FEDERAL COURT OF AUSTRALIA
Nichia Corporation v Arrow Electronics Australia Pty Ltd (No 4) [2017] FCA 864
ORDERS
Applicant | ||
AND: | ARROW ELECTRONICS AUSTRALIA PTY LTD ACN 065 151 626 Respondent | |
AND BETWEEN: | ARROW ELECTRONICS AUSTRALIA PTY LTD ACN 065 151 626 Cross-Claimant | |
AND: | Cross-Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. Each party provide a draft of the orders it proposes (including on costs) to give effect to the reasons published today as Nichia Corporation v Arrow Electronics Australia Pty Ltd (No 4) [2017] FCA 864.
2. The applicant provide its draft orders to the Associate to Yates J by 4.00 pm on 14 August 2017.
3. The respondent provide its draft to the Associate to Yates J by 4.00 pm on 21 August 2017.
4. Leave be granted to each party, when submitting their proposed orders, to make supporting submissions in writing, limited to three pages.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
YATES J:
1 The applicant, Nichia Corporation, is the patentee of Patent No. 720234 (the patent). It sues the respondent, Arrow Electronics Australia Pty Ltd, for infringement of claim 3 of the patent. Claim 3 is dependent on claim 1.
2 Claim 1 is:
A light emitting device, including a light emitting component and a phosphor capable of absorbing a part of light emitted by the light emitting component and emitting light of wavelength different from that of the absorbed light; wherein said light emitting component comprises a nitride compound semiconductor and said phosphor contains a garnet fluorescent material including at least one element selected from the group consisting of Y, Lu, Sc, La, Gd and Sm, and at least one element selected from the group consisting of Al, Ga and In, and being activated with cerium.
3 Claim 3 is:
A light emitting device according to claim 1, wherein the phosphor contains fluorescent material represented by a general formula (Rel-rSmr)3(Al1-sGas)5O12:Ce, where 0≤r<l and 0≤s≤1 and Re is at least one selected from Y and Gd.
4 The respondent denies infringement and has cross-claimed seeking revocation of claims 1 and 3 under s 138(1) of the Patents Act 1990 (Cth) (the Act) on the grounds that, at the priority date:
the invention, as claimed in each claim, was not novel;
the invention, as claimed in each claim, was obvious and did not involve an inventive step;
the invention, as claimed in each claim, was not a manner of manufacture within the meaning of section 6 of the Statute of Monopolies;
the invention, as claimed in each claim, was not fairly based on the matter described in the specification; and
the invention, as claimed in each claim, was not defined and was not clear.
5 The relevant specification (AU 199736355 C) is entitled “Light emitting device and display device” (the specification).
6 The priority date for each claim is, relevantly, 29 July 1996 (the priority date). The priority date was determined as a separate question: Nichia Corporation v Arrow Electronics Australia Pty Ltd [2015] FCA 699 (Reasons 1).
7 For the reasons that follow, I have concluded that the applicant’s case on infringement has not been established. I have also concluded that the respondent’s case on invalidity has not been established.
8 At the present hearing, the applicant adduced evidence from:
Kenneth Scott Alexander Butcher;
Andries Meijerink;
Genichi Shinomiya; and
Michael Kramer.
9 The respondent adduced evidence from:
Eric Colin Bretschneider;
Kuang-Mao Lu; and
Stefan Richter.
10 Dr Butcher, Professor Meijerink and Dr Bretschneider were called as experts. They made affidavits and gave concurrent evidence. Their affidavits were read subject to agreed rulings. Each deponent was separately cross-examined. I summarise aspects of their evidence in later paragraphs of these reasons. Their evidence was directed primarily to the respondent’s case on invalidity, in particular its case on obviousness. For present purposes, I record the following background matters concerning each expert.
11 Dr Butcher is the President and Chief Scientist of a Canadian corporation, Meaglow Ltd, which he founded in 2009 to develop Migration Enhanced Afterglow (MEAglow) and plasma-based technology for use in the semiconductor industry. He holds the degrees of Bachelor of Applied Science in Physics (Second Class Honours, Division 1), which was conferred by the University of Technology, Sydney in 1985, and Doctor of Philosophy, which was conferred by Macquarie University in 1997. His research for his doctoral degree was in the area of nitride compound semiconductors.
12 Between 1999 and 2005, Dr Butcher was a Research Fellow, and then an Australian Postdoctoral Fellow, in Macquarie University’s Physics Department. His principal area of research was the growth and characterisation of nitride semiconductors and their fabrication into test devices. He established Macquarie University’s Low Temperature Nitride Growth Facility.
13 Between 2005 and 2008 he founded a number of start-up companies to commercialise his research into the growth of nitride semiconductors at low temperatures and to develop plasma source systems for nitride film growth.
14 In 2009, he moved to Canada. Between 2010 and 2014 he was an Adjunct Professor in the Electrical Engineering Department of Lakehead University in Thunder Bay, Ontario, where he is now located.
15 Dr Butcher is the author of more than 100 refereed journal articles and conference papers. He is named as an inventor or co-inventor in seven international patent applications.
16 Professor Meijerink holds the Chair of Solid State Chemistry in the Department of Chemistry at Utrecht University in the Netherlands. Amongst his academic qualifications, Professor Meijerink holds the degrees of Master of Science cum laude and Doctor of Philosophy cum laude. These degrees were conferred on him by Utrecht University in 1986 and 1990, respectively. As part of the work undertaken for his Master’s degree, Professor Meijerink conducted detailed research on Y2O3:Eu3+, the red phosphor used in fluorescent tubes. As part of his doctoral degree, Professor Meijerink conducted research on X-ray storage phosphors used for digital x-ray imaging.
17 Professor Meijerink has had over 30 years’ experience as a researcher, author, teacher and inventor in the field of luminescence spectroscopy, with a particular focus on phosphor materials.
18 Professor Meijerink has been retained by the applicant in proceedings conducted in the Federal Patent Court in Germany (the FPC). I have referred to these proceedings in Nichia Corporation v Arrow Electronics Australia Pty Ltd (No 3) (2016) 240 FCR 13; [2016] FCA 466 (Reasons 3), when dealing with the admissibility of certain evidence sought to be adduced by the respondent. In broad terms, the European patent in the FPC proceeding corresponds to the patent in this proceeding, at least insofar as it claims, amongst other things, an LED containing YAG:Ce, which is capable of emitting white light.
19 Dr Bretschneider is a chemical engineer and the Chief Technical Officer of EB Designs & Technology LLC, which Dr Bretschneider described as a company which specialises in the design and development of innovative solid-state lighting technology. He holds the degrees of BSE in Chemical Engineering, which was conferred by Tulane University in 1989, and Doctor of Philosophy, which was conferred by the University of Florida in 1997.
20 Following his graduation from Tulane University, Dr Bretschneider commenced postgraduate research at the University of Florida. His research investigated the use of ZnSe as a semiconductor for the emission of blue light for use in LEDs. The research for his doctoral degree was in red light emitting ZnS Si semiconductor structures.
21 From 1989 to 1996, he trained graduate students and postdoctoral researchers in MOCVD (metal organic chemical vapour deposition) growth systems, and the technology and theory behind those systems. MOCVD is a method of growing semiconductor layers prior to processing (fabricating) those layers into semiconductor chips.
22 In 1990, Dr Bretschneider was a visiting researcher at the AT&T Bell Labs Holmdel Complex in New Jersey (AT&T).
23 From around 1993 to 1996, Dr Bretschneider worked on a variety of different projects through the Phosphor Technology Center of Excellence, a consortium between several universities in the United States of America and private enterprise. These projects included the development of phosphors for plasma televisions and the synthesis of phosphors for electroluminescence research and backlights for liquid-crystal displays.
24 Dr Bretschneider said that his fields of interest before the priority date included the fabrication of new kinds of semiconductor materials and their use as LEDs.
25 Dr Bretschneider has been retained by companies, including Everlight Electronics Co., Ltd (Everlight), to provide expert evidence in proceedings in which those companies were or are parties opposed to the applicant.
26 I should also record that evidence from another expert, Roger John Reeves, was received at the hearing of the separate question. Professor Reeves is a Professor in the Department of Physics and Astronomy, University of Canterbury, Christchurch, New Zealand. He made three affidavits (9 April 2015; 14 May 2015 and 10 June 2015). Certain parts of his first affidavit, and his second and third affidavits, were read at the hearing of the separate question and are referred to at [120]-[127] of Reasons 1. His evidence was taken as being before me at the present hearing.
27 Mr Shinomiya is the applicant’s Managing Director. He is also the Deputy Operating Manager of the Development Division of the applicant’s Optoelectronics Business Unit as well as the General Manager of the applicant’s Yokohama Technology Center and Suwa Technology Center. He made an affidavit concerning the applicant’s development of the white LED. Mr Shinomiya was cross-examined through an interpreter.
28 Dr Kramer is the Managing Director of LED Linear GmbH, a company located in North Rhine-Westphalia, Germany. In 2000, he was appointed as the co-Managing Director of Vossloh-Wustlich Opto GmbH & Co. KG (Vossloh-Wustlich) following the acquisition by Vossloh AG (a German manufacturer of lighting components and transport technology) of Wustlich Mikro-Elektronik GmbH (Wustlich Mikro) and Wustlich Opto-Elektronik GmbH (Wustlich Opto).
29 He made an affidavit in which he deposed to certain events related to the proceeding in the FPC to which I have referred including, in particular, a conversation he had with his co-Managing Director, Hans-Dieter Wustlich, concerning certain correspondence which is important to the respondent’s case that claims 1 and 3 of the patent are invalid on the ground that the invention was not novel at the priority date. I refer to this evidence in greater detail below. Dr Kramer was cross-examined. Part of his oral evidence was given with the aid of an interpreter.
30 Mr Lu is a chemical and materials engineer who is employed by Everlight. Everlight made the products which, it is alleged, the respondent supplied in infringement of claim 3 of the patent. He made an affidavit in which he identified and described the fluorescent materials present in the products concerned. Mr Lu was not cross-examined.
31 Dr Richter is an attorney-at-law admitted to practise in Germany. His firm acts for Everlight in the FPC proceeding and in related appeal proceedings. He made an affidavit in which he described certain events relating to the FPC proceeding. Dr Richter was not cross-examined.
32 At [9]-[33] of Reasons 1, I provided a brief scientific background based on a primer that had been prepared by the parties for use in determining the separate question. The parties have made some amendments to the primer to provide greater precision and clarity, and have added some sections that are pertinent to understanding the scientific issues that arise in determining the remaining questions in the proceeding. The supplementary primer has been admitted into evidence. It is reproduced in the Schedule to these reasons. The parties accept that the amendments to, and additions in, the supplementary primer do not affect the question of the correct priority date that has been determined. These reasons proceed on an assumed knowledge and understanding of the supplementary primer (the primer).
33 At this point, it is convenient to draw attention to two of the ways in which white light can be characterised.
34 The first is colour temperature. The colour temperature of a light source refers to the temperature at which a black-body radiator (see paragraph 69 of the primer) radiates visible light to which the colour of the light source can be compared. By this measure, white light can be neutral (3,500-4,000 degrees Kelvin (K)), cool-white (a bluish white) (5,000 K or higher), and warm-white (a yellowish white) (2,700-3,000 K).
35 The second is the colour rendering index (CRI). This is a quantitative measure of the ability of a light source to reveal the colour of an object compared with an ideal, natural light source. It allows one to say how “natural” colours look when viewed under the light source. The maximum CRI is 100. For industrial lighting, a CRI of >60 is usually acceptable; >70 is usually acceptable for street lighting, and >80 is usually acceptable for house lighting. A CRI of ≥90 is used for specialty applications, such as museum display lighting and medical equipment.
36 The specification discloses that various attempts have been made to make white light sources using light emitting diodes. It is common to refer to light emitting diodes as LEDs. I will do so in these reasons, although I note that the specification uses “LED” as the acronym for a “light emitting device”. The specification uses the expression “light emitting device” to refer to a phosphor in combination with a “light emitting component” where the phosphor of the device converts the wavelength of the light emitted by the light emitting component. In other parts, the specification appears to use the expression “light emitting device” synonymously with a “light emitting diode”. I do not think that anything turns on this. The specification identifies the “light emitting component” of the device as a nitride compound semiconductor.
37 The specification refers to the advantages of LEDs as a light source: they are compact and emit light of a clear colour with high efficiency; they are free from “burn-out” and have good initial drive characteristics; they have high vibration resistance, and high durability to endure repetitive on/off operations. The specification explains, however, that, although LEDs are effective as light emitting devices for generating monochromatic light (such as red, green and blue), a satisfactory light source capable of emitting white light using these components has not been obtained.
38 The specification discloses that the applicant had previously developed LEDs which use a fluorescent material to convert the colour of light that is emitted by the light emitting component. The specification identifies a number of patents held by the applicant which, the specification says, disclose LEDs that are capable of generating white light and other colours. The specification describes these LEDs as follows:
The light emitting diode … are made by mounting a light emitting component, having a large energy band gap of light emitting layer, in a cup provided at the tip of a lead frame, and having a fluorescent material that absorbs light emitted by the light emitting component and emits light of a wavelength different from that of the absorbed light (wavelength conversion), contained in a resin mold which covers the light emitting component.
The light emitting diode disclosed as describe above capable of emitting white light by mixing the light of a plurality of sources can be made by using a light emitting component capable of emitting blue light and molding the light emitting component with a resin including a fluorescent material that absorbs the light emitted by the blue light emitting diode and emits yellowish light.
(As in original.)
39 The specification says that these “conventional” LEDs have problems. These problems centre on the deterioration of the fluorescent material. The deterioration arises from various causes.
40 First, there is deterioration of the fluorescent material arising from the amount of light energy the material absorbs from the light emitting component. The specification discloses that this is a problem with organic phosphors and some inorganic phosphors ((Cd,Zn)S is exemplified). The deterioration of the fluorescent material leads to colour tone deviation of the emitted light. It also leads to darkening of the material, which results in lowered efficiency in terms of “extracting light”.
41 Secondly, there is deterioration brought about by the high temperature of the LED and heat transmitted from the external environment, such as sunlight when the device is used outdoors.
42 Thirdly, some fluorescent materials are subject to accelerated deterioration due to a combination of moisture (whether introduced from the outside or during the production process) and the light and heat transmitted from the light emitting component.
43 The specification also teaches that, where the fluorescent material is an organic dye, electrophoresis may result in a change in the colour tone of the emitted light.
44 The specification says that the invention aims to provide an alternative to known light emitting devices and displays, which alleviates at least one of the described problems to provide a light emitting device which experiences only extremely low degrees of deterioration in emission light intensity, light emission efficiency and colour shift over a long time of use, with high luminance.
45 The specification describes one aspect of the invention as a light emitting device that includes a nitride compound semiconductor component in combination with a phosphor that contains a garnet fluorescent material. The garnet fluorescent material must include certain identified elements. It is activated with Ce.
46 I pause to note that it is accepted that the nitride compound semiconductor emits blue light. The phosphor—the described garnet fluorescent material—is excited by the blue light emitted from the light emitting component, and emits yellow light. There is no dispute that blue light has a wavelength of approximately 450 nanometres (nm) and that yellow light has a wavelength in the range of approximately 500 to 700 nm. Thus, in the invention, the wavelength of the light emitted from the phosphor is longer than the wavelength of the light emitted from the light emitting component that is absorbed by the phosphor. The conversion of light from one wavelength to another is called spectral conversion.
47 The specification teaches that, generally, a fluorescent material which absorbs light of a short wavelength and emits light of a long wavelength (the specification is here discussing relativities) has higher efficiency than a fluorescent material which absorbs light of a long wavelength and emits light of a short wavelength.
48 The specification also teaches that it is preferable to use a light emitting component that emits visible light rather than ultraviolet (UV) light, because UV degrades the resin which is used as a moulding or coating material in the housing of the LED, which also includes the phosphor of interest embedded in the resin that coats the light emitting component. To this end, the specification teaches that it is preferable that the main emission peak of the light emitting component be set within a relatively short wavelength range of 400 nm to 530 nm, in the visible light region.
49 The blue light and the yellow light emitted by the light emitting component and the phosphor respectively, blend to produce white light. In its evidence and submissions, the applicant referred to a blue LED combined with fluorescent material to produce a white light source as a white LED. I will use the same expression.
50 For this embodiment, the specification expresses a preference for a phosphor that is an yttrium-aluminium-garnet fluorescent material (YAG phosphor) activated with Ce. The fluorescent material having the general formula in claim 3 is specifically referred to amongst other formulae: see [3] above. Although the specification uses a number of formulae, I will refer to the formula in claim 3 as the general formula.
51 The specification teaches that the wavelength of the light emitted from the YAG phosphor can be shifted to a shorter wavelength by substituting part of the Al in the phosphor with Ga. In this connection, the general formula comprehends the possibility of having only Al (and no Ga), or only Ga (and no Al), or a combination of both, in the phosphor.
52 Further, the general formula requires that the phosphor also include Y or Gd, or both. Sm can be present with the Y and/or Gd, but not alone. The specification teaches that the wavelength of the light emitted from the YAG phosphor can be shifted to a longer wavelength by substituting part of the Y in the phosphor with Gd.
53 Thus, the light colour emission can be changed continuously by changing the composition in the ways described immediately above. The specification also teaches that the addition of Sm will improve the efficiency of the light emission.
54 A second aspect of the invention is described in which a nitride compound semiconductor (represented by a given formula) is used in combination with a phosphor that contains one, two or more garnet fluorescent materials according to the general formula subject to the additional requirement that r≠0.
55 A third aspect of the invention is described in which a nitride compound semiconductor (represented by a given formula) is used in combination with a first fluorescent material (represented by a given formula) and a second fluorescent material (represented by another given formula).
56 A fourth aspect of the invention is described. It is a method of preparing a white light emitting device.
57 The following matters should be noted.
58 First, so far as fluorescent materials are concerned, the invention described in the specification is directed to the use of garnet fluorescent materials of a particular kind. Attention is directed to Y3Al5O12:Ce (YAG:Ce), or forms of that phosphor with substituents or additions, as the specified phosphor. Twelve examples of the use of such a phosphor are provided. In some examples, two such fluorescent materials are used. There are two comparative examples. The comparative examples do not use a garnet fluorescent material.
59 Comparative Example 1 concerns the use of cadmium zinc sulphide (Cd,Zn)S as the fluorescent material. As I have noted, the specification teaches that, in use, this material darkens and leads to lowered light-extracting efficiency. Nevertheless, the LED formed with this material showed, immediately after energisation, the emission of white light, albeit with low luminescence. Thus, it provided a white LED but not one that was satisfactory according to the teaching of the specification. I will refer again to this example when discussing the applicant’s work directed to developing a white LED.
60 Comparative Example 2 concerns the use of two organic dyes rather than garnet fluorescent materials. As I have noted, the specification teaches that when organic dyes are used, electrophoresis may occur, resulting in a change in the colour tone of the emitted light. It is implicit in the description of Comparative Example 2 that white light was emitted. A weatherability test (equivalent to irradiating the material with sunlight for one year) and a reliability test (energising the material to emit light at a constant temperature of 70° C while measuring luminance and colour tone at different times) were carried out. When the LED of Comparative Example 2 was compared with the LED of Example 9, which used a combination of garnet fluorescent materials, the LED of Example 9 experienced less deterioration. Once again it would seem that the specification does not accept that Comparative Example 2 provides a satisfactory white LED.
61 Secondly, although the general formula of claim 3 covers a phosphor containing Sm as well as Y and/or Gd, and Ga as well as, or in substitution for, Al, it includes the phosphor YAG:Ce. Therefore, it can be said that, at its simplest, the light emitting device claimed in claim 3 is one in which a nitride compound semiconductor (the light emitting component) is combined with YAG:Ce.
62 Thirdly, claim 3 does not, in terms, require the creation of white light, nor white light of any particular nature, quality or colour rendering. Nor does it require that the light emitting device be suitable for any particular application; there are no requirements of stability, durability, efficiency or performance expressed as essential features of the invention.
63 Nevertheless, the specification makes clear that the invention is a light emitting device that emits white light. The present case is an example of where the context provided by the specification rises up to insist that claims 1 and 3 are directed to such a device: International Business Machines Corporation v Smith, Commissioner of Patents (1992) AIPC 90-853 at 38,160-38,161. The specification teaches that the wavelength of the emitted light (and hence its colour rendering) can be varied by choices made within the scope of the general formula.
64 Further, the specification makes clear that an object of the invention is to provide a light emitting device with high luminescence, and which experiences only extremely low degrees of deterioration in emission light intensity, light emission efficiency and colour shift over a long period of use.
65 In this connection, the specification states:
The present applicant completed the present invention through researches based on the assumption that a light emitting device having a light emitting component and a fluorescent material preferably meets the following requirements to achieve the above-mentioned object.
(1) The light emitting component is preferably capable of emitting light of high luminance with light emitting characteristic which is stable over a long time of use.
(2) The fluorescent material being provided in the vicinity of the high-luminance light emitting component, preferably shows excellent resistance against light and heat so that the properties thereof do not change even when used over an extended period of time while being exposed to light of high intensity emitted by the light emitting component (particularly the fluorescent material provided in the vicinity of the light emitting component is exposed to light of a radiation intensity as high as about 30 to 40 times that of sunlight according to our estimate, and is required to have more durability against light as light emitting component of higher luminance is used).
(3) With regard to the relationship with the light emitting component, the fluorescent material is preferably capable of absorbing with high efficiency the light of high monochromaticity emitted by the light emitting component and emitting light of a wavelength different from that of the light emitted by the light emitting component.
(As in original.)
66 It can be taken that each embodiment of the invention that is claimed is a light emitting device (whether as an LED, a display device or some other light emitting device), or a method of preparing a light emitting device, that is directed to meeting these requirements. This is not to say that these requirements are imported as essential features of the invention that is claimed—as if the claims were limited by result. It does acknowledge, however, that the invention is directed to a white light emitting device that should attain these preferable (in the sense of desirable) attributes. These are the “promises” that the specification makes.
An issue of construction: infringement
67 There is an issue of construction concerning claim 3 which is determinative of the question of infringement.
68 Claim 3 requires that the light emitting device include a phosphor capable of absorbing a part of light emitted by the light emitting component and emitting light of a wavelength different from that of the absorbed light, “wherein the phosphor contains fluorescent material” (my emphasis) represented by the general formula.
69 The applicant submitted that, while the integers of claim 3 are essential features of the invention that is claimed, those integers are not exhaustive of the things that can make up the claimed light emitting device. The applicant argued that “contains” is used in claim 3 in an inclusive sense. Thus, the claimed device is not limited to the use of a single phosphor, or perhaps more accurately, a single fluorescent material. The applicant submitted that it is essential that the device includes fluorescent material of the stated formula, but other phosphor compounds can be present. Thus, if fluorescent material of the general formula is present, infringement cannot be avoided by adding other fluorescent material to the light emitting device.
70 The respondent submitted that, properly construed, claim 3 only permits a phosphor of the stated formula. It argues that “contains” must be construed in an exclusive sense. The respondent also argued that, even if the word “contains” is construed in an inclusive sense—so as to contain something other than the fluorescent material (such as the resin with which it is mixed)—the fluorescent material cannot contain fluorescent material other than the garnet fluorescent material of the general formula. The respondent submitted that this construction is consistent with the whole of the specification. It further argued that there is no description or suggestion of any embodiment in which the light emitting device contains any fluorescent material other than garnet fluorescent material. It argued that the specification makes plain that it is the use of garnet fluorescent material that will achieve the advantages promised for the invention in the specification.
71 When read in isolation, the word “contains” is capable of supporting either construction. The question is, in what sense is “contains” used in claim 3?
72 The parties referred to a number of standard authorities on claim construction.
73 The respondent referred to Welch Perrin & Co Pty Ltd v Worrel (1961) 106 CLR 588 at 609-610 for the proposition that the claims of a patent must be construed in the context of the specification as a whole.
74 The applicant emphasised that a patent should be given a purposive construction rather than a purely literal one: Decor Corporation Pty Ltd v Dart Industries Inc (1988) 13 IPR 385 at 400; Catnic Components Ltd v Hill & Smith Ltd [1982] RPC 183 at 242-243; H Lundbeck A/S v Alphapharm Pty Ltd (2009) 177 FCR 151 at [118]-[129]. The applicant submitted that a construction that would lead to a foolish result, or one that the patentee could not have contemplated, is to be avoided in favour of another construction that would lead to the opposite result: Electric & Musical Industries Ltd v Lissen Ltd [1938] 4 All ER 221 at 224-227. Relatedly, the applicant relied on Jacob J’s injunction in Tickner v Honda Motor Co Ltd [2002] EWHC 8 at [28] that, in determining the “purpose of the patent” one must be “fair to the patentee”. This observation must be seen in its context, where Jacob J was discussing “purposive construction”. The full passage should be quoted to understand what Jacob J meant by being “fair to the patentee”:
… You learn the inventor’s purpose by understanding his technical contribution from the specification and drawings. You keep that purpose in mind when considering what the terms of the claim mean. You [choose] a meaning consistent with that purpose – even if that involves a meaning which, acontextually, you would not ascribe to the word or phrase. Of course in this exercise you must also be fair to the patentee – and in particular must not take too narrow a view of his purpose – it is the widest purpose consistent with his teaching which should be used for purposive construction.
75 Here, the applicant said, the purpose the invention is encapsulated by the Abstract appended to the patent application, which refers to:
… a white light emitting diode having high luminance and a light emitting characteristic which is not deteriorated even when the diode is used for a long period of time….
