FEDERAL COURT OF AUSTRALIA
Bayer Pharma Aktiengesellschaft v Generic Health Pty Ltd [2017] FCA 250
ORDERS
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. The parties confer and submit agreed short minutes of order reflecting these reasons for judgment within 14 days, including directions if the issue of costs is not agreed.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
JAGOT J:
THE CLAIMS
1 The applicants (referred to below together as Bayer and separately as Bayer AG and Bayer Australia) claim damages against the respondents (together, Generic Health) for patent infringement.
2 In the reasons for judgment in Bayer Pharma Aktiengesellschaft v Generic Health Pty Ltd (No 2) [2013] FCA 279; (2013) 100 IPR 414 (the principal decision) I found that Generic Health had infringed claims 3 and 11 of Australian Patent No 780330 for a pharmaceutical combination of ethinylestradiol (EE) and drospirenone (DRSP) for use as a contraceptive. Pursuant to the patent, Bayer manufactured and sold in Australia the oral contraceptive known as Yasmin. Generic Health infringed the patent by manufacturing and selling the oral contraceptive known as Isabelle. I made orders on 8 May 2013 which included the following, but which were stayed pending the outcome of a foreshadowed appeal:
3. Generic Health (whether by itself, its directors, officers servants, agents or howsoever otherwise) be restrained from infringing claims 3 and 11 of the Patent by importing, selling, supplying, and offering to sell and supply the Isabelle Product (or keeping it for any such purpose) in Australia during the term of the Patent without the licence or authority of the Applicants.
4. Lupin (whether by itself, its directors, officers servants, agents or howsoever otherwise) be restrained from authorising any person to exploit the invention claimed in claims 3 and 11 of the Patent by importing, selling, supplying, and offering to sell and supply the Isabelle Product (or keeping it for any such purpose) in Australia during the term of the Patent without the licence or authority of the Applicants.
…
7. Generic Health and Lupin pay to the Applicants damages (including additional damages pursuant to section 122 (1A) of the Patents Act 1990, if any) or, at the Applicants' option, an account of profits, to be assessed pursuant to the procedure contemplated in order 10(a) or order 10(b).
3 A subsequent appeal was dismissed (Generic Health Pty Ltd v Bayer Pharma Aktiengesellschaft [2014] FCAFC 73; (2014) 222 FCR 336), as was an application for special leave to appeal to the High Court (Generic Health Pty Ltd v Bayer Pharma Aktiengesellschaft [2014] HCATrans 261).
4 Bayer elected for damages rather than an account of profits. Bayer’s total claim is for damages of $25,751,336 plus interest. This claim is based on an assessment of Bayer’s lost profits in which each sale of Isabelle and of Petibelle, a generic version of Yasmin which Bayer introduced after Isabelle was removed from sale, is taken to be a lost sale of Yasmin. Bayer’s lost profits are based on its standard costings for the production and sale of Yasmin including for determining its fixed and variable costs.
5 Generic Health contends that this amount would over-compensate Bayer on the grounds that:
(1) Section 115(1)(a) of the Patents Act 1990 (Cth) (the Patents Act) precludes any award of damages to Bayer for any infringements before 14 December 2012 (the date on which the patent was amended) because Bayer has not satisfied the Court that its original specification, before amendment, was framed in good faith and with reasonable skill and knowledge as required by that provision. The parties referred to this as the good faith issue.
(2) It should not be found that each sale of Isabelle and of Petibelle is a lost sale of Yasmin. At least some, and perhaps a substantial number, of sales of Isabelle were not in substitution for Yasmin. Further, the claim for damages in respect of Petibelle should be rejected based on the requirements of causation and remoteness of damage and, if maintainable at all, would relate to a relatively short period of time, measured in months not years. The parties referred to this as the one for one issue.
(3) Bayer’s standard costings should not be applied without also applying a discount for the risk that Bayer’s costs in producing the additional number of Yasmin tablets for sale would have been greater than the standard costings contemplate. The parties referred to this as the costings issue.
THE BASIC FACTS
6 The basic facts are not in dispute.
7 Yasmin was registered on the Australian Register of Therapeutic Goods (the ARTG) on 6 July 2001 and first sold in Australia in August 2002.
8 In September 2008 Bayer launched its Yaz oral contraceptive and diverted its marketing efforts from Yasmin to Yaz. Yaz is also based on a combination DRSP and EE, but the amount of EE in Yaz is less than in Yasmin and is in a different form. As a result, Yaz is not suitable for all women because the lesser amount of EE may give insufficient cycle control, which I infer is one reason that Yasmin remained (and remains) on the market.
9 On 8 April 2011 Bayer obtained a registration on the ARTG for Petibelle, a generic version of Yasmin, but did not put Petibelle on the market.
10 In October 2011 the Therapeutic Goods Administration (the TGA) issued a safety update to the effect that Yasmin may be associated with an increased risk of venous thromboembolism (VTE) compared to other combined oral contraceptives.
11 On 23 January 2012 Generic Health started to sell Isabelle in Australia in packets of 3 x 28 tablets. Isabelle was registered on the ARTG on the basis of bioequivalence to Yasmin.
12 On 15 February 2012 Bayer commenced this proceeding against Generic Health and applied for an amendment of the patent.
13 In February 2012, the initial stocks of Isabelle ran out and could not be replenished to fill back orders until around March/April 2012. Stocks of Isabelle ran low again in May 2012 and were not replenished until August 2012.
14 In September 2012 Bayer launched Yaz Flex and diverted its marketing efforts from Yaz to Yaz Flex. Yaz Flex is the same formulation as Yaz.
15 On 14 December 2012 Yates J allowed the amendment of the patent (Bayer Pharma Aktiengesellschaft v Generic Health Pty Ltd [2012] FCA 1510; (2012) 99 IPR 59 (referred to below as the amendment decision).
16 On 19 June 2014, after the appeal from my orders was dismissed, Generic Health was required to cease selling Isabelle immediately and did so.
17 On 26 June 2014 Bayer started to sell Petibelle in Australia. Bayer did not market Petibelle other than by a letter dated 20 June 2014 to doctors and pharmacists informing them of the availability of Petibelle.
THE GOOD FAITH ISSUE
Statutory provisions
18 Section 115(1) of the Patents Act provides that:
(1) Where a complete specification is amended after becoming open to public inspection, damages shall not be awarded, and an order shall not be made for an account of profits, in respect of any infringement of the patent before the date of the decision or order allowing or directing the amendment:
(a) unless the court is satisfied that the specification without the amendment was framed in good faith and with reasonable skill and knowledge; or
(b) if the claim of the specification that was infringed is a claim mentioned under subsection 114(1).
19 Section 115, in terms, qualifies the power of the Court under s 122(1) of the Patents Act which is as follows:
The relief which a court may grant for infringement of a patent includes an injunction (subject to such terms, if any, as the court thinks fit) and, at the option of the plaintiff, either damages or an account of profits.
20 Bayer does not press its claim for additional damages to be awarded under s 122(1A).
The amendments
21 In the amendment decision Yates J described the amendments at [24] in these terms:
The amendments, as they relate to claims 3, 11, 27 and 32:
(a) limit the dosage of drospirenone to 3 mg (the 3 mg feature);
(b) confine the composition or preparation to a tablet form (the tablet feature); and
(c) specify that the stated dissolution test is performed in 900 ml of water (the 900 ml feature).
22 The amendments to claims 3 and 11 of the patent which Yates J allowed on 14 December 2012 were as shown below:
3. A pharmaceutical composition in oral dosage form comprising;:
from 2 mg to 4 mg 3 mg drospirenone and 0.01 mg to 0.05 mg of ethinylestradiol, together with one or more pharmaceutically acceptable carriers or excipients, wherein the oral dosage form is a tablet, and
wherein at least 70% of said drospirenone is dissolved from said composition within 30 minutes, as determined by USP XXIII Paddle Method II using 900 ml of water at 37°C as the dissolution media and 50 rpm as the stirring rate.
…
11. A pharmaceutical preparation consisting of a number of separately packaged and individually removable daily dosage units placed in a packaging unit and intended for oral administration for a period of at least 21 consecutive days, wherein said daily dosage units are in tablet form and comprises a combination of 3 mg of drospirenone in an amount of from 2 mg to 4 mg and ethinylestradiol in an amount from 0.01 to 0.05 mg, wherein at least 70% of said drospirenone is dissolved from said dosage units within 30 minutes, as determined by USP XXIII Paddle Method II using 900 ml of water at 37°C as the dissolution media and 50 rpm as the stirring rate.
Principles
23 I accept many of the principles the parties proposed ought to be applied in resolving the issue whether I should be satisfied that “the specification without the amendment was framed in good faith and with reasonable skill and knowledge”, with the consequence that “damages shall not be awarded in respect of any infringement of the patent before” 14 December 2012, the date of the decision allowing the amendment of the patent.
24 I accept that the onus of proof lies on Bayer to satisfy the Court that the “specification without the amendment was framed in good faith and with reasonable skill and knowledge”. I do not accept Bayer’s submission that, as a principle of general application, good faith and reasonable skill and knowledge “is to be assumed in the patentee’s favour in the absence of internal or external evidence to the contrary”. The submission is based on an observation of Greene MR during the course of argument in Molins and Molins Machine Co Ltd v Industrial Machinery Co Ltd (1938) RPC 31 at 33. While it may be that the absence of internal or external evidence makes it appropriate to infer good faith and reasonable skill and knowledge having regard to all of the circumstances of the particular case, the exercise is one of inference, and not of assumption.
25 In common with Windeyer J in Pracdes Pty Ltd v Stanilite Electronics Pty Ltd (1995) 35 IPR 277 at 281, I do not consider General Tire & Rubber Co v Firestone Tyre & Rubber Co Ltd [1975] RPC 203 to stand for any contrary proposition. A number of important propositions were made in General Tire but they do not include a suggestion that under the equivalent provision in the UK (which is not in identical terms to s 115(1) of the Patents Act) the patentee did not bear the onus of proof. That said, a number of the observations in the Court of Appeal’s reasons in General Tire at 269-270 are relevant to the Australian context:
(1) It is not the case that the drafter of the patent necessarily must be called in order to discharge the onus of proof.
(2) “If a patent agent puts forward something of which he has no knowledge, which suffers from some fatal imperfection in the patent field we do not consider that, when the patent office accepts it without demur, it can be said that it was framed otherwise than in good faith. It is, after all, the function of a patent agent to argue in honesty for the width of the application”.
(3) “It is to be observed that the withdrawal of a claim does not necessarily involve that admission that that claim cannot be or could not ever have been sustained as valid. It is common practice to include in good faith a wider claim so as to have the benefit of a wider search in the Patent Office. It is also common practice for an applicant to decide that in terms of commerce a narrower claim suffices, and therefore to drop a wider claim which will not serve him commercially and may land him in opposition litigation. On the question of good faith it is not to be expected that the patentee should in evidence first raise up and then exorcise the ghost of every possible defect in the unamended claim: if he destroys the defects that are suggested, that suffices”.
26 This latter proposition must be right. If there is no suggestion that an unamended specification has been framed other than in good faith and with reasonable skill and knowledge, the onus does not mean that the patentee must adduce evidence attempting to guess in which respect it might be alleged that the court should not be satisfied under s 115(1)(a).
27 I accept also that by the terms of s 115(1)(a) it is the specification as a whole without the amendment which must be framed in good faith and with reasonable skill and knowledge.
28 Given that the framing of the specification in the present case involved work overseas and in Australia, I accept that the statutory question is to be answered considering the framing of the specification as a whole, both overseas and in Australia.
29 I accept that statements in UK decisions need to be understood in the different statutory context there applicable and, in particular, the fact that s 63(2) of the Patents Act 1977 (UK) included a provision that where a patent has been found to be “only partially valid”, the Court should not grant relief by way of damages “except where the plaintiff… proves that the specification for the patent was framed in good faith and with reasonable skill and knowledge”. There is no equivalent provision in the Patents Act.
30 Nevertheless, I also accept Bayer’s proposition based on Molins at 44 and General Tire at 269 that a patent invalid but for the amendments may nevertheless have been framed in good faith and with reasonable skill and knowledge. In Molins the unamended patent would have been invalid for lack of novelty by reason of an earlier patent described at 44-45 as a German document 48 years old at the date when the patent was applied for of which the patentee knew nothing. Greene MR said that if the patentee did not know anything about the prior art then the original claim was framed in good faith and with reasonable skill and knowledge. This said, it must also be the case that the validity of the unamended specification is at least relevant to the issues of framing in good faith and reasonable skill and knowledge.
31 I do not consider that a further submission of Generic Health may be taken at face value. The submission is this:
Secondly, consistently with s 140 of the Evidence Act 1995 (Cth), in order to meet the s 115(1)(a) test, clear and cogent proof is required. This is because of: (1) the nature of the cause of action – the amendment of a patent claim is an “indulgence to the patentee” (Les Laboratoires Servier v Apotex Pty Ltd (2016) 117 IPR 415 at [312] per Bennett, Besanko and Beach JJ), and having been granted that indulgence and having then obtained a finding of infringement of the amended patent, a patentee is then able to seek pecuniary remedies for infringement, subject only to the protection afforded by s 115; and (2) the nature of the claim – if Bayer is able to satisfy the s 115(1)(a) test, this is likely to sound in a substantial award of damages for the period before 14 December 2012 (ie millions of dollars).
32 First, given that s 115 is concerned only with monetary compensation for infringement, the size of any particular claim cannot affect the proper principles to be applied. Second, the reference to a need for “clear and cogent proof” may act as an inappropriate gloss on the ordinary civil standard of proof of on the balance of probabilities (s 140(1) of the Evidence Act 1995 (Cth)) and the capacity to take into account all relevant matters to determine whether that standard has been met in the particular case (s 140(2) of the Evidence Act). Third, references to the need for “clear and cogent proof” are common in the context of allegations such as fraud. As explained in Neat Holdings Pty Ltd v Karajan Holdings Pty Ltd [1992] HCA 66; (1992) 110 ALR 449 at 449-450 (footnotes excluded):
The ordinary standard of proof required of a party who bears the onus in civil litigation in this country is proof on the balance of probabilities. That remains so even where the matter to be proved involves criminal conduct or fraud. On the other hand, the strength of the evidence necessary to establish a fact or facts on the balance of probabilities may vary according to the nature of what it is sought to prove. Thus, authoritative statements have often been made to the effect that clear or cogent or strict proof is necessary “where so serious a matter as fraud is to be found”. Statements to that effect should not, however, be understood as directed to the standard of proof. Rather, they should be understood as merely reflecting a conventional perception that members of our society do not ordinarily engage in fraudulent or criminal conduct and a judicial approach that a court should not lightly make a finding that, on the balance of probabilities, a party to civil litigation has been guilty of such conduct.
33 It is not immediately apparent that the context established by s 115(1) of the Patents Act has much in common with an allegation of fraud or other dishonest conduct.
34 Other principles are relevant to the question of proof. In Australian Securities and Investments Commission v Hellicar [2012] HCA 17; (2012) 247 CLR 345 at [250], Heydon J summarised a number of propositions, which Generic Health set out as follows:
(a) In Blatch v Archer (1774) 1 Cowp 63 at 65, Lord Mansfield CJ stated:
“It is certainly a maxim that all evidence is to be weighed according to the proof which it was in the power of one side to have produced, and in the power of the other to have contradicted.”
(b) In Ho v Powell [2001] NSWCA 168; (2001) 51 NSWLR 572 at [14]–[15] Hodgson JA stated:
“in deciding facts according to the civil standard of proof, the court is dealing with two questions: not just what are the probabilities on the limited material which the court has, but also whether that limited material is an appropriate basis on which to reach a reasonable decision...
In considering the second question, it is important to have regard to the ability of parties, particularly parties bearing the onus of proof, to lead evidence on a particular matter, and the extent to which they have in fact done so”.
(c) In Shalhoub v Buchanan [2004] NSWSC 99 at [71] Campbell J stated:
“failure of a party who bears an onus of proof to call an available witness who could cast light on some matter in dispute can be taken into account in deciding whether that onus is discharged, in circumstances where such evidence as has been called does not itself clearly discharge the onus. This is an application of Lord Mansfield's maxim”.
(d) In Cook’s Construction Pty Ltd v Brown & Anor [2004] NSWCA 105; (2004) 49 ACSR 62 at [42], Hodgson JA stated:
“where a party has to prove something and prima facie has available evidence that would directly deal with the question, a court will be very hesitant in drawing an inference in that party's favour from indirect and second-hand evidence, when the party doesn't call the direct evidence that prima facie it could have called, at least unless some explanation is given, or the circumstances themselves provide an explanation”.
35 Further, in Kuhl v Zurich Financial Services Australia Ltd [2011] HCA 11; (2011) 243 CLR 361 the reasons of Heydon, Crennan and Bell JJ at [63] referred to the well-known observations of Handley JA in Commercial Union Assurance Co of Australia Ltd v Ferrcom Pty Ltd (1991) 22 NSWLR 389 at 418-419 as at least being authority for this proposition:
The rule in Jones v Dunkel [(1959) 101 CLR 298] is that the unexplained failure by a party to call a witness may in appropriate circumstances support an inference that the uncalled evidence would not have assisted the party’s case. That is particularly so where it is the party which is the uncalled witness. The failure to call a witness may also permit the court to draw, with greater confidence, any inference unfavourable to the party that failed to call the witness, if that uncalled witness appears to be in a position to cast light on whether the inference should be drawn. These principles have been extended from instances where a witness has not been called at all to instances where a witness has been called but not questioned on particular topics. Where counsel for a party has refrained from asking a witness whom that party has called particular questions on an issue, the court will be less likely to draw inferences favourable to that party from other evidence in relation to that issue. That problem did not arise here. The plaintiff's counsel did ask the plaintiff relevant questions.
36 I adopt Generic Health’s summary in respect of the good faith requirement as follows:
(a) In order to establish the good faith requirement, the plaintiff must prove that the specification was framed honestly with a view to obtaining a monopoly to which, on the material known to him or her, he or she believed he or she was entitled: Hallen v Brabantia [Hallen Co v Brabantia (UK) Ltd [1990] FSR 134] at 143; Chiron Corp v Organon Teknika Ltd [1994] FSR 458 at 467 (English Patents Court) (Chiron v Organon).
(b) The inclusion of something known by the framers of the patent specification to be detrimental to it may be evidence of a lack of good faith: General Tire at 269 per curiam (English Court of Appeal).
(c) The patentee has to establish that in the original specification it “meant and intended to claim that which [it] had invented and no more. I will not say that that is an exclusive definition, but that is an illustration”: Kane [Kane and Pattinson v J Boyle & Co (1901) 18 RPC 325] at 338.
37 I adopt also Generic Health’s summary in respect of the reasonable skill and knowledge requirement to the following extent (which excludes statements I consider to be no more than the result of the particular facts of the case):
(a) The words “reasonable skill and knowledge” require the specification as framed to be in the form in which a person, with reasonable skill in drafting patent specifications and a knowledge of the relevant law and practice, would produce given the patentee's knowledge of the invention: Chiron v Organon at 467-468.
(b) Where the draftsperson was not properly instructed, the “patentee cannot be in a better position than the patentee who properly instructs the draftsman”: Chiron v Organon at 468.
…
(d) Any mistake must be considered in the context of the whole specification, making allowance for any difficulty the drafter had and the importance of the passage containing the mistake: Hoechst Marion Roussel Ltd. & Ors v Kirin-Amgen Inc. & Ors [2002] EWHC 471 at [84]-[85], [87]-[89] (English Patents Court) (Kirin-Amgen (EPC) affirmed by the Court of Appeal in Kirin-Amgen Inc v Transkaryotic Therapies Inc [2002] RPC 31) (English Court of Appeal) (Kirin-Amgen (UKCA))).
(e) In Kirin-Amgen (UKCA) at [151]-[152], the UK Court of Appeal held that the relevant knowledge must encompass the knowledge of the person that formed the basis of the information in the specification. Thus where a specification contained an obvious mistake due to work done by a contractor, however skilled, the patentee had to shoulder the burden of establishing that the specification was framed with reasonable skill and knowledge.
38 Otherwise:
(1) I agree with Generic Health that the issue under s 115(1)(a) does not involve an exercise of discretion but is a question of fact – whether in all of the circumstances the court is satisfied that the specification without the amendment was framed in good faith and with reasonable skill and knowledge.
(2) The parties must be right that the patentee will not always need to call evidence from people involved in the drafting of an unamended specification. As Generic Health put it:
…the extent to which it is necessary to adduce evidence from those involved in drafting the pre-amendment specification depends on the circumstances of the case.
(3) It must also be right that the mere fact that those involved in drafting the unamended specification give evidence does not necessarily discharge the onus of proof. Again, as Generic Health put it:
… there are also cases in which the onus has not been discharged in circumstances where: (1) the patentee and the draftsperson have been called to give evidence (see e.g. Pracdes … and Rediffusion Simulation Ltd v Link-Miles Ltd [1993] FSR 369 at 381-385); and (2) where the draftsperson has been called to give evidence, and their instructions were also in evidence (see eg Ronson Products Ltd v Lewis & Co [1963] RPC 103). There is no “well-settled” principle on who should or should not be called to give evidence. That matter is heavily fact-dependent.
The earlier decisions
39 To the extent Bayer contended that Generic Health was estopped by the amendment decision from alleging that Bayer had not discharged its onus of proof under s 115(1)(a) of the Patents Act, I disagree. As Generic Health submitted, the amendment decision was not concerned with the question posed by s 115(1)(a). Justice Yates did not express any concluded view on the validity of the unamended claims of the patent (at [28]). Nor is it clear that his Honour’s conclusion that Bayer was not precluded from obtaining the amendment by reason of “covetous claiming” involved an essential finding on which his allowance of the amendment depended so as to give rise to an issue estoppel (Blair v Curran (1939) 62 CLR 464 at 532; see also EI Du Pont de Nemours & Co v Imperial Chemical Industries PLC [2007] FCAFC 163; (2007) 163 FCR 381 at [86]).
40 This said, the position of Bayer before Yates J is not irrelevant to the s 115(1)(a) issue. At [221] his Honour recorded that:
Bayer Pharma does not concede that, without amendment, the dissolution test claims of the Australian patent would not be fairly based on the matter described in the specification. Without deciding that question, I have accepted that Bayer Pharma’s stated position in that regard is one that is genuinely held by it on grounds that appear to be capable of being argued objectively and reasonably.
41 Given that finding, it would not be unreasonable to expect that if Generic Health, a party to the amendment decision, wished to assert in this proceeding that Bayer did not genuinely hold such a view it would take such evidentiary opportunities as were available to it to lay the foundation for that contrary proposition. As explained below, Generic Health did not do so.
42 Further, it is also not irrelevant that Yates J rejected an argument that Bayer had engaged in “covetous claiming”. As Yates J explained at [215]:
In the context of amendment applications, the notion of “covetous claiming” is properly understood as the deliberate obtaining or maintenance of patent claims where the patentee knows that those claims are of unjustified width…
43 At [218] (noting that Eremad Pty Ltd, like Generic Health, opposed the amendments) his Honour said:
I do not find it to be exceptionable that a patentee or patent applicant would seek to keep the scope of its patent claims as broad as possible in order to fully protect its invention as it perceives it to be. Similarly, I do not find it to be exceptionable that, for pragmatic reasons, a patentee or patent applicant might be prepared to compromise on, and resile from, its desired position and not seek to vindicate to the fullest its patent rights or entitlements, as it perceives them to be. The question is whether, in so doing, the patentee or patent applicant, improperly, has sought to prosecute or maintain claims of unjustified width. Contrary to the implication in Eremad’s submissions in this vein, I do not regard Bayer Pharma’s conduct, as revealed by the evidence, to involve it seeking to prosecute or maintain claims which it knows to be either impermissibly or indefensibly wide. In my view, none of the material relied upon by Eremad in this regard shows any untoward or inappropriate conduct on the part of Bayer Pharma which, on the present state of the evidence, would warrant criticism of Bayer Pharma by the court.
44 Test the proposition of irrelevance this way. Assume Yates J had found that Bayer engaged in covetous claiming. It is not difficult to imagine that Generic Health would have relied on that finding to support its case that the unamended specification was not framed in good faith. The fact that his Honour was asked but refused to make that finding is also potentially relevant to the s 115(1)(a) issue. Again, given the finding of no covetous claiming by Bayer in the amendment decision, if Generic Health wished to assert in this proceeding that Bayer had engaged in covetous claiming and for that reason had not framed the unamended specification in good faith, it would not be unreasonable to expect Generic Health to have taken such evidentiary opportunities as were available to it to lay the foundation for that proposition. And again, as explained below, Generic Health did not do so.
45 This said, Generic Health must also be right that the tests for “covetous claiming” and the requirement of good faith under s 115(1)(a) are not the same, so that the finding of Yates J cannot be taken to be a finding that the unamended specification was framed in good faith. Good faith is a broader concept than “covetous claiming”, not the least by reason of the compelling force of the observation in WAFV of 2002 v Refugee Review Tribunal [2003] FCA 16; (2003) 125 FCR 351 at [52] that “bad faith is not necessarily the obverse of good faith. Good faith requires more than the absence of bad faith”.
46 I also agree with Generic Health that its case under s 115(1)(a) does not involve an illegitimate attempt to re-argue the lack of fair basis case which was rejected in the principal decision. As Generic Health submitted, the principal decision concerned only the validity of the amended claims, not the unamended claims. Again, this said, I do consider that Generic Health’s submission that various circumstances related to the earlier decisions are irrelevant considerations, which can carry no probative value in resolving the issues under s 115(1)(a), goes too far.
47 Accordingly, it is not necessarily irrelevant that Generic Health did not challenge the unamended claims on the ground of lack of fair basis when it had the opportunity to do so and otherwise did challenge the claims. For example, depending on all of the circumstances, this fact may tend to support a finding that it was by no means obvious to any person, let alone to Bayer, that the unamended claims were invalid for lack of fair basis. For the same reason, the lack of any objection to the patent during Patent Office examination procedures is not necessarily irrelevant merely because “the test for fair basis (or the overseas equivalent) is different from the s 115(1)(a) test”. What Generic Health is right about is that these factors are not relevant by reason of any discretionary consideration. There is no discretion under s 115(1)(a) and thus Generic Health’s culpability or otherwise in entering the market knowing of the patent, and of the proposed amendments, is immaterial. These factors and others like them may be relevant, because they may tend to prove or disprove the relevant question of fact – whether the specification without the amendment was framed in good faith and with reasonable skill and knowledge. Beyond this, the language of relevant and irrelevant considerations is not well adapted to the issue to be resolved, because questions of fact usually involve the drawing of or refusal to draw inferences having regard to the entire evidentiary matrix.
