FEDERAL COURT OF AUSTRALIA
Australian Competition and Consumer Commission v Reckitt Benckiser (Australia) Pty Ltd (No 4) [2015] FCA 1408
IN THE FEDERAL COURT OF AUSTRALIA | |
AUSTRALIAN COMPETITION AND CONSUMER COMMISSION Applicant | |
AND: | RECKITT BENCKISER (AUSTRALIA) PTY LTD (ACN 003 274 655) Respondent |
DATE OF ORDER: | |
WHERE MADE: | HEARD IN SYDNEY (DELIVERED IN BRISBANE VIA video LINK) |
THE COURT ORDERS THAT:
1. In marketing and selling the current Nurofen branded products comprising:
Nurofen Migraine Pain ibuprofen lysine 342 mg tablet blister pack;
Nurofen Tension Headache ibuprofen lysine 342 mg tablet blister pack;
Nurofen Period Pain ibuprofen lysine 342 mg tablet blister pack; and
Nurofen Back Pain ibuprofen lysine 342 mg tablet blister pack;
(together, the Nurofen Specific Pain Range)
in the form of packaging comprising Annexures A, B, C and D to the Fast Track Statement filed on 4 March 2015, the Respondent, Reckitt Benckiser (Australia) Pty Limited, has in trade or commerce, from 1 January 2011:
engaged in conduct that is misleading or deceptive, or likely to mislead or deceive, in contravention of section 18 of the Australian Consumer Law (ACL) which is Schedule 2 to the Competition and Consumer Act 2010 (Cth) (CCA); and
engaged in conduct that is liable to mislead the public as to the nature, the characteristics or the suitability for their purpose of the products comprising the Nurofen Specific Pain Range within the meaning of section 33 of the ACL,
by representing that each product in the Nurofen Specific Pain Range:
was specifically formulated to treat the particular type of pain specified on the packaging relevant to that product; and
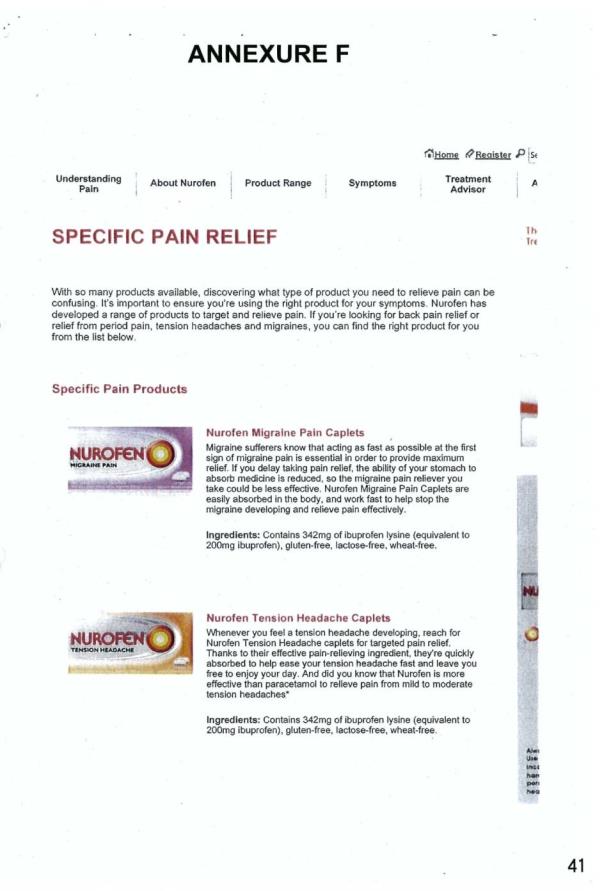
solely or specifically treated the particular type of pain specified on the packaging relevant to that product,
when in fact:
each product in the Nurofen Specific Pain Range contains the same active ingredient, namely ibuprofen lysine 342mg;
the Australian Register of Therapeutic Goods (ARTG) approved indications for each product in the Nurofen Specific Pain Range are the same;
each product in the Nurofen Specific Pain Range is of the same formulation; and
no product in the Nurofen Specific Pain Range is any more or less effective than the others in treating any of the symptoms shown on the packaging of the products in the Nurofen Specific Pain Range.
2. In the period between at least December 2012 and around May 2014, in publishing or causing to be published on the website www.nurofen.com.au:
a page with the heading “RELIEVE PAIN WITH THE RIGHT TYPES OF PAIN MEDICATION” (the Product Comparator Page); and
a page with the heading “SPECIFIC PAIN RELIEF” (the Specific Pain Relief Page),
a copy of which is set out in Annexures E and F to the Fast Track Statement filed on 4 March 2015, the Respondent, Reckitt Benckiser (Australia) Pty Limited has, in trade or commerce:
engaged in conduct that was misleading or deceptive, or likely to mislead or deceive, in contravention of section 18 of the ACL; and
engaged in conduct that was liable to mislead the public as to the nature, the characteristics or the suitability for their purpose of the products comprising the Nurofen Specific Pain Range within the meaning of section 33 of the ACL,
by representing that each product in the Nurofen Specific Pain Range:
was specifically formulated to treat the particular type of pain specified on the packaging relevant to that product; and
solely or specifically treated the particular type of pain specified on the packaging relevant to that product,
when in fact:
each product in the Nurofen Specific Pain Range contains the same active ingredient, namely ibuprofen lysine 342mg;
the ARTG approved indications for each product in the Nurofen Specific Pain Range are the same;
each product in the Nurofen Specific Pain Range is of the same formulation; and
no product in the Nurofen Specific Pain Range is any more or less effective than the others in treating any of the symptoms shown on the packaging of the products in the Nurofen Specific Pain Range.
3. The Respondent, Reckitt Benckiser (Australia) Pty Ltd be restrained for a period of three years from the date of this order, whether by itself, its servants or agents, from selling, marketing or promoting any product comprising the Nurofen Specific Pain Range:
(a) in the Nurofen Specific Pain Range Packaging;
(b) in packaging making any representation to the effect that:
(i) the product is specifically formulated to treat the particular type of pain specified on the packaging relevant to that product and not other types of pain; and/or
(ii) the product solely or specifically treats the particular type of pain specified on the packaging relevant to that product and not other types of pain.
4. The Respondent:
(a) within four weeks of the date of this order, cease or uses its best endeavours to procure the cessation of, any further shipment, distribution and sale of all Nurofen Specific Pain Range products packaged in the Nurofen Specific Pain Range Packaging; and
(b) within three months of the date of this order, procure, or uses its best endeavours to procure, the removal of all Nurofen Specific Pain Range products packaged in the Nurofen Specific Pain Range Packaging from display and sale in all retail outlets to which the Respondent supplies these products for sale.
5. The Respondent, at its own expense:
(a) within 14 days of the date of this order, and for a period of 30 days thereafter, publish on the Nurofen website, accessible at the URL www.nurofen.com.au, (Nurofen Website) a notice in the same terms and form as Annexure A to this order (Corrective Notice) and ensure that:
(i) the Corrective Notice is accessible by a prominent one-click hyperlink (Click-through Icon) displayed in the top third of the homepage of the Nurofen Website; and
(ii) the Click-through Icon:
(A) consists of a black bordered box at least 255 pixels wide by 60 pixels high;
(B) inside the box, has the title “Corrective Notice ordered by the Federal Court of Australia” in at least 14-point black, bold Arial font, on a white background and centred; and
(C) below the title, has the words “Click here for further information” in at least 14-point black, Arial font on a white background and centred; and
(iii) The Corrective Notice occupies the entire webpage that is accessible via the Click-through Icon.
(b) within 21 days of the date of this order, publish a corrective advertisement, in the form of Annexure A to this order in The Australian, and ensure that the advertisement:
(i) occupies no less than one third of a page of the newspaper;
(ii) is in a text which is Arial font and which is at least 12-point and bolded for the headline, and at least 11-point for the remaining text; and
(iii) is placed within the first 10 pages of the newspaper.
6. The Respondent, at its own expense:
(a) within 3 months of the date of this order ensure that its existing compliance program meets the requirements set out in Annexure B to this order (Consumer Protection Law Compliance Program); and
(b) maintain and administer the Consumer Protection Law Compliance Program for a period of 3 years from the date of these orders.
7. The Respondent pay the Applicant's costs of and incidental to these proceedings until and including 10 December 2015.


FORM OF COMPLIANCE PROGRAM (ANNEXURE B)
Reckitt Benckiser (Australia) Pty Ltd (RB) must establish a Consumer Protection Law Compliance Program in relation to packaging and promoting consumer products and concerning sections 18 and 33 of the Australian Consumer Law (ACL) which is Schedule 2 of the Competition and Consumer Act 2010 (Cth) (Compliance Program) that complies with each of the following requirements:
Appointments
1. Within three months of the date of this Order, RB must appoint:
(a) a director or a senior manager of the business as a compliance officer (Compliance Officer), with responsibility for ensuring the Compliance Program is effectively designed, implemented and maintained; and
(b) a suitably qualified, internal or external, compliance professional with expertise in competition and consumer law (Compliance Advisor).
2. RB must instruct the Compliance Advisor to conduct a consumer protection law risk assessment within three months of his or her appointment (Risk Assessment).
3. RB must use its best endeavours to ensure that the Risk Assessment covers the following matters, to be recorded in a written report (Risk Assessment Report):
(a) identifies the areas where RB is at risk of contravening relevant sections of the ACL;
(b) assesses the likelihood of these risks occurring;
(c) identifies where there may be gaps in RB's existing procedures for managing these risks; and
(d) provides recommendations for any action to be taken by RB having regard to the above assessment.
4. RB must, within one month of the date of this Order, issue a policy statement outlining RB's commitment to compliance with the ACL (Compliance Policy).
5. The Compliance Policy must contain:
(a) a statement of RB's commitment to compliance with the ACL;
(b) a requirement for all staff to report any Compliance Program related issues and ACL compliance concerns to the Compliance Officer; and
(c) a clear statement that RB will take action internally against any persons who are knowingly or recklessly concerned in a contravention of the ACL.
Complaints Handling System
6. RB must ensure the Compliance Program includes a consumer protection law complaints handling system capable of identifying, classifying, storing and responding to consumer protection law complaints (Complaints Handling System).
Staff Training
7. RB must ensure that the Compliance Program includes a requirement for regular (at least once a year) training for all employees of RB whose duties could result in them being concerned with conduct that may contravene relevant sections of the ACL.
8. RB must ensure that the staff training is conducted by a suitably qualified compliance professional or legal practitioner with expertise in the ACL.
9. RB must ensure that the Compliance Program includes a requirement that awareness of consumer protection compliance issues forms part of the induction of all new directors, officers and employees whose duties could result in them being concerned with conduct that may contravene relevant sections of the ACL.
Reports to Board/Senior Management
10. RB must ensure that the Compliance Officer reports to the Board and/or senior management every 12 months on the continuing effectiveness of the Compliance Program.
Provision of Compliance Program documents to the ACCC
11. RB shall, at its own expense, within 4 months of the date of this Order, cause to be produced and provided to the ACCC copies of the following documents constituting the Compliance Program:
(a) the Compliance Policy;
(b) an outline of the Complaints Handling System; and
(c) Staff Training materials and induction materials.
ACCC Recommendations
12. RB must implement promptly and with due diligence any recommendations that the ACCC (within 2 months of being provided the documents listed in paragraph 11 above) may make, and that the ACCC deems reasonably necessary to ensure that RB maintains and continues to implement the Compliance Program in accordance with the requirements of this Order.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
NEW SOUTH WALES DISTRICT REGISTRY | |
GENERAL DIVISION | NSD 180 of 2015 |
BETWEEN: | AUSTRALIAN COMPETITION AND CONSUMER COMMISSION Applicant |
AND: | RECKITT BENCKISER (AUSTRALIA) PTY LTD (ACN 003 274 655) Respondent |
JUDGE: | EDELMAN J |
DATE: | 11 DECEMBER 2015 |
PLACE: | HEARD IN SYDNEY (DELIVERED IN BRISBANE VIA video LINK) |
REASONS FOR JUDGMENT
1 In this proceeding, the applicant, the ACCC, alleged that the respondent, Reckitt Benckiser, contravened the Australian Consumer Law in (i) the packaging of various Nurofen products in the Nurofen Specific Pain Range, and (ii) the website description of those products.
2 The essence of the case against Reckitt Benckiser concerns the packaging and website descriptions of four Nurofen Specific Pain Relief products, respectively for back pain, period pain, migraine, and tension headache. In broad terms, Reckitt Benckiser represented that each Nurofen product was specifically formulated to treat that particular type of pain. In fact, each product contains the same active ingredient and is of the same formulation. None of the four products is any more or less effective than the others in treating any of the particular symptoms.
3 After an adjournment at the commencement of the trial, Reckitt Benckiser admitted various contraventions of ss 18 and 33 of the Australian Consumer Law. The ACCC did not press others. With further co-operation, the parties agreed to the terms of proposed orders, other than those concerning pecuniary penalties. I foreshadowed yesterday that I would make orders broadly in those terms, with some amendments which I explained during the course of the hearing. These are my reasons for making the orders which are broadly in the terms I had indicated.
The breaches by Reckitt Benckiser of ss 18 and 33 of the Australian Consumer Law
4 Nurofen is an over-the-counter medicine for the temporary relief of pain. Its active ingredient is ibuprofen or chemical equivalents of ibuprofen. It has been sold in Australia in pharmacies since the late 1980s and in grocery stores since the early 2000s.
5 Since 2006, Nurofen has been marketed and sold by Reckitt Benckiser. Four of approximately 50 stock keeping Nurofen units are the following products:
(1) Nurofen Migraine Pain ibuprofen lysine 342mg tablet blister pack;
(2) Nurofen Tension Headache ibuprofen lysine 342mg tablet blister pack;
(3) Nurofen Period Pain ibuprofen lysine 342mg tablet blister pack; and
(4) Nurofen Back Pain ibuprofen lysine 342mg tablet blister pack.
6 The Australian Register of Therapeutic Goods (ARTG), which registers all Nurofen products, has an approved indication for the four products above as follows:
The temporary relief of pain and/or inflammation associated with headache (including migraine and tension headache), dental pain, period pain, arthritis, aches and pains associated with the common cold or flu, backache, sinus pain, muscular and rheumatic pain. Reduces fever.
7 The characteristics of the packaging of each of these Nurofen Specific Pain Range products include the following matters. These can be seen in graphic form in annexures A, B, C, and D to these reasons.
8 Each product in the Nurofen Specific Pain Range:
(1) is coloured differently;
(2) refers to a different pain condition;
(3) bears the statement “FAST TARGETED RELIEF FROM PAIN”;
(4) bears a statement that the product “…IS FAST AND EFFECTIVE IN THE TEMPORARY RELIEF OF PAIN ASSOCIATED WITH…” the relevant pain condition;
(5) in the case of Nurofen Migraine Pain and Nurofen Tension Headache, contains an instruction in relation to the usage of the product that is specifically referrable to the symptoms of the relevant pain condition; and
(6) states, expressly on the front of the pack, that the active ingredient is “ibuprofen lysine 342 mg (equiv ibuprofen 200mg)”.
9 As to the packaging of Nurofen Migraine Pain (Annexure A to these reasons), it
(1) is predominantly violet in colour;
(2) bears the words “MIGRAINE PAIN” on the main, side and end panels of the packaging;
(3) bears the statement “FAST TARGETED RELIEF FROM PAIN”;
(4) bears the statement “NUROFEN MIGRAINE PAIN IS FAST AND EFFECTIVE IN THE TEMPORARY RELIEF OF PAIN ASSOCIATED WITH: MIGRAINE HEADACHE”; and
(5) contains the instruction “DOSAGE: Take with water at first onset of migraine”.
10 As to the packaging of Nurofen Tension Headache (Annexure B to these reasons), it
(1) is predominantly burgundy in colour;
(2) bears the words “TENSION HEADACHE” on the main, side and end panels of the packaging;
(3) bears the statement “FAST TARGETED RELIEF FROM PAIN”;
(4) bears the statement “NUROFEN TENSION HEADACHE IS FAST AND EFFECTIVE IN THE TEMPORARY RELIEF OF PAIN ASSOCIATED WITH: TENSION HEADACHE”; and
(5) contains the instruction “DOSAGE: Take with water at first sign of tension headache”.
11 As to the packaging of Nurofen Period Pain (Annexure C to these reasons), it
(1) is predominantly magenta in colour;
(2) bears the words “PERIOD PAIN” on the main, side and end panels of the packaging;
(3) bears the statement “FAST TARGETED RELIEF FROM PAIN”; and
(4) bears the statement “NUROFEN PERIOD PAIN IS FAST AND EFFECTIVE IN THE TEMPRARY RELIEF OF PAIN ASSOCIATED WITH: PERIOD PAIN”.
12 As to the packaging of Nurofen Back Pain (Annexure D to these reasons), it
(1) is predominantly green in colour;
(2) Bears the words “BACK PAIN” on the main, side and end panels of the packaging;
(3) bears the statement “FAST TARGETED RELIEF FROM PAIN”; and
(4) bears the statement “NUROFEN BACK PAIN IS FAST AND EFFECTIVE IN THE TEMPORARY RELIEF OF PAIN ASSOCIATED WITH: BACK PAIN”.
13 It is now common ground that the statements above involved two Packaging Representations. The common ground accords with the preliminary view I had formed prior to this trial and which I am now satisfied is correct. The common ground is that by making the packaging statements described above, Reckitt Benckiser represented that
(1) each product in the Nurofen Specific Pain Range is specifically formulated to treat the particular type of pain specified on the packaging relevant to that product; and
(2) the product solely or specifically treats the particular type of pain specified on the packaging relevant to that product and not other types of pain.
14 These two Packaging Representations contravene s 18 of the Australian Consumer Law because they are misleading or deceptive or likely to mislead or deceive. The Packaging Representations also infringe s 33 of the Australian Consumer Law because they are liable to mislead the public as to the nature, the characteristics, or the suitability for purpose of the Nurofen Specific Pain Range products.
15 The reason why Packaging Representations for each product in the Nurofen Specific Pain Range infringe ss 18 and 33 is because:
(1) each product contains the same active ingredient;
(2) the ARTG approved indications for each product is the same;
(3) each product is of the same formulation; and
(4) no product is any more or less effective than the others in treating any of the symptoms shown on the packaging.
16 Between at least December 2012 and May 2014, the Nurofen Website contained a page with the heading “RELIEVE PAIN WITH THE RIGHT TYPES OF PAIN MEDICATION” (Product Comparator Page).
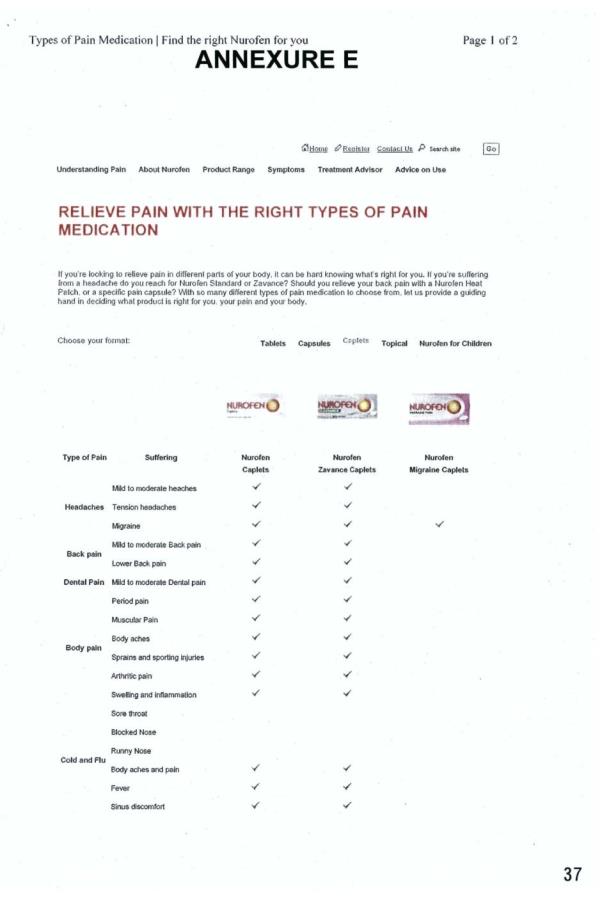
17 The Product Comparator Page can be seen in Annexure E to these reasons. As Annexure E shows, the Product Comparator Page contained:
(1) the statements “If you’re looking to relieve pain in different parts of your body, it can be hard knowing what’s right for you .... With so many different types of pain medication to choose from, let us provide a guiding hand in deciding what product is right for you, your pain and your body”; and
(2) a table which listed different types of pain and compared different Nurofen products, including the products in the Nurofen Specific Pain Range, for each type of pain (Product Comparator Table).
18 The Product Comparator Table contained the following:
(1) in the first and second columns under the headings “Type of Pain” and “Suffering” (respectively), a list of different types of pain and their descriptions, including:
(a) Headaches – mild to moderate headaches; tension headaches; migraine;
(b) Back pain – mild to moderate back pain; lower back pain; and
(c) Body pain – period pain; muscular pain; body aches; sprains and sporting injuries; arthritic pain; swelling and inflammation.
(2) in the fifth, sixth, seventh and eighth columns, the headings “Nurofen Migraine Caplets”, “Nurofen Tension Headache Caplets”, “Nurofen Back Pain Caplets”, and “Nurofen Period Pain Caplets” (respectively); and
(3) “tick” symbols where the following columns intersected with the following rows (respectively):
(a) “Nurofen Tension Headache Caplets” with Headaches/tension headaches;
(b) “Nurofen Back Pain Caplets” with Back pain/mild to moderate back pain/lower back pain;
(c) “Nurofen Period Pain Caplets” with Body pain/period pain; and
(d) “Nurofen Migraine Caplets” with Migraine.
19 Between at least December 2012 and May 2014, the Nurofen Website contained or displayed a page with the heading “SPECIFIC PAIN RELIEF” (Specific Pain Relief Page). An image of the Specific Pain Relief Page is annexed to these reasons as Annexure F.
20 It is common ground, again correctly, that the Nurofen website involved the same representations for the Nurofen Specific Pain Relief range as the packaging of the four products in that range (see [13] above). Again, those representations infringe ss 18 and 33 of the Australian Consumer Law for the reasons set out at [15] above.
Conclusion and the appropriate relief
21 The orders proposed by the parties, subject to some amendments made during the hearing, are appropriate. The declaratory relief is very closely tailored to the nature of the breaches. It serves to record the Court’s disapproval of the contravening conduct, vindicate the Commission’s claim, inform consumers of the contravening conduct and deter other corporations from contravention: see Australian Competition and Consumer Commission v The Construction, Forestry, Mining and Energy Union [2006] FCA 1730; [2007] ATPR 42-140 [6] (Nicholson J).
22 The injunctive relief – to restrain further breaches of this nature – is unambiguous. As senior counsel for Reckitt Benckiser cogently submitted, the injunction is appropriately confined to a period of three years for three reasons in combination: (i) the definite period avoids the potential consequences of contempt applying to Reckitt Benckiser indefinitely; (ii) corporate memory of an injunction and its terms will not be indefinite; and (iii) the nature of the breaches and my conclusions and reasons will be readily available for the foreseeable future.
23 The proposed corrective notice and corrective advertising and its terms are also appropriate. They have a clear nexus with the breaches. The corrective notice and advertising serves a protective purpose and an incidental educative effect: Medical Benefits Fund of Australia Limited v Cassidy [2003] FCAFC 238; (2003) 135 FCR 1, 20-22 [48]-[54] (Stone J). It is sufficient to fulfil the function of the notice and advertising, and consistent with other similar authorities, for
(1) the corrective advertising to be published in a single widespread national newspaper (the Australian newspaper) since the contravening conduct in this case is national and the publicity effect would not be vastly different from a considerably greater burden imposed on Reckitt Benckiser of advertising in separate newspapers in each State or Territory; and
(2) the format of the advertisement, involving a notice which is a third of a page with a 12 point headline and 11 point text is sufficient to bring the matter to the attention of consumers, particularly in circumstances where the notice is published in the first ten pages of the newspaper: Australian Competition and Consumer Commission v Actrol Parts Pty Limited [2015] FCA 312 [42] (Besanko J).
24 As to the order which amends Reckitt Benckiser’s existing compliance programme, this will reduce or remove the prospect of any future breach. It might be arguable, and was initially argued by Reckitt Benckiser, that there is no great utility in a compliance order in circumstances in which Reckitt Benckiser (i) has no history of non-compliance with the Australian Consumer Law, and (ii) has admitted the breaches in this case. However, there are four reasons why the orders are appropriate. First, the breaches in this case are not trivial or inconsequential. Secondly, there is no evidence that compliance orders will impose any substantial burden upon Reckitt Benckiser. Thirdly, Reckitt Benckiser has consented to such orders. Fourthly, the addition to Reckitt Benckiser’s existing compliance programme serves a prophylactic purpose which is based upon the circumstances of this case: Australian Competition and Consumer Commission v RL Adams Pty Ltd [2015] FCA 1016 [88].
I certify that the preceding twenty-four (24) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Edelman. |
Associate:
ANNEXURE A

ANNEXURE B

ANNEXURE C

ANNEXURE D

ANNEXURE E



ANNEXURE F