76 The applicant also called in aid a line of cases which are to the effect that infringement cannot be avoided by merely adding features to a claimed combination. The applicant placed particular significance on the following statement by the Full Court in Fresenius Medical Care Australia Pty Ltd v Gambro Pty Ltd (2005) 224 ALR 168; [2005] FCAFC 220 (Fresenius) at [70]:
… The inclusion of additional integers to a claimed combination does not necessarily avoid infringement. If those additional integers are properly characterised as inessential or do not make a new working of the combination and all of the essential integers of the claimed combination are present, there will be infringement. Where a patentee defines the claimed invention as consisting of a number of essential integers, it is no answer to infringement that the claimed combination is taken but additional integers are added that do not affect and are not part of the invention.
77 The applicant relied on similar statements in Seafood Innovations Pty Ltd v Richard Bass Pty Ltd (2011) 92 IPR 1; [2011] FCAFC 83 at [24] and Bitech Engineering v Garth Living Pty Ltd (2010) 86 IPR 468; [2010] FCAFC 75 at [30]. The applicant also referred to Bowen LJ’s aphorism in The Wenham Gas Company v The Champion Gas Lamp Company (1891) 9 RPC 49 that the “superadding of ingenuity to a robbery does not make the operation justifiable”.
78 The applicant’s reliance on this line of authority cannot be used to support its submissions on the question of construction. The question of claim construction is separate from, and anterior to, the question of infringement. Further, one does not construe the claims of a patent with one eye on the product that is said to be an infringement.
79 The light emitting device claimed in claim 3 is one in which the phosphor is a single fluorescent material represented by the general formula. Claim 3 does not claim a device in which the phosphor is the defined fluorescent material together with one or more other fluorescent materials.
80 The specification clearly points to this construction. In describing the invention, the specification takes as one of its starting points the fact that, even though LEDs of a “conventional” kind (i.e., as described in the specification) are capable of emitting white light by combining the blue light of the LED with a fluorescent material that absorbs this light and emits yellowish light, deterioration of the fluorescent material is a problem. The problem thus presented is one directed to the characteristics of the fluorescent materials that are used, whether those materials are organic or inorganic phosphors or some other similarly functioning material. Principally, the invention is directed to overcoming this problem.
81 As I have noted, so far as fluorescent materials are concerned, the invention described in the specification is directed to garnet fluorescent materials of a particular kind. The broadest definition of these materials is provided in claim 1. Claim 3 is directed to a more particular subset of these materials. The consistory statement for claim 3 (at page 8, lines 13-17) says:
In the light emitting device of the present invention, the phosphor may be a fluorescent material represented by a general formula (Re1-rSmr)3(Al1-sGas)5O12:Ce, where 0≤r<1 and 0≤s≤1 and Re is at least one selected from Y and Gd, in which case good characteristics can be obtained similarly to the case where the yttrium-aluminium-garnet fluorescent material is used.
82 A similar statement appears at page 11, lines 20-23 of the specification.
83 It is clear that each consistory statement is talking about specific fluorescent material. Each statement describes the phosphor as a fluorescent material of the stated formula. The consistory statements do not even suggest, let alone say, that the phosphor can be anything other than the specifically stated fluorescent material defined by the general formula.
84 Claim 3 should be read in that light. The person skilled in the art, reading the specification as a whole, with its description of various specific embodiments of the invention, would understand that claim 3 claims the light emitting device described in the consistory statement on which it is based.
85 Significantly, the consistory statement at page 8, lines 13-17 of the specification promises that, when this specific fluorescent material is used, “good characteristics can be obtained”. These characteristics are said to be similar to those obtained when YAG:Ce is used (it being remembered that YAG:Ce itself falls within the general formula of claim 3, although material other than YAG:Ce is covered).
86 In this connection, the specification teaches that YAG:Ce:
… has excellent resistance against light so that the fluorescent properties thereof experience less change even when used over an extended period of time while being exposed to light of high intensity. This makes it possible to reduce the degradation of characteristics during long period of use and reduce deterioration due to light of high intensity emitted by the light emitting component as well as extraneous light (sunlight including ultraviolet light, etc.) during outdoor use, thereby to provide a light emitting device which experiences extremely less color shift and less luminance decrease. The light emitting device of the present invention can also be used in such applications that require response speeds as high as 120 nsec., for example, because the phosphor used therein allows after glow only for a short period of time.
(As in original.)
87 This teaching strikes at the problems said to be associated with the “conventional” LEDs, which the invention seeks to overcome. The promise is that use of the fluorescent material defined by the general formula in claim 3 will achieve similarly good characteristics. The promised characteristics are secured by the word “contains” in claim 3.
88 In a later part of the specification (at page 36, lines 4-12), further reference is made to this embodiment and its promised advantages:
As for the fluorescent material, a fluorescent material represented by general formula (Re1-rSmr)3(All-sGas)5O12:Ce, may also be used as the phosphor. Here 0≤r<1 and 0≤s≤1, and Re is at least one selected from Y, Gd and La. This configuration makes it possible to minimize the denaturing of the fluorescent material even when the fluorescent material is exposed to high-intensity high-energy visible light emitted by the light emitting component for a long period of time or when used under various environmental conditions, and therefore a light emitting diode which is subject to extremely insignificant color shift and emission luminance decrease and has the desired emission component of high luminance can be made.
89 It will be noted that, in this embodiment, Re can be La. Nonetheless, the description of the fluorescent material plainly covers material of the general formula and speaks of the advantages of using that material.
90 In context, “contains”, as used in claim 3, can only be a reference to the defined fluorescent material as the phosphor of the claimed device, not some combination involving, for example, other fluorescent materials about which the specification says nothing and about which it can make no promises as to their characteristics when used in a light emitting device containing the nitride compound semiconductor as the light emitting component.
91 This construction is supported by other aspects of the description of the invention. In particular, when a specific embodiment of the invention can contain more than one fluorescent material as the phosphor, the specification says so; in these cases, the specification describes the materials that can be used. This assists with the meaning of “contains” when used throughout the claims.
92 In arguing for its construction, the applicant pointed to the use of the word “including” in claim 1 (on which claim 3 is dependent). The applicant submitted that the use of “including” shows that the essential integers of claim 3 are not exhaustive of the features of the device that is claimed.
93 I do not accept the purport of this submission. The word “including” shows that components of the light emitting device, other than the light emitting component and the phosphor, may be present. Indeed, it is accepted that other componentry must be present in order for the light emitting device to function. But claim 1 then proceeds with greater detail to specify the characteristics of the light emitting component and the phosphor. At this point, the effect of the word “including” is spent in relation to those materials: the light emitting component and the phosphor are defined. Claim 3 proceeds with even greater detail to specify the phosphor to be used by reference to the stated fluorescent material.
94 The applicant submitted that the construction I have found fails to give meaning to the distinction drawn in claim 3 between the phosphor and its constituent fluorescent material. I accept that claim 3 makes this distinction. I do not accept that the construction I have found fails to give meaning to it. The use of the phosphor in claim 3 is derived from the use of the same words in claim 1. Their use in claim 3 is integral to confining the phosphor of claim 1 to the specific fluorescent material stated in claim 3.
95 The applicant referred to claim 6 in aid of its construction. Claim 6 is:
A light emitting device according to claim 3, wherein the phosphor contains two or more fluorescent materials of different compositions represented by a general formula (Re1-rSmr)3(All-sGas)5O12:Ce, where 0≤ r<1 and 0≤s≤1 and Re is at least one selected from Y and Gd.
96 The applicant pointed to the fact that claim 6 is expressed to be dependent on claim 3 and, for that reason, must be narrower than claim 3. The applicant then argued that claim 6 had been narrowed “to exclude a phosphor containing only one fluorescent material”.
97 This submission does not assist the applicant’s construction of claim 3. Claim 6 confirms the construction I have found. The invention of claim 6 is distinguished from the invention of claim 3 in that, in claim 6, two or more fluorescent materials represented by the general formula are used as the phosphor. This confirms that, in claim 3, the words “fluorescent material” are used advisedly to refer to a single fluorescent material, being material represented by the general formula. The applicant’s construction, which proceeds on the basis that the phosphor merely include fluorescent material represented by the general formula, would suggest that claim 6 is largely redundant because, from the perspective of infringement, claim 3 would also do the work of claim 6. This cannot have been intended by the drafter of the specification. Claim 6 is an example of where the specification is specific when more than one fluorescent material can be used as the phosphor. Claims 7 and 8 provide further examples. Claim 3 confines the phosphor to a specific material (i.e., material within the general formula). Claims 6, 7 and 8 each confine the phosphor to specific materials (i.e., each material of a different composition but nevertheless within the general formula).
98 The applicant called in aid NV Philips Gloeilampenfabrieken v Mirabella International Pty Limited (1993) 44 FCR 239 (Philips (FCAFC)) at 259 to support its construction of claim 3 by relying on the Full Court’s construction of another claim in another patent, which concerned another light emitting device—a low-pressure mercury vapour discharge lamp. The applicant’s reliance on Philips (FCAFC) does not advance its case in this regard. In the present case, the task of the Court is to construe claim 3 in the particular context of the specification in suit. In any event, in construing the patent in Philips (FCAFC), Lockhart J (with whom the other members of the Full Court agreed) had regard to the fact that, if the luminescent layer of the discharge tube in that invention were to be confined to one phosphor or a single chemical compound, the object of the invention could not be achieved. There is no evidence before me of any similar consideration in this case. Indeed, the teaching of the specification is to the contrary.
99 The applicant also pointed to two passages in the specification which, it submitted, supported the contention that “contains” in claim 3 is used inclusively, so as not to exclude the presence of other fluorescent materials. These passages concern an embodiment of the invention which the specification refers to as “Embodiment 2” or “the second embodiment”.
100 The first passage is page 36, lines 15-19 of the specification:
Now the phosphor used in the light emitting component of the second embodiment will be described in detail below. The second embodiment is similar to the first embodiment, except that two or more kinds of phosphors of different compositions activated with cerium are used as the phosphor, as described above, and the method of using the fluorescent material is basically the same.
101 The second, earlier, passage is at page 35, lines 15-20 of the specification:
The light emitting diode of the second embodiment of the present invention is made by using an element provided with gallium nitride compound semiconductor which has high-energy band gap in the light emitting layer as the light emitting component and a fluorescent material including two or more kinds of phosphors of different compositions, or preferably yttrium-aluminum-garnet fluorescent materials activated with cerium as the phosphor. …
102 The applicant placed particular emphasis on the fact that the second passage expresses no more than a preference that YAG phosphor activated by Ce be used, at least in this embodiment. In essence, the applicant’s contention was that these passages show that more than one phosphor can be used in an embodiment of the invention and that, when using a combination of phosphors, YAG phosphors activated by Ce are preferred but not essential.
103 There is, of course, no denying that the word “preferably” is used in the second passage. Considered in isolation, it appears to give the words “… a fluorescent material including two or more kinds of phosphors of different compositions ...” wide scope. However, other passages of the specification dealing with the same embodiment make clear that this is an embodiment in which two YAG phosphors activated by Ce are used. I refer, in particular, to page 37, lines 11-24; page 37, line 25 to page 38, line 2; page 38, line 21 to page 39, line 6; page 39, lines 7-16; and page 39, line 24 to page 40, line 1. When these passages are read with the two passages quoted above, it is clear that the word “preferably” is used infelicitously and cannot be accorded its literal meaning. I am satisfied that the person skilled in the art would understand the second embodiment as one in which two YAG phosphors activated by Ce are used, not merely used preferentially. I am not persuaded, therefore, that these passages assist the applicant’s case on the proper construction of claim 3 which, in any event, claims a different embodiment of the invention based on other passages in the specification.
104 The applicant alleges, and the respondent accepts, that the respondent has exploited, in the patent area, the following white LED products manufactured by Everlight:
Model number 62-217D/QK2C-S5050R1R3B42Z15/2T (Device A);
Model number 62-217D/QK2C-S5757R1R3B42Z15/2T (Device B);
Model number 62-217D/QK2C-S6565R1R3B42Z15/2T (Device C);
Model number JU1215-KT507N7-12507-090T (Device D);
Model number 45-21S/KK2C-S5757L1L4B2Z3/2T (Device E); and
Model number 67-21/LK2C-BX5070B3B6B2/2T (Device F).
105 The only issue on infringement that divides the parties is this: although each product includes a fluorescent material that is YAG:Ce, which is within the general formula of claim 3, each product also includes other fluorescent material that is not within the general formula. Based on Mr Lu’s evidence (see [30] above), which I accept, Devices A to E include, in addition, a garnet fluorescent material that is not within the general formula and one or more other, non-garnet fluorescent materials. Exceptionally, Device F includes, in addition, only a non-garnet fluorescent material.
106 Given that each product includes YAG:Ce, does it follow that, in each case, infringement of claim 3 has been established? Based on the construction I have found, the answer to this question is “no”.
107 The applicant’s case is not advanced by reliance on statements on infringement such as that found in Fresenius at [70]. The question is whether all the essential features of the invention claimed in claim 3 are present in an accused product. All the essential features are not present because claim 3’s requirements as to the phosphor are not met.
108 As I have said, the light emitting device of claim 3 is one in which the phosphor is a single fluorescent material represented by the general formula. As a matter of definition, the light emitting device of claim 3 does not extend to one where the phosphor contains two or more fluorescent materials, even if one of them is fluorescent material represented by the general formula. The present case is an instance where full force is to be given to the principle that matter that is not claimed is disclaimed. Put simply, none of the accused products accords with the definition of the light emitting device claimed in claim 3.
109 This is not to give claim 3 an unduly narrow construction. Claim 3 is of a scope that provides for a choice in the composition of the fluorescent material whilst adhering to the general formula. In other words, the general formula permits adjustments and substitutions to be made. Thus, the invention can be practised in a way that allows the user, by permitted adjustments and substitutions, to control the wavelength of the emitted light of the device, whilst at the same time attaining the desirable attributes that are promised in the specification for this particular embodiment.
110 For these reasons, the applicant’s case on infringement has not been established and must be dismissed.
111 It is convenient at this point to say something about the construction of claim 1, even though the applicant does not put claim 1 in suit. The construction of claim 1 arises in the context of the respondent’s challenge to validity based on s 40 of the Act.
112 As I have noted, claim 1 contains the broadest definition of the phosphor that can be used. The phosphor is a garnet fluorescent material of a particular kind. Claim 2 confines the phosphor to YAG and, as discussed, claim 3 confines the phosphor even further to fluorescent material represented by the general formula (which, amongst other things, is activated by Ce).
113 Claim 5 is dependent on claim 2 and provides a more limited embodiment where the fluorescent material is two or more YAG materials of different composition, activated with Ce. Claim 5, like claims 6, 7 and 8, is another example where the specification is specific when one or more fluorescent materials can be used as the phosphor.
114 All these claims use the word “contains” in the exclusive sense I have described when construing claim 3. When “contains” is used with respect to the phosphor, it means that the phosphor in the light emitting device is the fluorescent material specified in the relevant claim, not some other phosphor or admixture of fluorescent materials.
115 I now turn to consider the respondent’s case on invalidity more generally. It is convenient to start with the evidence concerning the development of the claimed invention.
THE DEVELOPMENT OF THE INVENTION
116 In 1991, the applicant’s LED Development Team developed a gallium nitride (GaN) LED which emitted near-UV light and violet light. The luminance of the emitted light was not good. With the aim of improving the luminance, the LED Development Team combined the LED with fluorescent materials. This was the first time that this idea had been raised within the applicant. This work led to Japanese Patent 5-152609, filed on 25 November 1991. This patent is referred to on page 2 of the specification.
117 In November 1993, the applicant announced that it had developed the world’s first high-brightness blue LED using a GaN semiconductor. Before this announcement, high-brightness blue LEDs had been made available within the applicant for research purposes. The development of this LED was a significant scientific achievement. One of the inventors, Shuji Nakamura, received the Nobel Prize in Physics in 2014 for his work.
118 In 1993, the applicant established its Backlight Development Team. This team was to attempt to develop a white backlight (such as used in computer screens) by using a fluorescent material to convert the wavelength of the GaN blue LED. The team’s first efforts focused on the use of organic fluorescent materials. At that time, some established applications used organic fluorescent materials (such as organic dyes) which could be excited by visible light. As noted in paragraphs 61-62 of the supplementary primer, light that is capable of producing a visual sensation to the human eye ranges from about 360 nm to about 830 nm, and includes all colours from red to violet. However, it is common to say that the visible region of the spectrum ranges from 400 nm to 700 nm, as perception varies from person to person. The visible region of the spectrum is bounded by the UV (<400nm) and infrared (IR) (>700nm) regions. These regions are not precisely bounded, and may overlap into other regions.
119 The applicant was using organic fluorescent materials in other applications. In 1993, the LED Development Team developed a green LED using the combination of a blue LED and a green organic fluorescent material. This work led to Japanese Patent H07-99345, filed on 28 September 1993. This patent is also referred to on page 2 of specification. The lifetime of the developed green LED was only several hours because the green organic fluorescent material degraded due to the strong light and heat from the LED.
120 The first white backlight developed by the Backlight Development Team, in 1993, was a combination of a blue LED with red and green organic fluorescent materials disposed on a light guide plate. The Backlight Development Team developed an alternative combination of a blue LED with yellow and orange organic materials disposed on a light guide plate. This work led to Japanese Patent 7-176794, filed on 17 December 1993, and Japanese Patent 8-7614, filed on 17 June 1994. The developed white backlight was subsequently modified and put into practical use in 1994. This used a colour conversion sheet made of organic fluorescent materials which was placed on the light guide plate to dilute the strong light emitted from the blue LED. This resulted in a reduction of the light density because light from the blue LED was spread over the entire surface of the light guide. This helped to stabilise and reduce degradation of the organic fluorescent materials.
121 In March 1995, the applicant produced, by way of a trial, a disposable white LED for use as the light source for an endoscope. It used yellow and orange, or alternatively yellow and red, organic fluorescent materials. This application did not require the LED to have a long lifetime, simply because the light source was intended to be disposable.
122 To the present time, the applicant continues its research into organic fluorescent materials.
The development of a white LED
123 The applicant was, in 1996, and remains today, the world’s largest manufacturer of inorganic phosphors.
124 At the beginning of 1996, the President of the applicant, Mr Eiji Ogawa, gave an instruction that the applicant was to begin investigating the option of combining the blue LED with an inorganic phosphor to achieve a white LED.
125 A team was created, called the White LED Development Team. Mr Yori Shimizu, then the General Manager of the Engineering Department of the Second Division (Optoelectronics Products Business Unit), was appointed as team leader. His direct supervisor was Mr Shinomiya, who (as I have noted at [27] above) is now the Managing Director of the applicant. Mr Ogawa’s instruction was given directly to Mr Shinomiya, who shortly thereafter established the White LED Development Team.
126 In cross-examination, Mr Shinomiya gave an answer that indicated that Mr Ogawa’s instruction was given in April or May 1996, rather than at the beginning of 1996. I think that, in giving that evidence, Mr Shinomiya may well have mistaken this instruction with a later instruction given by Mr Ogawa concerning the need to extend the search for phosphors beyond the class of phosphors the applicant was investigating in April/May 1996: see below at [138]. I find that Mr Ogawa’s initial instruction was given at the beginning of 1996.
127 Mr Shinomiya said that he and Mr Shimizu “had previously been involved in many inorganic phosphor developments” for the applicant. He said that, because of that experience, he and Mr Shimizu were the people within the applicant who had the most technical knowledge concerning inorganic phosphors.
128 Mr Shinomiya said that, at this time, inorganic phosphors were classified according to their applications. There were three classifications: phosphors for televisions (electron beam excitation), phosphors for fluorescent lamps (UV-ray excitation) and phosphors for X-ray machines (X-ray excitation). Mr Shinomiya said that, despite the technical knowledge he had gained concerning inorganic phosphors, he did not, at this time, know of an inorganic phosphor that could be excited by visible light. He said that Mr Shimizu did not suggest that he knew of such an inorganic phosphor.
129 Mr Shinomiya said that he and Mr Shimizu’s approach was to search for a phosphor within those already produced by the applicant. He said that he and Mr Shimizu could have considered searching in the scientific literature for phosphors that could be excited by visible light. However, they did not adopt that approach because, even if such a phosphor could be found, they would have to synthesise it if the applicant did not already produce it. Synthesising a new phosphor required at least one year’s work.
130 Mr Shimizu suggested that the search within the phosphors that the applicant already produced should be for those with a red, green or yellow body colour because a phosphor which is excited by blue light should show some body colour under natural light. Here, it is necessary to understand that, ordinarily, a phosphor will appear white to the human eye because it reflects visible light. A phosphor which absorbs visible light and emits light of a different wavelength should appear to the human eye as a colour other than white. Mr Shinomiya said that, carrying out an inspection of this type, would only enable an initial investigation of potential phosphor candidates, rather than a conclusion that a phosphor would in fact be excited by visible light. This is because phosphor body colour is influenced by reflection as well as luminescence.
131 Mr Shinomiya said that, in another conversation, Mr Shimizu suggested, as a possibility, zinc cadmium sulphide phosphor doped with (i.e., activated by) silver (ZnCdS:Ag). Mr Shimizu’s suggestion was based on the fact that ZnCdS:Ag phosphor has a strong yellow body colour. Mr Shinomiya said that, at the time, he was aware of this fact. He said that he was also aware that ZnCdS:Ag phosphor emits yellow light when excited by an electron beam. This phosphor was available to the applicant for use in monochrome cathode ray tube (CRT) display applications.
132 Mr Shinomiya said that, accordingly, the focus of the initial investigations was to identify inorganic phosphors having a body colour, such as red, green or yellow. In early 1996, Mr Shimizu selected about 20 sulphide phosphors as candidates, including ZnCdS:Ag, from the several hundred phosphors in the company. Mr Shimizu carried out testing on the sulphide phosphor candidates. He was assisted by Mr Toshio Moriguchi, who had previously been in the Backlight Development Team. Amongst other things, Mr Moriguchi’s role during this period was to investigate the sulphide phosphor candidates and narrow the list. Mr Kensho Sakano also assisted by preparing and testing specific LED samples as requested by Mr Shimizu.
133 Based on this work, ZnCdS phosphor was confirmed as the preferred candidate. Further specific development work was carried out, including determining an appropriate ratio of CdS to ZnS in the phosphor, and determining the preferred activator. Before this work was carried out, Mr Shimizu was aware that ZnS-based phosphors were susceptible to water. He nevertheless expressed the view to Mr Shinomiya that the effect of water could be excluded in an LED application because the phosphor would be sealed with a resin. Mr Shinomiya said that, based upon other work undertaken within the applicant, he was aware that ZnS-based phosphors had good stability in CRT applications where water was not an environmental factor.
134 Specific testing was done using ZnCdS:Ag phosphor. It was found that while the phosphor produced a white colour that was acceptable, it degraded within one day.
135 Mr Shinomiya said that, given its high reliability in the CRT environment, it was very surprising to him that ZnCdS:Ag phosphor would degrade so quickly in the LED environment simply because moisture was present. Mr Shimizu’s observation, as reported to Mr Shinomiya, was that, under strong light and heat, the phosphor seemed to be blackened by the very tiny amount of water that either remained in the resin or entered from outside the resin.
136 Further development work was carried out using a ZnCdS:Cu,Al phosphor. The LED also emitted white light of an acceptable colour. This LED is, in fact, used as Comparative Example 1 disclosed at page 49, line 18 to page 50, line 9 of the specification:
(Comparative Example 1)
Formation of a light emitting diode and life tests thereof were conducted in the same manner as in Example 1 except for changing the phosphor from (Y0.8Gd0.2)3Al5O12:Ce to (ZnCd)S:Cu, Al. The light emitting diode which had been formed showed, immediately after energization, emission of white light but with low luminance. In a life test, the output diminished to zero in about 100 hours. Analysis of the cause of deterioration showed that the fluorescent material was blackened.
This trouble is supposed to have been caused as the light emitted by the light emitting component and moisture which had caught on the fluorescent material or entered from the outside brought about photolysis to make colloidal zinc to precipitate on the surface of the fluorescent material, resulting in blackened surface. Results of life tests under conditions of energization with a current of 20mA at 25°C and 20mA at 60°C with 90% RH are shown in Fig. 13 together with the results of Example 1. Luminance is given in terms of relative value with respect to the initial value as the reference. A solid line indicates Example 1 and a wavy line indicates Comparative Example 1 in Fig. 13.
(As in original.)
137 The “life test” referred to in this disclosure involves testing the product under harsher conditions than normal, for example at a temperature of 60°C and 90% humidity. The purpose is to test how the phosphor performs under conditions which accelerate its life cycle. In his affidavit, Mr Shinomiya gave the following evidence:
The problems the White LED Development Team experienced with the ZnS phosphor highlight one of the fundamental challenges involved with phosphor research. Even when a phosphor can be successfully employed in one operating environment, it cannot be assumed that it will have sufficient durability in another environment. This means that any candidate must be tested to ascertain whether or not it will actually have the required luminance and durability in a new environment. The light, heat and moisture in the LED environment can be particularly challenging in terms of durability.
138 In about late April or May 1996, Mr Ogawa visited Mr Shinomiya and Mr Shimizu. Mr Ogawa said that there should be many other phosphors that can emit yellow light like the ZnS-based phosphors. He inquired whether the White LED Development Team had “tested all of them”.
139 In his affidavit, Mr Shinomiya placed this event after the testing of the ZnCdS phosphors. In cross-examination, he accepted the possibility that the testing of the ZnCdS phosphors took place at the same time as the testing of YAG:Ce (which I describe in the following paragraphs). The documents indicate that this is likely to be the case — at least there appears to have been an overlap in the testing of different classes of phosphors. However, I do not understand Mr Shinomiya to have accepted that the testing of the ZnCdS phosphors and YAG:Ce was conterminous. In cross-examination, Mr Shinomiya also accepted the possibility that ZnCdS phosphors were being tested for the purpose of comparing them with YAG:Ce. Whilst Mr Shinomiya accepted this possibility, I do not understand his evidence to go so far as accepting that to be the fact. Also, his evidence was given with respect to a particular test. As I understood Mr Shinomiya’s evidence in this regard, he did not know whether the proposition put to him was a fact, so far as that particular test was concerned.
