FEDERAL COURT OF AUSTRALIA
AFT Pharmaceuticals (AU) Pty Ltd v Reckitt Benckiser (Australia) Pty Ltd [2021] FCAFC 222
ORDERS
AFT PHARMACEUTICALS (AU) PTY LTD Appellant | ||
AND: | RECKITT BENCKISER (AUSTRALIA) PTY LTD Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The appeal be allowed.
2. Orders 1 to 10 and 12 to 17 made in proceeding NSD 252 of 2019 on 24 September 2020 be set aside and in lieu thereof the following declarations and orders be made:
(a) declarations that:
(i) there is an adequate scientific foundation for the following statements as presented in, and in the context of, the advertisement attached as Annexure A:
A. “maxigesic GET BETTER1 & FASTER1 PAIN RELIEF THAN PARACETAMOL OR IBUPROFEN ALONE1”;
B. “A new clinical study of moderate to severe dental pain shows that Maxigesic ® provides better and faster pain relief than both paracetamol and ibuprofen alone, at maximum dosage1”;
C. “Faster onset of meaningful pain relief than for Paracetamol or Ibuprofen alone1”;
D. “Maxigesic ® is a unique Paracetamol + Ibuprofen combination that provides superior analgesic efficacy for patients than either Paracetamol or Ibuprofen alone1”;
E. “PROVEN TO PROVIDE MORE EFFECTIVE PAIN RELIEF IN A NEW CLINICAL STUDY”; and
F. “36% MORE EFFECTIVE THAN IBUPROFEN1 & 78% MORE EFFECTIVE THAN PARACETAMOL1”,
(together, the Maxigesic Statements),
(ii) there is an adequate scientific foundation for the Maxigesic Statements as presented in, and in the context of, the training aid attached as Annexure B;
(iii) there is an adequate scientific foundation for the following statement as presented in, and in the context of, the point of sale material attached as Annexure C:
A. Maxigesic Based on a new study1 GET BETTER PAIN RELIEF than paracetamol or ibuprofen alone1; and
(b) orders that:
(i) the cross-claim dated 1 March 2019 be dismissed; and
(ii) the respondent pay the applicant’s costs of the proceeding.
3. The respondent pay the appellant’s costs of the appeal.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.













THE COURT:
1. Introduction
1 In its originating application the appellant, AFT Pharmaceuticals (AU) Pty Limited, sought a declaration that there is an adequate scientific foundation for a list of claims which appear in an advertisement (new advertisement) concerning an analgesic product that AFT markets and sells in Australia called Maxigesic. The respondent, Reckitt Benckiser (Australia) Pty Limited, disputed AFT’s entitlement to the declaration on the basis that the claims made in the new advertisement did not have an adequate scientific foundation and filed a notice of cross-claim seeking declarations that, by publishing and distributing the new advertisement, AFT had made a number of representations that were false and/or misleading in breach of ss 18, 29(1)(a), 29(1)(g) and 33 of the Australian Consumer Law (being Schedule 2 of the Competition and Consumer Act 2010 (Cth)) (ACL), injunctions restraining their further publication, corrective advertising and damages.
2 Three forms of Maxigesic advertisements were in issue before the primary judge (referred to collectively as the 2019 marketing materials). They are described at [12] below. However, for present purposes it is sufficient to refer only to the new advertisement, a copy of which appears as annexure 1 to these reasons.
3 The commencement of the proceeding below by AFT is to be understood against the following background.
4 In an earlier proceeding this Court found that AFT had contravened provisions of the ACL by publishing somewhat different advertisements for Maxigesic which lacked an adequate scientific foundation: see Reckitt Benckiser (Australia) Pty Limited v AFT Pharmaceuticals (AU) Pty Limited [2018] FCA 1552 (2018 judgment); upheld on appeal: AFT Pharmaceuticals (AU) Pty Limited v Reckitt Benckiser (Australia) Pty Limited [2020] FCAFC 45 (appeal judgment).
5 On 29 November 2018, following publication of the 2018 judgment, the Court made orders (November 2018 Orders) including, as Order 12, a permanent injunction restraining AFT from making a representation (among others) that, when taken at their respective maximum daily doses, Maxigesic provides stronger and more effective relief from pain than over the counter (OTC) doses of either paracetamol or ibuprofen alone without an adequate scientific foundation for doing so.
6 In late 2018, after publication of the 2018 judgment, an additional scientific study by Daniels et al., entitled “Analgesic Efficacy of an Acetaminophen/Ibuprofen Fixed-dose Combination in Moderate to Severe Postoperative Dental Pain: A Randomized, Double-blind, Parallel-group, Placebo-controlled Trial” (2018) 40(10) Clinical Therapeutics 1765 (Daniels 2018 study) was published. This was the second study that recorded data about the comparative efficacy of Maxigesic. In the 2018 judgment a finding was made that the first such study, the Merry Study, was unreliable.
7 On 31 January 2019 AFT filed its notice of appeal appealing from the November 2018 Orders and the findings made in the 2018 judgment that the wider body of science, excluding the Merry study, did not provide an adequate scientific foundation in scientific knowledge for comparative claims of superiority of Maxigesic that were made by AFT.
8 The advertising campaign which comprised the 2019 marketing materials began prior to the commencement of the proceeding below. Before the primary judge AFT contended that the Daniels 2018 study, and a substantial body of scientific evidence consistent with it, provided the scientific foundation for its claims in the 2019 marketing material. Evidently, AFT sought the declaratory relief described at [1] above before the primary judge in the context of it seeking to ensure that the claims that it makes in the new advertisement would not fall foul of the November 2018 Orders, including relevantly Order 12 thereof (see [5] above).
9 The primary judge found that some of the claims were not the subject of adequate scientific foundation, and that a number of the representations alleged by Reckitt in its cross-claim were false or misleading: see AFT Pharmaceuticals (AU) Pty Ltd v Reckitt Benckiser (Australia) Pty Ltd [2020] FCA 672 (judgment). AFT then sought leave to re-open the case on the basis that the primary judge had misapprehended that it had accepted or conceded that most of the representations pleaded by Reckitt had been made in the new advertisement in an unqualified manner. The primary judge rejected that argument: see AFT Pharmaceuticals (AU) Pty Ltd v Reckitt Benckiser (Australia) Pty Ltd (No 2) [2020] FCA 1092 (re-opening judgment). There then arose a contest over the form of orders that were appropriate to make having regard to the judgment, which her Honour resolved in AFT Pharmaceuticals (AU) Pty Ltd v Reckitt Benckiser (Australia) Pty Ltd (No 3) [2020] FCA 1352 (relief judgment) by declarations and orders made on 24 September 2020.
10 AFT now appeals against those declarations and orders. In its notice of appeal it contends that the primary judge erred in finding that:
(1) the new advertisement and the training aid conveyed the five representations alleged by Reckitt in its notice of cross-claim, the point of sale (POS) materials conveyed the first and fifth of those representations and the 2019 marketing materials conveyed the “restrained representation” (see [18] below);
(2) AFT had accepted in the running of the trial that those representations had been conveyed in a manner that was not qualified by the other content and context of the 2019 marketing materials in which they appeared;
(3) the new advertisement and the training aid conveyed the representation that Maxigesic provides “faster pain relief” rather than “faster onset of meaningful pain relief”;
(4) by reason of grounds (1)-(3) AFT had engaged in conduct in contravention of the ACL; and
(5) declarations should be made to the effect that AFT had acted in breach of Order 12 of November 2018 Orders.
11 At the hearing of the appeal, senior counsel for AFT accepted that if ground 2 of the appeal failed then ground 1 could not succeed.
2. The decisions of the primary judge
2.1 The judgment
2.1.1 The advertisements, the claims and the representations
12 The 2019 marketing materials which were before the primary judge comprised: the new advertisement, which was published in pharmacy and dental trade journals in February and March 2019; a training aid used by AFT’s sales staff in their dealings with pharmacies; and various POS materials displayed in pharmacies. The new advertisement and the training aid were relevantly the same and were directed to health professionals, whereas the POS materials were directed to a wider audience, including consumers: see judgment at [10].
13 At [12]-[13] the primary judge set out the claims in the new advertisement and the training aid that AFT sought to justify by reference to scientific evidence:
12 The new advertisement and the training aid contain the following statements, for which AFT contends that it has an adequate scientific basis:
(1) “maxigesic GET BETTER1 & FASTER1 PAIN RELIEF THAN PARACETAMOL OR IBUPROFEN ALONE1”;
(2) A new clinical study of moderate to severe dental pain shows that Maxigesic® provides better and faster pain relief than both paracetamol and ibuprofen alone, at maximum dosage1;
(3) “Faster onset of meaningful pain relief than for Paracetamol or Ibuprofen alone1”
(4) “Maxigesic® is a unique Paracetamol+ Ibuprofen combination that provides superior analgesic efficacy for patients than either Paracetamol or Ibuprofen alone1”
(5) “PROVEN TO PROVIDE MORE EFFECTIVE PAIN RELIEF IN A NEW CLINICAL STUDY”; and
(6) “36% MORE EFFECTIVE THAN IBUPROFEN1 & 78% MORE EFFECTIVE THAN PARACETAMOL1”.
Footnote
13 AFT pleads that the reference denoted by “1” in both the new advertisement and the training aid reads as follows:
(a) (in the middle of the page) “Daniels study results assessed on the intent-to-treat (ITT) population with adjustment for the use of rescue medication. Sensitivity analysis confirms primary endpoint independent of rescue medication adjustment1.” (mid-page footnote); and
(b) (at the foot of the page) “References: Daniels SE, Atkinson HC, Stanescu I, and Frampton C (2018), “Analgesic Efficacy of an Acetaminophen/Ibuprofen Fixed dose Combination in Moderate to Severe Postoperative Dental Pain: A Randomized, Double-blind, Parallel-group, Placebo-controlled Trial”, Clinical Therapeutics 40 (10): 1765 – 1776. Result achieved in a trial of post-operative pain relief after removal of at least 2 impacted third molars using Maxigesic® fixed-dose combination (Paracetamol 975mg/Ibuprofen 292.5mg) compared with Paracetamol 975mg or Ibuprofen 292.5mg alone 4 times a day (Paracetamol 3900mg or Ibuprofen 1170mg per day). Maxigesic® 975/292.5mg combination is bioequivalent to Maxigesic® 1000/300mg Australian combination at full dose (Aitken et al, J Bioequiv Availab, 10:5). Research sponsored by AFT Pharmaceuticals…” (bottom page footnote)
14 There was little contest as to the way in which the footnotes would be treated by readers of the new advertisement. In relation to the mid-page footnote the primary judge found that its font is not so small as to be unreadable and that a reasonable reader of the new advertisement would be likely to read out the mid-page footnote when considering the footnoted statements in the new advertisement. In relation to the bottom page footnote the primary judge recorded AFT’s acceptance that the matter should be approached on the basis that some readers, likely the majority, would not read the footnotes. In light of that, her Honour considered the meaning of the new advertisement on the basis that the information in the footnotes would not necessarily form part of the context in which statements in the new advertisement are to be understood: see judgment at [16]. Relevantly, the primary judge recorded AFT’s submission that the footnoted content was of “little relevance to the identification of the representations conveyed”, observing that the statements which convey the disputed representations are not found in the footnotes, but rather in the body of the various advertising materials: see judgment at [18].
