claims 1 to 17 of the method patent are invalid and an order revoking those claims. The Full Court also set aside other orders I made insofar as necessary including the release of Wyeth and Wyeth Australia from the undertakings as to damages. The Full Court stayed two of its orders (revoking the claims and requiring rectification of the Register of Patents to reflect the revocation) to permit Wyeth and Wyeth Australia to seek special leave to appeal from the High Court, Wyeth and Wyeth Australia giving certain further undertakings.
101 Wyeth and Wyeth Australia applied for special leave to appeal to the High Court on 2 December 2011.
102 On 1 April 2012, following applications made in November and December 2011, the following generic equivalents to Efexor-XR were listed on the PBS:
(1) Apotex 75mg and 150mg products under brand names Apo-VenlafaxineXR, ChemmartVenlafaxineXR, and Terry White Chemists Venlafaxine XR;
(2) Generic Health 75mg and 150mg products under brand name Venlafaxine GENERICHEALTH XR;
(3) Alphapharm 75mg and 150mg products under brand name Enlafax-XR;
(4) Spirit Pharmaceuticals Pty Limited 37.5mg, 75mg and 150mg products under brand name Elaxine SR;
(5) Sandoz Pty Limited 75mg and 150mg products under brand name Venlafaxine Sandoz XR;
(6) Ascent Pharma Pty Ltd 75mg and 150mg products under brand name Venlexor XR; and
(7) Ranbaxy Australia Pty Ltd 37.5mg, 75mg and 150mg products under brand name Venla RBX.
103 The High Court refused to grant special leave to Wyeth’s application on 11 May 2012.
104 On 15 October 2009, Pfizer Inc. completed its acquisition of Wyeth LLC and Wyeth Australia Pty Limited (ACN 000 296 211), the respondents in the proceedings.
105 On 19 December 2005, Sigma Company Limited (ACN 004 132 923) merged with Arrow Pharmaceuticals Limited (ACN 008 417 403). The merged company was renamed Sigma Pharmaceuticals Limited (ACN 008 417 403) (now known as Sigma Healthcare Limited) and listed on the ASX.
106 At that time, Sigma Pharmaceuticals Limited became the parent company of Sigma Company Limited (ACN 004 132 923).
107 Sigma Pharmaceuticals (Australia) Pty Ltd (004 118 594), referred to in these reasons as Sigma, is the applicant in proceeding no. VID195/2009. Sigma was the subsidiary of Sigma Company Limited.
108 On 31 January 2011, Sigma Company Limited sold Sigma to Aspen Asia Pacific Pty Ltd (ACN 146 444 484) under a share sale agreement between, Sigma Pharmaceuticals Limited, Sigma Company Limited (as vendor), Aspen Asia Pacific Pty Ltd (as purchaser) and Aspen Pharmacare Holdings Limited (a South African registered company and the ultimate parent company of Aspen Asia Pacific Pty Ltd).
109 Sigma was renamed Aspen Pharma Pty Ltd on 31 January 2011.
110 On 31 August 2015 Sigma’s generic business was sold to Arrow Pharmaceuticals Limited. Sigma’s claims extend to alleged losses up to 31 August 2015.
Alphapharm
111 Alphapharm Pty Ltd is the applicant in proceeding no. NSD596/2009. Alphapharm is an (indirect) subsidiary of Mylan, Inc.
112 Mylan N/V is the ultimate holding company of Mylan, Inc.
113 Mylan Ireland Limited (Mylan Ireland) is also an (indirect) subsidiary of Mylan, Inc.
114 Generics (UK) Ltd is an indirect subsidiary of Mylan, Inc. Mylan Ireland took the place of Generics (UK) Ltd under the agreements for supply to Alphapharm with Pharmathen.
115 Since September 2010, Generic Health Pty Ltd has been a subsidiary of Lupin Limited. Lupin Holdings B.V., which is wholly owned by Lupin, currently holds 100% of the issued capital of Generic Health.
Pharmathen
116 Pharmathen S.A., a company incorporated in Greece, is the holding company of the Pharmathen group of companies and is also known as Pharmathen Pharmaceuticals S.A.
Alembic
117 On 24 January 2011, Alembic Limited was demerged and its obligations in relation to the manufacture and supply of pharmaceutical products were transferred to Alembic Pharma Limited.
118 On 12 March 2011, Alembic Pharma Limited changed its name to Alembic Pharmaceutical Limited.
Principles relating to the undertakings
119 The terms of the undertakings which Wyeth gave on the grant of the interlocutory injunctions were the same in each case, namely:
to submit to such order (if any) as the Court may consider to be just for the payment of compensation, to be assessed by the Court or as it may direct, to any person, whether or not a party, adversely affected by any operation of the orders below or any continuation (with or without variation) thereof.
120 The undertakings reflect the Court’s then Practice Note No 3 which refers to the “usual undertaking as to damages”. The description, the “usual undertaking as to damages”, in turn, reflects the principles most conveniently described by Brooking J in National Australia Bank v Bond Brewing Holdings Ltd [1991] 1 VR 386 at 599-602 including the following:
When a court of equity is asked to grant an interim or interlocutory injunction it will consider whether it should take steps, as it ordinarily will, to protect the party enjoined against the danger of injury resulting from the injunction. It has been doing this for the past 200 years.
…
The power of a court of equity to require, as a condition of the grant of an injunction, that a bond be given, or that an undertaking (which may or may not be required to be buttressed by security) be given, is an inherent power which derives from the court's discretion to grant or refuse the injunction sought, a power exercised for the purpose of effecting justice between the parties… By giving the undertaking the litigant has put himself under the power of the court; the order later made in an appropriate case for an inquiry and payment of the damages is an appendage to the undertaking…
It is because damage flowing from the act of the court is not - unless one of the recognised causes of action exists - compensable in damages that equity requires its undertaking or bond or other appropriate safeguard. Time and again we are told that this is equity's way of avoiding injustice. It is never said that equity insists on an undertaking or bond merely in order to prevent arguments and provide a summary remedy. Implicit in the judgments is the idea that without the undertaking there will be no remedy, so that injustice will result…
Equity has its own means - an anticipatory one - of avoiding injustice, by refusing to grant an injunction or appoint a receiver by interim or interlocutory order except on terms. The applicant can take the order or leave it, but if he takes it he does so on terms which make clear, once recourse is had to the authorities, the extent to which he is at risk in relation to damages.
121 It was the Court’s making of the interlocutory orders which called forth the undertakings from Wyeth. By the undertakings Wyeth obtained the interlocutory orders and by the undertakings Wyeth submitted to the making of such order, if any, as the Court may consider to be just for the payment of compensation to any person “adversely affected by any operation of the [interlocutory] orders…or any continuation (with or without variation) thereof”.
122 In Coshott v Principal Strategic Options Pty Ltd [2004] FCAFC 50 at [18] the Full Court referred to Air Express Ltd v Ansett Transport Industries (Operations) Pty Ltd [1981] HCA 75; (1981) 146 CLR 249 as authority for these propositions:
(a) the court has a discretion not to enforce an undertaking as to damages, but unless the respondent has been guilty of conduct that would render it inequitable to enforce the undertaking it will be just, speaking generally, for an applicant who fails on the merits to recompense the respondent for the damage suffered by him or her as a result of the making of the interlocutory order (see Gibbs J at 311 – 312);
(b) it is necessary to draw a distinction between results which are caused by the making of the interlocutory order and those which flow from the fact of the litigation itself (see Barwick CJ at 310; Gibbs J at 312 and Stephen J at 315);
(c) generally speaking, the damages must be confined to loss which is the natural consequence of the interlocutory order under the circumstances of which the applicant for the order had notice (see Gibbs J at 312 and the authorities there cited and Stephen J at 319);
(d) the making of the interlocutory order must have been a cause without which the damage would not have been suffered (Gibbs J at 313 and Stephen J at 320); and
(e) the onus of proof in respect of the damage claimed lies on the respondent who asserts that he or she sustained damage by reason of the making of the interlocutory order (see Gibbs J at 313 and Stephen J at 316 and 320).
123 The importance of proposition (b) accords with the fact that the contingency which enlivens the power to award compensation is that a person has been adversely affected by the operation of the interlocutory order. Thus, at 310 in Air Express, Barwick CJ said:
But the adoption of that view makes it the more imperative to maintain the distinction between results which are caused by the grant of an injunction and those which flow from the fact of the litigation itself…
124 The “view” to which his Honour was referring is as stated in Griffith v Blake (1884) 27 Ch D 474 that the damages recoverable on the undertaking relate to the action of a court in granting the injunction, and not the action of a party in seeking the injunction.
125 At 312-313 in Air Express Gibbs CJ said:
However, it is perfectly clear, and it appears from the words of the undertaking themselves, that the only damages to which a defendant is entitled are those which he has sustained by reason of the grant of the injunction. The generally accepted view is that the damages must be confined to loss which is the natural consequence of the injunction under the circumstances of which the party obtaining the injunction has notice.
…
In a number of authorities the court has distinguished between loss which was caused by the injunction and loss which arose from the litigation…
There is no reason to doubt that it is correct in principle to draw such a distinction if the facts warrant it. If the pendency of the litigation, rather than the making of the order, was the cause of the plaintiff's loss, the terms of the undertaking have no application, since the plaintiff has not sustained loss by reason of the order. Moreover, except in certain cases analogous to malicious prosecution, a defendant is not entitled to recover damages for loss resulting from legal proceedings brought against him — the only liability of the unsuccessful plaintiff is to pay costs. The court should no doubt scrutinize with care an assertion by a plaintiff that loss which has been suffered by a defendant has resulted from the litigation rather than from the making of the interlocutory order, since a plaintiff should not be allowed to evade payment of the price which he has agreed to pay for the grant of the injunction. In the end, however, the question becomes one of fact: did the making of the order cause the loss? The onus of proof must, in accordance with general principles, lie on the defendant who asserts that he sustained damage by reason of the order.
It was submitted on behalf of the appellant that it is enough that the making of the order should have been a cause of the damage, so that if both the making of the order and the continuance of the litigation are concurrent causes the undertaking will be applicable. However, in almost every case in which an injunction is granted the injunction will play some part in causing the party bound by it to act in accordance with its terms. To order a plaintiff to pay damages where it appears that the party bound by the injunction would have acted as he did even if the injunction had not been granted, would be to give the undertaking an effect obviously not intended. The party seeking to enforce the undertaking must show that the making of the order was a cause without which the damage would not have been suffered. It was further submitted that the onus lies on the plaintiff, against whom the undertaking is sought to be enforced, to disentangle any damage arising from the litigation from that which was caused by the making of the order. However, the onus of proof does not shift in this way; the defendant, who seeks to enforce the undertaking, must prove that the damage he has sustained was caused by the making of the order.
126 At 320 Stephen J said:
From this it can be seen that it will only be if damage is suffered because of the grant of the injunction, and would not have been suffered but for it, that the court should compensate a defendant who claims damages under the undertaking. Its grant must be shown to be the causa sine qua non of the damage complained of before the defendant can be entitled to be compensated for what turns out to be the erroneous grant by the court of the injunction against it….
It follows that it is for the claimant under an undertaking to establish by evidence, or by inference from evidence, a prima facie case both that the grant of the injunction was a cause of his damage and that, but for it, he would not have suffered that damage.
127 Mason J, in dissent in the result, said this at 324-325:
The distinction between damage caused by the injunction and damage which flows from the litigation is, I think, well founded on the language in which the usual undertaking as to damages is expressed. The party seeking damages must show that he has sustained damage “by reason of the order”. The words connote a causal connection between the damage and the interim injunction.
English law has not adopted a uniform approach to causation. Instead, it has tended to take refuge in the notion that causation is very largely a question of fact. But the many statements to this effect which are to be found in the decided cases do not attempt to deny the fact that the common law has applied a variety of theories and standards of causation in each instance applying that which is in point of policy the most apt or appropriate to the question which arises for decision.
For this reason little is to be gained in the present case from an examination of the myriad authorities which deal with causation of damage in contract, tort and other situations many of which were pressed upon us in argument. We are better advised to look to the purpose which the undertaking as to damages is designed to serve and to identify that causal connection or standard of causal connection which is most appropriate to that purpose. The object of the undertaking is to protect a party, normally the defendant, in respect of such damage as he may sustain by reason of the grant of the interim injunction in the event that it emerges that the plaintiff is not entitled to relief. It is no part of the purpose of the undertaking to protect the defendant against loss or damage which he would have sustained otherwise, as for example, detriment which flows from the commencement of the litigation itself. That is loss or damage which the defendant must bear himself, as he does when no interim injunction is sought or granted. Consequently, it is for the party seeking to enforce the undertaking to show that the damage he has sustained would not have been sustained but for the injunction.
128 A number of the claimants in the present matter stressed the word “sought” in Mason J’s reference to the words “when no interim injunction is sought or granted” to support the proposition that all actions taken in anticipation of the interlocutory injunctions should be disregarded. In some cases, as will be seen, this extended to propositions that anything said or done, even by third parties not the subject of the interlocutory orders, should be disregarded if what was said or done was or might have been “tainted” by anticipation of the interlocutory orders.
129 I do not consider that such an approach reflects either the terms of the undertakings or the principles which are otherwise clear from Air Express. It is the operation of the interlocutory order which is the focus of the undertaking, not events leading up to the making of the interlocutory order. Assume, for example, a person cancelled a shipment of goods anticipating that an interlocutory injunction would be granted but the anticipated interlocutory injunction either was not sought or was not made. The person might well suffer loss, but there would be no undertaking as to damages; without some other cause of action, the loss would lie where it falls. For this reason, I consider that to the extent the claimants approach the matter on any other basis, the approach is wrong in principle. Because most of the claimants claim under each undertaking it is also often difficult to work out what interlocutory order is said to be relevant. As a result, I have dealt with many of the submissions on the facts and within the conceptual framework of the claimants’ submissions, but where I do so it should not be assumed that I accept that conceptual framework.
130 European Bank Ltd v Robb Evans of Robb Evans and Associates [2010] HCA 6; (2010) 240 CLR 432 does not support a contrary proposition. French CJ and Gummow, Hayne, Heydon and Kiefel JJ, after referring to Mason J’s observations about causation, said:
[17] A party seeking an equitable remedy is required to “do equity” and this is the origin of the requirement that the party giving an undertaking as to damages submit to such order for payment of compensation as the court may consider to be just. Given its origin and application to varied circumstances in particular cases, the process of assessment of compensation cannot be constrained by a rigid formulation.
[18] These considerations, bearing upon the interests of justice in the particular circumstances of the litigation, support the following statement by Aickin J in Air Express [(1981) 146 CLR 249 at 266–267], made with respect to interlocutory injunctions, but applicable to the interlocutory order made by the Court of Appeal against European Bank. His Honour said:
In a proceeding of an equitable nature it is generally proper to adopt a view which is just and equitable, or fair and reasonable, in all the circumstances rather than to apply a rigid rule. However the view that the damages should be those which flow directly from the injunction and which could have been foreseen when the injunction was granted, is one which will be just and equitable in the circumstances of most cases and certainly in the present case.
The phrase “could have been foreseen” should be noted.
131 At [29] their Honours identified the relevant questions as:
(1) What is the loss that is now alleged?
(2) Did that loss flow directly from the interlocutory order?
(3) Could the loss sustained have been foreseen at the time of that order?
132 Their Honours were not suggesting that loss other than that caused by the operation of the interlocutory orders, such as loss caused by the existence of the litigation or mere anticipation of an interlocutory order, would be compensable. The point being made was that in determining the loss caused by the interlocutory orders, reasoning by analogy to the damages recoverable under other causes of action, such as in tort or contract, may be inapt. So much is apparent from the fact that the “purpose which the undertaking as to damages is to serve” has never been to protect a person from the exigencies of litigation which include the risk of acting in anticipation of an interlocutory injunction which might never be sought or made, in which event there will be no undertaking. The purpose is only to protect those who may suffer loss from the operation of an order made by a court before the rights of the parties are able to be fully determined.
133 It follows that for causation, whether it be Wyeth’s approach based on the statements in Air Express at 313, 320 and 325 (“the order was a cause without which the damage would not have been suffered” per Gibbs J, the order must be shown to be “the causa sine qua non of the damage complained of” per Stephen J, or “it is no part of the purpose of the undertaking to protect the defendant against loss or damage which he would have sustained otherwise” per Mason J) or the approach of some of the claimants (a “non-trivial contribution” to the loss will suffice), the relevant contingency is confined to the operation of the interlocutory orders.
134 To be specific to the present case, the purpose of undertaking has nothing to do with protecting any person from loss resulting from the existence of the method patent, decisions taken by a person as a result of the existence of the method patent, the existence of the litigation or decisions taken by a person as a result of the existence of the litigation, which include the anticipation of an interlocutory injunction against them. It does not matter that the reason one claimant (for example, Alphapharm) may have anticipated an interlocutory injunction against it is because of the Sigma interlocutory injunction. That anticipation was not a result of the operation of the Sigma interlocutory injunction which did not bind Alphapharm. It was a result of the anticipation by Alphapharm of the litigation against it, including one of the potential exigencies of litigation, which is an interlocutory injunction. As a result, Alphapharm’s claim based on the Sigma interlocutory injunction is misconceived. The same approach must be taken to Generic Health’s claim based on the Alphapharm interlocutory injunction which did not bind Generic Health and did not affect Generic Health in any way other than in the sense that it caused Generic Health to anticipate the likely course of anticipated litigation against it, including an interlocutory injunction. This anticipation was not a result of the Alphapharm interlocutory injunction but of the anticipated litigation against Generic Health including one of the exigencies of that anticipated litigation which was an interlocutory injunction.
135 One of the many factual complexities in the present matter is that Generic Health was wearing multiple hats. It was a supplier of its own branded generic pharmaceutical products to pharmacists (in common with Sigma and Alphapharm) but was also a supplier to Sigma of Sigma’s venlafaxine products sourced from Alembic and a potential supplier to other generics of venlafaxine products. As such, it is apparent that the operation of the Sigma interlocutory injunction could have had an adverse effect on Generic Health (and thus Alembic). But the same cannot be said in respect of the Alphapharm interlocutory injunction for the reasons given. Mere anticipation of the exigencies of litigation as a result of an interlocutory injunction which binds another party is not causally connected to the operation of the interlocutory injunction in the relevant sense. To adopt such an approach would be to assess compensation not by reference to the operation of the interlocutory injunction but by reference to mere litigation, actual or anticipated. This would be contrary to principle. The undertaking is not given as the price of the capacity to commence or prosecute the litigation.
136 This proposition also explains why I do not accept the approach proposed by some of the claimants that for the purpose of assessing loss I should postulate that “Wyeth sought the injunctions and the applications were refused on the basis that the patent claims were invalid as determined by the Full Court” or that Wyeth is “not permitted to assert (including by seeking the injunctions and threatening and commencing litigation) the invalid patent claims”. These approaches conflict with the purpose of the undertaking which is confined to protecting persons from loss caused by the operation of the interlocutory orders. The effect of the claimants’ approaches would be to assess causation and loss on the basis that the method patent and/or the litigation about the method patent did not exist and, thereby, to compensate the claimants for the mere existence of the method patent and the litigation. As discussed, these approaches are far removed from the terms of the undertakings and their purpose.
137 The claimants submitted that the two reasons Wyeth proposed for a different approach, that there was no appeal against the interlocutory injunctions and it would remove the effect of the method patent and the litigation, were not valid. As to the first, I agree. The fact that there were no appeals against the interlocutory injunctions is immaterial. As to the second, I disagree for the reasons given above.
138 To take one example of the difficulties in the claimants’ approaches, Generic Health submitted that it was “natural” that Wyeth should be taken to have applied for and failed to obtain the interlocutory injunctions on the grounds which ultimately found favour in the Full Court. As Generic Health put it, I should hypothesise, contrary to the fact, that Sundberg J (and then I) refused each of the interlocutory injunctions on the basis that there was not a sufficient prima facie case for Wyeth to obtain interlocutory relief on the same basis as the Full Court ultimately found, that the claims were not fairly based on the document from which the method patent claimed priority. If this approach were to be taken, however, the generics would be attributed with knowledge in 2009 that they did not in fact possess until 28 October 2011, namely that the view of the Court, even if only a prima facie view, was that the relevant claims of the method patent said to be infringed were invalid as they were not novel at the claimed priority date.
139 In my view, this is not a “natural” hypothesis, but is irreconcilable with the authorities to which I have referred. The task is to assess compensation considered to be just (if any) to any person adversely affected by any operation of the orders. The task would be corrupted by positing that, instead of the interlocutory orders being made, the generics obtained the benefit of an interlocutory judgment refusing to grant interlocutory relief on the very ground on which the Full Court held the method patent should be revoked. It is or should be obvious that this would largely remove the method patent and the litigation from the relevant circumstances despite, as Generic Health put it, the judgment being interlocutory only so that the risk on a final hearing remained. The hypothesis bears no resemblance to the position the generics would have been in if the interlocutory orders are disregarded. If this proposed approach were to be accepted, I consider that the generics would be compensated not for any adverse effect on them by the operation of the interlocutory injunctions, but for the adverse effect on them of the mere existence of the method patent and the litigation, which is impermissible.
140 In summary, I do not accept any approach to the present claims which would involve compensating the claimants for anything other than the operation of the interlocutory orders. The fact that the origins of the undertaking as to damages is equitable, and that considerations of fairness mean that a rigid approach to compensable loss would be inappropriate, do not expand the scope of compensable loss beyond the terms of the undertakings. The method patent did exist. Wyeth did threaten proceedings to enforce the rights it claimed under the method patent, including by seeking an interlocutory order. But the focus of the undertakings remains the operation of the interlocutory orders.
141 It may be accepted that the effect of an order revoking a patent under s 138(3) of the Patents Act 1990 (Cth), as for the predecessor provision, means that “the patent registration is revoked ab initio”: Envirotech Australia Pty Ltd v Enviroclear Co Inc (1987) 10 IPR 657 at 659. But this does not mean that causation and loss are to be assessed as if the method patent, and thus the litigation, never existed. To do so would be contrary to principle. Nor can it be the case that the parties are to be attributed with knowledge (specifically, that the Full Court would hold the method patent to be invalid) which they did not have. To do so would have the effect of converting the terms and purpose of the undertakings as to damages from protecting a person from the Court’s act in making the interlocutory order to protecting a person from the existence of the subject-matter of the litigation and the litigation itself. No recourse to notions of equity, justice or fairness can have that transformative effect on the terms or the purpose of the undertakings. My approach to the required analysis, in which I often deal with the claimants’ propositions at the level of fact, should not be understood to be a departure from these principles.
142 Some of the claimants submitted that everything Wyeth said about enforcing its rights under the method patent involved the making of unjustified threats within the meaning of Pt 3 of Ch 11 of the Patents Act and that, accordingly, Wyeth should not be permitted to take the benefit of having made those threats when it comes to assessing compensation. Again, this involves an impermissible attempt to broaden the terms and the purpose of the undertakings, the focus of which begins and ends with the operation of the interlocutory orders. None of the generics brought proceedings under s 128 of the Patents Act for unjustifiable threats. They are also not seeking damages for unjustifiable threats as provided for in s 128(1)(c). They are seeking compensation under the undertakings. The claims are to be assessed in that context alone.
143 There is another unspoken but persistent theme informing many of the submissions for the claimants, to the effect that Wyeth merits some form of punishment for having invoked the jurisdiction of the Court and ultimately failed. This is not so. Wyeth was entitled to come to the Court seeking to enforce its rights under the method patent. Having failed, the method patent was revoked. The concept of punishment of Wyeth is foreign to the purpose of the undertakings. As Barwick CJ said in Air Express at 310, a party in Wyeth’s position is not to be discouraged from nor visited with a penalty for having invoked the Court’s jurisdiction.
144 To return now to the issue of causation, again, the terms of the undertakings must be given effect. To have a claim under the undertaking, the person must prove to the ordinary civil standard of proof that they have been “adversely affected by any operation of the orders …or any continuation (with or without variation) thereof”. If the person would have been in the same position irrespective of the orders then the person has not proved that they are “adversely affected by any operation of the orders”. This is not to suggest that the orders must be the sole cause of the loss, nor that the “but for” test is an exclusive criterion for assessing causation. But it is to say that the subject of compensation is no more and no less than the adverse effect, if any, of the operation of the interlocutory injunctions.
145 Apart from this it may safely be said that compensation should generally be confined to kinds of direct losses which were reasonably foreseeable at the time the interlocutory orders were made. This is another reason for excluding compensation for losses said to result from the mere anticipation of an interlocutory injunction by reason of the grant of an earlier interlocutory injunction. Losses of this kind are not a direct, natural or ordinary consequence of the grant of the earlier interlocutory injunction.
146 Given the admonitions against reasoning by rigid analogy to other causes of action in this area of discourse, I do not consider it appropriate to apply the distinction between reasonable foreseeability for the purpose of tortious liability and reasonable contemplation for the purpose of contractual liability: see the summary in Alexander v Cambridge Credit Corporation Ltd (1987) 9 NSWLR 310 at 364-366. It is sufficient to say that there must be some limit on the potential liability of a party under an undertaking given that the person’s submission is to “such order (if any) as the Court may consider to be just for the payment of compensation”. It would be unlikely to be just to order compensation for kinds of losses which could not have been reasonably foreseeable by Wyeth at the time it gave the undertakings. Similarly, to subject a party to indeterminate liability for indirect economic losses would be inconsistent with the development of principle in other areas of the law and thus would also be unlikely to be just.
147 These considerations also explain why it is important that the claimed loss be identified with precision. In the present case, the claimants pleaded different kinds of losses.
148 For Sigma and Alphapharm, the primary pleaded loss was the loss of opportunity to supply their products to pharmacists.
149 For Generic Health, one pleaded loss was the loss of opportunity to supply its products to pharmacists. Another pleaded loss was the loss of opportunity to supply the Sigma products to Sigma. A further pleaded loss was the loss of opportunity to supply generic venlafaxine products to other generics. These are different kinds of losses which must be considered separately. As will become apparent, the opportunity for Generic Health to supply its own products was within its own control. Its opportunity to supply Sigma depended on Sigma ordering products under back-to-back supply agreements with Alembic and Sigma which existed before the Sigma interlocutory injunction. Its opportunity to supply other generics depended on the decision of other generics to enter into supply agreements with Generic Health (which Apotex, Sandoz and Ascent did but after the Generic Health interlocutory injunction) and to order products under those agreements. These are important differences.
150 For Alembic, the primary pleaded loss was the loss of opportunity to supply Sigma’s products, which Alembic manufactured, to Generic Health for supply to Sigma under the back-to-back supply agreements between Sigma, Generic Health and Alembic. Alembic’s opportunity thus depended on Sigma placing orders for its products with Generic Health.
151 For Pharmathen, the primary pleaded loss was in three categories. It had a supply agreement with Alphapharm which pre-dated the Alphapharm interlocutory injunction and its opportunity to supply under that agreement depended on orders from Alphapharm. It had a supply agreement with Generic Health which pre-dated the Generic Health interlocutory injunction and its opportunity to supply Generic Health’s branded products under that agreement depended on orders from Generic Health. It had an opportunity to supply to Generic Health other generic venlafaxine products if Generic Health secured supply agreements with other generics and thus its opportunity to supply those products depended on the actions of Generic Health and other generics and those other generics placing orders for the products with Generic Health.
Principles relating to proof of loss by the generics – supply by them to pharmacists
152 All of the parties accepted that the generics had to prove some loss on the balance of probabilities. Thereafter, consensus ended.
153 Wyeth proposed that the generics had to prove on the balance of probabilities that, without the interlocutory injunctions, they would have sought and obtained the listing of their products on the PBS (the agreed effect of which would have been to trigger an automatic 12.5% reduction in the Approved Price to Pharmacist of Efexor-XR) and/or would have sold their products to pharmacists outside the PBS and thus on the private market. Leaving aside an argument about the terms of the Sigma interlocutory injunction, Wyeth’s position about causation was that if the generics (or one or other of them) proved on the balance of probabilities that they would have sought and obtained the listing of their products on the PBS then they would be entitled to compensation of 100% of the loss to them from having been prevented from doing so by the interlocutory injunctions, subject only to discounting for the risks in all of the circumstances. If, however, they did not prove on the balance of probabilities that they would have sought and obtained the listing of their products on the PBS then there can be no compensation on account of not having done so and thus not having made any PBS sales. Further, if the generics (or one or other of them) proved on the balance of probabilities that they would have sold their products outside of the PBS on the private market, they would be entitled to compensation of 100% of the loss to them from having been prevented from doing so by the interlocutory injunctions, subject only to discounting for the risks in all of the circumstances. If, however, they did not prove on the balance of probabilities that they would have sold their products outside of the PBS on the private market then there can be no compensation on account of not having done so and thus not having made any private market sales.
154 In other words, on Wyeth’s approach, the adverse effects of the interlocutory orders, if any, are the inability to supply under or outside of the PBS and each of these opportunities must be proved to have been lost on the balance of probabilities. If neither claimed adverse effect is so proved (not seeking and obtaining listings on the PBS and making PBS sales and/or not selling the products into the private market) there can be no compensation for the possibility that a generic might have sought and obtained listing on the PBS and made PBS sales or might have sold its products into the non-PBS private market.
155 The generics’ approach was ultimately one of having their cake and eating it. They contended, albeit with numerous variations, that they would prove on the balance of probabilities that the interlocutory injunctions prevented them from obtaining PBS listings for their products and thus prevented them from making PBS sales and also prevented them from selling their products on the private market, both or either of which they otherwise would have done, so that they are entitled to 100% of the loss caused by them of not having done so (that is, their estimated losses are to be treated as certain). They also said, however, that if they failed to prove either or both of these matters on the balance of probabilities then, in any event, provided they proved some loss, even if it be only the loss of some opportunity of some value (even the loss of opportunity to decide whether or not to apply to obtain PBS listing and make PBS sales or to sell into the private market), they were entitled to compensation based on an assessment of the possibilities of them having done so. The incoherence of these fundamentally inconsistent approaches is explained below.
156 Much of the debate focused on Sellars v Adelaide Petroleum NL [1994] HCA 4; (1994) 179 CLR 332 and Badenach v Calvert [2016] HCA 18; (2016) 257 CLR 440. Sellars was a claim for damages under s 82(1) of the Trade Practices Act 1974 (Cth) (which referred to “loss or damage by conduct of another person that was done in contravention of a provision…”). Mason CJ, Dawson, Toohey and Gaudron JJ held at 348 that an applicant under s 82(1) can only recover for loss actually incurred, in contrast to potential loss. They explained at 349 that, in the law of contract, where the contractual promise is to provide a chance:
…where there has been an actual loss of some sort, the common law does not permit difficulties of estimating the loss in money to defeat an award of damages. The damages will then be ascertained by reference to the degree of probabilities, or possibilities, inherent in the plaintiff's succeeding had the plaintiff been given the chance which the contract promised.
157 Their Honours noted at 349 that this approach was not confined to games of chance, sporting contests or competitions. Accordingly, at 349-350 they said:
…there can be no doubt that a contract to provide a commercial advantage or opportunity, if breached, enables the innocent party to bring an action for damages for the loss of that advantage or opportunity. So, in The Commonwealth v Amann Aviation Pty Ltd [(1991) 174 CLR 64], Mason C.J. and Dawson J., Brennan J. and Deane J. concluded that a lost commercial advantage or opportunity was a compensable loss, even though there was a less than 50 per cent likelihood that the commercial advantage would be realised. Damages for breach of contract were assessed by reference to the probabilities or possibilities of what would have happened.
Damages in tort have also been assessed by reference to the probabilities or possibilities of what will happen or what would have happened. That approach has been frequently adopted in the assessment of damages for personal injuries where a court has been called upon to assess future possibilities and past hypothetical situations. In Malec v J.C. Hutton Pty Ltd [(1990) 169 CLR 638], this Court drew a distinction between, on the one hand, proof of historical facts - what has happened - and, on the other hand, proof of future possibilities and past hypothetical situations. The civil standard of proof applies to the first category but not to the second, particularly when it is necessary to determine future possibilities and past hypothetical situations for the purpose of assessing damages. In Malec, Deane, Gaudron and McHugh JJ explained the way in which the matter is to be approached in these terms:
“If the law is to take account of future or hypothetical events in assessing damages, it can only do so in terms of the degree of probability of those events occurring. ... But unless the chance is so low as to be regarded as speculative - say less than 1 per cent - or so high as to be practically certain - say over 99 per cent - the court will take that chance into account in assessing the damages. Where proof is necessarily unattainable, it would be unfair to treat as certain a prediction which has a 51 per cent probability of occurring, but to ignore altogether a prediction which has a 49 per cent probability of occurring. Thus, the court assesses the degree of probability that an event would have occurred, or might occur, and adjusts its award of damages to reflect the degree of probability.”
158 At 355 in Sellars, they continued:
Notwithstanding the observations of this Court in Norwest [Norwest Refrigeration Services Pty Ltd v Rain Dawes (WA) Pty Ltd (1984) 157 CLR 149], we consider that acceptance of the principle enunciated in Malec requires that damages for deprivation of a commercial opportunity, whether the deprivation occurred by reason of breach of contract, tort or contravention of s. 52(1), should be ascertained by reference to the court's assessment of the prospects of success of that opportunity had it been pursued. The principle recognized in Malec was based on a consideration of the peculiar difficulties associated with the proof and evaluation of future possibilities and past hypothetical fact situations, as contrasted with proof of historical facts. Once that is accepted, there is no secure foundation for confining the principle to cases of any particular kind.
On the other hand, the general standard of proof in civil actions will ordinarily govern the issue of causation and the issue whether the applicant has sustained loss or damage. Hence the applicant must prove on the balance of probabilities that he or she has sustained some loss or damage. However, in a case such as the present, the applicant shows some loss or damage was sustained by demonstrating that the contravening conduct caused the loss of a commercial opportunity which had some value (not being a negligible value), the value being ascertained by reference to the degree of probabilities or possibilities. It is no answer to that way of viewing an applicant's case to say that the commercial opportunity was valueless on the balance of probabilities because to say that is to value the commercial opportunity by reference to a standard of proof which is inapplicable.
159 Commonwealth v Amann Aviation Pty Ltd [1991] HCA 54; (1991) 174 CLR 64 involved the repudiation of a contract under which there was no promise that Amann would obtain a further contract but there was a promised opportunity to seek a further contract which the repudiation prevented. Deane J said this at 118-119, in the context of cases about the loss of a chance:
There are, however, cases where considerations of justice or the limitations of curial method render ultimate findings, about what would have been or will be, impracticable or inappropriate. In such cases, damages must be assessed on some basis other than findings about what would have ultimately happened if the repudiation or breach had not occurred or about the precise ultimate implications of the situation which exists after the repudiation or breach. In particular, it may be appropriate that damages be assessed by reference to the probabilities or the possibilities of what would have happened or will happen rather than on the basis of speculation that probabilities would have or will come to pass and that possibilities would not have or will not. If, for example, what the plaintiff has lost by reason of the defendant's repudiation or breach of contract is a less than 50 per cent but nonetheless real and valuable chance of winning some contest or prize, of being the successful tenderer for some commercial undertaking or of deriving some other advantage, in circumstances where a court can decide that a proportionate figure precisely or approximately reflects the chance of success but can do no more than speculate about whether, but for the defendant's wrongful act, the plaintiff would have actually won the contest, prize or tender or derived the advantage, it would affront justice for the court to hold that the plaintiff was entitled to no compensation at all for the lost chance of competing or striving or for the wasted expenditure which was incurred in obtaining or performing the contract. In such a case, considerations of justice require that the plaintiff be entitled to recover the value of the lost chance itself and that the defendant be not allowed to take advantage of the effects of his own wrongful act to escape liability by pointing to the obvious, namely, that it is theoretically more probable than not that a less than 50 per cent chance of success would have resulted in failure. Thus, for example, a plaintiff whose action against a third party has become statute-barred by reason of a defendant solicitor's breach of contract may recover damages by reference to the court's assessment of what the chance of success in the action against the third party would have been even though that assessment is 50 per cent or less.
160 Fundamental considerations of fairness thus inform the approach to damages in cases of the loss of a chance.
161 Norwest Refrigeration Services Pty Ltd v Rain Dawes (WA) Pty Ltd [1984] HCA 59; (1984) 157 CLR 149 concerned an opportunity to avoid loss by having obtained a different insurance policy. The High Court held that to succeed the plaintiff had to prove on the balance of probabilities that it could have secured effective cover. Whether Norwest would have been decided in the same way after Sellars is unclear. What is clear and relevant is the High Court’s acceptance of (i) the difficulty of proof of past hypothetical facts, (ii) for a loss of opportunity case, the need for proof on the balance of probabilities only of some loss; and that (iii) these principles are not confined to cases of tort, contract or misleading and deceptive conduct.
162 Badenach v Calvert involved the tort of negligence. A solicitor had not advised a client about the risk of a family provision claim relating to a will. The beneficiary sued claiming, at least on appeal, loss of the opportunity to take steps to avoid such a claim. The beneficiary failed because of a failure to prove on the balance of probabilities that the client would have done anything different had the kind of advice contended for been given. French CJ and Kiefel and Keane JJ said that:
[38] It has been explained that to speak of loss as the loss of a “chance” distorts the question of causation. It involves the application of a lesser standard of proof than is required by the law and, it follows, by s 13(1)(a). It confuses the issue of the loss caused with the issue of assessing damages which are said to flow from that loss. In that assessment a chance may be evaluated.
[39] The respondent’s case on causation is not improved by seeking to equate the chance spoken of with an opportunity lost. It may be accepted that an opportunity which is lost may be compensable in tort. But that is because the opportunity is itself of some value. An opportunity will be of value where there is a substantial, and not a merely speculative, prospect that a benefit will be acquired or a detriment avoided.
[40] It remains necessary to prove, to the usual standard, that there was a substantial prospect of a beneficial outcome. This requires evidence of what would have been done if the opportunity had been afforded. The respondent has not established that there is a substantial prospect that the client would have chosen to undertake the inter vivos transactions. Therefore, the respondent has not proven that there was any loss of a valuable opportunity.
[41] The onus of proving causation of loss is not discharged by a finding that there was more than a negligible chance that the outcome would be favourable, or even by a finding that there was a substantial chance of such an outcome. The onus is only discharged where a plaintiff can prove that it was more probable than not that they would have received a valuable opportunity…
163 The explanation identified in [38] of Badenach v Calvert is provided in Kiefel J’s judgment in Tabet v Gett [2010] HCA 12; (2010) 240 CLR 537 at [142] which was based on these propositions:
(1) “What cases in contract, such as Commonwealth v Amann Aviation Pty Ltd and Sellars v Adelaide Petroleum NL, have in common is that the commercial interest lost may readily be seen to be of value itself”: at [124]
(2) “Sellars v Adelaide Petroleum NL confirms that the general standard of proof is to be maintained with respect to the issue of causation and whether the plaintiff has suffered loss or damage. In relation to the assessment of damages, as was said in Malec v J C Hutton Pty Ltd, “the hypothetical may be conjectured.” The court may adjust its award to reflect the degree of probability of a loss eventuating. This follows from the requirement that the courts must do the best they can in estimating damages; mere difficulty in that regard is not permitted to render an award uncertain or impossible”: at [136].
(3) “Thus in the case of the loss of a commercial opportunity, the plaintiff must first establish the fact of the loss, for example by reference to the fact that it had a commercial interest of value which is no longer available to be pursued because of the defendant’s negligence. The damages assessed of that loss, the estimation of its value, reflect the chance, often expressed in a percentage, that the opportunity would have been pursued to a successful outcome. The award is proportionate in that sense”: at [137].
164 In Mal Owen Consulting Pty Ltd v Ashcroft [2018] NSWCA 135 at [18] Basten JA saw no inconsistency between Sellars and Badenach v Calvert. Macfarlan JA at [62], in dissent, found the decisions “difficult to reconcile”. Barrett AJA, having referred to Sellars at [98], said:
[99] The court’s task, in relation to the issue of causation, is thus to assess the prospects of success of the opportunity had it been pursued. Such an assessment depends on proof by a plaintiff according to the balance of probabilities that he or she has sustained some loss or damage because deprived of an opportunity having value beyond merely theoretical or negligible value. As French CJ, Kiefel and Keane JJ pointed out in Badenach v Calvert (2016) 257 CLR 440; [2016] HCA 18 at [40], there must be a determination, according to the balance of probabilities, whether there was a substantial prospect of a beneficial outcome; and the plaintiff’s onus in that respect is discharged only by proof that it was more probable than not that an opportunity of value would have been received but for the defendant’s negligence.
[100] The process just described goes to the issue of causation. It represents the first of what the joint judgment in Sellars v Adelaide Petroleum NL identifies as two distinct stages relevant to the resolution of a case such as the present. At that first stage, causation must be proved on the balance of probabilities… The second stage becomes relevant only if causation is established at the first. The issue at the second stage is the assessment of damages; and the focus then is upon the actual value of the lost opportunity which, to that point, has been appraised only as not merely theoretical or negligible. Value must be ascertained at the second stage by reference to “the degree of probabilities, or possibilities, inherent in the plaintiff’s succeeding had the plaintiff been given the chance” of which the plaintiff has been deprived. These are again words used in the joint judgment in Sellars v Adelaide Petroleum NL.
[101] At each of the two stages, therefore, attention must be given to a question relevant to the value of the lost opportunity. At the first stage concerned with causation, the task is no more than to confirm that the value is not in the realms of the merely theoretical or negligible - in other words, to establish, according to the balance of probabilities, that there is some colour of value to the lost opportunity. It is only if the second stage is reached (after causation is established at the first) that anything approaching particular quantification is required. An assessment made at the second stage by reference to the degree of probabilities and possibilities of factual hypotheses may require a process of estimation extending even to a degree of guesswork and may lie at any point within a broad range.
165 With these principles in mind, it can be seen that on Wyeth’s approach the generics had two separate commercial opportunities. One to obtain listing of their products on and thereafter to sell their products under the PBS and another to sell their products outside of the PBS on the private market. It is implicit in Wyeth’s approach that the only potential value of either opportunity relates to profits from the acts of supply. Thus, Wyeth’s case is that the generics have to prove on the balance of probabilities the loss of either or of both of these opportunities. For there to be an adverse effect from the interlocutory injunctions, accordingly, the generics must prove on the balance of probabilities they would have listed on the PBS to be eligible for compensation in the form of lost profits from a putative PBS listing and/or prove on the balance of probabilities they would have sold their products on the private market to be eligible for compensation in the form of lost profits from these kinds of sales. If not, according to Wyeth, the position of the generics would be the same irrespective of the interlocutory injunctions.
166 In their primary cases, the generics contend that they will prove both of these matters on the balance of probabilities and thus are eligible for (indeed, entitled to) compensation assessed by reference to 100% of the lost opportunities (PBS listing and private market or private market alone). In their alternative cases, the generics contend that they had an opportunity or opportunities which was or were of value and which they lost because of the interlocutory injunctions. They described the opportunity or opportunities in various ways ranging from an opportunity to sell their generic venlafaxine products to pharmacists generally in which the PBS/private distinction dictates only the size of the market to an opportunity to decide whether or not to sell their generic venlafaxine products to pharmacists. Thus, there is a corresponding range of facts the generics say they must prove on the balance of probabilities from, for example, proof that they would have supplied on the PBS and/or private market, to proof they would have supplied on the private market only which will suffice for all issues to be determined on the probabilities and possibilities (including the possibility of PBS listing and supply), to proof that they had the capacity to supply as that capacity itself carried non-negligible value. Whichever way the opportunity is described, the generics say that the opportunity had real value and, on the balance of probabilities, was an opportunity lost because of the interlocutory injunctions, so that the quantum of loss is to be assessed by reference to the probabilities and possibilities of the opportunity, with all its potential variations (PBS or private market) having been pursued.
167 As noted, however, in their primary case the generics claimed 100% of their putative loss even if they prove only a 51% (on the balance of probabilities) likelihood that they would have obtained PBS listing for their products and sold them on the PBS. Alternatively, they want 49% of their putative loss if they prove a 49% likelihood that they would have obtained PBS listing for their products and sold them on the PBS. And they claim 100% of their putative loss even if they prove only a 51% (on the balance of probabilities) likelihood that they would have sold their products on the private market. Alternatively, they want 49% of their putative loss if they prove a 49% likelihood that they would have sold their products on the private market.
168 It is not necessary to attempt to resolve every permutation of the arguments which were put. Despite the claimants’ submissions that the injunction is akin to a promise, this not a case of breach of a contractual promise to give the claimants an opportunity of value: Chaplin v Hicks [1911] 2 KB 786 and Sellars at 359. Nor is it a case of misrepresentation or negligent failure to advise about the existence of an opportunity of value, both of which could only cause loss if an alternative opportunity could and would have been pursued absent the wrong: Gates v Mutual Life Assurance Society Ltd [1986] HCA 3; (1986) 160 CLR 1; Sykes v Midland Bank Executor & Trustee Co Ltd [1971] 1 QB 113 and Badenach v Calvert at [34] and [41] (French CJ, Kiefel and Keane JJ) and [97]-[99] (Gordon J). But it is a case in which compensation is sought for the allegedly adverse effect of the operation of the interlocutory orders, a context in which it has been said that the purpose of the undertakings is to be kept in mind, which is to protect people who may be adversely affected by the Court’s act in granting an interlocutory order.
169 As disclosed in the statement of agreed facts in Schedule 2, products approved by the TGA and thus registered on the ARTG may be supplied to pharmacists and others whether or not the product is listed on the PBS. The opportunity to obtain PBS listing may or may not exist for a product once a product is registered on the ARTG. In the present case, the opportunity for PBS listing of the generic venlafaxine products existed because Efexor-XR was listed on the PBS, but it was an opportunity that depended on obtaining ARTG registration of the generics’ venlafaxine products. In 2009, the generics alone possessed that opportunity.
170 As noted, under Div 1 of Pt 3-2 of the Therapeutic Goods Act, it is an offence, amongst other things, to import into, manufacture or supply in Australia therapeutic goods unless, relevantly, the goods are registered or listed goods in relation to the person (see, for example, s 19B). To obtain ARTG registration the generics had to apply under Div 2 of Pt 3-2 of the Therapeutic Goods Act. The goods the subject of an application may or may not be registered, relevantly to the present case, under s 26. To obtain registration, the goods must satisfy a range of requirements, depending on the kind of good in question. As noted in the statement of agreed facts in Schedule 2, clinical studies proving bioequivalence of the generics’ products to Efexor-XR was required to obtain listing. The evidence discloses that to obtain ARTG registrations involved the generics in significant effort, time and expense.
171 Sigma, Alphapharm and Generic Health are in the business of supplying generic products to pharmacists. Once they obtained ARTG registrations of their products (which involved substantial effort, time and expense), the fact of supply to pharmacists on the private market was a commercial decision for them alone. The extent to which pharmacists might have purchased the products may be open for debate, but not the fact that the generics would have been able make their own commercial decision about supply and that some pharmacists would have purchased the products if available. This forms an important part of the context relevant to the kind of loss being claimed and how it must be proved. Similarly, it was for the generics alone to decide if they wished to apply for PBS listing of their products once they had obtained the ARTG registrations. The ARTG registrations meant that the acts of supply, be they on the private market or on the PBS if PBS listing was obtained, would not be a criminal offence, as would otherwise be the case under Div 1 of Pt 3-2 of the Therapeutic Goods Act.
172 The Therapeutics Goods Regulations also enabled the relevant person in relation to whom goods were registered or listed to transfer or assign the registration or listing. In 2009, for example, r 10A provided:
If:
(a) a person agrees to dispose of a business relating to the manufacture, distribution or sale of therapeutic goods; and
(b) it is agreed that the disposal of that business is to include a transfer of the registration or listing of therapeutic goods;
then:
(c) the person who acquires that business is taken to be the person in relation to whom the therapeutic goods are registered or listed; and
(d) that person must, not later than 3 months after the transfer, notify the Secretary that the person has, by reason of that agreement, become the person in relation to whom the goods are to be registered or listed.
173 In summary, once a generic had obtained TGA approval and thus registration of their products on the ARTG, the generic had all of the rights associated with ARTG registration, specifically the right to supply their products to pharmacists and others in Australia. I consider that rights of this kind have value to the holder which would not be characterised as negligible or theoretical, excluding perhaps some particular circumstance which meant that none of the rights could or would be exercised. And as to the latter potential qualification on value, it cannot be assumed that a decision not to exercise supply rights in one set of circumstances would have been made in another. Nor can it be assumed that a decision, once made, would be irrevocable. This is part of the value of supply rights afforded by ARTG registration. The rights can be exercised as the relevant person in relation to whom the goods are registered sees fit from time to time. Such rights can be held, exercised, or transferred so another person may exercise them in response to circumstances as they develop (such as Sigma’s transfer of its ARTG registrations to its parent company). If held, the rights have value because they can be exercised or transferred so another person may exercise them.
174 On the reasoning in Sellars and Badenach v Calvert, which I do not consider to be inconsistent, my view is that the generics will have proved on the balance of probabilities that they lost an opportunity of some value as a result of the interlocutory injunctions if before the interlocutory injunctions they could exercise the rights afforded by ARTG registration and after the interlocutory injunctions they were prevented from exercising these rights.
175 I do not accept Wyeth’s characterisation of the alternative cases as involving “the loss of an opportunity to consider whether or not to pursue an opportunity”, which Wyeth described as a “second generation loss of opportunity case”. To oversimplify, the generics’ basic opportunity was to supply their products and make a profit doing so. This opportunity was not one shared by the world at large in 2009 or 2010. It depended on ARTG registration of the products and the generics’ own decision-making. ARTG registration gave rise to a valuable opportunity, assuming the rights associated with ARTG registration could be exercised. If an interlocutory injunction removed that opportunity so that the products could not be supplied, the valuable opportunity is necessarily lost. The value of the ARTG registrations, be it from holding, exercising or transferring them, was necessarily eliminated or substantially reduced for the period during which the interlocutory injunctions operated. On this construct, the operation of each interlocutory injunction has adversely affected the generic bound by the injunction. Further, on this construct, the question whether a generic would have supplied its products does not relate to the existence of the loss of opportunity of some value but, rather, the extent of the value of the opportunity lost. In other words, the alternative cases do not involve a loss of opportunity to pursue an opportunity, but the loss of a valuable opportunity in which the extent of the value of the lost opportunity will depend on a wide range of circumstances including the probability or possibility of supply having been pursued.
176 To my mind, this approach accords with what was said in Badenach v Calvert at [40] that it is “necessary to prove, to the usual standard, that there was a substantial prospect of a beneficial outcome”. For a generic, an ARTG registration of its product, excluding some circumstance which meant that it could not exercise the rights afforded by ARTG registration, involves a substantial prospect of a beneficial outcome, being the prospect of supply of its product for the purpose of making a profit.
177 I thus accept the generics’ submissions about their alternative cases. Alphapharm’s reply submissions conveniently summarise those submissions which apply equally to all the generics, namely:
[10] At WWCS [Wyeth’s closing submissions] [133], Wyeth impliedly seeks to characterise, so as to dismiss, the generics’ contention on loss of opportunity as “nothing more than the opportunity to consider whether or not it will pursue a new opportunity”. That is not a fair characterisation of Alphapharm’s submission. Alphapharm accepts that it is required to prove on balance probabilities that it has lost a valuable opportunity, not merely an opportunity to consider a valuable opportunity.
[11] However, Wyeth’s criticisms of Alphapharm’s submissions on the appropriate approach to proof of loss (including the specific criticism in WWCS [135]) are not warranted. First, the criticisms do not appreciate the way in which Alphapharm’s submissions are advanced in the alternative.
[12] Secondly, as Alphapharm submitted in AWCS [Alphapharm’s closing submissions] [2.13] – [2.14], in the circumstances of this case there can be no real doubt that a loss of opportunity analysis (that is, where causation of some loss is established and the estimation of loss may be carried out by assessing the probabilities and possibilities) is readily available because it is clear that the claimants have shown (on the balance of probabilities) an actual loss, in the nature of a lost commercial advantage or opportunity. Actual loss was occasioned by injunctive orders that explicitly removed any potential for the opportunity described in the orders to be taken up. The principles in Sellars at 349 and 355 apply. In other cases there might be more reasonably contestable evidence about whether an applicant has proven on the balance of probabilities that it has suffered “some loss or damage” by way of “the loss of a commercial opportunity which had some value (not being a negligible value)”; but here Alphapharm’s loss of opportunity is very clear. The Court may therefore address damages by reference to the degree of probabilities or possibilities inherent in Alphapharm succeeding if it had been given the chance to supply Enlafax XR.
178 This being so, none of the parties can or should be permitted to escape the effect of the fact that the claims are to be assessed as lost opportunities. Alphapharm noted that in AstraZeneca AB v KRKA dd Novo Mesto [2015] EWCA Civ 484 at [14], because the trial judge found on the balance of probabilities that the generic would have entered the market with “full force and effect”, the English Court of Appeal concluded that it was not necessary for the trial judge to “assess the loss of the chance of the defendants taking this course or of taking it in a particular way”. Rather, the trial judge was required to evaluate “the degree of success they would have achieved and the profits they would have made but for the injunction, and in carrying out that exercise to take into account all significant factors but to ignore speculative possibilities” and if necessary, to “apply a discount to reflect the various uncertainties inherent in the assessment of this counterfactual scenario”.
179 It may be that whether the uncertainties are weighed as part of the hypothetical analysis or as an overall ultimate single discount, the result is the same. But, assuming a loss of a valuable opportunity is proved, it is because the common law treats a finding on the balance of probabilities about a past event as a certainty one way or another (that is, 100% or 0%), that the High Court in Malec v J C Hutton Pty Limited [1990] HCA 20; (1990) 169 CLR 638 at 643 considered that an assessment of loss by reference to “hypothetical events” required a different approach, referring to the need for a court to assess “the degree of probability that an event would have occurred, or might occur”.
180 There are other statements of principle to the same effect. In Tranquility Pools & Spas Pty Limited v Huntsman Chemical Company Australia Pty Limited [2011] NSWSC 75 at [380] Einstein J summarised principles relevant to the assessment of damages on the basis of a hypothetical, what would have occurred in the pools business of the claimant but for the wrong, including the following:
…
(2) The process of weighing the many possibilities involves the assessment of many variables. The court is nevertheless, by the nature of the task, required to form a single view about the quantum of loss. As has been repeatedly recognised this will involve a degree of speculation.
(3) Once causation is established, although the plaintiff retains an evidentiary onus, the balance of probabilities has no role to play and assessing the facts upon which the hypothetical (whether past or future or, as in this case, both) on that basis would be an error of law: Fightvision Pty Ltd v Onisforou (1999) 47 NSWLR 473 at [137]–[144] per Sheller, Stein & Giles JJA.
(4) In the great majority of cases, a scientific or actuarial approach is, given the imponderables, an exercise in spurious precision, and the proper approach is for the court, having weighed all the possibilities, to settle on a single hypothesis on a qualitative and intuitive basis…
181 Consistent with these observations, a finding of a 51% probability that a generic would have obtained PBS listing and supplied their products under the PBS does not mean that the generic is eligible for compensation assessed by reference to 100% of the profits it would have obtained if it had done so. Despite having proved the loss of that opportunity on the balance of probabilities the generic would be eligible for compensation assessed by reference to the 51% probability of that opportunity being taken and the likely results of it being taken. The generic would only be eligible for compensation quantified by reference to 100% of the lost profits if it is a practical certainty (99% or higher) that it would have obtained PBS listing and supplied their products under the PBS. To the same effect, however, if there is a 49% possibility that a generic would have obtained PBS listing and supplied their products under the PBS, compensation is not to be assessed as zero. The generic would be eligible for compensation assessed by reference to the 49% possibility of that opportunity being taken. The reference to a generic being “eligible” here is because there are numerous arguments in play in this matter and these observations are directed only to the questions of causation and assessment of loss as a matter of principle, and not to the facts of any particular claim.
182 I should say that Sigma, at least, recognised the force of these observations by noting in its closing submissions that:
Sigma has pleaded damages on a loss of opportunity basis as well as on a balance of probabilities basis. Indeed, even if Sigma had not pleaded such damages, on one view of the authorities the only appropriate approach to assessing loss in this type of claim is to do so by reference to the chances of particular future hypothetical events occurring and reflecting those chances in the damages award.
183 There are other reasons to consider this approach best accords with the purpose of the undertaking.
184 As will become apparent, once they discovered the method patent, the generics assumed they would be subject to applications for interlocutory injunctions and ordered their affairs accordingly. In the event, their beliefs proved well founded as interlocutory injunctions were granted. By so observing I am not suggesting that the generics are to be compensated for the way in which they ordered their affairs before the interlocutory injunctions by which they were bound were granted. As discussed above, any compensation must relate to the operation of the interlocutory injunctions. The issue, however, is one of evidence and proof. What the generics would or might have done had the interlocutory injunctions not been granted necessarily involves proof of a hypothetical fact. Given the complexity of the circumstances in which actions of one person and their timing necessarily affect the actions of others, the question of what the generics would or might have done had they not been enjoined is not well suited to the all or nothing approach which ordinarily applies to proof. But without the interlocutory injunctions, what the generics would have done in the face of the method patent and the litigation would be known. Having taken the benefit of the interlocutory injunctions at the price of the undertakings, it is not for Wyeth to rely on the necessarily evaluative character of the required exercise as a reason for resisting the claims against it. Provided that it is proved on the balance of probabilities that the interlocutory injunctions prevented the generics from exercising or realising the value of the rights afforded by the ARTG registrations of their products when but for the interlocutory injunctions they could have exercised those rights, an approach based on an evaluation of the inferred range of possibilities to which the evidence gives rise (excluding mere speculative possibilities) better reflects the purpose of the undertakings than the primary approaches of any of the parties.
185 The primary approaches of the parties would also be unlikely to yield a result just to either the party which gave the undertakings (Wyeth) or the claimants restrained by the interlocutory injunctions.
(1) From the perspective of the generics, in a world of multiple possibilities given their businesses, they will succeed or fail by reference to two questions (on the balance of probabilities would they have listed on the PBS and/or on the balance of probabilities would they have supplied their products on the private market) when the interlocutory injunctions, from their grant, meant that these decisions were no longer theirs to make. Leaving aside an issue about the Sigma interlocutory injunction (which did not in terms prevent Sigma from seeking to list its products on the PBS), no generic had the occasion to decide what it would do about PBS listing and supply after the interlocutory injunctions were granted. What they would have done would inevitably have been affected by a range of circumstances, including hypothesised circumstances. Once we are in the realm of hypothesised action inferred from hypothesised circumstances, as we shortly will be given the inter-relationship between the claims, then the artificiality of an exercise which might depend on the difference between a 49% and 51% probability of what the generics would have done seems obvious.
(2) From the perspective of Wyeth, in the same world of multiple possibilities, a 51% chance of a PBS listing would make the generics eligible for 100% of the potential profits from a PBS listing in circumstances where one thing is clear – it was supply under the PBS which involved by far the greatest potential profits. On a rough and ready basis, it is apparent that the private market claims of the generics range are about one third of their PBS market claims.
186 Nothing I have said in this section should be understood as proposing that a claimant is eligible to be compensated under the undertakings if the claimant is in the same position the claimant would have been in irrespective of the undertakings. In any such case, the claimant would not be a person who has been adversely affected by the operation of the interlocutory injunctions. Nor am I suggesting that it is not necessary for a loss of some valuable opportunity to be proved on the balance of opportunities. Nor should mere speculative possibilities be compensable. I am saying, however, that in the context of the present case and insofar as the generics’ claims concern their lost opportunity to supply their products to pharmacists:
(1) there is not a loss of a valuable opportunity to a generic only if a generic proves on the balance of probabilities that they would have obtained PBS listing and supplied under the PBS and/or would have supplied their product on the private market;
(2) excluding some circumstance such as an irrevocable decision not to supply or seeking withdrawal of a product from the ARTG, there is a loss of a valuable opportunity of supply to a generic if a generic proves on the balance of probabilities that, before an interlocutory injunction, they could have exercised the supply rights given by ARTG registration and, as a result of an interlocutory injunction, they could not exercise those supply rights;
(3) the value of the opportunity lost under (2) is then to be determined as a matter of the probabilities and possibilities. The generics do not get to choose between their primary and alternative cases opting for the primary case if they happen to prove what they would have done at a percentage above 50% and the alternative cases if they do not; and
(4) this approach best reflects the purpose and the terms of the undertakings, whereas the primary approaches of the generics runs the risk of real injustice to Wyeth and Wyeth’s approach runs the risk of real injustice to the generics.
187 This said, I propose to make findings in a way which will disclose whether or not I consider that the various hypothesised facts have been established on the balance of probabilities (that is, with a probability of greater than 50%).
188 I should also record that if my approach is wrong, and what must be proved on the balance of probabilities is that the generics would have supplied their products under the PBS or on the private market, then I would not accept the submissions of some of the claimants that if they prove on the balance of probabilities that they would have supplied only to the private market then they can nevertheless recover compensation assessed by reference to the possibility that they would have supplied under the PBS. This is because, on this construct, the opportunity is only capable of being lost if, but for the interlocutory injunctions, the generics would have supplied their products. Of necessity, the question then concerns how they would have supplied their products. The evidence establishes (and it was not in serious dispute) that there are fundamental differences between supply in the private market and supply under the PBS. Supply under the PBS involves contingencies and considerations which do not come into play for supply in the private market. The generic must decide to seek PBS listing knowing that, if listed, there would be an automatic price reduction of 12.5% on the price for supply of Efexor-XR to pharmacists (which, in the present case, each generic believed it would most probably be liable for if it was the first to obtain PBS listing and the method patent was valid). The product must be listed on the PBS, which involves a decision of the Minister or Minister’s delegate which is not under the control of the generic. The generic must also be able to provide the guarantee of supply at the time of PBS listing.
189 Sigma submitted that the difference between the private market and PBS market was comparable to the different markets considered in Norris v Blake (No 2) (1997) 41 NSWLR 49 and Fightvision Pty Ltd v Onisforou [1999] NSWCA 323; (1999) 47 NSWLR 473. Those cases involved an actor and a boxer who were already successful in Australia and had substantial prospects of success in the USA. They had each already pursued opportunities in the USA. The circumstances are not comparable to the present case. The generics had not entered the PBS market at all. They could not do so without PBS listing. The first generic to do so would trigger the 12.5% price reduction in Efexor-XR and all of the generics proceeded on the basis that the first generic to obtain PBS listing would be liable to Wyeth for the price reduction if the method patent was valid. PBS listing depended on a Ministerial decision.
190 For these reasons, if my approach to proof of loss as a result of the operation of the interlocutory injunctions is wrong, the loss of the distinct opportunities represented by the private market and PBS market would each have to be proved on the balance of probabilities. As Wyeth said:
If the commercial opportunity which the generics have been deprived of is found to be launching on the private market – because not engaging in that activity is the “difference” (see Tabet v Gett per Hayne and Bell JJ) between how they behaved in the real world and how they would have behaved if the Interlocutory Injunctions had not been made – then the assessment of damages should involve quantifying the value of that lost opportunity. Adding an amount to reflect the possibility of those generics also listing on the PBS would be to compensate the generics for a loss which they did not suffer.
191 The same considerations do not arise on the approach which I consider should be applied.
192 I said above that it will not be possible in this matter to settle on “a single hypothesis”: Tranquility Pools at [380]. This is because of the inter-relationship between the claims. For example, we know that Alphapharm would not have been the first generic to apply for and obtain PBS listing of its generic venlafaxine products. Alphapharm was not prepared to take the risk of being liable for the 12.5% price reduction in Efexor-XR under any circumstances so that, even if none of the interlocutory injunctions were granted, Alphapharm was not going to seek PBS listing until another generic had obtained PBS listing and triggered the 12.5% price reduction in Efexor-XR (for which it assumed that generic, and not Alphapharm, may then be liable). It follows that the assessment of the degree of probability that Sigma or Generic Health would have sought and obtained PBS listing of their products, and when they would have done so, necessarily affects what Alphapharm would have done.
193 I made clear to the parties that compensation could not be assessed on the basis of inconsistent hypotheses. There could be disputes about the construction of the applicable hypotheses but, once constructed, the hypotheses must be consistent across all claims. Otherwise no determination of compensation could be just, at least not to Wyeth. To give an example, the generics and the manufacturers/suppliers did not agree about hypothetical supply prices. Because the claims of the manufacturers/suppliers depend on the generics, there cannot be inconsistent hypothesised supply prices between them.
194 Another matter needs to be considered. In its closing submissions Wyeth contends that the generics did not plead its case in this way. Wyeth referred to Barnes v Forty Two International Pty Ltd [2014] FCAFC 152; (2014) 316 ALR 408 at [119]-[123] in which Beach J (with whom Siopis and Flick JJ agreed) concluded that an alternative scenario which had not been “pleaded, opened or run” could not found relief. The pleaded case was that the claimant would not have entered into an agreement but for the alleged wrong. The alternative scenario was that the claimant lost the opportunity to negotiate a different agreement.
195 The present case is different.
196 Sigma pleaded the interlocutory injunction in terms (para 11). It pleaded its ARTG registrations (para 28). It pleaded that it was restrained from supplying (etc.) its products by reason of the interlocutory injunction (paras 36 to 41). Each of these paragraph is expressed by reference to what Sigma “would have” done but for the interlocutory injunction (e.g. listed on the PBS and supplied its products). Sigma pleaded that it suffered loss by, amongst other things, the operation of the interlocutory injunction, as particularised in the Appendix to the statement of claim (para 43) and that such loss flowed directly from the operation of the interlocutory injunction (para 44). In the Appendix the loss is said to be a “loss of opportunity”, amongst other things, to continue to sell its products in the private market, to obtain PBS listing, and to sell its products under the PBS.
197 Alphapharm’s pleading is to the same general effect insofar as it alleges that the interlocutory injunctions caused it loss as particularised in the Appendix to its statements of claim which identifies the loss as a “loss of opportunity”, amongst other things, to sell its products in the private market, to obtain PBS listing, and to sell its products under the PBS.
198 Generic Health’s pleading also characterised its relevant loss in this regard as a “loss of opportunity” to supply its products, be it on the private market or under the PBS.
199 Accordingly, the generics all pleaded their loss as a loss of opportunity to supply. Wyeth’s point, as I understand it, is that the opportunities said to be lost, in the body of the pleading, are framed by reference to what the generic “would have” done so the opportunity claimed to be lost is confined to that which the generics would have done. The difficulty with this is that the pleading’s purpose is to identify the material facts. By the pleadings Wyeth was on notice that the generics claimed that they lost the opportunity, in effect, of supply (including supply on the PBS). The fact that the pleadings are framed by reference to what the generics would have done is unremarkable. But in so framing their pleadings the generics could not have been binding themselves to any particular legal proposition about causation and proof. Provided the opportunities said to be lost remain the same, causation and proof involve legal principles. In their alternative cases, the generics do not suggest any new kind of opportunity lost. They say only that given the nature of the pleaded opportunities and of the effect of the interlocutory injunctions they need not prove on the balance of probabilities that they would have taken those opportunities. This is because, whether or not the rights would have been exercised, the rights prevented from being exercised by the interlocutory injunctions had value in and of themselves.
200 Perhaps most importantly, in Barnes the alternative scenario (the loss of the opportunity to negotiate a different agreement) was different from the pleaded case (the claimant would not have entered into the agreement). In the present case, loss of opportunity to supply was pleaded. The generics are not contending in their alternative cases that they would have done something different from what they pleaded. They are contending that those same pleaded facts and the same pleaded loss can be determined by reference to different legal principles from those identified in their opening submissions. And I am saying that the generics were right to plead their loss as a loss of opportunity. Further, having done so, the first legal question is whether they have proved on the balance of probabilities the loss of the opportunity to supply because of the interlocutory injunctions. The first legal question is not whether they have proved on the balance of probabilities that they would have supplied their products but for the interlocutory injunctions (as their primary cases and Wyeth would have it).
201 It is this distinction, whether they have proved on the balance of probabilities the loss of the opportunity to supply because of the interlocutory injunctions as opposed to whether they have proved on the balance of probabilities that they would have supplied their products but for the interlocutory injunctions, which is critical. Conceived of as a loss of opportunity case, as pleaded, provided a loss of opportunity having some value is proved to the civil standard, that standard is exhausted and has no further work to do in the analysis. It is not surprising that this distinction is not disclosed in the pleadings. It is not orthodox to identify in a pleading the standard of proof. There is also no difference in the material facts relevant to the different conceptions of the opportunities lost.
202 Of course, the question of the degree of probability that the generics would have supplied their products is still relevant. It is that degree which will inform the assessment of the amount of compensation. But the proper starting point of the legal analysis, in my view, is better reflected by the generics’ alternative cases rather than their primary cases.
203 It is true that the generics did not open their cases by reference to Sellars or similar authorities. It would be fair to say that despite their pleadings referring to their loss as the loss of opportunity to supply their products, the opening submissions appeared to assume that the generics needed to prove on the balance of probabilities that they would have taken the opportunities claimed to be lost. However, the matter was heard from 4 June to 13 July 2018. The openings occurred on 4 and 5 June. The first witness, Mr Heine (Sigma’s then sales manager), gave evidence on 7 June. Before the next witness, Mr Ellis, gave evidence, I said that I had forgotten to say three words at the close of opening submissions, those three words being “loss of opportunity”. I then said:
I wouldn’t want to get to the end of this case without hearing from people about the relevance of any loss of opportunity doctrines and things like loss of a chance, all those sorts of things. I am just not sure they really are grappled with in the opening submissions.
204 While it appears from this part of the transcript that Wyeth may have had a different view from the conclusions I have reached above about the authorities (specifically, what opportunity must be proved to be lost on the balance of probabilities), Wyeth did not suggest that the generics had not pleaded a loss of the opportunity to supply their products by reason of the interlocutory injunctions. Wyeth did not make that contention until its closing submissions and, in so doing, Wyeth did not suggest that they had been taken by surprise by some new fact. Its submissions were directed at issues of legal principle.
205 Finally, I should note that I gave Wyeth leave to amend its defences after the hearing had started. I did so on the basis that I did not consider that there would be any irremediable prejudice to the other parties from Wyeth being granted leave (and I refused leave to some amendments where I considered that there would be irremediable prejudice if leave to amend were granted). If greater clarity was required in the generics’ pleadings then I would have permitted amendments by the generics provided that the identified opportunities said to be lost remained the same. In my view, the generics’ alternative cases do not introduce any new opportunity said to be lost. The alternative cases merely take a different view of the principles relevant to causation and loss in respect of the same opportunities as pleaded.
Principles relating to proof of loss by the manufacturers/suppliers
206 As noted, in addition to its claims relating to the supply of its products to pharmacists, Generic Health also claims for the loss of opportunity to supply Sigma’s products to Sigma and the loss of opportunity to supply Pharmathen’s venlafaxine products to Apotex, Sandoz and Ascent. These claims also differ from each other in that Generic Health had an existing supply contract with Sigma and Alembic relating to supply of the Sigma products at the time of the Sigma interlocutory injunction. Generic Health did not have a contract with Apotex, Sandoz or Ascent (or with Pharmathen in respect of Apotex, Sandoz or Ascent) before the grant of any of the interlocutory injunctions.
207 Alembic was the contracted manufacturer of and supplier to Generic Health of Sigma’s generic venlafaxine products which Generic Health was then to supply to Sigma. Alembic claims for the loss of opportunity to make profits by supply of Sigma’s products to Generic Health.
208 Pharmathen was the contracted manufacturer of and supplier to Alphapharm and Generic Health of their respective generic venlafaxine products. Pharmathen claims for these lost opportunities of supply (which were the subject of contracts entered into before the interlocutory injunctions), as well as the lost opportunity of Generic Health supplying Apotex, Sandoz and Ascent (which were not the subject of contracts before the interlocutory injunctions).
209 Alembic and Pharmathen contended that their opportunity was a single opportunity to supply their customers under the contracts of supply. From their perspective, the question whether the generics would have applied for and obtained PBS listing and supplied under the PBS or would have supplied on the private market alone is relevant only to the value of the opportunity lost. Provided they prove on the balance of probabilities that they would have sold some (or some additional) venlafaxine products to the generics, their quantum of loss is to be determined by reference to the probabilities and possibilities including of both PBS listing and supply and private market supply.
210 However, there is an important difference between Alembic and Pharmathen (and Generic Health to the extent its claims relate to supply to Sigma and other generics) and the generics. Alembic and Pharmathen (and Generic Health to the extent its claims relate to supply to Sigma) did not have the rights associated with ARTG registration. The interlocutory injunction did not prevent them from exercising such rights. Their only opportunity was to supply under the contracts. As they accepted, they must prove some loss on the balance of probabilities. For the commercial opportunity represented by the existing contracts to have any value, they must prove on the balance of probabilities that they would have supplied some product or some additional product if not for the interlocutory injunctions. As they submitted, if some loss is proved on the balance of probabilities, the magnitude of the value lost is to be assessed as a matter of the probabilities and possibilities. To prove that they would have supplied some product or some additional product if not for the interlocutory injunctions on the balance of probabilities requires proof to that standard that the generics would have sold their products or more of their products if not for the interlocutory injunctions whether that be by supply on the private market or the PBS.
211 It will be apparent that the different opportunities of the generics and the manufacturers/suppliers (and Generic Health to the extent its claims relate to supply to Sigma and other generics) means that different considerations will apply to their claims of loss.
212 For those claims where there was no existing contract of supply (of Generic Health and Alembic in relation to Apotex, Sandoz and Ascent), the same conclusion must follow. Without proof on the balance of probabilities that there would have been some supply a loss of opportunity of some value has not been proved.
213 Wyeth submitted that this difference, which it seemed to accept was difficult to escape from given the principles identified in the authorities, exposed that the alleged losses of the manufacturers/suppliers were indirect consequential economic losses which are not or should not be recoverable under the undertakings for that reason alone. I will deal with this issue shortly.
Coherence of the above approaches
214 I consider that the different approaches discussed above are the result of the terms of the undertakings which are concerned with the adverse effect (if any) of the operation of the interlocutory injunctions. In all cases, the claimed adverse effect must be identified with precision. Once identified, if it is apparent that the claimed adverse effects are different, as I consider to be the case here, then a principled approach may well require different putative facts to be proved to different standards.
215 For the generics, if (as I consider) they held valuable rights by reason of ARTG registration which the interlocutory injunctions prevented them from exercising then loss is necessarily proved on the balance of probabilities, and the extent of the loss is to be assessed with reference to the possibilities and probabilities that the rights could be exploited for profit in the hypothetical future whether through private market or PBS supply.
216 For the manufacturers/suppliers, what is being claimed is the lost opportunity to supply venlafaxine. This opportunity depended on the generics actually going to market; a hypothetical fact relevant to causation which must be proved to the usual civil standard. If no generic would have gone to market irrespective of the interlocutory injunctions then no supply would have been made in any event so the loss could not be said to be caused by the injunctions. But if venlafaxine would have been supplied then the question becomes how much and at what price, and that depends on the possibilities and probabilities associated with private market or PBS supply.
Remoteness of damage
217 Wyeth contended that the losses claimed by the manufacturers/suppliers were not reasonably foreseeable and are too remote to support an order for compensation. I will consider the issue of reasonable foreseeability separately on the facts. In this part of the reasons I will deal only with issues of principle about remoteness of damage.
218 Wyeth’s essential contention was that the claimed losses of the manufacturers/suppliers did not “flow directly” from the interlocutory injunctions. The contention reflects the words of Aickin J in Air Express at 266-267 (see above) that the “view that the damages should be those which flow directly from the injunction and which could have been foreseen when the injunction was granted, is one which will be just and equitable in the circumstances of most cases and certainly in the present case”. As also noted above, the High Court endorsed this view in European Bank at [29], framing the second of the three necessary questions as “[d]id that loss flow directly from the [interlocutory] order?”
219 The concept of loss flowing directly from an interlocutory injunction reinforces the necessity of focusing on the allegedly adverse effects of the interlocutory orders alone, rather than the potentially adverse effects of the litigation. Wyeth’s submissions, however, suggest that the concept does more than this. I say “suggest” because Wyeth’s submission on this point were not straightforward. On one view, it was contending that claimable losses are confined to those losses suffered by the party against whom the interlocutory order was made. On another view, it was contending that claimable losses are confined to those losses suffered by a person who, if not a party, was bound by the interlocutory orders. Leaving aside the fact that it is arguable that the suppliers/manufacturers would have been bound by the orders not to arrange or facilitate the importation of the generic products into Australia (as to which see Warner-Lambert Company LLC v Apotex Pty Limited (No 2) [2018] FCAFC 26; (2018) 129 IPR 205), Wyeth’s contentions are inconsistent with the terms of the usual undertakings which Wyeth gave which refer to “any person, whether or not a party, adversely affected by any operation of the order below”. The interlocutory orders may have had a foreseeable and direct adverse effect on a person who was neither a party nor bound by the orders.
220 Wyeth’s final alternative submission focused on the elusive concept that the losses claimed by the manufacturers/suppliers were merely consequential upon the effect of the orders on the generics and thus could not be said to be losses flowing directly from the orders. The description of the manufacturers/suppliers’ losses as consequential is accurate. If the generics are right that the orders prevented them from supplying their products when they otherwise would have done then the orders necessarily had consequential impacts on the manufacturers/suppliers. But this description does not answer the question whether the claimed loss of the manufacturers/suppliers flowed directly from the interlocutory orders or are too remote to be the subject of a just order for compensation.
221 Other causal metaphors have been used to describe the requisite relationship between the interlocutory orders and the claimed loss. As noted above, both Gibbs J and Mason J in Air Express (at 312 and 323 respectively), referring to Smith v Day (1882) 21 Ch D 421, framed the causal requirement in terms of loss which is the natural consequence of the injunction. The concept of “natural” loss, as explained in European Bank at [13], is derived from the law of contract and focuses attention upon damages “as arise naturally, that is, according to the usual course of things, from the breach of contract, or such damages as may reasonably be supposed to have been in the contemplation of both parties concerned at the time they made the contract as the probable result of the breach”.
222 It is apparent that losses which may fairly be described as unexpected, unanticipated or out of the ordinary course of business given the circumstances as they existed when the interlocutory injunctions were granted or which would depend on future negotiations have been held to be too remote. Aickin J reviewed a number of cases giving examples of this principle in Air Express. Accordingly, in Smith v Day the lost benefit of a potential lease was held to be too remote. The lease would have been over part of a building that an injunction prevented from being built as planned but it was still being negotiated when the injunction was granted. Even if the lease had existed when the injunction was granted, the lessee would not ordinarily have required a building of the kind the injunction prevented. At 428 Brett LJ said:
If any one obtains an injunction preventing another from proceeding with a building, he must be taken to have notice of everything in the building contract, and all liabilities which the person stopped incurs to his contractor by reason of the stoppage, are a natural and immediate consequence of the injunction. But the fact that the injunction prevents the carrying out of an entirely independent agreement as to the property is too remote.
223 At 430 Cotton LJ said:
It is not proved that there was a binding agreement to take a lease, and I agree with Lord Justice Brett that if there had been one, damages could not be recovered on that ground. I think that the damages must be confined to loss which is the natural consequence of the injunction under the circumstances of which the party obtaining the injunction has notice, as for instance a claim by the builder in consequence of the injunction compelling the Defendant to break his contract with him.
224 In Ex parte Hall; In re Wood (1883) 23 Ch D 644 it was held that damages could not be claimed for wrongful acts of a receiver outside of the scope of restraining orders, even though the wrongful acts could not have occurred but for the restraining orders.
225 In Re an Arbitration between Pemberton and Cooper (1912) 107 LT 716 two farmers claimed on an undertaking for the lost profits they would have gained from ploughing up pasture land and planting corn, which an interlocutory injunction sought by their landlord restrained, and for the deterioration in a flock of sheep they kept on the pasture land instead of allowing the land to go derelict. At 718 Banks J held that both kinds of loss “necessarily and naturally flowed from the course which the landlord compelled them to adopt”.
226 Reasoning by analogy, to the extent that the claims of the manufacturers/suppliers are based on contracts for supply of the products to Sigma, Alphapharm and Generic Health which existed before the interlocutory injunctions were granted, I am unable to characterise the claimed loss from the generics not having ordered products as anything other than loss which is the direct and natural consequence of the interlocutory orders which prevented supply. The products as supplied to the generics were the complete products in packages ready for supply to pharmacists. The fact of supply to the generics or not was a natural and ordinary consequence of and directly related to their sales to pharmacists. If restrained as they were by the interlocutory injunctions, it was inevitable that the generics would not place orders with their suppliers.
227 However, to the extent that Generic Health and Pharmathen claimed for loss of the opportunity to supply other generics with the products pursuant to potential contracts which did not exist at the time the interlocutory injunctions were granted and which would have depended on future negotiations, the position is different. Even if those contracts would have existed at an earlier time but for the injunctions, the loss was not a direct or natural consequence of the injunctions. The putative contracts remained dependent on the future negotiations and the unrestricted choices of Generic Health and those other generics.
228 I will explore the facts relating to these claims in greater detail separately but, for present purposes, it is sufficient to say that the cases indicate that alleged losses of this kind are too remote to be recoverable.
Adverse effect of the interlocutory orders
229 There is another aspect of the matter which should be considered. It is that while Sigma has claimed under the Sigma interlocutory injunction alone, Alphapharm and Generic Health have claimed under each of the interlocutory injunctions. To the extent that Generic Health was Sigma’s supplier of the Sigma products, this makes sense. Otherwise, the claims are of a different kind. The claims are to the effect that the interlocutory injunctions which were granted but which did not bind them caused Alphapharm and Generic Health to anticipate that they too would be the subject of an interlocutory injunction by Wyeth and modified their conduct accordingly, as a result of which they suffered loss.
230 I do not accept that action taken in mere anticipation of an interlocutory injunction application can engage the undertaking which in terms concerns only whether a person has been “adversely affected by any operation of the order”. An order cannot operate before it is made. Things done in mere anticipation of an interlocutory injunction application are part of the ordinary exigencies of the existence of litigation and losses which result are therefore not within the scope of the undertaking. Nor do I accept that a person merely anticipating an interlocutory injunction because an interlocutory injunction has already been granted againt another person can be an adverse effect of the operation of that earlier granted interlocutory injunction.
231 This said, the fact that the Sigma interlocutory injunction was granted first, the Alphapharm interlocutory injunction second, and the Generic Health interlocutory injunction third raises a different issue. Is it necessary or appropriate to disregard the grant of Sigma interlocutory injunction when considering Alphapharm’s position had the Alphapharm interlocutory injunction not been granted? And is it necessary or appropriate to disregard the grant of Sigma and Alphapharm interlocutory injunctions when considering Generic Health position had the Generic Health interlocutory injunction not been granted?
232 In my view, these two questions must be answered yes, as otherwise it is not practically possible to construct consistent hypotheses of what would or might have occurred in any case. For example, if when considering Sigma’s position it is taken that Sigma would not be subject to the Sigma interlocutory injunction but when considering Alphapharm’s position it is taken that Sigma was subject to an interlocutory injunction, then the inevitable consequence is that the hypothesised market for Alphapharm is distorted from the outset. The hypothesised market on this latter approach would contain only Alphapharm when, in fact, it is known that Sigma was and would have been the first to market.
233 The parties all assumed this approach to be necessary (correctly in my view) but it has practical consequences. It means that the initial probabilities and possibilities are fixed by Sigma’s position because it was first to obtain ARTG registrations and first to the market. What Alphapharm and then Generic Health would or might have done is to be assessed by reference to the probabilities and possibilities for Sigma. Accordingly, if there are two possibilities for Sigma from any time, private market supply or PBS supply, which have different probabilities of having occurred, then the probabilities and possibilities for Alphapharm and then Generic Health should be assessed by reference to both possibilities for Sigma. This approach does not mean, however, that conduct in mere anticipation of litigation or even an interlocutory injunction may be treated as an adverse effect within the scope of the undertakings given that the undertakings are confined to the adverse effect of the interlocutory orders themselves.
Interlocutory injunctions not wrongly granted?
234 Wyeth submitted that because it succeeded at first instance it could not be said the interlocutory injunctions were wrongly granted and that:
If the result of the trial justifies the making of the interlocutory injunction (and assuming there is no separate challenge to the initial process of making the injunction), the injunction will not have been wrongly granted in the relevant sense and damages will not be payable under the undertaking.
235 Sigma described this submission as “startling”. I agree. It is without merit and not much needs to be said about it.
236 The applicable principle in this regard is identified in Abbey Forwarding Ltd (in liq) v HM Revenue & Customs [2015] EWHC 225 (Ch); [2015] Bus. L.R. 882 at [85]:
In determining whether the order was wrongly made, the court uses the full benefit of hindsight and asks itself in effect whether in the light of subsequent events it can be seen that the order was wrongly made. The court is not asking itself whether the order was wrongly made in the sense of whether, on the evidence and submissions available at the time, the judge making the order was then wrong to do so. Nor is an order for an inquiry in any sense dependent on the existence of any fault on the part of the applicant at the time. It is a matter of justice that where there has not been a final determination of the matters on which the order is made the applicant should compensate the respondent in the event that it transpires that the order was wrongly made.
237 The “full benefit” of hindsight in the present case includes the Full Court’s decision that the method patent was invalid. The question whether an interlocutory injunction was wrongly granted is not answered by reference to the outcome of the trial if, on appeal, orders are made setting aside the orders at first instance. The orders made on appeal are then the orders of the Court. There can be no doubt that, having regard to the Full Court’s decision, all of the interlocutory injunctions were wrongly granted because they effectively assumed the method patent was valid when it was invalid.
The effect of the final orders
238 On 8 November 2010 I made final orders. In the Sigma proceedings I made orders 6 and 7 that:
6. Orders 1 and 2 made on 25 August 2009 be discharged and the Respondents / Cross-Claimants be released from the undertakings given by them to the Court in relation to those orders, being undertakings:
…
7. The Applicant / Cross-Respondent (whether by itself, its directors, servants, agents or otherwise) be restrained from infringing claims 1, 4, 5, 8, 9, 10, 15, 16 and 27 of the Patent, including, without limitation, by, in Australia, during the term of the Patent and without the licence or authority of the First Respondent / First Cross-Claimant:
(a) importing, marketing, taking orders for, selling, supplying or offering to supply the Evelexa XR Products (or any other product comprising the same generic extended release formulation of venlafaxine hydrochloride) for use by persons in need of venlafaxine;
…
239 In the Alphapharm and Generic Health proceedings I also made orders 6 and 7 to the same effect as orders 6 and 7 in the Sigma proceeding, albeit referring (as relevant) to the Alphapharm and Generic Health interlocutory injunctions and to Alphapharm and Generic Health.
240 On 21 December 2011 the Full Court, amongst other things, set aside order 7 in each proceeding and set aside order 6 “in so far as it releases the respondents from the undertakings as to damages given by them to the Court on” the relevant dates: Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 3) [2011] FCAFC 165.
241 The issues in dispute are sufficiently exposed through Wyeth’s contentions as follows:
(1) the only relevant periods of time for assessing whether or not the claimants have established loss or damage are the periods of the interlocutory injunctions which ended on 8 November 2010;
(2) any loss suffered by the claimants during the period of operation of the final injunctions was not a loss that could be said to “flow directly” from the operation of the interlocutory injunctions, but was loss caused by the final injunctions;
(3) the terms of the interlocutory injunctions are clear. In particular, the words “pending the determination of the proceeding” mean the first instance proceeding and not the appeal proceeding as submitted by the claimants;
(4) given the purpose of an interlocutory injunction and the associated undertaking as to damages, it would be wrong in principle to infer, speculate or assume that but for the interlocutory injunctions the generics would or might have obtained a stay of the final injunctions so that the claimed adverse effect of the interlocutory injunctions may be said to have continued after 8 November 2010;
(5) alternatively, the generics would not have obtained a stay because Wyeth would have been entitled to the fruits of its victory, the generics’ infringing conduct would have been undertaken with their “eyes wide open” to the risk that they would be restrained on a final basis if they failed at the trial, and as the Court found when rejecting Alphapharm’s application for a stay, a stay would have permitted other generics to enter the market: Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 2) [2010] FCA 1212; (2010) 88 IPR 633 at [35]; and
(6) the generics’ evidence about applying for a stay did not consider that they would have had to “be prepared to assume liability for the damage that could be caused to Wyeth not only by their own conduct during the period of any stay, but also by the conduct of each other and any other generic suppliers in the venlafaxine market during that period”.
Discussion
242 I will deal with the construction issues first.
243 The interlocutory injunctions were each made “[p]ending the determination of the proceeding or further order”. Wyeth’s undertakings operate in relation to any person “adversely affected by any operation of the order below or any continuation (with or without variation) thereof”.
244 One argument the generics put is that “the proceeding” includes any appeal so that they are entitled to claim compensation under the undertakings up to the date on which the High Court rejected Wyeth’s application for special leave to appeal.
245 I disagree. It is not to the point that s 4 of the Federal Court of Australia Act 1976 (Cth) defines “proceeding” as follows:
proceeding means a proceeding in a court, whether between parties or not, and includes an incidental proceeding in the course of, or in connexion with, a proceeding, and also includes an appeal.
246 The relevant issue is the meaning of the interlocutory injunctions which is to be considered having regard to the purpose of an interlocutory injunction. As Wyeth submitted, the only reason interlocutory injunctions exist is because it is not always possible for a court to make final orders on the day that a proceeding is commenced. If, for example, it had been possible for Sundberg J to hold a final as opposed to an interlocutory hearing on 22 May 2009, then there would have been no interlocutory injunction against Sigma and no undertaking from Wyeth. The final orders would have had effect pending an appeal. If the final orders were wrong, Sigma could have had no claim against Wyeth for the effect of the final orders. Final orders which are subsequently overturned on appeal are part of the exigencies of litigation for which there is no remedy except for costs.
247 Construed against this background, the generics’ construction of the interlocutory injunctions is not open. “Pending the determination of the proceeding or further order” meant nothing more than pending the grant or refusal of final relief. This occurred on 8 November 2010. It is apparent that Sigma, Alphapharm and I assumed this to be the case in Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 2) [2010] FCA 1212; (2010) 88 IPR 633 when I said this:
[10] … Accordingly, if Sigma and Alphapharm succeed in the foreshadowed appeals the undertaking as to damages Wyeth gave in respect of the interlocutory injunctions will operate in respect of loss by reason of those interlocutory orders, but no such undertaking will be in place in respect of loss by reason of the final orders pending the appeal.
[11] Wyeth also refuses to undertake not to market and sell a generic extended release venlafaxine hydrochloride product of its own in Australia. Sigma and Alphapharm, in these circumstances, are concerned that they will be exposed to a change in position from the time at which they were enjoined (when there were no generic extended release venlafaxine hydrochloride products on the market, with Sigma being the first and Alphapharm the second to obtain ARTG registration). In short, they contend that without undertakings or orders to protect them from a hiatus in Wyeth’s undertaking as to damages and what may be described as a change in the circumstances in which they otherwise would have entered the market except by reason of the interlocutory injunctions against them, they are exposed to an injustice which the Court should not permit.
…
[23] Continuing the interlocutory injunctions and refusing to release Wyeth from its undertakings will not assist because Wyeth’s undertakings relate only to the consequences of the interlocutory orders.
248 If the interlocutory injunctions and undertakings operated as the generics now contend then the argument before me could not have been that there would be an hiatus in Wyeth’s undertaking as to damages.
249 It seems that the Full Court must have been operating on the same assumption because the Full Court’s orders of 11 November 2011 set aside my order 6 only to the extent that it released Wyeth from the undertaking as to damages. My order 6 in each proceeding discharged each interlocutory injunction. If the generics’ submissions are right, the interlocutory injunctions should never have been discharged at all. Further, given that my order 6 in each proceeding discharging the interlocutory injunctions has never been set aside, it is not clear how the generics can maintain that those orders continued after that date.
250 Contrary to the generics’ submissions nothing the Full Court said in Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 3) [2011] FCAFC 165 should be understood as suggesting to the contrary. The Full Court disagreed with the substantive conclusions I reached and disagreed with my view that, given the discharge of the interlocutory injunctions, Wyeth should be released from its undertakings. It also disagreed (as do I) with Wyeth’s proposition that there could be no claims on the undertaking merely because I made final orders in Wyeth’s favour. This is Wyeth’s argument to the effect that because I made final orders in Wyeth’s favour the interlocutory injunctions were not wrongly granted and could not be called upon irrespective of my final orders being overturned on appeal. It is this argument that the Full Court said was unsupported by authority: at [13]. It is this argument I also have rejected above.
251 The Full Court at [10]-[14] was not suggesting that the interlocutory injunctions could or did operate after 8 November 2011. The Full Court’s point was only that the order releasing Wyeth from its undertakings had to be set aside so they “may operate in accordance with their terms”; at [14]. The terms of the undertakings relate to any operation of the order below or any continuation (with or without variation) thereof” and the “order” in question is the interlocutory injunction in each case which operated pending the determination of the proceeding. It is consistent with the Full Court’s reasoning and orders that the “proceeding” means the first instance proceeding only.
252 As Hely J succinctly put it in Red Bull Australia Pty Limited v Sydneywide Distributors Pty Limited t/as Sydneywide Bottlers Australia [2001] FCA 1750 at [9]:
The undertaking is required in the case of an interlocutory injunction, as interlocutory relief is granted on the basis that there is a triable issue, before any determination of the merits: American Cyanamid Co v Ethicon Ltd [1975] AC 396, 406, 409.
253 Beecham Group Ltd v Bristol Laboratories Pty Ltd [1968] HCA 1; (1968) 118 CLR 618 at 623 is not authority to the contrary. The High Court there said:
It is of course to be remembered that if an injunction be granted it will be upon terms of the plaintiff submitting, in the event of his ultimately failing, to such order as to damages as the Court may make in order to compensate the defendant for any injury caused by the injunction
254 The word “ultimately” here does not support the generics’ argument. It means only a determination on the merits.
255 Another argument that the generics put is that my order 7 in each matter made on 8 November 2010 was a “variation” of the interlocutory injunction orders which thereafter continued as varied. Again, I do not consider this construction to be open. The final interlocutory injunctions were new orders, not a variation or continuation of the interlocutory orders. The interlocutory injunctions were not varied and did not continue. I discharged them. Once a matter has been heard and determined, there is no scope for the continuation of interlocutory orders pending that determination. This is so irrespective of the determination. Parties may agree or the Court may decide that other interlocutory orders should be made, for example pending the determination of an appeal, but whatever the form those orders take they are new orders and any undertaking given in exchange for those new orders would be a new undertaking.
256 Sigma submitted this:
The authors of Spencer, Bower and Turner “The Doctrine of Res Judicata” [2nd Ed] note at p.59 that, where an appellate tribunal reverses the order of the court of first instance, and refuses the relief granted below, the former decision, until then conclusive, disappears altogether, and is replaced by the appellate decision.
Accordingly, the effect of the Full Court’s order was as if Jagot J had not discharged the undertaking on 8 November 2010 nor granted the permanent injunction on that day in lieu of the interlocutory injunction granted on 3 June 2009.
257 The submission proceeds on the false premise that the Full Court set aside my orders discharging the interlocutory injunctions. It did not. The terms of the Full Court’s orders in this regard are clear. The orders were:
Sigma proceeding
3. Order 6 made by the primary judge on 8 November 2010 in the Sigma Proceeding be set aside in so far as it releases the respondents from the undertakings as to damages given by them to the Court on 3 June 2009.
Alphapharm proceeding
3. Order 6 made by the primary judge on 8 November 2010 in the Alphapharm Proceeding be set aside in so far as it releases the respondents from the undertakings as to damages given by them to the Court on 25 August 2009.
Generic Health proceeding
3. Order 6 made by the primary judge on 8 November 2010 in the Generic Health Proceeding be set aside in so far as it releases the respondents from the undertakings as to damages given by them to the Court on 10 November 2009.
258 The inescapable fact is that the interlocutory injunctions were discharged on 8 November 2010. They were not revived by the Full Court. The Full Court’s orders are consistent with the orthodox principle that once final relief is determined at first instance the function of an interlocutory injunction pending the determination of the proceeding is exhausted. The interlocutory injunctions also would have been discharged if the generics succeeded before me. The generics did not succeed until the Full Court’s decision, the consequence of which was that the final orders I made had effect until the Full Court’s orders. Apart from the stay issue discussed next, it is not suggested that the interlocutory injunctions had some adverse effect after 8 November 2010. If the final injunctions had not been made, it is possible to conceive of circumstances in which the discharged interlocutory injunctions continued to have an adverse effect after 8 November 2010. But that is not what occurred.
259 The generics’ other argument, based on the posited stay, involves different considerations. The argument is that if not for the interlocutory injunctions they would have been selling their products on the market so when they came to apply for a stay of the final orders I made on 8 November 2010 the relevant status quo would have been different from what it was when, in fact, Alphapharm applied for such a stay before me (which I refused in Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 2) [2010] FCA 1212; (2010) 88 IPR 633) and Sigma applied for a stay before Jacobson J pending the determination of Sigma’s appeal which he also refused: Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2010] FCA 1258. The argument is that the operation of the interlocutory injunctions had an adverse effect on the generics within the meaning of Wyeth’s undertakings in that the generics were not supplying their products which deprived them of the opportunity to apply for a stay on the basis of a different status quo from the status quo in fact.
260 I do not consider this kind of lost opportunity can or should be the subject of an order for compensation.
261 No such lost opportunity was pleaded.
262 Further, as a matter of principle, the kind of adverse effects which may result in compensable loss are those which were reasonably foreseeable at the time the interlocutory orders were made and which may fairly be characterised as the direct and natural consequence of the operation of the interlocutory orders. The adverse effects which the generics are claiming in this respect, in my view, cannot fairly be characterised as the direct and natural consequence of the operation of the interlocutory orders. To explain, as will be apparent, I accept that a direct and natural consequence of the operation of the interlocutory orders was that the generics could not supply their products. I have concluded that it is highly likely that, but for the interlocutory injunctions, the generics would have supplied their products on the private market. It follows from what I have said that it was a direct and natural consequence of the operation of the interlocutory orders that the generics were not supplying their products as at 8 November 2010. It is after this point that, in my view, the steps in the chain of reasoning break down.
263 I do not consider that it can be said that a direct and natural consequence of the operation of the interlocutory orders was that the generics were deprived of an opportunity to obtain a stay that they might otherwise have obtained. There are simply too many other imponderable factors in play to reach this conclusion. The grant of a stay involves an exercise of judicial discretion in all of the relevant circumstances. The need to speculate about what I might have done or Jacobson J might have done or some other hypothetical judge might have done discloses that we are dealing with claimed loss of a kind which might arise other than as a direct and natural consequence of the interlocutory orders. The claimed loss depends on a contingency, the grant of a stay of final orders, which depends on a decision of a judge. The case is not like Air Express in which the posited decision of the Secretary of the relevant department was itself the subject of the interlocutory order.
264 I am not even persuaded that loss of the kind claimed would have been reasonably foreseeable at the time the interlocutory injunctions were granted. On what basis could Wyeth have foreseen that if it succeeded before me and obtained final orders the generics would be able to claim that as a result of the interlocutory injunctions (i) they lost the opportunity to supply their products; (ii) but for the interlocutory injunctions they would have been supplying their products; (iii) had they been supplying their products they would have applied for and obtained a stay of the final orders so that they could continue to supply their products despite it having been found that in so doing they were and would be infringing the method patent (which are necessary aspects of this hypothesis); and (iv) as a result, Wyeth’s undertaking would extend to the value of the lost opportunity to obtain a stay of the final orders? To my mind, the steps in this asserted causal chain indicate that this kind of alleged loss is outside the boundaries of reasonably foreseeable kinds of loss.
265 Nor do I consider that rational speculation about the generics’ prospects of having obtained such a stay is possible. Their arguments to the effect that fact of supply would have involved a different status quo which the Court would then have preserved by ordering a stay are deceptively simple. The reality is more complex.
266 For example, to foreshadow some matters dealt with later in these reasons, the conclusions I have reached will involve compensating the generics for their lost opportunity of supply on the hypothesis that they alone would or might have been supplying generic venlafaxine products to pharmacists before the Full Court’s decision. It is apparent that the prospect of other generics being able to enter the market if the claimant generics were not restrained was an important factor to both the grant of the interlocutory injunctions and the refusal of the applications for a stay. In Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 2) [2010] FCA 1212; (2010) 88 IPR 633 at [35] I noted that if the stay Alphapharm sought was granted then Generic Health and other generics would be able to enter the market and the undertaking Alphapharm offered was confined to loss Wyeth might suffer as a result of Alphapharm being able to sell its products and did not extend to other loss Wyeth might suffer from other generics being able to enter the market. The evidence is no different at the present time about what would or might have occurred if Alphapharm had been supplying its products. That is, the evidence does not suggest that Alphapharm might have been willing to offer an undertaking of any broader scope than that which it in fact offered. If this is so it is by no means certain or almost certain, as the generics would have it, that they would have obtained a stay if they had been supplying their products. And if an allowance was made for this possibility, it would also be necessary to factor that consideration into the resolution of the prospect of supply by other generics (which I have not done). The compensation to the generics would thus be reduced because I have accepted that the size of the market would have remained the same and, on the necessary hypothesis this approach requires, the other generics would be inferred to be in the market taking their market share from all other market participants.
267 Further, Sigma’s position in both Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (No 2) [2010] FCA 1212; (2010) 88 IPR 633 and Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2010] FCA 1258 is another confounding factor. Sigma, apparently recognising that Wyeth’s undertaking did not operate after 8 November 2010, sought a regime in which Wyeth would undertake not to de-list Efexor-XR (which Wyeth was willing to do) and would undertake not to release its own generic product (which Wyeth was not willing to do) and Sigma would give an undertaking as to damages but only in exchange for Wyeth giving a fresh undertaking as to damages for the period up to determination of the appeal. Wyeth refused to do so. See [2010] FCA 1212 at [35] and [2010] FCA 1258 at [2], [10], [15], [21], [27] and [31]. It is apparent that Sigma was never willing to offer an unconditional undertaking as the price of a stay. Why would it be inferred, speculated or assumed that Sigma’s position would or might have been any different had it been supplying its products and was seeking a stay of the final orders on that basis? Again, this is not confronted in Sigma’s evidence but undermines the submission that it would have been certain or almost certain that Sigma would have obtained a stay if it had been supplying its products.
268 Generic Health’s position is even more difficult. It did not seek a stay of the final orders. The idea that it would have been willing to give an undertaking as the price of a stay is irreconcilable with all of its conduct. Its evidence does not suggest any chance existed that it would have been willing to give the kind of undertaking that would have been required as the price of a stay.
269 All of these matters may be another way of saying that I am unable to escape the conclusion that the generics’ arguments would have the effect of compensating them for the fact that final orders were made against them which were subsequently overturned on appeal. Wrongly granted final orders are part of the exigencies of litigation. The undertaking required to obtain an interlocutory order does not extend to compensation for the adverse effects of the litigation, yet the effect of what the generics seek is compensation for the effect of my final orders. While characterised by the generics as a loss of an opportunity to obtain a stay (which was not a pleaded loss of opportunity in any event), it is the operation of the final orders which caused the generics loss after 8 November 2010. I am unable to see how it could be just to impose liability for those losses upon Wyeth.
270 Generic Health submitted that:
The prospect of the trial judge being overturned on appeal is and was an ordinary incident of litigation; it was part and parcel of the risks that Wyeth took on when it gave the undertakings. By giving the undertakings, Wyeth obtained court orders which prevented the generics from entering the market up to the decision at trial, irrespective of what that decision was. Moreover, by obtaining that relief and keeping the generics from the market in that period, it increased the prospect that irrespective of the decision at first instance, it would still be able to keep the generics from the market up to resolution of the appeal. If the first instance decision was against Wyeth, the fact that the generics had not entered the market would tend in favour of a continuation of the interlocutory injunctions pending the appeal. If the first instance decision was in favour of Wyeth, the fact that the generics had not entered the market would tend against a stay of the final injunction. In other words, irrespective of the outcome at trial, the status quo would be no generic entry and the likelihood is that the status quo would be maintained up to resolution of the appeal.
Having obtained a benefit which extended beyond trial and all the way to the resolution of the appeal, it is not just and equitable to restrict the quid pro quo given by Wyeth to the outcome at first instance.
271 In my view, this submission exposes that the generics are seeking compensation for the effect of the wrongly granted final orders when their claims must be confined to the adverse effect of the operation of the interlocutory injunctions. In particular, Wyeth did not undertake to pay compensation if the Court’s final orders at first instance turned out to be wrong. That was not part of the risks Wyeth took on when it gave the undertakings. It is not the case that the interlocutory injunctions might have continued if Wyeth had failed at first instance. If Wyeth had failed it could have sought interlocutory injunctions pending the determination of the appeal but to do so it would have had to offer undertakings as the price for those interlocutory orders. It could have decided if it was willing to pay that price in all of the circumstances at the time. But whatever the form of the order, in substance, it would not have involved a continuation of the existing interlocutory injunctions or of the existing undertakings. Any benefit Wyeth obtained from the operation of the interlocutory injunctions which extended beyond 8 November 2010 was subsumed by the final orders.
