Federal Court of Australia
Ariosa Diagnostics, Inc v Sequenom, Inc [2021] FCAFC 101
ORDERS
Appellant | ||
AND: | Respondent | |
VID 879 of 2019 | ||
| ||
BETWEEN: | SONIC HEALTHCARE LIMITED Appellant | |
AND: | SEQUENOM, INC Respondent | |
VID 910 of 2019 | ||
| ||
BETWEEN: | CLINICAL LABORATORIES PTY LTD Appellant | |
AND: | SEQUENOM, INC Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The parties confer and within 14 days file an agreed minute of orders, including as to costs, reflecting the reasons of the Court, or, in default of agreement:
(a) within 14 days of the date of these orders, the appellants are to file and serve submissions of no more than 5 pages in support of their draft form of orders;
(b) within 14 days thereafter, the respondent is to file and serve submissions of no more than 5 pages in response; and
(c) within 7 days thereafter, the appellants are to file and serve any submissions in reply of no more than 2 pages.
2. Unless otherwise requested, any outstanding issues between the parties be determined on the papers.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
[1] | |
[7] | |
[9] | |
[10] | |
[13] | |
[17] | |
[22] | |
[30] | |
[30] | |
[65] | |
[76] | |
[76] | |
[94] | |
[95] | |
[108] | |
[168] | |
[168] | |
[179] | |
[181] | |
[185] | |
[185] | |
[204] | |
[206] | |
[219] | |
[227] | |
[228] | |
[228] | |
[233] | |
[234] | |
[236] | |
[244] | |
[244] | |
[245] | |
[249] | |
[250] | |
[252] | |
[272] |
THE COURT:
1 The present appeals concern a ground-breaking discovery by Professor Yuk-Ming Lo that cell-free foetal DNA could be detected in the blood plasma and serum of pregnant women. It led to the grant of Australian patent No.727919, entitled “Non-invasive prenatal diagnosis”, claim 1 of which is:
A detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample.
2 The respondent, Sequenom, Inc, is the patentee. Ariosa Diagnostics Inc conducts and licenses others, including Sonic Healthcare Ltd and Clinical Laboratories Pty Ltd (collectively, the appellants) in Australia, to conduct a non-invasive prenatal test called the Harmony Test. Sequenom sued the present appellant’s alleging that the Harmony Test infringed claims 1, 2, 3, 5, 6, 9, 13, 14, 22, 23, 25 and 26 of the patent. The appellants cross-claimed seeking the revocation of those claims. They failed to establish invalidity in respect of the grounds of lack of manner of manufacture, lack of inventive step, lack of utility, lack of sufficiency and false suggestion, but succeeded in relation to claim 26 in their allegations of lack of fair basis. The respondent succeeded in its infringement case on all but claim 26: Sequenom, Inc. v Ariosa Diagnostics, Inc. [2019] FCA 1011; 143 IPR 24 (Beach J).
3 The appellants now appeal against the decision of the primary judge on the bases that he erred in his conclusions in relation to lack of manner of manufacture (appeal grounds 1 to 5), lack of fair basis (appeal grounds 6 to 9), lack of sufficiency (appeal grounds 10 to 11) and aspects of the infringement findings (appeal grounds 12 to 15). In the present appeals, the appellants challenge the validity of claims 1, 2, 3, 5, 6, 9, 13, 14, 22, 23 and 25 (relevant claims).
4 The priority date of the patent is 4 March 1997. It was filed on 4 March 1998 and granted on 19 April 2001. The relevant legislation applicable to the issues in suit is the Patents Act 1990 (Cth) in the form that it took at the priority date.
5 For the reasons set out in more detail below, we dismiss appeal grounds 1 to 13, but allow the appeal insofar as it challenges the primary judge’s findings concerning infringement by use of the “send out test” as identified in grounds 14 and 15 of the appeal.
6 There are divided views as to whether the correct spelling is “foetus” or “fetus” (and its derivatives). In these reasons we adopt the former.
2. BACKGROUND COMMON GENERAL KNOWLEDGE
7 The primary judge commenced his judgment with a glossary of some relevant terms in the field of prenatal diagnosis and then set out a summary of the common general knowledge in the fields of foetal medicine and molecular genetics as at the priority date. He found that the relevant person skilled in the art to whom the patent is addressed is a team comprising a person with experience in foetal medicine and in particular an interest in prenatal screening and diagnosis, and a person with experience in standard molecular genetics techniques, preferably including some practical experience of the techniques involved in laboratory-based genetic analysis of patient samples in a clinical context. The findings that he made in respect of these subjects are not challenged on appeal.
8 The following matters are relevant background to understanding the disclosure of the patent and have been extracted from the glossary of terms and the primary judge’s findings of common general knowledge as at the priority date.
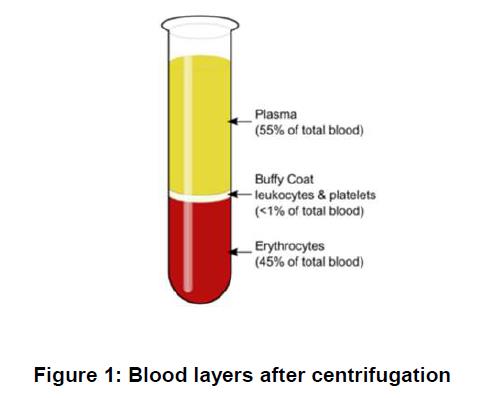
9 It was known in the field that foetal cells were present in the mother’s blood. Plasma makes up 55% of the blood. It contains water, blood plasma proteins (including clotting factors), minerals and dissolved nutrients and waste products. Serum is the name given to plasma which has had the clotting factors removed. Whole blood can be separated by centrifugation into three layers: (a) the upper plasma layer; (b) the “buffy coat” layer which contains leukocytes and thrombocytes; and (c) the lower layer which contains the erythrocytes which may be depicted as follows:
10 “Cell free DNA” is non-cellular DNA (i.e. DNA that is outside a cell). “Cell free foetal DNA” (cffDNA) is non-cellular DNA from a foetus.
11 The idea that foetal cells might be present in the mother’s blood had been first proposed in 1969. The possibility of being able to access whole foetal cells by taking a maternal blood sample was of great interest because it would allow analysis of the foetal genome to be carried out without the need for invasive testing, which typically involved extracting a sample with a needle inserted through a mother’s abdomen or cervix, and the problems associated with those invasive techniques such as an increased risk of miscarriage. Instead, all that would be needed was a blood sample from the mother’s arm. As a result, a substantial amount of work was carried out in this area through the 1980s and the 1990s. By the priority date it was known that several different types of foetal cells were present in maternal blood during pregnancy, and that these foetal cells had the potential to be used for prenatal testing.
12 From at least the late 1980s there was research being done by a number of groups on processes of recognition and enrichment of foetal cells. The aims of this research included investigating the use of foetal cells isolated from maternal blood to provide a non-invasive means of diagnosis of Down syndrome, to determine the blood type of the foetus and enable the pregnancy to be managed accordingly, to provide a way to determine the sex of the foetus (which would be useful when trying to identify foetuses with possible sex-linked disorders), and to diagnose single gene disorders. However, isolating foetal cells was not easy because they were known to occur only rarely in maternal blood and were vastly outnumbered by maternal cells in the sample.
13 The human genome represents the complete set of inherited instructions encoded in DNA in a human cell. Genetic disorders are caused by changes to the genome. They may be caused by a range of mechanisms such as deletions, duplications, rearrangements and chemical modifications affecting anything from a single base to a whole chromosome or even the whole genome. Those caused by a defect in only one gene, for example cystic fibrosis, are classed as “single-gene disorders”. Other disorders may be caused by one of a number of genes. Although the spectrum is a continuum, disorders involving larger regions such as partial or whole chromosomes, for example Down syndrome, are generally referred to as “chromosomal” whilst those involving changes at the nucleotide level are generally classed as “molecular”. Genetic disorders may be inherited or arise “de novo” in the germ cell or developing embryo.
14 Before the priority date, prenatal testing was available for some single gene disorders, such as sickle cell anaemia, thalassemia and cystic fibrosis. Disorders can be inherited in an X chromosome-linked fashion in which a male will be more likely to have the disease phenotype as they only have one copy of the X chromosome.
15 A number of diseases were known to be caused by a defective gene on the X chromosome, e.g. haemophilia. In many cases, these diseases primarily affect male foetuses because female foetuses will have another, non-defective, copy of the X chromosome. Work was on-going at the priority date to try to find ways to identify foetuses with possible sex-linked disorders. There was, therefore, a desire to develop a way to identify the sex of the foetus quickly, accurately and as early as possible in the first trimester. Further, in cases of congenital adrenal hyperplasia, which is not an X-linked condition, treatment with dexamethasone is needed to prevent virilisation in girls. This medication can be stopped once the foetus is known to be male. In severe X-linked diseases for which the parents may wish to elect termination, having a diagnosis as early as possible in the first trimester is valuable. DNA testing for diseases in such cases, which often took a week or longer, would only be done after the sex determination and could be omitted if the foetus was known to be female. Gender determination by ultrasound only became reliable for establishing the sex of a foetus from 18 weeks onwards.
16 As at the priority date, the sex of a male foetus would be apparent if a particular molecular test involved the amplification of sequences known to be from the Y chromosome. In other words, if one could detect a Y-specific fragment, it was most likely that the foetus was male. This required sequence information for the Y chromosome to be known to allow for the design of primers to result in the Y-specific polymerase chain reaction (PCR) products. Further, the loci would need to be known to be unique to the Y chromosome. As at the priority date, there were Y-specific sequences published in the literature which could be used as PCR primers.
2.4 Molecular genetic diagnosis
17 Molecular genetic clinical diagnosis first became possible in the 1980s following the discovery of genetic markers, that is, known sites of variation between individuals within a population located on a particular chromosome, otherwise known as genetic polymorphisms.
18 The process of working out which allele (marker) a person had at a particular position or set of positions on a chromosome, that is, determining the genetic make-up of the alleles at the relevant loci on an individual’s chromosomes was called genotyping.
19 At the priority date the vast majority of genotyping methods depended on either detecting differences by length of genetic variation, sequence, position, conformation, or methylation.
20 Genetic markers used to detect human DNA per se could be as simple as detecting any one of the many repeat element families in the human genome e.g. Alu repeats. An alternative approach would be to test for the presence of a single copy gene. A more refined method of detection would rely on demonstration of differences based on polymorphism such as restriction fragment length polymorphisms (RFLPs) detected either by genomic Southern blotting, or by PCR. Other markers commonly used at the time included short tandem repeats (STRs), variable number of tandem repeats (VNTRs), and other genetic variants. These markers and others, along with the determination of single or multiple copy sequences relative to a standard, were used to quantify DNA.
21 In 1997 thousands of markers that could be used for human molecular genetic testing, including STRs, VNTRs, RFLPs and markers of structural variants, were known. Of the variants, those used for human molecular screening/diagnosis would detect or be closely linked to the mutations underlying the specific genetic disease. Such markers would ideally be validated to document sensitivity, specificity, positive and negative predictive values. A wider number of markers may be required for diagnostic testing compared to screening.
22 By the priority date there was a range of molecular techniques and technologies available. These included gel electrophoresis, PCR (including nested PCR, and quantitative PCR techniques) and ligase chain reaction (LCR). For present purposes it is sufficient to describe PCR.
23 PCR is a standard molecular biology technique that involves the amplification of specific sequences of DNA using repeated cycles of denaturation, primer annealing and extension, resulting, in theory, at least in exponential accumulation of DNA fragments.
24 A primer is a short nucleic acid sequence that serves as a starting point for DNA synthesis. It is required for DNA replication because the enzymes that catalyse this process, DNA polymerase, can only add new nucleotides to an existing strand of DNA. DNA primers must be designed to bind to opposite DNA strands and flank the target of interest and initiate synthesis of a new DNA strand complementary to the target sequence of each template strand. These primer pairs are commonly referred to as the forward and reverse primers.
25 PCR requires the following components:
(a) A DNA template which is the gene or DNA sequence to be amplified.
(b) Primers, which are short single stranded pieces of DNA that have been synthesised to be complementary to a section of DNA. Two primers flank the gene or DNA fragment (in opposite orientation) to be amplified.
(c) DNA polymerase, being an enzyme that recognises primers and mediates the replication of the DNA template between the two primers starting from the 5' end to the 3' end (being the two ends of a strand of DNA) by adding a complementary base to the growing strand. The most commonly used of these enzymes was Taq DNA polymerase which was derived from the heat-resistant bacteria Thermus aquaticus. As it is heat resistant it is suitable for use in PCR, which relies on thermal cycling.
(d) Nucleotides (dNTPs or deoxynucleotide triphosphates), being single units of the bases A, T, G, and C or in other words the “building blocks” for new DNA.
26 In broad terms, PCR involves the following steps:
(a) Denaturation where the sample DNA is heated to break the weak bonds between the nucleotides of each strand so that the DNA separates into two separate strands.
(b) Annealing. PCR does not copy all of the DNA in the sample but only a very specific gene or DNA sequence targeted by the primers. In this step, the primers bind, or anneal, to their complementary sequences on the DNA strands, marking the beginning of the sequence to be copied in the following step. Annealing occurs at lower temperatures than denaturation, and in this respect a balance needs to be achieved between a higher temperature which achieves greater specificity / lower mismatches, and the risk of denaturing the primers at those higher temperatures which prevents binding.
(c) Extension where the reaction tube is heated to facilitate the extension of the nucleotide chain. From the start of the regions marked by the primers, nucleotides in the solution are added to the annealed primers by DNA polymerase to create a new strand of DNA complementary to each of the single DNA template strands. After this step, two identical copies of the original target DNA sequence are made. The extension must continue past the position of the primer on the complementary strand, so that in the next PCR round that new copy serves as a template for further amplification.
(d) Repetition of the above steps about 30 to 35 times to produce a sufficient amount of DNA copies of the original DNA target sequence. Too many amplification cycles beyond this leads to a building up of PCR “artefacts”, which create extra bands and impede analysis.
27 PCR relies on the design of primers that will amplify a sequence of interest. As at the priority date, a number of issues needed to be considered in primer design, which sometimes required multiple rounds of optimisation, including limited sequence information and manual primer design. The Gene Mutation Database and Human Genome Database were used at the priority date to design primers for use in DNA amplification methods for human molecular genetic testing. Therefore, it was wise to validate any chosen marker. Both of these databases were searchable for specific DNA loci and the specific sequences could be downloaded and employed for primer design.
28 Quantitative PCR (qPCR), is a broad term that is used to refer to PCR methods which enable the products of a conventional PCR reaction to be quantified. By the priority date, amplification of sequences that were the targets of qPCR could be detected using a number of methods including agarose gels (a form of gel electrophoresis), fluorescent labelling of PCR products and detection with laser-induced fluorescence using capillary electrophoresis or acrylamide gels and plate capture and sandwich probe hybridisation.
29 One type of qPCR method that was available by the priority date was real-time quantitative PCR (real-time qPCR). Real-time qPCR involved a PCR reaction during which accumulation of the amplified PCR product was monitored by measuring a signal created by either fluorescent dyes or fluorescent probes in the reaction sample to generate an amplification curve. That is, real time qPCR is a development of standard (or end-stage) PCR, and uses fluorescent reporter molecules to monitor the amounts of PCR product present after each PCR cycle. This allows the generation of a growth curve and enables the quantification of DNA in the exponential phase by determining the number of amplification cycles necessary to achieve a specified fluorescence level.
30 The patent commences by identifying the field (page 1 lines 1 to 5):
This invention relates to prenatal detection methods using non-invasive techniques. In particular, it relates to prenatal diagnosis by detecting foetal nucleic acids in serum or plasma from a maternal blood sample.
31 The patent continues by referring to known methods for detecting foetal abnormalities (page 1 lines 7 to 11):
Conventional prenatal screening methods for detecting foetal abnormalities and for sex determination traditionally use foetal samples derived by invasive techniques such as amniocentesis and chorionic villus sampling. These techniques require careful handling and present a degree of risk to the mother and to the pregnancy.
32 The patent then turns to techniques for predicting abnormalities (page 1 lines 12 to 14):
More recently, techniques have been devised for predicting abnormalities in the foetus and possible complications in pregnancy, which use maternal blood or serum samples. Three markers commonly used include alpha-foetoprotein (AFP- of foetal origin), human chorionic gonadotrophin (hCG) and estriol, for screening for Down’s Syndrome and neural tube defects.
33 The patent continues (page 1 lines 18 to 28):
The passage of nucleated cells between the mother and foetus is now a well-recognised phenomenon (Lo et al 1989, Lo et al 1996). The use of foetal cells in maternal blood for non-invasive prenatal diagnosis (Simpson and Elias 1993) avoids the risks associated with conventional invasive techniques. WO 91/08304 describes prenatal genetic determination using foetal DNA obtained from foetal cells in the maternal blood. Considerable advances have been made in the enrichment and isolation of foetal cells for analysis (Simpson and Elias 1993; Cheung et al 1996). However, these techniques are time-consuming or require expensive equipment.
34 The specification then describes a breakthrough discovery at page 2 lines 5 to 9:
It has now been discovered that foetal DNA is detectable in maternal serum or plasma samples. This is a surprising and unexpected finding; maternal plasma is the very material that is routinely discarded by investigators studying non-invasive prenatal diagnosis using foetal cells in maternal blood.
35 It goes on to describe some benefits of the discovery, including that using serum or plasma results in higher detection rates than comparable volumes of blood, suggesting enrichment of foetal DNA in maternal plasma and serum. At page 2 lines 12 to 16 the specification explains that:
… the concentration of foetal DNA in maternal plasma expressed as a % of total DNA has been measured as from 0.39% (the lowest concentration measured in early pregnancy) to as high as 11.4% (in late pregnancy), compared to ratios of generally around 0.001% and up to only 0.025% for cellular fractions (Hamada et al 1993).
36 In what may be described as a broad characterisation of the invention the specification then says (page 2 lines 20 to 23):
This invention provides a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention thus provides a method for prenatal diagnosis.
37 The term “prenatal diagnosis” is defined in the passage that follows (page 2 line 24 to page 3 line 4):
The term “prenatal diagnosis” as used herein covers determination of any maternal or foetal condition or characteristic which is related to either the foetal DNA itself or to the quantity or quality of the foetal DNA in the maternal serum or plasma. Included are sex determination, and detection of foetal abnormalities which may be for example chromosomal aneuploidies or simple mutations. Also included is detection and monitoring of pregnancy-associated conditions such as pre-eclampsia which result in higher or lower than normal amounts of foetal DNA being present in the maternal serum or plasma. The nucleic acid detected in the method according to the invention may be of a type other than DNA e.g. mRNA.
38 The specification explains aspects of how the method may be performed, both in the examples and also in the general description. In the latter, it explains first that a maternal serum or plasma is “derived” from the maternal blood, and that as little as 10 µl of serum or plasma can be used (page 3 lines 5 to 6).