Australian patent attorneys – common ground
48 With two reservations, I accept that the evidence given by the Australian patent attorneys, Dr McCann and Ms Sinclair, about the functions of a patent attorney provides a useful context for the assessment of the issues under s 115(1)(a) of the Patents Act.
49 The first reservation is this. The specification does not state that side effects will be avoided at the maximum dosage level. It says:
it has surprisingly been found that a hitherto undisclosed minimum dosage level of drospirenone is required for reliable contraceptive activity. Similarly, a preferred maximum dosage has been identified at which unpleasant side effects, in particular excessive diuresis, may substantially be avoided.
50 To the extent that Ms Sinclair’s evidence assumed, or was to the effect, that the patent promised that side effects would be avoided at the maximum dosage level, I disagree. The use of “may” and “substantially” in this part of the specification qualifies the promise. Side effects may substantially be avoided, no more and no less.
51 The second reservation is this. In the principal decision I concluded that the skilled addressee would read the dosage range of 2 to 4mg of DRSP as subject to the accepted variance in quantities for pharmaceutical products as in force at the priority date (at [104]). The accepted variance was ± 7.5% (at [102]).
52 Subject to this, I generally accept many of the submissions of both parties about the effect of the evidence of Dr McCann and Ms Sinclair about the functions of a patent attorney. The following propositions, which I accept, are based on and adopt parts of those submissions.
53 Patent drafting is a collaborative process between the inventors or client and the patent attorney, who each bring different skills and information to the process. As Ms Sinclair said, the drafting of a patent is a “balancing act” between the commercial, legal and technical position of the patent applicant. As Dr McCann said, claim drafting involves an exercise in judgment as to how broadly an invention can be defined.
54 It is common for patentees to seek to claim a range of the dosage of an active pharmaceutical ingredient (API).
55 With respect to technical matters such as the appropriate dosage range for a pharmaceutical it is typical and reasonable for a patent attorney to rely on information provided to him and her by the inventor/client. That is not to say that a patent attorney will “simply parrot the instructions one is given” and, as Ms Sinclair said, instructions should be reviewed “with a critical eye to make sure that they’re not going to potentially cause issues in the future”. However, there is no doubt that a patent attorney also, as Dr McCann said, must:
rely on the client because they have the expertise. You have the scientific background, but they have the detailed expertise, and so you have to rely heavily on the client. That’s usually the case…
56 When a range is claimed, the patent attorney expects that the client will have a sound basis for an honest belief in the range claimed. The belief may be based on experimental evidence or a plausible expectation of the skilled addressee that the invention would work throughout the range defined. Ms Sinclair’s conventional practice is to try to understand whether any trial data meets this objective and, in the event of doubt, she “would go back to my client and ask the question of whether or not they wanted to include that within the range, given that it may have legal consequences at a later date if they did”. Similarly, Dr McCann considered it prudent for a patent attorney to “understand fully the appropriate width of the invention”. As he put it, “somebody” had to form the “state of mind” necessary to establish a “reasonable or sound basis” for a “prediction” that an outer limit of a range has the “appropriate properties” of the invention. However, generally, that is for the client as it is not up to a patent attorney, when drafting a patent specification, to make “sound predictions” of a technical nature unless they have particular technical expertise in a specific area,
57 I take from this evidence that it necessarily follows that the concepts of reasonable skill and knowledge are to be assessed, recognising that competent practice and the application of reasonable skill and knowledge to the framing of a specification as a result of the interactions between patent attorney and client themselves involve a range. The standard set by s 115(1)(a) for the framing of the specification is one of reasonable skill and knowledge, not the highest possible level of skill and knowledge on the part of either the attorney or the client.
58 Dr McCann and Ms Sinclair are highly experienced patent attorneys.
59 Dr McCann has been a registered Australian patent and trade mark attorney since 1988 and currently is the lead lecturer for three patents related subjects in the Master of Intellectual Property at the University of Technology, Sydney. He has worked in the patents area since 1982 first as a technical assistant and then as a principal of Spruson and Ferguson, a patent and trade mark attorneys firm in Sydney. Before this, he worked as a research and development chemist. He holds a PhD completed in 1979, his PhD thesis focusing on photoelectrochemistry. After he completed his PhD he worked as a research associate primarily in the field of photoelectrochemistry at semiconductor electrodes.
60 Ms Sinclair holds a Bachelor of Science majoring in organic chemistry. She has been registered as a patent attorney in Australia since 1994. She is currently a principal of Watermark Patent and Trade Mark Attorneys. She has been an Examiner for the Professional Standards Board for Patent and Trade Marks Attorneys in the subject Patent Systems. She has also been a Fellow of the Academy of Education of the Institute of Patent and Trade Mark Attorneys. Since 2013 she has been a Senior Fellow in the Faculty of Law, University of Melbourne, and teaches aspects of the course Patent Practice and Interpretation and Validity of Patent Specifications. She was appointed to the Professional Standards Board for Patent and Trade Marks Attorneys for two terms between 2008 and 2013.
61 Given the high levels of expertise of Dr McCann and Ms Sinclair, including that of Dr McCann as a research and development chemist, it is not necessarily the case that the standards they apply to their own practice (that is, their answers to question of what they would consider or might do) set the boundaries of competent practice for a patent attorney. If anything, their personal practices are likely to exceed the minimum standards of competence.
The 2-4mg range
Generic Health’s case
62 According to Generic Health, the documents discovered by Bayer do not disclose what occurred in respect of the selection of the dosage range of 2-4mg of DRSP in the unamended specification (subsequently amended to 3mg of DRSP). Dr Broesamle (an internal witness called by Bayer) has remained silent about the selection of the 2-4mg dosage range, and Dr Ulrich, who worked with Dr Broesamle and was involved in communications with the European patent attorneys, Plougmann and Vingtoft (P&V), has not given evidence. Given the lack of explanation of the documents and their ambiguity, no inference that might be drawn in Bayer’s favour from the face of the documents should be drawn. As Generic Health put it:
The “most natural inference” when a party fails to obtain evidence in chief from a witness on a topic is “that the party fears to do so” because it “would have exposed facts unfavourable to the party”: see eg Communications, Electrical, Electronic, Energy, Information, Postal, Plumbing and Allied Services Union of Australia v ACCC (2007) 162 FCR 466 at [230] (Weinberg, Rares and Bennett JJ); in Zaccardi v Caunt [2008] NSWCA 202 at [27] per Campbell JA (Allsop P (as his Honour then was) and Barr J agreeing); Commercial Union Assurance Company of Australia Limited v Ferrcom Pty Limited (1991) 22 NSWLR 389 at 418E per Handley JA. That form of inference has frequently been drawn by this Court. Indeed, as has been quoted with approval in Ferrcom at 419 per Handley JA and by the Full Court in Braverus Maritime Inc v Port Kembla Coal Terminal Ltd [2005] FCAFC 256 at [160] (Tamberlin, Mansfield and Allsop JJ),
“the omission to interrogate a friendly witness in respect to facts presumably within his knowledge is more significant than the failure to call such a person as a witness, and that the presumption that the testimony would not have been favourable to the party’s case is stronger than the one which arises from the failure to produce such a person as a witness.”
Given the sparse evidence in chief provided by Dr Broesamle concerning the framing process, the Court should draw an inference that such evidence as he could have given would not have assisted Bayer.
Further, given: (1) Dr Broesamle’s silence in his evidence-in-chief, including with respect to the Broesamle Notes, the other documents in evidence in respect of whom the author has not been identified and the framing process generally; (2) that matters concerning the framing process are peculiarly within Bayer’s knowledge; and (3) that matters concerning the framing process may have more readily been addressed by witnesses who did not give evidence (including, perhaps, Ms Ulrich), no criticism can be levelled at Generic Health for not cross-examining Dr Broesamle on his recollection of the framing process or the various documents before the Court: Ozecom v Hudson Investment Group [2007] NSWSC 1441 at [56].
63 As such, there is (said Generic Health) an “evidentiary vacuum” about the 2-4mg range. Given that “Generic Heath’s power to contradict the version of events put forward by Bayer depends wholly on, and is constrained by, the documents, and witnesses, that Bayer has put forward”:
… the forensic difficulties lie with the party bearing the onus of proof; it is a “legal error” to attempt to place at the other party’s “doorstep” the “difficulties flowing” from the evidentiary vacuum: McHugh v Australian Jockey Club Ltd (2014) 314 ALR 20 at [20] per Perram J, White J agreeing.
64 According to Generic Health, amongst other things:
(1) The documents produced by Bayer are ambiguous and incomplete. In particular, a “fax with details regarding DRSP” sent by Ms Ulrich between 17 March 1999 and 26 August 1999 – a potentially important document given the chronology set out above – is not in evidence
(2) There is no evidence that Bayer had relied upon any testing for dosages above 3mg for the purposes of formulating the patent specification.
(3) There is no evidence that the framers of the patent at P&V were advised by Bayer personnel that the therapeutically effective range for DRSP was 2 to 4mg or that relevant Bayer personnel formed a state of mind that there was a reasonable or sound basis for this dosage range.
(4) There is nothing to suggest that a report known as the 9274 report was considered in the process of framing the specification for the patent.
(5) The documentary record indicates a concern within Bayer as to whether side effects could be avoided at a dosage range of 4mg of DRSP.
(6) The evidence indicates Bayer’s doubts about the efficacy of the dosage amount of 2mg of DRSP.
Evidentiary assessment
65 In deciding questions of proof, context is critical. It is not possible to weigh up the capacity of a party to adduce evidence or whether the evidence that has been adduced is an appropriate basis upon which to reach a reasonable decision that the burden of proof has been discharged, without understanding the context. Aspects of the evidence, particularly but not exclusively those which are not in dispute, provide important contextual information in this case which assist in a rational assessment of issues of proof.
66 Generic Health’s submissions gloss over certain important matters and, as a result, present an unrealistic picture of the kind of evidence which Bayer might have adduced. It is necessary that those important matters be identified.
67 First, Bayer is a large international pharmaceutical group which manufactures, distributes and markets a range of pharmaceutical products across many countries including Australia. Yasmin, for example is manufactured in Germany by Bayer AG and marketed in Australia by Bayer Australia. The Bayer group of companies includes Schering AG following a merger in 2006.
68 Second, and as a result of the first proposition, in his former role as Senior Patent Counsel employed by Bayer Intellectual Property GmbH (and formerly by Schering AG) Dr Broesamle was responsible for the managing, filing and prosecution of “a significant number of patents within [Bayer’s] patent portfolio around the world”, one only of which was International Patent Application No PCT/ICB00/01213 filed on 31 August 2000 (the PCT application) from which the patent took its priority date and which included the claims which were subsequently amended.
69 Third, Dr Broesamle was a highly qualified chemist and patent attorney well before the PCT application was prepared. He holds a PhD completed in 1983 and had worked as a patent attorney at Schering AG since November 1987. Moreover, since that time his particular responsibility has included fertility control and hormone replacement therapy, with his work in respect of DRSP starting in 1989, 10 years before the PCT application was being prepared.
70 Fourth, to this must be added the fact that Schering AG (and thus, relevantly, Bayer, given the merger between the two entities) had been working with DRSP since 1976. For example, use of DRSP as a diuretic was disclosed in Schering AG patent specification no. DE2652761. Schering AG patent specification no. DE3022337 referred to the “gestagen-like activity of [DRSP] and its consequent utility as a contraceptive agent is described at dosage levels of 0.5-50mg of DRSP per day. It is also noted that the mechanism of action of the compound is very similar to that of the natural corpus luteum hormone progesterone, and that it does not give rise to increased blood pressure…”. Schering AG’s research in respect of DRSP continued with numerous studies and patents involving DRSP thereafter involving numerous dosage ranges for various purposes. Dr Broesamle, as noted, became responsible for this patent portfolio in 1989.
71 Fifth, Dr Broesamle’s job description of Senior Patent Counsel was accurate. Despite Generic Health’s contentions to the contrary about the role of Dr Ulrich, Dr Broesamle’s evidence was consistent. He, not Dr Ulrich, was in charge of the managing, filing and prosecution of Bayer’s patents. Dr Ulrich and he worked as a team but he was senior to Dr Ulrich. As he put it:
…definitely I was, you know, the more experienced ... senior part, because I worked on the drospirenone business from the beginning and Dr Ulrich, she only stepped in here for some years
72 This is consistent with Dr Broesamle’s unchallenged affidavit evidence to this effect:
The final decision concerning any issue in relation to the patent family was…made by me in consultation with … [Bayer’s] Head of Patents and Licensing…
73 He also gave evidence that since 2000 a substantial proportion of his work involved DRSP.
74 Sixth, the claims of the patent were based on those in the PCT application, which was itself based on two priority documents, European and US patent applications, filed on 31 August 1999. The framing of the PCT application and the priority documents on which it was based thus occurred in 1999/2000, some sixteen years ago. In these circumstances, and given the significant number of patents for which he was responsible, the fact that Dr Broesamle could not recall why a letter from P&V was addressed to Dr Ulrich rather than him is to be expected. Consistent with his lack of recall of the circumstances surrounding the letter from P&V to Dr Ulrich in August 2009, Dr Broesamle said in his affidavit of 25 August 2016:
Whilst I remember attending a number of meetings and telephone discussions with P&V in relation to the Yasmin invention, I did not have a clear recollection of attending a meeting on 14 January 1999 or what was discussed at that meeting…
75 Seventh, one thing Dr Broesamle could recall and gave positive evidence about was that he decided to amend the patent because he formed the view that it was desirable to do so for “an abundance of caution” given Generic Health’s challenge to the validity of the patent based on lack of fair basis. The amendments were not sought because of any view that the claims might be invalid. Dr Broesamle repeated this in his oral evidence and it was not put to him that the amendments were sought because Bayer believed the unamended claims were, or might be, invalid.
76 Some other matters also need to be taken into account:
(1) When asked why the letter from P&V sent in August 1999 was addressed to Dr Ulrich, Dr Broesamle could not remember why that might be so. He said he might have been on vacation but rejected the suggestion that his focus might have shifted away from the PCT application and reiterated that he was more senior to Dr Ulrich and Dr Ulrich and he worked as a team. This is consistent with his unchallenged evidence that any “final decision concerning any issue in relation to the patent family was…made by [him] in consultation with … [Bayer’s] Head of Patents and Licensing”.
(2) Generic Health did not challenge Dr Broesamle’s lack of recall of the meeting of 14 January 1999. Nor did it suggest to Dr Broesamle that he had any recollection of any of the “number of meetings and telephone discussions with P&V in relation to the Yasmin invention” which he said he attended. Nor for that matter did it suggest to Dr Broesamle that he had not in fact attended or been privy to a number of meetings and telephone discussions with P&V in relation to the Yasmin invention.
(3) Generic Health did not challenge Dr Broesamle’s status as a highly qualified chemist and patent attorney who, since 1987, was specifically responsible within Schering AG (and then Bayer) for fertility control and hormone replacement therapy and, since 1989, had been involved with Schering AG’s work and had been responsible for its DRSP patents. In the face of unchallenged evidence about his expertise and experience, Generic Health did not suggest to Dr Broesamle that in his role as Senior Patent Counsel he failed himself to act as a competent patent attorney in respect of the framing of the PCT application
(4) In the face of Dr Broesamle’s unchallenged evidence that he considered P&V to be a highly reputable patent attorney firm which, in his experience, had completed all work with a high level of skill and care and in accordance with his instructions and expectations, Generic Health did not suggest to Dr Broesamle that in his role as Senior Patent Counsel he failed to hold P&V to the high standards of competence which he expected from them in respect of the PCT application.
77 Given this, what inferences ought to be drawn? Before this question is answered, it should be said that some other principles, identified by Bayer, are also relevant but appear not to have been given any weight in Generic Health’s contentions.
78 First, a patent is to be construed through the eyes of the skilled addressee. As such, it cannot be said that something has been “omitted” or is “missing” from a patent if the skilled addressee, relying on the common general knowledge, would supply the feature.
79 Second, a patentee is entitled to base a claim on a sound prediction. As Bayer said, referring to Olin Mathieson Chemical Corp v Biorex Laboratories Ltd [1970] RPC 157 at 193:
It is settled that a patentee is free to select the boundaries of its invention and that a selection will be sound and valid provided that a challenger is not able to discharge its onus of demonstrating that the range includes embodiments that are not useful or are old or obvious.
80 Third, it is obvious from the internal Bayer reports discussed below that, as in Asahi Kasei Kogyo Kabushiki Kaisha v W R Grace & Co (1991) 22 IPR 491 at 520, we are not dealing with “a field of science where relevant properties suddenly emerge or disappear as a precise numerical point is reached”.
81 Fourth, in NV Philips Gloeilampenfabrieken v Mirabella International Pty Ltd (1992) 24 IPR 1 at 27 it was said that:
It is not a legitimate objection that the specification does not disclose the experimental and statistical basis for the chosen STD and e.n values. It does not have to. The obligation of the patentee is to instruct the skilled addressee how to perform the invention. The patentee is not obliged to explain how the invention was made or the theoretical basis underlying any stipulated integer: see Blanco White, Patents for Inventions, 5th ed, 1983, para 4-505.
82 I also accept Bayer’s submission that:
It cannot be a requirement that for a claim to be “framed in good faith and with reasonable skill and knowledge”, the drafter must frame a claim to a higher standard than the Court requires for the claim to be valid.
83 I turn now to certain inferences which I consider should be drawn on the evidence.
84 Dr Broesamle must have been involved in giving instructions on numerous pharmaceutical compositions over many years. Dosage ranges are commonplace in patents for pharmaceutical compositions, as the Schering AG patents involving DRSP before the PCT application was prepared, disclose. The notion that any person, Dr Broesamle or Dr Ulrich or anyone else, could reliably recall instructions to a patent attorney about aspects of one patent application from sixteen years ago would stretch credulity.
85 Given his role and expertise, it is difficult to imagine that Dr Broesamle did not familiarise himself with the research and patents obtained by Schering AG for DRSP when he became involved with the work on DRSP in 1989 or at least before giving instructions to P&V about the priority documents for the PCT application in 1999. While he did not say so expressly, Dr Broesamle himself is a patent attorney and was responsible for all final decisions about the DRSP patent family (in consultation with the Head of Patents and Licensing). As such, I have no difficulty inferring that Dr Broesamle, consistently with the evidence given by Dr McCann and Ms Sinclair about competent patent attorney practice, would have familiarised himself with the information about DRSP available which included all of the earlier Schering AG research and patents. Even if this specific inference is not drawn, as explained below, there are a number of reasons not to assume or infer that Dr Broesamle, as the responsible person, either gave no instructions to P&V or gave instructions knowing nothing of the research which Schering AG had completed before Dr Broesamle requested P&V to prepare the priority documents for the PCT application.
86 Generic Health’s contentions to the contrary are highly improbable once the full context of the evidence is understood.
87 In particular, it cannot fairly be said that Dr Broesamle “has chosen to remain silent on the critical issues in the framing process” when the compelling inference from the full context is that Dr Broesamle could recall only attending numerous meetings and having numerous discussions with P&V about the Yasmin invention and no more. While Dr Broesamle said this expressly about only one meeting, on 14 January 1999, this must be considered with:
his evidence of lack of recollection about the August 1999 letter;
his role in Bayer, as described;
his responsibility for fertility control since 1987;
his work with DRSP since 1989;
his evidence about the substantial number of patent applications for which he was responsible;
the fact that since 2000 the DRSP family of patents (around the world) has been a substantial part of his work;
Generic Health not challenging his expertise, experience, competence, responsibilities, or views about P&V’s high competence and skills as patent attorneys;
Generic Health not challenging the fact that he attended or was involved in numerous discussions with P&V about the PCT application;
Generic Health not challenging his evidence that he did not recall what was discussed at the meeting on 14 January 1999; and
Generic Health not putting to Dr Broesamle its interpretation of notes of apparent meetings and discussions with P&V about the PCT application.
88 When these matters are considered it is apparent that the suggestion that it should be inferred that Dr Broesamle deliberately remained silent about things because his evidence would not have assisted Bayer, is in the realm of far-fetched and fanciful. Moreover, Generic Health never put to Dr Broesamle the proposition that he was effectively withholding relevant evidence from the Court or had deliberately remained silent or recalled more than he had said in his affidavits. Generic Health sought to justify this on the ground that a cross-examiner is only obliged to cross-examine about the evidence a witness actually gives. This may be accepted. But the first problem for Generic Health is that if it is going to be submitted that a witness has deliberately withheld information in evidence or had deliberately remained silent or recalled more than he had said in his affidavits because what he recalled would not assist the case, then at least that proposition must be put. It was not put. The second problem for Generic Health is that the evidence which Dr Broesamle actually gave in this case supports the inference that the reason he did not give evidence about the instructions he gave or what the documents meant was what would be expected in the ordinary course - he had no independent recollection of the framing of one patent application amongst many some sixteen years ago and thus could add nothing useful to the documents themselves which he had located and produced. Indeed, it might be thought that if Dr Broesamle had purported to give evidence about what instructions he gave in 1999 and what documents created in 1999 were intended to mean, such evidence would have been of dubious value given the well-known problems associated with attempts to refresh memory and avoid reconstruction, the latter with its inevitable tendency towards the self-serving no matter the honesty of the witness.
89 In the circumstances identified I do not accept that it was essential for Bayer to have sought leave to adduce further evidence in chief from Dr Broesamle by asking him whether he recalled anything about the preparation of the PCT application other than what was contained in his affidavit. Dr Broesamle was called on the first day of the hearing. Nothing to that time would have suggested to Bayer that Generic Health’s case would involve a contention that Dr Broesamle could give any useful evidence beyond that which he had given in his affidavits but had deliberately remained silent for fear that any evidence he gave, about either his instructions or the documents, would not assist Bayer’s case. That submission was only made in closing by Generic Health. The submission is to be weighed, not only in the context set out above, but also against the fact that Bayer ensured Dr Broesamle was available for cross-examination on his affidavits, which included an affidavit as to discovery, despite the need for Dr Broesamle (who, by the time of the hearing had retired from Bayer) to travel from Germany to do so. To the contrary, in all of the circumstances, the proper inference to be drawn is that Generic Health deliberately did not ask Dr Broesamle anything about his recollection or what the documents (including those he had prepared) meant for fear that Dr Broesamle’s memory might be revived in some respect and Bayer’s case thereby assisted.
90 I also reject Generic Health’s submissions to the effect that I should not draw any inference from any document that might favour Bayer because Dr Broesamle (or others) did not give evidence about the meaning of the document. The fact that the document was created at least 16 or more years ago provides a rational basis for inferring that nothing Dr Broesamle or anyone else could have said about the document would be other than potentially self-serving reconstruction. Further, Generic Health never put to Dr Broesamle, the person who gave evidence, that he was responsible for managing the PCT application and all patents within the DRSP family, any suggestion either that the documents which he had located and produced had any meaning different from that on their face or that he was refraining from giving evidence about any document for fear it would not assist Bayer’s case. As a result, Generic Health’s contentions that I should infer that any evidence that could have been given about various documents would not have assisted Bayer’s case, cannot be accepted. Contrary to Generic Health’s submission, the present case is not comparable to Gilead Sciences Pty Ltd v Idenix Pharmaceuticals Ltd [2016] FCA 169; (2016) 117 IPR 252. As I noted in Gilead at [515] Idenix did not call any witness who had any involvement in the relevant process in that case. Bayer, however, has called the person who was responsible for managing the preparation, filing and prosecuting the PCT application on which the patent was based.
91 This is not to say that I would construe any document more favourably to Bayer than might otherwise be the case. It is to say only that, despite Dr Broesamle’s silence about his instructions and the meaning of documents, I do not accept that the proper inference, as Generic Health would have it, is that the reason for “Bayer’s failure” to obtain evidence in chief from Dr Broesamle on the topic is that Bayer “fears to do so” because it “would have exposed facts unfavourable” to it. That inference against Bayer is not available in circumstances where Dr Broesamle was called, he was undoubtedly the relevant witness to give evidence having regard to his role, and nothing to that effect was put to him. The inference also becomes improbable in the face of the substantial number of patents for which Dr Broesamle was responsible, his particular responsibility for all fertility and hormone patents, his unchallenged expertise in chemistry and patents law, his unchallenged evidence of P&V’s expertise in pharmaceutical patents, his unchallenged evidence of attending many meetings and having numerous discussions with P&V, and his unchallenged and unsurprising evidence of lack of any memory of the circumstances surrounding the August 1999 letter from P&V and of the meeting of 14 January 1999.
92 Where this leaves me, of course, is with the documents themselves. Consistent with my observations above, the documents are to be approached mindful of the overall context. There is no basis to construe the documents in a manner for or against Bayer in the event of ambiguity. There is also no basis to draw or not draw inferences for or against Bayer from the documents. In the circumstances of this case, the meaning of and inferences to be drawn from the documents are not to be approached with any predisposition of the kind which informed my reasoning in Gilead.
93 Nor, for the same reasons as given above, can any significance be given to the fact that Dr Ulrich (who returned to Bayer and currently works there) was not called to give evidence. Dr Broesamle was the person who made the decisions about the PCT application, not Dr Ulrich. The submission of Generic Health that Dr Broesamle did not suggest that Dr Ulrich was “incapable of handling the matters in his absence” is beside the point. There is no evidence that Dr Broesamle was absent during any particular period. The fact that correspondence was sent between Dr Ulrich and P&V is immaterial given Dr Broesamle’s evidence that he, not Dr Ulrich, was the responsible decision-maker for the entire patent family (in consultation with a person other than Dr Ulrich), and that while he and Dr Ulrich worked as a team, he was Dr Ulrich’s senior.
94 Further, given that Bayer called Dr Broesamle there is no scope for the submission that Bayer has not explained why it did not call Dr Ulrich. Bayer did not have to explain not calling a person who reported directly to Dr Broesamle given that he was called. Thus, there was no “failure” to call Dr Ulrich. There is no reason to infer that Dr Ulrich would be in any better position than Dr Broesamle to give evidence about the PCT application (or priority documents leading up to it). The fact that Dr Ulrich was the recipient of the August 1999 letter is of no significance once it is accepted that Dr Broesamle had the senior role and was the relevant decision-maker and that Dr Ulrich worked with Dr Broesamle as a team.
