FEDERAL COURT OF AUSTRALIA
Apotex Pty Ltd v Les Laboratoires Servier [2013] FCA 1426
FEDERAL COURT OF AUSTRALIA
Apotex Pty Ltd v Les Laboratoires Servier [2013] FCA 1426
CORRIGENDUM
1. The original medium neutral citation “Apotex Pty Ltd v Servier Laboratories (Aust) Pty Ltd [2013] FCA 1426” on the cover page was incorrect and has been replaced with “Apotex Pty Ltd v Les Laboratoires Servier [2013] FCA 1426”.
2. In paragraph [50] “Prof Evans is” has been replaced with “Until he was recently appointed Provost, Prof Evans was”.
3. In paragraphs [49], [60], [75], [107], [183], and [213] “Bryn” has been replaced with “Byrn” wherever occurring.
4. In paragraph [87] “bytylamine” has been replaced with “butylamine”.
5. In paragraph [109] “burylamine” has been replaced with “butylamine”.
6. “Aequous” has been replaced with “aqueous” wherever occurring.
7. In paragraph [146] “which had a molecular weight of 44.15 mmol” has been replaced with “(44.15 mmol)” and “which had a molecular weight of 41.94 mmol” has been replaced with “(41.94 mmol)”.
8. In paragraph [148] “paramaters” has been replaced with “parameters”.
9. In paragraph [150] “The salt break” in the third sentence has been replaced with “The method”.
10. In paragraph [151] “with a molecular weight of 32.56 mmol” has been replaced with “(32.56 mmol)” and “with the same molecular weight” has been replaced with “(32.56 mmol)”.
11. In paragraphs [181] and [182] “Illonois” has been replaced with “Illinois”.
12. In paragraph [181] “Fireball” has been replaced with “Firebelt”.
13. In paragraph [186] “vaguaries” has been replaced with “vagaries”.
14. In paragraph [240] “hygroscopity” has been replaced with “hygroscopicity”.
15. In paragraph [250] “germaine” has been replaced with “germane”.
16. In paragraph [268] “dessicant” has been replaced with “desiccant”.
17. In paragraph [289] “Coverysl” has been replaced with “Coversyl”.
|
I certify that the preceding seventeen (17) numbered paragraphs are a true copy of the Corrigendum to the Reasons for Judgment herein of the Honourable Justice Rares. |
Associate:
Dated: 10 April 2014
|
IN THE FEDERAL COURT OF AUSTRALIA |
|
|
APOTEX PTY LTD ACN 096 916 148 Applicant | |
|
AND: |
First Respondent SERVIER LABORATORIES (AUST) PTY LTD Second Respondent |
|
DATE OF ORDER: |
|
|
WHERE MADE: |
THE COURT ORDERS THAT:
1. The parties confer and prepare draft orders, including as to costs, to give effect to the reasons for judgment published today and in the event that the parties are unable to agree on such orders:
(a) on or before 31 January 2014, the applicant file and serve its draft orders, any further evidence and written submissions limited to 10 pages;
(b) on or before 10 February 2014, the respondents file and serve their draft orders, any further evidence and written submissions limited to 10 pages.
2. The proceedings stand over to 14 February 2014 for directions or the making of orders.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
|
NEW SOUTH WALES DISTRICT REGISTRY |
|
|
GENERAL DIVISION |
NSD 51 of 2012 |
|
BETWEEN: |
APOTEX PTY LTD ACN 096 916 148 Applicant |
|
AND: |
LES LABORATOIRES SERVIER First Respondent SERVIER LABORATORIES (AUST) PTY LTD Second Respondent |
|
JUDGE: |
RARES J |
|
DATE: |
24 DECEMBER 2013 |
|
PLACE: |
SYDNEY |
REASONS FOR JUDGMENT
1 These proceedings concern the validity of Australian patent AU 2003200700 (the patent) which has the priority date of 18 April 2002. The patent makes claims for an invention of a new salt of the compound perindopril, called perindopril arginine. Perindopril, itself, had been patented much earlier. Perindopril is used to lower blood pressure in patients with high blood pressure (or hypertension).
2 Les Laboratoires Servier is the registered proprietor of the patent and its wholly owned subsidiary, Servier Laboratoires (Aust) Pty Ltd, is the exclusive licensee (collectively Servier). There is no relevant need to distinguish between those two companies in these reasons.
3 Servier had an earlier patent for the salt known as perindopril erbumine (an abbreviation for perindopril tert-butylamine). Between 1992 and 2006 Servier marketed that compound in Australia and internationally in a tablet form known under the brand name Coversyl. Commercially, Coversyl has been a very successful drug product for Servier. When the earlier patent expired, Servier began to market its new tablets containing perindopril arginine under the Coversyl brand name. At the same time Apotex Pty Ltd began marketing a generic version of perindopril erbumine.
4 Apotex brought these proceedings to challenge the validity of the patent because it wishes to enter the market with a generic version of perindopril arginine. It claimed that the patent should be revoked on six grounds. Broadly, those grounds are that, based on s 138 of the Patents Act 1990 (Cth) (the Act) (as in force at 18 April 2002):
the invention was not a patentable invention on the bases that :
(1) it was not novel when compared to the prior art base, because one or both of two earlier patents had claimed the invention of a method for synthesis of perindopril and its pharmaceutically acceptable salts (s 7(1), 138(3)(b)) (the novelty issue); or
(2) it lacked an inventive step because it would have been obvious to a person skilled in the art, in light of the existing common general knowledge to try making a salt of perindopril with arginine (ss 7(2), 138(3)(b)) (the inventive step issue);
the claims in the patent were not fairly based on the matter described in the specification (ss 40(3), 138(3)(f)) (the fair basis issue);
the patent did not describe the best method known to Servier of performing the invention (ss 40(2)(a), 138(3)(f)) (the best method issue);
the patent had been obtained by false suggestions or misrepresentations (s 138(3)(d)) (the false suggestion issues).
5 I will describe first, some of the relevant pharmaceutical concepts, secondly, the legislative scheme, thirdly, the patent, and fourthly, each issue in turn together with its factual matrix and the parties’ arguments on that issue. I think that this will aid understanding of the separate factual and legal questions pertinent to each issue.
6 Perindopril is an amino acid that is commonly prescribed to lower a patient’s blood pressure. It acts as an angiotensin converting enzyme (ACE) inhibitor. It is usually administered in small dosage tablets taken once each day. The tablets comprise of a compound consisting of a salt form of perindopril, which is its active pharmaceutical ingredient (API), and excipients. The salt may dissociate into its ionic components when it is added to an aqueous medium. Such a reaction can occur in the human stomach. The salt is converted in the patient’s body by hydrolysis into the biologically active compound, perindoprilat. The hydrolysis results from the perindopril molecule reacting with water to produce the perindoprilat and ethanol.
7 A salt is a compound that contains ionic components, one of which, called a cation, is positively charged, and another, called an anion, which is negatively charged. The cation lacks one or more electrons while the anion has one or more extra electrons. The cation is attracted to the anion and an electrostatic interaction occurs between them so that they form an ionic bond in which the charges are in equilibrium. A salt of an organic molecule consists of the ion of the organic molecule and a counter-ion from the reagent, relevantly here, an acid or base with which it reacts. Typically, the acid is defined as a proton donor (being positively charged) and the base as a proton acceptor (being negatively charged).
8 When a pharmaceutical salt is formed, a proton, being a hydrogen ion, transfers from the acid to the base. This results in an ionic pair consisting of both a cation and an anion. The general salt forming equilibrium reaction can be summarised as follows, where B is the base, HA is the acid or proton donor, BH+ is the cation, A– is the anion and the salt is comprised of both the cation and anion:
B + HA ⇌ BH+ + A–
9 Perindopril is known as a zwitterion because it can form a salt with a counter-ion that is either an acid or a base. That is because perindopril contains both an acidic (carboxylic acid) group that can lose a proton to form a salt with a base and a basic (amine) group that can gain a proton to form salts with an acid. Perindopril may also be crystallised in a non-salt form if both its acidic and basic groups interact with each other. The acidic and basic groups of perindopril are shown on the figure below:
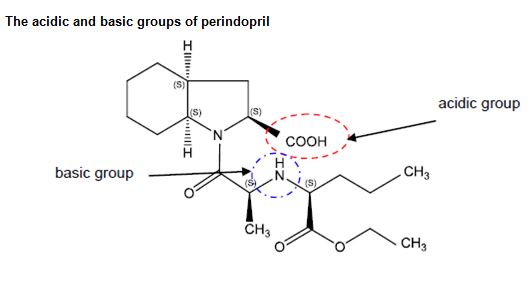
10 The effectiveness of a drug can be affected by its physicochemical properties. Those physicochemical properties can be altered and, possibly, optimised by the creation of one or more salt forms of the drug substance. Typically, the solid state properties of salts of a drug substance will differ from those of the substance itself: i.e. when it is in its free acid or free base form. Those properties can ultimately affect the biological and pharmacokinetic profile of the drug substance.
11 Salts can be used to improve the properties of a drug substance in a number of ways, including improving solubility, tailoring and adapting the drug to the therapeutic use and pharmaceutical dosage form, modifying, if need be, the drug’s pharmacokinetic properties (i.e. the body’s absorption, distribution, metabolisation and excretion of the drugs), improving or preserving the drug’s chemical and/or physical stability, and facilitating or enhancing its industrial processing.
12 An API is formulated with excipients into a tablet or composition. The excipients are inactive compounds that usually contribute to the drug delivery profile, or way and time in which the drug substance will arrive at the place in the patient’s body where it is effective. Thus, excipients can cause the drug substance to be released immediately, gradually at or during a particular time period after the patient takes the tablet. In addition, the formulation of the tablet and the process by which it is made can affect the shelf-life of the drug product.
13 Tablets are made by mixing their ingredients as intimately and evenly as possible and then compressing these in a die in a tablet press. The evenness of the mixture of the API with the excipients is a critical part of the process to produce tablets. The three main methods of tablet production are:
• wet granulation: the ingredients are mixed with water or another solvent, granulated, dried and compressed into tables;
• dry granulation: the granulation occurs without addition of water or another solvent;
• direct compression: the ingredients are mixed and directly compressed into tablets without a granulation step.
14 Thus the creation of a salt of a drug substance can be an important means to bring it to market as a medically and commercially useful product.
15 The Therapeutic Goods Administration (TGA) is the Australian regulator with the responsibility for medicines and the administration of the Therapeutic Goods Act 1989 (Cth). The TGA publishes and applies regulatory guidelines for drugs or prescription medicines that are based, to a large extent, on international standards. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) is the international body responsible for establishing a unified global regulatory rÉgime. It recommends harmonised guidelines that can be adopted by local regulators. The European Medicines Agency (EMEA) is the European regulator and the Food and Drug Administration (FDA) is the regulator in the United States of America. In general, the TGA does not directly adopt ICH guidelines. Rather, the TGA commonly adopts the guidelines published by the EMEA that, in turn, adopt those of the ICH.
16 The TGA, or other regulator, assesses a shelf-life and appropriate storage conditions for a pharmaceutical substance when it has been developed to the point that it is ready for commercial sale. This is necessary because the API or the tablet or other composition in which the API is administered to the patient may be susceptible to degradation or deterioration over time or in particular temperatures or other storage conditions. That is, the pharmaceutical product, in the form it is to be sold, may or may not be stable.
17 There are two aspects of the stability of both drug substance and a drug product that the regulator, and no doubt a manufacturer, must address, namely its chemical and physical stability. Chemical stability concerns whether the chemical content of the drug substance itself remains constant or degrades. Physical stability concerns changes to the physical form of the product (e.g. whether the form is, or varies to, polymorphic, solvate or hydrate) or its appearance and to its handling or storage. Ideally, the drug substance and product should remain chemically and physically unchanged for many years under typical ambient storage conditions.
18 Tests to evaluate which of the number of counter-ions are appropriate for further testing as a suitable salification (salt making) agent are called salt-screens. One element considered in salt-screening is the stability of the salt produced. Stability of a salt is important in considering its effect on the shelf-life of any developed product utilising the salt. This consideration is important in assessing the commercial viability of any product that utilises the salt. In general, the longer the shelf-life of a drug product the greater the manufacturer’s flexibility to deal with unused or unsold but older or dated stock. The ICH Stability Guideline defines shelf-life of a pharmaceutical product as:
“The time period during which a drug product is expected to remain within the approved shelf-life specification, provided that it is stored under the conditions defined on the container label.”
19 An expiry, or use-by date, is the usual means of stating the shelf-life of a commercial pharmaceutical product. However, a retest date, as opposed to an expiry date, is sometimes given for an API. That is because the API will usually remain under the control of the drug manufacturer which will need to retest the API at, or after, the given date in order to see if it remains well within specifications and so is suitable to use in manufacturing a drug product. The ICH Stability Guideline defines “retest period” as:
“The period of time during which the drug substance [API] is expected to remain within its specification and, therefore, can be used in the manufacture of a given drug product, provided that the drug substance has been stored under the defined conditions.”
20 Drug manufacturers must undertake extensive stability testing of each of an API and a drug product to satisfy a regulator of the appropriateness of its particular given shelf-life. Ordinarily, the regulator will only approve a shelf-life for a drug product on the basis of extensive testing of the stability performance of its dosage form, as packaged, in particular controlled conditions, including conditions of identified temperature and humidity. This is replicated in Australia in the TGA Stability Guideline that requires testing of the exact formulation and final packaging intended for the marketing of the drug product in this country.
21 From 1996 until October 2005, the ICH recognised the following four climatic zones in the table below for its requirements for pharmaceutical products:
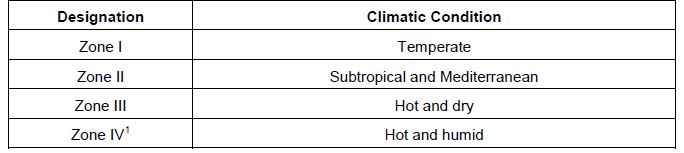
22 Australia is in zones III and IV while Europe is in zones I and II. Therapeutic goods Order No 69: General requirements for labels for medicines provides for the standard permitted storage temperatures for pharmaceutical products as:
“(a) Store below -18°C (Deep freeze);
(b) Store below -5°C (Freeze);
(c) Store below 8°C (Refrigerate);
(d) Store at 2°C to 8°C (Refrigerate. Do not freeze.);
(e) Store below 25°C; and
(f) Store below 30°C.”
23 While the TGA can approve a different storage temperature for a pharmaceutical product, in general 30°C was, relevantly, the maximum storage temperature for pharmaceutical products marketed in Australia.
24 Packaging can play a significant role in the approval of a shelf-life for a pharmaceutical product. Packaging has two principal purposes in the pharmaceutical industry, namely presentational and functional. Packaging enables, first, the product to be presented to and identifiable by users (such as pharmacists, doctors and patients) and to convey product information, including as to storage and shelf-life, evidence of no prior tampering or use and features to make the product visually or tactilely attractive. Secondly, packaging can serve a number of functions, some of which can overlap. These functions include providing a convenient and reliable way of dispensing a consistent dose, child proofing, a means of maximising shelf-life by protecting the product from humidity and or air, as well as protecting it during transport, warehousing or other storage.
25 The sponsor (or proponent) of a pharmaceutical product, such as each of the parties to these proceedings, must provide the regulator, as part of the marketing approval process, with details of the packaging in which the product is proposed to be marketed. Such products are typically marketed in bottles or blister packs. Since before 2002, bottles used for pharmaceutical products have been made of glass or a plastic, such as high density polyethylene (HDPE). A desiccant will often be placed in a bottle if the drug product can absorb water from the atmosphere or the air in the bottle.
26 Blister packs can consist of (1) a sheet of polymer formed into a series of wells into which individual tablets are placed and (2) a sheet of aluminium foil, the edges of the abutting side of which has a coating of heat-seal lacquer, placed over the open wells containing the tablets. Then, usually for less than a second, the two sheets are pressed together and heated to between 140°C and 160°C to enable the lacquer to bond. This type of packaging is known as aluminium/PVC or PVC/Alu. However, if the application of heat may affect the drug product, it is possible to use lacquer that can adhere at a lower temperature or a more expensive pressure sensitive adhesive.
27 A second form of blister packaging, known as Alu/Alu can be made using aluminium laminate as the welled base and aluminium lidding foil. These are sealed using a heat seal lacquer in the same way as aluminium/PVC blisters are.
28 A third form of blister packing, known as tropicalised packaging, consists of a aluminium/PVC blister overwrapped with an aluminium foil bag that contains a desiccant. Servier used tropicalised packaging for its perindopril erbumine Coversyl products that it sold in Australia at all relevant times.
29 The parties agreed that the relevant provisions of the Patents Act were those as in force when Servier filed the provisional specification for the patent in France on 18 April 2002 (which did not mention hydrates at all) and when the complete specification was filed here on 27 February 2003. The Act remained unamended between 1 April 2002 and 4 July 2003. I have used that version of the Act in these reasons.
30 Any person could apply for an order revoking the patent (s 138(1)). Critically, s 138(3) provided:
“(3) After hearing the application, the court may, by order, revoke the patent, either wholly or so far as it relates to a claim, on one or more of the following grounds, but on no other ground:
…
(b) that the invention is not a patentable invention;
(d) that the patent was obtained by fraud, false suggestion or misrepresentation;
…
(f) that the specification does not comply with subsection 40(2) or (3).”
31 Apotex raised two issues under s 18(1)(b) in support of the ground in s 138(3)(b), namely novelty and inventive step. Those issues were governed by ss 18(1)(b) and 7(2), which for present purposes provided:
“18 Patentable inventions
Patentable inventions for the purposes of a standard patent
(1) Subject to subsection (2), an invention is a patentable invention for the purposes of a standard patent if the invention, so far as claimed in any claim:
…
(b) when compared with the prior art base as it existed before the priority date of that claim:
(i) is novel; and
(ii) involves an inventive step;
7 Novelty and inventive step
Novelty
(1) For the purposes of this Act, an invention is to be taken to be novel when compared with the prior art base unless it is not novel in the light of any one of the following kinds of information, each of which must be considered separately:
(a) prior art information … made publicly available in a single document or through doing a single act;
…
Inventive step
(2) For the purposes of this Act, an invention is to be taken to involve an inventive step when compared with the prior art base unless the invention would have been obvious to a person skilled in the relevant art in the light of the common general knowledge as it existed in the patent area before the priority date of the relevant claim …”
The Dictionary in Sch 1 of the Act relevantly provided:
“prior art base means:
(a) in relation to deciding whether an invention does or does not involve an inventive step or an innovative step:
(i) information in a document that is publicly available, whether in or out of the patent area; and
(ii) information made publicly available through doing an act, whether in or out of the patent area.”
32 Apotex also raised two issues under s 40(2)(a) and (3), namely whether the specification described any, or the best, method known to the patentee of performing the invention and whether the claims in relation to hydrates of perindopril arginine were fairly based on the matter described in the specification. Relevantly, s 40 provided:
“40 Specifications
(1) A provisional specification must describe the invention.
(2) A complete specification must:
(a) describe the invention fully, including the best method known to the applicant of performing the invention;
…
(3) The claim or claims must be clear and succinct and fairly based on the matter described in the specification.”
33 The Commissioner of Patents had to accept a patent request and a complete specification relating to an application for a standard patent (such as the patent in issue) if she were satisfied that the invention, so far as claimed, satisfied the criteria in s 18(1)(b) and she considered that there was no other lawful, unresolved ground of objection to the request and specification (s 49(1)). Any person could oppose the grant, relevantly, on the grounds that the invention was not a patentable invention or the specification filed in respect of the complete application did not comply with s 40(2) or (3) (s 59).
The patent
34 The specification in the patent began by stating that the invention related to a new salt of perindopril and pharmaceutical compositions containing it. The specification stated that perindopril had previously been described in a European patent (EP 0 049 658) that had mentioned, as was usual, that compounds of the invention might be presented in the form of addition salts with a pharmaceutically acceptable, mineral or organic, base or acid. It stated that the compounds described in the European patent were in a non-salt form and “primarily, when addition salts with a pharmaceutically acceptable base or acid are mentioned by way of example, the sodium salt or the maleate are given” (p 1 lines 12-14). The specification continued:
“In the development of that product, however, it has proved very difficult to find a pharmaceutically acceptable salt having not only good bioavailability but also adequate stability to be suitable for the preparation and storage of pharmaceutical compositions.” (p 1 lines 15-17)
35 The specification noted that the non-salt form had been studied, as well as the maleate and the sodium salt, and that in the course of temperature and humidity stability studies these had been found not to be suitable because the sodium salt was immediately converted into an oil on contact with the atmosphere and the non-salt form and maleate degraded rapidly under higher temperature conditions. The specification continued:
“The tert-butylamine salt was thus alone in exhibiting the best stability compared to the other forms studied. However, in view of the intrinsic fragility of perindopril, the tert-butylamine salt has not been capable of providing a complete solution to the problems of the product’s stability to heat and humidity. Indeed, for marketing, tablets of perindopril tert-butylamine salt must, in certain countries, be protected with additional packaging measures. Moreover, even for temperate-climate countries, that instability has made it impossible to obtain a shelf-life of more than 2 years for the tablets. Finally, for marketing of the tablets, they have to be marked “to be stored at a temperature less than or equal to 30 degrees”.
These constraints are, of course, onerous especially in terms of organisation and cost, and it has appeared especially useful to try to develop a new perindopril salt in order to reduce the constraints due to the tert-butylamine salt.” (p 1 line 23-p 2 line 9)
36 The specification then said that numerous salts had been studied and that those customarily used in the pharmaceutical sector had proved to be unusable and continued:
“On the other hand, and in surprising manner, it has been found that the arginine salt of perindopril, besides being new, has entirely unexpected advantages over all the other salts studied and, more especially, over the tert-butylamine salt of perindopril.” (p 2 lines 12-14)
37 The specification then said that the present invention related to the arginine salt, its hydrates and also to the pharmaceutical compositions comprising it. It identified the preferential form of the salt as being natural arginine (L-arginine) and said that the pharmaceutical compositions according to the invention comprised the arginine salt together with one or more, non-toxic, pharmaceutically acceptable and appropriate excipients. The specification then identified a variety of possible pharmaceutical compositions and various administration and dosage methods. It stated that the pharmaceutical compositions according to the invention would preferably be immediate release tablets and that the useful dosage varied according to the age and weight of the patient, the nature and severity of the disorder and to the administration route (p 2 line 15, p 3 line 5).
38 The specification identified the amount of arginine salt contained in the compositions according to the invention, including the preferred quantities between 1 to 10 mg and that those compositions were of use in the treatment of hypertension and heart failure. It continued:
“The basic characteristics of this salt are very great stability to heat and to humidity compared to the tert-butylamine salt.
Long-term stability studies carried out under very precise temperature and humidity conditions have yielded the results indicated in the Table hereinbelow.
In that study, perindopril was assayed by inverse-phase high-pressure liquid chromatography using, as eluant, an aqueous phase (comprising sodium heptane-sulphonate, and the pH of which is 2) and acetonitrile (67/33). Detection of the product was carried out by UV (215 nm).