15 At [39] under the heading “Representations” the primary judge observed that AFT accepted a number of the representations that were pleaded by Reckitt (accepted representations), saying:
AFT accepted that the following representations were conveyed by the advertising materials:
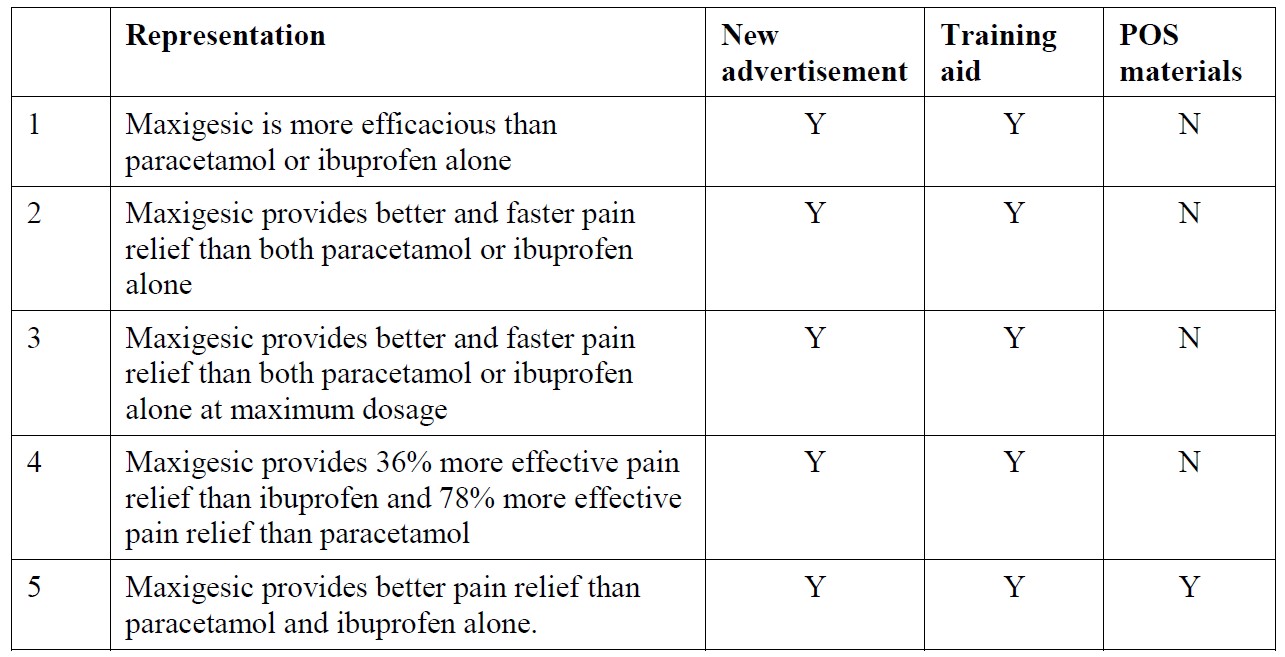
16 The circumstances in which AFT advanced these representations and whether it accepted that they were in fact made in an unqualified way, having regard to the content of the 2019 marketing materials, are considered in the context of ground 2 of the appeal.
17 At [40] and [41] respectively, the primary judge recorded Reckitt’s contentions that the first representation is also conveyed by the POS materials; and that two additional representations were made: that Maxigesic provides stronger and more effective relief from pain than OTC doses of either paracetamol or ibuprofen alone when taken at their respective maximum recommended daily doses, referred to in the judgment as the restrained representation and so named because of Order 12 of the November 2018 Orders which restrained AFT from making such a representation (see [5] above); and that Maxigesic provides faster onset and more meaningful pain relief than for paracetamol and ibuprofen alone.
18 There was a contest about these representations. In relation to the POS materials the primary judge found that a reasonable consumer is likely to understand the claim “better pain relief” as a representation that Maxigesic is more efficacious: see judgment at [45]. Turning to the two additional representations, in relation to the restrained representation the primary judge considered the language of the new advertisement and the competing expert evidence of Professor Keech and Dr Russo and concluded that a reasonable interpretation was that the 2019 marketing materials include a claim in terms of the restrained representation: see judgment at [53]. However, the primary judge rejected the contention that the second additional representation was conveyed by the new advertisement and the training aid: see judgment at [56]. In doing so her Honour rejected the representation pleaded by Reckitt at [23(f)] of its statement of cross-claim.
19 It is material to note in this context that the primary judge understood that AFT had agreed to the terms of the accepted representations and so did not proceed to consider whether they could or should be derived from the new advertisement.
2.1.2 Review of the scientific evidence
20 The primary judge next considered the scientific evidence in some detail.
21 In relation to the Daniels 2018 study her Honour found that its primary end point “was the time-adjusted sum of pain intensity difference over 48 hours (SPID48). The authors of the Daniels 2018 study concluded (at p 1768) that analysis of the time-adjusted SPID48 ‘... showed that [Daniels] FDC 975/292.5 provided more effective pain relief than placebo, acetaminophen or ibuprofen’”: see judgment at [64].
22 At [67] her Honour referred to the secondary endpoints identified in the Daniels 2018 study, quoting the following from it:
Secondary end points of the study included the following: time to perceptible and meaningful pain relief using the two-stopwatch method; maximum VAS [visual analogue scale] pain intensity score after the first dose of study medication; percentage of patients requiring rescue medication, time to first doses of rescue medication, and cumulative consumption (milligrams) of rescue medication; percentage of patients that achieved <50% reduction in baseline VAS pain before the consumption of rescue medication (response rate), time to peak response (time to maximum percentage reduction in VAS or time to first rescue medication use, whichever is shorter); and the categorical global pain relief rating.
23 The primary judge observed at [72]-[75] that, of these, Dr Russo considered that the three most clinically relevant were:
(1) median time to meaningful pain relief, which the Daniels 2018 study reported as being quicker for a fixed dose combination of 975mg of paracetamol and 292.5mg of ibuprofen (Daniels FDC) (42.98 minutes) than any of the other study arms (ibuprofen, 61.86 minutes and paracetamol, 46.62 minutes) (Table II on p 1771);
(2) mean maximum VAS pain score, which the Daniels 2018 study reported to be significantly lower for the Daniels FDC group (55.06mm) than the placebo group (72.05mm) and paracetamol group (64.46mm), and lower than the ibuprofen group (61.28mm); and
(3) mean consumption of rescue medication (mg), where the Daniels 2018 study reported that the requirement for supplementary analgesia with oxycodone was lower in the Daniels FDC group compared with the other treatment groups (see p 1770). The mean amount of oxycodone consumed by participants in the Daniels FDC group was 3.7mg, compared with 7.1mg in the ibuprofen group, 11.0mg in the paracetamol group and 17.9mg in the placebo group.
24 Her Honour also observed at [76] that Dr Russo referred to the significantly fewer patients in the Daniels FDC group requiring rescue medication (compared to monotherapy groups) as providing clinically meaningful information regarding the comparative strength of analgesia of the Daniels FDC and the monotherapies.
25 At [77] the primary judge recorded the agreement of the experts that:
(1) the study employs the Dental Impaction Pain Model (DIPM) which is “a validated method for evaluating efficacy of treatments for acute pain”;
(2) the primary and secondary end points used in the Daniels 2018 study are commonly evaluated measures in the DIPM;
(3) the primary end point “provides information about the extent of pain relief, but does not provide information concerning time to pain relief”;
(4) “Each of the measures identified as primary or secondary end points in the Daniels 2018 study was identified as such in the protocol and in the statistical analysis plan, both of which were finalized prior to completion of the trial, locking of the database, and unblinding the resulting data”; and
(5) for the primary end point and a range of secondary end points, including time to meaningful pain relief, “the reported p-value for comparing the Daniels FDC to each of the paracetamol, ibuprofen and placebo was less than 0.05, thereby meeting the stated criterion for superiority ...”.
And at [80] their further agreement that:
(1) (Except Professor Gebski for whom DIPM is outside his sphere of expertise) DIPM does not assess effectiveness at controlling chronic pain, and one cannot extrapolate effectiveness in pain control from acute pain models such as DIPM to chronic pain settings.
(2) The Daniels 2018 study is not directly informative concerning degree of pain relief or onset of pain relief in contexts other than the acute pain dental impaction setting.
(3) The Daniels 2018 study does not evaluate the Maxigesic formulation being commercially marketed in Australia.
26 The primary judge next considered whether the Daniels 2018 study provides support for any claim of efficacy over a 24 hour period (rather than 48 hours). After considering the expert evidence, her Honour concluded that it did, even though the study did not specifically test for it, because it provided a rational basis to found an opinion based on the data provided: see judgment at [88]. The primary judge concluded that a claim that it is highly likely that patients would experience greater improved pain relief with the Daniels FDC over the first 24 hours compared with monotherapies is supported: see judgment at [89].
27 The primary judge then turned to the use that could be made of the secondary end point data in the Daniels 2018 study and the testing reported that was relevant to a claim for “faster pain relief”. In relation to the latter, the primary judge referred to the experts’ agreement that a superiority claim could not be made unless an end point reaches statistical significance, and that a claim of faster onset of perceptible pain relief would not be supported by the Daniels 2018 study: see judgment at [111].
28 The primary judge noted that the bottom page footnote in the new advertisement makes a claim to bioequivalence, in effect, between the Daniels FDC and Maxigesic. AFT submitted that this claim should be understood to refer to the fact that the Daniels FDC and Maxigesic achieve essentially the same clinical outcome. Her Honour rejected that submission and considered that, to the extent that it is relevant to consider how the bottom page footnote to the new advertisement may be interpreted, the words “(Aitken et al, J Bioequiv Availab, 10:5)” signify that the claim of bioequivalence is based on the Aitken study. In that regard, after considering the expert evidence, the primary judge concluded at [127]:
Ultimately, the experts were not in agreement as to whether it is appropriate to describe Daniels FDC and Maxigesic as bioequivalent where the products satisfied seven of the eight tests for bioequivalence set in the Aitken study. Whatever the better position, the Aitken study does not provide unqualified support for a conclusion that the two combination products are bioequivalent because the study included a test for bioequivalence of Cmax in fasting conditions, which test was not passed.
29 The primary judge then considered the wider body of scientific evidence upon which AFT relied in support of its statement that Maxigesic “provides better pain relief and faster pain relief”.
2.1.3 Consideration of the claims and the representations
30 Under the heading “Adequate scientific foundation for claims and representations” the primary judge first considered the topic whether “Maxigesic provides better pain relief”.
31 At [176(1)] of the judgment her Honour records the submission advanced by AFT as follows:
The primary end point of the Daniels 2018 study (SPID48) provides a foundation for these claims. Ultimately, there appeared to be little dispute about this among the experts. The SPID6 results reported in the Daniels 2018 study also provide support these claims. Further, several of the secondary end points of the Daniels 2018 study, including mean consumption of rescue medication (mg) and percentage of patients requiring rescue medication, provide further support for the claims. The results of the Daniels 2018 study can be read directly on to Maxigesic. There is common ground between the experts that, to the extent that the fasted Cmax difference is of any relevance, its relevance would bear upon to the time to perceptible pain relief, rather than the extent of relief. That does not concern the primary end point or AFT’s claims in the new advertisement insofar as they claim “better” relief.
32 Her Honour rejected this submission, noting that the experts’ agreement was more specific than AFT acknowledged. However, more significantly, at [178]-[179] the primary judge made a finding as to the manner in which the case was argued:
178 The new advertisement can be read as a set of claims about the results of the Daniels 2018 study. As I understood the evidence of Dr Russo and Professor Thisted, each of them read the advertisement in that manner. I accept that a reasonable reader may read the new advertisement in that way, particularly because of the extensive use of footnotes and the reference, in the body of the advertisement, to a “new clinical study of moderate to severe dental pain” which “shows that Maxigesic provides better and faster pain relief …”. On that reading of the new advertisement, I accept, for example, that the primary end point of the Daniels 2018 study (SPID48) provides a foundation for the claim that Maxigesic provides better pain relief than paracetamol or ibuprofen alone. On that reading, it is implicit that the claim of better pain relief is limited to better pain relief as measured by the Daniels 2018 study, extrapolated onto Maxigesic on the basis of the Aitken study.
179 However, the case proceeded on the basis that the advertising materials conveyed much broader representations. For example, the first agreed representation, that Maxigesic is more efficacious than paracetamol or ibuprofen alone, is unrestricted as to the nature of the pain treated, the dosage of the drugs taken and the duration of treatment. There was no expert evidence that the results of the Daniels 2018 study could be extrapolated to an unqualified statement that Maxigesic is more efficacious than paracetamol or ibuprofen alone in all contexts. To the contrary, the experts agreed that the Daniels 2018 study is not directly informative concerning degree of pain relief in contexts other than the acute pain dental impaction setting. The experts also agreed that one cannot extrapolate effectiveness in pain control from acute pain models, such as was used in the Daniels 2018 study, to chronic pain settings.
(Bolded emphasis added.)
33 At [180] the primary judge also considered the submission advanced by AFT (at [176(2)] of the judgment) to the effect that the wider body of scientific literature provides strong support for the conclusion that the combination of 400mg ibuprofen/1000mg paracetamol will provide better pain relief than 400mg ibuprofen or 1000mg paracetamol as monotherapies, finding:
Similarly, neither the experts collectively, nor Dr Russo individually, gave evidence that the implications of the wider body of scientific literature are as far reaching as AFT now contends. The broadest statement is in the Acute Pain Management text but, on closer examination, that does not support an unqualified claim that Maxigesic provides better pain relief than either paracetamol or ibuprofen alone.
(Emphasis added.)
34 Finally, the primary judge considered the contention advanced by AFT (at [176(3)] of the judgment) that, insofar as the claims refer to Maxigesic being 36% more effective than ibuprofen and 78% more effective than paracetamol, those claims are based on the primary end point of the Daniels 2018 study and the derivation of the percentages is a matter of mathematics. The percentages are not to be understood as absolute figures, but rather the percentages achieved in the Daniels 2018 study itself. It can be recognised that a different study may achieve different percentages, but that is not the point. At [181]-[182] her Honour addressed that submission as follows:
181 As to the claims referring to Maxigesic being 36% more effective than ibuprofen and 78% more effective than paracetamol, I accept that those claims are based on the primary end point of the Daniels 2018 study and the derivation of the percentages is a matter of mathematics.
182 However, since the Daniels FDC contained different quantities of paracetamol and ibuprofen from Maxigesic, the relative effectiveness of Maxigesic to corresponding treatment with ibuprofen and paracetamol separately cannot be assumed to match the figures for the Daniels FDC. Professor Keech’s view that the true numbers would be different by an unknown amount, even though we would expect them to be very similar. Professor Thisted agreed that the results would not be identical if the Daniels 2018 study was replicated using Maxigesic, but held “that the percentage differences in pain relief should be substantially similar and the 36% and 78% figures would not misrepresent them”.
35 The primary judge then turned to consider whether Maxigesic provides faster pain relief. Her Honour recorded the parties’ competing submissions on the subject, which concerned the relevance of the secondary end point of the Daniels 2018 study and the wider body of scientific literature (most particularly the Mehlisch and Daniels 2011 study), before concluding at [189]-[190] that:
189 I am satisfied that the Daniels 2018 study secondary end point of median time to meaningful pain relief provides an adequate scientific foundation for the statement that Maxigesic provides “Faster onset of meaningful pain relief than for paracetamol or ibuprofen alone”, where it is implicit that the statement is limited to speed of onset as measured by the Daniels 2018 study, extrapolated onto Maxigesic on the basis of the Aitken study.
190 Otherwise, I am not satisfied that the single secondary end point provides an adequate scientific foundation for a claim of faster pain relief because the Daniels FDC results did not achieve statistical significance for all secondary end points related to time to pain relief.
36 Her Honour rejected the contention that the other studies provided support for the claim: see judgment at [192]-[193].
37 The primary judge concluded at [194] that AFT had an adequate scientific foundation, in the Daniels 2018 study, read with the Aitken study, for the following statements which appear in the new advertisement (being claims (3)-(6) recorded at [13] above), “to the extent to which they are read as reporting the results of the Daniels 2018 study” (emphasis added):
(1) “Faster onset of meaningful pain relief than for Paracetamol or Ibuprofen alone1”;
(2) “Maxigesic® is a unique Paracetamol+Ibuprofen combination that provides superior analgesic efficacy for patients than either Paracetamol or Ibuprofen alone1.”
(3) “PROVEN TO PROVIDE MORE EFFECTIVE PAIN RELIEF IN A NEW CLINICAL STUDY”; and
(4) “36% MORE EFFECTIVE THAN IBUPROFEN1 & 78% MORE EFFECTIVE THAN PARACETAMOL1”.
38 However, “even reading the new advertisement as making statements that can only be understood by reference to the Daniels 2018 study” her Honour was not persuaded that claims (1) and (2) (see [9] above), concerning faster pain relief, had adequate scientific foundation: see judgment at [196].
39 Insofar as Reckitt’s cross-claim was concerned, the primary judge concluded, on the basis of her findings, that the five unqualified representations i.e. the accepted representations (see [15] above) did not have an adequate scientific foundation. Her Honour said at [198] that:
The reason for these conclusions is that each of the representations is unlimited as to nature of pain, dosage and treatment duration. As explained above, the scientific evidence is addressed to much more specific comparisons and the expert evidence does not support a conclusion that there is an adequate scientific basis for extrapolating from the Daniels 2018 study and the wider body of scientific evidence to the unqualified representations. Even the evidence of Dr Russo and Professor Thisted, that the Daniels 2018 study provides an adequate scientific foundation for the statements in the new advertisement, must be understood in the context of their agreement that the study is not directly informative concerning degree of pain relief in contexts other than the acute pain dental impaction setting. As I understood their evidence on this point, it was predicated upon an assumption that the claims in the advertisement are addressed to the treatment of acute pain.
(Emphasis added.)
40 The primary judge found that there was also no adequate scientific foundation for making the restrained representation: see judgment at [199].
2.2 The re-opening judgment
41 AFT brought an application for leave to re-open pursuant to r 39.04 of the Federal Court Rules 2011 (Cth) on the basis that the primary judge had misapprehended “that AFT accepted during the running of the trial that the impugned statements could be assessed on the basis that they had been made in an unqualified manner and without appropriate reference to the Daniels 2018 study”: see re-opening judgment at [12]. AFT submitted that it had never made the concession that the accepted representations were unqualified.
42 The primary judge considered AFT’s submission in the context of two documents which had been provided to the Court by AFT in the course of the hearing.
43 The first document was provided by AFT during opening submissions and is in the following terms:
Representations which the Applicant accepts arise
1. Maxigesic is more efficacious than paracetamol or ibuprofen alone.
2. Maxigesic provides better and faster pain relief than both paracetamol or ibuprofen alone.
3. Maxigesic provides better and faster pain relief than both paracetamol or ibuprofen alone at maximum dosage.
4. Maxigesic provides 36% more effective pain relief than ibuprofen and 78% more effective pain relief than paracetamol.
5. Maxigesic provides better pain relief than paracetamol and ibuprofen alone.
We will refer to this document in these reasons as the Representations Document.
44 The second document was handed up by AFT at the commencement of its closing submissions and is reproduced in the judgment at [39] (see [15] above). This document contains the same representations as in the Representations Document but also notes in which of the 2019 marketing materials AFT says those representations arise.
45 After quoting various exchanges between the Court and counsel for AFT, which we address in further detail below, the primary judge noted that in considering a case such as the present, it is conventional to adopt two stages: first, to consider whether each alleged representation was conveyed; and secondly, whether such representation as was conveyed was misleading or deceptive or likely to mislead or deceive, citing Australian Competition and Consumer Commission v Dukemaster [2009] FCA 682 at [61]; Australian Competition and Consumer Commission v Telstra Corporation Ltd [2007] FCA 1904 at [14]-[15]; and Novartis Pharmaceuticals Australia Pty Ltd v Bayer Australia Ltd [2015] FCA 35 at [200].
46 In the present case, her Honour found that AFT made a forensic decision to accept that specified representations were made, with the result that the main issue at trial was whether those representations were misleading or deceptive. As a matter of principle, the identification of the representations was separate from and not “yoked” to the question of whether there was an adequate scientific foundation. If AFT’s position was that the five representations, which it accepted to arise, did not arise in “the context of the ad”, then its acceptance was meaningless because Reckitt only alleged that the representations were made in that context: see re-opening judgment at [34].
47 Accordingly, the primary judge did not accept that the Court had misapprehended AFT’s case and the application to re-open was refused.
2.3 The relief judgment
48 The parties disagreed about the final orders to be made based on the judgment. In relation to AFT’s claim, AFT proposed that the Court make two declarations while Reckitt proposed that AFT’s application be dismissed. In relation to Reckitt’s cross-claim, Reckitt proposed declarations, injunctive orders and orders for corrective advertising. There was also disagreement as to the costs orders to be made.
49 Putting to one side costs, the primary judge refused to make the two declarations sought by AFT, made the declarations and injunctive orders sought by Reckitt on its cross-claim and made an order dismissing AFT’s application.
3. The appeal
50 As set out at [10] above AFT raises five grounds of appeal, which we address below.
3.1 Grounds 1 to 4
51 Grounds 1 to 4 are interdependent and, in broad terms, concern an allegation by AFT that the primary judge failed to consider the representations as a whole and in context. Ground 1 concerns what AFT says is an error of principle on the part of the primary judge, ground 2 concerns what AFT says is an error of fact on the part of the primary judge, ground 3 concerns the primary judge’s approach to “faster” pain relief and ground 4 only arises if AFT succeeds on grounds 1 to 3.
52 In addressing these grounds it is convenient to commence with consideration of ground 2. It concerns whether the primary judge misunderstood the manner in which AFT put its case at trial. In particular, whether, as AFT contends, her Honour made an error of fact in making the findings at [197] to [199] of the judgment by proceeding on the basis that she could assess any representation conveyed by the 2019 marketing materials in an unqualified manner, divorced from the content and context of the 2019 marketing materials and, in particular, the Daniels 2018 study.
3.1.1 Ground 2 - the parties’ submissions
53 AFT submitted that it is evident that the primary judge made the error for which it contends from the following matters.
54 First, AFT submitted that the pleadings and evidence concerned the 2019 marketing materials, the Daniels 2018 study and, to a lesser extent, the Aitken study. AFT contended that it would be incongruous for it to accept that the Court could adjudicate the case based on unqualified representations, divorced from the matters referred to in the pleadings and the evidence. It noted that, consistent with the pleadings and the evidence, its opening and closing submissions were concerned with the content of the 2019 marketing materials.
55 Secondly, AFT submitted that it would have been illogical and unprincipled for it to have accepted that the primary judge could assess any representation conveyed by the 2019 marketing materials in an unqualified manner divorced from the content and context of those materials. It contended that, when considered as unqualified representations, it becomes apparent that the representations cannot logically be divorced from the qualifying statements that accompanied them. By way of example AFT referred to the purported unqualified representation that “Maxigesic provides 36% more effective pain relief than ibuprofen and 78% more effective pain relief than paracetamol”. It said that self-evidently this statement is derived from research, referring to the judgment at [181], and submitted that it would be illogical for it to treat the specific percentage results from a single study as unqualified general statements of fact.
56 Thirdly AFT submitted that, had it accepted the position attributed to it by the primary judge, it would have represented a dramatic shift in the case, which is not discernible from the transcript or either party’s written or oral closing submissions. AFT contended that such a concession would have amounted to a complete capitulation at odds with the pleadings, the way the case was opened and run and, significantly, the correct approach to construing advertising material.
57 Reckitt submitted that AFT now seeks to qualify its agreement at trial that the accepted representations were conveyed by the 2019 marketing materials by alleging that, irrespective of its agreement in this regard, the primary judge was obliged to somehow assess those representations by reference to the wording, content and context of that material. Reckitt contended that it is not readily apparent what AFT proposes the primary judge ought to have done and what the alleged wording, content and context of the 2019 marketing material would have done to the meaning of the accepted representations.
58 Reckitt said that AFT had two opportunities during the hearing to qualify the accepted representations in writing. It submitted that simply stating that the accepted representations had to be understood in context is, of itself, meaningless absent an expression of how the representations would materially change in effect by virtue of that context. Reckitt contended that clearly the context is the 2019 marketing materials by which the accepted representations are conveyed and that the Court took that context into account as well as the fact that Reckitt clearly considered and argued that the accepted representations were relevantly unqualified.
59 Reckitt submitted that AFT made a forensic decision to draft the accepted representations in the fashion handed to the Court. It said that when the matter was heard in July 2019, a Full Court of this Court had not yet heard the appeal from the 2018 judgment, which was heard in August 2019 with judgment delivered in May 2020. Reckitt contended that if AFT had been able to succeed in its claim in respect of the accepted representations on the basis that there was an adequate foundation in scientific knowledge for the unqualified representations, then it would have rendered that appeal somewhat otiose and AFT would have been able to engage in further comparative advertising on the basis of the accepted representations. Reckitt submitted that accordingly there were clear commercial reasons why AFT would be prepared to seek a determination of the validity of the accepted representations. It said that having made the forensic decision and having unsuccessfully sought leave to re-open the case to challenge the accepted representations, AFT should be bound by its forensic decision.
3.1.2 Ground 2 - consideration
60 In order to consider this ground it is necessary to review the way in which AFT presented and argued its case before the primary judge, commencing with the pleadings and then over the course of the trial.
3.1.2.1 The pleaded case
61 As set out at [1] above, in its originating application AFT sought a declaration that there is an adequate scientific foundation for six statements (see [13] above) which appear in the new advertisement. The prayer for relief is framed in a way that might suggest that AFT sought a declaration on a broad basis so as to be able to make the statements divorced from the 2019 marketing material. However, as AFT submitted, that was not its intention. What it sought, as is apparent from other material referred to below, was a declaration in relation to those statements for the purpose of and as part of the 2019 marketing material.