95 The same reasoning applies to Generic Health’s submission that Bayer failed to call other people involved in the drafting process, including people from P&V. The answer to the submission is that the most relevant person was Dr Broesamle because it was he who was responsible within Bayer for managing the PCT application (and related priority documents) and it was he who had the final decision-making capacity in consultation with Bayer’s Head of Patents and Licensing, and Dr Broesamle was called. The question why Bayer did not call other people to give evidence about the same issue does not arise in these circumstances.
96 Nor do I consider it open to Generic Health to submit that a document which has not been produced (a facsimile from Dr Ulrich to P&V referred to in the August 1999 letter from P&V to Dr Ulrich) founds an inference against Bayer in respect of the missing document having regard to Jones v Dunkel (1959) 101 CLR 298 at 303, 312 and 320-332. This is because Dr Broesamle’s unchallenged evidence was that he had made reasonable enquiries and that there were no other documents than the documents discovered, which included the March and August 1999 letters but not the missing letter from in between these dates. While it is true that Dr Broesamle subsequently located some further documents, he explained that they had been inadvertently overlooked. In the face of this unchallenged evidence it cannot be submitted that the failure to tender the missing document is “unexplained” or that Bayer did not tender it for fear it might harm its case, and thus there can be no inference that the missing document would not have assisted Bayer. It is simply a missing document.
97 Other than this it is apparent from the evidence that Dr Broesamle’s view of the high level of skill and competence of P&V was justified. As Dr McCann said:
The choice of the combination of Ms Birgit Thalso-Madsen and Dr Marianne Johansen, given their combined backgrounds in organic chemistry and pharmaceutical sciences, together with their patent qualifications and experience as European Patent Attorneys, to draft the priority documents appear to me to be one that falls into an ideal overall combination of technical backgrounds, competency and experience for the subject matter in suit, being broadly speaking, a pharmaceutical formulation comprising a combination of two organic chemicals for contraception.
98 The next issue is the documents which are in evidence.
Documentary evidence
99 As explained below there is force in Bayer’s submission that Generic Health’s case “is based on the flawed premise that the universe of information available to those involved in the process of drafting the Patent priority documents is solely that contained in the summary reports summarised in” a table which was prepared within Bayer immediately before instructions were given to obtain patent protection for the Yasmin formulation. As Bayer also said:
Such an approach is artificial and ignores the reality of the drug development process at a pharmaceutical company. It overlooks the background research work against which the invention was made and the accumulated knowledge of the properties and characteristics of DRSP known to the drug discovery team members resulting from an ongoing 10 year research and development project investigating potential uses of DRSP including as an OC or in Hormone Replacement Therapy (HRT).
100 The documents fall into three categories, the documents relating to the preparation of the PCT application (and related priority documents), other documents created within Bayer which pre-date the PCT application, and the PCT application itself (including documents referred to in the PCT application).
101 Documents relating to the preparation of the PCT application: Dr Broesamle explained that in late 1998 the then Head of Patents at Schering AG directed him and Dr Ulrich to engage P&V to obtain patent protection for the Yasmin formulation of DRSP and EE. Dr Broesamle believed that P&V had particular expertise in the preparation of pharmaceutical formulation patents and he had confidence in the quality and soundness of P&V’s work, his experience always having been that P&V complete their work with a high level of skill and care and in accordance with his instructions.
102 The PCT application (on which the Australian patent was based) was based on two priority documents, being US and European patent applications which were filed on 31 August 1999. To prepare those priority documents, Dr Broesamle instructed P&V, but others from Schering AG were also involved in the process including Dr Ulrich and three of the named inventors, Mr Hilmann, Dr Heil, and Dr Lipp. The PCT application based on the priority documents was filed on 31 August 2000. In accordance with its instructions P&V then instructed Australian patent attorneys (FB Rice & Co initially and then Davies Collison Cave) to obtain patent protection in Australia. The Australian application was filed on 14 March 2002 and granted on 30 June 2005 and was based on the PCT application, subject to an amendment made in 2004, before the grant of the patent, to ensure consistency with the European Patent application then also being prosecuted based on the PCT application. The amendment is relevant to the dissolution test issue which is dealt with separately below.
103 The first document which I infer is of direct relevance to the preparation of the priority documents and thus the PCT application is dated August 1998. It is a table identifying five reports in respect of different formulations of DRSP and EE including 0.5, 1, 2, and 3mg of DRSP. From the fact that the table identifies the status and availability of each report, I infer that Schering AG was collating information it considered of obvious relevance to the patents for DRSP for which it subsequently applied. I infer that Schering AG was at least aware of all of the information in these five reports when it gave instructions to P&V for the framing of the specifications for the priority documents and PCT application.
104 Another document headed “Open questions for patent application regarding DRSP” contains the following:
1) DRSP: EE (ratio) contraceptive
[Dr Hethecker]
DRSP: EE (ratio) hormonal disorders
[Dr Schurmann]
- therapeutic effect (optimal)
- side effects (minimal)
105 From this I infer that Schering AG was considering the ratio of, relevantly, DRSP to EE to achieve the optimal therapeutic effect and minimal side effects for contraceptive purposes (and separately for hormonal disorders). I infer that they were doing so for the purposes of seeking patent protection for the Yasmin formulation given the timing and the content of the document.
106 There is a typed document dated 13 November 1998 which appears to have been prepared by Mr Hilmann and Dr Heil, two of the three named inventors. It refers to an active ingredient of drospirenone in the range of 0.5 to 7.0mg, with a handwritten note beside this stating “lower limit 2mg for ovulation inh” which must mean 2mg as the lower limit for ovulation inhibition (a figure to be expected once the reports, discussed below, are considered). I infer from this that Schering AG was considering the dosage range and held the view that 2mg was the lower limit for ovulation inhibition.
107 There is also a handwritten document which identifies the structure and characteristics of DRSP and contains other information which resembles the information in the priority documents and PCT application. It identifies the invention as involving:
(a) Amount of drospirenone (and estradiol) for therap. effect (contraceptive without increased blood pressure).
(b) quick release in gastiric environ of sparingly sol.subst…
108 As both of these concepts find their way into the priority documents and PCT application, I infer that these notes were prepared for the purpose of instructing P&V and that the substance of them, if not the notes themselves, was communicated to P&V.
109 After summarising a number of the reports referred to in the table discussed above, the notes record this:
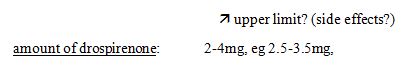
110 There was debate about the significance of the arrow and question marks in this note. The real significance of the document is that it discloses active consideration by Schering AG of the dosage range of DRSP, I infer, for therapeutic effect and of the upper limit consistent with the objective of minimising side effects. The range being considered to achieve those objectives was 2 to 4mg. Insofar as the arrow might be of any relevance, there is good reason to reach the same conclusion as Dr McCann that, in the context of the research about DRSP and the intention to obtain protection for the Yasmin formulation, it is likely that what was being asked was whether side effects could be avoided at a dose of about 4mg. This is consistent with a subsequent suggestion by P&V for a higher (5mg) dose as the upper end of the range which, it may be inferred, Schering AG subsequently rejected. It is also consistent with Dr McCann’s evidence that a patent attorney, properly, would always look for the higher range. As Dr McCann put it:
…But there’s another way that a patent attorney would normally look at it – is, “Can we get higher than four milligrams?” Right? The scientist has said, “Look, the range is two to four milligrams”, and the patent attorney says, “Well, can we get six milligrams?”, or, “Can we get five milligrams?”.
Yes?---Is there basis for going further than that? And so the question marks there that I – the way I would, sort of, look at them is more in that light as a patent attorney, rather than there’s a question mark about four milligrams necessarily. You know, I – as a patent attorney, I would say, you know, “Can we get more, and is there basis for getting more without getting – without – that we can we support?”, if you like.
111 One document that Dr Broesamle located after he completed his discovery affidavit is an undated two page handwritten document. Dr Broesamle did not write these notes but recognised that the paper on which they are written was commonly used at Schering AG at the relevant time (14 January 1999). The notes are in two columns, one headed “Anti Contraceptive” and one headed “Hormonal Replacement”. Both sides refer to DRSP and EE. Under the first column there is stated:

112 From this I can comfortably infer at least the following:
(1) The document relates to the preparation of the priority documents for the PCT application.
(2) The document was created by someone within Schering AG or P&V.
(3) Schering AG or P&V was considering the optimum dosage of DRSP and EE for two compositions, the first being contraceptive and the second hormonal.
(4) The dosage range for DRSP being considered in order to achieve the optimum effect and the optimum side effect level was 2 to 4mg or 3mg.
113 Dr Broesamle then located some notes in his own handwriting immediately behind a letter from P&V dated 19 January 1999. Dr Broesamle’s handwritten notes, partly in German, include the following:
DRSP – Meeting
3 mg DRSP
…
OC | HRT |
DRSP 3mg - (4-2) | DRSP 2,3,4mg |
… | … |
114 Given that this is in Dr Broesamle’s handwriting this document confirms that he was considering the dosage range for DRSP for contraceptive and hormonal replacement therapy purposes. Given the similarity between this and the document above I infer that they are notes of the same meeting. I thus infer that Dr Broesamle was considering 2 to 4mg or 3mg as the dosage range for DRSP for contraceptive purposes for optimum effect and side effect (which must mean minimised) level.
115 The next document is a letter from P&V to Dr Ulrich and Dr Broesamle dated 14 January 1999 which thanks them for a “pleasant and interesting meeting in Berlin on 14 January 1998 [sic]”. The letter continues:
We look forward to cooperating with your [sic] regarding the drawing up of a new patent application and expect to receive further material from you by the end of January.
116 I thus infer that the handwritten notes relate to a meeting between Dr Broesamle, Dr Ulrich and P&V and that at that meeting Dr Broesamle informed P&V of his consideration of the possible dosage for DRSP for contraceptive purposes for optimum effect and side effect level to be 2 to 4mg or 3mg. I infer this because, as the patent attorneys agreed, it is essentially a matter for the client to instruct the patent attorney about such matters as dosage range, and Dr Broesamle was the representative of the client. From this, it must also be inferred that Dr Broesamle was aware of some or perhaps all of Schering AG’s research about DRSP. He could not have considered any range in a vacuum and Schering AG had already carried out substantial research about, and obtained other patents involving, DRSP. Dr Broesamle’s handwritten notes contain other details which are inconsistent with any inference other than that as at 14 January 1999 Dr Broesamle was well aware of DRSP, its, solubility, operation, and potential dosage levels for optimum contraceptive effect and side effect levels. As noted, given his responsibility for fertility and hormone patents, the inference I would draw is that, as any competent patent attorney would, he familiarised himself with the information available within Schering AG about DRSP (and EE) as part of the briefing of P&V.
117 There is a facsimile from Schering AG (copied to Dr Ulrich, Dr Broesamle, Dr Heil, Dr Lipp and Mr Hilmann amongst others) to P&V dated 18 January 1999 enclosing the list summarising “open questions” dated 13 November 1998 and the material needed to answer those questions in order to prepare a first draft (I infer, of the priority documents) and a statement that Schering AG will try to provide this information to P&V by the end of the month.
118 On 17 March 1999, P&V sent a letter to Dr Ulrich and Dr Broesamle about the “Patent application for DRSP” enclosing a first draft of the specification including claims and ending with the statement:
We are looking forward to further discussions with you on this matter. In particular, your comments on the claims as well as our questions will be greatly appreciated.
119 Given the detail already within the first draft of the specification and the questions posed by P&V, I infer that Schering AG must have sent to P&V a considerable amount of material relevant to the proposed patent application, consistent with the terms of the 14 January 1999 letter.
120 The draft specification includes the following:
The claimed pharmaceutical composition is novel because it cites a narrower dosage range than that indicated in the prior art. The lower limit has been set to 2 mg per dosage unit/day because it is indicated to be the lowest dosage required to obtain ovulation inhibition. The upper limit has arbitrarily been set at 5 mg per dosage unit. Is that realistic, or could the limit be higher without causing adverse effects? What are the adverse effect[s] at higher doses?
This dosage level has been found to be particularly advantageous for the purposes of contraception as well as for other therapeutic uses. Is this correct, or are the indicated dosage levels inappropriate for the therapeutic uses of the compound?
121 The draft claims include as claim 1:
A pharmaceutical composition comprising, as an active agent, 6β, 7β; 15β, 16β-dimethylene-3-oxo-17α-pregn-4-ene-21, 17-carbolactone (drospirenone) together with one or more pharmaceutically acceptable carriers or excipients, the amount of drospirenone corresponding to a daily dosage, on administration of the composition, of from about 2 mg to about 5 mg (what is a suitable upper limit of the dosage – that is, one at which the severity of adverse effects is not prohibitive?).
122 From this I infer that P&V had considered the material about DRSP which had been provided to them and was querying whether the dosage range of DRSP could be more than 4mg (as Schering AG had indicated in the 14 January 1999 meeting) and up to 5mg without causing adverse side effects. No question was raised about the lower, 2mg, dosage range. From this I infer that, having reviewed the available material, P&V accepted what had been discussed with Schering AG on 14 January 1999, that 2mg was the lowest dose for optimum therapeutic effect for contraceptive purposes, but queried whether the maximum dosage range which could be claimed was 4mg as had been discussed or could be 5mg or more without adverse side effects.
123 On 26 August 1999 P&V sent a letter to Dr Ulrich which thanked Dr Ulrich for a fax with “details regarding DRSP”. This is the fax which has not been found. The letter asked for comments on the draft claims. Draft claim 1 refers to a dosage for DRSP from about 2mg to about 4mg.
124 From this it is clear that Dr Ulrich had sent to P&V further information about DRSP. It is also apparent that either in discussions or in the fax from Dr Ulrich, Schering AG had instructed P&V that the dosage range for DRSP which was to be claimed was about 2 to about 4mg. As the patent attorneys said, P&V would not have taken it upon itself to make this decision. I also do not infer that Dr Ulrich was responsible for making this decision. In accordance with the discussion above, it was Dr Broesamle, in consultation with the Head of Patents and Licensing, who was responsible for deciding what P&V should be instructed. Even if the instruction was contained in the missing fax, Dr Ulrich would have been conveying instructions upon which Dr Broesamle, in consultation with the Head of Patents and Licensing, had decided. I also have no difficulty inferring that the instruction was given with the objective of achieving an optimal therapeutic effect and to minimise side effects, consistent with the objectives which had been discussed between Schering AG and P&V since 14 January 1999. Given the contemporaneous references to optimal therapeutic effect and optimal (minimal) side effects, and the question raised by P&V about a possible 5mg dosage for the upper end of the range in the context of side effects, I consider that there is no reason to infer other than that Schering AG gave this instruction to P&V honestly believing that the instructed range would achieve these objectives. To infer otherwise, would suggest that Schering AG and P&V were in the business of making inaccurate and misleading contemporary notes of their discussions, in effect, identifying objectives of the dosage range which neither of them believed they were seeking to achieve in the patent specification and claims. This is nonsensical.
125 On 30 August 1999 Dr Ulrich sent to P&V the draft specification for the priority documents which said it enclosed “our comments” (not, I note, “my comments”). This is consistent with my conclusions above that Dr Ulrich was not the relevant decision-maker. While involved in the process, she was communicating the comments of Schering AG which, as I infer, Dr Broesamle authorised, given his role. The comments written on the draft specification include details about prior Schering AG patents (which, as I would expect, means that those involved in preparing the priority documents for the PCT application knew the details of those earlier patents and, necessarily given the detail of the comments, the research which underlay those patents), the dissolution test (discussed below) and other matters. There is no comment, however, about the range of DRSP in claim 1 (or otherwise) of 2 to 4mg. From this I infer that Schering AG was satisfied that the 2 to 4mg range for DRSP was appropriate and fulfilled the initially identified objectives of optimum therapeutic effect and optimum (meaning minimal) side effects.
126 In February 2002 P&V instructed FB Rice & Co, patent attorneys, to prepare the Australian patent application based on the priority documents (which had been filed on 31 August 1999) and the PCT application. Dr Warner of FB Rice & Co amended the claims in accordance with her usual practice to ensure satisfaction of the requirements of Australian patents law. FB Rice & Co filed the Australian patent application on 14 March 2002. Davies Collison Cave took over the Australian patent application from FB Rice & Co thereafter as Bayer had decided to consolidate its pharmaceutical patent portfolio in Australia. The patent, as noted, was granted on 30 June 2005.
127 At least insofar as the 2 to 4mg dosage range is concerned (and perhaps otherwise as well) there is no suggestion that the preparation, filing or prosecution of the Australian patent (in contrast to the PCT application and the priority documents on which it was based) was itself carried out other than in good faith and on the basis of reasonable skill and knowledge. No such suggestion could be made given the evidence. As Bayer submitted:
Dr Jacqueline Warner also gave written and oral evidence. Dr Warner is an Associate at FB Rice and a Registered Patent Attorney. Dr Warner obtained a PhD in 1993 from the University of Sydney after graduating from the University of Sydney in 1988 with a First Class Honours Degree in Science, double majoring in organic chemistry.
Dr Warner’s PhD involved research on biosynthetic models for the formation of naturally occurring anti-malarial and prostaglandin-like compounds. Dr Warner did post doctoral research in the organic chemistry department at Cambridge University, England on the topic of mannosidase inhibitors for use in enzyme purification.
In 1996, Dr Warner completed a Graduate Diploma in Legal Studies at the University of Technology in Sydney and in 2000 qualified as an Australian patent attorney. Dr Warner works in all areas of chemistry, including pharmaceutical chemistry.
Dr Warner was responsible for the prosecution of the Australian application from about February 2002 to March 2003.
Dr Warner considers that she handled the prosecution of the Application in good faith with all due skill and knowledge in accordance with accepted Australian patent attorney practice. Dr Warner was also, at all relevant times, well qualified to handle the prosecution of the Application in Australia.
128 Insofar as Davies Collison Cave are concerned:
Mr Mark Roberts gave written and oral evidence. Mr Roberts has been a principal of Davies Collison Cave Patent and Trade Mark attorneys since 2002. Mr Roberts has a first class Honours Bachelor of Science degree. His honours studies were in the field of molecular radiation biology at the Peter MacCallum Cancer Institute.
Although Mr Roberts joined DCC as a technical assistant in the chemical/ biotechnology group in 1994, he also has experience in the pharmaceutical industry. In 1998 Mr Roberts undertook a month’s work experience in the Corporate Patent department of BASF Algin Germany. From 1998 to 1999 Mr Roberts was employed as a patent advisor within the Global IP department at Glaxo Wellcome PLC in the UK. In that role Mr Roberts was involved in the preparation and prosecution of European patent applications.
As a principal at DCC, Mr Roberts specialises in the drafting, preparation and prosecution of patent applications, including for patents for pharmaceutical inventions. As a result of his time at BASF and Glaxo Wellcome, Mr Roberts has a knowledge and understanding of European patent law and practice.
Mr Roberts briefly handled the prosecution of the Australian patent in the late Dr Peter Stearne's absence in late 2004, including by preparing and sending a detailed response to the examination report that was issued by the APO in relation to the Patent Application, which ultimately led to acceptance of the Application.
It is Mr Roberts' opinion that the Australian application was framed in good faith and with reasonable skill and knowledge and prosecuted by appropriately qualified attorneys with all reasonable care and skill and in accordance with accepted and usual Australian patent attorney practice.
129 Otherwise, as Bayer submitted about Dr McCann’s evidence relating to the actions of the Australian patent attorneys:
(a) the Australian Application was at all times prosecuted by registered Australian patent attorneys with a scientific background appropriate for the subject matter of the Patent, namely Dr Jacqueline Warner, the late Dr Peter Stearne and Mr Mark Roberts, and that the Australian attorneys all possessed more than sufficient skill and knowledge to prosecute the Australian Application;
(b) those involved in the prosecution of the Australian Application did not take a course of action that would have been inconsistent with Australian practice in 2002 to 2004 in an effort to obtain claims of a wider breadth than what they would otherwise fairly be entitled to;
(c) the professionals involved in the drafting and prosecution of the Australian Application acted fairly, reasonably and sought to obtain, in good faith, the scope of protection for which Bayer was entitled….
130 Having regard to these matters it will be apparent that I do not accept the overall approach of Generic Health to the documents. Generic Health’s approach seems to be to assume that Schering AG did not intend to identify a range which would meet the objectives of optimum therapeutic effect and minimal side effects. Generic Health’s approach is disclosed by its submissions about the note containing the statements:
(a) Amount of drospirenone (and estradiol) for therap. effect (contraceptive without increased blood pressure).
(b) quick release in gastiric environ of sparingly sol.subst…
…
amount of drospirenone: 2-4mg, eg 2.5-3.5mg,
131 Generic Health made this submission:
Generic Health submits that this involves a reference to the possibility of adverse side effects at a dosage of 4 mg and that the inference that should be drawn is that the author, likely someone involved in the “framing” process, harboured concerns about the side effects arising from a dosage of 4 mg.
132 A more natural reading of the notes is that 4mg was being considered as the upper limit at which side effects might be largely avoided. This reading is consistent with the subsequent P&V draft (see below) which raised the upper limit, arbitrarily, to 5mg and asked Schering AG if that would involve side effects. The fact that the next version of the specification went back to 4mg strongly suggests that Schering AG instructed P&V that the upper limit to achieve its object of minimal side effects was 4mg, not 5mg.
133 Generic Health dismissed the other notes as nothing more than “records of a discussion about different compositions”. The documents are more than this. They show active consideration of a dosage range to achieve the stated objectives of optimum therapeutic effect and minimal side effects.
134 The fact, which I accept, that Schering AG had not reached a concluded view about at least the upper dosage range by the time of the 14 January 1999 meeting (in the sense that it considered P&V’s arbitrary upper range of 5mg thereafter) is beside the point. It is plain Schering AG did reach that view before any patent application was lodged because it must be inferred that Schering AG made P&V change the maximum dosage back to 4mg. And why might Schering AG have done so? For the obvious reason that it believed that the dosage range of 2 to 4mg satisfied its stated objectives of optimum therapeutic effect and minimal side effects. Accordingly, I find unpersuasive Generic Health’s submission that:
…it can be inferred that the upper limit of 4 mg was selected some time between 17 March 1999 and 26 August 1999, albeit by persons unknown and for reasons unknown.
135 The persons who decided are not unknown. They must have been Dr Broesamle in consultation with the Head of Patents and Licensing because there was no challenge to Dr Broesamle’s evidence that this was how all final decisions about the patent family were made. The reasons for the decision are not unknown. The reasons must have been to achieve the identified objectives of optimum therapeutic effect and minimal side effects because these were the only objectives for the range identified.
136 Similarly, the fact that side effects are not expressly referred to in the inventor’s typed notes of 13 November 1998 does not mean that the dosage range of 0.5 to 7mg was recorded without any regard at all to possible side effects. Schering AG had been researching DRSP and EE for many years and had carried out multiple studies of its effects. While Schering AG did not select this range of 0.5 to 7.0mg for the Yasmin patents family, I infer that this was because it did not achieve the goals of optimum therapeutic effect and minimal side effects in the context of contraception. It cannot be inferred, as Generic Health would have it, that this range was merely arbitrary. Given all the work that had been done, no such inference would be drawn about any range proposed by Schering AG in this period.
137 Other documents which pre-date the PCT application: As noted above, it is apparent that Schering AG provided P&V with information about DRSP on at least two occasions, before 17 March 1999 and 26 August 1999 respectively. I would infer that, at the least, the material provided to P&V included the five reports which Schering had included in the table it prepared in August 1998. It is obvious from the documents referred to above that Schering AG considered those reports in the context of the preparation of the priority documents and the PCT application. The P&V patent attorneys involved were themselves highly qualified chemists with specialist expertise in the preparation of pharmaceutical patents. They must have had access to substantial information about DRSP to prepare even the first draft of the specification. It is rational to infer that this information included these reports which Schering AG had identified as relevant to DRSP only a few months before P&V were instructed. The information also necessarily included the earlier patents referred to in the specification itself, considered separately below.
138 There is one other report which must be considered. It is report No. 9274 entitled “PK/PD study of the combination DRSP/EE after repeated oral administration” dated 1 July 1993, which relates to 2mg and 4mg formulations of DRSP (the 9274 report). This document is an internal Schering AG document consisting of multiple “fly sheets”. Generic Health submitted that the 9274 report cannot shed any light on the s 115(1)(a) issue because there is no proof that it was relied upon (or even known to) those involved in the framing of the patent, and in particular those involved in the selection of the 4mg upper limit. I disagree. While I accept that Dr Broesamle does not explain where or how he located the report, this has to be weighed along with a number of other factors. The report records a trial conducted by Schering AG. The report, on its face, discloses the existence of a sophisticated system for information control and retention. For example, the report is signed by multiple persons in respect of compliance with specific study standards and quality assurance requirements. The report records that the clinical study, which it summarises, took place over many years and was subject to quality assurance audits on no less than six occasions between 12 December 1989 and 2 June 1993. The report records how many pages it contains in total (185) and the extracts available are consecutively page numbered. The clinical study with which the report is concerned was a randomised double-blind study comparing two doses of DRSP and EE with Microgynon, a commercially available contraceptive. As such, the clinical study must have involved more than a trivial cost to Schering AG. Schering AG was in the business of developing innovator pharmaceutical products. Patent protection is an integral part of that business. Against these factors (which would weigh against the report having been overlooked for the Yasmin patent), is to be weighed the lack of direct evidence about the relevance of the 9274 report to the preparation of the patent and its absence from the table and handwritten notes discussed above (which refer to the other reports).
139 Importantly, the 9274 report deals expressly with the aldosterone antagonist effect of DRSP. The patent also refers to DRSP being an “aldosterone antagonist” which has diuretic properties and “is therefore suitable for counteracting the water-retentive properties of ethinylestradiol”.
140 While, unfortunately, there is no short statement of the relevance of aldosterone to contraceptive activity, there is evidence that aldosterone is a hormone which retains sodium in the body and is thus involved in control of blood pressure. Hormones associated with fertility, estrogens and progesterone, affect sodium and water retention in the kidneys but these two hormones, in a layperson’s terms, counteract each other. If levels of these hormones are not regulated throughout the contraceptive cycle, levels of aldosterone can increase causing sodium retention and increased blood pressure.
