FEDERAL COURT OF AUSTRALIA
Novartis Pharmaceuticals Australia Pty Ltd v Bayer Australia Ltd [2015] FCA 35
IN THE FEDERAL COURT OF AUSTRALIA | |
NOVARTIS PHARMACEUTICALS AUSTRALIA PTY LTD (ACN 004 244 160) Applicant | |
AND: | BAYER AUSTRALIA LIMITED (ACN 000 138 714) Respondent |
DATE OF ORDER: | |
WHERE MADE: |
THE COURT ORDERS THAT:
1. On or before 20 February 2015, the parties are to identify which parts of the reasons they contend should not be published because of confidentiality and file a proposed form of orders.
2. The parties are to endeavour to agree the figure to be included in [366] of these reasons.
3. If the applicant wishes to contend that costs should not follow the event, it should file and serve by 20 February 2015 a written submission of no more than three pages in support of that contention. If the applicant files such a written submission, the respondent is to file and serve by 28 February 2015 its written submission of no more than three pages in response.
4. The proceedings be listed at 9.30 am on 6 March 2015 for the making of orders.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
NEW SOUTH WALES DISTRICT REGISTRY | |
GENERAL DIVISION | NSD 314 of 2013 |
BETWEEN: | NOVARTIS PHARMACEUTICALS AUSTRALIA PTY LTD (ACN 004 244 160) Applicant |
AND: | BAYER AUSTRALIA LIMITED (ACN 000 138 714) Respondent |
JUDGE: | ROBERTSON J |
DATE: | 6 FEBRUARY 2015 |
PLACE: | SYDNEY |
REASONS FOR JUDGMENT
Introduction
1 These proceedings concern two medicines for the treatment, by intra-vitreal injection, of neovascular (wet) age-related macular degeneration (wet AMD or wAMD), a medical condition which results in loss of vision. Each medicine involves ongoing treatment.
2 Lucentis® (Lucentis), the Novartis product, has been available for sale in Australia since 2007. It is only available on prescription and is, and has been since 2007, administered only by ophthalmologists. Its active ingredient is ranibizumab and it is sometimes referred to by that name.
3 Eylea® (Eylea), the Bayer product, has been marketed and sold in Australia since 2012. It also is only available on prescription and is administered only by ophthalmologists. It too is administered via intra-vitreal injection. Its active ingredient is aflibercept and it is sometimes referred to by that name.
4 It is common ground that Lucentis and Eylea are in direct competition with each other as prescription-only medicines indicated for use in Australia for the treatment of wet AMD.
5 In February 2007, Lucentis was registered on the Australian Register of Therapeutic Goods and in August 2007 it was listed on the Schedule of Pharmaceutical Benefits (PBS Schedule).
6 On 7 March 2012, Eylea was registered on the Australian Register of Therapeutic Goods. On 1 December 2012, Eylea was listed on the PBS Schedule.
7 Novartis pleads that in approximately 46 acts of publication, which I list below, and in detailing conduct, Bayer made the following representations:
(a) that in clinical use, in accordance with the approved dosage, the Novartis product, Lucentis, is administered monthly for all patients (First Representation);
(b) that, for each and every patient, Lucentis will only be a clinically effective treatment if administered monthly (Second Representation);
(c) that the Lucentis Product Information specified, without qualification, that it was to be administered monthly (Third Representation);
(d) that in accordance with approved dosages, all Eylea patients receive fewer injections than all Lucentis patients (Fourth Representation).
In its reply submissions, Novartis said that the heart of the case centred on the Fourth Representation.
8 Bayer denies that the representations were conveyed but admits that, if they were, each is false — that is, an incorrect statement of fact — but denies that they were misleading or deceptive or likely to mislead or deceive.
9 Novartis claims that Bayer breached ss 18, 29(1)(a) and/or 29(1)(g) of the Australian Consumer Law, which sections were as follows:
18 Misleading or deceptive conduct
(1) A person must not, in trade or commerce, engage in conduct that is misleading or deceptive or is likely to mislead or deceive.
(2) Nothing in Part 3-1 (which is about unfair practices) limits by implication subsection (1).
Note: For rules relating to representations as to the country of origin of goods, see Part 5-3.
…
29 False or misleading representations about goods or services
(1) A person must not, in trade or commerce, in connection with the supply or possible supply of goods or services or in connection with the promotion by any means of the supply or use of goods or services:
(a) make a false or misleading representation that goods are of a particular standard, quality, value, grade, composition, style or model or have had a particular history or particular previous use; or
…
(g) make a false or misleading representation that goods or services have sponsorship, approval, performance characteristics, accessories, uses or benefits; …
…
Section 29 was in Part 3‑1—Unfair practices: see s 18(2) above.
10 By way of example, the content of one of Bayer’s acts of publication, by baby banner (second form), was:

11 The central issues for decision are as follows:
(i) Were the four pleaded representations conveyed by the acts of Bayer? As I have said, Novartis pleaded approximately 46 acts of publication and detailing conduct, occurring on various dates up to mid-May 2013, but with one further publication in June 2013;
(ii) If so, was that conduct misleading or deceptive or likely to mislead or deceive, or false or misleading?
(iii) If so, what is the appropriate remedy, if any? In particular, has Novartis proved that it suffered loss or damage because of Bayer’s conduct and, if so, in what amount, and is other relief, in the form of declarations, injunctions and corrective advertising, appropriate?
12 It is necessary to approach these issues with an appreciation of the context, in particular the regulatory context, and the identification of the relevant class of addressees and the education, training, experience and state of knowledge of a (hypothetical) reasonable member of that class.
The words used and the acts of publication
13 The acts of publication were alleged to have been by poster, which was a large banner designed to stand on the floor, being approximately 2.13 m wide and 2.19 m high; by baby banner which was smaller than a poster, say for a desk top, being approximately 40 cm wide and 95 cm high; by detailing conduct which meant the detailing, i.e. communications by Bayer company representatives with ophthalmologists and/or optometrists, during which the ophthalmologists and/or optometrists were shown Detail Aids (on an iPad), which included words in terms substantially similar to those otherwise complained of by the applicant Novartis; advertisements in the journal Clinical & Experimental Ophthalmology and in mivision magazine; and by displaying and disseminating an attendee workbook at the Eylea product launch events in Sydney, Melbourne, Brisbane and Adelaide. Bayer accepts that it published magazine advertisements, published and had on display certain posters and baby banners at meetings and events, and that its sales representatives engaged in certain detailing conduct with an electronic document on an iPad. It also accepts that it provided the attendee workbook.
14 The magazine mivision is a monthly industry publication which is provided as a free subscription to ophthalmologists and optometrists and their practices in Australia and New Zealand 11 times a year on the first Monday of every month (except for January). In September 2012 the audited distribution for the magazine was 7265, made up of optometrists and optical shops (3793 recipients), ophthalmologists (1176 recipients) and ophthalmologists’ practices and staff (216 recipients). The journal Clinical & Experimental Ophthalmology is a clinical journal produced by the Royal Australian and New Zealand College of Ophthalmologists (RANZCO) and distributed to RANZCO members, ophthalmologists, as part of their membership.
15 I next set out the words used of which complaint is made.
16 It is alleged that, by displaying a poster, Bayer published, amongst other words, the following words:
“EYLEA® HELPS YOU AND YOUR
PATIENTS WITH FEWER INJECTIONS
WHEN USED ACCORDING TO THE APPROVED
DOSAGE COMPARED TO MONTHLY RANIBIZUMAB 1, 2
…
References: 1. EYLEA® Product Information. 2. LUCENTIS® Product Information.”
17 This was the form for the poster (first form), the baby banner (first form), the Detail Aid and the attendee workbook.
18 It is next alleged that Bayer caused advertisements to be published in the same form.
19 This was the form used for the advertisements (first form).
20 Another form (second form) is alleged as follows:
“EYLEA® FOR YOU AND YOUR PATIENTS
WITH FEWER INJECTIONS WHEN USED
ACCORDING TO THE APPROVED DOSAGE
COMPARED TO MONTHLY LUCENTIS®1, 2
…
References: 1. EYLEA Product Information. 2. LUCENTIS Product Information.”
21 This was the form for the poster (second form), the baby banner (second form) (see [10] above) and the advertisements (second form).
22 The references to first form are to “Eylea helps …” (larger font) “when used …” (smaller font), and the references to second form are to “Eylea helps/for you and your patients …”, “when used … ” (same font).
23 Next I set out in chronological order the numbered acts of publication contended for by the applicant.
Date | Form of publication and event | |
1. | 28 July 2012 | Baby banner (first form) displayed by Bayer at the Sydney Eye Hospital Alumni Associate’s Ninth Biennial Conference held at the Sofitel Wentworth, Sydney |
2. | 3, 4 August 2012 | Poster (first form) displayed at Bayer’s trade display booth at a meeting of the Queensland branch of RANZCO |
3. | 6 August 2012 | Baby banner (first form) displayed by Bayer at an educational meeting held at Hobart Eye Surgeons, Hobart |
4. | September 2012 | Advertisement (first form) in Volume 40, Issue 7 of the journal Clinical & Experimental Ophthalmology |
5. | October 2012 | Advertisement (first form) in Issue 73 of mivision magazine |
6. | 24 October 2012 | Attendee workbook (first form) displayed and disseminated at the Eylea product launch hosted by Bayer at Four Points by Sheraton, Sydney |
7. | 24 October 2012 | Poster (first form) displayed at Eylea product launch at Four Points by Sheraton, Sydney (respondent denies and says it displayed the baby banner (first form)) |
8. | 24 October 2012 | Attendee workbook (first form) displayed and disseminated at the Eylea product launch hosted by Bayer at the Sebel & Citigate Albert Park, Melbourne |
9. | 24 October 2012 | Attendee workbook (first form) displayed and disseminated at the Eylea product launch hosted by Bayer at the Sebel & Citigate King George Square, Brisbane |
10. | 24 October 2012 | Attendee workbook (first form) displayed and disseminated at the Eylea product launch hosted by Bayer at Hotel Grand Chancellor on Hindley, Adelaide |
11. | 24 October 2012 | Baby banner (first form) displayed by Bayer at the launch event at the Sebel & Citigate Albert Park, Melbourne |
12. | 24 October 2012 | Baby banner (first form) displayed by Bayer at the Sebel & Citigate King George Square, Brisbane (not admitted by respondent) |
13. | 27 October 2012 | Baby banner (first form) displayed by Bayer at a meeting attended by ophthalmologists at the Hotel Grand Chancellor, Hobart |
14. | November 2012 | Advertisement (first form) in Volume 40, Issue 8 of the journal Clinical & Experimental Ophthalmology |
15. | November 2012 | Advertisement (first form) in Issue 74 of mivision magazine |
16. | 24 to 28 November 2012 | Poster (second form) displayed at Bayer’s trade display booth at a national meeting of RANZCO |
17. | December 2012 | Advertisement (second form) in Issue 75 of mivision magazine |
18. | December 2012 | Advertisement (first form) in Volume 40, Supplement S1 of the journal Clinical & Experimental Ophthalmology |
19. | December 2012 | Advertisement (second form) in Volume 40, Issue 9 of the journal Clinical & Experimental Ophthalmology |
20. | January 2013 | Advertisement (second form) in Volume 41, Issue 1 of the journal Clinical & Experimental Ophthalmology |
21. | 9, 10 January 2013 | Baby banner (second form) displayed by Bayer at a training conference for registrar ophthalmologists held at the Sydney Eye Hospital |
22. | February 2013 | Advertisement (second form) in Issue 76 of mivision magazine |
23. | 1, 2 February 2013 | Baby banner (second form) displayed by Bayer at a meeting of the Tasmanian branch of RANZCO held at the Henry Jones Art Hotel, Hobart (not admitted by respondent) |
24. | 12 February 2013 | Baby banner (second form) displayed by Bayer at an educational event held at the Lions Eye Institute, Nedlands, Western Australia |
25. | 26 February 2013 | Baby banner (second form) displayed by Bayer at an optometrists’ referral meeting held at venue of the Victorian Eye Surgeons, Paisley Street, Footscray |
26. | March 2013 | Advertisement (second form) in Issue 77 of mivision magazine |
27. | 5 March 2013 | Baby banner (second form) displayed by Bayer at a referral meeting held at the Empire Grill, Geelong |
28. | 22, 23 March 2013 | Poster (second form) displayed by Bayer at its trade display booth at a meeting of the New South Wales branch of RANZCO |
29. | April 2013 | Baby banner (second form) displayed by Bayer at a Luxottica educational meeting attended by optometrists held at the Novotel in Darling Harbour, Sydney (not admitted by respondent) |
30. | April 2013 | Advertisement (second form) in Volume 41, Issue 3 of the journal Clinical & Experimental Ophthalmology |
31. | April 2013 | Advertisement (second form) in Issue 78 of mivision magazine |
32. | 3 April 2013 | Baby banner (second form) displayed by Bayer at an educational meeting for Luxottica optometrists held at the Mercure Hotel, Perth |
33. | 5-7 April 2013 | Poster (second form) displayed at Bayer’s trade display booth at the Australian Vision Convention, a meeting of the Optometrists’ Association of Australia held on the Gold Coast |
34. | 10 April 2013 | Baby banner (second form) displayed by Bayer at an optometrist referral meeting held at the Norwest Eye Clinic, Norwest, NSW |
35. | 10 April 2013 | Baby banner (second form) displayed by Bayer at an educational meeting attended by optometrists hosted by Outlook Eye Specialists held at the Vibe Hotel, Surfers Paradise |
36. | 10 April 2013 | Baby banner (second form) displayed by Bayer at an educational meeting for Luxottica optometrists held at the Amora Hotel, Richmond, Melbourne (not admitted by respondent) |
37. | 16 April 2013 | Baby banner (second form) displayed by Bayer at an educational meeting for Luxottica optometrists held at the Mercure Hotel, Geelong |
38. | 17 April 2013 | Baby banner (second form) displayed by Bayer at an educational meeting attended by optometrists held by Luxottica at the Old Woolstore Hotel, Hobart, Tasmania |
39. | 26 April 2013 | Baby banner (second form) displayed by Bayer at a referral meeting attended by optometrists held at the consulting rooms of the Northern Eye Consultants, Victoria |
40. | 29 April 2013 | Baby banner (second form) displayed by Bayer at an educational meeting attended by optometrists held by Luxottica at the Lion Hotel, North Adelaide |
41. | 30 April 2013 | Baby banner (second form) displayed by Bayer at a retinal meeting held at the University Club at the Crawley campus of the University of Western Australia |
42. | May 2013 | Advertisement (second form) in Issue 79 of mivision magazine |
43. | 3 May 2013 | Baby banner (second form) displayed by Bayer at an educational meeting attended by optometrists hosted by Central Queensland Eye held at the Mercure Hotel, Barney Point, Queensland |
44. | 7 May 2013 | Baby banner (second form) displayed by Bayer at an optometrist referral meeting held at the Metwest Eye Clinic Blacktown, NSW |
45. | 14 May 2013 | Baby banner (second form) displayed by Bayer at an educational meeting attended by optometrists held by Luxottica at the Mantra in Chatswood, NSW |
46. | June 2013 | Baby banner (second form) displayed by Bayer at a Luxottica educational meeting attended by optometrists held at the Mantra Hotel in the Australian Capital Territory (not admitted by respondent) |
Various Dates (Novartis) Between May 2012 and May 2013 (Bayer) | Detailing Conduct (first form) |
24 I note that the first form ceased to be published at or about the time Eylea was listed on the Pharmaceutical Benefits Scheme (PBS) and therefore became, practically, available for prescription. Novartis submitted that the first form applied at the critical time leading up to and through the national product launch, when minds were being won. On or about 15 May 2013, Bayer gave an undertaking that it would cease publishing or otherwise communicating the promotional material on a without admissions basis.
25 I consider at [227]–[233] below the disputed acts of publication.
The regulatory context
26 Under s 25(4) of the Therapeutic Goods Act 1989 (Cth) (the TGA), as in force at the relevant time, the Secretary had a discretion to register therapeutic goods after an evaluation under s 25. If the Secretary decided so to register the goods and they were “restricted medicine” as defined, he or she was required to approve product information in relation to the medicine. Section 3(1) of the TGA defined “product information” in relation to therapeutic goods to mean: “information relating to the safe and effective use of the goods, including information regarding the usefulness and limitations of the goods.”
27 Under s 7D(1) of the TGA, a form was approved for providing product information to accompany the application for registration in accordance with s 23(2)(ba) of the TGA. That form required product information set out under certain specified headings including clinical trials and dosage and administration.
28 Under the Therapeutic Goods Regulations 1990 (Cth) (the TG Regulations), by reg 9A the sponsor of therapeutic goods (specified in Part 1 of Schedule 10) must not supply the goods if the sponsor does not supply with the goods written information about the goods that meets the requirements for a patient information document set out in Schedule 12 to the TG Regulations. By reg 9A(2), information must be provided in the primary pack in which the therapeutic goods are supplied or in another manner that will enable the information to be given to a person to whom the goods are administered or otherwise dispensed.
29 Schedule 12 to the TG Regulations provided, so far as relevant:
A patient information document about a medicinal product must be:
…
• consistent with product information about the product.
A patient information document must include the following:
1. Identification
The name of the medicinal product, which is the name given to the product by the sponsor, including or followed by the non-proprietary name(s) of the active ingredient(s) and the dosage form or strength, or both, of the product.
A statement of the active ingredients expressed quantitatively and excipients expressed qualitatively, using their common names, in the case of each presentation of the product.
The pharmaceutical form and the contents by weight, volume or number of doses of the product, in the case of each presentation of the product, together with its identifying Australian Register number.
2. What the product is used for and how it works
The therapeutic indications, unless a competent authority determines that dissemination of such information may have serious disadvantages for the patient.
The pharmaco-therapeutic group, or type of activity, if there is a term that is easily comprehensible for the patient. If not, a simple description of what the medicinal product is for and how it works, in 1 or 2 sentences.
3. Advice before using the medicinal product
A list of factors that are useful to consider before taking the medicinal product, including, if appropriate:
• contraindications, including consideration of whether the patient has experienced previous allergic reactions
• precautions for use, taking into account the particular condition of certain categories of users, such as the elderly, children, infants, pregnant or breastfeeding women, persons with specific pathological conditions
• potential effects of the medicinal product on the ability to drive vehicles or to operate machinery
• interactions with other medicinal products or other forms of interaction (for example with alcohol, tobacco, foodstuffs) which may affect the action of the product
• special warnings, such as effects on sensitivity to sun exposure.
4. How to use the medicinal product properly
The necessary and usual instructions for proper use of the medicinal product, in particular:
• the dosage, together with an indication that this may not always apply and may be modified by the prescriber
• the method and, if necessary, route of administration
• the frequency of administration, specifying, if necessary, the appropriate time at which the medicinal product should or must be used
In addition, depending upon the nature of the therapeutic goods:
• the duration of treatment, if it should be limited
• the expected effect of using the medicinal product
• what to do if 1 or more doses have not been taken
• the way the treatment should be stopped, if stopping the treatment may lead to withdrawal or other adverse effects. …
30 In order for a medicine to be listed on the PBS, the company must lodge a submission with the Pharmaceutical Benefits Advisory Committee (PBAC) in accordance with the PBAC Guidelines: Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee. The Guidelines applicable at the relevant time ran for nearly 300 pages. The overview of a major submission required: section A to establish the context for the submission, including a description of the proposed drug, its intended use on the PBS, and the therapies that would be co-administered or substituted (the therapy likely to be most replaced by prescribers in practice being the “main comparator”); section B to provide the best available evidence comparing the clinical performance of the proposed drug with that of the main comparator, preferably from direct randomised trials, and details to be provided about the trials, the section concluding with a comparative therapeutic assessment of the proposed drug; section C to describe the methods used in pre-modelling studies to translate the results of the evaluation of the clinical studies to the context of the requested listing; section D to provide an economic evaluation focused on changes in health outcomes and in the provision of healthcare resources due to the proposed drug; section E to include financial analyses for the PBS/Repatriation Pharmaceutical Benefits Scheme and government health budgets; and section F (optional) to present any additional information of relevance to the major submission.
31 In the PBS Schedule the “dispensed price for maximum quantity” (the amount the Commonwealth government pays for the medicine so as to make it available at a government-subsidised price) was $1431.37 for Eylea and also for Lucentis. From 1 January 2013, the co-payment amount by a patient was $36.10 or, if the patient had a pensioner concession card, $5.90.
32 The Medicines Australia Code of Conduct edition 16 came into effect on 1 January 2010 (the Code). It stated it should be viewed as the minimum set of standards required to promote prescription products in Australia and did not in any way prohibit more stringent and comprehensive requirements being applied by individual companies. The Code complements the requirements of the TGA and the TG Regulations. Both Bayer and Novartis were members of Medicines Australia.
33 It was common ground that under the law in Australia it was illegal to advertise prescription-only medicines to the general public.
34 As I have said, Lucentis has been available for sale in Australia since 2007. It was approved by the Therapeutic Goods Administration and was registered on the Australian Register of Therapeutic Goods in February 2007. From August 2007, Lucentis was listed on the PBS Schedule.
35 The Product Information for Lucentis contained the following:
Treatment of Wet AMD
The recommended dose of Lucentis is 0.5 mg (0.05 mL) or 0.3 mg (0.03 mL) given as a single intravitreal injection.
Lucentis is given monthly. The interval between two doses should not be shorter than 1 month. Although less effective, treatment might be reduced to one injection every 3 months after the first three injections (e.g. if monthly injections are not feasible) but, compared to continued monthly doses, dosing every 3 months may lead to an approximate 5-letter (1-line) loss of visual acuity benefit, on average, over the following nine months. Patients should be evaluated regularly.
36 The Consumer Medicine Information for Lucentis stated:
The usual dose is 0.05mL or 0.03mL
(equivalent to 0.5mg or 0.3mg). The
interval between two doses should
not be shorter than one month.
If you are treated for wet age-related
macular degeneration, the injection is
given once a month. If given less
frequently, the full benefit may not
be obtained or benefits already
obtained might be lost (that is your
vision might be less sharp or even).
37 On 7 March 2012, Eylea was registered on the Australian Register of Therapeutic Goods.
38 Between March and April 2012, Bayer sales representatives attended an 8 week training course including training in relation to anatomy, macular degeneration and wet AMD treatment options. On 23 March 2012, there was a “Marketing strategy & activities 2012” presentation to Bayer sales representatives.
39 Between May 2012 and May 2013, there was detailing conduct.
40 Eylea was launched on 24 October 2012.
41 In December 2012, VIEW 1 and 2 year 1 trial results were published in Heier et al, ‘Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration’, Ophthalmology 2012; 119: 2537–2548. The summary objective, results and conclusions were stated as follows:
Objective: Two similarly designed, phase-3 studies (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD [VIEW 1, VIEW 2]) of neovascular age-related macular degeneration (AMD) compared monthly and every-2-month dosing of intravitreal aflibercept injection (VEGF Trap-Eye; Regeneron, Tarrytown, NY, and Bayer HealthCare, Berlin, Germany) with monthly ranibizumab.
…
Results: All aflibercept groups were noninferior and clinically equivalent to monthly ranibizumab for the primary end point (the 2q4, 0.5q4, and 2q8 regimens were 95.1%, 95.9%, and 95.1%, respectively, for VIEW 1, and 95.6%, 96.3%, and 95.6%, respectively, for VIEW 2, whereas monthly ranibizumab was 94.4% in both studies). In a prespecified integrated analysis of the 2 studies, all aflibercept regimens were within 0.5 letters of the reference ranibizumab for mean change in BCVA; all aflibercept regimens also produced similar improvements in anatomic measures. Ocular and systemic adverse events were similar across treatment groups.
Conclusions: Intravitreal aflibercept dosed monthly or every 2 months after 3 initial monthly doses produced similar efficacy and safety outcomes as monthly ranibizumab. These studies demonstrate that aflibercept is an effective treatment for AMD, with the every-2-month regimen offering the potential to reduce the risk from monthly intravitreal injections and the burden of monthly monitoring.
42 On 1 December 2012, Eylea was listed on the PBS Schedule.
43 The product information for Eylea contained the following:
Pharmacodynamic effects
…
In patients treated with EYLEA (one injection per month for three consecutive months, followed by one injection every 2 months), retinal thickness decreased soon after treatment initiation, and the mean CNV lesion size was reduced, consistent with the results seen with ranibizumab 0.5 mg every month.
Dosage regimen
The injection volume is 50 μL of EYLEA (equivalent to 2 mg aflibercept).
EYLEA treatment is initiated with one injection per month for three consecutive months, followed by one injection every two months. (See CLINICAL TRIALS for dosing experience).
The clinical trials referred to were the VIEW 1 and VIEW 2 studies, which were summarised at pages 5 to 8 of the Eylea product information.
44 The Consumer Medicine Information for Eylea contained the following:
The recommended dose of EYLEA
is 50 μL (microlitre).
The interval between two doses
should not be shorter than one
month.
If you are being treated for wet
AMD:
The injection is given once a month
for the first 3 months followed by
one injection every 2 months.
The witnesses
45 The applicant relied on the evidence of the following witnesses:
(a) three affidavits of Ms Sweta Ghelani, affirmed 2 May 2013, 24 July 2013 and 30 August 2013;
(b) two affidavits of Dr Benjamin Waterhouse, sworn 14 August 2013 and 13 March 2014;
(c) two affidavits of Mr Paul Hodgkinson, sworn 23 August 2013 and 14 March 2014;
(d) four affidavits of Mr David Moore, health economist, affirmed 26 August 2013, 10 January 2014, 17 March 2014 and 12 April 2014; and
(e) three affidavits of Mr Tony Samuel, chartered accountant, affirmed 11 September 2013, 23 December 2013 and 17 March 2014.
46 The respondent relied on the evidence of the following witnesses:
(a) an affidavit of Dr Allan Ared, optometrist, affirmed 28 June 2013;
(b) two affidavits of Dr Paul Mitchell, retinal ophthalmologist, affirmed 28 June 2013 and 3 March 2014, the latter being supplemented by a report in the form of a letter to the respondent’s solicitors dated 10 April 2014;
(c) two affidavits of Dr Andrew Crawford, ophthalmologist, affirmed 28 June 2013 and 21 February 2014, and a report in the form of a letter dated 24 March 2014;
(d) an affidavit of Dr Jan Twomey, affirmed 8 November 2013;
(e) an affidavit of Ms Emel Elibol, affirmed 8 November 2013;
(f) an affidavit of Ms Radmila Grabovac, sworn 8 November 2013;
(g) an affidavit of Ms Naomi Leonard, sworn 8 November 2013;
(h) an affidavit of Ms Michele Doyle, sworn 8 November 2013;
(i) an affidavit of Mr John Burstow, sworn 8 November 2013;
(j) an affidavit of Mr Larry Hodges, affirmed 8 November 2013;
(k) an affidavit of Ms Cecilia Martinek, sworn 8 November 2013;
(l) two affidavits of Ms Susan Adams, affirmed 8 November 2013 and 19 December 2013;
(m) an affidavit of Ms Alena Reznichenko, affirmed 8 November 2013;
(n) an affidavit of Mr Andy Kuo, sworn 19 November 2013;
(o) an affidavit of Dr Philip Williams, economist, sworn 17 February 2014; and
(p) an affidavit of Mr Terence Potter, chartered accountant, sworn 14 February 2014.
47 Ms Ghelani was the marketing manager (wAMD) for Lucentis and had held that position since September 2012. In that role she was responsible for the oversight and coordination of the Lucentis marketing team. In July 2011, she began working as a member of the Lucentis cross-functional team in the role of Senior Brand Manager with her main responsibility being to optimise the commercial potential of Lucentis, including the development of marketing plans and sales material. In her affidavit of 24 July 2013, Ms Ghelani gave more detailed evidence of her experience as a pharmacist and in the marketing of pharmaceutical products. She has a Masters of Business (specialising in Marketing) from RMIT University, which she obtained in 2003. She said that as of April 2012, the International Council of Ophthalmology estimated on its website that there were approximately 763 ophthalmologists in Australia.
48 Much of her evidence was not controversial.
49 She gave her understanding of Lucentis as an anti-vascular endothelial growth factor (anti-VEGF) therapy and said that it was the only such therapy available for sale in Australia during the period 2007 to 2012. During the period 2007 to November 2012, Lucentis was the only anti-VEGF product available for sale in Australia which was registered on the Australian Register of Therapeutic Goods, listed on the PBS, and administered through intra-vitreal injection.
50 Ms Ghelani gave the monthly ex-factory sales of Lucentis by unit for the period January to November 2012. She then gave the sales figures for Lucentis for the period December 2012 to April 2013 following the launch of Eylea. It was in October 2012 that the Minister for Health announced that the proposed PBS listing for Eylea would be amended to allow the switching of existing Lucentis patients to Eylea. She also set out a table showing the relative market share (as measured by sales from wholesalers to pharmacies) for Lucentis and Eylea determined by reference to the data contained in a report by IMS, a company which provided industry market data. The figures for the monthly ex-factory sales of Lucentis by unit were substantially lower following the launch of Eylea and so was the share of the age-related macular degeneration (AMD) market in Australia held by Lucentis. A comparison of the monthly ex-factory sales of Lucentis by unit (vials) was 17,000 units in April 2012 against 8574 units in April 2013, and the market share for Lucentis in October 2012 was 99.9% (19,136 units) against 58.3% (9916 units) in February 2013, with Eylea having a market share in February 2013 of 41.7% (7090 units).
51 In her third affidavit, Ms Ghelani deposed that optometrists may detect evidence of wet AMD during a routine eye examination, and a patient who was having difficulty with their vision will often present to an optometrist, but optometrists may not diagnose or treat the condition. Consequently, they refer their patients to ophthalmologists who may make a diagnosis and then treat wet AMD, sometimes with intra-vitreal anti-VEGF injections such as Lucentis or Eylea. Both the Lucentis product information and the Eylea product information stated that the medicine must only be administered by a qualified ophthalmologist or physician experienced in administering intra-vitreal injections. The relevant entries on the PBS Schedule required that the intra-vitreal injections be administered by an ophthalmologist in order for reimbursement to be made. Consequently, Novartis focused its advertising, promotion and visits to optometrists on education in relation to identification of wet AMD, so as to facilitate diagnosis, and the importance of early referral to an ophthalmologist for definitive diagnosis and treatment as appropriate. For ophthalmologists, Novartis focused more directly on Lucentis and the science surrounding its features and would delve more deeply into the scientific literature in that regard.
52 Ms Ghelani deposed that after the initial scientific studies which established the safety and efficacy of Lucentis, many of the subsequent studies had attempted to determine whether, and the extent to which, the interval between injections of Lucentis could be extended beyond monthly.
53 In her oral evidence, Ms Ghelani said that most clinicians would know about the clinical trials in the product information document. She was speaking specifically of the ANCHOR and MARINA studies referred to in the Lucentis product information. She agreed that the exchange of scientific information with the clinician occurred very frequently. Ms Ghelani agreed that, in her perception, the ophthalmologists and optometrists she was visiting for Lucentis were aware in 2011 of Eylea on the horizon and that they were aware of clinical studies for Eylea either taking place or being reported as a precursor to the PBS launch of Eylea. Her understanding was that ophthalmologists and optometrists had their own sources of information about the coming of the Eylea product, including the obtaining by them of clinical study material and other scientific papers about aflibercept and its qualities. Before the first publication in issue in the proceedings, before July 2012, her understanding was that many ophthalmologists and optometrists already had a great deal of detailed information about the qualities of Eylea that was coming to market.
54 Ms Ghelani also agreed that Novartis’ marketing for Lucentis very regularly adopted the practice of footnoting a claim and the footnotes referred to product information documents as well as other clinical trials. She said whatever the claim, it must be supported by a form of evidence and it was usually either a clinical paper or the product information. It was her understanding that ophthalmologists and optometrists understood the procedure of footnoting product information documents.
55 More generally in relation to the product information document, her working assumption in her role was that the marketing of Lucentis had as one of its main facts that it conveyed to ophthalmologists what the approved product information document said about dosage and administration.
56 Ms Ghelani was taken in cross-examination to a Novartis marketing document prepared some time in the period April to July 2011 which contained the statement “A MONTHLY REGIMEN OF LUCENTIS DEMONSTRATED THE BEST VA OUTCOMES IN THE CLINICAL TRIALS”, with a footnote reference to a study published in 2009. She accepted that it was her understanding that the intention of the statement was to encourage a monthly regimen of Lucentis because it gave the best results and that if doctors took up the suggestion of a monthly regimen it would be good for sales as patients who are dosed monthly consume more vials and are materially more profitable to Novartis than patients who have intra-vitreal injections less frequently.
57 Ms Ghelani agreed that at the conferences there was a variety of information, including clinical studies and product information, which was available to conference delegates and that material was distributed as ophthalmologists or optometrists expressed an interest in it or they would sometimes pick it up. It was standard practice to have such material for people to pick up. As part of the Code, the product information must be available on the stand and the sponsor may also have available a clinical paper.