The study was carried out using immediate-release tablets containing either 2.4 mg of the arginine salt of perindopril or 2.0 mg of the tert-butylamine salt of perindopril (each of the two tablets containing 1.7 mg of perindopril). The tablets were assayed 6 months after the start of storage of the tablets at different temperatures and different relative humidities (% R.H.).
The arginine salt used in this study is the L-arginine salt. It has been prepared according to a classical method of salification of organic chemistry.
|
Conditions 6 months |
tert-Butylamine salt of perindopril Percentage remaining(%) |
Arginine salt of perindopril Percentage remaining (%) |
|
25°C 60% R.H. |
101.0 |
99.5 |
|
30 °C 60% R.H. |
94.4 |
98.1 |
|
40°C 75% R.H. |
67.2 |
98.6 |
The results presented in the Table above show extremely clearly the very great stability of the arginine salt compared to the tert-butylamine salt. Indeed, after 6 months, practically no degradation of the arginine salt has taken place whereas the tert-butylamine salt exhibits a degradation rate of approximately 33%.” (p 3 line 9-p 4 line 5)
39 The specification then asserted that the results were entirely unexpected and could not have been deduced from or suggested by the teaching of the literature on the product and concluded by stating:
“The results allow us to consider less onerous constraints with respect to the packaging of the pharmaceutical compositions and also to obtain a shelf-life of at least three years for our pharmaceutical compositions.” (p 4 lines 8-10)
40 The patent asserted 11 claims defining the invention but, as noted above, only the following five are relevant:
“1. The arginine salt of perindopril and its hydrates.
2. Pharmaceutical composition comprising, as active ingredient, the arginine salt of perindopril and its hydrates, in combination with one or more pharmaceutically acceptable excipients.
3. Pharmaceutical composition according to claim 2, characterised in that it is presented in the form of an immediate-release tablet.
4. Pharmaceutical composition according to either claim 2 or claim 3, characterised in that it contains from 0.2 to 10 mg of the arginine salt of perindopril.
…
6. A method of treatment or prophylaxis of hypertension and heart failure comprising administering to a patient in need of such treatment or prophylaxis an efficacious amount of the arginine salt of perindopril and its hydrates of claim 1, or a pharmaceutical composition as claimed in any one of claims 2 to 4.”
41 Claims 8-11 referred to descriptions of the invention in “the Examples”, but there were no examples set out in the patent and so those claims could not be sustainable.
Some general background
42 In late 2006 Servier ceased to supply perindopril erbumine tablets under the Coversyl brand name and began selling perindopril arginine tablets under it instead. The perindopril arginine tablets are made by wet granulation and then packaged in HDPE bottles with a desiccant. They have a TGA approved shelf-life of three years when marked “store below 30°C”.
43 Also in late 2006, Apotex began selling its perindopril erbumine tablets. Those are made by direct compression, marketed in Alu/Alu (or cold form foil) packaging and initially had a TGA approved shelf-life of two years when marked “store below 25°C”. Pharmacists can substitute Apotex’ perindopril erbumine tablets for patients who present prescriptions for Servier’s perindopril arginine tablets under the National Health Act 1953 (Cth). In 2007, the TGA approved a longer shelf-life of three years for Apotex’ perindopril erbumine tablets when marked “store below 25°C”.
44 In January 2012, Apotex informed Servier that it had applied to the TGA for registration of generic perindopril arginine products on the Pharmaceutical Benefits Scheme once they were approved by the TGA. Apotex commenced these proceedings on 16 January 2012. Servier obtained an interlocutory injunction in March 2012 restraining Apotex from selling perindopril arginine effectively until final judgment. Apotex did not oppose that course. Apotex accepts that if claims 1, 2, 3, 4 and 6 of the patent are valid, it would infringe them. Servier does not seek relief on the basis of any apprehended infringement of claims 5 and 7 on the basis that Apotex does not allege that those two claims are independently invalid. The parties did not present argument about the detail of how the individual claims may be infringed, no doubt because of Apotex’ concession that if its invalidity arguments fail, unless further restrained, it will infringe the patent by going to market with its new generic.
45 The parties called six experts in chemistry with expertise in pharmaceutical matters. There was no issue that each of these experts was appropriately qualified. I found their evidence of considerable assistance. I will very briefly summarise their qualifications which, however, are much more extensive and impressive than is necessary to record in these reasons.
46 Apotex relied on Dr Peter Spargo, Dr Desmond Williams and Associate Professor Michael Perkins. Dr Spargo is currently a consultant. He had worked in the field of chemistry research and development in the pharmaceutical industry seeking to develop new chemical entity therapeutic agents since he began his career working for Pfizer Ltd in the United Kingdom in 1988. He had particular experience and expertise in solid form (including salt and polymorph) selection.
47 Dr Williams is currently the program director, pharmaceutical science in the School of Pharmacy and Medical Sciences and supported researcher in the Sansom Institute for Health Research at the University of South Australia. He is also a registered pharmacist and chartered chemist. His experience included pharmaceutics (i.e. the formulation of drug substances, such as APIs, into drug products suitable for, and adapted to, effective delivery to the intended site within the human body). He had worked for a number of large pharmaceutical companies and had considerable experience in dealing with regulators, including the TGA, and has been a member of the pharmaceutical subcommittee of the TGA’s Advisory Committee on Prescription Medicines since 2005.
48 Prof Perkins is currently an Associate Professor in the School of Chemical and Physical Sciences at Flinders University, South Australia. His experience is broadly in organic chemistry and natural product chemistry, with particular expertise in synthetic organic chemistry, including the discovery, action and metabolism of drugs.
49 Servier relied on Professor Stephen Byrn, Professor Alan Evans and Dr Angelo Morella. Prof Byrn has had an academic career at Purdue University, Indiana in the United States of America since 1972, becoming a Professor of Medicinal Chemistry in 1981 and being head of the Department of Industrial and Physical Pharmacy, School of Pharmacy and Pharmacal Sciences there between 1994 and 2009. He is an accomplished scientific author, having written a text book and many peer-reviewed journal articles. He co-founded a teaching program for sustainable medicines in Africa that seeks to address the lack of accessibility to high quality medicines there. In 1991, he co-founded SSCI Inc (an acronym for “Solid State Chemical Information”) and was its study director till 2006 when it was taken over by Aptuit Inc. He has since then been a consultant to SSCI. From 1993, SSCI has been both a professional development provider of educational services in the pharmaceutical industry and a commercial provider of contract research including formulation development, salt and polymorph screening on potential drug candidates and existing drugs. By 2006, SSCI employed over 100 persons. Prof Byrn had conducted, supervised or controlled over 100 salt-screens and over 200 polymorph screens as at April 2002.
50 Until he was recently appointed Provost, Prof Evans was Professor of Pharmaceutics at the University of South Australia and currently its Pro-Vice Chancellor and Vice President, Division of Health Sciences. He was head of that university’s School of Pharmacy and Medical Sciences from 2004 to 2009. His primary research specialty is pharmacokinetics and biopharmaceutics. He also worked as a retail pharmacist for seven years to 1989. He has been involved in consulting for the pharmaceutical industry, in particular in comparative bioavailability studies between different salts in the development of pharmaceutical compounds.
51 Dr Morella is currently a drug delivery and pharmaceutical consultant. He had worked from 1985 to 2012 with FH Faulding and Company Limited and with Mayne Pharma International, following its takeover of Faulding, in various roles relating to drug development, his final position there being General Manager R & D. He is a named inventor on over 100 patents. He had responsibilities at Faulding and Mayne that required him to be aware, in general terms, of formulation and packaging requirements for countries, including those in ICH climate zones III and IV, to which his employers exported drug products.
52 The experts prepared two joint reports and gave their oral evidence concurrently. The joint reports were very helpful in distilling a number of matters that were either agreed by all relevant experts or on which they disagreed. The concurrent evidence lasted for four and a half days and allowed each expert to articulate the substantive points on which he was in agreement or at issue with any of his colleagues. This greatly assisted in identifying the real issues.
53 At the trial Apotex relied on the disclosure of perindopril and its pharmaceutically acceptable salts in two Australian patents published before 18 April 2002, that being the agreed priority date of the claims under s 43(2)(a). Those were:
Australian patent 200124847 entitled “Method for synthesis of perindopril and its pharmaceutically acceptable salts” that had a journal publication date of 1 November 2001 (the synthesis patent);
Australian patent 2001276418 entitled “A crystalline form of perindopril tert-butylamine salt” that had a journal publication date of 14 February 2002 (the crystalline form patent).
54 The synthesis patent claimed a process for the industrial synthesis of formulae of “perindopril and pharmaceutically acceptable salts thereof”. Its specification noted that perindopril and its pharmaceutically acceptable salts “and more especially the tert-butylamine salt thereof, have valuable pharmacological properties”. That specification also referred the capacity for one formula to be “converted, if desired, into a pharmaceutically acceptable salt, such as the tert-butylamine salt”.
55 The crystalline form patent claimed a new Α crystalline form of the compound formula for the tert-butylamine salt and also a process for its preparation as well as compositions containing it. The specification again recited that “Perindopril and its pharmaceutically acceptable salts, and more especially its tert-butylamine salt, have valuable pharmacological properties”. It went on to say that perindopril, “its preparation and its use in therapeutics have been described in European Patent specification EP 0 049 658”.
56 Critically, neither the synthesis nor the crystalline form patents mentioned any specific salt of perindopril other than the tert-butylamine salt, except in the generalised unspecific references to “pharmaceutically acceptable salts”. That is, nothing in those two patents taught the skilled addressee, or the lay reader, what salts of perindopril, other than perindopril erbumine, were pharmaceutically acceptable or anything about them. They did not suggest that any such salt had been made, far less identify what it might be. Nothing in either of those patents referred to arginine at all, let alone, its potential to create a pharmaceutically acceptable salt of perindopril. On the other hand, both patents left open and unconfined their disclosures that perindopril’s pharmaceutically acceptable salts (whatever they might be in addition to the tert-butylamine salt) had pharmacological uses.
57 The parties agreed that the common general knowledge in Australia prior to 18 April 2002, included the existence of perindopril erbumine and Coversyl, and that perindopril erbumine was the API in Coversyl, each of perindopril (and Coversyl containing it) was used as an ACE inhibitor, being an active ingredient in pharmaceuticals for the treatment of heart failure and hypertension.
58 In addition, Apotex contended that a skilled addressee would have been aware, as part of the common general knowledge, of a reference source for counter-ions that could be used to create a pharmaceutically acceptable salt. That source was a paper by Stephen M Berge, Lyle D Bighley and Donald C Monkhouse entitled “Pharmaceutical Salts” published in the January 1977 edition of the Journal of Pharmaceutical Sciences (the Berge paper).
59 The Berge paper surveyed literature over the preceding 25 years and identified from that exercise potentially useful counter-ions with which to make pharmaceutical salts. It then divided the results into three tables which were subdivided into anions (acids) and cations (bases) and listed the percentage of the instances of use of each substance. Tables I and II of the Berge paper listed all counter-ions used to make salts of organic compounds that were commercially marketed up to 1974. Table I comprised all counter-ions that had been used in products approved by the FDA, while Table II comprised counter-ions that had been used in products marketed in other countries, but had not been approved by the FDA. The paper explained that its authors had considered only salts of organic compounds in Tables I and II because most drugs were organic substances. Table III comprised other counter-ions that had been “reported to be potentially useful [scil: to form] pharmaceutical salts”. Arginine was listed, with lysine, in Table III. Both of those substances were amino acids that occur naturally in the human DNA. The Berge paper listed well over 100 counter-ions that could provide a relatively discrete source of reference for persons skilled in the art of creating salts if they were aware of that publication.
60 Both Dr Spargo, an Englishman, and Prof Byrn, an American, were aware of the Berge paper. Of the Australian witnesses, Dr Evans was aware of the information concerning FDA approved commercially marketed salts in Table I of the Berge paper, but not its source. His awareness came from his familiarity with the pharmacy textbook: Remington: The Science and Practice of Pharmacy (19th ed). Dr Morella was also aware of the Berge paper but, like Prof Byrn, both in their work, did not use counter-ions that were not on Table I because those substances had not created salts that the FDA had approved. Prof Perkins did not recall being aware of the Berge paper prior to 2002 and I am not satisfied that he was aware of it. Dr Williams was aware of the Berge paper from his doctoral studies in Kansas in 1979. The experts agreed in their first joint report that it is not possible to predict whether a salt-screen would make a crystalline salt and what properties any such solid would have.
61 Apotex argued that the prior art before the priority date of the patent in suit consisted of, first, the disclosures in the synthesis and crystalline form patents, that there were other, albeit unidentified pharmaceutically acceptable salts of perindopril in addition to the tert-butylamine salt and, secondly, the common general knowledge that arginine was a counter-ion listed in the Berge paper as a substance that could be used to make a pharmaceutically acceptable salt form. Apotex submitted that the Berge paper disclosed a finite range of candidate counter-ions that the person skilled in the art would be aware of as part of common general knowledge in Australia before the priority date. Thus, it contended, such a person having read either or both of the disclosures about perindopril and its pharmaceutically acceptable salts in the synthesis and or crystalline form patents, would understand that matter to refer to the limited number of counter-ions that can be used to form salts listed in the Berge paper as including arginine. For that reason, so the argument ran, the two earlier patents anticipated all pharmaceutically acceptable salts including the arginine salt in the patent in suit.
62 Apotex contended that if the skilled addressee initially did not read either of the two prior art patents as revealing the arginine salt as a pharmaceutically acceptable salt of perindopril, he or she would have arrived at that conclusion using the Berge paper as part of the addressee’s common general knowledge. That paper was the only item of common general knowledge on which Apotex relied in aid of its challenge based on the patent’s lack of novelty. In essence, Apotex’ argument was that the skilled addressee on reading either or both of the synthesis or crystalline form patents, and using common general knowledge derived from the Berge paper’s list of potential counter-ions, would understand that patent to contain a direction to make, at least, the arginine salt and so infringe a claim or claims in that patent.
(b) Who is the skilled addressee?
63 In my opinion, for the purposes of these proceedings, the hypothetical person skilled in the art or, the skilled addressee, was a non-inventive chemist with scientific qualifications working in a drug development context whose routine work involved the formulation and making of salts for use in pharmaceutical products.
64 It is likely that such a person would have worked in a team. Such a person would be conversant with the principles and general practicalities of salt making and salt selection as well as the methods of pharmaceutical tablet formulation and composition. The perspective of “a person skilled in the relevant art” was that of a hypothetical non-inventive worker in the field who was equipped with the common general knowledge in Australia (being the patent area) before the priority date of 18 April 2002: Lockwood Security Products Ltd v Doric Products Pty Ltd [No 2] (2004) 235 CLR at 197 [56]. Relevantly, the evidence did not demonstrate that the common general knowledge at that date differed from the position at 27 February 2003.
(c) Consideration
65 An invention is presumed to be novel unless, relevantly here, when compared separately to each piece of prior art information made publicly available in a single document (here each of the synthesis and crystalline form patents), the comparison would demonstrate to a skilled addressee that the invention had been disclosed in that earlier information (s 7(1)(a)).
66 The skilled addressee’s consideration of the prior art for the purposes of s 7(1) can be at two levels, the first that simply takes account of what information the prior art conveyed to him or her; the second, that is aided by his or her use of what was “common general knowledge” in Australia at the relevant time (here 27 February 2003), in the sense of that expression as explained by Aickin J, with whom Barwick CJ, Stephen, Mason and Wilson JJ agreed in Minnesota Mining and Manufacturing Co v Beiersdorf (Australia) Ltd (1980) 144 CLR 253 at 292 and 295, namely (see at 292):
“The notion of common general knowledge itself involves the use of that which is known or used by those in the relevant trade. It forms the background knowledge and experience which is available to all in the trade in considering the making of new products, or the making of improvements in old, and it must be treated as being used by an individual as a general body of knowledge.” (emphasis added)
67 There is nothing in either of the synthesis or crystalline form patents beyond the words “pharmaceutically acceptable salts” that deals with the creation or use of any possible salt of perindopril, other than the tert-butylamine salt, in connection with the claims. In the first joint experts report, Prof Perkins and Drs Spargo and Williams said:
“One would have an expectation to form one or more crystalline salts, which could be subjected to further testing with regard to their suitability as pharmaceutical salts.”
68 Profs Byrn, Evans and Perkins, and Drs Morella, Spargo and Williams agreed that there was no certainty that any counter-ion would produce a salt, or, if it did, what its properties and suitability would be. They accepted, as the Berge paper itself noted, that “choosing the appropriate salt, however, can be a very difficult task, since each salt imparts unique properties to the parent compound” (emphasis added). And they all agreed that one would need to do one or more salt-screens and undertake a process of trial and error in order to ascertain what particular counter-ion or counter-ions would yield, first, a salt and secondly, one that was pharmaceutically acceptable. The experts all accepted the correctness of the Berge paper’s statement that:
“Furthermore, even after many salts of the same basic agent have been prepared, no efficient screening techniques exist to facilitate selection of the salt most likely to exhibit the desired pharmacokinetic, solubility, and formulation profiles.”
69 Each of the parties’ arguments on novelty had a logical tension. Servier contended that the two patents that Apotex asserted were anticipations of perindopril arginine did not give any instruction as to that substance’s formulation and so it was outside their claims. Apotex argued that Servier would have sued, for infringement of those two patents, anyone who created any new pharmaceutically acceptable salt, such as the arginine salt, because such a salt was within the claims in each of those patents.
70 It is necessary that the prior art (here one or both earlier patents) disclose an invention that, if performed, would necessarily infringe the patent in suit: H Lundbeck A/S v Alphapharm Pty Ltd (2009) 177 FCR 151 at 194 [189] per Bennett J, with whom Middleton J agreed. Her Honour added (at 194 [189]-[190]):
“Once the very subject matter of the invention has been disclosed, the person skilled in the art is assumed to be willing to make trial and error experiments to get it to work ... where the prior publication is of the subsequently claimed invention, that is sufficient. Where the prior disclosure falls short of a complete disclosure, the question of the sufficiency of that disclosure arises. It is there that consideration must be given to the quality of a disclosure to the skilled addressee armed with common general knowledge … the Court, armed with the evidence of the skilled addressee as to terms of art and the nature and extent of the disclosure in the prior art document, must determine whether the prior disclosure is sufficient to enable the skilled addressee to perceive, understand and, where appropriate, apply the prior disclosure necessarily to obtain the invention.” (emphasis added)
71 The invention will be disclosed to a skilled addressee by the prior art provided that any trial and error experimentation needed is a standard or ordinary procedure or something that is part of common general knowledge as a practical means of performing the invention: Lundbeck 177 FCR at 190 [173]. However, if all the prior art does is to describe the earlier invention without disclosing the effective means by which it could be produced, it will not anticipate a subsequent invention: Olin Corporation v Super Cartridge Co Pty Ltd (1977) 180 CLR 236 at 261 per Stephen and Mason JJ, Barwick CJ agreeing on this point at 239. For an anticipation of a subsequent discovery, the prior art, coupled if need be with common general knowledge, must disclose to the skilled addressee “a practicable mode of producing the result which is the effect of the subsequent discovery”: Olin Corporation 180 CLR at 261. And as Bennett J observed in Lundbeck 177 FCR at 192 [181]:
“If the prior art discloses some but not all integers of a claimed patent to a product, such as a combination, there is anticipation if the skilled addressee would add the missing information as a matter of course and without the application of inventive ingenuity or undue experimentation (Nicaro [Holdings Pty Ltd v Martin Engineering Co (1990)] 91 ALR [513] at 530-531).”
72 In ICI Chemicals & Polymers Ltd v The Lubrizol Corporation Inc (2000) 106 FCR 173 at 230 [51] Lee, Heerey and Lehane JJ applied the “flag metaphor” used by Sachs, Buckley and Orr LJJ in General Tire & Rubber Co v Firestone Tyre & Rubber Co Ltd [1972] RPC 457 at 485-486 to illustrate the concept of anticipation. Their Lordships said:
“To anticipate the patentee's claim the prior publication must contain clear and unmistakable directions to do what the patentee claims to have invented: Flour Oxidizing Co Ltd v Carr & Co. Ltd. (1908) 25 RPC 428 at 457, line 34, approved in BTH Co Ltd v Metropolitan Vickers Electrical Co Ltd (1928) 45 RPC 1 at 24, line 1). A signpost, however clear, upon the road to the patentee's invention will not suffice. The prior inventor must be clearly shown to have planted his flag at the precise destination before the patentee.”
73 The Full Court in Lubrizol 106 FCR at 230 [51] added that anticipation was not proved if the skilled addressee had to rummage through the prior inventor’s flag locker to find a flag there that could have been (but was not) planted.
74 I am of opinion that the synthesis and crystalline patents, when read by a skilled addressee and using common general knowledge, that included the Berge paper, did not anticipate the invention of the arginine salt claimed by the patent in suit. Those patents contained no directions whatever as to the production of any salt, other than the tert-butylamine one, in the catch-all words “pharmaceutically acceptable salts”. The Berge paper told the skilled addressee that the FDA had approved salts made from the counter-ions listed in Table I. That paper said that counter-ions listed in Table III, such as arginine, had been reported to be “potentially useful” in creating pharmaceutical salt forms. At best, if the Berge paper were common general knowledge of a skilled addressee, it gave arginine as an ambiguous signpost, obscured by its status in Table III, upon the road to the invention of the arginine salt.
75 Ordinary trial and error experimentation would have involved using counter-ions in Table I of the Berge paper for the reasons that Prof Byrn and Drs Evans and Morella gave in the first joint experts report for not trying arginine. Indeed, Dr Williams said that as “there are no obvious precedents, then arginine would not have been high on the list of options to try”. Ordinary trial and error experimentation does not extend to prolonged research, inquiry or experiment: cf TA Blanco White, Patents for Inventions (4th ed: 1974) Stevens & Sons: London) at [4-504]; see too (5th ed: 1983) at [4-504]. All the experts agreed that they would not try arginine in a first salt-screen to make a new perindopril salt. The unpredictability of successfully making a salt with any counter-ion, let alone one that is pharmaceutically acceptable, requires a person skilled in the art to engage in a selection process. Inventions are born of insights that make actual things that, beforehand, were potentially possible, albeit sometimes highly unlikely. As Prof Byrn, whose business had involved salt making, explained, if the first salt-screen or screens using counter-ions in Table I failed, and if the skilled addressee had additional time and money, he or she might go to Tables II or III in the Berge paper but would not be likely to try arginine.
76 The necessity to choose the appropriate counter-ion or ions to make a pharmaceutically acceptable salt and the absence of either any, let alone a clear, description of the arginine salt or any instructions on how to make it, satisfy me that there was no disclosure in the anodine wording of the synthesis or crystalline form patents (even if read in light of the Berge paper): Apotex Pty Ltd v Sanofi-Aventis (2009) 82 IPR 416 at 439-440 [136]-[139] per Bennett and Middleton JJ. Here, the skilled reader of either of those patents could not predict that any particular acid or base, and especially arginine, used as a counter-ion for perindopril, would result in a pharmaceutically acceptable salt. Gyles J, as the trial judge, expressed the test graphically in Apotex Pty Ltd v Sanofi-Aventis (2008) 78 IPR 485 at 525 [91]:
“Anticipation is deadly but requires the accuracy of a sniper, not the firing of a 12 gauge shotgun.”
