FEDERAL COURT OF AUSTRALIA
Australian Competition and Consumer Commission v Reckitt Benckiser (Australia) Pty Ltd [2016] FCAFC 181
ORDERS
AUSTRALIAN COMPETITION AND CONSUMER COMMISSION Appellant | ||
AND: | RECKITT BENCKISER (AUSTRALIA) PTY LTD ACN 003 274 655 Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
2. Order 1 of the orders made on 29 April 2016 be set aside.
3. In lieu thereof, for the contraventions of s 33 of the Australian Consumer Law (Schedule 2 to the Competition and Consumer Act 2010 (Cth)) the respondent pay to the Commonwealth of Australia within 30 days a pecuniary penalty of $6,000,000.
4. The respondent pay the applicant’s costs of the appeal and of the hearing below, as agreed or taxed.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
THE COURT:
Introduction
1 This is an appeal by the Australian Competition and Consumer Commission (ACCC) from orders made by a judge of this Court imposing a civil penalty of $1.7 million on Reckitt Benckiser (Australia) Pty Ltd (Reckitt Benckiser) for conduct in trade or commerce liable to mislead the public as to the nature, characteristics or suitability for purpose of pain killers known as the Nurofen specific pain range.
2 The maximum penalty for each contravention of s 33 of the Australian Consumer Law (ACL, set out in Schedule 2 to the Competition and Consumer Act 2010 (Cth) (CCA)) is $1.1 million (s 224(3)).
3 Over a period of nearly five years from 1 January 2011 to 10 December 2015, Reckitt Benckiser repeatedly engaged in the contravening conduct, selling 5.9 million packets of the so-called Nurofen specific pain range products (the conduct had in fact started in 2007, but the contravention period started on 1 January 2011 due to the limitation period in s 228(2) of the ACL). Accordingly, Reckitt Benckiser must have engaged in the proscribed conduct at least 5.9 million times. As such, the theoretical maximum penalty for the contraventions, as the primary judge said, was “many, many millions of dollars”. As we discuss below, in a practical sense, the overall maximum penalty was so great that there was no maximum penalty.
4 For the reasons that follow, the appeal must be upheld, the penalty imposed by the primary judge set aside, and in lieu thereof Reckitt Benckiser must pay a pecuniary penalty of $6 million for the contraventions.
Overview
5 The proceeding concerned conduct by Reckitt Benckiser consisting of representations made on product packaging and two webpages about a purported range of four ostensibly different Nurofen pain medications said to be “targeted” to treat four different types of pain, namely “migraine pain”, “tension headache”, “period pain” or “back pain”. In fact, there was no difference between any of the four products. The products all provided in the human body a dose of active ingredient equivalent to 200mg of ibuprofen. The only difference was the packaging and marketing. Moreover, the products were sold at about double the price of standard Nurofen which also provided a dose of 200mg of ibuprofen. The price premium of the products above standard Nurofen was part and parcel of Reckitt Benckiser’s overall marketing of the products.
6 Contrary to the representations, ibuprofen does not “target” any particular kind of pain. It treats all types of pain in precisely the same way. Any representation to the effect that a medication in which the sole active ingredient is (or is the chemical equivalent of) ibuprofen “targets” pain is inherently misleading, at least when combined with any reference to any specific type of pain. This is because the overall effect is to convey the false impression that the medication is capable of treating the specific type of pain differently from any other type of pain.
7 With 5.9 million sales of the four products over five years (yielding revenue to Reckitt Benckiser of about $45 million), millions of consumers were liable to be misled by the representations on the packaging that each product was targeted to treat a particular type of pain when, in fact, they were all identical products. An unknown number of consumers were also liable to be misled over at least 18 months by the representations on the webpages purporting to help consumers to “choose” which of the four products was “right” for treating different types of pain. In fact, there was no choice involved because there was no difference between the four products beyond their packaging and marketing.
8 Reckitt Benckiser made very late admissions, only at trial, to civil contraventions for misleading or deceptive conduct under s 18 of the ACL, as well as civil penalty contraventions under s 33 of the ACL for conduct liable to mislead the public as to the nature, character or suitability for purpose of the four identical products. This appeal relates only to the civil penalty contraventions under s 33 of the ACL, and ultimately only in relation to the quantum of the penalty imposed by the primary judge.
9 Our reasons for determining that the appeal must be allowed and a penalty of $6 million imposed on Reckitt Benckiser for the contraventions follow.
The packaging and website representations
10 In the statement of agreed facts and the reasons for judgment of the primary judge (Australian Competition and Consumer Commission v Reckitt Benckiser (Australia) Pty Ltd (No 4) [2015] FCA 1408 in respect of liability and Australian Competition and Consumer Commission v Reckitt Benckiser (Australia) Pty Ltd (No 7) [2016] FCA 424 in respect of penalty), the four products are referred to as the Nurofen “specific pain relief range” or the Nurofen “specific pain relief products”. While so identified for convenience, the references disclose the misleading character of the packaging and the webpages. The products masquerade as pain relief for specific pain (“migraine pain”, “tension headache”, “period pain” or “back pain”), but they are not specific in any way. They are all the same product delivering the same dose of ibuprofen in the same way.
11 It was an agreed fact that the active ingredient of all of the products, ibuprofen, acts on pain in the body by inhibiting the production of pain causing chemicals known as prostaglandins which occur in the central nervous system and at peripheral sites around the body. When there are locally elevated levels of prostaglandins, the nerve endings in that area trigger pain signals to the central nervous system. Ibuprofen acts generally to inhibit prostaglandin production irrespective of the location of the pain (for example, back pain) or the type of the pain (for example, migraine pain, tension headache, or period pain).
12 The packaging of each of the products is shown below.


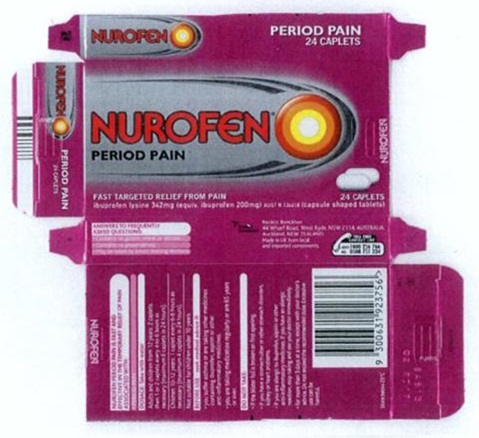

13 It will be seen that each package identifies itself as “Nurofen”, underneath which is the identification of the purported specific pain type (“migraine pain”, “tension headache”, “period pain” or “back pain”). Underneath this again is the statement “fast targeted relief from pain”. Each package also contains a statement that the product “is fast and effective in the temporary relief of pain [“and/or inflammation” in the back pain product] associated with”, followed by the purportedly relevant specific pain type. The Nurofen migraine pain product also contains an instruction “Dosage: Take with water at first onset of migraine”. The Nurofen tension headache product contains an instruction “Dosage: Take with water at first sign of tension headache”.
14 The webpages are annexed to these reasons as Annexure A. They included statements “Relieve pain with the right types of pain medication”, “Specific pain relief”, and “targeted relief”. As one example, the Nurofen migraine product is shown as part of a table compared to Nurofen caplets (referred to as standard Nurofen, a product the active ingredient of which is 200 mg of ibuprofen) and Nurofen Zavance, a product with a different chemical, sodium ibuprofen dehydrate, about which there is no evidence except it is said on the packaging of that product to be equivalent to 200mg ibuprofen and to be “Absorbed up to twice as fast as standard Nurofen”. The standard Nurofen product is shown with ticks against various pain “types” whereas the Nurofen migraine product is shown with a single tick against “Migraine”. In another comparison table, the Nurofen Tension Headache product is shown with a tick against only “Tension headaches”, the Nurofen Back pain product is shown with ticks against only “”Mild to moderate Back pain” and “Lower Back pain”, and the Nurofen Period Pain product is shown with a tick against only “Period pain”. No ticks appear against any other “pain type” in the table, such as “body aches and pain”, an omission that enhanced the misleading impression by suggesting a material difference when there was none.
15 In his liability judgment, the primary judge found that by its packaging of the products Reckitt Benckiser from 1 January 2011 had represented that:
…each product in the Nurofen Specific Pain Range:
• was specifically formulated to treat the particular type of pain specified on the packaging relevant to that product; and
• solely or specifically treated the particular type of pain specified on the packaging relevant to that product,
when in fact:
• each product in the Nurofen Specific Pain Range contains the same active ingredient, namely ibuprofen lysine 342mg;
• the Australian Register of Therapeutic Goods (ARTG) approved indications for each product in the Nurofen Specific Pain Range are the same;
• each product in the Nurofen Specific Pain Range is of the same formulation; and
• no product in the Nurofen Specific Pain Range is any more or less effective than the others in treating any of the symptoms shown on the packaging of the products in the Nurofen Specific Pain Range.
16 The primary judge also found in the liability judgment that, by its webpages, Reckitt Benckiser between at least December 2012 and around May 2014 had represented that:
each product in the Nurofen Specific Pain Range:
• was specifically formulated to treat the particular type of pain specified on the packaging relevant to that product; and
• solely or specifically treated the particular type of pain specified on the packaging relevant to that product,
when in fact:
• each product in the Nurofen Specific Pain Range contains the same active ingredient, namely ibuprofen lysine 342mg;
• the ARTG approved indications for each product in the Nurofen Specific Pain Range are the same;
• each product in the Nurofen Specific Pain Range is of the same formulation; and
• no product in the Nurofen Specific Pain Range is any more or less effective than the others in treating any of the symptoms shown on the packaging of the products in the Nurofen Specific Pain Range.
Key events
17 The products were registered on the Australian Register of Therapeutic Goods (ARTG) between 2003 and 2007. From the last registration of the Nurofen Back Pain product in 2007 Reckitt Benckiser marketed and sold the purported “Nurofen specific pain range”.
18 In 2010, consumer advocacy group Choice “awarded” Reckitt Benckiser a “Shonky Award” for “pain in the hip pocket” for the purported range. Details of the Shonky Award included the following information disclosed at [74]-[75] of the penalty judgment:
Nurofen
Shonky for pain in the hip pocket? Nurofen
Got a headache?
Backache? Neck ache?
A trip to your pharmacy or supermarket reveals there are specific painkillers for all sorts of pains: back pain, tension headache pain, migraine pain, period pain, osteoarthritis pain, neck pain, little toe pain… Panadol has a few pain-specific products, but Nurofen has more, with a range of caplets for migraine, back, tension headache and period pain. Yet a closer look at the ingredients shows they’re identical from product to product.
So does the back pain version somehow magically go straight to your back – and only your back – as soon as you’ve swallowed it? Could you, say, choose to treat only your back pain while keeping your headache? If you want to treat both, do you need to take a dose of each? The answers are no, no and definitely no. When you take these pain killers, the active ingredient spreads through your whole body, attacking whatever pain it comes across, wherever it is. Filling up your medicine cabinet with different painkillers for every type of pain is unnecessary, not to mention wasteful, should they expire before you’ve used them all. But the shonkiest aspect of this type of marketing is that the fast-acting painkillers labelled for specific pain types are more expensive – costing almost twice as much in some stores we surveyed – than their “all-pain” fast-acting equivalent, Zavance caplets, which contain a comparable fast-acting form of ibuprofen. Our advice? Stick with Zavance – and see your doctor if pain persists. See the video.
19 In his penalty judgment at [76], the primary judge rejected Reckitt Benckiser’s submission that it was not aware of the Shonky Award it received for the purported Nurofen specific pain range. That conclusion was not challenged on appeal. Nor were the further adverse findings referred to below.
20 Reckitt Benckiser continued to market and sell the products without any material change after the Shonky Award.
21 In 2012, two complaints about the purported Nurofen specific pain range were made to the Therapeutic Goods Administration (TGA). These complaints were referred to the TGA’s Complaints Resolution Panel.
22 Reckitt Benckiser published the webpages from at least December 2012 to May 2014, and continued to market and sell the products without any material change.
23 On 18 April 2013, the purported Nurofen specific pain range featured on the television show “The Checkout”. The show was critical of Reckitt Benckiser for selling products that claim to work on specific pain at a higher price than other products. At [83] of the penalty judgment, the primary judge rejected Reckitt Benckiser’s submission that it was not aware of the show, saying that:
It is a very simple inference to conclude, as I do, that Reckitt Benckiser was aware of the program about which it had been contacted and to which it responded through a public relations company.
24 Reckitt Benckiser continued to market and sell the products without any material change thereafter.
25 On 12 June 2013, the TGA’s Complaints Resolution Panel made findings that:
in the absence of clear and prominent statements to the contrary, the use of the descriptive names Nurofen Back Pain, Nurofen Migraine Pain, Nurofen Period Pain, and Nurofen Tension Headache Pain in the advertisement would convey to an ordinary and reasonable consumer that:
a. The products so named were different in their ingredients or effects, and did not differ solely because of the consumer to which advertisements about them were directed;
b. The advertised products would have an effect in the named area or site of pain, and would not have an effect on other pain or act elsewhere in the body other than in the named area.
26 Shortly after this time, one of Reckitt Benckiser’s senior management, the Australian Regulatory and Medical Affairs Director, consciously decided to continue to sell the products under the same packaging and website representations pending a review of this decision by a delegate of the Secretary of the Department of Health.
27 Between July 2013 and October 2014, the ACCC requested documents and information from Reckitt Benckiser in respect of the ACCC’s investigation of the products. Reckitt Benckiser complied with these requests.
28 On 11 April 2014, the Secretary of the Department of Health ordered Reckitt Benckiser to withdraw any representations that implied that any two or more Nurofen products that contain equivalent ibuprofen quantities and include the same product specific indications on the Australian Register of Therapeutic Goods; (i) are effective only in treating a particular condition or conditions or pain in a particular part or parts of the body; or (ii) are not effective in treating other conditions or pain in other parts of the body. As the primary judge noted at [79] of the penalty judgment the delegate explained her reasoning in part as follows:
In order to ensure that consumers are not misled, I consider that it is necessary to order [Reckitt Benckiser] to withdraw any representations, including implied representations, that imply that any two or more Nurofen products that contain equivalent quantities of ibuprofen and include the same product indications, are effective only in treating particular conditions or pain in particular parts of the body and/or are not effective in treating other conditions or pain in other parts of the body.
29 Reckitt Benckiser removed the webpages within a month of these orders but continued to market and sell the products without any material change.
30 On 17 December 2014, in the context of the ACCC’s investigation, Reckitt Benckiser wrote to the ACCC. This letter records that:
Reckitt Benckiser’s point of view is that the labelling of each product, considered alone, is accurate.
31 On 4 March 2015, the ACCC filed its fast track application commencing this proceeding.
32 The hearing on liability was scheduled for 9 and 10 December 2015. Reckitt Benckiser, by its response filed on 22 April 2015, denied the substantive allegations against it and that the ACCC was entitled to any relief.
33 Throughout the proceedings and until 10 December 2015, Reckitt Benckiser continued to market and sell the products without any material change.
34 On the first day of the liability hearing, 9 December 2015, Reckitt Benckiser indicated for the first time that it would make admissions and consent to the making of orders against it to the effect it had contravened the ACL (ss 18 and 33) and was liable to a civil penalty under s 33 for conduct liable to mislead the public as to the nature, character or suitability for purpose of the four identical products. The admissions were set out in an amended response to the fast track application dated 10 December 2016, which was filed on the second day of the hearing on liability. At the same time, to enable current stock of the products to continue to be sold, Reckitt Benckiser and the ACCC agreed on an interim amendment to the packaging of the products (affixed by means of a sticker).
In the court below
35 As noted, on 10 December 2015 Reckitt Benckiser admitted that both the packaging and website representations constituted:
(1) misleading or deceptive conduct contrary to s 18 of the ACL; and
(2) conduct that was liable to mislead the public as to nature, characteristics and/or suitability for purpose contrary to s 33 of the ACL.
36 Reckitt Benckiser did not admit that the packaging and webpage representations were in fact false or misleading with respect to the performance characteristics, uses and/or benefits of the four identical products contrary to s 29(1)(g) of the ACL, another civil penalty provision. Upon the ss 18 and 33 contraventions being admitted, the ACCC did not press the s 29(1)(g) allegations. Accordingly, the civil penalty case was brought upon the basis that the representations were liable to mislead in contravention of s 33 of the ACL, rather than that they were in fact false or misleading representations in contravention of s 29(1)(g) of the ACL.
37 Acceptance of a guilty plea to a lesser offence is well known to the criminal law, as are the consequences for sentencing in any such case. A sentencing court cannot impose a sentence for a greater offence than that charged and either admitted or proven: R v De Simoni (1981) 147 CLR 383 at 389. Whether or not the principle in De Simoni applies to civil penalty proceedings (an issue not addressed by the parties and which we do not decide), we proceed on the basis that the primary judge’s task was to decide the appropriate penalty in respect of the contravening conduct – being the conduct liable to mislead the public as to nature, characteristics and/or suitability for purpose contrary to s 33 of the ACL.
38 On 11 December 2015, the primary judge made orders including declarations of contravention, injunctions restraining like conduct for a period of three years, orders requiring corrective adverting and website notices, and in respect of Reckitt Benckiser’s compliance programs.
39 On 12 April 2016, a contested penalty hearing took place, with further submissions provided after the hearing.
40 It is common ground that the primary judge correctly identified the requirements of s 224 of the ACL (penalty judgment at [21]) by which the court may order payment of a pecuniary penalty for contravention of, relevantly, s 33 of the ACL. Section 224(2) of the ACL provides that:
In determining the appropriate pecuniary penalty, the court must have regard to all relevant matters including:
(a) the nature and extent of the act or omission and of any loss or damage suffered as a result of the act or omission; and
(b) the circumstances in which the act or omission took place; and
(c) whether the person has previously been found by a court in proceedings under Chapter 4 or this Part to have engaged in any similar conduct.
41 On 29 April 2016, the primary judge ordered Reckitt Benckiser to pay the Commonwealth within 30 days a pecuniary penalty of $1.7 million for the contraventions of s 33 of the ACL, and provided reasons.
42 For the purposes of the penalty hearing, the parties signed a statement of agreed facts and admissions pursuant to s 191 of the Evidence Act 1995 (Cth), with numerous attachments. A number of other documents were also tendered.
43 The ACCC submitted, and it is the fact, that the unchallenged findings of the primary judge included the following matters (the references to PJ being to the penalty judgment):
(a) the contraventions continued for a period of almost 5 years (PJ [47]);
(b) the contraventions were made as part of a general marketing approach to profit (PJ [47]);
(c) the contravening conduct was widespread (the Nurofen Specific Pain Relief products were available at 5,500 pharmacies and 3,000 other retail outlets) (PJ [48]);
(d) in the period of contravention, the respondent sold approximately 5.9 million units of Nurofen Specific Pain Relief products (PJ [66]);
(e) the respondent was a large corporation with a large share of the market for oral analgesics in Australia (PJ [50]);
(f) the respondent earned revenues of $45 million … from the sale of the Nurofen Specific Pain Relief products in the contravening period (PJ [66]);
(g) the marketing strategy was developed and implemented by senior management including personnel in regulation, sales and marketing and legal (PJ [71]);
(h) the respondent’s compliance programs failed at basic levels (PJ [70]) and its compliance program made no reference to the ACL (PJ [73]);
(i) there were obvious warnings of the likelihood of the products misleading consumers arising from criticisms by consumer groups, TGA complainants and the media, and from decisions of the TGA itself, and the respondent was aware of those warnings (PJ [74] – [83]);
(j) the respondent continued to sell the Nurofen Specific Pain Relief products in their contravening packaging despite those obvious warnings and despite the ACCC commencing these proceedings; and
(k) the respondent had previously contravened s 18 of the ACL in respect of a false representation on the packaging of a different product (PJ [89] – [90]).
Nature and scope of appeals to a Full Court and the need for error to be demonstrated
44 Given the range of issues raised in this appeal, it is convenient to restate the governing principles. This is especially so because this is an appeal concerning the imposition of a civil penalty, which has certain features in common with criminal sentencing, but as noted in Commonwealth v Director, Fair Work Building Industry Inspectorate [2015] HCA 46; (2015) 326 ALR 476 (the CFMEU civil penalty case) at [51]-[61], there are some important and fundamental differences, especially in relation to the purpose behind imposing a civil penalty. The dominant common feature is that determining both a sentence and a civil penalty usually involves, and in this case did involve, a difficult and complex process of multi-factorial decision-making, where the result is arrived at by a process of “instinctive synthesis”, addressing many conflicting and contradictory considerations (Wong v The Queen [2001] HCA 64; (2001) 207 CLR 584 at [74]-[76]). Those paragraphs were quoted with approval in Markarian v The Queen [2005] HCA 25; (2005) 228 CLR 357 at [37]. This integrated and holistic approach, requiring the weighing up of all relevant factors into a final result, often makes the process of appellate review difficult, particularly for an appellant seeking to identify and establish error in the reasoning process and outcome, or sometimes the outcome alone.
45 Appeals to this Court are by way of a rehearing (Branir Pty Ltd v Owston Nominees (No 2) Pty Ltd [2001] FCA 1833; (2001) 117 FCR 424 at [20]). An appeal by way of rehearing requires this Court to decide the case for itself as to both facts and law and give effect to its own judgment (Warren v Coombes (1979) 142 CLR 531 at 552). However, this does not remove the need to find error on appeal before intervening (Branir at 435 [21]). In practice, the application of these principles may involve accepting the findings of the trial judge, especially factual findings, including as to the reliability and credit of witnesses and the weight that should be given to competing evidence, unless shown to be wrong (Cabal v United Mexican States [2001] FCA 427; (2001) 108 FCR 311 at [223]-[224], quoted with approval in Branir at [23]). However it should be observed that as there was no oral evidence in this case and all the representations were before this Court in documentary form, this Court is under no disadvantage compared to the primary judge in relation to consideration of the facts and evidence. The requirement for error to be established nevertheless remains.
46 In Costa v Public Trustee of New South Wales [2008] NSWCA 223; (2008) 1 ASTLR 56 at [101], Basten JA said:
Decisions with respect to discretionary powers may fall into various categories. One category involves a determination of where, within a range, the result properly lies. In such a case, as with the exercise of the sentencing discretion, the principles in House v R may properly be applied. Examples in the civil jurisdiction include the assessment of damages in personal injury cases and the valuation of property.
47 The High Court’s statement in House v The King (1936) 55 CLR 499 at 504-505 is that:
The manner in which an appeal against an exercise of discretion should be determined is governed by established principles. It is not enough that the appellate court consider that, if they had been in the position of the primary judge, they would have taken a different course. It must appear that some error has been made in exercising the discretion. If the judge acts upon a wrong principle, if he allows extraneous or irrelevant matters to guide or affect him, if he mistakes the facts, if he does not take into account some material consideration, then his determination should be reviewed and the appellate court may exercise its own discretion in substitution for his if it has the materials for doing so. It may not appear how the primary judge has reached the result embodied in his order, but, if upon the facts it is unreasonable or plainly unjust, the appellate court may infer that in some way there has been a failure properly to exercise the discretion which the law reposes in the court of first instance. In such a case although the nature of the error may not be discoverable, the exercise of the discretion is reviewed on the ground that a substantial wrong has in fact occurred.
48 In Park Trent Properties Group Pty Ltd v Australian Securities and Investments Commission [2016] NSWCA 298, Leeming JA, with whom McColl and Gleeson JJA agreed, said:
[51] Two things of present importance emerge from the reasons of Gummow ACJ, Kirby, Hayne and Heydon JJ in Macedonian Orthodox Community Church St Petka Inc v His Eminence Petar (2008) 237 CLR 66; [2008] HCA 42. The first is the proposition accepted at [120] that:
when a court is invited to make a discretionary decision, to which many factors may be relevant, it is incumbent on parties who contend on appeal that attention was not given to particular matters to demonstrate that the primary judge’s attention was drawn to those matters, at least unless they are fundamental and obvious.
[52] The second is the explanation of the nature of the “orthodox approach to appellate intervention in relation to discretionary decisions” described at [137]–[138]. There it was pointed out that the expression “balancing exercise” is one to be employed with care, and that where (as in the present case) no statute mandates that particular weight be given to any one factor:
[T]he question of what weight the relevant factors should be given or what balance should be struck among them is for the person on whom the discretion is conferred, provided no error of law is made, no error of fact is made, all material considerations are taken into account and no irrelevant considerations are taken into account, subject to the possibility of appellate intervention if there is a plain injustice suggesting the existence of one of the four errors just described even though its nature may not be discoverable, or if there is present what has come to be known as ‘Wednesbury unreasonableness’.
[53] The same passage confirms that it is wrong to apply the words from House v R in isolation, as if they were not qualified by an absence of reasons explaining how the decision was reached. Park Trent’s selective statement of the principle upon which it relied has a tendency to dilute the test. The entire relevant passage from House v King, which was restated in the passage from Macedonian Orthodox Community Church St [P]etka Inc v His Eminence Petar, was as follows:
It may not appear how the primary judge has reached the result embodied in his order, but, if upon the facts it is unreasonable or plainly unjust, the appellate court may infer that in some way there has been a failure properly to exercise the discretion which the law reposes in the court of first instance. In such a case, although the nature of the error may not be discoverable, the exercise of the discretion is reviewed on the ground that a substantial wrong has in fact occurred.
Of course, that is not the present case, where the reasons of the primary judge are elaborate.
49 This is not to say that elaborate reasons are immune from appellate review. In the absence of specific error, the outcome reached either will or will not be one which was reasonably open. If not reasonably open, elaborate reasons will not protect the result from appellate intervention.
50 Accordingly, error is not involved merely because an appeal court would have reached a different conclusion.
51 Error may be specific, in the sense of apparent on the face of the reasons given, such as by application of a wrong principle in reaching the result (which may be evident by the primary judge addressing the wrong question), reaching the result by taking into account something that should not have been considered or by failing to take into account something that should have been considered, or by making a determinative error on the facts in the sense that the factual finding was not properly available to be taken into account in a way that affected the outcome.