141 Weighing up all of the circumstances, I am unable to accept that in instructing P&V, Schering AG had forgotten the information in the 9274 report and overlooked the existence of that report. Information control must be vital to an innovator pharmaceutical company. Schering AG was working on DRSP for years, carrying out various studies, and filing various patents along the way. Yet Generic Health would have it that a clinical trial directed at the specific quality of the aldosterone antagonist effect of DRSP compared to another contraceptive which concluded that the combination of DRSP and EE tested had a clearly demonstrable aldosterone antagonist effect, was not considered by Schering AG when it instructed P&V to prepare a patent application for the same compounds (DRSP and EE) claiming the very same aldosterone antagonist effect. The notion that Schering AG would overlook one of its own clinical trials, let alone one that was concerned with comparing DRSP with a commercially available contraceptive to test the aldosterone antagonist effect of DRSP when it was arranging for a patent to be prepared for DRSP as a contraceptive with an aldosterone antagonist effect, is one I am unable to accept.
142 In case this inference is incorrect, I reach conclusions below on the two bases – the first being that Schering AG did consider the 9274 report when instructing P&V and the alternative being that Schering AG did not consider the 9274 report when instructing P&V.
143 Assuming Schering AG did consider the 9274 report, I am also unable to accept Generic Health’s proposition that the 9274 report does not provide proper scientific support for the 4mg upper limit. The submission is based on a misconception that aldosterone antagonist effect and diuresis are unrelated concepts. As the patent records, an aldosterone antagonist effect is related directly to diuresis (because it counteracts water retention which EE promotes). In other words, and again in a layperson’s terms, EE acts to increase water retention and thus possibly blood pressure but DRSP acts to reduce water retention by increasing urine production. The ratio of DRSP to EE was important to Schering AG’s objective of minimal side effects because the two components counteracted the tendencies of each other.
144 Thus, to submit as Generic Health does that the 9274 report says nothing about diuresis, when the report is concerned with the aldosterone antagonist effect of DRSP, is to miss the point of the report. The 9274 report reported that both dosages (2mg DRSP + 30µg EE; 4mg DRSP +30µg EE compared to Microgynon which has 0.15mg levonorgestrel + 30µg EE) were “well tolerated, subjectively and objectively”, with “no adverse drug reactions” and the aldosterone antagonistic effect was clearly demonstrated for DRSP in both doses with initial increased sodium excretion being later neutralised by the body’s counter regulation. No one with the expertise within Schering AG or P&V would have failed to appreciate that the report was dealing with water retention and removal by diuresis. The dosages being well tolerated must have meant that the water retention effects of EE were effectively countered by the DRSP without excessive diuresis at either dose.
145 While it is true that the 9274 report does not involve testing doses higher than 4mg of DRSP, Generic Health’s submission that the “document says nothing about dosages above 4 mg, nor does it assess why 4 mg is a maximum” is based on another misconception. The submission appears to assume that rather than predicting the effect of a dose which is likely to achieve therapeutic efficacy and minimise side effects (which must include avoiding excessive diuresis) and then testing the prediction, the only way a pharmaceutical innovator, in good faith and with reasonable skill and knowledge, can decide upon a maximum dosage range to achieve these objectives is to conduct a test which will show adverse side effects and then, in effect, conduct a precise titration exercise, lowering the dose by degrees to find the highest absolute dose at which the effect is avoided. This approach has nothing to commend it. It assumes that dosages are not subject to any possible variance when this is not how a person skilled in the art approaches a dosage range. It rules out the role of sensible prediction by those skilled in the art. It would involve subjecting people to the risk of the very adverse side effects which are sought to be avoided.
146 Generic Health’s other objections to the weight which might be given to the 9274 report are also without substance. The fact that the study on which it reports involved a sample of 27 women, when other Schering AG research involved more women, is immaterial. It does not mean that the 9274 report did other than demonstrate that the range of 2 to 4mg of DRSP was well tolerated which, in the context of the 9274 report, must include that the water retention effects of EE were effectively countered by the DRSP without excessive diuresis at either dose.
147 The 9274 report also said that the ovulation inhibitory effect of the formulations had not been conclusively proved but that the results indicated that ovulation was probably inhibited by both formulations. However, inhibition of ovulation was dealt with in the other reports identified in the table discussed above.
148 I also find Generic Health’s approach to those other reports unpersuasive. Generic Health submitted this:
As to the selection of the lower range of 2 mg of drospirenone, the evidence indicates Bayer’s own doubts about the efficacy of that dosage amount. See: (1) the 9693 Report (CB3.585), which concludes, in relation to a comparison between a tablet containing 2 and 3 mg drosprinenone, “a 3 mg-ZK 30.595-version should be developed further because an ovulation-inhibiting effect was demonstrable under the 3-mg version in all cases examined. It is to be expected that the contraceptive reliability of a 3 mg version will be greater than that of a 2 mg version on widespread use of the preparation”: (2) the A892 Report (CB3.590-1), which describes trials using 0.5, 1, 2 and 3 mg drospirenone, states on page 2 (CB3.590), “The present results confirm what already been shown in earlier studies: with 2 mg, the dose of DRSP lies in the threshold range of inhibition of ovulation” and concludes on page 3 (CB3.591) that the research “demonstrates that the DRSP dosage of 3 mg DRSP provides a safety margin and can be recommended for use as an oral contraceptive in combination with an estrogen”. No contemporaneous witness has been called by Bayer to explain these documents. In the circumstances, the appropriate inference to draw is that Bayer had doubts about the efficacy of a dosage of 2 mg of drospirenone.
149 This submission does not reflect the substance of the other reports.
150 The 9692 report dealt with a study of formulations of 2 and 3mg of DRSP (with 30µg EE in each formulation). It found a distinct aldosterone antagonist effect for both formulations (see the discussion above). It said that “clinically, no differences worth mentioning were observed between the two trial preparations” and that both were equally well tolerated so that other criteria would be needed to be employed to select a candidate for future development.
151 The 9693 report related to a trial for ovulation inhibition of the 2 and 3mg DRSP formulations (with 30µg EE in each formulation). The results in respect of tolerance were consistent with the 9692 report. In respect of ovulation, it was reported that follicular ripening occurred in several cases under both preparations. Further, although three ovulations were diagnosed under the 2mg preparation, one was “equivocal” and another involved “tablet-taking error”, and “no differences were demonstrated statistically…between the two preparations as regards the hormones LH, FSH, estradiol and progesterone” and the parameter “ovulation during the treatment cycles”. Further, “in keeping with the hormones, cervical function was greatly limited…under both trial preparations”. The conclusions of the 9693 report include that:
• The present results confirm what has already been demonstrated in earlier studies: with 2 mg, ZK 30.3595 is in the threshold region of ovulation inhibition.
• Although no differences were demonstrable between the two preparations (p > 0.05), a 3-mg-ZK 30.595-version should be developed further because an ovulation-inhibiting effect was demonstrable under the 3-mg-version in all cases examined. It is to be expected that the contraceptive reliability of a 3-mg-version will be greater than that of a 2mg-version on wide-spread use of the preparation.
152 Dr McCann gave this evidence which I accept:
I guess, I mean, the way I look at these reports is that they were trying to – and if you look at one of the earlier ones they’re trying to work out whether to go with the two or the three milligrams because both of them had contraceptive properties and one of the earlier reports they just couldn’t tell the – there was a difference between two or three whatsoever…
But subsequent reports – there were what you call equivocal results with the two, and so they thought from a commercial perspective it would be better to go with the three, but it still worked within the statistical significance with the two which, to me, as a patent attorney – the experimental results show it works. Statistically, you couldn’t – they couldn’t really tell the difference between the two…
As a patent attorney, I – to be honest, I would probably put two as the lower range and have a backup 2.5, if you like, as my fall-back position on the basis of that information.
153 It was not suggested to Dr McCann that selecting 2mg as the lower end of the range would lack good faith or reasonable skill and knowledge given the reports available. Nor, given those reports, could it be.
154 The 9970 and A187 reports concerned trials in which all formulations included 3mg of DRSP but the dosage of EE was varied. The A892 report concerned a comparison between formulations containing 0.5, 1, 2, and 3mg DRSP. This report includes the following:
Adequate ovulation inhibition was found only under SH T 470 C and SH T 470 N. The present results confirm what had already been shown in earlier studies: with 2 mg, the dose of DRSP lied in the threshold range of inhibition of ovulation.
…
Body weight and blood pressure were stable and the tolerance was good. No pregnancy, no unexpected and no serious adverse events occurred.
The aldosterone antagonist effect of DRSP could be demonstrated for all dose ranges investigated….
…
Conclusions
• The investigation on the ovulation inhibiting effect reported here showed adequate ovulation inhibition only under SH T 470 C and SH T 470 N. This is in agreement with earlier studies in which the threshold dose for ovulation inhibition was 2 mg. This also demonstrates that the DRSP dosage of 3 mg DRSP provides a safety margin and can be recommended for use as an oral contraceptive in combination with an estrogen.
• The aldosterone antagonistic effect of DRSP could be demonstrated for all dose ranges investigated…
155 Based on the substance of the reports I do not accept that Bayer (or Schering AG at the time) “had doubts about the efficacy of a dosage of 2 mg of drospirenone”. To draw this inference would be to place excessive weight upon a layperson’s approach to the notion of a “threshold dose” and to ignore the clinical and statistical analyses which demonstrated not that the 2mg dosage of DRSP was unreliable, but that it was the lowest dose at which ovulation was adequately inhibited. Given this, Generic Health’s submission that “in order for Bayer to discharge its onus of proving that that lower limit was selected in good faith and with reasonable skill and knowledge, an explanation from a witness in Bayer’s camp would be expected” must be rejected.
156 I also do not accept Ms Sinclair’s evidence about the lower end of the range. Ms Sinclair said that “…it is questionable whether a claim is framed in good faith if it includes a range that is not efficacious, in its contraceptive reliability, in all circumstances, when this is the promise of the invention”. As Bayer said in response:
(1) The patent states at p. 2, lines 25 to 26 that it has surprisingly been found that “a hitherto undisclosed minimum dosage level of drospirenone is required for reliable contraceptive activity”.
(2) The statement must be read (through the eyes of the person skilled in the art) in the context of the specification as a whole.
(3) The context includes example 5 in the patent, which reproduces the conclusions drawn by Bayer in relevant experimental reports and openly repeats much of the text of the 9692 report.
(4) “There is no (nor can there be) [a] suggestion that Bayer misrepresented the performance characteristics of the 2mg dose or that it did not honestly believe that a dose of 2mg would relevantly work”.
(5) As Dr McCann said, no contraceptive is 100% reliable. Nor does utility require 100% effectiveness (see Apotex Pty Ltd v Warner-Lambert Company LLC (No 2) [2016] FCA 1238 at [170]).
157 Documents referred to in the PCT application: As noted, the PCT application (and the Australian patent) referred to the disclosure in numerous Schering AG patents related to DRSP. Schering AG patent specification no. DE2652761 disclosed DRSP as a diuretic. Schering AG patent specification no. DE3022337 disclosed DRSP as involving gestagen-like activity and utility as a contraceptive agent at dosage levels of 0.5-50mg of DRSP per day, that the mechanism of action of the compound is very similar to that of the natural corpus luteum hormone progesterone, and does not give rise to increased blood pressure. Schering AG patent specification no. EP398460 (for which Dr Broesamle drafted the priority document) disclosed the use of DRSP for the treatment of androgen-induced disorders at dosage levels of 0.5-50mg, preferably 1-10mg per day. Schering AG patent specification for DE3051166 disclosed the use of DRSP for the treatment of gynaecological irregularities and for contraception at a dosage level of 0.5-50mg per day. All of these disclosures are identified in the specification for the unamended patent (and the PCT application on which it was based). Given his role, Dr Broesamle must have been involved in the preparation of all of these patents. The unamended specification also contains Example 5 which contains information from the 9693 report. As I do not accept Generic Health’s submissions about that report, I also do not accept its submission that Example 5 does not provide support to Bayer’s contentions.
158 It necessarily follows that the specification for the unamended patent was framed having regard to all of this information, in addition to the other documents to which I have referred.
The Australian patent attorneys
159 It will be apparent from the discussion above that I have not placed weight on the evidence of Ms Sinclair which went beyond a description of the standard procedures of a competent patent attorney. Dr McCann and Ms Sinclair gave evidence which might be described as going to the ultimate issue whether they would infer that the specification for the patent was framed in good faith and with reasonable skill and knowledge. While the ultimate issue rule has been abolished (s 80 of the Evidence Act), Ms Sinclair’s evidence dealing directly with inferences about good faith and reasonable skill and knowledge was not particularly helpful for a number of reasons. Ms Sinclair’s academic qualifications were confined to an undergraduate degree in science majoring in organic chemistry. In contrast, Dr McCann held the PhD qualification as discussed above. Ms Sinclair had not worked as a research scientist, whereas Dr McCann had. Ms Sinclair went from her undergraduate degree to work in a patent attorney’s office. She had no familiarity with the development of oral contraceptives and no knowledge of any particular biochemical mechanisms associated with oral contraceptives. She was unfamiliar with the concept of an aldosterone antagonist effect, whereas Dr McCann, although he accepted he was not an expert in the subject, was aware of the relationship between this effect and diuresis. Ms Sinclair had not considered any of the documents referred to in the patent specification. Ms Sinclair agreed, as was appropriate, that she did not have sufficient expertise to construe the reports in many respects and if she had been involved in drafting the specification she would have closely considered the project and taken advice from the relevant clinicians. That she could not do so for the purpose of giving evidence is obvious, but the consequence is that her opinions about lack of good faith and reasonable skill and knowledge are based on lack of relevant scientific expertise, incomplete information, and inability to consult with those with relevant expertise when, had she been drafting the patent as the P&V patent attorneys were, she would not have been in that position. Given this, it cannot be inferred that the P&V patent attorneys were in the same position of lack of relevant scientific expertise, incomplete information, and inability to consult with those with relevant expertise as Ms Sinclair. They plainly were not and must be inferred to have done precisely what Ms Sinclair says she would have done had she been drafting the patent (namely, place themselves in a position where they understood the science sufficiently, familiarise themselves with as much information as they could, and consult with the relevant clinicians).
160 For these reasons I am unable to accept Ms Sinclair’s evidence insofar as it extended beyond that part of her description of competent patent attorney practice which was to the same effect as the evidence of Dr McCann.
Conclusions
161 For the reasons given I do not accept Generic Health’s contention that Bayer has not discharged its onus of proof to establish that the unamended specification was framed in good faith and with reasonable skill and knowledge insofar as the 2 to 4mg range of DRSP is concerned.
162 Further, as Bayer submitted, Generic Health’s case, consistently with Ms Sinclair’s evidence, assumed that the promise of the unamended specification was to avoid side effects at the maximum dosage of 4mg of DRSP, but this is not what the patent says. The promise is “qualified by both the use of the words ‘may’, ‘preferred’ and ‘substantially’, and in relation to a particular side effect, excess diuresis”.
163 I accept Bayer’s submissions that:
(a) (at least) Bayer was in possession of the 9274 report demonstrating the effectiveness and safety of drospirenone within the range of 2 to 4mg. This information would have been known to the inventors;
(b) Bayer and P&V were in possession of numerous experimental reports demonstrating that there was no statistically significant difference between drospirenone doses of 2 and 3mg;
(c) Dr Broesamle met with P&V and discussed a dosage range of 2 to 4mg;
(d) P&V initially proposed a dosage range of 2 to 5mg and queried whether the upper limit could be increased; and
(e) Bayer reviewed the draft and a decision was made to reduce the claimed range to 2 to 4mg, as had been discussed at the initially meeting referred to above.
164 Even if Bayer had somehow forgotten one of its own clinical trials as reported in the 9274 report (which seems highly improbable), it had available to it a wealth of information enabling it to make a sound prediction as to the upper limit of the dosage range of 4mg. The fact that P&V tried to put the range up to 5mg, which Schering AG must have rejected, is irreconcilable with any suggestion of lack of good faith or reasonable skill and knowledge in the framing of the unamended specification. As Bayer said, the evidence demonstrates “that the bounds of the range were determined very conservatively within the extensive knowledge accumulated by Bayer over the period of time its invention had been developed”.
The 900ml, 3mg and tablet feature
165 It will be recalled that claim 3 was also amended by addition of the words “wherein the oral dosage form is a tablet” and claim 11 was amended by adding in references to 3mg DRSP, 900ml for the amount of water, and the dosage units being in tablet form. Claim 3, in its form before the amendments, reflected the terms of the PCT application.
166 In my view Generic Health’s approach to these issues is confounded by the same kinds of problems discussed above. It relied on Ms Sinclair’s evidence when she had no expertise in dissolution testing and thus was not the skilled addressee of any claim relating to dissolution testing. It relied on a selective approach to the evidence. It relied on Jones v Dunkel inferences which may not be drawn.
167 By far the best qualified person in respect of dissolution testing was Professor Dressman, pharmaceutical scientist and Professor and Director of the Institute of Pharmaceutical Technology at the JW Goethe Institute in Frankfurt am Main. Professor Dressman’s evidence disclosed what the skilled addressee of a patent involving a dissolution test would have known as part of the common general knowledge. As Bayer submitted:
Professor Dressman’s evidence was that of the three commonly used dissolution volumes 900ml was at the priority date the typical volume used for USP type II dissolution tests. She herself would use a volume of 900ml unless it was specified otherwise. Professor Dressman also noted that volumes less that 900mls were generally only used for highly soluble drugs (whereas DRSP is sparingly soluble in water). Professor Dressman did not expect to see a difference in dissolution rate if 1000mls were used rather than 900ml.
Professor Dressman’s evidence was also that the dissolution rate specified in claim 1 could be met by a formulation that was a capsule or a tablet.
168 Further, and again as Bayer submitted:
(1) “The specification clearly says that the formulation can be prepared in different forms, including but not limited to a tablet, and it was therefore competent and prudent for the dissolution test claims not to be limited to a tablet”.
(2) “The contemporaneous drafting records provided to P&V from Schering also make it clear that the inventors did not consider that the invention was limited to “tablet” formulations”.
(3) “As at the priority date, the USP II specified the routine laboratory steps that should be taken to test a variety of oral dosage forms including tablets and capsules and a skilled person would be able to perform the test on such forms”.
(4) A skilled person would understand that a formulation would fall within the scope of the claims (and possess the advantageous bioavailability profile) as long as it met the dissolution criteria specified.
(5) A skilled person would understand that they could vary a number of parameters (e.g. type of dosage form and particle size) with a view to achieving the advantageous dissolution profile.
(6) A skilled person would know how to perform the claimed dissolution test using a variety of oral dosage forms including tablets, soft and hard gelatine capsules, coated tablets and granules.
(7) Given that the unamended specification referred to a dosage range of 2 to 4mg of DRSP it was appropriate for the dissolution test parameters to also refer to these amounts of DRSP.
169 Generic Health’s submissions to the contrary are unconvincing.
170 Professor Dressman gave this evidence:
(1) “…there are over 600 dissolution tests in the United States Pharmacopeia that are product specific, and most of them use 900 mils as their dissolution medium if the dissolution test is done in either a type 1 or a type 2 tester”.
(2) “900 mils is far and away the most commonly used”.
(3) The volume of water to use in a dissolution test of this kind is “a simple selection procedure”.
(4) “You could use 1000 mils but… as I said also in my affidavit, I wouldn’t really expect to see a difference”.
(5) “I understand how, and would be able, to perform the Dissolution Test on a tablet, capsule or other dosage form”.
(6) “It is not necessary for the Dissolution Test to specify that the oral dosage form is a “tablet”. As at the priority date, the USP [USP XXIII Paddle Method] specified the routine laboratory steps that should be taken to test a variety of oral dosage forms including tablets and capsules”.
(7) As all the stressors are fixed, “I do not agree that the Dissolution Test “does not provide sufficient or complete parameters for the testing of the solubility characteristics claimed”.
171 As Bayer submitted:
Professor Dressman’s evidence is supported by the Bayer dissolution test reports which report that testing comparing the dissolution rates of:
(a) 3mg v 6mg of DRSP; and
(b) 500ml v 900ml of water,
using USP II paddle method apparatus gave identical dissolution profiles.
172 Dr Broesamle gave evidence that:
(1) “… the skilled addressee, after having consulted his specification of the patent, knows that drospirenone is a sparingly soluble compound. And then the skilled person will know that this test has to be performed at the volume at the upper end, because it has – these are so-called sink conditions, is the term, because, of course, all the drospirenone in this test has to be able to go into solution”.
(2) Apart from the skilled addressee knowing that an amount of water at the upper end of the range (1000ml) should be used “the skilled addressee can easily do a test there, before he makes the final dissolution test, to make sure that he will have the – the best volume to do the test”.
173 Dr Wagner, the Head of the Pharma & Chemistry section and the Head of Opposition and Litigation of P&V, holds a PhD from the Technical University of Denmark in the area of protein chemistry. His Master of Science was in Chemical Engineering. He also has experience in the pharmaceutical industry, having been an in-house patent attorney at the Danish pharmaceutical company Novo Nordisk, where he drafted patents for inventions relating to genetically modified enzymes. Having had access to P&V’s files, Dr Wagner concluded that the P&V attorneys involved in the drafting, prosecution and defence of the Yasmin patents acted professionally and in accordance with good European patent attorney practice and exercised all due skill and care. He also gave this evidence (adopting a summary Bayer provided), which I accept:
(a) the specification describes a formulation with 2-4mg DRSP and it was therefore competent and prudent to draft the dissolution test claims to specify a dosage range of 2-4mg;
(b) the specification says that the formulation can be prepared in different forms, including but not limited to a tablet, and it was therefore competent and prudent for the dissolution test claims not to be limited to a tablet;
(c) the specification is directed to formulations with DRSP in a rapidly dissolving form. The specification refers specifically to the dissolution of a composition in the form of a tablet containing 3mg DRSP, however Dr Wagner does not interpret the specification as restricting the application of the dissolution test for this composition. It was therefore competent and prudent for the claims to refer to the application of the dissolution test to any composition described in the specification;
(d) 900ml is the standard volume that is used in USP XXIII dissolution testing (being the version of the USP that was current and official in August 1999);
(e) the International Preliminary Examination Authority (IPEA) did not object to the dissolution test claims in the PCT Application on the basis that the claims did not include the 3mg, tablet and 900ml features;
(f) the EPO accepted the European Parent Application for grant with dissolution test claims not including the 3mg, tablet and 900ml features; and
(g) the Australian Patent Office granted the Australian patent with dissolution test claims not including the 3mg, tablet and 900ml features.
174 Generic Health’s submissions fail to confront the overall effect of this evidence and the vast distance between it and the contention that, in framing the unamended specification, there was a lack of good faith and/or reasonable skill and care. Given that the skilled addressee would have understood that the dissolution test for this sparingly soluble compound ought to be carried out in 900ml of water in accordance with standard practice for the USP XXIII Paddle Method and would have been capable of carrying out the dissolution test on a range of dosage forms without any difficulty, the suggestion that the unamended specification was framed other than in good faith and/or without reasonable skill and care seems to me to be untenable. Not being told what volume to use, in these circumstances, is immaterial. The skilled addressee would know how to work the dissolution test. The fact that the dosage forms were not specified for the dissolution test is also immaterial. The dosage form might affect the working of the test, but there is no doubt on the evidence that the skilled addressee would have been more than capable of working the test across the range of possible dosage forms.
175 Nothing in the evidence supports Generic Health’s contention that the unamended specification might lack clarity. The contention is based on the unproven possibility that the composition in question, in its particular form, might pass the “composition test” at 1,000ml but not at 900ml thereby giving rise to a risk of lack of claim clarity. The submission is without any real evidentiary foundation and lacks merit. Nor does a mere risk of lack of claim clarity necessarily suggest a lack of good faith or reasonable skill and care in framing the specification.
176 Given Dr Broesamle’s unchallenged evidence that Bayer did not consider the unamended claims invalid and did not amend the specification because it did so, but merely wished to avoid an argument, it is also not open to Generic Health to submit, as it did, that:
Bayer’s decision to amend the claims provides a “reliable and expert opinion on the question” of whether the Original Claims were susceptible to a fair basing attack: see by analogy Halliday & Nicholas v Corsiatto [2001] NSWCA 188 at [17]. It also provides a “reliable and expert opinion” that there was a defect in how the Original Claims were drafted, that is, the omission of the tablet and 900 ml features.
177 To make this submission Generic Health had to challenge Dr Broesamle’s evidence that invalidity was not the reason for the amendments. Dr Broesamle’s evidence was not merely that “amendments were made to bring the Original Claims “into a better form”, being one that was “narrower” and “in closer alignment with what was in the specification”, as Generic Health would have it. Dr Broesamle said:
(1) “The claims were amended to have the patent in a better conversation, yes, and to defend it. But not because the claims had been invalid in the broad version”
(2) “It was to bring it into a better form, but this was not true, and this was not due to the reason that it was invalid before”.
(3) “And why was that a better version, as you put it? It’s narrower a bit”.
178 I do not see how this evidence can be said to support the proposition that there was any defect in the unamended specification. I do not accept that any of the cases to which Generic Health referred are analogous to the present matter where the skilled addressee would have had no difficulty in carrying out the dissolution test in the unamended specification. This is not a case of clear error in the specification as in Rediffusion Simulation Ltd v Link-Miles Ltd [1993] FSR 369 at 381-385. It is not a case of the patent attorney misunderstanding the client’s instructions as in Ronson Products Ltd v Lewis & Co [1963] RPC 103. It is not a case where the unamended claims involved are of such a breadth and vagueness as to justify a conclusion of lack of reasonable skill and knowledge as in British United Show Machinery Co Ltd v Gimson Shoe Machinery (No 2) (1929) 46 RPC 137. Generic Health accepted that it was not necessary or appropriate to run a hypothetical fair basis case to determine the s 115(1)(a) issues. I agree. The problem for Generic Health is that it is not apparent from any of the evidence that anything at all was wrong with the unamended specification. In particular, it is not accurate to say that “Bayer needs to explain how and why the 900ml and tablet features came to be omitted from the Dissolution Claim and, ultimately, the Original Claims”. There is no evidentiary basis to support the notion of any omission of this material. It is merely that, the claims having been amended in other jurisdictions and validity having been challenged, Bayer took the opportunity, as it was entitled to do, to seek to amend the claims.
179 The suggested risk of lack of fair basis also does not support a conclusion of lack of good faith or reasonable skill and care in framing the specification. The risk is said to arise from disconformity between the unamended claim and p 4 of the specification which states that:
It has surprisingly been found that when drospirenone is provided in micronized form… in a pharmaceutical composition, rapid dissolution of the active compound from the composition occurs in vitro (“rapid dissolution” is defined as the dissolution of at least 70% over about 30 minutes, in particular at least 80% over about 20 minutes, of drospirenone from a tablet preparation containing 3 mg of drospirenone in 900 ml of water at 37°C determined by the USP XXIII Paddle Method using a USP dissolution test apparatus 2 at 50 rpm).