39 Secondly, it refers to the preparation of the serum or plasma (using “standard techniques” which are expanded upon in the examples) and, normally, the process of nucleic acid extraction (page 3 lines 11 to 23):
The preparation of serum or plasma from the maternal blood sample is carried out by standard techniques. The serum or plasma is normally then subjected to a nucleic acid extraction process. Suitable methods include the methods described herein in the examples and variations of those methods. Possible alternatives include the controlled heating method described by Frickhofen and Young (1991). Another suitable serum and plasma extraction method is proteinase K treatment followed by phenol/chloroform extraction. Serum and plasma nucleic acid extraction methods allowing the purification of DNA or RNA from larger volumes of maternal sample increase the amount of foetal nucleic acid material for analysis and thus improve the accuracy. A sequence-based enrichment method could also be used on the maternal serum or plasma to specifically enrich for foetal nucleic acid sequences.
40 Thirdly, it explains (at page 3 lines 24 to 28) that the amplification of foetal DNA sequences in a sample is normally carried out, and describes “standard nucleic acid amplification systems” that can be used as including PCR, LCR, nucleic acid sequence based amplification, branched DNA methods “and so on”. However, preferred amplification methods involve PCR.
41 The specification then sets out one use to which the method of detection may be put (page 3 line 29 to page 4 line 1):
The method according to the invention may be particularly useful for sex determination which may be carried out by detecting the presence of a Y chromosome. It is demonstrated herein that using only 10 µl of plasma or serum a detection rate of 80% for plasma and 70% for serum can be achieved. The use of less than 1ml of maternal plasma or serum has been shown to give a 100% accurate detection rate.
42 As examples of detection methods within the invention, the specification describes each of (page 4 lines 5 to page 5 line 2): foetal RhD status determination in rhesus negative mothers; the detection of haemoglobinopathies; and the detection of paternally-inherited DNA polymorphisms or mutations. The techniques so described concern the qualities of the foetal genes detected.
43 The patent then says (page 5 lines 3 to 6):
The plasma or serum-based non-invasive prenatal diagnosis method according to the invention can be applied to screening for Down’s Syndrome and other chromosomal aneuploidies.
44 It explains two possible ways in which this might be done by consideration of the quantity of foetal cells in the maternal blood or the quantitation of foetal DNA markers on different chromosomes. A further application of the accurate quantitation of foetal nucleic acid levels is then said to be the monitoring of certain placental pathologies such as pre-eclampsia, because in that condition the concentration of foetal DNA in maternal serum and plasma is elevated (page 5 line 28 to page 6 line 2).
45 As we note in section 5 below, the distinction between qualitative and quantitative methods is relevant to the sufficiency challenge advanced by the appellants based on s 40(2)(a) of the Act.
46 The patent then illustrates the invention by reference to five examples.
47 Example 1 provides an analysis of cffDNA for sex determination, a qualitative test. The example describes the patients being pregnant women from whom maternal peripheral blood was collected, centrifuged and the plasma and serum separately extracted. Detail in the example addresses sample preparation and DNA extraction from plasma and serum as follows (page 7 lines 6 to 26):
Sample preparation
Maternal blood samples were processed between 1 to 3 hours following venesection. Blood samples were centrifuged at 3000g and plasma and serum were carefully removed from the EDTA-containing and plain tubes, respectively, and transferred into plain polypropylene tubes. Great care was taken to ensure that the buffy coat or the blood clot was undisturbed when plasma or serum samples, respectively, were removed. Following removal of the plasma samples, the red cell pellet and buffy coat were saved for DNA extraction using a Nucleon DNA extraction kit (Scotlabs, Strathclyde, Scotland, U.K.) The plasma and serum samples were then subjected to a second centrifugation at 3000g and the recentrifuged plasma and serum samples were collected into fresh polypropylene tubes. The samples were stored at -20°C until further processing.
DNA extraction from plasma and serum samples
Plasma and serum samples were processed for PCR using a modification of the method of Emanuel and Pestka (1993). In brief, 200 µl of plasma or serum was put into a 0.5ml eppendorf tube. The sample was then heated at 99°C for 5 minutes on a heat block. The heated sample was then centrifuged at maximum speed using a microcentrifuge. The clear supernatant was then collected and 10 µl was used for PCR.
48 Next, the example describes the DNA extraction from amniotic fluid. It then describes the use of PCR (page 8 lines 7 to 20):
Polymerase chain reaction (PCR)
The polymerase chain reaction (PCR) was carried out essentially as described (Saiki et al 1988) using reagents obtained from a GeneAmp DNA Amplification Kit (Perkin Elmer, Foster City, CA, USA). The detection of Y-specific foetal sequence from maternal plasma, serum and cellular DNA was carried out as described using primers Y1.7 and Y1.8, designed to amplify a single copy Y sequence (DYS14) (Lo et al 1990). ... The Y-specific product was 198 bp. Sixty cycles of Hot Start PCR using Ampliwax technology were used on 10 µl of maternal plasma or serum or 100ng of maternal nucleated blood cell DNA. ... Forty cycles were used for amplification of amniotic fluid.
49 The PCR products then were analysed using “agarose gel electrophoresis and ethidium bromide staining”. Gel electrophoresis is a technique used to separate and visualise molecules like DNA based on their molecular weight.
50 The specification then reports the results, saying at page 9 lines 1 to 5 that of the 30 women bearing male foetuses, Y-positive signals were detected in 24 plasma samples and 21 serum samples, when 10 µl of the respective samples was used for PCR.
51 By contrast, the results using cellular DNA were less accurate (page 9 lines 6 to 7): “When nucleated blood cell DNA was used for Y-PCR, positive signals were only detected in 5 of the 30 cases”.
52 The specification concludes at page 9 lines 10 to 13:
Accuracy of this technique, even with serum/plasma samples of only 10 µl, is thus very high and most importantly it is high enough to be useful. It will be evident that accuracy can be improved to 100% or close to 100%, for example by using a larger volume of serum or plasma.
53 Example 2 is entitled “Quantitative analysis of foetal DNA in maternal serum in aneuploid pregnancies”. The specification explains that prenatal screening and diagnosis of foetal chromosomal aneuploidies is an important part of modern obstetric care, and that much effort has been devoted to the development of non-invasive screening methods. The two main such methods are maternal serum biochemical screening and ultrasound examination for nuchal translucency, but both are associated with significant false positive and negative rates (page 9 lines 18 to 26). The specification notes that recent studies have demonstrated that there is increased foetal nucleated cell number in maternal circulation when the foetus is suffering from a chromosomal aneuploidy (page 10 lines 2 to 5).
54 The primary judge describes the process used in the example at [260]:
In this example, the inventors used 400 to 800µL of plasma/serum samples derived from the blood of pregnant women, extracted DNA from those samples and subjected the extracted DNA to real-time qPCR using the methods described in Heid et al 1996 (discussed above) and an SRY TaqMan system consisting of amplification primers for the SRY region on the Y chromosome and a dual-labelled fluorescent TaqMan probe. The primer/probe combinations were designed using the Primer Express software and Sequence data for the SRY gene were obtained from the GenBank Sequence database (page 11, lines 19 to 21 of the Patent).
55 In the discussion of the results the specification says (page 14 lines 18 to 25):
In this study we demonstrate that the concentration of foetal DNA in maternal serum is elevated in aneuploid pregnancies. These results indicate that foetal DNA quantitation has the potential to be used as a new screening marker for foetal chromosomal aneuploidies. A large scale population-based study could be carried out to develop cutoff values for screening purposes. It would also be useful to investigate the correlation of foetal DNA concentration with the other biochemical markers for maternal serum biochemical screening.
56 Example 3 is entitled “Non-invasive prenatal determination of foetal RhD status from plasma of RhD-negative pregnant women”. It is a qualitative test.
57 By way of background, the Rhesus factor (also known as the RhD antigen) was known to be a protein found on the surface of red blood cells in Rh positive individuals. Rh negative individuals lack this protein, because of a mutation of the gene. Rh disease can cause haemolytic disease of the newborn and foetus and typically arises in the second or subsequent pregnancies when a Rh negative mother is carrying a Rh positive foetus, and the foetus expresses the Rh factor on its red blood cells. During pregnancy and birth the mother may be exposed to those red blood cells, and then her immune system mounts a response to Rh factor, which it identifies as foreign, and that response sensitises her immune system to Rh factor. That sensitisation may result in the destruction the red blood cells of a Rh positive foetus in subsequent pregnancies. It was routine to test mothers for Rh status and to treat mothers identified as Rh negative with anti-Rh factor antibodies to prevent an immune response being raised. This was inefficient, because it involved treating Rh negative mothers who did not need it because they were not carrying a Rh positive foetus.
58 The specification says that a number of groups have investigated the possibility of using foetal cells in maternal blood for the determination of foetal RhD status, but that the system is not sufficiently reliable without foetal cell enrichment or isolation procedures, which are tedious and expensive to perform (page 15 line 26 to page 16 line 3). It says (page 16 lines 4 to 6):
Our discovery of the presence of foetal DNA in maternal plasma and serum offers a new approach for non-invasive prenatal diagnosis.
59 The primary judge summarised the method then described at [272]:
In the project the subject of Example 3, blood samples were taken from 21 serologically RhD negative pregnant women, including some who were in the second trimester of pregnancy just prior to amniocentesis and others who were in the third trimester just before delivery (page 16, lines 13 to 17). No blood or DNA samples were taken from fathers. 800µL of plasma was used for cffDNA analysis (page 17, line 5), which was performed as described in Example 2 with modifications relating to the primer/probe sets. The Example 3 method also included a non-fetal specific beta-globin TaqMan system, which acted as a control for the presence and amplifiability of cell free DNA in the plasma samples (page 19, lines 1 to 3).
60 The discussion of the example provided in the specification says (page 19 lines 6 to 13):
In this study we have demonstrated the feasibility of performing non-invasive foetal RhD genotyping from maternal plasma. This represents the first description of single gene diagnosis from maternal plasma. Our results indicate that this form of genotyping is highly accurate and can potentially be used for clinical diagnosis. This high accuracy is probably the result of the high concentration of foetal DNA in maternal plasma.
61 Example 4 is entitled “Elevation of foetal DNA concentration in maternal serum in pre-eclamptic pregnancies” and focuses on a quantitative test.
62 It was known before the priority date that pre-eclampsia is a pregnancy disorder which affects about 6% of pregnancies and is characterised by high blood pressure and elevated protein levels in the maternal urine. Pre-eclampsia generally occurs 24 to 26 weeks after fertilisation and often increases in severity until birth. Untreated, pre-eclampsia may lead to eclampsia (convulsions), bleeding in the mother’s brain and death of the mother. The early forms of pre-eclampsia are often associated with foetal growth restriction due to placental dysfunction.
63 In this example, the inventors used a real-time qPCR assay to show that the concentration of foetal DNA in the serum of women suffering from pre-eclampsia was elevated compared to normal pregnancies. Y chromosomal sequences from male foetuses were used as a foetal marker and a proxy for the total level (concentration) of foetal DNA (page 21 lines 5 to 9). Serum/plasma samples were taken from women with and without pre-eclampsia and real-time qPCR was performed as described in example 2 (page 22 line 6 to 7). The results indicated that the concentration of foetal DNA is higher in pre-eclamptic compared with non-pre-eclamptic pregnancies and that foetal DNA concentration measurement in maternal plasma may be used as a new marker for pre-eclampsia (page 22 lines 20 to 23). The specification says that compared with other markers, foetal DNA measurement is a genetic marker, which has the advantage that it is completely foetal-specific (page 22 lines 24 to 28).
64 Example 5 is entitled “Quantitative analysis of foetal DNA in maternal plasma and serum”. It commences by citing an article by Lo et al, 1997 for the proposition that the inventors have demonstrated that foetal DNA is present in maternal plasma and serum, with the detection of foetal DNA sequences in 80% and 70% of cases using only 10 µl of boiled plasma and serum respectively (page 23 lines 18 to 21). It says that these observations indicate that maternal plasma/serum DNA may be a useful source of material for the non-invasive prenatal diagnosis of certain genetic disorders, but that foetal DNA needs to be shown to be present in sufficient quantities for reliable molecular diagnosis to be carried out, and data on the variation of foetal DNA with regard to gestation age is required to determine the applicability of the technology to early prenatal diagnosis (page 23 lines 22 to 30).
65 For convenience we repeat claim 1, which provides:
A detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample.
66 Claim 2 is the method according to claim 1, “comprising amplifying the foetal nucleic acid to enable detection”.
67 Claim 3 is the method according to claim 2, “wherein the foetal nucleic acid is amplified by the polymerase chain reaction”.
68 Claim 5 is relevantly the method according to any one of claims 1 to 3, “wherein the foetal nucleic acid is detected by means of a sequence specific probe”.
69 Claim 6 is relevantly the method according to any one of the above claims, “wherein the presence of a foetal nucleic acid sequence from the Y chromosome is detected”.
70 Claim 9 is the method according to any one of claims 1 to 5, “wherein the presence of a foetal nucleic acid from a paternally-inherited non-Y chromosome is detected”.
71 Claim 13 is relevantly the method according to claim 6, “for determining the sex of the foetus”.
72 Claim 14 is relevantly the method according to claims 6 or 9, which comprises “determining the concentration of the foetal nucleic acid sequence in the maternal serum or plasma.”
73 Independent claim 22 is “A method of performing a prenatal diagnosis, which method comprises the steps of”:
(i) providing a maternal blood sample;
(ii) separating the sample into a cellular and a non-cellular fraction;
(iii) detecting the presence of a nucleic acid of foetal origin in the non-cellular fraction according to the method of any one of claims 1 to 21;
(iv) providing a diagnosis based on the presence and/or quantity and/or sequence of the foetal nucleic acid.
74 Claim 23 is the method according to claim 22, “wherein the non-cellular fraction as used in step (iii) is a plasma fraction”.
75 Independent claim 25 is:
A method of performing a prenatal diagnosis on a maternal blood sample, which method comprises removing all or substantially all nucleated and anucleated cell populations from the blood sample and subjecting the remaining fluid to a test for foetal nucleic acid indicative of a maternal or foetal condition or characteristic.
4.1 The decision of the primary judge
76 The primary judge commenced by referring to s 18(1)(a) of the Act and then providing a detailed review of the law concerning patentable subject matter, commencing with the decision of the High Court in D’Arcy v Myriad Genetics Inc [2015] HCA 35; 258 CLR 334. It is convenient here to set out the reasoning of the plurality in Myriad at [28]:
A number of factors may be relevant in determining whether the exclusive rights created by the grant of letters patent should be held by judicial decision, applying s 18(1)(a) of the Act, to be capable of extension to a particular class of claim. According to existing principle derived from the NRDC decision, the first two factors are necessary to characterisation of an invention claimed as a manner of manufacture:
1. Whether the invention as claimed is for a product made, or a process producing an outcome as a result of human action.
2. Whether the invention as claimed has economic utility.
When the invention falls within the existing concept of manner of manufacture, as it has been developed through cases, they will also ordinarily be sufficient. When a new class of claim involves a significant new application or extension of the concept of "manner of manufacture", other factors including factors connected directly or indirectly to the purpose of the Act may assume importance. They include:
3. Whether patentability would be consistent with the purposes of the Act and, in particular:
3.1. whether the invention as claimed, if patentable under s 18(1)(a), could give rise to a large new field of monopoly protection with potentially negative effects on innovation;
3.2. whether the invention as claimed, if patentable under s 18(1)(a), could, because of the content of the claims, have a chilling effect on activities beyond those formally the subject of the exclusive rights granted to the patentee;
3.3. whether to accord patentability to the invention as claimed would involve the court in assessing important and conflicting public and private interests and purposes.
4. Whether to accord patentability to the invention as claimed would enhance or detract from the coherence of the law relating to inherent patentability.
5. Relevantly to Australia's place in the international community of nations:
5.1. Australia's obligations under international law;
5.2. the patent laws of other countries.
6. Whether to accord patentability to the class of invention as claimed would involve law-making of a kind which should be done by the legislature.
Factors 3, 4 and 6 are of primary importance. Those primary factors are not mutually exclusive. It may be that one or more of them would inform the "generally inconvenient" limitation in s 6 of the Statute of Monopolies. It is not necessary to consider that question given that no reliance was placed upon that proviso. They are nevertheless also relevant to the ongoing development of the concept of "manner of manufacture".
77 The primary judge relied upon and repeated his reasoning in Meat & Livestock Australia Limited v Cargill, Inc [2018] FCA 51; 354 ALR 95 (MLA (No 1)) for a general exposition of the relevant law. He then summarised in detail the submissions advanced on behalf of the appellants before turning to his analysis. He observed that in Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd [2013] HCA 50; 253 CLR 284 Crennan and Kiefel JJ at [224] noted that:
In Australian law, the starting point is the recognition in the NRDC Case that any attempt to define the word “manufacture” or the expression “manner of manufacture”, as they occur in s 6 of the Statute of Monopolies, is bound to fail.
78 The primary judge noted that it was significant in the present case that there is no claim to the product or presence of cffDNA, but rather to a method by which the discovery of the existence of cffDNA can be put to practical use. In this way, his Honour considered that the subject matter of the relevant claims can be seen to fall within the principles of National Resource Development Corporation v Commissioner of Patents [1959] HCA 67; 102 CLR 252 (Dixon CJ, Kitto and Windeyer JJ) (NRDC) and affirmed in Myriad, whilst not falling within the impermissible product claims rejected in Myriad (at [463]).
79 The primary judge noted that pursuant to Myriad the starting point for the resolution of the issue is the identification of what in substance each claim is for. In this regard the primary judge first noted:
[467] ... the Patent is entitled “Non-invasive prenatal diagnosis”. The first paragraph of the Patent provides that the invention described and claimed therein “relates to prenatal detection methods using non-invasive techniques” and “[i]n particular, it relates to prenatal diagnosis by detecting foetal nucleic acids in serum or plasma from a maternal blood sample.”
[468] The Patent describes (page 1) the prenatal testing methods used before the priority date of the invention, including CVS and amniocentesis, being techniques requiring careful handling and that present a degree of risk to the mother and to the pregnancy, and the use of fetal cells in maternal blood.
80 The primary judge then quoted the passage at page 2 lines 5 to 19 of the specification (which we have summarised and quoted at [35] above), and at [470] said that the patent specification confirms at page 2 lines 20 to 23 that the invention is:
… not simply the presence of cffDNA in maternal serum or plasma but rather, “a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample”. It is explained that the claimed method thus provides a “method of prenatal diagnosis”, a term defined at page 2, line 24 to page 3, line 4.
81 Earlier, at [215], the primary judge had characterised the invention:
The inventors named in the Patent, Dr Dennis Lo and Dr James Wainscoat had discovered the presence and utility of cffDNA. The invention described and claimed in the Patent is the practical application of this discovery by a new method of detection of fetal DNA, which unlike previously disclosed cellular methods, involved human mediated discrimination between cell-free maternal and fetal DNA in an artificially prepared plasma or serum sample extracted from a pregnant female.
(emphasis in original)
82 He then identified at [472] to [476] that there are four classes of claims. The first is a detection method as set out with increasing detail in independent claim 1 and dependent claims 2, 3, 5, 6 and 9. The second is a detection method within the first class for determining the sex of a foetus in dependent claim 13. The third is a method of the first class which further comprises determining the concentration of foetal nucleic acid sequences in the maternal serum or plasma sample in dependent claim 14, and the fourth is the provision of a “prenatal diagnosis” following the performance of the method in the first class in independent claim 22 and dependent claims 23, 25 and 26.
83 The primary judge distinguished the present case on its facts from Myriad finding (at [477]):
It should be well apparent that unlike Myriad, this case does not concern whether product claims to naturally occurring nucleic acid molecules per se or naturally occurring genetic information encoded by such sequences are patentable. Rather, like MLA (No 1), this case concerns method claims (MLA (No 1) at [409] and [462]). Both MLA (No 1) and the present case involve claims to methods of identifying or detecting a nucleic acid having a particular characteristic, not the nucleic acids or the information encoded by such nucleic acids per se.
(emphasis in original)
84 The primary judge noted that the detection method of claims 6 to 9 inherently involve at least artificial steps requiring human action (at [480]) and that a person seeking to detect cffDNA from maternal serum or plasma would necessarily be deliberately employing the method invented and claimed. As such, a method of detection would involve the use of artificial human action, such as PCR in the case of claim 3 or probes in the case of claim 5 (at [482]).
85 In relation to the inventive concept underlying the invention as disclosed in the specification the primary judge said at [481]:
Further, all uses of the relevant method(s) would be commensurate with the scope of the inventor’s claimed new method of prenatal detection involving the detection of cffDNA from a maternal serum or plasma sample, rather than existing methods which all focused on more problematic cellular (as distinct from non-cellular) samples of DNA. The claims cover what the inventors of the Patent invented. In other words, the inventive step or concept, that is, that paternally inherited cffDNA is detectable in maternal plasma or serum, is the subject of and is carried out in, for example, claims 6 and 9.
(emphasis in original)
86 In this regard at [484] the primary judge drew upon the evidence in the joint expert report, where the experts agreed that “detect” in context means:
... demonstrating the presence of non-maternal nucleic acid that is inferred to be of fetal origin. It is difficult to see how it could sensibly be said that such a method does not involve artificial human action to discriminate or distinguish between nucleic acids of maternal origin and non-maternal (i.e. fetal) origin. Moreover, without such a step, one cannot detect the presence of non-maternal (i.e. fetal) nucleic acid.
87 The primary judge accepted the submission advanced by the respondent that the substance of the method applies and follows from, but is different to, the identification of a natural phenomenon, namely the presence of cffDNA in maternal blood. This, he considered, is made clear by page 16 lines 4 to 6 of the specification which explain that the discovery “offers a new approach for non-invasive prenatal diagnosis”. The primary judge found at [485]:
Thus, the invention builds on, uses, practically applies and reduces to practice a discovered substance found in nature, namely, cffDNA in maternal blood, to provide a new, inventive, useful, artificial method of detection of cffDNA, and where the method is of economic significance.
88 The primary judge then addressed claims 13 and 14 and concluded that they provide for the practical application of the discovery of cffDNA in maternal plasma and that claims 22, 23, 25 and 26, founded as each is on “prenatal diagnosis”, are on any view a manner of manufacture (at [486], [487]).
89 The primary judge next turned to what he described as the “inventive concept”. After quoting from NRDC at 262 and 264, he said at [491]:
Now in my view there is no requirement that the inventive concept be seen to make a contribution to the essential difference between the product and nature. But even if I were to conclude otherwise, the inventive concept of the Patent does so. In nature, the presence of cffDNA in the maternal blood has not and cannot be detected without human action. Accordingly, unlike the claims considered in Myriad, the invention claimed adds to human knowledge and involves the suggestion of an act to be done which results in a new result, or a new process.
90 The primary judge considered the question of whether the claimed invention was for an artificially created state of affairs. In a passage criticised by the appellants on appeal the primary judge said at [494]:
In the present case the relevant claims involve human interaction and the creation of an artificially created state of affairs discernible by an observer. The artificially created state of affairs is the detection of cffDNA in the tested sample. This “product” is, by definition the result of human action and is not naturally occurring. The inventive method does not simply produce an abstract, intangible situation. It is not just the “information” encoded by the naturally occurring cffDNA itself. Moreover, production of the result requires at least:
(a) The taking of a maternal blood sample from a pregnant female.
(b) The separation of the maternal blood sample into its component parts, namely, plasma, white blood cells and red blood cells. It is obvious that such samples do not exist in the natural world. They must be artificially created by centrifuging a sample of blood to remove all cells in the presence of an anti-coagulant in the case of plasma, or without an anti-coagulant but after clotting in the case of serum.
(c) The extraction of nucleic acids from the plasma component of a maternal blood sample.
(d) A step to discriminate between maternal and cffDNA in the sample, such as, the targeting and amplification by PCR of paternally inherited sequences from the Y chromosome in the case of claim 6 and a non-Y chromosome in the case of claim 9.
91 Next, the primary judge concluded that the invention is of economic significance, again distinguishing Myriad, because unlike in that case here the claim is not to the nucleic acid isolate or merely a step along the way to another method claimed (at [499]), but a method that provides a significant advantage over existing foetal DNA detection methods (at [500]). His Honour continued at [503]:
In my view the claimed invention falls clearly within the concept of manner of manufacture described by the High Court in NRDC, satisfies the first two criteria identified in Myriad at [28] and is therefore patentable. But even if the substance of the invention is considered to fall outside the established boundaries of patentable subject matter, consideration of the non-exhaustive list of additional factors outlined in Myriad confirms my opinion of the invention’s patentability.
92 Despite his finding that the invention fell clearly within the first two factors identified in Myriad, his Honour nevertheless turned to consider the other factors. In relation to factor 3, he considered that there would be no chilling effect as the boundaries of the claims are clear and commensurate with the invention described in the patent, and there was no risk that someone could infringe the patent without knowing it (at [506]-[509]). In relation to factor 4, his Honour noted that his findings were consistent with MLA (No 1) and would accordingly promote consistency in the application of the law (at [511]-[517]). In relation to factor 5, at [519] his Honour noted that in corresponding proceedings in the United Kingdom Carr J held that claims equivalent to claims 6 and 9 were patentable (Illumina, Inc v Premaitha Health Plc [2017] EWHC 2930 (Pat) at [184] to [189]), but also observed at [522]-[525] that in the corresponding United States proceedings the majority of the Supreme Court took a different view (Ariosa Diagnostics Inc. Sequenom, Inc. 788 F3d 1371 (3d Cir 2015) (Ariosa (US)). The primary judge distinguished the US court’s findings on the basis that the court had approached the claims by dissecting integers, which was not the correct approach under Australian law.
93 The primary judge concluded on the subject of manner of manufacture at [526]:
The Patent does not simply claim the discovery of cffDNA in maternal blood. Rather, it claims a new and inventive practical application of the discovery comprising a method requiring human action to detect, in an artificially created sample of maternal plasma or serum, a DNA sequence as being of fetal rather than maternal origin. And prior to the invention, no-one had worked or was working a method comprising the detection of cffDNA in plasma or serum samples extracted from pregnant females.
94 The appellants contend broadly in ground 1 of the appeal that the primary judge erred in finding that the relevant claims are for an invention that is a manner of manufacture within s 6 of the Statute of Monopolies 1624 (21 Jac c 3) and s 18(1)(a) of the Act. They elaborate in grounds 2 to 5 by contending that the primary judge ought to have found that the end result of each claim does not, at all or sufficiently, involve an artificial effect or an artificially created state of affairs, but rather involves the detection of what is naturally occurring, and that in substance each of the relevant claims is to a mere discovery. The appellants further contend that the primary judge erred in finding that the alleged invention does not concern a new class of claim and that accordingly there was no need to apply the “other factors” identified by the plurality in Myriad, and in finding in any event (at [506]-[525]) that those factors confirm the patentability of the invention claimed.
95 The appellants submit that the invention claimed in the relevant claims does not involve a manner of manufacture because what is claimed is a mere discovery of a naturally occurring phenomenon, and not a method involving a practical application that in substance goes beyond the discovery itself, and as a result there is no artificially created state of affairs. In a related submission, the appellants contend that properly understood the end result of each claim is information only and, accordingly, not an artificially created state of affairs.
96 The appellants submit that as at the priority date it was known that foetal DNA could provide valuable information about the foetus, and it was known that foetal DNA for analysis could be obtained by an invasive sampling method, such as amniocentesis or CVS. Another known source of foetal DNA was the foetal cells that were circulating in the mother’s blood, albeit in very low concentration. The cffDNA from maternal plasma or serum, once its existence was discovered, was another source of foetal DNA from these other known sources.
97 The appellants submit that the primary judge’s findings at [494] (set out in section 4.1 above) miss the point, because the claims are not directed to a new method of obtaining a plasma or serum sample from maternal blood. Rather, in this case, although human action may be involved in the performance of the claimed methods, the end result or outcome of the method as claimed is simply the information, namely the presence of cffDNA discovered by Professor Lo. They summarise the reasons of the plurality in Myriad as, in characterising an invention as a manner of manufacture, requiring consideration of the question “whether the invention as claimed is for a product made, or a process producing an outcome as a result of human action” (at [28]), which is to be answered by reference to whether the invention yields an “outcome which can be characterised, in the language of NRDC, as an ‘artificially created state of affairs’” (at [6]). In this regard the appellants also rely on the decisions of the Full Court in Research Affiliates LLC v Commissioner of Patents [2014] FCAFC 150; 227 FCR 378 (Kenny, Bennett and Nicholas JJ) at [8], [92], [95], [103], [106] and [115], Commissioner of Patents v RPL Central Pty Ltd [2015] FCAFC 177; 238 FCR 27 (Kenny, Bennett and Nicholas JJ) at [95] and Commissioner of Patents v Rokt Pte Ltd [2020] FCAFC 86; 379 ALR 86 (Rares, Nicholas and Burley JJ) at [89].
98 The appellants submit that in the present case the substance of what has been invented is simply the information about the DNA of the foetus, which is no more than naturally occurring information. By focussing on the process used to obtain the information the appellants submit that the primary judge failed to look at the “end result” and ask whether that end result involved an artificial effect. In this regard, the primary judge is said to have failed to apply the reasoning of Gageler and Nettle JJ in Myriad at [152].
99 The appellants contend that while the claims of the patent are drafted as methods of detection or diagnosis, in substance none does more than Professor Lo’s discovery that cffDNA is detectable in maternal serum or plasma. In this respect, claim 1 does no more than claim Professor Lo’s discovery that cffDNA is detectable in maternal serum or plasma by “detecting the presence of a nucleic acid of foetal origin”. The discovery only of the presence of a naturally occurring phenomenon, being the presence of foetal DNA in maternal plasma or serum, is not an invention. The appellants emphasise that there is no claim in the patent to any new method for detecting cffDNA once it is discovered to be present in the plasma or serum, it being accepted in the statement of common general knowledge in these proceedings that the methods so used were well-known by the priority date.
100 The appellants submit that unlike the claims in MLA (No 1), the claims here do not claim any specific or new method of applying the detectability of cffDNA in maternal plasma or serum, but simply claim the identification or discernment of the naturally occurring phenomenon of the presence of cffDNA in maternal plasma or serum. The dependent claims are merely broad examples of how to detect cffDNA in maternal plasma, using well-understood and conventional activity.
101 Each of claims 6 and 9 specifies the chromosome from which the foetal nucleic acid to be detected is derived, but taken together they cover all foetal chromosomes that are paternally-inherited and which may therefore be distinguishable from the maternal DNA if a sequence can be identified that is not possessed by the mother. So considered, claims 6 and 9 do not effectively limit the scope of claims 1 to 3 or 5, they only identify the subsets of foetal nucleic acids which might be detected in those latter claims.
102 The appellants further submit that claim 13 is the first and only asserted claim that involves a specific application of the discovery, involving a method “for determining the sex of the foetus”, but this conclusion follows so directly from the discovery itself, that it involves nothing more than the discovery; knowing that foetal DNA is present, a known probe is used to determine whether sequences from the Y chromosome are present or not. Claim 14 requires the concentration of the foetal nucleic acid in the maternal serum or plasma to be determined. It requires detecting the presence of foetal nucleic acid and measuring how much is there. The claim is not directed to any application of that information and so similarly does not give rise to a manner of manufacture. The appellants submit that independent claims 22 to 26 involve making a prenatal diagnosis, and whilst in form these claims might appear to involve a practical application of the discovery, in substance these claims are still merely for the identification or discernment of the naturally occurring phenomenon because the claims say nothing as to the method of arriving at a diagnosis, nor as to the diagnosis to be made, and consequently provide no relevant limitation. In substance they are to the discovery itself.
103 The appellants submit that even if the claims would satisfy the threshold requirements of NRDC the claims are still not patentable as they are not within the established boundaries of what constitutes patentable subject matter, having regard to the additional factors identified by the plurality in Myriad at [28].
104 The respondent distinguishes the decision in Myriad by noting that the relevant claims are not product claims to isolated nucleic acids, or naturally occurring “genetic information” encoded by such sequences. Nor do they claim cffDNA itself. Rather, the claims concern the application of the discovery of the existence of cffDNA by the inventors, and their invention of new methods of non-invasive detection of foetal DNA from a maternal serum or plasma sample and prenatal diagnosis. The respondent submits that the claimed method must be considered as a whole. It is not to the point that once it was discovered that foetal DNA was present in extracellular maternal serum or plasma samples such DNA could be detected using known biotechnical methods. The steps involved in implementing the method were correctly found by the primary judge to be part of the claimed method, and cannot be disaggregated from it. Prior to the invention, skilled persons sought to detect foetal DNA by invasive methods or by seeking to extract foetal DNA from foetal cells within the cellular component of maternal blood, but before the priority date no one had amplified and detected cffDNA in maternal plasma and serum samples.
105 The respondent submits that for a conclusion that a claim is the proper subject matter for a patent it will normally suffice to find that the claim is for an invention that gives rise to “something brought about by human action” (citing Myriad at [6] (plurality)) or a discernible artificially created state of affairs (NRDC at 277, MLA (No 1) at [455]) or something of economic significance. The present case falls within these categories and it is unnecessary to consider whether the claims concern a new class of claim requiring consideration of the “other factors” identified by the plurality in Myriad at [28].
106 The respondent contends that the reasons in Myriad support the contention that detection and diagnosis methods are patentable subject matter, and submits that the substance of the invention is a method resulting in an artificially created state of affairs producing an economically significant outcome. It submits this in relation to each of the asserted claims, but advances a composite exemplar detection method claim to demonstrate its point, being claims 6 and 9 when dependent on claims 2, 3 and 5:
A method of detecting in a maternal plasma sample prepared from blood extracted from a pregnant female, the presence of a foetal nucleic acid sequence [claim 6: from the Y chromosome or claim 9: from a paternally-inherited non-Y chromosome], wherein the foetal nucleic acid is amplified by the polymerase chain reaction and the foetal nucleic acid is detected by means of a sequence specific probe.
107 After defending the primary judge’s reasoning in respect of each of the asserted claims, the respondent turns to the “other factors” identified by the plurality in Myriad at [28] and contends that, to the extent that it is necessary to apply them, the primary judge correctly considered that they pointed in favour of patentability.
108 Fundamental to the appeal is the contention that what is claimed is a mere discovery of a naturally occurring phenomenon, and not a method involving a practical application that goes beyond the discovery itself. Here, the appellants identify the discovery as the fact that cffDNA from maternal plasma or serum was a source of foetal DNA. They submit that there is no artificially created state of affairs arising from the invention as claimed. Furthermore, they submit that the “end result” of each claim results in information only, being the detection of cffDNA. The appellants rely substantially on the decision in Myriad in support of their appeal.
109 For an invention to be patentable, it must meet the relevant requirements of the Act including s 18(1)(a) which requires that the invention, as claimed, be a manner of manufacture within the meaning of s 6 of the Statute of Monopolies. Schedule 1 to the Act defines “invention” as “any manner of new manufacture the subject of letters patent and grant of privilege within s 6 of the Statute of Monopolies, and includes an alleged invention”. This element of patentability was considered in NRDC, Apotex and more recently in Myriad.
110 According to NRDC, as explained in Myriad, the relevant question to be posed is: “Is this a proper subject of letters patent according to the principles which have been developed for the application of s 6 of the Statute of Monopolies?” (Myriad at [18] (plurality), citing NRDC at 269).
111 The common law has developed standards by which patentable subject matter may be assessed by reference to the determination of what is a manner of manufacture. In NRDC Dixon CJ, Kitto and Windeyer JJ emphasised the long-established requirement that a patentable invention must involve more than mere discovery of a scientific fact or law of nature. However, at 263-264 they counselled caution in too quickly using such distinctions, quoting Frankfurter J in the United States Supreme Court decision Funk Bros. Seed Co v Kalo Inoculant Co (1948) 333 US 127 at 134, 135 (92 Law Ed 588, at 591), where his Honour said:
It only confuses the issue ... to introduce such terms as ‘the work of nature’ and the ‘laws of nature’. For these are vague and malleable terms infected with too much ambiguity and equivocation. Everything that happens may be deemed ‘the work of nature’, and any patentable composite exemplifies in its properties ‘the laws of nature’. Arguments drawn from such terms for ascertaining patentability could fairly be employed to challenge almost any patent.
112 Their Honours continued in NRDC at 264:
The truth is that the distinction between discovery and invention is not precise enough to be other than misleading in this area of discussion. There may indeed be a discovery without invention – either because the discovery is of some piece of abstract information without any suggestion of a practical application of it to a useful end, or because its application lies outside the realm of “manufacture”. But where a person finds out that a useful result may be produced by doing something which has not been done by that procedure before, his claim for a patent is not validly answered by telling him that although there was ingenuity in his discovery that the materials used in the process would produce the useful result no ingenuity was involved in showing how the discovery, once it had been made, might be applied. The fallacy lies in dividing up the process that he puts forward as his invention. It is the whole process that must be considered: and he need not show more than one inventive step in the advance which he has made beyond the prior limits of the relevant art.