77 The expression “pharmaceutically acceptable salts” used in the two prior art patents lacked any substantive precision. The first joint expert report explained that it is not possible to predict with certainty what both the outcome of any salt-screen process and the properties of any particular salt produced will be. The two prior art patents did not give any hint of what, if any, pharmaceutically acceptable salts existed other than perindopril erbumine.
78 Leaving aside the tert-butylamine salt, those patents did not describe how to make any other salt that would have the characteristic of pharmaceutical acceptability or provide any examples of such a salt. They “taught” the reader nothing about any pharmaceutically acceptable salt of perindopril other than perindopril erbumine.
79 Apotex correctly argued that the prior art merely needed to disclose, not teach, the invention: Lundbeck 177 FCR at 192 [178]. However, the prior art (in light of common general knowledge) must really disclose the invention. Here, the “disclosure” was of unspecified “pharmaceutically acceptable salts of perindopril”, and the prior art gave no direction how to perform that invention. In a real sense that phrase was devoid of content in that context, even with knowledge of the Berge paper. Table III of the Berge paper merely identified arginine as “potentially useful”, as opposed to a substance that ordinary trial and error experiments would involve in salt-screens. I am not satisfied that a skilled addressee would be able to perceive, understand and apply the disclosure relied on by Apotex in either of the two earlier patents, in light of common general knowledge including the Berge paper necessarily to obtain the invention: Lundbeck 177 FCR at 194 [190]. The quality of the “disclosure” relied on by Apotex was insufficient to amount to a disclosure of the invention of perindopril arginine. The skilled addressee would have had to engage in much more than ordinary trial and error experimentation to think of, or produce, the arginine salt. He or she would not have seen any flag to try arginine as a counter-ion in the two earlier patents that Apotex relied on as anticipations and the common general knowledge.
80 In any event, I am not satisfied that the Berge paper was common general knowledge of the ordinary skilled addressee or had been generally accepted and assimilated by the class of skilled addressees in Australia as at 27 February 2003: Lockwood [No 2] 235 CLR at 197 [55]-[56] per Gummow, Hayne, Callinan, Heydon and Crennan JJ; Minnesota Mining 144 CLR at 292; Alphapharm 217 CLR at 426-427 [31]. In Lubrizol 106 FCR at 232 [57] the Full Court acted on the findings of the trial judge, Emmett J, that common general knowledge was the technical background to the hypothetical skilled worker in the relevant art. Emmett J said that it was not limited to material that might be memorised and retained in the front of that worker’s mind but included material in the field in which he or she worked that the worker “knows exists and to which he [or she] would refer as a matter of course”. Here two of the four Australian experts, Dr Evans and Prof Perkins, were unaware of the Berge paper while Dr Williams knew of it adventitiously because, while studying in Kansas, his thesis supervisor drew it to his attention. As a result of his lack of awareness, Prof Perkins could not have taught his university students about the Berge paper before he knew of its existence as a result of his involvement in these proceedings.
81 Dr Spargo and Prof Byrn were the most experienced salt-makers of the expert witnesses. However, because each of them did not live or work in Australia prior to 27 February 2003 their knowledge of the Berge paper does not establish any state of relevant common general knowledge in this country, as was required by ss 7(1)(a) and 18(1)(b)(i): Minnesota Mining 144 CLR at 292, 295.
82 Accordingly, I am not satisfied that Apotex has established that the invention of perindopril arginine was not novel in light of either of the two prior art patents for the purposes of s 7(1)(a) of the Act. Apotex did not prove that, even when read with common general knowledge of the Berge paper, that either the synthesis or the crystalline form patents disclosed the arginine salt at all or as a pharmaceutically acceptable salt of perindopril. Nor did it prove that the Berge paper was common general knowledge in Australia at 27 February 2003.
(a) Principles
83 The word “obvious” in s 7(2) of the Act means “very plain”. It is a question of fact whether an invention is “obvious”. However, that assessment involves a question of degree and this often will be by no means easy: Lockwood [No 2] 235 CLR at 195 [51]. The parties agreed that no question arises under s 7(3) in these proceedings. In Aktiebolaget HÄssle v Alphapharm Pty Ltd (2002) 212 CLR 411 at 427-433 [33]-[53] Gleeson CJ, Gaudron, Gummow and Hayne JJ discussed the law in respect of obviousness and the requirement of an inventive step as it had developed up to the Patents Act 1952 (Cth) (the 1952 Act). Subsequently, the Court said that that discussion was relevant and applicable to the current Act: Lockwood [No 2] 235 CLR at 193 [46]. The Court observed that (at 195 [52]):
“obviousness and inventiveness are antitheses and the question is always “is the step taken over the prior art an ‘obvious step’ or ‘an inventive step’?” ... A “scintilla of invention” remains sufficient in Australian law to support the validity of a patent.” (citations omitted, emphasis added)
84 There is no unique approach to ascertaining whether any invention claimed involves “an inventive step” for the purposes of s 18(1)(b)(ii). Sometimes, the question of whether an invention is obvious when compared to the prior art base, in light of common general knowledge, can be approached by a “problem and solution” analysis. But, this has its limitations, as the Court explained in Lockwood [No 2] 235 CLR at 200-201 [65]-[66]. They held that such an approach could be unfair to an inventor of a simple solution adding (at [65]):
“… especially as a small amount of ingenuity can sustain a patent in Australia. Ingenuity may lie in an idea for overcoming a practical difficulty in circumstances where a difficulty with a product consisting of a known set of integers is common general knowledge. This is a narrow but critical point if, as here, the circumstances are that no skilled person in the art called to give evidence had thought of a general idea or general method of solving a known difficulty with respect to a known product, as at the priority date.” (citations omitted, emphasis added)
85 The Court cautioned against treating a specification that described a problem and solution as a conclusive admission by the patentee. That was because the patentee makes such statements from the vantage point of knowing the solution. Their Honours said that statements of this type must be weighed with any evidence given by persons skilled in the art of their perception, before the priority date, of any problem: i.e. before those witnesses had been exposed to the solution contained in, or provided by, the invention (235 CLR at 211 [105]). The concept of whether a step is inventive (235 CLR at 213-214 [111]):
“… will turn on what a person skilled in the relevant art, possessed with that person's knowledge, would have regarded, at the time, as technically possible in terms of mechanics, and also as practical. That is the sense in which an idea can involve an inventive insight about a known product. A court cannot substitute its own deduction or proposition for that objective touchstone, except in the rarest of circumstances, such as where an expressly admitted matter of common general knowledge is the precise matter in respect of which a monopoly is claimed. Even if an idea of combining integers, which individually may be considered mere design choices, is simple, its simplicity does not necessarily make it obvious. Older cases concerning simple mechanical combinations illustrate this point. Common general knowledge has negative as well as positive aspects. Practical and technical issues can affect the means by which a concept may be implemented in respect of an already known vendible product, and scepticism can inhibit recognition of the utility of applying a concept or idea to a known set of integers. These are matters within the knowledge of relevant witnesses.” (citations omitted, emphasis added)
86 Thus, the practical considerations that a person skilled in the art would take into account in seeking to address a problem may affect the characterisation of a step as inventive or not. In Alphapharm 212 CLR at 432-433 [50]-[53] Gleeson CJ, Gaudron, Gummow and Hayne JJ discussed the difference between what a person skilled in the relevant art, in light of common general knowledge, might do to lead to the alleged invention by way of a series of steps or experiments that were routine as opposed to doing something that amounted to an inventive step. They drew on what Aickin J had said, with the agreement of Gibbs ACJ, Stephen, Mason and Wilson JJ, in The Wellcome Foundation Ltd v VR Laboratories (Aust) Pty Ltd (1981) 148 CLR 262 at 280-281 and 286. Aickin J referred to evidence of what the inventor did, for example by way of experiment, as one means of establishing or negating the involvement of an inventive step. He said (212 CLR at 432-433 [51]):
“It might show that the experiments devised for the purpose were part of an inventive step. Alternatively it might show that the experiments were of a routine character which the uninventive worker in the field would try as a matter of course. The latter could be relevant though not decisive in every case. It may be that the perception of the true nature of the problem was the inventive step which, once taken, revealed that straightforward experiments will provide the solution. It will always be necessary to distinguish between experiments leading to an invention and subsequent experiments for checking and testing the product or process the subject of the invention. The latter would not be material to obviousness but might be material to the question of utility.” (original emphasis)
Aickin J also said (148 CLR at 286):
“It is still correct to say that a valid patent may be obtained for something stumbled upon by accident, remembered from a dream or imported from abroad, if it otherwise satisfies the requirements of the legislation. What is important is that the patent itself should involve an inventive step, whether or not it was consciously taken by the patentee and whether or not it appeared obvious to the patentee himself. The test is whether the hypothetical addressee faced with the same problem would have taken as a matter of routine whatever steps might have led from the prior art to the invention, whether they be the steps of the inventor or not.” (emphasis added)
87 Thus, steps that lead to the alleged invention that a hypothetical non-inventive worker in the field would have taken as a matter of routine negate the involvement of an inventive step. That is because steps of a routine character that the worker would try as a matter of course would demonstrate obviousness: Alphapharm 212 CLR at 433 [52]. In the modern context of large pharmaceutical companies, the correct approach is to ask a question along the lines of that described as the reformulated “Cripps question” (which was in an early example of the use of a written submission by Stafford Cripps KC of counsel, as Sargant LJ explained in Sharp & Dohme Inc v Boots Pure Drug Company (1928) 45 RPC 153 at 176 and see at 173 per Lord Hanworth MR; cf Blanco White, op cit at [4-211]): (I have adapted and added emphasis to this question below.)
“Would the notional research group at the relevant date, in all the circumstances, (including a knowledge of all relevant prior art and of the facts of the nature and success of the existing drug or formulation [here, the tert-butylamine salt of perindopril] be led directly, as a matter of course, to try the new or substituted drug or formulation [here, the arginine salt] in substitution in the expectation that it might well produce a useful alternative to or a better drug than [perindopril erbumine] or a body useful for any other purpose?”
88 If a hypothetical formulator or skilled addressee must pursue a complex, detailed and laborious course of action, involving a good deal of trial and error, dead ends and retracing of steps, he or she will not have taken routine steps leading to an invention as a matter of course: Alphapharm 212 CLR at 436 [58]. There Gleeson CJ, Gaudron, Gummow and Hayne JJ accepted what Maugham J had said in In re IG Farbenindustrie AG’s Patents (1930) 47 RPC 289 at 322 that mere verification was not invention. They approved his analogy advertising to the capture of the citadel in the passage below. The latter case concerned a selection patent i.e. one where, like here, a putative inventor selects, from one or more candidate substances or compounds, something to use as a substitute or derivative for an earlier component or ingredient of an original compound. There can be many possible candidates for such combinations (47 RPC at 321). His Lordship propounded a corollary to the Cripps’ question, namely, that if for practical purposes it is not obvious to skilled chemists, in the state of chemical knowledge existing at the (priority) date, that the selected components possess a special property then there is, or may be, a sufficient “inventive step” to support the patent. He said that in this respect the workings of the inventor’s mind were not usually material. Rather, the Court was “concerned, so far as subject-matter is concerned, only with the results. The invention must, of course, add something of a substantial character to existing knowledge; but the Courts do not inquire into the way in which the conquest achieved. If the language of metaphor may be used, the citadel may be captured either by a brilliant coup-de-main or by a slow and laborious approach by sap and mine according to the rules of the art; the reward is the same.
89 It is not appropriate to analyse whether a claimed invention, at least in the cases of selection or combination patents, involves an inventive step on the basis that it will not if the selected answer was “worth a try” or “obvious to try” or resulted from an exercise in trying out various known possibilities until a correct solution emerged: Alphapharm 212 CLR at 441-443 [72]-[76], [78]. Secondary evidence such as commercial success, the satisfaction of a long felt but unsolved want or need and the failure of others to find a solution to the problem is also relevant, but not necessarily determinative, of the question whether the invention is obvious: Lockwood [No 2] 235 CLR at 215-216 [115]-[116], [119].
90 The purpose of considering the prior art base, in assessing whether the invention involves an inventive step, is to look forward to see from it what a skilled addressee “is likely to have done when faced with a similar problem which the patentee claims to have solved with the invention” (235 CLR at 218 [127]). Each claim in a patent must be examined independently of the others in assessing whether an alleged invention involves an inventive step. Also in addressing the “jury” question of whether the claimed invention involves an inventive step, a judge must be very careful to avoid the wisdom of hindsight: Alphapharm 212 CLR at 443 [78].
91 The specification in the arginine patent described perindopril as being intrinsically fragile (p 1 line 24). It noted that perindopril and its non-salt compounds had previously been described in European patent EPO 049 658 (the compound patent) and that this had given examples of addition salts, with a pharmaceutically acceptable salt, that had both good bioavailability and adequate stability to be suitable for the preparation and storage of pharmaceutical compositions. The specification identified the earlier conclusion that perindopril erbumine had adequate qualities for the development of the product and was currently being marketed (p 1 lines 12-17). Then the specification explained that the tert-butylamine salt had not been capable of providing a complete solution to the problems of the product’s stability to heat and humidity. That was because of the constraints that required perindopril erbumine to use additional and more costly packaging, and that limited its shelf-life (p 1 line 24-p 2 line 7).
92 A substance is hygroscopic if it physically absorbs water. It may do so without changing its chemical structure. In that case, the substance will gain weight, being a reflection of the amount of water absorbed, thus affecting its physical stability. On the other hand, if the substance is hygroscopic and reacts with water so that its chemical structure changes, it also will gain weight but will not be chemically stable.
93 Apotex and Servier are at odds over what was the starting point identified in the specification identified for the purpose of evaluating, under s 7(2) of the Act, whether the invention involved an inventive step. Apotex said that the skilled addressee would have proceeded, as the patent explained, namely by immediately considering whether a new salt could be developed to meet the issues created by the problems with perindopril erbumine’s stability to heat and humidity. Thus, Apotex’ expert witnesses (Drs Spargo, Williams and Prof Perkins) were instructed to address what they would do, as Dr Spargo explained: “… if I was tasked with developing a new salt of perindopril which was more stable to heat and humidity than the tert-butylamine salt of perindopril.” Servier contended that the skilled addressee would see the starting point, or problem, as addressing the problems with the more general issues of perindopril’s stability to heat and humidity, including those problems experienced with the tert-butylamine salt. I will consider the correct characterisation of what the skilled addressee would have taken as the starting point later in these reasons.
94 I do not accept as reliable Prof Perkins’ evidence that, prior to the priority date, he would have considered using arginine as a counter-ion to make a salt with perindopril. His discussions with Apotex’ lawyers prompted his thought process, that led to his including arginine in his considerations. Servier did not suggest that that prompting occurred in any improper way. Rather, Prof Perkins’ cross-examination revealed how his identification of arginine as a potential counter-ion in his affidavit came about as:
“ASSOC PROF PERKINS: It was a natural progression of the discussions of the basic basicity and acidity strengths of the various functional groups and the ideas behind salt formation.
MR BANNON: And the natural progression, the progression of the discussions was prompted by the lawyers, I take it?
ASSOC PROF PERKINS: The series of questions were set by the lawyers, yes.”
95 I did not find of assistance his evidence as to how the skilled addressee, prior to 18 April 2002, would have approached dealing with making improvements to perindopril erbumine or creating a new salt. That is because I think that he approached the issue affected by the apparently accidental prompting and, once having thought of arginine in this context he, not unnaturally, continued to be influenced by this. Prof Perkins did not do any research into what information a skilled addressee may have found about salt forms that were used in other ACE inhibitors at or before 18 April 2002. He accepted that the results he could have discovered in such research may have led him down one research path rather than others. Moreover, Prof Perkins accepted that he had no expertise in 2002 as to whether a salt was suitable in a pharmaceutical context.
96 Only Prof Evans, Drs Morella and Spargo were familiar in 2002 with the pain relief substance, ibuprofen. It was marketed in a lysine salt formulation. Prof Evans had written his thesis on that substance and was aware that some research was being conducted at around that time as to whether the active moiety could also make a salt with arginine to speed up its absorption in patients. He said that this was illustrative of how the choice of the counter-ion could have pharmacokinetic and or pharmacological effects on the active moiety that would require appropriate studies to satisfy regulatory authorities as to the properties of the API. I am not satisfied that ibuprofen or its formulation as a salt with lysine, or any research involving it in salt formulation with arginine, was general common knowledge at the priority date.
97 The person skilled in the art would have recognised that perindopril erbumine was unstable in higher temperatures and humidity levels, as the experts agreed. They all suggested, in no particular order, that a number of options could be explored including development of more stable forms of perindopril such as new salts, polymorphs (i.e. crystal structures, solvates or complexes, reformulating perindopril erbumine compositions using a range of excipients or new formulations, improving the packaging or tablet manufacturing processes, developing derivatives of perindoprilat, including new prodrugs or analogues, or changing the chemical structure of the active moiety, for example, by identifying another “pril” with a more stable chemical structure than the compounds based on the active moiety, being perindoprilat.
98 As Prof Byrn said, there were at least five methods of crystallisation that a person skilled in the art could use and these involved potentially many different variables such as the choice of one or more solvents, heating rate, maximum temperature, whether, and how long, to cool the mixture, the time allowed for crystals to form, type of crystallisation vessel, its surface volume and the mixing rate, to name only some. Each variable can affect the result.
99 Thus, the selection and development of a new salt of perindopril was one of a range of reasonably open choices that the person skilled in the art would have considered. As Prof Byrn explained, if one tries to create new hydrates, polymorphs or other crystal structures in which a salt exists, each crystal structure can affect the salt’s stability characteristics in a way that is not predictable. Prof Byrn said that when the patent used the concept of “perindopril arginine” and its hydrates it was referring to a solid material (the salt) that can be in a solvent and that the solvent could be water (and then it would be a hydrate - i.e. it would contain, but not be chemically bonded to, water). He explained that differences in the form of polymorphs or hydrates can also affect the stability of the substance. He illustrated how the form can affect stability by the example of the minerals, diamond and graphite, each of which is different solid form of carbon and has very distinct properties from the other. He said that a person skilled in the art could try to make a new polymorph of perindopril erbumine to solve the then stability problem with its correct form. He pointed out that one would need to do empirical studies of whatever new form or forms of polymorph or hydrate came about in order to determine what, if any, difference(s) in their properties had occurred.
100 As I have explained above, there is no certainty that, first, any particular counter-ion will be able to make a salt with an API and, secondly, if it does what the properties of that salt will be.
101 If, and it is not certain that this would necessarily occur, a person skilled in the art decided before or after trying other alternatives, to experiment with creating a new salt, it would be necessary to do one or more salt-screens. The person skilled in the art would have selected a number of counter-ions for a first salt-screen from Table 1 in the Berge paper. That is because the counter-ions in Table 1 have been used to make salts that the FDA previously had approval for pharmaceutical use. The source of the common general knowledge for counter-ions in that table in Australia for persons skilled in the art was, as Prof Evans explained, the standard text, Remington and, on the evidence, they would not have known of, or gone to, the Berge paper itself.
102 If the first salt-screen failed to identify a suitable counter-ion, the person skilled in the art would be likely to have undertaken a second salt-screen. That is the more likely because of the significant commercial value promised by a successful and patentable improvement that a more stable composition of perindopril offered. Dr Williams, who knew of the Berge paper from his Kansas education, said that it was hard to say if he would have tried arginine at all. All the experts agreed that, as a natural substance, a salt made with arginine was likely not to have toxicity issues. Prof Evans and Dr Morella considered that the lack of commercial precedents for arginine as a counter-ion in a pharmaceutical salt would discourage and make unlikely its selection as a candidate in a salt-screen. Drs Morella and Williams also made the significant point that the economics of the selection process would affect its comprehensiveness and duration.
103 One of the difficulties in evaluating the “jury” question of whether an alleged invention involves an inventive step, is that the expert witnesses, in a case like this, will often not be the ordinary, non-inventive person skilled in the art. Here, each expert was distinguished and accomplished in his field. Nonetheless, the evidence of Prof Evans, Drs Morella and Williams left me with the firm impression that none of them would have included arginine in a second salt-screen.
104 Both Prof Byrn and Dr Spargo had much more extensive practical experience of salt-screens than the Australian expert witnesses. Prof Byrn said that he would not have tried arginine since it is complex and could catalyse reactions. He illustrated this on a whiteboard as I explain below at [113]-[115]. Only Dr Spargo said that he would have tried arginine if his first salt-screen failed to produce a suitable salt. He said that he had not treated Table 1 in the Berge paper as prescriptive during his professional career. He said that he would not go through all of the counter-ions listed in it before branching outside to try other ones and would look at other literature for a potential candidate. However, he acknowledged that Prof Byrn had a different, and acceptable, perspective on this issue. Dr Spargo had used arginine, unsuccessfully, once in a salt-screen in the mid-1990s. He was aware of, and used, the Berge paper at that time.
105 In his affidavit evidence, Dr Spargo hypothesised that he would have tried both basic and acidic counter-ions from Table 1 in his first salt-screen to take account of zwitterionic nature of perindopril. He said that he would have assumed that Servier had tried these and failed to make a salt before it had succeeded with tert-butylamine since the latter was an unusual choice of counter-ion and not in Table 1. His first 12 screen selections would have been simple metal (basic) cations such as sodium, potassium, magnesium and calcium, and the most common (acidic) anions such as hydrochloride, maleate, citrate and mesylate. He could not say whether any of those counter-ions could have made a salt with perindopril, including the ones that Servier had unsuccessfully tried: sodium, hydrochloride and maleate. That was because different techniques in salt making can produce different results and, as all the experts agreed, successful salt making is sometimes dependent on the maker’s intuitions and interventions with stirring or leaving the mixture. Dr Spargo accepted the description that salt making was “something of a dark art and you can do it at different times and get different results”.
106 In contrast, Prof Perkins said that he was not aware of the Berge paper before the priority date. He said that he would only have tried arginine if there was (and he was not aware before the priority date of any) a scientific reason in literature evidence that one or more potentially pharmaceutically acceptable arginine salts had been prepared previously.
107 I prefer the more convincing evidence of Prof Byrn, Drs Evans and Morella to that of Dr Spargo on this issue. I find that the skilled addressee at the priority date would not have tried to make a salt with arginine because it was outside the field of candidates that he or she would have considered for this purpose for the reasons given by Prof Byrn, Drs Evans and Morella that I have referred to above. I am not satisfied that, before the priority date, the skilled addressee, in Australia could be reasonably expected to have ascertained, understood and regarded the Berge paper and, in particular the contents of Table III, as relevant to work in relation to creating a pharmaceutically acceptable salt of perindopril. That paper was not part of the common general knowledge: Firebelt Pty Ltd v Brambles Australia Ltd (t/as Cleanaway) (2002) 188 ALR 280 at 289 [36] per Gleeson CJ, McHugh, Gummow, Hayne and Callinan JJ.
108 Once a salt has been made, the maker then must subject it to stability studies that usually (and for perindopril would) include exposing it to extreme conditions of temperature and humidity. Those studies potentially could be significant in order to assess whether the new salt offered the promise of improved stability over that of perindopril erbumine. If, on the initial stability studies, the new salt performed similarly to, or its results were ambiguous in comparison to, the existing one, Dr Spargo thought that it would probably not be worthwhile subjecting it to longer term stability studies. That was because the aim of the search was to obtain a new salt that was clearly better. Dr Spargo said in one affidavit that, having reviewed Servier’s internal study reports for the stability testing of the tert-butylamine and arginine salts:
“I do not consider that one is clearly better than the other based on these data. These data do not suggest to me that there are insurmountable problems with perindopril tert-butylamine or perindopril arginine with respect to heat and humidity. Accordingly, these data would not discourage me from selecting either salt.” (emphasis added)
109 He said that a skilled addressee considering those results would “say it’s a tough call” as to whether to subject the arginine salt to more extensive testing. He said that the arginine salt was more hygroscopic than the tert-butylamine. He said that this might be balanced against arginine being a natural substance and probably less toxic. Dr Spargo observed that based on those initial results:
“these things are a balance and there would not necessarily be a clear view. In the end somebody has to make a decision. And that’s a business decision. It would not be purely a scientific decision ...”
110 Dr Spargo said that even if there were a decision to proceed with further tests, until tested in tablets one would not know if the new salt would be an improvement on the existing one. Moreover, he agreed that changes in formulation in the salt formation process or of the tablets could produce different results and considerations of both time and money would affect whether such changes would be exposed. Dr Spargo said that in arriving at the selection of counter-ions for a second salt-screen he, or a person skilled in the art, might, or might not, have selected arginine. In his career he had only done so once in a salt-screen and that had not resulted in a successful salt. He said that he could not say that he, or a skilled addressee, would have actually tested arginine, because some other counter-ion might have produced success earlier. He gave this evidence that I accept:
“MR BANNON: But you would accept, would you not, that there would be others of ordinary skill who would quite reasonably, in the circumstances, not choose arginine.
DR SPARGO: Well, clearly. Professor Byrn said he wouldn’t, Dr Evans and Dr Morella have said they wouldn’t.
MR BANNON: And you would accept that they were reasonable views as to what a skilled addressee would do, albeit not the one you would prefer; correct?
DR SPARGO: Well, I respect their views because they are their views and I respect these gentlemen.”
111 However, I do not accept his evidence immediately after he said this that sought to qualify the last answer in which he seemed to disagree with the proposition that the views of Prof Byrn, Dr Evans and Dr Morella were reasonable. Dr Spargo, in trying to justify his disagreement with the three other experts, gave this evidence:
“I wouldn’t talk myself out of doing it and, in fact, that’s one of the things that you know, the poorer scientists talk themselves out of doing experiments because they think they’re clever enough to know and actually you don’t know until you try, as we’ve said.
MR BANNON: Just to make it clear, to make sure I understand your position on this, what I’m suggesting to you is: Do you agree, notwithstanding what you say that you think you would have done, that an ordinary skilled worker in similar circumstances could quite reasonably have chosen not to take the arginine route?
DR SPARGO: What, given that all of the more common counter-ions have failed, I don’t think I can answer that question with a yes or no answer because I actually think the context and the circumstances are very, very important and they influence what level of risk and the new ground you break when you’re doing this.” (emphasis added)
112 I prefer Prof Byrn’s evidence that in a real world study only 6 to 12 salts would be tested and the skilled addressee would use counter-ions in Table 1 of the Berge paper. He had never encountered or heard of a salt-screen that worked through all or most of the counter-ions in the three Tables in that paper. As he said:
“It’s so unpredictable that I’m not sure it helps you to go to unusual salts. I didn’t say I would never, ever. I think what I said was if I had unlimited money and time I might go on to Berge 2 and 3, but in a practical sense I don’t think I really would.”
113 Profs Byrn and Perkins together with Dr Spargo drew, and commented on, a whiteboard illustration of how a perindopril molecule could form a salt with alternately the guanidino group in a side chain of arginine as part of a (positively charged) cation, or acid, on the one hand, or a (negatively charged) carboxylic acid anion or base, on the other hand. Potentially, two amino acids with acidic side chains, aspartic acid and glutamate acid, could form a salt with perindopril, and there are three amino acids with basic side chains that could do so, namely in lysine, histidine and arginine. Another amino acid, glycine, is a zwitterion. Arginine is the strongest of those bases with the best chance of making a salt in solution. Though as Prof Byrn noted, there is no certainty that a crystalline salt will form from that solution. Dr Spargo considered that glycine could be a candidate although he considered that it was a more complex question whether it would be successful in making a salt.
114 Prof Byrn considered that the anion, or acid, would be likely to form a stronger iconic bond with perindopril because it had two ionisable groups with which to form a resonance structure (or ionic bond). He said that perindopril also had three ionisable groups with which it could form a resonance structure with a cation, like arginine, but that this was likely to make a more complex and weaker bond and, thus it was less likely to form a crystalline salt. Prof Evans agreed.
115 Drs Spargo, Williams and Prof Perkins disagreed that those differences in the ionic bonding characteristics affected the predictability of whether a crystalline salt would form. Dr Morella observed that the theoretical discussion on this point emphasised the unpredictability of salt formation and of the characteristics of any salt that did form, including whether it would crystallise.
116 These matters would be known to a skilled addressee. However, he or she would only give consideration to them if, and when, he or she had moved beyond the routine salt-screen candidate selection process.
117 Apotex criticised Prof Byrn’s evidence that he would not have screened arginine because, it contended, he had been aware of a paper by Kenneth R Morris and others entitled: “An integrated approach to the selection of optimal salt form for a new drug candidate” published in the International Journal of Pharmaceutics in 1994 (the Morris article). Dr Morris became, and has remained, a colleague of Prof Byrn’s at Purdue University and SSCI since about 1996. Another co-author of the paper, Dr Ann W Newman, worked for SSCI for about 10 or so years before leaving in around 2012.
118 The Morris paper discussed a general method that the authors had employed to select an optimal salt for a carboxylic acid, HMG-CoA reductase inhibitor. As Prof Byrn explained, however, that substance is quite a different molecule to that of the zwitterionic structure of perindopril. Perindopril has a carboxylic acid limb that can be used as a base to form a salt. The Morris paper referred to the Berge paper and discussed the (Morris) authors’ development of a three tiered procedure for salt selection. The authors chose to study five bases from Table 1 of the Berge paper together with two bases from Table III, arginine and lysine. The first tier of the study considered the hygroscopicity of each of the seven salts which led to the rejection of four (sodium, potassium, calcium and zinc) because they took up too much moisture in the range of anticipated humidity present in pharmaceutical manufacturing plants. The remaining three salts were tested in tier 2 for changes in their crystalline structure in respect of humidity and for their aqueous solubility in the gastrointestinal pH range. This led to the exclusion of the magnesium salt. The authors found that the crystalline structures of the arginine and lysine salts were resistant to change under extremes of humidity conditions and had high aqueous solubility. They were tested in tier 3 of the screen to assess their chemical stability in respect of temperature, humidity and the presence of excipients. Following this, the authors chose the arginine salt for development. They said that the process could be completed within four to six weeks. However, that salt was never taken to the market. Prof Byrn explained that even if a compound or a salt appeared to perform well in experiments it may not be able to be successfully made on a larger scale for commercial use. Pros Evans and Perkins, and Drs Morella and Williams were not aware of the Morris paper before the priority date.
119 In or after 2009, Prof Byrn used the three tier study described in the Morris paper in a presentation he gave to the Boston Solubility Conference. Prof Byrn accepted that the Morris study described an intelligent and tenable salt selection process. Although he was aware of that study from around the time it was published, he never tried arginine in any salt-screens he conducted, preferring instead to work with counter-ions in Table 1 of the Berge paper that he considered appropriate for the particular compounds with which he was trying to make salts. He also had used a tiered approach when screening salts from about 1993 but with different criteria at different stages. He had developed the protocols for his method while he was working extensively in dealings with the FDA. Usually, he looked for crystallinity in the first tier, solubility in the second and hygroscopicity in the third, but his could vary depending on what requirements SSCIs customer had.
120 Apotex argued that the weight of Prof Byrn’s evidence that he would not have tried arginine to form a salt with perindopril should be discounted because he knew of the Morris paper in which arginine had been successfully used and had used it himself much later in the conference presentation he gave in or after 2009. It contended that neither the Morris paper, nor Prof Byrn’s subsequent use of it in the presentation, had referred to arginine’s complexity as a reason for not trying to make a salt with it. Apotex submitted that the skilled addressee, knowing of the commercial potential and importance that a new salt form of perindopril could offer, would be motivated to go to additional lengths to find an alternative salt to erbumine. It contended that while those additional lengths, of themselves, did not support a finding of obviousness, nonetheless the testing of a range of counter-ions to see if a crystalline salt could be formed is a routine part of drug development. Apotex argued that verification of whether a salt was suitable did not amount to invention.
121 The skilled addressee would have been aware that arginine was a possibility to use in a salt-screen when exploring an improvement over perindopril erbumine. However, I am of opinion, there was no reason to suggest that the skilled addressee would have selected or considered arginine as a practical choice for such a salt-screen or as a matter of routine or as a matter of course. At best arginine was a very remote possibility. It was in Table III in the Berge paper and, if chosen and pursued would have required considerable additional testing to ensure that it achieved regulatory approval. That would have made other candidates in Table 1 more attractive for testing well before the skilled addressee would contemplate arginine or trying it.
122 Apotex submitted, and I accept, that Prof Byrn and Dr Spargo had the greatest experience of the six expert chemists in relevant respects. I consider that Prof Byrn’s evidence and reasoning for not doing so was convincing. I accept his evidence that by far the greatest significance to him of the Morris paper was its description of the tiered approach to salt selection, as opposed to the illustration it gave of such an approach using arginine and lysine. Prof Byrn also said, and I accept, that he focused in his work on Table 1 in the Berge paper and, “I can’t be sure what I would have thought in 2002, but all I can say is that I wrote a lot of salt-screens and I didn’t use arginine”. For the reasons above, I was not persuaded by Dr Spargo’s evidence that the skilled addressee would have chosen to use arginine in a second or subsequent salt-screen.
123 Moreover, despite the notorious commercial success of Coversyl when formulated with perindopril erbumine, and the disclosed properties of perindopril, no-one else came up with a new pharmaceutically acceptable salt or the arginine salt before the patent in suit was filed. If routine or obvious salt making was all that was involved, as Apotex contended, neither it nor any other of Servier’s commercial competitors found the arginine salt, even though there was a clear commercial incentive to look for an alternative to the expensive packaging and limited shelf-life of perindopril erbumine: Lockwood [No 2] 235 CLR at 215-216 [115]-[116], [119]. Indeed, there is no evidence of any pharmaceutical product using an arginine salt being sold anywhere in the world other than perindopril arginine.
124 There are many pitfalls and blind alleys in the selection and making of pharmaceutically acceptable salts. That does not mean that the making of every new such salt will confer on its creator a patentable right. I am not satisfied that the selection of arginine as a counter-ion to use in making a salt of perindopril or the invention of perindopril arginine as claimed in the patent was obvious to a skilled addressee in light of the common general knowledge at the priority date.
125 The consistory clause in the specification stated:
“The present invention accordingly relates to the arginine salt of perindopril, its hydrates and also to the pharmaceutical compositions containing it.”
(p 2 lines 15-16, emphasis added)
126 Apotex contended that although the claims in the patent included hydrates of perindopril arginine nothing else in the specification indicated how any hydrates were part of the invention or how a skilled addressee would make any such hydrate.
127 A hydrate is a compound that includes water either as a part of the compound or within its structure. Thus, the water can either alter the physical structure of the compound or, in some cases, it can simply lodge within gaps or spaces of the structure. For example, a hydrate can be formed when a compound is crystallised out of water or when a hygroscopic compound absorbs water from the atmosphere. Hydrates can have fixed or variable ratios of water to their other components. There are many stable hydrated salts and many of these are on the market. Some are so stable that they will not lose water until they melt at a boiling point well above that of water.
128 The experts agreed that it can be advantageous if a hydrated salt is highly crystalline and has good manufacturing properties, including a lack of hygroscopicity. And, so long as the hydrate remains stable and does not degrade chemically, the fact that it absorbs water is not necessarily a disadvantage. The experts agreed that a number of commercial solid dosage form products, such as tablets and capsules, contain APIs that are hydrates.
129 They also agreed that although not obligatory, usually a proponent seeking approval of a product will provide to the regulator, such as the TGA, information that, first, describes any known hydrate or polymorphic forms of a drug substance and, secondly, indicates the particular solid form (i.e. hydrate, polymorph, etc) of the API that will be formulated into the drug product. However, that information relates to the particular form of the active moiety that is used in the proposed drug product. Where a pharmaceutical salt could have more than one form, the properties of each form will only be known after empirical studies or testing of the compound.
130 If perindopril and arginine are subjected to a classical method of salification, the nature of the precipitate that will form (i.e. the form of the crystalline salt, whether it is anhydrous, a monohydrate, dehydrate, polymorph etc) could be influenced by the solvent or solvents used and temperatures of the process. And, as Prof Evans pointed out a hydrate could form after salification while the salt was being stored. If water were the solvent, or was present, the resulting salt could be a hydrate. The terminology is inexact and, as Prof Byrn explained, the FDA calls hydrates “polymorphs”.
131 Importantly, Dr Williams said of claim 1 (for “the arginine salt of perindopril and its hydrates”): “That does not suggest that there are hydrates.” He said that it was common for a patent to claim hydrates as a precaution against someone taking out a subsequent patent for a hydrate form that frustrated the patentee’s attempt to exploit a more narrowly framed claim. Drs Morella and Spargo, Profs Byrn and Perkins agreed.
132 Apotex argued that the specification in the patent did not make any real and reasonably clear disclosure in respect of the claims to the invention of the hydrates of the arginine salt. That was because, Apotex contended, the specification was silent about hydrates except for the bare and unexplained words “its hydrates” in the consistory clause (p 2 line 15). Yet, the argument ran, the words “its hydrates” appeared in claims 1, 2, 6, 7 and 11 as part of the definition of the monopoly that the patentee claimed. Apotex argued that the expression “its hydrates” was a classic “stray phrase” in the patent. It submitted that the specification did not disclose how any hydrate of the arginine salt had the advantages asserted for the salt itself of “very great stability to heat and to humidity compared to the tert-butylamine salt”. Apotex argued that this was because, as Prof Byrn had observed in his written evidence, “the inventor did not consider the particular hydration state of the salt as a feature of the invention”. Apotex contended that since hydrate forms have different properties to those of the salt compound itself, the specification had to disclose what, if any, differences existed between the salt and each hydrate form.
133 This issue concerns whether the claims made, because they extend to hydrates, are “fairly based on the matter described in the specification” within the meaning of s 40(3) of the Act or whether the claims travel beyond that matter: Kimberly-Clark Australia Pty Ltd v Arico Trading International Pty Ltd (2001) 207 CLR 1 at 12 [15] per Gleeson CJ, McHugh, Gummow, Hayne and Callinan JJ approving what Barwick CJ had said in Olin Corporation v Super Cartridge Co Pty Ltd (1977) 180 CLR 236 at 240. The complete specification must not be read in the abstract. Rather, it must be construed by the Court from the vantage point of a skilled addressee in light of the common general knowledge and the art at the priority date: Kimberly-Clark 207 CLR at 16-17 [24]-[25].
134 A consistory clause in a specification in a patent is a general description of what the invention is said to consist of: Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (2004) 217 CLR 274 at 282 [10] per Gleeson CJ, McHugh, Gummow, Hayne and Heydon JJ (Lockwood [No 1]). The expression “the matter described in the specification” as used in s 40(3) refers to the invention as discussed in the specification (217 CLR at 293-294 [53]- [54]). The Court must evaluate whether the claims assert a monopoly over something wider than the invention as it was really and reasonably clearly disclosed in the specification in the sense in which a skilled addressee would read it (217 CLR at 300-301 [68]-[69], 306 [83]-[85]). However, a claim that is couched in the same terms as the description of the invention in the specification, is not necessarily “fairly based” on that description because, as the Court explained in Lockwood [No 1] 217 CLR at 306 [87]:
“A “coincidence of language” between a claim and part of the body of a specification does not establish fair basing if that part of the language of the specification does not reflect the description of the invention in the light of the specification as a whole.” (emphasis added)
135 Thus, if a specification described an apparatus limited to a particular use as the invention, a claim for the apparatus itself, without the limitation described, would not be fairly based on the more limited description and, so, would travel beyond the specification (217 CLR at 311 [101]). However, if the words of the claim reflect, even in the same language, what the specification discloses in real and reasonably clear words as the invention, it will be fairly based. The requirement of s 40(3) is distinct from that in s 40(2)(a) for the specification to describe the invention fully (217 CLR at 292 [49], 301 [70]). The Court held in Lockwood [No 1] 217 CLR at 310 [99] that:
“Doric submitted that Olin decided that a claim based on a consistory clause cannot be fairly based. It did not. Rather, as the Patentee submitted, the correct position is that a claim based on what has been cast in the form of a consistory clause is not fairly based if other parts of the matter in the specification show that the invention is narrower than that consistory clause. The inquiry is into what the body of the specification read as a whole discloses as the invention [Welch Perrin & Co Pty Ltd v Worrel (1961) 106 CLR 588 at 612-613]. An assertion by the inventor in a consistory clause of that of which the invention consists does not compel the conclusion by the court that the claims are fairly based nor is the assertion determinative of the identity of the invention. The consistory clause is to be considered by the court with the rest of the specification.” (emphasis added)
136 As Bennett J put it in Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth [2011] FCAFC 132 at [90] (and see too at [169] per Nicholas J, [243], [249] per Yates J):
“… it is not simply a matter of matching a consistory clause or other stray phrases with a claim. It is a question of comparing the invention claimed with the invention disclosed. Frequently, indeed most often, there is no real difference; sometimes there is.” (original emphasis)
137 The skilled addressee would understand that the patent was directed generally to the salt and its hydrates. The skilled addressee would know that classical salification can use water and that could result in a hydrate form of salt, as could the storage conditions of a crystalline salt.
138 Nothing in the specification narrowed the scope of the invention disclosed in the consistory clause or otherwise qualified it so as to suggest that any of the claims overreached that matter. The claims were congruent with and matched the disclosure in the specification, namely that the hydrates of the arginine salt were part of what was both claimed and disclosed as the invention; nothing more nor less.
139 Apotex’ argument that the specification did not teach or refer to the advantages of hydrates could not support its contention that the claims were not fairly based under s 40(3). In substance, the argument attacked the sufficiency of the description of the invention so far as it related to the hydrates. However, an objection as to sufficiency can only arise under s 40(2)(a); it has nothing to do with the comparative construction required by s 40(3): Lockwood [No 1] 217 CLR at 292-293 [49].
140 A skilled addressee reading the specification as a whole would understand that the patentee was making a real and reasonably clear disclosure that the invention consisted of the arginine salt, the various forms that it could take, namely hydrates, as well as the salt and its hydrates in pharmaceutical compositions. And, the claims were fairly based on that disclosed invention. That is why Prof Byrn, correctly, appreciated, as a skilled addressee, that the inventor had not disclosed the, or any particular, hydration state of the salt as being a feature of the invention. The hydration state was not such a feature. Rather, the invention that the specification, relevantly, really and reasonably clearly disclosed, in the consistory clause, was the salt in all of its potential hydration states generally including a state where it was not a hydrate at all.
141 The specification disclosed that the arginine salt was prepared according to a classical form of salification of organic chemistry (p 3 lines 22-23). Prof Byrn and Dr Spargo agreed that a skilled addressee knew that a salt could be produced in this way using more than one such method. They also agreed that the resulting substance could be a hydrated form, depending on the precise method used, but that it would be necessary to test the substance to ascertain whether it was a hydrate and what its properties were. The invention disclosed by the consistory clause’s expression “the arginine salt of perindopril, and its hydrates” was, as Prof Evans said:
“… irrespective of whether or not there are different crystal forms of a salt, it is still the salt. Perindopril arginine is perindopril arginine. It could come in several forms, in theory …” (emphasis added)
142 The first joint expert report explained that once the skilled addressee read that perindopril arginine could be produced by a classical method of salification, first, he or she would expect to be able to make the salt and, secondly, that there are many parameters that could influence the solid form of the salt that might be obtained from such a classic salification. I infer that the skilled addressee would understand that the consistory clause when read with the specification as a whole in light of the common general knowledge, disclosed that the form of the salt so produced (i.e. whether a hydrate or not) made no difference. Hence, the claims for the hydrates were fairly based on the matter described in the specification.
143 Section 40(2)(a) requires that the complete specification must “describe the invention fully, including the best method known to the applicant of performing the invention”. The complete specification was filed on 27 February 2003. The relevant descriptions concerning performance of the invention that are contained in the patent are:
(in the consistory clause), “[t]he present invention relates to the arginine salt of perindopril, its hydrates and also to the pharmaceutical compositions comprising it” (p 2 lines 15-16);
that arginine salt is “preferentially the salt of natural arginine (L-arginine)” (p 2 line 17);
the pharmaceutical compositions “comprise the arginine salt of perindopril together with one or more, non-toxic, pharmaceutically acceptable and appropriate excipients” (p 2 lines 18-20);
those compositions will preferably be immediate release tablets (p 3 lines 1-2);
the amount of the salt contained in the compositions is between 0.2 and 10 mg, preferentially from 1 to 10 mg (p 3 lines 6-8); and
“[t]he arginine salt used in this study is the L-arginine salt. It has been prepared according to a classical method of salification of organic chemistry” (p 3 lines 22-23).
144 Importantly, the claims included the arginine salt of perindopril and its hydrates (claim 1) and pharmaceutical compositions comprising, as active ingredient, that salt and its hydrates in combination with one or more pharmaceutically acceptable excipients (claim 2) presented in the form of an immediate release tablet (claim 3).
145 The inventor, Gerard Damien, and Servier knew that perindopril arginine had been prepared in 1986 and 1991 using slightly different classical salifications. Apotex had obtained leave to amend its particulars of invalidity to rely on the allegation that the complete specification did not describe the best method known to the patentee on the basis that it could not rely at the hearing on evidence other than the face of the specification and the evidence of Servier’s internal methods. That is why Apotex pleaded that the specification failed to describe, first, either of those two methods to prepare the invention each of which, clearly enough, was a method known to the patentee and, secondly, a means to prepare a pharmaceutical composition containing perindopril arginine (including which form or forms to use in that composition) so as to achieve the results of the long term stability study described in the table in the specification.
146 Mr Damien was a research scientist who worked principally in senior roles in Servier’s research laboratories in France. He said that by around 1984 or 1985 in evaluating perindopril erbumine in the course of his work, he realised that the salt caused stability issues in tablets. One day, while he was engaged in an unrelated discussion with a colleague, the subject of arginine came up and his knowledge of its properties caused him to wonder whether it might be a possible alternative to erbumine. He thought that arginine, being a natural amino acid, was likely to be safe and while both were amines, arginine was not volatile, unlike erbumine. In March 1986, Mr Damien directed one of his assistants to make a small quantity of perindopril arginine. The assistant chose to utilise a two stage process to do so. The first stage involved a salt-break in which perindopril erbumine was converted, or broken down, so as to extract a quantity of 16.268 gm of perindopril in a white crystalline form. The second stage immediately used the freeform perindopril (44.15 mmol) with 7.306 gm of L-arginine (41.94 mmol) and 50 ml of permuted water. Those three ingredients were mixed in a 100 ml spherical flask resulting in a semi-limpid solution. This was stirred for 15-20 minutes and evaporated until dry, producing a crystalline residue. The residue was retaken in a trituration using 150 ml of anhydrous ether.