180 The unreality of Generic Health’s propositions is exposed by submissions such as this:
In relation to the water quantity, it cannot be assumed that Schering/Bayer or its patent attorneys deliberately chose to keep the claim unlimited so as to obtain a wider monopoly. It is not apparent what purpose that would serve, or how such an omission would give the client any greater protection. Nor has there been evidence as to what was known or considered about how the claim would be read. Nor has there been evidence about whether it was anticipated that the claim would be read down by reference to the specification or, if so, why regard to the specification would not highlight the disconformity between the dissolution test on page 4 of the specification and the dissolution test in the claim, and how the reader of the patent was anticipated to resolve that issue. This is thus a very different question from some general issue of the patent attorney or the client wanting to preserve the broadest form of claim.
181 A number of points can be made.
182 First, it is true that it cannot be assumed that Bayer (in fact, Schering AG) was deliberately attempting to obtain a wider monopoly than it knew it had a right to. This cannot be assumed because it was never put to Dr Broesamle (and, I note, is also contrary to the finding against covetous claiming by Yates J as between the same parties which, irrespective of issue estoppel, would weigh against making a contrary finding based on no new evidence). This, however, is a fact in favour of Bayer when it comes to issues of good faith and reasonable skill and knowledge in the framing of the unamended specification.
183 Second, the proposition that there has been no evidence about how the claim would be read overlooks the evidence of Dr Broesamle and Professor Dressman, who represent the skilled addressee of this patent, that the skilled addressee would know exactly how to read the claim and, in doing so, would assume the 900ml feature and that the dissolution test could be made to work across dosage forms (assumptions which, on the evidence of Dr Broesamle and Professor Dressman would be valid even if some routine trial might be required for the dosage forms).
184 Third, evidence about the alleged disconformity between the claim and the dissolution test on p 4 would not have assisted a fair basis challenge. Once it is accepted (as it must be on the evidence) that the common general knowledge of the skilled addressee was to the effect described above, there is no disconformity. Further, beyond this, fair basis is not a matter for evidence in any event.
185 In the circumstances described above, I do not accept that any inference can be drawn against Bayer that the features in question “were left out in error (i.e. absence of ‘reasonable skill’)”. It cannot be a lack of reasonable skill to draft a patent on the conventional basis that it will be construed through the eyes of the skilled addressee possessing the common general knowledge relevant to the area of discourse. Nor can Generic Health’s submission that “Bayer’s silence also leaves open the prospect that the Original Claims were initially drafted as they were in an attempt, impermissibly, to broaden Bayer’s monopoly beyond that which defines the invention (i.e. absence of ‘good faith’)”. This submission is directly inconsistent with Generic Health’s other submission that “it cannot be assumed that Schering/Bayer or its patent attorneys deliberately chose to keep the claim unlimited so as to obtain a wider monopoly”. It is also wrong to characterise Bayer as having been “silent” when the evidence it did call disclosed how the skilled addressee would have approached the dissolution test in issue.
186 Having regard to the evidence, I am more than satisfied that Bayer has discharged its onus of proving that the unamended claims and specification as a whole were framed in good faith and reasonable skill and knowledge, “notwithstanding that they omitted the 900 ml, 3 mg and tablet features”. In truth, there was no omission given the common general knowledge about dissolution testing and the 2 to 4mg range which otherwise appears in the unamended specification (dealt with above).
Conclusions
187 For the reasons given I accept Bayer’s submission that “Generic Health’s s115 contentions founder by reason of a combination of”, at the least:
(a) its reliance on the opinions of a witness as to what can be drawn from the isolated information in the summary reports, when that witness is plainly wholly unqualified to express such opinions…; and
(b) its failure to put any of its suggestions of lack of good faith to Dr Broesamle, the highly qualified senior patent counsel of Bayer who as at the priority date had extensive experience in DRSP and was not only involved in the drafting process, including attending the relevant 14 January 1999 meeting, but the senior person within Bayer undertaking those tasks.
188 I accept also Bayer’s submission that the suggestion of a lack of good faith on the basis of nothing more than the existence of the onus ought not to be encouraged. In Bayer’s words:
The mere fact of the amendment does not justify a hindsight conclusion that the unamended claim was drafted other than in good faith or without reasonable skill and knowledge. Such an approach would be a requirement that claims at all times be drafted perfectly. This has never been the law.
189 With the benefit of hindsight, and to assist in future s 115 cases, I would agree with Bayer that a party putting good faith in issue ought to be made to particularise its contentions so that the patentee is protected from any suggestion that it should “in evidence first raise up and then exorcise the ghost of every possible defect in the unamended claim” (General Tire at 269-270).
190 That said, such ghosts as were raised by Generic Health have been effectively exorcised by Bayer. No conclusion is open other than that the specification without the amendment was framed in good faith and with reasonable skill and knowledge.
191 As a result, the prohibition on the award of damages for Generic Health’s infringements of the patent before 14 December 2012 is not engaged.
DAMAGES
Principles
192 The parties emphasised different aspects of the relevant principles, as suited their respective contentions.
193 Bayer noted that damages for patent infringement are compensatory. It emphasised that:
(1) Estimating damages is “not an exercise required to be undertaken with mathematical precision and … the court must do its best on the evidence before it” (Advanced Building Systems Pty Ltd v Ramset Fasteners (Aust) Pty Ltd, [2001] FCA 1098; (2001) 52 IPR 305 at [29]).
(2) The exercise is “a matter which is to be dealt with in the rough – doing the best one can, not attempting or professing to be minutely accurate – having regard to all the circumstances of the case, and saying what upon the whole is the fair thing to be done. … but dealing with the matter broadly, and as best we can as men of common sense” (Meters Ltd v Metropolitan Gas Meters Ltd (1911) 28 RPC 157 at 161-162).
(3) The task is to “form an estimate, recognising that precision, or even near precision, is impossible of attainment”… “in short one cannot expect much in the way of accuracy when the court is asked to re-write history” (Gerber Garment Technology Inc v Lectra Systems Ltd (1995) 112 RPC 383 at 395).
(4) In performing this task, the court should adopt the approach of Stirling J in British Motor Syndicate Ltd v Taylor & Sons Ltd (1900) 17 RPC 189 at 196 who said that it must be remembered that as he was “dealing with wrongdoers” he was “entitled to fix the damages on a liberal scale...”. the same point was made by Wilberforce LJ in General Tire and Rubber Co v Firestone Tyre and Rubber Co Ltd (1976) 93 RPC 197 at 212.
194 Generic Health agreed that the purpose of an award of damages for patent infringement is compensatory, rather than punitive (Seafolly Pty Ltd v Fewstone Pty Ltd [2014] FCA 321; (2014) 313 ALR 41 at [506]). As such, the appropriate quantum of damages is, so far as possible, “that sum of money which will put the injured party in the same position as he would have been in if he had not sustained the wrong”: (Seafolly at [510]). The process, Generic Health submitted, is as described in Pacific Enterprises (Aust) Pty Ltd v Bernen Pty Ltd [2014] FCA 1372; (2014) 321 ALR 715 in that the award of damages should restore the claimant to the position it would have been in had the infringements not occurred (at [5]), which generally requires the assessment of damages by reference to a common sense causal link between the infringing conduct and the loss suffered (at [6]).
195 Generic Health emphasised, however, that Bayer bears the onus of proof (Gerber Garment Technology Inc v Lectra Systems Ltd [1995] RPC 383 at 393 cited in Paramount Pictures Corporation v Hasluck [2006] FCA 1431; (2006) 70 IPR 293 at [34] and Seafolly at [508]). Accordingly, in Aristocrat Technologies Australia Pty Ltd v DAP Services (Kempsey) Pty Ltd (in liq) [2007] FCAFC 40; (2007) 157 FCR 564 at [96] Rares J said:
But it was not for the alleged infringers to make the case against themselves. That was the onus cast on Aristocrat if it wanted to recover compensation by way of damages.
196 Otherwise, the parties were at issue concerning the relevant counter-factual hypothesis, with Bayer contending that every sale of Isabelle and Petibelle (the latter to June 2016 only) was a lost sale of Yasmin and Generic Health contending that not all, or even necessarily most, sales of Isabelle and no sales of Petibelle could be characterised as a lost sale of Yasmin. As this involves an issue of fact to be resolved by reference to the applicable principles, I deal with it below in that part of the reasons considering the one for one issue. Bayer also contended that if some sales of Isabelle and Petibelle were not lost sales of Yasmin then, in any event, Bayer must be compensated for the infringement of its proprietary rights (by application of the so-called user principle). Generic Health contended that this would involve impermissible “double dipping”. This issue is also best resolved in the relevant factual context, to which I now turn.
Factual context
197 The following summary is based largely on Bayer’s submissions which, in this regard, are amply supported by the evidence.
198 Bayer submitted that:
The OC market in Australia comprises two market segments:
(a) OCs listed on the Pharmaceutical Benefits Scheme (PBS), which are then sold to customers at a Government-subsidised price (the PBS market); and
(b) OCs purchased on private prescriptions (the private market).
199 Having regard to the whole of the evidence I would prefer to recognise that there are two aspects of a single market. The first aspect involves PBS prescription OCs in which the cost of the OC to the consumer is subsidised under the PBS. The second involves private prescription OCs where the cost of the OC is not subsidised under the PBS.
200 All OCs require a prescription from a doctor.
201 PBS prescriptions relate to first and second generation OCs which generally contain EE and norethisterone (first generation progestogen) and EE and levonorgestrel (second generation progestogen). These OCs have a substantial share of the market by use and volume.
202 For PBS prescriptions the cost to the consumer is around $19 to $24 for a packet of four cycles (i.e. four months). The maximum cost for any OC listed on the PBS is about $36 for a four month supply (leaving aside concession card holders).
203 Private prescriptions relate to some second generation and all third generation OCs. Third generation OCs contain EE with the progestogens desogestrel, gestodene, nomegestrol, cyproterone or DRSP.
204 Second generation OCs not listed on the PBS are generally sold to customers at a price of $35 to $40 for a packet of three cycles (i.e. three months).
205 Third generation OCs are marketed for their side effect profiles and benefits, including lighter bleeding (Zoely and Qlaira), better management of androgenisation such as facial hair and acne (the cyproterone containing combined OCs) and reduced weight gain, fluid retention and effect on mood (Yasmin, Yaz and Yaz Flex).
206 Third generation OCs are priced at a premium with the prices (leaving aside Isabelle and Petibelle) ranging from around $71 to $84 for a packet of three cycles (i.e. three months).
207 Doctors generally give a prescription for a single packet for three or four cycles plus two repeat prescriptions of a similar size. As such, in the usual course, a patient (or at least one who is already taking the same OC as is being prescribed) is given an initial prescription for three or four months plus repeat prescriptions for six or eight months of supply to give sufficient tablets to last up to 12 months before she will need to return to the doctor.
208 Doctors prescribe OCs using originator brand names and are not generally aware of generic versions of brands unless and until a patient specifically requests them. Hence, before Isabelle was introduced (and, on the evidence, also after), doctors would have prescribed “Yasmin” if the doctor wished the patient to take that particular formulation of EE and DRSP.
209 When prescribing an OC doctors consider:
(a) Whether the patient has a contra-indication that would make it unsafe to take any COCP [combined oral contraceptive] such as migraine with aura, history of thromboembolic disease or significantly elevated blood pressure;
(b) The effectiveness of that formulation in that woman. Generally all COCPs are all equally effective as contraceptives in all women with the exception of women taking other medications that alter the metabolism of the COCP e.g. women taking medications for epilepsy. In these women higher dose COCPs or alternative forms of contraception may be required.
(c) Other problems that may be effected (helped or hindered) by the COCP including acne, weight gain, fluid retention, menstrual cycle control; and
(d) Patient preference – side effect profile, cost, their friends’ recommendations.
210 There is evidence that insofar as cost is relevant, the issue of primary concern to doctors should be that it is desirable to “maximise the likelihood of maintaining long term affordability” (Exhibit 22: “Contraception: common issues and practical suggestions”, Australian Family Physician, 41(10), October 2012) so that, in the first instance and subject to the issues above including patient preference, “choosing a PBS subsidised pill initially is essential, however attractive it might seem to give one or two ‘free’ samples of an unsubsidised agent as a starter”. That said, there is evidence that a number of women who obtained private prescriptions for Yasmin were surprised at its high cost which suggests doctors do not always or even routinely discuss cost in any detail with a patient seeking an OC. Overall the evidence supports Bayer’s submission that insofar as cost is relevant to the prescribing doctor at all the main issue would be the long-term affordability of PBS prescriptions as against the capacity of the patient over the long term to pay for a private prescription.
211 Typically OCs are taken by otherwise healthy patients daily for an extended period of time. All OCs are reliable, if used as directed, in acting as a contraceptive, but have different side effects on different individuals. Women are reluctant to change from one OC to another if they are happy with the OC which they are taking in terms of side effects. While women do change OCs, that is usually due to a material change of circumstances such as returning to an OC after having a baby. Generally speaking, however, women do not “chop and change” OCs; they tend to stick with the same OC unless some major life event results in a change of circumstances. In this sense, there is high “brand” or “molecule” loyalty by women to their OC (although I doubt consumers think in terms of molecules, each brand does represent a different molecule).
212 Yasmin consists of 3mg of DRSP and 30µg of EE. It was registered on the ARTG on 6 July 2001. It was sold in Australia from August 2002 as a private prescription OC in boxes containing one cycle (28 tablets) or three cycles (3 x 28 tablets). It has never been listed on the PBS.
213 Yasmin involved a unique molecule. When first supplied in Australia in late 2002, it was the first and only OC to contain DRSP as the progestogen component. The pharmacological profile of DRSP meant that Yasmin offered non-contraceptive benefits which were not otherwise available in the one OC including by reason of DRSP’s aldosterone antagonist effect thereby avoiding increased plasma volume and body weight and symptoms such as breast tension, headache and bloating and in some cases increased blood pressure.
214 Bayer marketed Yasmin to doctors only. Bayer Australia sold Yasmin mainly to wholesalers. Bayer Australia did not provide a recommended retail price for the retail sale of Yasmin by pharmacists. Yasmin was sold by pharmacists to customers for around $71 to $73 for a three month supply and around $28 to nearly $30 for a one month supply.
215 Isabelle is a generic form of Yasmin. Isabelle was registered on the ARTG based on bioequivalence with Yasmin (that is, the relevant molecule is the same, 3mg DRSP and 30µg EE). From January 2012 Yasmin and Isabelle were the only products on the market in Australia containing 3mg DRSP and 30µg EE.
216 Generic Health marketed and sold Isabelle to pharmacies in Australia from January 2012. Isabelle was sold by pharmacists to customers for around $60 to $61 for a three month supply and around $25 to $26 for a one month supply.
217 Because doctors prescribe on the basis of the originator brand, doctors continued to issue prescriptions for Yasmin after Isabelle was introduced. There is no evidence any doctor issued a prescription for Isabelle. Provided the prescribing doctor had not ticked the box “brand substitution not permitted” (in which event the pharmacist could not supply Isabelle on that prescription), a pharmacist could supply a customer with Isabelle on a prescription for Yasmin. A pharmacist could not supply Isabelle in response to a prescription for any OC apart from Yasmin.
218 Generic Health continued to sell Isabelle until 19 June 2014 when it was permanently restrained from so doing.
219 As Bayer noted:
The Generic Health order form sent to pharmacists for the launch of Isabelle in January 2012 noted that the originator brand was Yasmin, that Isabelle was “the only generic available in Australia” and highlighted Isabelle as “another profit opportunity from Generic Health” and “the easiest conversion opportunity”. Initially only a 3 x 28 tablet Isabelle pack was offered by Generic Health.
Generic Health later introduced a 1 x 28 packet of Isabelle in about August 2012, some six months after the commencement of the infringement litigation, so that it supplied Isabelle in the same two forms (1 and 3 x 28 tablets) as Yasmin.
220 In respect of Petibelle, as Bayer submitted:
Bayer obtained an ARTG listing for their own generic version of Yasmin, “Petibelle”, on 8 April 2011. This was in keeping with Bayer Australia’s usual strategy where if a third party started selling a generic, it may launch its own generic brand of that product.
Following the awarding of a permanent injunction restraining Generic Health’s sales of Isabelle in June 2014, Petibelle was launched. Stocks of Petibelle were released to wholesalers on 26 June 2014.
Bayer derived and derives a lower gross margin on the sale of a unit of Petibelle than it does from the sale of a unit of Yasmin.
Other than sending the initial letter advising of Petibelle’s availability to doctors and pharmacists on 20 June 2014, Bayer did not undertake any specific marketing of Petibelle.
221 Petibelle was sold by pharmacists for about $66 for a three month supply and nearly $22 for a one month supply.
The one for one issue
Some further principles
222 Generic Health noted that where parties compete in the same market and the claim is for lost profits, it is necessary to consider the counterfactual position, that is, what the applicant would have sold if not for the infringement and the costs associated with those sales. In TS & B Retail Systems Pty Ltd v 3Fold Resources Pty Ltd (No 3) [2007] FCA 151; (2007) 239 ALR 117 at [207], a copyright case but the principle is of general application, Finkelstein J said this:
The plaintiff must show that he has lost sales to the defendant as a result of the infringement and quantify the loss suffered. This requires the court to explore the counterfactual hypothesis of the contracts the plaintiff would have obtained absent the infringement and the costs associated with them. Necessarily the process will involve a degree of speculation, but that is no bar to recovery. The claim is not for loss of revenue but for loss of profits. The profits to be calculated are the lost net profits. By net profits I mean revenue less all costs including variable and indirect costs, but not including income tax. Care must be taken to ensure that costs savings are brought to account. If a plaintiff sells less of his products he will have less costs and that should be treated as a gain to be offset against the lost revenue which forms the basis of the computation of lost profits. The plaintiff is also entitled to recover indirect losses (such as damage to goodwill) as long as the cause is the infringement, the loss is foreseeable and is not unduly speculative. It will often be impossible to be precise in the calculation of lost profit. If needs be, the calculation can be rough and ready, with the benefit of any doubt favouring the plaintiff.
223 Generic Health also referred to Seafolly at [574] in which Dodds-Streeton J said:
The respondent‘s sales multiplied by the applicant‘s profit margin is rarely an accurate reflection of the applicant’s loss. It is usually necessary to discount that figure in recognition that not all sales made by a respondent can be regarded as sales lost by an applicant.
224 As Generic Health submitted:
This discounting typically involves a five-step approach, being: (1) examine the number of sales made by the Respondent; (2) assume that the Respondent was trying to capture sales from the Applicant, the market leader; (3) assume that the number of sales made by the Respondent is equal to the number of sales lost by the Applicant; (4) discount the number in (3) to reflect the fact that not all sales made by the Respondent can be considered sales lost by the Applicant; and (5) apply any further discount necessary in the circumstances of the case: Seafolly at [518]; Elwood Clothing Pty Ltd v Cotton On Clothing Pty Ltd (2009) 81 IPR 378 at [11] and [13] per Gordon J; Norm Engineering Pty Ltd v Digga Australia Pty Ltd (2007) 162 FCR 1 (Norm Engineering) at [266]-[271].
225 Generic Health also noted the observation in Seafolly at [592] that a difference in price might justify a significant discount. Generic Health said:
The discount factor should be “determined according to proper principles, so as to reflect a proportionate sense of the impact upon the applicant of the respondent’s conduct”: Norm Engineering at [270]. For example, in Norm Engineering at [292] to [296] Greenwood J applied a 50% discount even though there was evidence that both parties operated in a similar market and the respondent was actively seeking to divert sales from the applicant as the market leader.
226 The principles which Generic Health has identified may be accepted as appropriate in their context. For example, Seafolly involved copyright in designs used on swimwear. The damages claim was for lost profits as a result of the sale of swimwear which had the infringing designs imprinted upon them. Elwood Clothing Pty Ltd v Cotton On Clothing Pty Ltd [2009] FCA 633; (2009) 81 IPR 378 concerned damages for copyright infringed by the sale of T shirts. Norm Engineering Pty Ltd v Digga Australia Pty Ltd [2007] FCA 761; (2007) 162 FCR 1 concerned copyright in an industrial bucket. Damages were sought for lost profits on the basis of the sales of the infringing buckets.
227 The context of the present case is different. A person wishing to buy swimwear, a T-Shirt or an industrial bucket is not constrained in any relevant way. The person is free to buy whichever swimwear, T-Shirt or industrial bucket best meets their current needs and circumstances. A woman who wishes to use an OC is not in that position. Every woman who wishes to use an OC must do so under prescription from a doctor. As Yasmin was the only formulation of 3mg DRSP and 30 µg EE on the market for some 10 years before Isabelle entered the market, all sales of Yasmin represent a prescription given by a doctor to a woman for Yasmin. Having received that prescription, a woman could obtain only Yasmin from a pharmacist. If the woman did not fill that prescription (because of price or for some other reason), her choice was to not use any OC or to return to a doctor to obtain a prescription for a different OC. If she returned to her doctor, she would be informed that Yasmin was the only OC containing 3mg DRSP and 30 µg EE available and that this formulation had (or at least was said to have had, based on clinical trials) a unique suite of benefits in terms of not causing weight gain, bloating, breast tenderness and perhaps increased blood pressure. Further, given that women generally do not change their OC other than by reason of some major change in circumstances, it may be inferred that women who had been prescribed Yasmin in the past would continue to be prescribed and use Yasmin in the future unless subject to a major life event which may cause them to interrupt their use of an OC, to cease using an OC altogether, or to change their OC.
228 When Isabelle entered this market, it did so on the basis that it was bioequivalent to Yasmin. On the evidence, doctors would not prescribe by reference to the brand “Isabelle”. Doctors would continue to prescribe Yasmin but, provided the doctor did not tick the “brand substitution not permitted” box on the prescription, a pharmacist could supply Isabelle instead of Yasmin. Accordingly, a woman could obtain Isabelle only if she held a prescription for Yasmin. A woman with a prescription for any OC other than Yasmin could not, on that prescription, obtain Isabelle. As a result, it is accurate to say that the only OC for which Isabelle could be substituted was Yasmin.
229 These are different circumstances from the markets for items of clothing or industrial equipment. They give a different context. The context for clothing and equipment (and many other consumables) is one of consumer choice and substantial substitutability. This is not the context for the OC market in which the product can be obtained only on prescription, the prescription will be for a specific brand, the consumer will take advice from a doctor as to the brand which best meets the consumer’s requirements, brand substitution may be permitted or prohibited, the fact that consumers do not change from one OC to another without good reason and, in the case of Isabelle, the only brand for which it could be substituted was Yasmin.
230 Accordingly, it is at least clear that every sale of Isabelle represents a prescription for Yasmin which resulted in Isabelle rather than Yasmin being prescribed. This is because the evidence was that doctors generally prescribe by reference to the originator brand, there was evidence of pharmacists dispensing Isabelle in respect of a prescription for Yasmin, and there was no evidence of any prescription for Isabelle. This proposition is not the same as Bayer’s proposition that every sale of Isabelle (and Petibelle) must be a lost sale of Yasmin. It is, however, the necessary starting point for the consideration of the issue of Bayer’s lost profits. Against this starting point, Generic Health’s contentions can be assessed.
231 Generic Health contends that it should be inferred that some sales of Isabelle were not lost sales of Yasmin in that there are likely to have been:
(1) Women with a prescription for Yasmin who would not have purchased or continued to purchase Yasmin due to the high price but purchased Isabelle because of its lower price.
(2) Women who obtained a prescription for Yasmin in order to purchase the lower priced Isabelle but who would not have purchased Yasmin.
(3) Women who changed from another OC to a prescription for Yasmin because they could purchase the lower price Isabelle but who would not have purchased Yasmin.
232 It should be said that the hypothetical circumstances in which a woman might have come to purchase Isabelle are capable of infinite variation. Nothing turns on the description of the circumstances. The key criterion is that the woman purchased Isabelle when she would not otherwise have purchased Yasmin.
233 Bayer’s position is that while it is possible that some sales of Isabelle were made in these circumstances, there is no evidence of any such sales and that any such sales, on the evidence, are likely to be immaterial. As such, there should be no discount for the possibility of sales of Isabelle (and Petibelle) being other than lost sales of Yasmin or, if there is to be a discount, it should be less than 1%.
Mr McCann’s evidence
234 Mr McCann is a pharmacist (and is a different person from Dr McCann, patent attorney, discussed above). Mr McCann’s pharmacy, it should be noted, is located in an area he described as “… poor – we’re in a lower socioeconomic area of New South Wales”. This is relevant because there were only two things which distinguished Isabelle from Yasmin from a consumer’s perspective. The first was price (Isabelle was about 14 % to 18% or about $10 to $13 cheaper than Yasmin over three months). The second was brand, Yasmin being produced by the originator brand Bayer and Isabelle being akin to a generic.
235 As Mr McCann put it, a woman with a Yasmin prescription had three options. She could purchase Yasmin from a pharmacist. If she did not raise any concern about price, Mr McCann would not raise the possibility of purchasing Isabelle instead of Yasmin. If she raised any concern about price, Mr McCann would provide the woman with information about Isabelle, to the effect that there was a less expensive brand available which had the same therapeutic effect as Yasmin. The third possibility was a woman who “just can’t afford it at all because they might be on a health care card of concession or a single mum with three kids or whatever else and they ask, “Please, what’s an alternative, you know, that I can receive.” Mr McCann would give that woman information about OCs generally and note that she could go back to her doctor to discuss. He also noted that about a third of all prescriptions issued (not just prescriptions for OCs issued) are never filled. Mr McCann also said that his dispensing practice did not provide him with a financial incentive to switch a woman from Yasmin to Isabelle because he supplied both products based on a “standard dispensing fee” that he applied on top of the “net in store cost to the pharmacy in order to calculate the retail price”.
236 However, contrary to Generic Health’s submission, there is evidence from which it would be inferred that some pharmacists would have had a financial incentive to switch women from Yasmin to Isabelle. Generic Health’s own marketing of Isabelle to pharmacists said:
“another profit opportunity from Generic Health”;
“Isabelle – originator brand – Yasmin”;
“Easiest conversion opportunity”; and
“Save your customers more and generate more profit”,
and included a table comparing Yasmin with Isabelle in terms of cost to the pharmacist and customer, with Isabelle yielding “$12.81 more profit” per pack of three courses. This table is set out below:
Brand | Isabelle | SAVING | |
RRP [recommended retail price] | $74.95 | $64.95 | $10 for customers |
NIS [net in store cost] | $66.37* | $43.56 | $22.81 |
Profit | *8.58 | $21.39 | $12.81 more profit |
237 In the face of its own marketing, Generic Health’s submission that there was no evidence that “pharmacists had a financial incentive to switch customers to Isabelle” is wrong and its submission that “Bayer has not even put on any evidence which establishes that Bayer actually lost sales as a result of Isabelle” is specious. Generic Health produced a product, Isabelle, that could be substituted only for Yasmin. It marketed Isabelle to pharmacists on the basis that conversion from Yasmin to Isabelle presented a “profit opportunity” for the pharmacist of $12.81 per pack of three courses. While it lasted, Isabelle sold well, despite stocking issues (for at least two periods, Isabelle stocks ran low generally). Given these circumstances, that submission that there is no evidence that Bayer lost Yasmin sales to Isabelle is unmeritorious.
