FEDERAL COURT OF AUSTRALIA
Australian Competition and Consumer Commission v Pfizer Australia Pty Ltd [2015] FCA 113
IN THE FEDERAL COURT OF AUSTRALIA | |
AUSTRALIAN COMPETITION AND CONSUMER COMMISSION Applicant | |
AND: | PFIZER AUSTRALIA PTY LTD (ACN 008 422 348) Respondent |
DATE OF ORDER: | |
WHERE MADE: |
THE COURT ORDERS THAT:
1. The Amended Originating Application filed by the Australian Competition and Consumer Commission is dismissed.
2. The Applicant is to pay the costs of the Respondent.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
NEW SOUTH WALES DISTRICT REGISTRY | |
GENERAL DIVISION | NSD 146 of 2014 |
BETWEEN: | AUSTRALIAN COMPETITION AND CONSUMER COMMISSION Applicant |
AND: | PFIZER AUSTRALIA PTY LTD (ACN 008 422 348) Respondent |
JUDGE: | FLICK J |
DATE: | 25 FEBRUARY 2015 |
PLACE: | SYDNEY |
REASONS FOR JUDGMENT
1 On 13 February 2014 the Australian Competition and Consumer Commission (the “ACCC”) filed in this Court an Originating Application and a Statement of Claim. On 8 September 2014, the ACCC filed an Amended Originating Application and an Amended Statement of Claim. The Respondent was Pfizer Australia Pty Ltd (“Pfizer”).
2 Pfizer has been a long-time participant in the supply of pharmaceutical products in Australia. It is ultimately owned by Pfizer Inc. Pfizer Inc is listed on the New York Stock Exchange and has its headquarters in New York. Pfizer researches, develops, manufactures and markets human prescription medicines as well as over-the-counter healthcare products and animal health products. Its human prescription medicines consist of:
medicines that are protected by patents; and
generic prescription medicines.
3 The pharmaceutical which assumes centre stage in the present proceeding is atorvastatin. Atorvastatin functions by blocking an enzyme in the liver which the human body uses to make cholesterol and results in lower levels of cholesterol.
4 Warner Lambert LLC was the registered owner, within the meaning of the Patents Act 1990 (Cth) (the “Patents Act”), of the atorvastatin patent. That company has been owned by Pfizer since about 2000. The patent was in effect from 18 May 1987 to 18 May 2012. From 1988 until 2000 Warner Lambert LLC utilised the Australian patent and supplied a pharmaceutical product under the name “Lipitor atorvastatin” (“Lipitor”) to community pharmacies. From 2000 Pfizer was the exclusive supplier of Lipitor.
5 The patent gave Pfizer exclusive rights prior to its expiration, but other manufacturers of pharmaceutical products could manufacture and supply their own generic atorvastatin once the patent expired. Not surprisingly, there were many documents circulating within Pfizer modelling the effect of the expiration of the patent upon the revenue of Pfizer. One such document in June 2009 estimated that the value of its sales of Lipitor would fall from $771 million in 2011 to $70 million in 2015. The document revealed its projected loss of net sales and revenue and the increased impact of generic manufacturers of atorvastatin over that five year period as follows:
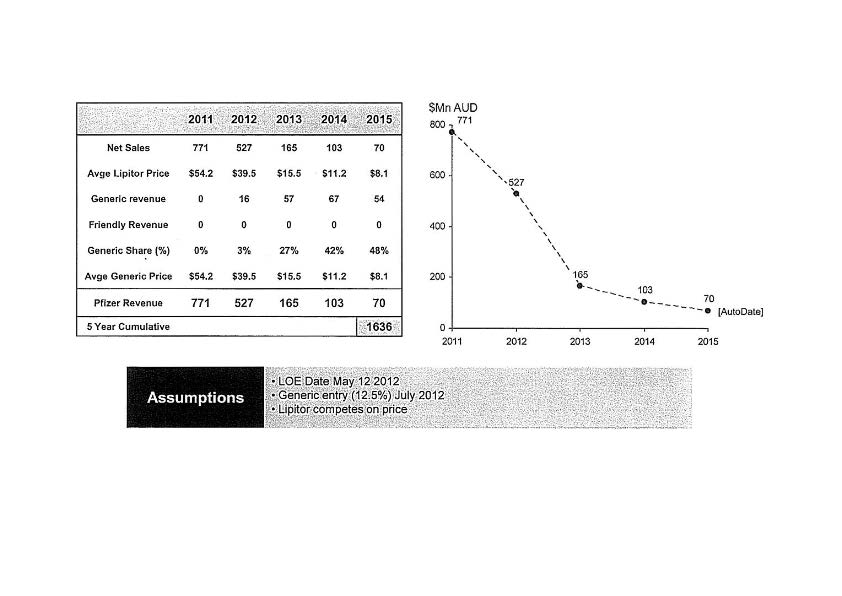
Immediately prior to the expiration of its patent, the only other supplier of atorvastatin was Ranbaxy Australia Pty Ltd (“Ranbaxy”). Ranbaxy was able to supply atorvastatin as from 19 February 2012 as the result of a settlement agreement arising out of unrelated proceedings. Also of concern to Pfizer was the fact that a number of patents in respect to other pharmaceutical products which it had the right to exploit were also coming to an end in the immediate future.
6 In anticipation of the competition it would face in 2012, Pfizer took a number of steps. In very summary form, those steps included:
the announcement in December 2010 that it would cease supplying prescription pharmaceuticals through wholesalers and would itself commence marketing and supplying prescription pharmaceuticals exclusively direct to community pharmacies (the “Direct-to-Pharmacy Model”);
the establishment in January 2011 of an accrual funds scheme whereby funds would accrue to each community pharmacy to which it supplied prescription pharmaceuticals (the “Accrual Funds Scheme”). A percentage of the price of purchases of Pfizer pharmaceutical products was credited to an account created for each pharmacy to be rebated on terms which would be announced at a later date; and
the making of an offer in January 2012 to all, or virtually all, community pharmacies as to the terms upon which it would supply Lipitor and its own generic atorvastatin (Atorvastatin Pfizer) which, inter alia, tied the rebates that were available from the accrual fund to the quantity of Atorvastatin Pfizer that the pharmacy purchased.
The strategy developed by Pfizer became known as “Project LEAP”. Although the emphasis placed upon protecting the “branded” product of Lipitor – as opposed to promoting Atorvastatin Pfizer – was the subject of continued consideration during the evolution of Project LEAP, a continuing concern of Pfizer throughout was the manner in which it would manage the inevitable “switching” of a patient’s use of atorvastatin from Lipitor to Atorvastatin Pfizer and from one generic version of the pharmaceutical to another. Intense competition from the generic manufacturers was inevitable; a principal concern within Pfizer was how best to manage the erosion of the revenue it enjoyed from sales of atorvastatin whilst it enjoyed exclusive rights by reason of its patent.
7 The ACCC claims that the conduct engaged in by Pfizer contravened ss 46 and 47 of the Competition and Consumer Act 2010 (Cth) (the “Competition and Consumer Act”). In very summary form, the ACCC claims (inter alia) that Pfizer held a substantial degree of market power and took advantage of that power for a purpose proscribed by s 46(1)(c). The ACCC further claims that Pfizer engaged in a course of exclusive dealing pursuant to s 47(1)(d) and (e) and did so for the proscribed purpose of lessening competition. Declaratory relief is sought together with orders for the imposition of pecuniary penalties.
8 The hearing in October 2014 dealt solely with whether a contravention of the Competition and Consumer Act had occurred. Any question as to penalty was deferred.
9 That hearing took some 14 days.
10 There was, not unexpectedly, a frequently recurring debate as to the definition of the “market” in respect to which the further findings required by ss 46 and 47 were to be made. The ACCC maintained that the “market” was the Australia-wide “market” for the supply of atorvastatin to, and the acquisition of atorvastatin by, community pharmacies (the “atorvastatin market”). The Pfizer submission was that the “market” was one for the wholesale supply of pharmaceutical products and over-the-counter products to community pharmacies (the “generics market”). Separate from the expected debate as to the proper characterisation of the market, however, was a further issue which fundamentally divided the parties – namely, the period of time under scrutiny for the purposes of ascertaining whether a contravention had occurred. The ACCC maintained that the period of time extended from December 2010 to at least May 2012; Pfizer maintained that the period of time had been confined by paragraphs [67] and [67A] of the Amended Statement of Claim to the period from January to May 2012.
11 It has been concluded that the ACCC definition of the relevant “market” should be accepted, namely that the “market” is the “atorvastatin market”. Pfizer’s submission that the “market”, defined in its Defence as the “Wholesale Market” – but also variously referred to as the “generics market” – is rejected. The “atorvastatin market” was the relevant “market” – irrespective of whether the inquiry is focussed on the period spanning December 2010 to at least May 2012 or that period of time between January and May 2012.
12 The fundamental division between the ACCC and Pfizer as to the period of time at which various factual inquiries were to be resolved made the balance of the fact-finding exercise in respect to both ss 46 and 47 more complex. Given this division, it has been considered prudent to make relevant findings which seek to address the principal further submissions advanced on behalf of both the ACCC and Pfizer. Within that context, it has been thus concluded that:
if the period of time to be considered is that from December 2010 through to about December 2011, Pfizer had a substantial degree of market power in the atorvastatin market and took advantage of that market power by implementing its Direct-to-Pharmacy Model, establishing the Accrual Fund Scheme but not in offering Atorvastatin Pfizer on the terms that it did;
and further concluded that:
the market power possessed by Pfizer during the period from January to May 2012 was significantly less than that which it previously enjoyed and that as from January 2012 Pfizer’s power in the atorvastatin market could no longer be described as “substantial”.
13 Whatever be the period of time properly the subject of inquiry, however, and whatever may have been the degree of market power it possessed, it has been further concluded that Pfizer did not exercise that power for a purpose proscribed by ss 46 or 47.
14 But all such findings in respect to s 46 potentially assume only secondary importance because of the manner in which the ACCC pleaded the “contraventions” of s 46 in paragraphs [67] and [67A] of the Amended Statement of Claim. Those paragraphs formed the basis of the Pfizer submission:
that the period of time the subject of inquiry in this proceeding had been confined to January to May 2012; and (more importantly)
that the manner in which the “contraventions” had been pleaded was “legally incoherent”.
The latter submission is well-founded. As pleaded, it has been further concluded that, even if the material facts pleaded in paragraphs [67] and [67A] of the Amended Statement of Claim were proved, they cannot constitute a contravention of s 46 as pleaded.
15 It was, nonetheless, considered inappropriate to resolve the ACCC’s contentions regarding the alleged contraventions of s 46 on a “pleading point”. The factual and legal issues were pursued and addressed in detail during the course of the proceeding. To a significant extent, the findings of fact which have been made as to the two periods of time contended for by the ACCC and Pfizer have really assumed lesser significance by reason of the findings made as to whether or not Pfizer was pursuing a proscribed “purpose”. But different minds may reach different conclusions both in respect to the manner in which the case was confined by paragraphs [67] and [67A] and in respect to the period of time properly the subject of inquiry. Although it is readily recognised that the present reasons for decision could have been much shorter, the course of making more findings of fact than is strictly necessary may ultimately prove to be of some future assistance to the parties.
16 However the claims advanced on behalf of the ACCC are to be approached, it is concluded that the Amended Originating Application should be dismissed with costs.
17 In explaining the reasons for reaching this conclusion it is necessary at the outset to outline both:
the terms of ss 46 and 47 and the general principles to be applied in respect to those provisions, together with other relevant statutory provisions; and
the manner in which pharmaceutical products are supplied in Australia.
It is thereafter necessary to:
set forth the manner in which Pfizer developed its strategy to respond to the looming loss of exclusivity on a number of its patented products, including Lipitor.
It is against this background:
that findings will be made in respect to both the contraventions alleged in respect to both ss 46 and 47.
The Competition and Consumer Act – Sections 46 & 47
18 The provisions of the Competition and Consumer Act which are alleged to have been contravened are ss 46 and 47. Penalties are sought pursuant to s 76.
19 There was no real dispute between the parties as to the meaning and ambit of operation of these provisions. The real dispute was whether the facts fell within these provisions. It nevertheless remains important to set forth some accepted principles in respect to each provision.
20 In respect to both ss 46 and 47 the onus lies upon the ACCC to prove the contraventions alleged: cf. John S Hayes & Associates Pty Limited v Kimberly-Clark Australia Pty Limited (1994) ATPR 41-318 at 42-237 per Hill J. See also: Stirling Harbour Services Pty Ltd v Bunbury Port Authority [2000] FCA 38 at [115], (2000) ATPR 41-752 at 40,732 per French J (as his Honour then was).
Section 46 – misuse of market power
21 Section 46 provides in relevant part as follows:
Misuse of market power
(1) A corporation that has a substantial degree of power in a market shall not take advantage of that power in that or any other market for the purpose of:
(a) eliminating or substantially damaging a competitor of the corporation or of a body corporate that is related to the corporation in that or any other market;
(b) preventing the entry of a person into that or any other market; or
(c) deterring or preventing a person from engaging in competitive conduct in that or any other market.
Section 46(6A) and (7) give further content to s 46(1):
(6A) In determining for the purposes of this section whether, by engaging in conduct, a corporation has taken advantage of its substantial degree of power in a market, the court may have regard to any or all of the following:
(a) whether the conduct was materially facilitated by the corporation's substantial degree of power in the market;
(b) whether the corporation engaged in the conduct in reliance on its substantial degree of power in the market;
(c) whether it is likely that the corporation would have engaged in the conduct if it did not have a substantial degree of power in the market;
(d) whether the conduct is otherwise related to the corporation's substantial degree of power in the market.
This subsection does not limit the matters to which the court may have regard.
(7) Without in any way limiting the manner in which the purpose of a person may be established for the purposes of any other provision of this Act, a corporation may be taken to have taken advantage of its power for a purpose referred to in subsection (1) notwithstanding that, after all the evidence has been considered, the existence of that purpose is ascertainable only by inference from the conduct of the corporation or of any other person or from other relevant circumstances.
Further, Section 46(3) provides:
In determining for the purposes of this section the degree of power that a body corporate or bodies corporate has or have in a market, the court shall have regard to the extent to which the conduct of the body corporate or of any of those bodies corporate in that market is constrained by the conduct of:
(a) a competitors, or potential competitors, of the body corporate or of any of those bodies corporate in that market; or
(b) persons to whom or from whom the body corporate or any of those bodies corporate supplies or acquires goods or services in that market.
Reference should also be made to s 46(3A), (3B) and (3C) which provide additional guidance on determining the degree of power in a market that a body holds.
22 The provision which assumes central relevance to the ACCC’s case is s 46(1)(c).
23 At the heart of s 46 is the need to identify the “market” under consideration.
24 It is thereafter necessary to determine whether a corporation has “power in a market”, whether that power is “substantial” and whether the corporation has taken “advantage of that power” for a proscribed “purpose”.
25 Each of these elements of s 46 has attracted its own learning.
Section 47 – exclusive dealing
26 Section 47 relevantly provides as follows:
Exclusive dealing
(1) Subject to this section, a corporation shall not, in trade or commerce, engage in the practice of exclusive dealing.
(2) A corporation engages in the practice of exclusive dealing if the corporation:
(a) supplies, or offers to supply, goods or services;
(b) supplies, or offers to supply, goods or services at a particular price; or
(c) gives or allows, or offers to give or allow, a discount, allowance, rebate or credit in relation to the supply or proposed supply of goods or services by the corporation;
on the condition that the person to whom the corporation supplies, or offers or proposes to supply, the goods or services or, if that person is a body corporate, a body corporate related to that body corporate:
(d) will not, or will not except to a limited extent, acquire goods or services, or goods or services of a particular kind or description, directly or indirectly from a competitor of the corporation or from a competitor of a body corporate related to the corporation;
(e) will not, or will not except to a limited extent, re-supply goods or services, or goods or services of a particular kind or description, acquired directly or indirectly from a competitor of the corporation or from a competitor of a body corporate related to the corporation; or
(f) in the case where the corporation supplies or would supply goods or services, will not re-supply the goods or services to any person, or will not, or will not except to a limited extent, re-supply the goods or services:
(i) to particular persons or classes of persons or to persons other than particular persons or classes of persons; or
(ii) in particular places or classes of places or in places other than particular places or classes of places.
…
(10) Subsection (1) does not apply to the practice of exclusive dealing constituted by a corporation engaging in conduct of a kind referred to in subsection (2), (3), (4) or (5) or paragraph (8)(a) or (b) or (9)(a), (b) or (c) unless:
(a) the engaging by the corporation in that conduct has the purpose, or has or is likely to have the effect, of substantially lessening competition; or
(b) the engaging by the corporation in that conduct, and the engaging by the corporation, or by a body corporate related to the corporation, in other conduct of the same or a similar kind, together have or are likely to have the effect of substantially lessening competition.
Section 47(13) goes on to provide the following definitions:
In this section:
(a) a reference to a condition shall be read as a reference to any condition, whether direct or indirect and whether having legal or equitable force or not, and includes a reference to a condition the existence or nature of which is ascertainable only by inference from the conduct of persons or from other relevant circumstances;
(b) a reference to competition, in relation to conduct to which a provision of this section other than subsection (8) or (9) applies, shall be read as a reference to competition in any market in which:
(i) the corporation engaging in the conduct or any body corporate related to that corporation; or
(ii) any person whose business dealings are restricted, limited or otherwise circumscribed by the conduct or, if that person is a body corporate, any body corporate related to that body corporate;
supplies or acquires, or is likely to supply or acquire, goods or services or would, but for the conduct, supply or acquire, or be likely to supply or acquire, goods or services; and
(c) a reference to competition, in relation to conduct to which subsection (8) or (9) applies, shall be read as a reference to competition in any market in which the corporation engaging in the conduct or any other corporation the business dealings of which are restricted, limited or otherwise circumscribed by the conduct, or any body corporate related to either of those corporations, supplies or acquires, or is likely to supply or acquire, goods or services or would, but for the conduct, supply or acquire, or be likely to supply or acquire, goods or services.
Section 4G further explains the meaning of the statutory concept of “lessening of competition” as follows:
Lessening of competition to include preventing or hindering competition
For the purposes of this Act, references to the lessening of competition shall be read as including references to preventing or hindering competition.
27 The provisions which are of central importance to the ACCC’s case are s 47(2)(d) and (e).
28 Section 47(1), it should be noted at the outset, “appears to have a wide application”: Parmalat Australia Pty Ltd v VIP Plastic Packaging Pty Ltd [2013] FCA 119 at [25], (2013) 210 FCR 1 at 13 per Collier J.
The market
29 It is the identification of the relevant “market” which sets the perimeters for the balance of the requirements of s 46 and which also assumes central relevance to s 47.
30 The Trade Practices Act 1974 (Cth) (the “Trade Practices Act”), when first introduced, simply defined a “market” as meaning “a market in Australia”. It was the Trade Practices Amendment Act 1977 (Cth) which inserted a form of the definition carried forward into s 4E of the Competition and Consumer Act. That provision was subsequently amended by the Trade Practices (Misuse of Trans-Tasman Market Power) Act 1990 (Cth). It now provides as follows:
For the purposes of this Act, unless the contrary intention appears, market means a market in Australia and, when used in relation to any goods or services, includes a market for those goods or services and other goods or services that are substitutable for, or otherwise competitive with, the first-mentioned goods or services.
The section does not purport to provide an exhaustive definition of the term “market” – “it merely states that a market includes goods and services substitutable for, or otherwise competitive with, the goods or services under consideration”: SPAR Licensing Pty Ltd v MIS Qld Pty Ltd (No 2) [2012] FCA 1116 at [42], (2012) 298 ALR 69 at 80 per Griffiths J. This decision was reversed by the Full Court but without any comment on the accuracy of the statement: SPAR Licensing Pty Ltd v MIS Qld Pty Ltd [2014] FCAFC 50, (2014) 314 ALR 35.
31 Given its central relevance to trade practices and consumer protection legislation, there have been repeated judicial expositions regarding the concept of a “market”. Notwithstanding the number of occasions upon which the concept has been discussed, a brief reference to some of these authorities serves as a useful reminder of the task to be undertaken by a court when resolving a question as to the definition of a “market” in any particular case.
32 Thus, for example, in an oft-cited passage, the Trade Practices Tribunal in Re Queensland Co-Operative Milling Association Ltd (1976) 25 FLR 169 at 190, 8 ALR 481 at 517, said:
… We take the concept of a market to be basically a very simple idea. A market is the area of close competition between firms or, putting it a little differently, the field of rivalry between them. (If there is no close competition there is of course a monopolistic market.) Within the bounds of a market there is substitution – substitution between one product and another, and between one source of supply and another, in response to changing prices. So a market is the field of actual and potential transactions between buyers and sellers amongst whom there can be strong substitution, at least in the long run, if given a sufficient price incentive. Let us suppose that the price of one supplier goes up. Then on the demand side buyers may switch their patronage from this firm's product to another, or from this geographic source of supply to another. As well, on the supply side, sellers can adjust their production plans, substituting one product for another in their output mix, or substituting one geographic source of supply for another. Whether such substitution is feasible or likely depends ultimately on customer attitudes, technology, distance, and cost and price incentives.
It is the possibilities of such substitution which set the limits upon a firm's ability to “give less and charge more”. Accordingly, in determining the outer boundaries of the market we ask a quite simple but fundamental question: If the firm were to “give less and charge more” would there be, to put the matter colloquially, much of a reaction? And if so, from whom? In the language of economics the question is this: From which products and which activities could we expect a relatively high demand or supply response to price change, i.e. a relatively high cross-elasticity of demand or cross-elasticity of supply?
See also: Australian Competition and Consumer Commission v Australia and New Zealand Banking Group Limited [2013] FCA 1206 at [554] per Dowsett J.
33 In Queensland Wire Industries Proprietary Limited v Broken Hill Proprietary Company Limited (1989) 167 CLR 177 at 187-188 Mason CJ and Wilson J observed:
The analysis of a s. 46 claim necessarily begins with a description of the market in which the defendant is thought to have a substantial degree of power. In identifying the relevant market, it must be borne in mind that the object is to discover the degree of the defendant’s market power. Defining the market and evaluating the degree of power in that market are part of the same process, and it is for the sake of simplicity of analysis that the two are separated. Accordingly, if the defendant is vertically integrated, the relevant market for determining degree of market power will be at the product level which is the source of that power: see the discussion of market power below. After identifying the appropriate product level, it is necessary to describe accurately the parameters of the market in which the defendant’s product competes: too narrow a description of the market will create the appearance of more market power than in fact exists; too broad a description will create the appearance of less market power than there is.
Section 4E directs that a market is to be described to include not just the defendant’s product but also those which are “substitutable for, or otherwise competitive with”, the defendant’s product. This process of defining a market by substitution involves both including products which compete with the defendant’s and excluding those which because of differentiating characteristics do not compete.
Deane J said of the concept of “market”:
The identification of relevant markets and the definition of market structures and boundaries for the purposes of determining [the case then under consideration] involves value judgments about which there is some room for legitimate differences of opinion. The economy is not divided into an identifiable number of discrete markets into one or other of which all trading activities can be neatly fitted. One overall market may overlap other markets and contain more narrowly defined markets which may, in their turn, overlap, the one with one or more others. The outer limits (including geographic confines) of a particular market are likely to be blurred: their definition will commonly involve assessment of the relative weight to be given to competing considerations in relation to questions such as the extent of product substitutability and the significance of competition between traders at different stages of distribution. While actual competition must exist and be assessed in the context of a market, a market can exist if there be the potential for close competition even though none in fact exists. A market will continue to exist even though dealings in it be temporarily dormant or suspended. Indeed, for the purposes of the Act, a market may exist for particular existing goods at a particular level if there exists a demand for (and the potential for competition between traders in) such goods at that level, notwithstanding that there is no supplier of, nor trade in, those goods at a given time — because, for example, one party is unwilling to enter any transaction at the price or on the conditions set by the other. It is, however, unnecessary to pursue that question for the purposes of the present appeal: (1989) 167 CLR at 195-196.
In the same case, Dawson J made the following observations:
Lying behind [the questions regarding a defendant’s degree of market power and what would constitute taking advantage of that market power] is the concept of the market, a concept which is sometimes dealt with in a more complex manner than is necessary. A market is an area in which the exchange of goods or services between buyer and seller is negotiated. It is sometimes referred to as the sphere within which price is determined and that serves to focus attention upon the way in which the market facilitates exchange by employing price as the mechanism to reconcile competing demands for resources … In setting the limits of a market the emphasis has historically been placed upon what is referred to as the “demand side”, but more recently the “supply side” has also come to be regarded as significant. The basic test involves the ascertainment of the cross-elasticities of both supply and demand, that is to say, the extent to which the supply of or demand for a product responds to a change in the price of another product. Cross-elasticities of supply and demand reveal the degree to which one product may be substituted for another, an important consideration in any definition of a market … : (1989) 167 CLR at 198-199.
His Honour continued:
Important as they are, elasticities and the notion of substitution provide no complete solution to the definition of a market. A question of degree is involved — at what point do different goods become closely enough linked in supply or demand to be included in the one market — which precludes any dogmatic answer … The process is an inexact one as may be illustrated by reference to the concept of a sub-market which has been employed from time to time. In Re Queensland Co-operative Milling Association Ltd … the Trade Practices Tribunal observed:
“The distinction between markets and sub-markets can be merely one of degree. Sub-markets are the more narrowly defined, typically registering some discontinuity in substitution possibilities. Where the defining feature of a market is the existence of close substitutes (whether in demand or supply), the defining feature of a sub-market is the existence of still closer and more immediate substitutes. Sub-markets may be especially useful in registering the short-run effects of change; but they may be misleading if used uncritically to assess long run competitive effects.”
Too rigid an approach in defining a market is apt to lead to unrealistic results … But the existence or non-existence of sales of a product cannot conclude whether a market exists or not. It must be sufficient to constitute a market that there is a product for exchange, regardless of whether exchange or negotiation for exchange has actually taken place.
In truth, the need to define the relevant market arises only because the extent of market power cannot be assessed otherwise than by reference to a market: (1989) 167 CLR at 199-200.
34 Similarly, Wilcox J in Trade Practices Commission v Australia Meat Holdings Pty Ltd (1988) 83 ALR 299 at 317 adopted the submission which defined a “market” as follows:
“A market is the field of activity in which buyers and sellers interact, and the identification of market boundaries requires consideration of both the demand and supply side. The ideal definition of a market must take into account substitution possibilities in both consumption and production. The existence of price differentials between different products, reflecting differences in quality or other characteristics of the products, does not by itself place the products in different markets. The test of whether or not there are different markets is based on what happens (or would happen) on either the demand or the supply side in response to a change in relative price.”
35 Finally, reference should also be made to Australian Gas Light Company v Australian Competition and Consumer Commission [2003] FCA 1525, (2003) 137 FCR 317 at 426-427 where French J (as his Honour then was) observed:
[378] The concept of market describes, in a metaphorical way, an area or space of economic activity whose dimensions are function, product and geography. A market may be defined functionally by reference to wholesale or retail activities or a combination of both. The concept of product encompasses goods and services and, having regard to the definition of “market” in s 4E, includes the range of goods or services which are substitutable for or competitive with each other.
[379] The process of market definition was expounded in QCMA where the Tribunal defined “market” as the area of close competition between firms and observed that substitution occurs within a market between one product and another, and between one source of supply and another in response to changing prices (at 190):
So a market is the field of actual and potential transactions between buyers and sellers amongst whom there can be strong substitution, at least in the long run, if given a sufficient price incentive.
In Re Tooth & Co Ltd (1979) 39 FLR 1, the Tribunal identified the task of market analysis as involving:
1. Identification of the relevant area or areas of close competition.
2. Application of the principle that competition may proceed through substitution of supply source as well as product.
3. Delineation of a market which comprehends the maximum range of business activities and the widest geographic area within which, if given a sufficient economic incentive, buyers can switch to a substantial extent from one source of supply to another and sellers can switch to a substantial extent from one production plan to another.
4. Consideration of longrun substitution possibilities rather than shortrun and transitory situations recognising that the market is the field of actual or potential rivalry between firms.
5. Selection of market boundaries as a matter of degree by identification of such a break in substitution possibilities that firms within the boundary would collectively possess substantial market power so that if operating as a cartel they could raise prices or offer lesser terms without being substantially undermined by the incursions of rivals.
6. Acceptance of the proposition that the field of substitution is not necessarily homogeneous but may contain submarkets in which competition is especially close or especially immediate. This is subject to the qualification that competitive relationships in key submarkets may have a wide effect upon the functioning of the market as a whole.
7. Identification of the market as multidimensional involving product, functional level, space and time.
Of particular importance in respect to each of these expositions of the concept of “market” are the continued references to goods or services which are “substitutable for or competitive with each other” and the focus upon the identification of a “field of actual and potential transactions between buyers and sellers amongst whom there can be strong substitution, at least in the long run …”. See also: Karsh, ‘The Role of Supply Substitutability in Defining the Relevant Product Market’, (1979) 65 Virginia L Rev 129.
36 However the concept of a “market” may be defined, it must be recognised that “a market is not an artificial economic construct but rather a place, actual or nominal but recognisable not just by economists but also by its participants, be they suppliers or consumers, in which forces of supply and demand interact in the conduct of trade, a profession or commerce”: Australian Competition and Consumer Commission v Flight Centre Ltd [2013] FCA 1313 at [108], (2013) 307 ALR 209 at 234 per Logan J.
37 With specific reference to the terms of s 4E, it has long been recognised that the section refers to both a market in which goods are “substitutable” and “otherwise competitive with” other goods. The “better view” as to the construction of s 4E, it has been said, “is that s 4E addresses constraints upon the supply or acquisition of the relevant goods or services” and in that context “the word ‘substitutable’ is used in a narrow sense whilst the words ‘or otherwise competitive with’ include degrees of ‘substitutability’”: Seven Network Ltd v News Ltd [2009] FCAFC 166 at [621], (2009) 182 FCR 160 at 295 per Dowsett and Lander JJ. However construed, s 4E confirms that the concept of substitution is basic to the process of market definition: Australian Competition and Consumer Commission v Air New Zealand Limited [2014] FCA 1157 at [213] per Perram J.
A substantial degree of power in a market – s 46(1)
38 Section 46(1) places constraints on a corporation which “has a substantial degree of power in a market…”.
39 “Market power”, it has been said, “means capacity to behave in a certain way (which might include setting prices, granting or refusing supply, arranging systems of distribution), persistently, free from the constraints of competition”: Melway Publishing Pty Limited v Robert Hicks Pty Limited [2001] HCA 13 at [67], (2001) 205 CLR 1 at 27 per Gleeson CJ, Gummow, Hayne and Callinan JJ.
40 One of the considerations relevant to determining whether a corporation has “market power”, indeed a “primary consideration”, is whether there are “barriers to entry”: Eastern Express Pty Limited v General Newspapers Pty Limited (1992) 35 FCR 43 at 62. Lockhart and Gummow JJ there observed:
Market power is concerned with power which enables a corporation to behave independently of competition and of the competitive forces in a relevant market.
The primary consideration in determining market power must be taken to be whether there are barriers to entry into the relevant market. …
The existence of a patent is but one of the factors that may create a barrier to entry: cf. Australian Competition and Consumer Commission v Boral Ltd [1999] FCA 1318 at [140], (1999) 166 ALR 410 at 438 per Heerey J.
41 Although the meaning of the term “substantial” may still remain the subject of “inconclusive debate” (cf. Rural Press Ltd v Australian Competition and Consumer Commission [2002] FCAFC 213 at [98], (2002) 118 FCR 236 at 264 per Whitlam, Sackville and Gyles JJ), the same term as used in s 45D of the Trade Practices Act was the subject of the following comments by Bowen CJ in Tillmanns Butcheries Pty Ltd v Australasian Meat Industry Employees' Union (1979) 42 FLR 331 at 338-339:
The next question is whether the conduct was engaged in for the purpose of causing substantial loss or damage to the business of Tillmanns. To constitute a contravention of s. 45D the purpose of causing substantial loss or damage must exist. …
The word “substantial” would certainly seem to require loss or damage that is more than trivial or minimal. According to one meaning of the word the loss or damage would have to be considerable … However, the word is quantitatively imprecise; it cannot be said that it requires any specific level of loss or damage. No doubt in the context in which it appears the word imports a notion of relativity, that is to say, one needs to know something of the circumstances of the business affected before one can arrive at a conclusion whether the loss or damage in question should be regarded as substantial in relation to that business.
Deane J there also observed:
The word “substantial” is not only susceptible of ambiguity: it is a word calculated to conceal a lack of precision. In the phrase “substantial loss or damage”, it can, in an appropriate context, mean real or of substance as distinct from ephemeral or nominal. It can also mean large, weighty or big. …
In the context of s. 45D (1) of the Act, the word “substantial” is used in a relative sense in that, regardless of whether it means large or weighty on the one hand or real or of substance as distinct from ephemeral or nominal on the other, it would be necessary to know something of the nature and scope of the relevant business before one could say that particular actual or potential loss or damage was substantial. As at present advised, I incline to the view that the phrase, substantial loss or damage, in s. 45D (1) includes loss or damage that is, in the circumstances, real or of substance and not insubstantial or nominal. It is, however, unnecessary that I form or express any concluded view in that regard since the ultimate conclusion which I have reached is the same regardless of which of the alternative meanings to which reference has been made is given to the word "substantial" in s. 45D (1): (1979) 42 FLR at 348.
42 A corporation does not, however, have a substantial degree of power in a market if it is unable to sustain its conduct over a reasonable period of time. Thus, for example, in Boral Besser Masonry Limited v Australian Competition and Consumer Commission [2003] HCA 5, (2003) 215 CLR 374 at 467-468 McHugh J observed:
[287] … Firms only have a substantial degree of market power when they can persistently act in a way over a reasonable time period unconstrained by the market's forces of supply and demand. Firms that do not have “the power to raise price above cost without losing so many sales as to make the price rise unsustainable” do not have market power. Cutting prices is not evidence of market power. Any firm can do that. Market power is an economic concept and should be given its ordinary meaning. As Professors Krattenmaker, Lande and Salop point out:
“When economists use the terms ‘market power’ or ‘monopoly power,’ they usually mean the ability to price at a supracompetitive level.”
[288] Market power also includes the power to sell less in terms of quality or quantity at the same price or to sell products on terms and conditions which a firm without market power would not be able to enforce — this being an element of market power that arises in conduct other than “predatory pricing”. But market power is not equivalent to the mere cutting of prices.
A little later his Honour further observed:
[293] The concept of “market power” in s 46 shows that the section is not concerned with a one-second snapshot of economic activity. Market power can only be determined by examining what a firm is capable of doing over a reasonable time period. Whether a firm has market power — whether it has the ability to act unconstrained by competition, whether it can raise prices above competitive levels — requires an examination of the existing structure and the likely structure of the market if competitors are removed or prices rise to supra-competitive levels. Such an analysis requires an examination of the business structure and practices of the alleged offender and its competitors, their market shares and the barriers to entry (if any) into the market …: (2003) 215 CLR at 470.
Gleeson CJ and Callinan J had previously made the same point as to an ability to cut prices not necessarily being an exercise of market power as follows:
[139] … There can be circumstances in which price-cutting may be undertaken by a powerful firm, or combination of firms. But the ability to cut prices is not market power. The power lies in the ability to target an outsider without fear of competitive reprisals from an established firm, and to raise prices again later.
Before citing the observations of McHugh J at [287] and [293], Wilcox, French and Gyles JJ in Universal Music Australia Pty Ltd v Australian Competition and Consumer Commission [2003] FCAFC 193 at [158], (2003) 131 FCR 529 at 568 observed:
… Market power is judged by reference to persistent rather than temporary conditions …
Purpose – ss 46(1) & 47(10)
43 Common to both ss 46(1) and 47(10) is the reference to “purpose”. Section 46(1)(c) proscribes (inter alia) a corporation taking advantage of its market power “for the purpose of deterring or preventing a person from engaging in competitive conduct …”; s 47(10) provides that certain forms of exclusive dealing by a corporation are prohibited (inter alia) if the conduct “has the purpose … of substantially lessening competition”.
44 Section 4F further defines what is meant by “purpose”. That section provides as follows:
References to purpose or reason
(1) For the purposes of this Act:
(a) a provision of a contract, arrangement or understanding or of a proposed contract, arrangement or understanding, or a covenant or a proposed covenant, shall be deemed to have had, or to have, a particular purpose if:
(i) the provision was included in the contract, arrangement or understanding or is to be included in the proposed contract, arrangement or understanding, or the covenant was required to be given or the proposed covenant is to be required to be given, as the case may be, for that purpose or for purposes that included or include that purpose; and
(ii) that purpose was or is a substantial purpose; and
(b) a person shall be deemed to have engaged or to engage in conduct for a particular purpose or a particular reason if:
(i) the person engaged or engages in the conduct for purposes that included or include that purpose or for reasons that included or include that reason, as the case may be; and
(ii) that purpose or reason was or is a substantial purpose or reason.
(2) This section does not apply for the purposes of subsections 45D(1), 45DA(1), 45DB(1), 45E(2) and 45E(3).
45 The “purposes” referred to in ss 46(1) and 47(10) are nevertheless differently expressed and invite different inquiries.
46 In respect to both provisions, however, “purpose” is to be ascertained subjectively and not objectively: ASX Operations Pty Ltd v Pont Data Australia Pty Ltd (No 1) (1990) 27 FCR 460 at 474-475. Lockhart, Gummow and von Doussa JJ there observed:
There was no dispute that in s 46 “purpose” was to be ascertained “subjectively” rather than “objectively”. Reliance was placed by ASX and ASXO upon what was said by Toohey J in Hughes v Western Australian Cricket Association (Inc) (1986) 19 FCR 10 at 37-38 where, after reviewing the authorities, his Honour said:
“I accept the view that it is the subjective purpose of those engaging in the relevant conduct with which the court is concerned. All other considerations aside, the use in s 45(2) of ‘purpose’ and ‘effect’ tends to suggest that a subjective approach is intended by the former expression. The application of a subjective test does not exclude a consideration of the circumstances surrounding the reaching of the understanding.”
47 “Purpose” is concerned with the “intent” of the corporation engaging in the impugned conduct and is not concerned directly with the effect of conduct: Dowling v Dalgety Australia Limited (1992) 34 FCR 109 at 143. Lockhart J there referred to a number of authorities, including ASX Operations, and observed:
The determination of purpose for the purposes of s 46 is to be ascertained subjectively, in the sense of ascertaining the intent of the corporation in engaging in the relevant conduct … “Purpose” in s 46 is not concerned directly with the effect of conduct, but with purpose in the sense of motivation and reason, though, as mentioned earlier, purpose may be inferred from conduct …
The same proposition was repeated by Lockhart and Gummow JJ in Eastern Express Pty Limited v General Newspapers Pty Limited (1992) 35 FCR 43 at 66 as follows:
If a corporation has a substantial degree of power in the relevant market the question then arises whether the corporation has taken advantage of that power for one or other of the purposes proscribed by s 46(1)(a), (b) or (c). It is permissible to infer the relevant purpose under s 46: s 46(7). Further, a corporation shall be deemed to have engaged in conduct for a particular purpose if it engaged in conduct for purposes that included that purpose, and that purpose is a substantial purpose: s 4F(b). The determination of purpose for the operation of s 46 is to be ascertained subjectively, in the sense that what is to be ascertained is the intent of the corporation engaging in the relevant conduct: … “Purpose” in s 46 is not concerned directly with the effect of conduct, but with "purpose" in the sense of motivation and reason, although, as mentioned earlier, purpose may be inferred from conduct …
Lockhart J had previously observed in Dowling that purpose “will generally be inferred from the nature of the contract, the circumstances in which it was made and its likely effect”: (1992) 34 FCR at 134.
48 What needs to be proved is the actual purpose of the relevant respondent: Universal Music Australia Pty Ltd v Australian Competition and Consumer Commission [2003] FCAFC 193 at [255], (2003) 131 FCR 529 at 588. Wilcox, French and Gyles JJ there went on to observe:
[256] Ascertaining the purpose for which conduct is engaged in by a party stands on quite a different footing. It would make no sense to consider that issue without paying regard to the direct and indirect evidence as to the actual intentions and purposes of the party. Of course, proof of the required purpose is not limited to direct evidence as to those purposes. Further, the court is not bound to accept such evidence. Indeed, it will normally be critically scrutinised; it is often ex post facto and self-serving. …
See also: Parmalat Australia Pty Ltd v VIP Plastic Packaging Pty Ltd [2013] FCA 119 at [28], (2013) 210 FCR 1 at 14 per Collier J.
49 More recently, the difference between “purpose” and “motive” has, perhaps, been slightly differently expressed. Gleeson CJ in News Limited v South Sydney District Rugby League Football Club Limited [2003] HCA 45 at [18], (2003) 215 CLR 563 at 573 observes:
[18] … The distinction between purpose and effect is significant. … In a case such as the present, it is the subjective purpose … that is to say, the end they had in view, that is to be determined. Purpose is to be distinguished from motive. The purpose of conduct is the end sought to be accomplished by the conduct. The motive for conduct is the reason for seeking that end. The appropriate description or characterisation of the end sought to be accomplished (purpose), as distinct from the reason for seeking that end (motive), may depend upon the legislative or other context in which the task is undertaken. …
See also: Seven Network Ltd v News Ltd [2009] FCAFC 166 at [851]-[858], (2009) 182 FCR 160 at 355-356 per Dowsett and Lander JJ.
50 One manner in which “purpose” may well be established is if a corporation exerts pressure so as to defeat a new entrant’s attempt to gain market share and a place in the market: Australian Competition and Consumer Commission v Liquorland (Australia) Pty Ltd [2006] FCA 826, (2006) ATPR 42,123. Allsop J (as his Honour then was) there observed:
[807] There is a danger in disembodying the debate about purpose from the evidence that is available. Even if a market is workably competitive or highly competitive, the appearance of a new entrant actively engaging in the winning of market share and recognition is the working of the competitive process. The effect of a new entrant may have a detrimental effect on the business and turnover of incumbents. That, of itself, will create competitive pressures and close competition to defeat the new entrant’s attempt to gain market share and a place in the market. There may be cases where a firm acting to prevent a new entrant can explain that by a desire divorced from competition and the competitive process. If a firm has a purpose to impede or prevent the entry of a new competitor into a market lest that new entrant conduct itself competitively to wrest business from the incumbent and so damage its business, that purpose involves the process of competition. It involves preventing entry into the market and preventing a state of affairs of lost sales through additional competitive activity. Such lost sales and damage to business will, in the ordinary course call for steps, if available, in response to meet the challenge of any new entrant. The available steps may be marginal if the market is already highly competitive. To say as much, however, is only to posit even closer, or more fierce, competition. If a purpose is to prevent or impede market entry and so to prevent competitive activity, that is sufficient it seems to me to amount to a purpose directed to the competitive process. One does not need to superadd a further purpose that the success of that purpose is to affect the degree of competitive activity as opposed merely to preserving the firm’s share of revenue in a mercantilist sense. The entry of competitors is an essential attribute of the competitive process. It is the means of access for competitive trading and for pressure on incumbent firms, through their revenue and profitability, to offer more or charge less in order to retain their places in the competitive (on this hypothesis increasingly competitive) market. If the grant of new licences were seen as a competitive threat they were so seen because they were a threat to business through competitive activity. A purpose to prevent or impede such competitive activity is a purpose concerned with the process and conduct of competition: (2006) ATPR at 45,303.
51 A proscribed purpose may be made out even though a corporation maintains that it is simply seeking to “win as much business as possible”: Australian Competition and Consumer Commission v Baxter Healthcare Pty Ltd (No 2) [2008] FCAFC 141 at [381], (2008) 170 FCR 16 at 100 per Gyles J. The Baxter corporation was the manufacturer and supplier of sterile fluids and PD fluids. It negotiated and tendered for long-term contracts with the governments of four States and the Australian Capital Territory. It required those governments to acquire all of their sterile fluids and between 90 and 92.5% of their PD fluids from Baxter. The “bundled” prices were significantly lower than the item-by-item tender. The Commission alleged that Baxter had taken advantage of its market power in the sterile fluids market because otherwise it would not have been able, under competitive conditions, to force the States to take the bundled offer by threatening prohibitive prices for sterile fluids. The primary Judge concluded that there had not been a contravention of s 46 but that there had been a contravention of s 47. On appeal it was concluded that Baxter had a “substantial degree of power” in the Australia-wide sterile fluids market. Mansfield and Gyles JJ further concluded that Baxter’s conduct fell within s 46(1)(c). In rejecting the argument that Baxter’s conduct did not fall within s 46(1)(c), Gyles J concluded:
[381] Baxter contends that its aim was solely to win as much business as it could across the board and that its conduct was directed to achieving that result rather than harming competitors. In my view, that confuses purpose with motive. Baxter's method of winning business in the PD market was to prevent its potential competitors from competing in a meaningful way in that market because of its leverage from the substantial degree of market power it held in the sterile fluids market. The objective of maximising profit can only be achieved if there is compliance with the Act. Since Queensland Wire Industries Pty Ltd v Broken Hill Pty Company Ltd (1989) 167 CLR 177, it has been recognised that the conduct of a party with substantial power in a market may be subject to restraints to which others without power are not (see NT Power Generation Pty Ltd v Power and Water Authority (2004) 219 CLR 90 per McHugh ACJ, Gummow, Callinan and Heydon JJ at [72], [76], [85], [125] and [126]). Even if Baxter did have the purpose of winning as much business as possible, it also had the purpose of deterring or preventing others from engaging in competitive conduct in the PD market. The latter purpose was substantial (s 4F(1)(b)).
Mansfield J recited the finding of the primary Judge as to the purpose being pursued by Baxter as follows:
[179] Based upon the evidence, his Honour found that the “core … of the purpose of the bundle or tie”, or more broadly the alternative offer strategy, was to foreclose the likelihood or restrict the possibility of the Gambro or Fresenius bids for PD fluids having any realistic prospect of success. That was, he said, illustrated or reflected in the “stubbornness” of Baxter’s attitude to the request by SA for a tender for the long-term exclusive supply of sterile fluids, because a discount for exclusive supply of a volume of sterile fluids only would have exposed the Gambro and Fresenius bids for PD fluids to realistic prospects of acceptance. Baxter’s attitude was to prevent those prospects and so to protect its PD fluids revenue stream.
His Honour thereafter continued on to reach the same conclusion as that reached by Gyles J, saying:
[187] Baxter, having a substantial degree of power in the sterile fluids market, took advantage of that power by the impugned conduct. It did so to secure as much of the supply of sterile fluids and PD fluids as it could. It did so for obvious commercial reasons. In doing so, it had the purpose of shutting out Gambro and Fresenius from being able to put forward bids for the supply of PD fluids which could be accepted by the SPAs except at a significant cost penalty. The significant cost penalty existed by reason of the bundled offers, and the fact that Baxter in the sterile fluids market had a substantial degree of power, and took advantage of that power by its alternative offer strategy. Baxter did not compete separately with Gambro and Fresenius in the PD fluids market, whether by price or quality of product or service or otherwise. It sought to avoid competing with them directly by taking advantage of its market power in the sterile fluids market. Its purpose, as the primary judge found, was to deter or prevent Gambro and Fresenius from engaging in competitive conduct in the PD fluids market by shutting them out from effectively being able to compete in that market.
[188] I do not accept Baxter's alternative contention. The primary judge found, upon analysis and assessment of the evidence, that Baxter's purpose by its impugned conduct was to shut out Gambro and Fresenius from being able to effectively compete in the PD fluids market in relation to the tender invitations from the SPAs. The outcome of its alternative offer strategy shows that its purpose was fulfilled. The fact that both Gambro and Fresenius were, before the impugned conduct, its competitors in the PD fluids market and remained its competitors in that market after the impugned conduct does not mean that Baxter did not have the purpose referred to in s 46(1)(c). Nor does the fact the (sic) Gambro and Fresenius tendered in various ways for all or part of the PD fluids supply contracts available in the tendering invitations of the SPAs. Nor does the fact that, in the period of time after the impugned conduct, Gambro and Fresenius may have increased their respective market shares in the PD fluids market.
Although in dissent as to whether the conduct whether there had been a hindering of competition, Dowsett J had there also observed:
[329] Purpose, effect and likely effect are quite distinct concepts. To establish that Baxter had a proscribed purpose, it was necessary to show an actual, subjective intention. As always, purpose must be distinguished from motive, but subjective purpose must still be demonstrated. Purpose may be inferred from statements and actions in the light of common experience. In the present context, effect is to be distinguished from likely effect. The effect of conduct is its outcome as demonstrated by evidence concerning actual events. Likely effect involves a prediction as to whether particular events will flow from relevant conduct. Likely effect may vary, depending upon the time at which the prediction is made. It may be made before, at the time of, shortly after, or long after the relevant causal conduct.
An application for special leave to appeal was refused but without prejudice to the ability to institute a further application at the conclusion of the litigation: Baxter Healthcare Pty Limited v ACCC [2009] HCATrans 20.
52 Just as “purpose” must be distinguished from “motive” (as referred to by Dowsett J in Baxter), “effect” must also be distinguished from “motive”. Thus, for example, in Universal Music Australia Pty Ltd v Australian Competition and Consumer Commission [2003] FCAFC 193, (2003) 131 FCR 529 at 587 Wilcox, French and Gyles JJ observed:
[249] We turn to the subject of purpose. A person may have the purpose of securing a result which it is, in fact, impossible for that person to achieve. That no doubt explains the reference to purpose, in para (a) of s 47(10) of the Act, as an alternative to effect and likely effect. The paragraph is satisfied if the relevant corporation has the requisite purpose, regardless of whether or not that purpose has been, or was or is likely to be, achieved. It may conceivably be satisfied even in a case where the court finds the purpose could never in fact have been achieved; although that finding would be relevant in determining whether to infer the proscribed purpose.
Purpose -v- taking advantage – s 46
53 Section 46(1) provides that a corporation that has a substantial degree of power in a market “shall not take advantage of that power … for the purpose of …”. And s 46(6) elaborates upon those matters to which a Court may have regard when determining whether a corporation has taken advantage of any such power.
54 These references in s 46 to “purpose” and to the “taking advantage” of power are, self-evidently, distinct elements. A corporation may pursue conduct which takes advantage of its market power without intending to injure a competitor. Caution should thus be exercised to avoid equating a finding that a corporation has taken advantage of its substantial market power with a conclusion that it did so for a purpose proscribed by s 46.
55 The overlap between “purpose” and the “taking advantage” of market power is nevertheless demonstrated by the facts in Melway Publishing Pty Limited v Robert Hicks Pty Limited [2001] HCA 13, (2001) 205 CLR 1. Melway was a publisher of a street directory in Melbourne. It held in excess of 80-90% of the retail market. For a number of years it had adopted a distribution system which divided the retail market into segments and had selected wholesale distributors allocated to defined segments. Melway decided to terminate the appointment of Robert Hicks Pty Ltd as a distributor. Robert Hicks thereafter sought to purchase 30,000-50,000 directories from Melway. Melway refused. Litigation followed. The primary Judge found for the distributor: Robert Hicks Pty Ltd (t/as Auto Fashions Australia) v Melway Publishing Pty Ltd (1998) 42 IPR 627. In doing so, his Honour concluded:
… It is only by virtue of its dominant position in the Melbourne directory market and the absence of a competitive market that Melway can afford, in a commercial sense, to withhold from supplying 30,000—50,000 directories at its usual wholesale price and terms to Auto Fashions. If Melway lacked substantial market power — in other words, if it were operating in a competitive market — it is highly unlikely that it would stand by, without any effort to compete, and allow Auto Fashions to secure its significant supply of directories from a competitor. Put another way, one would not expect to observe a refusal to supply 30,000—50,000 directories in a competitive market. Accordingly, in refusing supply Melway has taken advantage of its market power: (1998) 42 IPR at 641.
An appeal was dismissed by a majority: Melway Publishing Pty Ltd v Robert Hicks Pty Ltd [1999] FCA 664, (1999) 90 FCR 128. The appeal to the High Court was allowed: Melway Publishing Pty Limited v Robert Hicks Pty Limited [2001] HCA 13, (2001) 205 CLR 1. In setting out the facts, Gleeson CJ, Gummow, Hayne and Callinan JJ said:
[30] In the present case, there was no suggestion of a purpose of preventing the respondent from becoming a wholesaler of street directories. It was never suggested that Melway had any concern, for example, to prevent the respondent from distributing the products of one of its competitors. What Melway intended to do, and did, was to terminate the respondent's Melway distributorship, with the necessary consequence that it would cease to be a wholesaler of Melway street directories. Melway was not the only possible source of supply of Melbourne street directories. It was the only possible source of Melway street directories, but that would have been the case if it only had 10 per cent of the market, or if it had no substantial degree of market power. Its ability to stop the respondent becoming a wholesaler of Melway directories resulted from the fact that it was Melway, and could appoint, or not appoint, distributors as it saw fit in its commercial interests.
[31] … [T]here are cases in which it is dangerous to proceed too quickly from a finding about purpose to a conclusion about taking advantage. That is especially so when, in a context such as the present, the purpose as referred to in s 46 is relatively narrow. The purpose presently in question is that of deterring a person from engaging in competitive conduct in a market. If a manufacturer supplies to a single distributor, or a limited number of distributors, then, from one point of view, turning down an application from a person who wishes to become an additional distributor will have the effect of preventing that person from engaging in competitive conduct. Purpose, in this connection, involves intention to achieve a result. Where distributorship arrangements are concerned, an intent to give a particular distributor exclusivity may constitute a very insecure basis for concluding that there had been a taking advantage of market power.
In reversing the decision of the primary Judge, their Honours held:
[57] There are a number of difficulties about that process of reasoning. First, it appears to assume that the 30,000—50,000 directories in question would be sales lost to Melway, and gained by its competitors, if Melway were operating in a competitive market and, in that context, the respondent sought supply. But the decision not to supply the respondent was made in a situation where the respondent was primarily seeking to take business away from existing distributors. A second, and related, difficulty is that the reasoning fails to address the question of the nature of the wholesale distribution arrangements, both of Melway and its competitors, that would exist in a competitive market. Why, for example, might there not be a competitive market for Melbourne street directories in which Melway and/or its rivals supplied direct to retailers, or in which each operated through an exclusive distributor, or a fixed number of distributors? In such a case, as in the present case, a refusal to supply Melway directories to a wholesaler, or to another wholesaler, might be regarded by Melway as unlikely to result in any reduction in total Melway sales. In a competitive market, a manufacturer does not necessarily increase total sales by selling to everyone who seeks wholesale supply, or lose market share by selling to only a small number of wholesalers or, for that matter, by selling all its product directly to retailers. Thirdly, the focus of the question is too narrow. It is only as a manifestation of Melway's wholesale distribution system that the anti-competitive effect of the refusal to supply the respondent can be judged. What must be asked is whether Melway's wholesale distributor system, involving, as it did, restriction of competition at the wholesale level, amounted to taking advantage of its market power.
[58] The most likely explanation of the assumption that, in a competitive market, a refusal of the respondent's application for supply would be a loss of 30,000—50,000 sales to Melway is that it was thought that s 46 required that assumption. But the hypothesis that Melway lacks a substantial degree of power in the market does not require the assumption that the distribution arrangements or practices of Melway and its competitors are such that they are all commercially obliged to supply anyone who seeks to become a wholesaler, or that, at the wholesale level in the market, there exists a state of perfect competition, or that a decision to confine supply to one or a small number of wholesalers will result in a loss of sales. The only purpose of the hypothesis is to seek to test whether Melway has taken advantage of its degree of market power. It is one thing to compare what it has done with what it might be thought it would do if it lacked that power. It is a different thing to compare what it has done with what it would do in circumstances that are completely divorced from the reality of the market.
Their Honours ultimately concluded:
[61] Bearing in mind that the refusal to supply the respondent was only a manifestation of Melway’s distributorship system, the real question was whether, without its market power, Melway could have maintained its distributorship system, or at least that part of it that gave distributors exclusive rights in relation to specified segments of the retail market.
See: Wright and Painter, ‘Recent developments in the application of s 46: Melway and Boral considered’ (2001) 21 Aust Bar Rev 105.
56 The expression “take advantage of that power” in s 46 limits the kind of connection between market power and purpose: Rural Press Limited v Australian Competition and Consumer Commission [2003] HCA 75, (2003) 216 CLR 53 at 76. Gummow, Hayne and Heydon JJ there observed:
[51] Conclusion on s 46. The words “take advantage of” do not extend to any kind of connection at all between market power and the prohibited purposes described in s 46(1). Those words do not encompass conduct which has the purpose of protecting market power, but has no other connection with that market power. Section 46(1) distinguishes between “taking advantage” and “purpose”. The conduct of “taking advantage of” a thing is not identical with the conduct of protecting that thing. … If a firm with market power has a purpose of protecting it, and a choice of methods by which to do so, one of which involves power distinct from the market power and one of which does not, choice of the method distinct from the market power will prevent a contravention of s 46(1) from occurring even if choice of the other method will entail it.
57 The overlap between an analysis of “purpose” and the “taking advantage” of power nevertheless remains. One of the central difficulties inherent in s 46 is well recognised, namely the tension between pursuing conduct for the legitimate commercial objective of being competitive as opposed to the proscribed objectives. Thus, for example, in Top Performance Motors Pty Ltd v IRA Berk (Queensland) Pty Ltd (1975) 5 ALR 465 at 468 Joske J observed:
So far as concerns the construction of s 46, the submission put forward on behalf of the applicant, which is in effect that to exercise a power is to take advantage of a power, is, in my opinion, not the proper construction of the section. In my view, exercise of its contractual right to terminate a contract for the genuine purpose of protecting legitimate trade and business interests is not taking advantage of a power of controlling a market within the meaning of s 46, and providing that there is this genuine purpose, that is enough, though it may be there would always be people who would not regard it as reasonable to exercise the power in the circumstances of any particular case. Unreasonable behaviour may go to show absence of bona fides, but it goes no further than this.
It was there concluded that the termination of a dealership agreement for the sale of Datsun motor vehicles on the Gold Coast in Queensland was for the genuine purpose of protecting legitimate trade and business interests and was not the taking advantage of market power for the purposes proscribed by s 46. A contrary conclusion was reached on the facts in Mark Lyons Pty Ltd v Bursill Sportsgear Pty Ltd (1987) 75 ALR 581.
58 Where conduct undertaken for legitimate commercial purposes ends and conduct undertaken for a proscribed purpose or purposes commences may be difficult to determine.
59 One test that may be applied to determine whether a corporation has taken advantage of its market power requires a comparison between what it has in fact done and what it would rationally do if it lacked substantial market power: Rural Press Ltd v Australian Competition and Consumer Commission [2003] HCA 75 at [52], (2003) 216 CLR 53 at 76 per Gummow, Hayne and Heydon JJ; Seven Network Ltd v News Ltd [2009] FCAFC 166 at [971]-[975], (2009) 182 FCR 160 at 381-382 per Dowsett and Lander JJ.
60 Further assistance as to whether a corporation has taken advantage of its market power is also provided by the following observations of Greenwood J in Australian Competition and Consumer Commission v Cement Australia Pty Ltd [2013] FCA 909, (2013) 310 ALR 165 at 509-510:
[1899] In examining the evidence, I ask whether a profit maximising firm operating in a workably competitive market could in a commercial sense profitably engage in the conduct in question having regard to the business reasons identified by the witnesses, assuming such a firm is confronted with similar circumstances to those confronting Pozzolanic of asking itself whether it should enter into the original Millmerran Contract on 30 September 2002 (and later engage in the further steps of maintaining the contract).
[1900] In answering that question, a practical judgment must be brought to bear having regard to the identified so-called legitimate or ordinary business rationale informing the decision-making of the firm in question in all the circumstances. Those similar circumstances in which a hypothetical firm might be called upon to decide the questions confronting Pozzolanic will, however, reflect a hypothetically competitive market in which all aspects or sources of Pozzolanic’s substantial degree of market power are stripped away so as to neutralise its market power. In all other respects, the hypothetical market will reflect the circumstances of the actual market: Commerce Commission v Telecom Corp of New Zealand Ltd [2011] 1 NZLR 577; [2010] NZSC 111.
[1901] … Judgments about such a firm engaging in the conduct must be made in circumstances where a profit maximising firm would need to take account of the constraints imposed by workable competition.
[1902] The question, put simply, is whether a firm profitability could have engaged in the conduct in question in the absence of a substantial degree of power in the relevant market. Because that question involves a hypothetical construct it must be answered by applying an objective test but one which takes into account the legitimate business reasons identified by the firm for engaging in the conduct.
Purpose of deterring or preventing – s 46(1)(c)
61 Section 46(1)(c), the provision of relevance to the present dispute, refers to the proscribed purpose of “deterring or preventing a person from engaging in competitive conduct in that or any other market”.
62 In Australian Competition and Consumer Commission v Baxter Healthcare Pty Ltd (No 2) [2008] FCAFC 141, (2008) 170 FCR 16 at 82 Dowsett J said of this phrase:
[317] The words “deterring or preventing” also require attention. To “deter” is to “(r)estrain or discourage (from acting or proceeding) by fear, doubt, dislike of effort or trouble, or consideration of consequences”: see the Shorter Oxford English Dictionary. The same dictionary defines the verb “prevent” as to “[s]top, hinder avoid. Forestall or thwart by previous or precautionary measures … Frustrate, defeat, make void (an expectation, plan, etc.). … Stop (something) from happening to oneself; escape or evade by timely action. … Cause to be unable to do or be something, stop (foll. by from doing, from being)”. The combined effect of the words “deterring” and “preventing” includes persuading a person to decide to withdraw from, not to enter or not to compete in a market, as well as making it difficult or impossible for that person to do so.
The co-existence of market power, conduct and proscribed purpose – s 46(1)
63 Section 46 “requires, not merely the co-existence of market power, conduct and proscribed purpose, but a connection such that the firm whose conduct is in question can be said to be taking advantage of its power”: Melway Publishing Pty Limited v Robert Hicks Pty Limited [2001] HCA 13 at [44], (2001) 205 CLR 1 at 21 per Gleeson CJ, Gummow, Hayne and Callinan JJ.
64 Similarly, s 47(10) relevantly provides that s 47(1) does not apply to the practice of exclusive dealing unless the engaging in that practice “has the purpose, or has or is likely to have the effect, of substantially lessening competition”.
Condition – s 47(2)
65 Section 47(2) provides that a corporation engages in the practice of exclusive dealing if it supplies or offers to supply goods or services on one or other of the “conditions” set forth in s 47(2)(d), (e) or (f).
66 The term “condition” appearing in s 47 does not require that the condition be legally binding; but it must have “attributes of compulsion and futurity”: SWB Family Credit Union Ltd v Parramatta Tourist Services Pty Ltd (1981) 48 FLR 445 at 464 per Northrop J.
67 But the mere fact that a “likely consequence” is exclusion will not be sufficient: Monroe Topple & Associates Pty Ltd v Institute of Chartered Accountants in Australia [2002] FCAFC 197 at [71], (2002) 122 FCR 110 at 130 per Heerey J. There in issue was the manner in which the Institute provided training to persons who wished to become members. It was argued that the Institute had the power to affect competition in the support materials market for persons trying to become accredited as Chartered Accountants by reason of its exclusive right to enrol and assess candidates. Contraventions of ss 45, 46, 47 and 51AC of the Trade Practices Act were alleged. In rejecting the contention that there had been a contravention of s 47 and that the Institute had imposed a “condition” on the supply of its services, Heerey J concluded:
[104] The answer to this question must be in the negative, for two reasons. First, although “condition” in s 47(2) need not necessarily have legal or equitable force (see s 47(13)(a)), as Deane J said in Re Ku-ring-gai Co-operative Building Society (No 12) Ltd (1978) 36 FLR 134 at 168:
“The section does not look at the origin of the condition upon which there is supply of services. The section looks at the supply of services on that condition.”
And in SWB Family Credit Union Ltd v Paramount Tourist Services Pty Ltd (1980) 48 FLR 445 at 454 Smithers J said:
“It is clear that the act of exclusive dealing contemplated by the section is the supply of services by A to B in circumstances such that is it a correct assessment of the facts, as at the moment of supply, to say that the act of supply as been performed pursuant to a transaction, the terms of which include some sort of arrangement between A and B, to which of course, B is a party which gives to the supply the character of being made on the condition that B will acquire the services from C, or to which some external factor is applicable which gives that character to the supply. Supply in other circumstances might be a supply on the hope or even on the expectation that B would acquire the services from C, but it could not be a supply ‘on the conditions’ that B will acquire such service”.
There is no evidence of any condition which would have the effect that when Candidates obtained support services from the Institute they were in any way inhibited from obtaining similar services from MTA or anybody else. …
[105] This approach confuses the issues of purpose and effect with that of condition. It really alleges no more than, in the words of Smithers J, hope or expectation on the part of the Institute that Candidates will not purchase support material from other suppliers, including MTA. Even making allowances for the extended meaning given by s 47(13)(a), this is not the condition of which s 47(2) speaks. At the time of supply of services to Candidates they are perfectly free to decide whether or not to also acquire support material from MTA. Their decision would presumably be made on comparisons of the benefit to be derived from the extra cost. For a trader to offer products A and B for a single price which might make the consumer more inclined to purchase the package and not buy a competing trader's product B is not to impose any sort of condition within the meaning of s 47(2): (2002) 122 FCR at 138-139.
Similarly, Tamberlin J concluded:
[160] This claim fails because it is not a condition of the acquisition of support services that a candidate will not acquire support services from any other suppliers of those services or suppliers of materials. Although the practical consequence of the requirement to acquire, as part of the enrolment process, may in fact lead to applicants not acquiring support services from other suppliers it is not possible to point to any condition which mandates that result: (2002) 122 FCR at 149.
Black CJ agreed with Heerey and Tamberlin JJ.
Substantially lessening competition – s 47(10)(a)
68 Section 47(10)(a) refers to the purpose of “substantially lessening competition …”. The same phrase, it may be noted, is also employed in s 45.
69 The term “substantially” in s 47 has a similar meaning to the term “substantial” in s 46: Universal Music Australia Pty Ltd v Australian Competition and Consumer Commission [2003] FCAFC 193 at [241], (2003) 131 FCR 529 at 585 per Wilcox, French and Gyles JJ. Competition, it was there pointed out, “is a means to the end of protecting the interests of consumers rather than competitors in the market”: [2003] FCAFC 193 at [242], (2003) 131 FCR 529 at 585. It is only when competitive trading has been substantially interfered with that the public will suffer.
70 In Outboard Marine Australia Pty Ltd v Hecar Investments No 6 Pty Ltd (1982) 66 FLR 120 there had been a refusal to supply outboard marine engines to one retailer. In respect to the phrase “substantially lessening competition”, Bowen CJ and Fisher J observed:
The market in question is geographically wide. There seems to be no evidence that O.M.A.'s refusal to supply Hecar had or would be likely to have the result of altering the market structure so as to produce an anti-competitive effect, for example, there is no evidence that the barriers to entry have been raised, nor that price competition has been reduced. Although the competitive position of Hecar as an individual retailer may be affected in the future, it is unlikely that this would have such a dramatic effect as to lessen substantially competition in the retail market which extends from Forster to Umina … : (1982) 66 FLR at 125.
Fitzgerald J similarly observed:
It would, I think, be an unusual and exceptional case in which it could be shown that competition in a generally competitive market was or was likely to be substantially lessened by a refusal to supply one of a number of competitive retailers in the market with a product otherwise freely available and competitively marketed. Further, where there is a market which is generally competitive, it plainly does not follow that conduct which affects the balance of competition by advantaging or disadvantaging a particular dealer or dealers or a particular product or products necessarily lessens the competition in the market: (1982) 66 FLR at 134.
71 And, as Collier J recognised in Parmalat Australia Pty Ltd v VIP Plastic Packaging Pty Ltd [2013] FCA 119 at [28], (2013) 210 FCR 1 at 15, Smithers J in Dandy Power Equipment Pty Ltd v Mercury Marine Pty Ltd (1982) 64 FLR 238 also directed his attention to the requirement that there be a “substantial lessening” of competition in a market. Smithers J had there observed:
… To my mind competition in a market is the sum of activity engaged in by persons in promoting the sale to potential buyers of the goods with which that market is concerned … To apply the concept of substantially lessening competition in a market, it is necessary to assess the nature and extent of the market, the probable nature and extent of competition which would exist therein but for the conduct in question, the way the market operates and the nature and extent of the contemplated lessening. To my mind one must look at the relevant significant portion of the market, ask oneself how and to what extent there would have been competition therein but for the conduct, assess what is left and determine whether what has been lost in relation to what would have been, is seen to be a substantial lessening of competition. I prefer not to substitute other adverbs for “substantially”. “Substantially” is a word the meaning of which in the circumstances in which it is applied must, to some extent, be of uncertain incidence and a matter of judgment. There is no precise scale by which to measure what is substantial. I think in the context, particularly the penalty and other remedies for contraventions of the Act, and the nature of trade which is the subject of the Act, the word is used in a sense importing a greater rather than a less degree of lessening. Accordingly in my opinion competition in a market is substantially lessened if the extent of competition in the market which has been lost, is seen by those competent to judge to be a substantial lessening of competition. Has competitive trading in the market been substantially interfered with? It is then that the public as such will suffer … : (1982) 64 FLR at 259-260.
See also: Australian Competition and Consumer Commission v Baxter Healthcare Pty Ltd (No 2) [2008] FCAFC 141 at [340], (2008) 170 FCR 16 at 89 per Dowsett J.
72 When addressing a comparable provision to s 47(10)(a), namely that found in s 45(2) of the Trade Practices Act, Gummow, Hayne and Heydon JJ in Rural Press Ltd v Australian Competition and Consumer Commission [2003] HCA 75, (2003) 216 CLR 53 observed:
[41] … The relevant questions in this case are whether the effect of the arrangement was substantial in the sense of being meaningful or relevant to the competitive process, and whether the purpose of the arrangement was to achieve an effect of that kind.
When addressing the comparable provision found in the Trade Practices Act, Burchett and Hely JJ in Stirling Harbour Services Pty Limited v Bunbury Port Authority [2000] FCA 1381, [2000] ATPR 41-783 observed at 41,267:
[12] There was no dispute but that in determining whether the proposed conduct has the purpose, or has or is likely to have the effect, of substantially lessening competition in the relevant market, the Court has to:
– consider the likely state of future competition in the market “with and without” the impugned conduct; and
– on the basis of such consideration, conclude whether the conduct has the proscribed anti-competitive purpose or effect
Dandy Power Equipment Pty Ltd v Mercury Marine Pty Ltd (1982) ATPR ¶40-315 at 43,887; (1982) 64 FLR 238 at 259; Outboard Marine Australia Pty Limited v Hecar Investments No 6 Pty Limited (1982) ATPR ¶40-327 at 43,982; (1982) 44 ALR 667 at 669-670. The test is not a "before and after" test, although, as a matter of fact, the existing state of competition in the market may throw some light on the likely future state of competition in the market absent the impugned conduct.
The licence to supply towage services in the Port of Bunbury was there terminable upon two years’ notice. The Port Authority gave that notice and advertised for tenders for an exclusive licence for a term of five years. Declaratory relief was sought that the proposed tender would be contrary to ss 45, 46 and 47 of the Trade Practices Act. At first instance, the trial judge refused relief. The appeal was dismissed. The tender, it was concluded, would elicit competitive bids. Competition, it was concluded, would be promoted and not lessened. See: Ergas, ‘Stirling Harbour Services v Bunbury Port Authority: A review of some economic issues’ (2002) 10 CCLJ 27.
Lessening competition – ss 47(10) & 4G
73 Section 47(10) refers to a “lessening” of competition and s 4G expands upon that statutory expression by deeming it to include “preventing or hindering competition”.
74 The phrase “preventing or hindering”, as employed in s 4G is to be given a “broad construction”.
75 When rejecting a submission that the phrase should be construed as “directly hinders or prevents”, Mason CJ in Devenish v Jewel Food Stores Pty Limited (1991) 172 CLR 32 at 45-46 concluded:
Further, it cannot be, and indeed was not, suggested in argument that the notion “direct” is inherent in either of the words “hinder” or “prevent”. “Hinder” has been construed in England “in the general sense of in any way affecting to an appreciable extent the ease of the usual way of supplying the article” (emphasis added): Tennants (Lancashire) Ltd. v. C.S. Wilson and Co. Ltd., per Lord Dunedin; Peter Dixon & Sons Ltd. v. Henderson, Craig & Co. Ltd. What was there said in relation to hindrance of supply would apply with equal force to hindrance of acquisition. The comments of Gibbs J. relating to the words “prevent or hinder” in Reg. v. Bell; Ex parte Lees, must be seen in the context of that case. As his Honour observed, a broad construction would have effected a very drastic interference with ordinary civil rights. There is no similar reason for rejecting a broad interpretation of those words in this case. As has been said, such an interpretation is entirely consonant with the purpose of the section.
Hely J expressed the following similar observations in Australian Wool Innovation Ltd v Newkirk [2005] FCA 290, (2005) ATPR 42-053:
[34] “Prevents” suggests a total cessation of dealings between the third person and the target; “hinders” suggests that they have been made more difficult: J D Heydon, Trade Practices Law, Lawbook Co, Sydney, 2001 at [10.130]. “Hinder”, in the context of s 45D has received a broad construction, as in any way affecting to an appreciable extent the ease of the usual way of supplying or acquiring goods or services: Devenish v Jewel Food Stores Pty Ltd (1991) ATPR ¶41-098 at 52,563; 172 CLR 32 at 45–46 (Mason CJ); Australian Builders’ Labourers’ Federated Union of Workers — Western Australian Branch v J-Corp Pty Ltd (1993) ATPR ¶41-245 at 41,308; 42 FCR 452 at 460: (2005) ATPR at 42,671.
Section 51
76 A further provision of the Competition and Consumer Act which ultimately assumes little relevance to the present proceeding (given the conclusion that s 47 has not been contravened) should nevertheless be mentioned.
77 It is s 51(3). In relevant part, that sub-section provides as follows:
A contravention of a provision of this Part other than section 46, 46A or 48 shall not be taken to have been committed by reason of:
(a) the imposing of, or giving effect to, a condition of:
(i) a licence granted by the proprietor, licensee or owner of a patent, …
to the extent that the condition relates to:
(iii) the invention to which the patent or application for a patent relates or articles made by the use of that invention;
…
This provision previously existed in relevantly identical terms in the Trade Practices Act.
78 Section 51 addresses, at least in part, the potential for a tension between (for example) the Patents Act and trade practices legislation. See: Gummow, ‘Abuse of Monopoly: Industrial Property and Trade Practices Control’, (1976) 7 Syd L Rev 339. Although s 51 thus puts the stated intellectual property rights to which it refers in a “special position” in relation to claimed breaches of s 47, it does not apply to s 46: ASX Operations Pty Ltd v Pont Data Australia Pty Ltd (No 1) (1990) 27 FCR 460 at 471 per Lockhart, Gummow and von Doussa JJ.
79 Section 51(3) – and the use of the phrase “relates to” – should not be given any “narrow construction”: Transfield Proprietary Limited v Arlo International Limited (1980) 144 CLR 83. Arlo International was there a licensee of a patented process for the manufacture and erection of a steel pole, the “Arlo PTL pole”. It granted an exclusive sub-licence to Transfield. Clause 7 of the sub-licence provided as follows:
The Licensee covenants during the period of the Power Transmission Line Licence at all times to use its best endeavours in and towards the design fabrication installation and selling of the Arlo PTL pole throughout the licensed territory and to energetically promote and develop the greatest possible market for the Arlo PTL pole.
In issue was whether this clause was unenforceable because it had the purpose, or had or was likely to have the effect of substantially lessening competition. On its proper construction, this argument was rejected. Views were nevertheless expressed as to s 51(3) of the Trade Practices Act. Mason J thus observed:
The second issue is whether cl. 7 of the Agreement is rendered unenforceable by s. 45 of the Trade Practices Act. The appellant has not shown that cl. 7, on the construction I have given it, tends to lessen competition so as to come within the terms of s. 45 (1). Likewise, the appellant has failed to show that cl. 7, on that construction, is within s. 45 (2).
In any event, I take the view that s. 51 (3) (a) (i) and s. 51 (3) (a) (iii) of the Trade Practices Act would provide a defence to a case of contravention of s. 45 (2) arising out of the presence of cl. 7 in the Agreement. Section 51 (4) extends this defence to the determination of whether cl. 7 is unenforceable by reason of s. 45 (1) …
The appellant submitted that cl. 7 of the Agreement does not only relate to “the invention” or to “articles made by the use of that invention” but it goes beyond the terms of s. 51 (3) (a) (iii) and relates to other products, that is, it relates to not using any other poles. This submission in part reflects an interpretation of cl. 7 which I have rejected and in part attributes to the word “relates” a meaning which is too narrow, thereby giving s. 51 (3) an overly restrictive operation.
In bridging the different policies of the Patents Act and the Trade Practices Act, s. 51 (3) recognizes that a patentee is justly entitled to impose conditions on the granting of a licence or assignment of a patent in order to protect the patentee's legal monopoly. Even under American antitrust law, where there is no equivalent exception to s. 51 (3), the patentee is entitled to exercise some measure of control over the licensee consistent with the scope of the patent monopoly, though there has been some controversy as to the scope of permissible control …
Section 51 (3) determines the scope of restrictions the patentee may properly impose on the use of the patent. Conditions which seek to gain advantages collateral to the patent are not covered by s. 51 (3) ... : (1980) 144 CLR at 102-103.
Pecuniary penalties – s 76
80 A further statutory provision which ultimately assumes no relevance given the conclusions that there has been no contravention of either s 46 or s 47 is the provision empowering the Court to order the payment of a penalty, namely s 76. Its terms should nevertheless be mentioned (albeit briefly) and solely for the purpose of emphasising the prospect that any contravention may ultimately have given rise to the imposition of a substantial penalty.
81 That section relevantly provides as follows:
Pecuniary penalties
(1) If the Court is satisfied that a person:
(a) has contravened any of the following provisions:
(i) a provision of Part IV (other than section 44ZZRF or 44ZZRG);
…
the Court may order the person to pay to the Commonwealth such pecuniary penalty, in respect of each act or omission by the person to which this section applies, as the Court determines to be appropriate having regard to all relevant matters including the nature and extent of the act or omission and of any loss or damage suffered as a result of the act or omission, the circumstances in which the act or omission took place and whether the person has previously been found by the Court in proceedings under this Part or Part XIB to have engaged in any similar conduct.
(1A) The pecuniary penalty payable under subsection (1) by a body corporate is not to exceed:
…
(b) for each act or omission to which this section applies that relates to any other provision of Part IV–the greatest of the following:
(i) $10,000,000;
(ii) if the Court can determine the value of the benefit that the body corporate, and any body corporate related to the body corporate, have obtained directly or indirectly and that is reasonably attributable to the act or omission–3 times the value of that benefit;
(iii) if the Court cannot determine the value of that benefit–10% of the annual turnover of the body corporate during the period (the turnover period) of 12 months ending at the end of the month in which the act or omission occurred; and
…
(3) If conduct constitutes a contravention of two or more provisions of Part IV (other than section 44ZZRF or 44ZZRG), a proceeding may be instituted under this Act against a person in relation to the contravention of any one or more of the provisions but a person is not liable to more than one pecuniary penalty under this section in respect of the same conduct.
(4) The single pecuniary penalty that may be imposed in accordance with subsection (3) in respect of conduct that contravenes provisions to which 2 or more of the limits in paragraphs (1A)(aa), (a) and (b) apply is an amount up to the highest of those limits.
…
82 The principles regarding the imposition of penalties have been reviewed in many decisions: e.g. NW Frozen Foods Pty Ltd v Australian Competition and Consumer Commission (1996) 71 FCR 285; Australian Competition and Consumer Commission v Qantas Airways Ltd [2008] FCA 1976 at [16]–[27]; (2008) 253 ALR 89 at 105–108 per Lindgren J. Branson, Sackville and Gyles JJ in Minister for Industry, Tourism & Resources v Mobil Oil Australia Pty Ltd [2004] FCAFC 72, (2004) ATPR 41-993 referred to the decision in NW Frozen foods and continued:
[51] The following propositions emerge from the reasoning in NW Frozen Foods:
(i) It is the responsibility of the Court to determine the appropriate penalty to be imposed under s 76 of the TP Act in respect of a contravention of the TP Act.
(ii) Determining the quantum of a penalty is not an exact science. Within a permissible range, the courts have acknowledged that a particular figure cannot necessarily be said to be more appropriate than another.
(iii) There is a public interest in promoting settlement of litigation, particularly where it is likely to be lengthy. Accordingly, when the regulator and contravenor have reached agreement, they may present to the Court a statement of facts and opinions as to the effect of those facts, together with joint submissions as to the appropriate penalty to be imposed.
(iv) The view of the regulator, as a specialist body, is a relevant, but not determinative consideration on the question of penalty. In particular, the views of the regulator on matters within its expertise (such as the ACCC’s views as to the deterrent effect of a proposed penalty in a given market) will usually be given greater weight than its views on more “subjective” matters.
(v) In determining whether the proposed penalty is appropriate, the Court examines all the circumstances of the case. Where the parties have put forward an agreed statement of facts, the Court may act on that statement if it is appropriate to do so.
(vi) Where the parties have jointly proposed a penalty, it will not be useful to investigate whether the Court would have arrived at that precise figure in the absence of agreement. The question is whether that figure is, in the Court’s view, appropriate in the circumstances of the case. In answering that question, the Court will not reject the agreed figure simply because it would have been disposed to select some other figure. It will be appropriate if within the permissible range.
These principles continue to apply: Australian Competition and Consumer Commission v Titan Marketing Pty Ltd [2014] FCA 913 at [17] per Rangiah J.
83 In the present proceeding, the ACCC foreshadowed that if it was successful in establishing a contravention of either s 46 or s 47, reliance would be placed upon s 76(1A)(b)(iii), namely a penalty would have been be sought not exceeding 10% of “the annual turnover” of Pfizer Australia. Given that the present hearing has been to-date confined to the question of whether there has been a contravention of ss 46 and/or 47 – and is to be resolved in advance of any hearing as the quantum of any penalty that may be imposed if a contravention is established – there is no evidence as to that “annual turnover”. Upon the evidence that there is presently available, however, namely the annual income derived by Pfizer from atorvastatin alone, it may be expected that 10% of the “annual turnover” would have been a considerable sum. But no contravention of either ss 46 or 47 has been established and any musings as to any penalty that may otherwise have been appropriate need not be further considered.
A single course of conduct - the same conduct: s 76(3)
84 Section 76(3), it may further be briefly noted, provides that “a person is not liable to more than one pecuniary penalty under this section in respect of the same conduct”.
85 It was common ground that a penalty could not be imposed for each act which constitutes but a single course of conduct or “in respect of the same conduct”.
86 Thus, for example, in Singtel Optus Pty Ltd v Australian Competition and Consumer Commission [2012] FCAFC 20 at [53], (2012) 287 ALR 249 at 262 Keane CJ, Finn and Gilmour JJ cited, with approval, the following summary of principles set forth by Middleton J in Australian Competition and Consumer Commission v Telstra Corporation Ltd [2010] FCA 790, (2010) 188 FCR 238 at 277:
[231] … [I]t is useful to refer to the Trade Practices Commission v Bata Shoe Company of Australia Pty Ltd [1980] ATPR 40-161 at 42,277 where Lockhart J talked in terms of the “same episode” when considering an appropriate penalty:
Guidance is given in the field of sentencing for criminal offences by the well-known principle that where several offences are heard together and arise out of the same transaction it is a sound working rule that the sentences imposed for those offences should be made concurrent; it is inappropriate to sentence consecutively when the offences were all really involved in the same episode: see R. v. Duff a decision of the full court of this court, judgment delivered 6 December 1979; R. v. Walsh (1965) 109 Sol. J. Pt. 1 150; R. v. Melville (1956) 73 W.N. (N.S.W.) 579; R. v. Hussain Crim. L.R. 712; R. v. Hally (1965) 58 Q.R. 582 and Re: P.J. Kastercum (1972) 56 Cr. App. R. 298.
[232] Justice Lockhart considered at 42,277 that the contraventions arose out of:
the one course of conduct in that it was directed to Woolworths and reflected the adherence by the respondent to a policy of engaging in resale price maintenance in relation to Woolworths.
[233] He then said at 42,277:
I accept that the contraventions arose out of the one course or pattern of conduct. Although it is necessary to look at each contravention separately, nevertheless consideration must be given to the facts common to each contravention.
[234] In this way, Lockhart J regarded the seven contraventions as falling into three categories relating to the conversations giving rise to the contraventions, although from “one course or pattern”. From there, consideration was given to the facts common to each contravention. However, Lockhart J did not treat the case as involving only one contravention even though arising from “one course or pattern”, namely the adherence to a policy of engaging in resale price maintenance in relation to Woolworths.
Whether the conduct of Pfizer constituted a single course of conduct, similarly, is not an issue which arises for consideration.
Penalties – the standard of proof
87 The standard of proof to be applied when imposing a penalty is the civil standard and not the criminal standard of proof: The Heating Centre Pty Ltd v Trade Practices Commission (1986) 9 FCR 153 at 160. Pincus J there relevantly observed:
Whatever may be the reason for the distinction, the position is that the Act clearly characterises proceedings under s 76 as civil: see s 78 and contrast with s 79, while equally clearly characterising proceedings for a penalty in respect of a breach of Pt V of the Act as criminal proceedings. In so doing, Parliament must be taken to have intended that the court would apply the respective standards of proof applicable to each category. It is, of course, an attribute of civil proceedings that the necessary facts must be proved on the balance of probabilities, but, of course, taking into account the gravity of the matters alleged: Briginshaw v Briginshaw (1938) 60 CLR 336; Helton v Allen (1940) 63 CLR 691.
88 But all such questions as to any penalty that may have ultimately been applicable and the principles to be applied may presently be left to one side.
The Patents Act
89 A provision which also assumed some relevance during the course of the hearing was s 13 of the Patents Act.
90 That section provides as follows:
Exclusive rights given by patent
(1) Subject to this Act, a patent gives the patentee the exclusive rights, during the term of the patent, to exploit the invention and to authorise another person to exploit the invention.
(2) The exclusive rights are personal property and are capable of assignment and of devolution by law.
(3) A patent has effect throughout the patent area.
The term “exploit” is defined within the Dictionary in Schedule 1 of the Patents Act as follows:
exploit, in relation to an invention, includes:
(a) where the invention is a product–make, hire, sell or otherwise dispose of the product, offer to make, sell, hire or otherwise dispose of it, use or import it, or keep it for the purpose of doing any of those things; or
(b) where the invention is a method or process–use the method or process or do any act mentioned in paragraph (a) in respect of a product resulting from such use.
This definition is inclusive and not exhaustive: Seafood Innovations Pty Ltd v Richard Bass Pty Ltd [2010] FCA 723 at [29], (2010) 87 IPR 380 at 384 per Spender J; Damorgold Pty Ltd v JAI Products Pty Ltd [2014] FCA 150 at [69], (2014) 105 IPR 60 at 70 per Middleton J. The definition includes the exclusive right to “sell’ the invention. But the reach of the definition of “exploit” is a matter “open to debate”: Otsuka Pharmaceutical Co Ltd v Generic Health Pty Ltd [2012] FCA 239 at [87], (2012) 291 ALR 763 at 778 per Yates J. The “exclusive rights” of the patentee are the rights to “exclude” others from exploiting the invention or authorising another person to exploit the invention: Inverness Medical Switzerland GMBH v MDS Diagnostics Pty Ltd [2010] FCA 108 at [193], (2010) 85 IPR 525 at 568. See also: Grain Pool of Western Australia v Commonwealth [2000] HCA 14 at [83]-[85], (2000) 202 CLR 479 at 513-514. Bennett J went on to observe that s 13 “looks to whether the product being exploited infringes the claimed invention and not whether the person authorising that conduct intends to authorise infringement or knows that the product will infringe”.
91 Whether the supply of a product constitutes infringement is determined by s 117 which provides as follows:
Infringement by supply of products
(1) If the use of a product by a person would infringe a patent, the supply of that product by one person to another is an infringement of the patent by the supplier unless the supplier is the patentee or licensee of the patent.
(2) A reference in subsection (1) to the use of a product by a person is a reference to:
(a) if the product is capable of only one reasonable use, having regard to its nature or design–that use; or
(b) if the product is not a staple commercial product–any use of the product, if the supplier had reason to believe that the person would put it to that use; or
(c) in any case–the use of the product in accordance with any instructions for the use of the product, or any inducement to use the product, given to the person by the supplier or contained in an advertisement published by or with the authority of the supplier.
92 The term “authorise”, at least as it has been construed in the Copyright Act 1968 (Cth), means to grant or purport to grant to a third person the right to do an act, whether the intention is that the grantee shall do the act on his own behalf or only on account of the grantor: Roadshow Films Pty Ltd v iiNet Ltd [2012] HCA 16 at [126], (2012) 248 CLR 42 at 84-85 per Gummow and Hayne JJ.
The Supply of Pharmaceutical Products
93 The evidence relevant to determining whether the conduct of Pfizer contravened ss 46 and/or 47 of the Competition and Consumer Act canvassed the manner in which pharmaceutical products are sold and regulated in Australia, including the manner in which other manufacturers marketed their own products.
94 The manner in which Pfizer was making its own branded atorvastatin (Lipitor) and its own generic atorvastatin (Atorvastatin Pfizer or Atorva as it was known at one point prior to launch) available for sale necessarily has to be considered against the backdrop of the market in which it was operating prior to Pfizer’s loss of exclusivity over atorvastatin and the way in which it was proposing to operate in the market once that loss of exclusivity occurred.
95 Findings must necessarily be made – primarily as to the dimensions of the “market”, as that term is employed in both ss 46 and 47, and each of the other elements of those two sections.
96 It is prudent to:
identify the evidence relied upon by the parties;
outline the relevant provisions of the Pharmaceutical Benefits Scheme, at least in summary form; and
outline the significance of the sales of atorvastatin in Australia.
Thereafter it is prudent to address:
the relationship between the generic manufacturers and many of the community pharmacies in Australia – including the close “vertical integration” between some generic manufacturers and many pharmacies.
It is also prudent to consider in some degree of detail the manner in which one or other of the generic manufacturers and suppliers of pharmaceutical products operated. Evidence as to those matters included details regarding the nature of the relationships between generic manufacturers, wholesalers and community pharmacies and the discounts and rebates offered to pharmacies by those manufacturers. Such evidence was directed primarily to the operations of:
Apotex Australia Pty Ltd (Apotex);
Alphapharm Pty Limited; and
Ranbaxy Australia Pty Ltd (Ranbaxy).
97 The matters of particular significance raised by that evidence included the extent of the commercial influence that could be exerted by manufacturers upon pharmacists and the extent of the discounts and rebates that could commercially be offered by manufacturers. Of particular concern to the manufacturers was their ability to convince patients using Lipitor to switch (or “convert”) to a generic atorvastatin after 19 May 2012. Analysis was being undertaken by each of the generic manufacturers as to how best to market their own generic atorvastatin.
The evidence filed by the parties
98 In support of its case, the ACCC relied upon a large number of Pfizer’s own internal documents and upon the following affidavits either sworn or affirmed by:
Mr Roger Millichamp, the Regional Managing Director (Australia and New Zealand) of Apotex;
Mr Stephen Fraser, the Managing Director of Alphapharm;
Mr Robert King, a pharmacist who was a partner in and manager of five pharmacies (four of which are located in the Central Coast region of New South Wales);
Ms Nancy Carter, the Purchasing Manager employed by Chempro (Qld) Pty Ltd;
Mr Michael Karsz, a pharmacist employed by Jon Ravech’s Chemsave Pharmacy;
Mr Reza Safaei-Hosseinpour, a Pharmacy Manager employed by VietPhuoc Pty Limited, trading as Curry Chemist Hornsby; and
Mr Alexander Evans, the Managing Director of Ranbaxy.
The expert relied upon by the ACCC was:
Dr Christopher Pleatsikas.
With the exception of Ms Carter, each of these witnesses was cross-examined. Ms Carter was unavailable for cross-examination. It was nevertheless agreed between the parties that subject to the “weight” to be given to her evidence, her affidavit could nevertheless be read.
99 In support of its case, Pfizer relied upon affidavits either sworn or affirmed by:
Ms Jennifer Alltoft, the General Manager, Global Established Pharma, Australia and New Zealand employed by Pfizer Australia;
Mr Mark Crotty, the General Manager of the Established Products business unit for Australia and New Zealand at Pfizer Australia between January 2009 and April 2011;
Ms Sharon Brady, the Business Operations Manager, Strategic Planning and Operations, Established Products employed by Pfizer Australia;
Mr David Gledhill, a Senior Finance Manager who joined Pfizer Australia in April 2010;
Mr Michael Held, Pfizer Australia’s National Key Accounts Manager, a position he has held since July 2011;
Mr Deran Bagdadi, the Trade Marketing Manager, Established Products employed by Pfizer Australia;
Mr David Penny, Pfizer Australia’s Regional Sales Manager for Victoria and Tasmania;
Mr John Latham, now retired but a former Managing Director of Pfizer Australia.; and
Mr Gary Cooper, the Commercial Manager, Pharmacy in Pfizer Australia’s Established Products division between August 2009 and April 2013.
The expert witness relied upon by Pfizer was:
Dr Sumanth Addanki
100 The course pursued by the parties, which considerably facilitated the orderly conduct of the hearing, was to prepare in advance proposed rulings as to objections to evidence and agreements as to the use to be made of certain evidence. The (generally agreed) rulings proposed were made at the outset when each of the affidavits were sought to be read.
101 Notwithstanding the number of witnesses called, the case for the ACCC turned – at least initially – upon the contents of documents circulating internally within Pfizer and the inferences to be drawn from those documents. Not surprisingly, the Pfizer officers were cross-examined largely upon one or other of those documents. That cross-examination, however, was on occasions hampered by the fact that the officer giving evidence had never seen the document before, or was not the author of the document, or by the fact that the witness had not participated in a meeting or conference at which decisions were made or issues canvassed for consideration. Any finding of fact to be made in respect to some issues necessarily has to take into account both the difficulties experienced by the witness and the difficulties confronting the cross-examiner.
The Pharmaceutical Benefits Scheme
102 The supply of certain pharmaceutical benefits in Australia is regulated by Part VII of the National Health Act 1953 (Cth) (the “National Health Act”).
103 Legislative instruments made pursuant to that Act list pharmaceutical benefits available to be dispensed to members of the general public at government-subsidised prices.
104 The process for determining the pricing of pharmaceuticals subsidised under the Pharmaceutical Benefits Scheme changed on 1 October 2012. For present purposes it is sufficient to refer to the position prior to that date. The pricing of pharmaceuticals was, at the relevant time, determined by reference to the “Approved Price to Pharmacist” (or “Chemist List Price”). That price was determined either through:
agreement between the supplier and the Minister for Health and Ageing; or
a price determination made by the Minister.
The “Approved Price to Pharmacist” consisted of the “ex-manufacturer price” plus a fixed “wholesale mark-up”. This is the price at which pharmaceuticals were provided to pharmacists.
105 The remuneration provided to a pharmacist under the Pharmaceutical Benefits Scheme covered three items. First, the “Approved Price to Pharmacist” (or “Chemist List Price”). Second, a “mark-up” of 4-15% of the “Approved Price to Pharmacist” to cover the pharmacist’s costs in handling and storing the pharmaceutical. Third, a “pharmacy dispensing fee”. Between January 2011 and 30 June 2012, the “pharmacy dispensing fee” for atorvastatin was $6.42.
106 Pharmacists then received payment for the pharmaceuticals they dispensed in two forms: a “Patient Co-Payment” and a “PBS Subsidy”. The “Patient Co-Payment” is an amount paid by the patient to the pharmacist at the point of sale. Throughout 2012, this amount was capped at $35.40. The remainder of the cost (comprising the “Approved Price to Pharmacist”, the “pharmacy dispensing fee” and the “mark-up”) was covered by the “PBS Subsidy” – an amount paid to the pharmacist by the government.
Atorvastatin
107 Atorvastatin was (and remains) one of the prescription pharmaceuticals subsidised under the Pharmaceutical Benefits Scheme.
108 The amount of money expended annually upon atorvastatin in Australia inevitably attracted the attention of generic manufacturers who wished to participate in the supply of that pharmaceutical upon the expiration of the Pfizer patent.
109 That amount of money was, to say the least, not insignificant.
110 The Australian Government’s pharmaceutical benefits expenditure on an accrual accounting basis for the year ending 30 June 2012 totalled $9,193.7 million, compared with $8,872.7 million for the previous year. For the year ending 30 June 2012, the three drugs with the highest cost to government were:
Atorvastatin ($593.3 million)
Rosuvastatin ($359.2 million)
Ranibizumab ($307.8 million)
For each of the financial years ending June 2010, 2011, 2012 and 2013 atorvastatin was the highest selling prescription pharmaceutical in Australia. The value of sales for the financial years ending June 2010, June 2011 and June 2012 exceeded $700 million. As at January 2012 the atorvastatin molecule was the highest-selling in terms of volume and value of any molecule under the Pharmaceutical Benefits Scheme in Australia.
111 Not surprisingly, the expiration of Pfizer’s patent on 18 May 2012 was a much anticipated event by generics manufacturers who wanted to sell their own atorvastatin products.
112 By mid-2011 a number of generic manufacturers had already listed their impending atorvastatin products on the Australian Register of Therapeutic Goods (the “ARTG”). These generic manufacturers were the following:
Brand | Manufacturer or importer | Date listed on ARTG |
Lorstat | Alphapharm Pty Ltd | 18 December 2009 (10mg & 40mg) 23 December 2009 (20mg & 80mg) |
APO-Atorvastatin | Apotex Pty Ltd | 22 January 2010 |
Chemmart Atorvastatin | Apotex Pty Ltd | 22 January 2010 |
Terry White Chemists Atorvastatin | Apotex Pty Ltd | 22 January 2010 |
Atorvastatin Sandoz | Sandoz Pty Ltd | 5 January 2011 |
Atorvastatin SCP | Dr Reddy’s Laboratories (Australia) Pty Ltd | 15 January 2011 |
Atorvachol | Ascent Pharma Pty Ltd | 16 September 2011 |
Atorvastatin SZ | Sandoz Pty Ltd | 23 September 2011 |
Trovas | Ranbaxy Australia Pty Ltd | 23 September 2011 |
Atorvastatin GH | Generic Health Pty Ltd | 15 November 2011 |
STADA Atorvastatin | STADA Pharmaceuticals Australia Pty Ltd | 15 November 2011 |
Torvastat | Aspen Pharma Pty Ltd | 15 November 2011 (80mg film coated tablets blister pack) |
Well prior to 2012, it was thus clear that generic manufacturers were preparing to offer their own generic products to the Australian community as soon as they could lawfully do so.
Australian manufacturers and importers – “vertical integration”
113 At all relevant times there were in Australia manufacturers and importers of pharmaceutical products and wholesalers of pharmaceutical products.
114 At a very general level, the manufacturers fall into either of two categories – they are either “originator manufacturers” (being those investing in research and development) or “generic manufacturers” (being those manufacturers producing bioequivalent alternatives).
115 The major “generic manufacturers” are Alphapharm, Aspen, Apotex and Sandoz. These manufacturers are said to account for at least 85% of generic sales in Australia.
116 The manufacturers supply the wholesalers. The three major wholesalers are Australian Pharmaceutical Industries, Sigma Pharmaceuticals Limited and Symbion Pharmacy Services. Prior to 2011, these three wholesalers controlled over 90% of the distribution of prescription medicines in Australia. Smaller wholesalers included Central Hospital Supplies and CH2.
117 It was the wholesalers who supplied pharmaceutical products to the community pharmacies.
118 In early 2012 there were approximately 5,000 – 5,200 community pharmacies in Australia and about half of these were members of so-called “banner groups” such as Terry White, Chemists Warehouse, Amcal, Guardian, Priceline and Pharmacists Advice. In addition to these so-called “banner groups”, there are also about 500 “buying groups”, which purchased products collectively so as to increase their bargaining power. Of the remaining pharmacies (about 2,500) many have preferred supply agreements with wholesalers.
119 Some of the major “banner groups” are either owned by or have major commercial alignments with the major pharmaceutical wholesalers and manufacturers. One Pfizer document (being the final version of the Project LEAP: Review dated 14 September 2011) depicted this “vertical integration” then in place between the manufacturers and the wholesalers as follows:
This table exposes the “vertical integration” between the pharmacy groups identified and their manufacturers, Aspen, Alphapharm and Apotex.
120 The influence which could be exerted by these wholesalers was not inconsiderable. One instance of the manner in which this influence could be exerted – and exerted to the detriment of a competitor was provided by the evidence given about Pfizer’s experience with the anti-depressant, sertraline (originally branded as Zoloft), when its patent expired. There, Pfizer had entered into an agreement with the generic manufacturer, Sigma, for Sigma to market, promote and sell the Pfizer generic Xydep. In June 2008, Sigma created and launched its own sertraline generic, Sertra and ceased selling Xydep despite the agreement with Pfizer remaining in place. All Sigma customers, it was reported, were encouraged to switch patients from Xydep to Sertra. As the following graph demonstrates, the impact upon the sales of Pfizer’s product, Xydep, was significant:
The graph depicts not only the influence that vertical integration can have upon the sale of a product; the graph also depicts the ability to effect a change in the generic products sold. A not insignificant number of customers obviously switched from Xydep to Sertraline. Whether the change was entirely attributable to Sertraline or other generics, matters not; what remains significant is the extent of the change following the introduction of Sertraline.
Apotex
121 One of the manufacturers participating in the supply of generic pharmaceutical products in Australia is Apotex.
122 Apotex Australia Pty Ltd is a wholly owned subsidiary of Apotex International Inc. All are part of a group of companies, the ultimate holding company of which is Apotex Inc, a Canadian pharmaceutical company founded in 1974. The Apotex Group has operations in Canada, the United States, Australia and a number of European countries. Measured by sales, Australia is the third largest source of revenue for the Apotex Group. Apotex does not manufacture pharmaceutical products in Australia, but rather sources them from overseas suppliers.
123 Apotex is the exclusive supplier of (inter alia) the Chemmart and Terry White banner groups’ own-branded prescription generic products. An “Apotex International FY 13 Budget”, prepared in mid-to-late 2011, analysed its “generic market competitors” and ranked them and assessed each competitor’s market share. The competitors were there described as including Alphapharm, Aspen, Sandoz, Hospira, Ranbaxy and Ascent. The estimated rank and market share, perhaps, matters not. It is sufficient to note that Apotex considered itself one of the more successful generic manufacturers.
124 One of the pharmaceutical products in which Apotex expressed considerable interest was atorvastatin. Mr Millichamp acknowledged that he “… had been aware for a number of years prior to 2012 that the expiry of Pfizer’s patent over atorvastatin and the subsequent introduction of generic atorvastatin products in Australia was keenly anticipated by community pharmacies”. Mr Millichamp stated that “Apotex started planning the development of its generic atorvastatin products about five years before the atorvastatin product was due to expire, and started manufacturing its generic atorvastatin products in or around November 2011 in Canada for ultimate distribution in Australia”.
125 Of present concern, however, is the manner in which Apotex supplied its pharmaceutical products within Australia. The overwhelming majority – over 90% – of its supplies to community pharmacies in Australia are made through wholesalers. A small proportion of Apotex’s sales are made directly to buying groups and banner groups.
126 The commercial relationships as between Apotex and:
one of its “buying groups”, namely the Catalyst Group (that group comprising approximately 30 chemists);
Symbion; and
Chemmart (that “banner group” comprising approximately 260-270 chemists)
were explored in the cross-examination of Mr Millichamp.
127 The Trading Terms Worksheet with the Catalyst Group in evidence was expressed to be for a period of three years. The detail of that Worksheet changed from time to time. But its general structure remained the same. The detailed content of the Worksheet attracted a legitimate claim for commercial confidentiality. The general structure, however, can be disclosed. The Worksheet disclosed that the relationship between Apotex and the Catalyst Group was structured in a manner whereby Apotex offered the Catalyst Group:
a discount and rebate on the products it supplied; and
a “Head Office Rebate”.
An attached Schedule set forth the various percentage discounts off the “Chemist List Price” for each of the products offered and any specific rebate that was also offered. The “Head Office Rebate” was expressed as a percentage of total purchases. After a period of time, the percentage of the “Head Office Rebate” payable depended upon a “compliance level”. The greater the “compliance level” the greater the payment. In addition to these discounts and rebates being offered, the Trade Terms Worksheet also showed that Apotex would offer “Bonus stock”, an annual payment to each “compliant member” and a “sign-on” bonus. The “Bonus stock” was the provision of free stock – a greater amount being provided for the “first year of contract” and a lesser amount for each subsequent year. Staff training was also offered and assistance was provided to achieve store “conversion” to customers purchasing products from the Apotex range.
128 The agreement between Apotex and Symbion was also explored in cross-examination, albeit to a much more limited extent.
129 The agreement between Apotex and Chemmart similarly revealed Apotex offering “Store Rebates” and a “Head Office Rebate”.
130 The various discounts and rebates offered by Apotex could be considerable. Thus, for example, with respect to one product explored in cross-examination the following calculation was exposed:
Chemist List Price | $15.38 | |
Discount (65% of CLP) | - | $10.00 |
_______ | ||
Net Into Store Price | $5.38 | |
Product Rebate (20% of CLP) | - | $3.08 |
_______ | ||
“Net net” price (factoring in product rebate) | $2.30 (85% off CLP) |
In respect to this product at that time, the Commonwealth Government would pay $15.38 and the pharmacy would (in effect) pay Apotex $2.30. The patient would pay a sum of approximately $6.00. If the value of any “Bonus Stock” (for example) is also taken into account when considering the value of the terms being offered by Apotex, the terms are even more commercially attractive to the pharmacy than the above calculation would indicate.
131 Apotex sold its pharmaceutical products as a “range” of generic products. This was not uncommon. Mr Millichamp thus recognised that a “very common way” of conducting the business of supplying pharmacists was to have a “range” of products. In his cross-examination he was taken to an e-mail from Apotex to a pharmacist referring to “the offer that we have in place for your group [delivering] significant profit across all Apotex molecules” and the following exchange then occurred:
Right. And that was, in effect, your position in relation to how you – that you sought to compete by offering across the range? --- Absolutely.
Yes? --- Absolutely. That - - -
That’s the only sensible way to conduct this business? --- Well, it’s not – I wouldn’t put it as strongly as that. Not the only sensible way. There are a number of ways. But it is a very common way of Apotex and other generic companies to compete in the market by offering a range of products, absolutely.
Apotex itself resisted a course whereby pharmacists could “cherry pick” one molecule from its range. In explaining the reason for Apotex’s “strenuous” resistance to “cherry-picking” and the insistence upon supplying its “range” of generic products, Mr Millichamp was taken to an e-mail from himself to Mr Cuthill, described by Mr Millichamp “an important pharmacist as part of the Capital Chemist Group”. The e-mail stated in part as follows:
Although we may not be the cheapest on every molecule in the market, you do get the very significant additional benefits across all your Apotex lines.
In cross-examination, Mr Millichamp was taken to this passage and his questioning continued:
In effect, you were saying, “If you want – you can’t – you just can’t assess what one’s dealing with here on a molecule by molecule basis. It’s not commercially sensible,” correct? --- That’s correct. And if I may help the court – we call this kind of approach – I know it’s a bit basic, but we call it cherry-picking in the industry. A customer looking around and saying, “Citalopram at 85 per cent discount from company X. I want 85 per cent as well.” Our standard response is, “We’ve got a range of products. You need to look at the whole range. You need to look at the services, the clinics, the net rebate, the bonus stock,” whatever else we’re offering, as we’ve gone through in Catalyst terms – well, as we went through in the Catalyst term agreement, and we look at the deal as a whole.
If you departed from that – if it became known in the market that you were up for departing from that approach, they would tear apart your business model before too long, wouldn’t they, if you were available to be cherry-picked easily; that’s correct, isn’t it? --- If we were, yes. If we were – if we – how can I explain it best? Yes, if we didn’t have – if we didn’t structure our terms, conditions, trading terms and go-to market strategy in a way that protected the range, we would leave ourselves open to cherry-picking, absolutely.
Right. And your organisation resists that strenuously, correct? --- We do resist cherry-picking strenuously, yes.
132 Another feature of the manner in which generic manufacturers operated was to encourage a “sell-in” of its own generic product the very moment a patent expired. When addressing the anticipated loss of exclusivity on the atorvastatin patent, the following exchange thus occurred between Mr Millichamp and his cross-examiner:
Well, you would want to enter – your practice was to be selling and delivering into the marketplace as soon as loss of exclusivity took place, correct? --- We would absolutely want to be selling the minute the patent expired, absolutely.
…
And I mean, your organisation is a very successful promoter of generics, is it not? I mean, it’s successful – it’s a major organisation? --- Look, I would rather someone else judge how successful we are, but we certainly made a – we’ve got very good market share in the – in the market and got a lot of customers. So on that basis, we’re reasonably successful. Yes.
And once this window was part of the market, it was obvious to a person of your sophistication that there was a commercial benefit to pharmacies and to Apotex that there be significant sell-ins of product within that month? --- Yes.
Correct? --- Yes.
Just obvious? --- Well, I can say it’s obvious to me.
Yes. Because – and – because the pharmacist buying significant volumes is apt thereby to protect the chemist list price for a longer period than if they buy outside that period? --- What you’ve explained is – is correct in terms of the principle. To try and get every – every pharmacist to align with that is – is difficult. …
Apotex thus wanted to enter the market with its own generic product “the minute” a patent expired. And it was in the interests of both Apotex and the pharmacist for Apotex to “sell in … significant volumes” of its own generic product soon thereafter.
133 In anticipation of the volume of its own generic atorvastatin that would be required to supply its own customers once the Pfizer patent expired, Apotex carried out its own modelling. In mid-2011 that modelling anticipated that Apotex would capture 22% of the entire atorvastatin market by December 2011. The target launch date was identified as 19 May 2012, the day after the expiration of the Pfizer patent. In May 2011 it placed an order to satisfy the anticipated demand. At least initially, the product was being sourced from overseas and packaged in (for example) the Terry White or Chemmart boxes.
134 Apotex was planning a large sell-in of its generic product. An e-mail sent to Mr Millichamp on 18 October 2011 thus stated in part:
... We need all the stock at once in order to meet market demand and optimise the price disclosure free period available for selling. There will be more than 7 players launching at the same time so we cannot afford to be late.
Apotex was not only formulating its own strategy to combat Pfizer; it was also monitoring the steps being taken by Ranbaxy. The Apotex assessment in November 2011 as to the steps being undertaken by Ranbaxy was that Ranbaxy was “pushing for a 15 month commitment on their stock with an offer of 85% discount at a group level”.
135 By March 2012 Apotex revised its forecast as to the volume of product needed. An e-mail sent on 16 March 2012 recorded that “… many of our customers have advised us that they have signed deals with other suppliers”. Apotex was also holding discussions with its “key customers” as to the arrangements to be put in place after the Pfizer patent had expired on 18 May 2012. An e-mail from Mr Millichamp to Dr Barry Sherman (described as the “number one person in Apotex”) sent on 16 March 2012 stated in part as follows:
• Pfizer has already launched their own generic and Ranbaxy has also launched.
• The compound patent expires May 18th.
• If we bring in inventory and /or make overt representations in the market before then Pfizer will almost certainly sue us and we will be subject to an interlocutory injunction until patent expiry.
• We have been talking with our key customers (obviously no emails or details on paper) “behind the scenes” and secured their commitment to take our product when available.
• Most of our key customers are now taking the Pfizer generic and then switching to Apo - Atorvastatin after June 1st.
The reference in the e-mail to the prospect of being “sued” by Pfizer is presumably a reference to the constraints imposed by s 13 of the Patents Act and the exclusive right conferred upon the holder of a patent to “exploit” a patent whilst it remains current. The e-mail went on to further state that “we have secured our business with key customers”. Whatever was meant by “secured” and “behind the scenes” and whether the reference to “key customers” included “all key customers” or only some of them, matters not. The substance of the e-mail was an assurance provided to Dr Sherman that as at 16 March 2012 Apotex had at least “secured” a commitment from a not-insignificant number of its “key customers” to take the Apotex generic product. How the assurances were consistent with an e-mail dated 15 March 2012 explaining that “there are several majors who unfortunately will not be on board” was not really explained.
136 Immediately before the expiration of the Pfizer patent, Apotex perceived that circumstances had changed significantly. Mr Millichamp was thus reporting on 8 May 2012, inter alia, that “[s]ubstitution rates [had] dramatically increased and products that used to be difficult to substitute no longer carry that status”. This was said to be attributable to “the latest round of PBS reforms” at the beginning of April 2012 and the fact that there was, accordingly, “now huge pressure on Pharmacists to substitute to ensure financial viability”. The switch to generic atorvastatin was said to have been so successful that Pfizer “needed to place national newspaper ad’s (sic) explaining that Lipitor is still available”.
137 What is known, however, is that at the time of the Apotex launch on 19 May 2012 two “deals” were being offered to pharmacies by Apotex, namely:
Deal 1: which provided for a percentage discount of 90% “off invoice”, that offer being “based on a 9 month order at a 60% substitution rate”; and
Deal 2: which provided for a lesser discount, but which stated that Apotex would “cover the Pfizer rebate”. “Upon presentation of their Pfizer statement”, this deal provided that Apotex “would organise a credit for their wholesale account”.
A 90% discount, it was envisaged by Apotex, would at least meet any offer to be made by Pfizer.
138 As at the date of the Apotex launch, “deals” had been struck with at least:
Blooms – The Chemist;
Discount Drug Stores;
Chemmart; and
Terry White.
Each of these deals also had their own further terms and each offered substantial discounts.
139 The extent of competition in the market after the expiration of the Pfizer patent was such that Apotex considered that at least one invitation to tender was not worthwhile pursuing. In May 2012 National Pharmacies (a Friendly Society of pharmacies in South Australia comprising about 40 stores) invited tenders for the supply of atorvastatin. An email sent on 29 May 2012 circulated within Apotex was brief; namely:
This is not worth going for is it? Ranbaxy will offer their product for free.
Apotex apparently did not participate in the tender.
140 And, within Apotex, Pfizer was not perceived as one of the “main aggressors” after the expiration of its patent. In a “Business Review” presentation carried out regarding the first quarter of the 2013 Financial Year, the “Main Aggressors” were identified as:
Ranbaxy;
Alphapharm/Aspen; and
Sandoz.
The same presentation recorded that a very high rate of substitution from Lipitor, the Pfizer product, to the Apotex generic atorvastatin, had been achieved. It was also noted that Chemist Warehouse had advertised “generic lipitor” supplied by Sandoz for $0 (subject to terms and conditions).
141 By 8 June 2012 internal Apotex emails were recording that “[d]espite Ranbaxy and Pfizer having a 2-3 month head start on us, not to mention Ranbaxy giving the product away for free, we have done a fantastic job selling in our Atorvastatin and securing orders”.
Alphapharm
142 Alphapharm is an Australian pharmaceutical company that was founded in 1982. It is the largest supplier of pharmaceuticals by volume to the Pharmaceutical Benefits Scheme with annual sales exceeding $350m.
143 Alphapharm is owned by Mylan Inc, a pharmaceutical group based in the United States. Mylan is one of the largest generic pharmaceutical companies in the world, with worldwide revenues of $6.8 billion in 2012. It operates globally with a workforce of more than 20,000 and provides a portfolio of approximately 1,300 products to customers in approximately 140 countries and territories.
144 Of the generic manufacturers, Alphapharm offered the largest range of generic products.
145 Like Apotex, Alphapharm had arrangements in place with community pharmacies. And, again in a manner similar to Apotex, those arrangements offered varying degrees of discounts and rebates on its products.
146 Consistent with the evidence of Mr Millichamp, the Managing Director of Alphapharm Pty Limited (Mr Stephen Fraser) said in his affidavit that the loss of exclusivity “over atorvastatin was a long-awaited event in the Australian generic industry due to the revenues and high profile associated with atorvastatin and was monitored closely by Alphapharm and other generic suppliers”. He said that “Alphapharm expected that all the major generics suppliers in Australia would launch their own generic atorvastatin after” the loss of exclusivity.
147 Alphapharm began the development of its own generic atorvastatin product, Lorstat, in around October 2006. Testing was undertaken to determine, for example, the active pharmaceutical ingredient in atorvastatin. Steps also had to be taken to make submissions to the Therapeutic Goods Administration for registration of its own product. The estimated cost of developing Lorstat was approximately $500,000.
148 As Lorstat is manufactured overseas by Mylan, Alphapharm was required to place orders for its forecasted volume of atorvastatin in or around November 2011. Once this initial order for Lorstat was placed, it was unable to adjust the order given the fact that Mylan had a fixed six month lead time for atorvastatin product orders.
149 In or around December 2010, Mr Fraser became aware of “rumours” that Pfizer would supply pharmaceutical products direct to pharmacies. He thought that it was a lot easier and more commercially viable for manufacturers and suppliers to use wholesalers to distribute their pharmaceutical products. But he also recognised that “Pfizer was in a different position to generic manufacturers and suppliers in that it had a large number of patented originator pharmaceuticals which pharmacies needed to stock to supply customers and for which there were no available substitutes”.
150 Mr Fraser also became aware of what was referred to within Alphapharm as the “Pfizer Bank” in early December 2010. This was a reference to the Accrual Funds Scheme proposed to be offered by Pfizer. He became aware in about March 2011 of the fact that Pfizer had listed its generic version of Lipitor, then known as Zarator, on the Australian Register of Therapeutic Goods. The launch of generic atorvastatin, he considered, was the biggest launch in dollar terms of a generic pharmaceutical to date in the Australian market.
151 The assessment within Alphapharm was that the terms of Pfizer’s offer of generic atorvastatin to community pharmacies would effectively block competition from generic suppliers for up to a 12 month period.
152 The competitive response of Alphapharm was threefold, namely:
to offer a higher discount on Lorstat at the time of its launch, being a discount of 90% to pharmacies;
to not require of pharmacies any minimum purchase commitment; and
to exploit what it saw as negativity towards Pfizer – being discontent among pharmacies about Pfizer’s direct distribution model; the lack of transparency with the “Pfizer Bank” accrual scheme and the lack of support Pfizer had shown pharmacies when compared to Alphapharm’s long standing support.
This strategy was developed at a conference held in Hobart in mid-January 2012. With reference to the discounts it proposed, one of the slide-presentations at the Hobart conference stated:
“When we launch our atorvastatin product, it will be at a higher discount rate than either of Pfizer’s products. If you commit to purchasing 6 to 12 months’ worth of stock from Pfizer now, you will miss your opportunity to take full advantage of Alphapharm’s higher discount on atorvastatin at launch.”
Ranbaxy
153 Ranbaxy Australia Pty Ltd is the Australian subsidiary of Ranbaxy Laboratories Limited, a research-based international pharmaceutical company serving customers in more than 150 countries. Ranbaxy Laboratories Limited has its global headquarters in India, has operations in 43 countries and 21 manufacturing facilities in 8 countries.
154 Australia ranks in the top 15 markets for the pharmaceuticals produced by the Ranbaxy Group.
155 Ranbaxy has been a supplier of generic pharmaceuticals and commenced supplying generic pharmaceuticals in Australia since late 2006. Given the established relationships between the major generic suppliers in Australia with wholesalers and “banner groups”, Ranbaxy focussed its efforts upon developing relationships directly with community pharmacies.
156 Although there was some uncertainty as to the precise number of molecules offered by Ranbaxy in its range of products offered to community pharmacies, its products comprise 2% of generic sales in Australia. It began developing its generic atorvastatin product some 10 years prior to the expiration of the Pfizer patent. Its atorvastatin generic product was supplied under the brand name Trovas. Ranbaxy’s commercial advantage was that it could supply its generic products at a lesser price than its rivals. Ranbaxy could sell Trovas at a 95% or 96% discount and break-even.
157 Unlike other generic manufacturers, Ranbaxy was able to supply atorvastatin in Australia from 19 February 2012. It was able to do so by reason of the settlement of various patent infringement and invalidity proceedings between Pfizer Inc and Ranbaxy Laboratories and its related entities.
158 The ability of Ranbaxy to supply atorvastatin as from 19 February 2012 gave it a three-month window of opportunity not available to other generic manufacturers.
159 As at late 2011, Ranbaxy was making forecasts about its potential Trovas sales in the 2012 calendar year. This forecast was founded upon:
the estimated size of the atorvastatin market in 2011;
an expected growth rate;
an estimate as to the share of the market to be retained by Pfizer; and
an estimate as to the market share to be captured by generic suppliers.
160 In late 2011 at least two things were happening within Ranbaxy, namely:
detailed consideration was being given to the proposed “pricing structure” of Trovas and the discounts that could be offered to pharmacies; and
pharmacies were being visited by a sales force with a view to (at the very least) informing them about the forthcoming launch of Ranbaxy’s own generic, Trovas.
161 One internal e-mail sent to its Managing Director, Mr Evans, on 23 November 2011 stated (without alteration) as follows:
Will we be in a position to submit Trovas discount to Chemmart this Friday? If not in writing, perhaps verbal?
Would could go aggressive to make it difficult for Apotex as well as decision makers at Symbion, Chemmart, TWC & Pharmacy Choice.
We need an initial pricing structure for major groups, then later change the structure based on how many come on board early enough: ie., If we get “enough” groups at higher discount comitted early enough, then reduce discount for others.
Suggestion for min 12mth contract –
1. 90%: Chemist Warehouse & Nationals. Nationals = direct.
2. 88% or 90%??: All Wholesaler banners
3. 88%: Chemplus (direct), T&L (direct), Scaffidi, PAL, IPG, DDS, Good Price (?), & other similar size groups.
4. Other Groups = 85%
5. Independents: 85%??
6. Arana & SWAPS: Give 86% or 87% to members, 2% /3% to Arana & SWAPS?
The number of pharmacies to be embraced by this “suggestion” were considerable: Chemplus (for example) comprising some 70 pharmacies; T & L comprising some 30-40 pharmacies; PAL – being Pharmacy Alliance – comprising some several hundred pharmacies; DDS – being Discount Drug Stores – comprising some 100 pharmacies; Good Price comprising some 30-40 pharmacies; SWAPS – being a Western Australian wholesaler/distributor – supplying some 400-500 pharmacies.
162 Whatever else was being said by the Ranbaxy sales force in late 2011, there is no doubt that they were extensively canvassing pharmacies throughout Australia. One e-mail, for example, sent on 25 November 2011, recorded an assessment of the responses of pharmacies in Victoria.
163 There was a prolonged exchange between Mr Evans and his cross-examiner as to whether the sales force went beyond indicating to the pharmacies that were visited the terms of offers that would be made (including the price at which Trovas would be offered) or whether they “slipped into” making offers that were capable “then and there” of acceptance by those pharmacies. Mr Evans repeatedly denied that any offers were made prior to 19 February 2012.
164 The cross-examination was such that a concern was raised by the Court as to whether consideration should be given to s 128 of the Evidence Act 1995 (Cth) and the need to advise Mr Evans as to the privilege against self-incrimination. The concern was whether or not the conduct being pursued by Ranbaxy in advance of 19 February 2012 could involve a contravention of s 13 of the Patents Act. Although some doubt is expressed at the proposition that this conduct fell within the ambit of s 128, both Senior Counsel for the ACCC and Pfizer agreed that it was appropriate to bring the potential availability of that privilege to Mr Evans’ attention. That was done. Mr Evans indicated that he did not wish to have a short adjournment to consider his position and to consider whether he wished to invoke any available privilege. He decided to voluntarily proceed with the giving of his evidence.
165 Notwithstanding the denial of Mr Evans, it is concluded that from late 2011 through to early 2012 – and prior to 19 February 2012 – the Ranbaxy sales force were visiting pharmacies throughout Australia and making offers as to the terms upon which Ranbaxy would be making its own generic product available as from 19 February 2012. Whatever may be the debate as to the outer “reach of the definition of ‘exploit’…”, (Otsuka Pharmaceutical Co Ltd v Generic Health Pty Ltd [2012] FCA 239 at [88], (2012) 291 ALR 763 at 778 per Yates J), the conduct of Ranbaxy fell within the term “exploit” and was an encroachment upon the “exclusive right” of Pfizer to “exploit” its patent. The terms of those offers included both an identification of discounts that would be offered and the prices at which the Ranbaxy generic would be sold as from that date. That conclusion is founded upon:
the language of internal e-mails within Ranbaxy which repeatedly and over a prolonged period referred to “offers”;
the fact that such language was being employed by persons who were conversant with the commercial significance of making an “offer”;
the fact that comparisons were being made between (for example) the offers being made by Ranbaxy and the terms of the offers being made by Pfizer, the terms of the Pfizer offers being known as from the beginning of 2012; and
the fact that orders were being placed.
Moreover:
in none of the documents to which reference was made was there any qualification expressed as to the “offers” being made to suggest that the “offers” were (for example) merely expressing an “indicative” price.
Whatever may have been the qualifications expressed by the members of the Ranbaxy sales team as to how certain the terms of any “offer” may have been, there can be no questioning the fact that those customers saw those terms as sufficiently certain as to be capable of acceptance and sufficiently certain as to enable them to place orders for the purchase of the Ranbaxy generic atorvastatin being offered prior to 19 February 2012.
166 There were repeated e-mails which referred to “offers” having been made. Those e-mails included e-mails from or to customers of Ranbaxy and internal e-mails within Ranbaxy. Thus, and by way of example, it would appear that an officer of Ranbaxy (Mr Raj) was in Adelaide in January 2012. In e-mails from Mr Raj to different groups of pharmacies in Adelaide, Mr Raj referred to “our atorvastatin offer…”. An e-mail addressing the training to be given to a distributor in Western Australia referred to “the structure of the deal with Trovas …”. A later e-mail from Mr Raj sent on 16 January 2012 addressed the training to be given and referred to “our atorvastatin offer…”.
167 There was sufficient certainty as to the “offers” being made that a firm of chartered accountants, Vincents, was apparently retained by a pharmacy group to prepare a “Comparison between Pfizer and Ranbaxy Atorvastatin Offers” in about February 2012. Similarly, an e-mail “copied” to Mr Raj on 1 February 2012 stated in part (without alteration):
… As you can see, even with your Pfizer rebate, Ranbaxy’s offer will still add over $130,000 to your bottom line. For the purpose of this analysis I have used 88% as the Ranbaxy discount. However your groups discount will be finalised in our next meeting. This information is provided to you in strict confidence. …
It is, with respect, difficult to see how comparisons could be made between the offers being made by Pfizer and Ranbaxy without there being considerable certainty as to the prices at which the two generic products were being or were to be sold.
168 One instance of an “order” being placed occurred in an exchange of e-mails between a collection of pharmacies and Ranbaxy on 19 January 2012. Those e-mails were expressed in the language of an “18 month order qty’s below”.
169 The evidence of Mr Evans is rejected that prior to 19 February 2012:
no “offers” had been made;
and that:
no “orders” had been placed.
Offers had been made and orders placed. There was nothing “indicative” about the terms of those offers or orders. In many respects the evidence of Mr Evans was open to reservation. Thus, and by way of example, an internal e-mail within Ranbaxy forwarded on 6 February 2012 by the Ranbaxy Sales Team Leader referred to Chemsave having been “aapproached (sic) with our offer by” Mr Raj. The e-mail stated that Chemsave had decided to only look at “short term offers of Three Months”. The e-mail continued on to state (without alteration):
However we have decided that you should ring eash of the pharmacies listed in the attachment and give them the oppertunity to independently choose the very profitable Ranbaxy 12 month deal based on a min 85% - indicate it will probably be more discount.
Although Mr Evans was not the author of the e-mail, it was difficult to accept his explanation that the term “probably” indicated that price (as opposed to the discount) had not been fixed.
170 For the purposes of this proceeding, it matters not that Ranbaxy was engaging in conduct which would appear to be a contravention of s 13 of the Patents Act. What matters is the fact that Ranbaxy was pursuing a course of conduct to secure sales of its generic atorvastatin, Trovas, at a point in time in early 2012.
171 The willingness of pharmacies to obtain supply from Ranbaxy was perhaps equivocal. As explained by Mr King, a pharmacist called by the ACCC, he never considered using Ranbaxy products in his pharmacies because “its products are sometimes regarded as not being of high quality” and because “the differences in the packaging, size, shape and colour of Trovas compared to Lipitor was likely to reduce the capacity of the Relevant Pharmacies to substitute patients onto Trovas”. Moreover, Mr King perceived that “Ranbaxy does not provide the same level of service as other suppliers…”. Notwithstanding these remarks, Mr King in a subsequent affidavit nevertheless went on to say that he had in fact obtained a supply of Trovas from Ranbaxy.
Other manufacturers – Ascent and Sandoz
172 In early 2012 Ascent was also canvassing with its own pharmacies its own generic version of atorvastatin, Atorvachol. An email sent on 18 January 2012 showed that the following standard-form e-mail had been prepared for distribution to a number of its pharmacies. That email provided (without alteration):
Dear Valued Customer
A number of customers have contact me recently regarding an Atorvastatin generic offer they received from a competitor. Many customers do not feel comfortable with this offer, which I understand is quite restrictive and onerous. However, most customers also understand that the offer is not a one-off opportunity.
As you are a valued customer, I wanted to personally share with you (as I have with those I’ve spoken to) that waiting to see what else is on offer for your business, and not concerning yourself with a one molecule deal, is probably the most prudent course of action.
I hope that our partnership to date has proven that we place your business first when building sales programs to drive conversion and profitability, and we will continue to do so during this very important first half of 2012.
Ascent will be launching an industry first initiative in March 2012 along with the full basket of major first to market generic products during this time. In the interim, please do not hesitate to call me or any of my team to discuss any offers you receive.
We will be in touch shortly to showcase our 2012 initiatives.
173 Even though the Pfizer patent did not expire until 18 May 2012, there was thus significant consideration being given by both manufacturers of generic atorvastatin and their aligned pharmacies to the offers being made by Pfizer and the ability of the generic manufacturers to match or better those offers. Thus, for example, with respect to Alphapharm and Ascent, Chemsave forwarded to its members on 27 January 2012 an “analysis” of the “atorvastatin deals” and setting forth reasons as to “Why you should reject Pfizer and Ranbaxy Offers”. The “analysis” of those “deals” sets forth information which it may be accepted contains (or at least then contained) confidential commercial information. But the substance of the message being communicated sufficiently emerges from the following extract:
We have completed our analysis of the Atorvastatin deals that have been offered by both Pfizer and Ranbaxy, and compared those deals against our expected Chemsave deals from Alphapharm and Ascent. This analysis is for a 12 month period, as this is the stock and deal commitment that both Pfizer and Ranbaxy are asking for.
Our analysis shows you will be worse off by supporting Pfizer and Ranbaxy, and as a consequence we recommend that you reject both the Pfizer and Ranbaxy offers.
Our analysis is shown on the accompanying Excel spreadsheet (‘Atorvastatin Deals Analysis’ – also attached as a PDF). To give a full and true analysis, the spreadsheet takes into account both Atorvastatin generic and Lipitor discounts that you will receive depending on which supplier you support. We have used industry average figures for our analysis. You can use the spreadsheets to insert your own actual figures and expected conversion rates (in the yellow highlighted cells), and you should get essentially the same end result, just as I did for each of my 3 pharmacies.
The analysis shows the following:
1. The Pfizer and Ranbaxy offers are no better than our expected offers from our preferred generic suppliers, Alphapharm and Ascent, taking into account both Atorvastatin generic and Lipitor discounts.
2. Not only are the Pfizer and Ranbaxy offers no better than our expected Alphapharm and Ascent offers in terms of discounts, but both the Pfizer and Ranbaxy offers carry a heavy cost and high risk in terms of having to purchase up to 12 months worth of stock upfront …
There then followed an “analysis” of (inter alia) the costs to pharmacies in accepting one or other of the offers made.
174 A further standard-form e-mail was sent to a large number of the Ascent-aligned pharmacies on 8 February 2012. This latter e-mail, in addition to being in similar terms to the earlier e-mail forwarded on 18 January 2012, also contained this paragraph (without alteration):
As one of our most valued customers I wanted to personally share with you (as I have with those I’ve spoken to) that waiting to see what else is on offer for your entire business, and not succumbing to one deal, on one molecule, is the most prudent decision to make at this time. I hope that our partnership to date has proven that we place your business and it’s sustainability first when building programs to help drive conversion and profitability, and on that basis guarantee that we will continue to do the same during this very important time in our industry.
Ascent will be launching an industry first initiative in March 2012, however in the meantime we are happy to discuss any competitor offers with you or alternatively please call me personally at any time.
175 Ascent proceeded to launch Atorvachol on 18 May 2012.
176 Sandoz was also engaged in active communications with its aligned pharmacies. One communication prepared in mid-February 2012 for the purposes of distribution referred to the expiration of the Pfizer patent on 18 May 2012 and to the registration of its own generic atorvastatin products and continued:
We understand that you may have received offers requiring you to commit to
– purchases of minimum volumes of generic atorvastatin products at fixed prices
– and/or certain levels of substitution
– and/or a long-term exclusive contract.
If you find any such offer rather complex, Sandoz encourages you to seek independent advice from your accountant and/or lawyer before signing up.
We also encourage all Sandoz customers to carefully consider whether the acceptance of any such offer could prevent you from taking advantage of Sandoz’s most exciting and competitive offers which your Sandoz representative will discuss with you immediately after 18th May 2012.
In this context we also recommend reading the very interesting “Weekly Comment” by Bruce Annabel in Pharmacy Daily on 30th January 2012 titled “Proceed with Caution”.
Pfizer – The Need for Change
177 Pfizer traditionally focussed on supplying its patented products and researching and developing new medicines.
178 As at 2009 it had little experience in marketing and supplying generic products.
179 The looming expiration of a number of its patented products, however, dictated the need for Pfizer to give consideration to what position it would occupy in the market once its key patents expired.
180 The atorvastatin patent was not the only Pfizer patent which was due to expire. Between 2010 and 2014 the patents for an unusually high number of other important pharmaceuticals also expired, namely, the patents over the molecules used in Exefor, Caduet, the Xalabrands (Xalatan and Xalacom), Aricept, Viagra and Celebrex.
181 In 2007, there were sales of approximately $9 billion of pharmaceuticals under the Pharmaceutical Benefits Scheme. Of those, approximately $2.4 billion were sales of products whose patent expired in the period 2010-2014.
182 There can be no doubt that Pfizer had to take some steps to combat the competition which it would confront when the atorvastatin patent expired in May 2012.
183 To assist them in their development of a strategy, Pfizer formally retained Sinapse Consulting in September 2009 (“Sinapse”). Their appointment had been recommended by Mr Crotty “based upon their practical experience in retail pharmacy and direct distribution”. Mr Crotty explained the reasons for selecting Sinapse, in preference to two other groups who had recognised “consulting experience” or a recognised “theoretical viewpoint” as follows:
… What criteria were you looking to determine which of those three was the most appropriate to work with you on this exercise? --- Yes. So look, what was interesting is that both PCG – BCG and Pricewaterhouse had, you know, very – you know, well known consulting-type experience. They knew the sector from – from a theoretical viewpoint. Sinapse obviously had a lot of people with them in their organisation who had worked in generic and wholesaling and branded pharma business, so they had, I suppose, more real world practical experience, and so as subject matter experts, that’s what we found them to be. I suppose the difference between the other two large companies.
Sinapse was seen as possessing the expertise that Pfizer lacked – namely, experience in the generic and “real world” experience.
184 From about mid-2009 Pfizer (or Sinapse) was conducting market research and giving detailed attention as to the manner in which it would combat the forthcoming changes. In a paper prepared in August 2009 Pfizer identified the “three key drivers for change” as follows:
These three “drivers for change” were to be repeatedly referred to by Pfizer throughout 2010 and 2011.
185 Market research at that time carried out by Sinapse also directed attention to the “key factors” which influenced the purchasing behaviour of pharmacies. One presentation of that research was as follows:
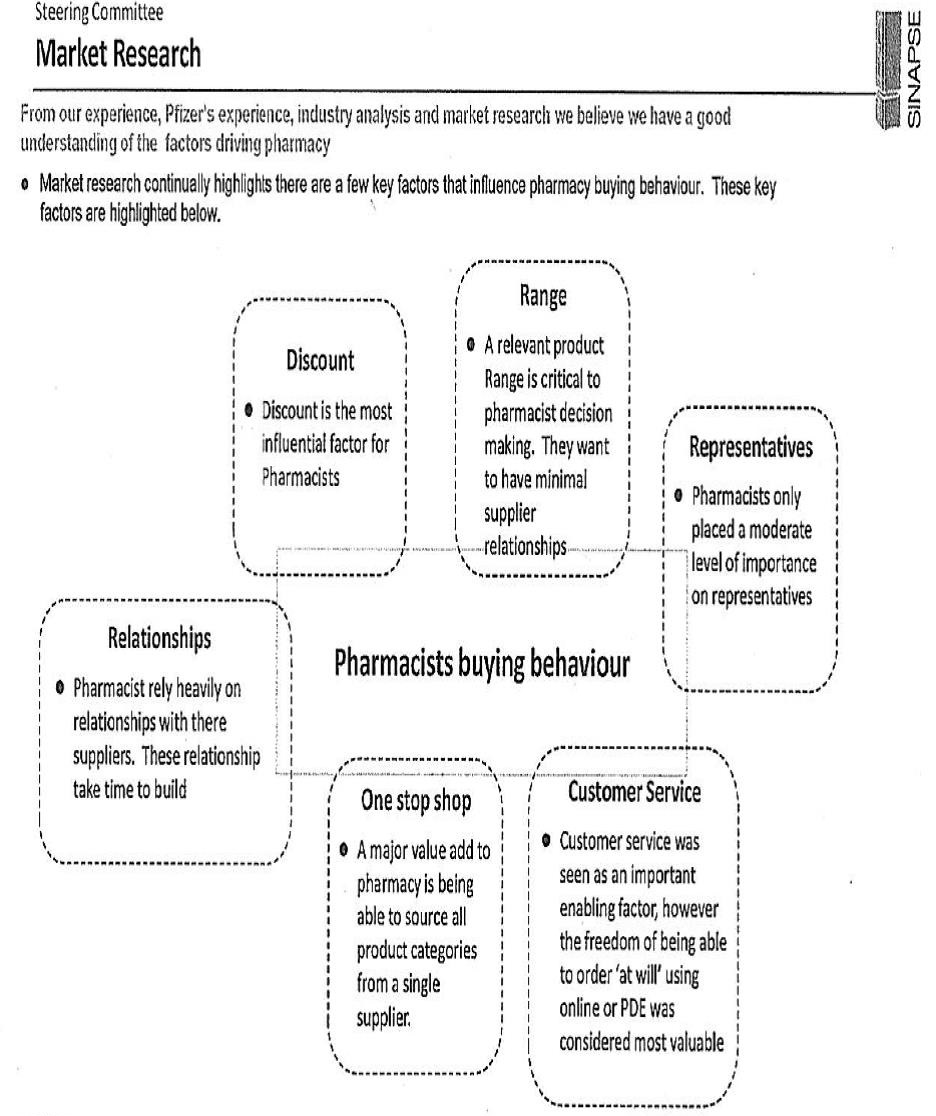
186 The market research undertaken either by Pfizer or on its behalf consistently emphasised the importance placed by pharmacists upon “product range” and “discount”. The Final Report of Project LEAP prepared in March 2010 again depicted these factors as follows:
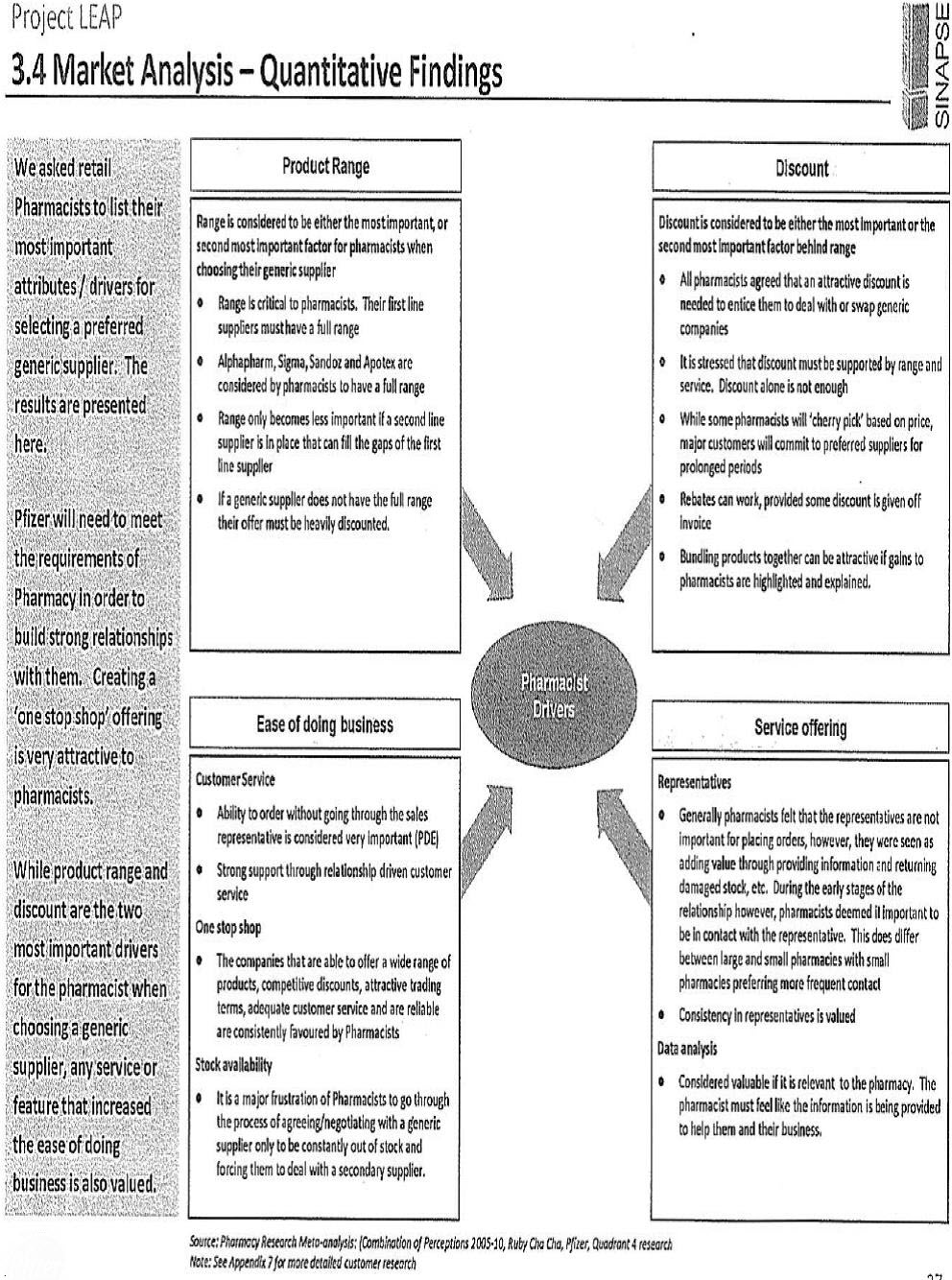
The Executive Summary at the outset of the Final Report also referred to the vertical integration of “traditional wholesalers … with generic companies”. The emphasis repeatedly placed by Pfizer upon marketing a “product range” was an emphasis also adopted by (for example) Mr Millichamp in his evidence that Apotex strenuously resisted pharmacists “cherry picking” by buying only one molecule from the Apotex “range”.
The Pfizer strategy – Project LEAP
187 The plan to combat these changes came to be known as “Project LEAP”.
188 As explained by Mr Crotty, he perceived the main features of Project LEAP to be:
the supply by Pfizer of pharmaceutical products directly to retail pharmacies, bypassing wholesalers;
the development of a dedicated sales field force and customer service support teams;
the development of a range of Pfizer Australia generics – or what it termed “price fighters”;
the development of a range of non-Pfizer generics;
the offering of an immediate trading term discount of 5% off the “Chemist List Price” on pharmaceuticals listed on the Pharmaceutical Benefits Scheme and sold to community pharmacies;
the offering of commercial proposals at the time Pfizer Australia launched a generic product in the future that was competitive with those being offered by other manufacturers; and
the creation of an accrual scheme to fund rebates on seven major Pfizer Australia products – including Lipitor – as they approached and entered the post patent expiry phase of their lifecycle.
189 The aspects of Project LEAP that the ACCC takes issue with in this proceeding involve three “platforms”, namely:
the Direct-to-Pharmacy Model, involving the sale and distribution of its products directly to pharmacies;
the Accrual Funds Scheme, involving the accrual of 5% of a pharmacy’s purchase of Pfizer’s patented products (including Lipitor) from 31 January 2011 to be credited as a rebate on conditions; and
a “bundled offer” to pharmacies which (inter alia) tied the prices upon which the branded product, Lipitor, was to be provided to the amount of Pfizer’s generic atorvastatin that the pharmacy agreed to purchase.
The first reference in the documentary evidence to Project LEAP appears in a Feasibility Study forwarded by Sinapse to Pfizer in September 2009.
190 The content of the plan, including each of the three “platforms”, evolved from its outset in 2009. Issues which were canvassed over time and changes which took place included:
whether the Direct-to-Pharmacy Model should in fact be pursued or whether there were other options open to Pfizer;
the quantum of the Pfizer generic atorvastatin required to be purchased by a pharmacy in order to “unlock” the rebate which had been accruing in that pharmacy’s “bank”; and
the date upon which Pfizer would launch its own generic atorvastatin.
There was also a change in the manner in which one or other of the objectives sought to be achieved were expressed. There was also, perhaps not surprisingly, differing views being expressed within Pfizer.
191 Although each of these “platforms” was separately considered, they each inter-related one with the other. Thus, for example, a table included with the Project LEAP Overview: Primary Care in February 2010 provided as follows:
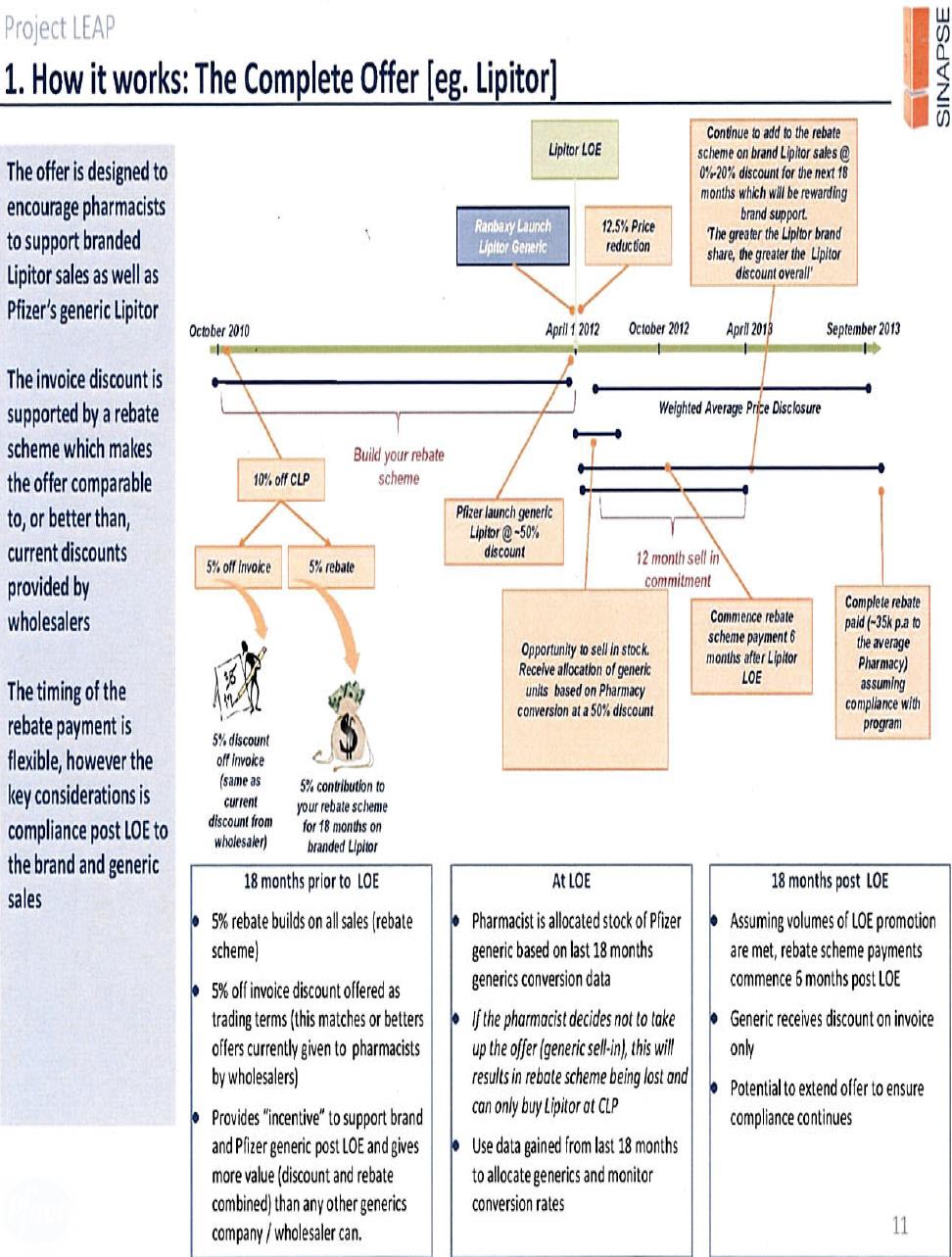
This table was reproduced from time to time in slightly different forms. Earlier variants emerged (for example) in December 2009 and January 2010. Without being exhaustive, the relevant features in this table, and other variants of it, are:
the 5% discount – both in respect to the invoice price and the separate contribution to the pharmacist’s “bank”;
the reference to the expected date of Ranbaxy’s generic atorvastatin “launch”;
the proposed timing of the Pfizer launch; and
the proposed “12 month sell in”.
192 The detailed evolution of Project LEAP from 2009 through to 2012 was largely tracked by the documents being circulated within Pfizer. Caution must be exercised, however, in deciding what inferences should be drawn from them. There were various drafts of documents and some uncertainty as to whether a document labelled “draft” was in fact the final version. The language, terminology and views expressed in some documents – not surprisingly – simply communicated the views or thinking of the author of the document and did not necessarily expose the views or thinking which was ultimately embraced when Project LEAP was approved in its final form. Indeed, some proposals were raised for consideration but later abandoned. Within Pfizer itself there were also different corporate divisions each having different responsibilities and priorities. Some documents produced within one division may not have been considered or agreed upon by another division.
193 The documents circulating internally within Pfizer nevertheless remain an invaluable source of information disclosing the consideration being given by Pfizer as to how it would combat a very changed market when its patent on atorvastatin – and its patents over other products – expired.
194 Within Australia, the development of Project LEAP was under the supervision of a Steering Committee comprised of Messrs Latham and Crotty – but it was Mr Crotty who had the more “hands-on” role. It was the case that Mr Crotty in some cases had far more detailed knowledge of the evolution of the project than Mr Latham. There was, with respect, uncertainty on the part of Mr Latham in respect to some of those documents upon which he was cross-examined as to whether he had even seen one or other of those documents before. In the case of those documents he had seen, there was uncertainty as to whether the document he was being questioned about was the version of the document that he had seen; or whether he had even read a particular document. Given his seniority within Pfizer, his comparative lack of “hands-on” familiarity with the documents was understandable. But that uncertainty and lack of familiarity made Mr Crotty the more reliable source of explanation in respect to many of those documents. However, Mr Latham’s evidence as to the policy or strategy being pursued by Pfizer, and the reasons for the making of centrally relevant decisions, nevertheless remained reliable.
195 As the substance of Project LEAP evolved it became apparent that some elements of the proposed project commended themselves more to Mr Crotty than to Mr Latham.
196 At a very general level, an overview of the steps taken along the way in developing the strategy involved at least the following:
the preparation in June 2009 of a Long Range Forecast review;
a number of meetings in September 2009 at which there was a presentation of a Feasability Project;
the preparation of a Commercial Offer Assessment in November 2009;
a meeting of the Steering Committee on 17 December 2009;
a further meeting of the Steering Committee in January 2010;
the preparation of a Final Report for Project Leap in March 2010;
a video conference in April 2010 in which there was a presentation of Project Leap to Mr John Young and the responses to a series of questions which were then raised;
a presentation in Australia in late May 2010 of Project Leap to Mr Simmons the Global President of Established Products;
the preparation of a “Briefing document” on about 9 June 2010 for a presentation on 10 June 2010;
the preparation on 8 July 2010 of an “Approval document for the ELT” (namely the “Executive Leadership Team”) for the purposes of a presentation in New York on 14 July 2010.
The final approval of Project LEAP was communicated to Mr Crotty on 14 July 2010. The project as finally approved obviously went through a process of review both within Australia and ultimately in New York. As Mr Crotty observed, “this [was] probably the most approved project I’ve ever been involved in”.
197 The ACCC’s case focussed (at least in part) on the language used to express Pfizer’s objective within a number of internal Pfizer documents and the various changes to the manner in which elements of Project LEAP were to be structured and implemented throughout the development process.
198 It is unnecessary to trace each of these elements from the outset through to the final approval of Project LEAP in New York in July 2010. It is equally unnecessary to canvass each of the occasions and the context in which each of these elements was considered. It is sufficient to make reference to a limited number of discrete occasions upon which one or other of these elements arose for consideration.
199 Such references are sufficient to give context to both the inferences sought to be drawn from the documents on the part of the ACCC and to the explanations sought to be propounded by the Pfizer witnesses during their cross-examination by Senior Counsel for the ACCC.
200 And, although it is convenient to direct separate attention to each of the three elements of Project LEAP which were said by the ACCC to culminate in contraventions of ss 46 and 47 of the Competition and Consumer Act, within Pfizer it was manifestly apparent that a consideration of these elements progressed on many occasions in tandem. Focussed attention upon one or other of these three elements on occasion exposes parallel consideration being given to one or more of the other elements.
201 Subject to those reservations, it is nevertheless convenient to consider the evolution of each of these three “platforms” relied upon by the ACCC separately.
Direct-to-Pharmacy Model
202 One central “platform” of Project LEAP focussed upon the manner in which Pfizer was contemplating distributing its products to pharmacies.
203 This “platform”, as it was ultimately approved, came to be known as the Direct-to-Pharmacy Model.
204 Historically, Pfizer had distributed its products solely through wholesalers.
205 In September 2007, however, it established a parallel distribution division called Pfizer Direct. Pfizer Direct gave Pfizer the capability to distribute products directly to pharmacies. Originally, the major interest for Pfizer Direct was for Viagra, which at the time was a private medicine not listed on the Pharmaceutical Benefits Scheme. Pfizer Direct was, apparently, launched “in a ‘soft’, non-aggressive-way…”. It sought to get pharmacies to order their Pfizer range of products through a web-based ordering facility. But it struggled to penetrate the market. As at mid-2010 it represented about 2-3% of Pfizer Australia’s annual turnover.
206 A variant on this was what emerged in mid-2009 through Project LEAP.
207 In June 2009, a paper titled “Established Products Australia: Strategic Framework” referred to the growing acceptance within Australia of the market for generic products and anticipated “Government regulation reforms” which would reduce the profitability of the pharmaceutical industry. The Executive Summary went on to state (without alteration):
An exclusive, direct to pharmacy model will optimize “One Pfizer’ and EP revenues
– Unique opportunity for commercial offering to pharmacists to retain loyalty at LOE
– Retention of wholesaling margin provides funding for commercial offering
– Direct relationship with pharmacist allows tailored support
“EP revenues” was a reference to “Established Products”, one of the corporate divisions within Pfizer. “LOE” is an acronym used to denote “loss of exclusivity”. The paper canvassed “three scenarios” – one scenario being a focus by Pfizer upon its “branded” products (eg Lipitor); the second scenario being one in which Pfizer would maintain its own “branded” products but also selectively grant a licence to one or multiple competitors the right to manufacture its product; and the third scenario being one in which it would exclusively supply all of its products direct to the pharmacies. Modelling was undertaken to attempt to forecast the impact upon the Pfizer revenue of these scenarios. Although the detail of the graph would change over time, it was then forecast that the Direct-to-Pharmacy Model would considerably ameliorate the decline in Pfizer revenue. The graph then depicting the impact of this model upon Pfizer revenue was as follows:
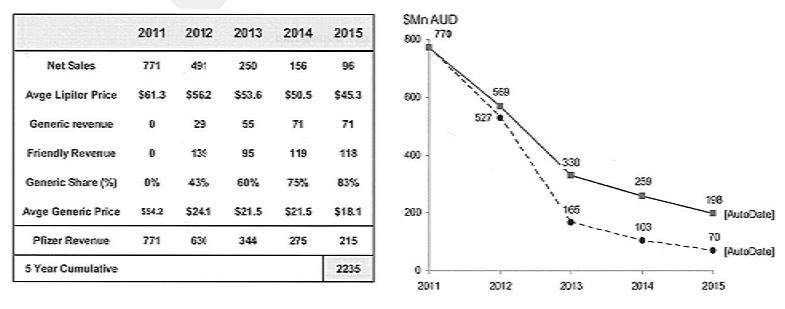
208 The document entitled “Case for Change – Strategic Review” dated 27 July 2009 concluded in its Executive Summary that “[a]n exclusive direct-to-pharmacy model appears a financially and strategically attractive response to … market challenges, with four sources of value identified”, namely:
it would “capture … the wholesaler margin”;
it would provide an “improved ability to defend volume and price erosion” upon loss of exclusivity;
it would provide “access to detailed customer purchasing information”; and
it would provide the “potential for other innovator companies to distribute their products through the model”.
209 The “Feasibility Study” forwarded by Sinapse to Pfizer in September 2009 set forth the case for a “direct to pharmacy” model as follows:
Case: Direct-to-Pharmacy
Background
Company established a direct to pharmacy distribution model. The key business drivers were:
• accessing the wholesaler margin,
• protecting off-patent product revenue and
• to generating new revenue from generic product sales
The paper went on to identify as follows the “operational attributes” that were to be taken into account as part of the “strategy” being propounded:
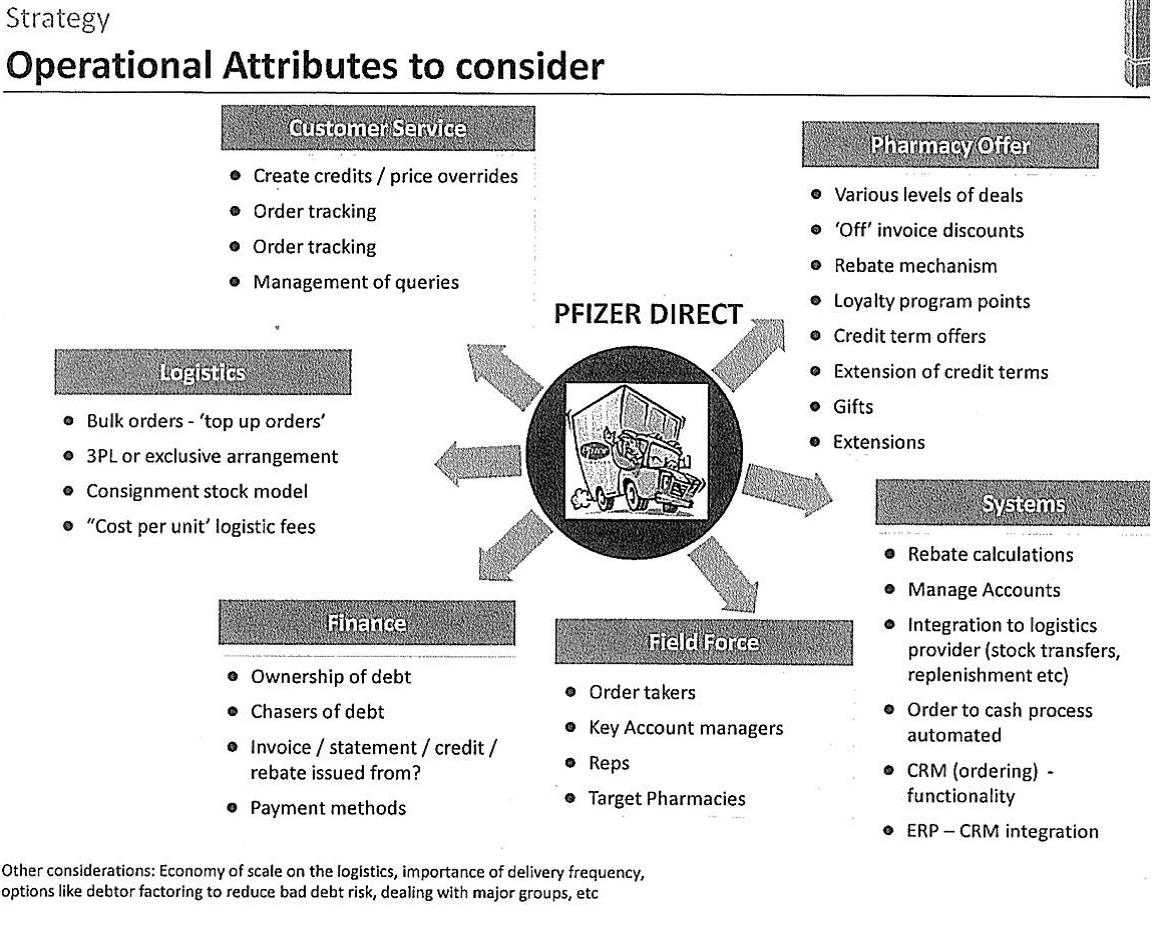
210 The advantages perceived by Pfizer in implementing its Direct-to-Pharmacy Model were referred to repeatedly. One further instance is in a Regional Presentation of Project LEAP that took place in February 2010 when it was again being propounded that that model would “optimize ‘One Pfizer’ revenues” and would provide a “platform to defend LOE revenue erosion…”.
211 But the Direct-to-Pharmacy Model was not the only option being pursued. The second of the “scenarios” canvassed in the June 2009 paper “Established Products Australia: Strategic Framework” remained under active consideration. This was the strategy whereby Pfizer would enter into a licensing agreement with one or more of the existing generic manufacturers. One of the advantages of this strategy was perceived to be that Pfizer could “leverage their existing relationships with pharmacy & hospital, giving Pfizer [market share] protection”.
212 Following the presentation to Mr Young in April 2010 and up to mid-May 2010 there were internal deliberations within Pfizer in respect to what was referred to as “a Plan B should we not be able to go with the exclusive DTP strategy”. In an e-mail sent from Mr Latham to Mr Young on 13 May 2010 Mr Latham attached “the updated actions (sic) points and follow up from our telecon of 21 April …”. The attached materials presented the comparative merits of the “Fallback plan to DTP” as follows:
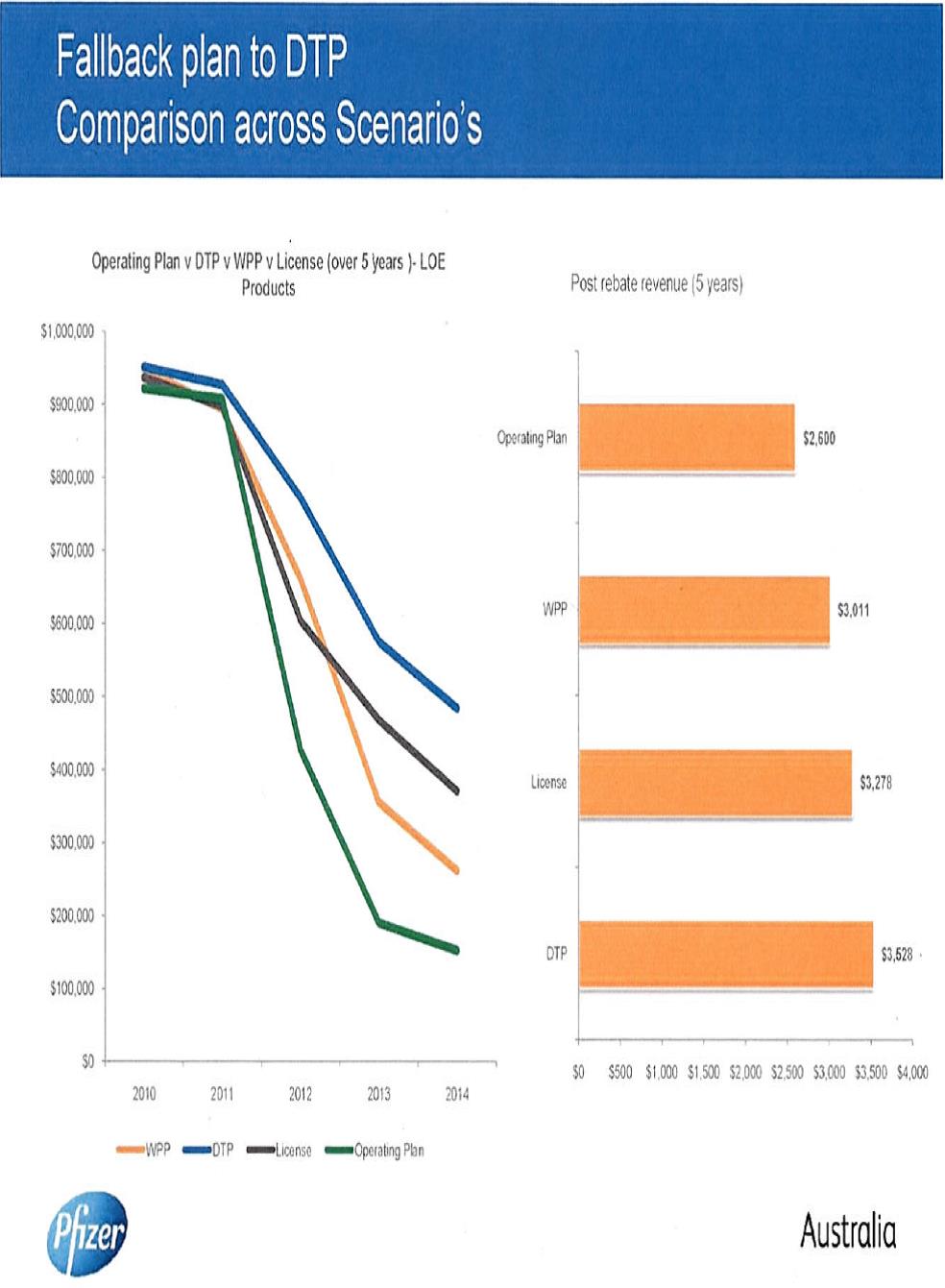
The reference in that graph to “WPP” was understood to be a reference to the position which would prevail if Pfizer continued to distribute through the existing wholesalers.
213 This case was also put to Mr David Simmons, the Global President of Established Products, in a presentation by Mr Crotty in late May 2010. Mr Crotty could not recall this part of the presentation. The presentation itself, however, included the following table and graph:
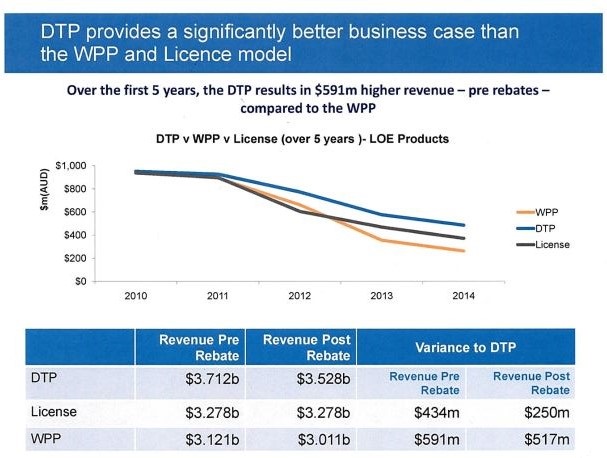
What is revealed by the table and graph, self-evidently, is that it was then perceived that the Direct-to-Pharmacy Model produced a better commercial outcome to Pfizer than the licensing arrangement. In the May 2010 presentation to Mr Simmons, he was advised that Pfizer had considered “three alternative models” but that the “DTP model [provided] the best business case”.
214 As at June 2010, it was said that “DTP is the enabler, not the nucleus of the strategy. It is the peri LOE offering and entitlement that differentiates project Leap”.
The rebates and the Accrual Fund Scheme
215 The second of the three “platforms” relied upon by the ACCC in advancing its case against Pfizer focussed attention upon the rebates being offered by Pfizer and the terms upon which such rebates were offered.
216 The prospect of Pfizer being able to offer discounts and rebates to pharmacies was contemplated from the outset.
217 The proposal that Pfizer would deliver its products directly to the pharmacies enabled it to offer a 5% “discount” off the invoice price of its products. This represented an approximate amount which had previously been incurred by the wholesalers responsible for the delivering of product to pharmacies.
218 The 5% “rebate” was a separate amount. It, too, was related to the Direct-to-Pharmacy Model.
219 In an Executive Summary of Project LEAP circulated in June 2010 the operation of the “rebate” was explained as follows (without alteration):
Project Leap aims to create Pfizer and brand loyalty in the 18 months leading up to LOE. Building a relationship with Pharmacy is key to our strategy. Accordingly, a pharmacy field force will be recruited at the end of 2010.
Pfizer and brand loyalty will be achieved via a two pronged approach. Firstly, a 5% reduction from Chemist’s List price will form our net invoice price.
Secondly, each pharmacy will start to accrue a rebate “bank” of 5% on all purchases of an LOE product 18 months out from LOE. That is, purchases of Lipitor will start to accrue a rebate before Caduet, Xalabrands, and there will be overlap. Due to the launch date of Feb 1 2011, Lipitor will only have 12 months before LOE.
That rebate accrual is analogous to a frequent flyer scheme, whereby the pharmacy will know each month how their rebate bank is accruing. The pharmacy representative will have that accrual on their CRM screens each month, and AR will have it noted on the monthly debtor statements.
Once LOE occurs, we will require the pharmacies to continue to meet preconditions related to purchase of Pfizer branded products.
If successfully meeting those purchasing conditions for 6 months post LOE, the rebate “bank” will be paid to the pharmacy via credit on their AR account in quarterly instalments over a 12 month period.
Access to dispensing data will enable measurement of the purchasing behaviours.
Should a pharmacy fail to meet their purchasing levels, they will lose their “bank” and the accrual will be reversed. For modeling purposes we have assumed 87.5% of pharmacies will actually be paid, the balance finding generic competitor’s offerings more attractive.
220 In the “Briefing document for the WBB ELT” prepared in June 2010 the loss of exclusivity of the Pfizer patents over the forthcoming years was referred to. The “opportunities in Australia” were set forth, one of which included “[d]eveloping a platform to defend LOE revenue erosion”. A “Solution” was set forth. It again referred to the role played by the “rebate” as follows:
The Solution
The Project Leap commercial offer aims to create Pfizer and brand loyalty in the 18 months leading up to LOE. The intended launch date of the commercial offer is Feb 1 2011 and building a relationship with Pharmacy is key to the strategy. Accordingly, a pharmacy field force will be recruited at the end of 2010.
…
Secondly, each pharmacy will start to accrue a rebate “bank” of 5% on all purchases of an LOE product 18 months out from LOE. That is, purchases of Lipitor will start to accrue a rebate before Caduet, Xalabrands, and there will be overlap. Due to the launch date of Feb 1 2011, Lipitor will only have 12 months before LOE.
That rebate accrual is analogous to a frequent flyer scheme, whereby the pharmacy will know each month how their rebate bank is accruing. The pharmacy representative will have that accrual on their CRM screens each month, and AR will have it noted on the monthly debtor statements.
But the offer of a mere “rebate” was not without its own consequences.
221 It was a term of Pfizer’s offer that pharmacists accept a specified volume of Pfizer’s generic atorvastatin. An incentive for the sell-in of a large volume of stock before 30 April 2012 lay in the one-month grace period available under the statutory price disclosure regime then in operation. For the first month after the listing on the Pharmaceutical Benefit Scheme Schedule of a generic product, sales of that product were excluded from calculations of the weighted average disclosed price.
222 There were within Pfizer two schools of thought as to whether pharmacists should be encouraged to take delivery of generic stock in advance of patent expiry. One school of thought was that having generic stock on pharmacist’s shelves would only accelerate erosion by encouraging pharmacists to sell through their generic stock quickly at the expense of Lipitor. That scenario would have an adverse effect on Lipitor sales and on Pfizer’s revenue given that Atorvastatin Pfizer was less profitable than the brand, Lipitor. The other school of thought was that selling in stock would not be perceived favourably by pharmacies and could affect the take of up of Pfizer’s atorvastatin offer.
223 Four levels of offer were developed – the Platinum Offer, the Gold Offer, the Silver Offer and the Alternate Offer.
224 One internal Pfizer document dated 16 November 2011 entitled “Project LEAP Update” referred to the Platinum, Gold and Silver Offers. This document referred to the Platinum Offer as “requiring a commitment on the generic of 12 months at 70% support and carries the highest levels of discount on the brand and the generic version”.
225 The terms upon which discounts would be offered and rebates offered was finally settled in January 2012. It provided for Platinum, Gold, Silver and Alternate Offers – each dependent upon the volume of Pfizer’s generic atorvastatin purchased by the pharmacy and the time over which that quantity was to be used. The final rebate and discount structure is summarised by the following table:
Offer Type | Relevant Proportion of Accrual Fund Released | Nominated Volume of Atorvastatin Pfizer | Nominated Conversion Rate | Atorvastin Pfizer Discount | Lipitor Discount |
Platinum Offer | 100% | 75% of 12 months’ anticipated generic atorvastatin | <60% | 60% | 20% |
60-75% | 70% | 10% | |||
>75% | 75% | 5% | |||
Gold Offer | 75%, with an opportunity to receive the balance later | 75% of 9 months’ total anticipated generic atorvastatin | <60% | 60% | 15% |
60-75% | 65% | 10% | |||
>75% | 70% | 5% | |||
Silver Offer | 50%, with an opportunity to receive the balance later | 75% of 6 months’ total anticipated generic atorvastatin | <60% | 55% | 10% |
60-75% | 60% | 8% | |||
>75% | 65% | 5% | |||
Alternate Offer | 0% | No minimum volume requirement | 40% | 1.5% |
This Table distinguishes between (inter alia) the generic product (Atorvastatin Pfizer) and Lipitor. Pursuant to this offer, pharmacists were invited (for example) to purchase under the Platinum offer enough Atorvastatin Pfizer to fulfil 75% of their anticipated demand for generic atorvastatin for a period of 12 months. The Gold Offer was to purchase 75% of their anticipated demand for generic atorvastatin for 9 months; the Silver Offer was to purchase 75% of the anticipated demand for a period of 6 months.
The “bundled offers”
226 The final “platform” relied upon by the ACCC was what was referred to as the “bundled offers”.
227 An aspect of the strategy pursued by Pfizer from the outset was that it would sell both Lipitor and its own generic atorvastatin to pharmacies. It would sell its atorvastatin – namely, its Lipitor and its generic atorvastatin - as a “bundle”.
228 It was only Pfizer that could sell both branded and generic atorvastatin. In this regard, its position was “unique”. Pfizer recognised the position it held and thus (for example) sought to exploit the “premium image” it held in respect to Lipitor by marketing its generic atorvastatin in almost identical packaging. It sought to market its generic atorvastatin as an “Identical Product from the same factory.”
229 In “bundling” its branded and generic atorvastatin it was doing something which no other manufacturer could offer. And, in doing so, some documents circulating internally within Pfizer stated that the stocking of pharmacy shelves with its own product would effectively “block” the ability of pharmacies to obtain and sell the generic products of other manufacturers.
230 This was an important element of the strategy under consideration by Pfizer.
231 In late November 2009, for example, a document entitled “Commercial Offer Assessment” (apparently in draft form) recorded as a “Key Explanation Point” of “Scenario 2: Become a Generics Player”:
• Second brands will be sold into pharmacy in bulk to ‘block’ other generic offers in the market.
• Second brands will be launched before LOE on key molecules to allow them to gain maximum market-share before other generic brands enter the market.
One of the assumptions relied upon was referred to as “Sell-in execution”. The “Sell-in execution” was described as follows (without alteration):
It is possible to sell in over 6 months worth of stock into a pharmacy. They will accept this.
The Project LEAP Steering Committee had its attention directed back to this aspect of the strategy at its meeting in December 2009 when a further presentation stated in respect to the “blended scenario”:
“Leverage discount on patent products to support exposed brands post LOE.”
The same “PowerPoint” presentation stated that a “Key strength” of the “blended” scenario was that it provided a:
“[g]reater chance of locking in market-share when second brands are launched before LOE.”
232 At the February 2010 meeting of the Steering Committee it was being said that:
The benefit of this scenario is we delay market share loss for year one and this benefit carries through the subsequent years as we are starting from a higher market share. We can look at strategies for year 2 and beyond down the track.
233 A presentation emailed to Mr Latham in March 2010 indicated that this scenario, which was then referred to as the “Complete Package”, was the “preferred offer”. The presentation stated:
Create generic (second brands) for Pfizer LOE brands and a full generic range of key molecules (non Pfizer products) and discount competitively. Support generic with discount on brand (pre and post LOE), offering ‘total molecule solution’ for pharmacy.
Mr Latham was also presented with the following graph:
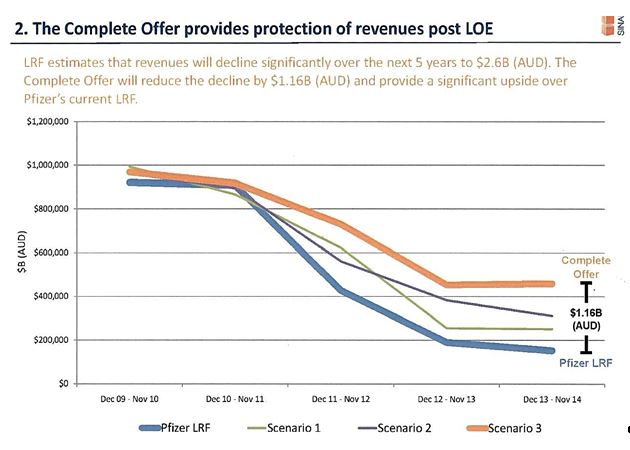
The advantage of the “complete offer” was thus presented as providing a better commercial outcome for Pfizer than if the company did nothing. The “complete offer” was recorded in this graph as showing greater “protection of revenues” than the “LRF” predicted, namely the long range forecast, if Pfizer did nothing.
234 The consideration of the strategy to be pursued by Pfizer was obviously taken against the backdrop of a consideration of the strategies that could be pursued by its competitors. Thus, for example, at a “Lipitor Scenario Planning Day” in March 2011 there were “numerous risks identified”. The first of those risks was the risk that Ranbaxy, either with or without a “co-licensing partner” would “flood [the] market in Month 1 …. blocking entry to Pfizer Atorva generic…”. The note recording the “summary outcomes” for that day records that Pfizer needed “to have a compelling offer that is sold into pharmacy EARLY. Pfizer has the advantage of being able to formulate deals and sign contracts before competitors”.
235 This “unique” position occupied by Pfizer was repeatedly acknowledged. Thus, for example, it was stated in the draft September 2011 “Project LEAP: Review” that “Pfizer is in a unique position – they are the only company selling both the brand and generic”. The same Review also stated:
There are many factors that determine the market share of generic entrants post LOE. Two of the most crucial factors are:
1. First or equal first to market, and
2. Authorised / licensed generic
Being first to market allows the generic to maximise the initial patient switch. It also provides the opportunity to employ ‘blocking’ strategies against yet to be launched generics by taking up valuable shelf space, limiting the ability for subsequent companies to sell their product. It was assumed that Pfizer would launch its 2nd brand equal first to market.
Later in this draft Review it was explained as follows how a promotional “sell in” would become “an effective blocking strategy”:
Explanation: How a promotion sell in becomes an effective blocking strategy
___________________________________________________________________________
• Pfizer will be able to ensure it is first, or equal first, to market on the launch of 2nd brands for its LOE range
• For Lipitor, there will only be one competitor – Ranbaxy – for the first 3 months post LOE
• This is due to the Government regulations regarding when a generic product can be listed (currently it is not monthly)
• Ranbaxy are seen as a weak competitor given their existing market share is estimated at around only 1%
…..
• This 3 month window presents Pfizer with a unique opportunity to gain market share
• A promotional sell in would be aimed at putting in stock longer than the 3 month window – this serves two purposes:
• Blocking shelves with Atorvastatin to reduce motivation of the pharmacist to purchase another suppliers molecule when they come to market
• If sold within the first month, the sale is excluded from Price Disclosure calculations
This draft version of the Project LEAP: Review was superseded by at least two further versions. One version dated 14 September 2011 and labelled “DRAFT ONLY” deletes the reference to the term “blocking”. A further version, labelled “FINAL Version 11, Dated 14/9/11” also deletes the term “blocking”. There were also changes to the text of the document. Part of the final version was as follows:
5.5.6 Explanation: How a promotion sell in becomes an effective 2012 strategy
___________________________________________________________________________
• Pfizer will be able to ensure it is first, or equal first, to market on the launch of 2nd brands for its LOE range
• For Lipitor, there will only be one competitor – Ranbaxy – for the first 3 months post LOE
• This is due to the Government regulations regarding when a generic product can be listed (currently it is not monthly)
• Pharmacies are aligned to each of the generic companies, selecting one of them as their ‘preferred’ supplier
• This relationship ensures that the best discount is on offer to pharmacy, in return for support of the generic partner range
• If the generic partner cannot offer a generic molecule, the pharmacy is free to source it from another supplier
• This small time window presents Pfizer with an opportunity to gain market share
• A promotional sell in would be aimed at putting in stock longer than the 3 month window – this provides an important benefit in that if the stock is sold within the first month, the sale is excluded from Price Disclosure calculations
236 The language of “blocking” nevertheless persisted. In a document prepared in about November 2011 and intended to be used at the “Atorva Launch – January 2012” (Atorva being an earlier proposed name for Pfizer’s generic atorvastatin) it was stated that the “deal” to buy 12 months stock “blocks competition”.
237 The first approaches to Community Pharmacies occurred in about December 2010. The first few months evidenced pharmacy resistance to Pfizer Australia. The Regional Sales Manager for Victoria and Tasmania, Mr David Penny, maintained that the period from December 2010 to March 2011 “were the four hardest months of [his] professional life”. It was his view that the anger expressed towards Pfizer Australia by the pharmacies was “created largely because the pharmacists were concerned that Pfizer DTP would take business away from the wholesalers and would threaten the viability of the Australian wholesaler model if other originators followed Pfizer's lead”.
The reaction of the pharmacists
238 The strategy developed and implemented by Pfizer met with some reservation from pharmacists in respect to both:
the Direct-to-Pharmacy Model; and
the manner in which rebates were linked to a commitment to purchase future product.
239 Mr King, for example, is a partner in and the manager of 5 pharmacies. He was approached by a Pfizer representative who “offered deals on Pfizer’s products”. The Direct-to-Pharmacy Model, in Mr King’s opinion, had two implications, namely:
wholesalers were no longer able to supply several high-volume lines of pharmaceuticals manufacturer by Pfizer; and
Pfizer offered fewer free deliveries than wholesalers – Pfizer only offered two free deliveries a week whereas wholesalers generally offered five to six free deliveries.
Mr Karsz, a pharmacist employed by Jon Ravech’s Chemsave Pharmacy, expressed the same concerns as to the limited deliveries from Pfizer.
240 When an offer was made to Mr King, he discussed it with his partners. They decided in late February 2012 to recommend they accept the Pfizer offer because:
by that stage they had accumulated “tens of thousands of dollars in rebates” which they would lose if the offer was not accepted; and
Atorvastatin Pfizer was likely to be the only generic atorvastatin which was the same size and shape as Lipitor with similar packaging.
The similarity of the packaging and physical appearance of Atorvastatin Pfizer and Lipitor was an important consideration to Mr King because he believed:
his pharmacies would be able to convert more customers to an atorvastatin product manufactured by the original supplier than to any other brand of generic atorvastatin; and
it would be less confusing to customers to switch from Lipitor to a generic also manufactured by Pfizer – customers frequently relying upon the colour, shape and branding of tablets and packaging to identify which pharmaceutical they are meant to take.
241 The account given by Mr King was substantially the same as that given by Mr Karsz. In February 2012 a representative of Pfizer attended upon Mr Karsz and Mr Ravech, Mr Karsz’s superior. The representative apparently said words to the effect:
You can accept a certain type of offer depending on the amount of Atorvastatin Pfizer you agree to purchase from Pfizer.
At that time Pfizer was the only supplier of atorvastatin. The offer made meant that the pharmacy could purchase the pharmaceutical at about $3 and sell at a “significant margin”. The rebate offered was the “main reason” the offer was accepted. The pharmacy also received a 20% discount off the price of Lipitor and a 60% discount off the price of Atorvastatin Pfizer. A further reason for accepting the offer, as explained by Mr Karsz, was that the pharmacy made “higher margins from the sale of generic brands than it does from originator brands”.
242 Ms Carter, another of the pharmacists who had prepared an affidavit at the request of the ACCC but who was unavailable to be cross-examined, also commented upon the fact that the Direct-to-Pharmacy Model impacted on the frequency at which Chempro could offer Pfizer products. Ms Carter also gave the following account of her reaction to the offer being made by Pfizer by one of its representatives, Ms Saunders:
When Ms Saunders presented the Pfizer Offer to me, I recall thinking that the quantity of Atorvastatin Pfizer that Chempro would be required to order if it accepted any of the offers was ‘mind boggling’. I also recall thinking that the discounts offered on Atorvastatin Pfizer as a term of the offers were not very attractive in comparison to discounts Chempro usually receives on other generic molecules and in comparison to Ranbaxy’s offer for atorvastatin …
She also gave evidence that the Pfizer response to the failure of the Chempro Group to reach its required “substitution” rates was swift and immediate. Ms Carter went on to give the following account of her conversation with Ms Saunders, a Pfizer sales representative:
Saunders: “Your atorvastatin substitution rates are not where they need to be and I can tell by your attitude that you are not interested in doing anything about it so I am cutting your discount on Lipitor.”
Carter: “So you’re not going to give us an opportunity to try to increase the substitution rate?”
Saunders: “I can tell by your attitude that you are not interested in doing that”.
Carter: “When will the discount be removed?”
Saunders: “Tomorrow”.
Ms Carter’s affidavit went on to note:
The conversation ended shortly thereafter. I recall that immediately following this discussion I started to place an order for Lipitor on Pfizer’s online ordering system and found that the discount recorded in the system was at the reduced rate of 1.5% advised by Ms Saunders. I didn’t continue with this order and didn’t complete the “checkout”.
243 Common to the accounts given by the pharmacists called by the ACCC to give evidence was that the following facts were key to their reasons for accepting the Pfizer offer:
the fact that each had accumulated a rebate which they did not wish to lose;
the fact that atorvastatin was a significant part of their business, both in terms of volume and value; and
the fact that the generic atorvastatin supplied by Pfizer (Atorvastatin Pfizer) was available in advance of other alternative generics.
Sales of atorvastatin after 18 May 2012
244 Although the ACCC’s case focussed particular attention upon the period up to the expiration of the Pfizer patent on 18 February 2012, there was some evidence as to the events that unfolded thereafter.
245 Those later events established:
the fact that the introduction of generic atorvastatin immediately impacted upon the 100% market share previously enjoyed by Pfizer;
the fact that there was a significant shift from persons who previously took Lipitor to a generic atorvastatin; and
the fact that people purchasing atorvastatin “switched” from one generic atorvastatin to another generic.
246 The impact of the availability of generic atorvastatin entering the market was significant. The following table exposes the percentage share of the market of Lipitor as against all generics before and after 1 April 2012 (being the date upon which Pfizer and Ranbaxy’s generic atorvastatin products were first able to be supplied under the Pharmaceutical Benefits Scheme):
Substitution of generics for brand | ||
(Percentage shares of all atorvastatin dispensed) | ||
Lipitor | All generics | |
Jan-2012 | 100% | 0% |
Feb-2012 | 100% | 0% |
Mar-2012 | 100% | 0% |
Apr-2012 | 32% | 68% |
May-2012 | 25% | 75% |
Jun-2012 | 23% | 77% |
Jul-2012 | 23% | 77% |
Aug-2012 | 22% | 78% |
Sep-2012 | 22% | 78% |
Oct-2012 | 22% | 78% |
Nov-2012 | 21% | 79% |
Dec-2012 | 22% | 78% |
Jan-2013 | 22% | 78% |
Feb-2013 | 22% | 78% |
Mar-2013 | 22% | 78% |
Apr-2013 | 22% | 78% |
May-2013 | 22% | 78% |
Although the percentage share of the market for “all generics” included within this table included the Pfizer generic atorvastatin, the immediate impact upon the market of the listing of other generic atorvastatin products nevertheless remains.
247 The experience of the extent to which atorvastatin users would “switch” from one generic atorvastatin to another generic varied from pharmacy to pharmacy. At one pharmacy, being the pharmacy run by Mr King at Walcha in New South Wales, the following table exposes both the “switch” from Lipitor to a generic and the “switch” from Atorvastatin Pfizer to another generic:
Substitution of generics for brand | Conversion from one generic to another | ||||||
(Percentage shares of all atorvastatin dispensed) | (Percentage shares of all generic atorvastatin dispensed) | ||||||
Lipitor | All generics | Atorvastatin Pfizer | Torvastat | Trovas | Atorvastatin APO | ||
Pfizer | Aspen | Ranbaxy | Apotex | ||||
Jan-2012 | 100% | 0% | |||||
Feb-2012 | 100% | 0% | |||||
Mar-2012 | 100% | 0% | |||||
Apr-2012 | 5% | 95% | 99% | 0% | 1% | 0% | |
May-2012 | 5% | 95% | 100% | 0% | 0% | 0% | |
Jun-2012 | 9% | 91% | 100% | 0% | 0% | 0% | |
Jul-2012 | 6% | 94% | 100% | 0% | 0% | 0% | |
Aug-2012 | 10% | 90% | 100% | 0% | 0% | 0% | |
Sep-2012 | 10% | 90% | 100% | 0% | 0% | 0% | |
Oct-2012 | 8% | 92% | 98% | 2% | 0% | 0% | |
Nov-2012 | 11% | 89% | 69% | 31% | 0% | 0% | |
Dec-2012 | 14% | 86% | 40% | 60% | 0% | 0% | |
Jan-2013 | 12% | 88% | 31% | 69% | 0% | 0% | |
Feb-2013 | 19% | 81% | 27% | 73% | 0% | 0% | |
Mar-2013 | 11% | 89% | 16% | 84% | 0% | 0% | |
Apr-2013 | 17% | 83% | 17% | 83% | 0% | 0% | |
In the pharmacy managed by Mr Safaei-Hosseinpour, the switch from Atorvastin Pfizer to the Sandoz generic was even more dramatic. By September 2012 100% of generic atorvastatin sold at that pharmacy was supplied by Sandoz.
Sections 46 and 47
248 In order to bring these disparate facts within the reach of ss 46 and/or 47 of the Competition and Consumer Act, the ACCC submits that from 2010 onwards Pfizer embarked upon a strategy to avert impending losses which it foresaw would flow from the loss of its exclusivity on patented products, including Lipitor. The strategy, or so it was submitted, sought to delay its exposure to the full force of competition by entering into long-term supply agreements with community pharmacies before its competitors could effectively compete for sales.
249 The conduct of Pfizer relied upon, cumulatively, by the ACCC to bring the facts within the reach of s 46(1)(c) of Competition and Consumer Act include:
(i) the commencement in January 2011 of an exclusive supply arrangement for the supply of pharmaceuticals (including Lipitor) directly to community pharmacies – the Direct-to-Pharmacy Model;
(ii) the allocation to an accrual fund, as from January 2011, of 5% of all Lipitor sales, to be used as a rebate which could be “unlocked” or accessed by those community pharmacies that accepted Pfizer’s offers for its generic atorvastatin; and
(iii) the making of “bundled offers”, prior to 18 May 2012 – the date that the atorvastatin patent expired.
This conduct, the ACCC further submits, was pursued by Pfizer for the substantial purpose of deterring or preventing:
(a) all competitors, other than Ranbaxy, from supplying atorvastatin to a substantial proportion of community pharmacies from 19 May 2012; and
(b) Ranbaxy (and potentially one licensee from Ranbaxy) from supplying atorvastatin to a substantial proportion of community pharmacies from 19 February 2012.
To bring the facts within the reach of s 47(2)(d) and/or (e), the ACCC further submits that atorvastatin was supplied by Pfizer on a “condition” and for the purpose of “substantially lessening competition” (see s 47(10)(a)).
250 The submissions advanced on behalf of the ACCC that Pfizer thereby contravened ss 46 and/or 47 of the Competition and Consumer Act are rejected.
251 For the purposes of s 46 of the Competition and Consumer Act, it is concluded that:
(i) the relevant market is the Australia-wide market for the supply of atorvastatin to, and acquisition of atorvastatin by, community pharmacies;
(ii) until late 2011 Pfizer had a substantial degree of market power in that market and took advantage of that market power by both distributing its products through the Direct-to-Pharmacy Model and establishing the Accrual Funds Scheme whereby “rebates” would accumulate in a “bank” for the benefit of each pharmacy; and
(iii) Pfizer took advantage of such limited market power as it retained in making the Platinum, Gold and Silver Offers in January 2012;
but that:
(iv) as from January 2012 Pfizer did not have a substantial degree of market power;
and that:
(v) at no point of time did Pfizer engage in conduct for a purpose proscribed by s 46(1)(c).
Separate from any of these conclusions, and on one view the only conclusion that need be reached, is that:
(vi) the manner in which the ACCC pleaded the contravention of s 46 was “legally incoherent” such that the entirety of its s 46 claim could have been summarily dismissed.
252 For the purposes of s 47 of the Competition and Consumer Act, it is concluded that:
(i) the market is again the Australia-wide market for the supply of atorvastatin to, and acquisition of atorvastatin by, community pharmacies;
(ii) only one condition – namely a condition found within the Pharmacy Acceptance Form completed by pharmacies – was a “condition” that fell within s 47(2);
but that:
(iii) Pfizer did not engage in conduct for the purpose of “substantially lessening competition”.
SECTION 46
253 Section 46 focusses attention, relevantly for present purposes, upon:
the correct identification of the “market”; and
the degree of power Pfizer possessed within that “market”.
It is only if Pfizer possessed a “substantial degree of power” in a market that it becomes relevant to determine:
whether Pfizer “took advantage” of that power; and
the “purpose” for which it “took advantage” of that “power”.
The “market”
254 There was common ground between the ACCC and Pfizer that – whatever may be the “market” – that “market” was an Australia-wide market. Unlike the question which arose in Australian Competition and Consumer Commission v Air New Zealand Limited [2014] FCA 1157 as to whether there was a market in Australia, there was agreement in the present case that any market was Australia wide.
255 The division between the parties was – within Australia – how the “market” was to be defined and over what period of time.
256 Paragraph [27] of the Amended Statement of Claim pleads that “… at all material times there was an Australia-wide market for the supply of atorvastatin to, and acquisition of atorvastatin by, Community Pharmacies (Atorvastatin Market)”. No alternative market was pleaded by the ACCC.
257 The Defence to the Amended Statement of Claim denies this allegation. Pfizer contends that “… at all material times there was a market for the wholesale supply of pharmaceutical products and over-the-counter products to Community Pharmacies in Australia (the Wholesale Market)”.
258 When considering the proper characterisation of the “market” for the purposes of resolving the present dispute, there were a number of relevant points of agreement as between the ACCC and Pfizer, including agreement that:
the definition of the “market” in question is a factual inquiry which is to be approached in a “purposive” manner;
the “market” could potentially change over time; and
the “market” was the same for the purposes of both ss 46 and 47.
There was disagreement, however, between the parties – largely caused by the manner in which paragraphs [67] and [67A] of the Amended Statement of Claim were pleaded – as to the period of time during which the market was to be determined. On behalf of the ACCC, it was contended that the Amended Statement of Claim identified that period as:
the period “from at least 1 December 2010 to 18 May 2012”;
and that the period was not confined to:
the period from 16 January 2012 to 18 May 2012.
Pfizer contended that the relevant period was from 16 January 2012 to 18 May 2012.
259 Whatever be the period of time in question, the resolution of this divergence of views as to which constituted the “market” necessarily involved a factual inquiry. The factual nature of the inquiry to be undertaken, it is well recognised, is fundamental. Thus, for example, in his treatise on Trade Practices Law (at [30.245]), J D Heydon writes as follows:
The dimensions of a market are real, not theoretical. To define those dimensions the best evidence will come from the people who work in the market: the marketing managers and salesmen, the market analysts and researchers, the advertising account executives, the buyers or purchasing officers, the product designers and evaluators. Their records will establish the dimensions of the market; they will show the figures being kept at competitors’ and customers’ behaviour and the particular products being followed. They will show the potential customers whom salesmen are visiting, the suppliers whom purchasing officers regularly contact, products against which advertising is directed, the price movements of other suppliers which give rise to intra-corporate memoranda, the process by which products are bought, what buyers must seek in terms of quantities, delivery schedules, price flexibility, why accounts are won and lost.
This passage has been referred to with approval: eg, Australian Competition and Consumer Commission v Metcash Trading Ltd [2011] FCAFC 151 at [312], (2011) 198 FCR 297 at 358 per Yates J. The identification of the relevant market “involves fact-finding together with evaluative and purposive selection”: Singapore Airlines Limited v Taprobane Tours WA Pty Ltd (1991) 33 FCR 158 at 174 per French J (as his Honour then was).
260 For the purposes of the present proceeding, it is further concluded that:
the “market” is the market contended for by the ACCC;
there has been no change in the “market” during the period from December 2010 to May 2012;
and that:
Dr Pleatsikas was correct in his conclusion that “the economic principles relevant to defining the dimensions of a market for the purposes of” ss 46 or 47 “are essentially identical in general”.
In so concluding it is nevertheless recognised that the “market” was in a state of flux from at least January/February 2012. Whether the perimeters of the “market” remained the same later in mid-to-late 2012 was not a question which was expressly addressed in the evidence and which, accordingly, could be resolved. Whether there was a “generics market”, for example, in 2012/2013 is not a question presently in need of resolution. It is to be recalled that “there can be overlapping markets with blurred limits and disagreements between bona fide and reasonable experts about their definition”: NT Power Generation Pty Ltd v Power and Water Authority [2004] HCA 48 at [68], (2004) 219 CLR 90 at 117 per McHugh A-CJ, Gummow, Callinan and Heydon JJ.
The expert evidence: a fundamental difference in approach
261 Expert evidence may serve a useful role in the definition of a market. An expert economist can assist the Court “by expressing in his or her own words, what the human underlying facts reveal to him or her as an economist and what it reflects to him or her about underling economic theory and its application”: Australian Competition and Consumer Commission v Liquorland (Australia) Pty Ltd [2006] FCA 826 at [840], (2006) ATPR 42-123 at 45,308 per Allsop J (as his Honour then was). His Honour there further observed:
[837] In this context, it is appropriate to say something of the place and role of expert witnesses in cases such as this. In giving reasons for rulings on some of the expert evidence ([2005] FCA 630) I identified some aspects of the presentation of expert evidence in competition cases. If I may repeat, by way of paraphrase, part of what I there said in the context of ruling on evidence in the following.
[838] In cases such as this dealing with a social science, the views of Professor Brunt expressed, if I may respectfully say so, with her customary clarity in chapter 8 of the helpful compendium of her work Economic Essays on Australian and New Zealand Competition Law, illuminate one aspect of the helpful, indeed essential, role for expert evidence in this field. In that chapter, Professor Brunt quoted Keynes at page 358, where that learned economist said:
The Theory of Economics does not furnish a body of settled conclusions immediately applicable to policy. It is a method rather than a doctrine, an apparatus of the mind, a technique of thinking, which helps its possessor draw correct conclusions.
[839] The “economic” questions here involved the assessment of the purposes of humans working in a commercial environment and the appropriate economic framework in which to discuss them.
[840] With the taxonomy of expert evidence of fact, assumptions, reasoning process and opinions as an accepted (indeed necessary) framework, one then comes to the role of the economist in a case such as this. Because it is a social science, and because it is a way of approaching matters and a way of thinking about matters, there is a role, for the economist to assist the court …
262 In the present proceeding, the expert relied upon by the ACCC was Dr Christopher Pleatsikas. Dr Pleatsikas is a Director at Berkeley Research Group, a business strategy consulting firm headquartered in California. That group provides economic and litigation support services. Dr Pleatsikas holds a PhD in regional economic analysis from the University of Pennsylvania.
263 The expert relied upon by Pfizer was Dr Sumanth Addanki. Dr Addanki is a Senior Vice President at National Economic Research Associates, Inc. Dr Addanki holds a PhD in economics from Harvard University.
264 Dr Pleatsikas was presented with a number of assumptions and formed the conclusion in his report that the relevant market was the market for the supply of atorvastatin. He further concluded that Pfizer operated “as a vertically integrated supplier of atorvastatin drugs that manufactures … these drugs and operates as a wholesaler…”. He concluded that “several significant factors favor defining a relevant market that includes the manufacturing functional level…”.
265 Dr Addanki reached the opposite conclusion. Dr Addanki concluded that “Pfizer Australia’s conduct that is the subject of the ACCC’s Statement of Claim occurred in the market for the supply of pharmaceuticals to community pharmacies in Australia”.
266 The approach of the two experts was significantly different.
267 Indeed, when pursuing the differences between the approach of Dr Pleatsikas and that of Dr Addanki, Dr Pleatsikas maintained in the following passages in his cross-examination that Dr Addanki had undertaken the analysis of market “backwards” (without alteration):
Right. And you see, what you had to look at to satisfy yourself about the restraining capacity of atorvastatin was conduct going forward to at least September 2012. That’s right, isn’t it? --- I – I would disagree with that characterisation.
And what you didn’t do, looking forward, is considered the behaviour and the constraining effect upon the price of atorvastatins of the behaviour of the major suppliers of generic products, did you? --- I defined a product market and then I looked at the constraints within the product market. You’re trying to do it backwards. You’re trying to say, okay, let’s look at the constraints first and then define the product and that’s not the way it’s done.
It’s an iterative process, isn’t it? You look at the – the product – start with the product and then start identifying operative constraints and, through an iterative process, work out whether you’re dealing with the correct product market. That’s correct, isn’t it? --- Not necessarily, no.
It may be, mightn’t it? --- In some circumstances, yes. In this circumstance, that wasn’t necessary because atorvastatin has no substitutes. None at all.
And that pure physical fact is the beginning and end of your inquiry, isn’t it? --- No.
But you don’t look to the behaviour of other participants and how they seek to constrain the price of atorvastatin over the long term, do you? --- Yes. The fact that they – that they operate as constraints on the price of atorvastatin doesn’t mean that other non-substitutable items suddenly become substitutable for atorvastatin. That’s not the way it works.
Right. But you may be looking at the wrong product, mightn’t you? And the true product is ranges of pharmaceuticals offered in the Australian market. That’s correct, isn’t it? --- That’s wrong.
Right. But you understand that’s what Dr Addanki has done? --- I understand - - -
That’s correct ---? - - - I understand that Dr Addanki did it backwards. He started with market participants and defined a market – he actually didn’t define a product market and didn’t use a methodology to do it.
What he found – what I want to suggest to you – is the true close constraints upon the behaviour of Pfizer which are relevant to the subject matter of the inquiry in this case. That’s correct, isn’t it? --- I disagree with that and I disagree that he even looked at the relevant conduct ---
Yes? --- - - - to try to figure out what the relevant constraints were.
In addition to disagreeing with the approach of Dr Addanki, Dr Pleatsikas also disagreed with the conclusion that the market was a market for a “range” of pharmaceutical products.
268 Central to the reasoning of Dr Addanki was his conclusion that there was no doubt as to the product dimension of the market and his conclusion that the product – atorvastatin – was supplied as part of a “bundle”. He thus rejected as follows the proposition of his cross-examiner that the “initial candidate” for determining the product dimension of a market was the product itself:
Now, you would agree that for the purposes of identifying the product dimension of the market, it would not be relevant to focus on the conduct or presumed conduct of other market participants? --- No, I think that’s incorrect.
What I want to suggest that for the product dimension of the market, the initial candidate market would be the product supplied by the firm pursuant to the conduct in issue. You would agree with that, wouldn’t you? --- If there were any doubt about the product dimension of the market and one were engaged in a serious inquiry in order to resolve that doubt, one might proceed on those lines, but that simply isn’t the case here.
Shortly thereafter he explained as follows his reasons:
… Now, in undertaking your market definition exercise, I suggest you have not focused on the smallest market that would satisfy the hypothetical monopolist test in terms of the product dimension, have you? --- I have not used a hypothetical monopolist test on the product dimension, because I didn’t need to. Pharmacies need to buy atorvastatin. The question is how did they buy it and what constraints the – the putative exercise of market power by a supplier of atorvastatin, and that’s the question we addressed and I came up with the close comparative constraints that define the relevant market.
Now, when you said “we address”, who is “we”? --- We is me. Sorry. I.
Dr Addanki returned to this theme a little later when the following exchange occurred:
Now, again, I want to suggest to you that the product dimension of the market is a different exercise to determining who are the participants in a market; would you agree? --- Again, if there were any doubt, and there is sometimes doubt about the product dimension, you may embark on an inquiry to resolve that doubt. When you know that the product being transacted in the first instance is atorvastatin, there is no doubt about that. The question is what exactly actually happens in the transacting that leads to pharmacies buying atorvastatin.
Now, you say there’s no doubt that it’s clearly atorvastatin, but you don’t identify an atorvastatin market; is that fair? --- I identify a market that represents the actual economic realities of the competitive constraints of a supplier of atorvastatin.
And what I want to suggest to you, that market definition is concerned not with competitive constraints, but rather with close substitutes. What do you say to that? --- At the end of the day, there is no recipe or formula. What we’re looking for is what constrains the potential exercise of market power – that is the relevant market – and all of these thought experiments and aids to analyses perform – simply perform the function of organising your thinking in getting to the right solution on that question.
The atorvastatin market
269 It is respectfully concluded that many facts dictate the conclusion that the “market” is the market for atorvastatin as identified by the ACCC.
270 Notwithstanding the morass of factual detail as to the manner in which manufacturers supply their pharmaceutical products and the manner in which pharmacies purchase those products, the conclusion that the market was a market for atorvastatin is a conclusion dictated by the evidence informed by the views of Dr Pleatsikas. At all times a pharmacist presented with a prescription for atorvastatin was required to supply atorvastatin. In the language of an economist, throughout that period there was:
no demand-side substitution; and
no supply-side substitution.
271 In the first of his two reports, Dr Pleatsikas concluded:
[55] … that Pfizer operates as a vertically integrated supplier of atorvastatin drugs that manufacturers (or contracts for the manufacture of) these drugs and operates as a wholesaler (through a logistics arrangement with DHL) of atorvastatin drugs to Community Pharmacies based in Australia. As such, it competed (but to a limited extent during the Relevant Period – and only with supplies from Ranbaxy) both with generic makers of atorvastatin products and with wholesalers that could supply atorvastatin products to Community Pharmacies. The relevant conduct relates to both manufacturing and wholesale/distribution functions.
[56] However, given that the objective of market definition analysis is to evaluate whether and to what extent any market power may exist that could facilitate or otherwise enable the conduct at issue, several significant factors favor defining a relevant market that includes the manufacturing functional level. First, and most important, the patent protection on atorvastatin precluded supply-side substitutability, absent a licence from Pfizer (which, during the Relevant Period, was not available except to Ranbaxy for a short time just before the end of the Relevant Period). This patent protection is most relevant to the manufacturing function. Second, once prescribed, there could be no demand-side substitution between atorvastatin and other molecules. This lack of demand-side substitutability for atorvastatin appears to be the result of regulatory rules and is not a function of market forces in any market. Third, the wholesaler margins under the PBS were relatively small (7.52 percent up to a maximum of $69.94). The PBS wholesale margin limits would likely constrain the ability of wholesalers to exercise or counterbalance any substantial market power. Fourth, Pfizer was able to completely bypass the major wholesalers during most of the Relevant Period in order to implement the Atorvastatin Conduct and the Atorvastatin Supply Conduct.
Concurrence is expressed with these conclusions. No matter how other generic manufacturers may have been supplying their own “range” of generic products and no matter what may have been the terms and conditions of supply arrangements between those manufacturers and their aligned pharmacies, the fact remained that throughout the period from December 2010 through to May 2012 a prescription for the supply of atorvastatin could only be supplied by the atorvastatin pharmaceutical supplied by Pfizer. The availability to secure product from Ranbaxy for a limited period towards the end of the period, it is respectfully concluded, does not alter that conclusion.
272 Expressed differently, but still with the emphasis upon whether there was substitutability in respect to the product dimension of market definition, it is not without relevance to note:
the volume and value of atorvastatin supplied to the Australian community under the Pharmaceutical Benefits Scheme. Prior to 1 April 2012, Lipitor was the only atorvastatin product available on a subsidised basis;
the fact that atorvastatin was a significant, if not key, pharmaceutical product essential to the practice of an Australian pharmacy – it was a product which contributed significantly to the income of pharmacies. As Ms Carter expressed it: “every pharmacy has to stock” atorvastatin; and
the fact that there was a demand for atorvastatin from a considerable number of patients – it was a pharmaceutical product prescribed by medical practitioners for a considerable number of patients.
Moreover:
when comparing offers, pharmacists compared the prices at which atorvastatin was being offered. According to Ms Carter, “the most important factor for Chempro in determining which supplier of generics (sic) pharmaceuticals … to use to supply a particular pharmaceutical is always price …”.
Additional matters include the fact that:
the generic manufacturers long before 18 May 2012 were contemplating how they would supply atorvastatin to the Australian community;
Apotex, in structuring how it would supply its own generic atorvastatin perceived its challenges to include the ongoing need for pharmacies to continue stocking Lipitor;
the fact that Pfizer had offered and was offering significant discounts on Lipitor and that pharmacies had built up over time considerable sums in Pfizer’s “bank”;
Alphapharm, in structuring how it would supply its generic atorvastatin perceived that it was unable to respond to the Pfizer offers and devised its own “three-pronged strategy” which focussed on “requiring no long term purchase commitments and exploiting [their] understanding of the pharmacies (sic) negative views of Pfizer…”;
Ranbaxy offered discounts on its own generic atorvastatin – Trovas – even though it did not generally offer rebates or discounts to customers which were tied to the bundling of more than one product; and
there was considerable competition for the supply of atorvastatin – with a considerable number of people switching from Lipitor to Atorvastatin Pfizer and a considerable number of people switching from one generic product to another.
Such facts, it is considered, support a conclusion that atorvastatin was being seen to be – and being marketed as – a separate pharmaceutical product in its own right for which there was no substitute throughout the period in question. Although the generic manufacturers may have marketed their own generic range of pharmaceuticals, and resisted attempts by pharmacies to “cherry-pick” individual products from within their ranges, atorvastatin had long been regarded as a truly unique product. It was a product which pharmacies had to stock and was a considerable source of their income.
273 Even if it is accepted that the period of time during which the “market” is to be determined is that period between January/February and May/June 2012, no different conclusion would be reached.
274 Although it may readily be accepted that the dimensions of a market may change over time, and even though it may well be the case that the “market” as between January/February and May/June 2012 may have been in a state of flux, it is nevertheless concluded that the dimensions of that “market” had not changed by mid-2012.
275 On the case advanced by Pfizer, two factors assumed particular relevance as at 16 January 2012, if not much earlier, namely:
the loss of exclusivity on the Pfizer atorvastatin patent was a “massive event” – it was an event which had long been anticipated. The total volume of sales of atorvastatin whilst under patent and the value of those sales, was considerable. Prospective competitors, it was submitted (and the evidence demonstrated), were anticipating gaining their own share of such a valuable source of income. Prospective competitors were anticipating that they could make their own generic atorvastatin available at discounts at rates of up to 85 to 90% off the “Chemist List Price”;
and contrary to the case being advanced by the ACCC:
it was the other generic manufacturers who were best placed to take advantage of the loss of exclusivity which would occur on 18 May 2012.
But neither factor, it is respectfully concluded, dictates any different conclusion in respect to the definition of the “market” – even if attention be confined to the first half of 2012.
A generics market - a view which does not prevail
276 In reaching this conclusion, the contrary position advanced on behalf of Pfizer is necessarily rejected. This contrary position was that there was “a market for the wholesale supply of pharmaceutical products and over-the-counter products to Community Pharmacies in Australia”.
277 The contrary argument advanced on behalf of Pfizer was intensely fact-oriented. This contrary argument initially focussed upon:
the dominant role played by the Commonwealth Government via the Pharmaceutical Benefits Scheme in the provision of prescription pharmaceuticals.
The focus of submissions thereafter shifted to (for example):
the fact that generic manufacturers considered that they competed in a generics market and that Pfizer was a new entrant to that market in early 2012;
the agreements between the generic manufacturers and their aligned pharmacies and the terms of those agreements;
the fact that generic manufacturers generally compete with each other on range of products supplied, price and supply terms; and
the fact that long-term preferred supplier agreements between generic manufacturers and pharmacies typically offer discounts, rebates and other benefits across a range of products.
These are but some of the facts which Pfizer claimed were relevant to a conclusion that the market was the generics market for the supply of pharmaceutical products and over-the-counter products.
278 Whatever may be the “range” of other pharmaceutical products offered by the generic manufacturers, and whatever may be the manner in which pharmacies purchase a “range” of products from the generic manufacturers or wholesalers, the fact nevertheless remains (for example) that a prescription issued by a medical practitioner for atorvastatin cannot be “filled” by the supply of any other product. As maintained by Mr King: “[a]s atorvastatin is a prescription pharmaceutical … a pharmacist is not permitted to supply any other pharmaceutical product other than atorvastatin when filling the customer’s atorvastatin prescription”. The fact that atorvastatin may now – and the fact that other pharmaceutical products have long been offered as but a part of a range of other products – does not detract from the conclusion that atorvastatin prior to mid-2012 formed its own market.
279 Whether the intense competition for the “switching” from one generic version of atorvastatin to another and whether the manner in which atorvastatin is now marketed would lead to a different definition of “market” as at (for example) mid-2013 is a question which need not be pursued. Whether atorvastatin has, for example, now entered a more generalised “wholesale market” may be a question for another day.
Substantial market power?
280 Within the atorvastatin market, the ACCC thereafter maintained that Pfizer had a substantial degree of power in that market.
281 That period of time during which the ACCC maintains that Pfizer had such market power was variously expressed. The Amended Statement of Claim thus maintained that Pfizer:
had a “substantial degree of market power” as from “in or around June 2000 until at least 18 May 2012” (para [61]); and
contravened s 46 “in that during the period between at least 1 December 2010 and 18 May 2012 [it] held a substantial degree of market power in the Atorvastatin Market and took advantage of that power …” (para [67]).
The Amended Originating Application sought a declaration in respect to s 46 in terms that “in the period between at least 1 December 2010 and 18 May 2012” Pfizer held a substantial degree of market power and took advantage of that power.
282 Separate from its contention that the “market” was the market for the supply of atorvastatin to community pharmacies in Australia, Pfizer further contended that any market power it exercised varied over time. Whatever may have been its market power at a point of time long before there was any real prospect of a generic atorvastatin being supplied by competitors, Pfizer submitted that its market power became ever increasingly exposed to threat the more imminent its loss of exclusivity became. The “barrier” to the entry of other suppliers of atorvastatin erected by the exclusive rights conferred by its patent was gradually being eroded.
283 On the case advanced on behalf of Pfizer, its market power as at:
January 2011 – namely at that point of time when the Direct-to-Pharmacy Model was implemented and its rebate scheme established
was very different to the market power it possessed:
between January and May 2012.
Although the Pfizer submission as to the definition of “market” has been rejected, it is at that point of the analysis where attention is focussed upon “market power” that the division between the parties as to the period of time the subject of inquiry potentially becomes more pressing.
284 Prior to late 2011, it is respectfully considered that no conclusion is open other than that Pfizer possessed both “market power” and that such power as it possessed was truly “substantial”. If the market be correctly identified as the atorvastatin market, Pfizer had long been the sole supplier of atorvastatin. The fact that the price at which it could sell that product may have been subject to some limited degree of regulation pursuant to the Pharmaceutical Benefits Scheme is not sufficient to render its market power anything other than “substantial”.
285 Well prior to the expiration of its patent in May 2012, the other generic manufacturers began planning their onslaught upon the market. From at least 2010, the established generic manufacturers were planning their future sale of atorvastatin. But – and despite the fact that the other generic manufacturers were circling the prey from an early date – Pfizer retained substantial market power up to late 2011.
286 But Pfizer’s market power gradually decreased the more imminent the expiration of its patent became. Notwithstanding this reduction in its power, it is nevertheless respectfully concluded that Pfizer maintained some degree of market power up to May 2012. It retained its unique ability to exploit, for example, the marketing of Lipitor at a premium price and to package its generic atorvastatin in a manner identical with or substantially similar to the packaging of the established brand, Lipitor. But as from January 2012 it is concluded that the market power Pfizer retained was not “substantial”.
287 There is, of course, no clear or definitive point of time at which Pfizer’s market power ceased to be “substantial”. It matters not that that point of time may have been reached by September or October 2011 rather than December 2011. What potentially matters is the finding that as from January 2012 Pfizer no longer possessed “substantial” power in the market for the supply of atorvastatin.
288 Relative to the forthcoming competition from the generic manufacturers of atorvastatin, the power that Pfizer had once exercised had waned (Tillmanns Butcheries Pty Ltd v Australasian Meat Industry Employees' Union (1979) 42 FLR 331 at 348 per Deane J). And the power it retained was no longer “enduring”; that power could not be “sustained” throughout the period from January to May 2012 (Boral Besser Masonry Limited v Australian Competition and Consumer Commission [2003] HCA 5 at [287] and [293], (2003) 215 CLR 374 at 467-468 and 470-471 per McHugh J; Universal Music Australia Pty Ltd v Australian Competition and Consumer Commission [2003] FCAFC 193 at [158], (2003) 131 FCR 529 at 567-568 per Wilcox, French and Gyles JJ).
289 There are many factors which dictate such a finding. From late 2011, Ranbaxy had (for example) been promoting the sale of its own generic atorvastatin at discounts of 85% off the “Chemist List Price”. And Ranbaxy was able to enter the market on 19 February 2012. The impact of Ranbaxy’s entry into the market alone had the potential to significantly diminish the market power once held by Pfizer.
290 Irrespective of the constraints placed upon Pfizer by the conduct of Ranbaxy, however, other factors which also support the conclusion that Pfizer’s market power had decreased such that it lacked substantial market power during the period from January to May 2012 include:
the fact that a number of other generic manufacturers had listed their impending atorvastatin products on the Australian Register of Therapeutic Goods, with some registrations taking place in late 2009 to early 2010 but with a number occurring in the period from September to November 2010.
Moreover:
Apotex was holding discussions with its “key customers” from at least March 2012 regarding the arrangements it would put in place for the supply of atorvastatin after the Pfizer patent expired. It had arranged for its stock of atorvastatin sourced from Canada to be flown into Australia by two transport aircraft the very day the Pfizer patent expired;
and
Alphapharm had told its customers in January 2012 that it would beat Pfizer’s offer and encouraged them to take an interim deal and buy 12 months’ worth of Alphapharm’s products.
The influence that such large generic manufacturers could exert on the market cannot be underestimated. Approaches to pharmacies canvassing the relative merits of what was being offered by Pfizer at January 2012 and what other manufacturers were anticipating could be on offer after 18 May 2012 were not confined to Apotex and Alphapharm. Thus:
in early February 2012 Ascent was communicating to its aligned pharmacies in respect to the Pfizer offers which had by then been announced and urging them not to succumb “to one deal, on one molecule …”; and
Sandoz was also writing to its own aligned pharmacies in mid-February 2012 encouraging “all Sandoz customers to carefully consider” whether to accept the Pfizer offers, to seek “independent advice” and to consider whether acceptance of any such offer “could prevent you from taking advantage of Sandoz’s most exciting and competitive offers” which were to be discussed “immediately after 18th May 2012”.
Chemsave was also:
writing to its members in late January 2012 recommending that they not accept the Pfizer or Ranbaxy offers.
Within Pfizer:
it was anticipated, of course, that the other generic manufacturers would all enter the market immediately upon the expiration of its patent.
Taking advantage
291 The many permutations as to the manner in which the ACCC’s Amended Statement of Claim may be construed and resolved – and the question as to the period of time during which findings of fact are of relevance – again potentially affects the relevance of those findings of fact as to whether Pfizer has “taken advantage” of its market power.
292 The ACCC allegation that Pfizer took advantage of its substantial degree of market power was set forth at paragraphs [62] to [65B] of the Amended Statement of Claim. Those paragraphs, including the underlined amendments, provided as follows:
Taking Advantage
62. Pfizer could not have established the Pharmacy Supply Arrangements to the extent that they included the supply of Lipitor and offered significant discounts on the purchase of Lipitor without:
(a) its exclusive right to supply Lipitor in Australia;
(b) being the only source from which Community Pharmacies could acquire Lipitor in the Atorvastatin Market;
(c) there being sustained demand from Community Pharmacies for Lipitor; and
(d) its discretion as to what price it charged for Lipitor and its ongoing ability to charge a substantial premium in its price for Lipitor relative to the prices for Generic Atorvastatin;
63. Pfizer could not have:
(a) established the Accrual Funds and accumulated the Lipitor Rebates;
(b) limited access to the Lipitor Rebates to only Community Pharmacies that accepted a Platinum Offer within specified time periods;
(c) provided access to only a proportion of the Lipitor Rebates to Community Pharmacies that accepted a Gold Offer or Silver Offer within specified time periods; and/or
(d) precluded access to the Lipitor Rebates to Community Pharmacies that did not accept any Atorvastatin Pfizer or only accepted an Alternate Offer,
without:
(e) its discretion as to what price it charged for Lipitor in the period from at least the establishment of the Accrual Funds to the loss of exclusivity for Lipitor;
(f) its power and discretion over access to the Lipitor Rebates; and
(g) its power and discretion over discounts from the price of Lipitor.
64. Pfizer could not have included the Terms and Conditions or the Late Acceptance Terms and Conditions in the Atorvastatin Pfizer Offers, at the time they were made, without:
(a) Pfizer being the only supplier of Lipitor to Community Pharmacies;
(b) prior to 1 April 2012, Lipitor being the only atorvastatin product that could be supplied by Community Pharmacies to general members of the public through the PBS;
(c) Pfizer being the only firm offering, and capable of offering, a discount off the price of Lipitor;
(d) Pfizer being the only firm offering, and capable of offering, a Community Pharmacy the benefit of the Lipitor Rebates;
(e) Pfizer being initially the only firm (and from 18 February 2012 to 18 May 2012, one of only two firms) offering, or capable of offering, to supply a Generic Atorvastatin to Community Pharmacies;
(f) Ranbaxy Australia not being able to lawfully market or supply atorvastatin to the Atorvastatin Market until 18 February 2012;
(g) Other Generics Suppliers not being able to lawfully market or supply atorvastatin to the Atorvastatin Market until 19 May 2012; and
(h) from 1 April 2012 until 1 June 2012, Atorvastatin Pfizer and Trovas being the only Generic Atorvastatin brands that could be supplied by Community Pharmacies to general members of the public under the PBS.
65. By reason of the matters pleaded in paragraphs 62 to 64 above, Pfizer has taken advantage of its substantial degree of market power in the Atorvastatin Market in engaging in the conduct pleaded in paragraphs 43 to 59 above.
65A. By structuring at least the Platinum Offer to include the Lipitor Rebates, discounts on Lipitor and discounts on Atorvastatin Pfizer, Pfizer offered and supplied Atorvastatin Pfizer to Community Pharmacies pursuant to the Terms and Conditions and the Late Acceptance Terms and Conditions below Pfizer’s forecast cost of supplying Atorvastatin Pfizer, and in some cases below a zero price, in that the sum of:
(a) the Lipitor Rebates;
(b) the incremental discounts on Lipitor (relative to the discounts offered pursuant to the Alternate Offer); and
(c) the discounts on Atorvastatin Pfizer,
offered pursuant to the Terms and Conditions and the Late Acceptance Terms and Conditions of the Platinum Offer resulted in Pfizer offering and supplying Atorvastatin Pfizer to Community Pharmacies below Pfizer’s forecast cost of supplying Atorvastatin Pfizer, and in some cases below a zero price.
Particulars
i. Document named ‘Lipitor Forecast New Structure 20120104.xlsx’, January 2012.
ii. The ACCC repeats the particulars pleaded in relation to paragraphs 18(g) and 51 above.
65B. Further, or in the alternative, to paragraph 65 above, by reason of the matters pleaded in paragraph 65A above, Pfizer has taken advantage of its substantial degree of market power in the Atorvastatin Market in engaging in the conduct pleaded in paragraphs 50 to 59 above.
293 If the Pfizer submission as to the adequacy of the pleadings set forth in paragraphs [67] and [67A] of the Amended Statement of Claim is again presently left to one side, the conclusion that Pfizer did not have a substantial degree of market power during the period from January to May 2012 again makes it somewhat potentially academic to determine whether Pfizer took advantage of such power as it then possessed during that period of time.
294 But the diametrically opposed positions of the ACCC and Pfizer on this matter again suggests that it is prudent to make separate findings on these issues.
The Direct-to-Pharmacy Model – January 2011
295 There is no question but that the Direct-to-Pharmacy Model could not have been successfully implemented by Pfizer without the position it occupied as the sole supplier of atorvastatin prior to 19 February 2012. The pharmacies did not like the Direct-to-Pharmacy Model – but they could not obtain atorvastatin (at least prior to 19 February 2012) from any other source. Although Ranbaxy was able to supply pharmacies with atorvastatin after 19 February and before 1 April 2012, the amount of atorvastatin it supplied during this period was minimal. It was only from 1 April 2012 that Ranbaxy’s atorvastatin became available on the pharmaceutical benefits scheme.
296 Mr Crotty accepted as follows his cross-examiner’s suggestion that Pfizer could not have implemented its Direct-to-Pharmacy Model without a patent over atorvastatin and the exclusive rights that the patent conferred:
Now, what I want to put to you is that, unless you had patent protection over the molecules the subject of that arrangement, an exclusive direct to pharmacy arrangement would not have been commercially viable, as you understood it, for Pfizer to pursue. That’s the case, isn’t it? --- Well, yes. If you didn’t have patent protection, then the customer – the pharmacist – would have had a choice of – of other sources, and if they didn’t want to go with you and you only had a direct arrangement then they could make that decision.
And the benefit of having the patented molecules, particularly for Lipitor, was that you appreciated the pharmacy wouldn’t have any choice. They would have to take the molecules from you, wouldn’t they? --- That’s right.
297 When Pfizer implemented its Direct-to-Pharmacy Model in January 2011 it had a substantial degree of market power and took advantage of that power in order to implement that model.
The Accrual Funds Scheme – January 2011
298 Considerable care needs to be exercised when considering the allegation in the Amended Statement of Claim that Pfizer took advantage of its market power in the “establishment of the accrual fund scheme”. The “establishment” of that scheme took place in January 2011. Paragraph [63(a)] of the Amended Statement of Claim seems to allege that the establishment of the scheme in itself constitutes a “taking advantage” of market power. Paragraph [63(b) and (c)] then proceed to link the establishment of the Accrual Funds Scheme to the ability to “access” the rebates by the acceptance of a Platinum, Gold or Silver Offer. But those offers were not made until January 2012. Prior to the terms of the offers being announced, there remained considerable uncertainty as to how any pharmacy could access the rebates that had accumulated in its account or “bank”.
299 Separate from any question as to the adequacy of the pleading of a contravention of s 46 is the question as to the manner in which it is suggested by the ACCC “took advantage” of its market power in respect to the Accrual Funds Scheme.
300 Notwithstanding the uncertainty as to the precise terms upon which a pharmacist could access its “rebate” until January 2012, it is nevertheless concluded that:
Pfizer took advantage of its market power in establishing the Accrual Funds Scheme in January 2011; and that
that taking advantage of power falls within paragraphs [63(a)] of the Amended Statement of Claim – subparagraphs (a), (b), (c) and (d) being pleaded either conjunctively or disjunctively. Paragraph [48] pleads as a material fact, being a pleading later included with the pleading as to the taking advantage of power (paragraph [65]), the fact that prior to 16 January 2012 Pfizer had not advised pharmacies as to “how they could use or redeem their Lipitor Rebate”.
301 The accruing rebate upon the sale of its patented products inevitably provided an incentive to pharmacies to accept Pfizer’s offers to supply generic atorvastatin. Such a result necessarily followed from Pfizer’s position in the market as the only supplier of a product over which it had a patent and the linking of the rebate to the quantity of its generic atorvastatin that the pharmacy purchased. Mr Crotty accepted such a connection in his cross-examination:
So you were able to plan to use some of the profit that you obtained on the sale of your patented products for the purpose of creating an incentive to encourage pharmacies to purchase a Pfizer generic following loss of exclusivity? --- Yes. It would provide some of the funding towards a generic, yes.
302 In offering a rebate on its products, Pfizer may well have been pursuing no different strategy than was open to any other manufacturer of pharmaceutical products. Pfizer may well have been the only entity that could offer a rebate on Lipitor (and its other patented products). But any other manufacturer could also offer a rebate on any of the products they supplied to pharmacies. To offer a rebate on products does not itself involve or require the taking advantage of any market power.
303 But the taking advantage of market power is exposed when consideration is given to the fact that:
Pfizer could establish the Accrual Funds Scheme even in the absence of any certainty on the part of the pharmacists at the outset as to how or when the rebates in their own “banks” could be accessed; and
the ability to access the rebates that had accrued in each of the “banks” was linked to the commitment to purchase Pfizer’s generic atorvastatin.
304 Even though prior to January 2012 there was no certainty about the terms on which a pharmacy could access its rebate, there was nevertheless an expectation on the part of both Pfizer and the pharmacies it supplied that the rebate would not be appropriated by Pfizer to its own financial ends. It is concluded that the requirement imposed by s 46 as to a corporation taking advantage of power is satisfied in the particular circumstances of the present case by creating an expectation that rebates that had accumulated would be made available upon terms and conditions to be announced in the future. It was only by reason of the market power possessed by Pfizer in January 2011 that it could announce a rebate scheme without at the same time telling pharmacies how they could recover the monies that were accumulating for their benefit.
305 As at April 2012 the amount of money that had accumulated was considerable. As at that date the total value of the rebate was $35,608,484.72 held in respect to approximately 5,272 community pharmacies.
306 The manner in which Pfizer could potentially exercise its market power is exposed by the consideration being given by Pfizer to the terms and conditions upon which the rebates would be released to pharmacies. The proposed terms underwent many changes. But an instance of the manner in which Pfizer could take advantage of its market power in structuring those terms emerged in mid-2011. In May 2011 one issue being then debated was the ability of a pharmacy to access its rebate and thereafter quickly move to a competitor generic product. In an e-mail dated 18 May 2011, for example, Mr Gledhill wrote to Mr Deran Bagdadi:
… You would not want to allow the pharmacy to use its accrued rebate bank for the first 2 months of stock because they could then break the agreement and move to an alternate generic supplier. You would want to spread the usage of the rebate bank over the 6-9 month period.
A presentation later in May 2011 also referred to the strategy of releasing the accrued rebate over time. One proposal was to release 25% of the rebate in four stages when orders were made for the forthcoming three month period. The “risk” that the pharmacy may choose “to purchase generic from another supplier at any time” was identified. An alternative proposal was to release increasing percentages of the rebate depending upon the volume of stock purchased by the pharmacy.
307 Of present importance are not the terms then under consideration; of importance is the fact that Pfizer was throughout 2011 developing the manner in which it could take advantage of the market power it had in respect to Lipitor and the rebate that had accrued in respect to purchases of that product. It was developing the manner in which it could best take advantage of that power in securing the greatest commitment of pharmacies to purchase its generic atorvastatin.
308 It is of importance, however, to not focus attention exclusively upon the terms upon which the rebate could be accessed to the exclusion of the other terms of the offers being made by Pfizer. Those terms were but a part of the offer being made and but a part of the overall strategy being developed by Pfizer.
309 According to Mr Cooper, the terms upon which the rebate was to be released to pharmacies was important – “but not that important.” One exchange between Mr Cooper and his cross-examiner went as follows:
And the precise terms upon which the rebate was to be released was a very important part of the incentives, I suggest, that you were seeking to put in place to encourage community pharmacies to accept the Pfizer generic offer; that’s fair, isn’t it? --- Yes, but not that important.
When you say “not that important”, what was more important? --- The molecule solution around the brand the discount. The rebate was more a sales and marketing tool that we had sold for the 12 months prior to get them to build their relationship in a hostile environment and, financially, the rebate was not significant compared to their spend.
The cross-examination continued:
Mr Cooper, what you were seeking to do while you were developing the commercial offer was to come up with an offer that Pfizer could make that would be more attractive than what you anticipated your competitors would do for generic atorvastatin; that’s the case, isn’t it? --- No.
Were you trying to come up with an offer that was less attractive than what your competitors were likely to be able to offer? --- We were trying to come up with an offer that was competitive.
And by “competitive’ what you mean is more attractive, don’t you? --- No.
Do you seriously suggest that you were anticipating that your offer to community pharmacies would be portrayed by your sales team as nothing more than competitive with your opposition? --- No. In the selling process of it, we would try and maximum our deal.
And one way you were going to maximum that was to be able to say to community pharmacies, “This is how you get access to your Lipitor rebate; accept our offer”; that’s the case, isn’t it? --- Yes, but the rebate was only one component.
And it was one component, but I suggest, as you understood it, a very important component of the attractiveness of the commercial offer that Pfizer was proposing to make to community pharmacies, wasn’t it? --- It was – no. It’s a component, but it was actually more psychological. We used it as a mechanism to build the relationship in a period where we couldn’t trade, and we wanted the pharmacists to relate to the rebate, and consider it was their money and that they wanted to access it, but in the scheme of their purchases and the overall deal, the rebate was not the main driver.
Mr Cooper also claimed, in respect of the Accrual Funds Scheme, that “it was the only thing we had to reinforce our relationship [with the pharmacies] on the lead up to loss of exclusivity”.
310 Although it is concluded that Pfizer was took advantage of its market power in establishing the accrual funds in January 2011, it is also further concluded that the value of the rebate was but a part of the overall strategy being developed by Pfizer. It would be a mistake to look simply to the dollar amount of any individual rebate and to divorce that value from the overall value of the terms upon which Lipitor and the Pfizer generic atorvastatin were being offered.
311 It has proved unnecessary in reaching these conclusions regarding whether Pfizer took advantage of its market power in establishing the accrual funds to form any view as to whether Pfizer had been selling Lipitor at “supra-competitive prices.” The ACCC submission in this respect was that Pfizer had “used the supra-competitive profits it derived from the sale of Lipitor while it had a statutory monopoly over the supply of atorvastatin in order to finance the accumulation of the accrual funds”. Had it been necessary to resolve this further plank to the ACCC case, it would most probably have been rejected. The earning and use of “supra-competitive profits” had not been pleaded. Even more relevant, however, is the fact that the evidence would most probably not have supported any such finding being made. Any correlation, for example, between the accrual of the rebate and the profits being earned on the sale of Lipitor was rejected by Mr Latham in the following exchange with his cross-examiner:
… Now, you weren’t able to accrue that rebate, I suggest, if you weren’t making a significant profit on the sale of Lipitor pursuant to the statutory monopoly that you enjoyed prior to loss of exclusivity; that’s the case, isn’t it? --- No, I don’t think it has got anything to do with the profitability of the product.
If you weren’t making any profit on the sale of Lipitor prior to loss of exclusivity, how would you be able to accrue a five per cent rebate on all the sales? --- They’re mutually exclusive, I think.
Nor did the cross-examination of Mr Latham which immediately followed go anywhere near establishing that “supra-competitive profits” were being earned in respect to the sale of Lipitor, namely the following exchange:
… the point I’m trying to make to you, Mr Latham, is that what gave you the financial ability to accrue a five per cent rebate on Lipitor was the fact that you were making a very significant profit on the sale of Lipitor prior to loss of exclusivity, weren’t you? --- It was – it was a five per cent – it was a five per cent accrual, yes.
And you were able to - - -? --- We were - - -
Sorry? --- We were making more than five per cent profit on – on Lipitor, yes.
Significantly more than five per cent profit, weren’t you, Mr Latham? --- Significantly, yes.
The offers in their final form
312 Given the conclusion that Pfizer did not have a substantial degree of market power in early to mid-2012, it is unnecessary to reach any conclusion as to whether it took advantage of any market power it retained in making the Platinum, Gold and Silver Offers in January 2012.
313 It is thus unnecessary to express any concluded view in respect to the ACCC submission that Pfizer took advantage of its market power in making its offer, a submission which it was said was supported by the opinion of Dr Pleatsikas that Atorvastatin Pfizer was effectively being offered below cost.
314 But the submissions should be briefly addressed.
315 It may be accepted at the outset that in determining whether a corporation has taken advantage of a substantial degree of market power it is relevant to have regard to whether the corporation would have engaged in the conduct under scrutiny if it did not have that power: Competition and Consumer Act s 46(6A). As Gleeson CJ, Gummow, Hayne and Callinan stated in Melway Publishing Pty Limited v Robert Hicks Pty Limited [2001] HCA 13, (2001) 205 CLR 1 at 23-24:
[52] … To ask how a firm would behave if it lacked a substantial degree of power in a market, for the purpose of making a judgment as to whether it is taking advantage of its market power, involves a process of economic analysis which, if it can be undertaken with sufficient cogency, is consistent with the purpose of s 46. But the cogency of the analysis may depend upon the assumptions that are thought to be required by s 46.
316 In considering the manner in which Pfizer behaved in making the offers it did, Dr Pleatsikas invoked the economic tool of the discount attribution test and concluded:
147. The results of the discount attribution test are unambiguous – Atorvastatin Pfizer was being effectively offered below cost (and in some cases below a zero price – i.e., Community Pharmacies are effectively being paid to take Atorvastatin Pfizer at the margin). This is not consistent with behaviour in which a profit-maximising firm without substantial market power would likely have engaged because a profit maximising firm without substantial market power would not have been able to recover those losses with additional (supracompetitive) profits on other products.
This conclusion of Dr Pleatsikas follows an analysis of two scenarios depending (at least in part) upon the projected conversion rate of a particular pharmacy from Lipitor to a generic atorvastatin. On one scenario, Dr Pleatsikas concludes that “under the discount attribution test, the Pfizer Platinum Offer would always result in a net cost of Atorvastatin Pfizer of less than zero (i.e., effectively Community Pharmacies would be paid to take Atorvastatin Pfizer)”. Dr Pleatsikas continues on to observe that at “lower conversion rates than 50%, the effective price of Atorvastatin Pfizer declines even further (is even more negative)”. A similar result follows in respect to the second scenario addressed by Dr Pleatsikas.
317 Had it been necessary to express a concluded view, the submission of the ACCC would have been rejected for a number of reasons.
318 First, the mere fact that goods may be sold at below cost does not necessarily expose any anti-competitive conduct. There was agreement between Dr Addanki and Dr Pleatsikas on this point. Thus, Dr Addanki observed:
85. Moreover, in any event, a price below “cost” does not by itself indicate anything sinister or anti-competitive about the offer, and Dr. Pleatsikas is wrong in suggesting that economics teaches us otherwise. Indeed, the very source he cites for his proposition that pricing below cost is, by itself, anticompetitive, contradicts his position. It is easy to see why as a matter of economics, pricing below cost need not be anticompetitive. For example, it is common practice for firms entering a market to offer very low pricing – even below cost – in order to induce consumers to switch away from their existing suppliers. This tactic is sometimes referred to as market penetration pricing. A new restaurant offers customers coupons; a new store has a huge opening sale. These are everyday examples of how firms may price below their cost in order to “jumpstart” their new business venture. Here, a below-cost price results “not from a low price for the traditional product purchase, but from the high ‘price’ that consumers ‘charge’ to induce them to try the product.”
Dr Pleatsikas agreed that you have to know “something more” before a view could be expressed as to whether selling below cost was anti-competitive.
319 Second, during the “launch phase” of a new pharmaceutical product the evidence was that it could be sold below cost. Mr Millichamp accepted that that could “happen from time to time but it [was] not common place”. But it does happen. Ranbaxy, for example, at one point of time was supplying its generic atorvastatin free of any charge if certain conditions were met. And Dr Pleatsikas also accepted in the following exchange during his cross-examination that a new product could be sold at below cost:
In launch phases of products, organisations sell below cost regularly, don’t they? --- They often do, yes.
It’s no indication of using market power, is it? --- Well, it may be in some circumstances. It may not be in others.
It is completely unspeaking as to whether one’s taking advantage of market power, isn’t it? --- It depends on the circumstances, so I don’t think it’s completely unspeaking unless you’re just talking about a general – well, if you sell below cost, is that automatically anti-competitive? And I would certainly agree with you it’s not.
You’ve got to know something more beyond selling below cost, and a lot more, to know whether it’s anti-competitive. That’s correct, isn’t it? --- You have to know something more, yes.
And of itself it does not constitute a taking advantage of market power, does it? --- Well, in the sense that we’re talking about taking advantage as an economist.
Yes? --- - - - would view it, I would say that merely selling below cost is not something that I would automatically characterise as taking advantage of market power. I want to abstract away from the legal test.
Exactly. And as an economist, there are concepts such as recoupment that have to be considered, correct? --- You know, when you’re talking about bundling and loyalty rebates, recoupment has a different meaning than it does in a predatory pricing context.
What you’ve got to do is have regard to other economic considerations if you’re looking at selling below cost from an economic point of view, correct? --- I think you have to have consideration to other circumstances. I wouldn’t necessarily confine them to economic considerations.
Quite. And you think that applying this formula – this test is of assistance in determining a question of whether one is taking advantage of market power, correct? --- I think in this circumstance it’s of assistance.
Do you say that merely satisfying the test, that is, establishing a below cost sale, constitutes taking advantage of market power in this case? --- Well, given the other facts and circumstances, I do.
If attention is focussed for present purposes upon the time during which the Pfizer offers were being made and the duration of those offers, it is difficult to resist the conclusion that the offers were being made during the “launch phase” of the Pfizer generic atorvastatin. Assuming that Pfizer was in fact selling generic atorvastatin during its “launch phase” at prices below cost, it would have been concluded that such conduct did not amount to the taking advantage of market power.
320 One area of disagreement between Dr Pleatsikas and his cross-examiner ultimately turned in part upon the length of time during which a supplier would continue to sell at below cost and whether the Pfizer offers extended over a significant period of time: On this issue the cross-examination continued:
Now, the offers which were open of this variety were really only open for a very short period of time, weren’t they, not for long term? --- You mean the Pfizer offers?
Yes? --- I think they were open for several months.
Well, that’s not the long term, is it? --- You know, depends on what you think of as the long term. That’s a fairly long period for offers to be open of this kind. But the way I think of the long term, generally, as an economist, it’s generally more than a year. So in that sense, it would not be the long term.
It was a short term launch phase offer. That’s correct, isn’t it? --- It was certainly a short term offer - - -
Yes? --- - - - in terms of only being several months.
And the sort of conduct a short term offer at a – making the assumptions you make with respect to the discount attribution test – at a loss, in a launch phase, is something that a company without market power would regularly do? --- I don’t agree with that. I don’t believe that people regularly sell large proportions of their output for a lengthy period of time below cost.
What about new entrants into a field who are trying to overcome barriers to entry? They might commit substantial amounts in capital to get a foothold, mightn’t they? --- It depends on what you mean by substantial. I still don’t believe they would sell product at a loss for a long period of time, you know, that covered a significant portion of their output.
Had it been necessary to do so, it would have been concluded that the duration of time over which the Pfizer offers were made and the duration of the terms consequent upon accepting one or other of those offers was a relatively short period of time. The conclusion as to the time being relatively short would have been considerably influenced by the facts and circumstances surrounding the expiry of the Pfizer patent and the attempts being made by the other generic manufacturers to enter the atorvastatin market.
321 Third, the discount attribution test employed by Dr Pleatsikas as an economic tool is more useful when deployed for the long term analysis of a market than to the short term effect of certain conduct.
322 Fourth, and without meaning any disrespect to Dr Pleatsikas, he failed to elaborate on or explain what was the “something more” that he indicated to his cross-examiner would be needed before a conclusion could be reached that sales made below cost were anti-competitive. The further cross-examination of Dr Pleatsikas and the text of his report exposed two potentially relevant matters, namely:
the answer provided in his cross-examination that “you’re talking about a very large proportion of output for a very long period of time”; and
the statement in his report that selling below cost “is not consistent with behaviour in which a profit-maximising firm without substantial market power would likely have engaged…”.
Pfizer submits that there is a relevant lack of analysis on the part of Dr Pleatsikas.
323 The lack of analysis is exposed in part by the failure on the part of Dr Pleatsikas to consider the overall profitability of Pfizer. On the case advanced by Dr Pleatsikas, the bundled sale of Lipitor and generic atorvastatin is only below Pfizer’s actual costs where conversion rates are less than 54%. As submitted on the part of Pfizer, “[i]n order to express a view as to the overall profitability to Pfizer of the conduct, Dr Pleatsikas would have had to analyse the profit received from supplying to pharmacies converting above 54% and the losses incurred from supplying to pharmacies converting below 54%, in order to work out whether the conduct, overall, was profit-maximising”. And, with reference to whether “you’re talking about a very large proportion of output”, there is no consideration by Dr Pleatsikas of the fact that for nominated conversion rates below 54% the quantity of Atorvastatin Pfizer that a pharmacy would need to acquire was relatively small.
324 Had it been necessary to do so, it would thus have been concluded that the Pfizer offers when first made did not involve the taking advantage of any market power that Pfizer may have retained. Even if it be assumed that Pfizer was supplying the generic atorvastatin at below cost, the offers it made were but offers made during the launch phase of a new product and for a relatively short period of time. And the quantum of any losses being incurred relative to the profit that Pfizer may have been making were not addressed.
Purpose
325 The case advanced on behalf of the ACCC in respect to both ss 46 and 47 was relevantly confined to establishing a proscribed “purpose”. No case was sought to be advanced, for example, that the “effect” of Pfizer’s conduct was to lessen competition within the meaning of s 47(10)(a).
326 With reference to s 46, paragraph [60] of the Amended Statement of Claim identifies that “purpose” as follows:
A substantial purpose of Pfizer in engaging in the conduct pleaded at paragraphs 43 to 59 above, was to hinder, deter or prevent other suppliers of Generic Atorvastatin, including:
(a) Ranbaxy Australia, from 18 February 2012; and
(b) the Other Generics Suppliers, from 18 May 2012,
supplying Generic Atorvastatin to a substantial proportion of Community Pharmacies …
This is then defined to mean the “Intended Foreclosed Supply”. Paragraph [60] then goes on to refer to the conduct of Pfizer going back to 2010.
327 Conclusions have already been reached as to whether Pfizer had:
a substantial degree of market power;
and whether Pfizer had:
taken advantage of any such power;
both in respect of the period from:
January 2011 to December 2011;
and from
January 2012 to May 2012.
328 Left to one side has been the question as to the “market” in respect to which the “purpose” must be directed in order to make out a contravention of s 46.
329 Pfizer contends:
that there is a large gap in the case sought to be advanced by the ACCC pursuant to s 46 – namely, the fact that the “ACCC has simply not proved, or even attempted to prove, that the Atorvastatin Market existed or was likely to exist after 18 May 2012”;
and
that there has been a failure to prove the existence of any proscribed purpose.
Again, the period of time in respect of which any analysis as to “purpose” must be directed – be it from December 2010 to May 2012 or from January to May 2012 – potentially complicates matters.
330 However the matter be approached, it is respectfully further concluded that the ACCC has failed to establish that Pfizer undertook the conduct complained of for any proscribed purpose.
The market … or any other market – s 46(1)(c)
331 The Pfizer contention that the ACCC has failed to prove any market after 18 May 2012 has as its origins s 46(1). The terms of s 46(1) should be repeated. That sub-section provides as follows:
A corporation that has a substantial degree of power in a market shall not take advantage of that power in that or any other market for the purpose of:
(a) eliminating or substantially damaging a competitor of the corporation or of a body corporate that is related to the corporation in that or any other market;
(b) preventing the entry of a person into that or any other market; or
(c) deterring or preventing a person from engaging in competitive conduct in that or any other market.
The distinction drawn between “that market” as opposed to “any other market” should be noted. As, too, should be ACCC pleading at para [60(b)] that a purpose of Pfizer was to hinder, deter or prevent other suppliers of generic atorvastatin “from 18 May 2012”.
332 Section 46(1) thus contemplates that the market in which a corporation engages in conduct may be different to the market at which its conduct is directed. The two “markets” may be different. Thus, in Australian Competition and Consumer Commission v Australian Safeway Stores Pty Ltd [2003] FCAFC 149, (2003) 129 FCR 339 at 410 Heerey and Sackville JJ observed:
[338] … Section 46(1)(c) explicitly contemplates that the purpose of the proscribed conduct might be to deter or prevent conduct in a market quite different from that in which the firm holds a substantial degree of power.
By way of illustration, in Queensland Wire Industries Proprietary Limited v Broken Hill Proprietary Company Limited (1989) 167 CLR 177 BHP was found to have a substantial degree of market power in the making and supply of steel and contravened s 46 by exercising that power to prevent Queensland Wire from entering or engaging in competitive conduct in (inter alia) the rural fencing market. As Deane J concluded:
… It is unnecessary to seek to identify the precise structure and boundaries of what should be seen, for the purposes of the present case, as what I have described as “the steel market”. Regardless of the more precise definition of that market, B.H.P. has a substantial degree of power in it. … Its refusal to supply Y-bar to Q.W.I. otherwise than at an unrealistic price was for the purpose of preventing Q.W.I. from becoming a manufacturer or wholesaler of star pickets. That purpose could only be, and has only been, achieved by such a refusal of supply by virtue of B.H.P.’s substantial power in all sections of the Australian steel market as the dominant supplier of steel and steel products. In refusing supply in order to achieve that purpose, B.H.P. has clearly taken advantage of that substantial power in that market …: (1989) 167 CLR at 197-198.
333 In the present proceeding, it has been concluded that the “market” was the atorvastatin market – and that the dimensions of that market remained constant from December 2010 to mid-2012. It has been further concluded that as at mid-to-late 2011 Pfizer held a substantial degree of market power in the atorvastatin market; but as at January 2012 Pfizer no longer held a substantial degree of power in that market.
334 Pfizer’s final written submissions proceeded upon the basis of a different conclusion having been reached in respect to market definition. Its underlying concern nevertheless remains – the closer to the end of any period expiring in May 2012, the greater becomes the need to identify the “market” to which the alleged purpose is directed. Pfizer accepted the inevitable fact that as from May 2012 it would face competition from the other generic manufacturers in respect to atorvastatin.
335 If it be correct to conclude that during the period from January to May 2012 Pfizer lacked a substantial degree of market power in the atorvastatin market, it is thereafter largely irrelevant to further inquire as to whether the “market” may have changed in mid-to-late 2012. Whether the “market” be the same in early 2012 or a different “market” later in 2012, Pfizer lacked a substantial degree of power. If the “market” did in fact then change into a generics market, as Pfizer contended, the market power possessed by Pfizer would inevitably have been even less.
336 Although Pfizer is correct to submit that it suited the ACCC case to identify the market as the atorvastatin market during the period from (for example) January to May 2012, it then became necessary to prove something as to the nature and dimensions of the “market” to which the Pfizer conduct was directed after its patent expired. Pfizer is also correct in submitting that the ACCC failed to direct specific attention to the market to which any conduct being pursued by Pfizer was directed “from May 2012…”. Dr Pleatsikas, for example, was only requested to consider the market up to 18 May 2012. Dr Pleatsikas was only requested by the ACCC to express an opinion in respect to matters during the “relevant period”, a period defined in his Report to the period from 3 December 2010 to 18 May 2012.
337 But such difficulties in identifying the “market” in which Pfizer had a substantial degree of power and the potentially different “market” to which its conduct was directed can be left to one side.
The absence of a purpose of deterring or preventing competitive conduct
338 The proscribed purpose that Pfizer is said to have had is that proscribed by s 46(1)(c): “deterring or preventing a person from engaging in competitive conduct …”.
339 The ACCC has failed to establish that Pfizer took advantage of its market power for a purpose proscribed by s 46(1)(c) by reference to either events:
extending back to December 2010;
or by reference to events confined to:
the period from January to May 2012.
340 In so concluding, it is recognised that the search for the “purpose” of Pfizer is a search for its subjective purpose – and in doing so must search for what Pfizer “had in view” or the “end sought to be accomplished”: News Limited v South Sydney District Rugby League Football Club Limited [2003] HCA 45 at [18], (2003) 215 CLR 563 at 573 per Gleeson CJ. Purpose, it is accepted, is not to be determined by reference to Pfizer’s “motive”.
341 With reference to the “purpose” pleaded in both paragraphs [60] and [66] of the Amended Statement of Claim, the ACCC further pleads in summary form that:
(i) the advice provided to pharmacies by Pfizer in late 2010 of its intention to commence manufacturing and supplying generic brands and its intention to offer pharmacies a discount on the supply of generic brands (para [43]);
(ii) the announcement by Pfizer in December 2010 of its intention to change its distribution arrangements with pharmacies and that it would commence marketing and supplying prescription pharmaceuticals directly to pharmacies (paras [44] to [46]);
(iii) the establishment of the “Accrual Funds Scheme” in January 2011 (paras [47] to [49]); and
(iv) the terms upon which it made offers, commencing in January 2012 (paras [50] to [60])
were pursued by Pfizer:
(v) “for the purpose, or for purposes that included the substantial purpose, of deterring or preventing other suppliers of Generic Atorvastatin, including Ranbaxy Australia and Other Generic Suppliers, from engaging in competitive conduct in the Atorvastatin Market”.
Pfizer’s defence to the allegation of “purpose” was, in essence, to deny that it had the “purpose” alleged.
342 Realistically, it is concluded that any case should be rejected which sought to contend that Pfizer had a purpose of “preventing” other generic manufacturers from supplying atorvastatin to community pharmacies – even in the short to medium term. To so contend, with respect, would be commercial naivety. It would also be a finding contrary to the evidence. The value of the atorvastatin market made competition inevitable once Pfizer’s patent expired. At a point of time, at the very latest, shortly after the expiration of the Pfizer patent, competition was inevitable. However, it was plainly open to contend that Pfizer’s conduct was undertaken for the purpose of “deterring” the entry of other generic manufacturers – and for the purpose of “deterring” the entry of other generic manufacturers for as long a period of time as possible.
343 Short of a submission that Pfizer’s purpose was to “prevent” other generic manufacturers from supplying atorvastatin, the alternative manner in which the ACCC maintained that Pfizer had a proscribed “purpose” was put with a degree of flexibility. One of the ways in which the ACCC sought to establish a proscribed “purpose” focussed upon the “sell-in” by Pfizer of a large volume of stock. The volume of stock sold-in, it was submitted, necessarily had the intended consequence of reducing the demand for supply from competitors.
344 It is respectfully concluded, however, that several factors provide a firm foundation for the conclusion that Pfizer was not engaging in conduct for a purpose proscribed by s 46(1)(c). Although Pfizer accepted that its conduct would provide an incentive for pharmacies to accept its offers, it is concluded that Pfizer pursued its conduct for the substantial purposes of:
ensuring that it remained a supplier of pharmaceutical products, including both Lipitor and Atorvastatin Pfizer; and
ensuring that it remained competitive in the atorvastatin market.
Rejected is the allegation that Pfizer:
engaged in conduct for the purpose of “deterring or preventing” other generic manufacturers from engaging in competitive conduct.
Also rejected is the submission that Pfizer pursued its conduct for the purpose of:
“blocking” competition.
Even if it could be said that Pfizer’s desire to gain a commercial advantage or make it harder for a generic competitor to succeed were “purposes” which could fall within s 46(1)(c), it is further concluded that any such “purposes” were not a “substantial” purpose.
345 The factors which it is respectfully concluded ultimately demand the rejection of the case advanced on behalf of the ACCC that s 46 has been contravened emerged from a number of discrete themes pursued by Senior Counsel for the ACCC in his cross-examination of the more senior officers within Pfizer.
346 Given a tension between the competing inferences which could be drawn from the documents circulating internally within Pfizer between 2009 and 2012, it is necessary to express some brief observations as to the credibility of the Pfizer witnesses. Left unexplained, those documents unquestionably provided a platform from which the ACCC could argue that the impugned conduct was undertaken for a proscribed purpose. Expressed as a general conclusion, the Pfizer witnesses satisfactorily answered the inferences which were otherwise available from the documents. To a considerable extent, the explanations are to be found in the terms of the evidence provided and in the consistency of the explanations provided by the Pfizer witnesses. But to a considerable extent the explanation is also to be found in the integrity with which they gave that evidence. The manner in which they presented their evidence provided, with respect, considerable comfort in accepting their explanations in the face of the inferences otherwise available.
347 Although the reasons or purposes pursued by Pfizer were differently expressed by different officers within Pfizer, and although it is somewhat artificial to seek to divorce one reason or purpose from another where reasons or purposes appear interrelated, a number of discrete themes can conveniently be addressed separately.
Direct-to-Pharmacy Model – a myriad of reasons
348 The scheme by which Pfizer was to distribute its pharmaceutical products directly to the community pharmacies, and not by means of wholesalers, was announced in December 2010.
349 The Direct-to-Pharmacy Model was not well-received by the pharmacies.
350 The Direct-to-Pharmacy Model nevertheless put Pfizer in the position that made it the sole supplier of atorvastatin to pharmacies until February 2012.
351 Documents internal to Pfizer threw light on the objectives sought to be achieved by the Direct-to-Pharmacy Model. Both the July 2009 “Strategic Review” and the “Feasability Study” forwarded by Sinapse to Pfizer in September 2009 referred (for example) to the model as a means of protecting the volume of sales of Lipitor upon loss of exclusivity and “off-patent product revenue”. A central concern of Pfizer was to protect its “branded” product.
352 Other models of distribution, it is to be recalled, were also being discussed – including the prospect of Pfizer entering into a licensing agreement with one or other of the existing generic manufacturers in order to “leverage” that other manufacturer’s relationship with pharmacies.
353 Following the presentation to Mr Young in April 2010 a series of questions were then raised. One of the questions raised concerns about the prospect of other competitors “adopting the same scheme and essentially accelerating price erosion”. The response to that question provided in part as follows:
... In Australia a pharmacy must stock both the branded and generic product, so the strategic sweet spot for Pfizer is that, under an exclusive distribution model, only Pfizer can sell their branded product. This provides a competitive advantage, as competitors will only be able to offer discounts on generics whereas Pfizer will be able to offer discounts on both the branded and generic Pfizer molecules. Due to the timing of the brands LOE and the accumulation of the rebates (due to overlapping patent expiries), this will add a level of complexity in a pharmacy evaluating the best deal. This is a classic incumbent strategy as it makes it hard for the customer to work out whether a competitor offer is better value to their business, which in turn lowers their motivation to switch away from their current deal/supplier. Further, the rebate scheme builds loyalty with Pfizer prior to LOE, as the first rebate payment only occurs 6 months post-LOE, and, in order for a pharmacist to receive their rebate, they must remain supportive to the Pfizer generic/offer (total molecule solution with the pharmacy is 36 months).
The response was part of the information provided to Pfizer’s senior management in order for a final decision to be made as to whether (inter alia) an exclusive direct-to-pharmacy distribution arrangement should be approved.
354 But the Direct-to-Pharmacy Model was the strategy ultimately decided upon. The evolution of the model need not be repeated.
355 Of present relevance are the reasons provided for ultimately deciding upon that model.
356 The reasons for ultimately approving the Direct-to-Pharmacy Model are many. However, none of those reasons was the desire to deter or prevent other generic suppliers from entering the market for the purposes of s 46(1)(c).
357 Mr Latham, for example, maintained that his “objectives” in supporting the Pfizer proposal “were to promote Pfizer Australia’s generic products business and develop a close relationship with community pharmacies …”. And the complexity of the decision-making process was emphasised by Mr Latham when he was being cross-examined as to the prospect of entering a licence arrangement with a generic manufacturer rather than pursuing the Direct-to-Pharmacy Model. In that context, the following exchange occurred:
But my question, Mr Latham, was directed to Lipitor and generic atorvastatin, not some dream of establishing a generics business? --- But once again you’re asking me to make a decision on – on one product, when I have seven products, over $1 billion, coming off patent. And it’s not just Pfizer Australia. It’s around the world. And to try to get the best business organisation that’s going to deliver continuing operations through those generic products, plus, they have these additional benefits of being closer to pharmacy. Going through the licensee doesn’t tick that important box.
This questioning followed cross-examination directed to the graph forwarded to Mr Young by Mr Latham in late April 2010. That graph depicted, inter alia, the fact that there was a “fallback plan” still then being considered – namely, the “fallback” of Pfizer licensing another manufacturer to produce atorvastatin. Indeed, that graph depicted the “licence” scenario as a plan still protecting substantial Pfizer revenue – albeit not to the same extent as the Direct-to-Pharmacy Model. The line of inquiry pursued with Mr Latham was that the “fallback plan” as at late April remained a plan still under active consideration. But that proposition was rejected during the following exchange (without alteration):
But you weren’t ruling out in your mind at this time the possibility that if you weren’t able to, for whatever reason, proceed with that strategy an alternative would be a licence type arrangement as described in the slides that you sent to Mr Young? --- I think at this stage we were convinced that the DTP was the best way.
But if you weren’t able to proceed, for whatever reason, with the DTP you considered at the time that an alternative which would deliver a better result over doing nothing was the licence arrangement as set out in the your slides to Mr Young. That’s the case, isn’t it? --- I – there was a lot more involved than just these dollar sales. What we’re saying there is that the dollar sales is 250 million greater over five years. But I think there were lots of other reasons for us to go to pharmacy, and from primary care getting closer to pharmacy was important to me, as pharmacy paid an increasingly larger part of health care. If you just go through other generic companies you’re not going to have that closeness to pharmacy that you do get under DTP. So DTP wasn’t just – wasn’t just generics. It was more than that.
But, Mr Latham, I’m not suggesting that you thought at the time that the licence arrangement was superior to DTP; all I’m suggesting is that at this time, that is at least in April/May 2010 you considered a commercially viable but less attractive alternative to the DTP was a licence arrangement. That’s the case, isn’t it? --- No. And that’s why I was taken aback when I saw that in here, that this thing had been put up as a second – as a second suggestion.
Why would you have put to Mr Young a proposal that you didn’t regard as commercially feasible? --- Well, I – I didn’t prepare this; right. I’m not saying that – I’m not saying that going through a licensee doesn’t have financial implications. It does have financial implications. But if you want – I’m working on the basis that if you try to set up your own generics business then probably the fourth one or sixth one on the list would be a licence. And I think it’s – I think it’s incorrect to put in there to say that that’s what we’re recommending to him if we don’t go ahead with the DTP.
There were, accordingly, many reasons in Mr Latham’s mind as to why he was supporting the Direct-to-Pharmacy Model and why he would not support a model which included Pfizer entering into a licensing arrangement with another manufacturer to produce generic atorvastatin.
358 Although Mr Crotty left Pfizer Australia in April 2011 to become Vice President of Established Product for Pfizer in South Europe, his evidence as to the thinking behind the strategy being developed prior to that date nevertheless remains of assistance. With respect to the Direct-to-Pharmacy Model, Mr Crotty maintained his:
… reason for supporting the direct to pharmacy model stemmed from the vertical alignments between generic manufacturers, wholesalers and pharmacies which meant that Pfizer Australia could not rely on generic manufacturers and wholesalers to supply Pfizer Australia’s off-patent products. I thought that the direct to pharmacy model would provide the platform to enable Pfizer Australia to establish relationships with pharmacies which would provide Pfizer Australia with a greater opportunity to be able to sell its generic products, including atorvastatin, to those pharmacies at the time of loss of exclusivity.
He also thought that the exclusive Direct-to-Pharmacy Model provided an improved ability to defend volume and price erosion upon loss of exclusivity. The model established a direct platform with pharmacies that would facilitate a successful commercial offering.
359 Mr Crotty did not agree with the suggestion that Pfizer was pursuing “a classic incumbent strategy”. After being taken to the note containing that suggestion, the following exchange occurred:
And that’s a statement you agreed with at the time these questions were answered, wasn’t it? --- Look, I don’t agree with that statement.
Do you think that statement at the time it was made was wrong, do you? --- I don’t think it’s a classic incumbent strategy. As I said before, I think that there is – there are differences in the offers that Pfizer was putting out and differences that the generic companies were able to put out. One of the things which a lot of our work had revealed is that the deals that the generic companies were putting up there were very complex. And whilst you need a degree of complexity because of – to ensure compliance, you also had to be mindful to keep things as clear as possible, so.
The exchange continued as follows:
… I will put it this way. But you would agree, Mr Crotty, that it was a classic incumbent strategy that you were aware of at this time to make an offer in a way that would be difficult for the person to whom the offer was made to determine which offer was better, and in those circumstances, would be more likely to stick with their current supplier rather than move to a different supplier. You would agree that that was a classic incumbent strategy at that time? --- Well, we weren’t the incumbent. We – you know, we were entering the generic space.
I’m sorry, Mr Crotty. You were the only supplier of atorvastatin at this time, weren’t you? --- We were the only supplier of atorvastatin – of Lipitor, yes. Yes.
And what you were seeking to do through this strategy was to protect your share of the market for sales of atorvastatin, weren’t you? --- We were trying to minimise the erosion once the product came off patent.
And you were minimising the erosion from sales of atorvastatin, weren’t you? --- Yes.
So you were the incumbent with respect to atorvastatin, weren’t you? --- From – from that perspective, yes.
360 It is concluded that the reasons of Pfizer in establishing the Direct-to-Pharmacy Model were as explained by Messrs Latham and Crotty. That evidence should be accepted. Uppermost in the minds of the Pfizer officers was the need to establish a close relationship with pharmacies and the Pfizer objective of creating a greater opportunity to sell its own generic products. The explanation provided by Mr Latham in respect to the “fallback plan” as depicted in the internal Pfizer documents also provides, only by way of further example, reason for caution in too readily drawing inferences from documents drafted in some cases by unknown persons and divorced from the input of those responsible within Pfizer for making decisions. On many occasions the author of the documents upon which cross-examination proceeded was unknown – the author may have been an officer of Pfizer or (indeed) Sinapse. But it was not the subjective purpose of any individual author which mattered; it was the purpose of those who were responsible for making the ultimate decision that mattered. The views being expressed by those various authors may be a source upon which the views of those responsible may be questioned. Ultimately, however, it was the purpose of the decision-maker that remains of primary importance for the purposes of s 46(1).
361 It was not a substantial purpose of Pfizer, in establishing that Model, to hinder, deter, or prevent other generic manufacturers from supplying their own generic atorvastatin to community pharmacies.
362 The mere fact of implementing a Direct-to-Pharmacy Model, it should be noted, could not of itself necessarily expose any proscribed anti-competitive purpose. Such a model may be implemented for purposes free of any anti-competitive purpose. Thus, for example, Ascent also implemented such a model. One of its reasons for doing so focussed on “customer relationships and control over customer service”. In implementing that model it was said that “Ascent interacts directly with its pharmacy customers across the entire chain from promotion and order taking through to product support and customer queries”. Such was also part of the reasoning of Pfizer.
363 Although Pfizer’s implementation of its Direct-to-Pharmacy Model was potentially capable of supporting the ACCC submission as to purpose, neither the implementation of that Model alone – nor in combination with other factors – made good that submission.
The sell in – a question of volume & timing
364 The date upon which Pfizer intended to “sell-in” its generic atorvastatin changed over time.
365 As with many aspects of the strategy being pursued by Pfizer, one particular aspect of a decision – for example, the date upon which it would “sell-in” its generic atorvastatin to pharmacies – cannot be considered in isolation from other considerations relevant to the decision to be made. One such factor to be taken into account when making that decision was the volume of generic atorvastatin that would be “sold-in” and the terms and conditions upon which it would be sold.
366 It should immediately be noted that any suggestion on the part of Pfizer that the timing of its launch of its atorvastatin offers was simply an exercise of the exclusive rights provided for by its patent as opposed to an exercise of whatever market power it retained is rejected. An exercise of intellectual property rights may also involve an exercise of market power: NT Power Generation Pty Ltd v Power and Water Authority [2004] HCA 48, (2004) 219 CLR 90 at 136. McHugh A-CJ, Gummow, Callinan and Heydon JJ there observed:
[125] … [T]o suggest that there is a distinction between taking advantage of market power and taking advantage of property rights is to suggest a false dichotomy, which lacks any basis in the language of s 46. … [P]roperty rights can be a source of market power attracting liability under s 46 and intellectual property rights are often a very clear source of market power.
367 If attention is thus presently confined to the reasons motivating the selection of the relevant date, there can be no doubt that the date upon which Pfizer proposed to “sell-in” its generic atorvastatin was a matter given some detailed consideration. It was a decision taken with reference to the prospect that an early “sell-in” of product could prejudice the commercial position of Pfizer by reducing profits from the sale of Lipitor.
368 It was originally intended, for example, that Pfizer would “sell-in” its generic atorvastatin in November 2011. But that date exposed Pfizer to a number of potential difficulties. Those difficulties were explored with Mr Cooper by his cross-examiner in the following exchange:
And you appreciated, didn’t you, that one of the cons – the negatives of the November 2011 sell-in was that selling in in November 2011 would allow time for your competitors to react and unravel the Pfizer deal? --- Yes. It gave them more time to review it and work out what they would do to combat it. Yes.
And another disadvantage of a November 2011 sell-in was that pharmacies wouldn’t commit to the Pfizer deal and would use the Pfizer offer as leverage to negotiate with other generic suppliers? --- Yes.
And another disadvantage of a November 2011 sell-in was that it might trigger generic competitors to start commercialising their deals early? --- Yes.
In fixing a date for the proposed “sell-in” of its generic atorvastatin, Pfizer was thus giving active consideration to the potential courses which could be pursued by its competitors and to the risks that it would face.
369 The date was changed from November 2011.
370 The case sought to be advanced on behalf of the ACCC, for present purposes, had at least two strands to it, namely:
the purpose of placing on the shelves of pharmacies as much stock of the Pfizer generic atorvastatin as possible such as to reduce the “motivation” of pharmacists to purchase generic atorvastatin from another supplier;
and – but perhaps differently expressed:
to “block” the ability of a competitor to supply a pharmacy with generic atorvastatin.
Although both strands may be but a means of conveying a single message, each should be separately addressed.
371 Any consideration of the significance to be attached to the strategy of selling in large quantities of generic atorvastatin upon its launch – or the motivation or ability of pharmacies to order a generic atorvastatin from a manufacturer other than Pfizer – must necessarily recognise at the outset that there were many reasons why Pfizer was pursuing a strategy of encouraging pharmacies to purchase large quantities of its generic atorvastatin.
372 As explained by Mr Latham during the course of his cross-examination, those reasons were expressed as follows:
It was your view, Mr Latham, I suggest, that unless Pfizer included an upfront bulk purchase in its offers it would be unlikely to gain any significant ongoing custom for Atorvastatin Pfizer. That’s correct, isn’t it? --- It was unlikely that we would get ongoing unless we had a - - -
Unless the offers included an upfront bulk purchase? --- It’s – it’s a benefit to Pfizer to have an upfront offer. Absolutely. There was significant demand from the pharmacies. Pharmacies were expecting – were wanting – to have this ability to buy as much as they could during this grace period. So not only do you have Pfizer wanting to sell as much as we can, you also have the pharmacists wanting to purchase as much as they can in this grace period. You have the Pharmacy Guild doing road shows around Australia, every capital city, talking to pharmacies about this. About the opportunity, this window, where they can buy as much as they can in the grace period and substitute as much as they can to generics to offset the $600 million that they were losing on 1 April from price decreases. So as I said, there was demand there for this buy-in as well as from Ranbaxy and Pfizer, and also a subsequent buy-in from Alphapharm as well too. That’s also excluded from the 13 month period that they go to. So, you know, this – Pfizer wasn’t – Pfizer wasn’t generating this demand. This demand for a big buy-in was there, and certainly surprised us the amount of that demand, for sure.
373 Those reasons were also explained by Mr Crotty as follows:
And you thought that that was a relevant strategy for Pfizer to consider in the context of any exclusive direct to pharmacy case scenario, didn’t you, at this time? --- This – yes, this was a – a part of the strategy that we were considering.
And an important part of the strategy that you were considering, wasn’t it? --- The sell-in – you know, for a company that had no, I suppose, experience with pharmacy, it did require a sell-in over the period prior to and post-patent expiry.
Mr Crotty continued on to say:
Now, you mentioned a moment ago that one of the reasons why you thought the sell-in was appropriate, was that Pfizer didn’t have any pharmacy experience? --- That was one of the reasons, yes.
Why was the lack of any experience with pharmacies a reason to have a sell-in? --- Well, first and foremost, there’s a number of reasons for the sell-in. The first is that you’re allowed to under the PBS rules – the first month didn’t count towards price disclosure. The second one is that we needed to – we needed to have a go at competing in that marketplace, and so we needed to have stock, basically, in the pharmacies upon both the entry of Ranbaxy and also when the other generic companies would come in. If we didn’t have any stock, given the very tight vertical arrangements between the generic companies, the pharmacists and the wholesalers, we would have got absolutely slaughtered. So it was a way of having relevance.
So, your view, as early as June 2009, was that in order to have relevance, you had to get your stock in the shelves of the pharmacies before the generic companies were able to market any generic atorvastatin product? --- Well, for a start, there’s a distinction between Ranbaxy and the licence they could grant and when a – the other generic companies could come in. So we had a decision to make. Did we compete with Ranbaxy and one other generic company prior to patent expiry, the three month period – and if we did, I think we would have been out of the game – or did we compete with them first and so we had to have a sell-in then, or that was the plan, and then once the other companies came into the marketplace, we obviously had to have stock on shelf then. So there was more than one reason why we had to have a sell-in.
And, in respect to the sell-in, there was what was referred to as the “first mover advantage”. Pfizer, according to Mr Crotty, was of the view that “a sell-in would provide Pfizer with an ability to get the stock in the shelves of the chemist before the other generic manufacturers other than Ranbaxy were able to market their generic product”.
374 The prospect of one manufacturer selling in large quantities of product and thereby placing at risk the ability of another to supply its own product was not confined to Pfizer. As at May 2011 one risk to Pfizer envisaged by Ms Brady, for example, was that Ranbaxy could supply its generic atorvastatin for free. If Ranbaxy did so, it would place at “risk” the ability of Pfizer to supply its own generic atorvastatin to those pharmacies that accepted any such offer from Ranbaxy.
375 The ACCC proposition that Pfizer was pursuing a strategy of selling in large quantities of its own generic atorvastatin upon launch for the purpose of – or with the intention of – discouraging other competitors from supplying their own product was thus not borne out by the evidence of the other Pfizer witnesses. Pfizer did so for the purpose of protecting its own commercial position – and did so in a manner consistent with the practice pursued by other manufacturers upon the release of a new pharmaceutical product.
376 The ACCC proposition was also rejected by Ms Brady. Her response in cross-examination was as follows:
Another objective of the bulk sell-in, as you understood that Pfizer was seeking to pursue, was to discourage those pharmacies that accepted a platinum offer from taking generic atorvastatin from your competitors for a significant period of time, wasn’t it? --- I don’t believe it was an objective, no.
What you were – what Pfizer, as you understood it, was seeking to achieve by the bulk sell-in was to discourage those pharmacies who had accepted the Pfizer generic offer from taking generic atorvastatin from your competitors during the period at least of the sell-in stock; would you agree? --- I can’t say I agree that it was the intention, no. I believe that that is a likely result, but I don’t believe that it’s the intention.
And when you say “intention”, you’re talking about the intention of senior management within Pfizer? --- Well, I’m not referring to any specific people.
It was your view that the result of the sell-in would be that community pharmacies that accepted the platinum offer would be most unlikely to have any incentive to acquire generic atorvastatin from your competitors, that is Pfizer’s competitors, until at least the stock and the sell-in had been sold? --- Until they started to dispense the atorvastatin Pfizer stock, they were unlikely to take a volume stock from a competitor, yes.
377 Ms Alltoft was also taken to the reasons pursued by Pfizer in seeking a bulk “sell-in” of product. When questioned by her cross-examiner, Ms Alltoft responded as follows:
Because the idea of the bulk sell-in was that you hoped it would improve, first brand retention? --- The idea of the bulk sell-in was the bulk sell-in was a volume incentive and as a result of that bulk sell-in we would take advantage of price disclosure for that volume not counting in the first month of price disclosure, and the second was we were hoping that it would mean that pharmacies would actually support us in the longer term because that was our intention in the marketplace was to be a competitor in the post-patent marketplace. So having volume sell-in that gave greater commitment to pharmacies to actually support us long-term, that was the purpose of the bulk sell-in.
And beyond the term of the bulk sell-in, wasn’t it? --- Beyond the term of the - - -
The long term support that you were looking for to achieve by the bulk sell-in wasn’t limited to the term of the bulk sell-in, was it? --- We wanted long – we wanted to be a player in the post-patent marketplace and so we wanted customers to support us not only for the short term on the deal but for the long term.
378 The explanations provided by these witnesses, it is respectfully concluded, should be accepted.
379 It is concluded that Pfizer recognised that a consequence of selling-in large quantities of its generic atorvastatin at launch – and in “incentivising” pharmacies to commit to take specified percentages of their future requirements – was that those pharmacies which did may well have little need to source generic atorvastatin from a Pfizer competitor. But it is further concluded that it was not the intention, purpose or objective of Pfizer in attempting to “sell-in” large quantities of atorvastatin to shut out its competitors or to discourage pharmacies from sourcing product from its competitors.
The sell in as a “blocking” strategy
380 Although, taken by itself, the objective of Pfizer in seeking to “sell-in” large quantities of its generic atorvastatin may not have itself fallen within any purpose proscribed by s 46(1)(c), the ACCC submitted that the “sell-in” took on a different complexion when viewed against a background of Pfizer pursuing an alleged course of “blocking” other competitors from supplying their own generic atorvastatin.
381 This submission was that the “sell-in” by Pfizer of large amounts of generic atorvastatin would effectively “block” the ability of the generic manufacturers to get their products on the shelves of pharmacies. And this, so contended the ACCC, was what Pfizer intended to do. Pfizer, it was submitted, intended to “block” or shut out its competitors. Until the pharmacies had “sold through” the Pfizer generic atorvastatin, there would be limited shelf space to stock any other generic atorvastatin and little incentive on the part of pharmacies to purchase more generic atorvastatin from a rival manufacturer.
382 Considerable reliance was thus placed by the ACCC upon the many references in the internal Pfizer documents –extending throughout 2011 – to “blocking”.
383 Notwithstanding the various reasons why a “sell-in” of product upon launch may have commercial benefits to both the pharmacy and the supplier, there nevertheless remained a question as to what was meant to be conveyed in those Pfizer documents that referred to “blocking”.
384 It was obviously a term which attracted some particular attention within Pfizer. The draft version, for example, of the September 2011 Project LEAP: Review referred to a promotional “sell-in” becoming “an effective blocking strategy”. The final version of that Review dated 14 September 2014 deleted the term “blocking” and simply referred to a promotional sell in becoming “an effective 2012 strategy.”
385 The use of such language provides at least a platform for an adverse inference to be drawn as to the purpose sought to be pursued by Pfizer. But the use of particularly colourful language has to be understood in context: Seven Network News Ltd v News Ltd [2007] FCA 1062 at [2490]. Sackville J there observed:
The difficulty of showing that a desire to cause harm to a competitor is necessarily indicative of an anti-competitive purpose is illustrated by the observation of an American commentator. Professor Hovenkamp, a leading antitrust scholar, argues that jury trials in the United States are an unfortunate way to decide contested issues in complex anti-trust cases. The reason he gives is this:
‘Jurors are often unskilled in business and have a difficult time distinguishing aggressive competitive intent from anticompetitive intent. In the minds of many jurors “intending” to knock out a rival sounds evil. The fact is that such intentions are the subject of hundreds of business seminars every day. In markets of every type sales personnel are urged to “destroy” or “kill” the competition or to sink new rivals before they have a chance. But this is nothing more than the rhetoric of competition, which anti-trust seeks to preserve but jurors often misinterpret’.
H Hovenkamp, The Antitrust Enterprise: Principle and Execution (Harvard University Press, 2005), at 61.
386 In the present proceeding, various explanations were provided by the Pfizer witnesses for the usage of the terminology of “blocking”.
387 Mr Crotty gave evidence that one of the reasons for the “sell-in” was the lack of experience Pfizer had with respect to pharmacies and the desire on the part of Pfizer to continue to have “relevance” with pharmacies. After giving this evidence it was thereafter put to Mr Crotty that he wanted to have a “sell-in” of Pfizer products before the other generic manufacturers were able to make offers to pharmacies. But he rejected that suggestion as follows (without alteration):
And you wanted to build market share by getting pharmacies to accept your product for up to 12 months, didn’t you, at the time of loss of exclusivity? --- That’s right.
And you wanted to have the opportunity to do that sell-in before your generic competitors for generic atorvastatin were able to make their offers to the market? --- That’s not correct. So Ranbaxy and one other had three months on the marketplace, and we saw Ranbaxy as probably a key competitor, because as I said before, Ranbaxy probably didn’t have the alliances, so it’s going to be Lipitor, the aligned generic, and either Ranbaxy or Pfizer. So we were obviously looking at a sell-in period initially while we were competing with Ranbaxy and one other, and then we needed to have stock in the store from 1 June, given those alliances, or else we would have been, as I said, slaughtered.
Mr Crotty also recognised that the inevitable effect of the “sell-in” was that a pharmacy would buy less of a competitor’s generic atorvastatin. The cross-examiner was thus content to conclude with the following exchange:
If a pharmacy accepted a significant supply of generic Atorvastatin from Pfizer wouldn’t you accept that the logical consequence of that was that it was less likely to need to acquire generic Atorvastatin from a competitor at least for the period during which that pharmacy sold through Pfizer Australia stock? --- Well, we definitely had the intention of – of wanting to compete and that they would buy less of our competitor’s range.
You realised that would be the consequence of the sell-in, didn’t you? --- We wanted to compete and we wanted to make sure that we had a foothold in pharmacy that there was a reason for our reps to go in and continue to sell through the stock, so if that meant that our competitors didn’t get as much share as they hoped then that is the – it’s the consequence.
You would accept that was the effect that you sought to achieve through the sell-in, wasn’t it? --- The effect we sought to achieve is to compete, to have a chance because if we didn’t have stock in the pharmacy on either 19 February when Ranbaxy came in or 1 June we would have got slaughtered.
Now, your motivation, I suggest, was to enable Pfizer to compete with the generic manufacturers for the supply of Atorvastatin post-loss of exclusivity, wasn’t it? --- Yes - - -
And the way you were going to? --- - - - and to sell Lipitor.
Sorry. Yes, and sell Lipitor, sorry. And the way you were going to achieve that object was to sell-in your stock so the pharmacy would be much less likely to want to take stock from your competitors. That’s the case, isn’t it? --- Of course.
Your Honour, I have no further questions.
The explanations provided by Mr Crotty are accepted. His view that Pfizer took the steps that it did to avoid being “slaughtered” was a view oft-repeated in his evidence. It was a view, with respect, which he genuinely and passionately held.
388 The reasons for the bulk “sell-in”, it is thus concluded, were driven by a recognition that it was in the commercial interests of both the pharmacy and Pfizer to take advantage of the first month’s stock not being included in the subsequent calculation and adjustment of price under the Pharmaceutical Benefits Scheme and a desire to secure the long-term support of pharmacies. It was not a purpose of the bulk “sell-in” to deter or prevent other generic manufacturers – or to “block” those manufacturers – from supplying their own products to pharmacies.
The Platinum Offer – a requirement to take 100% of generic for 12 months
389 Nor do the particular terms of the offers made by Pfizer dictate any different conclusion as to purpose.
390 It will be recalled that the terms upon which the Platinum, Gold and Silver Offers were to be made varied over time. Those terms, as ultimately approved and implemented, required consideration to be given to the volume of atorvastatin to be supplied and the period of time over which stock was to be provided. Those terms included requirements imposed upon pharmacies as to the projected sales of Atorvastatin Pfizer (or “conversion rate”).
391 The evolution of those terms need not be traced in any great detail.
392 However, the evolution of the Platinum Offer should be briefly considered. An early proposal for the Platinum Offer suggested that a pharmacy be required to take 100% of its projected requirements of generic atorvastatin to unlock 100% of its accrued rebate. In late 2011/early 2012 a number of the documents circulating within Pfizer, and documents used at various presentations of the then forthcoming launch of the Pfizer generic atorvastatin, recorded this suggested requirement.
393 But that proposed requirement was quickly scotched by Mr Latham. He claimed to be the person who made the decision to reduce that requirement to 75% – even though it was anticipated that such a decision could reduce Pfizer’s revenue by about $30 million. The anticipated loss of revenue was highlighted in an e-mail to Mr Latham forwarded on 10 January 2012 which stated in relevant part:
… For each level platinum, gold and silver, we will require pharmacies to take 75% of their generic stock requirements to obtain the discounts, previously this requirement was 100%. This has a potential revenue risk of ~ $30M to PC in 2012.
The prospect of making a decision which so significantly impacted upon the revenue of Pfizer, not surprisingly, attracted the attention of the cross-examiner.
394 The reasons for making the decision were explained by Mr Latham as follows in his cross-examination:
… Why did you put at risk $30 million by restating the offer to be 75 per cent of 100 rather than 100 per cent of a 100? --- It was important to me to make sure that Pfizer was making sure that the other competitors were able to sell their products. Okay. If I’m – if I’m going to say that you have to sell 100 per cent of your product that’s fine I can do that, and I can – I can put my deal accordingly, but to me it was important as a principle that pharmacists were able to buy from more than one supplier.
Taken by itself, some reservation may have been experienced as to whether this explanation should have prevailed.
395 But Mr Latham’s account was supported by the evidence of Ms Alltoft. In a series of questions and answers Ms Alltoft repeatedly emphasised that the decision to reduce the requirement from 100% to 75% had been taken by Mr Latham and that she had not participated in the decision-making process. She was nevertheless pressed as to whether there was any difference between a requirement to take 75% of an anticipated 12 months stock of atorvastatin as opposed to 100% of 9 months stock. In terms of the “number of units” required to be purchased, she accepted that “those two circumstances are the same”. But to her mind there remained a difference. One of the exchanges between Ms Alltoft and her cross-examiner was the following (without alteration):
So the commercial effect of that was they were going to have to take nine months supply of generic atorvastatin from Pfizer if they were to be able to take up the terms of the platinum offer. That was the case, wasn’t it? --- For the platinum offer, yes, 75 per cent of their 12 month is nine months stock.
So what was the difference between requiring a pharmacy, as you understood it, to take 12 months supply on the one hand, and on the other hand take nine months’ supply? --- Because of their anticipated 12 months’ supply, we would supply 75 per cent of that and 25 per cent of that would then be available for them to choose a second supplier if they so desired to do so.
But you knew as a commercial person, I suggest, Ms Alltoft, that this was effectively smoke and mirrors as far as requiring pharmacies to only take a percentage of their anticipated requirements, didn’t you? --- I don’t agree with you in smoke and mirrors that it ---
And the smoke and mirrors, I suggest, is telling a commercial person as a condition of an offer that you have to take 75 per cent of your 12 month anticipated supply on the one hand or 100 per cent of nine months anticipated supply on the other hand, meant exactly the same thing to the pharmacist, didn’t it? --- Taking – to a pharmacist, taking 12 months is 12 months of anticipated supply at 100 per cent and of their 12 months only needing to take – is not the same thing.
Of importance is Ms Alltoft’s understanding that the reason for the reduction from 100% to 75% was to enable a pharmacy “to choose a second supplier if they desired to do so”. Even though Ms Alltoft may not have participated in the decision and was not aware of the reasons for the decision, her understanding as to how the changed requirement would operate in practice is of significance. It is evidence of a recognition within Pfizer at the time that the consequence of the decision was to permit supply from a supplier other than Pfizer.
396 The explanation advanced by Mr Latham – which was supported by Ms Alltoft – as to why the requirement was reduced from 100% to 75% is accepted. It is accepted that Pfizer made that decision so as to permit a pharmacy the opportunity to purchase generic atorvastatin from a supplier other than Pfizer.
The delaying of the date for the acceptance of the Pfizer offer
397 The date upon which it was proposed that pharmacies needed to respond to the offers made by Pfizer and, correspondingly, the date of any “sell-in” of the new generic product changed over time. The date was originally proposed to be 15 February 2012 and then was changed to 17 February 2012. It was then finally changed to 24 February 2012.
398 The ACCC sought to ascribe some significance to the proposed 15 and 17 February 2012 dates – those dates being before Ranbaxy could lawfully launch its own atorvastatin generic. Although the Ranbaxy generic could not be placed upon the Pharmaceutical Benefits Scheme prior to April 2012, it could supply its own generic on “private scripts” as from 19 February 2012.
399 Not surprisingly, different officers within Pfizer placed differing levels of emphasis upon the value of requiring pharmacies to respond to the Pfizer offer prior to Ranbaxy being able to lawfully enter the market.
400 There can be no question but that the date upon which Ranbaxy could lawfully make offers for the supply of atorvastatin assumed importance in the minds of some Pfizer officers. Thus, for example, Mr Cooper was taken to an e-mail in late September 2011 which referred to a “Deal Deadline Feb 15 – No rebate”. The following exchange then occurred during his cross-examination (without alteration):
Now, was the “deal deadline Feb 15 – no rebate” a reference to the anticipated deadline at that time by which community pharmacies had to indicate whether or not they were willing to accept the Pfizer generic atorvastatin offer? --- Yes.
Do you recall where the date of 15 February came from? --- We – we worked on a number of arrangements for the date and took into account how long it would take us to process the orders, etcetera. And mid-February or thereabouts was a convenient date for us.
The cross-examination continued:
There was something else that really drove 15 February, wasn’t there? --- Yes. I was also aware of the Ranbaxy date, and commercially for me on the sales process it was advantageous to have a date pre Ranbaxy joining the market.
Why was that? --- From a – I mean, my job is to selling the offer and run my team, and I will try and – executing every advantage I can.
And why was that an advantage? --- Well, it actually wasn’t because they were already out there, but it would, in theory, give us the ability to present our offer and have them accept before they would legitimately be selling their offer.
It was a very important part of the planning of your team at this time, I suggest, Mr Cooper, that the deadline for the acceptance of the Pfizer generic atorvastatin offers was before the date on which Ranbaxy was able to legally make offers to community pharmacies, wasn’t it? --- Yes, from – for what I wanted to achieve, yes.
Ms Brady was also of the view that the 18 February 2011 date was fixed by reference to a concern within Pfizer about the ability of Ranbaxy to enter the market. When taken to an e-mail sent on 5 May 2011, the following exchange thus occurred:
And was that your understanding, at least as at May 2011, that the objective of Pfizer, in connection with the Project LEAP strategy, was to aim to get as many orders as possible placed before Ranbaxy was able to make offers legally to community pharmacies on 18 February 2012? --- As per this email, yes.
Yes. And that was a strategy that you understood was at the heart of what you and others were working towards while you were working on the implementation of Project LEAP, wasn’t it? --- I’m sorry. Can you rephrase that?
What you were seeking to do in working on Project LEAP and its execution was to seek to secure for Pfizer as many customers as possible before Ranbaxy was able to legally launch its offer from 18 February 2012? --- Well, as of 5 May 2011, then I believe that was the intention.
401 Notwithstanding the initial importance ascribed to a requirement that pharmacies respond to the Pfizer offer prior to the introduction of Ranbaxy into the atorvastatin market, it was also understood within Pfizer that Ranbaxy had been extensively canvassing pharmacies and making known the prices at which it would supply its own generic well prior to February 2012.
402 Whatever may have been the thinking of these persons within Pfizer, the decision to change the date for acceptance of the offers to 24 February 2012 was also taken by Mr Latham. He accepted in his cross-examination that “a benefit of a cut-off date of 18 February to Pfizer would be that the community pharmacies were required to commit to [Pfizer’s] before Ranbaxy was able to legally make offers to community pharmacies …”. His reasons for extending the date to 24 February 2012 were, obviously enough, a matter which attracted the attention of his cross-examiner. An initial venturing into this area was the following exchange (without alteration):
Now, looking at this document, do you maintain your evidence that it was you who made the decision to move to 24 February? --- Yes.
And do you say the reason why you moved it to 24 February was to allow established products more time for logistics and rebate calculations? --- No. The – the move – the move from 18 February for 24 February was to allow pharmacists time to assess the offers and everything that were being made by – by Ranbaxy. The reason it’s not later than 24 February, like 7 March or 15 March, is that you have to put everything in place to process the orders and to make – make the deliveries. Remember, the stock has to be there on the shelves in the pharmacies ready for sale on 1 April.
The notion that Pfizer wanted to extend time to pharmacies to “assess the offers” attracted further attention in the following exchange occurring a little later:
… In order to give community pharmacies five business days to consider any offers that Ranbaxy will make, we’re going to move the offer cut off date to 24 February? --- Yes, that is correct.
You’re not making this up as you’re going along, are you, Mr Latham? --- No, I’m – I’m not making this up as I go along.
Why did you want to give community pharmacies five business days to consider the Ranbaxy offer, Mr Latham? --- Well, I suppose, to a certain extent, it’s optical. We already knew that Ranbaxy were out there. We already knew that Ranbaxy were talking to pharmacists. We already knew that Ranbaxy were talking to the groups. We already had evidence that – that other generic manufacturers were out there talking to the groups as well, too. However, it would appear to be improper, incorrect, to ask pharmacies to make a decision before the time that legally Ranbaxy were entitled to be out there.
Why were you concerned about that? --- About the optics?
Yes. Why were you concerned about – weren’t you out there to try and make money? --- No. But it would appear to me that the pharmacists have to have time to legally assess the offers made by Ranbaxy.
Why? --- Otherwise we would be in the situation of getting pharmacists to sign up when, in fact, legally they had not been able to have any offers made to them by Ranbaxy or everybody else, even though we knew – and we have evidence – and we have documented evidence, that there was significant activity in the marketplace, as far as terms and conditions were concerned.
But, Mr Latham, you had known for some time, I take it, that the original proposal had been to have a cut-off date of 18 February? --- That’s right. And then it was brought to my attention, okay, that that does not give – when Ranbaxy are not legally allowed to talk to anybody legally until 18 February, it looks a bit silly getting pharmacists to make a decision to accept your offer when they haven’t legally had a chance to listen to Ranbaxy. That makes sense to me.
403 Taken by itself, there may well have been good reason to also question this evidence of Mr Latham. Against the background of the repeated references in the internal Pfizer documents to (for example) the “blocking strategy”, considerable reservation may have been expressed as to whether Pfizer’s reasons were as altruistic as suggested.
404 But such reservation is, it is concluded, removed when consideration is given to the further evidence of Ms Alltoft. By the time she had arrived at Pfizer, the decision to extend the date to 24 February 2012 had already been taken. Her evidence can, accordingly, not go to any finding of fact as to the reasons being pursued by Mr Latham on behalf of Pfizer. Her evidence can, however, assist in an understanding of what Pfizer was seeking to achieve in the longer term. Although her understanding as to the reasons for extending the date for acceptance of the offers may have been a matter left unexplored in cross-examination, Senior Counsel did pursue this issue with her. She explained her understanding as follows:
And why was, as you understood it, the fact that Ranbaxy was able to make offers legally to community pharmacies from 18 February relevant to the decision as to when Pfizer was going to impose a cut-off date for community pharmacies to accept the Pfizer offer? --- It was one of the relevant pieces because it was the other competitor in the market at the time, and we wanted pharmacies to be able to consider our offer and competitor offers.
Why did you want to do that? --- We have to have the pharmacy to be able to consider all offers that were in the market at the time and make an informed decision about whose offer they wanted to go with.
She returned to the same explanation a little while later in her evidence when the following exchanged occurred:
Because, prior to 24 February, you wanted pharmacists to believe that if they didn’t accept the offers that you were making prior to that date, they would have to wait for some later time to get access to their accrual funds with respect to Lipitor. That’s the case, isn’t it? --- We wanted to have a date where there was a – a target to actually gain acceptances by pharmacies.
And you wanted them to provide those acceptances before they had had any significant period of time to consider the terms of any Ranbaxy offer? --- As I said before, I think we believed that Ranbaxy was out talking about their deal, and that by giving them till 24 February gave them time to consider the Ranbaxy offer.
405 Founded upon such evidence, it is concluded that the reason for extending the date for acceptance of the Pfizer offers was that propounded by Mr Latham. In fixing the various dates upon which offers could be accepted, it was no part of Pfizer’s purpose to deter or prevent the other generic manufacturers from engaging in competitive conduct in the atorvastatin market.
406 Rejected is the submission of the ACCC that “[t]he effect that Pfizer sought to achieve by setting a deadline for acceptance of 24 February 2012 for the initial Atorvastatin Offers was to secure supply arrangements with as many Community Pharmacies as possible before they were able to receive or adequately assess definitive offers from other suppliers or potential suppliers of generic atorvastatin including Ranbaxy”. Also rejected is the similar submission advanced by the ACCC in respect to setting the deadline for acceptance of the late acceptance terms and conditions of 10 March 2012 and 15 April 2012.
Purpose overall?
407 Any finding as to the “purpose” being pursued by Pfizer in the present case – and whether any such “purpose” or “purposes” was the “substantial” purpose for its conduct – necessarily has to take into account the entirety of both the evidence given by the individual witness and that evidence in the context of the inferences to be drawn from documents and the evidence of other witnesses. Part of that evidence is to be found not only in the documents themselves and in the oral evidence of the Pfizer witnesses; it is also to be found in the factual content of the conduct pursued. Thus, for example, one fact was that Pfizer did “bundle” both its branded product with its generic atorvastatin; another fact is that the “sell in” of product necessarily had the effect that pharmacies would have less shelf-space for other competitive generic products.
408 Any finding must also necessarily take into account the suggestions made by Senior Counsel for the ACCC from time-to-time that one Pfizer witness was making it up as he went along or was “deliberately avoiding” answering a question or making a concession.
409 Left unexplained, the inferences which may otherwise have been drawn from the documents circulating internally within Pfizer and the objective facts flowing from the conduct of Pfizer may well have supported a finding that its purpose fell within s 46(1)(c). But such inferences were not left unexplained.
410 Notwithstanding the terminology employed in some of those internal Pfizer documents, and (indeed) the changes in terminology in various versions of the same document, it is concluded that each of the Pfizer witnesses were persons of commercial integrity who sought to do no more than give an honest account as to the “purposes” or “reasons” behind the decisions being taken. The witnesses who gave evidence regarding the “purpose” sought to be pursued by Pfizer were not the authors of many of the statements relied upon by the ACCC, although they may have been persons to whom these documents or e-mails containing these statements had been sent. These witnesses, on some occasions, rejected the appropriateness of the manner in which some of those statements had been expressed.
411 A corporation may, of course, have many disparate purposes in engaging in conduct. The fact that a corporation may have a substantial purpose does not preclude that it may also be simultaneously pursuing another purpose which may also be characterised as a “substantial purpose”.
412 Whatever other purposes Pfizer may have been pursuing between December 2010 and at least May 2012, it is concluded overall that a substantial purpose of the conduct complained of was not the purpose of either “deterring or preventing a person from engaging in competitive conduct in” the atorvastatin market or any other market (s 46(1)(c)) or the purpose of lessening competition (s 47(10)(a)).
413 Pfizer unquestionably knew that it would face intense competition as from either February or May 2012. Its revenue would unquestionably be impacted. As explained by Mr Crotty:
Yes. Look, our strategy was we knew – when you have 100 per cent of a market because you’re patent protected, there’s only way to go, down, and our strategy was to manage that market share erosion as best we could.
That objective was variously expressed. At all material times Pfizer was seeking to position itself to remain a viable supplier of atorvastatin into the future.
414 The reliance sought to be placed by the ACCC upon the decision in Baxter is, with respect, misplaced. Central to the decision in that case was the finding of fact that the “core … of the purpose of the bundle or tie” by Baxter of its tender for the supply of the sterile fluids and the PD fluids was to foreclose tenders from its competitors having any realistic prospect of success: Australian Competition and Consumer Commission v Baxter Healthcare Pty Ltd (No 2) [2008] FCAFC 141 at [179], (2008) 170 FCR 16 at 54-55 per Mansfield J. Pfizer pursued no such comparable purpose. And, contrary to the facts in Baxter, in the present proceeding it was inevitable that there would be intense competition for the supply of generic atorvastatin to community pharmacies after May 2012 (if not earlier).
Browne v Dunn
415 One final submission advanced by Pfizer should be raised to be rejected.
416 This submission was that it was not put to Mr Latham that his purpose was “to deter or prevent other suppliers of generic atorvastatin from supplying generic atorvastatin to a substantial proportion of Community Pharmacies”.
417 The submission was presumably founded upon the following observations of Lord Herschell LC in Browne v Dunn (1893) 6 R 67 at 70-71:
Now, my Lords, I cannot help saying that it seems to me to be absolutely essential to the proper conduct of a cause, where it is intended to suggest that a witness is not speaking the truth on a particular point, to direct his attention to the fact by some questions put in cross-examination showing that that imputation is intended to be made, and not to take his evidence and pass it by as a matter altogether unchallenged, and then, when it is impossible for him to explain, as perhaps he might have been able to do if such questions had been put to him, the circumstances which it is suggested indicate that the story he tells ought not to be believed, to argue that he is a witness unworthy of credit. My Lords, I have always understood that if you intend to impeach a witness you are bound, whilst he is in the box, to give him an opportunity of making any explanation which is open to him; and, as it seems to me, that is not only a rule of professional practice in the conduct of a case, but is essential to fair play and fair dealing with witnesses.
Reference may also be made to the like observations of Lord Halsbury: (1893) 6 R at 76-77.
418 More recently, in MWJ v The Queen [2005] HCA 74, (2005) 80 ALJR 329 at 339 Gummow, Kirby and Callinan JJ described the rule thus:
[38] We should next say something about the rule in Browne v Dunn … The rule is essentially that a party is obliged to give appropriate notice to the other party, and any of that person’s witnesses, of any imputation that the former intends to make against either of the latter about his or her conduct relevant to the case, or a party’s or a witness’ credit.: (2005) 80 ALJR at 339.
419 The “rule” in Browne v Dunn, however, is essentially a rule of procedural fairness. Where it is readily apparent that the account of a witness is in question, the “rule” does not require a contrary proposition to be formally put to a witness: cf. Trade Practices Commission v Mobil Oil Australia Ltd (1984) 3 FCR 168 at 179-181 per Toohey J. Nor does any failure to comply with the “rule” require the evidence of a witness to be disregarded: cf. Payless Superbarn (NSW) Pty Ltd v O’Gara (1990) 19 NSWLR 551 at 556 per Clarke JA.
420 In the circumstances of the present case, it can – with respect – not be seriously contended that Mr Latham was not aware that the “purpose” being pursued by Pfizer was an issue of central relevance. Such cross-examination of Mr Latham which merely suggested that (for example) the bulk “sell-in” made it “less likely” that pharmacies would be interested in acquiring generic atorvastatin from other suppliers does not detract from the weight to be given to his evidence as to “purpose”. Nor did the manner in which the cross-examination proceeded cause any prejudice to Pfizer or unfairness to Mr Latham.
421 The evidence as to “purpose” has, in any event, been accepted. Any failure on the part of the ACCC to “formally” put more directly a challenge to this evidence has not worked any procedural unfairness.
The contravention of s 46 as pleaded – paragraphs [67] and [67A]
422 Given the conclusion that the ACCC has failed to establish a contravention of s 46, it is unnecessary to resolve a further, more broadly expressed, submission advanced on behalf of Pfizer.
423 The submission, however, should be addressed.
424 Pfizer maintained at the outset that the case mounted against it by the ACCC was “legally incoherent”. What was intended to be conveyed by such a characterisation of the ACCC claim was – perhaps – not fully articulated at the outset of the case. But the submission was advanced at the outset and more fully developed in final submissions.
425 The submission focussed attention upon the terms in which declaratory relief sought in the Amended Originating Application and the manner in which the claimed “contraventions” of s 46 were pleaded in the Amended Statement of Claim. The Amended Originating Application sought three declarations – two of those declarations mirrored paragraphs [67] and [67A] of the Amended Statement of Claim. Paragraphs [67] and [67A] were in substantially identical terms – with the exception that paragraph [69(c)] identified the period as being “from on or about 16 January 2012”; paragraph [67A(c)] identifying the period as “from at least on or about 24 February 2012”. The relevance of the former date is that 16 January 2012 was the date upon which the original Pifzer offers were made; the latter date was the start of the period in which the Pfizer offers which included the late acceptance terms and conditions were made. It is sufficient for present purposes to refer to paragraph [67] because [67A] does not differ materially in the way it is pleaded. Paragraph [67] provided, with the amendments made after the commencement of the proceeding being underlined, as follows:
By reason of the matters pleaded in paragraphs 42 to 53, 55 to 59, 60(a) to 60(r), 60(t) to 60(v) and 61 to 66 above, Pfizer has engaged in conduct in contravention of section 46(1) of the CCA, in that during the period between at least 1 December 2010 and 18 May 2012 Pfizer held a substantial degree of market power in the Atorvastatin Market and took advantage of that power by its conduct which cumulatively comprised:
a. the implementation of the Pharmacy Supply Arrangements;
b. the establishment of the Accrual Funds Scheme, including the Lipitor Rebates; and
c. from on or about 16 January 2012 offering to supply and subsequently supplying atorvastatin to Community Pharmacies pursuant to the Atorvastatin Pfizer Offers which included the Terms and Conditions,
for the purpose of deterring or preventing other suppliers of generic atorvastatin from engaging in competitive conduct in the Atorvastatin Market.
426 The Pfizer submission seized upon the term “cumulatively”. The submission was that each of those elements set forth in paragraphs (a), (b) and (c) were alleged by the ACCC to “cumulatively” constitute the contravention of s 46(1); there being no allegation that one or other of those elements alone constituted a separate contravention.
427 Chronologically:
the Direct-to-Pharmacy Model was implemented in January 2011;
the Accrual Funds Scheme was established in January 2011; and
the making of the atorvastatin offers took place between 16 January 2012 and 18 May 2012.
Confined to this time scale, Pfizer maintained that the only two contraventions of s 46 being advanced for resolution focussed attention upon the two periods, one period being “from on or about 16 January 2012”; the other period being “from at least on or about 24 February 2012”. That was the case, Pfizer submitted, it had come to meet. Forensic decisions had been taken based upon this construction of the contravention of s 46 alleged.
428 If the Pfizer submission be accepted:
whether the implementation of the Direct-to-Pharmacy Model; and/or
the establishment of the Accrual Funds Scheme;
could have constituted a contravention of s 46 as at January 2011:
was not the case it came to meet.
Indeed, if this submission be accepted:
evidence as to the purpose sought to be achieved by Pfizer in implementing its Direct-to-Pharmacy Model or the Accrual Funds Scheme prior to January 2012 was irrelevant to the issues to be now resolved.
“Section 46 of the Act”, Pfizer correctly emphasised, “requires, not merely the co-existence of market power, conduct and proscribed purpose, but a connection such that the firm whose conduct is in question can be said to be taking advantage of its power”: Melway Publishing Pty Limited v Robert Hicks Pty Limited [2001] HCA 13 at [44], (2001) 205 CLR 1 at 21 per Gleeson CJ, Gummow, Hayne and Callinan JJ. Indeed, on this approach:
even the question as to whether the atorvastatin offers made between January and May 2012 contravened s 46 was not a question which arose for resolution – that not being a contravention taken in isolation which had been pleaded by the ACCC.
It was the conduct identified in paragraph [67(a), (b) and (c)] which “cumulatively” was the conduct pleaded by the ACCC which constituted the two contraventions of s 46.
429 No application was made during the hearing on the part of the ACCC to further amend paragraph [67] to confront or otherwise address the Pfizer submission. Indeed, shortly before the close of final submissions in reply, Senior Counsel for the ACCC again adhered to the position that no application to amend would be made.
430 Notwithstanding considerable reservation, it is ultimately concluded that the Pfizer submission would have been accepted. The submissions should prevail both by reason of:
the manner in which the contraventions of s 46 were pleaded; and
the manner in which the evidence unfolded and submissions advanced on behalf of the ACCC.
The submission should, accordingly, not simply be resolved “as a pleading point”.
431 Confined to the pleadings, the Pfizer submission that the ACCC is “incoherent” is accepted. Paragraph [67(a) and (b)] focusses attention upon the “implementation” of the Direct-to-Pharmacy Model and the “establishment” of the Accrual Funds Scheme. Both events took place in January 2011. Whatever “market power” Pfizer may then have possessed and whatever may have been the “purpose” it was then pursuing matters not. No contravention is separately alleged in respect to such conduct as otherwise falls within paragraph [67(a) or (b)]. The pleading that “during the period between at least 1 December 2010 and 18 May 2012 Pfizer held a substantial degree of market power” is confined by reference to the “cumulative” conduct thereafter identified and confined to the period (in respect to paragraph [67]) “from on or about 16 January 2012 …”. Given the need for there to be a “co-existence” of each of the requirements of s 46 at the time of contravention (Melway Publishing Pty Limited v Robert Hicks Pty Limited [2001] HCA 13 at [44], (2001) 205 CLR 1 at 21 per Gleeson CJ, Gummow, Hayne and Callinan JJ), a contravention of s 46 “from on or about 16 January 2012” cannot be made out by reference to conduct pursued in January 2011.
432 Reservation is necessarily exercised in many cases before upholding a “pleading point”. Even a shunned opportunity (or several shunned opportunities) to amend does not thereafter provide a licence to too eagerly uphold a “pleading point” if sense can otherwise be made of a pleading such that litigation can proceed upon a basis where the issues are reasonably capable of identification.
433 But what may otherwise have been regarded simply as a “pleading point” gains substantive force when reference is made to the manner in which the case was conducted. Consideration as to whether the “market” may have changed as between January 2011 and January 2012 may be left to one side. But what cannot be left to one side is the prospect that the “market power” possessed by Pfizer unquestionably changed as between January 2011 and January 2012, let alone February 2012. As the expiration of its patent for atorvastatin was becoming more imminent, Pfizer’s “market power” was ever-declining. What conduct it may have been able to pursue in January 2011 was conduct that may not have been open to it a year later. But such questions were left unaddressed. The focus of much of the evidence was directed to ascertaining the “market power” Pfizer possessed as at January 2011 and the “purpose” of Pfizer’s conduct as at January 2011. The evidence, of course, was not confined to early 2011. The scrutiny of the internal deliberations of Pfizer extended from 2009 through to 2012. But there was no mistaking the emphasis placed by the ACCC upon the “purpose” being pursued by Pfizer as at January 2011 in implementing its Direct-to-Pharmacy Model and its Accrual Funds Scheme. Such an emphasis was, of course, consistent with the manner in which paragraph [67(a) and (b)] were pleaded – to focus on the time when the Direct-to-Pharmacy Model and the Accrual Funds Scheme were “implemented” and “established”. That emphasis was also mirrored in the ACCC’s final written submissions.
434 Had it been necessary to resolve the Pfizer submissions as to the confined manner in which paragraphs [67] and [67A] of the Amended Statement of Claim were pleaded, those submissions would have been resolved in its favour.
435 Even had the “pleading point” been resolved in favour of the ACCC the further difficulty in its path to success would have emerged from:
the fact that any contravention as pleaded could only have arisen in January 2012 (at the earliest); and
the fact that by that date Pfizer lacked any substantial degree of market power.
436 This “pleading point” was confined to the alleged contravention of s 46; the submission did not extend to the manner in which the contravention of s 47 had been pleaded.
SECTION 47
437 The ACCC’s case that Pfizer contravened s 47 of the Competition and Consumer Act appears in paragraphs [68] to [72] of the Amended Statement of Claim.
438 Those paragraphs provide, in part, as follows (without alteration):
68. Pfizer:
(a) formulated and made the Atorvastatin Pfizer Offers; and
(b) offered to and did supply Lipitor and Atorvastatin Pfizer to Community Pharmacies in accordance with the terms of the Atorvastatin Pfizer Offers,
in trade or commerce within the meaning of section 47(1) of the CCA.
69. In making the Atorvastatin Pfizer Offers to Community Pharmacies, Pfizer:
(a) offered to give or allow discounts on Atorvastatin Pfizer and Lipitor pursuant to the terms and conditions of the Atorvastatin Pfizer Offers; and
(b) offered to give or allow rebates or credits through the Accrual Fund Scheme pursuant to the terms and condition of the Atorvastatin Pfizer Offers,
in relation to the proposed supply of Atorvastatin Pfizer and Lipitor to Community Pharmacies.
70. In supplying Atorvastatin Pfizer and Lipitor to Community Pharmacies pursuant to the terms and conditions of the Atorvastatin Pfizer Offers, Pfizer:
(a) gave or allowed discounts being the discounts offered on Atorvastatin Pfizer and Lipitor pursuant to the terms and conditions of the Atorvastatin Pfizer Offers; and
(b) gave or allowed rebates or credits through the Accrual Fund Scheme pursuant to the terms and conditions of the Atorvastatin Pfizer Offers,
in relation to the supply of Atorvastatin Pfizer and Lipitor to Community Pharmacies.
71. Pfizer offered, gave or allowed the discounts referred to in paragraphs 69(a) and 70(a) above, and the rebates or credits referred to in paragraphs 69(b) and 70 (b) above, on the condition that Community Pharmacies would not, except to a limited extent, for specified periods of up to 12 months:
(a) acquire atorvastatin directly or indirectly from a competitor of Pfizer in the Atorvastatin Market; or
(b) re-supply atorvastatin acquired directly or indirectly from a competitor of Pfizer in the Atorvastatin Market.
Paragraph [72] goes on to allege that the relevant conduct was engaged in by Pfizer for the purpose of substantially lessening competition. That paragraph provides as follows:
By reason of the matters pleaded at paragraph 60 above, the purpose or a substantial purpose of Pfizer engaging in the conduct pleaded at paragraphs 68 to 71 above, was to cause a substantial lessening of competition in the Atorvastatin Market by:
(a) preventing or hindering other suppliers of Generic Atorvastatin, including Ranbaxy Australia and the Other Generics Suppliers, from supplying Generic Atorvastatin to a substantial proportion of Community Pharmacies; and thereby
(b) preventing or hindering other suppliers of Generic Atorvastatin, including Ranbaxy Australia and the Other Generics Suppliers, from competing with Pfizer for the Intended Foreclosed Supply.
Pfizer’s Defence admits paragraphs [68], [69] and [70]. Pfizer admits that it offered and gave the specified discounts “on the condition that Community Pharmacies would not except to a limited extent re-supply atorvastatin acquired directly or indirectly from a supplier other than Pfizer” but otherwise denies paragraph [71]. Pfizer also denies it had the purpose alleged in paragraph [60] (which is relied upon in paragraph [72]).
439 There are three discounts or rebates which could potentially fall within s 47, namely:
(i) the discount off the “Chemist List Price” in respect to Atorvastatin Pfizer – that discount varying between 55% and 75% under the Platinum, Gold and Silver Offers;
(ii) the release of all or part of the Lipitor rebate given in respect to the purchase of Atorvastatin Pfizer under the Platinum, Gold and Silver Offers; and
(iii) the on-going discount off the “Chemist List Price” given on Lipitor under the Platinum, Gold and Silver Offers
440 Two of the principal issues to be confronted in resolving the ACCC case in respect to s 47 are:
whether Pfizer’s conduct in supplying or offering its atorvastatin products for sale was “on the condition” set forth in either s 47(2)(d) or (e);
and, if so:
whether that condition was imposed for the purpose of substantially lessening competition within the meaning of s 47(10)(a). The ACCC does not allege or otherwise rely upon the other limbs of s 47(10)(a), that the conduct “has or is likely to have, the effect of substantial lessening competition”.
It is unnecessary to resolve a preliminary submission advanced on behalf of Pfizer that the ACCC contention in respect to s 47 could not be sensibly resolved in the absence of any consideration being given to the “with and without test” (Stirling Harbour Services Pty Limited v Bunbury Port Authority [2000] FCA 1381 at [12], (2000) ATPR 41-783 at 41,267 per Burchett and Hely JJ). That test, Pfizer contended, required an assessment of whether the end Pfizer sought to achieve would lead to a substantial lessening of competition and that required an assessment of competition over the long term. Dr Pleatsikas, the ACCC’s expert, had only been asked to – and only gave – an opinion in respect to the period up to 18 May 2012 whereas the market for the purposes of s 47 required a consideration of the market extending well beyond that date. It is unnecessary to resolve this preliminary submission because it is respectfully concluded that the ACCC has not established that any of the pleaded conditions were imposed for the “purpose” of substantially lessening competition sufficient to give rise to a contravention of s 47.
On the condition
441 The first of the two issues relevant to the application of s 47 to the facts focusses upon the need to identify the “condition” upon which goods were supplied.
442 Paragraphs [68] to [71] of the Amended Statement of Claim identifies the terms and conditions relevant to the alleged contravention of s 47 of the Competition and Consumer Act as those to be found within the “Atorvastatin Pfizer Offers”.
443 The ACCC maintains that the particular “condition” of present relevance is to be found either within the final form of the brochure setting forth the terms upon which offers would be made or in the “Pharmacy Acceptance Form” provided by Pfizer to the pharmacies for completion. The “condition” as set forth in the final form of the brochure in respect to the Platinum Offer provided as follows:
To be eligible for the Platinum offer, you must comply with purchasing & dispensing at least 75% of your generic Atorvastatin requirements from Pfizer. You are free to purchase more – it’s your choice. Alternate offers also available.
The relevant “condition” as set forth in the “Pharmacy Acceptance Form” provided as follows:
Lipitor discounts are subject to first line support of Atorvastatin Pfizer – at least 75% of your total generic atorvastatin volumes dispensed must be Atorvastatin Pfizer. This requirement applies from the date of your first delivery of Atorvastatin Pfizer until you have dispensed the total agreed Atorvastatin Pfizer volume. If you do not meet this requirement, your Lipitor discount will revert to 1.5%.
444 In its final written submissions the ACCC maintained (footnotes excluded):
The bundled offers for Lipitor and Atorvastatin Pfizer constituted exclusive dealing for the purposes of s 47 of the CCA for 2 reasons. These offers included conditions that: first, had the effect of effective precluding Community Pharmacies from acquiring more than 25% of their anticipated requirements for the supply of generic atorvastatin for specified periods from Pfizer’s competitors; and second, re-supplying atorvastatin from Pfizer’s competitors if the re-supply would exceed 25% of the total generic atorvastatin dispensed by that pharmacy. The practical effect of each of the Platinum, Gold and Silver Offers was that the Community Pharmacies would not, or would not except to a limited extent, acquire or re-supply generic atorvastatin from other suppliers. Moreover, it was readily apparent that acquiring 75% of 12 months anticipated requirements for generic atorvastatin was equivalent to 100% of 9 months anticipated requirements of generic atorvastatin. Pfizer certainly modelled the effects of the 75% requirement on this basis. These supply conditions contained in the offers fit squarely within the terms of s 47(2) of the CCA and contravene s 47 if they satisfy the requirements of s 47(10).
445 Pfizer accepts that the ongoing discount given on Lipitor was given on a “condition” that falls within s 47(2)(e). That condition, it is accepted by Pfizer, was imposed by that condition in the “Pharmacy Acceptance Form” linking Lipitor discounts to “first line support of Atorvastatin Pfizer”.
446 But the other two discounts or rebates, Pfizer contends, were not given on any “condition” that fell within s 47. The pharmacies, it is said, remained free to purchase generic atorvastatin from other suppliers. The fact that any particular pharmacist may have been less likely to buy as much generic atorvastatin from another supplier as he would have had he not accepted the Pfizer offer does not, so it is submitted on behalf of Pfizer, mean that it engaged in the practice of exclusive dealing. These “conditions” may have had the “effect” or may have the “practical consequence” that a pharmacy may been less “inclined” to purchase generic atorvastatin from a supplier other than Pfizer (Monroe Topple & Associates Pty Ltd v Institute of Chartered Accountants in Australia [2002] FCAFC 197 at [105], (2002) 122 FCR 110 at 138-139 per Heerey J, [2002] FCAFC 197 at [160], (2002) 122 FCR 110 at 149 per Tamberlin J). But no “condition” was imposed on the pharmacies inhibiting their freedom to acquire generic atorvastatin from other suppliers.
447 These submissions are accepted.
Purpose
448 There remains outstanding, however, the question of whether the one “condition” which it is accepted by Pfizer as falling within s 47 was a “condition” imposed for a purpose proscribed by s 47(10).
449 Whereas s 46(1)(c) of the Competition and Consumer Act expresses the requisite purpose as being the purpose of “deterring or preventing a person from engaging in competitive conduct”, s 47(10)(a) expresses the requisite purpose as being for the purpose of “substantially lessening competition…”. Section 4G expands upon the meaning of this phrase as including “preventing or hindering competition…”. Again, it was not part of the ACCC’s pleaded case that any of the other limbs of s 47(10)(a) had been made out.
450 The relevant pleading as to Pfizer’s “purpose” is that set forth in paragraph [72] of the Amended Statement of Claim. The reference in that paragraph to paragraph [60] is, perhaps, curious. Paragraph [60] pleads that certain conduct was undertaken for a “purpose” proscribed by s 46(1)(c). But that reference can presently be left to one side. What is presently relevant is the pleading in paragraph [72] that Pfizer engaged in exclusive dealing for the proscribed purpose of causing “a substantial lessening of competition in the Atorvastatin market…”.
451 It is respectfully concluded that the ACCC case founded upon s 47(10) fails for either of two reasons, namely:
Pfizer’s “purpose” in pursuing the conduct that it did was to ensure its own corporate “survival”; and/or
more specifically, and of more immediate relevance to s 47, the ACCC has failed to establish that Pfizer’s “purpose” was to cause a “substantially lessening of competition”.
The latter reason, it may be noted, was contributed to by the failure of the ACCC to direct any real attention to the likely state of future competition in the market “with and without” Pfizer’s impugned conduct.
452 The evidence called by Pfizer propounded a “purpose” for its conduct which was not satisfactorily displaced by the ACCC. Mr Latham deposed in his affidavit that he approved the condition requiring a certain dispensing rate of Atorvastatin Pfizer in order for the pharmacy to unlock the higher Lipitor discount “in order to maximise Pfizer’s Australia’s sales of Atorvastatin Pfizer”. Mr Bagdadi also gave evidence as to the reasons for including this term or condition in the offers made to pharmacies. One of the reasons he gave was expressed as follows:
I was concerned that the strength of the arrangements between generic manufacturers, wholesalers and retail pharmacies would render Pfizer’s atorvastatin products irrelevant come 1 June 2012. I considered that encouraging ‘sell-through’ of Pfizer’s generic atorvastatin product, would make Pfizer’s generic products relevant to both pharmacies and patients, thereby improving the chances of those pharmacies continuing to deal with Pfizer in the future rather than returning immediately to their preferred generic supplier.
This evidence regarding the reasons of Pfizer for imposing the relevant condition is accepted. It was no part of the “purpose” of Pfizer in imposing this condition to substantially lessen competition.
453 Albeit directed to a different “purpose”, the earlier findings as to the “purpose” being pursued by Pfizer in respect to s 46 – and the observations made in respect to the acceptance of the honesty and integrity of the Pfizer witnesses – can be taken as repeated in respect to the analysis of “purpose” in respect to s 47.
Section 51 – an exception
454 Pfizer further contends that s 51(3)(a)(i) and (iii) of the Competition and Consumer Act provides an exception to s 47.
455 Given the conclusion that the ACCC has failed to establish the requisite “purpose” to make out a contravention of s 47, this further defence on the part of Pfizer need not be resolved.
456 But it should, perhaps, be briefly addressed.
457 The gist of Pfizer’s submission was that – but for a licence from Pfizer – the sale by pharmacies of the patented atorvastatin would be a contravention of s 13 of the Patents Act. The sale by the pharmacist, it was submitted, would be a breach of the exclusive right of Pfizer to “exploit” its patent, namely the right to “sell or otherwise dispose of …” the pharmaceutical product. To address this prospect of breach on the part of the pharmacist, it is then submitted that “… the law treats the sale without express restrictions as involving the grant of a licence from the patentee authorizing such future use of the goods as the owner for the time being sees fit”: Interstate Parcel Express Co Proprietary Limited v Time-Life International (Nederlands) BV (1977) 138 CLR 534 at 549 per Stephen J.
458 If this be correct, the condition which would otherwise offend s 47 of the Competition and Consumer Act, is said on behalf of Pfizer to be a condition which “relates to … the invention to which the patent relates … by the use of that invention” for the purposes of s 51(3)(a)(iii).
459 Had it been necessary to resolve this argument, it would have been rejected for either of two reasons, namely:
the sale of the atorvastatin by Pfizer to the pharmacies would not have been held to involve the granting of any “licence”;
and, perhaps more relevantly:
the “condition” otherwise contained within any such licence would not have been held to constitute a “condition” to which s 51(3) applied. Section 51(3), it has been said, “determines the scope of restrictions the patentee may properly impose on the use of the patent. Conditions which seek to gain advantages collateral to the patent are not covered by s 51(3)”: Transfield Proprietary Limited v Arlo International Limited (1980) 144 CLR 83 at 103 per Mason J.
The relevant “conditions”, it would have been concluded were “conditions” which sought to gain “advantages collateral to the patent…”.
Conclusions
460 The central facts relevant to the resolution of the present dispute, once stripped from the necessary but possibly distracting detail, are within a narrow compass.
461 The situation confronting Pfizer in Australia as at 2009 was that a number of its patents over its pharmaceutical products were coming to an end. Its exclusive rights to distribute those products would therefore be lost. Amongst those patents was the patent over atorvastatin, a pharmaceutical product which had long contributed immensely to the revenues of Pfizer. Its “brand” product, Lipitor, would continue to be available. But it would inevitably confront competition from the existing generic manufacturers, and possibly new generic manufacturers, who would wish to establish their own share of a lucrative source of income.
462 Pfizer had decisions to make. One option was to simply continue to manufacture and sell its “branded” product. Another option was to manufacture and market its own generic atorvastatin. That was the path it decided to go down. But, in so deciding, it inevitably had to confront the reality that there were well-established arrangements between the generic manufacturers and many of the pharmacies. Those arrangements were such that the pharmacies were likely to sell the generic product supplied by the manufacturer with which it had an arrangement. In entering the generic market, Pfizer was entering a market in respect to which it had relatively no prior experience.
463 The strategy that it developed was to depart from its traditional method of distributing its products to pharmacies through wholesalers. To confront the challenges faced by existing generic manufacturers and many of the pharmacies, Pfizer decided to implement what it described as a Direct-to-Pharmacy Model whereby it would sell and deliver its products direct to the pharmacies. It also developed a scheme to give a “rebate” on its branded product which could be “unlocked” by pharmacies accepting one or other of the offers it made. But those offers were dependent upon supplying substantial quantities of its generic atorvastatin. Discounts on the sales of Lipitor were “bundled” with Pfizer’s generic product.
464 It has been concluded (inter alia) that the ACCC has failed to establish that Pfizer:
for the purposes of s 46 pursued its course of conduct “for the purpose of deterring or preventing a person from engaging in competitive conduct”; and
for the purposes of s 47(10)(a) pursued its course of conduct for the purpose of “substantially lessening competition”.
Rather than pursuing its course of conduct for either of these purposes, Pfizer (in summary form) did so for the purpose of seeking to remain competitive in the market. Although Pfizer recognised that in pursuing that course, the incentives it offered to pharmacies to take its atorvastatin products – including both Lipitor and its own generic atorvastatin – made it harder for the other manufacturers to compete, it did not engage in the conduct in question for any substantial purpose of deterring or preventing the other generic manufacturers from entering the market.
465 The Amended Originating Application filed on 8 September 2014 should be dismissed.
466 There is no reason why the usual rule as to costs should not apply such that ACCC should pay the costs of Pfizer.
The Orders of the Court Are:
1. The Amended Originating Application filed by the Australian Competition and Consumer Commission is dismissed.
2. The Applicant is to pay the costs of the Respondent.
I certify that the preceding four hundred and sixty-six (466) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Flick. |
Associate:




