FEDERAL COURT OF AUSTRALIA
Otsuka Pharmaceutical Co., Ltd v Generic Health Pty Ltd (No 2)
[2016] FCAFC 111
ORDERS
OTSUKA PHARMACEUTICAL CO., LTD First Appellant BRISTOL-MYERS SQUIBB COMPANY Second Appellant | ||
AND: | GENERIC HEALTH PTY LTD ACN 110 617 859 Respondent | |
DATE OF ORDER: |
THE COURT ORDERS THAT:
2. The appellants pay the respondent’s costs of the appeal.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
BESANKO AND NICHOLAS JJ:
Introduction
1 This appeal concerns a carbostyril compound called aripiprazole and its use in the treatment of the disorders of cognitive impairment caused by treatment-resistant schizophrenia, cognitive impairment caused by inveterate schizophrenia, and cognitive impairment caused by chronic schizophrenia.
2 The appellants are Otsuka Pharmaceutical Co., Ltd and Bristol-Myers Squibb Company. Otsuka Pharmaceutical Co., Ltd is the registered owner of Patent No 2005201772, titled “Substituted carbostyril derivatives as 5-HT1A receptor subtype agonists” (“the 722 Patent”), and Bristol-Myers Squibb Company is a licensee of the 722 Patent. The priority date of the 722 Patent is 29 January 2001 and the patent was granted on 6 September 2007. The respondent to the appeal is Generic Health Pty Ltd and it is a pharmaceutical company that supplies generic pharmaceuticals and other products. The respondent obtained registration of a number of aripiprazole products on the Australian Register of Therapeutic Goods. These products are registered under either the ARIPIPRAZOLE GENERICHEALTH label or the ARIPIPRAZOLE GH label. For present purposes, only the GH products are relevant. They are registered for the treatment of schizophrenia, including maintenance of clinical improvement during continuation therapy.
3 The appellants brought a proceeding against the respondent for infringement or threatened infringement of claims 1 and 7 of the patent. The respondent brought a cross-claim in which it alleged that the claims were invalid on various grounds, including a lack of novelty and lack of an inventive step. A key issue before the primary judge was the proper construction of the claims which his Honour resolved in a manner which we will identify later in these reasons. The primary judge then addressed the issue of infringement and he held that, assuming the claims to be valid, the respondent had infringed or threatened to infringe the claims. However, his Honour held that the claims were invalid for lack of novelty and lack of an inventive step. He rejected the other grounds of invalidity raised by the respondent. On 16 July 2015, the primary judge made orders that claims 1 and 7 of the 722 Patent be revoked and that the appellants’ claims for infringement of the 722 Patent be dismissed (Otsuka Pharmaceutical Co., Ltd v Generic Health Pty Ltd (No 4) (2015) 113 IPR 191; [2015] FCA 634).
4 In the appellants’ written outline of submissions in chief, they submitted that the primary judge had made six errors. Four of the alleged errors relate to the primary judge’s construction of the claims. The fifth alleged error related to the primary judge’s decision when considering inventive step to combine two pieces of prior art for the purposes of s 7(3) of the Patents Act 1990 (Cth) (“the Act”). That challenge was abandoned during the course of the appellants’ oral submissions. The sixth alleged error is that, as a result of the primary judge’s erroneous construction of the claims, his Honour’s findings on novelty and inventive step were flawed. Subject to one matter, this submission must fail if his Honour’s construction of the claims is upheld. It follows that the proper construction of the claims is central to the determination of the appeal.
5 In addition to resisting the appeal, the respondent has filed a notice of contention in which it challenges the primary judge’s conclusions with respect to infringement and contends that, even if the appellants succeed on the issue of the proper construction of the claims, their case on infringement must fail.
6 For the reasons which follow, the primary judge’s conclusions about the proper construction of the claims are correct as are his conclusions about lack of novelty and lack of an inventive step. The appeal must be dismissed. It is not necessary to consider the respondent’s submissions with respect to infringement of the claims.
The Claims in the 722 Patent
7 It will be necessary to consider the provisions of the specification of the 722 Patent in detail later in these reasons. However, at this stage, it is convenient to set out the claims, the features of the claims which raise the points of construction and the primary judge’s characterisation of the claims. Claim 1 of the 772 Specification is as follows:
Use of a carbostyril compound of [a given structural formula] … or a pharmaceutically acceptable salt or solvate thereof, for the production of a medicament, effective in the treatment of disorders of the central nervous system associated with [the] 5-HT1A receptor subtype, which disorder
(i) is selected from cognitive impairment caused by treatment-resistant schizophrenia, cognitive impairment caused by inveterate schizophrenia, or cognitive impairment caused by chronic schizophrenia, and
(ii) fails to [respond] to antipsychotic drugs selected from chlorpromazine, haloperidol, sulpiride, fluphenazine, perphenazine, thioridazine, pimozide, zotepine, risperidone, olanzapine, quetiapine, or amisulpride.
8 Claim 7 of the 772 Specification is as follows:
A method for treating a patient suffering from disorders of the central nervous system associated with [the] 5-HT1A receptor sub-type, which disorder
(i) [is] selected from cognitive impairment caused by treatment-resistant schizophrenia, cognitive impairment caused by inveterate schizophrenia, or cognitive impairment caused by chronic schizophrenia, and
(ii) fails to [respond] to antipsychotic drugs selected from chlorpromazine, haloperidol, sulpiride, fluphenazine, perphenazine, thioridazine, pimozide, zotepine, risperidone, olanzapine, quetiapine, or amisulpride,
comprising administering to said patient a therapeutically effective amount of a carbostyril compound of [the given structural formula] … or a pharmaceutically acceptable salt or solvate thereof.
9 The primary judge noted that it was not in dispute that, in one form, the carbostyril compound, referred to in each claim, is aripiprazole.
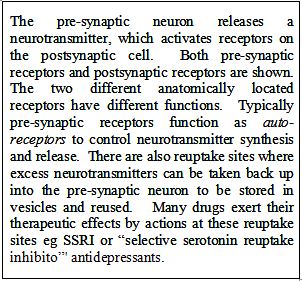
10 The first and major point of construction relates to that feature in the claims which describes the named disorders as associated with [the] 5-HT1A receptor subtype. We will refer to this as the association feature. The second point of construction relates to that feature in the claims which refers to the failure to respond to antipsychotic drugs selected from the drugs named in the claims. We will refer to this as the failure to respond feature.
11 The primary judge characterised claim 1 as a Swiss type claim. He noted that the generalised form of a Swiss type claim is “the use of compound X in the manufacture of a medicament for a specified (and new) therapeutic use”. His Honour discussed the history of such claims and he made reference to the decision of the Enlarged Board of Appeal of the European Patent Office in EISAI/Second Medical Indication (G05/83) [1979-85] EPOR B241 and the decisions in Bristol-Myers Squibb v Baker Norton Pharmaceuticals Inc [1999] RPC 253 (“Baker Norton”), and Actavis UK Ltd v Merck & Co Inc [2009] 1 WLR 1186. His Honour noted that the legal position in Australia was different from that in the United Kingdom and he referred to the decision of the High Court in Apotex Pty Ltd v Sanofi-Aventis Australia Pty Limited [2013] HCA 50; (2013) 304 ALR 1 at [50] per French CJ; at [286] per Crennan and Kiefel JJ; and [314] per Gageler J. No aspect of this part of his Honour’s analysis has been challenged on the appeal.
12 The primary judge considered whether an invention defined by a Swiss type claim is properly characterised as a product claim or as a method or process claim and, further, whether either of these characterisations are apt for a Swiss type claim. The appellants’ case before the primary judge was that Swiss type claims are process claims, whereas the respondent’s case was that they are neither method or process claims nor product claims. His Honour said that the words “method” and “process” were to be given their ordinary English signification, and that for the purpose of the question he was considering, there was no difference between the two. He reached the conclusion that “method or process” were apt to describe the content and subject matter of the Swiss type claim to which he had referred. He said that this approach accorded with the authorities which he identified (at [119]-[120]).
The Medical and Scientific Evidence
13 Each party put forward a considerable volume of expert evidence. There was also some lay evidence, but it is not necessary to refer to that evidence for the purposes of the appeal.
14 The appellants called Professor Bruce Sugriv Singh who swore four affidavits, Associate Professor Jonathan Phillips who swore one affidavit, and Associate Professor Trevor Ronald Norman who swore three affidavits.
15 Professor Singh is a consultant psychiatrist in private practice at the Melbourne Clinic and a senior consultant psychiatrist at the Royal Melbourne Hospital. He was Deputy Dean of the Faculty of Medicine, Dentistry and Health Sciences at the University of Melbourne, where he has been a Professor of Psychiatry since 1991. He has been a Fellow of the Royal Australian and New Zealand College of Psychiatrists (“RANZCP”) since 1979.
16 Associate Professor Phillips is a consultant psychiatrist in private practice. He is an Associate Professor at the University of New South Wales, the University of Adelaide and James Cook University. He is a Fellow of the RANZCP and was the President of the College from 1998 to 2000.
17 Associate Professor Norman is an Associate Professor in the Department of Psychiatry at the University of Melbourne. He was an Adjunct Professor in the School of Behavioural Sciences at La Trobe University and President of the International Society for the Investigation of Stress from 2002 to 2004.
18 The respondent called Professor Patrick Dennistoun McGorry who swore four affidavits, Dr Jeremy Francis O’Dea who swore six affidavits, and Professor Iain Stewart McGregor who swore one affidavit.
19 Professor McGorry is a consultant psychiatrist and Professor of Youth Mental Health at the University of Melbourne. He is an Executive Director of the Orygen Youth Health Research Centre and has been a Fellow of the RANZCP since 1986.
20 Dr O’Dea is a consultant psychiatrist in public and private practice and a Conjoint Lecturer in the School of Psychiatry at the University of New South Wales. He has been a Fellow of the RANZCP since 1991.
21 Professor McGregor is Professor of Pharmacology at the University of Sydney. He is a Professorial Fellow of the Australian Research Council.
22 The parties prepared an agreed primer of the medical and scientific background relevant to the case for the use of the primary judge. This primer was prepared from the affidavit evidence given by the expert witnesses. His Honour described a number of the medical and scientific concepts in detail in his reasons. In order to properly understand the issues of construction raised on the appeal, it is necessary for us to summarise a number of the medical and scientific concepts, although we do not need to do so with the same level of detail adopted by the primary judge.
23 Schizophrenia is a serious mental disorder affecting 1% of the population worldwide. Its main features are disturbances in the ability to experience reality, a lack of capacity to engage with others in the outside world, and disturbances in thinking, behaviour and emotional responses. Standard definitions of schizophrenia state that the symptoms must last for at least six months for the diagnosis to be made. The cause of schizophrenia is not known, but both genetic factors and environmental stressors operating prenatally and in childhood may play a part.
24 The symptoms of schizophrenia can be classified according to separate dimensions: positive and negative. Positive symptoms include hallucinations (the perception of a sensory experience in the absence of a source), delusions (fixed false beliefs not shared by others), and disorganised speech. Negative symptoms include blunting of emotional response, apathy, amotivation and poverty of thought.
25 More recently, some have classified the symptoms of schizophrenia by reference to two additional dimensions: cognitive and mood. Cognitive symptoms refer to a range of impaired higher cognitive functions including problems with attention, long-term memory, abstraction and planning. Mood symptoms can include anxiety and depression.
26 The course of schizophrenia can be variable. Acute schizophrenia is schizophrenia that lasts for more than six months and then remits. Chronic schizophrenia is schizophrenia which lasts significantly longer than six months and usually for many years. A chronic schizophrenia patient may have his or her positive symptoms under control, but negative symptoms persist. Treatment-resistant schizophrenia is a particular form of chronic schizophrenia where the patient’s positive symptoms, in addition to negative and cognitive symptoms, are unresponsive to antipsychotic medication.
27 Schizophrenia is treated with antipsychotic medications described as being either “typical” or “atypical” antipsychotics. Typical antipsychotics were developed in the 1950s and 1960s and include chlorpromazine, haloperidol, sulpiride, fluphenazine, perphenazine, thiroidazine, pimozide, and zotepine. Atypical antipsychotics, currently used more widely in Australia than typical antipsychotics, block serotonin as well as dopamine receptors and include risperidone; olanzapine; quetiapine; and, amisulpride.
28 Some patients may not respond well or experience unacceptable side effects to a particular medication that is first prescribed. It is common to then try prescribing a different medication for those patients and this change to a different medication is known as “switching”.
29 The transmission of nerve impulses in the brain and peripheral nervous system takes place by means of specific chemical agents called neurotransmitters. Transmission within the peripheral nervous system is important not only for everyday processes necessary for survival (i.e., breathing, heartbeat, movement, etc.), but also for functions such as thinking (cognition) and feeling (emotion).
30 Neurotransmission is the process by which neurotransmitters transmit signals (electrical impulses) from one neuron to the next. Neurotransmission usually occurs when an electrical impulse is initiated in a neuron (known as the pre-synaptic neuron). The impulse arrives at the nerve terminal and causes the release of neurotransmitters. The neurotransmitters bind with receptors on another neuron (known as the post-synaptic neuron). The term ‘affinity’ refers to the strength of binding to the receptor.
31 A receptor is a protein molecule consisting of chains of amino acids and typically consists of three parts. The first part is an extra-cellular portion which protrudes above the cell membrane, such that it may receive signals from nearby cells. The second part is a transmembrane-spanning domain located within the cell membrane, arranged as a series of helical shapes which give the receptor its shape. The third part is an intracellular domain which interacts with intracellular elements to produce changes in second messenger systems.
32 For some receptors, the second part of the neurotransmitter will act as a ligand. The term “ligand” refers to a molecule that will bind to a receptor to induce a conformational change (i.e., a change in shape) and bring about an alteration in the function of the receptor. Neurotransmitters and hormones are endogenous ligands (i.e., they are present in the body). Drugs are exogenous ligands (i.e., they are introduced to the body).
33 An agonist is a drug that mimics the effects of an endogenous neurotransmitter by bringing about a similar conformational change (and therefore a similar biological response) in the receptor as that brought about by the endogenous ligand. An antagonist is a drug that binds to the receptor but does not bring about a biological response. Its effect is to block the action of the endogenous ligand. A partial agonist is a drug that binds to and activates a given receptor but has only partial efficacy at the receptor in that it elicits less than maximal response from the receptor.
34 Serotonin (5-hydroxytryptamine or “5-HT”) is a neurotransmitter intimately connected to neuropharmacology and found throughout the body, including in many cells that are not neurons. The serotonin system in the brain is perhaps one of the most complex due to the multiplicity of receptors with which serotonin can interact. 5-HT1A receptors are the best characterised of the 5-HT1 family of receptors and they have high affinity for serotonin.
35 The primary judge did not make specific findings about dopamine receptors. It is sufficient to note that Associate Professor Norman said that it has been inferred that the positive symptoms of schizophrenia arise because of over-activity of dopamine in the mesolimbic dopamine pathways of the brain. Professor McGregor agreed that medications that are effective in alleviating the positive symptoms of schizophrenia usually have some action as antagonists at dopamine D2 receptors, but did not agree that schizophrenia is in part a dopamine D2 disorder.
The Primary Judge’s Reasons
The Construction of the Claims
36 The primary judge set out the principles applied in the construction of claims in a way which is not controversial. The construction of a patent specification and of prior art documents is a matter for the Court. However, those documents are construed through the eyes of the hypothetical person skilled in the art. The words in the documents are to be given the meaning which the person skilled in the art would attach to them in light of common general knowledge and what is disclosed in the document itself (Osram Lamp Works Ld v Pope’s Electric Lamp Company Ld (1917) 34 RPC 369 at 391 per Lord Parker; Root Quality Pty Ltd and Another v Root Control Technologies Pty Ltd and Others [2000] FCA 980; (2000) 49 IPR 225 (“Root Quality”) at [49] per Finkelstein J; Kinabalu Investments Pty Ltd v Barron and Rawson Pty Ltd [2008] FCAFC 178 (“Kinabalu Investments”) at [45]). The “person” skilled in the art might be a team of persons (General Tire & Rubber Company v Firestone Tyre and Rubber Company Limited and Others [1972] RPC 457 at 485 per Sachs LJ).
37 The primary judge said that there were three issues of construction.
38 The first issue of construction concerned the meaning of the association feature and whether the feature was an essential feature of the claims or purely descriptive. The appellants submitted that the association feature was a “broad requirement” that meant no more than that the 5-HT1A receptor was implicated in, or played a role in, the identified disorders. The primary judge set out the following passage from the appellants’ closing written submissions at trial:
The necessary relationship will be found to exist even if the 5-HT1A receptor is not faulty or defective in a patient suffering from the claimed disorder. There will also be the necessary relationship notwithstanding the 5-HT1A receptor is not solely responsible for the claimed disorder. Moreover, the necessary relationship will be satisfied notwithstanding the 5-HT1A receptor is not directly responsible for the claimed disorder.
(Original emphasis.)
39 His Honour identified (without setting out) a number of other passages in the appellants’ closing written submissions at trial and said that the appellants’ submission was that the evidence supported the conclusion that the 5-HT1A receptor is “relevantly associated” with cognitive impairment in chronic and treatment-resistant schizophrenia (at [133]). His Honour said the purpose of the appellants’ cross-examination of the experts was to demonstrate “the involvement, in some way, of the 5-HT1A receptor in cognition, the extent of any such involvement, the action of certain antipsychotic drugs on the 5-HT1A receptor and other receptors, and the effect that such drugs have or might have in improving impaired cognition” (at [139]).
40 The respondent’s submission was that the association between the disorders named in the claims and the 5-HT1A receptor subtype needed to be “a significant and consistent connection”, and that the evidence did not demonstrate that connection.
41 His Honour said that both parties treated the described association as one that required scientific proof and that each party said that this association was, in and of itself, an essential feature of the invention claimed. His Honour commented on the forensic reasons each party may have taken this approach and, in the case of the appellants, he suggested that they had taken the approach they had because it would make it more difficult for the respondent to prove its case on invalidity. At all events, his Honour summarised the appellants’ submission as being to the effect that it is only necessary to show that the 5-HT1A receptor is implicated in, or plays a role in, the disorders.
42 The primary judge said that he would not attempt to decide what he described as, in effect, a scientific controversy based on the literature in evidence and the witnesses’ opinions on that literature. He did not need to decide the scientific controversy because of the construction he placed on the claims. His Honour’s construction of the claims was that the association feature was but part of the description of an essential feature, namely the disorders to be treated (at [145]). His Honour said that the feature did not define a free-standing essential feature of the invention that added, meaningfully, to the identification of the specific forms of cognitive impairment ([at 146]).
43 The second issue of construction concerned the meaning to be given to the reference to cognitive impairment as a disorder caused by schizophrenia. His Honour referred to the evidence of the experts to the effect that cognitive impairment can be a symptom of schizophrenia. However, he noted that, properly described, it is not a disorder caused by schizophrenia. His Honour reached the conclusion that the 722 Specification made it tolerably clear that it is speaking of cognitive impairment as a symptom of schizophrenia, and that the claims should be understood accordingly. His Honour’s decision on this issue of construction is not challenged on the appeal.
44 The third issue of construction concerned the meaning of the failure to respond feature. The issue in relation to this feature was whether it meant that there has been a failure to respond to one or more such drugs (second line or later line treatment) or two or more such drugs (third line or later line treatment). His Honour construed the failure to respond feature as meaning a failure to respond to two or more such drugs (i.e., third line or later line treatment), having regard to the use of the plural “antipsychotic drugs” in the claims and the examples given in the 722 Specification.
Infringement
45 The primary judge’s construction of the association feature meant that when he came to consider the issue of infringement, he proceeded on the basis that “use of the compound to produce a medicament for (claim 1), or use of the compound as a method of treating (claim 7), one of those forms of cognitive impairment is use of the medicament or the method to treat a disorder for the central nervous system that is associated with the 5-HT1A receptor” (at [146]).
46 There were other issues in relation to infringement which the primary judge addressed. The respondent intended to market and supply the GH products in Australia and an issue arose as to whether that was a threatened infringement of claim 1 in circumstances where the method or process claimed in claim 1 is performed outside the patent area. His Honour considered a patentee’s exclusive right to exploit the invention in the patent area (s 13(1) of the Act). His Honour decided that the respondent’s intention to market and supply the GH products was a threatened exploitation of the invention and would have been a threatened infringement of the claim had it been valid (at [174]). The respondent challenges this conclusion in its notice of contention (ground 1(b)(i)). The appellants’ case in relation to the infringement of claim 7 was based on s 117 of the Act. The primary judge held that the GH products were not staple commercial products within s 117(2)(b), which is a conclusion challenged by the respondent in its notice of contention (ground 1(b)(ii)). His Honour then went on to consider if there was reason to believe that the GH products would be used in a way that infringed claim 7. He held that there was and that the appellants’ case on infringement of claim 7 would have been made out had the claim been valid.
Validity
47 The primary judge identified the prior art. For the purposes of the appeal, there are only four pieces of prior art which need be identified. There were other pieces of prior art put forward by the respondent at trial, but these were, for one reason or another, rejected by the primary judge as being relevant to the respondent’s invalidity claim.
US 528/EP 141
48 The 722 Patent identifies US Patent No. 5,006,528 (“US 528”), European Patent No. 367,141 (“EP 141”) and another patent, as related art containing “the same chemical structural formula as the carbostyril derivatives in the present invention”. Before the primary judge, it was accepted that the disclosures in US 528 and EP 141 are materially the same.
49 US 528 describes the field of invention in the following terms:
The present invention relates to novel carbostyril derivatives. More particularly, the invention relates to novel carbostyril derivatives and salts thereof, processes for preparing said carbostyril derivatives and salts thereof, as well as pharmaceutical compositions for treating schizophrenia containing, as the active ingredient, said carbostyril derivative or salt thereof.
50 In the section of US 528 which describes the invention, it is said that schizophrenia is the most common type of psychosis caused by an excessive neurotransmission activity of the dopaminergic nervous system in the central nervous system. The observations continue as follows:
Heretofore, a number of drugs, having the activity for blocking the neurotransmission of dopaminergic receptor in the central nervous system, have been developed, the example for said drugs are phenothiazine-type compounds such as Chlorpromazine; butyrophenone-type compounds such as Haloperidol; and benzamide-type compounds such as Sulpiride. These known drugs are now used widely for the purpose of improving so-called positive symptoms in the acute period of schizophrenia such as hallucinations, delusions and excitations and the like ….
51 The observations continue with a reference to, among other things, the negative symptoms of schizophrenia:
However, many of these drugs are considered as not effective for improving so-called the negative symptoms which are observed in the chronic period of schizophrenia such as apathy, emotional depression, hypopsychosis and the like. In addition to the above, these drugs give important side-effects such as akathisia, dystonia, Parkinsonism dyskinesia and late dyskinesia and the like, which are caused by blocking the neurotransmission of dopaminergic receptor in the striate body. Furthermore, other side-effects such as hyperprolactinemia and the like given by the drugs are become [sic] other problems …
52 The specification continues with the observation that, under the circumstances described, the development of drugs for treating schizophrenia having safety and clinical effectiveness, have been “eagerly expected”. The observations continue:
The present inventors have made an extensive study for the purpose of developing drugs for treating schizophrenia, which would be not only effective for improving the negative symptoms, but also effective for improving the positive symptoms of schizophrenia, furthermore such drugs would have less side-effects as compared with those shown by drugs known in prior art. As the result, the present inventors have successfully found carbostyril derivatives having strong activity for blocking neurotransmission of dopaminergic receptor. As to the side-effects given by known drugs for treating schizophrenia are for example, in the case of phenothiazine-type drugs, the orthostatic hypotension and hypersedation on the basis of strong a-blocking activity; and in the case of drugs having strong activity for blocking neurotransmission of dopaminergic receptor, the side-effects are so-called extrapyramidal tract syndromes such as catalepsy, akathisia, dystonia and the like caused by the blocking neurotransmission of dopaminergic receptor in the atriate body.
53 The objects of the invention in US 528 are said to be as follows:
(1) to provide novel carbostyril derivatives and salts thereof;
(2) to provide processes for preparing said carbostyril derivatives and salts thereof; and
(3) to provide a pharmaceutical composition for treating schizophrenia.
54 The claims in US 528 include the following claims:
16. A pharmaceutical composition for treating schizophrenia containing, as the active ingredient, a carbostyril compound or pharmaceutically acceptable salt thereof of claim 1 and a pharmaceutically acceptable carrier.
17. The pharmaceutical composition of claim 16, wherein the carbostyril compound or salt thereof is [aripiprazole].
55 The primary judge noted that EP 141 contained a similar claim and that, in addition, EP 141 contained, in claim 32, a Swiss type claim directed to the use of aripiprazole for the preparation of a drug useful for the treatment of schizophrenia.
Serper
56 Serper is an article entitled “Novel Neuroleptics Improve Attentional Functioning Schizophrenic Patients: Ziprasidone and Aripiprazole” by Serper et al. This article was published in CNS Spectrums 2(8) (1997). Under the heading “Overview”, the authors state:
Attentional dysfunction has long been recognized as a core feature of schizophrenic (SZ) illness. It has been consistently found that SZ patients manifest significant deficits in their ability to sustain their attention over time, as well as their ability to focus their attention on target stimuli when distractors are present. SZ attentional deficits are found across all developmental periods and clinical states including in children at high risk for the disorder, acutely ill patients, and remitted outpatients. These cognitive deficits, however, have been shown to improve over the course of neuroleptic treatment.
Numerous studies have confirmed that SZ performance, particularly on the digit span distraction task (a measure of selective attention) and the Continuous Performance Test (CPT: a measure of sustained attention), is highly responsive to classical neuroleptic treatment. For example, Oltmanns et al found that neuroleptic-withdrawn SZ patients showed a significant decline in their selective attention performance on the digit span distraction task from their stabilized medication baseline. Additionally, SZ patients’ digit span attentional performance has been found to be associated with higher serum neuroleptic levels in patients who were receiving neuroleptic treatment.
…
The finding that antipsychotic drugs improve attentional processes has led researchers to hypothesize, in much the same way they did for psychotic symptoms, that dopamine (DA) dysfunction underlies SZ attentional deficits. A recent model, for example, postulated that SZ attentional impairment is attributable to hypodopaminergic functioning in the prefrontal regions of the cortex.
Novel neuroleptics hold great promise in achieving further significant improvement in SZ attentional functioning because they preferentially increase prefrontal DA activation. Few studies to date have examined SZ attentional functioning during the course of novel neuroleptic treatment. Therefore, we compared attentional functioning of SZ patients receiving either typical or novel neuroleptics (ie, aripiprazole or ziprasidone) at both medication-free baseline and 30 days after receiving novel or classical neuroleptic treatment. We hypothesized that SZ patients receiving novel neuroleptics would demonstrate significant improvement in attentional functioning compared with patients receiving typical neuroleptic treatment.
(Citations omitted.)
57 The primary judge found, and it is not in dispute, that the reference in these passages to “neuroleptic” treatment, is to treatment with antipsychotic drugs, and that the reference to “classical neuroleptic” is to typical antipsychotic drugs, and the reference to “novel neuroleptic” is to atypical antipsychotic drugs.
58 The primary judge said that Serper addressed a study involving the treatment of 21 patients who presented with an acute episode of schizophrenia. Five of the patients received aripiprazole and four received ziprasidone. The remaining 12 patients received an unidentified typical antipsychotic drug. The primary judge found that patients in both groups suffered chronic schizophrenia. In dealing with the results of the study, the authors state:
Consistent with the study hypothesis, the present results found that SZ patients receiving novel neuroleptic treatment showed superior performance in CPT reaction time and in digit span immediate recall compared with SZ patients receiving traditional neuroleptic therapy. The present study also found that all patients receiving classical or novel neuroleptic therapy showed significant improvement in selective and sustained attentional functioning. These results are consistent with past investigations examining SZ attentional improvement following typical neuroleptic treatment and further support the notion that SZ attentional impairment, like many SZ symptoms, is mediated by mesostriatal DA pathways.
(Citations omitted.)
59 The authors conclude as follows:
Overall, the present results suggest that enhanced attentional performance obtained while on novel neuroleptic medication may enable SZ patients to further advance their functioning in important life domains.
Saha
60 Saha is an article entitled “Safety and Efficacy Profile of Aripiprazole, A Novel Antipsychotic” by Saha et al. The article was published in 1999 in Schizophrenia Research 36(1-3). The article states that aripiprazole is a novel antipsychotic which was going through worldwide Phase 3 development. The article states that a unique feature of aripiprazole is its agonistic effect at presynaptic dopamine receptors with a postsynaptic dopamine D2 antagonistic effect. The article refers to a Phase 2 study which was a completed double-blind, four-week, placebo-and-haloperidol-controlled study conducted in a total of 307 acutely relapsing hospitalised schizophrenia patients. The article states:
In this study aripiprazole fixed doses of 2 mg, 10 mg, and 30 mg per day were administered. Based on the last observation carried forward (LOCF) analysis, aripiprazole was superior to placebo in improving the Positive and Negative Syndrome Scale (PANSS)‒total for each dose group, and the 30-mg dose showed significant effect also for PANSS-negative score. The related items of PANSS were clustered and were analysed (LOCF) to determine the effect of aripiprazole on the following dimensions: excitement, depression, and cognitive function. On each of these dimensions the aripiprazole doses showed improvement over baseline, starting from week 1 to the last visit. In addition, at last visit, improvement for excitement and depression was statistically significant compared to placebo for each aripiprazole dose group; and for cognition, the aripiprazole 30-mg dose also showed statistically significant improvement over placebo.
61 The primary judge said that PANSS is used to evaluate the severity of the symptoms of schizophrenia in patients, and the efficacy of medications used to treat schizophrenia. The scale includes a list of seven positive symptoms, seven negative symptoms and 16 general psychopathology symptoms. The primary judge then referred to the evidence before him and, in particular, the evidence of Professor Singh and Dr O’Dea. The authors of the Saha article expressed the following conclusion:
An impressive effectiveness and a favourable safety profile indicate that aripiprazole should be a substantial addition to the new generation antipsychotics.
62 Keefe is an article entitled “The Effects of Atypical Antipsychotic Drugs on Neurocognitive Impairment in Schizophrenia: A Review and Meta-analysis” by Keefe et al. The article was published in Schizophrenia Bulletin, Volume 25, No 2 (1999).
63 The abstract in the article is as follows:
Cognitive deficits are a fundamental feature of the psychopathology of schizophrenia. Yet the effect of treatment on this dimension of the illness has been unclear. Atypical antipsychotic medications have been reported to reduce the neurocognitive impairment associated with schizophrenia. However, studies of the pattern and degree of cognitive improvement with these compounds have been methodologically limited and have produced variable results, and few findings have been replicated. To clarify our understanding of the effects of atypical antipsychotic drugs on neurocognitive deficits in patients with schizophrenia, we have (1) reported on newly established standards for research design in studies of treatment effects on cognitive function in schizophrenia, (2) reviewed the literature on this topic and determined the extent to which 15 studies on the effect of atypical antipsychotics met these standards, (3) performed a meta-analysis of the 15 studies, which suggested general cognitive enhancement with atypical antipsychotics, and (4) described the pharmacological profile of these agents and considered the pharmacological basis for their effects on neurocognition. Finally, we suggest directions for the development of new therapeutic strategies.
64 Keefe contains a discussion on the positive relationship between cognitive improvement and drugs that bind to serotonin receptors which “may facilitate or impair certain cognitive functions, depending on the location and subtype of the affected receptors”.
65 Serper is one of the 15 studies which were the subject of the meta-analysis performed by Keefe. Serper is referred to in the body of the article. Keefe reports that none of the 15 studies that were reviewed met all of the recently developed standards for the assessment of cognitive change in schizophrenia. It notes that most importantly, only three of the 15 studies used double-blind methodology.
66 Keefe drew the following conclusions from the meta-analysis and review of the studies:
Despite a conservative statistical approach, correcting the results of each study for the number of statistical comparisons made, the meta-analysis conducted in this study suggests that atypical antipsychotics, when compared with conventional antipsychotics, improve cognitive functions in patients with schizophrenia. Verbal fluency, digit-symbol substitution, fine motor functions, and executive functions were the strongest responders to novel antipsychotics. Attention subprocesses were also responsive; learning and memory functions were the least responsive.
Novelty
67 The primary judge noted that the respondent relied on each of EP 141/US 528, Serper and Saha as novelty-defeating.
68 The primary judge referred to the reverse infringement test and the need for a clear description of, or a clear direction, recommendation or suggestion to do or make, something that would infringe the patentee’s claim if carried out after the grant of the patentee’s patent, in order for the prior art to be anticipatory. There is no dispute about the accuracy of his Honour’s statements of principle.
69 The primary judge recorded the respondent’s submission to be as follows. The use of aripiprazole for the treatment of schizophrenia, as taught in the relevant prior art documents, would have resulted, inevitably, in the treatment of a patient’s cognitive impairment associated with schizophrenia, including in the circumstances referred to in claim 7 of the 722 Patent and, according to the teaching in the Patent, this would have involved the 5-HT1A receptor subtype. It followed (so the submission proceeds) that it is not necessary for any of the prior art documents to make reference to an association between the 5-HT1A receptor subtype and the treatment of cognitive impairment in schizophrenia in order for the prior art document to constitute an anticipation.
70 The primary judge acknowledged that there were limitations on the “inevitable result” test, particularly where the invention claimed is a new method of medical treatment involving the administration of a known compound for a hitherto unknown and unexpected, but nevertheless useful, therapeutic use. In this context, his Honour referred to the influence of the decision of the Enlarged Board in MOBIL/Friction Reducing Additive (G02/88) [1990] EPOR 73 (“Mobil”) on the question of anticipatory disclosure in United Kingdom patent jurisprudence. In Mobil, the issue was the patentability of an additive for oil that was known for rust prevention in engines. The patentee was allowed to claim the use of the known additive for a new purpose, namely, friction reduction. The new purpose and the undisclosed technical effect was sufficient to confer novelty on the invention as claimed.
71 The primary judge then referred to two United Kingdom cases which had identified limits to the Mobil principle (Baker Norton; Actavis UK Ltd v Janssen Pharmaceutical NV [2008] FSR 35 (“Actavis”)). More information about the old use (Baker Norton at [277] per Jacob J) or merely explaining the mechanism which underlies a use already described in the prior art (Actavis at [99] per Floyd J) is not of itself sufficient to give rise to novelty. In Baker Norton, Jacob J said (at 277):
It is implicit in what Laddie J said, I think, that Mobil has its limits. It should be remembered that Mobil was treated as a case where the new use was different from the old. Perhaps a clearer example is BASF/Triazole derivatives (T231/85) [1989] OJ EPO 74 of the discovery that a particular compound (previously used for influencing plant growth) also controlled fungi. One can imagine cases where it was used for one purpose or the other. The purposes do not necessarily overlap. That is simply not the case here. All you have is more information about the old use. In due course no doubt more information about the exact mode of action of taxol will emerge. No-one could obtain a patent for its use simply by adding “for” at the end of the claim and then adding the newly discovered details of the exact mode of action.
In Actavis, Floyd J said (at [99]):
In my judgement, merely explaining the mechanism which underlies a use already described in the prior art cannot, without more, give rise to novelty. In MOBIL, the technical effects which underlay the new and old uses were different and distinct. So also in Mr Alexander’s example about disease X and disease Y. It is not the case that every discovery about the mode of action of a drug can be translated into a new purpose and claimed as such.
72 The primary judge said that these United Kingdom cases provided valuable insights in analysis which assist in considering closely similar questions arising under Australian patent law.
73 The primary judge found that EP 141/US 528 disclosed the following:
(1) aripiprazole, amongst other carbostyril derivatives, was used as a drug for the treatment of schizophrenia generally and it was a useful alternative to earlier generation drugs, including three mentioned in claims 1 and 7, being chlorpromazine, haloperidol and sulpiride, including in the chronic period of the illness; and
(2) at the priority date, the person skilled in the art would have understood that when EP 141/US 528 refer to aripiprazole’s utility in treating negative symptoms, those symptoms included at least some of the symptoms which claims 1 and 7 of the 722 Patent characterise as cognitive impairment.
74 The primary judge found that EP 141/US 528:
(1) did not disclose that aripiprazole is a partial agonist at the 5-HT1A receptor;
(2) makes no reference to 5-HT1A receptors or the action of aripiprazole on 5-HT1A receptors or the serotonergic system more generally; and
(3) makes no express reference to switching or the use of aripiprazole as third line treatment where a patient’s cognitive impairment has failed to respond to other identified antipsychotics.
75 The primary judge said that if he followed the reasoning in the United Kingdom cases of Baker Norton and Actavis, he would conclude that neither the association feature nor the failure to respond feature were sufficient to give rise to novelty. He considered that it was appropriate to follow the reasoning in those cases. The essence of his Honour’s approach was to hold that (as he put it) claim 7 merely partitioned something that is old under the guise that the part it takes and claims as an invention is new. He said that claim 7 is directed to an old therapeutic use, not a new one. His Honour said that claim 7 claims a then known substance, aripiprazole, for its then known therapeutic purpose, the treatment of schizophrenia, with only some of the symptoms of schizophrenia and only some occasions of use referred to in the claim. His Honour said that the same was true of claim 1. His Honour said the association feature was new information about the disorders, but that the disorders were part of the then known symptoms of schizophrenia. The association feature was no more than an elucidation of the action of the known carbostyril compounds, including aripiprazole, in treating schizophrenia, and a contribution to knowledge of the possible aetiology of those particular symptoms. These “accretions to knowledge” without more, do not provide novelty of invention (at [321]).
76 The primary judge said that, in any event, he did not accept that the knowledge provided by the association feature defined a free-standing essential feature of the invention which is to be considered as meaningfully adding to the identification of the specific forms of cognitive impairment referred to. The association feature could not confer novelty (at [322]).
77 The primary judge held that the failure to respond feature did not confer novelty. The disclosures in EP 141/US 528 did not limit the use of carbostyril compounds, including aripiprazole, to a particular line of treatment and there would be no reason why a person skilled in the art at the priority date and therefore aware that switching was a feature of clinical practice would read the disclosures down.
78 The primary judge tested his conclusion by asking whether EP 141/US 528 contained a clear description of, or a clear direction, recommendation or suggestion to do or make, something that would infringe the patentee’s claim if carried out after the grant of the patentee’s patent. He answered that question in the affirmative (at [326]).
Serper
79 We do not need to set out in any detail his Honour’s conclusions in relation to Serper. His Honour concluded that it was an anticipation and the important point is that his reasoning in relation to the association feature and the failure to respond feature was materially the same as it was in relation to EP 141/US 528 (at [332]).
Saha
80 The primary judge first considered a contention by the appellants that Saha did not disclose the effectiveness of treating cognitive impairment. He rejected that contention for the reasons he gave and which we do not need to repeat. Thereafter, he applied similar reasoning in relation to Saha as he had in relation to EP 141/US 528 (at [337]).
Inventive Step
81 The primary judge held that the invention as claimed lacked an inventive step, having regard to the common general knowledge at the priority date and Saha, or Keefe and Serper, treated as combined information. It is not necessary to summarise in detail the primary judge’s reasons for reaching this conclusion because (subject to one matter which we will identify) the appellants’ grounds of challenge to his reasons in relation to inventive step are limited to the consequences of his “flawed” construction. As we have said, the complaint by the appellants that the skilled person would not have combined Keefe and Serper was abandoned during their oral submissions.
82 The primary judge set out the respondent’s formulation of the Cripps question in relation to claim 7 as follows:
[W]hether the person skilled in the art (a team including a psychiatrist and if necessary the medicinal chemist and pharmacologist suggested by the Applicant), in light of the common general knowledge combined with s 7(3) information would have been led as a matter of course to try the method as claimed in claim 7 (to administer aripiprazole) in the expectation it would have been beneficial in treating a patient with chronic or treatment-resistant schizophrenia (or one of the other disorders listed in the claim) with cognitive impairment symptoms that had failed to respond to two or more of the other listed antipsychotics.
83 He said that the appellants formulated the Cripps questions with reference to both claims 1 and 7 and that he did not think that, as a matter of substance, the appellants’ formulations differed in any way that would lead to a materially different inquiry.
84 We are not sure his Honour is correct in viewing the appellants’ Cripps questions as being materially the same as the respondent’s (see Appellants’ Closing Submissions paragraphs 194 and 195), but at all events, the appellants submit on the appeal that the Cripps question in relation to claim 7 should have been as follows:
Would the notional research group at 29 January 2001, in the light of the common general knowledge alone or combined with s 7(3) information, directly be led as a matter of course to try aripiprazole for a method of treating a patient suffering from:
(a) disorders of the central nervous system associated with [the] 5-HT1A receptor sub-type,
(b) which disorder
(i) is selected from cognitive impairment caused by treatment-resistant schizophrenia, cognitive impairment caused by inveterate schizophrenia, or cognitive impairment caused by chronic schizophrenia, and
(ii) fails to [respond] to antipsychotic drugs selected from chlorpromazine, haloperidol, sulpiride, fluphenazine, perphenazine, thioridazine, pimozide, zotepine, risperidone, olanzapine, quetiapine, or amisulpride,
comprising administering to said patient a therapeutically effective amount of aripiprazole, in the expectation that it might well produce a useful result?
(Emphasis in original.)
85 The primary judge said that the invention is for the treatment of at least one of the identified forms of cognitive impairment and necessarily, for a medicament containing aripiprazole that had been produced for such treatment. Again, he made the point that the discovery that aripiprazole’s action at the 5-HT1A receptor is the reason or one of the reasons for that utility cannot provide an inventive step when Saha had already disclosed that aripiprazole was useful in treating cognitive impairment in patients suffering from chronic schizophrenia. His Honour repeated the point he had previously made that this discovery provided no more than an elucidation of why aripiprazole was useful and, perhaps, an explanation of why it had the beneficial effects reported in Saha (at [439]). His Honour’s analysis in relation to Serper/Keefe was relevantly the same (at [458]).
86 The appellants’ submission is that their construction of the claims is correct and it means that their formulation of the Cripps question rather than that adopted by the primary judge is the correct one. The appellants’ submission is that the correct formulation of the Cripps question raised a specific problem and that there was no evidence before the primary judge that any person skilled in the art would try aripiprazole, or even if they might have, that they would have done so with any expectation that it might well be useful.
87 As far as the failure to respond feature is concerned, the primary judge rejected the suggestion that there was anything inventive about the feature. His Honour made findings as to the relevant common general knowledge at the priority date which were based on the respondent’s submissions and which he records as not being contested by the appellants. The common general knowledge at the priority date included the following:
(1) that some patients suffered from refractoriness in which positive, negative and neurocognitive symptoms did not respond to treatment with antipsychotics;
(2) that a number of treatment-resistant and treatment-refractory schizophrenic patients displayed symptoms that did not respond adequately to a variety of known effective classes and doses of typical or atypical antipsychotic drugs;
(3) the switching of patients undergoing treatment with antipsychotic drugs to other atypical antipsychotic drugs;
(4) switching antipsychotics in an attempt to improve cognitive impairment in schizophrenic patients; and
(5) switching the medication of a patient with chronic or treatment-resistant schizophrenia, where the patient’s symptoms and signs of schizophrenia had failed to respond to two or more existing antipsychotics.
88 The primary judge concluded that, in light of that common general knowledge, a person skilled in the art and with the benefit of the information in Saha (at [440]) or Keefe/Serper (at [458] and [466]) would have been directly led as a matter of course to try aripiprazole as a method of treatment, with the expectation that it might well be useful, where the patient’s cognitive impairment had failed to respond to previous medication. The invention as claimed, therefore, lacked an inventive step.
Issues on the Appeal
89 As we have said, the appellants contend that the primary judge made five errors. The first alleged error relates to the primary judge’s construction of the association feature and the failure to respond feature. The second and third alleged errors relate to the primary judge’s construction of the association feature. The first three alleged errors, insofar as they relate to the association feature, overlap and it is convenient to deal with them together.
90 The first alleged error is that the primary judge gave no effective meaning to the association feature and, thus, notionally put it to one side. The second alleged error is that the primary judge gave no effective meaning to the association feature because he proceeded on the premise that all of the disorders named in the claims are disorders of the central nervous system, associated with the 5-HT1A receptor subtype when (so the appellants contend) that premise was contrary to the teachings of the 722 Patent and the evidence. The third alleged error is that the primary judge should have resolved the question of whether, and the extent to which, the disorders named in the claims are in fact “disorders of the central nervous system associated with [the] 5-HT1A receptor subtype” and that he should have done that by reference to the “extensive body of scientific evidence put forward by both parties”.
91 As we understand it, the steps in the appellants’ argument are as follows. First, the claims should have been construed such that the association feature was an essential feature or integer of the claims. The reason for this is that not all cases of cognitive impairment caused by the disorders named in the claims are disorders of the central nervous system associated with the 5-HT1A receptor subtype. Contrary to his Honour’s conclusion that the teaching in the 722 Patent was to the effect that the forms of cognitive impairment were disorders of the central nervous system associated with the 5-HT1A receptor subtype, in fact, the teaching in the 722 Patent did not proceed on the basis that all forms of cognitive impairment resulting from the named disorders are disorders of the central nervous system associated with the 5-HT1A receptor subtype. Secondly, even if the teachings in the 722 Patent are not as the appellants contend or not necessarily as the appellants contend, the primary judge needed to determine the factual question of whether all forms of cognitive impairment resulting from the named disorders are disorders associated with the 5-HT1A receptor subtype. The appellants contend that the teachings in the 722 Patent are only one item of evidence, a point made by the High Court, albeit in the context of admissions as to prior art and common general knowledge in Lockwood Security Products Pty Ltd v Doric Products Pty Ltd [No 2] [2007] HCA 21; (2007) 235 CLR 173 at [105]-[111]. The appellants contend that the primary judge needed to decide the question otherwise his construction of the claims would proceed on an incorrect factual premise. This, they contend, is in fact what happened in this case. Thirdly, the appellants contend that the primary judge should have decided the factual question in the negative, that is to say, that not all forms of cognitive impairment resulting from the named disorders are disorders of the central nervous system associated with the 5-HT1A receptor subtype. Had the primary judge addressed the factual question and had he decided in the way in which the appellants contend that he should have decided it, then he would have construed the claims so that the association feature was an essential feature or integer. Furthermore, had the primary judge identified the association feature as an essential feature or integer, then the prior art would not be novelty defeating and the Cripps question (as reformulated by the appellants), would be answered in the negative. The final step in the appellants’ argument is that any difficulty for them in proving infringement if the association feature is an essential feature is more apparent than real. In view of the restriction in the claims to the use of aripiprazole as a third or later line treatment (assuming the primary judge’s construction) the effectiveness of aripiprazole as such treatment after other antipsychotic drugs which target different receptors have been tried is sufficiently likely to be due to an association between the disorder and the 5-HT1A receptor subtype as to justify an injunction.
92 The arguments advanced by the appellants on the appeal involve a departure from the way in which the appellants conducted their case in the Court below. We accept that in the Court below, the appellants argued for a broad meaning to be given to the word “association” in the claims, but nevertheless they did argue that there is a relationship between the disorders named in the claims and the 5-HT1A receptor subtype, albeit that it is sufficient that it is “implicated in, or plays a role in, the disorders” (at [141]). The respondent’s case below was that the association or link or connection could not be established. The appellants’ submission on the appeal seems to be raising a third alternative, that is, that the association exists in some cases and not in others. We would also make the observation that it is hardly surprising that the primary judge reached the view that the teachings of the 722 Patent support the conclusion that the claims take the particular forms of cognitive impairment as being disorders of the central nervous system associated with the 5-HT1A receptor subtype, having regard to the appellants’ arguments in the Court below.
93 The approach to the construction of claims in a patent specification is well-established.
94 We start with the well-known passage in the speech of Lord Russell of Killowen in Electric and Musical Industries, Ltd and Boonton Research Corporation, Ltd v Lissen, Ltd and another (1939) 56 RPC 23 (“EMI v Lissen”) at 39:
The function of the claims is to define clearly and with precision the monopoly claimed, so that others may know the exact boundaries of the area within which they will be trespassers. Their primary object is to limit, and not to extend, the monopoly. What is not claimed is disclaimed. The claims must undoubtedly be read as part of the entire document, and not as a separate document. Nevertheless, the forbidden field must be found in the language of the claims, and not elsewhere. It is not permissible, in my opinion, by reference to some language used in the earlier part of the specification to change a claim which by its own language is a claim for one subject-matter into a claim for another and a different subject-matter, which is what you do when you alter the boundaries of the forbidden territory. A patentee who describes an invention in the body of a specification obtains no monopoly unless it is claimed in the claims. As Lord Cairns LC said, there is no such thing as infringement of the equity of a patent (Dudgeon v Thomson, L.R. App Cas. 34).
95 In Martin v Scribal Proprietary Limited (1954) 92 CLR 17 at 97, Taylor J said:
Plain language must be given its plain meaning, and clear words in a claim must not be tortured into an unnatural meaning by importing passages from the body of the specification. (See Lord Russell’s speech in Electrical & Musical Industries, Ltd. v Lissen, Ltd.). The claims also must be construed without an eye on the alleged infringer’s acts. (So said Greene LJ in R.C.A. Photophone Ltd. v Gaumont British Picture Corporation). On the other hand, it is right to construe a claim with an eye benevolent to the inventor and with a view to making the invention work — this is an application of the old doctrine ut res magis valeat quam pereat — and it is illustrated in Nobel’s Case; and, where the language of a claim is obscure or doubtful, the doubt may sometimes be resolved by referring to words in the body of the document to explain it. This is known as the dictionary principle. (See Lord Haldane’s Speech in British Thomson- Houston Coy., Ltd. v Corona Lamp Works, Ltd.).
(Citations omitted.)
(This passage was referred to with approval by Gummow J in Sartas No 1 v Koukourou & Partners Pty Ltd and Another (1994) 30 IPR 479 at 486.)
96 The Full Court in Kinabalu Investments at [45] provided the following summary of the applicable principle:
• a specification should be given a purposive construction rather than a purely literal one;
• the hypothetical addressee of the specification is the non inventive person skilled in the art before the priority date;
• the words used in a specification are to be given the meaning the hypothetical addressee would attach to them, both in the light of the addressee’s own general knowledge and in the light of what is disclosed in the body of the specification;
• as a general rule, the terms of the specification should be accorded their ordinary English meaning;
• evidence can be given by experts on the meaning those skilled in the art would give to technical or scientific terms and phrases, and on unusual or special meanings given by such persons to words which might otherwise bear their ordinary meaning;
• however, the construction of the specification is for the court, not for the expert. In so far as a view expressed by an expert depends upon a reading of the patent, it cannot carry the day unless the court reads the patent in the same way.
97 Lord Tomlin made the following observations about the proper role of expert evidence in determining issues of construction in British Celanese, Ltd v Courtaulds, Ltd (1935) 52 RPC 171 (“British Celanese”) at 196:
The area of the territory in which in cases .of this kind an expert witness may legitimately move is not doubtful. He is entitled to give evidence as to the state of the art at any given time. He is entitled to explain the meaning of any technical terms used in the art. He is entitled to say whether in his opinion that which is described in the specification .on a given hypothesis as to its meaning is capable of being carried into effect by a skilled worker. He is entitled to say what at a given time to him as skilled in the art a given piece of apparatus or a given sentence on any given hypothesis as to its meaning would have taught or suggested to him. He is entitled to say whether in his opinion a particular operation in connexion with the art could be carried out and generally to give any explanation required as to facts of a scientific kind.
He is not entitled to say nor is Counsel entitled to ask him what the Specification means, nor does the question become any more admissible if it takes the form of asking him what it means to him as an engineer or as a chemist. Nor is he entitled to say whether any given step or alteration is obvious, that being a question for the Court.
98 The appellants began their submissions by referring to the fact that, although it is open to a court to hold that an express feature is not an essential feature, that in fact is done very rarely and the Court must be cautious in holding that an express feature is an inessential feature or integer (Blanco White TA, Patents for inventions and the protection of industrial designs (5th ed, Stevens, 1983) at [2-111]); Fresenius Medical Care Australia Pty Ltd v Gambro Pty Ltd and Another [2005] FCAFC 220; (2005) 67 IPR 230 at [87]; Root Quality at [43] per Finkelstein J; EMI v Lissen at 39 per Lord Russell of Killowen). We do not question that proposition, but it seems to us that it really is a question of characterisation. It might be one thing to say that a limitation is not essential. However, it seems to us that to say something is descriptive or an addition to existing knowledge is in a different category. Cases such as Baker Norton and Actavis make that clear. The other point to note is that the primary judge did not ignore the association feature. His Honour referred to the feature as part of the description of an essential feature, being the disorders to be treated. The disorders and the association with the identified receptor defined one essential feature, they are to be read together and not treated as separate features or integers (at [145]).
99 The starting point in a construction issue is the words of the claims. As we understand it, the appellants’ case is that the claims should be read so as to recognise two classes of cognitive impairment caused by the forms of schizophrenia identified, namely those associated with the 5-HT1A receptor subtype and those not associated with the 5-HT1A receptor subtype. We do not think that that is a construction of the claims which immediately suggests itself, having regard to the structure of the claims. The wording of the claims suggests to us that (to take one example) treatment-resistant schizophrenia resulting in (i.e., as a symptom) cognitive impairment is a disorder of the central nervous system associated with the 5-HT1A receptor subtype and not that the reference to association with the 5-HT1A receptor subtype is intended as a limitation. In other words, we would read the reference as descriptive of the disorders identified. Had the intention been to specify a further limitation or restriction on the disorders which are the subject of the claims, then one might have expected to see an additional subparagraph rather similar to the way the appellants seek to formulate the Cripps question (at [84] above). The wording of the claims supports the primary judge’s construction, but it is also appropriate to consider the teachings of the 722 Specification.
100 The teachings in the 722 Specification are as follows:
(1) The invention as claimed is said to relate to a method of treating a patient suffering from a disorder of the central nervous system associated with the 5-HT1A receptor subtype.
(2) The related art is said to include US Patent No 5, 006, 528 and European Patent No 367, 141.
(3) It has been reported that aripiprazole binds with high affinity to dopamine D2 receptors and with moderate affinity to dopamine D3 and 5-HT7 receptors and that aripiprazole possesses presynaptic dopaminergic autoreceptor agonistic activity, postsynaptic D2 receptor antagonistic activity, and D2 receptor partial agonistic activity.
(4) It has not been reported that compounds in the invention as claimed have agonistic activity at 5-HT1A receptor subtype.
(5) It has been reported that:
- therapeutic interventions using 5-HT1A receptor ligands may be useful drug treatments for alcohol abuse;
- 5-HT1A agonist drugs may be useful for the treatment and/or prophylaxis of disorders associated with neuronal degeneration resulting from ischemic events in mammals;
- 5-HT1A receptor hypersensitivity could be the biological basis for the increased frequency of migraine attack in stressful and anxious conditions;
- that another compound that is a 5-HT1A receptor agonist has neuroprotective, anxiolytic and anti depressant-like effects in animal models;
- that 5-HT1A receptor agonists appear to be broad spectrum antiemetic agents.
(6) Serotonin plays a role in several neurological and psychiatric disorders.
(7) Buspirone which is a 5-HT1A receptor agonist is efficacious in treating a variety of symptoms associated with attention deficit hyperactivity disorder (“ADHD”), and that combined use of a D2 receptor agonist and 5-HT1A agonist provides effective treatments for ADHD and Parkinson’s disease.
(8) That 5-HT1A agonists or partial agonists have been found to be effective in the treatment of:
- cognitive impairment in Alzheimer’s disease, Parkinson’s disease or senile dementia;
- Alzheimer’s disease by improving memory;
- short term memory in patients in need of treatment;
- depression and certain primary depressive disorders;
- motor disorders such as neuroleptic induced parkinsonism and extrapyramidal symptoms.
(9) Aripiprazole, because of its potent, partial agonistic activities at D2 and 5-HT1A receptors, can be used to manage psychosis in geriatric patients, Alzheimer’s disease, Parkinson’s disease or senile dementia, and may improve extrapyramidal symptoms.
(10) Typical antipsychotic drugs, such as chlorpromazine and haloperidol, are effective in treating the positive symptoms of schizophrenia which include hallucinations, delusions and the like. Atypical antipsychotic drugs, such as clozapine, risperidone, olanzapine and quetiapine have other activities in addition to their DA-receptor blocking activities and less extrapyramidal side effects. In contrast to typical antipsychotic drugs, it has been reported that atypical antipsychotic drugs are more effective against the negative symptoms and cognitive impairments associated with schizophrenia.
(11) There are patients who have schizophrenia who do not respond adequately to a variety of known effective classes and doses of typical or atypical antipsychotic drugs (treatment-resistant, treatment-refractory, inveterate or chronic schizophrenic patients).
(12) Cognitive impairment exists separately from the psychic symptoms in a schizophrenic individual and may disturb the socially adaptable behaviour of these individuals. Therefore, medical treatment is quite important.
(13) Clozapine is an antipsychotic drug that is effective against treatment-resistant schizophrenia and it has been reported to be effective against cognitive impairments in treatment-resistant schizophrenics. The 5-HT1A receptor has been demonstrated to play a role in the therapeutic efficacy of clozapine against treatment-resistant schizophrenia and cognitive impairments.
(14) There is a passage which refers to the role of the 5-HT1A receptor in treatment-resistant schizophrenia and cognitive impairments. Although lengthy, it is important, and we set it out in full:
Further, in accordance with progress in molecular pharmacology, it is clearly understood that 5-HT1A receptor agonistic activity or 5-HT1A receptor partial agonistic activity plays an important role in treatment-resistant schizophrenia and cognitive impairments (A. Newman-Tancredi, C. Chaput, L. Verriele and M. J. Millan: Neuropharmacology, Vol. 35, pp. 119, (1996)). Additionally, it was reported that the number of 5-HT1A receptor is increased in the prefrontal cortex of chronic schizophrenics who were classified treatment-resistant. This observation was explained by a compensatory process where by the manifestation of severe symptoms of chronic schizophrenia are a result of impaired neuronal function mediated by hypofunctional 5-HT1A receptors (T. Hashimoto, N. Kitamura, Y. Kajimoto, Y. Shirai, O. Shirakawa, T. Mita, N. Nishino and C. Tanaka: Psychopharmacology, Vol. 112, pp S35, (1993)). Therefore, a lowering in neuronal transmission mediated through 5-HT1A receptors is expected in treatment-resistant schizophrenics. Thus the clinical efficacy of clozapine may be related to its partial agonist efficacy at the 5-HT1A receptors (A. Newman-Tancredi, C. Chaput, L. Verriele and M. J. Millan: Neuropharmacology, Vol. 35, pp. 119, (1996)). 5-HT1A receptor agonistic activity may be related to the clinical effects of clozapine, and this hypothesis is supported by a positron emission tomography study in primates which showed that clozapine interacts with brain 5-HT1A receptors at a therapeutically effective dose (Y.H. Chou, C. Halldin and L. Farde: Int. J. Neuropsychopharmacol., Vol. 4 (Suppl. 3), pp. S130, (2000)). Furthermore tandospirone, which is known as a selective 5-HT1A receptor agonist, improved cognitive impairments in chronic schizophrenic patients (T. Sumiyoshi, M. Matsui, I. Yamashita, S. Nohara, T. Uehara, M. Kurachi and H.Y. Meltzer: J. Clin. Pharmacol., Vol. 20, pp. 386, (2000)). While, in animal tests, all reports do not always suggest that 5-HT1A receptor agonist activity may be related to cognitive impairment, however, 8-OH-DPAT (8-hydroxy-2-(di-n-propylamino) tetralin), which is known as a selective 5-HT1A receptor agonist, improves learning and memory impairments induced by scopolamine known as a muscarinic receptor antagonist, suggesting a relationship between 5-HT1A receptor agonistic activity and improvements in cognitive impairments (M. Carli, P. Bonalumi, R. Samanin: Eur. J. Neurosci., Vol. 10, pp. 221, (1998); A. Meneses and E. Hong: Neurobiol. Learn. Mem., Vol. 71, pp. 207, (1999)).
(15) Two atypical antipsychotic drugs which were marketed after clozapine are risperidone and olanzapine and as to those drugs:
(a) it has been reported that they improve treatment-resistant schizophrenia or cognitive impairments in treatment-resistant schizophrenics;
(b) in contrast to clozapine, which it had been reported was moderately effective against treatment-resistant schizophrenia, risperidone and olanzapine were not consistently superior to typical antipsychotic drugs in their effectiveness against treatment-resistant schizophrenia. Thus, risperidone and olanzapine bind with lower affinity to human 5-HT1A receptors at clinical effective doses.
(Our emphasis.)
(16) Therefore, at present, it is understood that clozapine is effective against treatment-resistant schizophrenia.
(17) The Patent provides:
As explained above 5-HT1A receptor agonistic activity is important for improving treatment-resistant schizophrenia or cognitive impairment caused by treatment-resistant schizophrenia.
(18) Clozapine, although effective against treatment-resistant schizophrenia, has severe side-effects and the development of a safe antipsychotic drug with potent, full or partial agonist activity at 5-HT1A receptors is “earnestly desired”.
(19) Aripiprazole binds with a high affinity and displays a potent, partial agonist activity at the 5-HT1A receptors and it has higher intrinsic activity (about 68%) as compared with that of clozapine. Therefore, the compound in the present invention has a 5-HT1A receptor agnostic activity that is more potent than the agnostic activity of clozapine.
(20) Aripiprazole may be “a potent and safer drug therapy” and “a potent and highly safe drug therapy” where a patient fails to respond to other typical or atypical antipsychotic drugs and there are 12 drugs listed.
101 The 722 Specification is replete with references to the association, link or connection between the 5-HT1A receptor and cognitive impairment in treatment-resistant schizophrenics. We emphasise, in particular, the reference we have summarised in paragraph (13) (the 5-HT1A receptor has been demonstrated to play a role in etc.), paragraph (14) (5-HT1A receptor agnostic activity or 5-HT1A receptor partial agnostic activity plays an important role in etc.), and paragraph (17) (5-HT1A receptor agnostic activity is important for improving etc.). There is very little to the contrary. There is the reference in the passage we have set out in paragraph (14) to reports in the case of animal tests not always suggesting that 5-HT1A receptor agonist activity may be related to cognitive impairment. However, we do not think in referring to inconclusive evidence or even evidence pointing in a different direction, the 722 Specification was teaching that there were two classes of the disorders, being those associated with the 5-HT1A receptor subtype and those not associated with that receptor subtype. A good deal of the appellants’ arguments centred on what we have summarised in paragraph (15) above. We do not think it correct (as the appellants contended) to read those references as saying that risperidone and olanzapine have low activity at the 5-HT1A receptor, but they are effective in the treatment of cognitive impairment in treatment-resistant schizophrenia, and that it follows from that, that the 722 Specification is teaching that there are circumstances in which cognitive impairment in treatment-resistant schizophrenia is not associated with the 5-HT1A receptor subtype. It seems to us that such an interpretation would ignore the sequence in which matters appear in the 722 Specification. The first statement is simply that something had been reported i.e., that risperidone and olanzapine improve treatment-resistant schizophrenia or cognitive impairments in treatment-resistant schizophrenics. A little later, it is stated that risperidone and olanzapine were not consistently superior to typical antipsychotic drugs in their effectiveness against treatment-resistant schizophrenia and, thus, (confirming, not contradicting the teachings of the 722 Specification), “these drugs cannot clearly perform activities through human 5-HT1A receptors at clinical effective doses”. Therefore, clozapine (which clearly does operate at the 5-HT1A receptor) is, at present, understood to be effective against treatment-resistant schizophrenia.
102 The primary judge’s construction of the claims by reference to the text of the claims and the teachings of the 722 Specification as a whole, was correct. It is appropriate to take into account the expert evidence to identify common general knowledge, explain terms and address the other general matters referred to by Lord Tomlin in British Celanese and the Full Court in Kinabalu Investments. However, there was no need for the primary judge to resolve the scientific controversy. There was no need for the primary judge to make findings about the evidence now identified by the appellants in paragraph 48 of their Outline of Appellants’ Submissions in Chief. We note, in passing, that a good deal of this evidence was evidence of their own witnesses in cross-examination, emphasising the fact that they have changed tack on the appeal. Irrespective of that, it seems to us that this was a case where there was a scientific controversy and the 722 Specification was premised on taking one side of that scientific controversy.
103 The appellants submit that the primary judge made two errors in relation to the failure to respond feature. First, he erred in construing it as applying to third line or later line treatment and not second line or later line treatment (the fourth alleged error). Secondly, (although the appellants accept that it is not entirely clear the primary judge did this) he erred in treating the failure to respond feature as having no effective meaning and, thus, notionally deleting it or putting it to one side (part of the first alleged error). It is convenient to deal with the issues in that order.
104 The appellants submit that there were two reasons why the primary judge’s construction of the failure to respond feature was erroneous. First, they submit that the primary judge did not give adequate weight to the expert evidence to the effect that the requirement related to second line or later treatment. Both Dr Singh and Associate Professor Norman gave evidence that that was how they interpreted the claims. We do not think that this is of any particular significance. As we have already said, it is well-established that ultimately the issue is one for the Court and the Court is not bound to accept the views of an expert. Secondly, the appellants point to a passage in the 722 Specification on page 12 where it is claimed that aripiprazole may represent a more potent and highly safe drug for curing the named disorders, “as compared with other currently available pharmacotherapeutic treatments”. The appellants submit that this meant “which fail to respond adequately to currently available antipsychotics such as the antipsychotic drugs listed in the claim”. We infer that the thrust of this submission is that the key point is the failure to respond, not the failure to respond to any particular number of drugs.
105 We reject these submissions. The primary judge’s two reasons for construing the failure to respond feature as applying to third line or later line treatment support his construction. The fact that the claims refer to antipsychotic drugs supports his construction, although it is not a strong indicator. However, the examples given in the 722 Specification are a strong indicator. They are broadly as follows:
(1) failure to respond to both of one to three typical antipsychotic drugs and one atypical antipsychotic drug;
(2) failure to respond to both of two typical antipsychotic drugs and one atypical antipsychotic drug (there are two examples of this in the specification each involving different classes of drugs); and
(3) failure to respond to both of one to two typical antipsychotic drugs and one atypical antipsychotic drug.
106 After the primary judge had addressed this issue of construction of the failure to respond feature, he noted that the 722 Specification did not provide any reason for limiting claims 1 and 7 to third line or any other line of treatment. He said that the specification did not teach that aripiprazole will only be effective where other drug treatments have failed (at [160]). That conclusion is correct.
107 The primary judge held that, although the limitation in claims 1 and 7 stood as an essential feature of the invention as claimed, it appeared to be “nothing more than one that had been arbitrarily imposed” (at [161]). His Honour went on to say the following:
the 722 Specification does not teach against using aripiprazole as first line or second line treatment;
there is no reason a person skilled in the art reading the 722 Specification would think that aripiprazole would not be equally efficacious in the treatment of a patient’s cognitive impairment if used as first line or second line treatment;
none of the expert witnesses gave evidence that as they read the 722 Specification, aripiprazole would only have utility in the treatment of cognitive impairment associated with schizophrenia if used as third line or later line treatment; and
such an understanding would have been at odds with the prescribing practices of a number of experts in relation to aripiprazole.
108 The primary judge did construe the failure to respond feature as an essential feature, but he said that he considered it an arbitrary limitation. The latter conclusion was relevant when he came to consider the respondent’s case that the invention as claimed lacked novelty and an inventive step (at [162]).
109 The primary judge’s conclusions as to the proper construction of the claims were correct. Subject to one matter which we deal with below, that means that the appellants’ challenge to his conclusions about novelty and inventive step based on his alleged “flawed” construction of the claims must fail.
110 The appellants submit that the failure to respond feature is not arbitrary and they suggested this was relevant to the primary judge’s conclusions in relation to novelty and inventive step. They point to evidence given by the experts which suggests that switching antipsychotics could lead to a worsening as well as an improvement of symptoms. That could be due to a non-response or a delayed response to the new drug and the fact that the patient’s response rate diminishes with the more drugs tried. That would apply to aripiprazole, even though it was disclosed to be effective in treating schizophrenia generally. However, contrary to that expectation, the invention claims that aripiprazole would still be effective as a second, third or later line treatment where other medications have failed. Furthermore, the appellants’ submission that the failure to respond feature is not arbitrary is supported (so it is argued) by Dr Singh’s evidence that when prescribing a different medication to the one that the patient is already on, he would try a medication which has a different receptor profile to a drug previously prescribed.
111 There are a number of answers to these submissions. The primary judge was aware that switching of antipsychotics could lead to a worsening of symptoms and of a patient’s diminishing response rate (at [52]-[53]). However, he made clear findings of the practice at the priority date of switching antipsychotics in an attempt to improve cognitive impairment in schizophrenic patients (at [388]). Neither of the matters identified by the appellants overcomes the primary judge’s finding that there would be no reason to read down the prior art to exclude any particular line of treatment, including third or later line treatment, where the patient fails to respond to two or more of the identified antipsychotic drugs (at [324]). It seems to us that the position is a fortiori in the case of inventive step. The primary judge said when considering Saha (at [440]):
The person skilled in the art would not have understood Saha to be disclosing that aripiprazole has a limited utility that is dependent on the medication that had previously been administered to the patient or on the number of times the patient’s previous medication had been switched. No witness suggested otherwise. The person skilled in the art would have understood from Saha that aripiprazole could be used in a method such as that claimed in claim 7 or for the production of a medicament such as that claimed in claim 1.
Conclusions
112 The primary judge’s conclusion that claims 1 and 7 of the 722 Patent were invalid was correct and the appeal must be dismissed. There is no need for this Court to consider the respondent’s challenge to his Honour’s conclusions with respect to infringement. The matters relating to the definition of “exploit” and s 117 of the Act were matters this Court could have addressed had it been necessary to do so. It would have been more difficult for this Court to address the infringement issue had the Court decided that the appellants’ construction of the association feature had been the correct one. It seems to us that it would have been appropriate to remit the issue to the primary judge who heard the evidence and had a full appreciation of the complexities it raised. However, it is not necessary to pursue that matter. The appellants must pay the respondent’s costs of the appeal.
I certify that the preceding one hundred and twelve (112) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justices Besanko and Nicholas. |
Associate:
REASONS FOR JUDGMENT
BEACH J:
113 I have had the advantage of considering the reasons in draft of Besanko and Nicholas JJ. I agree with their principal conclusions, but would express my reasoning differently concerning the construction and significance of the phrase “disorders of the central nervous system associated with [the] 5-HT1A receptor subtype” in claims 1 and 7 of the specification. Their Honours have described this as the “association feature”. I have nothing to add on what they have described as the “failure to respond feature”.
114 Initially I was impressed with the force of the contention advanced by Mr Tony Bannon SC, counsel for the appellants, that this phrase embodied a separate and essential integer. But on reflection, the specification, its context and the relevant science now dissuade me from accepting that submission.
115 But even if I had accepted the appellants’ construction, the patent would still be invalid for, inter alia, lack of novelty. By the use of the said phrase, assuming that on this hypothesis it constituted a separate integer, claims 1 and 7 would be infected with what patent lawyers would diagnose as parametritis. This affliction involves an attempt to re-patent the prior art by limiting claims by reference to a series of parameters not mentioned in the prior art (Raychem Corporation’s Patents [1998] RPC 31 at 37 per Laddie J) The parameter could be something measured on test equipment, which equipment did not exist at the time of the prior art. In the present case, the parameter is a statement in essence of a scientific theory positing a link between relevant disorders of the central nervous system and the 5-HT1A receptor subtype. But to inject this parameter adds nothing to the invention. It does not create a new process or method. It does not create a new use of an old product. In substance, one still has an old use of an old product or a more limited class of an old use of an old product. Scientific knowledge may have been enhanced by the identified association, whatever “association” or “associated” means, between the receptor subtype and the disorder. But there is no new invention by the addition of such knowledge to the claim language or using it as a limitation.
116 Indeed, the artificiality of such a result and the nebulous verbiage of the claim language gives me confidence in my first conclusion that the phrase “disorders of the central nervous system associated with [the] 5-HT1A receptor subtype” is not a separate and essential integer.
117 Let me make one other point before descending into the detail. I am still unclear as to what is precisely meant by “associated” in the relevant phrase and what the appellants contend its boundary constraints and content to be. What causative role is the 5-HT1A receptor subtype said to play? What is the mechanism? Direct or indirect? Separate operation? In combination with other factors, and if so what? And should one be looking at the presence of other factors or their absence? Further, does the 5-HT1A receptor subtype need to be faulty or unusually variant? Further, does one generalise across all individuals or only a sub-set, and if so what? Moreover, if one puts to one side what is embraced by the notion “association”, is it strong association or weak association? The expert evidence adduced before the primary judge was opaque on these matters; I will discuss the appellants’ expert evidence later.
118 The appellants had no satisfactory answers to any of these matters. Indeed, their position also seemed to be quite fluid. But what these vagaries point against is treating the relevant phrase as a separate and essential integer. If it was a separate integer, it would have to have meaningful boundaries and content such that one could know, in order to avoid infringing the claim(s), what was within and what was outside the claim(s). The relevant phrase has no such meaningful boundaries and content such that one could carve out a subset of the disorder(s) satisfying inter alia condition (i) in each of claims 1 and 7 by reference to any such hypothesised separate integer. This all suggests that the relevant phrase is not to be construed as having independent work to do. Rather it is part of the description of the integer “which disorder: (i) is selected from …”.
119 It is appropriate to discuss the text and context of the specification and then the science.
THE SPECIFICATION
120 Claim 1 of the specification provides as follows:
1. Use of carbostyril compound of formula (1):
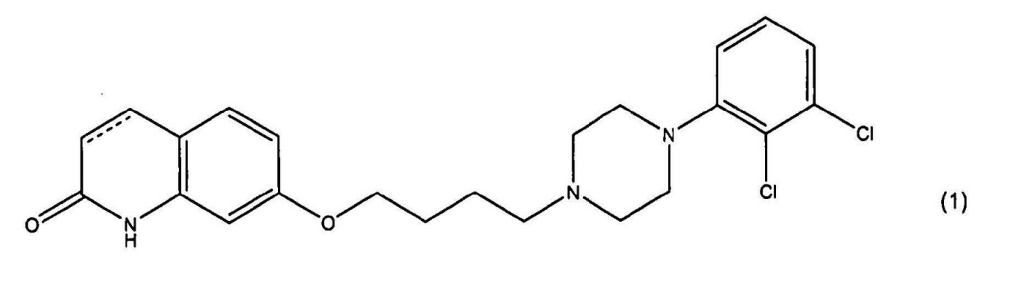
wherein the dotted line represents a single or a double bond, or a pharmaceutically acceptable salt or solvate thereof, for the production of a medicament, effective in the treatment of disorders of the central nervous system associated with 5-HT1A receptor subtype, which disorder
(i) is selected from cognitive impairment caused by treatment-resistant schizophrenia, cognitive impairment caused by inveterate schizophrenia, or cognitive impairment caused by chronic schizophrenia, and
(ii) fails to respond to antipsychotic drugs selected from chlorpromazine, haloperidol, sulpiride, fluphenazine, perphenazine, thioridazine, pimozide, zotepine, risperidone, olanzapine, quetiapine, or amisulpride.
121 For present purposes, it is not necessary to set out claim 7.
122 There are a number of observations that can be made as to the text of the claims and their context.
123 First, the expression, “which disorder: (i) is selected from …” sets out the boundaries and content of the disorders to be treated. Moreover, the reference to “which disorder…” is a reference back to the disorders identified in the phrase “the treatment of disorders…”. So, the structure of the claim is identifying to the reader that anything falling within the phrase “which disorder: (i) is selected from …” meets or is to be taken as meeting the composite and prefatory language “disorders of the central nervous system associated with [the] 5-HT1A receptor subtype”.
124 Second, if the appellants’ construction was correct, the claim would have been expected to have had a different structure with three conditions. It should have read:
… effective in the treatment of disorders of the central nervous system, which disorder:
(i) is associated with the 5-HT1A receptor subtype;
(ii) the present (i); and
(iii) the present (ii).
125 But it is not so structured.
126 Third, the nebulous concept of “associated” is confirmatory of the position that one is dealing with prefatory language when one is considering “associated with [the] 5-HT1A receptor subtype”.
127 Generally, it may be said that one does not need to look further into the body of specification. The claim language is clear from its text and structure. Moreover, the primary judge’s construction does not involve ignoring an essential integer or ignoring words of the claim. The prefatory phrase is part of the description of the essential integer “which disorder: (i) is selected from …” and is given meaning in that context. But for completeness, let me delve further into the body of the specification and the contextual scientific theories (as at the priority date).
128 Besanko and Nicholas JJ have analysed the specification in some detail in assessing the context of the association feature. I agree with their observations, but would make some additional observations. If the appellants’ contention was correct, namely, that the words “disorders of the central nervous system associated with [the] 5-HT1A receptor subtype” constituted a separate and essential integer (and in effect a third condition and limitation on the present conditions (i) and (ii)), one would have expected the body of the specification to be replete with references supporting such a position. It is not.
129 Indeed, the following principal passage at pages 12 and 13 of the specification (putting to one side the relevance of this passage to the failure to respond feature) does not support such a separate integer:
The carbostyril compound in the present invention binds with high affinity and displays a potent, partial agonist activity at the 5-HT1A receptors and it has higher intrinsic activity (about 68%) as compared with that of clozapine. Therefore, the compound in the present invention has a 5-HT1A receptor agonistic activity that is more potent than the agonistic activity of clozapine. Thus, the present carbostyril compound may represent a more potent and highly safe drug for curing treatment-resistant schizophrenia, cognitive impairments caused by treatment-resistant schizophrenia, inveterate schizophrenia, cognitive impairments caused by inveterate schizophrenia, chronic schizophrenia, cognitive impairments caused by chronic schizophrenia and the like, as compared with other currently available pharmacotherapeutic treatments. That is, the compound in the present invention may prove to be a potent and safer drug therapy for treatment-resistant schizophrenia, cognitive impairments caused by treatment-resistant schizophrenia, inveterate schizophrenia, cognitive impairments caused by inveterate schizophrenia, chronic schizophrenia, or cognitive impairments caused by chronic schizophrenia, etc., which fail to respond adequately to currently available antipsychotic drugs such as chlorpromazine, haloperidol, sulpiride, fluphenazine, perphenazine, thioridazine, pimozide, zotepine, risperidone, olanzapine, quetiapine, amisulpride, etc.
In particular, the carbostyril compound in the present invention may be a potent and highly safe drug therapy against treatment-resistant schizophrenia, cognitive impairments caused by treatment-resistant schizophrenia, inveterate schizophrenia, cognitive impairments caused by inveterate schizophrenia, chronic schizophrenia or cognitive impairments caused by chronic schizophrenia, etc. which fail to respond adequately to both of 1 to 3 typical antipsychotic drugs selected from the group consisting of chlorpromazine, haloperidol and perphenazine, and one atypical antipsychotic drug selected from the group consisting of risperidone, olanzapine, quetiapine and amisulpride.
130 What is apparent from this passage is that it is disclosed or asserted that the carbostyril compound of the invention, which includes aripiprazole, has been shown to bind with high affinity and to display a potent, partial agonist activity at the 5-HT1A receptor subtype. So, it is then concluded that “the present carbostyril compound may represent a more potent and highly safe drug for curing treatment-resistant schizophrenia…” (see line 15 on page 12). What then follows are the general descriptors of disorders including the cognitive impairments generally described in condition (i) of claim 1. This passage does not support the proposition that the relevant phrase is a separate and essential integer in and of itself. It is the carbostyril compound that is reported to be the partial agonist at the 5-HT1A receptor subtype. And it is said to be suitable to treat the generally expressed cognitive disorders, not a narrower subset thereof.
131 Further, reference is made on page 16 at line 23 to the fact that “[t]he potent, partial 5-HT1A receptor agonist in the present invention is useful for various disorders …”. But again this is a reference to the carbostyril compound. Further, it is true that the same passage goes on to identify the disorders in terms “various disorders of the central nervous system associated with the 5-HT1A receptor subtype …”. But it then relevantly and generally describes on page 17 at lines 14 to 19:
… treatment-resistant inveterate or chronic schizophrenia, (which fail to respond adequately to currently available antipsychotic drugs); cognitive impairments caused by treatment-resistant schizophrenia, inveterate schizophrenia or chronic schizophrenia and the like”.
132 Again, these disorders are described generally (as they are in condition (i) of claim 1). There is no attempt to define or refine any sub-set carved out of the general set.
133 In summary, there is little, if anything, in the body of the specification which supports the appellants’ construction. Indeed, the central passages are consistent with the textual analysis that I have set out earlier. It is now appropriate to turn to the science.
SOME NEUROCHEMISTRY
134 The following description is taken from the primary judge’s reasons (at [64] to [77]), the agreed statement on the relevant science put before the primary judge, some additional aspects of the evidence of Associate Professor Trevor Norman not reflected in that statement, and enhanced versions of key diagrams set out in that material. The hypothetical skilled addressee as at the priority date can be taken to have had knowledge of the following matters.
Neurotransmission and receptors – an overview
135 The transmission of nerve impulses takes place by means of specific chemical agents called “neurotransmitters”. This transmission occurs in the brain, other parts of the central nervous system and the peripheral nervous system. Transmission within the peripheral nervous system is important for everyday processes without which a human could not survive, eg breathing, heartbeat, movement and generally every important bodily function. This transmission is coordinated by electrical signals (nerve impulses) to and from the central nervous system in which the brain and spinal cord are key components. In addition to coordinating these everyday functions, the central nervous system subsumes other roles such as thinking (cognition) and feeling (emotion).
136 Neurotransmission is the process by which small molecules (neurotransmitters) transmit signals (electrical impulses) from one neuron to the next. Neurotransmission takes place at a “synapse”, ie where two axons (ie neurons) are in close proximity but not touching one another. These are the sites of communication between neurons in the central nervous system. The gap between the two adjacent neurons is the “synaptic cleft” or “synaptic gap”. It is across this gap that impulses are transmitted by the use of specialised chemicals known as neurotransmitters. When the nerve impulses arrive at a synapse, neurotransmitters are released, which in turn can influence another neuron, either by inhibitory or excitatory impulses. Further, the receiving neuron may “on-transmit” to many other neurons which then influence these neurons and so on. The neurotransmitters are released by a “presynaptic neuron” which bind to and activate “receptors” as simplistically portrayed below.
|
|
137 Neurotransmission occurs when an action potential (ie an electrical impulse) is initiated in a neuron and arrives at the nerve terminal of the pre-synaptic neuron. The mechanism of the action potential is a function of the electrical potential across part of the neuron, being the axon. It involves “travelling” switching polarity across the membrane of the axon in one direction only mediated by the action of the opening and closing of voltage gated ion channels. The action potential or electrical impulse when it arrives at the nerve terminal causes the release of chemical neurotransmitters. Neurotransmission then takes place, but in one direction, ie from the pre-synaptic cell to the post-synaptic cell. When neurotransmitters bind to their receptors on the postsynaptic neuron this may result in short term changes, such as changes in the membrane potential (ie the electrical charge contained by that postsynaptic neuron) called a postsynaptic potential; this may trigger a further action potential. Such a binding process can also cause longer term changes caused by the activation of G-protein coupled second messenger systems and their associated signalling cascades, which I explain later.
138 Neurons are arranged in the form of networks (neural networks) through which signals can travel. Information arrives at each neuron from many others. The human brain has approximately 100 billion neurons which make about 100 trillion synapses. Information is transferred in the brain, and signals are propagated throughout the body, by way of nerve impulses. Signals are sent to and from the central nervous system by efferent (ie conducting away) and afferent (ie conducting to) neurons in order to coordinate functions essential for survival.
Serotonin - a neurotransmitter
139 Serotonin (5-hydroxytryptamine; 5-HT) is an indoleamine neurotransmitter intimately connected to neuropharmacology. The reason for this is that it has certain chemical structural resemblances to the hallucinogenic compound LSD. Serotonin is synthesised from the amino acid tryptophan by a series of enzymatically catalysed steps; there are several steps needed to synthesise serotonin, with each of these steps requiring specific enzymes or catalysts to make the process happen along with other factors to provide energy to the process. Serotonin is found throughout the body and in many cells that are not neurons, such as platelets, mast cells (part of the immune system) and enterochromaffin cells of the intestinal mucosa.
140 Serotonin has long been implicated in mental illness, particularly to unusual levels of feeling of well-being. Only a small percentage of total body serotonin is synthesised in serotonergic neurons of the central nervous system. 90% of serotonin is made in the gut. Physiologically, serotonin is thought to play a role in the regulation of mood, appetite and sleep as well as in cognitive functions, such as memory and learning. Most classes of psychotherapeutic medications have some interaction with receptors for serotonin. Some antidepressants, selective serotonin reuptake inhibitors (SSRIs), are believed to bring about their therapeutic effects as a consequence of the ability to increase serotonergic neurotransmission by inhibiting serotonin reuptake at the pre-synaptic nerve terminal.
141 The 5-HT1A receptor subtype is one of a number of receptors which use serotonin as their neurotransmitter as a means of communication in the brain and elsewhere.
Receptors - general
142 Receptors are a type of protein molecule consisting of chains of amino acids; there are usually 500-700 such amino acids connected in a specific order. Receptors are located partially within neuronal cell membranes. They generally consist of three parts (as shown below):
(a) First, an “extracellular” portion which protrudes above the cell membrane such that it may receive signals from nearby cells.
(b) Second, the “transmembrane” spanning domain located within the cell membrane. This is arranged as a series of helical shapes within the membrane and gives the receptor its shape. For some receptors, the ligand-binding domain is located within this transmembrane portion of the receptor. “Ligand” comes from the Latin word meaning binding; it usually refers to a small molecule (eg a neurotransmitter) which will bind to the receptor to induce a shape (conformational) change in the receptor and bring about an alteration in the function of the receptor. Neurotransmitters and hormones are endogenous ligands (ie present in the body) while drugs are exogenous ligands (ie introduced to the body).
(c) Third, the “intracellular domain” or “cytoplasmic loops”, which interact with intracellular elements to produce changes in second messenger systems. So-called “G-proteins” are one form of second messenger which, when activated, can initiate a cascade of events within the cell. This can ultimately lead to interactions with the DNA (genome) causing events such as new protein to be synthesised. G-proteins couple to the cytoplasmic loops for some receptors and form an integral part of signal transduction. An example of a G-protein coupled receptor is illustrated below, highlighting the three specific domains of the receptor.
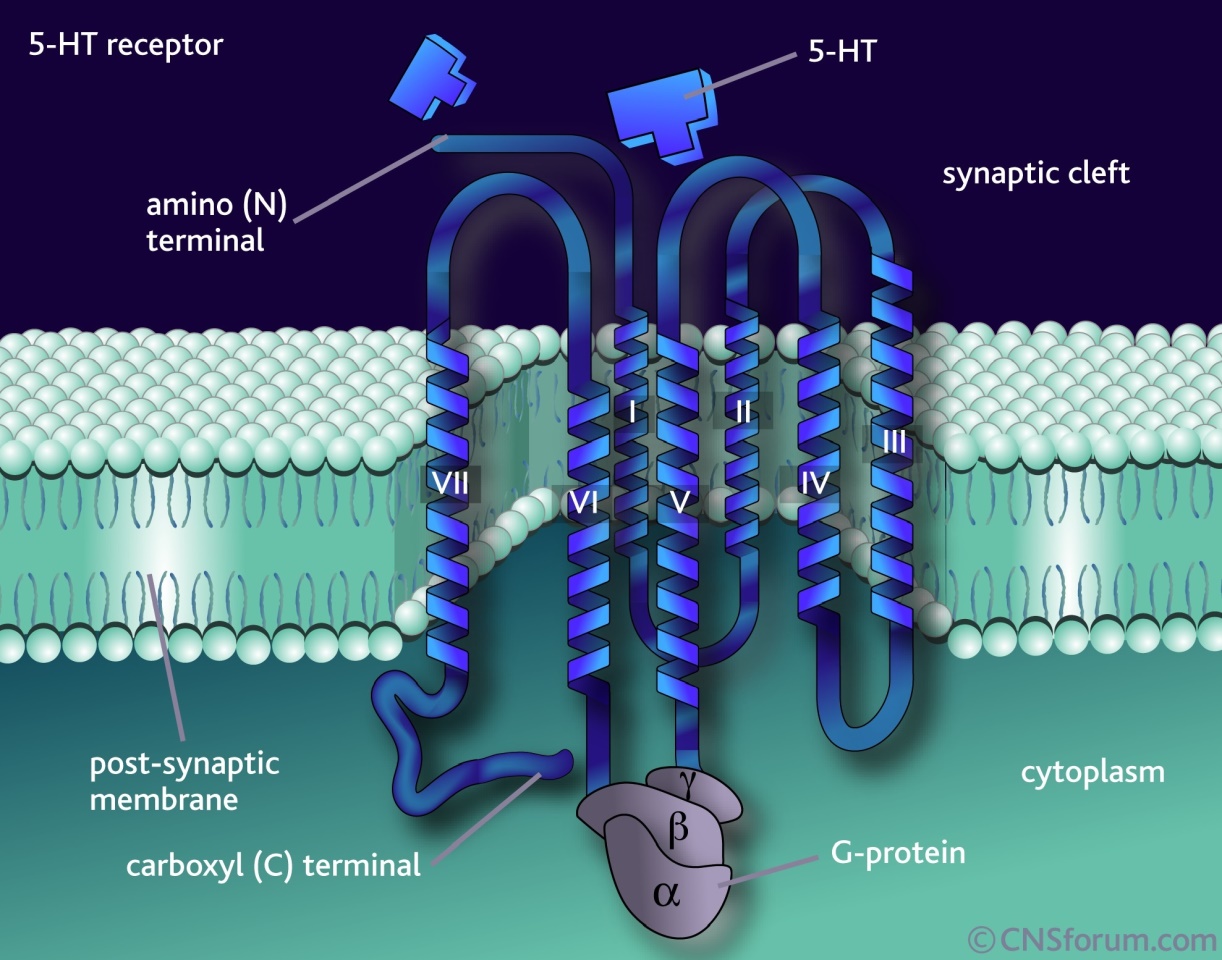
There are four broad 'superfamilies' of receptor: (1) the channel-linked (ionotropic) receptors; (2) the G-protein coupled (metabotropic) receptors; (3) the kinase-linked receptors; and (4) receptors that regulate gene transcription. The 5-HT1, 5HT2, 5HT4, 5HT5, 5HT6 and 5HT7 receptors belong to the G-protein coupled super-family. They are membrane receptors that have 7 trans-membrane spanning a-helices. 5-HT binding to the 'binding groove' on the extracellular portion of the receptor activates the G-proteins, which initiate secondary messenger signalling pathways. The downstream effect is either inhibitory or stimulatory, depending on the type of G-protein linked to the receptor - 5-HT1 receptors are linked to inhibitory G-proteins, whereas 5HT2, 5HT4, 5HT5, 5HT6 and 5HT7 are linked to stimulatory G-proteins. |
143 Within a cell, several receptor types may be found. Each type may be coupled to a particular biochemical pathway. The various receptors recognise and bind only with certain ligands. Simplistically, one might think of the receptor as the lock and the ligand as the key. Thus, the binding of a selective ligand to its receptor activates or inhibits a specific biochemical pathway. When the ligand binds to its receptor, the receptor can be stabilized in a specific conformation (ie the three-dimensional shape). This may be associated with gain or loss of function, usually leading to a response within the cell. Changes in receptor conformation, induced by ligand binding, result in cellular changes which constitute the biological activity of the ligands.
144 The physical relationship between a drug and its target receptor is described by binding studies. However, such studies do not assess the consequences of such an association. There are three distinct aspects of drug binding: affinity, efficacy and potency.
(a) “Affinity” describes the strength of the binding between the drug and its receptor. A strong affinity implies an ability of a drug to compete with or displace other drugs, or neurotransmitters such as serotonin itself, that bind at the same receptor.
(b) “Efficacy” describes the nature of the biological effect elicited by that binding.
(c) “Potency” describes the amount of a drug required to produce an effect of a given intensity. The potency of a drug at a receptor depends on both its affinity and efficacy.
145 Drugs generally interfere with the binding of the neurotransmitter (the endogenous ligand) to its receptor where they are said to compete for the receptor. Drugs have markedly different effects with respect to potency and efficacy. Two traditional categories of drugs have been recognised: “agonists” and “antagonists”. An “agonist” mimics the effects of the endogenous neurotransmitter by bringing about a similar conformational change in the receptor and hence a similar biological response to that of the endogenous ligand. On the other hand, an “antagonist” binds to the receptor and does not evoke a biological response. In other words, an antagonist is inert at the receptor and brings about its effect by blocking the action of the endogenous ligand (neurotransmitter). The differences in efficacy of agonists and antagonists at the receptor are independent of their affinity and potency for the receptor.
146 A third category of drug is the “partial agonist”. These drugs also bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist, ie they elicit less than the maximal response from the receptor.
147 Partial agonists may exert both agonist and antagonist effects under certain conditions. At high doses, a partial agonist may antagonise the effects of a full agonist if its affinity for the receptor is greater than that of the full agonist. This is sometimes referred to as mixed agonist-antagonist actions.
The 5-HT1A receptor subtype
148 The serotonin system in the brain is complex due to the multiplicity of receptors with which serotonin can interact. At least 14 unique receptor subtypes have been identified which use serotonin as their neurotransmitter, all of which are denoted “5-HT”. The function of these receptors is not certain because drugs which act specifically at the different subtypes have not been synthesised. Nevertheless, the roles of some subtypes in various physiological processes have, to some extent, been delineated.
149 The 5-HT1A receptor subtype is the best characterised of the 5-HT1 family of receptors. It has high affinity for serotonin as well as for the arylpiperazine class of anxiolytic agents (buspirone, gepirone, ipsapirone). The 5-HT1A receptor subtype is hypothesised as playing a role in neuroendocrine function and thermoregulation, memory, immune function, depression and anxiety (amongst other hypothesised functions).
150 The 5-HT1A receptor subtype is a 7-transmembrane-spanning, G-protein coupled receptors (see below). Studies in humans have shown the presence of the receptor in significant levels in almost all parts of the brain. The highest level of expression of the receptor has been found in the hippocampus, cingulate cortex, septum, and intra-limbic cortex, with the lowest levels in the cerebellum.
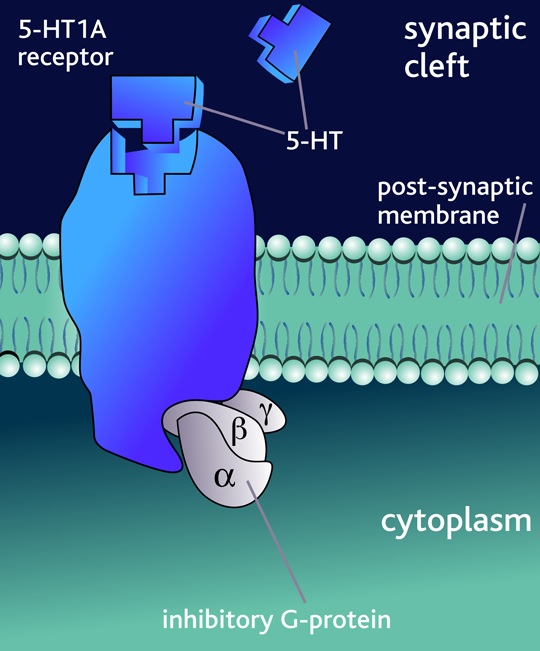
The structure of the 5HT1A receptor. The 5-HT1 receptors are classified into A, B and D subtypes, which are found in the central nervous system and blood in vessels. Coupled to inhibitory G-proteins, the 5-HT1A receptors have an inhibitory effect on neurotransmission when an agonist binds to the receptor. |
151 5-HT1A receptors function both as pre-synaptic auto-receptors (ie controlling their own firing rate and therefore the release of serotonin) and as postsynaptic receptors (ie they receive the neurotransmitter released and, as a consequence of the changes in the receptor shape, they alter biochemical processes within the postsynaptic neuron). 5-HT1A auto-receptors are mainly located on the cell body (or soma) and dendrites (branched projections of neurons which receive the input from other adjacent neurons) of 5-HT neurons in the raphe nuclei (dorsal and median raphe nuclei are located in the brain stem and form the main cell bodies in the brain which contain serotonin). Hence these receptors are sometimes referred to as somato-dendritic receptors. On the other hand, postsynaptic 5-HT1A receptors exist on postsynaptic membranes of neurons or nerve terminals (heteroreceptors). Stimulation of postsynaptic 5-HT1A receptors inhibits the firing of target neurons through G-protein-mediated mechanisms. Put another way, the major electrical effect brought about by interaction with postsynaptic 5-HT1A receptors on neurons involves attenuation of the firing of neurons, thus resulting in decreased release of neurotransmitters from the synaptic ends of these neurons.
Association between the 5-HT1A receptor and cognitive impairment in schizophrenia
152 Associate Professor Trevor Norman, an expert called by the appellants, gave evidence of the following matters. I have referred only to his evidence as it is the high point for the appellants’ case. I will also assume in favour of the appellants that this can be taken to be the state of knowledge of the skilled addressee as at the priority date. If that assumption is not good and the state of knowledge was even more rudimentary as at the priority date, then the strength of my conclusions set out in the next section would only be enhanced.
153 Of approximately 14 serotonin receptors, four 5-HT receptor subtypes are most prominently involved in cognitive function – the 5-HT1A, 5-HT2A, 5-HT6, 5-HT7 receptors. Serotonin receptors other than those noted could also be important in cognitive function but there is little information due to the lack of drugs which target these receptors and which are suitable (ie not toxic) for administration in humans.
154 Associate Professor Norman has theorised the following. From cell bodies in the dorsal raphe nuclei (in the brain stem), serotonin neurons (axons) project to areas of the brain (such as the hippocampus) that are thought to be important for determining various aspects of cognitive function (eg in the case of the hippocampus, memory formation). The amounts of serotonin released at the end of these projections (the so-called terminal fields) are controlled, in part, by the auto-receptors, ie 5-HT1A receptors located in the raphe nuclei (cell bodies). It can be inferred from this distribution that among the many other effects that serotonin has in the brain, it will influence aspects of cognitive function. Given that these are very long axons, there is an opportunity for other neurons to synapse along the serotonin projections and therefore also influence the activity (release) of serotonin at the terminal fields. Both 5-HT1A partial agonists and full antagonists have shown cognitive enhancing effects in animals, probably due to their different anatomical sites of action; partial agonists possibly act on terminal projections (ie where the axons or neurons end) while antagonists act at auto-receptors in the raphe.
155 Associate Professor Norman has theorised that given the anatomical location of 5-HT1A receptors in the brain, alteration of the frequency of firing (modulating the tone) of 5-HT1A receptor-dependent neuronal activity might affect memory, cognition or both, and there is pre-clinical evidence suggesting that 5-HT1A-active drugs may enhance memory or cognition.
CONCLUSIONS ON THE ASSOCIATION FEATURE CONSTRUCTION
156 A number of matters may be noted in terms of the state of knowledge that could be attributed to the skilled addressee as at the priority date.
157 First, although there was considerable knowledge of the operation of the serotonin system in the brain, the system was complex. 14 unique receptor subtypes had been identified which used serotonin as their neurotransmitter (the 5-HT series). But the precise function of the receptor and the subtypes was unclear.
158 Second, four 5-HT receptor subtypes had been identified as being involved in cognitive function. But the association, let alone the precise mechanism, linking the 5-HT1A receptor subtype and cognition was unclear, save that this receptor subtype had a higher affinity for serotonin.
159 Third, there was some evidence linking schizophrenia with alterations in the 5-HT1A receptor function, with the further hypothesised link to cognitive function. But the hypothesis could not be said to be robust. The mechanism was unclear. Perhaps this might explain the nebulous language that “alterations in the 5-HT1A receptor are associated with cognitive impairment in schizophrenia” (Associate Professor Norman’s reply evidence).
160 Fourth, it was a matter of speculation as to whether partial 5-HT1A agonists, such as aripiprazole, may be useful in the treatment of cognitive impairment in schizophrenia.
161 Fifth, and relatedly, how speculative this all is can be demonstrated by the appellants’ reply evidence as at 13 June 2013 and a fortiori as at the priority date. Associate Professor Norman said at [10] to [12] in his affidavit of that date:
The reason why I describe the proposition that partial 5-HT1A agonists may improve cognition as a “theory” is that it has not been conclusively proven that it is the 5-HT1A activity of these drugs which improve cognition and indeed, based on the current state of knowledge about the human brain, it would be impossible to “prove” a causal link between the 5-HT1A receptor and cognition because the brain is such a complex area and there is still so much unknown about how it works…
Professor McGregor has criticised my evidence for use of the term “associated with” in the context of the link between the 5-HT1A receptor and cognition (at paragraph 45 of his affidavit) and the link between the 5-HT1A receptor and MDD (at paragraph 67 of his affidavit). I used the word “association” and “associated with” because, as noted above, there is no conclusive proof that the two are causally connected. However, based on the studies which find a change in a patient’s behaviour and/or physiological response (for example, hormone responses) after being treated with a drug which acts at that receptor, I believe it is appropriate to state that there is an “association” between that receptor and the behaviour/physiology, even if it is not currently possible to prove the causal connection between them.
I am not aware of any drug available for clinical use which targets exclusively the 5-HT1A receptor. Accordingly, as all drugs which act at the 5-HT1A receptor also act as other receptors, it is not possible to conclusively prove that it is the 5-HT1A activity which brings about the physiological effect. Conversely, if a study purports to show that a 5-HT1A partial agonist drug does not improve cognition, it is impossible to conclude that there is no causal connection between the 5-HT1A receptor and cognitive improvement as it may be the effect of the drug at other receptors acting against the effects which may otherwise be caused as a result of the 5-HT1A activity.
162 Generally and with that background knowledge, if one was the skilled addressee as at the priority date turning his or her mind to what was meant by the phrase “disorders of the central nervous system associated with [the] 5-HT1A receptor subtype”, and whether that was to be taken to be a separate and essential integer such as to carve out a subset of disorders from condition (i) of claim 1, the skilled addressee would likely have concluded that there was no such separate integer.
163 First, the skilled addressee would have appreciated that the words “associated with” manifested imprecision in thought and a lack of understanding of the precise scientific mechanism and causal connection between the relevant receptor subtype (and action thereon) and the relevant disorders.
164 Second and relatedly, the skilled addressee would then have concluded that the phrase was not intended to be anything more than a prefatory description. The skilled addressee would have appreciated that the relevant disorders were properly identified in condition (i) of claim 1. Moreover, the skilled addressee would have appreciated that none of the identified disorders in condition (i) could confidently be said not to be associated with the 5-HT1A receptor subtype. In other words, all of the disorders in condition (i) could be said to be associated with that receptor subtype. Neither the teaching in the specification nor the skilled addressee’s relevant knowledge would have suggested otherwise. And if that be so, then that supported the construction that the relevant phrase was not a separate integer carving out a sub-set.
165 Third, the grammatical structure of the claim did not support such a separate integer for the reasons that I have set out earlier.
166 Fourth, the words “associated with” were too imprecise to delineate let alone apply the relevant phase as a separate integer to determine whether something was within or outside the claim. Moreover, the body of the specification did not assist to provide precision to “associated with”. Indeed, it reinforced the proposition that, in general, all of the categories of disorders referred to in condition (i) of claim 1 could, in the loose language of the claim, be said to be “associated with” the 5-HT1A receptor subtype. Such matters pointed against the relevant phrase being a separate integer.
167 Fifth, some individuals suffering from one or more of the disorders referred to in condition (i) of claim 1 might not in an idiosyncratic case have their disorder associated with their 5-HT1A receptor subtype. But to admit of such possibilities takes the appellants nowhere on construction. One is looking at a method or process claim in general. Further, for any one individual taking the relevant carbostyril compound, the precise neurochemical pathway could not be known until after the method had been applied, and more likely never known at all. It cannot reasonably be contended that in a method claim not defined by reference to the result, that such idiosyncratic differences in terms of actual causes of the specified disorders could drive the construction question. They may never be known, but on any view could only be known after the act of infringement.
168 Sixth, in the above discussion concerning the association feature I have discussed the relevant phrase and condition (i), but left to one side condition (ii). Condition (ii) may be separate in form but its inclusion is artificial in substance. But putting that point to one side, its inclusion provides no support for treating the association feature phrase as itself a separate integer. Condition (ii) deals not with the categories and subject matter of the disorders (condition (i)), but rather with the different question of whether such disorders (condition (i)) have failed to respond to certain treatments; in other words condition (ii) proceeds on whatever fits within condition (i) without changing the subject matter identified in condition (i).
169 For the foregoing reasons, the primary judge was correct on the construction question dealing with the association feature.
INVALIDITY
170 In my view, and for the reasons given by the primary judge, the patent is invalid for lack of novelty and lack of inventive step. But that conclusion is based upon the correctness of his Honour’s construction on the association feature and the failure to respond feature.
171 But let me assume for the sake of argument the scenario in which his Honour was incorrect in his construction of the association feature such that the phrase “disorders of the central nervous system associated with [the] 5-HT1A receptor subtype” did constitute a separate and essential integer; for present purposes I am still proceeding on the basis that his Honour was correct on the failure to respond feature. In my view the patent is still invalid in this alternative scenario for inter alia lack of novelty given the prior disclosures in US Patent No. 5,006,528 (US 528) and European Patent No. 367,141. For present purposes, it is only necessary to consider US 528.
172 Now as to US 528, it may be accepted that it does not disclose that aripiprazole is a partial agonist at the 5-HT1A receptor subtype. Further, as the primary judge accepted (at [311]), there is no reference to 5-HT1A receptors or the action of aripiprazole on 5-HT1A receptors or the serotonergic system more generally. But as his Honour rightly pointed out, US 528 teaches that the carbostyril derivatives it describes, including aripiprazole, are alternative to a range of other drugs, and are useful for improving both the positive and negative symptoms of schizophrenia (at [312]). It was accepted by the parties that a person skilled in the art at the priority date would have understood the significant overlap between the negative and cognitive symptoms of schizophrenia (at [313] and [317]).
173 At [318] to [321], the primary judge said the following:
For these reasons, I am satisfied that EP 141/US 528 disclose the use of aripiprazole, amongst other carbostyril derivatives, as a drug for the treatment of schizophrenia generally, which is a useful alternative to earlier generation drugs such as, for example, chlorpromazine, haloperidol and sulpiride, including in the chronic period of the illness. I am also satisfied that, at the priority date, the person skilled in the art would have understood that when EP 141/US 528 refer to aripiprazole’s utility in treating negative symptoms, those symptoms included at least some of the symptoms which claims 1 and 7 of the 772 patent categorise as cognitive impairment.
The following question arises: When the invention claimed in each of claims 1 and 7 of the 772 patent is compared with the prior art information in EP 141/US 528, is the invention, as so claimed, novel because no disclosure is made with respect to the action of aripiprazole on 5-HT1A receptors (whether due to its partial agonism or otherwise) or the use of aripiprazole specifically as third line treatment where the patient has failed to respond to two or more of the identified prior art drugs? If the present case were to be decided by reference to the reasoning in the United Kingdom cases such as Actavis [2008] EWCA Civ 444; [2009] 1 WLR 1186 and Baker Norton, the answer to that question, it seems to me, would be “no”. For one thing, the invention, as clamed in each claim, would not be directed to a new use of the known substance. Is that reasoning, or similar reasoning, applicable in the Australian legal context? In my respectful view, it is.
The test provided by s 7(1) of the Act is directed to whether an invention, as claimed, is novel. This raises a question of substance, not one of mere claim form. Once the claim is properly construed, the question of substance is whether the invention, as so defined, is novel in the sense of providing an invention (here, a method of treating schizophrenia) that is new. Properly understood, claim 7 of the 772 patent is directed to the use of aripiprazole to treat a subset of the symptoms of schizophrenia in certain patients on certain occasions, when it is already known that aripiprazole can be used to treat schizophrenia generally. Claim 7 merely partitions something that is old under the guise that the part it takes and claims as an invention is new. However, such an “invention” is not new. Claim 7 is not directed to a new therapeutic use, but an old one. The forms of cognitive impairment referred to in claim 7 were, at the priority date, but some of the known symptoms of schizophrenia. If, at that time, aripiprazole had been used in a therapeutically effective amount to treat schizophrenia, it would inevitably have treated cognitive impairment of the kind referred to in claim 7, including if used as third line or later line treatment in those patients whose symptoms had failed to respond to two or more of the antipsychotic drugs identified in the claim. In substance, claim 7 claims a then known substance, aripiprazole, for its then known therapeutic purpose, the treatment of schizophrenia, only that some of the symptoms of schizophrenia and only some occasions of use are referred to in the claim. The same is true of the invention claimed in claim 1, being the use of aripiprazole in the production of a medicament for treating patients in respect of the same symptoms in the same circumstances.
Novelty of invention is not provided merely because information given as part of the definition of the invention in a claim is new information. With specific reference to claims 1 and 7, new information is provided by the feature that aripiprazole can be used to treat a patient suffering from disorders of the central nervous system associated with the 5-HT1A receptor. However, the disorders referred to were part of the known symptoms of schizophrenia. The provision of information that certain of the then known symptoms of schizophrenia are also associated with the 5-HT1A receptor is really no more than an elucidation of the action of the known carbostyril compounds, including aripiprazole, in treating schizophrenia, and a contribution to knowledge of the possible aetiology of those particular symptoms. These accretions to knowledge, without more, do not provide novelty of invention. They are simply aspects of knowledge, albeit new information, about the then known therapeutic use (the treatment of schizophrenia) of a then known compound.
174 Now just stopping at this point, the primary judge was prepared to conclude this even accepting that the relevant phrase that I have discussed as the association feature could be considered in one sense separate. I say that because the primary judge then went on to deal with the case where the relevant phrase was not a free standing feature and said at [322]:
Moreover, as I have explained, I do not accept, in any event, that the reference in claims 1 and 7 to this knowledge defines a free-standing, essential feature of the invention which is to be considered as meaningfully adding to the identification of the specific forms of cognitive impairment referred to: see [146]. Thus, the further description in claims 1 and 7 of the disorders as “disorders of the central nervous system associated with [the] 5-HT1A receptor subtype ...” cannot confer novelty. It is merely supplementary information about the symptoms of cognitive impairment associated with schizophrenia generally and, more specifically, the particular forms of schizophrenia identified in the claims.
175 With respect, I agree with his Honour’s reasons at [318] to [321]. On that foundation, even if the appellants are correct on their association feature construction, they still fail on lack of novelty.
176 Like the primary judge, I agree that novelty is not conferred merely by:
(a) providing more information about an old use;
(b) explaining the scientific theory for the mechanism which underlines a use already described in the prior art; or
(c) claiming a narrower use of an old product, where that narrower use fits within the broader use for the old product already described in the prior art.
177 In this respect I agree with the observations in Actavis UK Ltd v Janssen Pharmaceuticals NV [2008] FSR 35 at [99] and [100] per Floyd J and Bristol-Myers Squibb Co v Baker Norton Pharmaceuticals Inc [1999] RPC 253 at [59] per Jacob J. The addition of the embryonic scientific hypothesis referring to the 5-HT1A receptor subtype is hardly the obtaining of a new technical effect or a new use. Once one accepts the significant overlap between negative and cognitive symptoms of schizophrenia, all that the patent in suit has done is to take a subset of the use and method disclosed in US 528 and to justify the narrowing based on a scientific theory as at the priority date that could hardly be said to be robust. In essence this is just a variant form of parametritis.
178 For the foregoing reasons, the claims fail for lack of novelty even if the appellants’ association feature construction was to be accepted.
179 Generally, I otherwise agree with the conclusions of Besanko and Nicholas JJ and their proposed orders.
I certify that the preceding sixty-seven (67) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Justice Beach. |
Associate:
Dated: 24 August 2016