238 The fact that Generic Health chose to adduce evidence from a pharmacist who services an area he described as “poor” and who charges a standard dispensing fee, in a case dealing with one of the most expensive OCs then on the market which was not subsidised by the PBS, does not establish that pharmacists had no financial incentive to switch customers from Yasmin to Isabelle. Generic Health was banking on switching by pharmacists and was providing a large financial incentive to pharmacists to do so. Its pricing of Isabelle discloses this strategy. The recommended price of Isabelle to customers was well above the price of PBS prescriptions ($64.95 for three months compared to a range of $19 to $24 for four months) and above the price for second generation OCs not on the PBS ($35 to $40 for three months) but below the price of Yasmin (by about $10 for three months) and also below the price of any non-PBS third generation OC.
239 The obvious inference available, and which should be drawn from Generic Health’s own marketing to pharmacists, is that it wanted pharmacists to switch customers from Yasmin to Isabelle. Mr McCann had no financial incentive to do so but Generic Health was relying on other pharmacists having a real financial incentive to encourage switching. It was also relying on the fact that women willing and able to spend the kind of money necessary to obtain Yasmin would also be willing to substitute it for a bioequivalent product. This is not to say that, for a multitude of reasons, a woman who was taking an OC or was new to OCs might have purchased Isabelle when she would not have purchased or continued to purchase Yasmin. The complexities of life are such that these possibilities must exist, at least as possibilities. But it is to say that Generic Health, which must be inferred to have known how the OC market operates (including the PBS and non-PBS components and the reluctance of women to change from a brand with which they are happy), also knew that it needed to get pharmacists to sell Isabelle to women who held a prescription for Yasmin. In this context, the submissions that Bayer has “made no attempt” to prove its loss and that it is not for Generic Health to make the case against itself, do not engage with the proven circumstances of this case.
240 To return to the evidence of Mr McCann, it is not unexpected that in a pharmacy servicing an area he described as “poor”, women were “shocked” at the price of Yasmin, and expressed their shock, at which point Mr McCann would raise the possibility of a substitution by Isabelle. This evidence is also contrary to another of Generic Health’s propositions, that cost is (rather than should be) an important prescribing matter for doctors. If cost was an important prescribing factor, it is unlikely that doctors would prescribe Yasmin to a woman who could not afford it. Yet Mr McCann dealt with a number of women who had been prescribed Yasmin who could not afford it and did not fill their prescription at all. According to Mr McCann, while the difference in price between Yasmin and Isabelle might not seem large (about $10 over three months), this difference was “just impetus to get that – you know, the couple [of] people using it.” This last observation must involve a degree of conjecture on Mr McCann’s part as nothing in his evidence suggested that he asked any customer who purchased Isabelle whether they would or would not have filled their prescription for Yasmin but for the availability of Isabelle. It must also be the case that a woman shocked at the price of Yasmin could never have purchased it before as Yasmin was always priced at the premium level. A woman who had purchased Yasmin previously (of whom there must have been many given the sales of Yasmin before Isabelle appeared on the market) would not be shocked by the price. Such a woman would know the price. She would have paid the price previously. Without a change in circumstances affecting her price sensitivity, but for Isabelle, such a woman should be inferred to have been willing to continue to pay the Yasmin price. But if such a woman was offered a bioequivalent product for $10 less per packet, it would be rational for that woman to change from Yasmin to Isabelle. Only a woman also sensitive to pharmaceutical brand (for example, one who might think Bayer equated with quality or who saw no reason to risk changing a product affecting their hormonal status despite bioequivalence) or wholly indifferent to price would not consider switching from Yasmin to the bioequivalent Isabelle.
241 Mr McCann’s evidence focused on the newcomer to Yasmin, shocked at the price, who would not fill a prescription at all for Yasmin but might do so for Isabelle. While I accept the possibility of such women, I do not think that Mr McCann’s evidence can be taken to represent the general position for a number of reasons.
242 First, as I have said, no woman who had purchased Yasmin before could be shocked at the price so this evidence must be confined to a newcomer to Yasmin.
243 Second, if a woman was so shocked at the price that she would not fill the prescription at all, then it seems likely that such a woman had not previously purchased a third generation OC, none of which are subsidised by the PBS and all of which cost far more than a PBS prescription.
244 Third, Yasmin had been on the market for 10 years before Isabelle entered the market so, leaving aside women being prescribed Yasmin for the first time, its customer base (which was large) already knew its price.
245 Fourth, for a pharmacy in an area Mr McCann described as “poor”, it would not be unexpected that a woman who had been prescribed Yasmin for the first time, when she had either never been prescribed an OC before or had never been given a prescription for a non PBS subsidised OC before would be shocked at the price of Yasmin and not fill the prescription. If she had been prescribed OCs before she would know the price of PBS subsidised OCs which were far less than Yasmin. If she did not know that price range, her pharmacist could inform her. Either way, a very price sensitive woman would have a far greater incentive to obtain a PBS prescription than to save $10 by purchasing Isabelle rather than Yasmin.
246 If Isabelle sales had been dependent on women described by Mr McCann (that is, shocked at the price of Yasmin but willing to pay for Isabelle rather than getting a PBS prescription) then the market for Isabelle, I infer, would have been absurdly narrow. Isabelle’s market, it seems obvious to me, depended on women who were willing to pay the kind of price that third generation OCs commanded. This, of course, is the very market on which Generic Health’s marketing to pharmacists focused – converting women on Yasmin to Isabelle.
247 In other words, Generic Health’s marketing seems to me to have been inherently rational and to reflect the proven circumstances of the OC market in Australia. Its submissions in this case, suggesting that there was a substantial segment of the market consisting of women who would not pay the price for Yasmin but would pay the price for Isabelle (at a difference of about $10 over three months), when PBS subsidised prescriptions could result in a saving of around $40 per month compared to Isabelle, does not correspond well with the evidence. It is possible that there were some women, as Mr McCann said, who wanted the benefits of Yasmin but could not afford to pay for it but could afford to pay for Isabelle. If such women existed they were more likely than not to be in an area such as that serviced by Mr McCann’s pharmacy. Yet Mr McCann’s evidence did not identify any actual woman who purchased Isabelle who would not have purchased Yasmin.
248 I also consider that Generic Health’s focus on Mr McCann’s evidence about price sensitivity to prescription medicines generally is to be treated with care. Generic Health summarised that evidence in these terms:
His evidence was that: (1) price influences whether a patient will fill a prescription (McCann 1 [27]), and that price was a key issue for premium price products for a proportion of the population: xxn T237.45-7; (2) patients frequently ask whether there is a generic brand of a medicine: McCann 1 [28]; (3) many patients will choose to take a generic product because of the cost saving – if the cost saving is $10 or more, they usually will: McCann 1 [30]; and (4) “A third of all prescriptions never get filled or never get dispensed”, including because of the price of filling the script: xxn T232.16-18.
249 This evidence did not relate to the OC market but to prescription medicines generally. The evidence of all relevant people was to the effect that women did not “chop and change” their OC because, in effect, it was regulating hormones and thus affecting a woman’s sense of wellbeing as a whole. Given this, it may be inferred there would be more perceived risk in changing from one OC (using one molecule to provide contraceptive effect) to another OC (using a different molecule) compared to some other prescription medicines. Also, this evidence is about swapping to a bioequivalent product, not swapping from one OC to another different OC. The evidence that people will usually swap from a brand to a generic for a saving of $10 or more is consistent with my view that many women who had purchased Yasmin, when presented with bioequivalent Isabelle, would swap to Isabelle. What this evidence does not support is the notion that a woman who had been taking Yasmin would readily swap to another OC altogether but for Isabelle being available.
250 Nor does the decline in sales which Yasmin experienced support Generic Health’s case that there was a material or substantial proportion of women who were taking Yasmin but would have ceased doing so and swapped to another OC but for Isabelle entering the market or who obtained a prescription for Yasmin for the first time intending to purchase Isabelle when they would not have purchased Yasmin. There are other obvious explanations for the decline in sales of Yasmin unconnected to the existence of the particular kind of price sensitivity in women that Generic Health proposes – the kind that would make them not buy Yasmin at all if Isabelle was not available but obtain a prescription for Yasmin and buy Isabelle instead because of the saving of $10 per month (as opposed to the kind of women that Bayer proposes – women who would have bought Yasmin but were more than happy to take a $10 saving over three months for a bioequivalent product). To my mind, the latter kind of price sensitivity seems far more likely than the former in the OC market.
251 To this must be added that the obvious reasons for the decline in sales of Yasmin were unconnected with price. First, Bayer was not marketing Yasmin once it introduced Yaz and then Yaz Flex because the latter were Bayer’s “successor” brands to Yasmin (Yaz from September 2008 and Yaz Flex from September 2012). It may be inferred that Bayer wanted women whose cycles could be effectively controlled by the lesser dose of EE to switch from Yasmin to Yaz and Yaz Flex (also premium priced products). It must be understood, however, that this was not encouraged by price (Yaz and Yaz Flex were as expensive, or more expensive, than Yasmin). Second, in 2011 the Yasmin molecule received the kind of adverse publicity (about deep vein thrombosis risks greater than some other OCs) which would be expected to depress sales of all forms of the molecule – Yasmin and Isabelle.
252 In other words, while I accept there is price sensitivity for women purchasing OCs it is a sensitivity mediated through a number of other considerations. There is the doctor prescribing the product who, it seems, should consider long-term financial capacity to pay for an OC, but the evidence suggests does not in fact do so routinely. There is the fact that the market involves radically different price points – a low price point for PBS prescriptions and a high price point for private prescriptions of third generations OCs. There is the fact that women do not usually change from one OC to another OC involving a different molecule unless there is good reason to do so. There is the fact that Yasmin had been on the market for 10 years at a premium price so only newcomers to Yasmin (or private prescription OCs) could have been surprised at the price of Yasmin. There is the fact that OCs could not readily be seen as optional by women dependent on an OC, in contrast to some other prescription medicines. All of these considerations tend to support the inference that Isabelle’s most likely source of sales was women who had been taking Yasmin and would have continued to do so until a good reason arose to change to another molecule.
Mr Peace’s evidence
253 It should be said that the discussion above is largely consistent with evidence given by Mr Peace, Bayer Australia’s former Business Unit Manager Women’s Health Care Unit. Mr Peace was responsible for marketing decisions about Bayer’s OCs including Yasmin and Petibelle. He had been involved in the OC market for many years and must be taken to have had intimate knowledge of how it functions. According to Mr Peace:
(1) The OC market in Australia is crowded.
(2) The market consists of PBS and non PBS products. There is a large demand for cheaper OCs as shown by the sale of PBS subsidised OCs.
(3) Yasmin was in the non PBS part of the market and was accepted as offering a unique suite of non-contraceptive benefits, due to its unique formulation using DRSP.
(4) Women have high brand loyalty to an OC which they feel works for them in terms of non-contraceptive benefits. They do not like to change their OC if it has been working for them in non-contraceptive terms but there are always women who are new to the market or who decide to take a break from OCs or who otherwise have a change in circumstances which affects the advice they might get from a doctor about the OC appropriate for them.
(5) Yasmin had done extremely well as a premium priced product, not subsidised on the PBS (about 35% by value of the market before Isabelle became available).
(6) If a woman had been prescribed Yasmin it would be for a reason and if a woman “still wanted those features and benefits in the main they would have to go back and use Yasmin, because cyproterone acetate would only offer them the skin comparison”, and not the other benefits of Yasmin. Also “generally speaking, the literature would support the notion that from a general tolerance point of view, drospirenone, containing oral contraceptives, are better than CPA containing pills”.
(7) A woman buying Isabelle is still buying a premium priced pill so that while price would be a factor for some people, a woman taking the Yasmin molecule in Yasmin or Isabelle was “paying a premium price because they knew that this particular product was unique in what it offered”.
(8) He decided to offer Petibelle not because he thought substantial or material numbers of women would switch to a CPA product. As he put it, he “very carefully made sure and you will see in the strategy that the recommended retail price which was accepted commercially by pharmacy and competitively they set that price was such that it offered something for the returning patient for those that – those small few that may be concerned that price was an issue. But as far as I was concerned, I believed, and I think the figures bear it out, that a significant number of women returned to Yasmin when Isabelle was taken off the market”.
(9) While he accepted that some women bought Petibelle instead of Yasmin this was after Isabelle had been on the market. However, this did not mean that other than a small proportion of women might have bought Isabelle but would not have bought Yasmin if prescribed Yasmin.
254 Mr Peace was cross-examined about an internal document he prepared dealing with options if Bayer succeeded or failed in having Isabelle removed from the market. Under the heading “Outcome/behaviour” in this document Mr Peace posited that if Bayer won, resulting in Isabelle being removed from the market then:
• Pharmacy offer Yasmin at normal price or more likely suggest a switch to a CPA brand.
• Will be Q4 ex IMS [which gives information about pharmacy purchases from wholesalers ] before we see which way the switch goes.
• Expect publicity with potential negative focus on DRSP/Yasmin and VTE [venous thromboembolism] issue.
255 Mr Peace explained that the “switch” is the switch back from Isabelle to Yasmin. He was aware that a “CPA” brand (cyproterone acetate such as Diane) was not substitutable for Yasmin at pharmacy level but was identifying the concern that pharmacists might take it upon themselves to suggest a different OC altogether which he described as an expression of his “callousness of the commercial possibilities”. This concern, of course, is inconsistent with Mr McCann’s evidence about how he behaves, and how pharmacists should behave, but the fact that Mr Peace raised the issue in a document dealing with “win/lose scenarios” to provide information about the possibilities to his General Manager, is not unexpected. Mr Peace was identifying the range of possibilities, and this was one despite the fact that prescribing and advice of this kind are a matter for the doctor not the pharmacist. Identifying this possibility in such a document is not inconsistent with Mr Peace’s evidence that he did not accept that the price difference between Yasmin and Isabelle meant that a material number of women who would never have bought, or could not continue to buy, Yasmin, would buy Isabelle. Rather, Mr Peace accepted that women who bought Yasmin would happily buy the bioequivalent Isabelle at the $10 or thereabouts discount, which was a different phenomenon. Mr Peace gave this evidence:
• Q And you will agree, won’t you, that for some women, that would enable them to purchase the Yasmin composition at a price they could afford when Yasmin itself might have been out of reach?
A I don’t think so. I think those women were already motivated to buy Yasmin, and I think it’s just common sense that if they’ve then got a chatty pharmacist saying they’re going to give you the same thing for $10 difference, they would take it. Some would take it; some wouldn’t.
• But a lot of women, particularly with oral contraceptives, if they know it’s working for them and they’re happy on it and they’re settled on it [they] don’t like messing around with the name on the box
• Q You would accept also, wouldn’t you, that there would be women out there who would now be able to afford a product that was previously slightly out of reach?---
A No, I don’t think so. I think these premium products are in a class where – when you’re talking about the features and benefits that they offer, they’re in a zone of pricing which I don’t think $10 or $5 or $20 is going to make much difference. If they’re motivated, they will walk – they will shop around i.e. for the same product but at a discounted price such as at Chemists Warehouse].
• Q …it offers you a suite of benefits and features that you otherwise can’t get, you might be prepared to look at your budget hard and see if you can afford it. If you have a cheaper version, it makes that decision easier and you might be able to take it, whereas you can’t quite stretch for the Yasmin product; that’s correct, isn’t it, Mr Peace?
A I think these women that you’re referring to have already shopped around. I think they’ve done their homework. At the end of the day, I think it’s a persuasive pharmacist. These are women who are already having Yasmin or convinced of the benefits of Yasmin. I think the entire motivation to switch or to substitute rather is with the pharmacist.
256 Ultimately, Mr Peace gave this evidence about the potential for a woman, who would never have bought Yasmin or could no longer continue to buy Yasmin, to buy Isabelle (my emphasis):
Yes? I think you’re talking about existing patients who have gone to a pharmacy who have been exposed to the potential offer of a cheaper alternative.
Well, there’s a number of possibilities. One is people who have Isabelle can talk to their friends, for example, and people ? Sure.
become aware there’s a product called Isabelle? Yes.
It’s a bit cheaper than Yasmin, and that might be an attractive product for them; that’s a possibility? It is a possibility.
Yes? I think it’s a small possibility, but it’s a possibility.
Yes. And there are also people who, on taking a new script, might get to the pharmacy and find that it’s quite expensive. Those people might not fill their script if they’re only – they only have Yasmin; whereas they might fill it if they have Isabelle? I think those patients – if they got to – in my view, if they got to pharmacy and said – and the pharmacy said that will be $75 or $80 or whatever, I don’t think it would make much difference if they said 50 or 60 dollars. I think they’re going to go, “You’ve got to be joking. I’m going back to my doctor to discuss this again.”
Well, somebody who wanted to pay $20, that’s clearly right, but ? $20 is different because you’re now entering into the zone under which PBS pills are priced.
Yes, quite? They’re somewhat differently packaged, but into the zone of where they’re priced.
Yes, but you’re not saying that for everyone there’s no difference between 75 and 60, are you? I’m saying that for someone who is aware of the features and benefits of that particular formulation after having that discussion with their doctor – I believe that that range of pricing would be – you know, if that was important to them, they would have done their walking around already.
Yes, but it’s not just the one price, is it; it’s every time you have to renew something, being a product that you’re taking ? Sure.
on a long-term basis ? Sure.
it does add up over time, doesn’t it, Mr Peace? I mean, I can’t deny that’s a possibility, of course.
Yes. Yes? But small, in my view, given that kind of product.
…
Well, let’s put it another way. There would be some people who would buy Petibelle who would not buy Yasmin if Yasmin was the only product available? I can’t deny that that’s possible.
Yes? I can’t – I again think it would be very small.
257 This evidence carries weight because of Mr Peace’s substantial experience in and knowledge of the Australia OC market and the general consistency of his opinions with the other evidence about OCs in Australia.
258 Generic Health’s submissions about Mr Peace’s evidence were not convincing. Mr Peace did not accept that the class of consumers who could not afford Yasmin “would have considered a cheaper alternative (i.e. Isabelle)”. To the contrary, he thought that the class of women who could not afford Yasmin would be purchasing PBS subsidised OCs, not private prescription OCs, be it Yasmin or Isabelle. As Mr Peace repeatedly made clear he thought the class of women who purchased Isabelle who would not have purchased Yasmin was a “very small” possibility.
259 Mr Peace did agree that “as sales of Yasmin were declining, it is difficult to ascertain and separate out from market share graphs/data whether, in fact, some of the Isabelle share of the market was new business” but also repeated his view that:
But I would think it’s very, very small. As far as I’m concerned, an Isabelle sale was a lost sale of Yasmin. I said that at the beginning, I say it now.
Mr Houston’s and Mr Williams’s evidence
260 Mr Houston holds a BSc (Economics). For two years he worked as a financial economist in a bank. He then worked as an economist for NERA Economic Consulting before setting up his own economic consulting business. He has given expert economic evidence in numerous hearings and inquiries and spoken at many conferences on economic issues. His curriculum vitae discloses one publication (other than speeches, advices to clients and expert reports and evidence).
261 Dr Williams holds a Masters and PhD in Economics. Between 1978 and 2002 he taught economics at the University of Melbourne and was Professor of Law and Economics in the Melbourne Business School at this University. He has given expert evidence in various matters and has also published widely including books and articles in a wide range of journals both in Australia and internationally. He is currently the leader of the Competition and Legal Group of a firm of consultant economists.
262 Neither Mr Houston nor Dr Williams had any particular knowledge of the OC market in Australia or about OCs generally before this case.
263 Generic Health relied heavily on Mr Houston’s evidence to support its case that it could not be inferred that every sale of Isabelle (or Petibelle) was a lost sale of Yasmin. Bayer relied on Dr Williams’ evidence, in effect, to refute many aspects of Mr Houston’s analysis.
264 I found important parts of Mr Houston’s evidence unpersuasive.
265 Mr Houston said that Bayer’s introduction of Petibelle was consistent with an expectation that pharmacists would be likely to suggest to a woman that she switch to a different OC altogether once Isabelle was no longer available. This is not the effect of the evidence. Mr McCann said a pharmacist would not recommend to a woman that she switch OCs altogether. This is not the role of a pharmacist. Mr McCann, who had no direct financial incentive in switching a woman from Yasmin to Isabelle, would discuss Isabelle (a product bioequivalent to Yasmin) if the woman was concerned about cost. As noted, I infer from Generic Health’s marketing that other pharmacists would have been more proactive in encouraging a switch from Yasmin to Isabelle as a bioequivalent product. However, there is no evidence to suggest that a pharmacist would encourage a woman to switch from one OC to a different OC involving a different molecule. The evidence of Mr McCann, pharmacist, is to the contrary and indicates such advice would be inappropriate. The evidence of Mr Peace indicates that his identification of the risk that some pharmacists might try to do so in the context of setting out the commercial consequences if Bayer succeeded in removing Isabelle from the market does not mean that pharmacists, in fact, would give this kind of advice. Further, the reluctance of women to change an OC with which they are happy unless their circumstances have materially changed, also suggests to the contrary.
266 No doubt Bayer wanted to retain every sale it could and was concerned about reputational damage if, by reason of its successful action against Generic Health, women who had been taking the Yasmin formulation in Isabelle had no option but to pay more for Yasmin. This is Mr Peace’s explanation for his decision to introduce Petibelle on the market. In his words:
• …we introduced this product [Petibelle] as a convenience to ensure that there was minimal disruption for whatever reason a patient may have had in front of the pharmacist. We wanted to make sure there was no reputational damage to Bayer, so that’s why we introduced this second brand, if you like, but had there been no Isabelle, as far as I’m concerned, we would have not introduced that product.
• There’s no commercial basis for launching it. Our mission was to bring Yaz and YAZ Flex – these are successive brands to Yasmin – into the market at a premium price.
• Petibelle was only there, as I’ve mentioned, once Isabelle was withdrawn.
• …there was no commercial imperative there for a pharmacist to carry Petibelle, because the margins were no better then they were on Yasmin. So that is why that product was brought and offered to the marketplace as a product to minimise any damage to reputation, as I’ve said before. There’s no commercial imperative to carry.
• The only gain was that for some women who came back, that was a closer price to Isabelle, but also closer to Yasmin, and it was a move by Bayer to try and minimise the risk of a woman not continuing on that particular moment with contraception. Otherwise you’ve got a woman who’s suddenly confused, and before you know it, she’s not taking the pill when she should be.
267 This explanation is also consistent with the evidence of the nature of the OC market in Australia, as discussed above. It is inconsistent with the notion that Bayer was concerned that a substantial or even material number of women might refuse to pay the higher price for Yasmin once Isabelle was no longer available. As with all of the witnesses, Mr Peace and Dr Williams accepted the possibility that some women might fall within this category. As Mr Peace put it, he would expect the possibility of women in this category to be “very small”. Mr Houston, however, appears to have proceeded on the basis that Bayer expected that pharmacists would be likely to suggest to a woman that she switch to a different OC altogether once Isabelle was no longer available when I consider that, on the evidence, this was the merest possibility and Bayer had no such expectation. Mr Peace was doing no more than identifying possible risks to management and, when it came to deciding to introduce Isabelle, was overwhelmingly aiming to protect Bayer from reputational damage and smooth the transition, for women who had been taking Isabelle, back to Yasmin. Bayer was not expecting that a material proportion of women would no longer be willing to pay the higher price for Yasmin but accepted that women who had been paying the lower price for Isabelle might well be unhappy or confused about Isabelle no longer being available. Mr Peace pitched Petibelle at a price designed to smooth the path for these women and to minimise cannibalisation of Yasmin by Petibelle. Accordingly, and contrary to Generic Health’s submission, there is no inconsistency or paradox in Bayer’s case given its introduction of the lower priced Petibelle after Isabelle had entered and been removed from the market.
268 Mr Houston said that doctors discuss cost with patients and take this into account in prescribing decisions. From the evidence it appears to be the case that doctors should discuss cost with patients but, if Mr McCann’s pharmacy experience is relevant (and I consider it is), generally do not do so. The evidence of Dr Warner, emphasised by Generic Health, mentioned cost as a relevant factor, but did not give it anything resembling the prominence that Mr Houston’s evidence indicated, Mr Houston being neither a doctor nor a pharmacist, nor a person who knew anything about OCs before this case.
269 Mr Houston said customers would be expected to know the options available to them and their related costs. This was not the effect of Mr McCann’s evidence as an experienced pharmacist. The basis for this view of Mr Houston’s is unclear.
270 Dr Walker’s evidence that Yasmin tended to be used by younger women with higher disposable incomes who wanted the non-contraceptive benefits it gave them does not mean that cost was material for Yasmin users, as Mr Houston said. Rather, it suggests (not unexpectedly) that women with high incomes were the ones who were users of Yasmin because they could afford its premium price, as a result of which these women were likely to be less price sensitive than women buying first or second generation OCs. In other words, if anything, women who were buying Yasmin at a premium price, absent some change in financial circumstances, were likely to be the least price sensitive consumers in the OC market. If they were price sensitive, they would not have been purchasing Yasmin given the many cheaper alternatives. As Mr Peace said, it seems obvious that these women valued the non-contraceptive benefits Yasmin gave to such an extent that they did not mind paying more than three times as much for Yasmin compared to many PBS subsidised OCs. Mr Houston thus appears to have proceeded on an important premise fundamentally inconsistent with the reality of the OC market in Australia. It is these kinds of issues which persuade me that Mr Houston’s opinions in this case lack sufficient foundation in the reality of the OC market in Australia.
271 Mr Houston identified a series of “economic principles”, which may be accepted in the abstract, but which cannot necessarily be applied mechanically to a market such as that for OCs in Australia which has the characteristics discussed above. The principles were that: (i) consumers’ willingness to pay changes over time, (ii) the volume of sales will increase as prices fall, and (iii) more profit can sometimes be made by charging two prices for the same product. In the context of the OC market the extent to which these principles hold good would need to be tested because: (i) women are reluctant to change their OC, (ii) market shares by volume do not seem to reflect price, and (iii) in the case of Yasmin, but for Isabelle, there was no incentive for Bayer to sell Petibelle because Bayer alone controlled the DRSP molecule in OCs and was in the processing of encouraging women whose cycle could be controlled by a lower dose of EE to use Yaz and then Yaz Flex rather than Yasmin (that is, Yaz and Yaz Flex would be the product of first choice and, if cycle control was not achieved, Yasmin would be used).
272 Mr Houston, however, appeared to proceed on the basis that these economic principles necessarily held good for the OC market. For example, Mr Houston said:
(1) A patient’s desire to switch away from Yasmin or Isabelle will depend on the price they are asked to pay. I disagree. A patient taking Yasmin or Isabelle is paying a premium price. If there is no material change in financial circumstances, then their desire to switch away would not be based on price but continued therapeutic effectiveness.
(2) The fact that Bayer recognised that Yasmin was entering a crowded market does not mean, as Mr Houston seems to suggest, that Bayer thought women readily switched OCs. Bayer knew the opposite to be true and depended upon the unique suite of Yasmin’s benefits overall (not just skin related benefits) to persuade women to switch to Yasmin. Further, Bayer’s anticipation of switching women from Yasmin to Yaz also does not mean this. Yaz and Yaz Flex use the same DRSP formulation as Yasmin. Bayer, it must be inferred, wanted women to take the lowest appropriate dose, which would have been Yaz or Yaz Flex but recognised that a number of women would require the higher dose of EE in Yasmin to control their cycle. Switching from one kind of OC containing DRSP to another also containing DRSP is not the same as switching to an OC using a different progesterone altogether. Mr Houston appeared not to be aware that Yasmin, Yaz and Yaz Flex are all the only OCs, but for the market intrusion by Isabelle, that are based on DRSP and EE.
(3) Against this background, Mr Houston’s statement that Isabelle reduced the saving a woman could have made by switching from Yasmin to another OC so that fewer numbers would have switched as a result of Isabelle being available, is a kind of syllogism based on a series of premises which do not accord with the OC market or Yasmin’s place in it as a premium priced product. As Bayer submitted, the thesis appeared to “rely heavily on unfounded assumptions that ‘price’ is an important consideration for patients within the private OC market and that the difference in retail price between Yasmin and Isabelle of less than $4 a month would cause new and existing OC patients who would otherwise have not purchased Yasmin to purchase Isabelle”.
(4) For the same reasons Mr Houston’s expectation that Bayer could earn more by selling Yasmin and Petibelle than it could earn from selling Yasmin alone seems to miss the point that the only reason Bayer had for introducing Petibelle was to ameliorate the harm caused by Isabelle.
273 As Bayer submitted, Mr Houston did not grapple with the evidence indicating that “choices between third generation, private OCs are based upon side effect profile and non-contraceptive benefits” not price sensitivity (as they are all expensive compared to PBS prescriptions). As between third generation OCs and PBS OCs, however, price sensitivity is obviously relevant. Bayer submitted, and I accept, that Mr Houston’s evidence was “divorced from the factual realities” including:
(a) that the price difference between Yasmin and Isabelle is much less significant than the price difference between private and PBS market OCs;
(b) that truly “cost sensitive” patients could not afford Yasmin or Isabelle;
(c) the high level of product and brand loyalty within the OC market;
(d) the fact that patients are prescribed Yasmin for a specific reason, namely its unique benefits and side effect profile; and
(e) the fact that Mr McCann never suggested Isabelle to a customer who wished to purchase an OC other than Yasmin (together with the fact that doctors would not be aware of the existence of Isabelle on the market and would not recommend the product to their patients).
274 Section 4 of Mr Houston’s report is headed “Evidence is consistent with my expectation”. This discloses that Mr Houston approached the evidence, and his analysis, by reference to a series of expectations which, as I have indicated above, do not reflect the reality of the market for OCs in Australia. When evidence is approached on the basis of general expectations in this case unconnected to the reality of a particular situation, the risk of false conclusions seems high. It seems to me that this risk has been realised in Mr Houston’s analysis and conclusions. I do not accept any part of his econometric analysis, the criticisms of which by Dr Williams are well-founded.
275 First and foremost, as Dr Williams said:
The detection of the magnitude of substitution between products can only be undertaken by means of empirical analysis. However, in this case, even a full-blown empirical analysis of demand is unlikely to reveal any significant substitution of Isabelle for oral contraceptives (OCs) other than Yasmin because of other effects that were operating at the same time. These other effects so dominated any possible substitution in favour of lower-priced products, that the net effect was a reduction in sales coinciding with the period in which Isabelle was in the market.
276 This criticism applies to all of Mr Houston’s analysis which, as I have said, seems to have started with some expectations of dubious accuracy insofar as the OC market and Yasmin’s place in it are concerned and ended with some modelling based on limited information which was taken by Mr Houston to confirm his expectations. While I do not doubt the genuineness of Mr Houston’s aims, I do not see this as a methodology likely to yield reliable conclusions.
277 Dr Williams’ approach gave greater weight to the nature of the OC market than that of Mr Houston. Dr Williams noted that prescription medicines have special characteristics which limit the extent to which patients respond to relative prices. These include that while the doctor writes the prescription, the patient pays the bill. Further, economic principle also distinguishes between consumption caused by changes in constraints and by changes in taste, which Mr Houston has not taken into account. Dr Williams noted also that there is extensive literature about the preference many people have for an originator over generic brands (and for which they are prepared to pay a price premium) which would be relevant to any analysis of Yasmin and Petibelle, given that Petibelle appeared to consumers to be a generic brand.
278 Dr Williams, rightly, made the point that it is not possible for Isabelle to have “reduced the savings” that a woman could make by switching from Yasmin to another OC. The potential saving always existed irrespective of Isabelle. Mr Houston can only have meant that a woman on Isabelle would save less by switching to a cheaper brand than a woman on Yasmin would save. But, in any event, why this necessarily means that more women would have switched from Yasmin to another OC if Isabelle had not been available is unclear. As Dr Williams said, there is no suggestion in the economic literature that brand loyalty is less for more expensive brands. Dr Williams thought Mr Houston probably meant that some sales of Isabelle would otherwise have been sales of an OC other than Yasmin, which might be true, but did not follow from economic theory. Such an effect would need to be empirically demonstrated.
279 The following observations of Dr Williams also have force:
…Mr Peace states that, as at December 2011, there were 32 brands indicated for use as OCs on the Australian market; and, of these, Yasmin had the highest market share by value (39.43%), followed by YAZ (at 20.80%) – making a total share of 60.23 per cent.
Furthermore, Bayer achieved this position of market leadership despite charging a significant premium over the prices paid by consumers for OCs listed on the PBS Schedule. The combination of market leadership with charging a significant price premium implies that many doctors were prescribing Yasmin because it had benefits that were not matched by other OCs. In the language of economic, Yasmin was highly differentiated from other OCs.
Mr Peace gives reasons for this product differentiation. He states that Yasmin provides a complete package of benefits, namely:
a. weight loss rather than weight gain;
b. dermatological benefits;
c. reduced premenstrual symptoms; and
d. an overall feeling of improved well-being.
The market leadership of the Yasmin product while being priced at a significant premium to most other OCs indicates the degree to which Bayer had succeeded in developing and marketing a product that doctors regarded as differentiated from other OCs.
Mr Houston downplays the extent to which Yasmin was differentiated from other OCs in support of his first expectation. He states:
Although there was some differentiation with other COCs, they were still considered to be competitors. Further, the advice available to doctors in relation to prescribing COCs included comparison of a wide range of products, including Yasmin. This also indicates that there was a choice between Yasmin and a wide range of other COCs.
There may have been ‘a choice’ for some doctors in the prescribing for some patients. However, the evidence of premium pricing combined with high market share indicates a high degree of product differentiation. The precise degree of product differentiation could only be established by means of empirical analysis.
280 In common with Dr Williams, I consider that Mr Houston has inappropriately downplayed the significance of this product differentiation.
281 I also accept Dr Williams’s scepticism about Mr Houston’s analysis purporting to show that Petibelle increased the overall sales of the molecule. As Dr Williams said:
It is hard to accept that, without the intervention of Isabelle, Bayer would have earnt greater profits by adding Petibelle to its product line. This insight that Mr Houston claims to have gained from economic theory (that Bayer would have been able to earn greater profits from selling both Yasmin and Petibelle than from selling Yasmin alone) seems not to have occurred to Bayer in the decade between its launch of Yasmin in 2002 and the launch of Isabelle in 2012. It seems unlikely that a consulting economist could know better than Bayer where its profit-maximising interests lay.
282 I accept Dr Williams’s conclusions about Mr Houston’s predictions based on economic principles. Dr Williams said:
Mr Houston claims to derive two predictions from his three economic principles.
The first seems to be that Isabelle drew some sales from combined oral contraceptives (COCs) other than Yasmin.
The second is that Bayer would have been able to earn greater profits from selling both Yasmin and Petibelle, than from selling Yasmin alone.
Neither of these conclusions can be inferred from economic theory. Both can only be established from empirical analysis undertaken on the facts. Mr Houston has not attempted the empirical analysis in accordance with the principles and practices established in the economics literature.
283 In respect of Mr Houston’s analysis of market trends, Dr Williams’ criticisms also carry weight. Dr Williams explained:
Mr Houston reaches the conclusion that the sales statistics support his first theoretical conclusion by means of the following steps:
a. he removes seasonal variation in the data through standard methods that he describes on pages 30 and 31 of Appendix A2;
b. he fits a trend line to the (deseasonalised) sales of Yasmin for the six months prior to January 2012;
c. he extrapolates this trend up to the end of 2012 – reasoning that these would have been the sales of Yasmin but for the introduction of Isabelle; and
d. he compares the forecast but-for sales of Yasmin in 2012 with the actual combined sales of Yasmin and Isabelle (as depicted in Figure 4.2)
284 However, as Dr Williams said, the predicted trends are highly contingent on the period of data upon which the trends are based.
285 Mr Houston’s prepared revised analyses, intended to demonstrate that Dr Williams’ criticisms were not valid. Again, however, I consider that this further work supports Dr Williams’ overall opinion that Mr Houston’s attempt at statistical analysis is flawed because it depends on insufficient data. Mr Houston’s revised table depicting trends depending on the months of data used to prepare the trend is set out below.
Table 3.1: The linear trend of Yasmin sales by period used to calculate the trend
Number of months in pre-January 2012 period | Change in sales per day (old version) | Change in sales per day (corrected) |
4 months | -57.47 | -48.34 |
5 months | 5.57 | -18.52 |
6 months | 2.60 | -21.99 |
7 months | -89.28 | -85.11 |
8 months | -60.55 | -60.90 |
9 months | -27.80 | -27.26 |
10 months | -43.76 | -39.50 |
11 months | -40.91 | -38.36 |
12 months | -29.62 | -27.69 |
286 The variation depending on the data set shown in this table, in my view, lends weight to Dr Williams’ opinions that Mr Houston’s statistical exercise is of dubious value.
287 In their joint report, Mr Houston calculated the sales of Yasmin and Isabelle together which, on his analysis, were not lost sales of Yasmin (that is, by comparing the actual sales to his predicted low, medium and high trends for Yasmin but for the introduction of Isabelle). According to this calculation, on the high trend for Yasmin, Isabelle increased the overall sales of the molecule by 12%. On the medium trend for Yasmin, Isabelle increased the sales of the molecule by 28%. On the low trend for Yasmin, Isabelle increased the sales of the molecule by 74%. In yet a further analysis, which sought to exclude the effect of outlier sales levels in April and August 2012 likely to be a result of Isabelle stocks having run out, Mr Houston extrapolated trends resulting in another set of percentages. On this on the high trend for Yasmin, Isabelle increased the overall sales of the molecule by 19%. On the medium trend for Yasmin, Isabelle increased the sales of the molecule by 45%. On the low trend for Yasmin, Isabelle increased the sales of the molecule by 90%. This further exercise itself reveals the enormous sensitivity of Mr Houston’s analysis to the inputs used and the way in which the inputs are capable of manipulation.
288 Leaving aside the magnitude of these percentages by which Isabelle is said to have increased the sales of the molecule, I consider that the variance between them shows their lack of utility. Mr Houston acknowledged that the low trend could probably be disregarded or given little weight, but the results are of dubious relevance for at least two reasons (putting to one side Dr Williams’ criticisms).
289 First, without any statistical analysis at all, the range of sales of Isabelle which would not have been in substitution for sale of Yasmin is between 0% and 100%. Mr Houston’s analysis takes the range to between 12% (or 19%) and 74% (or 90%) which is too wide to be of any real use. An analysis which gives outcomes across such a range, without sufficient information to enable a rational selection of the underlying criteria which causes the variation, is unhelpful.
290 Second, the results are inconsistent with the facts about the OC market in Australia and Yasmin’s place within it. Despite all of the circumstances discussed above being: (i) the reluctance of women to change their OC if they are happy with it, (ii) Yasmin having been on the market for 10 years before Isabelle, (iii) Yasmin having always been a premium priced product in a market in which many much cheaper alternatives were available given the distinction between PBS subsidised OCs and private prescription OCs, (iv) the Yasmin molecule offering a suite of non-contraceptive benefits which could not be obtained from other OCs, (v) Isabelle itself being a product priced well above the price of PBS and second generation OCs, (vi) the price difference between Isabelle and Yasmin being about $10 over three months, (vii) Isabelle being bioequivalent only to Yasmin, and (viii) Isabelle only being obtainable by a woman who held a prescription for Yasmin, Mr Houston’s analysis is that 12, 28 or 74 or 19, 45 or 90 women out of every 100 who bought Isabelle would not have otherwise purchased Yasmin. To my mind, all of these results are untenable. They are based on an exercise incapable of yielding anything meaningful and have not yielded anything meaningful.
291 I thus accept Bayer’s submission that Mr Houston’s figures:
find no support at all in the market based evidence of Mr McCann and Mr Peace. Mr McCann's evidence, as has been noted, was emphatic, that he had never supplied Isabelle as a substitute for an OC other than Yasmin and his sales figures suggested a very small number of customers. The evidence of Mr Peace was that if there were any customers in the classes suggested by Mr Houston, they would be “very, very small.” Although the possibility of such customers was put as a matter of theory to Dr Williams, he too, thought they would be immaterial. Thus, the danger of adopting Mr Houston’s modelling is that its inherent unreliability as identified by Dr Williams involves risks because of the complete absence of evidence of even a single example of a sale of Isabelle deriving from a customer who would not have purchased Yasmin in the absence of Isabelle. Thus, the suggestion by Generic Health that there ought to be a very substantial discount of millions of dollars on the basis that the Court should not accept a 1 for 1 substitution, is premised on a completely speculative hypothesis. This is an inappropriate basis for the Court to reduce the damages payable by Generic Health a wilful infringer of Bayer's long standing patent rights.
292 Nothing in the oral evidence given by Mr Houston and Dr Williams in concurrent sessions suggested to me that any different conclusion should be reached. While that evidence covered many topics which I do not consider it necessary to deal with in detail (such as seasonal adjustments and other impacts on Mr Houston’s trends), it confirmed in my mind the validity of Dr Williams’ opinion that, while Mr Houston had done his best in good faith with the information he had, the kind of analysis he had undertaken could not yield results of sufficient reliability to be useful. Dr Williams reiterated his concerns about:
(1) The insufficient data points used to conduct the analysis.
(2) The enormous sensitivity of the results to the data points selected.
(3) The difficulty of extrapolating a trend given (1) and (2).
293 As Dr Williams explained, and I accept:
• …this dataset shows enormous variability from month to month so fitting a trend to data that’s jumping all around is fraught. It’s very dangerous to infer a long term trend from data that’s jumping all around that seems to show no particular pattern just to the eye. Of course, if you manipulate data a lot, you can extract something, but there is – it’s very dangerous to detect or infer trends when you can’t even see them with the naked eye.
• It’s not clear to me that we were in a period of falling sales of the molecule prior to January 2012. After January 2012, it’s quite clear sales of the molecule were falling, but before that date, it’s not clear from that 24 months of data… there’s so much variability in that data in that 24 months prior. It’s very hard to draw that inference.
• It’s always better to have more data than less. Always. And the way you have to cope with other things that are changing is to control for them and that’s one problem that we both agree with; this data ..... is that we have no ability to control for these things that we know are affecting the data [for example, Bayer marketing Yaz and Yaz Flex, not Yasmin and adverse publicity about the Yasmin molecule].
• …it’s perfectly standard – there are three reasons why we observe variability in the data over time. One is long-term trend; one is seasonality factors; and the other is just ups and downs, random bumps for one reason or another. Now, what you have to do if you’re trying to isolate one rather than the other is you try and decompose the trend into the – sorry, the data set into those three components. So if there are seasonal factors, I agree with Mr Houston. You should try and extract them before you undertake the trend.
• [Dr Williams was] happy to accept that there might be somebody that price may be a relevant factor in determining such a person switching [that is, to Isabelle when the woman would not have purchased Yasmin, but the relevant issue] is about quantum. That … is it de minimis or is it something that’s material.
294 Dr Williams also said and I accept:
• there’s no evidence that the availability of Isabelle increased the sales of the molecule…
In fact, the sales of the molecule decreased with the introduction of Isabelle. One cannot appeal to the law of demand to infer that the availability of Isabelle caused sales of the molecule to increase. The law of demand is merely an empirical generalisation. It is not derived from economic theory. It is generally true. I agree with Mr Houston about that. However, in certain circumstances, it does not hold and in other circumstances it may hold but the reduction in price is likely to cause such a small increase in the quantity of sales that the effect can be ignored for practical purposes.
• With the introduction of Isabelle in January 2012, a lower-priced version of the molecule was available. Mr Houston claims that the law of demand means that the availability of this lower-priced version must have increased sales of the molecule.
For these increased sales to have occurred, they must have come from either increased sales of oral contraceptives as a whole and/or substitution of the Yasmin molecule for other forms of oral contraceptives. As I understand, there was no evidence of such forms of substitution and, as I state in my report, I doubt whether a statistical study could detect any such forms of substitution.
• It is my opinion that for the period after June 2014 it is appropriate to assume that every cycle sold of Petibelle was one less cycle sold of Yasmin. The sales data suggests that after June 2014 some customers who had previously purchased Isabelle moved back to Yasmin, whereas others moved to Petibelle. The availability of Petibelle does not seem to have increased the sales of the molecule if you just look at the raw data compared either with what would have been achieved had only Yasmin been available or compared with what would have been achieved had both Yasmin and Isabelle been available. Mr Houston claims that Bayer’s decision to introduce Petibelle was justifiable by considerations independent of the prior availability of Isabelle. This can only be true if two assumptions hold.
The first is that the producer can limit the extent to which consumers switch from the more expensive product to the less expensive product. Secondly, that the offering of the lower-priced version leads to a substantial increase in sales.
• In my opinion, if there were any increase in sales of the molecule caused by the introduction of Isabelle or Petibelle, it must have been very small indeed. And, for this reason, it is my opinion that, in estimating the loss for the period after June 2014, one should assume that each cycle sold of Petibelle is one less cycle sold of Yasmin.
295 Dr Williams’ opinion was consistently to the effect that:
The available evidence (including the evidence of Peace, Walker and McCann) suggests that the best available assessment of Bayer’s claim for damages would be by treating each sale of Generic Health’s Isabelle product as a lost sale of Bayer’s Yasmin product.
296 Generic Health’s submissions, however, do not reflect the full effect of Dr Williams’ evidence. It is plain from the whole of Dr Williams’ evidence, written and oral, that while he accepted the likelihood of some women who might be prepared to pay the Isabelle price but not the Yasmin price as a possibility, he considered in terms of quantum that the best approach was to treat this possible class as immaterial. As such, the suggestion that Bayer’s approach differs from that of Dr Williams is incorrect. In both his main report, the joint report, and his oral evidence Dr Williams gave his opinion that the best assessment in the circumstances of this case was to accept one for one substitution. As Dr Williams put it in his oral evidence:
…my opinion concerning to both periods comes down to this, that the availability of the lower-priced option – whether Isabelle or Petibelle – the availability of that option had no perceptible effect on the volume of sale of the Yasmin molecule. This ..... period – first of all, for the period January 2012 to June 2014. It is my opinion that for that period it is appropriate to assume that every cycle sold of Isabelle was one less cycle sold of Yasmin.
297 For the reasons given above I do not accept Generic Health’s submission that:
Ultimately, given the declining share of the molecule, in order to determine the impact of Isabelle on the sales of the molecule as a whole it is necessary to look whether sales have risen relative to the trend of decline, that is, whether the fall in the market for the molecule was made more shallow by Isabelle's presence on the market.
298 This is because it is not possible to identify any significant declining trend before Isabelle was introduced, if there was such a trend it may well have been caused by other factors which cannot be controlled for, and it is impossible given the available data to extrapolate any reliable trend which would enable any meaningful assessment of “whether the fall in the market for the molecule was made more shallow by Isabelle's presence on the market”.
299 I do not accept that the approach of Bayer and Dr Williams was “to put any attempts to quantify its lost sales in the ‘too hard basket’ and seek to fall back on the assumption that each and every sale was a lost sale of Yasmin”. If quantification is not possible given the available data it is not helpful to make the attempt and give unreliable outcomes. The one for one proposition is not a mere assumption in any event. It is an expert opinion of Dr Williams and Mr Peace (from different perspectives) by reference to non-quantitative but detailed data about the OC market in Australia and the place of Yasmin (and Isabelle) within that market. The fact that the opinions are based on non-quantitative data does not make them mere assumptions.
300 Generic Health’s submissions about Petibelle that “Bayer itself – by its Petibelle strategy – implicitly recognised the price sensitivity of patients in respect of Yasmin”, failed to recognise that Bayer’s concern was that Isabelle had disrupted the market possibly introducing a price sensitivity that would not have existed but for Isabelle.
301 It is not the case that given “its onus and quantification burden, Bayer’s approach is not of assistance to the Court”. The onus of proof can be discharged in many ways. I am satisfied that no discount should be made in the particular circumstances of this case in order to allow for the possibility that some women bought Isabelle but would not have bought Yasmin. Apart from the fact that I agree with Mr Peace and Dr Williams that this possible class of women is likely to be very small, to the point of immateriality, it is relevant that Generic Health was the wrongdoer so that the assessment of damages ought to be liberal, not miserly. There is doubt whether such a class actually exists; it is a mere possibility. There is doubt that such a class, if it exists, in fact acted on price signals in the way Generic Health’s case would have it. All of these very real doubts are to be resolved in favour of Bayer and not the wrongdoer.
302 This approach reflects the reality that every assessment of damages is an estimate which is imprecise in a multitude of respects. In a case such as the present, where the evidence supports the conclusion that sales of Isabelle which were not lost sales of Yasmin is likely to represent nothing more than a very small possibility, there is no reason to make an adjustment of 1% (as Bayer suggested as a possible adjustment in the alternative to its principal position). While 1% may sound trivial, it represents not only one woman in every 100 who purchased Isabelle but, as Generic Health posited, one woman in every 100 who purchased Isabelle and had the particular form of price sensitivity which meant she would not have purchased Yasmin if Isabelle had not been available. It also represents not insubstantial monetary sums given the overall sales and period of infringement. Given that Generic Health is the wrongdoer I see no reason to make such an adjustment given the evidence which I have accepted in the circumstances of this case (which, as I have said, bears no resemblance to that of other consumables) and that, on any view, Bayer is entitled to the benefit of doubt, not Generic Health.
303 I do not consider it necessary to descend into detail about a number of Dr Williams’ criticisms of Mr Houston’s work, for example, whether seasonal adjustment was or was not appropriate, it does not alter the matters discussed above. For these reasons I am unable to accept that “Mr Houston’s approach is of assistance to the Court in showing that there was a trend by which Isabelle increased sales of the molecule beyond what they were otherwise projected to be”.
Petibelle
304 Generic Health made other submissions about Petibelle which require consideration. Generic Health described Bayer’s claim for damages in respect of Petibelle unusual in that “Petibelle was only introduced after Isabelle had been withdrawn, and therefore never competed against Isabelle”. This is so but I accept Mr Peace’s evidence that, but for Isabelle, Bayer would never have put Petibelle on the market.
305 Bayer’s claim in respect of Petibelle is not to the date of judgment but until June 2016, just over two years after Isabelle was removed from the market.
306 Generic Health summarised Mr Peace’s reasons for introducing Petibelle as follows:
(a) At T140.10-12: “Petibelle was introduced because we believe we had a responsibility to the marketplace, to consumers. We were concerned about company reputation, so that product was – we informed the market.”
(b) At T141.3-6: “As I said, we introduced this product as a convenience to ensure that there was minimal disruption for whatever reason a patient may have had in front of the pharmacist. We wanted to make sure there was no reputational damage to Bayer, so that’s why we introduced this second brand.”
(c) At T145.20-23: “it was a move by Bayer to try and minimise the risk of a woman not continuing on that particular moment with contraception. Otherwise you’ve got a woman who’s suddenly confused, and before you know it, she’s not taking the pill when she should be.”
(d) At T149.17-25: “Well, as I say, the idea of Petibelle was that if the patient came back that was an Isabelle user, presumably that pharmacist has still got Yasmin on the shelf, and now they may or may not have Petibelle on the shelf, and it was on the basis that if they did have a patient that came back who said this was an issue for them now or stressed them in some way or if they took umbrage to the fact that the one that they were taking for is suddenly removed and what’s going on, the idea was to ensure that we minimised the risk of any reputational damage. It was also a service to the pharmacist to be able to accommodate the patient with another choice.”
(e) At T141.37-40: The concern, if an injunction were granted, that there could “a very small number of people making a lot of noise” about pricing.
307 Generic Health described this as a “jumble of explanations”. I do not accept that these explanations are inconsistent with Bayer’s case that Isabelle had potentially or actually fractured the price/value perception of Yasmin. It is obvious that Bayer was concerned about reputational damage, women not continuing contraception, and pharmacists being able to offer an option to women closer in price to Isabelle because of the risk or reality that Isabelle had, as Bayer put it, “damaged the market acceptance over the 10 years between 2002 and 2012 of the high premium price Bayer charged for Yasmin”. Far from undermining this submission, Mr Peace’s evidence strongly supports it. This is the risk which underlies all of the considerations Mr Peace was describing. But for that risk, the other concerns would not have existed.