272 As a result, in the present case no compensation should be ordered for the period after 8 November 2010.
Some further matters relating to proof
273 To the extent that the evidence concerned what people would have done had circumstances been different from what they were, some general observations apply.
274 In another context, dealing with people’s attempts to recall what was said when the outcome of litigation might depend on the precise words used, McLelland CJ in Equity in Watson v Foxman (1995) 49 NSWLR 315 at 319 said:
…human memory of what was said in a conversation is fallible for a variety of reasons, and ordinarily the degree of fallibility increases with the passage of time, particularly where disputes or litigation intervene, and the processes of memory are overlaid, often subconsciously, by perceptions of self-interest as well as conscious consideration of what should have been said or could have been said. All too often what is actually remembered is little more than an impression from which plausible details are then, again often subconsciously, constructed. All this is a matter of ordinary human experience.
275 The wisdom of these observations has been repeatedly proved.
276 What then does ordinary human experience tell us about people’s attempts to give evidence, on which litigation may depend, not about what they recall, but about what they say they would have done in circumstances different from those which actually occurred? In Asden Developments Pty Ltd (in liq) v Dinoris (No 3) [2016] FCA 788 Reeves J summarised the observations from the authorities in these terms:
[156] A plaintiff’s subjective evidence about what he or she would, or would not, have done if the negligent conduct in contention had not occurred is now inadmissible under the Civil Liability legislation in force in many jurisdictions in Australia: see s 5D(3)(b) of the Civil Liability Act 2002 (NSW); s 11(3)(b) of the Civil Liability Act 2003 (Qld); s 5C(3)(b) of the Civil Liability Act 2002 (WA); and s 13(3)(b) of the Civil Liability Act 2002 (Tas). The hindsight bias inherent in such evidence was one of the main reasons underpinning the introduction of these provisions. So much appears from the Final Report of the Review of the Law of Negligence (Commonwealth of Australia, Canberra, September 2002), where the authors observed (at para 7.40):
The enormous difficulty of counteracting hindsight bias in this context undermines the value of such testimony. In practice, the judge’s view of the plaintiff’s credibility is likely to be determinative, regardless of relevant circumstantial evidence. We therefore recommend that in determining causation, any statement by the plaintiff about what they would have done if the negligence had not occurred should be inadmissible.
(Emphasis added)
[157] Similar reservations about the value of such evidence have been expressed in medical negligence cases involving a doctor’s failure to warn a patient about the risks of surgery. For example, in Rosenberg v Percival (2001) 205 CLR 434; [2001] HCA 18 (Rosenberg), Callinan J observed (at [221]):
It is perfectly understandable that a person who has suffered what the respondent suffered here would say, and might also even have come to believe implicitly that she would not have had the operation had she known of the risk which has in fact materialised. That would usually be, and it probably was here an honest belief on the part of the respondent at the time that she gave her evidence. However, the true position is much more likely to be, no matter what a plaintiff may have honestly come to believe, that she cannot really say, in an absolute way, that she would have not had the operation. The much more likely position is that perhaps she might not have.
See also [15]–[17] per Gleeson CJ; [44]–[45] per McHugh J; [86]–[87] per Gummow J; and [157]–[158] per Kirby J.
277 These cautionary observations have particular significance for the assessment of evidence about asserted past hypothetical facts in the present matter. Irrespective of the credibility of any witness the following matters must be acknowledged.
278 The hearing was in 2018. The witnesses were being asked about what they think they would have done or would have occurred between 2009 and 2011. The amount of time which has passed is a sufficient reason, of itself, to cast significant doubt on the reliability of the evidence no matter how honest and careful the individual witness.
279 For reasons which need not be identified here, this matter took a long time to get to hearing. Many of the affidavits in which the witnesses gave their principal evidence date from 2015 and 2016. Even the period from 2009 to 2015/2016 would exceed the bounds of ordinary human recall beyond generalities and exceptional events. And the period between 2015 and 2018 itself might be sufficient to infer that what was most likely occurring was some witnesses were giving evidence about what they thought they had said in their affidavits (and, even then, it was unsurprising that a few witnesses had lost the logical underpinnings of their own affidavit by reason of the passage of time).
280 To add to the overall likelihood of such evidence being inherently unreliable, the key affidavits were all based on alternative scenarios which had been expressed in a form which managed to be simultaneously general, de-contextualised and extraordinarily compressed. For example, Sigma witnesses were asked “what Sigma would have done if the Federal Court refused to grant an interlocutory injunction against Sigma”. Wyeth witnesses were asked the same question. But no context, either of time or circumstance, qualified the questions, leaving the witness to fill in or assume the context, presumably from their own memory (as it existed in 2015 or 2016) of the situation in 2009. This observation involves no criticism of those who posed the questions or of the witnesses who answered. It is simply that the task which was given to the witnesses was artificial in the extreme and fraught with the risk of hindsight being brought to bear even from the most scrupulous and cautious of witnesses.
281 Other aspects of ordinary human experience must be factored into the equation. There are many kinds of pressures to which a person may be subject in giving evidence, whether consciously or not. These pressures are likely to hold less sway if the person is being asked to recall what happened. When a person is being asked instead to conjecture what would have happened, the constraints imposed by the usual nature of a witness’s task, to recall things, are removed. When this is combined with the magnitude of the claims in this case it does not take much to infer that a more reliable guide to what would or might have happened is inference from available contemporaneous material and objective contemporaneous circumstances assuming rational commercial decisions rather than the evidence of witnesses speaking many years later in the context of litigation.
282 For the witnesses called by the generics, there was an added risk. For the generics, the expiry of the compound patent on 6 December 2008 presented a rare opportunity. Venlafaxine was PBS listed and the biggest selling anti-depressant in Australia worth more than $100 million a year to Wyeth. Before they became aware of the method patent, the generics planned to seek PBS listing after ARTG registration and to supply their products on the PBS. They had each expended significant amounts in order to fulfil this intention. Then they discovered the method patent and their plans were thrown into chaos. They all received some kind of legal advice, of varying degrees of formality, which, whatever its details and howsoever it may ultimately be characterised, gave them reason to believe that the method patent might well be invalid. They were each the subject of an interlocutory injunction which prevented them from supplying their products until 8 November 2010 when I granted the final injunctions. It was not until 28 October 2011 and the Full Court judgment that the generics knew they would be able to supply their products albeit that Wyeth’s application for special leave to the High Court remained for determination. From 28 October 2011, in effect, the generics were vindicated. The method patent was invalid. They had been held out of one of the apparently most valuable generic opportunities of its time for more than two years. As a result, from 28 October 2011 until the date the witnesses provided their affidavits, they knew of their ultimate vindication.
283 In these circumstances, the generics having each planned to seek PBS listing and supply under the PBS before they became aware of the method patent and the witnesses having known the ultimate vindication achieved on 28 October 2011, to ask the witnesses to project themselves backwards to 2009 or 2010 and say what they would have done or recommended but for the interlocutory injunctions is to invite inherently unreliable evidence, tainted by hindsight. To say now what the person would have done and recommended is effectively risk free. And, as noted, apart from the extent to which witnesses could recall what was going on at the time, the evidence is virtually context free. But at the time in 2009, acting and recommending would have occurred in highly specific and ever changing circumstances and would have carried all kinds of potential risks and rewards, not just the risk of a 12.5% automatic price reduction of Efexor-XR for which the generics assumed they would be liable if they were the first to obtain PBS listing and the method patent was valid. Many years later, insofar as liability for patent infringement from mere PBS listing alone is concerned, this assumption would be falsified by the decision in Warner-Lambert Company LLC v Apotex Pty Limited [2017] FCAFC 58; (2017) 249 FCR 17 but that is beside the point. The assumed exposure existed at the relevant time.
284 These observations underpin my approach to much of the evidence about alleged past hypothetical facts.
285 There is another observation which it is convenient to make now. In Les Laboratoires Servier v Apotex Inc [2008] EWHC 2347 (Ch); [2009] FSR 220 Norris J said this at [9]:
…whilst it is for Apotex [the generic] to establish its loss by adducing the relevant evidence, I do not think I should be over eager in my scrutiny of that evidence or too ready to subject Apotex' methodology to minute criticism.
That is so for two reasons, quite apart from an acceptance of the proposition that the very nature of the exercise renders precision impossible. (a) Whilst, in order to obtain interlocutory relief, Servier will not have had to persuade Mann J. that it was easy to calculate Apotex’ loss in the event of the injunction being wrongly granted, it will have had to persuade him that that task was easier than the calculation of its own loss in the event that the injunction was withheld. The passages I have cited from its skeleton argument and evidence show that it did so. Having obtained the injunction on that footing it does not now lie in Servier’s mouth to say that the task is one of extreme complexity and that the court should adopt a cautious approach. Having emphasised at the interlocutory stage the relative ease of the process, it should not at the final stage emphasise the difficulty. (b) In the analogous context of the assessment of damages for patent infringement, in General Tire and Rubber Co v Firestone Tyre and Rubber Co Ltd (No.2) [1975] 1 WLR 819; [1975] FSR 273; [1976] R.P.C. 197 HL at 212 Lord Wilberforce said:
“There are two essential principles in valuing the claim: first, that the plaintiffs have the burden of proving their loss: secondly, that the defendants being wrongdoers, damages should be liberally assessed but that the object is to compensate the plaintiffs and not to punish the defendants.”
The principle of “liberal assessment” seems to me equally applicable in the present context. Although a party who is granted interim relief but fails to establish it at trial is not strictly a “wrongdoer”, but rather one who has obtained an advantage upon consideration of a necessarily incomplete picture, he is to be treated as if he had made a promise not to prevent that which the injunction in fact prevents. There should as a matter of principle be a degree of symmetry between the process by which he obtained his relief (an approximate answer involving a limited consideration of the detailed merits) and that by which he compensates the subject of the injunction for having done so without legal right…
286 It must immediately be acknowledged that it is not the law in Australia that compensation under this kind of undertaking is assessed as if the undertaking contained a contractual promise not to prevent that which an interlocutory injunction presents. Nor is Wyeth a wrongdoer in any sense and, as Barwick CJ noted in Air Express at 310, it is no part of the process to discourage persons from invoking the jurisdiction of the Court including to seek interlocutory relief. Wyeth had the method patent. It was entitled to exercise rights under it just as the generics were entitled to challenge the method patent’s validity. But the fact that Wyeth had the benefit of the interlocutory injunctions based on what it believed would happen if it did not obtain them and the generics were the subject of the interlocutory injunctions is relevant for a number of reasons. One, it suggests that the need for estimation of the value of the proven lost opportunity is not a reason to refrain from ordering compensation. Two, it suggests that the traditional liberal approach to the assessment of the quantum (in contrast to the right to) compensation should be kept in mind. Three, it suggests that contemporaneous evidence is a more reliable source of evidence than subsequent conjecture in the context of making and resisting claims under the undertakings.
The generics’ preclusion arguments
Wyeth’s contentions said to be precluded
287 Alphapharm took the helm on these arguments on behalf of the generics. It sought orders at the start of the hearing to the effect that Wyeth be prevented from adducing evidence about or cross-examining in relation to the following matters:
(a) Alphapharm would have been prevented from selling its generic venlafaxine products on the private market and/or on the PBS market by Wyeth seeking and obtaining an interlocutory injunction against Alphapharm based on alleged infringement of copyright in the Efexor-XR PI and CMI.
(b) Alphapharm would not have made sales of Enlafax XR on the private market in the period between 27 August 2009 and 11 November 2011.
(c) Sigma/SPAL and/or Generic Health would not have caused their generic venlafaxine products to list on the PBS and would not have sold their venlafaxine products on the PBS market.
(d) Alphapharm would not have caused its venlafaxine products to list on the PBS and would not have sold its venlafaxine products on the PBS market (following the listing of another generic company’s venlafaxine products on the PBS).
288 I declined to make these orders. Any such application had to be made well in advance of the hearing to be the subject of a separate order as Alphapharm proposed. The issues involved were too complex to deal with on the run during the hearing. Alphapharm submitted that in any event Wyeth should not be permitted to make these contentions.
289 Sigma and Generic Health submitted to the same effect that Wyeth was precluded from putting these arguments. They also submitted that Wyeth should not be permitted to contend against them that Generic Health’s importation and supply to Sigma of Sigma’s products was (and would have been) in contravention of s 19D of the Therapeutic Goods Act so that Generic Health and Sigma should not obtain compensation for lost profits that would have been tainted by illegality.
290 To explain further, issue (a) above, the copyright issue, is described in this way in Wyeth’s submissions:
First, Wyeth contends that the Generics would not have launched their products or listed them on the PBS due to the risk of being held liable for infringement of copyright in the Efexor-XR PI and CMI documents. In this regard, Wyeth contends that, in the counterfactual world, if there had been no interlocutory injunctions based on the Method Patent, it would have brought claims against the Generics for infringement of copyright in those documents on a final basis.
Secondly, Wyeth contends that, in the counterfactual world, if there had been no interlocutory injunctions based on the Method Patent, it would have sought and obtained interlocutory injunctions against the Generics on the basis of their threatened infringement of copyright in the Efexor-XR PI and CMI.
Thirdly, Wyeth contends that it would not be reasonable or equitable to compensate the Claimants having regard to the fact that, if the Generics had launched their products or listed them on the PBS, this would have involved, inter alia, the Generics infringing Wyeth’s copyright in the Efexor-XR PI and CMI.
291 Issues (b) to (d) above concern the supply by generics to pharmacists of their products either on the PBS (as a result of PBS listing) or on the private market. Wyeth contended that if not for the interlocutory injunctions none of the generics would have sought or obtained PBS listing of their products, Sigma’s private market supply would have continued but failed over time, and Alphapharm and Generic Health would not have supplied their products at all (or, if they did, their supply also would have failed over time).
292 The s 19D issue is put by Wyeth against Sigma and Generic Health in this way:
(1) As to Sigma:
… any counterfactual sales made by SPAL in the PBS or private markets would have been in respect of generic venlafaxine products imported by Generic Health and supplied by it to SPAL in contravention of s.19D of the TG Act. These sales would, therefore, have been made by SPAL as a consequence of conduct that was “illicit” or “illegal” in the sense referred to in the authorities, including Columbia Picture Industries [[1987] Ch 38] and Servier [Les Laboratoires Servier v Apotex Inc [2014] UKSC 55; [2015] AC 430]. In this regard, such conduct meets the requirements imposed by the Supreme Court in Servier. Section 19D is directed at enforcing the public interest and creates significant civil penalties. It involves quasi-criminal conduct of a kind that comes within the principles espoused by the Supreme Court. A fortiori, of course, such conduct is also relevant to the exercise of the Court’s discretion to grant relief pursuant to the Undertakings…
(2) As to Generic Health:
In April/May 2009, Generic Health imported 326,919 units of generic venlafaxine products manufactured by Alembic and supplied them to SPAL in May 2009. For the reasons outlined …above in relation to SPAL, in doing so, Generic Health contravened s.19D of the TG Act. Generic Health received payment for that order, and Wyeth understands that it does not form part of Generic Health’s claim.
However, any counterfactual sales made by Generic Health of additional product sourced from Alembic would similarly have been in contravention of s.19D of the TG Act, and thereby ought not attract an order for compensation for the same reasons.
293 The generics submitted that various legal doctrines, abuse of process, Anshun estoppel (Port of Melbourne Authority v Anshun Pty Ltd [1981] HCA 45; (1981) 147 CLR 589), the principles against approbation and reprobation, and/or the principle that a person seeking equity must do equity, operated to prevent Wyeth from making any of the contentions described above.
294 It is helpful to group Wyeth’s contentions so that the generics’ preclusion arguments against them can be better understood. The following groupings may be helpful:
(1) The copyright issue has two aspects:
(a) the first aspect, said to be relevant to the hypothetical analysis, concerns what Wyeth would or might have done if not for the interlocutory injunctions. Wyeth said if not for the interlocutory injunctions it would have sought and obtained interlocutory and final relief in 2009 and 2010 respectively based on the generics infringing its copyright in the Efexor-XR PI and CMI documents. As a result, the generics would not have been able to supply their products in any event; and
(b) the second aspect, said to be relevant to the question whether it is just that the undertakings be enforced against Wyeth, concerns the character or nature of the hypothesised profits of the generics. Wyeth said that any such profits would be tainted by illegality because they would have been obtained as a result of infringement of Wyeth’s copyright in the Efexor-XR PI and CMI. This second aspect does not depend on proof that Wyeth would or might have done anything if not for the interlocutory injunctions. It depends on Wyeth now proving that the generics’ hypothetical profits would have been illegal due to copyright infringement.
(2) The s 19D issue is said to be relevant to question whether it is just that the undertakings be enforced against Wyeth and concerns the character or nature of the hypothesised profits of the generics. Wyeth alleged that hypothesised profits of Sigma and Generic Health in respect of the Sigma products would be tainted by illegality because they would have been obtained as a result of contravention of s 19D of the Therapeutic Goods Act.
(3) The supply issue concerns what the generics would or might have done if not for the interlocutory injunctions. The generics’ argument is that in order to obtain the interlocutory injunctions Wyeth contended that, if interlocutory orders were not made, the generics (variously) would apply for and obtain PBS listing of their products and/or supply their products on the private market. Having obtained the interlocutory injunctions on that basis, the argument is that Wyeth should not now be permitted to put arguments to the contrary.
295 A risk with the way in which the generics dealt with these arguments is that the essential difference between the copyright issue and the other issues might be overlooked. It is easy to succumb to the idea that as it is necessary to work out what the probabilities and possibilities would have been if not for the interlocutory injunctions, all of Wyeth’s potential hypothetical actions are of the same character and all may be raised by Wyeth in defence of the claims for compensation. However, it is necessary to consider the copyright issue separately because, assuming Wyeth has copyright in the Efexor-XR PI and CMI, copyright was a legal right of Wyeth’s to assert or not against the generics when it had the opportunity to do so, which was before the first instance final hearing in April to May 2010. I turn to this issue below.
The copyright issue
296 The generics submitted:
(1) given that Wyeth would have been the subject of an Anshun estoppel or other procedural preclusion (in effect, by operation of ordinary principles of case management) had it attempted to raise new causes of action to seek to obtain an interlocutory or final injunction on different causes of action, Wyeth should not now be permitted to run those allegations indirectly as steps it says it would have taken in the hypothetical world;
(2) Wyeth ought not now be in a better position because it seeks to rely on those claims in these very proceedings indirectly, in the hypothetical analysis, still without providing any explanation as to why they were not brought earlier; and
(3) alternatively, as Wyeth cannot now prove the new claim could have been brought due to various procedural preclusions (be it Anshun estoppel if the claims had been made in separate proceedings or case management principles if the claims had been attempted to be made in these proceedings), Wyeth cannot now be permitted to advance those claims in the hypothetical world.
297 In its reply submissions Alphapharm also made a different point in these terms:
The sense in which the counterfactual differs from the real world must be related to the purpose for which the counterfactual is employed. Here, the counterfactual is designed to determine what loss was suffered by the generics as a result of the injunctions in the real world. The counterfactual that the interlocutory injunction was neither sought (in the sense of filed) nor obtained, is closely correlated to the event which created legal liability (the seeking and granting of the interlocutory injunction). It is a common form of analysis to create a counterfactual by removing the event creating the legal liability…
But Wyeth’s introduction of its copyright claim seeks to make the counterfactual differ from the real world in a way which has nothing whatsoever to do with the event creating legal liability. It introduces a supervening, unrelated counterfactual event. If Wyeth’s approach to counterfactuals were correct, it would be open to a negligent bus company in a case for personal injury to a pedestrian to attempt to prove that, had the plaintiff not been injured by the defendant bus company driving negligently, and then been taken to hospital, he would have continued on his journey to work, and then fallen down the stairs when he arrived at work and suffered the same injury. Of course, such a plaintiff [sic] would not be permitted to attempt to prove that matter, because rather than simply removing the event creating a legal liability (the negligent driving), it adds a new supervening event to the counterfactual. That is what Wyeth effectively seeks to do here by adding its copyright claim to the counterfactual.
298 Wyeth submitted that the copyright issue arises as part of a consideration of what would have occurred in the ongoing proceedings, if the interlocutory injunctions had not been granted. According to Wyeth it does not offend principles of finality or fairness for Wyeth to now make contentions as to what it would have done had it been confronted with a different set of circumstances from those which in fact existed.
299 The generics’ submissions over-complicate the critical point. The critical point is that these are the same proceedings as the proceedings against the generics for interlocutory and final relief which were heard and decided at first instance in 2010 and were the subject of an appeal in 2011. Wyeth knew about but chose not to assert its alleged copyright in the Efexor-XR PI and CMI against the generics at any time other than in response to the generics’ claims for compensation under the undertakings. In this context, the issue is not about what Wyeth would or might have been confronted with had it tried to assert copyright at any time after 22 May 2009 (when the Sigma interlocutory injunction was granted) and before the first instance final hearing in 2010. Wyeth did not do this when it had the chance to do so and thus the issue is whether a party which made a deliberate forensic decision not to assert a legal right (which the evidence will show Wyeth did) in a proceeding against parties can conduct an interlocutory and final hearing and an appeal on the basis of that forensic decision and subsequently in the same proceeding against the same parties assert that legal right for any purpose. It may be accepted that the claim is now for compensation but the claims are in the same proceedings and are made under the undertakings in those proceedings. To enter into debates about supervening causes and Anshun estoppel or case management principles as part of the hypothetical analysis involves the wrong framework of reference. The relevant framework of reference is that these are the same proceedings and as between Wyeth and the generics involves the same parties and that Wyeth has had a full hearing on the merits of its legal claims against the generics in respect of their venlafaxine products both at first instance and on appeal without asserting copyright against them.
300 Unlike the other issues which Wyeth is seeking to raise as relevant to the hypothetical analysis and the justice of any order being made against it, the copyright issue involves a legal right of Wyeth. Assuming Wyeth owned the copyright in the Efexor-XR PI and CMI (which is disputed except by Generic Health), no-one but Wyeth could bring the claim for copyright infringement. Copyright was Wyeth’s right alone to assert. Wyeth, as the evidence will show, chose in 2009 not to seek interim or final relief based on copyright. Wyeth thus made a forensic decision in these proceedings. It maintained that forensic decision throughout the first instance proceedings and on appeal to the Full Court and when it tried and failed to get special leave to appeal to the High Court. Now, in the context of these same proceedings, Wyeth is asserting claims based on copyright. In my view having made forensic decisions to seek interlocutory injunctions and final relief on the basis only of patent infringement Wyeth cannot now assert in the same proceedings legal claims which it had available at the relevant time but deliberately chose not to assert.
301 Wyeth’s response that the necessary analysis is hypothetical is no answer to the problems with the copyright issue. The claims for compensation are under the undertakings. They are claims in the same proceedings in which the undertakings were given. The so-called “counter-factual” approach is merely a tool to assess whether the operation of the interlocutory injunctions has caused loss. The proceedings, however, are the same proceedings. As between the generics and Wyeth, the parties are also the same, the other claimants’ claims deriving from those of the generics. In these proceedings, Wyeth chose to assert some legal rights (patent) and not others (copyright) for the purposes of interlocutory and final relief. All issues of relief have been finally determined. The remaining issue in these proceedings is the compensation claims under the undertakings but the context remains these proceedings and the task is to assess compensation in that context. The task is not to assess compensation in the hypothesised context of some other litigation in which Wyeth asserted different rights and succeeded rather than failed. Accordingly, a wrongly granted interlocutory injunction on the basis of the legal rights actually asserted cannot be transformed into a correctly granted interlocutory injunction on the basis of legal rights never asserted.
302 Wyeth must remain bound by its forensic decisions. It cannot assert legal claims against the generics which it did not assert when it had the opportunity and the time was ripe to do so. Describing the claims as being part of the required “counter-factual” analysis or as relevant to the issue of illegality or discretion does not change the fact that these are the same proceedings in which Wyeth, at the time when it was able to seek interlocutory and final relief against the generics based on copyright infringement, chose not to do so.
303 The nature and purpose of the undertakings supports these conclusions. The undertakings were given as the price of the interlocutory injunctions. These undertakings are able to be called upon only because the interlocutory injunctions were wrongly granted. Whether or not the interlocutory injunctions were wrongly granted depends on the legal rights the moving party chose to assert. To ascertain loss for the purpose of a claim under undertakings of this kind it will generally be necessary to consider what the position would have been if not for the interlocutory injunctions. If the party who gave the undertaking and had the benefit of an interlocutory injunction and a full hearing on the merits in respect of the legal rights the party chose to assert can later assert in the context of a claim for compensation under the undertaking that if not for the interlocutory injunction the position would have been the grant of an interlocutory injunction on the basis of some other right (as Wyeth seeks to do) the entire history of the proceedings is set at naught. The fundamental premise of the wrongly granted interlocutory injunction is undermined. Apart from this being wrong in principle, there are other undesirable consequences. The first is regression because it would also have to be posited that undertakings would have been given as the price for those hypothetical interlocutory injunctions based on other legal rights never asserted. The second is that to escape from regression there must be a case within a case (or, worse, as here, cases within a case). Thus, in the present matter, Wyeth seeks to prove that it owned copyright in the Efexor-XR, the generics infringed that copyright, and that all of the generics’ profits would have been payable to Wyeth in the form of damages. It also seeks to prove that but for the interlocutory injunctions it would have sought and obtained interlocutory and final relief based on copyright. The first case involves a determination now. The second case involves a projection back into 2009 and 2010. As a result, it is even possible that the outcomes of these cases within the case might be different. That Wyeth’s conduct relating to its copyright claim, if permitted, would place the other parties and the Court in an untenable position should be readily apparent.
304 In any event, the inescapable fact is that these are the same proceedings and as against the same parties Wyeth is now seeking to assert its alleged copyright deliberately chose not to make that assertion when it had the opportunity to do so. Had Wyeth made its copyright claims when it had the opportunity to do so (be it for interlocutory and final relief or only final relief) then all of the complex issues of fact and law to which the copyright issue gives rise would have been decided in the ordinary course of the litigation. Having chosen not to do so, if Wyeth is permitted to assert copyright now the same complex issues now arise indirectly in two potentially inconsistent exercises, hypothetical and actual. The issues arise, moreover, in circumstances where it can never be known what the generics could, would or might have done had Wyeth asserted copyright against them in the context of its claims for interlocutory and final relief.
305 This may be contrasted with the other issues raised by Wyeth. All of those other issues, including what the generics would have done, Wyeth’s generic defence strategy, and whether the Minister’s delegate would have listed the generics’ products on the PBS given the litigation, never arose because of the interlocutory injunctions, not because of a forensic decision by Wyeth about what rights it would and would not assert. Because the interlocutory injunctions were granted, how those issues would have played out is necessarily unknowable. The copyright issue is different. The copyright issue did not arise before or after the interlocutory injunctions because Wyeth decided not to raise the issue. Had it chosen differently we would have known whether it would have obtained interim or final relief based on copyright infringement allegations. The copyright issue was not necessarily unknowable. Wyeth made it so by its forensic choice.
306 It may not be necessary to characterise these circumstances as giving rise to an abuse of process to conclude that Wyeth should not be permitted to raise the copyright issue but I would be prepared to reach that conclusion. The doctrine of abuse of process is not confined to the commencement of proceedings. It extends to any step in the proceedings having the effect of bringing the administration of justice into disrepute or imposing unjustifiable oppression on other parties: Batistatos v Newcastle City Council [2006] HCA 27; (2006) 226 CLR 256 at [14]-[15].
307 It would bring the administration of justice into disrepute for a party in Wyeth’s position to make a forensic decision not to assert its alleged copyright for the purpose of interlocutory and final relief and then in the same proceedings years later to assert copyright for the purpose of defeating a claim on the undertaking as to damages by parties in the position of the generics who were bound by the interlocutory orders in exchange for which Wyeth gave the undertakings. To permit this course would make a mockery of principles essential to the administration of justice including the finality of litigation. That principle has many manifestations of which one is that parties are bound by their forensic decisions. It is difficult to imagine a case which calls for the application of this principle more powerfully than the present in which Wyeth chose in 2009 what right it wished to assert against the generics, being the method patent, and chose not to assert another right, copyright in the Efexor- XR PI and CMI, obtained interlocutory injunctions on the basis of the patent right and had a full hearing on the merits both at first instance and on appeal on that basis.
308 In Tomlinson v Ramsey Food Processing [2015] HCA 28; (2015) 256 CLR 507 at [24] the High Court (per French CJ, Bell, Gageler and Keane JJ) said that the doctrine of abuse of process is informed by considerations of finality and fairness. While the context of claimed abuses of process is often an attempt to re-litigate an issue which was litigated in earlier proceedings or should have been so litigated, it is the same considerations of finality and fairness which underlies the principle that in a proceeding a party is generally bound by its forensic decisions. For example, in Metwally v University of Wollongong [1985] HCA 28; (1985) 60 ALR 68 at 71 the High Court said:
It is elementary that a party is bound by the conduct of his case. Except in the most exceptional circumstances, it would be contrary to all principle to allow a party, after a case had been decided against him, to raise a new argument which, whether deliberately or by inadvertence, he failed to put during the hearing when he had an opportunity to do so.
309 In Coulton v Holcombe [1986] HCA 33; (1986) 162 CLR 1 at 7-8 Gibbs CJ, Wilson, Brennan and Dawson JJ said:
It is fundamental to the due administration of justice that the substantial issues between the parties are ordinarily settled at the trial. If it were not so the main arena for the settlement of disputes would move from the court of first instance to the appellate court, tending to reduce the proceedings in the former court to little more than a preliminary skirmish. The powers of an appellate court with respect to amendment are ordinarily to be exercised within the general framework of the issues so determined and not otherwise. In a case where, had the issue been raised in the court below, evidence could have been given which by any possibility could have prevented the point from succeeding, this court has firmly maintained the principle that the point cannot be taken afterwards
310 While the context of Metwally was an application to vary a perfected order and of Coulton v Holcombe was raising a new issue in an appeal the guiding principles of finality and fairness are the same.
311 Further, it almost goes without saying that, analysed in what I consider to be the proper context, it would be “unjustifiably oppressive” to the generics to permit Wyeth to now make the copyright claims in these circumstances: Tomlinson at [25]. They responded to Wyeth’s assertion of its patent right in 2009 by claiming that the method patent was invalid. They defended Wyeth’s infringement claims. They did so at first instance, on appeal to the Full Court, and in respect of Wyeth’s application for special leave. They were vindicated. The right Wyeth chose to assert against them did not exist. Now, when they claim compensation under the wrongly granted undertakings, Wyeth chooses to assert against them copyright in the Efexor-XR PI and CMI. They not only have to defend against copyright cases within a case but are burdened by having to both dispute what Wyeth contended it would have done but for the interlocutory injunctions and by attempting to prove what they and the Commonwealth would have done in response. It is difficult to overstate the degree of prejudice, vexation and oppression which this involves to the generics.
312 The same conclusions apply to both aspects of the copyright issue. Whether it be for the purpose of the hypothetical analysis or assessing the justice of enforcing the undertakings, Wyeth is asserting copyright against the generics in circumstances where it chose not to do so at any time before the final resolution of its case against the generics. In my view having had its case against the generics based on the asserted patent rights heard on the merits and ultimately decided against it on appeal Wyeth cannot now assert copyright in these proceedings for any purpose. To do so involves an abuse of process which must not be permitted. If not an abuse of process, it is nevertheless an exercise which would undermine the principles of the finality of litigation and that a party must be bound by its forensic decisions. As a matter of discretion this should not be permitted.
313 No different conclusion applies to the other claimants. Their claims derive from the generics’ claims. They were not parties to the proceedings as originally constituted. But if as I consider to be the case Wyeth cannot assert copyright in these proceedings then it cannot do so for any purpose. In any event given the derivative nature of the other claimants’ claims, copyright is irrelevant as against them.
314 Equitable principles provide another perspective about the same issue. The interlocutory injunctions are the result of the fact Wyeth wanted to keep the generics out of a market it believed was protected by the method patent. For that purpose Wyeth had both the patent and copyright infringement claims available to it at all times. It could have sought interlocutory orders on both grounds. It chose not to do so. Further, after the grant of the interlocutory injunctions, Wyeth could have applied to amend its claim to include the copyright infringement case but again chose not to do so. Having taking the benefit of the equitable remedy of interlocutory injunctions as against the generics why would Wyeth still not be bound to do equity to the generics in the context of the generics’ claims for compensation under the undertakings given as the price for the interlocutory injunctions? If so bound is Wyeth not acting against good conscience by now contending that it would have made a claim that it had an opportunity to make and which it did not make? In my view, it is not.
315 Wyeth’s submissions do not grapple with these abuse of process issues other than to assert that because the question is hypothetical the fact that it decided not to raise the copyright issue is immaterial, the question being what it would have done if not for the interlocutory injunctions. This approach disregards the actual course of the litigation (contrary to Wyeth’s correct insistence that this cannot be disregarded for the purposes of causation) and treats the required hypothetical analysis as if it is its own separate cause of action when it is nothing more than a tool to assess if the interlocutory injunctions caused loss and, if so, to what extent.
316 Whatever way in which the issue is approached I consider that Wyeth should not be permitted to assert copyright in the Efexor-XR PI or CMI in these proceedings. The time for doing so was 2009 or 2010. That time has long since passed.
Supply by the generics
317 The generics’ argument is that to obtain the interlocutory injunctions in 2009 Wyeth contended and proved to the requisite standard for interlocutory relief that the generics would have sought and obtained PBS listing of their products and then supplied their products on the PBS and that, if their products were not PBS listed, they would have supplied on the private market. The corollary is said to be that Wyeth cannot now be permitted to attempt to prove or submit to the contrary.
318 The generics submitted in support of these arguments that:
(1) the interlocutory character of the decisions which led to the interlocutory injunctions is insufficient to deprive Wyeth’s present contentions of the character of an abuse of process;
(2) it would tend to bring the administration of justice into disrepute for the Court to permit Wyeth to escape liability to pay compensation under the undertakings on the basis that the generics have not proven they would have supplied their products as such a decision would be directly inconsistent with the decisions which led to the grant of the interlocutory injunctions;
(3) Wyeth making one submission to achieve one result and being able to now make another submission as to witnesses’ faulty recollections after nine years have passed in an attempt to achieve the opposite result is oppressive and unfair, particularly given that the generics now only find themselves in the “in the position of needing to bring [their] present claim in these long and complicated proceedings, is the fact that Wyeth incorrectly asserted an invalid patent against [them] and obtained an interlocutory injunction on the basis of that invalid patent”;
(4) the finality of judicial determination will be undermined in that the Court will be put in a position where the Court is asked to embark on a process of reaching and recording a different result from that previously the subject of a considered and published judgment;
(5) Wyeth took the benefit of the interlocutory injunctions and cannot now controvert a fundamental premise upon which the interlocutory injunctions were given; and
(6) it would be inequitable and unjust to allow Wyeth to defend this application on a basis that departs from its cases for the interlocutory injunctions.
319 I do not find these submissions persuasive to the extent that they are relied upon to preclude Wyeth from making these contentions. This is not to say that a party such as Wyeth should be permitted to say one thing to obtain an interlocutory injunction and then another to defend against a claim under the undertakings should it fail to obtain final relief. Inconsistent assertions of that kind would justify close scrutiny and a degree of scepticism because inconsistency may involve permitting a party who has obtained equity not to do equity. In this sense, the concept of symmetry to which Norris J referred in Les Laboratoires Servier v Apotex Inc [2008] EWHC 2347 (Ch); [2009] FSR 220 at [9] is apt. The present issue, however, involves other more complex considerations. As will become apparent, there was considerable forensic manoeuvring occurring between Wyeth and the generics in 2009 including about the question of the generics supplying their products and how they might do so. In this context, the assertion of inconsistency is not straightforward. Other considerations are also engaged.
320 First, the supply issue is different from the copyright issue. The supply issue does not concern a right which Wyeth had and could have asserted against the generics in 2009 but chose not to assert. It concerns what the generics would or might have done if not for the interlocutory injunctions which is a necessary component of assessing whether and to what extent the interlocutory injunctions caused them loss. To preclude Wyeth from responding to the generics’ claims about what they would or might have done if not for the interlocutory injunctions would be for the mere grant of the interlocutory injunctions to determine the issues of causation and loss.
321 Second, in 2009 Wyeth could only act on the evidence it had which consisted of representations by the generics about their intentions. The Court also determined the interlocutory injunctions on that basis. The context of a claim for an interlocutory injunction is different from the present context. In the context of the interlocutory injunctions Wyeth would not have been permitted to attempt to go behind the generics’ representations about their intentions. It could only take what the generics said at face value and decide to apply for interlocutory relief or not on that basis. No discovery or cross-examination seeking to challenge the generics’ representations would have been permitted.
322 Third, the generics which defended against the interlocutory injunctions, Sigma and Alphapharm, did not choose simply to rely on their evidence from 2009. Their claims for compensation are based on further evidence from them about what they would or might have done but for the interlocutory injunctions. The generics also chose to tender additional documents to advance their claims for compensation. Having done so it is not apparent to me as a matter of principle why Wyeth would be precluded from doing the same. The result is that the evidentiary landscape of the current claims is different from and far more extensive than the evidentiary landscape in 2009 for the purpose of the interlocutory injunctions. The evidentiary landscape shows the extent to which the parties’ conduct was affected by forensic considerations.
323 Fourth, there was no evidence, hearing or judgment in respect of the Generic Health interlocutory injunction. Wyeth and Generic Health consented to the orders being made. Accordingly, in my view, none of the principles on which the generics relied can be engaged in respect of Generic Health.
324 Fifth, insofar as Sigma is concerned, whatever the true positon about Sigma’s offer not to seek PBS listing (that is, whether it was conditional on Wyeth giving an undertaking as to damages or not), that issue was not part of the dispute between the parties in the context of the Sigma interlocutory injunction. It is apparent that the balance of convenience was decided in the context of Sigma’s private market supply only, not any potential PBS listing and PBS supply. As such, there can be no inconsistency or inequity or the like about that issue.
325 Sixth, insofar as Alphapharm is concerned, there was no issue in the context of the Alphapharm interlocutory injunction about Alphapharm’s evidence that it would not be the first generic to obtain PBS listing of its products. The Court was not required to decide whether Alphapharm would have applied for PBS listing. Again, there can be no inconsistency or inequity or the like about that issue. The issue of forensic manoeuvring is also particularly acute in respect of Alphapharm’s private supply.
326 Seventh, for the issue of supply by the generics, the relevant context is hypothetical. The question is the degree of probability or possibility of something occurring which did not or could not occur because of the interlocutory injunctions. In that context I cannot see how the doctrines of abuse of process, Anshun estoppel, approbation and reprobation or the like have any role to play. One circumstance is factual (the interlocutory injunctions were granted) and one is hypothetical (the interlocutory injunctions were not granted). There is no relationship between the two circumstances upon which these legal doctrines can operate. Again, however, I stress that contemporaneous evidence is likely to be the best or at least useful evidence for various purposes. Wyeth’s evidence from 2009 about what it thought the prospects of Sigma and Alphapharm successfully obtaining market share from the supply to the private, non-PBS market, is evidence of this kind.
327 Further, I accept Wyeth’s submissions as follows, subject to a few additional comments as noted in parentheses:
(1) when an interlocutory injunction is granted, a court does not decide any right, but only determines on what footing matters shall stand until the trial. At the trial the rights of the parties are finally determined;
(2) at the interlocutory injunction stage, a court’s assessment of the balance of convenience involves an assessment of the likelihood or risk of certain eventualities, by reference to the evidence which is then available;
(3) the issues arising for determination at the interlocutory injunction stage and the issues arising for determination when there is a later claim pursuant to an undertaking as to damages, while related, are different and proceed by reference to different bodies of evidence (albeit that I would add that the evidence adduced in respect of the interlocutory injunctions is relevant);
(4) there is nothing unorthodox about a court revisiting the question of whether conduct enjoined by an interlocutory injunction would in fact have occurred if the injunction had not been granted; and
(5) by the generics’ reasoning, the mere grant of an interlocutory injunction would be treated as giving rise to a final entitlement to damages based on a hypothesis of adverse affectation rather than any factual reality (although I would not adopt the phrase “factual reality” but say instead the “assessment in all of the circumstances as now known of the degree of probability that asserted events would have occurred”).
328 These submissions support my conclusion that principles which the generics called in aid of their preclusion arguments are not engaged in respect of the issue of supply.
329 In these circumstances I consider that there is no unfairness, oppression, inconsistency or potential embarrassment to the administration of justice, still less any form of impermissible approbation or reprobation or even any lack of symmetry arising from Wyeth responding to the current claims about what the generics would have done in terms of supply of their products by reference to all of the available material and on the necessary hypothesis that the interlocutory injunctions had not been granted. I am unable to see how excluding Wyeth from testing the generics’ contentions and adducing evidence relevant to what it says the generics would have done if not for the interlocutory injunctions can be reconciled with the terms of the undertakings which require the Court to make such order as it considers just for compensation.
330 Otherwise, I agree that the approach to the assessment of compensation should be liberal, provided that it has been proved on the balance of probabilities that the operation of the interlocutory injunction had an adverse effect on the claimant.
The s 19D issue
331 I do not see the s 19D issue in the same way as the copyright issue. Wyeth’s contention is not that it would have asserted against the generics any right it had under s 19D of the Therapeutic Goods Act. The section did not vest any right in Wyeth. It is a provision which creates a contravention of the law attracting a civil penalty. Wyeth could not have raised s 19D against the generics at any earlier time. It is only potentially relevant to the claims for compensation by Generic Health and Sigma.
332 The present issue is not whether the issue has any merit. It is whether Wyeth should be permitted to raise the issue at all. I am unable to discern any principled basis for concluding that Wyeth should not be permitted to contend that the importation and supply of Sigma’s products by Generic Health for the purpose of supply to Sigma contravened and would have contravened s 19D so that their hypothetical profits from such conduct was and would have been tainted by illegality so that it would not be just to order compensation in their favour on that account.
333 In my view all of Wyeth’s contentions against the generics involving copyright constitute an abuse of process or conduct which should not otherwise be permitted as a matter of discretion. Wyeth’s other contentions do not engage any of the principles of preclusion on which the generics relied.
334 Sigma’s claim is made under Wyeth’s undertaking in respect of the Sigma interlocutory injunction. Sigma claims compensation for the value of Sigma’s lost opportunity to supply the Sigma products to pharmacists by way of wholesale.
Some uncontroversial facts
335 The following summary includes extracts from Sigma’s closing submissions to the extent that they record facts which appear to be uncontroversial and otherwise includes undisputed facts apparent from other parts of the evidence.
336 Sigma entered the Australian generic pharmaceuticals market after Sigma Company Limited, the parent of Sigma, merged with Arrow Pharmaceutical Limited in December 2005. The merger enabled Sigma Company Limited to take over Arrow’s substantial generic product portfolio and establish a new generic division within Sigma Company Limited conducted by Sigma as a subsidiary of Sigma Company Limited.
337 In the 2008/2009 financial year Sigma was the third largest supplier of products, by script volume, to the PBS. Sigma offered a range of over 12,000 product lines and distributed products to over 4,000 retail pharmacies daily from its 15 Australian distribution centres. It had external sales revenue of over $3 billion and employed over 1800 people in 19 locations across Australia.
338 In 2008-2009 Sigma was the “first-line” generics supplier to about 1,500 of around 5,000 retail pharmacies in Australia, in the sense that those pharmacies stocked most or all of Sigma’s generic pharmaceutical range and over-the-counter ranges. Approximately 1,000 of the 1,500 pharmacies to whom Sigma was the “first-line” generics supplier were members of Sigma’s “Embrace” support and loyalty program. This program worked so that the more of Sigma's products and services a pharmacy used, the better its trading terms were across all areas of Sigma’s business. Pharmacies who participated in the Embrace program could access benefits such as preferential trading terms, prioritised delivery runs, preferential access to scarce stock, preferential treatment of credits and returned stock, a brokerage service, free catalogue deliveries and the like. About 1,500 further pharmacies stocked some, but not all, of Sigma’s products.
339 Sigma had a team known as the Generics Team headed by Mr Heine, as National Sales Manager, Generics, consisting of about 40 sales representatives including seven managers who reported directly to Mr Heine. The primary target for the generics team was retail pharmacies. Members of the generics team personally visit around 4000 pharmacies per month across Australia.
340 By May 2009 Sigma and Arrow had been the first to market with generic versions of five so-called “blockbuster” drugs (a product with Australian sales in excess of $100 million per year) because this facilitated Sigma establishing a market share that it considered other generics would have difficulty eroding and increased its overall sales.
341 Sigma also had experience in selling generic versions of drugs which were not PBS listed. Given that there was no PBS listing for the originator drug, such sales had to be private market sales. Most of the 3,000 to 3,500 retail pharmacies to whom Sigma sold products purchased both “generics” (that is, generic versions of PBS listed drugs) and “privates” (that is, drugs not listed on the PBS).
342 Where an originator drug had a PBS listing, Sigma had previously launched a number of generic products in two stages with a private market launch and supply first to be followed by supply under the PBS once PBS listing had been obtained. Other evidence discloses that this two stage approach is not uncommon for generics but the time period between the private supply and the PBS supply commencing is usually in the order of up to but no longer than three or four months. Neither Sigma nor any other generic had supplied a generic version of a PBS listed drug on the private market without also anticipating PBS listing of the generic product within the immediately foreseeable future. It would be fair to describe such an approach as revolutionary or unprecedented.
343 In 2007 Sigma and Arrow Group ApS, a Danish company which through various subsidiaries operated an international generic pharmaceuticals business, entered into a further agreement in respect of 80 “pipeline” drugs which Arrow Group was developing for launch including an extended release formulation of venlafaxine hydrochloride. Sigma’s intention was to launch its generic venlafaxine hydrochloride products in Australia after the expiry of the compound patent in December 2008. Sigma was unaware of the method patent when it made these arrangements and formed this intention.
344 Sigma relied on patent clearance searches conducted by Arrow Group for “pipeline” products and other generic products that originated with Arrow Group. These searches did not disclose the existence or relevance of the method patent to Sigma’s plans for generic venlafaxine hydrochloride products.
345 Sigma took steps after 2007 to ensure it could launch its generic venlafaxine hydrochloride products as soon as possible after expiry of the compound patent. As summarised in Sigma’s closing submissions:
(a) in August 2007 “Venlafaxine SR” was recorded in Sigma’s “Product Pipeline” with a patent expiry date of 16 December 2008, a TGA estimated approval date of 1 December 2008, a “product available” date of February 2009, and a PBS listing estimate of 1 April 2009;
(b) from about 2007 onwards Sigma took steps to have a generic venlafaxine hydrochloride XR product registered by the TGA on the ARTG, including licensing data from other companies and retaining a consultant to conduct, prepare and submit a bioequivalence study;
(c) on 8 July 2008 Sigma amended its agreement with its supplier, Generic Health, to include the manufacture, packaging and supply of Evelexa XR, and in October 2008 and February 2009, in anticipation that it would obtain ARTG registration, Sigma placed significant orders for Evelexa XR with Generic Health amounting to about $3 million;
(d) from late 2008 Mr Heine undertook various tasks to prepare Sigma’s launch strategy for Evelexa XR, including reviewing trends for sales of the Efexor-XR Products, developing marketing support materials and programs, preparing internal budget forecasts, developing sales strategy and tactics, training Sigma’s generic sales team, establishing incentive programs for the generic sales team, developing aids to assist in communicating the benefits to a pharmacy of switching to Evelexa XR, and actively selling Evelexa XR to key national retail pharmacy groups;
(e) by the end of 2008 the design of the external packaging and blister packs for Evelexa XR had been finalised;
(f) In February and March 2009 Sigma prepared a dispensing history for Efexor-XR Products for a large number of retail pharmacies across Australia, so that Sigma’s generic sales team could tailor offers to pharmacies based on their actual historical dispensing of Efexor-XR;
(g) Sigma encountered difficulties in obtaining ARTG listing for Evelexa XR in late 2008 and early 2009… but on 6 February 2009 the TGA notified Sigma that …approval would be recommended. Mr de Alwis described the TGA’s decision to recommend approval as “great news for us” and Mr Smith described it as “awesome news”.
346 Sigma’s strategy was to be the first generic entrant on the market which Sigma expected would enable it to obtain at least a three month first mover advantage before another generic entrant.
347 In order to get Evelexa XR onto the market as quickly as possible (rather than waiting for PBS listing to launch the product) and enjoy a first-mover advantage for the maximum possible period, Sigma planned a marketing strategy for Evelexa XR. This was to launch the product on the private market to non-concessional (or general) patients for a period of 3 months commencing on 1 May 2009 before it was listed on the PBS on 1 August 2009. To secure its first mover advantage Sigma’s strategy involved:
(a) getting Evelexa XR on to the market as quickly as possible by launching first on the private market and not waiting for PBS listing; and
(b) maximising Sigma’s first mover advantage by achieving a forward sell-in to the pharmacies so that they had so much stock on hand that when a competitor entered the market, the pharmacy would have no need to order more venlafaxine hydrochloride XR. A further benefit of the forward sell-in was that Sigma would sell as much product as it could in the first month, to avoid the statutory price disclosure requirements, which do not operate during the first month of the first reporting period.
348 Sigma believed that the private market for venlafaxine hydrochloride XR was close to 50% of patients taking Efexor-XR. Sigma considered that it was well placed to capture 50% of the available sales to general patients and, therefore, about 20-25% of the overall Australian market for venlafaxine hydrochloride XR.
349 On 26 February 2009 the TGA notified Sigma by letter that it had approved Evelexa XR for registration on the ARTG. The ARTG records that Evelexa XR 150, Evelexa XR 75, and Evelexa XR 37.5 were entered on the Register on 5 March 2009, in each case on the basis that it was bioequivalent to one of the Efexor-XR products, and was indicated for treatment of the same range of depressive illnesses. In his affidavit in 2009 Mr de Alwis explained that Sigma had taken approximately three years to demonstrate bioequivalence of its products with Efexor-XR in order to obtain these ARTG registrations. The ARTG registrations thus represented the culmination of a substantial investment of time and money by Sigma and put Sigma in a unique position as at 5 March 2009, it then being the only generic which could lawfully supply its venlafaxine products in Australia.
350 In early 2009, Sigma Company’s Group Commercial Manager (Finance Division), Mr Paul Meulblok, forecast the total expected gross profit to be generated by Evelexa XR for the 2009/2010 financial year (from 1 February 2009 to 31 January 2010) on the basis of Sigma listing Evelexa XR on the PBS on 1 August 2009. Mr Meulblok estimated that:
(a) the total market for venlafaxine hydrochloride XR was $105,000,000;
(b) Sigma would obtain 20% of the market for venlafaxine hydrochloride XR;
(c) Sigma would achieve sales totalling $10.5 million in that year, with a 40% margin; and therefore
(d) Sigma would achieve a gross profit of $4.2 million.
351 In February 2009 Mr de Alwis presented to Sigma Company’s board its budget for the 2009/2010 financial year, which included revenue and profit forecasts in respect of anticipated sales of Evelexa XR. Consistent with Mr Meulblok’s forecast, the budget assumed a PBS listing date of 1 August 2009 and forecast revenue of $10.5 million and profit of $4.2 million for the relevant financial year (being 1 February 2009 to 31 January 2010) making Evelexa XR Sigma’s most profitable generic product.
352 On 2 March 2009, Sigma applied for listing of the Evelexa XR Products on the PBS. It requested to the Department of Health that the products be listed on the PBS effective on 1 August 2009. On 6 March 2009, Sigma sent a further letter to the Department in which it requested that the Evelexa XR Products be listed on the PBS before 1 August 2009.
353 Sigma then became aware of the method patent on or about 6 March 2009.
354 Apart from these undisputed matters, it is apparent that on 29 April 2009 Sigma decided to proceed only with its planned private launch of its products to general patients and took steps to ship its stock of Evelexa XR which was being held in Singapore to Australia. Sigma also substantially revised its launch strategy and budget on the basis that its launch should be confined to the private market. Sigma subsequently launched its products on the private market. The launch process involved the following:
(a) The extensive Sigma generics sales team visiting pharmacies to inform them of the availability of Evelexa XR. The material highlighted that Sigma was “first-to-market” and the only generic registered with the TGA.
(b) On 4 May 2009 Sigma sent a fax to 4500 pharmacies announcing the availability of Evelexa XR, noting it to be the “first-to-market Generic” and its availability in 37.5mg, 75mg and 150mg doses.
(c) The Sigma generic sales team approached pharmacies in order of importance. Sigma’s most important customers were visited first. The detail aid which Sigma’s generic sales team used to detail Evelexa XR to the pharmacies set out the two phase launch process. Phase 1 being the launch on the private market – “prescription launch now” and Phase 2 “PBS launch” at a later date.
(d) In early May 2009, the Sigma generic sales team commenced taking orders for Evelexa XR, on the basis that the product would be delivered to pharmacies for sale by 1 June 2009.
(e) By 12 May 2009, the generics team had visited approximately 800 pharmacies and received orders from 550 pharmacies for Evelexa XR products.
(f) On 22 May 2009, Sigma wrote to the TGA providing Commencement of Supply forms in relation to Evelexa XR indicating that the products would be made available as private prescriptions on 1 June 2009.
(g) On 22 May 2009, Sigma notified the TGA that it had begun marketing production batches of Evelexa XR on 1 May 2009.
(h) By 3 June 2009, Sigma had received orders from 2,300 pharmacies for over 53,000 units of Evelexa XR.
The competing cases – preliminary points
355 Much was said by Sigma and Wyeth about what occurred between Sigma discovering the method patent and the grant of the Sigma interlocutory injunction on 3 June 2009.
356 Sigma’s principal case can be boiled down to these propositions. While Sigma considered that it had good arguments the method patent was invalid it anticipated that the prospect of a 12.5% price reduction from PBS listing of its generic product would make an interlocutory injunction almost an inevitability. It decided to take that risk out of play and thus withdrew its PBS listing application in an attempt to increase its prospects of resisting an interlocutory order preventing it from supplying at least on the private market. Had there been no interlocutory injunction it would have reverted to its original plan for private market sales from 1 May 2009 and PBS listing by 1 August 2009 to enable supplies under the PBS thereafter. Neither the existence of the method patent nor the litigation would have deterred it from implementing its plans because it believed it had good prospects of having the method patent declared invalid.
357 Sigma said that its conduct in anticipation of being restrained by an interlocutory injunction does not reflect what would have occurred but for the interlocutory injunctions and thus must be disregarded. Further, Wyeth’s conduct threatening an interlocutory injunction application must also be disregarded. I have rejected these submissions above because I consider that they do not reflect the terms of the undertakings and would eliminate the existence of the method patent and the litigation from the assessment, which is impermissible. If I am wrong about this, the only material difference would be one of timing in relation to the prospect of PBS listing and PBS supply in that instead of that prospect relating to the date of 1 December 2009 for Sigma it would relate instead to the date of 1 August 2009. This would have a consequential effect for the prospect of PBS listing and PBS supply by Alphapharm and Generic Health – the relevant date for them for PBS listing and PBS supply would become 1 December 2009 rather than 1 March 2010.
358 To return to Sigma, however, Wyeth’s principal case can be boiled down to these propositions. When it discovered the existence of the method patent Sigma decided to withdraw its application for PBS listing. Whether it did so in anticipation of an interlocutory injunction or for fear of being liable for a 12.5% price reduction in Efexor-XR is immaterial as the position would have been the same if no interlocutory injunction had been granted. If the PBS listing was withdrawn to ensure that Sigma would at least be able to supply the private market then assuming no interlocutory injunction Sigma would have achieved that objective of ensuring it could supply on the private market. It would not then be inferred that having achieved its objective Sigma would change its mind and re-apply for PBS listing at the risk of prompting the very thing it has successfully avoided, an interlocutory injunction preventing supply at all. If it was done for fear of being liable for a 12.5% price reduction in Efexor-XR, that fear would have remained operative at all times pending the final injunctions being granted. It is also convenient to record here that insofar as Sigma’s private market launch is concerned, Wyeth accepted that Sigma would have continued with its private market supply but contended that Sigma achieved its sales based on an anticipated PBS listing which would not have been forthcoming. Pharmacists would not have been able to sell the stock, the stock would have been returned to Sigma, and Sigma’s private market supply would have been unsuccessful.
359 As a matter of principle Wyeth said that as the undertakings are concerned with allegedly adverse effects of the interlocutory injunctions it is not legitimate to disregard what actually occurred leading up to the making of the interlocutory injunctions. All of what in fact occurred must be taken to have occurred. The only thing that is removed from the analysis is the grant of the interlocutory injunction. Insofar as Mason J in Air Express at 325 described the postulate as the injunction not being sought or granted, this cannot be understood as requiring actual events occurring before the injunction was granted to be disregarded. This would broaden the scope of the undertaking beyond its terms which focus on any adverse effect by operation of the interlocutory order. Accordingly, Mason J’s reference to “sought” is best understood as meaning sought or applied for on the day the Court was asked to make the interlocutory orders. On this basis everything that occurred up to 22 May 2009 (the date on which Sundberg J heard Wyeth’s interlocutory application against Sigma) is to be taken into account. The difference commences only with the hypothesis that on that day Wyeth did not apply for the interlocutory injunction.
360 As discussed above, I consider that Wyeth’s approach to the start of the hypothetical analysis is to be preferred because it ensures that the terms of the undertaking, which focus on whether a person has been “adversely affected by any operation of the order”, remain the relevant contingency. This said, and also as noted above, I do not accept submissions that were put, for example by Generic Health, that this approach means that it should be assumed the injunctions were sought but not granted on the same basis that the Full Court found the method patent to be invalid, more than two years later. Generic Health’s submissions were in these terms:
Hypothesising that the injunctions were not sought leaves unanswered the question of why they were not sought. In the real world, Wyeth was not prepared to not to seek the injunctions because it did not want to take the risk of erosion of its monopoly position. It is far from axiomatic that the hypothetical world should be constructed so that Wyeth takes the very risk it was not prepared to take in the real world.
The other option is that Wyeth seeks the injunctions but they are refused. There is no reason why the outcome of the injunctions applications in the hypothetical cannot be different to the outcome in the real world. What is involved is a hypothesis that the judges hearing the injunction applications in the hypothetical world reach a view on the strength of the patent (ie prima facie case), consistently with the ultimate decision of the Full Court, which means that the injunction applications are refused.
That second option would… provide an appropriate counterbalance to the damages threat which Wyeth relies upon…
361 As explained, I disagree with these submissions. Wyeth had the benefit of the method patent. The method patent gave Wyeth the exclusive rights of exploitation provided for in s 13 of the Patents Act (to exploit the invention and to authorise another person to exploit the invention). Unless and until the method patent was revoked Wyeth continued to have those rights. Threats to seek interlocutory relief are part of the ordinary exigencies of the existence of litigation. If Wyeth had withdrawn its interlocutory application on 22 May 2009 its only exposure to liability would have been to costs. It would not have been liable to Sigma for any loss Sigma might have suffered as a result of Sigma taking steps in anticipation of the hearing of the interlocutory application. It also would not have given the undertaking and would not be exposed to a claim for compensation under the undertaking by Sigma. These considerations support the view that everything that occurred up to 22 May 2009 is taken to have occurred for the purposes of the analysis.
362 Apart from this, as I have said, the notion that it is to be assumed contrary to the fact that the interlocutory injunction was refused let alone that it was refused on the ground that founded the Full Court’s orders is irreconcilable with the repeated emphasis in Air Express on the requirement that any compensation relate to the effect of the interlocutory orders, not the litigation. To assume otherwise would effectively remove the majority of the risk which the method patent presented to the generics. If the analysis proceeds on that basis, the inevitable tendency would be to compensate the generics for the existence of the method patent and the litigation which is impermissible.
363 It is because the notion of the Court refusing Wyeth’s application for the interlocutory injunction gives rise to the question of the grounds of refusal that the better course is to adopt Wyeth’s approach and assume no more than that on 22 May 2009, contrary to the fact, Wyeth did not prosecute its application for interlocutory relief. I accept that this leaves unanswered the question of why Wyeth did not do so. In my view, however, this is exactly the position the generics would have been in had Wyeth not prosecuted its application for interlocutory relief on 22 May 2009. I say generics rather than Sigma here because it is clear that within a relatively short amount of time the generics all knew what was happening about the method patent litigation.
364 If Wyeth had not sought the interlocutory injunction on 22 May 2009 Wyeth would not have been obliged to explain itself. The mere fact of Wyeth not prosecuting its interlocutory application would have given the generics no clue as to why Wyeth had so decided. One thing that cannot be doubted is that Wyeth would not have said or done anything which might have given the generics any comfort. If it had not prosecuted its interlocutory application on 22 May 2009 Wyeth would have made it clear, as it otherwise had always maintained, that in its view the method patent was valid and it intended to enforce its rights. The generics all had legal advice. The lawyers all would know that there are a multitude of reasons why a party might not seek interlocutory relief. At the least they would know that by not seeking interlocutory relief Wyeth was not waiving its rights to claim that the method patent was being infringed and seek relief in the form of final injunctions and for damages.
365 The difficulty with the contrary approach of hypothesising that the Court refused Wyeth’s application is exposed in some of the evidence. For example, Mr de Alwis said in an affidavit that he had been asked “what Sigma would have done if the Federal Court refused to grant an interlocutory injunction against Sigma”. Mr de Alwis said:
If the Federal Court refused to grant an interlocutory injunction against Sigma and Sigma was free to launch both on the private and PBS markets, I would have perceived this as a very strong indication from the Judge that he considered the Patent was invalid and that Sigma had excellent prospects of revoking the Patent in the substantive proceeding. This is because I understood that the balance of convenience was weighted strongly in favour of the originators due to the introduction of the mandatory price reduction in 2005, and that since then, courts had routinely granted interlocutory injunctions in favour of originators.
366 In other words, Mr de Alwis has assumed a judgment which disclosed that Sundberg J considered the method patent to be invalid so that Sigma had excellent prospects of revoking the patent. This hypothesis would have the impermissible effect of removing the existence of the method patent and the litigation from the analysis. This also exposes the artificiality of the exercise the witnesses were asked to undertake. Presumably because the question lacks context (why was the application refused) Mr de Alwis has taken it upon himself to supply the most favourable context he can conceive of which was that Sundberg J concluded that there was such a strong case the method patent was invalid interlocutory relief had to be refused. This confirms my view that for this and many other reasons inferences about commercially rational behaviour from objective circumstances are a far better guide to the probabilities and possibilities of what might have occurred but for the interlocutory injunctions.
367 The hypothetical starting point I prefer is that on 22 May 2009 Wyeth did not prosecute its application for interlocutory relief. The method patent existed. The litigation existed. Everything that had occurred up until 22 May 2009 had occurred. The generics could surmise as much as they might wish as to why Wyeth did not prosecute its interlocutory application on 22 May 2009 but any such surmise would be in the context of the fact that Sigma had filed its application for revocation of the method patent on 1 April 2009, Wyeth had filed its cross-claim including an application for an interlocutory injunction on 1 May 2009 and on the scheduled hearing of the interlocutory injunction application on 22 May 2009 Wyeth did not prosecute its interlocutory application. Wyeth’s cross-claim against Sigma for infringement and final relief including an injunction and damages otherwise remained.
368 It is necessary to consider the events leading up to 22 May 2009 to test Wyeth’s proposition that Sigma decided to withdraw its application to list its products on the PBS because it feared triggering the 12.5% price reduction and/or because if as Sigma contended it was attempting to avoid a complete interlocutory restraint on supply and was thus prepared to give up PBS supply to increase its chance of private market supply then Sigma would have realised on 22 May 2009 that its strategy had worked and would not thereafter have risked prompting a further application for interlocutory relief by changing its mind and again seeking PBS listing. To the extent Wyeth’s submissions rely on Sigma’s attempts to avoid an interlocutory injunction it would be wrong to assume that Wyeth in not prosecuting its interlocutory application did anything more than not prosecute its interlocutory application. Wyeth’s submissions in this regard appear to assume that it would be common ground between it and Sigma that it was not prosecuting its interlocutory application because Wyeth had accepted Sigma’s offer not to list its products on the PBS and to confine its launch to the private market only.
369 The first point is that this is part of a hypothetical analysis. In fact, Wyeth did not accept Sigma’s proposal and was not prepared to risk Sigma being permitted to supply on the private market. The second point is that once the point at which the hypothetical analysis starts there is no better reason to assume in Wyeth’s favour that it did not seek interlocutory relief because it was prepared to accept Sigma’s offer than there is to assume that Wyeth did not seek such relief because it considered the method patent to be weak and likely to be declared invalid. Both assumptions skew the analysis in favour of one party or the other whereas, in my view, the better starting point is as neutral as possible. Wyeth had not accepted Sigma’s offer. Thus Wyeth is not to be assumed at the start of the hypothetical to have communicated that it was not prosecuting its application for interlocutory relief because it was content for Sigma not to PBS list its products and instead supply them on the private market. Wyeth was not so content. Nor is to be assumed that Wyeth communicated any concern about the validity of the method patent. It simply did not prosecute its application for interlocutory relief on the day it was listed.
370 The problem with Wyeth’s approach is that it assumes contrary to the fact that it had accepted Sigma’s offer not to list on the PBS and to confine its supply to the private market pending the final hearing. The terms of this offer, specifically whether it was conditional on a cross-undertaking from Wyeth as to damages, were in dispute. But irrespective of any confusion about the terms, Wyeth did not accept the offer. Wyeth’s approach then assumes that, in the hypothetical analysis, it said that it was not seeking interlocutory relief because it accepted Sigma’s offer not to list on the PBS. The path of potentialities then opens up. Would Sigma have insisted that its offer was conditional on a cross-undertaking from Wyeth? If so, we have a hypothetical cross-undertaking under which Wyeth would be exposed to the same claims as Sigma now brings. If not, why not? Whatever the reason, the hypothetical would be skewed immediately in Wyeth’s favour.
371 From the as neutral as possible starting point I consider appropriate, the following matters are reasonably clear. Having not accepted Sigma’s offer and not prosecuted its application for interlocutory relief for alleged infringement of the method patent on the day it was listed for hearing, Wyeth and Sigma would both assume that Wyeth would not again apply for interlocutory relief based on the method patent. Wyeth had had its chance to do so and had not taken it. They would operate on the basis that at least insofar as the method patent was concerned, the litigation would run its course to final hearing. Sigma would make its decisions about its products in the context of the existence of the method patent and the litigation. Wyeth would make its decisions about what it should do on the basis that it had decided not to prosecute its application for interlocutory relief based on the method patent when it had the opportunity to do so.
372 For these reasons I consider much of the debate about the terms of Sigma’s offer not to list its products on the PBS immaterial. No aspect of Sigma’s conduct between 6 March and 22 May 2009 irrevocably bound it not to exercise the full range of rights which ARTG registration of its products gave it. This said, it is time to move to those events.
Events between 6 March and 22 May 2009 (and the evidence of Mr de Alwis and Mr Ellis)
373 To recap briefly, Sigma had placed its first order for its products from Generic Health on 4 September 2008 and was invoiced by Generic Health for that order on 24 October 2008. It placed its second order on 12 February 2009 and was invoiced for its second order on 23 February 2009.
374 The TGA advised Sigma that it has approved registration of Sigma’s products on the ARTG on 26 February 2009. On 27 February 2009 Sigma applied for PBS listing of its products. On 2 March 2009 Sigma informed the Department of Health that it wished to obtain PBS listing on 1 August 2009. Sigma’s products were registered on the ARTG on 5 March 2009. On 6 March 2009 Sigma informed the Department that it wished the Department to consider listing its products on the PBS before 1 August 2009. On 6 March 2009 Sigma also became aware of the method patent.
375 It may be assumed that the discovery of the method patent was a significant blow to Sigma. The Sigma group was under serious financial pressure by 2009. As Wyeth noted from the annual financial report of Sigma Pharmaceuticals Limited for the year ending 31 January 2010 the Sigma group had:
(a) a loss of net profit before tax of $389 million (even taking into account $237 million raised by SPL through a capital raising in September 2009 to improve or secure its financial stability ) (pp. 3, 4);
(b) the loss of net profit was primarily driven by an impairment of goodwill of $424 million. This was due to “a progressive deterioration in competitive conditions and a changing regulatory environment … [a]dditionally, the synergies expected from the merger of Sigma Pharmaceuticals Limited and Sigma Company Limited in 2005 have not been achieved… (p. 4)”.
376 For Sigma itself, referred to as the pharmaceuticals division, the report showed an EBIT (earnings before interest and tax) amount excluding a goodwill impairment of $59.8 million and a goodwill impairment of $184.4 million. The EBIT reflected Mr de Alwis’s criminal conduct in what would colloquially be described as “cooking the books” of Sigma Pharmaceuticals Limited (and having cooked them Mr de Alwis then lied to the auditors when asked about the falsifications).
377 Mr de Alwis, the CEO and a board member of Sigma Pharmaceuticals and managing director of Sigma in 2009, committed four offences to which he later pleaded guilty which Wyeth summarised as follows:
(a) first charge: conduct in connection with the purchase of Livial and products and the accounting treatment thereof that resulted in the falsification of Sigma’s financial report for the half year ended 31 July 2009 in that Sigma’s revenue and income for that half year was overstated by $3,500,616.43 and inventories were overstated, contrary to section 1307(1) of the Corporations Act 2001 (Corporations Act);
(b) second charge: conduct in connection with the purchase of Livial, Ozmep, Sozol and Clovix products and the accounting treatment thereof that resulted in the falsification of Sigma’s full year financial report for the financial year ended 31 January 2010, and that Sigma’s other revenue and income for that financial year was overstated by $15,500,616, inventories were overstated by $11,313,224, prepayments were overstated by $2 million, and total equity and profit after tax were overstated by $9,599,000, contrary to section 1307(1) of the Corporations Act;
(c) third charge: giving information to PricewaterhouseCoopers, the auditor of SPL, which he knew was false or misleading in a material particular, or had omitted from it matters the omission of which rendered the information misleading in a material respect contrary to section 1309(1) of the Corporations Act;
(d) fourth charge: giving information to the directors of SPL which he knew was false or misleading in a material particular, or had omitted from it matters the omission of which rendered the information misleading in a material respect, contrary to section 1309(1) of the Corporations Act.
378 There were seven directors on Sigma Pharmaceuticals’ board apart from Mr de Alwis who were given the false financial information by Mr de Alwis. In 2015 Mr de Alwis was convicted. As Wyeth recorded, “[i]n relation to the first charge, Mr de Alwis was sentenced to imprisonment for nine months; for the second charge he was sentenced to imprisonment for nine months; for the third charge he was sentenced to imprisonment for one year; and for the fourth charge he was sentenced to imprisonment for one year. He was, however, released on a recognisance release order with a good behaviour bond and payment of a pecuniary penalty of $25,000”.
379 So we have Sigma under substantial financial pressure, a CEO who was falsifying the accounts to overstate revenues, Sigma anticipating making substantial money out of its generic venlafaxine products, and Sigma having ordered and paid about $3 million for its generic venlafaxine products from Generic Health (327,000 units) when it discovers the method patent. To this might be added that, as Mr de Alwis said in one of his affidavits from the interlocutory injunction proceeding in 2009, this stock had a two year shelf life. It does not take much to infer that threatened with an interlocutory injunction which if granted would preclude supply of any stock at least until final judgment at first instance, Sigma knew it was at serious risk of being stuck with $3 million worth of stock it could not sell and presumably ultimately would have to destroy. Mr de Alwis said this in his principal affidavit in 2009 as a reason against the grant of an interlocutory injunction. Against this, Sigma’s plans for launch were tied to expiry of the compound patent. Sigma was not intending to launch at risk of infringement and liability for damages when it made its plans. It was intending to launch without risk based on the expiry of the compound patent.
380 Wyeth emphasised that this reflected Sigma’s overall policy recorded in one of Sigma’s documents that:
Sigma’s policy and history is not to infringe any patents. R Beveridge stated the policy was launch generics as soon as possible legally. For example, Sigma and Arrow intend to go to market as soon as a relevant known patent has expired, engineer their products around other patents.
381 That this was Sigma’s policy may be accepted. But it would be wrong to assume that the policy automatically meant that Sigma would simply shelve its plans and do nothing for the eight years until the method patent expired (and Wyeth does not suggest this to be the case, as it accepted that Sigma would have continued with its private supply). Sigma did not do nothing. This is not unexpected. It was confronted with a difficult, perhaps unprecedented, situation. Whatever its general policy it could not take the same approach it did with the compound patent, simply to await is expiry, and it did not do so. Accordingly, this policy is of limited weight because it reflects a general approach only. The same applies to Mr Ellis’s evidence that Sigma’s policy was to launch when “it was legally free to do so or with negligible legal risk.” A general policy is one thing. Being confronted with unexpected and exceptional circumstances is another.
382 Sigma’s concern about being stuck with $3 million of stock it could not sell because of Wyeth obtaining an interlocutory injunction becomes immediately apparent. Mr Ellis contacted a solicitor and then emailed Mr de Alwis saying:
If we launch now Wyeth are likely to seek an injunction on the basis of this patent. The “advantage” of launching now (pre-PBS) is that they are less likely to get an injunction particularly as there appears to be reasonable grounds to mount an invalidity action and the 12.5 % has not been triggered. Once the 12.5 % is triggered it is most likely an injunction would be granted as this is currently irrevocable and not considered by the courts to be remedied by undertakings or guarantees. If we launch and were unsuccessful in a revocation action the worst case outcome would be all costs (approx. $1M each side) and damages/accounting of profits, significantly more than $3M (emphasis added).
383 In other words, Sigma was weighing the risk of costs and damages against the $3 million it had paid for stock. Mr Ellis confirmed in oral evidence that he had estimated Sigma’s exposure if it listed its products on the PBS and caused the 12.5% reduction in the price of Efexor-XR as in the order of in excess of $100 million (that is, he assumed Sigma was likely to be liable for this amount if the method patent was valid). This amount reflects a 12.5% price drop on Efexor-XR over the remaining eight years of the method patent on the basis Wyeth was selling around $110 million of Efexor-XR annually. Mr de Alwis, in contrast, agreed at one point that he was aware that the PBS listing could expose Sigma to a damages claim in excess of $100 million. Later, Mr de Alwis said “I don’t agree with the $100 million. If you are saying it’s what Sigma would have had to pay Wyeth, it may be correct. If you’re saying that’s the cost to Sigma, it’s completely wrong”. And then there was this exchange:
And ..... eight times about $100 million plus market is causing a loss on that scenario – if you lose the revocation case, you have in excess of $100 million? In calculating what it would have cost the company, that is certainly one factor I would have taken into account. It wouldn’t have been 100 million; it would have been discounted back. I wouldn’t have paid for ..... million for eight years out. It would have been discounted back to today’s value. And I would have also offset against that the benefits that we would have gained as a result of launching Evelexa.
384 Sigma submitted that Mr de Alwis was not challenged about this evidence, but he was. The exchange continued:
You say discounted back to today’s date. When did you think a final hearing on damages would have been heard and determined? Whenever it was.
It could be five or six - - -? 2010 was mentioned.
Yes. Well, that would be just be the revocation suit, wouldn’t it? Yes. Well, it didn’t matter. The principal is still the same. It doesn’t matter the date.
So about five years down the track, you might have a damages – the damages hearing would be heard. Correct? Yes, but as I said, I would have offset that. I did offset against the potential payment the benefits that Sigma would have achieved, not just from the sale of Evelexa, which was significant, but from all the other benefits that we would have accrued as a result of being first to market with a blockbuster drug.
And where were the calculations of that you made? I certainly made them myself.
In your head? Well I don’t know that’s in my head. I’m sure it was – my mental arithmetic probably isn’t that up to speed, but I would have run pen on paper.
385 This is unconvincing evidence. It had the hallmarks of Mr de Alwis (who holds accounting and finance qualifications) attempting to claw back ground from an admission (Sigma would be exposed to in excess of $100 million in damages of the method patent was valid and Sigma listed on the PBS) he realised would weigh against his evidence that he would not have been deterred from PBS listing Sigma’s products. It is apparent that Mr de Alwis was attempting to convey that Wyeth’s potential loss over eight years would be assessed in 2010 or before the method patent expired and its loss would therefore be calculated on a net present value basis. If Mr de Alwis had actually done such a sophisticated exercise in 2009 and could now recall what he had done as he was suggesting then he would have known that the 2010 date was the date for hearing of the revocation and infringement claims. He would know that damages would be assessed at a later time if the method patent was valid and was infringed (and, in this regard, Sigma was effectively assuming its products did infringe the method patent, which was a proper assumption in the circumstances). He would know that whatever loss Wyeth would have suffered before judgment on damages would not be discounted back to the date of judgment and that only future losses would be discounted back. Holding accounting and finance qualifications, he also would have anticipated liability for interest. In my view, Mr Ellis’s evidence is far more likely to be reliable than that of Mr de Alwis in this regard. Accordingly, Sigma estimated that if it listed its products on the PBS and the method patent was valid it would be liable for damages to Wyeth in the excess of $100 million.
386 In these circumstances and given the $3 million it had paid for stock it is not unexpected that Sigma’s immediate focus was to ensure it could avoid an interlocutory injunction. Mr Ellis met with Griffith Hack on 10 March 2009. Mr Ellis sent Mr de Alwis a note about the meeting which recorded:
Will probably have to give an undertaking not to list on the PBS to avoid injunction being granted …
worst case, avoid injunction but loose [sic] revocation case – costs against us and damages or accounting of profit. If we are marketing as a private and case runs for 18 months and we gained 10% of the market this could be in the order of $15M.
387 For what it is worth, there is no suggestion that the undertaking would be conditioned on a cross-undertaking from Wyeth, which I find unsurprising because what was being proposed was a strategy to avoid an interlocutory injunction altogether on the basis that Sigma would be free to supply on the private market.
388 Griffith Hack’s file note of the same meeting also does not mention any cross-undertaking from Wyeth, which is consistent with the view that what was being devised was a strategy to avoid any interlocutory injunction by Sigma agreeing not to apply to list its products on the PBS and Wyeth agreeing not to try to restrain Sigma from supplying its products on the private market only. Otherwise the file note records what Sigma’s plans and expectations had been before it became aware of the method patent and notes, as Sigma submitted:
Gilbert + Tobin (G + T) were acting for Wyeth. “[copyright] issue on PI likely to be thrown in”. “[D]ifficult for Wyeth to get an int[erlocutory] inj[unction] in relation to the [copyright] issue” and it “should be fixed by spring session of Parliament”.
389 This confirms Sigma’s awareness of the copyright issue and that the issue, in any event, would not have had any effect on Sigma’s plans.
390 The note also recorded:
- don’t take orders
- earliest it might be is 4 weeks.
- need to know by 1 May re PBS listing.
391 In other words, until Sigma had decided what to do about PBS listing it should not take orders from pharmacists and it would not know Wyeth’s position for at least four weeks.
392 There can be no doubt from this and other evidence that Sigma believed that the only chance it had of avoiding being restrained altogether by an interlocutory injunction was not to have its products listed on the PBS. Sigma was thus never in fact called upon to consider if it would list its products on the PBS if no interlocutory injunction was sought on 22 May 2009.
393 Sigma’s fears of Wyeth seeking interlocutory relief were well-founded. On 12 March 2009 Gilbert & Tobin, Wyeth’s solicitors, wrote to Sigma saying that “Wyeth is concerned that the supply and use of the EVELEXA XR Products may infringe the [method patent] and in those circumstances it may have the right to obtain relief from the Federal Court in relation to such infringement”. As Sigma also noted:
On 19 March Sigma was served with various affidavits filed by the Wyeth Parties in a preliminary discovery application brought by the Wyeth Parties against the TGA in respect of potential infringement of the Patent. Those affidavits included definitive statements about the Wyeth Parties’ intentions to seek an interlocutory injunction to restrain asserted infringement of the Patent.
394 Another Griffith Hack file note of 20 March 2009 says “if have to give undertaking not to list on PBS”. This means if Sigma had to undertake not to seek PBS listing in order to avoid an interlocutory injunction, it would do so. Beyond this, the note is neutral. It does not mean, as Wyeth would propose, that this also would have been Sigma’s position if Wyeth had not prosecuted its application on 22 May 2009. It also does not mean that Sigma had decided to proceed with its PBS listing unless restrained by an interlocutory injunction. The note is recording only a strategy for the purpose of trying to avoid an interlocutory injunction. As Sigma said, what can be inferred is that PBS listing remained “on the table”. The note also records “Sigma needs advice on prospects of success to decide what to do”. As Sigma said, this is inherently plausible although I would not go so far as to say the advice “drove its decision-making”. But the advice was in fact an important factor and, it may be inferred, legal advice would remain an important factor after 22 May 2009.
395 The legal advice from Griffith Hack was received on 30 March 2009. Sigma stressed those parts of the advice which recorded that an “interlocutory injunction is expected to be filed by Wyeth shortly regarding importation and supply in Australia by Sigma of capsules containing venlafaxine hydrochloride” and:
It appears likely that Sigma products would infringe at [sic] some of the granted claims … However, in view of the strong grounds for invalidity of these claims, the difficulty of proving infringement of at least some of these results based claims and non-infringement of at least some of the claims, several important ‘balance of convenience’ factors weight in Sigma’s favour.
396 Wyeth emphasised that this advice as to “strong grounds for invalidity of these claims” related to the context of an interlocutory injunction and not final relief, and referred to Mr Ellis’s evidence that he understood this to be so. Consistent with my reasoning above about the likely unreliability of evidence about what a person would have done or recommended nine years ago, I am also of the view that evidence of this kind is inherently unreliable even if it is on one view contrary to the interests of the party calling the witness. Mr Ellis seems a paradigm example of this unreliability despite my view that he was giving honest evidence as carefully as he could. For example, Mr Ellis struck me as a cautious man. In his affidavit he gave evidence including the following:
(1) “I also recall that in March or April 2009 I undertook rough calculations as to what Sigma’s damages exposure was likely be if it launched on the private market and also listed on the PBS so as to trigger the mandatory price drop. I recall that these calculations took into account Wyeth’s lost profits arising from the mandatory price drop and the sales lost to Sigma, as well as the profits that Sigma would make. I cannot now recall the figures that I calculated but I recall that they were significant”.
(2) “Although I assessed that Sigma’s damages exposure if it listed on the PBS and triggered the mandatory price drop would be significant, I considered that Sigma’s risk of having to pay out damages was low given that the prospects of revoking the ‘586 Patent were high… On that basis, I believed that Sigma should proceed to launch EVELEXA XR at risk and list on the PBS if it could avoid an interlocutory injunction and I made my view known to Mr de Alwis”.
(3) “I also understood that if Wyeth sought an interlocutory injunction the prospects of Sigma being able to avoid the anticipated interlocutory injunction without undertaking not to list on the PBS (in exchange for the usual undertaking) were slim. Accordingly, I thought it was sensible for Sigma to take the approach of planning to launch in the private market first and then list on the PBS only if, and when, it was free to do so. I understood that to be the approach that was being taken throughout April and May 2009”.
(4) “If Wyeth had not sought an interlocutory injunction, I expect that I would have taken any remaining necessary steps to assist Sigma to continue with its original plan, which was to launch EVELEXA XR privately in May 2009 and list on the PBS on 1 August 2009. I would have discussed the commercial and legal risks with Mr Condon, Ms Morgan and Mr de Alwis, and taken direction from Mr de Alwis. At that time, I considered that Sigma had high prospects of revoking the patent and therefore that the risk of having to pay damages was low. If Wyeth had not sought an interlocutory injunction, I would have considered that it was likely that Wyeth had obtained advice that the ‘586 Patent may be invalid and that it would be unlikely to succeed in an infringement action against Sigma. At that time, the ultimate decision would have been made by Mr de Alwis”.
397 In his oral evidence, Mr Ellis, for example, said that:
I formed the view fairly early on that we had a high chance of success, and that continued to improve – my confidence continued to improve as we became aware and were advised by our both legal and technical experts.
…
I can vividly recall my confidence building and certainly being comfortable with the evolution that happened in terms of our confidence of being successful.
…
I pushed him [Sigma’s lawyer] further and I said what do you feel our chances of success are and he said 80 per cent.
I think he said it was “my best estimate was 80 per cent”.
I interpreted it to be very low risk, that we had a high probability of winning… I saw that as being a low risk.
The 80 per cent, what was that referring to? Revocation of the patent.
In the final hearing? Yes.
…
And you give some evidence to say the decision as to whether or not to PBS list after interlocutory hearing or after May you say was ultimately a decision for Mr De Alwis? It was.
But you know that he would have had to go to the board, wouldn’t you? He should have gone to the board.
He should have gone to the board. He would have needed to go to the board, as you understood it, to seek an approval in relation to taking a risk of more than $100 million? That’s what should – in that scenario, that’s what should happen.
398 Thus, apart from Mr Ellis having said PBS listing was Mr de Alwis’s decision when in fact he believed Mr de Alwis should have gone to the board, the overall impression from Mr Ellis’s evidence is that he was confident that Sigma would ultimately be successful, believed the risk of Sigma having to pay Wyeth damages of over $100 million was low, and thus would done all he could to facilitate PBS listing and supply under the PBS if not for the Sigma interlocutory injunction. Yet there was one piece of evidence which Mr de Alwis gave about Mr Ellis which I accept. Mr de Alwis said:
I can recall a number of discussions we had internally with Mr Ellis, myself and Ms Morgan. I got to a stage where I felt that Mr Ellis, in particular, was – was seeking to take an easy option or saying let’s not launch this, because we avoid all this hassle. …
Now, you said today you felt Mr Ellis, in particular, was seeking to take an easy option or saying, “Let’s not launch this because we avoid all this hassle.” Do you remember saying that this morning? Yes.
His view was you shouldn’t launch at all, wasn’t it? Sorry, his view was
His view was you shouldn’t launch at all private or otherwise? Yes. That was his view, yes, which I disagreed with.
And that was his view, having heard whatever the lawyers had said to him, as you understood it? Well, I know it was his view. But, at the end of the day, it was not a decision he was going to make.
That was his view because he thought it was too risky, as you understood it? He didn’t tell me the reasons why. He just thought that it was, you know – my perception, rightly or wrongly, was that he was trying to find an easier course of action than go through what we had to go through in order to launch.
399 As I have said, I formed the view that Mr Ellis was an honest and careful witness. But his evidence about what he believed at the time and would have done is irreconcilable with Mr de Alwis’s evidence about what Mr Ellis did do and say at the time. I do not consider that this part of Mr de Alwis’s evidence was false. First, irrespective of the fact that he was no longer employed by Sigma, Mr de Alwis was not inclined to concede any point against his perception of Sigma’s interest. Second, the evidence accords with the overall impression which I formed of Mr Ellis as a cautious man. The best explanation for the inconsistency, in my view, is that Mr Ellis’s beliefs about what he thought at the time have been largely shaped by hindsight, particularly the knowledge that Sigma was ultimately successful. The entire period between 2009 (the period to which Mr Ellis’s evidence relates) and 2011 (when Sigma was successful in the Full Court) is so long ago that even the evidence of an honest and careful witness like Mr Ellis about what he believed and said at the time is unreliable. When the task is to go further and say what the witness would have done eliminating the effect of hindsight, the task is almost impossible even for a witness like Mr Ellis.
400 I would not describe Sigma’s other main witness about what he would have done but for the interlocutory injunction, Mr de Alwis, as a careful witness and, perhaps, not even an honest witness. Apart from the matters discussed above, including his proven willingness to lie to the face of Sigma’s auditors about his criminal conduct, I note the following which I consider support the inference that Mr de Alwis was not a credible witness when it came to any contested issue (or issue he perceived might be contested):
(1) Despite the obvious importance of Sigma not being stuck holding $3 million of stock it could not sell, this does not feature as one of Mr de Alwis’s key concerns in his 2015 affidavit.
(2) In his 2015 affidavit Mr de Alwis said that in mid-April 2009 he believed the strategy of not listing on the PBS to avoid an interlocutory injunction was a good approach because, amongst other things, if the Court ultimately found in favour of Sigma it would be able to list its products on the PBS and recoup its losses from Wyeth for being kept out of the PBS market when, in fact, Mr de Alwis had been advised in conference with senior counsel that it was unlikely that such recoupment would be possible if Sigma voluntarily undertook not to seek PBS listing to enable it to launch at least on the private market.
(3) Although he said in his 2015 affidavit that he would instruct Mr Ellis and Ms Morgan to obtain legal advice about Sigma’s prospects of success in the revocation proceedings and the damages which Sigma would face if unsuccessful for the purpose of presenting that information to the board, he appears in his affidavit to have assumed that irrespective of the exposure he would have recommended a launch on the PBS at risk and that the board would have treated the decision as an “operational” one for him, because the board was not experienced with generics and had never acted contrary to is recommendation. This lack of apparent care about what the exposure might be is irreconcilable with the contemporaneous evidence where Mr de Alwis was concerned to limit Sigma’s risk even on a private market launch.
(4) In any event, as noted, Mr de Alwis’s evidence about what he would have done assumed he would have received a judgment from the Federal Court effectively advising him that the method patent was so likely to be invalid, that interlocutory relief should not be granted. For example, Mr de Alwis said:
In presenting the decision to list EVELEXA XR on the PBS, I would have put forward to the Board the ‘best’ and ‘worst’ case scenarios, with the worst case scenario including the potential damages which Sigma would be required to pay if Sigma was not ultimately successful in revoking the Patent. I would have emphasised that the risk of the worst case scenario eventuating was extremely low, given that the Court's refusal to grant an interlocutory injunction sent a very clear message that the Patent was likely to be invalid, a message which Arrow Group had also consistently communicated to Sigma. I also would have emphasised the importance of obtaining a first-mover advantage, keeping Sigma's promises to pharmacies to list on the PBS if an injunction against Sigma were not granted and maintaining the morale of Sigma's sales representatives.
I believe there was a high probability that the Board would have accepted without objection my proposed course of action to launch EVELEXA XR at risk.
This evidence cannot be given weight because it assumes a fact (a Federal Court judgment in 2009 to the effect the method patent is invalid) which cannot be assumed for the purpose of the required analysis. Having expressed these views in 2015, Mr de Alwis was unlikely to alter his views when confronted with different assumptions.
(5) Mr de Alwis repeated his views about most of the board being “relatively new” to generics but Sigma had taken over Arrow’s substantial generics portfolio in December 2005. It is also apparent that a decision to launch at risk on the basis of PBS listing and supply, in Sigma’s mind, would have exposed it to an extremely large claim for damages. For a company with a policy and history of not risking patent infringement, which had planned its launch to coincide with the expiry of the compound patent, the idea that the decision would have been that of Mr de Alwis or that the directors of a publicly listed company would not bring their own independent scrutiny to bear is untenable. As Wyeth submitted:
Mr de Alwis would have gone to the SPL’s Board so that it could determine whether or not it was willing for SPAL to launch on the PBS market at risk. The evidence is clear that the approval of the Board was required: “I had to prepare a paper to the board where I justified my decision”. Whether Board approval is characterised as a condition precedent or a condition subsequent to a proposed course of action or “decision” is irrelevant to the substantive requirement of Board approval.
(6) Despite my view that Mr de Alwis must have known at the time at least that Sigma had decided to not proceed with PBS listing whether or not Wyeth gave a cross-undertaking, Mr de Alwis was prepared to describe contemporaneous Sigma documents to that effect as a mistake or wrong in order to shore up the evidence he had given that Wyeth giving a cross-undertaking was always fundamental to Sigma’s decision. The reality is that Sigma would hardly have withdrawn its PBS listing application as Mr de Alwis had instructed be done on or about 31 March 2009 when he decided the launch should be confined to the private market without Wyeth having given a cross-undertaking (as was the case) if the cross-undertaking was seen to be necessary (in contrast to desirable once it had been conceived of). Yet Mr De Alwis was determined to stick to his version of events as recreated in 2015, saying (contrary to the fact) that “our offer to Wyeth was always conditional to getting a cross-undertaking from them” and this requirement appeared “in every single letter” (when it did not).
(7) Mr de Alwis’s attempt to explain his comment recorded in one of the file notes of the 31 March 2009 conference about keeping the discounts small to limit Sigma’s exposure as not reflecting his contemporaneous concern about the amount of risk he was prepared to expose Sigma to, but instead being about his “desire to minimise the cost to the company in the event that we had to pay damages”, involves a distinction without a difference and discloses Mr de Alwis’s unwillingness to concede what must have been obvious at the time.
(8) Despite having said in his 2015 affidavit that he would have instructed Mr Ellis and Ms Morgan to obtain legal advice including about Sigma’s potential damages exposure Mr de Alwis would not agree that in or around 2009, assuming no interlocutory injunction, he did not have sufficient information of this kind and instead said he “could have made a decision with what I had, but there was no down side to seeking further information”.
(9) Mr de Alwis said that “we felt the probability of losing that case was, based on the advice I had got, was very small” but even the most bullish verbal advice Sigma received put the chance of success at around 80%. Given the potential liability, a 20% risk of losing could not have been seen at the time as “very small”.
401 These matters weigh heavily against accepting anything Mr de Alwis said about what he would have done or believed would have occurred if not for the interlocutory injunction.
402 To the extent Mr Heine gave evidence about what Sigma would have done if not the subject of the interlocutory injunction, no more need be said than my general observations about the inherent unreliability of such evidence and the fact that, in contrast to Mr de Alwis (and, to some extent, Mr Ellis) Mr Heine’s role within Sigma does not indicate that he would have been involved in the process of informing the board of Sigma’s options other than, perhaps, being a source of information for Mr Ellis and Mr de Alwis. As a result, these aspects of Mr Heine’s evidence also should not be given weight.
403 To return to the chronology of events, the idea that Mr Ellis dissected the legal advice into advice about Sigma’s prospects of establishing the method patent was prima facie invalid for the purpose of the interlocutory injunction hearing in contrast to invalid for the purpose of the final hearing is incredible. As would be expected by people like Mr Ellis given his role, the advice is framed in terms of prospects of success and in the context of the immediately pressing issue of Wyeth seeking an interlocutory injunction. It is not the kind of advice that would have been relied upon for Sigma to decide whether or not to list on the PBS. But it did convey that there were grounds upon which the method patent was likely to be invalid. Thus, this was said:
(1) Inventive step: “[w]e consider that inventive step is the preferred ground on which to base a successful challenge to the validity of AU 2003259586… We therefore consider that a successful challenge under this ground is likely providing expert opinion is obtained to support the above position”; and
(2) Fair basis: “[w]e recommend challenging the patent on the ground of fair basis because we consider the method claims covering amendments filed on 20 December 2006 are not fairly based on the matter described in the complete specification as originally filed”.
404 On 31 March 2009 there was a conference with senior counsel. Wyeth contended that the file notes of that conference disclose that Sigma had decided not to launch on the PBS market, was prepared to give an undertaking to that effect, and had decided to launch on the private market only. So much may be accepted at least in the context of Sigma’s wish to avoid being restrained on an interlocutory basis from supplying its products at all. But it is also apparent that the legal advice Sigma received was there were “good grounds” that the method patent was invalid. One of the file notes records “unlikely the court will allow Sigma to recoup loss if forego listing on PBS. Not a lot of precedent”. In other words, if Sigma volunteered not to seek PBS listing in order to enable its private market launch, it was unlikely Wyeth would be required to give an undertaking to compensate Sigma for any loss Sigma might suffer as a result of its voluntary actions.
405 Other important aspects of this evidence include that part of one of the file notes says “impact of not launching is very significant – particularly not launching on PBS (fully)” and that “launching with a private prescription basis, discount less attractive, wants to launch ASAP”. In other words, not being able to PBS list and PBS supply would have a substantial impact on Sigma and confining the launch to the private market would mean that the discount Sigma could offer pharmacists would be less attractive. One of the notes also recorded this:
If defeat int[erlocutory] inj[unction]: can go to market at risk earlier
- but is likely on private market.
406 Sigma submitted that read in the context of the earlier advice given by Griffith Hack and recorded in contemporaneous documents the statement “is likely on private market” can only mean that Sigma was being advised (again) that in practical terms it would need to undertake not to list on the PBS in order to avoid being enjoined from selling altogether. I disagree. In my view, the note was recording that it was likely Sigma’s launch would be confined to the private market even if it was not enjoined. Further, the same note discloses that one powerful reason Sigma wanted to launch on the private market was because it was holding $3 million of stock and that it had no concern about such a launch (on the private market) at risk because its liability would be confined to the stock it sold and would not extend to the price reduction in Efexor-XR which PBS listing would cause. Sigma’s immediate interest in the face of an anticipated interlocutory injunction was to try to salvage its first mover advantage by being the first generic to the market, albeit the private market only. But even for its proposed private market launch, Sigma is recorded as having noted that it wanted to keep its discounts “small” to limit its exposure to a damages claim from Wyeth. Moreover, these views about confining its exposure are attributed in the file note to Mr de Alwis, not the cautious Mr Ellis. I should also note here that in one of his contemporaneous affidavits from 2009 Mr de Alwis said Sigma would not encourage pharmacists to sell Sigma’s products at below the general co-payment contribution and expected pharmacists to sell Sigma’s products at the same price as Efexor-XR. Given that Mr de Alwis’s evidence related to a private market launch only by this stage, this supports my view that Sigma was concerned to limit its potential exposure to a damages claim from Wyeth despite believing it had good prospects of having the method patent declared invalid.
407 In other words, far from Sigma being willing to expose itself to a very substantial damages claim if it triggered a 12.5% price drop on Efexor-XR and supplied on the PBS, Sigma wanted to ensure that it was not left holding $3 million worth of stock it could not supply at all, was willing to forego PBS listing to ensure it could do so and, even for its private market launch, wanted to confine its exposure to damages by ensuring the discounts it offered were small. This was so despite Sigma believing that it had good prospects of establishing the method patent was invalid. This contemporaneous evidence confirms my view that Sigma’s witnesses were giving evidence as a result of the overwhelming effect of hindsight – a risk which in the generics’ case is accentuated by reason of the facts that their plans to launch on the PBS were created before they became aware of the method patent, they ultimately succeeded in 2011 and the time between 2009 to 2011 and the date on which the witnesses were asked to attempt to re-create what they believed and would have done (in 2015 at the earliest) is simply too long after the events for the evidence to be reliable.
408 The evidence that Sigma was concerned to limit its exposure to liability even on a confined private market launch is contemporaneous evidence which tends to suggest that it is unlikely that Sigma would have been willing to trigger a 12.5% price drop by listing on the PBS had there been no interlocutory injunction. The argument that the risk might be greater but so would be the reward overlooks the fact that Sigma already believed the risk to be low because it had good prospects of invalidating the method patent. The relevant point is not so much that Sigma “gave up on” PBS listing (it did give up on PBS listing as a method to try to avoid an interlocutory injunction), but that the contemporaneous records show a company that, understandably, did not want to be stuck holding $3 million worth of stock (which was not a mere risk, but a fact) and despite believing it had good prospects of invalidating the method patent was going to ensure that even its planned private market launch did not increase its exposure to damages claim by Wyeth by offering aggressive discounts. It is this kind of company which existed and would have existed even if Wyeth had not sought an interlocutory injunction on 22 May 2009.
409 It is true, as Sigma said, that:
there was no need for Sigma to make a final decision whether or not to list on the PBS before the injunction hearing – the logical time to finally make that decision would have been if, and after, it managed to avoid the looming injunction and then only if, contrary to what it had been led to expect was the likely position, it was still free to list on the PBS.
410 My point is that it is not obvious that the kind of company which believes it has good prospects of having the method patent declared invalid yet is concerned not to engage in aggressive discounting for even a confined private market launch because it wants to limit its exposure to potential damages is the kind of company that but for the interlocutory injunction would have been willing to trigger the 12.5% price drop on Efexor-XR in the face of the method patent and the litigation. This would be particularly so if the company could, by launching on the private market: (i) resolve its immediate problem of holding $3 million of stock, (ii) be the first generic to market, (iii) confine its exposure to lost sales to Wyeth by not triggering the 12.5% price drop, and (iv) confine its exposure to damages for those lost sales by keeping its discounts small. In contrast, in the hypothetical world, this company would be confronted with an opportunity for PBS listing and supply but an attendant risk if unsuccessful of liability not only for the 12.5% automatic price drop of Efexor-XR, but all of the price drops associated with Wyeth attempting to compete with Sigma’s generic products.
411 On 1 April 2009 Sigma filed an application to have the method patent revoked. It received legal advice not to launch on the private market as planned until the proceedings for revocation were filed as this would be seen to be coming to the Court with clean hands. It is apparent from the file note of 1 April 2009 that it was also seen as important to present Sigma as a company whose policy and history was not to infringe patents. The file note discloses, as I accept to be so, that Sigma saw venlafaxine as the “jewel in the crown” of its generic portfolio and the most significant product for Sigma in the last four years and that its first to market opportunity was important and unquantifiable whereas Wyeth’s damage was quantifiable and “even triggering the 12.5% price reduction is quantifiable, …just not reversible”. I would not take this last observation as evidence of Sigma changing its interlocutory injunction strategy or as undermining Sigma’s desire not to be exposed to a damages claim any larger than it could possibly avoid whilst enabling it to dispose of its $3 million of stock and be first to market. It is a lawyer’s observation about the importance to an interlocutory injunction of evidence relevant to the balance of convenience.
412 Further communications between the lawyers followed.
413 On 14 April 2009 Griffith Hack wrote to Wyeth’ lawyers saying that Sigma was intending to import and supply its products from 1 May 2009 but would undertake not to “make any application for listing of the EVELEXA XR products on the Schedule of Pharmaceutical Benefits, unless it has provided your client with 30 days’ notice prior to making such application for listing”. I agree with Wyeth that it is most likely the letter was framed in this way (recalling that Sigma had already applied for PBS listing on 2 March 2009) because Sigma had decided to withdraw its PBS listing application. I agree with Sigma that this decision was taken in order to try to forestall an interlocutory injunction restrain all supply by Sigma but it also discloses that Sigma was not of the mindset of “PBS supply or nothing”. I do not accept Sigma’s contention that “Sigma was evidently positioning itself to try to get in a position where it could list on the PBS before the validity issue was determined”. This contention is inconsistent with Sigma’s strategy albeit that the strategy was focused on avoiding an interlocutory injunction.
414 On 21 April 2009 Mr Ellis spoke with Griffith Hack and confirmed to Mr de Alwis and others that the legal advice was to “wait until close of business today and if there has been no response from Wyeth activate our launch plans as a private tomorrow”. This is also consistent with Sigma having decided to withdraw its PBS listing application as part of its interlocutory injunction strategy. On the same day, 21 April, Gilbert + Tobin responded noting that it was “not clear from the terms of the undertaking above whether you client intends to apply for listing of the EVELEXA XP products on the PBS on 1 August 2009” and requiring undertakings not to launch until “final resolution of…revocation action and any infringement action brought by our client”. The letter said that if the undertakings were not given Wyeth would commence proceedings for infringement including an application for interlocutory relief.
415 Mr Ellis then instructed Griffith Hack that Sigma was “[h]appy to give undertaking in relation to PBS not to seek listing until completion of the revocation proceedings” and “[w]e…give Wyeth until 28 April to commence interlocutory proceedings”. Wyeth is correct to point out that none of the events so far involved Sigma seeking a cross-undertaking from Wyeth in exchange for Sigma undertaking not to list on the PBS. Again, I find the evidence of witnesses about how important this always was from Sigma’s perspective unconvincing because it is inconsistent with the contemporaneous records. Having voluntarily decided it was going to withdraw its PBS listing application in order to try to avoid being restrained from a private market launch and having been advised in conference with senior counsel that Sigma would not be entitled to a cross-undertaking from Wyeth if Sigma volunteered not to list on the PBS, the far more likely position is that Sigma was working on the assumption it could not obtain any such undertaking at this point and, importantly, it was willing not to try to obtain PBS listing on that basis (that is, no cross-undertaking). The asserted importance of the undertaking from Wyeth is another example of hindsight under the pressure of litigation many years after the event. As we will see, Sigma subsequently thought it worthwhile to try to obtain a cross-undertaking, to which it might be said, why not, but it is plain that Sigma’s decision not to list on the PBS to try to avoid being enjoined altogether was not contingent on such a cross-undertaking.
416 I also do not accept Sigma’s contention that as Sigma had always planned to launch on the private market first (on 1 May 2009) and then to launch on the PBS market after obtaining PBS listing on 1 August 2009, I should infer that Sigma’s plans had not changed because of its discovery of the method patent. This is wishful thinking of a high order. The contemporaneous evidence shows that Sigma had changed its plans. To avoid an interlocutory injunction, Sigma was willing to give up PBS listing until the proceedings were finally resolved. The subsequent letter from Sigma’s lawyers to Wyeth’s lawyers of 22 April 2009 raising the cross-undertaking for the first time does not suggest anything different. In my view, Sigma had changed its position and was willing to volunteer not to list on the PBS whether or not it obtained a cross-undertaking from Wyeth because its real object to was to try to safeguard the private market launch so it would not be stuck with $3 million of stock and would still be the first generic to market. The 22 April 2009 letter, which introduced a requirement for a cross-undertaking from Wyeth, in my view, was an attempt to obtain what Sigma knew would be unobtainable. Sigma had already decided to withdraw its PBS application of its own volition. But I do not consider there is anything improper in Sigma having sought a cross-undertaking. At worst it is regrettable that the letter did not disclose the true position that Sigma had already applied for PBS listing and would withdraw the application but there are many possible explanations for the error. Trying to get a cross-undertaking for a voluntary undertaking not to list on the PBS could not be improper in circumstances where Sigma’s decision not to seek PBS listing was in anticipation of an interlocutory application to restrain it from supply of any kind.
417 Sigma submitted this:
…the letter of 22 April 2009 is not consistent with Sigma having given up on PBS listing prior to determination of the validity of the Patent. If it had given up, why was it insisting on a cross-undertaking as to damages? Such an undertaking would have been of no value to it.
418 In my view, given its belief that Wyeth would succeed in enjoining it from any supply on an interlocutory basis if it did not undertake not to seek PBS listing, Sigma had given up on PBS listing prior to determination of the validity of the method patent. It was seeking a cross-undertaking because its decision was a result of the rightly anticipated interlocutory injunction application. But Sigma’s decision was not irrevocable. If Wyeth did not seek an interlocutory injunction, Sigma could revisit its decision.
419 It will be apparent that I do not accept Sigma’s submission that:
None of the documents referred to above give even the slightest impression that, absent the looming Sigma Interlocutory Injunction, Sigma would have even considered, let alone decided, to give up on its PBS listing.
420 The documents did suggest that even if Wyeth failed to obtain an interlocutory injunction, it is likely Sigma’s launch would be confined to the private market. I accept that this is an indication only, but it undermines Sigma’s apparent case thesis that but for the interlocutory injunction it would have blithely proceeded to PBS listing and supply on 1 August 2009 as it had originally planned.
421 In a letter of 24 April 2009 Wyeth’s lawyers noted the undertaking about the PBS but conveniently ignored the requested cross-undertaking and noted also the lack of any undertaking about the private market launch. Sigma’s lawyers, on the same day, repeated that Sigma’s undertaking was conditional on a cross-undertaking. On 28 April 2009, Wyeth’s lawyers notified Sigma’s lawyers that as Sigma had not undertaken not to launch at all it would be commencing proceedings for infringement of the method patent including a claim for interlocutory relief.
422 On 29 April 2009 Sigma decided that it would proceed with a private launch only which meant that the PBS listing application then had to be withdrawn. Sigma also adjusted its launch material. That material identified why the launch was to be private only noting:
1. Wyeth’s key formulation patent expired December 2008.
2. Sigma commenced legal proceedings against Wyeth to invalidate additional divisional patent.
3. Sigma has provided an undertaking not to launch on PBS until proceedings are resolved.
423 As with everything else that has occurred and no matter what Mr Ellis said in oral evidence this was in the context of the anticipated interlocutory injunction. Again, however, it is revealing that Sigma did not see PBS listing as essential to its position. It believed it could move its $3 million of stock and be first to market without PBS listing. It was also evidently willing to give up PBS listing to avoid being enjoined altogether whether or not Wyeth gave a cross-undertaking.
424 On the same day, Mr Beveridge sent an email to numerous people within Sigma, including Mr Ellis, Mr Heine, Mr Smith and Ms Morgan saying:
We are launching Evelexa-XR as a private prescription immediately.
The sales team will be taking orders from tomorrow, and we have no plans at this stage to apply for PBS [listing]. Please take whatever steps are require to load this product as a private prescription item as CSO contribution will not be claimed on Evelexa-XR. It is very important that pharmacy under no impression that this item is a PBS item and we are only supplying as a private prescription medication.
Stock will arrive in the country towards the end of May, and the team will be putting the product on back-order as orders are taken during May for dispatch at a later date.
425 This is consistent with the conclusions reached above and inconsistent with Sigma’s case theory. This said, the decision of 29 April 2009 does not make good Wyeth’s case because Sigma would have been free to re-visit its decision from 22 May 2009 but for the interlocutory injunction.
426 As noted, Wyeth filed its cross-claim for infringement and interlocutory relief on 1 May 2009. On the same day Sigma launched its products on the private market. It took orders from pharmacists between 1 and 22 May 2009.
427 Griffith Hack provided further advice to Sigma on 5 May 2009. The advice remained that Sigma had strong grounds for challenging the validity of the method patent. Again, there is no reason to infer that Sigma would have read the advice as being about the concept of prima facie invalidity for the purpose of an interlocutory injunction even though the interlocutory injunction was the context. I generally accept the evidence from the Sigma witnesses that they understood that they had good prospects of the method patent being declared invalid. I consider that they also understood that litigation is an inherently risky process and that having good or strong prospects did not mean that it was certain or guaranteed Sigma would succeed. After all, Sigma believed it had good prospects early on but was still determined not to give other than small discounts in the private market in order to limit its exposure to Wyeth.
428 Sigma’s preparedness to launch at risk on the private market in circumstances where it would otherwise be holding $3 million of stock says little if anything about whether it would be prepared to apply again for PBS listing after 22 May 2009 (assuming Wyeth had not prosecuted its application for interlocutory relief on that day). The risks involved in PBS listing and supply would be of a different order entirely. For the reasons given above I do not accept any of the evidence to the extent it suggested that Sigma would have simply proceeded to seek PBS listing as originally planned if on 22 May 2009 Wyeth had not prosecuted its application for interlocutory relief.
429 One, it is clear that Mr de Alwis would have had to take such a decision to the board of the ultimate parent company. The boards of the subsidiaries were the same as that of the parent. Sigma’s submission that Mr de Alwis would have made such a decision personally are untenable in light of all of the evidence. Mr de Alwis’s evidence as a whole can only be understood as saying that he would have formed a view based on information he would have obtained and would have taken his view to the board which he expected to accept his recommendation because it had not rejected any operational decision he had made in the past.
430 Two, despite Mr de Alwis’s cavilling in oral evidence it would have been necessary to identify in some detail not only the potential profits from a PBS listing and supply compared to private market supply but also the potential exposure to Wyeth from the 12.5% price drop in Efexor-XR over the eight year life of the method patent as well as the lost sales to Wyeth at the margins Wyeth could have been expected to be garnering (which given the evidence of the different business models of originators and generics would be expected to be materially greater than Sigma’s margins). And a lawyer asked in 2009 to give legal advice knowing it was to be used by a board deciding whether or to seek PBS listing and trigger a 12.5% price drop in Efexor-XR would have ensured that the advice was subject to all the usual caveats one sees in the advices which Griffith Hack in fact gave.
431 What this means is that Sigma was never going to get legal advice saying anything other than it had good prospects of success or that its prospects of success were strong but litigation is an inherently risky endeavour. Further, Sigma was always going to be told that the amount it could hope to make by PBS listing would be dwarfed by the amount Wyeth would lose and that its potential liability on PBS listing and PBS supply would not be confined to what Sigma made but would most likely extend to Wyeth’s losses – of Wyeth’s sales of Efexor-XR at Wyeth’s margins before the 12.5% price drop and any further discounting by Wyeth to compete with Sigma. This is the reality that is not confronted squarely in the evidence of Sigma’s witnesses because of the confounding effect of the passing of the best part of a decade and hindsight.
432 Sigma’s private market launch appears to have been treated as an operational decision which Mr de Alwis was free to make and did make. But as I have said, the evidence that a decision to list on the PBS and supply under the PBS would have been taken by Mr de Alwis to the board is overwhelming. This also discloses the fundamental difference between taking steps to ensure that Sigma was not stuck with $3 million of stock knowing that the discounts and thus potential exposure to Wyeth could be kept confined (which Sigma did and which I consider a rational business decision in the circumstances Sigma was in) and the obtaining of a listing on the PBS and PBS supply triggering the 12.5% price reduction in Efexor-XR (which would have confronted Sigma with entirely different issues from those raised by the anticipated interlocutory injunction).
433 On 8 May 2009 Sigma belatedly withdrew its application for PBS listing. Mr de Alwis intended this to occur as early as his decision not to launch on the PBS on 31 March 2009 and instructed that it be done at least around 29 April 2009 but the withdrawal of the application appears to have been overlooked until 8 May 2009. In its letter to the Department of Health Sigma said:
[Sigma] wish to advise that we will not be able to supply the above products under the Pharmaceutical Benefits Scheme from 1st August 2009 (PBS listing date) due to legal reasons. The products will be available as Private Script Items from June 2009.
Therefore, Sigma wishes to withdraw the application dated 2nd March 2009 for 1st August 2009 PBS listing for the above products.
Please find attached to this letter the completed PBS Notification form required under Section 99AEG.
434 Section 99AEG is the provision of the National Health Act which makes it an offence for the responsible person for a guaranteed brand not to notify the Minister as soon as reasonably practicable if “during the guaranteed period for a guaranteed brand of a guaranteed item, the responsible person for the guaranteed brand of the guaranteed item forms the belief that the person will fail to supply, or will be unable to supply, the guaranteed brand of the guaranteed item in the period”. While the “guarantee period” would not have commenced until the day of PBS listing, Sigma’ letter reflects its view that the anticipated interlocutory injunction would prevent it from supplying the products as required.
435 On 11 May 2009 Griffith Hack wrote to Wyeth’s lawyers saying:
I am instructed by my client, Sigma Pharmaceuticals (Australia) Pty Limited that an application to list its venlafaxine hydrochloride on the Pharmaceutical Benefits Register was made shortly after obtaining the ARTG registration of its EVELEXA XR product on 26 February 2009.
I am instructed a subsequent decision was made by my client to withdraw the application to list the product on the Pharmaceutical Benefits Register but that, through inadvertence, that withdrawal was not communicated to the relevant authority. My instructions are to the effect that the withdrawal of the application has now been formally communicated to the relevant authority.
I reconfirm my client’s commitment to the undertaking previously communicated to you which is to the effect that our client does not intend to make application to list EVELEXA XR on the Pharmaceutical Benefits Register until final resolution at trial of our client’s revocation proceeding and your client’s cross-claim for infringement or until further order of the Federal Court.
436 The lack of importance of a cross-undertaking is apparent from the fact it is not mentioned in this letter. The lack of mention is not a mistake. In my view, the evidence discloses that Sigma had decided not to PBS list whether or not Wyeth gave a cross-undertaking because it thought this gave it a chance of avoiding being enjoined altogether when it needed to sell its $3 million of stock on the private market. The sporadic attempts to obtain a cross-undertaking were opportunistic but not improper because Sigma was acting in the context of an anticipated interlocutory injunction. Sigma had not decided, outside the context of an anticipated interlocutory injunction, that it would not PBS list. Thus, I do not accept Wyeth’s submission that Sigma’s “decision not to cause its generic venlafaxine products to list on the PBS until the final resolution of the proceeding was entirely independent of the SPAL Interlocutory Injunction”. And I do not consider the issue of the cross-undertaking by Wyeth resolves what Sigma would have done but for the interlocutory injunction.
437 Sigma’s private market launch of a generic version of a PBS listed product when no PBS listing of the generic product could be anticipated was unprecedented. Mr Heine says he was confident of success. Sigma also had a $3 million incentive to sell its products and thus, as might be expected, brought to bear all of its experience in its unprecedented private market only strategy.
438 I should record here that it is apparent that Sigma did not and would not have given weight to any of the public health interest arguments that Wyeth raised. To explain, pharmacists have ethical obligations under which in the event of conflict with their own interest, the interest of the patient must come first. Insofar as prescription medicines are concerned this means that in practice pharmacists do not substitute a patient from an originator brand to a generic if the pharmacist believes that substitution would be contrary to the patient’s interest. As generic products are bioequivalent to and cost less than originator brands usually it would only be contrary to a patient’s interest to suggest substitution if there is a risk that the patient’s compliance with medicine taking requirements may be undermined, for example, by confusion. It does not take much to imagine that some patients may have trouble keeping track of their medicines if they are used to taking one brand and are substituted to another brand with a different name and appearance. The evidence indicates that pharmacists generally would not attempt to switch such a patient to a generic. Depression requiring medication such as Efexor-XR is also a serious illness where pharmacists would not want to create a risk of lack of compliance with medication taking or confusion. As such, pharmacists generally would take care not to switch a patient suffering such a condition to a generic if it would create the risk of non-compliance or confusion. The evidence also indicates that, for obvious reasons of continuity of medicine taking and compliance, pharmacists prefer to switch a patient to a generic only once. Pharmacists try to avoid more than one switch because of the non-compliance and confusion risks.
439 These circumstances led Wyeth to contend that in the context of a generic drug for depression facing patent infringement proceedings, pharmacists would be reluctant to switch patients to the generic due to the risk of having to switch the patient back again to the originator brand (that is, if the generic product was found to infringe the patent and the generic product had to be removed from the market). I do not accept that a generic company such as Sigma (or the other generics) would have given this consideration separate from their consideration of their overall prospects of market success (which, in Sigma’s case, were and would have been subsumed into its need not to be stuck holding $3 million of stock). As explained later, in any event, the significance of pharmacists’ ethical obligations should not be given too much prominence. The real risk which would motivate pharmacists not to switch a patient is confusion. If that risk existed and could not be ameliorated through discussion with the patient the pharmacist would be unlikely to switch the patient regardless of the particular illness the medication was treating. Excluding that risk and assuming the no brand substitution box was not ticked on the prescription (which prevents switching), the pharmacist would have no ethical constraint preventing them from raising with the patient the issue of switching to a cheaper bioequivalent generic. The patient would then be able to decide on substitution. As the pharmacist Mr Chami said, as long as there was no negative impact on treatment, providing patients with their medication at the cheapest possible price is generally in the patient’s interest because “the cost of medicines can be a significant contributor to non-compliance by patients”.
440 From Mr Chami’s evidence, and the other evidence from various witnesses about pharmacists, I do not consider that pharmacists would attempt to predict the outcome of patent litigation. If a supplier such as Sigma was offering a product, the pharmacist would assume that the product would be likely to continue to be available. They would not feel constrained in offering the generic version because of a fear that the generic product might become unavailable due to patent litigation. As such, they would treat the product as they would any generic product, encouraging switching if it was in their financial interest and would not negatively impact on the patient’s treatment because the patient also has an interest in being offered the cheapest brand of the bioequivalent medicine available.
441 The result of this digression is this. Sigma launched its products on the private market without giving this issue any apparent consideration. It must also be inferred that it would not have given any weight to this concern about pharmacists not being able to switch patients to its products on an ethical basis because of the possibility (which Sigma thought to be low) of them having to be switched back. Indeed, none of the generics gave any weight to this concern. Its relevance must be confined to the likely success of supply rather than the likelihood of supply by the generics.
442 Wyeth would have it that because Sigma’s success was surprising (yet, according to Wyeth was also somehow simultaneously “underwhelming”), it should be inferred for that reason that Sigma misled pharmacists about PBS listing. As will be explained later, the evidence does not support any such inference.
443 While Sigma’s private launch was not reaching the levels it had budgeted for in its revised forecasts (which were aggressive), the evidence indicates that it was having far more success than might be expected for an unprecedented strategy. As Sigma submitted the evidence shows that:
As at the date of the Interlocutory hearing on 22 May 2009, Sigma had taken significant and concrete steps and invested considerable time and money in preparing for the Evelexa XR launch. This included having purchased $3,316,357 of Evelexa XR Products that would have to be written off if an injunction were to be granted…
Mr de Alwis emailed Mr Beveridge, copying in Mr Smith and Mr Heine, on the morning of 21 May 2009, noting that a low stock on hand report recorded “$5.1 million of Evelexa orders in the system already!! Great boost to sales as this will go out in May.” The private prescription launch was evidently perceived as a success by senior management right up to the hearing of the Sigma Interlocutory Injunction.
444 Even if Wyeth’s description of the private market strategy having sold 53,000 units from a stock of 327,000 units and obtained orders from only 2300 out of the 3000 targeted pharmacies is accepted, there can be no doubt that Sigma was committed to its private market supply, was doing everything it could to achieve its sales targets in the private market, and was enjoying a considerable measure of success for the short period it was on the market.
The effect of the interlocutory injunction on Sigma?
445 The hearing before Sundberg J was on 22 May 2009. It must be hypothesised that on that date Wyeth did not press its application for interlocutory relief. In fact, Wyeth did so and Sundberg J granted the interlocutory orders on 3 June 2009. As noted, the injunction restrained Sigma, pending the determination of the proceeding, from marketing, taking orders for, selling, supplying, offering to supply or otherwise exploiting in Australia the products listed on the ARTG under the name Evelexa XR or any other product comprising the same generic modified release formulation of venlafaxine hydrochloride, without the licence of or authority of Wyeth.
446 Wyeth would have it that the injunction did not prevent Sigma from obtaining PBS listing because irrespective of the injunction Sigma had made plain that it did not intend to list its products on the PBS pending final resolution of the proceeding. It may be accepted that the focus of the evidence and the hearing before Sundberg J was Sigma’s private market supply because Sigma had said it undertook not to list on the PBS. As I have explained, whether this was conditional on a cross-undertaking from Wyeth or not (and I do not consider it was) is not determinative. If, as must be posited, Wyeth simply did not apply for an interlocutory injunction on 22 May 2009 not because it accepted Sigma’s offer (which it did not) but for reasons unknown, it is inconceivable that Sigma would not have revisited a strategy that it developed in response to the anticipated interlocutory injunction.
447 It may be accepted that the interlocutory injunction did not in terms prevent Sigma from seeking PBS listing. But it cannot be doubted that the operation of the order had this effect. Sigma would not revisit PBS listing because being restrained from marketing, taking orders for, selling, supplying, offering to supply or otherwise exploiting in Australia meant that PBS listing would serve no practical purpose other than to unnecessarily expose Sigma to a claim for damages by Wyeth for triggering a 12.5% price drop. It is artificial in the extreme for Wyeth to suggest that the injunction did not prevent Sigma from seeking listing on the PBS. The operation of the interlocutory injunction prevented Sigma from continuing to sell its products on the private market. It prevented Sigma from exercising any of the rights that registration of its products on the ARTG gave it. As a result, Sigma had no occasion to re-consider whether to seek PBS listing of its products.
448 But for the interlocutory injunction Sigma would have had all of the options afforded by ARTG registration. It could have continued with its private market supply. It could have re-considered its position about applying for PBS listing depending on the success or otherwise of its private market supply. Even if it had decided not to again apply for PBS listing, it could continue to monitor the situation and make its decisions accordingly. Given that it had taken substantial effort for Sigma to obtain its ARTG registrations, it is my view that all of these opportunities had value and all were lost as a result of the interlocutory injunction. That is, on the balance of probabilities (in fact, as a certainty), Sigma suffered losses of opportunity of some value by reason of the operation of the interlocutory injunction.
449 For the reasons given, I do not accept Wyeth’s submissions that Sigma necessarily would have made the same decision but for the interlocutory injunction as it made in anticipation of the interlocutory injunction. If Wyeth had not applied for interlocutory relief on 22 May 2009 Sigma would have immediately re-considered its decision to withdraw its PBS listing application. For that purpose, I consider Sigma would have sought further legal advice. The lawyers would have been asked to advise both about prospects of success and Sigma’s potential exposure. As foreshadowed, I do not think any reasonable lawyer (and Griffith Hack, Sigma’s lawyers, were reasonable and had relevant expertise) knowing that the advice would be relied upon for such a serious decision would have gone further than saying that Sigma’s products infringed the method patent but there was a good or strong prospect of the Court declaring the method patent invalid. Any such advice also would have been qualified to the requisite degree to the effect that all litigation is inherently risky, not all events in litigation can be anticipated, the risk of Sigma not succeeding in having the method patent revoked could not be described as trivial, negligible or insignificant, and that if Sigma succeeded an appeal by Wyeth would be expected. At the same time, the advice would have made it clear that the risks associated with sales on the private market were of a different order of magnitude from those associated with PBS listing. For private market sales, Sigma could be liable for more than its own profits due to Wyeth having a likely greater margin than Sigma and the possibility Wyeth would discount in response to Sigma’s entry, but liability would largely be related to Sigma’s own sales. On PBS listing, the price of Efexor-XR would drop by 12.5% for the life of the method patent. Sigma’s potential liability would not be related to its own sales but would relate to Wyeth’s previous 100% of sales at the higher price. There would also be a greater likelihood of Wyeth having to discount its products further to compete with Sigma, including the risk of Wyeth cannibalising Efexor-XR sales by itself introducing a generic version of Efexor-XR. All of those risks would have been taken into account by the lawyers and would have been exposed in the advice. As Wyeth contended, Sigma would have been advised that if it obtained PBS listing and triggered the 12.5% price reduction and supplied on the PBS and if the method patent was not invalid Sigma would be exposed to a damages claim of over $100 million.
450 Wyeth submitted that:
(1) “Once he obtained the required advice Mr de Alwis would have taken the decision to Sigma’s board which comprised eight highly regarded and well qualified individuals”.
I agree. This was the effect of Mr de Alwis’s evidence even on the basis of his assumption that he would have had the benefit of a judgment from Sundberg J to the effect the method patent was invalid (which he would not have had).
(2) “Sigma called only one board member, Mr de Alwis, a convicted criminal”.
I agree but this does not mean I am unable to draw inferences about what the board might have decided to do from time to time having regard to the objective contemporaneous evidence and circumstances.
(3) “Mr de Alwis gives no evidence as to what he would have done in the absence of any interlocutory application for an injunction. His counterfactual evidence only addresses a circumstance in which the interlocutory injunction application was heard and refused on the basis that the method patent was so likely to be invalid that Wyeth could not be granted interlocutory relief”.
I agree.
(4) “The counter-factual evidence Mr de Alwis gave exposed that he perceived it to be of critical importance to any hope of persuading the board to approve a PBS listing, that there be an interlocutory judgment to the effect that the method patent was invalid. Absent such a clear indication from the Court, the inference is that he could not have persuaded the board to approve a PBS listing”.
I agree. As Wyeth said, the fact Mr de Alwis’s evidence was framed in this way weighs heavily against a conclusion that Sigma would have sought to list its products on the PBS after 22 May 2009 so as to trigger the 12.5% price reduction in Efexor-XR.
(5) “The contemporaneous evidence that in fact Mr de Alwis was concerned to limit Sigma’s exposure damages in a private launch as reflected in the Griffith Hack file note of 31 March 2009 (“If lose, only lose the amount they sold. Keep discounts small so on worst case, not a large exposure”) is contraindicative of a preparedness to expose the company to the even greater price drop risk of a PBS listing”.
I agree. Again, as Wyeth said, this weighs heavily against a conclusion that Sigma would have sought to list its products on the PBS after 22 May 2009 so as to trigger the 12.5% price reduction in Efexor-XR.
451 Wyeth submitted that, as a result, Sigma would not have taken the commercially irrational approach of exposing itself to a liability in excess of $100 million given the financial pressure it was under, its history and policy of taking on only negligible risks of legal liability, and its decision not to obtain PBS listing but instead to confine its launch to the private market and try to obtain an early final hearing.
452 Sigma submitted that Sigma’s perception that it had “strong grounds” to invalidate the method patent and the magnitude of the perceived commercial opportunity presented by Evelexa XR and its importance to Sigma in achieving its budget would favour PBS listing notwithstanding an appreciation of damages exposure by reason of the alleged infringement of the method patent.
453 In my view, as the evidence discloses, Sigma’s immediate concern when confronted by the method patent was not being stuck with $3 million of stock. This is why it adopted the strategy it did for the interlocutory injunction. Had it been determined to implement its original plan despite becoming aware of the method patent, it would not have given up the PBS listing opportunity so readily no matter what it thought of its prospects of success at an interlocutory hearing. Further, the short-term benefit of “achieving its budget” would not have outweighed the enormity of the potential exposure of PBS listing. In contrast, not having wasted $3 million provided a compelling incentive for the commercially rational decision to still be the first generic to the market but confine supply to the private market and thereby confine Sigma’s likely exposure to damages to sales lost by Wyeth to Sigma albeit at Wyeth’s likely greater margin.
454 It should also be noted that, unlike Alphapharm, Sigma was not concerned at that time that Wyeth might switch patients to Pristiq for the purpose of de-listing Efexor-XR. As will be seen, even though it believed Wyeth would do this, Alphapharm was still not willing to risk being the first to list its products on the PBS. Sigma did not hold this concern and, as a result, it would not have weighed in Sigma’s consideration of the competing opportunities and risks in mid-2009.
455 Sigma submitted that Sigma regarded Evelexa XR as a very important opportunity and understood that it would lose a substantial part of the benefit if it did not list on the PBS. This is true but it overlooks the enormity of the potential exposure and the fact that the private market gave Sigma a way of selling the $3 million of stock it held and being the first generic on the market in circumstances where Sigma would know that if another generic took the risk of PBS listing Sigma could always re-apply for PBS listing itself. And if no generic took that risk, again, Sigma would have given itself the best chance of selling the $3 million of stock and would still be the first generic on the market.
456 Sigma submitted that the critical question whether Mr de Alwis would have been deterred by the litigation was not explored with him. It did not need to be given that the evidence he gave assumed that Sundberg J would have refused to grant an interlocutory injunction on the ground that the method patent was prima facie invalid, thus giving Mr de Alwis confidence that Sigma’s legal advice was right. The assumption is unfounded. In any event, I have explained above why the hypothetical evidence, and particularly that of Mr de Alwis, is unreliable.
457 If Sigma had to prove on the balance of probabilities that but for the Sigma interlocutory injunction it would have sought and obtained PBS listing so as to supply under the PBS then I would have found that Sigma had failed to discharge that burden of proof. Apart from the unreliable non-contemporaneous evidence of the witnesses, the evidence, properly assessed, does not support the inference that it is more likely than not Sigma would have sought PBS listing after 22 May 2009 if in so doing Sigma would be the first to list its generic products on the PBS and would trigger the 12.5% price reduction which would have been perceived to expose it to such enormous potential liability (as would have been the case at this time).
458 Sigma’s contrary submissions that PBS listing was more likely than not seem to me to be divorced from the reality with which Sigma would have been confronted. It had purchased $3 million of stock and was understandably desperate not to be left holding it. It believed that it could sell the stock on the private market and thereby limit its exposure to Wyeth and thus was willing to withdraw its PBS listing application. When confronted with the method patent, it almost immediately developed this manifestly commercially sensible strategy and was willing to implement it before Wyeth took any unequivocal step. If free to re-consider its position from 22 May 2009 because Wyeth withdrew its application for interlocutory relief on that day, Sigma would still have believed it could sell its $3 million of stock on the private market. It would still be the first generic on the market and thus obtained the “first mover” advantage it wanted. It would have known that if another generic did obtain PBS listing, it could then re-apply for PBS listing itself without being the one who had triggered the 12.5% price reduction and thus without such enormous potential exposure to Wyeth. Even given its belief that it had good or strong prospects of success of having the method patent revoked, Sigma would have known from further legal advice that the risk of failing in the litigation could not be eliminated and could never be negligible, trivial or insignificant.
459 Confronted with the magnitude of the potential exposure and without the assumed benefit of a judgment from Sundberg J to the effect that the method patent was invalid, in my view it is not likely that Mr de Alwis would have recommended to Sigma’s board in favour of PBS listing. Even if he had done so, he would have presented all the information to the board and the board would have made its decision in light of all of that evidence. The idea that it is more likely than not that the board would have taken the risk of seeking PBS listing before the final judgment in the circumstances with which it would have been confronted is neither manifestly commercially rational conduct nor readily reconcilable with the objective contemporaneous evidence.
460 Sigma submitted that Mr de Alwis gave effectively unchallenged evidence as to how he assessed the risk of the litigation concluding that “taking all those into account, and also based on the advice I had received, concluding that the probability of losing such a case was low, I felt very comfortable to go ahead with it”. This, however, was a reference to the private market launch and, yet again, Mr de Alwis has left out of his account the pressure he must have felt for Sigma not to be left holding the $3 million of stock. As I have said, Mr de Alwis’s evidence is not a reliable foundation for any inference in Sigma’s favour for all of the reasons already given.
461 Sigma discounted what it described as Mr Ellis’s confused evidence which is fair enough and involves no criticism of Mr Ellis, but then sought to bolster its case by reference to Mr Heine’s evidence when Mr Heine had no decision-making role in respect of PBS listing or not. The same observation applies to Ms Morgan. As I have said, I am not suggesting Mr Ellis, Mr Heine or Ms Morgan were being untruthful. But their evidence about what Sigma would have done or they would have done about PBS listing is immaterial and, as with nearly all of the hypothetical evidence, is unreliable.
462 Sigma submitted that Wyeth asked the Court to find that the board would have intervened to thwart the plans of Sigma’s senior management without any evidence to that effect in circumstances where is no suggestion that the board was not aware of what was going on. However, for the purpose of seeking a PBS listing in the face of the method patent and litigation the board would not have been intervening. It is clear that Mr de Alwis would have taken the issue to the board (which had the same members as Sigma’s parent).
463 However, because I do not consider that Sigma had to prove that it would have listed its products on the PBS it is necessary to consider the possibility it would have done so. Given the evidence to which I have referred, I consider the likelihood of Sigma deciding to re-apply for PBS listing to be relatively low but not merely speculative. Overall, I consider that there was a real chance that Sigma’s board might have given more weight to what would have been perceived as the good prospects of having the method patent declared invalid than the magnitude of the perceived financial risk if the method patent was not declared invalid.
464 Assigning a specific percentage to this possibility is not a straightforward task. The best way to explain my conclusions on the whole of the evidence is to consider the range of real possibilities. Having particular regard to the fact that Sigma would have been the first mover into the generic market, considered venlafaxine to be a rare significant opportunity for it and, on the evidence, needed a “win” in respect of venlafaxine, I am not persuaded that the chance of PBS listing was merely speculative or less than 1%. A 1 in 20 or even 1 in 15 prospect of PBS listing, in my view, is too low and would be below the bottom end of the range. A 1 in 3 or even a 1 in 4 prospect of PBS listing, in my view, is too high and would be above the top end of the range. The range which may be inferred to be reasonable from the evidence therefore lies between these two extremes. Given the considerations to which I have already referred supporting a more liberal approach to compensation, I have concluded that I should accept a figure towards the top end of my range of reasonable inference from the evidence. On this basis, I conclude that there was a 20% chance that Sigma would have re-applied for PBS listing of its products.
465 In respect of the 20% possibility of Sigma having re-applied for PBS listing, there is also a timing issue. On my approach, Sigma would have been re-considering its position (which was that it was undertaking the supply of its products on the private market) from 22 May 2009. To do so, it would need to obtain comprehensive legal advice which would take some time to prepare. To obtain a PBS listing on 1 August 2009, Sigma would have to have re-applied by 1 May 2009. This would have been impossible. It follows that the 20% possibility of applying for PBS listing would have led to listing on 1 December 2009. To obtain PBS listing on 1 December 2009 Sigma would have had to apply by 1 September 2009. This would have been achievable and should be applied to the Sigma 20% possibility. The issue whether the Minister’s delegate would have PBS listed Sigma’s (or any generic’s product) before the first instance judgment about the method patent will be considered separately.
466 As a result, the 20% possibility of Sigma having applied for PBS listing operates in respect of a potential PBS listing on 1 December 2009. For the period from the date of Sigma’s actual launch of its products on the private market (1 May 2009) to 1 December 2009 it must be inferred that Sigma would have continued to supply its products on the private market. In the circumstances I have identified but for the interlocutory injunction there was no chance that Sigma would have decided to cease supplying its products on the private market unless the venture ultimately proved to be financially unfeasible. The $3 million of stock it was holding would have compelled it to continue with its private market supply. Sigma needed a “win” in the market. It had the opportunity to be the first to market albeit on the private market and had $3 million of stock to move. I consider that the chance of Sigma having not continued is private market supply should be assessed to be less than 1% and thus disregarded. Thus for the period from 1 May 2009 to 1 December 2009 the hypothetical fact of Sigma continuing private supply is to be treated as certain (100%).
467 Given my conclusion about the 20% prospect of Sigma having applied for PBS listing in time for its products to be listed on 1 December 2009, it follows that from that time there would have been an 80% probability of Sigma continuing with its private market supply of its products but for the Sigma interlocutory injunction.
468 As I have said if, contrary to my view, Sigma’s conduct in anticipation of the Sigma interlocutory injunction should be disregarded, the practical effect is that the notional PBS listing date would be 1 August 2009 and not 1 December 2009.
469 I have concluded below that the prospect of another generic first obtaining PBS listing should be disregarded. In case this is incorrect I note my view that if another generic obtained PBS listing for its products before Sigma then the dynamic would have changed. Sigma would believe that the first generic to obtain listing alone would be responsible for the 12.5% price reduction. Sigma would also have known that the first mover advantage it had obtained by launching on 1 May 2009 might well be undermined. The motivation to protect its first mover advantage would have been overwhelming weighed against the perceived risk of ultimate liability to Wyeth which, under this hypothesis, would not include liability for the 12.5% price reduction. In any such case, Sigma would almost certainly have decided that it could not afford not to be in the PBS market despite the risk of increased exposure from increased sales and discounting. In the event of another generic listing on the PBS first, I would assess the probability of Sigma having then applied for PBS listing to be an effective certainty (100%). I can see no chance that Sigma would have been prepared to see its first mover advantage undermined if another generic had obtained PBS listing and thus removed from Sigma the perceived risk of liability for the 12.5% price reduction of Efexor-XR. Even if its private market supply had sustained losses, the prospect that Sigma would not have applied for PBS listing as soon as possible to commence supply under the PBS, in the event of another generic having obtained PBS listing, must be assessed to be so negligible (less than 1%) that it should be disregarded.
470 In respect of the private market supply, as noted, Wyeth contended that Sigma’s strategy would have ultimately failed and that it would not have ordered any more stock than the $3 million it held, in effect, because the pharmacists who purchased stock would not have understood that PBS listing was not anticipated in the reasonably foreseeable future, would not have been able to sell the stock purchased, and ultimately would have been looking to Sigma to replace the stock with equivalent value products the pharmacists could sell (which, according to Ms McTavish, was Sigma’s policy). This aspect of the argument concerns the value of the opportunity Sigma lost which I will deal with separately in the context of the industry, econometric and accounting evidence.
471 The assessment above does not factor in the potential actions of Wyeth, other generics or the Department’s response to an application for PBS listing. I deal with those issues separately.
472 I am satisfied that no anticipation by Sigma of what Wyeth might do in competitive response to Sigma’s supply or otherwise would have weighed on Sigma’s mind. Nor would any concern about the Department’s potential response to an application to list the products on the PBS have been taken into account by Sigma. Further, but for the interlocutory injunction, Sigma would have been able to give the required assurance of supply on PBS listing and would have no reason to be concerned about the statutory guarantee of supply provisions (a matter also discussed separately below).
Summary of interim conclusions - Sigma
473 My interim conclusions about Sigma, accordingly, may be summarised as follows:
(1) Sigma has proved on the balance of probabilities that it suffered the loss of an opportunity of some value associated with the ARTG registration of its products because the injunction prevented it from exercising, from time to time and as it saw fit, the rights associated with ARTG registration. The value of this opportunity depends on the probabilities and possibilities of what Sigma would or might have done.
(2) If Sigma needed to prove on the balance of probabilities that it would have re-applied for PBS listing of its products but for the interlocutory injunction, then Sigma has failed to prove that putative fact.
(3) If Sigma needed to prove on the balance of probabilities that it would have continued with its supply of its products on the private market but for the interlocutory injunction, then Sigma has proved that putative fact.
(4) If, as I consider, Sigma does not need to prove (2) or (3) because it has proved (1) on the balance of probabilities, then the probabilities and possibilities are as follows:
Between 1 May and 1 December 2009
(a) Sigma would have re-applied for PBS listing in time for listing on 1 August 2009 for the purposes of supply under the PBS: 0%.
(b) Sigma would have continued its supply its products on the private market: 100%.
(c) Sigma would not have continued its supply of its products: 0%.
From 1 December 2009
(d) Sigma would have re-applied for PBS listing by 1 September 2009 in time for listing on 1 December 2009 or at some later time before the Full Court judgment in October 2011 for the purposes of supply under the PBS: 20%.
(e) Sigma would have continued to supply its products on the private market after 1 December 2009: 80%.
(f) Sigma would not have continued to supply its products on the private market from 1 May 2009: 0%.
(5) If another generic had obtained PBS listing on 1 August or 1 December 2009 or thereafter the probabilities and possibilities are:
(a) Sigma would have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could: 100%.
(b) Sigma would not have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could: 0%.
Facts and contentions
474 Wyeth contended that in or about October 2010 Sigma ceased to be a person who could be adversely affected by the Sigma interlocutory injunction because of events connected to the sale of shares in Sigma to Aspen Asia Pacific Pty Ltd. It contended in the alternative that Sigma ceased to be such a person on 28 January 2011 when it transferred its ARTG registrations to Sigma Company Limited in connection with the sale of shares in Sigma to Aspen Asia.
475 The relevant facts are these. In August 2010 Aspen Pharmacare Holdings Limited reached an in-principle agreement with Sigma Company Limited that Aspen Pharmacare’s subsidiary Aspen Asia Pacific Pty Ltd would purchase all of the shares in Sigma Company Limited’s subsidiary, Sigma. This transaction was completed on 31 January 2011 pursuant to a share sale agreement.
476 I published reasons for judgment to the parties on 23 September 2010 to the effect that the method patent was valid and infringed or threatened to be infringed by the generics for the purpose of them identifying any confidential matters. In late October 2010 Sigma informed Aspen Asia that it proposed to offer an undertaking in exchange for interlocutory orders pending an appeal restraining Wyeth from making any application to de-list Efexor-XR from the PBS, marketing or allowing any other person to market any generic venlafaxine product, and applying to amend the method patent otherwise than in this Court. This prompted Aspen Asia to propose in an email of 5 November 2010 an arrangement whereby Sigma Company Limited would “retain the risks and rewards associated with the Wyeth litigation as if it does so and is successful, then it will have an asset (in the form of the product) which it will either be entitled to sell or commercialise on a royalty basis through a third party distributor, which may or may not be Aspen”. This was said in the email to reflect Aspen’s policy of not being litigious and its focus on its core business.
477 As part of a restructuring in anticipation of the sale of shares in Sigma to Aspen Asia, on 28 January 2011 Sigma transferred its ARTG registrations for Evelexa XR to its parent company, Sigma Company Limited.
478 The share sale agreement between Sigma Pharmaceuticals Limited, Sigma Company Limited, Aspen Pharmacare Holdings Limited and Aspen Asia Pacific Pty Ltd reflected an arrangement under which, in effect and to the extent relevant, all of Sigma’s generic pharmaceuticals business would be transferred as part of the share sale other than in respect of two products which were the subject of litigation, one of which was Sigma’s venlafaxine products. Pursuant to the restructuring arrangements the products themselves (given the transfer of the ARTG registrations from Sigma) and risks and rewards of the Excluded Litigation would remain with Sigma Company Limited.
479 Accordingly, cl 1.1 contains definitions of Excluded Litigation (defined to include the Wyeth Litigation), Excluded Product (defined to include the Venlafaxine Products), Excluded Product Registrations (defined to include the Venlafaxine Product Registrations), and Litigation Benefits (defined to mean any amount or benefit received or to which a Group Company or Remaining Group Company becomes entitled).
480 By cl 11.3(a)(i) Sigma Company Limited as Vendor “will have control of the Excluded Litigation”.
481 By cl 11.3(e), all Litigation Benefits for the Excluded Litigation, whether realised before or after completion, belong to the Vendor, Sigma Company Limited. By cl 11.3(f) the Purchaser, Aspen Asia, must pay to the Vendor, Sigma Company Limited, “immediately following receipt by [Sigma]…the amount of any Litigation Benefits in respect of the Excluded Litigation”.
482 Clause 11.7 records that pursuant to a restructure deed all Excluded Product Assets will be held by the Vendor or a Remaining Group Company “and that after Completion neither the Purchaser nor any Group Company will have any right or interest in respect of those assets”.
483 As I understand it, Wyeth’s reference to October 2010 as the date on which Sigma ceased to be a person who could be adversely affected by the Sigma interlocutory injunction is intended to reflect the date on which the parties entered into the share sale agreement. Wyeth submitted that Sigma was not a person adversely affected by the Sigma interlocutory injunction after October 2010 because:
…a claim pursuant to the SPAL Undertaking only has content when linked to the “Excluded Product Assets”, including the Wyeth Litigation; it attaches to and runs with those assets. But in this case SPAL (now owned by Aspen) divested itself of the risks and rewards associated with the Wyeth Litigation.
484 Sigma’s response was to focus on the terms of the share sale agreement. Sigma submitted that:
Importantly, the definition of “Excluded Product Assets” does not purport to pick up every right associated in any way with, relevantly, the Venlafaxine Products and Venlafaxine Product Registrations, but is confined to the “Intellectual Property Rights” in the Venlafaxine Products, the “Venlafaxine Product Registrations” and “all product dossiers and other records relating wholly or predominantly to any of the [Venlafaxine Product Registrations] or other assets referred to in paragraphs (a) to (d) inclusive of this definition”.
By cl 11.7 the Purchaser (AAP) acknowledged that all Excluded Product Assets would be held by the Vendor (SCL) and/or a “Remaining Group Company” (defined to mean other subsidiaries of SCL not being sold to AAP) and that “after Completion neither the Purchaser nor any Group Company (defined to mean subsidiaries of AAP) will have any right or interests in respect of those assets”.
Accordingly, although the Share Sale Agreement contemplated that Sigma’s intellectual property rights in Evelexa XR would be assigned to SCL or a subsidiary of SCL, it did not contemplate an assignment away from Sigma of its choses in action – including its rights against the Wyeth Parties in the present proceedings. Indeed, it is perfectly clear that the Share Sale Agreement cannot have effected such an assignment because Sigma was not party to it.
That is further confirmed by the specific provisions of the Share Sale Agreement dealing with the present proceedings. “Wyeth Litigation” was defined to mean the present proceedings. “Excluded Litigation” was defined to include the “Wyeth Litigation”. By cl 11.3 the parties acknowledged and agreed that the Vendor (SCL) was to have “control of the conduct of the Excluded Litigation” (cl 11.3(a)). By clauses 11.4(b)-(c) read together with cl 11.5, SCL agreed to indemnity SCL in respect of any losses, liabilities and so forth in the Wyeth Litigation.
Wyeth places reliance on clauses 11.3(e) and (f) of the Share Sale Agreement, which provide:
“(e) All Litigation Benefits in respect of each Litigation, if realised:
(i) before Completion, will belong to the Vendor; or
(ii) on and from Completion, will belong to the Purchaser,
except:
(iii) to the extent such benefits relate to Liability of a Remaining Group Company for which it is not indemnified in respect of the Litigation under this agreement; and
(iv) that all Litigation Benefits in respect of the Excluded Litigation, regardless of whether those benefits are realised before, on or after Completion, will belong to the Vendor.
(f) To give effect to the principles set out in clause 11.3(e):
(i) the Purchaser must pay to the Vendor, immediately following receipt by SPAL [being Sigma] (or any other Group Company), the amount of any Litigation Benefits in respect of the Excluded Litigation;
(ii) the Vendor must pay to the Purchaser, immediately following receipt by any Remaining Group Company, the amount of any Litigation Benefits in respect of the Litigation to the extent these belong to the Purchaser under clause 11.3(e); and
(iii) the Purchaser must pay to the Vendor, immediately following receipt by any Group Company, the amount of any Litigation Benefits in respect of the Litigation to the extent these belong to the Vendor under clause 11.3(e).”
Those clauses confirm that Sigma remains the proper party to bring the claim on the Sigma Undertaking in these proceedings. It is apparent from cl 11.3(f)(i) that Sigma – referred to as SPAL in the Share Sale Agreement – was anticipated to realise the “Litigation Benefits” (if any) of the Venlafaxine Litigation and AAP was obliged to pay to SCL “the amount of” any such benefits upon their “receipt” by Sigma. The word “belong” in cl 11.3(e) can only sensibly be read as reflecting an intention that, as between AAP and SCL, SCL was to be entitled to the “Litigation Benefits”. Its precise legal effect (including whether it creates or contemplates a trust in favour of SCL in respect of Litigation Benefits realised in these proceedings, or merely a personal obligation on the part of AAP to pay an equivalent amount to SCL) does not matter because it only affects the position as between SCL and AAP.
Critically, cl 11.3(e) plainly does not purport to assign, and in any event could not be effective to assign, Sigma’s rights against the Wyeth Parties under the Sigma Undertaking or otherwise in these proceedings to any other person. Contrary to the Wyeth Parties’ submission, AAP’s obligation to pay over to ACL the amount of any “Litigation Benefits” under cl 11.3(f)(i) realised by Sigma does not mean Sigma is not the proper party to claim for, and receive, those benefits – rather, the contractual provisions confirm that it is.
485 Sigma submitted that Wyeth’s alternative argument relating to the 28 January 2011 date was also misconceived. According to Sigma:
Wyeth’s submission assumes that the approach taken to the Evelexa XR ARTG registrations in late October and November 2010 in the actual world would also have been taken in the counterfactual scenario where Sigma had been on the market selling Evelexa XR (either the private market only or also listed on the PBS) since May 2009. That is not the correct approach.
Conjecturing as to what would have happened in respect of a single component of a large and complex business transaction in late 2010 is exceedingly difficult. It is, however, properly understood as being part of the exercise of quantifying Sigma’s loss. It is something the Court must attempt even though it involves a high degree of speculation and uncertainty. Difficulty in quantifying Sigma’s loss is no reason to deny it recovery...
While acknowledging that the exercise is difficult and requires speculation about the actions that would have been taken by both AAP and SCL, Sigma submits that, having regard to Mr Ziman’s evidence and the objective probabilities as established by the contemporaneous documents (most importantly, the terms of the Share Sale Agreement that were actually negotiated…), the likelihood is that the Evelexa-XR ARTG registrations would not have been transferred away from Sigma. Alternatively, Sigma submits that there is a greater than negligible chance that the Evelexa-XR ARTG registrations would not have been transferred away from Sigma and that chance must be taken into account in quantifying Sigma’s loss…
The short point is that if Evelexa-XR had been on the market in October-November 2010 it would have been commercially rational – both for ACL and AAP – for the Evelexa-XR ARTG registrations to remain with Sigma, so that it could continue to sell Evelexa-XR, with AAP appropriately protected from litigation risk by indemnities and Sigma appropriately compensated by a payment conditional on it succeeding in the litigation. Such an arrangement would have been possible and consistent with the commercial objectives of the parties in the real world.
486 Sigma relied on three matters said to support these conclusions.
487 First, while Sigma accepted that it was clear from Mr Ziman’s evidence that Aspen Asia would not have accepted any financial risk from the Wyeth litigation he also said that:
…if there was a contracted outcome whereby Aspen was completely protected from any such liabilities, I may have recommended Evelexa XR still be included in the Transaction, thus leaving Aspen with no damages/liability exposure. However, given that the negotiation of a complex transaction has number of moving parts, it is difficult to speculate now what would have occurred.
488 Second, Sigma submitted that the terms of the share sale agreement also indicated what might have occurred. In particular, cl 11.8 provided that if the Wyeth litigation was settled or judicially determined in such a way that the Venlafaxine Products could be commercialised “without risk of suit for breach of patent or other Intellectual Property Rights (as agreed by the parties, each acting reasonably)” then Aspen Asia and Sigma Company Limited would negotiate in good faith a sale of the Excluded Product Assets in respect of the Venlafaxine Products which would have included a transfer of the ARTG registrations for Evelexa XR from Sigma Company Limited to Aspen Asia. Sigma Company Limited could do what it wished with the Venlafaxine Products only after 60 days from the failure of such negotiations. According to Sigma, this discloses that the parties had the common objective, if possible, of ensuring that the Evelexa XR products were kept as part of Sigma’s generic portfolio of products. To this should be added that:
…if Evelexa XR had been on the market the approach taken in the real world would not have been open. Sigma ceasing to sell Evelexa XR after 18 months on the market would have been disastrous for both Sigma, pharmacies and patients... It would therefore have been necessary for the parties to devise an arrangement whereby:
(a) Sigma was able to continue selling Evelexa XR;
(b) Aspen was protected from the litigation risk which, it must be accepted, from 8 November 2010 onwards would have included the risk relating to an undertaking in support of a stay of the Sigma Final Injunction to keep Evelexa XR on the market; and
(c) SCL received monetary compensation for Evelexa XR.
489 Third, Sigma submitted that “any number of arrangements could have been devised which enabled the three objectives identified above to be realised”, including making part of the purchase price for Sigma payable only on a successful outcome of the Wyeth litigation and an indemnity from Sigma Company Limited if the outcome was not successful. Sigma Company Limited gave an equivalent kind of indemnity under cll 11.4(d) and 11.5 of the share sale agreement.
490 Wyeth contended to the contrary that Mr Ziman’s evidence that “Aspen was and is generally risk averse to material known legal and financial exposures. In that counterfactual situation I would not have agreed that Aspen carry this risk exposure for this product (Evelexa XR)” indicated that the Sigma interlocutory injunction made no difference to the arrangements and agreements about the sale of shares in Sigma.
Discussion
491 In my view, the October 2010 date is immaterial but not for the reasons Sigma gave.
492 Sigma’s submissions may be accepted insofar as they accurately explain the effect of the share sale agreement and record that nothing in that agreement means that Sigma is not a proper claimant on the undertaking Wyeth gave as the price of the Sigma interlocutory injunction. Wyeth’s points are to a different effect and involve different considerations.
493 The relevant point which answers Wyeth’ first contention about October 2010 being a cut-off date for any potential adverse effect on Sigma is this. While the agreements and arrangements reflected the common intention of the parties to the share sale agreement that the ARTG registrations for Evelexa XR would be transferred from Sigma to Sigma Company Limited and that Sigma Company Limited would retain the risks and rewards of the Wyeth litigation, nothing in those agreements and arrangements prevented Sigma from exercising the rights of supply of Evelexa XR which the ARTG registrations gave to Sigma at any time before the transfer of the registrations. Wyeth did not identify anything in the agreements and arrangements which would have had that effect before the transfer of the ARTG registrations on 28 January 2011. I am unable to identify anything which would have had that effect. The thing which prevented Sigma from exercising its rights of supply remained the Sigma interlocutory injunction. Accordingly, the adverse effect of the Sigma interlocutory injunction on Sigma remained at least until 28 January 2011.
494 Sigma’s approach to the 28 January 2011 date involves more difficult considerations. I have found that if not for the Sigma interlocutory injunction the relevant hypothesis involves an 80% probability that Sigma would have been selling its Evelexa XR products on the private market and a 20% possibility that it would have applied for and obtained PBS listing of Evelexa XR on 1 December 2009 so that from that date it would have been supplying the products under the PBS. As a result of the Sigma interlocutory injunction, however, it was prevented from supplying the products on and from 22 May 2009. This fact, of non-supply, is the context within which the arrangements and agreements in respect of the sale of shares in Sigma to Aspen Asia were negotiated and concluded.
495 Wyeth’s approach to Mr Ziman’s evidence cannot be accepted. Mr Ziman explained in his second affidavit that he had misunderstood the question that was put to him (hardly surprising, in my view). In proposing what Aspen Asia would have done if the Sigma interlocutory injunction had not been granted and Sigma had been selling Evelexa XR since May 2009, he had assumed that “there was no litigation in any form with regards to Evelexa XR” and Sigma, the company being acquired “had no exposure to any form of patent or financial liability whatsoever (contingent or otherwise) by including Evelexa XR in the acquisition basket”. In my view, this confirms the difficulty in asking a witness to give evidence about what could or might have occurred but for the interlocutory injunction. Mr Ziman’s misunderstanding is not apparent on the face of his first affidavit yet the effect of his evidence had it not been corrected would have been to ignore the existence of the method patent and the litigation which is impermissible. In his second affidavit Mr Ziman corrected his misunderstanding and added in another fact which existed (and which, despite Generic Health’s submissions to the contrary, cannot be wished away). That fact is that on 8 November 2010 I made orders in accordance with my conclusions that the method patent was valid, Sigma had infringed and was threatening to infringe the method patent, and granted final injunctions. Mr Ziman said in his second affidavit:
I now understand that the hypothetical situation being put to me was that [Sigma] would have been found guilty of patent infringement in November 2010 ie approximately two (2) months before the Transaction closed and therefore Aspen would have known that [Sigma] was potentially exposed to damages liability of many millions of dollars.
496 Mr Ziman then said this:
Aspen was and is generally risk averse to material known legal and financial exposures. In that counterfactual situation I would not have agreed that Aspen carry this risk exposure for this product (Evelexa XR).
However, if there was a contracted outcome whereby Aspen was completely protected from any such liabilities, I may have recommended Evelexa XR still be included in the Transaction, thus leaving Aspen with no damages/liability exposure. However, given that the negotiation of a complex transaction has a number of moving parts, it is difficult to speculate now what would have occurred.
497 What is Sigma’s claimed loss on and from 28 January 2011? As I understand it, the claim is that as a result of the Sigma interlocutory injunctions it did not supply Evelexa XR, because it did not supply Evelexa XR the context within which the negotiations about the sale of Sigma occurred would have been different and, had they been different there is a chance that Sigma would have retained the ARTG registrations so that Sigma’s loss of opportunity to supply Evelexa XR continued after 28 January 2011.
498 The first difficulty is that the required focus remains the effect of the Sigma interlocutory injunction on Sigma, relevantly, supplying the Evelexa XR products “whether by itself, its directors, officers, servants, agents or otherwise”. The Sigma interlocutory injunction thus changed one thing only – the fact of supply of Evelexa XR. It did not change the fact of the existence of the method patent. It did not change the fact of the existence of the litigation. It did not change the fact that I found in favour of Wyeth and against Sigma and made orders accordingly on 8 November 2010. The arrangements and agreements would still have been negotiated in the context of all of these matters. Sigma’s submissions do not confront these matters or the fact that the negotiating parties, which did not include Sigma itself, were free to reach whatever deal they chose. The Sigma interlocutory injunction could not and did not act upon the terms of the deal that was reached about the sale of the shares in Sigma.
499 The second difficulty is that the evidence does not support the suggestion that the approach of Aspen Asia was in any way influenced by the issue of the supply or non-supply of Evelexa XR. Aspen Asia’s approach thus had nothing to do with the Sigma interlocutory injunction. Its approach was based on the existence of the litigation. It may be accepted that it would have been possible for any number of different arrangements and agreements to have been negotiated for the sale of shares in Sigma. The fact is all of the approaches which Sigma has identified in its submissions were available irrespective of the Sigma interlocutory injunction but the one taken involved the ARTG registrations for Evelexa XR being transferred from Sigma to Sigma Company Limited.
500 The focus must remain the effect of the Sigma interlocutory injunction. Whether or not Sigma was supplying Evelexa XR, it is apparent that Aspen Asia’s sole focus was the risks associated with the litigation. The deal which was done reflected its response to those risks at the time. Nothing in the evidence supports the suggestion that the deal that was done, at least to the extent it involved the transfer of the ARTG registrations to Sigma Company Limited, was because of the fact that the Sigma interlocutory injunction had prevented the supply of Evelexa XR. As a result, it cannot be inferred, speculated or assumed that the Sigma interlocutory injunction had any effect on the agreements and arrangements about Evelexa XR.
501 The third difficulty is that Sigma’s posited “disaster” depends on the premise that if Sigma had been selling Evelexa XR for 18 months, the only choice would have been between Sigma continuing to sell Evelexa XR and Evelexa XR not being sold at all. Leaving aside the effect of the final injunctions which I granted, the deal that was done discloses that these would not have been the only choices available. Clause 11.8 of the share sale agreement recognises that the ARTG registrations gave Sigma Company Limited an asset which it could commercialise, including by transfer to third parties depending on the outcome of the litigation. This would have been the same position irrespective of the Sigma interlocutory injunction. Further, the “disastrous” consequences identified by Sigma if Evelexa XR ceased to be available altogether is not mentioned by Mr Ziman as relevant in any way to what would or might have occurred, confirming that from Aspen Asia’s perspective the only relevant issue was and would have been the risks of the litigation.
502 The fourth difficulty is that Sigma’s submissions about this issue traverse the same kind of territory as the submissions of Generic Health about the possibility that if not for the interlocutory injunctions it might have been able to reach agreements about supply with other generics at an earlier time. I concluded that these kinds of losses would not be a direct and natural consequence of the interlocutory injunctions. They would not be losses which arise in the usual course of things as a result of the interlocutory injunctions. As such, they are too remote to be compensable. The same conclusion must be reached about Sigma’s claimed losses after 28 January 2011. As noted, the parties to the Sigma share sale agreements and arrangements were free to reach whatever negotiated outcome they chose. The Sigma interlocutory injunction did not constrain their negotiating positions in any way. Sigma was not a party to those negotiations but was the subject-matter of them. Sigma had no role to play in the negotiations. The deal that was done reflected the “mere unrestricted volition” of the negotiating parties (as in Chaplin v Hicks [1911] 2 KB 786 at 792-793). As a result of the deal, Sigma no longer held the ARTG registrations. Accordingly, it could no longer lawfully supply the products and thus could not be adversely affected by the Sigma interlocutory injunction. Alternatively, in common with my conclusion about the claims of Generic Health based on other generics, I consider Sigma’s claimed losses after 28 January 2011 are too remote to be recoverable or, if not too remote, should not be the subject of any order for compensation as a matter of discretion in the circumstances I have described.
503 For these reasons I also consider that Sigma’s claim to the extent that it extends beyond 28 January 2011 should not be accepted.
504 Alphapharm is an Australian-based company which manufactures and supplies pharmaceutical products in Australia and exports Australian-made pharmaceuticals to other countries around the world. Alphapharm is part of the Mylan group of companies whose parent company is Mylan Inc in the United States. It was one of Australia’s largest suppliers of generic products, with an estimated total market share of all generic products of around 40%. It counted about 38% of all pharmacists as Alphapharm “supporters”, meaning that the pharmacies purchased all of Alphapharm’s products.
Alphapharm’s claims
505 Alphapharm claims to have been adversely affected by all three interlocutory injunctions. As noted, the Sigma interlocutory injunction was granted on 3 June 2009, the Alphapharm interlocutory injunction on 25 August 2009, and the Generic Health interlocutory injunction on 10 November 2009.
506 As will become apparent, Alphapharm’s pleadings and submissions were extraordinarily complicated and convoluted (and in my view unnecessarily so).
507 For example, it is not apparent to me how the Generic Health interlocutory injunction could have had any adverse effect on Alphapharm (or at least not any adverse effect which was not also an effect of the Alphapharm interlocutory injunction). Insofar as Alphapharm is concerned, the Generic Health interlocutory injunction is a distraction. It cannot be material one way or another. Alphapharm sought to make it material by presenting a multiplicity of hypothetical probabilities and possibilities if the various interlocutory injunctions had not been sought or granted. For example, according to Alphapharm if the Generic Health interlocutory injunction had not been granted, then the Sigma interlocutory injunction and the Alphapharm interlocutory injunction would have been discharged. It then posits when such discharge would have occurred and what would have occurred on discharge of the interlocutory injunctions.
508 None of this was necessary or helpful. As a result, I have had to spend time rejecting numerous submissions of Alphapharm when ultimately I accept that if Sigma was supplying its products on the private market there is a high likelihood that Alphapharm would have followed suit and if Sigma (or another generic) had obtained PBS listing first then, again, there is a high likelihood that Alphapharm would have done so at the first opportunity thereafter.
509 Alphapharm’s principal witness was Mr Hurley, a former Executive Director of Alphapharm who gave evidence in 2009 for the purpose of defending the Alphapharm interlocutory injunction and evidence about these claims.
510 I have explained above that I consider the hypothetical evidence is likely to be unreliable for numerous reasons. Mr Hurley’s evidence discloses the problems with evidence of this kind. Mr Hurley’s evidence also disclosed other issues which diminish the weight which his evidence should be given about contested issues.
511 Mr Hurley gave unconvincing evidence to the effect that his position between 2008 (when Alphapharm was unaware of the method patent and proposed to obtain PBS listing and only supply to pharmacists under the PBS) and 2009 (when Alphapharm became aware of the method patent, decided it was unwilling to trigger the price reduction in Efexor-XR by obtaining PBS listing, and instead decided to confine its launch to the private market) changed. Mr Hurley accepted that his view in 2008 was that pharmacists would have no interest in the private supply of a generic when the originator product was PBS listed. He said his view changed in 2009. Because pharmacists would then know they could not obtain a PBS listed product from Alphapharm he believed that pharmacists would be interested in Alphapharm’s private supply of Enlafax XR despite Efexor-XR being PBS listed.
512 His evidence that his position in fact changed was unpersuasive. Mr Hurley could not suggest that pharmacists would have any interest in such a product in 2008 because Mr Hurley had prepared a document at that time which disclosed his view that if Alphapharm tried to supply before PBS listing of its products it would achieve “minimal sales” because the PBS subsidy would not be available. No doubt this is why Alphapharm never had in mind a two stage, private then PBS launch, as Sigma had proposed. Consistent with his own document, Mr Hurley said:
It was my view that in 2008 and up to the time that we realised that we weren’t going to get PBS listing in 2009, that pharmacists would not be interested in buying private prescription products prior to the PBS launch.
513 Minimal sales on the private market, of course, would be contrary to Alphapharm’s interests in this case as it accepts that it would not have sought PBS listing before any other generic had obtained PBS listing. Mr Hurley did not accept that insofar as his state of mind was concerned at least nothing in fact changed in 2009. Mr Hurley’s explanations for his allegedly changed view included:
Something changed, in that we couldn’t achieve a PBS launch of venlafaxine, so we had to find an alternate strategy.
…they [pharmacists] would understand that they could not get a PBS listed product for venlafaxine. We would present them with an opportunity where they could make a margin, a higher margin than they did on Efexor. And, you know, that was what we did. We presented profit opportunities to pharmacies and the pharmacists, we believed, would come in and take that strategy on board.
514 After an adjournment requested by Alphapharm’s counsel relating to an objection to a question of Mr Hurley about the same issue, Mr Hurley agreed that the view he held in 2008 that pharmacists would have no interest in a private supply of venlafaxine unless the product would shortly be listed on the PBS was also the view he held in 2009. I do not doubt this was Mr Hurley’s view in 2009. The strength of this view is apparent from other material which disclosed that Alphapharm believed that Sigma’s sales representatives must have been leading pharmacists to believe Sigma’s products would be PBS listed for Sigma’s private supply to be succeeding (as it apparently was). As Mr Hurley later said about pharmacists, “[t]hat’s the world they live in. Everything is PBS listed”.
515 Mr Hurley prepared a document about Sigma’s private market supply saying that Alphapharm should “make it clear to our customers that Sigma’s action is likely to fill them up with stock that is not currently ‘a’ flag [that is, PBS listed] and, as a result, can’t be substituted”. This document continued “[t]he purchase risk sits with the pharmacist. They may care to ask Sigma if they will refund them for costs, not replacement stock, if it turns out they are significantly delayed or unable to substitute the Sigma brand”, and “[p]harmacists should remember they have previously been bitten by Alendro, and Sigma’s venlafaxine offer may place pharmacists in a similar position”. The document also said “Alphapharm’s registration could allow us to launch our venlafaxine XR. However, our position is not to launch at present. It is our view that it is in the best interests of our pharmacy customers not to serve venlafaxine XR until we can confirm a PBS date”.
516 Despite this, when the topic again arose during his evidence on the following day, Mr Hurley gave this evidence:
… We weren’t going to list on the PBS unless somebody else did it first. So we changed our strategy and decided to develop a private market strategy. That is what has changed.
These private markets still remained the same, didn’t it? I don’t understand your question.
Well, what I’m suggesting to you is – I won’t go back and put it again. I will do it a last time. If there was a risk they couldn’t shift it in a private market in May, the same risk obtained in July and August, so far as you were concerned. Do you agree or not? No, I think that we’re mixing up situations here. In May, the risk was if the product was not listed on the PBS on a promise of being listed on the PBS, then it would be a problem for the pharmacists because they may commit to a lot of stock on the basis of that belief that it’s going to be listed. In August, we would go to them with a totally different offer.
Let’s assume, on your answer there, they commit to stock. The stock – the reason they would be stuck with it is because they couldn’t move it in a private market? We believed that in July/August they could have moved it in a private market.
But nothing had changed about the private market in July or August compared to the private market in May, had it? Only the mindset we would have to convince the pharmacists about the mindset and how they were to approach the patients.
So it was really your change of mind, not the change of ? This is
what you thought the pharmacists might do? But this is what – this is what we do. I mean, Alphapharm took the substitution rate up over five years, nearly double the substitution rate in the market, through training and education of pharmacists. That’s what we did.
517 As best as I can explain it, it seemed that Mr Hurley did not want to say anything that might support Wyeth in establishing that Alphapharm’s private market launch of its products in July 2009 was not genuine. As will be explained, this concern was unnecessary because the primary issue is what Alphapharm would or might have done if not for the Alphapharm interlocutory injunction. What Alphapharm did in July 2009 is not necessarily what it would have continued to do if not for the Alphapharm interlocutory injunction.
518 I consider that the facts are these. Alphapharm was extremely concerned that during the anticipated litigation Wyeth would implement a strategy of encouraging doctors to switch patients from Efexor-XR to Pristiq (a compound which was patent protected) and then de-list Efexor-XR from the PBS, which would mean that no generic venlafaxine brand could be listed on the PBS. Alphapharm also believed private market supply of a generic when the originator brand was PBS listed would not be in the best interests of pharmacists and would be unlikely to succeed (a not unreasonable view given this kind of supply was unprecedented). But Alphapharm had to launch its products on the private market in any event because otherwise it had no chance of getting what it wanted from Wyeth, which was an undertaking from Wyeth not to de-list Efexor-XR as part of the price Wyeth would have to pay to obtain an interlocutory injunction. Knowing as it did that Wyeth would obtain an interlocutory injunction if it applied for it, whether private market supply would have succeeded or not was simply not critical for Alphapharm. There was no need for Alphapharm to give serious consideration to whether private market supply would succeed or not. Faced with the method patent and Wyeth’s allegations of infringement, Alphapharm knew that it could get what it wanted if it launched on the private market (an undertaking from Wyeth not to de-list Efexor-XR as the price of an interlocutory injunction as well as an undertaking as to damages) and would be restrained quickly by an interlocutory injunction from continuing its private market supply. Alphapharm’s concerns about the problems confronting private market supply were thus not material to its strategy. Further, insofar as pharmacists were concerned, Alphapharm’s policy was “sale or return” (that is, return with a refund) so Alphapharm was also not risking its relationship with its customers by doing what it did. This is why, as we will see, there is not a single piece of paper about Alphapharm’s launch, not a single document recording Alphapharm’s projections or budget for the launch, or even a document recording a single order from the launch.
519 Adding weight to these inferences is the fact that Alphapharm knew it would not seek PBS listing unless and until another generic obtained PBS listing. It was thus dependent on the decision-making of another generic and had no control over that decision-making. If Efexor-XR did not remain PBS listed, everything Alphapharm had done would have been wasted and the substantial opportunity it believed existed after expiry of the compound patent would be lost. Ensuring Efexor-XR remained PBS listed was fundamental for Alphapharm’s long-term interests.
520 I will return to the issue in more detail, but should say now that contrary to Wyeth’s contentions none of this means that Alphapharm was engaged in a sham (which is the only meaning I can give to Wyeth’s contention that the launch was not “genuine”). Alphapharm did launch its products on to the private market albeit in a confined manner and in the expectation (and, indeed, given the circumstances as they existed, I would infer hope) of being quickly restrained so it could obtain the undertakings it wanted. Alphapharm’s beliefs, intentions and strategies are relevant to what Alphapharm would have done if confronted by everything as it was in 2009 but for the interlocutory injunctions, but this does not mean that if Wyeth had not sought the interlocutory injunction against Alphapharm that Alphapharm would have continued with a confined private market supply, unsupported by any budgets, marketing, training or the like. What is obvious that, but for the interlocutory injunctions, Alphapharm would have been confronting a venture which it had never attempted before and had serious concerns about and that Alphapharm did not see Sigma’s example as any comfort because it thought Sigma’s sales representatives might have been holding out the carrot of PBS listing to pharmacists.
521 It should also be apparent from this why Alphapharm’s preclusion arguments about its supply of its products should not be accepted. In a case claiming equitable compensation under Wyeth’s undertaking, Wyeth cannot fairly be prevented from making the case that Alphapharm’s launch was the result of forensic considerations and does not represent what it would have done had the Alphapharm interlocutory injunction not been granted.
522 The other point which should be recorded immediately is that Wyeth’s contention concerned Alphapharm alone. It did not suggest, and nor could it, that Alphapharm’s lawyers did anything other than properly act on the instructions they received from Alphapharm. Alphapharm submitted that this of itself should put paid to Wyeth’s contention that Alphapharm’s launch was not genuine but I do not doubt Mr Hurley was well able to understand the effect of legal advice, formulate strategies to suit what he perceived to be in Alphapharm’s best interests and give instructions to Alphapharm’s lawyers accordingly.
523 For example, Mr Hurley agreed that any launch of an Alphapharm product involved the preparation of written material. He then appears to have appreciated where this line of questioning was heading because the fact is there is not a single document relating to Alphapharm’s private market launch of its products in July 2009. Mr Hurley then clarified that there would be written material if “sales representatives were launching the product” but not necessarily if sales managers did so because they could be given verbal instructions. This exchange ensued:
Even sales managers needed to know (a) what the product was which was being sold, didn’t they? Sales managers can be – there are fewer of them. They’re more in tune with the environment. And you can talk to them and communicate verbally. You don’t necessarily have to put things in writing.
You have to show them something, wouldn’t you? Not necessarily.
A picture of the product? Not necessarily.
Are you answering these questions in the way you are because you know that not a single piece of paper was produced in relation to the Alphapharm private launch? Yes, that’s true.
So there’s not one single piece of paper to confirm, is that right, that there was, in fact, any private launch of venlafaxine by Alphapharm? I don’t know whether there was a single sheet of paper or not, but there was no promotional material or written material provided to the sales manager.
You say that’s normal, do you? No. I’m saying that in this situation, we were trying to keep it close.
524 Again, I consider that Mr Hurley was concerned to shore up Alphapharm’s case.
525 In his affidavit from 2009 Mr Hurley referred to Alphapharm having received one order as a result of its private market launch. The order was not annexed to his affidavit. It has never been produced. Mr Hurley agreed that a company like Alphapharm kept records of orders but appeared incapable of agreeing to some basic propositions which he must have believed would be contrary to Alphapharm’s interests. This evidence was given:
So may we take it in the ordinary course if an order was received, there would be a document recording the order? There may or may not be put in the system at that time. The order was received by a sales manager. He may have not put it in the order system.
It’s not like going into a grocery shop and say, “Can I have a packet of Smarties”. That’s not how you order these sort of products, they are not verbal orders, are they? In the circumstance we are talking about here where he is sitting across the customer from key customers, with a leadership customers, they may, in fact, give him a verbal order.
… And then it’s up to the sales manager and go back and potentially put it in the system.
526 Not annexing an order to an affidavit defending an application for an interlocutory injunction is one thing. The evidence in this case, that no such order can be found, is another. This evidence then emerged:
May we take it you don’t remember looking at a document before you saw that affidavit recording an order? Well it depends on your definition of an order. You are saying an order is something that is recorded in our computer system. I am saying an order is something that can be verbally given to a sales manager who is having further negotiations with the customer to maybe try to increase that order.
So it may not have even been a firm order. Is that right? I understand at that time, it was a firm order.
And that’s an understanding based on what somebody told you? Yes.
Do you remember who it was who told you? I – the key account manager for Chemist Warehouse. I think it might have been Tim Burrows.
What did he say to you? That he had received an order for – I think it was eight thousand five hundred and something thousand dollars.
On what terms? Did he tell you what the terms were, date of delivery? Was it dependent on PBS? No. It wasn’t dependent on PBS listing. There was no communication about PBS listing when we talk to the customers about private launch.
How do you know? You weren’t there? Because that was an instruction.
Who gave to whom? I gave to the sales managers.
How do you know they followed it? Well, that was their own – the responsible people within the company, and that’s why I restricted the launch to the sales managers because they could communicate more effectively with the customers and more likely to follow direction.
527 The important point for present purposes is that Mr Hurley disclosed none of this in his affidavit in 2009. In 2009 he represented that an order had been received. On the basis of the evidence that Alphapharm has no record of any such order and that Mr Hurley appears to have been relying on a conversation alone, I would not accept that Alphapharm in fact received an order in 2009.
528 When asked whether any modelling of Alphapharm’s private launch had been done, Mr Hurley said “I may have done it on a scrap of paper” but “I wouldn’t keep such things”. This was a launch which Mr Hurley described in his affidavit in 2009 in these terms:
Alphapharm launched Enlafax through a process by which four of its National Account Managers informed eleven key customers in verbal discussions of the availability of Enlafax XR. Under my supervision, the Account Managers were instructed to advise those customers that Enlafax XR would not be immediately listed on the PBS, but that it would be a non-PBS, or a "private launch".
…
529 The idea that Alphapharm would launch a product to 11 key customers without recording what results it expected to obtain from the launch other than on a scrap of paper which would not be retained is untenable. This confirms that Alphapharm’s launch on the private market to its 11 key customers bore no resemblance to what would ordinarily be understood to be a launch of a new generic product by a generic company in the expectation of significant profits. None of these matters were conveyed in Mr Hurley’s 2009 affidavit.
530 Mr Hurley’s tendency to convey incomplete or inaccurate material when he thought it in Alphapharm’s interests to do so is also apparent from the fact that he gave instructions for Alphapharm’s first PBS listing application to state that “we will forward as soon as possible, or by the 15th January 2009 at the latest, the following documents in support of our application”, which included the TGA approval and thus ARTG registration of the products. However, Alphapharm did not know it would receive TGA approval by 15 January 2009. Mr Hurley was “very hopeful” and “pushing hard” for that to occur, which may be accepted. But he knew of the difficulties Alphapharm was experiencing in achieving TGA approval of its products and nevertheless instructed that a clear representation be made to the Department on a serious matter, that Alphapharm would be able to forward such an approval by 15 January 2009 at the latest, when he could not possibly have been certain this would be so. Alphapharm could not do so and thus its PBS listing application lapsed.
531 Mr Hurley also said in in his 2009 affidavit, I infer at a time when he believed the legal advice would not be disclosed, that:
Once Alphapharm became aware of the Method Patent, it determined that the patent was invalid and not infringed by its proposed product. Accordingly, Alphapharm took steps to convince Wyeth that its product did not infringe. Ultimately Alphapharm commenced proceedings seeking to have the Method Patent revoked.
532 In the context of Alphapharm’s claims under the undertakings, the legal advice has now been disclosed. The summary of the advice was in fact to this effect:
There are several arguments against the validity of the broad method claims of the Method Patent, but we can anticipate potentially plausible counter-arguments to most of them. We rate the prospects of demonstrating invalidity, based on the information we have considered, and without having spoken to an independent expert, as better than even. In other words, although there are certainly lines of attack on the validity of the broad method claims, the vagaries of patent litigation are such that there is a substantial risk that Alphapharm will not be able to invalidate the method claims.
533 When shown the advice, without having been reminded of what he said about it in 2009, Mr Hurley gave this evidence:
…you didn’t understand, but the – according to this advice, at least, that Alphapharm could proceed in relation to validity on anything other than that it was potentially finely balanced issue. Mallesons thought Alphapharm had the better of the argument but the vagaries of litigation were unpredictable? Yes.
Is that a reasonable summary? Yes.
534 In short, what Mr Hurley said about the advice in his 2009 affidavit bears little resemblance to the advice Alphapharm had in fact been given.
535 Mr Hurley instructed Alphapharm’s lawyers to send a letter to Wyeth’s lawyers on 8 July 2009 saying that Alphapharm “intends to begin supplying Enlafax XR shortly”. At the time Alphapharm had no stock in the country but Mr Hurley was keen to point out in oral evidence that it could have shipped stock in very quickly (a fact which I accept). Mr Hurley instructed that a further letter be sent on 17 July 2009 saying that Alphapharm had “today launched its products”. I have explained above the circumstances in which these instructions were given (namely, Alphapharm knowing it had to get Wyeth into court so that it could obtain the undertakings it wanted and hoping to be restrained quickly, so it did not matter whether the private market supply would succeed or not).
536 The relevant point for present purposes is that despite this, Mr Hurley repeatedly refused to agree to the obvious proposition that in order to achieve his objective of getting Wyeth to undertake not to de-list Efexor-XR, Alphapharm needed to get Wyeth into court. These exchanges occurred:
And what I’m suggesting to you is you understood that you needed to get Wyeth into court in order to extract from them the one protection you really wanted, namely, a protection against delisting. That’s right, isn’t it? That was an outcome of the interlocutory injunction, yes.
But that was what you understood you needed – I will put it again. You understood you needed to get Wyeth into court to secure the one protection you thought you really needed, namely, it was an undertaking from Wyeth not to delist. Do you agree? I agree that that was an outcome of it. Yes.
What I’m putting to you is you understood you needed to get Wyeth into court in order to get that protection? I would have preferred to launch the product and take the product to market and generify the product. I didn’t want an interlocutory injunction.
You would have preferred to have got that protection without having to go to court; that’s right? You would have preferred to get the protection of an undertaking from Wyeth not to delist without going to court? I don’t think they would give us such an undertaking, no.
Without it being a condition of interlocutory relief? I presume so.
…
And see, what I’m suggesting to you is what you were told by the lawyers was that in order to get an undertaking from Wyeth not to delist, you would have to persuade Wyeth that you were going to launch and get them take you to court and secure an interlocutory injunction. You understand what I’m saying? I understand what you’re saying.
And do you agree with me? Part of it.
Which part? That the advice was that if the interlocutory injunction was sought in the court, that we could get a guarantee or a potential guarantee not to delist venlafaxine from the PBS or Effexor from the PBS.
That was only likely to happen if you could persuade Wyeth that you were actually going to launch, as you understood it? I don’t know that.
…
what I’m suggesting to you is you were only going to get the guarantee if you could persuade Wyeth that you were actually going to launch because that would get Wyeth into court and you could get the undertaking from them. You understood that, didn’t you? I – do I have a document that says that? I don’t understand necessarily that. I mean, Wyeth could have sought an interlocutory injunction without us attempting to launch.
Don’t worry about what documents you may have or may not have. I’m just going you whether you agree with me with this proposition: you understood, I suggest, that Wyeth needed to be persuaded that you were threatening to launch in order to get Wyeth into court so you could achieve from them the undertaking not to delist. Do you agree with that? I don’t know that I understood that I had to convince Wyeth that I was going to launch for them to seek an interlocutory injunction.
Can I suggest to you you did understand that. You don’t agree with me? I don’t recall understanding that.
So it’s a possibility, you don’t deny you had that understanding but you don’t simply recall having that understanding? Yes.
537 I am prepared to accept that Mr Hurley did not recall what he understood in 2009, but I do not doubt he understood in 2009 that the only way of getting Wyeth to undertake not to de-list Efexor-XR was for Alphapharm to threaten and, if necessary, launch its products to prompt an interlocutory injunction application by Wyeth. But what is presently relevant is Mr Hurley’s repeated refusals to engage with the questions being put to him and preference to state nothing more than that if the method patent did not exist and Wyeth did not assert infringement of it Alphapharm would have done what it originally intended to do which was to apply for PBS listing and supply its products under the PBS (which I do not doubt but is irrelevant).
538 Mr Hurley’s evidence in response to questions about other aspects of the interlocutory injunction hearing in 2009 also appeared to be self-serving (or Alphapharm-serving). Ms Braithwaite of Wyeth gave affidavits in support of Wyeth’s interlocutory applications. In her affidavit about Alphapharm she noted that despite Alphapharm saying to Wyeth it had launched its products Wyeth’s sales representatives and other sources of information were unaware of “any commercial activity in the marketplace associated with Alphapharm’s Enlafax XR products”. When asked only if he understood Ms Braithwaite was saying that Wyeth had seen no evidence of Alphapharm attempting to sell its products, Mr Hurley volunteered this:
The representation that was made for the Alphapharm product was made to the key account groups at head office level, firstly, and therefore, you know, the chat around pharmacies may have been limited at that point of time. Secondly, Wyeth’s sales representatives, I would submit, would be GP sales representatives which were bobbing in and out of pharmacy without any particular relationship with the pharmacists and may not have got a truthful or a reasonable response from a pharmacy.
539 The more likely explanation is that Alphapharm’s launch consisted of the minimum activity it believed it could conduct whilst still being able to say that it had launched its products.
540 There was also evidence about Mr Hurley’s approach to serious commercial dealings in respect of instructions to Alphapharm’s lawyers resulting in letters of 31 July and 6 August 2009. In those letters Alphapharm said that it would undertake not to apply to list its products on the PBS unless it was possible to do so without triggering the 12.5% price reduction in Efexor-XR in exchange for Wyeth undertaking not to de-list Efexor-XR from the PBS. This exchange occurred, which again discloses the self-serving quality of much of Mr Hurley’s evidence and a lack of appreciation that what he saw as standard commercial operating mode others might see as approaching sharp practice:
And over the page, second paragraph from the top of that page. So, again, would you agree there’s an emphasis coming from the Alphapharm side through Mallesons on that delisting undertaking? Yes, there’s a trade-off against committing not to – for Alphapharm not to list on the PBS.
Yes, which you weren’t going to do anyway? They didn’t know that.
I beg your pardon? Wyeth wouldn’t have known that.
And you weren’t going to tell them? Of course not.
You weren’t going to tell them because you wanted to extract the undertaking not to delist; that’s right? No.
I beg your pardon? No, I wouldn’t discuss with anybody whether I was going to list a product – a competitor whether I was going to list a product on the PBS.
541 It is not that Alphapharm was obliged to let Wyeth know its intentions. It is that in giving his evidence Mr Hurley appears to have seen nothing even potentially amiss with Alphapharm seeking to obtain a valuable benefit from Wyeth based on what might have been a misapprehension about Alphapharm’s intentions confirmed and perhaps even induced by Alphapharm. This observation does not involve any criticism of Alphapharm’s lawyers. Alphapharm alone knew what is intentions were and the lawyers acted on Alphapharm’s instructions. The observation also does not involve any criticism of the terms of the letter.
542 There was then this exchange, which again shows Mr Hurley consistently answering questions by adding comments in a manner which served Alphapharm’s interests:
And what I want to suggest to you you understood the only leverage you had over Wyeth to extract from them an undertaking to delist was a threat to privately launch. Did you – do you understand what I’m saying? I understand what you’re saying.
And do you agree with me that that’s what you understood was the only leverage you had out of – over Wyeth to get a delisting undertaking – no delisting undertaking? No, I would prefer that Wyeth didn’t issue an interlocutory injunction, and we could get on in the marketplace and sell privately.
543 Apart from it being gratuitous, I do not accept that this was Mr Hurley’s preference in the actual circumstances with which Alphapharm was confronted in 2009. While his undoubted preference would be for the method patent and the anticipated infringement litigation not to exist so Alphapharm could seek PBS listing and supply under the PBS in the ordinary course, given the strength of his concern about Wyeth switching patients to Pristiq and de-listing Efexor-XR (let alone his belief that private market supply would probably be unsuccessful), the unavoidable inference is that his preference in 2009 given that the method patent did exist was for Alphapharm to do what it needed to do to get Wyeth into court, where the inevitable result would be for Alphapharm to be restrained, and Alphapharm could get the undertakings it wanted.
544 Mr Hurley’s evidence about how the products were launched to the 11 key customers also exposed difficulties. Mr Hurley refused to accept that he knew it was inevitable or highly probable that if Alphapharm launched Wyeth would apply for an interlocutory injunction (despite it having done so in respect of Sigma) but was forced to agree that he thought this was probable. This evidence was then given:
Did you say to the key customers, “Now, our – we have been told that we have to threaten to launch in order to get Wyeth into court. That’s why we are approaching you”? Of course not.
You didn’t say that? Of course not.
Did you tell them that, “We want you to acquire some product but, look, they’re almost – they’re probably going to injunct us, and we won’t be able to supply them”. Did you tell them that? Of course not.
Of course not? No.
Wouldn’t it be misleading to approach the customers, if you didn’t tell them that? We wanted to launch the product privately in the market to our key customers, and that’s what we – that was our undertaking at that point of time. I’m not talking about the legal case or injunctions.
But presumably, if you were engaging in a proper private launch, you would be telling them, “We are offering to supply you a product”? Yes.
All right? Yes.
So at the same time as the – let’s assume those words were coming out of the mouth of a sales representatives for one of these 11 key customers ? Yes.
You knew that it was probable ? Probable.
that there would be an injunction application, and if there was, the inevitable outcome was that the sales representative would not be able to honour what he was saying to the one of the 11 key customers, namely, “We will supply product”? Yes.
And what you say that you let – you sent people out into the marketplace to convey the message of an offer of supply without letting the key customers know that it was highly likely that the supply would not be able to be provided? Of course we didn’t say that to them.
Can I suggest – I suggest to you this idea of a private launch just didn’t happen in the way you suggest? I suggest to you that it did.
See, what I want to suggest is you understood that you needed to have some sort of launch in order to trigger an interlocutory injunction application, but it wasn’t a serious endeavour to try and sell a product? It was, because we approached our key customers, our major customers. We didn’t approach small pharmacies in the back of nowhere. This was the head office of our major customers.
Well, if they were key customers, they might understand your predicament and understand if you gave them a full explanation as to why you were approaching them? They would understand the predicament the product wasn’t going to be listed on the PBS.
545 What Alphapharm’s sales managers were instructed to say to the 11 key customers say will remain a mystery, but whatever was said I consider that it did not result in a firm order for the products. Mr Hurley gave this evidence:
Your evidence is that this private launch proceeded for a period of middle of July to about 25 July, which is about – 25 August, about six weeks? Yes.
On the working assumption that they could return it if they couldn’t move it and they weren’t told that there was an impending injunction application and they may not be able to sell it. That’s your evidence to the court? Yes.
And with all those – in all those circumstances, according to your evidence you managed to get just one order? Yes.
Of $8,512 which I think the price was $16, you assumed? Yes.
That is 532 units? Is it? Okay.
546 When asked how this was to be reconciled with his estimate first given in 2009 that Alphapharm’s maximum market penetration if no other generics were in the market was 13.9% of the total market, Mr Hurley said that Alphapharm had not “applied any marketing program against this, at this point of time”. This again discloses that what Alphapharm did in 2009 is not a guide to what it would or might have done but for the interlocutory injunctions.
547 In conclusion, Mr Hurley’s evidence about contested issues or what he perceived might be contested issues contained so many difficulties that, contrary to Alphapharm’s submissions, it cannot be given weight. As a result, it is not fruitful to deal in detail with Alphapharm’s submissions to the extent they relied on Mr Hurley’s evidence about contested issues. His evidence is not a reliable foundation for any inference about a contested issue.
Evaluation of the probabilities and possibilities
548 Alphapharm was unaware of the method patent when it decided to pursue the opportunity it perceived would exist to supply generic extended release venlafaxine products on expiry of the compound patent in December 2008. In September 2007 Alphapharm applied for ARTG registrations for its products, which were to be sourced directly by Alphapharm from Pharmathen. In the ordinary course Alphapharm proposed to apply for its products to be listed on the PBS after they had been registered on the ARTG and to supply its products only under the PBS. Unlike Sigma, Alphapharm did not plan to launch its products in two stages, first on the private market for a period of some months, to be followed by PBS listing and supply under the PBS. To this end Alphapharm applied for PBS listing on 1 December 2008 but this application lapsed because Alphapharm had not obtained ARTG registration of its products. Because Alphapharm believed that the only impediment to its plans was the compound patent scheduled to expire in December 2008 it did not need to seek the approval of Mylan to apply for PBS listing because it did not perceive its launch to be at risk.
549 To obtain ARTG registrations for its products Alphapharm was required to submit a bioequivalence study. It did so and, after substantial time, effort and expense, obtained ARTG registrations for its Enlafax XR products, 150mg and 75mg, on 30 April 2009.
550 In late March 2009, however, Alphapharm became aware of the method patent.
551 Alphapharm accepted that once it became aware of the method patent there was no prospect that it would have been the first to apply to list its generic products on the PBS. It was not willing to trigger the 12.5% price reduction of Efexor-XR by being the first to obtain PBS listing or by obtaining PBS listing at the same time as any other generic. Alphapharm could hardly contend to the contrary given that in his 2009 affidavit Mr Hurley had said that because Wyeth alleged that Alphapharm’s products infringed the method patent:
Alphapharm has decided that it cannot proceed with a PBS listing which would lead to a 12.5% price reduction being required of Wyeth under the PBS. Were Wyeth to be successful in these proceedings, the possibility that Alphapharm may have to compensate Wyeth for that price reduction represents a significant commercial threat to Alphapharm and an amount which would exceed the likely profits which could be made from sales of Enlafax.
552 It will be apparent that it was the mere allegation of infringement which caused Alphapharm not to seek PBS listing, which has nothing to do with the interlocutory injunctions.
553 Wyeth submitted this:
The evidence of Alphapharm’s Chief Executive Officer at the relevant time, Mr Montgomery, is also specific on this point. He did not want Alphapharm to trigger the 12.5% price reduction for being the first generic listed on the PBS (or indeed be an “equal first generic” and share that responsibility with other suppliers), “and so [he] would not have caused Alphapharm to proceed to list on the PBS”. Mr Montgomery quantified the extent of the risk at “12.5 million per year until patent expiry” (based on annual sales of $100 million and the mandatory price reduction of 12.5%). The Method Patent was not scheduled to expire until 2017, meaning that the potential exposure was in the order of $100 million. Mr Montgomery’s position was, therefore, that even a proportion of that amount was an unacceptable level of exposure.
554 Perhaps uniquely in terms of the generics’ evidence about PBS listing, Mr Montgomery’s evidence accords with commercial rationality. The fact that he assessed the potential liability of PBS listing to be in the order of $100 million also undermines Mr de Alwis’s unconvincing attempts to avoid this proposition. Further, it supports my conclusions that the evidence (particularly of Mr de Alwis and Mr Upiter) that Sigma and Generic Health would have sought PBS listing but for the interlocutory injunctions should not be accepted. The fact that Sigma stood to gain more benefit than Alphapharm from PBS listing may be accepted but confronted with a potential $100 million exposure, this evidence about Alphapharm’s position in 2009 is strong contemporaneous evidence of the reasoning process of a commercially rational decision-maker.
555 The chance that Alphapharm would have sought PBS listing of its products at any time before the Sigma interlocutory injunction, accordingly, depends on Sigma or some other generic having first obtained PBS listing. To foreshadow further conclusions below about Generic Health and other generics having sought PBS listing at any time earlier than they did, I consider that Alphapharm’s prospect of having sought PBS listing for its products before the Full Court’s judgment in October 2011 depends solely on the possibility that Sigma might have obtained PBS listing on 1 December 2009. It necessarily follows that if Alphapharm needed to prove that it would have listed its products on the PBS at any time before it in fact did so on the balance of probabilities, Alphapharm’s claim based on PBS listing and supply of its products must fail. If, as I consider to be so, the prospect of Alphapharm applying for PBS listing after Sigma had obtained PBS listing is the relevant issue then that prospect must be assessed along with Alphapharm’s claim that in any event it would have supplied its products on the private market but for the interlocutory injunctions.
556 I accept Wyeth’s proposition that contrary to Alphapharm’s submissions it “is clear that Alphapharm had not previously undertaken a private launch of a product that was PBS listed by the innovator”. Wyeth provided a convenient summary of the evidence disclosing Alphapharm’s concerns about private market supply and what Sigma was doing which I adopt as follows:
(a) An email chain dated 4 May 2009 (originating with a pharmacist and reaching upper levels of Alphapharm management, including Mr Hurley) contained commentary on SPAL’s private market offer, which was critical of Alphapharm’s competitor for using language that “conjures up the image of PBS listing” where none was imminent, and suggested that “Pharmacists should be asking [SPAL] specifically when PBS listing will be”.
(b) An email dated 5 May 2009 from a State Sales Manager to her sales team members suggested that they tell Alphapharm’s customers to ask SPAL questions directed to the true nature of its private launch, which presumably it was hoped would make the take-up of an offer from SPAL less appealing:
1. What is the date for Sigma[’]s product to be PBS listed?
2. Who carries the risk associated with any inventory should the product not be PBS listed?
3. Is the product A flagged?
(c) A document that was prepared for Alphapharm sales representatives emphasised that “Sigma is selling this product into Pharmacy with NO confirmation of a PBS listing. (You may care to ask your customer if Sigma can confirm or promise a date for PBS listing)” and, in contrast, stated that “Alphapharm’s registration could allow us to launch our ENLAFAX-XR, however our position is not to launch at present. It is our view that it is in the best interests of our Pharmacy customers not to sell ENLAFAX_XR until we can confirm a PBS date”.
(d) An Alphapharm “coaching tool” for sales representatives (which was distributed in early May 2009), similarly stated that “Alphapharm could have also launch[ed] our ENLAFAX-XR today however we took the decision not to launch as it is our view that it is in the best interest of our Pharmacy customers not to sell in the stock until we are able to confirm a PBS date. That way you are able to best manage your stock and prepare to substitute EFEXOR XR aggressively when the time comes”.
(e) An email chain dated 13 May 2009, which originated with an Alphapharm sales representative and was escalated by a State Sales Manager to the Director of the Medical Division (Mr Robert Richardson), included bemusement at the idea that SPAL would structure a private offer to substitute a PBS listed product (“However stupid that seems!”), and an encouragement of patience in the face of SPAL’s efforts (“I am frustrated by this as well, but we are following a strategy that we believe is correct, so it is important we hang in there”).
557 The strategy that Alphapharm believed to be correct at this time, May 2009, was not to move immediately to private market supply without knowing when PBS listing could be obtained. This necessarily would have been Alphapharm’s position under any relevant hypothesis because Wyeth did not seek the interlocutory injunction against Sigma until 22 May 2009. Even if Wyeth’s threats to seek interlocutory relief could all be disregarded (which I do not accept), this would have been Alphapharm’s position because at the time it believed that what Sigma was doing was contrary to the best interests of pharmacists and would give Sigma no longer-term advantage because the pharmacists would be stuck with stock they could not sell.
558 The following propositions by Wyeth should also be accepted about Alphapharm’s change of mind in July 2009 to launch its products on the private market, all of which are in stark contrast to Alphapharm’s usual practice when launching a new product:
(a) First, there was no strategy document setting out the benefits and challenges for the unprecedented private launch, or addressing the circumstances that had caused Alphapharm to reconsider the stance it had taken in the face of its principal competitor’s marketing efforts over several months….
(b) Second, no modelling was done of the financial impact of a private launch of venlafaxine….
(c) Third, there was no promotional documentation produced to support sales representatives or sales managers in altering the message that had been conveyed on Alphapharm’s behalf in the period up to and including May 2009…
(d) Fourth, there was no documentary record of a positive reception from pharmacy customers to Alphapharm’s supposed change of position, and valuable private market offer. In particular, there are no documents or records substantiating any order placed in response to the verbal offers said by Mr Hurley to have been made by Alphapharm’s sales managers…
559 Wyeth also noted that Mr Montgomery’s evidence was that all launches at risk required Mylan’s approval but there is no evidence that Alphapharm sought or obtained Mylan’s approval for its 2009 private market launch. Alphapharm submitted that this proposition could not be advanced by Wyeth because it was not put to Mr Montgomery or Mr Hurley. I disagree. Mr Montgomery and Mr Hurley did say that all launches at risk required Mylan’s approval but this evidence was evidence about the general policy position. They did not suggest that they had sought and obtained Mylan’s approval for what occurred in July 2009. There is no evidence of any of the kind of information that Mr Montgomery identified as necessary to submit to Mylan for approval for an at risk launch when, had approval been sought and obtained, such evidence would be expected in the ordinary course. Mr Montgomery said that the process from 2007 when Mylan acquired Alphapharm was “a more comprehensive, formal process for the approval of product launches by Alphapharm” than previously and, in particular at risk launches “required that Alphapharm set out the risks and benefits of the launch”. There is no evidence suggesting this occurred in 2009.
560 Wyeth submitted that:
…the evidence before the Court does not support a finding that in mid-2009 Alphapharm was preparing to make, or had made, a private launch of its generic venlafaxine as a serious commercial endeavour. On the contrary, the absence of documentary support for the case Alphapharm now seeks to advance undermines the veracity of that core contention.
561 If by “serious commercial endeavour” Wyeth means an endeavour in the hope that the supply could continue unrestrained by Wyeth and in the expectation that Alphapharm would make material profits from the endeavour, I agree. This does not mean, however, that Alphapharm necessarily would have taken the same approach if on 22 May 2009 Wyeth had not sought an interlocutory injunction against Sigma and, as relevant on my approach, on 14 August 2009 had not sought the interlocutory injunction against Alphapharm. At least one reason for this is that Sigma would have been selling its products on the private market leading to a high likelihood of increasing queries from Alphapharm’s customers about Alphapharm’s position and associated commercial pressure on Alphapharm.
562 Alphapharm’s first order for generic venlafaxine products from Pharmathen was made on 22 May 2008 which pre-dates Alphapharm’s discovery of the method patent. A second version of the order form (which removed a dosage strength for which Alphapharm did not receive ARTG approval) re-scheduled delivery for 4 July 2009. That order must have been deferred on Alphapharm’s instructions before 4 July 2009 (and thus before Alphapharm was subject to an interlocutory injunction) because the products under that order did not arrive until 20 December 2011. It is not clear whether the order was deferred before or after 22 May 2009. If before it was not caused by Wyeth having sought interlocutory relief against Sigma on 22 May 2009. Nor was it caused by the Sigma interlocutory injunction granted on 3 June 2009 and it could not have been caused by Wyeth seeking the Alphapharm interlocutory injunction on 14 August 2009 or the operation of that interlocutory injunction which was granted on 25 August 2009. Mr Williams, Vice President Global Business Development of Pharmathen, said in an affidavit that “Alphapharm advised Pharmathen that it was not in a position to take delivery of the Product due to litigation with Wyeth”.
563 I consider it overwhelmingly likely that the order was deferred because of the mere existence of the method patent and anticipated litigation rather than the Sigma interlocutory injunction (let alone the operation of the Sigma interlocutory injunction which had nothing to do with Alphapharm’s products). But, as will be explained, this does not mean that Alphapharm’s claim for the products it had to destroy must fail. It means only that Alphapharm did not have stock available for immediate supply to pharmacists when it launched its products in July 2009 and would not have had stock available then irrespective of the interlocutory injunctions. But for the Alphapharm interlocutory injunction, however, Alphapharm would have been free to consider its position from time to time in response to circumstances and necessarily would have done so given Sigma’s continuing private market supply up to 1 December 2009.
564 Wyeth submitted that:
Alphapharm’s contentions that it did in the real world make, and would in the counterfactual world have made, a genuine attempt to launch its generic venlafaxine products on the private market should be rejected. Both contentions fail on the evidence, which rises no higher than self-serving assertion by Mr Hurley, not properly corroborated by documentary evidence, and contradicted by a careful reading of Mr Montgomery’s evidence.
565 I have already accepted above that what Alphapharm did in 2009 was not done in the hope that the supply could continue unrestrained by Wyeth and in the expectation that Alphapharm would make material profits from the endeavour. As such, I agree that it is not a reliable evidentiary foundation for what Alphapharm would or might have done if on 22 May 2009 Wyeth had not sought an interlocutory injunction against Sigma and on 14 August 2009 had not sought an interlocutory injunction against Alphapharm so that Sigma would have been continuing its private market supply with apparent success.
566 Alphapharm submitted that six matters supported the inference that it would have continued to sell Enlafax XR privately.
567 Alphapharm said that:
First, on 19 June 2009 Alphapharm filed proceedings NSD 596 of 2009 seeking revocation of the Method Patent. That indicates a firm intention to sell Enlafax XR and the belief that the Method Patent was invalid.
568 It may be accepted that this indicates a firm intention to sell Enlafax XR if the method patent was declared invalid. It does not say anything about Alphapharm’s intentions before such a declaration.
569 Alphapharm said that:
Second, Alphapharm contested the application for the AII [the Alphapharm interlocutory injunction]. It is a necessary premise of Wyeth’s submission that Alphapharm would not have sold Enlafax XR even in the absence of the AII, that Alphapharm went to the expense of contesting the AII without intending to sell Enlafax XR.
570 However, Alphapharm needed to contest the interlocutory injunction in order to ensure that its primary concern that Wyeth not de-list Efexor-XR would be addressed by Wyeth giving undertakings to that effect. Sigma had not obtained such an undertaking. Alphapharm had tried and failed to negotiate a deal to this effect with Wyeth. There would be no incentive for Wyeth to offer an undertaking not to de-list Efexor-XR unless it was forced to do so. From Alphapharm’s perspective, the only way to force Wyeth to do so was to defend the interlocutory injunction application.
571 Alphapharm said that:
Third, Mr Hurley’s understanding that Alphapharm’s assessment that the Method Patent was invalid and not infringed was vindicated in so far as invalidity was determined by the Full Court.
572 Vindication by the Full Court cannot be relevant to what Alphapharm would or might have done in 2009. In any event, on 7 May 2009, a file note of a discussion with Alphapharm’s lawyers says “much better non-infringement” than the lawyers had initially believed due to the hydrogel technology argument, but another file note of the same meeting records a need to “ask a formulator”. A file note of 28 May 2009 records no more than that “we say non-infringement” but this was “not necessarily enough to outweigh” Wyeth’s case for an interlocutory injunction as the Court would not want to deal with that argument in the context of an interlocutory injunction, and the result may be different from the Sigma interlocutory injunction due to “good non-infringement/validity” but “can’t guarantee”. Another file note of the same meeting records only that there was an “arguable”, “strongly arguable even” case that the method patent was invalid. Subsequently, Alphapharm had counsel’s advice that the arguments would not avoid an interlocutory injunction. In any event, it may be accepted that by July 2009 Alphapharm believed it had a good case of non-infringement and invalidity of the method patent but the legal advice it received, as would be expected, never suggested that it was certain to succeed and was always subject to the kind of caveats that lawyers routinely include in advice given the inherent risks and uncertainties of litigation.
573 Alphapharm said that:
Fourth, Alphapharm was sufficiently confident in its assessment that the Method Patent was invalid, such that even after being unsuccessful on this ground at first instance, it went to the considerable expense of pursuing an appeal
574 Again, this says little if anything about what Alphapharm would or might have done in 2009 but for the interlocutory injunctions.
575 Alphapharm said that:
Fifth, after being successful on the appeal, Alphapharm immediately commenced selling Enlafax XR, and listed Enlafax XR on the PBS on the first available date, namely 1 April 2012, notwithstanding that an application for special leave had been foreshadowed and filed by Wyeth. In that period, the Court would infer from the fact of selling on the private market and listing on the PBS that Alphapharm was prepared to and did place itself at risk of being liable to Wyeth for damages for patent infringement in the event that the High Court granted special leave to appeal and allowed the appeal.
576 As discussed elsewhere, this risk is of a different kind altogether from the risk as it existed and would have existed in 2009. Alphapharm had the benefit of a fully reasoned judgment from the Full Court. It knew that there was no right of appeal. It would have rightly assessed the risk after the Full Court’s judgment as negligible or trivial which it could not have done in 2009 (and the same will be said for Generic Health which put a similar submission).
577 Alphapharm said that:
Sixth, Alphapharm’s position in relation to the risk of damages is inherently plausible. Alphapharm’s position, apparent from its evidence filed in these proceedings and by inference from the objective steps that it took in the course of the litigation, reflects a nuanced cost benefit analysis which indicated that the benefit of selling Enlafax XR, including making PBS sales, outweighed the cost/risk in circumstances where Alphapharm did not trigger the 12.5% price drop by being the first generic to list.