58 Ms Ghelani was taken to a Novartis document dated February 2013 referring to most clinics where clinicians practice (centres) having trialled aflibercept (at different rates), “mainly switching existing LUC[entis] patients not responding and/or patients who cannot be extended out further than 4–5 weeks [with or without] fluid on the retina”. Ms Ghelani accepted that in this analysis in the summary, the point was being made that two reasons were put forward for clinics switching existing Lucentis patients. She added there was a third reason given being extending out further than four or five weeks. She said that: “[i]n some cases as the extension occurs, sometimes the fluid returns. But in some cases clinicians may have wanted to extend the patient out further for other reasons, such as where they lived, or impact on carers to bring them into the clinic. So there potentially are other reasons other than purely efficacy.” She added it may be fluid was one of the reasons that the clinician did not want to extend the patients out but it may also be that the clinician just wished to extend the patient out further if they could and the clinician would have a clinical discussion with the patient around: “you might not get as great vision, but we’re going to extend you out to eight weeks because we know that you are coming from Dubbo into the clinic, and that’s a long way for someone to come for an injection in the eyeball.” She said her understanding of the reason an ophthalmologist would regard an existing Lucentis patient as not being able to be extended, and therefore should be switched, was that it could be the visual acuity outcomes were not as great or it could be fluid. She said that she knew that ‘treat and extend’ was the most predominant regimen in Australia and that had been verified by a number of quantitative sources of data as well, but accepted that she had not addressed that question or brought forward material to support it in her affidavits. In re-examination, she said that the Commonwealth’s drug utilisation sub committee in 2012 showed that in the first year, Lucentis patients were on 7.42 injections, which, she said, clearly indicated that those patients were not being dosed monthly. She also referred in re-examination to a touch responder pad of about 100 retinal specialists at a weekend conference, held usually in June of most years. Their response was that around 80% of clinicians used treat and extend, 10% use a PRN (i.e. as required) and the balance would be monthly.
59 She agreed that the marketing document attributed the switching as mainly being for the two reasons of non-responsiveness of Lucentis patients to Lucentis, on the one hand, and those who could not be extended without fluid on the retina on the other. She added another reason as well, being just simply not being able to extend the patient out. The same document set out qualitative feedback from the top ten prescribing doctors and in the headline stated that usage of aflibercept was mostly in non-responders and patients on four weekly Lucentis. Ms Ghelani said there were varying definitions as to what constituted a non-responder. She agreed that the reasons that Novartis was provided at the time by these clinicians were good clinical reasons for adopting Eylea as a treatment for wet AMD. The same document contained a statement that the sales of aflibercept for the first few months from December 2012 were identical to Lucentis at launch for the first few months from August 2007. The document also contained pages setting out “qualitative field insights and Novartis response”, being information that had become apparent to Novartis employees, predominantly the sales team, reporting back to Novartis. The document described three complaints and none of them was to the effect that Bayer’s advertising said of Lucentis that it was administered monthly for all patients. Ms Ghelani’s recollection was that there was no complaint at all about any reference to monthly Lucentis. In re-examination she was asked whether her thinking changed from the views there expressed and she said that what changed was that the switching did not just stop at patients at four weeks but patients at 5 to 11 plus weeks were being switched to Eylea and this was not anticipated: certainly people eight weeks and more were not anticipated by Novartis in its marketing assumptions. Novartis did not anticipate that clinicians would switch patients who had been extended and who were not having high volumes of injections.
60 Ms Ghelani was taken in cross-examination to a printout of the digital screens from the iPad sales aid deployed by sales and marketing representatives of Novartis in their meetings one-on-one with ophthalmologists and optometrists. She accepted that in Novartis’ promotion to ophthalmologists Novartis always put the particular mandatory statement as to the ANCHOR and MARINA trials, which involved Lucentis administered on a fixed monthly basis. The sales aid also included the statement “less than monthly dosing* can sustain VA improvements in some patients vs baseline at 12 months … *Lucentis® is given monthly. Although less effective, treatment might be reduced to one injection every three months after the first three injections (e.g. if monthly injections are not feasible).” Ms Ghelani did not accept that the iPad sales aid involved the regular frequent presentation to ophthalmologists of the proposition coming from Novartis that Lucentis was given monthly. She said she thought the page was about less than monthly dosing sustaining VA improvements but Novartis had “put the mandatory there because we want to be clear about what’s in our label”. She accepted that what she called “the mandatory” was something that appeared frequently and regularly in the sales aid iPad material that Novartis was providing to ophthalmologists in 2012 and 2013.
61 Ms Ghelani agreed that the Consumer Medicine Information for Lucentis included the words: “[i]f you are treated for wet age-related macular degeneration, the injection is given once a month. If given less frequently, the full benefit may not be obtained or benefits already obtained might be lost (that is your vision might be less sharp or even)” and was included in that Consumer Medicine Information at all times during 2012 and 2013.
62 Ms Ghelani was taken to a March 2013 report and accepted that, as there stated at that time, the uptake of Eylea in Australia was similar to Japan on the basis of comparing units and the uptake in Australia was similar to the United States on the basis of comparing patients. She accepted that the uptakes in Japan and in Australia in those initial months were similar and the uptakes in the United States and Australia were not dissimilar.
63 Subject to one exception, I accept Ms Ghelani’s evidence. In particular, I give weight to her understanding that most clinicians would know about the clinical trials in the product information document; that the exchange of scientific information with the clinicians occurred very frequently; her perception that the ophthalmologists and optometrists she was visiting for Lucentis were aware in 2011 of Eylea on the horizon and that they were aware of clinical studies for Eylea either taking place or being reported as a precursor to the PBS launch of Eylea; and her understanding that ophthalmologists and optometrists had their own sources of information about the coming of the Eylea product, including the obtaining by them of clinical study material and other scientific papers about aflibercept and its qualities, such that before 28 July 2012, the date of the first publication in issue in the proceedings, many ophthalmologists and optometrists already had a great deal of detailed information about the qualities of Eylea that was coming to market.
64 I also accept Ms Ghelani’s evidence as to the role of optometrists in relation to the detection of wet AMD, that is, that a patient having difficulty with their vision will often present to an optometrist, but optometrists may not diagnose or treat the condition. Consequently they refer their patients to ophthalmologists who may make a diagnosis and then treat wet AMD, sometimes with intra-vitreal anti-VEGF injections such as Lucentis or Eylea. This evidence is entirely consistent with that of Dr Ared, an optometrist called by the respondent. It establishes, and I so find, that any impact of the respondent’s conduct on optometrists is at best indirect because optometrists do not make any choice between Lucentis and Eylea. My finding is confirmed by Ms Ghelani’s evidence that Novartis focused its advertising, promotion and visits to optometrists on education in relation to identification of wet AMD, so as to facilitate diagnosis, and the importance of early referral to an ophthalmologist for definitive diagnosis and treatment as appropriate.
65 The exception to my acceptance of Ms Ghelani’s evidence, to which I have referred at [63] above, is that I do not accept that the Novartis sales aid was about less than monthly dosing sustaining VA improvements: in my opinion the statements in the sales aid should be taken as conveying, to those who read them, that the product information stated that Lucentis was given monthly and, although less effective, treatment might be reduced to one injection every three months after the first three injections (e.g. if monthly injections are not feasible).
66 Dr Jan Twomey was the medical director for Bayer Healthcare Pharmaceuticals, a division of Bayer, since August 2010. She graduated from medicine at Flinders University at the end of 1991. She had full general registration as a medical practitioner and had held this continuously since 1993. In 2004, she was appointed Acting Medical Director for the phase 1 clinical trials unit at GlaxoSmithKline Limited for a short period before holding the position of Associate Medical Director at Wyeth Limited, now part of Pfizer Limited. In August 2005, she was appointed Area Medical Director/Director of Global Clinical Operations for Australia and New Zealand at Schering Plough and held that position until February 2010. As Medical Director she was responsible for: Medical Affairs (which was responsible, amongst other things, for compliance review of all promotional material for pharmaceutical products); Medical Information (which was responsible for providing medical information requested by internal staff, healthcare professionals and consumers); Pharmacovigilance; Regulatory Affairs; and Clinical Operations.
67 Dr Twomey had ultimate autonomy and authority to review, reject and, if appropriate, approve marketing materials. She said it was her practice to approve marketing materials only if she was satisfied that they complied with all applicable Australian or New Zealand medical and compliance requirements, including the scientific veracity of any such campaigns. This involved ensuring such promotional materials met the relevant requirements set out in the Code and the TGA and the TG Regulations.
68 Dr Twomey said that clinical papers and academic articles formed an important part of the way that Bayer promoted prescription medicines, including Eylea, to doctors and educated doctors on the mechanism of action, efficacy and dosage strength and regimen for medicines. She said that part of the training of Bayer’s Eylea sales representatives included training given by Bayer’s medical affairs department on eye diseases and the structure and mechanism of action of Eylea, including the associated clinical trials and academic papers. As part of the training, sales representatives were instructed to take copies of clinical papers and academic articles to meetings with ophthalmologists or arrange for a copy to be sent to a doctor who asked for a paper or article the sales representative did not have to hand.
69 Dr Twomey listed a number of such clinical papers and academic articles which were referred to in the process of preparing the marketing materials for Eylea, including:
(a) VEGF-Trap: A VEGF blocker with potent antitumor effects, 99(17) Proceedings of the National Academy of Sciences of the United States of America (2002), August 2002, Holash et al (2002) (Holash et al);
(b) VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade, 104(47) Proceedings of the National Academy of Sciences of the United States of America (2007), November 2007, Rudge et al (Rudge et al);
(c) Predicted biological activity of intravitreal VEGF Trap, 92 British Journal of Ophthalmology (2008), March 2008, Stewart et al;
(d) VEGF Trap-Eye for Exudative AMD, Retinal Physician (2009), April 2009, Heier (Heier);
(e) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab, Angiogenesis (2012), February 2012, Papadopoulos et al (Papadopoulos et al);
(f) Intravitreal aflibercept injection for neovascular (wet) age-related macular degeneration, 13(4) Expert Opinion on Pharmacotherapy (2012), March 2012, Ohr et al (Ohr et al);
(g) Intravitreal aflibercept (VEGF Trap-Eye) in wet Age-related macular degeneration, 119(12) Ophthalmology (2012), December 2012, Heier et al (Heier et al, elsewhere referred to as the VIEW study);
(h) Different antivascular endothelial growth factor treatments and regimens and their outcomes in neovascular age-related macular degeneration: a literature review, 0:1-11 British Journal of Ophthalmology (2013), August 2013, Lanzetta et al (Lanzetta et al);
(i) Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration, 33(8) The Journal of Retinal and Vitreous Diseases (2013), September 2013, Kumar et al (Kumar et al); and
(j) Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration, American Journal of Ophthalmology (1 July 2013), Yonekawa et al (Yonekawa et al).
70 Dr Twomey said she was responsible for approving and overseeing the approval of the marketing and educational materials for the Eylea campaign. She said this included a wide range of materials. She summarised examples of some of those materials, all of which were approved either by her or by staff within the medical department acting under her supervision. Her summary included: the first Detail Aid (from about May 2012 to around late June 2013); the second Detail Aid (from or around late June 2013); a document titled ‘Attendee Workbook’ that was to be made available for ophthalmologists attending the Eylea product launches in Sydney, Melbourne, Brisbane and South Australia on 24 October 2012; various speeches and presentations made at the Eylea product launches on 24 October 2012; and a large pull-up banner for use by the territory managers. I have reproduced this banner at [110] below.
71 Dr Twomey gave her understanding of the term ‘me too’. She said it referred to a medicine that was a copy of an existing drug with only minimal differences — i.e. structurally very similar, with a very similar mechanism of action, dosing regimen, method of administration and therapeutic effect to the existing medicine. In particular, it offered no real clinical advantage or disadvantage over the existing medicine. She said that Eylea was not a ‘me too’ medicine in relation to Lucentis, in particular: it had a different active ingredient; it had a different mechanism of action; and it had a different dosing regimen.
72 In cross-examination, Dr Twomey agreed that the clinical papers she had listed — set out at [69](g), (h), (i) and (j) above — post-dated the particular set of marketing materials she had referred to in her affidavit and so were not referred to or considered for the purposes of approving those marketing materials. She said she had identified those four papers because they had been used in ongoing preparation of marketing materials.
73 Dr Twomey was asked about the term ‘me too’. She agreed it was a term used in pharmaceuticals but did not have a technical or fixed meaning. She agreed that one would not describe a generic drug as a ‘me too’ drug: it would be called a generic drug. She agreed that ‘me too’ was an expression used to describe similar drugs, but minds could legitimately differ in terms of the spectrum of similarity. She said that some people might have a different interpretation of whether a drug is ‘me too’ or not and agreed that the core concept was similarity and the area for different opinions was on the extent of similarity.
74 Very little of Dr Twomey’s affidavit evidence was challenged in cross-examination. There was some question as to how much of the materials for the Eylea campaign Dr Twomey approved or was responsible for approving. In her affidavit, at [39], she said she was responsible for approving and overseeing the approval of the marketing and educational materials for the Eylea campaign and said that she summarised examples in the following paragraphs. But the details of her approval or responsibility for or oversight of the approval of most of the material at issue in these proceedings was not tested. Her evidence was given in a matter of fact and responsive manner. I accept Dr Twomey’s evidence. In particular, I find that the emphasis of the training of Bayer’s sales representatives given by Bayer’s medical affairs department was on eye diseases and the structure and mechanism of action of Eylea, including the associated clinical trials and academic papers. I also find that Eylea was not a ‘me too’ drug in the sense that it was not relevantly the same as Lucentis: it had a different active ingredient; it had a different mechanism of action; and it had a different standard dosing regimen.
The Bayer sales representatives
75 I come now to the written and oral evidence of the sales representatives called by Bayer.
76 The first Bayer sales representative to give oral evidence was Ms Radmila Grabovac, the territory manager for the city of Melbourne and north-west Victoria. She had tertiary qualifications in business marketing. There were approximately 140 ophthalmologists in her territory and of those about 45 were injectors. She was given extensive product training on Eylea, and on anatomy and macular degeneration. She also gave evidence that she read textbooks on her own as well as having face-to-face training. She spent most of April and May 2012 in training including a few weeks in Sydney, training as a team with other territory managers. She also received training on how to use a presentation about Eylea on her iPad (Detail Aid). She said that she was aware that the proceedings involved a dispute about two pages of the Detail Aid (the disputed pages). She also referred to a database used by Bayer, called Cortex, to record sales calls. She said that whenever she made a sales call to an ophthalmologist or optometrist, she entered details of the call into Cortex. It was her practice to put in “the main crux” of her discussion with the doctor, anything interesting that they mentioned and the objective of the next call. She exhibited these notes to her affidavit. She said that she only used the Detail Aid in an arranged face-to-face meeting, called a “sit down call”, and then where she had the opportunity unless there was a time restriction or the ophthalmologist made it clear they were not interested in seeing it. She said that in the time between approximately May and September 2012 the only other information she had to use was the Eylea product information. After using the Detail Aid once or twice in the first or second call with a doctor, she did not generally use it again. Occasionally she used it to stress or explain a particular point in relation to clinical information or to answer administration questions on storage or vial size, for example. She estimated that the number of ophthalmologists to whom she showed the Detail Aid was between 63 and 72, but that figure may have been too high. She said it was rare for her to show the disputed pages to ophthalmologists, and an ophthalmologist would only have seen those slides by accident and in passing on one of the rare occasions when she opened the Detail Aid during the actual sales call instead of having it already open at the MOA (mode of action) tab where it was her practice to have the Detail Aid ready. She estimated that this would have happened with a maximum of 3 to 4 ophthalmologists. She did not think that any doctor would have seen only the disputed pages. Once the VIEW study clinical paper was published in December 2012/January 2013, she said it was her practice to build on the information about that study in the Detail Aid by referring to the actual VIEW study clinical paper and handing it out. She also handed out other clinical studies to ophthalmologists in sales calls. Those studies included: Ohr et al; Holash et al; Kumar et al; and Yonekawa et al.
77 In her oral evidence, Ms Grabovac said there was a national sales manager and there were eight representatives who reported to the national sales manager directly. Each of them worked solely in relation to the selling of Eylea. During her training she was not provided with a sales manual or any document identifying key selling messages and there were not key selling messages she took into the field. She was familiar with the phrase but said “we were not given key selling messages for the Eylea role”. She said she had a training manual that looked at anatomy, clinical papers, a lot of clinical information because “our role was to provide clinical information to doctors.” She disagreed that her role was to sell as many products as possible. She said there were no specific messages she was given to give the doctors regarding Eylea. The training was based around product information, the VIEW study, predominantly, and that was the main crux of the information that the sales representatives were giving to doctors. She denied that she had in mind when she met doctors in the field a key selling message or key selling messages in respect of Eylea. When taken to a reference to “key selling messages” in the notes of one of her sit down calls, on 10 May 2013, Ms Grabovac said “key selling messages” would have referred to reducing the burden for patients, which appeared in the VIEW study in several cases, reducing the burden for patients with less injections, and that would be supported with information, and perhaps she would have spoken about less visits and less chance of an adverse event, which was also highlighted and supported by the VIEW study and product information. She said she believed she would have spoken about key selling messages but there would have been a lot of other clinical information that was delivered and she believed that she spoke about the way the doctor treated and also the growth of his practice and how he managed patients. Ms Grabovac was next taken to another reference in her Cortex notes to key selling messages. She was asked what the key selling messages were and she said that to the best of her recollection “I would have assumed that it would be reducing — perhaps reducing the burden, which is also in the VIEW paper; providing, perhaps, less injections, as demonstrated in the VIEW study; perhaps less cost for the patient. And … Less visits … and less cost.” She agreed that the benefits she had identified all resulted from fewer injections. She agreed that one of her responsibilities was to grow sales. She also agreed that when she actually had a product, at the beginning of December 2012, she did have a sales target and one of her responsibilities was to achieve that sales target. She said she did not remember a clear marketing message being given based on anything outside the clinical information. She said she was told to discuss information that was in the product information and the VIEW paper because there was not a product for the first year. She denied that she had been seeking to convince ophthalmologists about the benefits of fewer injections of the product since at least May or June 2012. She said the discussion she had with ophthalmologists initially, from what she recalled, was information gathering, discussing the product information and developing relationships. She was taken to her Cortex notes for a sit down call on 3 July 2012 with an ophthalmologist. She agreed that what she did with that ophthalmologist, after discussing the MOA, efficacy and administration was to discuss the benefits of Eylea for patients and less injections. She agreed that what she was doing from July 2012, as one facet of what the role entailed, was laying the platform for future sales. She also agreed that one of the ways she sought to lay the platform was to impart the message of less injections. She said the comparison was with Lucentis, because in the VIEW study that was the comparison.
78 Ms Grabovac was next taken to a sit down sales call for an ophthalmologist on 21 May 2012. The notes recorded: “[w]ent through MOA, efficacy, admin and discuss benefits to the patient of less injections but with optimal treatment.” After first denying that she was seeking to convince ophthalmologists of the merits of Eylea over Lucentis, she accepted that that was one of the components of her discussions. She said she would never say to a doctor “Eylea helps you and your patients with fewer injections” without drawing reference to a clinical paper or product information because that was actually contrary to Medicines Australia. She agreed it was part of her task to talk about clinical equivalence based on the VIEW study because the VIEW study did demonstrate clinical non-inferiority to the current standard of treatment and it was equivalent in efficacy and safety.
79 Ms Grabovac said she called on optometrists on a couple of occasions only, although she went to lots of meetings where ophthalmologists were presenting to optometrists. She agreed that some optometrists wanted to know about treatment options for discussion with their patients. She also accepted that she spent time with an optometrist and she had a hope of a referral meeting. She said the reason there were referral meetings was not always about education of Eylea: it was relationship building and it was also educational support for doctors, ophthalmologists and their referrers. She accepted that she would be happy for optometrists to discuss the product because optometrists on occasion have close relationships with ophthalmologists.
80 Ms Grabovac was asked for her understanding on whether ophthalmologists were not in fact required to administer either Eylea or Lucentis in accordance with what was said about dosing in the product information. She was aware of the practice of treating other than on guidelines. She said this seemed to be the practice of therapy that was tailored specifically towards the needs of the patient in terms of interval. She needed to discuss Eylea treatment intervals on label which was what was approved in TGA guidelines. If the doctor chose to treat at different intervals, that was his or her choice. Some doctors did PRN, some did treat and extend, and some treated on label, whether it be monthly or two monthly. It was really a tailor-made approach for each patient based on the discussion and the evaluation the doctor would make with his or her patient. It was their choice how they treated their patient. Some had the discussion that they extended out over eight or 12 weeks. There was a range of practices.
81 In relation to the Detail Aid (iPad) Ms Grabovac’s evidence was that it was rare for her in sales calls to show the disputed pages (which were under the “Home” and “Summary” tabs). To the question whether the mode of action of Eylea was being explained to explain why there were fewer injections required with Eylea, Ms Grabovac answered “On occasion, yes”. She could not estimate the number of such occasions.
82 It is not a straightforward matter to evaluate Ms Grabovac’s evidence. Plainly she was nervous and uncomfortable giving evidence and volunteered that it was her first time in a court. Also she had some difficulties in understanding the more complex questions she was asked. On occasions she appeared reluctant to accept the obvious and was overly cautious in her answers. She was overly keen, in my view, to emphasise the informational aspects of her work rather than the selling of Eylea. But I find that she was truthful and I accept her evidence. In particular, I find that in her dealings with ophthalmologists and optometrists Ms Grabovac did not refer to fewer injections in the abstract but with reference to the product information, or a clinical paper, and the VIEW study paper once it became available. As to the disputed pages of the Detail Aid, I find that three or four ophthalmologists saw those pages in passing in the course of Ms Grabovac’s visits but in the context of the balance of the pages.
83 The second Bayer sales representative to give oral evidence was Ms Elibol. In her affidavit she said her sales territory was eastern Victoria. She replaced Ms Reznichenko as the Eylea territory manager for eastern Victoria when Ms Reznichenko was promoted. (Ms Reznichenko also gave evidence, which I consider below.) Ms Elibol said there were approximately 128 ophthalmologists in her territory and she shared approximately 15 of those with Ms Grabovac. She has a Bachelor of Science, a Diploma of Health — Medical Laboratory, and a Graduate Diploma in Business Systems. She said she had two weeks of training, including formal training from Bayer. She was shown how to use the Detail Aid on the iPad. From December 2012 to January 2013 she said the vast majority of her time spent with ophthalmologists and clinic support staff was spent answering logistical questions from doctors and clinic support staff such as: (a) How do I obtain Eylea? (b) How do I write a prescription for Eylea? (c) How many repeats do I need to prescribe? (d) How do I fill out the Medicare form? (d) What temperature is it stored at? and (e) What is Eylea’s shelflife/expiry date? Ms Elibol also annexed to her affidavit her Cortex notes. Her Cortex summary showed that she visited a total of 60 ophthalmologists and had at least one sit down call with each ophthalmologist during the period from 7 May 2012 to 21 June 2013. She said she did not recall using the Detail Aid at all during her first six months with Bayer from October 2012 to March 2013. She estimated she had used the Detail Aid approximately 3 to 4 times in sit down calls with ophthalmologists since March 2013. She did not recall ever using the “Summary” or “Home” tabs or the disputed pages within them. She said it was her practice only to use the Detail Aid in a reactive way, that is, if an ophthalmologist had a question. It was her practice to use the product information and clinical and academic papers about Eylea to answer any questions asked of her by ophthalmologists. She said she used the following clinical and academic papers as they became available: Holash et al; Ohr et al; Heier et al; Kumar et al; Yonekawa et al; and Lanzetta et al.
84 Ms Elibol estimated that approximately half the ophthalmologists in her territory injected for wet age-related macular degeneration. She said she did not conduct sales calls on optometrists and therefore did not show the Detail Aid to any optometrist.
85 In cross-examination, Ms Elibol was asked whether the statement “Eylea helps you and your patients with fewer injections” was the key marketing message for the sale of Eylea. She said she could not really answer that with a full yes or no because when she spoke about key messages she spoke about higher binding affinity, different molecule, that the eight weekly dose gave the same results as the four weekly dose. She said the key message of fewer injections with Eylea did not make sense to her. She said she would not discuss and say just there were fewer injections with Eylea. That needed an explanation. It depended entirely on the doctor and what the doctor was interested in: whether he or she wanted to know about the molecule, the mode of action, the trial design, the type of patients he was treating. The relevant transcript was as follows:
Q … So what you needed to do in order for ophthalmologists to consider switching over to your company’s product, EYLEA, was to point to some key difference in the products which he or she might find persuasive, a persuasive reason to change their existing practices. Do you agree with that?
A Because you mentioned key differences – I’ve pointed out key differences, because there has been patients that don’t respond to Lucentis that can respond to EYLEA because of difference in molecule design, difference in higher binding affinity, longer duration. So it’s - - -
Q Yes. Yes. Longer duration – can I ask you about longer duration? … If you pointed out longer duration, what you’re pointing out is that there would need to be fewer injections, because it lasts longer? Is that right?
A Yes, because it has got a higher binding affinity. It has got a lower dissociation constant … So once it binds, it doesn’t let go.
Q Yes. So did – can I just suggest to you - - -?
A But not in all patients. I’ve got to just – it’s not in all patients. It’s just what’s in the study…
Q But you did point out from time to time, can I suggest to you, to ophthalmologists, that this product requires fewer injections than Lucentis?
A Whenever I have pointed that out, it has been in reference to the VIEW trial or to the product information.
Q Yes. Yes. Yes. I’m just asking whether you did point that out?
A Yes. It’s in the trial – yes … It’s mentioned several times in the trial.
Q Yes. But you did point it out regularly, did you not, in your interactions with ophthalmologists, that EYLEA meant fewer injections for the ophthalmologist’s patients?
A Did I mention it regularly? … How would you define regularly? Sorry. Because I really concentrate on – I concentrate on my trial design, I talk about, again, the molecule design, it’s a human fusion protein, I – it’s level 1 evidence, the type of patients that were involved in the trial, how it might work for some patients where Lucentis doesn’t respond. So – I mean, I wouldn’t just go in there and just talk about fewer injections, fewer injections – it would have to come after a discussion.
Q Yes. And you’re not saying, are you, to his Honour that you never told a single ophthalmologist that EYLEA meant fewer injections – “Helps you and your patients with fewer injections”?
A Have I not – have I - - -
Q You’re not saying, are you, that you never said that to an ophthalmologist?
A No, I have said it.
…
Q Just focusing on the words, “EYLEA helps you and your patients with fewer injections.” Is it your evidence, Ms Elibol, that that statement – just that statement, “EYLEA helps you and your patients with fewer injections,” is misleading?
A Just that sentence, is that misleading? “EYLEA helps you and your patients with fewer injections.”
…
A What’s my belief? Like, what I think it is?
Q Yes. Yes?
A “EYLEA helps you and your patients with fewer injections,” I would like to know what is that compared to, personally, myself, if I saw that statement. Fewer injections compared to what?
Q Yes. Well – Lucentis?
A Okay. Fewer – in what way, I would want to know. Like, fewer injections in what way? How do you – what do you mean by fewer injections? That’s what I would want to know.
Q All right. Thank you. Because that statement may be misleading?
A Well, it just wouldn’t - - -
…
Q The statement, “EYLEA helps you and your patients with fewer injections,” just that statement?
A And so don’t look at the other thing – when you used according to – don’t look at that?
Q Yes. Yes?
A It wouldn’t make sense to me.
Q Right. Why?
A Because I - - -
Q Because you don’t know what it’s comparing it to?
A Yes. Like – yes. Comparing it to and how dosing - - -
Q All right. Well, just let me ask you this: it would be obvious, wouldn’t it – no. I will just leave that. I will leave that topic.
86 Ms Elibol was asked whether it was her understanding that a key message to communicate to physicians was that Eylea had comparable efficacy and safety to ranibizumab with fewer injections and she answered “Yes”.
87 Ms Elibol was asked whether she became aware of the summary results of the VIEW extension study soon after she started with Bayer. She said only from what she heard a few times from doctors from what they had heard from Novartis.
88 I found Ms Elibol to be a frank, forthright and truthful witness. I accept her evidence. In particular, I find that whenever she pointed out that Eylea required fewer injections than Lucentis it was with reference to the VIEW trial or to the product information. As to her use of the disputed pages, I find that she did not show the disputed pages to the ophthalmologists she visited and those ophthalmologists would not have seen those pages during her visits to them.
89 Ms Susan Adams was the third Bayer sales representative to give oral evidence. She was employed by Bayer as an Eylea territory manager between May 2012 and July 2013. Her territory was southern New South Wales and the ACT. Her customer base was made up of all the retinal specialists in her territory and some general ophthalmologists who managed patients with wet AMD. She has a Bachelor of Science and a Diploma of Education. She said she did not receive the same level of formal training as the rest of the team because she joined late and already had a very good knowledge of the treatment of wet AMD from her previous employment. She received an iPad with information about Eylea on it (Detail Aid) and she thought she received some training on the iPad but did not remember the details. She also exhibited to her affidavit her Cortex notes. She said that she made sales calls on 86 different ophthalmologists. She listed 24 where she was fairly confident that she did not show the Detail Aid to them and of the remaining 62 ophthalmologists she could not recall how many had seen the iPad presentation, although it was certainly not all. She said that the retinal specialists she dealt with were very well informed about Eylea and the timelines for its availability from data presented at congresses, international educational meetings and journal papers. She said their general reaction to Eylea was that they were pleased to have more than one drug available so as to have an alternative to Lucentis. She said she was told by some ophthalmologists that between “about 10% and 30% of their patients” were confined to monthly Lucentis and could not be “stretched out”. She was informed by retinal specialists that they were looking forward to the possibility of being able to extend treatment for these patients with Eylea. In relation to the Detail Aid she said to the best of her recollection she did not open the “Summary” tab but ophthalmologists may have seen pages under the “Home” tab when she was opening up the presentation, but that was rare and never intentional. To the best of her recollection, no doctor only saw the disputed pages. Ms Adams made a second affidavit as to her possession and use of the two baby banners.
90 In cross-examination, Ms Adams was first asked about the use of the poster and the baby banners.
91 As to her period in a sales role, she agreed that she had key performance indicators and agreed there were specific sales targets in terms of units sold. She agreed that one of the aspects of her job was to secure as many new or initiating patients to use Eylea and another aspect was to convince ophthalmologists to use Eylea instead of Lucentis with their existing cohort of patients. She said her approach was not to seek to achieve switches irrespective of whether the patients were being treated on a four weekly interval or an inject-and-extend basis or a PRN basis. She said she was not out to switch all patients, that was not her approach to the role. She agreed that in her training, her briefing from people from marketing involved discussion of such things as a key selling message. The witness was asked the following questions and gave the following answers:
Q … the key selling message was that EYLEA helps you and your patients with fewer injections?
A When used according to the approved dosage.
Q Well, I – yes, I - - -?
A Yes. It’s not just - - -
Q I see you looking at the poster. My proposition, I – what I’m putting to you was that marketing people explained to you that the key selling message was that EYLEA – EYLEA’s key point of differentiation from Lucentis was that it involved delivery of fewer injections?
A Compared to the standard of care, which is monthly Lucentis.
Q Well, fewer injections than Lucentis?
Q No, compared to the standard of care.
Q Right. You knew, didn’t you, from your long experience in this very specific AMD field, that many ophthalmologists did not inject on a monthly basis beyond the initial three months. Correct?
A Yes.
Q Yes. Thank you. Many engaged in the inject-and-extend method?
A Yes.
Q Yes?
A I would say there would be quite a few, yes.
Q Yes. And extended their patients out for the interval between injections. Extended out - - -?
A If appropriate, yes.
Q - - - if appropriate, sometimes to eight or more weeks. Correct?
A Yes.
92 Ms Adams agreed that there was a growing trend from 2007 that a greater number of ophthalmologists were treating the disease of wet AMD. There was a growing number of injectors. She agreed there was a cross-section of the ophthalmic community that injected with either Lucentis or Eylea. She agreed that in her experience many ophthalmologists would have professional relationships with particular optometrists who referred patients to them.
93 The following exchanges occurred:
Q … now, Ms Adams, it was a constant theme of your interactions with ophthalmologists, wasn’t it, that EYLEA required fewer injections than Lucentis?
A Yes, in the context of the VIEW study. Yes.
Q Yes. And another aspect which could fairly be described as a constant theme of your interactions with ophthalmologists is that fewer injections had real benefits for both patients, carers and clinicians?
A Yes.
Q And when you said in the context of the VIEW study you thought that that study established that fewer injections were required?
A When compared to – monthly with Lucentis, when compared to the standard of care, yes. Yes.
Q You were looking at the poster when you gave that answer?
A I’m looking at that. Yes.
Q And reading out the third and fourth lines of the poster?
A Yes. Yes.
Q Right. When – in this market, for all practical purposes, given that although there was a drug called Avastin, it wasn’t PBS listed, when you used the expression “Fewer injections,” you were speaking, at least in many cases, to an educated audience of ophthalmologists. Correct?
A Yes.
Q And to your understanding, when you used the expression or words to the effect of fewer injections, it would have been obvious to them that you meant fewer injections compared to Lucentis, because it was the only other or the dominant alternative on the market, don’t you agree?
A No. Compared to monthly Lucentis, because - - -
Q Pardon?
A No. Compared to monthly Lucentis, the ... required fewer injections.
Q Yes. Yes. Sticking to the - - -?
A You cannot apply that for all patients, because that’s the truth. You can’t say “EYLEA requires fewer injections” because every patient responds differently to the drug.
Q Yes. Yes. But you routinely tell ophthalmologists that EYLEA required fewer injections than Lucentis, didn’t you?
A No. When it’s dosed monthly.
Q Are you able to point to any entry in your Cortex notes where you refer to fewer injections, which make it clear that you spelt out “When it’s dosed monthly”?
A Probably not, because these notes are a summary. They were my summary. I was not aware anyone was going to be looking at them. And I know that I always use this in the context of the VIEW study. I’m not going to write that in every – every time I write a summary of the Cortex notes. So no, it won’t appear there.
Q You knew, didn’t you, that the first year of the two VIEW studies had as part of the protocol that Lucentis was, in that first year, administered monthly?
A Yes.
Q Correct?
A Yes.
Q Yes. You also knew, based on your experience – I think you’ve already accepted this – that many ophthalmologists did not inject Lucentis on a monthly basis after the first three months?
A Yes.
Q Right. Thanks. Now, it’s the case, isn’t it, to your understanding, that there’s no legal requirement or obligation for ophthalmologists to administer according to or in line with the product information for a particular product? Do you agree?
A Yes.
94 Ms Adams was asked a series of questions as to whether or not she had access to information about the second year of the VIEW studies and whether or not she discussed that information with the doctors on her calls. It appears that on at least one occasion a doctor was putting to Ms Adams what had been raised with him by the Novartis sales representative. The following exchange occurred concerning an entry in Ms Adams’ Cortex notes:
Q The reference to the ... to the number of injections of Lucentis and EYLEA being the same is a shorthand reference by you to the results of the second year of the VIEW study, isn’t it? Because that’s what those results showed: similar outcomes for the same number of injections?
A Yes. It could have been but, again, in the context, it would have been they were taking that second year in isolation and, again, that’s inappropriate. We need to look at the first year as well.
Q And to the extent there was a differentiation in the first year, that was driven entirely by the protocol, wasn’t it, which mandated that Lucentis would only be injected monthly. Do you agree?
A Which, ethically, was required because it is the standard of care; it is the best treatment regimen for Lucentis. It gives the best outcome.
Q It wasn’t the typical treatment regimen, though, in Australia, was it?
A It was the standard of care at the time the trial was set.
Q The standard of care – you mean, by reference to product information?
A Yes, and also evidence based as well. But yes, it was the standard of care at the time.
95 In my view, Ms Adams was a careful and impressive witness. I accept her evidence. In particular, I find that she did not say to the doctors she visited as a Bayer sales representative that, in the abstract, Eylea meant fewer injections for patients but spoke in terms of the product information and the VIEW study. I also find that any sighting by those doctors of the disputed pages of the Detail Aid were, at most, fleeting.
96 Ms Naomi Leonard was the fourth Bayer sales representative to give evidence. Her sales territory was Western Australia and, as of August 2013, the Northern Territory. Before her current role she had, amongst other things, been employed in various sales roles by Pfizer for twelve-and-a-half years and for five of those years she sold glaucoma medication. Before working in the pharmaceutical industry she was a registered nurse. In or around March to May 2012 she completed about eight weeks of training with the other Eylea territory managers employed by Bayer. During the course of the training she received an iPad containing information about Eylea (Detail Aid). She said that on or around 7 May 2012, she began visiting ophthalmology clinics with the Detail Aid and the product information for Eylea and two clinical papers: Ohr et al; and Holash et al. She referred also to leaving behind with ophthalmologists other clinical papers, those by Kumar et al and by Yonekawa et al. She also exhibited to her affidavit her Cortex notes. She used the Detail Aid both in desk calls, which were unscheduled meetings, and sit down calls, which were scheduled appointments. She estimated that she used at least part of the Detail Aid with about 48 of the 60 ophthalmologists she called on. She did not remember showing any ophthalmologist the disputed pages. She said that most of the doctors she saw only wanted to see how the molecule worked, the results of the VIEW study, and safety information. She said that to the best of her recollection, it was highly unlikely that any doctor saw just the disputed pages and no other pages of the Detail Aid. Her Cortex notes showed that she visited one optometrist, who worked in an ophthalmology clinic. She said it was likely she showed the Detail Aid to that optometrist. She could not be sure whether he saw the disputed pages.
97 In cross-examination, Ms Leonard accepted that her job was to sell a product for her company and it was all about differentiating Eylea from Lucentis. The following exchanges occurred:
Q And it was important in your sales campaign, if EYLEA was to establish a foothold in the market, that it be differentiated in a clear and decisive way from Lucentis. Correct?
A And that’s what it was developed for, yes.
Q Yes?
A Yes.
Q And the VIEW studies really established that it couldn’t be differentiated for clinical efficacy or safety, because it was – the result showed non-inferiority, so you had to distinguish on some other basis other than clinical efficacy or safety ... suggest, and that was fewer injections?
A Based on – yes, the efficacy – the efficacy having different end – different usage to times, so the eight weeks versus the four weeks, yes, that’s correct.
Q Fewer injections?
A Yes, that’s one of the – the differences.
Q Yes. Well, it’s the principal difference, isn’t it? As presented by you to ophthalmologists in the field?
A Yes, one of the differences, yes.
Q It’s the principal, is – it’s the principal difference, isn’t it?
A Yes, it’s – it’s – based on the VIEW data.
…
Q Ms Leonard, you, in your interactions with ophthalmologists, thought that the VIEW study established that fewer injections were required with EYLEA than Lucentis and that’s what you told clinicians?
A Yes, where appropriate. You know, depending on the patient.
98 Ms Leonard agreed that she had regular catch-up meetings with her national colleagues and had telephone hook-ups as well as physical catch-ups. She agreed that they discussed what key messages were resonating with ophthalmologists and she agreed that the key message which was resonating was fewer injections.
99 At the end of Ms Leonard’s cross-examination the following exchange occurred:
A … We were discussing and had been given the information for efficacy and safety based on VIEW first year data. And, actually, most of the time, we had the other papers and product information.
Q And when the VIEW study for the first year became available, you gave that out?
A Yes.
Q And you said it demonstrated that EYLEA meant fewer injections?
A When used according to the product information, yes.
Q Yes?
A Because it’s eight-weekly versus the four-weekly.
100 Ms Leonard gave her evidence in a frank, straightforward and truthful manner. I accept her evidence. In particular, I find that she did not say to the doctors she visited that, in the abstract, Eylea meant fewer injections for patients but spoke in terms of the product information, the VIEW study and the papers to which she referred in her evidence. I also find that any sighting by those doctors of the disputed pages of the Detail Aid were not isolated from the balance of the material in the Detail Aid.
101 Ms Michele Doyle was the fifth sales representative called by Bayer. Her territory, from 12 March 2012, was South Australia and Tasmania. Her responsibilities as a territory manager included promoting and selling Eylea for wet AMD, conducting educational meetings on the management of wet AMD and managing her territory to enable adequate coverage of all retinal specialists and their clinics. She said there were approximately 84 registered ophthalmologists in her sales territory. In Tasmania there were six retinal specialists and three general ophthalmologists who injected for wet AMD. In South Australia there were 29 injecting ophthalmologists. She had been a registered nurse for 20 years and after that she had also been a representative of a number of pharmaceutical and medical companies from 1988. She received eight weeks’ training in March and April 2012 along with other members of the Eylea sales team. She also received four days’ practical training from retinal specialists at the Adelaide Eye & Retina Centre and Hobart Eye Surgeons. She received training for an additional day at the Launceston Eye Institute. In her last week of training she was given an iPad by Bayer and an application known as the eDetailer was installed onto the iPad (Detail Aid).
102 Ms Doyle also exhibited her Cortex notes to her affidavit. According to that summary, she had sit down calls with 59 ophthalmologists from 7 May 2012 to 21 June 2013 inclusive. She said that all the ophthalmologists she called on to discuss Eylea told her that he or she had heard about Eylea at a conference they had attended overseas and that he or she was aware of the VIEW studies. She said the ophthalmologists she visited were aware of the VIEW studies before they had been published. She said she could not recall showing the disputed pages to any ophthalmologists. She said she did not like using those pages as she considered them to be too commercial. She said that she had observed that many doctors had made comments to the effect of “don’t show me the glossies” when she had attempted to show them the commercial advertising in a detail aid. Based on that experience, she said, she was aware that doctors were only interested in evidence-based medicine and clinical data showing the results. She gave as examples of the questions she was asked: “When is Eylea going to be available?”; “When is Eylea going to be PBS listed?”; and “I want to be able to switch my non-responders to Eylea, can I do that?” After her initial appointments, she said, the subject matter of her discussions with ophthalmologists moved from Eylea’s product characteristics and the results of the VIEW studies to more practical issues. She gave as examples: why Eylea was not approved for switch patients until just before the launch in October 2012; the letter or email written by Dr Beaumont saying that there was an increased risk of stroke for patients over 85 using Eylea; and when Eylea was first PBS listed, Medicare only reimbursed patients for three injections in the first six months. Bayer covered the cost of the fourth injection until Medicare changed its policy, which took a number of months.
103 Ms Doyle said it was also her practice to refer to the following materials in sales calls with ophthalmologists: the Eylea product information and clinical papers as they became available, including the Ohr et al, Heier et al, Kumar et al and Lanzetta et al papers.
104 In cross-examination, Ms Doyle was taken to her experience of product launches and differentiating the product from any existing product. She said that success of a product launch involved not only differentiating the product but it also involved credibility, product knowledge, knowledge of the diseased state and good communication with the prescriber. She agreed that there needed to be good clear communication of a good clear message about the product. The following exchanges occurred:
A … The sales department, obviously, always work closely with marketing. Marketing obviously set the – the selling message and it’s up to the sales force to interpret and – and put it forth to the prescriber.
Q And that’s – I take it that answer occurred here in the context of your interaction with the marketing department – the identification by the marketing department of the message to be taken forward by the sales team?
A Always based on the results of the VIEW study.
Q Yes. Yes?
A So based on the VIEW study, equal efficacy, equal safety as Lucentis but fewer injections.
Q Thank you. Now – and that, in a nutshell, is the message you sought to take into the market?
A In – yes.
105 Ms Doyle agreed that her key roles were to promote and sell Eylea for neovascular wet AMD.
106 She agreed that in theory one would expect ocular nurses, orthoptists and optometrists to discuss with ophthalmologists the benefits of Eylea. She said, however, that from her experience that was not how it worked. As far as the orthoptists and ocular nurses went, her selling job was made easy. When she first got out in the field, everybody knew about Eylea because the retinal specialists had heard about it from overseas conferences and they would come back and talk about it. They were waiting for it. She discounted the effect of the mivision magazine. She said she did not visit optometry rooms. She said that it was the specialists who advertised their clinics to optometrists because they knew it was the optometrists who referred patients to them. She said she believed it was important for optometrists to learn about the benefits of Eylea. Optometrists should know what options were available for their patients. She agreed that it was important for optometrists to know about the particular benefits of Eylea because she would expect them to discuss those benefits of that treatment with their patients.
107 Ms Doyle said she found the Bayer marketing department very different from marketing departments in other companies. At Bayer, there had never been the hard push, the hard sell or the single selling message. She said that she had not discussed at the meetings between members of the marketing and sales departments selling messages but obviously she had discussed the benefits of Eylea, all based on the clinical studies. She agreed that the principal benefit as she understood it was the fewer injections proposition. She said that she did not remember as a topic selling messages being part of what was said at meetings with the marketing department of Bayer. By this she meant there was never a session on selling messages but there was discussion about selling messages.
108 In my assessment, Ms Doyle was a senior and experienced representative with the additional qualification of many years’ experience as a nurse. She was frank and direct in her answers to questions. I accept her evidence. In particular, I find that she did not say to the doctors she visited that, in the abstract, Eylea meant fewer injections for patients but spoke in terms of the VIEW study. I also find that she did not show those doctors the disputed pages of the Detail Aid and they did not thereby see them.
109 The sixth Bayer sales representative to give oral evidence was Mr John Burstow. He had been an Eylea territory manager since March 2012. His sales territory included Southern Queensland, northern New South Wales and the cities of Cairns, Mackay, Rockhampton and Gladstone in northern Queensland. He has an applied science degree and was studying for an MBA. He was completely new to the field of ophthalmology when he started as a territory manager. The induction training that he was given lasted for eight weeks. It included training about general ophthalmology, the history of macular degeneration, Eylea and other treatment options for wet AMD. He was given an iPad and was able to access information about Eylea on the iPad via the eDetailer software application (Detail Aid). He also exhibited to his affidavit his Cortex notes. He said he used the Detail Aid in sit down calls. He had sit down calls, to his recollection and according to his Cortex notes, with 48 ophthalmologists between 7 May 2012 and 21 June 2013. He did not recall ever using the disputed pages. Initially, the only other material that he referred to was the product information for Eylea. As various clinical papers and review articles relating to Eylea were published, it was his practice to discuss them in his sales calls with ophthalmologists and leave a copy behind if requested. The clinical papers and review articles included those by Heier et al, Lanzetta et al, Kumar et al and Yonekawa et al. He visited a total of four optometrists and had at least one sit down call with each during the period 7 May 2012 to 21 June 2013. He estimated that he showed two of those four optometrists the Detail Aid. He did not recall ever using the disputed pages with the optometrists. On 3 and 4 August 2012 he attended the Queensland RANZCO conference on the Gold Coast.
110 In cross-examination, the following exchange took place:
Q … you knew, didn’t you, that the protocol – tell me if I’m using the wrong language – but the VIEW 1 and VIEW 2 studies had a protocol which required Lucentis to be, for the purposes of the trial, administered on a monthly basis?
A Yes, that’s correct. For the first 12 months.
Q The first year. You knew in practice, didn’t you, from your work in the field and your education that, in fact, a lot of clinicians, in fact, use Lucentis on an inject-and-extend basis?
A I would say there’s a lot who use it to the label who do use it monthly but there are some who use it less than monthly for a number of reasons.
Q Yes?
A But, yes, there are certainly – who follow the guidelines, the recommended dosing schedule and others who use it less frequently.
Q Yes. Yes. Either inject-and-extend or PRN?
A Yes. But there are a lot who treat to the label as well.
Q I know. You’ve said that three times. I’m asking you about inject-and-extend and PRN?
A Yes. There’s certainly clinicians who do that as well.
111 Mr Burstow was then asked questions about the sheet which was the approved artwork for a large pull-up banner, and about the banner itself, the banner being displayed at the meeting of the Queensland branch of RANZCO on 3 and 4 August 2012. The sheet was as follows:
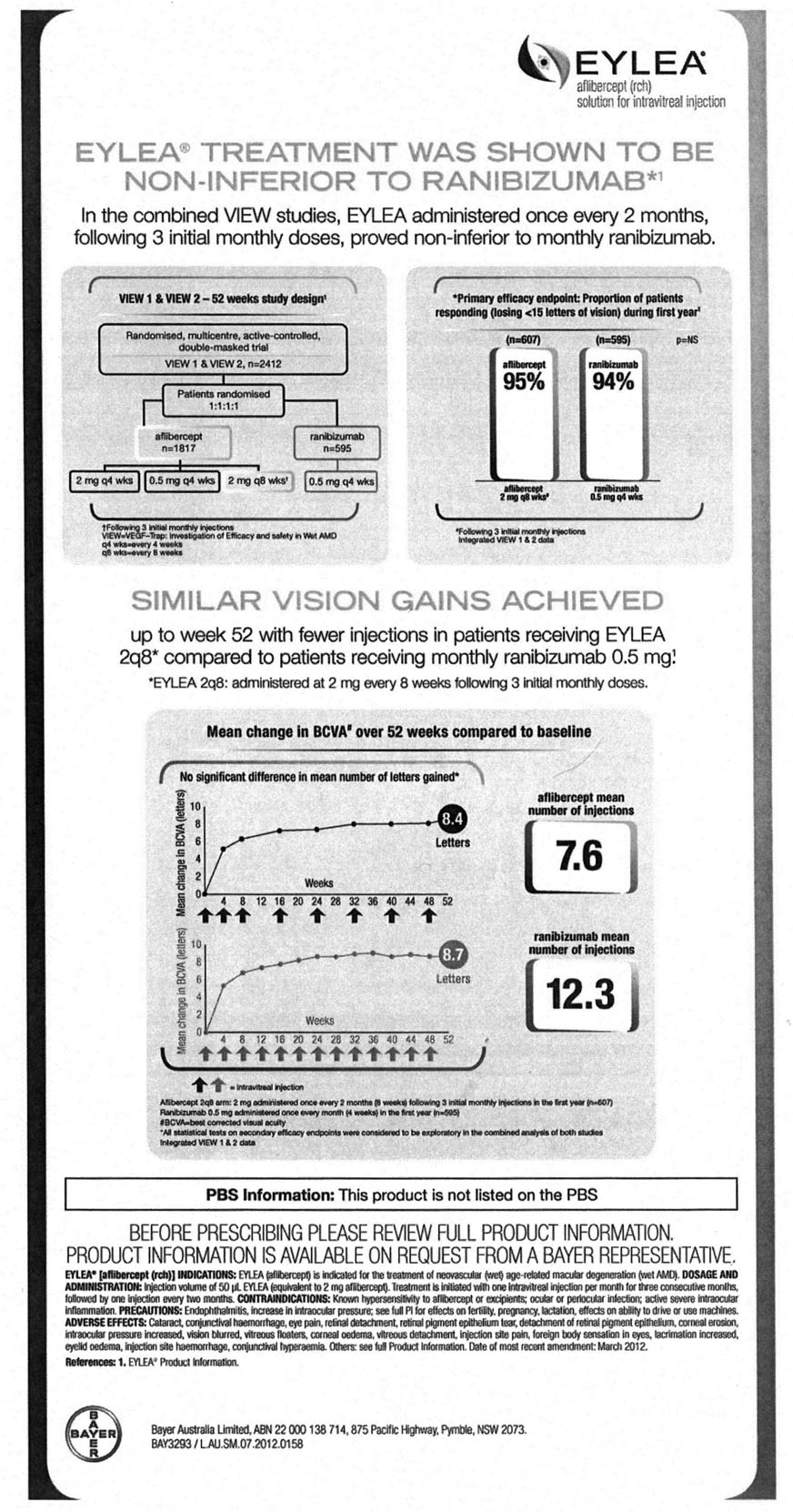
112 He was asked and answered as follows:
Q … where on the sheet does it indicate that the VIEW study mandated, as part of its protocol, that – for the purposes of the trial, Lucentis was to be injected monthly?
A Well, you can see the treatment design.
Q Pardon?
A So if you look at the top right – sorry, top left-hand box, you will see the study design. And you can see down the bottom right-hand corner in that box you’ve got ranibizumab and you can clearly see “Administer” .5 milligrams Q4, which every doctor would understand to be a monthly treatment.
Q Yes. Yes. But where does it say that that was mandated by the protocol of the study?
A Well, I guess, to my understanding that is the design of the trial so - - -
Q No. But - - -?
A - - - you know, that’s clearly showing the design of the trial, which every clinical paper would do – would have a methodology and, you know, you can clearly see the different four arms – the four main arms of the trial.
Q Yes. But is there anything else on this page which you say indicates that the protocol for the study - - -?
A Monthly, yes - - -
Q - - - dictated - - -?
A Well, you can see prove non-inferior, it’s a monthly ranibizumab - - -
Q Yes. Yes. But - - -?
A - - - up the top, again, like I said, in the top left-hand box.
Q Yes?
A The box next to it, the top right-hand box, you’ve got the 94 per cent box which – down the bottom – says, again, Q4, which is understood as monthly.
Q Yes?
A So, yes, there’s quite a few, actually.
Q Yes. But where does it say here that the study required, as part of its scientific protocol, that Lucentis only be injected monthly in the first year?
A Yes. Well, to me, it clearly identifies that in the top left-hand box. So you’ve got – I will take it – I will take you through it. So you’ve got – all the patients are randomised, one to one to one, and then you’ve got an aflibercept arm and then you’ve got a ranibizumab arm.
Q Yes?
A And then you can clearly [see] that. Then the aflibercept was divided into three groups and two milligrams administered monthly, .5 milligrams administered monthly and then 2 milligrams administered Q8.
Q Yes. Yes. Yes?
A And then you’ve got, in the ranibizumab arm, .5 milligrams administered, you know, Q4, so monthly as well. So every clinician – I would, you know, be very comfortable to say – who attended that meeting would read that – would have a 100 per cent clear idea about that’s the monthly treatment for Lucentis.
Q Yes. Yes. I’m not asking about whether it was a monthly treatment. Where does it show - - -
…
that that was mandated by the protocol of the study?
A Are you asking me – so you get my point it’s clear that it was monthly. Are you asking me - - -
Q Where does it indicate that - - -?
A Why it was designed or - - -
Q - - - that was a requirement of the study?
A I go back to that same box.
Q Right. Okay?
A You know, so every patient is in one to one to one, they’re either in four groups – they’re in the EYLEA two milligrams monthly, .5 monthly, two milligrams over eight weeks after three monthly injections or the ranibizumab group, Lucentis, .5 monthly.
113 He was then asked questions about whether, by the time of the Queensland RANZCO conference, the results of the second year of the VIEW 1 and 2 studies were known to Bayer. He agreed that by the time of the conference, the results of the second year of the VIEW 1 and 2 studies might have been known but said they were not published. The essence was as follows:
Q … Are you in a position, Mr Burstow, to explain why this information shown on the pull-up screen omitted any reference to the second year of the VIEW 1 and VIEW 2 studies, given that the results were known to Bayer by that time?
A Sure. I guess in Australia we have a very strict, you know, code of compliance with what we can talk about and not talk about. And, you know, studies, you know, once the results are known they have to go through – you know, be published and there’s a process in which that takes – so, I guess, at that moment we were able to use, you know – at that time, we were able to use, you know, the VIEW 52 week’s data and then, you know, as we have done now, you know, we incorporate the 96 week data as well. Additionally to your question, the second part of that data – so the 96 – that second year which you’re referring to wasn’t, you know, I guess – the design protocol wasn’t something that’s probably, you know, similar to how the doctors would use a drug in Australia. So, you know, at the time of the conference, that’s the only data which we could actually put forward, because the rest of it wasn’t published. And, you know, that’s the gold standard of treatment, you know, which the trials were comparing to, so that’s what we had to present at that time.
…
What Mr Burstow said on this issue of compliance was his understanding.
114 Mr Burstow agreed that it was important, in his opinion, that the benefits of Eylea be explained to optometrists as well as ophthalmologists. In his opinion, it was important for the optometrist to understand the drugs that were being used to manage the patients and build the relationship with the ophthalmologist as well so they could co-manage the patients. He agreed that there was a lot of co-management that happened in Queensland where optometrists and ophthalmologists were working closely together with particular patients and part of their working relationship involved discussing between themselves different available treatments for a particular ophthalmic issue. That was one of the reasons why time was invested by Bayer in facilitating referral meetings and education meetings between ophthalmologists and optometrists.
115 Mr Burstow was asked the following questions and gave the following answers:
Q And one of the reasons you take the baby banners along to those meetings is because so far as you understood it, they captured quite succinctly the key benefit of EYLEA?
A Yes, it’s just, I guess, raising awareness of EYLEA.
Q Yes?
A Initially, you know, a lot of ophthalmologists – sorry, optometrists were really interested in the drug, so we’re keen to get some information about it.
Q Yes. Yes. But the message on the banners, you understood to be the – no, I withdraw that. You certainly were of the view, weren’t you, that the principal benefit of EYLEA was that it would permit fewer injections which would reduce the patient burden than would be involved with Lucentis?
A I think that’s a key – a key point amongst sellers.
Q Yes?
A Yes.
Q Yes. It’s – but really is the key – was the key point, wasn’t it, in the whole campaign, the fewer injections message?
A I wouldn’t say it’s the only key, but I would say it’s an important, you know, point.
Q It’s one of the most, if not the most, important benefit, isn’t it, as you understood it?
A Yes, it’s an – it’s an important point, you know, amongst others.
Q But well, because, this is correct isn’t it, because fewer injections ease the burden for patients and that was a very powerful message, as you understood it?
A Yes, that is, absolutely.
Q Yes. And that’s the message you took around Queensland as you sought to promote this new product that there are great benefits, particularly for patients, but also for carers and doctors with fewer injections. And the product used, if you wanted fewer injections, was EYLEA. Do you agree?
A Yes, we took that message, but we also took other messages as well.
Q Yes. But what I’ve put to you is one of the central messages?
A It was certainly one of the central messages amongst others, as I said.
Q Yes?
A Yes.
116 Mr Burstow was taken to one of his Cortex notes in relation to a sit down call with an ophthalmologist on 20 May 2013. The note read, in part:
… he has used but has not seen much of a difference btw both drugs – asked to explain – we discussed VIEW trials show no diff btw outcomes just that these outcomes can be achieved with less injections – he has been able to extend a few pxs out a bit further but still early days, discussed molecule design, mathematical model and reasons why it lasts longer in the eye, discussed medicare resolution and can px monthly if required – flexibility
The cross-examination was as follows:
Q … That’s not atypical summary of the type of discussion you had with many of the ophthalmologists you saw in the course of the campaign?
A I guess it’s hard to say that they’re all similar, like every conversations, quite different.
Q Yes. But the general thrust of what you recorded - - -?
A Of that conversation?
Q Yes, is – was not unusual type of discussion you would have in the context of your interactions with ophthalmologists?
A Yes, absolutely, you know, amongst other things. It’s obviously, I guess to me, what I’m saying there is – yes, the doctor is not seeing any efficacy differences, but, you know, then I’m obviously talking about you can achieve the same efficacy with less injections, you know, which is what is reflected within the VIEW trials.
Q Yes. All right. Now, there was a bit of a concern, wasn’t there, around the time of the PBS listing. I don’t know whether it was before or after, perhaps you will be able to know better than me. There was a bit of a concern, wasn’t there, that some – probably after the PBS listing actually, that – that some doctors were finding they had to – had to inject EYLEA more frequently than once every eight weeks and – but that the PBS approval only allowed the subsidised price on a eight week cycle rather than a six week cycle, correct?
A Do you mean after the initial three monthly loading doses?
Q Yes. Yes?
A Yes. There was some doctors who found that and now, you know, Medicare has, you know, the PBS system has – has – has changed all that.
Q Yes. So the – and that was a bit of an issue, wasn’t it, across the – around the count[r]y. You understood it, including in your own region, where doctors were reporting that well, they actually got a finding that they were needing to inject having switched to EYLEA or initiated with EYLEA - - -?
A Yes.
Q - - - or finding they had to inject more frequently than once every eight weeks after the initial three month period?
A Yes, there was – there was some doctors who found that.
Q Yes?
A I guess within medicine, you know, every patient is – is different. The VIEW trials were set up with naïve patients, so they – they hadn’t ever been treated before.
Q Yes?
A Whereas a lot of these doctors initially were obviously switching existing patients who had maybe potentially years of treatment, you know, chronic patients, you know, I guess the – the most severe sufferers and they potentially needed some more aggressive therapy within the eight week period.
Q But some of the naïve patients, the doctors were finding that eight weeks was not sufficient even with some of the naïve patients?
A There were – there were some.
Q Yes. Yes?
A And I guess, you know, that even reflected in the VIEW trials, you know, 95 per cent were able to – to achieve that.
Q Yes?
A But there’s still that five per cent.
117 Mr Burstow agreed that most doctors would understand that a comparison in relation to the number of injections was with Lucentis.
118 Mr Burstow gave his evidence competently, responsively and truthfully. I accept his evidence. In particular, I find that he did not say to the doctors he visited that, in the abstract, Eylea meant fewer injections for patients but spoke in terms of the VIEW study. I note that the form of questioning of Mr Burstow included as an abstract proposition that Eylea would permit fewer injections, which is not the same thing. I also find that he did not show the doctors he visited during his sit down calls the disputed pages of the Detail Aid and they did not thereby see them.
119 The seventh sales representative called by Bayer was Mr Larry Hodges. He had been employed as an Eylea territory manager sales representative since March 2012. Previously he had worked for Allergan in a sales capacity, where he developed relationships with ophthalmologists prescribing drugs for use in the treatment of glaucoma. He had also previously worked as a sales associate for Eli Lilly Australia between 2002 and 2006, and as a medical representative for that company from 2001 to 2002. He has a Bachelor in Health Sciences. He was one of the two Eylea territory managers in Queensland. His territory included the Brisbane CBD and ran north to Cairns, including the Sunshine Coast, Bundaberg and Townsville. There were approximately 90 ophthalmologists in his territory. He received about eight weeks’ training in total after first starting work with Bayer. The training was a combination of home study and sessions at head office. He also spent time at two or three ophthalmology surgeries observing the clinical process of treating patients with injections for wet AMD. In early May 2012, he was given an iPad containing a Detail Aid in relation to Eylea. He was trained on how to use the Detail Aid.
120 Mr Hodges exhibited his Cortex notes to his affidavit. Those notes showed that he visited a total of 75 ophthalmologists and he had at least one sit down call with each person. He said in around May to August 2012 he first visited the ophthalmologists in his territory to gather information about their practices and establish relationships with them. Most of the ophthalmologists he visited, he said, made comments to him which showed they knew aflibercept had been launched in the United States a year earlier, were aware of the clinical trials for aflibercept and were waiting for its introduction in Australia. He estimated about three of the ophthalmologists he visited saw the disputed pages on the Detail Aid. He said that no doctor would only have seen the disputed pages as part of his presentation of the Detail Aid. He said that in his experience ophthalmologists were high level technical customers who kept up-to-date with developments in their field. He said the available information about Eylea and Lucentis was already known to most of them when he began making sales calls in May 2012. At that time, the questions he got on Eylea were about efficacy and safety, or “Does it work?” and “How safe is it?”
121 As clinical trials were published, Mr Hodges took the papers with him and discussed them with the ophthalmologists and left them a copy after his sales calls. They included the Kumar et al and Yonekawa et al papers.
122 He said that all of the 17 optometrists to whom he made sales calls were clinical optometrists working in ophthalmology practices. He also met regularly with other members of the clinical teams in ophthalmology practices including orthoptists and ocular nurses. In relation to the eight or nine optometrists with whom he would have used at least part of the Detail Aid, he did not believe any of them saw the disputed pages.
123 In cross-examination, Mr Hodges agreed that he was trying to convince, as part of his job, as many ophthalmologists as possible to use Eylea in respect of both any new patients those doctors had and any existing patients those doctors had. He agreed that one of the benefits of both Eylea and Lucentis was that, because of the relative ease of administration through injection, a broader cross-section of ophthalmologists was able to deliver the treatment for the disease. He agreed he had an interest in the context of promoting Eylea also to try and convince some ophthalmologists who were not injecting, or who were thinking of injecting, to begin doing so and to do so with Eylea. He agreed that he saw real value in marketing Eylea and speaking to its benefits to optometrists as well as ophthalmologists in practices where there were both. Mr Hodges agreed that he certainly appreciated by the time he started with Bayer the need for a clear message or communication about the merits of a new product compared to any established product on the market. He was taken to one of his Cortex notes of a sit down call with an ophthalmologist on 10 May 2013 which read:
used eDetailer to position eylea’s ability to reduce burden. stated that he is using. starting to feel they are the same. used the efficacy page to show they get the same results in different ways. eylea less frequently leading to better patient and clinical outcomes.
The questions and answers which followed were in essence:
Q … Your pitch, if [I] can – you understand that word, your pitch, you know that expression, I take it.
A Yes.
Q Your pitch was same results but with fewer injections. Correct?
A Partially.
Q Pardon?
A Partially.
Q Right. I’m not suggesting that was the entirety of your pitch, but your pitch to him, as recorded in this note, for example, was what I put to you, wasn’t it? Fewer results with less frequent or fewer injections – sorry, same results but with less frequent injections or fewer injections. Correct?
A What’s your question? I’m sorry.
Q The pitch, as recorded in this note, was same results but with fewer injections, with benefits to patients. Correct?
A That’s the correct – that’s correct.
Q Right. And that was typical of your pitch to many of the ophthalmologists who you visited throughout Queensland. Correct?
A Not correct.
Q Not correct. All right. It was similar, wasn’t it, to your general pitch to ophthalmologists throughout Queensland?
A I don’t really have a pitch as you’re stating.
Q All right. Okay?
A I think, if I understand what you’re saying – is that there’s a message or a pitch that I go in with. And I don’t work like that. So - - -
Q All right. Well, I will come back to that. Thank you for that. Do you see your note for the next visit? Sorry. So you thought this ophthalmologist, MD, because of what he said to you – and as you recorded it in the third and fourth line – you see what you record in the third and fourth line – you saw that he might be wavering, for example, in relation to his views about your product. Correct?
A No.
…
Q Okay. You see in the third line he has told you – this is your record of what he told you – you stated that he is using – now, that, I take it, is EYLEA, correct? And he’s starting to feel they are the same. Do you see that? They - - -?
A That’s correct.
Q That they - - -?
A I see.
Q You were referring to EYLEA and Lucentis, correct?
A That’s correct.
Q You didn’t need to say Lucentis because everybody knew, to your understanding, that there are really only two products in the game. Correct?
A That’s correct.
Q The one which had been around for about five years and EYLEA, correct?
A That’s correct.
…
Q He said what we agree you’ve recorded there, he’s starting to feel they are the same, and you’ve responded to that – according to your note – by seeking to differentiate between the two in terms recorded in the next sentence. Correct?
A Correct.
…
Q Yes. So to distinguish EYLEA from the existing product, there had to be something else able to be pointed to to differentiate or to mark out your product from the existing product. Correct?
A That’s correct.
Q And what it was is captured in that sentence, “Less frequently, leading to better patient and clinical outcomes.” That’s what you used to differentiate the product?
A I use many different things to differentiate the product.
Q You didn’t use efficacy, did you?
A No, I didn’t.
Q No. And you didn’t use safety? … and you used injection frequency?
A In these – in this small set of notes there’s injection frequency.
Q Yes. Yes?
A But I use many different things to differentiate EYLEA.
Q Yes?
A So in this brief synopsis that you’re pulling here - - -
Q The principal point of differentiation which you used generally in your field visits to differentiate the two products was injection frequency. That’s what I suggest to you?
A Is that a question? I’m sorry.
Q Yes?
A No. That’s not correct.
Q You regularly used the difference in injection frequency to differentiate the products in your field visits, didn’t you?
A Say that again.
Q You regularly used difference in injection frequency to differentiate the products in your field visits, didn’t you?
A Amongst other things, yes.
Q Yes. And I’m putting to you that that was – that difference being injection frequency, was the predominant point of differentiation you pointed out in your field visits?
A Not correct.
Q Pardon?
A Not correct.
Q It was – if it was one of a number of points of differentiation, it was an important point of differentiation from your point of view, wasn’t it?
A I would agree with that.
Q Yes. And being important, I take it, you pointed it out to your customers. Correct?
A Yes, amongst other things.
Q Yes. But being important, can I suggest to you, you always pointed it out to your customers?
A That would be incorrect.
Q Would it? So there was an important – are you saying to his Honour that as an experienced, successful sales representative, you had a view that injection frequency was an important point of differentiation, but you didn’t always draw that to the attention of the people you visited?
A That’s correct.
Q Right. In the overwhelming majority of cases you did though, didn’t you?
A Amongst other things, yes.
Q Thank you. Now, just staying on this note at the bottom of 156, you say:
Go over differences with Mark on next visit. Really bring home difference in treatment burden due to lower injection rate in most patients.
Can I suggest to you that whilst you have identified differences, plural, in your note, the one you have singled out is injection frequency. Do you see that?
A Yes.
Q And you agree that’s a fair reading of your note?
A Yes.
Q Yes. And the reason you singled that one out is because in your mind that was the most important point of differentiation or difference between the two products?
A Not correct.
Q No. Some of the other differences you have in mind in your answers, things like benefits to patients, easing the burden on carers, easing the burden on practices, are they the differences – examples of the other differences you had in mind and pointed out?
A That amongst the mechanism of action against efficacy - - -
Q Yes?
A Yes, yes.
Q Yes, but - - -?
A So numerous.
Q Sorry, so you’re saying the three I identified – easing the burden on patients, easing the burden on carers that have to – and easing the burden on practices – they were three of the other points you had in mind together with mechanism and action?
A That’s correct.
Q Yes. Now, those three points, the first of those three points – easing the burden on patients, easing the burden on carers and easing the burden on practices – all follow, don’t they, logically, from fewer injections?
A I would agree with that.
Q Yes. It’s really another way of expressing the fewer injections message, isn’t it, in that they are the consequences of fewer injections; correct?
A I wouldn’t put it that way.
Q Well, they are the consequences of fewer injections, aren’t they?
A Yes, they - - -
Q That patients are less burdened?
A They would be. That’s correct.
Q That carers are less burdened?
A Yes.
Q And that practices are less burdened?
A I would agree with that.
Q Yes. They’re not the consequence of anything else other than fewer injections, are they?
A I would agree with that.
Q And so when you pointed out in your visits – and I accept you say you didn’t do it all the time – but in many cases when you were pointing out the importance of fewer injections, you would also point out the benefits of easing the patient burden, easing the carer burden, and easing the burden on a practice; correct?
A Correct.
Q And those last three points really reinforce the attractiveness of fewer injections; correct?
A That’s correct.
…
Q Yes. That message of fewer injections with the benefits of easing patient care, easing carer burden, easing burden on practices, that, from a seller’s perspective, or a marketing or selling perspective, was a very, very powerful message in support of the product you were promoting; correct?
A I would agree with that, amongst other things.
124 I have set out this transcript at some length in order to illustrate the point Mr Hodges was asked about lower injection rate or fewer injections in the abstract without context. This was made clear in two ways. First, later in the cross-examination the following exchanges took place:
Q All right. What I’m suggesting to you is that the message which is encapsulated in this – the second half of that column:
Keep focused on the saving that EYLEA will offer patients with less injections, such as lower risk of endothermic injection anxiety and less travel.
That was typical of the message you communicated to the ophthalmologists and optometrists to whom you were trying to sell this new product?
A Well, if you put – if you just look at that second half, then yes. But if you look at the entire thing, there’s other – you can’t just go into a doctor with – with these doctors with just the message there’s fewer injections. You can’t do that. You really have to talk about safety, you really have to talk about efficacy, its effectiveness, how it works.
Q Yes?
A So you just can’t go into a doctor and say, “Less injections.” I’m sorry.
Q Yes. All right. I accept that. Thank you. And when you – so there’s a broader context – and when you talk to the doctors about safety, you said that the VIEW study showed no difference in adverse outcomes, same safety profile?
A Yes.
Q Right. That’s - - -?
A That would be one part of the conversation.
Q One part. And another part would say clinical efficacy, the same results achieved in different ways?
A Yes.
Q Correct?
A Yes.
Q And then you would come to this, would you not, but fewer injections with all of these benefits. Correct?
A Using the VIEW data, which demonstrated that. Yes.
125 Secondly, in re-examination Mr Hodges was asked about his answer “Partially” at the beginning of the extract of his cross-examination I have set out above. His answer was as follows:
EYLEA was – gives the same results with fewer injections. So everything that I do is based on the clinical data, so the PI or the VIEW trials. So it’s a case of looking at the efficacy, and in the VIEW studies you had different arms – some arms of the EYLEA product was monthly, just as the Lucentis product, and there was ... was eight weeks, so when you look at the efficacy across the different arms, they’re similar, so the results show that you get the same results with efficacy if you were to treat monthly with Lucentis or EYLEA, but you also get the same results if you treat bi-monthly with EYLEA that you would get if you treated with Lucentis. So then you – there’s other things that you discussed as well. The mechanism of action, its binding affinity to the molecule, the fact that it binds on both arms, so you can’t just go in and talk to these – they’re really highly technical doctors – just with a – you can’t do a pitch, because then you lose your credibility, you lose your integrity, and once you’ve lost that, you – you won’t be visiting these doctors …
126 In my opinion, Mr Hodges was an impressive witness who considered his answers with appropriate care. I accept the truth of his evidence. In particular, I find that Mr Hodges did not refer in his communications with doctors to “Eylea means fewer injections” in the abstract but did so by reference to the product information or the VIEW trials. I also find that three of the ophthalmologists he visited saw the disputed pages on the Detail Aid but that those doctors did not see only the disputed pages as part of Mr Hodges’ presentation of the Detail Aid.
127 The eighth Bayer sales representative called was Ms Cecilia Martinek. She joined Bayer as an Eylea territory manager in New South Wales on 5 March 2012. Prior to joining Bayer she worked at Biogen Idec for 8 months as an area business manager. Before that she was at Allergan for almost 5 years as a territory manager working in the field of glaucoma and dry eye. Her customers at Allergan were ophthalmologists. She was a medical representative at AstraZeneca between 2003 and 2006. She has a Bachelor of Science, majoring in Pharmacology and her postgraduate studies included a certificate in marketing. Her territory was Chatswood, the inner and outer west of Sydney, parts of the Hills District, Orange and Dubbo. There were approximately 61 injectors in her territory.
128 She spent her first two months at Bayer, in March and April 2012, in training. She also did preceptorships, educational sessions, with ophthalmologists which continued past the training period. She went to ophthalmology clinics to get deeper insight, particularly into how the retinal specialists worked, and watched them give injections for wet AMD.
129 During the course of her training she was given an iPad containing a presentation on Eylea (Detail Aid). In the week beginning 7 May 2012 she began visiting ophthalmologists and other clinic staff with the Detail Aid and the recently released Eylea product information.
130 She exhibited her Cortex notes to her affidavit. Those notes showed she visited a total of 72 ophthalmologists and had at least one sit down call with 58 of them.
131 She said that after her first appointments she recalled that academic and clinical papers about Eylea started to become available. She recalled using the Holash et al paper and the Ohr et al paper in her discussions. The VIEW paper was published in January 2013. Her practice was to discuss these documents with the ophthalmologists and talk to some of the key points. It was also her practice, she said, to leave these documents with ophthalmologists so they could review them in their own time. Since then there had been some studies, such as Kumar et al in 2013, that she had discussed and left behind with ophthalmologists.
132 After the VIEW paper was published she rarely used the Detail Aid. She estimated the number of ophthalmologists who saw at least part of the Detail Aid would be nine. She did not think the disputed pages were helpful as, given her experience presenting to ophthalmologists, they did not speak to what was relevant. In her estimation perhaps 1 to 2% of the nurses or other medical staff she showed the Detail Aid to saw the disputed pages. She did not use the disputed pages with ophthalmologists.
133 Ms Martinek said the ophthalmologists in her territory had been using Lucentis for five years when she started to visit them. She understood that they were highly skilled and qualified professionals, with a sophisticated level of technical knowledge and practical experience. Even for those she showed the Detail Aid to, she did not need to show it twice, particularly after the VIEW data was published. In the two or three months leading up to the PBS listing of Eylea in 2012, it was her experience during appointments that generally ophthalmologists were more interested in practical issues such as whether Eylea could be used in switch patients, when it would be PBS listed and how many injections would be reimbursed by Medicare.
134 She said she had no sales calls with optometrists recorded on Cortex. She did not specifically target optometrists because they could not prescribe Eylea. Her only contact with optometrists was through referral meetings and optometrists working in ophthalmology clinics.
135 In cross-examination, Ms Martinek agreed that product differentiation was important in selling and marketing and when asked whether that was particularly important in the case of a new product being launched into a market where there was a well-established incumbent, she replied that most important was the evidence and what the evidence showed and the differentiation that the evidence showed. She said that the objective of her job at Bayer with Eylea was to present the evidence for Eylea: doctors were interested in the evidence and that was the objective, in her recollection. She said that education was a big part of her role. The outcome was to sell or convince ophthalmologists to use Eylea rather than Lucentis.
136 Ms Martinek, in my view, gave her evidence frankly, truthfully and efficiently. I accept her evidence. I note she was not asked questions in any detail about her dealings with ophthalmologists. I find she relied on the product information and later on the VIEW study as well in her communications with ophthalmologists. I also find she did not show the disputed pages to ophthalmologists and they would not have thereby seen those pages.
137 The ninth and last sales representative called by Bayer was Ms Alena Reznichenko. She had been a sales representative at Bayer since 12 March 2012. From 12 March 2012 to 22 October 2012 she was employed as the territory manager for eastern Victoria. Her territory included the parts of Melbourne east of Victoria Parade, out to Heidelberg, north to Albury and down to Wonthaggi and Mornington. She preceded Ms Elibol as territory manager for this territory. On 23 October 2012, she was promoted to the role of medical advisor. From January 2013 she was the medical advisor for Eylea. On 16 September 2013, she was promoted to the role of medical affairs manager, so that other medical advisers reported to her as well.
138 Before working at Bayer, she was a manager and senior clinical optometrist at the Epworth Eye Centre for three years. Prior to that she was a dispensing optician at Optik Eyecare for one year. Prior to that she was a consultant and medical advisor for the Royal New Zealand Foundation of the Blind for four years. She also practiced as an ophthalmologist for five years in Russia. She has a Diploma in Medicine, a Masters of Science in Medicine (specialising in Ophthalmology), a Bachelor of Optometry, a postgraduate certificate in Public Health and a Bachelor of Science in Software Engineering.
139 She said she received eight weeks’ training at Bayer when she started as a territory manager. During her training, she spent one and a half days training with Associate Professor Wilson Heriot in his ophthalmology practice. Towards the end of her training she was given an iPad by Bayer and received training on how to operate the Eylea detailing aid (Detail Aid). She exhibited her Cortex notes to her affidavit.
140 She used the Detail Aid in sit down calls. The Cortex notes showed she had at least one sit down call with 68 ophthalmologists. She said it was likely that she showed the Detail Aid to each of the 68 ophthalmologists with whom she had a sit down call. She said, based on her experience as an ophthalmologist, the scientific material meant more when speaking to ophthalmologists. The ophthalmologists she visited showed the most interest in the information in the MOA tab. She rarely used the Detail Aid during subsequent sit down calls with an ophthalmologist. The discussions she had with the ophthalmologists she visited focused on the scientific material.
141 She estimated that she showed the pages under the “Home” and “Summary” tabs (including the disputed pages), to approximately 50% of the ophthalmologists who saw the Detail Aid. If she had ever shown the disputed pages she said she would always have shown other pages from the Detail Aid.
142 Ms Reznichenko said that, in addition to using the Detail Aid, she always brought copies of the product information for Eylea with her to meetings with ophthalmologists. If she had not met with the ophthalmologist before, it was her practice to give to, and leave with, him or her a copy of the product information. The results of the VIEW studies (the Phase 3 clinical trials conducted using Eylea) had not yet been published when she was visiting ophthalmologists and she did not recall any other clinical papers being available. She did not conduct sales calls on optometrists.
143 In cross-examination, she accepted that she received some material from the Bayer marketing department as part of her two-month training program with other sales representatives. The following summarises her evidence:
Q … Did it – did it involve, for example, some suggested key messages about the product which might be useful in the – in the interaction with the future customers?
A Yes, and those key messages, they were all taken from the PI, from the product information.
Q Yes. What – yes?
A So they were the attributes of the product.
Q Yes. And the key message that was communicated by the marketing department to you, in the course of your – your training, was that EYLEA meant fewer injections for doctors and patients. Correct?
A That’s correct, because EYLEA means fewer injections.
Q Thank you. And that crisp message, “EYLEA means fewer injections,” formed – I’m not suggesting it was the only thing you said to ophthalmologists when you visited them, but that was the clear message that the marketing department had suggested could be imparted?
A That wasn’t the only message.
Q No?
A Fewer injections, it’s one of attributes of EYLEA. EYLEA has many more other attributes.
Q Yes?
A And they are all taken from the clinical trials which is described in the product information.
Q But the – and other messages include benefits to patients, benefits to carers, benefits to eye practices. Correct?
A What do you mean?
Q That EYLEA - - -?
A What other – yes.
Q - - - would deliver benefits to patients. Benefits to their carers, benefits to practices because of the need for fewer injections. Correct?
A I don’t understand what you’re saying. You’re asking me the other messages imply, that’s what you say.
Q Yes. All right?
A Can you just rephrase that.
Q Yes. Did the marketing department press upon you that – or did you understand – that fewer injections would mean benefits for patients?
A As an ophthalmologist, I see that.
Q Yes. And benefits for their carers, in terms of losing the burden of getting them – those who need assistance, getting them to and from surgery?
A You could make that assumption, yes.
Q Yes. And benefits to practices, either by reducing the burden of injections or opening up the opportunity for more patients?
A For other patients to receive timely treatment.
Q Yes?
A Yes, you can make that assumption as well.
Q And those benefits, those three benefits, benefits for patients, benefits for their carers, and benefits for practices were all, can I suggest to you, features of EYLEA which you understood?
A These benefits come out of effect that EYLEA requires fewer injections, as described by the protocol of a study as described by the product information.
Q Yes?
A Which is approved by the Therapeutic Goods Administration.
Q Yes?
A Yes.
Q But those – you certainly, when you went out in the field speaking to ophthalmologists and optometrists, identified to them those benefits. Correct?
A I will just correct you, that I did not speak with optometrists.
Q Okay. Yes?
A I spoke with ophthalmologists, and I did communicate that EYLEA requires fewer injections when used according to the label.
Q Yes?
A Yes.
144 Ms Reznichenko made clear in re-examination what she meant by the expression “according to the label”. The relevant questions and answers were as follows:
Q Ms Reznichenko, in one of your answers you said that you communicated to ophthalmologists that EYLEA requires fewer injections when used according to the label; what does the expression “according to the label” mean in the way you used it in that answer?
A EYLEA is approved to be used in Australia every two months following three loading monthly injections. That’s what the label of EYLEA says.
Q And when you use the word “label” what are you referring to?
A That’s what it’s approved by the Therapeutics Goods Administration which is the registration board of Australia - - -
Q Yes?
A - - - that registers products in Australia for the use.
…
Q So is there a physical thing called a label that you’re referring to?
A So when a product is tested and you do clinical trials, then that information is submitted to the Therapeutics Goods Administration, which is the TGA for short, and the TGA assesses all the information and then approves a certain label of application of a product which is the indication of how it’s going to be used. And EYLEA has been approved to be used every two months following three monthly injections, and that’s based on the clinical trial information. So that’s considered to be label of EYLEA which is - - -
Q And when you use the word “label” are you referring to a physical document? Something on a piece of paper?
A Yes. Which is the product information. Each pharmaceutical product in Australia has a product information, and that’s where the label is stated.
145 Ms Reznichenko was self-evidently a highly qualified witness. She had an impressive grasp of the subject matter. I accept her evidence. In particular, I find that she did not say that Eylea meant fewer injections in the abstract but referred to the product information and the clinical trials there described. As to the use of the disputed pages, I find Ms Reznichenko showed those pages to approximately 50% of the ophthalmologists who saw the Detail Aid but that those pages were shown with the other pages from the Detail Aid.
146 In respect of the sales representatives taken together, I find that although there were selling messages, those messages did not include “Eylea means fewer injections” in the abstract but in connection with the product information and the clinical trials and, later, the VIEW study.
147 As to the Cortex notes themselves, in my view the relatively infrequent references to “fewer injections” did not qualify or derogate from the context in which those references were made, as I have set out above.
148 Mr Kuo’s affidavit was formal and concerned the Cortex material. It is not necessary to summarise his evidence. He was not required for cross-examination.
The expert witnesses
149 On this branch of the case the experts were called by the respondent Bayer.
150 Professor Paul Mitchell had practiced as a medical retina specialist since 1983 and estimated that since 2007 he had treated approximately 800 patients for wet AMD. As at June 2013, he was treating approximately 450–500 patients for wet AMD with either Lucentis or Eylea. That number was expanding by around three to five new patients per week. Professor Mitchell’s clinic, and Professor Mitchell as Principal Investigator, had enrolled patients into many of the major pivotal trials testing different drugs for wet AMD, including ranibizumab and aflibercept. He had also been involved in academic work in relation to wet AMD and stated that he was one of a few Australian experts on the occurrence, risk factors and treatment for wet AMD. Professor Mitchell’s evidence was that once he had defined and diagnosed whether the patient had wet AMD he determined whether the patient was likely to respond to treatment. Once he had decided that treatment was appropriate for the particular patient, the next decision was which agent/drug to use. The decision as to whether to treat with Lucentis or Eylea, Professor Mitchell said, was based on:
(a) his experience using the drug on patients in terms of the responsiveness and the treatment burden of the drug. He had treated patients with Lucentis since 2007 and with both Lucentis and Eylea since December 2012 and had gained considerable understanding about the two drugs from observing his patients’ responses to treatment. He drew on this understanding and knowledge and applied it to the particular patient he was treating in terms of choosing which drug should be used;
(b) what he had learnt from the peer-reviewed medical literature about wet AMD, including the data from the randomised clinical trials. He had experience in having patients enrolled in some of those studies, including the VISION, ANCHOR SUSTAIN and VIEW 2 trials. He said he knew the literature very well and used what he had learnt in those studies to guide his decisions about which drug to prescribe and administer for individual patients. He said that ophthalmology was very much an evidence-based speciality. He, like other ophthalmologists, relied on the peer-reviewed evidence about the safety and efficacy of drugs;
(c) the product information for Lucentis and Eylea. He had read this on numerous occasions and knew the contents;
(d) potential durability/duration of action of the drug;
(e) any credible reports of adverse events in the use of either of the drugs.
Professor Mitchell’s understanding was that both general ophthalmologists and retinal specialists adopted a somewhat similar decision-making process to his own, except that many did not have the same level of academic background knowledge that he had. He gave the basis of his understanding, being his regular collaboration with colleagues and discussions with them as to what they were doing in practice in relation to wet AMD, which confirmed to him that they adopted a relatively similar decision-making process to that used by him, and other ophthalmologists’ discussion of their practices at symposia, conferences and College and Society meetings which he attended. In particular, his colleagues asked questions at those events which indicated that they had reviewed and understood the medical literature about wet AMD and that this peer-reviewed literature played a substantive role in their decision-making processes, as it did in his.
151 Dr Andrew Crawford had training and experience as a general ophthalmologist and had managed wet AMD patients since 1999 using most of the techniques available at the relevant times. Once intra-vitreal injection treatment became well established in Australia he sought relevant training and commenced to perform injections at the end of 2010, continuing ever since. The decision-making process that he followed as a matter of practice when deciding whether to prescribe and administer Lucentis or Eylea was as follows:
(a) he gathered information about the use, efficacy, burdens and hazards of each drug. The sources of information that he relied on included, in no particular order:
(i) medical literature, especially reputable peer-reviewed journals. He evaluated items in the medical literature by their content and also by the reputation and affiliation of their authors;
(ii) medical conferences and meetings, where he also evaluated presentations by that content and by the reputation and affiliation of presenters;
(iii) discussion with his peers;
(iv) auditing of his own patients’ outcomes;
(v) information disseminated by pharmaceutical companies including product information, advertising materials and presentations by sales and scientific representatives;
(b) he also gathered information about the specific pathology presented by the patient and the progress of the pathology once treated;
(c) he also gathered information from the patient;
(d) he combined the information from (a), (b) and (c) in order to make an individualised recommendation for treatment to each patient. The recommendation might be revisited from time to time during treatment if he believed that clinical progress suggested that an alteration would be beneficial or if the patient changed their attitude to treatment.
152 In cross-examination, the evidence of Professor Mitchell and the evidence of Dr Crawford on these points was not challenged. I accept their evidence. Novartis accepted, and I find, that ophthalmologists were aware of the dosage regime of Lucentis and Eylea set out in the product information I have set out at [34] and [42] above, respectively.
153 Professor Mitchell confirmed that he published fairly extensively in the area of wet AMD and that probably no one in Australia or possibly globally had been as prolific in terms of publications. He qualified this answer by referring to Professor Robyn Guymer in Melbourne and said that she could have more publications in this particular field than he had. It was put to Professor Mitchell that he was atypical amongst retinal specialists in terms of the extensive list of his publications and his international profile but he said that in terms of the clinical aspects of treating patients he was the same as most other retinal specialists and he spent a lot of time talking to his colleagues and he was among many others who treated the large number of people who developed macular degeneration. He agreed he was a full-time academic so that put him apart from some others but said clinical practice was a fair component of his work. In re-examination he made it clear that his clinical practice was about 60% of his work, seeing patients for most of three days a week.
154 He confirmed that he was an investigator in the VIEW 1 and 2 trial and he had, he thought, 11 patients in that study. He said the VIEW trial was divided into two studies as mandated by the United States Food and Drug Administration: the VIEW 1 was conducted in the United States and the VIEW 2 in the rest of the world, including Australia, so his patients were in the VIEW 2 trial.
155 In relation to the Australia and New Zealand Journal of Ophthalmology, Professor Mitchell said it was the only local peer-reviewed publication specialising in ophthalmology. He said the Journal went to all members of the Australia and New Zealand College of Ophthalmologists approximately once a month. He agreed it was an important publication and that most practitioners would look through it each month when they got it. He agreed that sometimes there would be a supplementary edition of the Journal in the month prior to a conference whereby Fellows received a copy of the abstract journal which covered the abstracts from the papers being presented at the national conference. They received it when they registered for the conference. He said the mivision magazine was not peer-reviewed and was more like a trade magazine.
156 Professor Mitchell was taken to slides he used at the national product launch of Eylea. He was first taken to a slide presenting the 52 weeks results in terms of the primary efficacy endpoint. He said that the VIEW studies were non-inferiority studies: aflibercept was being compared to ranibizumab and the aim of the study was to demonstrate that aflibercept was non-inferior to ranibizumab in terms of efficacy for the primary endpoint, which was preventing a serious loss of vision. That primary endpoint was chosen because it was the same in the Lucentis pivotal studies: the ANCHOR and MARINA studies. Professor Mitchell was taken to the slides which followed, dealing with clinical equivalence and safety. He was taken to slide 29 on which he said were the conclusions which, in a limited time, were important to highlight. They were as follows:
VIEW Studies: Conclusions
• Aflibercept 2mg given every 2 months* was shown to be noninferior to ranibizumab 0.5mg given monthly
• Aflibercept 2mg every 2 months* provided similar efficacy to ranibizumab 0.5mg monthly in maintaining vision (95.3% vs. 94.4%, avoiding a 3-line loss, respectively)
• Results of secondary endpoint analyses of aflibercept and ranibizumab treatments were comparable
• Aflibercept and ranibizumab also had similar safety profiles
*After 3 initial monthly doses
(Footnotes omitted.)
Professor Mitchell said the first bullet point gave an overview and the second bullet point gave the specifics.
157 Professor Mitchell said that the VIEW studies did have aflibercept given every month as one of the arms of the trial but because there was no difference between monthly and two monthly, they had done the comparison for two monthly. Professor Mitchell agreed that there were three aflibercept protocols, two different doses and two different regimens, and there was the one protocol for Lucentis, which was protocol dictated, and it was also because it was the result of the pivotal ANCHOR/MARINA studies which treated patients monthly.
158 Professor Mitchell was asked about his understanding of the phrase ‘me too’. He agreed it was not a scientific term or a term with a technical scientific definition. He agreed that minds could legitimately differ in terms of the relative significance of particular criteria such as mode of action, mode of administration, efficacy and safety and that minds could legitimately differ as to the degree of similarity between two products within any one criterion.
159 I accept Professor Mitchell’s evidence. Plainly he was eminently qualified and gave his evidence in a clear and frank manner.
160 I have set out above the parts of Dr Crawford’s affidavit evidence which I consider to be significant. I also note that he annexed to his second affidavit a second report dealing with further questions he had been asked concerning: detailing in the period May 2012 to June 2013 and discussions with Bayer representatives in that period; ‘me too’ pharmaceuticals and his understanding of that phrase and whether, in his opinion, Eylea was a ‘me too’ pharmaceutical in relation to Lucentis; medical literature; events; and referrals. There was also in evidence a short letter Dr Crawford wrote to the respondent’s solicitors on 24 March 2014 stating that his opinion in relation to the ‘me too’ issue was based upon knowledge of published, peer-reviewed studies that described the results of trials where patients had been treated sequentially with Lucentis then Eylea and direct clinical experience of patients under his treatment who had been switched from ranibizumab to aflibercept, or vice versa, with differences in clinical outcome.
161 In cross-examination, Dr Crawford was asked questions about the Yonekawa trial to which he had referred in his 24 March 2014 letter and agreed it involved a modest number of patients and was a retrospective study which involved limitations.
162 Dr Crawford was also asked about the VIEW 1 and VIEW 2 studies and agreed that they were important but he said that he did not believe there was a leading study of Lucentis and Eylea.
163 Dr Crawford was asked about a presentation he had given at a referral meeting attended by optometrists in which he used a series of slides, one of which referred to “reduced injection burden” in respect of Eylea. He said reduced injection burden referred to the VIEW study. He agreed it was a point the Bayer sales representatives had no doubt raised in meetings with him.
164 As to ‘me too’ drugs, Dr Crawford agreed that while a generic was an exact copy, a ‘me too’ may be a drug that was similar but different and minds could legitimately differ as to the extent of similarity required for somebody to form a view that a particular drug was a ‘me too’.
165 Dr Crawford was taken to the statement in his second report: “… the clinically effective outcome of each drug has been shown to be different if administered sequentially to the same patient.” He said that statement was not in his slide presentation because the VIEW trial did not sequentially administer the two drugs to the same patient so that answer could never have been contained in his summary of the VIEW trial data.
166 Dr Crawford emphasised in his evidence that he was a general ophthalmologist and not an academic ophthalmologist and that his practice was in the clinical, not the academic, sphere. Having said that, he gave his evidence in a direct and straightforward manner and I accept it.
167 Dr Ared was an optometrist and had nearly 20 years of experience as a clinical optometrist in direct patient care. He did not directly treat patients with wet AMD but had been co-managing patients with wet AMD with his ophthalmology colleagues for over five years. He had not been involved with clinical trials in relation to wet AMD and he had not been involved in any academic work in relation to wet AMD. He was not permitted to prescribe or administer Lucentis or Eylea as an optometrist. His understanding was that only ophthalmologists were permitted to prescribe and administer intraocular injections with anti-VEGF therapy such as Lucentis and Eylea. He said he did not himself treat patients who had wet AMD other than to co-manage the patient. In deciding whether to refer a patient who presented with symptoms of wet AMD, Dr Ared said his referral decision was based on a patient’s signs and symptoms. He determined a patient’s signs and symptoms by taking a detailed patient history and undertaking a clinical examination. For that purpose he used a diagnostic eye scanning imaging instrument known as an OCT (Optical Coherence Tomography). He said that information about patient treatment options came to him by way of a number of sources such as: direct reports from a treating ophthalmologist, industry meetings, magazines and trade exhibitions, company-sponsored continuing education evenings, ophthalmic literature articles and independently peer-reviewed randomised clinical studies. He drew on those sources of information when making a decision about whether a patient needed a referral for wet AMD.
168 Dr Ared said he did not attend any of the meetings where the relevant materials were displayed nor did he see any of the advertisements in the mivision magazine.
169 In cross-examination, Dr Ared agreed that there was a broad section of optometrists in terms of age, experience and interest. He said that ophthalmology, like all disciplines of medicine, was becoming a more sub-specialised profession, and the role of the optometrist was becoming a bit like a GP for eye care. He said that he did not treat wet AMD but he detected and referred, or diagnosed and referred. He agreed that many of his patients would ask about what treatments were available but said it was not his role as an optometrist to suggest to them what they may or may not end up with at their consultation with the specialist. He said the standard of care would be to get them to the ophthalmologist and then the ophthalmologist could have that discussion with the patient. Dr Ared accepted that if a patient of his asked him what the range of treatments available was, he would tell them and he would not be breaking any legal requirement or medico-legal requirement he was aware of in telling them that information. He said he could discuss the treatment options and he could tell the patients the various modalities but really it was trying to get the patient to the specialist. If he were asked by a patient whether the injection was just a one-off or did the patient have to keep having an eyeball injection and how frequently, he would answer that question by telling them it was up to the specialist to tell you how often. Dr Ared agreed that in the areas of practice where he also dealt with ophthalmologists, he also discussed developments in the field, new treatments and new drugs with them. He agreed that the role optometrists play in the broader eye care profession was valued by ophthalmologists, especially because in Australia the optometrists saw 80% of the patients for initial primary care.
170 I accept Dr Ared’s evidence. It is relevant to identifying the relevant class of addressees. It throws light on the relationship or interrelationship between optometrists and their work and ophthalmologists and their work.
Consideration
171 I have set out the four pleaded representations at [7] above.
172 For the reasons which follow, I accept that there is a class of addressees and the relevant class is ophthalmologists who are qualified to prescribe and inject Eylea and Lucentis.
173 Professor Paul Mitchell, whose expertise I accept, said he was not aware whether the prescription of Eylea and Lucentis was formally restricted to ophthalmologists but in practice only ophthalmologists prescribed those drugs because the PBS authority prescription forms required a statement from a medical practitioner that the patient had neovascular AMD and evidence to support that diagnosis, which was typically photographs from a fluorescein angiogram, that needed to be sent to Medicare, and other details of the clinical aspects of wet AMD cases. Such a diagnosis required specialist training that only ophthalmologists would have. For similar reasons, in practice in Australia Lucentis and Eylea were only administered by ophthalmologists.
174 To similar effect was the evidence of Dr Andrew Crawford, whose expertise I also accept, who said that Lucentis and Eylea could be prescribed by any medical practitioner but PBS benefits were only available from ophthalmologist prescriptions and therefore, in practice, only ophthalmologists tended to prescribe Lucentis and Eylea. Similarly, in practice administration of Lucentis and Eylea was only by, or directly under the control of, ophthalmologists.
175 In my opinion, the relevant class of addressees is ophthalmologists, as it is they who prescribe either Lucentis or Eylea. I accept that optometrists have an important role in referring the patients they see to an ophthalmologist but on the evidence they do not participate in or affect the prescription decision. I accept the applicant’s submission that the Court should infer that optometrists were targeted in the way they were because Bayer assessed that there was real value to it in doing so, notwithstanding that optometrists do not prescribe or inject Eylea, but this does not mean that that targeting had a relevant effect. That is, it is correct to say that the words used were directed at and were seen by optometrists as well as ophthalmologists but this was of no relevant consequence. The effect on optometrists was an effect of general awarenesss of Eylea as a newly available drug for the treatment of wet AMD by intra-vitreal injection. I refer here in particular to the evidence of Dr Ared at [167]–[170] above as to his practice of provisional diagnosis and referral to an ophthalmologist. Any such understanding did not affect an ophthalmologist in deciding whether to prescribe Eylea, either at all or in preference to Lucentis: see my findings in relation to the ophthalmologists’ decision-making processes at [150]–[152]. Any effect of the words used on an hypothetical optometrist was too general or diffuse to be taken into account in terms of the representations conveyed, causation or loss. I therefore accept the respondent’s submission that because the evidence does not support the conclusion that optometrists had either a direct or indirect role in the process of selection of Eylea, rather than Lucentis, as the medicine with which to treat a particular patient, optometrists should not be regarded as a relevant part of the audience. I find that optometrists did not suggest or advocate that an ophthalmologist should prescribe a particular wet AMD drug for the patient as part of any referral of a patient. I therefore refer to the class of addressees as ophthalmologists. I include in this class not only the approximately 25–50% of ophthalmologists who prescribed and injected the wet AMD drugs but also those likely to prescribe and inject such drugs, Lucentis or Eylea. The length of experience will, of course, vary but in each case the ophthalmologist will be a highly trained and knowledgeable specialist doctor making a serious decision and taking into account factors similar to those of which Professor Mitchell and Dr Crawford gave evidence.
176 My general observation is that the pleaded representations seek to give to the words used in any of the forms involved in the acts of publication (the words used) a meaning of invariability which the words do not convey to their audience, particularly given the inherent variability of patients and their conditions and the professional judgment involved. The gist of the First Representation is “for all patients”; of the Second Representation is “for each and every patient”; of the Third Representation is “without qualification”; and of the Fourth Representation “all Eylea patients” and “all Lucentis patients”.
177 In construing the words used and deciding whether the representations were conveyed, I take into account the evidence adduced by Bayer as to how an ophthalmologist would decide to prescribe and administer one of these drugs to a patient. I also take into account that ophthalmologists were aware of the respective dosage regimes of Lucentis and Eylea: see [152] above. A high level of interest in and awareness of the relevant scientific information is also evidenced by a series of letters Bayer sent to ophthalmologists in response to enquiries made by them to Bayer sales representatives as to particular aspects of the operation of the medicine. I have also taken into account that the words used were comparative rather than unilateral so there is no room for resort to notions of mere advertising puffery. The same approach is required by the specific nature of the words used. I accept the submission of the applicant that I should proceed on the basis that the words were put forward deliberately and with some precision. I also accept the submission of the applicant that those who engage in comparative advertising ought take particular care to ensure that the comparisons are accurate because errors made in comparative advertising may have a greater potential to mislead ‘consumers’ than statements made in unilateral advertisements.
178 In addressing these issues I have consciously avoided poring over the material divorced from the context in which it would have been seen and have assessed the words used in the circumstances in which they were read and seen, perhaps fleetingly, by their audience. The posters were displayed at professional meetings of ophthalmologists where other medical and scientific information about both Eylea and Lucentis was also available. These professional meetings were primarily scientific presentations of interest to ophthalmologists where both Novartis and Bayer promoted their products by reference to scientific and clinical material. Each of the advertisements was published in issues of either mivision or the Journal of Clinical & Experimental Ophthalmology. Baby banners were generally displayed at seminars for optometrists where the only ophthalmologists who attended were those presenting. The Detail Aid was used in some of the detailing visits, as set out above. Within the Detail Aid only 1 page within the “Home” tab (not being the first page) and 1 page within the “Summary” tab (not being the first page) contained the words about which Novartis complains. The attendee workbook was an eight page booklet available at the launches in Sydney, Melbourne, Brisbane and Adelaide, page eight of which contained the material about which Novartis complained.
179 I have also taken into account the principles in the authorities relied on by the parties, as follows.
180 At the forefront of the applicant’s submissions was Australian Competition and Consumer Commission v TPG Internet Pty Ltd [2013] HCA 54; (2013) 250 CLR 640 (TPG Internet). That case concerned an offer to consumers of an attractive price for the ADSL2+ service which TPG Internet Pty Ltd (TPG) supplied. The advertisements prominently displayed the offer to supply broadband internet ADSL2+ service for $29.99 per month. Much less prominently, the advertisements qualified this offer, stating that it was made on the basis that the ADSL2+ service was available only when bundled with a home telephone service, provided by TPG through landline technology, for an additional $30 per month (with a minimum commitment of six months). In addition, there was a setup fee of $129.95, plus a deposit of $20 for telephone charges. The primary judge upheld the Australian Competition and Consumer Commission’s claim that the advertisements were misleading and deceptive by reason of the disparity between the prominent headline offering the service at an attractive price and the less prominent terms qualifying that offer. The primary judge found that TPG’s target audience consisted of “the broad class of Australian consumers around mainland capital cities who were users or potential users of broadband internet services”. The primary judge found that there should not be imputed a high level of knowledge about broadband internet to the ordinary or reasonable consumer. The target audience included first time users of ADSL2+ services, the primary judge found, and the ordinary or reasonable consumer would not have any starting assumption as to whether TPG’s offering was of a separate or bundled service, and would rely on the advertisement for information as to the service offered. The primary judge found that each advertisement had the same dominant message namely: “Unlimited ADSL2+ for $29.99 per month.” The ordinary or reasonable consumer taking in only the dominant message would have the impression that the entire cost of the service was $29.99 per month, with no other charges and no obligation to acquire another service and the balance of the advertisement which contained that information was not given sufficient prominence to counter the effect of the headline claim. The dominant message was false because to acquire unlimited ADSL2+ for $29.99 per month, the consumer was also obliged to rent a home telephone line from TPG and to pay an additional $30 per month for it. The bundling condition operated to double the headline advertised monthly charge and for many consumers would involve the acquisition of a service extra to the service they were interested in acquiring. In those circumstances, the primary judge concluded that the information about TPG’s bundling condition needed to be quite clear and prominent if it was to correct the misleading impression of the message. Similar findings were made about the setup condition. The primary judge imposed a pecuniary penalty of $2 million, amongst other orders.
181 On appeal to the Full Court of the Federal Court, all but three of the primary judge’s findings of misleading conduct were set aside and the pecuniary penalty was reduced to a total of $50,000 in respect of the findings of infringement which were upheld. The Full Court held that the revised television advertisement, initial and revised radio advertisements, initial and revised newspaper advertisements, initial and revised online advertisements and public transport advertisements were not misleading. The High Court held that their Honours’ conclusion reflected differences in point of principle with the approach taken by the primary judge, particularly in relation to the primary judge’s view that the “dominant message” of the advertisements was of critical importance in determining whether they were to be characterised as misleading.
182 The High Court held, Gageler J dissenting, that the Full Court erred in setting aside the findings of the primary judge as to the extent of TPG’s contraventions of the Trade Practices Act 1974 (Cth). The primary judge’s assessment of the appropriate pecuniary penalty of $2 million was restored.
183 After noting, at [39], that there must be a sufficient causal link between the conduct and the error on the part of persons exposed to it, French CJ, Crennan, Bell and Keane JJ said, at [45], that the Full Court erred in holding that the primary judge was wrong to regard the “dominant message” of the advertisements as of crucial importance. Further, the Full Court erred in failing to appreciate that the tendency of TPG’s advertisements to mislead was not neutralised by the Full Court’s attribution of knowledge to members of the target audience that ADSL2+ services may be offered as a “bundle”. At [47], the plurality distinguished Parkdale Custom Built Furniture Pty Ltd v Puxu Pty Ltd (1982) 149 CLR 191 (Parkdale) on the basis, first, that TPG’s target audience did not consist of potential purchasers focused on the subject matter of their purchase in the calm of the showroom to which they had come with a substantial purchase in mind. The audience could not have been expected to pay close attention to the advertisement; certainly not the attention focused on viewing and listening to the advertisements by the judges obliged to scrutinise them for the purposes of the proceedings. Secondly, the plurality said, at [48], the Full Court did not recognise that the tendency of the advertisements to mislead was to be determined not by asking whether they were apt to induce consumers to enter into contracts with TPG, but by asking whether they were apt to bring consumers into negotiation with TPG rather than with one of its competitors on the basis of an erroneous belief engendered by the general thrust of TPG’s message. Thirdly, the plurality said, at [51], this was not a case where the tendency of TPG’s advertisements to lead consumers into error arose because the target audience might be disposed, independently of TPG’s conduct, to attend closely to some words of the advertisements and ignore the balance. The tendency of TPG’s advertisements to lead consumers into error arose because the advertisements themselves selected some words for emphasis and relegated the balance to relative obscurity. The Full Court erred in failing to appreciate the implication of its finding that “many persons will only absorb the general thrust”, which was to recognise the effectiveness of the selective presentation of information by TPG. The plurality said at [52]:
It was common ground that when a court is concerned to ascertain the mental impression created by a number of representations conveyed by one communication, it is wrong to attempt to analyse the separate effect of each representation. But in this case, the advertisements were presented to accentuate the attractive aspect of TPG’s invitation relative to the conditions which were less attractive to potential customers. That consumers might absorb only the general thrust or dominant message was not a consequence of selective attention or an unexpected want of sceptical vigilance on their part; rather, it was an unremarkable consequence of TPG’s advertising strategy. In these circumstances, the primary judge was correct to attribute significance to the “dominant message” presented by TPG’s advertisements.
(Footnote omitted.)
Justice Gageler dissented, not because he disagreed with the statements of legal principle of the plurality but because he could not read the reasons of the Full Court of the Federal Court as having ignored them: see [72].
184 In my opinion, while accepting, of course, the statements of principle by the High Court in TPG Internet, there is no real similarity between the facts and circumstances of that case and those of the present case. First, the class of addressees is entirely different. Secondly, the content of the material in question is quite different as I do not read the present materials as having a separate dominant message. In part this is because of the form of the representations but also because of their meaning. Thirdly, the circumstances in which a member of the class of addressees would or might be influenced by the material is very different from a representation apt to bring a ‘consumer’ into negotiation with Bayer rather than with Novartis on the basis of an erroneous belief engendered by the general thrust of Bayer’s message. Fourthly, due account must be taken of the different media involved: multimedia, including television and radio, in TPG Inernet compared with print (or its equivalent in the case of the Detail Aid) in the present case. I do not therefore accept the importance placed by Novartis on [55] of TPG Inernet which concerned the drawing of an inference of an effect from a representation made in terms apt to create a particular mental impression and intended to do so.
185 The applicant also referred to Australian Competition and Consumer Commission v Jewellery Group Pty Ltd [2012] FCA 848; (2012) 293 ALR 335 (Jewellery Group); Apotex Pty Ltd (formerly GenRx Pty Ltd) v Les Laboratoires Servier (No 2) [2008] FCA 607; (2008) 77 IPR 1 (Apotex); and Sidhu v Van Dyke [2014] HCA 19; (2014) 251 CLR 505 (Sidhu).
186 Jewellery Group related to six catalogues and a flyer which promoted 44 separate items of jewellery the subject of the proceedings. There was dual pricing, consisting of “strikethrough/sale pricing” and “was/now pricing”. The applicant’s case was that by making the statements of price in respect of each of the items of jewellery in the catalogues and the flyer, the respondent represented that consumers would save an amount of money, being the difference between the strikethrough price and sale price or the “was” price and the “now” price if the items were purchased during the relevant catalogue sale period. Justice Lander considered the audience, which he held to be very wide and to consist of aware and unaware customers, and the representation or representations conveyed by the catalogues and the flyer which, his Honour said at [26] required considering the words used in the advertisements in their context and in their entirety, and giving those words a fair and reasonable meaning. It was not appropriate to give the words a strained, false or unreasonable meaning. Rather, the words ought to be understood in their ordinary sense. The words would not be studied closely by the reasonable reader, Lander J said, but they would be read by that reader for the purpose of determining what it was that the retailer was offering. If the words were qualified in any way, then that qualification must be recognised and given effect to as an ordinary and reasonable reader would. The words may have more than one meaning. If they did, the question to be determined was whether each meaning that was reasonably open amounted to a representation that was misleading or deceptive, or likely to mislead or deceive. The applicant Novartis also relied on the following paragraphs, [61]–[62], where Lander J said:
I also reject the respondent’s second contention, namely that the applicant failed because it did not lead any evidence from a consumer from the class of persons to whom the representations were made (that is readers of the catalogues and the flyer) that the consumer understood the dual pricing in the catalogues and the flyer as the applicant contended. The respondent contended that the evidence it led from Mr Murphy, the chief executive officer of the respondent, was that he was not aware of any consumer complaints concerning the respondent’s pricing, which meant that the applicant’s failure to call any evidence as to the understanding of the audience leads to the conclusion that the applicant’s case was bound to fail.
The applicant is not bound to call the evidence contended for by the respondent, but may of course do so if the evidence is available and if the applicant considers that the evidence would assist the court in reaching its decision as to whether the representations amount to misleading or deceptive conduct: [Taco Co of Australia Inc v Taco Bell Pty Ltd (1982) 42 ALR 177] at 202 per Deane and Fitzgerald JJ. The applicant is entitled to ask the court to make a finding as to what a reasonable member of the class of persons identified by the court would make of the advertisement and would understand the representation contained in the advertisement to mean. The court must determine, objectively, the representation that was conveyed, and whether the representation is misleading or deceptive, or likely to mislead or deceive. Evidence that members of the public have actually been mislead (sic) is neither conclusive nor necessary: [Australian Competition and Consumer Commission v Ascot Four Pty Ltd (2008) 250 ALR 467; [2008] FCA 1295] at [90] and [97] per Mansfield J; [Ascot Four Pty Ltd v Australian Competition and Consumer Commission (2009) 176 FCR 106; [2009] FCAFC 61] at [34](iii); Parkdale at CLR 198–9 per Gibbs CJ; Taco Co at 202 per Deane and Fitzgerald JJ. The applicant proceeded on that basis. It follows then that the evidence led by the respondent from Mr Murphy is not dispositive.
I accept these statements of principle.
187 As I understood it, the point sought to be derived by Novartis from Apotex was that advertisements containing representations may be found to mislead or deceive or be likely to mislead or deceive medical practitioners. I accept that. Everything depends on the facts.
188 I also note that, for example, in relation to the stamp representations in Apotex, Bennett J held at [57] that the sections of the public to whom the representations were directed were medical practitioners (doctors), pharmacists and patients: no reason was advanced, her Honour said, to subdivide those groups into any subclasses or areas of specialisation. Her Honour went on to assess the reactions or likely reactions of the members of each class to whom the conduct was addressed: see [59]–[63] and [65]–[81] in relation to doctors and pharmacists.
189 In relation to the advertisements held misleading or likely to mislead medical practitioners, Bennett J held, at [119]–[120], that reference to “improved stability” in the first whole-page advertisement represented that there was a genuine improvement or benefit, or superior product performance of new Coversyl over its generic substitutes and that this was a reason for the doctor to prevent generic substitution. The other representations in the first whole-page advertisement were not misleading as alleged.
190 For the second whole-page advertisement, Bennett J held that the reference to “now indicated for stable coronary artery disease” was misleading and, by linking that statement to perindopril arginine (new Coversyl), the advertisement represented that perindopril erbumine was not so indicated and that also amounted to a misrepresentation as perindopril erbumine had been so indicated since 2005.
191 As I have said, I accept, of course, that representations may mislead or deceive medical practitioners. I note, however, that Bennett J at [130], in relation to the second whole-page advertisement, referred to ordinary and reasonable doctors, including general practitioners not specialised in hypertension and the mechanisms of blood pressure regulation, and was not satisfied that ordinary and reasonable doctors would generally have been aware of the Europa study and so discount the message otherwise conveyed by the advertisement. That is not the position in the litigation with which I am concerned.
192 Sidhu was referred to, first, for its citation of Gould v Vaggelas (1984) 157 CLR 215 at 236, where Wilson J, with whom Gibbs CJ and Dawson JJ agreed, speaking of an action in deceit, said:
If a material representation is made which is calculated to induce the representee to enter into a contract and that person in fact enters into the contract there arises a fair inference of fact that he was induced to do so by the representation.
Secondly, the respondent relied on Sidhu in relation to the second of four reasons given by the plurality for concluding that the respondent in that case discharged her onus of proof. The second reason was that it was not necessary that the conduct of the party estopped should be the sole inducement operating on the mind of the party setting up the estoppel. As elaborated on by Gageler J in a separate concurring judgment, the respondent did not need to establish that the belief to which she was induced by the appellant’s representations was the sole or predominant cause of the course of action or inaction she took but, in the language of Rich, Dixon and Evatt JJ in Newbon v City Mutual Life Assurance Society Ltd (1935) 52 CLR 723 at 735, she did need to establish that the belief was a “contributing cause”.
193 The respondent relied on AstraZeneca Pty Ltd v GlaxoSmithKline Australia Pty Ltd [2005] FCA 1645. That case concerned Seretide advertisements which included text such as “Imagine if you had the power to totally control asthma …” and “Symptomatic Patients” – “Looking for control?12 Switch to Seretide”. The target audience was general practitioners: see [39]. The claim, set out at [7] of the judgment, was that in each of the Seretide advertisements, the respondent made representations to the effect: (1) that all or virtually all asthma patients will achieve 100% control or total control of all asthma symptoms by using Seretide; (2) alternatively, that a majority (i.e. greater than 50%) of all asthma patients will achieve 100% control or total control of all asthma symptoms by using Seretide; (3) alternatively, that a sufficiently high proportion of all asthma patients will achieve 100% control or total control of all asthma symptoms by using Seretide to justify the use of the brand slogan “Seretide Total Control”; (4) that 41% of all patients taking part in the study known as the “Gaining Optimal Asthma Control” study published in the American Journal of Respiratory & Critical Care Medicine in 2004 (GOAL Study) achieved 100% control of all asthma symptoms by using Seretide; (5) alternatively, that 41% of all patients taking part in the GOAL Study achieved “total control” (as that term was defined in the Seretide advertisements) of all asthma symptoms by using Seretide; (6) that 71% of all patients taking part in the GOAL Study achieved “well controlled asthma” (as that term was defined in the Seretide advertisements) of all asthma symptoms by using Seretide; and (7) that the patient results of the GOAL Study identified in (4), (5) and (6) (sic) would be achieved or would be likely to be achieved in clinical practice.
194 At [112], Edmonds J said that a difficulty for AstraZeneca’s case was that it led no evidence to suggest that general practitioners relied on the information/representations made in advertisements of the kind comprising the Seretide advertisements, and appearing in publications of the kind in which some of those advertisements appeared, to prescribe medication for those of their patients suffering from the symptoms of asthma. Indeed, such evidence as was led indicated that general practitioners placed no reliance on such advertisements in prescribing medications for their patients with symptoms of asthma: that they would merely regard such advertisements as part and parcel of wider marketing campaigns undertaken by drug companies to promote their products, and not as a source of information by reference to which they prescribed medications for their patients. At [113], Edmonds J said that the evidence indicated that general practitioners relied on sources of information, including their own familiarity with and experience in the form of patient acceptance and results achieved in prescribing Seretide, other than any representations conveyed by the Seretide advertisements, as a basis for prescribing medication to their patients suffering from the symptoms of asthma. Seretide was first available in Australia from August 2000 and had been extensively prescribed prior to the publication of the Seretide advertisements. At [114], Edmonds J said that in the absence of any evidence that any representations conveyed by the Seretide advertisements would be relied upon by general practitioners in prescribing medication for their patients suffering from the symptoms of asthma, it was impossible to find any causal connection between the conduct complained of and any inferential error or erroneous assumption of the kind suggested. That would be sufficient, Edmonds J said, to dispose of AstraZeneca’s case. At [123], Edmonds J said this:
Even assuming that one or more of the representations complained of can be made out as being conveyed by the GOAL Mailer, the logical causal connection between the representations and the inferred error is so tenuous that it is not possible to arrive at any finding that the representation is false and misleading. This conclusion is supported by the following matters:
(1) The balance of the advertisement, viewed as a whole, …
(2) The qualifying footnotes at all levels …
(3) The truth of the extract from the GOAL Study, with or without the omitted words …
(4) The audience to which the advertisements was directed …
(5) The prescribing practises of GPs …
(6) The experience of GPs in dealing with asthma symptoms and their familiarity and experience in dealing with Seretide and Symbicort …
I understood Bayer to be submitting that there were factual similarities between the case before Edmonds J and the present case.
195 I accept whether or not the relevant audience was or was likely to be led into error must be an evidence-based conclusion rather than a conclusion based simply on the fact that advertising is taking place. In my opinion, Novartis’ proposition that advertising seeps through to ophthalmologists may be accepted but it does not follow that it affects ophthalmologists in deciding to prescribe a drug for the treatment, by intra-vitreal injection, of wet AMD.
196 As to Novartis’ proposition that the Court need only conclude that it is “reasonably open” that a representation is conveyed, Bayer submitted that the issue was not a lawyer’s test but the effect or likely effect of conduct on the minds of the relevant class of recipients of the communication and whether the misconceptions or deceptions alleged to arise or to be likely to arise were properly to be attributed to the ordinary or reasonable members of the relevant class: Campomar Sociedad, Limitada v Nike International Ltd [2000] HCA 12; (2000) 202 CLR 45 (Nike) at [105]. Bayer also submitted that a Full Court of this Court had made clear that the issue could not be considered in the abstract but needed to be considered from the perspective of the section of the public to whom the promotional material was directed, namely, ophthalmologists. In that respect Bayer relied on Apotex at [32] and Astrazeneca Pty Ltd v GlaxoSmithKline Australia Pty Ltd [2006] FCAFC 22; [2006] ATPR 42-106 at [35], [42] and [49]. I agree.
197 In Nike, at [105], the High Court said that in an assessment of the reactions or likely reactions of the “ordinary” or “reasonable” members of the class of prospective purchasers of a mass-marketed product for general use, the court may well decline to regard as controlling the application of s 52 those assumptions by persons whose reactions are extreme or fanciful. The initial question which must be determined was whether the misconceptions, or deceptions, alleged to arise or to be likely to arise were properly to be attributed to the ordinary or reasonable members of the classes of prospective purchasers.
198 I have already considered Apotex. The paragraph relied on by the respondent, Bayer, makes the point, in the context of that case, that it was the doctor who made the initial decision whether to permit substitution of a generic product for a branded product and the pharmacist who then decided, if permitted to do so, whether to substitute a generic product for a branded product.
199 In Astrazeneca Pty Ltd v GlaxoSmithKline Australia Pty Ltd [2006] FCAFC 22; [2006] ATPR 42-106 at [42] the Full Court viewed each of the flyers and the advertisements as marketing documents directed by the respondent to ordinary or reasonable members of the community of general practitioners throughout Australia in the context of the product and sales history which the Court had set out. The Full Court was unable to find that any of them made the representations alleged and which I have set out above in relation to the first instance decision of Edmonds J. Earlier, at [35], the Full Court said that such representations as may have been made in the flyers and the advertisements were not made to identified individuals nor to the public at large. What the Full Court said, at [49], does not involve any question of principle but was a finding as to the flyers or advertisements in question. I also observe that, at [52], the Full Court noted the primary judge’s reference to AstraZeneca’s omission to lead evidence to the effect that general practitioners relied upon the representations said to be made by the respondent in prescribing medication for use by the asthma sufferers. The Full Court observed that the lack of such evidence would not be determinative of a case for alleged contravention of s 52.
200 I test the matter by reference to the steps identified by Gordon J in Australian Competition and Consumer Commission v Telstra Corporation Ltd [2007] FCA 1904; (2007) 244 ALR 470 at [14]–[15], where her Honour said that a two-step analysis is required. First, it is necessary to ask whether each or any of the pleaded representations is conveyed by the particular events complained of: Nike at [105]; National Exchange Pty Ltd v Australian Securities and Investments Commission [2004] FCAFC 90; (2004) 49 ACSR 369; (2004) 61 IPR 420 at [18] per Dowsett J (with whom Jacobson and Bennett JJ agreed); Astrazeneca Pty Ltd v GlaxoSmithKline Australia Pty Ltd [2006] FCAFC 22; [2006] ATPR 42-106 at [37]. That is, do the events singularly or collectively convey any of the representations? Second, it is necessary to ask whether the representations conveyed are false, misleading or deceptive or likely to mislead or deceive. This is a “quintessential question of fact”: Australian Competition and Consumer Commission v Telstra Corporation Ltd [2004] FCA 987; (2004) 208 ALR 459 at [49]. Her Honour was concerned with ss 52, 53(aa) and/or 53(c) of the Trade Practices Act.
201 I also refer to Parkdale, at 199, where Gibbs CJ said with reference to s 52:
… the section must in my opinion by regarded as contemplating the effect of the conduct on reasonable members of the class. … What is reasonable will or [sic] course depend on all the circumstances. The persons likely to be affected in the present case, the potential purchasers of a suite of furniture costing about $1,500, would, if acting reasonably, look for a label, brand or mark if they were concerned to buy a suite of particular manufacture.
The conduct of a defendant must be viewed as a whole. It would be wrong to select some words or act, which, alone, would be likely to mislead if those words or acts, when viewed in their context, were not capable of misleading. It is obvious that where the conduct complained of consists of words it would not be right to select some words only and to ignore others which provided the context which gave meaning to the particular words. The same is true of acts.
See also per Mason J at 209. I take the former of the propositions stated by Gibbs CJ to mean that the seriousness or importance of the purchase to the purchaser is relevant to the assessment of the respondent’s conduct.
202 In my opinion, a reasonable member of the class of addressees would not have understood the words used by Bayer to have the meanings of any of the four alleged representations (see [7] above) and none of the pleaded representations was conveyed by the particular events of which the applicant complains. This is not to say that ophthalmologists are immune or effectively immune from the effects of advertising but that they will, first, understand and, secondly, be affected by advertisements in the light of their training, knowledge and experience. I do not accept that a reasonable member of the class of addressees would stop at the words “fewer injections”. Nor do I accept that there is any material difference between the first form and the second form of the words used. In each case a reasonable member of the class would read the words as a single claim. In my view, the font size and colour difference in the first form is minor and insignificant in relation to the content, grammar and placing of the words the subject of those differences. These are factual matters and decided cases can only provide examples of what the courts have found to be conveyed in different factual circumstances.
203 As to the First Representation, in my opinion the words used do not mean that in clinical use, in accordance with the approved dosage, Lucentis is administered monthly for all patients. I am not persuaded that the (hypothetical) reasonable member of the class of ophthalmologists would take from the words, in context, the meaning of this alleged representation. In my opinion, the words used would be understood to mean, in accordance with the product information, that Lucentis is given monthly: the words say nothing, in my view, about Lucentis being administered in practice monthly for all patients, which is the gist of this alleged representation. The First Representation depends on an inference as to clinical practice which does not find a foothold in the language of the publication and which I do not draw. Neither do I draw the inference that the language refers to all patients. This allegation, correctly in my view, takes the words “in accordance with the approved dosage” to qualify both Eylea/aflibercept and Lucentis/ranibizumab.
204 As to the Second Representation, in my opinion the words would not be understood to mean that for each and every patient Lucentis will only be a clinically effective treatment if administered monthly. The words used are addressed ultimately to an ophthalmologist and I would not take them as having the meaning that for every patient Lucentis would only be a clinically effective treatment if administered monthly. There is no basis for concluding that the (hypothetical) reasonable ophthalmologist would proceed on the basis that each one of his or her patients was relevantly identical. I am not satisfied that such an ophthalmologist would assume that each and every one of their patients would require the same frequency of injection. Furthermore, Lucentis had been available for sale in Australia since 2007. The product information stated: “Lucentis is given monthly. The interval between two doses should not be shorter than 1 month. Although less effective, treatment might be reduced to one injection every 3 months after the first three injections (e.g. if monthly injections are not feasible).” In my opinion, such an ophthalmologist would understand the words used in light of that experience, including the product information of which he or she was aware so far as concerns the dosage regime: see [152] above. The question is an objective one so I do no more than note that if there was an ophthalmologist who had a different understanding, whether from recently becoming an “injector” or otherwise, he or she did not give evidence.
205 As to the Third Representation, my conclusion is the same and for similar reasons. This representation accepts that the words used direct the reader’s attention to the Lucentis product information but puts that those words misstate the effect of that product information, that is, that Lucentis was to be administered monthly “without qualification”. I am not satisfied that the (hypothetical) reasonable ophthalmologist would understand the words on the basis that the product information specified that Lucentis was to be administered monthly without qualification. I am not persuaded that such an ophthalmologist would consider that the words used stated that the product information would so specify, given the inherent variability of cases. Ophthalmologists are aware of the dosage regime in the product information: see [152] above. In my opinion, such an ophthalmologist would understand the words used in light of his or her experience, including his or her awareness of the product information. If there was an ophthalmologist who had a different understanding, whether from recently becoming an “injector” or otherwise, he or she did not give evidence.
206 As to the Fourth Representation, in my opinion this involves propositions in relation to the Second Representation and the Third Representation which I have rejected. First, I would not conclude that the (hypothetical) reasonable ophthalmologist would proceed on the basis that each one of their patients was relevantly identical. Secondly, Lucentis had been available for sale in Australia since 2007 so it is unlikely that such an ophthalmologist would consider that all Lucentis patients were alike and that the approved dosage was fixed. Thirdly, such an ophthalmologist was aware of the dosage regimes set out in the product information for Lucentis and Eylea respectively and understood what product information was. Fourthly, such an ophthalmologist would know that he or she had and exercised professional judgment by reference to the particular patient and the condition of that patient from time to time. In my opinion, such an ophthalmologist would understand the words used in light of his or her knowledge and experience. In summary, in my view the words do not mean that in accordance with approved dosages every Eylea patient receives fewer injections than every Lucentis patient. The thrust of the pleaded Fourth Representation is that the words used misstate the effect of the product information by misstating that the product information means that all Eylea patients receive fewer injections than all Lucentis patients. In my view such an ophthalmologist would not read the words used as so misstating the effect of the product information and as operating to deny professional judgment and the inherent variability of patients and their conditions.
207 The words “monthly Lucentis” were a key theme in the applicant’s representation case but, in my opinion they do not, in context, have the meaning that Lucentis is always injected monthly. Further, “monthly Lucentis” was a description applied to Lucentis in material produced by Novartis. The Lucentis product information indicated that it was to be given once a month, as did the ‘patient information document’ which was included in every box of Lucentis. Novartis also produced a brochure which referred to a “monthly regimen of Lucentis” demonstrating the best visual acuity outcomes in the clinical trials. The versions of the e-detailer in use by Novartis from July 2012 onwards contained multiple pages with the headline: “Rapid and sustained improvements in vision with monthly injections”. I accept Bayer’s submission that a comparison of the Novartis e-detailer with the Bayer Detail Aid shows that the concept of “monthly” Lucentis is given much more prominence in the Novartis e-detailer. As I have said, at [202] above, neither am I satisfied that the words “Eylea helps you and your patients with fewer injections” would have been read as by themselves bearing a meaning without the words which immediately followed them, whether in the first form or in the second form. I consider the meaning is the same and the form constitutes a single statement, notwithstanding the differences in the size and colour of the characters.
208 I have considered the applicant’s emphasis on the written materials of the respondent’s marketing department. The approach of the applicant in its written submissions was to place great emphasis on what Bayer’s marketing department developed as a strategy and to do so before focussing on the words, in context, of the alleged misrepresentations. The addressees would not, of course, have been aware of this material. Thus, contrary to the submissions on behalf of the applicant, it does not seem to me to be of overwhelming significance “that there was a key message imparted by the marketing department to the sales team and that that key message coincided with the first two and most prominent lines of the advertisements that were placed … in a variety of different publications”. So far as concerns the written representations of which Novartis complains, I prefer to read the words used in the context in which they were used rather than place weight on material that was not communicated to the addressees. So far as concerns the detailing, the one-on-one communications between the sales representatives and the ophthalmologists, I prefer the direct evidence of the sales representatives, both as to what they said and the context in which they said it. The marketing material was of course useful to the applicant for the purpose of cross-examining the respondent’s sales representatives but it rose no higher than that. I do not draw any Jones v Dunkel inference from Bayer’s failure to call the marketing personnel. Also, as I have set out at length, I accept the evidence of the respondent’s sales representatives.
209 One example of the marketing material emphasised by the applicant is a document prepared for Bayer by Ipsos Healthcare (International Custom Healthcare) dated 11 May 2012 and entitled “Developing and Optimizing Detail Aid for VEGF Trap Eye Communication”. The business objectives were described as being “To develop, test and refine the EYLEA detail aid, which will be used to optimise Bayer Healthcare’s communication strategy thus maximising its successful launch” and the research objectives were to “Evaluate the detail aid and make improvements with the objective of supporting the brand position and promoting uptake of VEGF Trap Eye vs. competing agents” and “Build a storyline/detail aid flow that best helps support the winning positioning”. The methodology was to interview 12 ophthalmologists described as being either retinal surgeons, specialists or ophthalmologists with a sub-speciality in retina. The key findings were stated to be simplicity, patient orientated outcomes, and data issues. Under “A simple story is needed” it was said: “The compelling story behind the product is essentially a simple one, care must be taken to avoid over-complicating the core brand story and diluting it.” Then, hightlighted as the key message to communicate to physicians was: “EYLEA has comparable efficacy and safety to ranibizumab with fewer injections”. The authors recommended amending the MOA section to further simplify the story”: the recommendations were that a summary of the MOA could be included in the dosing section to provide context to its inclusion; detail currently included in the opening section could be moved to an appendix or be part of a leave piece for those desiring further information – only the most scientifically minded would be interested; and if the MOA section was to be shown in full it required further context. Comparision to the MOA of ranibizumab would highlight the relevance of stronger binding and fewer injections.
210 I have set out this material at some length because the applicant relied on it strongly. However, in my view, what was recommended cannot be a substitute for; the results of the recommendation, the Detail Aid as in fact used, with its two disputed pages; or the circumstances in which the Detail Aid was deployed; and the context in which the disputed pages were seen. It would be a mistake, in my opinion, to conduct the objective exercise of giving meaning to the representations from this starting point.
211 I turn now to a bundle of documents on which Novartis placed great emphasis. I should say by way of preface, although nothing turns on it, that I do not accept that these documents were belatedly produced by Bayer, as submitted by Novartis, if by that it is intended to mean that Bayer was under an obligation to produce these documents earlier.
212 First was a document entitled “Marketing strategy & activities 2012, Field Force Training 23 March 2012 - Sydney”. The document is of some 80 pages. The key messages were stated to be: the market opportunity in wAMD in Australia is significant; regulatory approval granted; reimbursement expected by October 2012; relationships with stakeholders established; patient familiarisation and patient support programs well underway; and organisational build up of capabilities newly completed. The following pages deal with: Unique market — major opportunity; Sales Development wAMD market 2007–2011; Actual patient numbers for wAMD from Medicare Australia confirm our estimations; In Australia, patients are mainly seen & treated in private practices; In Australia, optometrists play an important role in the referral process; Australia has the highest treatment rate, with 95% of eyes that will receive treatment after diagnostic: Australian physicians have a high rate of “treat and extend” (47% versus 53% for “fixed intervals”); In Australia, visual acuity and evolution is mainly measured in “letters lost”; In Australia, Lucentis leads the market with almost 90% injection shares; There is a high frequency of injections in Australia with injections performed every ~8 weeks in year 1; XXXXXXXXXXXXXXXXXX XX XXX XXXXXX XXXXX XXXXXX XXXX XXXXXX XXXXXXXX XXXXXXXXX XXX XXXX XXXX XXXX XXXXXXXXX XXXX XXXXXXX XXX XXXXXXXXXX XXXX XXXX XXXXX XXXXXXXXX XXXX XXXXXXXXXX XXXXXXXXX XXXXXXX XXX XXX XXXXXXXXX XXXXXXX XXX XXXXX XXXXX XXXXXXX XX XX XXXX XXXX XXXXX XXXX XXXX XXXXXX XXXXXXXX XXXXXX XXXX XXXXXX XXXXXXXXXX XXXXX XX. There was then a tactical plans overview with a key activities timeline for 2012 and key marketing activities. Many pages later, following the page titled “Promotional Materials”, there is reference to: product information and consumer medicine information; reprints of published papers including those by Papadopoulos et al and Ohr et al; Research Review; CLM — Focus on e-detailer; and Sneak Preview … There is then a section entitled “Special Projects” and within that some pages directed to product familiarisation programs (PFP). The Overview referred to: Ophthalmologists in Australia are asking for access to EYLEA; The PFP is an unique opportunity to provide Eylea to treaters prior to reimbursement under controlled condition; The PFP will generate early prescriber experience with EYLEA ensuring treaters trial the drug prior to reimbursement; Establish Byer’s leader image despite new comer to the market; Ensure good sales from day 1 as patients will be prescribed reimbursed product as of listing; Objective: maximise uptake of EYLEA upon PBS listing; From drug availability (1 July 2012?) until PBS listing (1 Oct 2012); Limit 10 pts per prescriber under MA Code; Maximise prescriber enrolment (~200) to maximise patient numbers; 1500-2000 patients in PFP prior to PBS launch; Territory Managers will enroll (sic) retina specialists and ophthalmologists with retina interest and ensure clinic staff buy in; Marketing agency developing materials & forms; External data management; and DHL distribution. On the sixtieth page of the document there is a reference to “Every second month administration: less frequent injections and cost”. There is then a reference to “Narrative wheel workshop (11 Jan 2012)” where there appears: “The cross functional team developed a draft core story for EYLEA that will be the foundation for all communication” and, on the next page: “The workshop team identified responses to key issues and interests of each audience”. The former page includes a reference to “Current treatments help sustain vision but require monthly injections in the eye” and, in relation to Eylea, “It is a significant improvement that delivers the benefits seen with existing treatments but requires less frequent injections.” The latter, at least under the legible wheel, included similar words but also a statement: “All information is contained in the PI”.
213 The next Bayer document from the marketing department entitled “Other resources” dated 20 April 2012 contains a summary of Lucentis trials. Under the heading “Pivotal Trials” ANCHOR/MARINA were described as: three monthly injections followed by monthly injections for one year; nearly all patients with monthly injections maintained vision (lost less than 15 letters) for up to 2 years and vision improved by at least 15 letters in up to 40% of patients. Up to 42% achieved 20/40 or better at 2 years.
214 The third Bayer document from the marketing department was entitled “Q&A and objection handling”, dated 20 April 2012. One of the possible questions was “Year two data shows no difference to Lucentis”, with one of the suggested answers being “It is off label for us to discuss”.
215 The fourth Bayer document from the marketing department was entitled “Key Selling Messages Q2-Q3 2012” dated 20 April 2012. It contains a similar “Narrative wheel workshop (11 Jan 2012)” to the one which I have referred to above. It also repeats, with reference to the VIEW studies, although not on every page, “Dosing: Fewer injections”.
216 The fifth Bayer document from the marketing department was a “Marketing Update”, dated 8 June 2012. It contained a responder pad survey of attendees at the Australian and New Zealand Society of Retinal Specialists conference in relation to their anti-VEGF treatment pattern and also a responder pad survey of current practices against the question “If EYLEA was introduced, estimate the decrease in current injections vs Lucentis”.
217 The sixth Bayer document from the marketing department was entitled “Cycle Meeting: Marketing update”, dated 19 September 2012. A key message was stated to be “Predictable: less frequent dosing”. There were other references in the document to less frequent injections, one of which was followed by a statement suggesting that the VIEW trials provided evidence for the assertion.
218 The seventh Bayer document from the marketing department was entitled “Market overview and competition”, dated 7 February 2013. XXXXXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXX XXXXX XXXXXXXXXX XXXXXX XXXXXX XXXXXXXX XXXXXX XXX Answers were suggested with reference to the clinical studies, including the VIEW trials.
219 The eighth Bayer document from the marketing department was entitled “Session 4: 2013 Tactical Plans”, dated 7 February 2013. Nothing of significance was said to flow from this document.
220 The ninth Bayer document from the marketing department was entitled “Strategy and Messaging” and dated 7 February 2013. It contains a reference to a key message of “Predictable: less frequent dosing” but contains frequent and extensive references to the VIEW studies. One page was entitled “Burden of Treatment with Current Standard of Care – Lucentis”, stating that a “fixed” regular administration of Lucentis was supported by the findings from pivotal trials: once monthly and referred by name to the various trials. The next page was headed “Reduced Burden of Treatment with EYLEA®” and referred to a predictable regimen with fewer office visits for treatment and other associated tests.
221 The tenth and last Bayer document from the marketing department was entitled “Cycle Meeting: Marketing Update” dated 18 March 2013. The first substantive slides dealt with the awareness of Eylea among respondents in Australia and the Eylea market share two months post launch. It referred to an overall higher rating than Lucentis; differentiated from Lucentis on “therapeutic effect for 8 weeks” and “lower number of injections”. The next page referred to the top messages recalled for Eylea including “labeled dosing”; 31% do not recall discussing about “fewer visits” and 77% about “no requirement for monitoring between injections”. The other relevant page was a reference on page 30 of this 50 page document to all of the following being Eylea’s key selling messages: Eylea maintains eyesight with a lower cumulative risk of injection related adverse events; unique MOA; and reduce burden and a predictable treatment.
222 In my opinion, these internal marketing materials of Bayer were remote from what I have found to have been said by the Bayer sales representatives to ophthalmologists. I do not find there was a single isolated key selling message in those materials. Nor do I find that those materials prove the intention of Bayer to convey an isolated message of fewer injections or that there was an intention to mislead by publishing that message. These marketing materials were not put to the witnesses called by Bayer and no application was made by Novartis to recall any of those witnesses for that purpose.
223 I do not accept the submission put by Novartis that “fewer injections” became the bedrock for the promotion of Eylea from the first half of 2012 and into 2013, nor the associated submission that unsurprisingly the key message was squarely represented in the impugned publications. Neither do I accept that the “fewer injections” message was deployed divorced from the context of the product information, for each of Lucentis and Eylea, and the VIEW studies, which is the burden of the applicant’s written submissions at [38]–[42]. The basis for my conclusion will be apparent from the review in these reasons of the evidence of the Bayer sales representatives. In particular, in that connection, I do not accept the construction of the evidence of Ms Reznichenko for which Novartis contends at [38] of its written submissions. As I have set out at [143]–[145] above, Ms Reznichenko’s evidence was that only one of the attributes of Eylea was fewer injections and all of these were taken from the clinical trials described in the product information.
224 I reject the submission put on behalf of Novartis that “the consistent and central message” inculcated in the sales representatives was one of “fewer injections”, which message was not tethered to the product information. As I have said, that submission is inconsistent with the evidence of the Bayer sales representatives and is also inconsistent with the tenor of the Cortex notes which were a more or less contemporaneous summary of their individual communications with ophthalmologists and optometrists.
225 In my opinion, messaging or dominant messaging should not be used as a substitute for the meaning conveyed by the words used, by which I mean all of the words used in their context. TPG Internet does not require or support that approach. Neither, in my view, do the other authorities relied on by the applicant Novartis. The starting point for a conclusion based on dominant messaging, as explained in Telstra Corporation Ltd v Optus Communications Pty Ltd [1996] FCA 1035; (1996) 36 IPR 515 at 524, is that a false dominant impression is conveyed: see also Singtel Optus v Telstra [2004] FCA 859 at [40], on which the applicant Novartis relied. Global One Mobile Entertainment Pty Ltd v Australian Competition and Consumer Commission [2012] FCAFC 134 at [84]–[106] concerned television advertisements to consumers of mobile telephone premium services. Further, the form and terms of the advertisements were quite different to the impugned publications in the present case.
226 I have also taken into account the particular circumstances of each act of publication as set out above at [23] and [178].
227 I next deal with the disputed acts of publication of which there were nominally six, those numbered 7, 12, 23, 29, 36 and 46 in the table at [23] above.
228 As to 7, alleged to be a poster (first form), Novartis relies on the oral evidence of Ms Adams and Ms Martinek. Bayer contends that it displayed the baby banner (first form) at the Eylea product launch at Four Points by Sheraton, Sydney on 24 October 2012. In her first affidavit, Ms Adams deposed that she attended the official launch on that date in Darling Harbour but did not recall “whether there were any baby banners on display at that meeting.” In her oral evidence, Ms Adams agreed that it was conventional for Bayer to make use of marketing materials on significant occasions where there were lots of ophthalmologists being gathered and, although she could not recall any posters being used at all, she could not give a reason why the poster would not have been on display at the launch. Her evidence was she could not recall it being used. Ms Martinek’s oral evidence was that she thought the big poster was there at the launch of Eylea and she could not think of any rational reason why it would not have been there. I find that a poster, rather than a baby banner, was used by Bayer. It is not necessary to decide whether a baby banner was used as well at this launch on 24 October 2012 as there is no allegation of such an act of publication.
229 As to 12, a baby banner (first form) at the Eylea launch event in Brisbane on 24 October 2012, Novartis relies on the evidence of Mr Hodges, one of the two Eylea territory managers in Queensland. Mr Hodges attended the Eylea launch meeting on 24 October 2012. He annexed to his affidavit a copy of a baby banner which he deposed he thought was displayed at the launch meeting. In cross-examination, he agreed that he used the baby banner and took it to the product launch. In my opinion, it is more probable than not that the baby banner (first form) was displayed at the launch meeting on 24 October 2012 and I so find.
230 As to 23, a baby banner at the Tasmanian RANZCO meeting, Novartis relies on the affidavit of Ms Doyle, employed by Bayer as the territory manager, ophthalmology for South Australia and Tasmania. She attended the RANZCO meeting on 1 and 2 February 2013 and she recalled a large Eylea branded banner set up behind the trestle table at Bayer’s trade booth at the conference. She was responsible for setting up the booth. In her oral evidence, Ms Doyle said there was not a big poster at the trade booth but her recollection was that the Eylea baby banner was displayed, sitting on the trestle table. I find that a baby banner was displayed and it was the baby banner in the second form given the date of this event, that is, 1 to 2 February 2013.
231 As to 29, a baby banner at the Luxottica educational meeting in Darling Harbour, Novartis relies on the affidavit and oral evidence of Ms Adams, at the time an Eylea territory manager. Ms Adams deposed to having attended the April 2013 Luxottica educational meeting and to displaying a baby banner on a registration table outside the lecture room. I find that a baby banner was displayed. On the evidence, I am not able to say which form of baby banner was used at this meeting: Ms Adams had both forms in her possession; she could not recall which form she used; and as at April 2013 there was no reason for her to prefer one over the other.
232 As to 36, a baby banner at an educational meeting for Luxottica optometrists, Novartis relied on the affidavit of Ms Elibol, at the time an Eylea territory manager in eastern Victoria. She deposed to attending an educational meeting for Luxottica optometrists on 10 April 2013 and setting up a baby banner, she cannot recall in which form, inside the meeting room on a table located at the front right of the room. I find that a baby banner was displayed and it was probably the baby banner in the second form given the date of this event, 10 April 2013.
233 As to 46, a baby banner at the Luxottica educational meeting in the ACT, Novartis relies on the affidavit evidence of Ms Adams, at the time an Eylea territory manager in southern New South Wales and the ACT. She deposed in her first affidavit that she attended a meeting held by the Luxottica optometry group in or about June 2013 and said: “I cannot recall where I placed a baby banner.” In her second affidavit, she made clear that she did use the baby banner at that meeting but she could not recall which baby banner she used. I find that a baby banner was displayed and it was the baby banner in the second form since Ms Adams gave evidence that her understanding was that there was one baby banner that she should not use, which was replaced with one that she could use.
234 Since I have found the representations were not conveyed, it is not necessary to consider the question of whether or not the conduct was misleading or deceptive or likely to mislead or deceive. Nevertheless I should deal with the submissions in the alternative, that is, on the basis that the representations were conveyed.
235 Bayer admitted that, if conveyed, the representations were false but were not misleading or deceptive or likely to mislead or deceive. This raises two questions, one in relation to s 18 and another in relation to s 29.
236 As to the s 18 question, Bayer submitted in effect that even if the representations were conveyed, and accepting for this purpose that they were incorrect, the conduct, in the circumstances, did not lead, and was not capable of leading, a reasonable member of the class of addressees into error: see Australian Competition and Consumer Commission v Dukemaster Pty Ltd [2009] FCA 682 (Dukemaster) at [10(1)] and the authorities there cited by Gordon J. I consider each of the four representations in turn. As to the First Representation, in my opinion no (hypothetical) reasonable member of the class of ophthalmologists was or could have been led into thinking that in clinical use Lucentis was administered monthly for all patients. As to the Second Representation, in my opinion no such member of the class of ophthalmologists was or could have been led into thinking that for each and every patient, Lucentis would only be a clinically effective treatment if administered monthly. As to the Third Representation, in my opinion no such member of the class of ophthalmologists was or could have been led into thinking that the Lucentis product information specified, without qualification, that it was to be administered monthly. As to the Fourth Representation, in my opinion no such member of the class of ophthalmologists was or could have been led into thinking that in accordance with approved dosages, all Eylea patients received fewer injections than all Lucentis patients. My reasons for so concluding that the reasonable member of the class was not led and was not likely to have been led into error by the conduct are the level of training, information and experience and the nature of the decision-making brought to bear on the very serious nature of the question of prescribing an expensive drug for injection into a patient’s eyeball to retain vision. I rely here particularly on the evidence of Professor Mitchell and Dr Crawford. I find that a reasonable member of the class of ophthalmologists had a high level of training, knowledge and expertise. Secondly, I find that the prescription by ophthalmologists of a drug for injection into a patient’s eyeball was serious, complex and specific to the condition and needs of the particular patient. It is also expensive, although not to the prescriber or, except to a limited extent, to the patient. Thirdly, I do not accept that ophthalmologists considering prescribing a drug for injection into a patient’s eyeball would consciously or unconsciously take into account, even as a contributing factor, the slogan or message contended for by the applicant Novartis. Rather, I find an ophthalmologist would make his or her own decision based on their own experience with the relevant studies and articles and what they knew from their experience. I find also that clinical data, what they heard at overseas conferences with their peers, and their awareness of the VIEW clinical trials would have been the cause or causes of the prescription of Eylea to the exclusion, as a contributing cause, of the impugned conduct. I find that the decision-making of specialist doctors in these circumstances would not have been altered or affected by a sentence in any or in all of the impugned publications.
237 Similarly, I am not persuaded that Novartis suffered loss because of the conduct of Bayer. I am not persuaded that a reasonable member of the class of addressees did, or was likely to, prescribe Eylea rather than Lucentis by reason of the impugned conduct, assuming the representations were conveyed and were false. I approach the question of “by reason of” in ss 236 and 237 of the Australian Consumer Law, set out at [246] below, by reference to whether or not the impugned conduct was a contributing cause. I repeat what I have said in [235] above. I find that the decision-making of specialist doctors in these circumstances would not have been altered or affected by a sentence in any or in all of the impugned publications. I find that the impugned conduct was not and was not likely to have been a contributing cause in the prescription of Eylea.
238 As to the s 29 question, this arises because s 29(1)(a) and s 29(1)(g) each refer to a false or misleading representation. Thus Bayer’s admission that, if conveyed, the representations were false may mean, on the present hypothesis that the representations were conveyed, that Bayer would have breached s 29, assuming causation. Any additional work done by these provisions was only lightly developed in argument. This is against the background, as Gordon J noted in Dukemaster at [14], that the vast majority of cases that discuss an alleged breach of the predecessor provision couple it with a breach of s 52 (now s 18) and deal with the “false or misleading” and “misleading or deceptive” aspect of the conduct mutatis mutandis.
239 For those reasons, and in light of my findings on other issues, it is not appropriate to deal with the particular s 29 questions extensively but I shall indicate my views in a summary way.
240 Novartis submitted in its written closing submissions on this question that the making of a false representation will amount to a contravention of s 29(1) without the need to establish separately misleading or deceptive conduct. Bayer did not seem to dispute this. Bayer submitted, assuming everything else against it, that Novartis had not advanced specific submissions in support of its contention that one or more of the representations was a representation “that goods are of a particular standard, quality, value, grade, composition, style or model or have had a particular history or particular previous use” (within the meaning of s 29(1)(a)), or that the goods “have sponsorship, approval, performance characteristics, accessories, uses or benefits” (within the meaning of s 29(1)(g)). Bayer submitted that the First and Fourth Representations were not within the ambit of s 29, since those representations were directed to the conduct of doctors in clinical practice, not any quality or characteristic of Lucentis. The Third Representation was not within the ambit of s 29, since it was a representation directed to what the product information document provided with the goods specified, not any quality or characteristic of Lucentis. As to the Second Representation, Novartis had not specified the way in which s 29 was alleged to apply to that representation. In its written reply submissions Novartis submitted that the First, Second and Fourth Representations related to the frequency with which Lucentis was injected, which was an attribute, or performance characteristic, of Lucentis, citing Instant Colour Pty Ltd v Canon Australia Pty Ltd [1996] FCA 763. The term “particular quality” in s 29(1)(a) extended to include the attributes and properties of the goods, citing Nick Scali Ltd v Super A-Mart Pty Ltd [2011] FCA 751 at [14]–[16]. Similarly, a “performance characteristic” signified something that the goods can do, or something that can be done with the goods, citing Australian Competition and Consumer Commission v Pest Free Australia Pty Ltd [2004] FCA 527. Finally, the Third Representation was a representation that Lucentis had a particular approval within the meaning of s 29(1)(g).
241 The subject matter of s 29 is one or more representations. Thus, on the present hypothesis, the issue in respect of the First Representation is whether a representation that in clinical use, in accordance with the approved dosage, the Novartis product, Lucentis, is administered monthly for all patients was a representation that Lucentis was of a particular quality. In my view it was such a representation, even though it was as well a representation directed to the conduct of doctors in clinical practice. The issue in respect of the Second Representation is whether a representation that, for each and every patient, Lucentis will only be a clinically effective treatment if administered monthly was a representation that Lucentis was of a particular quality. For the same reasons, I conclude that it was. The issue in respect of the Third Representation is whether a representation that the Lucentis Product Information specified, without qualification, that it was to be administered monthly was a representation that Lucentis had a particular approval. In my view, it was not as the representation is insufficiently connected with an approval. It was not submitted by Novartis that the Third Representation was a representation that Lucentis was of a particular quality. The issue in respect of the Fourth Representation is whether a representation that in accordance with approved dosages, all Eylea patients receive fewer injections than all Lucentis patients was a representation that Lucentis was of a particular quality. Again, for the same reasons as for the First and Second Representations, I conclude that it was.
Remedies
242 The applicant sought declarations, an injunction, the publication of corrective statements and damages pursuant to s 236 of the Australian Consumer Law or compensation pursuant to s 237.
243 The declaration sought was that the respondent had engaged in conduct contravening ss 18, 29(1)(a) and/or 29(1)(g) of the Australian Consumer Law by publishing words conveying the four representations I have set out at [7] above.
244 No question of a declaration or an injunction or corrective statements arises in light of the conclusions I have reached.
245 In case I am wrong in those conclusions and because there was a deal of evidence in relation to the claim for damages or compensation I should make findings and express conclusions on those issues in the alternative.
246 Sections 236 and 237 provided:
236 Actions for damages
(1) If:
(a) a person (the claimant) suffers loss or damage because of the conduct of another person; and
(b) the conduct contravened a provision of Chapter 2 or 3;
the claimant may recover the amount of the loss or damage by action against that other person, or against any person involved in the contravention.
(2) An action under subsection (1) may be commenced at any time within 6 years after the day on which the cause of action that relates to the conduct accrued.
237 Compensation orders etc. on application by an injured person or the regulator
(1) A court may:
(a) on application of a person (the injured person) who has suffered, or is likely to suffer, loss or damage because of the conduct of another person that:
(i) was engaged in a contravention of a provision of Chapter 2, 3 or 4; or
…
make such order or orders as the court thinks appropriate against the person who engaged in the conduct, or a person involved in that conduct.
Note 1: For applications for an order or orders under this subsection, see section 242.
Note 2: The orders that the court may make include all or any of the orders set out in section 243.
(2) The order must be an order that the court considers will:
(a) compensate the injured person, or any such injured persons, in whole or in part for the loss or damage; or
(b) prevent or reduce the loss or damage suffered, or likely to be suffered, by the injured person or any such injured persons.
…
247 Novartis’ case on damages or compensation was that it sought damages in accordance with scenario 1 which, including tax gross up and interest up to 31 March 2014, gave a figure of $64,375,660 for the total loss. This was its preferred scenario.
248 The claim was founded on the following evidence.
249 Dr Waterhouse was the Managing Director of Model Solutions Pty Ltd, a company he founded in 2007 and which consisted mainly of himself, that provides economic modelling services as well as analysis of drug utilisation under the PBS to pharmaceutical companies. To his first affidavit, he annexed the treatment and clinical criteria required for reimbursement of Lucentis and Eylea, that is, the criteria which must apply to a particular patient in order for that patient to obtain the drug at the reduced rate on the PBS. For both, authority was required under s 85 of the National Health Act 1953 (Cth). Both Lucentis and Eylea are s 85 authority drugs with the same authority criteria.
250 Dr Waterhouse said that the PBS makes available for sale to companies such as his a 10% sample of the PBS data as at the date on which the claim for reimbursement is submitted. The sample is updated quarterly. Having received the 10% sample from the PBS, Dr Waterhouse extrapolated it to represent 100% of the market, including making allowance for reimbursement under the separate Repatriation Pharmaceutical Benefits Scheme. Dr Waterhouse prepared a confidential exhibit entitled “Lucentis New PBS data report 2013Q1”, being at that time the most recent drug utilisation analysis he had prepared for Novartis in relation to Lucentis.
251 In his second affidavit, Dr Waterhouse exhibited a more recent confidential exhibit entitled “Lucentis New PBS data report 2013Q3.xlsx”, being the most recent drug utilisation analysis he had prepared for Novartis in relation to Lucentis. This data accounted for all prescriptions processed by the PBS as at the end of November 2013. However, in Dr Waterhouse’s opinion, it appeared as though there was at that time a significant delay in the processing of all prescriptions by the PBS and he did not believe the data for August and September 2013 contained in his second confidential exhibit was reliable.
252 Dr Waterhouse was cross-examined but, in my view, the cross-examination did not cast doubt on the reliability of his figures.
253 It is the evidence of Dr Waterhouse which establishes the actual frequency of injections of patients using Lucentis. I accept Dr Waterhouse’s evidence.
254 Next is the evidence of Mr Hodgkinson, who was the Chief Financial Officer for the Novartis group of companies in Australia and New Zealand, the group including the applicant.
255 In his first affidavit he deposed that the applicant company was a wholly-owned subsidiary of Novartis Australia Pty Ltd. The financial year for both Novartis Australia Pty Ltd and the applicant company was the year ending 31 December. In cross-examination, Mr Hodgkinson confirmed that the applicant was an Australian company, wholly owned by Novartis Australia Pty Ltd, also an Australian company, and Novartis Australia Pty Ltd in turn was wholly owned by the Swiss company Novartis Pharma AG.
256 XX XXXXXXXXXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXX XXXXXXXX XXXXXXXXXXXX XXXXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXX XXXXXXX XXXXX XXXX XXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXX XXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXX XXXXXXXXX XXXXXXXXXX XXXX XXXXXXXX XXXXXXXXXX XXXXXXX
257 X XXXXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXX XXXXXXX XXX XXXX XXXX XXXXXXXXXXXX
XXXXXXXXXXXXXX XXXXXXXXXXXXXXXXXXXXX XXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXX XXXXXXX XXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXX XXX XXXX XXXXXXXXXXXXXXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXX XXXXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXX XXX XXXXXXXX XX XXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXX XXXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXX XXXXXXXX XXXXXX XXXXXXX XXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXX XXXXXXXXXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXX XXXXXXX XXXXXX XXXXXXXX XXXX X XXXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXXXXX XXXXXXX XXXXXXXX XXXXXXXXX XXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX.
258 X XXXXXXXXX XXXXXXX XXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXX XXX XXXXXX XXXXXXXXX XXXXXXXX XXXXXXX XXXXXXXX XXXX XXXXXXXXX XXXXXXXXX XXXXXXXX XXXXX XXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXX XXXXXXX XXXXXXXX XXXXXXXXX XXXXX XXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXX XXXXXXXXX XXXXXXXX XXX XXXXX XXX XXXXX XXX XXXXX XXX XXXXXX XXXXX XXX XXXXXXXXXX XXXX XXXXXX XXX XXXXXXXX XXXXXX XXXXXXXXX X XXXXX XXXXX XXXXXXXXX XXXXXXXX XXXXXXX XXXXXX X X XXXXXXX
259 X X XXX XXXXXXX XXXXXX XXXXXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXX XXXXX XXXX XXXXXXXXX XXX XXXXXX XXXXX XXX XX XXXXXXX XXXXXXXXX XXXXXXXXX XXXXXX XXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXX
260 X XX XXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXX XXXXX XXX X XXXX X XXXX X XXXXXXX XXX X XXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXX XXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXXXXXX XXXXXXXXX XXXXXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXXX XXXXXXX XXXXXXXXX XXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX
261 X XXXXXX XXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXX XXXXXXXX XXXXXXXXXXX XXXXXXXXXXXX XXXXXX XXX XXXXX XXXXX XXXX XXXXXXXXXX XXXXXXXX XXXX XXXXXXX
262 X XXXXXX XXXXXXXX XXXXXXX XXXXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXXXX XXX XXXXXXXXX XXXX XXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXX XXXXXXXXX
263 XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXXX XXXX XXXXX.
264 Mr Hodgkinson annexed a copy of the Novartis Australia Pty Ltd special purpose annual report for the year ended 31 December 2012. Those statutory accounts showed the results for Novartis Australia Pty Ltd and its subsidiaries but they did not record sales results in respect of particular products. Mr Hodgkinson, in the balance of his first affidavit, sought to demonstrate, amongst other things, the sales results for the applicant for the financial year ended 31 December 2012 as a whole and on a product by product basis.
265 He first referred to a journal entry processed at year end as a result of the operation of the supply agreement XXXXX XXXXXXXXXX XXXXXXXXXX XXXXXX XXXXXXX XXXXXX X
266 X XXXXXXXXXXX XXXXXXXXXXXXX XXXXXXXXXXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXX XXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXX XXXXXXXX XXXXXXXXXX XXXXXX XXXXXXX XXXX XXXXX XXXX XXXXXXXX XXXXXX XXXXXXX XXX XXXX XXXXXXXXXXX XXXXXXX XXXXXXXX XXXXX XXXXXXX X XXXXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXXXX XXXXXXX XXXXXXXXXXX XXXXXXXX XXXXXX XXXXXXXXXX XXXXXXX XXXXX XXXXXXX XXXXXX XXXXXX XXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXX XXXXXXX XXXXXXXXX XXXXX XX XXX XXXXXX XXXXX XXXX XXXX X X XX XXXXXXXXX X X XXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXX XXXXXXXXXXX XXXXXX XXXXXXXXX XXXXXXXXX XXXXXXX XXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXX XXXXXXX XXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXX XXXXXXXX XXXXXX XXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXXX XXXXXXX
267 Mr Hodgkinson said that he caused a report to be generated using the applicant’s management account system to demonstrate its actual net sales for financial year 2012 by product.
268 He also caused a trial balance for the applicant to be prepared for the period 1 January 2013 to 30 June 2013 and a report to be generated to demonstrate the applicant’s actual net sales for the period 1 January 2013 to 30 June 2013 by product.
269 Turning to Lucentis’ specific reports, Mr Hodgkinson caused a report to be prepared recording the following information for Lucentis during various periods in 2012 and 2013:
(a) sales quantity, being the volume of ex-factory sales (measured in units) of Lucentis by the applicant during the respective period;
(b) gross sales, being the gross sales of Lucentis by the applicant during the respective period;
(c) XX XXXXX XXXXXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXXXXXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXX X
(d) other adjustments, being additional, immaterial, expenditure accrued by the applicant in respect of Lucentis during the respective period (for example, the provision of free stock);
(e) net sales, being gross sales of Lucentis by the applicant during the respective period less rebates accrued during the respective period and other adjustments;
(f) cost of goods sold, being the inventory cost to the applicant for Lucentis sold during the respective period.
(g) margin, being the margin on the Lucentis product calculated as the difference between net sales and cost of goods sold during the respective period; and
(h) XX XXXXX XXXXXXX XXXXXXXX X XXXXXXXXXXX X XXXXXXX X XXXXXXX X XXXX X XXXXXXXXXX X XXXXXXXXXX XX XXXXX X XXXXXXXXXX X XXXXXXXXX X XXXXXXXXX X XXXXX XXXXXXXXXX X XXXXXXXX XXXXX XX XXXXXXXXXXX XXXXXXXX X XXXXXXXXX X XXXXXXXXXX X XXXXXXXXX Figures were also given for the applicant’s budgeted marketing and sales expenditure for the financial year ending 31 December 2013 and what the applicant had either accrued or budgeted for marketing and sales expenditure pursuant to the latest outlook for the financial year ending 31 December 2013.
270 Mr Hodgkinson was cross-examined. XXXXXXX XXXXXXXX XXXXXX XXX XX XXXXXXXXXX XXXX XXXXXXXXXXX X XXXXXXXXXXXXXXXXX X XXXXXXXXXXXX X XXXXXXXXXXX X XXXXXXXXXXX X XXXXXXX X XXXXXXXX X XXXXXXXXXX
271 Mr Hodgkinson agreed that the applicant was a distributor of Lucentis, which received the products it distributed, including Lucentis, in a finished and packaged form. It was not the manufacturer of Lucentis nor the owner of any of the primary patents, trade marks or other intellectual property in Lucentis. XXXXX XXXXX XXXX XXXXX XXXX XXXX XXXX XXXX XXXXX XXXXXX XXXXXX XXXXX XXXXXX XXXXXX XXXXX XXXXXX XXXXXX XXXXXXX. The applicant did not on sell to patients or doctors but sold to the pharmaceutical distribution companies, the wholesalers.
272 XX XXXXXXXXX XXXXXXXXXXX X XXXXXXXX X XXXXXXXX X XXXXXXX XXXXXXXX XXXXXX X XXXXX X XXXXXXXX X XXXXXX XXXXXXXX XXXXXXXX X XXXXXXX XXXXXXX XXXXXXXXXXX XXXXXXX X XXXXX XXXXX XXX XXXXXX XXXXXXXX XXXXXXXXX XXXXXXX XXXXX XX XXXXXXXX XXXXX XXXXX XXXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXX
273 XX XXXXX XXXXXXXXXX X XXXXXXXXX X XXXXXXX X XXXXXXXXXX X XXXXXXX X XXXXX XX XXXXXXXXXX X XXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXX
274 XX XXXXXXXXX XXXXXXXXXX XXXXXXXXX X XXXXXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXXX X XXXXXXXXXXX X XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXXXXX XXXXXXXX
275 XXXXXXXXXXXXXXXX XXXXXXXXXXXXXXXX XXXXXXXXXXXXXXXX XXXXXXXXXXXXX XXXXXXXX XXXXXXXXXXXX XXXXXXX XXXXXXX XXXXXXX XXXXXXXXX XXXXXX XXXXXXX XXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX
276 XXXXXXX XXXXXX XXXXXXXX XXXXXXXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXXXXX XXXXXXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXX XXXX XXXXXX XXXX XXXXX XXX XXXXX XXXXXXXXXX XXXXX XXXXXX XXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXXX XXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXX XXXX
277 XXX XXXXXXXXXXXXX XXXXXXXXXXX XXXXXXXX XXXXXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXX XXXXXXX XXXXXXX XXXX XXXXXXXX XXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXX XXXXX XXXXX X XXXXXXXXXXXXXXX XXXXXX XXXXXXXXX XXXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXXX XXXXXXXXXX XXXXXX XXXXXXX XXXXXXXX XXXXXX XXXXXX XXXXX XXXXXXXXXXX XXX XXXXXXXXXX
278 Although I accept Mr Hodgkinson’s evidence as to the applicant’s accounts and the operation of the supply agreement, I have formed my own opinion on the proper construction of the supply agreement: see [134] below.
279 The proper construction and application of the supply agreement was the fundamental point on which the accountants, Mr Samuel and Mr Potter, disagreed.
280 Mr David Moore was an expert called by Novartis to quantify Novartis’ lost sales caused by the alleged misrepresentations. He set out in his first report dated 19 August 2013 his relevant training, study and experience in the New Zealand health sector. He had 18 years’ experience in the Pharmaceutical Management Agency in New Zealand (PHARMAC) as a former General Manager and Director. PHARMAC is a Crown agency that decides, on behalf of District Health Boards, which medicines and related products are subsidised to use in the community and in public hospitals in New Zealand. He set out in his fourth affidavit further details of his relevant qualifications and experience. He stated that, in addition to his role with PHARMAC, he was a Regional Director of the Transitional Health Authority from 1997 to 1998, and in 1998 founded the Health Funding Authority’s Personal Health Operating Group, which funded all hospital and primary care services in New Zealand, including referred services such as laboratory and pharmaceutical services. He was later appointed its acting co-Chief Executive. As to the Australian market, when he was in a management role Mr Moore had ongoing meetings, telephone calls, emails and social connections with the Commonwealth Department of Health and Ageing and, less frequently, with the PBAC. He said he did not think the differences between the Australian and New Zealand pharmaceutical markets were significant for present purposes. He noted that New Zealand allowed direct-to-consumer advertising but said that, because it was a very political issue, the extent of that advertising was very limited and it was tightly self-regulated by the industry. He annexed a 1999 article of which he was a co-author: ‘Managing Pharmaceutical Expenditure while Increasing Access — The Pharmaceutical Management Agency (PHARMAC) Experience’, PharmacoEconomics, 16(6): 649–660.
281 He was asked to assume that the four representations pleaded were in fact conveyed by the promotional material. At the time of Mr Moore’s first report the promotional material in question was the six acts of publication then annexed to the defence to the further amended statement of claim. He said his opinion was based on the effect of the promotional material and the representations which he had been asked to assume were conveyed. Also admitted into evidence as an assumption by him was that documents discovered by Bayer from Ipsos Healthcare suggested that messaging in substantially similar terms to that in the promotional material may have been used by Bayer as part of a broader marketing campaign, in particular, in detailing. Admitted as a further assumption was that the central theme of the representations, both on their own and collectively, was that Lucentis needed to be injected more frequently than Eylea, or to put it another way, that Eylea will require “fewer injections” than Lucentis.
282 Mr Moore was asked to provide a report containing his opinions on the following:
(a) The likely impact of the entry of Eylea into the market for anti-VEGF drugs in Australia on the sales of Lucentis, including in relation to:
(i) the factors which impact upon sales with the entry of a competitor;
(ii) any peculiarities of the market for pharmaceutical products in Australia, and anti-VEGF drugs in particular, which bear on these factors and/or his opinion in this regard.
(b) The actual impact of the entry of Eylea into the market for anti-VEGF drugs in Australia on the sales of Lucentis, including, if applicable, a further consideration of the matters set out in [282](a)(i) and (a)(ii).
(c) The difference, if any, between the anticipated and the actual impact of the entry of Eylea into the market for anti-VEGF drugs in Australia (as set out in [282](a) and (b)).
(d) If there was a difference between the anticipated and actual impact of Eylea on the sales of Lucentis (as set out in [282](c)), his opinion as to the factors which may have been relevant to that difference, including, but not limited to, a consideration of the effect, if any, of the promotional material.
283 There was a consistent increase in the use of both Lucentis and Eylea (i.e. growth in the market) despite a number of barriers to that usage, in Mr Moore’s opinion.
284 In figure 1 of his first report, Mr Moore summarised Eylea’s penetration since being made available on the PBS. By March 2013, four months after being listed on the PBS, Eylea had captured: almost 60% of initiations (patients new to treatment and patients returning after more than six months); almost 30% of the patient population Lucentis held before Eylea entered the market through switches (i.e. net switches — switches from Lucentis to Eylea less any switches from Eylea to Lucentis); and between 40% and 50% of monthly injection sales.
285 Mr Moore deposed that pharmaceutical markets were highly regulated and entry barriers were substantial. Third-party payers pay the bulk of the cost, excepting some element of patient co-payment. In Australia, many pharmaceuticals, including both Lucentis and Eylea, were listed on the PBS and were funded by the Commonwealth government. Marketing was largely to the prescriber and other allied health professionals, largely without reference to price. Competition was generally based on non-price characteristics.
286 Mr Moore referred to “significant evidence that advertising, detailing and other promotion influences prescribing behaviour.” He referred to a review by Spurling GK et al, ‘Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: A systematic review’ (2010) PLoS Medicine 7(10): E 1000352, and Azoulay P, ‘Do Pharmaceutical Sales Respond to Scientific Evidence’ (2002) Journal of Economics and Management Strategy 11(4): 551 (the Azoulay Study) in relation to anti-ulcer pharmaceuticals. He gave as his opinion that the market share elasticities were informative as to the potential impact of marketing generally and specific types of marketing and he based his opinion in his report, he said, on the effect of the promotional material. He recognised that the market for anti-ulcer pharmaceuticals was clearly different from that of anti-VEGF pharmaceuticals: the latter were prescribed by ophthalmologists and the former by a broader community of prescribers, including general practitioners. However, he said there were also considerable similarities between the markets: the overarching nature of regulation; subsidisation; and information asymmetries (prescribers knowing more than patients). He added that the relationship between advertising and clinical trial information was also important. The Azoulay Study also found that pharmaceutical companies increased advertising in response to the release of positive clinical trial results.
287 Mr Moore set out what he described as a commonly accepted framework for analysing the rate of adoption. He referred to the study by Dunn AG et al, ‘Nation-scale adoption of new medicines by doctors: an application of the Bass diffusion model’ (2012) BMC Health Services Research 12:248 (the Dunn Study) which modelled the pattern of adoption for 103 commonly prescribed pharmaceuticals in Australia. Mr Moore set out in his table 5 some of the data from the study for some selected groups of pharmaceuticals which would have initially been prescribed largely by specialists and then increasingly by general practitioners. Clearly some pharmaceuticals in the table found their peak quickly and others did not. He said that in comparison to the modelled adoption rates in the table, Eylea’s penetration had been rapid. Based on the modelled results, Eylea having captured more than 40% of the monthly market for injections within five months was faster than any of the 103 pharmaceuticals modelled in the Dunn Study. Mr Moore also referred to the study by Sweeny K, ‘Trends and Outcomes in the Australian Pharmaceutical Benefits Scheme’ (Working Paper No. 36, Pharmaceutical Industry Project Working Paper Series, Centre for Strategic Economic Studies, Victoria University of Technology), December 2007 (Sweeny Study) which, he said, analysed the impact of the entry of generic pharmaceuticals. Mr Moore assumed that Eylea was more analogous to a generic entering a market than most ‘me toos’.
288 This expression he defined in his first report as meaning “[a] common term for pharmaceuticals that have the same mechanism of action and similar therapeutic effect to an existing pharmaceutical (in this case Eylea)”, and then used it as applied to a pharmaceutical similar to the incumbent with the same therapeutic effect and in the same class. In his oral evidence, Mr Moore said that the correct definition should refer to there being the same or similar therapeutic effect. In his third report, the definition was “[a] common term for pharmaceuticals that have the same or similar therapeutic effect irrespective of the chemical entity or mechanism of action”.
289 Mr Moore then turned to what he described as perceived relative advantage, stating that one of the most important and relevant conclusions from the literature was that the key variable (as commonly categorised) influencing the rate of adoption of ‘me too’ imitators was their “perceived relative advantage”. In the absence of a strong relative advantage — which may be “perceived” or actual — a ‘me too’ pharmaceutical will fail to take significant market share over the innovator and take a significant time to penetrate the market.
290 Mr Moore then referred to a key finding from the diffusion of innovation literature, being that the rate of adoption of an innovation was generally governed by the social nature of diffusion, whereby the rate of adoption increased as penetration increased due to the influence of existing adopters. This diffusion pattern was used to explain the typical “S-curve” adoption pattern whereby the rate of adoption was initially slow, rapidly progressed and then slowed again once the market saturated. He said a simple and extensively used model for describing this diffusion pattern was the Bass Diffusion Model (BDM). The BDM estimated the number of new adopters by the additive effects of two influences: an “external” influence, which referred to influences that did not depend on other adoptees (such as advertising, detailing and, in the case of pharmaceuticals, published research); and an “internal” influence which reflected the influence from existing adoptees (such as the influence of key opinion leaders or simply the effect of previous trialling of the innovation on other patients). Mr Moore said that the literature and data suggested that there was a significant internal influence effect operating within the Eylea and Lucentis market. He said the BDM showed that establishing an early foothold through external influence was critical in increasing market penetration in the longer term via internal influence as that message was spread by key opinion leaders and became entrenched. He said that it was the perception of relative advantage that was important. He assumed that the key differentiating factor between Lucentis and Eylea was the perception of fewer injections and the implications of that for the patient experience.
291 To estimate the impact of the promotional material, Mr Moore developed a model of Eylea and Lucentis sales, in terms of new patients and switches of existing patients, that estimated the numbers of patients by pharmaceutical. He applied that model from the time that Eylea was reimbursed on the PBS to December 2016. In addition to accounting for repeat sales to treat existing patients, it was necessary to consider patient sales past the time when the promotional material occurred because of the delayed direct effect of advertising and the delayed indirect effects (the internal influence effects).
292 He assumed that the prospect of fewer injections had a charismatic appeal that would attract attention given the nature of delivery of the pharmaceutical and due to the considerable positive impact for patients that ophthalmologists would seek from this elderly population. He also assumed this was supported by the centrality of the message in the promotional material.
293 He set:
(i) at 10% the lower limit of the range of the boost to sales;
(ii) at 75% his most likely estimate which implied that in the counterfactual Eylea would have in 2016 obtained 30.1% of initiations and by December 2016 captured 23.7% of patients. He said he considered this assumption (estimate) to be reasonable on the basis that a significant adjustment was required because Eylea’s penetration into the market had been significantly more rapid than either the generics in the Sweeny Study or any of the 103 pharmaceuticals in the Dunn Study; and
(iii) at 350% the upper limit, making use of the results of the Sweeny Study, which implied that the originator would lose no more than 5 to 6% of the market in the first year to generic competitors. An assumption (estimate) of 350% implied that in the counterfactual Eylea would have in 2013 obtained 6% of initiations; and by December 2013 captured 5.4% of patients.
He said these calculations were consistent with the findings of the Azoulay Study which estimated elasticities of advertising “that implied that a 1% increase in journal advertising led to a 0.627% increase in market share and a 1% increase in detailing led to a 1.242% increase in market share. These elasticities implied that a doubling (i.e. 100% increase) in advertising would lead to a 62.7% to 124.2% increase in market share.” Consequently, Mr Moore said he considered a 75% boost to sales to be reasonable in the circumstances.
294 To December 2016, Mr Moore’s estimate of the impact to Lucentis in terms of lost injections was 297,346, of which 275,346 were lost between July 2013 and December 2016. The estimate did not include any lost sales of injections post 2016. The total number of injections in the counterfactual was 832,654. This was a loss of 36% of the counterfactual.
295 For his second report, Mr Moore was asked to make further assumptions. These assumptions were that, in addition to the original six acts of publication, during the course of 2012 and/or 2013 Bayer company representatives: engaged in detailing to ophthalmologists and/or optometrists during which they were shown Detail Aids (in the form of the eDetailer) which contained words in substantially similar terms to those in the first acts of publication and which conveyed the same representations; distributed attendee workbooks to ophthalmologists; and displayed baby banners at conferences attended by ophthalmologists and optometrists which contained such words. He was also asked to assume that the representations were not only conveyed but were misleading or deceptive or likely to mislead or deceive. He was also given affidavits sworn or affirmed by Bayer’s sales representatives for the purposes of the proceedings.
296 He applied the model used in his first report, the analysis depending on a number of assumptions, in particular two material assumptions regarding the peak direct effect of the promotional material and the degree of internal influence. Based on the additional materials he had received, in his second report Mr Moore modified his assumptions as to the peak direct effect but retained all the other assumptions in his first report.
297 Mr Moore had previously concluded that the peak direct effect of the promotional material was most likely to have been a 75% boost to sales. His review of the additional materials led him to increase his estimate of the minimum peak direct effect from 10% to 20%. Also having reviewed the additional materials, he increased his most likely estimate to 90%. In summary, he doubled his initial estimate of 75% to 150% but, given the evidence of the Bayer sales representatives as to the limited way in which they used the pages of the Detail Aid, he reduced the figure of 150% to 90%, which he considered to be more reasonable in the circumstances. In terms of impact on injection numbers the summary results were that in Mr Moore’s estimate there were 22,484 patients lost and 319,879 injections lost by Lucentis to December 2016 which, against the figure of 854,892 injections in the counterfactual, was 37% of the counterfactual lost.
298 In his third report, Mr Moore reviewed and replied to an expert report provided by Dr Williams. Mr Moore concluded there was nothing in the Williams Report that gave him reason to reconsider any of his opinions and analysis in his first and second reports. He gave detailed reasons. In addition, revised PBS data had become available and Mr Moore updated the model he developed for his first report. His estimate of the injections lost by Lucentis to December 2016 fell from 319,879 to 303,705.
299 Mr Moore also prepared a joint report with Dr Williams, as directed by the Court, setting out areas where those experts agreed and areas where they disagreed. As to the broad approach for estimating loss, the experts agreed that using a model of three series was reasonable, those series being: (a) a baseline market for Lucentis and Eylea patients; (b) a baseline of patients using Eylea (which included actual data up to and including July 2013); and (c) a counterfactual of patients using Eylea. The experts also agreed that the numbers in series (a) were reasonable, that is, Mr Moore’s estimates of the total number of patients treated (and from that, the total number of injections sold) in the market for Eylea and Lucentis, the total being calculated for each month using actual data up to (and including) July 2013 and forecast data from August 2013 to December 2016. The last area of agreement was that the numbers in series (b) were reasonable, that is, Mr Moore’s estimates of the total number of patients treated using Eylea. That total number of patients was calculated for each month using actual data up to (and including) July 2013 and forecast data from August 2013 to December 2016.
300 The joint report stated that the approach to the estimate of the loss of sales to Novartis had not been disputed, that is: Mr Moore’s estimates of Novartis’ loss of patients as the difference between the patients being treated by Eylea under the baseline and counterfactual scenarios; and Mr Moore’s estimates of Novartis’ loss of injections by converting patients to injection numbers based on the historical pattern of injections per patient per month.
301 There was no agreement on method or on numbers in calculating the counterfactual, that is, the estimation of Eylea’s counterfactual share of the market.
302 By the time Mr Moore gave his oral evidence, he had had the opportunity to review the cross-examination of the Bayer sales representatives.
303 In cross-examination, Mr Moore was taken to the description and application of the BDM in one of the papers to which he referred: Mahajan V, Muller E and Bass FM, ‘Diffusion of New Products: Empirical Generalizations and Managerial Uses’ (1995) Marketing Science 14(3): G79. He agreed that the BDM constituted an empirical generalisation. He said that in preparing his reports he made the assumption that each time Eylea was used it was like the first purchase of a new product and he agreed that as far as Eylea or Lucentis were concerned the purchasing decision was a regularly and repeatedly made decision in respect of the product. Mr Moore agreed that the central proposition of the BDM was that the probability of adoption at time t, given that adoption had not yet occurred, equalled p + q cumulative fraction of adopters at time t. He did not accept that the effect of his application of the BDM, in applying it in the circumstances, was inconsistent with that central proposition. Mr Moore did not agree that each prescription, sale and injection of either Eylea or Lucentis after the first one that the patient was given did not meet the criterion that adoption had not yet occurred. Mr Moore said the point was that the patient was the point of adoption not the injection. Mr Moore said that one of his modifications to the BDM was applying it not to products but to patients in respect of whom products were prescribed. Mr Moore agreed that the BDM was designed to apply to initial purchase (adoption) of the products and not to apply to replacement demand but did not accept that he had applied it to a circumstance that involved both the initial purchase of Eylea product and to replacement demand purchases of the Eylea product. Mr Moore said that in this terminology the purchase is the purchase of the treatment course. Mr Moore did not accept that what was involved in his application of the BDM was to take account of replacement demand but said that in his model he was applying the framework of the BDM to generate a counterfactual of what might have happened if the innovation process was as was seen for other pharmaceuticals. Mr Moore accepted that ophthalmologists consider the question and assess the condition of their patients before making each and every injection of Eylea and that the ophthalmologist applied a process of professional judgment before each and every prescription of Eylea and before each and every injection of Eylea and that ophthalmologists did not set a patient on a course of injection of a fixed duration without regard to the condition of the patient. It was not his interpretation that the prescription and purchase and injection of Eylea involved replacement demand in the sense described in the Mahajan et al paper.
304 Mr Moore said that it was the patient that was modelled, not the injections. Had the injections been modelled he would agree that it would be inappropriate to apply the BDM so as to regard a course of injections as equivalent to an initial purchase or adoption of a product: that would look quite different. But he had not modelled the injections, he had modelled the patients. He said there was not an issue of replacement in the model, in the sense that it was meant in the Mahajan et al paper. He accepted that if there were such a thing as replacement demand in this market then data based on replacement demand would seriously contaminate his application of the BDM but there was not, Mr Moore said, replacement demand in the Eylea/Lucentis market. Mr Moore accepted that there was no reference in his reports to any scientific article or research article that supported the application of the BDM by reference not to individual prescriptions and sales of the product but by reference to the commencement of a patient on a new course of treatment. He said he had no academic or research studies which had applied the BDM as he had done it.
305 Mr Moore was taken to the statement in paragraph 2.2 of the Mahajan et al paper that the model applied to the generic demand for a product category but that, in at least some instances, the model would apply to the demand for individual brands and to niches within a category, as well as to the category adoption patterns. He said that in his use of the BDM he had simply taken as a given that it applied to the drug categories that he worked with, that is, individual pharmaceuticals within categories, and he did not accept that that approach was not within the description of the authors of the paper. He said that the take-up of Eylea was the take-up of a product as it was being launched and within that the innovation applied to the product and not to the product category. Mr Moore accepted that he had applied the model to a specific product within a product category but did not agree that the statement by the authors in paragraph 2.2 that the model applied to generic demand for a product category was something entirely different. He did not agree that it was irrational to suggest that because the BDM described an S-curve for a generic demand for a product category, it therefore also described an S-curve for the particular demand of an identified product within a category. As to the proposition in the Mahajan et al paper that “[f]orecasting adoption timing prior to product introduction will obviously require guesses of parameters”, Mr Moore initially accepted that as an accurate statement, saying you would be trying to guess the internal influence and that formally generating a forecast, the estimation is necessarily going to have assumptions about p and q. Whether they were guesses or whether they were based on evidence was another matter but he would not say “guess” or “guesses” of p and q, he would say “an estimate”.
306 In relation to product sampling as part of a marketing campaign, Mr Moore accepted that he was not aware of the extent of the sampling but agreed it would certainly be a factor in the take-up of Eylea by ophthalmologists as it would go to the ability of the prescribers to trial and to experience the pharmaceutical. He agreed with the statement of the authors of the Mahajan et al paper that “[w]hen sampling is to be employed, key questions concern the number of samples to distribute and to whom.” Mr Moore also agreed he had no evidence about the scale of sampling in relation to Eylea and he had no information to quantify it.
307 In relation to the Dunn Study, which Mr Moore saw as a very relevant paper which he used as giving an indication of the range of what the factors q and p might be, he accepted that the conclusions expressed in the study were retrospective and not predictive or prospective. The conclusion stated in the study’s abstract was: “[t]he Bass diffusion model may be used to retrospectively represent the patterns of adoption exhibited in prescription volumes in Australia, and distinguishes between adoption driven primarily by external forces such as regulation, or internal forces such as social contagion.” Mr Moore also agreed that the aim of the study was not to apply the BDM to individual medicines or products. He also agreed that neither Lucentis nor Eylea were in the group from which the 103 drugs referred to in the study were chosen, that is, drugs commonly prescribed in Australia other than over-the-counter drugs. Mr Moore agreed that the Dunn et al result was really at the aggregate level and that the most that could be said about the Dunn Study was that it concluded that the BDM appeared to work considering the whole of the landscape, that is, all of the drugs taken together and their adoption rates, not individual drugs or individual classes of drugs. He did not accept, however, that it was impossible to conclude from taking a wide view of the modelling of averages of 103 drugs that that modelling was representative of what might happen to a particular drug in particular circumstances. He said it was instructive on both the q and p, the internal and the external, effects that were active in each of the markets and he would expect to see a high degree of variability in those markets. Mr Moore accepted that the performance of individual drugs within a particular class can vary enormously from class to class and he concluded his answer to the question “It’s impossible to predict the way a particular class will operate, isn’t it?” by saying that the central point of the Dunn Study was that all of the drugs followed an innovation process, which was what he looked through with the BDM. He said he used the Dunn Study as an example of the range of possible factors for the internal effect and the external effect, so there was not a direct link between the Dunn Study and the results. He said the average of 12.7 was calculated from a meta-analysis (Sultan et al (1990)) of different rates of diffusion and calculations of the q and p effect, the particular figures being .38 for q and .03 for p. When .38 is divided by .03 it produces 12.7. In answer to the question: “Do you regard the Bass diffusion model as a problematic one?” Mr Moore answered: “I think the application of the Bass diffusion model and essentially managerial processes takes a lot of information which often isn’t available. So, yes, it can be problematic to do so. But I feel very comfortable with the method that we’ve applied here.” It is also worth recording the immediately following question and answer:
Q It’s the case, isn’t it, that in your evidence, these reports, you have picked up the Bass diffusion model, modified it extensively, and applied it for a purpose for which it was never designed; namely, the prediction of adoption rates of a repeatedly prescribed medicine? Do you agree?
A No. I – I don’t agree with that. What I’ve done is I have used a tried and true model, and I’ve applied it in the situation to give insight on what might be the effect of the promotional materials.
308 In relation to the Spurling review, Mr Moore was taken to the conclusions which stated, in part:
The limitations of studies reported in the literature mentioned above mean that we are unable to reach any definitive conclusions about the degree to which information from pharmaceutical companies increases, decreases, or has no effect on the frequency, cost, or quality of prescribing. In theory, advertising may be beneficial in several ways: by distributing information and thus improving the quality of prescribing, by reducing costs through increasing price-elasticity, by increasing prescribing of drugs that provide better health outcomes, or by improving the cost-effective use of healthcare resources. Because of the limitations of both the included studies and this review we have not disproved those theories but we have found little evidence to support them and have found some evidence of increased costs and decreased quality of prescribing.
(References omitted.)
In answer to the question that it provided no support at all for a proposition that marketing can have a significant influence on prescribing behaviour, Mr Moore replied that it provided quite a good landscape report and within that pointed to the difficulties of figuring out whether or not there is a significant impact.
309 Mr Moore accepted that pharmaceutical companies in Australia were not permitted to make statements directly to patients and he agreed that the ophthalmologist was the person who prescribes. There were the following questions and answers:
Q So does that mean that you’ve assumed that ophthalmologists make statements of a particular type to their patients in respect of products that are consistent with the statements to which you refer in paragraph 24(a) [of your third report]?
A I think it’s - - -
Q Is that what you’ve done?
A I think within the social – no, no. This is just – it would be my view that within, you know, the social contagion theories that underlie the innovation that, although they may not see the poster, that there will be an internal effect and there will be some understanding of that with patients. For instance, when a patient is referred by an optometrist, there are two agents. When the patient then comes through to an ophthalmologist the optometrist will field the nature of the referral and the ophthalmologist may be taking the prescribing decision, but they would generally do that with some consultation with the patient, particularly around issues of efficacy and side effects. So in some sense the message won’t be marketed directly to patients, but the message will, through the process of diffusion, follow through those three groups.
310 As to the Azoulay Study, Mr Moore agreed that it did not deal with the nature of false claims but dealt only with the nature of detailing and advertising and its effect on prescribers for one group of pharmaceuticals. It did not dwell at all on the nature of a false claim. He also agreed that the Azoulay Study did not consider or analyse the differentiation between statements made in marketing campaigns and the broader marketing campaign of which particular statements form a part. In answer to the question whether he agreed that Azoulay did not deal with the effect of marketing claims in respect of products where the marketing claim was always accompanied by reference to the product information, Mr Moore said it was unclear to him whether in America the same rule applied or not.
311 As to Mr Moore’s opinion that the take-up of Eylea was very rapid, he agreed that he had not done the same process with the take-up of Lucentis in its first six months: in his reports he did not consider the innovation process underlying Lucentis. In cross-examination, figures in relation to Lucentis were put to Mr Moore and, while agreeing that the rate of growth of Lucentis was approximately as rapid as for Eylea, he did not accept that this showed error in testing Eylea’s rate of adoption by reference to the median drug adoption rate of 4% of peak sales adopted in the Dunn Study. He said all that it showed was that both Eylea and Lucentis had a very rapid takeoff. He said the comparable very rapid movement of Lucentis when it entered the market would imply that both of the curves were exhibiting a very strong internal effect — the diffusion effects seen by Lucentis and seen by Eylea were in both instances very strong indeed.
312 In relation to his predictions going out to December 2016, Mr Moore said he had assumed that Eylea and Lucentis would continue to be marketed to ophthalmologists, adjusted based on what he observed in the statistics. He had also assumed that through to December 2016 there would be no new product available to ophthalmologists whether produced by Novartis or Bayer that treated wet AMD.
313 In relation to his estimated range of effect of Bayer’s marketing of Eylea, being a boost to sales of between 20% and 350%, Mr Moore agreed that that was a very wide range and indicated that, in his view, there were very significant uncertainties in his suggested process of estimation. As to his “most likely” estimate of 75% he agreed that he did not explicitly set out in writing in his reports any explanation of why the best estimate that he settled on was 75%, updated to 90%. He said he gave a point estimate based on the effect of the promotional material and the effect of the repeat sales, the internal influence effect and the reliance on the data report. He considered his figure of 75% to be reasonably conservative as in this market the internal influence could have been bigger but he relied on the statistic of 12.7, a widely used benchmark, because of the uncertainties. There followed these questions and answers:
Q Mr Moore, is it right that the main item of evidence or support upon which you say that you’ve settled on your most likely estimate of 75 per cent is the Dunn study and the comparison that you draw between the pharmaceuticals in the Dunn study and EYLEA’s comparatively faster penetration into the market?
A I exercised my opinion on the 75 per cent based on my view of the particular circumstances. What I say is the assumption produces a result; that is consistent with the findings of Dunn and Sweeny. I used Sweeny to determine the bottom right-hand corner on page 14, and I used Dunn to give an indicative range up to very high with Q over P of 58.8. So my choice is consistent with those. I don’t use those to directly determine the 75 per cent. For the 75 per cent, that is purely my opinion.
Q Yes. And there’s no explanation in writing in your reports of why you settle on 75 per cent rather than, say, 150 per cent, is there?
A The 75 to – the answer is no, and the 75 per cent to 90 per cent is – and to give it common language, the term is approximately half the sales, in my view, would fall due to the importance of this message, and I give a thorough description of why I arrive at that opinion.
314 It is appropriate here to refer to the evidence of Dr Philip Williams, the economist called as an expert by the respondent.
315 In summary, Dr Williams’ opinion was that the inferences and conclusions that Mr Moore drew from published studies and other materials could not be justified and that Mr Moore’s reliance on the paper by Spurling et al in support of his argument that Bayer’s conduct caused Novartis’ loss of Lucentis sales could not be justified. Dr Williams’ opinion was that Mr Moore’s estimates of the loss in sales suffered by Novartis as a result of the alleged misleading conduct were derived from three series: (i) a monthly series of total sales of Lucentis and Eylea; (ii) a monthly series for the sales of Eylea in the factual (that is, given the alleged misleading conduct); and (iii) a monthly series for the sales of Eylea that would have occurred but for the alleged misleading conduct. None of these series was estimated by statistical inference. Each of them was produced by an informal combining of information from published sources and from Novartis. In Dr Williams’ opinion, Mr Moore’s estimates of the sales of Eylea that would have occurred but for the alleged misleading conduct were particularly troubling. They were derived by adjusting his estimates of “factual” sales based on three published studies of rates of sales of other drugs — Dunn, Azoulay, and Sweeny. These studies could not be relied upon for the purposes to which they were put by Mr Moore because: (a) the Dunn and Sweeny studies were not concerned with the effects on sales of a new entrant drug in the same therapeutic class using a new molecule; and (b) even if there were good evidence concerning entry by a new molecule in the same therapeutic class (as contained in the Azoulay Study), one could not infer patterns of substitution between two particular drugs from patterns of substitution between other drugs. Dr Williams’ conclusion was as follows:
79 Mr Moore’s estimate of the loss of patients caused by the impugned conduct is based principally on facts that he infers from his experience and from published studies. Apart from his data of (i) total sales of the therapeutic group up to March 2013; (ii) factual sales of Lucentis up to March 2013; and (iii) factual sales of Eylea up to March 2013 (for which published data are available), Mr Moore relies for data on his experience and on inferences from published studies of other drugs. In particular, he does not infer estimates by means of statistical inference.
80 I am in no position to assess Mr Moore’s ability to make inferences about factual matters based on his experience. However, for the reasons given in this report, it is my opinion that the inferences of factual propositions concerning Lucentis and Eylea that he draws from published studies of other therapeutic groups cannot be justified.
316 In cross-examination, Dr Williams agreed that none of his articles concerned the pharmaceutical industry, none of them were on the topic of medicinal health economics, and none of them concerned the economics of health care. The same applied for the chapters in books which he had identified in his curriculum vitae. None of his formal study concerned itself with the pharmaceutical industry, with health economics or the economics of health care. None of the cases in which he had given evidence concerned with the Trade Practices Act concerned the pharmaceutical industry but he had given evidence to do with the pharmaceutical industry in other cases. One instance was in Canada about whether damages could be calculated. He had given advice, but not evidence, in a couple of other matters in Australia to do with calculation of damages in cases where there was a second drug coming in. Dr Williams agreed that he did not have any particular knowledge of any of the 103 pharmaceuticals referred to in the Dunn Study but proceeded on an assumption in his report that the drugs considered in the Dunn Study were all distinct molecules. He also agreed that he knew almost nothing about the prescribing behaviour of physicians or any difference between the prescribing behaviour of general physicians and those who were more specialised. He agreed he had made no study of, and had no expertise in, the effect of marketing on prescribing practices for pharmaceuticals. He had made no study of, and had no familiarity with, the impact of advertising on physicians. He claimed no expertise in anything to do with health economics in particular, as distinct from economics in general. He had been on the boards of two hospitals for many years but he claimed no knowledge of medicine apart from his experience as a hospital board member. Prior to preparing his report, he was not familiar with any of the Azoulay, Dunn, Spurling, Sanson-Fisher or Sweeny articles or studies. He did not identify in his report any relevant study or article in addition to those identified by Mr Moore: his only references had been to those that Mr Moore referred to. He had never used the BDM and was not familiar with it before obtaining his present instructions. In preparing his report he read quite a few papers about the BDM and cases where people had used the BDM to see what it was and how widely it was used and in what circumstances. The BDM was mainly used in the marketing literature and not in economics.
317 In [56] of his report, Dr Williams said that the time it takes to gain market share may be quite different from the time it takes to reach 95% of the monthly maximum prescription rate. One simply could not infer one from the other. At [57], he said this could be seen from some examples offered by the Azoulay Study which, he said, referred to three cases in which second-comers quickly gained sales at the expense of their competitors. Dr Williams was taken to [57] of his report in cross-examination. He accepted that the word “quickly” in [57c] was incorrect and that [57c] was not accurate and agreed that in respect of [57b], because that drug did not appear in the Dunn Study, there was no data about the speed of its uptake in Australia. Dr Williams said he was not comparing the speed in America with the speed in Australia but was comparing the speed with which you gain market share and the speed with which you reach maximum sales. His general point was that these two things were quite conceptually distinct. He used the examples in [57a] and [57b] to show that people can quickly gain sales. When comparing the [57a] and [57c] figures in respect of the Australian market and some conceptual ideas of speed of uptake in the American market, Dr Williams did not do anything in particular to inform himself about regulatory similarities or differences between those two markets and he agreed that those considerations may have a significant impact. He said the points in [57] were merely meant to illustrate the general conceptual point that there was a difference between the time it takes to reach maximum sales and the speed with which you gain market share. Ultimately, Dr Williams agreed that it was only [57a] that was an illustration of the point he was trying to make. Dr Williams reiterated that in light of the joint report he had no fundamental disagreement with Mr Moore on the total market and on what Mr Moore called his factual market, the first two series Mr Moore estimated.
318 As will appear below at [358]–[364], I have significant doubts about the reliability and applicability of Mr Moore’s estimates. Dr Williams did not have the pharmaceutical expertise of Mr Moore, but his evidence did not, as I find Mr Moore’s evidence did, incline to shoehorn the facts into a model and thus to overstate the applicability of the model and to discount the facts particular to the introduction and uptake of Eylea.
319 It was Mr Moore’s estimates of lost sales which underpinned the accounting evidence to which I now turn.
320 Mr Tony Samuel’s evidence set out his opinion as to the appropriate methodology for calculating the quantum of loss and damage suffered by Novartis “due to an advertising campaign run by Bayer”, which he had been asked to assume was false, misleading and deceptive, and the quantum of any such loss and damage under various scenarios. A summary of the more important assumptions he was instructed to make was that: the conduct of Bayer caused, and would cause, the lost sales of Lucentis identified in Mr Moore’s report dated 26 August 2013; XXXXXX XXXXXXXX XXXXXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXXXX and that the marketing and sales expenditure incurred by Novartis in marketing and selling Lucentis for the financial year 2013 had not decreased as a result of the lost sales of Lucentis and would not have increased had sales of Lucentis been higher.
321 For his first report, Mr Samuel was instructed to calculate the loss and damage suffered by Novartis under 25 loss scenarios. The only difference between each scenario was the units he was instructed were lost during 1 November 2012 to 31 July 2013 (the Historical Loss Period) and 1 August 2013 to 31 December 2016 (the Future Loss Period). In each case the lost units had been calculated by Mr Moore and set out in Appendix E to Mr Moore’s report. Each loss scenario was calculated on the assumption that the units recorded in the Moore report had been and would now be lost as a result of Bayer’s false, misleading and deceptive advertising.
322 The summary of Mr Samuel’s methodology in his first report was as follows:
(a) the appropriate methodology for calculating the loss and damage was to calculate the difference between the net cash flows Novartis would have received absent the false, misleading and deceptive advertising (the counterfactual or hypothetical scenario) and the net cash flows Novartis actually received and would now receive as a result of the false, misleading and deceptive advertising (the actual scenario);
(b) the most appropriate way to calculate that difference was to multiply the unit sales that were lost by the net cash per unit that would have been made by Novartis on each additional unit it would have sold;
(c) the best estimate of the net cash per unit in any one month during the Historical Loss Period was the actual profit per unit made by Novartis in that month, adjusted for timing of cash flows; and
(d) the lost cash flows were discounted to allow for the uncertainty inherent in forecasting hypothetical cash flows and a discounted cash flow approach was appropriate. Under this approach, the cash flows were discounted back to 1 November 2012 as Mr Samuel was instructed that Novartis lost sales of Lucentis from November 2012.
323 The summary of his conclusions as to the quantum of loss and damage in Mr Samuel’s first report was as follows:
Scenario 1
In my opinion, the loss suffered by Novartis before interest and tax gross up is XXXXXX comprising XXXXXXX for the Historical Loss Period and XXXXXXX for the … Future Loss Period.
The lost sales volumes were as set out in Mr Samuel’s instruction letter. Sales per lost unit were based on the average net sales and Mr Samuel’s instruction that the net sales for the Future Loss Period should be based on the actual net sales achieved. He also assumed an average net sales price during the Future Loss Period of XXX being the average net sales price per unit XXXXXXX XXXXX XXXXX XXXX The cost per unit of XXXX was based on XXXXXX XXXXXXXX XXXXXX XXXXX XXXX XXX and Mr Samuel’s instruction that the cost of sales applicable to the Future Loss Period should be based on the average cost of sales for the period December 2012 to July 2013. A discount rate of 10.73% applied to lost cash flows in the Hisotrical Loss Period and a rate of 13.43% applied to lost cash flows in the Future Loss Period. Assuming any award to Novartis was taxed as income, it would be necessary to gross up any after-tax calculation of loss to allow for the tax consequence of the award. The tax gross up on the loss in scenario 1 was XXXXXXXXX at a tax rate of 30%. A further amount would need to be added for interest on the award. Mr Samuel estimated the amount from November 2012 to 31 August 2013 to be XXXXXXX
324 As to the other 24 scenarios, the assumption that varied for each loss scenario was the number of lost unit sales. All other assumptions were the same as used for scenario 1.The table set out the loss calculated for each of the scenarios including the tax gross up and interest components. The total principal loss varied from XXXXXXXXX in scenario 1, to a low of XXXXXXXXXX in scenario 2, and up to XXXXXXXXX in scenario 25. With the tax gross up the figures were XXXXXXXX in scenario 1, XXXXXXXX in scenario 2, and XXXXXXXX in scenario 25.
325 XXXX XXXXXXXXXXX XXXXXXXXX XX XXXXXXXXXXXX XXXXXXXX XX XXXXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXX XXXXXXXXXX XXXXXXXXXX XXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXX XXXXXXX XXXXXXXX XXXXXXXX XXXXXXX XXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXXXXX XXXXXXXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXXXXX XXX
326 In his second report, Mr Samuel updated aspects of his first report having regard to the second report of Mr Moore and to Mr Samuel’s instructed assumptions. The additional assumption was that the representations were conveyed and were misleading and deceptive and likely to mislead and deceive.
327 The corresponding calculations in Mr Samuel’s second report as updated were that in scenario 1 the loss suffered by Novartis before interest and tax gross up was XXXXXXXX comprising XXXXXXXXX for the Historical Loss Period and XXXXXXXX for the Future Loss Period. The tax gross up on the loss in scenario 1 was XXXXXXX The total loss assuming tax gross up but before interest was therefore XXXXXxX. The corresponding figures in scenario 2 were a total principal loss of XXXXXxX and, assuming tax gross up but before interest XXXXXXX. In scenario 25 in table 1 in Mr Samuel’s second report the figures were the same as in Mr Samuel’s table 1 in his first report.
328 Mr Samuel’s third report was in response to instructions to: provide his opinion on the views expressed in the report of Mr Michael Potter (whose report had been filed on behalf of the respondent); update any aspect of his second report which he considered necessary having regard to the third report of Mr Moore, the report of Mr Potter and the two affidavits of Mr Hodgkinson; calculate Novartis’ loss on the assumption that Mr Potter’s opinion as to the effect of the supply agreement was correct; and calculate Novartis’ loss having regard to the third report of Mr Moore and the report of Mr Potter on the assumption that the cost of sales per unit for the Historical Loss Period and the Future Loss Period were from XXX to XXX at intervals of XXX up to XXX.
329 Using the same methodology as in his first report, Mr Samuel updated his loss calculations as a result of the updated figures contained in the third report of Mr Moore. In scenario 1, Mr Samuel’s opinion was that the loss suffered by Novartis before interest and tax gross up was XXXXXXX, comprising XXXXXXX for the Historical Loss Period and XXXXXXX for the Future Loss Period. The tax gross up was XXXXXXXX The total loss assuming tax gross up but before interst, was therefore XXXXXXX In scenario 2, the total principal loss was XXXXXXX and the tax gross up was XXXXXXX giving a total, before interest, of XXXXXXX In scenario 25, the total principal loss was XXXXXXXX and the tax gross up XXXXXXX giving a total, before interest, of XXXXXXX.
330 In his third report, Mr Samuel also calculated the loss using Mr Potter’s methodology, updated for the lost units calculated in the third report of Mr Moore.XX XXXX XXXXXXX XXXXXX XXXXXXXX XXXX XXXXXXX XX XX X XXX XX X XX X XX XXXXX XX XXXXXXXX XXXXX XXXXXXXXXX XXXXXXX XXXXXXXXXX XXXXXXXXX XXXX XXXXXXXXXX XXXXXX XXXXXXXXX XXXXXXXXX XXXXXX
331 Mr Potter, an expert accountant called by the respondent, was asked for his opinion on two matters.
Question 1: What is the effect of the Supply Agreement on the quantum of loss and damage calculated by Mr Samuel?
Question 2: Irrespective of his opinion in respect of Question 1, calculate Novartis’ alleged loss using Mr Samuel’s loss methodology, but on the assumption that the cost of sale per unit for both the Historical and Future Loss Periods range XXXXXXXXXXX
332 As to Question 1, Mr Potter agreed that damages could be assessed on an incremental basis, as Mr Samuel had done, but disagreed with Mr Samuel’s application of the methodology as it did not take into account the terms of the supply agreement. Whereas Mr Samuel concluded that the supply agreement was not relevant to the assessment of damages, Mr Potter considered that, from an accounting perspective, the supply agreement was relevant to the assessment of the damage claimed to have been suffered by Novartis for the reasons that:
(a) the damages claim related to the incremental sales that were assumed would have been made by Novartis in circumstances where the alleged misleading and deceptive advertising of Bayer had not occurred;
(b) the impact of obtaining additional sales was that Novartis would generate an additional operating margin on those sales;
XXXXX XXX XXXXXX XXXXXXXX X XXXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXX XXXXXX XXXXXXXX XXXXXX XXXXXXXX XXXXXX XXXXX XXXX X XXX X XX X XXXXXXXXXXXX XXXXXXXXX X XXXXXXXXXX XX XXXXXXXXXX X XXXXXXXXX X XXXXXXXXX X XXXXXXXXXX X XXXXXXXXX X XXXXXXXX X XXXXXXXXX X XXXXXX X XXXXXXX XXXXXXX X XXXXXXXXXX X XXXXXXX X XXXXXX X XXXXXXXXXX XXXXXX X XXXXXXXXXXXXX XXXXXXXXXX X XXXXXXXXXXXXX X XXXXXXXX X XXXXXXX X XXXXXXX X XXXXXX XXXXXXXXXXXX XXXXXX XXXXXXXXXXXXXXXXX XXXXXXX XXXXXXXXX XXXXXXX XXXXXX
333 XX XXXX XXXXXXXXX X XXXXXXXX XXXXXXXX XXXXXXXXX X XXXXXXXXXXXX XXXXXXX XXXXXXXX X XXXXXXXX X XXXX X XXXXXX X XXXXXXX XX XXX XX XXXXXXX XXXXXXXX XXXXXXX XXXXXX X XXXXXXXX XX XXXXXXX XXXXXXXX XX XXXXXXXXX XXXX XXXXXX XXXXXXXXX XXXXX XXXXXXX XXXXX XX XXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXX XXX XX XXXX X XXX X XXX X XXXXX X X X X X XXXXXXXXXXXXXXXXXXXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXX XXXXXXXX XXXXXXXXXXX XXXXXXXX XXXX XXXXXXXXXXX X XXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXX XXX X XXXXXXXXXXXX XXXXXX XXXXXXX XXXXXXXXX XXXX XXXXXX XXXXXXXXX XXXXXX XXXXXXX X XXXXXX X XXXXXXXX XXXXXXXXX XXXXXXXX X XXXXXXX X XXXXXXXXXXX X XXXXXX X XXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXXX X XXXXXXX XXXXXXXXXX X XXXXXXXXX
334 As to the second matter, Mr Potter had an alternate calculation of damage claimed to have been suffered by Novartis under Mr Samuel’s method (on the assumption that the supply agreement did not apply) but adopting a range of alternate costs per unit ranging from XXXXXX to XXX increasing at intervals of XXX and also a scenario using a cost per unit of XXX in respect of the amount that Novartis would pay XXXXXXXXXX for Lucentis. The quantum of damage in scenario 1 was in the range of XXXXXXXXXXXXXXXXXXXXXXXXXX XXX XXXXX (calculating that Novartis would have reported a loss of XXXXXXX assuming a cost of XXX per unit for Lucentis, meaning that no damage was suffered). The cost per unit for Lucentis had a material impact on the calculation of the damage suffered, adopting the loss model prepared by Mr Samuel.
335 The joint report of Mr Samuel and Mr Potter, directed by the Court, noted three matters on which they agreed. First, as set out above, Mr Samuel assessed damages on an incremental basis, meaning the incremental profit that would have been made on the lost sale had Novartis made the sale. Mr Potter agreed that damages could be assessed on an incremental basis, although he did not prefer this approach. Secondly, Mr Potter did not comment on the assumptions that Mr Samuel had made or been instructed to make regarding the integers applied in the damages model but had accepted those assumptions for the purposes of preparing an alternative assessment of loss and also adopted the damages model. Thirdly, each expert had reviewed and agreed with the mathematical accuracy of each other’s calculations.
336 The joint report of Mr Samuel and Mr Potter noted a large number of matters, some 11 issues or sub-issues, on which they disagreed but they said the only significant matter on which they disagreed was the application of the supply agreement to the calculation of damages. XXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXX XXXXX XXXXX XXXXXXXX X XXXXXXXXXXXXX X XXXXXXXXXXX XXXXXX XXXXXX X XXXXXXXXXX X XXXXXX X XXXXXXXXX X XXXXXXX X XXXX X XXXXXXXX XXXXX XXXXXXXXX XXXXXXXXX X XXXXXXXXXX XXXXXXXXX XXXXXXX XXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXXX XXX XXXXXXXXXX XXXXXXX XXXX XXXXXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXX X XXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXX XXXXXXX XXXXXXXXX XXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXX XXX XXXXXXXXXXXXXXX XXXXXX XXXXXXXX XXXXXXXXX XXXXXX XX XXXXXXXXXXXX X XXXXXXXXXXX XXXXXX XXXXXXXXXXXXX X XXXXXXXXXXXXXXX X XXXX X XXXXXXXXX XXXXXXXX X XXXXXXXXXXX XX XXXX XXXXXX XXXXX XXXXXXXXXXX XXXXXXXXXXX XXX XXXXXXXXXX XXXXXXXXX XXXXXXX XXXXXXXXX XXXXXXX XXXXX X XXXXXXXXXX X XXXXXXXXX XX XXXXXXXXXX XXXXXXXXX XXXXXXXXXXX X XXXXXXXXXXXXXX XX XXXXXXXXXXX XXXXXXX XXXXXX XXXXX X XXXXXXXXX XXXXXXXXX XXXXXXX XXX XXXXX XXXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXX XXXXXXXXXX XXX XXXXX XXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXX XXXXXXXXX X XXXXXXXXXXX XXXXXXXXXX XXXXXXXXX XXXX XXXXXXXX XXXXXXXXXXXX XXX XXXXXXXXXXXXXX X XXXXXXXXXXX X XXXXXXXXX XXXXXXXXXX XXXXXXXXXX X XXXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXXX X XXXXXXXXXX XXXXXXXX
XX XXXXX X XXXXXX X XXXXXXXX X XXXXXXX X XXXXXXXXXXX X XXXXXXX X XXXXXX X X X XXXXXXXXXXX X XXXXXXXX XXXXX X XXXXXXX X XXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXXXX XXXXXXX XXXXX XX XXXX X XX XXXXXXXXXXXX XXXXXX X XXXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXXXXX X XXXXXX X XXXXXXX X XXXXXXXXXX X XXXXXXXXX X XXXXXXXXX X XXXXXXXX X XXXXX XXXXXXXX X XXXXXXXXXX X XXX XXXXXXX XX XXXXX XXXXXX XXXXXXX XXXXXXXXXX X XXXXXXXXXXXX X XXXXXXXXX X XXXXXXX X XXXXXXXXXX X XXXXXXXXXXX X XXXXXXX X XXXXXXX X XXXXXXXXX XXXXXXXXXXX XXXXXXXX XXXXX X X XXXXXXXXXXXXXXXX XXXXXXX XXXXXXXXXXXXXX X XXXXXXXXXX X XXXXXX X XXXXXX X XXXXXX
337 The oral evidence of Mr Samuel and Mr Potter was given concurrently with both experts first giving a brief summary of their evidence, an overview of the joint report and a summary of the issues that remained in dispute. It is not necessary to set out the detail of it, although I should note that the issues between these experts became clearer in the course of it. XX XXXXXXXXXXX XXXXXXXXX XXXXXXXXXXXXXXXX X XXXXXXXXXXXX X XXXXXXXXXX X XXXXXX XXXXXXXXX XXXXXXXX XXX X X XX X X XXXXXXXXXXXXXXXX X XXXXXXXXXXXX XXXXXXXXX X XXXXXXXXXXX X XXXXXXX X XXXXXXXXXXXX X XXXXXXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXXX X XXXXXXXXXXX X XXXXXXXX XXXXXXXXX XXXXXXXXX X XXXX X XXXXXXX XXXXXXXXXX X XXXXXXXXXX X XXXXXXXXXXXXX XXXXX XXXXXXXX X XXXXXXXXXXXX X XXXXXXXXXXXXXX X XXXXXXXXXXXXXX XXXXXXX XXX XXXXXXXX XXXXX X XXXXXXXXXXXX XXXXXXXX XXXXXXX X XXXXXXXXXXX XXXXXXXXXX X XXXXXXXXXXX XXXXXXXX XXXXXXXX XXXXXXXXXX XXXXXXXX XXXX X XXXXXXXXXXX X XXXXXXXXXXXX XX XXXXXXXX XXXXXXXX X XXXXXXXXXX XX XXX XXXXXXX XXXXXXXXX XXXXXXXXXX XXX XXXXXXXX X XXXXXXXXXX XXXXXXX XXXXXXX XXXXXXXXX XXXX XXXXXXXXXX X XXXXXXXXXX X XXXXXXXXX X XXXXXXXXXX X XXXXXXXXX X XXXXXXXXXX X XXXXXXXXXXX X XXXXXXXXXXXX
338 Mr Potter agreed that had the hypothetical incremental sales in fact taken place they would have been accounted for in exactly the same way as the sales that did take place and also agreed that, at the point of each of the sales taking place, Novartis had the benefit of a profit margin of approximately XXX.
339 Mr Potter and Mr Samuel appeared to agree that Mr Hodgkinson’s evidence that the cost reimbursement related more to expenses than it did to products did not matter.
340 As I have said, the essential difference between Mr Samuel and Mr Potter was the point at which the supply agreement should be applied. XXXXXXX XXX XXXX XXXXXX XXXXXX XXXXXX XXXXXXXX XXXXX XX XXX XXXXX XXXXXX XXXX XXXXX XXXXXX XXXX XXXXXXXXX XXXXXXXX XXXXX XXXXXXXXX XXXXXXXXX XXXXXXX XXXXXXX XXXXX XXXXXXXXX XXXXXXXXX XXXXXXXXXXXXXXX XXXXXX XXXXX XXXX XXX XXXXXXXX XXXXX XXXXXX XXXXXXXXXXXXXX XXXXXXX XXXX XXXXX XXXXXX XXXXXXXXXXXXXX XXXXX XXXXXXXX XXXXXX XXXXX XXXX XXXXXXXX XXXXXXXXXXX XXXXX. XXXXX XXXXX XXXXXXX XXXXX XXXXXXXXXXX XXXXX XXXXXX XXXXXXXXXX XXXXXX XXXX XXXXX
341 XXXXXX XXXXXXXX XXXXXX XXXXXXXX XXXXXXXXXXXX XXXXX XX XXXX XXXXXXXXXXXXX XXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX XXXXXXX XXXXXXX XXXXXX XXXXXXXXXX XXXXXXX XXXXXXX XXXX XXXXXX XXXXXXXXX XXXXXXXX XXXXXXXX XXXXXX XXXXXX XXXX XXXX XXXXXXXX XXXXXXX XXXXXX XXXXXXXXXX XXXXX XXXXX XXXXX XXXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXXXX XXXXXXXX XXXX XXXXXXXXXXXXXXXX XXXXXXXXX XXXXXXX XXX XXXXXXXXXX XXXXX XXXXXXXXXX XXXXXXXXXXXXXX XXXXXXXXX XXXXXX XXXXXX X XXXXXXXXXXXXXX XXXXX XXXXXXXX XXXXXXXXXXXXX XXXXXXXXX XXXXXXX XXXXXXXXXX X XXXXX XXXXXX XXXXXX XXX XXX XXXX XXXXXX XXXXXXXX XXXXXXXX XX XXXXXX XXXXXXXXXXXXXX XXXXXX XX XXXXXXXX XXXXXX XXXXXXX XXXXXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXXXXXX XXX XXXXXXXXXXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXX X XXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXX XX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXX XXXXXXX XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXX XXXXXXXXXXXX XXX XXXXX XXXXXXX XXXXXXXXXXX XXXXXXXX XXXXXXXXXXXXX XXXXXX XXXXXXXXXX XXXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXXX XXXXXXXXXX. XXXXXXX XXXXXXXXX XXXXXX XXXX XXXXXXXXXXXXXXX XXXXXXXXXX I prefer the evidence and conclusions of Mr Samuel. XXXX XXXXXXX XXXXX XXXXXXXX XXXX XXXXX XX XXXXX XXXXXXXXXXX XXXXXXXXXXX XXXX
342 As I have said, the calculations of the accountants are, however, based on the numbers of “lost units” of Lucentis set out by Mr Moore in his reports. The real issue is the calculation of lost units.
343 Novartis submitted that the normal approach to damages in cases of comparative advertising which is misleading or deceptive is to assess the impact of the advertising campaign in the market. The quantum of the loss is assessed by calculating the value of the sales that the applicant would have made had the misleading or deceptive advertising not occurred. In the present case, Novartis’ loss and damage is its loss of profits arising from the fact that patients who would otherwise have been prescribed Lucentis were prescribed Eylea, the cause of which was the contravening conduct.
344 As I have said, Mr Moore developed an economic model of sales of Lucentis and Eylea which was his modified version of the BDM. In his modelling of the anticipated impact of the entry of Eylea into the market, Mr Moore set the lower end at 10% and the upper limit at 350%. He then selected a point estimate of 75% as the most likely peak boost to sales. Subsequently, as I have set out, Mr Moore factored in a wider range of materials, arrived at a 150% boost to sales but discounted it to 90% in light of the affidavit evidence of the Bayer sales representatives.
345 The applicant submitted that this was far from guesswork. The applicant submitted the evidence demonstrated that Mr Moore was eminently qualified to undertake the task of calculating the volume of lost sales caused by Bayer’s contravening conduct. Mr Moore used a paper on which Bass was a co-author and which in turn referred to an analysis of 213 studies in which the BDM was applied, to arrive at an average q/p ratio of 12.7, which he described as a point estimate of the empirically measured average. Moreover, the applicant submitted, Mr Moore took great care to ensure that he read not only the Bass paper, but also the underlying meta-analysis and cited both. Mr Moore used those integers to model 25 different scenarios of the impact of the message providing a percentage boost to sales. In the applicant’s submission, the criticisms made by Dr Williams and in cross-examination of Mr Moore concerning his reliance on, and deployment of, key literature studies were demonstrated to be without foundation. The applicant submitted that Mr Moore provided careful and considered responses under cross-examination and Mr Moore’s evidence was of great assistance to the Court and was effectively uncontested as Bayer did not identify any alternative studies or proffer or develop any alternate loss model.
346 The total loss figure of XXXXXX (scenario 1) was based on the direct effect of 90% but with a q/p ratio of 12.7.
347 The respondent submitted that Novartis had failed to prove that the impugned conduct caused it to suffer the loss or damage which it alleged it had or will in future suffer. Bayer referred to a number of considerations which it submitted supported this conclusion.
348 First, Bayer submitted that Novartis had not adduced any direct evidence that it had lost any Lucentis sales because of the representations. Novartis had been unable to procure evidence from a single ophthalmologist (or optometrist, pharmacist, executive or salesperson) that supported an alleged connection between the impugned conduct and a single lost sale.
349 Secondly, even if the representations were to arise from the promotional materials, Bayer submitted that they would have no relevant impact or effect on the prescribing by ophthalmologists of either Eylea or Lucentis. The impugned promotional activities could not be supposed to have changed the mind of, or materially influenced, a specialist doctor by a single sentence in an advertisement. Bayer submitted the impugned conduct was an inconsequential aspect of a much wider process of dissemination by Bayer and others of medical information to ophthalmologists.
350 Thirdly, Bayer submitted, Novartis had itself identified a series of reasons which explained Eylea’s gain in market share in Australia and the reduction in sales of Lucentis after Eylea became available. Novartis’ contemporaneous internal documents considered that there were three key products differentiations: (a) the retention of fluid on the retina associated with Lucentis; (b) patients who were considered to be non-responders to Lucentis; and (c) patients on Lucentis who could not be extended out further than four or five weeks. Bayer also referred to ophthalmologists becoming familiar with Eylea and its characteristics by being provided with free samples of Eylea.
351 Fourthly, Bayer submitted, the uptake of Eylea was consistent with other markets. Novartis acknowledged in its internal analysis, Bayer submitted, that the rate of uptake of Eylea in Australia was similar to the experience in other countries. A comparison of sales rates indicated that the growth of sales in Australia was not atypical and in need of an explanation or cause outside the usual range of ordinary market forces.
352 Fifthly, Bayer submitted, ophthalmologists had detailed knowledge of the comparative science in the VIEW clinical study: it was to be expected that the results of the VIEW clinical study would lead to a rapid gain of market share by Eylea. The VIEW study was the centrepiece of the launch of Eylea on 24 October 2012. It also featured extensively in the Eylea product information. Ophthalmologists gave presentations at educational events which contained extensive information about the VIEW study. Bayer undertook a nationwide detailing campaign in which its sales representatives built on the positive clinical information in the VIEW study. 522 ophthalmologists were visited by Bayer’s sales representatives between May 2012 and June 2013 with the total number of sales visits being 4170, of which 3235 were sit down sales calls. The primary focus of the Detail Aid was the results of the VIEW study data. Furthermore, ophthalmologists knew Eylea was coming and had their own sources of information about it, including clinical study material and other scientific papers.
353 Sixthly, Bayer submitted, Novartis itself was the source of extensive information to ophthalmologists over many years that Lucentis is given monthly. The regularly deployed approved Lucentis product information said as much. The Lucentis patient information document, included in every box of Lucentis, stated that for wet AMD the injection of Lucentis was given once a month. A brochure disseminated to ophthalmologists said that a monthly regimen of Lucentis demonstrated the best visual acuity outcomes in the clinical trials. Novartis sales representatives conveyed the same information including by means of the Novartis eDetailer.
354 Seventhly, large amounts of other information about Lucentis and Eylea were available to, and provided to, the relevant addressees.
355 Bayer also submitted the reports of Mr Moore were deeply flawed and could not be accepted as reliable or accurate. The entire premise of his reports was an assumption that the pleaded case involved representations which could be taken together to be a “fewer injections” representation. The basis of Mr Moore’s analysis was different from, and inconsistent with, Novartis’ pleaded case. What Mr Moore purported to assess was not the effect of the representations but the promotional effect of a different and much broader message. Mr Moore’s incorrect premise infected the whole of his three reports. Also, he seemed to assume that pharmaceuticals could be marketed directly to patients in Australia, as they can be in New Zealand. An additional vice in Mr Moore’s analysis, Bayer submitted, was that the four pleaded representations were not the only meanings that could be attributed to the promotional material, which meant that a “fewer injections” message must include both legitimate and illegitimate meanings on Novartis’ case. Mr Moore’s reports were incapable of measuring the effect of the impugned representations in the marketplace for this additional reason. Bayer next submitted that large parts of Mr Moore’s reports were based on an assumption that Eylea had no actual advantage over Lucentis because it was either a ‘me too’ product, akin to a generic, or was otherwise therapeutically similar to Lucentis. Bayer submitted that the incorrect assumption that Lucentis was a ‘me too’ product was not made out and Mr Moore’s assumption, which represented the foundation of his reasoning, was entirely inconsistent with the evidence. Bayer also submitted that Mr Moore’s early foothold assumption was not made out. Next Bayer submitted that Mr Moore’s assumption that Novartis’ loss should be calculated out to December 2016 was unsupportable. Ophthalmologists learned through experience with the product they prescribed and made repeated prescribing decisions over time. Given Bayer’s undertaking on or about 15 May 2013, it was entirely unrealistic to suggest the period of any loss suffered by Novartis would extend even to 2015, let alone until the end of 2016. If ophthalmologists had been misled by the promotional material one would expect to see a swing in sales back to Lucentis after the cessation of the relevant promotional activities but there had been no such swing.
356 Bayer also submitted that the methodology used by Mr Moore was deeply flawed for other reasons. Mr Moore applied his own personally modified version of the BDM and in doing so misunderstood and misapplied the BDM in a number of ways, Bayer submitted. First, Lucentis did not follow an S-curve adoption pattern when launched in 2007 but, like Eylea, had a sharp uptake in its first few months after launch. Secondly, the central proposition of the BDM was that adoption had not yet occurred and in this respect Mr Moore assumed that each time an ophthalmologist prescribed Eylea it was like the purchase of a new product. However, “purchasing” decisions for Eylea were made regularly and repeatedly over time by the same ophthalmologist. Thirdly, Mr Moore applied the BDM to a specific product within a product category but the model only applied to the generic demand for a product category. Fourthly, applying the BDM in the real world to forecast adoption rates involved guesses of the key parameters. On top of that, Mr Moore made three extensive mathematical “modifications” to the model, which modifications were not supported by any published paper or article and were inconsistent with the model itself. Fifthly, the Mahajan, Muller and Bass article made it clear that before applying the model in the real world key questions arose as to the number of samples being offered to the market. Mr Moore agreed that he had no evidence or understanding about the scale of sampling undertaken by Bayer in relation to Eylea. The adjustment factors that Mr Moore employed to move from his estimate of Eylea factual sales to Eylea counterfactual sales were not derived from any statistical analysis that he had undertaken. The adjustment factors involved pure guesswork by Mr Moore. No reasoning was given to justify the lower limit figure of a 10% boost in sales and no reasoning was exposed as to how the figure of 350% was arrived at. Mr Moore exposed no reasoning as to why he selected 75% but confirmed that it was purely his opinion. Neither the Sweeny Study nor the Dunn Study supported Mr Moore’s assertion that his choice of a 75% boost in sales (within the range of a 10% to 350% boost in sales) was consistent with the findings of those studies. The Azoulay Study provided no support for the 75% figure chosen by Mr Moore. Mr Moore’s explanation of the increase from 75% to 90% was entirely inadequate as the explanation of an increase that was a very significant factor in the eventual assessment of the alleged number of “lost sales”.
357 In Novartis’ reply submissions, the rate and extent of Eylea’s gain of market share was emphasised. It also submitted that the existence of a number of causal elements in play in relation to a shift to Eylea did not mean that the misleading and deceptive conduct was not a contributing cause. The critical point was the impact of misleading and deceptive conduct on sales. It was in this context that Mr Moore’s evidence was especially significant because his analysis sought to identify what proportion of total lost sales were a result of the misleading conduct. Novartis submitted that the more similar the products and the more established the incumbent, the more difficult market penetration would be. Significant market penetration by a new entrant in such circumstances must be accounted for by way of some actual or perceived advantage. There was no material advantage but the potent source of the perceived advantage was the impugned publications. They must have contributed significantly to what Mr Moore opined, by reference to other published work and his own experience, was an atypical rate and depth of market penetration by Eylea.
358 Novartis submitted that the figure of 75% provided by Mr Moore was not an opinion given in the abstract or in a vacuum, indeed it was conservative. Mr Moore’s use of the 12.7 q/p ratio was also conservative. Novartis recognised that the Court may disagree with Mr Moore’s best estimate and therefore presented an additional 24 scenarios that could be used by the Court to estimate damages if it formed the opinion that other figures were more appropriate. Novartis submitted that Mr Moore’s evidence was not the only evidence before the Court which demonstrated the impact of the misleading conduct. Eylea’s entry into the Australian market was faster and deeper than any of the 103 other pharmaceuticals analysed in the Dunn Study. There was also market research evidence by Novartis that supported the view that the impugned publications had a potent impact on the market. Further, patients who were switched from Lucentis to Eylea included not only non-responders but also patients who were receiving very infrequent dosing of Lucentis.
359 In my view, the live issue is the number of sales Novartis had lost as at 31 March 2014 and would lose because of the conduct of Bayer, assuming the representations were made and assuming some causation. The monetary value of those lost sales may then be calculated in accordance with my findings as to the effect of the supply agreement between Novartis and Novartis Pharma AG and the evidence of Mr Samuel.
360 The difficulty lies in assessing on the balance of probabilities the causative effect of the conduct. As Mr Moore’s evidence, taken at its highest, demonstrates, no precision is available and the range is very wide.
361 Given the circumstances in which a patient’s condition is revisited and the individualised professional judgment of an ophthalmologist would be re-applied for each prescription decision and that the ophthalmologist would at that point, if not before, have actual experience of Lucentis or Eylea or both, I am not persuaded that the effect of Bayer’s conduct would continue far past the time the false representations ceased to be made in mid-May 2013. In my opinion, any causative effect of the representations would have ceased by the end of December 2013. This would permit prescriptive conduct by an ophthalmologist based on experience in relation to his or her patients, that conduct being no longer affected, on this hypothesis, by the impugned conduct.
362 I then turn to the circumstances in which this particular drug was introduced into Australia. I take into account: the influence of the overseas information, including the VIEW studies, and the consequent expectations of ophthalmologists; the state of knowledge of ophthalmologists apart from the impugned conduct, in particular the Lucentis product and other information and the ophthalmologists’ prescribing practices in terms of frequency of injection in the years Lucentis had been available for prescription; that pharmaceuticals in general are goods that need to be used by prescribers and trialled with patients before the physician is satisfied about their effects, such that prescribers will learn over time how they work; the similarity between the content of the VIEW studies and the impugned conduct, that is, that the falsity of the material, on the present hypothesis, consisted of divorcing the results of the VIEW studies from the VIEW studies themselves and from the Lucentis product information; the provision of samples of Eylea to ophthalmologists; the rate of uptake of Eylea in the overseas markets of Japan and the United States, which was not dissimilar to the rate in Australia (although there was no evidence as to what advertising of Eylea had occurred in those countries); and regard should also be had to the rate of penetration of the market of Lucentis from when it first became available in Australia.
363 These particular considerations must affect markedly what might otherwise flow from the modelling conducted by Mr Moore. A particular difficulty, in my view, with Mr Moore’s evidence was its focus on “fewer injections” rather than on the representations which, on the present hypothesis, were false. Also, in my view, there was an overemphasis on generalised models without sufficient attention being paid to the particular circumstances surrounding the introduction of Eylea. As to the Azoulay Study, I find to be unconvincing the similarities between that study and the introduction of Eylea to which Mr Moore referred and which I have set out in the last three sentences of [284] above. Rather, as I have set out at [308] above, the study did not deal with significant aspects particular to the introduction of Eylea. As to the Mahajan et al paper, I adopt the criticisms I have set out at [302]-[303] above and I do not regard that application of the BDM as providing support for Mr Moore’s conclusions. That is, I find to be unpersuasive the application of the BDM to yield an estimate rather than a guess by reference to the commencement of a patient on a new course of treatment and by reference to a specific product within a product category. As to the Dunn Study, in my opinion Mr Moore’s use of it as I have set out at [285] above involves a clear case of preferring theory to empirical data. Much more relevant to the adoption of Eylea were: the facts as to the adoption of Lucentis; the facts relevant to the adoption of Eylea in the United States and Japan; the perceived differences between Eylea and Lucentis; and the amount of information available about Eylea apart from the impugned conduct which went also to the profession’s expectations surrounding Eylea’s introduction. Also, as I have noted at [305] above, Mr Moore accepted that the aim of the study was not to apply the BDM to individual medicines and the conclusions expressed in the study were retrospective not predictive.
364 I also find that Mr Moore’s definitions of ‘me too’ drugs played an important part in his analysis but his application of the concept in his assessment of Novartis’ lost sales discounted perceived differences between Lucentis and Eylea, those differences being in relation to the retention of fluid on the retina associated with Lucentis; patients who were considered to be non-responders or sub-optimal responders to Lucentis; and patients on Lucentis who could not be extended out further than four or five weeks. His application of the ‘me too’ concept also discounted that: Eylea had a different active ingredient; it had a different mechanism of action; and it had a different standard dosing regimen. This makes unreliable, in my view, Mr Moore’s estimates of Novartis’ lost sales of Lucentis. The point is not the content of the expression ‘me too’ but Mr Moore’s assumption that there were no relevant differences between Lucentis and Eylea. The Sweeny Study, on which Mr Moore relied, was directed to the entry of a generic pharmaceutical into a market.
365 In light of these matters, a figure of a 20% boost to sales should be used, up to the end of December 2013. Further, I would assess the internal influence, that is the behaviour of the ophthalmologists due to the influence of existing adopters, as not having been established and the q/p ratio to be zero.
366 Damages should therefore be calculated as per Mr Moore’s scenario 2 using the historical loss figure but with future loss limited to the end of December 2013. That figure is XXXXXXXX plus a tax gross up of XXXXXXX; it does not include a sum for pre-judgment interest.
Conclusion
367 Having made my reasons available to the parties I will stand the matter over for four weeks to enable them to identify which parts of the reasons should not be published because of confidentiality and to arrive at a proposed form of orders. The parties should also agree the figure to be included, even if not published, in the immediately preceding paragraph of these reasons. At that time I will make final orders dismissing the application. If the applicant contends that costs should not follow the event, it should file and serve within 14 days of the date of these reasons a written submission of no more than three pages in support of that contention. The respondent should file and serve within 21 days of the date of these reasons its written submission of no more than three pages in answer to any such submission of the applicant.
I certify that the preceding three hundred and sixty-seven (367) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Robertson. |
Associate:
Dated: 26 March 2015