140 The day after the conversation with Mr Ogawa, Mr Shinomiya and Mr Shimizu went to an inspection room located in the applicant’s Inspection Building, where the applicant’s phosphor reference samples were stored. Mr Shinomiya said that he and Mr Shimizu carefully observed the body colour of several hundred sample phosphors, one by one. Mr Shinomiya gave this evidence:
The samples were stored in a variety of ways; some were stored in plastic bags, others were in transparent bottles. If a phosphor was in a black plastic bag or in a bottle with a cover to prevent exposure to light, we opened them so the phosphors were exposed to light during our observation. We were in the inspection room for more than two hours to observe all of the samples there. We did not look at any specification sheets, just the phosphor samples themselves.
141 Mr Shinomiya said that one of the phosphor samples that he and Mr Shimizu observed was YAG:Ce. Its body colour was slightly yellowish in appearance. Mr Shinomiya said that, at this time, he knew of this phosphor, but he did not know until then that its body colour was slightly yellow (or that it had a body colour other than white). YAG:Ce was available at Nichia because it had been used in electron beam excitation applications to emit green light, and for flying spot scanners. Mr Shinomiya said that, at that time, he had “never seen a real visible light emission of YAG phosphor in a flying spot scanner”.
142 Mr Shinomiya said that, at this time, he also knew that YAG:Ce was used as an optical crystal for a laser oscillation. In cross-examination he accepted that, at that time, he knew that the phosphor was very stable when used in that application.
143 Mr Shinomiya said that not all of the phosphors owned by the applicant were stored in the inspection room. Mr Shinomiya gave an instruction that other phosphor samples held by the applicant were to be collected for inspection.
144 Mr Shinomiya said that, following his and Mr Shimizu’s inspection of samples in the inspection room, YAG:Ce was identified as the preferred candidate for further testing because of its slightly yellow body colour. In late May 1996, the White LED Development Team produced LED samples using YAG:Ce and managed to create an LED that emitted a weak greenish white colour. This confirmed that the light emission had a broad emission spectrum when excited by a blue light. It also meant that there was a possibility that a white LED suitable for various applications could be obtained. At this juncture, it should be recalled that YAG:Ce falls within the definition of the phosphor of claim 3 of the patent.
145 Mr Shinomiya said that while YAG:Ce was stable in a laser application, it was not known whether the phosphor powder, having a diameter of only several micrometres, would have sufficient durability when moved to the LED environment. He said that this was particularly so in light of the team’s experience with ZnCdS. Experiments were carried out on a test sample. The experiments showed that the phosphor had good durability in the LED environment.
146 I note that various test reports are in evidence, including a test report dated 30 May (known to be in 1996) and 6 June 1996 that concern the testing and comparison of YAG:Ce and ZnCdS phosphors.
147 Once YAG:Ce had been confirmed as the lead candidate, the remaining problem was to modify its greenish white colour to achieve a more desirable tone of white. Mr Shinomiya said that this could be achieved by reducing the colour temperature of the phosphor. Mr Shimizu requested that experiments be performed by the applicant’s Phosphors Group. This stage of the work commenced in early June 1996. It was carried out by Mr Noguchi. Mr Shimizu’s idea was to create a more desirable white LED by adding a red light component. He proposed introducing a co-activator which emitted red light, such as Ce with Sm3+, Ce with Eu3+ or Ce with Mn. The work with the coactivators was not successful.
148 Mr Shinomiya said that Mr Noguchi took it upon himself to prepare YAG:Ce with some of the Y substituted with Gd in order to make the wavelength of the emitted light longer. Mr Noguchi’s plan was to change the matrix of the phosphor and adjust the content of Ce as an activator. The test with the modified YAG:Ce showed very good results. It produced desirable white light and had the flexibility of shifting the colour temperature in a broad range.
149 In June 1996, a decision was made by the applicant to file a patent application in respect of the work undertaken by the White LED Development Team. The patent in this proceeding derives from one of a number of applications that were made.
150 As is well-recognised, the person skilled in the art is, for the purposes of the Act, a legal construct that sets the standard against which questions posed by the Act are to be answered. One such question is posed by s 7(2). That provision has been amended from time to time. The form of the provision applicable to the present case is shown in Reprint 2 of the Act:
For the purpose of this Act, an invention is to be taken to involve an inventive step when compared with the prior art base unless the invention would have been obvious to a person skilled in the relevant art in the light of the common general knowledge as it existed in the patent area before the priority date of the relevant claim, whether that knowledge is considered separately or together with either of the kinds of information mentioned in subsection (3), each of which must be considered separately.
151 Although identified as a single person, it is understood that the person skilled in the art may be a composite entity, frequently referred to as a team of persons: General Tire & Rubber Company v Firestone Tyre & Rubber Company Limited [1972] RPC 457 (General Tire) at 485. In the present case, the respondent contended that the person skilled in the art is a team; in effect, the applicant contended otherwise.
152 The respondent says that the person skilled in the art is, relevantly, the person who, at the priority date, had knowledge of and experience in LEDs, phosphors and lighting together with knowledge of the basic physics and chemistry used in this field.
153 On the other hand, the applicant says that the person skilled in the art is the person who, at the priority date, was skilled in semiconductors and not a person skilled in phosphors. The applicant says that a person skilled in the art of phosphors would only be involved, if at all, at a later stage, after attempts to produce a white LED using only semiconductors had failed and the idea to use phosphors with a blue LED was conceived.
154 In my view, the respondent’s contention is correct. Leaving aside the question of what is, and what is not, common general knowledge, the evidence establishes that, well before the priority date, the use of phosphors in lighting was ubiquitous. For the proof of that proposition, one need only look to fluorescent lighting. However, the evidence went much further than this.
155 Blasse G & Grabmaier B C, Luminescent Materials, Springer-Verlag, Berlin, 1994, is a standard textbook that provides an introduction to luminescent materials and solid state physics. A copy was available in the library of the University of Technology Sydney as at 17 November 1994. Parts of this work—referred to below as the Blasse extract—were tendered as prior art information available under s 7(3) of the Act. Leaving aside the question of whether it is available for that purpose, the Blasse extract illustrates various applications of luminescent materials, including as lamp phosphors, CRT phosphors, and X-ray phosphors. In the context of lamp phosphors, the Blasse extract discloses that luminescent lighting using lamp phosphors started even before the Second World War. The Blasse extract also includes a discussion on semiconductors, and on electroluminescence involving LEDs and semiconductor lasers. In its introduction to electroluminescence, the Blasse extract states:
When a luminescent material can be excited by the application of an electric voltage, we speak of electroluminescence. In order to convert electric energy from the applied voltage into radiation, three steps have to be considered: excitation by the applied field, energy transport to the luminescent center, and emission from this center. According to the voltage applied, one can distinguish between low-field or high-field electroluminescence. Light emitting diodes, where energy is injected into a p-n junction, are typical of low-field electroluminescence.
156 The Blasse extract goes on to discuss the use of LEDs with a phosphor to produce visible radiation.
157 The present significance of this evidence is that it shows not only the use of phosphors in lighting, but also, in that context, the use of LEDs with phosphors, and that these matters were discussed in a standard textbook, such as one would find in a university library in Australia before the priority date.
158 Dr Bretschneider gave evidence that in the 1960s and 1970s, phosphors were first used with LEDs to modify the light emitted by the LED. He gave the example of IR GaAs-based LEDs used with rare earth phosphors that absorb IR light and emit visible light (so-called “up-converting” phosphors). The same example is given in the Blasse extract. The use of GaAs-based LEDs with up-converting phosphors is also described in the 1984 IES Lighting Handbook (IES refers to the Illuminating Engineering Society of North America). Dr Bretschneider said that these LEDs had been commercialised for segment LED displays before the priority date. There was no challenge to the correctness of this evidence.
159 In his cross-examination, Mr Shinomiya gave evidence that the applicant itself produces phosphors and LEDs. The effect of his evidence was that the applicant started producing fluorescent material from about 1996 and that it commenced its research into LEDs from about 1975. I have referred to part of that work when summarising the steps taken by the applicant to produce a white LED. The applicant’s own published work shows that, before the priority date, it was using LEDs in conjunction with phosphors to produce visible light. Mr Shinomiya accepted that companies such as OSRAM, Philips and Cree were likewise producing phosphors and LEDs before the priority date.
160 The applicant’s case, directed to identifying the person skilled in the art, was based on the proposition that work in the field of semiconductors (including LEDs) and work in the field of phosphors were separate and distinct endeavours which, in practice, did not overlap.
161 This position was based, substantially, on Professor Meijerink’s evidence that, before the priority date, the fields of LEDs and phosphors were “largely separate”. In this connection, Professor Meijerink said that, in his extensive experience, including his contact, internationally, with those at universities and in industry working in luminescence spectroscopy and with phosphor materials, he was not aware of anyone who worked in both fields at the priority date. This may be so. But I did not understand Professor Meijerink to suggest that, in the field of lighting, appropriately skilled persons in the field of phosphors were not working as part of a team that would include appropriately skilled persons with knowledge of various lighting sources. Indeed, in his evidence in chief, Professor Meijerink discussed how, before the priority date, luminescent materials had been used to achieve spectral conversion in light emitting devices: see [200]-[201] below.
162 Dr Butcher gave similar evidence. He said that, based on his experience in the LED field in Australia before the priority date, “phosphors were an unrelated field”. I have no hesitation in accepting that, in Dr Butcher’s particular field of study before the priority date—which was directed mainly towards fundamental material studies predominantly with aluminium nitride semiconductors without any specific commercial application in mind—“phosphors were an unrelated field”. But I do not understand Dr Butcher’s evidence to be directed to the field of lighting generally. In this connection, he said that it was well-known to him before the priority date that phosphors were used in mercury vapour discharge lamps (of which fluorescent lamps are one type) with UV light. He also said that he learned about phosphors from undergraduate courses, although this was not taught in relation to LEDs.
163 In my view, the evidence plainly shows that, before the priority date, the field of lighting embraced the use of phosphors in combination with electrical sources to produce light, including (as it happens) the use of phosphors with LEDs. This was certainly the case with the up-converting phosphors excited by IR emissions from LEDs. I do not think that, for the purposes of determining the characteristics of the person skilled in the art in Australia, these realities can be ignored. It does not matter that, for example, the use of an LED with a phosphor or phosphors to produce white light had not been attempted before the priority date.
164 There is another matter. It is not to the point that, before the priority date, there was not, in fact, a team of skilled persons in Australia working on LEDs and phosphors. The relevant question is: what is the notional research team that would be put together at the priority date to attempt to make a white light emitting device. This question is not answered by choosing the narrowest possible team, as the applicant would seem to have it. Knowledge in the field of lighting, where the use of phosphors had been established for many decades, would require the team to include a person skilled in the art of phosphors. Such knowledge could not be ignored. It would be unrealistic to proceed otherwise, unless one were looking to minimise the team’s chances of success when carrying out the notional research task.
165 This finding is relevant to a submission advanced by the applicant based on certain observations made by Jacob LJ in Schlumberger Holdings Ltd v Electromagnetic Geoservices AS [2010] RPC 33; [2010] EWCA Civ 819 (Schlumberger). In that case, Jacob LJ canvassed the possibility that the notional research team—standing as the person skilled in the art—might not be the same team for all questions arising in respect of a given patent. In other words, the person skilled in the art might not be invariant. In answer to the proposition that the person skilled in the art, and hence the notional team, must be invariant, Jacob LJ said (at [63]-[65]):
63 I think the flaw in that is to assume that “the art” is necessarily the same both before and after the invention is made. The assumption may be correct in most cases, but some inventions are themselves art changing. If a patentee says “marry the skills of two different arts to solve a problem,” marrying may be obvious or it may not. If it is not, and doing so results in a real technical advance then the patentee deserves and ought to have, a patent. His vision is out of the ordinary.
64 This is not because a different construction is being given to the phrase “person skilled in the art” in the different Articles. It is because the phrase is being applied to different situations. Where the issue is claim construction or sufficiency one is considering a post-patent situation where the person skilled in the art has the patent in hand to tell him how to perform the invention and what the monopoly claimed is. But ex-hypothesi the person skilled in the art does not have the patent when considering obviousness and “the art” may be different if the invention of the patent itself is art changing.
65 In the case of obviousness in view of the state of the art, a key question is generally “what problem was the patentee trying to solve?” That leads one in turn to consider the art in which the problem in fact lay. It is the notional team in that art which is the relevant team making up the person skilled in the art. If it would be obvious to that team to bring in different expertise, then the invention will nonetheless be obvious. Likewise if the possessor of the “extra expertise” would himself know of the other team's problem. But if it would not be obvious to either of the notional persons or teams alone and not obvious to either sort of team to bring in the other, then the invention cannot fairly be said to be obvious. As it was put in argument before us the possessors of the different skills need to be in the same room and the team with the problem must have some reason for telling the team who could solve it what the problem is.
166 In an earlier passage, Jacob LJ referred to the need, when considering obviousness, to have regard to “the reality of the position at the time”. In this connection, he said (at [42]):
What the combined skills (and mind-sets) of real research teams in the art is what matters when one is constructing the notional research team to whom the invention must be obvious if the patent is to be found invalid on this ground.
167 The starting point for the applicant’s reliance on Jacob LJ’s observations in Schlumberger is that, in the present case, according to the applicant, the invention in suit was art-changing in that it involved putting together two arts that were disparate at the priority date. The applicant argued that, before the priority date, the occasion would not have arisen to put together one person practised in the art of LEDs with another practised in the art of phosphors. According to the applicant, this would only have occurred “once the hypothetical person skilled in the semiconductor art was directly led as a matter of course to attempt to solve the problem of producing white light by the particular choice of the use of phosphors in combination with a semi-conductor producing blue light”. On the evidence before me, that argument cannot be accepted. It fails at the starting point: as a matter of fact, the invention was not art-changing in the sense described by Jacob LJ.
168 As a final matter on this topic, I note that determining the identity of the person skilled in the art at the relevant time is an inquiry that is separate to and distinct from determining the state of the common general knowledge as it relates to a consideration of obviousness. It is only after the person skilled in the art has been conceptualised that one can turn to consider the state of the common general knowledge. This is because the state of the common general knowledge depends on the identity of the person skilled in the art. In the context of an inquiry as to obviousness, it is quite meaningless to speak of the common general knowledge without this frame of reference. It is also an error to think that the identity of the person skilled in the art can be determined by reference to some notional, ill-defined body of common general knowledge. When one is considering how a notional research team might be put together, one simply looks to the realities, as revealed by the evidence. As I have said, knowledge in the field of lighting, where the use of phosphors had been established for many decades before the priority date, would require the team in the present case to include a person skilled in the art of phosphors.
The issue
169 The applicant acknowledged that if the respondent’s conception of the person skilled in the art were to be accepted, then the information in the primer—other than the information in paragraph 36 (last two sentences), paragraphs 46 to 49 and 51 to 53; and paragraphs 58 to 60—could be taken as information comprising part of the common general knowledge before the priority date.
170 For its part, the respondent did not accept that the information in paragraphs 71 to 76 of the primer (dealing generally with thermal quenching) was part of the common general knowledge before the priority date. It is not necessary for me to resolve that particular question. Although relevant to a debate between Professor Meijerink and Dr Bretschneider about whether thermal quenching is a problem with YAG:Ce and whether that problem is demonstrated in a prior art document advanced by the respondent, that debate itself does not need to be resolved. This is because, having raised the matter, Professor Meijerink, who is undoubtedly an expert in the field of inorganic phosphors, said that he was not aware of a thermal quenching problem with YAG:Ce before the priority date.
171 The respondent specifically advanced the following information as part of the common general knowledge:
YAG:Ce absorbed blue light and emitted yellow light;
YAG:Ce was a stable, inorganic phosphor with a garnet structure;
YAG:Ce was a commercially available phosphor that had been used in a variety of applications, such as in CRTs, discharge lamps and mercury vapour lamps;
there were problems with organic phosphors; and
there were problems combining red, green and blue LEDs to create a source of white light.
172 The last two propositions require further explanation.
173 As to organic phosphors, Dr Bretschneider gave evidence that it was known before the priority date that organic phosphors had problems with stability. Specifically, he said that it was known that organic phosphors could react with resin material and degrade; it was known that they could be affected by electrophoresis; and it was known that they could degrade when exposed to blue light of a relatively high energy.
174 Professor Meijerink also gave evidence that organic phosphors had stability problems, although he said the problems varied considerably between these phosphors. He said that organic phosphors are generally less stable than inorganic phosphors. He said that, at the priority date, organic phosphors were known to have limited thermal and optical stability, particularly in high photo flux and temperature environments. He gave evidence that research in organic phosphors has gone on since 1996, and is still going on, to ameliorate these problems.
175 As to combining red, green and blue LEDs, Dr Bretschneider and Dr Butcher both gave evidence of difficulties in providing such LEDs in one “package”. Dr Bretschneider said that the chromaticity and brightness of an LED changes with operating temperature. These changes occur at different rates for each individual colour chip. As a result, a white LED using red, green and blue LEDs will change in both intensity and colour as operating temperature changes. To prevent these changes occurring with temperature, a separate power supply for each LED is required. Dr Bretschneider also referred to the fact that, because of a difference in materials and manufacturing processes, red, green and blue LEDs will age differently over time, leading to colour deviation over time. Thus, further componentry would be required to ensure that changes, including in respect of drive current, are made in real time to maintain a constant chromaticity for such a device.
176 Dr Butcher accepted that combining red, green and blue LEDs into one package to produce white light presented the kinds of challenges to which Dr Bretschneider referred. He said that producing such a device would require three power sources or one power source with three different branches or currents, which would have been inefficient. Whilst acknowledging these challenges, I think it is fair to say that Dr Butcher considered them to be no more problematic than the challenges presented by other means of producing a white LED using a blue LED as a component.
177 The respondent also submitted that three publications referred to below—Blasse and Bril I, Blasse and Bril II and the Blasse extract—were also common general knowledge in Australia before the priority date.
Conclusion and reasons
178 One difficulty attending the respondent’s case is that it called no direct evidence on the state of knowledge in Australia before the priority date concerning phosphors in lighting applications or, indeed, in other applications. Rather, it relied, firstly, on the very general proposition that “phosphors” was an international field of science. So much may be accepted. But it does not materially advance the respondent’s case concerning the state of the common general knowledge relevant to the present case.
179 Next, the respondent asserted that there was “a global pool of common general knowledge on issues relevant to the invention”. This proposition was not established on the evidence. The applicant accepted in opening that it was not contending that Australia was “a technical backwater”. It also said that it was not seeking to put the person skilled in the art in Australia in “unreal isolation”. However, these concessions are far removed from an acceptance that there was a global pool of common general knowledge on issues relevant to the invention. Indeed, on more than one occasion the applicant pointed to what it considered to be significant gaps in the respondent’s evidence in this regard.
180 The applicant did call some direct evidence on the question. Professor Meijerink gave evidence of his membership of international professional bodies and societies relevant to his work in the field of luminescence spectroscopy and his research on phosphor materials. He said that he has been regularly involved in activities such as organising and attending the meetings of these bodies and societies, selecting the location of conferences, advising local organising committees on the selection of topics and speakers, and chairing and leading discussions at the conferences. He gave evidence of his regular exposure to the research, ideas and people working in the fields of luminescence spectroscopy and phosphors internationally since 1988. In light of this knowledge and background, Professor Meijerink said:
Based on my experience … including my regular attendance at international conferences such as the International Conference on Dynamical Processes in Excited States of Solids in Australia in 1995, the level of phosphor knowledge in Australia was much lower than in Europe, the United States of America and even New Zealand. There is no strong tradition of research on phosphor materials for lamps and displays in Australia, and as at the Priority Date the leading optical materials groups in Australia concentrated on hole-burning (related to optical memories), luminescence for geological identification/dating and the luminescence of semiconductors. I was not aware of any group in Australia that was, as at the Priority Date, working on phosphors for application in lamps or displays. As far as I am aware, the same is true today.
181 Given the state of the evidence, I am not prepared to assume that the knowledge of phosphors that Dr Bretschneider and Professor Meijerink had before the priority represents the state of knowledge that the person skilled in the art in Australia would have had in the same period. This is not to place the person skilled in the art in Australia at the relevant time in “a technical backwater” or “in unreal isolation”. It simply acknowledges that, on the state of the evidence before me, and relevantly to the present case, the state of knowledge in Australia on the use of phosphors in lighting devices was not the same as in other countries.
182 In these circumstances, I am only prepared to accept that, before the priority date, the common general knowledge in Australia on phosphors, as it relates specifically to this case, was no greater than the applicant conceded it to be. Thus, I would accept that YAG:Ce was known and was part of the common general knowledge of the person skilled in the art. I would also accept that it was known, and part of the common general knowledge of the person skilled in the art, that:
YAG:Ce absorbed wavelengths of light that correspond to exciting Ce electrons to higher energy states, including blue light of 450 nm; and
after being excited by the absorption process the Ce electrons return to a lower energy state by the complementary process of light emission, with the emitted light being of a different wavelength than the absorbed light.
183 I am not prepared to accept that the problems with organic phosphors to which Dr Bretschneider and Professor Meijerink referred were part of the common general knowledge of the person skilled in the art in Australia.
184 As to combining red, green and blue LEDs into one package to produce white light, I accept that the common general knowledge of the person skilled in the art in Australia before the priority date would have included knowledge of the challenges to which Dr Bretschneider and Dr Butcher referred.
185 Turning now to Blasse and Bril I, Blasse and Bril II and the Blasse extract, neither the respondent’s evidence nor its submissions explained how these documents and the information in them would have entered the common general knowledge of the person skilled in the art in Australia before the priority date. I have noted that the textbook from which the Blasse extract is taken was in at least one Australian university library before the priority date, but this does not establish that the extract was part of the common general knowledge at that time.
186 Nonetheless, in closing submissions, the applicant accepted that, if the respondent’s conception of the person skilled in the art were to be accepted then Blasse and Bril I and Blasse and Bril II would have been common general knowledge. So much was made clear by the applicant’s written submissions.
187 Based on the same assumption, the applicant accepted that the person skilled in the art would have known of the textbook from which the Blasse extract was taken, and would have had resort to it. However, the applicant was clear that I should not assume that all the information in the textbook or the extract was part of the common general knowledge at the relevant time. However, the applicant did accept that the person skilled in the art would have known that YAG:Ce was a phosphor with garnet structure that absorbs blue light and converts it with high efficiency into a yellow emission, as recorded on p 124 of the text. The applicant also accepted that the person skilled in the art would have known of tricolour lamps of the kind mentioned in the Blasse extract.
188 Thus, I accept that the person skilled in the art would have known of Blasse and Bril I and Blasse and Bril II before the priority date. I am also prepared to accept that the Blass extract was part of a textbook whose existence was part of the common general knowledge of the person skilled in the art before the priority date and that such knowledge included tricolour lamps of the kind mentioned, as well as the absorption and emission characteristic of YAG:Ce recorded at p 124 of the text to which I have referred.
The evidence of the expert Witnesses
189 In response to certain matters raised by the applicant’s solicitors, Dr Butcher discussed whether he was aware, before the priority date, of efforts to make a white LED; how, before the priority date, he would have gone about developing a white LED, if asked to do so; and how, before the priority date, he would have gone about finding information relevant to “a white LED project”.
190 After he had performed this task, he was given the prior art documents discussed below at [293]-[324] (the prior art documents) and asked a number of questions, including whether he was aware of each document before the priority date and, if not, whether he thought that he would have found the document conducting a search of the kind described by him.
191 As to how he would go about developing a white LED, Dr Butcher said that he became aware of the possibility of producing white light from LEDs at around the time that Mr Nakamura’s work on the first blue GaN device was published in 1991. He said that, at that time, people working in the nitride semiconductor field were mentioning the possibility of producing white light from LEDs, specifically by mixing red, green and blue LEDs to produce the white light. Dr Butcher said that, at this time, he was aware that this was “how a television screen worked”.
192 Dr Butcher said that he first became aware of the use of phosphors in combination with a blue LED to create a white LED “probably shortly after this work was published by Nichia”. He said that he was “blown away” by this information, which he considered to be something “out of the blue”.
193 Dr Butcher said that, if he had been asked to try to develop a white LED before the priority date, his starting point would have been to mix red, green and blue LEDs. He was also aware that nitride semiconductors with defect emission bands could be mixed to create white light. Dr Butcher said that, at the time, he would have explored these defects even though, in retrospect, this research path would not have been effective to create a useful white light. In cross-examination, he accepted that while the exploration of such defects was “an interesting line of research”, it was “probably not” a practical solution that could have been applied to obtain white light.
194 In relation to the task of finding information relevant to “a white LED project”, Dr Butcher was also asked to perform a literature search using certain keywords he had identified in earlier parts of his evidence, restricted to publications available before the priority date. Dr Butcher reported on the results of his investigation and summarised the different ways in which, based on his investigation, research teams were trying to achieve a white LED before the priority date. He also discussed how this information, if known to him before the priority date, would have impacted on the likely direction he would have taken in the hypothetical task he had been set to develop a white LED. In essence, Dr Butcher said that he would not have been deflected from pursuing the two paths he had identified. He added that, based on one publication he had discovered, he may also have looked at using the near-UV emission from a GaN or GaN/InGan diode (or blue LED) to excite separate red, green and blue phosphors. He doubted, however, that this publication would have been available in Australia before the priority date with the consequence that, at the relevant time, he would not have been aware of this potential research path.
195 Dr Butcher dealt with a number of other topics, which I need not discuss at the present time. He commented on certain parts of Dr Bretschneider’s second affidavit, discussed below.
196 As with Dr Butcher, the applicant’s solicitors asked Professor Meijerink to discuss whether he was aware, before the priority date, of efforts to develop a white LED; how, before the priority date, he would have gone about developing a white LED, if asked; and how, before the priority date, he would have gone about finding information relevant to “a white LED project”.