308 Mr Peace did not reject the “notion that Petibelle was introduced because price was a factor to a number of Yasmin purchasers, and said it was about Bayer company reputation”. He said that he set the price point for Petibelle carefully so that “those small few that may be concerned that price was an issue” would have an alternative to Yasmin. This does not mean that he was not concerned that Isabelle had damaged Bayer’s reputation with women generally which Petibelle would ameliorate to some extent. While Dr Williams properly refrained from expressing opinions about consumer psychology, it is not difficult to infer that there were women who would have paid the Yasmin price without demur when Isabelle was not an alternative, would also have happily taken the benefit of the $10 discount when Isabelle was available given its bioequivalence, and would later feel aggrieved by having to pay the full price for Yasmin again. Bayer was entitled to take reasonable steps to rectify the brand damage which Isabelle’s unlawful entry into the market had caused Bayer. The introduction of Petibelle to rectify the damage, to the extent it could be rectified, was a reasonable response to the difficult commercial position in which Generic Health’s infringing conduct had placed Bayer. Bayer did not need to adduce expert or survey evidence to enable this inference to be drawn given the wealth of evidence available about the OC market in Australia and Yasmin’s place within it. Mr Peace’s evidence disclosed that but for Isabelle, Bayer would not have introduced Petibelle and that Bayer’s reasons for so doing were reasonable in the circumstances.
309 It is not necessarily the case that the loss to Bayer caused by the introduction of Petibelle is indirect loss but, assuming that characterisation to be correct, as was said in TS & B at [207]:
The plaintiff is also entitled to recover indirect losses (such as damage to goodwill) as long as the cause is the infringement, the loss is foreseeable and is not unduly speculative. It will often be impossible to be precise in the calculation of lost profit. If needs be, the calculation can be rough and ready, with the benefit of any doubt favouring the plaintiff.
310 I am satisfied that the requirement of causation is met in the present case, whichever way the issue is approached. As noted, in Comcare v Martin [2016] HCA 43; (2016) 339 ALR 1 at [42], applying Travel Compensation Fund v Tambree (trading as R Tambree and Associates) [2005] HCA 69; (2005) 224 CLR 627 at [28] to [36] and [45] to [50], “[c]ausation in a legal context is always purposive”. In the context of the Patents Act, relief by way of damages is for infringement of a patent. In the present case, but for the infringement, Bayer would not have introduced the lower priced Petibelle. Nothing Generic Health has said provides any cogent reason for concluding that damages for patent infringement, for which s 122 of the Patents Act expressly provides, should not extend to loss resulting from the patent holder’s reasonable and timely actions to redress the effects of the infringement, particularly where, as in the present case, those actions would themselves have been reasonably foreseeable to the infringer while the infringing acts were occurring.
311 Not all voluntary acts break the chain of causation. In the pharmaceutical market, the introduction of a generic version by an originator of the brand is, as Mr Peace’s evidence indicated, the natural and obvious consequence of the entry of a generic version into the market. The first thing Bayer considered once Isabelle entered the market was to launch its own generic version of Yasmin. Bayer had Petibelle registered before Isabelle entered the market because this is what originator pharmaceutical companies do in case a generic enters the market so that, if appropriate, they can move quickly to introduce their own generic. It must be inferred that Generic Health, as a part of this market, was aware that its introduction of Isabelle might have a range of natural and direct consequences including the possible reduction in price of Yasmin and the introduction by Bayer of its own cheaper version of Yasmin either immediately on Isabelle entering the market or at some later time. The fact that Bayer introduced Petibelle immediately after Isabelle was withdrawn supports the existence of a sufficient causal connection between it and the infringement.
312 The statement in Fabio Perini SpA v LPC Group plc [2012] RPC 30 at [106] that “[c]ausation questions (like assessment questions) have to be approached in a commercially realistic way and with the ultimate object of yielding compensation that is fair (but no more than fair) for the wrong suffered”, cited by Generic Health, also supports Bayer’s claim. It would not be commercially realistic, or consistent with any apparent purpose of s 122 of the Patents Act, to deny relief by way of damages for loss resulting from the patent holder’s reasonable and timely actions to redress the effects of the infringement, particularly where, as in the present case, those actions would themselves have been reasonably foreseeable to the infringer while the infringing acts were occurring..
313 I do not accept Generic Health’s submissions to the contrary. In particular, I do not accept that “reputation-related pricing issues relate to Bayer’s strategic decisions; they fall well outside the scope of intellectual property rights protection”. Provided the requirements of causation and foreseeability are satisfied the loss is not too remote and is compensable. As noted, the requirements are satisfied in the present case.
314 Generic Health’s submission that Bayer intended to capitalise on Isabelle’s removal from the market misses the point. The point is that it was reasonable of Bayer to try to respond to Isabelle’s unlawful disruption of the market. It was foreseeable that Bayer would respond to the infringement in various ways including the introduction of its own generic version at a cheaper price than Yasmin. This was a natural and direct consequence of the introduction of Isabelle. The fact that Bayer waited until it had succeeded in having Isabelle removed from the market does not undermine either causation or foreseeability. The manifest temporal connection between the removal of Isabelle and the introduction of Petibelle (within the week of Isabelle’s removal Petibelle was on sale) confirms the causal connection. If, for example, there had been substantial time between the removal of Isabelle and the introduction of Petibelle, the result might well have been different. As it is, however, it cannot be said that the infringement was merely a background circumstance of, or set the scene for, the introduction of Petibelle. The infringement was the direct and obvious cause of the introduction of Petibelle. The fact that Dr Williams agreed that “it would only be profitable if the loss in margin on the units that switched from Yasmin to Petibelle would be made up for in extra volume on the sales of the Petibelle” goes nowhere given that Bayer’s reasons for introducing Pettibelle were not to increase its profits (and Dr Williams concluded that there was no financial incentive for Bayer to introduce Petibelle as it would not make more money by so doing). As such, the submissions that “Bayer clearly understood the risks to the volume of Yasmin sales which were associated with the introduction of Petibelle. Its reasons for doing so cannot be dissociated from its commercial motivations. Its introduction is Bayer’s cross to bear, not that of Generic Health” are unpersuasive. It is the infringement that created the risks which Bayer reasonably addressed. As such, it is Generic Health’s cross to bear.
315 Generic Health’s submission that the loss is too remote because originator companies usually introduce their own generic while the competing generic is on the market does not mean that Bayer’s decision, to put Petibelle on the market immediately Isabelle had been removed, involves loss that is other than reasonably foreseeable and thus is too remote.
316 Nor do I accept that the claim period, of two years, would involve compensating Bayer for loss that is too remote to be recovered. Bayer’s reasons for introducing Pettibelle were multiple but all involved trying to ameliorate the harm done to the market which the infringement caused. It is true that Bayer cannot recover the loss resulting from the cheaper price of Petibelle in perpetuity but the period of two years is reasonable. There is no basis in the evidence for Generic Health’s submission that “a loss (if any) resulting from Petibelle would be a short-term loss or no more than a month or two” or would be rectified on the first pharmacy visit to obtain Yasmin or Petibelle. Again, any doubt in this regard should be to the account of Generic Health, not Bayer. I can see no reason why the claim over two years offends the requirement that loss be compensated only if it satisfies the test of causation, foreseeability and is not otherwise speculative.
317 Generic Health’s final submission about Petibelle was that:
As observed by Dr Williams, it was only rational to introduce Petibelle to deal with customers who would not otherwise purchase Yasmin, and only if they outweighed the cannibalisation effect on potential sales of Yasmin: T 542.45 – 543.25. Many of the Isabelle customers switched back to Yasmin. Therefore, the Petibelle customers will disproportionately consist of those who were never prepared to pay the Yasmin price. Thus, the discount to the Petibelle claim (if allowable, and for whatever period allowable) should be further discounted by at least 50%.
318 Dr Williams was dealing only with the issue of profitability. He was not dealing with any of the other considerations with which Bayer had to contend. The fact that many women switched back from Isabelle to Yasmin rather than to Pettibelle does not mean that Petibelle customers will disproportionately consist of those who were never prepared to pay the Yasmin price. They may well have been prepared to pay the Yasmin price but for Isabelle and then but for Petibelle, but as Bayer recognised for women to be forced to do so because of the lack of an alternative (when the infringement had given an alternative) also may well have damaged its brand over the longer term. As a result, I also do not accept this submission for further discount in respect of Petibelle.
The user principle
319 Almost as an afterthought, Bayer contended that if any substantial discount was to be made on the basis of a finding that some sales of Isabelle (or Petibelle) would not have been sales of Yasmin, it was nevertheless entitled to compensation for the unlawful use of its property (the user principle). Generic Health submitted that the claim based on the user principle could not be made and that, in any event, “the proceedings have not been conducted on the basis of a ‘user pays’ damages claim and Bayer should not be permitted to run that case at this late stage”.
320 Generic Health’s submissions about this issue raise a number of important points of principle which, given the way in which the claim was articulated at a late stage, have not been thoroughly explored in this case. Justice Yates explored the principle in detail in Winnebago Industries Inc v Knott Investments Pty Ltd (No 4) [2015] FCA 1327; (2015) 241 FCR 271 at [12] to [99]. The principle, as Yates J explained at [13], is that:
…a plaintiff is entitled to recover, by way of damages, a reasonable sum from a defendant who has wrongfully used the plaintiff’s property. The plaintiff may not have suffered actual loss from the use, and the wrongdoer may not have derived actual benefit. Nevertheless, under the principle, the defendant is obliged to pay a reasonable sum for the wrongful use. The reasonable sum is sometimes described as a reasonable rent, hiring fee, endorsement fee, licence fee or royalty (amongst other expressions), depending on the property involved and the nature of the wrongful use.
321 Winnebago, however, was a case in which the plaintiff could not prove loss by any other means. The plaintiff was not in the Australian market and thus did not suffer lost profits. Its only measure of damages was the sum assessed to be reasonable for the use, on the assumption that the plaintiff would never have permitted its property to be used for free. A similar approach was taken in Pearce v Paul Kingston Pty Ltd (1992) 25 IPR 591 where a lost profits assessment proved impossible, so that an assessment based on an assumed royalty was conducted. The point Generic Health makes in the present case is that Bayer has claimed lost profits and will be compensated for those lost profits. Any discount applied would be to ensure that Bayer was not over-compensated. As a matter of principle, therefore, Bayer cannot also recover under the user principle any part of the amount discounted from its lost profits claim. To do so must over-compensate Bayer.
322 According to Generic Health:
The correct principle is that if the patentee claims damages, and the patentee has suffered loss by reason of the infringements in question and that loss is able to be calculated, then the calculated loss is the measure of recovery. If the patentee is not able to calculate loss, then some other form of recovery may be utilised.
323 Generic Health referred to Winnebago as follows:
[35] In General Tire & Rubber Co v Firestone Tyre & Rubber Co Ltd [1975] 1 WLR 819 (General Tire), Lord Wilberforce (with whom Viscount Dilhorne, Lord Diplock and Lord Kilbrandon agreed) identified three main groups of reported cases which exemplify the approach of courts when assessing damages for patent infringement.
[36] The first group relates to manufacturers who exploit an invention by making articles or products which they sell for profit. Lord Wilberforce said that, in these cases, if the patent is infringed, the effect of the infringement will be to divert sales from the patentee to the infringer. Therefore, normally, the measure of damages will be the profit which would have been realised by the patentee if the sales had been made by it.
[37] The second group relates to inventions that are exploited through the granting of licences in return for royalty payments. Lord Wilberforce said that, in these cases, if the infringer uses the invention without a licence, the measure of damages will be the sums which the infringer would have paid by way of royalty if it had acted legally.
[38] Lord Wilberforce identified a third group where it was not possible to prove a normal rate of profit (as in the first group) or a normal or established licence royalty (as in the second group)…
324 The present case, said Generic Health, is in the first group. The second and third groups are inapplicable. In particular, as to the third group, this is not a case where “it was not possible to prove a normal rate of profit”. While more than one measure of damages may be used in an appropriate case (such as in Watson, Laidlaw & Co Ltd v Pott, Cassels and Williamson (1914) 31 RPC 104 where business was conducted in one part of a territory and not in another), this is not such a case given that Bayer and Generic Health were competing in the same market and Generic Health’s product diverted sales from Bayer’s product, the only issue being the extent of the diversion.
325 Given that this issue need not be resolved in the present case and arose so late in the piece, I prefer not to express any concluded views about it.
The costings issue
326 The issues between the accountants were significantly narrowed during the course of their joint report and concurrent evidence. Mr Stone did not consider any discount for risk was necessary to take into account any potential inaccuracies in Bayer AG’s standard costings. Mr Samuel considered that a discount for that risk was required. Mr Stone also did not deduct tax from Bayer’s damages for the purpose of calculating interest, whereas Mr Samuel did.
327 The differences between the accountants are apparent from their oral evidence.
328 Mr Samuel’s evidence included the following:
(1) “…we’re in danger of assuming the Bayer AG loss calculation is not a hypothetical position. It is. It’s a hypothetical result. It’s relying on standard costs which are an estimate; not actual costs. We are, in effect, estimating or forecasting what those costs would have been between 2012 and 2016. The fact we were having to estimate for historical dates instead of future dates, in my opinion, doesn’t change the fact that it is still an estimate. And I’ve been estimating damages for about 25 years in hundreds of cases and I would say the circumstances in which a hypothetical is not discounted is the exception, not the norm”.
(2) “…the fundamental valuation principle [is] that uncertainty means risk and risk means a discount”.
(3) “I proposed in my report an adjustment of about [$]406,000 for variable costs… That represents 2.8 per cent of the Bayer AG loss… So if you were to accept that adjustment, that creates a minimum position. And then we have three further uncertainties that we’ve been discussing yesterday being not being able to investigate before costs have been included due to the lack of a reconciliation, not being able to investigate if the standard ..... cost allocation is reasonable and not being able to identify the consequences of the variances from standard costs”.
(4) “Now, if you take all that together, that would be about four per cent and might suggest a range of three to five or whatever it might be. Now, all of this, I want to emphasise, is that we’re dealing with uncertainty. We just don’t know. There may be a big error. There may be a big omission. We just don’t know because we haven’t had the opportunity to check it. There may be no error or no omission. So it’s really about trying to come up with a percentage for uncertainty. Ultimately, it has to be a matter of judgment and I will leave it there”.
329 Mr Stone’s evidence included these statements:
(1) “I think it’s fair to say that there is an asymmetry in that risk. It is much more likely that a fixed cost should be treated as variable than a variable cost should be treated as fixed..”.
(2) “…, the only element that has risk associated with it is the incremental costs [that is, the additional costs of Bayer AG producing the additional Yasmin pills it would have been able to sell but for Isabelle], so from my perspective, it really is a question of who bears the estimation risk of that and has that – has sufficient information been provided”.
(3) “To my mind, Mr Samuel has identified two or three different ways of identifying where an error may exist but, ultimately, the risk is that one element is understated or overstated, that risk being the incremental cost. Now, that may come about through a variety of means. It may come about because fixed costs have been – well, costs should have been treated as variable when they had been treated as fixed and we have some small examples of that…
It may be that there were costs omitted altogether but we don’t know that. It may be that there is a variance. We do know that variances overall exist. We just don’t know whether they are fixed or variable in their nature, so we don’t know whether they should be taken into account”.
(4) “Simply put, the risk is that the costs are understated. How that comes about may happen in a series of ways but the idea I do agree with is that we are talking about a very small level of risk compared with the overall level of damages and the idea that there is a three to five per cent risk is difficult to get away from. However, a phrase that I often use in trying to look at damages is the concept of spurious accuracy because it’s tempting to suggest that we can get this down to the nearest dollar or cents and it is – as I said at the beginning of my opening, it’s an estimation process.
So do we know enough to be able to say that this really is three per cent or is it three and a half or is it five, especially when there may be other uncertainties associated with it which are of a greater magnitude or a potentially greater magnitude. But the idea that there is a small risk is something that doesn’t sound abhorrent to me. An unquantified discount was something that I felt was difficult to get my head around and, to my mind, it is additive”.
(5) “…if it is decided that, well, actually, in these circumstances, they’ve put up enough information. It’s not perfect, but it’s reasonable to do an estimation process and, therefore, no further discount is required. That, to my mind, is not an accounting question and… that’s a question for your Honour… I have some difficulty with saying no lower than three per cent, but certainly the idea that it’s no higher than five per cent would make sense”.
330 Given this evidence, the issue of discounting for uncertainty in Bayer’s standard costings need not involve extensive consideration of the detail of the standard costings or the treatment of fixed and variable costs. The existence of uncertainty indicates a discount is appropriate for the reasons given by Mr Samuels. The magnitude of the discount between the target of 0% and 5% as the accountants discussed is ultimately a matter for judgment. A number of factors suggest that a discount at the lower end of that range is appropriate. Bayer is a large company which relies on standard costings throughout its entire business. The standard costings must be of sufficient accuracy for Bayer to be confident that it can run its business on the basis of those costings. There is evidence that Bayer reviews its standard costings. Further, they are used as the source value for the cost of goods sold in Bayer's audited financial statements. As such, the risk of material line items being simply omitted seems speculative. In the words Bayer used in its submissions:
The data used by Bayer, a large, sophisticated, multinational company, to support their claim is derived from a standard SAP system with Product Cost Controlling Information System (CO-PC) module and activated Material Ledger functionality to calculate the manufacturing costs and the periodical actual costs for each of their products and was prepared as a management tool for purposes unrelated to this proceeding. The methodology of this system is relied upon for Bayer's Inventory Valuation and determination of Cost of Goods Sold used in Bayer Pharma AG's Financial Statements as audited by PwC, globally, for management purposes.
Mr Murray has explained that Bayer's standard costing system is also "part of the accounting process to appropriately cost inventory" and that from an external reporting or audit perspective, it is an important part of Bayer's financial systems.
331 Mr Stone is also right that while the uncertainty can be described in a multitude of ways, there is only one source of uncertainty – inaccuracy in the additional costs. The most likely source of this inaccuracy is the allocation of variable and fixed costs, with variable costs being characterised as fixed when they ought to be variable (and thus would reduce Bayer’s profit if it produced more pills). Bayer submitted, and I accept that:
Whether a cost is fixed or variable depends on the nature of the cost and the circumstances…
The allocation of fixed and variable costs is a matter of judgment where reasonable persons may differ as to how to treat particular cost items in the circumstances for a particular purpose over a particular period of time.
Mr Stone and Mr Samuel agree that the volume of the production Bayer Pharma AG lost to Isabelle in this case would not cause fixed costs to become variable costs…
Bayer Pharma AG's costs were allocated as fixed or variable in accordance with Bayer's Costing Manual.
332 Bayer also noted Mr Murray’s evidence to this effect, which supports the submission that the appropriate discount would be at the lower end of the range of 0% to 5%. Mr Murray said:
I think it’s reliable to the extent that over a three year period also the revisions to the standard cost have not moved substantially so I don’t believe there’s any inherent errors in the standard cost.
333 Mr Samuel’s deduction of 2.8% from the fixed costs was arbitrary (representing a 50% overstatement of fixed costs compared to variable costs). As Mr Stone said, this figure also involves spurious notions of accuracy, as if the discount can be estimated to one decimal place.
334 The other factor to which I must give weight is the principle that doubt is to be resolved in favour of Bayer not Generic Health, the wrongdoer. As a result, I am not satisfied that I should adopt the arbitrary figure of 2.8% or that Mr Samuels’ approach of accreting for different kinds of potential uncertainty is appropriate. I consider that there should be one discount to account for the uncertainty about costings. The discount should be small for the reason given by Mr Stone. As Mr Stone said, 3% is not the lowest limit of any appropriate discount. And as Bayer submitted:
…as the risk relates to Bayer Pharma AG's cost element, which amounts to approximately only 3.7% of its revenue, any discount must be relatively small in the context of the estimated loss of Bayer Pharma AG. As illustrated by Table 5 of the Second Financial JER [joint experts report], applying a discount of 2% to Bayer Pharma AG’s claim translates to a significant 50% increase of Bayer's stated variable cost figures. A 4% discount would effectively double Bayer's variable cost figures.
335 It is not necessary to deal in detail with Generic Health’s submissions about this issue given where the accountants ended up in their evidence. It is sufficient to say that Mr Stone did not in fact agree that a discount was necessary. Mr Stone did not agree that the uncertainty was all in one direction – to increase Bayer’s claim against what it ought to be. In fact Mr Stone pointed to contrary examples in his evidence and said:
They [the standard costings] were prepared at the time, we’ve got no reason to suggest that they are materially wrong. They may have errors in them, but those errors may go both ways. That’s my perspective. Mr Samuel’s perspective is I can see some unexplained issues. I don’t know quite how it has been put together and therefore, there is a risk that they are incorrect and this is how I’m going to deal with that risk. It really comes down to, in my mind, who gets to wear that risk and at what point do you say, well, there has been a reasonable provision of information.
336 Mr Stone did not agree that the appropriate range was 3% to 5%. He agreed that 5% was the top of the range but thought no discount or 0% could also be appropriate and any discount should be small. The figure of 5% is not the appropriate discount. It is the top of Mr Samuel’s range when doubts should be resolved in Bayer’s favour. A discount of less than 3% is reasonable in the circumstances. Apart from what I have already said, Mr Stone agreed that Mr Samuel’s calculation equated to 2.8% of Bayer AG’s claimed loss. He did not agree that Bayer AG’s claimed loss should be reduced by 2.8%. Mr Stone could not make any meaningful comment about Mr Samuel’s 50% adjustment of fixed costs to variable costs because 50% was arbitrary. A 50% adjustment is large given that Bayer relies on its allocation of costs for all management purposes and thus has good reason to ensure their accuracy.
337 Having regard to these matters I would allow a discount of 2% in all of the circumstances.
338 The only other issue between the accountants related to interest, it being agreed that interest before judgment is payable there being no good reason not to order payment of interest (s 51A of the Federal Court of Australia Act 1976 (Cth)). Generic Health accepted that the pre-judgment interest rate should reflect the provisions of the Court’s Practice Note Interest on Judgments Practice Note (GPN-INT), which is a rate 4% above the cash rate last published by the Reserve Bank of Australia before the relevant period. The issue is whether interest should be calculated on Bayer’s pre or post-tax losses.
339 Generic Health submitted that interest should be calculated on post tax losses. Mr Samuel said:
I was instructed to calculate interest. In doing so, I recognise, of course, that interest is a matter of discretion for the court. However, being asked to calculate it, I’ve done so by applying the rates to an after tax loss, not a pre-tax loss, and I’ve done that because awarding interest on a pre-tax loss would, in my view, result in putting a claimant in a better position than they would have been absent the infringements.
…
And this happens because when a claimant receives an award, they would typically pay tax on it and certainly, the profits they missed out on, they would have paid tax on, and therefore, they would only have had the after tax figure available to them, so that’s the actual funds they’ve lost, but perhaps for a small timing difference in when they receive the profits and when they pay tax. And so just to give an example of how that would work, if the lost profit was $100 and the after tax figure is 70, the statutory rates would award in 2013, an interest of six and a half or six and three quarter per cent on the full 100, whereas in practice, a company wouldn’t have any interest on 30 of that 100, so that’s the basis for doing that.
340 In Hungerfords v Walker [1989] HCA 8; (1989) 171 CLR 125 Brennan and Deane JJ explained the distinction between an award of damages on a judgment sum pursuant to a statutory power and an award of damages to compensate for the loss of the capacity to use money by reference to the interest that could have been earned on the money had it been available. The purpose of the latter is compensatory in the orthodox sense which applies in respect of damages for the infringement of an intellectual property right. The former is an exercise of discretion pursuant to a statutory power. While the overall object remains compensatory and not punitive, the context weighs against Mr Samuel’s approach. There is no good reason to manipulate the pre-judgment interest payable so that it relates only to post tax earnings when, as Bayer said:
(a) there is no guidance in the Act nor the Practice Note as to whether interest is to be applied to pre-tax or post-tax sums;
(b) it is relatively rare for parties to adduce evidence on the potential taxation implications of damages which would be sufficient to allow the complex post-tax calculation to be undertaken;
(c) any damages payable to Bayer Australia as a lump sum (including an interest component) will itself be separately subject to income tax (at the same rate as would have been paid if Bayer Australia had made the sales it is now claiming); and
(d) any damages payable to Bayer Pharma AG as a lump sum (including an interest component) would be subject to income tax (i.e. corporate income tax plus surcharges and trade tax) in respect of any damages awarded in this case which would be taxable to the same extent if Isabelle had not been launched and Bayer Pharma AG had made the sales it is now claiming.
341 Griffiths J declined to undertake an analogous exercise in RPR Maintenance Pty Ltd v Marmax Investments Pty Ltd [2014] FCA 514 at [5] to [10]. In Australian Mud Company Pty Ltd v Coretell Pty Ltd (No 7) [2016] FCA 991 Barker J also declined to use post tax profits as the basis for the assessment of interest (at [838 to [842]).
342 Given these considerations, I see no reason to engage in the exercise of assessing interest based on post tax profits, and good reason to adopt the same approach as in Marmax and Coretell.
Mr Murray’s evidence
343 Given the accounting evidence, there is no need to discuss the evidence of Mr Murray, Bayer Australia’s Head of Accounting. Mr Murray calculated the profit that Bayer would have derived had all sales of Isabelle been sales of Yasmin and all sales of Petibelle up to 30 June 2015 had been sales of Yasmin. Ultimately, nothing Mr Murray said was materially in dispute, the calculations depending on the answers to the issues dealt with above.
CONCLUSIONS
344 Bayer has established that the specification for the patent before its amendment was framed in good faith and with reasonable skill and knowledge. As a result, the prohibition on an award of damages in respect of infringement of the patent before the amendment in s 115(1)(a) of the Patents Act is not applicable.
345 Bayer has established that its loss is best assessed on the basis that every sale of Isabelle is a lost sale of Yasmin. It has also established that its loss is best assessed on the basis that every sale of Petibelle over the period from the introduction of Petibelle until 30 June 2016 is a lost sale of Yasmin.
346 In respect of the costings used to assess Bayer AG’s loss, the only discount should be in the amount of 2% to account for the risks associated with uncertainty about Bayer’s costings.
347 Interest should be assessed on Bayer’s pre-tax losses rather than its post-tax losses.
348 The parties should confer and submit agreed short minutes of order reflecting these reasons for judgment within 14 days. In the ordinary course, Bayer should obtain the usual order as to costs. If there is any reason to make some other order as to costs, the parties are to agree and submit directions within the same 14 day period.
I certify that the preceding three hundred and forty-eight (348) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Jagot. |
Associate: