FEDERAL COURT OF AUSTRALIA
GlaxoSmithKline Australia Pty Ltd v Reckitt Benckiser Healthcare (UK) Ltd [2016] FCAFC 90
ORDERS
DATE OF ORDER: |
THE COURT ORDERS THAT:
1. As to the First Product (defined in the orders made by the Court at first instance on 19 June 2015), the appeal be dismissed, other than in respect of the finding of infringement of claim 9 of Australian Patent No. 2003283537 (the patent).
2. As to the Second Product (defined in the orders made by the Court at first instance on 19 June 2015), and the First Product in relation to the finding of infringement of claim 9 of the patent, the appeal be allowed.
3. Within 21 days, the parties bring in short minutes that provide for:
(a) the revocation and variation of orders made by the Court at first instance on 19 June and 22 September 2015; and
(b) the compilation of a compendious form of orders conformable with these reasons, including the question of the undertaking as to damages and costs.
4. Should the parties not agree on said orders, within 28 days each side file and serve draft short minutes and submissions of no more than three pages in support thereof.
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
THE COURT:
Introduction
1 This appeal is from orders made by the primary judge on 19 June 2015 and 22 September 2015, reasons for judgment having been given on 20 May 2015: Reckitt Benckiser Healthcare (UK) Ltd v Glaxosmithkline Australia Pty Ltd (No 5) (2015) 112 IPR 273; [2015] FCA 486 (the reasons).
2 The proceeding concerns the appellants’ infringement of the first respondent’s Australian Patent No. 2003283537 (the patent) entitled “Improvements in and relating to liquid dispensing”, by the supply of two over-the-counter paediatric medicinal products. The second respondent is the exclusive licensee of the patent.
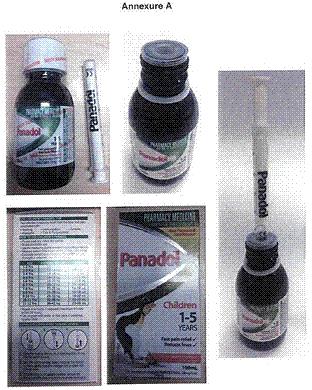
3 In the orders of the primary judge, the “First Product” is defined to mean Children’s Panadol 1-5 years (of any flavour or size) incorporating the liquid dispensing apparatus comprising a bottle, a bottle neck liner and a syringe, as depicted in Annexure A and in the form of Exhibits B, D and J.
4 The “Second Product” is defined to mean Children’s Panadol 1-5 years (of any flavour or size) incorporating the liquid dispensing apparatus comprising a bottle, a bottle neck liner and a syringe, as depicted in Annexure B and in the form of Exhibits C, D and J.
5 The First Product is depicted in Annexure A to the orders as follows:
6 The Second Product is depicted in Annexure B to the orders as follows:

7 Permanent injunctions were made by the primary judge restraining the appellants from using the First Product and the Second Product. The first appellant (then the only party against whom infringement was alleged) denied that it was liable for any infringement and sought an order under s 138(3) of the Patents Act 1990 (Cth) (the Act) that the patent be revoked. In this connection, the first appellant alleged that the invention claimed in each of claims 1 to 6 and 9 is not a manner of manufacture; is obvious and does not involve an inventive step; was obtained on a false suggestion or misrepresentation; and is not fairly based on the matter described in the specification. The first appellant also alleged that the first respondent is not entitled to the patent to the extent that it includes the invention claimed in claims 1 to 6 and 9. The primary judge rejected these grounds and upheld the validity of each of the relevant claims. There is no appeal from his Honour’s findings in that regard, other than in respect of the ground of fair basis, as noted below.
8 The amended notice of appeal dated 12 October 2015 claims that the primary judge erred:
In finding that the Second Product infringes claim 9 of the patent.
In finding that the First Product infringes claims 1, 2 to 6 and 9 of the patent.
In finding that claim 9 of the patent, on the construction adopted, is fairly based on the matter “disclosed” in the specification.
9 The appellants seek orders setting aside the injunctions and ancillary orders, and the certification that claims 1 to 6 and 9 of the patent are valid. The appellants seek orders that claims 1 to 6 and 9 be revoked and that the proceeding be remitted to determine (a) the basis upon which the costs of the proceeding are to be paid, and (b) any claim on the usual undertaking as to damages given on 28 May 2013 and 17 July 2013.
10 By their amended notice of contention, the respondents claim that the judgment relating to the Second Product should also be affirmed on the ground that the syringe of the Second Product is a flat-nosed syringe as claimed in claim 1 of the patent and, therefore, that the Second Product also infringes claims 1 to 6 of the patent.
The complete specification
11 The drafting of the complete specification (the specification) proceeds in an orthodox fashion. After identifying the field of the invention and providing a background section which describes, amongst other things, a number of problems associated with the prior art methods of withdrawing liquids from bottles (including using syringes to withdraw medicine), the specification provides a consistory statement directed to a liquid dispensing apparatus comprising a bottle, a bottle neck liner and a flat-nosed syringe (identified as the first aspect of the invention). Claim 1 of the specification (see [23] below) corresponds to this consistory statement. However, by way of further description (at p 4, line 23 to p 5, line 7), the specification provides definitions of terms used in the consistory statement. There is no reason to doubt that these definitions apply to other parts of the description of the invention in the specification and are carried into the claims themselves:
By “syringe” we mean a syringe comprising a hollow syringe barrel in which is located, or arranged to be located, a reciprocating plunger, the syringe barrel having a dispensing aperture, through which a liquid may be drawn, then discharged.
By “flat-nosed syringe” we mean a syringe whose barrel ends in a generally flat distal end which is perpendicular to the barrel axis, and in which the dispensing aperture is formed. Preferably there is no part of the distal end which extends beyond the bore.
By “sealingly” we mean that under conditions of normal use liquid cannot flow or leak between the respective parts, that is, between the bottle neck and the bottle neck liner, and between the bore and a syringe barrel.
(Emphasis added.)
12 The specification discloses a number of preferred, non-essential features of the apparatus. Save in one respect referred to at [19] below, it is not necessary to descend to the detail of those features.
13 The specification then describes the second (and remaining) aspect of the invention, namely a method of dispensing a liquid using the liquid dispensing apparatus of the first aspect of the invention. Claim 7 is directed to this method. This claim has no particular significance to the issues arising in this appeal. We mention it only to give a complete picture of the drafting of the specification.
14 The following disclosure is made at p 13, lines 4-21 of the specification. It has some importance for the respondents’ submissions in this appeal:
Thus a bottle is provided from which liquid can be dispensed in a conventional manner, and also prescribed amounts of liquid can be delivered using a syringe; and in which no components need to be inserted into or removed from the bottle or bottle neck in order to switch between the dispensing methods. The use of a flat-nosed syringe and an inward step in the bottle liner also minimises the contact of the liquid with the syringe barrel, thereby also minimising the scope for contamination of the liquid from microorganisms on the syringe barrel. The outside of the syringe barrel is not coated in the liquid, thus minimising cleaning of the syringe, and dripping of liquid from it. As the bottle liner is such that the barrel of the syringe fits sealingly into the sleeve, substantially no liquid leaks from the bottle when the syringe is inserted and the bottle tilted or inverted. The only exit route for liquid is the through bore of the liner, by one or other of the methods described above.
15 Under the heading “Brief Description of the Drawings”, the specification states (at p 13, line 25 to p 14, line 17):
In order to better understand the various aspects of the invention, and to show embodiments of [scil: how] the same may be put into effect, the invention will now be described by way of example, with reference to the accompanying drawings in which:
Figure 1 illustrates a side elevational view of a preferred embodiment of a bottle neck liner of the first aspect of the invention;
Figure 2 illustrates a side sectional view of the bottle neck liner of Figure 1;
Figure 3 illustrates the bottle neck liner of Figures 1 and 2 inserted into the bottle neck of a bottle;
Figure 4 illustrates a flat-nosed syringe, arranged in use to be inserted into the bottle neck liner of Figures 1 and 2;
Figure 5 illustrates the syringe of Figure 4 inserted into the bottle of Figure 3; and
Figure 6 illustrates a side sectional view of the syringe, bottle neck liner and bottle taken through the line AA of Figure 5.
16 The figures are as follows:
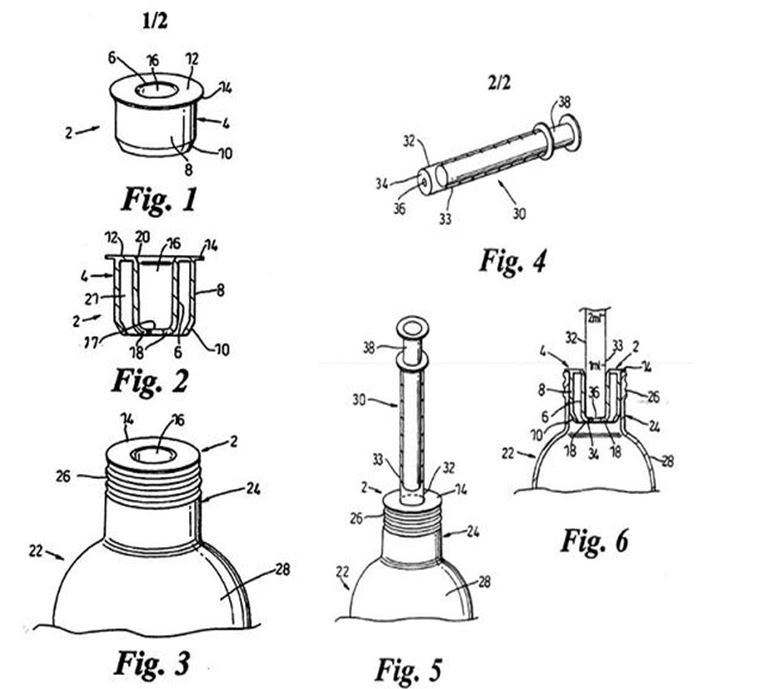
17 In this example, the bottle neck liner is described as a one-piece injection moulding made from a resilient plastics material. As depicted in Figures 1 and 2, it comprises a sleeve (4) with a circularly cylindrical wall (6). The outer body of the sleeve (8) has a taper or chamfer (10). The top wall of the sleeve (12) has an outwardly protruding annular flange (14). This is best seen in Figure 2. The sleeve has a through bore (16) and, at its lower end, an inward step (18). The inward step is an annular formation protruding inwardly from the interior surface of the sleeve, which terminates in a circular aperture (17). The sleeve is inwardly bevelled at its upper end (20). Figure 2 shows an annular space (21) between the outer body (8) and the sleeve (4). The outer body and sleeve are connected only at the upper end of the liner by the top wall (12).
18 Use of the bottle neck liner is described by reference to Figures 3 to 6 (at p 15, lines 14-32):
… The bottle neck liner 2 is inserted into a bottle 22, which is preferably a medicine bottle for dispensing a viscous liquid medicine. The bottle is formed at its upper end with a cylindrical narrowed bottle neck 24, which is itself formed with an external screw thread 26. The bottle neck liner 2 is push fitted into the bottle neck 24 until the flange 14 of the bottle neck liner 2 abuts the rim of the bottle neck 24. At this point the bottle neck liner cannot be pushed further into the bottle neck 24. The bottle neck liner is dimensioned such that the sleeve 4 does not protrude past the bottle neck 24 and into the main body 28 of the bottle 22. The resilient nature of the plastics material of the bottle neck liner 2 means that as the liner is pushed into the bottle neck 24 the outer body 8 is deformed inwardly, and provides a sealing fit between the outer body 8 and the bottle neck 24. Thus, if the bottle 22 is inverted, no liquid can flow between the outer body 8 and the internal surface of the bottle neck 24.
19 In an earlier passage dealing with the preferred, non-essential features of the apparatus (at p 8, lines 23-29), the specification makes the following related disclosure with respect to the bottle neck liner:
Preferably the cylindrical body is slightly oversized relative to the bottle neck into which it will be fitted. The intention is to achieve a firm interference fit, such that the parts will not separate in use. Thus, the force required to withdraw the liner from the bottle neck preferably exceeds the force required to withdraw a syringe barrel from the sleeve into which it is inserted.
20 Figure 4 depicts the flat-nosed syringe, described as follows (at p 16, lines 11-19):
… a flat-nosed syringe 30 for use in the present invention. The flat-nosed syringe 30 includes a hollow circularly cylindrical syringe barrel 32 having a distal end region 33 to be received in the liner, and terminating in a perpendicular, circular face at its distal end 34, formed with a centrally located dispensing aperture 36. The syringe 30 also includes a plunger 38 arranged to move under reciprocal motion within the syringe barrel 32.
21 This description must be understood within the confines imposed by the definition of “flat-nosed syringe” (see [11] above). The consistory statement of the first aspect of the invention itself imposes constructional limitations on the flat-nosed syringe, including that it has a generally flat face having a diameter corresponding to the diameter of the syringe barrel: see also in that connection, claim 1 reproduced below at [23]. This is plainly depicted in Figure 4.
22 In explaining Figure 6, the specification states (at p 16, line 25 to p 17, line 23):
As the distal end region 40 [quaere, 33] of the barrel is inserted into the bottle neck liner 2, it pushes slightly against the sleeve 4, the cross-section of the bore 16 of the latter being slightly smaller than the cross-section of the barrel. Good sealing against the passage of liquid between the barrel and the sleeve is thereby provided. The insertion continues until the distal end 34 of the syringe barrel 32 abuts the inward step 10 of the sleeve 6 (see Figure 6). In this position, the distal end region of the syringe barrel is a tight sealing fit within the sleeve inside the bottle neck 24, but the rest of the syringe barrel - the larger part - is not; it stands outside the bottle. Thus, graduations on the syringe barrel 32 can still be seen by a user. The seals between the liner 2 and the bottle neck 24, and the syringe barrel 32 and the sleeve 4, prevent leakage of liquid between such parts if the bottle is tilted or inverted. The dispensing aperture 36 of the syringe barrel 32 is located centrally and contiguously with the opening within the inward step 10 of the sleeve 4.
In use, in order to accurately measure and dispense an aliquot of liquid from the bottle 22, the bottle is tilted, or preferably inverted, and the plunger 38 of the syringe 30, previously stowed inside the barrel, is withdrawn until the prescribed aliquot of liquid has been drawn from the bottle 22, via aperture 17, into the syringe barrel 32, as measured by the graduations on the syringe barrel 32. The bottle may then be placed upright again, and the syringe 30 withdrawn from the bottle neck liner 2 in order to dispense the prescribed quantity of liquid now present in the syringe barrel 32.
The claims
23 The claims defining the invention are, relevantly:
1. A liquid dispensing apparatus comprising a bottle, a bottle neck liner and a flat-nosed syringe having a plunger and a barrel, the barrel terminating at its distal end in a generally flat face having a diameter corresponding to the diameter of the syringe barrel and being perpendicular to the longitudinal axis of the barrel, the bottle having a bottle neck in which is located the bottle neck liner having a cylindrical body sealingly engaged inside the bottle neck such that liquid cannot flow between the bottle neck liner and the bottle neck, the bottle neck liner comprising a sleeve comprising at its lower end an inward step located within the bottle neck, an aperture being defined inwardly of the inward step, wherein the cylindrical body and the sleeve are connected together with a web of material only at the upper end of the cylindrical body and of the sleeve, wherein the sleeve is formed with a flared portion at its upper end into which the distal end of the syringe barrel passes; wherein when the syringe barrel is inserted into the sleeve the inward step prevents the syringe barrel from protruding past the step and liquid cannot flow between the sleeve and the barrel, but can leave the bottle only via the aperture and thence the syringe.
2. A liquid dispensing apparatus as claimed in claim 1, wherein the aperture is pre-formed and permanently open.
3. A liquid dispensing apparatus as claimed in Claim 1 or 2, wherein the sleeve comprises a resilient material.
4. A liquid dispensing apparatus as claimed in any preceding claim, wherein the liner comprises an outwardly protruding flange extending around at least a portion of an end of the liner and abutting the rim of the bottle neck into which the liner is inserted.
5. A liquid dispensing apparatus as claimed in any preceding claim, wherein the bottle contains liquid medicine.
6. A liquid dispensing apparatus as claimed in any preceding claim, wherein the bottle includes a closure member which can be secured over the bottle neck.
…
9. A liquid dispensing apparatus, substantially as described with reference to the drawings and/or examples.
The primary judge’s reasons
24 So far as relevant to the appeal, the reasons of the primary judge are as follows.
25 The primary judge found, at [151]-[152] of the reasons, that the First Product infringes claim 1. His Honour noted that it was not in dispute that, if the First Product infringes claim 1, it also infringes each of claims 2, 3, 4, 5 and 6. His Honour also found that the First Product infringes claim 9.
26 The primary judge found that the Second Product does not infringe claims 1, 2, 3, 4, 5 or 6 but does infringe claim 9.
27 The primary judge commenced his consideration by dealing with the following questions of construction, amongst others.
28 With respect to claim 1, the primary judge noted (at [91] of the reasons) that the first critical question of construction was whether the barrel of the syringe must be of a uniform diameter throughout its length.
29 At [65] of the reasons, the primary judge recorded the respondents’ submission that claim 1 only requires the diameter of the flat nose of the syringe to correspond with the diameter of the barrel at its distal end, regardless of whether the shape or configuration of the barrel above its distal end remains the same or changes. At [71] of the reasons, the primary judge recorded the first appellant’s submission that claim 1 requires the barrel of the syringe to be of a uniform diameter along its length; claim 1 does not claim an invention in which the syringe has “more than one barrel or cylindrical portions of different diameters”.
30 In considering this question, the primary judge noted (at [91] of the reasons) that the experts had expressed diametrically different views, although they agreed that, near its proximal end, the barrel of a syringe will, ordinarily, flare out to enable the plunger to be inserted. However, his Honour appeared to accept that this flaring does not affect the concept that the “thing” described is a “barrel”.
31 At [96] of the reasons, the primary judge rejected the respondents’ construction, reasoning (at [94] of the reasons) as follows:
I am of opinion that a skilled addressee would understand that the barrel shape that claim 1 specified had a generally uniform diameter along its length, although that addressee would realise that, at its proximal end, the barrel would need to be slightly flared in order to allow the plunger to enter. Such an understanding would be confirmed by the shape of the barrel in the syringe in figure 4 in the patent. The patentee used its own clear language in claim 1 to identify the characteristic of the barrel having a diameter that is both uniform along its length and corresponds to the flat face at the distal end.
32 His Honour noted (at [95] of the reasons) that this construction is also consistent with the narrowing of the claims undertaken by the first respondent in amendments made to the specification after March 2006.
33 With respect to the features of the bottle neck liner, the primary judge said (at [97] of the reasons) that claim 1 requires the following features:
(1) the liner has an outer cylindrical body;
(2) the outer cylindrical body of the liner would sealingly engage with the inside of the bottle neck;
(3) that engagement has to produce the result that liquid cannot flow between the wall of the cylindrical body, contiguous with the inner wall of the bottle neck;
(4) the liner comprises a sleeve that:
(a) is located inside the cylindrical body;
(b) the upper end of the inner sleeve is connected to the outer cylindrical body by a web of material;
(c) the sleeve descends from the web of material to an inward step that has an aperture opening into the sleeve;
(d) the sleeve is formed with a flared portion at its upper (web of material) end into which the distal end of the syringe barrel passes;
(e) when the syringe barrel is inserted into the sleeve the inward step prevents:
(i) the barrel going past the step; and
(ii) liquid flowing between the sleeve and the barrel except through the aperture into the barrel.
34 As to the expression “sealingly engaged” as used in claim 1, the primary judge said (at [98] of the reasons) that the definition of “sealingly” in the specification (see [11] above) means that, under conditions of normal use, liquid cannot flow or leak between the respective parts. The requirement is that the cylindrical body of the liner is “sealingly engaged inside the bottle neck such that liquid cannot flow between the bottle neck liner and the bottle neck”. Thus, his Honour said, claim 1 specifies the degree of engagement between the liner and the bottle neck that must be achieved, namely that liquid cannot flow between them.
35 At [99]-[100] of the reasons, his Honour said:
99 The skilled addressee reading the complete specification, including the claims, (as a whole) would have understood that the apparatus claimed had to be capable, in conditions of normal use, of leaving the liner in place sealingly engaged inside the bottle neck in the manner stipulated in claim 1, for at least as many occasions as the user took to dispense all of the liquid contents of the bottle. In other words, the skilled addressee would have understood that there would or could be a number of occasions on which the user would insert a syringe, draw up liquid and then withdraw the syringe, and that, in conditions of normal use, the liner had to continue to remain “engaged” with the bottle neck so as to prevent leakage for, at least, the duration of those occasions of use.
100 Importantly, claim 1 specified that the sealing engagement had the purpose of preventing the escape of liquid as opposed to retaining the liner in place. Ordinarily, the skilled addressee would understand that both results could be achieved by a firm interference fit. However, the skilled addressee would know that there were other means of keeping the liner in place over multiple uses. He or she would realise that claim 1 was directed to making essential the nature of the seal achieved between the liner and the bottle neck, not the mechanism by which the liner could be held in place over multiple uses. The claim required the sealing engagement between the liner and the bottle neck to prevent liquid flowing between the two.
36 Accordingly, his Honour rejected the appellants’ construction that the sealing engagement required by claim 1 has to be a firm interference fit so as to prevent the liner being dislodged when the syringe is withdrawn from it. At [101] of the reasons, the primary judge noted that the requirement in claim 1 that the liner being “sealingly engaged” is distinct from the functionality of the preferred embodiment referred to in the specification at p 8, lines 23-29 (quoted at [19] above), which speaks of a firm interference fit between the cylindrical body of the liner and the bottle neck. His Honour noted that the function of an interference fit is to keep the liner in place, not simply to prevent leakage, which, in claim 1, is the characteristic provided by the defined meaning of “sealingly”.
37 With respect to claim 9, the omnibus claim, the primary judge reasoned (at [108] of the reasons) that it seeks to bring within the patentee’s monopoly any liquid dispensing apparatus “substantially as described with reference to the drawings and/or examples”. His Honour said that this form of claim does not limit the claimed apparatus to the precise form of the description, drawings or examples. In this connection, his Honour referred to Radiation Limited v Galliers & Klaerr Proprietary Limited (1938) 60 CLR 36; [1938] HCA 17 (Radiation) at 41, 46 and, per Dixon J, at 51-52; Lewis v Hall (2005) 68 IPR 89; [2005] FCAFC 251; (Lewis v Hall) at 97 [31]-[32]; and Raleigh Cycle Co Ltd v H Miller and Co Ltd (1948) 65 RPC 141 (Raleigh Cycle) at 157, per Lord Morton of Henryton. At [112] of the reasons, his Honour said:
In my opinion, claim 9 in the patent should be given a construction that reflects its use of the word “substantially” in the same way as Lord Morton and Dixon J did in the passages that I have set out above. That is, the claim extends to the substantial idea disclosed by the specification and shown in the drawings, but is not limited to the exact expression or illustration of that idea in the patent.
38 The primary judge then went on to consider the question of infringement. At [113] of the reasons, his Honour identified four issues. The first two issues were common to both accused products and concerned two features of the bottle neck liner used by the appellants which remained unchanged, namely whether the liner has, first, a cylindrical body on the sleeve in the lower portion of the centre (as opposed to the innermost) sleeve and, secondly, a flared portion. The third issue concerned only the Second Product namely, whether the syringe in that product is a flat-nosed syringe within the meaning of claim 1 of the patent. The fourth issue was whether the First Product and the Second Product infringe claim 9. Only some of those issues are relevant to this appeal.
39 The first issue considered by the primary judge that is relevant to this appeal concerns the cylindrical body of the liner. It relates to the requirement in claim 1 that the cylindrical body be “sealing engaged” inside the bottle neck. His Honour noted (at [133] of the reasons) that the experts had agreed that the appellants’ liner “is sealingly engaged with the inside of the bottle neck”. This was due to the functionality of that part of the cylindrical body of the appellants’ liner (above the taper of the centre sleeve or wall of the liner) which is contiguous to the inner wall of the bottle neck.
40 In this connection, the experts agreed that the apparent design purpose of this part was to stop liquid leaking out of the bottle. Robert Tiller, an expert called by the respondents, tested these co-operating parts in the two accused products for leakage. He found that no liquid leaked between the touching surfaces. William Hunter, an expert called by the first appellant, did not dispute these observations. He said that this part of the centre sleeve is “a size for size fit”.
41 At [140] of the reasons, the primary judge concluded that the appellants’ liner has a cylindrical body as required by claim 1. His Honour also found that the liner “engages sealingly so as to prevent liquid flowing between the liner and the bottle neck above the lower taper of the centre sleeve”. We note that, earlier (at [132] of the reasons), the primary judge found that the liner of the Second Product “has the features of claim 1 and is substantially as described with reference to the drawings as in claim 9…”. As we have noted (at [38] above), at trial the bottle neck liner of each accused product was considered to be the same. We understand the primary judge’s finding at [132] of the reasons to be an affirmation that the liner in each product has the essential features of the bottle neck liner defined in claim 1 and by claim 9.
42 It is convenient to record at this juncture another finding concerning the appellants’ liner. The appellants’ liner has a snap lock feature. At [120] of the reasons, the primary judge recorded Mr Hunter’s evidence that this is provided by “the small outer wall of the … liner that has a ridge or clip in effect locking the liner in place over the outer rim of the top of the bottle neck …”. This snap lock prevents the liner dislodging from the bottle neck when the syringe is removed. This feature assumes importance in the appellants’ submissions on appeal, particularly concerning whether the First Product infringes the patent.
43 The second issue in relation to infringement relevant to this appeal that was considered by the primary judge concerns the syringe in the Second Product, which the primary judge called “the alternate syringe”. The primary judge found (at [126] of the reasons) that “the alternate syringe does not have a generally uniform diameter along its length. That is because the indented section at the distal end is of a significantly smaller diameter than the major portion of the syringe.” We observe that this is apparent from the depiction of the Second Product at [6] above. On the basis of this finding, it followed that the Second Product does not infringe claims 1 to 6, and the primary judge found accordingly.
44 This finding, however, left open the question of whether the Second Product infringes claim 9. This raises the third issue in relation to infringement relevant to this appeal which concerns, specifically, the shape and configuration of the alternate syringe.
45 At [144] of the reasons, the primary judge found that the flat face of the alternate syringe and its tip operate “exactly as described in the specification and drawings in the patent …”. His Honour did not accept that certain functional differences between the alternate syringe and the flat-nosed syringe described in the specification, as propounded by Mr Hunter, made any difference. His Honour described these as distinctions without a substantive difference. Placing emphasis on functional equivalence, his Honour said (at [149] of the reasons):
The alternate syringe has exactly the same function as that described in the patent and the drawings. The alternate syringe is a flat-nosed syringe that has a distal end that fits into the liner and achieves a good seal with it so that it can draw up liquid without leaking from the bottle or the syringe. The mere fact that there is a corresponding tip on both the barrel and the reciprocating plunger used in the alternate syringe in the second product complained of should not be allowed to disguise that that product has taken the substantial configuration resulting from the patentee’s invention and its character for the dispensing of liquids from bottles without mess using an apparatus with a flat-nosed syringe: Radiation 60 CLR at 52; Raleigh 65 RPC at 160. The alternate syringe, as incorporated into the second product complained of, is not a substantially new or different combination: Clark v Adie (1875) LR 10 Ch App 667 at 675 per James LJ; Minnesota 144 CLR at 286.
46 Thus, the primary judge concluded (at [150] of the reasons), “[t]he alternate syringe takes the substance of the flat-nosed syringe described in the patent and drawings as stated in claim 9”. This conclusion was, no doubt, informed by his Honour’s approach to the construction of claim 9, namely that the claim extends to the “substantial idea” disclosed in the specification and is not limited to the precise form of the description, drawings or examples there given.
47 With respect to the question of fair basis, the primary judge concluded (at [238]-[240] of the reasons) that, based on the construction that his Honour had given to claim 9, and in particular the word “substantially”, claim 9 is fairly based on the matter described in the specification. His Honour said that he had reached this conclusion “for the reasons I have given as to why the alternate syringe and the second product complained of infringes claim 9”.
The Appellants’ submissions
Claim 9 and infringement
48 The appellants submit that the primary judge erred in construing claim 9 as covering any apparatus with the same functions as the combination described in the patent, with the result that the primary judge erred in finding that the First Product and the Second Product infringe claim 9. The appellants submit that the primary judge fell into this error in six respects. As developed in the appellants’ written submissions, the points, in summary, are as follows.
49 First, an “omnibus claim”, such as claim 9, is to be construed to determine its scope like any other claim. However, where it follows after broad claims, such as claims 1 to 6, it is to be taken as an attempt to claim the features (other than trivial ones) of the (or a) preferred embodiment.
50 Secondly, the words “substantially as described with reference to the drawings and/or examples” means it is a claim to “the invention” by way of example. In context, “the invention” referred to is the invention which is described in the immediately preceding section entitled “Summary of the Invention”. That section describes the invention, in its broadest form, in the consistory clause, which is in terms identical to claim 1. The description of the example which then follows could not, as a matter of construction, be broader than the invention of which it is, expressly, an example. The primary judge found that the Second Product infringes claim 9, at [149]-[150] of the reasons. The primary judge had earlier found, at [127] of the reasons, that the Second Product does not include a flat-nosed syringe, with the result that it does not infringe claims 1 to 6. The effect of these two findings is that the primary judge gave a broader construction to claim 9 than he did to claim 1.
51 Thirdly, and more specifically, claim 9 is limited by reference to the description of the example and drawings which have the effect of incorporating the drawings and their descriptions into claim 9.
52 Fourthly, the word “substantially” in a claim merely shows an intention that the claim should not be read too literally. It cannot broaden the claim to substitute for an essential feature of the described apparatus, something different. In Fresenius Medical Care Australia Pty Ltd v Gambro Pty Ltd (2005) 67 IPR 230; [2005] FCAFC 220 at 245 [87] the Full Court said that “to characterise a feature set out in the claim as not an essential feature of that which is claimed and consequently to disregard it in determining the scope of the claim, is rare”. In the context of modern claim construction, it would be a most unusual case where a feature expressly set out in the claim is not essential.
53 Fifthly, to give a purposive construction to a patent specification and its claims is not to extend the patentee’s monopoly to the “ideas” disclosed in the specification. In other words, purposive construction does not extend the patentee’s monopoly to products that the patentee did not, by the claims, define as the invention, even if the products perform the same function as the invention. There is no principle of “non textual” infringement.
54 Sixthly, contrary to the suggestion of the primary judge at [147] of the reasons, what is left unclaimed, by the wording of the claim properly construed, is not within the patentee’s monopoly. Contrary to the reasons of the primary judge at [112] and [148], neither the Full Court in GlaxoSmithKline Australia Pty Ltd v Reckitt Benckiser Healthcare (UK) Ltd (2013) 103 IPR 487; [2013] FCAFC 102 at 503 [60] nor Dixon J in Radiation at 51 gave the primary judge a licence to depart from the above principles and interpret claim 9 as a claim to the “substantial idea” or “substance” of the invention. As Dixon J said in Radiation at 51, in considering infringement, it is “the substantial idea disclosed by the specification and made the subject of a definite claim” (emphasis added) that must be considered.
55 Accordingly, the appellants submit, the primary judge ought to have construed claim 9 by reference to the drawings (Figures 1 to 6) and their descriptions at p 13, line 25 to p 18, line 26 of the specification, and thereby construed the claim as requiring:
(a) a “flat-nosed” syringe, being one whose barrel terminates at its distal end in a flat face that has a diameter corresponding to the diameter of the syringe barrel and is perpendicular to the barrel axis, and in which the dispensing aperture is formed, as shown in Figures 4, 5 and 6 of the patent;
(b) a bottle neck liner comprising an outer body, with a circularly cylindrical through bore, where the interior surface of the sleeve at its upper end is inwardly bevelled, as shown at item 20 in Figure 2; and
(c) a bottle neck liner that is push fitted into the bottle neck, such that the outer body of the liner is deformed inwardly so that it cannot be separated from the bottle neck when in use.
56 The appellants submit that, had the primary judge properly construed claim 9, he would have found that the Second Product does not infringe the claim because:
(a) as found by the primary judge at [126]-[127] of the reasons, the barrel of the syringe of the Second Product is not a flat-nosed syringe in that it does not terminate at its distal end in a flat face that has a diameter corresponding to the diameter of the syringe barrel: see also Figure 4;
(b) as found by the primary judge at [141] of the reasons, the web (the top wall (12)) of the bottle neck liner falls into the sleeve on a gradual, descending angle, rather than at a right angle; and
(c) consistently with the primary judge’s findings at [115], [117] and [120] of the reasons (concerning the presence in the bottle neck liner of the accused products of a snap lock fastening), the bottle neck liner is not push fitted into the bottle neck so that it is deformed inwardly, thereby achieving the result that the liner cannot be separated from the bottle neck when the apparatus is in use.
57 The appellants submit that, had the primary judge properly construed claim 9, he would have also found that the First Product does not infringe claim 9 because the same bottle neck liner is used in the First Product: see features (b) and (c) described immediately above.
Claim 9 and fair basis
58 The appellants submit that if the primary judge’s construction of claim 9 is correct, then his Honour erred in finding that claim 9 is fairly based on the matter described in the specification, as required by s 40(3) of the Act . His Honour ought to have held that claim 9 is invalid and liable to be revoked under s 138 of the Act.
Claim 1 and infringement
59 The appellants submit that the primary judge failed to give meaning to the word “engaged” in claim 1. In particular, the appellants submit that his Honour failed to find that claims 1 to 6 require the cylindrical body of the bottle neck liner and the bottle neck to be “engaged” such that the liner and the bottle neck are prevented from separating when in use. The appellants submit that, in effect, the word “engaged” requires a firm interference fit between the cylindrical body of the bottle neck liner and the bottle neck itself. The appellants argue that, had the primary judge construed the integer in this way, his Honour would have found that the First Product does not infringe claims 1 to 6 of the patent, because the product does not have this feature. Rather, they argue, the bottle neck liner of the First Product has a snap lock fastening to hold it securely on the lip at the top of the bottle, which prevents the liner from dislodging from the bottle neck when the syringe is removed. The appellants say that, as the Second Product has the same bottle neck liner, this is an additional reason why the primary judge ought to have found that the Second Product does not infringe claims 1 to 6 of the patent.
60 The appellants submit that, in this regard, the primary judge fell into error in three respects. As developed in the appellants’ written submissions, the points, in summary, are as follows.
61 First, the primary judge failed to construe the word “engaged” in isolation from his Honour’s consideration of the bottle neck liner of the accused products. When construing a claim, the Court should disregard an alleged infringing article.
62 Secondly, the words of a claim are in the patentee’s own choosing and fix the boundaries of the monopoly. The appellants submit that the primary judge treated the verb “engaged” as entirely confined by the adverb “sealingly”, thus leaving “engaged” with no work to do. His Honour’s finding ought to have been that the word “sealingly” and the word “engaged” were both used carefully to do separate work. As developed in oral submissions, the appellants argue that “sealingly” speaks about preventing fluid getting through, but “engaged” brings with it the concept of an “interference fit”. The contrast in the evidence was between a size-for-size fit which could be achieved that would be “sealing” but not “engaged”. The reason for this, articulated in the evidence, is that, when one inserts the syringe into the sleeve, there is a tight fit between the syringe and the liner, so that the syringe can be used to safely draw out the medicine. But when the syringe is pulled out from the liner, there is plainly a force applied. What is necessary for this to work is the ability of the liner to stay in position, notwithstanding the forces that are applied to pull out the syringe.
63 Thirdly, a claim must be construed in a practical, common sense manner with an eye to the utility of the claimed invention. The primary judge failed to construe claim 1 in this way. The primary judge erroneously rejected the appellants’ construction of claim 1 in favour of a construction that left claim 1 inutile because it contained no means of preventing the liner and the bottle neck separating when in use.
The respondents’ submissions
64 The respondents support the primary judge’s findings and conclusions on infringement but, by an amended notice of contention, contend that, contrary to [94] and [126]-[127] of the reasons, the Second Product also infringes claims 1 to 6 of the patent.
Claim 9 and infringement
65 The respondents submit that claim 9 uses “common language” and that the general principles of patent construction apply to omnibus claims. The respondents say that further guidance is given in decisions in which the word “substantially” has been used in a similar context. The respondents submit that the appellants’ arguments are based on the false premise that the primary judge construed claim 9 as covering any apparatus with the same functions as the combinations described in the patent. According to the respondents, the primary judge did not construe the claim in this way. The respondents say that the primary judge construed claim 9 in accordance with well-established principles of construction. They point to the primary judge’s reliance on Radiation and Raleigh Cycles and say that his Honour correctly held (at [112] of the reasons) that claim 9 extends to “the substantial idea disclosed by the specification and shown in the drawings” but is not limited to “the exact expression or illustration of that idea in the patent”. The respondents submit that no essential feature of the invention is missing from the Second Product. The respondents point to p 13, lines 4-21 of the specification (quoted at [14] above) and argue that the essential features of the invention are broadly described as the combination of a bottle, bottle neck liner and a flat-nosed syringe, the liner having an inward step and being such that the barrel of the syringe fits sealingly into the sleeve of the liner so that no liquid leaks from the bottle. The respondents submit that each of these features is present in the alternate syringe which, the respondents say, was intended as a “design-around”. In this connection, the respondents characterise the alternate syringe as a “subterfuge” that has been made in “an attempt to take full advantage of the invention while avoiding trespass upon the literal meaning of the claim by a modification so small as to be insignificant and to have no material effect upon the way the invention as claimed…works” (Rehm Pty Ltd v Websters Security Systems (International) Pty Ltd (1988) 11 IPR 289; [1988] FCA 232 at 301 per Gummow J citing Commonwealth Industrial Gases Limited v MWA Holdings Pty Limited (1970) 180 CLR 160; [1970] HCA 38 at 167 per Menzies J), and as a “clumsy infringement” (Rotocrop International Limited v Genbourne Limited [1982] FSR 241 at 257 per Graham J).
Claim 9 and fair basis
66 The respondents submit that the appellants’ fair basis argument is circular. They say that the primary judge had rejected the appellants’ argument (at [240] of the reasons) in light of his Honour’s construction of the omnibus claim, particularly the word “substantially”. The respondents submit that the appellants’ argument conflates issues of construction/infringement and fair basis. They argue that the only question is whether the claims are fairly based on the matter described in the specification and that the test, in this regard, is whether there is a real and reasonably clear disclosure of that which is claimed. The respondents say that claim 9 is “traditional” and cannot be said to be not fairly based because it extends beyond exact reproduction of the examples and/or drawings.
Claim 1 and infringement
67 The respondents submit that the claims of a patent must be read at the relevant date through the eyes of the skilled addressee in the context of the whole of the specification in which they appear, in the same way as any other commercial document is read. The respondents say that, in accordance with these principles, the primary judge properly construed the words “sealingly engaged” in the context in which they appear in claim 1. In this connection, the respondents refer to the reasons at [98], [135] and [140]. The respondents submit that the appellants’ assertion that the primary judge failed to give meaning to the word “engaged” as used in claim 1 is no more than an attempt to shift focus away from the word “sealingly” and say that the primary judge did not make the errors alleged by the appellants.
Notice of contention
68 The respondents submit that the primary judge ought to have found that the alternate syringe is a flat-nosed syringe as claimed in claim 1, contrary to [94] and [126]-[127] of the reasons. As developed in the course of oral submissions, the respondents’ point is that the claim does not specify that the barrel of the syringe must have a uniform diameter along its length. Thus, the barrel, as it exists in the alternate syringe, is not excluded from the claim. The respondents submit that when the words in claim 1 are properly construed, they refer to the diameter of the barrel at the flat face (ie, at the distal end of the barrel). According to the respondents, it does not matter that the barrel has, for example, an expanded diameter, such as in the alternate syringe, “above the distal end”.
Consideration
Claim 9 and infringement
69 The principles of claim construction—including the construction of omnibus claims such as claim 9—are not controversial and need not be repeated in these reasons. It is sufficient to note for present purposes that, when an omnibus claim is in issue, greater attention is generally placed by the words of the claim on the body of the specification to provide the necessary definition of the invention as required by s 40(2)(b) of the Act. Typically of omnibus claims, claim 9 in the present case draws attention to “the drawings and/or examples”. These are to be found in that part of the specification that appears under the heading “Brief Description of the Drawings” to which we have referred at [15]-[22] above.
70 It is convenient at this point to note the following matters with respect to that description.
71 First, as the prefatory statement at p 13, lines 25-29 of the specification makes clear, the example and accompanying drawings are given to “better understand the various aspects of the invention”. As we have already noted (see [11] and [13] above), the invention is said to have two aspects—a particular apparatus (the first aspect) and a method using that apparatus (the second aspect).
72 Secondly, the drawings (represented by Figures 1 to 6) are of the one, preferred embodiment. This is made clear by the description of the drawings at p 13, line 31 to p 14, line 17 of the specification: see [15] above. Thus, only one example is given.
73 Thirdly, as the description of the drawings makes clear, the example given is that of the first aspect of the invention. This focuses attention on the description of the invention given by the consistory statement, which is to be found at p 3, line 30 to p 4, line 21 of the specification.
74 Thus, the example given does not represent, and should not be construed as, a departure from that which the specification has previously described as the first aspect of the invention. Properly understood, the example falls within the confines of that description.
75 When regard is had to that description, there can be no doubt that the syringe comprising part of the liquid dispensing apparatus is not simply a flat-nosed syringe. For whatever reason—and there may be, for example, sound legal reasons (see the observations of the Full Court in Fisher & Paykel Healthcare Pty Ltd v Avion Engineering Pty Ltd (1991) 22 IPR 1; [1991] FCA 566 (Fisher & Paykel) at 20, lines 4-14)—the consistory statement for the first aspect of the invention characterises the flat-nosed syringe as one where the diameter of the flat “face” corresponds to the diameter of the syringe barrel. Contrary to the respondents’ submission, this characterisation of the diameter of the flat “face” is not one by reference to the distal end of the barrel. It is a reference to the diameter of that part of the syringe that constitutes its “barrel”, not some part of the barrel. The only reasonable conclusion is that the barrel has one diameter (in the words of the consistory statement “…the diameter of the syringe barrel…” (emphasis added)), aside from what the experts considered to be a necessary degree of flaring required at the proximal end of the barrel to enable the plunger to be inserted.
76 As we have previously remarked, the consistory statement for the first aspect of the invention finds expression in claim 1. As the primary judge correctly found (at [94] of the reasons), the skilled addressee would understand that the barrel shape that claim 1 specifies is one in which the barrel has a generally uniform diameter along its length, apart from the necessary flaring at the proximal end. The alternate syringe does not have that shape. It is not, therefore, a flat-nosed syringe for the purposes of claim 1. Thus, the primary judge was correct to conclude that the Second Product does not infringe claims 1 to 6 of the patent.
77 Given that the consistory statement for the first aspect of the invention finds expression in claim 1, and given that the specification makes clear that the example given is of the first aspect of the invention, it must follow that the invention defined in claim 9 cannot be wider in scope than the invention defined in claim 1. Thus, the primary judge’s finding that the Second Product does not infringe claims 1 to 6 because the alternate syringe is not the flat-nosed syringe claimed in claim 1, should have led his Honour to conclude, also, that the Second Product does not infringe claim 9. The primary judge erred in finding otherwise.
78 As we have observed, claim 9 draws attention to the “drawings and/or examples” which stand as the reference point for the scope of the claim, to be read with the description to which we have referred. The example given, with reference to Figure 4, is plainly of a liquid dispensing apparatus in which the barrel of the flat-nosed syringe has a generally uniform diameter along its length. This is consistent with the description of the syringe in the consistory statement and confirms that the Second Product cannot infringe claim 9.
79 In our opinion, contrary to the conclusion of the primary judge, the use of the word “substantially” in claim 9 in the expression “substantially as described with reference to the drawings and/or examples” does not extend the definition of the invention to “the substantial idea” disclosed by the specification and shown in the drawings.
80 The word “substantially” provides no warrant for departing from what the specification itself mandates to be the essential features of the invention. A flat-nosed syringe dimensioned as described in the consistory statement is one of the essential features of the invention. Thus, whatever work the word “substantially” is to perform in claim 9, it cannot transform a feature made essential by the description of the invention into one that is now inessential. Put another way, an embodiment that does not possess the essential features of the invention as described, cannot be one that is “substantially as described”. Thus, the word “substantially” in claim 9 does not do the work which the primary judge held that it did.
81 We would add that, once the proper scope of claim 9 is understood, we do not, with respect, read the authorities on which the primary judge relied (Lewis v Hall at 97; Raleigh Cycle at 157 and Radiation at 51-52) as supporting his Honour’s conclusion. Indeed, in our opinion, those authorities provide greater support to the appellants.
82 Further, as the Full Court remarked in Fisher & Paykel at 20, there is need for caution in accepting in an infringement suit the suggestion by the patentee that what appears to be a limitation upon the claimed subject matter is merely a “slip of the pen” and not to be held too readily against it: see also in this regard, Lord Upjohn’s observations in Rodi and Wienenberger AG v Henry Showell Ltd [1969] RPC 367 at 392 about the caution with which a court should approach the construction of claims which, on a superficial reading, might appear to be unnecessarily circumscribed.
83 For these reasons, the appellants have established error and, to this extent, their appeal in respect of the infringement of claim 9 by the Second Product succeeds.
Claim 9 – the bottle neck liner
84 We note that, in their submissions, the appellants raise two additional reasons as to why they say the Second Product does not infringe claim 9. Both concern the accused bottle neck liner.
85 First, the appellants say that the primary judge’s finding at [141] of the reasons (that “[t]he web falls into the sleeve wall on a gradual descending angle, rather than on a right angle”) means that, apparently contrary to his Honour’s later conclusion at [142] of the reasons, the liner does not have, substantially, “the features of the cylindrical body described in the specification and illustrated in the drawings of the liner”. Here, the point appears to be that the sleeve of the liner depicted in Figure 2 is described in the specification (at p 15, line 2) as being inwardly bevelled (see item 20).
86 Secondly, the appellants say that the accused liner is not push fitted into the bottle neck such that it is deformed inwardly and cannot be separated from the bottle neck when in use.
87 The appellants submit that these two additional reasons also stand as reasons why, contrary to the primary judge’s finding at [151] of the reasons, the First Product does not infringe claim 9, given that the liner in the two products is the same.
88 The respondents argue that these alleged differences are not raised in the amended notice of appeal. We do not think that, strictly speaking, this is correct. Ground 1(e) (with respect to infringement by the Second Product) and ground 2(e) (with respect to infringement by the First Product) allege that the primary judge erred in that his Honour should have found that the bottle neck liner was not “substantially as described” with reference to the drawings and examples. We accept, nevertheless, that the alleged differences are not particularised in the amended notice of appeal, when they should have been. However, nothing turns on that failure in the present appeal.
89 In light of our finding at [83] above, it is not necessary to consider these alleged differences with respect to the infringement of claim 9 by the Second Product. However, it is necessary to consider them with respect to the infringement of claim 9 by the First Product.
90 As to the first matter, the consistory statement provides that the sleeve of the liner is formed with a flared portion at its upper end into which the distal end of the syringe barrel passes. The specification explains (at p 6, lines 15-17) that the flared portion provides a lead-in to aid the engagement of the syringe barrel into the through bore. As we have noted, item 20 in Figure 2 is described in the specification as showing that the sleeve is inwardly bevelled to provide this flared portion.
91 The primary judge (at [104] of the reasons) described item 20 in Figure 2 as “indicating a very modestly sized flared portion” that “creates a gradual transition in the shape of the top of the sleeve”. The primary judge’s later finding (at [141] of the reasons) that, in the accused liner, the web falls into the sleeve wall on a gradual descending angle, rather than at a right angle, must be read with his Honour’s perception of the geometry of the transition provided by item 20 in Figure 2, as noted immediately above, and his Honour’s conclusion (at [142] of the reasons) that, with respect to the flared portion, the accused liner “has substantially the features of the cylindrical body described in the specification and illustrated in the drawings of the liner”.
92 His Honour’s findings in this regard are the result of an evaluative judgment with which an appellate court would not readily interfere: see, in this connection, the observations of Lord Hoffmann in Biogen Inc v Medeva plc [1997] RPC 1 at 45, quoted with approval by the Full Court in Esso Australia Resources Ltd v Commissioner of Taxation (1998) 84 FCR 541; [1998] FCA 851 at 554 and in S & I Publishing Pty Ltd v Australian Surf Life Saver Pty Ltd (1998) 88 FCR 354; [1998] FCA 1463 at 359-60. We are not persuaded that the appellants have demonstrated error in the primary judge’s findings in this regard. Moreover, in the case of an article claimed as in claim 9, it is not necessary that every detail of the drawings be taken in order for infringement to be found: see, for example, Multisteps Pty Ltd v Source and Sell Pty Ltd (2013) 214 FCR 323; [2013] FCA 743 at 372-73 [291].
93 The second matter stands in a somewhat different light. As we have recorded at [18] above, the specification gives a specific description of the bottle neck liner depicted with reference to Figures 3 to 6. The liner is characterised as one that is push fitted into the bottle neck in a way in which the outer body of the liner is deformed inwardly. This feature is said to provide a sealing fit between the outer body and the bottle neck. This part of the specification cannot be read apart from the earlier passage in the specification (which we have quoted at [19] above) which speaks, preferably, of the presence of a firm interference fit between the liner and the bottle neck, such that the liner will not separate from the bottle neck when the apparatus is in use and the syringe barrel is withdrawn from the sleeve of the liner. In later paragraphs of these reasons (see [97]-[102] below), we consider the question of whether this feature is an essential feature of the apparatus claimed in claim 1. For present purposes we note that it does not appear to be disputed that the accused liner does not possess this feature. Rather, the liner is retained in the bottle neck substantially by means of the snap lock feature to which we have referred.
94 In our view, the significance given in the example and drawings to the feature that the liner is push fitted into the bottle neck to give a firm interference fit cannot be overlooked when assessing whether each of the accused products is “[a] liquid dispensing apparatus substantially as described with reference to the drawings and/or examples” within the meaning of claim 9. The primary judge dealt with the appellants’ argument concerning the need for an interference fit when considering the meaning of “engagement” as used in claim 1. However, his Honour does not appear to have considered that argument when dealing with the infringement of claim 9. His Honour erred in not doing so.
95 Given the absence of this feature in the accused liner, it follows that the First Product does not infringe claim 9. The absence of this feature also stands as an additional reason why the Second Product does not infringe claim 9.
Claim 9 and fair basis
96 In the particular circumstances of this case, we do not need to deal with the issue about whether claim 9, on the construction adopted by the primary judge, is fairly based on the matter described in the specification. As we understand it, this point was deployed by the appellants to give emphasis to their submissions concerning the error made by the primary judge in his construction of claim 9, principally with respect to the features of the flat-nosed syringe. As we have explained, that error has been made out. The question of fair basis, as articulated by the appellants, falls away accordingly. We would only add that it is difficult to see how an omnibus claim, such as claim 9, properly understood, could fail for want of fair basis in the required sense. Generally speaking, one would think that an apparatus that is “substantially as described with reference to the drawings and/or examples” is one in respect of which it could also be said that there has been a “real and reasonably clear disclosure” in the specification: Lockwood Security Products Pty Limited v Doric Products Pty Limited (2004) 217 CLR 274; [2004] HCA 58 at 300-1 [69]. The real question raised in this appeal is the proper construction of claim 9.
Claim 1 and infringement
97 The only issue on appeal in respect of claim 1 is whether the apparatus represented by each accused product is one in which the bottle neck liner is “sealingly engaged” with the bottle neck. The issue really boils down to whether there are two separate objectives dealt with by those words, or whether the words have a single meaning, as found by the primary judge. The question is one of construction.
98 As we understood it, the appellants do not dispute that the engagement between the cylindrical body of the liner and the bottle neck is such that liquid cannot flow between the liner and the bottle neck, but they do dispute that the liner and the bottle neck are, relevantly, “engaged”. The essence of the appellants’ argument is that, in order for the liner and the bottle neck to be “engaged” as required by claim 1, there has to be an interference fit between the liner and the bottle neck. They argue that, as this feature is not present in the accused products—the liner is held in place by the snap lock mechanism—the apparatus cannot infringe claim 1. Similarly, they argue that the apparatus cannot infringe claims 2 to 6. Thus, they submit, the primary judge erred in concluding that the First Product infringes claims 1 to 6. The appellants also argue that this stands as an additional reason why the Second Product does not infringe claims 1 to 6.
99 We see no error in the construction given by the primary judge to the phrase “sealingly engaged”, especially at [99]-[100] of the reasons, which we have quoted at [35] above and with which we respectfully agree.
100 We reject the appellants’ construction of “engaged”, which seeks to draw on other aspects of the description in the specification (particularly with respect to preferred embodiments) but fails to have due regard to the immediately preceding word “sealingly” which plainly qualifies the nature of the engagement that is required.
101 We also reject the appellants’ submission that to construe “engaged” as the primary judge did, is to construe claim 1 in a way that would promote inutility. The function of a claim is to define the invention. It is an essential feature of the invention claimed in claim 1 that the bottle neck liner be sealingly engaged inside the bottle neck such that liquid cannot flow between the liner and the neck. The manner in which the liner otherwise engages the neck, or the bottle more generally, is not a matter that is essential to the invention as so claimed. Indeed, it is a matter on which claim 1 is entirely agnostic. Any appropriate means, if required in practice, can be employed, whether that means be an interference fit, a lock mechanism (such as used by the appellants) or some other means. The specification expresses a preference for an interference fit between the liner and inside of the bottle neck. But that feature is not essential to the working of the invention claimed in claim 1. In contrast, the apparatus of claim 9 requires the additional feature that the bottle neck liner be push fitted into the bottle neck such that the outer body of the liner is deformed inwardly: see [93] above. The point of present significance is that, in order to ensure claim validity, it is not necessary for a claim to specify all the constructional features that might be required for the person skilled in the art, exercising the ordinary skill of the calling, to produce a working embodiment of the invention that is claimed.
102 Accordingly, this aspect of the appeal fails.
The notice of contention
103 The respondents’ notice of contention may be dealt with shortly, especially having regard to the conclusion we have expressed at [76] above. In light of the characterisation of the “flat-nosed syringe” in the consistory statement and in claim 1, the primary judge did not err in concluding that the Second Product does not have the syringe that claim 1 defines.
Conclusion and orders
104 The outcome of the appeal is that the First Product infringes claims 1 to 6, but not claim 9 and the Second Product does not infringe any claim. The validity of the patent has not been put in issue in the appeal, other than in the limited way in which the argument on fair basis was advanced. The legal and commercial reality is that the respondents were entitled to injunctive and other relief in respect of the First Product, though not on every ground upon which the relief was claimed. Thus, the respondents on appeal (and as applicants below) should have their costs of the prosecution of that claim. The first appellant sought to circumvent the patent by a design change that saw the Second Product introduced. Before the primary judge that change was seen as an inessential device to circumvent the patent and was made the subject of relief; we disagree. The Second Product does not infringe. The appellants should therefore have their costs below and on appeal in respect of the Second Product.
105 The costs of the attack on the validity of the patent below should be paid by the appellants (respondents below).
106 Unless the parties wish to put to us contending overall percentages of costs, we are of the view that these parameters should guide the orders as to costs.
107 The substantive orders of the primary judge were made on 19 June 2015. Those orders were made only against the first respondent (which became the first appellant) GlaxoSmithKline Australia Pty Ltd. Orders were made on 22 September 2015 (by consent) joining GlaxoSmithKline Consumer Healthcare Pty Ltd to the proceeding as second respondent (which became the second appellant) and making the same orders against it as were made against the first respondent.
108 Consequent upon our reasons, amendments need to be made to the orders of 19 June 2015 as amended by the orders of 22 September 2015. Orders 4, 5, 8 (and probably 9) should be set aside. The existing orders should be put in a form that provides for the subjection of both respondents/appellants to injunctive and other relief in relation to the First Product. The inquiry as to damages flowing from the undertaking in relation to the Second Product needs to be provided for; as do costs orders in accordance with the above outline.
109 The parties should bring in short minutes, and if there is any debate, the draft orders can be accompanied by short submissions.
110 The orders we would make are:
1. As to the First Product (defined in the orders made by the Court at first instance on 19 June 2015), the appeal be dismissed, other than in respect of the finding of infringement of claim 9 of Australian Patent No. 2003283537 (the patent).
2. As to the Second Product, (defined in the orders made by the Court at first instance on 19 June 2015), and the First Product in relation to the finding of infringement of claim 9 of the patent, the appeal be allowed.
3. Within 21 days, the parties bring in short minutes that provide for:
(a) the revocation and variation of orders made by the Court at first instance on 19 June and 22 September 2015; and
(b) the compilation of a compendious form of orders conformable with these reasons, including the question of the undertaking as to damages and costs.
4. Should the parties not agree on said orders, within 28 days each side file and serve draft short minutes and submissions of no more than three pages in support thereof.
I certify that the preceding one hundred and ten (110) numbered paragraphs are a true copy of the Reasons for Judgment herein of the Honourable Chief Justice Allsop and Justices Yates and Robertson. |