52 Alternatively, error may be inferred from a result that cannot have been arrived at without some kind of operative error. The influence of the reasons given for the result arrived at on this process will vary. Reasons are not to be ignored, but nor do they necessarily confine in a rigid or inflexible way the scope of the appellate inquiry. It may be legitimate to have regard to what was said and not said in order to identify how the asserted erroneous result was reached. But for error to be inferred from the result, the result must be one which was not open on the evidence or facts found or agreed.
53 In all cases of specific error, the error must have either caused or materially contributed to the result. An error which has not in some material way affected the outcome will ordinarily result in the appeal court declining to intervene, at least as to the result.
54 In this case, the ACCC advances eight grounds of appeal. The first seven grounds may be seen as within the first category of asserted specific error. Thus for each or any of those grounds to succeed in relation to the appeal, the ACCC must not only establish the asserted error, but also that the error was material to the penalty imposed. Any errors in reasoning or approach falling short of this are insufficient for such grounds to succeed.
55 The ACCC’s eighth ground, asserted manifest inadequacy, is ordinarily asserted as an error of the second kind described in House v The King: inferred error. As Gleeson CJ and Hayne J observed in Dinsdale v The Queen [2000] HCA 54; (2000) 202 CLR 321 at [6]:
Manifest inadequacy of sentence, like manifest excess, is a conclusion. A sentence is, or is not, unreasonable or plainly unjust; inadequacy or excess is, or is not, plainly apparent. It is a conclusion which does not depend upon attribution of identified specific error in the reasoning of the sentencing judge and which frequently does not admit of amplification except by stating the respect in which the sentence is inadequate or excessive. It may be inadequate or excessive because the wrong type of sentence has been imposed (for example, custodial rather than non-custodial) or because the sentence imposed is manifestly too long or too short. But to identify the type of error amounts to no more than a statement of the conclusion that has been reached. It is not a statement of reasons for arriving at the conclusion. A court of criminal appeal is not obliged to employ any particular verbal formula so long as the substance of its conclusions and its reasons is made plain. The degree of elaboration that is appropriate or possible will vary from case to case.
56 A finding of manifest inadequacy (or excess) can be supported by reference to specific errors. In that event, even if the asserted specific error is not established as a separate basis upon which the appeal must be allowed, it may nonetheless help to explain the overall result said to be erroneous. While Dinsdale makes it clear that the appeal court does not have to attribute identified specific error in the reasoning of the sentencing judge, it is not precluded from doing so. This is especially so if a combination of such reasoning is asserted to produce or contribute to a manifestly inadequate (or excessive) result. It follows that the conclusion reached by this Court about grounds 1 to 7 may properly inform and support the conclusion properly to be reached about ground 8, whether or not our conclusions about any one of grounds 1 to 7 are themselves sufficient to require the primary judge’s order as to penalty to be set aside.
The purpose of a civil penalty
57 The appeal grounds, in particular ground 8 which asserts manifest inadequacy of the penalty, are informed by the purpose of civil penalty provisions. It is sufficient to refer to the CFMEU civil penalty case. French CJ, Kiefel, Bell, Nettle and Gordon JJ explained that:
[55] …whereas criminal penalties import notions of retribution and rehabilitation, the purpose of a civil penalty, as French J explained in Trade Practices Commission v CSR Ltd [(1991) ATPR 41–076 at 52,152], is primarily if not wholly protective in promoting the public interest in compliance:
Punishment for breaches of the criminal law traditionally involves three elements: deterrence, both general and individual, retribution and rehabilitation. Neither retribution nor rehabilitation, within the sense of the Old and New Testament moralities that imbue much of our criminal law, have any part to play in economic regulation of the kind contemplated by Pt IV [of the Trade Practices Act]. … The principal, and I think probably the only, object of the penalties imposed by s 76 is to attempt to put a price on contravention that is sufficiently high to deter repetition by the contravenor and by others who might be tempted to contravene the Act.
58 The primary judge recognised this purpose in the penalty judgment as follows:
39 It is well established that considerations of specific and general deterrence are vital in the assessment of pecuniary penalties. In Singtel Optus Pty Ltd v Australian Competition and Consumer Commission [2012] FCAFC 20; (2012) 287 ALR 249, 265 [62]-[63], the Full Court of the Federal Court said that:
There may be room for debate as to the proper place of deterrence in the punishment of some kinds of offences, such as crimes of passion; but in relation to offences of calculation by a corporation where the only punishment is a fine, the punishment must be fixed with a view to ensuring that the penalty is not such as to be regarded by that offender or others as an acceptable cost of doing business… those engaged in trade and commerce must be deterred from the cynical calculation involved in weighing up the risk of penalty against the profits to be made from contravention.
40 These comments were reiterated with approval in Australian Competition and Consumer Commission v TPG Internet Pty Ltd [2013] HCA 54; (2013) 250 CLR 640, 659 [66] (French CJ, Crennan, Bell and Keane JJ).
Appeal grounds 1 and 2: loss to consumers and causation
59 Grounds 1 and 2 challenge the approach of the primary judge on the issue of loss to consumers in different ways. They may be summarised as follows.
60 Ground 1 asserts that his Honour erred in concluding that any attempt to quantify profits caused by Reckitt Benckiser’s contravening conduct and losses suffered by consumers as a result of that conduct would be impossible, so speculative as to be useless, of no assistance and neither necessary nor appropriate (penalty judgment [5], [53] and [66]), and thereby failed to take into account or give adequate weight to the statutory mandatory consideration of losses suffered by consumers in s 224(2)(a) of the ACL.
61 Ground 2 asserts that in considering the losses suffered by consumers, the primary judge should have found not only that that the products were the same, but also that the products were relevantly the same as standard Nurofen, the only difference between them (apart from the specific pain range costing about twice as much as standard Nurofen) being the conduct liable to mislead the public; that is, that the products were specifically formulated to treat, and in fact solely or specifically treated, the particular type of pain specified on the packaging relevant to each product when they were not and did not. Accordingly, this ground asserts that his Honour should have concluded that the contravening conduct was a material (and the primary) contributing cause of consumers choosing to purchase the more expensive products instead of the standard product, with the consequence that a reasonable estimate of the loss suffered was an amount of about half of the retail sales revenue during the contravening period (the sum calculated by the ACCC being some $26.25 million).
62 In the penalty judgment at [51]-[66], the primary judge was critical of the approach taken by Reckitt Benckiser (that it had not profited at all from the contravening conduct) and, to a lesser extent, the ACCC’s response of attempting to demonstrate the existence, and calculate the extent, of profit. In relation to the ACCC, his Honour noted that a substantial part of the evidence and submissions on sales, profit and potential losses to consumers and competitors focused upon an “elaborate exercise, based on volumes of evidence” directed to estimating the profit made as a result of the contravening conduct. This produced a result of some millions of dollars, but less than $10 million (penalty judgment at [51]). His Honour described the ACCC as carrying out “a labour of Hercules”, impossible to carry out in the absence of expert evidence and even then at best an “extremely rough approximation” and at worst an “informed guess”. His Honour accepted a submission by Reckitt Benckiser that any attempt to quantify profits caused by its contravening conduct would be either an impossible task or so speculative as to be useless, one reason being that it involved proof of a counterfactual, it being insufficient merely to show that the contraventions contributed to the profit made. His Honour rejected Reckitt Benckiser’s submission that it should be found it had not made any profit from the contravening conduct.
63 We agree with the primary judge that the ACCC’s approach, over-complicated though it might have been, rebutted Reckitt Benckiser’s proposition that it had derived no financial benefit from the contravening conduct (penalty judgment at [53]). Notably, Reckitt Benckiser continued to propound this proposition in this appeal on the basis that if by reason of error by the primary judge this Court had to determine penalty, it should do so on the basis that Reckitt Benckiser had derived no financial benefit from the contravening conduct. As discussed below, we reject this proposition.
64 An important part of the ACCC’s challenge to the reasoning of the primary judge concerned his Honour’s finding (at [60] of the penalty judgment), built on the counterfactual approach which his Honour considered was required, that “there may be consumers who were prepared to pay a substantial price premium for the products compared, for example, to standard Nurofen, for reasons such as ease of selection for the relevant condition, product placement, advertising and the like”. In this regard, and by way of example, the packaging of the products was brightly coloured and Reckitt Benckiser’s marketing provided for the products to be grouped together in prominent and easily accessible locations. We infer that these are the kinds of considerations the primary judge had in mind at [60] of the penalty judgment.
65 The primary judge, at [66] of the penalty judgment, considered it was sufficient to refer to the recommended retail prices in evidence before his Honour and the volume of sales over the five year period. This enabled total revenue from the sale of the products for the period 2011 to 2015 (the relevant contravening period, although the products were all sold from 2007 onwards) to be determined as $45 million. His Honour did not take the additional step of subtracting from that figure what the revenue would have been if the same sales had taken place of standard Nurofen. Nor was his Honour invited to do so by the ACCC. That additional step was only taken by the ACCC in further developing ground 2 of this appeal in its reply submissions. Calculations based on this approach produced a figure of aggregate additional sums paid by consumers over the five years of $26.25 million. Reckitt Benckiser did not dispute the accuracy of the calculations. This calculation also accords with the undisputed fact of the impugned products being sold for just over double the price of the standard product.
66 Reckitt Benckiser maintained that the approach advocated by the ACCC on appeal was not open, and there was no error by the primary judge. Reckitt Benckiser made the following three points in support of its position.
67 First, in respect of alleged error by the primary judge, his Honour did not fail to consider consumer loss. Rather, his Honour set out reasons, commencing at [51], why the evidence did not permit him to make any findings about the extent to which consumers had been induced by Reckitt Benckiser’s conduct to purchase any of the four products. So much is apparent, Reckitt Benckiser submitted, from the heading before [51] of the penalty judgment which is “Sales and profit from contravening conduct, and potential losses to consumers and competitors” (our emphasis), as well as the primary judge’s statement at [66] that:
For these reasons it is neither necessary, nor appropriate, in this case to attempt to engage in an exercise of attempting to quantify any amounts of (i) profit to Reckitt Benckiser from the contravening conduct, (ii) loss to consumers, or (iii) loss to competitors (our emphasis).
68 Second, the underlying premise of the calculation is incorrect. It cannot be assumed that the products are the same as standard Nurofen. This was not part of the contravening conduct found. The products contain 342mg of ibuprofen lysine which is equivalent to 200 mg of ibuprofen. Standard Nurofen contains 200mg of ibuprofen. Nurofen Zavance contains 256mg of sodium ibuprofen dehydrate which is equivalent to 200mg of ibuprofen but is absorbed twice as fast as standard Nurofen. Accordingly, it cannot be assumed that the products are the same as standard Nurofen or other products (for example, of competitors) containing 200mg of ibuprofen which cost less than the purported specific pain range products. This point was related to another submission by Reckitt Benckiser which the primary judge appears to have accepted at [61] of the penalty judgment that:
… it was not alleged and has not been found that any representation was made that, based on the packaging and higher pricing, the Nurofen Specific Pain Range products are more effective than other Nurofen pain relief products.
69 Third, the primary judge was correct at [60] that the counterfactual approach depended on excluding consumer choices based on factors other than the contravening conduct such as “ease of selection for the relevant condition, product placement, advertising, and so on”. His Honour was also correct at [60] and [61] to accept Reckitt Benckiser’s submission that minor changes to the packaging removed the misleading representations with the consequent conclusion that the contraventions may well have yielded little profit to Reckitt Benckiser.
70 We should reiterate that the ACCC’s case before the primary judge focused on Reckitt Benckiser’s alleged profit as a result of the contravening conduct and his Honour was not invited to quantify consumer loss in the manner which the ACCC has now identified. The ACCC nevertheless maintained before his Honour that consumers had suffered loss as a result of the contravening conduct and his Honour concluded that the extent of such loss could and should not be assessed (at [66]). For the reasons given below, we consider that the primary judge’s approach to consumer loss was in error and, despite the ACCC’s focus on profits before his Honour, the error was material to the outcome.
71 It may be accepted that the contravening conduct involved the purported specific pain range, not standard Nurofen or any competitor product containing the equivalent active ingredient (ibuprofen) and dose (200mg). It may also be accepted that the contravening conduct did not involve a misrepresentation that any of the purported specific pain range products was more effective in treating pain than another product. However, these matters do not lead to the outcomes for which Reckitt Benckiser contended.
72 The task under s 224(2)(a) is to have regard to the nature and extent of the contravening conduct and the loss or damage suffered as a result. If the assessment of the loss involves facts other than those comprising the contravening conduct, those facts must be considered. In the present case, the agreed facts and other evidence disclosed that ibuprofen lysine is the chemical equivalent of ibuprofen, the purported specific pain range products contained 342mg of ibuprofen lysine which is the equivalent of 200mg ibuprofen, the active ingredient of standard Nurofen is 200mg ibuprofen, and the purported specific pain range products cost about twice as much as standard Nurofen.
73 In particular, the agreed facts included these paragraphs:
7. The active ingredient of all Nurofen products is ibuprofen or chemical equivalents of ibuprofen (for example, ibuprofen lysine and sodium ibuprofen dehydrate).