147 The first joint expert report explained that the process of trituration can be used if no direct crystallisation or precipitation of a salt occurs after the initial mixing of ingredients (here, perindopril and arginine) in the solvent (here permuted water). The resulting solution is evaporated to a low volume or until it is dry. That may yield either a solid or a concentrate. The concentrate may have the form of what the experts described as a “gum” a “glass” or a “foam”. Trituration is used with the intention of converting such a concentrate into a solid that is potentially useable. Trituration involves mixing the concentrate into a solution with a new, volatile solvent that “retakes” the concentrate.
148 The concept of “retaking” works as follows. For example, after the initial mixing, the resulting solid or concentrate may stick to the sides of the mixing flask. The introduction of a new solvent “retakes” that substance into a liquid form. This can separate out impurities that could have impeded the formation of a crystalline salt, so that when the new solvent evaporates, the scientist hopes to achieve a useful crystalline salt. If different solvents are used in triturations, different solid forms of the salt may result, such as a polymorph, hydrate or solvate. The experts also noted that in a classical salification there are many parameters, including the choice of solvent, that could influence the solid form of any perindopril arginine that might be obtained from such a process.
149 Notably, as Dr Spargo explained, with the agreement of the other experts, while this technique may be useful in laboratory conditions, trituration and scraping out flasks is not practicable on a large scale or in commercial production. The trituration step used in the 1986 salification involved stirring the ether solution overnight. Then the product was filtered under a vacuum, washed again with anhydrous ether and dried again in a desiccator under another vacuum. This produced 21.29 gm of a white crystalline substance, being perindopril arginine.
150 When he revisited the question of possible salts for perindopril in 1991, Mr Damien asked his assistants to prepare another salt-screen, including perindopril arginine. The notes recording the making of the 1991 batch also detailed a preliminary salt break to extract perindopril from the erbumine salt. The method used on this occasion was different from that in 1986 because it involved lyophilisation, another standard procedure. Lyophilisation, or freeze drying, is a way of removing water from material. The material is frozen, placed in a vacuum and the water, in ice crystal form, separates from the other substance with which it was mixed. A familiar example of the result of such a process is freeze dried instant coffee granules.
151 The 1991 salification used 12 gm of perindopril (32.56 mmol), 5.67 gm of L-arginine (32.56 mmol) and 150 ml of permuted water. Thus, the quantities and proportions of perindopril and L-arginine in this salification were different from the earlier one. The assistants mixed the three ingredients, dissolving the two solids in the water and then filtered the mixture on a sintered filter to eliminate insoluble particles. The filtrated solution was then lyophilised (freeze dried). The residue was taken up again in 150 cc of anhydrous ethyl ether, stirred for two hours and passed through a sintered filter again before being dried in a desiccator. This resulted in a white crystalline product weighing 16.58 gm.
152 Mr Damien said that for the purposes of conducting the 2000 study, he prepared perindopril arginine with the same excipients and in the same quantities as were used in the commercial Coversyl product in which perindopril erbumine was the API. The identity and quantity of excipients used in Coversyl were contained in its TGA approved product information document that was readily available to skilled addressees and others. There was no clear evidence of the details of the salification ingredients or method used in preparing the perindopril arginine used in the 2000 study. However, it is likely that the method repeated that used in 1991, it being the more recent laboratory formulation that Mr Damien required and the study being also a laboratory exercise.
153 As I have explained above, the experts agreed that the selection of different methods and parameters can influence the precipitation or crystallisation of a salt as the outcome of the various classical methods of salification. Thus, there was no certainty that any particular method within the range of classical methods, or the variety of choices, such as solvents, duration, or temperatures within a selected method would necessarily produce a crystalline salt or a particular salt form (such as a hydrate) that could be used in a pharmaceutical composition.
154 The experts also agreed that if an API is hygroscopic and takes up water there is potential for its crystal structure to change to one or more new crystalline forms (i.e. a hydrate or hydrates) that will have different physiochemical properties to the anhydrous form. Those differences can be large or small, but could affect the substance’s dissolution and solubility. This, in turn, has the potential to affect the substance’s bioavailability. However, it is also possible that water sorption will have no effect whatever and so will not cause any problem.
155 As Dr Spargo said, most chemists would know that arginine is very soluble in water and this was also stated in the standard pharmaceutical text, Martindale: The Extra Pharmacopoeia (13th ed: 1993) The Pharmaceutical Press: London. That text observed that arginine was sparingly soluble in alcohol and insoluble in ether. Similarly, a skilled addressee would be aware that perindopril would have good water solubility.
156 The experts agreed that Mr Damien’s assistants used standard classical procedures for each of the salifications in 1986 and 1991. The first joint experts report also stated that the formulation of an API into a pharmaceutical product introduces factors that may compromise the final product’s stability. Thus, the ingredients and method used to make a pharmaceutical composition with an API can have significant consequences for the end product’s stability.
157 Servier argued that it was essential for Apotex to prove that the alleged non-disclosed best method provided a result that was better than the result of the method disclosed in the complete specification. It relied on the fact that Apotex accepted that the patent did disclose a form of the invention that worked to fulfil the promise in the complete specification: i.e. Apotex did not contend that the patent was revocable for lack of utility. Hence, Servier argued that a method was disclosed and Apotex had to prove that one or both of 1986 or 1991 method(s) of salification was a better method than that.
158 Servier pointed to the differences between the 1986 and 1991 methods, uses of, among others, reagents, ratios, amounts of permuted water and evaporation techniques (the first using rotary and the second lyophilisation) and lengths of stirring times (2 hours and overnight). However, Servier argued that Apotex had not established that any of the variations between those two methods, had had any effect at all on the resulting perindopril arginine each had produced.
159 Moreover, Servier contended that there was no evidence of any use of a classical method of salification that had produced an arginine salt of perindopril that was in any way materially different or worse than the salts produced by the 1986 or 1991 salifications. It argued that the experts’ unanimous opinion, that differences in classical methods of salifications and parameters used in them might produce different results, was an unproven hypothesis. Rather, Servier submitted, Apotex had led no evidence of any experiment to prove the validity of that hypothesis. It pointed to Mr Damien’s unchallenged evidence that an inherent property of arginine was that it was not volatile and that he understood that perindopril arginine was chemically stable, as compared to perindopril erbumine, and that that stability was not affected by perindopril arginine being anhydrous or in any particular hydration state.
160 Servier contended that there was no evidence that the methods it had used to prepare the arginine salt were special or particular. It argued that there was no suggestion of any material difference between the perindopril arginine produced by the 1986 and 1991 methods, being the only methods in evidence. It submitted that this supported Prof Byrn’s evidence that “[u]sually or often there’s one most stable form. So classical salification would tend to make that form”. Servier also argued that there was no evidence, apart from the first joint experts report’s hypothesis, that a skilled addressee using classically salified perindopril arginine with known excipients would produce a composition that was any worse or had relevantly different properties to that of the tablet produced by Servier for the 2000 study.
161 Servier also argued that an applicant had no obligation to disclose the best method of making, as opposed to performing, the invention. It submitted that the complete specification did not need to disclose the method of making a particular form of the invention within a particular claim, that is known to the patentee to exist and to be the best form, “if it is sufficiently described in the sense that a skilled addressee could make it having been told what the form is”. Servier contended that the invention claimed in the patent was not limited to a particular form of perindopril arginine and that, accordingly, the complete specification did not have to describe any form.
162 Servier contended that an applicant for a patent had no obligation to disclose a method of performing the invention beyond providing a full description sufficient to enable a skilled addressee to make something within each claim and that “the only obligation to disclose a ‘best method of performing the invention’ arises where there is in fact a ‘best method’ and it is ‘known to the applicant’.”
163 I reject Servier’s argument. It ignored the express requirement in s 40(2)(a) that the description in the complete specification include the best method of performing the invention known to the applicant. The statute does not give the applicant any option not to comply with that requirement. If the applicant knows only one method, that is the method that must be described; if more than one, the applicant must include the best one known to him, her or it.
164 The requirement imposed by s 40(2)(a), that a complete specification describe the best method known to the applicant for performing the invention, is a fundamental aspect governing the grant of a patent. It supplements the co-ordinate requirement in s 40(2)(a) that the complete specification also describe the invention fully. Relevantly, the obligation on the applicant is to describe the best method that the applicant knows. The applicant cannot leave to chance that a skilled addressee could readily ascertain that method, or some essential feature of that method by omitting from the complete specification a sufficient description of it. However, as French and Lindgren JJ said in Pfizer Overseas Pharmaceuticals v Eli Lilly & Co (2005) 68 IPR 1 at 77 [374], with Crennan J agreeing on this issue at 83 [408], the best known method need not be identified as such in the complete specification. Rather, the requirement in s 40(2)(a) is that that method be disclosed in the complete specification.
165 Thus, it does not matter that the applicant does not identify any particular method in the complete specification as the best method, and s 40(2)(a) will be satisfied so long as the best method known to the applicant is disclosed there. The requirements of disclosure in s 40(2)(a) is specifically linked to the performance of the invention: Firebelt Pty Ltd v Brambles Australia Ltd (2000) 51 IPR 531 at 544[51]-[53] per Spender, Drummond and Mansfield JJ. They said (51 IPR at 544-545 [53]):
“The requirement of s 40(2) of the Act is that the patentee is required to give the best information in his power as to how to carry out the invention. That requirement is ordinarily satisfied by including in the specification a detailed description of one or more preferred embodiments of the invention offered, with reference to drawings of specific mechanisms or structures or examples of specific process conditions or chemical formulations, depending on the field of the invention and the nature of the instruction to be conveyed. It is necessary to have regard to what is the invention claimed in the petty patent. The invention here claimed is not a particular type of lid opening device operating at any particular time. It is only if it were such a claim that there might be a failure such as the primary judge found.” (italic emphasis in original, bold emphasis added)
166 Thus, it is crucial, first, to identify the invention claimed in order to determine whether the complete specification discloses the best method of performing that invention to the applicant. The purpose of this obligation of disclosure is to ensure that the patentee observes good faith in his, her or its application for the grant of the monopoly. It is also to protect the public against a patentee deliberately withholding from the public, in the complete specification, something novel and not previously published that the patentee knows or has found out gives the best results without giving to the public, as consideration for the grant of the monopoly, that knowledge of the best method of performing the invention: Firebelt 51 IPR at 544 [48], Pfizer 68 IPR at 77 [374]. In Firebelt 51 IPR at 544 [49]-[50] the Full Court identified how the requirement of disclosure of the best method should be understood by quoting with approval from a well-known text as follows:
“Blanco White in Patents for Inventions, 4th ed, Stevens, London, 1974 at para 4-502 notes:
To be proper and sufficient, the complete specification as a whole (that is, read together with the claims [Evans v Hoskins and Sewell (1907) 24 RPC 517 at 522 (CA)], and in the light of the drawings, if any [Bloxam v Elsee (1827) 1 C & P 558 at 564]) must in the first place contain such instructions as will enable all [Knight v Argylls (1913) 30 RPC 321 at 348 (CA)] those to whom the specification is addressed to produce something within each claim “by following the directions of the specification, without any new inventions or additions of their own” [R v Arkwright (1785) 1 WPC 64 at 66; Otto v Linford (1882) 46 LT 35 at 41 (CA); No-Fume v Pitchford (1935) 52 RPC 231 at 243 (CA)] and without “prolonged study of matters which present some initial difficulty” [Valensi v BRC [1972] FSR 273 at 311 (CA)].
Of the objection based on the provisions of the United Kingdom legislation, Blanco White says at para 4-516:
There would seem to be no obligation under this provision to include information not strictly relating to “the invention”, however necessary to anyone needing to work the invention.
The learned author continues:
Thus it would seem unnecessary to disclose how starting materials for a process are to be obtained [American Cyanamid's (Dann) Patent [1971] RPC 425, ante, §4-507]; while it has been held that the patentee of a new article need not disclose the best method of making it, “performing” here going only to the design of the article and not to techniques for manufacturing it [Illinois Tool Works v Autobars [1972] FSR 67 at 71–2. But cf ante, §4-502].” (emphasis added)
167 A complete specification must disclose each essential element or feature for performing the invention, even if a skilled addressee would know or could readily ascertain that element: Norton and Gregory Ltd v Jacobs (1937) 54 RPC 271 at 277 per Greene MR, Romer and Scott JJ; see too per Clauson J (as the trial judge) at 54 RPC 58 at 74-75; Colgate-Palmolive Co v Cussons Pty Ltd (1993) 26 IPR 311 at 355 per Sheppard J. Importantly, the requirement in s 40(2)(a) is to describe every essential element or feature for performing the invention, as opposed to what a skilled addressee would know from the description actually given in the complete specification, about details such as well-known analytical agents, commonly used methods, well-known terms of art or a description of machinery in standard use: Expo-Net Danmark A/S v Buono-Net Australia Pty Ltd (No 2) [2011] FCA 710 at [14] per Bennett J.
168 The party seeking revocation must prove that the patentee knew of a better method than was disclosed at the time of filing of the complete specification: Expo-Net [2011] FCA 710 at [15]. Her Honour found useful the analysis of an analogue of s 40(2)(a) by Harms J, the Supreme Court of South Africa, Transvaal Provincial Division in Enka BV v E I Du Pont de Nemours & Company 1987 BP 13 at 22-23 (Ackerman and Van Zyl JJ concurring). Bennett J summarised that analysis as follows at [16]:
“Justice Harms said that an applicant for revocation must show that:
(a) the method which the patentee failed to disclose is a method of performing the invention;
(b) the method is in fact a better method of performing the invention than the method disclosed in the specification;
(c) the method was known to the patentee at the time when the application for the patent was lodged at the Patent Office;
(d) the method is not disclosed in the specification; and
(e) the patentee knew that the method was better than the method(s) described in the specification.”
169 The patentee’s subjective state of mind must be proved by a person seeking revocation of a patent. That is because s 40(2)(a) prescribes that the best method that the specification must prescribe is “known to the patentee”. The parties debated the significance of Graham J’s decision in Illinois Tool Works Inc v Autobarn Co [1974] RPC 337 at 369 (also partly reported in [1972] FSR 67). However, that decision is of little assistance on the construction of s 40(2)(a) because Graham J did not discuss what was involved in the requirement to describe the best method.
170 The complete specification is not read in the abstract when considering whether it satisfies s 40(2)(a). It must be read from the vantage point of the skilled addressee, i.e. a person acquainted with the surrounding circumstances as to the state of the art and manufacture in light of the common general knowledge and the art immediately before the priority date: Kimberly-Clark 207 CLR at 16 [24]. Gleeson CJ, McHugh, Gummow, Hayne and Callinan JJ formulated a test at [25] after citing with approval what Romer LJ had said in No-Fume Ltd v Frank Pitchford & Co Ltd (1935) 52 RPC 231 at 243 of a requirement that the complete specification “sufficiently and fairly describe and ascertain the nature of the invention and the manner in which the invention [was] to be performed”, namely:
“[I]n other words, [it is essential] that the patentee should disclose his invention sufficiently to enable those who are skilled in the relevant art to utilise the invention after the patentee's monopoly has come to an end. Such disclosure is, indeed, the consideration that the patentee gives for the grant to him of a monopoly during the period that the patent would run.
…
It is not necessary that he should describe in his specification the manner in which the invention is to be performed, with that wealth of detail with which the specification of the manufacturer of something is usually put before the workman who is engaged to manufacture it.”
171 Immediately after setting out those remarks the High Court enunciated the test under s 40(2)(a) in Kimberly-Clark 207 CLR at 17 [25]:
“The question is, will the disclosure enable the addressee of the specification to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty [Blanco White, Patents for Inventions, 5th ed (1983), §4-502]?”
172 Disputed questions of fact in litigation must be decided on the evidence actually adduced at the hearing and not on speculation as to what other evidence might possibly have been led. Thus, the Court can draw more confidently an inference in favour of one party for which there is a basis in the evidence when a person, whose evidence presumably might put a true complexion on facts is, without a sufficient explanation, not called as a witness by the opponent: Australian Securities and Investments Commission v Hellicar (2012) 247 CLR 345 at 412-413 [165]-[167] per French CJ, Gummow, Hayne, Crennan, Kiefel and Bell JJ.
173 In essence the question is whether Servier’s description in the complete specification of preparing perindopril arginine from L-arginine and perindopril according to a classical method of salification of organic chemistry (p 2 line 17, p 3 lines 22-23) satisfied the requirement in s 40(2)(a) to state the best method of performing the invention known to it. The invention was identified in the claims. The claims were for, among others, the arginine salt of perindopril and its hydrates, and pharmaceutical compositions comprising that salt and its hydrates, as active ingredient in combination with one or more pharmaceutically acceptable excipients (claims 1 and 2).
174 I reject Apotex’ argument that the description of the best method here had to include the initial salt break that Mr Damien’s assistant had used to obtain pure perindopril from the tert-butylamine salt. The skilled addressee would know that perindopril was unstable and that when performing a salification he or she needed first to obtain the critical ingredients, namely perindopril and arginine. How one obtained those ingredients formed no part of the invention and required no description in the complete specification.
175 I accept Servier’s argument that whatever the particular crystalline form of the arginine salt (including whether it was hydrated) was produced was intended to be part of the invention and that there was no evidence that it had investigated the precise nature of the forms produced in 1986 , 1991 or 2000. But, that still left the question about what choice of parameters the skilled addressee had to make to get a result within the ambit of the asserted monopoly.
176 The skilled addressee would have understood that the complete specification disclosed that the excipients used in the study were the same for the two salts and that the only variable was the quantity included of different salts as described in the patent (p 3 lines 17-19). That is how the experts approached it in their first joint report. However, the patent’s disclosure that the arginine salt used in the study had “been prepared according to a classical method of salification” was pregnant with ambiguity. It left the skilled addressee to speculate as to which of the variety of classical methods the patentee had used. Each of the two, broadly similar, methods that Mr Damien’s assistants used for the 1986 and 1991 salifications worked to produce the invention. But, as the first joint experts report stated the result that will be obtained from classical salification is sensitive to the choices of solvent and many other parameters. Thus, while each method used in 1986 and 1991 produced a useful result, the lack of detail of either method in the complete specification left the skilled addressee to speculate about what parameters, including solvents, he or she might use to perform the invention.
177 I reject Servier’s argument that Apotex had to prove that a skilled addressee using another classical method of salification would have achieved, or did achieve, a worse result than in the 1986 or 1991 salifications. Servier was aware, when it filed the complete specification, of the vagaries of classical salification identified by the experts in their first joint report, for this was basic science. The selection of the parameters, including solvents, could affect the result. The complete specification essentially failed to disclose any detail sufficient to provide a skilled addressee with the directions necessary to perform the invention without undertaking potentially extensive trial and error experimentation in refining the choices of parameters and methodologies of classical salification (see [170]-[171] above).
178 I do not accept Servier’s arguments that, first, the complete specification described the best method of performing the invention known to it simply because it referred to preparation using a classical method of salification and, secondly, the opinion of the six experts that the variety of choice as to methods parameters available to a skilled addressee for selection classical salification was merely a hypothetical, but unproven, lacuna in the disclosure made in the complete specification of the best method.
179 I am of opinion that the expert evidence revealed that the mere reference to utilising a classical method of salification was wholly inadequate to describe the best method, or any substantive content of any particular classical method that the patentee knew of performing the invention. The generalised and unspecific description of the method in the complete specification left too much to chance for a skilled addressee to select from in order to perform the invention. While the patentee need not disclose the best method of making an article provided that the complete specification describes its design, and hence performance, the patent for an article ordinarily will illustrate and provide detail of that design from which a skilled addressee will be able to deduce how to manufacture the invention: Firebelt 51 IPR at 544 [49]-[50]. The bare description “a classical method of salification” does not allow the skilled addressee to follow a routine process of deduction from that description because it leaves open too many variables.
180 Servier’s argument was that because the patentee disclosed in the complete specification a method of performing the invention and that method, by its generality, included both of the actual methods it used in 1986 and 1991, Apotex had the onus of proving that those methods were better than the general one. That argument was syllogistic. The mere fact that a complete specification described a method for performing the invention so that sufficient information was conveyed to a skilled addressee to enable him or her to work it, does not necessarily satisfy the patentee’s additional obligation to describe the best method he, she or it knows. Mr Damien did not know, and was indifferent to, whether the 1986 or 1991 methods created a hydrate or not. But he did know that, those methods created the arginine salt in a useable form, and as a person skilled in the art, he knew that there were many alternatives available from which to choose and that some were likely not to be as good as others.
181 The teaching in the patent of how to perform the invention was unlike that in cases involving articles such as Firebelt 51 IPR 531 and Illinois Tool Works [1974] RPC 337. In Illinois Tool Works [1974] RPC at 369 Graham J said:
“… in the case of an article claim which is directed to the shape of that article, the method of performance means the production of an article in the shape described, irrespective of how it may in fact have been made.”
182 The inventions claimed in the patent were, first, the arginine salt in all its forms and, secondly, that salt in pharmaceutical compositions. Thus, the inventions concerned not simply a new salt but also one that was useful in pharmaceutical compositions. The description in a patent of the shape of an article by reference to illustrations, such as the paper cup in Illinois Tool Works [1974] RPC 337, will expose to the skilled addressee what he or she can produce by working the invention. There, the machinery needed to manufacture the cup was not part of the invention. But here, the very general description of one method (classical) of salification that itself afforded many possible means of performance that could involve considerable trial and error is not the same as a description of the best method that Servier knew.
183 I am of opinion that the 1986 and 1991 methods are better than that disclosed in the patent because they eliminated the risks as to time and resources needed in the trial and error experimentation potentially involved in a skilled addressee following the variety of possible, but unspecified, methods of classical salification. Prof Byrn gave this evidence that makes the point:
“PROF BYRN: Yes. Now, again, I don’t – I think when you say “classical salification” you mean that a lot of those different methods will produce a salt.
MR CATTERNS: They’re all within the umbrella, as it were, of classical salification, are they?
PROF BYRN: Yes, and I think when a patent says classical salification, [it] means it’s relatively easy or easy to form a salt.” (emphasis added)
184 Clearly enough, the monopoly claimed is of the salt and its hydrates prepared by whatever methods of salification exist. There are many potential choices available in classical salifications that may, but need not, lead to the successful preparation of a crystalline arginine salt or hydrate. As Prof Byrn said, the method described in the patent was sufficient for a skilled addressee to produce something within each claim without prolonged study and some initial difficulty, i.e. it was relatively easy to form a salt : Kimberly-Clark 207 CLR at 17 [25]. However, that fell short of disclosing the best method of doing so: i.e. the method known to the patentee that yielded an arginine salt that could be used in a pharmaceutical composition.
185 Servier had used a particular method or methods of classical salification and parameters that produced a guaranteed result. The complete specification described a broad and very general method of performing the invention that left to chance whether the skilled addressee would choose, from among the very large range of variables identified by the first joint expert report, the method (or one of the 1986 or 1991 methods) that the patentee knew actually worked to enable the API to be used in a tablet form. The variety of choice left open by the complete specification’s generalised identification of “a”, and not a particular, method of classical salification, coupled with the omission of the description of the method that the patentee had used and which it knew worked, was analogous to Sir Winston Churchill’s description in October 1939 of the future behaviour of the Soviet Union in World War II:
“I cannot forecast to you the action of Russia. It is a riddle, wrapped in a mystery, inside an enigma; but perhaps there is a key. That key is Russian national interest.”
186 Here, the only key to the riddle of producing a salt in the complete specification is the reference to a classical salification. But, for the reasons given by the experts, the best solution to the riddle known to the patentee was wrapped in the mystery of choices of such salifications and the enigma of choices of parameters to apply. These vagaries occur in science before the accepted use of the chemist’s or skilled addressee’s “dark art” in actually getting crystallisation to occur, which the patent need not describe.
187 By omitting a sufficient description of the or one successful method it had employed, the patentee failed to describe in the complete specification the best method known to it of performing the invention. Hence, the complete specification did not satisfy one of the essential requirements of s 40(2)(a).
(5) The false suggestion issues
188 Apotex pleaded 10 instances of what it alleged were false suggestions or misrepresentations in the patent as grounds for its revocation under s 138(3)(d). It alleged that these consisted of two representations as to the problem that the patent addressed (the problem representations), two as to the solution that it provided (the solution representations), one as to each of the shelf-life of and the storage temperature for, perindopril erbumine (the shelf-life representation and the temperature representation), three as to the cost-savings offered by the invention (the cost-savings representations) and one as to the nature and presentation of the results of the study (the study representation). Apotex also alleged that the patent’s discussion concerning the study conveyed, by implication, four additional representations (the additional study representations). Apotex also relied on the cumulative effect of all or any combination of those representations as being material to the Commissioner’s consideration as to whether to grant the patent. I will deal with each of the claimed representations below. There are some areas of overlap in these which I will also discuss. However, before doing so I will address the construction of this ground of revocation under s 138(3)(d).
(b) The construction of s 138(3)(d) – What is false suggestion or misrepresentation?
189 Counsel were unable to identify any case that had examined the individual application of each of the three bases for revocation specified in s 138(3)(d) namely that, “the patent was obtained by fraud, false suggestion or misrepresentation”.
190 “Fraud” was not a basis for revocation in s 100(1) of the 1952 Act which provided “that the patent was obtained on a false suggestion or representation”. Thus, the 1990 Act used three different expressions to those used by its predecessor: the word “by” replaced “on”, “fraud” was added and “representation” became “misrepresentation”. In Project Blue Sky Inc v Australian Broadcasting Authority (1998) 194 CLR 355 at 382 [71] McHugh, Gummow, Kirby and Hayne JJ said that a court construing a statutory provision must strive to give meaning to every word of the provision. It follows that each of the three bases, fraud, false suggestion and misrepresentation, should be given its own work to do, if possible. The nature of the circumstances comprising or surrounding the fraud, false suggestion or misrepresentation that is proved may be relevant to the court determining whether to exercise the discretion to revoke the patent.
191 Clearly enough, “fraud” has its common law meaning. It includes conduct (usually in the form of a representation) by the patentee that is engaged in so as to induce a state of mind in the Commissioner as to a matter concerning the patent or the application for the patent that the patentee either knows is untrue or is recklessly indifferent as to its truth or falsity. The conduct can include omissions. Fraud can be practised, as cases over centuries have shown in many ways. One example was given by Lord Blackburn in Smith v Chadwick (1884) 9 App Cas 187 at 201. He explained:
“And if with intent to lead the plaintiff to act upon it, they put forth a statement which they know may bear two meanings, one of which is false to their knowledge, and thereby the plaintiff putting that meaning on it is misled, I do not think they can escape by saying he ought to have put the other. If they palter with him in a double sense, it may be that they lie like truth; but I think they lie, and it is a fraud. Indeed, as a question of casuistry, I am inclined to think the fraud is aggravated by a shabby attempt to get the benefit of a fraud, without incurring the responsibility.” (emphasis in original)
192 The next question is whether the word “false” qualifies only “suggestion” or whether it also extends to “misrepresentation”. It is difficult to think of a meaningful and practically applicable difference between a fraud and a false misrepresentation as a basis for obtaining the grant of a patent. On the other hand, there are other instances in the law in which a misrepresentation, that is not fraudulent, can provide a basis for relief. These include negligent, misleading and innocent misrepresentations. In my opinion, the word “misrepresentation” as used in s 138(3)(d) should bear its natural and ordinary meaning, of an objectively incorrect statement, unqualified by the word “false”. It is likely that the Parliament intended the Court to have a discretion to revoke a patent that the patentee obtained by a misrepresentation, although the circumstances, materiality and nature of the misrepresentation may affect the exercise of the discretion.
193 The doctrine that a patent obtained by a false suggestion is void or may be avoided predates the Statute of Monopolies 1623 (UK). It is based on a common law principle that was stated in Arthur Legat’s Case (1612) 10 Co Rep 448; 77 ER 1093 (quaintly subtitled by Sir Edward Coke “In Subversion of pestilent Patents of thievish Concealers”). That case concerned the royal grant of land by letters patent that were alleged to have been obtained on a false suggestion, on the following principle (10 Co Rep at 456; 113b):
“… and it is a high and great prerogative which the King has, that when he makes any grant upon such false suggestions as aforesaid, they are void in law; so when upon false insinuations or pretences, he makes any grant as of any monopoly, &c. which in truth is to the prejudice of the King and commonwealth, the King jure Regio shall avoid such grants, and such letters patent by judgment of law shall be cancelled. And it was said that perpetuities, monopolies, and patents of concealments were born under an unfortunate … constellation; for as soon as they have been brought in question, judgment has always been given against them, and none at any time given for them; and all of them have two inseparable qualities, sc. to be troublesome and fruitless.” (emphasis added)
194 The words “of concealments” in the passage above probably mean “affected by concealments” in modern parlance. That principle appears to have been that if the sovereign was deceived by a suggestion as to his or her title to, or to value of, the thing granted in the letters patent (including monopolies), the grant would be found to be void. By the early 19th century, the common law had developed this principle, in its application to patents for inventions by reference to the statements that formed the consideration inducing the grant of the patent. In Brunton v Hawkes (1821) 4 B & Ald 541 at 558; 106 ER 1034, Best J explained that where a number of statements were made in support of the grant of a patent and these constituted an entire consideration, if one were wrong, the whole grant failed.
195 On the other hand, Best J also reasoned that if there were multiple grants in the same instrument, each based on a separate consideration, only the grant affected by the void consideration was bad and the others remained effectual (see too Bovill v Finch (1870) LR 5 CP 523 at 532 per Keating, Montague Smith and Brett JJ). Best J said (and see too at 552 per Abbott CJ, 553 per Bayley J):
“… the patentee has claimed the mode of avoiding welding as a new discovery. That is not a new discovery, and he has therefore taken out his patent for more than he is entitled to; and I am of opinion, that that avoids the patent in toto. For the king is deceived; the patentee is represented to have the merit of inventing two things, whereas he has discovered only one; and the crown might have considered the discovery, as to both, a sufficient ground for granting a patent, when it would not have thought so of the discovery of one alone.” (emphasis added)
196 The principle on which a false suggestion operated to avoid a patent required identification of the consideration proffered for the grant of the letters patent, including whether it was an entire or indivisible consideration. An entire obligation is one in which the consideration for the payment of money or the rendering of some other counter-performance (such as the grant of a patent) is entire and indivisible: Baltic Shipping Co v Dillon (1993) 176 CLR 344 at 350 per Mason CJ; see too Cutter v Powell (1795) 6 Term Rep 320; 101 ER 573.
197 Parke B took up this concept of the principle in delivering the judgment of the Court of Exchequer in Morgan v Seaward (1837) 2 M & W 544 at 561-562. He said that where the patentee had suggested that the invention was novel because of an improvement that was part of the consideration for the grant of the patent “… we must reluctantly hold, that the patent is void, for the falsity of that suggestion”. However, he went on to note that the Attorney-General then had a statutory discretion to remove that consequence so as to save the patent, no doubt because of the relative insignificance of the false suggestion (2 M & W at 562).
198 In Prestige Group (Australia) Pty Ltd v Dart Industries Inc (1990) 26 FCR 197 both Gummow J, with whom Northrop J agreed, and Lockhart J discussed the expression “false suggestion or representation” as used in s 100(1)(k) of the 1952 Act. They identified two instances of conduct that could fall within that provision, namely, first, statements in the patent itself and, secondly, conduct of the patentee during the process of application for grant, including whether the concept of “file wrapper estoppel” existed in Australian law (26 FCR at 199, 218). I note that the present proceedings involve only the first of those instances, namely statements in the grant itself. Gummow J examined the principles upon which a writ of scire facias was formerly granted for repeal of a patent. He observed that Morgan 2 M & W 544 was decided before development of both the more modern principles concerning claims made in patents and a system of investigation before grant (26 FCR at 215). He said that it was not necessary to prove common law fraud, sharp practice, or a deliberate intent to deceive, in order to establish a false suggestion or representation under s 100(1)(k) of the 1952 Act.
199 Both Lockhart J and Gummow J held that equitable fraud, accident or mistake could suffice (26 FCR at 201, 216, 218). They concluded that to make out a case for revocation under s 100(1)(k), conduct constituting a false suggestion or representation had to be a material inducing factor that had led to the grant of a patent (26 FCR at 200-201, 218). Gummow J observed that it would be a most unusual case where the alleged false suggestion or representation was relied on as having been made only in the specification (26 FCR at 218); see too Lubrizol 106 FCR at 243 [88].
200 I am of opinion that Lockhart J correctly identified the rationale for the ground of revocation based on false suggestion as having been expressed by Parker J in Re Alsop’s Patent (1907) 24 RPC 733 at 752-753. That was synthesised by Lord Birkenhead LC in Hatmakers v Joseph Nathan & Co Ltd (1919) 36 RPC 231 at 237, namely:
“[Patent] protection is purchased by the promise of results. It does not, and ought not to, survive the proved failure of the promise to produce the results.”
201 I am of opinion that similar principles apply to the construction of s 138(3)(d) in the Act. The essence of each of a false suggestion or misrepresentation by a patentee in the patent is a promise that the invention will produce a particular result or results for which he or she seeks the protection of a limited monopoly. As Parker J explained, this promise can be distinguishable from incorrect assertions by the patentee of one or more useful purposes to which the result obtained by invention can be applied. In other words, if the invention actually produces the substantive result or results asserted by the patentee in the specification, the mere fact that one or more additional purposes, embodiments or examples is wrong, ordinarily, will not amount to a false suggestion. It is only if what is asserted is of such a nature that the Court finds that the Commissioner has been deceived by it, as a material inducing factor, into making the grant, that the discretion to revoke will arise.
202 The use of the word “may” in the chapeau in s 138(3) creates a discretionary power in the Court to order revocation of a patent or a part of it relating to a claim. Apotex’ argument that revocation is ordered as of right, must be rejected. In Owners of the Ship “Shin Kobe Maru” v Empire Shipping Company Inc (1994) 181 CLR 404 at 421 Mason CJ, Brennan, Deane, Dawson, Toohey, Gaudron and McHugh JJ said:
“It is quite inappropriate to read provisions conferring jurisdiction or granting powers to a court by making implications or imposing limitations which are not found in the express words.” (citations omitted)
203 No doubt in exercising its discretion, the Court will be mindful of the proved ground for revocation as a significant factor in support of an order for such a revocation. However, there may be other considerations that will justify withholding that relief, including the materiality and circumstances of the proved ground of revocation and that the patent could be amended to remove or correct whatever was proved as a ground for revocation. The fact that s 105 expressly contemplates that in revocation proceedings the Court may by order direct the amendment of the patent, the patent request or the complete specification, subject to the prohibitions in s 102, demonstrates that the word “may” in s 138(3) creates an effective and important discretion to enable the Court to, as it were, save an otherwise revocable patent.
204 In this exercise of discretion a deliberate fraudster may fare less well than a patentee who made an accidental or relatively trivial misrepresentation. In the end, all that can be said is that the exercise of the discretionary power to revoke a patent under s 138(3) must be considered judicially having regard to all relevant considerations. Indeed, the harsh consequences that would flow from automatic revocation of a patent when any ground for revocation has been proved have be capable of amelioration at least since the 19th Century as Parke B noted of the then discretion of the Attorney-General in Morgan 2 M & W at 562.
(c) The problem representations
205 Apotex alleged that the problem representations, as contained in the patent, were:
(1) “In the development of [perindopril], however, it has proved very difficult to find a pharmaceutically acceptable salt having not only good bioavailability but also adequate stability to be suitable for the preparation and storage of pharmaceutical compositions”; (p 1 lines 12 to 14)
(2) “... in view of the intrinsic fragility of perindopril, the tert-butylamine salt has not been capable of providing a complete solution to the problems of the product's stability to heat and humidity.” ( p 1 line 24 to p 2 line 1)
206 Apotex claimed that the problem representations were false because of:
(a) the agreed facts that:
Servier had sold its flagship products, Coversyl and Coversyl plus, in Australia which contained perindopril erbumine between 1992 and 2006, when the patent for that salt expired;
in late 2006, Servier substituted perindopril arginine for perindopril erbumine as the API in its Coversyl tablets and continued using that brand name;
in 2006, Servier’s revenue from sales in Australia of Coversyl was about $72 million and Coversyl plus was about $41 million;
(b) the statement in the crystalline form patent for perindopril erbumine: see [53]-[55] stated (p 4 lines 3-4) that:
“The form thereby obtained is sufficiently stable to allow its storage for long periods without particular requirements for temperature, light, humidity or oxygen level.”
207 Apotex pleaded that the patentee knew, when making the problem representations, that there was no problem in Australia with the stability of perindopril erbumine and particularly that that salt first, had good bioavailability and adequate stability so as to be suitable for preparation and storage of pharmaceutical compositions, secondly, had undergone extensive stability testing before it had been authorised to be marketed and sold here and, thirdly, had no problems with its stability.
(d) Apotex’ submissions on the problem representations
208 In its closing submissions, Apotex argued that the problem representations were calculated to convey to the Commissioner that:
although perindopril erbumine was able to be marketed, it did not have “adequate stability”;
the inadequacy of the stability of perindopril erbumine was reinforced by the data in the table on p 4 of the patent showing its poor result in tablet form at 30oC.
209 Apotex contended that the skilled addressee would understand the patent to be describing the problem with perindopril erbumine as the API alone. It asserted that this followed from claim 1 (being for the arginine salt alone), and the specification’s references to the erbumine salt in discussing the original studies (p 1 lines 18-22), preparation of pharmaceutical compositions (p 1 lines 12-14) and to the two salts in the tablet forms or compositions (p 2 lines 1-2, p 3 lines 1-2, 6).
210 Dr Martin Ehlert, Vice President, Research and Development of Apotex Pharmachem Inc, Canada, gave unchallenged evidence that the erbumine salt, as an API, was stable for at least five years. In addition Apotex relied on Servier’s admission that the erbumine salt:
“… had good bioavailability and also adequate stability to be suitable for the preparation and storage of pharmaceutical compositions [and] that perindopril erbumine had undergone extensive stability testing before being authorised to be marketed and sold in Australia.”
211 Apotex contended that at the time that the patentee filed the complete specification, Servier had been selling Coversyl as a commercial product for over 10 years with an approved shelf-life of three years at 30°C. It submitted that Servier had not proved that the cost of tropicalized packaging, by which that shelf-life approval was obtained, was of such overall significance as to make the stability of perindopril erbumine inadequate. Indeed, it argued, Servier’s stability data showed that tablets composed with perindopril erbumine had an average degradation in assay (for the API) of about 1% over four years when stored in tropicalized packaging at 30°C and 75% RH. Apotex extrapolated from the agreed fact, that a three year shelf-life was preferred to a two year one for commercial and or marketing reasons, that a four year shelf-life was advantageous. Therefore, Apotex argued, the erbumine salt could support a four year shelf-life and could not fairly be described as having inadequate stability. Apotex argued that in substance there was no problem or substantial disadvantage with the stability of perindopril erbumine for which the arginine salt provided a solution as represented to the Commissioner in the patent.
(e) Consideration of the problem representations
212 I am of opinion that a skilled addressee would understand that the patent, including the specification, was discussing a problem “in certain countries” that had been encountered with the stability, to heat and humidity, of pharmaceutical compositions that used perindopril tert-butylamine (p 2 lines 1-2). The skilled addressee would understand that the problem was manifested in the need for additional packaging “in certain countries”, the impossibility of obtaining a shelf-life of more than two years for the tablets in temperate countries and the need to mark them as requiring storage at no more than 30°C (p 2 lines 2-5). The skilled addressee would understand that because the discussion in the specification concerned the stability of perindopril erbumine tablets to heat and humidity, the assertion that it was “impossible to obtain a shelf-life or more than 2 years for the tablets” (p 2 line 4) referred to regulator’s approval for such a shelf-life in conventional PVC/Alu blister packs. That is because the specification also identified the need for additional packaging measures in, what the skilled addressee would understand, were hotter or more humid, non-temperate climate countries. The specification also highlighted that the stability constraints imposed greater organisational requirements (such as appropriate storage) and cost in packaging.
213 In the first joint experts’ report, Profs Byrn, Evans and Williams, Drs Morella and Spargo, together with Apotex’ packaging expert, Jean Pierre Pienaar, explained that ordinary packaging consists of packaging that the majority of pharmaceutical manufacturers across the world would use within cost constraints, namely, among others, PVC/Alu blisters and containers such as HDPE, PP and glass. They agreed that specialised packaging, such as tropicalized packaging and Alu/Alu cold form blisters, is more expensive and requires additional equipment. They said that product formulation directly influences the choice of packing that is used. In addition, they agreed that that choice is influenced by marketing and commercial considerations including the stability of the particular product.
214 In their second joint report, Pros Byrn and Evans and Drs Morella, Spargo and Williams agreed that, based on the data submitted to the TGA by Servier for approval of perindopril erbumine tablets, the TGA:
was not prepared to grant a two year shelf-life for the tablets in PVC/Aluminium blisters at either 25°C and 60% RH or 30°C and 65% RH;
suggested a shelf-life of 18 months at 25°C, but only if Servier tightened its release specification to a minimum API assay of 97.5% (i.e. no less than 97.5% of the API dosage amount would be contained in manufactured tablets batches before they were to be sold) and explained to the TGA certain other matters (concerning mass balances that are not relevant here);
required that, for any shelf-life to be assigned to the tablets in PVC/Aluminium blister packaging, the lower assay limit on release had to be increased from a value of 95% to at least 97.5% so as to allow for up to 5% assay degradation between release and (shelf-life) expiry.
215 The experts explained the reason that the TGA imposed the minimum assay of 97.5%, instead of the minimum assay of 95% that Servier had used in its data. This was because the assay value for one batch in the data had deteriorated by 5.25% over two years stored at 25°C and 60% RH, even though that, and all other batches in the data, had remained within specification. However, if tablets that contain a only minimum assay of 95% at release experienced degradation in their API assay level of more than 5% over a two year period, such as a loss of 5.25%, that would result in them having an API assay of less than 90%. The experts had not seen perindopril erbumine tablet stability data for two years at 30°C and 65% RH. But, they agreed that it was reasonably likely that the minimum shelf-life assay for tablets in PVC/Alu blisters in those conditions would be breached. This was based on their extrapolation of the results for the tablets for 6 and 12 months stored at 30°C and 60% RH.
216 I accept Mr Damien’s evidence that he perceived that there was a problem with the tablets formulated using perindopril erbumine and that this led him to embark on the studies in 1998 and 2000, the results of which were summarised as those of a single study in the table in the patent. Over the years prior to 1998, his colleagues in the manufacturing section of Servier had directed criticism to him about the need to use tropicalized packaging for Coversyl in countries with climates in zones III and IV (as described by the ICH (see [21]-[22] above)). Thus, in 1998 he devised a study to demonstrate that tropicalized packaging was the only suitable form for tablets made with the erbumine salt in hot and humid countries. He said that his aim in the 2000 study was to see if perindopril arginine could solve the issue of stability in hot and humid countries.
217 I reject Apotex’ argument that the patent identified a problem with the stability of perindopril or perindopril erbumine alone as opposed to its stability after incorporation into a pharmaceutical composition. A skilled addressee, reading the patent as a whole, would understand it to be identifying that, in tablet compositions, the erbumine salt had particular stability problems that the arginine salt in tablet compositions ameliorated. Read literally and out of context, some parts of the specification could support the construction that Apotex posited. However, I am not persuaded that the skilled addressee would have arrived at that conclusion.
218 The two passages in the specification that Apotex relied on for its case of false suggestion or misrepresentation based on the problem representations must be read, as a skilled addressee would read them, in the context of the patent as a whole. When so read, the skilled addressee would realise that, first, perindopril is unstable, hence the need to prepare pharmaceutical compositions of it using a salt form, and secondly, the tert-butylamine salt, while able to be incorporated in a commercially marketable tablet form, had then to be stored in particular conditions with a limited shelf-life and sold in special and more costly packaging in certain countries. The skilled addressee would appreciate that the patent and the invention it propounded was addressing those stability problems that the tert-butylamine salt had not resolved. However, contrary to Apotex’ submissions, the skilled addressee would not read the patent as identifying, as relevant, stability problems with perindopril or the erbumine salt on their own, as opposed to when these had been incorporated in commercially marketable tablets.
219 Moreover, the patent made clear that tablets comprising perindopril erbumine were on the market and so, the API was sufficiently stable to be used for such a product. The skilled addressee would read and understand the patent as identifying that the arginine salt, when used in tablets, resulted in a significant and unexpected improvement in the existing, commercial product’s stability to heat and humidity in the way it explained. The skilled addressee would regard as unrealistic, however, a suggestion that the patentee was saying in the patent that it did not regard perindopril erbumine as sufficiently stable to use in a marketable product. Rather, the skilled addressee would have understood that the patent had identified practical difficulties posed by the tert-butylamine salt form in tablet compositions that amounted to limitations and constraints that the claimed invention overcame in the respects it explained. I accept the following evidence of Prof Evans as indicative of the skilled addressee’s approach to reading the patent and the problem it addressed:
“… the problem that is being spoken about is not the erbumine or the arginine as an API. The fact that the erbumine has been formulated into a tablet form suggests that there isn’t a problem. The problem is in the use of erbumine in a way that is convenient for patient use and distribution manufacturing. I think that’s the problem that was being addressed, and the stability data that’s provided is the stability data that is relevant to that problem, the stability of the API in the tablet.
…
... the whole patent talks about a stability issue in relationship to perindopril tert-butylamine. That’s the problem. And if the claim is that the salt has very great stability to heat and humidity compared to the other compound, there’s only one piece of data, really, that suggests. [It] improves [stability to] heat [and] humidity and that is the form of the API in the tablet.” (emphasis added)
220 Apotex which, of course, carried the onus of proof of falsity and misrepresentation of the matters it alleged did not establish that the problem representations were false or conveyed a misrepresentation in the way which it had alleged. I am of opinion that no such false suggestion or misrepresentation was contained in or conveyed by the patent to the Commissioner or a skilled addressee (as she is taken to be for this purpose).
(f) The solution and study representations
221 The solution and study representations were pleaded separately but had some common issues and it is convenient to deal with them as a group.
222 Apotex alleged that the solution representations as contained in the patent were. Apotex alleged that the solution representations as contained in the patent were:
“6 … At page 1 line 24 to page 2 line 1 the [patent] states that “the tert-butylamine salt has not been capable of providing a complete solution to the problems of the product's stability to heat and humidity”. In that context:
(a) at page 2 lines 12 to 14 the [patent] states that "On the other hand, and in surprising manner, it has been found that the arginine salt of perindopril, besides being new, has entirely unexpected advantages over all the other salts studied and, more especially, over the tert-butylamine salt of perindopril”: and
(b) at page 3 lines 9 to 10 the [patent] states that “The basic characteristics of this salt are very great stability to heat and to humidity compared to the tert-butylamine salt”.
7 By reason of the false suggestions or misrepresentations referred to in paragraphs 6(a) and 6(b) above and the Problem Representations, the [patent] expressly or impliedly represented that the perindopril arginine salt provided a complete solution, or a materially superior solution, or a solution, to the alleged problems of perindopril’s stability to heat and humidity.” (original emphasis)
223 Apotex pleaded that the solution representations were false because:
the arginine salt did not provide a complete, or materially superior, or any solution of perindopril’s stability to heat and humidity;
the arginine salt is, first, hygroscopic, secondly, significantly more hygroscopic than the erbumine salt and, thirdly, the patentee’s studies prior to the patent application showed this.
224 Importantly, Apotex qualified these allegations and those it made for the study representations as follows:
“For the avoidance of doubt, by this paragraph 9 and paragraph 43 [which related to the study representations], Apotex does not allege that perindopril arginine is less stable than perindopril erbumine (or vice versa). …
The Solution Representation simply asserts that the perindopril arginine salt was hygroscopic and therefore, not a complete solution to the stability problems with perindopril erbumine highlighted in the [patent]. The Solution Representation does not put the improved stability of perindopril arginine in issue.” (emphasis added)
225 In its written submissions Apotex explained of the solution representations that:
“This ground is confined to hygroscopicity. The experts agreed that hygroscopicity is an aspect of stability to humidity.”
226 Next, Apotex alleged that the study representations, as contained expressly or by implication in the patent were:
“the [patent] was obtained by the following false suggestions or misrepresentations: at page 3, lines 11 to page 4, line 5 the [patent] states that:
“Long- term stability studies carried out under very precise temperature and humidity conditions have yielded the results indicated in the Table hereinbelow.
In that study, perindopril was assayed by inverse-phase high-pressure liquid chromatography using, as eluant, an aqueous phase (comprising sodium heptane-sulphonate, and the pH of which is 2) and acetonitrile (67/33). Detection of the product was carried out by UV (215 nm).
The study was carried out using immediate-release tablets containing either 2.4 mg of the arginine salt of perindopril or 2.0 mg of the tert-butylamine salt of perindopril (each of the two tablets containing 1.7 mg of perindopril). The tablets were assayed 6 months after the start of storage of the tablets at different temperatures and different relative humidities (% R.H.).
The arginine salt used in this study is the L-arginine salt. It has been prepared according to a classical method of salification of organic chemistry.
|
Conditions 6 months |
tert-Butylamine salt of perindopril Percentage remaining (%) |
Arginine salt of perindopril Percentage remaining (%) |
|
25°C 60% R.H. |
101.0 |
99.5 |
|
30 °C 60% R.H. |
94.4 |
98.1 |
|
40°C 75% R.H. |
67.2 |
98.6 |
The results presented in the Table above show extremely clearly the very great stability of the arginine salt compared to the tert-butylamine salt. Indeed, after 6 months, practically no degradation of the arginine salt has taken place whereas the tert-butylamine salt exhibits a degradation rate of approximately 33%.”
…
28. The Study Representation impliedly represents that:
(a) the results in the Table support the conclusion that the perindopril arginine salt (as an API alone) shows very great stability compared to the perindopril erbumine salt (as an API alone);
(b) the results in the Table are the outcome of a single study;
(c) the results in the Table are the complete results from the study; and
(d) the results in the Table are a fair and accurate summary of the entire results of the tests conducted in the study.”
227 Apotex pleaded that the study representations were false because the results in the table:
(1) did not support the assertions that:
the arginine salt, as an API alone, showed very great stability compared to the tert-butylamine salt (as an API alone);
after six months there is a degradation of approximately 33% of the tert-butylamine salts;
(2) showed no clear difference in stability for 25°C at 60% RH and 30°C at 60% RH;
(3) did not show the amount of relevant salt (i.e. assay) present at the start of the study, indicated one measurement at only one point in time, namely after six months, and did not indicate that the differences in assay values for the two salts were statistically significant;
(4) related to a comparison only of immediate release tablets and not the two salts as API’s alone, and did not indicate whether the formulations of the immediate release tablets were identical other than for the differences in the salt;
(5) were not the result of a single study but of two separate studies, one for tablets containing the erbumine salt and the other for tablets containing the arginine salt; were not the complete results of the studies, did not include all the test results; and did not reveal the significantly greater hygroscopicity of the arginine salt as compared to the erbumine salt and so were not a fair and accurate summary of the entire results of the studies.
(g) The scope of Apotex’ case in respect of the solution and study representations
228 The way in which Apotex pleaded the falsity of the solution and study representations generated a significant issue between the parties. Servier argued that because of the qualification that Apotex made set out in [224] above the pleading created a non-sequitur that resulted in Apotex’ case going nowhere.
229 Apotex pleaded that the patent did not reveal the fact that perindopril arginine was hygroscopic. However, it did not plead that any particular problem or difficulty arose in respect of the salt. Rather, Apotex contended that the omission of any reference to the hygroscopicity of perindopril arginine in the patent gave rise to a material false suggestion or misrepresentation in one or more ways, namely:
• the arginine salt provided a complete, or materially superior, or any, solution to the alleged problem of perindopril’s stability to heat and humidity;
• the study results represented in the table in the specification, first, supported a conclusion that as against each other, as APIs alone, the arginine salt showed very great stability compared to that of the erbumine salt, and, secondly, were a fair or accurate summary of the entire results of the tests in the study or studies.
230 I consider that those matters can only be assessed on the limited basis that Apotex pleaded, namely that it did not contend that perindopril arginine was less stable than perindopril erbumine but only asserted that the omission from the patent of the hygroscopicity of perindopril arginine was a material matter. Apotex confined its attack on the solution representation to the fact that the arginine salt was hygroscopic and so was not a complete solution.
231 During the hearing I rejected attempts by Apotex to tender evidence as to how hygroscopicity affected perindopril arginine because that issue was outside its pleading. Apotex argued that despite the unqualified statement in its pleading that it did not allege that perindopril arginine was less stable than perindopril erbumine, it could contend that the arginine salt was less physically stable because of its greater hygroscopicity. That is, Apotex argued that its pleading merely removed the issue of chemical stability of perindopril arginine from the forensic battlefield.
232 I do not accept that argument. The expert evidence called by both parties elaborated at length on the relevance of hygroscopicity in the selection of a salt and its potential effects on both a salt and a substance that contained a salt. Apotex’ pleading did not put in issue the improved stability of perindopril arginine. Its concession could not be understood to relate only to the chemical, as opposed to the physical, stability of perindopril arginine. That construction of Apotex’ pleading is not open on its express terms. Rather, I consider that the pleading asserted that although perindopril arginine has improved stability as compared to perindopril erbumine, the mere fact that it is hygroscopic is the only reason why perindopril arginine is not a complete solution to the stability problems with perindopril erbumine, as identified in the patent.
233 Apotex did not allege that some consequence flowed from the mere fact that the arginine salt was hygroscopic other than that this meant it was, by that fact alone, not a complete solution. That is, although it had improved stability over the erbumine salt, the arginine salt was not perfect because it could take up water.
234 Apotex contended that had the hygroscopicity of the new salt been explicitly stated in the patent, the Commissioner is likely to have required Servier to satisfy her about that matter. Apotex did not contend that this omission amounted a failure to describe the invention fully (s 40(2)(a)). Rather, it complained that the omission was a material matter for the Commissioner in considering whether to grant the patent. Yet, Apotex’ pleading asserted no more than the omission. Critically, Apotex did not plead that the hygroscopicity had some particular consequence or effect that created a false suggestion or misrepresentation in the patent, far less how that was material.
235 Importantly, claim 1 in the patent is solely for the salt, perindopril arginine. Professors Byrn and Evans, and Drs Morella, Spargo and Williams stated in the second joint expert report that “perindopril arginine API [i.e. the salt] takes up water [at the rate of] at least one molecule of water per molecule of perindopril under certain conditions of humidity”. However, they reported that they had seen no evidence that indicated that hygroscopicity was a problem in tablets containing perindopril arginine. It was common ground that perindopril arginine itself is hygroscopic. Mr Damien acknowledged in his evidence that it was “a little” or “slightly” hygroscopic. He accepted that his 1991 study showed that perindopril arginine was more hygroscopic than perindopril erbumine and much more so when tested in unprotected conditions of 25°C at 90% relative humidity.
236 Professors Byrn and Evans and Drs Morella, Spargo and Williams, together with (as to the first two points below only) Prof Perkins agreed in the first report that:
• hygroscopicity is a non-specific term that indicates that water is absorbed by the solid, but the term does not indicate whether the absorbed water resides in the crystal lattice (hydrate) or is “free”;
• the majority of APIs will absorb water if the relative humidity is sufficiently high. Accordingly, the level of relative humidity at which the API absorbs water is important in evaluating the consequences of water sorption;
• hygroscopicity may be a problem if the absorbed water:
(1) is not bound into the crystal lattice and that results in chemical instability: i.e. it causes degradation of the API to create unwanted impurities because the water either reacts with the API or creates an environment in which it is more reactive;
(2) results in the API becoming “sticky” so that it does not flow and blend well during tablet manufacture;
(3) has the potential to change the crystal structure of the API to one or more new crystalline forms (i.e. crystalline hydrates) with different physicochemical properties compared with the anhydrous form(s). The extent of those differences may be large or small and may affect dissolution and solubility. That in turn may affect the bioavailability of the API;
however, water sorption can also have no effect whatsoever and be no problem;
• hygroscopicity is a typical criterion in salt selection, on the basis that the less hygroscopic a salt is, the less likely any of the foregoing potential issues will occur. The importance of this criterion in salt selection can vary considerably depending on the system and intrinsic stability of the compound or formulation for use which a salt is being considered;
• the hygroscopicity of a salt is a disadvantage if it causes chemical degradation. However, the mere absorption of water by a salt, without chemical degradation, is not necessarily a disadvantage, for example, where a stable hydrate is formed that is a preferable form for the API in tablet manufacture;
• it is the usual practice in regulatory submissions to provide regulators, such as the TGA, with information that, first, describes different known polymorphic and hydrated forms of a drug substance (i.e. API) and, secondly, indicates the solid form (i.e. whether it is a hydrate or polymorph) of the API that is to be formulated into a drug product. Because differences in the form of the drug substance can, in some cases, affect the quality or performance of the new drug product, the relevant ICH guideline provides that the appropriate solid state of the drug substance should be specified where differences exist between forms that have been shown to affect drug product performance, bioavailability or stability.
237 The circumstances in which Apotex obtained the amendments to its pleading on 2 August 2013 involved it giving the pleaded qualification (set out at [224]) that the relative stability of perindopril arginine was not in issue and that it only relied on the actual hygroscopicity of the salt to assert that it was not a complete solution to the stability problems with perindopril erbumine highlighted in the patent.
238 The expert evidence showed that hygroscopicity had the potential to, but would not necessarily, create a problem with a salt. Apotex did not allege in its pleading what effect any manifestation of that potential had in respect of perindopril arginine. The reason for this is not far to seek. Servier had opposed Apotex’s original attempt to amend to rely on the issue of hygroscopicity because of the need to obtain evidence to meet such a case, including the performance of experiments and obtaining of further expert evidence. That is why the consent order permitting the current form of the pleading contained the statements concerning the relative stabilities of the two salts and the limited reliance that Apotex placed on perindopril arginine’s hygroscopicity.
239 The experts agreed that hygroscopicity is not always a disadvantage, and that its impact on the selection or performance of a salt depends on the circumstances. Thus, for the omission of reference to perindopril arginine’s hygroscopicity to have been material to the grant of this particular patent, Apotex had to propound a case that demonstrated its materiality to the stability of tablet compositions containing the arginine salt. It did not do so and cannot be allowed to now.
(h) The solution representations – consideration
240 I am not persuaded that, fairly read, the patent makes any representation that the arginine salt provided a “complete solution” to the stability problems of the tert-butylamine salt. There is no evidence to show that when he or she read it, a skilled addressee would understand the patent to convey any of the solution representations. Apotex accepted that perindopril arginine had improved stability over perindopril erbumine. No doubt that is one powerful commercial motivation for Apotex engaging in this litigation to seek revocation of the patent so that, as it concedes, it can manufacture and sell generic tablets using the arginine salt. Apotex has not alleged or proved what, if any, effect hygroscopicity actually has, as opposed to what potential it might have, on perindopril arginine as a salt or API alone or in a pharmaceutical composition.
241 For the reasons I have given above at [216]-[219], the skilled addressee would understand that the stability problems referred to in the patent concerned the stability of tablets or pharmaceutical compositions containing perindopril erbumine and not to the intrinsic stability of any of perindopril or the two salts itself.
242 The mere possibility, on the expert and other evidence that, because of its hygroscopicity, there may be problems with perindopril arginine as a salt, including that it became sticky at 25oC in 90% RH, did not establish that the patent was obtained by material false suggestion or misrepresentation. Moreover, the experts agreed that there was no evidence that hygroscopicity was a problem in tablets containing the arginine salt. It follows that the patent does not convey to a skilled addressee any false suggestion or misrepresentation in terms of the solution representations that Apotex has proved to be false.
(i) The study representations – Apotex’ submissions
243 Apotex argued that the way in which the patent discussed the results of the study involved the patentee using the results that showed perindopril erbumine performing worst in comparison to perindopril arginine. It contended that that presentation was not fair and accurate because the results had not been obtained in a single study, excluded details of the accuracy and statistical significance of the figures in addition to not referring to the hygroscopicity of the APIs. It also contended that the patent did not identify the packaging used in the tests and left unexplained the relevance of the 40°C condition results.
244 In its closing submissions, Apotex argued that the patent represented that stability at temperatures above 30°C was important. It argued that the table represented that arginine salt was significantly more stable than the tert-butylamine one at 30°C at 60% RH, being the lightest condition permitted for pharmaceutical storage in Australia.
245 Apotex put a number of other matters in its closing submissions that Servier contended were outside the pleaded particulars of invalidity. Those were Apotex’ submissions that:
the table showed that after six months at 30°C at 60% RH, the remaining API assays of perindopril arginine and perindopril erbumine respectively were 98.1% and 94.4%. Apotex submitted that if these rates of degradation were extrapolated to 12 months the remaining API assays would be respectively more than 96% and less than 90%;
it contended the use of the 40°C at 75% RH results did not necessarily reflect that perindopril erbumine was less stable at a lower temperature because different kinetics were involved, and that the use of those results represented that stability at temperatures over 30°C was important;
the two studies had measured the hygroscopicity of the two APIs but those results were omitted from the patent, despite the importance of hygroscopicity in selecting a pharmaceutically acceptable salt to use;
HDPE bottles with a silica gel desiccant was the packaging used in the studies and this information was relevant to the Commissioner in assessing the reported results of the studies, particularly since perindopril erbumine was not marketed in that form of packaging. Apotex submitted that, had this been disclosed, the Commissioner would have understood that in other packaging perindopril erbumine could have achieved superior stability results. It contended that the use of the silica gel desiccant rendered the results for perindopril erbumine worse than if no desiccant had been used at all. Had that been done, Apotex submitted, the Commissioner would have appreciated that the study results presented in the patent merely compared the stability of tablets of perindopril arginine and perindopril erbumine in a packaging condition that favoured the former.
246 Apotex relied on Mr Damien’s evidence that the study of the arginine salt in 2000 was not intended to compare packaging. It contended that the patent failed to disclose there were two different studies done two years apart on two individual APIs by different personnel. It also argued that although the table in the patent presented results to three significant figures, and thus implied accuracy of +/- 0.05%, in fact, as Mr Damien said, the accuracy was no better than +/- 2% and there was no statistical analysis. Apotex submitted that this meant that it was not possible to conclude that, in conditions of 30°C at 60% RH, perindopril arginine had better stability than perindopril erbumine or vice versa. It argued that this was because the range of the possible correct assay levels overlapped and that Servier had not put the comparative degradation data in evidence.
247 Apotex argued that the data presented in the patent did not support its general assertion that the arginine salt had better stability than the tert-butylamine salt. It contended that the study results only supported such a conclusion on the limited basis in respect of tablets of a particular formulation of each salt if stored at 40°C at 75% RH, when Servier knew that the conditions used in the study would be a problem for perindopril erbumine.
(j) The study representations – consideration
248 I reject Apotex’ argument that any of the implied representations that it pleaded in [28] of its further amended particulars of invalidity set out at [226] above was made in the patent. For the reasons I have given above ([216]-[219]), a skilled addressee would understand that the discussion of the study in the specification concerned the two APIs in tablets and not their respective results when they were not in tablet or pharmaceutical compositions. Indeed, the specification said as much at p 3 line 17. The results in the table were clearly explained as results derived from a study of immediate release tablets. The only sensible reading of the table, the specification’s discussion of it and the study in the patent is that the results reflect assays of the API present in the tablets after storage over the periods and in the conditions specified. There is no suggestion, and a skilled addressee would not understand, that the study compared the APIs standing alone.
249 Apotex correctly contended that the specification represented that there was one study, when there were, in fact, two separate studies that were undertaken about two years apart and were not intended to be comparative. However, the patentee’s presentation in the patent of the results obtained in those studies accurately reflected the substantively comparable performance of tablets containing the two APIs in the same conditions and the same packaging. No doubt the patent could have presented the circumstances better. But, Apotex did not establish that any material inaccuracy existed in what the patent said were the comparative results. The second joint expert report stated the agreement of Profs Byrn and Evans, and Drs Morella, Spargo and Williams that the results in the table were “essentially consistent” with the underlying data in the two studies. They said: “the stability trends are similar and the results can be considered consistent.”
250 The data from the study presented in the patent did not purport to be exhaustive or non-exhaustive. It was presented to reflect the stability of the APIs when used in tablet compositions in the same particular conditions and packaging. The hygroscopicity of the APIs standing alone was not germane to the greater stability that perindopril arginine showed over perindopril erbumine when the salts were in the tablets or pharmaceutical compositions in the comparable conditions and packaging. Apotex argued that the hygroscopicity of perindopril arginine was evident from Servier’s data for the 2000 study that showed an increase of about 50% in water content in the pure API used in testing the assay content of the tablets.
251 It is important to understand for the purposes of the present issue how the assay is measured. The assay is ascertained using a chromatograph. That machine measures the amount (or assay) of various substances in a solution by recognising what each substance is against what it knows as a standard consisting of a pure form of each substance. The standard is placed in a solution and measured first. The technician must measure the standard accurately, identifying in the result any known impurity or extraneous matter, such as water. Thus, the technician who obtained the data for the 2000 study measured the amount of water in the perindopril arginine samples before putting these in solution and having the chromatograph measure the pure API without (or adjusting for) the water it had absorbed when weighed. This measurement of what the pure undegraded form of API looks like provides the chromatograph with a standard. The machine uses that result to measure the remaining API and degradation by-products present in the tablets that have been taken out of storage in the various controlled conditions and placed in a solution for the chromatograph to measure.
252 However, as Dr Morella pointed out there was no evidence of the storage conditions of the API used as the standard. It did not matter that it had absorbed water over time because, first, the water does not cause any degradation to occur in perindopril arginine and, secondly, the purpose of measuring the water content was only for the chromatograph.
253 The parties agreed the fact that by 9 August 2013 Servier had provided to Apotex all its relevant data concerning the water contents of the API samples and other analyses in the two studies that Dr Williams had referred to in his first affidavit. Apotex did not file any evidence in relation to those documents, including about the significance, if any, of the differences in the sample API’s water content used in the two studies. Apotex had the onus of proving that any differences in the 1998 and 2000 studies, not disclosed in the patent, had any, or any material, impact on the patent’s inaccurate references to their having been a single study. Apotex failed to prove that this inaccuracy (about a single study) affected in any material way the presentation of the results from the studies in the patent.
254 Importantly, Apotex pleaded the ways in which it alleged that the study representations were false as I have summarised at [227] above. I am not satisfied that any of those matters of falsity has been proved for the following reasons.
255 As to [227] (1): The skilled addressee would understand that the results in the table represented the position in respect of tablets and not the APIs alone. The first joint experts report stated that the patent “shows that perindopril erbumine tablets are unstable at 30°C/60% RH and 40°C/75% RH after storage for 6 months”. The experts confirmed this in their second joint report after considering full versions of data in the 1998 and 2000 studies by Servier. The only statement that there was a degradation of approximately 33% in the tert-butylamine salt after six months appeared in the results for conditions of 40°C at 75% RH. There was no evidence that this reporting of that particular result was other than accurate.
256 As to [227] (2): The two joint experts reports, as noted immediately above, confirmed the substantial accuracy of the results for perindopril erbumine stated in the table in the patent. The first report stated that the perindopril erbumine tablets were stable at 25°C at 60% RH. As Apotex alleged, the results in the table for perindopril arginine tablets in the same conditions and packaging did not show a clear difference in stability of the two salts in the tablets in those circumstances. However, the assertions in the patent concerning the comparatively very great stability of the arginine salt compared to that of the tert-butylamine one in tablets had to be read in the context of what the patent presented in the table as their justification. The skilled addressee would not read one line in the table in isolation from the rest.
257 There was no suggestion in the patent of a problem with the stability of perindopril erbumine tablets in conditions of 25°C at 60% RH. It was obvious from the table that the results for 25°C at 60% RH were similar for tablets with both salts, but in two succeeding conditions the results showed that perindopril erbumine was, as the experts agreed, significantly unstable. The experts agreed, in their first joint report, that the results in the table suggested an advantage for perindopril arginine tablets in terms of stability. In the second joint report, they said that the stability of perindopril erbumine tablets was poorer at 30°C/60% RH and markedly poorer at 40°C/75% RH when stored under the same conditions as the perindopril arginine tablets.
258 As to [227] (3): Drs Spargo and Morella said that, first, the skilled addressee would infer that the percentage of API present in the tablet at the commencement of the six month study was 100% and, secondly, the study documentation confirmed that it was. Indeed, the conclusions as to stability of the tablets in the joint expert reports as to what the study showed in the table in the patent must have proceeded on their common understanding that the percentage remaining columns began at 100%. The table only reported the results after six months. Apotex did not demonstrate how the accurate reporting of those six months results gave rise to any false suggestion or misrepresentation in or by the patent. As noted above, in their first joint report the experts agreed that the results in the table suggested an advantage in stability.
259 That brings me to Apotex’ argument that the patent did not indicate whether the differences in assay results for the two tablets were statistically significant and that while the table suggested the reported results were accurate to +/- 0.05%, Mr Damien had said that they were only accurate to about +/- 2%.