62 In its statement of claim AFT:
(1) at [12] referred to Order 12 of the November 2018 Orders i.e. the restrained representation;
(2) at [13] referred to the new advertisement that it had recently caused to be published in pharmacy and dentistry trade journals;
(3) at [14] set out the six statements contained in the new advertisement (see [13] above);
(4) at [15] recorded what was denoted by the footnote reference “1” in the new advertisement (see [13] above);
(5) at [16]-[17] pleaded that:
16 The [2018 Daniels study] referred to in the [new advertisement] was published in the October 2018 issue of Clinical Therapeutics and was not considered by the Court in the Proceeding which concerned earlier advertisements.
17 There is an adequate scientific foundation for each of the statements set out in paragraph 14 above.
(6) at [18] referred to a letter received from Reckitt’s lawyers (referred to as the HWL Letter) in relation to the new advertisement;
(7) at [19] pleaded that in the HWL Letter Reckitt alleged that:
(a) the [new advertisement] conveys the [restrained representation];
(b) AFT’s use of the [new advertisement] is a breach of Order 12; and
(c) AFT’s use of the [new advertisement] contravenes ss 18, 29(a) and (g) and 33 of the ACL.
(8) at [21] denied Reckitt’s allegations in relation to the new advertisement and recorded that it had declined to provide the undertaking sought by Reckitt that it cease to use the new advertisement.
63 In its defence to AFT’s statement of claim Reckitt:
(1) pleaded at [15] that:
In answer to paragraph 15 of the Statement of Claim, [Reckitt]:
(a) admits that the text set out in paragraph 15 of the Statement of Claim appears in very small font on the face of the advertisement;
(b) says that the text is so small of itself and/or by comparison to the surrounding text and/or is placed in such a position on the advertisement that it would not be read by a casual or reasonable observer of the advertisement and/or otherwise would not and does not operate to qualify the statements to which the reference denoted by “1” is affixed in the advertisement;
(c) otherwise does not admit the allegations in the paragraph.
(2) at [16] admitted the allegations at [16] of AFT’s statement of claim, but at [17] denied the allegations at [17] of AFT’s statement of claim;
(3) at [19] admitted the allegations pleaded at [19] of the statement of claim and said that:
(a) the [new advertisement] that [AFT] created and caused to be published conveys the [restrained representation];
(b) [AFT’s] conduct in creating, publishing and/or otherwise using the [new advertisement] constitutes a breach of order 12 of the [November 2018 Orders];
(c) [AFT’s] conduct in creating, publishing and/or otherwise using the [new advertisement] contravenes, inter alia, ss 18, 29(a) and (g) and 33 of the [ACL].
64 As we have already observed, Reckitt filed a notice of cross-claim in which it sought, among other things, the following declarations:
1. A declaration that [AFT], in trade or commerce, by publishing, distributing exhibiting, displaying or communicating to the public, or causing to be published, distributed exhibited, displayed or communicated to the public:
(a) the material in Annexure A;
(b) publishing any matter to the effect that:
a. that Maxigesic is more efficacious than paracetamol or ibuprofen alone;
b. Maxigesic provides better and faster pain relief than both paracetamol or ibuprofen alone;
c. Maxigesic is better and faster for pain relief than both paracetamol or ibuprofen alone for all pain
d. Maxigesic provides better and faster pain relief than both paracetamol and ibuprofen alone at maximum dosage;
e. when taken at their respective maximum recommended daily doses, Maxigesic provides stronger and more effective relief from pain than OTC doses of either paracetamol or ibuprofen alone;
f. Maxigesic provides faster onset and more meaningful pain relief than for paracetamol and ibuprofen alone
g. Maxigesic provides 36% more effective pain relief than ibuprofen and 78% more effective pain relief than paracetamol;
(the New AFT Representations);
has:
(c) engaged in conduct that was misleading or deceptive, or likely to mislead or in contravention of section 18 of the [ACL];
(d) made false representations in contravention of sections 29(1)(a) and 29(1)(g) of the ACL;
(e) engaged in conduct that was misleading or liable to mislead in contravention of section 33 of the ACL.
2. A declaration that by publishing the advertisement at Annexure A in medical, pharmacy and dental trade journals, [AFT], in trade or commerce, also made misleading representations that there was a current adequate foundation in scientific knowledge for each of the New AFT Representations, and has thereby engaged in conduct that was misleading or deceptive, or likely to mislead or deceive, in contravention of section 18 of the ACL.
3. A declaration that by publishing the [new advertisement] [AFT] has breached Order 12 of the [November 2018 Orders] in the proceedings Reckitt Benckiser (Australia) Pty Ltd v AFT Pharmaceuticals (AU) Pty Ltd (NSD 1542 of 2017).
65 In its statement of cross-claim:
(1) among other things, Reckitt pleaded that:
New AFT Advertisement
22. From a date presently unknown to [Reckitt], but at least since February 2019, [AFT] has in trade or commerce, has undertaken an advertising and promotional campaign in Australia comprising the material at Annexure A.
…
New AFT Representations
23. By the [new advertisement], [AFT] has conveyed the following representations:
a. that Maxigesic is more efficacious than paracetamol or ibuprofen alone;
b. Maxigesic provides better and faster pain relief than both paracetamol or ibuprofen alone;
c. Maxigesic is better and faster for pain relief than both paracetamol or ibuprofen alone for all pain;
d. Maxigesic provides better and faster pain relief than both paracetamol and ibuprofen alone at maximum dosage;
e. when taken at their respective maximum recommended daily doses , Maxigesic provides stronger and more effective relief from pain than OTC doses of either paracetamol or ibuprofen alone;
f. Maxigesic provides faster onset and more meaningful pain relief than for paracetamol and ibuprofen alone;
g. Maxigesic provides 36% more effective pain relief than ibuprofen and 78% more effective pain relief than paracetamol;
(the New AFT Representations )
…
24. In relation to each New AFT Representation there is a further representation that:
a. there is a current adequate foundation in scientific knowledge for that representation;
b. the representation is supported by the [Daniels 2018 study].
25. In fact:
a. there is no current adequate foundation in scientific knowledge for the New AFT Representations; and/or
b. the [Daniels 2018 study] does not support the New AFT Representations made by AFT.
Particulars
(i) The [Daniels 2018 study] tested the efficacy of a combination treatment with the monotherapy treatment over a period of 48 hours and not 24 hours as the words “at maximum dosage” suggest.
00 The statements “36% more effective than ibuprofen” and “78% more effective than paracetamol” are made in respect of the results of the primary endpoint test, which was carried out over a 48 hour period. The statements are are (sic) made in the context of a “maximum dosage” which is either perceived to be a single dose or a maximum dose over a period of 24 hours and not over a period of 48 hours.
(Hi) The statement “faster” pain relief than the monotherapies is misleading as the [Daniels 2018 study] notes the similar time to perceptible pain relief.
(iv) The [Daniels 2018 study] exclusively tested dental patients who underwent molar extraction surgery only. In fact, the authors of the study themselves acknowledge the existence of several pain studies in emergency department patients that do not show superiority of analgesic combination therapy over monotherapy.
M The [new advertisement] compares the efficacy of Maxigesic (1000mg paracetamol/300mg ibuprofen per 2 tablet dose) to the monotherapies. The [Daniels 2018 study] tests the efficacy of a combination analgesic with a slightly different composition to Maxigesic (975mg paracetamol/292.5mg ibuprofen per 3 tablet dose).
(vi) A representation is made that the two combination treatments are bioequivalent however that is not supported by the clinical study referred to for that purpose in the [new advertisement].
(vii) There is an element of “sponsor bias” involved in the [Daniels 2018 study]. AFT sponsored the [Daniels 2018 study], and three of the four authors of the study are shareholders and/or employees and/or consultants of AFT.
26. By reason of the matters set out in paragraphs 23 to 25 above, the New AFT Representations and the representations set out at paragraph 21 abpve (sic), all made by AFT in the advertisement at Annexure A are:
a. False; and/or
b. Misleading or deceptive, or likely to mislead or deceive.
27. By reason of the matters pleaded in paragraphs 23 to 26 above and its continuing conduct generally, AFT has:
a. Engaged in conduct in contravention of Section 18 of the ACL; and/or
b. Engaged in conduct in contravention of Section 33 of the ACL; and/or
c. Made false or misleading representations in contravention of Section 29(1)(a) or Section 29(1)(g) of the ACL.
28. Further or in the alternative, AFT has breached Order 12 of the [November 2018 Orders].
(2) commencing at [29] Reckitt addressed matters under the heading “Future Conduct” by reference to the HWL Letter. At [34] it pleaded that:
[Reckitt] is concerned that AFT will continue to publish the advertisements at Annexure A, or make some or all of the New AFT Representations in other forums.
66 Before proceeding further, we observe two matters: first, the references to the “material at Annexure A” and the “advertisement at Annexure A” in the notice of cross-claim and statement of cross-claim are references to the new advertisement; and secondly, Reckitt abandoned [23(c)] of the statement of cross-claim on the first day of the trial. As to the latter, AFT submitted that by Reckitt abandoning that representation the parties proceeded on the common ground that they were dealing with the usual “dental type study for non-chronic pain”.
67 In its defence to Reckitt’s statement of cross-claim, AFT:
(1) in response to [22] of Reckitt’s statement of cross-claim pleaded that it:
(a) admits that it has caused the [new advertisement] to be published in dental and pharmacy trade journals in Australia;
(b) admits that its sales representatives have been provided with the [training aid];
(c) admits that, through its sales representatives, it has caused stickers in the form attached as Annexure 2 to this Defence (POS Stickers) to be placed on point of sale materials for its Maxigesic product;
(d) otherwise denies the allegations made in that paragraph.
(2) in response to [23] of Reckitt’s statement of cross-claim pleaded that the new advertisement contained the statements which are set out at [13] above, that the reference denoted by “1” read as set out at [13] above, that “the statements made in the [new advertisement] are to be read and understood in the context of the advertisement as a whole” and otherwise denied the allegations in that paragraph;
(3) in response to [24] of Reckitt’s statement of cross-claim, pleaded as follows:
(a) admits that the [new advertisement] conveys that there is an adequate scientific foundation for the statements made in that advertisement;
(b) says that the statements made in the New AFT Advertisements are said to be supported by the Daniels and Aitken studies referred to in paragraph 23(b)(ii) above;
(c) otherwise denies the allegations made in that paragraph.
(4) denied the allegations in [25] to [28] of Reckitt’s statement of cross-claim; and
(5) in response to [34] of the statement of cross-claim admitted that, subject to the orders of the Court, it intended to continue to use the new advertisement, the training aid and the POS stickers in the course of promoting its Maxigesic product and otherwise denied the allegations made in that paragraph.
68 It is clear from its pleaded case that AFT sought relief in relation to the new advertisement only. While the relief sought by Reckitt in its cross-claim appeared to go beyond the new advertisement (see [64] above), it is apparent when the cross-claim is considered as a whole, that it was focussed on the New AFT Representations insofar as they were contained in the new advertisement and the other 2019 marketing materials.
69 That that was so was also evident from the parties’ written opening submissions to which we now turn.
3.1.2.2 Opening submissions
70 AFT’s written opening submissions included at [2]-[3]:
2. In February 2019, AFT started using a new series of advertising claims in relation to Maxigesic (New Maxigesic Claims). In summary, the New Maxigesic Claims make claims to the effect that Maxigesic provides “better and faster” pain relief than paracetamol or ibuprofen alone. The New Maxigesic Claims rely on the Daniels Study and the Aitken Study. We set out the New Maxigesic Claims and details of where they have been made in Annexure A.
3. AFT seeks declaratory relief to the effect that there is an adequate scientific foundation for the New Maxigesic Claims. [Reckitt] alleges that, by making the New Maxigesic Claims, AFT has engaged in contraventions of ss 18, 29(1)(a) and (g) and 33 of the ACL. The basis of [Reckitt’s] allegations is that there is no adequate scientific foundation for the New Maxigesic Claims.
(Footnotes omitted.)