197 Professor Meijerink said that, at the priority date, he had only a basic understanding of how LEDs worked, based on reading general textbooks on solid state physics. He said that he was aware of the existence of LEDs capable of emitting red light, green light or infrared light, specifically through their use as indicator lights in consumer electronics and, in the case of infrared diode lasers, in CD players. He said that, even though he had had extensive knowledge of phosphors and luminescence spectroscopy in the years leading up to the priority date, he was not aware of efforts to develop a white LED at that time.
198 Professor Meijerink said that the first time he became aware of the use of a luminescent material for spectral conversion in an LED was in 1999 when his colleague Dr Hintzen and one of Dr Hintzen’s PhD students discussed the use of oxynitrides doped with Ce3+ or Eu2+ to convert blue LEDs into white LEDs.
199 Professor Meijerink said that, at the priority date, he was aware that luminescent materials were used for spectral conversion in several industrial and commercial applications. But because different luminescent materials have different physical and chemical properties, and because their suitability varies considerably depending on the particular application and operating conditions in which they are used, it would have been impossible to have known whether a given luminescent material could have been used for spectral conversion in any other application under other conditions, unless extensive research and testing of the material had confirmed its suitability for that purpose. He stressed that even though a luminescent material could be used for spectral conversion in one application under certain conditions, it did not follow that the same material would operate in the same way or, indeed, that it would operate at all, in another application under different conditions.
200 Professor Meijerink referred to and discussed CRTs, low-pressure mercury discharge lamps (which are commonly used as fluorescent tubes and compact fluorescent tubes), X-ray imaging, and black and white laser projection displays as examples of industrial and commercial applications in which, before the priority date, luminescent materials were used for spectral conversion. This evidence illustrated the different operating conditions in which luminescent materials can be used, and the different physical and chemical properties required of them for these applications.
201 In relation to how, before the priority date, he would have gone about developing a white LED, Professor Meijerink said that, as he had only a basic understanding of LEDs at that time, he could only have been involved in such an effort if part of the research group included a person working in the field of LEDs. On that assumption, and on the further assumption that he had been aware of blue LEDs before the priority date, Professor Meijerink said that, in the first instance, he probably would not have considered using a luminescent material with a blue LED to achieve white light by spectral conversion. This was because the conditions for operating a blue LED are different to those needed for the other known industrial and commercial applications which Professor Meijerink discussed in his affidavit. He drew particular attention to the high operating temperatures, high photon flux and long operating time of LEDs, which led him to conclude that the search for a suitable phosphor for spectral conversion in conjunction with a blue LED would have been a difficult and time-consuming task.
202 Professor Meijerink said that, at the time, rather than using a luminescent material for spectral conversion, he would have considered combining red, green and blue LEDs, which he said would have been “the most logical way to create white light”. In saying this, Professor Meijerink also made clear that, at the time, he was not aware that this method (which he called the RGB method) had a number of drawbacks, such as problems with colour stability and colour reproducibility.
203 Professor Meijerink said that if, hypothetically, he had discarded the RGB method, and had considered spectral conversion using a luminescent material with a blue LED, he would have considered three options, in the following order of preference.
204 First, he would have considered applying one or more phosphors to a blue LED to generate white light by converting part of the blue light to green and red light, thus creating a tri-colour spectrum. Professor Meijerink said that this would be his first choice based on his knowledge, at the priority date, of lighting and fluorescent lamps, where a high colour rendering and high efficiency required the combination of red, green and blue light.
205 Secondly, he would have considered applying one or more phosphors to a blue LED to generate white light by converting part of the blue light to yellow/orange light. Professor Meijerink raised this option even though, in his view, it would have resulted in poor colour rendering, which might be a serious drawback when considered against existing commercial light sources.
206 Thirdly, Professor Meijerink said that he would have considered applying one or more phosphors to an LED capable of emitting UV light—once again to create a tri-colour spectrum. Professor Meijerink raised this option because, at the priority date, he was aware that shifting the output of the LED to shorter wavelengths would provide more opportunities for the creation of a white LED, because UV excitation provides a wider range of phosphors to achieve efficient spectral conversion to green and red light. He noted, however, that UV LEDs were not well-developed at the priority date.
207 Professor Meijerink said further that if, before the priority date, he had considered using a phosphor to convert a blue LED to a white LED, there were a large number of known organic and inorganic materials that, potentially, could have achieved the desired spectral conversion. Professor Meijerink discussed these materials, which he classified as organic fluorescent dyes, organometallic complexes, inorganic phosphors, and hybrid systems containing both organic fluorescent dyes and inorganic phosphors. Professor Meijerink also referred to the possibility of creating a new inorganic phosphor.
208 Professor Meijerink said that if, before the priority date, he had considered using a phosphor to convert a blue LED into a white LED, he would have researched and tested red and green phosphors that were excited in the blue spectral region, and later yellow/orange emitting phosphors, in the following order of preference:
silicates, halosilicates or aluminates doped with Eu2+;
CaS,SrS, (Zn,Cd)S or thiogallates doped with Eu2+,Ce3+ or Cu2+ and co-dopants;
phosphors doped with Mn2+, possibly in combination with Eu2+;
silicates, halosilicates or aluminates doped with Ce3+ (a category which includes YAG:Ce);
f-f emission from lanthanide ions like Eu3+, Tb3+, Sm3+ and Pr3+;
Mn4+ phosphors;
quantum dots; and
organic dyes.
209 Professor Meijerink said:
Researching, selecting and testing the most suitable luminescent material would not have been a trivial exercise, and it would not have been clear to me which luminescent materials would work and whether any luminescent material could be found that would create a white light source that could compete with existing light sources, such as compact fluorescent tubes, as a commercial product. While the properties of a luminescent material on paper or in another application would have provided a starting point, that would have by no means have guaranteed successful spectral conversion in a Blue LED.
210 Professor Meijerink said that, at the priority date, he was aware that YAG:Ce had strong absorption around 460 nm and efficient yellow emission at around 550 nm. He said, however, that those spectral properties would not have made YAG:Ce an obvious choice for application as a spectral converter when used with a blue LED.
211 In this connection, Professor Meijerink explained that, in addition to the general matters I have summarised above concerning the difficulty in predicting the suitability of luminescent materials in applications involving different operating conditions, doping specific host lattices, such as YAG, with Ce3+ has the problem that Ce3+ has a higher energy position than, say, Eu2+. This makes it harder to shift the emission to the desired longer wavelengths. He said that the selection of host lattices in which Ce3+ has emissions in the required spectral range is much more limited than for Eu2+.
212 Professor Meijerink also explained that, at the priority date, the performance of YAG:Ce under the high irradiance conditions present in a blue LED was not known to him. He said that, at the time, he would not have assumed, based on its performance in lower photon flux environments (such as in fluorescent tubes and blue laser displays), that YAG:Ce would have remained stable under such extreme conditions.
213 Professor Meijerink also advanced thermal quenching, even at room temperature, as a problem with YAG:Ce, although, importantly, he was not aware of this problem at the priority date. He attributed the problem to high Ce3+ concentrations and defects. As I have already noted, it was a matter of debate between Professor Meijerink and Dr Bretschneider as to whether thermal quenching is a problem with YAG:Ce.
214 In summary, Professor Meijerink said:
In my opinion, using YAG:Ce in a Blue LED to create an efficient White LED with a long enough operating time for use in industrial or commercial applications was an important and ground-breaking development. Given the physical and chemical properties of YAG:Ce and the challenging and unique operating conditions in a Blue LED, I would have tried many other phosphors over many years before settling on YAG:Ce as a spectral converter to create White LEDs. The discovery of using YAG:Ce to create White LEDs marked a breakthrough in the history of lighting and, almost 20 years after the Priority Date, YAG:Ce is still the most widely applied White LED phosphor with a better overall performance than other phosphors for converting the wavelength of blue light in LEDs to yellow. The resulting white light is, however, a cool white and for that reason YAG:Ce is sometimes used in combination with other phosphors to adapt the spectrum to generate a warmer White LED.
215 Like Dr Butcher, Professor Meijerink dealt with a number of other topics, which I need not discuss at the present time. He, too, commented on certain parts of Dr Bretschneider’s second affidavit.
216 Dr Bretschneider made two affidavits (1 June 2015 and 12 January 2016). Certain parts of Dr Bretschneider’s first affidavit were read at the hearing of the separate question and are referred to at [107]-[119] of Reasons 1. This evidence was taken as being before me at the hearing to which these reasons relate.
217 In his second affidavit, Dr Bretschneider discussed the sources of knowledge and information that were available to him before the priority date. He gave an account of the basic principles relating to LEDs and their design. He discussed the use of phosphors, with particular reference to the lighting industry. In the course of this discussion, Dr Bretschneider said that he knew of instances where down-converting phosphors had been used with blue LEDs to obtain white light. He said that the usefulness of such devices was limited by the poor efficiency of the blue LED. Curiously, Dr Bretschneider did not identify these instances in his affidavit or his oral evidence. In concurrent evidence, Professor Meijerink disputed the fact. He said that, to his knowledge, there were no publications, and no research had been done, on phosphors that had been used to convert blue LED light to make a white light source: “white LED phosphors were not known”. Dr Bretschneider responded by referring to a number of publications. None of them feature in the respondent’s pleaded case on invalidity. What these publications disclose is simply not known. The first disclosure of a down-converting phosphor with an LED revealed in the evidence is one of the applicant’s patents referred to on page 2, lines 13-19 of the specification.
218 Dr Bretschneider was asked by the respondent’s solicitors to discuss how, before the priority date, he would conduct a project to develop “a new LED for commercial use that was better than, or an alternative to, the existing blue LED”. He also commented on certain prior art documents and the patent itself.
219 In the context of the present proceeding, the project that Dr Bretschneider was asked to discuss is an odd one, given his extensive experience as an expert witness for the opposing side in proceedings involving the applicant. As Dr Bretschneider explained, in the course of his work as an expert witness in these other proceedings, he reviewed copies of patents granted in the United States of America and the United Kingdom which, on his understanding, were equivalent to the patent in the present proceeding. He volunteered that he was “generally familiar with the issues associated with the equivalent US and UK patents”. Against this background, the generality of the project he was asked to discuss has an air of artificiality. It is little wonder that, with the knowledge he had as an expert witness in the other proceedings, Dr Bretschneider suggested, in response, that one project of interest to him “would have been to look at developing a white LED”.
220 Dr Bretschneider said that, before the priority date, a white LED could have been made by combining three separate red, green and blue LEDs into a single package. However, because of certain disadvantages and complexities which he discussed, Dr Bretschneider said that, at the priority date, he would not have considered such a system in order to produce white light unless there was a specific need to alter the colour emitted from such a light source. Dr Bretschneider identified similar problems with a white LED constructed from a blue chip and a yellow chip.
221 Dr Bretschneider also considered the possibility of using a UV LED with a phosphor mix (either a red, green and blue phosphor mix or a blue and yellow phosphor mix) to convert the UV to white light. He noted, however, that, at the priority date, UV LEDs had been mentioned in the literature but were not commercially available.
222 Having effectively rejected these possibilities, Dr Bretschneider said that he would have selected a blue GaN LED which, he said, would have been an obvious choice, for reasons he gave. Then, having decided to use a blue LED, he said that he would have selected a suitable phosphor or phosphors. He said that, initially, he would have preferred to use the simplest phosphor mix possible. Indeed, he said that he would have preferred to use a single phosphor rather than a mix of phosphors, for a number of reasons.
223 Having then moved to a single phosphor, Dr Bretschneider said that he would explore how the wavelength of that phosphor might be varied “…to fine tune the device so that the most desirable light could be obtained …”
224 Dr Bretschneider said that he knew, before the priority date, that blue light and yellow light could be combined to give white light. He said that, with this knowledge, he would next consider the identification and selection of a suitable yellow phosphor—that is, one that would be excited by blue light and, in response, emit yellow light. He said that he would have begun by looking at the existing phosphors he knew of at the priority date and then, if he was not sure of a suitable phosphor, consulting commonly used text books and the literature to identify existing candidates.
225 In this connection, he said that he would have used the Commission Internationale de l’Eclairage (CIE) chromaticity diagram to plot the x, y co-ordinates for the blue LED and a target white light: see paragraph 68 of the primer. Dr Bretschneider said that plotting a line through these points would have helped him to identify the x, y co-ordinates for the target yellow phosphor.
226 Dr Bretschneider said that, before the priority date, he was familiar with a number of potentially suitable yellow phosphors, including YAG:Ce. He said that he would have selected YAG:Ce as being, potentially, the most suitable phosphor.
227 Dr Bretschneider then said that it would have been a relatively simple task for someone like him to construct the LED and test the phosphor in conjunction with the chip. He said that, with his experience, it would have taken him about one day to prepare about 1,000 chips mounted on headers. Having prepared the chips, he said that he would then have mixed the resin with the phosphor and applied the resin to the chips, coating at least 300 to 500 chips per day. He said that once this had been done, he would have been able to evaluate chip performance and colour “quite quickly”. He said that he would have expected that a combination of a blue LED with YAG:Ce would have produced white light based on a simple application of the principle that blue light combined with yellow light produces white light.
228 In summary, Dr Bretschneider said:
Based on my knowledge of Ce-activated YAG phosphors and GaN-based semiconductor LEDs, including their structure and properties, the outcome of the combination of these materials (to give a white light source) is the natural consequence of the materials behaving as expected, according to scientific principles. In particular, my knowledge of Ce-activated YAG’s structure and composition, how it would behave, and its uses in other applications at the Priority Date would have told me that it could convert light emitted by a GaN-based semiconductor material.
229 In their affidavits, Dr Butcher and Professor Meijerink criticised and challenged some aspects of Dr Bretschneider’s reasoning. Professor Meijerink, in particular, considered Dr Bretschneider’s knowledge and experience in the field of phosphors as “very limited”.
230 One particular challenge, in this regard, was to the facility with which Dr Bretschneider would have chosen to use a yellow phosphor with a blue LED.
231 In this connection, Dr Bretschneider had said:
At the Priority Date, to give white light, research had shown that the combination of blue light and yellow light would be the most efficient combination of colors, and would provide better color rendering, thus making that combination the preferred one for artificial light sources.
232 Dr Butcher commented that it was not clear what research Dr Bretschneider was referring to. Whilst acknowledging that blue light and yellow light can provide white light, Dr Butcher said it was not clear on what basis it was said that this combination provided better colour rendering than a combination of red, green and blue light.
233 Professor Meijerink said that Dr Bretschneider’s statement was incorrect. Professor Meijerink said that, at the priority date, it was widely known in the phosphors field that the colour rendering of blue and yellow was inferior to the colour rendering of a combination of red, green and blue light at wavelengths close to maxima for eye sensitivity.
234 In this connection, Professor Meijerink referred to certain literature concerning halophosphate lamps (the Blasse extract discussed below at [300] – [312]) which, before the priority date, indicated the possibility that such a combination may achieve high colour rendering and high brightness, but this was with specific rare earth activated phosphors with narrow band blue emission (from Eu2+), narrow line green emission (from Tb3+) and narrow red emission (from Eu3+). Professor Meijerink said that the broad band yellow/orange emission from Ce3+ (the activator in YAG:Ce) combined with blue emission would have had the same problem as lamps with the halophosphate phosphor.
235 In the next stage of his affidavit, Dr Bretschneider was asked to put to one side his personal knowledge of YAG:Ce and assume that this phosphor was “not commonly known within the industry” at the priority date. He was then asked to describe the steps he would have taken to identify a suitable phosphor.
236 Here, Dr Bretschneider said he would have begun by reviewing phosphor catalogues and spoken to major phosphor suppliers to see what they might have been able to suggest and that, after identifying a candidate phosphor, he would have delved deeper into its chemical, physical and optical properties, and any other information that might tell him if it was suitable as a blue light absorbing, yellow light emitting phosphor. I note that Dr Bretschneider said that his goal would have been to identify materials in the yellow emitting range. Dr Bretschneider said that, as organic phosphors can have a variety of disadvantages, he would not have explored them if other options were available. He said that he would not have explored sulphide phosphors if YAG:Ce was available, because sulphide phosphors are not suitable for use in a resin coating. Dr Bretschneider said that his work, in this regard, would have been routine for those in “the LED industry”.
Obviousness: The respondent’s primary case
237 The respondent’s primary case is that the invention claimed in each of claims 1 and 3 would have been obvious to the person skilled in the art based on the common general knowledge in the patent area before the priority date.
238 The respondent submitted:
A finding of obviousness will follow if it can be shown that the hypothetical person skilled in the relevant art would have been led directly as a matter of course to try a particular experiment or act in the expectation that it might well produce the desired outcome.
239 In putting the matter this way, the respondent was seeking to paraphrase Graham J’s reformulation of the “Cripps question” in Olin Mathieson Chemical Corporation v Biorex Laboratories Ltd [1970] RPC 157 at 187-188—an approach which the High Court said should be accepted: Aktiebolaget Hässle v Alphapharm Pty Limited (2002) 212 CLR 411; [2002] HCA 59 at [53].
240 With reference to DSI Australia (Holdings) Pty Ltd v Garford Pty Ltd (2013) 100 IPR 19; [2013] FCA 132 at [275], the respondent also submitted that there would be no inventive step if all that is involved is a selection and combination of components that were within the ordinary perception and competence of the person skilled in the art who is seeking to achieve a desired result. Similarly, the respondent submitted that there will be no inventive step if “one who takes the ordinary route will be likely to come upon it”: Elconnex Pty Limited v Gerard Industries Pty Limited (1991) 32 FCR 491 at 507.
241 So far as relevant to the present case, the respondents submitted that the common general knowledge included knowledge of LEDs (including the blue GaN LED), the absorption, excitation and emission of light, the combination of colours of visible light (including blue and yellow) to produce white light, the use of phosphors as substances that exhibit luminescence when suitably excited, and the use of fluorescent materials to convert the wavelength of light. In respect of phosphors, the respondent placed particular emphasis on YAG:Ce and its basic properties.
242 Returning to the modified “Cripps question”, the respondent posed the relevant question as whether, at the priority date, the person skilled in the art, being a person with knowledge of and experience in LEDs, phosphors and lighting, equipped with the common general knowledge, would have been led directly as a matter of course to try YAG:Ce with a blue GaN LED in the expectation that it might well produce white light?
243 The respondent submitted that this question should be answered affirmatively because, at the priority date, it was well-known that blue light and yellow light would combine to yield white light. In this vein, the respondent submitted that given a blue LED, the task for the person skilled in the art was to select a suitable yellow phosphor (ie, one that was excited by blue light and emitted yellow light). The respondent submitted that YAG:Ce was not only part of the common general knowledge, it was also an obvious choice. The respondent submitted that the person skilled in the art would choose a single yellow phosphor in preference to a combination of red and green phosphors, because a single phosphor was “a simpler solution” for use with a blue GaN LED. The respondent argued that, although it was known before the priority date that a combination of red, green and blue LEDs could create white light, the person skilled in the art would not have pursued that route because “it was known to be very complicated”.
244 The respondents sought to illustrate the correctness of this reasoning by relying on Dr Bretschneider’s evidence as to the steps he would have taken to develop a white LED. According to the respondent, Dr Bretschneider was the only witness with all the qualifications of the person skilled in the art. The respondent advocated Dr Bretschneider’s approach as that of a practical person working in the industry before the priority date, based on his actual knowledge at that time.
245 The respondent accepted that Professor Meijerink was a highly qualified phosphor expert, but argued that his outlook was really that of an academic with an interest in potential areas of research rather than what might be a practical solution for industry. The respondent pointed to Professor Meijerink’s acceptance that his approach to developing a white LED was an academic approach “to make new materials, provide understanding”. In this connection, Professor Meijerink said:
… concerning my approach, my approach is an academic approach where I indeed want to make new materials, provide understanding. I have not objections against industrial approach, which is fast, the way you make an invention by in a faster way combining known phosphors that are available with the blue LED to make white light. That is also a valid approach to make an invention. In industry the object is to make a new product, to invent something quickly. The objective of an academia is to provide understanding and that is a different approach, so my approach would have been slower and I would have missed the YAG cerium.
I think Dr Bretschneider’s approach by only going to YAG cerium, discarding all the sulphides and all the materials, well, history is proving him wrong because later the sulphide, calcium sulphide europium was successfully applied in an LED. An award winning LED was a colour rendering of 91, a very high colour rendering …
(As transcribed.)
246 In this section of his evidence, Professor Meijerink was explaining why, unlike Dr Bretschneider, he would not have immediately arrived at using YAG:Ce as the phosphor of choice. His point was that, contrary to Dr Bretschneider’s approach, there were other approaches to obtaining white light using a blue LED that could not have been simply disregarded. Nonetheless, he said:
… You would go to the commercial phosphor suppliers, but I think it’s a valid approach to go in there from an industry with the objective to make an invention, to make the white LED and not take my approach, which is a more academic approach, searching literature, finding what is known about luminescent ions that can convert blue into green, yellow, red, orange and go from there.
(As transcribed.)
247 The respondent criticised Professor Meijerink’s approach because the options he described for developing a white LED were not only based on his knowledge before the priority date, but also on what he had learned subsequently from literature searches he conducted for the purposes of the FPC proceeding in Germany and, later, for the present proceeding.
248 The respondent also relied on Professor Meijerink’s reasonable concession that it was hard for him to say what he would have done in his white LED project before the priority date. Professor Meijerink explained that the focus in his evidence on the use of Eu2+ could have arisen because of his intensive work on this ion between 1986 and 1995.
249 The respondent also criticised Professor Meijerink’s dismissal of YAG:Ce as an obvious choice as a spectral converter when used with a blue LED. The respondent’s submissions contested the various reasons advanced by Professor Meijerink for his views in this regard.
250 The respondent also submitted that much of Professor Meijerink’s evidence was directed to producing a white light for commercial applications requiring acceptable colour rendering, colour tuning, stability and performance which, the respondent argued, were not relevant to claims 1 and 3 of the patent: see, in that regard, my comments at [62] above.
251 The respondent submitted:
In summary, Professor Meijerink’s evidence is not directed to the practical path that would have been taken to create a white LED. First, Professor Meijerink canvases various options from the point of view of a pure academic not an industry person. Secondly, Professor Meijerink’s evidence is directed to a commercial product, not the creation of white light using a blue GaN LED. To the extent that it is relevant, his evidence is consistent with the conclusion that YAG:Ce was the obvious choice for an industry person. It absorbed blue light (at the right wavelength) and emitted yellow light and it was known to be stable.
252 The respondent criticised Dr Butcher’s evidence on the basis that he was not the person skilled in the art. The respondent argued that, although he was an expert in the growth of nitride semiconductor materials, Dr Butcher did not design, make or test LEDs before the priority date. The respondent submitted that Dr Butcher’s lack of practical interest in the areas of lighting was demonstrated by (what the respondent said was) his lack of basic knowledge of colour mixing, chromaticity and colour temperature.
253 The respondent also criticised the literature search undertaken by Dr Butcher in respect of his white LED project. It is not necessary for me to descend to the detail of those criticisms.
254 In support of its case, the respondent made specific reference to the work carried out by the applicant to develop a white LED. The respondent submitted that the invention was conceived no later than 30 May 1996 by reference to a test report of that date. The respondent argued that the subsequent work carried out by Mr Noguchi was no more than subsequent checking and testing for the purpose of producing a commercial product.
255 By way of summary, the respondent submitted:
The evidence as to what was done at Nichia is consistent with the conclusion that the invention was obvious … Once the blue GaN LED was developed, all that remained to be done was to find the right phosphor, which absorbed blue light and emitted yellow light. Mr Shinomiya said that the only thing they did not know was how to find the right phosphor. That simply required going to the sample room at Nichia and turning the lights on. They found YAG:Ce because it was yellow. Once they decided to visit the inspection room, it only took an hour or two. This accords with the path that Dr Bretschneider would have taken based on his knowledge and experience before July 1996. Unlike [Aktiebolaget Hassle v Alphapharm Pty Limited], there was a clear path forward.
256 The applicant commenced its submissions by expressing its agreement with the respondent’s position that the inventive step in question starts with the prior knowledge of the blue GaN LED and, as the applicant put it, “the desire for white light in numerous applications”.
257 Adopting the reformulation of the “Cripps question”, the applicant submitted that, from this starting point, the person skilled in the art “would…directly be led as a matter of course to try” combined red, green and blue LEDs to create a white LED. Despite the challenges presented by such a combination, the applicant argued that such challenges are “the nature of science”. This was the approach that Dr Butcher—who the applicant advanced as the exemplar of the person skilled in the art in Australia at the priority date—would have taken; it was also the approach that Professor Meijerink advocated, even though he only had a basic understanding of how LEDs worked at the priority date. In support of its submission that this was the approach that would have been taken at the priority date, the applicant also pointed to a press release by Cree (a manufacturer of LEDs) on 29 June 1995 that announced the release of its new “super blue” LEDs which, it said, could be combined with already available high brightness red and green LEDs to create clusters capable of producing all colours of the visible spectrum, including white.
258 The applicant submitted that this approach—“the most likely approach”—would either succeed or fail. If it failed, there would be a “retracing of steps” in the sense that “the team would be reconstituted with different expertise, and work commenced from the start”. The applicant submitted that it was only at this point that the person skilled in the art would include a “phosphor expert”.
259 My acceptance of the respondent’s conception of the person skilled in the art stands as a rejection of this last-mentioned submission. There are further difficulties with it. The question is whether the invention in suit was obvious at the priority date. This question is not directly concerned with previously failed attempts at an invention, although I would accept that evidence of failed attempts might be relevant to whether or not the invention in suit was obvious at the priority date. Further, the alleged obviousness of an invention is not judged according to whether there was an equally or more obvious solution within the sight of the person skilled in the art at the priority date. Once again, the question is the obviousness of the invention in suit, not some other “invention”, failed or otherwise.
260 The applicant submitted that the conception of using a phosphor to convert the blue light of the GaN LED is part of the invention. The applicant accepted that, if a phosphor expert were on “the team”, the person skilled in the art would have known that red, green and blue light, as well as blue and yellow light, could be used to make white light. Thus, the applicant accepted that either red and green phosphors, or a yellow phosphor, would be used in combination with the blue GaN LED.
261 The applicant stressed, however, that red, green and blue combinations would have predominated and would have been the first choice, in part because these combinations were known to give a better colour rendering. The applicant referred to the use of red, green and blue phosphors in tricolour lamps, including in fluorescent lighting. It relied on the fact that the use of three phosphors would have been Professor Meijerink’s choice (in this scenario) rather than use of a yellow or yellow/orange phosphor, which would have been, according to him, an inferior option.
262 The applicant submitted that even if the person skilled in the art would have been directly led as a matter of course to use YAG:Ce, it does not follow that he or she would have done so with an expectation of success. The applicant referred to Professor Meijerink’s evidence that it was impossible to know whether luminescent material could be used for spectral conversion in any given application under any given conditions, unless extensive research and testing of the material had confirmed its suitability. The applicant also referred to Professor Meijerink’s evidence that, given the challenging and unique operating conditions in a blue LED, it would have been extremely difficult before the priority date to predict the stability and performance of any given luminescent material in the blue LED.
263 Next, the applicant referred to its own work to develop a white LED. It criticised and disputed various aspects of the respondent’s submissions in this regard. I do not need to record those matters. The summary I have provided of the applicant’s work at [116]-[149] above, stands as my findings of fact in this regard.
264 The applicant then criticised Dr Bretschneider’s evidence, noting the reliance placed on it by the respondent. The applicant submitted that Dr Bretschneider’s evidence was unavoidably tainted by hindsight, especially by the evidence he had given in foreign proceedings concerning corresponding patent claims, which had been informed by documents that were irrelevant to the case on obviousness in the present proceeding. The applicant submitted that “the project” set by the respondent for Dr Bretschneider was artificial, given that Dr Bretschneider already knew the solution to the problem he had been set. The applicant submitted that this artificiality was exacerbated by the fact that, in his second affidavit, Dr Bretschneider provided comments on the primer (including comments on the general role of phosphors used with LEDs and YAG:Ce) before discussing what he would have done to create a white LED. As an illustration of operative hindsight, the applicant also pointed to the fact that Dr Bretschneider expressed his expectation that the combination of a blue LED with YAG:Ce would have produced a white light, even though Dr Bretschneider had not worked with YAG:Ce before the priority date.
265 Next, the applicant submitted that Dr Bretschneider did not represent the person skilled in the art in Australia at the priority date. The applicant submitted that Dr Bretschneider was unlike anyone who worked in the relevant field in Australia because he had experience with both semiconductors and phosphors. These submissions were directed to the identity of a person skilled in the art in Australia before the priority date. Once again, I have rejected the applicant’s submissions in that regard.
266 Relatedly, the applicant submitted that Dr Bretschneider, unlike Dr Butcher and Professor Meijerink, did not directly address the state of knowledge in Australia at the priority date. I have already dealt with that submission in earlier paragraphs of these reasons: see [?]-[?] above. The applicant’s central argument was that the state of the common general knowledge is a matter of proof. I accept that submission. I also accept that the respondent’s evidence does not establish the state of the common general knowledge in Australia at the priority date beyond the matters conceded by the applicant.
267 Finally, before addressing certain criticisms made of Professor Meijerink’s evidence and Dr Butcher’s evidence, the applicant advanced a number of matters that were critical of the way in which Dr Bretschneider gave his evidence. It is not necessary for me to detail any of these matters.
Conclusion and reasons
268 The case on obviousness occupied much of the hearing. I have set out, in some detail, the parties’ respective cases, including the evidence of the expert witnesses. Having considered all the matters that were raised, I have reached the conclusion, which I firmly hold, that the invention claimed in claim 1 and claim 3 was not obvious when measured against the common general knowledge in Australia before the priority date. My reasons can be stated relatively briefly.
269 As I have noted, both parties accepted that the blue GaN LED should be taken as the starting point. The respondent’s case was that, given this starting point, the person skilled in the art would be directly led as a matter of course to try YAG:Ce. As I have noted, YAG:Ce falls within the scope of claim 1 and claim 3. If the case on obviousness can be made out on the basis of the routine adoption of YAG:Ce in combination with the blue GaN LED, then each claim would fall.
270 I am not persuaded that, at the relevant time, the person skilled in the art would have been directly led as a matter of course to try YAG:Ce with the blue GaN LED to the exclusion of other avenues of choice that presented possibly effective solutions. I can see no reason why these other avenues of choice would be disregarded. They would only be disregarded if I were to accept Dr Bretschneider’s evidence over all the other evidence in the case. I do not do so. I treat Dr Bretschneider’s evidence with considerable caution because I am satisfied that his analysis of the pathway that would have been chosen at the priority date to develop a white LED was unavoidably and irremediably tainted by hindsight, despite the efforts he said he made to put out of mind the substantial body of actual knowledge he had of the solution provided by the invention. On final analysis, the pathway presented by Dr Bretschneider was really a highway. I do not think that his analysis truly reflects the state of affairs that would have been presented to the person skilled in the art at the priority date.
271 The evidence shows, persuasively, that one branch of research that would have been worthwhile trying, was the combination of different coloured LEDs to produce white light—the so-called “package” of LEDs. Another branch of research that would have been worthwhile trying was the conversion of part of the wavelength of the light emitted by the blue GaN LED and the combination of that converted light with the blue light from GaN LED that had not been absorbed in the conversion process. The evidence indicates that spectral conversion of this kind might be achieved by a fluorescent material, but then there would be choices as to which materials, and which colour combinations, should be tried.
272 Dr Bretschneider’s evidence was that a yellow light-emitting phosphor would be chosen over a combination of red and green light-emitting phosphors to work in combination with the blue light of the GaN LED. His reason was that use of a single phosphor would provide ease of manufacture of the white LED. Inevitably, this led him to conclude that the phosphor would be a yellow light-emitting phosphor and, ultimately, YAG:Ce.
273 Dr Bretschneider’s explanation may provide a reason for choosing one phosphor, but Professor Meijerink’s evidence was that mixing two or more phosphors in a slurry for commercial scale manufacture does not present significant problems. In this connection, he provided the example of CRTs, where slurries of typically three or four phosphors are used without issue. Professor Meijerink expressed a preference for using red and green light-emitting phosphors with the blue light to provide a tricolour spectrum. Once again, he referred to the example provided by fluorescent lamps where high colour rendering and high efficiency could only be provided by this combination. Further, Professor Meijerink said that, based on the example of halophosphate lamps, combining blue light with yellow/orange light was likely to result in poor colour rendering.
274 In my view, the evidence shows no good reason why a yellow light-emitting phosphor would have been chosen over a combination of red and green light-emitting phosphors. I am satisfied that, at the priority date, the person skilled in the art would have seen both as potentially viable pathways to be explored.
275 The respondent criticised the applicant’s case as presenting the false image of a bewildering range of choices for the person skilled in the art. Whilst I do not accept that the person skilled in the art would have been likely to pursue all the options presented in the applicant’s evidence (for example, the use of defect emission bands, as discussed by Dr Butcher), I am satisfied that the person skilled in the art would have had available the broad options I have described above and that these would have presented the person skilled in the art with a number of realistic choices to pursue that might yield effective solutions.
276 However, the question of obviousness is not directly concerned with choices of this kind. The question is whether the pathway chosen by the inventor is one which the person skilled in the art, equipped with the common general knowledge before the priority date, would have been directly led as a matter of course to try. If obvious, a solution does not become less obvious simply because the person skilled in the art might have been presented with other possibly effective solutions of the kind I have described: see, for example, the related comments made by Gageler and Keane JJ in AstraZeneca AB v Apotex Pty Ltd (2015) 323 ALR 605; [2015] HCA 30 at [115]. Still less will that be the case where these other possibly effective solutions are said to be more obvious.
277 The question thrown up in the present case is whether the person skilled in the art would have been directly led as a matter of course to try the combination of the blue GaN LED (which, on the respondent’s and applicant’s respective cases, is a given) with YAG:Ce to produce a white LED. Other pathways, which might be more or less “obvious”, can be put to one side.
278 Given the applicant’s acceptance that YAG:Ce was part of the common general knowledge (having regard to my finding on the identity of the person skilled in the art), and given the other matters of common general knowledge referred to in the primer, I am satisfied that YAG:Ce was certainly an option and that the person skilled in the art would have been directly led as a matter of course to try it in order to produce a white LED. YAG:Ce was a known phosphor with known spectral properties.
279 But it is not enough that the person skilled in the art would consider it worthwhile to try YAG:Ce in combination with the blue GaN LED. More needs to be established in order for it to be concluded that the invention claimed in claim 1 and claim 3 is obvious in light of the common general knowledge. This is provided by the requirement in the reformulated “Cripps question” that the person skilled in the art must be led to try the posited solution “in the expectation that it might well produce” the outcome that is sought.
280 The expression of expectation is important. It signifies the likelihood, as anticipated by the person skilled in the art, that an event will occur or that a result will be achieved. On the other hand, as Jacob LJ explained in Saint-Gobain PAM SA v Fusion Provida Limited, Electrosteel Casting Limited [2005] EWCA Civ 177 at [35]:
… Mere possible inclusion of something within a research programme on the basis you will find out more and something might turn up is not enough. If it were otherwise there would be few inventions that were patentable. The only research which would be worthwhile (because of the prospect of protection) would be into areas totally devoid of prospect.
281 In my view, it is at this point that the respondent’s primary case on obviousness, based on the common general knowledge, fails. I am satisfied that the person skilled in the art would have considered it worthwhile to explore the use of YAG:Ce in combination with the blue light of the GaN LED, but I am not persuaded that he or she would have done so with the requisite expectation of success.
282 Based on Blasse and Bril I and Blasse and Bril II, the person skilled in the art would have known that YAG:Ce could be used in flying-spot CRTs, especially for colour television. But, at the priority date, YAG:Ce had not been used in an application that had operating conditions comparable to those of the blue GaN LED.
283 It certainly would not have followed from the fact that YAG:Ce could be used in flying-spot CRTs that it could also be used satisfactorily with a blue GaN LED. As Professor Meijerink pointed out in concurrent evidence, the power levels in the two applications are “extremely different”. Moreover, in flying-spot CRTs the laser contacts the phosphor for only a short period of time.
284 By way of example, Professor Meijerink pointed out that, even though ZnS phosphor is extremely stable in a CRT, the applicant’s own experiments showed that it was insufficiently stable when used with a blue GaN LED. Relatedly, Dr Butcher gave evidence that quite often the luminescence of a given material will change depending on the level of intensity of the power source that is used to create that luminescence.
285 Further, Blasse and Bril I teaches that, in the CRT application, the use of YAG:Ce with efficient blue phosphors is required: see below at [297]. The person skilled in that art would not have known, and would not have been able to predict, whether a similar mixture would be required when YAG:Ce was used with a blue GaN LED. I do not accept that the person skilled in the art would have dismissed this possibility simply because the GaN LED itself emitted blue light.
286 Thus, there were a number of unknowns concerning how YAG:Ce with the blue GaN LED would perform, including whether it would produce white light at all. I accept Professor Meijerink’s evidence that YAG:Ce’s stability and performance with a blue GaN LED could not have been predicted, particularly having regard to the high operating temperatures, high photon flux and long operating times of LEDs. These matters would need to be investigated and ascertained by experimentation.
287 Evidence of the patentee’s own experiments is admissible on the question of obviousness and was admitted without objection in the present case: Wellcome Foundation Limited v V.R. Laboratories (Aust.) Proprietary Limited (1981) 148 CLR 262 280-281. The applicant’s own work supports my conclusion concerning the absence of the requisite expectation of success.
288 There can be no doubt that the applicant possessed expertise in the field of inorganic phosphors and other fluorescent materials, such as organic and inorganic dyes and pigments. It had carried out work using such materials with LEDs. Its own work led to the invention of the blue GaN LED. Yet even armed with this expertise and know-how, it was necessary for it to carry out a research programme in order to find a satisfactory way to make a white LED using the GaN LED.
289 I would add that its work in this regard went beyond simply using YAG:Ce as the fluorescent material. The description in the specification, and the claims of the patent, are significantly broader than simply providing a light emitting device using a blue GaN LED with YAG:Ce.
290 Thus, I do not accept the respondent’s submission that the invention was made no later than 30 May 1996. Nor do I accept the respondent’s submission that the applicant arrived at the invention in a short period of time that was indicative of no more than routine work and testing to arrive at the white LED.
291 The applicant selected YAG:Ce because it was worthwhile trying. But even with its own considerable expertise, it simply did not know whether it would work with the blue GaN LED to produce a satisfactory white LED. The level of its expectation of success may be measured by the fact that it concentrated its first efforts on ZnS-based phosphors. Along the way, it experienced failure. It was only after Mr Ogawa’s instruction to test all the yellow light-emitting phosphors that the applicant undertook work on YAG:Ce. It was only through its research work testing YAG:Ce that it arrived at the invention. If the applicant had thought that YAG:Ce had greater prospects of success, then its selection and pursuit of ZnS-based phosphors as the preferred route was nonsensical. I do not accept that the applicant would have acted in such a way.
292 For these reasons, the respondent has not established that, at the priority date, the invention claimed in claims 1 and 3 was obvious and lacked an inventive step by reference to the common general knowledge.
Obviousness: The prior art Documents
Introduction
293 The respondent also relied on the information in the following documents as prior art information for the purposes of s 7(3) of the Act:
G Blasse and A Bril, “A New Phosphor for Flying-Spot Cathode Ray Tubes for Color Television: Yellow Emitting Y3Al5O12-Ce3+”, Applied Physics Letters, Vol 11, No 2, 15 July 1967 (Blasse and Bril I);
G Blass and A Bril, “Investigation of some Ce3+ Activated Phosphors, Journal of Chemical Physics, Vol 47, No 12, 15 December 1967 (Blasse and Bril II);
Blasse G & Grabmaier B C, Luminescent Materials, Springer-Verlag, Berlin, 1994—the parts relied on are the Table of Contents, Chapters 1, 3, 6 and pp 144, 210-219 (the Blasse extract);
W W Holloway Jr and M Kestigian, “On the Fluorescence of Cerium-Activated Barnet Crystals”, Physical Letters, Vol 25A, No 8, 21 September 1967 (Holloway and Kestigian I);
W W Holloway Jr and M Kestigian, “Optical Properties of Cerium-Activated Crystals”, Journal of the Optical Society of America, Vol 59, No 1, January 1969 (Holloway and Kestigian II);
M V Hoffman, “Improved Color Rendition in High Pressure Mercury Vapor Lamps”, Journal of the Illuminating Engineering Society, Vol 6 No 2, January 1977 (Hoffman);
United States Patent No. 4,727,283 titled “Los pressure mercury vapour discharge lamp” (the 283 patent); and
United States Patent No. 3,699,478 titled “Display system” (the 478 patent).
294 As I have noted, having regard to my finding as to the identity of the person skilled in the art, the applicant accepts that Blasse and Bril I and Blasse and Bril II, and certain information in the Blasse extract were part of the common general knowledge.
295 This publication reports on YAG:Ce. It discloses that Ce3+-activated phosphors are usually characterised by an emission band with a maximum in or near the UV region with a very short decay time. It reports that YAG:Ce has emissions at considerably longer wavelengths, in particular a bright yellow emission under excitation with cathode rays as well as with blue radiation.
296 The authors state:
…For the moment we conclude that Y3Al5O12 -Ce3+ is an exceptional Ce3+ phosphor with an emission at relatively long wavelengths and a high efficiency under cr [cathode ray] excitation.
Therefore this phosphor can be of use for flying-spot cathode-ray tubes, especially for color television …
297 In this connection, the authors also speak of a need to use efficient blue phosphors (for example, Ca2Al2SiO7:Ce) in combination with YAG:Ce, which does not emit blue light. Thus, Blasse and Bril I teaches the use of YAG:Ce as part of a mixture of two phosphors which, the authors say, “will suffice” for the application at hand, namely use in flying-spot CRTs.
298 This publication discusses and describes the fluorescence of a number of new Ce3+-activated phosphors, including YAG:Ce. The authors state that the “peculiar behaviour” of YAG:Ce on which they had earlier reported (Blasse and Bril I) prompted them to study the Ce3+ fluorescence in other oxides of the trivalent lanthanides (La3+, Gd3+, Y3+ and Sc3+).
299 The publication includes a table (Table I) showing the efficiencies for UV and cathode ray excitation, positions of emission and excitation bands, and the Stokes shift of some of the phosphors studied, including YAG:Ce. The authors sate that YAG:Ce is the only Ce3+-activated phosphor that has a yellow, not a white, body colour (although the host lattice itself is white).
300 Chapter 1 of this publication provides a general introduction to luminescent materials.
301 Chapter 3 provides a discussion on the emission of light from luminescent materials, including emissions from rare earth ions. The latter discussion relates to rare earth ions exhibiting line emission—Gd3+, Eu3+, Tb3+, Sm3+, Dy3+ and Pr3+; and the rare earth ions exhibiting band emission—the trivalent ions Ce3+, Pr3+ and Nd3+, and the divalent ions Eu2+, Sm2+ and Yb2+.
302 With respect to Ce3+, the Blasse extract discloses that, usually, emission is in the UV or blue spectral region. However, in YAG:Ce the emission is in the green and red spectral region and, in CaS:Ce, the emission is in the red region. The implication from the fact that YAG:Ce emissions are in the green and red spectral areas is that the peak emission would primarily be in the yellow region.
303 Chapter 3 also includes a discussion on luminescent semiconductors. The publication makes clear that it does not aim to treat semiconductors in any detail.
304 Chapter 6 concerns lamp phosphors. It contains a general discussion on luminescent lighting, including the following:
According to the principles of colorimetry, each color can be matched by mixing three primary colors. It is possible to represent colors in a color triangle [2]. Most currently used is the chromaticity diagram standardized by the Commission Internationale d’Eclairage. It is depicted in Fig. 6.2. For a definition of the color coordinates x and y, see Refs. [2] and [3]. The real colors cover an area enclosed by the line representing the spectral colors and the line connecting the extreme violet and the extreme red. The points within this area represent unsaturated colors.
The color points corresponding to Eq. (6.1) are given by the black body locus (BBL). Colors lying on the BBL are considered to be white. White light can be generated in different ways. The simplest one is to mix blue and orange. However, it is also possible to mix blue, green and red. Blending a number of emission bands into a continuous spectrum also yields, of course, white light. All these examples of color mixing are used in lamps, as we will see below.
Apart from the color point, there is another important lamp characteristic, viz. the color rendition. This property depends on the spectral energy distribution of the emitted light. It is characterized by comparing the color points of a set of test colors under illumination with the lamp to be tested and with a black body radiator. The color rendering index (CRI) equals 100 if the color points are the same under illumination with both sources. Under illumination with a lamp with low CRI, an object does not appear natural to the human eye.
305 Professor Meijerink accepted that the reference to mixing blue and orange would comprehend the mixing of blue and yellow because “orange and yellow are very close”. The discussion in Chapter 6 includes reference to the halophosphates (Sb3+ - and Mn2+ - activated calcium halophosphate). The Blasse extract teaches that, by carefully adjusting the ratio of the Sb3+ and Mn2+ ion concentrations, a white-emitting phosphor can be obtained with colour temperatures ranging between 6500 and 2700 K. The authors note, however, that a large drawback of halophosphate lamps is the fact that it is impossible to have simultaneously high brightness and high colour rendering. If the brightness is high, the CRI is of the order of 60. The CRI can be improved up to 90, but then the brightness decreases. The authors state that the use of rare earth activated phosphors has made it possible to achieve the combination of high efficacy (brightness) with a high CRI value.
306 Chapter 6 then turns to a discussion of such phosphors in the context of the realisation of a tricolour lamp, which uses three phosphors which emit in narrow wavelength intervals centred around 450, 550 and 610 nm. The individual phosphors for the tricolour lamp are discussed in separate sections, namely red-emitting phosphors, blue-emitting phosphors and green-emitting phosphors.
307 There is also a section dealing with phosphors for special deluxe lamps, which the authors introduce as follows:
Tricolor lamps show only emission in restricted wavelength intervals. For objects with a reflection spectrum peaking outside these regions the color appearance under illumination with a tricolor lamp will differ from the one under illumination with a black body radiator. Although a CRI of 85 quarantees a normal appearance for most objects, some typical colors will look unnatural under illumination with a tricolor lamp. For certain applications, therefore, a higher CRI is required. Examples of such applications are museum illumination and flower displays. For this purpose special deluxe lamps were developed with a CRI of 95. Simultaneously we have to accept an efficacy drop to 65 lm/W [3].
308 In this section, the authors state that a higher CRI can be obtained using a blue-emitting phosphor with an emission maximum at 490 nm. They state that a further increase of the CRI can be obtained by using band instead of line phosphors for the red and green. In this way, “a more or less continuous spectrum extending from the blue to the red is obtained”. The authors exemplify a phosphor with suitable blue emission and a phosphor with suitable red emission. They state that an efficient broad-band green-emitting phosphor is not known, but can be simulated by combining the Tb3+ emission with the Mn2+ emission of the halophosphate phosphor.
309 The authors then exemplify a special deluxe lamp containing blue-, red-, and green-emitting phosphors. The emission spectrum of this lamp is illustrated in Fig. 6.16 which “shows on the short wavelength side the blue mercury lines”. In the course of that discussion, the authors state that these lines can be efficiently suppressed by adding YAG:Ce. The authors state:
This phosphor, with garnet structure, absorbs blue light and converts it with high efficiency into yellow emission …
310 Chapter 6 goes on to describe and discuss other lamp phosphors. It ends with a section titled “Outlook”, which states:
It will be clear from this chapter that the introduction of rare-earth activated luminescent materials has drastically changed the situation. Apart from the cheaper halophosphate phosphors, we have now available a family of rare-earth activated phosphors which make luminescent lighting ideal. Not only the light output is high, but also the color rendering is excellent. For even better color rendering, the special deluxe lamps give a very good solution, although the light output is lower. The maintenance of these tricolor lamps is also very good. It is not realistic to anticipate important breakthroughs in this field.
311 Chapter 7 deals with cathode ray phosphors. YAG:Ce is mentioned in that context:
In order to transmit pictures in color, the emission of the phosphor should cover the whole visible area. For this purpose a mixture of Y2SiO5 : Ce3+ and Y3Al5O12 : Ce3+ has been used. The former emits in the blue, the latter in the green and red [13]. The reason that Ce3+ in Y3Al5O12 emits at such long wavelengths is due to the extended crystal-field splitting of the excited Ce3+ ion …. This was considered in Sect. 3.3.3.a.
312 Here, the authors note that YAG:Ce was originally developed for the flying-spot scanner tube but that its application today (1994) lies in the special deluxe lamp discussed in Chapter 6. The context in which YAG:Ce is discussed in the Blasse extract is important. In the context of lamps, its use is confined to special deluxe lamps of the tricolour variety, to suppress short wavelength “blue mercury lines”.
313 This publication concerns the fluorescence profiles and other optical properties of three Ce-activated garnet crystals—YAG:Ce, Lu3Al5O12:Ce and Y3Al2Ga3O12:Ce. The authors state that the optical properties of rare-earth doped garnet materials have been extensively investigated because of their importance in laser applications. The authors’ investigation was of the three phosphors under excitation by “commercial long-wavelength u.v. sources”. Blasse and Bril I is cited.
314 This publication refers to the report of preliminary data on the fluorescence of Ce-activated garnet crystals in Holloway and Kestigian I. In relation to the work undertaken in Holloway and Kestigian I, the authors state that a bright-yellow fluorescence was observed in several garnet host materials. They also state that the fluorescence profiles were shown to be host-dependent and, at low temperatures, partially resolve into two components.
315 Holloway and Kestigian II reports on additional measurements of the fluorescence spectra of Ce3+ in garnet materials. Absorption data were also measured in an effort to clarify the nature of the fluorescent site. The authors state their assumption, following Blasse and Bril I that the observed optical spectra arise from the presence of Ce3+ in the garnet materials. They report that the most striking feature of the data they obtained was that the absorption bands were shifted to longer wavelengths. It appears that the phosphors were excited by the same sources used for Holloway and Kestigian I.
316 As the title of this publication makes clear, it deals with improving the colour rendition in the output of high pressure mercury discharge lamps. The publication discloses that YAG:Ce is useful in improving colour rendition by absorbing the blue Hg radiation and adding to the total emission of such a lamp by converting blue radiation into emissions centred at 560 nm. The author states that YAG:Ce can be combined with the Y(VP)O4:Eu emission, effectively changing the colour of the lamp.
317 The author notes that YAG:Ce was developed for its emission characteristics under cathode ray excitation and cites Blasse and Bril I and Holloway and Kestigian II for this proposition. In this connection, the author notes that YAG:Ce is “strongly absorbing in the blue … and is also excited by this radiation”.
318 The author also states:
As with most phosphors, the specific conditions of the lamp determine its applicability, and the composition can often be altered according to its use. In the YAG:Ce phosphor, the cerium concentration is the critical factor. Both the absorption at 436 nanometers and the emission intensity increase with the cerium concentration with the brightness reaching a maximum at about one- to two-atom percent and the absorption at about three-atom percent when measured at 25° C. At the temperature of operation of the lamp, the phosphor is less efficient, and brightness measurements made at 300° C show that lower cerium concentrations are desirable. As shown in Fig. 5, a rather narrow range of cerium content must be maintained for the best conversion of 436 radiation to emission at 560 nm in the lamp.