113 The Court went on to explain this point by reference to the distinction between a mere idea and its application by reference to Watt’s invention of the use to which steam may be put (at 264):
This is perhaps nowhere more clearly put than it was by Fletcher Moulton LJ in Hickton’s Patent Syndicate v Patents and Machine Improvements Co Ltd when he said of Watt’s invention for the condensation of steam, out of which the steam engine grew: “Now can it be suggested that it required any invention whatever to carry out that idea when once you had got it? It could be done in a thousand ways and by any competent engineer, but the invention was in the idea, and when he had once got that idea, the carrying out of it was perfectly easy. To say that the conception may be meritorious and may involve invention and may be new and original, and simply because when you have once got the idea it is easy to carry it out, that that deprives it of the title of being a new invention according to our patent law, is, I think, an extremely dangerous principle and justified neither by reason nor authority”.
(citations omitted)
114 Three points of emphasis may be noted from these passages. First, the distinction between mere discovery and an invention lies in its practical application to a useful end. Secondly, it is important that the invention be considered as a unitary concept, not segregated artificially into parts. The invention may arise from an idea and then be applied in a perfectly well known way, and yet the combined effect of the idea and its application may result in patentable subject matter, as arose from the example described in Hickton’s Patent Syndicate v Patents and Machine Improvements Co Ltd (1909) 26 RPC 339. The approach of the appellants in the present appeal, which seeks to disaggregate the discovery of cffDNA in maternal plasma or serum from the method used to harness that discovery, tends to overlook these matters. Thirdly, an invention may reside in an abstract idea, such as the condensation of steam that is then put to a useful end, even though the way of putting it to that end can be carried out in many useful ways, all of which are otherwise known.
115 In Apotex the High Court considered whether methods of treatment of the human body using a known substance would amount to a manner of manufacture. At [230] Crennan and Kiefel JJ referred to an earlier passage at 264 in the NRDC case:
In determining that a novel use of known substances (for the eradication of weeds from crops) was a patentable invention, this Court (Dixon CJ, Kitto and Windeyer JJ) decided that it was not essential that a process produce or improve a vendible article. Their Honours explained, by reference to the doctrine of analogous uses set out in BA's Application:
"If ... the new use that is proposed consists in taking advantage of a hitherto unknown or unsuspected property of the [known] material ... there may be invention in the suggestion that the substance may be used to serve the new purpose; and then, provided that a practical method of so using it is disclosed and that the process comes within the concept of patent law ultimately traceable to the use in the Statute of Monopolies of the words 'manner of manufacture,' all the elements of a patentable invention are present ... It is not necessary that in addition the proposed method should itself be novel or involve any inventive step".
(emphasis added, citations omitted)
116 Later, their Honours considered and approved the reasoning of Lockhart J in Anaesthetic Supplies Pty Limited v Rescare Limited [1994] FCA 304; 50 FCR 1 at 19. At [240] their Honours said:
In the majority, Lockhart J found that once the notion of the necessity for a vendible product (as in Re C & W's Application) is eliminated (as it was in the NRDC Case), there is no distinction in principle between a product for treating humans and a method for treating humans. ... His Honour said:
“I see no reason in principle why a method of treatment of the human body is any less a manner of manufacture than a method for ridding crops of weeds as in NRDC. Australian courts must now take a realistic view of the matter in the light of current scientific development and legal process; the law must move with changing needs and times ...
If a process which does not produce a new substance but nevertheless results in ‘a new and useful effect’ so that the new result is ‘an artificially created state of affairs’ providing economic utility, it may be considered a ‘manner of new manufacture’ within s 6 of the Statute of Monopolies”.
(emphasis added in Apotex, citations omitted)
117 Claim 1 in issue before the Court in Apotex was for a method of preventing or treating psoriasis, and claimed a previously unknown therapeutic use of a pharmaceutical substance which was known for other therapeutic uses disclosed in an earlier patent (at [276]). The Court rejected a submission that the subject matter of the claim was “essentially non-economic” (French CJ at [50], Crennan and Kiefel JJ at [277], Gageler J at [314]). Crennan and Kiefel JJ provided seven reasons for this conclusion at [278]-[285] (with which Gageler J agreed at [314]). Two aspects are presently material. In the first, their Honours noted at [278] that the requirement that an invention have “economic utility” raises the same considerations as those applicable in the UK and Europe that an invention be susceptible or capable of industrial application. This was, they said, apparent from the definition of “exploit” in the Act.
118 The reference to that definition serves to emphasise the distinction between product and method claims and, in respect of method claims, to demonstrate that a “product” in the sense of a tangible thing is not required for patentability. In this respect, the plurality in Myriad noted at [16] that the definition of "exploit" distinguishes “between an invention which is a product and an invention which is a method or process which may or may not yield a product” (emphasis added). They noted that in Northern Territory v Collins [2008] HCA 49; 235 CLR 619 at [18] Gummow ACJ and Kirby J “linked that distinction to the way in which, over time, the expression ‘manner of manufacture”’ had been construed to include the practice and means of ‘making’, as well as its product, which would include an economically useful outcome effected by an inventive method” (emphasis added).
119 In the fourth aspect discussed by Crennan and Kiefel JJ, their Honours said at [282]:
... the subject matter of a claim for a new product suitable for therapeutic use, claimed alone (a product claim) or coupled with method claims (combined product/method claims), and the subject matter of a claim for a hitherto unknown method of treatment using a (known) product having prior therapeutic uses (a method claim), cannot be distinguished in terms of economics or ethics. In each case the subject matter in respect of which a monopoly is sought effects an artificially created improvement in human health, having economic utility. It could not be said that a product claim which includes a therapeutic use has an economic utility which a method or process claim for a therapeutic use does not have. It could not be contended that a patient free of psoriasis is of less value as a subject matter of inventive endeavour than a crop free of weeds. Patent monopolies are as much an appropriate reward for research into hitherto unknown therapeutic uses of (known) compounds, which uses benefit mankind, as they are for research directed to novel substances or compounds for therapeutic use in humans. It is not possible to erect a distinction between such research based on public policy considerations.
(emphasis added)
120 In their concluding remarks in relation to patentability, Crennan and Kiefel JJ noted at [287] that the expression “essentially non-economic” came from the requirement that the subject matter of a patent must have useful application in the sense that it must be capable of being practically applied in commerce or industry in the sense of being “susceptible or capable of industrial application”. These observations serve to reinforce the proposition that it is not necessary for a method or process claim to have as its outcome or result a tangible product or output in order to be a method that produces an economically useful outcome. What is necessary is that there be “a new and useful effect”.
121 The decision of the High Court in Myriad concerned the inherent patentability of a claim to an isolated DNA sequence that mirrored naturally occurring genetic information. Although three claims were in issue, it is sufficient to refer for present purposes to claim 1, which was in the following terms:
An isolated nucleic acid coding for a mutant or polymorphic BRCA1 polypeptide, said nucleic acid containing in comparison to the BRCA1 polypeptide encoding sequence set forth in SEQ.ID No:1 one or more mutations or polymorphisms selected from the mutations set forth in Tables 12, 12A and 14 and the polymorphisms set forth in Tables 18 and 19.
122 The reference to “coding for a mutant or polymorphic BRCA1 polypeptide” was to an isolated nucleic acid molecule which, when compared to the standard reference sequence set forth in SEQ.ID No:1, exhibits one or more of the 54 mutations or polymorphisms delineated in Tables 12, 12A, 14 and 18 (Gageler and Nettle JJ at [115]) and which were said to be present in women with familial breast cancer (at [117]).
123 Before the Full Court of the Federal Court in D’Arcy v Myriad Genetics Inc [2014] FCAFC 115; 224 FCR 479 (Allsop CJ, Dowsett, Kenny, Bennett and Middleton J) (Myriad (Full Court)), Ms D'Arcy submitted that isolated nucleic acid was not materially different to cellular nucleic acid and that naturally occurring DNA and RNA, even in isolated form, are products of nature that could not form the basis of a valid patent: Myriad at [82] (plurality); Myriad (Full Court) at [162]. Myriad, on the other hand, contended that its claims were for a product consisting of an artificial state of affairs providing a new and useful effect of economic significance, and that isolated nucleic acid differed from the nucleic acid found in a human cell chemically, structurally and functionally: Myriad at [82] (plurality), Myriad (Full Court) at [163]. Myriad failed.
124 In the High Court the plurality accepted that claim 1 was formally expressed as a product claim (at [6], [86]) but found that at the outset the invention as claimed must be construed having regard to the breadth and substance of the claims in issue (at [87]). It found that the code claimed refers to the sequence of nucleotides which, in a cellular environment, can ultimately be translated into the BRCA1 polypeptide, and that that sequence can properly be described as “information” which is stored in the sequence of nucleotides coding for the mutated or polymorphic BRCA1 polypeptide. That is the same information as that contained in the DNA of the person from which the nucleic acid was isolated. The plurality concluded at [89] that:
It is the existence of that information which is an essential element of the invention as claimed. The product is the medium in which that information resides. That characteristic also attaches to cDNA, covered by the claims, which is synthesised but replicates a naturally occurring sequence of exons.
125 The plurality found that whilst there were chemical, structural and functional differences between the isolated nucleic acids and nucleic acids found in the cellular environment, none of those played any part in the definition of the invention as claimed in the claims. The focus of the claims was on genetic information: at [90], [91]. So characterised, the plurality considered that the fact of the existence of the requisite mutations or polymorphisms was a matter of chance, it was not “made” or “artificially created” (an apparent reference to factor 1 set out at [28] of the plurality’s reasons): at [91]. Nor was the isolated BRCA1 gene to be considered of economic significance necessary to satisfy factor 2, the plurality saying at [85]:
The economic significance necessary to the patentability of an “artificially created state of affairs” in the sense used in NRDC is not demonstrated by stating that the artificially created state of affairs is a step along the way to a process or method itself claimed as an artificially created state of affairs of economic significance.
126 In this context the plurality rejected the finding of the Full Court that the isolation of the nucleic acid also led to an economically useful result, because the claim was not to the method of treatment of breast and ovarian cancers, but was a claim to the isolated gene used to identify an individual’s susceptibility to such cancers.
127 Having so characterised the invention, the plurality found that this subject matter lay at the boundaries of the concept of manner of manufacture (at [93]). It was not an artificially created state of affairs of economic utility. They considered that the very large number of the isolated nucleic acids that could fall within the claim raised the risk of a chilling effect upon legitimate innovative activity outside the boundaries of the monopoly and risked creating a penumbral de facto monopoly impeding the activities of legitimate improvers and inventors (factor 3) (at [93]). Furthermore, they considered that to extend the concept of manner of manufacture to include the invention claimed would not contribute to the coherence of the law, having regard to Apotex (factor 4) (at [94]). Nor did Australia’s international obligations suggest otherwise (factor 5) ([94]).
128 The decision of the plurality may be understood to endorse the long-established approach to patentable subject matter as it was considered in NRDC, noting the importance of factors 1 and 2 which will ordinarily be sufficient to determine the question (at [28]). Where a new class of claim involves a significant new application of the concept of manner of manufacture, it will be necessary to have regard to other factors, including those identified as factors 3 to 6 at [28] of Myriad.
129 It is apparent from the foregoing that the facts upon which the decision in Myriad was based are materially different to the present case, most particularly because the claim in issue there was for a product, being the isolated BRCA1 gene sequence. Other claims in the patent did concern methods of use, and the specification referred to these, but, as the plurality noted at [71] those claims were not in issue.
130 However, some indications as to the approach to be taken in assessing whether a method claim is a manner of manufacture may be seen from the reasons of the other members of the High Court.
131 Gageler and Nettle JJ considered that for a claimed invention to qualify as a manner of manufacture it must be something more than a mere discovery, and that the essence of invention inheres in its artificiality or distance from nature (at [126]). They said at [128]:
Relevantly, the artificiality of a product may be perceived in a number of factors, including the labour required to create it and the physical differences between it and the raw natural material from which it is derived. Regardless, however, of the amount of labour involved or the differences between the product and the raw natural material from which it is derived, it is necessary that the inventive concept be seen to make a contribution to the essential difference between the product and nature.
(citation omitted)
132 The reference here to “inventive concept” is not to be understood as a disaggregation of the integers of the invention as claimed, or a departure from the approach in NRDC at 264, but rather to the inventive concept apparent from the invention claimed as a whole.
133 Their Honours’ reasoning at times alluded to method claims, and their likely patentability, by way of contrast with the product claim. Their Honours noted at [137] that the application of naturally occurring phenomena to a particular use may be a manner of manufacture if it amounts to a new process or method of bringing about an artificially created state of affairs of economic significance. They said “[e]ven so, the inventor cannot claim to have invented the naturally occurring product as opposed to the process of application”. Their Honours distinguished between on the one hand what they perceived may be patentable subject matter in the form of a method of using the discovery of a connection between the BRCA1 gene in a person and a medical condition, and on the other, a claim to the BRCA1 gene sequence itself (at [147]):
It was not disputed that the first respondent might justly lay claim to the discovery that, if an isolated fragment comprising the BRCA1 gene is found upon examination to exhibit the specified mutations and polymorphisms, their presence is or may be indicative of particular kinds of malignancy in the cell. Nor was it disputed that a process or method of using known technology to isolate a sequence of nucleic acid comprising the BRCA1 gene and examining it for the presence of the specified mutations and polymorphisms for the purpose of detecting or predicting malignancy might be patentable. But, as has been observed, the discovery of a natural correlation is not patentable as such and its discovery does not entitle the first respondent to patent the BRCA1 gene as a product, whether or not afflicted by the specified mutations and polymorphisms.
(emphasis added, citations omitted)
134 Gageler and Nettle JJ concluded that although claim 1 was restricted to isolated nucleic acid comprising the mutated BRCA1 gene, it was “in truth a claim for a monopoly over the right to apply long-established methods for the isolation and amplification of specific nucleotide fragments to the isolation and amplification of a patient’s naturally occurring BRCA1 gene, where and if it is found upon subsequent examination that the patient’s BRCA1 gene happened to be afflicted by any of the specified mutations and polymorphisms” (at [160]). This was not a manner of manufacture.
135 Relevant to the current inquiry, their Honours said:
[168] It is not disputed that a process or method of detecting the increased likelihood of certain kinds of malignancy by isolating the BRCA1 gene and examining it for the presence of any of the specified mutations and polymorphisms may be patentable subject matter as a process [NRDC at 262; Apotex; Hickton’s Patent Syndicate] (subject to considerations of novelty and inventive step when compared to the prior art base). But, to repeat, claim 1 is not a claim for any such process. It is a claim for a monopoly over such isolated fragments of naturally occurring DNA as comprise the BRCA1 gene as are found upon examination to contain the (naturally occurring) specified mutations and polymorphisms.
[169] In the result, the claim extends too far. The difficulty for the first respondent is that, having discovered a presumably good and perhaps ground-breaking process for detecting the probability of certain kinds of malignancy by reference to the presence of particular mutations and polymorphisms in the BRCA1 gene, the first respondent has attempted to patent those sequences of the gene themselves notwithstanding that, even when isolated, they are naturally occurring and therefore not new.
(emphasis added, citation omitted from [169])
136 Gordon J at [252] also cited Fletcher Moulton LJ’s reasoning in Hickton’s Patent Syndicate where he said at 348:
In my opinion, invention may lie in the idea, and it may lie in the way in which it is carried out, and it may lie in the combination of the two; but if there is invention in the idea plus the way of carrying it out, then it is good subject-matter for Letters Patent."
(emphasis added by Gordon J)
137 Her Honour characterised Myriad’s idea, concept or principle as being that specific mutations or polymorphisms in the BRCA1 gene sequence suggest a predisposition to breast cancer and ovarian cancer (at [254]). But that was not the idea carried out in claim 1 which was the product claim. It is instructive to note her Honour’s reasoning also contrasted the result as it applied to the product claim with her view of the correct conclusion in relation to the method claims:
[256] What then did Myriad do? It took the idea, concept or principle that specific mutations or polymorphisms in that sequence suggest a predisposition to breast cancer and ovarian cancer and moved to carry out that idea, concept or principle, or embody it in a manner of new manufacture, in claims 4-30. The validity of those claims is not in issue.
[257] Claim 4 may be taken as an example. In simple terms, it comprises a nucleic acid probe in which the nucleotide sequence is a portion of an isolated nucleic acid with the characteristic identified in claim 1. In general terms, a probe is a fragment of isolated nucleic acid of variable length which is used to detect the presence of complementary nucleotide sequences and to investigate tissue samples to see whether particular genes are being expressed. A probe for BRCA1 alleles may be derived from sequences of the BRCA1 region or its cDNA. Probes are usually constructed artificially and have a radioactive label attached.
[258] The invention in claim 4 carried into effect the idea that specifically identified mutations or polymorphisms in a sequence of the BRCA1 gene suggest a predisposition to breast cancer and ovarian cancer by testing for the presence of one or more of the specifically identified mutations or polymorphisms. That is an invention.
138 By contrast, claim 1 was not to a means of carrying out the invention but to the isolated sequence.
139 As Myriad makes clear, it is of importance first to consider what in substance is the invention as claimed. This depends on the construction of the claim, when read in in the context of the specification as a whole and in the light of the common general knowledge as it stood at the priority date.
140 In our view, the authorities demonstrate that the relevant claims of the patent in suit here represent a manner of manufacture, and that the primary judge did not err in so concluding.
141 One commences with consideration of the substance of the invention having regard to the disclosure of the specification. The specification informs the skilled reader that the invention “provides a detection method performed on a maternal serum or plasma sample from a pregnant female”, which comprises detecting the presence of nucleic acid of foetal origin in the sample, thereby “provides a method of prenatal diagnosis” (page 2 lines 20 to 24, emphasis added). This explanation is given after the revelation of the discovery that the answer to the search for non-invasive prenatal diagnosis techniques using foetal cells in maternal blood lies in the maternal plasma, the very material that those investigating a non-invasive technique had been throwing away in their investigations (page 2 lines 5 to 9).
142 In this context, the invention described may be seen to be a new means by which foetal DNA may be detected which does not involve dangerous invasive sampling or encounter the difficulty involved in using maternal blood which has very low concentrations of foetal DNA. Instead, it supplies a method of taking maternal blood, separating from it the plasma or serum and then using tools to detect the presence of a nucleic acid of foetal origin. The specification supplies examples of how to perform these steps and how to detect the foetal nucleic acid. The person skilled in the art could be in no doubt about the use to be made of the invention so described. Such a person would understand it to be a method whereby the secrets of cffDNA, valuable for the diagnosis of foetal abnormalities, could be unlocked without the problems of using invasive techniques and without the complications associated with detecting and then amplifying DNA found in the scarce foetal cells in maternal blood. The specification provides a number of examples of contexts in which the method may be used, such as in sex determination or determining the RhD status of a foetus.
143 Against this background one now turns to consider the substance of the invention claimed in the relevant claims.
144 Claim 1 commences by identifying that it is for a “detection method performed on a maternal serum or plasma sample from a pregnant female” (emphasis added). The primary judge found at [1272] that “detect” in this context is not a term of art but has its ordinary English meaning of “[t]o uncover, lay bare, expose, display (something covered up or hidden)” and “[t]o discover, find out, ascertain the presence, existence, or fact of (something apt to elude observation)”. He also found (at [484]) that the experts agreed that “detect” here means demonstrating the presence of non-maternal nucleic acid that is inferred to be of foetal origin.