578 In fact, and as explained, Alphapharm did no cost-benefit analysis of its 2009 launch at all but if not for the interlocutory injunctions I consider it would have done so as part of an overall analysis of what it should do. If the Sigma interlocutory injunction had not been sought on 22 May 2009 then I cannot imagine that Alphapharm would have conducted itself as it did in July 2009 with its confined private market launch of its products, with no modelling, no analysis, no written documents, no marketing, and as I have said no serious expectation that it was undertaking a commercial endeavour that would continue or yield material profits.
579 If, as I consider, the seeking of the Sigma interlocutory injunction cannot be disregarded then while Alphapharm would have acted as it did up to 14 August 2009 it must also be posited that on 14 August 2009 Wyeth did not prosecute its claim for interlocutory relief against Alphapharm. In other words, Alphapharm would have been in the same position one way or another albeit a month or two earlier but for the Sigma interlocutory injunction. That is, it would have to decide what to do in circumstances where Wyeth asserted infringement, was litigating to that end on the basis it would claim final injunctions and damages against Alphapharm, Alphapharm was not willing to take the risk of PBS listing and believed that private market supply (as Sigma would have been undertaking) would not be in the interests of its customers, and Alphapharm could not negotiate a deal with Wyeth about not supplying in exchange for undertakings including an undertaking by Wyeth not to de-list Efexor-XR.
580 Before discussing this central issue of what Alphapharm would have done in these circumstances I need to deal with some of Alphapharm’s other submissions. Alphapharm submitted that exhibits 44, 45 and 46 would not have existed but for Wyeth seeking the Sigma interlocutory injunction. These are the documents disclosing Alphapharm’s concerns about what Sigma was doing. They are dated around 13 May 2009. The Sigma interlocutory injunction hearing was on 22 May 2009 and the Sigma interlocutory injunction was not granted until 3 June 2009. Contrary to Alphapharm’s submissions, it follows that there is no relevant hypothesis available before 22 May 2009. There is only what in fact occurred. Submissions to this effect overlook the fact the sole focus of the undertakings is the operation of the interlocutory injunctions. While I have been prepared to take into account the seeking of the interlocutory injunction on the day each was heard, the focus of the undertakings on the operation of the interlocutory injunctions must always be kept in mind.
581 Alphapharm submitted this:
Further, the circumstances in May 2009 were significantly different from the circumstances that Alphapharm faced in the real world after 3 June 2009. After the SII [Sigma interlocutory injunction] had been granted, it was certain that Sigma would not PBS list in the short or medium term. As Alphapharm was not willing itself to trigger the 12.5% price reduction, it was also then a certainty that Alphapharm would not PBS list in the short or medium term unless another generic PBS listed, and there were none on the horizon at that time to Alphapharm’s knowledge.
582 I accept that after 3 June 2009 and when it obtained counsel’s advice Alphapharm would have known that it had no real prospect of avoiding an interlocutory injunction if Wyeth sought one.
583 Alphapharm submitted that:
Although a private launch with no immediate prospect of a PBS listing was not the optimal outcome for both Alphapharm and pharmacists, in Alphapharm’s judgment that option was much better than no launch at all.
584 This assumes that Alphapharm in fact made such a judgment in 2009 when I do not accept that it did. Alphapharm, as noted, did no analysis at all before its 2009 launch, I infer because it knew that it would not be supplying for long.
585 Alphapharm’s submissions about its mid-2009 launch were unconvincing. I deal with the material points below, but none of this means that Alphapharm’s case must fail.
586 Mr Hurley did not give “clear evidence about his recollection of Alphapharm receiving orders for its generic venlafaxine products and that those orders were not dependent on Alphapharm listing its products on the PBS”. He recalled one order of which there is no record in circumstances where Alphapharm had no product available and could not give a supply date.
587 Alphapharm also submitted this:
To take an example, Wyeth’s position at all times has been that it could not maintain an interlocutory injunction against one generic if another generic was on the market. Accordingly, in the counterfactual that no interlocutory injunction was sought or obtained against Sigma, Wyeth would never have sought, still less obtained, an interlocutory injunction against Alphapharm. Thus, in that counterfactual the detailed correspondence between Mallesons and Gilbert + Tobin would never have occurred.
588 This, however, assumes away far too much given that the undertakings under which compensation is sought concern only the operation of the interlocutory orders.
589 Alphapharm submitted that “if Alphapharm’s private market launch was merely some kind of ruse, it could have achieved such a ruse without exposing itself to the potential embarrassment of selling to its 11 key customers. The ruse could presumably have been achieved by going to one minor customer”. I have already said that what Alphapharm did was not a ruse or sham but that does not mean it was undertaking a serious commercial endeavour to supply its products in the expectation of being able to continue to do so for material profit.
590 Alphapharm submitted that:
…there is absolutely no evidentiary basis for the suggestion that Mallesons (or any other adviser) ever told Alphapharm “that we have to threaten to launch in order to get Wyeth into court”. No such suggestion was put to Ms O’Connell, who was cross-examined. Alphapharm’s legal advice which raises the issue of seeking those undertakings from Wyeth, was given in a context where it was assumed that Wyeth would seek an interlocutory injunction against Alphapharm. There was no suggestion in that legal advice that Alphapharm should induce Wyeth to seek an interlocutory injunction.
591 This submission conflates a number of matters. One, I agree it was not and could not be suggested that Alphapharm’s lawyers suggested Alphapharm should induce Wyeth to seek an interlocutory injunction. Two, an interlocutory injunction would not be ordered absent a real threat of allegedly wrongful conduct. While a party may consent to such orders being made against it, orders would not be sought or made without evidence of a genuine threat. Everything Alphapharm’s lawyers said must have been premised on that fundamental proposition. In 2009 Alphapharm would have known or assumed that if it could not negotiate a deal with Wyeth about not de-listing Efexor-XR it had to present a credible threat of allegedly wrongful conduct before Wyeth could or would move for interlocutory relief and thus Alphapharm could address the risk which was its primary concern, of Wyeth de-listing Efexor-XR.
592 Alphapharm submitted that:
…the objective implausibility of Alphapharm undertaking such a complicated strategy of a ruse, and the seriousness of the allegation, tends strongly against it being true. It is a serious allegation to suggest that Alphapharm knowingly instructed its solicitors to misrepresent the position to Wyeth in relation to its launch plans in the letter of 22 July 2009 for strategic reasons. There can be no suggestion that Alphapharm’s solicitors would have knowingly participated in making misleading statements (and no such proposition was put to Ms O’Connell, the responsible partner in 2009, in the course of her cross-examination), and nor is there any basis for thinking that Alphapharm would have kept its solicitors less than fully informed about its true plans.
593 Alphapharm is not to be criticised for responding to Wyeth’s contentions and Wyeth did contend that Alphapharm’s launch in July 2009 was not genuine. As discussed, however, Wyeth’ description is a mischaracterisation. Alphapharm did what it did. It said it launched its products and it did so. However, I consider that it must be inferred that it did not necessarily do so expecting that it would be able to continue to do so in that confined and artificial manner for the purpose of making a profit. That inference is not only objectively plausible but in my view unavoidable on the evidence. As noted, it had no budget, no training material for pharmacists, and no marketing. However, what it did was not a ruse or sham and thus issues of misrepresentation and seriousness of the allegation do not arise.
594 Alphapharm submitted that:
In Ms Braithwaite’s affidavit of 30 July 2009, she says:
“I am aware from my knowledge that Alphapharm is also an established and significant manufacturer of generic medicines in Australia and has a large salesforce, divided into three teams that market to retail pharmacists, doctors and hospitals. As such it also has the capacity to quickly achieve significant market share for venlafaxine products as are discussed in paragraph 56 of my first affidavit, with the consequences which are discussed in paragraphs 57 and following of my first affidavit.”
In her affidavit of 12 August 2009, prepared by her after reading Mr Hurley’s affidavit of 10 August 2009 and learning that there was a private market only launch in which 11 key customers had been contacted, and only one order had been finalised, Ms Braithwaite nevertheless maintained her evidence from her 28 July 2009 affidavit that Alphapharm’s private market activities would cause Wyeth irreparable harm unless Alphapharm was restrained… In other words, Wyeth’s evidence, prepared in the knowledge of and in response to Alphapharm’s evidence that it had contacted 11 key customers, was that Alphapharm’s anticipated private sales would cause Wyeth irreparable harm. Indeed, Ms Braithwaite expressly contested Mr Hurley’s proposition that on the private market launch Alphapharm would be likely to attract a maximum of 30% of the non-concession market. Ms Braithwaite maintained the view in her earlier evidence that Alphapharm could achieve half of the total market.
595 As will be explained later, Ms Braithwaite’s evidence is important but this aspect of it is difficult to follow. For present purposes, it is sufficient to say that it must be inferred that Ms Braithwaite was assuming that Alphapharm would engage in a full-scale commercial supply of its products in 2009 if not restrained, despite her apparent belief that Alphapharm’s launch was not of that kind (as it was not), and that other generics would then also enter the market. Ms Braithwaite also plainly did not consider that a private market supply was likely to fail. To the contrary, she believed it would succeed in taking significant market share from Wyeth.
596 Further, Wyeth had to assume that Alphapharm was or if not restrained would be engaged in a serious commercial endeavour and that if there were no generic competition Alphapharm was operating on the basis that it could achieve a maximum market share of 13.5%. This was what Mr Hurley said in his 2009 affidavit. It cannot be doubted that if this occurred Wyeth’s belief that it would suffer irreparable harm was reasonable. This does not mean that the evidence now available is to be disregarded.
597 Alphapharm submitted that:
Despite Wyeth being willing to put an incomplete picture to Mr Hurley in cross-examination as to what Ms Braithwaite might have thought, Ms Braithwaite was never called. The Court can safely assume that had she been called, Ms Braithwaite would not have advanced what is now put as Wyeth’s case. The appropriate inferences include that Ms Braithwaite would not have indicated that she had been misled about the scope of Alphapharm’s private launch plans, but rather that (as her affidavits said in 2009) she considered that a launch to 11 key customers and the taking of one order was the first stage of an activity which, if not enjoined, would lead to extensive sales on the private market by Alphapharm.
598 This again must be unravelled. In 2009 Ms Braithwaite had no option other than to believe that Alphapharm was or if not restrained would be engaged in a serious commercial endeavour and that if there were no generic competition Alphapharm was operating on the basis that it could achieve a maximum market share of 13.5%.
599 Alphapharm submitted this:
Wyeth’s contention is audacious and wrong. By that contention, Wyeth seeks to impeach the judgment it obtained on the interlocutory application, without calling a single witness who swore an affidavit on the interlocutory application to suggest that in light of new facts known to them, the evidence they gave in support of the interlocutory application would have been different.
600 I do not agree that this has anything to do with impeaching the Alphapharm interlocutory judgment. It was not a sham for Alphapharm to tailor its launch of its products to the circumstance of knowing it would be restrained. Its launch was not a hoax or sham. However, it was not what it would have done but for the anticipated interlocutory injunction against it. Alphapharm did what it did and Wyeth and the Court acted on that basis. In 2009 Alphapharm was never called upon to decide what it should do but for the interlocutory injunctions. This is a matter of inference from the evidence. Evidence about Alphapharm’s strategies, beliefs and intentions in 2009 is part of that evidence. Alphapharm’s suggestion that Wyeth should have called Ms Braithwaite to say what evidence she would have given in 2009 if she knew in 2009 about all of the evidence now available concerning Alphapharm’s strategies, beliefs and intentions in 2009 is particularly baffling. One, the starting point for this kind of analysis is that Wyeth did not seek the interlocutory injunction on 14 August 2009. Two, Alphapharm’s approach in 2009 was based on the correct belief that it would be restrained. What it would have done but for the interlocutory injunctions is not necessarily the same as what it did. As such, this kind of evidence, in effect what would Ms Braithwaite now says she would have said in 2009 if she knew in 2009 about the evidence available only now, is irrelevant.
601 Alphapharm referred to various aspects of Mr Hurley’s evidence as if they all supported the proposition that he had decided a full-scale commercial supply on the private market would be in Alphapharm’s best interests. In fact, the evidence quoted was directed to Alphapharm’s preference not to have to confront the litigation. Even the last piece of evidence that, “I would have preferred to launch the product and take the product to market and generify the product. I didn’t want an interlocutory injunction”, is not evidence that Mr Hurley wanted to supply on the private market only. What he undoubtedly wanted was to be able to “generify” venlafaxine by PBS supply in the ordinary course but he could not do that because of the litigation (rather than the anticipated interlocutory injunction).
602 Alphapharm submitted that:
Wyeth approaches the matter as though Alphapharm acted by unitary motivation. In fact, like all sophisticated and well advised commercial parties, Alphapharm had several motivations. The primary, and most compelling motivation, as indicated by Mr Hurley’s answers extracted above, and as reflected in any rational commercial assessment of the situation before and after it knew about the existence of the Method Patent, was to enter the market both privately and on the PBS. After Sigma was enjoined, and it became clear Alphapharm would not list on the PBS, its primary motivation was to sell privately (and to list on the PBS once another party had listed).
603 I agree that multiple motivations are likely. Otherwise, however, this submission is simply wrong. Alphapharm’s rational commercial assessment after the method patent was that it was not going to seek PBS listing unless and until another generic had obtained PBS listing. It also made no rational commercial assessment about its private market launch in 2009 because it did not have to, knowing (as it did) that it would be quickly restrained. Accordingly, its primary motivation in fact was not and could not have been to supply on the private market. Its primary motivation in fact was to commence supply on the private market to prompt Wyeth into seeking interlocutory relief so that it could argue that the price of any interlocutory injunction should be for Wyeth not only to give the usual undertaking as to damages but also to undertake not to de-list Efexor-XR. If interlocutory relief had not been sought on 14 August 2009, however, Alphapharm would then have had to re-consider its position having regard to all of the circumstances at that time. Alphapharm would have been in the same effective position (albeit earlier in time) had Wyeth not sought the Sigma interlocutory injunction on 22 May 2009.
604 Alphapharm submitted that:
Mr Hurley was cross-examined further to the effect that fighting the interlocutory injunction from Alphapharm’s point of view had the potential to serve two useful purposes for Alphapharm, one of which was said to be extracting an undertaking in relation to delisting Efexor XR in a form which Alphapharm wanted. Mr Hurley candidly admitted that was a relevant purpose. It was neither improper nor unexpected to attempt to make the best of an adverse situation in which interlocutory relief was sought against the company and was likely to be granted. However, it was never put to Mr Hurley that the cross-undertaking not to delist was the sole useful purpose of Alphapharm fighting the interlocutory injunction, and his answer at T582.1 – 11 could not be so taken, particularly in light of his answer at T582.20 which rejected the second suggested useful purpose offered by the cross examiner. Further, Mr Hurley consistently gave evidence of the kind he gave at T582.25 – 27:
But I’m suggesting your defence of the interlocutory injunction proceedings, as you understood it, wasn’t because you had any serious intention to pursue in any concerted fashion a private launch?---We did have that intent.
Again, there is no reason why that evidence would not be accepted, and every reason why it would be accepted, having regard to its consistency with Mr Hurley’s contemporaneous sworn affidavit evidence in 2009.
On any view, Alphapharm’s primary purpose of contesting the interlocutory application was the prospect, however slim, that Alphapharm would succeed in resisting the interlocutory injunction and be free to sell Enlafax XR on the private market (in the absence of a competitive generic product), even if the legal reality was that it was highly likely not to succeed.
605 I agree that Alphapharm did not act improperly. I disagree that it had to be put to Mr Hurley that Alphapharm’s sole purpose in commencing private supply was to extract the undertakings. Wyeth’s focus, properly, was on Alphapharm’s real, substantial or moving purpose. I have also explained why Mr Hurley’s evidence is unreliable. Apart from this, the submission is internally inconsistent. Alphapharm’s primary purpose would hardly have been private market supply of its products if, as the submission appears to accept, it knew that it was going to be restrained from doing so. It is for this reason, as I have said, that the evidence of what Alphapharm did in 2009 is not a reliable foundation for inferring what it would or might have done but for the interlocutory injunctions other than to the extent that it shows that Alphapharm would not have been the first to apply for PBS listing and, unlike Sigma, had concerns about supplying its products on the private market pending the resolution of the litigation.
606 There are other inconsistencies between Alphapharm’s submissions and Mr Hurley’s evidence. As noted, Mr Hurley refused to acknowledge that he knew that it was inevitable, certain or even highly probable that Wyeth would seek interlocutory relief if Alphapharm commenced the private supply of its products. Mr Hurley would accept only that the thought this was “probable”. Yet Alphapharm submitted, as was the fact, that “there is no doubt that Wyeth was aggressively asserting its purported legal rights”. In this context, Mr Hurley’s insistence that he did not think it was inevitable, certain or even highly probable that Wyeth would seek interlocutory relief if Alphapharm commenced the private supply of its products did not enhance the apparent reliability of his evidence about issues he perceived to be in contest.
607 Alphapharm submitted that “Wyeth’s present contention seems to be that anything short of Alphapharm consenting to the injunction, in the light of the advice it had received, evidences a collateral purpose”. This is not Wyeth’s contention. Nor is it my conclusion. Indeed, the whole notion of “collateral purpose”, to my mind, is a distraction. So too is the issue whether the launch was “genuine”. If not a sham (which it was not), it is not clear what this means. In any event, Wyeth is entitled to contend that, but for the interlocutory injunctions, Alphapharm would not have supplied its products at all, just as Alphapharm is entitled to contend to the contrary. And both are entitled to refer to all available evidence in support of their contentions and to argue that the evidence supports different inferences about Alphapharm’s strategies and intentions in 2009.
608 Alphapharm submitted that:
The flaw at the heart of Wyeth’s approach of unduly focusing on litigation strategy and ignoring the underlying commercial motivation is pointed out in the English decision of AstraZeneca Ab v KRKA dd Novo Mesto [2015] EWCA Civ 484 (Court of Appeal). In that case, the recognition by a generic party of the legal reality that an injunction would be granted, to the point of consenting to the interlocutory injunction, did not deprive the party of any right to recover. That was a litigation decision which did not undermine the underlying commercial motivation of the party, which was to get onto the market. Here Alphapharm contested the interlocutory injunction, and legitimately sought to obtain concessions from Wyeth that were necessary to preserve to the extent possible the commercial benefit that might eventuate at the conclusion of the litigation. That is incapable of undermining Alphapharm’s primary commercial motivation to get on the market.
609 There is no doubt Alphapharm wanted to preserve the potential for PBS supply of its products pending the resolution of the litigation which depended on Wyeth not de-listing Efexor-XR. The issue, however, is not what Alphapharm’s commercial position would have been but for the method patent and the litigation. The answer to that question is obvious in that but for the method patent and the litigation Alphapharm would have supplied its products on the PBS as soon as it could have done so and would not have bothered with private market supply. The issue is what Alphapharm’s commercial position would have been in the face of the method patent and the litigation and but for the interlocutory injunctions (or, in my view, but for the Alphapharm interlocutory injunction). My conclusion is that what Alphapharm did in 2009 was directed to ensuring Wyeth did not de-list Efexor-XR so that the commencing of private supply of its products must be understood as a means to achieve that end and not as reflective of its commercial motivations in the face of the method patent and the litigation. This conclusion is not inconsistent with the Alphapharm interlocutory injunction. It engages none of the doctrines of preclusion on which Alphapharm relied. And it does not mean Alphapharm was perpetrating a sham on Wyeth.
610 Alphapharm submitted that:
The matters set out in Mr Hurley’s first affidavit state the sworn evidence of Mr Hurley in 2009, upon which he was not challenged in 2009. They are divorced from and unaffected by the present forensic contest. They provide a reliable framework for the court to find that in the absence of the AII [Alphapharm interlocutory injunction], Alphapharm would have continued to make private sales and listed on the PBS as soon as it could do so without triggering the 12.5% price drop.
611 Things Mr Hurley said in his affidavit in 2009 (for example, about the legal advice and the order Alphapharm received) have been exposed to be inaccurate or materially incomplete. Things he said in affidavits thereafter are unreliable for the reasons given. In these circumstances, where he would not have expected to be tested on his 2009 affidavit because it concerned an interlocutory application only and where Alphapharm had its own forensic objectives in 2009, it is difficult to give weight to any of Mr Hurley’s evidence.
612 Alphapharm referred to a series of matters it said all supported the inference that but for the interlocutory injunctions it would have supplied its products privately and obtained PBS listing provided another generic had obtained it first. The matters, most of which have been discussed above, are:
(a) Alphapharm made an application for registration of Enlafax XR on the ARTG in or around September 2007, in three strengths, 37.5mg, 75mg and 150mg;
(b) the application for registration on the ARTG was delayed by the fact that the TGA did not accept Alphapharm’s request for a bio-study waiver on the 37.5 mg Enlafax XR product, and as a result the ARTG listing was not complete when the Molecule Patent expired in December 2008;
(c) however on 1 December 2008, Alphapharm applied for listing of Enlafax XR on the PBS on 1 April 2009, and indicated that Alphapharm would forward as soon as possible the approval letter from the TGA, and by no later than 15 January 2009;
(d) as at 1 January 2009, the Molecule Patent had expired, Mr Hurley was not aware of any other Patent potentially preventing the launch of Enlafax XR, and he anticipated that Alphapharm would obtain TGA and PBS approvals and launch Enlafax XR on the PBS on 1 April 2009;
(e) by 15 January 2009, the TGA approval had not been provided and accordingly the first PBS application effectively lapsed on that day – Alphapharm did not receive an ARTG registration for the Enlafax XR products until the end of April 2009, following a decision to abandon the 37.5 mg strength product which was delaying the application;
(f) in March 2009, in the course of Wyeth’s preliminary discovery application against the Department, Mr Hurley became aware that Wyeth was the owner of the Method Patent and was attempting to obtain information about the identity of those who had registered generic venlafaxine products on the ARTG, which enlivened for him the risk of Alphapharm being liable for damages arising from the 12.5% price decrease if Alphapharm was the first to list on the PBS, and Enlafax XR infringed the Method Patent;
(g) given he understood the market was worth about $100 million in annual sales, a liability of 12.5% of that figure per year was a very high risk when weighed against the returns Alphapharm could potentially expect from sales of its own product;
(h) however, Mr Hurley considered that if another generic company had already listed on the PBS and triggered the price decrease, Alphapharm would not be potentially liable for those “price reduction” damages on the originator’s own product, but only for sales lost by the originator to Alphapharm;
(i) Mr Hurley formed the view that Wyeth was likely to take steps to assert its patent through legal action, irrespective of Alphapharm’s view of the validity or otherwise of Wyeth’s patent;
(j) in March 2009, Mr Hurley became aware that Sigma had filed revocation proceedings in respect of the Method Patent, which caused Mr Hurley to consider that there was a reasonable likelihood that Sigma had filed an application for PBS listing prior to 1 May 2009 for a 1 August 2009 listing date;
(k) on 4 May 2009, Alphapharm received a letter from Wyeth’s lawyers, Gilbert + Tobin, regarding Enlafax XR, and informing Alphapharm that Wyeth had filed a cross-claim against Sigma for infringement of the Method Patent, and that Wyeth was seeking an interlocutory injunction against Sigma;
(l) the Gilbert and Tobin letter of 4 May 2009 asserted infringement of the Method Patent by Enlafax XR, and sought an undertaking that Alphapharm would refrain from importing supplying or offering to supply the Enlafax XR products in Australia, with the threat that Alphapharm would be sued for patent infringement, including an application for interlocutory relief, unless that undertaking was provided;
(m) on 1 June 2009, Alphapharm sent a letter to the PBS enclosing a PBS listing application, requesting listing on 1 August 2009. Whilst Mr Hurley was unable to locate the final version of the letter of 1 June 2009, he was able to locate a draft, which is Exhibit MH-20, and the version of the letter received by the Commonwealth conforms with the substance of MH-20;
(n) importantly for present purposes, the letter of 1 June 2009 made it clear that Alphapharm’s application for PBS listing was:
“made on the condition that it can be withdrawn by Alphapharm prior to 1 August 2009 without triggering a price reduction of 12.5% for the originator product, should Alphapharm consider that withdrawal necessary.
This condition is critical to Alphapharm. If the PBS is not able to accept the application on that condition, please inform us immediately”;
(o) while the application was filed out of time to achieve listing on 1 August 2009, the condition is consistent with Alphapharm having not yet made a decision as to whether it would be willing to trigger the 12.5% price reduction, but confirms that it was reserving to itself the ability to withdraw the application if it decided it did not wish to do so;
(p) on or shortly after 3 June 2009, Mr Hurley became aware of the SII [Sigma interlocutory injunction], as a result of which it became obvious to him that Sigma would not trigger the price decrease on 1 August 2009 as any application it may have submitted would now have to be withdrawn, and that if no other generic company had applied for PBS listing on that date, and Alphapharm proceeded to list on that date, Alphapharm’s listing would have been the sole trigger of the 12.5% price decrease; and
(q) Mr Hurley formed the view very shortly after becoming aware of the SII, that it would not be advisable for Alphapharm to trigger the 12.5% price drop by being the first, or equal first, to list an extended release venlafaxine product on the PBS.
613 As already discussed, these matters disclose that but for the method patent and the litigation Alphapharm would have proceeded with its proposed PBS listing and supply of its products. These matters also disclose Alphapharm’s unwillingness, faced with the method patent and the litigation, to be the first generic to list its products on the PBS due to the fear of being liable to Wyeth for the 12.5% price reduction. These matters do not show that but for the interlocutory injunctions Alphapharm would have been willing to accept the risks of liability associated with supply or that Alphapharm would have pursued supply, particularly not mere private supply when it perceived this to be unprecedented and contrary to the best interests of pharmacists (and, I infer, potentially contrary to its own interests given its sell or return policy). Rather, if confronted by the circumstances that would have existed but for the interlocutory injunctions Alphapharm would have to have done what it did not have to do in 2009 – assess all of the potential risks and benefits it might hope to obtain by supply, private supply only if no other generic obtained PBS listing and under the PBS if and when another generic obtained PBS listing. Alphapharm could do so on only the basis of legal advice of its prospects and exposures and advice about its likely market success, as well as with Mylan’s approval for a launch at risk (which, tellingly, I infer it did not obtain in 2009 because it knew it would shortly be restrained and thus, in truth, its launch was not at risk in any material sense).
614 I will not deal with all of Alphapharm’s other submissions about Mr Hurley’s evidence. I have given sufficient reasons already for rejecting that evidence. What is now relevant is to record that irrespective of my views about the reliability of his evidence I have no doubt that, in common with the relevant people at Sigma (and Generic Health), Mr Hurley knew his business well and knew how to formulate strategies which would be in the best interests of Alphapharm given that Alphapharm’s business was making profits from supplying generic products in a highly competitive market.
615 Otherwise, I accept Alphapharm’s submissions that the following matters would be relevant to what Alphapharm would or might have done but for the interlocutory injunctions:
(1) Sigma was Alphapharm’s biggest competitor and Alphapharm had received questions about its intentions from pharmacists aligned to it given Sigma’s private market supply;
(2) “…if Sigma was able to offer a full range of products including venlafaxine, but Alphapharm was not, Sigma would have an opportunity to take customers and business from Alphapharm” because of the practice of generics to bundle products and the nature of the market in which many pharmacists were wholly aligned with one or other generic (in the sense that the pharmacist or group of which the pharmacist was part would buy all of a generic’s range and hold only some products from other generics). I accept that the risk that Alphapharm might lose ground to Sigma if Sigma continued with its private market supply (or PBS supply if Sigma obtained PBS listing) would be a very important factor in Alphapharm’s consideration of its positon;
(3) “if Sigma launched a venlafaxine product and Alphapharm did not, it was less likely that patients who were satisfied on Sigma’s product would switch to an alternative generic” at some later time; and
(4) “it was…important to Alphapharm to be seen as a market leader” so that if Sigma was supplying venlafaxine on the private market Alphapharm would have to give real consideration to its position about private market supply (and equally if Sigma was supplying venlafaxine under the PBS after PBS listing, Alphapharm would have to give real consideration to its position about PBS listing and PBS supply). This is particularly so in circumstances where Sigma and Alphapharm were both large competitors with extensive sales forces and also were months ahead of their nearest competitor in obtaining ARTG registrations (Generic Health, which was a small player and not in a comparable position to Sigma or Alphapharm in terms of capacity to penetrate the market).
616 These are the objective contemporaneous circumstances in which Alphapharm would have found itself in mid-2009 but for the interlocutory injunctions. The only material difference between my approach (the operation of the Alphapharm interlocutory injunction alone may be disregarded) and the generics’ approach (even the threat of the interlocutory injunctions must be disregarded) is one of timing, the relevance of which effects the notional PBS listing dates.
617 While I also accept that if “both Alphapharm and Sigma had been on the PBS in 2009, this would have created a thriving PBS market for generic venlafaxine which would have made it nearly commercially impossible for Wyeth to remove Efexor XR from the PBS”, we know that Alphapharm was dependent on Sigma or another generic first obtaining PBS listing before it would consider obtaining PBS listing for the purpose of PBS supply.
618 Alphapharm also referred to Mr Hurley’s evidence that “an additional benefit of a private market launch was that it would have been difficult for Wyeth to withdraw Efexor XR from the PBS as part of its strategy to switch the market to Pristiq, because Wyeth would have effectively given up any patients that were prescribed venlafaxine to generic venlafaxine”. In other words, PBS listing and the associated 12.5% price reduction, which created such an enormous exposure for the first generic to obtain PBS listing, was not essential to achieving the objective of making it difficult for Wyeth to de-list Efexor-XR. No doubt Alphapharm would have preferred for it be impossible for Wyeth to do so, but this is exactly the kind of strategic thinking I would expect such a person as Mr Hurley to have considered if Alphapharm had not been restrained on 14 August 2009. I thus accept that its fear of Wyeth switching patients to Pristiq provided Alphapharm with some incentive to proceed with private market supply on a commercial basis if not enjoined.
619 However, I do not accept that the fact that Mr Joscelyne prepared financial forecasts between 22 April and 28 April 2009 “is a powerful contemporaneous indicator that in the absence of the interlocutory restraints Alphapharm would have sold Enlafax XR on the venlafaxine market with the expectation of reaching those forecasts”. As noted, the forecasts assumed PBS supply. Alphapharm decided it would not pursue PBS supply because of the method patent and anticipated litigation. Accordingly, in the absence of the interlocutory injunctions, it cannot be the case that Alphapharm would have sold its products on the private market with the expectation of reaching those forecasts. The evidence relates only to Alphapharm’s intentions and expectations leaving aside the method patent and the litigation, which is an impermissible hypothesis.
620 What I do accept is that but for the interlocutory injunctions Mr Hurley would have obtained similar forecasting from a person such as Mr Joscelyne about private market supply but did not do so before the launch in mid-July 2009 because he knew Alphapharm would be restrained. This confirms that the mid-July 2009 launch was far from Alphapharm’s usual practice and bears no resemblance to what Alphapharm would have done but for the interlocutory injunctions. But for the interlocutory injunctions, before any private market launch or before any “ramping up” of private market supply after mid-July 2009, Alphapharm would have financially modelled the expected outcomes over the longer-term on the basis that if it wished to compete with Sigma it would have to bring to bear all of its expertise and capacities.
621 Mr Montgomery, Alphapharm’s CEO, made decisions in conjunction with Alphapharm’s senior leadership team the most senior member of which (apart from Mr Montgomery) was Mr Hurley. Mr Montgomery said:
…it was Mark Hurley and I who primarily drove the strategy and execution of the project. Mark Hurley was heavily involved in the decisions I made regarding venlafaxine, and he and I liaised very closely together on this project. I respect Mark’s strategic decision-making very highly. It was not just a matter of me taking notice of his views on such matters either - Mark and I discussed these types of decisions extensively with each other; we talked through the factors relevant to the decision, we debated them and, ultimately, we nearly always ended up coming to a common view.
622 Mr Montgomery assumed that if the method patent was valid Wyeth’s damages would be greater than Alphapharm’s profits whether or not Alphapharm had triggered the 12.5% price reduction (which it would not do). Again, I accept this and consider it discloses the position any commercially experienced decision-maker would have reached.
623 Mr Montgomery said that:
…after Mylan acquired Alphapharm in 2007, a more comprehensive, formal process for the approval of product launches by Alphapharm was introduced. Any launch which was considered to carry a risk of legal proceedings being brought against Alphapharm was considered to be "at risk". All at risk launches required Mylan approval. When seeking Mylan approval for an at-risk launch, it was required that Alphapharm set out the risks and benefits of the launch. On the risk side, the proposal which Alphapharm would put to Mylan assumed that Alphapharm would launch and sell its product, and that it would not be prevented from doing so by a Court injunction. Thus the risk, as I understood it, and which Alphapharm assumed when putting its proposal to Mylan, was Alphapharm's exposure, if it was subsequently found liable for infringement of a third party's patent rights, to having to pay the patentee's damages, or handing over Alphapharm's profits.
624 Accordingly, it must be inferred (contrary to the fact) that Mr Montgomery and Mr Hurley would have had available to them all relevant information about the potential risks and benefits to Alphapharm of whatever decision Alphapharm would or might have been contemplating. In this regard, unless and until another generic obtained PBS listing Alphapharm would not be contemplating PBS listing. Because of the overwhelming likelihood (indeed, the certainty in my view) that Sigma would be supplying on the private market, a necessary inference is that Alphapharm would be contemplating private market supply. And indeed it was in fact. There is no reason to infer that there was any chance Alphapharm would have acted differently from how it did act which was to do nothing until 22 May 2009 because at that time it believed that Sigma’s private market supply was not in the best interests of pharmacists and, in effect, may ultimately fail once pharmacists worked out that no PBS listing was to be forthcoming in the near future.
625 If Wyeth had not applied for interlocutory relief against Sigma or it, Alphapharm would have had to carry out the full risk-benefit analysis needed for Mylan to review any private market launch. Mr Montgomery and Mr Hurley would have decided what to recommend to Mylan based on the circumstances at the time and the results of the analysis in the context of Alphapharm being and wishing to remain a leader in a highly competitive market.
626 In contrast to Mr Hurley, Mr Montgomery was not subject to extensive cross-examination. In particular, his opinions about what he was likely to have recommended and his beliefs about Mylan’s likely decision-making were not directly challenged. While I still prefer to give the most weight to the objective contemporaneous evidence, Mr Montgomery appears to possess a capacity unique amongst the witnesses called by the generics, to give evidence about hypothetical facts which makes sense in the context the generics would have been in but for the interlocutory injunctions.
627 As noted, Mr Montgomery would not have recommended to Mylan that Alphapharm be the first generic to obtain PBS listing due to the potential exposure to liability to Wyeth in the order of $100 million if Alphapharm triggered the price reduction in Efexor-XR. Mr Montgomery also would not have recommended that Alphapharm risk listing on the same day as another generic for this reason. In other words, he would not permit Alphapharm to apply for PBS listing unless and until another generic had obtained PBS listing. As a result, he would not have sought Mylan’s approval for any launch “at risk” based on Alphapharm being the first generic to obtain PBS listing of its products. This was so irrespective of the strength of legal advice about Alphapharm’s prospects of having the method patent declared invalid or its products not infringing the method patent. As discussed, this position not only reflects Alphapharm’s evidence in 2009 but also commercial sense. It is powerful evidence of what a responsible decision-maker would have done in 2009 in all of the circumstances including the drive to compete and make profits from the supply of generic products.
628 Mr Montgomery said that if Sigma had obtained PBS listing then he would have recommended to Mylan that Alphapharm also apply for PBS listing because:
(a) Alphapharm’s exposure to damages would be lower because it would not have been responsible for triggering the 12.5% price reduction for Wyeth products;
(b) as Alphapharm would have been later in the market than Sigma, Sigma would have captured a greater market share and hence Alphapharm’s damages exposure (in terms of the sales it would have taken from Wyeth) would have been lower than if Alphapharm had been the first to enter the market; and
(c) at the time, he believed that Wyeth was undertaking a deliberate strategy of undermining the market for venlafaxine by promoting Pristiq and putting no promotion into Efexor.
629 Mr Montgomery considered that in these circumstances:
…there was a better than even chance that Mylan would have accepted my recommendation to launch Enlafax XR and list it on the PBS after Sigma had definitely listed its product on the PBS and been responsible for triggering the 12.5% statutory price reduction.
630 If, however, Alphapharm could not apply for PBS listing because Sigma (and no other generic) had first obtained PBS listing, Mr Montgomery said he would have sought Mylan’s approval for a private supply of Alphapharm’s products at risk involving, in effect, a ramping up of the launch in July 2009 to a full-scale commercial supply on the private market. Mr Montgomery believed that, in these circumstances, “there was a high likelihood that Mylan would have accepted my recommendation to conduct a private launch of this kind”.
631 But for the interlocutory injunctions I consider that the position would have been this. Alphapharm would still have deferred its first May 2008 order of stock because of the mere existence of the method patent and the litigation. At this time it would have still considered Sigma’s private market supply unprecedented and likely to leave pharmacists with stock they could not sell because Alphapharm considered that pharmacists would not be interested in purchasing generic venlafaxine for private market supply given that Efexor-XR was PBS listed. Alphapharm would still have its sale or return with a refund policy so would have been concerned to financially model the likely costs and benefits of a private market supply of venlafaxine. It would still have decided that by reason of the mere existence of the method patent and the litigation it would not under any circumstances risk triggering the 12.5% price reduction in Efexor-XR by being the first generic to obtain PBS listing of its products or obtaining PBS listing at the same time as another generic. As such, it would not apply for PBS listing until another generic had obtained PBS listing. Its focus, as a result, would be Sigma’s continuing private market supply and its apparent success.
632 Alphapharm’s immediate response to Sigma’s continuing private market supply would have been exactly the same as Alphapharm’s actual response to Sigma’s private market supply before 22 May 2009. Alphapharm would consider it unprecedented and likely to leave pharmacists with stock they could not sell. However, it would not have considered the issue of likely success in the terms framed by Wyeth relating to the ethical obligations of pharmacists. Confronted by Sigma’s ongoing private supply of Sigma’s product from May 2009 onwards, however, Alphapharm necessarily would have been forced to confront the question whether or not it should continue with its view that only PBS supply would be sensible. Showing the same care for its own commercial interests as it had consistently demonstrated, Alphapharm would ensure that it made the best informed decision it could on the basis of up to date and comprehensive legal and financial advice including advice about the potential success of private market supply if Alphapharm brought to bear all of its expertise and about its potential exposure to Wyeth. The legal advice, which would have been to the general effect that Alphapharm had good prospects of success at least in respect of the invalidity of the method patent, would have made clear that the prospects could not be put any higher than good and that litigation is inherently risky.
633 Alphapharm was not and would not have been in the same position as Sigma which was effectively driven to supply on the private market due to the $3 million of stock it had purchased. Alphapharm had purchased stock (far less than Sigma) but had deferred delivery in circumstances where payment was not due until delivery. Further, Alphapharm had never planned to supply on the private market only whereas Sigma had always planned on private market supply followed by PBS supply within three months. The change in position for Alphapharm was thus greater than it was for Sigma. Alphapharm also doubted that Sigma’s apparent success reflected supply on the basis of no PBS listing in the reasonably foreseeable future and was determined that it should not do what it thought Sigma may well be doing (that is, supplying on the basis that the carrot of PBS listing was reasonably foreseeable). Alphapharm also knew it would not be the first to market as Sigma had already gone to the market, but the evidence shows that the earlier a generic can make it to the market the better its prospects of securing market share and being the second to market would have been important to Alphapharm. Alphapharm was not subject to the same kinds of general financial pressures to which Sigma was subject but Alphapharm no doubt also wanted a “win” in the sense that a company in a highly competitive industry always wants a win. Sigma had also committed itself to a full-scale supply of its products on the private market whereas Alphapharm had not.
634 Taking into account all of the circumstances identified, I consider that there is one factor which would have outweighed all others from Alphapharm’s perspective in any hypothetical analysis. It is that Sigma would have been continuing to supply pharmacists on the private market when Alphapharm also would have been able to do so. To my mind, in the context of this industry and Alphapharm’s place within it, this fact alone would have made it highly likely that Alphapharm would have decided it needed to compete with Sigma so that Mr Montgomery would have made the recommendation to Mylan that Alphapharm enter the private market. In circumstances where Alphapharm would have been advised that its ultimate prospects of not being liable to Wyeth were good albeit not without risk and Sigma was continuing to supply, Alphapharm would have perceived there to be a strong imperative for it also to supply its products on the private market. While Mylan would have been the final decision-maker, faced with these circumstances, it is difficult to conclude that Mylan would not have approved a launch at risk on the private market to enable Alphapharm to compete with Sigma.
635 I accept that I cannot exclude the possibility that Alphapharm’s assessment might have been that there was a real risk that it might not be able to interest pharmacists in private market supply of its venlafaxine products if, as Alphapharm would have insisted, its sales representatives made clear to pharmacists that it could not be assumed that PBS listing would be achieved in the foreseeable future due to the patent litigation. I do not consider, however, that Alphapharm would have been deterred by the prospect of pharmacists having to switch patients back to Efexor-XR if the method patent was valid and infringed by Alphapharm’s products. Nor, as I have said, do I accept that pharmacists would have given weight to this risk. What must be accepted is that, confronted by continuing supply by Sigma, Alphapharm would perceive a strong commercial need for it to compete given that it had ARTG registrations and could supply its products by arrangement with Pharmathen. What must also be accepted is that Alphapharm would have taken steps to put itself in the best position it could to maximise its chances of success using all of its capacities to do so. As noted, Mr Hurley’s capacity for strategic thinking cannot be doubted. Given its sales and marketing resources and experience, Alphapharm would have been able to put forward a strong case to Mylan to support entering the market in competition with Sigma. Mylan thus would have made its decision on this basis.
636 As a result, I would assess the likelihood of Alphapharm, with Mylan’s approval, committing to or scaling up its mid-July private market launch to be 90%. I accept that the possibility Alphapharm would not have done so cannot be excluded as merely speculative as Alphapharm might have considered the prospects of successful private market supply not worth the continuing effort or might have failed to persuade Mylan to approve the full-scale commercial supply on the private market because it seemed to be contrary to conventional wisdom in the generic industry. In my view, however, the prospect that Alphapharm would have ceased further supply from that date would not be assessed to be higher than 10%. The success or otherwise of this private market venture would have been assessed on a continuous basis but some short-term losses would not have caused Alphapharm to cease supply if Sigma was continuing to supply.
637 In terms of the timing issue, Alphapharm in fact launched its products on a confined basis on 17 July 2009. The hearing of the Alphapharm interlocutory injunction occurred on 14 August 2009. In these circumstances and consistent with the discussion above to the effect that it is the Alphapharm interlocutory injunction alone which had a potential adverse effect on Alphapharm relevant to the terms of the undertakings, it should be inferred that there would have been a 90% probability that Alphapharm would have been supplying its products on the private market on a full scale commercial basis from 14 August 2009. As will be explained below, the econometric analysis assumes that Alphapharm commenced supply of its products on the private market on 22 July 2009. In the overall context of this matter and given that Alphapharm in fact launched on 17 July 2009, I consider it appropriate to adopt the start date for commercial supply to the private market by Alphapharm of 22 July 2009.
638 In respect of PBS listing, Alphapharm would not have applied for PBS listing until another generic had obtained PBS listing. However, if another generic had obtained PBS listing then Alphapharm would have known that it was not going to be responsible for the 12.5% reduction in the price of Efexor-XR, would have been concerned to protect its competitive position, and would still believe the risk of liability to Wyeth was low. Again, these objective circumstances also accord with Mr Montgomery’s assessment that he would have been willing to recommend to Mylan PBS listing and PBS supply in these circumstances.
639 I have concluded above that there was a 20% chance that Sigma would have applied for PBS listing in time to obtain PBS listing of its products on 1 December 2009. If this conclusion is maintained (as it must be for this hypothesis), there must also then have been a substantial prospect that Alphapharm would have applied for PBS listing as soon as possible after 1 December 2009. Mr Montgomery considered that the prospect of Mylan approval for a PBS listing application to be “better than even”. Given the objective circumstances, I consider the prospect highly likely. I cannot accept that, in such a case, Alphapharm would merely continue private market supply, allowing Sigma to reap the rewards of PBS supply.
640 Having regard to the objective circumstances, on these hypothetical facts, I would assess the likelihood of Mylan approving Alphapharm seeking PBS listing for the purpose of PBS supply to be high. If Alphapharm was on the private market, my estimate is that there was a 90% probability that in the event of another generic first obtaining PBS listing Alphapharm also would have applied for PBS listing as soon as it could thereafter for the purpose of PBS supply and a 10% possibility that it would not have done so but elected instead to continue with its private market supply.
641 If Alphapharm had not been supplying its products on the private market, I would still estimate the probability of it having applied for PBS listing in the hypothesised circumstances as high given my view that the material factors which would have weighed on Alphapharm’s mind were the need not to trigger the 12.5% price reduction (which would be irrelevant on this hypothesis) and the competitive need to enter the market against Sigma and be early to do so compared to other generics (which would be the driving factors on this hypothesis). In these circumstances, I would estimate the probability of it having applied for PBS listing in the hypothesised circumstance of Alphapharm not supplying its products on the private market to be 80% and the probability of it having continued not to supply its products to be 20%.
642 In respect of the timing issue relating to PBS listing for Alphapharm, on the basis of the 20% chance of Sigma having applied for and obtained PBS listing on 1 December 2009, it should be taken that the probabilities discussed above relate to Alphapharm’s products being been added to the PBS list on 1 March 2010 (reflecting a three month period from a posited application date of 15 December 2009 as referred to in paras 24 and 25 of the statement of agreed facts). Alphapharm had already applied for PBS listing of its products before it became aware of the method patent. It would have immediately become aware of PBS listing of Sigma’s products. There is no reason to think Alphapharm would not have been able to submit its listing application by 15 December 2010 which would have enabled it to obtain PBS listing of its products on 1 March 2010. As noted, if Alphapharm’s mere anticipation of an interlocutory injunction because of Wyeth’s threats or the Sigma interlocutory injunction may be disregarded (contrary to my view), then the prospect of Alphapharm obtaining PBS listing would operate from the earlier date of 1 December 2009 because, on this approach, it would have had to confront Sigma obtaining PBS listing on 1 August 2009, rather than 1 December 2009.
643 As with Sigma, Alphapharm would not have been concerned its products might not be listed on the PBS. It would not have been concerned by the need to give an assurance of supply and would have done so. It would have no concern about the statutory guarantee of supply provisions. Further, no anticipation of what Wyeth might do in competitive response to generic supply or otherwise would have weighed on Alphapharm’s mind.
Summary of interim conclusions – Alphapharm
644 My interim conclusions about Alphapharm, accordingly, may be summarised as follows:
(1) Alphapharm has proved on the balance of probabilities that it suffered the loss of an opportunity of some value associated with the ARTG registration of its products because the injunction prevented it from exercising, from time to time and as it saw fit, the rights associated with ARTG registration. The value of this opportunity depends on the probabilities and possibilities of what Alphapharm would or might have done.
(2) If Alphapharm needed to prove on the balance of probabilities that it would have re-applied for PBS listing of its products so as to be the first generic to obtain PBS listing but for the interlocutory injunctions, then Alphapharm has not proved (or sought to prove) that putative fact.
(3) If Alphapharm needed to prove on the balance of probabilities that it would have supplied or ramped its supply of its products on the private market but for the interlocutory injunctions, then Alphapharm has proved that putative fact.
(4) If, as I consider, Alphapharm does not need to prove (2) or (3) because it has proved (1) on the balance of probabilities, then the probabilities and possibilities are as follows:
(a) Alphapharm would have re-applied for PBS listing before another generic had PBS listed its products: 0%.
(b) Alphapharm would have supplied its products on the private market from the assumed date of 22 July 2009: 90%.
(c) Alphapharm would not have supplied its products on the private market from the assumed date of 22 July 2009: 10%.
(5) Assuming Sigma had obtained PBS listing on 1 December 2009 the probabilities and possibilities are:
(a) if Alphapharm was supplying its products on the private market (the 90% probability):
(i) Alphapharm would have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could (in my view, in time to obtain PBS listing by 1 March 2010): 90%; and
(ii) Alphapharm would not have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could but would have continued to supply its products on the private market: 10%;
(b) if Alphapharm was not supplying its products on the private market (the 10% possibility) :
(i) Alphapharm would have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could (in my view, in time to obtain PBS listing by 1 March 2010): 80%; and
(ii) Alphapharm would not have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could but would have continued not to supply its products: 20%.
645 The other aspect of Alphapharm’s claim relates to the value of the stock it ordered in 2008 which it received in 2011, part of which had to be destroyed due to the limited shelf life of the products. Alphapharm submitted this about the products it ordered:
The Court would find that Alphapharm paid Pharmathen on 18 November 2009 and 29 January 2010, during the currency of [the Alphapharm interlocutory injunction]. That undoubtedly evidences a loss suffered by Alphapharm, given that the product was not delivered until 2011, by which time portions of that delivery were too close to their expiry dates to sell. The fact that Alphapharm may not have been strictly contractually obliged to pay Pharmathen prior to delivery, if commercially it decided to do so, does not prevent it from suffering loss in that respect. Any amounts it paid early discharged its liability to Pharmathen. The proportion which it paid representing destroyed stock is recoverable by Alphapharm. Alphapharm paid Pharmathen 450,725 Euro for 77,322 packs of 75mg and 150mg venlafaxine (including packaging costs). 43,273 packs (55.96%) were destroyed due to the need to defer delivery of the stock to 20 December 2011 and the fact that the stock that had been ordered in 2008 had limited shelf life. Alphapharm should be awarded compensation to reflect that loss.
646 As discussed, the interlocutory injunctions operated until 8 November 2010. The final injunctions operated from that date until the Full Court’s orders in November 2011. As a result, on one view, the operation of the final injunctions caused this loss to Alphapharm. But for the final injunctions, Alphapharm would have been able to obtain delivery of its products 12 months earlier than it did when, I would infer, it would not have had to destroy any products due to their shelf life. However, this view overlooks the operation of the Alphapharm interlocutory injunction which is the relevant question. The final injunctions, on analysis, are nothing more than a subsequent potential cause of the loss. But for the Alphapharm interlocutory injunction, I have found that there was a high likelihood that Alphapharm would have supplied its products on the private market. It necessarily follows that Alphapharm would have re-visited its decision to defer delivery and would have received the products it ordered in 2008 in or about August 2009 when there was no risk of it having to destroy any products due to their shelf life.
647 Accordingly, another adverse effect of the Alphapharm interlocutory injunction was that Alphapharm could not take delivery of the products it had ordered in or about August 2009 when it would not have had to destroy any products. Alphapharm should be compensated for this additional loss. The most practical approach is simply to allow for 90% of the cost of the destroyed products having regard to my conclusions above. This amount will have to be calculated in Australian dollars.
648 Generic Health claims to have been adversely affected by all three interlocutory injunctions in terms of the lost opportunities of sales of Generic Health’s generic venlafaxine products, sales to Sigma of Sigma’s generic venlafaxine products, and sales to Apotex, Sandoz and Ascent of generic venlafaxine products under arrangements by which those companies ultimately obtained their own or had assigned to them one or more of Generic Health’s ARTG registrations.
649 As discussed, I consider that these different claims involve different considerations of causation of loss. For its own products, the ARTG registrations gave Generic Health all rights associated with such registration which could be held, exercised or transferred from time to time as Generic Health saw fit. The Generic Health interlocutory injunction prevented the exercise of those rights. Generic Health thus necessarily lost an opportunity of some value as a result of the operation of the Generic Health interlocutory injunction whether or not it would have exercised those rights. As such, loss of an opportunity of some value has been proved on the balance of probabilities.
650 In contrast, by the Sigma interlocutory injunction, Generic Health lost the opportunity to supply Sigma under its supply contract with Sigma, but that opportunity only had value if there would have been some additional supply under that contract. As such, the putative fact of some additional supply to Sigma must be proved on the balance of probabilities. Proof of this fact depends on what Sigma would have done which I have found would have been to continue its private market supply and the likely success of Sigma’s continuing private market supply.
651 In common with Alphapharm, Generic Health’s case was unnecessarily convoluted. In particular, from Generic Health’s perspective, the Alphapharm interlocutory injunction cannot have caused any direct adverse effect on Generic Health. In common with the purported effect of the Sigma interlocutory injunction on Alphapharm, all the Alphapharm interlocutory injunction did to Generic Health was make it anticipate that Wyeth would apply for interlocutory relief against Generic Health and would succeed if it did so, which is one of the ordinary exigencies of litigation which cannot be disregarded. Again, however, only the practical difference this makes is to the timing of any notional PBS listing by Generic Health (1 December 2009 rather than, as I have concluded, 1 March 2010).
652 The following summary includes extracts from Generic Health’s closing submissions to the extent that they record facts which appear to be uncontroversial and otherwise includes undisputed facts apparent from other parts of the evidence.
653 Generic Health was a relative newcomer to and a minor player in the generic pharmaceuticals industry, having been founded by Mr Upiter in 2004. In 2009 it had 8 sales representatives who would visit around 200 pharmacies each year, a much smaller product range than Sigma and Alphapharm, and relationships with fewer pharmacies across this smaller product range. Given its limited product range in 2009 Generic Health could not have supplied any pharmacist with all of its generic product requirements. As such, it was not a “first-line” supplier in the same sense as Sigma and Alphapharm.
654 Generic Health was also unprofitable. As Wyeth submitted:
For the June 2008 financial year, Generic Health’s gross loss was $740,118 and its EBITDA (earnings before interest, tax, depreciation, amortisation) was negative $4,511,059. For the June 2009 financial year, Generic Health’s gross profit was $980,362 (a shortfall of $1,201,876 from a budgeted gross profit of $2,182, 258) and its EBITDA was negative $3,050,795. In the month of June 2009 alone, Generic Health had made a gross loss of $235,586 (a shortfall of $467,726 from a budgeted gross profit of $252,138). For the June 2010 financial year, Generic Health’s total gross profit was $1,870,101 (a shortfall of $167,444 from a budgeted total gross profit of $2,037,546) and its EBITDA was negative $852,009. The 2010 financial management accounts noted that there were “very difficult trading conditions.”
655 As Wyeth also noted, Generic Health was being confronted by liquidity concerns. The board papers for a meeting on 28 April 2009 recorded:
Cash flow on working capital requirements
As communicated to the board earlier, discussions with NAB have failed to secure an increase in the amount of working capital available for the company. The company is required to repay $200,000 to NAB in order to reduce the amount of working capital available from $1.5m to $1.3m. This payment was made at the end of March.
A further repayment of $500,000 is due at the end of June 2009, and no directions has [sic] been provided by the bank as to whether or not this payment will be required to be made or allowed to be deferred.
…
…We also assume that no further repayments will be required to be repaid to NAB. In other words, the cash flow projections assume the NAB continues to provide a working capital facility of at least $800,000 to December 2009.
The future liquidity of the company is dependent on the availability of the $3 million working capital facility for Max Pharma.
656 Mr Upiter said that Generic Health’s assets were subject to a charge or other security in favour of NAB and that in referring to the company’s future liquidity being dependent on the provision of $3 million he meant that the company might be forced into insolvency but for that capital injection. The source of the funds was Lupin, a shareholder (and from 2010 the owner) of Generic Health.
657 The expiry of the compound patent represented a significant opportunity for Generic Health which was struggling to establish itself in the highly competitive generic pharmaceuticals market which in 2009 was dominated by, amongst others, Sigma and Alphapharm. Generic Health did not know about the method patent and applied for registration on the ARTG of its various venlafaxine products in April 2008 hoping to achieve registration by proving the bioequivalence of its products by 1 May 2009, to be followed by an application to list on the PBS and PBS listing on 1 August 2009. All of these dates turned out to be over-optimistic and necessarily would have been irrespective of the interlocutory injunctions.
658 On 7 January 2009 Generic Health ordered products from Pharmathen, the supplier to it of its proposed Generic Health venlafaxine products, conditional on ARTG registration. In fact, Generic Health’s products were not registered on the ARTG until 6 August 2009. Because a product cannot be listed on the PBS until has been registered on the ARTG, Generic Health could never have achieved its goal of PBS listing on 1 August 2009. The best Generic Health could have done was to apply for PBS listing by 1 September 2009 to obtain PBS listing on 1 December 2009. The possibility of Generic Health obtaining PBS listing on 1 August 2009, accordingly, may be disregarded.
659 In July 2008 Generic Health agreed an amendment to its existing supply agreement with Sigma to include Evelexa XR as a product to be supplied by Generic Health to Sigma. On 1 August 2008 Generic Health entered into an agreement with Alembic for the manufacture and supply to it of Evelexa XR for the purpose of sale to Sigma. In September 2008 Generic Health entered into an agreement with Sigma and Alembic about good manufacturing practice (or GMP) for Sigma’s Evelexa XR products. On 2 September 2008 Generic Health ordered three strengths of Evelexa XR from Alembic for supply to Sigma, receiving the formal order from Sigma for those products on 4 September 2008. On 12 February 2009 Generic Health received a further purchase order from Sigma and itself issued a purchase order to Alembic for that quantity of Evelexa XR. Generic Health invoiced Sigma on 23 February 2009. On 28 February 2009 Generic Health also received an invoice from Alembic. Both of these invoices were paid and no claim is made in these proceedings by Generic Health in respect of its payment to Alembic. Sigma placed a third order on 11 March 2009.
660 Mr Upiter became aware of the method patent in April 2009 as a result of Sigma filing its application to revoke the method patent which occurred on 1 April 2009.
The competing cases – preliminary points
661 In common with its approach to Sigma’s position, Wyeth contended that the steps Generic Health took in anticipation of launching its products and to supply to Sigma were immaterial because they were done in ignorance of the method patent and that when it became aware of the method patent Generic Health changed its plans and would have done so irrespective of the interlocutory injunctions.
662 This, however, is an over-simplification. Insofar as Generic Health is concerned what must be posited is that on 22 May 2009 Wyeth did not prosecute its application for an interlocutory injunction against Sigma, on 14 August 2009 Wyeth did not prosecute its application for an interlocutory injunction against Alphapharm, and on 10 November 2009 Wyeth did not prosecute its application for an interlocutory injunction against Generic Health. Otherwise, I accept that the litigation must be assumed to be as it was in fact. Sigma filed proceedings to revoke the method patent on 1 April 2009. Wyeth filed a cross-claim for infringement including for interlocutory relief against Sigma on 1 May 2009. Alphapharm filed proceedings to revoke the method patent on 19 June 2009. Wyeth filed a cross-claim for infringement including for interlocutory relief against Alphapharm on 23 July 2009. Generic Health filed proceedings to revoke the method patent on 6 October 2009. Wyeth filed a cross-claim for infringement including for interlocutory relief against Generic Health on 9 November 2009 (noting that, on 15 October 2009, the sale of Wyeth to Pfizer was completed). All of these things occurred and cannot be disregarded. The Generic Health interlocutory injunction was made without contest by Generic Health on 10 November 2009. It is this which must be disregarded.
663 In these circumstances, it cannot be said that the steps Generic Health took before becoming aware of the method patent are immaterial. For one thing, I have found it certain that Sigma would have continued with its private market supply if on 22 May 2009 Wyeth had not prosecuted its application for interlocutory relief against Sigma. Accordingly, Generic Health would have continued to supply Sigma with Evelexa XR under its agreement with Sigma, the volume of supply depending on Sigma’s degree of success in converting patients from Efexor-XR to Evelexa XR. For another, Generic Health had obtained ARTG registration of its products by 6 August 2009 and from that time had all of the rights afforded by ARTG registration. As for Sigma and Alphapharm, the Generic Health interlocutory injunction on 10 November 2009 prevented the exercise of those rights which necessarily involved a lost opportunity of some value.
664 Generic Health would be in the same position as Sigma and Alphapharm in other respects. It would not know why Wyeth had not sought interlocutory relief against Sigma, Alphapharm or itself. Its lawyers would know that there might be many reasons for Wyeth not to have done so. Wyeth would have made it clear to Sigma, Alphapharm and Generic Health that Wyeth maintained the method patent was valid and would prosecute its proceedings for infringement against them.
665 The facts as they existed and as I consider they would have existed (as described above for Sigma and Alphapharm) must be considered in this context.
666 Apart from this, the different claims of Generic Health under the different interlocutory injunctions must be kept in mind. To the extent it claims for the lost opportunity to supply Sigma, it is the operation of the Sigma interlocutory injunction which is relevant. To the extent it claims for the lost opportunity to supply its own products, it is the Generic Health interlocutory injunction which is relevant, although Generic Health also seeks to claim under the Alphapharm interlocutory injunction for an adverse effect in having made decisions as a result of that interlocutory injunction (an approach I consider impermissible but with which I deal below on the facts). The relevant point presently is that it is the adverse effect of each interlocutory injunction which must be kept in mind.
667 Generic Health’s principal witness, Mr Upiter, is in no different position from the other witnesses giving hypothetical evidence. For all of the general reasons already given, Mr Upiter’s evidence about what he would have done and recommended in 2009 is unreliable.
668 There were other problems with Mr Upiter’s evidence. It is apparent that he did not have access to all of the relevant documents when preparing his affidavits. He said in his first affidavit that he planned for Generic Health to apply for PBS listing despite being aware of the method patent because he believed that Generic Health’s products did not infringe the method patent as they were based on hydrogel technology (unlike Sigma’s products but in common with Alphapharm’s products). However, the contemporaneous documents show a far more nuanced and changing picture than Mr Upiter presented in his affidavit. Mr Upiter’s affidavit expressed the view that he would be “happy” for Generic Health to trigger the 12.5% price drop because he believed Generic Health’s products did not infringe the method patent so the risk to Generic Health was “very low”. It necessarily follows that contemporaneous documents about the perceived risk of infringement over time are relevant, yet Mr Upiter expressed opinions without having seen all such documents.
669 The way in which Mr Upiter’s affidavits were prepared made it impossible to know what documents he had seen at what time. As a result, Mr Upiter could not recall what documents he had seen before preparing his first affidavit and what additional documents he had seen when preparing his second affidavit. He had seen some but by no means all relevant documents. He had more documents available when he provided his second affidavit, some years after his first affidavit. He then looked at yet more documents before giving evidence. As a result, the process by which his affidavits were prepared undermined his potential to give reliable evidence about what he knew and believed at the time, let alone what he thought he might have done or said at the time had circumstances been different from what they were.
670 Mr Upiter also could not recall the “timing” of things which is an important part of the context. He could not recall what he thought about various important documents at the time and was capable only of attempting to reconstruct from the documents themselves. In particular, I infer that when preparing his affidavits he had (unsurprisingly) forgotten that the legal advice to Generic Health about non-infringement changed after the Alphapharm interlocutory injunction. His attempts to suggest that in his second affidavit when he used the expression “fall foul” (that Generic Health would not “fall foul” of the method patent) he did so deliberately to cover both non-infringement and invalidity due to confusion about the two and their timing were unconvincing. Until he saw the email of 26 August 2009 which disclosed that the non-infringement advice had changed (from good to not good prospect of success for Generic Health), the sole focus of his evidence was non-infringement. Confronted with the email, invalidity then emerged. I infer that having forgotten and then been reminded that the advice about non-infringement changed, Mr Upiter was concerned in oral evidence that his affidavit not be exposed as a reconstruction based on incomplete documents. This kind of evidence further undermined Mr Upiter’s reliability as a witness.
671 Mr Upiter also said that:
As a result, after I became aware of the Method Patent, I recommended to the board of Generic Health that it continue with its preparation to launch the GH Venlafaxine Products and the board agreed with that recommendation. I can recall that after discussion of the board in or around early June 2009, the board agreed with my recommendation. However, this was on the proviso that it would have final approval prior to an “at risk” launch being made.
672 The contemporaneous records are by no means as clear as Mr Upiter suggests.
673 According to Mr Upiter, he recommended that Generic Health not launch its products once the Alphapharm interlocutory injunction was granted because the products both used hydrogel technology and he “concluded that if Alphapharm was unsuccessful in resisting the injunction application brought against it, then Generic Health would no doubt also be restrained”. The contemporaneous records disclose a more complex picture than this, however.
674 Mr Upiter said that if Sigma and Alphapharm had not been subject to interlocutory injunctions then the opportunity and the risk for Generic Health would have been bigger as it would have been the only generic to have a PBS listed extended release venlafaxine product. This evidence does not follow from the facts. He acknowledged that “there certainly would have been some discussion or evidence put around the size of the opportunity and – and the risks that the company would – would have been taking in that scenario” and that the decision would have been that of the board. As Mr Upiter put it:
I certainly would have brought to the attention, as I said, a commercial opportunity, so the size – the size of the market. I would have made estimates as to what the market share that we would have gained would have been and, therefore, commercial return for the company. I would have presented what our buying price was, our selling price would have been and, therefore, some type of profitability calculations. I would have presented some type of the marketing plan in that scenario. And I would also have presented some estimates of the type of the quantum of damages, that if – if we – if we were not successful in eventually defending the patent or defending our position in the patent, what those were – would likely be.
675 Subsequently, it emerged that Mr Upiter also would have appreciated that if Generic Health was the first to list its products on the PBS not only would it be responsible for triggering the 12.5% price drop of Efexor-XR and consider that it would be liable for that price drop if the method patent was valid, but also that it knew that it would enjoy the advantage of being the only generic to have a PBS listed extended release venlafaxine product for only a short period as other generics would then also seek to list on the PBS without the risk of being responsible for triggering the 12.5% price reduction. In other words, Mr Upiter’s advice to the board would have been that if the method patent was valid Generic Health would then be solely responsible for triggering the 12.5% price reduction over the life of the method patent if it sought and obtained PBS listing on 1 December 2009 and, at best, would have enjoyed its status as having the only generic product on the PBS until the next PBS listing opportunity.
676 Mr Upiter accepted that he would have obtained further legal advice and prepared detailed information about the potential benefits and risks to Generic Health for the board to consider (including that if Generic Health listed its products on the PBS first, other generics would also be likely to seek to list their products on the PBS) and that his own views, both as to what he would have recommended to the board and decided as a director, may be qualified or influenced by this exercise and that he had not done this exercise before giving his evidence. Despite this, Mr Upiter refused to accept the proposition that not having done the exercise then or now for the purpose of preparing his evidence it was difficult for him to say what he would have done in 2009. Instead Mr Upiter said:
I don’t think it’s difficult as to how I would have reacted, because my position was clear then. I know how I would have reacted. I was constantly focused on balancing those risks and my view that I formed was that it was worth going ahead with this launch, despite not having gone and prepared each of those three pieces of paper. I was informed to an extent on all of those matters around the risks, the potential upside. As a managing director of a generics company, having analysed these numbers in deciding on spending the money on a product like this, I was well aware of the market potential of this product and I was aware of the type of market penetration that we could get. So in my mind it was very clear what the potential gain was, if not to an number in an order of magnitude, the risks in an order of magnitude would have been clear to me at that time, and the advice that I had been given had gotten me to form the view that we had very strong chances in the patent case, and for those reasons I’m confident about what I would have done.
677 The unreliability of this evidence, in my view, is clear. One, it is being given by a person who formed his plans before being aware of the method patent. Two, it is being given by a person who knows that in 2011 the method patent was held to be invalid. Three, it is being given by a person nearly a decade later. Four, it is being given by a person who had not seen all of the relevant documents which show that some of his key premises, particularly about Generic Health’s products not infringing the method patent because they used hydrogel technology, were not accurate. Five, it is being given by a person who disclosed a willingness to tailor his oral evidence to suit what he perceived to support what he had said in his affidavits and thus Generic Health’s case.
678 Again, for these reasons, inferences about rational commercial behaviour informed by the objective contemporaneous evidence and in light of all of the circumstances as they existed are a better guide to what would have occurred but for the interlocutory injunctions than Mr Upiter’s unreliable evidence nearly a decade after the events and with the benefit of hindsight that the Full Court in 2011 would find the method patent to be invalid.
679 In April 2009 Generic Health would have been in the same position it was in fact in at that time. Thus, Generic Health would have become aware of the method patent and the Sigma proceedings and would have done what it did, which was for the board to consider its position as the board did on 28 April 2009. The board paper records that:
Sigma has begun patent revocation proceedings against the innovator (Wyeth) and an additional directions hearing is scheduled for 1st May 2009. Alphapharm’s strategy is unclear at this point.
…
The company is evaluating the implications of a number of options as below:
1. Monitor Sigma’s progress and defer any decision either to launch or join patent revocation proceedings.
2. Join Sigma in revocation proceedings and share costs/benefit. This could cost Generic Health anywhere from $250,000 to $450,000 and could potentially be funded by selling Sigma our “right” to supply this product to a third party (currently in discussion with Genepharm and Sandoz). Patent revocation proceedings could take anywhere from 12 to 24 months to resolve in the courts. If Generic Health chooses to sell product while pursuing revocation proceedings, the company would also need to ensure sufficient funds are available to defend against an infringement case simultaneously.
3. Negotiating a commercial deal with the innovator.
Venlafaxine is forecast to contribute $50,000 per month to GM on average between July and December 2009 with an expected cash outlay of around $500,000 for stock in July 2009.
It should also be noted that as Generic Health has facilitated the manufacture and importing of Sigma’s brand of Venlafaxine, the company may be sued for infringement once the product arrives in Australia. The product is currently being held in Singapore under instruction from Sigma.
Recommendation
• That the board members provide any input or comments on the matter.
680 Of particular relevance here are two matters. First, it is apparent that Generic Health’s parlous financial state was relevant to its capacity to respond to circumstances. It had to consider carefully expenditure of as relatively little as $250,000 to $450,000 and in so doing had to consider how it might fund that limited expenditure (by, for example, selling Sigma the right to supply to others, a right which, it should be noted, would depend on ARTG registration of Generic Health’s products). In oral evidence Mr Upiter agreed that $250,000 to $450,000 was a significant amount of money for Generic Health. Second, Mr Upiter, no doubt informed by the financial realities, had little immediate appetite for a fight with Wyeth. The immediate suggestions he made included “wait and see” and negotiating a deal with Wyeth.
681 Generic Health submitted that:
Three points should be made about the forecast. First, it was short term. Secondly, an additional $50,000 per month in gross margin in that period was very significant for Generic Health given its financial position at the time. Thirdly, it was likely to have been conservative. By this point the original timeframe which was based on TGA approval in March 2009 had been proven to be overly optimistic. In a situation where by April, TGA approval still had not been obtained, there is a reasonable prospect that the forecast sales from July 2009 to December 2009 assumed only, or predominantly, private sales on the footing that the next available PBS date was probably November or December of that year. The short-term forecast was not purporting to quantify the full extent of the benefits expected by Generic Health.
682 These propositions may be accepted. My point is that despite the undoubted importance of the opportunity venlafaxine represented to Generic Health, Mr Upiter’s recommendation to the board was not to the effect that the opportunity was of such importance that Generic Health should simply plough on with its plans made before it became aware of the method patent. His recommendations were measured and shaped by the fact that Generic Health had little money to spare and that a fight with Wyeth would mean Generic Health would have to spend money it did not have readily available.
683 The board minutes for the 28 April 2009 meeting record:
Discussion held on the status of venlafaxine registration and patent registration and various options open to the company, it was agreed the best option seemed to be to align with Sigma to invalidate the patent together and this option should continue to be pursued. It was also felt the company should wait until the company’s brand is approved by the TGA before deciding any litigation strategy.
684 Consistent with my general approach, the fact that Generic Health may be anticipating an interlocutory injunction at this stage (which is unclear) does not mean that these matters may be disregarded. Generic Health would have been in the same position on 28 April 2009 as it was in on that date. Further, the minutes reflect the fact that without ARTG registration of its products Generic Health held nothing of value it could assign or exploit (leaving aside its contract with Sigma).
685 As noted, Wyeth cross-claimed against Sigma for infringement on 1 May 2009 and also sought interlocutory relief. This occurred and cannot be disregarded. It is to be assumed, however, that on 22 May 2009 Wyeth did not press its application for interlocutory relief and thus Sundberg J did not grant the Sigma interlocutory injunction on 3 June 2009.
686 Generic Health’s next steps, to check if it was using the same or a different formulation from Sigma, would have been taken by Generic Health in any event. Generic Health would have wanted to know if there was available to it an argument that appeared not to be available to Sigma, that Generic Health’s products did not infringe the method patent as they used hydrogel technology. Similarly, Generic Health would have believed it to be “good news” that its products to be sourced from Pharmathen used hydrogel technology because the specification for the method patent, arguably, disavowed hydrogel technology.
687 This would have been relevant to Generic Health irrespective of the Sigma interlocutory injunction because Generic Health would still have been confronted by the fact that Wyeth was pursuing its infringement proceedings against Sigma. Knowing, as it did through its connection with Lupin, the steps Wyeth had taken internationally to enforce the method patent, Generic Health would have anticipated that it too would be confronted by infringement proceedings if and when it obtained ARTG registration and was in a position to supply its products.
688 Generic Health submitted that the important point about these actions is that:
Wyeth had acted to restrain Sigma and was therefore likely to act to restrain Generic Health. The focus is on avoiding an injunction. There is no suggestion in that email exchange that Generic Health should or would not supply because of the existence of the patent or the threat of damages.
689 In my view, as I have said, whether or not Wyeth had applied for interlocutory relief on 22 May 2009 Generic Health would have explored the issue of its likely exposure to an infringement suit by Wyeth including the risk of an interlocutory injunction. It might well think that the risk of an interlocutory injunction was low if Wyeth had not sought interlocutory orders against Sigma on 22 May 2009 but it would still know that Wyeth was very likely to bring an infringement suit against it if and when Generic Health was in a position to supply its products. As such, the hydrogel technology issue would have been just as relevant albeit that Generic Health’s focus may have been its exposure to final relief in the form of a final injunction and damages rather than an interlocutory injunction. However, in any realistic hypothetical, as in fact, Generic Health was not yet in a position to supply as it did not have ARTG registration so (consistent with the board’s decision) it was not required to decide what it would do at this time.
690 Mr Upiter sent an email to the board on 18 June 2009 saying:
Based on our discussion with solicitors today, our strategy for Venlafaxine would rely on our ability to avoid an injunction. In this case we must be able to show an ability to pay damages and quarantine profits until the outcome of the revocation proceedings. Obviously, Wyeth will challenge our ability to do this based on the fact that we are not yet profitable and will not benefit via the venlafaxine sales as these will be quarantined.
We would therefore need to seek some type of loan against the quarantined profits. The loan would be further supported by a penal convertible note or charged over the business in the event that the case is lost or quarantine profits are required to be paid to Wyeth. The risk for the lender is mitigated largely due to the fact that the loan only comes into place if the injunction is not granted. If the injunction is not granted the courts have obviously found a very strong case for invalidity.
Our case would also be based on limiting the overall market size by focusing on private scripts, thereby limiting the maximum lost profits to about $5 million.
We are waiting on further information from the solicitors on the strategy…
691 It is apparent that this email anticipates an interlocutory injunction application by Wyeth against Generic Health. This would have been the same irrespective of the Sigma interlocutory injunction and, as discussed, cannot be disregarded as a matter of principle. The dire financial position of Generic Health is also apparent which also would have been the same irrespective of the Sigma interlocutory injunction.
692 Apart from this, it is notable that Generic Health immediately conceived of the same strategy as Sigma to confine Wyeth’s potential lost profits, being private market supply rather than PBS supply.
693 It may be accepted that uppermost in Generic Health’s mind at this time was avoiding an interlocutory injunction but, as with Sigma and Alphapharm, this does not mean what occurred is irrelevant or tainted. What occurred shows at least three things.
694 First, Generic Health (like Sigma and Alphapharm) was acting in a commercially rational manner to protect itself as best it could from an anticipated interlocutory injunction.
695 Second, Generic Health (like Sigma and Alphapharm) did not consider PBS listing and supply had to be achieved at any cost.
696 Third, (like Sigma and Alphapharm) Generic Health believed that PBS listing would involve potential liability to Wyeth of a different order of magnitude compared to private supply.
697 This concordance between the generics is unsurprising. Although Generic Health was in a different market position from Sigma and Alphapharm these companies all knew the business. They understood the benefits and risks. Their managers were canny strategists keenly aware of risk-reward analysis. Confronted by the method patent and the litigation (anticipated or actual) none of them simply ploughed on determined to obtain PBS listing. They all immediately appreciated that being the generic which triggered the 12.5% price reduction in Efexor-XR involved a risk of potential liability to Wyeth of an entirely different kind from the risk associated with supply of their products on the private market.
698 Mr Upiter met with Generic Health’s solicitors, Middletons, on 18 June 2009. A file note of that meeting records that Generic Health was not profitable, the generics market was highly competitive, Generic Health was not growing as fast as it would like so it “needed new products and fast”, and venlafaxine was “one product we need”. In this sense, Generic Health was in a similar position to Sigma but, of course, stood to gain far less than Sigma might from venlafaxine because of Sigma’s size, product range and arrangements with pharmacies. It also cannot be doubted that Alphapharm’s drive to obtain a “win” would have been no different, despite its less parlous financial position.
699 In another email of 18 June 2009 Mr Upiter advised Middletons that the cost to Generic Health under two options should be looked at. The email said that in respect of the private only launch “we would expect to earn $2m GP [gross profit] from direct sales and a further potential $1m from an increase in sales of other products”. In respect of the PBS and private launch the email said “[i]f we sell on PBS and privately we would likely be selling with other generic players, but have access to the whole market. In this case we expect we would earn between $2.5m and $3.5m GP from direct sales and probably minimal from increased sales of other products”.
700 This email discloses an important fact. Generic Health’s upside from PBS supply was not as great as might be expected because it was a much smaller supplier than Sigma or Alphapharm and, as noted, it also knew that if obtained PBS listing the other generics would also be supplying on the PBS. Generic Health submitted that given Generic Health’s poor financial position the difference in potential benefit would have been “very significant”. The total difference is $500,000 gross profit. I accept this was a significant amount for Generic Health but this needs to be measured against the fact that Generic Health knew being the first to obtain PBS listing would have exposed it to a risk of liability of around $100 million. As should be apparent, even believing the risk of liability to be low the relativities of benefit and risk involve an enormous disparity.
701 The matter was put before the board again on 23 June 2009. The board papers record the fact of the Sigma interlocutory injunction, the anticipation of ARTG registration of Generic Health’s products within three weeks, and that the strategy was to “build a case supporting the argument that Generic Health is likely to be able to sell and is likely to avoid being injuncted [sic]”, and that this case would be presented to Wyeth before Wyeth was aware of the ARTG registration of Generic Health’s products. The options presented were “move to sell and face an injunction hearing”, reach a commercial outcome with Wyeth, and withdraw from any further activity. A solicitor was to be engaged with the focus being an approach to Wyeth for a commercial outcome with the “risks and costs” of each option to be analysed. A table identifying outstanding legal matters identified the Wyeth method patent and that Generic Health’s exposure “may be significant”.
702 Generic Health submitted that as it would struggle to pay any amount of damages to Wyeth, the materiality of Generic Health’s appreciation that it could be exposed to significant damages should not be over-estimated. In effect, what was being put is that Generic Health might as well have exposed itself to the maximum extent given that even a relatively small liability to Wyeth most likely would have exceeded its capacity to pay. This is an unpersuasive submission. It assumes that the board members of Generic Health were willing to expose the company to potential liabilities it had no hope of paying. It thus assumes that the board members were willing to disregard the kind of obligation that would attach to a director of a company which was already at risk of insolvency and which was considering exposing the company to a risk which, if it crystallised, would be certain to make the company insolvent. Wyeth noted that directors in such a positon would be bound to consider the interests not only of the company but also of existing and future creditors: Kinsela v Russell Kinsela Pty Ltd (In liq) (1986) 4 NSWLR 722 at 730; Nicholson v Permakraft (NZ) Ltd (in liq) [1985] 1 NZLR 242 at 249; Lewis (as liquidator of Doran Constructions Pty Ltd) v Doran [2005] NSWCA 243; (2005) 219 ALR 555 at [145]; Sunburst Properties Pty Ltd (In Liq) v Agwater Pty Ltd & Ors [2005] SASC 335 at [154]-[155]; Jeffree v National Companies & Securities Commission (1989) 15 ACLR 217 at 221 citing Winkworth v Edward Baron Development Co Ltd [1987] 1 All ER 114 at 118. Whether the directors would have been aware of the precise legal implications or not, they would have known that the continued existence of the company may depend on the decision they made. They would have known that their decision-making would be exposed to close scrutiny in the event of insolvency. They would have known that their personal reputations in the industry may depend on what occurred.
703 Wyeth correctly stressed the importance of Mr Upiter’s evidence as follows:
Mr Upiter agreed that he understood at the relevant time that as a Director, that the question of solvency of any company was an important matter and that “where the solvency of the company was at risk, to take into account the interests of creditors”. Mr Upiter also agreed that “there was a risk that a director might become personally liable for taking a decision which causes the company to … go insolvent, but there may be a defence to that [claim]”. He acknowledged that it was, however, “a risk which any director in 2010, as far as [he was] concerned, would have to take into account”.
Mr Upiter also agreed that “from a reputational point of view [he] regarded it as important, as [his] own reputation as a director, not to be a director of a company which ultimately was forced into insolvency” as “it might affect your ability to get directorships on other companies” and “may even affect your ability to attract investment if you were to set up a future company.” He also accepted that “taking a decision which might result in a company becoming insolvent … might … have a negative impact on creditors” and “a negative impact on employees” (“who may have long service leave entitlements”) and “could have a negative impact on trade creditors” and “shareholders who might lose their investment”.
704 As Wyeth also noted:
Lupin was a shareholder and creditor of the company for a facility of $3 million. The Directors representing Lupin had an interest in that shareholding and that debt not being set at nought. The other Directors were investors in the company themselves. Again, they had a clear interest in their shareholding not being rendered worthless by a commercially irrational decision and plainly they had no interest in exposing themselves to personal liability.
705 This was disclosed in Mr Upiter’s second affidavit. There were eight members of Generic Health’s board. Mr Upiter explained:
Dr Mumtaz, Mr Dhawan and Mr Sharma were nominees of Lupin Pharmaceuticals, a global generic pharmaceutical company, which was a large minority shareholder in Generic Health in 2009. The other members of the board were investors in Generic Health.
706 In this context, to see what actually happened, which is Generic Health consistently taking positions which effectively exposed it to no risk at all, is instructive. The positions actually taken by Generic Health are irreconcilable with the submission that its parlous financial state meant that but for the interlocutory injunctions it would have risked its existence on not infringing the method patent or the method patent being invalid. The fact that Generic Health was in dire financial circumstances may be accepted. But it was managing and had been managing since 2004. It had liquidity issues but was not at immediate threat of insolvency. To knowingly take an existential risk when it could be avoided by confining its supply to the private market or by not supplying its products until the finalisation of the litigation would be conduct of a different kind from anything that Generic Health had previously done. No doubt this explains why Mr Upiter’s first suggestion to the board involved exactly the same strategy as Sigma adopted, to confine supply to the private market.
707 The board minutes record that the issue was discussed on 23 June 2009 and:
…concerns were highlighted about limiting the company’s potential upside to only the private market. It was also considered necessary to consider further whether a second legal opinion should be required based on the importance of the case.
The board resolved to set up a committee comprising GU [Mr Upiter], VD [Vinod Dhawan, another Lupin nominee] and SM [Dr Mumtaz, a Lupin nominee] to review the strategy and decide on costs.
It was agreed that legal fees should be capped…
Board members agreed that the company should hold off sublicensing to other parties…to ensure the company’s strategy regarding Venlafaxine is not constrained in any way…
708 The board, it seems, was not as keen as Mr Upiter on immediately adopting the strategy of avoiding an interlocutory injunction by confining the supply to the private market. Generic Health said this showed the board’s appetite for risk and was inconsistent with the board not being willing to launch in the face of an exposure to damages, but the fact is the board made no decision at this time. It did not resolve to seek PBS listing immediately on the ARTG registration of its products. Rather, and as might be expected from commercially rational decision-makers, it wanted legal advice. If Generic Health had a voracious appetite for risk and was as determined to proceed with PBS listing and supply as now suggested, it would not have taken the cautious and commercially rational approach it did. It is understandable that Generic Health required formal legal advice because this was the responsible thing to do and Dr Mumtaz, one of the Lupin representatives on the board, believed that in the USA the disavowal of hydrogel technology would have meant that the Generic Health products would not infringe the method patent.
709 Generic Health’s position would have been the same irrespective of the Sigma interlocutory injunction. The board would have wanted formal legal advice before making any decision. In any event, the issue here (and up to this point) is Generic Health’s supply of its own products which was not subject to the operation of the Sigma interlocutory injunction. Thus, everything that happened is to be taken as having happened. Nothing may be disregarded or discounted as immaterial.
710 Middletons gave written advice on 29 June 2009. Middletons said that Generic Health “has very good prospects of establishing that [its venlafaxine product] does not infringe” the method patent but it was likely that Wyeth would seek an interlocutory injunction and would obtain it because Generic Health could not prove it would be able to compensate Wyeth should it be held to infringe the method patent. Middletons recommended a strategy to obtain an undertaking as to damages from Wyeth without Generic Health contesting interlocutory relief and to seek to have the issue of non-infringement by Generic Health determined separately and speedily. Insofar as Generic Health’s own products are concerned, this is the same advice Middletons would have given disregarding the Sigma interlocutory injunction. If the Sigma interlocutory injunction may be disregarded for this purpose, Middletons would not know if Wyeth would or would not seek interlocutory relief against Generic Health. Further, it would still have been in Generic Health’s interests not to contest any interlocutory application by Wyeth and to seek an early hearing on the issue whether it infringed the method patent or not given the belief that there were very good prospects Generic Health’s did not infringe.
711 What is important here is that this strategy involved Generic Health negotiating an undertaking as to damages from Wyeth in exchange for Generic Health not supplying its products in Australia pending a final hearing which, it believed, would result in a finding that its products did not infringe the method patent. This is the strategy of a company that did not have the money for an interlocutory fight it did not think it could win and which, at the time, was focused on proving that its products did not infringe the method patent. This has nothing to do with the Sigma interlocutory injunction. It is a strategy that Generic Health came up with in its own interests which would have been the same irrespective of the Sigma interlocutory injunction.
712 Generic Health acted in accordance with this advice and also would have done so irrespective of the Sigma interlocutory injunction. Through its solicitors, and more than once, it then asserted to Wyeth that its products did not infringe the method patent. It also asserted that it intended shortly to begin supply of its products in Australia albeit that in fact the board had not decided to do so. Its purpose in so doing was to get Wyeth to give an undertaking to pay Generic Health compensation if Generic Health’s products did not infringe the method patent. It also wanted confirmation from Wyeth that Generic Health’s products did not infringe the method patent because that would remove the risks associated with supply.
713 Mr Upiter was criticised for giving instructions to send letters which said that the company was about to commence supply when, in fact, the board had made no such decision. Mr Upiter said that this was his intention, subject to obtaining board approval. It is sufficient to say the criticism of Generic Health (not, I should say, its lawyers) was not without justification. The instructions resulted in a letter which represented as a fact something which was not a fact. Generic Health’s purpose was to obtain a benefit from Wyeth, the undertaking, when such an undertaking would not ordinarily be given unless there was a genuine threat of supply. Conduct of this kind is fraught with risks which need not be identified here. Generic Health’s submissions that Dr Mumtaz and Mr Dhawan agreed with the communications to Wyeth do not alter the fact that Generic Health had not decided to supply when the communications were sent. Nor is it the case that the “commercial reality is that the board would not have permitted that [the arrangements for supply] to occur if there was a real prospect it would not approve launch”. Generic Health’s real object was not launch or at the least not launch other than as might be necessary to prompt action by Wyeth so it could avoid any wasted expenditure and have the benefit of an undertaking from Wyeth.
714 Otherwise, the first relevant point is that this is what occurred and it cannot be disregarded and would have been no different if the Sigma interlocutory injunction had not been sought by Wyeth on 22 May 2009. And Wyeth would have responded as it did on 3 August 2009, in effect, by seeking further information. The second is that Generic Health wanted Wyeth to confirm either that it would be seeking interlocutory relief or that Generic Health’s products did not infringe the method patent. Generic Health was not willing to rely on its own legal advice. Its aversion to potentially wasted expense was such that it wanted, indeed needed, express confirmation from Wyeth before it decided what to do.
715 Also on 29 June 2009 Generic Health had received an email from Paul Jones of Freehills Patent & Trade Mark Attorneys to the effect that while Generic Health had a very strong case on non-infringement and invalidity it would nevertheless be subject to an interlocutory injunction because the Court was “extremely pro-patent” in the context of interlocutory relief. Mr Jones said that he would not “not recommend launch until the current dispute is resolved”. There is no need to enter the debate about whether Mr Upiter knew about this at the time or before he gave evidence. What is relevant is that, as noted, it is apparent that Mr Upiter’s evidence was given without his memory having been refreshed by consideration of all of the relevant documents.
716 Wyeth also stressed that Mr Upiter agreed that he understood the reference in this email to the current dispute being resolved to mean final resolution not resolution of an interlocutory injunction. Generic Health submitted this must be in error. Given my view that none of the witnesses could have a clear recollection of what they thought in 2009 let alone what they would have thought in different circumstances (including those like Mr Upiter who said to the contrary) nothing much depends on this debate. In any event, a lawyer could only advise about the risks of launch before the final resolution of the litigation and the prospects of success in the context of interlocutory and final relief. Whether to launch at risk or not was ultimately a commercial, not a legal, decision.
717 On 4 August 2009 Mr Upiter sent an email to Dr Mumtaz noting that if Generic Health supplied on the private market without Alphapharm also supplying (and, of course, without Sigma supplying, as it had already been restrained) it might have four weeks before Wyeth obtained an interlocutory injunction in which it could expect gross profit of between $500,000 to $900,000. But for the interlocutory injunctions, however, Sigma and Alphapharm both would have been supplying on the private market and it must be inferred that the potential financial benefit to Generic Health would have been far less than $500,000 to $900,000.
718 Consistent with the overall object of minimising the potential for wasted expense, on 5 August 2009 Generic Health sent an email to its supplier, Pharmathen, saying that “we would like to order products for launch. However, due to possible injunction, we would like to keep the initial order to a very minimal quantity. Please let us know [whether] the order of 1,000 packs per strength of 75mg and 150mg will be acceptable or not”. The purpose of this inquiry was to see if Generic Health could do as little as possible in order to prompt an interlocutory injunction application by Wyeth.
719 Wyeth’s response to Middletons did not give Generic Health either the undertaking or comfort it was after so Middletons sent another letter on 7 August 2009. Again, the letter said that Generic Health expected to release its products in Australia “in the very near future” when the board had made no such decision and was hoping to secure a deal in which Generic Health would not have to supply at all in order to prompt an application for an interlocutory injunction by Wyeth. And again Generic Health sought confirmation from Wyeth that it either would be seeking interlocutory relief or that Generic Health’s products did not infringe the method patent. This consistent request exposes the extent of Generic Health’s aversion at the time to making any decision which could expose it to the risk of wasted expenditure.
720 Generic Health also became aware around this time of Wyeth’s claim for interlocutory relief against Alphapharm and that Alphapharm was going to run an argument that because its products used hydrogel technology they did not infringe the method patent. This too cannot be disregarded. It happened and Generic Health would have acted as it did in response. Having not managed to prompt action by Wyeth and the undertaking it was after, Generic Health noted that it was considering ordering stock (1000 units of each strength) from Pharmathen to “trigger an action from Wyeth”. In the same email of 10 August 2009 Mr Upiter again mentioned the possible second strategy of launching prior to the outcome of an interlocutory injunction hearing, but said this was “unlikely to proceed based on the downside risks”.
721 The potential “downside risks” included the potential wasted expense of a launch onto the private market that would likely be restrained. In other words, the short-term benefit of receiving $500,000 to $900,000 in gross profit was not seen as sufficient to warrant the possibility of being stuck with stock that could not be sold because of an interlocutory injunction. It also cannot be doubted that if Sigma and Alphapharm were both supplying the private market (as would be the case on my hypothetical analysis) the potential benefit to Generic Health would have been far less than $500,000 to $900,000, albeit that Generic Health also would then have perceived it as far less likely that Wyeth would seek interlocutory relief against it.
722 The important point is this, however. The same kind of risk-benefit analysis would have applied even if Generic Health believed that Wyeth would not seek an interlocutory injunction, because the anticipated interlocutory injunction was simply one part of the anticipated litigation in which Wyeth would seek final relief including injunctions and damages against Generic Health. The kind of risk-benefit analysis which Generic Health was engaging in is the same kind of analysis it would have done irrespective of an anticipated interlocutory injunction. And because the costs and exposures would be far greater than those associated with a mere four weeks supply, as Mr Upiter accepted, a far more detailed and formal process analysing all potential benefits and risks would have been required, as would have board approval of any strategy to be adopted.
723 The hearing of the interlocutory injunction application against Alphapharm took place on 14 August 2009.
724 In a letter of 17 August 2009 Wyeth’s solicitors confirmed that it considered Generic Health’s products infringed the method patent, refused to be drawn into making a threat about an interlocutory injunction, and instead said any actions by Generic Health would be at its own risk. Of course, this is the position Generic Health would have been in had Wyeth never threatened or sought an interlocutory injunction against any of the generics. It was not a satisfactory position for Generic Health who was so averse to the potential it might spend money that would be wasted that it was after the equivalent of a guarantee that nothing it did might involve that risk.
725 In an email of 20 August 2009 Mr Upiter informed Dr Mumtaz and Mr Dhawan that he had instructed Middletons to send another letter to Wyeth’s solicitors. That letter, which was sent on 21 August 2009, repeated Generic Health’s request that Wyeth inform it either that it would seek interlocutory relief or that it accepted that Generic Health’s products did not infringe the method patent. This was the third attempt by Generic Health to obtain the level of certainty it wanted before it would decide what it would do. Again, Generic Health’s determination not to expose itself in any way to wasted expenditure is apparent.
726 On 20 August 2009 Mr Upiter sent an email to Pharmathen requesting urgent confirmation as to the date for stock under the two scenarios noted above. The email stated “[t]his is critical for our planning to enter the market by potentially avoiding an injunction or achieve declaration of non-infringement”. On the same day Mr Upiter advised Mr Dhawan and Dr Mumtaz that “[w]e have also planned launch activities to be ready to cover about 2000 pharmacies by phone and over 5000 by fax over two days and are prioritizing and grading the highest potentials. Tony [Middletons] said that we can expect the judges [sic] decision any day”. Mr Upiter acknowledged that this option was available only if Alphapharm succeeded on its non-infringement argument and thus was not subject to an interlocutory injunction. Generic Health submitted the relevance of this qualification should not be over-stated as:
For example, it was not put to Mr Upiter that the proposal would not have been activated if the Court had refused the injunction on the basis that although the Alphapharm products infringed the patent claims if they were valid, those claims in fact were not valid. Such a distinction is not plausible in the circumstances.
727 Generic Health also submitted that:
The email is significant because it is a plan by Generic Health to launch on the private market if the Alphapharm injunction is refused, notwithstanding that the risk of being liable to Wyeth for damages at a final hearing remained. In other words, the email demonstrates that Generic Health were prepared to launch at risk.
728 In my view, it is this submission which is both inaccurate and over-stated. Generic Health did not just want to know that it could avoid an interlocutory injunction. It also wanted the comfort of the Court refusing to enjoin Alphapharm because of a prima facie conclusion that hydrogel technology would not infringe the method patent. This is consistent with Generic Health’s repeated but failed attempts to extract such a confirmation from Wyeth. As I have described it, at this time Generic Health wanted the equivalent of a guarantee that it would not be exposed to the risk of wasted expenditure either in one form from Wyeth or in another form from the Court before it would decide to risk supplying its products.
729 For example, Mr Upiter gave evidence in this exchange:
In any event, can I suggest that the – you were seeking to have potential preparatory steps for a commercial quantity against the possibility that there was a sufficiently favourable outcome in the Alphapharm injunction hearing which meant, effectively, you were free to launch? Correct.
Courtesy of, effectively, a blessing of the court? Correct.
Is that right? Yes.
But if that didn’t happen, then you weren’t going to proceed with a commercial quantity, as far as you were concerned at least, anyway? Yes. Yes, if that didn’t happen we couldn’t proceed.
…
Your reference to planned launch activities was dependent upon the court in the Alphapharm proceedings indicating that hydrogel technology didn’t infringe? Yes. We had made these plans in anticipation of the decision being that that product – that the product did not infringe in the Alphapharm hearing, yes.
730 The board papers of 25 August 2009 record that the Alphapharm interlocutory injunction issue was key to Generic Health’s strategy because Alphapharm was running the non-infringement argument due to the use of hydrogel technology. The papers also refer to Generic Health’s strategy of getting an undertaking as to damages from Wyeth noting that stock was required “either to release into the market to force Wyeth to seek an injunction or to launch commercial quantities”. Again, the latter option concerned the potential strategy only if Alphapharm succeeded before the Court in its non-infringement argument.
731 Generic Health submitted that there:
…is nothing inconsistent between a strategy of attempting to obtain undertakings in a real world in which an interlocutory injunction is the overwhelmingly likely outcome and the proposition that in the hypothetical world Generic Health would have launched. It simply represents a pragmatic view in the circumstances of the real world. It does not bespeak any lack of desire or motivation actually to launch.
732 Generic Health noted that:
The board meeting on 25 August 2009 occurred prior to Generic Health receiving notice of the outcome of the Alphapharm injunction application. The minutes from the meeting record that Mr Upiter presented an outline of the venlafaxine litigation as per the board paper and the board discussed the timing of first stock availability.
At that point, Generic Health was still trying to find a way to supply to the market, but recognised that the outcome of the Alphapharm injunction application would determine whether or not Generic Health would be restrained. There is no suggestion any decision has been made not to launch because of the damages liability to Wyeth. Rather, the discussion centred on the timing of stock availability, presumably for the two scenarios referred to. All this conduct suggests that the board did not think it unlikely that a launch would be approved.
733 It may be accepted that Generic Health did not in fact decide not to launch because of the potential liability to Wyeth. However, what occurred shows a company which consistently decided not to expose itself to any potentially wasted expense if possible. Its strategy was not to expose itself to any expense, in effect, by volunteering not to launch its products provided it could get an undertaking from Wyeth. This is a form of “no risk” strategy. If its products did infringe, it would not be liable to Wyeth because it would not have sold any products. If its products did not infringe, Wyeth would be liable to it under the undertaking (or so it believed). It may be accepted that this strategy was adopted in the belief that it would be subject to an interlocutory injunction if it did launch its products, but it is apparent that this was a company unable and thus unwilling at this time to risk wasting a cent. It would not commit to launch its products to prompt an interlocutory application by Wyeth unless forced to do so. To do so would involve expense. So instead it represented that it was about to launch to try to prompt an interlocutory application by Wyeth. Having not managed to prompt Wyeth into action, it was forced to consider some kind of confined launch but it still did not want to expose itself to a full launch on the private market unless it had the comfort of knowing that Alphapharm’s non-infringement argument had succeeded.
734 To my mind, it is plain that this is not the kind of company that volunteers to be the first to list its products on the PBS and trigger the 12.5% price reduction in Efexor-XR knowing that if it did so other generics would follow suit shortly and thereby get the benefit of PBS listing and supply without taking on the risk of being liable to Wyeth, a risk which if it eventuated for Generic Health would almost certainly mean insolvency.
735 Generic Health submitted that “all that Mr Upiter cared about was that the legal advice at all stages was saying that Generic Health had a strong case that supply of its products would not be in breach of Wyeth’s patent. As he said in cross examination, his understanding was that “our product was not going to cause us an issue with the patent that was in existence””. This submission is divorced from the reality exposed by the contemporaneous records. Mr Upiter and Generic Health had initial advice that there were good prospects of both non-infringement and invalidity but were still motivated not to expose the company to any risk of wasted expenditure by committing to supply. It is obvious that this was the concern driving Generic Health. It did not want to spend any money launching its products if its expenditure would then be wasted because of an interlocutory injunction. It preferred the “no risk” approach of being restrained by consent in exchange for an undertaking. Why would it be inferred that the same company would have been willing to put its existence on the line by being the first to obtain PBS listing of its products? The answer that it did what it did because it believed an interlocutory injunction to be inevitable does not change the fact that everything it did was directed towards minimising and if possible eliminating any potentially wasted expenditure. Such a company is hardly likely to have indulged in the greatest potential waste of all by threatening its solvency for a short-term gain which it would have known would be likely to be quickly eroded by Sigma and Alphapharm. After all, but for the Sigma interlocutory injunction Sigma would have been supplying in the private market and but for the Alphapharm interlocutory injunction Alphapharm also would have been supplying in the private market. Generic Health would thus have known that it would not be the only generic on the private market. It would have known also that neither Sigma nor Alphapharm had obtained PBS listing on 1 August 2009 so that if Generic Health obtained PBS listing on 1 December 2009 it would have been solely or jointly responsible for the 12.5% price reduction in Efexor-XR. And it would have known that if it obtained PBS listing, then Sigma and Alphapharm would soon also obtain PBS listing, substantially reducing the potential upside to Generic Health.
736 On 25 August 2009 I granted the Alphapharm interlocutory injunction. For the reasons already given, if Wyeth had not applied for the Alphapharm interlocutory injunction on 14 August 2009 Generic Health would not have been in a different position than the position it was in. Generic Health would have it that the Alphapharm interlocutory injunction caused it not to seek PBS listing for its products and not to supply its products. It should be apparent that this is unsustainable. It was not the operation of the Alphapharm interlocutory injunction that caused Generic Health to act as it did. It had already decided that if Alphapharm’s non-infringement argument did not succeed it would do no more than the minimum necessary to prompt an interlocutory application by Wyeth. If Wyeth had not applied for interlocutory relief against Alphapharm on 14 August 2009, Generic Health would not have had the hoped for comfort of the Court refusing interlocutory relief against Alphapharm because of the strength of the argument of non-infringement of the method patent by hydrogel technology. It also would not have had the comfort from Wyeth that it was seeking either that Wyeth accepted that Generic Health’s products did not infringe or that Wyeth would be seeking interlocutory relief against Generic Health. Accordingly, if the Alphapharm interlocutory injunction is to be disregarded as against Generic Health (contrary to my view), Generic Health would have been confronted with the need to make a commercial decision for itself – it could still try to obtain an undertaking from Wyeth in exchange for not supplying its products or it could try to reach a deal with Wyeth enabling supply (the “no risk” approaches) or it could launch on the private market. PBS supply was not then an option because Generic Health could not have obtained any such listing before 1 December 2009 given that its products were not registered on the ARTG until 6 August 2009.
737 Given Generic Health’s actions to this point it is overwhelmingly likely (indeed in my view a certainty) that its first option would have been to try to reach a deal with Wyeth of some kind. I have no doubt based on the contemporaneous evidence that faced with only the method patent and the litigation Generic Health would have exhausted the possibility of a deal with Wyeth before it was willing to commit itself to any risk of wasted expenditure.
738 What actually occurred, of course, is that Generic Health did try to negotiate a deal with Wyeth. The only material difference is that as a result of the Alphapharm interlocutory injunction Middletons revised its view about the non-infringement argument but remained of the opinion that there were good prospects that the method patent would be held to be invalid. There was a dispute about what Mr Upiter understood at the time about infringement as opposed to invalidity of the method patent which, in my view, need not be resolved. I do not accept that Mr Upiter could recall one way or another what he thought about these kinds of details at the time so nothing is to be gained by exploring the substance of that issue. As discussed, even Mr Upiter’s insistence that he had in mind invalidity as well as infringement when preparing his affidavits was unconvincing.
739 Generic Health submitted that:
The position throughout August was that Generic Health was prepared to launch commercial quantities if the Alphapharm injunction was refused. That bespeaks a hope that Alphapharm might succeed in resisting the interlocutory injunction on the non-infringement grounds that Alphapharm and Generic Health had in common. That is, notwithstanding the view that it would be difficult to resist an interlocutory injunction on non-infringement grounds, both Generic Health and Middletons held out some hope on those grounds. The effect of the decision granting the injunction against Alphapharm was to remove that hope. Accordingly, Mr Watson was must less confident on the non-infringement angle as a basis to resist the injunction.
More importantly, this is another example of the grant of the injunctions altering the real world. The event that caused Mr Watson to be less confident was the interlocutory decision restraining Alphapharm. That event would not have taken place in the hypothetical.
Moreover, even if Mr Upiter understood that Mr Watson was less confident on “non-infringement” overall, he still plainly understood, consistently with all the advice he had received, that Generic Health had a strong case that it could supply its product without being liable to Wyeth for breach of patent. That is entirely consistent with the file note. Mr Watson’s advice, as recorded in the file note, was that Generic Health would likely win on invalidity and “could just launch”. However, doing so in a context where Alphapharm had just been restrained would encounter the downside that Mr Upiter identified in his email of 10 August 2009 (referred to above), namely the overwhelming likelihood was that Wyeth would apply for and obtain an injunction against Generic Health, such that Generic Health would be in a position where it had committed to purchase product it would be unable to sell.
In the hypothetical world in which Alphapharm had not been restrained, the overwhelming likelihood referred to above would not have existed and the prospect that Generic Health “could just launch” would not have had the downside that it did in the real world.
740 These submissions overlook the fundamental fact that Generic Health’s willingness to undertake a full-scale launch on the private market was premised on having received comfort from the Court about the non-infringement argument as a result of the Alphapharm injunction having been refused. There is no relevant hypothesis in which this would have occurred. Further, if Wyeth had not sought the interlocutory injunction against Alphapharm on 14 August 2009 (which is a relevant hypothesis) a full-scale private launch would still have downsides for Generic Health. It would know that, as it had for Alphapharm, Wyeth would seek final relief against it for infringement of the method patent. As such, it would have had to confront the risk it had not had to confront because of the anticipation of being subject to an interlocutory injunction – the risk that it would commit itself to substantial expenditure and make money in the short-term but then be subject to a final injunction and exposure to damages.
741 As Mr Upiter acknowledged, if confronted by the need to decide what to do Generic Health would have required more detailed legal advice and that advice with additional information about all of the potential benefits and risks would have been put to the board for decision. Accordingly, the submission for Generic Health that but for the Alphapharm interlocutory injunction there would have been no downside to a “just launch” approach is contrary to the evidence and to commercial common sense. Mr Upiter, for example, acknowledged that he would need information of this kind and that the board would have been informed that its potential exposure to Wyeth if it was first to obtain PBS listing for its products would include the effect of the 12.5% price reduction in Efexor-XR for the remaining eight years of the method patent. He acknowledged that this risk, if it eventuated, would put Generic Health into insolvency. To suggest there was no downside to a “just launch” approach in the face of the evidence is untenable.
742 As Wyeth also submitted:
…the evidence demonstrates that Lupin was not even prepared to provide an open ended guarantee in support of private launch. The likelihood that it would have risked its investment and its $3 million loan on a first PBS listing is remote in the extreme. The putative benefits compared to the clear damages exposure would render such a decision for any Director commercially unwise to say the least. The evidence demonstrates that the [company] was conservative, concerned as to saving money at every step and concerned about risk taking.
743 This submission must be accepted given the evidence. The notion of Generic Health as a company with a voracious appetite for risk in 2009 cannot be reconciled with the company’s actual conduct in 2009 which was not only about avoiding an interlocutory injunction but was also about avoiding any risk of potentially wasted expenditure if at all possible because it knew that it could not afford it.
744 On 27 August 2009 Wyeth’s lawyers wrote to Generic Health’s lawyers referring to the Alphapharm judgment and repeated that any steps by Generic Health to market or supply its products or to sell or assign its ARTG registrations would be done in “full knowledge” of the method patent and at Generic Health’s own risk. If there had been no Alphapharm judgment Generic Health’s lawyers may well still have believed the non-infringement case to be good but this is immaterial because in any event they believed the invalidity case also had good prospects. The Alphapharm interlocutory injunction thus could not have affected anything apart from Generic Health’s view about its prospects of avoiding an interlocutory injunction. But even in that regard Generic Health had been advised by two different law firms that it would not be able to avoid an interlocutory injunction. The Alphapharm interlocutory injunction merely confirmed that advice. Further, had Wyeth not sought the Alphapharm interlocutory injunction on 14 August 2009 Wyeth’s lawyers would still have been saying to Generic Health that Generic Health’s products infringed the method patent and that if Generic Health marketed or supplied its products or sold or assigned its ARTG registrations Generic Health would be acting in “full knowledge” of the method patent and at Generic Health’s own risk.
745 On 31 August 2009 Middletons wrote to Wyeth’s lawyers saying that Generic Health expected to be in a position to commence selling its products in Australia in mid-September 2009 and based on Wyeth’s position, the contents of the correspondence between the lawyers, and the Alphapharm judgment, Generic Health assumed that Wyeth would apply for an interlocutory injunction if Generic Health commenced supply. As Generic Health anticipated that an interlocutory injunction would be granted against it based on the Alphapharm judgment (which, in fact, Generic Health had anticipated in any event) it offered to be restrained on an interim basis in exchange for an undertaking as to damages. Mr Upiter confirmed that the board had still not decided to supply its products and that this offer reflected Generic Health’s object of avoiding expenditure on litigation.
746 Given that everything Generic Health had done was about avoiding potentially wasted expenditure if at all possible, it follows that the position would have been the same on 31 August 2009. Irrespective of the Alphapharm interlocutory injunction Generic Health would have tried to extract a “no risk” deal from Wyeth in preference to taking even the perceived low risk of commencing supply only to be ultimately restrained and liable to Wyeth for damages. In my view, the only thing that ultimately would have forced Generic Health’s hand is the necessary assumption that, despite its best efforts, Wyeth would not do a deal with Generic Health. Generic Health would then be forced to decide for itself whether it was prepared to launch at risk. These efforts, of course, would have taken time. Generic Health, it is clear, would not have lightly given up on trying to do a deal with Wyeth. This said, if anticipating an interlocutory injunction can be disregarded, I do not doubt that Generic Health would have been canny enough to hedge its bets in the sense that, if it knew that Sigma had obtained PBS listing on 1 August 2009 (my conclusion if the generics are right that conduct anticipating an interlocutory injunction must be disregarded), it would have protected its position by applying for PBS listing for 1 December 2009 knowing that such an application would be withdrawn if it did a deal with Wyeth.
747 To return to actual events, however, Mr Upiter’s subsequent email of 3 September 2009 to Mr Dhawan and Dr Mumtaz said that there had been no response from Gilbert & Tobin to the letter of 31 August and that Mr Watson “has been preparing to issue proceedings to begin formal court proceedings in order to secure the undertaking to damages ASAP”. It is the last part of this email which is telling. It is consistent with my view that Generic Health was focused on a deal and not supply because it needed to avoid potentially wasted expense.
748 Generic Health submitted this:
Mr Upiter was asked in cross examination to confirm that the purpose of the proceedings was to secure the undertaking and that there was no board approval to launch at that point in time: Tr 744.35-47. Although Mr Upiter answered yes to the first question, in fact the purpose of the proceedings could only be to challenge the validity of the patent (as occurred subsequently). Those proceedings would then provide a forum in which Wyeth could cross claim for infringement (as they did against Sigma and Alphapharm) and obtain the injunction. The fact that no board approval to launch was in place at that time does not take matters very far. Once the Alphapharm injunction was granted, Generic Health understood that there was no prospect that it would not be restrained provided Wyeth sought to do so, which Generic Health fully expected. The immediate focus was therefore to put an end to the waiting by procuring Wyeth to seek an injunction. It does not address what would have happened in a hypothetical world in which the Alphapharm injunction had either not been sought or had been sought and refused.
749 As discussed, it cannot be assumed that the Alphapharm interlocutory injunction would have been refused. Further, the submission fails to recognise that if Wyeth had not sought the Alphapharm interlocutory injunction Generic Health would still have been in the same position of needing to avoid any potentially wasted expenditure. The litigation itself created this risk. This is why Generic Health would have sought to do a deal with Wyeth regardless of the Alphapharm interlocutory injunction. Accordingly, leaving aside the Sigma interlocutory injunction’s impact on Generic Health’s supply to Sigma, Generic Health was in materially the same position up to this time as it otherwise would have been in.
750 Gilbert & Tobin’s response on 4 September 2009 was that Generic Health’s proposal appeared satisfactory save for some matters including the need for proceedings to be commenced. Consistent with its object of avoiding expense if at all possible, Middletons noted to Generic Health in an email of 15 September 2009 that the Alphapharm and Sigma proceedings were fixed for hearing in April 2010, the invalidity case appeared to be “quite strong”, and ideally the questions of validity and non-infringement would be decided in the Alphapharm proceedings and Generic Health would not need to prepare evidence or be involved. Rather, it would seek discovery and mediation in its proceedings. The consistent focus on avoiding expenditure in the litigation is again obvious.
751 There was subsequent correspondence between the lawyers negotiating the terms of Generic Health’s proposed undertakings which need not be discussed other than to say it is necessary to assume that, ultimately, the deal which caused Generic Health not to defend Wyeth’s interlocutory application and to enter into consent orders on 10 November 2009 would not have been done.
752 An email from Mr Upiter of 1 October 2009 said that others within the company “would be aware that a court date has been set for April 2010 to hear the Alphapharm and Sigma proceedings and this will dictate our outcome as well”. This would undoubtedly have been the same view Generic Health would have held irrespective of any application by Wyeth for an interlocutory injunction.
753 In early October 2009, Generic Health cancelled the orders with Pharmathen on the basis it had reached agreement for an injunction and cross-undertaking. The report to the board for October 2009 recorded the agreement with Wyeth and the interlocutory orders made on 10 November 2009 reflected the terms of that agreement. This report again reflected Generic Health’s consistent focus on avoiding expenditure if possible. It also reflected Generic Health’s intention of seeing whether it could negotiate a deal with Wyeth about the litigation overall, referring to its proposal to seek mediation and its object if the matter did not settle at mediation of having its proceedings deferred until after the Sigma and Alphapharm proceedings had been decided (which, of course, was yet another costs savings measure). The board minutes record the decision to ensure all expenses associated with the litigation were “maintained” (meaning recorded), again reflecting the reality of Generic Health’s position that no money could be spent if there was any possible way of avoiding it.
754 All of this supports my conclusions that disregarding the interlocutory injunctions Generic Health would have been in materially the same position in respect of its products as it was in at this time – it would be trying to negotiate a resolution of the litigation with Wyeth without exposing itself to the risk of any wasted expenditure.
755 What are the ultimate possibilities and probabilities for Generic Health in circumstances where its business was to make profits from generic pharmaceuticals and it received ARTG registrations for its products only on 6 August 2009? This question is to be answered in circumstances where:
(1) Generic Health was in a parlous financial position so that the kind of exposure being the first generic to obtain PBS listing would involve would make it insolvent if the method patent was valid;
(2) all of Generic Health’s actions since it became aware of the method patent show that it was unwilling to take a risk of potentially wasted expenditure if possible and that it saw the best option for avoiding potentially wasted expenditure as doing a deal with Wyeth in relation to both interlocutory and final relief;
(3) Generic Health could not obtain immediate supply of its products from Pharmathen and, at the earliest, might have been able to import its products from mid-September 2009;
(4) Generic Health would know that Wyeth had filed proceedings against Sigma and Alphapharm for interlocutory and final relief (in the form of final injunctions and damages) although it is also to be assumed that Wyeth had not prosecuted its applications for interlocutory relief on 22 May 2009 against Sigma or on 14 August 2009 against Alphapharm;
(5) Generic Health would thus know that if it moved towards supply Wyeth would file infringement proceedings against it at least seeking final relief;
(6) Generic Health would not have had the benefit of an interlocutory judgment in favour of Alphapharm giving it comfort that its products did not infringe the method patent, indeed, there would be no interlocutory judgments;
(7) Generic Health was aware that the Sigma and Alphapharm proceedings would be heard in April 2010 and that the Alphapharm proceedings would decide both the infringement and validity issue for Generic Health;
(8) it must be assumed that Generic Health would be aware that Sigma and Alphapharm were supplying their products on the private market so that Generic Health would be competing with them for a share of the private market in circumstances where it would have entered the market later and was a far smaller player in the generics industry ;
(9) Generic Health knew that if it was the first generic to obtain PBS listing at least Sigma and Alphapharm would then obtain PBS listing about three months later;
(10) Generic Health’s board would have detailed legal advice to the effect that its prospects of success about avoiding liability to Wyeth were good based on either or both of the non-infringement and invalidity arguments but that advice would have also made it clear that the course of litigation can never be predicted with certainty, there could be no guarantee of success, the risk of liability could not be described as trivial, negligible or insignificant, and if Generic Health succeeded it could be expected that Wyeth would appeal. This advice would have estimated Generic Health’s potential exposure if the method patent was valid as in excess of $100 million if it was the first to list its products on the PBS. The advice also would have provided a detailed estimate of legal costs, including of any appeal, which would have been many hundreds of thousands of dollars if the proceedings could not be settled with Wyeth. The board also would have had an analysis of the potential financial returns to Generic Health of supply estimated on the basis that Sigma and Alphapharm were already in the private market and would have captured shares of it before Generic Health could enter and would seek PBS listing if Generic Health obtained PBS listing so that any benefit to Generic Health of being the only generic on the PBS would be short-lived; and
(11) Generic Health held eight ARTG registrations. It was actively considering selling some of those ARTG registrations and, indeed, did so in 2010 when it assigned two to Apotex.
756 Generic Health submitted that:
Generic Health’s strategy simply involved avoiding spending unnecessary money and they saw an opportunity where it didn’t need to spend money… To the extent the cross-examination [of Mr Upiter] was directed at suggesting that in the hypothetical world, Generic Health would not have spent money necessary for it to launch and vindicate its rights against Wyeth, such a suggestion should be rejected.
757 According to Generic Health:
Initially Generic Health had a plan to launch venlafaxine on the private and PBS markets. It had spent money on obtaining bioequivalence studies and had obtained supply. There is no doubt it would have launched on the private market and listed on the PBS in accordance with the plan… Three things supervened, namely discovery of Wyeth’s patent, commencement of litigation and the obtaining of the injunctions. Following those supervening events, Generic Health did not launch. Neither the discovery of the patent nor the commencement of litigation prevented Generic Health’s launch; the injunctions did.
758 These submissions cannot be reconciled with the evidence. Nor can Generic Health’s case theory that because it was not profitable and had ongoing liquidity problems it should be inferred that the potential benefits of supplying both on the private market and as soon as possible under the PBS would have caused it to do so irrespective of whether it would be first to obtain PBS listing. Generic Health never acted in any way that exposed it to a risk of even some wasted expenditure if it could avoid it, no matter what the possible short-term benefit. The idea that it would have acted so as to expose itself to even a perceived low risk of an existential threat by reason of insolvency is untenable.
759 Generic Health submitted that:
As to detriment, given Generic Health’s financial state, it may be accepted that it would have difficulties meeting a damages liability to Wyeth under any of the scenarios and certainly would not be able to meet a damages liability to Wyeth if it triggered the PBS price drop. One consequence is that in practical terms the potential detriment to Generic Health was the same for the greater benefit of PBS listing as it was for the comparatively smaller benefit of a private only launch.
760 Generic Health repeated this submission in these terms:
Sigma and Alphapharm were large, established and profitable companies. There was a difference for those companies between paying say a few millions of dollars in damages versus potentially paying more than one hundred million dollars in damages. In Generic Health’s case, liability of either order of magnitude would likely be beyond Generic Health’s capacity to pay. From Generic Health’s perspective, the increased likely reward from a PBS listing comes without additional risk. This is not to suggest that Generic Health adopted a cavalier attitude to creditors; but rather to recognise that if the same risk of infringement applies to the chance to obtain a larger benefit for the same practical downside if the risk eventuates, then a rational commercial entity will take the chance for the larger upside.
761 I have already rejected that approach above. It is not a form of thinking which Mr Upiter adopted. Nor would it be the thinking of any reasonable director and certainly not directors who represented investors in Generic Health. It may be accepted that, as was submitted, “the primary purpose Generic Health was to compete and grow in the generic pharmaceutical industry and become profitable so as to generate return for its shareholders”. But Generic Health’s further submission that “[h]aving invested in a start-up generic, they were obviously not risk averse. More fundamentally, it would be contrary to the very purpose of the investment to sit back and let rare opportunity to enter early to market with a blockbuster drug simply slide away” is unpersuasive, at least to the extent it is said to support the notion of Generic Health being willing to be the first to obtain PBS listing of its products. The risk of investing in a start-up company is one thing. The risk of deliberately taking any course of action which meant the company would be insolvent if the method patent was valid is another. Moreover, it was not the purpose of Generic Health to knowingly expose itself to damages and wasted expenditure by potentially infringing patents.
762 Similarly, it may be accepted that “there is no principle of law to the effect that the fact that a business decision could result in insolvency if it goes badly means that the business decision cannot be taken or that directors breach their duties if they take the decision and the risk eventuates”, just as it may be accepted that a “a sensible evaluation of the magnitude of the reward and risk and the probabilities of either eventuating” is required. But where the risk is to the company’s solvency and is avoidable, as it was here, the approach Generic Health now says it would have taken is far removed from commercial sense.
763 I have explained already why Mr Upiter’s evidence is unreliable. I also do not accept that the fact that Dr Mumtaz and Mr Dhawan were aware of the legal advice to the effect that Generic Health’s ultimate prospects were good would have caused it to “just launch”. The submission that they reviewed the Middletons’ letter of 29 July 2009 and agreed it reflected Generic Health’s position and could be sent and thus agreed that Generic Health’s position was that it intended to launch unless Wyeth sought to restrain it from doing so should not be accepted. The instructions to Middletons were disingenuous. Generic Health had not decided to launch and Mr Upiter knew that board approval was required to do so. It is obvious from the evidence that Generic Health was fishing for an undertaking as to damages from Wyeth without being willing to commit itself to doing anything (in contrast to Alphapharm which was willing to enter the market in support of its launch representations to Wyeth albeit in a confined manner).
764 It is also inaccurate to suggest that the fact that Mr Dhawan and Dr Mumtaz did “not express any resistance to, or concern or surprise about, Generic Health’s plan in late August 2009 to launch on the private market in the event that the Alphapharm interlocutory injunction was refused” is material. That plan was predicated on the Court having decided that hydrogel technology would not infringe the method patent. Generic Health’s submission that they or Mr Upiter would understand that this would have been an interlocutory decision only so that there was still risk attached to the plan fails to grapple with reality. They and Mr Upiter understood that if the Court rejected Wyeth’s application for interlocutory relief against Alphapharm because its products used hydrogel technology then it was a virtual guarantee that Generic Health’s products would also be found not to have infringed the method patent. Mr Upiter in fact said that he understood such an interlocutory judgment to be the equivalent of a “declaration of non-infringement”. A declaration of non-infringement would have been taken as a virtual guarantee that Generic Health’s supply would be risk free but Generic Health would never have been in that position before the Full Court’s decision in October 2011.
765 Accordingly, there is no basis to infer that “Dr Mumtaz and Mr Dhawan would have communicated their views about the strength of Generic Health’s position to the board in unison with Mr Upiter”. They were members of a board that repeatedly sought to avoid the risk that the company might expend money that could be wasted if possible.
766 It is also not the case that if Sigma and Alphapharm were supplying their products and had not been restrained by Wyeth, Generic Health would believe that “Wyeth was not confident enough in its own patent to apply to restrain entry into its market”. On my analysis, up to 1 December 2009 Sigma and Alphapharm’s supply would be in the private market only so that at this time Generic Health would have known that in the face of the method patent Sigma and Alphapharm were not prepared to take the risk of triggering the 12.5% price reduction in Efexor-XR. Further, the litigation in which Wyeth was suing for infringement of the method patent existed. Generic Health also would not have received legal advice that Wyeth must believe the method patent to be invalid.
767 Contrary to Generic Health’s submissions, the objective evidence is overwhelmingly against its overall “just launch” case theory. I respond as follows to the points it made in closing submissions.
768 Generic Health said that “the concern at the 23 June board meeting was with limiting the upside rather than exposure to potential downside. That is, consistently with the position of Generic Health as a start-up player in the highly competitive generic pharmaceutical industry, the stated concern of the board was to maximise the potential for benefit to Generic Health rather than minimise the potential for harm”.
769 In fact, the concern of the board at this time, understood in context, was not to confine its options until it had legal advice. This was commercially sensible. For Generic Health to be the first to obtain PBS listing, in contrast, would have been commercially irrational.
770 Generic Health said that “in the period immediately prior to finding out the outcome of the Alphapharm injunction, Generic Health was in fact planning a full launch on the private market (by promoting to 2000 pharmacies by phone and 5000 pharmacies by facsimile)”.
771 As noted, this plan was on the basis that if Alphapharm succeeded in resisting the interlocutory application, Generic Health would have the benefit of an interlocutory judgment confirming its non-infringement argument. Generic Health would never have had this benefit.
772 Generic Health also submitted that the “refusal of the Alphapharm interlocutory injunction would not have eliminated the risk that at a final hearing Wyeth might prevail. That risk would have remained notwithstanding any interlocutory decision the Court might make. And yet the plan (in respect of which neither Mr Dhawan nor Dr Mumtaz raised a word of caution) was to take the risk of being liable at final hearing and launch immediately on the private market”.
773 This submission does not engage with the reality that Generic Health was only considering this strategy if it achieved, as Mr Upiter put it on 20 August 2009, a “declaration of non-infringement”. In other words, a favourable decision from the Court about hydrogel technology not infringing the method patent was essential to this option. It was essential because Generic Health saw this as something which would make its decision the equivalent of risk free which it never would have been. As also discussed, Generic Health would never have received what it described as a “judicial green light”, at least not until the Full Court’s decision on 28 October 2011.
774 Generic Health submitted that it “did in fact launch on the private market in November 2011 and commenced selling on the PBS on 1 April 2012. Each of those dates was after the Full Court had allowed the appeal but at a time when the revocation orders had been stayed pending the determination of the application for special leave to appeal to the High Court (which occurred on 11 May 2012). Accordingly, at that time, Generic Health was prepared to launch and list on the PBS notwithstanding that the risk that it would infringe valid patent claims still remained. The risk was probably small given the Full Court’s decision and the vagaries of special leave applications, but it still existed”.
775 However, there is no meaningful comparison between the risk of supplying after a fully reasoned judgment of the Full Court holding the method patent to be invalid and the small prospect of a special leave application being granted (let alone an appeal succeeding) and the risk with which Generic Health would have been confronted in 2009 and 2010. The Full Court judgment would have given Generic Health the confirmation that it was seeking from the outset, first from Wyeth and then from the Alphapharm interlocutory injunction, that it would be at effectively no risk if it commenced supply or a risk so low that it could be described as trivial. This is confirmed by the position of Alphapharm. As we know Alphapharm was unwilling to take on any risk of triggering the 12.5% price reduction in Efexor-XR in the face of the method patent and anticipated litigation. Yet after the Full Court’s decision in October 2011 Alphapharm also applied for PBS listing of its products and, along with Generic Health and other generics, Alphapharm’s products were listed on the PBS on 1 April 2012. Alphapharm thus perceived that there was effectively no risk after the Full Court’s decision. Generic Health must be inferred to have held the same view.
776 Generic Health submitted that “in January 2012, Generic Health did a full commercial launch of the drug Yasmin notwithstanding that there was an originator patent which it knew about. The result was that Generic Health was found liable to damages for patent infringement. No doubt there are differences between that case and this case. In particular, Yasmin was not a PBS listed drug so the magnitude of the risk and potential gain were likely different from that for venlafaxine. Nevertheless, the evidence is probative of a corporate mindset which does not shy away from risk of patent infringement if justified by the risk/reward calculus”.
777 This submission is without merit. I have no idea what Generic Health believed about Yasmin or the generic version supplied by Generic Health. Generic Health might have believed that the risk to it about its product was non-existent or trivial. The conduct was also in 2012. Mr Upiter left Generic Health in 2012. It is not clear that Mr Upiter had anything to do with Yasmin. It is also apparent that Yasmin was not listed on the PBS. All supplies were in the private market.
778 In the circumstances identified above, I consider that the Alphapharm interlocutory injunction made no material difference to Generic Health. Generic Health’s consistent and overwhelming objective was to avoid the risk of wasted expenditure if possible. Its preferred course of doing a deal with Wyeth would have been pursued by it irrespective of the interlocutory injunctions.
779 Nevertheless, on my approach, it must be assumed that on 10 November 2009 the deal was not made and Wyeth did not prosecute its application for interlocutory relief against Generic Health. Generic Health would thus then know that it would not be the subject of an interlocutory injunction but also would not have the benefit of an undertaking as to damages from Wyeth. In my view, given the evidence, there is no possibility that Generic Health would not have pursued this objective of doing a deal with Wyeth up to 10 November 2009. This necessarily means that Generic Health would not have decided to supply its products at any time before 10 November 2009.
780 As a result, Generic Health’s claims under the Alphapharm interlocutory injunction undertaking must fail on the facts (and also should fail as a matter of principle, as discussed above).
781 Given the conclusions I have reached, it is thus necessary to deal with Generic Health’s position from 10 November 2009 until 1 December 2009 and from 1 December 2009 having regard to the possibility that Sigma would have obtained PBs listing of its products on that date.
782 Having regard to the matters discussed above and leaving aside the prospect of Sigma having obtained PBS listing on 1 December 2009, it is effectively certain that Generic Health would not have applied for PBS listing given that its overwhelming objective was to do a deal with Wyeth to resolve the litigation altogether if necessary through a process of mediation within the context of the anticipated (or, by mid October 2009, actual) litigation. This conclusion is reinforced by the fact that Generic Health knew that the Sigma and Alphapharm proceedings would be heard in April 2010 and the Alphapharm proceedings would decide Generic Health’s case for it, believed that if it was held liable to Wyeth for infringement and had caused the 12.5% price reduction in Efexor-XR it would become insolvent, would know that it was not the first generic to the market (Sigma and Alphapharm would have been supplying on the private market), and would know that any benefit to it of PBS listing would be short-lived because Sigma and Alphapharm at least would rapidly follow (and would also be protected from the potential liability of triggering the price reduction). I consider that every action Generic Health took after it became aware of the method patent is irreconcilable with the notion that but for the interlocutory injunctions it would have sought to list its products on the PBS if, in so doing, it would or might cause the 12.5% price reduction in Efexor-XR. In other words, the chance of Generic Health being the first to obtain PBS listing, in my view, is merely speculative and less than 1% and must be disregarded.
783 The unreality of Generic Health’s case theory to the contrary is apparent from submissions such as this:
The fact that Sigma and Alphapharm were unwilling to take the risk [of PBS listing] (if that were the case) would not have dissuaded Generic Health from taking the risk. Quite the opposite. It would have provided Generic Health with an opportunity to be first to PBS list and therefore gain additional profit and stature within the market without increased practical risk. If anything, Generic Health would have been more likely to PBS list in circumstances where Sigma and Alphapharm had not done so.
The probability is that the continued inaction of Wyeth in the face of an initial supply by Generic Health would have emboldened Generic Health to launch commercial quantities. It could scarcely have had any other effect. Once Generic Health decided to launch commercial quantities, it would have decided to list on the PBS because the additional rewards of a PBS listing would come at no greater commercial risk for Generic Health.
784 I consider this submission to be contrary to the evidence and commercial sense at every level. Generic Health would hardly gain stature by being the company that first listed so as to trigger the 12.5% price reduction in circumstances where it was obvious that the generics who had already captured greater shares of the private market than Generic Health could hope to do (Sigma and Alphapharm) would swiftly follow and obtain the benefit of what could only have been perceived as a form of irrational self-sacrifice by Generic Health. Any additional profit to Generic Health also would have been reduced by the fact that Sigma and Alphapharm had already been supplying on the private market for months and would soon also PBS list if Generic Health did so. Inaction by Wyeth would have been confined to not seeking the interlocutory injunction on 10 November 2009, but Generic Health would have been left in no doubt that Wyeth considered the method patent to be invalid and infringed and would be seeking to enforce it against Generic Health. Further, it is apparent that nothing in fact “emboldened” Generic Health to take any risk when it thought it might be able to avoid it.
785 There is another way of looking at this issue. Generic Health could not have obtained PBS listing on 1 August 2009 because it did not obtain its ARTG registrations until 6 August 2009. At best, it could have applied by 1 September 2009 to obtain PBS listing on 1 December 2009. In all of the circumstances can it rationally be concluded that the chance of Generic Health being the first to seek PBS listing on 1 December 2009 was greater than the chance of Sigma having done so? If the answer to this question is no (as I consider it must be) then the issue of Generic Health seeking PBS listing is also moot as it could never have obtained PBS listing before Sigma. It is only if it can rationally be concluded that the chance of Generic Health having obtained PBS listing on 1 December 2009 was greater than the chance of Sigma having done so that this issue is relevant at all.
786 Further, if I am right that the chance of Sigma obtaining PBS listing on 1 December 2009 was 20% then it seems apparent that the prospect that Generic Health would have done must have been far less, and at least approaching negligible. After all, Sigma had purchased and paid for stock, was desperate for a “win”, was the first mover in the generic market, had the expertise, resources and market reach to capture and (unlike Generic Health) retain material market share from Wyeth, and had far more financial depth than Generic Health despite its financially poor performance. In contrast, Generic Health also needed a “win” but was not holding stock it had paid for, was not the first or even the second mover into the market, was small, was in a parlous financial position, and could not have retained market share from PBS listing once Sigma and Alphapharm entered the PBS market.
787 For these reasons, the entire issue of Generic Health having been the first to obtain PBS listing of its products is beside the point. It could never have done so by 1 August 2009. The idea that the chance of it having done so by 1 December 2009 exceeded the chance of Sigma having done so by the same date is a nonsense in the circumstances as they existed or would have existed. None of the evidence supports the inference.
788 What then of private supply by Generic Health from 10 November 2009 to 1 December 2009? Everything that had occurred up to that point reflects Generic Health’s risk avoidance strategy. This is because up to 10 November 2009 Generic Health perceived, and would have perceived in any relevant hypothetical circumstance, that it could avoid any risk by reaching a deal with Wyeth. From 10 November 2009, however, the position should be inferred to be different. From that time, it should be inferred Generic Health knew that it could not do a deal with Wyeth. It could then do nothing and watch the value of its investment in its products erode as Sigma and Alphapharm continued to supply their products. Or it could enter the market itself and do what it was in business to do, supply generic products and compete with Sigma and Alphapharm as best as it could. While the matters referred to above might be seen as weighing against the inference that Generic Health would have decided to supply its products on the private market from 10 November 2009 that conclusion would be unfair to Generic Health. The circumstances existing before 10 November 2009 allowed it to avoid the risk of wasted expenditure. But by 10 November 2009 it must be inferred that Generic Health would have exhausted the option of a potential deal with Wyeth. On that date, it must be taken that Generic Health would know that there would be no interlocutory injunction, no undertaking as to damages, and no risk-free deal with Wyeth.
789 Generic Health submitted that:
If neither Sigma nor Alphapharm had launched, Mr Upiter’s evidence is that he would have caused a small quantity of product on to the private market in order to trigger a reaction from Wyeth: Upiter2, [48]. The cross-examination did not attempt to controvert that evidence. The [this] was the strategy in place in the real world if Wyeth did not otherwise apply for an interlocutory injunction against Generic Health. There was no resistance to that strategy in the real world and there would have been none in the hypothetical world.
790 This submission misses the point that the reaction Generic Health wanted from Wyeth at the time was to do a deal which would remove the prospect of a launch at risk altogether. Further, in any relevant hypothesis, it must be taken that at this time Sigma and Alphapharm would have launched and would have been supplying to the private market for months.
791 However, to adapt from another submission put for Generic Health, knowing that its preferred option of doing a deal with Wyeth was impossible from 10 November 2009, would Generic Health then have “sat on the sidelines and watched Sigma and Alphapharm take advantage of the opportunity” when it had ARTG registrations for its products, would believe that provided it was not the first generic to obtain PBS listing it could not be liable for the associated price reduction in Efexor-XR, and with the benefit of legal advice would have assessed its risk of liability to be low, and for private market supply would quantify its risk as being related to the sales it would make (even if at Wyeth’s higher margin)?
792 In all of the circumstances, I consider that the dominating factor for Generic Health would be similar to that for Alphapharm. These companies were in the business of competing for market share for those products which they could supply. Generic Health had made a substantial investment of time and money in its generic venlafaxine products. If Sigma and Alphapharm were supplying their products on the private market then I consider that once it was forced by circumstance to make a decision about supply, as it would have been on 10 November 2009, it is more likely than not that Generic Health would have followed suit. In all of the circumstances as identified, and weighing up the position of Generic Health compared to Alphapharm (that is, Generic Health was smaller, under financial pressure and later to the market), I would assess the probability of Generic Health having launched its products on the private market from 10 November 2009 to be 80%. This is because by then it would be out of options other than watching Sigma and Alphapharm supply and the drive to compete when Sigma and Alphapharm would have been in the market would have been strong. Combined with the perceived low risk of liability to Wyeth, I consider that when forced to do so as it would have been from 10 November 2009, Generic Health would have done what it was in business to do, supply its generic products for the purpose of making a profit albeit confined to the private market. Accordingly, I assess the chance that Generic Health would do nothing to be 20%.
793 Further, if Sigma had obtained PBS listing on 1 December 2009 then Sigma, not Generic Health, would have triggered the 12.5% price reduction. As such, one of the primary risks of PBS listing would not have existed for Generic Health. I consider that Generic Health’s position would then be equivalent to that of Alphapharm. It would be strongly motivated to seek PBS listing and it should be inferred that it would have taken the first opportunity to do so after 1 December 2009. This inference is supported by the fact that in all likelihood Generic Health would have a good idea what Sigma was up to given it was Sigma’s supplier. In common with Alphapharm’s position, I assess the likelihood of Generic Health seeking PBS listing in time to be listed on 1 March 2010 as higher if it had been supplying its products in the private market than not. In these hypothesised circumstances I can see no operative difference between the positions of Generic Health and Alphapharm and thus I assess the relevant possibilities of Generic Health seeking and obtaining PBS listing on 1 March 2010 as 90% if it had been supplying its products in the private market and 80% if it has not been supplying its products in the private market.
794 As noted, to the extent it was submitted for Generic Health that but for the interlocutory injunctions it should be assumed that the first instance judgment would have been different, I disagree. Nothing supports that submission. There is no reason to assume or infer that anything would have happened other than that I would have provided the same reasons for judgment to the parties as I did on 23 September 2010 and would have published the same reasons as I did on 8 November 2010.
795 Apart from this I consider that Generic Health would otherwise have been in the same position as Sigma and Alphapharm. It would not have been concerned its products might not be listed on the PBS. It would not have been concerned by the need to give an assurance of supply and would have done so. It would have no concern about the statutory guarantee of supply provisions. No anticipation of what Wyeth might do in competitive response to generic supply or otherwise would have weighed on Generic Health’s mind.
Generic Health’s supply to Sigma
796 Insofar as the Sigma interlocutory injunction is concerned, Generic Health would be a person adversely affected by the interlocutory injunction only if it is proved on the balance of probabilities that Sigma would have ordered some additional products from Generic Health. This in turn depends on the likely success of Sigma’s private market supply of its products which is discussed separately below.
Generic Health’s supply to other generics
797 I also deal separately with Generic Health’s claims in relation to the lost opportunity of potential supply to Apotex, Sandoz and Ascent.
Summary of interim conclusions
798 My interim conclusions about Generic Health, accordingly, may be summarised as follows:
(1) Generic Health has proved on the balance of probabilities that the Generic Health interlocutory injunction caused it to lose various opportunities of some value associated with the ARTG registration of its products because the injunction prevented it from exercising, from time to time and as it saw fit, the rights associated with ARTG registration. The value of this opportunity depends on the probabilities and possibilities of what Generic Health would or might have done.
(2) If Generic Health needed to prove on the balance of probabilities that it would have applied for PBS listing of its products but for the Generic Health interlocutory injunction, then Generic Health has failed to prove that putative fact.
(3) If Generic Health needed to prove on the balance of probabilities that it would have supplied its products on the private market but for the Generic Health interlocutory injunction, then Generic Health has proved that putative fact.
(4) If, as I consider, Generic Health does not need to prove (2) or (3) because it has proved (1) on the balance of probabilities, then the probabilities and possibilities are as follows:
(a) Generic Health would have applied for PBS listing of its products before another generic had PBS listed its products: 0%.
(b) Generic Health would have supplied its products to pharmacists on the private market from 10 November 2009: 80%.
(c) Generic Health would not have supplied its products on the private market from 10 November 2009: 20%.
(5) Assuming Sigma had obtained PBS listing on 1 December 2009 the probabilities and possibilities are:
(a) if Generic Health was supplying its products on the private market (the 80% probability):
(i) Generic Health would have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could (in my view, in time to obtain PBS listing by 1 March 2010): 90%; and
(ii) Generic Health would not have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could but would have continued to supply its products on the private market: 10%;
(b) if Generic Health was not supplying its products on the private market (the 20% possibility):
(i) Generic Health would have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could (in my view, in time to obtain PBS listing by 1 March 2010): 80%; and
(ii) Generic Health would not have re-applied for PBS listing for the purposes of supply under the PBS as soon as it could but would have continued not to supply its products: 20%.
The competing claims about other generics
799 Generic Health and Pharmathen contended that but for the interlocutory injunctions Generic Health would have supplied Apotex, Sandoz and Ascent with products sourced from Pharmathen.
800 All of the generic claimants also contended that if another generic had obtained PBS listing of venlafaxine products they would have applied for and obtained PBS listing of their own products as soon as possible thereafter for the purpose of supplying their products under the PBS (a proposition which I accept). The primary position of Sigma and Alphapharm, however, was that there was no evidence to support the inference that any other generic would have been on the market but for the interlocutory injunctions with the consequence that the compensation payable to them should be assessed on the basis of competition between Wyeth, Sigma, Alphapharm and Generic Health alone for the period before the Full Court’s orders in November 2011.
801 Wyeth’s position was not straightforward. Its primary contention was that if, as it contended, Sigma’s private market supply would have failed over time and Sigma would not have applied for PBS listing for the purpose of PBS supply and Alphapharm would not have entered the market at all (or only to a desultory extent in the private market which also would have failed over time) and Generic Health would not have entered the market at all, then it must also be inferred that no other generic would have entered the market before the Full Court’s judgment in October 2011. According to one of Wyeth’s submissions, there is no evidence to support a contrary inference that any other generic would have entered the market before the Full Court’s judgment in October 2011. However, it also contended that if contrary to its primary position Sigma, Alphapharm, and/or Generic Health would have successfully supplied their products either privately or under the PBS then it should be inferred that other generics would also have entered the market, albeit not necessarily by supply arrangements with Generic Health.
Evidence
802 No other generic has claimed under the undertakings. There is no direct evidence from any other generic about what it might have done but for the interlocutory injunctions. The available evidence, which is scant, is conveniently summarised by Wyeth as follows:
As to third party suppliers of generic venlafaxine products, see:
(a) Generic Health’s Board paper for the meeting to be held on 28 April 2009 records discussions with Sandoz indicating interest in the market: Exhibit 145; CB 40 at TB-GH-174.
(b) Attachment 26 to the Statement of Agreed Facts lists a number of generic suppliers’ ARTG registrations which were made in 2009 and 2010: CB 34 at CB-MISC-116.
(c) Ms McTavish’s opinion was that Apotex and Sandoz could have obtained PBS listing and supplied product from 1 March 2010: McTavish 1 at [3.8]; CB 28 at CB-COM-9935.
(d) Ms McTavish explained how both SPAL and Alphapharm lost market share and pharmacy customers primarily to Apotex, and to a lesser extent to Sandoz and Ascent, in the period from 2009 to 2011: McTavish 1 at [8.16]-[8.18]; CB 28 at CB-COM-9960.
(e) Ms McTavish’s first report records Generic Health as having entered into supply agreements for generic venlafaxine products with Apotex on 25 March 2010 and Sandoz on 1 October 2010: McTavish 1 at [3.7]; CB 28 at CB-COM-9934.
(f) Those supply agreements have been tendered by Generic Health: CB 40, Tabs 71 and 74. The Apotex supply agreement includes as a recital that “Apotex had planned to obtain PBS Listing and launch the Product in Australia by 1 October 2010”: CB 40 at TB-GH-366.
(g) Statement of Agreed Facts [102] records that, “[a]s at 11 November 2011, there were nine sponsors of generic modified release venlafaxine hydrochloride products registered on the ARTG”: CB 34 at CB-MISC-17.
Further, in the real world, numerous other brands of generic venlafaxine were in fact listed on the PBS from 1 April 2012, when the Alphapharm and Generic Health brands were listed: McNeill 1 at [121], [122], [132]; CB 11 at CB-COM-194, 199; Annexure FM-50; CB 12 at CB-COM-1004-1006. These included Apotex and Sandoz brands, and the Elaxine-SR brand supplied by Aspen Pharma. (By this time, the Evelexa-XR brand the subject of SPAL’s original plans was defunct.)
803 In addition to the supply agreements for generic venlafaxine products with Apotex (25 March 2010) and Sandoz (1 October 2010), Generic Health entered into a similar agreement with Ascent on 25 November 201.
804 Importantly, the supply agreements between Generic Health and Pharmathen had to be amended to deal with each new generic as and when Generic Health entered a supply agreement with that generic. The existing agreements dealt only with the supply by Pharmathen to Generic Health of Generic Health’s own branded products. It did not provide for the supply by Pharmathen to Generic Health of venlafaxine products branded for other generics.
805 For example, in relation to Apotex, Generic Health and Pharmathen entered into a deed of variation of 7 April 2010 which referred to the original supply agreement between Generic Health and Pharmathen and recorded that Generic Health wished to sub-licence and supply Pharmathen’s generic venlafaxine products to Apotex and that Pharmathen consented to this sub-licence. The deed varied the original agreement to provide for such supply. The necessary arrangements included Pharmathen consenting to Apotex acting as a sponsor of the products and agreeing to supply:
13.1 Product in the form of bulk tablets directly to Generic Health for Generic Health to package itself or via a Third Party, for supply to Apotex; or
13.2 Product packed in Apotex’s livery to Generic Health for Generic Health to supply to Apotex.
806 I do not doubt that it was reasonably foreseeable to Wyeth that Generic Health might wish to transfer one of its surplus ARTG registrations to another generic to facilitate it entering into a supply agreement with that other generic. There is ample evidence to support that conclusion, not the least of which is that the very issue is dealt with in the Generic Health interlocutory injunction. The terms of the injunction reflect Wyeth’s awareness of the risk of Generic Health transferring some of the ARTG registrations, a necessary corollary of which is that Wyeth must have foreseen that Generic Health might have a role as an intermediate supplier to other generics. This much is also apparent from a letter from Wyeth’s lawyers of 17 August 2017 dealing with the same issue. But as discussed in the context of the issue of remoteness, this does not mean that it would be just to compensate Generic Health or Pharmathen for any loss caused by such a loss of opportunity.
807 The conclusions I have reached above also defeat these claims on the facts. The generic claimants had ARTG registrations of their products in 2009. No other generic had its products registered on the ARTG at this time. Sigma and Generic Health adduced evidence about the likelihood of them being willing to be the first to obtain PBS listing of their products. In effect, I rejected that evidence. I concluded that there was a relatively low possibility (20%) that in its unique circumstances as the first mover in the generic market which had to obtain a “win” on venlafaxine, Sigma would have been willing to be the first generic to obtain PBS listing on 1 December 2009. Those unique circumstances applied only to Sigma. The question which arises is in these circumstances how could I infer, estimate or even speculate that there was any chance of another generic having been willing to be the first to obtain PBS listing or, indeed, to supply its products at all?
808 First, in contrast to the position for Sigma, Alphapharm and Generic Health, I have no knowledge of the circumstances of those other generics. I have not seen any evidence of their internal deliberations or intentions. The fact that three entered into supply agreements with Generic Health in 2010 says nothing about their actual intentions to supply. For example, the recitals to the Apotex agreement can be read in more than one way. On one view, they may disclose Apotex’s intention to commence supply on 1 October 2010 but for the interlocutory injunctions. On another view, particularly given that the first instance hearing would be heard shortly after the agreement was executed, the recitals may represent an attempt to shore up a potential claim by Apotex as a person adversely affected by the interlocutory injunctions. Without any evidence from Apotex about what it intended, there is no rational foundation for an inference one way or another of for inference, estimation or speculation about what Apotex might have done other than that if the chance Sigma would have applied for PBS listing is only 20%, the chance any other generic might have done so at some later time (as they could apply only once they held an ARTG registration) must be or be approaching the merely speculative.
809 Second, the evidence about Alphapharm’s position is relevant. Alphapharm would not have sought PBS listing unless another generic had already obtained listing and triggered the 12.5% price reduction for Efexor-XR. It also initially doubted the sense of supply confined to the private market. This was Alphapharm’s position despite it having made a substantial investment in its generic venlafaxine products and having obtained ARTG registrations which would have enabled it to be the second onto the market. There is no evidence that any other generic had made a similar investment and was preparing to launch in 2009. Apart from the claimant generics, the next earliest ARTG registrations are those of Ranbaxy on 22 January 2010, with Spirit Pharmaceuticals following with its ARTG registrations on 30 July 2010 (a date after the first instance hearing). These circumstances are inconsistent with an inference that any generic which might have been ready to enter the market in 2010 would or might have done so, still less that such a generic would or might have been the first to obtain PBS listing.
810 Third, the evidence about Aspen’s position in relation to its acquisition of Sigma is relevant. Aspen had no interest in acquiring Sigma’s rights in relation to its generic venlafaxine products due to the mere existence of the litigation. In an email of 23 September 2010 it was said that this accorded with Aspen’s policy of not being litigious and focusing on its core business. It is also apparent that none of the claimant generics intended to infringe any patent. They each invested in their proposed launches of generic venlafaxine without being aware of the method patent. Given that other generics would have known about the method patent as a result of the Sigma interlocutory injunction and the evidence indicating that at that time none had invested in the launch of a proposed generic venlafaxine product, the obvious inference to draw is that no other generic would have chosen to involve itself in the litigation by launching a product before the Full Court’s decision and orders in late 2011.
811 Further, it cannot be inferred (or even rationally posited) that Generic Health’s supply agreements with the other generics would have been executed at an earlier time but for the interlocutory injunctions. The Apotex agreement is only a month before the first instance hearing, the Sandoz agreement is after the first instance hearing, and the Ascent agreement is later again in 2011. Given that I have no evidence of their actual intentions, why would I infer that there was any chance that these other generics were willing to do anything before the first instance judgment and the resolution of the appeal to the Full Court?
812 As a result of these conclusions, it is not necessary to resolve the debate about Ascent in any greater detail. However, the submissions about Ascent expose the need for some restraint on the positing of hypotheses in a case such as this.
813 Wyeth submitted this about Ascent:
As to sales resulting from on-supply by Generic Health to Ascent, the assertion that Ascent would have acquired a generic venlafaxine product from Generic Health and launched that product in the counterfactual world is contrary to the evidence of Mr Cooper. Mr Cooper gave evidence of the contract entered into between Pfizer and Ascent (also known as Genepharm) in the real world in 2009, of Ascent’s PharmAnnuity program, of the inclusion of Efexor-XR amongst the products that were included in that program, and that it and a second brand of venlafaxine would have been included in that program earlier in the counterfactual world. Ms McTavish gives expert evidence that she considers that this was, and would have been, the case. Additionally, in the real world, Generic Health’s supply agreement with Ascent was entered on 25 November 2011, that is, after the Generic Health Interlocutory Injunction was discharged, and the Final Injunctions were ordered.
814 Pharmathen submitted that the Pfizer and Ascent contract was “tainted by the interlocutory injunctions that had already been granted against Sigma and Alphapharm at that time and the known imminence of the interlocutory injunction to be granted against Generic Health, the making of which had already been agreed to by the parties”. Pharmathen continued:
It is reasonable to infer that but for those interlocutory injunctions, Genepharm (Ascent) would have entered into a supply agreement with Generic Health for venlafaxine of the kind they ultimately entered into so as to pursue sales of its own venlafaxine product.
815 The submission that the 2009 agreement between Pfizer and Ascent was “tainted” by the interlocutory injunctions is meaningless. What is being said is that it should be inferred that Ascent knew that Generic Health would be restrained and thus chose to enter into a contract with Pfizer when otherwise it would have entered into a contract with Generic Health and that, somehow, this should be treated as an adverse effect on Generic Health of the operation of the Generic Health interlocutory injunction despite that injunction not existing at the time of the agreement between Pfizer and Ascent. To my mind, this is all far removed from ascertaining the adverse effect of the Generic Health interlocutory injunction. Even if were not, again, there is no rational foundation for inference, estimation or speculation about why Ascent entered into a contract with Pfizer when it did. Equally, there is no rational foundation for inference, estimation or speculation as to whether Ascent would have entered into a contract for supply of venlafaxine with Generic Health at any time earlier than it in fact did.
816 Otherwise, as I have said, any loss to Generic Health (and thus Pharmathen) by reason of Generic Health’s lost opportunity to supply other generics would depend entirely on the “mere unrestricted volition” of those other generics: Chaplin v Hicks [1911] 2 KB 786 at 792-793 and Fink v Fink [1946] HCA 54; (1946) 74 CLR 127 at 143. The loss is simply too remote to be compensable.
817 There is another issue in respect of Pharmathen’s claim about other generics. Pharmathen did not hold any ARTG registrations. It did not have the opportunity Generic Health had to do a deal with other generics by reason of ARTG registrations. All Pharmathen objectively could have had (and subjectively it may not have had even this) is a hope that Generic Health of its mere volition might be able to do a deal with another generic of its mere volition, as a result of which Pharmathen would be a consequential beneficiary due to Generic Health placing increased orders for the products.
818 In these circumstances I consider that it would be inconsistent with the purpose of the undertakings for any order to be made compensating Generic Health or Pharmathen for the loss of the opportunity associated with the supply of other generics.
819 If any of these conclusions are incorrect as a matter of principle, I would not have made any order for compensation in favour of Generic Health or Pharmathen in respect of these claims in any event on a discretionary basis because of the fact that they wholly depend on the unrestricted volition of third parties.
820 It also follows that the prospect of another generic being the first to obtain PBS listing of generic venlafaxine products must be treated as non-existent. Given my interim conclusions, this means that the only prospect of PBS listing was the 20% chance that Sigma would have applied in time to obtain PBS listing on 1 December 2009.
Summary of interim conclusions – other generics
821 My interim conclusions about the claims of Generic Health and Pharmathen about the loss of opportunity to supply other generics, accordingly, may be summarised as follows:
(1) There should be no order for compensation in favour of Generic Health or Pharmathen on account of the opportunity to supply other generics with their products, because either the value of that lost opportunity should be treated as zero or because no order should be made on a discretionary basis.
(2) The prospect of another generic obtaining PBS listing of generic venlafaxine products before the Full Court’s judgment in October 2011 or supplying its products in the private market should be disregarded.
Alembic’s case
822 Alembic’s claim is for compensation under Wyeth’s undertaking given on 3 June 2009 in respect of the Sigma interlocutory injunction. Its primary claim is for the lost profit opportunity of supplying Evelexa XR products to Generic Health for the purpose of on-supply to Sigma. It is apparent that the assessment of this claim depends on what Sigma would have done and the success or otherwise it would have experienced.
823 Alembic makes a subsidiary claim for a small amount (US$10,500) for the cost of Evelexa XR products manufactured by it before the Sigma interlocutory injunction was granted which it could not then supply thereafter.
824 The following summary is based on those parts of the submissions for Alembic which are not in dispute.
825 Alembic is and was an experienced manufacturer of pharmaceutical products, including venlafaxine. It is a corporation registered in India under the Corporations Act 1956.
826 In July 2008 Sigma and Generic Health amended their supply agreement to provide for Generic Health to supply Sigma with the venlafaxine product, Evelexa XR. In August 2008 Generic Health then entered into an exclusive contract manufacturing agreement with Alembic which at that time was manufacturing generic versions of venlafaxine products in 37.5mg, 75mg and 150mg strengths for the European market. Under this agreement Alembic agreed to manufacture for and supply to Generic Health venlafaxine products to be branded Evelexa XR in three strengths, 37.5mg, 75mg and 150mg. Clause 14.1 of this agreement provided for a term two years from the date of first delivery to Generic Health of products made by Alembic, with the option to renew by consent of the parties.
827 As Alembic put it:
The intent of the commercial arrangements between Sigma, Generic Health and Alembic was that Alembic would make and supply EVELEXA-XR to Generic Health, and that Generic Health would then on-supply and sell the product to Sigma, and that Sigma would then supply the product in the Australian marketplace. That intent was confirmed by all three parties in a “GMP [Good Manufacturing Practice] Agreement” entered in August 2008. The GMP Agreement set out the respective roles of each party in respect of EVELEXA-XR, identifying Sigma as the sponsor, Generic Health as the supplier, and Alembic as the manufacturer of the product.
828 Alembic had approval from the TGA to manufacture solid unit dosage forms (tablets and capsules) and was also the notified manufacturer for each of Sigma’s ARTG registrations of generic venlafaxine products.
829 Generic Health placed its first order for Sigma’s products with Alembic (in response to an order from Sigma to Generic Health) in September 2008. The order was in the amount of US$1,443,294.50 for 19,642 packs of 37.5mg strength, 136,000 packs of 75mg strength, and 170,000 of 150mg strength Evelexa XR. Alembic shipped the goods and invoiced Sigma on 28 February 2009. Sigma paid for these products. They arrived in Australia via Singapore in May 2009. As Wyeth noted, Alembic suffered no loss on that account.
830 Generic Health placed its second order for Sigma’s products with Alembic (in response to an order from Sigma to Generic Health) in February 2009. The order was in the amount of US$113,000. These goods were never shipped.
831 While Sigma placed a third order with Generic Health in March 2009, Generic Health did not place an equivalent order with Alembic for these products.
Application of principles to Alembic’s case
832 I have explained above that Alembic must prove on the balance of probabilities that but for the Sigma interlocutory injunction it would have supplied some additional product to Sigma. If this is proved then Alembic has necessarily lost an opportunity of some value, so that the quantum of its loss must be assessed on the probabilities and possibilities. I thus accept Alembic’s submission as follows:
Given the circumstances of the present case, the three-step approach identified in European Bank is to be read in light of the abovementioned dicta in Sellars. That is, Alembic submits the principled approach that emerges, applicable to the present case, is that the claimants must each establish as a matter of causation (on the civil standard) that it has suffered some foreseeable loss or damage by having been deprived a commercial opportunity, which loss or damage has a value beyond merely theoretical or negligible value. The value to be attributed to that loss of opportunity is then to be assessed and ascertained by reference to the degree of probabilities or possibilities as to the hypothetical circumstances which would have arisen in the event the interlocutory injunction had not been sought.
833 In circumstances where there were back-to-back supply agreements between Alembic, Generic Health and Sigma and Alembic in fact supplied products to Sigma, it is difficult to conclude that Alembic has not proved some loss on the balance of probabilities. Wyeth’s contrary argument that Sigma would not have placed any more orders because its private market supply would have failed is difficult to accept given that Sigma would have wanted to continue supplying in the private market. Sigma would have considered ceasing to supply only if supply became financially unfeasible. Apart from that prospect, however, Sigma would have had every reason to continue its private market supply which would have necessitated obtaining more of its products from Alembic.
834 Apart from this, I have also explained above why I consider that the kind of loss Alembic claims must be seen as a direct and natural consequence of the Sigma interlocutory injunction. It is true that Alembic was not supplying Sigma directly. It was supplying Generic Health for supply to Sigma. But this does not make the loss indirect. A derivative claim is not necessarily an indirect claim. In a case such as the present the proper boundary for considerations of remoteness is to be set recognising that Alembic was not a mere supplier of some constituent part of Sigma’s product. It was manufacturing and supplying the product, Evelexa XR, to Generic Health for the purpose of supply to Sigma under supply arrangements which existed before the Sigma interlocutory injunction was granted.
835 The kind of loss Alembic claims was also reasonably foreseeable to Wyeth at the time the undertaking was given. Alembic’s submissions to that effect are compelling.
836 The question of foreseeability is to be assessed at the time Wyeth gave the undertakings: Air Express at 266-267. The question is not the reasonable foreseeability of the actual loss but whether the kind of loss subsequently claimed could have been reasonably foreseen: European Bank at [29]. As Alembic also noted, the test of reasonable foreseeability is not as confined as that of reasonable contemplation which is engaged in the law of contract: Alexander v Cambridge Credit Corporation Ltd (1987) 9 NSWLR 310 at 365.
837 I also accept Alembic’s submissions as follows:
(1) The undertaking, in terms, is not confined to parties. Wyeth must be taken to have been aware of this fact.
(2) It must have been reasonably foreseen by Wyeth that Sigma would have a supplier of its products. Dr Mannion, who had the day-to-day carriage of the litigation in 2009 for Wyeth, gave evidence that she was aware that Sigma might be sourcing its venlafaxine products from a third party manufacturer.
(3) While Dr Mannion and Mr Manspeizer expressed surprise in their affidavits about the claims of the suppliers, this evidence is immaterial. The relevant issue is whether Wyeth could have reasonably foreseen claims of the kind brought by the suppliers in respect of the lost opportunity to supply products for the generics to sell to pharmacists on the Australian market. It is impossible to conclude that claims of this kind were not reasonably foreseeable.
(4) There is no evidence suggesting that Wyeth was advised at the time the undertakings were given that a supplier would not be able to make such a claim.
(5) Mr Cooper gave evidence that commercial arrangements between third party manufacturers and generic companies were well known including the kind of back-to-back arrangement into which Sigma, Generic Health and Alembic had entered. Wyeth must have foreseen that if Sigma could not sell its products the entity or entities supplying Sigma with the products could not supply Sigma for that purpose.
Wyeth’s other answers to Alembic’s claims
838 Wyeth contended that Alembic failed to prove that the second order was cancelled due to the Sigma interlocutory injunction. I agree. The second order was placed in February 2009 before Sigma became aware of the method patent and was due for delivery on 29 May 2009. The order was cancelled before shipment. Alembic did not invoice Generic Health for the products, its practice being to invoice on the date of shipment. However, it is not apparent that the cause of cancellation was the Sigma interlocutory injunction as opposed to the mere existence of the method patent and the litigation.
839 In respect of the claim for US$10,500, however, there is unchallenged evidence that because of the Sigma interlocutory injunction Alembic had to destroy some materials that could only be used to produce Evelexa XR. Provided Sigma would have placed some additional orders with Generic Health for its products, this loss is recoverable.
840 Wyeth also contended that Alembic has not proved that it had the manufacturing capacity to fulfil any additional orders by Sigma. Given that Alembic, Sigma and Generic Health had entered into arrangements for Alembic to supply Sigma with all of the products it might require for a proposed PBS supply of Evelexa XR, this submission has little immediate attraction. It would not lightly be inferred that one of Australia’s largest generic pharmaceutical companies entered into a contractual arrangement intended to enable it to supply under the PBS a drug of the importance to it of Evelexa XR without satisfying itself that the supplier had the capacity to manufacture the required quantities of the drug as and when required.
841 As might be expected, the substance of Wyeth’s submission is spurious. It is that:
…the evidence adduced by Alembic does not establish that it had excess capacity that would have enabled it to produce the additional venlafaxine products on any particular day, week or month during the relevant period. Mr Ghosal asserts generally that no additional property, equipment, or personnel would have been needed to fulfil the hypothetical orders. However, the only direct evidence about capacity is a spreadsheet showing annual calculations he conducted for the years 2008-2017. This evidence is not sufficient to show excess capacity for venlafaxine for a number of reasons. First, it appears to show capacity for the entire plant, and does not differentiate for individual products. Secondly, it shows annual capacity, not daily, weekly, or monthly capacity. Thirdly, Mr Ghosal does not explain how the excess supply of venlafaxine could have been accommodated at any given time in relation to the actual capacity at which the plant was operating at (which in some years is said to be around 80% capacity).
842 As Alembic noted:
(1) The proposition that Alembic could not have met its contractual obligations was never put to Mr Ghosal.
(2) The proposition is that Alembic committed to contractual arrangements it could not fulfil or would inevitably breach. Any such proposition ought to have been put to Mr Ghosal, but was not.
(3) There was no evidence that Alembic had at any time defaulted in its contractual obligations as a contract manufacturer of pharmaceutical products, whether in Australia or elsewhere.
(4) It was not necessary for Alembic to establish that on any given day during the relevant period it had “excess capacity” to enable it to fulfil hypothetical contractual obligations to Generic Health. Mr Ghosal’s evidence shows Alembic’s capacity to “increase, in significant amounts, the annual production of tablets during the entirety of the loss period”, which is:
powerful evidence demonstrating Alembic’s ever-increasing production capacities, which implicitly establishes Alembic’s growth in the world market as a manufacturer of pharmaceutical products, and also implicitly establishes its ongoing capability to expand its manufacturing business and meet the ever increasing requirements of its contractual obligations to customers. That evidence is sufficient to demolish instantly Wyeth’s proposition raised at the heel of the hunt.
(5) The evidence about plant utilisation percentages confirmed that Alembic had excess capacity in each financial year.
(6) The minimum number of tablets to be manufactured and supplied under the agreement with Generic Health was a miniscule percentage (2%) of the total tables manufactured by Alembic.
(7) Sigma would hardly have entered into agreements for manufacture and supply of a product of such importance to it with an entity that was incapable of fulfilling Sigma’s demands for the product.
843 Accordingly, Wyeth’s lack of manufacturing capacity argument is without foundation.
844 Some other points made by Alembic may also be accepted. In its defence Wyeth pleaded that Alembic should be denied compensation as a matter of discretion and that it would not be reasonable or equitable for Alembic to be compensated. As Alembic noted:
…no factual allegations are specifically pleaded against Alembic. That is, it is not alleged that Alembic engaged in any particular conduct which might support these contentions. Rather, the contentions are made with respect to alleged conduct of Sigma or Generic Health. The contentions are therefore entirely derivative in nature.
845 It is not apparent why it would not be reasonable or equitable for Alembic to recover for adverse effects on it of the operation of the Sigma interlocutory injunction. The fact that its claim is derivative does not mean that any conduct of Generic Health or Sigma which would weigh against recovery on a discretionary basis must also weigh against Alembic. In any event, as will become apparent, I have not found in favour of Wyeth’s discretionary arguments.
Summary of interim conclusions – Alembic
846 In my view:
(1) To be a person adversely affected by the Sigma interlocutory injunction, Alembic must prove on the balance of probabilities that Sigma would have ordered additional products from Generic Health.
(2) While this issue cannot be finally assessed other than in the context of the likely success of Sigma’s continued private market supply, it would not readily be inferred that Alembic has suffered no loss given Sigma’s determination to supply its products.
(3) Subject only to (2), it is just that Alembic be compensated by Wyeth for its losses as expressly contemplated by the undertaking Wyeth gave to obtain the Sigma interlocutory injunction.
847 Pharmathen’s claim is for compensation under all of Wyeth’s undertakings. Its claim is for the lost profit opportunity of supplying their respective venlafaxine products to its customers, Alphapharm and Generic Health. As noted, the primary part of the claim relating to Generic Health concerns the supply of products to Generic Health for the purpose of Generic Health’s wholesale supply to pharmacists. The subsidiary part of the claim relating to Generic Health concerns the supply of products to Generic Health for the purpose of wholesale supply to other generics, Apotex, Sandoz and Ascent. I have rejected the subsidiary part of the claim above in respect of both Generic Health and Pharmathen.
848 The following summary is based on those parts of the submissions for Pharmathen which are not in dispute.
849 Pharmathen is a company incorporated in Greece and is a manufacturer and supplier of generic pharmaceutical products worldwide. The products it manufactures are supplied in bulk semi-finished and/or in fully finished (packaged for sale in accordance with the supplier’s requirements) form. Pharmathen’s customers include the Mylan Group (of which the Australian company Alphapharm and the company Generics (UK) Ltd, form part) and Generic Health. It manufactures its products in Greece.
850 Pharmathen began working on a generic sustained release venlafaxine product in the early 2000s. The generic sustained release venlafaxine formulation developed by Pharmathen comprises small tablets (mini or micro tabs) filled into a capsule which differs from Wyeth’s Efexor-XR product which is a capsule containing pellets.
Agreements with Alphapharm
851 In 2004 Pharmathen and Generics (UK) Ltd (a company related to Alphapharm) entered into a licence and commercial supply agreement in respect of generic venlafaxine. In anticipation of supply in accordance with those agreements Pharmathen sent pricing information to Alphapharm on 24 April 2008. Alphapharm sent a supply order to Pharmathen on 22 May 2008. These products could not be shipped to Australia unless Alphapharm obtained ARTG registrations for them, which occurred on 30 April 2009. Pharmathen manufactured the products but Alphapharm deferred the order due to the litigation (not the interlocutory injunctions) and Pharmathen stored the products which were then delivered to Alphapharm in December 2011.
852 As explained for Pharmathen:
Until the end of 2011, Alphapharm issued purchase orders for venlafaxine to, and paid, Pharmathen directly. From the start of 2012, Mylan Ireland ordered from Pharmathen and paid for venlafaxine on Alphapharm’s behalf, Pharmathen delivered such stock to Alphapharm and Mylan Ireland subsequently invoiced Alphapharm for the same.
853 The supply agreements remain on foot but were amended in 2013 and 2016 including in respect of prices. The 2013 amendment meant that the supply arrangement with Pharmathen was no longer exclusive but Alphapharm has continued to acquire all of its venlafaxine products from Pharmathen.
Agreements with Generic Health
854 In 2007 Pharmathen and Generic Health entered into a licence and commercial supply agreement in respect of generic venlafaxine.
855 Generic Health issued a purchase order on 7 January 2009 which was conditional on ARTG registration of its products which was not obtained until 6 August 2009. The nature and circumstances of Generic Health’s subsequent dealings with Pharmathen are described above. It will be apparent from that discussion that I do not accept Pharmathen’s submission that Generic Health’s second purchase order on 5 August 2009 was cancelled due to the Generic Health interlocutory injunction (or, for that matter, the Alphapharm interlocutory injunction). It was cancelled because of the anticipated litigation against Generic Health in circumstances where the non-infringement argument based on Alphapharm’s products using hydrogel technology had not been accepted in the Alphapharm interlocutory judgment on 25 August 2009. As such, this loss is outside the terms of the undertakings.
856 The agreements between Pharmathen and Generic Health were varied on 10 June 2010 to permit supply Generic Health to supply Apotex, on 7 September 2010 to permit Generic Health to supply Sandoz, and on 1 November 2012 to permit Generic Health to supply Ascent. I have also rejected above any claim based on the lost opportunity to supply these generics with their products via Generic Health.
Application of principles to Pharmathen’s case
857 Pharmathen must prove on the balance of probabilities that it would have supplied some additional product to Alphapharm and Generic Health.
858 Given my conclusions above about Alphapharm and Generic Health, Pharmathen’s position is the same as that of Alembic in respect of Sigma’s products. Wyeth’s contrary argument that any private market supply would have failed is difficult to accept given that Alphapharm and Generic Health would have wanted to continue supplying in the private market. Like Sigma, Alphapharm and Generic Health would have considered ceasing to supply only if supply became financially unfeasible. Apart from that prospect, however, Alphapharm and Generic Health would have had every reason to continue their private market supply which would have necessitated obtaining products from Pharmathen.
859 Apart from this, Pharmathen is in no different (and perhaps a better) position than Alembic in terms of the reasonable foreseeability and directness of is claimed loss given that there was no supplier interposed between Pharmathen and Alphapharm or Pharmathen and Generic Health. While Mylan Ireland was involved from 2012 onwards the products were nevertheless delivered directly from Pharmathen to Alphapharm. In other words, there was one step in the supply process between Pharmathen and each of Alphapharm and Generic Health.
860 In response to Pharmathen’s contentions, I otherwise accept that:
Loss of the kind suffered by Pharmathen, that is, loss of profit in manufacturing venlafaxine as a result of Alphapharm and Generic Health being restrained from supplying the product in Australia, was foreseeable. That was so whether such manufacturing was to be undertaken by Alphapharm and Generic Health (in which case the loss of profit in respect of both manufacture and supply would be suffered by the same party), or by a third party manufacturer.
861 I also accept that Mr Hurley’s 2009 affidavit in respect of the Alphapharm interlocutory injunction disclosed to Wyeth that Alphapharm was sourcing its products from a third party manufacturer (indeed, it specifically disclosed to Wyeth’s lawyers that the supplier was Pharmathen) and Dr Mannion of Wyeth was aware of the fact of a third party manufacturer (albeit not that it was specifically Pharmathen due to confidentiality claims). It does not matter that Dr Mannion also said she understood this to mean an entity at arm’s length from Alphapharm. Pharmathen was such an entity. Wyeth also knew that Alphapharm would be importing its products which carries with it the necessary implication that it knew that Alphapharm was not manufacturing the products and thus must have had contractual supply arrangements with another company. The suggestion that it was not foreseeable that the interlocutory injunctions would prevent Alphapharm purchasing products from the third party manufacturer is not sustainable.
862 I accept that the position is no different in terms of Pharmathen’s arrangement with Generic Health at least insofar as supply of Generic Health’s own products are concerned. As Pharmathen noted, Wyeth was aware that Pharmathen was the manufacturer of Generic Health’s products, having been informed to that effect by communications on 7 August and 27 October 2009. It follows that it was reasonably foreseeable that the Generic Health interlocutory injunction would prevent Generic Health from purchasing products from Pharmathen.
863 The fact that Pharmathen did not notify Wyeth that it would or might be adversely affected by the interlocutory injunctions before they were granted is immaterial. The relevant consideration is the reasonable foreseeability of the kind of loss at the time the undertakings were given, not whether notice of a potential claim was given.
864 To the extent that the Sigma interlocutory injunction can be relevant (which is not apparent to me given my conclusions above), I agree with Pharmathen that any suggestion of delay by it in making its claim is unsustainable.
Wyeth’s other answers to Pharmathen’s claims
865 Wyeth contended that Pharmathen has not proved that it had the capacity to manufacture the additional products the subject of the claims.
866 I consider Wyeth’s submissions misconceived. As with Alembic above, it would not lightly be inferred that Alphapharm or Generic Health would have entered into supply agreements with an entity that was incapable of fulfilling their requirements for supply. To the contrary, compelling evidence would be required to draw that inference. In other words, the fact of the supply agreements it itself good evidence that Pharmathen had the capacity to supply all of the requirements of Alphapharm and Generic Health.
867 Apart from this, Wyeth has misunderstood the evidence which relates to Pharmathen’s annual production capacity of venlafaxine products. Further, as Pharmathen submitted:
Mr Spyropoulos was not required for cross-examination. It was therefore not put to Mr Spyropoulos that the Pallini facility did not have sufficient capacity to meet the additional volume of hypothetical supply required by Alphapharm and Generic Health.
868 In common with Alembic’s position it is not apparent why it would not be reasonable or equitable for Pharmathen to recover for adverse effects on it of the operation of the interlocutory injunctions. The fact that its claim is derivative does not mean that any conduct of Alphapharm or Generic Health which would weigh against recovery on a discretionary basis must also weigh against Pharmathen.
Summary of interim conclusions – Pharmathen
869 In my view:
(1) To be a person adversely affected by the Alphapharm and Generic Health interlocutory injunctions Pharmathen must prove on the balance of probabilities that Alphapharm and Generic Health would have ordered additional products from it.
(2) While this issue cannot be finally assessed other than in the context of the likely success of the continued private market supply by Alphapharm and Generic Health, it would not readily be inferred that Pharmathen has suffered no loss given my conclusions that they would have been supplying their products.
(3) Subject only to (2), it is just that Pharmathen be compensated by Wyeth for its losses as expressly contemplated by the undertaking Wyeth gave to obtain the Alphapharm and Generic Health interlocutory injunction.
Wyeth’s case
870 Wyeth contended that if it had not sought the interlocutory injunctions on the days in question (22 May, 14 August, and 10 November 2009) then:
(1) it would have brought claims against the generics for infringement of copyright in the Efexor-XR PI and CMI documents on a final basis;
(2) it would have sought and obtained interlocutory injunctions against the generics on the basis of their threatened infringement of copyright in the Efexor-XR PI and CMI; and
(3) “it would not be reasonable or equitable to compensate the Claimants having regard to the fact that, if the Generics had launched their products or listed them on the PBS, this would have involved, inter alia, the Generics infringing Wyeth’s copyright in the Efexor-XR PI and CMI”.
871 I have decided above that Wyeth cannot raise the copyright issue. On one view, that should be the end of the matter. It would be wrong to decide issues which I consider to constitute an abuse of process. This said, dealing with at least some of the aspects of the copyright issue will expose the difficulties to which it gives rise and supports my conclusion that Wyeth should not be permitted to raise the issue.
872 Wyeth also contended that if not for the interlocutory injunctions it would have taken steps to defend the market share of Efexor-XR if (contrary to its arguments) the generics would have made material inroads into the market. Wyeth said it would have implemented a “generic defence strategy” consisting of two main aspects, the promotion of Efexor-XR and the launch of a second brand of the molecule at an earlier time than it did so.
873 Wyeth further contended that if any generic had applied for PBS listing of their generic versions of Efexor-XR, the Minister’s delegate would not have listed the products on the PBS in the face of the unresolved litigation about the method patent.
The copyright issue
PIs and CMIs
874 The Commonwealth’s submissions explained the relevant statutory regimes.
875 The regime for CMIs was (and is) in the Therapeutic Goods Regulations 1990 (Cth), reg 9A in particular. By reg 9A(1):
The sponsor of therapeutic goods that are specified in Part 1 of Schedule 10 must not supply the goods if the sponsor does not supply with the goods written information about the goods that meets the requirements for a patient information document set out in Schedule 12.
876 Part 1 of Sch 10 included therapeutic goods which contain a substance mentioned in Sch 4 of the Poisons Standard, which included venlafaxine.
877 Regulation 9A(2) provided:
For the purposes of subregulation (1) or (1A), information must be provided:
(a) in the primary pack in which the therapeutic goods are supplied; or
(b) in another manner that will enable the information to be given to a person to whom the goods are administered or otherwise dispensed.
878 Schedule 12 required the document to be:
…consistent with product information (within the meaning of section 9D of the Act) about the product.
879 Section 9D(5) of the Therapeutic Goods Act defined product information as follows:
product information, in relation to therapeutic goods, means information relating to the safe and effective use of the goods, including information regarding the usefulness and limitations of the goods.
880 As the Commonwealth explained in relation to ARTG registration:
In 2009, the guidelines followed by the TGA required an applicant for registration of a generic version of an existing registered medicine to submit a draft PI document. There was no legislative obligation to do so until changes were made to the TG Act in January 2011.
…
…the act of registration has a statutory basis that is not affected by the nature of the documents submitted as part of the application for registration: an application under section 23 of the TG Act leads to evaluation of the goods under section 25(1). At the time, part of that evaluation involved whether “the presentation of the goods” (as defined in section 3) “is acceptable” under section 25(1)(g). That part involves evaluation of the PI document, but as a matter of TGA guidelines only, and not as a requirement of statute. The evaluation did not check PI documents for copyright issues. At that time, following the evaluation, the Secretary had to make a decision on the evaluation under section 25(4), and, if the decision was to register the therapeutic good, the Secretary entered that good onto the ARTG under section 25(4)(d).
…
…although draft PI documents are evaluated by TGA Evaluators as part of their evaluation of the entire[ARTG application, under section 25 of the TG Act the Secretary must make a decision to accept or reject the application for registration within 255 days from the date of application – this is the application as a whole, including the draft PI. Once a product had been registered, the sponsor could apply for approval of a proposed amended PI…
881 As the Commonwealth explained in relation to PBS listing:
Under the Listing Unit Requirements in force as at 1 April 2009 and 1 August 2009, an applicant for PBS listing was required to supply “a copy of the current approved Product Information”. There was no requirement to supply a CMI document…
The requirement to supply a PI document was removed from the guidelines that took effect on 1 September 2009; after that date, a PI document did not need to be provided for new brands of existing listed items, such as the generic venlafaxine products.
882 Further:
The TG Act was amended with effect from 28 January 2011 to include a definition of “product information” and to create the concept of approved production information. For existing registered products, a new section 25AA(2) provided that where the notice of the Secretary’s decision under section 25(4) “specified the product information that was approved by the Secretary in relation to the medicine”, that product information became the “approved product information” for the medicine.
However, the amending legislation still did not introduce any obligation to make the approved product information available to any person.
The Copyright Act was amended as of 27 May 2011 to insert a new section 44BA which provided that certain acts were not infringements of copyright. In particular, “supplying, in Australia, some or all of any product information that is approved under [section 25AA] in relation to medicine” (section 44BA(2)(a)), or reproducing, publishing, communicating or adapting, in Australia, “some or all of” any product information that is approved under section 25AA, “to the extent that the supply, reproduction, publication, communication or adaptation is for a purpose related to the safe and effective use of the medicine” the subject of the approved product information (section 44BA(2)(b)-(e)).
883 Apart from evidence about the factual and legal basis for Wyeth having copyright in the Efexor-XR and CMI and the generics’ substantially reproducing those documents without its authority, Wyeth relied on evidence from Dr Mannion and Mr Manspeizer about what it would have done in respect of copyright but for the interlocutory injunctions. The general observations I have made above about the unreliability of this kind of evidence apply. The fact that I consider that both Dr Mannion and Mr Manspeizer were honest and careful witnesses yet their evidence about the copyright issue was utterly unconvincing again demonstrates the difficulty of asking a witness nearly 10 years after the events in question what they think would have happened in hypothetical circumstances. As the cross-examination disclosed, and as would be expected, Dr Mannion and Mr Manspeizer recalled very little of what occurred in 2009. Yet they were asked to hypothesise about what they thought would have happened in a virtual contextual and temporal vacuum. They are not to be criticised for having answered the questions put to them, but as will become apparent nothing they said could go any higher than the obvious proposition that as employees of Wyeth they would have acted in what they perceived to be Wyeth’s best interests to the best of their abilities at all times.
884 In her first affidavit Dr Mannion said that in 2009 as Wyeth’s Director of Patents and Trade Marks she was responsible for making day-to-day decisions for Wyeth in relation to the Efexor-XR litigation. She reported to Mr Manspeizer, Wyeth’s Vice President - Intellectual Property and Associate General Counsel who in turn reported to Lawrence Stein, Wyeth’s Senior Vice President and General Counsel. She was required to take any significant strategic decision to Mr Manspeizer and Reem Jishi, Wyeth’s Vice President and Deputy Chief Patent Counsel. As Dr Mannion put it and I accept she, Mr Manspeizer and Ms Jishi “would work together to decide on a course of action that we believed to be in Wyeth's best interests, taking all of the circumstances we were aware of into consideration”. They would so with “input from external legal counsel in the jurisdiction to which the decision related” and from other employees of Wyeth as required. Once they had decided what to do about a significant strategic decision Mr Manspeizer or Mr Jishi would present their views to Mr Stein or to Wyeth’s Chief Executive Officer, Bernard Poussot for approval.
885 Dr Mannion said that if the Court had refused to grant the Sigma or Alphapharm interlocutory injunctions she would have sought advice from Wyeth’s external lawyers in Australia “as to whether there were any alternative legal options that may have allowed Wyeth to maintain exclusivity in the Australian extended release venlafaxine market” and discussed the options with Ms Manspeizer and Ms Jishi and others as required. Dr Mannion said:
If any other legal rights that may have enabled Wyeth to maintain exclusivity in the Australian extended release venlafaxine market had been identified and determined to have reasonable prospects of success, then I expect that Wyeth would have asserted those rights.
886 Given that Wyeth’s lawyers had also represented the Sanofi-Aventis companies in their litigation against Apotex about leflunomide in which Sanofi-Aventis obtained an interlocutory injunction based on infringement of copyright in Sanofi-Aventis’s PI document, Dr Mannion expected that had she sought advice about other options as a result of the Federal Court refusing Wyeth’s applications the advice would have been that it was likely Wyeth could obtain interlocutory relief based on copyright. Dr Mannion said that in “this counterfactual scenario I believe it is highly likely that Wyeth would have decided to pursue this cause of action”.
887 In her second affidavit Dr Mannion noted that she had received emails from Wyeth’s lawyers for the Efexor-XR litigation about the copyright issue and that she “was not attracted to the idea of a copyright infringement claim at the time in circumstances where Wyeth’s position was that it had a valid patent and could rely on that to obtain an interlocutory injunction against Sigma on the basis of patent infringement by Sigma”, as she “did not see the benefit of committing Wyeth to additional legal fees and work relating to a copyright infringement claim provided that an interlocutory injunction against Sigma on the basis of patent infringement was granted”. Subsequently in around late April 2009, and after a call between the external lawyers and Bret Parker, Wyeth’s Vice President responsible for trade marks and copyright matters, Wyeth decided not to pursue a copyright infringement claim. It is this evidence which is the basis for my description above of Wyeth having made a deliberate forensic decision in this proceeding not to assert copyright as the foundation for relief against the generics.
888 The relevant emails disclose that Gilbert & Tobin, Wyeth’s external lawyers, had not carried out the kind of work that would have been necessary to provide formal advice about the copyright issue. By this I mean that I infer from the nature of the emails that the lawyers simply assumed that Sigma’s PI for its venlafaxine products had been copied from the Efexor-XR PI because this was known to be standard industry practice. They had not researched issues such as Wyeth’s capacity to prove that it owned the copyright in the Efexor-XR PI or compared the PIs in the kind of detail that would be required to give formal legal advice.
889 The emails also disclose that Gilbert & Tobin appreciated that there was a timing issue. In an email of 21 April 2009 the lawyers said:
If Wyeth wishes to assert a claim for copyright infringement (to form part of the interlocutory orders sought), we will need to put Sigma on notice of this claim as a matter of urgency
890 This reflected the fact that Sigma had filed its proceedings claiming the method patent was invalid on 1 April 2009 and Wyeth was preparing to file its proceedings for patent infringement including a claim for interlocutory relief which it filed on 29 April 2009. Gilbert & Tobin, as would be expected, were operating on the sound assumption that all of Wyeth’s legal claims against Sigma should be brought at the one time because otherwise the additional claim may not have been permitted to be brought at all.
891 Dr Mannion also said that she would have considered a copyright injunction application “far more favourably” if grounds for applying for the patent injunction did not exist, Wyeth had decided not to apply for a patent injunction, or the Court had not granted the patent injunction. Further, and assuming certain legal advice would have been received, if Wyeth had not sought an interlocutory injunction on copyright grounds and the generics had not been restrained by an interlocutory injunction on patent grounds, Dr Mannion said that adding a claim for final relief on copyright grounds would have been attractive “because it would have strengthened the claim for final relief, but also provided an additional reason why the generic party would not take the risk of entering the market or seeking PBS listing”, so that “is very likely that [she] would have recommended that Wyeth pursue this option because it would have increased the likelihood of Wyeth maintaining its position in the market and the Efexor price under the PBS, pending the final determination of the proceedings”.
892 The assumed legal advice in Dr Mannion’s mind was advice to the effect that:
(a) Wyeth had a good argument that the relevant generic party was infringing Wyeth’s copyright in the Efexor product information and consumer medicines information;
(b) the generic party may not be able to solve the copyright infringement issue by rewriting its product information and consumer medicines information, and even if it was achievable any such solution would take several months at least to achieve; and
(c) the damages payable by the relevant generic party if copyright infringement was established would include the profits lost by Wyeth as a result of sale of the generic venlafaxine product, including because of the statutory price reduction under the PBS (if PBS listing was obtained).
893 Mr Manspeizer’s two affidavits, insofar as the copyright issue is concerned, are in very similar terms and to the same effect as Dr Mannion’s affidavits.
The problems with Wyeth’s evidence
894 It emerged from the cross-examination of Dr Mannion and Mr Manspeizer that, unsurprisingly, they recalled very little at all about events in 2009. They had given their affidavits in a near factual and contextual vacuum. As a result, their evidence about what they would or might have done is effectively worthless.
895 Further, and as discussed, the best construct to apply is that on 22 May 2009, 14 August 2009, and 10 November 2009 Wyeth did not prosecute its applications for interlocutory relief but otherwise events that occurred cannot be disregarded. Having taken this approach for the generics (an approach for which Wyeth advocated) it cannot now be abandoned to suit Wyeth. The result is that it must be taken that Wyeth would have done what it did which is to decide in late April 2009 not to pursue a copyright infringement claim against Sigma. It would have maintained that position until at least 22 May 2009 when it must be assumed that it did not press its application for interlocutory relief against Sigma. It must also be accepted, for example, that Sigma would have continued its private market supply. In other words, the context in which Wyeth would have been operating is not the apparent vacuum in which Dr Mannion and Mr Manspeizer were asked to give their affidavits. Until 22 May 2009 Wyeth’s lawyers would have had no reason to consider the copyright issue in detail and would not have done so. Wyeth would have applied for interlocutory relief based only on patent infringement which it then would not have pursued. Sigma would be supplying in the private market. This is the context in which Wyeth would or might have been re-considering the copyright issue.
896 Assume that Wyeth would have re-considered its decision. We know from Dr Mannion’s evidence that Wyeth believed the method patent to be valid and infringed by Sigma. We know also from the evidence that Wyeth also believed that Alphapharm’s products (of which Wyeth was aware by this time) infringed the method patent. Given this and that Wyeth must be assumed not to have sought interlocutory relief based on patent infringement what logical basis is there to posit that it would have sought interlocutory relief based on copyright infringement?
897 Further, given that it believed the method patent to be valid and the generics were infringing the method patent so that if it did not obtain interlocutory orders based on the patent the generics would be liable to it in damages including damages for “the profits lost by Wyeth as a result of sale of the generic venlafaxine product, including because of the statutory price reduction under the PBS (if PBS listing was obtained)”, why would Wyeth have not reached precisely the same decision as it did reach about final relief – not to add the copyright case? What would the claim have added apart from increased cost and complexity given Wyeth considered the method patent to be valid and infringed? The answer is nothing once hindsight is excluded.
898 It is also apparent that the key person within Wyeth who either made the actual decision about not asserting copyright in 2009 or made that recommendation to his superiors was Mr Parker. There is no evidence from Mr Parker. We do not know and have no evidentiary basis to speculate about why Wyeth decided not to pursue copyright in 2009. Dr Mannion and Mr Manspeizer had nothing to do with that decision. The possibilities are endless. The external legal advice might have been that Wyeth’s prospects of interlocutory or final relief based on copyright were poor. It might have been that it would involve so much work that it would detract from the preparation and prosecution of the case based on the method patent. Mr Parker might have held the view that Wyeth should not assert copyright in respect of possible infringements of copyright in PIs and CMIs in Australia as a matter of policy given that it was well-known in the industry that the relevant Commonwealth bodies considered it to be in the public health interest for these documents to convey the same information and that the leflunomide injunction based on copyright was seen to raise important policy issues. There is no evidence about why Wyeth decided not to pursue copyright claims in 2009 but whatever the reasons they may have applied with equal force if Wyeth had not obtained the interlocutory injunctions. All we do know is the critical fact that in 2009 Wyeth made a deliberate forensic decision not to assert copyright against the generics to which it must be held.
899 Apart from these imponderable matters, it must be recalled that it is Wyeth’s case that the only matter to be disregarded is the seeking of the interlocutory injunctions on the day on which each was sought. I have accepted that approach above. In these circumstances why would it be inferred or assumed that Wyeth’s external lawyers would have advised Wyeth that having not prosecuted each of its interlocutory injunction applications on 22 May, 14 August and 10 November 2009 against Sigma, Alphapharm and Generic Health respectively, it would have good prospects of obtaining interlocutory relief based on copyright? It is obvious that Gilbert & Tobin knew that if it did not bring forward any copyright claim as the basis for interlocutory relief at the same time as its patent claim Wyeth would not necessarily be able to claim such relief at a later time. Gilbert & Tobin appreciated that if it did not make all its claims for interlocutory relief at the same time Wyeth would be confronted by a range of arguments against it subsequently obtaining interlocutory relief on a new ground including unjustifiable vexation, oppression and delay. To this must be added that Gilbert & Tobin would be advising in circumstances where Sigma would have been continuing its private supply in the context of Wyeth not having pursued its application for interlocutory relief for some period before Gilbert & Tobin would have the opportunity to provide the kind of formal advice that Wyeth would have required before deciding to assert copyright. Indeed, by the time such advice could have been provided and considered by Wyeth, Alphapharm too would have been supplying its products on the private market. Gilbert & Tobin’s advice about the prospects of Wyeth obtaining interlocutory relief would have had to take these matters into account.
900 Further, it cannot be inferred or assumed that Gilbert & Tobin’s advice would have been to the effect assumed by Dr Mannion and Ms Manspeizer for the purposes of their evidence. Gilbert & Tobin’s advice would have had to grapple with many complex legal and factual issues, not just the problems of having sought but belatedly not prosecuted applications for interlocutory relief and delay. Using some points made in Alphapharm’s submissions as a framework and on the basis that the same propositions apply to all of the generics, the following matters may be noted, which I accept:
(1) “It cannot be assumed that the assembled evidence in relation to copyright which is now available to Wyeth (and which in any event is inadequate for the reasons discussed above), which it has had since 2012 to prepare, would have been readily available to Wyeth in 2009. There is no evidence to that effect. Rather, such evidence as there is, points the other way. Even in these proceedings, Wyeth did not complete its evidence in relation to copyright matters until weeks into the trial. Mr Konnaris’ second affidavit contained documents which on any view were crucial to attempting to prove that various employees of various companies in the Wyeth group of companies were authors of the relevant PI and CMI. Mr Konnaris also gave evidence that many of the documents, some dating back to 1996, were hard to find and took time and effort to extract from Wyeth's archives”.
(2) “Whatever may have been said about whether Wyeth could prove ownership and subsistence to the level of a serious question to be tried, Wyeth would have needed to know its position in relation to proof at a final hearing before seeking the interlocutory injunction. Otherwise it would have been assuming an unknown exposure on the claim on the undertaking as to damages”.
(3) “…while Mr Manspeizer gave evidence that Wyeth had pursued a claim of copyright infringement in Singapore or Taiwan prior to 2009, there was no evidence that such a claim had ever been pursued in Australia. Further, there was no evidence from anyone at Wyeth Australia (which Wyeth now appears to be putting forward as a joint owner of copyright, although its position is not clear), that such a strategy would have been pursued in Australia. The evidence of Mr Konnaris was that so far as he was aware, such a strategy was never discussed within Wyeth Australia in 2009. Wyeth Australia, via Mr Konnaris, also knew that the TGA had a policy of requiring generics to have PIs and CMIs in the same form as the originators. Thus, Wyeth Australia would have known that pursuing such a claim would be directly in the face of the TGA’s policy, a government authority which it needed to deal with day-to-day in its business”.
(4) It cannot be assumed that the legal advice would not have been to the effect that damages would be an adequate remedy for the alleged copyright infringement. There was no Wyeth copyright in the generic venlafaxine products themselves or the packaging in which they were sold. The copyright claims concerns the associated PIs (which are not part of the product or packaging sold) and the CMIs (which are inside each packet). The PIs and CMIs, at best, are ancillary (or even peripheral) items to the products so to prevent sale of the products based on copyright, as opposed to use of the copyright material itself, would be unorthodox. Nor can it be assumed that, in these circumstances, the damages for copyright infringement would include the 12.5% price reduction (which would have been triggered only by PBS listing not ARTG registration of the generic products) or Wyeth’s lost profits from the lost sales of Efexor-XR. While Wyeth could have made arguments to this effect the issues are by no mean straight-forward and it cannot be inferred or assumed that Gilbert & Tobin would have given such advice.
Other problems for Wyeth about copyright
901 There are yet further problems for Wyeth. Product information documents were required to obtain ARTG registration. They were not otherwise legally required but there was a practice supported by the Commonwealth of ensuring that the product information document was publicly available to assist prescribing doctors. Sigma’s products were registered on the ARTG on 6 March 2009, Alphapharm’s on 30 April 2009, and Generic Health’s on 6 August 2009. By the time that Wyeth would have been considering asserting copyright the ARTG registrations had already been obtained. As Alphapharm submitted:
The PI is only relevant in determining whether “the presentation of the goods” (as defined in sec 3) “is acceptable” under sec 25(1)(g) of the Therapeutic Goods Act. That part involves evaluation of the PI document, but as a matter of TGA guidelines only, and not as a requirement of statute. The evaluation did not check PI documents for copyright issues. Following the evaluation, the Secretary must make a decision on the evaluation under sec 25(4) of the Therapeutic Goods Act, and, if the decision is to register the therapeutic good, that good is entered onto the ARTG under sec 25(4)(d) of the TGA.
…
The requirement to supply a PI document for the purposes of obtaining PBS listing was removed from the PBS guidelines that took effect on 1 September 2009; after that date, a PI document only needed to be provided where a new item was listed on the PBS after a PBAC recommendation. That was not the case for the listing of any of the generic venlafaxine products. That means that any interlocutory injunction restraining acts comprised in the copyright in the PI would not have prevented Alphapharm from listing Enlafax XR on the PBS on and after 1 September 2009…
… although the CMI must be made available to the public, the CMI is a straightforward document which could be easily redrafted, in a matter of days, to be non-infringing with reference to publically available CMI templates, including one which had existed since 1997 specifically for anti-depressant medicines (that is, Ex. 105).
902 All of the generics also adduced evidence to the effect that if Wyeth had raised the copyright issue they would have been undeterred (leaving aside actually being enjoined on that basis) and, in any event, would have taken steps to avoid the issue including by re-writing their PIs and CMIs and lobbying the Commonwealth to accelerate its response to the copyright issue including by threatening to or actually supplying without a PI being available for doctors (a strategy which Apotex ultimately proposed in respect of leflunomide).
903 As to this last matter, supply without a PI, it may be accepted that for the purpose of this proceeding the generics admitted that in order to supply their products they would have caused a PI to be available, but these admissions related only to a PI not the allegedly infringing PIs. Further, the mere threat of supply of a generic venlafaxine without a PI at all, on the evidence, would have been of serious concern to the Commonwealth. To add yet more complexity still to this (in my view, impermissible) case within a case, the Commonwealth adduced evidence to the effect that if confronted by the copyright issue in the context of Efexor-XR (a widely used anti-depressant) it would have accelerated its legislative response to the issue by enacting the Therapeutic Goods Legislation Amendments (Copyright) Act 2011 (Cth) at an earlier time. That Act commenced on 27 May 2011. As the Commonwealth noted, the amendment inserted a new s 44B providing that certain acts do not infringe copyright including:
“supplying, in Australia, some or all of any product information that is approved under [section 25AA] in relation to medicine” (section 44BA(2)(a)), or reproducing, publishing, communicating or adapting, in Australia, “some or all of” any product information that is approved under section 25AA, “to the extent that the supply, reproduction, publication, communication or adaptation is for a purpose related to the safe and effective use of the medicine” the subject of the approved product information (section 44BA(2)(b)-(e)).
904 All of the imponderable issues discussed above arise because Wyeth decided not to assert copyright against the generics in these proceedings in 2009 and only to assert copyright only in the context of the claims under the undertakings. As I have said, in my view this is a clear abuse of process or, if not, is so unjustifiably oppressive and vexatious to the generics to require exclusion of the issue on a discretionary basis.
905 Apart from these matters it is apparent that, as was submitted for the generics:
(1) much of the relevant version of the Efexor-XR PI (itself a vexed issue) is a “cut and paste” job from earlier versions and other studies and reports of which the only witness as to authorship, Mr Konnaris, was not the author of either the earlier versions of the PI or the studies and reports;
(2) none of those other unknown authors have been called to give evidence;
(3) there is no evidence of joint authorship or collaboration. Rather different individuals prepared different versions of the Efexor-XR PI over different periods of time; and
(4) at the least it is not apparent that the authors of the other studies and reports were employees of Wyeth Australia.
906 Wyeth’s submissions did not confront these fundamental difficulties other than in the most superficial way. Wyeth could not have taken that course in 2009 had it raised the copyright issue and expected to obtain any kind of relief as a result, interlocutory or final. For the reasons given it cannot do so now. No further attention should be given to the issue.
Wyeth’s generic defence strategy
907 Wyeth contended that if faced with generic competition “Wyeth and (its successor in interest) Pfizer would have mounted a successful defence of their share venlafaxine market, by the promotion of Efexor-XR and the launch of a second brand of the molecule”, as well as discounting the price of Efexor-XR.
908 Wyeth relied on various aspects of evidence to support this contention.
909 For example, Mr Braithwaite in her July 2009 affidavit in respect of the Alphapharm interlocutory injunction said that:
Given the entry of Alphapharm into the market, it is my opinion that it is likely that Wyeth Australia will be forced to take steps in an effort to match the commercial position established by Alphapharm including discounting the price of Efexor XR to wholesalers or direct to pharmacies by reducing the ex-factory price and providing free stock to pharmacies. It is also my opinion that it is likely that Wyeth Australia will be forced to consider applying discounts to other products supplied to pharmacy in order to sustain the commercial position in relation to Efexor XR. It is also my opinion that it will become necessary for Wyeth Australia to invest in marketing and sales activities to support Efexor XR including healthcare professional mailings, advertising, public relations activities, development of sales materials, and the use of representatives to call on either doctors or pharmacies. All of this will be new activity funded by diverting both human and financial resources away from other products and commercial opportunities.
910 Ms Braithwaite gave evidence to the same effect in her May 2009 in support of the Sigma interlocutory injunction.
911 What will be noted about Ms Braithwaite’s evidence is that, contrary to the submissions for Wyeth, she does not mention the launch of a second brand of Efexor-XR. She mentions only the discounting and promotion of Efexor-XR.
912 Wyeth noted that Mr Hurley said in cross-examination that if Wyeth had faced generic competition he expected that it would have considered and if appropriate pursued launching a second brand venlafaxine product, marketing and sales activities to promote the Efexor-XR brand including encouraging doctors to tick the brand substitution not permitted box, and providing attractive offers to pharmacists which could include bundling Efexor-XR, discounts on other products, and competitive pricing. As Wyeth put it:
Mr Hurley also agreed with the range of marketing techniques that Mr Cooper and Ms Braithwaite suggested would or could have been employed by Wyeth and Pfizer. In his view, they comprised “the standard suite of response” that an originator pharmaceutical company might employ as part of an overall defence strategy against generic competition”, which he would have expected Wyeth and Pfizer to employ.
913 Wyeth noted also that Mr Cooper of Pfizer which acquired Wyeth on 15 October 2009 said he believed that Pfizer would have developed and implemented the following strategies:
(a) Launching a second brand of venlafaxine;
(b) Creating an improved “value proposition” for pharmacies by reducing the price of Efexor-XR and/or the second brand of venlafaxine, and/or a bundle of products;
(c) Partnering with Ascent (formerly Genepharm) or another generic pharmaceutical company to promote and distribute a bundle of Pfizer products, including Efexor-XR and/or the second brand of venlafaxine;
(d) Utilising Pfizer’s pharmacy field force to promote and distribute a bundle of products, including Efexor-XR and/or the second brand of venlafaxine; and
(e) Focusing on encouraging doctors prescribing Efexor-XR to tick the brand substitution not permitted box.
914 Similarly, Mr Dick, the expert with substantial experience in the originator pharmaceuticals industry, said that it would have been logical for Wyeth to take action to protect its market share in Efexor-XR.
915 Wyeth also submitted:
(a) Pfizer had great human and financial resources which could have been directed to support its major antidepressant franchise;
(b) A likely strategy by Pfizer would have been to launch a second brand of venlafaxine. Ms McTavish estimated that the earliest date for PBS listing of second brand of venlafaxine by Wyeth or Pfizer would have been 1 April 2010, and stated that:
In the absence of its own generic sales team, the agreement with Ascent gave Pfizer immediate access to a sales team which could implement pharmacy strategies.
(c) Pfizer would have implemented a strategy whereby an improved “value proposition” for pharmacies was offered by reducing the price of Efexor-XR, the second brand of venlafaxine and/or a bundle of products;
(d) In a private market scenario, Pfizer would have been in a position to provide competitive offers in relation to every venlafaxine prescription dispensed, although the experts differ as to the timing of the commencement of an aggressive commercial strategy to promote Efexor-XR. Mc McTavish estimated the market share which the generics may have obtained in the private market in Counterfactual Scenario C, and considered that the rate of generic substitution would decline after Pfizer adopted its aggressive strategy.
916 As with all of the evidence of this kind, however, nothing is as straightforward as it might first seem. The evidence on which Wyeth relied lacks context and when context is added the unreliability of the evidence is exposed.
917 First, as Sigma put it:
Overarching the consideration of Wyeth Australia and Pfizer Australia’s likely defensive strategies in the counterfactual world, is the acquisition of Wyeth by Pfizer. The global acquisition was announced in late January 2009, and completed on 15 October 2009. Pfizer Australia assumed control of Efexor XR on ‘Day 1’ which was 16 October 2009. Thus Wyeth Australia’s likely defensive strategies are only relevant until Day 1 (16 October 2009) when it handed over responsibility of Efexor XR to Pfizer Australia. Equally Pfizer Australia was constrained by the terms of the acquisition as to what it information it had and what actions it could take prior to Day 1.
918 While I do not doubt that Wyeth’s employees would have diligently discharged their duties at all times up to 15 October 2009, the sale of Wyeth would have acted as an inevitable constraint on Wyeth’s decision-making. It should be inferred that it would not have taken any decision that might materially prejudice Pfizer’s future options other than after serious consideration and only if considered necessary. Launching its own “generic” version of Efexor-XR or substantially discounting the price of Efexor-XR would have been irrevocable decisions significantly constraining Pfizer’s future options. This may explain why Ms Braithwaite did not mention this option as one within Wyeth’s contemplation in response to generic entry into the market in her 2009 affidavits.
919 Second, Wyeth’s contemporaneous documents apart from Ms Braithwaite’s affidavits from 2009 also do not suggest that its first options in any generic defence strategy would have included launching its own generic version of Efexor-XR. Rather, Wyeth was focused instead on Pristiq which was launched in February 2009 and which Wyeth was positioning as a superior product to Efexor-XR. It is the existence of Pristiq which explains why Wyeth ceased promoting Efexor-XR by the end of 2008 and focused all of its marketing efforts on Pristiq. As Sigma also noted:
Mr Dick agreed that Wyeth Australia had stopped promoting Efexor XR in late 2008 and that in the real world there was no marketing support for Effexor XR for the balance of the period until Day 1. In his view if Wyeth was facing potential generic entry, it made no sense to continue promotion of Efexor XR as otherwise the generics would benefit from a share of any new prescriptions gained by Wyeth Australia’s promotional effort.
920 Third, Wyeth could have sought to register a generic version of Efexor-XR on the ARTG as a preliminary step in a defensive strategy without constraining Pfizer’s future options but it did not take even this step. Again, this is explained by the fact that its primary interest and strategy was focused on promoting Pristiq which was registered on the ARTG in August 2008 and listed on the PBS on 1 February 2009.
921 In these circumstances, the prospect that Wyeth would have taken any material irrevocable step in respect of Efexor-XR before the completion of the sale to Pfizer is negligible. In particular, Wyeth was not contemplating launching a second brand of Efexor-XR in 2009 as a response to generic entry into the market. It was contemplating that it might have to discount the price of Efexor-XR in response to generic entry but, in the context of the sale to Pfizer, it must be inferred that it would not lightly have done so and in any event it would have carefully tailored any discounting to the competitive circumstances from time to time. It follows that the issue of discounting by Wyeth is better considered in the context of the likely success or otherwise of the generic entry into the market.
922 In respect of Pfizer, the evidence is that Pfizer had its own people and its own plans. Pfizer did not continue to employ the Wyeth employees dealing with Efexor-XR. Pfizer had just acquired an international business of which Efexor-XR in Australia formed one minor component. As Sigma noted, an internal Pfizer email of 22 October 2009 said:
Efexor (Aus)
• 50% Chance of retaining Efexor patent until 2017 – Wyeth has no strategies in place to manage brand if patent is held.
• No promotion since Oct/Nov 2008
• Pristiq strategy is to grow initiations only and not switch to Efexor
• No second brand of Efexor registered with TGA.
923 There is no reason to infer that the position would have been any different if not for the interlocutory injunctions. Pfizer was and would have been starting from scratch in respect of Efexor-XR. It was also confronted with the same commercial issue with which Wyeth had to grapple. There was a new product, Pristiq, on which Wyeth had been focused and no benefit could be obtained by sales of the higher priced and long-term patent protected Pristiq being cannibalised by sales of a lower priced authorised generic version of Efexor-XR. Given that it was only recently released, the performance of Pristiq was not so poor that the entire strategy of focusing all marketing efforts on it rather than Efexor-XR would have been lightly abandoned.
924 Mr Cooper’s evidence that if not for the interlocutory injunctions everything Pfizer ultimately did to protect the market share of Efexor-XR would have been done much earlier suffered from the same kinds of problems as all of the other evidence of this kind (extending to the bizarrely specific proposition that this all would have happened because amongst other things he would have moved from New Zealand to Australia at an earlier time but for the interlocutory injunctions). In any event, as Mr Dick noted, planning a generic defence strategy was a complex and time consuming exercise requiring skill and expertise. No matter what its resources Pfizer could not have worked out its strategy or been in a position to implement it for many months after 15 October 2009. A Pfizer document from November 2009, for example, estimated that it would take 6 months to obtain all approvals necessary to launch a second brand of Efexor-XR and that promotion of Efexor-XR and Pristiq had to be co-ordinated. These were not straightforward issues and no matter what might now be said years after the events in question, in the context of a global acquisition and global re-focus of Pfizer’s business to develop a generic capacity, Pfizer would not have rushed its decision-making about a second brand of Efexor-XR, particularly not if it could protect its position by a less radical option such as discounting the price of Efexor-XR.
925 At best it should be accepted that PBS supply by generics would have caused Pfizer to try to accelerate its decision-making processes, but as Sigma submitted (and as I accepted for the generics and thus must accept also for Pfizer):
In the counterfactual world Pfizer Australia would have analysed all the different scenarios and come up with the best commercial outcome. However, Mr Cooper agreed that any plan of attack depended on the advice that he got from legal and regulatory, and he says that in terms of the counterfactuals he can’t tell what the advice would have been and what Pfizer Australia would have done.
926 Further, as Alphapharm submitted (with some amendments to reflect my views):
(1) in 2009 Mr Cooper had little experience relevant to selling generic products on the Australian market;
(2) the type of salesforce employed by Pfizer was one developed for the purpose of selling to doctors, comprising highly trained and often university educated representatives, some of them doctors themselves, not experienced in selling to pharmacies;
(3) Pfizer entered into a deal with Genepharm to sell to pharmacies shortly after the closing on 27 October 2009 because it did not have the capability to sell to pharmacies itself;
(4) the Genepharm pharmacy field force was very small compared to Sigma’s and Alphapharm’s;
(5) Pfizer needed time to consider and make a decision whether to continue with the Wyeth strategy of promoting Pristiq before deciding whether to launch its own venlafaxine generic, because the strategy of increasing the market for Pristiq would be undercut by the launch of Pfizer’s own generic, which would have the effect of maintaining rather than reducing the market for venlafaxine;
(6) the launch by Pfizer of its own generic before waiting to see the final decision in the litigation would also have involved Pfizer in imposing downward price pressure on Efexor-XR in circumstances where the primary objective of continuing with the patent litigation to a final hearing in the counterfactual would have been to attempt to re-achieve exclusivity in the venlafaxine market; and
(7) Pfizer would have preferred certainty about the final outcome of litigation, not just the interlocutory stages of litigation, before deciding whether to launch its own generic.
927 I also accept Alphapharm’s submission that the course of Mr Cooper’s oral evidence including his re-examination suggested a considerable degree of confusion about the context and internal logic of his evidence. As I have said repeatedly this was a hallmark of much of this kind of evidence in the present case and it is hardly surprising that Mr Cooper had a tenuous grasp on the exercise he had been asked to undertake. Mr Cooper’s confusion about venlafaxine being “off patent” is an example of this. As Alphapharm said:
The attempt to fix this misunderstanding in re-examination by reference to the material in Mr Cooper’s affidavit, and by suggesting that the cross examiner had confused Mr Cooper, only served to illustrate that the affidavit evidence was not properly understood by Mr Cooper. That lack of understanding explains why Mr Cooper did not give any weight in his affidavit evidence to the plainly overwhelming commercial factor that Pfizer would not launch its own generic during the litigation whilst there was any possibility of it retrieving its position of exclusivity in the venlafaxine market. It also explained why Mr Cooper was so adamant that Pfizer would in the counterfactual launch its own generic immediately – obviously in the off patent situation, as opposed to the no interlocutory injunction situation, Pfizer would have nothing to lose by launching its own generic. Accordingly, Mr Cooper’s evidence in relation to the counterfactual scenarios should be given no weight.
928 I accept also that is relevant that, as Alphapharm put it:
…even though an appeal was lodged immediately upon Wyeth being successful in November 2010, and was heard in February 2011, by May 2011, Pfizer still did not have in place any plans for a pharmacy field force to promote Efexor and there was no generic venlafaxine product in Genepharm’s agreement. By May 2011, some three months after the Full Court hearing, Pfizer could not have reasonably assumed that any decision by the Full Court would extend beyond 2011… In those circumstances, the lack of any mature contingency plans in May 2011 to launch its own generic confirms that Pfizer’s approach was to wait and see whether it could achieve its ideal outcome (i.e. winning the litigation finally), which would involve maintaining exclusivity in the venlafaxine molecule. There is no reason to think its conduct would have been any different in the counterfactual.
929 As Alphapharm also said (in submissions which sound increasingly similar to those Wyeth put about the generics’ hypotheses and which I also accepted):
… while Mr Cooper confidently asserted in his affidavit that he could have launched a Pfizer generic venlafaxine product in 2009, his affidavit indicates that he would have relied on modelling before making any such decision. He did not exhibit to his affidavit any modelling, whether modelling which was actually done in the real world, or modelling of the kind which might have been done in the counterfactual world. Thus a fundamental underpinning of his opinion is absent.
930 Pfizer’s decision-making also would have been constrained by the fact that it was in the early phases of introducing its overall generic strategy. It is contrary to commercial sense to infer that a global strategy would have been driven by one product in Australia. Pfizer did not have a pharmacy sales force until late 2010 or early 2011. It did not have the kinds of relationships with pharmacies the generics had. Its primary relationships were with doctors. It initially outsourced its pharmacy sales force work to Genepharm. While Mr Cooper would have it that Pfizer would have rapidly created a pharmacy sales team if not for the interlocutory injunctions, this too is difficult to accept as a realistic prospect.
931 As Sigma submitted, to the extent that Pfizer would have pursued a “tick the box” strategy for doctors:
Mr Dick’s opinion was that ‘tick the box’ non substitution campaigns are ‘hopeless’ and that he did not place weight on them as a defensive strategy as ‘very few doctors actually tick the box, even if you tell them to, and I’m not entirely sure that pharmacists would respect the box being ticked.’ Ms McTavish also considered that tick the box campaigns were of limited success.
932 Mr Cooper also agreed that Pfizer’s strategies would be tailored to the particular market within which it was competing. As Sigma submitted:
In the private market scenario where the generics were only in a position to target a subset of the market (40%) Mr Cooper expected that Pfizer’s discounting would be less aggressive. Mr Dick’s evidence was consistent with Mr Cooper’s, he agreed that the smaller the market share of the generics, the less aggressive the originator would discount.
933 For these reasons I do not consider that I should give any weight to the possibility that Wyeth or Pfizer might have launched a second brand of Efexor-XR at any time earlier than it did. Wyeth’s actions would have been heavily constrained by the sale to Pfizer. Pfizer would have considered discounting the price of Efexor-XR if considered necessary to respond to competitive pressures from time to time. However, Pfizer’s willingness to discount also would have been constrained to some extent by the practically irrevocable nature of such a decision given the ultimate objective of retaining the monopoly by reason of the method patent. The issue of discounting is best dealt with in the context of the econometric evidence. The prospect of Pfizer having launched an authorised generic at any time earlier than it did involves pure speculation and thus should be disregarded.
The PBS listing issue
934 I have made findings above about the chance of any generic applying for listing of their venlafaxine products at any time earlier than the Full Court’s decision in October 2011, assuming the interlocutory injunctions had not been sought and granted. This part of these reasons concerns the posited response of the Minister’s delegate to any such application for listing. Wyeth would have it that the products would not be listed on the PBS because of the litigation. As explained below, this contention is without merit.
935 For the purpose of resolving this issue it is necessary to infer and on the evidence I would infer that had they decided to seek PBS listing of their products the generics would have done so in accordance with all applicable requirements including the provision of the assurance of supply. As noted, to obtain PBS listing the generics would have been required by the policy of the Department of Health to submit a written assurance of supply that “sufficient stock of the product to meet anticipated demand will be available at the time of listing on the PBS”. As the necessary construct is that the interlocutory injunctions would not have been sought or granted, the requirement for this assurance would not have caused any difficulty. The generics would not have anticipated any difficulty with their capacity to supply at the potential PBS listing dates of 1 December 2009 and 1 March 2010 if not for the interlocutory injunctions because the final hearing had been fixed for a later time. Nor do I accept that the statutory guarantee of supply provisions would have been a material factor for the generics’ decision-making.
936 Accordingly, if it must be posited that on each of the relevant dates (22 May, 14 August and 10 November 2009) Wyeth did not prosecute its applications for interlocutory relief then it follows that each generic would have known that it could provide the assurance of supply which related only to the date of PBS listing.
937 Insofar as the statutory guarantee of supply provisions are concerned, as explained below, the statutory scheme does not in fact require supply to be maintained. It requires notice to be given if the responsible person believes that supply will not be maintained and then empowers the Minister, amongst other things, to de-list the brand in question. Given their belief that they had good prospects on the issue of final relief, the generics would have had no reason to believe that they “will fail to supply, or will be unable to supply, the guaranteed brand of the guaranteed item” unless and until they were the subject of a final injunction. The assurance of supply and statutory guarantee of supply issues, accordingly, would have been immaterial to the question of the generics’ conduct if Wyeth had not sought the interlocutory injunctions.
Discussion
938 The first relevant matter is that no-one, and particularly not Wyeth, suggested at any time before these claims for compensation were made that if a generic had applied to list its venlafaxine products on the PBS, the products would not have been listed due to the litigation.
939 It must be inferred from all of the evidence that in 2009 and at all times and for all purposes other than for the claims under the undertakings Wyeth and the generics were operating on the basis of a common assumption that if the generics applied for PBS listing they would obtain PBS listing. There is never a hint in any of the evidence from 2009 that Wyeth or Wyeth’s lawyers or the generics or the generics’ lawyers contemplated that the mere existence of the method patent and the litigation meant that if the generics applied for PBS listing the Minister’s delegate would or even might refuse to list the products.
940 For example, there is never a suggestion in any document, be it an internal or external document of Wyeth or the generics or their respective lawyers, that:
(1) the generics would not be able to make a PBS listing application including providing the guarantee of supply because Wyeth might obtain final relief as a result of which the generic products could no longer be supplied;
(2) any guarantee of supply by a generic as part of a PBS listing application would or might be false, misleading or deceptive because Wyeth might obtain final relief as a result of which the generic products could no longer be supplied;
(3) the Department would or might consider that any such guarantee of supply would or might be false, misleading or deceptive because Wyeth might obtain final relief as a result of which the generic products could no longer be supplied;
(4) the Minister’s delegate would or might refuse to list any generic venlafaxine product on the PBS because Wyeth might obtain final relief as a result of which the generic products could no longer be supplied;
(5) Wyeth need not be concerned about the potential for a generic to obtain PBS listing of any generic venlafaxine product for any reason of a kind similar to (1) to (4) above; or
(6) Wyeth need not obtain orders to prevent Alphapharm and Generic Health from applying for PBS listing for their generic venlafaxine products for any reason of a kind similar to (1) to (4) above.
941 This is in circumstances where Wyeth, to the generics’ knowledge, was determined to do everything it could to protect the monopoly to which it believed it was entitled by reason of the method patent.
942 These matters are relevant because it must be inferred that Wyeth, the generics and their respective lawyers were familiar with the practices of the Department of Health and its various branches and with the exercise of power under s 85(6) of the National Health Act by the Minister’s delegate. Given that it must be inferred that no-one believed at the time that the mere existence of the method patent and litigation would or might mean that the generics’ products would not be PBS listed if they applied, Wyeth’s current contentions to the contrary effect are inherently unpersuasive. I would not go so far as to say Wyeth’s contention is an abuse of process or should not be permitted on a discretionary basis (as for the copyright issue) but the contention is inconsistent with the shared assumptions within which Wyeth sought and obtained the interlocutory injunctions and thus deserves short shrift.
943 Another relevant matter is that the Commonwealth has a clear incentive to manage the PBS scheme in a financially sustainable manner. The scheme was required to be administered in the known context that generic products are an essential aspect of the financial sustainability of the PBS scheme which provides Australians with access to Commonwealth subsidised pharmaceutical products.
944 A further relevant matter is the guarantee of supply provisions which were introduced into Div 3C of Pt VII of the National Health Act in 2007. It may be accepted that, as Wyeth said:
A concern to protect against unfairness underpinned the 2007 reforms…The purpose of the supply guarantee, as revealed from the terms of Part VII and the secondary materials (see Revised Explanatory Memorandum to the National Health Amendment (Pharmaceutical Benefits Scheme) Bill 2007, p 2), is to discourage listing when there is no “guarantee” of supply for a period of up to two years. This in turn avoids adverse consequences in terms of pricing and payments under the PBS, in circumstances where the generic cannot meaningfully participate in the market. It also prevents supply disruptions for patients and pharmacists.
These principles and concerns are reflected in the Department’s own publications. The Department published a PBS Reform Fact Sheet which relevantly stated:
The guarantee of supply provisions will deter suppliers from entering the Australian market without a viable business model able to support their long term participation in the market. Interruptions to supply are disruptive for patients, prescribers and pharmacists. The provisions also ensure that the government has as much notice as possible about supply failures so that their impact on patients, prescribers and pharmacists can be minimised.
945 The unfairness was that without a guarantee of supply the listing of a first generic product on the PBS would cause the automatic 12.5% price reduction for the originator product in circumstances where the generic product would not create effective competition because it was not available for supply.
946 The essence of the current debate is this. It is the generics’ position that the Commonwealth’s undoubted intention to ensure that PBS listed products could and would be supplied as required was given effect by requiring that the responsible person submit with the PBS listing application the “written assurance” that “sufficient stock of the product to meet anticipated demand will be available at the time of listing on the PBS” as provided for in the applicable guidelines. If that assurance was given, in effect, the supply issue would be taken to be addressed.
947 It is Wyeth’s position that in order to make a rational and lawful decision, notwithstanding the provision of the written assurance:
If the decision-maker has reason to conclude that the sponsor is not, or should not be, “confident of their ability to supply the brand in the longer term”, this is a matter required to be taken into account by the decision-maker in considering such an application.
948 In other words, as Wyeth would have it, the delegate could not and would not accept the assurance of supply at face value. In the context of an application to list generic venlafaxine products on the PBS in the face of the unresolved patent litigation in 2009 to 2011, Wyeth contended the delegate would not have proceeded to list the products.
949 In my view, to the extent it deserves any attention, Wyeth’s approach involves a misconception about the statutory scheme. Assuming the power under s 85(6) is administrative rather than legislative (an issue not raised by the parties) the sole source of any mandatory relevant consideration for the exercise of that power is the provisions, objects and purpose of the National Health Act. Under the statute, there are no identified matters which the Minister (or Minister’s delegate) must take into account. The exercise of power, if it occurs, takes place in the context of a statute which has its own regime, Div 3C of Pt VII, regulating the issue of supply. Under that regime, the responsible person must supply and must notify the Minister if the person cannot supply which then enables the Minister to take certain actions, including de-listing. There is no basis to conclude that the Minister or Minister’s delegate in exercising the power in s 85(6) is intended to attempt to second-guess the issue of supply which is regulated by Div 3C of Pt VII. The fact that the Department sensibly implemented procedures to obtain from the responsible person an assurance of supply at the date of PBS listing does not mean that the Minister or delegate is bound to (or even could) try to second-guess the capacity of the responsible person to comply with its statutory obligations.
950 As the Commonwealth submitted:
(a) the scheme does not create a “guarantee” of supply in a strict sense with freestanding enforceable obligations at general law;
(b) rather the scheme imposes on the responsible person of a guaranteed brand an obligation to report a failure or inability to supply, whilst also conferring upon the Minister certain discretionary powers that may be exercised if and when there is a failure or inability to supply the guaranteed brand; and
(c) the consequences of an inability or failure to supply are primarily to be determined if and when the inability or failure occurs, or where the supplier of the brand actually forms a belief that the failure or inability will occur.
Thus, the mere possibility of an inability or failure at some point in the future has no consequences under the NHA. Rather, the NHA contemplates that the Minister or delegate can deal with the issues surrounding the failure or inability to supply if and when it occurs and depending on whether the circumstances warrant the exercise of the discretionary powers in section 99AEG. The risk that a generic brand will become unavailable as a result of the determination of patent proceedings at some unknown time in the future does not dictate (or even suggest) that the brand should not be listed in the first place.
951 I also agree with the Commonwealth’s submissions as follows:
It is not possible in this case to derive a mandatory requirement to consider the capacity to meet the obligation to supply in section 99AEB of the NHA by “implication from the subject matter, scope and purpose” of the NHA [citing, in support, Minister for Aboriginal Affairs v Peko-Wallsend Ltd [1986] HCA 40; (1986) 162 CLR 24, 39-42]. The Minister’s powers in section 99AEH suggest that the consequences of a failure to meet the obligation of supply are to be determined if and when the failure occurs, rather than pre-emptively at the point of the determination to list a particular brand. As the Full Court held in Warner-Lambert Company LLC v Apotex Pty Limited [[2017] FCAFC 58; (2017) 249 FCR 17] at [18], “the guaranteed supply provisions do not operate on the making of an application”. This is confirmed by the discretionary nature of the powers in section 99AEH. The Minister has the power to permit a brand to remain listed notwithstanding a failure to fulfil the obligation of supply. This tells strongly against the contention that the Minister must consider a responsible person’s capacity to fulfil the obligation of supply at the point of giving consideration to the application to list.
The facts of this case demonstrate that it is extremely unlikely that the Parliament intended that the Minister must consider the capacity to meet the obligation of supply in every case when determining a listing application. The Minister is not in a position to determine the merits of a patent or copyright dispute; nor can she predict the future. A disputed patent may or may not be held to be valid; supply of a generic brand may or may not be an infringement. The question of whether a patent holder’s asserted monopoly will at some future point in time operate to restrict a responsible person’s ability to supply cannot be determined at the point of listing simply by reason of the fact that there is pending litigation. Wyeth’s contention misconstrues the obligation placed upon certain responsible persons to supply a brand upon listing in section 99AEB as a duty upon the Minister to determine, in advance and with unachievable certainty, whether that obligation will be fulfilled.
952 In any event, Wyeth’s contentions are contrary to the evidence of Ms McNeill, an Assistant Secretary of the Pharmaceutical Evaluation Branch between 4 January 2010 and 27 September 2010 and thereafter First Assistant Secretary of the Pharmaceutical Benefits Division (PBD) within the Department until June 2015.
953 Wyeth’s submissions about Ms McNeill’s evidence were unconvincing. It may be accepted that Ms McNeill was not involved in the workings of the Department before 4 January 2010. As noted, however, even on the generics’ best case the earliest possible listing date of any generic product was 1 August 2009. This is a mere seven months before Ms McNeill took up her role. Further, I have concluded that the relevant possibility of first PBS listing was Sigma on 1 December 2009, a mere month before Ms McNeill took up her role in which she held the relevant delegation from the Minister. As a result of her role Ms McNeill was required to become (and on her evidence did become) familiar with all of the applicable policies, guidelines and practices of the Department about, amongst other things, PBS listing. Given this, Ms McNeill was able to give probative evidence about the Department’s policies, guidelines and practices relating to PBS listing of generic brands for all potentially relevant periods between 2009 and 2012. Wyeth’s submission to the contrary depends on the misconception that the Department lacked any kind of practical and procedural continuity. This proposition is illogical and contrary to the evidence. It would have been impossible for Ms McNeill to fulfil her function if she had not obtained knowledge of the Department’s policies, guidelines and practices in the weeks and months from January 2010. Those policies, guidelines and practices did not emerge from nowhere. They had been developed within the Department over time. There is no evidence of any relevant material change between 2009 and 2010 and, indeed, the evidence is to the contrary. As the Commonwealth submitted:
The close temporal connection between those dates [August or December 2009] and the commencement of her employment [January 2010] allows the Court to draw an inference of continuity of practice.
954 In these circumstances, the submission that Ms McNeill could not give probative evidence about the relevant policies, guidelines and practices in 2009 is without merit. Similarly, the submission that the Commonwealth could have called evidence from people who held the relevant delegation to exercise the power in s 85(6) of the National Health Act in 2009 is beside the point. Ms McNeill held the relevant delegation from 4 January 2010. She was capable of giving probative evidence about the policies, guidelines and practices of the Department in relation to PBS listing for the whole of the relevant period. There is no unexplained failure to adduce relevant evidence and thus no inference that such evidence would not have assisted the claimants’ case can arise.
955 The dispute about whether Ms McNeill was an expert in respect of the Department’s relevant policies, guidelines and practices is also beside the point. Ms McNeill held the relevant delegation from January 2010. There can be no doubt that for that period onwards she was capable of giving relevant evidence about how she exercised her powers in accordance with the Department’s relevant policies, guidelines and practices. It is not suggested, and nor could it be, that Ms McNeill exercised her powers as a delegate in some arbitrary, capricious or idiosyncratic way. To the contrary, the evidence shows that the exercise of powers by a delegate was carried out within a confined framework of specific policies, guidelines and practices. Her evidence is thus also evidence of how a delegate is likely to have exercised powers. As discussed above, where the evidence is also to the effect that there was no material change in the Department’s relevant policies, guidelines and practices between 2007 and 2012 it must be inferred that Ms McNeill’s evidence is as probative of the likely facts in 2009 as it is for the subsequent periods. Wyeth’s submissions to the contrary are divorced from reality.
956 It may be accepted that Ms McNeill never made a listing decision of the kind that would have been required in the present case had any generic applied for PBS listing of their generic venlafaxine products after Wyeth is to be taken not to have prosecuted its claims for interlocutory relief. This does not mean that Ms McNeill was unable to give cogent evidence about how she and thus by inference other delegates would have approached the same task be it in 2009, 2010, 2011 or 2012. It is also not to the point that the relevant guidelines and policy documents do not deal with the specific circumstances which would have arisen if any generic had applied for PBS listing of their generic venlafaxine products before the Full Court’s decision in October 2011. As a result of her role from January 2010 Ms McNeill must have been steeped in the Department’s culture, practices and procedures. While it is theoretically possible that the Commonwealth might have called Ms McNeill’s predecessor for the 2009 period and then Ms McNeill for the period from January 2010 onwards it is difficult to imagine a person better placed to give evidence about these issues than the person filling this role. There was also evidence that Ms Mc Neill’s predecessor was no longer an employee of the Department and was unwilling to give evidence. Ms Mc Neill’s evidence was thus the best possible in the circumstances.
957 Ms McNeill’s evidence was also of a different character from the other hypothetical evidence in this proceeding. The other hypothetical evidence was from witnesses suggesting what commercial recommendation or decision they would have made or reached in circumstances which did not exist and the complexity of which for the most part the witnesses had not taken into account. The most helpful parts of Ms McNeill’s evidence were not of this kind. It was her evidence about her knowledge of and experience as a delegate in the Department which was the most useful.
958 Wyeth said that the effect of Ms McNeill’s evidence was that “as the decision-maker, her only concern would have been to ensure that an assurance of supply had been given by the sponsor, whether or not that assurance of supply is considered to be reliable”. This is an over-simplification of the whole of Ms McNeill’s evidence. As, for example, Wyeth noted:
Ms McNeill was asked to consider a scenario in which there was an application for PBS listing of a trigger brand in circumstances where there was an unresolved patent dispute, no interlocutory injunction and a trial coming up: T938.8-44. Ms McNeill stated “as long as I have the assurance of supply from the generic provider and until such time as they withdraw it I proceed as proposed”: T938.47-T939.2. The following exchange then took place, at T939.4-42 (emphasis added):
So if there’s a situation where the generic provider has provided an assurance of supply but there is material such as – well, if there’s material that makes you think that there is a significant risk that they won’t supply, do you defer your judgment as to whether or not they will supply to what they say? But what type of supply are you talking about?
The supply of their – of their new – newly listed first generic product, so somebody applying for a trigger brand says “We give you the assurance of supply” but they say “Look, there is a risk, there’s a significant risk we could lose this litigation but we’re prepared to take that risk.” Now, my question is in circumstances like that do you just not exercise your own judgment as to whether the risk will come. You just accept their judgment as to whether they’re prepared to accept the risk? I’m exercising my judgment but I’m very clear that it is up to the generic company to tell me, they must fulfil the assurance of supply. They are the ones that actually have to tell me that and thereafter if they do not feel – if they feel they will fail to supply they can then issue me a statement under the guarantee of supply provisions up under the National Health Act. But at the point I can’t be second-guessing what a court may or may not decide so therefore I proceed.
Can you be second-guessing what the person who gives you the piece of paper that’s called an assurance of supply would decide. Can you second-guess that? I expect that when people complete that form that they understand the intentions behind that and they proceed. And I – my experience with how these have been approached in the past and how I dealt with them once I was in the position is that the generic companies were very clear of providing assurance of supply, they were very clear in providing conditional assurance of supply and opportunities when they would choose to withdraw based on court hearing times. I had examples where we were proceeding with a – a listing and they said that because a court date had changed they withdrew their assurance of supply and their listings. So that is the basis on which I give you my advice.
Yes. They are all scenarios where they’ve given either conditional assurance of supply or an assurance of supply and withdrawn if they think they’re going to be unable to supply? Mmm.
But what I’m putting to you is a situation where they are prepared to take the risk that they will be unable to supply even though they acknowledge that there’s a risk. Do you at that point second-guess them? I consider what’s put before me and I proceed with the listing until such time as they tell me otherwise.
959 Wyeth described this evidence as a “doggedly formalistic approach to the assurance of supply” which should not be accepted to represent a “reasonable and lawful approach to the administration” of the National Health Act. It said that “it would be absurd for a delegate to rely on an assurance of supply as indicating that there will in fact be supply on and from the date of listing if the delegate knows that the assurance is not reliable and there is reason to doubt the capacity of the sponsor to supply”. It said such an approach would be unreasonable and involve legal error.
960 The problem with all of these submissions is that they do not confront the entirety of Ms McNeill’s evidence nor the fact that the relevant issue concerns the particular circumstance of unresolved patent litigation where (as must be posited) the interlocutory injunction applications would not have been pursued by Wyeth.
961 As noted, Ms McNeill confirmed that she had not made a decision in the circumstances that would have applied in the present case had a generic made a PBS listing application for generic venlafaxine products before the Full Court’s decision in October 2011. Nor was she aware of any other delegate having done so. But these are not reasons to conclude that her evidence was unreasonable or still less would have involved legal error. Apart from the fact that this evidence accords with what must be inferred to have been the common understanding of Wyeth and the generics in 2009 (that is, that if any generic had applied for PBS listing as required and when required the product would have been listed on the PBS in the ordinary course) the evidence also accords with the legislative scheme and common sense.
962 As the Commonwealth noted, Ms McNeill’s evidence was to the effect that:
(a) she is not aware of any delegate proposing to list a generic brand having conducted, or requested, any searches or inquiries as to whether the application (or the subsequent listing of the generic brand) would infringe a patent;
(b) she has not conducted or requested such searches or inquiries, or received a request from another delegate to do so;
(c) she is not aware of any evidence of a delegate taking into account patent matters when making a determination to list a first generic brand on the PBS between 1 August 2007 and 1 April 2012;
(d) since she joined the Department in January 2010, PEB has not questioned assurances of supply given by any generic applicant prior to listing – the assurance is generally taken at face value; and
(e) based on her knowledge and experience, the same approach in respect of the assurance of supply was taken during the period 2007 to 2009, such that if an applicant did not provide a written assurance of supply, or if a generic applicant withdrew the assurance, PEB would not request the delegate to list the applicant’s generic brand. Conversely, if an assurance of supply was provided and not withdrawn before listing, it was taken at face value.
963 There is good reason for the Department to have adopted an approach of not attempting to second-guess what might happen in an unresolved patent dispute in circumstances where there is no interlocutory injunction preventing supply. Insofar as the assurance of supply is concerned, the required assurance was that “sufficient stock of the product to meet anticipated demand will be available at the time of listing on the PBS”. The primary purpose of the guarantee of supply is to ensure that the responsible person is and will be capable of supplying the product to be listed on the PBS for the required minimum period. Insofar as the legislation is concerned, the person “must supply” as required and must notify the Minister as soon as possible if the person cannot supply as required in which event de-listing may occur: ss 99AEB, 99AEG and 99AEH. Being unable or failing to supply is not an offence but failing to notify the Minister as required is an offence: s 99AEG(3). Further, the Minister may (amongst other things) increase the approved ex-manufacturer’s price or AEMP (formerly the APP) if a guaranteed brand is de-listed: s 99AEI.
964 In circumstances where the statutory scheme discloses the Commonwealth’s objective in ensuring supply of PBS listed products and the financial sustainability of the PBS, for the Minister or Minister’s delegate to frame a PBS listing decision-making by reference to the possible outcome of patent disputes would be likely to result in serious maladministration. As discussed, the National Health Act cannot be construed as requiring the decision-maker to consider that matter and a rational decision-maker would not undertake that task. Some potential difficulties and impracticalities of the Department adopting such an approach to its listing powers are obvious. For example:
(1) At what point would the Minister or Minister’s delegate be taken to have knowledge of the patent dispute so as to engage its obligation of consideration of this matter? One answer might be the date on which the Secretary of the Department of Health receives a certificate under s 26C of the Therapeutic Goods Act but the Minister or Minister’s delegate might be aware of a potential patent dispute before any such certificate is lodged.
(2) How would the Minister or Minister’s delegate give weight to the allegedly mandatory consideration? Would Minister or Minister’s delegate be bound not to PBS list because of knowledge of a patent dispute? Would Minister or Minister’s delegate be bound to consider the extent of the risk to supply which in turn would depend on which party might win the patent dispute? If so, how is Minister or Minister’s delegate meant to assess which party might win the patent dispute? Is Minister or Minister’s delegate meant to obtain legal advice on the prospects of the patent owner of obtaining an injunction to prevent supply? How would Minister or Minister’s delegate do so given that it is not a party and has no access to the claims, contentions or evidence?
(3) Why would the obligation of consideration be limited to a threat to supply from a patent dispute? Why would the obligation not extend to any potential threat to supply? How would the Minister or Minister’s delegate assess such potential threats?
965 What these problems disclose is that the assurance of supply was not concerned with the potential outcomes of patent disputes. It could not be concerned with this issue because there was no way the Minister or Minister’s delegate could rationally take that issue into account. The assurance of supply was concerned with the practical capacity of the responsible person to supply the product not whether the person might be restrained from supplying in the future. As Ms McNeill’s evidence disclosed, in such a case, the Department’s expectation would also be that the responsible person would withdraw the assurance of supply (which was what Sigma effectively did in anticipation of the interlocutory injunction). Given the provisions of Div 3C of Pt VII this expectation is reasonable and appropriate and Ms McNeill’s evidence is that responsible persons acted consistently with this expectation. It may be accepted that if the Department knew a product could not be lawfully supplied in Australia the delegate would not exercise the power to list the product under s 85(6) even if the assurance of supply had not been withdrawn. But this is a world away from Wyeth’s case that mere knowledge of unresolved patent litigation would or might prevent PBS listing.
966 It is not productive to consider the many hypothetical examples that were put to Ms McNeill. The effect of her evidence, insofar as relevant, remained the same. That evidence, moreover, was relevant to the Department’s overall process not just the so-called listing decision. It may be accepted that, as Wyeth put it, issues with products were resolved before the delegate considered the exercise of power under s 85(6). If relevant issues remained, in effect, the delegate would not be asked to exercise the power in relation to the product. This does not mean, however, that the effect of Ms McNeill’s evidence was confined to the exercise of power. Her evidence concerned the way in which the Department dealt with the issue as a whole.
967 Nor is it helpful to consider the dispute about the extent to which other examples of products might or might not provide meaningful analogies to the PBS listing of generic extended release venlafaxine products. I record only that although the circumstances are not identical to the present case (or posited case if the interlocutory injunctions were not granted) the examples are consistent with the conclusions which I have reached. The Commonwealth thus noted:
(a) two instances of applications for listing of first generic brands where patent proceedings were on foot, interlocutory injunctions were either not sought or not granted, and the first generic brands were listed: perindopril with indapamide (listed 1 August 2007); and docetaxel (listed 1 March 2011);
(b) two instances where applications for generic listing were made while patent litigation was on foot, and in the appellate stages, but where there were no orders in place restraining the generics brands, and the generic brands were listed while the litigation was ongoing: venlafaxine (listed on 1 April 2012); rosuvastatin (listed on 1 June 2013); and
(c) several examples (the collection is not exhaustive) where the Department received, and started processing applications to list first generic brands, despite patent litigation, and only ceased to do so when notified by the generic applicant that the application was withdrawn (following the grant of an interlocutory injunction): gemcitabine (2008); leflunomide (2008); fentanyl (2009); aripiprazole (2009); olanzapine (2009); and rosuvastatin (2012).
968 Otherwise, I reject Wyeth’s submission that Ms McNeill was advocating the Commonwealth’s case. As the Commonwealth responded:
First, the submission unfairly mischaracterises Ms McNeill’s evidence. Her answers were responsive and showed considerable knowledge of the Department’s policies and practices, as well as the rationale which underpinned those matters.
Secondly, it was never put to Ms McNeill that she was not giving truthful evidence or that she was tailoring her evidence to suit the Commonwealth’s forensic purposes.
Thirdly, it should not be inferred that the Commonwealth somehow chose Ms McNeill to give evidence to gain a forensic advantage. For reasons advanced in the Commonwealth’s closing submissions and explained in the affidavit of Mr Korbel, the delegate responsible for listing determinations in August and December 2009, Ms Jackson, was not willing to assist the Commonwealth. At the time Ms McNeill swore her first affidavit, she was the First Assistant Secretary of the Pharmaceutical Benefits Division and had been a delegate for more than five years. During that period she had been responsible for a majority of listing determinations in that period. Given those circumstances she was the obvious witness for the Commonwealth to call in relation to listing decisions.
969 Even without Ms McNeill’s evidence and the examples of other products, two matters would lead me to the conclusion that Wyeth’s contention about PBS listing should not be given any weight.
970 The first matter, as noted, is that the contention is inconsistent with what I infer was the common assumption of Wyeth and the generics about PBS listing in 2009.
971 The second matter is the nature of the statutory scheme and the administrative arrangements which the Department has put in place to administer that scheme as described above. Within that context for a delegate to act as Wyeth proposes would be irrational.
972 Having regard to that statutory scheme I also do not accept Wyeth’s submission that the Commonwealth was or believed it would have been exposed to a risk of a damages claim by Wyeth if the generics’ products had been PBS listed before the Full Court’s decision in October 2011. Had that been so then it would be inferred that Wyeth would have put the generics and the Commonwealth on notice of such a risk, but it did not do so or suggest so. Instead, Wyeth relied on Departmental documents from 2012 after the Full Court’s decision. The first said:
I am assuming (but am not sure) that as a result of this undertaking, the generic companies can seek to market and list on the PBS their venlafaxine products during the course of the stay on the Court Order. However, if Wyeth is eventually found to have the patent claims, Wyeth could take action in relation to any breach of their patent … the question is whether it is OK for us to list (if asked) or if this exposes us to a claim for ‘relief’ by Wyeth/Pfizer. I am not confident to say that Pfizer could not pursue us for relief in relation to any patent infringement. The related question concerns the consequences under the Act if the generic companies can PBS list now and then Wyeth is found by the High Court to have their patents. If any company with guarantee of supply obligations fails to supply (eg: they are no longer allowed to supply) and a 16% reduction and F1 to F2 movement was based on their listing, then both the 16% reduction and the F2 move can be reversed (and the 16% can be applied again later when a new brand lists). Any new generics confident about what happens if a later generic listing occurs (say a month or two after the first ones. That later listing extinguishes the ‘guarantee of supply obligations’ for the initial trigger brands. Then, if the later listing (and earlier listing) generics become unable to supply because of patent findings, then I am not sure we have the power to reverse the 16% and F2 moves because the only brand with guarantee of supply obligations did not trigger those changes. I think I previously raised this issue, but am not sure if legal advice was sought on it.
973 An email in response from the Director of the Pricing Section in PBD said:
Do you think we should get legal advice on the issue you have raised re reversing the 16% SPR etc in anticipation of the likely listing of further brands?? If the advice is that this is an issue we would presumably have to consider refusing to list further brands until the legal action is over, or change the NHA.
974 Wyeth said:
This email exchange…confirms that the Court should reject altogether Ms McNeill’s evidence that those responsible for processing and determining PBS listing applications did not, and would not have, paid any attention to patent litigation. To the contrary, the email demonstrates responsible officers having regard to precisely the kind of discretionary considerations that Wyeth submits would have been considered in 2009, had an application for listing been made by SPAL or Generic Health (see 729 above). It may be that the Department and the relevant delegate were ultimately persuaded that these concerns were not a sufficient reason to refuse to list generic venlafaxine products in the particular circumstances that existed in 2012. However, the significance of the evidence is to demonstrate that equivalent officers faced with applications for PBS listing in 2009 would have raised similar concerns and would not have simply listed the items “as a matter of course”, as the Commonwealth and Ms McNeill would have it. Those concerns would have been far more acute in 2009, when the matter had yet to been tried.
975 To the contrary, on the necessary hypothesis in 2009 Wyeth would not have been granted the interlocutory injunctions. The Department and delegate would have been confronted with nothing more than patent litigation. There was nothing more than a risk that in the future there might be a final injunction granted the effect of which would prevent supply (a position the Department had confronted before and which did not prevent PBS listing). Equally, there might not be any final injunction. The Department could not know one way or another. After the Full Court’s decision the Department was confronted by a more complex situation. I had found the method patent to be valid and granted final injunctions against the generics. The Full Court had allowed the generics’ appeal against my orders. The Full Court had found the method patent to be invalid and set aside my orders including the injunctions. The Full Court also made an order revoking the method patent but stayed that order during the maintenance of any special leave application and if special leave was granted until any appeal was determined. Generic Health on 29 November 2011 and Alphapharm on 1 December 2011 applied to list their products on the PBS (Sigma had been sold by this time). Wyeth applied for special leave to appeal to the High Court on 2 December 2011. A number of generic venlafaxine products were listed on the PBS on 1 April 2012 (the products of Generic Health and Alphapharm, and also of Apotex, Spirit Pharmaceuticals, Sandoz, Ascent and Ranbaxy). The statutory price reduction for Efexor-XR, by then 16%, occurred. Wyeth’s application for special leave was heard and refused later on 11 May 2012.
976 Thus the fact is despite the possibility of Commonwealth liability having been raised in the complex circumstances that existed in late 2011 and early 2012 Ms McNeill exercised the power in s 85(6) of the National Health Act to list generic venlafaxine products on the PBS. Even if the same concern had been raised in 2009 or 2010 there is no reason to infer other than that delegate (who was Ms McNeill in 2010) would have done anything differently.
977 To the extent it was pressed, for the same reasons I do not accept Wyeth’s contention that the Minister could not reasonably or in the proper exercise of discretion have made a determination under s 85(6) of the National Health Act to list Sigma, Alphapharm or Generic Health’s brands on the PBS in 2009.
978 For the same reasons Wyeth’s submission that it should be inferred that the generic venlafaxine products would not have been PBS listed before the Full Court’s decision in October 2011 due to negative public health impacts from the risk of patients being switched and then having to be re-switched between brands cannot be accepted. The submission again depends on the notion that the delegate might be able to second-guess the outcome of patent litigation. The delegate could not do so and thus could not weigh any potential health impacts on a rational basis.
979 In the circumstances described above it would be unfair, unreasonable and inequitable to impose any discount or deduction or make any adjustment to the compensation that might otherwise be payable to the generics (or the parties whose claims depend on the generics) on account of the chance that had the generics applied for PBS listing of their products the Minister’s delegate would have refused to do so because of the litigation about the method patent. The contention is inconsistent with the common assumption shared between Wyeth and the generics at the time of the interlocutory injunctions. It is inconsistent with the statutory scheme as a whole. Even if these conclusions are incorrect on the evidence the chance of any contention such as Wyeth identified having operated to prevent PBS listing is negligible.
The statutory provisions, facts and contentions
980 Wyeth contended that any sales Sigma would have made but for the Sigma interlocutory injunction would have been sales of products imported and supplied by Generic Health to Sigma in contravention of s 19D of the Therapeutic Goods Act. As a result the Court would not order Wyeth to pay Sigma or Generic Health compensation in respect of the posited supply by Generic Health and sale by Sigma of those products. Wyeth relied on Columbia Pictures Industries Inc v Robinson [1987] Ch 38; [1986] 3 WLR 542 to support its position and submitted that Les Laboratoires Servier v Apotex Inc [2014] UKSC 55; [2015] 1 AC 430 did not support a contrary conclusion.
981 Section 19D of the Therapeutic Goods Act provided that:
(1) A person contravenes this subsection if:
(a) the person does any of the following:
(i) imports into Australia therapeutic goods for use in humans;
…
(iv) supplies in Australia therapeutic goods for use in humans; and
(b) none of the following subparagraphs applies in relation to the goods:
(i) the goods are registered goods or listed goods in relation to the person;
(ii) the goods are exempt goods;
(iii) the goods are exempt under section 18A;
(iv) the goods are the subject of an approval or authority under section 19;
(v) the goods are the subject of an approval under section 19A.
Maximum civil penalty:
(a) for an individual—5,000 penalty units; and
(b) for a body corporate—50,000 penalty units.
(2) Subsection (1) does not apply if the person proves that he or she was not the sponsor of the goods at the time of the importation, exportation, manufacture or supply, as the case may be.
982 By s 3 of the Therapeutic Goods Act:
sponsor, in relation to therapeutic goods, means:
(a) a person who exports, or arranges the exportation of, the goods from Australia; or
(b) a person who imports, or arranges the importation of, the goods into Australia; or
(c) person who, in Australia, manufactures the goods, or arranges for another person to manufacture the goods, for supply (whether in Australia or elsewhere);
but does not include a person who:
(d) exports, imports or manufactures the goods; or
(e) arranges the exportation, importation or manufacture of the goods;
on behalf of another person who, at the time of the exportation, importation, manufacture or arrangements, is a resident of, or is carrying on business in, Australia.
983 Section 25(1) of the Therapeutic Goods Act is also relevant. It provides that “an application is made for the registration of therapeutic goods in relation to a person”. It is that person alone who can ensure that the other requirements of s 25 are satisfied. For example, if an application is made for registration, amongst other things, the Secretary must evaluate “(e) whether the presentation of the goods is acceptable”, “(g) if a step in the manufacture of the goods has been carried out outside Australia - whether the manufacturing and quality control procedures used in the manufacture of the goods are acceptable” and “(ja) whether all of the manufacturers of the goods are nominated as manufacturers of the goods in the application”. By s 25(3) the Secretary decides to register or not to register the goods which in context must mean, in relation to the person.
984 Pursuant to the contractual arrangements between them Generic Health imported 326,919 units of Evelexa XR manufactured by Alembic and supplied the products to Sigma in May 2009. Sigma sold 53,000 units of Evelexa XR on the private market before the Sigma interlocutory injunction prevented further sales.
985 Wyeth argued that as Generic Health imported the goods it was the “sponsor” of the goods for the purpose of importation and the phrase “on behalf of” in the definition of “sponsor” refers only to a principal/agency relationship and Generic Health was not Sigma’s agent for that purpose so was not excluded from the definition of “sponsor” and was not the person in relation to whom the goods were registered on the ARTG. As a result, the exceptions to liability in s 19D(1)(b)(i) and (2) did not apply to Generic Health. Further, as against Sigma, Wyeth argued that the products in its hands as imported by Generic Health were also tainted by Generic Health’s illegality.
986 Wyeth submitted that:
(1) In undertaking the importation and supply of Evelexa XR Generic Health was not acting “on behalf of” Sigma because, under the relevant supply agreement between them dated 8 July 2008, there was no relationship of principal and agent. In this regard, Wyeth referred to various provisions of the supply agreement including, in particular, cl 20 to the effect that nothing in the agreement “will be construed so as to place the parties in the relationship of principal, partner, joint venturer or legal representative of any other party” and that the “parties expressly agree and acknowledge that each of the parties is an independent contracting party and does not, unless expressly provided, have the authority or power for or on behalf of the other to enter into any contract, to incur debts, to accept money, to assume any obligations or to make any warranties or representations”.
(2) The fact that Sigma and Generic Health were not in a principal and agent relationship is supported by other facts including:
(a) Generic Health placed the first order with Alembic on 8 August 2008, before Sigma placed its first order with Generic Health on 4 September 2008; and
(b) under the manufacturing agreement dated 1 August 2008 between Alembic and Generic Health it is clear that title to the Evelexa XR products passed from Alembic to Generic Health before supply to Sigma.
(3) The tripartite GMP [Good Manufacturing Practice] agreement between Sigma, Generic Health and Alembic covers only the “GMP requirements and responsibilities for the manufacture, testing and packaging” of the Sigma products. Wyeth is not alleging that Generic Health was the sponsor in relation to the manufacture of these products but only in relation to their importation and supply to Sigma.
(4) Accordingly, Sigma obtained its products by reason of Generic Health’s contravention of s 19D of the Therapeutic Goods Act.
987 Wyeth referred to Hui v Lane [2003] SASC 401 which involved s 20(1A) of the Therapeutic Goods Act. The structure of that provision is equivalent to the structure of s 19D. Mullighan J said at [2]:
All of the elements of the offences were established by the agreed facts and the only issue is whether the appellant had proved that she was not the sponsor of the goods at the relevant time. She was in the process of exporting, or arranging the exportation of, the goods from Australia. There is no suggestion that she was doing so on behalf of another person. She falls within the definition of “sponsor” in s 3 of the Therapeutic Goods Act 1989 (Cth).
988 His Honour also made the point at [3] that “there is no reason to construe s 20(1A) so that there can only ever be one sponsor of the goods for all time”.
989 Debelle J said this:
[12] The definition of “sponsor” makes it clear that a sponsor is the actual exporter, importer or manufacturer of the therapeutic goods except if that person is exporting, importing or manufacturing the goods on behalf of another person who at the relevant time is a resident of Australia or is carrying on business in Australia. Thus, those who are acting as agents of another do not come within the definition of “sponsor”. The definition might also extend to include independent contractors of those who are exporting, importing or manufacturing therapeutic goods but it is unnecessary to determine that issue now. It is apparent from the terms of the definition that the Act proceeds on the footing that there is a sponsor whenever therapeutic goods are exported, imported or manufactured. The plain intent of the definition of “sponsor” is that there will be a sponsor at the time of import, at the time of manufacture and at the time of export. Subject to the exception provided in the definition of “sponsor”, the sponsor will be either the exporter, importer or manufacturer. Thus, the scheme of the Act is that, unless the therapeutic goods fall within any of the listed exceptions, the sponsor is guilty of the offence.
990 Gray J said:
[36] The Therapeutic Goods Act seeks to regulate the conduct of persons having control of therapeutic goods at any particular time. The Act regulates the conduct of manufacturers, importers, exporters and suppliers.
…
[38] The provisions of the Act apply to corporations which import, export, manufacture or supply therapeutic goods and persons who import, export, trade interstate or provide therapeutic goods to the Commonwealth. The national control of therapeutic goods is effected through the determination of standards, the establishment of an Australian Register of therapeutic goods which have been approved for import, export and supply and the licensing of Australian manufacturers of therapeutic goods. The central control mechanism is the register. The register consists of two parts, registered goods and listed goods. Registered goods are those which require an evaluation of their quality, safety and effectiveness before being approved. Listed goods are those of a less hazardous nature which require an assessment of their quality and compliance with any official standard or other specific requirement.
…
[40] During the course of argument counsel for Ms Hui submitted that there was uncertainty about the proper interpretation of s 20(1A) of the Act. Section 20(1A) was introduced into the Act by the Therapeutic Goods Amendment Bill of 1996. The explanatory memorandum provided:
Offences under subsection 20(1) of the Act apply only to sponsors. Sponsors can be broadly described as principal importers, exporters and manufacturers, and the definition of “sponsor” does not extend to include those who act on behalf of the principles, except where the principals operate offshore. Currently, in proceedings under this provision, to establish that a person is a sponsor the Crown is required to show, among other things, that there is no agency arrangement. However it is not possible to establish something that does not exist and a fact that is within the knowledge of the sponsor.
The effect of the changes made by Item 11 will be to require a “sponsor” in such a situation to establish that there was an agency arrangement, and that therefore the person did not act as a principal in unlawfully importing, exporting or manufacturing therapeutic goods.
The principal/agent relationship, particularly in cases not involving major corporations, can only be ascertained conclusively through confidential commercial arrangements known only to the parties concerned, to which the Commonwealth is not privy and often precluded from discovery for the purposes of establishing who committed an offence under section 20. Until the identity of the sponsor can be established, it is not possible to lay charges under section 20 of the Act so as to effectively preclude the exportation, importation, supply and use within Australia of unapproved therapeutic goods, including counterfeit drugs.
No change has been made to the nature of the offences under section 20, which apply only to “sponsors”, as defined in subsection 3(1) of the Act.
…
[45] The appropriateness of the literal or ordinary meaning of “sponsor” is confirmed by the explanatory memorandum identifying the mischief addressed by s 20(1A) of the Therapeutic Goods Act. As earlier noted the purpose of introducing s 20(1A) into the legislation was to provide a defence to a person acting as “sponsor’s” agent. The section provides that a defendant carries the onus of establishing agency. The mischief to be addressed specifically by this section was the difficulty faced by the Crown in proving agency when the relevant information lay solely within the knowledge of a defendant.
991 According to Wyeth:
The legislative reforms serve the important purpose of ensuring that… only persons in respect of whom the goods are registered or listed or their agents can undertake the activities regulated in s.19D(1)(a). This allows the TGA through other mechanisms within the TG Act (such as the imposition of conditions) to be able to regulate those activities: the sponsor of registered or listed therapeutic goods must comply with the TG Act and will be in a position to control the conduct of their agents. The section reflects a clear legislative choice that these activities cannot lawfully be undertaken by others, with the imposition of significant penalties for each act of inter alia importation. It is entirely consistent with the purpose of the TG Act and this provision in particular that arrangements for importation that contravene s.19D should be illegal and unenforceable.
Discussion
992 It is not in dispute that the enforcement of an undertaking as to damages involves an exercise of discretion. This has been repeatedly stated. A necessary consequence of the equitable foundations of the requirement to give the usual undertaking as the price of interlocutory relief is that all circumstances relevant to the justice, fairness and reasonableness of enforcement of an undertaking may be taken into account: see, for example, Air Express at 261-262 and European Bank at [18].
993 In CT Bowring & Co (Insurance) Ltd v Corsi & Partners Ltd [1994] 2 Lloyd’s Rep 567 at 582, Sir Michael Kerr said that, an undertaking as to damages:
…is given to the court, not to any opposite party. No action, set-off or counterclaim can be founded upon it. In relation to such undertakings the court acts or declines to act in its own right, not merely as an umpire in an adversarial process between the parties, though obviously having full regard to the position of the parties and to the interests of justice. In deciding how to deal with such an undertaking the court exercises a broad equitable jurisdiction: see Cheltenham and Gloucester Building Society v Ricketts [1993] I WLR 1545 (CA).
994 The starting point for resolving this issue should be the agreed statement of facts to which Wyeth is a party. The following facts appear in the agreed statement of facts:
2. The person or company holding the registration of a pharmaceutical product registered on the ARTG is called the sponsor of the relevant product. A sponsor may offer to supply, and supply, that therapeutic product to pharmacies, pharmaceutical distributors/wholesalers, government authorities and private or public hospitals in Australia when the product has been registered on the ARTG.
3. To register a therapeutic product on the ARTG the sponsor must obtain approval from the TGA.
4. If a pharmaceutical product is approved by the TGA, the brand name and the active ingredient of the product are entered on the ARTG, along with the name of the sponsor, the approved indication(s), the dosage form and the pack sizes for which the product may be supplied.
…
42. The ARTG records that the following products (the Evelexa XR Products) were entered on the Register on 5 March 2009 (see public summaries of ARTG entries in Attachment 3):
42.3 Evelexa XR 150 venlafaxine (as hydrochloride) 150mg extended release capsule blister pack under number 146744 (Evelexa XR 150);
42.4 Evelexa XR 75 venlafaxine (as hydrochloride) 75mg extended release capsule blister pack under number 146729 (Evelexa XR 75);
42.5 Evelexa XR 37.5 venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack under number 146645 (Evelexa XR 37.5).
43. As at 5 March 2009 Sigma Pharmaceuticals (Australia) Pty Ltd was the sponsor of each of the Evelexa XR Product registrations.
995 It must be inferred that Sigma was the “sponsor” of Evelexa XR as defined in s 3(1) of the Therapeutic Goods Act because as provided for in (c) of the definition it was the person who, in Australia, arranged for another person to manufacture the goods for supply in Australia. This accords with the facts, as Sigma pointed out, that:
(1) Sigma’s application for registration of the Evelexa XR products on the ARTG (originally lodged in the name of Arrow Pharmaceuticals Pty Ltd) nominated the manufacturer of the products as Alembic.
(2) At the time it manufactured the Evelexa XR tablets, Alembic had GMP clearance approval from the TGA to manufacture solid unit dosage forms – tablets and capsules, and it was the notified manufacturer for each of the Evelexa XR ARTG registrations.
(3) The Evelexa XR was imported into Australia in packaging which, in compliance with schedule 12 of the TG Regulations, displayed Sigma’s name and address, the name of the manufacturer (Alembic), and the appropriate Sigma ARTG registration number for the packaged dose of Sigma’s registered Evelexa XR.
996 While it would be contrary to the reasoning in Hui v Lane there is much to be said for Sigma’s submission that a construction which allows for more than one sponsor in respect of a registered therapeutic good at any one time is inconsistent with the overall statutory scheme. Provisions of the Therapeutic Goods Act which support this view of a single sponsor at one time include s 25(1) (discussed above), s 28(6), s 30(2)(c), s 31A(1), s 41JD, and s 42E(3)(f). Indeed, it is difficult to see how the scheme can operate if there is not a single sponsor of goods at any one time (albeit that a registration may be transferred from one person to another). As Sigma put it:
…in the case of Evelexa XR, Sigma was the registered sponsor for each ARTG registration. It would be contrary to the scheme of control established under the TG Act (and confusing to patients) for there to be multiple [unregistered] sponsors in respect of a drug registered on the ARTG.
997 It is apparent that this assumption underlies the facts agreed between the parties. If this is correct there can be no doubt that as the agreed facts record there was only one sponsor of the Evelexa XR products being Sigma. Generic Health was not the sponsor of those products merely because it imported them pursuant to contractual arrangements with Sigma and Alembic.
998 I will proceed, however, on the basis that Wyeth is able to assert that because it imported the Evelexa XR products Generic Health was the “sponsor” of the Evelexa XR products despite the agreed facts recording only one sponsor, Sigma. Either way, Wyeth’s approach involves the unappealing proposition that despite the contractual arrangements between Sigma, Generic Health and Alembic, Generic Health was on a frolic of its own when it imported Sigma’s products for the purpose of supply by Sigma and was not acting on behalf of Sigma in so doing.
999 I see nothing in the statutory scheme to support the conclusion that Generic Health was importing the Evelexa XR products other than “on behalf of [Sigma] who, at the time of the exportation, importation, manufacture or arrangements, [was] a resident of, or [was] carrying on business in, Australia”.
1000 In my view, it does not matter how Generic Health and Sigma chose to describe their relationship in their contracts if objectively and within the context of the Therapeutic Goods Act Generic Health was acting on behalf of Sigma when it imported the Evelexa XR products. If necessary to so conclude I would say that Generic Health was acting as Sigma’s agent for the purpose of the act of importing the Evelexa XR products into Australia, whatever the terms of the contractual arrangements between them.
1001 Further, in Hui v Lane the meaning of “on behalf of” in the definition of “sponsor” in the TGA did not need to be decided. In my view, Sigma’s submissions as follows should be accepted to the effect that something less than a principal and agent relationship will suffice. Sigma noted this:
The definition of sponsor, refers to a person who is performs specified acts ‘on behalf of another person’. In R v Portus, Ex parte Federated Clerks Union of Australia [[1949] HCA 53; (1949) 79 CLR 428] at 435, Latham CJ observed that the expression “on behalf of” is “not an expression which has a strict legal meaning”. In the same case, Dixon J noted that the phrase has no single and constant significance. Instead it may be used in conjunction with a wide range of relationships.
In [R v Toohey; Ex parte The Attorney-General for the Northern Territory of Australia (1980) 145 CLR 374] at [386] Stephen, Mason, Murphy and Aickin JJ stressed the many possible relationships to which the words “on behalf of” may be applied, saying of the expression: “… it may be used in conjunction with a wide range of relationships, all however in some way concerned with the standing of one person as auxiliary to or representative of another person or thing.” [In Otzen v Beabout [1947] HCA 49; (1947) 75 CLR 116 at 122 Latham CJ, Dixon, McTiernan and Williams JJ stated that the words] “do not necessarily imply that the transaction was with the actual authority of the person represented”.
1002 R v Portus, Ex parte Federated Clerks Union of Australia [1949] HCA 53; (1949) 79 CLR 428 concerned the phrase “on behalf of the Crown” and Otzen v Beabout [1947] HCA 49; (1947) 75 CLR 116 concerned liquor licensing laws.
1003 Generic Health referred to Colonial Mutual Life Assurance Society Ltd v Producers and Citizens Co-Operative Assurance Co of Australia Ltd [1931] HCA 53; (1931) 46 CLR 41 at 50 in which Dixon J referred to the difference between common conceptions of “agency” and the legal relationship of principal and agent, noting that expressions such as “on behalf of” are frequently used to describe services which “although done for the advantage of the person who requests them, involve no representation”.
1004 In R v Toohey; Ex parte The Attorney-General for the Northern Territory of Australia (1980) 145 CLR 374 at 386 the High Court said:
The phrase “on behalf of” is, as Latham C.J. observed in R v Portus; Ex parte Federated Clerks Union of Australia, “not an expression which has a strict legal meaning”, it bears no single and constant significance. Instead it may be used in conjunction with a wide range of relationships, all however in some way concerned with the standing of one person as auxiliary to or representative of another person or thing.
1005 Otzen is particularly instructive. The law authorised the licensee and the licensee’s agents to serve liquor. It was an offence to sell liquor at a price greater than the maximum price. The person who had served liquor at the greater than maximum price was an employee of the company running the premises, not the licensee. The High Court held that the person was the agent of the licensee whether or not the person had the licensee’s actual authority to act as the person did. Thus for the purpose of the relevant regulation the licensee was a person on whose behalf goods had been sold at greater than the maximum price. While as between the company and the licensee the person was the company’s employee, for the purpose of the legislation which concerned sales to the public the licensee was the principal and the person serving liquor was the licensee’s agent.
1006 By analogy in the present case, as between themselves, Generic Health and Sigma did not intend their relationship to be that of principal and agent. For the purposes of the Therapeutic Goods Act, however, what Generic Health did was necessarily “on behalf of” Sigma because Sigma was the only person in relation to which the products were registered. As Sigma put it:
By reason of the terms of the Generic Health agreement, and the packaging of the Evelexa XR, which in accordance with the TG Regulations bore Sigma’s name and address, and Sigma’s ARTG registration number, the Evelexa XR products could not be supplied by Generic Health to anyone but Sigma. Generic Health was acting on behalf of Sigma when it imported the Evelexa XR into Australia and supplied it to Sigma pursuant to the terms of the Generic Health agreement. Thus the defence in s19D(2) is enlivened and Generic Health was not in contravention of s19D(1) when it imported and supplied the Evelexa XR on behalf of Sigma.
1007 Given these conclusions it is not necessary to consider Generic Health’s alternative argument that the Sigma products were also registered on the ARTG “in relation to” Generic Health.
1008 For these reasons I do not accept the premise of Wyeth’s argument based on s 19D of the Therapeutic Goods Act. In my view Generic Health was importing the Evelexa XR products on behalf of Sigma and Sigma was supplying, and intending to supply those products, as the person in relation to whom those goods were registered. Accordingly, nothing that occurred contravened s 19D of the Therapeutic Goods Act.
1009 If the above conclusions are incorrect and Generic Health contravened s 19D of the Therapeutic Goods Act then in any event I would not accept Wyeth’s contentions that this circumstance, of itself, could or should disentitle Sigma or Generic Health from an order for compensation.
1010 Sigma was the person in relation to which the Evelexa XR products were registered. It was lawfully able to import and supply those products being within the exception in s 19D(1)(b)(i). If Generic Health was not acting “on behalf of” Sigma within the meaning of the definition of “sponsor” in s 3(1) of the Therapeutic Goods Act then, nevertheless, Generic Health was hardly on a frolic of its own. It was acting pursuant to contractual arrangements with Sigma the person in relation to whom the goods were registered. In this context the posited contravention of s 19D(1) is of the most technical nature. It bears no resemblance to the case on which Wyeth relied, Columbia Pictures, in which the stock of video tapes the subject of the injunction was largely pirated tapes the copyright in which was owned to persons other than the proprietor of the business. In Columbia Pictures Scott J said at 88:
Further, every sale of every video tape from the Mill Street shop of which evidence has been given in this case seems to have been the sale of a pirate tape. The prospect of an inquiry as to the damage caused by the Anton Piller order to such a business brings to my mind the application by the highwayman against his partner for an account. The court would not countenance that application and I do not think I should countenance an inquiry into the damage caused by the order to the business of the Mill Street shop.
1011 In Les Laboratoires Servier v Apotex Inc [2014] UKSC 55; [2015] 1 AC 430 at [27] Lord Sumption (with whom Lords Neuberger and Clarke agreed) described the reasoning about this issue in Columbia Pictures as “both brief and cryptic”. His Lordship continued saying:
The judge appears to have reached his conclusion on two grounds. The first was that under the then law the pirated tapes which were the stock-in-trade of the defendant’s business belonged to the copyright owners, so that the defendant’s inability to sell them caused him no loss. The second was that the defendant’s business was dishonest (the judge thought the case analogous to the Highwaymans’ Case). By this I think that he must have meant that any sales that the defendant would have made but for the Anton Piller order would have been made by dishonestly misleading his customers about the origin of the videos. It is I think only on that footing the judge’s second reason can be justified. Scott J was not suggesting that a breach of copyright was in itself a sufficient basis on which to raise the illegality defence.
1012 If this is so, the reasoning in Columbia Pictures cannot apply to Generic Health’s important of Sigma’s Evelexa XR products. Under the contractual arrangements Sigma was bound to pay Generic Health for the products and Generic Health was bound to pay Alembic. The products did not belong to a third party. Further no one could have been misled about the products which were required to be packaged as Sigma products.
1013 In Les Laboratoires Servier v Apotex Inc [2014] UKSC 55; [2015] 1 AC 430 Lord Sumption concluded as follows:
[28] … In my opinion the question what constitutes “turpitude” for the purpose of the defence depends on the legal character of the acts relied on. It means criminal acts, and what I have called quasi-criminal acts. This is because only acts in these categories engage the public interest which is the foundation of the illegality defence. Torts (other than those of which dishonesty is an essential element), breaches of contract, statutory and other civil wrongs, offend against interests which are essentially private, not public. There is no reason in such a case for the law to withhold its ordinary remedies. The public interest is sufficiently served by the availability of a system of corrective justice to regulate their consequences as between the parties affected.
1014 It may be accepted that the public interest is protected by s 19D and that contravention of the provision involves quasi-criminal conduct. What I do not accept is that Wyeth’s contentions, if legally correct, support the conclusion that the undertaking should not be enforced against it. As I have said if contrary to my view the arrangements between Sigma, Generic Health and Alembic meant that Generic Health was the sponsor of the Evelexa XR products for the purpose of importation then the contravention would have been of the most technical kind. At worst Sigma and Generic Health omitted to say in their contractual arrangements that in importing the goods, for the purpose of the definition of “sponsor” in s 3 of the Therapeutic Goods Act, Generic Health was acting “on behalf of Sigma as the person in relation to whom the goods were registered on the ARTG”. No moral turpitude was involved on the part of Sigma or Generic Health. No public safety implication could arise. On the evidence such arrangements are also common place in this industry.
1015 Generic Health referred to Fitzgerald v FJ Leonhardt Pty Ltd [1997] HCA 17; (1997) 189 CLR 215 in which McHugh and Gummow JJ at [41] said:
[41] In Nelson v Nelson [(1995) 184 CLR 538 at 604-605], McHugh J referred to the dictum of Lord Mansfield in Holman v Johnson [(1995) 184 CLR 538 at 604-605] that no court would lend its aid to a plaintiff founding the cause of action upon an immoral or illegal act…
[43] …the courts should not refuse to enforce contractual rights arising under a contract, merely because the contract is associated with or in furtherance of an illegal purpose, where the contract was not made in breach of a statutory prohibition upon its formation or upon the doing of a particular act essential to the performance of the contract or otherwise making unlawful the manner in which the contract is performed. Rather, the policy of the law should accord with the principles set out by McHugh J in Nelson v Nelson. His Honour said [at 613]:
“Accordingly, in my opinion, even if a case does not come within one of the four exceptions to the Holman dictum to which I have referred, courts should not refuse to enforce legal or equitable rights simply because they arose out of or were associated with an unlawful purpose unless: (a) the statute discloses an intention that those rights should be unenforceable in all circumstances; or (b)(i) the sanction of refusing to enforce those rights is not disproportionate to the seriousness of the unlawful conduct; (ii) the imposition of the sanction is necessary, having regard to the terms of the statute, to protect its objects or policies; and (iii) the statute does not disclose an intention that the sanctions and remedies contained in the statute are to be the only legal consequences of a breach of the statute or the frustration of its policies.”
1016 Consistently with the submissions for Generic Health I see no reason why the same approach by analogy would not be applied to a contention of illegality to defeat a claim on an undertaking as to damages. As Generic Health said:
The conclusion that Generic Health should not be compensated for profits it would have derived from the contractual arrangements referred to above can only be based on a proposition that had it imported and delivered the Sigma products as per those agreements, it would not have been able to recover the contract price from Sigma. To reach such a conclusion, the Court would have to answer in the affirmative (a) or each of (b)(i) to (iii) identified by McHugh J in Nelson.
1017 On this basis it is apparent that s 19D is a civil penalty provision. It does not suggest that a contract between the person in relation to which goods are registered and an importer would be unenforceable merely because the contract omitted to say that for the purposes of the definition of “sponsor” in the Therapeutic Goods Act the importer was importing the goods on behalf of the sponsor of the goods. Any such sanction would have been disproportionate to the seriousness of the conduct. The imposition of that sanction would not be necessary to protect the objects or policies of the Therapeutic Goods Act. The Therapeutic Goods Act does disclose an intention that the sanctions and remedies contained in the statute are to be the only legal consequences of a breach of the statute or the frustration of its policies.
1018 For these reasons if my legal conclusions are wrong then in any event, it would not be just or equitable to deny Sigma or Generic Health compensation by reason of the operation of s 19D of the Therapeutic Goods Act.
1019 Wyeth’s contentions about s 19D of the Therapeutic Goods Act should not be accepted. Section 19D is immaterial to the question of compensation orders.
Delay
1020 Wyeth pleaded that the claimants did not bring their claims for compensation within a reasonable time but effectively disavowed this contention in its submissions. The contention is untenable on the facts. The Full Court made orders on 11 November and 21 December 2011. Wyeth’s special leave application was dismissed on 11 May 2012. The principal interlocutory applications were filed by the generics on 28 May 2012 (Generic Health), 5 June 2012 (Sigma), and 7 June 2012 (Alphapharm). There was no delay of a kind that would make it inequitable to require Wyeth to pay compensation pursuant to its undertakings.
1021 Otherwise, Wyeth submitted this:
A further discretionary answer to the claims for compensation relates to the Generics’ time-consuming pursuit of issues on which they failed. If they had confined their defence of the proceedings to the only issue on which they ultimately succeeded, the trial would have been determined very promptly and it may be assumed in the same manner in which it was in fact determined. If this had occurred, the likelihood that any interlocutory restraint would have had any serious financial consequence is so low as to be ignored. The Generics brought on themselves the “delay” in obtaining a final judgment at trial by the pursuit of issues on which they ultimately lost. It is not just that they be compensated for that “delay”.
1022 It may be accepted that the circumstances of the case may make it unjust for a party which proffered an undertaking to be ordered to pay compensation for the whole or part of a period in which an interlocutory injunction was in force. Similarly, delay in making a claim on the undertaking is a relevant consideration and may preclude the grant of compensation: Air Express at 261.
1023 Wyeth referred to Barratt Manchester Ltd v Bolton Metropolitan Borough Council [1998] 1 All ER 1 and Abbey Forwarding Ltd (in liq) v HM Revenue & Customs [2015] EWHC 225 (Ch); [2015] Bus. L.R. 882. These cases, in common with others concerning delay in this context, involved delay in applying for damages or compensation pursuant to the undertaking. In that context, of delay in applying for damages pursuant to the undertaking, this was said in Abbey Forwarding at [130]:
It is a question of discretion in each case, on the particular facts of the case, whether any delay is such as to make it inappropriate to order an inquiry. The existence or otherwise of good reasons for the delay will clearly be a material factor, as also will be any prejudice caused by the delay, although as previously stated the court may refuse to order an inquiry even where no prejudice has been shown.
1024 Wyeth also referred to Computer Accounting and Tax Pty Ltd v Professional Services of Australia Pty Ltd [No 5] [2012] WASC 382; (2012) 92 ACSR 1, another case where the alleged delay was in claiming pursuant to the undertaking, in which Simmonds J said:
[100] There is strong authority that a delay in applying to enforce a claim for compensation under an undertaking may justify the court in refusing enforcement of the undertaking. See Newcomen [Newcomen v Coulson (1878) 7 Ch D 764] (765–766) (Malins VC: 11 months after discontinuance of action “during which negotiation probably went on” not such delay); Smith v Day (1882) 21 Ch D 421 at 426 (Jessel MR: eight months after dismissal of main action “too long”), 427 (Brett LJ: claim should be brought within a “reasonable time”), and 430 (Cotton LJ: not so long a delay as “of itself” to be “fatal to the application”; but delay “unexplained”); Ex parte Hall; Re Wood (1883) 23 Ch D 644 at 650 and 651–652 (Baggallay LJ: delay of nearly four years after case against claimant failed; delay not accounted for; claim should fail), 652 (Cotton LJ: delay “entirely unexplained”, “so unreasonable” that undertaking should not be enforced), 653 (Bowen LJ: “marvellous” that appellant “if he had any real claim to damages” would not have brought it before “more than three and a half years” had elapsed); and see Ansett (261) (Aickin J, referring to first and third of these authorities; not considered by members of the High Court upholding his decision).
[101] I note that these authorities do not appear to stipulate that the other side has to show prejudice from delay. Such a showing is of course required for the defeat of an equitable claim by laches not amounting to acquiescence. See Meagher RP, Heydon JD and Leeming MJ, Meagher, Gummow & Lehane’s Equity: Doctrines & Remedies (4th ed, 2002) [36-020].
1025 Wyeth did not identify any case in which compensation had been refused on the ground that the substantive hearing took longer to complete than it otherwise might had the party claiming under the undertaking not relied on all of the grounds on which it did rely. I would not exclude the possibility that in all of the circumstances of a particular case delay by a party in the conduct of the substantive proceeding might lead to that party’s claim under an undertaking being refused or adjusted to take into account the delay. If the effect of the delay is to extend the period for which the interlocutory orders have effect it is possible that it may not be fair for the party who gave the undertaking to be liable to the party who caused the delay.
1026 This is not such a case.
1027 First, it is relevant that Wyeth accepted that the generics had not acted unreasonably in any respect in making their case that the method patent was invalid. While I accept that this may not be a determinative factor it is relevant and entitled to substantial weight.
1028 Second, as Sigma submitted, the ultimate controlling consideration is whether the generics’ conduct of the substantive proceeding “would render it inequitable to enforce the undertaking”: Air Express at 311-312. It is not suggested that they conducted the substantive proceeding other than with due expedition. Further, as Wyeth acknowledged, none of the generics’ contentions lacked apparent merit. Nothing the Full Court said in Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2011] FCAFC 132; (2011) 119 IPR 194 supports a contrary view.
1029 In these circumstances I am unable to conclude that it would be inequitable to enforce the undertakings merely because the Full Court found in the generics’ favour on the ground of lack of fair basis. In particular, what must be taken into account is that it was Wyeth who sought and obtained the interlocutory injunctions and the generics who were wrongly held out of the market by the interlocutory injunctions from the date of their grant until the date on which I made final orders, 8 November 2010. If at any time Wyeth considered that it did not wish to take the continuing risk of exposure under the undertakings Wyeth could have applied for a release from the undertakings from that time on, the effect of which would have been the discharge of interlocutory injunctions. It must be inferred that Wyeth did not do so because it continued to be willing to take the benefit of the interlocutory injunctions and thus must also accept the continued burden of the undertakings.
Discretion overall
1030 Wyeth submitted, and I accept that:
… where a number of matters are raised going to discretion, the Court must consider them cumulatively and not simply individually. That is, the Court does not ask whether each matter in isolation is a sufficient ground to exercise a discretion to refuse compensation, but must exercise its discretion having regard to all those matters considered together: Co-operative Insurance Society Ltd v Argyll Stores (Holdings) Ltd [1998] AC 1 at 16; Australian Workers’ Union v Yallourn Energy Pty Ltd [2000] FCA 65 at [80].
1031 However, as none of the discretionary factors on which Wyeth relied are sustainable, considering those factors cumulatively adds nothing. The copyright issue cannot be asserted by Wyeth because it involves an abuse of process. If this is wrong, the copyright issue should not be permitted to be asserted because in the circumstances of this case, the belated assertion involves unreasonable oppression of the generics. If my legal conclusions about the s 19D issue are wrong then in any event it cannot be concluded that an object of the statutory provision would be to relieve a party such as Wyeth of potential liability pursuant to its undertakings. There was no material delay in the making of the claims for compensation. There was no material delay in the conduct of the substantive proceedings. There is nothing inequitable in the claimants seeking compensation pursuant to the undertakings.
1032 To the contrary, all discretionary considerations weigh in the claimants’ favour. Wyeth took and had the benefit of the interlocutory injunctions. As a result of the grant of the interlocutory injunctions any risk to Wyeth’s monopoly was removed from the date of grant until the date I made final orders on 8 November 2010. While Wyeth was entitled to assert the method patent and to seek interlocutory relief to vindicate its rights under the method patent Wyeth made the forensic decision to obtain interlocutory relief. It did not have to do so. It could have decided that damages would be an adequate remedy. It could have decided that it was unwilling to take the risk of liability pursuant to the undertakings. Having obtained interlocutory orders it could have applied to discharge the interlocutory injunctions and to be released from the undertakings from that time onwards. Wyeth chose for its monopoly to be protected by the interlocutory orders for their duration. In so choosing it must be taken to have accepted the equivalent burden of the undertakings.
1033 Wyeth’s assertions of copyright involve an abuse of process and should not be permitted. Alternatively, the copyright contentions involves such unjustifiable oppression of the generics that Wyeth should not be permitted to assert copyright as a matter of discretion.
1034 I do not consider that there should be any allowance made by way of deduction, discount or adjustment to the compensation that might otherwise be payable pursuant to the undertakings on account of Wyeth or Pfizer possibly launching a second brand of Efexor-XR at an earlier time if not for the interlocutory injunctions.
1035 The issue of discounting of Efexor-XR should be considered further in the context of the likely success of generic entry into the market.
1036 There should not be any allowance made by way of deduction, discount or adjustment to the compensation that might otherwise be payable pursuant to the undertakings on account of the possibility that had any generic applied for PBS listing of a generic version of Efexor-XR before the Full Court’s decision in October 2011 the Minister’ delegate may not have exercised the power under s 85(6) of the National Health Act to list those products on the PBS.
1037 I am also satisfied that Wyeth’s other contentions that it would not be just or equitable for orders for compensation to be made against it should not be accepted.
Overview of approach to market shares
1038 Quantifying the claimants’ loss as a result of the lost opportunities of supply involves two essential questions which should be answered having regard to two overriding considerations.
1039 The essential questions are, first, if not for the interlocutory injunctions how much of their generic venlafaxine products would the generics have sold in the relevant period and, secondly, what profit would the generics and the manufacturers/suppliers (including Generic Health as an intermediate supplier to Sigma) have made from the supply of their generic venlafaxine products in the relevant period? The relevant period for each generic, as noted, begins on the date the interlocutory injunctions were granted (except for Alphapharm where the date of 22 July 2009 should be applied because that has been used in the econometric analysis and Alphapharm did launch its products on 17 July 2009, before the grant of the Alphapharm interlocutory injunction) and ends on 8 November 2010.
1040 The first overriding consideration is that the task of assessment of quantum is not intended to be an exercise in certainty and precision. The task requires a broadly evaluative approach, doing the best I can on all of the material. The second overriding consideration is that given that Wyeth had the benefit of the wrongly granted interlocutory injunctions where doubt exists across a reasonable spectrum the doubt should be resolved in the claimants’ favour.
Contemporaneous evidence relevant to generic market shares
1041 I have referred above to the fact that at least insofar as Wyeth, Sigma and Alphapharm are concerned there is evidence disclosing their views about the possible or likely sales of generic venlafaxine products if not restrained by the interlocutory injunctions. The evidence Wyeth called to support its cases against Sigma and Alphapharm that if interlocutory injunctions were not granted Wyeth would suffer irreparable harm should be given substantial weight for a number of reasons.
1042 First, it was evidence Wyeth called in 2009 to persuade the Court to grant the interlocutory injunctions. The equitable context of both Wyeth’s applications for the interlocutory injunctions and the claimant’s applications pursuant to the undertakings and the maxim that the party which sought and obtained equity (Wyeth) must do equity indicates that Wyeth should not be allowed to suggest that its own evidence in 2009 about the market shares the generics might have achieved if unrestrained is unreliable.
1043 Second, Ms Braithwaite must have been authorised by Wyeth to provide the affidavit. Her views must be taken to have been made with Wyeth’s authority. As a result, in my view Ms Braithwaite’s statements are admissions which bind Wyeth: s 87(1)(a) and (b) of the Evidence Act 1995 (Cth). It does not matter that the statements are opinions: s 81(1) of the Evidence Act.
1044 Third, the person who gave the evidence on Wyeth’s behalf, Ms Braithwaite, must be inferred to have been available to give evidence in relation to the present claims as Wyeth filed an affidavit from her but did not call her to give evidence. This supports the inference that nothing Ms Braithwaite could have said would have undermined the effect of her evidence in 2009.
1045 Fourth, Ms Braithwaite gave her evidence in 2009 in her capacity as Wyeth Australia’s Pharmaceuticals Business Director and thus was well-placed to give evidence about the market shares she considered the generics would be likely to obtain if not restrained.
1046 Fifth, the fact that Ms Braithwaite would have had no reason to minimise the potential market shares the generics would have been likely to gain in 2009 means that her evidence if not constituting admissions against interest by Wyeth nevertheless accords with the principle that the assessment of compensation should be approached on a liberal basis.
1047 In her 11 May 2009 affidavit in support of Wyeth’s application for an interlocutory injunction against Sigma Ms Braithwaite explained that she was responsible for Wyeth Australia’s sales and marketing activities for all pharmaceutical products in Australia. In that capacity she said she was aware of the “the business models and pricing structures of pharmacists, including their commercial responses when a new generic drug becomes available”. She also understood the diagnosis and treatment of depression, as well as the PBS scheme, the market for Efexor-XR, and the discounting strategies of generics. In other words, Ms Braithwaite was saying that she was well-placed to give evidence about the effect of generic entry into the extended release venlafaxine market in which Wyeth held a monopoly by reason of the method patent.
1048 Ms Braithwaite was aware of the ARTG registrations for Sigma’s products and Alphapharm products (which were the only generic registration at the time of her affidavit). She anticipated more would follow and enter the market so the “competition for market share for venlafaxine products in Australia is likely to be intense”. She also assumed, based on the communications between the respective lawyers, that Sigma intended to confine its entry into the market to the private market and did not intend to supply under the PBS. It is not clear whether Ms Braithwaite assumed that any other generics entering the market would also confine their supply to the private market (the most logical assumption given the assumption about Sigma) but, in any event, Ms Braithwaite considered that Wyeth would rapidly lose market share to generics.
1049 Ms Braithwaite said this:
In my opinion there is likely to be a significant impact on monthly sales of Efexor XR within the first three months of launch of the Sigma products to the extent that sales of Efexor XR might be reduced by up to half of the normal monthly sales level.
1050 It may be accepted that the 50% estimate was expressed as a possibility and not probability. The evidence is also difficult to follow unless understood as representing the total potential generic share of the market (as opposed to Sigma’s potential share) as it seems to overlook the fact that the general non-concessional market which Sigma could target by private market supply represented only 48% of the overall market. Accordingly, the 50% estimate cannot represent Sigma’s possible market share because it would involve Sigma capturing virtually 100% of the general non-concessional market. Sigma recognised this estimate could not be correct in an email of 14 May 2009.
1051 What is relevant and not in doubt is that Wyeth adduced evidence in support of the grant of the interlocutory injunction to the effect that Sigma’s private market supply would have a significant impact on Wyeth’s sales. The only way Sigma could do this was by putting a deal together which was attractive to pharmacists and by pharmacists switching patients from Efexor-XR to Sigma’s brand. In particular, Ms Braithwaite did not suggest that pharmacists would not be attracted to Sigma’s product or would be so concerned about switching patients given the litigation that Sigma’s private market supply would soon fail and cease.
1052 Ms Braithwaite identified that in order to defend Efexor-XR’s position in the market against Sigma’s private market supply it was likely that Wyeth would be forced to discount the price of Efexor-XR and divert resources to the promotion of Efexor-XR. Ms Braithwaite considered that Sigma would be able to offer discounts enabling pharmacists to sell at below the patient co-payment amount with the result that “a significant number of patients will be induced to change brands”, as a price difference of even $1 would be enough to induce a patient to change brands.
1053 Based on this evidence Wyeth submitted in support of the Sigma interlocutory injunction that Sigma’s private market supply of its products meant that Wyeth was “at serious risk of rapidly losing market share for Efexor-XR which will continue to decrease over time. Experience shows that such loss of market share is very significant over the first year of generic competition”.
1054 In his reasons for judgment explaining the grant of the Sigma interlocutory injunction, Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2009] FCA 595; (2009) 81 IPR 339, Sundberg J said:
[51] It is likely that Wyeth Australia (and thus Wyeth) will suffer a loss of market share from Sigma’s possible acquisition of up to 50% of the market for venlafaxine products at the expense of Efexor‑XR…Ms Braithwaite, Wyeth Australia’s Pharmaceutical Business Director, said that in view of the size of Sigma’s sales force and the presence it has at the pharmacy level, which is where the decision and incentive to substitute a generic product for Efexor‑XR lies, Sigma’s product has the ability to quickly take market share from Efexor‑XR, “with consequent considerable and irreparable damage to Wyeth’s business”.
[52] In addition Wyeth Australia is likely to be forced to reduce the price at which it sells Efexor‑XR due to discounting by Sigma.
[53] Wyeth challenges the adequacy of Sigma’s undertaking not to seek to have Evelexa‑XR listed on the PBS. See [47(a)]. It points out that Sigma intends to supply the product to pharmacies outside the PBS with the expectation that it will compete with Efexor‑XR, and may be provided to patients in place of Efexor‑XR. According to Ms Braithwaite, this creates the serious risk that Wyeth Australia will rapidly lose significant market share for Efexor‑XR, which will continue to decrease over time. She says that loss of market share for originator brands in the first year of generic competition can be as much as 40 per cent.
[54] Wyeth contends that it is likely that Sigma will supply Evelexa‑XR to pharmacies at prices that will enable them to offer Evelexa‑XR as a cheaper alternative brand, thus inducing patients to change brands. Ms Braithwaite says that even a price which is cheaper by $1 can make a difference. This she says has the potential to affect the 45 per cent of sales of Efexor‑XR made to general benefit patients (ie non healthcare card holders) and which currently have a value in excess of $50 million.
1055 In her 28 July 2009 affidavit in support of the Alphapharm interlocutory injunction Ms Braithwaite gave evidence to the same effect as she gave in her 11 May 2009 affidavit. Again she said this about Alphapharm’s private market launch before the anticipated launch by any other generics:
In my opinion there is likely to be a significant impact on monthly sales of Efexor XR within the first three months of launch of the Alphapharm products to the extent that sales of Efexor XR might be reduced by up to half of the normal monthly sales level.
1056 In her affidavit of 30 July 2009 in support of the Alphapharm interlocutory injunction, in which she dealt with the prospect that the refusal to enjoin Alphapharm would lead to the discharge of the Sigma interlocutory injunction, Ms Braithwaite said (again on the basis of private market supply by the generics):
Sigma is the leading generic pharmaceutical company in Australia.
[Sigma’s extensive marketing and sales teams and relationships with pharmacies meant that Sigma] has the capacity to quickly take market share from Wyeth’s Efexor-XR.
Alphapharm is also an established and significant manufacturer of generic medicines in Australia and has a large sales force…As such, it also has the capacity to quickly achieve market share for venlafaxine products.
…having both Sigma and Alphapharm on the market will create a significantly increased competitive environment in the market.
1057 In her affidavit of 12 August 2009 in support of the Alphapharm interlocutory injunction, Ms Braithwaite noted that Alphapharm was the largest supplier in Australia of prescription products by volume for 2008. Ms Braithwaite disagreed with Mr Hurley’s assessment that Alphapharm would obtain a maximum of 30% of the general market for venlafaxine as she anticipated that other generics would be entering the market if Alphapharm was not restrained. As discussed, this supports my view that whatever the literal meaning of her affidavits Ms Braithwaite was intending to convey that in the event of full generic competition Wyeth would stand to lose up to 50% of its market share. Again, however, for present purposes the relevant point is that Ms Braithwaite evidently did not consider that Alphapharm’s private market supply would fail. She believed it would succeed.
1058 Based on this evidence Wyeth submitted in support of the Alphapharm interlocutory injunction that Alphapharm’s private market supply of its products meant that “[s]ales of Enlafax-XR have the potential to rapidly take market share from Efexor-XR”.
1059 The matters discussed above have a number of consequences. They support my conclusion that if not for the Alphapharm interlocutory injunction there was a high likelihood that if it carried out the necessary analysis Alphapharm would have concluded that private market supply could succeed. They support the evidence of Sigma that it achieved sales of its products on the private market on the basis of its instructions to its sales team that PBS listing was not anticipated in the foreseeable future due to the litigation. They indicate that Wyeth’s reliance on Alphapharm’s perception that Sigma may well have been misleading pharmacists is misplaced. They undermine Wyeth’s contentions that pharmacists would not have been interested in the private supply of venlafaxine products for any of the reasons Wyeth put forward (such as ethical concerns about switching patients, safety net issues, inability to persuade patients to switch, practical inability to later raise prices once products were PBS listed). Ms Braithwaite did not consider that any of these matters would mean that the supply of generic venlafaxine products on the private market would fail. She believed such supply would succeed and that Wyeth would have to respond by discounting and promoting Efexor-XR.
1060 These matters should be given substantial weight as they were an important part of the evidence Wyeth adduced to obtain the interlocutory injunctions against Sigma and Alphapharm. I would not go so far as to say it is impermissible for Wyeth now to contend to the contrary but these opinions of Ms Brathwaite given with Wyeth’s authority to support the grant of the interlocutory injunctions must be taken to represent Wyeth’s views about the likely impacts on its sales of Efexor-XR if Sigma continued its private market supply and Alphapharm undertook private market supply on a full commercial basis. This evidence discloses Wyeth’s views assuming private market supply on a commercial basis with each of Sigma and Alphapharm bringing to bear all of their experience and resources and being willing to apply their full armoury of generic marketing and sales techniques, including such matters as adjusting discounts in response to competitive pressures. My conclusions about the high likelihood of private market supply by the generics reflect the same kind of considerations Ms Braithwaite believed to be the case and Wyeth adduced as support for its claims for interlocutory injunctions.
1061 As such, Wyeth’s contentions to the effect that Sigma’s private market supply would have failed and Alphapharm would have decided not to supply on the private market or, if it did, its private market supply would have failed are unpersuasive.
1062 While Ms Braithwaite’s evidence did not extend to the Generic Health interlocutory injunction which was uncontested her evidence shows that there was money to be made by the private market supply of generic venlafaxine products. As such, it also supports my conclusion that there was a high likelihood that Generic Health would have launched its products as soon as it became apparent that it could not do a deal with Wyeth.
Overview of the econometric evidence
1063 Professor Hausman is the MacDonald Professor of Economics at the Massachusetts Institute of Technology in Cambridge, Massachusetts. He has nearly 45 years’ experience in economics, specialising in microeconometrics and applied microeconomics. Professor Hausman explained that:
Microeconometrics is a statistical and mathematical approach applied to economic data analysing activities of individuals, such as consumers, and the activities of firms. Applied microeconomics is the application of economic analysis to a wide range of activities and sectors, and includes industrial organisation, public, health, labour, and environmental economics.
1064 He has published over 200 research articles in leading economic journals including the American Economic Review, Econometrica, and the Rand (Bell) Journal of Economics and in numerous academic books. He is among the most highly cited economists in the world, and has been recognised for his work by distinctions too numerous to mention.
1065 He has conducted extensive research into pricing and marketing by pharmaceutical companies since the mid-1990s. He has also consulted in the pharmaceutical industry for over 20 years in relation to pricing strategies adopted by pharmaceutical companies, including the effects of the regulation of prices by numerous foreign governments. He has written academic papers that study competition in the pharmaceutical industry and the effect of generic entry including “Characteristics of Demand for Pharmaceutical Products: An Examination of Four Cephalosporins” with S. Fisher Ellison, I. Cockburn, and Z. Griliches, Rand Journal of Economics vol. 28, 1997.
1066 He was retained by the generic claimants to provide an opinion about the market shares and units sold of venlafaxine that each would have obtained in the Australian market for extended release venlafaxine if injunctions restraining each from launching a generic extended release venlafaxine product had not been granted, including the likely discount that would have been offered by each.
1067 For the purpose of preparing his evidence Professor Hausman was instructed to assume that the Australian pharmaceutical market, including the operation of the Australian PBS, operates as described in the affidavit of Ms McNeil sworn on 23 September 2015, the affidavit of Ms McNeil sworn on 10 November 2016 and the statement of agreed facts. This source information about the Australian pharmaceutical market and PBS is accurate. Following his review of this and other material Professor Hausman considered that he was “sufficiently informed as to the operation of the Australian pharmaceutical market to be able to perform the” task requested and give the opinions which he provided.
1068 Professor Hausman explained the concept of regression analysis as a tool to investigate functional relationships among variables. Regression models require data for each of the variables, including the dependant variable, which are the subject of the analysis. As he put it:
Once a model is estimated that establishes a relationship between the dependent variable (output) as each other variable (input) is changed, that model can be used to predict what the dependent variable would be for a given set of (new) inputs. This approach allows for the creation of counterfactuals that track relationships between variables that are derived from data.
Although actual output data is used to initially estimate the relationship between the output and the input, that relationship is then used to predict output (for which there is no actual data) from given inputs so as to make use of the estimated relationship. The given inputs may reflect a counterfactual world, such that when those inputs are used, the model will then predict output for that counterfactual world.
1069 A standard regression model is represented by the following equation:
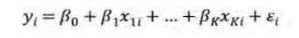
1070 There are two sets of variables in a regression model as disclosed in this equation. The “left-hand-side variable” is typically some measure of an outcome or result. The explanatory or “right-hand-side variables” are factors that may have an effect on the left-hand side variable. The i subscripts in the above equation indicate that when estimating a regression model there are multiple observations on the left-hand-side and right-hand-side variables and that the equation holds for each observation i. Professor Hausman continued:
The remaining components of the regression model are the unknown coefficients β0 through βk and the error term ε. Each coefficient βk measures how the left-hand-side variable changes, on average, in response to a change in the right-hand-side variable when all other right-hand-side variables are held constant. Thus, if βk is greater than zero, increases in xk, holding other variables constant, tend to increase y, while if βk is less than zero, increases in xki, holding other variables constant, tend to decrease y. The error term ε represents the influence of other factors affecting the left-hand-side variable that cannot be measured, and reflects the fact that even if we knew the true values of the coefficients β0 through βk, we would not be able to predict perfectly the lefthand-side variable.
Although the true values of the coefficients β0 through βk are unknown, they can be estimated using the observed values of the left-hand-side and right-hand-side variables. Although various methodologies to estimate the coefficients are available, the most commonly used estimation methodology is known as the “least squares” methodology. In this methodology, the estimated values of the coefficients are those that minimize the sum of the squared residuals, where the residual is the difference between the (actual) left-hand-side variable and the value of the left-hand-side variable that is predicted by the right-hand-side variables and the coefficients.
…
An estimation methodology such as least squares produces not only estimates of the coefficients, but also measures of how precisely the coefficients are estimated. One measure of precision is what is known as the standard error of the coefficient. Small standard errors indicate that a coefficient is measured precisely. A related measure of precision is what is known as the p value of the test that a coefficient is equal to zero. This p value is the probability of observing a coefficient at least as large as the estimated value of the coefficient if the true value of the coefficient were actually zero. Put another way the p value is indicative of the probability that the value would be observed by pure chance. If the p value is sufficiently small (typically below 0.05), the estimated coefficient is said to be statistically significant. If the estimated coefficient has a p value less than or equal to 0.05, the interpretation of the result is that the variable associated with that coefficient is statistically significant in determining the output variable. This approach is a standard framework used in undergraduate economic text books.
…
The predicted values from a regression model can be compared to the actual values to calculate what is known as the coefficient of determination, or R2. R2 is a measure of the explanatory power of a regression model (that Is, how accurately the model predicts known left hand side variables given any particular set of right hand side variables), and measures how much of the variation In the left-hand-side variable is accounted for by variation in the right-hand-side variables. R2 lies between 0 and 1, with numbers close to 1 indicating that the model has high explanatory power.
1071 Professor Hausman also explained the logit demand model, a very widely used technique in microeconomics. The logit demand model is a model of product choice by consumers (or firms). Consumer i is assumed to derive utility from product j according to the following equation:

1072 Under the assumptions of the logit demand model the market share of product j is given by:
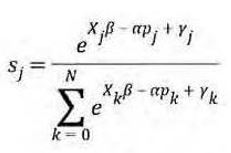
1073 Professor Hausman developed a model to estimate the market shares the generic claimants would have obtained under various assumptions about their entry into the market but for the interlocutory injunctions. He obtained a list of molecules in respect of which there has been generic entry since 2003 in Australia excluding injectables and non-prescription drugs. He obtained information about variables he considered would be useful for the logit demand model including therapeutic class, date of first generic entry, the name/s of the first generic entrant, the names of the generic entrants (and corresponding dates of launch) in the first 18 months post generic entry, and the total sales (in units and dollars) of each branded product during the year prior to generic entry. He excluded two products from the 121 products these inquires yielded on the basis that they were not pills (extended release venlafaxine being available in tablet/capsule form only). He excluded products that experienced generic entry between 2004 and 2015 whose sales in the year prior to generic entry (in the oral solid form) were less than $25 million because research shows that market size is a primary determinant of generic entry and the market for extended release venlafaxine was $114 million per year. He chose the time period of 2004 to 2015 because it encompasses the period of interest and is broad enough to ensure that the number of drugs in the sample is large enough to obtain sufficiently precise estimates. This process gave a subset of 45 products. He obtained further IMS sales (value and volume) data about these 45 products.
1074 On this basis Professor Hausman modelled eight scenarios.
1075 In scenario 1:
Sigma launches on the private market on 1 May 2009.
Sigma applies by 1 May 2009 to list on the PBS on 1 August 2009.
Alphapharm launches on the private market on 22 July 2009.
Alphapharm applies on 1 June 2009 to list on the PBS on 1 September 2009.
Generic Health launches on the private market on 9 August 2009.
1076 Further, in scenario 1, Professor Hausman was instructed to assume that various other generics would have obtained PBS listing of their products at various times earlier than those generics in fact did including in the period before the Full Court’s orders in November 2011 and that Pfizer would have obtained PBS listing of its second brand of extended release venlafaxine in March 2011.
1077 Scenario 2 to scenario 7 involve variations on these private market and PBS market entry dates, assuming other generics and Pfizer with its second brand obtained PBS listing and entered the market when they in fact did so. Given my conclusions above it is relevant to note that scenario 5 involves these hypothesised facts:
Sigma launches on the private market on 1 May 2009.
Sigma applies by 1 September 2009 to list on the PBS on 1 December 2009.
Alphapharm launches on the private market on 22 July 2009.
Alphapharm applies by 15 December 2009 to list on the PBS on 1 March 2010.
Generic Health launches on the private market on 9 August 2009.
Generic Health applies by 1 September 2009 to list on the PBS on 1 December 2010.
1078 It will be apparent that, insofar as Sigma and Alphapharm are concerned, scenario 5 accords with my conclusions. The scenario does not accord with my conclusions in respect of Generic Health, one, because it has Generic Health launching on the private market on 9 August 2009 and, two, because it has Generic Health, in effect, co-listing with Sigma on the PBS on 1 December 2009. I do not accept the first proposition because, as I have explained, as a matter of principle and of fact the only interlocutory injunction which had an adverse effect on Generic Health in respect of its own products was the Generic Health interlocutory injunction which was not granted until 10 November 2009. As a result, I have concluded that the relevant hypothesised fact involves the probability (80%) that Generic Health would have commenced supply on the private market from 10 November 2009. I do not accept the second proposition because I have concluded that there was no chance that Generic Health would have been the first (or joint first) to obtain PBS listing of its products. Its only prospect of obtaining PBS listing would be after Sigma obtained PBS listing on 1 December 2009. Nevertheless, scenario 5 most closely accords with my conclusions and, given my other conclusions that the overall size of the market would not have increased and the participants in the market would have been confined to Wyeth, Sigma, Alphapharm and Generic Health, I anticipate that it might be possible to re-allocate Generic Health’s sales from scenario 5 as required to the other participants on a suitable proportional basis.
1079 Scenario 8 involves:
Sigma launches on the private market on 1 May 2009.
Sigma applies by 1 December 2010 [sic 2011] to list on the PBS on 1 April 2012.
Generic Health launches on the private market on 9 August 2009.
Generic Health applies by 1 December 2010 [sic 2011] to list on the PBS on 1 April 2012.
Alphapharm launches on the private market on 22 July 2009.
Alphapharm applies by 1 December 2010 [sic 2011] to list on the PBS on 1 April 2012.
1080 It will be apparent that the PBS listing dates in scenario 8 reflect the actual PBS listing dates which occurred after the Full Court’s orders of November 2011. Again, but for the date from which Generic Health would have supplied its products on the private market, this accords with my conclusions about the more likely than not hypothetical facts.
1081 Professor Hausman was also instructed to assume that Generic Health and Alphapharm would have launched 75 mg and 150 mg products, that Sigma would have launched 37.5 mg, 75 mg and 150 mg products, and that the other generic suppliers would have launched the 37.5 mg, 75 mg and/or 150 mg products that they did in the real world. These assumptions are appropriate and should be adopted. There is no reason to infer that at any earlier time any of the claimant generics would have entered the market with different products from their actual products.
1082 Professor Hausman carried out a three step analysis. He analysed the total unit sales of venlafaxine in the actual world. Using data for 34 drugs he considered appropriate, he estimated econometric models of total generic market share and average generic price. Using the same set of 34 drugs, he analysed price differences among generic products and estimated a model of demand for individual generic products. In performing this analysis, he noted that generic entry into the market in 2012, in fact, had almost no effect on total venlafaxine unit sales. As a result, he proceeded on the premise that the total sales would have been the same as they were in fact. Professor Hausman described this as a conservative assumption as the total sales might have been greater if the generics had entered the market earlier given the impact of Pristiq, which meant that the sales of Efexor-XR levelled off after Pristiq was introduced at the beginning of 2009.
1083 Professor Hausman used two regression models (total generic share and average generic price) to estimate how factors such as time since entry, size of the branded market, and number of products affect share and price. In the first regression model, the variable to be modelled (known as the “left-hand-side variable”) is the total generic share of the market. In the second regression model, the left-hand-side variable is a measure of average generic price. The measure used is the generic price ratio which is the average generic price in a given month divided by the branded price in the month prior to generic entry. He included a number of variables as right-hand-side variables in the regression models for both share and price ratio based on his expertise in economics and experience in relation to the pharmaceutical industry, which he identified and explained as follows:
(a) The number of months since generic entry, and the number of months squared…
(b) The natural logarithm of the number of generic products on the market… I include this variable because, based on standard economic theory and my previous experience, when there are more generic products on the market for a given drug, generic share is likely to be higher and generic prices likely to be lower…
(c) The natural logarithm of sales of the branded product in the month prior to generic entry, in millions of dollars… include this variable because in my previous experience when the branded drug has more sales, typically, more generics are attracted into the market which leads to increased competition and therefore generics tend to obtain a greater total generic share and/or charge lower prices.
(d) The fraction of generic products that are listed on the PBS at any given month… include this variable because I would expect that being listed on the PBS increases demand for a product (because of the price subsidy effect), and thus I would expect generic share to be higher when more of the generic products are PBS listed.
(e) Indicator variables for the therapeutic class of the drug, as measured by the first level of the Anatomical Therapeutic Chemical (ATC) Classification System. I include these variables because based on my previous experience I would expect that the overall level of generic share and price may vary depending on therapeutic class…
1084 Professor Hausman also included statutory and to be expected price reduction variables in the price ratio regression model.
1085 Professor Hausman also explained:
Because generic share and generic price ratio are generally between zero and one, I use a regression method known as a fractional logit, which is designed to model lefthand-side variables that are between zero and one. When the purpose of the model is to obtain predictions of the left-hand-side variables for different values of the right-hand-side variables (as in the present situation), the fractional logit is preferred over other regression methods such as least squares because the fractional logit is guaranteed to produce predicted values that are between zero and one, while least squares is not.
1086 Dr Jenkins was retained by Wyeth to review Professor Hausman’s results. Dr Jenkins is the Managing Partner of Oxera Consulting LLP, an economics and finance consulting company headquartered in Oxford, UK. Dr Jenkins holds hold an MPhil and DPhil in Economics from the University of Oxford. She is as an empirical micro-economist with particular experience in the pharmaceutical sector, built on 19 years of providing advice to government and to suppliers of branded medicines and generic products. She led the Fundamental Review of Arrangements for the Supply of Generic Medicines to the UK National Health Service (NHS), a two-year investigation into the full supply chain of generic medicines, from manufacturer to patient, including market authorisation, production, wholesaling and distribution, prescribing, dispensing and reimbursement. She led a project in 2001 that assisted the UK Department of Health in designing processes for monitoring the supply and distribution of generic medicines in the UK in order to encourage entry and to identify market characteristics that hamper entry. In so doing, she developed a detailed understanding of how expected market size, access to active pharmaceutical ingredients, product formulation and therapeutic need affect the incentives of branded and generic businesses to enter and compete. She is advising the British Generic Manufacturers Association in its negotiations with the UK Department of Health on changes to the regulatory arrangements for reimbursement of generic medicines, focusing on products where there are limited suppliers despite the patent having expired. She has also advised pharmaceutical businesses (both branded and generics) in the context of competition authority investigations and commercial disputes. Dr Jenkins is one of the competition economists internationally recognised in The International Who’s Who of Competition Lawyers & Economists and has been since 2001. She is also co-author of Economics for Competition Lawyers, a textbook on the application of economics to market assessment and antitrust issues, now in its second edition and published by the Oxford University Press.
1087 Dr Jenkins familiarised herself with the pharmaceutical market and the workings of the Australian PBS before forming her opinions.
1088 In her principal report Dr Jenkins made a number of points about Professor Hausman’s analysis including those summarised below.
1089 Dr Jenkins considered that the total size of the venlafaxine market was likely to have decreased but for the interlocutory and final injunctions because Wyeth otherwise would have decreased its promotion and marketing of Efexor-XR to doctors. I am unable to accept this proposition because it is contrary to the contemporaneous evidence, first, that Wyeth in fact ceased promoting Efexor-XR from 2009 in any event because of its focus on Pristiq and, second, as Ms Braithwaite said, that it would have to put resources into promoting Efexor-XR if the Sigma and Alphapharm interlocutory injunctions were not granted. I thus consider Professor Hausman’s approach to the size of the total market to be sound.
1090 Dr Jenkins considered that Professor Hausman’s econometric framework for the second step in his analysis, the evolution of the total generic share and average generic price in the venlafaxine market, was useful and informative but flawed because of his approach to excluded and included molecules, a lack of direct accounting for the potential impact of originators’ reactions to generic entry, particularly the pricing of the originators’ second brand, and aspects of the market that potentially influence generic share and price, such as the use of brand premia by originators, the changing market share positions of Alphapharm and Sigma in the Australian market, and changes in wider regulatory conditions.
1091 Further, in respect of scenario 8 Dr Jenkins said:
Professor Hausman’s approach and the available data cannot be used to assess Hausman Counterfactual Scenario 8, in which the Generic Claimants are listed on the PBS in April 2012, almost three years after the date of the first generic launch (May 2009). This is because Professor Hausman has not taken appropriate account of the dynamics of generic competition in the private market, especially given that, in the large majority of the comparators he uses to estimate his econometric model, PBS listing of generic products occur within six months of launch. The unsuitability of Professor Hausman’s analysis for Hausman Counterfactual Scenario 8 is evident from the impossibly high market shares predicted by the model for this scenario.
1092 Dr Jenkins also considered that the third step in Professor Hausman’s analysis was flawed in that his modelling “does not account for a number of market realities that affect the shares of specific firms, for example their position as first- or second-line supplier, as well as specific aspects of the relevant molecule, such as the therapeutic indication, which influences pricing strategies”. In particular, he assumed that “the average discount that each Generic Claimant offered across his 34 comparators is the best estimate of the discount that would be offered by each Generic Claimant in respect of venlafaxine” which Dr Jenkins did not consider a good predictor for the relevant discounts for venlafaxine.
1093 Dr Jenkins developed econometric models which:
a. are based on a different comparator set, comprising molecules that treat chronic symptomatic conditions, as these are therapeutically more similar to venlafaxine than those used by Professor Hausman. In my comparator set, I include molecules that have faced an injunction and/or those with a market value in the year before generic entry of less than $25 million, and explicitly account in my models for any differences in how these molecules might evolve in the market;
b. account for the competitive dynamics between the originator and the generic companies after entry, specifically controlling for price reactions from the originator and the introduction of an authorised generic product; and
c. account for other market-specific factors, such as regulatory or other changes over time.
1094 However, Dr Jenkins remained of the view that no models of this kind could be used to predict outcomes in scenario 8 as the data set involves molecules which were launched on the private market with PBS listing within 6 months and scenario 8 involves private market supply between 2009 and 1 April 2012 until PBS listing was obtained.
1095 To the extent Dr Jenkins otherwise criticised Professor Hausman’s approach, the issues are sufficiently exposed in Professor Hausman’s response.
1096 In his response, Professor Hausman explained that he remained of the view that the exclusion of molecules below $25 million and subject to an injunction was appropriate. In so concluding he said he used the Chow test, which tests whether the relationship between the left-hand side and right-hand side variables differ between two groups of observations (which Dr Jenkins considered inapt for the circumstances). He also used the Chow test to assess other aspects of Dr Jenkins’ approach including the distinction Dr Jenkins drew between molecules for treating chronic symptomatic conditions (such as depression which venlafaxine treats) and non-chronic symptomatic molecules. He also noted that his model did take into account the response of originators such as discounting. As he put it:
All originators have an economic incentive maintain the profits of their product in the face of generic entry and hence the effect of such originator response is implicit in the relationships estimated in my models. Thus Dr Jenkins criticism is valid only if the originator in the case of venlafaxine would have responded differently to generic entry than is typical.
1097 He also tested the possibility that including a Pfizer molecule would change the results based on Pfizer being a more aggressive discounter of its molecules. He found that Pfizer being the originator has no effect.
1098 Professor Hausman disagreed that he should have confined his modelling to independent generics and thus should have excluded authorised generics (that is, authorised by the originator). His analysis showed that demand for authorised generic products was not significantly different from the demand for other non-first line generic products.
1099 He considered Dr Jenkins’ concerns about scenario 8 in which there is a gap of 35 months between the time the first generic product launches and the time PBS listing is obtained. He accepted that the data set involves much shorter time periods between launch and PBS listing, with the longest gap being four months. He explained that because there are no molecules in the comparator set with a gap comparable to the scenario 8 gap it is not possible to perform a Chow test to ascertain the reliability of the results. He also agreed that his approach to the total private market in scenario 8 was incorrect because he had overlooked the fact that it represented no more than 42% of the total market for venlafaxine (because, as the generics accepted, the private market could only target non-concessional patients who represented 42% of the total market).
1100 Professor Hausman also agreed with a number of Dr Jenkins’ other propositions. He thus revised his model in response to Dr Jenkins’ observations including by taking into account the effect of magnitude of the brand price premium and including year indicator variables in both the price equation and the share equation (which he found to be statistically significant variables that should be addressed).
1101 Professor Hausman explained that, overall, he had made four revisions to his model as a result of Dr Jenkins’ work, being (i) adding a relative brand price premium variable to the share equation; (ii) adding year indicator variables to the share equation and year indicator variables and year interactions to the price equation; (iii) assuming that olanzapine was subject to an injunction reducing his sample from 34 to 33 molecules; and (iv) using the statutory price reduction and price disclosure eligibility dates in Annexure HJ-13 rather than the ones he had used in JAH-16.
1102 Otherwise, he noted that his model was not unreliable merely because it “overpredicts total generic share for venlafaxine in the factual” (that is, the model predicts generic shares greater than the shares they in fact achieved when they when they entered the market after PBS listing in 2012). He explained that the evidence indicates that generic share is suppressed for molecules subject to an injunction and the model is predicting shares for molecules that are not subject to an injunction. Accordingly, as venlafaxine was in fact subject to an injunction, the fact that the shares predicted by the model exceed the actual generic shares achieved from 2012 is to be expected.
1103 Professor Hausman also considered that selecting four molecules and demonstrating over and under prediction for those molecules, as Dr Jenkins had done, was inappropriate. As he explained:
Because all real world regression models overpredict for some observations and underpredict for other observations cherry picking four molecules out of 34 that exhibit overprediction or underprediction provides no evidence about the reliability of the model…
…the goal of regression analysis is not to predict each individual observation with perfection but instead to make accurate predictions on average. Thus in evaluating the reliability of a regression model it is appropriate to use averages to evaluate the reliability of a model. An average includes all molecules in the sample and is not subject to the cherry picking problem from which Dr Jenkins method suffers… As demonstrated in Annexure JAH33 when all molecules are taken into account the predicted values are on average close to the actual values. The R2 between the actual and predicted generic shares set out in Annexure JAH33 is 97.1% which as discussed above indicates that the model has a high degree of explanatory power and is highly reliable.
1104 The R2, it will be recalled, is the coefficient of determination which is a measure of the explanatory power of a regression model between 0 and 1 (so that the higher the number, the greater the accuracy of the model in predicting known left hand side variables given any particular set of right hand side variables.
1105 For the same reason Professor Hausman disagreed with Dr Jenkins’ selection of two molecules to test for the effect of an injunction. He said:
The fact that my models predicted share is close to actual shares for these two injunction molecules does not provide evidence as to whether there is a difference for injunction molecules overall. To answer this question it is necessary to consider all eight injunction molecules in my dataset. The document annexed to this affidavit and marked Annexure JAH34 sets out my analysis which for all eight injunction molecules in my dataset compares the average actual generic share to the average generic share predicted by my mode. As Annexure JAH34 illustrates the actual share for the injunction molecules is below the predicted share for almost the entire time period. This result is consistent with both the theory that an injunction suppresses generic share and the results of the Chow Test and further supports my decision to exclude injunction molecules from my analysis. As noted …above my model is designed to predict non injunction molecules venlafaxine in the counterfactual world not injunction molecules.
1106 Professor Hausman noted that although Dr Jenkins criticised his analysis of the specific shares of the individual generic claimants she had not provided any predictions of her own in this regard. Further, despite the complexities involved economists commonly use the methods he used in his analysis to estimate product specific shares.
1107 Professor Hausman also revised his logit demand model to take into account Dr Jenkins’ concerns including indicator variables about major generic suppliers most likely to be first line suppliers (Alphapharm, Sigma, Sandoz and Apotex) as well as for authorised generics. He then evaluated whether his logit demand molecule provides a reliable estimate of the shares obtained by the generic claimants by comparing for each of the generic claimants the average actual share of the generic market each claimant obtained to the average share of the generic market each claimant is predicted to obtain by his logit demand model. This shows the following:
Generic claimant | Actual | Predicted | Difference |
Alphapharm | 22.42% | 23.56% | 1.14% |
Sigma | 19.62% | 19.03% | -0.60% |
Generic Health | 1.76% | 1.72% | -0.04% |
1108 Professor Hausman said that this table shows that his logit demand model reliably predicts the average share each generic claimant obtains of the generic market as the differences between actual and predicted shares are small.
1109 Professor Hausman did not dispute Dr Jenkins’ claim that discounting behaviour can vary across molecules and over time, but disagreed that this made his analysis unreliable. He said:
Taking into account such factors in a precise and comprehensive manner would require information on how each generic supplier would have discounted each product in each month in each counterfactual scenario. Given the subjectivity that would be inherent in such an exercise I believe it is more reliable to use my approach which is based on each suppliers typical behaviour.
1110 He also carried out further analysis which showed that the median price ratios for the supplier groups change very little when the sample of molecules used to calculate them changes.
1111 Professor Hausman also reviewed Dr Jenkins’ model and concluded it was unreliable. He said:
Dr Jenkins has 4 endogenous variables in her share equation price ratio price ratio squared market size times price ratio and months times price ratio. As instruments for these endogenous variables Dr Jenkins uses 19 variables the instrumental variables that are included in the price ratio equation but not the share equation. Because Dr Jenkins uses more instrumental variables than she has endogenous variables her model is what econometricians call an overidentified model. When a model is overidentified it is possible to test whether the instrumental variables are valid here whether the instrumental variables do not affect generic share directly by performing what is known as an overidentification test. Dr Jenkins fails to perform an overidentification test on her model. I perform the overidentification test and find that the validity of Dr Jenkins instrumental variables is rejected at a high degree of statistical significance (p<0.0001). I thus conclude that Dr Jenkins approach is not reliable in estimating her share model because of the failure of the test of overidentification.
1112 Professor Hausman and Dr Jenkins also prepared a joint report identifying the matters about which they agreed and disagreed. I deal with that evidence, to the extent necessary, in the context of the discussion below.
1113 In their concurrent oral evidence Professor Hausman explained that:
The regression method finds the best fit which minimises the deviation between the model prediction and the actual data; thus, it minimises the area of prediction. Regression models use a property of conditional expectations, which is just a fancy word to say average, to achieve the best fit of the model to real world data. The model can be used to predict what the deep end variable would be for a given set of new inputs. This approach allows for the creation of counterfactuals that tracks relationship ... variables that arrive from data. The property of the regression model carries over the prediction. It will minimise the expected error of prediction in the counterfactual world. … It provides a scientific approach rather than relying totally on personal judgment to choose an analogue molecule. With all regression models, choices must be made on the appropriate specification of the model, which means a sample used to estimate the model and the choice of explanatory variables. Dr Jenkins and I disagree on a number of these issues, but this disagreement does not lead to a large difference in our results.
1114 He also explained why he considered his analysis to be more reliable than that of Dr Jenkins.
1115 Dr Jenkins said this:
I would agree with Professor Hausman about the fact that we both adopt an econometric approach and the reason for that being that we are seeking to predict a counterfactual outcome which by definition is something that we don’t know what would have happened in the venlafaxine market absent the injunction, and that’s the task that we have. The advantage of regression is exactly as Professor Hausman was outlining, that rather than as the market experts do, you select one comparator, you are able to pick a selection of comparators that you think will be useful in helping understand how venlafaxine would have evolved in the counterfactual, but what’s really important is that we know that every one of these molecules does have specific aspects, specific elements that are actually quite different from one another.
So when you extend your comparator set you need to make sure you’ve got relevant controls in your regression analysis that can control for the differences, compared with your counterfactual venlafaxine. And I think most of the differences between Professor Hausman and myself are around that piece of judgment, right, which is about is the set that I’ve got the best comparator set in order to predict this counterfactual feature.
… And with Professor Hausman’s revised report, my view is that actually now the root mean square errors, the predictions within sample of our two models are now very similar, and whereas for his first report, my view was that my report was a significant improvement within sample, I would say now that within sample our reports are very similar.
1116 The first point is that in circumstances where, as here, I am satisfied on the balance of probabilities that the interlocutory injunctions caused the claimants some loss it would be unjust and inequitable to shirk from the task of assessing the quantum of their loss merely because it involves a degree of estimation and even some speculation. The cause of uncertainty is the interlocutory injunctions Wyeth obtained to enforce a monopoly to which it turned out it had no right. It is not now for Wyeth to complain that the task of assessing quantum is confounded by too many uncertainties. The task must be approached recognising that accuracy and precision are impossible and inexactitude inevitable.
1117 My general conclusions are these.
1118 First, the econometric approach is more likely to yield robust results than an approach based on a comparator molecule or molecules.
1119 Second, it cannot be doubted that Dr Jenkins’ review of Professor Hausman’s work caused him to make material changes which significantly improved the reliability of his models.
1120 Further, at least insofar as scenario 1 is concerned (which is the scenario Dr Jenkins ran through her model), Wyeth acknowledged that Professor Hausman’s results could be accepted. Apart from scenario 8, the other scenarios (including scenario 5) use the same models on the basis of different assumptions which are ultimately a matter for me. In other words, if the outputs of scenario 8 are acceptable to Wyeth as sufficiently reliable calculations, then the same must follow for scenario 5. The real dispute as between scenarios 1 to 7, accordingly, is not Professor Hausman’s models, but the inputs into them.
1121 Wyeth described scenarios 2 to 7 as “unrealistic and implausible”, as they:
… represent an opportunistic attempt by SPAL, Alphapharm and Generic Health to divide the counterfactual generic market amongst themselves, in circumstances where the commercially rational conclusion to draw is that, had (contrary to Wyeth’s case) one of the Generics listed its venlafaxine products on the PBS, multiple other suppliers (including Pfizer with its authorised generic) also would have entered the PBS market.
1122 As I have explained above, I have concluded that compensation should be assessed on a loss of opportunity basis in which the probabilities and possibilities are assessed. I have assessed the chance of any generic having obtained PBS listing before 1 April 2012 to be confined to the 20% chance of Sigma obtaining PBS listing on 1 December 2009 and a dependent chance thereafter for Alphapharm and Generic Health to obtain PBS listing on 1 March 2010. On this basis and given my conclusions about the position of other generics, I do not accept that it is unrealistic and implausible for compensation for that loss of opportunity to be assessed on the basis that no other generic would have entered the market and Pfizer would not have launched its second-brand at any earlier time than they in fact did. To the contrary, on the evidence proceeding on this basis in the circumstances of my conclusions involves a reasonable and appropriate approach to compensation.
1123 I do not doubt that it would always be possible for an analysis of the kind Professor Hausman undertook to be improved. But this does not mean that it must be improved before it may be relied upon for the purpose of this matter. Wyeth accepted Professor Hausman’s approach for scenario 1. Leaving aside scenario 8, it presented no reason why his overall approach would not be acceptable to estimate generic shares, total and individual, for another set of assumed circumstances, including those represented by my conclusions.
1124 The criticisms, overt or implied, of Professor Hausman’s analysis in cross-examination do not suggest to me that a contrary conclusion would be appropriate. I deal briefly with these matters below.
1125 The fact that none of the molecules in Professor Hausman’s data set include molecules which were on the market during a period where there was extant unresolved litigation may be accepted but this did not cause Dr Jenkins to suggest the econometric approach was unreliable. As explained above, at least insofar as pharmacists are concerned, who are the generics’ customer, I consider the entire issue would have been immaterial. And insofar as the generics are concerned, the issue has been considered and evaluated in the context of my primary conclusions about the probabilities and possibilities. As a result, it cannot be material to Professor Hausman’s work.
1126 As to the shares allocated to each generic claimant, Professor Hausman explained:
In these models, if you’re a bit low for one of the claimants, you will be higher for the others because the shares add up to one for the entire market. So in other words, the error terms are negatively correlated, you know, because high for one is going to be low for another.
… that’s why in my second report, I looked at the average across all molecules, and the model does a very good job for each of the claimants of prediction because sometimes it’s going to be positive, and sometimes it’s going to be negative, but you do need to take the negative correlation into account. So it’s not as if the defendant here, Pfizer or Wyeth or whoever they are, is going to end up paying too much because these errors are going to negatively balance out. And over time, what my second report shows is you get an unbiased prediction so that on average, you’re going to get it correct for shares. But I do agree that you can always find and pick a molecule and say look how badly it’s doing, especially if it’s tiny, like this molecule is for Generic Health. That’s well known in ..... models. When you have very, very small shares like this, it is extraordinarily difficult to predict well. But this is just one. If you look across all of them, which is my second report, on average, it’s just about spot on.
1127 I found this evidence of Professor Hausman persuasive. As a result, in circumstances where the total market is taken not to have increased and the market participants are taken to be confined to Wyeth, Sigma, Alphapharm and Generic Health, it does not matter (at least, not to Wyeth) if there is an over or under allocation as between Sigma, Alphapharm and Generic Health. It does matter as between Sigma, Alphapharm and Generic Health and it was apparent that Generic Health considered it was being harshly treated in the results. In my view, however, the results reflect the different market position of Generic Health compared to Sigma and Alphapharm. In 2009 and 2010, Generic Health was small and had a small product range. It was not remotely comparable to Sigma and Alphapharm. It was also the last of the three to obtain ARTG registrations and thus could never have made it to the market before Sigma or Alphapharm in circumstances where early entry is key to obtaining market share.
1128 Wyeth submitted that Professor Hausman’s evidence about scenario 8 is “beset by so many difficulties that it should not be relied on at all”. It may be accepted that the econometric analysis involved in scenario 8 is likely to be less robust than for scenarios in which PBS listing is assumed. As Professor Hausman acknowledged there are no molecules in the data set involving a 35 month gap between private market supply and PBS listing. It may also be accepted that this caused Dr Jenkins to conclude that the data did not enable scenario 8 to be assessed. Yet what remains is this. On the evidence it must be inferred that Sigma was convinced it could undertake private market supply successfully and Sigma did manage to sell a material portion of its stock before being restrained. Wyeth’s Ms Braithwaite believed that if Sigma and Alphapharm were not restrained from selling their stock on the private market they would make significant inroads into Wyeth’s market share. In common with Sigma, on learning of the existence of the method patent as a result of the Sigma litigation, Mr Upiter immediately thought of private market supply. Only Alphapharm held real doubts about the prospects of successful private market supply but I am satisfied that it would have changed its mind under the perceived commercial pressure from Sigma’s supply and that it too would have been more than capable of formulating strategies enabling it to undertake a successful private market supply.
1129 Further, as Professor Hausman noted in oral evidence, some of the molecules in his data set were sold on the private market before PBS listing. In both situations the pharmacist is trying to earn a profit. There is plenty of margin within which a pharmacist could make a profit on a generic version because the cost of generic drugs is usually low. Generics are able to adjust their prices to make sure the pharmacy finds it in their best interest to sell the drugs “otherwise the generic company is not going to make much money”. As Professor Hausman put it:
…these drugs are sold into a world market. I’ve had experience in this type of matter for 30 years and they’re always sold at a very low price because so long as they get government approval, usually FDA approval in the United States, they’re all selling exactly the same drug and the lowest price wins for the manufacturer.
1130 When asked about the additional time and training that may be required for a pharmacist to switch patients Professor Hausman said:
Yes, but remember this drug venlafaxine, people take pretty much forever and so it’s not just a one-time thing that the pharmacy is going to incur costs. In my experience, it’s true everywhere that patients, unless they move, don’t typically change pharmacies, so yes, it would be a one-time cost, but that patient – she’s going to be buying from you for the next 10 years so it’s really going to be in the pharmacy as best economic interests to switch a patient.
1131 Professor Hausman agreed that the financial incentive would have to come out of any margin that the pharmacist might otherwise make but said:
It’s not that the pharmacist has a pot of money. The generic – the generic distributor sets the margin for the pharmacy by saying here’s the price. Now, you could be right if the margins were very low and you bought it abroad but they’re just not, they’re very large. And if the generic company knows what it’s doing, which they do because that’s why the stay in business and make a lot of money, they’re going to set the margin for the pharmacy to make it economically worthwhile for the pharmacy to carry and sell the drug, because if they don’t, the pharmacy – excuse me, the generic company is not going to make any money. So the only situation where this is not going to happen is if the cost of the drug bought abroad – you know, I’m just using India for an example – is close to the price in Australia and that’s just not going to be the case.
1132 Professor Hausman disagreed that his approach to scenario 8 was bad economics. He explained:
…what I’m saying is the pharmacies can make money and the patients can save dollars. Both of those are an economic incentive for the private market to operate. I have yet to hear you posit any set of assumptions that says that private market won’t operate because profits are a powerful economic incentive and saving money for patients has been shown time and time again if they can save a dollar or two, not all of them but a significant percentage are going to switch.
You know, some people who don’t have a lot of money, saving a dollar or two every month is worthwhile. That’s why people will drive around and try to find – I don’t know in Australia but at least in the US will drive around and try to find cheap petrol, which I – if that’s the term you use here – cheap gasoline and that, again, you know, has been shown. So I’m just saying this is how people behave with respect to economic incentives…
The United States – not all because of Medicare, but the large majority of the prescription market is private, you know, non-government, and it has been shown time and time again that when you have generic competition, the lowest price wins, and, you know, the price often goes down by a very large amount after the substantial generic entry. Why is that? Because customers or patients, depending on what you want to call them, want to save money.
1133 When the issue of the safety net was raised as a basis for suggesting that patients would not switch because they may then not reach the safety net they otherwise would have reached, Professor Hausman said this type of “cliff behaviour” requires the patient to be good forecaster which, it turns out, few people are. I note also that Ms McTavish said the number of people who achieve the safety net is small. Further, Sigma’s internal documents suggest no more than 7% of general non-concessional patients would be able to access safety net benefits for Efexor-XR.
1134 Professor Hausman also said about scenario 8:
I think where we differ is Dr Jenkins is saying, well, the longest period you have is four months and this will be 36 months and she thinks that you can’t extrapolate that long. I say, well, I would attempt it if I could but, of course, we don’t have any data that long. But… I think that the economic incentives to sell into the private market are quite strong for the pharmacies and, of course, for the generic companies, otherwise they’re going to not make any money and for the patients they will switch.
Not all patients will switch, I want to emphasise that. You find that in any market but a significant percentage will switch to save money. So I certainly agree that I can’t test it for what would happen for 36 months. If I had the data I would. But I think the underlying economics remain very similar, not exactly the same but very similar. And the only thing that I have heard is it’s going to cost some money for the pharmacies to shift patients in terms of training staff or whatever and I say since this is a chronic disease and we, of course, agree on that, that that cost is amortised over a long period of time, it could be 10 years or whatever.
And so a profit-seeking pharmacy is going to find it worthwhile to do to, to increase its profits. I mean, the idea that they would not sell into the private market, I – you know, I just can’t accept because they’re leaving money on the table, you know, and in economics we don’t expect profit-seeking enterprises to be leaving money on the table.
1135 I consider Professor Hausman’s evidence persuasive. As explained below his views about the private market are also supported by ample evidence of those with specific expertise in the Australian industry.
1136 I do not disregard Dr Jenkins’ observations leading her to the view that the scenario 8 analysis involved an “heroic assumption” but when I weigh all of the evidence which I have identified above I am not persuaded that it is so unreliable that it should not be applied as the best evidence available and perhaps possible in all of the circumstances.
1137 On this basis the generics have proved on the balance of probabilities that this lost opportunity was a real one of substantial value. Professor Hausman’s results the best evidence available about private market supply. The fact that the models are not as robust for private market supply as they are for PBS supply does not mean that the evidence is unreliable or should simply be disregarded. In any event, it is difficult to conceive of any other evidence that it might have been within the power of the generics to call. In Placer (Granny Smith) Pty Ltd v Thiess Contractors Pty Ltd [2003] HCA 10; (2003) 196 ALR 257 at [38] the High Court said:
It may be that, in at least some cases, it is necessary or desirable to distinguish between a case where a plaintiff cannot adduce precise evidence of what has been lost and a case where, although apparently able to do so, the plaintiff has not adduced such evidence. In the former kind of case it may be that estimation, if not guesswork, may be necessary in assessing the damages to be allowed. References to mere difficulty in estimating damages not relieving a court from the responsibility of estimating them as best it can may find their most apt application in cases of the former rather than the latter kind.
1138 The generics have adduced the best evidence they can of the loss they sustained as a result of the interlocutory injunctions in respect of private market supply. Further, it must be recalled that Sigma and Alphapharm tried to do a deal with Wyeth to permit private market supply only and sought to defend the interlocutory injunctions on that basis. Wyeth refused to accept these proposals. It maintained that it would suffer irreparable harm if Sigma or Alphapharm were permitted to continue their private market supply. The Court accepted what Wyeth said. In these circumstances, any submission from Wyeth that the generics, who have done what it is within their power to do to prove this loss, have not proved loss due to the mere existence of some degree of uncertainty about the reliability of the models for private market supply given the available data should be (and is) firmly rejected.
1139 Professor Hausman was asked if he had looked at the actual margins which the generics were proposing before they were restrained. He was also asked, for example, if he knew that Sigma proposed that pharmacists would sell the product at the same price as the PBS-listed product, which he did not. Wyeth ultimately contended that there was no evidence from Sigma that it would have offered the prices upon which Professor Hausman based his analysis. This, however, is immaterial. It would have been commercially irrational for Sigma (and the other generics) to maintain unsustainable prices. It is clear from the evidence that the generics had substantial price flexibility with their suppliers and expertise in pricing their products so as to encourage generic substitution. Professor Hausman’s approach to pricing is the best evidence of what would have occurred in terms of prices and market shares but for the interlocutory injunctions because it is based on market evidence.
1140 Mr De Alwis’s evidence that Sigma would not have encouraged pharmacists to discount is also immaterial. Pharmacists were not bound by the generics’ recommendations and would act in their own best interests. Further, to the extent Wyeth’s submissions depended on the notion that pharmacists would not forego a certain profit today for the mere potential of greater profit in the future from PBS listing, I disagree. This was not consistent with Mr Chami’s evidence. It was not the effect of Professor Hausman’s evidence. It was not the effect of the evidence of Ms McTavish, discussed below. It was inconsistent with Sigma’s sales in the private market. It also does not accord with ordinary human experience. PBS listing could have been years away. Why would a pharmacist not be willing to make an extra dollar in 2009 for the mere chance of making more than an extra dollar in one, two or more years? A concern about not being able to later raise the price of the products would not carry the kind of weight Wyeth attempted to place upon it. One, prices can be raised over time. Two, pharmacists could not know how long they might have to wait for PBS listing, and many circumstances might have changed in the interim. In short, the certainty of extra money now is going to be more attractive to most people than the possibility of extra money at some unknown future time.
1141 As noted, Professor Hausman and Dr Jenkins prepared a joint report. To the extent I have not identified the issues in dispute between the experts above, I make the following comments.
1142 I do not consider it necessary or helpful to resolve the debate between the experts about aspects of the application of the Chow test. As explained below, I consider Professor Hausman’s approach in his revised work sufficiently robust to be accepted.
1143 I do not consider that Professor Hausman’s approach to molecules included and excluded from his comparator set undermines the validity of his work. He provided cogent explanations of his process of selection which satisfy me that his revised work is fit for the purpose of use in these matters.
1144 I accept Professor Hausman’s evidence that while his model does not include an explicit measure of originator response all originators have an economic incentive to maintain their profits in the face of generic entry and thus originator response is implicit in the model’s results. In my view, this provides support to the conclusion that no specific deduction, allowance or adjustment should be made for Wyeth or Pfizer having done anything they did not do or having done earlier that which they in fact did later. To the extent necessary, I am satisfied Professor Hausman’s models take into account originator responses because they are based on actual market shares obtained by generics on their entry into the market and, as Professor Hausman said, these shares necessarily reflect originator responses as all originators have the same interest in defending their market share to the extent that they are able to do so. I would no infer, in favour of Wyeth, that any other deduction, allowance or adjustment should be made to account for something Wyeth or Pfizer might have done over and above that which every originator may be inferred to have done for every molecule in the comparator set.
1145 In particular, and for example, I do not accept that it can be inferred that Wyeth or Pfizer would have discounted Efexor-XR in response to the private market supply by the generics in the same way as they in fact discounted in 2012 on full generic entry into the PBS market. In my view, taking into account the inexact nature of this exercise and my overall conclusions above, the best approach is not to require any adjustment to Professor Hausman’s model on account of originator discounting for private market supply. For PBS supply, I accept Professor Hausman’s evidence that originator responses which must include discounting are embedded within the comparator set so it is only if Wyeth or Pfizer would have been more aggressive discounters than the average originator that the issue arises. As noted, I would not draw such an inference in Wyeth’s favour. A number of considerations support this approach. One, as discussed, Wyeth would have done nothing which would materially confine Pfizer’s options. Two, Pfizer would not have been able to act immediately after acquisition of Wyeth. Three, Pfizer would not have acted to decrease the price of Efexor-XR if it believed, as on Dr Mannion’s evidence Wyeth did, that the method patent was valid and was being infringed given that the purpose of the litigation by Wyeth was to maintain its monopoly. Four, Pfizer would not have wished to undermine sales of Pristiq which had only been on the market since early 2009 and decreasing the price of Efexor-XR may well have cannibalised sales of pristiq. Five, in any event, a liberal approach should be taken to compensation of parties which have proved that they were adversely affected by wrongly granted interlocutory injunctions.
1146 I am not persuaded that any further adjustment to Professor Hausman’s revised work is required to take account of brand premium, the potential differences between independent and authorised generics, or price disclosure issues.
1147 I am not persuaded that Professor Hausman’s approach to determining individual generic shares and prices is inappropriate. No doubt different approaches could be taken, but nothing in the evidence leads me to conclude that Professor Hausman’s work is unfit for the purposes of this matter.
1148 For these reasons, and subject to one qualification, I consider Professor Hausman’s revised approach can appropriately be applied to the hypothesised factual circumstances which represent my conclusions for both the lost opportunities of private supply and of PBS supply.
1149 The qualification is this. In accordance with his instructions, Professor Hausman’s work disregards the interlocutory and final injunctions. The final injunctions made on 8 November 2010 cannot be disregarded. In his second report Professor Hausman was asked to respond to Dr Jenkins’ approach to isolating the effect of the interlocutory injunctions. Professor Hausman considered that the best approach would be to use his model assuming that sales decrease to zero in November 2010 and then re-start the model from the date of the Full Court’s orders. I am not persuaded that this approach would exclude the effect of the final injunctions made on 8 November 2010 because it does not recognise that, in this case, the final injunctions must have subsumed any effect of the interlocutory injunctions after 8 November 210. Further, Mr Samuel, accountant, provided a short report late in the hearing adopting a different approach which involved simply stopping his calculations based on Professor Hausman’s revised work on 8 November 2010. Wyeth accepted this approach as a matter of principle, as do I. In my view, Mr Samuel’s approach would properly reflect the terms of Wyeth’s undertakings and the principles which underlie the requirement that such undertakings be given as the price of interlocutory injunctions.
1150 I am satisfied that the generic claimants (and thus the manufacturers/suppliers from whom the generics would have ordered their products) suffered loss as a result of the operation of the wrongly granted interlocutory injunctions and that Wyeth’s undertakings should be enforced in all of the circumstances. The obligation in terms of assessment of the quantum of loss is thus to do the best that can be done on the evidence to ensure that such compensation as is ordered to be paid is just having regard to the nature and extent of the adverse effect of the interlocutory injunctions on the claimants. A robust and practical approach should be taken to the quantification of loss. In particular, in my view, further work of the econometric experts should be minimised or, if possible, eliminated. Mr Samuel’s approach to isolating the adverse effects of the interlocutory injunctions would have this effect. It is clear, robust and practical and I consider his methodology in this regard should be adopted.
1151 Further, it should not be thought that any of my conclusions require any further involvement of the econometric experts. In particular, for the opportunity of PBS supply, I note that scenario 5 involves Sigma supplying on the private market from 1 May 2009 and obtaining PBS listing on 1 December 2009, with Alphapharm supplying on the private market from 22 July 2009 and obtaining PBS listing on 1 March 2010 and Generic Health supplying on the private market from 9 August 2009 and obtaining PBS listing on 1 December 2009. As explained above, the small discrepancy between the Alphapharm private supply date in this scenario (22 July 2009) and the date of the Alphapharm interlocutory injunction (14 August 2009) is immaterial given Alphapharm’s actual launch on 17 July 2009.
1152 Insofar as Generic Health is concerned, my conclusions are that Generic Health’s private market supply prevented by the Generic Health interlocutory injunction would have commenced on 10 November 2009 (not 9 August 2009) and Generic Health would not have obtained PBS listing of its products until 1 March 2010, at the same time as Alphapharm. I anticipate that one consequence of this is that for the period up to 10 November 2009 the market shares of Wyeth, Sigma and Alphapharm will include a proportion of the share that on Professor Hausman’s results would have been allocated to Generic Health. Sigma’s share of PBS supply between 1 December 2009 and 1 March 2010 would also increase given my conclusion that Generic Health would not have sought PBS listing before Sigma obtained PBS listing on 1 December 2009. I anticipate that Mr Samuel may be able to ascertain a fair adjustment to the market shares of all putative participants in the market, Wyeth, Sigma, Alphapharm and Generic Health, to reflect my conclusions without additional econometric modelling.
1153 Further, no overall adjustments to sales volumes, prices or shares should be made to reflect these adjustments or the 8 November 2010 cut-off date given the final injunction (I return to aspects of this issue below in greater detail). A similarly robust approach should be taken to any other issue arising in relation to the quantification of sales volumes and prices in the PBS supply scenario. The differences between scenario 8 and my conclusions in relation to private supply should also be accommodated in the same manner if possible.
1154 Given that the need for these kinds of adjustments arises from my conclusions, the generic claimants will be given an opportunity to propose the best method to enable the sales volumes of their products to be ascertained in a manner which involves the least amount of further work. Directions to this effect and to allow the other parties also to be heard about these kind of adjustments will be made in consultation with the parties.
1155 Ms McTavish is an expert retained by the Commonwealth to provide evidence relevant to its claims. As the Commonwealth’s claim have been settled, I propose to deal with Ms McTavish’s evidence and the responsive evidence of Mr Dick only to the extent that their evidence remains relevant to the claims of the generics and manufacturers/suppliers. I will also consider Mr Heine’s evidence on behalf of Sigma in this section particularly as relevant to private market supply.
1156 Ms McTavish has over 20 years’ experience in the pharmaceuticals industry working for generic companies in Canada and Australia.
1157 Ms McTavish was asked to provide her opinion about the “market shares of pharmaceutical companies supplying venlafaxine, and the discounts and incentives which those companies would have offered to pharmacists in relation to their venlafaxine products” by reference to three scenarios (Scenario A - PBS listing of a generic brand and PBS supply from 1 August 2009, Scenario B - private market supply by generics from 3 June 2009 followed by PBS supply from 1 December 2009, and private market supply only from 3 June 2009 until 31 March 2012). Ms McTavish was asked to assume that for the purpose of PBS supply other generics (Apotex, Ascent and Sandoz) would have obtained PBS listing for their products by 1 March 2010 and Ascent, authorised by Pfizer, would have done so by 1 April 2010. These assumptions were not based on access to the internal documents or deliberations of these other generics and thus do not weigh against the conclusions I have reached that any prospect of entry to the market by those other generics is purely speculative and should thus be disregarded.
1158 In her first report Ms McTavish said that in the industry it is “common for supply contracts to have provisions for the re-negotiation of prices based on market conditions and the need for all companies in the supply chain to make a profit margin” supporting her assumption that “when market forces put downward pressure on net sale prices and therefore product profit margins, …suppliers could renegotiate their cost of goods”. I consider this assumption sound. It is consistent with Professor Hausman’s evidence based on his extensive experience working in the pharmaceutical industry that generic medicines have substantial price flexibility in terms of both supply prices to generics and by generics to pharmacists.
1159 Ms McTavish also concluded that it was “reasonable to assume that the volume of venlafaxine prescribed by doctors in the counterfactual scenarios I was asked to consider would not have been significantly different to the volume prescribed in the real world”. This too is consistent with the view of Professor Hausman and accords with the view I have reached. The fact that Ms McTavish considered this assumption to be reasonable provides further support to my conclusion that Professor Hausman’s opinion in this regard is to be preferred over that of Dr Jenkins.
1160 Ms McTavish’s approach to estimating market shares was based on the number of first line accounts a generic held. She noted Mr Heine’s evidence that in 2009 Sigma had 1500 first line pharmacies (or about 30% of the total, which she considered accurate) and estimated Alphapharm’s first line pharmacies as in the order of 35%. This supports the evidence I have accepted that Sigma and Alphapharm were major generic companies in Australia with the capacity to support unprecedented action such as private market supply of a PBS listed drug. Ms McTavish said that Generic Health’s first-line customer base in 2009 was less than 2% of pharmacies and it had to compete on price and flexibility. This supports the conclusion I have reached about the different position of Generic Health compared to Sigma and Alphapharm in 2009 and 2010.
1161 Ms McTavish estimated generic substitution rates for venlafaxine by reference to the trend for pantoprazole which first became subject to generic completion in April 2010. As explained above, I consider the econometric evidence provides a better foundation for the assessment of market shares and prices of generic and originator products than the analogue based model which Ms McTavish developed. As a result, I do not propose to discuss this part of Ms McTavish’s evidence except to the extent it assists in understanding the relevant issues more generally.
1162 Ms McTavish said this about Wyeth:
Wyeth’s evidence indicates that in the counterfactual world, if Wyeth expected to face generic competition for Efexor XR, it would have considered defensive strategies, such as revised commercial offers and discounting for Efexor XR. However, in my opinion it is unlikely that Wyeth would have responded in that way in mid-2009, including by discounting Efexor XR. That is primarily because in my opinion Wyeth did not, at that time, have a dedicated pharmacy sales force capable of implementing such an offer in pharmacies, and nor, given the impending completion of its acquisition by Pfizer, would it have been viable for it to establish such a team.
1163 This also accords with the conclusions I have reached about how Wyeth’s position should be treated. Ms McTavish took a different view about Pfizer, but as I have explained I am satisfied that Professor Hausman’s work in ascertaining total generic market share sufficiently allows for originator responses including originator discounts.
1164 In respect of private market supply Ms McTavish said:
The opportunity for pharmacists to dispense generic brands of venlafaxine in that way, in these scenarios, would have been limited to the 75mg and 150mg strengths of venlafaxine, because by 3 June 2009 the DPMQ [the PBS-published Dispensed Price for Maximum Quantity] for the 37.5mg strength of venlafaxine was already below the general patient co-payment. Accordingly, there were no general patients being subsidised for that product under the PBS who could have been attracted into the private market.
1165 Professor Hausman’s work does not support the conclusion that Sigma (which had an ARTG registration for a 37.5mg product) would have been unable to sell its 37.5mg product on the private market. Nor does the evidence of Mr Heine, considered below. For the reasons already given, Professor Hausman’s evidence is to be preferred.
1166 Ms McTavish considered that for a patient to be enticed to choose a private prescription the pharmacy would have needed to provide a discount of at least $1.00 to the general co-payment amount as otherwise there would have been insufficient financial incentive for a general patient to move from supply under the PBS to supply in the private market. Wyeth attempted to make much of evidence that Sigma, at least, did not propose in 2009 that pharmacists should discount Sigma’s venlafaxine products below the PBS general co-payment amount. Wyeth’s purpose was to support its contention that private supply would have ultimately failed. I do not accept Wyeth’s approach. It assumes the generics would have acted against their commercial interests by maintaining unsustainable pricing despite being confronted by evidence that a different pricing approach would or might have been required. The evidence is clear that the generics were highly skilled at adjusting their prices as required to maximise the opportunity for their pharmacist customers and themselves. The evidence about actual pricing is thus immaterial. It must be inferred that the generics would have priced their products as necessary to maximise their position from time to time including the potential for reasonable re-negotiation of the supply prices if necessary. To adopt Wyeth’ assumption would be contrary to commercial sense, a standard that I have applied to the issue of the likelihood of PBS supply and which must be applied consistently. Wyeth cannot seek to apply that standard to one critical issue, PBS supply, and then to disregard it for another, the pricing of the generic products if they had not been wrongly enjoined. Professor Hausman’s approach based on actual pricing of his comparator set provides the best guide to the range of likely prices.
1167 Ms McTavish calculated that pharmacists could make a profit out of the private supply of venlafaxine. This accords with Professor Hausman’s evidence, Mr Heine’s evidence, and the evidence of Sigma’s sales before being enjoined. Wyeth sought to use Ms McTavish’s calculations to prove that pharmacists would have been foolish to discount to the extent necessary to switch patients in the private market because the effect would be to deprive the pharmacists in the longer-term of profit that could be made for the PBS supply of the products. As discussed, I do not accept Wyeth’s approach. This would seem to be another example in which pharmacists would have to be excellent forecasters to hold any such concern. Further, on my conclusions, pharmacists would have no idea when the products might be PBS listed. I do not accept that pharmacists would be willing to ignore a short to medium term profit opportunity for the hope of ultimate gain at some unknown future time. Finally, the approach assumes prices can never be increased after a discount, another assumption I do not accept.
1168 In her initial report Ms McTavish identified a range of factors she believed would be likely to constrain the market share of the generics if their supply was confined to the private market, including PBS safety net considerations which would add complexity to private market sales, training and motivation of pharmacy staff, the extent of support given by the generic to the pharmacies, the originator response, and the unresolved litigation. She said that in her experience “pharmacists were generally only interested in stocking a generic product a few weeks before the product obtained PBS listing”. She estimated a low rate of substitution in pharmacies gradually increasing over time. Ms McTavish revised some of these views in her second report because of the success Sigma achieved in private market supply in May 2009.
1169 In her second report Ms McTavish responded to other reports and evidence, including the views of Mr Dick that she had over-estimated the market shares the generics would have achieved if they had been supplying their products on the private market. Consistent with my approach above I do not propose to discuss Mr Dick’s opinion that clopidogrel would have been a more suitable analogue than pantoprazole.
1170 In her second report Ms McTavish reinforced her view that Wyeth would not have been well-placed to defend Efexor-XR from generic competition for a number of reasons including the Pfizer purchase of Wyeth which had started by January 2009, Pfizer’s strategic focus away from “small molecules” (of which venlafaxine was one), the lack of any specific plans by Wyeth (which is correct on the evidence), and Wyeth’s lack of experience in generic defence strategies (which also accurately reflects the evidence). I consider Ms McTavish’s views are sound. Mr Dick’s contrary views based on Mr Cooper’s evidence and his own experience give insufficient weight to the position in which Wyeth and Pfizer would have been in throughout 2009 and 2010 by reason of the acquisition of Wyeth, Pfizer’s limited access to information before 15 October 2009, Pfizer’s limited relationship with pharmacies and lack of a pharmacy sales team, Pfizer’s lack of experience in respect of generic products and broader objectives, and the confounding factors of both Pristiq and the ultimate objective of the litigation being to maintain a monopoly over venlafaxine.
1171 I also agree with Ms McTavish that Mr Dick has under-estimated the incentive pharmacists would have had to switch patients to generic venlafaxine on a private market basis. As a general proposition I accept that both pharmacists and patients would have been likely to be attracted to generic venlafaxine for what may appear to be small profit increases (for pharmacists) and small price reductions (for patients) even if the short-term benefits, if analysed, may not outweigh potential long-term benefits. That is, I consider that people will take a known short-term gain in preference to waiting for a possible but not certain greater longer-term gain. This is consistent with Professor Hausman’s evidence which I accept. Further, as Ms McTavish noted there are “many examples of drugs that achieve relatively high rates of generic substitution where there is no brand price premium and therefore no price difference to the consumer, including both pantoprazole and clopidogrel”, but where there is a brand price premium a discount of less than $1 has been sufficient to encourage generic substitution. This is contrary to Wyeth’s insistence that the whole of Ms McTavish’s evidence depended on an assumed $1 discount to patients. The $1 discount was important to her market share projections but I am not adopting those projections and she gave other useful evidence which had nothing to do with this discount.
1172 Ms McTavish also gave this evidence in her second report which I accept:
While it is generally true that pharmacists prefer to stock a generic product only a few weeks before it is PBS listed, Sigma proved that it was possible to change what was previously a common practice in that regard.
1173 This evidence was based on the fact that between 1 May and 3 June 2009 Sigma had taken orders from 2,300 pharmacies for 53,000 packs of venlafaxine on the basis of private market supply. Ms McTavish said:
In my opinion, there are many factors that contributed to this success but the most significant are the strength of Sigma’s relationships with its customers, the training provided to its sales team, the marketing initiatives that were planned to support pharmacies in their efforts to convert patients from Efexor XR to the generic product and the trust that Sigma’s customer base had in the company.
1174 I agree. These conclusions are supported by Mr Heine’s evidence, discussed below, which I accept. Wyeth’s responses, that the sales achieved were underwhelming and that it should be inferred that pharmacists were misled by Sigma sales people, were unconvincing. Sigma was only on the private market for four weeks and achieved good results which must have reflected the pharmacists’ beliefs that they could achieve generic substitution in pharmacies. Sigma instructed its sales team that there was no known PBS listing date and the products were for private market supply only. The only evidence to the contrary, such as it is, is insufficient to draw the inference Wyeth seeks that pharmacists were misled. In particular, Alphapharm’s concerns are not reliable evidence that Sigma misled any pharmacist unintentionally or otherwise. Alphapharm’s documents are not admissions against Sigma. Alphapharm was surprised by Sigma’s success and believed at the time it would not be in pharmacists’ long-term interests but, as I have said, I do not doubt that Alphapharm would have been able to replicate Sigma’s success without misleading pharmacists about PBS supply.
1175 Further, as Ms McTavish noted, because the cost of generic goods generally decreases over time, generics can offer increasing discounts to pharmacists. I also give weight to Professor Hausman’s evidence that the cost of generic goods to the generic leaves sufficient room for discounting over time to ensure ongoing profitability.
1176 Although it is unnecessary to discuss the competing analogues used by Ms McTavish and Mr Dick it is relevant to note than in oral evidence it became clear that the analogue Mr Dick used, clopidogrel, involved a generic defence strategy carefully developed by an originator over about two years. For the reasons already given, this bears no resemblance to the position Pfizer would have been in in October 2009 when it completed the acquisition of Wyeth had the interlocutory injunctions not been granted. This example discloses why Mr Dick’s evidence about what Wyeth and Pfizer would have done should not be given material weight. It is evidence given without a proper context and without regard to the real constraints that would have acted on Wyeth and Pfizer.
1177 Mr Dick’s evidence also overlooked the effect of the litigation on the strategies open to Wyeth and Pfizer. Mr Dick said this:
But I need to comment on that because the first decision that you would make as a originator company is what kind of strategy you would pursue. Would you pursue a legal strategy and go for an injunction or go for a commercial strategy to defend the product? It’s clear from the evidence that Wyeth made the decision to pursue – in the real world to pursue a legal strategy. If they had not taken the legal strategy, then they would have had to, in the counterfactual world, take a commercial strategy.
1178 The problem with this evidence is that it disregards the litigation in which Wyeth and Pfizer were involved which is impermissible. Wyeth believed the method patent to be valid and infringed and invested in the litigation to retain its monopoly for the duration of the method patent not merely to obtain a temporary advantage from interlocutory injunctions. So much was clear from Mr Manspeizer’s evidence. Pfizer continued the litigation. The object of the litigation, to retain the monopoly for the duration of the method patent, would have constrained the available options for Wyeth and Pfizer. Mr Dick said this about an authorised generic:
You would not launch the product on the PBS until the legal strategy was resolved, but you would still consider applying in – for example, in March, if they had not have gone for the legal strategy and had decided to go with a commercial strategy, it would be logical that they would apply for the generic listing [meaning ARTG registration not PBS listing] at that point in time (my emphasis).
1179 In the present case the legal strategy was not resolved until the Full Court’s orders in November 2011 and even then Wyeth applied for special leave to appeal. In other words, properly understood, Mr Dick’s evidence supports my conclusion that Wyeth and Pfizer would not have launched a second brand of venlafaxine at any earlier time than in fact occurred if not for the interlocutory injunctions because to do so would be contrary to the objective of the litigation and also would potentially undermine the new product, Pristiq. Further, as Mr Dick also said:
The strategy was very clear that they stocked a generation of prescriptions for Efexor XR in October and has tried to transfer as much of the new patients that would have been Efexor’s to Pristiq. And so their focus was on generating prescriptions for Pristiq as opposed to generating more prescriptions on Efexor.
1180 This also supports the conclusions I have reached, as does Mr Dick’s evidence that (contrary to Ms Braithwaite’s views in 2009) that in fact it would make no sense for Wyeth or Pfizer to have promoted Efexor-XR if the interlocutory injunctions were not granted as Pristiq was not subject to generic competition. Wyeth (and Pfizer) would not have wanted patients to be prescribed Efexor-XR or an authorised generic version of it if, instead, they might be prescribed Pristiq.
1181 Mr Dick also agreed that if “generics gain a foothold in a market before an innovator can respond, it becomes more difficult for the innovator to engage in an effective defence”. Sigma and Alphapharm would have gained this foothold well before Pfizer could do anything to respond to generic competition. These observations reinforce my conclusion that Professor Hausman’s approach to both PBS and private market supply of generic venlafaxine is reasonable. Nothing Wyeth or Pfizer could or would have done would have prevented the generics from taking market share even if confined to private market supply.
1182 I do not accept Mr Dick’s evidence that there would have been no private market for the 75mg and 150mg generic venlafaxine products due to the DPMQ (PBS-published Dispensed Price for Maximum Quantity). Ms Braithwaite’s evidence in 2009 assumed the contrary. Ms McTavish’s evidence was to the contrary. Professor Hausman’s results are to the contrary. Mr Heine’s evidence was to the contrary. In any event, in oral evidence Mr Dick also said that he accepted that generics could sell these products for prices below the DPMQ and thereby create a private market for these products.
1183 Similarly, I do not accept Mr Dick’s evidence that pharmacies would need to invest “significant additional effort” to achieve private market sales which would not be worth their while. Every sale involves effort of some kind. The generics were well placed to provide support to pharmacies to ensure that their supply, PBS or private, would have succeeded if the interlocutory injunctions had not been granted. Mr Dick’s experience did not involve him in day-to-day dealings with pharmacists. Ms McTavish had more experience of this kind as did a number of other witnesses including Mr Heine. Mr Dick also accepted that as there are small margins on many pharmacy products so that pharmacists foster relationships with their customers and have a financial incentive (shared with patients) to encourage generic substitution. In this context, Wyeth’s emphasis on the additional burden of encouraging appropriate generic substitution as a disincentive was misplaced.
1184 I do not consider that the cross-examination of Ms McTavish undermined her views that Sigma had proved that private market supply of venlafaxine could succeed. What Sigma did was unprecedented. As I have said, it had an unexpected degree of success over a mere four weeks of supply. This cannot reasonably be attributed to misconceptions of pharmacists. It was a result of Sigma’s experience, expertise and relationships with pharmacists, all of which Alphapharm also possessed, and Generic Health, albeit without the market reach of Sigma and Alphapharm. When confronted with the evidence about Alphapharm’s initial scepticism in response to Sigma’s success, Ms McTavish said:
I’m not sure why Alphapharm decided not to sell in, and I don’t think it’s contrary to a customer’s interests to have a product that they could potentially make more margin on. I acknowledge the difficulties of trying to establish a product market on a product that is PBS listed because it had never been done before. So I can’t – I can’t say why Alphapharm chose to approach it this way and chose not to launch their product, but I certainly couldn’t say it’s not in a customer’s interests. I think the risk to the customer is if they couldn’t sell it into the private market, couldn’t put the processes in place in pharmacy, that they could be stuck with ... stock and then there’s the issues of what would Sigma do and it raises other questions.
…
…typically, certainly, Sigma and all the generic companies at the time would simply replace that stock or traded it for something else. So dealing with a big company and established customers and customers that use Sigma, they wouldn’t have seen that as a particularly big risk.
MR BANNON: Was it your experience that Sigma would take back stock that pharmacists couldn’t sell.
MS McTAVISH: Generally, yes, or replace it. I think more common practice was to replace it with something else or more of the same thing with better dating… I think they might have assumed that – at the worst-case scenario is that they would be able to return it once it expired.
1185 Alphapharm did launch its products, albeit in the confined manner discussed. This evidence supports the inference that Wyeth’s case that pharmacists would not be interested in generic venlafaxine for private market supply is unfounded. It also supports my conclusion that Alphapharm’s initial scepticism would have given way rapidly under the competitive pressure from Sigma, and Generic Health, a company also in the business of supplying generic products, most likely would have followed suit by private market supply once it had run out of other options to avoid potentially wasted expense.
1186 Ms McTavish also gave this evidence, which I accept:
MR BANNON: And the question I was asking you – I will frame it again – was that if that statement by Mr Held was responsive – I’m asking you to assume this – to an interest expressed by the pharmacies who ordered the stock as to when it would be PBS listed, such an interest so expressed by the pharmacy would be consistent with your view, the pharmacies acquire stock in anticipation of PBS listing.
MS McTAVISH: No, not in this case because Sigma had gone out with a different model and was promoting – you know, I would assume that while PBS listing would be important to Capital Chemists that they had purchased stocks not just in anticipation of PBS listing, but they had purchase it because they accepted that there was an opportunity to dispense it previously that would be a benefit to them.
MR BANNON: But you don’t know how exactly it was promoted to Capital Chemists, do you?
MS McTAVISH: No, but you asked me to assume that that was a response that was consistent with my prior statement, and I think my prior statement was that, prior to the Sigma launch, that was sort of common practice or commonly accepted that pharmacists weren’t interested until shortly before PBS listing. So I guess I would assume that while Capital Chemists would be – a pharmacy customer would be interested in the listing date and certainly want it to be PBS listed because it would make it easier, I would have to, at least, consider that it’s possible that Sigma said, “We have a model and an opportunity for you to make money on this product as a private product to roughly half of the – half of your customers, and we’re going to come in and we’re going to show you and we’re going to train you and we’re going to – give you all the material that we normally give you to run these sort of programs”, and I think that that is how Sigma would have operated.
1187 Ms McTavish’s hypothesis about how Sigma would have operated is consistent with the evidence. Given that there is evidence that Sigma’s sales team were instructed that this was a private market supply only with no known PBS listing date, the only speculation is that of Wyeth that pharmacists were misled. It was not necessary for Sigma to call evidence from its sales team from 2009 to negative mere speculation that they acted contrary to clear instructions. The fact that they may be inferred to have received incentives for sales, with the internal speculation of Alphapharm and a couple of emails from pharmacists inquiring about PBS listing, does not support the inference or even possibility for which Wyeth advocates.
1188 Ms McTavish also said that for general non-concessional payments she did not accept that the safety net was a major consideration. She said that for general patients the potential triggering of the safety net involves “a very small portion of customers”. This indicates that concerns about the safety net dissuading pharmacists from generic substitution should not be over-stated. The number of patients potentially affected will be small. The potential for the issue to arise would not have dissuaded pharmacist from generic substitution on a private market basis for most patients.
1189 Ms McTavish also gave evidence supporting my conclusion that Wyeth’s approach to supposedly rational conduct of pharmacists was unrealistic in this exchange:
MR BANNON: It would be sensible for a rational and economically thinking pharmacist to think making a few bucks on a few packs now with few complications to switch people might not make much sense, rather than making more dollars on the same product for many years afterwards if they get PBS listed. You agree that would be a sensible thing for a rational pharmacy to take into account?
MS McTAVISH: Well, I think that that would be the way some pharmacists would look at it, but I also know there are other considerations that go into a pharmacist’s decision based on their relationship with their generic company. So there are incentives beyond discounts that are put in place to encourage both volume and substitution and there are rebates and things at the back, both the – often, the pharmacy itself and also to the head office. So I also consider that some of these would be in place, depending on the importance of the product and company, that would also come into play in the situation. So I – I accept that it’s easy to isolate this product and say, on this one product, does it make sense that you’re going to make an extra 40 or 50 bucks a month. What are you risking longer term, but then I think there are other factors that go into that mix, and there are a fair number of variables that a – not just a pharmacist would consider, but that the pharmaceutical company or the generic company would certainly put to the pharmacist. So I think that’s not just one consideration.
MR BANNON: Well, I’m only addressing the one which you referred to, namely, the profit margin in – one that you refer to in your material.
MS McTAVISH: Yes, but you’ve said, “Is that a rational decision”, and I don’t think that those decisions are made in isolation of all factors.
1190 When confronted with Alphapharm’s apparent lack of success in its private market launch Ms McTavish said she found the sales of 532 units “surprisingly low for Alphapharm”. I need not repeat my conclusions that what Alphapharm in fact did in July 2009 bears no resemblance to what it would have done if not for the Alphapharm interlocutory injunction. Ms McTavish also said that she did not think Sigma would have been as successful as it thought it would be in respect of its private market supply but she plainly did not share Wyeth’s view that this supply simply would have failed. As Ms McTavish put it:
I think that they did incredibly well, but I do also think that they were highly motivated to create this market. I think that they needed a win. I think they were in a position where they had gone through changes in the company that I think left them in a position that they sort of needed to prove themselves, and I think this was a massive opportunity to do that and that’s what they set up to do. So I suppose that’s the other factor in the mix is that – is that they really needed this win and they needed to go out and make it happen…
1191 Ms McTavish agreed that pharmacists tended to be conservative and would be cautious about dispensing a product that might result in interrupted supply by reason of a court order. This may have some weight as a general proposition but must be tempered in the present case by three matters. One, the generics thought they had good prospects of success so if any pharmacist was interested in the litigation it is doubtful they would have been informed that the generics believed supply would be interrupted. Two, the returns policies of the generics would have provided pharmacists with a substantial degree of comfort. Three, there is evidence from Mr Chami that if he was aware of any risk to supply he would dispense the generic product and “see what comes around”. This makes sense. Supply of a product might be interrupted for all kinds of reasons not related to litigation. If pharmacists attempted to second-guess every potential interruption to supply they would not dispense any products. I do not accept that ethical obligations would have prevented supply in the circumstances of this case or would have weighed materially on the minds of pharmacists when considering whether it was appropriate to substitute a generic venlafaxine product. If they considered it inappropriate to substitute for a particular patient due to a risk of confusion the pharmacist would not substitute for that patient irrespective of concerns about supply interruption which, in my view, would not feature in the consideration of pharmacists.
1192 Ms McTavish also gave evidence in this exchange, which supports my view that the private market supply by the generics would not have failed:
MR BANNON: And on the scenario I’ve painted, you might have a great new private market collapsing in on itself very quickly, people reverting to normal practice; do you agree?
MS McTAVISH: No. I think that, again, that there’s other incentives in place, and I think that once people started down that road, they would be committed to it. I think if it were working for them, they would continue it. If people hadn’t been successful, then they might not bother trying. I think you would get a full range, there’s always a full range in the ..... and do very well at it and pharmacies would be very ..... at it.
MR BANNON: And you couldn’t assume it would be successful on the figures I – continue to be successful on the figures I’ve given to you, could you?
MS McTAVISH: Well, I think it would be less successful than what Sigma thought it would be.
MR BANNON: And as soon as you have an outcome where it’s less successful than both the pharmacists and Sigma thought it would be, there’s a prospect that it would not continue at all. That would be one of the prospects.
MS McTAVISH: Well, I think that’s a broad generalisation, and I think that’s not what practically happens. What practically happens is that some pharmacies would carry on and others wouldn’t bother.
MR BANNON: In any event, you haven’t built into any assessments a consideration of some pharmacists not bothering to continue because of poor conversion rates, have you?
MS McTAVISH: Well, I don’t think that they would stop trying because of poor conversion rates. I think if they had order to convert at 50 per cent and they only – of that population, they only converted at 20 per cent, that they would continue with those patients on what they started, and it would just impact their subsequent purchases of the product. They would just buy less. It doesn’t mean they would say, “I can’t be bothered”, and it’s – I think it’s unlikely they would revert back on patients that they had already changed.
MR BANNON: But you haven’t built that private scenario into your assessments, have you, as a possibility?
MS McTAVISH: I haven’t specifically built in an assumption that some people would start and then stop. I built in an assumption that the uptake would be very slow.
…
Well, just because – just because they’re not reaching a goal doesn’t mean they’re not going to stop. Every pharmaceutical – every generic pharmaceutical company wants pharmacies to hit 80 per cent in the first month, but that doesn’t happen. They – they get there over time and some get there more quickly than others, but just because they’re not as successful as maybe everybody wants them to be in the first month, doesn’t mean that everybody stops trying.
1193 Given my view that reliance should be placed on Professor Hausman’s revised method for both PBS and private market supply it does not matter that Ms McTavish did not allow for pharmacists to cease purchasing the products altogether. The relevance of this evidence is that it discloses her view that Wyeth’s case of private market supply simply failing and ceasing for any reason, including pharmacists’ asserted incapacity to switch patients, is unrealistic. When Wyeth again attempted to over-state the complications and under-state the potential benefits to pharmacists by referring to the “couple of extra bucks for a few months” a pharmacist might earn, Ms McTavish, consistently with the effect of Professor Hausman’s evidence, said:
I think the couple of extra bucks is part of a much bigger picture and a much bigger relationship that pharmacists have with their generic companies, and I go back to saying you can’t – you can’t simply consider that one molecule is going to behave sort of independently of that overall relationship. So I think that is a factor, one factor of many factors in looking at what a pharmacy would do with this or would have done with this molecule…
If I look at just a couple of bucks a month, there’s a lot of products that do really well with pharmacy a lot less than a couple of bucks a month that astound me. That – why – the products that are so minimally incrementally profitable, that I wonder why pharmacists still to it, but they do. So I don’t – because I think that a pharmacist, when they would be looking at this product, they wouldn’t be saying, “I’m going to make an extra two or $3.” They wouldn’t be looking at it in isolation. It would be part of their overall strategy. They would have either decided to do it or not do it, and the people that are then on the front line actually implementing it, for the most part, it wouldn’t be in their head, I’m going to do this because it’s an extra $2 or $4 or whatever it happens to be, they’re doing it because it’s part of their strategy.
MR BANNON: Okay. I will rephrase my question again. Would you agree that, in this particular case, if one was just focusing solely on the potential to make a few dollars on the number of packets, we’ve discussed before lunch, for a period of time before litigation is resolved in and of itself, and leaving aside relationships and wider rebates which you’ve discussed – you leave aside those things – would you agree, for those few dollars of those units in this circumstance would not have been enough in your assessment to drive a private market; do you agree?
MS McTAVISH: If there’s nothing else going on in a market and a pharmacy is only buying one product and this is – they have no other relationship, this is all they’re doing, yes, it would have been a less sensible decision.
1194 The last part of Ms McTavish’s answer shows how divorced from the likely position Wyeth’s contentions are in relation to the private market.
1195 Ms McTavish also said, as must be accepted, that pharmacies make their own price decisions. They are not bound to adopt the generic supplier’s recommendation. She said:
I think pharmacies absolutely make their own decision. I think that they would have taken that as a suggestion and in the end decided if they could make the sale without having to discount it, that would have – that would be what they would choose to do. So they certainly wouldn’t price it the way a generic company told them to price it. They would simply take that as a – as an idea that they could actually make more money by discounting the product. They still get to keep more. So, yes, they don’t do what they’re – necessarily what they’re advised to do – or what’s model, I suppose, because that was a model that Sigma put together to show their pharmacists how they could make more money and still offer the customer a better deal.
1196 I accept this evidence.
1197 The only other evidence which Ms McTavish gave that should be noted is her view that Generic Health could not have maintained any long-term advantage even if it had been the sole PBS supplier. She said Generic Health would not have held on to customers once other suppliers, Sigma and Alphapharm in particular, came onto the PBS market. This supports my view that Generic Health had nothing much to gain by being the first generic to obtain PBS listing.
1198 In the context of my reasoning, the main point to be taken from Ms McTavish’s evidence is that she considered that the private market supply would have succeeded as a profit making venture and would have continued. Mr Dick’s contrary evidence, for the reasons given, should not be accepted.
1199 Mr Heine, Sigma’s sales manager, also gave evidence relevant to the issue of the likely success of private market supply. Mr Heine said:
I have spent almost 10 years of my career working with, and catering to the needs of, retail pharmacies. During that time, I estimate that I have spent approximately 50% of my working time speaking with hundreds, if not thousands, of pharmacies about their generic pharmaceutical needs and the way they go about making their purchasing decisions in order to understand their needs and key drivers and ascertain how best to service those needs.
1200 While I do not accept his evidence insofar as it touched upon PBS listing, Mr Heine was an impressive witness. Given his experience Mr Heine’s evidence about how generic companies deal with pharmacists and the relationship between then should be given substantial weight. While on this topic I should record that many objections to evidence were taken on the basis that a witness could not give evidence about pharmacists in general, in contrast to the pharmacists with whom they had had dealings. For witnesses like Mr Heine and Ms McTavish (as well as Mr Hurley and Mr Upiter), who were in the business of understanding how to maximise sales to and by pharmacists, I disagree.
1201 Wyeth tried to suggest that Sigma’s offer in May 2009 was the equivalent of giving its venlafaxine products away but Sigma still did not manage to sell much of its stock before being enjoined. This misunderstands the relationship-based nature of Sigma’s generics business which Mr Heine, in particular, cogently explained.
1202 As Mr Heine said:
The significant majority of pharmacies in Australia purchased the majority of their generic product portfolio from one generic supplier which was often referred to as their first line supplier. A pharmacy purchased the majority of stock from its first line supplier because it generally obtained better pricing with the more stock it purchased. For example in 2009, Sigma offered the lowest pricing to the pharmacies for which Sigma was the first line supplier. It would also make available special bundling deals for its first line suppliers, which were designed to further cement Sigma’s relationship with those pharmacies.
1203 The same was true for Alphapharm which was also a major first-line supplier. While not a major first-line supplier Generic Health was also in the business of fostering overall relationships with pharmacies.
1204 Mr Heine’s evidence that pharmacists would not have been cautious about private market supply mere because it necessarily would focus on general non-concessional patients was based on his extensive experience dealing with pharmacies in 2009. This experience may be contrasted with the contrary evidence, for example, of Mr Cooper of Pfizer who at least in 2009 had little experience in dealing with pharmacists. Mr Heine said that pharmacists would not have been deterred from the private market supply of venlafaxine given that “venlafaxine XR was the number one anti-depressant in terms of market size”, “the vast majority of pharmacy sales staff did not differentiate between particular generic products and therefore would offer every customer the opportunity to take a generic product if it was available” and if “patients were served by casual pharmacy staff, the staff could ask each patient whether they wanted a generic version if available and the pharmacist would only fill the script with a generic product if the patient was a non-concessional patient” and that Sigma provided specific training to pharmacies to ensure they were well equipped to achieve effective and appropriate substitution. Mr Heine said:
Sigma’s sales representatives and I trained pharmacists to approach patients directly to provide education when a new generic product came on the market. In my visits to pharmacies, I observed pharmacists following our training method and educating patients directly…In launching EVELEXA XR, the Sigma sales representatives and I trained pharmacists to undertake this approach with respect to the dispensing of EVELEXA XR…In June 2009, the profit incentive for pharmacists to convert non-concessional patients to a generic venlafaxine XR product was compelling due to the considerable size of the non-concessional market for venlafaxine XR.
1205 I accept this evidence. It explains Sigma’s relative success in its short-lived private market supply. It supports my view that this supply would have continued successfully until 8 November 2010 but for the Sigma interlocutory injunction. It supports my conclusion that Alphapharm’s and Generic Health’s private market supply also would have succeeded up to 8 November 2010.
1206 Mr Heine otherwise gave evidence which I accept that Sigma was planning for its generic venlafaxine product to be the “jewel in the crown” of Sigma’s product range. While I have not accepted that this mean that it was more likely than not that Sigma would have been the first generic to seek PBS listing I do consider this means that it would done everything it could to ensure that its private market supply succeeded. The stock it was holding was an incentive for this but it cannot be doubted that Sigma intended to continue to supply its products on the private market and would have used all its resources to maximise its position in so doing if not restrained by the Sigma interlocutory injunction. Wyeth’s accepted that Sigma would have continued its private market supply but insisted that it would have failed, an insistence I reject on principle given that Wyeth was the beneficiary of the interlocutory injunctions and in any event find unpersuasive on the evidence.
1207 When Sigma became aware of the method patent and shifted its planned launch to a private market supply, Mr Heine was well placed to transform Sigma’s offer from then two stage launch (private market supply from 1 May 2009 and then PBS supply from 1 August 2009) into a private market supply only proposal. New budgets and marketing materials were quickly prepared. Mr Heine approached the launch “strategically by identifying the order of importance of pharmacies and ensuring that Sigma's most important customers were visited first and so on”. The marketing materials made it clear that this was a private market supply only (contrary to Wyeth’s suggestions). Mr Heine noted that by 3 June 2009 Sigma’s sales accorded with his expectations and that the 2,300 pharmacies which had ordered Sigma’s venlafaxine products “was well in excess of the 1,500 pharmacies to which Sigma was the first-line generics supplier”.
1208 Mr Heine considered that if the Sigma interlocutory injunction had not been granted and if Sigma had continued to be the only generic supplier (which it would not have been given Alphapharm would have entered the private market shortly after Sigma) then Sigma would have achieved a market share of about 12% to 14% of the total market. Contemporaneous Sigma documents from May 2009 indicate that Sigma considered a 12% market share to be an aggressive target. I do not find it surprising that Sigma set itself aggressive targets but this does not mean the targets could never be achieved. Given the availability of Professor Hausman’s evidence, I do not adopt Mr Heine’s estimated market penetration for Sigma, but I accept Mr Heine’s view that Sigma would have succeeded to obtain material market share even on the confined private market basis. I see no reason why Alphapharm’s position would not have been similar. One thing that cannot be doubted about Mr Hurley is that in 2009 he was well placed to ensure Alphapharm maximised the positions it had decided to take (and thus must be inferred to have been capable of maximising the hypothesised positions that I have concluded Alphapharm would have taken but for the Alphapharm interlocutory injunction). While not equivalent to Sigma and Alphapharm in terms of size, reach or experience, there is no reason to infer that Generic Health was incapable of maximising such opportunities as it had. Wyeth’s contrary suggestions based on Generic Health’s documents about its sales staff is an insufficient foundation to infer to the contrary, particularly given that Generic Health’s different market position compared to Sigma and Alphapharm is already reflected in its far smaller market shares.
1209 Mr Heine’s willingness to accept that he could not control what every sales person said to pharmacists at all times does not support Wyeth’s contention that Sigma achieved sales because pharmacists wrongly believed that Sigma’s products would be PBS listed in the not too distant future. It merely confirms Mr Heine’s credibility as a witness. I accept Mr Heine’s evidence that Sigma made it “reasonably clear” in all of its materials that the supply was confined to the private market with no set date for PBS listing or guarantee that Sigma’s products would be PBS listed. As a result Wyeth’s proposition that Sigma was effectively giving the stock away (which depended on pharmacists mistakenly believing that the products would shortly be PBS listed) should not be accepted. Mr Heine’s evidence remained cogent and rational. He accepted that some pharmacists would find Sigma’s offer more attractive if PBS listing was imminent (which may be accepted but goes nowhere). He refused to accept that pharmacists would have been deterred from substituting Sigma’s generic product merely because of the risk that supply might be interrupted (which is a sound view). As Mr Heine said:
So again I will refer there was 2300 customers who obviously were comfortable in placing that order, but for some customers that may have had that thought process in their mind for whatever reason, yes, that may have impacted those customers.
1210 Similarly, Mr Heine’s willingness to accept that the rate of sales was slowing towards the end of May 2009 does not suggest that his views about the likely success of Sigma’s private supply had it not been enjoined are unreliable. Nor does the fact that Sigma did not reach its own sales targets. Mr Heine described the target of 3300 pharmacies as a “stretch target” which I would understand to mean ambitious and in any event obtaining orders from 2,300 pharmacies, as Sigma did, was an excellent result for an unprecedented strategy. As Mr Heine also said, incremental improvements would always be expected month to month.
1211 Mr Heine properly accepted that the strategy was unique, but also said:
…so this was unique in the sense that it was a private launch of a PBS item, a generic that was a PBS item. We had at Arrow, then into Sigma, had launched many private products before into the marketplace, and it had been probably – in my opinion – the most successful company in doing that. And that’s in representing other companies’ products. It was revolutionary, but the one thing that Arrow/Sigma did in the marketplace, what I think better than anyone, is that we revolutionised how we brought products to market and got customers to come on a journey with us that they would not have done otherwise previously. So I agree at a base level that it was revolutionary. But I also, you know, look back on those times and think it was absolutely achievable that we could have hit substantial success numbers.
1212 Mr Heine accepted that his prior experience was with private market supply where PBS listing was expected shortly and without the products being subject to litigation. This does not mean his contemporaneous expectations or those of Sigma in 2009 could never be achieved, even though it may be accepted that the expectations were aggressive.
1213 Mr Heine also gave evidence providing reasons why Wyeth and Pfizer would not have been able to compete effectively with Sigma. It is not necessary to traverse this evidence (which provides nothing but further support for the conclusions I have already reached for other reasons). For present purposes what is relevant is Mr Heine’s evidence that Sigma was focused on its first mover advantage (which I have accepted) and was not concerned about any defensive strategy Wyeth might take (which I also accept). This evidence further reinforces that the approach I have taken to Wyeth’s “generic defence strategy” arguments is sound.
1214 The only other observation required is that Mr Heine’s evidence supports my view that once it saw Sigma’s success Alphapharm would have changed its initial position that private market supply was unlikely to be in the longer-term interests of pharmacists. For the reasons already given I do not accept that Alphapharm would have maintained its initial position to the contrary (as, indeed, it did not when it launched its products on 17 July 2009, albeit in a confined manner). Given their similar expertise and market positions it must be inferred that Alphapharm’s market position would be similar to Sigma’s albeit that it would not have the first mover advantage that Sigma had and would have continued to enjoy but for the wrongly granted Sigma interlocutory injunction.
1215 In its submissions Alphapharm identified a number of sources of evidence from which it would be inferred that its private market supply would have been successful. While focusing on its own position, the evidence applies equally to Sigma and Generic Health. Alphapharm pointed to the evidence of Mr Chami, pharmacist, to the effect that the private market supply would have been attractive. I accept that evidence. It pointed to Mr Cooper’s acceptance that pharmacists could have made more profit from selling generic venlafaxine products on the private market than from selling Efexor-XR on the PBS. It pointed to that part of Professor Hausman’s evidence in which he said:
Nothing I have seen causes me to expect that the amount of switching to generic venlafaxine will be less than if generic venlafaxine were PBS-listed holding other factors equal, which the regression model does, because pharmacies have an incentive to earn higher profits and patients have an economic incentive to shift to the lower priced product.
1216 It also pointed to Professor Hausman’s evidence that a significant percentage of patients would switch to a cheaper generic product. In Alphapharm’s words, which I accept:
While Professor Hausman agreed that not all patients would switch to the generic in scenario 8, the econometric model was designed precisely to estimate this proportion using data relating to the proportion of patients that switch to a generic in the case of other molecules.
1217 Alphapharm also noted that:
Consistent with the opinion of Professor Hausman, Ms McTavish’s evidence was that the same economic considerations apply to private products as they do to PBS listed products - that is, generic companies and pharmacies are driven to supply products and obtain market share irrespective of whether or not the product is listed on the PBS. This is particularly the case where, as Mr Dick acknowledges, the private venlafaxine market would represent around $50 million in circumstances where a very large number of pharmaceutical products sold in the Australian market have a total market value of less than $50 million.
1218 Alphapharm submitted this:
Too much should not be read into Ms McTavish’s statement that the situation of a generic launching only on the private market, in circumstances where there was a PBS-listed originator, would be “unprecedented”. That says nothing more than the counterfactual had never actually happened before, to Ms McTavish’s knowledge. It does not suggest that private market launch would not or might not succeed. Most importantly, it does not deny that the usual economic drivers of commercial life would apply, namely that it is to be expected in the ordinary course of business that many pharmacies will wish to increase profits and many patients will wish to accept a generic medicine if it costs them less money.
1219 I agree with Alphapharm’s submissions.
1220 To the extent that Wyeth suggested that the generics might somehow have benefited from the wrongly granted interlocutory injunctions by, for example, having been saved from a failed private market supply or from the need to refund pharmacists for stock that could not be sold (in cash or kind or by other rebates or discounts) or as a result of their sales teams having been freed up to sell other products or otherwise, I consider a different conclusion is required. The loss of the opportunity to supply venlafaxine up to 8 November 2010 undoubtedly caused the generics losses of various kinds quantifiable and unquantifiable. The ability to supply venlafaxine for the period up to 8 November 2010 would have provided the generics with a rare opportunity which they lost as a result of the wrongly granted interlocutory injunctions. Justice demands that they be compensated for their quantifiable losses.
1221 I have accepted that the final injunctions on 8 November 2010 must represent the date from which no compensation is payable. Those final injunctions, of course, were also wrongly granted but Wyeth’s undertakings did not extend to any effect of the final injunctions (and nor logically could they do so given the principles which found the requirement to give the usual undertaking as to damages as the price of interlocutory orders). Unfair as it no doubt appears to the claimants, losses sustained as a result of a wrongly granted final injunction must lie where they fall. However, to the extent Wyeth might have suggested that the final injunctions would have caused the generics loss, I should record that I do not consider that any separate discount, deduction or adjustment should be made to reflect the adverse effects of the final injunctions granted on 8 November 2010 to account for the risks that the generics might have faced at that time such as having to destroy stock supplied to the generics but not yet sold to pharmacies or having to provide refunds or replacement stock to pharmacists.
1222 First, it is one thing to reject the generics’ approach that they lost an opportunity to apply for a stay of the final injunctions made on 8 November 2009 (as I have done). It is another to speculate in favour of Wyeth, the party which obtained the wrongly granted interlocutory injunctions, that the generics would be left holding a material volume of stock they could not supply to pharmacist or would have had to refund pharmacists or provide them with alternative products for stock the pharmacists could not sell. In the circumstances, I am not willing to speculate in Wyeth’s favour in this way.
1223 Second, while I consider the generics’ stay argument is an impermissible means of disregarding the final injunctions granted on 8 November 2010 it would be wrong to ignore the fact that if the generics had not been wrongly enjoined on an interlocutory basis it would have been necessary for the operation of the final injunctions to be moulded to suit the circumstances. It is not necessary to speculate what form that exercise would have taken but it would have been guided by the principle that unnecessary harm to third parties such as pharmacists and patients should be minimised.
1224 Third, one thing that cannot be doubted on the evidence is that the relationship between the generics and their pharmacist customers was one which enabled the generics to tailor their offers to best suit the circumstances. This capacity would extend to the generics ensuring that their exposure to material costs by reason of the grant of the final injunctions on 8 November 2010 was minimised. The generics had a broad range of products and strong relationships with pharmacies. They would have known how to package offers to pharmacists which would have minimised their costs exposures.
1225 Fourth, I have accepted that the generics suffered unquantifiable losses as a result of the wrongly granted interlocutory injunctions. While those losses are unquantifiable I consider they would have been material.
1226 Fifth, it was Wyeth which obtained the benefit of the wrongly granted interlocutory injunctions and the generics which suffered the harm from them.
1227 All of these considerations weigh against any discount in Wyeth’s favour based on mere speculation.
1228 Finally, if (as is the case) the generics cannot be compensated for the adverse effect on them of the final injunctions, I cannot see why Wyeth can argue that the compensation to the generics should be reduced because of any such potentially adverse effect. To do so would be to impose a triple burden on the generics. They were the subject of wrongly granted interlocutory injunctions for which they should be compensated by Wyeth given the undertakings. They were the subject of the wrongly granted final injunctions in respect of which losses must lie where they fell. They should not also be subject to a reduction of their compensation to account for the hypothetical risk that the final injunctions would have had an adverse effect on them.
1229 None of the evidence which I have accepted supports the conclusion that the private market supply by the generics would have failed. As such, it does not follow that they would not have ordered further stock. Their ordering would have depended on their degree of success, which is best reflected in Professor Hausman’s analysis, scenario 5 (for the lost opportunity of PBS supply) and scenario 8 (for the lost opportunity of private market supply) adjusted as necessary to reflect my conclusions in particular that there can be no compensation for the period after 8 November 2010 because of the final injunctions I granted and in relation to the position of Generic Health. Those adjustments should not include any discount or deduction for the risk that the generics would have been left with stock they could not sell or would have had to refund pharmacists in one way or another.
General approach of accountants
1230 Mr Samuel and Mr Meredith are highly experienced forensic accountants. Their evidence ranged over many hypotheses as they were required to do but they agreed about the overall approach which should be taken. Mr Samuel used the results about sales volumes and prices produced by Professor Hausman’s work to develop hypothetical cash flows on a monthly basis identifying posited revenues, costs (including supply and distribution costs), profits and interest. Actual cash flows were then deducted from the hypothetical cash flows to identify the claimants’ losses other than for Pharmathen which adduced no accounting evidence and instead relied on calculations provided during the course of final submissions which it sought to characterise as “simple arithmetic” (a characterisation to which Wyeth took objection).
1231 Mr Meredith prepared cash flows based on Dr Jenkins’ modelling. For the reasons given above Professor Hausman’s results are to be preferred. As noted, Mr Samuel’s cash flows were based on Professor Hausman’s model outcomes. Accordingly, they are the focus of this discussion.
Supply prices to generics
The approaches of Mr Samuel and Mr Meredith
1232 Mr Samuel and Mr Meredith disagreed about the approach that should be taken when the cash flows showed that the generics would be losing money unless they re-negotiated the price of supply of their products to them. Mr Samuel considered that the generics would re-negotiate the supply price after three months of losses. Mr Meredith allowed losses to continue for 12 months.
1233 Mr Meredith said:
…what we both had to do is put ourselves – try to put ourselves in the shoes of a commercial manager at a pharmaceutical company with handling particular lines of drugs in a portfolio and how that manager might behave where a – you know, a line of drug starts to make negative gross margins. The view I formed is that a reasonable manager wouldn’t have gone beyond 12 months of gross margins. I think I’m right in saying that Mr Samuel feels that that’s too long a period, and he has elected for a period of three months. I’m comfortable with my view of 12 months.
1234 Mr Samuel said:
…there were two things I took into account in assuming three months – or three things, really. The first is that it would be really commercially irrational to operate at a gross loss. That, hopefully, is a matter of common sense. If you do that with all products, you will go bust. The only reason you would do it is if that product was a loss leader for other products, and I’m not aware of that being the case here.
The second is it seemed to me that three months was a reasonable period to allow for negotiation to take place between a party facing a negative margin, and whether it be the person they’re selling to or the supplier they’re getting it from, that would be a reasonable period for which to negotiate and then either stop selling altogether if you couldn’t find a solution or to come up with some renegotiated price.
And the third thing I looked at what actually happened, and the actual cash flows don’t show any circumstance in which we have negative cash flows for such a long period. There are some negative cash flows in some of the claimants for short periods, two, three months, five months, I think, at most at any one time, and I’m working at memory so that it might not be correct, but it’s never for that long a period, 12 months.
1235 Mr Samuel’s approach is to be preferred. His approach best accords with the evidence. There was an opportunity for all of the claimants, generics and manufacturers/suppliers, to make a profit from the supply of venlafaxine. The generics might have been prepared to accept short-term losses by reason of a mismatch between supply and sales prices but given the dynamics of the generics pharmaceutical industry this would not have been tolerated for any sustained period. Mr Samuel’s approach is reasonable and fair. It does no more than even out unrealistic peaks and troughs in the profit margin of the generics which result from the kind of timing mismatch which the hypothetical exercise should be expected to produce. It does not interfere with the work or Professor Hausman in any way. It does not change Professor Hausman’s prices at all. It merely adjusts the timing of applicable supply prices from the manufacturers/suppliers to ensure that wholesale supply to pharmacists did not unnecessarily and unrealistically become a loss making enterprise for more than three months. These adjustments accord with the evidence about the industry and give a best approximate fit between the hypotheticals and likely reality.
1236 The period of loss which Mr Meredith posits a generic company would have accepted before re-negotiating the supply price, 12 months, is unreasonable. The evidence, including that of Professor Hausman and Ms McTavish and the actual re-negotiation of supply prices when the generics were supplying their products after November 2011, supports the inference that the price of supply of their products to generics was readily able to be re-negotiated to reflect market conditions with the objective of ensuring it was worthwhile for the generics to continue to purchase the products.
1237 Mr Samuel also explained how he adjusted the supply prices. He said:
I adjusted the purchase price in all cases to reflect an actual purchase price that had been negotiated, but at a different date. So my general rule was to adopt the actual purchase prices that had been incurred, and if the margins looked too high as well as too low, I adjusted those from time to time to reflect an actual purchase price negotiated either prior to or after that date. And so I was always referencing an actual price that had been negotiated.
1238 Mr Samuel was asked whether he understood that the econometric modelling was intended to produce a best fit on average so that cash flows may be over-stated at one time and under-stated at another so that his adjustments would skew the outcomes towards only the “benefit of the good times, rather than the average bad times”. As Mr Samuel explained, however, the econometric model did not produce supply prices to the generics. It produced sales volumes and sales prices from the generics to the pharmacists. Further, he made adjustments in supply prices both up and down based on actual re-negotiated prices, the adjustment being confined to the time at which the price was applied. This exchange occurred:
MR BANNON: If the assumption is right that the model overstates cash flows at some points in the model and understates them at other points, with the consequence that there are larger profits that really occur at one point in time, but also losses at another point in time, for you to take steps which adjusted out the losses would have the result that only the – well, would get rid of that negative averaging aspect of the model.
MR SAMUEL: That’s incorrect at two levels, your Honour. Firstly, the econometric model is producing only a sales price, not a profit. The profit is then a consequence of then overlaying the purchase price assumptions. And it’s wrong at the other level because I do both. I allow for the higher cost when the margins are higher, and I allow for a lower cost when the margins are lower, so my approach for Alphapharm sort of balances it out.
MR BANNON: Well, you do your own balancing – but if you – but – what you’re saying if you left the prices and just had the actual costs and you had some profits at one point and negative losses at another point, if that was the correct approach to dealing with an econometric model, that’s not something you’re familiar with, I take it?
MR SAMUEL: I think you’re confusing what the econometric model does and what I’m doing. The econometric model is only giving us the prices, the sales prices. So what we’re doing to get profits is then a function of what Mr Meredith and I do with purchase costs.
MR BANNON: Put another way, you’re adjusting to make sure there were no losses at any point in time; that’s right, aren’t you?
MR SAMUEL: No, I do allow for some short periods of negative margins, and with other claimants, there are scenarios in which the trade stops altogether if the negative margin is going to continue. For Alphapharm, yes, I think there’s – I don’t – there’s no sustained period of loss.
…
MR BANNON: But if the model predicts a price which would produce a negative margin, and you say the market would react to ensure that you don’t have a negative margin by raising the price in that instance
MR SAMUEL: So we’re talking of two different prices. The model predicts a sales price.
MR BANNON: Yes.
MR SAMUEL: The figure I’m adjusting is the cost price, not the sales price. So the sales price is what it is, that comes from the econometric model, I do not adjust that at all, but if that produces negative margins based on actual costs
MR BANNON: Yes.
MR SAMUEL: I say that would not be sustainable. Either you would stop trading or you would renegotiate your supply price.
MR BANNON: Well, I’m putting to you is, at that point in time, if you’re using an actual cost and the putative price, hypothetical price is producing a negative margin and if it had been the case, during that period of time, Alphapharm was not, in fact, suffering a negative margin with that actual cost price, that would suggest, wouldn’t it, that the hypothetical price is wrong at that period of time? It’s a bad prediction.
MR SAMUEL: No, I don’t buy into that at all.
1239 Nor do I. As Mr Samuel noted, there is firm evidence that the price of supply to generics was re-negotiated when the price of sale from the generics to pharmacists decreased. Mr Samuel’s approach of adjusting supply prices to ensure that the generics do not suffer losses for a period greater than three months based on actual re-negotiated supply prices to the generics is sensible, fair, and in my view necessary. The fact that Professor Hausman’s work is capable of producing timing mismatches between posited sales prices to pharmacists and actual negotiated supply prices does not suggest that the model is unreliable. Mismatches of this kind are to be expected. Timing adjustments are required to give a commercially rational relationship between supply and sales price. Provided that the adjustments are done reasonably, which Mr Samuel has done, there is (or should be) no issue. Mr Meredith’s approach of allowing losses to be sustained for 12 months when (perhaps unbeknownst to him) there is ample evidence of the re-negotiation of supply prices to enable supply to continue as a profit-making enterprise for the manufacturers/suppliers and the generics is unreasonable. Mr Samuel’s approach of allowing losses to be sustained for up to three months and also adjusting periods of apparently excessively high margins downwards is reasonable.
Disputes between generics and manufacturers/suppliers
1240 The issue of the re-negotiation of supply prices, as might be anticipated, also generated disputes between the generics and the manufacturers/suppliers. It is convenient to resolve the issues about supply prices in this section.
1241 I encouraged the generics and the manufacturers/suppliers to resolve these disputes on some appropriate basis between themselves because they are a zero-sum game. The sales volumes to pharmacists which are an outcome of Professor Hausman’s work dictate the amount of product the generics would have ordered from the suppliers. Those sales volumes are related to the sales prices to pharmacists, another outcome of Professor Hausman’s work. The supply prices to the generics are not part of Professor Hausman’s work. They thus do not affect sales volumes in the model. In reality, however, the manufacturers/suppliers and the generics had both competing and mutual interests. They both wanted the generics to sell as much product as possible to pharmacists. The generics could only continue to do so, however, if the sales to pharmacists could be made at an acceptable profit. The supply prices to the generics were the main variable cost which enabled them to continue to tailor their offers to maximise sales. So the manufacturers/suppliers had an interest in the generics being able to continue to make a profit which (together with the low cost of the manufacture of generic drugs) explains why the evidence discloses supply prices to generics being re-negotiable and re-negotiated. Professor Hausman and Ms McTavish both recognised this dynamic as fundamental to the generic pharmaceuticals industry and the overall trend over time towards lower prices to consumers on the entry of multiple generic brands of a drug into a market.
1242 Despite their disputes being a zero-sum game, the generics and manufacturers/suppliers were unable to reach an agreement for the purpose of their hypothetical loss when the evidence shows that when required to do so by commercial circumstances they do reach agreements which ensure continued mutual profitability. This same principle of re-negotiation as and when required to ensure continued mutual profitability is the only sensible approach to these disputes.
1243 As between Sigma and Generic Health, a dispute arose because of exchange rate fluctuations affecting the supply of Sigma’s products by Generic Health to Sigma. The supply agreement between Sigma and Generic Health was based on Australian dollars. The supply agreement between Generic Health and Alembic for Sigma’s products was based on US dollars. As a result, the supply arrangements between Sigma and Generic Health provided for a re-negotiation of the supply price between them if the exchange rate from AUD to USD moved below 0.76 or above 0.86. Generic Health’s pricing email dated 13 February 2009 disclosed Generic Health’s approach to prices outside in the range 0.50 to 0.80. Based on this email, Mr Samuel said:
I have calculated the USD revenue to Generic Health at each AUD price and determined that the prices have been set so that the revenue to Generic Health remains at 2.40 USD for the 37.5mg product, 4.60 USD for the 75mg product, and 6.16 USD for the 150mg product. I have demonstrated this for the exchange rate range of 0.80 to 0.70 in the table below. My detailed calculations for the entire range are set out in Appendix G worksheet “Sigma GH pricing variations”.
1244 Mr Samuel then noted:
During the period June 2009 to July 2011 there were 21 months in which the actual exchange rate was both outside the range referred to in the Generic Health Supply Agreement (being 0.76 to 0.86) and outside the range referred to in the 13 February 2009 Generic Health Email and Pricing Attachment (being 0.80 to 0.50). I observe that there was no agreement or reference to the price that would apply if the exchange rate moved above 1 AUD/0.86 USD, and that “Both parties will agree to negotiate the price if the exchange rate from AUD to USD is below 0.76 or above 0.86”. I am unable to say what the parties would have negotiated, and it is possible the prices would not have changed, or would have changed in a step fashion instead of a linear fashion. However, I consider it reasonable to assume that as the exchange rate moved above 0.86 the AUD price would have continued to move so as to maintain the USD revenues to Generic Health as set out in Table 26 above. I consider this assumption to be reasonable as Generic Health’s agreement with its supplier, Alembic, was in USD.
1245 Not unexpectedly given that the situation is merely hypothetical, Generic Health did not consider it fair that for unanticipated movements in the exchange rate it be held to the revenues in USD it had adopted for anticipated movements in the exchange rate. To support its submission that Generic Health would have sought to keep at least some of the benefit of the increased exchange rate Generic Health relied on Mr Upiter’s evidence to this effect:
If the exchange rate from AUD to USD went above 0.86, Generic Health’s margins would increase, but Sigma’s margins would not decrease because Sigma was buying in AUD and selling in AUD. My approach to any negotiations that Sigma sought to trigger would depend on the circumstances. If Sigma was able to charge a relatively high price for its products due to a limited number of generics being in the market, I would have sought to maintain the existing supply prices (or discount as little as possible) on the basis that Sigma was making good profits and there was no reason to increase them further at Generic Health’s expense. If, on the other hand, Sigma was having to substantially discount its prices to maintain market share, then I would have been more open to discounting the supply price in order to allow Sigma still to be profitable in order to ensure the longevity of the supply arrangement.
1246 Again, I consider Mr Samuel’s approach is reasonable and fair and should be adopted. He has adjusted the supply price to Sigma to maintain Generic Health’s revenue in USD. In my view this reflects the reality of the commercial arrangements between Sigma and Generic Health. At least one of the reasons Sigma entered into the arrangement with Generic Health must have been to protect itself from risks created by changes in the exchange rate. Generic Health’s email of 13 February 2009 discloses the approach it took to pricing which was to maintain a particular revenue in USD irrespective of exchange rate fluctuations. Mr Samuel’s approach ensures that this level of revenue is maintained. It cannot be suggested that this would make supply for Generic Health unprofitable because the revenue level in USD its contemporaneous documents disclose it adopted is maintained.
1247 For the period after July 2011 (which is immaterial on my conclusions) Mr Samuel and Mr Meredith take a different approach to the supply price from Alembic to Generic Health. Mr Meredith adopted the price at which Sigma in fact purchased generic venlafaxine from Spirit Australia (Elaxine XR, a different brand from Sigma’s Evelexa XR products, recalling that the ARTG registrations for these products remained with Sigma Company Limited on the sale of Sigma) but did not adjust the price to reflect the price which spirit paid to Alembic. Mr Samuel, in contrast, proceeded on the basis that Sigma, Generic Health and Alembic would engage in negotiations to reach a set of pricing assumptions which would ensure each of them continued to be profitable for as long as possible. Mr Samuel’s approach again accords with commercial common sense and is fair and reasonable. It reflects the evidence of Mr Ghosal of Alembic that it would have would have decreased its supply price to the higher of the price at which it supplied Spirit Australia in the real world and a price which would allow Generic Health to maintain its margins. It reflects the evidence of Professor Hausman and Ms McTavish about the real world behaviour in the generic market. Accordingly, again, Mr Samuel’s approach is to be preferred to that of Mr Meredith.
1248 In respect of Generic Health’s possible supplies to other generics Generic Health noted that:
Mr Meredith was given an instruction not to model Generic Health’s loss in respect of supplies to the other generics. Accordingly, the only evidence on quantification of this loss is Mr Samuel’s reports. Mr Samuel quantifies the loss at about $180,000 (without regard to interest or discounting).
1249 For the reasons given there should be no compensation on account of these possible supplies. If I am wrong about this then Mr Samuel’s approach must be adopted.
1250 Pharmathen contended that as both Alphapharm and Generic Health had placed orders pursuant to their supply agreements with Pharmathen before the grant of the interlocutory injunctions against those generics the prices paid or payable for those products should be used as the supply price in Mr Samuel’s calculations.
1251 In respect of Alphapharm Pharmathen noted (and I accept) that it is clear that the supply price for Alphapharm’s initial orders of its products in 2009 are the revised floor prices set out in the email from Pharmathen to Alphapharm of 24 April 2008. Pharmathen then submitted that:
Rather than use the supply prices prevailing at the time of the interlocutory injunction for the hypothetical sales that would have incurred during the injunction period, Mr Samuel uses the lesser prices ultimately paid by Alphapharm by reason of a reconciliation exercise and retrospective adjustment to such prices undertaken in early 2013, over a year after the final injunction was dissolved. Mr Samuel assumes that such prices were applicable during the intervening period. However, there is no evidence that such prices were applicable until retrospectively adjusted as set out in the reconciliation table. Although that price adjustment applied to venlafaxine the subject of the first order and invoice, those prices obviously (by reason of the fact that they occurred over a year after dissolution of the final injunction) take into account the injunctions themselves and events subsequent thereto (being the first year or so of commercial activity after entry into the market, the participants in which and dynamics of which were, of necessity, changed by virtue of the interlocutory injunction).
In cross-examination, Mr Samuel acknowledged that if a different floor price prevailed at the time the interlocutory injunction was granted, it would be appropriate to use that other price…
For these reasons, the prevailing price actually agreed and paid at the time of the interlocutory injunction and whilst it was on foot, is the best evidence of the price that would have been paid but for the interlocutory injunction. In the event, however, that the Court is not satisfied that the price actually agreed and paid at and around the time of the interlocutory injunction is the best evidence of the price that would have been paid but for the same, then Pharmathen submits that the price set out in contemporaneous forecasts prepared by Alphapharm [those prepared by Mr Joscelyne in April 2009] should be preferred over the price which ultimately applied (albeit it was retrospectively applied to adjust the price paid in respect of the first order) as determined over a year after the final injunction was dissolved.
1252 Pharmathen recognised that for Alphapharm’s 150mg strength product this would mean that Alphapharm would have suffered negative margins for the first four to six months of supply but said this “is to be expected immediately on generic entry, with high initial discounting before sales price increases and stabilises”.
1253 Alphapharm contended that Mr Samuel’s approach was appropriate. It relied on the negative gross margins for the 150mg product and the evidence that, in fact, supply prices were routinely re-negotiated to ensure profitable supply by generics. It noted also that the agreement ultimately reached included the retrospective application of the new floor prices resulting in a credit note to Alphapharm. Further, and as a result, it submitted that:
…a court will take into account what occurred in the actual world (except where what actually occurred was affected by the imminent application for an injunction), and will use all available information, using hindsight if necessary, to best approximate what would have occurred in a causally coherent counterfactual world,… It is plain that the parties had no need to renegotiate the floor price in the actual world where no product was being sold during the period of the injunction. In the actual world, a new floor price was agreed and the parties agreed that it would be applied to sales that had occurred prior to that agreement. Moreover, as noted, the Pharmathen approach results in Alphapharm incurring negative gross margins for some periods in the counterfactual world and therefore should be rejected as not commercially realistic.
1254 Alphapharm also submitted that Mr Samuel’s oral evidence, on which Pharmathen relied, did not in fact support its case. The evidence was this:
MR DARKE: If you just assume that the floor prices in exhibit JH3 weren’t agreed until sometime in the fourth quarter of 2011 and that different floor prices applied in 2009 when the interlocutory injunction was granted, you would accept on those assumptions, wouldn’t you, that it would be more appropriate to use the floor prices at the time the interlocutory injunction was granted than those that were agreed in the fourth quarter of 2011?
MR SAMUEL: Well, I think the answer is in the question. You’re asking me to assume a different floor price. Then I would apply a different floor price.
1255 Alphapharm further submitted that Mr Samuel’s approach of not accepting hypothetical supply prices resulting in negative margins for more than three months was not subject to challenge by Pharmathen. The approach he took to supply prices during the period of the injunctions was consistent with the approach he took to the period after the injunctions had been discharged.
1256 Alphapharm then made this point, which I consider fundamental.
Pharmathen’s claim is derivative upon Alphapharm’s. Its claim is for lost profits on product it would have sold to Alphapharm by reason of Alphapharm on-selling Enlafax XR to pharmacies at the hypothetical sales prices and volumes output by Prof Hausman’s model, being volumes greater than that which Alphapharm sold in the actual world.
Accordingly, it is fundamentally inconsistent for Pharmathen to fail to take those hypothetical sale prices into account in the counterfactual world for the purposes of assessing the quantum of its loss. But for those sales prices, Alphapharm would not have sold in the volumes above that which it sold in the actual world. But for those increased volumes sold by Alphapharm, Pharmathen would not have a derivative claim. Alphapharm would not have agreed to Pharmathen charging the supply prices for which Pharmathen now contends, in circumstances where those supply prices would have resulted in Alphapharm making a negative gross margin.
…
…if the price had not been renegotiated as assumed by Mr Samuel, Alphapharm would have simply stopped taking supply from Pharmathen. It follows that Pharmathen’s submission is not just unrealistic, but ultimately self-defeating.
1257 I am satisfied that Alphapharm’s submissions should be accepted. It may be accepted that Alphapharm agreed to certain floor prices in 2008 and placed an order reflecting those prices. Insofar as Pharmathen is concerned it was paid for that order and subsequently agreed to credit Alphapharm for the difference between the supply price as paid and as revised and agreed to be applied retrospectively. The required exercise, however, is hypothetical. In that context, it cannot be inferred that the supply prices from July 2009 when Alphapharm would have been supplying its products would have remained as agreed in 2008 when supply at that price of the 150mg product would have been at a loss for Alphapharm. The revised prices agreed between the parties to be applied retrospectively are the best evidence of what would have occurred in 2009 for the purposes of the necessarily hypothetical exercise. It is no answer that these prices were affected by the injunctions. Pharmathen’s proposition that the prices agreed in 2008 and the subject of the order would have applied for the duration of the injunctions is similarly affected because we do not know what would have occurred had Alphapharm not been restrained.
1258 Pharmathen also proposed the use of the agreed supply prices as varied over time for Generic Health. Pharmathen did not suggest that this was contrary to Mr Samuel’s approach. It did not put anything to Mr Samuel about his approach to the supply prices he used in relation to Generic Health’s products. As a result there is no reason to depart from Mr Samuel’s approach in this regard.
Mr Samuel’s alternatives 1 and 2
1259 Mr Samuel calculated losses on the basis of two alternatives.
1260 Alternative 1 estimates losses by adding pre-judgment interest to all losses assessed in periods prior to the date of assessment (30 April 2018, a proxy for the date of judgment), and then calculated up to the date of assessment and discounting all future losses assessed in periods after the date of assessment using a weighted average cost of capital (WACC) to calculate a net present value (NPV) at the date of assessment. In other words, of particular relevance to my conclusions which confine the period of compensation to the period of operation of the interlocutory injunctions up to 8 November 2010 is that Mr Samuel’s approach does not involve a discount for risk for hypothetical historical losses. His discount for risk is applied only to future hypothetical losses.
1261 Mr Samuel provided alternative 2 in case it was considered that the hypothetical cash flows contain risks that have not been adequately allowed for. Alternative 2 estimates losses by calculating the NPV of all losses as at 3 June 2009 (the date of the first interlocutory injunction) and adds pre-judgment interest from 3 June 2009 up to the current date.
1262 I should record that the results from the different alternatives are not as great as might be expected for any claimant because, as Mr Samuels explained, it must be remembered that:
… under alternative 2, although you’re discounting back you’re now adding interest on again for a longer period because the interest now runs from June 2009 so the two factors are offsetting each other to a large extent.
1263 Mr Samuel considered that it was for the Court to assess the extent to which the risks in the cash flows have been adequately accounted for. Mr Meredith considered alternative 1 was to be preferred as “it is more reasonable to discount future losses at an applicable discount rate (Alternative One) than to assess losses at the start date of the loss period (Alternative Two)”.
1264 The accountants both acknowledged that pre-judgment interest was an issue for the Court to decide (which I deal with separately below).
1265 On my conclusions, there are no hypothetical future cash flows which must be discounted back to the date of judgment. This said, as a matter of principle, I do not consider it appropriate for loss in this case to be assessed on the basis of the NPV of the total losses as at 3 June 2009 (or, for the other claimants, the date of the other interlocutory injunctions as applicable). This approach does not reflect the nature of the adverse effect of the interlocutory injunctions on the claimants. They did not suffer a one-off loss at the date the interlocutory injunctions were granted. No cause of action accrued at that date. As Pharmathen submitted, if each interlocutory injunction had been discharged the day after it was made, what loss would have been suffered? What the claimants lost was the opportunity to supply their products from day to day over the period for which the interlocutory injunctions operated. Their loss is reflected in whatever profit they might have made on each lost sale over that period. Accordingly, if it is necessary to resolve the debate about alternative one or two, I would adopt alternative one.
1266 This does not, however, answer the question whether there should be any additional discount applied for risk in respect of hypothetical historical losses. The reason that Mr Samuel presented his two alternatives in this way, as I understand it, is because as he put it the “most commonly used methodology of discounting for risks” when calculating damages is:
(a) to discount the losses to the date of the first hypothetical cash flow, commonly referred to as a discounted cash flow (DCF) approach. This approach assumes each cash flow thereafter suffers a compound risk arising from the first cash flow. In other words, the cash flows in March 2012 are subject to the risks associated with estimating the cash flows for February 2012, and so on, back to 3 June 2009; and
(b) to use a discount rate based on the WACC reasonably attributable to those cash flows.
1267 Because I do not consider that the appropriate approach is to calculate the NPV of the total losses resulting from the interlocutory injunctions as at 3 June 2009, the DCF approach should not be adopted. There are other ways to account for risk, however, such as a simple percentage deduction from the total compensation. Wyeth contended that such a discount should be applied. I turn to that issue now.
Additional discounting for risk
1268 It is necessary to understand that as Mr Samuels explained:
The use of a WACC to discount losses back to the date of the first hypothetical cash flow is consistent with valuation theory for the purpose of determining a “market value”, or “fair value”, or “fair market value”, of a stream of cash flows as at a particular date. Those definitions of value do not allow for the use of hindsight when conducting a valuation. They are entirely forward looking, and therefore have to take into account all the risks associated with estimating future cash flows. However, for the purpose of estimating damages or compensation, the estimates of hypothetical cash flows in this report have had the benefit of hindsight including, for example:
(a) the identification of market size with a high degree of certainty based on actual data (see the Hausman Report);
(b) the use of actual production cost and/or purchase cost data based on actual transactions; and
(c) the knowledge of actual market events between 3 June 2009 and 31 August 2017 including matters which affect the sale and distribution of pharmaceutical products such as political decisions, inflation levels, foreign exchange rates, and the solvency or otherwise of the Applicants.
1269 Because future events are not known Mr Samuels and Mr Meredith agreed that the unadjusted WACC, agreed to be 9.8%, should be used as a discount rate for risk applicable to future cash flows (of which there are none on my conclusions).
1270 Mr Samuels and Mr Meredith also agreed that a discount rate of 7.8%, which reflects the relevant industry WACC adjusted to take into account the benefits of hindsight, should be used where hindsight is available (that is, for past events).
1271 To the extent Wyeth contended that a greater discount rate should be applied in Mr Samuel’s alternative two despite the availability of hindsight, I disagree. The contention was inconsistent with the position agreed between the accountants, which should be given determinative weight.
1272 To the extent Wyeth contended that the hypothetical historical cash flows should be subject to a non-compounded discount of 7.8% or some greater percentage (that is, a discount simply taken off the total compensation), I also disagree.
1273 First, the purpose of making any such adjustment would be to reflect the uncertainties inherent in Professor Hausman’s estimates of sales volumes and sales prices. Because his estimates are based on regression analysis applied to an assumed circumstance that the size of the total venlafaxine market would not have increased, there is no basis for knowing if is his results involve an over-estimate or under-estimate of the generics’ market shares. Nothing more can be said other than that it is just as likely his results under-estimated the generics’ market share as it is that they over-estimated the generics’ market share. If the exercise was to ascertain the market value of the hypothetical cash flows then, As Mr Samuels indicated, a discount would be applied. This is because, irrespective of the directionality of the uncertainty (an increase or decrease in value), the discount a hypothetical purchaser would make is in one direction only (down). The relevant exercise, however, is not to ascertain the market value of the hypothetical cash flows. The exercise is to identify the hypothetical historical cash flows as best as can be done on the available material. Unless something in the material indicates that, on balance, the cash flows represent an over or an under estimate, a discount would necessarily be arbitrary and impose an unfair burden on the claimants.
1274 Second, what is known is that by reason of the wrongly granted interlocutory injunctions Wyeth maintained its monopoly over venlafaxine between 3 June 2009 and 8 November 2010 when it had no right to that monopoly. By that monopoly Wyeth obtained 100% of the profits from venlafaxine for that period and the generics obtained 0%. I have no real doubt that but for the interlocutory injunctions the generics would have been on the market and taking a material part of Wyeth’s 100% share from it. To the extent that uncertainty remains about that share and what it would have been worth, the burden of uncertainty should fall on Wyeth not the claimants.
1275 The unprecedented nature of the private market supply does not persuade me to the contrary. For one thing, the size of the market in the private market supply scenario is confined to general non-concessional patients (42%). My evaluation of the probabilities and possibilities means that this confined market size largely determines the potential compensation payable to the generics. For another, the evidence that the private market supply would have succeeded including Wyeth’s own contemporaneous evidence is powerful. In these circumstances, to impose a discount for risk rather than accepting that the hypotheses which I have reached represent the best estimate that can be reached, would be unfair to the generics and confer an undeserved benefit on Wyeth.
Residual value analysis
1276 Mr Samuel projected sales volumes and prices beyond the end date of Professor Hausman’s analysis in 2016 because there remained a positive difference between the hypothetical and actual profit margins at that date. He said:
I observed from the econometric model we had where the hypothetical volumes and prices were at and the trends that they were exhibiting, and I projected those forward to a point – taken together my assumptions what was happening on the costs side, to the point at which the profit margins were equivalent between the hypothetical and the actual, and then all I had to allow for was the volume differential. So I took the econometric model position, which showed as – for the volumes in particular, largely flat market shares at that point, and, therefore, we could deal with the market volumes based on those largely flat market shares, and I assume that the prices would continue to decline in the same form that they had been declining up to that point in the econometric model.
MR BANNON: So you sort of did a bit of an add-on to the econometric model off your own bat, did you?
MR SAMUEL: Yes. I did.
…
MR BANNON: You didn’t think it would be appropriate to say, “Well, I can’t” – “I’m a forensic accountant. I haven’t – I don’t particularly – I’m not privy to the workings of the economic model. To project any further, I have to ask the econometricians to continue the model onwards”? You didn’t think to do that?
MR SAMUEL: Well, I had what I had, which was the econometric model. And I am a valuer. I thought what I did was a reasonable approach to come up with a reasonable estimation of loss, which is my task.
MR BANNON: And whether or not the model would have agreed with you if you kept going out, you’ve got no idea, have you?
MR SAMUEL: That’s correct.
1277 Mr Meredith did not undertake the same exercise because his approach resulted in negative margins (founded on an approach which I have rejected above). Mr Meredith said:
I can’t comment on the way Mr Samuel has addressed that calculation of residual value because I can’t remember how he has done it in this particular instance. I would say that if there was a situation of a positive gross margin, then I would consider the prospect of assessing a residual value, but I would assess it on its merits.
MR LANCASTER: But something would need to be done to capture that loss in an amount; you agree with that?
MR MEREDITH: Yes.
1278 If I am wrong in my conclusion that loss must be confined to the period during which the interlocutory injunction operated, then Mr Samuel’s approach to the residual value of the claims is fair and reasonable. Wyeth cannot escape the fact that Professor Hausman’s model produced positive results for the generic supply of venlafaxine. Nor can it escape the effect of Mr Meredith’s evidence that if the hypothetical profit margin exceeded the actual profit margin at the end of the assessment period, something would need to be done to capture that residual loss. As Mr Samuel also explained, if he did not project the analysis forward beyond the econometric model results, the same exercise would have to be undertaken but it would be more complicated as allowance would then have to be made for the volume differential.
Pharmathen
1279 As noted, Pharmathen did not adduce any accounting evidence. Rather, in closing submissions, it handed up a series of spreadsheets which it relied upon as calculations of Pharmathen’s losses. As a result of this approach, neither Wyeth nor its accountant, Mr Meredith, had any opportunity to consider the spreadsheets.
1280 While Pharmathen presented the spreadsheets as “simple arithmetic”, this approach denied Wyeth a reasonable opportunity to review the calculations. However, the fact is Wyeth acknowledged that it was likely, even inevitable, that further accounting work would need to be carried to reflect my conclusions. I also note, for example, that Wyeth’s submissions record that it had yet to review Mr Samuel’s calculations confining the losses to the period ending on the discharge of the interlocutory injunctions on 8 November 2010. Given that this material was provided on or about 8 July 2018, in the last week of the hearing, Wyeth must be given this opportunity. Mr Samuel’s 8 July 2018 calculations will have to be adjusted to take into account my conclusions. Pharmathen’s spreadsheets will also have to be adjusted for this purpose. As a result, Wyeth must and will be given a sufficient opportunity to review the final calculations adjusted to reflect my conclusions.
1281 All that needs to be said for present purposes is that I do not accept that Pharmathen should not be permitted to calculate its loss in the way it has proposed (that is, by calculations in spreadsheets using figures based on other evidence rather than by accounting evidence). It was not necessary for Pharmathen to adduce expert evidence from an accountant to present its calculations. Wyeth will have an adequate opportunity to consider and respond to the calculations (which will need to be adjusted in accordance with my conclusions). Nor am I concerned that Pharmathen has taken a different approach to interest than the other claimants (presumably because Mr Samuel’s approach to interest involves more than “simple arithmetic”). Mr Samuel has allowed for interest on each month’s lost profit from the time the loss was incurred. Pharmathen claims interest on its total annual losses. Subject to the issue of principle Wyeth raises about interest, both approaches are reasonable.
The interest issue
1282 The claimants sought interest under s 51A of the Federal Court of Australia Act 1976 (Cth) or as an aspect of the Court’s power to make such order as the Court may consider just for the payment of compensation in respect of the undertaking as to damages.
1283 Section 51A provides that:
(1) In any proceedings for the recovery of any money (including any debt or damages or the value of any goods) in respect of a cause of action that arises after the commencement of this section, the Court or a Judge shall, upon application, unless good cause is shown to the contrary, either:
(a) order that there be included in the sum for which judgment is given interest at such rate as the Court or the Judge, as the case may be, thinks fit on the whole or any part of the money for the whole or any part of the period between the date when the cause of action arose and the date as of which judgment is entered; or
(b) without proceeding to calculate interest in accordance with paragraph (a), order that there be included in the sum for which judgment is given a lump sum in lieu of any such interest.
1284 Pre-judgment interest under s 51A has been awarded in two cases in respect of compensation pursuant to undertakings as to damages, Principal Strategic Options Pty Ltd v Coshott [2003] FCA 736 and Specsavers Pty Ltd v The Optical Superstore Pty Ltd (No 3) [2012] FCA 504; (2012) 290 ALR 263. Neither case involved a dispute about the application of the provision.
1285 I am not persuaded by Wyeth’s submissions that s 51A does not apply.
1286 The Commonwealth made particularly helpful submissions about this issue. Those submissions form much of the basis for the following observations.
1287 Section 51A must be given as broad an operation as it may reasonably bear because its purpose is beneficial, to ensure that the Court can make monetary orders which, insofar as possible, do justice.
1288 The provision was introduced into the Act in response to State Bank of New South Wales v Commonwealth Savings Bank of Australia [1984] HCA 41; (1984) 154 CLR 579 in which Gibbs CJ at 585 recorded the submissions of the parties that the High Court and this Court lacked statutory power to award interest which is “regarded as a necessary adjunct of judicial power in almost every jurisdiction in Australia” so an order for remittal would be unjust. His Honour continued at 587:
I would add that it is anomalous, if it be correct, that neither this Court nor the Federal Court has the statutory power to award interest which most other superior courts in Australia possess. That, however, is no reason why the power of remitter should be used to increase the plaintiff's rights. I of course say nothing on the question whether any power exists to award interest under the general law, as the plaintiff will contend.
1289 In State Bank of New South Wales v Commissioner of Taxation (1995) 62 FCR 371 at 385 Wilcox J said:
…s 51A(1) is a facultative provision intended to confer power on the Court to do justice between parties in relation to pre-judgment interest; a matter of some importance in these days of high interest rates and extensive delays in finalising litigation. The subsection should be interpreted as widely as its language allows.
1290 The Full Court endorsed this principle in Elsinora Global Ltd v Deputy Commissioner of Taxation [2006] FCAFC 156; (2006) 155 FCR 413. In Elsinora the claim for interest was against the Deputy Commissioner in circumstances where there was no action against the Deputy Commissioner for the recovery of money and, accordingly, there was no order for the recovery of money from the Deputy Commissioner. Young J (with whom Gyles and Stone JJ agreed) said:
[39] In my opinion, the claim for interest under s 51A is fatally flawed. Section 51A authorises an award of interest only where there is a cause of action against a party for the recovery of money, and the claim for interest is made against the same party. In other words, the statute confers a power to award interest as an incident of a cause of action for the recovery of money that results in a judgment.
[40] This construction of s 51A is supported by the following passage from the joint judgment of Gleeson CJ, Gummow, Hayne and Callinan JJ in Victorian WorkCover Authority [Victorian WorkCover Authority v Esso Australia Ltd [2001] HCA 53; (2001) 207 CLR 520] at 538 [41]:
‘… the phrase [“any proceeding for the recovery of debt or damages”] should be understood as a composite expression. It embraces any proceeding in which a claim for money is made, in contrast to declaratory relief and claims for specific forms of relief such as mandatory injunctions, charging orders and orders for specific performance. The circumstance that relief of that description is sought in addition to a money claim does not deny the application of s.60 [of the Supreme Court Act (Vic)] in respect of that money claim.’
[41] Even stronger support is provided by the High Court’s decision in SCI Operations. In that case, the High Court held that importers had no entitlement to interest under s 51A(1) because there was no cause of action for the recovery of overpaid customs duty to which the claim for interest could attach.
1291 Young J then explained the context of the High Court’s decision in Commonwealth v SCI Operations Pty Limited [1998] HCA 20; (1998) 192 CLR 285 at [42]-[43], continuing as follows:
[44] …The common and decisive thread in the separate judgments of Brennan CJ and Gaudron J, and in the joint judgment of McHugh and Gummow JJ, is that the power to award interest under s 51A is closely linked to the existence of a cause of action for the recovery of what might be called the principal sum. In applying s 51A(1), their Honours said that it was necessary to determine the nature of the legal right or other entitlement of the importers to recoup the duty in question: at 295-296 [10]-[11] per Brennan CJ, at 303 [34] and 305 [40] per Gaudron J, and at 309 [56] per McHugh and Gummow JJ. Gaudron, McHugh and Gummow JJ accepted that the refund provisions of the Customs Act conferred a right of action that was enforceable by an action for debt: at 305 [40] per Gaudron J, and at 310 [59] and 313 [65] per McHugh and Gummow JJ. Brennan CJ said that it was unnecessary to determine whether the importers’ entitlement to a refund should be classified as a cause of action for debt or as an entitlement to a public law remedy compelling the making of a refund: at 295 [10]. Only Kirby J thought that the importers were confined to a public law remedy: at 327 [99]. Critically, Brennan CJ, Gaudron, McHugh and Gummow JJ held that there was no foundation for any application of s 51A because the duty was refunded on the day on which the concession order was made and there was no period in which debts in respect of the refunds were due and owing but unpaid. Thus, there was no period between the date when the cause of action arose and the date as of which judgment could be entered so as to satisfy the condition in par (a) of s 51A(1): at 296 [11] per Brennan CJ, at 306 [42] per Gaudron J, and at 314 [68] per McHugh and Gummow JJ. As there was no sum for which judgment could be given against the Commonwealth within the meaning of s 51A(1), there was no entitlement to interest.
[45] Gaudron J expressly rejected the Full Court’s view that interest could be awarded under s 51A(1)(b). Her Honour held that par (b) of s 51A(1) was a subsidiary provision that was not independent of subs (1)(a); it merely allowed a lump sum to be awarded in lieu of interest under par (a). More broadly, Gaudron J said that par (b) cannot be construed as conferring a discretion to award interest independently of the existence of a cause of action or for a period prior to the date on which the cause of action arose: at 299-300 [22].
[46] Kirby J decided the case on the distinct basis that the provisions of the customs legislation, properly understood, stated the entirety of the sums which may be recovered by an importer following the making of a concession order and ousted both the general power of the Federal Court to include interest on judgments and any power to do so under the general principles of the law of restitution or otherwise: at 320 [85]; cf McHugh and Gummow JJ at 313-314 [66]-[67].
[47] In my opinion, the High Court’s reasoning is fundamentally inconsistent with the argument advanced by the cross-appellants in this case. The thrust of the decision is that s 51A(1) cannot be construed as conferring a discretion to award interest independently of the existence of a cause of action or for a period prior to the date on which the cause of action arose. Of its very nature, interest is incidental to the recovery of a principal sum. It follows that s 51A(1) cannot be construed as conferring a discretion to award interest against a person against whom there is no cause of action for the recovery of the principal sum.
[48] This construction is also supported by the reference in s 51A(1)(a) to an order for interest being ‘included in the sum for which judgment is given’. This obviously refers to judgment on, or arising from, the cause of action that is referred to elsewhere in s 51A(1). Consequently, it reinforces the view that interest cannot be awarded against a party against whom there was no cause of action for the recovery of money.
…
[50] Nothing in the State Bank case, or in these passages in Gaudron J’s judgment in SCI Operations, provides any support for the cross-appellants’ argument. It is one thing to conclude that the early payment of money claimed in legal proceedings does not prevent the proceedings being characterised as an action for recovery of money or prevent the entry of judgment for costs and interest. It is another thing altogether to argue that s 51A empowers the Court to make an order against a third party against whom there is no cause of action for the recovery of money. Moreover, the language, structure and purpose of s 51A is inconsistent with any suggestion that it was intended to confer a free-standing right to the recovery of interest whenever it seems fair that one party, rather than another, should redress the consequences of a party being kept out of its money, regardless of whether the claimant can establish a cause of action for the recovery of the money from that party.
1292 Nothing in the language or context of s 51A suggests that “cause of action” was intended to take a narrow meaning. To the contrary, the better view is that the words “cause of action that arises after the commencement of this section” impose a temporal requirement. The words which control the character of the kind of claims within the scope of the section are “proceedings for the recovery of any money”. What is meant is that the proceedings must be for the recovery (meaning payment, not reimbursement) of money and that the legal foundation of the claim must have arisen after the section commenced.
1293 Similarly, the words “the date when the cause of action arose” in s 51A(1)(a) also involve a temporal requirement. If there is no period between the date on which the claim may be made for the payment of money and the date of judgment, then the provision cannot apply.
1294 In any event, in Port of Melbourne Authority v Anshun Pty Ltd (1981) 147 CLR 589 at 610 Brennan J said:
There is an imprecision in the meaning of the term cause of action, which is sometimes used to mean the facts which support a right to judgment (see per Williams J. in Carter v. Egg and Egg Pulp Marketing Board (Vict.) (1942) 66 CLR 557, at pp 600, 601); sometimes to mean a right which has been infringed (see Serrao v. Noel (1885) LR 15 QBD 549), and sometimes to mean the substance of an action as distinct from its form (see Krishna Behari Roy v. Brojeswari Chowdranee (1875) LR 2 Ind App 283 ).
1295 In CGU Insurance Ltd v Watson (as trustee of the deed of arrangement in respect of Greaves) [2007] NSWCA 301 Giles JA with whom Spigelman CJ and Basten JA agreed said at [44] that what is “meant by cause of action is notoriously difficult”. His Honour continued at [46]:
In Onerati v Phillips Constructions Pty Ltd (in liquidation) (1989) 16 NSWLR 730, in which a question was whether a proprietor’s second proceedings for breach of contract against the builder for defects discovered after judgment in the first proceedings was barred by res judicata, I said at 738-9 –
“Different forms of words have been used to describe what is meant by a cause of action. Brennan J referred to three descriptions; others are every fact which it would be necessary for a plaintiff to prove, if traversed, in order to support his right to a judgment (Read v Brown (1888) 22 QBD 128 at 131 per Lord Esher); the essential ingredients in the title to the right which it is proposed to enforce (Williams v Milotin (1957) 97 CLR 465 at 474 per Dixon CJ, McTiernan, Williams, Webb and Kitto JJ; Cartledge v E Jopling& Sons Ltd [1963] AC 758 at 783-784 per Lord Pearce); the act on the part of the defendant which gives the plaintiff his cause of complaint ( Jackson v Spittall (1870) LR 5 CP 542 at 552 per Brett J for himself, Bovill CJ and Keating and Montague Smith JJ; Bass v The King [1948] NZLR 777 at 781 per Gresson J; Distillers Co (Biochemicals) Ltd v Thompson [1971] AC 458 at 467 (PC); and rights which can be enforced, or liabilities which can be redressed, by legal proceedings: Sugden v Sugden [1957] P 120 at 133 per Denning LJ. I do not find minute examination of the verbal formulae particularly helpful. The form of words may vary according to the purpose for which the description is required, and in any event may not be illuminating…
1296 The source of Wyeth’s potential obligation to pay compensation is the undertakings. By the undertakings it submitted to “to such order (if any) as the Court may consider to be just for the payment of compensation…to any person…adversely affected by any operation of the order”. In my view, it can readily be concluded that a person adversely affected by the operation of the interlocutory injunctions has a cause of action against the party who gave the undertaking. The fact that enforcement of an undertaking involves an exercise of discretion does not change the fact that a claimant on the undertaking is seeking vindication of its asserted right to be compensated as a person adversely affected by the operation of the interlocutory injunction. It also cannot be doubted that the cause of action is one for the recovery of money. The order each claimant seeks is for the payment to them of money pursuant to an inquiry into the compensation payable on the undertakings. Further, the “essential ingredients” of the claimants’ rights to obtain compensation (in the sense that no such order may be made without these facts being proved) are that the operation of the interlocutory injunctions had an adverse effect for which compensation would be a just remedy.
1297 As discussed above, the adverse effect which the interlocutory injunctions caused in the present case was to prevent the claimants from supplying their products. From day to day throughout the period of the wrongly granted interlocutory injunctions they suffered loss in the form of not being able to sell the products they would or might otherwise have sold. As a result, there is necessarily a “period between the date when the cause of action arose and the date as of which judgment is entered” within the meaning of s 51A(1)(a). The period runs from the date of each hypothesised lost sale to the date of judgment. On each such date the claimants suffered an adverse effect by the operation of the interlocutory injunctions and there arose in them a contingent right to claim compensation under the undertakings. One contingency was the fact of each interlocutory injunction being wrongly granted. Another is that the Court considers it just to make the order for compensation. The fact that Mr Samuel calculates interest on a monthly (rather than daily) basis merely reflects common sense.
1298 I do not find Wyeth’s submissions to the contrary persuasive. Wyeth submitted that:
…a claim on an undertaking as to damages is not a “cause of action”. That position is clear on the authorities:
(a) “[T]he undertaking as to damages is … not a contract between parties or some other cause of action upon which one party can sue the other”: European Bank at [14] per French CJ, Gummow, Hayne, Heydon and Kiefel JJ (emphasis added);
(b) “The cross-undertaking in question is given to the court, not the party opposite, and may be enforced or discharged by the court in its discretion. The party seeking to enforce the undertaking has no cause of action. Although entitled to apply to enforce the cross-undertaking, he has no legal right to its enforcement or to damages”: Barratt Manchester Ltd v Bolton Metropolitan Borough Council [1998] 1 All ER 1 at 7 per Millet LJ, Dillon LJ and Sir Brian Neill agreeing (emphasis added); see also at 12 per Sir Brian Neill (emphasis added): “The undertaking, though described as an undertaking as to damages, does not found any cause of action”); and
(c) “Although the defendant is claiming monetary compensation for loss which it alleges it has sustained as a result of the injunction, it has no independent cause of action to recover such loss. It cannot bring separate proceedings, whether by writ or counterclaim in the existing proceedings. Its claim arises out of and is wholly dependent upon the plaintiff’s cross-undertaking”: CT Bowring & Co (Insurance) Ltd v Corsi Partners Ltd [1994] 2 Lloyd’s Law Reports 567 at [580] per Millett LJ
1299 These submissions, however, conflate the principles relevant to the nature of the undertaking with the proper construction of s 51A. None of these cases are considering the meaning of “cause of action” in s 51A which is the relevant question. Wyeth’s approach impermissibly gives an unduly narrow meaning to “cause of action” in s 51A and fails to recognise that what is meant is nothing more than the existence of the facts which found the claim for the payment for the payment of money. Thus it may be accepted that the claims arise from the undertakings and, in that sense, have been said not to involve an independent cause of action vested in the claimants, but that does not mean that claims for the payment of compensation pursuant to the undertakings are not “proceedings for the recovery of any money (including any debt or damages or the value of any goods) in respect of a cause of action that arises after the commencement of this section” as provided for in s 51A. The cause of action, in the sense of the facts which found the claim for the payment for the payment of money, existed in the present case from the date of each hypothesised lost sale. The rights of the claimants existed from that time but were contingent on the fact of the interlocutory injunctions being wrongly granted. This contingency was satisfied as soon as the Full Court made orders setting aside the final injunctions.
1300 Wyeth also submitted that there was no period between the date when the cause of action arose and the date as of which judgment is entered because there is no right to payment unless and until there is an order of the Court. Again, I consider this approach is unduly restrictive. If the criteria controlling the application of s 51A is that there must be a claim for the payment of money and that the essential facts founding the claim must exist before the date on which judgment is entered, then the requirements of the section are satisfied in the present case. As explained, this is because the essential facts are that the operation of the interlocutory injunction must have had an adverse effect on the claimant. The relevant right, to make the claim, then arises. As noted, it is a contingent right, but this does not mean that the essential facts founding the claim did not exist before judgment is entered.
1301 It follows that I reject this submission of Wyeth’s:
Until there is a determination by the Court that it is just to award compensation to a particular person, and an assessment in a specified sum of the amount of compensation, there is no obligation on Wyeth to pay any amount to anyone.
1302 Similarly, however, in every case for equitable compensation the Court must find it just for the order to be made in all of the circumstances, and unless and until the order is made the obligation of payment is merely contingent. I see nothing different in principle in the present case. From the moment it gave each undertaking Wyeth was subject to contingent obligations. By obtaining the wrongly granted interlocutory injunctions Wyeth kept the claimants out of money which they otherwise would have earned during the period of the interlocutory injunctions. The claimants have been out of their money since that time. They now claim compensation and interest is payable in the ordinary course.
1303 Wyeth noted that in Coshott Branson J awarded interest from the date of discharge of the interlocutory injunction. On my analysis, there is no reason why interest cannot be awarded under s 51A in precisely the manner Mr Samuel proposed which is from the date of each adverse effect of the interlocutory injunctions in the form of a lost sale (calculated on a monthly basis). No circumstance would warrant reducing the period over which interest is payable (in contrast, for example, to the circumstances described in H K Frost Holdings Pty Ltd (in liq) v Darvall McCutcheon (a firm) [1999] FCA 795 at [11]). If I am incorrect in this regard, then the next logical date for the interest calculation would be the date of the Full Court’s orders on 11 November 2011.
1304 On this basis, and as good cause has not been shown to the contrary, interest should be awarded pursuant to s 51A as calculated by Mr Samuel. The rate of interest is within the Court’s discretion (r 39.06 of the Federal Court Rules 2011 (Cth)), but the Court’s Interest on Judgments Practice Note (GPN – INT) is relevant to the discretionary exercise and informs parties that they should expect the Court to have regard to certain rates. Further, in 2009 when Wyeth sought and obtained the interlocutory injunctions and gave the undertakings Order 35 r 7A of the Federal Court of Australia Rules provided this:
If determining a rate of interest for an order under paragraph 51A (1) (a) of the Act, the Court or a Judge may fix the rate as:
(a) the cash rate of interest set by the Reserve Bank of Australia from time to time during the period mentioned in paragraph 51A (1) (a) of the Act, plus 4 per cent; or
(b) such other rate as the Court or Judge thinks fit.
1305 This is the same rate as set out in the current Practice Note.
1306 Accordingly, parties coming before the Court were on notice at all material times that if interest was claimed the Court would have regard to the cash rate of interest set by the Reserve Bank of Australia from time to time plus 4%. To my mind, this is a sufficient reason to make it just that this rate be applied in the present case. If this is an insufficient reason I would also rely upon the fact that Wyeth had the benefit of a monopoly on the sale of venlafaxine in Australia throughout 2009 and up to 8 November 2010 when it ought not to have done so. The claimants have suffered the adverse effects of the wrongly granted interlocutory injunctions since that time. They ought to be generously compensated by an award of interest at the rates published as relevant for the Court’s consideration.
1307 Given this, I consider it just that interest be awarded under s 51A at the rates specified in the Practice Note.
1308 I am also satisfied that interest should be awarded in the exercise of the Court’s inherent equitable jurisdiction.
1309 In its submissions the Commonwealth identified cases in which courts of equity have awarded interest on claims pursuant to the usual undertaking including De Mattos v Gibson (1860) 1 J & H 79 and Graham v Campbell (1878) 7 Ch D 490 at 494-495.
1310 In Graham v Campbell the English Court of Appeal said at 494:
As to the other subject of appeal, the inquiry as to damages, we think the Defendant Campbell is clearly entitled to have all damage sustained by him by reason of the injunction. The undertaking as to damages which ought to be given on every interlocutory injunction is one to which (unless under special circumstances) effect ought to be given. If any damage has been occasioned by an interlocutory injunction, which on the hearing is found to have been wrongly asked for, justice requires that such damage should fall on the voluntary litigant who fails, not on the litigant who has been without just cause made so. In this case we should therefore have granted the Defendant Campbell the inquiry he asks for as to damages. But, as the legal wrong which he has suffered was being kept many months out of his money, and as the law does not regard collateral or consequential damages arising from delay in the receipt of money, it is not right to direct an inquiry on a matter on which we can satisfy ourselves. He is entitled to interest at 5 per cent during the delay, minus any interest he may have made, and we will take his affidavit verifying that amount.
1311 The Commonwealth also noted that in Love v Thwaites (No 4) [2012] VSC 521 Dixon J said this at [91]:
The object of damages awarded by a court on an undertaking to pay should the applicant fail to justify a right to have obtained the injunction may, at its highest be restitutionary, having regard to the equitable nature of the remedy given, but in my view may be better described as compensatory, having regard to the historical object of providing a means of redress. These terms are, perhaps, unhelpful for the reasons given by the High Court in European Bank that I have set out. Having regard to the purpose the undertaking was intended to serve, I am not persuaded that s 60 determines the proper assessment of the interest component that may be awarded in this case. The proper consideration is for what period and at what rate is payment of interest by the injunctor considered just in order to meet the objective that the court had in mind when requiring that a party give to it such an undertaking.
1312 The order as to interest was confirmed on appeal: Love v Thwaites [2014] VSCA 56 at [51].
1313 Wyeth’s submissions to the contrary do not recognise that it is the terms of the undertakings themselves which enable an award of interest to be made if the Court considers it just to do so to compensate the claimants for the adverse effect of the operation of the interlocutory injunctions. It does not matter that the claimants sought interest “on” rather than as part of the damages or compensation payable. In making their claims, they sought interest. Hungerfords v Walker [1989] HCA 8; (1989) 171 CLR 125 at 152 does not assist Wyeth. The undertakings are the source of the power in the present case. If, as here, the claimants were adversely affected by the operation of the interlocutory injunctions the control on the compensation payable is what is considered just to compensate the claimants for those adverse effects. The claimants were not required to prove what they would have done with the money to obtain an award of interest.
1314 To adopt Wyeth’s submissions, it may be accepted that there has been no wrongdoing by Wyeth and that it is “merely required to honour its commitment to the Court, as determined and assessed”. Wyeth’s commitment to the Court was to pay the compensation the Court considers just to compensate any person adversely affected by the operation of the interlocutory injunctions. In my view, this commitment necessarily extends to a commitment to pay interest if the Court considers that is what justice requires (which I do).
1315 It may be accepted that the Court’s Interest on Judgments Practice Note (GPN – INT) does not apply to any award of interest other than that under s 51A. This does not mean, however, that the rates set out in the Practice Note are irrelevant. They disclose the rates which the Court is to consider when exercising its power under s 51A. The mere existence of the published rates is sufficient to make them relevant to the assessment of what justice might require in the circumstances of this case. When this is taken with the rates published in Order 35 r 7A I am persuaded that the same rate should be applied in the exercise of discretion under the Court’s inherent equitable jurisdiction.
Calculations of loss based on the probabilities and possibilities
1316 The issue of how the probabilities and possibilities should found the calculations of loss was touched upon only briefly in evidence (and only by Sigma in supplementary closing submissions). In his oral evidence Mr Samuels said that in a probability analysis:
…you would value the two streams of potential outcomes separately and you would apply the relevant probability to it. So if there is a 60 per cent of this cashflow stream happening and a 40 per cent chance of a different cashflow stream happening, you would value both of them and take 60 per cent of the first and add it to 40 per cent of the second.
1317 This sounds straightforward but it is not because there are inter-related probabilities. As a result, the parties will also be given an opportunity to consider what follows and to make further submissions about the most appropriate approach as part of the required re-calculation exercises. My present views are set out below as they may assist the parties in understanding the issues as I perceive them.
1318 As I have said, there are two fundamental requirements for the process of calculation. First, the world of possibility for each claimant at any one time must be treated as equivalent to 100% or 1. Second, the possibilities for each claimant must be consistent with the possibilities for each other claimant. One thing that this means is that on my conclusions it is the prospect that Sigma would have obtained PBS listing of its products on 1 December 2009 which determines the chances of Alphapharm and Generic Health having obtained PBS listing of their products on 1 March 2010.
1319 The effect of my conclusions may be summarised as follows:
(1) Sigma’s lost opportunity of supply should be valued on the basis of the following hypothesised facts:
(a) from 1 May 2009 until 1 December 2009:
(i) Sigma would have supplied its products on the private market: 100%;
(ii) Sigma would have supplied its products under the PBS: 0%; and
(iii) Sigma would not have supplied its products at all: 0%.
(b) from 1 December 2009 until 8 November 2010:
(i) Sigma would have supplied its products on the private market: 80%;
(ii) Sigma would have supplied its products under the PBS: 20%; and
(iii) Sigma would not have supplied its products at all: 0%.
(2) Alphapharm’s lost opportunity of supply should be valued on the basis of the following hypothesised facts:
(a) from 22 July 2009 until 1 March 2010:
(i) Alphapharm would have supplied its products on the private market: 90%;
(ii) Alphapharm would have supplied its products under the PBS: 0%; and
(iii) Alphapharm would not have supplied its products at all: 10%
(b) from 1 March 2010 until 8 November 2010 if Alphapharm was supplying its products on the private market (the 90% probability) and assuming the Sigma 20% possibility of PBS listing on 1 December 2009:
(i) Alphapharm would have continued to supply its products on the private market: 10%;
(ii) Alphapharm would have supplied its products under the PBS: 90%; and
(iii) Alphapharm would not have supplied its products at all: 0%.
(c) from 1 March 2010 until 8 November 2010 if Alphapharm was not supplying its products on the private market (the 10% possibility) and assuming the Sigma 20% possibility of PBS listing on 1 December 2009:
(i) Alphapharm would have supplied its products on the private market: 0%;
(ii) Alphapharm would have supplied its products under the PBS: 80%; and
(iii) Alphapharm would have continued to not supply its products at all: 20%.
(d) from 1 March 2010 until 8 November 2010 if Alphapharm was supplying its products on the private market (the 90% probability) and assuming the Sigma 80% probability of not PBS listing on 1 December 2009:
(i) Alphapharm would have supplied its products on the private market: 90%;
(ii) Alphapharm would have supplied its products under the PBS: 0% and
(iii) Alphapharm would not have supplied its products at all: 10%.
(e) from 1 March 2010 until 8 November 2010 if Alphapharm was not supplying its products on the private market (the 10% possibility) and assuming the Sigma 80% probability of not PBS listing on 1 December 2009:
(i) Alphapharm would have supplied its products on the private market: 0%;
(ii) Alphapharm would have supplied its products under the PBS: 0% and
(iii) Alphapharm would not have supplied its products at all: 100%.
(3) Generic Health’s lost opportunity of supply should be valued on the basis of the following hypothesised facts:
(a) from 10 November 2009 until 1 March 2010:
(i) Generic Health would have supplied its products on the private market: 80%;
(ii) Generic Health would have supplied its products under the PBS: 0% and
(iii) Generic Health would not have supplied its products at all: 20%.
(b) from 1 March 2010 until 8 November 2010 if Generic Health was supplying its products on the private market (the 80% probability) and assuming the Sigma 20% possibility of PBS listing on 1 December 2009:
(i) Generic Health would have continued to supply its products on the private market: 10%;
(ii) Generic Health would have supplied its products under the PBS: 90%; and
(iii) Generic Health would not have supplied its products at all: 0%.
(c) from 1 March 2010 until 8 November 2010 if Generic Health was not supplying its products on the private market (the 20% possibility) and assuming the Sigma 20% possibility of PBS listing on 1 December 2009:
(i) Generic Health would have supplied its products on the private market: 0%;
(ii) Generic Health would have supplied its products under the PBS: 80%; and
(iii) Generic Health would have continued to not supply its products at all: 20%.
(d) from 1 March 2010 until 8 November 2010 if Generic Health was supplying its products on the private market (the 80% probability) and assuming the Sigma 80% probability of not PBS listing on 1 December 2009:
(i) Generic Health would have supplied its products on the private market: 80%;
(ii) Generic Health would have supplied its products under the PBS: 0% and
(iii) Generic Health would not have supplied its products at all: 20%.
(e) from 1 March 2010 until 8 November 2010 if Generic Health was not supplying its products on the private market (the 20% possibility) and assuming the Sigma 80% probability of not PBS listing on 1 December 2009:
(i) Generic Health would have supplied its products on the private market: 0%;
(ii) Generic Health would have supplied its products under the PBS: 0% and
(iii) Generic Health would not have supplied its products at all: 100%.
1320 On this basis, the probabilities and possibilities may be represented by:
PBS + PM + N = 1
Where:
PBS is the chance of the generic supplying under the PBS
PM is the chance of the generic supplying on the private market
N is the chance the generic would not have supplied its products at all.
1321 The value of the generics’ lost opportunity being the profits they would or might have made from supply of their products but for the interlocutory injunction may then be represented by V as follows:
VPBS means the estimated profits from posited PBS supply.
VPM means the estimated profits from posited private market supply.
VN means the no supply position, which must always equal zero.
VT means the total profits from posited PBS and/or private market supply.
1322 My assessment for Sigma therefore may be represented as:
(1) from 1 May 2009 until 1 December 2009:
PM = 1
PBS = 0
N = 0.
(2) from 1 December 2009 until 8 November 2010:
PM= 0.8
PBS = 0.2
N= 0.
1323 Sigma’s lost profits would be calculated as follows:
Sigma VT for 1/5/09 to 1/12/09 = 1 x VPM + 0 x VPBS + 0 x VN.
Sigma VT for 1/12/09 to 8/11/10 = 0.80 x VPM + 0.2 x VPBS + 0 x VN.
Sigma total VT = Sigma VT for 1/5/09 to 1/12/09 + Sigma VT for 1/12/09 to 8/11/10.
1324 My assessments for Alphapharm and Generic Health require further explanation which is in Schedule 5. This further explanation focuses on the fact that the positions of Alphapharm and Generic Health are determined by independent anterior probabilities, being their own private market supply before 1 March 2010 (their hypothesised PBS listing date), Sigma’s prospect of PBS listing on 1 December 2010, and their own prospect of PBS listing on 1 March 2010 as a result of Sigma’s PBS listing.
1325 On the basis of Schedule 5 my assessment for Alphapharm may be represented as:
(1) from 22 July 2009 until 1 March 2010:
PM = 0.9
PBS = 0
N = 0.1.
(2) from 1 March 2010 until 8 November 2010:
PM = 0.666
PBS = 0.178
N = 0.156.
1326 On this basis, Alphapharm’s lost profits would be calculated as:
Alphapharm VT for 22/7/09 to 1/3/10 = 0.9 x VPM + 0 x VPBS + 0.1 x VN.
Alphapharm VT for 1/3/10 to 8/11/10 = 0.666 x VPM + 0.178 x VPBS + 0.156 x VN.
1327 On the basis of Schedule 5 my assessment for Generic Health may be represented as:
(1) from 10 November 2009 until 1 March 2010 as:
PM = 0.8
PBS = 0
N = 0.2.
(2) from 1 March 2010 until 8 November 2010:
PM = 0.528
PBS = 0.176
N = 0.296.
1328 On this basis, Generic Health’s lost profits would be calculated as:
Generic Health VT for 10/11/09 to 1/3/10 = 0.8 x VPM + 0 x VPBS + 0.2 x VN.
Generic Health VT for 1/3/10 to 8/11/10 = 0.528 x VPM + 0.176 x VPBS + 0.296 x VN.
1329 It should not be thought that because these assessments are to three decimal points that I am engaging in an exercise of “spurious precision”. This is a necessary consequence of the fact that the probability of some hypothetical scenario occurring must be certain (that is, equal 1). If rounded to fewer decimal places then the applicable hypotheses would equal more or less than 1 which would make no sense.
1330 The manufacturers/suppliers lost profits depend on the generics’ supply to pharmacists and thus may be calculated on the same basis.
1331 I consider that my overall approach appropriately reflects the terms and purpose of the undertakings which Wyeth gave as the price for the interlocutory injunctions to which in the event it was not entitled. The generics alone possessed the opportunity in 2009 to supply their generic venlafaxine products in Australia. Their opportunity was unique as a result of the generics’ ARTG registrations and the contracts for supply between the generics and the manufacturers/suppliers. The wrongly granted interlocutory injunctions deprived them of the opportunity to make profits from the supply of their products which, as the conclusions I have reached disclose, were opportunities of some (indeed, significant) value to each of them. As a result, justice demands that they be compensated having regard to the probabilities and possibilities of supply as found. The undertakings therefore should be enforced and orders for compensation made.
1332 Alphapharm claimed indemnity costs on the basis that it “has been required to incur the costs of these proceedings, thus adversely affecting it, as a reasonable attempt to recover its primary loss”. Alphapharm referred to James v Canadian Trust of the Church of Latter Day Saints [1998] OJ No 3924; (1998) 165 DLR (4th) 227 in support of this claim.
1333 I am not persuaded that the terms of the undertakings alone dictate that Alphapharm’s claim for indemnity costs must succeed.
1334 James also turned on its own facts. An order for indemnity costs was made because baseless allegations of serious misconduct were made and maintained. Finlayson JA said this:
[41] I want to be clear that I am not suggesting that costs on a solicitor-client basis must always be granted pursuant to the undertaking as to damages given by a plaintiff seeking injunctive relief. But this is not to say that they are not warranted in the particular case. I think the motions judge erred in not taking into consideration whether the full costs of retaining a solicitor fell within the ambit of the undertaking as to damages. That undertaking was given to the court to obtain the injunction.
[42] In my opinion, properly considered, this case more than warranted an award of solicitor-and-client costs. Since the respondents maintained to the end, including on this appeal, that the allegations were true, such an award is the only public rehabilitation of the appellants’ reputation.
1335 In my view, costs should be determined in the ordinary course, after all other issues in the matters.
1336 It is difficult to imagine that when Sundberg J and then I granted the interlocutory injunctions in 2009 we anticipated that if those injunctions turned out to be wrongly granted, the resulting exercise would bear any resemblance to this one. Hindsight makes one thing certain. Knowing what has occurred, it could never have been concluded, for example, that insofar as relevant to the balance of convenience it would be easier for the generics to prove their loss if the interlocutory injunctions were wrongly granted than for Wyeth to prove its loss if the interlocutory injunctions were withheld and the method patent was valid.
1337 This said, I am satisfied that it is just that Wyeth be ordered to pay compensation to each of the claimants for the adverse effect which the operation of the interlocutory injunctions had on them as explained in these reasons for judgment. This compensation should be assessed on the basis of the sales volumes and sales prices predicted by Professor Hausman and the calculations of Mr Samuel (and the equivalent calculations for Pharmathen) adjusted in the ways I have described above.
I certify that the preceding (1337) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Jagot J. |
ABBREVIATIONS AND BASIC CONCEPTS
API: active pharmaceutical ingredient.
APP: approved price to pharmacist.
ARGPM: the Australian Regulatory Guidelines for Prescription Medicines published by the TGA.
ARTG/Register: the Australian Register of Therapeutic Goods maintained by the TGA.
ARTG registration entry/number: a unique registration number for each pharmaceutical product which is specific to a particular dosage form (e.g. tablet, capsule, immediate release form, extended release form), formulation and strength.
bioequivalence: a generic product is considered to be bioequivalent to an originator product when it has “sufficiently similar plasma concentration/time profile” (see section 4.3.1 of the ARGPM) to that of the originator product: that is, when the API from the generic product is absorbed into the blood stream, metabolised and excreted similarly to the originator product such that its effect will essentially be the same as the originator product. To demonstrate bioequivalence, generally, the sponsor will need to submit to the TGA clinical (bioavailability) studies comparing the generic product to the originator product or a comparator which is identical to the Australian originator product.
CMI: consumer medicine information document which is a document providing information to consumers about the safe and effective use of a drug.
dossier: data supporting the safety, efficacy an quality of a pharmaceutical product which the sponsor of an application for registration on the ARTG of a product which has not previously been registered in the same dosage form, with the same active ingredient(s), and with the same route of administration must submit to obtain TGA approval (the sponsor of such a product being the “originator” in relation to that product).
Efexor-XR products: Wyeth Australia sponsored each of the Efexor-XR product registrations on the ARTG as follows:
Efexor-XR 150 venlafaxine (as hydrochloride) 150mg modified release capsule blister pack, on 11 May 1998 under number 60859 (Efexor-XR 150);
Efexor-XR 75 venlafaxine (as hydrochloride) 75mg modified release capsule blister pack, on 11 May 1998 under number 60858 (Efexor-XR 75);
Efexor-XR 37.5 venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack, on 14 October 2004 under number 99802 (Efexor-XR 37.5).
Enlafax-XR products: Alphapharm was the sponsor of registrations on the ARTG on 30 April 2009 of Enlafax-XR venlafaxine 150mg modified release capsule and Enlafax-XR venlafaxine 75mg modified release capsule.
Evelexa XR products: Sigma was the sponsor of registrations on the ARTG on 5 March 2009 of Evelexa XR 150 venlafaxine (as hydrochloride) 150mg extended release capsule, Evelexa XR 75 venlafaxine (as hydrochloride) 75mg extended release capsule, and Evelexa XR 37.5 venlafaxine (as hydrochloride) 37.5mg modified release capsule.
generic product/generic equivalent/generic: a bioequivalent version of a pharmaceutical product which is already registered on the ARTG (originator product) and which may be the subject an application that does not include all the information that was required of the originator to register the originator’s product.
generics/generic claimants: Sigma, Alphapharm and Generic Health.
IMS data: independent data concerning the pharmaceutical industry such as products sold, volume of sales and product prices published by a group of companies referred to as IMS and sold to IMS customers.
National Health Act: National Health Act 1953 (Cth).
Parties or claimants: the parties to or claimants in these proceedings:
Alembic: Alembic Pharmaceuticals Ltd, a supplier of generic venlafaxine products.
Alphapharm: Alphapharm Pty Ltd.
Generic Health: Generic Health Pty Ltd.
Pharmathen: Pharmathen SA, a supplier of generic venlafaxine products.
Sigma: Sigma Pharmaceuticals (Australia) Pty Ltd, also referred to as SPA or SPAL. Sigma was a wholly owned subsidiary of Sigma Company Limited (also known as SCL) until 31 January 2011 when it was sold by Sigma Company Limited to Aspen Asia Pacific Pty Ltd (also known as AAP or Aspen) and changed its name to Aspen Pharma Pty Ltd. The generics portfolio of Aspen Pharma Pty Ltd was sold to Strides (Australia) Pharma Pty Ltd (later known as Arrow) on 31 August 2015.
Wyeth and Wyeth Australia: Wyeth Inc and Wyeth Australia Ltd, a wholly owned subsidiary of Wyeth (sold to Pfizer Inc on 15 October 2009).
patient co-payment: the amount a patient must pay when purchasing a PBS-listed medicine, subject to certain “safety-net” provisions.
PBS: the Pharmaceutical Benefits Scheme which subsidises the cost to eligible patients of certain pharmaceutical products in Australia. A pharmaceutical product can only be listed on the PBS Schedule after the product has been registered on the ARTG.
PBS listing dates: under the policy of the Commonwealth, there are only three occasions per year on which a product whose listing on the PBS would result in a reduction in the APP of a pharmaceutical product (such as the first generic equivalent of an already-listed originator product) could be listed on the PBS (i.e. included on the PBS Schedule): on 1 April, 1 August and 1 December. The deadlines for listing on those dates are as follows:
an application to list a first generic equivalent on the PBS on 1 April must be filed by 1 December of the preceding year;
an application to list a first generic equivalent on the PBS on 1 August must be filed by 1 May of that year; and
an application to list a first generic equivalent on the PBS on 1 December must be filed by 1 September of that year.
Thereafter, a subsequent generic equivalent may be listed on the first calendar day of any month, with the application for listing to be submitted by the 15th day of the month which is three calendar months before the listing month.
PBS price: the price at which a PBS-listed medicine may be dispensed at retail pharmacy level as listed on the PBS schedule which is referred to in the PBS Schedule as the dispensed price for maximum quantity (DPMQ). The maximum quantity is also specified for each product on the PBS Schedule, as is:
the Approved Price to Pharmacist (APP/AP2P), which is the maximum price allowed to be charged to the dispensing pharmacist for the medicine under the National Health Act 1953 (Cth) and related legislative instruments (National Health Legislation) comprised of the ex-manufacturer price, which is the maximum price allowed to be charged by the sponsor or manufacturer of the medicine under the National Health Legislation and the wholesaler mark-up, which is the maximum mark-up that can be charged by a wholesaler under the National Health Legislation;
the pharmacist mark-up, which is the maximum mark-up that can be charged by a pharmacist under the National Health Legislation (giving effect to the Fourth and Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia);
a dispensing fee charged by pharmacies under the National Health Legislation (giving effect to the Fourth and Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia); and
The Approved Ex-Manufacturer Price (AEMP) which replaced the APP from 1 October 2012 and is the maximum wholesale price at which a manufacturer could supply to a wholesaler.
PI: product information document, which is a document providing healthcare professionals with a summary of scientific information relevant to the safe and effective administration of a particular drug.
price reduction: under Part Vll, Division 3A of the National Health Act when the first PBS listing of one or more generic versions of a pharmaceutical product that is already listed on the PBS Schedule occurs then, generally, there will be a price reduction of the APP for the originator brand. Under these arrangements for the period between 1 January 2009 and 31 January 2011, a PBS listing of the first generic version of an originator product would have triggered a 12.5% price reduction in the APP of the originator brand provided certain circumstances were met and any exceptions did not apply. The new APP would also have been applied to any other generic equivalent version subsequently listed unless the APP was otherwise further reduced by the time of their listing.
private dispensing: prescription products which are not listed on the PBS are dispensed privately. If a pharmacist dispenses a product privately the patient bears the full purchase price of the product as no Commonwealth subsidy is paid under the PBS. A pharmacist may dispense products privately, that is, not through the PBS, even where the products are PBS listed.
sponsor: the person or company holding the registration of a pharmaceutical product registered on the ARTG may offer to supply, and supply, that therapeutic product to pharmacies, pharmaceutical distributors/wholesalers, government authorities and private or public hospitals in Australia when the product has been registered on the ARTG.
TGA: the Therapeutic Goods Administration, a business unit of the Commonwealth Department of Health and Ageing which maintains a register of therapeutic goods (as defined in the Therapeutic Goods Act) that may be imported into, manufactured or supplied in, or exported from, Australia - the ARTG.
TGA approval/registration: if a pharmaceutical product is approved by the TGA, the brand name and the active ingredient of the product are entered on the ARTG, along with the name of the sponsor, the approved indication(s), the dosage form and the pack sizes for which the product may be supplied.
Therapeutic Goods Act: Therapeutic Goods Act 1989 (Cth).
venlafaxine: venlafaxine hydrochloride, a serotonin and noradrenaline re-uptake inhibitor (SNRI) which is thought to act by increasing the levels of these neurotransmitters in the brain.
Joint statement of agreed facts
Pursuant to order 5 of the orders made by the Court on 10 August 2012, Wyeth, Wyeth Australia Pty Ltd and the parties listed in the schedule to this statement who have filed an interlocutory application seeking an order for an inquiry pursuant to the usual undertaking as to damages in proceedings VID 195 of 2009, NSD 596 of 2009 and NSD 1124 of 2009 (together, the Proceedings) agree to the following facts in the Proceedings.
These agreed facts are for the purposes of the Proceedings only.
Further, these agreed facts are agreed between Wyeth, Wyeth Australia Pty Ltd and the parties listed in the schedule to this statement only. Wyeth and Wyeth Australia Pty Ltd and the parties listed in the schedule to this statement do not, by agreeing to the following facts or consenting to the filing of this statement, agree any fact with or make any admission in favour of any other person, including without limitation, the Commonwealth of Australia.
Pharmaceutical Marketing Approval
1. The Therapeutic Goods Administration (TGA) (a business unit of the Commonwealth Department of Health and Ageing) maintains a register of therapeutic goods (as defined in the Therapeutic Goods Act 1989 (Cth)) that may be imported into, manufactured or supplied in, or exported from, Australia – the Australian Register of Therapeutic Goods (ARTG). Subject to certain exceptions, a product for therapeutic use in humans may not be imported into, manufactured or supplied in, or exported from, Australia unless it is registered, listed or included on the ARTG, or otherwise approved or exempted by the TGA. Relevantly, a product containing the active pharmaceutical ingredient venlafaxine hydrochloride (venlafaxine) must be registered on the ARTG before it can be imported into, manufactured or supplied in, or exported from, Australia.
2. The person or company holding the registration of a pharmaceutical product registered on the ARTG is called the sponsor of the relevant product. A sponsor may offer to supply, and supply, that therapeutic product to pharmacies, pharmaceutical distributors/wholesalers, government authorities and private or public hospitals in Australia when the product has been registered on the ARTG.
3. To register a therapeutic product on the ARTG the sponsor must obtain approval from the TGA.
4. If a pharmaceutical product is approved by the TGA, the brand name and the active ingredient of the product are entered on the ARTG, along with the name of the sponsor, the approved indication(s), the dosage form and the pack sizes for which the product may be supplied.
5. An ARTG registration entry (designated by a unique registration number) for each pharmaceutical product is specific to a particular dosage form (e.g. tablet, capsule, immediate release form, extended release form), formulation and strength.
6. A pharmaceutical product in a different form, strength or formulation requires a separate ARTG registration entry/number.
7. The sponsor of an application for registration on the ARTG of a product which has not previously been registered in the same dosage form, with the same active ingredient(s), and with the same route of administration (the sponsor of such a product being the “originator” in relation to that product), has to submit a dossier of data supporting the safety, efficacy and quality of the product.
8. However, a sponsor wishing to supply a bioequivalent version (generic product) of a pharmaceutical product which is already registered on the ARTG (originator product) may make an application that does not include all the information that was required of the originator to register the originator’s product.
9. A sponsor of a generic product may rely on the safety and efficacy data submitted by the originator in respect of the originator product if the sponsor of the generic product demonstrates that the generic product is 'essentially similar' to the originator product.
10. As described in section 4.3.1 of the Australian Regulatory Guidelines for Prescription Medicines published by the TGA (ARGPM), a generic product is 'essentially similar' to an originator product if:
10.1 it has the same qualitative and quantitative composition in terms of active principles/substances; and
10.2 it is the same pharmaceutical form; and
10.3 it is 'bioequivalent',
unless it is apparent in the light of scientific knowledge that it differs significantly from the originator product as regards safety or efficacy.
11. A generic product is considered to be 'bioequivalent' to an originator product when it has "a sufficiently similar plasma concentration/time profile" (see section 4.3.1 of the ARGPM) to that of the originator product: that is, when the API from the generic product is absorbed into the blood stream, metabolised and excreted similarly to the originator product such that its effects will essentially be the same as the originator product.
12. In order to demonstrate 'bioequivalence' a sponsor of the generic product should normally conduct clinical (bioavailability) studies comparing the generic product to the originator product. The sponsor of the generic product is required to conduct such a study (or studies) with the Australian originator product or demonstrate that the comparator used in the studies (against which the generic product is compared) is identical to the Australian originator product.
13. Once a pharmaceutical product is registered on the ARTG, it can be sold, offered for sale and otherwise supplied in Australia, including to:
13.1 pharmacies;
13.2 pharmaceutical distributors/wholesalers;
13.3 government authorities (such as the Department of Defence); and/or
13.4 public and private hospitals.
14. Pharmacists may dispense a generic product registered on the ARTG, which is equivalent to the originator product (generic equivalent) when a doctor:
14.1 has prescribed the generic equivalent;
14.2 has prescribed the product by its pharmaceutical ingredient (API) name, such as venlafaxine;
14.3 has prescribed the originator product by brand name but does not identify on the prescription that substitution is not permitted and the patient consents; or
14.4 prescribes a different generic equivalent by brand name but does not identify on the prescription that substitution is not permitted and the patient consents.
The Pharmaceutical Benefits Scheme
15. The Commonwealth Government, through the Pharmaceutical Benefits Scheme (PBS) subsidises the cost to eligible patients of certain pharmaceutical products in Australia. Some of the features of that scheme are outlined in paragraphs 16 to 32.
16. The PBS Schedule is a list of all of the pharmaceutical products available to be dispensed to patients through the PBS at a Commonwealth-subsidised price. The PBS Schedule is usually updated and published on a monthly basis.
17. A pharmaceutical product can only be listed on the PBS Schedule after the product has been registered on the ARTG.
18. The PBS Schedule lists the price at which a PBS-listed medicine may be dispensed at retail pharmacy level (PBS Price), which is referred to in the PBS Schedule as the dispensed price for maximum quantity (DPMQ) (the maximum quantity is also specified for each product on the PBS Schedule). At the time of the proceedings, and until October 2012, the PBS Price comprised the following:
18.1 the Approved Price to Pharmacist (APP), which is the maximum price allowed to be charged to the dispensing pharmacist for the medicine under the National Health Act 1953 (Cth) and related legislative instruments (National Health Legislation), which in turn comprised:
18.1.1 the ex-manufacturer price, which is the maximum price allowed to be charged by the sponsor or manufacturer of the medicine under the National Health Legislation;
18.1.2 the wholesaler mark-up, which is the maximum mark-up that can be charged by a wholesaler under the National Health Legislation;
18.2 the pharmacist mark-up, which is the maximum mark-up that can be charged by a pharmacist under the National Health Legislation (giving effect to the Fourth and Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia); and
18.3 a dispensing fee charged by pharmacies under the National Health Legislation (giving effect to the Fourth and Fifth Community Pharmacy Agreement between the Commonwealth of Australia and the Pharmacy Guild of Australia).
19. The Pharmaceutical Benefits Pricing Authority (PBPA) recommends an APP to the Minister for Health and Ageing for new pharmaceutical items recommended for inclusion on the PBS. This process does not occur for generic equivalents. The most recent PBPA manual (“Policies, Procedures and Methods used in the Recommendations for Pricing of Pharmaceutical Products, April 2009) outlines the current process for pricing new brands of existing items, which is undertaken by the PBPA Secretariat.
20. When a patient purchases a PBS-listed medicine, subject to certain "safety-net" provisions, they are required to pay an amount known as the "patient co-payment". The maximum patient co-payment for a PBS-listed medicine is prescribed by the Commonwealth according to whether a patient holds a concession card or is a general benefit recipient. Currently, the maximum patient co-payment is set at $5.90 for patients who hold a concession card or $36.10 for patients who are general benefit recipients. Attachment 1 sets out the historical values of the maximum patient co-payment amounts, including since 2009. When pharmacists dispense a PBS-listed medicine whose PBS Price is above the patient co-payment, they receive the patient co-payment from the patient and, to the extent that the PBS Price exceeds the patient co-payment, a reimbursement of that difference by Medicare Australia.
21. Part VII, Division 3A of the National Health Act 1953 (Cth) sets out price reductions, which apply to pharmaceutical products that are already listed on the PBS Schedule, when the first PBS listing of one or more generic versions of that product (first generic equivalents) occurs. In summary, under these arrangements as applicable from 1 February 2011, a PBS listing of the first generic version of an originator product will, provided certain circumstances are met and any exceptions do not apply, trigger a 16% price reduction in the APP of the originator brand. This price reduction is first applied to the generic equivalent and flows on to the originator (and also any other generic equivalent versions subsequently listed unless the APP is otherwise further reduced by the time of their listing), though the price reduction for both the originator product and the generic product occurs on the same day. Relevantly, under these arrangements as applicable for the period between 1 January 2009 and 31 January 2011, a PBS listing of the first generic version of an originator product would have, provided certain circumstances were met and any exceptions did not apply, triggered a 12.5% price reduction in the APP of the originator brand (as a flow-on effect of the application of that 12.5% price reduction to the generic product). The new APP would also have been applied to any other generic equivalent version subsequently listed unless the APP was otherwise further reduced by the time of their listing.
22. Pursuant to a policy of the Commonwealth, during 2009 to the present time, there have been only three occasions per year on which a product whose listing on the PBS would result in a reduction in the APP of a pharmaceutical product of the kind referred to in the preceding paragraph (such as the first generic equivalent of an already-listed originator product) could be listed on the PBS (i.e. included on the PBS Schedule): on 1 April, 1 August and 1 December.
23. Each of the three listing occasions referred to in paragraph 21 above has a deadline by which the application for listing on that date must be filed. In particular:
23.1 an application to list a first generic equivalent on the PBS on 1 April must be filed by 1 December of the preceding year;
23.2 an application to list a first generic equivalent on the PBS on 1 August must be filed by 1 May of that year;
23.3 an application to list a first generic equivalent on the PBS on 1 December must be filed by 1 September of that year.
24. Once one or more first generic equivalents have been listed on the PBS, and an initial price reduction has been triggered and applied, subsequent generic equivalents may be listed on the 1st day of any subsequent month.
25. The application for listing of subsequent generic equivalents referred to in paragraph 24 above must generally be submitted by the 15th of the month which is three calendar months before the listing month. For example, an application for listing on 1 June in a given year must be submitted by 15 March in that year, except where 15 March is not a working day. The deadline then becomes the close of business on the working day prior.
26. An applicant for listing of a generic equivalent product on the PBS must provide, within 15 days of the relevant deadline referred to in paragraphs 22 and 25 above, a copy of the certificate from the TGA indicating that the product is registered on the ARTG (except where the 15th day is not a working day, in which case it must be provided on the working day prior). For example, for an application for listing on 1 June in a given year, the ARTG certificate must be submitted by 30 March in that year (provided 30 March is a working day).
27. The listing on the PBS of a generic equivalent annotated with "a-flagging" against the originator product means that pharmacists are able to dispense the generic equivalent to patients through the PBS when a doctor:
27.1 has prescribed the product by its API name, such as venlafaxine;
27.2 has prescribed the originator product by brand name but does not identify on the prescription that substitution is not permitted and the patient consents; or
27.3 prescribes a different generic equivalent by brand name but does not identify on the prescription that substitution is not permitted and the patient consents.
Additionally, the listing on the PBS of a generic product means that pharmacists are able to dispense that generic product through the PBS when a doctor prescribes that generic product by name.
28. A pharmacist may dispense products privately, that is, not through the PBS, even where the products are PBS-listed.
29. Prescription products which are not listed on the PBS are dispensed privately.
30. If a pharmacist dispenses a product privately the patient bears the full purchase price of the product as no Commonwealth subsidy is paid under the PBS.
31. The PBS Price and the ex-manufacturer price referred to in paragraph 18 above are maximum values, and a sponsor or manufacturer may elect to reduce the actual price it charges the pharmacist, at any time, by offering its products at a discount to the ex-manufacturer price component of the PBS Price.
32. In the case of PBS-listed medicines that are purchased by a pharmacist at the listed APP, the profit made by a pharmacist on the sale of those medicines comprises the set pharmacist mark-up and the dispensing fee. However, where a pharmacist is able to purchase a PBS-listed medicine at a price below the listed APP, an additional profit is earned which reflects the difference between their cost of goods and the listed APP. The greater the difference between the actual cost of goods and the listed APP, the greater the profit margin for the pharmacist.
First listing of a generic extended release venlafaxine hydrochloride product
33. Venlafaxine is a serotonin and noradrenaline re-uptake inhibitor (SNRI) which is thought to act by increasing the levels of these neurotransmitters in the brain.
34. Under the arrangements referred to in paragraph 21 above, the first listing of a generic venlafaxine hydrochloride product (equivalent to Wyeth’s originator product Efexor-XR) on the PBS in 2009 or 2010 would have (provided certain circumstances were met and any exceptions did not apply) triggered a reduction in the APP (and hence the PBS Price) of:
34.1 Efexor-XR (as a flow-on effect of the application of that reduction to the generic product); and
34.2 subsequent generic versions (unless the APP was otherwise further reduced by the time of their listing).
35. Under the arrangements referred to in paragraph 22 and 23 above, an application to list the first generic extended-release venlafaxine hydrochloride product (equivalent to Efexor-XR) on:
35.1 1 August 2009, would have required submission of the PBS listing application by the sponsor of the generic equivalent to be received by the Listing Unit of the Pharmaceutical Evaluation Branch on or before 1 May 2009;
35.2 1 December 2009, would have required submission of the PBS listing application by the sponsor of the generic equivalent to be received by the Listing Unit of the Pharmaceutical Evaluation Branch on or before 1 September 2009;
35.3 1 April 2010, would have required submission of the PBS listing application by the sponsor of the generic equivalent to be received by the listing unit of the Pharmaceutical Evaluation Branch on or before 1 December 2009.
36. Under the arrangements referred to in paragraph 21 above, in 2009 and 2010, upon the listing of the first generic equivalent of Efexor-XR, the reduction to the APP (of Efexor-XR and the first generic equivalent) would have been 12.5% (provided certain circumstances were met and any exceptions did not apply).
38. The ARTG records that the following products (Efexor-XR Products) were entered on the Register (see public summaries of ARTG entries in Attachment 2):
38.1 Efexor-XR 150 venlafaxine (as hydrochloride) 150mg modified release capsule blister pack, on 11 May 1998 under number 60859 (Efexor-XR 150);
38.2 Efexor-XR 75 venlafaxine (as hydrochloride) 75mg modified release capsule blister pack, on 11 May 1998 under number 60858 (Efexor-XR 75);
38.3 Efexor-XR 37.5 venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack, on 14 October 2004 under number 99802 (Efexor-XR 37.5).
39. Wyeth Australia Pty Ltd was the original sponsor of each of the Efexor-XR Product registrations. Pfizer Australia Pty Ltd is now listed as the sponsor of each of the registrations.
40. The 75mg and 150mg formulations of Efexor-XR were listed on the PBS on 1 February 1999, and in any event by 30 April 2009. The 37.5mg formulation of Efexor-XR was listed on the PBS on 1 December 2005, and in any event by 30 April 2009.
41. The Efexor-XR Products were the only products listed on the Schedule of Pharmaceutical Benefits containing venlafaxine hydrochloride as the active pharmaceutical ingredient during the period commencing 1 January 2008 to 31 March 2012.
42. The ARTG records that the following products (the Evelexa XR Products) were entered on the Register on 5 March 2009 (see public summaries of ARTG entries in Attachment 3):
42.3 Evelexa XR 150 venlafaxine (as hydrochloride) 150mg extended release capsule blister pack under number 146744 (Evelexa XR 150);
42.4 Evelexa XR 75 venlafaxine (as hydrochloride) 75mg extended release capsule blister pack under number 146729 (Evelexa XR 75);
42.5 Evelexa XR 37.5 venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack under number 146645 (Evelexa XR 37.5).
43. As at 5 March 2009 Sigma Pharmaceuticals (Australia) Pty Ltd was the sponsor of each of the Evelexa XR Product registrations.
44. Sigma Company Limited is now the sponsor of each of the Evelexa XR Product registrations.
45. Evelexa XR 150 was registered on the ARTG on the basis of being essentially similar to Efexor-XR 150, and is substitutable (including on the PBS) for Efexor-XR 150.
46. Evelexa XR 75 was registered on the ARTG on the basis of being essentially similar to Efexor-XR 75, and is substitutable (including on the PBS) for Efexor-XR 75.
47. Evelexa XR 37.5 was registered on the ARTG on the basis of being essentially similar to Efexor-XR 37.5.
48. As shown in the ARTG public summaries in Attachment 3, the Evelexa XR Products are indicated for treatment of major depression, including prevention of relapse and recurrence where appropriate, generalised anxiety disorder; social anxiety disorder and panic disorder; including prevention of relapse.
49. Sigma commenced proceeding VID 195 of 2009 on 1 April 2009.
50. On 1 May 2009 Wyeth filed a cross claim in proceeding VID 195 of 2009 including an application for an interlocutory injunction.
51. On 3 June 2009, Sundberg J made the orders in VID 195 of 2009 set out in Attachment 4, including order 1 (the Sigma Interlocutory Injunction).
52. In respect of order 1 referred to in the preceding paragraph, Wyeth and Wyeth Australia Pty Ltd gave the undertakings set out in Attachment 4.
53. On 8 November 2010, Jagot J made the orders in VID 195 of 2009 set out in Attachment 5.
Enlafax XR Products (Alphapharm)
54. The ARTG records that the following products (Enlafax-XR Products) were entered on the Register on 30 April 2009 (see public summaries of ARTG entries in Attachment 6):
54.3 Enlafax-XR venlafaxine 150mg modified release capsule blister pack under number 143552 (Enlafax-XR 150);
54.4 Enlafax XR venlafaxine 75mg modified release capsule blister pack under number 143533 (Enlafax-XR 75).
55. Alphapharm was and remains the sponsor of each of the Enlafax-XR Product registrations.
56. Enlafax-XR 150 was registered on the ARTG on the basis of being essentially similar to Efexor- XR 150, and is substitutable (including on the PBS) for Efexor-XR 150.
57. Enlafax-XR 75 was registered on the ARTG on the basis of being essentially similar to Efexor-XR 75, and is substitutable (including on the PBS) for Efexor-XR 75.
58. As shown in the ARTG public summaries in Attachment 6, the Enlafax-XR Products are indicated for the treatment of Major depression (including prevention of relapse and recurrence where appropriate) and Social anxiety disorder.
59. On 19 June 2009, Alphapharm commenced proceeding NSD 596 of 2009.
60. On 23 July 2009, Wyeth and Wyeth Australia Pty Ltd filed a cross-claim in proceeding NSD 596 of 2009.
61. On 25 August 2009, Jagot J made the orders in NSD 596 of 2009 set out in Attachment 7, including orders 1 and 2 (Alphapharm Interlocutory Injunction).
62. In respect of orders 1 and 2 referred to in the preceding paragraph, Wyeth and Wyeth Australia Pty Ltd gave the undertakings set out in Attachment 7.
63. On 8 November 2010, Jagot J made the orders in NSD 596 of 2009 set out in Attachment 8.
64. The ARTG records that the following products (Generic Health Products) were entered on the Register on 6 August 2009 (see public summaries of ARTG entries in Attachment 9):
64.3 (as now known) Venlafaxine Sandoz XR venlafaxine (as hydrochloride) 150mg modified release capsule blister under number 151875 (Venlafaxine Sandoz XR 150);
64.4 (as now known) Venlafaxine Sandoz XR venlafaxine (as hydrochloride) 75mg modified release capsule blister under number 151880 (Venlafaxine Sandoz XR 75);
64.5 Venlafaxine GENERICHEALTH XR venlafaxine (as hydrochloride) 150mg modified release capsule blister under number 151876 (Venlafaxine GENERICHEALTH XR 150);
64.6 Venlafaxine GENERICHEALTH XR venlafaxine (as hydrochloride) 75mg modified release capsule blister under number 151884 (Venlafaxine GENERICHEALTH XR 75).
65. Generic Health was and remains the sponsor of each of the Generic Health Product registrations.
66. Venlafaxine Sandoz XR 150 and Venlafaxine GENERICHEALTH XR 150 were registered on the ARTG on the basis of being essentially similar to Efexor-XR 150, and are substitutable (including on the PBS) for Efexor-XR 150.
67. Venlafaxine Sandoz XR 75 and Venlafaxine GENERICHEALTH XR 75 were registered on the ARTG on the basis of being essentially similar to Efexor-XR 75, and are substitutable (including on the PBS) for Efexor-XR 75.
68. As shown in the ARTG public summaries in Attachment 9, the Generic Health Products are indicated for "treatment of major depression, including prevention of relapse and recurrence where appropriate; generalised anxiety disorder; social anxiety disorder; and panic disorder, including prevention of relapse".
69. The ARTG records that the following products (Apotex Products) were entered on the Register (see public summaries of ARTG entries in Attachment 10):
69.3 On 6 August 2009:
69.3.1 (as now known) Apo-Venlafaxine XR venlafaxine (as hydrochloride) 150mg modified release capsule under number 151877 (Apo-Venlafaxine XR 150); and
69.3.2 (as now known) Apo-Venlafaxine XR venlafaxine (as hydrochloride) 75mg modified release capsule under number 151878 (Apo-Venlafaxine XR 75).
69.4 On 25 March 2011:
69.4.1 Apotex-Venlafaxine XR venlafaxine (as hydrochloride) 75 mg modified release capsule blister pack under number 177456 (Apotex-Venlafaxine XR 75);
69.4.2 Apotex-Venlafaxine XR venlafaxine (as hydrochloride) 150 mg modified release capsule blister pack under number 177457 (Apotex-Venlafaxine XR 150);
69.4.3 Chemmart Venlafaxine XR venlafaxine (as hydrochloride) 75 mg modified release capsule blister pack number 177458 (Chemmart Venlafaxine XR 75);
69.4.4 Chemmart Venlafaxine XR venlafaxine (as hydrochloride) 150 mg modified release capsule blister pack number 177459 (Chemmart Venlafaxine XR 150);
69.4.5 Terry White Chemists Venlafaxine XR venlafaxine (as hydrochloride) 75 mg modified release capsule blister pack number 177460 (Terry White Chemists Venlafaxine XR 75);
69.4.6 Terry White Chemists Venlafaxine XR venlafaxine (as hydrochloride) 150 mg modified release capsule blister pack number 177461 (Terry White Chemists Venlafaxine XR 150).
70. As at 6 August 2009, Generic Health was the sponsor of each of the Apotex Product registrations referred to in paragraph 69.1.
71. Apotex Pty Ltd is now the sponsor of each of the Apotex Product registrations.
72. Apo-Venlafaxine XR 150, Apotex-Venlafaxine XR 150, Chemmart Venlafaxine XR 150 and Terry White Chemists Venlafaxine XR 150 were registered on the ARTG on the basis of being essentially similar to Efexor-XR 150, and are substitutable for Efexor-XR 150 (including on the PBS where the Apotex product is listed on the PBS).
73. Apo-Venlafaxine XR 75, Apotex-Venlafaxine XR 75, Chemmart Venlafaxine XR 75 and Terry White Chemists Venlafaxine XR 75 were registered on the ARTG on the basis of being essentially similar to Efexor-XR 75, and are substitutable for Efexor-XR 75 (including on the PBS where the Apotex product is listed on the PBS).
74. As shown in the ARTG public summaries in Attachment 10, the Apotex Products are indicated for treatment of major depression, including prevention of relapse and recurrence where appropriate.
75. The ARTG records that the following products (Ascent Products) were entered on the Register on 6 August 2009 (see public summaries of ARTG entries in Attachment 11):
75.3 (as now known) Venlexor XR venlafaxine (as hydrochloride) 150mg modified release capsule blister pack under number 151874 (Venlexor XR 150); and
75.4 (as now known) Venlexor XR venlafaxine (as hydrochloride) 75mg modified release capsule blister pack under number 151885 (Venlexor XR 75).
76. As at 6 August 2009, Generic Health was the sponsor of each of the Ascent Product registrations.
77. Ascent Pharma Pty Ltd is now the sponsor of each of the Ascent Product registrations.
78. Venlexor XR 150 was registered on the ARTG on the basis of being essentially similar to Efexor- XR 150, and is substitutable (including on the PBS) for Efexor-XR 150.
79. Venlexor XR 75 was registered on the ARTG on the basis of being essentially similar to Efexor- XR 75, and is substitutable (including on the PBS) for Efexor-XR 75.
80. As shown in the ARTG public summaries in Attachment 11, the Ascent Products are indicated for "treatment of major depression, including prevention of relapse and recurrence where appropriate, social anxiety disorder".
81. On 6 October 2009, Generic Health commenced proceeding NSD 1124 of 2009.
82. On 9 November 2009, Wyeth and Wyeth Australia Pty Ltd filed a cross claim in proceeding NSD 1124 of 2009.
83. On 10 November 2009, Jagot J made the orders in NSD 1124 of 2009 set out in Attachment 12, including order 1 (Generic Health Interlocutory Injunction).
84. In respect of order 1 referred to in the preceding paragraph, Wyeth and Wyeth Australia Pty Ltd gave the undertakings set out in Attachment 12.
85. On 8 November 2010, Jagot J made the orders in NSD 1124 of 2009 set out in Attachment 13.
86. On 9 November 2010, Sigma filed an application in relation to order 7 of the orders made by Jagot J in proceeding VID 195 of 2009 on 8 November 2010.
87. On 12 November 2010, in relation to Sigma's application referred to in the preceding paragraph, Jacobson J made the orders set out in Attachment 14.
88. Sigma, Alphapharm and Generic Health appealed Jagot J's decision to the Full Federal Court and, on 28 October 2011, the Full Federal Court delivered its judgment.
89. On 11 November 2011, the Full Federal Court made the orders in NSD 1533 of 2010, NSD 1603 of 2010 and NSD 1644 of 2010 set out in Attachments 15, 16 and 17 respectively.
90. On 21 December 2011, the Full Federal Court made the orders in NSD 1533 of 2010, NSD 1603 of 2010 and NSD 1644 of 2010 set out in Attachments 18, 19 and 20 respectively.
91. Wyeth and Wyeth Australia Pty Ltd filed applications for special leave to appeal the Full Court's judgment to the High Court of Australia on 2 December 2011, which were amended on 27 February 2012.
92. On 11 May 2012 the High Court refused to grant Wyeth and Wyeth Australia Pty Ltd special leave to appeal (see Attachment 21).
Australian venlafaxine hydrochloride market
93. Immediately prior to 5 March 2009, Efexor-XR (Wyeth’s modified release venlafaxine hydrochloride) was:
93.3 the only brand of modified release venlafaxine hydrochloride on the ARTG;
93.4 the only brand of modified release venlafaxine hydrochloride on the PBS; and
93.5 the leading anti-depressant brand in Australia in terms of units sold and value of sales.
94. The ARTG records that the following products (Ranbaxy Products) were entered on the Register on 22 January 2010 (see public summaries of ARTG entries in Attachment 22):
94.3 Venlafaxine Ranbaxy venlafaxine (as hydrochloride) 150mg modified release capsules blister pack under number 149455;
94.4 Venlafaxine Ranbaxy venlafaxine (as hydrochloride) 75mg modified release capsules blister pack under number 149454; and
94.5 Venlafaxine Ranbaxy venlafaxine (as hydrochloride) 37.5mg modified release capsules blister pack under number 149432.
95. Ranbaxy Australia Pty Ltd is the sponsor of each of the Ranbaxy Product registrations.
96. The ARTG records that the following products (Spirit Products) were entered on the Register on 30 July 2010 (see public summaries of ARTG entries in Attachment 23):
96.3 Elaxine SR 150 venlafaxine (as hydrochloride) 150mg modified release capsule blister pack under number 160301;
96.4 Elaxine SR 75 venlafaxine (as hydrochloride) 75mg modified release capsule blister pack under number 160300; and
96.5 Elaxine SR 37.5 venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack under number 160299.
97. Spirit Pharmaceuticals Pty Ltd is the sponsor of each of the Spirit Products.
98. The ARTG records that the following products (SC Pharma Products) were entered on the Register on 30 July 2010 (see public summaries of ARTG entries in Attachment 24):
98.3 Stada Venlafaxine SR venlafaxine (as hydrochloride) 150mg modified release capsule blister pack under number 160295;
98.4 Stada Venlafaxine SR venlafaxine (as hydrochloride) 75mg modified release capsule blister pack under number 160294;
98.5 Stada Venlafaxine SR venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack under number 160293;
98.6 Venlafaxine SR SCP venlafaxine (as hydrochloride) 150mg modified release capsule blister pack under number 160298;
98.7 Venlafaxine SR SCP venlafaxine (as hydrochloride) 75mg modified release capsule blister pack under number 160297; and
98.8 Venlafaxine SR SCP venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack under number 160296.
99. SC Pharma Pty Ltd is the sponsor of each of the SC Pharma Products.
100. The ARTG records that the following products (Pfizer Products) were entered on the Register on 10 December 2010 (see public summaries of ARTG entries in Attachment 25):
100.3 Venlafaxine Wyeth venlafaxine (as hydrochloride) 150mg modified release capsule blister pack under number 171551;
100.4 Venlafaxine Wyeth venlafaxine (as hydrochloride) 75mg modified release capsule blister pack under number 171550;
100.5 Venlafaxine Wyeth venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack under number 171549;
100.6 Altven venlafaxine (as hydrochloride) 150mg modified release capsule blister pack under number 171537;
100.7 Altven venlafaxine (as hydrochloride) 75mg modified release capsule blister pack under number 171536; and
100.8 Altven venlafaxine (as hydrochloride) 37.5mg modified release capsule blister pack under number 171535.
101. Pfizer Australia Pty Ltd is the sponsor of each of the Pfizer Products.
102. As at 11 November 2011, there were nine sponsors of generic modified release venlafaxine hydrochloride products registered on the ARTG, the registration details of which are summarised in Attachment 26.
103. On 1 April 2012, the following generic versions of Efexor-XR were listed on the PBS:
103.3 Apotex 75mg and 150mg products under brand names:
103.3.1 "Apo-Venlafaxine XR";
103.3.2 "Chemmart Venlafaxine XR";
103.3.3 "Terry White Chemists Venlafaxine XR";
103.4 Generic Health 75mg and 150mg products under brand name Venlafaxine GENERICHEALTH XR;
103.5 Alphapharm 75mg and 150mg products under brand name Enlafax-XR;
103.6 Spirit Pharmaceuticals Pty Limited 37.5mg, 75mg and 150mg products under brand name Elaxine SR;
103.7 Sandoz Pty Limited 75mg and 150mg products under brand name Venlafaxine Sandoz XR;
103.8 Ascent Pharma Pty Ltd 75mg and 150mg products under brand name Venlexor XR;
103.9 Ranbaxy Australia Pty Ltd 37.5mg, 75mg and 150mg products under brand name Venla RBX.
Australian pharmaceutical market
104. Data Modelling Section Pharmaceutical Policy and Analysis Branch of the Pharmaceutical Benefits Scheme published a report on Expenditure and Prescription for each of the years ending 30 June 2008, 2009, 2010 and 2011 (PBS Expenditure Reports).
105. Extracts of the PBS Expenditure Reports recording PBS expenditure on venlafaxine hydrochloride for the years ending 30 June 2008, 2009, 2010 and 2011 are attached as Attachment 27.
106. The extracts of the PBS Expenditure Reports at Attachment 27 record PBS expenditure on venlafaxine hydrochloride for the years ending 30 June 2008, 2009, 2010 and 2011.
SECOND STATEMENT OF AGREED FACTS
Statement of Agreed Facts
The following facts are agreed for the purposes of the Proceedings only, subject to any further date restrictions specified below.
Further, these facts are agreed between Wyeth, Wyeth Australia Pty Ltd and the parties listed in the schedule to this statement only.
1. Until in or about 3 October 2016, IMS Health (IMS) was a global group of companies that collated information relating to the pharmaceutical industry in Australia and overseas and then provided this information to subscribers of its services.
2. IMS had operations in over 100 countries, including Australia.
3. Prior to being privatised in February 2010, IMS was a public company listed on the New York Stock Exchange.
4. IMS was independent of pharmaceutical companies (whether innovators or generics), independent of governments and independent of industry pressure or lobby groups.
5. IMS collected information such as products sold, volume of sales and product prices.
6. On or about 3 October 2016, IMS Health Holdings, Inc. (IMS Inc), the global parent of IMS, entered into a merger agreement pursuant to which IMS Inc. merged with Quintiles Transnational Holdings Inc., the global parent of the Quintiles group of companies, thus combining IMS and the Quintiles group of companies (Quintiles Group).
7. After 3 October 2016 and until 15 December 2016, the IMS Quintiles Group, including IMS Australia Pty Ltd, continued to collate and provide data in the same way as that data was collated and provided prior to 3 October 2016.
8. IMS collected data from a broad range of sources, including doctors, pharmacists, pharmaceutical wholesalers and hospitals and compiled it into a number of datasets. These datasets were stored in a database maintained by IMS, of which extracts were made available to customers of IMS.
9. IMS was a leading global provider of market and sales data in the pharmaceutical industry. IMS was considered to be the “industry standard market data provider” in Australia for the provision of the API and AMI data discussed below.
10. IMS data was used by academics, government and pharmaceutical companies in Australia, including, at various times prior to on or about 3 October 2016, both Alphapharm and Sigma.
11. IMS subscribers included pharmaceutical companies, (including, at various times prior to on or about 3 October 2016, Wyeth Australia Pty Ltd, and also Sigma/Alphapharm), hospitals, academics, allied health companies, manufacturers of over-the-counter products sold in pharmacies, and others.
12. These various groups and individuals used IMS data, amongst other data, and in combination with other data, for, among other things, research and development, marketing and marketing research, developing business strategy, and reporting to government organizations (such as the Pharmaceutical Benefits Pricing Authority).
13. During the period it was a subscriber to IMS, Wyeth Australia had access to a number of IMS datasets including the Australian Pharmaceutical Index (API), the Australian Hospital Index (AHI) and the Australian Medical Index (AMI). Wyeth Australia used data from these datasets, amongst other data, and in combination with other data, to assess the performance of its products relative to its own expectations and to relevant competitors, and to make decisions on questions such as sales force deployment, marketing and product development. Other pharmaceutical companies used IMS data in a similar way.
14. IMS data, amongst other data, and in combination with other data, was used by Wyeth Australia, and by other pharmaceutical companies, during the period that they subscribed as necessary to any such data sources, in order to prepare forecasts or projections for use in internal business planning and in dealings with external bodies such as the Pharmaceutical Benefits Advisory Committee.
15. The AHI and API datasets discussed below are materially accurate.
16. IMS databases contained information about many different pharmaceutical products.
17. The systems and procedures pursuant to which IMS data was gathered and made available were apt to ensure that, so far as is practicable, the data in the AHI and API datasets discussed below was reasonably accurate and useful.
18. IMS grouped products together according to their classification under a system commonly known as the ATC, developed by the European Pharmaceutical Market Research Association (EphMRA). This is distinct from the similarly named, and similar classification system developed by the WHO.
19. The EphMRA classification groups drugs together based on therapeutic ingredients and indication. This is a convenient way of arranging the data for the purpose of enabling ready comparison of the market performance of drugs used for treating similar conditions, although it is possible – provided the customer subscribes for the relevant data – to access relevant information relating to drugs which IMS has classified in different categories.
20. IMS created the following datasets which it maintained as part of its database:
20.1 the Australian Pharmaceutical Index (API), which recorded sales of prescription products to Australian retail pharmacies;
20.2 the Australian Hospital Index (AHI), which recorded sales of prescription products to Australian hospital pharmacies; and
20.3 the Australian Medical Index (AMI), which contained information regarding the prescribing of pharmaceutical products in Australia.
The Australian Pharmaceutical Index (API)
21. The API was a dataset that contained details of sales of pharmaceutical products to retail pharmacies in Australia. The API did not contain details of actual sales by retail pharmacies to patients.
22. In Australia, there are two channels through which pharmaceutical products are sold to pharmacies. The first channel is sales by wholesalers to pharmacies. (In Australia, there are three major wholesalers Symbion, Sigma and Australian Pharmaceutical Industries, and approximately eleven smaller wholesalers. The number of wholesalers has varied over time.). The second channel is sales direct from manufacturers to pharmacies.
23. The principal source of information utilised in order to create the API was actual sales data received directly from wholesalers and manufacturers regarding the number and value of sales to pharmacies. IMS estimated that such sales data received from wholesalers and manufacturers accounted for more than 90% of total sales of all pharmaceutical products sold to pharmacies. This estimate was made by comparing the results of their data collections from this method with their cross-check data collected in the manner described in paragraph 26 below, and by other means.
24. IMS entered into contracts with these wholesalers and manufacturers for the provision by them of details of their sales to pharmacies. The wholesalers or manufacturers provided information from their business records as to the number of sales of each relevant product. This information, which included invoice dates and numbers, account number and type, product number, quantity supplied, net sales value (after discount, before tax) and transaction type (invoice/credit) was treated as confidential to each supplier of information, but enabled IMS to aggregate the information of all such information suppliers into its databases.
25. In addition, IMS’s practice was to require any organisation that subscribed for a particular IMS service to provide IMS with its sales data in respect of that service. Accordingly, if a pharmaceutical manufacturer which subscribed for the API sold directly to pharmacies, IMS would require as a condition of that subscription that the manufacturer provide its sales data to IMS. Together with the information obtained from wholesalers, this resulted in IMS obtaining the great bulk of the information relevant to its datasets such as the API and AHI.
26. In some cases IMS was unable to obtain actual sales information (which occured, for instance, when a manufacturer sold directly to pharmacies and had not subscribed for an IMS product and therefore contracted to provide its own sales information to IMS, or where a pharmaceutical manufacturer prevented a wholesaler from disclosing sales data regarding its products). In such cases, IMS estimated such sales on the basis of data received from a stratified, randomized panel of approximately 100 pharmacies. Those 100 pharmacies were selected so that, in so far as is practicable, the pharmacies were representative of all Australian pharmacies, including in terms of the State or Territory in which the pharmacy was located, the size of the pharmacy and a number of other characteristics. Pharmacies in the sample provided their sales records to IMS, which were then utilised by IMS to project total sales for all Australian pharmacies. This information was used to cross-check and augment the actual sales data to produce the API.
27. Where IMS relied on a statistical sample as opposed to actual records, IMS determined the composition and size of the sample on the basis of recommendations from IMS's Global Statistical Services Office. The Global Statistical Services Office was a specialist division based in Germany with extensive statistical expertise.
28. The API dataset was stored in a database maintained by IMS for the purpose of its business. The dataset was regularly updated with additional information from the sources described above. IMS made copies and extracts of the database and made these available to relevant subscribers by providing the subscribers to the API with a set of disks, once a month, which contained the updated API data. Each disk contained data which included history of between 2 and 5 years, depending upon the deliverable. If a subscriber specifically requested additional data on an ad hoc basis (eg because the customer had not retained a disk, or because the information related to a period or database prior to or different from that for which the customer had subscribed), this specific, ad hoc, information could be provided - in the most appropriate form based on the subscriber’s use, for example, an Excel file.
29. Subscribers were provided either with IMS proprietary software, and/or were equipped with appropriate security (password) mechanisms which allowed them – and only them – to access the information which was provided to them on the disks provided by IMS. IMS aimed to limit access of the information provided, to those who had subscribed to it, because if this information were to become freely available to unsubscribing users, the value of IMS’s product – ie its ability to protect its intellectual property, and the correct billing for information services – would be undermined. It would also expose the company to potential breaches of its contractual obligations with other stakeholders, hence posing a risk to its data assets.
30. The API also showed volumes of drug sales expressed in dollar terms. These dollar values were calculated by IMS multiplying the number of pack sales by the “average wholesale price” as determined by IMS for the relevant packs.
The Australian Hospitals Index (AHI)
31. The AHI was similar to the API. The difference was that the AHI contained details of sales of pharmaceutical products to hospital pharmacies (as opposed to retail pharmacies). The AHI did not contain details of sales by hospital pharmacies to patients.
32. As was the case with the API, the principal source of information utilised in order to create the AHI was actual sales data received directly from wholesalers and manufacturers regarding the number and value of sales. IMS estimated (in the same way) that sales data received from wholesalers and manufacturers who sold directly to hospital pharmacies accounted for more than 90% of all sales of pharmaceutical products to hospital pharmacies.
33. As was the case with the API, where IMS was unable to obtain actual sales information, IMS estimated such sales on the basis of data received from a stratified, randomized panel of hospitals. The stratification of the panel ensured that, so far as was practicable, the hospitals were representative of all Australian hospitals, including in terms of the State or Territory in which the hospital was located, and the bed-size of the hospital (bed-size being the best indicator of turnover of pharmaceutical products). Hospitals in the sample provided their sales records to IMS, which were then utilised by IMS to project total sales for all Australian hospitals as a cross-check and to augment the actual data from wholesalers or direct-supplying manufacturers.
34. The AHI dataset was stored in a database maintained by IMS for the purpose of its business. The dataset was regularly updated with additional information from the sources described above. IMS made copies and extracts of the database and made these available to relevant subscribers by providing the subscribers to the AHI with a set of disks, once a month, which contained the updated AHI data. Each disk contained data which included history of between 2 and 5 years, depending upon the deliverable.
The Australian Medical Index (AMI)
35. The AMI contained information on, among other things, the number of prescriptions of pharmaceutical products by general practitioners to patients. The AMI constituted a projection based on information received from a sample of general practitioners. As outlined immediately below, the sample on which the AMI was based was collected in two ways.
36. First, IMS received, via the General Practice Research Network – Health Communication Network (a national network of Australian general practitioners), prescription information extracted electronically. Participating general practitioners had software which extracted anonymised prescription information.
37. Secondly, IMS received information from a sample of general practitioners who supplied paper information of prescription data. The set of doctors that provided information in this way as well as via the electronic method described in paragraph 36 above constituted a stratified random sample of the national universe of general practitioners. Approximately 25% of the panel was rotated annually.
38. The AMI dataset was stored in a database maintained by IMS for the purpose of its business. The dataset was regularly updated with additional information from the sources described above. IMS made copies and extracts of the database and made these available to relevant subscribers at various times prior to on or about 3 October 2016 by providing the subscribers to the AMI with a set of disks, once a month, which contained the updated AMI data.
SIGMA
Elmo de Alwis: the CEO of Sigma Pharmaceutical Limited and the managing director of its wholly owned subsidiaries, Sigma Company Limited and Sigma during the relevant period. Also a member of Sigma Pharmaceutical Limited’s board, which had the same members as Sigma’s board.
Robert Ellis: General Manager – Business Development at Sigma from April 2007 to March 2012.
Susan Morgan: Sigma Pharmaceutical Limited’s general counsel and company secretary during the relevant period.
Andrew Heine: National Sales Manager (Generics) at from January 2006 to August 2009.
Trevor Ziman: during the relevant period, a member of the team at Aspen Asia Pacific Pty Ltd which acquired Sigma and its generics pharmaceuticals business from Sigma Company Limited.
Paul Meulblok: Sigma Company Limited’s Group Commercial Manager (Finance Division) from October 2007 to October 2009.
Nicole Burrowes: Group Financial Controller at Aspen Pharmacare Pty Ltd, employed in that role since 2014.
Nathan Caldwell: Group Financial Controller at Sigma Company Limited.
ALPHAPHARM
Mark Hurley: from 2006 until July 2010, Executive Director of Alphapharm, whose parent company in 2007 became Mylan Inc.
Jalal Chami: pharmacist and pharmacy manager who, from August 2009 to October 2013, was employed by Alphapharm in a number of business positions.
John Montgomery: the CEO of Alphapharm from November 1999 to March 2010.
Max Joscelyne: Commercial Finance Manager at Alphapharm, working in a range of financial roles at Alphapharm during the relevant period.
John Hannon: Global Supply Chain Controller at Mylan Ireland Limited during the relevant period.
Lynette Adams: Senior Purchasing Officer, Supply Chain, at Alphapharm.
Elena Facchinetti: Senior Sales Finance Analyst and then Financial Controller at Alphapharm.
Kim O’Connell: a partner of King & Wood Mallesons, Alphapharm’s lawyers.
Maria Mollica: Business Unit Head – Prescription at Alphapharm.
Steven Flynn: Global General Counsel, Intellectual Property at Mylan Inc, Alphapharm’s parent company.
GENERIC HEALTH
Gavin Upiter: founder, CEO and managing director of Generic Health from 2004 to 2012.
Sudarshan Menon: Chief Financial Officer of Generic Health.
ALEMBIC
Amit Ghosal: Assistant General Manager of Business Development at Alembic from April 2015.
PHARMATHEN
Gareth Williams: since April 2017, Vice President Global Business Development of Pharmathen.
Anastasios Spyropoulos: Head of the Business Unit at Pharmathen’s Pallini plant since 2011.
Diomedes Vassiliou: since April 2016 Chief Financial Officer of Pharmathen.
THE COMMONWEALTH
Felicity McNeill: joined the Department of Health in January 2010, as the Assistant Secretary, Pharmaceutical Evaluation Branch, Pharmaceutical Benefits Division, a role she held until September 2010.
Phillipa Horner: Principal Legal Advisor to the TGA at the Commonwealth Department of Health from 2 February 2010 to 16 November 2016.
David Pearson: Director in the Pharmaceutical Policy Branch (PPB), a branch in the Pharmaceutical Benefits Division (PBD) of the Department of Health
Dr Mariana Gebara-Coghlan: Director in the Pharmaceutical Chemistry Section of the Scientific Evaluation Branch of the TGA. Employed at the TGA since September 2002, and during the relevant period held the position of Principal Evaluator.
Phillip Spann: Assistant General Manager in the Patents and Plant Breeder’s Rights Group, and is responsible for the Patent Opposition, Hearings and Legislation section at IP Australia.
Andrew Korbel: partner at Corrs Chambers Westgarth, the solicitors for the Commonwealth.
WYETH
Gary Cooper: Commercial Manager – Pharmacy of Pfizer Australia and Business Support Manager of Pfizer New Zealand, Pfizer being the company which acquired Wyeth in October 2010.
Candida Braithwaite: Pharmaceutical Business Director of Wyeth Australia during the relevant period who gave evidence in support of Wyeth’s interlocutory applications in 2009.
David Manspeizer: Vice President – Intellectual Property and Associate General Counsel at Wyeth during the relevant period.
Dr Sally Mannion: Director of Patent and Trade Marks at Wyeth during the relevant period.
Ioakim (Kim) Konnaris: Senior Regulatory Affairs Associate at Wyeth during the relevant period.
Katie Barclay: Human Resources Manager at Wyeth.
ECONOMETRIC EXPERTS
Professor Jerry Hausman: MacDonald Professor of Economics at the Massachusetts Institute of Technology in Cambridge, Massachusetts, engaged by the generics.
Dr Helen Jenkins: Managing Partner of Oxera Consulting LLP, an economics and finance consulting company headquartered, Oxford, UK, BEc (Hons) Science and Economics, from the Australian National University and an MPhil and DPhil in Economics from the University of Oxford.
INDUSTRY EXPERTS
Karen McTavish: has more than 20 years’ experience working in various sales and management roles in the pharmaceutical industry for generics in Australia and Canada, including as the Sales and Marketing Director of Apotex Pty Ltd and Managing Director of Actavis Pty Ltd, engaged by the Commonwealth.
Allan Dick: has more than 30 years’ experience working in various sales and management roles in the pharmaceutical industry for originators in Australia, including as the General Manager of Roussel and Hoechst Marion Roussel New Zealand, Director - Retail (Primary Care) at Sanofi Aventis Australia and the Commercial Director of Takeda Australia.
ACCOUNTANTS
Tony Samuel: accountant, engaged by the generics and Alembic.
Greg Meredith: accountant, engaged by Wyeth.
ALPHAPHARM AND GENERIC HEALTH CALCULATIONS
1. Alphapharm’s supply from 1 March 2010 to 8 November 2010 is dependent on two independent anterior circumstances:
(1) whether Sigma would have been supplying its products under the PBS from 1 December 2009 until 8 November 2010; and
(2) whether Alphapharm would have been supplying its products on the private market prior to 1 March 2010.
2. These circumstances (C) can be described as:
(1) C1 = Sigma PBS and Alphapharm PM
(2) C2 = Sigma PM and Alphapharm PM
(3) C3 = Sigma PBS and Alphapharm N
(4) C4 = Sigma PM and Alphapharm N
3. The probabilities of Alphapharm taking a particular course from 1 March 2010 to 8 November 2010 (PM, PBS or N) given each of those four circumstances, as I have found them, are as follows:
Alphapharm 1/3/10 to 8/11/10 | C1 | C2 | C3 | C4 |
PM | 10% | 90% | 0% | 0% |
PBS | 90% | 0% | 80% | 0% |
N | 0% | 10% | 20% | 100% |
4. To calculate the respective probabilities of Alphapharm’s supply from 1 March 2010, the joint probability of the two circumstances occurring together before 1 March 2010 must first be calculated and then multiplied by the probability that Alphapharm would have taken a particular course from 1 March 2010 in each of those circumstances.
5. But for the Sigma interlocutory injunction, the probability of Sigma supplying on the PBS at 1 March 2010 was 20% and on the private market was 80%.
6. The joint probability of Alphapharm being on the private market (90%) and Sigma listing on the PBS (20%) before 1 March 2010 can be calculated as follows:
Alphapharm PM and Sigma PBS = 0.9 x 0.20 = 0.18
7. This process if repeated for all four potential sets of anterior circumstances results in the following:
Before 1/3/10 | Alphapharm PM (90%) | Alphapharm N (10%) |
Sigma PBS (20%) | 18% or 0.18 (C1) | 2% or 0.02 (C3) |
Sigma PM (80%) | 72% or 0.72 (C2) | 8% or 0.08 (C4) |
8. The probabilities of Alphapharm’s position from 1 March 2010 in each of the four circumstances can be calculated in the same manner.
9. For example, the joint probability of Alphapharm listing on the PBS on 1 March 2010 (90%) and Sigma having listed on the PBS before 1 March 2010 and Alphapharm previously supplying on the private market can be calculated as follows:
Alphapharm PBS and C1 = 0.9 x 0.18 = 0.162.
10. Repeating this process yields the following table:
Alphapharm 1/3/10 – 8/11/10 | C1 | C2 | C3 | C4 | TOTAL |
PM | 1.8% | 64.8% | 0% | 0% | 66.6% or 0.666 |
PBS | 16.2% | 0% | 1.6% | 0% | 17.8% or 0.178 |
N | 0% | 7.2% | 0.4% | 8% | 15.6% or 0.156 |
TOTAL | 18% | 72% | 2% | 8% | 100% or 1 |
11. The overall probability of Alphapharm’s supply is the sum of all the probabilities in each of the four circumstances. For example, the probability that Alphapharm would supply on the private market from 1 March 2010 to 8 November 2010 can be calculated as follows:
Alphapharm PM = 0.018 + 0.648 + 0 + 0 = 0.666.
12. Repeating this process yields the following probabilities for Alphapharm:
PM = 0.666
PBS = 0.178
N = 0.156.
13. As required, these probabilities add up to 1.
Generic Health
14. The same exercise can be carried out for Generic Health.
15. The probabilities of Generic Health taking a particular course from 1 March 2010 to 8 November 2010 (PM, PBS or N) given each of the four circumstances, as I have found them, are as follows:
GH 1/3/10 to 8/11/10 | C1 | C2 | C3 | C4 |
PM | 10% | 80% | 0% | 0% |
PBS | 90% | 0% | 80% | 0% |
N | 0% | 20% | 20% | 100% |
16. The joint probabilities of Generic Health and Sigma making particular kinds of supply as at 1 March 2010 are:
Before 1/3/10 | Generic Health PM (80%) | Generic Health N (20%) |
Sigma PBS (20%) | 16% or 0.16 (C1) | 4% or 0.04 (C3) |
Sigma PM (80%) | 64% or 0.64 (C2) | 16% or 0.16 (C4) |
17. The probabilities for Generic Health thus are:
GH 1/3/10 – 8/11/10 | C1 | C2 | C3 | C4 | TOTAL |
PM | 1.6% | 51.2% | 0% | 0% | 52.8% or 0.528 |
PBS | 14.4% | 0% | 3.2% | 0% | 17.6% or 0.176 |
N | 0% | 12.8% | 0.8% | 16% | 29.6% or 0.296 |
TOTAL | 16% | 64% | 4% | 16% | 100% or 1 |
18. In other words, for Generic Health:
PM = 0.528
PBS = 0.176
N = 0.296.
19. As required, these probabilities add up to 1.
VID 195 of 2009 | |
WYETH AUSTRALIA PTY LTD (ACN 000 296 211) | |
ALEMBIC PHARMACEUTICALS LTD | |
Applicants | |
Fourth Applicant: | ALPHAPHARM PTY LTD (ACN 002 359 739) |
Fifth Applicant: | PHARMATHEN S.A. |
SCHEDULE OF PARTIES
NSD 596 of 2009 | |
Cross-Claimants | |
Second Cross-Claimant: | WYETH AUSTRALIA PTY LTD (ACN 000 296 211) |
Cross-Respondents | |
Second Cross-Respondent: | ALEMBIC PHARMACEUTICALS LTD |
SCHEDULE OF PARTIES
NSD 1124 of 2009 | |
Cross-Claimants | |
Second Cross-Claimant: | WYETH AUSTRALIA PTY LTD (ACN 000 296 211) |