319 I pause to note that light with a wavelength of 436 nm is in the violet-blue region of the spectrum, and light with a wavelength centred at 560 nm will be a yellow-green light.
320 Figure 5 in the publication shows emission intensity vs Ce content, measured at 25°C and 300°C with an excitation wavelength of 436 nm. Professor Meijerink said that this figure showed that the YAG:Ce was undergoing thermal quenching. Dr Bretschneider accepted that the figure showed some degree of thermal quenching, but that this was not a problem. Dr Bretschneider also remarked that 300°C was a very harsh or extreme temperature.
321 The 283 patent concerns a low pressure mercury vapour discharge lamp whose emission mainly lies in three spectral ranges, with the colour temperature of the emitted light in the range of 2000-3000 K. These are the tricolour lamps discussed in the Blasse extract. The specification states:
These lamps are commonly used in general illumination and have the advantage that they have both a good general colour rendition (colour rendition index R(a, 8) of at least 80) and a high luminous efficacy (up to values of 90 lm/W and higher). This is possible because the emission of these lamps is mainly concentrated in three comparatively narrow spectral bands. For this purpose the lamps contain a red luminescing material whose emission mainly lies in the range of 590-630 nm and a green luminescing material whose emission mainly lies in the range of 520-565 nm. The required emission in the third spectral range, i.e. the range of 430-490 nm, is supplied in many cases by a blue luminescing material. However, the visible radiation emitted by the mercury vapour discharge itself also provides a contribution (i.e. the emission of the 436 nm mercury line) in this spectral range. The lamps emit white light at a given colour temperature, that is to say that the colour point (x,y in the CIE colour coordinate diagram) of the emitted radiation lies on or near the line of the black body radiators. The colour point of fluorescent lamps of low colour temperature is generally chosen to lie preferably slightly above (for example about 0.010 in y coordinate) the line of the black body radiators.
322 Speaking as at 1986, the specification explains that, hitherto, incandescent lamps have been mainly used for interior lighting, where colour temperatures down to about 2000 K can be achieved. A disadvantage of compact fluorescent lamps is that, due to their compact construction, the luminescent layer is “heavily loaded”—meaning that the power consumed by the column during operation of the lamp is at least 500 W/m2 of the surface area of the luminescent layer. Further, the mercury vapour discharge is greater in lamps of this construction. The specification teaches that due to the intense blue mercury radiation of the lamps, they cannot be used in the frequently desired colour temperature range of about 2000 to 2700 K. The object of the invention is to obviate this disadvantage and, in general, to provide means for shifting the colour point of heavily loaded three-band fluorescent lamps to reduce colour temperature while maintaining good colour rendition.
323 The specification summarises the invention as follows:
A low-pressure mercury vapour discharge lamp of the kind described in the opening paragraph is characterized according to the invention in that the lamp is provided with an absorption layer comprising an aluminate activated by trivalent cerium and having a garnet crystal structure.
The said garnet is a known luminescent material (See for example J.O.S.A., 59, No. 1, 60, 1969), which absorbs short-wave ultraviolet radiation, but especially absorbs radiation having a wavelength between about 400 and 480 nm and converts it into radiation in a wide emission band (half-value width of about 110 nm) with a maximum at about 560 nm. It has been found that the use of such luminescent garnet in an absorption layer for three-band fluorescent lamps leads to a shift of the colour point of the radiation emitted by the lamp and allows for a reduction of the colour temperature of the lamp.
A reduction of the colour temperature in itself could be attained with any yellow pigment absorbing blue radiation. However, a yellow pigment leads to a reduction (unacceptable for this lamp type) of the relative luminous flux so that it cannot be used.
The use of the luminescent garnet in lamps according to the invention has the advantage that the absorbed radiation is no lost, but is converted with a high efficiency into visible radiation so that high relative luminous fluxes are obtained. In addition, the lamps according to the invention have high values of R(a, 8), which could not be expected because it is known for three-band fluorescent lamps that radiation in the range of 565-590 nm, in which a comparatively large part of the emission of the garnet is found, is detrimental to the colour rendition properties.
324 The 478 patent concerns laser projection display systems—primarily those producing black and white images. The specification states:
A laser display system results in a black and white image with a minimum of speckling. The system depends upon the use of a phosphorescent screen of cerium-activated garnet energized by a laser emitting in the visible at a somewhat shorter wavelength than the bulk of the emission from the screen. In a preferred arrangement yttrium aluminium garnet containing cerium is used. The characteristically yellowish cast of the emission from this phosphor as seen by the eye is adjusted to appear more nearly white by deliberate reflection of a portion of the laser emission.
Introduction
325 The extent to which the prior art information can be used for the purposes of s 7(2) of the Act, and thereby combined with the common general knowledge, is limited by s 7(3). Like s 7(2), that provision has also been amended from time to time. The form of the provision applicable to the present case is, once again, shown in Reprint 2 of the Act:
For the purposes of subsection (2), the kinds of information are:
(a) prior art information made publicly available in a single document or through doing a single act; and
(b) prior art information made publicly available in 2 or more related documents, or through doing 2 or more related acts, if the relationship between the documents or acts is such that a person skilled in the relevant art in the patent area would treat them as a single source of that information;
being information that the skilled person mentioned in subsection (2) could, before the priority date of the relevant claim, be reasonably expected to have ascertained, understood and regarded as relevant to work in the relevant art in the patent area.
326 The question of what the person skilled in the art could be reasonably expected to have ascertained, understood and regarded as relevant is to be determined on the evidence: Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (No 2) (2007) 235 CLR 173; [2007] HCA 21 at [153]; Otsuka Pharmaceutical Co Ltd v Generic Health Pty Ltd (No 4) (2015) 113 IPR 191; [2015] FCA 634 (Otsuka) at [399]. The “factual environment” in which the notional task presented by s 7(3) is to be undertaken is limited to the common general knowledge: AstraZeneca AB v Apotex Pty Ltd (2014) 226 FCR 324; [2014] FCAFC 99 (AstraZeneca) at [523]; Otsuka at [410].
327 The applicant submitted, and I accept, that, as with all other components of the obviousness inquiry, the question of what the person skilled in the art could be reasonably expected to have ascertained, understood and regarded as relevant must be determined without hindsight. Nevertheless, the intellectual exercise posited by s 7(3) requires that the person skilled in the art be taken as “giving attention to the subject concerned, in the sense of working upon, or at least thinking about, some possibilities for an improvement in the existing state of things, whether by the solution of a problem or otherwise”: AstraZeneca at [524]. As I will explain, the evidence given by Dr Butcher and Professor Meijerink on this subject was directed to this end.
328 In his affidavit, Dr Butcher discussed, amongst other things, the nature of journal searching available to him before the priority date. As I have noted, he was also asked how, before the priority date, he would have gone about finding information relevant to “a white LED project”. To this end, Dr Butcher described the searches he would have carried out. He then carried out searches using keywords chosen by him. After he had performed this task, he was given the prior art documents and asked a number of questions, including whether he was aware of each document before the priority date and, if not, whether he thought he would have found the document by conducting a search of the kind described by him.
329 As I have noted, Professor Meijerink, like Dr Butcher, was asked how, before the priority date, he would have gone about finding information relevant to “a white LED project”. To this end, Professor Meijerink described the searches he would have carried out. He was then given copies of the prior art documents and asked a number of questions, including whether he was aware of each document before the priority date and, if not, whether he thought he would have found the document conducting a search of the kind described by him. In this latter regard, Professor Meijerink reported on the results of various literature searches he conducted through the Web of Science. The Web of Science is a database which covers over 12,000 journals and over 160,000 conference proceedings. Professor Meijerink said that the database includes all the scientific journals and conference proceedings that are important in the field of luminescence spectroscopy.
330 In his second affidavit, Dr Bretschneider discussed the sources of knowledge and information that were available to him before the priority date. As I have noted, he also discussed how, before the priority date, he would conduct a project to develop “a new LED for commercial use that was better than, or an alternative to, the existing blue LED”.
331 Dr Bretschneider said that, if he were to begin a new research project, he would, amongst other things, conduct a literature search. As it happens, in this case, Dr Bretschneider did not carry out a literature search as part of the project he was set. He was, however, given the prior art documents and asked a number of questions, including whether he was aware of each document before the priority date and, regardless of the answer he gave, whether he considered he would have found the document when conducting a literature search of the kind referred to in his affidavit.
332 Although I have proceeded on the basis that Blasse and Bril I and Blasse and Bril II, and certain information in the Blasse extract, were part of the common general knowledge, I will nevertheless record the evidence that was given for the purposes of the s 7(3) inquiry in respect of these documents, as well as the other documents relied on for this purpose.
Blasse and Bril I
333 Dr Bretschneider knew of, and had read, Blasse and Bril I before the priority date. He had found this document from research by others with whom he was working, and had read it in the course of his work, at the Phosphor Technology Center of Excellence. He said that, had he not known of Blasse and Bril I before the priority date, he would have located it during a search for a suitable yellow emitting, blue light absorbing, phosphor based on the document’s title and its abstract which include the words “phosphor”, “yellow”, “fluorescence”, “550nm” and “color”.
334 Dr Bretschneider noted that Blasse and Bril I stated that, as the emission of YAG:Ce does not contain blue, it should be mixed with an efficient blue phosphor. Dr Bretschneider said that this statement was made in the context of CRT excitation and that a blue phosphor would not be required if there was a source of blue light available, such as a blue GaN-based LED.
335 Dr Butcher was not aware of Blasse and Bril I before it had been given to him for review. He said that, before the priority date, he would not have found it doing a search of the kind described in his affidavit. He said that, if he had found it, he would only have regarded it as relevant if he were looking for a phosphor solution to create a white LED. He said further that, even if he had been looking for such a solution, he would not have concluded from Blasse and Bril I that the combination of a blue light and yellow phosphor would have been sufficient to obtain a white light source. This was because Blasse and Bril I said that additional blue phosphors were needed in the “television context”. Dr Butcher said that, based on this, he would have assumed that a variety of phosphors were needed.
336 Dr Butcher disagreed with Dr Bretschneider’s opinion that an additional blue phosphor would not be needed if YAG:Ce were to be used with a blue LED. Dr Butcher said that it was not possible to form this conclusion based on Blasse and Bril I. He said that Blasse and Bril I specifically referred to the need for a blue phosphor in the context of CRT excitation and that, moving to the different context of a blue GaN LED, he would not have known whether or not an additional blue phosphor or phosphors would also have been required.
337 Professor Meijerink said that he was not aware of Blasse and Bril I before the priority date. He said that he did not think that, before the priority date, he would have found it doing a search of the kind he would have carried out. He said that, if he had found Blasse and Bril I, he would probably have considered it relevant to his white LED project because Blasse and Bril I reports on the optical spectra of YAG:Ce, which would have been an interesting candidate for spectral conversion in blue LEDs. Professor Meijerink said that Blasse and Bril I possibly would have increased his interest in luminescent materials with an unusually long emission wavelength for Ce3+.
Blasse and Bril II
338 Dr Bretschneider knew of, and had read, Blasse and Bril II before the priority date. He said that, had he not known of the document before the priority date, he would have located it during a search for information on YAG:Ce, based on the information appearing in the document’s title and abstract.
339 Dr Butcher was not aware of Blasse and Bril II before it had been given to him for review. He said that, before the priority date, he would not have found it doing a search of the kind described in his affidavit. He also said that, if he had found Blasse and Bril II, he would not have regarded it as relevant to his white LED project because it appears to use a UV light source and does not refer to blue excitation.
340 Although he could not recall, Professor Meijerink said that he was “probably aware” of Blasse and Bril II before the priority date because it provides a good overview of the luminescence of Ce3+ in various hosts and also discusses energy transfer from Ce3+ to other ions, a topic he was investigating before the priority date.
341 Professor Meijerink said that it was likely that others in the field of phosphors were aware of Blasse and Bril II before the priority date. He said it was the “standard paper” on YAG:Ce before the priority date. Professor Meijerink also said that it is likely that, before the priority date, he would have found Blasse and Bril II based on the searches he would have conducted for his hypothetical white LED project. Similarly to the comments he made with respect to Blasse and Bril I, Professor Meijerink said that he would have considered Blasse and Bril II to be relevant to his hypothetical white LED project because it reports on the optical spectra of some Ce3+-activated phosphors, which “would have made them interesting candidates for spectral conversion in blue LEDs”. Once again, he said that Blasse and Bril II possibly would have increased his interest in luminescent materials with an unusually long emission wavelength for Ce3+.
The Blasse extract
342 Dr Bretschneider knew of the textbook from which the Blasse extract was taken, before the priority date. He borrowed the textbook regularly from the University of Florida library. He said that, had he not known of the textbook before the priority date, it would have been one he would have considered in his searches described in his affidavit.
343 Dr Butcher said that he was not aware of the Blasse extract before it had been given to him for review. He said that, before the priority date, he would not have found the textbook if conducting a search of the kind described in his affidavit. He said that, if he had found it, he would not have regarded it as relevant to his white LED project unless he had also known at the time that blue light from an LED could excite phosphors—a fact that was unknown to him.
344 Professor Meijerink said that he regularly consulted the textbook before the priority date. He said it was well-known in the “luminescence community” before the priority date. Even so, Professor Meijerink said that nothing in the Blasse extract would have changed his order of preference in carrying out work in his white LED project (which I have discussed at [208] above).
345 As I have noted, a copy of the textbook from which the Blasse extract was taken was in the library of the University of Technology Sydney before the priority date.
Holloway and Kestigian I
346 Dr Bretschneider said that he knew of, and had read, Holloway and Kestigian I before the priority date. He said that he located the document as part of his general research at the Phosphor Technology Center of Excellence. He said that, had he not known of the document before the priority date, he would have located it during a search for information on suitable phosphors—specifically, YAG:Ce. He said that Physics Letters was a well-known journal which he read regularly before the priority date. He said that information appearing in the document’s title and abstract, particularly the words “fluorescence” and “cerium-activated garnet crystals” indicate to him that it is an article that he would have located in carrying out a search.
347 Dr Bretschneider said that Holloway and Kestigian I describes the emission spectra of different Ce-activated garnet crystals when excited by blue light.
348 Dr Butcher said that he was not aware of Holloway and Kestigian I before it had been given to him for review. He said that, before the priority date, he would not have found it doing a search of the kind described in his affidavit. Dr Butcher said that, if he had found Holloway and Kestigian I, he would not have regarded it as relevant to his white LED project because Holloway and Kestigian I refers to a UV light source, rather than a blue light source, to excite the phosphors. Here, Dr Butcher disagreed with Dr Bretschneider. Dr Butcher said that, while Holloway and Kestigian I mentions absorption features at 440 nm and 340 nm, these were not investigated; no blue light source was used. Dr Butcher said that there is no indication in Holloway and Kestigian I that “blue” absorption features at 440 nm were radiative. He said that the experiments might show that some of the phosphors “absorb in the blue and emit in the yellow” using a UV light, but there is no evidence in the document to support the proposition that blue absorption would result in the emission of yellow light.
349 Professor Meijerink said that he was not aware of Holloway and Kestigian I before the priority date. He said that it was very unlikely that, before the priority date, he would have found the document doing a search of the kind described in his affidavit. He based this opinion, in part, on a citation search he conducted which showed that Holloway and Kestigian I had only been cited once in the period 1990 to 1996. Professor Meijerink said that this indicated that there was little interest in the publication and, because it did not appear in the reference lists of other publications, people in the field of phosphors would have been unlikely to have found it by reading other publications.
350 Professor Meijerink said that, had he found Holloway and Kestigian I, he would not have considered the materials that were discussed as promising candidates for “spectral conversion in LEDs”. This was because, when reporting on the spectra of various Ce-activated garnet crystals, Holloway and Kestigian I incorrectly stated the assignment of the yellow emission in YAG:Ce. Specifically, Professor Meijerink said that Holloway and Kestigian I related the yellow emission to Ce3+ pairs, to Ce in a different valence state or to Ce3+ associated with a compensating site, rather than Ce3+ on a regular Y3+ lattice sight. Nevertheless, he said that Holloway and Kestigian I may have prompted him to investigate the materials further as yellow emission related to Ce3+ is “highly unusual”. However, this would not have changed his order of preference in carrying out work in his white LED project.
Holloway and Kestigian II
351 Dr Bretschneider knew of, and had read, Holloway and Kestigian II before the priority date. He said that, had he not known of the document before the priority date, he would have located it during a search for information on suitable phosphors, specifically YAG:Ce. He said that information in the document would have been of particular interest to him because of words like “luminescence”, “bright yellow fluorescence” and “cerium-activated garnet crystals” appearing in the document’s title and abstract.
352 Dr Butcher was not aware of Holloway and Kestigian II before it had been given to him for review. He said that, before the priority date, he would not have found it doing a search of the kind described in his affidavit. Dr Butcher said that, if he had found Holloway and Kestigian II, he would not have regarded it as relevant to his white LED project because Holloway and Kestigian II refers to a UV light source, rather than a blue light source, to excite the phosphors.
353 Professor Meijerink was not aware of Holloway and Kestigian II before the priority date. He said that it was unlikely that, before the priority date, he would have found the document doing a search of the kind described in his affidavit or that many other people in the field of phosphors would have found it. He based this opinion, in part, on a citation search he conducted which showed that Holloway and Kestigian II had only been cited once in the period 1990 to 1996.
354 Professor Meijerink said that, had he found Holloway and Kestigian II, he would probably have considered it relevant to his white LED project because it reports on the optical spectra of various Ce-activated garnet crystals which, once again, would have made them interesting candidates for spectral conversion in blue LEDs. He said that Holloway and Kestigian II may have increased his interest in luminescent materials with an unusually long emission wavelength for Ce3+.
Hoffman
355 Dr Bretschneider had not seen this document before the priority date. Nevertheless, he said that he would have located it during a search for information on suitable phosphors or YAG:Ce, based on the information appearing in the document’s title and abstract, namely “high pressure mercury vapor lamps”, “Y3Al5O12:Ce”, “phosphors” and “color correcting”.
356 Dr Butcher was not aware of Hoffman before it had been given to him for review. He said that, before the priority date, he would not have found it doing a search of the kind described in his affidavit. Dr Butcher said that, if he had found Hoffman, he would not have regarded it as relevant to his white LED project because Hoffman refers to the use of a broad spectrum UV light source. Dr Butcher said that, although Hoffman refers to the filtering of the blue components of the mercury vapour lamp’s spectrum, he would not have regarded that information as relevant.
357 Professor Meijerink was not aware of Hoffman before the priority date. He said that he had never heard of the Journal of the Illuminating Engineering Society in which Hoffman appears. He said that it is very unlikely that he would have found Hoffman doing a search of the kind described in his affidavit or that many other people would have found it. He based his opinion, in part, on a citation search which showed that Hoffman had only been cited once before the priority date. Even then, the citation was in 1980.
358 Nevertheless, Professor Meijerink said that, had he found Hoffman before the priority date, he probably would have considered it relevant to his white LED project because it shows spectra with absorption in the blue and emission in the yellow spectral region for YAG:Ce. In this connection, Professor Meijerink said that Hoffman may have increased his interest in YAG:Ce. However, according to Professor Meijerink, Hoffman also contains information on thermal quenching of the Ce3+ emission.
The 283 patent
359 During his time at AT&T, Dr Bretschneider looked at the patent literature and requested patent searches from AT&T’s IP Department. He said that, in industry-based research, patent searching is undertaken before embarking on a new research project to establish a starting point for research or to see if a particular area is already being investigated, which might indicate that certain work is not worth pursuing (because patents have already been granted in respect of that work). Additionally, Dr Bretschneider said that, before the priority date, it was common to come across references to patents in the literature. He said that, in cases where he thought a particular patent reference was important, he would obtain a copy of the patent.
360 Dr Bretschneider was not aware of the 283 patent before the priority date. However, he said that the 283 patent would have been located by a search of the patent literature for a suitable phosphor for the project he had been set or for information on YAG:Ce. He said that the abstract to the patent contained references to “aluminate”, “cerium” and “garnet”. He also noted that “there are references to YAG generally throughout the specification”.
361 Dr Bretschneider said that the 283 patent tells him that YAG:Ce and similar phosphors could be used as a luminescent coating on low pressure mercury vapour discharge lamps to convert light emitted by the mercury vapour discharge (typically comprised of wavelength lines in the UV and blue regions of the spectrum) to yellow light and that this light, combined with the blue from the vapour discharge would give white light with a number of advantages—in particular, improved colour rendering and a lower colour temperature. Dr Bretschneider said that the 283 patent teaches him that using a phosphor is a good option for ensuring the efficiency of the lamp is not compromised to obtain white light and that not only YAG:Ce but other related phosphors can be used.
362 In the period up to the priority date, Dr Butcher did not search for or review patents. He said that free online search engines for patent review were not widely available and accessible to him. He said that, if he had known of the existence of a particular patent that was of interest to him, he could have requested a patent search by the Macquarie University library staff using keywords chosen by him. He said that “the culture of checking patents before publishing and applying for patents yourself had not begun at Macquarie University at this time …”.
363 Dr Butcher was not aware of the 283 patent before it had been given to him for review. He said that, before the priority date, he would not have found it doing a search of the kind described in his affidavit.
364 Professor Meijerink was not aware of the 283 patent before the priority date. Before the priority date, he rarely searched the patent literature, although he had knowledge of some patents involving X-ray storage phosphors. He said that it was unlikely that he would have found this patent by doing a search of the kind described in his affidavit.
365 Professor Meijerink said that, had he found the 283 patent, he would have considered it relevant to his hypothetical white LED project because the patent discusses spectral conversion of blue/violet light of 436 nm to longer wavelengths (green, yellow) using Ce3+-doped garnet structures. He said that this would have increased his interest in YAG:Ce. He noted, however, that the irradiance for white LED phosphors would greatly exceed the 500 W/m2 for compact fluorescent tubes in which most of the radiation is UV radiation that is not absorbed by YAG:Ce. He said that no conclusions could be drawn from the 283 patent about the stability of YAG:Ce in white LEDs.
The 478 patent
366 Dr Bretschneider had read the 478 patent when he was working on ZnSe semiconductors. He said that one of the inventors, Dr Pinnow, was well-known in the field for his research on phosphor materials and lasers. He said that, if he had not known of the 478 patent before the priority date, he was “very confident” that he would have found it doing a patent search for a suitable phosphor, based on the reference in the patent abstract to “white”, “phosphorescent”, “cerium-doped yttrium aluminium garnet”, “yellowish”, “phosphor” and “blue”.
367 Dr Bretschneider said that the 478 patent “is consistent with the expectation that I would have had before the Priority Date that Ce-activated YAG phosphors could be used to design a white light LED”. In this connection, Dr Bretschneider said that the use of a laser as opposed to a GaN-based LED would not have been a significant difference to him because the wavelength of emitted light needed to stimulate the phosphor “is the same”. He said that the 478 patent is consistent with his expectation before the priority date that YAG was a stable, non-reactive compound. He also said that the phosphor was able to withstand irradiation from a laser beam which, for a low-powered industrial laser of 1W power, would have an intensity of at least 50 to 500 times that of sunlight—much higher than for a GaN LED.
368 Dr Butcher was not aware of the 478 patent before it had been given to him for review. He said that, before the priority date, he would not have found it doing a search of the kind described in his affidavit. However, he said that, had he found it, he would have regarded it as relevant to his white LED project because it shows that blue light excitation could be used to create a white light with yellow phosphors. Nevertheless, he noted that the excitation source was a laser and expressed a reservation as to whether a blue LED could be used in place of a blue laser to excite the yellow phosphors to a useful light intensity. In this connection, Dr Butcher said that, before the priority date, the known blue LEDs only claimed an output of 5 milliwatts (mW). He said it would have been unreasonable to expect a 5 mW LED to be able to achieve a similar result to a 1 W laser. He said that this would have suggested to him that the LED output would be insufficient to achieve any worthwhile emission.
369 Professor Meijerink was not aware of the 478 patent before the priority date. He said that it was unlikely that he would have found this patent by doing a search of the kind described in his affidavit. He noted that the papers in the open literature describing this invention were cited only nine times before the priority date, which suggested to him that it was unlikely that the 478 patent itself would be found.
370 Professor Meijerink said that, had he found the 478 patent, he would probably consider it relevant to his white LED project because it discusses the spectral conversion of blue light of 488 nm or 441 nm to yellow light using Ce3+-doped YAG to generate white light.
Conclusion and reasons
371 The position revealed by the evidence is that Dr Butcher did not know any of the prior art documents before the priority date, and he would not have found them using the literature searches he would have conducted to find information relevant to his white LED project.
372 Professor Meijerink knew of Blasse and Bril II and the Blasse extract, but none of the other documents. He expressed the view that it was unlikely that he would have found Blasse and Bril I, Holloway and Kestigian II, the 283 patent and the 478 patent—and very unlikely that he would have found Holloway and Kestigian I and Hoffman—using the searches he would have conducted to find information relevant to his white LED project.
373 On the other hand, Dr Bretschneider knew of all the prior art documents before the priority date, other than Hoffman and the 283 patent. He said that he would have found, or would have been very confident of finding, all the prior art documents if he had carried out a search.
374 On this aspect of the respondent’s case, I treat Dr Bretschneider’s evidence with the same considerable caution I expressed above. His evidence on what he would have found if he had conducted a literature search at the priority date is equally affected by hindsight and his knowledge of the invention. His evidence was no doubt influenced by his actual knowledge of most of the documents in question, which existed by reason of his own, particular circumstances which cannot be translated readily to the person skilled in the art. He had already commented on a number of the documents when giving his evidence in other, earlier proceedings. He expressed his views based on the titles in question and thus with actual knowledge of the result which the s 7(3) inquiry seeks to explore. As the applicant correctly submitted, this bespeaks hindsight of the highest order.