71 Similarly, Reckitt’s written opening submissions observed (at [2]) that the proceeding concerned “an advertising campaign for Maxigesic that AFT has already run and proposes to continue to run”. At [5]-[6] Reckitt submitted:
5. In February 2019, [Reckitt] became aware that AFT had commenced publishing a new advertisement (New AFT Advertisement) in the Australian Journal of Pharmacy that made a number of claims of superiority of Maxigesic over paracetamol or ibuprofen alone. These claims of superiority are alleged to be supported by a “new clinical study”: the Daniels Study. However, as will be explained below, the Daniels Study did not test the commercially available formulation of Maxigesic sold in Australia, but rather a materially different formulation. No reliable clinical study has ever analysed the commercially available formulation of Maxigesic sold in Australia. A prior study, the Merry Study, was relied upon by AFT to promote Maxigesic in the previous advertising campaign, but that study was found by this Court in the Previous Judgment to be unreliable and unable to provide an adequate foundation in science to support any claim of superiority of Maxigesic over paracetamol or ibuprofen alone (including, in particular, the third Maxigesic representation).
6. In light of the fact that the Court had already found that there was not an adequate foundation in science to support any claim of superiority of Maxigesic over paracetamol or ibuprofen alone, AFT commenced this proceeding to obtain declaratory relief to the effect that there is an adequate foundation in science for the express statements appearing in the New AFT Advertisement. [Reckitt] filed a responsive cross-claim to obtain injunctive and other relief with respect to AFT’s new conduct.
(Footnotes omitted.)
72 At [15] Reckitt referred to its cross-claim by which it said it sought various declarations including to the effect that AFT, “by publishing the [new advertisement] in various medical, dental and pharmacy trade journals, and by placing the POS stickers on point-of-sale materials for Maxigesic, has made representations about Maxigesic that are false, misleading or deceptive, or likely to mislead or deceive in contravention of ss 18, 29(1)(a), 29(1)(g) and 33 of the ACL”. At [16] Reckitt set out the “Representations” as recorded in its statement of cross-claim (see [64] above).
73 At [18]-[20] Reckitt submitted that:
18. The sole issue in the context of AFT’s application is whether there is an adequate scientific foundation for the Statements. AFT bears the onus of demonstrating such an adequate scientific foundation. The only real point of difference in the context of [Reckitt’s] cross-claim is whether the additional matters of implication contained in the Representations arise.
19. Two further matters warrant emphasis. First, the question whether there is an adequate scientific foundation for the Statements and Representations depends upon resolution of the following issues:
a) whether the Statements and Representations are supported by the [2018 Daniels study] (with the Aitken Study); and
b) whether, even if the [2018 Daniels study] (with the Aitken Study) supports the Statements and Representations (which is denied), “the totality of the scientific evidence available” enables the Court to conclude that the authoritative comparisons of alleged scientific fact in the [new advertisement] can be made by AFT.
20. Secondly, AFT, by its application, has assumed the onus of demonstrating that there is an adequate scientific foundation for the Statements. The failure of AFT to discharge this onus would permit the Court to be satisfied that AFT has contravened Order 12 of the [November 2018 Orders] and justify the relief sought in the cross-claim.
(Footnote omitted.)
The “Statements” were recorded by Reckitt at [9] of its submission and were those statements pleaded by AFT in its statement of claim (see [62(3)] and [13] above).
74 On the first day of the trial AFT opened its case. Relevantly the following exchanges took place between senior counsel for AFT, Mr Crutchfield QC, and the primary judge:
Mr Crutchfield: All right. Thank you for that indication. Can I just try and summarise, as best I can, what we understand to now be the key issues between the parties?
Her Honour: Perhaps I might mention at the outset one issue that I have, which is that your pleading is – or the relief you’re seeking is a declaration that there’s an adequate scientific basis for particular statements, as opposed to particular representations. Some of the six statements don’t actually make syntactical sense, read alone, and, so, I was wondering, do I not need to find what an ordinary - - -
Mr Crutchfield: Yes.
Her Honour: - - - reasonable reader would mean? And is it not possible for you to identify, within the representations that have been identified by Reckitt, the ones for which you contend?
Mr Crutchfield: We will certainly do that. We will prepare a note of what we say is conveyed by the advertisement, yes.
Her Honour: Yes.
Mr Crutchfield: It’s certainly not what Reckitt’s - - -
Her Honour: Mr Murray’s probably interested too.
Mr Crutchfield: Yes. And there’s an issue in the case about whether it’s referring to 24 hours - - -
Her Honour: Yes.
Mr Crutchfield: - - - which is how Professor Keech would read the ad. So we can do that. We can set out what we say is conveyed by the representations - - -
Her Honour: Yes.
Mr Crutchfield: - - - by the statements, I should say.
Her Honour: Yes.
And:
Her Honour: Well, can I indicate – having looked at the representations that are pleaded by Reckitt - - -
Mr Crutchfield: Yes.
Her Honour: - - - I think there are seven of them – my initial reaction was that the advertisement might convey representations 1, 2, 4 and 7, and I mention that because I think what you’re directing yourself to now is representation 6. So - - -
Mr Crutchfield: (a), (b) and (g).
Her Honour: Yes. Maxigesic is more efficacious than paracetamol or ibuprofen alone based on the Daniels study.
Mr Crutchfield: Yes.
Her Honour: Yes to the second. No to the representation, which includes for all pain, because the advertisement needs to be read in the context of the first bullet point.
Mr Crutchfield: Yes.
Her Honour: Yes to 4, which incorporates maximum dosage.
Mr Crutchfield: Yes.
Her Honour: No to 5, which is the restrained representation, on the basis that there’s no reference in the ad to “stronger”.
Mr Crutchfield: Yes.
Her Honour: And no to 6: faster onset and more meaningful pain relief.
Mr Crutchfield: Yes.
Her Honour: As a matter of construction of the second bullet point that you’ve just pointed to.
Mr Crutchfield: Thank you, your Honour.
Her Honour: And, then, yes to the seventh.
Mr Crutchfield: And what we will do, perhaps over lunchtime, is come back to your Honour as to whether or not we agree that they’re the representations, if we don’t agree if there’s anything else, or why we don’t agree. Yes. All right. I’m grateful for that.
Her Honour: Obviously, that’s subject to whatever Mr Murray also wants to say, but I just wanted to let you know my initial reading of the advertisement.
And in taking the primary judge to the joint experts’ report:
Mr Crutchfield: Now, 33 is important, and we will find out – no doubt this will be subject for discussion tomorrow. Even had the two formulations demonstrated bioequivalence for all eight tests, and they did for all of them except fasting Cmax paracetamol, Professor Keech’s view is that:
Extrapolating results from the Daniels study to Maxigesic would not be warranted because even bioequivalent drugs do not produce exactly the same results, even if the result they produce are very similar.
Specifically, Professor Keech holds that:
The true numbers representing the relative effectiveness of Maxigesic to corresponding treatment with ibuprofen and paracetamol separately would be different to 36 or 37 per cent respectively by an unknown amount –
Now, just to pause there, “an unknown amount”, when your Honour re-reads our friend’s submissions, that’s what they quote from this joint report. They stop the quote there. The report goes on to say:
… even though we would expect them to be very similar.
So again, to come back to the ad, and just read – sorry, your Honour. Read the rest of – if I could read the rest of 33:
Professor Thisted agrees the results would not be identical, as even a replication of the Daniels study would not produce the exact same numerical percentages due to variability between patients.
Well, that’s obvious. If you ran this study even with the same patients, your Honour, you bet your bottom dollar you wouldn’t get to the exact same percentages, because someone will forget to take one of the drugs after six hours or fall asleep, or they had more to eat the day before and the drug was less effective or whatever, which – this is why it comes back to adequate foundation. But in view of both the substantial bioequivalence of the two formulations and the transient effect on blood levels in the one situation, where they differ, Professor Thisted holds that:
The percentage differences in pain relief should be substantially similar.
Her Honour: I think my initial reaction to the ad, given that it is so clearly pinned to the study - - -
Mr Crutchfield: Exactly.
Her Honour: - - - is that the percentages in the ad need to be understood as a summary of - - -
Mr Crutchfield: Precisely.
Her Honour: - - - what is explained in a level of detail in the study.
Mr Crutchfield: That’s precisely right, we would submit. …
And:
Mr Crutchfield: … And your Honour rightly says, well, Reckitt has pleaded that we haven’t made good what we say the representations are, so we will clarify that. We do say, though, that Reckitt’s pleaded cross-claim in the expert evidence that they put forward focused on the alleged representations that they say arise from the ad. And in that regard we direct your Honour’s attention to Reckitt’s submissions, paragraph 19.
And we make the point that there’s no consumer – of course, there doesn’t have to be – but there’s no consumer evidence before the court identifying that such representations, in fact, arise – that is, consumer evidence in relation to the stickers, the point-of-sale material, or from health professionals. Can I hand forward to your Honour the representations – a page that shows – yes. Mr Merrick is feeling hard done by, because he did have a page that sets out what we say the representations are, but I might hold on to that and give it to you your Honour after lunch, while I’ve had a look at it.
But what we will give to your Honour is the representations that are conveyed by the advertisement and the training ad, which is to health professionals, as your Honour knows, and, very importantly, the different representations that are conveyed to the public by the stickers, and your Honour knows they’re much narrower. I will give that to your Honour after lunch.
75 Reckitt also made oral opening submissions. At the outset, senior counsel for Reckitt at the trial, Mr Murray SC, had the following exchange with the primary judge:
Mr Murray: Your Honour, the first matter of characterisation – to get very clear in this case – is that we’re really confronted with a conflict between marketer’s convenience and scientific precision, because, unlike the impression left by my learned friend’s opening, your Honour is not sitting in judgment on the Daniels paper. Your Honour is sitting in judgment on the advertisement, and it’s a question of whether the statements made in that advertisement are likely to mislead or deceive. And that must absolutely be the focus of the inquiry. Now, it’s understandable that our friends have this backwards. For example, the way in which their experts were briefed was to give them the Daniels paper first, so they’re fully loaded up with all of that information, then look at the ad. And they say, “Yes, that’s what I saw in Daniels.” Well, that’s not what the poor pharmacist running a busy practice does. They don’t have Daniels prepopulated in their mind. They look at an ad in a trade journal, and they take it at face value. And, so, what this case is about are not notions of whether it’s passing strange that your Honour might find a shortcoming in the Daniels study when it has been submitted to some other institution such as – as the FDA. The question is whether – is there a proper foundation for the statements made in the advertisement? Now - - -
Her Honour: But doesn’t the – I mean, as I read the advertisement, the thrust of it is the Daniels study says you can get better and faster pain relief.
Mr Murray: Now - - -
Her Honour: It’s really an advertisement of the Daniels study.
Mr Murray: But the – no one is – well, your Honour knows, it’s an advertisement for a product. It’s an advertisement that is trying to reassure pharmacists that they should promote, in their practice, Maxigesic, comparatively – to the comparative advantage over the monotherapies alone.
Her Honour: On the basis of the Daniels study. It’s very explicitly and closely linked to the Daniels study.
Mr Murray: Well, no question. Well, no question. We agree with that, your Honour. Yes. But that doesn’t mean you assume that the reader of the ad has read the Daniels study and, in fact, it would be wrong to do so. …
76 In addition:
(1) by reference to the authorities on comparative advertising senior counsel for Reckitt said:
And one of the things, as I will demonstrate to your Honour in due course, it’s the lack of qualification in this ad that causes a great deal of difficulty.
(2) in taking the primary judge to the new advertisement, her Honour observed that she thought that “the relevant footnote is the one in the middle of the page”;
(3) senior counsel for Reckitt submitted that:
… Your Honour, my submissions at the commencement of my opening around the focus being on the ad rather than on the study were really aimed at responding to my friend’s insistence that this is an extremely well-designed study, and there were some flourishes on top of that. It’s really not the question.
The question is: what did it show, and does the ad, in an accurate and appropriately qualified way, reflect what it showed? There is a further question that arises about, even accepting what it showed – what the Daniels study showed – how does that sit against other material? And your Honour will hear further about that.
And:
Your Honour, I will say this, hopefully, once – unless your Honour wants to revisit it – so I don’t have to do it again: this case has nothing to do with the ceiling and I wasn’t proposing to address any more; that was the last case. This case is all about AFTs conduct and its advertisement and what one can take from the ad and take from the study so as to justify the ad, if they’re able to do so.
77 At the conclusion of the parties’ oral opening submissions the following exchange took place between the primary judge, senior counsel for AFT and senior counsel for Reckitt:
Her Honour: All right. Thank you. Before lunch, I will raise three matters. Firstly, in relation to the question of the representations, I should also indicate that my provisional view was where I found that the representation was not made, my provisional view is also that there’s no adequate scientific foundation for that representation. What I would like the parties to do is to try and identify all of the representations that are in play, which, hopefully, will be somewhat less than seven, but I concede the possibility that it might be more than seven, and agree as far as possible in cases – as to the fact that there’s no scientific foundation for any representation that’s still in play. I haven’t expressed that well. But, for example, to give you an example: if the representation was made that Maxigesic is faster and better for pain relief for all pain - - -
Mr Crutchfield: Yes.
Her Honour: - - - which I think it wasn’t.
Mr Crutchfield: Yes. Sure.
Her Honour: Then there’s no adequate scientific foundation for that.
Mr Murray: I can shortcut that, your Honour. We don’t press that representation.
Her Honour: Thank you.
Mr Crutchfield: We understand, and what we understood – we’ve taken a note of what your Honour understood the representations or took from Reckitt’s pleading - - -
Her Honour: Yes.
Mr Crutchfield: - - - as a representation from the ad. For what it’s worth, they were pretty close to me as well, but we will have a look at that over lunch and see if we agree precisely they are the representations and if we’ve got any modifications and any other representations that we say are conveyed by the ad, and we will see if we can reach an agreed position in relation to that, your Honour, yes.
78 After the luncheon adjournment junior counsel for AFT, Mr Merrick, addressed the topic of the representations as previously raised by the primary judge in the following exchange:
Mr Merrick: Then, your Honour raised with Mr Crutchfield the issue of the representations. We’ve had a look at that over lunch. Could I pass forward – and I will pass my friends – two copies of a document which sets out a slight adaptation, as it were, of the four representations which your Honour referred to. And your Honour will see items 1 to 4 adopt the terminology from the paragraphs that your Honour identified. Item 4 is a slightly different form of words that’s not picked up from our friend’s document at all:
Maxigesic provides better pain relief than paracetamol or ibuprofen alone.
Maxigesic provides better pain relief than paracetamol or ibuprofen alone. There’s a couple of reasons for that. Most significantly, the POS materials, for example, make no reference whatsoever to “faster”, so insofar as in earlier iterations of the representation that our friends have identified “better and faster”, that’s not apposite for the POS materials. And, of course, we say that all of these representations, while we can record them on a piece of paper in this manner, they can’t be divorced from the context of the ad and the Daniels study for the trial.
Her Honour: Yes.
Mr Merrick: Appreciates that point.
Her Honour: Thank you.
(Emphasis added.)
79 The document handed up by Mr Merrick was the Representations Document (see [43] above). AFT’s position is that when the Representations Document was provided to the Court, it was common ground that each of the five representations identified in it was subject to a qualification, namely that each representation was to be read by reference to the Daniels 2018 study. Even if that is not so, it is clear that the Representations Document was provided to the Court on the basis that it could not be “divorced” from the new advertisement or the Daniels 2019 study. At the time Reckitt made no contrary submission.
3.1.2.3 Closing submissions
80 At the commencement of its oral closing submissions senior counsel for AFT handed up a table which he said “capture[s], we think, the representations which we say arise, which pick up the four representations that your Honour identified on day one. … Plus number 5, which is the one that arises from the POS material”. The table provided by AFT is set out at [39] of the judgment (see [15] above).
81 Senior counsel for AFT then addressed the first representation in the table that “Maxigesic is more efficacious than paracetamol or ibuprofen alone” saying:
Our friends’ pleading doesn’t say this but we accept what your Honour said on day 1. I won’t go to it. It was page 8, line 20 where your Honour said that that representation has to be based on the Daniels study. And that’s how we think the representation should be read. That is to say, by the new AFT advertisement, the representation is being made that Maxigesic is more efficacious than paracetamol or ibuprofen alone. Why? What’s the basis for that? The Daniels study.
The transcript, which includes page 8 at line 20, is set out at [74] above. The discussion which took place at that time and to which senior counsel for AFT adverted in closing was about the new advertisement and that it was said to be based on and referrable to the Daniels 2018 study.
82 Further submissions were made in the course of closing referred to the new advertisement and the Daniels 2018 study including:
Mr Crutchfield: It’s important to recognise you’ve got to read the ad as a whole. And it’s talking about a study of dental pain. It’s talking about a faster onset of meaningful pain relief. Now, a health professional is going to understand what that is. And there was bioequivalence in relation to meaningful pain relief as well.
But the ad is referring to faster pain relief, or faster meaningful pain relief when you read the whole thing. It’s based on the time to meaningful pain relief as identified in the Daniels study. Now, that endpoint is only measured following the first dose, which is at six hours. The reference to at maximum dosage, which is in the first bullet point, obviously refers to the maximum dosage regimen for the drug, for Maxigesic. It’s expressed as a daily dose in the footnote: two tablets, four times a day. That’s fairly obviously to identify to pharmacists that the Daniels study was undertaken using the maximum dosage rather than, for example, taking only one tablet every six hours.
The ad refers to and faithfully reports the sum of the pain intensity differences at 48 hours figures in the Daniels study. Dr Russo’s evidence is that he would expect pharmacists to understand that the results relate to the duration of the study, which they would expect to cover the totality of the pain experience. That was transcript 148, lines 6 to 42. Reckitt – again, your Honour, this is important, not because it’s a pedantic pleading point but again because it strongly suggests Reckitt knows there’s nothing in this point. they did not plead any representation that better or better and faster applies at the 24 hour mark as a result of this ad. There’s no representation to that effect. There’s no evidence from any pharmacist to that effect. And it’s, of course, impossible for the faster results to be assessed at that time point. These percentage figures are obviously – and a pharmacist is going to understand it that way. A health professional is going to understand it that way, that it’s talking about the results that were achieved in that particular study. You get that from the first bullet point where it says:
A new clinical study.
You get it from the bit in bold where it says:
Proven to provide more effective pain relief in a new clinical study.
And you get it from the percentages. …
At that point the primary judge inquired where she could find the percentages in the Daniels 2018 study and was directed by senior counsel for AFT to aspects of the study where they could be located.
83 At the commencement of its closing submissions senior counsel for Reckitt submitted that the case “identifies a conflict between the marketer’s convenience and scientific precision”. He said:
Reckitt’s case here is no more than prefer scientific precision over the expediency of advertising. Your Honour, the – in opening, I identified or I addressed particular emphasis to the principles well-known to your Honour concerning comparative advertising and the dangers of half-truth in that context. In addition to that, can I add, please, something that really – the way we all had to end up looking at the ad and the point of sale material revealed, which is how one deals with asterisks and footnotes and disclaimers. Because where we had one witness in the court really unable to read the footnote in the size at which it would be presented to the market, it really calls that issue into stark relief. Now, our friends have to decide, in a sense, whether they want the footnote as part of the analysis or not. …
The primary judge interjected at this point noting that she thought she would have to read it, i.e. the new advertisement, on both bases. Senior counsel for Reckitt then continued:
And that’s our submission. And that’s our submission. And so if there is a problem that’s cured only by the footnote, well, then there’s no cure, relevantly. And if there’s a problem that arises because of the footnote well, then our friends are burdened with it.
84 Senior counsel for Reckitt then referred to the “chart” handed up by AFT noting that there was agreement that the representations in 1 to 5 were made but that those for which Reckitt contended at [23(e)] and [23(f)] of its statement of cross-claim (see [65(1)] above) were in issue and turned to address each of those representations. When it came to the representation at [23(f)], that “Maxigesic provides faster onset and more meaningful pain relief than for paracetamol and ibuprofen alone”, the following exchange took place between the primary judge and senior counsel for Reckitt:
Mr Murray: … Now, particularly if one is not invoking the footnote there’s no evidence – so let me take it back a step. Even if you read the footnote, the advertisement is not saved by anything that’s in the study because it can’t be thought that every reasonable member of the class will go and read the study. So it needs to stand of its force.
Her Honour: The advertisement?
Mr Murray: Yes.
Her Honour: Well, let me just test you on that because just in the first half of the page there are seven footnotes.
Mr Murray: Yes.
Her Honour: What I understand those footnotes are as a footnote to the ref 1 in the middle of the page.
Mr Murray: Yes, your Honour.
Her Honour: And then the ref 1 has, yet, another footnote which I think takes the reader down to the bottom of the page, and that reader may or may not able to read that footnote.
Mr Murray: Yes.
Her Honour: But it seems to me that anyone reading this will see that this is an advertisement for a study.
Mr Murray: Yes. Yes.
Her Honour: So it’s not as though as a pharmacist is going to look at that and say, “I’m going to put my own spin on what ‘better’ might mean or what ‘faster’ might mean”.
Mr Murray: Our case is slightly different to that, your Honour. The pharmacist, the first thing, except for the particularly scholarly or diligent, not going to go and look up a study for themselves. It’s just unrealistic to think they would.
Her Honour: No. But they’re also not going to say to themselves, you know, “I am going to pluck my own notion of ‘faster’ out and read the advertisement like that”. I’m reading this as an advertisement of a study, and this says you get faster, footnote, pain relief”. So it’s faster as whatever the study says.
Mr Murray: Well, your Honour, that’s why I went to the authorities about disclaimers because the disclaimer has to have the effect of curing the ill. And that assumes the disclaimer is proximate enough and large enough to be able to be brought into the analysis. But assuming it is, the disclaimer must cure any ill. Now, assume for the - - -
Her Honour: Well, I think – sorry, I just want to make sure that I’ve fully articulated what I’m saying here. It may be the case that in some circumstances a disclaimer has to cure the ill, but in this case, if this is, as I say, an advertisement for a study, what is being conveyed here is there is a study which says you will get faster pain relief.
Mr Murray: Yes.
Her Honour: So it’s not as though there is any ill that needs to be cured. It’s more if you want to know precisely what we are relying on you can go and read the study.
The debate continued. Senior counsel for Reckitt submitted that:1
So you can’t be saved by having to go to the study, if it would fundamentally affect what the ordinary person would take from the face of the advertisement. And so in our written submissions, we will link the well-known authorities on disclaimers and the like to that proposition. …
And at a later point, senior counsel for Reckitt said:
So our proposition is this, your Honour: that you – if, on reading the study, you would form a different impression about the ad then you need to give a hint on the face of the ad. And when I say a hint, I mean a substantive indication of what it is that you’re talking about.
85 Relevantly, in making its submissions, Reckitt did not suggest to the primary judge that the views her Honour expressed about how to read the new advertisement by reference to or, as her Honour put it, “as an advertisement of” the Daniels 2018 study were of no consequence because AFT had accepted that representations 1 to 5 in the table were unqualified.
86 Finally in submissions in reply, among other things in addressing the representations at [23(e)] and [23(f)] of Reckitt’s statement of cross-claim, senior counsel for AFT submitted that:
Can we just say one needs to focus on what the representations are. And this is where Reckitt are conflating matters. We say what Dr Russo said and, with respect, what your Honour has said, you just don’t overcomplicate this. You look at the ad and you say, “What is this ad conveying”. It’s saying that this is an advertisement about what a study has shown. It’s just clear, we say. And it’s even clearer, perhaps, from the POS material which is also blown up. I don’t need to go to it all. But your Honour will recall it all says based on a new study. So:
Get better pain relief based on a new study.
So this material is plainly conveying, we say – and, of course, the pharmacists who are receiving this material are going to have that POS material in their pharmacy. It’s not complicated, your Honour. It’s saying these representations are made based on the study. And so the representations appear in the ad. The representations don’t appear in the footnotes. And that’s what Reckitt have tried to do, and why – well, the only reason we’re raising these pleading issues. Because they try and lift out of the footnotes what they say a representation is about bioequivalence. Just take bioequivalence as an example. That’s not what’s conveyed by the ad. The ad is you get better and faster pain relief in accordance with this – with the study. And if you want to know more, go and have a look at the study.
(Emphasis added.)
87 After the close of oral argument the parties filed written closing submissions.
88 AFT’s submissions included:
(1) under the heading “Overview”:
3. AFT seeks declaratory relief to the effect that there is an adequate scientific foundation for the New Maxigesic Claims. [Reckitt] alleges that, by making the New Maxigesic Claims, AFT has engaged in contraventions of ss 18, 29(1)(a) and (g) and 33 of the ACL. The basis of [Reckitt’s] allegations is that there is no adequate scientific foundation for the New Maxigesic Claims.