8. Ibuprofen is a non-steroidal anti-inflammatory drug that acts to inhibit pain causing chemicals (prostaglandins) responsible for tissue pain and inflammation. Inhibition of prostaglandin occurs at both peripheral sites in the body and in the central nervous system. Pain originates when locally-elevated concentrations of these prostaglandins sensitise the nerve endings found in tissue and trigger pain signals to the central nervous system. By blocking the production of prostaglandins, ibuprofen inhibits the sensitisation of nerve endings and prevents the transmission of signals.
74 As noted, the evidence included the packaging of the products in the purported specific pain range which contains the statement “equiv. ibuprofen 200mg”. The evidence also included the standard Nurofen packaging which identifies the only active ingredient of that product as ibuprofen 200mg. Reckitt Benckiser submitted that chemical equivalence did not mean that the active ingredients were the same. Literally, this must be true. But what is relevant for present purposes is that, at least insofar as the purported specific pain range products and standard Nurofen are concerned, the agreed facts and packaging demand the inference that they are relevantly the same, in that all of the products provide in the body a dose equivalent to 200mg of ibuprofen .
75 Contrary to Reckitt Benckiser’s submissions, the different chemical formulation of Zavance does not suggest any relevant difference between the purported specific pain range products and standard Nurofen. There is no evidence about how Zavance is absorbed more quickly than standard Nurofen but it is apparent the chemical formulation of the equivalent active ingredient is different from both standard Nurofen and the purported specific pain range products. Moreover, unlike Zavance, there is no suggestion on the packaging of the purported specific pain range products that they are anything other than equivalent to 200mg of ibuprofen or have any qualities (such as a faster rate of absorption) different from standard Nurofen. In any event, in the face of the agreed fact of chemical equivalence between the purported specific pain range and 200mg of ibuprofen, it was a matter for Reckitt Benckiser to call evidence to establish any proposition to the contrary. It did not.
76 The fundamental difficulty we have with the primary judge’s conclusion about the impossibility of assessing the extent of consumer loss is that it involved an implicit acceptance of the conceptual framework established by Reckitt Benckiser’s contravening conduct. In short, how can it be concluded that consumers might have been willing to pay a price premium for the purported specific pain range products “for reasons such as ease of selection for the relevant condition, product placement, advertising, and so on” without implicitly accepting that the products were different from each other (when they were not, the foundation of the contravening conduct) and relevantly different from standard Nurofen (when they were not, the foundation of any assessment of consumer loss)? There was, in truth, no “selection” involved. There was no “relevant condition” involved. The concepts of “selection” and “relevant condition” are constructs created by the contravening conduct. There was no difference between the products and thus, absent the contravening conduct, no rational reason for the different marketing to which his Honour referred. The marketing differences in the present case were part and parcel of the misleading character of the conduct.
77 Reckitt Benckiser submitted that the absence of any representation that the purported specific pain range products were more effective than any other ibuprofen product meant that there was no rational reason for a consumer to purchase the purported specific pain range products rather than any other product. We disagree. In Australian Competition and Consumer Commission v TPG Internet Pty Ltd [2013] HCA 54; (2013) 250 CLR 640 at [55] French CJ, Crennan, Bell and Keane JJ said:
It has long been recognised that, where a representation is made in terms apt to create a particular mental impression in the representee, and is intended to do so, it may properly be inferred that it has had that effect. Such an inference may be drawn more readily where the business of the representor is to make such representations and where the representor’s business benefits from creating such an impression.
78 The contravening conduct was apt to create in a consumer the impression that the purported specific pain range product had been formulated to and did in fact treat the specific pain type nominated. It must be inferred that Reckitt Benckiser engaged in the contravening conduct for its commercial benefit, encouraging consumers suffering from the nominated pain types to purchase one or more of the purported specific pain range products rather than a cheaper equivalent product. As such, why should it not be inferred that a consumer acted in accordance with the impression the contravening conduct was apt to create in a consumer’s mind? Contrary to Reckitt Benckiser’s submission, if suffering from back pain, it is rational for consumers to purchase a product that purports to treat back pain, without even turning their mind to concepts such as equivalent effectiveness.
79 For these reasons we do not accept another submission of Reckitt Benckiser that the primary judge’s orders with respect to liability mean that the products are liable to mislead the public only if considered as part of the purported specific pain range, and not if considered in isolation from one another. We do not consider that the ACCC’s case or his Honour’s findings and orders were confined in this way. It is apparent from the ACCC’s fast track statement that the contentions relate to each product within the so-called Nurofen Specific Pain Range. It was alleged that each product in that range contravened, relevantly, s 33 because of the “Packaging Statements” which were defined as statements made on each product. The primary judge’s orders also referred to each product in the so-called “Nurofen Specific Pain Range”. The words “in combination” or any like formulation do not appear. This said, it is true and apparently not disputed that the combined effect of the four types of packaging enhanced, and to that extent aggravated, their liability to mislead.
80 Test Reckitt Benckiser’s submission this way. Assume the only product on the market was the Nurofen migraine pain product. The packaging of that product included the following:
MIGRAINE PAIN
NUROFEN
MIGRAINE PAIN
FAST TARGETED RELIEF FROM PAIN
81 The primary judge’s orders included that Reckitt Benckiser, in carrying out the conduct of marketing and selling the so-called Nurofen Specific Pain Range, had represented that each product in that range was specifically formulated and solely or specifically treated the particular type of pain relevant to that product when it was not. Can it be maintained, as Reckitt Benckiser would have it, that if (contrary to the fact) only one product had been sold, the packaging of that product would not have represented that it was specifically formulated and solely or specifically treated the particular type of pain relevant to that product when it was not? The only possible answer to this question is “no”. Nor does anything in the liability judgment suggest this to be so. To the contrary, at [36] his Honour said this, which confirms the conclusion we have reached:
…the Packaging Representations which were admitted, and which I found had been made, concerned each product in the Nurofen Specific Pain Range. Nurofen’s representations were relied upon by the ACCC, and found by me to be contraventions, not merely for their character as representations on each of the four products but for their character as representations over the range of four products.
82 Accordingly, the fact that Reckitt Benckiser marketed four products which were all liable to mislead the public in the same way, and the ACCC’s fast track statement and primary judge’s orders reflect this fact, do not mean what Reckitt Benckiser apparently (and, it must be said, disturbingly) still maintains that there was nothing wrong with the packaging of each product considered in isolation.
83 Contrary to Reckitt Benckiser’s submission, these conclusions do not involve any expansion of the contravening conduct. The penalty is to be determined for the contravening conduct but the facts relevant to penalty (if agreed or proved) are not confined to the contravening conduct. Nor does this involve an assumption that, but for the contravening conduct, the products would not have existed at all. It does involve, however, acceptance of the proposition that one consequence of the contravening conduct was the illusion that the products involved a range specifically targeted to certain pain types when, in fact, they did not. This proposition, in our view, is a necessary consequence of the contravening conduct. And a necessary consequence of this proposition for the assessment of consumer loss is that consumers would have been induced to buy the products purporting to be specifically formulated to treat the type of pain from which they were suffering in preference to a product, such as standard Nurofen, which did not purport to be so specifically formulated.
84 For these reasons it is impossible to see how any features such as ease of product selection for the relevant condition, product placement, advertising and the like may be extracted for separate consideration from the misleading nature of the packaging representations, let alone be given any real weight. Each was part and parcel, or the result, of the contravening conduct. But for the contraventions, there was no range to inform the branding, advertising or placement of the products. The very notion of product selection is at the heart of the misleading character of the conduct and itself an illusion because all of the products, including standard Nurofen, were relevantly the same. The four products liable to mislead were identical.
85 Apart from the considerations which Reckitt Benckiser put forward and the primary judge accepted being tainted by the contravening conduct, any part they might have had to play in any purchase decision by a consumer, at best, was speculative. There was no material difference between the products and standard Nurofen. The obvious and expected consequence of the contravening conduct was to entice consumers to pay more for the products. Without compelling evidence to the contrary, there was no rational reason to speculate in favour of Reckitt Benckiser that consumers might have been willing to pay twice as much for the same product but for the contravening conduct. The same conclusion applies to the possibility that some consumers may not have cared about price. This is mere speculation by Reckitt Benckiser. The obvious, compelling inference, absent evidence to the contrary, was that the contravening conduct and its consequences for the branding and marketing of the products were the material reason that consumers – or at least the vast majority of consumers – purchased those products rather than standard Nurofen. Reckitt Benckiser’s contrary approach, largely accepted by the primary judge, assumes the existence of the range and of difference between the products, when this is the essence of the misleading character of Reckitt Benckiser’s contravening conduct.
86 We also do not consider that concepts such as elasticity of demand or cross-elasticity, which concerned the primary judge at [57]-[58] of the penalty judgment, were necessary components of the analysis in the circumstances of this case. The ACCC’s approach, as his Honour noted at [57], assumed in favour of Reckitt Benckiser that every person who purchased one of the purported specific pain range would otherwise have purchased another form of Nurofen. His Honour was concerned, however, that there were 50 different types of Nurofen so it was mere speculation to conclude that any person would have purchased any particular type. We disagree. The evidence was that these 50 types of Nurofen included different dosages, dosage forms, and different combinations of active ingredients. Within those types, it is the purported specific pain range and standard Nurofen (as well as Zavance, which is differentiated by apparent speed of absorption) which involved a dosage equivalent to 200mg of ibuprofen (for standard Nurofen whether it be in tablet, caplet or capsule form).
87 Again, absent compelling evidence to the contrary, the obvious and compelling inference, and also the inference most advantageous to Reckitt Benckiser, was that if not enticed to purchase the purported specific pain range product by reason of the contravening conduct, in the ordinary course a person would have purchased one of the standard Nurofen products. In the particular (and unusual) circumstances of this case, where the contravening conduct created the illusion of a range and thus a choice which did not exist, expert evidence about cross-elasticity in the market was unnecessary.
88 Nor do we accept that the lack of any representation that the products were more effective than any other Nurofen product was material to the assessment of consumer loss. The essence of Reckitt Benckiser’s contravening conduct as pleaded is not found in concepts of efficacy, but of different products for the purposes of different pain types when the products were the same and were, in terms of action within the body, indifferent to pain type or location. For these reasons Reckitt Benckiser’s submission, that no person could have purchased one of the specific pain range products believing the product to be more effective than standard Nurofen, is immaterial.
89 Insofar as Reckitt Benckiser relied upon the submission that minor changes to the packaging would mean that the packaging was no longer liable to mislead the public in contravention of s 33 of the ACL, which the primary judge accepted at [60]-[61], two additional problems arise.
90 First, at [60] his Honour referred to a “concrete example” which, he said, “illustrates the significance of this point to the impossibility of calculating profit from contraventions”. The example was Reckitt Benckiser’s new packaging (achieved by affixing a sticker on the products) which it had agreed as an interim arrangement with the ACCC after the liability judgment on 11 December 2015 and which enabled the existing stock to continue to be sold. However, the packaging which his Honour described was not the new packaging to which the ACCC agreed as an interim measure. It was the packaging which Reckitt Benckiser had put to the ACCC during the course of the investigation as a possible solution, but which the ACCC had rejected. It must be inferred that the ACCC rejected the proposal on the basis that it considered that the packaging continued to be misleading and deceptive or likely to mislead and deceive, or at least liable to mislead.
91 Contrary to Reckitt Benckiser’s submission, and for reasons set out more fully in respect of ground 3 below, this mistake was material. The terms of [60] and [61] of the penalty judgment confirm that the primary judge considered the new packaging to be important is plain. But there are material differences between the packaging proposal the ACCC rejected and the new packaging which it accepted as an interim measure. The latter did not bear a sticker at the top saying “suitable for general pain relief”. It bore a sticker beneath the nominated pain type, in equally prominent type, saying “equally effective for [all of the other nominated pain types] and general pain”. The differences between the agreed interim packaging and the contravening packaging are not minor.
92 Second, there was no evidence about the effect of the agreed interim packaging on consumer behaviour. It is not apparent why it would be inferred in favour of Reckitt Benckiser that the changed packaging had no effect on consumer behaviour when the change went to the heart of the branding of the purported specific pain range – that the products within the purported range were all the same and they were equally effective for all kinds of pain.
93 Accordingly, and contrary to the submissions made on behalf of Reckitt Benckiser, the mandatory consideration of consumer loss did not require precise causation or mathematical precision. It never required evidence from consumers on a “but for” basis or expert evidence. The primary judge was constrained by what was before him, but was required to do the best that he could with what was available, applying the orthodox “common sense” approach to causation of consumer loss which “requires no more than that the act or event in question should have materially contributed to the loss or injury suffered” (Henville v Walker [2001] HCA 52; (2001) 206 CLR 459 at [61] per Gaudron J citing Wardley Australia Ltd v Western Australia (1992) 175 CLR 514 at 525).
94 Considered other than through the distorting prism created by Reckitt Benckiser’s contravening conduct, the only reasonable inference available on the evidence was that a substantial number of the sales of the purported specific pain range products was caused by the contravening conduct but for which, at best for Reckitt Benckiser, consumers would have purchased a standard Nurofen product at half the price.
95 If not for the matters discussed above, in particular the implicit adoption of the concept of the products being formulated for and treating different pain types when they did not, it is unlikely that the primary judge would have concluded that the extent of the loss to consumers was beyond meaningful assessment.
96 Our final observation is that there is perhaps an easier way of looking at the problem of loss. With the benefit or advantage of conduct liable to mislead the public, namely by way of marketing representations that were deliberately made (putting to one side the issue of state of mind), 5.9 million packets of the purported Nurofen specific pain range were sold. As we have said, those representations were the only apparent reason for any rational consumer to buy the products at twice the price of standard Nurofen. The ordinary and predictable consequence of the conduct is not a circumstance of aggravation which the ACCC had to prove. Rather, if Reckitt Benckiser wished to have the Court accept that any of those 5.9 million sales were not substantially, if not overwhelmingly, influenced by the contravening conduct in a material way, the argument is one in mitigation which Reckitt Benckiser had to prove. There was no such evidence.
97 The primary judge started down the relevant path when he observed at [55] of the penalty judgment that Reckitt Benckiser engaged in its marketing and packaging of the purported specific pain range products, “with the intention of increasing profits”. Thereafter, his Honour’s acceptance of Reckitt Benckiser’s submissions also involved both an implicit acceptance of the concepts which gave rise to the contraventions and speculation about consumers’ behaviour. As a result, the primary judge was diverted from the drawing of obvious inferences which the evidence made compelling in the circumstances.
98 For these reasons, we consider that the primary judge erred on the question of loss to consumers. In our view, there was ample evidence to infer a direct causal relationship for sales of the more expensive four identical products. In the circumstances, that inference had to be drawn. No other reasonable inference could be drawn in the absence of evidence to rebut it. There was no reason not to draw that inference. In particular, there was no credible alternative explanation for the sales, or at least the vast bulk of them, taking place. Accordingly, a finding ought to have been made that a substantial proportion of the difference between the sales price of 5.9 million packages of the impugned products and of the equivalent number of sales of standard Nurofen had been lost to consumers as a result of Reckitt Benckiser’s contraventions of s 33 of the ACL between 2011 and 2015. Whether that be $26.25 million (as the ACCC has now calculated) or another amount (based on some refinement of the calculations), the loss was in the order of 50% of the total revenue from the sale of the impugned products, being $45 million.
Appeal ground 3: error in relation to replacement packaging
99 Ground 3 concerns the primary judge’s mistake in respect of the new packaging. As noted, his Honour mistakenly identified a packaging proposal rejected by the ACCC as the new packaging which involved “minor changes” and in respect of which the ACCC was said to have made no allegation that the new packaging was misleading (at [60] and [61] respectively; see also [4] and [5]). As also noted, Reckitt Benckiser accepted that the primary judge had identified the wrong packaging but argued that there was no material difference between the rejected proposed packaging and the interim packaging, nor any material consequence as a result of the mistake.
100 We do not accept Reckitt Benckiser’s submission for the following reasons.
101 Both before the primary judge and on appeal, Reckitt Benckiser contended that there was a very small difference in the packaging representations the subject of these proceedings, the alternative packaging initially proposed by Reckitt Benckiser but rejected by the ACCC, and the alternative packaging ultimately accepted by the ACCC as an interim measure by way of the addition of stickers so that existing stock would not be wasted. The essential differences were as follows, taking the so-called Nurofen “back pain” product as an example:
(1) The packaging representations giving rise to these proceedings:

(2) The mock-up packaging representations proposed by Reckitt Benckiser but rejected by the ACCC:

(3) The temporary packaging created by ACCC-approved stickers placed on existing stock:

102 Confined to the terms of s 33 of the ACL, the manner in which the contravening packaging was liable to mislead the public is manifest. The product is not specifically formulated for – and does not treat – any specific pain, be it back pain or otherwise. Accordingly, it is not “targeted” pain relief in the sense of being targeted to the type of pain nominated on the packaging.
103 The ACCC’s rejection of the proposed packaging secondly represented above is understandable. The added words (not prominent on the packaging as proposed) continue to represent that the product is formulated for and treats back pain, but also happens to be suitable for general pain relief. The proposal fell well short of ameliorating the misleading and deceptive character of the packaging.
104 The interim solution accepted by the ACCC, at the least, is better than the first two. The sticker is in type which has equivalent prominence to the words “back pain”. The sticker includes the important words “Equally effective for” followed by each of the nominated pain types on the purported specific pain range and the words “and general pain”. We do not accept that this was only a minor change from either the impugned packaging or the proposed replacement packaging rejected by the ACCC.
105 Before the primary judge, the ACCC did not suggest that the interim packaging to which it had agreed contravened the ACL. It follows that it cannot be said that the primary judge erred at [61] when he proceeded on the assumed (rather than proven) basis that the new interim packaging did not involve any contravention. The error, as we have said, was in mistakenly identifying the proposed but rejected packaging as the new interim packaging. The ACCC maintained its position in the appeal of refraining from any suggestion that the (actual) new interim packaging was liable to mislead the public. Given that the issue was not argued, it is not appropriate to venture into this area.
106 The primary judge’s mistake as to the relevant packaging was material to his conclusion that consumers might have been willing to pay a substantial price premium for the purported specific pain range products for reasons other than the contravening conduct. In turn, this conclusion was material to his Honour’s conclusion that it was neither necessary nor appropriate nor possible to attempt to engage in any exercise of, relevantly, assessing loss to consumers.
107 The fact that, in other cases, judges have reached the same conclusion on the evidence before them is immaterial. It may be accepted that in Australian Competition and Consumer Commission v Coles Supermarkets Australia Pty Ltd [2015] FCA 330; (2015) 327 ALR 540, Allsop CJ said at [54] that the “nature of the conduct involved in this case means that precise or even global assessment of any loss to consumers or competitors is difficult, if not impossible”. This was the ACCC’s position before Allsop CJ (see at [26]). And as Allsop CJ also said in Coles, referring to Perram J in Australian Competition and Consumer Commission v MSY Technology Pty Ltd (No 2) [2011] FCA 382; (2011) 279 ALR 609 at [77]–[79]:
[56] MSY Technology was a case where retailers were selling various computer-related goods and making representations which fell into three categories (at [57]):
… The first category consists of statements in which the respondents purported to disclaim or exclude their responsibility for providing warranties to consumers. The second consists of statements which purported to restrict the responsibility of the respondents for providing warranties to consumers in various ways. The third consists of statements suggesting that consumers were required to pay a fee for warranties beyond those provided by the manufacturer.
[57] The “commonsense” nature of his Honour’s suggestion is, with respect, far more apposite to a case such as the one before his Honour than it is in the present. It would be simple enough to adduce evidence of consumers who paid for additional warranties or who incurred expenses in the belief that they were not entitled to a warranty. The circumstances of that case were such that the misleading or deceptive conduct could clearly have a quantifiable and ascertainable pecuniary consequence for consumers. Here, the ACCC did not contend that the quality of the par-baked products was of any lower standard than goods baked from scratch. The primary source of any loss or damage to consumers was of a non-pecuniary nature. The fact that they had lost the opportunity to make a different purchasing choice that they may have made had they been provided with accurate information about the goods they were purchasing. In light of the period of time over which the conduct took place, I am not prepared to sentence on the basis that no one was in fact misled by Coles’ conduct.
[58] There is an alternative source of loss or damage, and that is loss or damage to competitors. Pecuniary damage might be proved here, but a number of other factors (such as those referred to in the affidavit of Mr Watson) may well be to blame for the loss. Demonstrating a causal link beyond a merely temporal connection is nearly impossible in these circumstances. This is not a situation like MSY Technology. That case involved a conclusion based on the specific factual circumstances of the case. Care must be taken to not elevate conclusions based on factual considerations to the status of rules of law.
108 There is no suggestion in Coles that the contravening product was the same as another product but sold at a price premium. Had that been the case then it cannot be assumed that the ACCC’s position would have been that loss could not be quantified, nor that Allsop CJ would have reached that conclusion.
109 We also do not accept that, in the circumstances of this case, an assessment of the loss to consumers as a result of Reckitt Benckiser’s contravening conduct involved undue speculation. The number of units of the purported specific pain range products sold in the relevant period was known. The chemical equivalence of the purported specific pain range products and standard Nurofen (and other products providing 200mg of ibuprofen) was known. The price premium for the purported specific pain range products was known. The real speculation involved was that of Reckitt Benckiser contending that:
(1) Consumers might have purchased the more expensive products for reasons such as “ease of selection for the relevant condition, product placement and advertising” when there was no “relevant condition” and the only reason for any difference in product placement and advertising was the contravening conduct.
(2) Consumers might not have purchased standard Nurofen but one of the other 50 Nurofen products when the relevant context was the purchase of a product providing 200mg of ibuprofen and no other active ingredient.
In any event, as discussed, the speculation involved in (2) above favoured Reckitt Benckiser because, but for the contravening conduct, a consumer might have purchased a competitor’s equivalent product (which the evidence indicated was available at prices less than those of standard Nurofen) and not Nurofen at all.
110 These conclusions support our view that the primary judge’s approach to consumer loss involved material error, requiring this Court to determine for itself the appropriate penalty to be imposed for the contravening conduct.
Appeal ground 4: types of harm
111 Ground 4 is that the primary judge erred by giving undue weight to the consideration that the harm caused to consumers by the contravening conduct was not physical and was only monetary (penalty judgment [97]). As argued, however, the ground was that this conclusion was simply incorrect.
112 It is apparent that this matter was significant to the primary judge. At [94] his Honour said that:
In this case, if it were not for three particular matters, the penalty would be far greater than that which I now impose.
113 The third of these matters was his Honour’s conclusion that as the products were effective to treat the pain that they represented they treated, the only potential effect on consumers was monetary.
114 We agree with the ACCC’s submission that the primary judge’s conclusion overlooked at least one readily apparent non-monetary effect of the contravening conduct: the loss or at least serious distortion of genuine consumer choice. This necessarily includes at least the risk of diverting a consumer from the choice of obtaining a product that was genuinely specifically formulated to treat, and in fact solely or specifically treated, a particular type of pain of the kind said to be targeted by the four identical products, be it migraine pain, tension headache, period pain or back pain. That choice involves being freely able, in the sense of not being misled, or liable to be misled, to choose a product which contained something more or other than ibuprofen, and therefore something potentially better for the particular type of pain (or more cost effective) than the ordinary ibuprofen in the four identical products. Further, the risk of the loss of consumer choice, if it materialised, created an additional risk of harm by reason of additional or prolonged duration of pain. As the ACCC submitted, Reckitt Benckiser’s conduct also created the risk of double-dosing. A person suffering both back pain and migraine pain, for example, might well have been induced to buy and take tablets from the purported Nurofen back pain and Nurofen migraine pain products believing they were different in that each was targeted to a different area of pain. Specific evidence of these risks was not required; the risks are the natural and ordinary consequence of the nature of the misleading character of Reckitt Benckiser’s conduct.
115 For these reasons, we consider that the primary judge’s conclusion at [97] of the penalty judgment involved error of a kind we would express as having reached a conclusion not open in the circumstances. As noted, given the terms of [94] of the penalty judgment, this conclusion was significant to his Honour’s determination of penalty. Accordingly, this is also sufficient to require this Court to determine for itself the appropriate penalty to be imposed for the contravening conduct.
Appeal ground 5: state of mind in relation to the contraventions
116 Reckitt Benckiser contended that the contraventions were not deliberate or even reckless, but rather were “innocent”. The primary judge accepted this view of the matter, commenting at [56] that:
This is not an [instance] where Reckitt Benckiser knew or was reckless that its conduct involved a contravention. It is simply that the conduct (however innocently committed) was intended to make a profit from consumers.
117 This absence of any pleaded intention or recklessness was the first of the three factors expressly relied upon by his Honour in his penalty judgment at [94] in arriving at what he evidently regarded as a penalty far more lenient than would otherwise have been the case (the third being the harm to consumers being only financial, as discussed above). His Honour found that Reckitt Benckiser’s conduct should be regarded as innocent because the ACCC did not plead any state of mind in relation to the conduct and because the ACCC did not make submissions as to any such state of mind existing.
118 By ground 5, the ACCC contends that the primary judge erred in relation to findings made about Reckitt Benckiser’s mental state in respect of the contraventions, and further erred in finding at [86] that TGA approval of the four identical products reduced concerns about the laxity of Reckitt Benckiser’s compliance program in relation to the ACL. There was some debate about what the correct mental state should have been found to be, but the ACCC’s final position fell short of asserting deliberateness as to the misleading character of the representations and instead was that it should have been found that Reckitt Benckiser had “courted the risk” that the representations had that character.
119 The first issue is whether, as the primary judge found, state of mind has to be pleaded when that is not an element of the provision giving rise to liability. At [67] of the penalty judgment the primary judge explained that:
There was no allegation in this case that the conduct by Reckitt Benckiser was deliberate or covert in the sense that the contraventions were made knowing that they were contrary to the Australian Consumer Law or that Reckitt Benckiser “courted the risk” of contravention. As I explained in relation to the discovery issues in Australian Competition and Consumer Commission v Reckitt Benckiser (Australia) Pty Ltd (No 5) [2016] FCA 167, even where liability is to be determined separately from remedy this is one matter which should be pleaded. It has the potential to affect dramatically the conduct of the case. No pleaded allegation concerning these matters was made by the ACCC at any stage of these proceedings. And, at a late stage in the proceedings after my decision on the discovery issues, the ACCC understandably did not seek to amend its pleadings.
120 In Australian Competition and Consumer Commission v Reckitt Benckiser (Australia) Pty Ltd (No 5) [2016] FCA 167 (the discovery judgment), his Honour identified that there were numerous matters about which Reckitt Benckiser ought to have been on notice by reason of the mere fact that the ACCC had sought a pecuniary penalty in its fast track application and fast track statement (at [13]). His Honour continued in these terms:
14 However, there is one exception which is perhaps the only exception. That exception is in relation to “intentionality” allegations, such as allegations that the contravention was deliberate (in the sense of known to be a breach), systematic, or covert, or whether Reckitt “took the odds” or “courted the risk” of engaging in contravening conduct.
15 Reckitt Benckiser submitted that these allegations of intentionality are equivalent to allegations of fraud. In Forrest v Australian Securities and Investments Commission [2012] HCA 39; (2012) 247 CLR 486, 501-502 [22], 502-503 [26], French CJ, Gummow, Hayne and Kiefel JJ characterised as fraud an allegation that a misleading statement was made knowingly or without an honest belief in its truth. The analogy with fraud is not precise. The ACCC’s characterisations of the basis for its discovery application do not rise to that level. But they are not distant from it. An allegation that misleading or deceptive conduct occurred deliberately (if, by this, it is meant that it occurred with knowledge that it was a contravention), covertly, or by “taking the odds” are extremely serious allegations. As I explained above, the more serious the allegation the more important it is for there to be clarity and particularised detail in a pleading (see, for instance, allegations analogous to fraud, in Idoport Pty Ltd v National Australia Bank Ltd [2000] NSWSC 599 [43] (Einstein J); Young Investments Group Pty Ltd v Mann [2012] FCAFC 107; (2012) 293 ALR 537, 540 [9] (the Court)).
16 Unlike issues described above at [11]-[13], allegations such as those “intentionality” allegations in this case will not often arise. Unlike the other issues, they are not matters that Reckitt Benckiser could fairly assume would be generally enlivened simply by the bringing of a penalty proceeding. The very serious nature of these allegations, coupled with the fact that these issues do not always arise, means that they are matters that should be properly pleaded and particularised.
121 In our view, his Honour was in error in concluding that because the ACCC did not plead any state of mind, penalty was to be assessed on the basis that the contravention was “innocent”, as characterised by the primary judge at [54] of the penalty judgment. By reason of the bringing of the proceeding for a penalty Reckitt Benckiser was fairly on notice that its state of mind, including that it might have acted intentionally or recklessly in carrying out the contravening conduct, was potentially in issue.
122 In [21] of the penalty judgment, the primary judge identified that there is long-standing authority that numerous factors are relevant for the assessment of penalty having regard to all relevant matters as required by s 224(2) of the ACL. His Honour said this:
There are numerous other factors which will commonly be relevant, and (where relevant) will be matters to which the Court must have regard. Those factors were described in the cases which I surveyed in Australian Competition and Consumer Commission v Woolworths Limited [2016] FCA 44 [124]-[126]. They include the following:
…
(2) the deliberateness of the contraventions and the period over which they extended;
…
(8) whether the contravening conduct was systematic, deliberate or covert;
123 In Australian Competition and Consumer Commission v Woolworths Limited [2016] FCA 44; [2016] ATPR 42-251 one of the decisions to which the primary judge referred was Trade Practices Commission v CSR Ltd [1991] ATPR 41-076 in which the deliberateness of the contraventions was identified as a matter relevant to penalty. Another was NW Frozen Foods Pty Ltd v Australian Competition and Consumer Commission (1996) 71 FCR 285, in which Burchett and Kiefel JJ at 292 approved the observations in CSR about matters relevant to penalty including the deliberateness of the contraventions and referred to the statements of Toohey J in Trade Practices Commission v Mobil Oil Australia Ltd (1985) 4 FCR 296 at 297-298 in which, again, the deliberateness of the contraventions was identified as a matter relevant to penalty.
124 In short, the deliberateness of the contraventions has always been a matter relevant to penalty for contraventions of consumer protection laws. As such, it is not possible to accept that procedural fairness required the ACCC in the present case to specifically plead that the contravening conduct involved any particular state of mind of Reckitt Benckiser.
125 This is reinforced by the fact that the fast track application did no more than claim a pecuniary penalty for the alleged contraventions (see para 5). The alleged contraventions (of s 29(1)(g) and s 33 of the ACL) did not require any particular state of mind to be proved. The fast track statement, as required, pleaded the facts material to the alleged contraventions and said in para 29 that:
The ACCC claims the relief specified in the Fast Track Application.
126 Reckitt Benckiser denied liability until the first day of the hearing on liability. Once it ultimately admitted liability, the only issue was the assessment of penalty in respect of which, in reality, there was no pleading. In these circumstances, it could not be assumed that there was any procedural unfairness to Reckitt Benckiser by the ACCC seeking to rely upon the evidence put forward by the parties relevant to penalty as supporting the proposition that Reckitt Benckiser courted the risk of the contraventions or was “objectively reckless” to that risk. Absent such procedural unfairness, there was no reason for the primary judge to characterise the conduct as “innocent”, merely because the ACCC confined its case to one of objective recklessness to an obvious risk, or “courting the risk”, or that Reckitt Benckiser should have known of the obvious risk (or whichever other formula is preferred). Further, and as discussed below, lack of intention, recklessness (in the conventional subjective sense), wilful blindness or negligence does not necessarily equate to “innocence”.
127 The second issue is whether the ACCC in fact put the proposition that Reckitt Benckiser courted the risk of the contraventions or was objectively reckless to that risk. There is no doubt that the ACCC put to his Honour that the risk of the conduct contravening the ACL was obvious and this should have been appreciated by Reckitt Benckiser’s senior management. Reckitt Benckiser submitted that this was not a submission about any state of mind of Reckitt Benckiser but about what its state of mind should have been.
128 Reckitt Benckiser’s submissions, insofar as they go, must be right. It is one thing to put that a person should have realised the risk of contraventions and another to put that the person in fact knew of, or was reckless to or wilfully blind to the risk. Each of the latter involves a particular state of mind which, in the ordinary course, would be seen as attracting a higher level of moral culpability than innocence or negligence. In this sense, it cannot be said that the primary judge was in error in saying that it was not submitted by the ACCC that the conduct involved an intentional or a reckless contravention.
129 Despite this, we consider that the reasons for judgment disclose error of the kind identified in ground 5 (which uses the phrase “objectively reckless” to describe the finding the ACCC says the primary judge ought to have made). As we have said, given [94] of the penalty judgment, there can be no doubt that his Honour considered the lack of any pleading or submission that the conduct involved an intentional or a reckless contravention to be of such significance that the penalty had to be far less than would otherwise be the case. But it appears from [56] that his Honour treated the deliberateness of the conduct as involving three possibilities, knowing contravention, recklessness or innocent contravention, rather than as a spectrum where, depending on the facts, the characterisation of kinds of conduct might involve more nuanced considerations than are capable of being conveyed by the limited concepts of knowing, reckless or innocent contraventions. In particular, the reference in [56] to the conduct being “however innocently committed” indicates that his Honour reasoned that because the ACCC had not submitted that the conduct was knowing or reckless, the conduct was necessarily “innocent”, thereby (at [94]) requiring a far lesser penalty than would otherwise be the case. Yet this approach is itself at odds with other findings of his Honour including:
(1) The rejection of Reckitt Benckiser’s submission that it was not aware of the 2010 Shonky Award (at [76]).
(2) The rejection of Reckitt Benckiser’s submission that it was not aware of the criticisms made of its purported specific pain range products on the television show “The Checkout” in 2013 (at [83]).
(3) Reckitt Benckiser’s knowledge of the complaints to the TGA and the findings of the TGA Complaints Resolution Panel in 2013 (at [78]).
(4) Reckitt Benckiser’s awareness of the orders of the Secretary, Department of Health in 2014 which the primary judge said must have been recognised by Reckitt Benckiser as having potential implications for its packaging (at [81]).
(5) The deliberate decisions made by Reckitt Benckiser’s senior management in 2013 and 2014 to continue with the same packaging (at [82]), characterised by the primary judge at [70] as decisions to “maintain the status quo”.
(6) The characterisation of the risks as “serious” and the above warnings Reckitt Benckiser received as “obvious” (at [73]).
130 We do not think it was reasonably open to his Honour to assess penalty on the basis that Reckitt Benckiser’s conduct was “innocent” or that because knowledge or recklessness had not been put the matters referred to above were not also relevant to evaluating the deliberateness of the conduct, and rendered it other than “innocent”.
131 If a contravention does not involve any state of mind then it is for the party asserting any particular state of mind (be it a deliberate flouting of the law, recklessness, wilful blindness, “courting the risk”, negligence, or innocence or any other characterisation of state of mind) to prove its assertion. If, in the event, neither party discharges its onus to establish any particular state of mind in relation to the contraventions, the Court determines penalty on no more than the fact of the proscribed nature of the conduct (see, by analogy see R v Olbrich [1999] HCA 54; (1999) 199 CLR 270 at [22]-[28]). However, if any degree of awareness of the actual or potential unlawfulness of the conduct is proved then, all other things being equal, the contravention is necessarily more serious. Such awareness may be able to be inferred from the very nature of the conduct or representations constituting the conduct. However absence of such proof does not establish a mitigatory state of mind (see, by analogy, R v Storey [1998] 1 VR 359 at 369, quoted with approval by the majority in Olbrich at [27]; see also [25]). It means only that the neutral state of mind required for liability has not been disturbed for the purposes of penalty. If a contravening party wishes to go beyond the neutral statutory state of mind for liability and positively assert a lack of consciousness of the character of the conduct for the purposes of penalty, that is a circumstance of mitigation which the contravening party must prove.
132 Ultimately, a judge must form his or her own views on whether and if so what state of mind existed on the evidence that is before the Court, provided that a party has been given an opportunity to be heard. To do otherwise is to require a judge to surrender an essential judicial function, even if it is not as clear-cut for civil penalty proceedings as it is for criminal proceedings: the CFMEU civil penalty case at [61]. The Court cannot surrender the ultimate responsibility for making the necessary findings leading to penalty, even if any penalty agreed between the parties cannot easily be departed from. The determination of state of mind, if any, is a central judicial function.
133 By accepting that the conduct was “innocent” merely because the ACCC did not submit that it was knowing or reckless in the conventional sense, the primary judge did not discharge this essential judicial function.
134 As we have already observed, the four products were the same and were relevantly the same as standard Nurofen. The purported differences were a fiction created by a marketing strategy by which, as the primary judge said more than once, Reckitt Benckiser wished to make a profit (at [6] and [56]). A marketing strategy designed around the creation and promotion of a fiction of difference and choice when none existed was inherently risky in terms of consumer protection laws. Deliberately persisting with it over a period of five years and until liability was ultimately admitted, despite pointed criticism on the basis of the very facts which established Reckitt Benckiser’s liability, cannot be described as “innocent” on any reasonable view.
135 Rather, to establish the innocence of the conduct Reckitt Benckiser would have had to call a sufficiently senior employee, probably the Australian Regulatory and Medical Affairs Director who decided in 2013 to continue with the marketing strategy, to give evidence explaining how such a state of mind was reached having regard to the objective facts that must have been known. Unsurprisingly, no such evidence was adduced by Reckitt Benckiser. Instead, the innocent state of mind was merely asserted, and accepted by the primary judge, apparently as a result only of the lack of any pleaded allegation of any particular state of mind.
136 In all of the circumstances, at the very least, Reckitt Benckiser did indeed “court the risk” of contraventions, at least to the extent that this concept embraces the notion of objective recklessness. It knew all of the facts that constituted contraventions. It knew that others with an interest in or responsibility for consumer protection considered the marketing misleading. It knew exactly why those others had reached that view. It knew that the facts the others had assumed for the purpose of reaching that view were true. This is more than sufficient to conclude that Reckitt Benckiser courted the risk of contraventions. This degree of aggravation is readily established. This aspect of this ground of appeal succeeds both as to principle and, given the weight which the primary judge placed upon it, as to the necessary effect on the outcome.
137 There is another aspect of ground 5 which concerns the primary judge’s finding at [86] that the TGA approvals of the purported specific pain range products “reduce the concerns about laxity of the compliance program” in relation to the ACL. We accept the ACCC’s submissions as follows:
First, under the Therapeutic Goods Act 1989 (TGA Act) the TGA is not the body responsible for enforcing compliance with ss. 18 or 33 of the ACL, and the TGA’s legislative focus was and is considerably more confined. Second, as observed by the trial judge, when evaluating therapeutic goods for registration, the TGA considers the goods and their packaging in isolation. Thus the issue of presentation and display of the Nurofen Specific Pain Relief products as a range did not arise because the products were registered separately at different times. Third, the respondent’s compliance program did not even reference the requirements of the ACL (PJ [73]). (footnotes removed and emphasis amended)
138 However, we are unable to conclude that these matters mean that the finding was not reasonably open to the primary judge. In any event, it is not apparent to us that the finding was material to his Honour’s assessment of penalty. As such, this aspect of appeal ground 5 should detain us no further.
Appeal ground 6: the course of conduct principle
139 The second principal reason the primary judge gave at [94]-[95] for the penalty imposed being far less than would otherwise have been the case was that his Honour accepted Reckitt Benckiser’s submission that the contravening conduct involved only two courses of conduct, one for the packaging representations, and one for the webpage representations (penalty judgment at [95]). While the primary judge did not regard this as imposing a cap of $2.2 million on the maximum penalty, he plainly regarded the characterisation of the contraventions as involving two courses of conduct as a significant factor in determining the appropriate penalty. It is apparent that the overall penalty imposed of $1.7 million falls within the aggregate limit of only two contraventions of $2.2 million, although one component for the packaging representations was $1.2 million and the other for the website representations was $500,000 (penalty judgment at [98]).
140 The ACCC’s case before the primary judge was that there were six courses of conduct, one for each of the four identical products and one each for each of the two webpages (penalty judgment at [32]). The ACCC sought a penalty of $6 million (penalty judgment at [91]). On appeal, the ACCC argued that his Honour misapplied the so-called course of conduct principle in various ways including by characterising the conduct as involving only two courses of conduct rather than six, giving inappropriate weight to a notional maximum penalty for two contraventions of s 33 of $2.2 million, focusing only upon the initial acts causing the contraventions rather than the contraventions continuing over nearly five years, and applying the principle in a manner that meant the overall penalty did not reflect the nature and extent of the conduct.
141 Whether this ground of appeal is subsumed into ground 8 (manifestly inadequate penalty) or not, we find the ACCC’s contentions generally persuasive. In particular, we accept the ACCC’s submissions as follows:
The penalty discretion must be guided first and foremost by the applicable statutory provisions. In that respect, s 224(1)(a)(ii) of the ACL provides that the Court may order a person who has contravened (relevantly) s 33 of the ACL to pay such pecuniary penalty, in respect of each act or omission by the person, as the Court determines to be appropriate. Section 224(3) provides that the maximum penalty for a body corporate for each act or omission to which s 224 applies that relates to (relevantly) s 33 of the ACL is not to exceed $1.1 million. It is uncontroversial that a contravention of s 33 occurs each time that a misleading representation is made to a person which, in the present case, involved millions of contraventions (PJ [24]).
As observed recently by Beach J in ACCC v Hillside (Australia New Media) Pty Ltd trading as Bet365 (No 2) [2016] FCA 698 at [24] – [25]:
“ … the “course of conduct” principle does not have paramountcy in the process of assessing an appropriate penalty. It cannot of itself operate as a de facto limit on the penalty to be imposed for contraventions of the ACL. Further, its application and utility must be tailored to the circumstances. In some cases, the contravening conduct may involve many acts of contravention that affect a very large number of consumers and a large monetary value of commerce, but the conduct might be characterised as involving a single course of conduct. Contrastingly, in other cases, there may be a small number of contraventions, affecting few consumers and having small commercial significance, but the conduct might be characterised as involving several separate courses of conduct. It might be anomalous to apply the concept to the former scenario, yet be precluded from applying it to the latter scenario. The ‘course of conduct’ principle cannot unduly fetter the proper application of s 224.” (emphasis and footnotes removed)
142 This said, we also accept Reckitt Benckiser’s submission that it cannot be said that the primary judge erred merely by characterising the courses of conduct involved as two in number. While we would have reached a different view, and accepted the ACCC’s submission that six courses of conduct were involved (one for each of the packaging representations given that there are four products constituting the range which came on the market at different times and one each for the two webpages), we do not suggest that it was not reasonably open to his Honour to characterise the courses of conduct as two in number, one relating to the packaging and one to the webpages.
143 We also accept that the primary judge made the important observations at [96] of the penalty judgment in these terms:
In this case, within the course of conduct concerning the Packaging Representations, the contraventions were not committed in identical circumstances. Later contraventions, where Reckitt Benckiser’s compliance procedures should have operated to require a review of its packaging, were more serious. And, as I have explained, the contravening conduct, although not a deliberate infringement, was part of a course of marketing designed for profit in relation to a range of products which led to $45 million in sales and many millions of dollars of profit from sales over the period of the Packaging Representations.
144 This must be so given that, as the years went by, Reckitt Benckiser’s awareness of the risk of contravention that it was courting must have increased by reason of the Shonky Award, the television show “The Checkout”, the complaints to the TGA, the decision of the TGA Complaints Resolution Panel, and of the Secretary of the Department of Health. Yet, as we have said, Reckitt Benckiser continued to make the packaging representations until it was unable to continue to do so by reason of the liability judgment.
145 Nevertheless, it is apparent from [94]-[95] and by inference from the total penalty imposed that the characterisation of the contraventions as involving two courses of conduct played a significant role in his Honour’s determination of penalty. The very fact that the penalty for the packaging representations was only $100,000 more than the maximum for one contravention and was $500,000 for the website representations, giving a total of $1.7 million, against a maximum of $2.2 million for two contraventions, to our minds, is indicative of error. To take the packaging representations as an example. Even characterised as one course of conduct, the representations continued for nearly five years, involved the sale of 5.9 million packages of the purported specific pain range products and necessarily was liable to mislead at least that number of consumers, and no doubt many more. As the ACCC said, consumers’ experience of the contraventions was not confined to the initial marketing decisions. Rather, the misleading character of the representations operated as contraventions each and every time a consumer saw the packaging. The conduct continued despite the events which should have made it obvious to Reckitt Benckiser that it was courting the risk of illegality. It continued as a result of at least one deliberate decision by Reckitt Benckiser’s Australian Regulatory and Medical Affairs Director. In these circumstances, characterisation of the contraventions as involving two courses of conduct only could not be entitled to the weight which the primary judge gave to that factor, as disclosed in [94] of his reasons.
Appeal ground 7: cooperation
146 The ACCC took issue with the primary judge’s conclusion at [68] of the penalty judgment that Reckitt Benckiser had provided “commendable and significant cooperation” by meeting with the ACCC before the proceedings began, making offers concerning changes to its packaging, proposing and attending a mediation (which did not succeed), making admissions of liability on the first day of the two-day liability hearing, agreeing to an interim packaging arrangement with the ACCC, and helping to devise a statement of agreed facts for the penalty hearing.
147 It was open to the primary judge to reach this conclusion as to cooperation. Nevertheless, given our conclusions above, we are called upon to determine penalty and in so doing we are not bound to make the same finding. In our opinion, while there was a degree of cooperation, including by admitting to contraventions of ss 18 and 33 of the ACL at the liability trial (but not sooner), this should not carry substantial weight. In particular, the admissions were very late and saved very little, if any, court time. The admissions can properly be seen as both recognising the inevitable, at least as to the contraventions admitted. The agreement about the interim packaging was a concession by the ACCC, not Reckitt Benckiser and it was to Reckitt Benckiser’s advantage.
Appeal ground 8: manifest inadequacy
148 The plurality in the CFMEU civil penalty case made it clear at [55] that the proper function of a civil penalty is “primarily if not wholly protective in promoting the public interest in compliance”, citing and quoting from CSR at 52,152. At [59], the High Court also affirmed the “essentially deterrent” purpose of civil penalties.
149 This case involves a clear example of the need for the application of the deterrence objective endorsed in the CFMEU civil penalty case. This is especially so given, first, that the potential gains from inducing consumers to buy a more expensive product with no additional benefit were very substantial (measured in the tens of millions of dollars) and, second, the potential distortion of competition in the market by gaining an unfair advantage over competitors who complied with the law. The need for deterrence in this case, both general and specific, was substantial. The notion of deterrence in the context of this case warrants some further elaboration. In our view, Reckitt Benckiser’s conduct was towards the high end of the range for s 33 contraventions.
150 The objective of any penalty in this case must be to ensure that Reckitt Benckiser and other “would-be wrongdoers” think twice and decide not to act against the strong public interest in consumers being able to making decisions about buying non-prescription medicines free from representations that are liable to mislead and thereby distort their decision-making processes.
151 All others things being equal, the greater the risk of consumers being misled and the greater the prospect of gain to the contravener, the greater the sanction required, so as to make the risk/benefit equation less palatable to a potential wrongdoer and the deterrence sufficiently effective in achieving voluntary compliance. Tipping the balance of the risk/benefit equation in this way is even more important when the benefit in contemplation is profit or other material gain. It is especially important if there are disadvantages, including increased costs or lesser sales or profits, in complying with legal obligations for those who “decide” to be law-abiding.
152 If it costs more to obey the law than to breach it, a failure to sanction contraventions adequately de facto punishes all who do the right thing. It is therefore important that those who do comply see that those who do not are dealt with appropriately. This is, in a sense, the other side of deterrence, being a dimension of the general deterrence equation. This is not to give licence to impose a disproportionate or oppressive penalty, which cannot be done, but rather to recognise that proportionality of penalty is measured in the wider context of the demands of effective deterrence and encouraging the corresponding virtue of voluntary compliance.
153 Breaches of the kind carried out by Reckitt Benckiser will usually involve a high degree of contemplation and choice. The involvement of Reckitt Benckiser’s Australian Regulatory and Medical Affairs Director in deciding to continue with the marketing of the products in 2013 is consistent with this likelihood. The right decision is more likely to be made if the sanction for the conduct is substantial relative to the possible gain to be made from it – a relationship which is closely related to, but not identical with, consumer loss. This critical importance of effective deterrence must inform the assessment of the appropriate penalty. It is the primary consideration in assessing the objective seriousness of Reckitt Benckiser’s conduct and in deciding whether the sanction imposed by the primary judge was manifestly inadequate.
154 In considering the sufficiency of a proposed civil penalty, regard must ordinarily be had to the maximum penalty. In Markarian, a criminal sentencing context, it was observed at [31] that:
careful attention to maximum penalties will almost always be required, first because the legislature has legislated for them; secondly, because they invite comparison between the worst possible case and the case before the court at the time; and thirdly, because in that regard they do provide, taken and balanced with all of the other relevant factors, a yardstick.
155 The reasoning in Markarian about the need to have regard to the maximum penalty when considering the quantum of a penalty has been accepted to apply to civil penalties in numerous decisions of this Court both at first instance and on appeal (Director of Consumer Affairs, Victoria v Alpha Flight Services Pty Ltd [2015] FCAFC 118 at [43]; Australian Competition and Consumer Commission v BAJV Pty Ltd [2014] FCAFC 52; (2014) ATPR 42-470 at [50]-[52]; Setka v Gregor (No 2) [2011] FCAFC 90; (2011) 195 FCR 203 at [46]; McDonald v Australian Building and Construction Commissioner [2011] FCAFC 29; (2011) 202 IR 467 at [28]-[29]). As Markarian makes clear, the maximum penalty, while important, is but one yardstick that ordinarily must be applied.
156 Care must be taken to ensure that the maximum penalty is not applied mechanically, instead of it being treated as one of a number of relevant factors, albeit an important one. Put another way, a contravention that is objectively in the mid-range of objective seriousness may not, for that reason alone, transpose into a penalty range somewhere in the middle between zero and the maximum penalty. Similarly, just because a contravention is towards either end of the spectrum of contraventions of its kind does not mean that the penalty must be towards the bottom or top of the range respectively. However, ordinarily there must be some reasonable relationship between the theoretical maximum and the final penalty imposed.
157 In this case, the theoretical maximum was in the trillions of dollars (some 5.9 million contraventions at $1.1 million per contravention). By way of example only, even if the appropriate penalty per contravention for each sale was $1, the penalty would approach $6 million. It follows that the assessment of the appropriate range for penalty in the circumstances of this case is best assessed by reference to other factors, as there is no meaningful overall maximum penalty given the very large number of contraventions over such a long period of time. Given this, we consider that, to the extent that the course of conduct principle had any meaningful work to do, the better way to look at it was in terms of each of the four “types” of packaging, each with its own consumer target audience. This proceeding really involves four types of contravention, with many individual contraventions each over the five years. The webpage contraventions can be viewed as one or two serious courses of conduct. But ultimately this discussion itself serves to demonstrate the limited utility of the course of conduct principle in the circumstances of a case such as the present, and why any such characterisation could not properly have the significance which the primary judge gave to it at [94]-[95] of the penalty reasons.
158 Overall, in the particular circumstances of this case we consider that one useful guide to the appropriate penalty range is loss to consumers. In this case, this loss may be assessed by reference to the extra amount paid by consumers as against a product that did not suffer from any of the impugned representations, such as ordinary Nurofen. This best approximates the impact on consumers, and thus the potential gains to Reckitt Benckiser. This gives a starting point of around $25 million and, on any view, more than $20 million (the price of the impugned products being about double that of standard Nurofen and the total revenue being $45 million). Regard must also be had to the following:
(1) Section 33 of the ACL proscribes conduct liable to mislead the public, rather the conduct that was in fact false or misleading (contrast s 29(1)).
(2) Reckitt Benckiser’s business includes the sale of consumer health care products and this part of its business may fairly be described as substantial. In particular, Reckitt Benckiser enjoyed a large share of the market for oral analgesics and the revenue of the Reckitt Benckiser Group worldwide between 2011 and 2015 was in the range of 8 to 10 billion GBP.
(3) The products are medicines and are used to treat pain.
(4) The products were available to the public at large, being sold in approximately 5,500 pharmacies and 3,000 other retail outlets across Australia.
(5) Reckitt Benckiser denied the contraventions until 10 December 2015, objectively indicating a need for specific deterrence.
(6) Reckitt Benckiser continued to sell the products well past the stage of negotiating with the ACCC and well after the proceedings commenced.
(7) An ordinary and natural consequence of the conduct was the real risk that some consumers did not buy an alternative product that was in fact formulated to treat their specific pain and of double-dosing by consumers suffering from more than one of the types of pain purported to be treated by the so-called Nurofen specific pain range.
(8) Reckitt Benckiser provided a measure of cooperation, albeit very late in terms of the admission of liability.
(9) There are competitors in the market for non-prescription oral analgesics, indicating the need for effective general deterrence.
(10) In 2012, Reckitt Benckiser was found to have contravened s 18 of the ACL in respect of the packaging of its Mortein product (penalty judgment at [88] – [90]).
159 The need for specific deterrence can be informed by the attitude of the contravener to the contraventions, both during the course of the contravening conduct and in the course of the proceedings both at first instance and on appeal. The analogy that can be drawn with criminal sentencing are the concepts of contrition or remorse, which conventionally give some comfort to a court that re-offending is unlikely and therefore specific deterrence less important.
160 Reckitt Benckiser continued to maintain in this Court, both at first instance and on appeal, that the representations on the individual packets for the four identical products viewed separately were not liable to mislead (described by their solicitors as late as 17 December 2014 as “accurate”), that the liability to mislead only arose from the combined effects of the four types of packets, that the packaging representations could not be shown to have any effect on purchasing decisions, and that minor changes by the addition of a few words in the packaging addressed the problem. We have rejected each of these propositions above. We find it difficult to reconcile these propositions with any genuine sense of remorse on the part of Reckitt Benckiser for having repeatedly contravened a basic requirement of the ACL (not to engage in conduct liable to mislead the public) for nearly five years for its own commercial benefit. This said, we did not put Reckitt Benckiser on notice that its submissions might be seen as demonstrating a lack of remorse and, as a result, do not propose to give weight to this factor when determining penalty.
161 A different consideration, and one which was within the control of Reckitt Benckiser alone, was that nothing apart from the agreed facts was put before the primary judge to indicate any remorse on the part of Reckitt Benckiser for its unlawful conduct. In the criminal context, in Barbaro v The Queen [2012] VSCA 288; (2012) 226 A Crim R 354 at [38], Maxwell P, Harper JA and T Forrest AJA said:
It follows, in our view, that a person wishing to rely on remorse as a mitigating factor needs to satisfy the court that there is genuine penitence and contrition and a desire to atone. In many instances, the most compelling evidence of this will come from testimony by the offender. A judge is certainly not bound to accept second-hand evidence of what the offender said to a psychiatrist or psychologist or other professional, let alone testimonials from family or friends, or statements from the Bar table.
162 We see no reason why a different principle ought to apply in the context of a civil penalty if a person wishes to rely on remorse or contrition as a mitigating factor. We thus agree with the observation of Mortimer J in National Tertiary Education Industry Union v Swinburne University of Technology (No 2) [2015] FCA 1080 at [109], where her Honour stated that:
If a respondent to a contravention wishes to take advantage of sentencing principles concerning remorse and contrition, there are plain and clear ways to achieve that through the tender of evidence with appropriate content. That has not occurred in this proceeding.
163 The same proposition applies to the present proceeding. As such, we do not consider that any additional discount for apparent remorse and contrition beyond that which is appropriate for the extent of co-operation Reckitt Benckiser did provide is warranted.
164 In all of the circumstances we are of the view that the penalty of $1.7 million cannot be viewed as substantial (as Reckitt Benckiser would have it) or as achieving the primary deterrence object of a civil pecuniary penalty. To the contrary, we consider that the penalty would reinforce a view that the price to be paid for the contraventions was an acceptable business strategy, and was no more than a cost of doing business. The public interest in consumers being able to making decisions about buying non-prescription medicines free from representations that are liable to mislead and thus distort decision-making processes would thereby be undermined rather than supported by the penalty that was imposed.
165 In all of the circumstances (including those set out below in the context of our assessment of the appropriate penalty), we are of the view that an appropriate penalty for the contraventions had to be not less than $6 million (and could have been many millions more, the ACCC’s figure of $6 million being at the bottom of the appropriate range in all the circumstances of this case). We do not consider it necessary or appropriate to divide the penalty between the courses of conduct given the lack of utility of this concept in this case for the reasons given above. It suffices to say that, in common with the primary judge, we consider the packaging representations, overall, to be more serious than the website representations and they account for $5 million of the minimum penalty we consider was reasonably open in the circumstances, and the website representations $1 million. As even the bottom of this range is more than triple the penalty the primary judge imposed, we are necessarily of the view that the penalty imposed by his Honour was manifestly inadequate. This conclusion is unavoidable in light of all the matters discussed in these reasons.
Exercising the penalty discretion afresh
166 As discussed, each of the individual packages in the so-called “Nurofen Specific Pain Range” – because there was in fact no range at all – constituted representations liable to mislead the public on their own. It follows that this Court is entitled to have regard both to the individual effect of each packaging representation, as well as their likely combined effect. The two webpages served to reinforce and amplify this effect, both as to the individual packaging and their combined effect. A consumer trying to ascertain what medication to take for a migraine (for example) cannot necessarily be assumed to have had any regard to the representations concerning back pain, but had the consumer done so, it would have reinforced the misleading effect of the representations relating to the Nurofen migraine pain.
167 The real vice in the combination of the use of the specific words in each of “migraine pain”, “tension headache”, “period pain” and “back pain”, with the prominent words “fast targeted relief from pain”, with an emphasis on the use of the word “targeted”, is the consequence that each individual product purported to be formulated for and to treat a particular type of pain when none of the products were formulated for or treated any specific kind of pain. Considered in isolation, the packaging of each product was liable to mislead in a fundamental and important respect: the nature of the product as a pain treatment. The simultaneous marketing of the four different “types” of products also meant that the individual conduct for any one product was significantly aggravated by the presence of each of the other three products.
168 The conduct liable to mislead for each of the product comparator webpages and the specific pain relief webpages, if anything, are more egregious, at least in terms of content, than the product packaging, albeit of the much shorter but still substantial duration of 18 months.
169 In relation to the product comparator page, it had a large print heading “RELIEVE PAIN WITH THE RIGHT TYPES OF PAIN MEDICATION”, which unavoidably represented that the information on the webpage related to different types of medication for different types of pain, when in fact the active ingredients are equivalent in every respect (save that one, Zavance, is said to enable faster absorption) and there is no relevant difference beyond packaging for the four identical products and standard Nurofen. The text below the heading then referred to:
(1) “knowing what’s right for you”, which necessarily represented that there is a material difference between the six products listed below the text;
(2) “with so many different types of pain medication to choose from” which, at least when read with “let us provide a guiding hand in deciding what product is right for you, your pain and your body”, represented that there was a material difference between the pain which the products listed below treated and a genuine choice involved for the type or kind of pain, when there was not.
170 The inherently misleading nature of the text is then aggravated by the layout below the text with six products listed across the top, a list of pain “types” or “sources” down the left, and the suggestion that, for example, for period pain, a customer could choose to use a standard or rapid acting general product (as listed for every type of pain treatable by ibuprofen) or choose to use one that was formulated specifically for the particular type of pain listed. As a result, and for example, the website representations had the effect that:
(1) The customer could choose to buy a general product or a product that was specific for the condition from which they were suffering. But, in truth, there was no product that was specific for the condition from which they were suffering.
(2) If the customer had more than one of the types of pain or was a member of a family where different family members suffered from different types of pain, it is not hard to imagine such a customer buying more than one of the four identical products to treat different types of pain. This was another risk inherent in the conduct.
(3) Having purchased a product that represented that it was formulated for and did treat the specific kind of pain from which the consumer or another family member was suffering, the consumer might well be induced not to purchase some other product which was genuinely specifically formulated for and did treat that kind of pain, whether a non-prescription or a prescription product. Again, no evidence of the potential for such consumer conduct was required. This potential is an obvious and natural consequence of the kind of conduct in which Reckitt Benckiser engaged over such a lengthy period.
171 Similar observations apply to the “Specific Pain Relief” webpage. Given the discussion above, it may be sufficient to observe only that this webpage includes the statement that “Nurofen has developed a range of products to target and relieve pain. If you’re looking for back pain relief or relief from period pain, tension headaches and migraines, you can find the right product for you from the list below”, with the products in the purported specific pain range depicted below. The products were not developed to target any of the kinds of pain described. There was no “right” product for any of the four pain types.
172 The approximately double price for the supposed “targeted” product would also have reinforced the misleading perception that the customer was getting something different from standard Nurofen or Nurofen Zavance because the product was formulated for and treated the specific pain from which the consumer suffered. Thus, the price difference between the various ibuprofen products is relevant not just because it enabled Reckitt Benckiser to make money (and necessarily more money than it could make from products sold for half the price), but also is a material circumstantial and contextual factor which exacerbated the likely misleading of consumers.
173 Reckitt Benckiser was on notice from numerous sources of obvious problems with the accuracy of their marketing strategy, starting before the pleaded five year contravention period commenced in 2011. Yet Reckitt Benckiser persisted. The only inference open is that it did so in its own commercial interests, no doubt because of the marketing advantage Reckitt Benckiser considered these representations yielded in terms of sales, market share and profit. It is difficult not to see Reckitt Benckiser’s conduct as a marketing ploy to create the appearance of difference when none in fact existed, intended to serve its own commercial interests, in which it persisted over many years despite knowing and being made aware of undisputed facts and circumstances from which it should have been aware that it was contravening the ACL. By persisting with its conduct, at least after the findings of the TGA Complaints Resolution Panel on 12 June 2013, Reckitt Benckiser plainly courted the risk of the contraventions.
174 The additional revenue Reckitt Benckiser obtained from the sale of the four identical products, compared to the revenue that it would have obtained if the same sales had been made of the chemically equivalent standard Nurofen product containing 200mg of ibuprofen was in the order of $25 million over the 5 years during which the relevant sales took place. Giving effect to the requirements of specific and general deterrence, one of the challenges of this appeal is to ascertain what relationship, if any, should exist between that amount of additional revenue, and therefore additional cost to consumers, and the pecuniary penalty that Reckitt Benckiser should be ordered to pay.
175 Judges imposing civil penalties, and in particular in arriving at a particular quantum, are in a broadly analogous position to criminal law judges imposing gaol terms. A complex set of facts and circumstances must ultimately be reduced, by a process of “instinctive synthesis”, to a single number. However it is desirable that the instinct involved is, and is seen to be, something more than a mere “gut reaction”, and that the final result is an outcome of a reasoned process. Importantly, that reasoning, at least in relation to criminal sentences, should not be mathematical in nature: Wong at [76]. It is not necessarily the case that such a mathematical prohibition so clearly applies to the ascertainment of the quantum of a civil penalty. However, this does not fall for determination in this case.
176 In this case, as noted, the single most important numerical benchmark or yardstick for the civil penalty to be imposed is the loss incurred by consumers by reason of Reckitt Benckiser’s conduct, which, for the reasons given, we accept to be a substantial proportion of some $25 million. The main reasons to have regard to loss are that it is not only an appropriate means of characterising, in a real and meaningful way, the extent of deterrence that might be appropriate, expressed in monetary units, but also is a mandatory consideration in assessing penalty by reason of s 224(2)(a) of the ACL.
177 The contraventions admitted to and accepted by the ACCC in the present case were the less serious “liable to mislead” contravention contrary to s 33 (in contrast to s 29(1)(g) which involves false or misleading representations). However, there was no mitigatory state of mind established (or, in our view, plausibly possible in the circumstances of this case). There are also the circumstances of limited assistance by Reckitt Benckiser and a very late admission of liability in the face of an overwhelming case with a virtual certainty of the contraventions of at least s 33 being established. Independently of the state of mind issue, Reckitt Benckiser had multiple warnings but decided to persist with its conduct.
178 It is also relevant to take into account the modest stance on penalty of the ACCC in seeking a penalty of $6 million. Sitting as trial judges, we would have been entitled to impose a considerably greater penalty, given the losses which we consider were occasioned by the conduct and that these were serious contraventions even within the spectrum of the liable to mislead category.
179 As we have said, the penalty the ACCC sought of $6 million was at the bottom of the appropriate range for the contraventions. For all of the foregoing reasons, and bearing in mind that we are exercising a discretion on appeal which usually calls for a measure of restraint, we consider that a penalty at the bottom of the range we have suggested should be imposed in lieu of that which the primary judge ordered, namely a penalty of $6 million.
Conclusion
180 The appeal is to be allowed. The pecuniary penalty of $1.7 million is set aside. Reckitt Benckiser is ordered to pay to the Commonwealth a pecuniary penalty of $6 million within 30 days.
181 Furthermore, Reckitt Benckiser must pay the costs of the ACCC of and incidental to this appeal and of the hearing below.
I certify that the preceding one hundred and eighty-one (181) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justices Jagot, Yates and Bromwich. |
Associate:
ANNEXURE A