375 I need not pursue the s 7(3) inquiry in respect of Blasse and Bril I and Blasse and Bril II given the applicant’s qualified concession that they were part of the common general knowledge. But even if that concession had not been made, I would find that Blasse and Bril I and Blasse and Bril II satisfy the test in s 7(3). Professor Meijerink said that it was likely that those in the field of phosphors were aware of Blasse and Bril II before the priority date and that others would have found it on a search of the kind he would have conducted to pursue a white LED project. He described it as the “standard paper” on YAG:Ce before the priority date. I am satisfied that if the person skilled in the art found Blasse and Bril II, he or she would also have found Blasse and Bril I, given that it is cited as prior work in Blasse and Bril II. However, my conclusion in no way alters the conclusion I have already expressed on the question of obviousness, which takes these documents into account as part of the common general knowledge.
376 I am satisfied that the Blasse extract satisfies the requirements of s 7(3). Professor Meijerink said that the textbook from which the extract is taken was well-known in the “luminescence community” before the priority and that he regularly consulted it. I have referred to the fact that a copy was located in the library of the University of Technology Sydney before the priority date.
377 The other prior art documents are problematic. Their inclusion is only supported by Dr Bretschneider’s evidence, on which I can place very little weight in this regard. Seen against Professor Meijerink’s evidence (it being noted that all are relevant to a phosphor-based solution, which would not have been the direction of Dr Butcher’s interest), I am not satisfied that the person skilled in the art could reasonably be expected to have ascertained them—although if ascertained, I would accept that the person skilled in the art would have understood them and considered them to be relevant to work in the art.
378 But even if I am wrong, and these documents stand as information that could be reasonably expected to have been ascertained by the person skilled in the art, I do not think that recourse to this information materially advances the respondent’s case on obviousness. Even though the information in these documents might possibly have heightened interest in investigating YAG:Ce as suitable fluorescent material, the person skilled in the art would be none the wiser as to whether YAG:Ce would work satisfactorily with the blue GaN LED. In short, even armed with this additional information, the expectation of success would be no greater.
379 For these reasons, the respondent has not established that, at the priority date, the invention claimed in claims 1 and 3 was obvious and lacked an inventive step by reference to the common general knowledge read with prior art information.
Obviousness: Secondary Indicia
380 The applicant sought to support its contention that the invention claimed in claims 1 and 3 was not obvious at the priority date by relying on the commercial success of its white LED products: Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (No 2) (2007) 235 CLR 173; [2007] HCA 21 at [115]. Its submissions were based on Mr Shinomiya’s evidence. However, Mr Shinomiya’s evidence did not distinguish sufficiently between light emitting devices claimed in the patent and successor products which were developments of the products and methods claimed in the patent. For example, when discussing commercial success, Mr Shinomiya’s evidence included the applicant’s “warm white” products which incorporate a “new red phosphor”. These products are covered by a different patent.
381 On the basis of this evidence, I am unable to come to any conclusion on whether the invention claimed in claims 1 and 3 has achieved commercial success of the kind that would support its validity in light of the respondent’s challenge on obviousness. However, in light of the findings I have made above, this is without consequence and need not be considered further.
382 In order to be patentable, an invention must be a manner of manufacture within the meaning of s 6 of the Statute of Monopolies: s 18(1)(a) of the Act.
383 In contending that the invention claimed in claims 1 and 3 is not a manner of manufacture, the respondent framed its case on NV Philips Gloeilampenfabrieken v Mirabella International Pty Limited (1995) 183 CLR 655 (Philips) where, at 663-664, Brennan, Deane and Toohey JJ said that the opening words of s 18(1) of the Act impose a threshold requirement that there be an “invention” and that, despite any assertion of newness in the specification, the requirement that there be an invention will not be satisfied:
… if it is apparent on the face of the relevant specification that the subject matter of the claim is, by reason of absence of the necessary quality of inventiveness, not a manner of manufacture for the purposes of the Statute Monopolies. That does not mean that the threshold requirement of “an alleged invention” corresponds with or renders otiose the more specific requirements of novelty and inventive step (when compared with the prior art base) contained in s 18(1)(b). It simply means that, if it is apparent on the face of the specification that the quality of inventiveness necessary for there to be a proper subject of letters patent under the Statute of Monopolies is absent, one need go no further.
384 The specific example of the absence of inventiveness considered in Philips was the one discussed in Commissioner of Patents v Microcell Limited (1959) 102 CLR 232 (Microcell): broadly expressed, there can be no invention in “the new use of an old substance” (there are significant qualifications to this aphorism).
385 In Microcell, the question was whether a self-propelled-rocket projector comprising a tube of synthetic resinous plastic material reinforced with mineral fibres was a patentable invention. With respect to that alleged invention, the High Court said (at 251):
We have in truth nothing but a claim for the use of a known material in the manufacture of known articles for the purpose of which its known properties make that material suitable. A claim for nothing more than that cannot be subject matter for a patent and the position cannot be affected either by the fact that nobody thought of doing the thing before, or by the fact that, when somebody did think of doing it, it was found to be a good thing to do.
386 In Advanced Building Systems Pty Limited v Ramset Fasteners (Aust) Pty Limited (1998) 194 CLR 171; [1998] HCA 19 (ABS), the High Court explained (at [36]-[40]), with reference to Philips, that the notion that a “new use of an old substance” cannot be patentable subject matter was a 19th century development of the law on obviousness and lack of inventive step. Where this lack of inventive step was admitted on the face of the specification, the grant of a patent could be refused in the first instance on the basis of the admission made. The admission disentitled the patent applicant to argue that even an alleged invention was disclosed. It should be noted here that Microcell makes clear (at 246) that an express admission is not necessary. What is important is the state of affairs shown on the face of the specification itself. In ABS, the High Court went on to explain that if the patent application had in fact proceeded to grant, the grant would then be liable to revocation on the ground of obviousness.
387 It will be appreciated that, in light of my finding that the invention claimed in claims 1 and 3 was not obvious at the priority date, the respondent’s case, framed on Philips, now sits rather oddly. It would be a strange outcome if, after a contested hearing in which it was found that the claimed invention was not obvious, the Court would nevertheless find that there is no patentable invention because a lack of inventiveness is manifest on the face of the specification.
388 In developing its case on this ground, the respondent said that the only invention claimed is the combination of the blue GaN LED and YAG:Ce (or modifications thereof). The respondent submitted that there is no assertion in the specification that this combination was “surprising or unexpected”. The respondent then submitted that the specification does not (and cannot) assert that, at the priority date:
blue GaN LEDs were new;
YAG:Ce was a new phosphor or that previously unknown properties of YAG:Ce were discovered;
the combination of blue light and yellow light to create white light was a discovery; or that
it was unknown that fluorescent materials could be used to convert the wavelength of light.
389 The respondent submitted that the patent claims no more than the new use of a known material (YAG:Ce) with blue GaN LEDs (well-known articles of manufacture) in the manufacture of known articles (lights combined with phosphors) for the purpose of which the known properties of YAG:Ce (it is excited by blue light and emits yellow light and is stable) made it suitable. The respondent submitted that the present case is analogous to Microcell and the claims lack the necessary quality of inventiveness.
390 I do not accept these submissions.
391 The respondent’s case cannot be made out on the basis that the specification does not or could not state the matters noted at [388]. The focus of attention must be what the specification does say or disclose, not what it does not say or disclose. Moreover, once one moves from the face of the specification to other information in order to consider inventiveness, the requirements of s 18(1)(b)(ii) are being addressed, not the threshold requirement of s 18(1): Bristol-Myers Squibb Company v FH Faulding & Company Ltd (2000) 97 FCR 524 at [30]; AstraZeneca AB v Apotex Pty Ltd (2014) 226 FCR 324; [2014] FCAFC 99 at [384]; Otsuka Pharmaceutical Co Ltd v Generic Health Pty Ltd (No 4) (2015) 113 IPR 191; [2015] FCA 634 at [356]-[358].
392 Further, there is no need—and certainly no legal requirement—for the specification to make some positive affirmation of inventiveness, such that the claimed inventive combination is, to take the respondent’s example, “surprising or unexpected”, in order to make out the threshold requirement of s 18(1) of the Act.
393 The present case is not of the kind considered in Microcell, where the High Court (at 248) gave the example of stainless steel:
If stainless steel and its properties were known, and many kinds of articles had been made of it, it would not be possible for a man to claim a monopoly for making kitchen sinks of stainless steel merely because he was the first man who ever thought of doing this.
394 In the present case, the GaN LED and YAG:Ce might have been known, but there is nothing on the face of the specification that indicates, contrary to the evidence, that YAG:Ce had ever been used with an LED to convert the wavelength of light or that it was known that it could be used for this purpose, let alone provide, successfully, a white light emitting device. The specification presents the phosphors of the invention as a novel, useful and inventive advance in the use of LEDs to provide such a device.
395 For these reasons, the respondent has not established that the threshold requirement of s 18(1) of the Act was not met at the priority date.
Introduction
396 An invention is taken to be novel when compared to the prior art base unless it is not novel in light of, amongst other things, prior art information made publicly available in a single document: s 7(1) of the Act.
397 The respondent alleges that the invention claimed in claims 1 and 3 was not novel at the priority date in light of the following prior art information, considered separately, which the respondent described as:
1. “enclosure with letter from Hans-Dieter Wustlich to Marcus Menden dated 22 September 1995 and posted in Germany” ; and
2. “2 page flyer entitled ‘White News (COB Technologie) 02/1995’ distributed by Wustlich Mikro-Elektronik GmbH and Wustlich Opto-Elektronik GmbH at least in Germany in around 1995”.
398 The first-mentioned prior art information in fact comprises a letter and two enclosures. I will refer to them as Document 1. The second-mentioned document in fact comprises a letter and an enclosure—the “2 page flyer”. I will refer to them as Document 2.
399 The applicant accepts that if either Document 1 or Document 2 was publicly available before the priority date, then the invention claimed in claims 1 and 3 would have been anticipated and claims 1 and 3 would be invalid. However, the applicant disputes the authenticity of each alleged disclosure.
The evidence of public availability
400 The respondent says that, on 22 September 1995, a letter bearing that date, which contained certain enclosures, was sent by Wustlich Mikro to Menden Buchstaben (Menden). The letter was addressed to Marcus Menden (the Menden letter). The respondent says that the Menden letter and its enclosures were received on 25 September 1995, and signed by Mr Menden on that day. The respondent says that the Menden letter and its enclosures were then collected by Mr Wustlich on 28 September 1995.
401 At the time, Wustlich Mikro was a German company located in Kamp-Lintfort which, together with Wustlich Opto, was involved in developing and marketing optoelectronics, in particular LEDs with metal and plastic casings and selected LED arrays and displays, as well as LCD backlight modules. The Managing Partner of Wustlich Mikro and Wustlich Opto (together, the Wustlich companies) was Hans-Dieter Wustlich. Menden was a German company located at Essen that was involved in illuminated advertising. Mr Menden was the Chief Executive Officer.
402 The Menden letter said (as translated):
Dear Mr Menden,
Thank you for our interest in our products from our 1994/95 catalogue page 4, especially product WU-M-001-B, but in white.
After extensive tests, from prototypes through to small and medium-sized series, we have found process reliability using a GaN chip and a covering of silicon and or epoxy mixed from yttrium aluminate: Ce (Y3Al5O12: Ce), to obtain, from the blue chip, a chip emitting white light.
Please decide for yourself whether the luminosity currently meets your requirements on the basis of the 150 samples which we are providing you from ongoing production.
In annex, we attach our preliminary data sheet in the chip on board technology with the conversion and use of the aforementioned material from the company Osram.
Furthermore, if you should not decide to use the chip on board technology, we can also offer from our own production our light-emitting diodes, as per page 27 of the same catalogue in white.
Here too, extensive tests of the same procedure have been completed.
Contrary to the COB technology, here we have only moulded light-emitting diodes – lead-frames – with a reflector and previously dispensed, via the blue chip, the converter material into the reflector, as in the COB technology.
The moulding material – materials from the company Dexter Hysol – has been successfully used by us for LED-production for over 5 years.
Here too we attach 120 white LED products from our own production.
In annex we also attach a type WU-1-62BC data sheet with the change to white LED.
We would be delighted if you were to opt for our white light-emitting diodes and our white COB technology and remain,
Yours sincerely,
Kamp-Lintfort, 22 September 1995
[signed]
Hans-Dieter Wustlich
CEO
403 Some of the evidence originally sought to be adduced by the respondent on this part of its case was objected to. At the request of the parties, a number of these objections were dealt with in advance of the hearing. I made a number of rulings, and deferred other rulings. In some instances, I said that I would admit the evidence provisionally: see Reasons 3. I will not repeat those rulings here. There is, however, one important matter of context concerning the objections that were made: Mr Wustlich had died some time before the hearing and Mr Menden had refused to give evidence. For the purpose of dealing with the objections, the parties accepted that Mr Wustlich and Mr Menden were each a person who, for the purposes of s 63(1) of the Evidence Act 1995 (Cth) (the Evidence Act), had made a previous representation and who was “not available to give evidence about an asserted fact”.
404 At the hearing, I admitted, as Exhibit 11, a redacted version of a verified declaration that had been made by Mr Wustlich in the FPC proceeding (the Wustlich declaration). The Wustlich declaration annexed a copy of the Menden letter and its enclosures. I made a ruling under s 136 of the Evidence Act in respect of one part of the declaration.
405 In the Wustlich declaration, Mr Wustlich said that one of the enclosures was a provisional data sheet that served as a substitute for the data sheet that was generally used by the Wustlich companies. Mr Wustlich said that the data sheet that was generally used was out of stock. The provisional data shows the structure of a chip on board (COB) module (i.e., a semiconductor mounted on a printed circuit board) and contains handwriting relating to the constituent materials of a mixture of epoxy or silicon and YAG:Ce over and around a blue GaN semiconductor chip. The YAG:Ce is shown as “L175(Y3Al5O12:Ce”. It was apparently sourced from OSRAM GmbH (OSRAM). Mr Wustlich said that the provisional data sheet was signed and dated by him on 22 September 1995, the same date as the Menden letter.
406 Mr Wustlich said that he also attached 120 white LED products “from our own production” and “a Type WU-1-62 BC data sheet with the change to white LED”. This data sheet shows an LED incorporating a GaN semiconductor chip with a mixture of epoxy or silicon and YAG:Ce. It describes the YAG:Ce as “CY3Al5O12:CE=L175”. In the Wustlich declaration, Mr Wustlich said that he could only imagine that the letter “C” before the YAG formula in this data sheet was a “typing or reading error”. He said that the formula was copied from a handwritten label on a bottle received by Wustlich Mikro from OSRAM. Mr Wustlich also said that the COB modules and the LEDs were no longer at the prototype stage; they came from ongoing production.
407 It is necessary to point out at this stage that the reference in the two data sheets to L175 is to OSRAM’s YAG:Ce phosphor. Each data sheet also refers to the phosphor as “yttriumaluminat: Ce”. This is important because OSRAM’s data sheets only referred to the L175 product as (when translated into English) either “Yttrium Aluminate: Ce” or “Yttriumaluminiumgarnet Ce”. The description “Yttrium Aluminate: Ce” was only used in a data sheet prepared by OSRAM in September 1996, after the priority date. From March 1989 until September 1996, customers who requested a data sheet from OSRAM for the L175 product received one which referred to the product as “Yttriumaluminiumgarnet Ce”.
408 In the Wustlich declaration, Mr Wustlich said that he visited Mr Menden on 28 September 1995 and got his “written quote” back with a receipt stamp dated 25 September 1995. He said that he also collected an order that Mr Menden had placed by hand.
409 In his affidavit, Dr Richter said that Mr Wustlich gave evidence in the FPC proceeding and that Document 1 was presented to him by the Presiding Judge and became an exhibit. Dr Richter also said that Mr Menden gave evidence in the FPC proceeding and referred to Document 1. He said that Document 1 was presented to Mr Menden by the Presiding Judge.
410 The respondent says that a letter dated 28 September 1995 and its enclosure were sent by Wustlich Mikro to Carl Brose GmbH (Brose). Brose was a German company located in Wuppertal. The letter was written to Christoph Schroeder (the Schroeder letter). From 1990 to 1998, Mr Schroeder was the technical manager of Brose with responsibility for its electronics development, mechanical construction and project management departments. The respondent says that the Schroeder letter was sent to Brose on 28 September 1995 and received on 2 October 1995.
411 Relevantly, the Schroeder letter said (as translated):
Catalogue/products – white
Dear Mr Schroeder,
Thank you for your interest in our Wustlich Mikro- und Optoelektronik GmbH products.
We are in the fortunate position of being able to inform you that we have already successfully set up our own production in COB technology for white luminous modules and have also increased our own rate of production of white light-emitting diodes. Production is currently dependent on the availability of blue GaN chips from the company Cree in the United States. We hope to increase intensities in the future, in order to be able to perform various applications.
Mr Wustlich already told you that our GaN chips in conjunction with the Osram converter [yttrium aluminate = (Y3 Al5 012:Ce=L175] and the mixture of silicon or epoxy dispensed over the chip led to a white success.
We attach our flyer from February 1995, which will give you a bit more of an understanding of the technology used. For review we attach 10 type WU-M-001 W products in white. Please see page 4 of our attached 1994/1995 catalogue for the mechanical measurements. We also attach to this letter in accordance with your wishes 1,500 5 mm white LEDs. For the mechanics please see page 27 of our 1994/1995 catalogue on type WU-1-61 BC. The difference is here only the fact that we have dispensed the L175 over the chip (blue GaN), as in the COB technology.
We would be delighted if you liked our products,
Yours sincerely,
Wustlich Opto-Elektronik GmbH
Wustlich Mikro-Elektronik GmbH
[signed]
Hans-Dieter Wustlich
(CEO)
Enc.
Flyer February 1995
10 WU-M-0001 W products
1,500 Wu-1-61 products
412 The Schroeder letter makes clear that, as at 28 September 1995, Wustlich Mikro was producing white LEDs. The letter indicates that the white LEDs were being manufactured using a Cree GaN chip. However, it is an accepted fact that Cree did not supply the Wustlich companies with blue GaN LED chips until 19 August 1996, after the priority date. Further, the Schroeder letter states that the white LEDs used YAG:Ce phosphor from OSRAM, described as “yttrium aluminate”. As I have noted, it was not until after the priority date that OSRAM stopped referring to the L175 product as “Yttriumaluminiumgarnet”.
413 The enclosure in the Schroeder letter is a document which is headed, on one page, “White-News (COB Technologie) 02/1995” and, on the other page, “White-News (LED Technologie) 02/1995”. The reference “02/1995” indicates that the document was current in February 1995.
414 The page headed “White-News (COB Technologie) 02/1995” is very similar to the provisional data sheet described by Mr Wustlich as having been enclosed with the Menden letter. It refers to the phosphor as “Yttriumaluminat: Ce” from OSRAM. The formula CY3Al5O12:Ce is given, as well as reference to the product number L175.
415 The page headed “White-News (LED Technologie) 02/1995” is similar to the other data sheet in the Menden letter. It also refers to “Yttriumaluminat: Ce” from Osram. Once again, the formula CY3Al5O12:Ce is given, as well as reference to the product number L175.
416 Some of the evidence originally sought to be adduced by the respondent on this part of its case was also objected to. My consideration of these objections is in Reasons 3. At that time, I rejected paragraphs 3 to 7 of an affidavit made by Mr Schroeder in which he sought to give evidence of his own testimony in the FPC proceeding concerning his receipt of Document 2. I rejected those paragraphs on the basis of hearsay and also on discretionary grounds: Reasons 3 at [42]-[44]. I should also record that, in a separate application made at the same time, I refused the respondent’s request that Mr Schroeder be permitted to give his evidence by video link: Reasons 3 at [53]-[82]. In part, I was not satisfied that the respondent had taken reasonable steps to secure Mr Schroeder’s personal attendance at the hearing. Notwithstanding that refusal, there remained, at that time, the prospect (however slim) that Mr Schroeder might give evidence in person at the hearing concerning his alleged receipt of the Schroeder letter.
417 I mention these matters because, in Reasons 3, I indicated that I would admit, provisionally, paragraph 32 of Dr Richter’s affidavit: Reasons 3 at [45]. In that paragraph, Dr Richter said that, during his evidence in the FPC proceeding, Mr Schroeder said that he received Document 2. Dr Richter also said that Document 2 was presented to Mr Schroeder by the Presiding Judge and became an exhibit in the FPC proceeding. I was satisfied as to the provisional relevance of Dr Richter’s evidence in this regard under s 57(1) of the Evidence Act. I was satisfied that, even though hearsay in nature, the reception of paragraph 32 was supported by s 64(3) of the Evidence Act, given that the prospect remained that evidence would be adduced from Mr Schroeder concerning this fact in issue, despite the fact that I had declined to permit Mr Schroeder’s evidence to be given by video link.
418 When Dr Richter’s affidavit came to be read at the hearing (on the last day for giving evidence), the admissibility of paragraph 32 was raised by the respondent, in whose case the affidavit was sought to be read:
MR BEVAN: Your Honour, I don’t know if it is caught by your Honour’s ruling in which your Honour rejected Mr Schroeder’s affidavit evidence as hearsay. If it is, I understand, of course, and I don’t want to cavil with that. If it’s not, I would like to be able to use it for its hearsay purpose.
HIS HONOUR: Well, in paragraph 52 of the rulings, I said that I will admit paragraphs 30 to 33 of Dr Richter’s affidavit provisionally under section 57(1) of the Evidence Act, other than the last sentence of paragraph 33 which I will reject. So I would provisionally admit them.
419 On reflection, I think that the better approach would have been to reject paragraph 32 of Dr Richter’s affidavit at that time. By then, there was no prospect of Mr Schroeder giving evidence of his alleged receipt of Document 2. In those circumstances, no support for the reception of paragraph 32 could have been provided by s 64(3) of the Evidence Act. At the time of the exchange quoted above, I overlooked that fact. In fairness to the respondent’s counsel, this lack of support might have been part of the reason why he raised the admissibility of this paragraph at that time.
420 Having considered the matter further, I will revisit the admissibility of paragraph 32 of Dr Richter’s affidavit and reject it. No procedural prejudice will be caused to the respondent by taking this course because, at the time the question of its admissibility was raised at the hearing, no step could have been taken by the respondent to support it or to adduce any other admissible evidence on the topic. In any event, for the reasons given below, I would place no weight on Mr Schroeder’s statement in the FPC proceeding that he received Document 2.
421 As I have noted at [28] above, in 2000 Dr Kramer was appointed co-Managing Director of Vossloh-Wustlich with Mr Wustlich, and remained employed by Vossloh-Wustlich in that capacity until May 2005.
422 Dr Kramer’s appointment came as a result of Vossloh AG (Vossloh) acquiring the Wustlich companies. Prior the acquisition, Dr Kramer was Vossloh’s Head of Corporate Development and Auditing. His responsibilities included mergers and acquisitions. In the course of Vossloh planning to acquire the Wustlich companies, Dr Kramer carried out a review of the Wustlich companies’ LED technology and know-how. He was provided with copies of the companies’ patents, including two patents registered by Mr Wustlich. Dr Kramer was also aware that the applicant had obtained a European patent—EP 0 936 682 (Nichia’s European patent).
423 On 22 May 2001, Vossloh-Wustlich filed an opposition to Nichia’s European patent. Vossloh-Wustlich deployed Document 1 and Document 2 in the opposition.
424 In 2002, Mr Wustlich gave Dr Kramer a copy of Document 1 and Document 2 (it seems, other than the Schroeder letter). Dr Kramer said that, when he reviewed these documents, he had some doubts about their authenticity. In his affidavit, he advanced two reasons for these doubts. First, he was surprised that Mr Wustlich had not applied for a patent for the product described in the documents. He had earlier reviewed the Wustlich companies’ patents and considered them to be distinguishable from Nichia’s European patent because the Wustlich companies’ patents covered backlight applications. Secondly, Dr Kramer found the documents to be unusual. There was, for example, a receipt stamp on every page of the Menden letter. He also noted that the drawings shown in enclosures had the same creation date (22 September 1995) as the Menden letter.
425 Dr Kramer said that, in 2002, Nichia was an important supplier of Vossloh-Wustlich and that, in his capacity as co-Managing Director, he considered it to be important to ensure that Vossloh-Wustlich’s opposition to Nichia’s European patent was based on “credible documents”.
426 Dr Kramer said that, in or around August 2002, he had a conversation with Mr Wustlich in Mr Wustlich’s office at Vossloh-Wustlich. Dr Kramer recounted the conversation in these terms:
I said: Dieter, I need to talk to you about the white light patent. As you are well aware, Nichia is an important business partner to us. We are trading Nichia products, which makes a significant portion of our sales revenue. As well, we like to use their blue chips for our own chip-on-board and backlighting modules. I am aware of the opposition to the Nichia Patent and I do have my doubts about those documents.
He said: Michael, what do you want from me?
I said: Dieter, I want to conduct my business in an ethically correct way. From the historic knowledge I have from the due diligence on the Vossloh buy-side, I got the impression that patents are very important to you. So, why didn’t you file a YAG white light conversion patent?
He said: I didn’t realise how significant it was at the time. I slept.
I said: The letter to Menden and the White Light Flyer don’t look authentic to me. They look so different to the regular way we do business. I also asked Mr Cladders what he thinks about the opposition. His answer was “make up your mind, you know Mr Wustlich”.
He said: Don’t you believe me?
I said: Yes, I think the documents are made up.
He said: I made the documents up.
I said: Why?
He said: As managing director, your priority is to protect the interests of our business. It is best for the company if we are able to produce our own white LEDs without getting licenses from Nichia. If you withdraw the opposition you have no clue what damage you are doing to the company. I think you are incapable as a managing director.
I said: If you think so, that is your opinion. Anyhow, I know what I have to do now.