145 The invention claimed in claim 1 involves the elements of two broad and simply described integers, being a detection method:
(1) performed on a maternal serum or plasma sample from a pregnant female,
(2) which method comprises detecting the presence of a nucleic acid of foetal origin in the sample.
146 Integer (1) involves the detection method being performed on maternal plasma or serum from a pregnant female. The means is not specified or limited. The common general knowledge at the priority date required that this involve obtaining the analysis material by extracting blood and then separating out the plasma and, if desired, obtaining the serum by removing clotting factors from the plasma.
147 Integer (2) involves the use of an unspecified technique to detect and distinguish nucleic acid of maternal origin from nucleic acid of foetal origin. One such technique is PCR. As his Honour said at [1274]:
Without such a step, one cannot detect or identify cell-free fetal DNA, as opposed to simply extracellular DNA from an unknown source, in the sample.
148 A simple example is the detection of a Y chromosome, which would assist in identifying whether the gender of the foetus is male. In the state of the art at the priority date the preferred means of doing so involved PCR, although other techniques could be used. Regardless of the technique, human intervention was undoubtedly required.
149 Professor Fisk, one of the experts called by the respondent, explained in his first affidavit (at [88]):
At the priority date, I would have understood that “detecting the presence of” would require discriminating between nucleic acids of fetal origin on the one hand and nucleic acids of maternal origin on the other (see, for example, Patent, page 4, line 30 to page 5 line 2 and the various references in the Patent to the “detection rate”). Without such a step, one would not be able to detect the presence of fetal DNA or know whether a given sample contains fetal nucleic acid and, at the priority date, the method, without such a step, would be of little or no apparent utility for prenatal diagnosis (being the subject matter of the Patent). At the priority date, one way of ascertaining the presence of fetal DNA in a given sample would be to take steps to try to probe or identify fetal sequences that are not possessed by the mother. The simplest case would be, as variously described in the Patent, specifically identifying sequences from the Y chromosome (for male fetuses), which would necessarily not be possessed by the pregnant female.
150 The person skilled in the art knew how to ascertain gender from nucleic acid. As the primary judge explained in a passage in the judgment setting out the agreed common general knowledge (at [109]):
As at the priority date, the sex of a male fetus would be apparent if a particular molecular test involved the PCR amplification of sequences known to be from the Y chromosome. In other words, if you could detect by PCR a Y-specific fragment, it was most likely that the fetus was male. This required sequence information for the Y chromosome to be known to allow for the design of primers to result in the Y specific PCR products. Further, the loci would need to be known to be unique to the Y chromosome. As at the priority date, there were Y-specific sequences published in the literature which could be used as PCR primers.
151 The steps involved in (a) to (d) of [494] of the primary judge’s reasons, set out in section 4.1 above, are not specified by claim 1, and accordingly the method so claimed is not limited to those steps. However, the primary judge found that in order to achieve the result of detection within integer (2) those steps were “at least” required. We do not consider that his Honour in so concluding was impermissibly adding integers to the claim. Rather, his Honour was demonstrating that although the method is broadly expressed, there was no means known within the common general knowledge whereby the method of the claim could be achieved without significant human intervention. The person skilled in the art would understand that to perform the detection method within what we have described as integer (1), plasma or serum needs to be separated from blood. To “detect” the nucleic acid of foetal origin within integer (2), analysis involving the use of a molecular technique such as PCR is required.
152 The appellants submit that the difficulty with this analysis is that despite the human mediation, “detection” is no different from the discovery that cffDNA is detectable. They involve the same thing. They submit that in substance there is no application of the discovery by simply claiming the detection of what has been discovered to exist.
153 However, such an approach tends to obscure the correct identification of the invention. It lies not in the mere observation that cffDNA is to be found in maternal plasma (or serum), but in the explanation as to how that knowledge may be unlocked for others to use it (that is, the explanation of how to extract the cffDNA from the plasma or serum). It is the idea coupled with a practical means of application that makes the invention.
154 The primary judge found at [494] that:
The artificially created state of affairs is the detection of cffDNA in the tested sample. This “product” is, by definition the result of human action and is not naturally occurring. The inventive method does not simply produce an abstract, intangible situation. It is not just the “information” encoded by the naturally occurring cffDNA itself.
155 We agree with that conclusion. Unlike the position in Myriad, claim 1 is not, as a matter of substance, directed to genetic information, but to a method involving the practical application of a means for identifying and discriminating between maternal and foetal nucleic acid. Although foetal nucleic acid occurs in nature, the substance of the invention is not cffDNA itself, but the identification of that particular nucleic acid as a part of a method. It is impermissible to disaggregate the integers of the method to point only to the cffDNA as the “invention”. Identification of the substance of the invention does not involve disregarding material aspects of the claim language. The invention as claimed is not merely output, but the detection process which yields an output. This is the very type of subject matter considered to fall on the correct side of the line between discovery of a scientific fact or law of nature and invention: NRDC at 264; Hickton’s Patent Syndicate; Apotex at [240]; Myriad at [137] (Gageler and Nettle JJ) and [252] (Gordon J).
156 The uses to which the method of claim 1 can be put are various. The primary judge’s summary of the common general knowledge at [108] (see section 2.3 above) provides some examples. In this context it is apparent that the detection of nucleic acid of foetal origin provides valuable insight for those in the art and is of economic utility.
157 As we have noted earlier, authorities from NRDC onwards demonstrates that a “vendible product” in the sense of a tangible thing is not required before a claimed invention can amount to a manner of manufacture (NRDC at 269), “manufacture” being a conception which as early as 1795 was considered to extend “to any new results of principles carried into practice ... new processes in any art producing results useful to the public”: NRDC at 270, citing Boulton v Bull (1795) 1 H Bl 493 at 492; 126 ER 651 at 666 (emphasis added). An artificially created improvement in human health brought about by applying a known pharmaceutical product for a new condition resulting in treatment of psoriasis satisfied the requirement in Apotex. The observation made by Crennan and Kiefel JJ at [282] of that case warrants repetition:
... Patent monopolies are as much an appropriate reward for research into hitherto unknown therapeutic uses of (known) compounds, which uses benefit mankind, as they are for research directed to novel substances or compounds for therapeutic use in humans.
158 In NRDC the Court contrasted abstract information (not patentable) with the use of such information in a practical application (patentable) (at 264). Notably, the definition of “exploit” in the Act notes that a patentee is given the exclusive right to “use the method”, and as the plurality observed in Myriad at [16], a method may or may not yield a “product”.
159 These matters compel the rejection of the appellants’ submission to the effect that the “output” of the method is mere information and accordingly cannot amount to a manner of manufacture. Whereas the invention lies in the idea that cffDNA may be detected in maternal serum or plasma, which may then be carried out using known means of detection, the method is not relevantly different to the process postulated by Gageler and Nettle JJ at [168] (see also [137], [147], [169]), or the use of the probe in claim 4 of the Myriad patent to convey the information that the presence of particular genes within complementary nucleotide sequences indicates a malignancy as postulated by Gordon J at [257]. Here, the invention as claimed carries into effect an idea that the presence of information within the naturally occurring code of a person will be useful. By a process of detection that information is yielded up. The claim construed as a whole necessarily involves an artificially created state of affairs yielding an outcome that is of economic utility. We reject the submission that because, disaggregated from the method, the result of the detection of foetal nucleic acid is information about a naturally occurring state of affairs, the requirements of Myriad factors 1 and 2 are not satisfied. The invention as claimed cannot be separated into discrete parts.
160 Nor do we accept that a conclusion of patentability as a manner of manufacture would be inconsistent with Research Affiliates, RPL Central or Rokt. The appellants seek to draw on these cases in support of their submission that although human action may have been involved in the performance of the method claims, the end result or outcome of the method is simply information, being the presence of cffDNA, which was discovered by Professor Lo. For the reasons given above, the characterisation of the outcome of the method as “information” does not disqualify the present invention. That is not to say that every method yielding information will be patentable. The consideration of patentable subject matter does not involve a one size fits all approach. In each of those cases the question for consideration was whether or not a mere scheme or plan was nonetheless a manner of manufacture because invention lay not only in the scheme but also the means by which it was realised using computerisation: Aristocrat v Commissioner of Patents [2020] FCA 778; 382 ALR 400 at [89] (Burley J). See RPL Central at [96]. The present case does not involve a scheme or plan implemented on the medium of a computer, which would involve different considerations.
161 It is appropriate to note that there is no real prospect of falling out of step with the international community of patent decisions in reaching this conclusion, because there are disparate approaches taken abroad to the question of patentable subject matter, involving different legal tests, none of which is in the same form as that which has developed in Australia.
162 One example arises by reference to the Illumina decision in the United Kingdom. In the United Kingdom ss 130(7) and 1(2) of the Patents Act 1977 (UK) are framed so as to have as nearly as practicable the same effect as the corresponding provisions of arts 52(2) and (3) of the Convention on the Grant of European Patents (opened for signature 5 October 1973, 1065 UNTS 199 (entered into force 7 October 1977)): Illumina at [184]. Those articles state that discoveries “as such” are not inventions.
163 In Illumina Carr J considered whether or not claim 1 of a patent (similar to claim 1 presently in suit, but which included the added words at its end “wherein said nucleic acid is a paternally inherited sequence which is not possessed by said pregnant female” (at [81])) fell within the prohibition that discoveries “as such” are not patentable inventions: at [184]. At [184] to [189] he identified the relevant legal test, drawn from Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2004] UKHL 46; [2005] RPC 9 at [77] (Hoffman LJ) and Tate & Lyle Technology td v Roquette Freres [2010] FSR 1 at [75] (Lewison J) as being whether, as a matter of substance and not mere form, the invention claimed “is a practical product or process, not information about the natural world”. This involved applying a four-part structured approach to objections to excluded subject matter set out by the Court of Appeal in Aerotel Ltd v Telco Holdings; Mcrossan’s Application [2007] RPC 7 and Symbian Ltd v Comptroller General of Patents [2009] RPC 1, involving: first, properly construing the claim; secondly, identifying the “actual contribution”; thirdly, asking whether the identified contribution falls solely within the excluded subject matter; and, finally, checking whether the actual or alleged contribution is “technical in nature” (at [186]). His Honour concluded that claim 1 was not to a discovery as such and accordingly was patentable subject matter (at [189]).
164 In the United States the Court of Appeals for the Federal Circuit considered a similar patent to the one in suit in Australia, where claim 1 was expressed as “a method for detecting a paternally inherited nucleic acid of foetal origin performed on a maternal serum or plasma sample from a pregnant female, which method comprises amplifying a paternally inherited nucleic acid from the serum or plasma sample and detecting the presence of a paternally inherited nucleic acid of foetal origin in the sample”: Ariosa (US) at 1373-1374. At 1375 the Court noted the established position that laws of nature, natural phenomena and abstract ideas are not patentable subject matter, and that in Mayo Collaborative Servicees v Prometheus Laboratories Inc 566 US 66, 132 S.Ct 1289 (2012) the Supreme Court set out a framework for distinguishing ineligible patents on the basis that they claimed such a law of nature, natural phenomena or abstract idea by first, determining whether the claims are directed to a “patent ineligible concept” and, secondly, if so, considering the elements of each claim both individually and as an ordered combination to determine whether additional elements “transform the nature of the claim” into a patent-eligible application. The second step is considered to be a search for an “inventive concept” that is sufficient to ensure that the patent in practice amounts to “significantly more than a patent upon the [ineligible concept] itself” (at 1375, quoting Mayo at 1294). The Court determined that by applying that specific test, Sequenom’s claim was not for patentable subject matter: at 1378.
165 Quite apart from the diverging approaches, and different results in respect of similar claims, it is apparent that the legal analysis developed over recent years in the United Kingdom (and Europe) and the United States diverges from that identified in the line of authorities identified in NRDC, Apotex and Myriad.
166 In our view the invention of claim 1 falls firmly within the concept of a manner of manufacture as that term is to be understood having regard to the authorities, being an artificially created state of affairs of economic utility (NRDC, Myriad at [28] (plurality)). We do not consider that it represents a new class of claim involving a significant new application or extension of the concept, such that factors 3 to 6 identified by the plurality in Myriad require consideration: see Commissioner of Patents v RPL Central Pty Ltd [2015] FCAFC 177; 238 FCR 27 at [118] - [119] (Kenny, Bennett and Nicholas JJ).
167 Having reached this conclusion in relation to claim 1, it follows that the same result applies in respect of each of dependent claims 2, 3, 5, 6, 9, 13 and 14 which explicitly add detail to the method of claim 1. Claims 22, 23 and 25 are methods for the provision of a “prenatal diagnosis” following the performance of the method in the first class (being claims 2, 3, 5, 6 and 9) and therefore are also a manner of manufacture for the reasons given. Grounds 1 to 5 of the appeal are accordingly dismissed.
5.1 The decision of the primary judge
168 Section 40(2)(a) of the Patents Act as it applied to the patent was as follows:
(2) A complete specification must:
(a) describe the invention fully, including the best method known to the applicant of performing the invention; and …
169 The primary judge provided a detailed survey of the law, noting (at [829]) that the following test for sufficiency was formulated in Kimberly-Clark Australia Pty Ltd v Arico Trading International Pty Ltd [2001] HCA 8; 207 CLR 1 at [25]:
The question is, will the disclosure enable the addressee of the specification to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty?
(citation omitted)
and (at [830]) that in Lockwood Security Products Pty Ltd v Doric Products Pty Ltd [2004] HCA 58; 217 CLR 274, the High Court said at [60]:
For the purposes of s 40(2)(a), it is not necessary for the inventor to disclose all the alternative means; it is enough that there is disclosure in the sense of enabling the addressee of the specification to produce something within each claim without new inventions or additions or prolonged study of matters presenting additional difficulty.
(citation omitted)
170 The appellants submitted before the primary judge that the test set out in Kimberly-Clark concerned the question of whether or not the person skilled in the art would be capable of producing something within each claim at the priority date or the filing date of the claim and that the focus of the test is on the practical requirement of enabling the production of something within the claim without invention or prolonged study. They submitted that the “invention” which must be described fully is the “embodiment which is described, and around which the claims are drawn”, citing Kimberly-Clark at [21]. The appellants submitted that there is a degree of tension between the concept of a single “embodiment” around which the claims are drawn, and the recognition by the High Court at [25] that the full description requirement is to be met in respect of each claim. The appellants submitted that the decision of the Full Court in Tramanco Pty Ltd v BPW Transpec Pty Ltd [2014] FCAFC 23; 105 IPR 18 (Nicholas J at [204]-[218], Allsop CJ and Greenwood J agreeing at [51] and [164] respectively) supports the propositions that:
(a) a specification may relevantly disclose more than one “invention” for the purposes of s 40(2)(a), involving more than one relevant “embodiment”;
(b) as a matter of substance, rather than merely form, a claim may be to more than one “invention”, each such invention relating to a different embodiment described in the specification; and
(c) where that occurs, the full description requirement in s 40(2)(a) must be satisfied for each “invention”, properly understood.
171 The primary judge accepted that the decision in Tramanco, and the reasoning of Nicholas J in Apotex Pty Ltd v Warner-Lambert Company LLC (No 2) (Apotex (No 2)) [2016] FCA 1238; 122 IPR 17 at [244] recognise that it is necessary to identify, as a matter of substance, whether the claim is directed to a single method of general scope, or whether it is directed to more than one such method. If the latter, then each method needs to be enabled (at [853]). However, his Honour found that leaving aside special classes of claims to methods producing one or more specified optional outcomes, it is well settled that only one embodiment within each claim need be enabled for sufficiency purposes. An invention is sufficiently disclosed if a skilled person could make a single embodiment of the invention which falls within the scope of the claims (at [855]).
172 The appellants submitted before the primary judge (and on appeal) that there were three embodiments within the patent, each representing a different invention, and each of which therefore need to be fully described to satisfy s 40(2)(a). The three embodiments according to the appellants were:
(a) the performance of a diagnosis by reference to the quality of the foetal DNA, specifically whether the maternal serum or plasma includes any targeted sequences not possessed by the mother (page 4 lines 5 to 7 of the patent) (qualitative method);
(b) the performance of a diagnosis by reference to the quantity of the foetal DNA, directed to determining the total levels of cffDNA (paragraph (a) on page 5 of the patent) (correlative quantitative method); and
(c) the performance of a diagnosis by reference to the quantity of the foetal DNA, directed to measuring the level of foetal DNA markers on different chromosomes (comparative quantitative method) (paragraph (b) on page 5 of the patent).
The appellants accepted that the qualitative method was sufficiently described, but submitted the other methods represent different inventions, neither of which satisfied s 40(2)(a).
173 The primary judge found that the appellants advanced cogent arguments on the basis of the science that neither of the quantitative methods were enabled (at [1012]), but ultimately rejected the argument on the basis of the construction of the specification:
[1013] In my view there is only one invention around which the claims are drawn. This is the method of detecting cffDNA in an artificially created plasma or serum sample isolated from blood extracted from a pregnant female. The claims are drawn around this invention to include particular applications, ranging from detection of Y sequences, detection of sequences for a particular purpose and quantification of the detected sequences. The examples are expressly stated to be examples of the one invention.
[1014] The relevant claims cover in substance and in form a method producing one outcome, namely, detection of a particular specified sequence, for example Y (claim 6) and non-Y (claim 9). Further, claims 1 to 3, 5, 6, 9 and 14 do not require a diagnosis to be made on the basis of a process of relative or absolute quantitation of cffDNA.
[1015] Further, the patent enables a person skilled in the art to detect cffDNA on Y and non-Y chromosomes for the purposes of, for example, sex and RhD status determination.
[1016] In my view the Patent provides sufficient information to enable a skilled person to produce something within each of the relevant claims without new inventions or additions, or prolonged study of matters presenting additional difficulty.
174 Against the prospect that he was wrong in reaching this conclusion his Honour considered in some detail whether the complete specification described the invention fully in relation to each of the quantitative methods. His Honour first considered the correlative quantitative method at [894]-[932]. This involved consideration of two aspects of page 5 of the patent. The first is the assertion in paragraph (a) that the total level of cffDNA is higher in pregnancies where the foetus has a chromosomal aneuploidy. He concluded, after reviewing in particular example 2 in the light of the expert evidence, that the preponderance of the evidence was that the suggestion that the level of cffDNA could be used as a screening test for aneuploidy pregnancies, which had been widely investigated over a period of more than 20 years, was not supported by any conclusive study, nor implemented in practice (at [918]).
175 The second is on page 5 line 27 of the patent and concerns elevated cffDNA in pre-eclamptic pregnancies, which his Honour found could be related back to paragraph (a) on page 5 (at [920]). After reviewing the specification, and in particular example 4 in the light of the expert evidence, his Honour concluded at [932] that the appellants’ submission that there was no evidence of any implementation of the method described in example 4 should be accepted. His Honour found that the evidence was that the asserted correlation between the higher concentration of cffDNA in maternal serum and pre-eclamptic pregnancies remains unproven, and no test using such a correlation had been used or existed, despite considerable efforts having been made.
176 The primary judge noted that the comparative quantitative method arose from the suggestion in paragraph (b) on page 5 of the specification that a further approach to quantitative screening for chromosomal aneuploidies might be to quantitate foetal DNA markers on different chromosomes (at [934]). The primary judge considered a dispute between the experts as to whether or not this might have been able to be implemented at the priority date for paternally inherited aneuploidies using suitable markers (at [937]). He agreed with the appellants that implementing the method proposed in (b) would have constituted a significant research project, noting that history showed that it was not until 10 years after the priority date, and the advent of new technology in the form of digital PCR and massive parallel sequencing, that the method was in fact implemented (at [961]). He considered that even with a significant amount of work, it is doubtful that the method could have been implemented in 1997 (at [987]).
177 In rejecting the legal arguments advanced by the appellants the primary judge said of the quantitation methods identified:
[1017] Now Ariosa’s lack of sufficiency attack has significantly focused on the question of whether a person skilled in the art could detect or diagnose aneuploidy and/or pre-eclampsia at the priority date. But the aneuploidy and pre-eclampsia examples are only two of a wide range of uses for the invention described and claimed in the Patent. By way of illustration, in the definition of prenatal diagnosis on page 2 of the Patent “aneuploidy detection” is listed as a subset of an example comprising the detection of fetal abnormalities and pre-eclampsia is the last mentioned example in the definition.
[1018] Further, the aneuploidy and pre-eclampsia examples of the Patent are described in very qualified terms as potential future applications requiring further investigation and development. Let me elaborate.
178 The primary judge then traced through the disclosure of the specification to demonstrate the point. Before addressing each of the asserted claims in turn, his Honour concluded by finding at [1025]:
I agree with Sequenom that even if Ariosa can establish that its asserted quantitative diagnosis embodiments were not feasible at the priority date, and I must say that Ariosa is strong on the science on this aspect, it does not follow that any of the relevant claims (as opposed to the specific aneuploidy claims 16 and 19 that are not asserted) lack sufficiency. A person skilled in the art could perform something falling within the scope of each of the relevant claims in any event.
179 In grounds 10 and 11 of the Notice of Appeal the appellants contend that the primary judge erred in concluding that the complete specification describes the invention fully. They contend that the primary judge ought to have found that each of the relevant claims is directed to more than one method, each of which needs to be described fully, and that in the case of one such method, being the method of diagnosis involving quantitating the level of cell-free foetal nucleic acid in maternal plasma or serum sample, the specification does not provide a description sufficient to enable the skilled addressee to perform the invention. The appellants challenge all of the relevant claims on this ground. They advance specific construction arguments in relation to claim 14 and then claims 22, 23 and 25.
180 The claim 14 construction argument overlaps with ground 13, which concerns non-infringement. It is therefore convenient to address ground 13 in this section also. In it, the appellants contend that the primary judge erred in finding that the term “concentration” in claim 14 includes relative concentration and is not limited to absolute concentration, and that in so concluding the primary judge erred in finding that the Harmony Test used by the appellants infringed the claim.
181 The appellants rely substantially on the same submissions they advanced before the primary judge. They contend that the reasoning of the High Court in Kimberly-Clark supports the proposition that the disclosure required to enable the addressee to produce something within each claim must be of the “invention” which is the “embodiment which is described and around which the claims are drawn” and is to be contrasted with the “invention so far as claimed in any claim” in s 18 of the Act. They submit that whilst courts have approached the sufficiency requirement on a claim-by-claim basis, the High Court’s observations draw attention to the invention described and claimed, and require analysis of the subject as a matter of substance, and not by reference to the scope of the broadest claim, citing Myriad. The appellants submit that it would be contrary to policy to allow a broader claim which extends both to insufficiently described means and sufficiently described means. In this context they rely on Tramanco, accepting that while in that case the Court was concerned with a different type of claim, the policy consideration that compelled the finding there was not confined, and should be applied in the present case.
182 On the facts, the appellants submit that the primary judge erred in his characterisation of the substance of the invention. They submit that contrary to his findings, it was not simply a detection method, rather it describes at pages 4 to 5 of the specification three broad ways by which the discovery of the ability to detect foetal DNA in serum or plasma of pregnant females may be applied to achieve a prenatal diagnosis, being what the primary judge defined as the qualitative method, the correlative quantitative method and the comparative quantitative method. Each of these involves a distinct application of the information about the detected foetal DNA. Each is, the appellants submit, a distinct “invention” or “embodiment” that must be enabled for the purposes of s 40(2)(a), whether considering the specification as a whole or on a claim by claim basis. The appellants submit that the findings of the primary judge are sufficient to support the proposition that neither of the quantitative methods are sufficiently described.
183 The appellants submit that the primary judge’s error lies in his mischaracterisation of the invention, contrary to Kimberly-Clark, and his failure correctly to apply the reasoning in Tramanco. The errors, they submit, are starkly illustrated by the primary judge’s observation at [1025] that claims 16 and 19 lacked sufficiency, those claims being dependent on earlier asserted claims. The appellants also address specific arguments to claim 14 and then claims 22, 23 and 25 to which we refer in more detail below.
184 The respondent defends the primary judge’s reasoning. It submits that it is well settled that a patentee will have given sufficient consideration for the grant of a limited monopoly provided for by a claim where it has enabled a skilled person to perform one common sense and substantive method within the scope of that claim and disclosed the best method known to it of doing so, and where the claim is otherwise fairly based on the matter described in the specification. The respondent submits that the primary judge found that the patent provides sufficient information to enable a skilled person to work a number of useful methods falling within the scope of each relevant claim, including to detect and amplify cffDNA from Y and non-Y chromosomes, diagnose RhD status, determine foetal sex and to quantify cffDNA. Crucially, it submits, the appellants do not challenge these findings, which the respondent submits plainly render the patent compliant with s 40(2)(a).
185 Section 40(2)(a) is directed to the disclosure of the specification and requires that it describe the invention fully. In Kimberly-Clark the requirement imposed by that section was explained at [25] by reference to the observations made by Romer LJ in No-Fume Ltd v Frank Pitchford & Co Ltd (1935) 52 RPC 231 at 243 in relation to the similar provision in the United Kingdom Patents and Designs Act 1907 (UK):
…
“[I]n other words, [it is essential] that the patentee should disclose his invention sufficiently to enable those who are skilled in the relevant art to utilise the invention after the patentee's monopoly has come to an end. Such disclosure is, indeed, the consideration that the patentee gives for the grant to him of a monopoly during the period that the patent would run …
It is not necessary that he should describe in his specification the manner in which the invention is to be performed, with that wealth of detail with which the specification of the manufacturer of something is usually put before the workman who is engaged to manufacture it.”
The question is, will the disclosure enable the addressee of the specification to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty?
(emphasis added)
186 The requirement that the patentee disclose the invention sufficiently to “utilise the invention” concerns the invention disclosed in the specification, being the embodiment which is described and around which the claims are drawn (Kimberly-Clark at [21]). However, contrary to the submissions advanced by the appellants, the italicised statement of the test or question at [25] of Kimberly-Clark (set out above) does not require an enabling disclosure across the breadth of the claim. That is because the High Court concluded at [25] that it was adequate consideration for the monopoly for the patentee to provide one means of utilising the invention, accepting that there may be several, or even many, others not disclosed. This approach was later affirmed by the High Court in Lockwood Security Products at [60] where it said that “it is not necessary for the inventor to disclose all the alternative means; it is enough that there is disclosure in the sense of enabling the addressee of the specification to produce something within each claim without new inventions or additions or prolonged study of matters presenting additional difficulty” (emphasis added). In that case the High Court rebuffed a contention that the approach described in Kimberly-Clark was unfair, saying at [103]:
One source of these unfairnesses was said to be the fact that s 40(2)(a), on the construction given by this Court in Kimberly-Clark, is complied with if the complete specification enables the addressee to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty: but Doric, whilst willing to attempt to sap life from Kimberly-Clark, prudently eschewed any attack upon that binding authority.
187 An approach that seeks to move from consideration of the extent to which the claim is enabled by the body of the specification to consideration of the question of whether more than one invention is disclosed in the body of the specification, tends to distract attention from the correct analysis. Such an approach perhaps seeks to draw into the form of the Act relevant to the current dispute the form that s 40(2)(a) now takes following the amendments brought about by the Intellectual Property Laws Amendment (Raising the Bar) Act 2012 (Cth).
188 The task mandated by authority involves consideration of the specification as a matter of substance. In our view, the appellants artificially seek to narrow the scope of the invention disclosed by segregating it into the three separate methods identified as the qualitative method the correlative quantitative method and the comparative quantitative method (as set out in section 3.1 above). In so doing, the appellants impermissibly develop the question of compliance with s 40(2)(a) by reference to the related, but conceptually different question of compliance with the requirement that the claims be fairly based on the specification within s 40(3). As Kimberly-Clark makes clear, the question of whether or not the invention is described fully within s 40(2)(a) is to be ascertained by having regard to the scope of the monopoly claimed in the claims. The test does not mandate an enquiry, as the appellants put it, to determine whether or not there is “more than one true embodiment” of the invention disclosed in the specification or more than one invention disclosed.
189 Nevertheless, to the extent that there is a conceptual basis for commencing the enquiry under s 40(2)(a) first by reference to the nature of the invention disclosed in the specification, the primary judge correctly concluded that the invention is of broader compass than that contended for by the appellants, and that there is in any event but one invention described.
190 The statement of the invention at page 2 lines 20 to 23 provides:
This invention provides a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention thus provides a method for prenatal diagnosis.
191 The breadth of the definition of “prenatal diagnosis” that follows this statement (at page 2 line 24 to page 3 line 4, set out in section 3.1 above) demonstrates that it is the method of detection that lies at the centre of the invention.
192 The description then elaborates on how detection of a nucleic acid of foetal origin may be used in several ways. In one it says that it may be “particularly useful for sex determination which may be carried out by detecting the presence of a Y chromosome” (page 3 line 29 to page 4 line 1). The specification provides for a further three uses on page 4 at line 5 to page 5 line 2:
The method according to the invention can be applied to the detection of any paternally-inherited sequences which are not possessed by the mother and which may be for example genes which confer a disease phenotype in the foetus. Examples include:
a) Foetal rhesus D status determination in rhesus negative mothers (Lo et al 1993). This is possible because rhesus D positive individuals possess the rhesus D gene which is absent in rhesus D negative individuals. Therefore, the detection of rhesus D gene sequences in the plasma and serum of a rhesus D negative mother is indicative of the presence of a rhesus D positive foetus. This approach may also be applied to the detection of foetal rhesus D mRNA in maternal plasma and serum.
b) Haemoglobinopathies (Camaschella et al 1990). Over 450 different mutations in the beta-globin gene have been known to cause beta-thalassaemia. Provided that the father and mother carry different mutations, the paternal mutation can be used as an amplification target on maternal plasma and serum, so as to assess the risk that the foetus may be affected.
c) Paternally-inherited DNA polymorphisms or mutations. Paternally-inherited DNA polymorphisms or mutations present on either a Y or a non-Y chromosome, can be detected in maternal plasma and serum to assess the risk of the foetus being affected by a particular disease by linkage analysis. Furthermore, this type of analysis can also be used to ascertain the presence of foetal nucleic acid in a particular maternal plasma or serum sample, prior to diagnostic analysis such as sex determination. This application will require the prior genotyping of the father and mother using a panel of polymorphic markers and then an allele for detection will be chosen which is present in the father, but is absent in the mother.
193 It is true that the inventors propose that there may be other ways in which the invention may be used, which are the correlative and comparative quantitative methods. Importantly, however, each is introduced in the following way (page 5 lines 3 to 6):
The plasma or serum-based non-invasive prenatal diagnosis method according to the invention can be applied to screening for Down’s Syndrome and other chromosomal aneuploidies. Two possible ways in which this might be done are as follows: ...
(emphasis added)
194 The language used is tentative and qualified. Unlike the four substantive examples of successful use of the invention described immediately before, screening for Down’s Syndrome and other chromosomal aneuploidies are raised as possibilities. Furthermore, in example 2, which further addresses the correlative quantitative method, the discussion concludes with the observation at page 14 lines 18 to 21 that:
In this study we demonstrate that the concentration of foetal DNA in maternal serum is elevated in aneuploid pregnancies. These results indicate that foetal DNA quantitation has the potential to be used as a new screening marker for foetal chromosomal aneuploidies ...
(emphasis added)
195 Example 4, which again concerns a quantitative method, similarly provides that foetal DNA concentration “may be used” (page 22 line 23) and that “further research will be required to investigate whether the level of foetal DNA is related to the severity of pre-eclampsia” (page 22 lines 29 to 30). The specification provides that “Our discovery also opens up research into the potential application of foetal DNA quantitation to predict the occurrence of pre-eclampsia, prior to the development of clinical signs such as hypertension and proteinuria” (page 22 line 30 to page 23 line 3). Example 5 concerns a quantitative method and is expressed in similarly tentative terms (page 33 lines 1 to 10).
196 The recognition that the invention may have several applications, some described more fully and others less fully, does not yield the result that the concept as set out in the embodiment of the invention around which the claims have been drawn must be sub-divided. The consideration for the monopoly is imparted by giving the person skilled in the art a means by which the embodiment may be performed. The standard set by s 40(2)(a) as described in Kimberly-Clark signifies that the statutory norm is satisfied by supplying a means of performing that invention, not every means.
197 The primary judge concluded that there is only one invention around which the claims are drawn, that being the method of detecting cffDNA in an artificially created plasma or serum isolated from blood extracted from a pregnant female (at [1013]). He compared at [1024] the tentative proposals for use that the inventors suggest in relation to the quantitative methods to which we have referred, with the uses to which the invention may be put:
Contrastingly, the following statements are made in the Patent regarding sex determination (relevant claims excluding claim 9) and RhD status (relevant claims excluding claim 6):
(a) the method according to the invention may be “particularly useful” for sex determination, “it is demonstrated herein that using only 10µl of plasma or serum a detection rate of 80% for plasma and 70% for serum can be achieved” (page 3, line 29 to page 4, line 4);
(b) “Sex determination has successfully been performed on pregnancies from 7 to 40 weeks of gestation” (page 6, line 5);
(c) “In [Example 3], we have demonstrated the feasibility of performing non-invasive foetal RhD genotyping from maternal plasma. This represents the first description of single gene diagnosis from maternal plasma” (page 19, lines 6 to 9);
(d) “The high concentration of foetal DNA in maternal plasma and serum has allowed us to reliably detect the presence of foetal genetic material” (page 30, lines 18 to 20); and
(e) “we were able to detect foetal SRY sequences as early as the 7th week of gestation” (page 31, lines 10 to 11).
198 Whilst the primary judge expressed doubts that the correlative quantitation method or the comparative quantitation method were sufficiently described in the specification, there is no challenge on appeal to his Honour’s finding that the so-called qualitative method was enabled. The distinction between these three methods adopts significance only if, assuming the correctness of an embodiment by embodiment approach (which we have rejected), it may properly be said that there was more than one invention around which the claims were drawn. For the reasons we have stated, we do not consider that his Honour’s reasons reflect error in that respect.
199 Having reached that conclusion, there can be no doubt that the primary judge correctly found that the disclosure of the specification supplied sufficient information to enable the person skilled in the art to produce something within each claim.
200 We do not consider that the reasoning of the Full Court in Tramanco suggests any different analysis. In that case Nicholas J at [207] (Allsop CJ and Greenwood J agreeing at [51] and [164] respectively) provided an example of a claim for a method that produces different outcomes. If the production of one or more of the alternative outcomes would amount to an infringement (as opposed to all of them together), his Honour considered that:
... it would seem to me to be wrong in principle to hold that the description of the invention is sufficient if the specification enables the use of the method to achieve outcome A, but not outcomes B or C. It would be inconsistent with the purposes of the Act to confer a monopoly on a patentee for a method of producing any of outcomes A, B or C, if the patentee’s disclosure only enabled the use of the method to produce some of those outcomes.
201 The Court in Tramanco did not eschew the requirement set out in Kimberly-Clark at [25] that the disclosure enable the addressee to produce something within each claim. Rather it considered that if a claim properly construed had the effect of claiming three distinct alternative outcomes, the position would not be any different to a case where each outcome was expressed in a separate claim. If the disclosure of the specification did not enable at least one method falling within each of the alternatives, then it would not conform with the requirement of s 40(2)(a). As a result, had the facts in Tramanco supported a conclusion that one of the three alternatives within claim 1 (involving the determination of the impact loading of a vehicle) could not be determined, then the invention claimed would not have been sufficiently described in the body of the specification. However, on its proper construction, claim 1 in Tramanco did not postulate different outcomes.
202 Nor did Nicholas J express any different view in Apotex (No 2). There, his Honour found, as a matter of substance, that the invention concerned the administration of the drug to humans, not rats (at [243]). Accordingly, had the person skilled in the art not had sufficient information to ascertain a dose regime in humans without new invention or prolonged study, then the claim to the dosage regime would not have been sufficiently described (at [247]). On appeal in Warner-Lambert Company LLC v Apotex Pty Limited (No 2) [2018] FCAFC 26; 355 ALR 44 (Jagot, Yates and Burley JJ) (Apotex (No 2) (Full Court)), the Full Court noted that in putting the matter in this way, it was apparent that Nicholas J had adopted the test in [25] of Kimberly-Clark (Apotex (No 2) (Full Court) at [27]-[28]). There was no challenge on appeal to his Honour’s characterisation of the invention as concerning the treatment of humans, rather than mammals (or rats) (Apotex (No 2) (Full Court) at [115]). Accordingly, the parties and the Full Court proceeded on the basis that the question on appeal was whether that invention was enabled within Kimberly-Clark. In so doing, unsurprisingly, the Full Court at [113] followed the test or “question” which the High Court stated in Kimberly-Clark at [25].
203 In the present case, the specification describes the embodiment around which the claims have been drafted in a broader form than that for which the appellants contend. The broader disclosure is sufficient, for the reasons that we have given, to account for the breadth of each of the asserted claims. As the primary judge found, something within each claim may be produced by the person skilled in the art without prolonged investigation or invention. That was not the case for dependent claims 16 and 19, which were of narrower scope than the other relevant claims, however that observation cannot disturb the correctness of the findings in relation to the other claims.
5.4.2 Claims 1, 2, 3, 5, 6 and 9
204 The appellants submit that claims 1, 2, 3, 5, 6 and 9 lack validity when tested against the standard of s 40(2)(a) because each includes within its scope the option between using a qualitative method, an absolute quantitative method or the two relative quantitative methods, which are, they submit “different methods that must be enabled”. Whilst they accept that none of these claims is in the form under consideration in Tramanco, nonetheless they submit that because the claims include within their scope options for their implementation, they also lack sufficient disclosure in the specification.
205 For the reasons set out above this argument has no merit. The primary judge found that there is disclosure in the sense of enabling the addressee of the specification to produce something within each of these claims. The appellants do not seek to disturb that finding. On orthodox principle, the challenge cannot succeed.
5.4.3 Claim 14 – construction, sufficiency and infringement
206 Claim 14 provides:
The method according to any one of claims 6 to 12, which comprises determining the concentration of the foetal nucleic acid sequence in the maternal serum or plasma.
207 The appellants submit that the reference to concentration here is to the absolute concentration which in the field of molecular biology can be expressed as an absolute percentage of mass in a given volume. Their primary submission is that the language of the claim precludes the reference to concentration from being a reference to relative concentration which is expressed as a fraction or percentage of another relevant parameter, and that the primary judge erred by concluding that claim 14 referred to either absolute or relative concentration. Alternatively, the appellants submit that if claim 14 includes the step of determining both the relative and the absolute concentrations, then the relative concentration step is not enabled. These arguments are also relevant to the appellants’ appeal in the context of infringement. In ground 13 of the appeal, the appellants contend that in finding that the term “concentration” is not limited to absolute concentration the primary judge erred in concluding that the appellants had infringed claim 14 of the patent.
208 The respondent contests the construction of claim 14 advanced by the appellants and submits that the argument based on lack of sufficiency within s 40(2)(a) is flawed because it seeks to circumvent the fundamental requirement in Kimberly-Clark that if one embodiment is enabled, the invention will be sufficiently described.
209 There is no dispute that if claim 14 is limited to a method involving determination only of the absolute concentration of foetal DNA in a volume of maternal serum or plasma, then the Harmony Test does not infringe, see [1229].
210 We commence by addressing the construction issue.
211 The appellants submit that the words “determining the concentration of the foetal nucleic acid sequence in the maternal serum or plasma” in claim 14 must be understood to refer to absolute concentration, because otherwise the emphasised words are redundant having regard to the dependency of claim 14 on earlier claims, including claim 1, which makes the same reference. Given their ordinary meaning, the emphasised words should be understood to refer to an amount of foetal nucleic acid in a volume of maternal serum or plasma. That is, the appellants submit, the ordinary meaning to be ascribed to those words, and was the meaning ascribed to those words by the experts in their joint expert report.
212 The difficulty for the appellants in this respect is that the primary judge, who had the benefit of hearing the evidence of the expert witnesses, took a different view which was entirely open to him when applying the well-established principles of claim construction, as to which see, for example, Jupiters Ltd v Neurizon Pty Ltd [2005] FCAFC 90; 222 ALR 155 (Hill, Finn and Gyles JJ) at [67].
213 In this regard his Honour:
(a) noted that both relative and absolute concentration are described and exemplified in the specification. An example of relative concentration is given on page 2 lines 12 to 16 (see section 3.1 above), and also example 5 which refers to methods to calculate both figures (at [1302]-[1303]);
(b) considered the construction of claim 14 in the context of the specification, including the balance of the claims. He noted that claim 20, which is dependent on the earlier claims including claim 14, provides for a method “wherein the sample contains foetal DNA at a fractional concentration of total DNA ...” (at [1305], our emphasis). He referred to the cross-examination of Professor Lovett, one of the expert witnesses called by the respondent, about claim 20, and his adherence to his view that whereas claim 14 refers to absolute or relative concentration, claim 20 is confined to relative concentration by the reference to “fractional” (at [1291]). In this context, the primary judge found that claim 20 provides a narrowing of the broader method of claim 14 (at [1306]), which is consistent with the construction that he preferred. We note that on appeal the appellants submit that when proper regard is had to the terms of claim 20 it is not a “narrower” iteration of claim 14, but instead stipulates a particular context in which each of claims 1 to 19 is performed. However, they cite no expert evidence for that proposition, or for their further proposition that the focus of the claim is on ensuring that the sample contains a minimum amount of foetal DNA relative to total DNA without subjecting it to foetal DNA enrichment; and
(c) noted that Professors Fisk and Lovett provided supplementary reports where they gave evidence that “concentration” in the context of claim 14 included both absolute as well as relative or fractional concentration (at [1285]-[1287]). The primary judge rejected the submission advanced by the appellants that it was to be inferred that this evidence was tainted by the witnesses having an eye to the alleged infringement, and he accepted that, first, claim 14 does not include any limitation to absolute concentrations but covers both absolute and relative or fractional concentrations and, secondly, in the field concentration can be expressed in either form (at [1299]-[1301]). We would not lightly circumvent his Honour’s acceptance of that evidence, and do not consider that in urging that we adopt an “ordinary meaning” to the language of the claim the appellants have provided a basis in the evidence for doing so in this appeal.
214 We see no error in the primary judge’s approach, or in the conclusion reached. Accordingly, we are not persuaded that the primary judge erred in his construction of claim 14.
215 We turn now to consider the sufficiency issue. The appellants contend that if claim 14 does include both absolute and relative concentrations, then the claim does not satisfy the requirements of s 40(2)(a) because it does not enable determination of the relative concentration within paragraph (b) on page 5 of the specification which involves the determination of the relative concentration of two foetal markers, and accordingly falls foul of the reasoning in Tramanco. As the appellants put it in oral submissions, the reasoning in Tramanco applies because:
... when met with claim 14 [one] has in substance the choice whether or not to employ the absolute concentration method, or the concentration method [in implementing the approach suggested in paragraph (b) on page 5 of the patent]. Alternatively, the concentration method in example 5, each being a very different species, each directed to a very different outcome ...
216 This argument fails at the threshold, because, consistently with Kimberly-Clark at [25], there is no dispute that the determination of absolute concentration is sufficiently described. Moreover, as the primary judge found at [1303], example 5 of the patent refers to both absolute and relative concentrations, and provides methods to calculate both figures. Professor Lovett gave evidence that it would have been straightforward at the priority date to use qPCR to quantify the relative amount of foetal DNA and the amount of total cell-free DNA by quantitative fluorescent PCR or real time qPCR, being the two means of quantification within claim 14. Professor Lovett also noted in his evidence that the skilled geneticist would not have viewed the distinction between absolute concentration and relative concentration to be technically important. As at the priority date it was well known that the qPCR used in the patent could either be used to determine the absolute concentration or the relative concentration of a DNA sequence in the sample. The quantification of the absolute concentration of a DNA sequence is more technically challenging as this requires the generation of reference plots. Furthermore, his evidence was that the skilled geneticist reading the patent would have recognised that if the absolute concentration was not required, the quantification of the relative concentration would have been a technically simpler alternative.
217 This evidence, which is relevant to his Honour’s reliance on the content of example 5 in the context of the construction of claim 14, serves to demonstrate that when “concentration” is understood as either absolute concentration or relative concentration, both are sufficiently described in the specification to enable a person skilled in the art to perform it.
218 As we have rejected the appellants’ contentions in relation to both the construction and sufficiency of claim 14, it follows that there is no need for us to consider further ground 13 of the Notice of Appeal insofar as it contends that the alleged erroneous construction of the term “concentration” led to an error in the primary judge’s infringement findings.
219 The appellants made specific submissions as to sufficiency in relation to claims 22, 23 and 25.
220 Claim 22 provides:
A method of performing a prenatal diagnosis, which method comprises the steps of:
(i) providing a maternal blood sample;
(ii) separating the sample into a cellular and a non-cellular fraction;
(iii) detecting the presence of a nucleic acid of foetal origin in the non-cellular fraction according to the method of any one of claims 1 to 21;
(iv) providing a diagnosis based on the presence and/or quantity and/or sequence of the foetal nucleic acid.
221 The appellants submit that this claim is analogous to claim 1 in Tramanco in that it involves within integer (iv) the identification of three different parameters upon which a diagnosis may be made (one qualitative method and two quantitative methods), with the consequence that if all three of the alternatives are not enabled then the invention claimed is not sufficiently described.
222 The same flows, it submits, to dependent claims 23 and 25.
223 We have noted that the appellants do not dispute that the qualitative method described and claimed in the patent is amply enabled in the body of the specification. We have set out above his Honour’s findings at [1024] concerning the adequacy of the description of that method.
224 As we have noted, where the body of the specification refers to diagnosis based on quantity, it concerns a quantity that moves beyond mere detection, and into the comparative and correlative quantitative methods, which the primary judge found not to have been described adequately.
225 Nevertheless, the person skilled in the art will understand that a prenatal diagnosis may be made on the basis of the presence of a single chromosome (for instance, RhD). The method of claim 21 is directed to a single outcome, namely a prenatal diagnosis. The alternatives in (iv) provide different means of achieving such an outcome. The obligation of the patentee pursuant to s 40(2)(a), as identified in Kimberly-Clark at [25], is to enable a single embodiment within each claim. In this case, by detecting a single chromosome a quantity of foetal nucleic acid will be detected. That the position would perhaps be different under the post-Raising the Bar test for disclosure under s 40(1)(a) does not assist the appellants. The validity of the claims must be assessed on the basis of the Act as it applies to the patent.
226 Accordingly, the challenge to claim 22, and the challenge to dependent claims 23 and 25, must fail. The learned primary judge was correct to conclude at [1036]-[1037] that this was so.
227 For the reasons set out above grounds 10 and 11 must be dismissed. Having regard to the conclusion that we have reached as to the construction of claim 14, ground 13 (which concerns infringement) must also be dismissed.
6.1 The decision of the primary judge
228 The primary judge commenced by observing that s 40(3) of the Act requires a real and reasonably clear disclosure in the body of the specification of what is claimed and noting that the language of s 40(3) points to a comparison between the claims and what is described in the body of the specification only. At [1044] his Honour cited the decision of the High Court in Lockwood Security Products at [68] and [69]:
[68] Erroneous principles. The comparison which s 40(3) calls for is not analogous to that between a claim and an alleged anticipation or infringement. It is wrong to employ “an over meticulous verbal analysis”. It is wrong to seek to isolate in the body of the specification “essential integers” or “essential features” of an alleged invention and to ask whether they correspond with the essential integers of the claim in question.
[69] “Real and reasonably clear disclosure”. Section 40(3) requires, in Fullagar J’s words, “a real and reasonably clear disclosure”. But those words, when used in connection with s 40(3), do not limit disclosures to preferred embodiments.
“The circumstance that something is a requirement for the best method of performing an invention does not make it necessarily a requirement for all claims; likewise, the circumstance that material is part of the description of the invention does not mean that it must be included as an integer of each claim. Rather, the question is whether there is a real and reasonably clear disclosure in the body of the specification of what is then claimed, so that the alleged invention as claimed is broadly, that is to say in a general sense, described in the body of the specification.”
Fullagar J’s phrase serves the function of compelling attention to the construction of the specification as a whole, putting aside particular parts which, although in isolation they might appear to point against the “real” disclosure, are in truth only loose or stray remarks.
(emphasis in original, citations omitted)
229 The primary judge identified two challenges brought by the appellants on this ground that are relevant to this appeal. The first was that the invention disclosed in the specification is at its broadest a method of prenatal diagnosis involving detecting cffDNA. Each of claims 1, 2, 3, 5, 6, 9 and 14 is not fairly based as none of those is limited in scope to use in a method of providing a prenatal diagnosis, but rather are claims to detection methods at large involving the detection of cffDNA (at [1046]). The appellants submitted below that there are cases where the detection of a nucleic acid of foetal origin does not provide any information about a condition or characteristic of the foetus or a pregnant woman (at [1047]). Accordingly, the appellants submitted before the primary judge, those claims are not fairly based. The second was that insofar as the patent discloses that a diagnosis may be undertaken by reference to detected cffDNA, the substance of what is disclosed as an invention in the specification does not extend beyond the identification of a DNA sequence possessed by the foetus, not by the mother, and the use of a primer or probe to target that sequence in order to detect the presence of the identified foetal nucleic acid (at [1048]). This was said to be underpinned by the examples (at [1049]) which impart an implied requirement that the sequence detected be known in advance – for instance by prior genotypic or phenotypic analysis – not to be possessed by the mother (at [1061(a)]). The appellants submitted below that claims 1, 2, 3, 5, 9, 14, 22, 23, 24, 25 and 26 include within their scope the detection of foetal nucleic acid even if that nucleic acid is possessed by the mother as well as the foetus (at [1050]).
230 The primary judge concluded that the disclosures in the specification generally supported the invention that he considered had been disclosed, and confirmed that the broadest claim is commensurate with and does not travel beyond the matters identified (at [1060]).
231 More specifically, in relation to the first challenge (namely that the patent at its broadest is directed to a method of prenatal diagnosis involving detecting cffDNA) the primary judge considered that there was a general disclosure in the body of the specification of a detection method not tied to the diagnosis of a foetal or maternal condition, citing passages on pages 1, 2, 4, 31 and 32 of the specification in support of this proposition (at [1072]). The primary judge further noted that the phrase “prenatal diagnosis” is broadly and inclusively defined on page 2 of the specification as covering the determination of any maternal or foetal condition or characteristic which is related to either the foetal DNA itself or to the quantity or quality of the foetal DNA in the maternal serum or plasma including the detection of simple mutations. The primary judge continued at [1073]:
In the context of this definition, the Patent also says at page 3 that “[t]he nucleic acid detected in the method according to the invention may be of a type other than DNA e.g. mRNA”. These references provide support for claims 1 to 3, 5, 9 and 14 of the Patent.
232 The primary judge also rejected the argument advanced by the appellants that an implied requirement should be distilled from the examples in the patent to the effect that a target sequence had to be known in advance not to be possessed by the pregnant female and that insofar as the claims are for an invention that does not involve knowledge of that sequence, they are not fairly based (at [1061]). He noted that in the joint expert report, the experts agreed that the patent does not require that “the nucleic acid of foetal origin” be known in advance to be of foetal origin (at [1064]) and that whilst some of the examples involve the detection of Y sequences known in advance of the test to not be possessed by the pregnant female or through prior genotypic or phenotypic analysis, the invention described and claimed is not so limited (at [1065]), the specification explicitly stating at page 6 lines 6 to 7 that the examples “do not in any way limit the scope of the invention”. The primary judge found that the appellants’ implied prior knowledge requirement was really one of enablement (at [1067]-[1071]), inapplicable in the context of a fair basis challenge.
233 Ground 6 is a general challenge to the primary judge’s finding that the relevant claims were fairly based on the matter described in the specification. In ground 7 the appellants contend that the primary judge ought to have found that the invention disclosed in the specification, properly read, does not extend beyond targeting a DNA sequence of a foetus which is known in advance not to be possessed by the pregnant female, so as to detect the presence of that sequence in the maternal plasma or serum. To the extent that the claims are not so limited, they are not fairly based. Ground 8 was abandoned by the appellants before the hearing. In ground 9, the appellants contend that the primary judge ought to have recognised that the person skilled in the art would have read the broad disclosures of the patent as being informed by the specification as a whole and accordingly subject to limitations pointed to in the context of the examples.
234 The appellants submit that the substance of what is disclosed as an invention in the specification does not extend beyond: (a) the identification of a DNA sequence that is possessed by the foetus but not by the mother. No method is described for detecting a DNA sequence that is possessed by both the mother and the foetus; and (b) the use of a primer or probe to target that sequence in order to detect the presence of the identified foetal nucleic acid. They submit that these disclosures underpin and are expressly described in each of the examples in the patent. The appellants submit that of the relevant claims, 1, 2, 3, 5, 9, 14, 22, 23 and 25 are not limited to such an approach, and are so broad as to include within their scope the detection of foetal nucleic acid (a) even if it is not known in advance not to be possessed by the mother, and (b) even if that nucleic acid is also possessed by the mother, being DNA which the foetus has inherited from the mother, which constitutes half of all foetal DNA. The appellants further submit that the primary judge at [1071] conflated the requirement that the invention be sufficiently described with the enquiry as to whether the claims are fairly based, notwithstanding that the High Court has cautioned against such an approach, citing Lockwood Security Products at [49].
235 The respondent defends the primary judge’s findings and submits that the appellants wrongly seek to confine the invention disclosed in the patent to the detection of DNA sequences that are “known in advance” not to be possessed by the mother, which is inconsistent with the expert evidence and the disclosure in the patent as a whole.
236 A claim will be fairly based within s 40(3) if there is “a real and reasonably clear disclosure in the body of the specification of what is then claimed, so that the alleged invention as claimed is broadly, that is to say in a general sense, described in the body of the specification”: Lockwood Security Products at [68]-[69].
237 The appellants do not, for these grounds of appeal, contest the general characterisation of the invention advanced by the primary judge at [1053], namely that the patent as a whole describes an invention comprising the detection of cffDNA in a plasma or serum sample prepared from blood extracted from a pregnant female. That characterisation is taken from the disclosure of the specification at page 2 lines 20 to 24, when understood in the context of the common general knowledge and the disclosure of the specification, which makes plain that the plasma or serum is only obtained by separating it from other parts of maternal blood. The appellants submit, however, that the general disclosure must be read down by reference to the more express disclosure in the examples. As the appellants put it in oral submissions, when the patentee talks about foetal DNA being detectable, the only way that it is described in the specification is “by looking for some sequence which you know for dead set certain the mother cannot have ...”. No other way is described in the specification. Although the general disclosure of the invention is not so constrained, in the appellants’ submission the person skilled in the art would only read the disclosure of the specification as being for an invention that involves detecting cffDNA in maternal plasma by sending in a probe that looks for some sequence, parentally inherited, that the mother does not have.
238 In our view, four points stand in the way of the appellants’ success on this ground.
239 First, the disclosure of the specification does not require advanced knowledge of the target sequence to detect the presence of the identified foetal nucleic acid. As the primary judge found, the broad description of the invention does not require that knowledge of the sequence be possessed: at [1055], [1072 (a) – (c)]. There is consistency between the broadest statement of the invention set out in the specification and the claims.
240 Secondly, it is not to be implied from the specific embodiments exemplified in the patent that the scope of the invention is narrower than the broader statement of the invention contained on page 2 of the specification. Nothing in the body of the specification suggests that this approach should be taken. To the contrary, the patentee states in terms that the examples “do not in any way limit the scope of the invention” (page 6 line 16).
241 Thirdly, the primary judge placed some weight on the views of the experts including, in particular, at [1064] on their answer to question 16 in the joint report. This answer was given in the context of the experts offering their understanding of the phrase “detecting the presence of a nucleic acid of foetal origin” in claim 1, which they considered to mean “demonstrating the presence of non-maternal nucleic acid that is inferred to be of foetal origin” (see primary judge at [1272]-[1277]). Question 16 asked whether the patent required that the “nucleic acid of foetal origin” is known in advance to be of foetal origin to which the experts answered “no”. It was suggested in submissions by the appellant that the answer given by the experts to question 16 was ambiguous but their answer was not explored in their oral evidence and, in the circumstances, we see no error in the use made of this answer by the primary judge.
242 Finally, we do not consider the criticism advanced by the appellants of the primary judge’s reasoning at [1071] as conflating the test for sufficiency within s 40(2)(a) with the enquiry as to whether the claims are fairly based is warranted. In that paragraph his Honour was expressing agreement with the submission advanced by the respondent that the appellants themselves had raised an enablement issue under the guise of a fair basis challenge: see [1067]-[1071]. His Honour was responding to the expert evidence provided by the appellants that the person skilled in the art would not be able to perform the invention insofar as the claims extended to detecting a nucleic acid of foetal origin in a serum or plasma sample without access to the maternal and paternal genomes. In so doing, his Honour correctly eschewed the approach there propounded by the appellants.
243 Accordingly, the fair basis grounds of appeal must be dismissed.
244 The primary judge dealt with a number of disputes arising in the context of the infringement allegations. Leaving aside the issue concerning the construction of claim 14, which we have addressed in section 5.4.3 above, the appeal, insofar as it concerns infringement, is limited to a challenge to the primary judge’s infringement finding in relation to the so called “send out” model for obtaining results from the testing performed by the appellants.
7.2 The decision of the primary judge
245 The primary judge noted that the respondent alleged that the Harmony Test conducted by Ariosa in the United States and licenced to Sonic and Clinical in Australia fell within the scope of claims 1, 2, 3, 5, 6, 9, 13, 14, 22, 23, 25 and 26 of the patent. The primary judge found that the Harmony Test analysed the cell free DNA present in the plasma of pregnant women to provide an estimate of the risk that the foetus was carrying an autosomal aneuploidy, and at the election of the patient it could also provide an estimate of the risk that the foetus was carrying a sex chromosome aneuploidy, and a determination of the sex of the foetus (at [1162]). The primary judge found that Sonic and Clinical had used the Harmony Test in Australia; in the case of Sonic, since September 2015, and in the case of Clinical since March 2016. He also found that they had been licensed to use the Harmony Test by Ariosa. After considering disputed questions of construction, the primary judge expressed his conclusions in respect of each of the relevant claims, finding all but invalid claim 26 to be infringed by the use of the Harmony Test by each of Ariosa, Sonic and Clinical (at [1459]). This finding is not challenged on appeal.
246 In findings that are now challenged, his Honour concluded that the “product” of the use of the impugned Harmony Test was the results of that test. They were typically communicated in writing as a report, either in electronic or paper form. A sample of such a report was included in the appeal book, and shows results for a foetus with a gestational age of 10 weeks, 3 days. The report provides an estimated level of risk of trisomy 21, a probability level (for example, less than 1/10,000 (0.01%)) and a recommendation such as “review results with patient”. However, the case as we understood it to have been presented before the primary judge did not turn on whether or not there was a physical output of the test results or whether that physical output was a “product”. Rather, the question was whether or not the information produced from the tests amounted to “a product resulting from such use”, being the output of the method of the invention claimed. As much is apparent from the primary judge’s reasons at [1475], where his Honour refers to obtaining results, including in electronic or paper form.
247 The infringement finding challenged by the appellants relates to a period of time before Sonic and Clinical performed the Harmony Test in Australia themselves. Between June and October 2015 (in the case of Sonic) and between December 2014 and March 2016 (in the case of Clinical), the appellants operated a “send out” model whereby they collected blood samples from pregnant women in Australia and sent those samples to Ariosa in the US. Ariosa then conducted the Harmony Test in the US and provided the results, in the form of a report made available by a file sharing platform, to Sonic and Clinical in Australia.
248 After setting out the definition of “exploit” in the Act, and considering the legal principles applicable his Honour found:
[1472] The term “product” is not defined in the Act. Accordingly and in context, the term is to be given its ordinary meaning, namely as covering anything resulting from the patented method that can be commercially exploited. Such an ordinary meaning was adopted in Northern Territory v Collins, where the Court was concerned with the interpretation of the word “product” in the context of s 117 of the Act; a term occurring in several places of the same statute should usually be similarly construed.
[1473] Further, it has been said that “[i]n relation to a process, the product is the state of affairs in which an effect may be observed” (Cancer Voices Australia v Myriad Genetics Inc (2013) 99 IPR 567 per Nicholas J at [88]) and that “vendible product” should be “understood as covering every end produced or artificially created state of affairs which is of utility in practical affairs and whose significance thus is economic” (CCOM Pty Ltd v Jiejing Pty Ltd (1994) 51 FCR 260 at 291 per Spender, Gummow and Heerey JJ, referring to NRDC at 276 to 277. Now I accept that these observations were made in a different context and with different qualifications. Nevertheless they provide a relevant analogy.
[1474] Moreover, interpreting the definition of “product” broadly limits the undesirable possibility that a potential infringer could utilise a patented process overseas and then import the resulting product into Australia, and so circumvent the monopoly granted by the method patent.
[1475] In my view, the reality is that the Harmony Test is carried out for the purpose of obtaining the results. The results are clearly commercially valuable; patients pay in the order of $400 to obtain them. The Harmony Test results including being recorded in electronic or paper form are the product of a process for the purposes of the definition of “exploit”. Moreover, by supplying the Harmony Test results to clinicians in Australia, Sonic and Clinical infringed the relevant claims during the send out period.
(emphasis in original)
249 The appellants advance four grounds of appeal relevant to the primary judge’s infringement analysis. As we noted earlier, we have addressed ground 13, which concerns claim 14, in section 5.4.3 above, which leaves three grounds for determination. Ground 12 is a general challenge to the primary judge’s findings on validity and the consequences of those findings for infringement. Having regard to our findings as to validity, it is unnecessary to consider this ground further. In ground 14 the appellants contend that the primary judge erred in finding that the Harmony Test results constitute a “product” resulting from the use of the method of the patent, and thereby erred in finding that the appellants infringed the relevant claims by importing, supplying, offering to supply or otherwise disposing of the Harmony Test results in Australia during periods from 2014 to 2017. In ground 15 they contend that the primary judge ought to have found that the Harmony Test results were fundamentally information and not a “product” within the definition of “exploit” in sch 1 of the Act.
250 The appellants submit that not every method or process claim will result in a “product” within the definition of “exploit”. The appellants submit that the primary judge erred by focussing at [1475] on the medium in which the Harmony Test results were produced, being in electronic or paper form, and that his Honour ought to have focused on the output of the infringing method as understood by reference to the claims, which the appellants submit result in “information” only. This finding led the primary judge to the conclusion that by supplying the results to clinicians in Australia, Sonic and Clinical had infringed the relevant claims in Australia. The appellants next submit that the primary judge erred in finding at [1472] that the word “product” in the definition of “exploit” should be given its ordinary meaning of “covering anything resulting from the patented method that can be commercially exploited”, citing for that proposition Collins at [34] (Hayne J). Furthermore, the appellants submit the primary judge’s approach would yield the outcome that if the results of the Harmony Test were communicated by telephone, rather than in writing, that telephone communication would similarly amount to an infringement. The primary judge did not address that issue.
251 The respondent supports the reasoning of the primary judge. The definition that he adopted, it submits, is consistent with Collins at [34] (Hayne J), and also with the characterisation of that word in the context of manner of manufacture in NRDC as an artificially created state of affairs which has economic significance. The respondent submits that the adoption of the ordinary meaning of “product” retains the balance struck by the legislature in relation to products resulting from patented methods or processes, ensuring that a potential infringer could not utilise a patented process overseas and then circumvent the monopoly by importing that product. It submits that there is no reason in principle or policy why “products” should be read down to exclude the results arising from use of the claimed methods.
252 For the following reasons, we respectfully disagree with his Honour’s conclusion that “product”, in the context of a product resulting from the use of a method or process within the definition of “exploit” covers “anything resulting from the patented method that can be commercially exploited” and that the send out model as used by Sonic and Clinical infringed the relevant claims.
253 Section 13 of the Act confers on the patentee the exclusive right to exploit the invention the subject of the patent throughout the patent area. Section 13 provides:
13 Exclusive rights given by patent
(1) Subject to this Act, a patent gives the patentee the exclusive rights, during the term of the patent, to exploit the invention and to authorise another person to exploit the invention.
(2) The exclusive rights are personal property and are capable of assignment and of devolution by law.
(3) A patent has effect throughout the patent area.
254 There is an intersection between the scope of the exclusive rights conferred upon the patentee “to exploit the invention” pursuant to s 13(1) of the Act and the inclusive definition of “exploit” in sch 1: Calidad Pty Ltd v Seiko Epson Corporation [2020] HCA 41; 34 ALR 577 at [25] (Kiefel CJ, Bell and Keane JJ).
255 The term “exploit” is defined in sch 1 as follows:
exploit, in relation to an invention, includes:
(a) where the invention is a product–make, hire, sell or otherwise dispose of the product, offer to make, sell, hire or otherwise dispose of it, use or import it, or keep it for the purpose of doing any of those things; or
(b) where the invention is a method or process–use the method or process or do any act mentioned in paragraph (a) in respect of a product resulting from such use.
256 The infringement aspect of the appeal turns on the correct understanding of the words in (b) of the definition of “exploit”.
257 The term “invention” is defined to mean “any manner of new manufacture the subject of letters patent and grant of privilege within section 6 of the Statute of Monopolies, and includes an alleged invention”: sch 1, definition of “invention”. An invention is disclosed in the complete specification of the patent (s 40(2)(a)). To be a patentable invention it must conform with the requirements of s 18.
258 A “patented product” means “a product in respect of which a patent has been granted and is in force” (sch 1, definition of “patented product”). A “patented process” means a process in respect of which a patent has been granted and is in force” (sch 1, definition of “patented process”).
259 However, the Act does not define “product” or what a product resulting from the use of a process or method will be. The meaning of that term in the context of the definition of “exploit” must therefore be ascertained having regard to the text of the provision and also consideration of its context, which includes the general purpose and policy of the provision, in particular the mischief it is seeking to remedy: Alcan (NT) Alumina Pty Ltd v Commissioner of Territory Revenue [2009] HCA 41; 239 CLR 27 at [47] (Hayne, Heydon, Crennan and Kiefel JJ). The primary object of statutory construction is to construe the relevant provision so that it is consistent with the language and purpose of all of the provisions of the statute. The meaning of the provision must be determined by reference to the language of the instrument viewed as a whole: Project Blue Sky v Australian Broadcasting Authority [1998] HCA 28; 194 CLR 355 at [69] (McHugh, Gummow, Kirby and Hayne JJ). In interpreting a provision of an Act, the interpretation that would best achieve the purpose or object of the Act (whether or not that purpose or object is expressly stated in the Act) is to be preferred to each other interpretation: Acts Interpretation Act 1901 (Cth) s 15AA. See also Collins at [16] (Gummow ACJ and Kirby J) and [99] (Crennan J).
260 The word “product” is ambiguous. In its primary definitions given in the Macquarie Dictionary (3rd ed, 1997, Sydney) it is either “a thing produced by any action or operation, or by labour; an effect or result” or “something produced; a thing produced by nature or by a natural process”. However, the context of (b) of the definition of “exploit” requires that a product result from the use of the method or process of the invention, suggesting that the method or process must be one that manufactures the product. Furthermore, the word “product” also appears in part (a) of the definition. There, where the invention is a product, it will be an act of exploitation to “make, hire, sell or otherwise dispose of” the product, or offer to do the same, or keep it for the purpose of doing those things. This language, and particularly notions of making, hiring, disposing of and keeping, most naturally connote acts done with tangible things.
261 Looking at the statutory purpose of (b) of the definition of “exploit” one may see that it defines two aspects of the exclusive rights to exploit a method or process patent. Of these, in Collins Crennan and Kiefel JJ said:
[124] The first aspect is the exclusive right to "use the method or process" per se. ... There was never any doubt that carrying out a patented method or process was an exercise of the invention: “[w]here the claim is for a process, any carrying out of the process is, of course, an exercise of the invention, unless purely experimental” (emphasis added) [Blanco White, Patents for Inventions, 4th ed (1974) at 96 [3-207]]. Accordingly, carrying out a patented method or process could constitute infringement of a patented method.
[125] The second aspect of the exclusive right to exploit a method or process patent is the exclusive right to do all the acts listed in par (a) of the definition of “exploit” in respect of “a product resulting from … use” of “a method or process”. In the absence of a product claim coupled with a method claim, there was often far less certainty about whether such acts would constitute an infringement of a patented method [see generally Blanco White, Patents for Inventions, 4th ed (1974) at 96-98 [3-208] and Walton and Laddie, Patent Law of Europe and the United Kingdom, (1983), vol 1 at II [2060]). The insertion of the second aspect into the statutory definition of “exploit” resolves such uncertainty [As do ss 60(1)(c) and 100(1) of the Patents Act 1977 (UK). See also Thorley, Miller, Burkill, Birss and Campbell, Terrell on the Law of Patents, 16th ed (2006) at 313 [8-28]]. …
[126] As a practical matter, it may often be easier to prove acts of infringement of a patented method by leading evidence of acts in respect of a product which results from use of a patented method, rather than seeking to prove use of the method per se [It can be noted that O 17 r 1 of the Federal Court Rules permits the Court to make orders for inspection of property including orders for “the observation of any process”].
(original emphasis in [124], emphasis added to [125])
262 It is apparent that the mischief that the words “product resulting from such use” in the definition of “exploit” addressed was the problem that a product resulting from a process might not be considered to infringe a patentee’s exclusive rights unless the form of claim was drafted so that the patent was for a product by process. That was a quirk of patent drafting that it was considered should not curtail the rights of patentees. It led to uncertainty, because there were three conflicting lines of authority (in the United Kingdom) as to the correct approach, as noted in Blanco White, Patents for Inventions (4th ed, 1974) at 96 at [3-208].
263 As appears from our reasoning in section 4.4 in relation to manner of manufacture, there is no necessity in principle for a method or process to yield a product in order for that method or process to amount to a manner of manufacture: Collins at [18] (Gummow ACJ and Kirby J); Apotex at [230] (Crennan and Kiefel JJ). However, the definition of product preferred by the primary judge tends to suggest the contrary.
264 A number of manner of manufacture cases indicate occasions on which various courts have considered that the use of a method or process has not yielded a product of any type. For instance, there was no “product” of the use of a selective herbicide which killed weeds other than a relatively weed-free field (NRDC), even though the outcome of the use of that invention is something resulting from the method that could be commercially exploited. The same may be said of the outcome of the method for the retrieval of graphic representations of desired characters for assembly of text (being pages of text in those characters) (CCOM Pty Ltd v Jiejing Pty Ltd [1994] FCA 396; 51 FCR 260), and the outcome of the method of treatment in Apotex, which led to healthier human patients.
265 In this context, we do not consider that the cases referred to by the primary judge at [1473] can be taken as providing support for the broad definition that he adopted. The passages in Cancer Voices Australia v Myriad Genetics Inc [2013] FCA 65; 99 IPR 567 (Nicholas J) and CCOM to which the primary judge referred do no more than acknowledge that an artificially created state of affairs of economic significance will be a manner of manufacture. The use of “product” in those cases was not by reference to the Act, but rather the older law on the subject of “vendible product” to which we have referred: see Cancer Voices at [87]-[88]; CCOM at 291. Those cases do not conclude that any outcome from a patentable method or process may correctly fall within the definition of “product”.
266 In our view, a construction of the word “product” in the context of the definition of “exploit” which recognises that not all methods or processes will led to a product resulting from their use is to be preferred.
267 Furthermore, the breadth of the definition proposed by the primary judge is likely to have results that are not sensible, one aspect of which is that if the outcome of the test is a “product” then the oral communication of that outcome can be an infringement, and a person who has heard the outcome (which may be as simple as “it’s a girl!”) and who flies into Australia with that information may infringe by importing that outcome.
268 We do not consider that the reasoning of Hayne J in Collins indicates that the word “product” should be given the meaning ascribed by the primary judge to it, in the context of its use in the definition of “exploit”. In the passage relied upon by the primary judge, Hayne J was concerned with the construction of that word within s 117 of the Act, which deals with contributory infringement. Unlike in that section, the definition of “exploit” in sch 1 is conditioned upon a requirement in each of (a) and (b) that the exploited product or method must be “an invention”. Those words serve to distinguish the usage in s 117 from that in the definition of exploit, a point that was made by Hayne J at [34] where he noted:
... the dictionary's treatment of the word “exploit” distinguishes between “where the invention is a product” and “where the invention is a method or process”. Read as a whole, s 117 can be seen to proceed on the footing that the word “product” has its ordinary meaning and is not confined to a patented product or a product that is itself the result of applying a patented method or process.
269 In our view it is more consistent with the purpose of the Act to conclude that the outcome that results from the use of the methods of detection or diagnosis of the relevant claims in the present case does not conform with the meaning of “a product resulting from such use” within the definition of “exploit”. A claim to mere information is not patentable: Grant v Commissioner of Patents [2006] FCAFC 120; 154 FCR 62 (Heerey, Kiefel and Bennett JJ) at [32]; Encompass Corporation Pty Ltd v InfoTrack Pty Ltd [2019] FCAFC 161; 372 ALR 646 (Allsop CJ, Kenny, Besanko, Nicholas and Yates JJ) at [94]. The fact that such information is derived from a patentable process or method cannot render the information itself patentable. In those circumstances, we do not consider that the word “product” in para (b) of the definition of “exploit” should be interpreted as extending the patentee’s monopoly to information which could not itself constitute patentable subject matter since it would have the unintended and odd consequence of permitting the patentee to obtain patent protection in respect of subject matter that has long been held to be unpatentable.
270 Accordingly, we consider that the primary judge erred in concluding that, during the limited periods between 2014 and 2016 when the Harmony Test was performed in California and the outcome of the tests communicated to Sonic or Clinical in Australia, those results constituted a product resulting from the use of the method of the patent. Grounds 14 and 15 of the appeal should be allowed.
271 We have found that the appellants have failed in relation to grounds 1 to 13 of their appeal but have succeeded in relation to grounds 14 and 15. The consequence is that the challenge to validity of the patent fails, but insofar as the primary judge found that the Harmony Test results infringed the patent during the limited period when the Harmony Test was conducted overseas and the results provided in Australia, his Honour’s findings and orders must be set aside.
272 The parties should confer and provide draft short minutes of order to the Court giving effect to these reasons including as to costs. To these extent that there is any dispute, the parties are to make submissions limited to no more than 5 pages, with the appellants to file and serve their draft within 14 days, the respondent to respond within 14 days thereafter, and the appellants to file a reply of no more than 2 pages within 7 days thereafter. Unless otherwise requested, we will determine any outstanding issues on the papers.
I certify that the preceding two hundred and seventy-three (272) numbered paragraphs are a true copy of the Reasons for Judgment of the Honourable Justices Middleton, Nicholas and Burley. |