4. As the case unfolded at trial, the dispute turns on the following issues:
(a) bioequivalence between the paracetamol / ibuprofen combination studied in the Daniels Study and the Australian formulation of Maxigesic;
(b) relevance of 24 hours data from the Daniels Study;
(c) is an adjustment to statistical significance for multiple comparisons warranted?;
(d) extent of scientific support for statements that Maxigesic provides better pain relief; and
(e) extent of scientific support for statements that Maxigesic provides faster pain relief.
(2) under the heading “Identifying the relevant representations”:
14. The impugned conduct of AFT concerns three types of promotional activity:
(a) an advertisement published by AFT in pharmacy and dental trade journals;
(b) a training aid used by AFT’s sales staff (but which is not in itself distributed within the trade); and
(c) point of sale (POS) materials.
These materials were attached as Annexure A to AFT’s opening submissions.
15. The [new advertisement] and training aid contain the following statements:
…
16. The reference denoted by “1” in these statements directs the reader to a text box in the middle of the page which reads as follows:
Daniels study results assessed on the intent-to-treat (ITT) population with adjustment for the use of rescue medication. Sensitivity analysis confirms primary end-point independent of rescue medication adjustment1.
17. The reference denoted by “1” in that text box in turn directs the reader to footnotes which read as follows:
…
18. The POS materials all contain the following statement:
GET BETTER PAIN RELIEF than Paracetamol or Ibuprofen alone1
Immediately adjacent to that statement appears a round badge which reads:
Based on a new study1.
The reference denoted by “1” directs the reader to footnotes which read as follows:
…
19. As is evident from paragraphs 14 to 18 above, there are important differences between (on the one hand) the [new advertisement] and training aid and (on the other) the POS materials. Further, the [new advertisement] and training aid are directed to health professionals, whereas the POS materials are directed to a wider audience including consumers.
20. In light of the above, AFT accepts that the following representations are conveyed by those promotional materials:
There followed the table set out at [15] above.
89 We pause to note that the reference to “the above” in para 20 is a reference to the matters set out in the closing submission at paras 14 and following. That is, necessarily AFT’s position of the extent to which the accepted representations were conveyed by the 2019 marketing materials was qualified or made in the context of its earlier submissions which described the 2019 marketing materials and their content in some detail.
90 AFT’s submissions continued:
21. During the trial, there was some debate about how the [new advertisement], training aid and POS materials would be read. AFT makes the following general submission as to that issue.
(a) A dominant, and unmissable, feature of the [new advertisement] and the training aid is the repeated and prominent references to the [Daniels 2018 study] or a “new clinical study”.
This occurs through references to a “new clinical study”, the “Daniels study” and the full reference to the [Daniels 2018 study] in the footnote.
(b) The advertisement and the training aid prominently identify that the study relied upon concerned “moderate to severe dental pain”.
(c) The advertisement and the training aid clearly identify that the claim that Maxigesic provides “faster” pain relief is a reference to “meaningful pain relief’.
(d) The advertisement and the training aid also make it clear that the New Maxigesic Claims are based on taking the “maximum dosage” of Maxigesic.
(e) The POS materials (unsurprisingly) convey less information. However, the statement “Based on a new study” (which is given further prominence in the POS materials) makes it clear that the claim to better pain relief is based on the results of a study. The details of the [Daniels 2018 study] are then set out in the footnote.
(f) Having regard to these matters, AFT submits that a reasonable reader of those promotional materials would understand that the New Maxigesic Claims convey (within the constraints of the advertising and marketing environment) the results of a new study (ie the [Daniels 2018 study]).
22. There was also some debate during the trial as to the role of the footnotes in the impugned materials. AFT submits that the Court should approach the matter on the basis that some readers (likely the majority) would not read the footnotes and some (likely the minority) would read the footnotes. Some readers (most likely health professionals) may be motivated to read the footnotes to identify and obtain further information about the study referred to in the New Maxigesic Claims. Ultimately, AFT submits that this is of little relevance to the identification of the representations conveyed by those promotional materials. The statements which convey the representations in dispute are not found in the footnotes, but rather in the body of the [new advertisement], training aid and POS materials.
23. As we discuss further below, the footnotes may be relevant to whether there is no adequate scientific foundation for the representations conveyed. Accordingly, it is useful at this point to note that the footnotes to the [new advertisement], training aid and POS material contain:
(a) a statement that in the [Daniels 2018 study], the participants took the fixed dose combination medicine four times a day;
(b) a statement based on the Aitken Study that a fixed dose combination of 975mg of paracetamol and 292.5mg of ibuprofen (ie the Daniels FDC) is bioequivalent to a fixed dose combination of 300mg of paracetamol and 300mg of ibuprofen (ie Maxigesic) at full dose; and
(c) in the context of product safety, a direction not to exceed the daily recommended dose.
D The applicable legal principles
24. The applicable legal test is well-established – it is whether there was no adequate scientific foundation for the New Maxigesic Claims having regard to the available body of scientific evidence. This test recognises that the Court may be asked to assess and balance competing scientific evidence and views. However, the test also recognises that absolute scientific certainty (an elusive concept) is not required. Rather, the test poses the following question:
Is it more probable than not that there is no adequate scientific foundation for the impugned representation having regard to the available body of scientific evidence?
(Footnotes omitted.)
91 Reckitt’s closing submissions included:
(1) under the heading “Introduction” after setting out the history including the findings made in the 2018 judgment:
6. In February 2019, AFT re-commenced comparative advertising claiming superior efficacy of Maxigesic over the monotherapies based upon the [Daniels 2018 study and claims of bioequivalence between Maxigesic and the Daniels FDC based upon the Aitken Study. The comparative advertising campaign (advertising material) involved: (a) the publication of a print advertisement in various medical, dental and pharmacy trade journals (New AFT Advertisement); (b) the distribution of a “training aid” to AFT sales representatives (Training Aid); and (c) the placing of “stickers” on point-of-sale materials for Maxigesic (POS Material). The advertising material included “claims to the effect that Maxigesic provides ‘better’ and ‘faster’ pain relief than paracetamol or ibuprofen alone”.
7. The representations of comparative superiority of Maxigesic over the monotherapies made by AFT in the advertising material are undoubtedly “unqualified and definitive statement(s) of scientific fact”. …
(Footnotes omitted).
(2) under the heading “Representations conveyed by the advertising material”:
29. AFT has conceded that the first 5 representations outlined below are conveyed by the advertising material. [Reckitt] contends that two further representations are conveyed by the advertising material – numbered 6 and 7 below and highlighted in bold.
There followed the table set out at [15] above with two additional representations as follows:
Representation | New AFT ad | Training Aid | POS Material |
6. When taken at their respective maximum recommended daily doses, Maxigesic provides stronger and more effective relief from pain than OTC doses of either paracetamol or ibuprofen alone. | Y | Y | Y |
7. Maxigesic provides faster onset and more meaningful pain relief than for paracetamol and ibuprofen alone | Y | Y | N |
(Footnotes omitted.)
(3) at paras 30 to 42 submissions in relation to whether representation 1 was conveyed by the POS material and whether representations 6 and 7, the additional representations set out above, were, as it contended, conveyed by the 2019 marketing materials.
3.1.2.4 Reckitt’s interlocutory application
92 On day three of the trial, at its conclusion, Reckitt pressed its application for an interlocutory injunction until the delivery of judgment in which it relevantly sought the following orders:
1. Upon the respondent by their Counsel giving the usual undertaking as to damages, an order that until further order, the applicant be restrained, whether by itself, its servants or agents or otherwise howsoever from publishing, distributing, exhibiting, displaying, broadcasting, or communicating to the public, or causing to be published, distributed, exhibited, displayed, broadcasted or communicated to the public each of:
a. the New AFT Advertisement (as defined in the Applicant’s Statement of Claim filed 21 February 2019 and appears at Annexure A to the Applicant’s Statement of Claim);
b. any matter which is substantially similar to, or substantially the same as, any of the above.
2. Upon the respondent by their Counsel giving the usual undertaking as to damages, an order that until further order, the applicant be restrained, whether by itself, its servants or agents or otherwise howsoever from publishing, distributing, exhibiting, displaying, broadcasting, or communicating to the public, or causing to be published, distributed, exhibited, displayed, broadcasted or communicated to the public each of:
a. any representation which conveys or substantially conveys that:
i. when taken at their respective maximum recommended daily doses, Maxigesic provides better, faster, stronger and/or more effective relief from pain than either paracetamol or ibuprofen alone (first Monotherapy Representation);
ii. Maxigesic provides better and faster pain relief than either paracetamol or ibuprofen alone for all pain (second Monotherapy Representation);
(together the Monotherapy Representations).
b. any representation that is substantially similar to, or to substantially the same effect as, the Monotherapy Representations.
93 The following exchange took place between the primary judge and senior counsel for Reckitt in relation to the form of relief sought by Reckitt in its interlocutory application:
Her Honour: Now, looking at the form of the relief that - - -
Mr Murray: Yes, your Honour. That was going to be my next point. The - - -
Her Honour: Well, can I put it this way?
Mr Murray: Yes.
Her Honour: Why would I make order 2, and what about the point of sale material?
Mr Murray: Your Honour would not make 2(a)(ii) because it picks up all pain, and I accept that we dropped that part of our case. Your Honour, 2(a)(i) is an amalgam of representations that are still in play. So there’s no drama because you know that better and faster are in play, and you know that efficacious, which is just another form of speech to effective, and your Honour knows from 23(e) that’s stronger.
Her Honour: But there’s no apprehended conduct.
Mr Murray: Other than - - -
Her Honour: The advertisement.
Mr Murray: Yes.
Her Honour: And the point of sale material.
Mr Murray: And the point of sale material. Yes. And so, we didn’t seek to finesse – we thought that prayer for relief sufficiently covered the point of sale material because it is all about better, as my friend accepted in argument today. And so 2(a)(i) is an appropriate reflection of the point of sale material. Sorry. I don’t – sorry. It doesn’t follow the point of sale material. It’s appropriate because of the threat posed by the point of sale material. Your Honour, the – of course, the other thing is the fact that your Honour disagreed with the precise drafting of an injunction that we see doesn’t mean if you were with us in all other respects, we lose. Your Honour, of course it’s completely a matter of your Honour’s discretion to sculpt the injunction as your Honour thinks necessary. But having – we accept 2(a)(ii) should fall away, but otherwise there is no vice in what’s put forward.
94 Ultimately, the primary judge was satisfied that the balance of convenience favoured granting an injunction in terms of prayer 1 of the interlocutory application, observing, in her view, that para (1)(b) covered the POS material. AFT submitted that once again this approach supports its contention that the issue before the primary judge was whether there was an adequate scientific foundation for the statements that it made in the new advertisement and the other 2019 marketing materials.
3.1.2.5 Did the primary judge err as alleged?
95 Having regard to the course of the trial before the primary judge, as illustrated by the matters set out above, it is apparent that the primary judge was mistaken when she said at [179] of the judgment that the “case proceeded on the basis that the [2019 marketing material] conveyed much broader representations”.
96 This was a proceeding by which AFT sought to vindicate its conduct in issuing the 2019 marketing materials. It was that material that included the statements referred to by AFT in its statement of claim (see [62(3)] above) and which, in turn, conveyed the representations alleged by Reckitt in its statement of cross-claim including the accepted representations. There was no dispute that, given the history of the litigation between them, AFT sought to obtain sufficient “clearance” from the Court by way of an appropriately fashioned declaration for its 2019 marketing materials which were based on the Daniels 2018 study. It did so, unsurprisingly, having regard to Order 12 of the November 2018 Orders which restrained AFT from making certain representations about the effectiveness of Maxigesic without an adequate scientific foundation.
97 Senior counsel for Reckitt, who did not appear below, submitted that AFT should be bound by its forensic decision in the running of the trial before the primary judge. However, that “forensic decision” was no more than the provision by AFT, in response to a request from the primary judge, of the accepted representations. They served to distil the representations which were said by AFT to be conveyed by the statements made in the new advertisement (and the other 2019 marketing materials) and thus to narrow the dispute about the representations conveyed by those materials. Critically, AFT provided the accepted representations on the basis that they were to be read as representations conveyed by the statements in the new advertisement and that they were made in that very context. That is, as we understand the way the case was conducted before the primary judge, and as is borne out by the exchanges and submissions set out above, AFT sought relief only in relation to the 2019 marketing materials and did not proffer the accepted representations on the basis that they were “broader representations” or “unqualified” such that they could arise outside of the 2019 marketing materials and absent reference to the Daniels 2018 study.
98 True it is that neither the Representations Document containing the accepted representations provided by junior counsel for AFT on the first day of the trial, nor the subsequent document provided in closing submissions, contained a statement qualifying the basis upon which those representations were made. With hindsight, it is evident that it would have been prudent to include such a statement. However, its absence does not mean, as Reckitt submitted, that the accepted representations were necessarily unqualified. Rather, as we have already observed, they ought to have been considered on the basis upon which they were first provided to the Court and thereafter on the basis on which the trial proceeded.
99 Senior counsel for Reckitt submitted that, had there been a qualifying statement or footnote to the accepted representations, Reckitt would not have acquiesced in the adoption of such an approach by AFT. But, that submission ignores the way in which the trial did in fact proceed without any objection being made by Reckitt at the time the accepted representations were first provided to the Court and when it was clearly submitted on behalf of AFT that they could not be “divorced from the context of the ad and the [Daniels 2018 study] for the trial”. It is impossible to know what the outcome may have been had Reckitt raised an objection at the appropriate time. It is simply the case that it did not.
100 It is difficult to reconcile [178] and [194] of the judgment on the one hand and [179] and [197]-[199] on the other. At [178] the primary judge accepted that the new advertisement could be read as a set of claims about the Daniels 2018 study and explained why that is so and at [194] her Honour returned to the new advertisement and accepted that AFT had an adequate scientific foundation for four of the statements said to be made therein to the extent to which they are read as reporting the results of the Daniels 2018 study. However, at [179] her Honour observed that the case proceeded on the basis that the 2019 marketing materials “conveyed much broader” representations and at [197]-[199] her Honour made findings that certain “unqualified representations did not have an adequate scientific foundation because they were unlimited as to “nature of pain, dosage and treatment duration”. If the primary judge made the latter findings because of the accepted representations, which we apprehend was the case, then her Honour did so having misunderstood the basis on which those representations were distilled and provided to the Court.
101 We have had regard to the re-opening judgment. There the primary judge considered whether she had proceeded on a misapprehension of the basis on which the Representations Document was provided by AFT to the Court, and the basis on which the case had been conducted. The trial judge’s views on such an issue would ordinarily be given significant weight. However, in this case it appears to us that her Honour’s analysis of the transcript, and the written submissions, was unduly narrow. In the circumstances, and in light of what emerges from our own analysis of the transcript and the written submissions, we think her Honour was mistaken in holding that the case was conducted on the basis that AFT accepted that statements appearing in the advertisement were to be construed without reference to qualifying statements or notes appearing elsewhere in the advertisement and in the training aid.
102 In the alternative, Reckitt submitted that, even if the Court accepted that the primary judge had misunderstood the way in which AFT put its case, there was no error because of the balance of the findings in [179] and following of the judgment. That is, Reckitt said that to the extent that there is no demonstrated error in the balance of [179], the findings there provide an independent basis upon which to dismiss this ground of appeal. The balance of [179] (see [32] above) concerns findings in relation to the first accepted representation that “Maxiegsic is more efficacious that paracetamol or ibuprofen alone” and the findings at [181]-[182] concern the fourth accepted representation that Maxigesic is 36% more effective than ibuprofen and 78% more effective than paracetamol. This submission was not developed in any detail. However, to the extent that the primary judge made findings, they were limited to findings about two of the five accepted representations and, in any event, those findings were made on the basis of her Honour’s understanding of the basis on which the case proceeded, namely that the 2019 marketing materials conveyed much broader representations.
103 It follows from the above that ground 2 of the appeal is made out and that her Honour proceeded on a misunderstanding of the basis on which the Representations Document was provided by AFT to the Court and the basis on which the case was conducted. That misunderstanding affected findings made by the primary judge including at [179]-[180] and [197]-[201] of the judgment.
3.1.3 Ground 1
104 Reckitt accepted that if AFT succeeded in establishing ground 2 of its appeal then it followed that it would succeed on ground 1. In proceeding on the mistaken basis identified above, the primary judge acted on the basis of a concession that was not made by AFT, namely, that each of the accepted representations would be understood by the relevant audience as unqualified by other statements and notes appearing in the new advertisement and the training aid: see Australian Competition and Consumer Commission (ACCC) v Coles Supermarkets Australia Pty Ltd [2014] FCA 634; (2014) 317 ALR 73 at [38]-[42].
3.1.4 Ground 3
105 By this ground AFT contends that the primary judge erred in finding that the new advertisement and the training aid conveyed the representation that Maxigesic provides “faster pain relief” rather than a representation that Maxigesic provides “faster onset of meaningful pain relief”.
106 AFT referred to [54]-[55] of the judgment where the primary judge found that a reasonable reader of the new advertisement and the training aid would understand the headline reference to “faster” pain relief to be qualified by the subsequent reference to “faster onset of meaningful pain relief”. AFT submitted that, despite that finding, at [190] and [196] of the judgment the primary judge found that the new advertisement (and thus the training aid) conveyed a representation as to Maxigesic providing “faster” pain relief, without being qualified as a reference to the “onset of meaningful pain relief”.
107 AFT submitted that those latter findings illustrate the errors of principle and fact made by the primary judge. AFT contended that they expose an error of principle because they do not reflect how a reasonable reader would understand the content of the new advertisement (and the training aid) and only arise because the primary judge disregarded that reading of the new advertisement and the training aid in favour of a decontextualised assessment of unqualified representations. AFT contended that the findings also expose an error of fact because the only apparent basis for them is the primary judge’s conclusion that AFT had accepted that an unqualified representation as to “faster” pain relief had been made, a position which it did not accept.
108 At [54]-[56] of the judgment under the heading “Faster onset and more meaningful pain relief” the primary judge said:
54 Reckitt argued that the references to “faster” pain relief are not limited by the bullet point which refers to “faster onset of more meaningful pain relief”. I do not accept this submission, having regard to the relevant audience for the new advertisement and the training aid which I take to be relatively discerning and likely to understand the banner claim to be qualified by reference to the bullet-pointed claims, especially having regard to the repeated use of footnotes in the banner claim. Despite the fact that the footnotes do not refer the reader to the bullet points, the multiple footnotes in the banner claim signify that the claim is not unqualified. In my view, even without looking at the content of the footnotes, the repeated use of footnotes emphasised that the general claim of “better and faster pain relief” is to be understood by reference to a particular study or studies.
55 I also do not agree that the relevant reader would understand “meaningful pain relief” to be a subcategory of “pain relief”, distinct from “perceptible pain relief”. Rather, the reader would understand that the words “faster onset of meaningful pain relief” describe the results of a study and, equally, that the claims made in the advertisement generally reflect the results of a study so it would not be appropriate to draw an inference of “faster onset and more meaningful pain relief”.
56 Accordingly, I do not accept that the new advertisement and the training aid convey a representation that Maxigesic provides faster onset and more meaningful pain relief than for paracetamol and ibuprofen alone.
(Emphasis in original.)
109 In doing so the primary judge was clearly addressing [23(f)] of Reckitt’s cross-claim where Reckitt alleged that by the new advertisement AFT conveyed, among others, the representation that Maxigesic provides faster onset and more meaningful pain relief than for paracetamol and ibuprofen alone (see [64] above). Her Honour found that the new advertisement and the training aid did not convey that representation.
110 At [196] of the judgment the primary judge, in addressing the statements which AFT said were made in the new advertisement, addressed those statements which claim “faster pain relief” namely that:
(1) “maxigesic GET BETTER1 & FASTER1 PAIN RELIEF THAN PARACETAMOL OR IBUPROFEN ALONE1”;
(2) A new clinical study of moderate to severe dental pain shows that Maxigesic® provides better and faster pain relief than both paracetamol and ibuprofen alone, at maximum dosage1;
111 There, her Honour found:
However, even reading the new advertisement as making statements that can only be understood by reference to the Daniels 2018 study, I am not persuaded that the statements which claim “faster pain relief” have an adequate scientific foundation because the Daniels FDC did not meet the stated “key secondary endpoint”, which concerned time to pain relief, to a statistically significant degree or, alternatively, because it did not meet the stated end point of time to perceptible pain relief to a statistically significant degree.
112 In both parts of the judgment referred to above, the primary judge addressed the claim to “faster” pain relief, albeit in the context of, on the one hand, Reckitt’s cross-claim and, on the other, AFT’s claim that there was an adequate scientific foundation for the particular statements. In the context of the former the primary judge made a finding that the repeated use of footnotes emphasised that the general claim of “better and faster pain relief” is to be understood by reference to a particular study or studies. AFT contended that it is difficult or reconcile that finding with the later finding at [196].
113 At [194] of the judgment, the primary judge accepted that AFT had an adequate scientific foundation (i.e. the Daniels 2018 study coupled with the Aitken study) for the statements which appear in the advertisement to the extent to which they are read as reporting the results of the Daniels 2018 study. Her Honour identified four specific statements at [194] that she found were supported by the Daniels 2018 study as those statements were presented in the advertisement. In particular, her Honour found that the statement “faster onset of meaningful pain relief” was supported by the result achieved in respect of that secondary end point. It follows that, if the “faster pain relief” statement appearing in the banner of the advertisement is to be understood as qualified by reference to the bullet pointed claim referencing “faster onset of meaningful pain relief” (as found by her Honour at [54]) then, on the basis of that finding (with which we agree) the “faster pain relief” statement as it appears in the banner when read with the reference to “meaningful pain relief” in the bullet pointed claim is supported by the Daniels 2018 study. For that reason we do not consider that the statement “faster pain relief” as it appears in the advertisement is misleading or deceptive or likely to mislead or deceive.
114 In [196] of the judgment, the primary judge refers to the fact that the Daniels 2018 study did not meet the “key secondary endpoint” concerning time to pain relief or another endpoint concerning time to “perceptible pain relief”. On that basis her Honour said she was not persuaded that the statement “faster pain relief” has an adequate scientific foundation.
115 With respect, we have some difficulty with this paragraph in her Honour’s reasons. If the paragraph is taken to be directed to the situation in which the “faster pain relief” statement is qualified by reference to the bullet pointed claim as found in [54] of her Honour’s judgment, then the conclusion expressed in [196] is in our view inconsistent with that finding. However, another possibility is that [196] of the judgment reflects what we have found was her Honour’s mistaken view as to the manner in which the case was conducted and that it is to be understood as a finding that an unqualified claim to “faster pain relief” did not have an adequate scientific foundation because there were a number of secondary endpoints relevant to an unqualified claim for “faster pain relief”, some of which were not met. Either way, it seems to us that when the statement “faster pain relief” is read in the context of the related bullet point (as we believe it should be) then the Daniels 2018 study, when read with the Aitken study, provides an adequate scientific foundation for the “faster pain relief” claim in the banner of the advertisement.
116 Ground 3 is made out.
3.2 Ground 4
117 By this ground AFT contends that by reason of the errors in grounds 1 to 3 the primary judge erred in finding at [197]-[201] of the judgment that in making the accepted representations and the restrained representation in the new advertisement and the training aid, and the first and fifth accepted representations and the restrained representation in the POS materials, AFT engaged in conduct in contravention of ss 18, 29(1)(g) and 33 of the ACL.
118 It follows from our conclusions in relation to grounds 1 to 3 that her Honour did so err and that those findings, which apparently followed from her Honour’s misapprehension of the basis on which the Representations Document was provided to the Court, and the basis on which the case was conducted, ought not to have been made.
3.3 Ground 5
119 By ground 5 AFT contends that the primary judge erred by making declarations that it had breached Order 12 of the November 2018 Orders by making the restrained representation in the 2019 marketing materials. However, given our findings in relation to grounds 1 to 4, it is not necessary to determine this ground.
4. Conclusion
120 Given the conclusions we have reached in relation to grounds 1 to 4, the appeal should be allowed and the declarations and the Orders made by the primary judge on 24 September 2020, with the exception of Order 11, should be set aside and in lieu thereof:
(1) declarations should be made to the effect that there is an adequate scientific basis for the statements included in the new advertisement and training aid and, to the extent they are so included, the POS materials. Those declarations should be framed so as to tie the statements to the new advertisement, training aid and/or POS materials and the context in which they appear; and
(2) orders should be made dismissing Reckitt’s cross-claim and for Reckitt to pay AFT’s costs of the proceeding before the primary judge.
121 Given AFT’s success, Reckitt should pay its costs of the appeal.
122 We will make declarations and orders accordingly.
I certify that the preceding one hundred and twenty-two (122) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justices Nicholas, Markovic and Burley. |
Annexure 1