427 Dr Kramer said that, in or around December 2002, he had a lengthy conversation with Mr Okude and Mr Sato of Japanese Matsushita Electric Works (a shareholder of Vossloh-Wustlich that had taken over the electrics division of the company) in which he urged them to withdraw Vossloh-Wustlich’s opposition to Nichia’s European patent, based on his strong belief that Document 1 and Document 2, which, as I have said, had been deployed in the opposition, were forgeries. The opposition was withdrawn on 29 January 2003.
428 Dr Kramer was cross-examined on this evidence. I found him to be a sincere and honest witness, who had a clear recollection of the events in question, notwithstanding that they occurred some years ago. I have no hesitation in accepting his evidence.
429 By 2 October 2001, oppositions to Nichia’s European patent had been filed by OSRAM, Toyoda Gosei Co, Ltd, and Wolfgang Grosse. These oppositions were withdrawn on 4 July 2002, 25 September 2002 and 30 September 2002, respectively.
The respondent’s submissions
430 The respondent advanced the authenticity of Document 1 and Document 2 as documents emanating from the Wustlich companies that had been signed by Mr Wustlich on the dates the documents bear. The respondent pointed to the nature of the Wustlich companies’ business in 1995 and submitted that it almost went without saying that the companies must have employed people with the necessary technical expertise and experience to produce LEDs. The respondent also submitted that, in these circumstances, it would not be surprising that the companies had technical drawings and information about white LEDs by at least the end of September 1995. The respondent placed reliance on the fact that the Menden letter and the Schroeder letter each bear receipt stamps, indicating the date on which Document 1 and Document 2 became publicly available.
Conclusion and reasons
431 I accept that Document 1 and Document 2 were prepared by Mr Wustlich. However, on the balance of probabilities, I am not persuaded that these are genuine documents that made the information in them publicly available on or around the dates which the documents bear.
432 On the evidence before me, I am satisfied that Document 1 and Document 2 were fabricated by Mr Wustlich after the priority date for the purpose of opposing the applicant’s European patent that corresponds to the patent in suit. In reaching this conclusion, I need go no further than Dr Kramer’s evidence of his conversation with Mr Wustlich, recorded above.
433 The respondent attacked Dr Kramer’s evidence on a number of bases. The respondent also suggested that, when he said that he had “made up” the documents, Mr Wustlich “was not being serious”. Having regard to the whole of the conversation, the circumstances in which it took place, and the steps taken following it, I find it impossible to accept that Mr Wustlich “was not being serious”. Dr Kramer did not accept that suggestion when it was put to him in cross-examination.
434 Further, as I have said, Dr Kramer was a sincere and honest witness, with a clear recollection of the events in question. This was an important conversation on a serious matter, where Dr Kramer confronted his co-Managing Director with his concerns about the falsity of the documents. I have no reason to doubt the honesty of Dr Kramer’s evidence or his ability to recall the general effect of that conversation. I do not propose to detail the respondent’s many criticisms of Dr Kramer’s evidence. It is sufficient for me to note that I am not persuaded that any of the matters raised stand as a reason to doubt Dr Kramer’s credibility.
435 There are other indications of falsity which support the conclusion that Document 1 and Document 2 are fabrications. I will refer to only some of them.
436 First, the Schroeder letter states that the Wustlich companies’ production of white LEDs was currently dependent on the availability of blue GaN LEDs from Cree. As I have noted, the accepted fact is that Cree did not supply the Wustlich companies with blue GaN LEDs until 19 August 1996, after the priority date. Thus, the statement about LEDs from Cree could not accurately convey the state of affairs as at September 1995. The respondent endeavoured to explain this away by suggesting that the Wustlich companies could have obtained blue GaN LED chips from another supplier, such as Toyoda Gosei, and that the Schroeder letter was only talking about future production using Cree LEDs. I do not think that this is a correct reading of the Schroeder letter. In my view, it is clearly talking about current production using Cree LEDs. Further, there is no evidence that would support the respondent’s supposition that the Wustlich companies had obtained blue GaN LED chips from Toyoda Gosei or any other company at any time relevant to this issue.
437 Secondly, Document 1 and Document 2 refer to the L175 phosphor as “ytrriumaluminat” whereas, in data sheets provided from March 1989 up to September 1996, OSRAM referred to the phosphor as “Yttriumaluminiumgarnet”. OSRAM only commenced using the description (as translated) “Yttrium Aluminate” in a new data sheet prepared in September 1996, after the priority date. The likelihood is that Document 1 and Document 2 were prepared by reference to the new data sheet.
438 Thirdly, contrary to the respondent’s suggestion, there is no evidence that would lead me to conclude that, as at September 1995, the Wustlich companies possessed the expertise or experience to produce white LEDs.
439 There are other peculiarities concerning Document 1 and Document 2 and the manner of their creation. Dr Kramer referred to a number of them. These matters no doubt contributed to his suspicions that Document 1 and Document 2 were fabrications. However, in light of the conclusion to which I have come, I do not propose to deal with these additional matters which, on the whole, would only provide more support for the conclusion that Document 1 and Document 2 are fabrications.
440 Apart from the markings on Document 1 and Document 2, there is no evidence of their receipt by any person, other than Dr Richter’s evidence concerning Mr Schroeder’s testimony in respect of Document 2. As I have said, I now reject Dr Richter’s evidence in this regard as inadmissible hearsay. In any event, I can place no reliance on this aspect of Dr Richter’s evidence because, regardless of what Dr Richter heard Mr Schroeder say in the FPC proceeding, I am satisfied that Document 2 was fabricated by Mr Wustlich after the priority date. I am satisfied that a document in the terms of Document 2 was not sent to Mr Schroeder before the priority date.
441 For these reasons, the respondent has not established that, at the priority date, the invention claimed in claims 1 and 3 was not novel.
s 40 matters: Lack of fair basis, definition and clarity
442 The respondent contended that, if the applicant’s construction of claims 1 and 3 were to be adopted, then the invention, as so claimed, would not be fairly based on the matter described in the specification and would not define the invention. The respondent also contended that the definition of the invention would not be clear.
443 I reject the respondent’s contentions that the invention, as so claimed, would not be defined or that the definition would not be clear. A claim does not lack definition, and the definition does not lack clarity, simply because the claim admits of two arguable constructions and one construction of the claim is chosen over another as the correct construction.
444 As to the question of fair basing, my conclusion on the construction of claims 1 and 3, which rejects the applicant’s construction, means that this ground of invalidity, as advanced by the respondent, does not arise for consideration.
445 The originating application and the cross-claim should be dismissed. I will hear the parties on the question of costs. Each party is to submit a draft of the orders it proposes (including on costs) in light of the findings I have made. Each party may make supporting submissions in writing, limited to three pages. The applicant should provide its draft orders and submissions to my Associate by 4.00pm on 14 August 2017. The respondent should provide its draft orders and submissions to my Associate by 4.00pm on 21 August 2017. I will then deal with the matter on the papers.
I certify that the preceding four hundred and forty-five (445) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Yates. |
Associate:
Supplementary Primer
Contents
4. Absorption and emission of light at different wavelengths 102
5. Nitride compound semiconductor 103
9. The general formula of claim 3 107
9.1 Structure of the general formula of claim 3 107
1. There are different processes that can lead to the emission of light. Incandescence refers to the process of emission of electromagnetic radiation from a hot body as a result of its temperature, also known as 'black body radiation'. Luminescence refers to the process of emission of electromagnetic radiation when the excitation process involves high energy photons, fast electrons, a chemical reaction or another mechanism different to black body radiation.
2. Photons are the elementary particles of electromagnetic radiation. Electromagnetic radiation is the technical name for the energy that is propagated by waves of electromagnetic fields. Electromagnetic radiation covers photon energies that are very high and have very short wavelengths such as x-rays, down to low energies in the form of microwaves and radio waves which have very long wavelengths.
3. The term light is often used to refer to electromagnetic radiation of energies (or wavelengths) that are visible to humans, and those adjacent to them (ultraviolet and infrared wavelengths).
4. Processes that can result in luminescence include bombardment by high energy electron beams (cathodoluminescence), electrical energy (electroluminescence), chemical reactions (chemiluminescence) and the absorption of photons (photoluminescence).
5. Photoluminescence is light (photon) emission after the absorption of photons (termed photoexcitation or excitation).
6. Common sources of luminescence are the relaxation transitions of excited electrons in atoms, ions or molecules. Electrons present in atoms, ions or molecules may exist in different energy states. The lowest energy state is termed the ground state. Higher energy states are referred to as excited states. In photoluminescence, when a photon is absorbed, the electron configuration is excited to a higher energy state. This is often referred to as an excited state.
7. Electrons can return to the ground state through different processes, the emission of light (that is, photons) is only one of these. There are different quantum mechanical pathways through which the electron may return to its ground state and light may be emitted, or the excited state energy may be dissipated as heat but no light is emitted.
8. In semiconductor materials, absorption of a photon may create a pair of negative and positive charge carriers that are electrons and holes, respectively. Recombination of an electron-hole pair may result in emission of light. Electrons and holes in semiconductors may be mobile and thus the energy of an absorbed photon may be distributed around a region, rather than confined to a single atom, ion or molecule.
9. A diode is an electronic component with two terminals. Most electronic diodes are made with semiconductor materials.
10. Diodes have low resistance to electrical current in one direction (the forward direction) and high resistance in the other direction (the reverse direction). In effect, this means they conduct electrical current predominantly in only one direction (the forward direction).
11. A light emitting diode, or LED, is a particular design of a diode where a portion of the forward current gets turned into light emission (see section 2 'light emitting diode'). This is achieved by the particular design of the semiconductor materials of the diode. Not all semiconductor diodes are LEDs.
12. Semiconductor materials have electrical conductivity between electrical insulators and conductors and are frequently used in electrically powered devices. Some of these semiconductor devices produce luminescence from the recombination of electrical charges in a diode (electroluminescence). These types of semiconductor devices are referred to as light emitting diodes or LEDs.
13. Semiconductors can be doped with specifically chosen atoms to tailor their electrical conductivity to be either p-type or n-type. In p-type material the electrical carrier is positively charged (called holes); in n-type it is negatively charged (called electrons).
14. The structure of an LED is commonly fabricated from the joining of a p-type semiconductor and an n-type semiconductor. The region where these two types meet is called the junction region (or, a p-n junction).
15. Light is most commonly emitted from an LED when electrons in the conduction band recombine with holes in the valence band. This recombination process occurs in the region of the junction. The electrons and holes are supplied from an electrical power source.
16. Electrical current flow, and thus light output, can only occur in an LED when the external power source is applied with the correct polarity (negative pole to n-type side, positive pole to p-type side). This is called forward bias polarity. In forward bias polarity the electrons from the power source are injected in the n-type side of the junction region. In reverse bias polarity, the negative pole of the power source is connected to the p-type side of the diode that is not conducting electrons, meaning no electrical current flow (and thus light output) can occur.
17. The relationships between light output, electrical current, applied voltage and applied polarity are distinctive features of a p-n junction, and thus a distinctive signature of the presence of p- and n-type semiconductors.
18. The spectrum of the light emitted from an LED is normally in the form of a peak or peaks at wavelengths characteristic of the semiconductor material. Refer also to section 5 ‘nitride compound semiconductor’. In addition to the material, the spectrum of the light emitted from an LED is also dependent on the structure of the LED (how its layers are arranged and what its crystal structure is).
19. A phosphor is a substance that exhibits a luminescence process when suitably excited.
20. Phosphors often have 2 components. The first is the luminescent centre (sometimes called the dopant or activator) and the second is the host matrix or host crystal lattice.
21. The activator is usually a particular element, which will often be in an ion form in the host matrix.
22. The general role of a phosphor when used with a light emitting diode is to change the overall emission wavelengths emitted from the integrated device. The phosphor changes the overall perceived colour of light emitted by absorbing some (but not all) of the light emitted by the semiconductor chip. It achieves this by the activator absorbing photons emitted by the LED semiconductor to produce excited states of the activator. The activator subsequently relaxes back to its original ground state by emitting photons of a different energy and hence a different colour to that absorbed. A key requirement of the phosphor is having an activator with absorption transitions that match the LED emission, and having emission transitions at the energies corresponding to the desired new colours.
4. Absorption and emission of light at different wavelengths
23. Every different type of dopant or activator has a fingerprint that comes from the individual ways that its electrons can be excited and relax. These transitions are called electronic transitions.
24. As mentioned in paragraph 6 an electron configuration goes to a higher energy state when light is absorbed. The wavelength of the absorbed light is determined by the energy difference between the two states of the transition.
25. Light may be (but is not always) emitted when an electronic state transitions to a lower electronic energy state. Light is emitted when the transition to a lower state is accompanied with the emission of electromagnetic radiation (photons). Similar to absorption, the wavelength of the emitted light is determined by the energy difference between the two states of the transition.
26. Usually, though not in every case, the light emitted is of a different wavelength to the light absorbed.
27. Light is not always emitted when there is a downwards transition. For example, the downwards transition may be in the form of heat, which is known as a nonradiative transition. The host matrix can have a role in determining the probability of light emission versus nonradiative transitions.
28. Light emission can be measured in an emission spectrum which can be represented as a graph of light intensity versus wavelength.
5. Nitride compound semiconductor
29. Compound semiconductors comprise two or more elements. The precise ratios of elements must remain uniform throughout the compound semiconductor. A particular compound can be a semiconductor even when the constituent elements are not.
30. A nitride compound semiconductor is linked to the family known as the group-III nitrides.
31. Aluminium (Al), gallium (Ga) and indium (In) are metal elements from group-III of the Periodic Table. They have similar but not identical chemical properties.
32. The nitride compound semiconductors form when Al, Ga, or In chemically bond to N in an approximate 1:1 ratio to form a crystal structure that has semiconducting properties.
33. In addition to the 3 binary compounds (AlN, GaN, InN), mixtures can be made. For example, InxGa1-xN is a nitride compound semiconductor, being a mixture of InN and GaN where the ratio of InN and GaN is determined by ‘x’. GaN LEDs can emit blue light.
34. Garnet is a general classification name for a class of natural gemstones that have oxygen forming tetrahedra with silicon (Si) at the centre. The general chemical formula for a natural garnet is X3Z2(SiO4)3.
35. In addition to natural garnets, a number of synthetic garnets have been fabricated following the same general chemical formula given above. One such synthetic garnet is YAG - an acronym for yttrium aluminium garnet. YAG is represented by the chemical formula Y3Al5O12 which can be written in the general garnet formula as Y3Al2(AlO4)3.
36. Garnets are crystalline materials. This means the elements in the garnet structure have a periodic arrangement. At the heart of a crystal structure is a single unit cell that represents the repeating pattern of the crystal. For the garnet structure the underlying unit cell is a cube that contains 8 formula units of X3Z2(SiO4)3. Thus the garnet crystal structure has a unit cell containing 160 atoms.
37. Fluorescence refers to a photoluminescence process in which a photon (electromagnetic radiation) is absorbed by a substance (for example an atom, ion or molecule) and a photon (electromagnetic radiation) is emitted from that substance. The wavelength of the emitted photon is usually longer than the wavelength of the absorbed photon, and the emission of light ceases immediately upon removal of the excitation source.
38. Luminescence can be divided into fluorescence and phosphorescence. In fluorescence, emission is an immediate process involving a spin-allowed transition. This differs from phosphorescence, which involves a spin-forbidden transition and has a longer life time, sometimes visible as afterglow.
39. At a basic level, a phosphorescent material will continue to emit light for a period of time after the excitation source is removed, whereas a fluorescent material will stop emitting light almost immediately after the excitation source is removed. In other words, a phosphorescent material has a long decay time, whereas a fluorescent material has a short decay time. Both are materials that absorb light of one wavelength and emit light of a different wavelength.
40. In order to act as a phosphor, a material is required to emit light when suitably excited. In general, the host matrix of a phosphor does not absorb and emit light and thus cannot act as a phosphor until it is “activated” by a suitable substance. The activator is deliberately chosen to have absorption and emission of light with photon energies required for the application. In general the activator does not influence the host matrix as it is included at low concentrations and is also chemically compatible with atoms of the host matrix.
41. The wavelengths of electronic transitions (see explanation in section 4) of activator atoms or ions is influenced by the host matrix.
42. The exact distance to, and bonding of, host matrix atoms or ions with the activator influences the energy levels of the activator.
43. The specific nature of atoms or ions of the host matrix influences the electronic energy levels of the activator.
44. Therefore, even for the same activator (for example cerium (Ce)), the exact wavelengths of absorption and emission change with different host matrixes.
45. The corollary is that all examples of the same host matrix and activator combination will exhibit the same wavelengths of absorption and emission, provided that the concentration of the activator is the same. Different concentrations of the activator can shift the wavelengths of absorption and emission, even with the same host matrix.
9. The general formula of claim 3
9.1 Structure of the general formula of claim 3
46. Claim 3 recites “fluorescent material represented by a general formula (Re1-rSmr)3(Al1-sGas)5O12 :Ce, where 0 ≤ r < 1 and 0 ≤ s ≤ 1 and Re is at least one selected from Y and Gd” (General Formula).
47. The General Formula represents a general description of the composition of a synthetic garnet structure. The formula unit identifies the ratio of oxygen ions to metal ions identified by “(Re1-rSmr)” to metal ions identified by “(Al1-sGas)”, being a ratio of 12 to 3 to 5.
48. The terminology of writing ":Ce" on the end is notation for cerium being a dopant replacing part of the Re ion in the matrix.
49. This formula represents a cerium-activated phosphor with a garnet structure.
50. In chemical notation:
(a) Y represents the element yttrium,
(b) Gd represents the element gadolinium,
(c) Sm represents the element samarium,
(d) Al represents the element aluminium,
(e) Ga represents the element gallium,
(f) O represents the element oxygen, and
(g) Ce represents the element cerium.
51. The General Formula includes (Al1-sGas)5 where 0 ≤ s ≤ 1. The parameter s describes the fraction of Ga present, e.g. s = 0.2 would mean 20 out of every 100 of (Al + Ga) would be Ga. The parameter s can be 0 or 1. This means that the formula covers the possibility of having only aluminium (and no gallium), or only gallium (and no aluminium) present in material represented by the formula.
52. The General Formula includes (Re1-rSmr)3 where 0 ≤ r < 1 and Re is at least one selected from Y or Gd. The parameter r represents the fraction of samarium present. It is indicated that r must be less than, and cannot be equal to, 1. As r cannot equal 1:
(a) material represented by the General Formula cannot contain only samarium, that is, crystals represented by the formula Sm3(Al1-sGas)5O12, are not included in this formula.
(b) there must always be some atoms of either Y or Gd (or both) present in material represented by the formula.
53. The inclusion of the words 'at least one' implies that both Y and Gd can be present. This means that possible combinations of elements represented by this portion of the General Formula are:
(a) Y,
(b) Gd,
(c) Y and Gd,
(d) Y and Sm,
(e) Gd and Sm, and
(f) Y, Gd and Sm.
9.2 Y3Al5O12:Ce
54. An example of a material represented by the General Formula is Y3Al5O12:Ce. This material may be identified as YAG:Ce.
55. Ce can be called the activator of the YAG:Ce phosphor.
56. YAG:Ce can absorb specific wavelengths of light that correspond to exciting cerium electrons to higher energy states. One wavelength for absorption in YAG:Ce is blue light of 450 nanometres. A nanometre (nm) is a unit of length that is 10-9 metre, or one billionth of a metre.
57. After being excited by this absorption process, the cerium electrons return to lower energy states by the complementary process of light emission (photoluminescence). This emitted light has a different wavelength than the absorbed light.
58. When YAG:Ce is excited by blue light, the phosphor emits an emission band that spans the wavelength range from around 500 to 700 nm. The human eye perceives this phosphor emission as yellow light, which can, when combined with blue light emitted by the LED, make the total light emitted appear to the eye as white light. Refer to section 10 for further information.
59. The diagram below illustrates how this works. Blue light at 450 nm enters the YAG:Ce phosphor, is absorbed, and yellow light is emitted. As the absorption by the phosphor is less than 100%, some 450 nm blue light is also transmitted through and around the phosphor.
60. The totality of the blue LED light exiting the device that is transmitted (the part not absorbed by the phosphor), and light in the wavelength range from around 500 to 700 nm from emission of the phosphor, constitute the white light emission of the device.
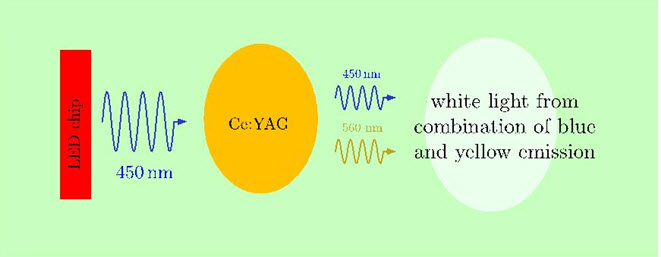
61. Light that is capable of producing a visual sensation to the human eye ranges from about 360nm to about 830nm and includes all colours from red to violet. It is also common to say that the visible region of the spectrum ranges from 400nm to 700nm, as perception varies from person to person.
62. The visible region of the spectrum is bounded by the ultraviolet (UV) (<400nm) and infrared (>700nm) regions. Regions are not precisely bounded and may overlap.
63. Visible light can be either natural or artificial. Sunlight and skylight are natural forms of light. Artificial forms of light include light produced by:
(a) incandescent sources, in which light is produced by a material heated to incandescence, typically having a very broad spectrum of emitted light (e.g. tungsten filament lamps and tungsten-halogen lamps);
(b) discharge lamps, in which radiation is produced by an electric discharge in a gas, typically emitting in a number of different and distinct spectral lines which may be in the UV or the visible and those in the UV require conversion to visible light by phosphors (e.g. fluorescent lamps);
(c) solid state devices, in which light is produced by a semiconductor material, typically emitting light in a particular range of one colour (e.g. LED semiconductors); and
(d) 'coherent' sources, in which light is produced within typically a very narrow spectral range (e.g. lasers).
64. The eye’s sensitivity to light varies depending on the wavelength of light. As the diagram below shows,1 the eye is most sensitive to light with a wavelength of around 555 nm, and the sensitivity to light drops off on either side of this maximum.
65. White light is a combination of other colours of visible light. It is a perception by the human eye of light when each of the three types of cone cells in the eye is similarly stimulated. Perceiving white light thus requires the presence of at least two different wavelengths of light.
66. Various colours of visible light can be combined to produce white light. For example:
(a) red, green and blue; and
(b) blue and yellow.
67. Based on the principles of colorimetry, every colour other than a primary colour can be realised by mixing two primary colours. The colours can be presented in a colour triangle known as a chromaticity diagram. The most widely used diagram is the one standardised in 1931 by the Commission Internationale d’Eclairage and is known as the CIE chromaticity diagram. With this diagram colours are defined by the colour coordinates x and y. From the emission spectrum of a light source the coordinates x and y can be determined and provide a quantitative representation of the colour.
68. In the diagram below,2 all possible colours of light are enclosed by a curved line representing saturated or 'pure' colours (single wavelength, in nm) of the electromagnetic spectrum and a line connecting the x and y coordinates for the extreme violet and extreme red.
69. Colours lying on the black-body locus (or Planckian locus) (which is the path or locus that the colour of an incandescent black-body radiator would take if it was heated) are typically considered to be white. A black-body radiator is an ‘ideal body’ that absorbs all incident electromagnetic radiation and, when heated, emits a broad spectrum of electromagnetic radiation with a colour that changes from red to yellow to white to bluish white as the temperature increases. It is the reference by which the whiteness of other light sources can be assessed. Typically, when the temperature of a black-body radiator is more than 2,500 Kelvin (K), it is considered white.
70. The colour ‘white’ is itself very difficult to define. Its definition depends on the application of the light source (e.g., in some fields, white is defined for applications in that field) and the adaptive state of the human eye (e.g., an incandescent house light will be perceived as a different hue of white when viewed indoors as opposed to outside under natural light).
71. When an ion has been excited to a high energy state it can return to the ground state by emitting light (the desired process in phosphors, called 'radiative decay') or it can return to the ground state by giving off the excess energy in the form of heat (undesired in phosphors, called 'non-radiative decay').
72. There are different mechanisms for non-radiative decay and typically they are thermally activated, which means that they require heat (high temperatures) to occur. The temperature at which the probabilities for radiative decay and non-radiative decay are equal is called the 'quenching temperature'. At this temperature, only half of the excited ions in a luminescent material emit light and the quantum efficiency of the luminescent material, defined as the number of photons (light particles) emitted divided by the number of photons absorbed, is 50%.
73. The quenching temperature for a specific luminescent material depends on many factors and is often not well understood. For example, it can depend on the type of luminescent ion, the type and exact chemical composition of the host material in which the luminescent ion is embedded, the concentration of the luminescent ion, the procedure for synthesising the luminescent material, the presence of impurity ions, and so on.
74. As a luminescent material reaches its quenching temperature, it starts to suffer from 'thermal quenching' and its emission intensity starts to decrease. Thermal quenching is a reversible process: once the material cools, its efficiency returns to its original level.
75. Thermal quenching is a common phenomenon in luminescent materials, and is caused by the opening up of non-radiative pathways for a luminescent ion to return to the ground state without emitting a photon at certain temperatures.
76. Thermal quenching is also known as 'temperature quenching' or 'luminescence temperature quenching'.
77. There can be Stokes phosphors and anti-Stokes phosphors.
78. A Stokes phosphor absorbs light of a short wavelength and emits light of a longer wavelength. A Stokes phosphor can also be referred to as a down-converting or a down-shifting phosphor because photon energy goes down.
79. An anti-Stokes phosphor absorbs light of a long wavelength and emits light of a shorter wavelength. Anti-Stokes phosphors, or up-converting phosphors, are not as efficient as Stokes phosphors (as more than one photon must be absorbed for each photon emitted and the process of up-conversion has more inherent loss mechanisms).
80. The Stokes shift is the energy difference between the maximum of the excitation band and the emission band of the same electronic transition.




