FEDERAL COURT OF AUSTRALIA
Australian Competition and Consumer Commission v Homeopathy Plus! Australia Pty Limited [2014] FCA 1412
IN THE FEDERAL COURT OF AUSTRALIA | |
AUSTRALIAN COMPETITION AND CONSUMER COMMISSION Applicant | |
AND: | HOMEOPATHY PLUS! AUSTRALIA PTY LIMITED First Respondent FRANCES MERCIA SHEFFIELD Second Respondent |
DATE OF ORDER: | |
WHERE MADE: |
THE COURT DECLARES THAT:
1) The First Respondent and the Second Respondent have in trade and commerce:
a) engaged in conduct that was misleading and deceptive or was likely to mislead and deceive, in contravention of section 18 of the Australian Consumer Law (“ACL”); and
b) in connection with the supply or possible supply of homeopathic treatments or products (“Homeopathic Treatments”), and in connection with the promotion of the supply of Homeopathic Treatments, made false or misleading representations that the vaccine publicly available in Australia for whooping cough (“Vaccine”) is of a particular standard or quality in contravention of sections 29(1)(a) and (b) of the ACL,
by publishing, or causing to be published, on the website www.homeopathyplus.com.au (“Website”):
c) from 1 January 2011 until around 26 April 2012, an article entitled “Whooping Cough – Homeopathic Prevention and Treatment” (the “First Whooping Cough Article”) in which a representation was made to the effect that the Vaccine is short-lived, unreliable and no longer effective in protecting against whooping cough;
d) from 11 January 2013 until around March 2013, an article entitled “Whooping Cough – Homeopathic Prevention and Treatment” (the “Second Whooping Cough Article”) in which a representation was made to the effect that the Vaccine may not be the best solution for, is of limited effect, and is unreliable at best, in protecting against whooping cough; and
e) from 3 February 2012 until around March 2013 an article entitled “Government Data Shows Whooping Cough Vaccine a Failure” (the “Government Article”) in which a representation was made to the effect that the Vaccine is largely ineffective in protecting against whooping cough;
when, in fact, the Vaccine is effective in protecting a significant majority of people who are exposed to the whooping cough infection from contracting whooping cough.
2) The First Respondent and the Second Respondent have in trade or commerce:
a) engaged in conduct that was misleading and deceptive or was likely to mislead and deceive, in contravention of section 18 of the ACL;
b) in connection with the supply or possible supply of Homeopathic Treatments, and in connection with the promotion of the supply of Homeopathic Treatments, made false or misleading representations that the Homeopathic Treatments are of a particular standard or quality in contravention of section 29(1)(a) and (b) of the ACL; and
c) in connection with the supply or possible supply of Homeopathic Treatments, and in connection with the promotion of the supply of Homeopathic Treatments, made false or misleading representations that Homeopathic Treatments have a use or benefit in contravention of section 29(1)(g) of the ACL,
by publishing, or causing to be published, on the Website:
d) the First Whooping Cough Article;
e) the Second Whooping Cough Article; and
f) the Government Article in conjunction with the Second Whooping Cough Article,
in which representations were made to the effect that there was a reasonable basis, in the sense of an adequate foundation, in medical science to enable it or them (as the case may be) to state that Homeopathic Treatments are a safe and effective alternative to the Vaccine for the prevention of whooping cough when, in fact:
g) there is no reasonable basis, in the sense of an adequate foundation, in medical science to enable the First Respondent and the Second Respondent to state that Homeopathic Treatments are safe and effective as an alternative to the Vaccine for the Prevention of Whooping Cough; and
h) the Vaccine is the only treatment currently approved for use and accepted by medical practitioners in Australia for the prevention of whooping cough.
THE COURT ORDERS THAT:
3) The matter is listed for directions at 9.30 am on Wednesday 4 February 2015 in order to set a timetable for any further evidence on the question of penalties and submissions including on the injunctive and other final orders sought by the Applicant.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
NEW SOUTH WALES DISTRICT REGISTRY | |
GENERAL DIVISION | NSD 256 of 2013 |
BETWEEN: | AUSTRALIAN COMPETITION AND CONSUMER COMMISSION Applicant
|
AND: | HOMEOPATHY PLUS! AUSTRALIA PTY LIMITED First Respondent FRANCES MERCIA SHEFFIELD Second Respondent |
JUDGE: | PERRY J |
DATE: | 22 DECEMBER 2014 |
PLACE: | SYDNEY |
REASONS FOR JUDGMENT
1 The first respondent, Homeopathy Plus! Australia Pty Ltd (“Homeopathy Plus”), is an Australian proprietary company limited by shares which was registered in New South Wales on 20 November 2008. It is not in dispute that, among other activities, Homeopathy Plus sells homeopathic products and treatments through its website at www.homeopathyplus.com.au (“the Website”).
2 The second respondent, Mrs Frances Sheffield, has been the sole director of the first respondent since 18 November 2009. It is common ground that she is the author of three articles which she uploaded onto the Website, namely:
(a) “Whooping Cough – Homeopathic Prevention and Treatment” (“First Whooping Cough Article”);
(b) “Whooping Cough – Homeopathic Prevention and Treatment” (“Second Whooping Cough Article”); and
(c) “Government Data Shows Whooping Cough Vaccine a Failure” (“Government Article”)
(collectively referred to as the “Three Articles”).
3 The domain name of the Website is registered to Mrs Sheffield and her husband.
4 The applicant (“the ACCC”) seeks declarations, injunctions, penalties and ancillary orders in respect of alleged contraventions by the Respondents of the Australian Consumer Law (“ACL”), being Schedule 2 to the Competition and Consumer Act 2010 (Cth) (“CCA”). The CCA and ACL came into force on 1 January 2011. The contraventions are said to arise from statements made in the Three Articles published on the Website.
5 This judgment addresses only the question of whether the alleged contraventions have taken place. As foreshadowed before the trial and confirmed at the hearing with the parties, the hearing covered all matters relating to the alleged contraventions of the ACL, the circumstances in which they occurred and the severity of those contraventions. The issues addressed by these reasons are similarly confined. That approach leaves open the option to the parties for evidence to be led otherwise in mitigation of penalty and the like, and for separate submissions to be made as to the pecuniary penalty, injunctive relief and other final orders sought by the ACCC aside from the declaratory relief sought which I will grant for the reasons I explain in the conclusion.
6 The dispute centres upon the proper characterisation of the representations made in the Three Articles and whether those representations were made in trade and commerce. The ACCC alleges that the Three Articles contained false, misleading and/or deceptive representations contrary to ss 18 and 29(1)(a), (b) and (g) of the ACL concerning:
(a) the effectiveness of the Vaccine publicly available in Australia for the prevention of whooping cough; and
(b) the safety and effectiveness of homeopathic treatments as an alternative to the Vaccine for the prevention of whooping cough.
7 No allegations are made with respect to any statements as to the effectiveness of homeopathic treatments for the treatment, as opposed to prevention, of whooping cough, or as a complementary treatment more broadly, or in respect of any vaccine other than that for Whooping Cough (“the Vaccine”).
8 Section 18 of the ACL provides that:
A person must not, in trade or commerce, engage in conduct that is misleading or deceptive or is likely to mislead or deceive.
9 Section 29 has more specific elements and relevantly provides that:
(1) A person must not, in trade or commerce, in connection with the supply or possible supply of goods or services in connection with the promotion by any means of the supply or use of goods or services:
(a) make a false or misleading representation that goods are of a particular standard, quality, value, grade, composition, style or model or have had a particular history or particular previous use; or
(b) make a false or misleading representation that services are of a particular standard, quality, value or grade; or
…
(g) make a false or misleading representation that goods or services have sponsorship, approval, performance characteristics, accessories, uses or benefits;…
10 The ACCC alleges that Homeopathy Plus and Mrs Sheffield engaged in the impugned conduct as principal contraveners. For the reasons I set out below, I find the contraventions of the ACL alleged by the ACCC to be established.
2.1 The ACCC’s primary contentions
11 The ACCC alleges that Homeopathy Plus and/or Mrs Sheffield, made representations to the effect that the Vaccine:
(a) is short-lived, unreliable and no longer effective in protecting against whooping cough (“the First Vaccine Representation”);
(b) may not be the best solution for, is of limited effect and is unreliable at best, in protecting against whooping cough (“the Second Vaccine Representation”); and
(c) is largely ineffective in protecting against whooping cough (“the Third Vaccine Representation”);
(together the “Vaccine Representations”).
12 The ACCC contends that the Vaccine Representations are false, misleading and/or deceptive in contravention of ss 18 and 29(1)(a) and (b) of the ACL because the Vaccine is, in fact, effective in protecting a significant majority of people who are exposed to whooping cough infection from contracting whooping cough.
13 The ACCC further alleges that, by publishing, or causing to be published, the First and Second Whooping Cough Articles and the Government Article in conjunction with the Second Whooping Cough Article, the Respondents made representations to the effect that there was a reasonable basis, in the sense of an adequate foundation in medical science, to enable it or them, as the case may be, to state that homeopathic treatments are a safe and effective alternative to the Vaccine for the prevention of whooping cough (the “Homeopathy Alternative Reasonable Basis Representation”). This representation is also alleged to be false, misleading and/or deceptive in contravention of ss 18 and 29(1)(a), (b) and (g) of the ACL on the grounds that:
(a) there is no reasonable basis in medical science to allow the Respondents to state that homeopathic treatments are safe and effective as an alternative to the Vaccine for the prevention of whooping cough; and
(b) the Vaccine is the only treatment currently approved for use and accepted by medical practitioners in Australia for the prevention of whooping cough.
14 In determining these claims, it was not in issue that the Court should construe the Three Articles, including the key notions said to be conveyed by them, in the plain English sense in which the public would understand them. In this regard, the ACCC submitted (and I do not understand it to be in issue) that, based upon the Oxford English Dictionary and Macquarie Dictionary (5th edition) definitions, the meaning of these key notions were as follows:
“Unreliable” means not able to be relied upon. “Reliable” in turn means consistently good in quality or performance, able to be trusted. “Ineffective” means not producing any or the desired effect. “Solution” means the act of solving a problem, or state of being solved; a particular instance or method of solving. “Short-lived” means lasting only a short time; or living or lasting only a little while.
15 In support of its contentions that each of the representations were false, misleading or deceptive, the ACCC relied upon the expert evidence of three witnesses:
a) Dr Nicholas Wood, Staff Specialist, General Medicine at the Children’s Hospital at Westmead and a Senior Research Fellow at the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases;
b) Dr Nigel William Crawford, Medical Head of Immunisation Services (which recommends and administers vaccines to high-risk children, including whooping cough, according to the schedule) and a Paediatrician at the Royal Children’s Hospital in Melbourne; andProfessor Kerryn Phelps AM, General Practitioner, who was the President of the Australian Medical Association from 2000-2003, and the President of the Australasian Integrative Medicine Association from 2009-2012.
16 Ultimately, no expert evidence was relied upon by the Respondents that materially contradicted the evidence of these witnesses; the evidence of Dr Mark Donohoe called by the Respondents being consistent with that of the experts called by the ACCC. Nor was the expertise of Dr Wood, Dr Crawford and Professor Phelps to give evidence on the matters addressed by them in their reports in issue.
17 In their defence, the Respondents contend that:
(a) the statements in the Three Articles were not made in trade and commerce but “were uploaded for general information and education purposes and were a contribution to an ongoing public debate of scientific and political interest which is an activity regularly undertaken by [Homeopathy Plus]”;
(b) the statements in the Three Articles were not false, misleading and deceptive as there is a reasonable basis in homeopathic science for the statements as to the effectiveness of homeopathy in the prevention of whooping cough; and/or
(c) the statements in the Three Articles were not false, misleading and deceptive even if measured against orthodox medical science.
18 The Respondents summarised the primary elements of their defence in their submissions in closing as follows:
I. When corporations conceived with an advocacy purpose participate in public debates about science and politics it does not necessarily follow that their conduct is commercial simply because they are corporations. The intention of the alleged contravener is important to assess during the trade and commerce test (i.e. the design of the representations). The representations were not centrally conceived or designed with a trading or commercial purpose and did not amount to conduct in trade or commerce. When qualified assertions, which are accurate summations of a position within a wider debate, are made not about ‘products’ but rather the roll-out of a complicated Government schedule and its effectiveness then those assertions bear a non-commercial character.
II. Those same qualified assertions, even if they were sufficiently commercial in character so as to attract the jurisdiction of the ACL, are factually well-founded insofar as they relate to the vaccines. This is based on a plain reading of the words themselves rather than an unnecessarily medico-technical one based on the insertion of concepts of efficacy foreign to the readers of the website.
III. The commentary on homeoprophylaxis has been shown to fall within the accepted definitions of that modality and cannot be considered misleading when read in their context as emanating from a homeopathy publication (the name Homeopathy Plus! across all banners and weblinks relevant to this proceeding is not merely suggestive, but overt, with regard to the framework within which the statements are made). There is a clear differentiation between the two epistemological frameworks and Homeopathy Plus! never passed off ‘mainstream acceptance’ as per the allegations of the Applicant.
(emphasis in original)
19 Preliminary issues arose as to the bulk of the expert evidence which the Respondents sought to lead in support of the second and third primary grounds of their defence. These are considered below.
3. The Respondents’ expert evidence
20 The Respondents initially sought to rely upon reports of Dr Isaac Golden, Dr Jurgen Schulte, and Dr Donohoe.
21 The ACCC objected to much of that evidence on the grounds that the expert opinions were irrelevant, conclusionary, argumentative, disclosed no reasoning, and were incapable of being tested intelligibly in cross-examination, and that the underlying studies were hearsay and not attached to the report.
22 The report of Dr Schulte was excluded in its entirety on the ground of relevance.
23 As a result of rulings at trial and of concessions made by the Respondents, substantial parts of the reports of Dr Golden and Dr Donohoe were also not received in evidence (albeit that some of that material was nonetheless received as submission). The reports were in patently inadmissible form with no apparent regard to the rules of evidence and, in particular, to s 79 of the Evidence Act 1995 (Cth) (“Evidence Act”). An application by the Respondents at trial under s 190(3)(b) of the Evidence Act to dispense with the rules of evidence insofar as they precluded the admission of substantial parts of Dr Golden’s report was also refused.
24 Dr Donohoe was a general medical practitioner with 26 years’ experience who retired in 2003. He did not purport to be an expert in the prevention of disease or in the prevention or treatment of whooping cough. He had little clinical experience with cases of infant pertussis in the past decade although he had seen many adult and adolescent cases in this period. Insofar as his evidence was admitted, Dr Donohoe’s evidence was largely consistent with that given by the experts called by the ACCC, as I later explain.
25 Dr Golden is an Honorary Research Fellow at the School of Science, Information Technology and Engineering at the University of Ballarat. He holds a Doctor of Philosophy from Swinburne University of Technology awarded in 2004 on the topic of Potential value of Homeoprophylaxis in the Long-Term Prevention of Infectious Diseases and the Maintenance of General Health in Recipients, together with diplomas in naturopathy and homoeopathy from the Melbourne College of Naturopathy in 1990 and the Melbourne College of Homoeopathy in 1989 respectively. To the extent that Dr Golden’s report was admitted, it was largely confined by orders under s 136 of the Evidence Act to a description of the philosophical approach of homeopathy to the treatment and prevention of disease as opposed to evidence on the effectiveness of homeopathy in preventing whooping cough. The closing submissions for the Respondents repeatedly overlooked the limited basis on which Dr Golden’s evidence was admitted, submitting that it showed that there is a reasonable basis in homeopathic science for the representations about homeoprophylaxis. However, given the terms of the order, his evidence simply could not be put to that use.
3.2 Admissibility of expert opinion evidence under the Evidence Act
26 Given the extent of the apparent difficulties with the Respondents’ expert evidence and the potential impact of excluding such evidence upon the Respondents’ case, I delivered detailed reasons in the course of the trial for ruling inadmissible sample passages from the report of Dr Golden. It is helpful to repeat the substance of those reasons here. These reasons were illustrative of the difficulties found in the bulk of the Respondents’ expert reports.
27 By s 76(1) of the Evidence Act, the general rule is that “evidence of an opinion is not admissible to prove the existence of a fact about the existence of which the opinion was expressed”. This rule is known as the “opinion rule”. Section 79 of the Evidence Act exempts evidence of certain opinions of experts from the general rule in s 76(1) and provides that:
If a person has specialised knowledge based on the person’s training, study and experience, the opinion rule [in s 76(1)] does not apply to evidence of an opinion of that person that is wholly or substantially based on that knowledge.
28 As Lindgren J held in Harrington-Smith on behalf of the Wongatha People v Western Australia (No 2) (2003) 130 FCR 424 (“Harrington-Smith”) at 427 (an authority relied upon by the Respondents):
By providing for an exception to the inadmissibility created by the opinion rule, s 79 goes to admissibility… the section poses an objective test; no discretion is involved; a party raising an objection to admissibility on the ground that the section is not satisfied is entitled to a ruling on the objection…
29 The test which must be applied in determining whether expert opinion evidence is admissible under s 79 was considered by the High Court in Dasreef Pty Limited v Hawchar (2011) 243 CLR 588 (“Dasreef”). The joint judgment in that decision of French CJ, Gummow, Hayne, Crennan, Kiefel and Bell JJ established in my opinion the following matters.
30 First, the expert opinion rule directs attention as to why the party tendering the evidence says that it is relevant, that is, as to the finding that the tendering party wishes the Court to make. Thus, in considering the operation of s 79(1), their Honours held at 602 [31] that it is “…necessary to identify why the evidence is relevant: why it is ‘evidence that if it were accepted, could rationally affect (directly or indirectly) the assessment of the probability of the existence of a fact in issue in the proceedings.’”
31 Secondly, as Gleeson CJ pointed out in HG v The Queen (1999) 197 CLR 414 at 427 [39] in a passage approved in Dasreeef at 604 [36], “…by directing attention to whether an opinion is wholly or substantially based on specialised knowledge based on training study or experience, [s 79] requires that the opinion is presented in a form which makes it possible to answer that question”. Thus, as their Honours in Dasreef later explained in their joint reasons, “[t]he point which is now made is a point about connecting the opinion expressed by a witness with the witness’ specialised knowledge based on training, study or experience.” (at 605 [41]).”
32 Thirdly, their Honours in Dasreef held that a failure to demonstrate that an opinion expressed by a witness is based on the witness’ specialised knowledge based on training, study or experience “…is a matter that goes to the admissibility of the evidence, not its weight.” (Dasreef at 605 [42]; see also Honeysett v The Queen (2014) 88 ALJR 786 (“Honeysett”) at 794 – 795 [44]-[46] (the Court). Thus in Dasreef, the evidence of the witness’ estimate as to the volume of respirable silica dust to which the plaintiff was exposed over time in the course of his employment lacked reasons. The absence of reasons in turn was held to point inexorably to the lack of any sufficient connection between a numerical or quantitative assessment or estimate, on the one hand, and relevant specialised knowledge, on the other hand (Dasreef at 605 [42]). In this regard, I reject the Respondents’ submission that the Court in Dasreef was not concerned with the absence of reasons in the expert’s report, but rather there had been a failure to demonstrate that the expert’s opinion had a foundation in his specialised knowledge in some other way, which the Respondents did not clearly articulate. In my view, the need to demonstrate the connection between the specialised knowledge and its application is inconsistent with the Respondents’ submission: see also e.g. Honeysett at 794–795 [44]–[46]. Reasons provide the mechanism, in other words, by which the connection is exposed. In this regard, Heydon J in his separate judgment in Dasreef also makes the point with respect to the need for the reasoning to be stated, that “[t]he opposing party is not to be left to find out about the expert’s thinking for the first time in cross-examination” (ibid at 623 [91]) and further that “[t]he requirement that the opinion be based wholly or substantially on specialised knowledge is an explicit precondition of admissibility. Like other preconditions under s 79, it is to be established by the party tendering the evidence. It is to be established in evidence in chief… not in cross-examination …” (ibid at 626 [99]).
3.3 Admissibility of the evidence
33 At its heart, the difficulty with Dr Golden’s report was that it cast no light upon the reasoning by which the opinions given were reached. Crucially, just as the figure reached in Dasreef as to the likely level of exposure to dust lacked reasons by which the connection between the specialised knowledge and the evidence was demonstrated, equally no such connection between the evidence as to the alleged efficacy of homeoprophylaxis in the prevention of whooping cough (or other diseases) and the application of specialised knowledge was identified by Dr Golden’s report. As such, the evidence fell well short of meeting the requirements of s 79 of the Evidence Act. In those circumstances, I had no discretion. The passages in question were not admissible.
34 The Respondents sought to rely upon the breadth of Dr Golden’s curriculum vitae, submitting generally that:
I would put it so high as to say this is one of the most eminently qualified homeopaths in Australia and he has got dozens and dozens of articles published, many of them are relevant to international trends and experience and I just wonder if we’re going to take the approach of seeing whether or not every single statement has a proper basis without reference to the curriculum vitae, then not only will it take a long time, but I will certainly feel, your Honour, that perhaps there has been some disenfranchisement going on here with the expert witness.
35 In a similar vein, the Respondents further submitted that:
…perhaps, my rather abstract and tangential opening submissions on the decision in Dorber, insofar as it may be that Dr Golden is being indirectly punished in the sense that the way he references in material, and the self-referential aspects like referring to his own studies do appear to be markedly from the approaches taken by Dr Woods and Dr Crawford.
In some sense, that’s the nub of the problem for our expert witness, is trying to feed their evidence through this orthodox medical paradigm and all I’m saying is let’s not confuse the orthodox medical paradigm with regard to how references are attached and how they’re referred to for the test under the Evidence Act, because they’re two separate things, and there is a danger of conflation insofar as you have eminent evidence-based medical practitioners appearing for the applicant and doing expert reports in the way you would expect of an orthodox evidence-based medical practitioner, as against a homeopath, I’m unsure of his experience with regard to building documents like this.
36 However, these submissions suffer from the same fallacies as those identified by Lindgren J in Harrington-Smith. Specifically, Lindgren J held at 427 that:
Unfortunately, in the case of many of the experts’ reports, little or no attempt seems to have been made to address in a systematic way the requirements for the admissibility of expert opinion. Counsel protested that, in order to ensure that the requirements of admissibility are met, lawyers would have to become involved in the writing of the reports of expert witnesses. In the same vein, counsel said in supporting the admission of certain parts of a report, that they were written in the way in which those qualified in the particular discipline are accustomed to write.
Lawyers should be involved in the writing of reports by experts: not, of course, in relation to the substance of the reports (in particular, in arriving at the opinions to be expressed); but in relation to their form, in order to ensure that admissibility is attracted by noting more than the writing of a report in accordance with the conventions of an expert’s particular field of scholarship. So long as the Court, in hearing and determining applications such as the present one, is bound by the rules of evidence, as the Parliament has stipulated … the requirements of s 79 (and of s 56 as to relevance) of the Evidence Act are determinative in relation to the admissibility of expert opinion evidence.
(emphasis in original)
37 Nothing in that approach is inconsistent with the decision in Dasreef; indeed, in my view, the decision in Dasreef emphasises the correctness of the approach.
38 In short, the reason why the evidence in question was excluded was not because it was product of, to use the words of counsel for the Respondents, a science which operates in a different paradigm from orthodox medical science. Nor was it excluded because, as the Respondents also submitted, “…automatic superiority and automatic preference [is] given to one paradigm over the other either at the admissibility level or the weight level …” The evidence was excluded because the Respondents’ evidence must comply with the same rules of evidence as those which apply to all parties under the Evidence Act, relevantly, the requirements for the admissibility of expert opinion evidence under s 79. Responsibility for ensuring that evidence is put before this court in admissible form lies with the legal representatives. It is no answer, as Lindgren J has said, to say that the expert is unaccustomed or untrained in presenting evidence in an admissible form; nor that the form in which the evidence is presented reflects the way in which material might customarily be presented within the field of expertise in question.
4. Background concepts and facts
39 The expert evidence led by the ACCC as to the nature and seriousness of whooping cough, the nature of the Vaccine and recommended schedule for its administration and the essential precepts of Evidence Based Medicine were not contentious.
4.1 Pertussis – the disease and potential complications
40 Pertussis, otherwise known as whooping cough, can be a serious respiratory disease and is caused by the bacterium Bordetella pertussis (“pertussis”). It is an exclusively human pathogen spread by respiratory droplets. It is highly infectious, spreading to 90% of “susceptible household contacts”, being individuals who are not immune and live in the same house as the infectious person. Pertussis is known for the uncontrollable and violent paroxysms (bouts) of coughing experienced by those suffering from the disease at the end of which, the patient needs to take a deep breath resulting in a “whooping” sound.
41 “Classical pertussis” typically has an incubation period of 7 to 10 days followed by three phases of illness:
(a) The first phase is catarrhal. This is a non-specific nasal congestion illness accompanied by a mild cough, lasting one to two weeks.
(b) The second phase is paroxysmal: namely, a spasmodic cough, post-cough vomiting and inspiratory whoop which lasts for four to six weeks or longer. Facial suffusion (redness/congestion) with prominent eyes and protrusion of neck veins may be seen during these paroxysms. It can also affect infant’s feeding.
(c) The final phase is convalescent during which the symptoms slowly improve over one to two weeks, although the phase may persist longer. The cough may persist for a number of weeks or months and future episodes of upper respiratory tract infections may restimulate the coughing paroxysms.
42 If untreated, a person with whooping cough is contagious for at least 21 days. Whooping cough is treated by ten day course of antibiotics, after the first five days of which there is minimal chance of transmitting the disease. This means, as Dr Crawford explained, that early treatment is an important public health measure for decreasing the risk of further contact cases. However the antibiotic treatment will not stop or improve the cough.
43 Professor Phelps explained that transmission of the disease from mother to child is thought to be responsible for 38% of cases of whooping cough in children, with fathers responsible for further 17% and siblings, 41%. Dr Wood also identified infected parents and siblings as the most important source of infection to infants, stating that:
Individuals with pertussis disease are most infectious during the initial catarrhal period and for the first 2 weeks of spasmodic cough, but can remain infectious for up to 6 weeks, especially in the case of non-immune infants. A probable scenario is that adults and adolescents, who become infected because of waning vaccine or disease induced immunity, act as reservoirs for infection and transmit infection to unvaccinated or partially vaccinated infants. In Australia, a national study identified a presumptive source of infection in 60% of 110 hospitalised infants, of whom 60% were parents. (Elliot E, McIntyre P, Ridley G et al. National study of infants hospitalised with pertussis in the acellular peturssis (sic) vaccine era. Pediatr Infect Dis J. 2004; 23: 246-252) In a recent study by Jardine et al in 2010, siblings have also identified as a source of infection for young infants. (Jardine A et al. Who gives pertussis to infants? Source of infection for laboratory confirmed cases less than 12 months of age during an epidemic, Sydney, 2009. Commun Dis Intell Q Rep. 2010 Jun;34(2):116-21).
44 The symptoms in older children, adolescents and adults can range from classic pertussis to a mild cough or even no cough. Young infants may present with apnoea only or an apparent life-threatening event characterised by shallow/absent breathing, slowing heart rate and cyanosis (i.e. appearing blue due to lack of oxygen).
45 Serious complications can arise from pertussis but are more common in non-immune young infants and very young infants. As Dr Crawford explained:
Respiratory complications are frequent with a pneumonitis (lung inflammation), commonly seen. The secondary pneumonia (chest infection) may also occur due to B.pertussis or a secondary bacterial infection. Occasionally the pneumonia may be of a severe necrotizing form, which is the major cause of death. Atelectasis (airway mucous plugging) may occur. Severe air leak complications relate to damage to the lungs following rupture of the alveoli. They include pneumothoraces, which is air between the lung and the pleura (lining of the lung) and if large may require intervention with an underwater sealed drain. Interstitial emphysema (damage to the lungs) and pneumomediastinum (air in the chest, but outside the lung) may also occur. Bronchiectasis or damage to the airways caus[ing] them to widen and become infected is a rare late complication. Su[b]conjunctival hemorrhages of the eye, rectal prolapse or inguinal (groin) hernia may occur due to the increase in intra-abdominal pressure. Cerebral anoxia (lack of oxygen), with associated convulsions, can occur in young children and an encephalopathy (global brain dysfunction) is seen in approximately 1 in 10,000 cases.
46 Younger infants under six months are more likely to have complications such as apnoea (breath-holding) and require hospitalisation. Most deaths secondary to pertussis also occur in children under six months of age and especially those under one month old. The infection can also result in death among adults, especially the very elderly.
47 Individuals can become immune to pertussis either by naturally acquiring pertussis or following receipt of a pertussis Vaccine.
48 All vaccines available in Australia, including that for pertussis, are approved for use and regulated by the Therapeutic Goods Administration (“TGA”) under the Therapeutic Goods Act 1989 (Cth). The stated object of the Therapeutic Goods Act is to create “a national system of controls relating to the quality, safety, efficacy and timely availability of therapeutic goods” that are, relevantly, used in Australia: s 4, Therapeutic Goods Act. It prohibits the importation, exportation, manufacture and supply of therapeutic goods in Australia for use in humans save where the goods are registered, listed, exempt, or approved/authorised under s19 or s 19A: see ss 19(4) and 19D(1). Therapeutic goods include goods for use in “preventing, diagnosing, curing or alleviating a disease, ailment, defect or injury in persons” (see the definition of “therapeutic good” and related definition of “therapeutic use” in s 3(1), Therapeutic Goods Act).
49 The TGA has published guidelines for the registration of prescription medicines, including vaccines, in the Australian Regulatory Guidelines for Prescription Medicines, June 2004.
50 Before a product can be registered under the Therapeutic Goods Act, the sponsoring company must make an application with data to support the quality, safety and efficacy of the vaccine for its intended use. Dr Wood explained that the data requirements are largely based on those applying in the European Union, supplemented where necessary by Australia–specific requirements. In the case of vaccines, the premarket evaluation data includes: the quality and quality control aspects of the manufacture; pre-clinical data designed to assess the toxicological profile of the vaccine, including safety when tested in animals: and clinical (i.e., human) trial data to support the safety and efficacy of the vaccine in humans. Information from equivalent regulators in other jurisdictions that undertake a similar approval process, such as the Food and Drug Administration in the United States, may also be requested.
51 In determining whether to allow an application for registration of the therapeutic product, the Secretary must evaluate the goods for registration having regard to a number of matters including “whether the quality, safety and efficacy of the goods for the purposes for which they are to be used have been satisfactorily established”: s 25(1)(d), Therapeutic Goods Act. The term “safety” has, in my view, been used in its ordinary meaning to refer to “the quality of being unlikely to cause hurt or injury; the quality of not being dangerous or presenting a risk’”: Aspen Pharmacare Australia Pty Ltd v Minister for Health and Ageing (“Aspen”) [2012] AATA 362 at [19]-[20]; see also the Oxford English Dictionary (1.a. The state of being protected from or guarded against hurt or injury; freedom from danger”); Macquarie Dictionary (6th Ed; 2013) (“the state of being safe; freedom from injury or danger”).
52 Similarly, I consider that the word “efficacy” is likely used in s 25(1)(d) in its ordinary sense, namely, the “‘ability to bring about the intended result’ … that is, effectiveness to bring about the intended therapeutic result for which the goods have been registered…”: Aspen at [21]; see also the Oxford English Dictionary (“1. Power or capacity to produce effects; power to effect the object intended”); and the Macquarie Dictionary (“capacity for serving to produce effects; effectiveness”). This view is also consistent with the statement in the Second Reading Speech for the Therapeutic Goods Bill 1989 (Cth) that, “[t]hree parameters are used to define the acceptability of a product for therapeutic use... The third parameter is the product’s efficacy, or its effectiveness in fulfilling its intended purpose” (Hansard 5 October 1989, page 1612) (emphasis added). It is true that in a technical medical sense, the term “efficacy” is used in the context of randomised control trials which constitute best evidence (as I later explain at [68]-[70]). However, it seems unlikely that the Parliament would have intended to limit the concept to its technical meaning in the context of the Therapeutic Goods Act given that other data may be relevant, including that obtained by subsequent observation of the use of the drug in other countries.
53 In undertaking the balancing exercise required by s 25(1), a high level of safety is required from vaccines, which unlike most medications, are generally given to healthy people, to prevent illness and death. Dr Crawford explained that a fever in the first twenty-four hours is an expected and common side effect from the administration of a vaccine because the vaccine operates to stimulate the system to produce a new protection.
4.3 Administration of the Vaccine under the National Immunisation Program
54 The Vaccine is administered in Australia as part of the National Immunisation Program (NIP), in common with the majority of vaccines. All of the pertussis vaccines under the NIP are acellular pertussis vaccines (“Pa”), containing three or more purified components of B.pertussis. The change from a whole cell pertussis to acellular vaccines took place in the late 1990s. The vaccine is injectable and usually given as part of a combination vaccine including diphtheria (D) and tetanus (T) (“DTPa”), and may also include hepatitis B, polio and Haemophilus influenzae type b.
55 In line with the requirements earlier outlined, the Vaccine had to be approved as a medication by the TGA before it could be included on the NIP. Dr Crawford explained the process following TGA approval by which a vaccine is added to the NIP:
The vaccine is then reviewed by the Australian Technical Advisory Group on Immunisation (ATAGI), who will make recommendations about the utility of adding a vaccine to the NIP. The vaccine then requires a cost effectiveness evaluation through the Pharmaceutical Benefits Advisory Committee (PBAC)… This assessment will take into account the efficacy/effectiveness of the vaccine on available evidence and the price proposed by the pharmaceutical company. These recommendations then go to the federal government for approval through the Department of Health in Canberra before a new vaccine can be added onto the NIP. State health departments are involved in the introduction of vaccines onto the NIP program.
56 The pertussis vaccine is generally included in a combined vaccine administered by injection, the type of which varies depending on the recipient’s age.
57 While pertussis vaccination schedules differ around the world, Dr Wood explained that none currently started earlier than six weeks of age. The schedule recommended by the World Health Organisation is six, ten and fourteen weeks for primary immunisation.
58 The recommended schedule in Australia is administration of the vaccine:
(a) at 6 – 8 weeks, 4 and 6 months of age (the primary schedule);
with boosters at:
(b) at 3.5 – 4 years; and
(c) during adolescence (11 – 13 years); and
(d) adulthood (recommended for those who wish to reduce the likelihood of becoming ill with pertussis, such as parents of young infants, and at 50 years of age);
(collectively, “the Vaccine”: see Australian Government Department of Health and Aging, The Australian Immunisation Handbook (10th Ed, 2013) at 4.12.7 (the “Australian Immunisation Handbook”)).
59 In relation to the recommended schedule, the Australian Immunisation Handbook advises that the booster dose at 3.5 years of age “is essential as waning of pertussis immunity occurs following receipt of the primary schedule.” The Handbook also advises that the optimal age for administration of the second booster dose is 11 to 13 years “due to waning antibody response following the 1st booster dose recommended at 4 years of age. This 2nd booster dose of pertussis – containing vaccine is essential for maintaining immunity to pertussis (and diphtheria and tetanus) into adulthood.”
60 In the case of adults, the Australian Immunisation Handbook recommends vaccination for any adult who wishes to reduce the likelihood of becoming ill with pertussis which is particularly important where an adult falls within a special risk group, such as health care workers and adults working with infants and young children aged less than four years. With respect to those in contact with infants, the Handbook advises that “[t]here is significant morbidity associated with pertussis infection in infants <6 months of age, particularly those <3 months of age, and the source of infection in infants is often a household contact.” As a consequence, pertussis vaccination of the close contacts of young infants (the “cocoon strategy”) is recommended for women planning pregnancy or during pregnancy and other adult household contacts and carers of infants less than six months of age. As Professor Phelps explained:
The recognition that pertussis immunity can wane with time and the urgency of protecting newborns from infection are the reasons that Australia has had active public health programs in place to encourage prospective parents, new parents and grandparents to make sure they have booster immunization to reduce the risk of exposure of unprotected infants.
61 Thus, for example, the Vaccine is available for women in public maternity units in New South Wales for the opportunistic postpartum (i.e. after childbirth) vaccination of new mothers, if they have not received a pertussis vaccine in the previous five years. The Vaccine is also available free in the Northern Territory to parents and close family members when administered within seven months of the birth of the child.
62 Dr Donohoe referred to additional strategies that may enhance the effectiveness of the Vaccine including removing susceptible children from exposure to the disease where a person such as a sibling or parent has been diagnosed with whooping cough, active surveillance of adults with children who may be carriers of pertussis, and higher awareness on the part of medical practitioners that mild forms of pertussis, even following vaccination, may provide a vector for transmission.
63 Evidence Based Medicine (“EBM”) is a movement which aims to increase the use of high quality clinical research in clinical decision-making. It has been described in the literature as the “conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research”: Sackett, DL, et al (1996) “Evidence based medicine: what it is and what it isn’t”, BMJ; 312:71-72 (as quoted and adopted by Professor Phelps in her report).
64 The Cochrane Collaboration was formed out of this movement and its systematic reviews of primary research in human health care and health policy are internationally recognised as the highest standard in evidence-based health care – the “gold standard in terms of evidence-based research in health care”, as Professor Phelps explained. The Cochrane Reviews (to which I return later) investigate the effects of interventions for prevention, treatment and rehabilitation. Dr Crawford explained that the Cochrane reviews “are a meta-analysis of robust clinical trials with clear justifications for studies included. If an individual study does not fully meet the rigorous criteria it will be excluded. In some instances, there will be insufficient data for the Cochrane review to make a firm statement on the effectiveness of an invention.”
65 The National Health and Medical Research Council (“NHMRC”), established under s 5B of the National Health and Medical Research Council Act 1992 (Cth) (“NHMRC Act”), is the national body responsible relevantly for raising public and individual health standards and fostering medical research: NHMRC Act, s 3(1). Its functions include responsibility for issuing guidelines, and advising the community, on the prevention, diagnosis and treatment of disease, and public health research and medical research: NHMRC Act, s 7(1)(a).
66 The NHMRC develops guidelines based on consultation with experts, expert bodies and the community in accordance with the requirements of s 13 of the NHMRC Act and does so in line with a rigorous nine step based approach. Given these matters and the statutory role of the NHMRC, I accept Dr Wood’s evidence that the NHMRC guidelines about levels of evidence for intervention are the accepted standard for clinical practice, including prevention such as vaccination, in Australia.
67 The standard taxonomy of levels of evidence for intervention studies based on the NHMRC guidelines and starting with the highest quality of evidence, is as follows:
Level I: evidence obtained from a systematic review of Level II studies;
Level II: evidence obtained from at least one properly designed randomised controlled trial of appropriate size;
Level III-1: evidence obtained from well-designed pseudo-randomised controlled trials;
Level III-2: evidence from comparative studies (including systematic reviews of such studies) with concurrent controls being a non-randomised experimental trial, a cohort study, an interrupted time series or matched case-controlled study;
Level III-3: evidence from a comparative study without concurrent controls, being a historical control study, two or more single arm studies (i.e. case series from two studies), or a well-designed interrupted time series trial without a parallel control group from more than one centre or research group or from case reports; and
Level IV: evidence obtained from a case series, either post-test or pre-test/post-test outcomes.
68 A “randomised controlled trial” is a trial designed to test the efficacy of a vaccine or medication by assigning the subjects of the trial randomly into those to whom the medication or vaccine is administered, on the one hand, and a control group, the members of which receive a placebo, on the other hand. The efficacy of the vaccine or medication can then be assessed by comparing the results of each group.
69 In this context, the term “efficacy” bears a technical meaning and is used in contradistinction to “effectiveness”. The difference between the two concepts was explained by Dr Crawford in the following terms:
Vaccine Efficacy (VE) is the percentage reduction in disease incidence attributable to vaccination. When this relative reduction is incidence measured in an individually randomised placebo-controlled clinical trial, the term vaccine efficacy is used. When it is measured through observational studies under program conditions (e.g. retrospective cohort or case control studies; or through outbreak investigations), the term vaccine effectiveness is used.
70 Thus, while “vaccine efficacy” is applied in the context of randomised controlled trials, “vaccine effectiveness” is concerned with how a vaccine is observed to perform in the real world. The differences between the two means of assessing effectiveness means that efficacy rates tend to exceed rates for vaccine effectiveness. This is because randomised controlled trials are conducted under very strict conditions with the subjects following a strict protocol, while the real world performance of a vaccine will be affected by variables which may impact adversely on its effectiveness, such as late compliance with the recommended NIP schedule or missed booster doses.
71 Homeopathy is said to be a system of treatment first practiced by the German physician and pharmacist, Dr Samuel Hahnemann, in 1783.
72 Dr Golden identified two principles as central to the practice of homeopathy:
a) the selection of medicines using the Principles of Similars (a matching of symptoms to be treated or prevented with the symptoms of the remedy which had been determined by controlled testing (provings) and/or clinical experience); and
b) the administration of medicines using the Principle of Minimum Dose (the use of the minimum amount of remedy needed to produce either a curative or a preventative effect).
73 These were described to be “the fundamental tenets of both homoeopathic practice as well as homoeopathic immunisation.” In short, as Burke J explained in Rose v Health Commission (NSW) (1986) NSWCCR 32, at [3], homeopathy can be described as a “method of treating disease by drugs, given in minute doses, which produce in a healthy person symptoms similar to those of the disease.”
74 Dr Golden explained that “homoeopathic medical science significantly differs from pharmaceutical medical science in that it is based on fundamental principles taken from a detailed and expert observation of the natural world by not only Dr Hahnemann but others before him such as Hippocrates.” On the basis that, unlike pharmaceutical medical science, “homoeoprophylaxis is based on observable and consistent Principles in the natural world and so its method of use has remained consistent over 200 years…”, Dr Golden argued that “information regarding homoeoprophylaxis [i.e. homeopathic treatment intended to prevent disease] needs to come from the homoeopathic medical literature, and not the pharmaceutical medical literature, as they relate to two completely different scientific paradigms”. The evidence of Dr Golden makes it clear that the principles underpinning the administration of homeoprophylaxis as explained by Dr Golden stand in stark contrast to the principles underpinning conventional, science-based medical treatment.
75 There are a number of homoeopathic professional bodies and groups within Australia. The single national registration body which is recognised by most homoeopathic professional associations is the Australian Register of Homoeopaths (“AROH”).
76 Finally, as at October 2013, there were no NHMRC levels of evidence regarding homeoprophylaxis. However, the NHMRC was reviewing the evidence for the effectiveness of homeopathy and a working group had been established to that end. Dr Wood explained that that review comprised “a systematic review of available systematic reviews on the effectiveness of homeopathy in treating a variety of clinical conditions in humans.”
5. Publication of the Three Articles
77 Homeopathy Plus was first registered as Homeolink Australia by Mrs Sheffield’s husband in around 2001, and re-registered from January 2009 under the name Homeopathy Plus. Mrs Sheffield became the sole director of Homeopathy Plus towards the end of 2009. I accept that one of the purposes of Homeopathy Plus is to advocate for homeopathy. More specifically, one of its main purposes is to change public health authority attitudes towards homeopathy prophylaxis and Mrs Sheffield has for several years promoted the prophylactic qualities of homeopathy through Homeopathy Plus. For example, at the time that the First Whooping Cough Article was published, the Website also called upon governments to look into the effectiveness of Vaccines as against homeopathy on a page headed “Immunisation Issues” under the heading “Isn’t it time?”. However, I do not accept that advocacy on such matters was the sole purpose of Homeopathy Plus. It is apparent from the Website that Homeopathy Plus was also engaged relevantly in the sale of homeopathy products.
78 Mrs Sheffield also runs a private homeopathy practice in her own name where she sees patients and provides homeopathy advice and treatment. She treats an average of 25 patients per week. It was her evidence that the “advocacy organisation” and the “homeopathic patient care business” with which she is involved “have distinct and separate purposes, even though there is a cross-over in the subject matter.”
79 Mrs Sheffield had also previously lobbied for changes in government policies in the homeopathy prophylaxis area as the founding member of a political lobby group and its spokesperson in 2007-2009. Further, Mrs Sheffield had written to state and federal members of parliament with an involvement in health policy on the issue in July 2007.
80 Mrs Sheffield has maintained the Website from around 2001. She collates the content for the Website and also contributes her own articles. While her husband has been a co-registrant of the Website, he does not have any involvement in producing or editing its content which is a task undertaken solely by Mrs Sheffield. She was responsible for all of the content uploaded to the Website and has promoted the prophylactic qualities of homoeopathy through Homeopathy Plus for several years.
81 Mrs Sheffield started offering free subscriptions to a homeopathy information newsletter several years ago, initially by posting hard copies and now by sending it online to subscribers using a group e-newsletter service. As at 28 June 2013, there were 12,041 subscribers to the email newsletter which contains articles written by Mrs Sheffield and others on the practice, science, history, current trends and politics around homeopathy.
82 The content and design on the Website as at the various dates on which the contraventions are alleged to have occurred are not in dispute.
5.2 Publication of the First Whooping Cough Article
83 Mrs Sheffield wrote the First Whooping Cough Article at some time during 2009. The article was published on a section of the Website entitled the “Treatment Room”. I accept that Mrs Sheffield intended in part that the Treatment Room section of the Website would educate visitors on how common homeopathic remedies are used for different symptom profiles and increase understanding of the discipline of homeopathy. I accept also that there were no direct links within the body of the text of the Three Articles to products available from the Online Shop. However, I do not accept that advertising had no role in the inclusion of information uploaded to the Treatment Room given that all such information, including the First Whooping Cough Article, were located on pages which included a menu with click-button access to the Online Shop and the sale of homeopathic products was one of the purposes of Homeopathy Plus. Furthermore, as Mrs Sheffield accepted, visitors to the Website could, by clicking through a series of two or three links from the First Whooping Cough Article commencing with the online shop button, reach a page where they could purchase Drosera which was referred to in the First Whooping Cough Article as a remedy for the prevention of whooping cough (see [148]). I do not, however, accept the ACCC’s submission that I should infer that Pertussinum was also available for sale through the Website shop. It would have been a simple matter for the ACCC to prove that fact, and its failure to do so cannot be remedied by evidence that a different product was available for sale through the Website.
84 On 13 April 2012, Ms White, who is an Assistant Director in the Enforcement Operations, at the ACCC, visited the Website and navigated to the section of the Website entitled “Treatment Room”. She then clicked on the First Whooping Cough Article and printed out a copy. The printout of the webpage where the First Whooping Cough Article was located included a toolbar which formed part of a constant frame within which all pages of the website were displayed. By clicking on one of the links on the toolbar, the user could navigate to other pages of the Website. Those links were displayed as follows:
Join Our Newsletter
Sign Up |
Main Menu |
Home |
Flu Watch |
Tutorials |
Answers |
Treatment Room |
Library |
Clinic Cases |
Immunisation Issues |
Research |
Political Issues |
Videos |
Agrohomeopathy |
Quick Tips |
Podcasts |
Your Story |
Latest News |
Contact Us |
Appointments |
Alerts |
Treatment for Autism |
Newsletter |
Newsletter Archives |
Letters to Editor |
Shop |
Simplex Remedies (Pills) |
Complex Remedies (Liquids) |
Kits and Bottles |
Agrohomeopathy Remedies |
Tissue Salts |
Bach Flowers |
Books |
Shop Notes |
Subscribe to our feed |
Search our Site |
Go! |
Follow Us On [facebook] [twitter] |
85 However, the physical printout of the webpage where the First Whooping Cough Article was located omitted to print the banner at the top of the webpage. It was common ground that at that time the banner read:

86 In contrast to the banner when the Second and Third Whooping Cough articles were published, the banner did not refer to the online shop.
87 From 20 April 2012, the ACCC corresponded with Mrs Sheffield for the purpose of requesting that the First Whooping Cough Article be removed from the Website. The First Whooping Cough Article was removed from the Website on 26 April 2012 following a verbal undertaking given by Mrs Sheffield to the ACCC in a telephone conversation earlier that day. In the course of that conversation she said words to the effect that “I will remove the article until I have time to check whether any of it needs to be changed. I will put it back up again when I am happy with it.” I also accept that she had always intended to re-upload the First Whooping Cough Article when she had undertaken further research.
5.3 Publication of the Second Whooping Cough Article
88 In the fortnight after removing the First Whooping Cough Article in April 2012, Mrs Sheffield read further material on the Vaccine, as well as prevention and treatment of whooping cough by homeopathic means.
89 At this time she asked her son, who assisted Homeopathy Plus with IT, to arrange for a “members’ area” to be added to the Website. There had been problems in the past with establishing such an area which was an “on-again and off-again project” during the second half of 2012 due to time constraints and their lack of familiarity with the software, WishList Member, which Homeopathy Plus had purchased for this purpose. Once WishList Member was ready to facilitate content uploads, Mrs Sheffield instructed her son to upload to the Website articles about homeopathy prophylaxis which had been temporarily removed pending establishment of the members’ area. The Second Whooping Cough Article was among several articles that her son was asked to upload. This was a revised version of the First Whooping Cough Article and contained direct links to two other articles under the heading “Further Reading”, namely, “Bordetella pertussis: Strains with increased toxin production associated with pertussis resurgence”, Emerging Infectious Diseases (2009) v 15(8) at 1206-1213 (the “2009 Emerging Infectious Diseases article”), and an online article by the Chicago based homeopath, Heidi Stevenson, entitled “Whooping Cough Outbreaks in Vaccinated Children Become More and More Frequent’ (the “Stevenson online article”) Mrs Sheffield’s son told her, and she believed, that he had placed the requested material within the members’ area. She therefore believed that the Second Article would not be accessed outside the members’ area on the Website.
90 Before being able to access material contained within the members’ area, a visitor was required to accept terms and conditions of access set out on a sign-up page. Mrs Sheffield compiled the terms and conditions for the members’ area after reviewing other medical sites on the internet and included those terms which she considered might be relevant and applicable to her Website.
91 The Website had been redesigned since publication of the First Whooping Cough Article and, at the time of the Second Whooping Cough Article, bore a different banner as follows which referred expressly to the online store:
“
92 Mrs Sheffield accepted that the words under the Homeopathy Plus banner were chosen as they were “the three encompassing things for websites”, and that those things included the provision of an online store.
93 The toolbar, which was previously located down the side of the page, was now placed horizontally below the banner but continued to form part of the frame within which all pages constituting part of the Website appeared. The toolbar provided the following links:
Latest News Tutorials Treatment Room Homeoprophylaxis/Vaccination Library Shop
Videos/Podcasts Contact Us What We REALLY said Members
94 Unlike the toolbar on the Website at the time that the First Whooping Cough Article was published, the toolbar no longer referred to a separate page for “Political Issues”.
95 On 15 January 2013, Ms White visited the Website and navigated to a page upon which the Second Whooping Cough Article appeared without having to accept the terms and conditions for the members’ area.
96 Following her discovery of the Second Whooping Cough Article on the Website, Ms White endeavoured to contact Mrs Sheffield between 15 and 17 January 2013 by telephone and email. Mrs Sheffield received an email from Ms White on 15 January 2013 referring to Mrs Sheffield’s agreement to remove the Second Whooping Cough Article and that she would attempt to revise the page in a manner that would satisfy the ACCC’s concerns about a breach of the ACL. In the email, Ms White advised that she had recently reviewed the “Whooping Cough – Homeopathic Prevention and Treatment” page and had some concerns which she would like to discuss.
97 The email from Ms White prompted Mrs Sheffield to visit the Website on 15 January 2013 where she saw that the Second Whooping Cough Article was not only visible in the members’ area, but also on the public area of the Website where it had been accessible for the past four days. On the same day, Mrs Sheffield asked her son to remove the Article from the public area of the Website so that it would be accessible only within the members’ area after viewing the front “disclaimer” screen. Mrs Sheffield then investigated the problem and found that in order to limit material to the members’ area, it was necessary to select a check box at the time of uploading an article. Mrs Sheffield’s son complied with her request as a result of which the article was accessible thereafter only in the members’ area of the Website after viewing a front page and accepting the terms and conditions. The front page contained text written by Mrs Sheffield and read:
“Oops! This Content is Members Only”
Looking for this content?
It’s now in our free member’s area – just follow the link.
Why has it been moved there?
Some people think you shouldn’t know about homeopathy – especially in relation to this particular problem.
They have lodged complaints with various government departments against Homeopathy Plus! and this website to stop the information entering the public domain.
We think this behaviour is silly, short-sighted and against the public interest.
It also makes it difficult for you to research potentially valuable information on homeopathy.
So, to reduce the complaints and protect your access to information we have moved this “shocking” content to a free member’s area where you can still read it and form your own opinion.
To access the information just click the link, accept the terms of membership and start reading.
98 A disclaimer (“the Stand Alone Disclaimer”) appeared beneath this text which is set out at [282] below.
99 The terms and conditions relevant to the current proceedings are set out at [286] below.
100 On 17 January 2013, Ms White spoke with Mrs Sheffield who advised that she intended to record the conversation. Ms White consented to that course. I accept Mrs Sheffield’s detailed evidence as to the content telephone conversation which ensued as follows:
[Ms White] said: I just wanted to check, I am not sure if you manage the website content yourself and whether you do know what the statements on the pages are at the moment?
[Mrs Sheffield] said: I went, when I got your email yesterday I went off and had a look at the article and it’s off in the member’s area. Now apparently when you came across it it wasn’t. Now these things happen sometimes because I have a web manager, and I said to put this, this, this and this article in the member’s area. And of course the whooping cough article wasn’t moved off into the member’s area, it was made public, for some reason or another, by accident of course, but it’s now in the member’s area now.
[Ms White] said: OK. But how can it be accessed in the member’s area? Can anybody find what can be accessed there?
[Mrs Sheffield] said: Yes, but they sign agreements going in that they are aware that it’s information that’s not accepted by the orthodoxy. It’s from the homoeopathy perspective only.
[Ms White] said: OK.
[Mrs Sheffield] said: And that it’s not to be removed out of that member’s area.
[Ms White] said: Right. Well, yeah, that’s what I thought may have been the case. It was on the page so I really just wanted to check that.
[Mrs Sheffield] said: Yeah, well thanks for that. I wasn’t… I went off and had a look and thought, what, well of all the articles that was the one that slipped through.
[Ms White] said: Yeah, [laughing], well OK at this stage that’s all I wanted to check with you. Um, well I think we still have to consider whether [inaudible] from our end, with the page, but I’ll give you a call.”
101 I accept Ms White’s evidence that at the end of the conversation she stated words to the effect that, while the content may be moved to another area, this would not necessarily allay the ACCC’s concerns over the potentially misleading nature of the statements made on the ‘Whooping Cough’ page. However, I am not prepared to find that Mrs Sheffield in fact heard anything further than that which is recorded in evidence as set out above.
102 Later on 17 January 2013, Ms White visited the Website and observed that the Second Whooping Cough Article did not appear and that the webpage directed readers to the “Members” area of the “Website”. She also observed that in order to sign up as a “Member”, readers had to agree to certain terms and conditions.
103 On the following day, another employee of the ACCC who is not Ms White, visited the Website again and signed up as a “Member” by inputting her name and email address on the Website and agreeing to the terms and conditions. She then accessed the Second Whooping Cough Article.
5.4 Publication of the Government Article
104 On 3 February 2012, Mrs Sheffield read an article entitled “Whooping cough in Australian children – how many were vaccinated?” by Greg Beattie. As a consequence of reading the article, Mrs Sheffield sent a newsletter “Alert” to the Homeopathy Plus subscription list. The content of that email was also uploaded to the Website on the same day and constitutes the Government Article. I accept that Mrs Sheffield’s motives in uploading the Government Article were in part to advocate for a change in the government’s approach to what she considered to be a whooping cough epidemic because in her view, “the current policies were inappropriately one sided”. However, I also consider that she was intending to promote her online shop.
105 Ms White visited the Website on 22 January 2013 and performed a search for “whooping cough” on the Website search engine. The website directed her to the Government Article which she printed. It was not necessary for Ms White to become a member of the Website before she was able to access the Government Article. It was freely accessible to any member of the public who visited the Website.
106 Subsequently, on 29 January 2013, Ms White placed an order for a product through the Website and received a tax invoice.
6. Elements of the statutory causes of action: overview
107 The ACCC alleges contraventions of ss 18 and 29(1)(a) and (b) of the ACL. I have earlier set out the terms of these provisions at [8] and [9] above. It is helpful, however, to briefly explain the elements of the two causes of action.
6.1 A person for the purposes of the ACL
108 There is no issue that each Respondent is “a person” within ss 18 and 29 of the ACL.
109 The ACL applies to the conduct of corporations by virtue of s 131 of the CCA and therefore to Homeopathy Plus.
110 Mrs Sheffield conceded that the ACL applied to her as an individual by operation of s 6(3)(b) of the CCA. Section 6(3)(a) provides (relevantly) that Parts 2-1 and 3-1 of the ACL, which include ss 18 and 29 respectively, apply as if:
(a) those provisions … were …. confined in their operation to engaging in conduct to the extent to which the conduct involves the use of postal, telegraphic or telephonic services … and
(b) a reference in those provisions to a corporation included a reference to a person not being a corporation.
111 The concession by Mrs Sheffield was correctly made, in my view, as the Three Articles were published on the internet and access to the internet involves the use of telephonic services: Seafolly Pty Ltd v Madden (2012) 297 ALR 337 at [76]-[79] (Tracey J); ACCC v Jutsen (No 3) (2011) 206 FCR 264 at 287 [100] (Nicholas J); ACCC v Jones (No 5) [2011] FCA 49 at [6] and [10] (Logan J).
112 Nor is it in dispute that both Respondents “engag[ed] in conduct” within s 18 of the ACL by making representations contained in the Three Articles and publishing those on the Website. By s 4(2)(a) of the CCA, a reference to “engaging in conduct” is to be read as a reference to “doing or refusing to do any act…”. Conduct can include making a statement which is misleading or deceptive: Global Sportsman Pty Ltd v Mirror Newspapers Pty Ltd (1984) 2 FCR 82 (“Global Sportsman”) at 88 (the Court). Any omission alleged to constitute “conduct” within s 18 must be deliberate, as s 4(2)(c) suggests in providing that the phrase includes a reference to “refraining (otherwise than inadvertently) from doing that act.”
113 The remaining elements that must be established under s 18 of the ACL are that:
(a) the conduct engaged in occurred “in trade or commerce”; and
(b) the conduct “is misleading or deceptive or is likely to mislead or deceive.”
114 The scope of s 29 of the ACL is more narrow. While certain elements overlap with those in s 18, s 29 is concerned with “representations” only. A representation is a statement made orally or in writing or which is implied from words or conduct (see Given v Pryor (1978) 39 FLR 437 at 440-441).
115 The elements specified in s 29 require that a representation is made “in trade or commerce” and is false or misleading, in common with s 18. In addition:
(a) the representation must be made “in connection with”:
(i) the supply or possible supply of good or services; or
(ii) the promotion by any means of the supply or use of goods or services; and
(b) the representation must be a representation of the kind caught by one of the subsections.
7. Were the alleged representations false, MISLEADING or deceptive?
7.1 What is meant by “misleading or deceptive”?
116 Sections 18 and 29 of the ACL are in effectively the same terms as their predecessor provisions, ss 52 and 53 of the Trade Practices Act 1975 (Cth) (“TPA”), save that the phrase “[a] person must not” is used in the ACL rather than the phrase “[a] corporation shall not” in the TPA. That difference is not of any relevant consequence here, as was also the case in Australian Competition and Consumer Commission v TPG Internet Pty Ltd (2013) 250 CLR 640 (“TPG Internet”) at 645 [11] (French CJ, Crennan, Bell and Keane JJ). As such, the consideration by authorities of whether conduct was “misleading or deceptive” conduct for the purposes of ss 52 and 53 of the Trade Practices Act is equally applicable to ss 18 and 29 of the ACL.
117 The principles to be applied in determining whether conduct is misleading or deceptive can conveniently be summarised as follows. In so summarising the relevant principles, it must be emphasised that they are interrelated.
118 First, in common with its predecessor provision, s 18 of the ACL:
… is concerned with the effect or likely effect of conduct upon the minds of those by reference to whom the question of whether the conduct is or is likely to be misleading or deceptive falls to be tested. The test is objective and the Court must determine the question for itself: Global Sportsman Pty Ltd v Mirror Newspapers Ltd (1984) 2 FCR 82 at 87 (the Court) (citations omitted).
119 As French CJ, Crennan, Bell and Keane JJ explained more recently in TPG Internet at [49], the characterisation of conduct as misleading or deceptive or as likely to mislead or deceive “generally requires consideration of whether the impugned conduct viewed as a whole has a tendency to lead a person into error” (citing Campbell v Backoffice Investments Pty Ltd (2009) 238 CLR 304 at 318 [24] (French CJ) with approval)). “That is to say…”, as their Honours had earlier explained in their joint reasons at [39], “there must be a sufficient causal link between the conduct and error on the part of persons exposed to it.”
120 Secondly, the test being an objective one, it follows that intention is not an element of s 18 (or s 29) of the ACL notwithstanding that intention may be relevant to the question of whether it may be inferred that a representation is misleading or deceptive or likely to be so: TPG Internet at [55]-[56] (French CJ, Crennan, Bell and Keane JJ).
121 Thirdly, conduct is “likely” to mislead or deceive if there is “a real or not remote chance or possibility regardless of whether it is less or more than fifty per cent’”: Global Sportsman at 87 (the Court) (citations omitted). This enquiry in turn focuses upon the time of publication: Australian Competition and Consumer Commission v Kaye [2004] FCA 1363 (Kenny J) at [105].
122 In the fourth place, where the impugned conduct is constituted by the making of a statement, the question of whether the statement has a tendency to lead into error can meaningfully be addressed only once the class of persons likely to be affected and their relevant attributes have been identified. Only then can the effect of the representations on persons acting reasonably in all of the circumstances be assessed. As Gibbs CJ held in Parkdale Custom Built Furniture Pty Ltd v Puxu Pty Ltd (1982) 149 CLR 191 (“Puxu”) at 199:
…consideration must be given to the class of consumers likely to be affected by the conduct. Although it is true, as has often been said, that ordinarily a class of consumers may include the inexperienced as well as the experienced, and the gullible as well as the astute, the section must in my opinion by [sic] regarded as contemplating the effect of the conduct on reasonable members of the class. The heavy burdens which the section creates cannot be have been intended to be imposed for the benefit of persons who fail to take reasonable care of their own interests. What is reasonable will or [sic] course depend on all the circumstances.
123 The last point – that what can reasonably be expected of the relevant class of consumers must be determined in all of the circumstances – is illustrated by the distinction drawn in TPG Internet between the circumstances in that case and those in the earlier decision in Puxu. Thus greater attention might be imputed to what might colloquially be called “small print” where the target audience consists of potential purchasers in the calm of a showroom who are visiting with a substantial purchase in mind (as was the case in Puxu) as opposed to that which might reasonably be expected of the target audience regarding representations and qualifications in advertisements that were an unbidden intrusion on the consciousness of the target audience intended to arrest its attention (as in TPG Internet). In the latter case, “…the attention given to the advertisement by an ordinary and reasonable person may well be ‘perfunctory’, without being equated with a failure on the part of the members of the target audience to take reasonable care of their own interests”: TPG Internet at [47]. In such a case, many members of the general public may absorb only the general thrust: ibid. Thus, the majority held in TPG Internet that “questions of carelessness by consumers in viewing advertisements may be relevant to that question of characterisation.” (at [49]). Relevantly also in TPG Internet, the tendency to lead potential customers into error arose because the advertisements themselves selected certain words for emphasis and relegated the balance to relative obscurity. Thus, in all of the circumstances, the majority held that carelessness in absorbing all of the detail was not an unreasonable response.
124 Fifthly, it is important to differentiate between a statement of past or present fact, on the one hand, and a statement as to the state of mind of the maker when the statement was made, on the other. In the former case, the maker’s belief in the accuracy of the statement is irrelevant to the question of whether the meaning conveyed by the statement is false. However, the position is different where the statement makes a representation as to the maker’s state of mind, such as where she or he purports to express an opinion. In the latter case, the maker’s state of mind may well be relevant. As Gordon J explained in her Honour’s helpful summary of relevant principles in Australian Competition and Consumer Commission v Dukemaster Pty Ltd [2009] FCA 682; [2009] ATPR 42-290 (“Dukemaster”) at [10]:
...
5. … A statement which involves the state of mind of the maker ordinarily conveys the meaning (expressly or impliedly) that the maker of the statement had a particular state of mind when the statement was made and, commonly, that there was a basis for that state of mind…
6. A statement of opinion will not be misleading or deceptive or likely to mislead or deceive merely because it turns out to be incorrect, misinforms or is likely to do so… An incorrect opinion does not of itself establish that the opinion was not held by the person who expressed it or that it lacked any or any adequate foundation… An expression of an opinion which is identifiable as an expression of opinion conveys no more than that the opinion is held and perhaps that there is a basis for the opinion. If that is so, an expression of opinion however erroneous misrepresents nothing...
7. However, an opinion may convey that there is a basis for it, that it is honestly held and when it is expressed as the opinion of an expert, that it is honestly held upon rational grounds involving an application of the relevant expertise. If the evidence shows that the opinion was not held or that it lacked any or any adequate foundation, particularly if the opinion was expressed as an expert, a statement of opinion may contravene s 52 of the TPA.
…
125 Finally, the question is not whether representations were apt to induce customers to enter into contracts with the maker of the representations, but rather whether they were apt to bring them into negotiations with that person on the basis of an erroneous belief engendered by the representations: TPG Internet at [48]. To say that consumers acting reasonably in their own interests could be expected to obtain a clear understanding of their rights and obligations before purchasing the services or items in question confuses the question of loss with the anterior question of whether the representation viewed as a whole, has a tendency to lead a consumer into error: TPG Internet at [49].
7.2 Identification of the class to whom the representations were directed and their characteristics
126 The first question, therefore, in assessing whether the statements were misleading and deceptive is to identify the class of persons to whom the representations were made and the characteristics of that class. Identification of this class is also relevant in determining whether the alleged representations are made insofar as the representations alleged are not express, but are said to be implied based on the persons to whom the representations are directed: Dynamic Lifter Pty Ltd v Incitec Ltd (1994) 30 IPR 198 (“Dynamic Lifter”) at 203 (Whitlam J).
127 All of the Three Articles were accessible to any user of the Internet. While the Second Whooping Cough Article, following changes to the Website on 15 January 2013, was accessible only to “members”, membership was open to any member of the public and access was free of charge. All that was required was the ticking of a box to indicate acceptance of terms and conditions by the person wishing to access the members only part of the Website. In those circumstances, I do not accept that there is any basis in the evidence to support Mrs Sheffield’s assertion in her evidence that, by creating the members area, the information accessible in that area would be confined to those with an understanding of homeopathy.
128 The breadth of persons accessing the Website is apparent from the fact that as at 28 June 2013 the Website was issuing a newsletter to 12,041 subscribers. As such, I accept the submission by the ACCC that the potential class of consumers viewing the Website should be presumed to include “the astute and the gullible, the intelligent and the not so intelligent, the well educated as well as the poorly educated, and men and women of various ages pursuing a variety of vocations”: Puxu at 93 (Lockhart J) Therefore, applying the principles earlier summarised, in determining whether the Three Articles were misleading or deceptive, it is necessary to assess at the time of publication, the likely effect of the representations in each of the Three Articles on members of this broad class acting reasonably in all of the circumstances. In this regard, it must be borne in mind that it is sufficient if there is a real chance only, as opposed to a probability, that the members of this class may be misled or deceived.
7.3 Characterisation of the alleged representations
129 The second question is whether the representations alleged by the ACCC are made in each of the Three Articles, either expressly or by implication. In determining this question, it may be necessary to have regard to the whole of the document in which the representations appear: Dynamic Lifter at 203 (Whitlam J).
130 It will be recalled that the ACCC contends that there were essentially misrepresentations of two kinds made in the three articles: the Vaccine representations concerning the effectiveness or lack thereof of the Vaccine in protecting against whooping cough; and the Homeopathy Alternative Reasonable Basis representation concerning the alleged effectiveness of homeopathic treatments as an alternative to the Vaccine for the prevention of whooping cough (see above at [6]).
7.3.1 The first alleged Vaccine representation in the First Whooping Cough Article
131 The first alleged Vaccine representation is said to arise from the First Whooping Cough Article. That article was entitled “Whooping Cough – Homeopathic Prevention and Treatment” and began:
[1] Whooping cough, or pertussis, is a highly contagious disease caused by the Bordetella pertussis bacterium. Worldwide, it results in about 300,000 deaths a year and leaves many surviving children with brain damage. Serious complications such as bleeding into the nose, eyes or brain, pneumonia, and hernias also occur.
[2] Most developed countries are currently in the grip of a whooping cough epidemic. To stop its spread, health officials are calling for the vaccination of adults as well as children. But is large-scale vaccination the best solution? Not only is protection from the current vaccine short-lived and unreliable, but side-effects are common. Recent research also suggests that the bacterium has mutated to a strain against which the vaccine is no longer effective.
[3] The homeopathic approach to this problem offers a safe and sensible solution. Homeopathy has a two hundred year history of treating and preventing whooping cough without the risk of dangerous side-effects. It can also be used as a second line of defence should a vaccine for whooping cough already have been given.
(emphasis and paragraph numbering added)
132 In its express terms, therefore, the First Whooping Cough Article represents that protection from the current Vaccine is “short-lived and unreliable”, and the Vaccine is ineffective because the disease has mutated. Nothing in the article qualifies those representations or expressly identifies their bases save that a hyperlink is given for an article, “Pertussis: the Fear Factor”, at the end of the First Whooping Cough Article under the heading “Further Reading”, which I consider later. In short, the overall impression is that there is little point in taking the Vaccine to protect against contracting the disease.
133 The Respondents allege that the representation that the bacterium has mutated to a strain against which the Vaccine is “no longer effective” must be read in the context of the sentence in which it appears as a whole, placing particular emphasis upon the qualification to the proposition imported by the use of the word “suggests”. I accept that the representation must be read in context but that context is not limited to the sentence in which the statement appears. As, for example, Gibbs CJ said in Puxu at 199, in determining what the words used convey:
[i]t would be wrong to select some words or act, which, alone, would be likely to mislead if those words or acts, when viewed in their context, were not capable of misleading. It is obvious that where the conduct complained of consists of words it would not be right to select some words only and to ignore others which provided the context which gave meaning to the particular words.
134 Read both in its internal context (i.e. within the body of the article) and its external context (including the Website where it appears), I consider that the sentence conveys the First Vaccine Representation alleged by the ACCC and, in particular, would be likely to be so understood by reasonable members of the public, for the following reasons.
135 First, the statement that the Vaccine is “no longer effective” is likely to be understood as a representation that it is no longer effective in protecting against whooping cough, i.e., against contracting the disease. This is a natural reading of the sentence which accords with the ordinary meaning of the concept of “vaccine”, vaccination being the action or practice of inoculating with a vaccine to induce or increase immunity against disease: see Oxford English Dictionary and Macquarie Dictionary definitions of vaccine, vaccination and inoculate. This meaning is also confirmed by the context in which the representation appears, with the questions of protection against the disease and how to stop its spread being the subject-matter of the paragraph where the representation is made.
136 Secondly, that the reference to “recent research” is made in the course of an explanation of Whooping Cough which is presented in its terms as an orthodox explanation, implies that the proposition that the Vaccine is no longer effective has an adequate, if not firm, foundation in medical science. This impression is reinforced by a number of factors.
a) The question posed in the second paragraph – “But is large-scale vaccination the best solution?” – is purportedly answered in the first instance by the alleged deficiencies with the Vaccine. Those deficiencies are presented as if they are established and accepted in orthodox medicine, in line with the impression conveyed by the preceding description of whooping cough, its complications, and the existence of an epidemic which are presented in an informative style. Nothing in that explanation or otherwise in the First Article suggests that the points made in the first two paragraphs were written from a homeopathic perspective, nor, as counsel for the respondents submitted, within a uniquely homeopathic paradigm.
b) The First Whooping Cough Article is found in the “Treatment Room” section of the Website, the title of which suggests that it is concerned with the giving of expert advice. That inference is borne out by the content of the article which, having discredited the Vaccine, focuses thereafter on providing advice in some detail about alternative “remedies” allegedly afforded by homeopathy for the prevention and treatment of Whooping Cough.
137 Thirdly, this impression is consistent with Mrs Sheffield’s evidence that the reason why she stated in the First Article that “[n]ot only is protection from the current vaccine short-lived and unreliable, but side effects are common” was because she believed that statement to be supported by “a growing body of emerging medical opinion confirmed by my own clinical experience” (emphasis added). In this regard, as I have already observed, intention can be relevant to inferring whether a representation is likely to be misleading or deceptive, notwithstanding that it is not an element of the causes of action in ss 18 or 29 of the ACL. As French CJ, Crennan, Bell and Keane JJ held in TPG Internet at [55]-[56], a case concerning an advertising campaign by TPG prominently displaying a discount offer to supply broadband but less prominently displaying terms and conditions:
[55] It has long been recognised that, where a representation is made in terms apt to create a particular mental impression in the representee, and is intended to do so, it may properly be inferred that it has that effect. Such an inference may be drawn more readily where the business of the representor is to make such representations and where the representor’s business benefits from creating such an impression.
[56] To say this is not to say that TPG acted with an intention to mislead or deceive: such an intention is not an element of the contravention charged against TPG, and there was no suggestion of such an intention in the ACCC's case. There can be no dispute, however, that TPG did intend to create an impression favourable to its offer in the mind of potential consumers; and that it did intend to emphasise the most attractive component of its offer in order to do so.
[57] It cannot be denied that the terms of the message and the manner in which it was conveyed were such that the impression TPG intended to create was distinctly not that which would have been produced by an advertisement which gave equal prominence to all the elements of the package it was offering to the public. In this regard, it is significant that, as the primary judge noted, TPG considered deploying just such an advertisement and chose not to adopt it, evidently opting to continue with its headline strategy.
138 Equally, in the present case it may more readily be inferred that the reference to “recent research” is likely to have created an impression in the reader that there was a sufficient foundation in medical science for the opinion that the vaccine was ineffective because that was the intention of the author. Furthermore, Mrs Sheffield’s business stood to benefit from creating that impression as it provided the basis on which the article then opined that homeopathic products afforded a better alternative for the treatment and prevention of whooping cough, one of which at least, Drosera, was available for sale on the Website.
139 Finally, Mrs Sheffield gave evidence that:
In the First Whooping Cough article I said, “Most developed countries are currently in the grip of a whooping cough epidemic. To stop its spread, health officials are calling for the vaccination of adults as well as children. But is large-scale vaccination the best solution?” I was inviting readers of my article to look into the subject further.
(emphasis added)
140 I do not accept that Mrs Sheffield was merely inviting readers to look into the subject further and did so as a part of a wider policy debate on mass vaccination. More importantly, however, I do not consider that this is what the article conveyed. For the reasons already given, the First Whooping Cough Article poses the question and then immediately provides an answer to it, which is presented as advice for which it is implied that there is a sound basis in medical science. Furthermore, the fact that at the same time as the First Whooping Cough Article, a message was published on other pages of the Website suggesting that it was time that governments looked into homeopathy, does not in my view affect the characterisation of the representations made in the context of the First Whooping Cough Article.
141 Nor did the First Article seek in any way to represent that there were different sides of a political debate. In this regard, in answer to the question of whether she attempted to reflect any pro-vaccine aspects of the public debate concerning whooping cough in the First Whooping Cough Article, she said “Only in part…” and could point only to the reference in the First Whooping Cough Article to homeopathy also being used as a second line of defence should a vaccine already been given. That statement, however, simply acknowledges the fact that a person may already have been vaccinated.
142 However, I do accept that Mrs Sheffield genuinely believed that protection from the Vaccine is short-lived and unreliable and that side effects are common, as she stated in the article, notwithstanding that it was apparent that she had been selective in the material upon which she relied and misinterpreted data (as I later explain).
7.3.2 The Alternative Homeopathy Reasonable Basis Representation in the First Whooping Cough Article
143 I also accept the ACCC’s contention that the First Whooping Cough Article made representations to the effect that there was a reasonable basis, in the sense of an adequate foundation in medical science, to enable the Respondents to state that homeopathic treatments are a safe and effective alternative to the Vaccine for the prevention of whooping cough.
144 In my view, having “debunked” the Vaccine, the article changes its tone at [3] to one of persuasion about the attributes of homeopathy as an alternative, advising that “the homeopathic approach to the problem offers a safe and sensible solution”, and in so doing, completes its answer to the question of whether large-scale vaccination is the best solution. As such, the passages quoted above set up, in my view, a comparison between:
(a) the approach to prevention adopted in orthodox medicine in the first two paragraphs;
(b) and the homeopathic approach, in the third paragraph;
suggesting that the homeopathic approach is effective, unlike the Vaccine.
145 The First Article then supports the proposition that homeopathy is the “solution” by alleging that it has a 200 year history of treating and preventing whooping cough without dangerous side-effects, in contrast to what has been said about the Vaccine. Furthermore, the sentence “[i]t can also be used as a second line of defence should a vaccine for whooping cough already have been given” (emphasis added) implicitly urges the use of homeopathy as a first line of defence against contracting whooping cough, as the ACCC submitted.
146 Again, there is nothing in that comparison or otherwise in the context of the First Article which suggests that the representations given in relation to the homeopathic approach emanate from a “different epistemological framework” as alleged by the Respondents in any relevant sense. The methods and approach adopted to treatment and prevention in homeopathy as against those adopted in orthodox medicine could fairly be described in such terms, as is evident from the Website and as Dr Golden’s evidence set out at [74] above makes clear. But the same cannot be said with respect to representations as to the results or outcomes achieved by applying those different methods and approaches. As to the latter, the article relevantly contrasts the Vaccine with the homeopathic approach using the same measure-stick or standard (effective protection/prevention and side effects), representing that homeopathy provides effective and safe protection against the disease whereas the Vaccine does not.
147 The Respondents’ submissions wrongly, in my view, conflated these different concepts. For example, in their submissions the Respondents explain what they identify as the “key feature of this case”, namely, the “two distinct epistemological frameworks”, as “a bifurcation of underlying methods/assumptions/practices”. They do not contend that a different epistemological framework exists with respect to ascertaining the results achieved by applying those different methods, assumptions or practices, i.e., their effectiveness to achieve the desired outcome. Similarly, in responding to the ACCC’s contention that the Three Articles suggest that there is a single framework or paradigm within which the Vaccine and alternative homeopathic prophylaxis can be assessed and compared, the respondents rely upon:
(1) the reference in all Three Articles to the “homeopathic approach”;
(2) the suggestion in the Second Article that “[p]erhaps it is time to revisit homeopathy – homeopaths certainly think so”; and
(3) the explanation of principles of homeopathic treatment in the Second Article that “[u]nlike conventional medicine, remedies are not prescribed according to the common symptoms of the disease but for those that are idiosyncratic to the sufferer…” (emphasis added).
148 However, each of those statements concern only the different methods or approaches applied by conventional medicine and homeopathy, as opposed to the means by which their effectiveness is measured or compared.
149 The First Article next introduces the homeopathic remedies, stating:
Introduction to the Remedies
[4] No two cases of whooping cough are exactly alike – the remedy needed by each sufferer will depend on their particular symptoms. Having said this, there are two remedies used more often than others for treatment of the early stage of whooping cough. For this reason, they are placed at the head of the list. They are Drosera and Pertussinum. These two remedies have also been used by medical and non-medical homeopaths over the last 200 years for whooping cough prevention.
[5] The remaining remedies are listed alphabetically and, though they form an incomplete list, are some of more commonly prescribed remedies. General information on the expected course of whooping cough its common symptoms can be found at the end of the article.
(emphasis and paragraph numbers added)
150 The statement that the Drosera and Pertussinum “have been used… for whooping cough prevention” and that they have been so used “by medical and non-medical homeopaths over the last 200 years” (emphasis added) carries with it the implication that their use has been effective in preventing whooping cough. The same implication arises from the inclusion of Drosera Rotundifolia (Dros) and Pertussinum (Pert) under next heading “Remedies for Early Treatment or Prevention”, as opposed to their inclusion in the list of different remedies under the heading “Frequently Used Remedies for Treatment” (emphasis added). The Article therefore distinguishes between remedies for treatment and prevention, on the one hand, and remedies for treatment on the other hand, identifying only Drosera and Pertussinum as remedies suitable for both applications. It will be recalled that Drosera was available for sale through the Website shop, the shop being accessible by left-clicking on the bar on the left-hand side of the article: see at [83] above.
151 It follows in my view that the First Whooping Cough Article suggests on an ordinary reading that there is a sound and rational basis for saying that these remedies are effective to prevent against the disease, being a foundation in medical science adequate to sustain the proposition. The inclusion of the article in the “Treatment Room” of the Website confirms this reading, as does the tenor of the article which conveys the impression that it is giving expert advice to readers of safer and more sensible ways in which to prevent (and treat) whooping cough.
152 Finally, while I address the significance of the issue later, it is apparent that the First Article is not formulated in a manner that suggests it is contributing to a political debate on whether or not the government should change its policies with respect to homeopathy. The Respondents’ submission that in asking whether large-scale vaccination is the best solution, and the article should be read in this manner is, with respect, untenable. The question is asked in the context of providing readers with advice about how to prevent and treat whooping cough.
7.3.3 The second alleged Vaccine representation in the Second Whooping Cough Article
153 The ACCC also contends that the Second Whooping Cough Article represents that the Vaccine may not be the best solution for, is of limited effect and is unreliable at best, in protecting against whooping cough (the Second Vaccine Representation).
154 The article begins by stating:
Whooping Cough – Homeopathic Prevention and Treatment?
[1] Many parts of the world are in the grip of a whooping cough epidemic.
[2] Whooping cough, or pertussis, is a highly contagious disease caused by the Bordetella pertussis bacterium. Worldwide, it is responsible for about 300,000 deaths a year and leaves many surviving children with brain damage. Serious complications such as bleeding into the nose, eyes or brain, pneumonia, and hernias also occur. Infants below the age of 12 months are particularly vulnerable.
[3] The characteristic symptoms of fully developed whooping cough are: rattling mucus; hard to expectorate mucus; nausea and vomiting; paroxysms of cough; exhaustion, and of course, the characteristic ‘whoop’ sound as the sufferer gasps for breath. Many of those affected by whooping cough have already been vaccinated. Recent studies show that the vaccine has limited effect.
[4] To stop the spread of the current epidemic, health officials are calling for the vaccination of adults as well as children. But is large-scale vaccination the best solution when protection from the current vaccine appears unreliable at best and side-effects are common. Perhaps it is time to revisit homeopathy – homeopaths certainly think so.
[5] Homeopathy offers an alternative or ancillary approach to whooping cough management. It has been used by medical and non-medical homeopaths during the past 200 years for that purpose and has an excellent safety record.
[6] Unlike conventional medicine, remedies are not prescribed according to the common symptoms of the disease but for those that are idiosyncratic to the sufferer; it is not the disease that is treated but the self-healing efforts of the individual’s body which are stimulated and strengthened.
[7] With the correct remedy – the one that matches the sufferers (sic) idiosyncratic symptoms – recovery is accelerated and the risk of super-imposed infections or complications minimised.
[8] Alternatively, prevention and protection of epidemic disease is possible by giving individuals a remedy before infection that best matches the common symptoms of that disease – those symptoms that most sufferers experience. This ‘genus epidemicus’ remedy reduces the susceptibility of vulnerable individuals to that infection.
Introduction to the Remedies
[9] As any affected family will tell you, no two cases of whooping cough are alike. And as homeopaths will tell you, the remedy needed by each sufferer depends on their unique and individual symptoms.
[10] Having said that, it has been frequently noted that two remedies are needed more often than others during the early stages of whooping cough. They are Drosera and Pertussinum. Both have been used extensively by homeopaths over the past 200 years for whooping cough treatment and prevention. For this reason, they have been placed at the head of the following list of remedies.
,,,
Remedies for Early Treatment or Prevention
[11] Drosera Rotundifolia (Dros)
…
(emphasis and paragraph numbers added)
155 For the reasons earlier given with respect to the First Vaccine Representation, I consider that the ACCC correctly contends that paragraph 3 of the article implies that the Vaccine “has limited effect”. The fact that those words are preceded by the words “[r]ecent studies show that [the Vaccine] has…” implies that the opinion expressed has an adequate foundation in orthodox medicine. The first three paragraphs of the article are no less informative in tone than the First Whooping Cough Article and convey the same impression in referring to “recent studies”.
156 Furthermore, as the ACCC submit, the question in paragraph [4] is posed in a rhetorical manner (albeit now without a question mark), thereby conveying in my view the message that vaccination may not be the best solution for the reasons stated in the remainder of the question. This is confirmed by the next sentence suggesting that it is perhaps time because of this to revisit homeopathy.
157 I also accept the ACCC’s submission that the article represents that the Vaccine is unreliable at best. A lawyer trained in the subtleties of words might place weight on the use of the word “appears” before the word “unreliable”, coupled with the suggestion that “perhaps” it is time to revisit homeopathy, to suggest that the proposition is a tentative one. However, it cannot be assumed that the audience, which extends as I have said to any member of the public, would pay such close attention to the niceties of expression. Nor that they would be unreasonable in doing so given that the overall impression given by the article is that the Vaccine is unreliable. This is particularly so where the preceding paragraph informed the reader that recent studies “show that the vaccine has limited effect”. Moreover, the proposition is not merely that the “vaccine appears unreliable” but rather that it appears unreliable “at best” (emphasis added). This suggests that the vaccine “appears unreliable” even if the most generous view of its effectiveness is adopted.
158 Before posing the rhetorical question, the tone of the article is that it is informing the reader of certain facts, implying that the information has a medical basis. There is no suggestion that that information is provided in any relevant sense from a homeopathic perspective. In common again with the First Whooping Cough Article, however, the tone of the article changes to one of persuasion and advice from the posing of the rhetorical question and representation that homeopathy offers “an alternative or ancillary approach to whooping cough management” (emphasis added). That the homeopathic alternative includes “prevention and protection” and not merely remedies for treatment is not only evident from its juxtaposition with the preceding discussion about the alleged deficiencies in the Vaccine, but also from [8] and [10] of the Second Article. Moreover, Drosera Rotundifolia (Dros) and Pertussinum again are listed under the heading “Remedies for Early Treatment or Prevention”, with both being described as “a major remedy for either the treatment or prevention of whooping cough”, while other remedies are separately listed under the heading “Frequently Used Remedies for Treatment”.
159 Furthermore, the representations compare the outcome or results of utilising the medical and homeopathic approaches and methodologies by the same yardstick: their alleged effectiveness and side-effects. The question of whether they emanate from different epistemological or philosophical frameworks is irrelevant to that comparison and to the characterisation of the representations: see above at [146]. There is no suggestion in the article that the outcomes achieved in terms of preventing whooping cough from the application of homeopaths’ homeopathic treatments are effective “for homeopathy” rather than in general and even to so state the proposition is to reveal its illogicality.
160 Finally, there is nothing in the Second Whooping Cough Article that suggests it is contributing to a political debate other than, at best, incidentally. In particular:
(a) While the article no longer appeared in the “Treatment Room” section of the Website, it appears in a section of the Website bearing the generic title, “Members”. There was no longer even a click through button to a section of the Website entitled “political issues”, in contrast to the position at the time of the First Whooping Cough Article.
(b) The title of the article is the same as that of the First Whooping Cough Article, namely, “Whooping Cough – Homeopathic Prevention and Treatment?” save for the addition of the question mark.
(c) The terms and conditions which a reader of the Second Whooping Cough Article was required to accept in order to access the article after 15 January 2013 (examined in more detail later in my reasons), do not limit the subject matter of the pages in the Members Area.
(d) The tenor of the article is that it provides information and advice about the means by which whooping cough can be treated and prevented and is directed towards persuading the reader to accept homeopathic treatments relevantly in the alternative to conventional vaccination. Detailed information is also provided about the individual remedies.
161 As such, the focus of the Second Whooping Cough Article remains essentially the same as the first: see at [152] above.
7.3.4 The Alternative Homeopathy Reasonable Basis representation in the Second Whooping Cough Article
162 I also accept that the Second Whooping Cough Article, in common with the first, conveys the representation that homeopathic remedies are a safe and effective alternative to the Vaccine for the prevention of Whooping Cough for which there is a reasonable basis, in the sense of an adequate foundation in medical science. It is true that [5] of the article states only that homeopathy offers “an alternative or ancillary approach to whooping cough management” (emphasis added) and that the term “management” in the context of a disease might be used to refer only to treatment of the disease in the individual case. However, read in context I consider that the statement conveys the impression that homeopathy offers “an alternative or ancillary approach to whooping cough management” in the sense of the treatment and prevention of the disease. This impression is conveyed by the Second Article given, in particular, the following considerations:
a) The title of the Article, “Whooping Cough – Homeopathic Prevention and Treatment?”
b) The immediate juxtaposition of the criticisms made of vaccination with the treatment derived from homeopathy.
c) The fact that in the context of the discussion (at [4]) of efforts to stop the spread of a whooping cough epidemic, the reference to the “whooping cough management” could reasonably be read as referring to the management of the spread of the disease.
d) The fact that the statement at [5] elaborates on the reasons why it is time to revisit homeopathy in answer to the question of whether large scale vaccination is the best solution when protection from the Vaccine appears unreliable.
e) The further elaboration in the Second Whooping Cough Article at [8] as to why prevention and protection is possible through the homeopathic approach.
f) The statement in the Article at [10] that both Drosera and Pertussinum “have been used extensively by homeopaths over the past 200 years for whooping cough treatment and prevention”, referring back to the earlier statement at [5] that homeopathy “has been used by medical and non-medical homeopaths during the past 200 years for that purpose [i.e. whooping cough management] and has an excellent safety record”.
g) The listing again at [11] of Drosera under the heading “Remedies for Early Treatment or Prevention” together with Pertussinum, as opposed to separate list of different remedies under the heading “Frequently Used Remedies for Treatment”.
7.3.5 The Third alleged Vaccine Effectiveness Representation in the Government Article
163 The Government Article reads that:
Government Data Shows Whooping Cough Vaccine a Failure
Australia, along with other countries, has seen a meteoric rise in the number of notified cases of whooping cough in the past few years.
Lack of vaccination is often blamed but now information from the Australian government shows that the whooping cough vaccine has been largely ineffective.
Between 2008 and 2010, of children aged 0-4 years whose vaccination status was known and who had contracted whooping cough, 75% were fully vaccinated and a further 14% were partly vaccinated. Only 11% were un-vaccinated.
Why was the Australian government so slow to release this information? Do records from other countries show this vaccine has been equally unsuccessful? What is the future recommendation from government about whooping cough prevention?
In the absence of an effective vaccine for this dangerous disease, it is also wise to know about homeopathy and whooping cough.
164 The article expressly represents, as the ACCC contends, that the Vaccine is “largely ineffective in protecting against whooping cough” (the Third Vaccine Representation). In contrast, however, to the other articles, the Government Article did not expressly mention any specific homeopathic products.
7.3.6 The Third alleged Alternative Homeopathy Reasonableness representation
165 The ACCC also contends that the Government Article in conjunction with the Second Whooping Cough Article conveyed the Homeopathic Alternative Reasonable Basis Representation.
166 As earlier mentioned, the Government Article did not refer to any specific homeopathic products, in contrast to the First and Second Whooping Cough articles. However, the impression given by the statement in the Government Article that “in the absence of an effective vaccine for this dangerous disease, it is also wise to know about homeopathy and whooping cough” is that the reader should look into the homeopathy as an alternative to the allegedly ineffective Vaccine. When read together with the Second Whooping Cough Article, the impression is that homeopathy provides an effective and safe alternative to the Vaccine.
167 In this regard, the Government Article was uploaded to the Website on 3 February 2012 and was therefore present on the Website when the Second Whooping Cough Article was uploaded to the Website on 11 January 2013. The toolbar above the Government Article lists click through buttons both to the “Treatment Room” and “Online Shop”, as well as a click through button headed “SEARCH THE SITE”, effectively inviting the reader to look further into the issue by reference to further information on other pages on the Website. If the reader were to do so, it can reasonably be inferred that the reader would be likely to access the Second Whooping Cough Article. That article, of course, mentioned the homeopathic products expressly, at least one of which could be purchased from the online shop which was also accessible by a click through button. Prior to 15 January 2013, the Second Whooping Cough Article could be accessed without viewing the disclaimer (the “Oops” page) and accepting the member’s terms and conditions: see at [97] above. After that date, the visitor would encounter first the disclaimer and then have to accept the terms and conditions: see at [97]-[98] above. However that could be done as I have earlier found by any member of the public without payment: see above at [105] and [127].
7.4 Were the three Vaccine Effectiveness Representations false, misleading or deceptive?
168 It follows from my findings above that the key notions conveyed by the Three Vaccine Representations are, as the ACCC contends, that the Vaccine is unreliable, ineffective, no longer effective or largely ineffective, not the best solution for whooping cough, and short-lived.
169 The ACCC contends that the Vaccine Effectiveness Representations are misleading and deceptive, or likely to mislead and deceive, and/or false or misleading representations that the Vaccine is of a particular standard or quality for the purposes of ss 18 and 29(a) and (b) of the ACL, because the Vaccine is effective in protecting a significant majority of people who are exposed to whooping cough infection from contracting the disease.
170 In this regard, I accept that it is unnecessary for the ACCC to prove that the Vaccine is infallible in order to negate the suggestion that it is “short-lived”, “unreliable” and “no longer effective”. Indeed, if this were the test, no vaccine would meet the test given Professor Phelps unchallenged evidence that no vaccine is 100% effective. Rather, I need be satisfied only that “administration of the Vaccine largely produces the desired effect. That effect comprehends prevention of the disease and amelioration of the symptoms of the disease if contracted.” The same would suffice, in my view, to counter the Vaccine Representations in the Second Whooping Cough Article that the Vaccine is of “limited effect” and that protection is “unreliable at best” or “largely ineffective”, notwithstanding that the deficiencies said to exist with respect to the Vaccine are slightly more qualified in the Government Article than those made in the First Whooping Cough Article.
171 The evidence clearly established that the Vaccine Representations were misleading, deceptive and false because the Vaccine is effective in protecting a significant majority of people who are exposed to whooping cough infection from contracting the disease and ameliorating the symptoms, if contracted. Each of the expert witnesses called by the ACCC gave evidence that the Vaccine is efficacious and effective in protecting against contraction of whooping cough and reducing the severity of the disease where it was contracted. Nor was any credible evidence led by the Respondents that cast doubt on the ACCC’s evidence as to the efficacy and effectiveness of the Vaccine. Indeed, Dr Donohoe largely agreed with critical aspects of the evidence of the expert witnesses called by the ACCC.
172 In particular, for the reasons I develop below:
(a) The efficacy of the Vaccine is supported by Level I and 2 scientific evidence, being evidence recognised as being of the highest quality.
(b) The Australian Immunisation Handbook, which represents current approved and accepted medical practice in Australia, accepts the efficacy and effectiveness of the Vaccine, as does the Center for Disease Prevention and Control in the United States.
(c) Real world observations bear out the effectiveness of the Vaccine in significantly reducing the number of cases of whooping cough and their severity, including in:
a. reducing the number of cases among those who are unvaccinated (such as infants under six weeks) or not fully vaccinated (such as infants under six months) by reason of by “cocooning strategies” and so-called “herd immunity” which is achieved where immunisation rates exceed 90%; and
b. reducing the severity of symptoms among infants even before completion of the primary vaccination schedule.
(d) It is misleading to suggest that the Vaccine is “short-lived” given that waning immunity is addressed by the administration of the Vaccine in accordance with the NIP, including boosters, by the aim through the NIP of achieving herd immunity, and by cocooning strategies.
(e) €The submission that the Vaccine representations are not misleading, deceptive or false if read in the context of a whooping cough epidemic for infants and toddlers is misconceived: it does not reflect the content of the First and Second Whooping Cough Articles; nor does the existence of the epidemic in 2008-2009/2010 mean that the Vaccine is “short-lived” and “unreliable”.
7.4.2 The efficacy of the Vaccine is supported by Levels 1 and 2 evidence
173 The most detailed evidence for the acellular vaccines currently used in Australia under the NIP to prevent whooping cough in children is The Cochrane Collaboration: Acellular vaccines for preventing whooping cough in children (Review) (2012; John Wiley & Sons, Ltd) (the “Cochrane review”) authored by Zhang, Prietsch, Axelsson, and Halperin. The review was initially published in 1999 and updated in 2009, with the latest review published in 2012. The revisions reflect the fact that the Cochrane Reviews are, as Professor Phelps explained, “a living document”. Specifically, they are updated on a regular basis by panels of international reviewers having relevant expertise so as to have regard to further studies of sufficient quality.
174 The objective of the Systematic Cochrane review is to assess the efficacy and safety of acellular pertussis vaccines in children. In this regard, Dr Crawford explained that the majority of the effectiveness data for whooping cough is in children under the age of six years who are most at risk of contracting the disease and of complications from it.
175 Dr Crawford also explained that it is more difficult to determine the effectiveness of booster vaccinations in adolescents and adults necessary to ensure ongoing protection, with effectiveness studies often relying on case-control studies following outbreaks of pertussis.
176 The Cochrane reviews are Level 1 and 2 scientific evidence (see at [64] above), with the authors selecting double-blind randomised efficiency and safety trials of aP vaccines (the type of vaccines used in Australia) in children up to six years old, with active follow-up of participants and laboratory verification of pertussis cases. The reliability of the Cochrane Review as a source of data concerning the efficacy of the Vaccine was accepted by Professor Phelps, Dr Crawford and Dr Wood.
177 The Cochrane Review summarised its main results as follows:
We included six efficacy trials with a total of 46,283 participants and 52 safety trials with a total of 136,541 participants. Most of the safety trials did not report the methods for random sequence generation, allocation concealment and blinding, which made it difficult to assess the risk of bias in the studies. The efficacy of multi-component (≥ three) vaccines varied from 84% to 85% in preventing typical whooping cough (characterised by 21 or more consecutive days of paroxysmal cough with confirmation of B. pertussis infection by culture, appropriate serology or contact with a household member who has culture-confirmed pertussis), and from 71% to 78% in preventing mild pertussis disease (characterised by seven or more consecutive days of cough with confirmation of B. pertussis infection by culture or appropriate serology). In contrast, the efficacy of one- and two-component vaccines varied from 59% to 75% against typical whooping cough and from 13% to 54% against mild pertussis disease. Multi-component acellular vaccines are more effective than low-efficacy whole-cell vaccines, but may be less effective than the highest-efficacy whole-cell vaccines. Most systemic and local adverse events were significantly less common with aP vaccines than with wP vaccines for the primary series as well as for the booster dose.
178 The rate of vaccine efficacy demonstrates, therefore, that for children under the age of six, a complete infant vaccine schedule provides:
a) 84%-85% protection from contracting typical pertussis infection (i.e. 21 consecutive days of paroxysmal cough); and
b) 71%-78% from contracting mild pertussis disease (seven or more days of cough with confirmed laboratory test);
179 Thus, as Dr Crawford explained, the likelihood of contracting whooping cough is decreased by 85% if the individual is fully vaccinated.
180 The evidence of Dr Donohoe for the Respondents as to the effectiveness of the Vaccine was to similar effect. Dr Donohoe accepted that the most significant adverse health effects of pertussis are usually seen in the very young and the very elderly. In his opinion, the estimated effectiveness of the pertussis vaccine following a full course of injections in childhood (two months, four months and six months) is approximately 80%, meaning that 4/5 infants will be effectively protected against the severe form of the disease, although only 65 to 70 percent will be protected against milder forms of the disease.
181 As to the quality of the evidence that supports these rates of vaccine efficacy, the authors of the Cochrane Review found that a meta-analysis of the efficacy data produced by the trials was inappropriate due to the small number of randomised controlled trials and the differences between them in dose schedule, vaccine characteristics, case definitions and background pertussis incidence. (The term “meta-analysis” describes an analysis of the combined data of a number of trials as opposed to an examination of a single trial, and can be a complex process particularly where the trials have been conducted differently). Nonetheless, at p. 17 of the Cochrane Review the authors concluded that:
the three large double-blind RCTs [i.e. randomised controlled trials] from Italy, Sweden, and Germany (Greco 1996; Gustafsson 1996; PVSG 1998) provide high level evidence about effectiveness of multi-component aP vaccines against whooping cough.
(emphasis added)
182 As to the safety profile of the acellular Vaccine, which is used in Australia, the authors found that:
meta-analysis of 52 studies [with a total of 136,541 participants] provides robust evidence about the superiority of aP vaccines over whole-cell vaccines.
(emphasis added)
183 Dr Donohoe also accepted on the basis of the Cochrane Review that the acellular pertussis vaccine is considerably safer than the whole cell pertussis vaccine, although its effectiveness is less.
184 The authors of the Cochrane Review concluded at p. 18 that “Multi-component acellular vaccines are effective against both typical whooping cough and mild pertussis disease, with a good safety profile.” (emphasis added)
185 In cross-examination of Dr Crawford and Dr Wood, the Respondents sought to undermine the conclusions reached by the Cochrane Review on the basis that there were no recent trials relied upon in the Review in support of the efficacy of the Vaccine. However, I accept the evidence of Dr Crawford and Dr Wood that the reason why there are no more recent randomised controlled trials of the Vaccine and, therefore, no new Level 1 or 2 evidence as to its efficacy, is because it would be unethical for trials of that nature to be undertaken today. A trial of that kind would require the creation of a “control” or placebo group of children who were deprived of the active component of the Vaccine in order to compare their results with those children who were administered the Vaccine, notwithstanding the proven efficacy and effectiveness of the Vaccine. Once the efficacy of a vaccine has been proven, the kinds of randomised trials that will be undertaken in the future are those which compare, for example, different vaccines (e.g. the whole cell whooping cough vaccine initially used as opposed to the acellular vaccine), or different administration schedules for a vaccine. None of this undermines the reliance on the randomised controlled trials relied upon as high level evidence of the efficacy of the Vaccine, notwithstanding that the trials were undertaken some time ago.
186 It follows, therefore in my view that the efficacy and effectiveness of the Vaccine is demonstrated by Level 1 and 2 scientific evidence which, it will be recalled, is the highest quality of evidence available.
7.4.3 The Australian Immunisation Handbook
187 The Australian Immunisation Handbook (10th Ed, 2013) accepted the rates of vaccine efficacy assessed in the Cochrane Review, stating at [4.12.4] that:
Pertussis vaccines provide good protection against severe and typical pertussis but substantially less against milder coughing illnesses. Vaccine efficacy of DTPa vaccines with three or more antigens has been reported as 71 to 78% for preventing milder symptoms of pertussis and 84% for preventing typical disease.
188 The Handbook further advised that “epidemiological data suggest that receipt of the 1st dose of the primary DTPa course significantly reduces the incidence of severe pertussis disease in young infants, as measured by hospitalisation rates.”
189 The Australian Immunisation Handbook is the product of a demanding process of research and review, and is regarded as providing the best advice to Australian doctors on administering vaccines against vaccine preventable diseases including whooping cough. While it no longer includes formal levels or grades of evidence or evidence tables, Dr Wood explained that it was developed using the highest quality of evidence available. Each chapter is researched and prepared by technical experts under the auspices of the Australian Technical Advisory Group on Immunisation (ATAGI), which also makes recommendations on the inclusion of vaccines on the NIP (see [55] above). Dr Wood, who assisted in preparing the Australian Immunisation Handbook as a technical writer for one of the chapters (albeit not on Pertussis), gave evidence as to the width of research undertaken in preparing a chapter of the Handbook. He explained that the research undertaken includes databases such as MEDLINE which reference primarily peer-reviewed articles, but also extends to so-called “grey literature” such as doctoral theses. Each chapter of the Handbook, in turn, is reviewed by ATAGI, the lead government advisory group on immunisation, and subsequently by the National Health and Medical Research Council (“NHMRC”), before the handbook is published and endorsed by the NHMRC. It was Dr Wood’s unchallenged evidence that, given the NHMRC endorsement, the Australian Immunisation Handbook (10th Ed) would be considered by the Australian community to reflect current approved and accepted medical practice.
7.4.4 The Center for Disease Control and Prevention
190 The Center for Disease Control and Prevention (“CDC”) is a federal agency under the Department of Health and Human Services in the United States. Its primary goal is to protect public health and safety through the control and prevention of disease, injury, and disability. It focuses national and international attention on developing and applying disease control and prevention. Professor Phelps gave evidence that the effectiveness of the Vaccine has also been accepted by the CDC which has stated that:
Pertussis vaccines are effective, but not perfect. They typically offer high levels of protection within the first 2 years of getting vaccinated, but then protection decreases over time. This is known as waning immunity. Similarly, natural infection may also only protect you for a few years.
In general, DTPa vaccines are 80-90% effective. Among kids who get all 5 doses of DTPa on schedule, effectiveness is very high within the year following the 5th dose – at least 9 out of 10 kids are fully protected. There is a modest decrease in effectiveness in each following year. About 7 out of 10 kids are fully protected 5 years after getting their last dose of DTPa and the other 3 out of 10 kids are partially protected – protecting against serious disease.
Our current estimate is that DTPa vaccination protects 7 out of 10 people who receive it. Since DTPa vaccines were only licensed in 2005, we don’t yet have results on long-term vaccine protection. We’re still working to understand how that protection declines over time or might differ based on which vaccine was received during early childhood (i.e., DTPa or DTP). CDC will be conducting an evaluation in collaboration with health departments in Washington and California to better understand how long DTPa vaccines protect from pertussis. The data from these evaluations will help guide discussions on how best to use vaccines to control pertussis.
7.4.5 Evidence of effectiveness in the real world
191 The burden of the disease continues to be monitored, including in Australia where any positive laboratory test of pertussis must be notified to the National Notifiable Diseases Surveillance System (“NNDSS”). The results of that monitoring bears out the effectiveness of the Vaccine and the NIP for the reasons I explain below.
7.4.5.1 Measuring effectiveness in the real world
192 As to the means by which effectiveness in the real world is measured, Dr Wood explained, first, that: “the ability of a vaccine to prevent disease effectively depends on its potency and proper administration to an individual. The success of vaccination performed under field conditions [or ‘disease burden’] may be assessed by measuring protection against clinical disease by epidemiologic means”, i.e., by counting the number of pertussis cases within a given population by reference to different levels of evidence. These levels range from those with a coughing illness for more than two weeks at the base of the “pyramid” hierarchy, to those with a coughing illness for more than three weeks and who have had contact with a person with whooping cough, and laboratory confirmation of the disease at its peak. Secondly, Dr Woods explained that vaccine effectiveness is measured:
…by calculating the cumulative incidence rates (attack rates) of disease among vaccinated and unvaccinated persons and determining the percentage reduction in the incidence rate of disease among vaccinated persons relative to unvaccinated persons. The greater the percentage reduction of illness in the vaccinated group, the greater the vaccine effectiveness.
The basic formula is written as:
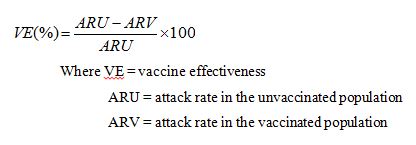
193 Dr Crawford expanded upon the strength of the evidence obtained through this method, described by him as the “case-control methodology”. Specifically, he explained:
We had 60,000 children born in Victoria every year and about 90 per cent of them are getting the vaccine, so we are monitoring [a] 50,000 cohort of children who have had the whooping cough vaccine and measuring the impact over time. So while there’s sufficient, big numbers in these studies, in actual fact, at a population level, we have a much better feel internationally and that data can be collated both in Australia, the United States, UK, other countries still have very large numbers of children being immunised and monitoring the amount of the disease. So while it’s ideal to do this randomised trials initially, it’s not the real world in terms of longer-term surveillance of these conditions.
…
And so what’s happening in the real world depends to a very large extent on the people doing the reporting on the ground, doesn’t it? So the notifications?--- As a crude measure of notification it is but you can also then do, is it [sic], outbreak investigation, you are monitoring for who had the vaccine and who didn’t. So you’re looking at a case-control-level analysis, which, again, is a very powerful epidemiology tool to work out how effective the vaccine may be in a certain population, because if you show that lots of people who had the disease were not immunised or not immunised for a long period of time, that gives you some interpretation of how effective the vaccine is in the setting of that outbreak of whooping cough in this example.
… So you can’t do a randomised controlled trial, but the case-control methodology is still a very strong way to measure effectiveness.
7.4.5.2 Effectiveness of the Vaccine and Herd Immunity
194 The evidence emphatically established that the Vaccine is effective. As would be expected given the results achieved in efficacy studies, population studies also demonstrated reduced incidence of pertussis, as well as reduced severity in the symptoms of the disease, where substantial numbers of people within the population have been vaccinated. In particular, the number of deaths occurring internationally from whooping cough has dramatically reduced, as it has in Australia where deaths are now rare.
195 Importantly, at the population level, widespread administration of the Vaccine not only affords immunity to those vaccinated but also gives rise to so-called “herd immunity”. As Dr Wood explained:
In general non-immune (either unvaccinated or no prior infection) people are more likely to contract an infectious illness, such as whooping cough, when exposed to it due to their lack of pre-existing immunity. This in part explains how herd immunity works. The overall decline in case numbers means that infants who are too young to be vaccinated are protected because there is less disease in the community. Herd immunity refers to this protective effect of a reduction in disease burden in a community due to widespread vaccination and therefore protection of those who are too young to be vaccinated.
196 The evidence of Professor Phelps and Dr Crawford was to similar effect.
197 In order to provide herd immunity, rates above 90% are required, as Dr Crawford explained. The achievement of these high vaccination rates within the community operates effectively to “cocooning” unvaccinated individuals against the disease, i.e., to reduce the risk that an unimmunised person will contract the disease, bearing in mind that the disease is highly infectious and spread by respiratory droplets from an infected person (see at [40] above). The importance of herd immunity is evident from the protection which it affords in particular to infants who cannot be administered the vaccine until 6 weeks. Nor can the primary schedule of three doses under the NIP affording maximum protection be completed until the child is six months of age although, as Dr Wood explained, “[i]n general infants who have had one or more doses of pertussis vaccine are partially protected from severe and complicated pertussis infections compared to infants who have had no pertussis vaccine doses”. Yet children are more likely to suffer classic pertussis and serious complications are most common in non-immune infants and very young infants, with most deaths occurring in children under six months and particularly under one month: see [45] above. As Dr Wood, Dr Crawford and Dr Donohoe explained, protection can also be afforded to infants between birth and six weeks through maternal transfer of antibodies. Hence the reason for maternal vaccination during pregnancy under programs in the United States and the United Kingdom, and the Australian cocoon vaccination program.
198 In this regard, one of the aims of the NIP is to provide high community levels of vaccine coverage, with the rates above 90% required to provide herd immunity. Statistics from the Australian Childhood Immunisation Register show that as at 31 March 2013 (age calculated as at 31 December 2012), the percentage of infants and children fully immunised in age groups spanning between 1 to 5 years of age was at least 91% for each respective group. Specifically:
Age | %DTP received | % fully immunised |
12-<15 months | 92.05% | 91.49% |
24-<27 months | 94.5% | 92.2% |
60-<63 months | 92.35% | 91.76% |
199 Dr Donohoe accepted the accuracy of these figures.
200 However, while the rate of vaccination for infants is high, Dr Crawford explained the school pertussis program in adolescence (12-16 years) is approximately 60 to 70% while the number of adults being vaccinated as part of the cocooning strategy is variable. As a result, he considered that coverage for adolescents and adults was not optimal for achieving herd protection.
7.4.5.3 The Vaccine Paradox: Why the number of vaccinated persons contracting the disease increases when vaccination coverage increases
201 One consequence of high vaccination rates is that the percentage of cases of whooping cough in vaccinated persons as opposed to those who are unvaccinated can be expected to increase. This does not, however, mean that the vaccine is ineffective. As Dr Wood explained, this phenomenon, commonly described as the “vaccine paradox” is often misunderstood:
Often anti-vaccine proponents argue that as a percentage more or equivalent cases of vaccine preventable diseases occur in those who are vaccinated compared to those who are unvaccinated and this means the vaccines are ineffective. However, this is not the case and can be easily explained by the vaccine paradox… The main point of the vaccine paradox is as vaccination coverage increases, the total number of cases is reduced, however the PROPORTION (or %) of cases who are unvaccinated increases. In general non-immune (either unvaccinated or no prior natural infection) people are more likely to contract an infectious illness, such as whooping cough, when exposed to it due to their lack of pre-existing immunity. This in part explains how herd immunity works. The overall decline in case numbers means that infants who are too young to be vaccinated are protected because there is less disease in the community. Herd immunity refers to this protective effect of a reduction in disease burden in a community due to widespread vaccination and therefore protection of those who are too young to be vaccinated.
202 The vaccine paradox is illustrated by Dr Woods in the following diagram:
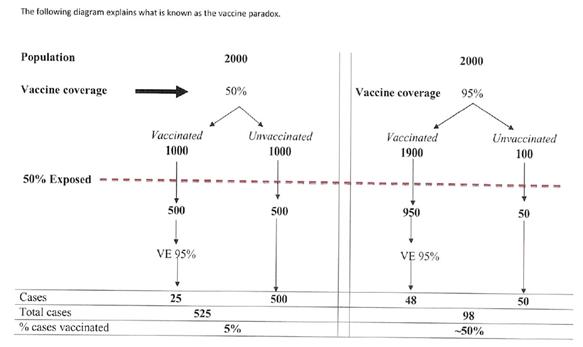
203 The diagram takes a hypothetical population of 2000 persons, 50% (i.e. 1000) of whom are exposed to the whooping cough disease. Vaccine effectiveness is assumed to be 95% for the purposes of the example. The percentage of those vaccinated and exposed to the disease who are likely to contract it will be a constant 2.5%. Where 50% of the population is vaccinated, this translates into 25 cases, and where 95% of the population is vaccinated, this translates into 48 cases. Equally, the percentage of those who are unvaccinated and exposed to the disease who are likely to contract it will remain a constant 50%. Where 50% of the population is vaccinated, this translates into 500 cases out of a population of 1000 unvaccinated people, and where 95% of the population is vaccinated, this translates into 50 cases out of a population of 100 unvaccinated people. The point is that, notwithstanding that the number of vaccinated persons contracting the disease has increased with increased vaccine coverage, the percentage remains the same, and overall the number of persons contracting whooping cough will have dropped from 525 where there is 50% vaccination coverage to only 98 cases where 95% of the population is vaccinated.
7.4.6 The failure to eradicate the disease does not mean that the Vaccine is ineffective
204 Contrary to the suggestion by the Respondents, the fact that the Vaccine has not resulted in the eradication of whooping cough does not mean that the Vaccine is ineffective even though vaccines for some other diseases such as measles and polio have achieved greater success, with there being no cases currently of polio in Australia. As Dr Crawford explained in answer to a question that, by contrast with whooping cough, there are no graphs that show a recent epidemic with polio in Australia:
… [w]e do surveillance for polio and there is no epidemic of polio but it’s a very different infection. So polio is infection where you present with acute paralysis and it’s a very clear cut timing and definition and cases are still seen internationally. We would like to eradicate it as of vaccine-preventable disease, if possible. There’s still isolated places. Whooping cough is very different. Whooping cough, you often don’t know you have it. You just have a chronic cough and you cough and splutter on everyone and you potentially given to a small baby who… is not protected, had not had immunisation or not had the complete course and is susceptible to getting severe infection. So it’s going to be something that… you’re going to struggle to eradicate because you get the illness and you don’t know about it until you’ve actually exposed a small, vulnerable infant.
205 Consequently, while the whooping cough vaccine has not achieved the same result as the polio vaccine, Dr Crawford explained that since the provision of the Vaccine in the 1950s:
… the number of deaths that have occurred internationally from whooping cough have dramatically reduced. So if you look that, you know, a century ago, the number of deaths from whooping cough was very substantial. It was hundreds of thousands of cases of people were dying secondary to whooping cough. The immunisation has definitely modified the illness and the amount of illness that we see. We rarely see deaths in Australia. We have hospitalisation but rarely deaths. So even if we have not eradicated whooping cough, it is very clear that the vaccine has been efficacious in reducing the number of cases and the cases that we do see, the severity is decreased through immunisation.
So do you say that the whooping cough vaccine and its introduction on the public health schedule has had an effect on the mortality of people dying from whooping cough? Do you say that the vaccine has had a big effect on that?--- Correct. There has been a decrease in the number of deaths in Australia secondary to the immunisation program with pertussis.
206 In this regard, Dr Crawford accepted that the intensive medical care available to infants who contract the disease clearly also has an impact on reduced mortality, immunisation including cocooning markedly decreases their chances of getting whooping cough in the first instance.
7.4.7 The multi-dose schedule does not mean that the Vaccine is ineffective
207 Finally, as Dr Crawford also explained, the complexity of whooping cough bacteria in terms of producing an immune response that affords longer-term protection means that it is likely that a vaccine for this disease will always require a multi-dose schedule. This does not, however, indicate that the vaccine is not effective. It is not unusual for a vaccine to require multiple doses. Even the polio vaccine requires four doses to be administered, while measles has a two dose schedule. Other diseases such as tetanus and diphtheria also require multiple doses to produce immune protection.
208 The Respondents also submitted that “‘short-lived’ is a phrase used by Homeopathy Plus! whereas the immunologists preferred terms like ‘waning’”, the suggestion being that the term “short-lived” conveys essentially the same meaning or impression as “waning”. On that basis, the Respondents contend that the representation that the vaccine “is short-lived” is supported by the ACCC’s expert evidence. The Respondents rely, in particular, upon evidence as to the need to adhere to the primary vaccination schedule for infants and young children to obtain protection, and for booster shots, given that the effectiveness of the vaccine “wanes” over time.
209 I reject that submission. The term “waning” is used in this context to refer to the diminishing level of antibodies against the disease over time. As Dr Wood explained, “…in general immunity is measured by presence of antibodies to pertussis on a blood sample from an individual. Over time antibodies acquired from either natural infection of vaccination wane and therefore previously protected individuals can become susceptible again.” However, the Vaccine accommodates “waning” in particular by:
(a) providing a schedule to protect against loss of immunity over time, particularly in the case of those most at risk (see above at [56]-[62]);
(b) recommending immunisation for those in respect of whom the vaccination may have waned who are likely, if they contract the disease, to transfer it to those most at risk of suffering the disease in a severe or life-threatening form (e.g. a mother to an infant), i.e. “cocooning” or prenatal transfer of antibodies (see above at [60]-[61]); and
(c) at a population level, through achieving high levels of vaccination so as to afford so-called “herd immunity” (see above at [198]-[196]).
210 To refer simply to the Vaccine as “short-lived”, therefore, ignores the NIP that is an integral part of the Vaccine, including through pursuing cocooning and herd immunity strategies. It follows, in my view, that it is misleading to suggest that the duration of protection afforded can accurately be assessed by considering the level of protection afforded by the Vaccine apart from the schedule for its administration and strategies and programs intended to address the phenomenon of waning. Yet this is the impression given by the unqualified statement that protection from the current vaccine is short-lived. By analogy, it would be misleading to suggest that a medication prescribed for treating a disease was ineffective or short-lived because it required multiple doses over a period of time.
211 Finally, while Dr Donohoe (called by the Respondents) gave evidence that “the immunity provided by the vaccine wanes more quickly than was previously thought”, I give that opinion no weight as, without any further elaboration, the opinion is too vague to be of assistance and Dr Donohoe did not disclose the basis of the opinion.
7.5 The contention that the representations are not misleading or deceptive if read in the context of an epidemic
7.5.1 The Respondents’ submission
212 The Respondents also contend that “[t]he evidence does not show that ‘short-lived protection from the vaccine is an inaccurate description of the problems faced during the epidemic, the period within which the [First Whooping Cough] article was published” and that “[d]uring the epidemic the vaccine was ‘short-lived’ and ‘unreliable’” among children aged between eighteen months to four years. Representations that protection from the vaccine is or appears to be “short-lived” and “unreliable” are made only in the First and Second Whooping Cough Articles. As such, the submission would seem to relate only to the First and Second Vaccination Representations.
213 The Respondents further submit that “[t]he evidence led in this proceeding actually substantiates the comment that the vaccine cannot be relied upon to prevent infection from whooping cough, especially during an epidemic.”
214 In effect, the submission is that the Vaccine Representations are not misleading or deceptive in the context of a whooping cough epidemic. The Respondents rely in this regard upon the evidence of Dr Wood that Australia has experienced a recent rise in pertussis incidence, as measured by data published on the Australian National Notifiable Diseases Surveillance System (“NNDSS”).
7.5.2 The evidence of an epidemic
215 The evidence established a high rate of notifications of the disease in Australia in or about 2008-2010 for infants and toddlers. With respect to the high rate of notifications noted in Australia in 2009-2010, the Australian Immunisation Handbook explained that: “…the highest notification rates in Australia were in children <10 years of age; the proportion of notifications in adults >20 years of age decreased to 57%. The greatest increase in notification rates occurred in 3-year-old children.” Based upon the NNDS data, Dr Wood observed that there had been a “recent rise in pertussis incidence” and “considerable rises in notifications” in children aged under 4 months and those aged 18 months to 4 years, in particular in 2008-2009, and that this is so notwithstanding a high degree of compliance with the NIP in the Australian community. The spike in notifications in 2008-2009 and earlier spikes are depicted in the graphs reproduced in Dr Wood’s report:
216 A similar spike in pertussis notifications in Australia since 2008, including in Victoria, was noted by Dr Crawford. Dr Crawford also observed that, while the number of pertussis cases started to decrease in mid-2012, high numbers of cases continued to be notified in Australia and internationally.
217 Increases in the incidence of pertussis of this kind in part reflect a recurring pattern with respect to whooping cough. As the Australian Immunisation Handbook states at [4.12.3], “[d]espite a long outstanding immunization program, pertussis remains wholly present in Australia and the least well-controlled of all vaccine – preventable diseases. Epidemics occur every 3 to 4 years.” Similarly, Dr Crawford explained that:
… pertussis often will increase. Every 3 to 4 years you will have a bit of an increase. The exact mechanism is not fully understood but it is a circulating bacteria that’s hard to eradicate and when it does start, it then does spread quite quickly. So if you look at a graph over history over time, there will be, every three to four years, a bit of an increase in pertussis cases seen in both Australia and also internationally. That’s sort of the nature of pertussis as a disease…
218 In this regard it will be recalled that the disease is highly infectious and a person contracting the disease remains infectious for at least 21 days, and possibly up to six weeks, if untreated: see at [40]-[42] above. Added to this, adults in particular may present with mild symptoms only as a consequence of which the disease may not be diagnosed or only diagnosed late in the progress of the disease. As Dr Crawford explained, a person with whooping cough may present, for example, only with a chronic cough as a result of which they seek assistance from their general practitioner only after the cough has persisted for some time.
7.5.3 Were the Vaccine representations misleading or deceptive if read in the context of the epidemic?
219 The first difficulty with the Respondents’ submissions is that neither the First nor the Second Vaccine Representations are limited to the context of an epidemic or to any specific age group. As such, the submission that the representations that the Vaccine is short-lived and unreliable should be read in this context fails to grapple with the fact that the reader is simply not informed of the limited context by reference to which it is now said that the representations should be understood.
220 In any event, even if the representations that the Vaccine is short-lived and unreliable are sought to be justified by reference to the existence of the epidemic (which seems to be the only way in which to make sense of the Respondents’ submission), they remain in my view misleading, deceptive and false.
221 First, even during the epidemic, the Vaccine has been effective in significantly reducing the incidence and severity of the disease, including the instances of mortality. As the Australian Immunisation Handbook states:
In unvaccinated populations, these outbreaks can be very large. In vaccinated populations, outbreaks are smaller, with greatly reduced mortality and morbidity, and may continue to occur every 3 to 4 years or be more widely spaced.
222 Thus notwithstanding the high rate of notifications in Australia in 2009-2010, the authors of the Australian Immunisation Handbook considered that:
In contrast to notifications, hospitalisation and death rates from pertussis in the most recent epidemic period has not increased substantially. A high proportion of hospitalisations, and almost all deaths, attributed to pertussis occur in infants too young to have received more than 1 dose of pertussis-containing vaccine.
223 The Respondents’ evidence was consistent with these views. I have already referred to the evidence of Dr Donohoe accepting the effectiveness of the Vaccine to protect the very young against whooping cough and against more severe forms of the disease, if contracted, when the Vaccine has been administered to them in accordance with the NIP schedule: see above at [171], [180] and [197]. Dr Donohoe also accepted generally that the course and severity of the illness is significantly altered by prior pertussis vaccination and/or prior infection with pertussis and, in either case, the severity is usually less than it would have been no prior exposure to the pertussis antigens. Consistently with this, he considered that complications arising from pertussis following vaccination would be expected to be less than complications arising from a first infection in an unvaccinated person. He did not differentiate in his evidence between periods in which there was an epidemic and other periods.
224 The impact of high rates of pertussis immunisation was also illustrated by Professor Phelps’ unchallenged evidence of reduced incidence of the disease in areas of high vaccination, namely that:
Epidemiological studies have established the effectiveness of pertussis vaccination programs, which rely on high uptakes of the vaccine to increase herd immunity. The most recent of these has recently been published in Pediatrics journal. The investigation into California’s 2010 pertussis outbreak, the state’s most severe in more than 60 years, shows a direct overlap between the geographical distribution of conscientious objectors and the spread of pertussis. After mapping the two, researchers concluded that clusters with high conscientious objection rates were 2.5 times more likely to be hit by pertussis than other areas. Individuals living in such a cluster had a 20% higher incidence than those living elsewhere. This is clear epidemiological demonstration of the success of high rates of pertussis immunization.
(references omitted)
225 I have already referred to evidence establishing that the case-control-level methodology, such as that applied in this example, provides a strong means by which to measure the effectiveness of a vaccine (see above at [193]).
226 Secondly, the reasons underlying the spike in notifications and pertussis incidence are multifactorial and only partially attributable to an increase in the number of cases of whooping cough. Thus, the Australian Immunisation Handbook considered that increased and more sensitive diagnostic testing using polymerase chain reaction (“PCR”) has contributed to the rise in notifications in 2009/2010. Dr Crawford expressed like views. In this regard, Dr Crawford explained that the use of PCR laboratory testing for whooping cough (which requires a sample to be taken within three weeks of the illness onset) is the more rapid test and the “gold standard”, as opposed to the serology for whooping cough used a cellular in the 1990s. The latter involved taking a blood sample and testing for the antibody against whooping cough in the blood with the results being problematic and hard to interpret. Dr Crawford also referred to increased testing for pertussis due to increased health professional awareness as contributing to the increase in notifications. Similarly Dr Wood considered that improved diagnostic techniques and reporting have made a major contribution to increased notification rates for pertussis. In his view, this is likely to be associated with a marked increase in the volume of tests performed primarily by laboratory method, with public funding in Australia for public laboratories to test specimens for pertussis under the Medicare Benefits Schedule having commenced in November 2005. He further considered that increased press reports and scientific literature on the “resurgence of pertussis” may have also increased clinician awareness and reporting, as well as the availability of laboratory based tests as a diagnostic tool.
227 Thirdly, the evidence established that the increased notifications are likely to be attributable also to an increase in the actual incidence of the disease. This accords with the typical pattern of the disease at a population level which is being observed internationally to which reference has already been made. There were a number of possible factors at play for the increase in the number of cases, including:
a) waning immunity following infection and vaccination: The Australian Immunisation Handbook observed that vaccine effectiveness among 3-year-old has been estimated at around 60%, consistent with waning of immunity following the primary DTPa course. Dr Wood also gave evidence that waning immunity following infant vaccination, was a contributing factor with estimates of the duration of protection following acellular pertussis vaccination being approximately 5-6 years. Furthermore, in his view, recent increased rates of pertussis in children aged 18 months to 4 years may be related to waning antibody levels in the second year of life following the removal of the Vaccine given at 18 months from the Australian vaccination schedule in 2003.
b) incomplete protection from vaccination: Consistent with the views expressed in the Australian Immunisation Handbook, Dr Wood also pointed to the fact that over half of the pertussis cases between 1994 to 2009 occurred in infants under 3 months, with infants under 4 months being too young to have received the two or more doses of the Vaccine needed for protection.
c) infection source for infants: Conversely, Dr Wood pointed to the fact that adults, particularly parents, and siblings of infants, may become infected because of waning vaccine or disease induced immunity, and thereby act as reservoirs for infection and transmit the disease to unvaccinated or partially vaccinated infants. This is potentially compounded by what Dr Crawford described as the “non-specific nature of the illness (prolonged cough) in older children, adolescents and adults”. In other words, persons infected with the disease in this category may be unaware that they have whooping cough but still highly infectious.
d) strain polymorphism: Finally Dr Wood explained that it has been suggested that Bordetella pertussis has adapted to express pertussis toxin and pertactin strains distinct from the vaccine strains. In other words, as Dr Wood explained in his evidence, while the Vaccine is intended to cover all of the different strains of the pertussis bacteria, each time the bacteria replicate, they can mutate or change raising the possibility of a strain that can evade vaccine-induced immunity and thereby become the dominant strain.
228 Aside from the last of these factors, administration of the Vaccine in accordance with the NIP schedule, including ensuring vaccination at levels to provide herd immunity and following the cocooning strategy, provides means by which these contributing factors can be addressed, as well as the kinds of practical measures referred to by Dr Donohoe to ensure early diagnosis with appropriate responses to minimise risk of infection. Thus, Dr Crawford explained that the public health response of most state health departments to this period of high disease burden of pertussis was to take steps to minimise the risk of infants contracting whooping cough. Specifically, he explained that:
On 15 June 2009, in response to the epidemic, the Victorian Government Department of Health introduced a time-limited free vaccination program for parents of new babies (the so called ‘cocooning’ strategy). All other states and territories (with the exception of Tasmania) implemented similar programs to parents of young children. This was administered to mothers as soon as possible after birth and to fathers during the pregnancy. Some states, such as the NSW, also funded the vaccine for grandparents.
229 The majority of states, including Victoria, ceased the program in 2012 as the number of whooping cough cases was decreasing.
230 The Vaccine also continues to be kept under review and updated by the Cochrane Review which may lead to adjustments to the NIP if considered likely to increase protection in light of further evidence. In this regard, Dr Crawford referred to current studies reviewing the impact that the state-funded parental programs had on the whooping cough epidemic.
231 As to the last factor in paragraph [227](d), despite evidence of polymorphism for pertussis toxin and pertactin in several countries, there was no challenge to Dr Wood’s evidence that no direct link to vaccination programs or efficacy has been proved. Similarly, Dr Crawford observed that “[s]ome changes to the pertussis strains [Octavia et al. Newly Emerging Clones of Bordetella pertussis Carrying pm2 and ptxP3 Alleles implicated in Australian Pertussis Epidemic in 2008-2010. J Infect Dis. 2012; 205(8): 1220-4.] have been detected in small numbers of samples and it’s role in the epidemic requires further research.”
232 It follows in light of these explanations, in my view, that it affords no answer to the misleading and deceptive nature of the Vaccine representations to point to the existence of the spike in notifications in 2008, 2009 and 2010. The Respondents seek, in effect, to rely upon the fact of the increase in incidence and notifications of the disease out of context, i.e., apart from the explanations, for the increase, divorced from the strategies that exist to respond to the increased incidence of the disease, and ignoring the significant impact that the Vaccine has had to reduce the incidence and severity of the disease during the epidemic in 2008, 2009 and 2010 and generally. When those matters are taken into account, in my view the evidence of the existence of the epidemic does not dispel the misleading, deceptive and false character of the Vaccine Representations.
7.5.4 The evidence relied upon by the Respondents as the basis for the Three Articles
233 Finally, none of the material on which Mrs Sheffield purported to rely provides any credible basis for the Vaccine Representations.
7.5.4.1 The First Whooping Cough Article
234 First, with respect to the First Whooping Cough Article, Mrs Sheffield explained that the article entitled “Pertussis: The Fear Factor” by Meryl Dorey (“the Fear Factor article”) which had appeared in the magazine Living Wisdom, “formed part of the basis my thinking when drafting the First Whooping Cough commentary [i.e., the First Whooping Cough Article]”. A hyperlink to that article was provided at the base of the First Whooping Cough Article under the heading “Further Reading”. Mrs Sheffield explained that:
Table 4 of the [Fear Factor] Article concerned me because it showed that prior to mass vaccination against whooping cough children were catching pertussis when they were older. The statistics in the article show that in Poland and the United States it was after mass vaccination started that children were getting whooping cough more often at an earlier age (infancy) which I know is the danger period for this infection.” (the text in italics was admitted for non-hearsay purposes only)
235 However, as Mrs Sheffield rightly conceded in cross-examination, the data in table 4 of the Fear Factor article said nothing about either the total number of cases of children getting whooping cough or the frequency of those cases. It purported to set out only the “percentage distribution of pertussis cases” among children of different age groups before and after widespread use of the vaccine in Poland in 1971 and 1991 and in the United States in 1918-1921 and 1980-1989. As such, it did not support the statement that children were getting whooping cough more often in infancy after the introduction of mass vaccination. Nor, as Mrs Sheffield properly conceded in cross-examination, did table 4 say anything about whether administration of the vaccine has modified the effects of the disease within any particular age group. Moreover when asked whether she had informed herself in drafting the First Whooping Cough Article as to the kinds of whooping cough vaccine administered in the US and Poland in the periods to which the data in table 4 related, Mrs Sheffield answered “Not especially because when I was reading that article, I was thinking it was in some way similar to what I was seeing happening in Australia”. Her answer demonstrates a preparedness to make assumptions without undertaking even basic inquiries or research.
236 Similarly, Mrs Sheffield gave evidence that “[i]n the First Whooping Cough commentary I said ‘side effects are common’ when discussing the vaccine. This conclusion sums up the information under the heading ‘A long history of side effects’ in the Fear Factor article.” However, as Mrs Sheffield conceded in cross-examination, the Fear Factor article does not state the actual occurrence of side effects from the use of the acellular Vaccine. It purports to reproduce only the list of potential side effects which are noted as a warning on the medication by the manufacturer. As such, the statement by Mrs Sheffield that “side effects are common” did not in fact sum up the information under the heading in the Fear Factor article.
7.5.4.2 The Second Whooping Cough Article
237 With respect to the Second Whooping Cough Article, Mrs Sheffield initially stated that she had undertaken what she described as “extensive reading and research” between uploading the First and Second Whooping Cough Articles. However, far from being an expert, she was not, even on her own admission, across the research or detail on vaccination.
238 Moreover, material relied upon by her was not accurately reflected in the article. In this regard, it will be recalled that the Second Whooping Cough article contained links under the heading “Further Reading” to two further articles, including the 2009 Emerging Infectious Diseases article: see at [89] above. Mrs Sheffield relied on the 2009 Emerging Infectious Diseases article as part of her “fact-checking” before uploading the Second Whooping Cough Article. Nonetheless, she properly accepted in cross-examination that the Second Article did not reflect the authors’ conclusion that their results suggest that “an effective way to control pertussis is the improvement of current vaccines” in the manner suggested.
239 Mrs Sheffield’s thinking on vaccine efficacy in preparing the Second Whooping Cough Article was particularly influenced by an ABC News radio story to which she listened in the months after the First Whooping Cough Article. She said that she remembered the story well because “a doctor from Westmead Children’s Hospital was asserting the same things in her interviews with the ABC journalist as I had been asserting in my original article which the ACCC had requested I take down.” However, while the doctor from Westmead Children’s Hospital referred to an increase in cases of whooping cough in Australia and to the possibility that one reason for that may be a new strain which is less affected by the Vaccine, she also said that “[t]here’s no doubt that the current vaccines have a significant protective effect.” As Mrs Sheffield acknowledged, that was not reflected in the Second Article. Indeed it is directly contrary to the Second Vaccine Representation. Similarly, in the same interview another doctor from New South Wales Health said that “… the vaccine, which has been around since the 50s, has continually improved in the last several years to make it more effective. And so while it’s not a perfect vaccine it has been effective in avoiding death from this nasty childhood disease.” Again, that statement found no reflection in the Second Whooping Cough Article, which Mrs Sheffield sought to explain simply by asserting that the statement was inaccurate.
240 In addition to relying upon these articles and the ABC News radio story, Mrs Sheffield read a number of other articles, referring in her evidence to seventeen by their title only as a sample of the kind of commentary read by her. While the Respondents tendered brief notes prepared by Mrs Sheffield in the course of her research for the revised article, I cannot afford any weight to Mrs Sheffield’s notes. The material to which the notes related was not tendered in evidence by the Respondents save for those referred to at the end of the Second Whooping Cough Article. Nor was any attempt made to demonstrate the standing of the authors or of the publication in which the articles appeared
7.5.4.3 The Government Article
241 Finally, Mrs Sheffield prepared, circulated as a newsletter alert, and uploaded, the Government Article to the Website on 3 February 2012 as a consequence of reading an article by Greg Beattie entitled “Whooping cough in Australian children – how many were vaccinated?” (the “Beattie article”) on the same day. She described the articles as “a statistical analysis of whooping cough notifications”, and said that it informed her drafting of the Government Article. The article was admitted subject to an order under s 136 of the Evidence Act limiting its use to non-hearsay purposes. The important point for present purposes is that the Beattie Article purported to analyse data obtained from the Department of Health and Aging which was reproduced in the Article as follows:
242 As Mrs Sheffield accepted, the caveat that “[f]ully and partially vaccinated are for age and do not necessarily mean the person is fully vaccinated according to the NIP” means that cases discussed in the table may be cases where the individual has not fully complied with the timing in schedule for the NIP and that that failure may affect the effectiveness of the vaccine for the individual. Yet no reference is made to any such caveat by Mrs Sheffield in the Government Article.
243 Furthermore, Mrs Sheffield said that one of the reasons why she said at the end of the article that “[i]n the absence of an effective vaccine for this dangerous disease, it is also wise to know about homeopathy and whooping cough” was that she believed that those under 6 months of age were at the highest risk of dying from the infection and it was impossible for children under 8 weeks of age to be vaccinated so that these children were, in her view “left vulnerable and exposed.” However, that view takes no account of herd immunity, cocooning strategies or pre-natal transfer of antibodies from an immunised mother: see at [195]-[198] above.
7.5.4.4 Conclusions on the material relied upon by Mrs Sheffield in support of the Three Articles
244 It is apparent for the reasons set out above that Mrs Sheffield’s evidence fell well short of providing any credible basis for the Vaccine Representations. The evidence also shows that Mrs Sheffield was selective in her use of some of the material which she identified as forming part of the basis of her thinking in writing the Three Articles, with only those aspects of the material in question which were “anti-vaccine”, that is, consistent with the Vaccine Representations, being reflected in the three articles.
245 In reaching this view, while intention is not a necessary element of the causes of action created by ss 18 and 29 of the ACL, I do not find that she intended to mislead or deceive by making the Vaccine Representations Rather, I consider that her admission that she is a “passionate advocate of homeoprophylaxis” and that this passion leads her to advocate against vaccination because she believes homeoprophylaxis is the better approach, are the reason why she consciously or subconsciously drew only upon those aspects of the material she read which supported her views. Added to this, she lacked any relevant expertise in vaccines, medicine and science. That lack of expertise, which is evident in her misconstruction of some of the material she read and in assumptions that she was prepared to make, demonstrates all too clearly the accuracy of her frank admission that:
I often tell other people that I am not an authority on all of the problems with vaccines; that’s not my area of expertise. I focus on homeoprophylaxis. When I have been approached by, let’s say, anti-vaccination groups, that’s what I always tell them, that if they want my input I can provide input in the area of homeoprophylaxis, but don’t expect it on the area of vaccines because that’s not my – while I am aware of the concerns, and I have some concerns as well, I am not into all of the research or the detail there. I read the literature but I don’t store it away and catalogue it. I read it enough to think, “Yes, I think homeoprophylaxis is a fine alternative.”
(emphasis added)
246 Mrs Sheffield’s evidence that she was incredibly busy at the time of preparing both the First and Second Whooping Cough Articles and wanted to get the information out quickly tends to suggest that she was rushed, and may also explain in part the erroneous assumptions made by her in her consideration of some of the material on which she relied.
247 However, the subject matter of the Vaccine Representations is a serious matter, and the publication of false, misleading and deceptive representations about their effectiveness has potentially very serious and dangerous consequences for those who may follow that advice and their families. These matters call for great care before representations are made to the general public about such matters where the representations imply, as here, that there is an adequate basis for the representations in medical science.
7.6 Is the Homeopathy Alternative Reasonable Basis representation misleading, deceptive or false?
7.6.1 The First and Second Whooping Cough Articles imply there is reasonable basis in medical science for the representation
248 It follows from my earlier analysis of the Homeopathy Alternative Reasonable Basis representations in the First and Second Whooping Cough Articles that the ACCC correctly submitted that, read in context, they imply that there is “one free-market paradigm within which both the vaccine and the alternative homeopathic prophylaxis can be assessed and compared,” at least insofar as the representations are concerned, and that homeopathy provides a safe and effective alternative: see above at [143]-[151] and [162] respectively. Equally, I have found that, when the Government Article is read together with the Second Whooping Cough Article, the impression is that homeopathy provides an effective and safe alternative to the Vaccine in medical science: see above at [165-166] above.
249 The position is therefore analogous to that in Noone v Operation Smile (Australia) Inc. (2012) VSCA 91 (“Noone”). In Noone, the Respondents operated a complementary medicine centre specialising in cancer treatment which contained statements regarding the efficacy of treatments offered by the clinic. The Director of Consumer Affairs (Victoria) alleged that, by making those statements, the Respondents had engaged in misleading or deceptive conduct in trade or commerce contrary to s 9(1) of the Fair Trading Act 1999 (Vic), namely, that they had falsely represented that the treatments in question were effective in treating cancer and secondly that they had scientific support. Nettle JA (with whose orders Warren CJ and Cavanough AJA relevantly agreed at [34]) found that, notwithstanding that parts of the Website in question distinguish between conventional medicine and the so-called “complementary therapies” offered, “the net effect of the first impugned statement [namely that the Hope Clinic ‘provides peer-reviewed and published methods for cancer treatment…’] in the context in which it appeared, was in my view plainly to imply that the methods of treatment offered by Hope Clinic were just as scientifically based and rigorously tested as those of conventional medicine.” (at [58])
7.6.2 Quality of evidence required
250 Given that the Homeopathy Alternative Reasonable Basis representations represent that there is a reasonable basis in medical science for the use of homeopathy as a safe and effective alternative to the Vaccine, I consider that evidence demonstrating a similar or better impact on reducing the incidence and severity of whooping cough would be necessary to support the efficacy of homeoprophylaxis as an alternative to the Vaccine, in line with the evidence of Professor Phelps. Further, that evidence would have to be evidence of the same quality as that supporting the efficacy of the Vaccine. This follows logically from the fact that whooping cough is a potentially life-threatening disease, particularly in infants and the elderly, for which an effective Vaccine is already in existence and used in Australia and internationally.
251 As Professor Phelps explained:
The current conventional pertussis vaccine has substantial population studies to show that children and adults who are protected by conventional pertussis vaccination have a far less likelihood of getting the disease. Given that it’s not a perfect 100 per cent prevention measure – it’s in the order of 80 to 90 per cent prevention – then the people who have been vaccinated have less severe disease, and I think we have to bear in mind that pertussis is a potentially life-threatening disease, particularly in babies and in older people. And we have population studies to demonstrate that you have less pertussis and it is less severe when substantial numbers of people in the population are vaccination (sic) and in individuals who have been vaccinated. On the contrary, when it comes to so-called homeopathic vaccination – in fact, I would dispute that there even is such a thing – there are no studies of efficacy in individuals or in populations of pertussis prevention using homeopathic remedies of any sort.
And what level of evidence do you believe such studies would have to reach in order to support a claim for the use of homeoprophylaxis for the prevention of whooping [cough]? What level of evidence? ---It depends on who you’re trying to convince. I think if you have a properly conducted trial, it would be difficult – you would need to prove that homeopathic vaccination in a population was able to have a similar impact on reducing the incidence and severity of pertussis. In doing that, you would need to find a population which didn’t have access to conventional immunisation but we do have proof that pertussis vaccination in conventional immunisation is effective. There is no proof at all that homeopathic remedies of any sort will have any impact on reducing the incidence or severity of pertussis.
7.6.3 No published evidence within accepted levels to support the alleged effectiveness of homeoprophylaxis to treat whooping cough
252 There is, however, no published evidence within the accepted levels of evidence based medicine to support the submission that homeopathic treatments are effective in preventing whooping cough. The only scientifically proven intervention for preventing whooping cough is immunisation by administration of a pertussis containing vaccine in accordance with the NIP or similar schedule. Indeed, no evidence before the Court supported the contention that any homeoprophylaxis specifically directed to the prevention of contraction of whooping cough has been subjected to any rigorous scientific testing. To the contrary, the evidence of Dr Wood, Professor Phelps and Dr Crawford established that no credible evidence supported the efficacy or effectiveness of homeopathic treatments for the prevention of whooping cough.
253 First, Dr Wood undertook a search of Embase and Medline, to identify any academic publications relating to the safety and effectiveness of pertussis homeoprophylaxis. Embase and MEDLINE are databases recognised and used by medical practitioners in Australia. MEDLINE is a United States government supported search engine of peer-reviewed journals of international repute in medicine, health and allied areas. Dr Crawford, who also undertook searches on Medline, considered that any scientific research being undertaken internationally would use MEDLINE is one of its main scientific research data bases.
254 Professor Phelps explained the significance attributed by practitioners operating in a scientifically-based manner of the results of appropriately conducted scientific trials being published in peer review journals, namely:
… the scientific journals where information is published is the dissemination of findings, the sharing of scientific findings based on properly conducted trials. Now, the significance of review by appropriately qualified professionals or peers is that you get independent eyes looking at the methods to make sure that the methods are robust; you get independent eyes looking at the conclusions to make sure that based on the method and the results that the conclusions can be drawn. And it’s important that you have that independence because you might pick up flaws, for example, in the robustness or the methodology that was used, and until you have a peer-review process then you can’t really be confident that, I guess, all of the glitches have been worked out of the research that is being done and questions need to be asked. And quite often a paper will be submitted to a journal and peer reviewed and then sent back to the original authors for clarification or for correction, and it’s a checks and balances process to ensure that material that is published has passed through independent eyes.”
255 Not surprisingly, therefore, the quality of the peer review undertaken by particular journal is one of the factors contributing to the regard in which a particular journal is held by the scientific community.
256 Dr Wood searched the databases using a variety of terms, including specific terms used by Homeopathy Plus, such as Drosera and Pertussinum. Medline was searched for articles relating to the pertussis homeoprophylaxis from 1946 until April (week 4) 2013, while Embase was searched for similar articles between 1974 and 1 May 2013. On the basis of the abstracts of articles located in the database searches, Dr Wood sought the full text of those articles which he considered may be useful on the question of the effectiveness of pertussis prophylaxis. He found a total of 20 articles as a result of the searches on Medline and 57 on Embase. Those literature searches failed to identify any level 1 or 2 studies comparing prophylaxis with placebo in a blinded manner.
257 Otherwise, Dr Wood located one abstract from the European Journal of Integrative Medicine (2012) that described a “single blinded clinical trial” of 112 children aged one to two months of age, divided into two groups of 61 participants each. The study was said to show that among a group prescribed homeopathic remedies, 22% of patients developed milder forms of certain diseases including measles, chicken pox, rubella, mumps and whooping cough while 84% of the allopathic group (i.e.: those administered a vaccine by injection) was said to have developed strong and/or severe forms of these diseases. However Dr Wood was unable to locate the complete paper as it was a publication from a conference. His evidence that it was a difficult abstract to understand and that he did not believe it presented convincing evidence of the effectiveness of pertussis homeoprophylaxis was unchallenged.
258 Dr Wood also located an article entitled, “Pertussin 30mpreventive for whooping cough” by J.M. English and published in the British Homeopathic Journal in 1987 (“English article”). He considered that the article was “inconclusive” on the use of pertussin 30 as a possible preventive for whooping cough. As the abstract of the article stated, “[i]t was recognized that neither the numbers involved, nor the method followed would of themselves be sufficient to prove efficacy, but it was hoped that a positive result would permit the conclusion to be drawn that a further study would be in the public interest.” This is consistent with the views expressed in the editorial on the article published in the same volume (exhibited to Dr Wood’s affidavit) which stated that:
It is far from clear whence the idea that Pertussin might be effective in the prophylaxis of whooping cough emanated; certainly no such claim is made in any of the main homeopathic material medicas.
…in this issue we publish the results of a survey by Dr John English on the effect of Pertussin 30 in whooping cough prophylaxis. Without in any way detracting from Dr English’s most important and timely effort, it is unfortunately true that the only firm conclusion that can be drawn from his study is that the resources available to him were not sufficient to allow any definite judgement on the efficacy or otherwise of Pertussin in whooping cough prophylaxis.
259 Dr Wood’s opinion regarding the lack of weight that could be given to these articles was not challenged. While Dr Golden referred to the English article in an appendix to his report, he did not annex it unlike other articles to which he referred. He accepted in cross-examination that he did not mention in his report any of the limitations or deficiencies in the methodology which qualified the reliability of the study as described by the author of the study in the article.
260 Furthermore, Dr Wood found no articles relating to the use of Drosera or Pertussinum in the prevention or treatment of pertussis in Medline Embase save for one mentioning “sundew” (drosera spp) as a treatment for pertussis. However, it lent no support to the Respondents’ case as it noted only that there is very little “clinical research on treatments for whooping cough and, as might be expected, published research on the botanical remedies for pertussis is lacking”.
261 Finally, Dr Wood searched the journal Homeopathy using the search term “pertussis” and found no articles published in the last fifteen years that specifically mentioned trials investigating pertussis prevention. He located, however, an article published in 1987 which mentioned the use of Pertussin and noted that claims that it was safe and effective for the prevention of pertussis are “unsupported”.
262 On the basis of his research, Dr Wood therefore did not consider that there was a reasonable basis in medical science to state that homeopathic treatments, including Drosera rotundiflora and pertussinum, are effective alternatives to the Vaccine.
263 Searches undertaken by Dr Crawford led him to the same view. He undertook a literature review of Medline for the period 1996-2013 regarding homeopathy and whooping cough, utilising appropriate search terms. He identified a total of forty-two studies, none of which had any evidence to support the statement that ‘homeopathy is a safe and effective alternative’ to the Vaccine. Nor did the systematic literature review of complementary medicine used in children by Hunt & Ernst et al. (Hunt K, Ernst E, The evidence base for complementary medicine in children: a critical overview of systematic reviews. Arch Dis Child. 2011; 96(8);796-76) cited by Dr Crawford find any evidence supportive of homeopathy in the management of illnesses such as pertussis. Dr Crawford concluded that in his opinion there is no reasonable basis in medical science to State that homeopathic treatments are safe and effective as an alternative to the Vaccine.
7.6.4 No support for the use of homeoprophylaxis to prevent whooping cough by peak medical and homeopathic bodies
264 In line with the lack of published evidence supporting the asserted effectiveness of homeopathic treatments for whooping cough, the consensus based on medical science is that homeopathic treatments are not an effective alternative to the whooping cough vaccine for the prevention of whooping cough.
265 This is the opinion of the Australian Medical Association. It is also the view expressed in the 2013 edition of the Australian Immunisation Handbook which states that “[h]omeopathic ‘immunisation’ has not been proved to give protection against infectious diseases; only conventional vaccination produces a measurable immune response.” Similarly, the Victorian Government has advised that: “[h]omeopathic immunisation is not a recognised form of immunisation and is not acceptable under the legislation, therefore cannot be listed on an immunisation status certificate.”
266 Nor do peak bodies for homeopathy recommend homeoprophylaxis as an alternative for the vaccination. The Australian Register of Homeopaths (“ARH”) has acknowledged that: “[n]o scientifically rigorous attempt has been made to compare the effects and effectiveness of HP (homeoprophylaxis) with those of immunization” and that patients should “understand that evidence for the effectiveness of HP is limited and is not accepted by public health authorities”. The ARH also advised in a statement given in August 2010 that “homeoprophylaxis should not be recommended as a substitute for immunisation”. Advice to the same effect from the Australian Register of Homeopaths is set out in a fact sheet published by the National Centre for Immunisation Research and Surveillance (“NCIRS”).
267 Similarly, the British Homeopathic Association recommends that people receive conventional immunisation and that homeopathic preparations “should not be recommended as a substitute for [conventional] immunization.” In cognate terms, the Council of the Faculty of Homeopathy, London, issued a statement in 1993 advising that it “…strongly supports the conventional vaccination program and has stated that vaccination should be carried out in the normal way, using the conventional tested and proved vaccines, in the absence of medical contraindications.”
268 In short, the only treatment currently approved for use and accepted by medical practitioners in Australia for the prevention of whooping cough is the combined pertussis vaccine. There is no evidence of any support even among peak homeopathic associations for the use of homeopathic treatments as an alternative to the Vaccine, nor any published literature that supports the efficacy or effectiveness of homeopathic treatments as an alternative to the Vaccine.
7.6.5 The leap of logic required by the “principle of similars”
269 Finally, Dr Golden described that the principles and practice of homeopathy encompassed prevention and treatment, and that the “principle of similars”, one of two fundamental tenets of homeopathy, could be stated in the following terms:
Treatment: A substance which is capable of producing symptoms in a healthy person can remove similar symptoms in an unwell person….
Prevention (may be stated in two ways):
(a) A substance which is capable of producing symptoms in a healthy person similar to the characteristic symptoms of an infectious disease, is capable of preventing symptoms similar to those characteristic symptoms in a previously unprotected person...
(b) A substance which is capable of removing the characteristic symptoms of an infectious disease in an infected person, is capable of preventing symptoms similar to the characteristic symptoms of the disease in the previously unprotected person.
270 In short, he explained that “[o]nce the method can be shown to be effective to prevent one disease then it follows that it will be similarly effective for the prevention of other diseases (provided the fundamental principles are correctly followed in the selection and administration of remedies).”
271 Despite this evidence falling far short of complying with the rules as to expert opinion evidence as I have earlier explained at [38] above, it was admitted subject to an order under s 136 of the Evidence Act limited to a description of the philosophical approach of homoeopathy to the treatment and prevention of disease.
272 In any event, as the ACCC submitted, no evidence supported the philosophical approach of homeopathy as described by Dr Golden, that once a method has been shown to be effective to prevent one disease, it follows that it will be similarly effective for the prevention of other disease. The proposition requires a leap of logic that cannot be made. It cannot obviate the need for evidence to demonstrate the effectiveness of a treatment to prevent a specific disease – especially where the symptoms of the disease are potentially severe and even life-threatening particularly to infants and an effective vaccine already exists. As Professor Phelps explained:
… if there is a report, for example, an isolated case report of apparent efficacy in one area of therapy that you cannot then make a leap of logic or a leap of faith that proof, no matter how limited or how extensive, of efficacy of one particular substance in one particular application can, indeed, be applied to another situation or another application. So that’s the leap of logic that cannot be made unless you have specific evidence for a specific product in a specific situation
273 That “leap of logic” is, as Professor Phelps further explained, analogous to assuming that, because an antibiotic is effective against a particular infectious disease, it can be used to treat other infectious diseases notwithstanding that there was no proof as to its efficacy for the treatment of those other diseases. As Professor Phelps said:
It requires a leap of logic at every level. It requires a leap of logic if you are assuming that one way of treating a – any disease will apply to every other disease. I mean if you look at medical science, we have multiple ways of approaching multiple different pathologies, and in the practice of any discipline in health care, there are many different ways and methods of treating disease and many different approaches that might be used, and so when you talk about a method, there are different methods that apply to different situations, but in this respect I took this to mean that any one treatment or any one method or any one approach to treating disease cannot be applied without going through the steps of logic to other diseases and without providing evidence of effectiveness….. there would need to be some scientific evidence, or again some technical evidence of the reasons why that approach would be said to be efficacious.
274 This is not to suggest, as the ACCC accepted, that no aspects of, or disciplines within, complementary and alternative medicine could ever qualify their claims within the hierarchies of evidence based medicine. Certain complementary medical treatments, such as St John’s Wort, have become more accepted where they have undergone a scientific evidence-based approach and the results have been published in peer review journals of repute. As Professor Phelps explained, “...you quite often will get experiential learning [i.e. from experience or observation], case reports that are published that raises the curiosity of the scientific researchers and then they set out to prove particular applications, but you again can’t make the leap of logic that because fish oil works for secondary prevention of heart disease in a certain number of people or it reduces the risk in a certain number of people… that it will prevent pertussis, for example.”.
7.7 Did the disclaimer in the member’s terms and conditions erase the misleading nature of the representations in these Second Whooping Cough Article?
275 As I have earlier found, after 15 January 2013 the Second Whooping Cough Article was accessible to any member of the public only in the Members Only section of the Website after viewing a front page entitled “Oops! This Content is Members Only” which contained the “stand-alone disclaimer” and agreeing to stated terms and conditions: see at [97] above. For the reasons explained below, neither the stand-alone disclaimer nor the disclaimers in the stated terms and conditions affect my conclusion as to the misleading, deceptive or false conduct constituted by the making of the Vaccine Representation or the Alternative Homeopathy Reasonable Basis Representation in the Second Article.
276 No disclaimer, as the ACCC submitted, necessarily affords absolution from committing a contravention of s 18 or 29 of the ACL. As Williams and Keane JJA held in Downey v Carlton Hotels Asia Pacific Pty Ltd [2005] QCA 199 (“Downey”) at [82] (citing Bowler v Hilda Pty Ltd (1998) 80 FCR 191 at 207), it is well established that:
… exclusionary and disclaimer clauses cannot override the statutory prohibition against misleading and deceptive conduct or prevent the grant of appropriate statutory relief where loss or damage is, as a matter of fact, caused by a contravention of the statute.
277 Rather, whether a disclaimer modifies the conduct alleged to be false, misleading and deceptive is a question of fact: Butcher v Lachlan Elder Realty Pty Ltd (2004) 218 CLR 592 (“Butcher”) at 613 [72]. Thus, Gleeson CJ, Hayne and Heydon JJ accepted in Butcher at 614 [72] that the conclusion was open to Burchett J in Benlist Pty Ltd v Olivetti Australia Pty Limited [1990] ATPR ¶41-043 at 51,590 to find as a matter of fact that a disclaimer in marketing material said to be misleading and deceptive had not modified the conduct in question, even though different conclusions were open in other cases. In the passage referred to, Burchett J had said:
It has been held on many occasions that the perpetrator of misleading conduct cannot, by resorting to such a clause, evade the operation of [the Act]. Of course, if the clause actually has the effect [of] erasing whatever is misleading in the conduct, the clause will be effective, not by any independent force of its own, but by actually modifying the conduct. However, I should think it would only be in rare cases that a formal disclaimer would have that effect. ...
In the present case, the suggestion of the suitability of the building for strata title conversion might continue to influence the mind of a prospective purchaser notwithstanding his awareness of the existence of a disclaimer clause, which did not single out the particular representation, but purported to apply generally to every detail stated in the investment report. If it were permissible to avoid the operation of [the Act] by such a clause, it would be all too easy to make representations in the confidence that they would be acted upon, and then withdraw them in the confidence (equally important for the securing of the desired business) that the withdrawal would not be acted upon.
(emphasis in orginal)
278 Similarly, McHugh J in Butcher at 640 [157] (in dissent but not relevantly on the matter of principle) referred with approval to the accepted line of Federal Court authority that:
…a disclaimer is only effective if it actually modifies the impugned conduct such that the conduct as a whole may be seen as not misleading, not because the disclaimer has any independent force of its own. … A disclaimer in a promotional brochure may purport to represent that the corporation does not accept responsibility for the accuracy of the information in that brochure, but it will only be effective if it operates to modify the corporation's conduct.
279 In this regard, his Honour also observed at [158] that “[t]he case law suggests that disclaimers that appear in small print at the foot of marketing brochures are rarely effective to prevent conduct from being found to be misleading or deceptive or likely to mislead or deceive.”
280 To similar effect, Williams and Keane JJA held in Downey at [83], that “…disclaimers can be effective ‘if the clause actually has the effect of erasing whatever is misleading in the conduct’” (emphasis added; citations omitted). Their Honours continued:
It has been recognised, however, in other words, if the effect of the disclaimer is to make clear something that, if allowed to remain vague or ambiguous, could have led a person into error. Disclaimers had this effect in Butcher where it was held that the effect of reading an entire brochure, including the disclaimers, was to make it clear that the survey report included in the brochure had not been prepared by the producer of the brochure but was simply being passed on without any representations being made as to its truth or falsity. It is apparent that if a disclaimer is to function in this way it must be worded unambiguously, feature prominently and it must be communicated to the reader that the disclaimer is relevant to the information it is seeking to qualify. As Jacobson and Bennett JJ noted in National Exchange [Pty Ltd v Australian Securities and Investments Commission [2004] FCAFC 90 at [55]; (2004) 49 ACSR 369 at 381]:
"Where the disparity between the primary statement and the true position is great it is necessary for the maker of the statement to draw the attention of the reader to the true position in the clearest possible way."
(emphasis added)
281 Consistently with the clarity and prominence required of a disclaimer to be effective to dispel otherwise misleading conduct, Wilcox J in Hutchence v South Seas Bubble Pty Ltd (1986) 64 ALR 330 observed (at 338) that:
There are occasions upon which the effect of otherwise misleading or deceptive conduct may be neutralized by an appropriate disclaimer.... But such cases are likely to be comparatively rare and to be confined to situations in which the court is able to reach satisfaction — the onus resting on the party relying upon the disclaimer — that the disclaimer is likely to be seen and understood by all those — leaving aside isolated exceptions — who would otherwise be misled before they act in relation to the relevant transaction.
(emphasis added; citations omitted)
7.7.3 Neither the stand-alone disclaimer or the terms and conditions of access to the Members’ Area erase the misleading, deceptive or false representations
282 The Stand-Alone Disclaimer which appeared at the end of the page entitled “Oops! This Content is Members Only” read as follows:
Disclaimer: All information, data, and material contained, presented, or provided on the Homeopathy Plus Website, or within its communications and newsletters, has been sourced from multiple authors and is for general information and education purposes only. The information is not to be construed necessarily as the opinions of the publisher and is not intended to replace from your homeopathic or doctor, or to be a substitute for a consultation with a trusted health care provider. All remedy-related information is drawn from homeopathic pharmacopoeias and materia medicas listed by the Therapeutic Goods Administration (Australia) and referenced worldwide. Serious injury or illness should not be treated without expert advice and we recommend you always seek medical help and diagnosis where appropriate.
(emphasis added)
283 Relevantly, the terms and conditions of access to the Members’ Area included the following:
Homeopathy Plus Website Member Area Terms and Conditions
PLEASE READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE ACCESSING THE MEMBER AREA OF THIS SITE
By accessing the Member Area you signify your assent to these Terms and Conditions. If you do not agree to all of these Terms and Conditions of use, do not access the Member Area! Homeopathy Plus may revise and update these Terms and Conditions of use at any time. Your continued usage of the Homeopathy Plus Member Area will mean you accept those changes.
…
The Homeopathy Plus Member Area Does Not Provide Medical Advice
The contents of the Member Area, such as text, graphics, images, information and other material contained in the Member Area (“Content”) are for informational purposes only. The Content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read in the Homeopathy Plus Member Area! If you think you may have a medical emergency, call your country’s emergency number immediately. Reliance on any information provided in the Homeopathy Plus Member Area is solely at your own risk.
284 I do not consider that either the Stand-Alone Disclaimer or disclaimer in the terms and conditions erase the false, misleading or deceptive nature of the information contained in the Second Whooping Cough Article and thereby modifies the conduct.
285 First, neither disclaimer is proximate to the representations or articles in which they are made. They are both in the nature of generic statements that appear on a different page at the point at which a member of the public is presented with the option of signing up for access to the Members Area of the Website. As such, it cannot be said that the relevance of the disclaimers to the representations made in the Second Whooping Cough Article has been effectively communicated to the reader; nor that the disclaimers featured prominently vis-a-vis the false, misleading or deceptive statements.
286 Added to this, the terms and conditions were exceedingly lengthy. The printout of the terms and conditions from the Website spanned some 4 and a half pages in minute font at 1 and a half line spacing. Those terms and conditions addressed many topics including terms and conditions of access to the Member Area, children’s privacy, use of the content of the Member Area, the liability of Homeopathy Plus for use of the Member Area and its content, passwords, responsibility of members for blogs on community and member to member areas of the Website and Homeopathy Plus’ rights to remove or edit such communications, disclaimer of liability for content of third-party websites to which links are provided, and jurisdiction for disputes with Homeopathy Plus. It seems highly unlikely that any visitor would trawl through these merely to get access to another part of the Website for free, although perhaps the more diligent might skim briefly through them.
287 Secondly, the terms and conditions stated that the information on the Website is “for informational purposes only”. Similarly, the Stand-Alone Disclaimer stated that information on the Website “is for general information and education purposes only.” As the ACCC submitted, however, this does not modify the fact that the information advanced as to the efficacy of homeoprophylaxis to prevent whooping cough has no foundation in medical science, nor the misleading statements as to the effectiveness or lack thereof of the Vaccine. Indeed, as the ACCC also submitted, “the nature of the disclaimers tends to aggravate the conduct, by suggesting that the statements have informative or educational content.” That impression is reinforced in the case of the Stand-Alone disclaimer in stating also that “[a]ll remedy-related information is drawn from homeopathic pharmacopoeias and material medicas listed by the Therapeutic Goods Administration (Australia) and referenced worldwide”.
288 Fourthly and most significantly, it cannot be said, in line with the authorities to which I have referred, that the disclaimers clearly bring to the attention of members of the public visiting the Website and reading the Second Whooping Cough Article the true position in clear and unambiguous terms so as to erase the otherwise misleading text. As the ACCC submitted:
A disclaimer of this kind would directly qualify the otherwise misleading text. It may comprise an accurate statement concerning the nature and limitations of the evidence base for statements about the efficacy of homeoprophylaxis, a statement concerning the care that should be taken in using homeoprophylaxis for any serious or life-threatening condition such as whooping cough, or something of a similar kind. It does not comprise a standard form disclaimer of the kind included in the Website.
8. Were the representations made in trade or commerce?
8.7.1 “in trade or commerce”: general principles
289 At all material times, as the ACCC submitted, the phrase “trade or commerce” has been defined in s 4(1) of the CCA to mean as “trade or commerce within Australia or between Australia and places outside Australia”. From the time of commencement on 1 January 2011, s 2(1) of the ACL has also contained a definition of “trade or commerce” which adds to the definition in the CCA the words “and includes any business or professional activity (whether or not carried on for profit)”
290 In so providing, the definition in the ACL would appear to have expressly adopted the Full Court’s construction of the words “trade or commerce” in the Trade Practices Act 1975 (Cth) (the predecessor Act to the ACL) in Re Ku-ring-gai Co-operative Building Society (No 12) Ltd (1978) 22 ALR 621 (“Ku-ring-gai”). The Court there held that the words “trade or commerce” were words of “the widest import” and were not restricted to activities for the making of a profit. Thus, in Ku-ring-gai, the applicants did not engage in lending moneys for profit or on the open market. Rather, they performed an important social function in making loans at low interest rates to members from moneys ultimately sourced in government funds or with the help of a government guarantee. Deane J (with whom Brennan J (as his Honour then was) relevantly agreed) held at 648-9 that:
The common dominant objective of trading and commercial dealings in the market place, namely profit, is lacking from their activities... Their motive for making such loans is to benefit the members to whom they lend. If the scope of the phrase “trade or commerce” in s 47 of the Act were restricted to ordinary trading and commercial activities in open markets, there would plainly be a great deal to be said for the applicants’ submission that their lending to their members is not in such trade or commerce. The phrase cannot, however, in my view properly be regarded as so restricted.
The terms ‘trade’ and ‘commerce’ are not terms of art. They are expressions of fact and terms of common knowledge. While the particular instances that may fall within them will depend upon the varying phases of development of trade, commerce and commercial communication, the terms are clearly of the widest import… They are not restricted to dealings or communications which can properly be described as being at arm’s length in the sense that they are within open markets or between strangers or have a dominant objective of profit-making.
291 Thus, his Honour found that, notwithstanding the altruistic character of the applicants’ activities, their lending to members was in trade or commerce: at 643.
292 While the words “trade and commerce” are widely construed, the plurality (Mason CJ, Deane, Dawson and Gaudron JJ) in Concrete Constructions (NSW) Pty Ltd v Nelson (1990) 169 CLR 594 (“Concrete Constructions”) held that requirement that the conduct be “in” trade or commerce qualifies the prohibition so as to limit it to “‘the central conception’ of trade or commerce and not to the ‘immense field of activities’ in which corporations may engage in the course of, or for the purposes of, carrying on some overall trading or commercial business” (at 603). Thus, consistently with Kur-ring-gai, their Honours held at 603-604 that:
…the section was not intended to impose, by a side-wind, an overlay of Commonwealth law upon every field of legislative control into which a corporation might stray for the purposes of, or in connection with, carrying on its trading or commercial activities. What the section is concerned with is the conduct of a corporation towards persons, be they consumers or not, with whom it (or those whose interests it represents or is seeking to promote) has or may have dealings in the course of those activities or transactions which, of their nature, bear a trading or commercial character. Such conduct includes, of course, promotional activities in relation to, or for the purposes of, the supply of goods or services to actual or potential consumers, be they identified persons or merely an unidentifiable section of the public.
(emphasis added)
293 In Concrete Constructions, for example, a purely internal communication from one employee to another employee in the course of ordinary activities about the construction of a building was held not to constitute conduct “in trade or commerce”. That decision in turn was subsequently approved by five members of the High Court (Gleeson CJ, Gummow, Hayne, Heydon and Crennan JJ) in Houghton v Arms (2006) 225 CLR 553 at 565 [33].
294 As the joint judgment in Concrete Constructions accepted, the dividing line between conduct that is or is not in trade and commerce applying this narrow approach will not always be readily drawn: see also Village Building Company Ltd v Canberra International Airport Pty Ltd (2004) 139 FCR 330 (“Village Building v CIA”) at 340 [48]-[49] (the Court). Later cases assist in illuminating the circumstances which will fall on one or the other side of the line.
295 Concrete Constructions was followed in Tobacco Institute of Australia Ltd v Australian Federation of Consumer Organisations Inc (1992) 38 FCR 1 (“Tobacco Institute”). That case concerned an advertisement published in a number of Australian newspapers authorised by the appellant, the Tobacco Institute, on the subject of passive smoking, headed “A message from those who do … to those who don’t”. The primary judge held that the statement in the advertisement that “…there is little evidence and none which proves scientifically that cigarette smoke causes disease in non-smokers” was misleading and deceptive contrary to then s 52 of the Trade Practices Act 1974 (Cth). While certain aspects of the appeal by the Tobacco Institute to the Full Court were successful, importantly for present purposes the Full Court upheld the primary judge’s finding that the relevant statements in the advertisement constituted a breach of then s 52 of the TPA. As Foster J held at 25:
The material was… published extensively nation-wide. The advertisement was prominent and eye-catching and described itself as an advertisement. Even the most cursory reading of it would, in my view, have been sufficient to convey to an ordinary reader a message favourable to the consumption of cigarettes as an article of commerce. The advertisement was persuasive in tone. It sought to allay fears which it suggested were commonly and erroneously held that the inhalation of tobacco smoke in the air could be harmful. The name of the appellant, appearing as the authoriser of the advertisement, would, in my view, when coupled with its obvious message, be quite capable of conveying to such a reader that the appellant had a commercial interest in assuaging community concerns about the harmful effects of inhaling environmental tobacco smoke. The general tenor of the advertisement, its wide exposure, and the name of the appellant combined to create an irresistible impression that it was promotional material designed to advance the course of cigarette smoking and to assist in the sale of cigarettes.
296 Similarly, in all of the circumstances, Hill J rejected the appellants’ submission that publication was a contribution to a very public debate on passive smoking occurring at the time. Rather, Hill J held at 43-44 that:
The advertisement was not intended as a learned contribution to a scientific debate. It was not published in the learned scientific journal. It was directed to the public at large, smokers and non-smokers. It was couched in language designed to persuade, rather than to instruct. It seems highly unlikely that a corporation, lacking a significant commercial interest in the tobacco industry, would have gone to the expense of publishing advertisements in various newspapers of national circulation merely to influence public opinion on a debate of health policy. Rather, it may be inferred, that the corporation which placed the advertisement did so to allay the fears of those who smoked and thus discourage them from quitting and to discourage those who did not smoke from applying pressure on those who did to reduce the occasions on which they smoked, or perhaps give up altogether. In either way the advertisement on its face was designed either to promote the further sale of cigarettes or to arrest a decline in such sales.
297 By contrast, the Full Court in Village Building v CIA held that forecasts of future aircraft noise levels in the vicinity of Canberra Airport published by the Airport in the context of a public debate concerning an application to rezone land south of the Airport and under the flight path as residential, were not made in trade or commerce. The Full Court (French, Sackville and Conti JJ) held that the representations were made by the Airport to members of the public and elected councillors and parliamentarians as part of a campaign to resist the application to rezone the area in question to facilitate residential development. “There was”, the Full Court held, “… no relevant trading or commercial relationship between CIA and the persons to whom the representations were made… The representations could not be described as promotional activities designed to persuade consumers to use the services offered at Canberra Airport.” (at 342 [51]).
298 The primary judge had characterised the Airport’s campaign to revisit the rezoning application as a political activity and therefore held that it was not conduct in trade or commerce. Importantly, the Full Court held (at 342 [54]) that “[t]he two categories are not however necessarily mutually exclusive”, explaining further that:
There may be activities which are “political” in the sense that they are designed to influence public opinion or achieving a particular regulatory outcome, but which might nonetheless form part of transactions bearing a commercial or trading character (as where a public relations company makes representations to a Minister on behalf of the client). The ultimate question is whether the impugned conduct has the requisite commercial or trading character.
8.7.2 Were the vaccine representations made in trade or commerce?
299 First, the Respondents contend that Mrs Sheffield’s evidence established that the articles were not intended for advertising or marketing but had an advocacy purpose, submitting that:
Mrs Sheffield’s unambiguous and passionate evidence about the design of the representations is that they were advocating a change in Government policy and not designed to persuade consumers to use the products offered by Homeopathy Plus! Design is left to the consumer and everything to do with the intention of the advocate. Mrs Sheffield gave compelling evidence that she was supremely uninterested in any sales gain that may come of her advocacy efforts.
(emphasis in original)
300 Thus the Respondents contend that “Mrs Sheffield’s intended purpose was to achieve a different policy outcome regarding the health officials’ approach to an epidemic and the commentary was not intended to have a consequence or impact on trading or commercial activities” (emphasis in original).
301 Secondly, the Respondents contend that the design of the Website does not imply that the articles should be considered as made in trade or commerce. In this regard, the Respondents submit that:
Much was made as the side-by-side ‘shop placement’ to the remedies listed in the ‘first whooping cough article’. However it does not appear there was any deliberate design to achieve that outcome. Mrs. Sheffield has other people upload articles to the website and design it. The shop sits within an unchanging ‘frame’ which is not manipulated depending upon the article content of particular page within the website… If the applicant is right, and the presence of the shop necessarily imputes a commercial character to any article sitting within the frame that would mean any article published by ‘a sometimes online seller’ no matter how political or non-commercial, would attract the jurisdiction of the ACL. The Respondent [sic] submits that this is an untenable and impermissible application of the Concrete Constructions principles (imagine political parties whose media release attracted ACL scrutiny because the Party’s website offered badges, T-shirts and bumper stickers bearing the same logos and colour scheme within a tab proximate to the advocacy content). (emphasis in original)
302 In essence, the Respondents’ contention is that the articles should be read apart from the ‘sidebar’ which comprises a permanent frame within which variable content of the Website, including the Articles, is depicted.
303 For these reasons, the Respondents submit that the statements were not made in trade and commerce but were uploaded for information and general educational purposes and constituted participation in, and a contribution to, an ongoing public debate.
304 I reject this submission. In my view, the publication of the Three Articles and making of the Vaccine Representations and Alternative Homeopathic Reasonable Basis Representations, the Respondents engaged in misleading, deceptive or false conduct, and made false or misleading representations, “in trade or commerce” for the purposes of s 18 and 29 of the ACL.
305 First, the question of whether or not the representations were made in trade or commerce does not turn upon whether they were made for the purpose of making a profit, as the definition of “trade or commerce” in s2(1) of the ACL makes clear: see at [289]-[291] above. Equally it must follow that the question of whether or not Mrs Sheffield was interested in any sales gains as a consequence of publishing the articles cannot be determinative of this question.
306 Secondly, in line with Full Court’s decision in Village Building v CIA, the fact that an activity may be political in the sense of advocating for a change of policy (or equally, that it is educational) does not necessarily mean that the activity is not in trade or commerce. The question remains whether the impugned conduct has the requisite commercial or trading character.
307 In this regard, I accept that one of the main purposes of Homeopathy Plus is to advocate for homeopathy. Nonetheless, I do not consider for the reasons earlier given at [140]-[141] and [152] and [160] that in the First or Second Whooping Cough Articles, Mrs Sheffield was merely inviting readers to look into the subject further as a part of a wider policy debate, nor that this is what the articles conveyed. Indeed, as I explain further below, it is doubtful whether the evidence established a policy debate about homeoprophylaxis in Australia, at least as an alternative to vaccination.
308 Nonetheless, I accept that the First Whooping Cough article was published in the Treatment Room” of the Website which was intended in part to educate visitors (at [83] above). However, despite the lack of hyperlinks within the text of the article to products available from the Online Shop, I consider that advertising also had a significant role in the inclusion of information uploaded to the treatment room, including the First Whooping Cough Article (at [83]).
309 In this regard, Homeopathy Plus is engaged in the sale of homeopathic products which activity is undertaken by it via the Website: see at [77] above. Moreover, as a director of Homeopathy Plus, Mrs Sheffield has statutory and common law obligations to act in the company’s best interests, including to optimise its trading activity: see ss 180-182, Corporations Act 2001 (Cth). She also maintains the Website and uploads material to it, including authoring and uploading the Three Articles. Both Mrs Sheffield and Homeopathy Plus are participants in the relevant trade or commerce, being the supply and promotion of homeopathic products.
310 It is this context – the supply and promotion of homeopathic products – that the representations in question were made. In this regard, contrary to the Respondents’ submission, I do not consider that the constant frame within which the First Article was published can be ignored. By a click through menu to the online shop, Drosera could be purchased, being one of the two remedies expressly mentioned in the First Whooping Cough article as a remedy for prevention of whooping cough. Furthermore, as in the Tobacco Institute case, the First Article was directed to the public at large and persuasive in tone. It sought to “debunk” vaccination as an effective means of preventing whooping cough and promote homeopathic remedies including Drosera as a safe and effective alternative. It was partial and was not intended as a learned contribution to a scientific debate, nor published in a learned scientific journal. In this regard, it is simply wrong to say, as do the Respondents, that the First Whooping Cough Article (and Second Whooping Cough Article) appear to be “…an exposition on competing schools of thought regarding the best way to tackle the whooping cough epidemic (the current Governmental approach or the homeopathic approach).” Nor even, was the article published in the “political issues” section of the Website, although even if it had been, I do not consider that I would have reached any different view. Given these features, in my opinion, the tenor of the article, coupled with ready access to the online Shop via the side menu for purchase of Drosera and other remedies, create an irresistible impression that the First Whooping Cough article was promotional material designed at least in part to persuade visitors to consider purchasing Drosera and other homeopathic remedies. The statements were made as part of “the mutual communings, the negotiations verbal and by correspondence, the bargain, the transport and the delivery which comprise commercial arrangements”: Ku-Ring-Gai at 139 (Bowen CJ). As such, the representations in the First Whooping Cough Article were plainly made in trade and commerce.
311 Essentially the same features are present in the Second Whooping Cough Article and lead, in my opinion, to the same conclusion. Added to this, the banner at the top of the Website which comprised part of the constant frame within which the Second Article was published, now referred expressly to the “Online Store” and Mrs Sheffield accepted that the online store was one of the three encompassing things for the website (see at [92] above). Nor was there any reference to a separate page for “political issues” in the toolbar. Again, the fact that the article may have been intended at least in part to educate visitors does not detract in my view from the fact that the representations nonetheless bore a trading and commercial character.
312 The Respondents also sought to make much of the fact that the second article poses a question in the title “Whooping Cough – Homeopathic Prevention and Treatment?”. That makes no difference in my view as the article then purports to answer the question by advocating for homeopathic prevention and thereby promoting remedies available for sale on the Website, including Drosera which again is expressly referred to as a remedy for the treatment or prevention of whooping cough in the body of the article.
313 I also consider that the Government Article bears a trading and commercial character. While I accept that the article was in part intended to advocate for a change in the government’s approach to the epidemic, I also consider that the article promoted the online shop, as I found at [104] above. It seeks also to promote homeopathic remedies in a partial and persuasive tone by inviting the visitor to know about them, having represented that the Vaccine is largely ineffective. I do not consider that the lack of a direct link to the Second Whooping Article mentioning Drosera or to the products themselves changes the character of the Government Article. The article appears within the same frame providing click-through access to the on-line shop and other parts of the Website, including to the Second Whooping Cough Article, together with a search engine, having invited the visitor in effect to seek out more information which the frame indicates can be found within the Website: see at [167] above.
314 The fact that the First and Second Articles do not expressly advertise that the products in question are for sale in the online shop, nor that the Government Article does not expressly mention specific products, does not lead me to any different view. As, for example, Hely J observed in Wool Innovation Network Ltd v Newkirk (No 2) [2005] FCA 1307 at [20], promotional activities may be in trade or commerce “even if they do not specifically refer to the goods or services in question. For example, promotion of BHP as ‘the big Australian’ might be conduct engaged in trade or commerce.” Equally, in Nixon v Slater & Gordon (2000) 175 ALR 15 at 22-23, Merkel J held that the circulation of booklets to medical practitioners providing information about the role of the law firm, Slater & Gordon, was a promotional activity in relation to the potential supply of legal services to clients, notwithstanding that it was not directed at consumers.
315 The Respondents also submitted that, if the Government Article were considered commercial, “the chilling effect of such a finding will make it increasingly difficult for anyone with a good faith alternative view (i.e. outside the prevailing orthodoxy accepted by government health departments) to disseminate information designed to inform that debate from the particular paradigm”. However, the argument would seem to be more in the nature of a policy argument rather than to have a legal foundation. In any event, the argument is met by the findings that the Articles made misleading, false or deceptive representations which were intended to promote the sale of particular products. Even if they were also intended to contribute to a policy debate, that does not absolve the makers from a contravention of the ACL.
316 Finally, the evidence establishes the existence of a public debate about the efficacy of homeopathy in the United Kingdom. In this regard, Professor Phelps explained that in the United Kingdom “[t]he debate is at Government level, in terms of funding through the National Health Service.… There is a debate within the medical profession and the medical and non-medical practitioners of homeopathy and, clearly, that spills either into a public debate because through the media the public become aware of debates that are being had at political and professional level and so it becomes a pervasive community debate about efficacy and also government funding.” I also accept that within Australia, there is a public debate about the use of homeopathy. Professor Phelps would seem to acknowledge this in her evidence even though she implies that the debate may be less widespread than in the United Kingdom, stating that:
There’s certainly a lot of questioning of the use of homeopathy in Australia at the moment. It’s far less popular in Australia than it is in some other countries, and there are other disciplines within the field of complementary medicine that are considered to be not only more efficacious but more plausible and certainly more popular with the public and with the medical profession. And so the international scene does vary from country to country.
317 However, I do not consider that the evidence supports the existence of any public debate about the use of homeoprophylaxis as an alternative to the vaccination for the prevention of whooping cough or generally. As the ACCC submitted, “the impugned conduct does not reasonably appear to promote or provide a contribution to, public debate concerning a current or proposed law or government policy, or another issue of general concern to the community.” Certainly Mrs Sheffield was an advocate and perhaps Dr Golden. However, there was no evidence of any widespread debate about the issue and in particular no evidence any homeopathic representative body considered that this was an issue. To the contrary, the Australian Register of Homeopaths, which is the peak body in Australia, has taken the position that homeoprophylaxis should not be recommended as a substitute for immunisation: see at [266] above.
318 It follows for these reasons that I find the contraventions of ss 18 and 29 of the ACL as alleged by the ACCC to be made out. First, by publishing the Three Articles containing the Vaccine Representations and the Alternative Homeopathy Reasonable Basis Representations, the Respondents engaged in conduct in trade or commerce that was misleading and deceptive, or likely to be so, in contravention of s 18 of the ACL. Furthermore, the Respondents in trade or commence made false or misleading representations that the Vaccine is of a particular standard or quality (essentially short-lived, unreliable and ineffective) contrary to ss 29(1)(a) and (b) of the ACL by publishing the Vaccine representations in the Three Articles when in fact the Vaccine is effective in protecting the significant majority of people exposed to the infection from contracting whooping cough. Finally, the Respondents also in trade or commerce made false or misleading representations in connection with the supply or possible supply of homeopathic products or treatments that these products or treatments have a particular standard or quality, contrary to s 29(1)(a) and (b) of the ACL, and have a use or benefit contrary to s 29(1)(g), namely: that there is an adequate foundation in medical science for the statement that homeopathic treatments are a safe and effective alternative to the Vaccine when no such foundation exists and the Vaccine is the only treatment currently approved for use and accepted by medical practitioners in Australia for the prevention of whooping cough.
319 I intimated at the end of the hearing that, if I should find a contravention, I may leave the terms of the declaratory relief to be matters for the conclusion of the case. However, in the circumstances, the terms of the draft declaratory relief sought by the ACCC simply reflect my findings. This is not a case where my findings would sustain only some, but not all, of the declaratory relief sought and where there may as a result be some room for argument as to how those findings might be reflected in the declarations. For this reason I consider it appropriate to make the declarations now in the terms sought. The parties will be heard on the question of the penalties sought by the ACCC and other final relief sought.
I certify that the preceding three hundred and nineteen (319) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Perry. |
Associate:




