FEDERAL COURT OF AUSTRALIA
Idenix Pharmaceuticals LLC v Gilead Sciences Pty Ltd [2017] FCAFC 196
ORDERS
DATE OF ORDER: |
THE COURT ORDERS THAT:
2. Within 14 days of the date hereof, the parties file and serve written submissions (limited to 3 pages) on the question of costs.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
THE COURT:
1 The appellants (Idenix) are the registered owners of patent no. 2003247084, titled “Modified 2' and 3'-nucleoside prodrugs for treating flaviviridae infections” (the Idenix patent). The Idenix patent relates to compounds for the treatment of flaviviridae infections, including the hepatitis C virus (HCV) infection. The Idenix patent was filed on 27 June 2003 and published on 19 January 2004, but claims earlier priority dates of 28 June 2002 based on US patent application 60/392,350 (the 350 application) or 14 May 2003 based on US patent application 60/470,949 (the 949 application).
2 The respondents (Gilead) are the registered owners of patent no. 2004253860 (the Clark patent). The Clark patent was filed on 21 April 2004 and published on 13 January 2005 but claims a priority date of 30 May 2003 based on the international patent application WO 2005/003147.
3 Gilead has sought to sell in Australia a pharmaceutical drug containing or comprising an effective amount of a compound called sofosbuvir to treat HCV. The disclosure of sofosbuvir was made in the Clark patent. Gilead commenced proceedings in this Court against Idenix seeking a declaration that claims 7 to 41 (inclusive) of the Idenix patent were invalid. It contended that the Idenix patent was invalid on the grounds that the patent did not disclose a manner of manufacture, lacked novelty, lacked fair basis, lacked utility, and lacked sufficiency. There was also an allegation of false suggestion.
4 In turn, Idenix brought a cross-claim alleging that Gilead threatened to infringe various claims of the Idenix patent through the supply of pharmaceutical compositions containing the compound sofosbuvir. Gilead conceded, subject to its invalidity assertions, that sofosbuvir infringed claims 7 and 8 as well as dependent claims 10 and 13 of the Idenix patent.
5 The primary judge found the Idenix patent to be invalid on the grounds of insufficiency in respect of claims 7 to 41 (Gilead Sciences Pty Ltd v Idenix Pharmaceuticals LLC (2016) 117 IPR 252; [2016] FCA 169). Her Honour also held claim 7 to be inutile. Otherwise, her Honour did not accept the balance of Gilead’s asserted grounds for invalidity. As a consequence of the primary judge’s conclusions, Idenix failed in its case of threatened infringement with the cross-claim being dismissed.
6 Idenix now appeals the primary judge’s decision on the basis that her Honour erred in her conclusions as to insufficiency, certain aspects of fair basis and inutility. Further and consequentially, Idenix contends that the primary judge erred in dismissing its cross-claim given that her Honour premised that dismissal on relevant claims of the Idenix patent being invalid.
7 Gilead has filed a notice of contention in which it challenges the primary judge’s conclusions that the Idenix patent was not invalid on the grounds of lack of novelty and other aspects of lack of fair basis.
8 For the following reasons, we would dismiss Idenix’s appeal. Moreover, if it is necessary to say so, we would also reject Gilead’s grounds of contention.
9 It is convenient to divide our discussion into the following sections:
(a) Relevant background science – [10] to [81].
(b) The patent specification – [82] to [101].
(c) Earlier applications – [102] to [123].
(d) The Clark patent – [124] to [131].
(e) Grounds of appeal 1 to 8; lack of sufficiency – [132] to [238].
(f) Novelty and lack of fair basis overview – [239] to [242].
(g) Grounds of appeal 9 and 10; lack of fair basis – [243] to [254].
(h) Grounds of appeal 11 and 12; lack of utility – [255] to [261].
(i) Notice of contention ground 1; lack of novelty – [262] to [312].
(j) Notice of contention ground 2; internal fair basis – [313] to [326].
(k) Conclusion – [327].
RELEVANT BACKGROUND SCIENCE
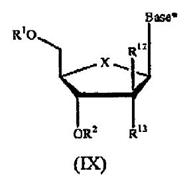
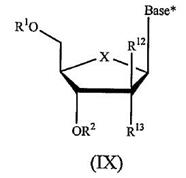
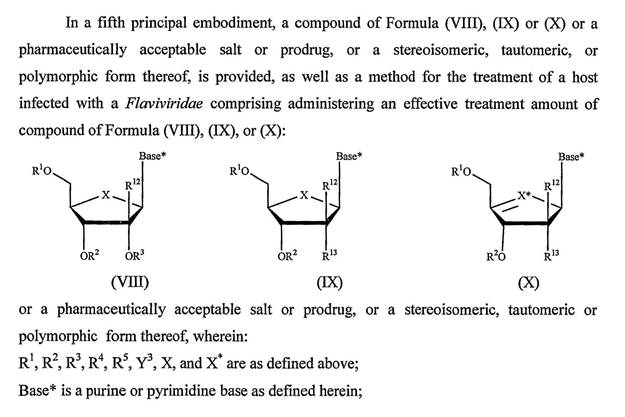
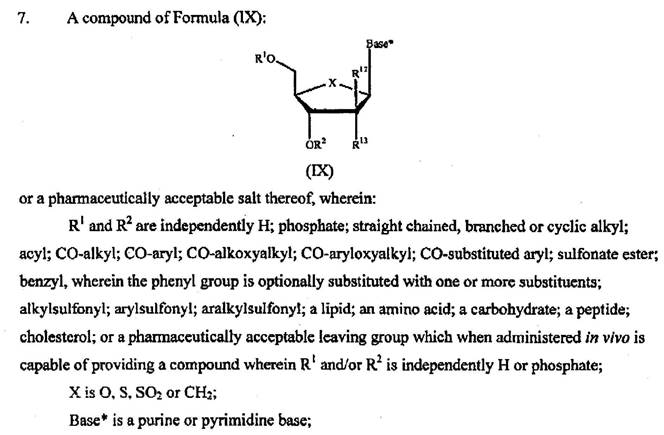
10 Claim 7 of the Idenix patent claims:
A compound of Formula (IX):
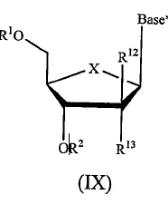
or a pharmaceutically acceptable salt thereof, wherein:
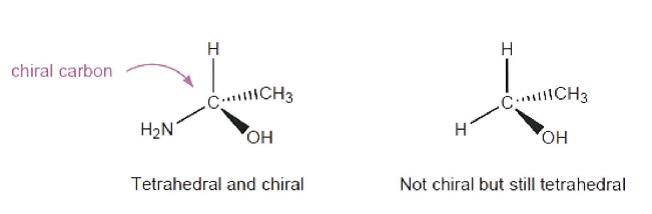
R1 and R2 are independently H; phosphate; straight chained, branched or cyclic alkyl; acyl; CO-alkyl; CO-aryl; CO-alkoxyalkyl; CO-aryloxyalkyl; CO-substituted aryl; sulfonate ester; benzyl, wherein the phenyl group is optionally substituted with one or more substituents; alkylsulfonyl; arylsulfonyl; aralkylsulfonyl; a lipid; an amino acid; a carbohydrate; a peptide; cholesterol; or a pharmaceutically acceptable leaving group which when administered in vivo is capable of providing a compound wherein R1 and/or R2 is independently H or phosphate;
X is O, S, SO2 or CH2;
Base* is a purine or pyrimidine base;
R12 is C(Y3)3; Y3 is independently H, F, Cl, Br or I; and
R13 is fluoro.
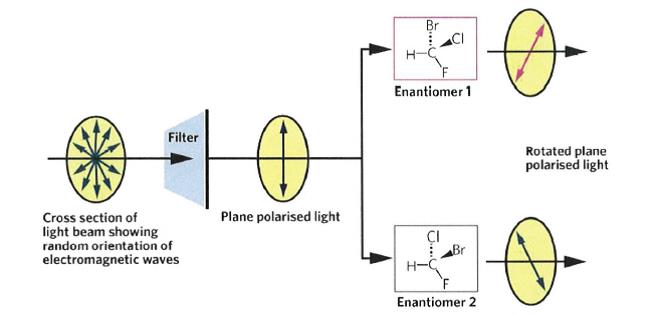
11 Claim 8 of the Idenix patent claims a compound of claim 7 wherein X is O and Y3 is H. Claim 9 claims a compound of claim 8 wherein R1 and R2 are H. It is not necessary to make reference to later dependent claims at this point.
12 In order to appreciate the nature of the composition(s) claimed including the potential synthesis thereof, and the parties’ submissions before us, it is necessary to set out the background science, a summary of which we have principally derived from the parties’ useful chemistry primers that were filed at first instance, the primary judge’s description and some additional matters drawn from the evidence before her Honour.
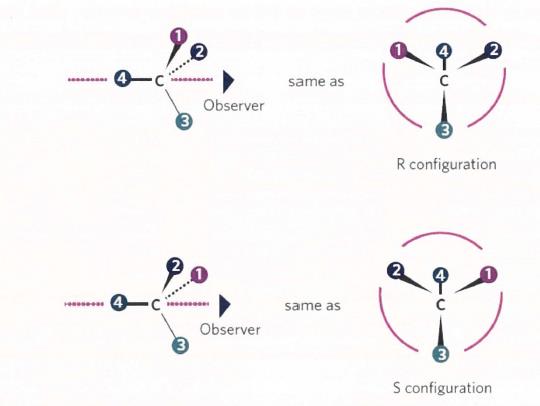
(a) Organic chemistry
13 It is useful to begin with some common terminology used in organic chemistry relevant to the present issues.
14 First, “analogue” in the context of organic chemistry is a molecule or compound to which at least a single chemical change has been made relative to a natural compound. A “conjugate” is the name often given to a molecule where two or more classes of molecules are linked together. For example, a peptide that is attached to a nucleic acid is a conjugate. We will discuss nucleic acids and peptides later. An “enzyme” is a biological molecule that catalyses or accelerates a chemical reaction. A “reagent” is a compound usually used to help bring about chemical change in other compounds.
15 Second, a “functional group” is the term used in organic chemistry to describe structural elements that display particular chemical behaviours or properties. For example and relevant to the present case, one common functional group is a hydroxyl. A “hydroxyl” (also referred to as a hydroxy group) is commonly represented by the shorthand letters “OH”. It consists of an oxygen atom bonded to a hydrogen atom. In an organic compound, a hydroxyl attached to a carbon atom is referred to as an alcohol. Hydroxyls are commonly referred to as primary, secondary or tertiary hydroxyls based on the classification of the carbon atom to which the OH substituent is bonded. Such a carbon atom is classified as a primary, secondary or tertiary carbon if it is bonded to one, two or three carbons, respectively. For example:
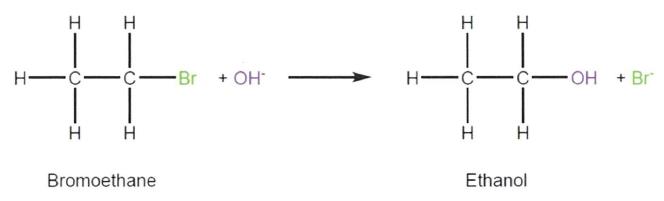
(a) a primary hydroxyl (1° hydroxyl) is bonded to a carbon atom that has one further carbon atom attached;
(b) a secondary hydroxyl (2° hydroxyl) is bonded to a carbon atom that has two further carbon atoms attached; and
(c) a tertiary hydroxyl (3° hydroxyl) is bonded to a carbon atom that has three further carbon atoms attached.
16 For completeness, a carbon is classified as a quaternary carbon when it is bonded to four other carbons. As such, a functional group such as a hydroxyl (which is not a carbon-containing group) cannot be attached to a quaternary carbon, given that a carbon atom only has four valence electrons and therefore can only form at most four covalent bonds. In other words, a quaternary carbon necessarily excludes the capacity to bond with a further functional group such as a hydroxyl.
17 Third, “heteroatom” is the term commonly used to describe any atom other than a carbon or hydrogen in an organic molecule. Further, a “ligand” is the name given to a molecule that binds specifically to a receptor site of another molecule. In other areas of chemistry ligands can be simple organic or inorganic molecules that coordinate (or bind) to metal atoms to form complexes.
18 Fourth, “polymorph” is a different crystalline form of the same compound. Polymorphs are not different compounds, just different solid states of a compound. The term “polymorphism” is the ability of a solid material to exist in more than one crystal structure. In pharmaceuticals, different polymorphs can have different pharmacokinetic profiles, often arising as a result of their different dissolution and absorption rates.
19 Fifth, a molecule is considered a “tautomer” (or “tautomeric”) if it can exist in another form of itself, simply by an internal rearrangement of electrons and bonds. A tautomer can freely interconvert between its tautomeric forms. In general, one tautomeric form of a molecule will predominate over the others. For example, the nucleobases adenine, cytosine, guanine, thymine and uracil exist as tautomers. We will elaborate on some aspects of stereochemistry in a moment.
20 Finally, there is other terminology that we should also briefly mention at this point as we use it later in our reasons. The term “alkyl” refers to a saturated straight, branched or cyclic hydrocarbon, typically a chain of C1 to C10 including CH3 (methyl) and so on. A halogen (halo) includes fluorine (F or Fluoro), chlorine (Cl or Chloro), bromine (Br or Bromo) or iodine (I or Iodo). A “substituent” is usually an element or group replacing a hydrogen on a part of a hydrocarbon chain. A “moiety” is best described as the characteristic part of a molecule. A “substrate” is a substance on which another substance acts and includes a substance affected by a catalyst or enzyme. The term “protected” refers to a process whereby a group is added to an oxygen, nitrogen or phosphorus atom to prevent further reaction. A “prodrug” is a chemical compound that on metabolism in the human body becomes active in a pharmaceutical sense. A Grignard reagent is an organometallic compound used in an organic synthesis. It usually takes the form RMgX, where X is a halogen atom, R is an organic radical and Mg is, of course, magnesium.
Drawing and naming organic molecules
21 The simplest method for drawing an organic molecule uses a structural formula or skeletal formula. A structural formula shows how the atoms are bonded and each bond is represented by a single line. For present purposes, we will only be referring to covalent bonds unless otherwise stated; hydrogen bonds and ionic bonds can be put to one side for the moment. A single line represents a single covalent bond (or a σ (sigma) bond). A double or triple line represents, simplistically speaking, double or triple covalent bonds (or π (pi) bonds) respectively. In a skeletal formula:
(a) the hydrogen and carbon atoms are not illustrated, but the carbon atoms are represented by the intersection (or vertices and termini) of carbon chains;
(b) the lines themselves represent the bond connecting the carbon atoms; and
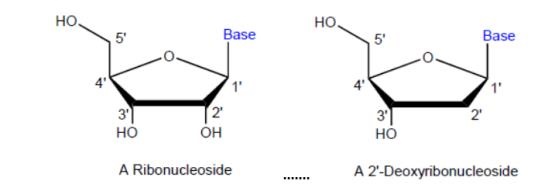
(c) any other non-hydrogen groups attached to the carbon atoms are illustrated.
22 Organic molecules can have the same molecular formula (i.e. have the same number of each type of atom) but may be different chemical substances; for example, the atoms are bonded to each other in a different order. Such molecules are called isomers. When two molecules have the same molecular formula but the atoms are connected differently, the molecules are known as structural isomers. Where two molecules have the same molecular formula and atoms are connected in the same way, but differ in their structural arrangement in space, the molecules are known as stereoisomers. Stereochemistry is concerned with the two-dimensional and three-dimensional shape of molecules. Enantiomers and diastereoisomers are two types of stereoisomers.
23 Because stereochemistry is an important concept in organic chemistry, a number of conventions have been devised in order to allow representation of 3D concepts on paper. The 3D stereochemistry of molecules can be illustrated on paper in 2D form by using the following generally accepted drawing conventions:
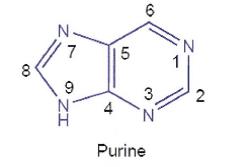
(a) a solid wedge indicates that the bond or group is projecting out of the plane of the page towards the observer;
(b) a broken hashed wedge indicates that the bond or group is pointing into the plane of the page away from the observer;
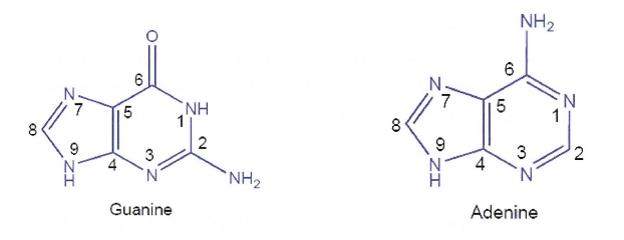
(c) a straight line indicates that the bond or group is in the plane of the page; and
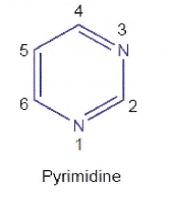
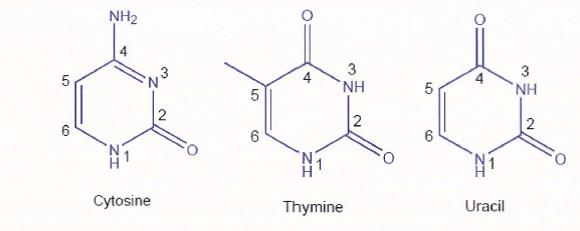
(d) a wavy (or wiggly) line indicates either: first, unknown or undefined stereochemistry; or second, a mixture of two stereoisomers but not necessarily a 50:50 mixture.
24 A “Haworth projection” is a common way of depicting a three-dimensional structure in two dimensions. Haworth projections are used to depict rings of atoms and provide an illustration of the stereochemistry of the groups attached to the ring. By convention, the thickened line at the bottom of a sugar ring indicates atoms which are coming out of the page towards the observer. In a Haworth projection the substituents are drawn in the “up” and “down” orientation, which illustrates that these substituents are either above or below the plane of the sugar ring, respectively. An example of a Haworth projection for glucose is illustrated below:
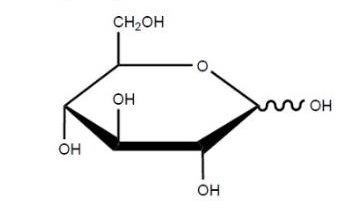
25 It is the common convention to show organic molecules without illustrating all of the C and H atoms, as illustrated above. An organic chemist well appreciates that both the C and H atoms are present even though they are not explicitly labelled as such. A Haworth projection for glucose and an example nucleoside showing the C and H atoms and their numbering conventions is illustrated below:
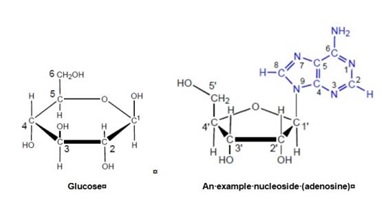
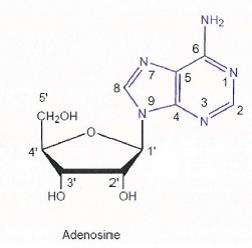
26 Carbon atoms on a sugar are numbered consecutively starting with number 1 which is assigned to the most highly oxidised carbon. In nucleoside chemistry, the numbers of the carbons of the sugar are designated using the symbol (') termed “prime”, and the positions on the nucleobase are numbered using non-prime numbering. Prime numbering can be written in a number of accepted ways including 1', C1', 1'C and 1'-C. The above diagram illustrates the different numbering systems used for carbohydrates (using glucose as an example) and nucleosides (using adenosine as an example).
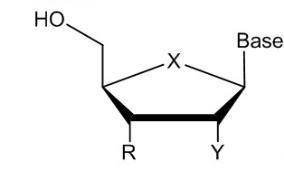
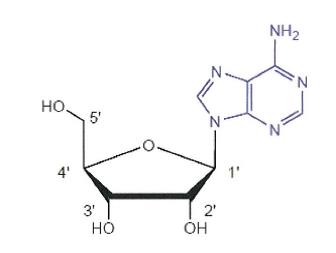
27 A Markush structure illustrates a group of compounds with common features, together with each of the variable groups which are typically labelled as R or other letters of the alphabet. Chemical elements may also be depicted such as X, Y and Z. A Markush structure can also be expressed in a Haworth projection to illustrate the stereochemistry features of the compounds of the Markush structure. A Markush structure in a Haworth projection for a nucleoside is illustrated below:
Shapes of molecules
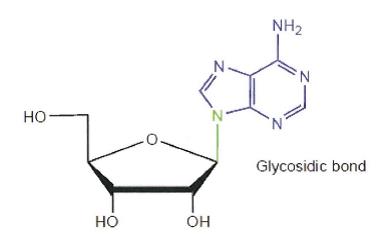
28 Where a carbon atom is bonded to four other atoms by single bonds, as illustrated below, the groups attached to the carbon atom are arranged in a tetrahedral arrangement that is 3-dimensional.
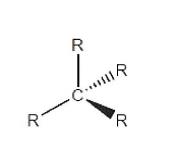
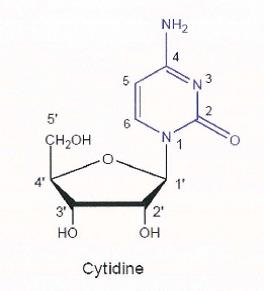
29 Where two carbon atoms are bonded to each other by a double bond, as illustrated below, the groups attached to the carbon atom are arranged in a planar arrangement that is 2-dimensional.
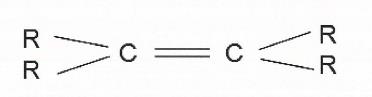
30 Where two carbon atoms are bonded to each other by a triple bond, as illustrated below, the groups attached to the carbon atom are arranged in a linear arrangement that is 1-dimensional.

Stereochemistry and chirality
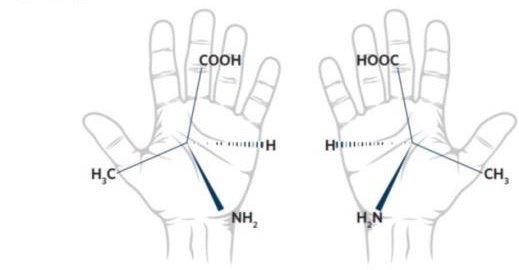
31 The concept of chirality is important to organic chemistry and stereochemistry. The word “chiral” is a Greek term meaning “handed”. A chiral molecule is a molecule that has a non-superimposable mirror image, analogous to the left and right hands. These forms are typically referred to as enantiomers. The figure below illustrates the enantiomers of the amino acid alanine which has one chiral carbon. One structure is the enantiomer of the other. In nature, one enantiomeric form may be more prevalent than the other.
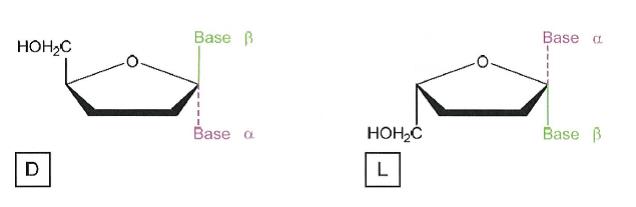
32 Carbon is a good example of an atom that in a molecular form can be chiral. A carbon atom can form four bonds. When a carbon atom has four groups attached to it through four single bonds, it is in a tetrahedral arrangement. If each of the four groups attached to the carbon atom through four single bonds are different, then the carbon is a chiral carbon (also known as a stereocentre), as illustrated below. Although carbon is one of the most common types of chiral atoms, other atoms, such as sulphur or phosphorus, which can also form four bonds to four different groups, can be chiral.
33 In the illustration above, the structure on the left is an example of a chiral molecule having four different groups attached to the central chiral carbon atom, wherein the CH3 group is projecting into the page, the OH group is projecting out of the page and the H atom and NH2 group (amine group) are in the same plane as the page. The structure on the right is not chiral because it does not have four different groups attached to the central carbon atom.
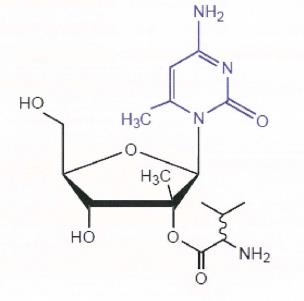
34 Many naturally occurring organic molecules are chiral in the sense that they have at least one chiral carbon. For example, amino acids, sugars and nucleosides are usually chiral molecules. A nucleoside is made up of a sugar and a nucleobase. Because the nucleobase is typically a 2D structure, the chirality of nucleosides and nucleotides and their analogues tends to reside in the sugar. In the nucleoside example illustrated below, each of the circled carbons are chiral carbons:
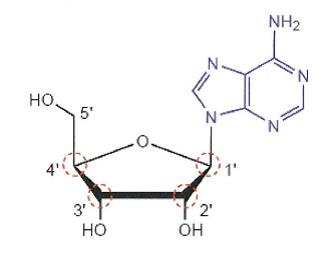
35 It is convenient at this point to make the following observations for illustrative purposes concerning the above diagram:
(a) First, the 1'-carbon is tetrahedral. It is also chiral because it is attached to four different groups: a nitrogen (N) within the nucleobase, an oxygen (O) (at the sugar ring oxygen position), a hydrogen (H) (at the 1' position in the “down” orientation which is not drawn) and a carbon (C) (at the 2' position).
(b) Second, the 2'-carbon is tetrahedral. It is also chiral because it is attached to four different groups: a hydroxyl (OH), a hydrogen (H) (at the 2' position in the “up” orientation which is not drawn), and two different carbons (at the 1' position and 3' position). Despite the 1'- and 3'- attachments both being carbon, the 1'-carbon and 3'-carbon are considered to be different carbons due to the different groups that are in turn attached to those carbons.
(c) Third, the 3'-carbon is tetrahedral. It is also chiral because it is attached to four different groups: a hydroxyl (OH), a hydrogen (H) (at the 3' position in the “up” orientation which is not drawn) and again two different carbons.
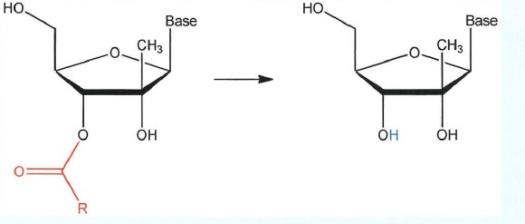
(d) Fourth, the 4'-carbon is tetrahedral. It is also chiral because it is attached to four different groups: an oxygen (O) (at the sugar ring oxygen position), a hydrogen (H) (at the 4' position in the “down” orientation which is not drawn) and again, two different carbons.
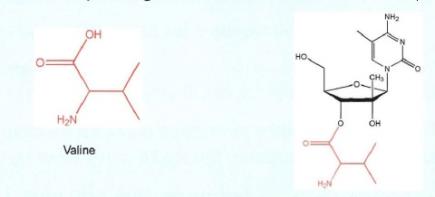
(e) Fifth, the 5'-carbon is tetrahedral but not chiral because it is not attached to four different groups. The 5'-carbon is attached to a carbon (C) (at the 4' position), a hydroxyl (OH), and two hydrogen atoms (H) (which are not drawn).
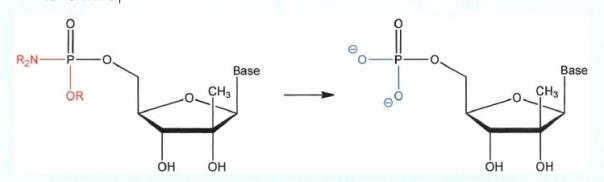
36 Two stereochemical configurations are possible for each chiral carbon atom. In most cases, the simple formula for calculating the total number of stereoisomers possible for any chiral molecule is 2N, where “N” is the number of chiral carbons. Using the diagram just discussed, a nucleoside containing four chiral carbons has 24 = 16 possible stereoisomers. A molecule such as a nucleoside having two or more chiral carbons can exist in a diastereoisomeric form (or as a diastereoisomer), being a particular type of one of these 16 possible stereoisomers.
37 Enantiomers have the same physical properties when analysed using non-chiral methods and can only be distinguished from each other in a chiral environment. The human body is an example of a chiral environment. Enantiomers rotate plane polarised light (light filtered to select only a single plane) to the same degree but in opposite directions as illustrated below. This ability to rotate plane polarised light is referred to as optical activity.
38 If a solution of a molecule fails to rotate plane polarised light, this could be due to a number of reasons. For example, the solution could contain non-chiral molecules or it could contain a racemate. A racemic mixture is a mixture that contains two enantiomers in equal proportions.
39 The stereochemical configuration of a chiral atom can also be communicated by the way the compound is named. A commonly used convention for describing the stereochemical configuration of a chiral atom is by using the letters R and S. One can determine whether a chiral atom is of an R or S configuration by ranking the four different groups attached to the chiral atom. Each group is ranked by the atomic number of the atom closest to the chiral carbon atom. The group with the highest atomic number is ranked first, the group with the second highest atomic number is ranked second, the group with the third highest atomic number is ranked third and the group with the smallest atomic number is ranked fourth.
40 The R and S configuration is then determined by orientating the molecule so that the group ranked fourth is pointing away from the observer. When orientated in this way, if the groups ranked 1, 2 and 3 travel in a clockwise direction the chiral centre is said to have an R configuration. If those groups travel in an anti-clockwise direction the chiral centre is said to have an S configuration.
41 The D and L terminology is another descriptor used in stereochemistry as it relates to a sugar or an amino acid. Designation of a sugar as D or L is based on the orientation of a particular substituent. In the context of a nucleoside, it is the 4' position which determines whether a nucleoside will be designated as D or L. If drawn as a Haworth projection and in the conventional orientation, when the substituent at the 4' position is orientated above the plane of the ring it is a D nucleoside, and when below the plane of the ring it is an L nucleoside.
Organic chemical reactions
42 There are many types of organic reactions that organic chemists take advantage of in order to synthesise new molecules. These reactions can be categorised into various classes including substitution reactions, elimination reactions and addition reactions.
43 In a typical substitution reaction, usually referred to as nucleophilic substitution and sometimes referred to as a displacement reaction, an atom or group of a molecule is replaced (substituted) with another atom or group. For example, one chemical atom or group attached to a tetrahedral carbon is replaced or substituted by another group. An example of a substitution reaction is the following:
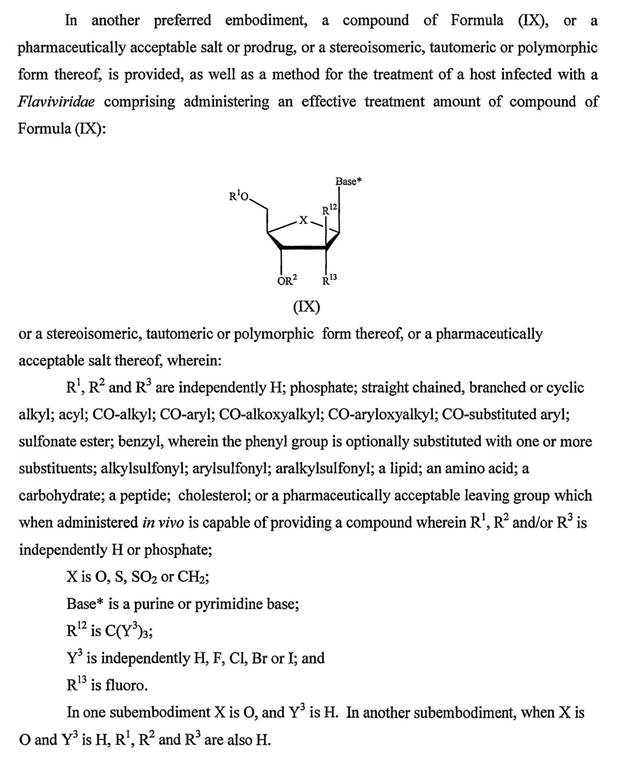
44 In a typical elimination reaction, a hydrogen atom attached to one carbon atom is removed by a base and a group on an adjacent atom is removed or eliminated. In elimination reactions, a new double bond is formed between the two participating carbon atoms. An example of an elimination reaction is the following:
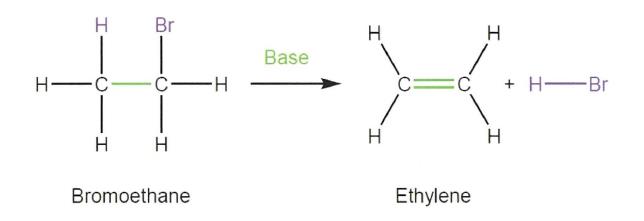
45 A typical addition reaction is the opposite of an elimination reaction. A carbon to carbon double bond is converted to a carbon to carbon single bond with an additional atom or group added to each of the participating carbon atoms. An example of an addition reaction is the following:
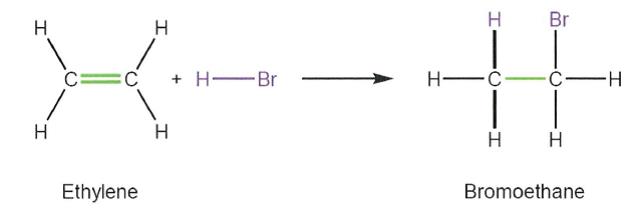
46 Nucleophilic substitution is a type of reaction between an electron donator (nucleophile) and an electron acceptor (electrophile). Nucleophiles can be described as electron-rich molecules. Because of their excess of electrons, they tend to react with electron-poor molecules and are therefore described as “nucleus loving”. Electrophiles are electron-poor molecules. Because of their lack of electrons, they tend to react with electron-rich molecules and are therefore described as “electron loving”.
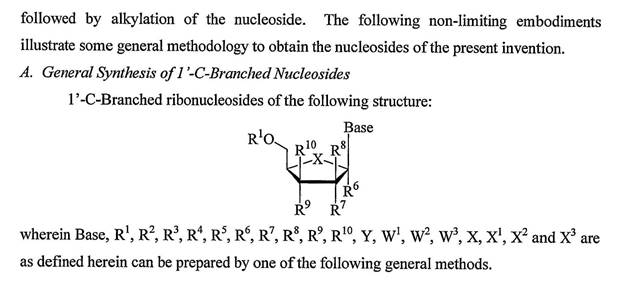
Organic synthesis
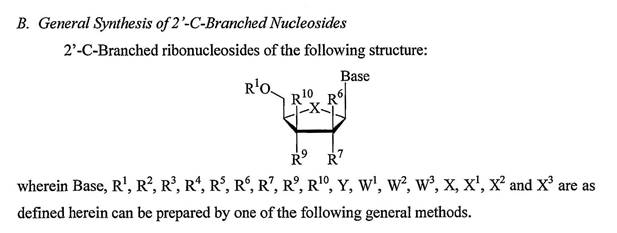
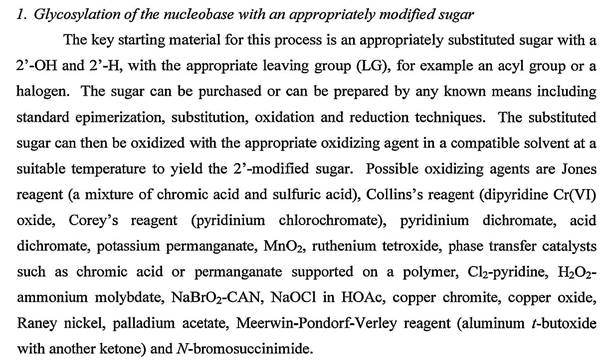
47 Generally speaking, synthesis is the process of using a series of chemical reactions to break down, build up or reconstruct molecules. Often starting with simple, commercially available materials, a series of controlled organic reactions or chemical transformations are used to turn those materials into a compound of interest. A reaction scheme is a standard tool used by organic chemists and represents a pictorial road map of the series of chemical reactions (i.e. reaction sequence) that are performed in a particular order to form a compound of interest. Reaction schemes are sometimes referred to as synthetic pathways or simply, schemes. In the laboratory, after each step in a reaction scheme it may be necessary to purify the intermediate product (that is, remove the product of interest from the surrounding reagents, solvents and other molecules which were used to perform the reaction step or were a by-product of it) before subjecting the product of interest to the next reaction step (purification) and also to use analytical chemistry techniques to characterise the intermediate and end product to confirm that the reaction step sought to occur did actually occur (characterisation). While in some cases, two or more synthetic steps may be carried out before purification and characterisation, this is less common.
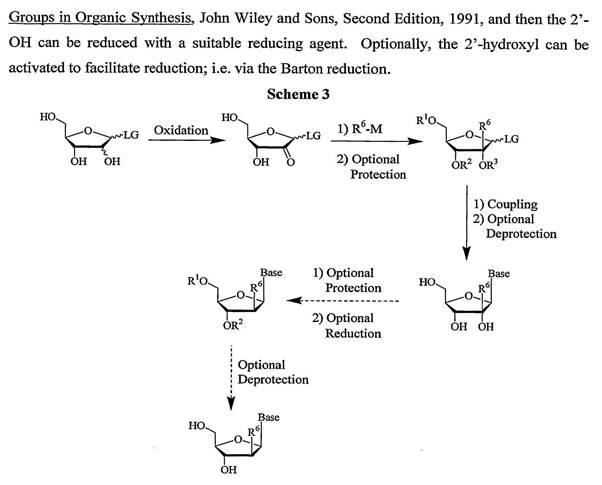
48 Reagents used in reaction schemes can selectively react with a particular group out of a number of chemically similar groups (chemoselective reagent) or particular molecules having a particular stereochemistry or react to give a particular stereochemistry (stereoselective reagents).
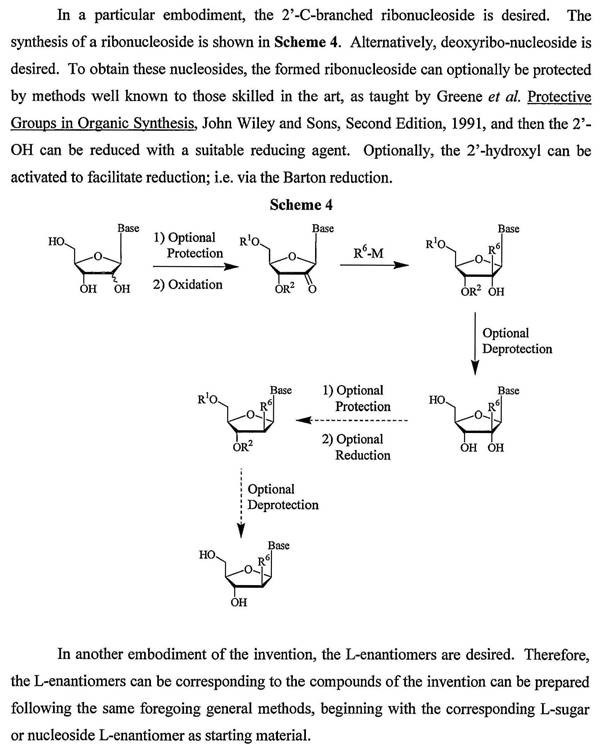
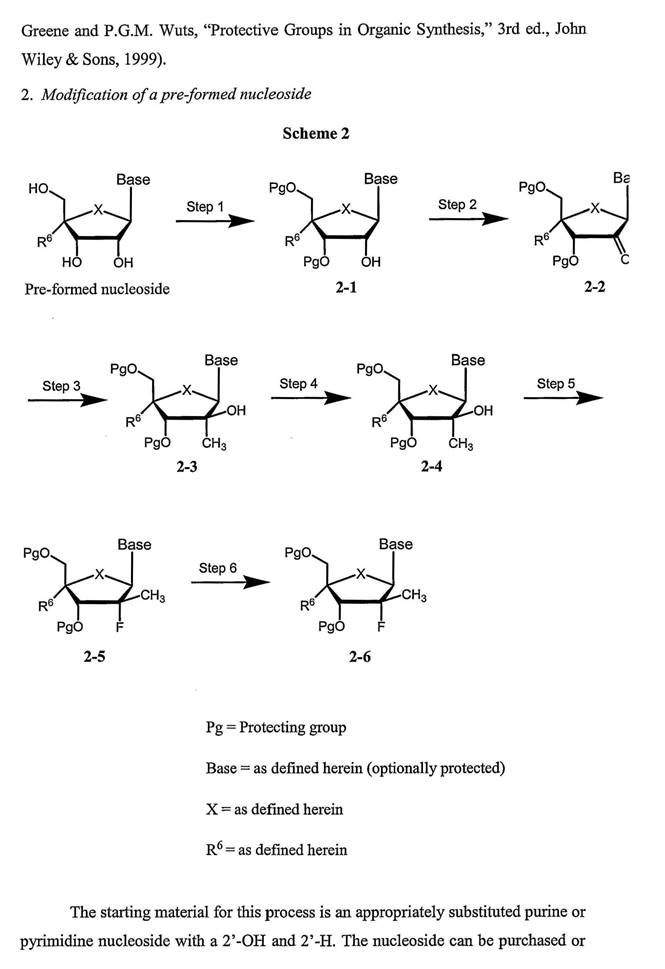
49 Analytical chemistry consists of qualitative and quantitative analysis. Qualitative analysis relates to the identification of a compound or substance in a given sample. Quantitative analysis relates to determining the relative amount or purity of a given sample. Some techniques used in analytical chemistry include spectroscopy and chromatography. Atoms and molecules absorb and emit different types of radiation in ways that are characteristic of their composition and structure. Spectroscopy is the science of using the absorption and emission of radiation by atoms and molecules to determine the structure of molecules or elements present. Chromatography describes a class of techniques used to separate different components of a mixture generally using a solid matrix and a fluid (or mobile) system. For the moment it is not necessary to delve into the detail of liquid or gas chromatography
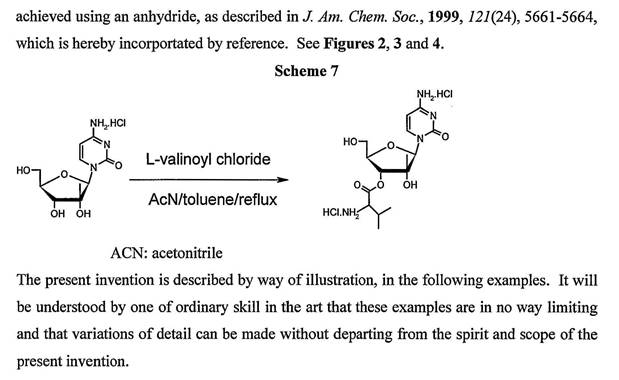
(b) Amino acids, peptides and proteins
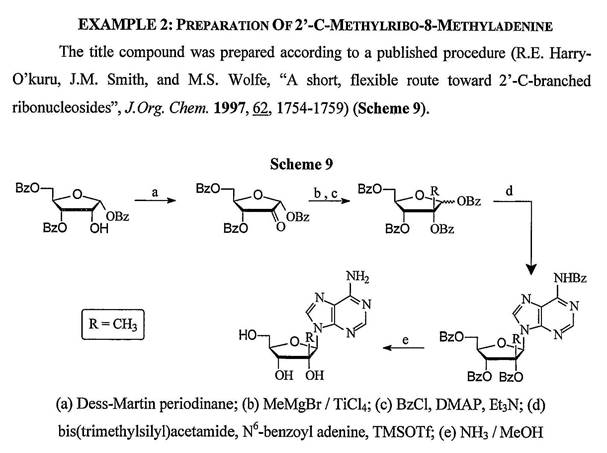
50 Amino acids are compounds that have a carboxyl group (-COOH, where C is carbon, O is oxygen and H is hydrogen) bound indirectly to an amino group (-NH2 where N is nitrogen and H is hydrogen) and a side chain (commonly referred to as R). Amino groups and carboxyl groups are examples of functional groups. The basic structure of a so-called α-amino acid can be represented pictorially as follows:
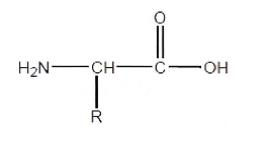
51 The amino acid shown above contains a central carbon (known as an alphacarbon) to which is bonded a hydrogen atom (H), an -NH2 group (amino group) and a -COOH group (carboxyl group). The remainder of the molecule is a variable group represented by the letter R. In this case R refers to a side chain group of the amino acid. It is important to note that each amino acid has a different side chain.
52 There are 20 different amino acids. Four nucleotides encode for these acids. DNA is read in blocks of three sequential nucleotides known as codons. Each codon codes for a particular amino acid, but most amino acids can be encoded by more than one codon. Each amino acid can be referred to by a single letter abbreviation or a three letter abbreviation of its name. Amino acids are the building blocks of peptides and proteins in nature. Peptides, polypeptides and proteins are chains of amino acids joined together by peptide bonds. Generally speaking, the order in which amino acids are connected to each other in a chain determines the function of that protein. A peptide bond is formed by the carboxyl group of one amino acid bonding to the amino group of a second amino acid. Accordingly, in a chain of amino acids, one of the terminal (or end) amino acids will have a “free” (unbound) amino group, with the amino acid at the other end of the chain having a “free” carboxyl group. The amino-group end of a protein, peptide or polypeptide is called the N-terminus and the carboxyl-group end of the protein, peptide or polypeptide is called the C-terminus. For illustrative purposes, a peptide bond formed between the amino acids, serine and glutamic acid is shown below:
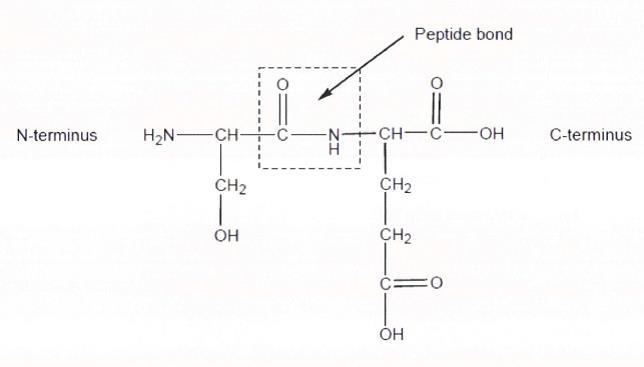
53 The terms protein and polypeptide are frequently used interchangeably, with the term protein more commonly used for a naturally occurring polypeptide. Proteins can be broken down or cleaved into fragments which may be termed peptides or polypeptides. Each of these fragments can be further broken down into their individual amino acids. Peptides, polypeptides and proteins can also be made by chemical synthesis or by recombinant methods.
(c) Nucleosides and nucleotides
54 Nucleoside chemistry refers to the study, manipulation and use of a class of molecules known as nucleosides. The term nucleoside chemistry is also colloquially used to describe the study, manipulation and use of either nucleosides or nucleotides.
55 A nucleoside is a chemical compound which is made up of two parts: (a) a heterocyclic base, which is typically called a base or more precisely, a nucleobase (both terms can be used interchangeably); and (b) a sugar, for example, ribose in ribonucleic acid (RNA) or a 2'-deoxyribose in deoxyribonucleic acid (DNA). The nucleobase and the sugar are linked by a glycosidic bond. A nucleotide is a chemical compound made up of three parts: (a) two parts consist of the nucleoside; and (b) the third part consists of a phosphate group replacing the hydrogen on the oxygen atom attached to the 5' carbon. For present purposes, we will in the main refer to nucleosides.
56 The structures of a naturally occurring ribonucleoside and deoxyribonucleoside (specifically a 2'-deoxyribonucleoside where there is no hydroxyl group on the 2' carbon on the sugar ring) are illustrated below:
57 A nucleobase is a core component of a nucleoside and a nucleotide. Nucleobases naturally occurring in 2'-deoxyribonucleosides and 2'-deoxyribonucleotides are adenine (A), guanine (G), thymine (T) and cytosine (C). Nucleobases naturally occurring in ribonucleosides and ribonucleotides are adenine (A), guanine (G), cytosine (C) and uracil (U) (instead of thymine which is present in 2'deoxyribonucleosides and 2'-deoxyribonucleotides only).
58 Positions on the nucleobase are distinguished from those on the sugar ring in a nucleoside by adopting non-prime numbering for the nucleobase and are defined according to agreed conventions. The nucleobases just mentioned fall into two groups: purine and pyrimidine.
59 By definition, a purine is a 5-membered ring fused to a 6-membered ring, with each ring containing 2 nitrogen atoms, wherein the nitrogen and carbon atoms are arranged such that:
(a) in the 6-membered ring the two nitrogen atoms are at the 1 and 3 position;
(b) in the 5-membered ring the two nitrogen atoms are at the 7 and 9 positions; and
(c) the carbon atoms are at the 2, 4, 5, 6 and 8 positions, as illustrated below:
60 Guanine (G) and adenine (A) are purines. The structure of guanine and adenine nucleobases are illustrated below:
61 By definition, a pyrimidine is a single 6-membered ring, which contains 2 nitrogen atoms wherein the nitrogen atoms are at the 1 and 3 position in a so-called 1, 3-relationship, and the carbon atoms are at the 2, 4, 5 and 6 position, as illustrated below:
62 Thymine, cytosine and uracil are pyrimidines. The structure of cytosine (C), thymine (T) (present in DNA only) and uracil (U) (present in RNA only) nucleobases are illustrated below:
63 Modifications can be made to the naturally occurring nucleobases to produce a nucleoside analogue or nucleotide analogue when the modified nucleobase is linked to the sugar, which itself may also be modified.
64 The sugar component of a nucleoside or nucleotide is a cyclic sugar. The sugar component of a natural ribonucleoside or ribonucleotide is ribose which is a 5 carbon sugar. The sugar component of a natural deoxyribonucleoside or deoxyribonucleotide is deoxyribose, specifically a 2'-deoxyribose which indicates (as we have already said) that no hydroxyl is present at the 2' position.
65 The ring of a ribose contains 4 carbon atoms and one oxygen atom. As noted earlier, the carbon atoms of the sugar in a nucleoside are identified using prime numbering and the 1'-carbon is known as the anomeric carbon. The remaining carbons on the sugar are then numbered consecutively in a clockwise manner according to the well-defined numbering conventions used by organic chemists, and as shown below for the nucleoside adenosine.
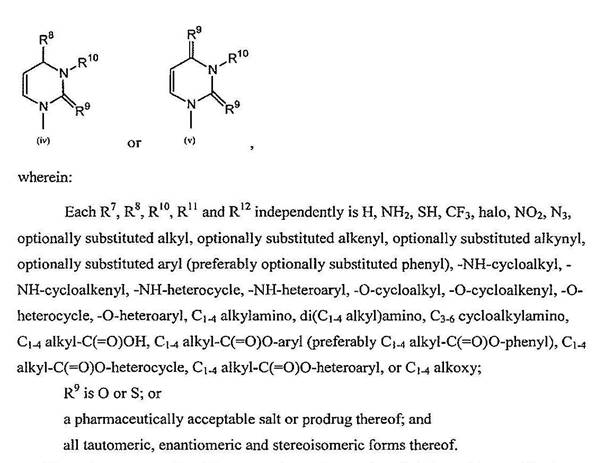
66 The oxygen atom naturally present in a sugar ring and commonly drawn at the top of chemical structures for the sugar ring is referred to as the ring oxygen.
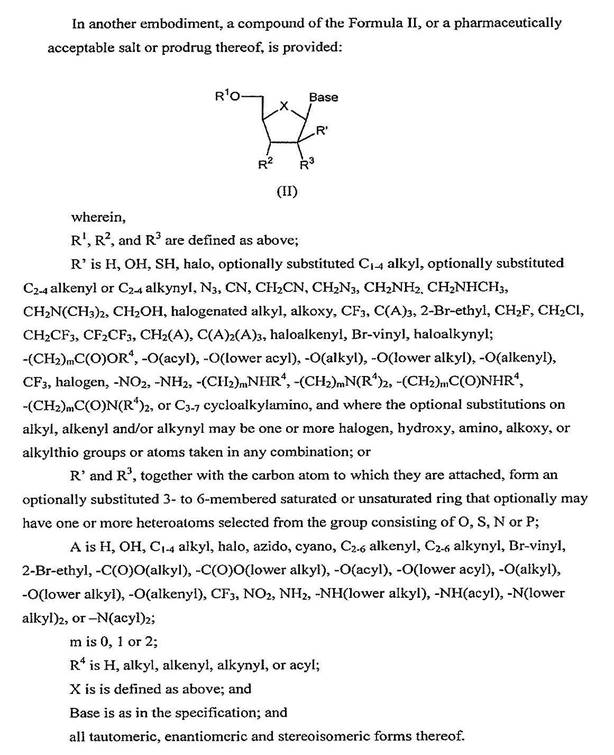
67 As illustrated below, the sugar and nucleobase of a nucleoside are connected by a chemical linkage referred to as a glycosidic bond. In the laboratory, the process used to create a glycosidic bond and thereby attach the sugar to the nucleobase is known as glycosylation. Glycosylation is a general term used to describe the attachment of a sugar to another molecule. Nucleosides and nucleotides and their analogues, in which the nucleobase is attached by a nitrogen (N) atom to the anomeric carbon (1'-carbon position) of the sugar ring (a N- C linkage), are referred to as N-nucleosides.
68 When the nucleobase is a naturally occurring pyrimidine (cytosine, thymine or uracil), the 1'-carbon of the sugar attaches to the nitrogen at the 1 position on the nucleobase which is referred to as N1, as illustrated below for cytidine:
69 When the nucleobase is a naturally occurring purine (guanine or adenine), the 1'-carbon of the sugar attaches to the nitrogen at the 9 position on the nucleobase which is referred to as N9, as illustrated below for adenosine:
70 The nucleobase and sugar can also be connected via a carbon-carbon linkage. That is, the 1'-carbon of the sugar and a carbon in the nucleobase. These nucleosides are referred to as C-nucleosides. It is not necessary to illustrate such an arrangement.
71 Modifications can be made to the naturally occurring sugar to produce a nucleoside or nucleotide analogue. As discussed, adenine, guanine, thymine, cytosine and uracil are naturally occurring nucleobases. The corresponding nucleosides containing adenine, guanine, thymine, cytosine and uracil nucleobases are referred to as adenosine, guanosine, thymidine, cytidine and uridine, respectively.
72 Generally speaking, the stereochemical configuration of nucleosides is described using D and L terminology and alpha (α) and beta (β) terminology. The categorisation of a nucleoside as D or L is determined by reference to the substituent (or group) at the 4' position. The α and β terminology refers to the orientation of the nucleobase at the 1' position relative to the orientation of the substituent (or group) at the 4' position. As illustrated below, where the nucleobase and substituent at the 4' position (which is CH2OH in the example above) are on the same side of the ring, a nucleoside is classified as a β-nucleoside. If they appear on opposite sides of the ring, a nucleoside is classified as an α-nucleoside.
73 Although the α and β terminology is based on the orientation of the nucleobase relative to the substituent on the 4' position, the α and β terminology is sometimes loosely used to describe whether substituents at any position on the sugar are on the α-face or β-face of the sugar ring.
74 The α and β terminology and the D and L terminology can be used together to describe the orientation and stereochemistry of nucleosides. For example in the illustration below, a nucleoside chemist understands that the name 2'-D or L-valine ester of β-D-2', 6-dimethyl-cytidine indicates the following:
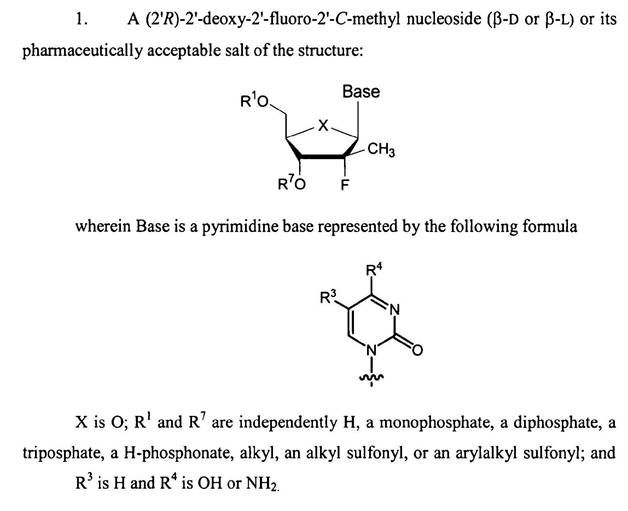
(a) The compound is derived from the nucleoside cytidine. The β-D indicates that the stereochemistry of the nucleoside is of a particular configuration: in this case it is in the configuration of the natural nucleoside.
(b) There are two methyl groups in the nucleoside: one at the 2' position on the sugar and one at the 6' position of the cytosine nucleobase.
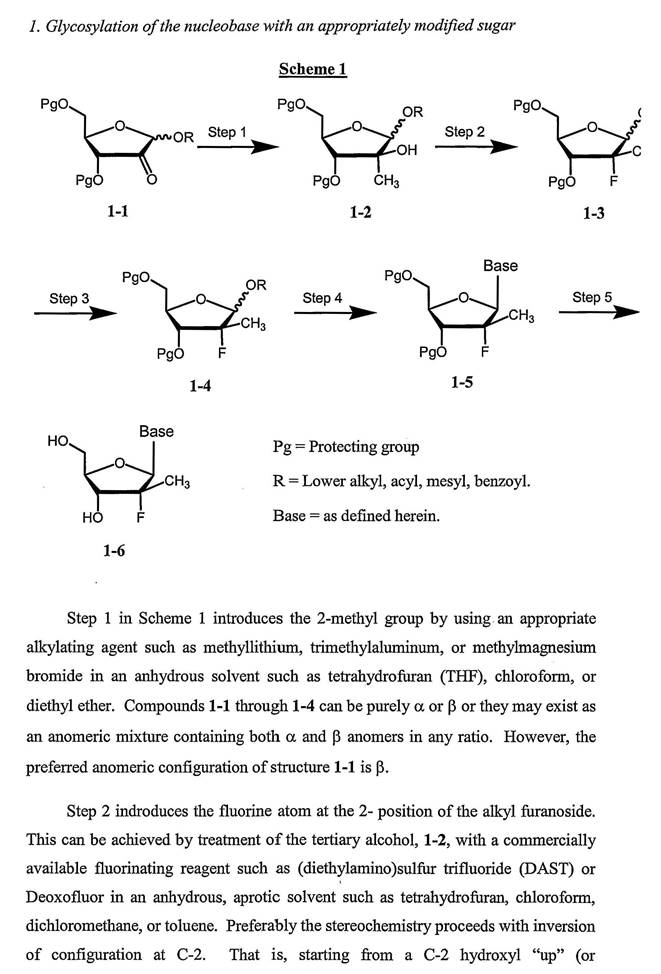
(c) The OH group normally present at the 2' position has been converted to a valine ester. The reference to 2'-D or L valine indicates that the stereochemistry of the valine ester is unspecified. That is, the valine may be in the D or L configuration.
(d) The use of nucleosides and nucleotides as antiviral drugs
75 In order to appreciate the invention claimed in the Idenix patent, it is convenient to now make brief mention of the use of nucleosides and nucleotides as antiviral drugs. We have conveniently drawn from the primary judge’s helpful description at [84] to [91] of her reasons.
76 HCV is a single-stranded RNA virus that infects human liver cells. HCV replicates using the intracellular machinery of its host cell. One of the key processes in the replication of HCV is the making of multiple copies of the HCV RNA. The enzyme responsible for this process is a viral RNA dependent- RNA-polymerase called NS5B.
77 Nucleoside analogues can act as antiviral drugs. They can do so by the inhibition of the relevant viral polymerase. In order to do so, the nucleoside analogue must be recognised by the relevant enzymes involved in the processes of phosphorylation (to be converted into the active triphosphate form) and RNA synthesis (to be incorporated into a growing RNA chain) so as to be a suitable substrate for those enzymes. The nucleoside analogue, once incorporated into the RNA chain, disrupts the growth of the chain. This results in chain termination. In the case of HCV, the nucleoside analogue (in the triphosphate form) must be recognised by NS5B as a substrate to be incorporated into the growing RNA chain.
78 If the nucleoside analogue does not possess a 3'-hydroxyl group, chain termination results because an “incoming” nucleotide cannot be attached to the RNA chain. But if the nucleoside analogue does possess a 3'-hydroxyl group, chain termination can still occur if the modification(s) to the nucleoside analogue prevents the formation of a 3',5'-phosphodiester bond with an incoming nucleoside. For example, the modification(s) may alter the conformation (shape) of the sugar ring so that the 3'-hydroxyl group is not correctly positioned for the formation of a 3', 5'-phosphodiester bond. Alternatively, the modification(s) may sterically hinder the formation of a 3',5'-phosphodiester bond.
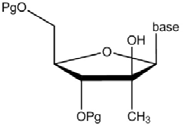
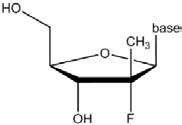
79 An example of a prodrug modification at the 3' position of a nucleoside is the addition of an acyl group. An acyl group is comprised of a carbonyl (C=O) attached to a carbon-containing group (R). When an acyl group is bonded to the oxygen atom of an hydroxyl group, the new group formed is called an ester. The structure of a 2'-methyl-“up” nucleoside analogue with a 3'-acyl (or 3'-ester) prodrug modification is shown below. This is an example of a nucleoside prodrug. After metabolism in the human body, the 2'-methyl-“up” nucleoside analogue is formed.
80 A specific example of an ester prodrug modification is the addition of valine. Valine is a naturally-occurring amino acid. As already explained, amino acids contain both an amino group (NH2) and a carboxylic acid group (COOH). The structures of valine and a nucleoside analogue with a 3'-valine prodrug modification are shown below.
81 An example of a prodrug modification at the 5' position of a nucleoside is the addition of a phosphoramidate group. A phosphoramidate group is comprised of a phosphorus atom attached to an oxygen atom via a double bond, two oxygen atoms via single bonds and a nitrogen atom via a single bond. The structure of a 2'-methyl-“up” nucleoside with a 5'-phosphoramidate prodrug modification is shown below. This is an example of a nucleotide prodrug. After metabolism in the body, the 2'-methyl-“up” nucleoside monophosphate analogue is formed.
THE PATENT SPECIFICATION
82 The specification begins by describing and incorporating by reference three earlier US provisional applications, two of which are the 350 application and the 949 application. We will elaborate on these two applications and their significance later in our reasons. The specification at page 1, line 9 to page 2, line 16 then describes the field of the invention and its background in the following terms:

83 In relation to the hepatitis C virus, the following description was given at page 3, line 22 to page 4, line 24:

84 In relation to some methods of treatment, page 7, lines 6 to 19 stated the following:
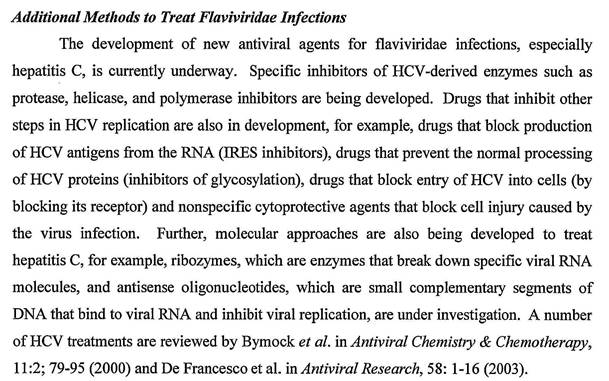
85 It was also said at page 9, line 27 to page 10, line 12:

86 The specification at page 12, lines 11 to 32 set out a summary of the invention in the following terms:

87 A more detailed description was given commencing at page 41, lines 20 to 30, the opening paragraph of which stated:

88 Various embodiments including preferred embodiments were then set out, including the following at page 91, line 19 to page 92, line 33:

89 We would note at this point that pages 91 and 92 of the 350 application include similar passages.
90 The specification at page 100, lines 6 to 29 also stated the following:
91 As we have said earlier, page 91 of the 350 application also described the compound of Formula (IX).
92 On pages 103 to 107 of the specification there are various definitions given for functional groups and other terminology. One that we would note at this point (page 104, line 14) is stipulated in the following terms: “The term ‘halo’, as used herein, includes chloro, bromo, iodo and fluoro”. It is also to be noted that there is no definition at this point for “base” (there are definitions elsewhere that define it to mean “purine or pyrimidine base”) or “nucleoside base” or reference to the adjective “natural” in combination therewith, although there is a discussion (page 104) of “purine” and “pyrimidine” bases.
93 At page 119, line 30 to page 120, line 7 of the specification, a section describing processes for preparation commenced with the following:

94 More relevant to the issues that concern us (as will become apparent later) is the synthesis of 2'-C-branched nucleosides with two general methods being discussed. Reference was made at page 122, line 21 to page 123, line 14 to the following:
95 This section continued at page 123, line 31 to page 124, line 5 in the following terms:

96 Thus far we have referred to what the specification described as glycosylation of the nucleobase with an appropriately modified sugar. The specification then goes on to talk about (page 124) the modification of a pre-formed nucleoside (still in the context of the discussion beginning on page 122 as to the general synthesis of 2'-C-branched nucleosides). In relation to that part of the specification dealing with the modification of a pre-formed nucleoside, the following was said at page 125, lines 7 to 20:
97 The specification at page 131, line 15 to page 132, line 9 also discussed the general synthesis of certain prodrugs in the following terms:

98 The specification describes 26 examples beginning with the discussion of Example 1 on page 132. What should be said at the outset by us is that 24 of the 26 examples purportedly set out examples for the preparation of relevant compounds and in that sense can be taken to describe methods for the synthesis of particular compounds. But what is noteworthy at this point is what is not said. There is no express disclosure of the 8 steps reaction scheme pathway to produce a compound within claim 7 (with the 2' position methyl “up” and the 2' position fluorine “down”) as propounded by Idenix both before the primary judge and before us, let alone the precursor identified by counsel for Idenix. At trial, emphasis seems to have been placed before her Honour on Example 2. Example 2 at page 135 is described as follows (in part):
99 It should readily be apparent that this is not a synthesis to produce a fluorinated 2' carbon with the required stereochemistry as stipulated for a compound falling within claim 7. Indeed it is a ribo-nucleoside rather than a de-oxyribo-nucleoside. Moreover, Scheme 9 (as is described) has the nucleobase added as step (d). Idenix’s 8 steps synthesis propounded to her Honour and us starts with step 1 as beginning from a “natural nucleobase”, whether purchased or synthesised.
100 The specification at page 157, lines 11 to 16 concludes (before the claims) with the following general statement:

101 The claims are set out commencing from page 158. It is only necessary to set out claims 7 to 13 at pages 189 to 190 which are in the following terms:

Earlier applications
102 As we have already identified, the Idenix patent claims (page 1) the benefit of priority from:
(a) US provisional application No. 60/392,350 (the 350 application) – priority date 28 June 2002; and
(b) US provisional application No. 60/470,949 (the 949 application) – priority date 14 May 2003.
103 Indeed the Idenix patent states (page 1, line 8) that “the disclosures of each … are incorporated herein by reference”. However, apart from this introductory statement in the specification, nothing further is said. It is necessary to elaborate further at this point on both the 350 application and the 949 application as they are relevant to later arguments that we will discuss concerning the issues of, inter-alia, external fair basis and internal fair basis.
(a) The 350 application
104 The primary judge discussed the 350 application at [280] to [391] of her reasons. We would incorporate by reference her Honour’s description as to what that application consists of and discloses, without the need to set it out.
105 Idenix’s contention before her Honour was that the invention claimed in claim 7 and dependent claims of the Idenix patent was in fact disclosed in Formula (IX) of the 350 application (see pages 91 and 92).
106 There is little difference between the description concerning Formula (IX) on pages 91 and 92 of the 350 application and the description at page 91 of the Idenix patent save in minor respects. For example, in the Idenix patent, the words before the three formulae are expressed differently with reference to “a pharmaceutically acceptable salt or prodrug”, although these words are after the formulae in each case in any event. But in the Idenix patent, reference is also made to stereoisomeric, tautomeric or polymorphic forms above and below the formulae. Further, there are references immediately after the formulae in the Idenix patent to “R4, R5, Y3 …and X* … as defined above”. But none of these differences are material for present purposes.
107 Now there is little doubt (and Gilead so conceded before her Honour) that the structural formula of Formula (IX) in the 350 application covers a compound within claim 7 of the Idenix patent. But the question for her Honour was whether there was a real and reasonably clear disclosure in the 350 application of a compound (fitting within claim 7 of the Idenix patent) where:
(a) The substituent at the 2' down position was fluorine;
(b) The base may be a natural base; and
(c) The compound was a 5'-only prodrug.
108 Her Honour held (at [329]) that there was a real and reasonably clear disclosure in the 350 application of a compound with fluorine in the 2' down position.
109 But her Honour held (at [345]) that there was no real and reasonably clear disclosure in the 350 application of a relevant compound with a natural base (as compared with a modified base).
110 Further, her Honour held (at [385]) that there was no real and reasonably clear disclosure in the 350 application of a relevant compound with a prodrug at the 5' position only.
111 We will leave for the moment passages from the 350 application dealing with the “base” question. As for the 5'-only prodrug question, we would note the following.
112 First, in the opening section (page 1) there is, in relation to the invention, a description of a “2' and/or 3' prodrug”.
113 Second, on pages 8-13 (before the first principal embodiment is discussed) there is reference to the following:
“2' and 3'-prodrugs”;
“2' or 3'-prodrug”;
“2'-prodrug [which] includes biologically cleavable moieties at the 2' and/or 5' positions”; so the option appears to be either a 2'-prodrug or a 2' and 5'- prodrug when read in context;
a “3'-prodrug [which] includes biologically cleavable moieties at the 3' and/or 5' positions”; so the option appears to be either a 3'-prodrug or a 3' and 5'-prodrug when read in context; and
other types being 2', 3' and 5' prodrugs.
114 We say in context, for although one grammatical reading of “and/or” where it appears might suggest a 5'-only prodrug, when you read the multitude of examples it is well apparent to us that there is no real and reasonably clear disclosure of a 5'-only prodrug. So much is apparent from the other passages in the 350 application. Indeed, parts of the 350 application point away from a 5'-only prodrug. So, for example at page 39, lines 15 to 28 the following is said:
The invention as disclosed herein is a compound, a method and composition for the treatment of a Flaviviridae infection in human and other host animals. The method includes the administration of an effective anti-Flaviviridae treatment amount of a 2' and/or 3'-prodrug of a 1', 2', 3' or 4'-branched β-D or β-L nucleoside as described herein or a pharmaceutically acceptable salt thereof, optionally in a pharmaceutically acceptable carrier. The compound of this invention either possesses antiviral (i.e., anti-HCV) activity, or is metabolized to a compound that exhibits such activity.
The 2' and/or 3'-prodrugs of a 1', 2', 3' or 4'-branched β-D or β-L nucleoside are acyl derivates of a secondary or tertiary alcohol alpha to a secondary or tertiary carbon. Due to the steric hindrance of these prodrugs over the 5'-prodrugs, an acyl derivative of a primary alcohol, these prodrugs differentially modulate the biological properties of the molecule in vivo. It has been discovered that the 2' and/or 3'-prodrugs of a 1', 2', 3' or 4'-branched β-D or β-L nucleoside can provide a drug with increased half-life and improved pharmacokinetic profile.
115 Further, if one considers the summary of the invention (pages 41 to 43), there is in our view no disclosure of a 5'-only prodrug. The summary is replete with references to a “2' and/or 3'-prodrug”. Further, it may be noted that there was a Table in evidence before her Honour (annexure RHF-12 to one of the affidavits of Professor Furneaux) well demonstrating that when one analysed all of the Examples 1 to 24, there was no 5'-only prodrug exemplified, whether alone or in combination.
116 Finally, we would make the point that various passages of the application are dealing with modified bases. For example, see page 7, lines 19 to 26, the bases described from page 48, reference to phraseology such as “Base is as defined herein” (page 57, line 21 and page 91, line 8) and Figure 1 (after page 5297 – that page reference indicating the sheer length of the application and the many permutations of the compounds claimed).
(b) The 949 application
117 The 949 application claimed as an invention methods and compositions for the treatment of infections caused by a coronavirus, togavirus or picornavirus including administering an effective amount of nucleosides of the disclosed formula. It relevantly discloses for present purposes a composition of Formula (AA). We note that claim 1 of the 949 application is a method claim which comprises administering a nucleoside analogue that has the structure of Formula (AA) or a pharmaceutically acceptable salt or prodrug thereof.
118 It is appropriate to set out Formula (AA) as described at pages 17-19 as follows:


119 It is also appropriate to set out Formula (II):
120 We would also note in relation to Formula (II) that the expression “R3 [is] defined as above” refers back to page 20 which provides a very substantial list of potential substituents including “halo”, which can be taken to include “fluoro” or a fluorine atom.
121 Her Honour discussed the 949 application at [392] to [417] of her reasons.
122 It is well apparent that a compound meeting the description of claim 7 of the Idenix patent is within Formula (AA) and Formula (II) of the 949 application.
123 Gilead contended before her Honour that there was no real and reasonably clear disclosure in the 949 application of a compound of claim 7 or the use of such a compound to treat a flaviviridae infection as claimed in claim 10. Her Honour rejected such arguments. Gilead’s notice of contention that we will discuss later addresses them.
Clark patent
124 Various issues concerning the question of sufficiency, which we will come to in a moment, require discussion of the Clark patent.
125 Application AU2004253860 was filed on 21 April 2004 as a PCT International Patent application in the name of Pharmasset Ltd, with the inventor being Jeremy Clark. It has a priority date of 30 May 2003.
126 Under the “Summary of the Invention” on pages 15 and 16, the following is said:

127 Claim 1 is in the following form:
128 But methods of synthesis are also claimed. For example, claim 13 comprises a method of synthesising a nucleoside of claim 1 comprising glycosylating a relevant pyrimidine. Claim 14 refers to a method of such synthesis comprising selective deprotection. Claim 20 refers to a method of synthesis with reference to the Examples.
129 Section VIII of the Clark patent sets out (as it is headed) a detailed Synthetic Protocol (pages 70 to 87). The Section opens with the following statement:

130 Pages 72 to 76 are in the following form:


131 Then there are detailed worked examples. Example 1 describes in detail the synthesis to get the target compound starting with a carbohydrate. Example 2 details a synthesis to get the target compound starting with cytidine. Example 3 starts with chloropurine riboside. Example 4 starts with chloro-2'-deoxy-2'-fluoro-2'-C-methylpurine.
grounds of appeal 1 to 8; lack of sufficiency
(a) Preliminary matters
132 Section 40(2)(a) of the Act at the relevant time provided:
Specifications
…
(2) A complete specification must:
(a) describe the invention fully, including the best method known to the applicant of performing the invention …
133 Gilead contended before the primary judge that the Idenix patent did not sufficiently describe how to synthesise compounds falling within claim 7. It was said that claim 7 did not describe how to produce a compound (sometimes referred to in these reasons as the target compound) that contained fluorine at the 2' down position of the sugar ring of the compound and a carbon-containing group, such as a methyl group, at the 2' up position on that ring. It was said that this was not routine chemistry as at the priority date(s) claimed for the Idenix patent. It was said that both the starting point and the method of synthesis to produce the target compound were not sufficiently described in the specification.
134 Gilead also contended before the primary judge that the Idenix patent did not identify which of the compounds within claim 7 were effective for the treatment of flaviviridae infections or HCV and did not describe how to identify such compounds.
135 It was common ground before her Honour that a compound that is within claim 7 is the 2'-methyl-“up”-2'-fluoro-“down” nucleoside with the structure set out in the projection on the right below labelled “target compound”. It was also common ground that the immediate precursor for a fluorination reaction to produce the target compound is the 2'-hydroxy-“up”-2'-methyl-“down” nucleoside of the structure set out in the projection on the left below labelled “precursor compound”:
Precursor compound | Target compound |
|
|
136 The hydroxyl group (OH) in the precursor compound is attached to a carbon further attached to three carbons and accordingly is a tertiary OH. By a chemical reaction with a fluorinating reagent, the hydroxyl group can be displaced by fluorine and the relative position on the 2' carbon inverted as appears in the target compound, such that the methyl group on the 2' carbon is “up” (previously “down”) and the fluorine is “down” (the hydroxyl group displaced by fluorine being previously “up”).
137 It was accepted before the primary judge that the precursor compound in the projection above was a potential precursor for a fluorination reaction to produce the target compound. But it was disputed before her Honour whether the skilled person would choose such an approach or start with or obtain the relevant precursor compound (as contended by Idenix) to then attempt the fluorination reaction to obtain the target compound.
138 It appears to have been accepted by the parties before her Honour and before us that the Idenix patent does not expressly describe any synthesis of a claim 7 compound. All that it appears to relevantly state is that “[t]he nucleosides of the present invention can be synthesized by any means known in the art” (page 119 lines 31-32, set out at [93] of these reasons above) (at [527] of her Honour’s reasons). Accordingly, the skilled addressee was not given any express method for producing the target compound (as we have described), but rather was left to construe the Idenix patent in light of the common general knowledge to develop such a method, including the starting point, steps in the synthesis and necessary precursor compounds.
139 The principal issue before the primary judge on this aspect was whether at the priority date (putting to one side for the moment the dispute between the parties as to the precise date), a person skilled in the art who was armed with the Idenix patent and relying only upon common general knowledge could have synthesised a compound of claim 7 (relevantly, for present purposes, the target compound that we have described) without new invention or addition or a prolonged study of matters presenting initial difficulty.
140 Now it was accepted in the evidence before the primary judge that by reacting the precursor compound with the reagent diethylaminosulfur trifluoride (DAST) (where fluorination would proceed with displacement and inversion) or Deoxo-Fluor (a DAST analogue with a similar function), and subsequent de-protection, one could obtain the target compound. For simplicity, we will describe this as the DAST route or DAST for short-hand. But DAST is not referred to in the Idenix patent, whether in the part relating to synthesis or otherwise. Idenix contended before her Honour that regardless of this absence of reference in the Idenix patent, DAST was within the common general knowledge of the skilled addressee at the priority date. Moreover, Idenix contended that the absence of specific reference to DAST as a method of synthesis did not justify a finding that claims 7 to 41 of the Idenix patent were invalid for insufficiency. Contrastingly, Gilead contended that DAST was not within the common general knowledge, let alone for the synthesis necessary or desirable to produce the target compound. Accordingly, it contended that the relevant claims of the Idenix patent were invalid for insufficiency.
141 The primary judge found that claims 7 to 41 of the Idenix patent did not comply with s 40(2)(a) of the Act and were therefore invalid for insufficiency on the basis that the specification did not enable the skilled addressee to make a compound within claim 7 at the priority date without new invention, addition or a prolonged study of matters presenting initial difficulty. Idenix has challenged that finding generally (ground 1) and specifically (grounds 2 to 8) in, if we may say so, an overly elaborate way and with a view, it would seem, to having us conduct a re-trial of the complex factual questions litigated before her Honour who had the benefit of, inter-alia, considering and reflecting upon the complex expert testimony adduced before her.
142 Idenix has contended before us that the primary judge’s findings on the insufficiency question were incorrect for the following reasons:
(a) First, it says that the primary judge erred in failing to find that the Clark patent admitted and demonstrated that applying the DAST route was within the common general knowledge of the skilled addressee (ground 2 with 8 sub-paragraphs of particulars).
(b) Second, it says that the primary judge erred (grounds 3 & 4) in the following respects (detailed particulars omitted):
3. The primary judge erred in finding that each of the synthetic routes recorded in the Idenix Documents (both tried and contemplated) (with the exception of the February 2003 Deoxo-Fluor Route (the Deoxo-Fluor Route)):
(a) involved an exercise of the common general knowledge at the priority date; and
(b) were relevant to, and indeed provided “a very strong basis” and "powerful evidence" of, the insufficiency of the Idenix Patent,
including by failing to consider, on a route by route basis, whether any evidence specific to that route supported a finding that the particular route was part of the common general knowledge: Reasons at [448]-[458], [506]-[509], [512] and related findings at [535] and [605].
4. The primary judge erred in making findings based on inferences drawn from the Idenix Documents as to, and in relation to, the content of common general knowledge to be attributed to the skilled addressee of the Idenix Patent and the work recorded in the Idenix Documents.
(c) Third, it says that the primary judge erred in her treatment of the expert evidence in determining the content of the common general knowledge of the skilled addressee of the Idenix patent and relevant priority documents (ground 5 with 9 sub-paragraphs of particulars). In essence, Idenix would have us engage in the task that on one view requires Idenix to demonstrate that her Honour’s evidentiary findings were “glaringly improbable”, “contrary to compelling inferences” or shown to be wrong by “incontrovertible facts or uncontested testimony”: Robinson Helicopter Company Inc v McDermott (2016) 331 ALR 550; [2016] HCA 22 at [43]. If that is the standard, we would say now that Idenix in its submissions before us has not come anywhere near to satisfying or meeting such a threshold. Indeed, the preponderance of evidence before her Honour well justified her factual findings. But as we will discuss in a moment, a more nuanced position dealing with the appropriate standard for appellate review is to be applied concerning evaluative questions the subject of expert evidence involving matters of degree and judgment.
(d) Fourth, Idenix says that the primary judge erred (ground 6) in the following respect:
6. The primary judge erred in finding that the following facts or matters were not part of the common general knowledge of the skilled addressee of the Idenix Patent and the priority documents as at the relevant priority date and in making the findings underpinning those conclusions:
(a) the facts and matters referred to in the Reasons at [221]-[249] and [552];
(b) the publications and review articles listed in sub-paragraphs (a) to (d) and (f) to (o) of paragraph 138 of Professor Meier’s second affidavit: Reasons at [218], [389];
(c) the Barros Paper and the Yang Paper: Reasons at [598]; and
(d) the Hudlicky Paper: Reasons at [599].
(e) Fifth, it says that the primary judge erred in finding that the skilled addressee could not make a compound within claim 7 without new invention or prolonged study of matters on the basis of the instruction in the Idenix patent using only the common general knowledge (ground 8 with 23 sub-paragraphs of particulars).
(f) Sixth, it says that the primary judge erred in failing to accept that based on the results of the AMRI experiments, it could be inferred that the target compound was made using the Deoxo-Fluor route (ground 7 with 4 sub-paragraphs of particulars). It says that the techniques adopted in the AMRI experiments were techniques used in the field of organic chemistry prior to June 2002.
143 In terms of the principal themes of Idenix’s criticisms, Idenix says that the primary judge erred in her conclusion that the DAST route was not within the common general knowledge for the following reasons. First, it says that the primary judge wrongly assumed that sufficiency could not be satisfied by mere disclosure of the invention itself plus common general knowledge. Second, it says that the primary judge wrongly construed the Idenix patent as teaching the skilled addressee nothing about making a claim 7 compound (at [537]) and as teaching “away” from such a compound (at [538] to [541]). Third, it says that the primary judge wrongly treated as relevant and “determinative” the evidence of Idenix’s internal failures to synthesise the target compound, without evidence that those failed methods were common general knowledge methods available to the skilled addressee. Fourth, it says that the primary judge wrongly treated Professor Furneaux as personifying or embodying the relevant skilled addressee and paid insufficient regard to French CJ’s description in AstraZeneca AB v Apotex Pty Ltd (2015) 257 CLR 356 at [23]; we would say now that there is no substance in that criticism.
144 In our view, the primary judge’s reasons involved no error. As her Honour correctly concluded at [615], the disclosure of the Idenix patent, when considered with the common general knowledge, did not meet the requirement of s 40(2)(a) applying the relevant test for sufficiency as expounded in Kimberly-Clark Australia Pty Ltd v Arico Trading International Pty Ltd (2001) 207 CLR 1. The relevant test as framed by the High Court in Kimberly-Clark is (see at [25]): “… will the disclosure enable the addressee of the specification to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty?” But this is precisely the question that was posed and applied by her Honour.
145 The principal question raised by s 40(2)(a) is whether the disclosure of the Idenix patent enabled the skilled addressee to make a compound within claim 7 “without new inventions or additions or prolonged study of matters presenting initial difficulty”. In our view there was clear and compelling evidence before the primary judge justifying her finding that the requirement was not met. Indeed, part of the evidence before her Honour was material demonstrating that Idenix was unable to obtain a claim 7 compound despite having a team of skilled scientists and external expert consultants engaged in a prolonged attempt to do so for a period of nearly three years. Idenix was apparently unable to produce any embodiment of the invention that it had claimed without new inventions or additions or prolonged study of matters presenting initial difficulty, even after having (on its own argument) supposedly a sufficient description of that invention in hand. Moreover, in terms of relative timing, it would seem that Idenix only succeeded in making the target compound after it obtained the Clark patent, which described and thus enabled such a synthesis. Now of course none of these matters are determinative of the question that her Honour had to address, but they are hardly helpful to Idenix’s position. The primary judge was correct to give some weight to this evidence and to find that in conjunction with the other evidence, proof of insufficiency was established.
146 We would make another general observation at this point. The primary judge was engaged in an evaluative exercise involving matters of degree and judgment that, in the context of the manner in which the case was conducted, has an affinity with the test for inventive step, notoriously a “jury question”. It involved the court placing itself in the position of the hypothetical skilled person as at the priority date in 2002 or 2003 in order to determine whether or not something within each claim could be made without new inventions or additions or prolonged study of matters presenting initial difficulty. In that task she had to consider and weigh the expert evidence before her. Generally speaking, an appellate court should not interfere with findings made in such a setting unless they are erroneous in principle or plainly and obviously wrong (Metricon Homes Pty Ltd v Barrett Property Group Pty Ltd (2008) 248 ALR 364; [2008] FCAFC 46 at [20]) or where there is a sufficiently clear difference of opinion (Branir Pty Ltd v Owston Nominees (No 2) Pty Ltd (2001) 117 FCR 424 at [29] per Allsop J (as he then was, Drummond and Mansfield JJ agreeing)). Perhaps the latter may be considered a lower threshold, although in that respect we are inclined to adopt the observations in Optical 88 Ltd v Optical 88 Pty Ltd (2011) 197 FCR 67 (Cowdroy, Middleton and Jagot JJ) at [25] to [34]. But on any view, and accepting that we must come to our own conclusion, after giving due respect and weight to her Honour’s findings we do not have any or any sufficiently clear difference of opinion on the evaluative questions that her Honour considered and determined. It may not unfairly be said that Idenix has used the opportunity on appeal before us simply to “put the dice into the box for another throw” (Optical 88 Ltd at [29] and the cases there cited).
147 Before proceeding further, there is one matter that it is convenient to dispose of now. Gilead has submitted that it is notable that similar findings to those made by the primary judge have been made in other jurisdictions where Idenix’s related patents were said to have been challenged on equivalent grounds. These jurisdictions apparently included the United Kingdom, Germany, Norway, Canada and the European Patent Office. Reference was made, for example, to Idenix Pharmaceuticals, Inc v Gilead Sciences, Inc [2014] EWHC 3916 (Pat) at [470] to [594] per Arnold J and on appeal [2016] EWCA Civ 1089 at [141] to [192] per Kitchin LJ; our attention was also drawn to his Lordship’s observations at [209] but on a separate issue that we are not presently discussing. It is also said that consistent factual findings have been made in Gilead’s favour in proceedings in the United States. Whilst we note such submissions, we have put such jury points to one side; they are not probative of the issue that her Honour had to resolve on the evidence before her and that we need to consider.
148 It is convenient at this point to now deal with the specific attacks made by Idenix concerning her Honour’s findings by reference to the specific grounds of appeal (grounds 2 to 8). It is convenient to re-order these grounds as we have done in the following discussion.
(b) Teaching of the specification and common general knowledge (ground 8)
149 The primary judge found that to obtain the target compound, the skilled addressee would undertake a “retrosynthetic analysis” (at [612]). Such analysis identifies the reaction route to the target compound, including the precursor in the penultimate step. Idenix says that DAST and Deoxo-Fluor were the most commonly known means for displacing with inversion a secondary hydroxyl group to introduce fluorine in a nucleoside. We have emphasised secondary at this point to contrast with what we have in the present context being the need to displace a tertiary hydroxyl group. It is said that the primary judge’s finding that it was not known to use DAST or Deoxo-Fluor on a tertiary hydroxyl group at the priority date involved error (at [229] and [605]); we note that her Honour in fact said that it was not a commonly known method to fluorinate and invert a tertiary hydroxyl. In any event, Idenix contends that regardless of that finding, any perceived uncertainty as to the likelihood of success of using DAST or Deoxo-Fluor to introduce fluorine in place of a tertiary hydroxyl group was not evidence that the skilled addressee was unable to produce the target compound employing common general knowledge. It is said that such a method was used by Dr Borthwick and Mr Clark on a tertiary hydroxyl group and it was asserted that this was the method that occurred to Professor Furneaux if he could not use knowledge obtained from non-common general knowledge research. Idenix contends, with respect rather superficially in our view, that the suitability and application of the DAST method was the same (in terms of common general knowledge) whether it was a secondary or tertiary hydroxyl group.
150 In our opinion, Idenix’s assertion that DAST and Deoxo-Fluor were the most commonly known means for displacing with inversion a secondary hydroxyl to introduce fluorine in a nucleoside did not accurately reflect the primary judge’s finding that the process was unpredictable with complicating and competing reactions (at [230]) and that “there was no commonly known method of introducing fluorine into a nucleoside at the 2'-position at all” (at [605]). Generally, Idenix’s submissions failed to take proper account of a range of relevant considerations including whether the proposed fluorination was of a secondary or tertiary hydroxyl group, whether the compound in question was a natural nucleoside or carbocyclic nucleoside (where a carbon atom has been substituted for the oxygen atom of the five-membered otherwise sugar ring), and also the position on the ring to be fluorinated. Just for completeness, we would note at this point that there was evidence before her Honour suggesting that a carbocyclic nucleoside was more stable as it was not subject to the action of nucleoside phosphorylase and hydrolase enzymes, which cleave the glycosidic linkage of natural nucleosides.
151 Idenix takes issue with what it says to be the primary judge’s reasoning that the skilled addressee would undertake a research project before attempting to synthesise the target compound and that such a research project necessarily involved non-common general knowledge material. Idenix says that the primary judge failed to address the question of whether the skilled addressee could make the target compound if confined to the common general knowledge. Idenix says that if the person skilled in the art was confined to common general knowledge, they could have employed known means, namely DAST or Deoxo-Fluor, to the precursor compound with a tertiary hydroxyl, regardless of any doubts. Idenix contends that, in essence, the gravamen of the primary judge’s reasoning was that it was “inventive” to try using DAST or Deoxo-Fluor on a tertiary hydroxyl (at [612]), which it says is a conclusion that was not supported on the evidence.
152 Idenix contends that the primary judge’s finding that the precursor could not be made by the skilled addressee using common general knowledge (at [612]) involved error and could not stand with evidence to the contrary. It is said that this finding was also inconsistent with her Honour’s findings that by inference, all of the internal Idenix work involved the use of common general knowledge, which work included the successful making of the precursor. It is also said that it was inconsistent of the primary judge to use Professor Furneaux’s evidence that the Idenix work was a “typical research project” as evidence of work using common general knowledge (at [453]) but using his evidence that he would undertake “research” to make the target compound as indicating his need to use non-common general knowledge (at [576(1)]).
153 Idenix says that contrary to the primary judge’s reasons, the Idenix patent is sufficient as the skilled addressee could make a compound within claim 7 simply by looking at the terms of the claim and armed only with the common general knowledge and without invention or prolonged study of matters presenting initial difficulty. The Idenix patent states that “[t]he nucleosides of the present invention can be synthesized by any means known in the art”. Idenix says that there is no statement in the Idenix patent that making the nucleosides of claim 7 would present any difficulty or that the skilled addressee would have to employ anything other than a known method to make a compound within claim 7. Accordingly, it is said that contrary to the primary judge’s reasons, the Idenix patent directs the skilled addressee to known processes, rather than to processes which require non-common general knowledge research. Further, it is said that it was common ground at trial and among the experts that none of the Schemes described in the examples set out in the Idenix patent were expressed to be directed specifically to making a claim 7 compound. Thus Idenix contends that no skilled addressee could have been misled onto an incorrect path by precisely following a scheme which they knew was directed to a different product.
154 But in our view the primary judge correctly recognised that the claims formed part of the description in the Idenix patent (Kimberly-Clark at [14]), and accepted Idenix’s identification of the target compound as a compound within claim 7 that the addressee would seek to make. The point made by her Honour was that Idenix’s formulation of the sufficiency test omitted the requirement that it is the disclosure in the complete specification that must enable the addressee to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty (at [436], [578] and [612]).
155 Further, in our opinion the statement in the specification “nucleosides of the present invention can be synthesized by any means known in the art” does not assist Idenix. First, “known” is not necessarily co-extensive with common general knowledge. Second, Idenix’s submission that such a stipulation directed the skilled addressee away from non-common general knowledge research is contradicted by the evidence. As Gilead correctly submitted, both Dr Borthwick and Professor Meier relied on sources outside common general knowledge, including but not limited to the Matsuda paper, in developing their target syntheses. Moreover, Professor Furneaux considered the synthesis to be complicated and would have had to have conducted significant research and experimentation. Moreover, Idenix’s team of skilled scientists and consultants carried out such research and experimentation over a lengthy period but without success. Generally, the evidence before her Honour well demonstrated that a route of synthesis of a claim 7 compound was not common general knowledge as at the priority date.
156 Idenix asserts that the primary judge erred in finding that Schemes 3, 4 and 9 of the Idenix patent teach nothing relevant to making a compound within claim 7. It says that such a finding was based on a flawed unstated premise, namely, that schemes directed to other end products could not contain any relevant information (at [537] to [539] and [543]); we would note at this point that Idenix’s description of her Honour’s reasons is, like some other characterisations of her Honour’s reasons by Idenix, not accurate. Idenix submits that the Idenix patent describes the synthesis of 2'-branched nucleosides at pages 122 to 125. Starting from either a sugar (the sugar route) or a nucleoside (the nucleoside route), it says that the Idenix patent discloses that these compounds can be synthesised by: (i) oxidising the 2'-OH group to yield the 2'-keto compound; (ii) reacting the 2'-keto compound with an organometallic reagent (for example, a Grignard reagent or an organolithium) to yield the 2'-alkylated compound (for example, the 2'-methyl-“down”-2'-hydroxyl-“up” nucleoside); and (iii) in the case of the sugar route, coupling the alkylated sugar to a nucleobase to yield the 2'-alkylated nucleoside. Idenix says that for such reaction steps, the Idenix patent teaches that the sugar or nucleoside can be “optionally protected with a suitable protecting group, preferably with an acyl or silyl group, by methods well known to those skilled in the art, as taught by Greene et al” (pages 123 to 124). It says that the Idenix patent then states that in particular embodiments the 2'-C-branched ribonucleoside is desired, that is, a 2'-branched methyl-“up”-2'-hydroxyl-“down” nucleoside. But it is said that those words do not limit the preceding text.
157 Idenix also contends that contrary to the primary judge’s finding at [612], the synthesis of the precursor was also a matter of routine. It says that the skilled addressee when reading the text and schemes as a whole would be able to arrive at the precursor. Specifically, it says that the skilled addressee could oxidise the 2'-OH group of a nucleoside to give the 2'-keto nucleoside, which would then be reacted with an organometallic reagent (such as a Grignard reagent) to give the precursor. It says that the skilled addressee would have expected the application of a Grignard reagent to the 2'-keto nucleoside in Scheme 4 (being an asymmetric cyclic ketone) to result in a mixture of both isomers, being the depicted 2'-methyl-“up”-2'-hydroxyl-“down” nucleoside as well as the un-depicted 2'-methyl-“down”-2'-hydroxyl-“up” nucleoside (precursor). It then says that the separation of individual isomers from the mixture was routine chemistry. Idenix submits that, contrary to the primary judge’s finding, the skilled addressee could have relied on Scheme 4, together with common general knowledge, to obtain the precursor.
158 Further, Idenix submits that the Idenix patent in Scheme 9 refers to the published procedure in the Harry-O’kuru paper, which it is said was incorporated by reference into the Idenix patent (page 157, line 11). It submits that the Harry-O’kuru paper gives detailed instructions as to how to prepare a 2'-methyl-“up”-2'-hydroxyl-“down” nucleoside; that is, a compound having precisely the same configuration as the target compound, with the exception that there is a hydroxyl, not a fluorine, at the 2'-“down”. Idenix submits that the evidence before her Honour was to the effect that the existence of that compound would have given the skilled addressee confidence that the target compound could be prepared, given what it asserts to be the “similarities” between a hydroxyl group and a fluorine atom; one might expect a lawyer more than a chemist to make such a simplistic assertion. Further, Idenix says that the primary judge erroneously failed to find that Scheme 9 represents a strong precedent for a compound which had been modified with the addition of a methyl group in the 2' up position together with a functional group in the 2' down position.
159 Further, Idenix says that the primary judge also erred in finding that Scheme 9 led the skilled addressee away from the routes that Dr Borthwick and Professor Meier adopted (at [539] to [540]). Idenix submits that the Harry-O’kuru paper at page 1754, lines 3 to 4 and therefore the Idenix patent tells the skilled addressee that the reaction of a 2'-keto nucleoside with an organometallic reagent (such as a Grignard reagent) usually produces a 2'-alkyl (e.g. 2'-methyl-“down”-2'-hydroxy-“up” nucleoside (i.e. a 2'-alkylarabinonucleoside) as the “major product” of the reaction. Idenix says that the Harry-O’kuru paper therefore provided the skilled addressee with “ample confidence” that they would be able to obtain the precursor following the guidance of, as opposed to steps illustrated in, Scheme 4.
160 We would reject Idenix’s submissions.
161 In our opinion, the primary judge’s treatment of Schemes 3, 4 and 9 was correct (at [537] to [541]). Her Honour did not find that these Schemes taught nothing relevant to the making of a claim 7 compound. Rather, her Honour correctly recognised that they did not describe a synthesis of the precursor compound let alone the target compound. Moreover, to the extent that any specific details were given in Scheme 9, the evidence disclosed that they led in a different direction. In fact, they provided a method of synthesis of a compound with the wrong stereochemistry to enable the production of a claim 7 compound.
162 Further, contrary to Idenix’s assertions, the preponderance of evidence before her Honour did not support the assertion that the skilled addressee could have relied on Scheme 4 and common general knowledge to obtain the precursor to the target compound that we have identified. Indeed, none of the experts adopted that approach. Further, in relation to the Harry-O’kuru paper, Idenix has erroneously disregarded the fact that that paper is referred to in Scheme 9 (not Scheme 4). Moreover, the reference is for the specific purpose of providing a selective method of synthesis of a compound with, in our context, the wrong stereochemistry at the 2' position. Moreover, Idenix has disregarded the evidence of Dr Borthwick, who accepted that Scheme 9 did not produce the precursor for that reason. As her Honour correctly identified, Dr Borthwick agreed that Scheme 9 did not provide any precedent for making the fluorinated target compound (at [560]). Further, and in any event, it would seem to us that Idenix’s reliance on the Harry-O’kuru paper was more a misplaced desire to pick up that paper’s footnoted references to the Matsuda papers that on no view were part of the common general knowledge at the relevant time (see for example Matsuda A, Takenuki K, Itoh H, Sasaki T and Ueda T, “Radical Deoxygenation of Tert-Alcohols in 2'-Branched-Chain Sugar Pyrimidine Nucleosides: Synthesis and Antileukemic Activity of 2'-Deoxy-2' (S)-Methylcytidine” (1987) 35 Chem Pharm Bull 3967; Matsuda A, Itoh H, Takenuki K, Sasaki T and Ueda T, “Alkyl Addition Reaction of Pyrimidine 2'-Ketonucleosides: Synthesis of 2'-Branched-Chain Sugar Pyrimidine Nucleosides” (1988) 36 Chem Pharm Bull 945).
163 Further, Idenix contends that the primary judge erred in finding that the Idenix patent did not disclose the use of DAST and points to the fact that the Idenix patent refers at page 10 to the Emory patent stating that it “discloses the use of certain 2'-fluoronucleosides to treat HCV”. It says that the Emory patent is incorporated by reference into the Idenix patent (page 157, line 11). Idenix says that if a skilled addressee is seeking to make a compound within claim 7 (which is a 2'-fluoronucleoside which may be used to treat HCV infection), the Emory patent is a particularly pertinent piece of information. It says that the Emory patent sets out a known method for the fluorination of nucleosides at page 33 lines 7-11, namely that “[f]luorine is usually introduced into these molecules through nucleophilic attack on an anhydro-nucleoside … or through replacement and inversion of a stereochemically fixed hydroxyl group with [DAST]”. Accordingly, Idenix says that, contrary to the primary judge’s reasons at [542], the Emory patent discloses DAST which consequently is disclosure as part of the Idenix patent, and that the use of DAST is a technique known in the art to achieve fluorination of nucleosides at the 2' position by displacement and inversion.
164 But we agree with Gilead’s submissions that the primary judge’s treatment of the Emory patent was correct (at [542]). As her Honour explained, the Emory patent does not teach the skilled person to use DAST to fluorinate the relevant precursor compound. It is merely one of more than 200 documents referred to in the Idenix patent. Moreover, it is referred to in the background section of the Idenix patent for a purpose unrelated to the synthesis of a claim 7 compound. Further, as is apparent from the evidence that was before her Honour, none of the experts had regard to it. It is appropriate to elaborate further.
165 The Emory patent is disclosed in the Idenix patent (page 10, line 11) with a simple and short reference that it “discloses the use of certain 2'-fluoronucleosides to treat HCV”. Nothing further is said about any method of synthesis. Moreover, it appears in a section headed “Additional Methods to Treat Flaviviridae Infections” (page 7) and is referred to as part of a class (class (10)) of drugs developed to treat such infections, that class being referred to on page 9 as “nucleoside analogs” developed for such treatment. A similar reference (page 115) to the Emory patent and in a similar context (i.e. use as part of a viral treatment) is made later in the Idenix patent although this time referred to as item (13). Clearly the Emory patent is not being cited for the purpose of incorporating by reference or disclosing a method of synthesis of a compound of interest. Its disclosure is made as an example of a compound being used as part of a method of treatment. We also do not consider that the boiler-plate provision, to the effect that all publications mentioned in the specification are incorporated by reference (page 157, line 11, see [100] of these reasons above), greatly assists when one considers the contextual framework within which the Emory patent has been referenced in the Idenix patent and the evident purpose of such references. Moreover, we do not consider that the Emory patent assists Idenix in any event. The references to fluorination for example at page 26, line 25 and page 27, line 13 of the Emory patent do not refer to using DAST. Moreover, they are referring to fluorination of a secondary substituent rather than a tertiary one. Further, although at page 33, line 10 there is a reference to DAST, it is not in the context where there is also a methyl group on the 2' carbon. Indeed, it is clear that this is not the relevant context when one considers the structure set out for the compound in claim 1, which only shows fluorine on the 2' carbon, i.e. no methyl group on the 2' carbon. In our view it is superficial to take a reference to DAST in one context and endeavour to transpose it to another context where the target compound and complexities of synthesis are quite different.
166 In our view, Idenix’s attempt to finesse relevant disclosure in the Idenix patent by reference to the Emory patent must be rejected.
(c) Clark patent (ground 2)
167 Idenix contended before the primary judge that the Clark patent “admitted” as routine chemistry a route of synthesis involving fluorinating a nucleoside with methyl-“down” and OH-“up” groups at the 2' position so as to obtain a nucleoside with methyl-“up” and fluoro-“down” groups. More generally, Idenix contended before her Honour that the Clark patent disclosed a reaction scheme which was within common general knowledge at the relevant time, i.e. that fluorination using DAST gave the necessary inversion.
168 But the primary judge found that the Clark patent did not disclose or demonstrate a route of synthesis of a compound within claim 7 of the Idenix patent which was within the common general knowledge of the skilled addressee at the priority date. Her Honour found that the Clark patent claimed the method of synthesis of the target compound and that the synthesis of the compound disclosed in the Clark patent was not routine (at [593] to [594]). Further, her Honour found that the Clark patent did not show that applying DAST to the appropriate precursor to produce the target compound for claim 7 of the Idenix patent was within common general knowledge at the priority date of the Idenix patent and that the reaction conditions for DAST were within such common general knowledge. Further, her Honour found that the Clark patent did not “admit” that the making of the precursor compound was within common general knowledge (at [596]).
169 Idenix now challenges these findings before us and has made the following submissions.
170 Idenix contends that the Clark patent provided objective evidence of the fact that methods of synthesis of the compounds within claim 7 (including applying DAST to the precursor) were common general knowledge around the priority date. It points to the following matters. First, at the relevant time, the named inventor Mr Clark was an employee of Pharmasset Inc, which was acquired by Gilead Sciences Inc in 2012. In or around late 2002 and early 2003, over the space of a few months, Mr Clark successfully synthesised 2'-methyl-“up”-2'-fluorine-“down” cytidine (a compound within claims 7, 8 and 9 of the Idenix patent). This compound was the subject of the Clark patent, granted in the name of Pharmasset Inc. Idenix asserts that “like the Idenix patent”, two general methodologies for the synthesis were provided by the Clark patent. The second approach, the nucleoside route, involved the modification of a pre-formed nucleoside. The fluorination reaction step involved the conversion of a 2'-methyl-“down”-2'-hydroxy-“up” nucleoside to a 2'-methyl-“up”-2'-fluorine-“down” nucleoside, as depicted in Scheme 2 of the Clark patent (page 74). Idenix says that after describing steps 1 to 3 in terms appropriate to their status as standard steps (and so it is said in similar terms to the corresponding steps in Scheme 4 of the Idenix patent), Scheme 2 of the Clark patent refers to the step to obtain the precursor in terms which do not instruct the skilled addressee how to obtain the precursor in a stereoselective way. Rather, it gives a choice of alkylating agents, including Grignard reagents (such as methylmagnesium bromide), methyllithium and trimethylaluminium, and leaves it to the skilled addressee to determine which one to use. Idenix says that it was not essential that the precursor be obtained in a stereoselective way after step 3, because if a mixture of isomers were to be obtained, which Idenix says is the normal position after a Grignard reaction, the skilled addressee could use routine chemistry to separate out the required precursor. Idenix says that the Clark patent does not suggest that there is anything novel or inventive about obtaining the precursor.
171 Second, Idenix says that the Clark patent then goes on to refer to “Step 5”, the fluorination step (pages 75 to 76), in what Idenix labels as “unremarkable terms”. Accordingly, so it is said, Mr Clark obtained the 2'-methyl-“up”-2'-fluorine-“down” compound by applying DAST to the appropriate precursor. In other words, fluorination of the precursor proceeded with displacement and inversion. It is said that this is the route that each of Dr Pankiewicz and Professor Patterson suggested in conversations with Mr Clark and that this merely “reflected the common general knowledge”. It is also submitted by Idenix that there is no suggestion in the Clark patent that any of this is anything other than a standard step. Now although Examples 2 and 3 of the Clark patent give examples of reaction conditions suitable to employ for Scheme 2, Idenix says that there is no suggestion in the Clark patent that it was necessary to follow the precise reaction conditions given in Examples 2 and 3 for any part of the reaction scheme set out in Scheme 2 to succeed. It is said that they were merely illustrative. Further, Idenix submits that the conditions given in the Examples and for Step 5 are unremarkable conditions for DAST proceeding with inversion. In this context, it refers for example to the Middleton paper and the Hudlicky paper. Further, Idenix submits that contrary to the primary judge’s analysis, no suggestion is made in the Clark patent that there is anything novel or unexpected about the DAST reaction conditions. Accordingly, so Idenix submits, contrary to the primary judge’s findings regarding the Clark patent and claims 13, 14 and 20 as granted, the Clark patent “admits” that it was routine chemistry to fluorinate a 2'-methyl-“down”-2'-hydroxyl-“up” compound so as to obtain a 2'-methyl-“up”-2'-fluorine-“down” compound, being the target compound under discussion concerning claim 7 of the Idenix patent.
172 Further, Idenix says that the Clark patent neither claims as inventive the fluorination step in the synthesis of the 2'-methyl-“up”-2'-fluorine-“down” nucleoside nor claims as inventive the synthesis of the precursor. Therefore, so it is submitted, by reason of it only claiming the glycosylation step on a pre-formed 2'-methyl-“up”-2'-fluorine-“down” sugar and the de-protection step on a pre-formed 2'-methyl-“up”-2'-fluorine-“down” nucleoside, the Clark patent “admits” (apparently implicitly according to Idenix) that there is no other relevant inventive step in the synthesis of the compounds. Accordingly, so Idenix submits, the Clark patent must be understood as asserting that the synthesis of the fluorinated nucleoside is a method known to the skilled addressee. Further, Idenix submits that contrary to the primary judge’s analysis at [593], claim 20 does not assert inventiveness in the fluorination step since its reference to claims 13 and 14 fixes on the post-fluorination steps set out in the Examples.
173 Further, Idenix says that it was open to Gilead to call persons involved in the Clark work, including the authors of the later Clark paper that was published in the Journal of Medicinal Chemistry (2005), to give evidence about that work including to rebut the inference that the steps of fluorination and inversion were routine. Idenix asserts that the primary judge ought to have inferred that any such evidence could not have assisted Gilead.
174 Further, it is said that the primary judge erred (at [587]) in treating Dr Pankiewicz’s “novel chemistry” reference as persuasive when the product sought to be produced was “novel” but not the means by which it was produced. We would just note at this point, contrary to Idenix’s emphasis, that Professor Patterson gave evidence that “[d]uring the conversation in which Dr Pankiewicz suggested to Mr Clark that he use DAST as a fluorinating reagent, Dr Pankiewicz said that he thought the application of DAST to a tertiary alcohol would be novel chemistry”.
175 Further, it is said that the primary judge erred in dismissing Professor Patterson’s reference to a host of literature to support tertiary fluorination (at [590]). It is said that Dr Borthwick’s evidence demonstrated that there was a “large volume of literature” relating to tertiary fluorinations. Again we would note that Idenix has not grappled with her Honour’s reasoning. Her Honour notes that Professor Patterson did not identify the “host” of literature and that it was not identified during the proceeding (at [590]). Further, Professor Patterson was not as experienced as Dr Pankiewicz. Moreover, Dr Borthwick’s references were not shown to be common general knowledge save as to one.
176 Finally, Idenix says that the primary judge erred (at [596]) in relying on publication of the Clark paper to support insufficiency when the paper’s purpose was to report the discovery of a potent anti-HCV agent, rather than to report an unclaimed mode of synthesis.
177 Generally speaking, we reject Idenix’s contentions. The primary judge correctly rejected Idenix’s reliance on so called “admissions” to be gleaned from the Clark patent. In our view, the Clark patent did not contain any admission (expressly or implicitly) that the synthesis of the compound that it disclosed was routine or common general knowledge. And that conclusion is notwithstanding the fact that the Clark patent did not include a claim directed to that synthesis. There is no requirement that a patent claim every aspect of an invention and it is misconceived to suggest otherwise. There is no implied admission that such aspects are routine if the patent does not claim them as part of the invention. Moreover, Pharmasset is a different legal entity to Gilead and in any event was not owned by Gilead at the time; it is incorrect to suggest that an admission was made by Gilead.
178 Further, far from admitting that the fluorination step involved no difficulty, the Clark patent described several methods of doing this and claimed such methods. Each step in the syntheses in the Examples is incorporated in claim 20; although claims 13 and 14 claimed glycosylation and deprotection, the nucleosides had to be fluorinated at the 2' carbon with a methyl also attached (and with inversion).
179 Further, as her Honour noted at [596], the Clark method was revealed in the Clark paper which was published in the Journal of Medicinal Chemistry, a peer-reviewed journal that published significant research. There was evidence before her Honour that suggested that the fluorination step in that method was considered by some chemists to be a novel piece of chemistry that was worthy of such publication.
180 Further, in relation to the Clark paper, its purpose was to describe both the synthesis and biological activity of 2'-deoxy-2'fluoro-2'-C-methyl cytidine as a potent anti-HCV agent. And in relation to synthesis, it was stated that “[t]he fluorination of tertiary alcohols using DAST has been reported, but the stereochemistry of such transformations is substrate-specific and often unpredictable”. It went on to state that: “Furthermore, dehydrations or eliminations, rearrangements and ring contractions are often pervading problems in the DAST fluorination of highly functionalized molecules”. We reject Idenix’s characterisation seeking to diminish the significance of the Clark paper, which in our opinion reports what could be described as a novel piece of chemistry relating to, inter-alia, synthesis of the compound then being discussed.
181 Further, the primary judge was correct to find that there was no admission in the Clark patent that the reaction conditions for DAST were common general knowledge (at [596]). The relevant parts of the Clark patent gave a detailed description of various synthetic routes, including via the use of DAST. But the reaction conditions varied from example to example. The relevant passages of the Clark patent were not capable of being read as any admission that the reaction conditions for the use of DAST to fluorinate a nucleoside at the 2' position with inversion of the methyl group were common general knowledge. Further, there was no admission in the Clark patent that the making of the precursor was common general knowledge or even “known”.
182 Further, as Gilead rightly points out, Idenix has emphasised the general methodology described on pages 74 and 75 of the Clark patent but has given little attention to the following detailed Examples. Of course, the Clark patent describes the Examples as “illustrative”, but it says that they “provide a further understanding of the present invention and further exemplify the general examples in Schemes 1 and 2”. On Idenix’s construction of the Clark patent, such further understanding and exemplification were arguably mere surplusage.
183 Further, Idenix’s proposition that there is no suggestion in the Clark patent that there is anything novel or inventive in the synthesis, is answered by saying that there is no requirement to expressly identify the respects in which an invention is novel or inventive. An invention is taken to be novel and inventive absent the contrary being shown. We agree with Gilead that there can be no admission, implicit or otherwise, from an absence of an unrequired statement of novelty or inventiveness.
184 Further, we also consider that the primary judge did not err in failing to draw a Jones v Dunkel inference with respect to the absence of a witness directly involved in the synthesis described in the Clark patent. First, in any event an inference is usually only drawn where a party is required or in the usual course of events expected to explain or contradict something. But Gilead was not required to do so with respect to the work of Pharmasset, which it subsequently acquired. That work was the subject of a valid and unchallenged patent and a publication in a peer-reviewed journal. Second, Professor Patterson, who was called by Idenix, demonstrated that the work was done by Mr Clark who does not appear to have been available. Third, Jones v Dunkel does not, as Gilead correctly submitted, otherwise enable Idenix to resort to its refuge to fill its own evidentiary holes, including the evidence of its own experts. Generally, the Clark patent does not assist Idenix to remedy any insufficiency in or of the Idenix patent.
185 Further, Idenix’s assertion that Dr Borthwick “demonstrated that there was a large volume of [literature and work prior to the priority date concerning] tertiary fluorinations” is incomplete and in relevant respects inapposite. His work with carbocyclic compounds was not common general knowledge as her Honour correctly found at [221] to [225] and [229]. And as for the literature, the vast majority was not common general knowledge. Apart from Idenix’s assertions before us, nothing tangible has been pointed to in the evidence before her Honour which undermines her statements in [229]. Idenix continually confused the scenario of using DAST to fluorinate a secondary hydroxyl group with that of a tertiary hydroxyl group and sought to glide over the complexities and subtleties that her Honour summarised at [230] and elaborated on in more detail later in her Honour’s reasons.
186 Finally, at this point in our reasons it is convenient to make reference to three items of literature that are relevant to the present section and the next section being:
(a) Middleton WJ, “New Fluorinating Reagents, Dialkylaminosulfur Fluorides” (1975) 40 J Org Chem 574. The parties at trial accepted that this was part of the common general knowledge (at [226]). It has been described in the primary judge’s reasons and our own as the Middleton paper.
(b) Hudlicky M, “Fluorination with Dialkylaminosulfur Trifluoride and Related Aminofluorosulfuranes” (1988) 35 Organic Reactions 513. Her Honour found that this was not part of the common general knowledge (at [599(3)]) and in any event it does not deal with the mechanism (or its genus) of interest.
(c) Biggadike K and Borthwick AD, “4'-Modification of Carbocyclic Nucleosides: Synthesis of 4'-α-Fluoro, 4'α-Hydroxy and 4',6'-Unsaturated Derivatives of the Antiviral Agent 2'-Ara-Fluoro Carbocyclic Guanosine” (1990) J Chem Soc Chem Commun 1380. Her Honour found that this was not part of common general knowledge (at [222]). In any event, it seems to concentrate upon modification of the 4' site and dealing with carbocyclic nucleosides; its focus is not on fluorinating a 2' carbon on a sugar ring with the 2' up position containing a methyl group and the 2' down position containing fluorine; admittedly, though, it does discuss a reaction of a tertiary alcohol with DAST and in the specific context before it does say that it “parallels the situation with secondary alcohols”.
187 In relation to the Middleton paper, and as the primary judge correctly found (at [557]), it does not assist Idenix. True it is that it discussed the fluorination of alcohols, but it did not deal with inversion. Moreover, although a small aspect thereof referred to tertiary alcohols, it was not dealing with tertiary hydroxyl groups on a sugar ring or carbocyclic ring (a stiffer or fixed structure) where steric hindrance and stereochemistry problems were likely to come into play, although we accept that to some extent this may have been more a yield question. And after all, if the Middleton paper being common general knowledge had shown the way, why then three years of failure by Idenix to synthesise the relevant compound? In truth, Idenix’s reliance upon the Middleton paper is exaggerated if not misplaced. In this context we note that at [559] her Honour rejected Idenix’s submission that the skilled addressee would simply rely on the Middleton paper and the March textbook. She observed that that submission failed to confront the problem that not only Dr Borthwick, but also Professor Meier and Professor Furneaux, Dr Lambert and the many chemists on the Idenix team did not adopt this course. In short, the course that Idenix asks the Full Court to take on appeal is one that is not supported by the evidence of any of the witnesses at trial, and which was rejected by the primary judge. We decline to do so.
188 We would also note that even if Dr Borthwick relied upon the Middleton paper (cf her Honour’s reasons at [559]), it was one of numerous references that he relied upon (see Exhibit 39 that we discuss later). As her Honour rightly found, he did not simply rely on the Middleton paper and the March textbook. Further, Idenix’s reliance upon the March textbook (Smith M and March J, March's Advanced Organic Chemistry: Reactions, Mechanisms and Structure (5th ed, John Wiley & Sons, 2001) ch 10, 519) can be similarly disposed of (as her Honour did at [558]).
(d) DAST was within common general knowledge (grounds 5, 6 and 7)
189 The primary judge stated at [206]:
… If the skilled addressee “is not an avatar for expert witnesses whose testimony is accepted by the court” but a tool for analysis (AstraZeneca AB v Apotex Pty Ltd (Matter No S54/2015) (2015) 323 ALR 605; 114 IPR 445; [2015] HCA 30 at [23]) then it cannot be correct to focus on whether one or other expert witness can be taken to represent the skilled addressee. The relevant issue is the common general knowledge that should be attributed to the skilled addressee given the area of discourse to which the relevant documents relate.
190 Undaunted by what her Honour said, Idenix contends that the primary judge erred in treating Gilead’s experts, Professor Furneaux and Dr Lambert, as representatives of the skilled addressee and in accepting that their knowledge both constituted the common general knowledge and represented its outer bounds. Relatedly, Idenix contends that her Honour erred in rejecting “every aspect” of the evidence of Idenix’s experts, Professor Meier and Dr Borthwick, as demonstrating additional knowledge beyond the common general knowledge. Idenix submits that the primary judge’s key finding to the effect that for a skilled addressee to use DAST to fluorinate with inversion a tertiary hydroxyl involved invention (at [612] and [615]), could not be supported in the evidence. It says that her Honour was required to undertake a careful balanced consideration of the evidence as a whole for the purpose of constructing, as a tool for analysis, an objective skilled addressee with no more human characteristics of volitional and purposive action than necessary. But accepting that to be so, that is what in our view her Honour did. None of Idenix’s criticisms of her Honour’s approach have merit.
191 In our opinion the primary judge correctly rejected Idenix’s contention that the Idenix patent was sufficient because the “DAST route” approach was a matter of common general knowledge. As her Honour rightly found, once one appreciates the scientific complexity, using the term “DAST route” disguises what was at the relevant date complex and uncertain chemistry, particularly for tertiary fluorinations with the correct stereochemistry at the 2' position of a nucleoside.
192 It is necessary for us to observe at this point that common general knowledge is background knowledge and experience which is available to all in the trade. It must be generally accepted and assimilated by persons skilled in the art and known and accepted without question by the bulk of those who are engaged in the particular art. Information is not common general knowledge merely because it might be found in a journal, even if widely read. In that conceptual framework, the primary judge correctly found that the hypothetical skilled addressee should not be treated as having available, as part of the common general knowledge, all of the very specific expertise and experience in the fluorination of nucleosides or carbocyclic compounds that Professor Meier and Dr Borthwick possessed by reason of their involvement in particular projects (at [218] to [225]). For example, Dr Borthwick relied substantially on his own experience and numerous non-common general knowledge publications in devising his target syntheses. In that regard, he was dependent upon his own experience in fluorinating a tertiary hydroxyl group at the 4' position of a carbocyclic compound. That work followed five years of research that he had already conducted, in which he had attempted and performed various different fluorination reactions and consulted the literature on the subject.
193 It is convenient to elaborate on some of Idenix’s specific arguments in respect of the expert evidence.
194 Idenix submits that before the priority date, Dr Borthwick used DAST as his first choice to fluorinate a tertiary hydroxyl on a nucleoside and achieved fluorination with inversion. It says that contrary to the primary judge’s analysis at [535], Dr Borthwick’s evidence could not be rejected as irrelevant and being “after the event” and “in wholly hypothetical circumstances”. It is said that his experience could not be dismissed as “unique” experience (at [207]) or involving “very particular knowledge” (at [554]) given that his work on the fluorination of two carbocyclic nucleoside analogues having a tertiary hydroxyl at the 4' position was reported in two peer-reviewed publications and was not suggested to be anything other than routine.
195 Further, Idenix submits that the Middleton paper and the March textbook formed part of the common general knowledge and that both disclosed the use of DAST to fluorinate tertiary alcohols. Idenix says that in the light of these texts, the idea of using or trying DAST to fluorinate a tertiary alcohol such as the precursor compound under present discussion could not be “inventive”. Further, Idenix criticises the primary judge’s finding that the skilled addressee “would know that the teachings of the Middleton Paper could not be applied to the compounds of” the Idenix patent (at [557]), with the apparent basis for that knowledge of the skilled addressee being the evidence of Dr Borthwick. But Idenix contends that that finding was erroneous because Dr Borthwick in 1990 did “exactly what the primary judge found he ‘knew’ he could not do”. Idenix submits that there was no basis for such a finding of “knowledge” of either Dr Borthwick or the skilled addressee. Further, it says that the primary judge also erroneously dismissed the Middleton paper by accepting Gilead’s submission that Dr Borthwick did not use the Middleton paper, but used the Matsuda paper (at [559]). Idenix contends that the Matsuda paper was a paper dealing with a specific form of the precursor and was available as part of the teaching of the Idenix patent; in any event it says that Matsuda said nothing about using DAST on the precursor compound. In any event it says that Dr Borthwick did not need to refer to either the Matsuda paper or the Middleton paper for the “common general knowledge idea” of using DAST on the precursor compound. Idenix further contends that the primary judge also dismissed the Middleton paper on the basis that none of Professor Meier, Professor Furneaux, Dr Lambert or the Idenix scientists relied on it (at [559]). But Idenix contends that the Idenix scientists did use the DAST analogue, Deoxo-Fluor, on the precursor compound in the only work Professor Furneaux identified as work he expected a skilled addressee to undertake. We would note at this point that Professor Furneaux’s evidence did not precisely say that this was the only work he would have identified, but that he did acknowledge under cross-examination that the Deoxo-Fluor route eventually undertaken by Idenix’s scientists was consistent with what he expected an ordinary skilled chemist to do (see the complete transcript of his cross-examination from T238 line 9 to T239 line 11). Further, Idenix contends that Professor Meier thought of using DAST. It was also contended that Dr Lambert was not a skilled addressee and could not assist on the question of what knowledge the skilled addressee could be taken to have possessed. Further, Idenix contends that the fact that the Idenix scientists thought of the method without having to refer specifically to the Middleton paper reinforces rather than detracts from its contention that using DAST on a tertiary alcohol was part of the common general knowledge.
196 We would reject Idenix’s submissions.
197 First, as Gilead points out, Dr Borthwick accepted that the material upon which he relied in developing his target syntheses included all of the material listed in Exhibit 39. But this included 19 journal publications for target synthesis 1 and those publications with additions for target synthesis 2. As part thereof, it involved a review by him of the Matsuda references to identify the state of the art in the synthesis of the desired precursor compound. With the exception of the Middleton paper, it would seem that none of the material listed in Exhibit 39 was common general knowledge at the priority date.
198 Further, Idenix has misdescribed the primary judge’s findings at [612] and [615] concerning “invention” in using DAST to fluorinate with inversion a tertiary hydroxyl. First, the primary judge’s findings were made with reference to the Kimberly-Clark test. The relevant question was not whether the idea of using DAST was inventive. Second, Idenix’s criticism of the primary judge’s reasons in relation to the Middleton paper does not pay proper regard to what her Honour reasoned at [557]. A similar error is made by Idenix with respect to the March textbook (at [559]) (see [187] and [188] of these reasons above). Third, Idenix’s assertion that Dr Borthwick did not need to refer to the Matsuda or Middleton papers is not supported by the evidence. He did so when asked whether he could develop a synthesis of a claim 7 compound. As we have said, he relied upon all of the material in Exhibit 39. Further, contrary to Idenix’s submission, the Matsuda paper was not part of the teaching of the Idenix patent. It was not common general knowledge. The reference to it was contained within the footnotes to the Harry-O’kuru paper which itself in any event was only part of the teaching in the Idenix patent for Scheme 9 therein.
199 Second, Idenix contends that the primary judge erred in treating as irrelevant Professor Meier’s evidence, who worked on two fluorination reactions on which he reported in 1999, that he would have adopted the DAST route. Idenix says that the primary judge at [207] wrongly “rejected” the evidence of Dr Borthwick and Professor Meier who were directly engaged in the field of the Idenix patent based on their unique or specialised experience. Idenix contends that such evidence was relevant to common general knowledge. Moreover, it is said that Professor Furneaux also had expertise and experience (albeit limited) in the fluorination of nucleoside analogues. Idenix contends that the distinction between Professor Furneaux’s expertise and that of Idenix’s experts and why the former but not the latter was relevant to the common general knowledge, was not explained by the primary judge. But in our opinion the primary judge correctly found that Professor Meier’s knowledge extended well beyond the common general knowledge. As her Honour correctly found, it was based on his own specific experience in relation to fluorination and literature searches he had conducted for the purposes of earlier Norwegian proceedings (at [218] to [219] and [548]).
200 Third, Idenix asserts that the primary judge erred in finding that Dr Lambert was representative of the skilled addressee. It submits that such a finding was inconsistent with her Honour’s finding that the skilled addressee would have some familiarity with fluorination reactions (at [219]). It is said that Dr Lambert did not have such familiarity and left such matters to synthetic chemists or nucleoside chemists engaged in the necessary synthesis. But we see no difficulty with her Honour’s conclusion that Dr Lambert was the kind of expert to whom the Idenix patent was addressed. Moreover, once again Idenix has not correctly described her Honour’s reasons; her Honour said at [219] that some familiarity with fluorination reactions might be imputed to the skilled addressee; further, as she explained, the 350 application and the Idenix patent were not “directed towards such a narrow topic as the fluorination of nucleosides”.
201 Fourth, Idenix submits that Professor Furneaux’s first thought when approached by Gilead’s lawyers to give evidence was to use DAST to fluorinate a precursor. It is said that he gave evidence that the use of DAST (or a two-step agent) on the precursor was “the first thing I would have conceived of” and “conceptually it would have been the obvious thing to conceive first … it was the first thing I thought of”. In the light of this evidence, Idenix contends that Professor Furneaux’s thoughts about perceived difficulties with using DAST in such a context could not detract from the fact that his first thought was to use DAST (or a two-step agent) on a precursor. Now the primary judge referred to evidence that Professor Furneaux thought that the DAST route had a high chance of not succeeding and “[b]ecause of this it was not his first thought in fact to use DAST on a precursor compound” (at [570]) Idenix contends that if her Honour’s finding is that it was not his first thought to use DAST, it was against the evidence. Further, Idenix contends that if it was found that he thought the chances of success were low, and therefore his view was that he would need to undertake further research, then such evidence or finding is “harmless but irrelevant”. Idenix submits that the important point is that it cannot rationally be “inventive” to try DAST on the precursor in the light of his evidence. It is said that the fact that Professor Furneaux might have had doubts as to success is not to the point. It is accepted by Idenix that any product of research he might have pursued may not have been part of the common general knowledge, but that it was common general knowledge to use or try the DAST route.
202 Before proceeding further it is worth us setting out what Professor Furneaux actually said (T252 line 31):
But just so we understand the sequence, you accept, don’t you, that your plan A, your first plan, would have been to apply DAST to an appropriately protected compound 6 in exhibit 8? --- It would have been the quickest concept to come up with, I accept that.
And that would be the first one you would try, but you would have a plan B ready to go? --- Not necessarily. As I think I explained, sir, the starting material required for that requires it to be made from – in a multistep process with selections for protection and possibly deciding the reaction conditions to get the compound with the right stereochemistry from something like a Grignard reagent. So it’s easy to write it on paper, but the chemistry still requires a number of steps.
I understand? --- So intellectually I would have planned out alternative routes and then considered which to undertake.
But doing the best you can, plan A was what you have identified? --- Yes, sir.
Namely, you applied DAST to an appropriately protected claim 6 – I’ll start again. Plan A would have been – taking into account everything you said, doing the best you can – would have been to apply DAST or the two-step, or both perhaps – I’ll start again. I’ll do it as clearly as I can. Is this right, doing the best you can, your plan A would have been to apply, with all the qualifications you’ve mentioned, DAST to an appropriately protected compound 6 that we see in exhibit 8? --- I will say yes, with one qualification.
Yes? --- And that is that plan A is not necessarily better than plan B or in sans C, it is simply named plan A.
Yes, but when you were answering this question yesterday you were understanding plan A to be the first thing you would try? --- Certainly that is what I’ve said here, sir, so I will correct, it would been the first thing I would have conceived of, but starting material wouldn’t have been something – or unlikely to have been something available in my laboratory. It would have required synthesis, it would have required a retrosynthesis plan to make it and I would have been at the same time considering other alternatives. So the step in deciding what to conduct first would have been on a rational basis of comparing alternatives and doing literature search and ---
But taking it step by step, do you agree that you were intending to convey in that answer at 230 that plan A, in the sense of DAST applied to a compound 6 precursor, was the first thing you’d likely try? --- No, sir.
Isn’t that what it says? --- I believe I’ve just said that I’m correcting that. I believe that’s correct, that’s what it says, sir, and I’m correcting that.
Yes. The answer you gave we’re looking at there which you wish to correct was that the first thing you would most likely have done, with all the qualifications, is to use DAST on a compound 6 exhibit 8 precursor. That’s what it says there, isn’t it? --- It says no, that I wouldn’t have necessarily considered DAST first. That I would have considered both reagents.
Sorry, but subject to that you agree with me? --- So conceptually it would have been the obvious thing to conceive first, but it was also the first thing – it was the first thing I thought of – but also I foresaw that it was, in my opinion, had a high chance of not working.
203 Further, Idenix submits that Professor Furneaux’s doubts as to the likely success of the DAST route related to potentially competing reactions. Idenix contends that before conducting searches of the Red Books (the Journal of Carbohydrate Chemistry) and the Pankiewicz Review, he thought it would be complicated chemistry and that there would be competing reactions, but that it would likely produce a range of reactions, one of which could reasonably include fluorination with inversion. Idenix contends that this evidence was quite consistent with his other evidence that inversion was not the only likely outcome of an attempted fluorination reaction of a tertiary hydroxy group in a sugar or nucleoside. It is also said that further oral evidence did not affect the import of that evidence. Idenix contends that the primary judge’s attempt to grapple with the ebbs and flows of his evidence does not detract from Professor Furneaux’s evidence that he thought that a reasonable possible outcome of the reaction would be the target compound. Idenix further submits that Professor Furneaux had claimed in a US patent application before the priority date a compound with a tertiary fluorine in the 2' down position on the basis that it could be made by methods analogous to known methods.
204 In our opinion, the primary judge correctly found that Professor Furneaux’s evidence did not support a finding of sufficiency (see [566] to [582] and especially [566] and [574]). Professor Furneaux was highly doubtful as to the utility of attempting to use DAST to obtain a claim 7 compound (at [570]). He thought DAST was highly unlikely to work, which led him to express the view, as her Honour found, that “several retrosynthetic steps would be required, including a literature search and experimentation, and that the chemistry would be complicated and unpredictable”. As Gilead correctly submitted, scepticism can inhibit recognition of the utility of applying a concept or idea. Moreover, Professor Furneaux in his evidence distinguished between what one may conceive of and what one may actually try. Although he conceived of the possibility of using the DAST approach, he thought it unlikely to work. Further, we have perused for ourselves US patent no. 5,047,518 (columns 9, 14, 33, 58 and 59) and the international PCT application WO 99/19338 (pages 16, 18 and 33) which in each case have Professor Furneaux as one of the inventors. Her Honour took these into account and in our view fairly dealt with them at [575(10)]. In any event, they were only a small part of the evidence considered on sufficiency.
205 Fifth and more generally, Idenix contends that common to the evidence of Dr Borthwick, Professor Meier and Professor Furneaux was their knowledge of DAST and Deoxo-Fluor and the use of such methods to fluorinate secondary alcohols, familiarity with that mechanism including inversion, and (as their first thought) the use of DAST to fluorinate a tertiary hydroxyl. Idenix also says that in 2002, DAST and Deoxo-Fluor were the most commonly known means of fluorinating a secondary hydroxyl in the relevant art. It points out that this was made clear in the Middleton paper and the March textbook. It is said that the primary judge erred in finding that there were other well-known means (at [605]). It is said that such other means were not specifically identified and that the evidence did not support such a conclusion.
206 Sixth, Idenix contends that contrary to the primary judge’s findings at [543], [577], [610] and [612], the evidence did not support a conclusion that the precursor could not be made by the skilled addressee with the benefit of the Idenix patent and the common general knowledge. It is said that Idenix so succeeded. Idenix asserts that it is “inconceivable” that there would have been a sufficiency attack if the compound in suit was the precursor. It is said that the primary judge failed to pay sufficient regard to the principles she herself expounded at [438(8)].
207 We disagree with both of these contentions. The generality at which Idenix puts these submissions, citing selective references from the extensive evidence, does not in our view do justice to the detailed and careful reasons of the primary judge. The primary judge correctly rejected Idenix’s case concerning the evidence of Dr Borthwick, Professor Meier and Professor Furneaux and the precursor. Her Honour’s factual findings on this issue have not been shown to be in error.
208 Finally, Idenix has contended that Gilead failed to demonstrate that if the skilled addressee had adopted the DAST route, they would not have obtained the target compound. It is said that the AMRI experiments and Mr Clark’s work prove that the skilled addressee would have. Now the AMRI experiments were conducted in 2014 and involved using the Deoxo-Fluor route. The product isolated in the AMRI experiments contained a compound having the same structure as the Clark compound 9, although the AMRI product was not completely pure. Idenix has contended that based upon the AMRI experiments, it could be inferred that Dr Griffon of Idenix created the target compound in February 2003 using the Deoxo-Fluor route. Further, Idenix has contended that it could also be inferred from the AMRI experiments that the reason the target compound was not isolated and identified was that Dr Griffon failed to analyse all of the reaction products. It is said that a skilled addressee would have done so and that such analysis of all the reaction products would have identified the target compound.
209 We agree with the primary judge (at [600]) that the AMRI experiments did not assist Idenix. Her Honour found that the AMRI experiments used more sophisticated techniques than those used by Dr Griffon. The evidence before her Honour well demonstrated such a finding. The AMRI techniques maximised the likelihood of the target compound being isolated and purified. Further, Dr Clemens used LC-MS, which is more sophisticated than TLC, as well as TLC to monitor the reaction. Further, Dr Clemens used HPLC, which is more sophisticated than column chromatography, which is what Dr Griffon used. In contrast, Dr Griffon used standard methods for the day. More generally, the AMRI techniques were neither reflective of the steps the skilled addressee would have taken at the time or that Dr Griffon in fact took. As such, her Honour correctly concluded that it could not be inferred that Dr Griffon would have found the target compound if he had tested all reaction products (assuming he had not) by the methods he in fact used. Further, we also see no difficulty with her Honour’s conclusion at [603] that she could not conclude that Dr Griffon had in fact made the target compound in any event. In that paragraph her Honour said:
… In this regard it must be recognised that Professors Furneaux and Barrett were drawing inferences. They were not expressing conclusions or opinions about facts they knew. Ultimately the drawing of any inference about whether Dr Griffon in fact obtained the target compound is a matter for the Court on the whole of the evidence and taking into account all circumstances including that Idenix chose not to call Dr Griffon to explain anything about what he did or did not do and why. There are so many imponderables in this regard and in the unexplained differences between the AMRI experiments and what Dr Griffon did, that I am not prepared to infer that Dr Griffon in fact obtained the target compound but just did not realise it. Even if this is incorrect, I am not prepared to infer that Dr Griffon would have been able to isolate and identify the target compound using the techniques that a person skilled in the art would have used in 2003 in the circumstances in which Dr Griffon was in at that time.
210 Idenix has submitted that the primary judge’s failure to find that those experiments obtained the target compound involved error and that her Honour preferred a judicial inference over unanimous expert scientific evidence to the contrary. But we reject that criticism for the reasons given. In any event the relevant and key question is whether the disclosure of the Idenix patent enabled the skilled addressee to make a claim 7 compound within what may informally be described as the Kimberly-Clark parameters taking into account common general knowledge at the relevant time. In that context the primary judge correctly considered the AMRI experiments not to be of much assistance (at [600] to [603]). In light of the significant differences between those experiments and the work of Dr Griffon, in particular the use by AMRI of more sophisticated techniques, and the absence of Dr Griffon as a witness, the primary judge has not been shown to be in error by finding that experimental and opinion evidence offered years later was insufficient to throw doubt on Idenix’s own documents, which we will discuss in a moment, disclosing and reporting at some length Dr Griffon’s failures.
(e) Idenix documents (grounds 3 and 4)
211 In evidence before her Honour and taken into account by her were a significant number of Idenix’s own documents recording Idenix’s attempts (indeed failures) to make a compound within claim 7 of the Idenix patent. The Idenix documents demonstrated that by the time Idenix applied for the Idenix patent in late June 2003, so far as it knew Idenix had been unable to make a compound within claim 7 despite considerable and lengthy efforts. Indeed, these failed attempts continued through until early 2005.
212 Some of the Idenix experimental material before her Honour and specifically drawn to our attention on the appeal that we have perused included the following:
(a) A memorandum from the inventors Storer and Gosselin dated 23 July 2002, dealing with a revised and updated research program referring to a “[n]ew synthetic priority” for putting a methyl group in the up position on the 2' carbon with fluorine already in the down position.
(b) An email from Griffon to Storer dated 12 September 2002, with a progress report showing failure in certain attempts for the synthesis of 2'-fluoro-2'-methyl-cytosine.
(c) A memorandum dated 31 December 2002, dealing with a meeting involving Storer and Gosselin discussing further ideas for a possible synthesis, including a failed attempt. There was also a progress report dated 31 December 2002 relating to Griffon discussing possible schemes for syntheses to be explored.
(d) A letter from Storer to Dr Paul Coe (an expert in the fluorination of organic compounds) dated 8 February 2003, stating: “We are OK with the nucleoside chemistry, it’s the fluorine chemistry we are struggling with and where your help will be valuable”. The letter attaches a memorandum titled “Fluorinated Targets for review by Dr Paul Coe” containing, in part, the following statement:
Nucleoside analogues
We have a number of nucleoside targets which contain fluorine most of which we are struggling with. The targets are all ribonucleosides of one sort or another and have an additional substituent, usually a methyl group, up at the 2' position.
Target 9 is an attempt to replace the tertiary OH of the ribo analogue with fluorine. We’ve tried a variety of procedures from the exocyclic methylene analogue in attempt to effectively add HF across the double bond. We had no success with that. We’re now looking at attempting to take the 2'-α methyl anhydro compound and open that with fluoride. I’m not too hopeful for success with that. Appendix 3 shows a summary of this. Your thoughts on how to introduce the tertiary fluoro substituent in compound 1 g would be appreciated.
(e) Dr Coe provided an undated response referring to problems and raising suggestions. He stated in part “… some of the route [sic] you have tried are OK except that I think you are using the wrong reagents, leaving groups and reaction conditions”.
(f) An email from Griffon to Storer dated 2 March 2003, attaching a progress report listing failures in the scheme of synthesis for 2'-fluoro-2'-methyl-cytosine. The report discusses further possibilities to be explored using different reagents and conditions.
(g) On 3 April 2003, Griffon emailed Storer attaching a progress report describing how what has been described before us (Gilead’s descriptions rather than Idenix’s contemporaneous descriptions) as strategies 2, 3, 4 and also 5 (elaborated on) had failed. The email said “… the compound I obtained after treatment with DeoxoFluor and deprotection was not the (very!) expected Fluoro derivative but the 2'-methylene derivative”. On 4 April 2003, there was a further communication between Griffon and Storer referring to Griffon’s attendance at a Scientific Update course on fluorination in Stratford-upon-Avon. Griffon said that the course “… allowed us to learn a lot about the last progress in the fields of nucleophile and electrophile fluorinating agents”. A progress report was attached discussing possibilities for the synthesis of 2'-azido and 2'-fluoro- 2'-methyl-cytosine.
(h) On 3 June 2003, Griffon emailed Storer attaching a further progress report. Reference was made to a failed strategy 6 (as described before us) using a procedure described by Dr Coe. A strategy 7 (as described before us) was also discussed.
(i) On 9 June 2003, Storer circulated a report on the Scientific Update course. Reference was made to a failed strategy 8 (as described before us) to get fluorine on the 2' down position and also a failed strategy, using DAST, to put fluorine on the 3' down position (apparently strategy 9).
(j) On 11 June 2003, a further revised research program was circulated with work by Griffon “in progress” being described as a high priority to achieve the 2' “down” fluorine and the 2' (presumably “up”) methyl group objective. On 7 August 2003 a further program was circulated. The objective was described as still a high priority. The following statements were made: “Up to now, all procedures starting from a nucleoside were unsuccessful”; and “[a] strategy starting from the corresponding fluorinated sugar might be the solution”; there was also reference to “… a last alternative using a nucleoside route: need to try the method described in the Fluorine Chemistry Course …”.
(k) At the end of June 2004, a report was prepared stating the following.
C. HCV Program: 2'-methyl “up”, 2'-fluoro “down” nucleosides project: “sugar strategies”
Opening of a spiro α-chloroepoxide (stand by)
Addition of an electrophilic fluorinating agent on a 1,2-double bond (stand by)
Total synthesis of the sugar synthon
[the parties before us have described the last possibility as strategy 10 and the second last possibility as strategy 11].
(l) After July 2004, a progress report was prepared again discussing a failure in adding fluorine and discussing a synthesis starting with a smaller molecule.
(m) In September 2004, Griffon (and Chappe) produced an Activity Report (September 2003 to 2004). It stated:
Different strategies were attempted in order to synthesize the 2'-methyl “up”, 2'-Fluoro “down” nucleosides:
• nucleoside strategies, starting from uridine: failed
• sugar strategies that failed so far.
Question: Due to the synthetic difficulties encountered, is it worthwhile to continue to consider this series as future targets?
(n) On 11 November 2004, Storer sent an email to Stewart and Moussa in part saying “A lot of things which look simple on paper in related systems have been tried and don’t work in this series. Having to make the tertiary fluoride is very different to having to make a secondary”.
(o) On that day Stewart responded to Storer in an email stating the following:
I’m going to ask George whether he’s already tried to invert the alcohol (step vi, Scheme G1) to make compound 10. If he’s already made the relevant azide (either compound 7 or 12) then that’d be great news…depending upon the yield. We had problems in the past where an azide had been successful when trying to introduce fluoride (see scheme), but could be very different at the now tertiary position. It’d be very handy if he’s got any of the useful intermediaries (2, 3 or 10) available, if we end up doing it rather than them.
Looks like the best approach so far. I was surprised that any formation of a tertiary fluoride from a tertiary alcohol had been reported until we found it yesterday, but I haven’t looked through literature that was ordered yet (in any case, no relevant reaction on sugar or nucleoside was reported). Hope you are well.
(p) Later on 11 November 2004, Stewart emailed Professor George Fleet at Oxford referring to the email Stewart had received from Storer and stating:
As you probably already know, we’d like to find a way to get a tertiary fluoride at the C-2 position (with stereochemistry as in compound 15 (Scheme G1)) to eventually form a nucleoside. Ben and I have been looking through the scheme and were wondering whether you’d had a chance to work on this route for the azides 8 and 13 (sounds as though you have, but I may not be thinking of the same azides or the right scheme!).
In particular we’re interested in whether you’d been able to invert the alcohol (from 3 to 10) and whether you’d had any success in introducing the azide at the tertiary position (3 to 7 or 11 to 12). Be great news if you were able to get that azide reaction to work, looks new to me. Any rough idea on yields for the steps in the scheme would be very useful as well. Although Chu-Yi and I ran into trouble with elimination for the lactones shown in the past work section, I imagine it could be a completely different story for the tertiary alcohol. I doubt you’ve tried SN2 displacement using fluoride?
(q) On 7 January 2005, Stewart sent to Moussa an email attaching a summary report which recorded the following: (a) an attempt at fluorinating a sugar ring using DAST which had the wrong stereochemistry; (b) an attempted benzoyl protection using DAST which had the correct stereochemistry but had “significant problems”; (c) a failed strategy 12 (as described before us) concerning a DAST reaction on a nucleoside; and (d) further proposals.
(r) On 12 January 2005, Stewart sent Storer an email in the following terms:
Thanks for that information from Gilles, could be very handy and might narrow things down a bit. I’m going to switch our focus straightaway onto the nucleoside that Jean-Francois provided and see whether we can avoid elimination in a few 50mg reactions by varying the fluorinating agent (changing from DAST to PBSF, Et3N.HF and HF.pyr) or temperature that I used the DAST (r.t. to -30 and -78°C).
I’m not sure if Adel mentioned it and I think he’s in a meeting so can’t ask him, but Alex did give us one piece of interesting information. The ring opening of anhydrouridine is apparently only possible using liquid HF in dioxane at 120°C under rigorous anhydrous conditions and doesn’t work with other fluorinating agents. Not sure whether Gilles got any more hints about the type of nucleoside that Pharmasset used, but if it were the C-2 methyl branched anhydrouridine then it would explain why Jean-Francois didn’t get any good results. Alex is going to send information about a company who specialise in this type of fluorination (as we couldn’t do it here), but I think it’s worth keeping in mind as an alternative approach once I’ve looked at the current routes…
[the Pharmasset work is a reference to the work described in the filed application concerning the Clark patent].
(s) On 19 January 2005, Stewart sent Storer an email showing that the reaction conditions were important.
(t) On 31 January 2005, a report was prepared by Stewart discussing 2'-methyl, 2'-fluoro nucleoside derivatives.
Reference was made on page 3 to the following:
Reaction of nucleoside 1 with DAST and DCM at room temperature gave a major product after 1h by t.i.c. analysis, as well as minor products which could not be separated by chromatography on the 100 mg scale used. Characterization of compound 2 was performed using both NMR (1H, 19F, 13C, COSY) and mass spectrometry. This compound corresponds to that obtained by J.-F. Griffon using Deoxyfluor in pyridine and DCM at -78°C. Attempts to carry out the experiment at O°C and without triethylamine proved to have little effect upon the reaction profile. The use of DAST at a lower temperature to increase the formation of the minor products was carried out by J. Wang. By comparison with subsequent discovery of the Pharmasset conditions, it seems that the theory being followed was correct and that the fluorinated nucleoside is present albeit in small yield.
(u) On page 5 it was said:
Investigation of Pharmasset Patent (US2005/0009737 A1) route
Following the publication of the Pharmasset patent it was decided that their route from cytidine 10 should be followed to make the required fluorinated nucleoside. Cytidine was selectively benzoylated on the base using benzoic anhydride in DMF in good yield (2 batches of 5g cytidine gave comparable yield). A change in solvent to dioxane/water was then studied since the scale-up would require removal of 2L of DMF (rather than a precipitation from dioxane) which would have been significantly more difficult. This first step in dioxane is currently being scaled up using 100g of cytidine. The TIDPS silyl protection at C-3' and C-5' was carried out on the DMF and dioxane batches in good yield and the oxidation at C-2' is currently under investigation.
…
Attempted direct fluorination of six-ring lactone using DAST
The reaction of 3,4-O-isopropylidene-2C-methyl-D-arabinono-1,5-lactone 13 has led to a fluorinated product, however, it has not been possible to characterize the mixture of products to date. Ongoing NMR experiments should resolve the matter; possible 14 or 15 may be present.
(v) Then on pages 6 and 7 there was a discussion involving an inversion of the stereochemistry of 2-C-methyl ribonolactone at the C-2 position and discussion of a DAST reaction on nucleoside 1464-JFN. It was said that “[a]lthough MS results strongly support the presence of product 8 [the target]”, fluorine and hydrogen NMR results of the mixture were “not very conclusive”.
213 Idenix accepts on appeal that it was legitimate for the primary judge to have regard to the Idenix documents in considering sufficiency, but contends she erred because it “might be inferred” that she placed determinative weight on those documents and because the primary judge ought first to have ascertained the specific content of the common general knowledge before proceeding to the Idenix documents.
214 In our view, Idenix’s apparent concession as to the relevance of its documents is plainly correct. The test as to whether the disclosure of the patent will enable the addressee of the specification to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty involves a factual analysis. In the present case, that analysis involves placing the court in the position of the person skilled in the art as at the priority date. Just as secondary evidence of the failure of others to find a solution to the problem at hand has a role to play in a case concerning lack of inventive step, so too may it play a role in applying the test. As the High Court said in Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (No 2) (2007) 235 CLR 173 at [116] and [119] (citations omitted):
[116] An Australian court should be slow to ignore secondary evidence or to rely on its own assumed technical expertise to reach conclusions contrary to such evidence. Australian courts have long recognised that the importance of such evidence and its weight will vary from case to case; it will not necessarily be determinative.
…
[119] … When skilled, non-inventive persons, and in this case also a skilled inventive person (Mr Garland), looking for improvements, fail to arrive at the invention, it is impossible to suggest that it would have been obvious to the skilled and not necessarily inventive person.
215 Before the primary judge Idenix contended that the Idenix documents were irrelevant because the evidence did not show that the routes that Idenix had identified in those documents would have been taken by the skilled addressee. The primary judge rejected Idenix’s contention. Her Honour found that the Idenix team were skilled in the art. She also found that if a team possessing not only the common general knowledge but also more highly specialised knowledge and skills directly relevant to the task at hand could not complete the task with the benefit of the Idenix patent, then that was strong evidence of the insufficiency of the Idenix patent. In so concluding, the primary judge was weighing up the extensive and complex evidence that was presented to her during the course of a lengthy hearing. An appellate court would be slow to displace a primary judge’s evaluation of the evidence with its own. In the present case there is no occasion to do so, because we agree with her Honour.
216 Before us, Idenix has contended that the primary judge placed what it labelled as “determinative weight” on the Idenix documents in finding insufficiency. Moreover, Idenix submits that her Honour inverted the relevant inquiry. It is said that her Honour ought to have ascertained the specific content of the common general knowledge from the expert evidence before then considering the Idenix documents. Further, it is said that Gilead failed to demonstrate that the work of the Idenix team was representative of the skilled addressee armed only with the common general knowledge. Accordingly, it is said that evidence of such work did not and could not inform the application of the correct test of sufficiency and that accordingly the primary judge erred.
217 But we agree with Gilead’s submission that her Honour did not treat the Idenix documents as “determinative”. In fact the primary judge said at [526] that the Idenix documents needed to be weighed with the other available evidence. Her Honour undertook such an assessment. Further, her Honour was correct to find that the Idenix documents provided a strong indication that the Idenix patent was in relevant respects insufficient. Further, her Honour did not invert the relevant inquiry. Her Honour ascertained the specific content of the common general knowledge from the expert evidence before considering this material in detail. Further, the Idenix documents provided probative evidence of what a team of skilled persons actually did at or prior to the relevant date when attempting to produce a claim 7 compound. In some respects, as Gilead correctly submits, such evidence was at least as probative, if not more so, as compared with hypothetical opinions expressed by experts years later, who did not undertake such a task either at that earlier time or even at or around the time of giving their evidence.
218 Further, the experts that the Idenix team consulted at that earlier time included Professor George Fleet, an expert in organic and synthetic carbohydrate chemistry, and Dr Paul Coe, to whom we have already referred. As Gilead points out, neither of them proposed the route that Idenix asserts would have been adopted as a matter of course by the skilled addressee from common general knowledge.
219 We agree with Gilead’s submission that the Idenix documents are littered with failed attempts and references to the difficulty of the task as her Honour found (see at [447] to [503]). Indeed, members of the Idenix team had to attend a specialist fluorination course to seek further instruction. Now before us, Idenix has contended that the core reasoning of the primary judge appears to have been that it was unlikely that Idenix scientists knew less than common general knowledge and that being so, the Idenix patent must be insufficient if they did not proceed at once to the DAST/Deoxo-Flour route. And that her Honour appears to have reasoned that although Idenix did employ the DAST/Deoxo-Fluor route, Idenix cannot invite the Court to “pretend” that that route was the first and only route employed. But Idenix submits that this reasoning ignores the need to identify different items of knowledge of chemistry or methodology, which individually may be inside or outside the common general knowledge. It is said that whatever other appeal such reasoning might have, sufficiency requires a consideration of what a skilled addressee would do armed only with the common general knowledge together with the patent. But it is said that the Idenix scientists were not bound in their endeavours by the rigour of any such legal principle. Contrastingly, so Idenix submits, the skilled addressee is notionally so bound for the purpose of the present question. It is said that having been told by the Idenix patent that known means will succeed, the skilled addressee would have chosen the DAST route and if need be retried it. It is said that other routes, on the evidence, were not “available” because they were beyond the common general knowledge.
220 We would reject Idenix’s submissions. There is an air of unreality to them. Idenix submits that evidence of the earlier attempts should be put to one side on the basis that, save for one of the many synthetic routes tried being the Deoxo-Fluor route attempted by Dr Griffon in February 2003, the work involved going beyond the common general knowledge. But that argument was correctly rejected by the primary judge for the following reasons.
221 First, the question is whether the disclosure enables the addressee to produce something within each claim without new inventions or additions or prolonged study of matters presenting initial difficulty. As her Honour correctly held, evidence that skilled persons resorted to information outside the common general knowledge in attempting to make an embodiment of the invention is probative to show that the disclosure is insufficient. Second, as her Honour correctly held, the Idenix team of scientists and consultants was a highly skilled team who necessarily possessed at least the common general knowledge, which was a general body of knowledge known and used by all those in the field. Her Honour rightly found that such knowledge was accordingly available to them and she was also entitled to find that they could be taken to have used it to the extent it could have assisted in the task in which they were engaged. Third, to the extent that the team possessed more than the common general knowledge, her Honour was entitled to infer that this could only have made it more likely that they would have succeeded without undue effort. But even then they did not succeed until 2005. Fourth, when the team in fact tried the Deoxo-Fluor route in February 2003, they did not obtain a claim 7 compound. Dr Griffon, who twice attempted that route, reported that it failed. Fifth, the Deoxo-Flour route was not the first thing that the Idenix team thought to try, but rather followed a number of other strategies all of which failed. Generally, her Honour’s conclusions and findings were apparent from or supported by Idenix’s documents and well open to her Honour to make. Moreover, Idenix did not call as a witness anyone involved during its three years of failure to explain that Idenix’s approach was idiosyncratic or not reflective of the ordinary skilled team or otherwise to contradict the inferences reasonably and, if we may say so, cogently drawn by her Honour.
222 Idenix also contends that the expert evidence before her Honour regarding the Idenix documents did not support a finding of insufficiency.
223 First, Idenix submits that Professor Furneaux did not attempt to identify any work recorded in the documents as work involving ordinary skill or common general knowledge. The primary judge at [453] characterised Professor Furneaux’s evidence as describing “Idenix’s work as appearing to be a typical research project” and found “this was a way of saying that he saw nothing out of the ordinary in what Idenix did”. Contrastingly, Idenix contends that his evidence was that the Idenix documents were typical of the documents created during a research project, in the sense of setting targets, assigning priorities and recording progress. Idenix contends that the primary judge’s characterisation of this evidence involved error.
224 Second, Idenix notes that Professor Furneaux gave evidence about the February 2003 Deoxo-Fluor route in which a precursor was reacted with Deoxo-Fluor. He said that that route was consistent with what he would expect an ordinary skilled worker to do. Idenix contends that this is in effect the DAST route. It further contends that Professor Furneaux said that the other routes disclosed in the Idenix documents were not routes that he could say would have occurred to him. It is said that there was no evidence upon which the primary judge could conclude that methods which would not have occurred to Professor Furneaux were part of the common general knowledge. It is said that at [452], the primary judge implicitly recognised this difficulty in finding “[i]f anything, this supports the inference that the Idenix team … was capable of exercising a greater level of skill” than Professor Furneaux. Accordingly, it is contended that if that be right, then the other Idenix methods could not reflect the common general knowledge.
225 Third, Idenix contends that in dealing with the evidence of Dr Borthwick and Professor Meier, the primary judge was rigorous in requiring satisfaction that their knowledge was common general knowledge. Yet, so it is contended, the primary judge dealt “quite inconsistently” with the Idenix scientists despite Professor Furneaux’s evidence and despite an absence of any attempt to identify with specificity any of the other Idenix methods as those which could be found, say in standard reference works. Thus, so it was submitted, it was found by her Honour that when Dr Borthwick used DAST on a tertiary hydroxyl, it was not considered useful evidence, but when Idenix attempted other routes it apparently became the “best guide” as to what a skilled addressee would do (at [455]). Further, Idenix submits that the primary judge erred in finding that the work of Dr Stewart and Ms Wang of Idenix in January 2005 was not independent of the work of Pharmasset (at [495] to [502]).
226 We reject these submissions.
227 It was not necessary for Professor Furneaux to characterise the work of the Idenix team as work skilled persons would undertake. They were skilled persons who actually undertook it. As Gilead contended, such evidence stood for itself. In any event, her Honour accepted Professor Furneaux’s evidence that the work was reflective of a typical research project (at [453]). Now this was not a finding that the methods used were necessarily common general knowledge. Rather, it was a finding that the work done was reflective of what the notional skilled person could be expected to have done in order to attempt to make a claim 7 compound. Moreover, if it involved resort to non-common general knowledge methods, then this was against Idenix’s case and bolstered the argument for insufficiency. In our view, there was no inconsistency in her Honour’s approach of the kind submitted by Idenix. Further, we agree with Gilead that it was not Professor Furneaux’s evidence that an ordinary skilled worker would simply employ the Deoxo-Fluor route instead of engaging in prolonged research and experimentation of the kind seen in the Idenix documents.
228 Further, her Honour was correctly cautious in not accepting Idenix’s submission that when it finally obtained a claim 7 compound in January 2005 it did so independently of Pharmasset (that is, the Clark patent). As her Honour held at [517], it was not necessary to decide this question, as by 2005 Idenix had engaged in a “prolonged study of matters presenting initial difficulty” on any view of the matter. But her Honour was entitled to infer that it was likely that the Pharmasset work (or Clark patent) may have assisted Idenix. Success was achieved by Idenix only after it had become aware of that work. Whether correlation or causation, her Honour did not need to finally decide. We do not perceive any error to have been made by her Honour in her analysis of the work of Dr Stewart and Ms Wang ([495] to [502]) or in her statements at [517]. Indeed, our own review of the Idenix documents (as we have detailed earlier) suggests some influence from the Pharmasset work later in the piece (early 2005).
(f) Other matters
229 We would conclude this part of our reasons by making four other observations.
230 First, we accept that the fact that a synthesis might be complex, time consuming and expensive does not necessarily entail that the steps required are not routine for the notional skilled person or team and we also accept that it must be assumed that “the notional skilled team will possess the will and resources necessary to perform the invention” (Apotex Pty Ltd v Warner-Lambert Company LLC (No 2) (2016) 122 IPR 17; [2016] FCA 1238 at [269] to [271] per Nicholas J). Relatedly, we would also accept, as her Honour did, that sufficiency is not necessarily undermined by the need for trial and error in the application of a method of synthesis (not described or disclosed in the specification) that was within common general knowledge. But accepting such propositions and making all necessary allowances, including for the fact that organic synthesis is of its nature usually complex and tricky, nevertheless insufficiency is established in the respects that we have discussed.
231 Second, we also accept the proposition that for patent validity the or a method of synthesis necessary to make the target compound does not need to have been actually performed by the inventor or associated persons prior to the priority date. There could be a sufficient description in a specification regardless of whether the synthesis had actually been carried out by the priority date. But in the present case, as we have said, there was no sufficient description.
232 Third, if Idenix is to be understood as contending that because claim 7 of the Idenix patent only claimed a compound, a sufficient description was necessarily given if the compound (or the result) was sufficiently described, we would reject such a proposition.
233 Finally, we cannot leave this part of our reasons without saying something further about Idenix’s “Example Synthetic Route”, which appears to have been Exhibit 8 before her Honour, and contained 8 steps. As to these:
(a) The Step 1 starting point was contestable. Who is to say that the skilled addressee knew or could have known or was directed to begin with a natural nucleoside? And if not, Steps 2 to 8 do not follow.
(b) As to Step 2, who is to say that the skilled addressee knew or could have known or was directed to taking steps at this point to protect groups at the 3' and 5' positions?
(c) As to Step 3, who is to say that the skilled addressee knew or could have known or was directed to producing a ketone “at the 2' position” at this point?
(d) As to Step 4 and alkylation, another series of contestable steps and options are proffered. But again, the specification was silent as to such matters. Moreover, Matsuda was not common general knowledge.
(e) As to Steps 5 and 6, they give rise to multiple possibilities, none of which were disclosed in the specification.
(f) As to Steps 7 and 8, they depended upon the precursor. But the precursor in turn depended upon the prior steps which were contestable.
234 In our view, Exhibit 8 can be compared with what was not disclosed in the Idenix patent. It in fact fortifies our view as to a lack of sufficiency. True it is that Dr Borthwick and Professor Meier suggested that the skilled addressee could have adopted such a route using common general knowledge. But that was debatable and in any event was not the correct question.
(g) Ground 1(b) of the notice of appeal
235 Ground 1(b) states as follows:
1. The primary judge erred in finding that the complete specification of Australian Patent No. 2003247084 (the Idenix Patent) does not comply with s 40(2)(a) of the Patents Act 1990 (Cth) (the Act) on the basis that it does not enable the skilled addressee to:
…
(b) identify a compound within claims 10, 12 and 13 (and dependent claims) at the priority date that has anti-viral activity such as to be effective for the treatment of Flaviviridae infections, including HCV infection: Reasons at [625].
236 The primary judge found that claims 10, 12 and 13 (and dependent claims) were insufficient for the additional reason that the Idenix patent did not enable a skilled addressee to identify a compound within those claims that would be effective for the treatment of flaviviridae infections including the HCV infection without new inventions or additions or prolonged study of matters presenting initial difficulty (at [620] to [625]). Later, the primary judge declined to make a finding that any compound within claims 7 or 8 was inutile pursuant to s 18(1)(c) of the Act because it was not so effective, adverting to deficiencies in Gilead’s evidence in that respect (at [648] to [654]).
237 Now Idenix contends that consistently with such findings in relation to claims 7 and 8, the primary judge ought to have found that Gilead failed to prove that any compound within claims 10, 12 and 13 (and dependent claims) were not effective in the treatment of at least one of the flaviviridae infections in at least one of a human or animal and hence failed to prove insufficiency. Idenix submits that the primary judge did not apply the sufficiency test correctly in relation to these claims. In making this submission, Idenix conflates the legal test for lack of utility and failure to describe the invention fully.
238 In our view, the primary judge’s reasoning and conclusion was correct (see at [620] to [625]). No error let alone any inconsistency has been demonstrated. Her findings were supported by the evidence of Dr Lambert and Professor Furneaux. Moreover, her Honour is not shown to be in in error in discounting the assertions of Professor Meier. Generally, her Honour rightly rejected the contention that “the skilled addressee, relying on the Idenix patent and the common general knowledge, could make any rational selection of classes of compounds from the trillions available to screen for anti-viral activity”.
NOVELTY AND LACK OF FAIR BASIS – overview
239 At trial, the issues of novelty and fair basis were intertwined. The 350 and 949 applications are priority documents that are relied upon by Idenix pursuant to s 43 of the Act and reg 3.12(1)(b) of the Patents Regulations 1991 (Cth) (as it then was) to yield the filing dates of one or other of those applications as the priority date for the claims in suit, rather than the later date of the filing of the Idenix specification (at [255]). Idenix concedes that if claim 7 is not fairly based on matter disclosed in either the 350 application or the 949 application, then claim 7 and all of its dependent claims are invalid for lack of novelty over the Clark Patent (at [252]).
240 The primary judge found that claim 7 was fairly based on the 949 application and so the novelty challenge failed. In ground 1 of its notice of contention Gilead challenges that finding. The primary judge found that claim 7 was not fairly based on the 350 application. In doing so she accepted one of Gilead’s arguments, but rejected two others. In ground 9 of its notice of appeal, Idenix challenges her Honour’s conclusion in relation to the 350 application, no doubt in order to protect its position in the event that Gilead’s contention in relation to the finding of fair basis on the 949 application is successful. Perhaps for similar reasons, in ground 1 of its notice of contention Gilead also argues that the primary judge was incorrect to accept only one of its arguments in relation to the 350 application.
241 Separately, Gilead argued at trial that claim 7 and its dependent claims are invalid because they are not fairly based on matter disclosed in the specification within s 40(3) of the Act. As we have noted, each of the 350 and 949 applications are incorporated by reference into the Idenix patent. Consistently with her findings in relation to external fair basis, the primary judge found that claim 7 was fairly based on the 949 application, but not the 350 application. As a result, her conclusion was that claim 7 is not invalid for want of fair basis. In ground 2 of its notice of contention Gilead challenges her Honour’s conclusions. In turn (unsurprisingly) in ground 10 of its notice of appeal Idenix challenges the finding in respect of the 350 application.
242 Below, we first consider grounds 9 and 10 of Idenix’s notice of appeal. Later we consider Gilead’s notice of contention.
grounds of appeal 9 and 10; lack of fair basis
243 Gilead contended before the primary judge that the relevant claims of the Idenix patent (claim 7 and all dependent claims) were not fairly based on the specification. In particular, Gilead contended that there was no real and reasonably clear disclosure in the specification of compounds which had a fluorine atom at the 2' down position together with a methyl at the 2' up position, a nucleobase that included a natural base and a prodrug element only at the 5' position. But it is said that such compounds were within claim 7 and dependent claims.
244 This issue was raised by Gilead in both the dimension of internal fair basis (incorporation of the 350 application and the 949 application into the body of the specification) and external fair basis, which was relevant to the priority date and accordingly the issue of an asserted lack of novelty. We will deal with external fair basis in more detail later when addressing Gilead’s notice of contention. Our following discussion in this section relates to fair basis and the incorporation of the 350 application in both the dimensions of internal and external fair basis in a narrower setting for the moment. Her Honour found that whether one was dealing with external fair basis (at [345] and [385]) or internal fair basis (at [423]), there was no real and reasonably clear disclosure in the 350 application of a claim 7 compound.
245 The primary judge considered whether claim 7 was fairly based on the 350 application in the context of Gilead’s argument that there was no real and reasonably clear disclosure in the 350 application of a compound where the substituent at the 2' down position was fluorine, the base was a natural base and there was a prodrug only at the 5' position (at [308]). Her Honour found that there had been no real and reasonably clear disclosure of the latter two elements. Before us, Idenix has contended that the primary judge erred in failing to find that claim 7 of the Idenix patent was fairly based on the 350 application.
246 First, the primary judge accepted that there was a real and reasonably clear disclosure of fluorine in the 2' down position because it was one of a number of possible substituents in Formula (IX) of the 350 application (at [329]). We can put that issue to one side for the moment and return to it in the context of Gilead’s first ground of contention.
247 Second, her Honour found that claim 7, insofar as it claimed a natural base, was not fairly based on the 350 application (at [345]).
248 Idenix contends that the primary judge accepted that Formula (IX) included as an integer a group identified by the term “Base*”, which term was defined to include natural bases. It is said that her Honour also recognised that not all of the formulae had that integer so defined, with others having the integer limited to non-natural bases. Idenix submits that Formula (IX) was introduced with the words “[i]n a fifth principal embodiment”, which is a reference to an embodiment of the invention, accepting that more than one invention may be disclosed. It is said that notwithstanding that, the primary judge found that claim 7 was not fairly based on Formula (IX) because it did not disclose a natural base. Idenix submits that her Honour’s conclusion involved giving precedence to general words in the 350 application over the clear indication in Formula (IX) that natural bases were included. It contends that the primary judge erred in determining there was ambiguity in Formula (IX) because of those general statements in circumstances where such ambiguity was entirely absent when the terms and integers of Formula (IX) were properly considered.
249 Third, the primary judge found that there was no disclosure of a compound with a prodrug only at the 5' position (at [346] and [385]). Her Honour accepted that Formula (IX) expressly included as an option a prodrug only at that position, but read in the context of the 350 application as a whole, nothing indicated that a compound within this formula should have a prodrug only at the 5' position.
250 Idenix has contended before us that the primary judge’s reading down of the formulae and in particular Formula (IX) involved error. It is said that the optional substituents in the formulae could not be treated as “stray phrases”. Idenix contends that the primary judge also accepted that various parts of the specification such as 2', 3' and/or 5' could embrace 5'-only prodrugs. Idenix contends that reasons would be needed to find that this did not include 5'-only prodrugs. Further, it says that the specification introduced each of the formulae as a “compound of Formula … or a pharmaceutically acceptable … prodrug thereof” with the effect that a prodrug element could be added at the 5' position only. Further, it says that as with other formulae, the available substituents at the 5' position of Formula (IX) (“R1”) included: (i) “H”; (ii) “phosphate (including mono-, di- or triphosphate…)”; (iii) “pharmaceutically acceptable leaving group which is capable of providing a compound wherein R1 … is … H”; and (iv) “phosphate (including … stabilized phosphate)” and “pharmaceutically acceptable leaving group which is capable of providing a compound wherein R1 … is … phosphate (including mono-, di- or triphosphate)”. Further, Idenix contended that the experts agreed that 5'-nucleotide prodrugs, being compounds in which the 5'-only phosphate group (be that a mono-, di- or triphosphate) of a nucleotide is masked or protected through chemical modification, are referred to as “stabilized phosphates”. Further, it is said that the primary judge accepted that some of the formulae specifically required 5'-only prodrugs but said they were not relevant because they had other elements. Idenix submits that the relevance of those formulae was that general words in the specification as to prodrug elements could not logically be used to qualify all or any specific formula, including Formula (IX). It is contended that there were no sound reasons to read “and/or” other than in the usual way. It is said that the primary judge erred in treating general statements, ambiguous at best, as modifying the clear disclosure of Formula (IX).
251 We would reject Idenix’s challenge.
252 Formula (IX) encompasses natural bases amongst the very broad range of bases defined by “Base*”. Moreover, the specification had to be read as a whole as the primary judge did. For example, the primary judge stated at [344]: “[w]hile the 350 application includes natural bases in its descriptions of Base … it is nevertheless doing so in the context of the disclosure of an invention or inventions in which the base is modified”. Indeed, the description of modified bases over more than 5000 pages of text and tables is not mere “general words”. It discloses the invention. The matching text or a generic formula that encompasses a narrower claim is not determinative of the nature of an invention. In Lockwood Security Products Pty Ltd v Doric Products Pty Ltd (2004) 217 CLR 274 (Lockwood (No 1)) at [99] it was stated:
… the correct position is that a claim based on what has been cast in the form of a consistory clause is not fairly based if other parts of the matter in the specification show that the invention is narrower than that consistory clause. The inquiry is into what the body of the specification read as a whole discloses as the invention. An assertion by the inventor in a consistory clause of that of which the invention consists does not compel the conclusion by the court that the claims are fairly based nor is the assertion determinative of the identity of the invention. The consistory clause is to be considered by the court with the rest of the specification. (footnote omitted)
253 Further, although Formula (IX) encompasses a nucleoside with a prodrug moiety only at the 5' position, as the primary judge held at [385], “[w]hen read as a whole the invention or inventions being disclosed in the 350 application necessarily involve nucleosides or nucleotides which have a prodrug at the 2' and/or 3' positions, optionally with a prodrug at the 5' position”. Further, the many pages of description of nucleosides with prodrug moieties only at the 2' or 3' position, or both, are not mere “general statements, ambiguous at best”. There was no ambiguity in the use of “and/or” once the context of its use was appreciated.
254 These grounds of appeal must be rejected. We will elaborate further on these matters when dealing with Gilead’s notice of contention.
grounds of appeal 11 and 12; lack of utility
255 Before the primary judge, Gilead contended that the Idenix patent claimed that the compounds described were useful in the prevention and treatment of flaviviridae infections including HCV, but the relevant claims encompassed compounds (or compositions or uses) that either could not be made (those where Y3 is bromine or iodine) or were inactive against flaviviridae infections including HCV or were toxic or both. Her Honour found that the promise of claim 7 was that compounds with a bromine or iodine at the Y3 position could be made (at [633]). Her Honour accepted Gilead’s contention that such compounds with bromine or iodine at the Y3 position could not be made and therefore claim 7 and the dependent claims were invalid on the ground of inutility.
256 Before us, Idenix has challenged such findings. Idenix contends that the evidence did not support a conclusion that compounds within claim 7 with a bromine or iodine at the Y3 position could not be made. It is said that there was no impediment to Gilead conducting experiments to prove the point and that the inference available from the absence of such evidence ought to have carried weight. Further, it is said that the evidence of the experts did not provide probative evidence that such compounds could not be made. It contends that their evidence was rather a purely theoretical expectation of difficulty rather than a “properly and empirically based” expert opinion as to impossibility, contrary to her Honour’s finding at [644]. Dependent claims 8 and 9 were in any event not affected.
257 But we reject Idenix’s arguments save the statement concerning claims 8 and 9. There is no requirement to prove inutility by experiment. Further, the primary judge rightly accepted the evidence of the experts, including Idenix’s expert Professor Meier, that some compounds within claim 7 could not be made. But of course we accept that claims 8 and 9 did not suffer from this vice as Y3 was stipulated as hydrogen for the purposes of those claims rather than as one of the halogens.
258 Dr Lambert gave evidence that based upon his understanding of the relative size of each of the halogens, he expected that not all combinations derived from C(Y3)3 could be successfully introduced at the 2' position in the up orientation. He then referred to difficulties with bromine and iodine.
259 Professor Furneaux said:
I do not have experience in attempting to introduce 3 chlorine atoms, 3 bromine atoms or 3 iodine atoms, or combinations of chlorine, bromine and iodine, to the same position on a complex molecule such as a sugar or nucleoside. I would expect an R12 substituent having one or more bromine and iodine atoms, due to their relative size, would be sterically hindered (or blocked) from approaching the 2'-position. Because of their size, I expect it would be particularly difficult to insert 3 iodine atoms or 3 bromine atoms at the R12 position. The Patent provides me with no guidance as to how to do so. I would not expect the reaction conditions to introduce 3 iodine atoms or 3 bromine atoms into the R12 position, if chemically possible, would be straightforward or predictable and I would need to undertake a substantial amount of experimentation to work out if this was in fact chemically possible.
260 Professor Meier gave the following evidence in cross-examination:
MR CATTERNS: Professor, I will ask that you be given a volume 1 of the hard copy we have of the 350 application. This has got the first thousand pages, but in particular it has the text of the first hundred and thirty or so pages, and I was going to go through it in some detail with you, but first would you mind going, please, to Formula I at page 13. We can see there in the 2’-(up) position the substituent C, and then it’s Y3 times 3. Is that right? --- Times, yes. That’s correct.
Thanks. And we can – I think we can see that – we see at various places that Y3 could be hydrogen or the halogens fluorine, chlorine, bromine, iodine. Is that right? --- Correct, yes.
Just as a matter of practicalities, would you agree that there would be an increasing degree of difficulty in synthesising a nucleoside of Formula I with chlorine, bromine or iodine in the position of being Y3? --- Yes.
And could you explain why, please? --- So the – the options that are given for Y3 can be subdivided into groups, if you want. So there is the hydrogen, as you mentioned, and the halogens. The separation or this differentiation can be made by the electronegativity of the halogens in contrast to hydrogen, and the halogens are – and the series of the hydrogens as they are given here, they are increasing in size from the fluorine, which is the smallest, going to iodine, which is the biggest, and then you – in order to synthesise compounds bearing these substituents, then you need to make compounds or make reagents that have this fragment already in.
That’s the fragment --- ? --- And this is getting – getting more and more difficult to – to make them.
As a practical matter, would it be impossible by the time one gets to the size of bromine or iodine? --- I think so, yes.
261 This ground of appeal must be rejected.
Notice of contention ground 1; lack of novelty
262 Gilead contended before the primary judge that the relevant claims of the Idenix patent were not fairly based on the 350 and 949 applications. In the context of the novelty question we, of course, are dealing with external fair basis. As such, it was contended that the Idenix patent was not entitled to the priority dates of 28 June 2002 or 14 May 2003, but was only entitled to the date on which the specification for the Idenix patent was filed being 27 June 2003. If this was so, as Gilead contended, then the relevant claims of the Idenix patent were not novel because the same invention was anticipated by the Clark patent, which was entitled to the priority date of 30 May 2003.
263 For present purposes, we adopt her Honour’s description of the applicable legislative regime at [253] to [255] and also the Full Court’s discussion of this topic in Multigate Medical Devices Pty Ltd v B Braun Melsungen AG (2016) 117 IPR 1; [2016] FCAFC 21 at [184] to [190] per Bennett, Yates and Beach JJ.
264 As we have discussed earlier, the primary judge held that claim 7 was not fairly based on the 350 application because there was no real and reasonably clear disclosure in the 350 application of a compound where the substituent at the 2' down position was fluorine, the base was a natural base and there was a prodrug only at the 5' position. The primary judge held at [329] that there was a real and reasonably clear disclosure of a compound with fluorine at the 2' down position, but that there was no disclosure of such a compound which also had a natural base and a prodrug only at the 5' position.
265 As for the 949 application, her Honour held at [413] that claim 7 was fairly based on the 949 application. Her Honour accepted that there was a real and reasonably clear disclosure in the 949 application of a compound of claim 7. Further, her Honour found that there was the relevant disclosure of the use of such a compound to treat flaviviridae as claimed in claim 10 (at [414]).
266 In summary, the primary judge found that claim 7 and the dependent claims of the Idenix patent were not entitled to the priority date of the 350 application. But her Honour found (at [417]) that claim 7 and the dependent claims were entitled to the priority date of the 949 application. Accordingly, her Honour found that the priority date of claim 7 and the dependent claims was the date of filing of the 949 application, which was 14 May 2003, being a date before the Clark priority date. Accordingly, her Honour concluded that the Clark patent did not deprive the Idenix patent of novelty. Thus, Gilead failed on this ground of invalidity.
267 Gilead in its notice of contention has submitted that the primary judge erred by failing to find that there was no real and reasonably clear disclosure in the 350 application of such a compound with a fluorine at the 2' down position, or of such a compound with a fluorine at the 2' down position in combination with a methyl group at the 2' up position; as we have already indicated, Gilead won on the other points concerning “natural base” and “5' only pro-drug” (which were the subject of Idenix’s notice of appeal at grounds 9 and 10 discussed above). In respect of the 949 application, Gilead contends that the primary judge should have found that there was no real and reasonably clear disclosure in that application of a compound of claim 7 or of the use of such a compound to treat a flaviviridae infection as claimed in claim 10. It is convenient to first deal with the issues concerning the 949 application and her Honour’s external fair basis finding sourced to the 949 application.
(a) Priority on the basis of the 949 application
268 The primary judge held at [417] that claims 7 to 41 of the Idenix patent were entitled to priority on the basis of the 949 application. At [413], her Honour said:
In respect of formula II (on which Idenix did rely, albeit belatedly), R3 is the 2' down position. It is said on p22 to be “defined as above”. This is a cross-reference to p20 in which, as Gilead said, amongst an even more expansive list of potential substituents than formula (AA) for this position, halo is mentioned, which is defined to include fluoro. However, consistent with my reasoning above about the 350 application and given that “halo” is defined to include fluorine I consider that the 949 application provides a real and reasonably clear disclosure of F at the 2' down position. Moreover, unlike the 350 application, nothing in the 949 application indicates that the invention disclosed is one that involves a prodrug at the 5' position only in combination with a prodrug at the 2' and/or 3' positions or that the base must be a modified base. On this basis, given that claim 7 is a claim to a compound only, I consider that claim to be fairly based on the 949 application.
269 Gilead points to the fact that the substituents of Formula (II) at the 2' up position include methyl (it falls within “C1-4 alkyl”) and many others. It also refers to the fact that the 2' down substituent, R3, is “defined as above”, which includes halo or halogen among at least 75 other possible substituents, but offers no preference for halo or halogen and does not even expressly refer to fluorine.
270 Gilead contends that there was no real and reasonably clear disclosure of fluorine in the 2' down position, i.e. R3 in Formula (II) of the 949 application.
271 Gilead submits that a broad generic disclosure, such as Formula (II), does not provide a fair basis for the limited species of the claims in suit. It is said that the concept of real and reasonably clear disclosure (as contrasted with “mere” disclosure) must be given substantive content. Gilead points out that the primary judge rejected Idenix’s submission that there is no scope to find a lack of fair basis if the subsequent embodiment is more specific than that in the priority document. Gilead says that this rejection was correct and based upon the authorities. Accordingly, it is said that her Honour ought to have held that this was just such a case, where a “more specific” embodiment lacks fair basis.
272 Gilead says that the 949 specification does not describe a compound of claim 7 with F at the 2' down position. Gilead submits that no rationale, direction or description is given by which the skilled reader can derive from the 949 application any disclosure of the narrower range of compounds claimed in claim 7. It submits that the primary judge erred in finding that the 949 application provides a real and reasonably clear disclosure of fluorine at the 2' down position.
273 Gilead says that her Honour said that she applied reasoning consistent with her reasoning in respect of the 350 application. Her Honour said at [329]:
Consistently with the ratio in Roche and the reasoning in Lockwood I consider that this issue must be answered in Idenix’s favour. By this I mean that by reason of F being one of the multitude of potential substituents at the 2' down position in formula IX it must be concluded that there is a real and reasonably clear disclosure of a compound with F at the 2' down position in the 350 application.
274 Gilead says that her Honour’s reasoning raises the question of whether a generic disclosure, such as by a broad and non-specific chemical Markush formula with a multitude of potential substituents can provide fair basis for a specific compound or group of compounds falling within the generic formula.
275 Now Gilead points to the fact that at [327] to [328], the primary judge referred to Novartis AG v Hospira Pty Ltd (2012) 98 IPR 185; [2012] FCA 1055 at [64] per Yates J (albeit only dealing with the context of an interlocutory injunction application) and Pfizer Inc v Commissioner of Patents (2005) 141 FCR 413 at [43] per Bennett J which stand for the proposition, which we accept, that when analysing fair basis, concepts such as “inventive selection” or “inventiveness” are not apposite. Applying Lockwood (No 1), the question is whether there is a real and reasonably clear disclosure, requiring a comparison between what is claimed and what has been described in the 949 application. Concepts of inventive step or merit have little to do with the inquiry.
276 But Gilead submits that it did not rely on such inapposite concepts. Its contention was that the broad generic disclosure of Formula (II), which encompassed fluorine at the 2' down position and CH3 at the 2' up position, each among a number of other possibilities, did not constitute a “real and reasonably clear disclosure” of the compounds claimed in claim 7 and dependent claims (Lockwood (No 1) at [69]).
277 Now her Honour held (at [413]) that the more specific claims were fairly based on the broad generic disclosure of Formula (II) as the compounds were included within the “even more expansive” formula. But Gilead submits that her Honour’s reasoning was erroneous. It contends that a narrower claim that falls within a broad disclosure is not, ipso facto, fairly based. Gilead says that the primary judge recognised this at [310] but failed to apply it at [413].
278 Further, Gilead submits that the reasoning that a compound must be fairly based if it falls within the scope of a broad formula is contrary to authority. Gilead points to Coopers Animal Health Australia Ltd v Western Stock Distributors Pty Ltd (1987) 15 FCR 382 at 389, where Fox J said, “A starting point is to ascertain the invention which is ‘described’ in the provisional specification”. Gilead contends that this is consistent with Lockwood (No 1). The solvent DGBE, an integer of the claim, was merely adverted to as one member of a class of solvent; it was not treated as part of the invention (at 390).
279 Gilead also points out that a submission that narrowing amendments must necessarily be fairly based was rejected at first instance in Apotex Pty Ltd v AstraZeneca AB (No 4) (2013) 100 IPR 285; [2013] FCA 162 at [424] to [428]. It is said that the narrower claims lost their priority date in that case. It is said that that holding was upheld in AstraZeneca AB v Apotex Pty Ltd (2014) 226 FCR 324 (AstraZeneca) at [231] to [247] and [253], stating at [244]:
A very general description of an invention in a specification before amendment might not contain a real and reasonably clear disclosure of more specific embodiments of the invention subsequently disclosed and claimed after amendment.
280 In the present case, Gilead submits that there is no rationale given in the 949 application or any other description or direction as to why the more specific embodiments of the invention subsequently claimed should be chosen. There was no real and reasonably clear disclosure of those embodiments. It is said that the primary judge ought to have concluded that claim 7 and dependent claims of the Idenix patent were not entitled to priority based on the 949 application.
281 Gilead has also made reference to the fact that at [270] to [271], the primary judge referred to passages in AstraZeneca (at [244], [285] and [286]) that were in the context of novelty “but nevertheless concerning the concept of disclosure”. Gilead contends that disclosure “with sufficient clarity” for novelty (ICI Chemicals & Polymers Ltd v The Lubrizol Corporation Inc (2000) 106 FCR 214 at [49]) has an affinity with “real and reasonably clear disclosure” for fair basis. Gilead submits that the relationship between novelty and priority (as reflected in the definition of “prior art base”) demonstrates an important aspect of the scheme of the Act. It is said that the researcher who files a patent application early, disclosing a genus encompassing millions of compounds, one of which might have particular therapeutic value, ought not to be able to prevent a competing researcher, who identifies a specific compound within the genus and determines that it has specific therapeutic effect, from securing patent protection for the specific compound. It is said that this policy objective is supported by rigorous tests for both priority and novelty. Further, it is said that a generic application that lacks a real and reasonably clear disclosure of the compound in question should neither anticipate the specific claim of the second researcher nor confer priority on a later complete specification filed by the first researcher that claims the particular compound.
282 We would reject this ground of Gilead’s notice of contention.
283 In our view the primary judge was correct to find that there was a real and reasonably clear disclosure of a fluorine atom in the 2' down position. Halo (halogen) is in the list of substituents for R3 and fluorine is a halo. Further, Professor Furneaux understood this definition of “halo” to include fluorine, amongst a finite number of other substituents, namely chlorine, bromine and iodine. As such, there was a real and reasonably clear disclosure of fluorine at the 2' down position.
284 It is worthwhile at this point to recall the principles set out in Lockwood (No 1), where the High Court said (citations omitted):
[68] Erroneous principles. The comparison which s 40(3) calls for is not analogous to that between a claim and an alleged anticipation or infringement. It is wrong to employ “an over meticulous verbal analysis”. It is wrong to seek to isolate in the body of the specification “essential integers” or “essential features” of an alleged invention and to ask whether they correspond with the essential integers of the claim in question.
[69] “Real and reasonably clear disclosure”. Section 40(3) requires, in Fullagar J’s words, “a real and reasonably clear disclosure.” But those words, when used in connection with s 40(3), do not limit disclosures to preferred embodiments.
The circumstance that something is a requirement for the best method of performing an invention does not make it necessarily a requirement for all claims; likewise, the circumstance that material is part of the description of the invention does not mean that it must be included as an integer of each claim. Rather, the question is whether there is a real and reasonably clear disclosure in the body of the specification of what is then claimed, so that the alleged invention as claimed is broadly, that is to say in a general sense, described in the body of the specification.
Fullagar J’s phrase serves the function of compelling attention to the construction of the specification as a whole, putting aside particular parts which, although in isolation they might appear to point against the “real” disclosure, are in truth only loose or stray remarks.
…
[71] … The correct way of answering the question is to examine the body of the specification in order to see what it describes as the invention.
285 The passage in [68] serves to address Gilead’s argument as to the affinity of this ground with novelty. The question concerns disclosure. It is a practical test. In the present context it is assisted by expert evidence. In that connection there is no doubt that the compounds falling within claim 7 are disclosed in the specification, or that the person skilled in the art would recognise that fact.
286 Now Gilead asserts (as we have said) that the primary judge erred at [413] when she stated that the compound is fairly based if it falls within the scope of a broad formula. It is said that so construed, this would be contrary to Lockwood and to Full Court authority. We reject these arguments.
287 First, Gilead mischaracterises the primary judge’s reasoning. The primary judge rejected in respect of the 350 application the pure ipso facto reasoning which Gilead submits was employed. The primary judge’s rejection was expressly applied to the 949 application at [413], as the final 2 sentences in [413] make clear. Gilead’s submission to the effect that in the same paragraph (at [413]), the primary judge failed to apply the reasoning at [310], ought not be accepted.
288 Further, the primary judge’s phrase at [413], “my reasoning above about the 350 application”, also picked up her reasoning on the 350 application at [327] to [329]. In this regard, the primary judge at [327] correctly followed Novartis at [63] and [64] in which Yates J referred to but declined to follow the following obiter statements of Gibbs J in F Hoffman-La Roche & Co AG v Commissioner of Patents (1971) 123 CLR 529 at 542 to 543:
It is crucial, in my view, that it is not suggested that the compounds the subject of proposed claims 2, 3 and 4 have been selected because they have any special utility. If a basic application disclosed a large class of compounds, all of which were claimed to be of pharmaceutical utility, and it were found that the claim was false, in that only some of the compounds were useful, or it appeared that some of the compounds had a particular and peculiar value, there would be much to be said for the view that a claim limited to those compounds selected for their utility or special value would not be fairly based on matter disclosed in the basic application, at least if the basic application did not itself provide a guide to that selection, and a fresh inventive step were necessary to enable it to be made. Here however a large class of compounds is disclosed, and clearly disclosed, in the basic application, and proposed claims 2, 3 and 4 in the complete specification are for compounds forming part of that class, but not selected because they alone are useful or because they have utility greater than that of other members of the class.
289 Yates J correctly indicated that he was not bound by such obiter remarks in preference to the statements in Lockwood (No 1) at [50] and [68] to [69]. Further, the primary judge at [328] explained that like Novartis, Bennett J in Pfizer at [43] indicated that such remarks of Gibbs J should not be followed in preference to Lockwood (No 1).
290 Now Gilead has sought to draw comfort from Coopers and AstraZeneca. But although Gilead appears to have disavowed the concept of “inventive selection” as part of its argument, Coopers (decided before Lockwood (No 1)) utilised the concept of inventive selection in a fair basis context. In Coopers, Fox J (with whom Spender J agreed) at 389 treated the obiter observations of Gibbs J in F Hoffman-La Roche at 542 as standing for the principle that the “selection of one compound with special qualities from a class of compounds disclosed in the provisional is not permissible”, and proceeded to apply that principle (at 390 to 391), using the language and themes of inventive step. But Coopers does not assist Gilead. It has been overtaken by Lockwood (No 1). Further, AstraZeneca does not stand as any contrary authority as we will explain in a moment.
291 Third, the primary judge stated at [329] that “[c]onsistently with the ratio in [F Hoffman-La Roche] and the reasoning in [Lockwood (No 1)] I consider that this issue must be answered in Idenix’s favour”. The ratio of F Hoffman-La Roche, as her Honour recognised by the primary judge, was in substance a test of “real and reasonably clear disclosure” as a matter of construction of the priority document. And when that test was applied to the priority document in that case, which disclosed a class of compounds (at 542 to 543), the result was that there was a real and reasonably clear disclosure of a compound within the class. This was how Pfizer at [73] applied Hoffman-La Roche, with Pfizer being undoubtedly a correct application of Lockwood (No 1). Similarly, in the present case, when the primary judge applied the test of real and reasonably clear disclosure to the 949 application, her Honour held that there was such a disclosure of fluorine in the 2' down position. This was because there was a clear disclosure of a class of compounds which included compounds with that configuration. Accordingly, there was no error made by her Honour at [413].
292 Fourth, there is no inconsistency between her Honour’s approach and that of the plurality (Besanko, Foster, Nicholas and Yates JJ) of the Full Federal Court in AstraZeneca. In AstraZeneca, the relevant claim was for a pharmaceutical composition comprising rosuvastatin or a pharmaceutically acceptable salt thereof as the active ingredient, and an inorganic salt in which the cation was multivalent. The claim was amended after filing to exclude inorganic salts where the counter anion was a phosphate and to make the same exclusion in the consistory clauses (at [34] and [232]). The plurality said (at [247]) of the specification prior to amendment:
However, the specification states that dibasic calcium phosphate and tribasic calcium phosphate are preferred inorganic salts, and tribasic calcium phosphate is the inorganic salt used in each of the Examples (Examples 1 to 4) of the pharmaceutical compositions of the invention described in detail in the specification. In each of these compositions the phosphate is the counter anion to the inorganic salt. This is fundamentally inconsistent with the revised form of claims and the additional matter introduced as a result of the 2005 amendments. Not only does the specification before amendment not suggest that phosphate not be used as the counter anion in the inorganic salts used in the pharmaceutical compositions of the invention, it positively recommends that it be used for that purpose. We are satisfied that the unamended specification does not contain a real or reasonably clear disclosure of what was claimed as a result of the 2005 amendments.
293 Accordingly, there was an inconsistency between the revised claims and the relevant “matter” with which it was being compared for the purposes of fair basis, being the specification prior to amendment. Inconsistency between the matter said to disclose the invention and the invention points against there being a real and reasonably clear disclosure of the invention. In Sigma Pharmaceuticals (Australia) Pty Ltd v Wyeth (2011) 119 IPR 194; [2011] FCAFC 132, claims unlimited to a particular type of extended release formulation of venlafaxine hydrochloride were found to be directly contradicted by unambiguous statements in the body of the specification that an extended release dosage form of venlafaxine hydrochloride was impossible to achieve or fruitless using hydrogel tablet technology (at [99] per Bennett J and [242] per Yates J). It was found that formulations produced by or using such technology were effectively disclaimed as being part of the invention (at [171] per Nicholas J and [247] per Yates J). In both Sigma and AstraZeneca, a significant inconsistency between the priority document and the invention claimed led to a lack of a real and reasonably clear disclosure. But in the case before her Honour (and now before us) there is no such inconsistency.
294 Further, the plurality in AstraZeneca at [244] drew a distinction between a case that involved a positive exclusion and another hypothesised case where there was a very general description of an invention before amendment and a more specific embodiment after amendment. It was said at [244]:
We do not accept that s 114(1) can never be engaged in circumstances where the claim in question claims less than what was described in the specification immediately prior to the amendment. A very general description of an invention in a specification before amendment might not contain a real and reasonably clear disclosure of more specific embodiments of the invention subsequently disclosed and claimed after amendment. Similarly, in the case of a claim to a pharmaceutical formulation, the description of the invention in the specification before amendment might allow for the use of particular classes of chemicals with which to make the formulation, while the amended claim might positively exclude their use for that purpose. Whether or not there is a real and reasonably clear disclosure in the specification before amendment of what is claimed in such circumstances is the question that arises in this case.
295 Gilead’s characterisation of the disclosure in the 949 application regarding fluorine as a “general description” is contestable. The disclosure of substituents of a chemical compound by way of a Markush structure within a Haworth projection is precise and specific for each of the relevant substituents and commonly used and so understood by chemists.
296 Fifth, Gilead’s contention that there is no rationale as to why the more specific embodiments of the invention subsequently claimed should be chosen, is essentially a repeat of Gilead’s arbitrary selection or arbitrary narrowing submission which was rejected by the primary judge at [416]. It is inconsistent with the test of real and reasonably clear disclosure set out in Lockwood (No 1). The asserted concept of needing to provide a guide to an inventive or at least non-arbitrary selection was part of the obiter observations of Gibbs J in F Hoffman-La Roche, which do not supplant Lockwood (No 1).
297 Finally and in any event, as Idenix points out, Formula (AA) of the 949 application provides an additional disclosure of the compounds of claims 7 to 9. Gilead accepted that claim 7 of the Idenix patent is within Formula (AA) (at [400]). The primary judge did not find to the contrary; the finding at [410] is restricted to claim 10 and dependent claims, each of which involve the treatment of a flaviviridae infection.
298 In summary, her Honour’s finding of external fair basis based upon the 949 application has not been shown to be in error.
(b) Priority on the basis of the 350 application
299 As we have earlier indicated, the primary judge identified three attributes that were in issue in relation to the fair basis of claim 7 (and dependent claims) concerning reliance on the 350 application. First, whether fluorine was at the 2' down position (in combination with methyl at the 2' up position). Second, whether there was a natural nucleobase. Third, whether there was a prodrug moiety at the 5' position only.
300 The primary judge held at [329] that there was no real and reasonably clear disclosure of a compound with fluorine at the 2' down position which also had a natural base and a prodrug only at the 5' position. She accepted though that there was disclosure of a fluorine at the 2' down position. Her Honour therefore held that the Idenix patent was not entitled to priority on the basis of the 350 application.
301 Gilead contends before us that her Honour erred in finding that there was a real and reasonably clear disclosure of a compound with fluorine at the 2' down position. It is said that in making that finding, her Honour in effect accepted that the invention claimed in claim 7 and dependent claims was disclosed in Formula (IX) of the 350 application. Claim 7 is a subset of Formula (IX).
302 As to the disclosure in the 350 application, the primary judge found at [320]:
(1) The 350 application does not describe a compound of claim 7 with F at the 2' down position.
(2) The approximately 5000 pages of tables of possible substituents and various combinations thereof in respect of each example (which, it is accepted, do not include an example based on formula IX) do not identify F at the 2' down position.
(3) Only formulas IV, V, VI, VII, IX, X, XI, XII and XIX permit F at 2' down (and, except for IV, this is among many other potential substituents).
(4) In formula IX, F at 2' down is allowed for among many (approximately 70) primary options at that position (and many more sub-options within the named possible substituents).
(5) The 350 application does not explain why, from the numerous potential substituents at the 2' down position, F would be preferred. No rationale for F being present at that position, other than its appearance amongst a multitude of other possible substituents, is provided.
303 Gilead submits that those findings should, in addition to the findings at [329], have led to the conclusion that there is no real and reasonably clear disclosure of the compounds of claim 7 and dependent claims in the 350 application. It is said that the compounds of claim 7 could not be observed in the 350 application; they must be derived by the skilled person and the specification provides no direction or assistance in this respect. It is said that no significance is attributed to having F at the 2' down position and that it is merely an arbitrary narrowing and not fairly based. It is said that the 350 specification describes a virtually infinite number of modifications of all positions of the nucleoside with particular focus, as the primary judge held, on modified bases and on prodrug moieties at the 2' or 3' positions, or both.
304 It is said that the 350 application provides a list of the substituents at the 2' down position (R13). At [324], the primary judge said:
As Gilead noted, given the sub-classes within a number of the potential substituents identified in the 2' down position, formula IX permits F at 2' down amongst thousands of possibilities. Nothing in the 350 application explains why F at 2' down might be preferred to any one or other of those thousands of possibilities.
305 It is pointed out by Gilead that the 350 application also provides a list of the substituents at the 2' up position (R12). There are 43 substituents or classes of substituents, with each class of substituent encompassing many different individual substituents. The combination with fluorine at 2' down multiplies the possibilities.
306 It is said that as the primary judge said at [320(5)], there is no rationale presented in the 350 application (nor is there any other substantive disclosure) by which the reader can perceive that the modified nucleoside with fluorine at the 2' down position is part of the invention or inventions disclosed. Gilead says that this is a significant finding and that the same applies to the choice of C(Y3)3 for R12 at 2' up and its combination with fluorine at the 2' down position (see [312]).
307 It is said that the primary judge reiterated this point at [326]:
Formula IX permits F at 2' down amongst many possibilities. Is claim 7, with F at 2' down, thereby fairly based? Is there a real and reasonably clear disclosure of a compound with F at 2' down in circumstances where there are thousands of potential substituents at that position, nothing in the 350 application discloses any reason for preferring F at 2' down over and above any of the other many potential substituents at this position in formula IX, and nothing in the common general knowledge would direct the skilled addressee to consider F over and above any of the other potential substituents? (emphasis added)
308 Finally, Gilead contends that the primary judge’s consideration of F Hoffman-La Roche, Novartis and Pfizer at [327] to [328] led her Honour to depart from the answer that her Honour might otherwise have given to the question posed at [326]. That is, by reason of fluorine being one of the multitude of potential substituents at the 2' down position in Formula (IX) it must be concluded that there is a real and reasonably clear disclosure of a compound with fluorine at the 2' down position in the 350 application (at [329]). Gilead submits that that departure reflects error. It is said that the broad generic disclosure of Formula (IX) did not provide a real and reasonably clear disclosure of the narrow species of claim 7 compounds.
309 We reject Gilead’s contentions.
310 Gilead’s submissions that the primary judge erred at [329] have sought to superimpose upon the concept of “real and reasonably clear disclosure” a requirement that the matter being compared with the claim provide some means to select the precise embodiment of the invention claimed in order for the claim to be fairly based upon it. In our view this approach was correctly rejected by the primary judge.
311 Further, although Gilead submits that F Hoffman-La Roche, Novartis and Pfizer led the primary judge to depart from the answer that (it appears from [326]) her Honour might otherwise have given, the ratio of each of those cases was consistent with Lockwood (No 1). The primary judge correctly applied them. The primary judge committed no error in dealing with those cases.
312 Finally, there was no dispute that fluorine was one of the substituents at the 2' down position in Formula (IX) of the 350 application. The finding by the primary judge at [329] that it must be concluded that there is therefore a real and reasonably clear disclosure of a compound with fluorine at the 2' down position in the 350 application was a correct application of Lockwood (No 1).
notice of contention ground 2; internal fair basis
313 Gilead contended before the primary judge that claim 7 of the Idenix patent and dependent claims were not “fairly based on the matter described in the specification” (the language of s 40(3) as it was at the time) because there was no real and reasonably clear disclosure in the specification of compounds within claim 7 which have fluorine at the 2' down position, a natural nucleobase and a prodrug at the 5'-only position (and not at the 2' and/or 3' positions).
314 The primary judge held at [431] that the embodiments on page 100 of the Idenix patent match or mirror claims 7, 8 and 9. First, her Honour stated that given that the terms of the Idenix patent also reflect the 350 application, which contains a number of ambiguities as she found in relation to external fair basis, it “might be concluded that whatever the Idenix patent discloses as the invention is not ‘reasonably clear’”. Second, her Honour found that in circumstances where the Idenix patent expressly incorporates by reference the 949 application (which her Honour had found provided external fair basis for the Idenix patent, given Formula (II)) and the Idenix patent also contains an express disclosure consistent with the disclosure in the 949 application at page 100, her Honour rejected Gilead’s argument that the Idenix patent did not contain a real and reasonably clear disclosure of the compounds of claim 7.
315 Gilead contends before us that the primary judge should have held that claims 7 to 41 of the Idenix patent were not fairly based. Gilead contends that the primary judge erred in holding at [431] that the “matching” preferred embodiments at page 100 of the complete specification provided fair basis for the claims, and that s 40(3) was therefore complied with. Further, Gilead contends that the primary judge erred in holding that because the 949 application was incorporated by reference in the specification, this also provided fair basis for the claims.
316 Gilead submits that the findings of the primary judge in the context of the 350 application, in relation to the lack of fair basis for the presence of a natural base and a prodrug moiety only at the 5' position, apply equally to the specification of the Idenix patent, both because the 350 application is incorporated by reference and because its text is largely reflected in the complete specification in any event. It is said that because the text of the 350 application is essentially the same as the Idenix patent, subject to the matching formula at page 100, her Honour’s conclusion at [329] should also have applied to the issue of internal fair basis. Her Honour said at [431] that given those matters, “it might be concluded that whatever the Idenix patent discloses as the invention is not reasonably clear” (emphasis added). Gilead submits that it should have followed that there was no fair basis.
317 Gilead submits that although the embodiments at page 100 “match claims 7, 8 and 9” (at [427]), it is not enough. It is said that the question is one of substance and not form. Gilead says that the Full Court in AstraZeneca rejected a submission that “the consistory clause was itself sufficient” (AstraZeneca at [417]).
318 Gilead submits that this is a case where the observations in Lockwood (No 1) at [87] are apposite, which are:
Finally, it is necessary to consider the trial judge’s citation of Atlantis Corporation Pty Ltd v Schindler for the proposition that to couch a claim “in the same terms as the description of the invention in the specification” did not of itself, by that mere “coincidence of language”, establish fair basing. That proposition is correct, but it is not fatal to the Patentee’s position in this case. A “coincidence of language” between a claim and part of the body of a specification does not establish fair basing if that part of the language of the specification does not reflect the description of the invention in the light of the specification as a whole. In the Atlantis Case, the specification, read as a whole, described an apparatus limited to a particular use as a sub-soil drainage system. The claims, however, were “pure apparatus” claims without that limitation on use. The Full Court of the Federal Court of Australia refused to construe them narrowly so as to conform with the description in the specification. A statement in the specification of a description of the invention in similar language to the first claim was not treated as the description of the invention. While the Full Court did not engage in close textual analysis, it did distinctly hold that the statement in the specification:
“should not be allowed to disguise the fact that the invention disclosed in the body of the specification is truly ‘a sub-soil drainage method based on a particular apparatus’ or ‘a particular apparatus in its application to sub-soil drainage’. The claims, however, are ‘pure apparatus claims’. They are not subject to any limitation as to use. They travel beyond, and are not fairly based on, the matter described in the specification.”
In short, the case is distinguishable. Here, the Patentee does not rely on mere “coincidence of language”: it contends that the language used, unlike that employed in the Atlantis Case, does describe the invention. (footnotes omitted)
319 It is said that the particular species described at page 100 do not “reflect the description of the invention in the light of the specification as a whole” and that the present case is a case of “mere coincidence of language”.
320 Gilead further contends that the incorporation of the 949 application does not improve the disclosure. It says that Formula (II) in the 949 applications appears in a different context when incorporated in the complete specification of the Idenix patent. It is said that the focus of the 949 application is on different viruses. Gilead submits that when the 949 application and 350 application are incorporated, the formula at page 100 as well as Formula (II) of the 949 application and Formula (IX) of the 350 application are several entirely unrelated formulae among thousands, with nothing to single them out from a multitude of compounds, described over thousands of pages. As such, Gilead submits that that cannot amount to a real and reasonably clear disclosure. As Lockwood (No 1) at [99] stated:
… The inquiry is into what the body of the specification read as a whole discloses as the invention. An assertion by the inventor in a consistory clause of that of which the invention consists does not compel the conclusion by the court that the claims are fairly based nor is the assertion determinative of the identity of the invention. The consistory clause is to be considered by the court with the rest of the specification. (footnote omitted)
321 Gilead submits that there is an absence of any rationale or direction or description from which the skilled addressee can identify the compounds of claim 7. Therefore claim 7 and the dependent claims do not comply with s 40(3) of the Act.
322 We reject these submissions.
323 First, the following table (provided to us by Idenix and which it is convenient to set out) demonstrates the clear disclosure of the compounds of claims 7 to 9 at page 100 of the Idenix patent. Further, the evidence of Professor Furneaux was that the passage at page 100 lines 6 to 27 of the Idenix patent is “almost identical” to claim 7 and the passage at page 100 lines 28 to 29 “correlates with” claims 8 and 9. In the following table, underlined is the text common to both claims 7 to 9 and the disclosure of Formula (IX) at page 100 of the Idenix patent.
Claim | Page 100 of the Idenix patent |
Claim 7 | Lines 6-27 |
A compound of Formula (IX):
or a pharmaceutically acceptable salt thereof, wherein: | In another preferred embodiment, a compound of Formula (IX), or a pharmaceutically acceptable salt or prodrug, or a stereoisomeric, tautomeric or polymorphic form thereof, is provided, as well as a method for the treatment of a host infected with a Flaviviridae comprising administering an effective treatment amount of compound of Formula (IX):
or a stereoisomeric, tautomeric or polymorphic form thereof, or a pharmaceutically acceptable salt thereof, wherein: |
R1 and R2 are independently H; phosphate; straight chained, branched or cyclic alkyl; acyl; CO-alkyl; CO-aryl; CO-alkoxyalkyl; CO-aryloxyalkyl; CO-substituted aryl; sulfonate ester; benzyl, wherein the phenyl group is optionally substituted with one or more substituents; alkylsulfonyl; arylsulfonyl; aralkylsulfonyl; a lipid; an amino acid; a carbohydrate; a peptide; cholesterol; or a pharmaceutically acceptable leaving group which when administered in vivo is capable of providing a compound wherein R1 and/or R2 is independently H or phosphate; | R1, R2 and R3 are independently H; phosphate; straight chained, branched or cyclic alkyl; acyl; CO-alkyl; CO-aryl; CO-alkoxyalkyl; CO-aryloxyalkyl; CO-substituted aryl; sulfonate ester; benzyl, wherein the phenyl group is optionally substituted with one or more substituents; alkylsulfonyl; arylsulfonyl; aralkylsulfonyl; a lipid; an amino acid; a carbohydrate; a peptide; cholesterol; or a pharmaceutically acceptable leaving group which when administered in vivo is capable of providing a compound wherein R1, R2 and/or R3 is independently H or phosphate; |
X is O, S, SO2 or CH2; | X is O, S, SO2 or CH2; |
Base* is a purine or pyrimidine base; R12 is C(Y3)3; Y3 is independently H, F, Cl, Br or I; and R13 is fluoro. | Base* is a purine or pyrimidine base; R12 is C(Y3)3; Y3 is independently H, F, Cl, Br or I; and R13 is fluoro. |
Claim 8 | Line 28 |
The compound of claim 7, … wherein X is O, and Y3 is H. | In one subembodiment [of Formula (IX) at lines 6-27] … … X is O and Y3 is H. |
Claim 9 | Lines 28-29 |
The compound of claim 8, wherein R1 and R2 are H. | In another subembodiment [of Formula (IX) at lines 6-27], when X is O and Y3 is H, R1, R2 and R3 are also H. |
324 Second, Formula (II) of the 949 application and Formula (AA) (in respect of claims 7 to 9) provide a clear disclosure of the compounds claimed. We agree with Idenix that because they are claims to compounds per se, the matters as to viruses relied upon by Gilead are irrelevant.
325 Third, the primary judge correctly considered page 100 of the Idenix patent and Formula (II) of the 949 application and in the context of the specification as a whole, properly applied in our view Lockwood (No 1) to find that there was a real and reasonably clear disclosure.
326 Fourth, the thrust of Gilead’s submission emphasises that the specification discloses many other compounds, including by reason of its incorporation by reference of the other application(s). Accordingly, it seems to contend that the express disclosures at page 100 of the Idenix patent and Formula (II) of the 949 application are somehow to be discounted or diluted so as to be treated as not real and reasonably clear. But Lockwood (No 1) does not justify such a position or support such a dilutive-type effect. Further, Lockwood (No 1) made clear that it was erroneous to isolate essential features from the body of the specification or to focus on preferred embodiments. There is no basis for saying that the requirement to consider the whole specification somehow warrants saying that some otherwise clear part of the specification is somehow to be outweighed or diminished by the number of other compounds disclosed in assessing the question of fair basis.
Conclusion
327 Idenix’s appeal must be dismissed. Gilead’s grounds of contention are also rejected. Prima facie, Gilead would ordinarily be entitled to costs, but that position may be affected in part by its lack of success on the grounds of contention. In the circumstances, we will give the parties the opportunity to file short submissions addressing that matter.
I certify that the preceding three hundred and twenty-seven (327) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justices Nicholas, Beach and Burley. |
Associate:
NSD 640 of 2016 | |
UNIVERSITE DE MONTPELLIER | |
Fifth Appellant: | IDENIX (CAYMAN) LIMITED |