260 I reject that argument. First, as Servier contended, no expert or other evidence suggested that the accuracy of the table was +/- 0.05%. Secondly, the degree of accuracy of the results did not cause the experts to qualify their conclusions as to the relative stabilities I have discussed above. Thirdly, the degree of accuracy of the apparent degradation results reported in the table can be assessed, as Mr Damien explained, by comparing them to the other results, showing degradation by-products, produced by the chromatograph. Thus, while it was correct for Apotex to say that Mr Damien acknowledged a prima facie accuracy for such HPLC being a form of (chromatographic) data of better than +/- 2%, he said that the rate of APIs degradation for a particular result is derived by considering the amount of degradation by-products present. That is because the API present in any particular tablet tested may not have started at 100%, but could be within the tolerances allowed, such as those for the release of commercial products with API components. If the initial amount of API present was more or less than 100%, the amount of degradation, if any after six months (or some other time) is the difference between, not an assumed 100%, but rather the starting figure and the subsequent result. Mr Damien, who gave his evidence through a French interpreter, said when shown the chromatograph results for 30°C at 60% RH:
“What I can see clearly in the chromatogram is that the arginine is a lot less degraded than the tert-butylamine salt. I need to make calculations, but visually you can already see the difference and confirm the difference.
MR BANNON: And I think you said yesterday that you checked – you were asked to check the figures in the patent before it was filed.
THE INTERPRETER: Yes.
MR BANNON: And did you check the figures?
THE INTERPRETER: Yes.
MR BANNON: And did you think they were accurate?
THE INTERPRETER: Yes. If I recall correctly, there was a very small error, maybe 0.1 per cent, but generally speaking that is correct.
MR BANNON: And at the time that you checked the figures, did you have all the information in relation to degradation products as well as … all the chromatograms including the chromatograms for the degradation products in relation to the relevant tests.
THE INTERPRETER: Yes. Of course, because as I said yesterday, in a stability study, to ensure that there is consistency, you need the degradation by-product as well as the amount of remaining product.”
261 I accept that evidence. Apotex did not file any expert evidence to demonstrate that the results in the table were not substantially accurate. The comparison between the API and degradation products assays could easily have been done by Apotex, if its argument had any real basis. In the absence of any degradation assay analysis, I am not satisfied that the results in the table were in any substantive way inaccurate to the degree of +/- 2%. The chromatography for the reported results in the table appear to support Mr Damien’s opinion that the degradation by-products in the tests for the arginine were minimal and those for the erbumine API were in the order of 4.5% to 5%.
262 As to [227] (4): For the reasons I have given, the skilled addressee would understand that the study related to tablets containing the two perindopril salts and their relative performance in the stated conditions. During their concurrent evidence, Profs Byrn and Evans and Drs Morella, Spargo and, more reluctantly, Williams all acknowledged that they and, I find, a skilled addressee, would have read the patent with the understanding that it was making a comparison between tablets in packaging and conditions with identical or sufficiently similar formulations except for the API to warrant the comparison to be meaningful. As Dr Morella put it, without any dissent from the other experts:
“I would agree with Dr Spargo; that one would assume that the compositions that were being tested were similar. It wouldn’t be essential that they were exactly the same. It’s a comparison test. You have two different salts in a container, in a tablet form, and you’re wanting to assess whether or not they’re the same or different. So in that sense you only need bottles and the compositions that are similar to perform that comparison, and I assume that they were similar.
HIS HONOUR: So that the excipients used in the formulation you would assume would be similar and that whatever form of packaging is being studied would be similar; is that what you’re saying?
DR MORELLA: That’s right, yes.”
263 In fact, Mr Damien used identical formulations, including the quantitive compositions of excipients in the 1998 and 2000 studies.
264 As to [227] (5): The mere fact that the 1998 and 2000 studies were separate had no effect on the substantive validity of the comparison of their results as presented in the patent. Apotex led no evidence to the contrary and, indeed, in his first affidavit, one of its experts, Dr Williams said as much. Moreover, the second joint experts report stated, without any qualification, that the results tabulated in the patent were essentially consistent with Servier’s data for the two studies. As I have noted above, although Apotex had access to all that data, it led no evidence to suggest that the results presented in the patent were in any way relevantly incomplete, misleading or otherwise unfairly selective. And, for the reasons I gave relating to the solution representation, Apotex has not established that the hygroscopicity of perindopril arginine created any false suggestion or misrepresentation in the patent.
265 As indicated at [245] above Servier complained that Apotex had sought to expand its pleaded case on the study representations in its final submissions. I am of opinion that to the extent I have not dealt with those matters in relation to the study representations above, Apotex cannot be permitted to go outside its pleaded case. Apotex had at least three opportunities, resulting in its further amended particulars of invalidity, to identify how it would make out its case. It did not apply to amend to add any new particulars to reflect these new matters on which it had led no evidence in chief. It gave no explanation why this extrapolation was being put forward only in closing address. It would be quite unfair to Servier to allow Apotex to rely on these new matters in the circumstances: Aon Risk Services Australia Ltd v Australian National University (2009) 239 CLR 175 at 214-215 [102]-[103], 217-218 [112], [114] per Gummow, Hayne, Crennan, Kiefel and Bell JJ; Dare v Pulham (1982) 148 CLR 658 at 664 per Murphy, Wilson, Brennan, Deane and Dawson JJ.
266 For example, Apotex’ attempt to extrapolate from the degradation of perindopril arginine over six months how it might further degrade over 12 or more months was not foreshadowed in its pleading. When Dr Morella was asked about this he observed that: “This data here is not designed to show stability other than at six months”. It was no part of Apotex’ pleaded case or the voluminous and detailed prepared expert evidence it led, that this extrapolation should be made or that it created a false suggestion or misrepresentation in the patent. This was not a case that Servier knew it had to meet.
267 Similarly, Apotex’ attempt to rely on the lack of relevance of results in conditions of 40°C was new and unpleaded. While Dr Williams briefly referred to some purposes of using 40°C testing in his first affidavit, that evidence did not suggest that testing stability at temperatures over 30°C was or was not important. He touched on this topic during the concurrent evidence, again without suggesting that testing stability above 30°C was not capable of being important. And Dr Byrn commented at the same time that the data in the table (in relation to perindopril erbumine) “suggests to me that there’s stability problems at 40° and also, for example, in Africa you probably couldn’t use this product. It’s just too unstable”. Once again, this was not an issue raised by Apotex before trial in its pleaded case far less as a false suggestion or misrepresentation. For the reasons above I do not consider that Apotex can be permitted to rely on it.
268 Last, Apotex contended that the failure to disclose the type of packaging, HDPE with a desiccant, used in the testing for the study would have misled the Commissioner. Once again this was not part of Apotex’ pleaded case and I am not persuaded that it can now rely on it. In any event, the Commissioner, as a skilled addressee, would have appreciated that the packaging used was different from that in the commercially marketed perindopril erbumine, was also cheaper, less constraining to use for commercial purposes and had the other promises foreshadowed in the specification. In that context, if Apotex had wanted to contend that the Commissioner had been misled in the new way it sought to raise in final address, it should have pleaded that.
(k) The shelf-life representation
269 Apotex pleaded that:
“the [patent] was obtained by the following false suggestion or misrepresentation: at page 2, lines 3 to 4 the [patent] states that “… even for temperate-climate countries, that instability [of perindopril erbumine] has made it impossible to obtain a shelf-life of more than 2 years for the tablets”;”
270 Apotex pleaded that the shelf-life representation was false because:
Servier applied to the TGA in May 1992 for a three year shelf-life for its 2 mg and 4 mg perindopril erbumine tablets (i.e. Coversyl) when stored at 30°C and, the TGA approved that application on 30 June 1992.
Accordingly, Servier knew, or reasonably ought to have known, that it was possible to apply for and obtain a shelf-life of more than two years for perindopril erbumine.
271 Relevantly, the patent discussed shelf-life in the following context, which includes the passage complained of:
“The tert-butylamine salt was thus alone in exhibiting the best stability compared to the other forms studied. However, in view of the intrinsic fragility of perindopril, the tert-butylamine salt has not been capable of providing a complete solution to the problems of the product’s stability to heat and humidity. Indeed, for marketing, tablets of perindopril tert-butylamine salt must, in certain countries, be protected with additional packaging measures. Moreover, even for temperate-climate countries, that instability has made it impossible to obtain a shelf-life of more than 2 years for the tablets. Finally, for marketing of the tablets, they have to be marked “to be stored at a temperature less than or equal to 30 degrees”. (p1 line 23-p2 line 6)
…
The results allow us to consider less onerous constraints with respect to the packaging of the pharmaceutical compositions and also to obtain a shelf-life of at least three years for our pharmaceutical compositions.” (p 4 lines 8-10)
(l) The shelf-life representation – Apotex’ submissions
272 Apotex argued that the views of expert witnesses could not provide any meaningful assistance as to how the Commissioner is likely to have understood the passages in the specification relevant to shelf-life. It submitted that this was a question solely for the Court and prayed in aid what the Full Court had said in PAC Mining Pty Ltd v Esco Corp (2009) 80 IPR 1 at 14-15 [29]. It pointed to the absence of any qualification for the assertion that the instability of the erbumine salt had “made it impossible to obtain a shelf-life of more than 2 years”, even in temperate climate countries, coupled with the unqualified assertion that the new results allowed the obtaining of a shelf-life of at least three years.
273 Apotex argued that those assertions would have conveyed to the Commissioner that, regardless of packaging, a shelf-life for perindopril erbumine of more than two years even for temperate climate countries was “impossible” and that the invention of perindopril arginine had removed this limitation and opined the possibility of a three year shelf-life. Apotex contended that the 1992 approval for Coversyl rendered those matters, and the representation in the patent, false to Servier’s knowledge. It submitted that there was no basis for Servier’s contention that the Commissioner would understand the patent to be discussing the impossibility of shelf-life of more than two years in a sense qualified by words to the effect “if additional packaging measures are not used”. That was because, Apotex submitted, the bold and unqualified language in the patent left no room for such a reading.
(m) Consideration of the shelf-life representation
274 The patent must be read from the perspective of the skilled addressee. After all, that is the hypothetical person to whom it is addressed. A lay person may understand the patent to make a false suggestion or misrepresentation that would never occur to a skilled addressee. It would be inappropriate to adopt Apotex’ argument that the views of experts are not relevant. That was, in fact, not supported by what Sundberg, Jessup and Middleton JJ said in PAC Mining 80 IPR at 14-15 [29]. One purpose of expert evidence in a case like the present is to place the Court in the position of a skilled addressee so that the Court can draw on a proper understanding of what the patent is saying.
275 While the construction of the patent is a matter solely for the Court, expert evidence enables the Court to be placed in the appropriate matrix of facts, with relevant knowledge of how a person skilled in the art will understand the patent. This is no different, in principle, to any other situation in which a court must construe a document or words. In order properly to understand what meaning words convey to a reader or listener, a court ordinarily must assess the objective characteristics of the person or class of persons to whom they are addressed. Thus, in a contractual situation such an approach to construction is clear, as Gleeson CJ, Gaudron, McHugh, Gummow and Hayne JJ explained in Royal Botanic Gardens and Domain Trust v South Sydney City Council (2002) 240 CLR 45 at 52-53 [10].
276 The same is so for the purposes of assessing whether a person has engaged in misleading or deceptive conduct or conduct that is likely to mislead or deceive: Campomar Sociedad Limitada v Nike International Ltd (2000) 202 CLR 45 at 84-85 [99]-[103] per Gleeson CJ, Gaudron, McHugh, Gummow, Kirby, Hayne and Callinan JJ. It is also so in cases of defamation where the matter complained may have a special or particular meaning to people with knowledge of a particular fact that is not conveyed to the ordinary reasonable reader, listener or viewer. In Reader’s Digest Services Pty Ltd v Lamb (1982) 150 CLR 500 at 504-506 where Brennan J, with whom Gibbs CJ, Stephen, Murphy and Wilson JJ agreed, explained the differences in establishing the natural and ordinary meaning, and an innuendo meaning (i.e. one conveyed to persons with particular knowledge that causes words to be understood in a special sense) of the same words: and see Mirror Newspapers Ltd v Harrison (1982) 149 CLR 293 at 300-301 per Mason J with whom Gibbs CJ, Wilson and Brennan JJ agreed.
277 We all understand words based on our knowledge and experience of human affairs. But, where words are used in a special way or in, for example, a technical or scientific document, the Court must be put in the position, by evidence, where it can read and construe those words in the sense in which persons to whom they are addressed will understand them. That is an objective exercise and is not aided by an expert explaining his or her understanding. Rather, the role of expert evidence in this context is to provide the Court with the knowledge or information that the addressee would use when reading the words so that by using that knowledge or information the Court can construe the document in context, objectively and accurately. Patents are no different to any other form of communication in this respect.
278 Here, the expert evidence provided the background knowledge and experience that can be employed in assessing how a skilled addressee in the position of the Commissioner would understand what the patent was conveying.
279 The first joint experts report said that the cost of manufacture of packaged product increased from the lowest, PVC blisters and bottles, to tropicalized packaging to Alu/Alu cold form blisters, with the last being at least twice as expensive as the first. They said that PVC blisters and bottles or containers (such as HDPE, PP and glass) are regarded as ordinary packaging and would be used by pharmaceutical manufacturers across the world. They also said that specialised packaging, such as tropicalized packaging and Alu/Alu cold form blisters, requires additional equipment and results in increased costs. The skilled addressee would know that it was likely that a longer shelf-life could be obtained by the use of either or both additional packaging or different temperature storage conditions, as Dr Morella said in his first affidavit.
280 I am of opinion that a skilled addressee (including the Commissioner) would understand the specification to be discussing the impossibility of obtaining a shelf-life greater than two years if only ordinary packaging were used for perindopril erbumine tablets. Apotex’ construction is a possible way that the patent could be read by the skilled addressee. However, it is much more likely that the skilled addressee would recognise that the patent’s promise of “less onerous constraints” in respect of packaging and the potential to obtain a shelf-life of at least three years (at p 4 lines 8-10), would transform the previously discussed impossibility of obtaining a shelf-life of more than two years: i.e. a shelf-life that could not be exceeded if ordinary packaging was used.
281 The skilled addressee would have understood that the additional packaging that had to be used for certain countries (referred to at p 2 lines 2-3) was not the packaging used in temperate-climate countries when the patentee sought to obtain a shelf-life greater than two years. Indeed, if it were relevant, I think that this is also the way in which an ordinary reasonable reader would understand the patent. Such a person would read it carefully as an important document and would understand that “additional packaging” was something beyond simple PVC blisters and perhaps more expensive bottles.
282 In addition, Servier knew that it had obtained an approved three year shelf-life in tropicalized packaging for the valuable product, Coversyl, that it was marketing at the time it applied for the grant of the patent. There is no reason to think that, in those circumstances, Servier would include an obviously false misrepresentation in the patent it was seeking, particularly when an important underlying commercial objective of that application was a further period of monopoly in which to sell its new perindopril arginine tablet product. It would have been obvious to Servier that any false statement of the nature alleged by Apotex, would have been exposed easily by a competitor who wished to challenge the patent. Accordingly, even if the construction at which I have arrived be wrong, I am satisfied that Servier did not understand the patent to have made the shelf-life representation.
(n) The temperature representation
283 Apotex pleaded that:
“The [patent] was obtained by the following false suggestion or misrepresentations: at page 2, lines 4 to 6 the [patent] states that “… for marketing of the tablets, they [perindopril erbumine tablets] have to be marked ‘to be stored at a temperature less than or equal to 30 degrees”.”
284 Apotex pleaded that the temperature representation was false because:
both the perindopril erbumine and perindopril arginine tablets sponsored by Servier have to marked “Store below 30 degrees Celsius”;
it followed that perindopril arginine did not provide a benefit or improvement over perindopril erbumine in relation to the temperature at which it can be stored.
285 Apotex pleaded the terms of Therapeutic Goods Orders Nos 48 and 69, the common general knowledge of persons involved in preparing pharmaceutical regulatory submissions in Australia that the maximum storage condition allowed by the TGA was at all relevant times “Store below 30°C”, and Servier’s awareness, as such a person, that it was not generally possible to obtain approval from the TGA for storage conditions above 30°C.
286 The specification continued with the following passage at p 2 lines 7-9 immediately after the temperature representation:
“These constraints are, of course, onerous, especially in terms of organisation and cost, and it has appeared especially useful to try to develop a new perindopril salt in order to reduce the constraints due to the tert-butylamine salt.”
(o) The temperature representation – Apotex’ submissions
287 Apotex argued that, in essence, the TGA requirement for tablets composed with perindopril erbumine to be marked with a condition they be stored at no more than 30°C was not a substantive or real constraint. It contended that no examples of a higher temperature storage specification were in evidence and that subsequently Servier had obtained only an approval from the TGA that its perindopril arginine products be stored below 30°C. Accordingly, it argued that that storage condition was not a problem or constraint at all.
(p) Consideration of the temperature representation
288 I reject Apotex’ argument. First, Australia has climates that fall within zones III and IV of the ICH designations and the TGA could approve a storage temperature greater than 30°C (see [22]-[24] above). Secondly, unlike tablets composed with perindopril arginine, those composed with perindopril erbumine were susceptible to degradation of their API at temperatures of 30°C and above (see [255] above). Thirdly, as Profs Byrn and Evans and Drs Morella, Spargo and Williams agreed in their first joint report, an exporter must show products intended for export to countries in climate zones III or IV to have long term stability at 30°C/65% RH and also provide accelerated data for them in conditions at 40°C/75% RH. Fourthly, the experience of Servier in obtaining regulatory approval for its perindopril arginine products, sometime after the filing of the complete specification in the patent, cannot be used show that earlier, in 2002 or 2003, the specification conveyed a false suggestion or misrepresentation. Fifthly, the potential for export was open to be relied on by the patentee. Dr Morella gave evidence that one Australian manufacturer, Faulding, exported products to countries with climates in zones III and IV.
289 The Australian regulator and market may offer only limited opportunities to sell products with storage conditions of no greater than 30°C. However, a product that, can be stored at higher temperatures is substantively less constrained than one that, because of its inherent characteristics, cannot be so stored. Regulatory requirements can change over the 20 year life of a patent. Moreover, as here, the potential for export of a new and more stable salt of a compound, perindopril, that had been commercially successful when sold as part of an API, namely, the erbumine salt, is self-evident. Blood pressure problems occur in humans throughout the world. Coversyl had been a highly successful brand for Servier at the time that the complete specification was filed and the arginine salt, if patentable, offered the potential for an extended monopoly for the new product. If it could be stored at higher temperatures in cheaper packaging, it offered commercial benefits to a manufacturer as compared to the constraints imposed by the fragility of the erbumine salt and comprising it.
(q) The cost-saving representations
290 Apotex pleaded:
“… the [patent] was obtained by the following false suggestions or misrepresentations:
(a) at page 2, lines 1 to 3 the [patent] states that “Indeed, for marketing, tablets of perindopril tert-butylamine salt must, in certain countries, be protected with additional packaging measures”;
(b) at page 2, lines 7 to 9 the [patent] states that “These constraints [being, among others, the constraint referred to in paragraph (a) above] are, of course, onerous, especially in terms of organisation and cost, and it has appeared especially useful to try to develop a new perindopril salt in order to reduce the constraints due to the tert-butylamine salt”; and
(c) at page 4, lines 8 to 10 the [patent] states that “The results allow us to consider less onerous constraints with respect to the packaging of the pharmaceutical compositions …”
291 Apotex pleaded that the cost-saving representations were false because Servier knew that it was possible and practicable “to use less onerous constraints” with respect to packaging of perindopril erbumine tablets without the need to change the salt form. Apotex’ particulars, that were pressed in its closing submissions, asserted that Servier had used tropicalised packaging for its erbumine salt Coverysl products in Australia up to 2006 and that it was common general knowledge, and Servier knew or should have known, that Alu/Alu blisters could have been used, were less expensive and onerous, and were the most protective and cost effective manner of packaging for a product that was sensitive to heat and humidity.
(r) The cost-saving representations – Apotex’ submissions
292 Apotex argued that Servier had used tropicalised packaging for its perindopril because it suited it to do so. That was because, Apotex argued, Servier could supply perindopril erbumine in blister packs in temperate countries and could over-wrap the blisters to achieve tropicalized packaging for supply to countries in climate zones III and IV, including Australia. Apotex submitted that the extra expense that Servier incurred in organising its packaging could not be used to establish that it was commercially reasonable for it to use the tropicalized packaging it did. It contended that stability data for Apotex’ generic erbumine salt product was sufficient to support TGA registration of a three year shelf-life at 25°C. Accordingly, Apotex contended, the cost-saving representations were not reflective of any real problem and were likely to have contributed materially to the Commissioner’s decision to grant the patent.
(s) Consideration of the cost-saving representations
293 I reject Apotex’ argument. The first joint experts report, in a section in which Mr Pienaar joined, explained that Alu/Alu cold form blister packaging was first, more expensive than tropicalized packaging and, secondly, about twice as expensive as PVC blister packaging. Accordingly, the premise for Apotex’ assertion of falsity of the cost-saving representations had no basis. Moreover, it did not call any evidence to contradict the joint experts report’s conclusions. Such evidence is likely, as Servier submitted, to have been readily available to Apotex based on its own experience as a manufacturer of pharmaceutical products.
294 In the second joint experts report, Profs Byrn and Evans, and Drs Morella, Spargo and Williams agreed that:
the TGA suggested that it would give Servier’s perindopril erbumine tablets packed in PVC/Alu blisters a shelf-life of 18 months if stored at 25°C but only if, among other changes, Servier tightened its release specifications to a minimum assay level of 97.5%;
the shelf-life of perindopril erbumine tablets can be extended by using more protective packaging, whereas the limited data for perindopril arginine tablets suggested that an equivalent shelf-life may be possible in less protective packaging and this would result in cost-savings;
the stability of perindopril erbumine tablets is worst in the least protective packaging HDPE bottles, better in PVC/Alu blisters, better still in Alu/Alu cold form blisters, and best in tropicalized packaging and that in the latter they achieved shelf-life of 3 years at 30°C/65% RH;
perindopril arginine tablets do not need protective packaging to achieve adequate shelf-life and stability at 30°C/65% RH (and can be supplied in less expensive HDPE bottles with a desiccant).
295 The parties spent considerable time debating whether Apotex’ generic erbumine salt tablets were entitled to their current shelf-life and the experts did not come to a joint position on this. However, it is not necessary to resolve this further dispute in an already over complex case.
Conclusion
296 Apotex has succeeded only in establishing that the specification in the patent did not describe the best method known to the applicant of performing the invention and so did not comply with s 40(2)(a). Accordingly, that raises the question of what relief should be granted under s 138(3) of the Act. Apotex’ many other challenges to the validity of the patent have all failed and those took up the bulk of the evidence and hearing. That failure should be reflected in costs. In addition, the parties should identify any errors or oversights in these reasons that may readily be corrected, including any argument or issue of substance that may not have been addressed so as to avert any unnecessary issue in any appeal.
297 I will hear the parties on the question of relief and on costs.
|
I certify that the preceding two hundred and ninety-seven (297) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Rares. |
Associate:




