FEDERAL COURT OF AUSTRALIA
D’Arcy v Myriad Genetics Inc [2014] FCAFC 115
| IN THE FEDERAL COURT OF AUSTRALIA | |
| Appellant |
| AND: | First Respondent GENETIC TECHNOLOGIES LIMITED Second Respondent |
| DATE OF ORDER: | |
| WHERE MADE: |
THE COURT ORDERS THAT:
Note: Entry of orders is dealt with in Rule 39.32 of the Federal Court Rules 2011.
| NEW SOUTH WALES DISTRICT REGISTRY | |
| GENERAL DIVISION | NSD 359 of 2013 |
| ON APPEAL FROM THE FEDERAL COURT OF AUSTRALIA |
| BETWEEN: | YVONNE D'ARCY Appellant |
| AND: | MYRIAD GENETICS INC First Respondent GENETIC TECHNOLOGIES LIMITED Second Respondent |
| JUDGES: | ALLSOP CJ, DOWSETT, KENNY, BENNETT & MIDDLETON JJ |
| DATE: | 5 september 2014 |
| PLACE: | SYDNEY |
REASONS FOR JUDGMENT
GENERAL INTRODUCTION
1 This case concerns the patentability of isolated nucleic acid sequences, that is, nucleic acid (DNA or RNA) that has been isolated from the cell nucleus. The primary judge stated the question at [1], as whether a patent may be granted for a claim that covers naturally occurring nucleic acid – either DNA or RNA – that has been “isolated”. His Honour said, that in this context, the word “isolated” implies that the naturally occurring nucleic acid found in the cells of the human body, whether it be DNA or RNA, has been removed from the cellular environment in which it naturally exists and separated from other cellular components also found there.
2 The particular gene with which Australian Patent No 686004 (the Patent) is concerned (BRCA1) is a human breast – or an ovarian cancer – disposing gene. Mutations that may be present in this gene have been linked to various forms of cancer, including breast cancer and ovarian cancer.
3 As Dixon CJ, Kitto and Windeyer JJ made clear in National Research Development Corporation v Commissioner of Patents (1959) 102 CLR 252 (NRDC), the field of patentability in modern legislation that is rooted in s 6 of the Statute of Monopolies is not ascertained by verbal or linguistic interpretation of the words and phrases therein: “manufacture” or “manner of manufacture”. Rather, the task is to ascertain whether what is claimed is a proper subject for monopoly by a patent according to the principles that have developed for, and informed, the application of s 6. That task will, of course, require explication of those principles.
4 As a matter preliminary to that task and to the task of deciding the appeal, it is worth stating that care should be taken in resort to metaphor in analysis in this field. Metaphor can assist thought, in particular, by the evocation of structure and form by imagination; but it can also blind the eye of the mind by oversimplification. It may risk blinding real illumination that is achieved through analysis of the facts, including the scientific principles involved, by the utilisation of a striking evocation of a simplified structure of analysis that is derived from the metaphor chosen, rather than from the facts as existing.
5 So, here, the whole process of isolation of the nucleic acid might be viewed as equivalent to the creation (by well-known means) of a metaphorical microscope enabling one to see into the BRCA1 gene in order to view the exon sequence in the subject person. That metaphor may seem apt because the desire is to find a way of knowing what the person’s gene sequence is, so that vulnerability or susceptibility to cancer can be assessed. A metaphor to see may thus be apt, the desire being to know what is present in the body. This may be seen to assist in persuasion that the differences between the isolated nucleic acid, and what is contained within the body before isolation, are functionally irrelevant; and that what is being sought to be patented is the human body itself.
6 The argument is not without its attraction. It lay at the base of the customarily persuasive (if we may respectfully say so) arguments of Mr Catterns QC. We should not, however, be taken as characterising all those arguments as dependent upon metaphor. The reasons that follow reveal their careful detail. (The metaphors used in discussion in the field are not limited to the microscope.)
7 The impugned claims in suit should not, however, be determined by oversimplified analysis. They are for a product set within a context of invention described in the specification: a context of development, through research and work, of the knowledge of the mutations or polymorphisms in question, and of the finding of the gene in question.
8 In that context, humans intervene to isolate the nucleic acid that is different in chemical composition from its state in the body, and to assess whether that which is present in that (different) isolated product by way of exon sequence coincides with what has been found, by work and effort, to be a sequence (derived itself from a human-made product, cDNA) that bespeaks susceptibility to cancer, and so to be bring about a useful effect, being a state of knowledge for the person upon which to contemplate, or assess, treatment.
9 What are the principles and considerations relevant to the applicability of s 6 of the Statute of Monopolies that inform the answer to the question whether the claims here are patentable? These are discussed more fully below, but the following are worthy of emphasis at the outset.
10 First, the boundaries of the conception of patentability are not dictated only by deductive logic from the linguistic premises formulated in the scientific knowledge of a particular age; rather, the boundaries must be such as to be apt to encompass the development of science and technology, and human ingenuity. This explains the broadening concept of patentability since the first quarter of the 17th century.
11 Secondly, human intervention that creates an artificial state of affairs that has some discernible effect is essential.
12 Thirdly, whilst notions of utility, ingenuity and invention have their place after one concludes that the claim is within the field of s 6, such notions also inform the context of analysis of patentability by assisting in describing the claims to processes or products that are claimed new results of principles carried into practice through human intervention and that create some claimed useful result by involving an artificial state of affairs.
13 Fourthly, expressions such as “the work of nature” or “the laws of nature” are not found in the statute; nor are they useful tools of analysis.
14 Fifthly, the distinction between discovery of a scientific principle or fact and a deployment of such to a useful end by a procedure is real.
15 These important informing principles and considerations assist in the conclusion that, for the reasons set out below, the relevant claims as analysed below are patentable as within the meaning and boundaries of s 6 of the Statute of Monopolies.
THE SCIENTIFIC BACKGROUND
16 The primary judge set out the scientific background, taken from undisputed expert evidence (at [10] to [54]) as follows:
The eukaryotic cell
17 The human body is a multi-cellular eukaryotic organism which consists of a large number of different types of eukaryotic cells. Eukaryotic cells are cells which contain a membrane-bound nucleus. These cells communicate and co-operate with each other for the common good of the organism. The process by which cells reproduce is known as “cell division”. This process is binary in the sense that each cell is able to separate into two daughter cells.
18 The human body can sense when high rates of cell division are necessary. For example, if a particular area of the body receives a severe cut with blood loss, the body can respond by producing a number of new blood cells to replace the cells that were lost. When the cut is healing, the body is able to decrease the production of blood cells to prevent over-supply. However, cells may sometimes divide in an abnormal or uncontrolled manner. The abnormal or uncontrolled division of cells is referred to as cancer.
The components of a human cell
19 Cells found in the human body consist of three main parts: the nucleus, the cytoplasm and the cell membrane. The cell membrane defines the outer boundary of the cell and separates its contents from the environment in which it exists. The nucleus of the cell appears as a cell within a cell. The boundary of the nucleus is defined by a nuclear envelope or membrane.
20 The cytoplasm comprises everything between the cell membrane and the nucleus. The majority of the cytoplasm is a liquid called cytosol which consists of water, salts and organic molecules. However, the cytoplasm also contains a number of components (including ribosomes) that have specific functions including protein and energy production.
21 The nuclear envelope separating the nucleus from the cytoplasm incorporates pores through which molecules may move between the nucleus and the cytoplasm.
22 DNA and RNA are molecules found within the nucleus of cells within the human body. DNA contains the genetic information that directs the growth, development, maintenance and reproduction of the human body. This information is made available for these purposes via RNA.
The chemical structure of DNA
23 Native DNA (genomic DNA) is an extremely long three-dimensional molecule consisting of a number of repeating monomeric units called nucleotides. These are linked end to end to form a strand (chain) of nucleotides (a polynucleotide chain). Each nucleotide is comprised of three separate chemical groups: a nitrogen-containing (nitrogenous) base, a phosphate group and a five-carbon sugar group comprising deoxyribose.
24 In DNA, nucleotides are linked to one another by covalent bonds running from the fifth carbon (5’) of the sugar group of one nucleotide to the third carbon (3’) of the phosphate group of the adjacent nucleotide. These bonds are referred to as phosphodiester bonds. They form the “sugar-phosphate backbone” of the DNA from which the nitrogenous bases protrude.
25 DNA chains have two distinctive ends. One end of the chain has a free 5’ on the sugar group, and the other end has a free 3’ on the phosphate group. By convention, DNA chains are usually depicted from left to right commencing at the free 5’ of the sugar group and ending at the free 3’ of the phosphate group.
26 There are four types of nitrogenous bases found in DNA. These nitrogenous bases (usually referred to by their initial letter) are adenine (A), guanine (G), cytosine (C) and thymine (T).
27 DNA chains contain repeating sugar-phosphate groups that are always linked together by phosphodiester bonds. However, the four bases of DNA (A, G, C, T) can be attached in any order along the sugar-phosphate backbone. The bases are covalently bonded to the sugar group.
28 In the cell nucleus, DNA almost always exists as a double helix formed by the intertwining of two polynucleotide chains. The two strands wind around each other to form the double helix. The sugar-phosphate backbone forms the outside of the double helix. The bases lie on the inside, in pairs, perpendicular to the axis of the double helix. They are paired along the length of the double helix and joined together by hydrogen bonds.
29 In DNA, G bonds with C, and A bonds with T. The pairing of G to C and A to T is referred to as base pairing. Base pairs can only form if two DNA strands are orientated in the opposite direction (anti-parallel) so that one strand runs in the 5’ to 3’ direction and the other in the 3’ to 5’ direction. The strand running in the 5’ to 3’ direction is often referred to as the “sense” or “coding” strand, as opposed to the “anti-sense” or “non-coding” strand, which runs in the 3’ to 5’ direction.
30 In DNA, if the sequence of one polynucleotide chain is known (e.g. ATCGG on the 5’ to 3’ strand), then that of the other polynucleotide chain (i.e. TAGCC on the 3’ to 5’ strand) may be inferred. These matching sequences are referred to as complementary sequences or complementary strands.
Nucleosomes, chromatin fibres and chromosomes
31 DNA is compacted in the nucleus in two main ways. First, the DNA double helix wraps around spooling proteins known as histones by way of hydrogen bonding to form complexes know as nucleosomes. Each nucleosome consists of a protein core around which double stranded DNA is wound. Second, nucleosomes are stacked on top of each other to form chromatin fibres which are organised into chromosomes.
32 In humans, the DNA in the nucleus is divided between two sets of chromosomes. There are 24 different chromosomes comprising 22 homologous chromosomes and two sex chromosomes. By convention, the homologous chromosomes are numbered from the largest (1) to the smallest (22), while the sex chromosomes are designated X and Y.
The chemical structure of RNA
33 RNA has a slightly different chemical composition to DNA. Unlike DNA, RNA consists of the sugar group ribose instead of deoxyribose, and the nitrogenous base uracil (U) instead of thymine (T).
34 RNA is much shorter in length than DNA. RNA is also single-stranded. Because of this, the nitrogenous bases of RNA are exposed which allows short stretches of these bases to form base pairs with other bases on the same strand resulting in folding of the molecule. RNA often takes the shape of a highly folded molecule.
35 There are a number of different species of RNA which perform a variety of biological functions. Those that are most relevant for present purposes are known as messenger RNA (mRNA) and pre-messenger RNA (pre-mRNA). Also relevant is RNA polymerase (RNApol), an enzyme that (in association with promoters and terminators in DNA), determines where transcription of a gene should start and finish.
The human genome
36 A gene is a functional unit of contiguous DNA which encodes a particular protein. It provides the chemical blueprint used by other parts of the cell to produce that protein. When a gene is “expressed” it will often result in the synthesis of a protein by other parts of the cell.
37 Human genes generally comprise sequences of DNA that specifically code for a particular protein, interspersed with sequences of DNA that do not code for a particular protein. Sequences of DNA coding for a particular protein are thought to account for approximately 1% of the human genome.
38 The sequences of DNA that comprise a gene are referred to as exons or exonic sequences. Most exonic sequences will code for a particular protein, but they also include other regulatory or non-coding regions that, although not coding for a particular protein, are important to the translation of mRNA. These non-coding sequences are referred to as untranslated regions (UTR) and occur at the 5’ end (5’ UTR) and 3’ end (3’ UTR) of the gene. Other sequences that do not code for protein, and which do not form part of the UTR of the gene, are referred to as introns or intronic sequences. Introns are found in DNA and pre-mRNA, but not in mRNA, which includes only the exonic sequences found in the DNA from which it is copied. Introns account for about 25% of the human genome. The remainder is made up of repetitive and other intergenic DNA.
39 The term “genome” refers to the entirety of the DNA sequence within an organism which, in a human, comprises approximately 3.2 billion individual nucleotides. The human genome comprises approximately 25,000 genes arranged onto chromosomes. In the absence of mutation, all nucleated cells in the human body contain the same genomic DNA sequences.
Proteins, polypeptides and amino acids
40 A protein is a polypeptide or a number of polypeptides consisting of a sequence of amino acids linked together by peptide bonds on a phosphate backbone. Amino acids act as the building blocks of proteins and each type of protein has its own unique amino acid sequence. There are 20 different amino acids known in nature and they are as follows:
| The 20 Amino Acids in Proteins | |
| Amino Acid | Three-Letter Abbreviation |
| Glycine | Gly |
| Alanine | Ala |
| Valine | Val |
| Isoleucine | Ile |
| Leucine | Leu |
| Serine | Ser |
| Threonine | Thr |
| Proline | Pro |
| Aspartic acid | Asp |
| Glutamic acid | Glu |
| Lysine | Lys |
| Arginine | Arg |
| Asparagine | Asn |
| Glutamine | Gln |
| Cysteine | Cys |
| Methionine | Met |
| Tryptophan | Trp |
| Phenylalanine | Phe |
| Tyrosine | Tyr |
| Histidine | His |
| [Reproduced from the table “The 20 Amino Acids in Proteins (James D Watson et al, Recombinant DNA (W.H. Freeman, 2nd ed, 1992)] | |
41 Proteins come in an immense variety of different shapes and sizes, and perform many different and complex functions. For example, some proteins act as enzymes, others generate movement, and others act to form structures (histones) used to pack DNA or complexes (ribosomes) that synthesise more proteins. There are also proteins that regulate cell division. When the DNA that encodes these regulatory proteins is mutated or damaged, abnormal or uncontrolled cell division may result.
The genetic code
42 The genetic code consists of groups of three nucleotides, each of which represents one amino acid. These nucleotide groups are referred to as codons or triplets. The grouping of four possible nucleotides in DNA (A,G,C,T) and RNA (A,G,C,U) into different codons permits 64 possible combinations of nucleotides.
43 There are a number of codons that code for the same amino acid (e.g. phenylalanine (Phe) – TTT, TTC, glutamine (Gln) – CAA, CAG). Indeed, most amino acids have multiple codons, which means that there are a number of different DNA or RNA sequences that can code for the same protein.
44 The codon ATG in DNA (AUG in RNA) codes for methionine (Met), but will frequently act as a “start” signal. A fixed point in a nucleotide sequence designated by a start codon establishes the groups (the reading frame) in which codons are translated. There are also a number of codons (in DNA; TAA, TAG and TGA, in RNA; UAA, UAG and UGA) that do not code for amino acids, but instead act as “stop” signals that terminate the process of translation.
45 The genetic code is usually presented in the form of a table of nucleotides. If the first, second and third bases in a codon are known, then the table can be used to predict the specific amino acid encoded by that codon. The table below is such an example:
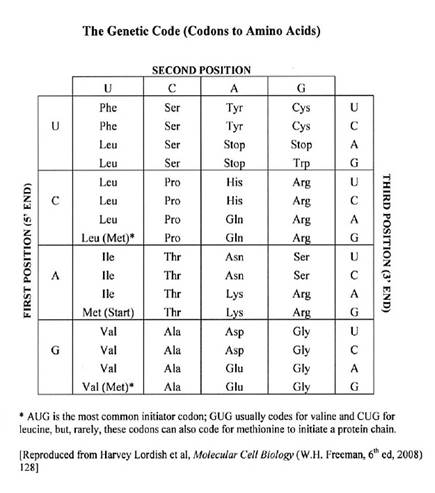
46 For example, if one wants to know what sequences of bases codes for glutamine (Glu), one can see from the table that there are two codons that do so: GAA and GAG. In the case of serine (Ser) one can see that there are six different codons that code for this amino-acid: UCU, UCC, UCA, UCG, AGU and AGC. As in the above table, the generic code is typically depicted as a table of RNA nucleotides. This table may be used to interpret DNA sequences by substituting T where U appears in the table.
47 Genetic information in DNA, in the form of sequences of codons that represent specific amino acid sequences, ultimately determines what particular protein will be synthesised in the cell.
The process of gene expression
48 The process by which a cell produces protein is referred to as “gene expression”. The production of pre-mRNA is the first step in the process of gene expression. This is followed by the production of mRNA. RNA plays a central role in gene expression through its involvement in the processes of transcription and translation.
Transcription
49 Transcription is a process that takes place within the nucleus of the cell whereby a portion of the DNA nucleotide sequence of a gene is copied into an RNA nucleotide sequence. Through this process, a single strand of the DNA double helix is used as a template (or, as it is sometimes called, the “sense”, or “non-coding”, strand) to synthesise a complementary strand of nascent mRNA known as pre-mRNA. Pre-mRNA includes both the exonic and intronic sequences of the gene transcribed from the DNA. The sequence of the nucleotide chain of the pre-mRNA strand is determined by base pairing with the DNA template (the “anti-sense”, or “non-coding” strand). Consequently, the nucleotide sequence of the strand of pre-mRNA transcribed from the DNA template strand will correspond to the non-template (the “sense” or “coding”) DNA strand.
50 During transcription, a chemical modification is made at the 5’ end of the transcribed sequence which results in the addition of a “cap”. The cap protects the molecule from enzymatic degradation and assists in the transport of the mature mRNA molecule to the cytoplasm. A further modification is made to the 3’ end of the sequence by the addition of a string of adenosine bases referred to as a poly-A tail.
51 Once the cap and poly-A tail have been added to the ends of the pre-RNA sequence the introns are removed and the exons joined together by a process known as RNA splicing. Splicing is a process performed by an enzyme complex referred to as the spliceosome. The pre-RNA transcript of exons and introns can be spliced to produce different polynucleotide sequences by a process referred to as alternative splicing.
52 Once splicing has occurred, the resulting mRNA molecule will consist of a complementary sequence of exons found in the DNA strand from which they were transcribed with a cap at the 5’ end and a poly-A tail at the 3’ end.
Translation
53 Once the process of transcription is complete, the mRNA molecule is transported through nuclear pores within the nuclear envelope into the cytoplasm where it is available for translation. Translation is a complex process by which the nucleotide sequence of an mRNA molecule is used as a template for the manufacture of the polypeptide chains which takes place in ribosomes located in the cytoplasm. For present purposes, it is sufficient to note that the ribosome manufactures the polypeptide chains in accordance with the mRNA template.
Isolation of DNA and RNA
54 As previously explained, an isolated DNA sequence is a sequence of DNA that has been removed from its normal cellular environment. Professor Rasko gave a detailed explanation of how DNA may be removed from its normal cellular environment. The following summary is drawn from his evidence.
55 Typically, DNA is obtained from cells removed from a sample of tissue or blood extracted from an individual. The tissue sample is broken down into clumps of cells or individual cells using enzymes or chemicals suitable for that purpose. In the case of a blood sample, the cells are already separated.
56 The bursting of the cell membrane or the nuclear membrane is referred to as cell lysis and can be achieved through techniques such as sonication (which involves the application of ultrasonic pressure waves) or grinding (which involves the application of physical disruptive forces). In this way the contents of the nucleus, including the DNA and RNA, can be released into a free-floating liquid suspension. Cell lysis results in the entire genomic DNA being released from the nucleus of the cell.
57 Proteins associated with DNA (including histones) are then degraded by the addition of enzymes known as proteases. This results in the destruction of the nucleosomes but does not eliminate all of the protein associated with the DNA.
58 A high salt solution is then added to precipitate the degraded proteins, including those which are still closely associated with the DNA. The degraded proteins are then separated from the DNA using a well-known chemical procedure that takes advantage of the fact that proteins are soluble in phenol, and DNA and RNA are not soluble in phenol, but are soluble in chloroform.
59 After centrifugation, the DNA and RNA are located in the interface between the phenol and the chloroform. Enzymes may then be applied in order to break down the RNA, leaving only purified DNA. The DNA can be precipitated from its soluble state into a solid state by the addition of ethanol or isopropanol. Further centrifugation results in a pellet of DNA.
60 Professor Rasko identified a number of techniques that may be used to create synthetic human DNA. For present purposes, that which is most relevant is a technique for template-based DNA synthesis that involves the use of mRNA as a template to create complementary DNA (cDNA). This technique is called “reverse transcription” because it involves the use of a particular enzyme (not naturally found in humans) known as reverse transcriptase.
61 The reverse transcription technique takes advantage of the existence of the poly-A tail on mRNA, allowing the mRNA to be isolated for use as a template for DNA synthesis. The result of the reverse transcription technique is to create an RNA-cDNA hybrid molecule that can then be converted to a double stranded DNA molecule using several different approaches. These hybrid molecules are better suited than mRNA molecules for use in molecular biology applications because mRNA is less stable than DNA. Nevertheless, it is clear that, like DNA, mRNA can also be isolated from the natural environment of the cell.
62 Dr Suthers explained that once a DNA sample has been isolated, the DNA sequence can be mapped using a variety of methods. Genetic testing is then completed by comparing the relevant DNA sequence of the sample to a normal reference sequence. The latter may be one of many reference sequences developed under the auspices of professional bodies or government agencies in the US or Europe. Of course, the goal of genetic testing is to determine what variations, if any, are present in a specific region of DNA and what their clinical significance is.
63 This concludes his Honour’s background material.
64 To reiterate, the following matters are of relevance:
A gene which encodes for a particular protein consists of exons which code for that protein and introns which are not translated. Introns account for about 25% of the genome.
Introns are found in DNA and pre-mRNA, but not in mRNA, which includes only the exon sequences. If the reading frame for the codons is altered, for example by commencing with a different nucleotide, a different protein will result.
The gene may also include regulatory regions that are important for the translation of mRNA. These occur at the 5’ end and 3’ end of the gene, respectively.
The three dimensional structure of genomic DNA is in part determined by the nucleotide bases, base pairing and the presence of histones.
DNA is isolated by disrupting the cell membrane such that the contents of the nucleus, including DNA and mRNA can be released into a free floating liquid suspension. After the processes described, purified DNA can be precipitated into a solid state and centrifuged.
Once a DNA sample has been isolated, the DNA sequence can be mapped using a variety of methods.
Genetic testing is completed by comparing the relevant DNA sequence of the sample to a normal reference sequence, such that variations can be determined in a specific region of DNA.
THE PATENT
The invention described in the Patent
65 It is only necessary to look to those parts of the specification that assist in the present analysis.
66 The title of the Patent is “In vivo mutations and polymorphisms in the 17q-linked breast and ovarian cancer susceptibility gene”. The invention is said to relate generally to the field of human genetics. Specifically, it is said to relate to methods and materials used to “isolate” and detect a human breast and ovarian cancer predisposing gene (BRCA1), some mutant alleles of which cause susceptibility to cancer, in particular breast and ovarian cancer. As used in this sentence, “isolate” is used in the sense of “locate”, not remove. An “allele” refers to an alternative form of the same gene. More specifically, the invention relates to germline (heritable) and somatic (non-heritable) mutations of the BRCA1 gene and their use, including in the screening process, in the diagnosis of a predisposition to breast and ovarian cancer. The invention is also said to relate to somatic mutations in the BRCA1 gene and their use in the diagnosis, prognosis and therapy of human cancers which have a mutation in the BRCA1 gene.
Background of the invention
67 As part of the background, the specification explains that previous work suggested that regions of chromosomal aberration may signify the position of important tumour suppressor genes involved both in genetic predisposition to cancer and in sporadic cancer. The mutation of one gene, BRCA1, was thought to account for approximately 45% of familial breast cancer, and at least 80% of familial breast and ovarian cancer. The background of the invention explains that “intense effort” to isolate the BRCA1 gene had proceeded since it was first mapped in 1990. In 1994 a second locus BRCA2 had been mapped which appeared to account for a proportion of earlier onset breast cancer roughly equal to BRCA1 but which conferred the lower risk of ovarian cancer.
68 Breast cancer had long been recognised to be, in part, a familial disease. Previous investigations showed that the data were most consistent with dominant inheritance for a major susceptibility locus or loci and that at least three loci existed which conveyed susceptibility to breast cancer as well as other cancers.
69 One of those loci is BRCA1. The specification sets out some of the theories or possibilities by which BRCA1 predisposing alleles function with respect to cancer.
70 While the linkage of BRCA1 was independently confirmed in three of five kindreds with both breast and ovarian cancer, the studies claimed to localise the gene within a very large region. Attempts to define the region further by genetic studies using markers proved unsuccessful. The specification explains that the size of the regions and the uncertainty associated with them had made it exceedingly difficult to design and implement physical mapping and/or cloning strategies for isolating the BRCA1 gene. It is stated that identification of a breast cancer susceptibility locus would permit the early detection of susceptible individuals and greatly increase the ability to understand the initial steps which lead to cancer.
Summary of the invention
71 The summary of the invention describes it in terms of methods and materials used to isolate and detect the BRCA1 gene, some alleles of which cause susceptibility to cancer, and also, more specifically, to the use of the gene in the diagnosis of predisposition to breast and ovarian cancer.
72 One of the figures of the invention includes the genomic sequence of BRCA1 with the intron sequences and exon sequences identified. Known polymorphic sites are identified.
Detailed description of the invention
73 Relevantly, the specification states:
The present invention provides an isolated polynucleotide comprising all, or a portion of the BRCA1 locus or of a mutated BRCA1 locus, preferably at least eight bases and not more than about 100 kb in length. Such polynucleotides may be antisense polynucleotides.
74 Also provided are methods of detecting a polynucleotide, comprising a portion of the BRCA1 locus or its expression product in an analyte. The portion of the BRCA1 locus may provide polynucleotides which are primers for amplification of that portion of the BRCA1 locus and which may be useful for diagnosis.
75 The invention is also said to provide methods of screening the BRCA1 gene by amplifying a portion of the BRCA1 locus. Again, these methods are said to be useful for identifying mutations for use in other diagnoses for predisposition to cancer or the diagnosis or prognosis of cancer. The invention is also said to provide the means necessary for production of gene based therapies directed at cancer cells. The specification suggests that therapeutic agents may also take the form of polypeptides based on either a portion of, or the entire protein sequence of, BRCA1, which may then functionally replace the activity of BRCA1 in vivo.
76 More generally the specification states:
It is a discovery of the present invention that the BRCA1 locus which predisposes individuals to breast cancer and ovarian cancer, is a gene encoding a BRCA1 protein, which has been found to have no significant homology with known protein or DNA sequences. This gene is termed BRCA1 herein. It is a discovery of the present invention that mutations in the BRCA1 locus in the germline are indicative of a predisposition to breast cancer and ovarian cancer. Finally, it is a discovery of the present invention that somatic mutations in the BRCA1 locus are also associated with breast cancer, ovarian cancer and other cancers, which represents an indicator of those cancers or of the prognosis of those cancers. The mutational events of the BRCA1 locus can involve deletions, insertions and point mutations within the coding sequence and the non-coding sequence.
77 The specification goes on to explain in some detail the methodology used to identify the locus. As a result of the work, two markers were discovered which represent physical boundaries of the BRCA1 locus. The use of genetic markers provided by the invention is said to allow the identification of clones which cover the region from a human yeast and a human bacterial chromosome library. This allowed the BRCA1 gene to be isolated. The inventors said:
… we have discovered that there are mutations in the coding sequence of the BRCA1 locus in kindreds which are responsible for the 17q-linked cancer susceptibility known as BRCA1. This gene was not known to be in this region. The present invention not only facilitates the early detection of certain cancers, so vital to patient survival, but also permits the detection of susceptible individuals before they develop cancer.
78 The specification states that a population group of Utah kindreds was used and that each large kindred independently provided the power to detect whether a BRCA1 susceptibility allele was segregating in that family.
79 It is not in dispute that the identification of the BRCA1 gene, its nucleic acid sequence and the characteristics and sites of the mutations identified involved an inventive step resulting from data collated from over 13,000 patients.
80 The specification explains that genetic mapping is usually an iterative process and that, as an initial step, recombination events, defined by large extended kindreds, helped specifically to localise the BRCA1 locus as either distal or proximal to a specific marker. As, until the disclosure of the present invention, the region surrounding BRCA1 was not well mapped and there were few markers, short repetitive sequences were analysed in order to develop new genetic markers. The process is set out in the specification. This resulted in a narrowing of the BRCA1 region to a small enough region to allow isolation and characterisation of the BRCA1 locus using techniques known in the art. Physical mapping and gene isolation were carried out and it is not suggested that the techniques there involved were other than well known in the field.
81 Under the heading “Testing the cDNA for Candidacy” the specification states that proof that the cDNA is the BRCA1 locus was obtained by finding sequences in DNA extracted from affected kindred members which create abnormal BRCA1 gene products or abnormal levels of BRCA1 gene product. The specification states that ‘the key is to find mutations which are serious enough to cause obvious disruption to the normal function of the gene product’. The mutations can take a number of forms. The specification then states that ‘according to the diagnostic and prognostic method of the present invention, alteration of the wild-type BRCA1 locus is detected’.
82 The methods of diagnosis are set out and the specification states that such methods are applicable to any tumour in which BRCA1 has a role in tumorigenesis. Further, the specification states that given the sequence of the BRCA1 open reading frame as shown in the Patent, the design of particular primers useful to facilitate cloning of amplified sequences, is well known within the art.
83 The specification explains that the inventors have discovered that individuals with the wild BRCA1 gene do not have cancer, but that mutations which interfere with the function of the BRCA1 protein are involved in the pathogenesis of cancer. The process of detecting a BRCA1 mutation is then summarised such that the mutant alleles are identified and sequenced to identify the specific mutation and that those which lead to an altered function of the BRCA1 protein are used for the diagnostic and prognostic methods of the invention.
84 The specification then sets out a number of definitions, specifically:
“Encode”. A polynucleotide is said to “encode” a polypeptide, if, in its native state or when manipulated by methods well known to those skilled in the art, it can be transcribed and/or translated to produce the mRNA for and/or the polypeptide or a fragment thereof. The anti-sense strand is the complement of such a nucleic acid, and the encoding sequence can be deduced therefrom.
“Isolated” or “substantially pure”. An “isolated” or “substantially pure” nucleic acid (e.g., an RNA, DNA or a mixed polymer) is one which is substantially separated from other cellular components which naturally accompany a native human sequence or protein, e.g., ribosomes, polymerases, many other human genome sequences and proteins. The term embraces a nucleic acid sequence or protein which has been removed from its naturally occurring environment, and includes recombinant or cloned DNA isolates and chemically synthesized analogs or analogs biologically synthesized by heterologous systems.
85 It is worth noting that the definition of “encode” is that the polynucleotide can be transcribed and/or translated.
86 The definition of “isolated” or “substantially pure” is in terms of a nucleic acid which is substantially separated from other cellular components and removed from its naturally occurring environment. That is, it is separated from other cellular components which naturally accompany a native human DNA sequence or protein. The material from which a substantially pure isolated nucleic acid is separated is said to be not only cellular material but also other human genome sequences. The specification elaborates the definition to make it clear that the terms, when applied to a nucleic acid, refer to a new nucleic acid which encodes the BRCA1 polypeptide, fragment, homologue or variant. Further:
The nucleic acids of the present invention will possess a sequence which is either derived from, or substantially similar to a natural BRCA1-encoding gene or one having substantial homology with a natural BRCA1 encoding gene or a portion thereof.
87 The coding sequence and the amino acid sequence are specified in tables in the Patent. Again, in further elaboration, the specification states that the polynucleotide compositions include RNA, cDNA, genomic DNA, synthetic forms and mixed polymers and include chemical or biochemical modifications. Also included are synthetic molecules that mimic polynucleotides in their ability to bind a designated sequence. It is stated that the invention provides recombinant nucleic acids comprising all or part of the BRCA1 region. Recombinant nucleic acid is a nucleic acid which is not naturally occurring or is made by the artificial combination of two otherwise separated segments of sequence.
88 The specification also sets out the nucleotide or codon length of the DNA sequences used in the invention. Those minimum lengths would seem to be the minimum length for a successful probe to hybridise. One or more introns may also be present.
89 Further definitions include the following:
BRCA1 protein or BRCA1 polypeptide ‘refer to a protein or polypeptide encoded by the BRCA1 locus, variants or fragments thereof’.
Ordinarily the polypeptides included within the definition which includes modification will be ‘at least about 50% homologous to the native BRCA1 sequence, preferably in excess of about 90% and more preferably at least about 95% homologous’.
90 The terms “isolate”, “substantially pure” and “substantially homogenous” are also defined but only in respect of proteins or polypeptides. It is stated that these terms are used interchangeably to describe a protein or polypeptide which has been separated from components which accompanied its natural state.
91 In the description of the method of use by way of nucleic acid diagnosis and diagnostic kits, the specification explains that in order to detect the presence of a BRCA1 allele predisposing an individual to cancer, a biological sample such as blood is prepared and analysed for the presence or absence of susceptibility alleles of BRCA1. Various methods of use are described in the specification, including peptide diagnosis and diagnostic kits, drug screening, drug design, gene therapy and peptide therapy. As to “industrial utility”, the invention is said to provide materials and methods for use in testing BRCA1 alleles of an individual and an interpretation of the normal or predisposing nature of the alleles. Various behavioural possibilities are suggested, including possible surgical procedures.
92 The DNA sequence, SEQ.ID No:1, represents the coding sequence of a nucleic acid (being cDNA) which encodes the BRCA1 polypeptide. It contains only the exon sequences (i.e. no introns) but includes the non-coding sequences that appear at the beginning and end of the exon sequence. The primary judge observed that a person skilled in the art would know that the corresponding RNA sequence may be obtained by substituting U for T.
93 Tables set out in the Patent identify mutations or polymorphisms by reference to the sequence listed in SEQ ID No:1. It is not in dispute that the identification of those mutations or polymorphisms was the work of the inventors and involved an inventive step.
94 SEQ.ID No:2 is a protein of 1864 amino acids in length.
95 That is, as the primary judge set out:
The invention is said to provide an isolated polynucleotide comprising all, or a portion of a mutated BRCA1 locus, preferably at least eight bases and not more than about 100 kb in length.
The invention also provides a recombinant construct suitable for expression in a transformed host cell.
The polynucleotide compositions of the invention are said to include RNA, DNA and cDNA.
The claim
96 The appeal focussed on claim 1, which is to:
An isolated nucleic acid coding for a mutant or polymorphic BRCA1 polypeptide, said nucleic acid containing in comparison to the BRCA1 polypeptide encoding sequence set forth in SEQ.ID No:1 one or more mutations or polymorphisms selected from the mutations set forth in Tables 12, 12A and 14 and the polymorphisms set forth in Tables 18 and 19.
97 It can be seen that the claim:
is to an isolated nucleic acid coding for a mutant or polymorphic protein;
characterises the nucleic acid by reference to the coding sequence of SEQ ID No:1 and containing one or more mutations set forth in the tables of the specification.
The decision of the primary judge
98 The primary judge observed that the reference to “a DNA coding” is a reference to the relevant DNA sequence that encodes for a relevant mutant or polymorphic polypeptide. While the word “coding” is not defined in the Patent, the word “encode” is defined by reference to the ability of a polynucleotide in its natural state or when manipulated by well-known methods to “encode” a polypeptide. A polynucleotide that codes for or encodes polypeptides is one that exhibits the sequence of bases that can, in the natural environment of a cell, result in its expression of such a polypeptide.
99 His Honour saw no relevant difference between “code” and “encoding” in the present context. Encoding sequences are, his Honour said at [71], those that code for polypeptides either in the natural environment of the cell or when manipulated by well-known methods.
100 In coming to the conclusion that each of the challenged claims in the Patent is to a manner of manufacture, the primary judge observed that (at [136]):
There is no doubt that naturally occurring DNA and RNA as they exist inside the cells of the human body cannot be the subject of a valid patent. However, the disputed claims do not cover naturally occurring DNA and RNA as they exist inside such cells. The disputed claims extend only to naturally occurring DNA and RNA which have been extracted from cells obtained from the human body and purged of other biological materials with which they were associated.
101 The primary judge concluded that:
Each of the disputed claims is to a chemical composition. The claims do not say anything about the length of the polynucleotide chains with which they are concerned.
There is nothing to suggest either in the claims or in the body of the specification that a complete nucleotide of DNA as originally found on chromosome 17 that has been isolated and that includes one or more of the relevant mutations, would be outside the scope of the disputed claims. The claims do not support the conclusion that every isolated DNA sequence within the scope of the claims must have had at least some covalent bonds broken as a result of the isolation process (the covalent bonds being bonds in the sugar phosphate backbone).
102 The primary judge referred to evidence to the effect that, in the process of isolation, it would be necessary to break hydrogen bonds between nucleoside bases and that there would need to be at least some breaks in the covalent bonds so that an extract could be removed. The experts agreed that while the breaking of covalent bonds could lead to a molecule of lower molecular weight, there may not have been any corresponding loss of information content, as what is removed could still retain enough of the coding sequence to define and code for a particular polypeptide. The primary judge concluded that not every isolated DNA sequence within the scope of the claim must have had at least some covalent bonds broken as a result of the isolation process. His Honour said that to imply such ‘would require [a need] to impose an impermissible gloss upon the words of the claim’.
103 The primary judge stated that there were two important points to make concerning the scope of the claims. First, the disputed claims are not to genetic information per se. They claim tangible materials. As they are not to information as such, his Honour observed that they could never be infringed by someone who merely reproduced a DNA sequence in written or digitised form.
104 Secondly, because each of the claims is to an isolated chemical composition, ‘naturally occurring DNA and RNA as they exist in cell are not within the scope of any of the disputed claims and could never, at least not until they had been isolated, result in the infringement of any such claim’.
105 After citing s 18(1) of the Patents Act 1990 (Cth) (the Act) and the definition of invention as contained in Schedule 1 to the Act, the primary judge turned to the relevant judicial considerations of manner of manufacture, in particular to the seminal consideration given to that topic by the High Court in NRDC and affirmed recently in Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd (2013) 304 ALR 1.
Legal Principles
NRDC
106 NRDC is the long accepted articulation of the principles to be applied to patentability and to the question of what is the proper subject matter for a patent. It is worth reciting the reasons in some detail.
107 The claim in issue in NRDC was to an agricultural process producing a commercially useful result. At 269, the High Court (Dixon CJ, Kitto and Windeyer JJ) stated that:
The word “manufacture” [in the expression “manner of manufacture”] finds a place in the present Act, not as a word intended to reduce a question of patentability to a question of verbal interpretation, but simply as the general title found in the Statue of Monopolies for the whole category under which all grants of patents which may be made in accordance with the developed principles of patent law are to be subsumed. It is therefore a mistake, and a mistake likely to lead to an incorrect conclusion, to treat the question whether a given process or product is within the definition as if that question could be restated in the form: “Is this a manner (or kind) of manufacture?”. It is a mistake which tends to limit one’s thinking by reference to the idea of making tangible goods by hand or by machine because, ‘manufacture’ as a word of everyday speech generally conveys that idea. The right question is: “Is this a proper subject of letters patent according to the principles which have been developed for the application of s 6 of the Statute of Monopolies?”
It is a very different question… a widening conception of the notion has been a characteristic of the growth of patent law.
108 Importantly, their Honours said at 271:
The truth is that any attempt to state the ambit of s 6 of the Statute of Monopolies by precisely defining “manufacture” is bound to fail. The purpose of s 6, it must be remembered, was to allow the use of the prerogative to encourage national development in a field which already, in 1623, was seen to be excitingly unpredictable.
109 In a passage that has often been cited, the High Court said (at 277):
[T]he view which we think is correct in the present case is that the method the subject of the relevant claims has at its end result an artificial effect falling squarely within the true concept of what must be produced by a process if it is to be held patentable.
110 In NRDC, the Commissioner argued that the claims in question were processes that were ‘dependent on the operation of natural laws or the natural properties of the materials involved’ and that ‘there is no process independent of the discovery itself’. The High Court explained (at 264) that:
… the distinction between discovery and invention is not precise enough to be other than misleading in this area of discussion. There may indeed be a discovery without invention – either because the discovery is some piece of abstract information without any suggestion of a practical application of it to a useful end, or because its application lies outside the realm of ‘manufacture’.
111 The distinction between discovery and invention was described by Buckley J in Reynolds v Herbert Smith & Co Ltd (1903) 20 RPC 123 at 126, in that discovery disclosed something which ‘before had been unseen or dimly seen’, whereas invention does not merely disclose something, it also involves ‘the suggestion of an act to be done, an act which results in a new product, or a new result, or a new process or a new combination for producing an old product or an old result’. Justice Whitford stated in Genentech Inc’s Patent [1987] RPC 553 at 556 that ‘if on the basis of that discovery you can tell people how it can be usefully employed, then a patentable invention may result’.
112 This statement was expressly approved by the House of Lords in Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2005] RPC 169 where Lord Hoffman said (at 77) that an invention is a practical product or process, not information about the natural world. The distinction between a discovery of one of nature’s laws and the application of that discovery to a new and useful purpose was also recognised by the High Court in Advanced Building Systems Pty Limited v Ramset Fasteners (Aust) Pty Limited (1998) 194 CLR 171 at [34]. An idea is not patentable; mere human discovery is unpatentable unless there is a practical means of carrying out that idea so as to add to the sum of human art (Ramset Fasteners at 34; Kirin-Amgen per Lord Hoffman (at 76)).
113 As the primary judge noted, the question whether a composition of matter is a “manner of manufacture” must be decided in Australia in accordance with NRDC, not applied as some statutory text, but as an explanation of the principles and concepts to apply to the question of what constitutes patentable subject matter.
114 There is no requirement for:
a consideration of whether the composition of matter is a “product of nature”; or
whether a microorganism is “markedly different” from something that already exists in nature.
115 Further, the High Court in NRDC stated a number of principles which can relevantly be summarised as follows:
As explained by Lord Buckmaster in Re BA’s Application (1915) 32 RPC 348 at 349:
… when once a substance is known, its methods of production ascertained, its characteristics and its constituents well defined, you cannot patent the use of that for a purpose which was hitherto unknown. That would give rise to analogous use for which the substance was already known.
While it is accepted that a patent is not available for something that is “nothing but a claim for a new use of an old substance”, emphasis is given to the expression “nothing but”. Invention may be found in a new method of using the material or some new adaptation of it so as to serve the new purpose. If the new use consists in taking advantage of a hitherto unknown or unsuspected property, there may be invention.
In contrast to a situation where the existence of a product is known and its characteristics and properties understood, for there to be a patentable invention there must be something which the alleged invention has super-added to the existing knowledge concerning the product.
Adopting the reasoning of Frankfurter J in Funk Brothers Seed Company v Kalo Inoculant Company, 333 US 127 (1948), it confuses the issue to use such terms as “the work of nature” and the “laws of nature”. It is not decisive or helpful to point out that the suggestion is that nature, in its newly ascertained aspect, be allowed to work in its own way. Expressions such as the “work of nature” or the “laws of nature” could fairly be employed to challenge almost any patent.
One can distinguish between discovery of a piece of abstract information without suggestion of a practical application to a useful end, and a useful result produced by doing something which has not been done by that procedure before. It is no answer to ingenuity in the discovery that the materials would produce a useful result to say that there was no ingenuity in showing how the discovery, once made, might be applied. It is only necessary to show one inventive step in the advance made beyond the prior limits of the relevant art.
A claim for a new use of an old substance is a claim which denies that the chemicals are old substances, in the sense in which the expression has been used. They are relevantly new and an applicant may have evolved a new and useful method by the application of scientific ingenuity.
The central question is whether the claimed process falls within the category of inventions to which, by definition, the application of the Act is confined. This necessitates an inquiry, not into the meaning of a word so much as into the breadth of the concept which the law has developed by its consideration of the text and purpose of the Statute of Monopolies.
116 Generally speaking, and in particular at 271, the High Court argued against any attempt to restrict the concept of what is encompassed by “manufacture”. It would be, their Honours said, ‘unsound to the point of folly’ to attempt to restrict or define the concept when science has made such extraordinary advances.
117 The High Court considered the case of Re Standard Oil Development Co’s Application (1951) 68 RPC 114, in which a patent was sought for selective herbicide. Justice Lloyd-Jacob had refused a patent, noting that the land itself remained unaltered. The High Court said at 274 ‘but it seems hardly sufficient… to dismiss [the case] by saying that, since the structure of the soil is unaffected by the killing of weeds, the process of converting a weed infested area into a weed free area is not within the notion of “manufacture”’ (citations omitted). The Court concluded that a process for improving land may be a “manufacture” in the relevant sense of the word, as an artificial process affecting the profitable use of land, positive in adding advantageous features or negative in eliminating what had formed a prejudicial element.
118 The High Court said (at 275) that a process, to fall within the limits of patentability, must be one that offers some advantage which is material in the sense that the process belongs to a useful art as distinct from a fine art and that its value to the country is in the field of economic endeavour. It noted that the exclusion of methods of surgery and other processes for treating the human body may lie outside the concept of invention because the subject is “conceived as essentially non-economic” (but see Apotex v Sanofi, discussed below). Their Honours affirmed that ‘although an inventor may use no newly devised mechanism, nor produce a new substance, nonetheless he may, by providing some new and useful effect, appropriate for himself a patent monopoly in such improved result by covering the mode or manner by means of which his result is secured’ (at 276).
119 Of course, in this case, we are not considering a process but a product. The High Court extended its reasoning to apply to a product (relevantly summarised by the Full Federal Court in Grant v Commissioner of Patents (2006) 154 FCR 62):
A product, in relation to a process, is ‘only something in which the new and useful effect may be observed’: that “something” need not be a “thing” in the sense of an article; it may be any physical phenomenon in which the effect, be it creation or merely alteration, may be observed (at 276).
Morton J’s ‘rule’ (the High Court’s inverted commas) may be accepted as long as ‘product’ is taken to cover ‘every end produced’ and ‘vendible’ as ‘pointing only to the requirement of utility in practical affairs’ (at 276).
The effect of the method is a ‘product’ because it consists of ‘an artificially created state of affairs’ (explained in the context of the growth of weeds and crops on sown land on which a method has been put into practice) (at 277).
120 The High Court in NRDC observed that patent law develops and necessarily must develop in a modern society, pointing out that the process in that case achieved a separate and additional result which possessed its own economic utility. The High Court saw no reason to exclude agricultural or horticultural processes simply by reason of the fact that they have been practised from the earliest of times.
121 From the High Court’s reasoning in NRDC, patentable subject matter covers both processes and products and extends ‘to any new results of principles carried into practice … new processes in any art producing effects useful to the public’.
Hill v Evans
122 Although the case was not relied upon by the parties, the reasoning of the High Court in NRDC is consistent with Hill v Evans (1862) 1A IPR 1, 45 ER 1195. Hill v Evans was not concerned with the subject of patentable invention; however, the case forms part of the bedrock of patent law. Lord Westbury LC, in considering want of novelty (as it then stood), said (at 6): ‘if something remains to be ascertained which is necessary for the useful application of the discovery, that affords sufficient room for another valid patent’. His Lordship said (at 7) that ‘apparent generality, or a proposition not true to its full extent, will not prejudice a subsequent statement which is limited and accurate, and gives a specific rule of practical application’. His Lordship also said (at 7):
The reason is manifest, because much further information, and therefore much further discovery, are required before the real truth can be extricated and embodied in a form to serve the use of mankind. It is the difference between the ore and the refined and pure metal which is extracted from it… The prior knowledge of an invention to avoid a patent must be knowledge equal to that required to be given by a specification, namely, such knowledge as will enable the public to perceive the very discovery, and to carry the invention into practical use.
(emphasis added)
Apotex v Sanofi
123 In Apotex v Sanofi, the High Court reconsidered the question of patentable invention in the context of whether a method of medical treatment of the human body can be a patentable invention, noting that, as here, ‘a clear, perhaps insoluble, conflict has emerged between two relevant competing considerations’ (at [223]). Justices Crennan and Kiefel noted differences between jurisdictions, in that some have patent legislation which, as in Australia, similarly defines invention by reference to the expression “manner of manufacture” in s 6 of the Statute of Monopolies (as in the UK until 1977 and New Zealand) whereas some define invention otherwise (as in the United States of America and Canada).
124 Importantly, the High Court reconsidered the principles applicable to the question of patentability and reaffirmed and restated concepts addressed in NRDC. In particular, their Honours traced the development of patent law and the consideration of what is meant by manner of manufacture in s 6. Justices Crennan and Kiefel:
noted that there has been continual widening of the concept of manner of manufacture, reflecting the growth of patent law and of scientific and technical developments (at [224]);
confirmed (at [237]) that there is no logical distinction to be made between a patent for a method or process for treatment of the human body and a product for the same;
affirmed that: ‘if a process which does not produce a new substance but nevertheless results in “a new and useful effect”’, so that the new result is ‘an artificially created state of affairs providing economic utility, it may be considered a “manner of new manufacture”’ within s 6 of the Statute of Monopolies (at [240]); and
commented (at [241]) on the relevance of the fact that Parliament had made a deliberate decision not to exclude methods of treatment so that courts had hesitated to introduce the exclusion.
125 Chief Justice French agreed with the reasons of Crennan and Kiefel JJ, as did Gageler J. The Chief Justice reviewed similar authorities as to the manner of manufacture question, observing that ‘the exclusion from patentability of methods of medical treatment represents an anomaly for which no clear and consistent foundation has been enunciated’ (at [50]). His Honour added that decisions of this kind, involving complex questions of public policy, are “best left to the legislature” (at [44]).
126 Justice Hayne disagreed that a method of preventing a human disease was a proper subject for the grant of a patent. His Honour reiterated the NRDC test, emphasising that the question of economic utility was whether a product or process had ‘utility in practical affairs’. He argued that a process to prevent human disease ‘produces no outcome which is capable of commercial exploitation’.
127 Justices Crennan and Kiefel noted aspects of the decision of Diamond v Chakrabarty 447 US 303 (1980), specifically that the implied exceptions to patentability were the laws of nature, physical phenomena and abstract ideas, and that a method or process that does no more than simply recite or describe, rather than apply, a law of nature is not patentable. Whereas a live human-made micro-organism was new and had ‘markedly different characteristics from any found in nature’.
128 Justices Crennan and Kiefel said at [282]:
Fourthly, and critically, the subject matter of a claim for a new product suitable for therapeutic use, claimed alone (a product claim) or coupled with method claims (combined products/method claims), and the subject matter of a claim for a hitherto unknown method of treatment using a (known) product having prior therapeutic uses (a method claim) cannot be distinguished in terms of economics or ethics. In each case the subject matter in respect of which a monopoly is sought effects an artificially created improvement in human health, having economic utility… Patent monopolies are as much an appropriate reward for research into hitherto unknown therapeutic uses of (known) compounds, which uses benefit mankind, as they are for research directed to novel substances or compounds for therapeutic use in humans. It is not possible to erect a distinction between such research based on public policy considerations.
129 Their Honours did note that in Association for Molecular Pathology v Myriad Genetics, Inc, 596 US 12-398 (2013), the United States Supreme Court had focussed on the genetic information encoded into genes associated with certain cancers, and had held that composition claims to a naturally occurring DNA segment fell within the exception to patentability. However, their Honours added the observation that this conclusion was reached ‘even though such important and useful genes had never before been located or isolated from surrounding genetic material’. With respect, that observation draws the important distinction between the newly isolated gene and the information it contains.
Association for Molecular Pathology v Myriad Genetics Inc
130 Justice Thomas delivered the opinion of the US Supreme Court. The Court characterised Myriad’s work. Myriad had identified the exact location of the BRCA1 and BRCA2 genes, allowing Myriad to determine their typical nucleotide sequence. That information in turn enabled Myriad to develop medical tests useful for detecting mutations in the genes and thereby assessing whether the patient has an increased risk of cancer.
131 The Court held that a naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated, because DNA’s information sequences and the processes that create mRNA, amino acids and proteins occur naturally within cells. cDNA was held to be patent eligible because it is not naturally occurring.
132 The claims under consideration in the Supreme Court were not identical to the claims of the Australian patent:
133 It is claim 2 of the US Patent to which attention should be directed. Claim 5 of the US Patent asserted a claim to any series of 15 nucleotides listed in a typical BRCA1 gene.
134 The approach of the Supreme Court was set out as follows (at 6):
Myriad’s patents would, if valid, give it the exclusive right to isolate an individual’s BRCA1 and BRCA2 genes (or any strand of 15 or more nucleotides within the genes) by breaking the covalent bonds that connect the DNA to the rest of the individual’s genome.
135 The reasoning of the Court, in summary, can be set out as follows:
Laws of nature, natural phenomena and abstract ideas are not patentable, as an implicit exception to patentability.
Products of nature are not created and manifestations of nature are free to all men and are reserved exclusively to none (Chakrabarty). However, the rule against patents on naturally occurring things is not without limits. All inventions, at some level, use or apply laws of nature.
Patent protection strikes a delicate balance between creating incentives and impeding the flow of information that might permit or spur invention. This “well established stand” must be used to determine the patentability of the Myriad claims.
The Myriad claims fall “squarely” within the law of nature exception. Myriad found the location of the BRCA1 and BRCA2 genes, but that discovery by itself does not lend to the BRCA genes new compositions of matter within § 101 of the US Act.
Myriad did not create or alter any of the genetic information encoded in BRCA1 and BRCA2 or the location and order of the nucleotides. It “found” an important and useful gene.
Separating the gene from its surrounding genetic material is not an act of invention.
Myriad’s extensive research efforts cannot be imported into a patentability inquiry.
The claims focus on the genetic information. They are not saved by the fact of isolation and the severing of chemical bonds, because Myriad’s claims are not expressed in terms of chemical composition nor do they rely in any way on chemical changes that result from the isolation.
The practice of the American Patent and Trade Mark Office is not relevant because it was not endorsed by Congress. Indeed, the US Government argued that isolated DNA was not patent eligible.
136 The Court recognised that the creation of a cDNA sequence from mRNA results in an exon-only molecule that is not naturally occurring. While it was argued that the nucleotide sequence of cDNA is dictated by nature, the Court said that mankind unquestionably created something new when cDNA was made. It was not thereby a product of nature and, accordingly, was held to be patent eligible. The exception was very short series of DNA that may have no intervening introns to remove when creating cDNA, where that short strand of cDNA may be indistinguishable from natural DNA.
137 The Court was careful to note that the patent claims were not to an innovative method of manipulating genes, that the processes used by Myriad to isolate DNA were well understood by geneticists at the time and that the case did not involve patents of new applications of knowledge about the BRCA1 and BRCA2 genes. The underlying conclusion was that ‘genes and the information they encode are not patent eligible under § 101 simply because they have been isolated from the surrounding genetic material’.
138 As the High Court observed, the reasoning focussed on the information contained in the nucleic acid sequences and not on the product itself. Also, in the United States, Congress had not considered the patentability of gene sequences.
Association for Molecular Pathology v United States Patent and Trademark Office and Myriad Genetics, Inc, 689 F.3d 1903 (2012)
139 It is worth also examining some of the reasoning in the Court of Appeals for the Federal Circuit, in particular because it contains a more detailed analysis of the underlying chemistry, which is not in dispute in this proceeding. The arguments before the Federal Circuit were similar to those presently advanced.
140 The question was, similarly, the extent to which isolated nucleic acid, whether limited to cDNA or not, falls within the patentability exception for products of nature.
141 In coming to the conclusion that isolated DNAs, including cDNAs, are patent eligible subject matter under § 101, Lourie J cited the US Supreme Court decisions in Chakrabarty and Funk Brothers.
142 His Honour decided that the relevant question was whether a change in the claimed composition’s identity compared to what exists in nature is such that when combined or altered in a manner not found in nature, the two compositions have similar characteristics or whether human intervention has given the composition ‘markedly different or distinctive characteristics’. This has some similarity to the reasoning in NRDC.
143 As his Honour observed, some derision had been directed to his reliance on the fact of the breaking of chemical bonds to conclude that the isolated nucleic acid is in fact a different compound. That, as we read his Honour’s reasons, does them injustice. The subject matter of the claims is a chemical compound, not pure information content. It cannot be inappropriate to view it as such. Judge Lourie said (at 1329) that a covalent bond is the defining boundary between one molecule and another, but that was not the sole basis for his Honour’s reasoning. His Honour’s conclusion was that, chemically, the isolated DNA molecule is a distinct chemical entity. It is not a purified form of a natural material. The claimed isolated DNA molecule does not exist as in nature. The point, as his Honour says at 1328, is that the claim is to a composition ‘having a distinctive chemical structure and identity’ from that of a native element, molecule or structure such that it has a markedly different chemical nature from the native DNA. In describing a distinction between an isolated gene and a leaf snapped from a tree, Lourie J incorporated matters that are reflected in NRDC, namely that isolated genes provide useful diagnostic tools and medicines – and so are within the concept of economic significance considered important by the High Court.
144 In dealing with the submission that the claims were to mere reflections of a law of nature, Lourie J said that they are not so any more than any product of man reflects and is consistent with the law of nature: ‘everything and everyone comes from nature, following its laws’, whereas these claims are to ‘the products of man’. These words bear resemblance to the High Court’s reasoning in NRDC.
145 Judge Moore was also alive, with respect, to the distinction between claims to subject matter that had previously existed in nature exactly as claimed, and the present case. Apart from citing Funk Brothers and Chakrabarty, her Honour referred to Parke-Davis & Co v HK Mulford Co, 189 F 95, 103 (SDNY 1911) where purified adrenaline was considered patentable subject matter because it was ‘for every practical purpose a new thing commercially and therapeutically’. Similarly, in Merck & Co. v Olin Mathieson Chemical Corp, 253 F.2d 156 (1958), the Fourth Circuit found purified vitamin B12 to be patentable, because it had ‘such advantageous characteristics as to replace [the naturally occurring] liver products. What was produced was, in no sense, an old product’; this was in contrast to “mere” purification, where the purified subject matter was of a naturally occurring element with inherent physical properties unchanged upon purification. Judge Moore applied Funk Brothers and Chakrabarty and said that she found ‘no reason to deviate from this longstanding flexible approach in this case’.
146 Again, turning to the chemistry, her Honour noted that DNA is a polymer, made up of repeating monomer units connected by chemical bonds to form one larger molecule. The process of polymerisation of the monomer units results in a new molecule, as polymerisation changes the monomers to result in a molecule with a different molecular charge, different chemical bonds and a different chemical composition as compared to the monomers in aggregate. A fragment of a DNA sequence has different properties to that of the parent molecule from which it is derived. These considerations led her Honour to conclude (at 1341) that just because the same series of amino acids appears in both the chromosome and an isolated DNA sequence does not mean that they are the same molecule. Her Honour said that man must create these isolated DNA molecules. This can be accomplished by constructing them using biochemical means, or by chemically altering the larger polymer to cleave off adjacent portions.
147 Her Honour pointed to other differences between isolated DNA and the nucleic acid sequence as it exists as part of the chromosome. Creating isolated DNA allows a scientist to remove potentially confounding sequences that are naturally present in a larger chromosome or polymer and instead to focus just on the sequence of interest (at 1342). Isolation also results in a substantially smaller molecule. Her Honour criticised a simple structural comparison as failing to recognise that chemical changes to the isolated DNA sequences, as compared to the natural state, could result in markedly different uses.
148 The removal of the DNA from the chromosome also has, as Moore J observed at 1342, important practical consequences leading to additional utility, for example, use of the DNA as a primer. Her Honour’s use of language is of some interest. cDNA has a unique sequence of DNA bases which is not actually present in nature and does not include introns. In discussing cDNA, her Honour recognised that it is “inspired by nature”, noting that naturally occurring RNA is the template upon which cDNA is constructed. However, the differences have a consequence even apart from the chemical structure to that of DNA. These include:
greater stability for the DNA sequences compared to the RNA sequence;
a distinctive name, character and use;
different chemical characteristics from either the naturally occurring RNA or in a continuous DNA sequence found in the chromosome; and
that cDNA sequences are the creation of man.
149 Judge Moore did not think that the differences in the chemical structure of isolated DNAs as compared to the corresponding native DNA was alone sufficient to make isolated DNA so markedly different from chromosomal DNA so as to be per se patentable subject matter (at 1343). Her Honour also said that the mere fact that the larger chromosome or polymer includes the same sequence of nucleotides as the smaller isolated DNA is not enough to make it per se a law of nature and to remove it from the scope of patentable subject matter. Judge Moore said that the appropriate course was to consider whether the differences impart a new utility which makes the molecules markedly different from nature. Judge Moore summarised the differences in function and utility of the isolated nucleic acids and the greater range of utility they provide as relevant to patent eligibility. Her Honour noted that the shorter isolated DNA sequences have a variety of applications and uses in isolation that are new and distinct as compared to the sequence as it occurs in nature. For example, they can be used as primers in diagnostic screening processes to detect gene mutations and as probes. As she noted, naturally occurring DNA cannot be used to accomplish these same goals because ‘unlike the isolated DNA, naturally occurring DNA simply does not have the requisite chemical and physical properties needed to perform these functions’ (at 1341).
150 Judge Bryson, in dissent, concluded that ‘Myriad is claiming the genes themselves’. His Honour looked to what he regarded as the only material change made to those genes, which he said was necessarily incidental to the extraction of the genes from the environment in which they are found in nature. He concluded that this meant that the isolated genes were not materially different than the native genes and drew on the metaphors of a “new mineral discovered in the earth”, “a new plant found in the wild” compared to a baseball bat that is “extracted” or “isolated” from an ash tree, necessarily changing the nature, form and use of the ash tree and thus results in a man-made manufacture, with a function entirely different from that of the raw material from which it was obtained and not a naturally occurring product.
151 The breaking of the chemical bonds was, in his Honour’s view, simply necessary to uphold the gene in its place in the body while the genetic coding sequence remained the same. The isolation process was, he said, ‘according to nature’s predefined boundaries; i.e., at points that preserve the ability of the gene to express the protein for which it is coded’ (at 1352). That is, his Honour held that they were not “the products of invention”. In that regard, he likened the new uses to which an isolated nucleic acid sequence could be put to extracting minerals or taking plant cuttings from wild plants.
152 Noting that the claim covers all isolated DNAs coding for the BRCA1 protein, with the protein being defined by the amino acid sequence encoded by the naturally occurring BRCA1 gene, Judge Bryson noted the very large number of molecules that were thereby claimed, which included variations that Myriad had not yet discovered. His Honour noted that the unifying characteristic was the naturally occurring BRCA1 gene. His Honour turned to the similarities and pointed out that the isolated genes and the naturally occurring genes have the same sequence code to the same proteins and represent the same units of heredity.
153 Judge Bryson focussed on the similarity in structure and the similarity in utility between the isolated nucleic acid and its naturally occurring counterpart, whereas Lourie and Moore JJ focussed on the differences between the isolated and naturally occurring DNAs. Judge Bryson formed the view that the informational content of the nucleotide sequences was the critical aspect of the molecules.
154 Judge Bryson agreed with the majority that cDNA was patent eligible despite the fact that, as his Honour characterised it, ‘that process occurs with natural machinery’. The end product was, in his Honour’s view, a human made invention with a distinct structure, with the introns that are found in the native gene removed from the cDNA segment and where the cDNA has additional utility.
155 With respect, we find the reasoning of Lourie J and Moore J, based on an analysis of the products as products and not on the information that they contain, to be consistent with patent law, and persuasive. Similarly, we agree that, consistent with NRDC and Australian law, the analysis should focus on differences in structure and function effected by the intervention of man and not on the similarities.
AUSTRALIAN LEGISLATIVE AND PUBLIC HISTORY
156 The primary judge looked at the legislative history of the Act to determine whether the conclusion to which he had come might for some reason be seen to be inconsistent with Parliament’s intentions.
157 His Honour turned to the decision in the Australian Patent Office in Kirin-Amgen Inc v Board of Regents of University of Washington (1995) 33 IPR 557, where the Deputy Commissioner of Patents observed that an objection of manner of manufacture might arise if the claims to a purified and isolated gene DNA sequence were directed to a mere chemical curiosity ‘but that is plainly not the case with this invention’. That decision was appealed to the Federal Court and then to the Full Court. No concern as to the patentability of the claimed DNA sequence was expressed and the question of manner of manufacture did not arise.
158 The Australian Law Reform Commission (ALRC) later published a report into gene patenting. The report stated that ‘there are attractive arguments for the view that such materials [isolated and purified biological materials] should not have been treated as patentable subject matter… however, the time for taking this approach… has long since passed’ ([6.51-6.52]). Even so, the ALRC did consider whether a new approach to the patentability of genetic materials was warranted (as at 2004). It concluded that it was not ([6.53]). It is worth setting out the reasons for that conclusion (as did the primary judge at [116]):
Nonetheless, the ALRC considers that a new approach to the patentability of genetic materials is not warranted at this stage in the development of the patent system, for the following reasons:
• It would represent a significant and undesirable departure from accepted international practice with respect to genetic inventions, and may adversely affect investment in the Australian biotechnology industry.
• It may fail to deliver the anticipated benefits because many pure and isolated genetic sequences do not exist in exactly the same form in nature – for example, patented sequences may not contain the introns that are found in the naturally occurring material.
• Claims to genetic materials in their natural form (that is, in situ) do not constitute patentable subject matter.
• Arguments that genetic materials are not patentable inventions do not always take adequate account of the fact that – in addition to the threshold requirement of ‘patentable subject matter’ – a number of statutory requirements must be satisfied for patent protection to be obtained. In particular, patent protection cannot be conferred over genetic materials unless a use for such materials has been identified and fully disclosed.
• It would be difficult, on any rational basis, to confine reform to genetic materials and technologies, yet the extension of the reform to other fields – where the patenting of pure and isolated chemicals that occur in nature is uncontroversial – may have unknown consequences.
159 Subsequently, in late 2010, a Private Members’ Bill (the Bill) was introduced into the Australian Senate which, if passed, would have excluded patents for ‘biological materials including their components and derivatives, whether isolated or purified or not and however made, which are identical or substantially identical to such materials as they exist in nature’. The term “biological materials” was defined to include DNA and RNA. The Legal and Constitutional Affairs Legislation Committee to which the Bill was referred for inquiry recommended by majority that the Senate not pass the Bill, which eventually lapsed.
160 The Australian Government’s response to the Bill and the ALRC report specifically accepted the ALRC recommendation that the Act not be amended to exclude genetic materials and technologies from patentable subject matter. It did make a number of recommendations, including stricter tests in relation to other patentability requirements and, importantly in the consideration of the balance between incentives and the flow of information (taken into account by the US Supreme Court), the introduction of a new “experimental use” defence. The recommendations resulted in the Intellectual Property Laws Amendment (Raising the Bar) Act 2012 (Cth).
161 While these legislative matters do not affect what constitutes patentable subject matter under the rubric of “manner of manufacture”, Parliament has considered, and has specifically declined, to exclude purified and isolated gene sequences from the scope of patentable subject matter.
THE DIFFERENT CHARACTERISATION OF THE PARTIES
162 The difference between the parties as stated by the primary judge is that the appellant submitted that isolated nucleic acid is not materially different to cellular nucleic acid and that naturally occurring DNA and RNA even in isolated form are products of nature that cannot form the basis of a valid patent.
163 The respondents contended that the claims were to a product that consists of an artificial state of affairs providing a new and useful effect that is of economic significance that is, that the product is a manner of manufacture within the meaning of NRDC. The respondents contended that isolated nucleic acid differs from nucleic acid found in a human cell chemically, structurally and functionally.
CONSIDERATION OF THE APPELLANT’S SUBMISSIONS
An artificial state of affairs and patentability
164 Ms D’Arcy submits that the fact that there is an artificial state of affairs is insufficient to constitute an invention or manner of manufacture within the Act and that the artificially created state of affairs discussed in NRDC was not the product per se but the effect that it produced, the end result. This, she says, contrasts with the isolated nucleic acid that has not been used to produce any artificial effect or artificially created state of affairs in the sense in which those words were used in NRDC.
165 Ms D’Arcy agrees that the isolation requires human intervention but says that it is not artificial and, if it is, it is not sufficient to fulfil the test in NRDC which does not stand, she says, for the proposition that an artificial effect is ‘the be all and end all’ of the question. That is, she submits that while the claimed invention has economic significance, it is not an artificial effect ‘in the relevant sense’ because the coding is the same as in nature, and that the intervention by man does not cause a change in the nucleic acid sequence, which she describes as simply taking the nucleic acid out of the cell.
166 This submission fails to recognise that the High Court has made it clear that the principles discussed in NRDC are equally applicable to products. Further, the isolated nucleic acid is itself an artificial state of affairs. It is removed from the genome and from the cell. In order to determine whether an invention claimed is a relevant product, the question is whether it consists of an artificially created state of affairs, not whether it produces or fails to produce an artificial effect.
167 NRDC does not, contrary to what Ms D’Arcy submits, require the claimed isolated nucleic acid to produce an artificial effect or an artificially created state of affairs. This submission represents an unduly narrow understanding of the reasoning in NRDC, which is not a statute nor is it to be narrowly construed. The “artificial effect” or “artificial state of affairs” to be considered in this case is the isolated nucleic acid itself, removed from its natural environment and from the cellular components that enable it to function in vivo.
168 That is not to say that merely demonstrating some sort of “artificial effect” always confers patentability. The principle to be taken from the High Court’s explanation in NRDC is in terms of the intervention of man to produce from, or by means of, a naturally occurring product or the laws of nature, something artificial or of an artificial effect that can bring benefit to mankind. That benefit generally has economic utility.
169 It was there said that an idea, even if original, is not patentable without some practical means of carrying it out, so as to add to the sum of human art and not merely human discovery. NRDC at 278 makes it clear that the discovery of the existing mutations and polymorphisms itself is not patentable but there is a distinction between the discovery of a law of nature and the application of such a law to a new and useful purpose (Ramset Fasteners at [34]).
170 The economic benefit of the claimed invention is not in issue. It is necessary to look at the substance of what is claimed to see to what extent it departs from unpatentable subject matter, such as an abstract idea. Such an approach has been adopted by the Supreme Court of the United States in Bilski v Kappos, 561 US 08-954 (2010).
171 Ms D’Arcy points out that it is common ground between the parties that the gene or sequence of nucleic acid that is the same as that occurs in nature is not an invention, such that the debate is restricted to the expression ‘isolated nucleic acid coding for’. She contends that there is no change to function on isolation, which is to ‘code for’ the BRCA1 polypeptide (or fragments of it).
172 This highlights the contention that there is no difference between “encode” and “code for” in the context of the claim, despite the different words being chosen in the claim. Ms D’Arcy’s submission assumes that “encode” equates to “code for” and means “possesses the code for” and relies on the assertion that “encode” encompasses a polynucleotide in its native state or when manipulated, which has the potential to produce the polypeptide, rather than looking to whether it can do so.
173 Ms D’Arcy describes the code as a set of rules by which the codons correspond to amino acids. She does not bring into that description a recognition that they are physically translated into the amino acids. The gene, she says, is the functional unit of contiguous DNA sequences that encodes the mutant protein. She says the words claimed and described relate to the function of coding and that all of the uses as described in the specification depend on the nucleotide sequence, but then ignores the functional aspects of the coding and the differences in the means by which it occurs in situ and in vitro.
174 This focusses on the information content of the nucleic acid sequence and not on chemical, structural or functional differences, which Ms D’Arcy says are irrelevant.
175 An alternative approach is to distinguish between those terms such that “code for” is understood as carrying the code (passive; having the potential to produce the polypeptide) and “encode” means actually to produce the polypeptide (the active). The definition of “encode” distinguishes between the polynucleotide in the native state, transcribed and translated without the intervention of man, and the polynucleotide, which needs to be manipulated to do so. If that approach is adopted, there is a difference because the nucleic acid sequence as it occurs in nature can code for the polypeptide due to its existence within the cell, which allows for transcription and translation. The isolated nucleic acid, removed from the cellular environment (e.g. ribosomes), cannot code for the polypeptide without further intervention.
176 The evidence is that in the cell, the genome beyond the actual BRCA1 gene is involved and controls the expression of proteins. Transcription and translation do not have an obvious or single outcome. The evidence is that the regulatory mechanisms and the environment of a cell can change the code of a set of nucleotides. Within the cell, the BRCA1 gene may result in the production of a number of different RNA or protein molecules. Isolated DNA cannot code, in the sense of being operated on by ribosomes to produce a protein or polypeptide, this being a function that occurs naturally within the cell. Isolated DNA cannot itself produce a polypeptide. In that sense it is inert, although it is capable of being manipulated to produce a protein but in a different way, by a different process to production from non-isolated genomic DNA.
177 An example of a structural difference is the addition of a cap and poly-A tail to naturally occurring mRNA, absent in isolated mRNA, which chemically alters that nucleic acid. The cap protects the mRNA molecules from any genetic degradation and assists in transport to the cytoplasm where translation occurs.
178 Further, Ms D’Arcy has not demonstrated that the genomic nucleic acid codes for fragments of the BRCA1 polypeptide, whereas the claimed nucleic acid sequences include sequences which may. Moreover, a fragment of a polypeptide is a different protein, so that a nucleic acid sequence that codes for a fragment of the BRCA1 polypeptide produces a different product to that produced by the gene sequence in the cell.
179 Ms D’Arcy does not challenge the claimed uses in later claims of polynucleotides with partial sequences as primers, probes, vectors and transformed cells. These consist of sequences that also, on her argument, exist in nature but do not code for the entire polypeptide. This seems to recognise that despite sequence identity, use and function differences from the naturally occurring genomic sequence are relevant to patentability. She submits that there cannot be a manner of manufacture because the sequence is a naturally occurring sequence. Recognising that cDNA, the sequence of SEQ.ID No:1, is an artificial construct, she points out that claim 1 is not so limited. She recognises that it encompasses a sequence that may be as short as five codons and submits that if it is a manner of manufacture, it is the coding sequence that equates to the naturally occurring coding sequence and is therefore unpatentable. This seems to conflict with her recognition that short sequences that equate to naturally occurring sequences and are used as probes are patentable. This makes her challenge to cDNA, an artificial sequence, more difficult to sustain.
180 The claimed nucleic acid can be as short as five codons (15 nucleotides) and one or more introns may be present, according to the specification. This may be, Ms D’Arcy says, genomic DNA which contains the introns which would then represent the precise gene of the person whose DNA, with the mutation, is reported in the specification – with the qualification, in passing, that it has been ‘isolated of course’. It may also be a sequence that mirrors the naturally occurring mRNA and a short sequence, even of a synthetic DNA, that is indistinguishable in sequence from naturally occurring DNA.
181 Again, the limitation in claim 1 to an “isolated” nucleic acid is the operative factor, even more so where a short nucleic acid which has no demonstrated function in the genome, is isolated and utilised.
Is the isolated nucleic acid precisely the same as the naturally occurring polynucleotide?
182 Ms D’Arcy submits that the patent claims the patient’s own mutation in her DNA and that ‘a human being’s DNA is not the thing we patent, unless isolation makes a difference’.
183 Myriad makes no assertion that it claims a person’s own DNA; to the contrary, it asserts that an isolated nucleic acid sequence is different. Ms D’Arcy’s submission assumes that there is no difference between the isolated nucleic acid and the nucleic acid sequence in the body. She says that the only part that matters is the coding sequence which, when the introns are removed, is identical. The claim, as she described it, extends to include any contiguous 15 codon exon which does naturally occur in nature, whether or not introns are present. On this basis, she says that the claim encompasses what corresponds with a portion of the coding sequence that occurs in a naturally occurring nucleic acid. In an RNA sequence, no introns are present and, she says, the only differences that can arise between a naturally occurring RNA and the isolated nucleic acid sequence are at the beginning and end of that sequence.
184 Ms D’Arcy submits that the polynucleotide sequences of the claims in suit (including cDNA) are not patentable unless their being isolated changes the position. She characterises the subject matter of claims 1-3 as the “isolated” native DNA (exonic sequence) and mRNA per se as varied in the tables of mutations and polymorphs that naturally occur in some people. The isolation of the polynucleotides is an important, but not the only important, factor. Ms D’Arcy’s characterisation of the DNA ignores the point that the exonic sequence does not exist as such in nature. Naturally occurring genomic DNA consists of both introns and exons.
185 Ms D’Arcy submits that the question for the Court is whether the isolation of a naturally occurring substance by conventional means can constitute an invention. Put another way, does that make it “artificial” and a manner or kind of manufacture as explained in NRDC? However, this focusses on the process of isolation rather than on the product the subject of the claim and tends to confuse manner of manufacture with inventive step.
186 In essence, Ms D’Arcy attacks the decision of the primary judge on a number of bases. She says that the mere presence of an artificial effect of economic utility is too broad a test and that an isolated nucleic acid as characterised in the claims is not an artificial effect in the sense referred to in NRDC. Ms D’Arcy contends that the correct approach is that adopted in Bilski and Mayo Collaborative Services, dba Mayo Medical Laboratories v Prometheus Laboratories, Inc, 566 US 10-1150 (2012) in the United States, that is, to look at the substance of what is claimed to see to what extent it departs from unpatentable subject matter, such as an abstract idea or a principle of nature. Isolation itself is, she says, insufficient for artificiality.
187 Ms D’Arcy turns to the primary judge’s description of the extent of human intervention by the isolation of the nucleic acid from other materials which were also present in the cell. She contends that this makes no relevant difference to what is claimed and that it is merely ‘the same thing in a different place’. Further, as the method of isolation was, on the evidence, routine and there was no claim to the contrary in the evidence or the specification (ignoring the agreed inventive step in the identification of the BRCA1 gene with the mutations or polymorphisms). Ms D’Arcy says that the primary judge’s reference to skill and effort was misplaced, being neither relevant nor the subject of evidence or argument.
188 Ms D’Arcy relies on the fact that the isolated polynucleotide possesses the code that, when translated in the body, results in the polypeptide. She says that the isolated polynucleotide can do this, but recognises that it can only do so when manipulated, albeit by well-known methods. She accepts that, as isolated, it is useful in various ways but says that that use is based on the same code, so that, ‘in that sense’ it is the same as the corresponding naturally occurring sequence, such as mRNA.
189 Ms D’Arcy is careful in her language about the isolated nucleic acid sequence being the same as the corresponding naturally occurring sequence. She recognises differences in function and usage and concentrates only on the sequence as a template. Her approach and the continued allusion to the naturally occurring structure for the purposes of, for example, transcription, can be tested by cDNA: it also codes for the polypeptide, but differs significantly from the naturally occurring structure.
190 The claims require comparison with the polypeptide encoding sequence set forth in SEQ.ID No:1, the cDNA sequence, which does not exist in nature. Ms D’Arcy says that ‘it corresponds precisely to the mRNA sequence and to the exons of the genomic DNA’. However, in nature, a continuous exon sequence of DNA coding for the polypeptide (cDNA) does not exist. Genomic DNA exists but with the exons separated by introns. mRNA, which is translated from genomic DNA exists, but it is not cDNA.
191 As the primary judge said (at [76]), the claims are for tangible materials. Genomic DNA sequence does not exist outside the cell and in that sense it can be said that an isolated chemical is not the same as the chemical in situ. One difference is that the isolated chemical can be manipulated and utilised in ways that the other cannot. That is, they may have different uses or functions, including those set out in the specification. Treating the claim as one to chemical entities, they are not the same as the nucleic acid sequence encoding the BRCA1 polypeptide in situ. They have different beginnings and different ends.
192 Ms D’Arcy accepts Professor Brown’s evidence that an isolated nucleic acid has properties which are useful in experimental circumstances not possessed by nucleic acid in its natural state but says that, as he accepted, each depends on the sequence or code. She does not challenge claims to such uses which depend on the isolated nucleic acid but maintains the challenge to the chemical entity that makes such uses possible. Each of those artificial uses utilises the coding sequence and arises from the isolation that is an essential part of claim 1.
193 Ms D’Arcy says that the primary judge’s statement that the isolated gene cannot code, in the sense of being operated on by ribosomes to produce the polypeptide, fails to consider that the claims are to nucleic acid which codes for the polypeptide which, she contends, simply means that it possesses the code that identifies the polypeptide and, in that sense, claims what is present in a cell.
194 Claim 1 is not to the genetic code. What is claimed is an isolated nucleic acid, a chemical molecule characterised in a certain way, which is chemically, structurally and functionally different to what occurs in nature. There is a distinction between a claim to an isolated nucleic acid comprised in part of a sequence of nucleotide bases and a claim to a written sequence of nucleotides which may be identical to the corresponding sequence in the natural cell. The claim is to be construed according to the normal principles of claim construction. To identify the invention as lying in the concept of information said to be embodied in a sequence of nucleotides ignores the language of the claim. The genetic code is not functionally a static sequence of nucleotides. It is a template for dynamic processes that result in the production of the polypeptide. The evidence is that the question of what polypeptides would be produced in the cell and in what quantity depends upon more than the sequence in which particular nucleotide bases are arranged.
Laws of nature
195 Ms D’Arcy’s proposition is that something that occurs in nature cannot be artificial but is a product of nature. It is not, she says a question of a “law of nature” (cf. the Supreme Court’s decision in Myriad per Thomas J). She seems to accept that cDNA is not a product of nature but points out that claim 1 is not so limited and includes “natural” as well as synthetic DNA. The case was contested before and decided by the primary judge on the basis that ‘naturally occurring DNA and RNA as they exist in the cells of the human body cannot be the subject of a valid patent’(at [136]); that is, it cannot be a manner of manufacture.
196 The isolated DNA can be characterised as material derived from naturally occurring material. This is not excluded from patentability within the reasoning of NRDC. The use of a living organism to produce a substance such as an antibiotic is patentable. It is not a question whether there is any overlap between what occurs in nature and that which is claimed. If so, all biological material would be inherently unpatentable.
197 Ms D’Arcy’s submissions can be summarised as follows:
An isolated polynucleotide may be the result of, or may constitute “an artificial effect” but that is not sufficient for patentability. There is nothing “artificial” in the isolated nucleic acid the subject of the claims.
The isolated nucleic acid of the claims is precisely the same as the naturally occurring polynucleotide in the sense that “coding for” is used in the claims, that is, the nucleic acid sequence of the claim has the ability in its natural state or when manipulated by well-known methods to “encode” the same polypeptide.
The claims arise out of a discovery of human biological material or “laws of nature”, which are not patentable.
198 From claim 1:
The nucleic acid is an isolated nucleic acid which, as defined, is substantially separated from other cellular components.
Nothing takes place in the cell.
The nucleic acid is not specified, so that it can refer to DNA, RNA and cDNA.
The nucleic acid “codes for” a mutant or polymorphic BRCA1 polypeptide. It has the potential to code for a protein.
The comparison is with the cDNA sequence in SEQ.ID No:1; that is, an artificial or not naturally occurring sequence.
The comparison is to identify one or more mutations or polymorphisms selected from the tables of the Patent, which information was determined after experimentation.
199 The nucleotide sequence with the mutation was present in a particular patient and the thesis of the Patent is that the mutation probably caused cancer. The sequence of an isolated nucleic acid is compared with the sequence of the Patent, as a “template”. As Ms D’Arcy points out, the claim is not to a process of comparison. The comparison is between the sequence of the isolated nucleic acid and the sequence of SEQ. ID No:1 which is, in turn, the BRCA1 encoding sequence which, in the cell, codes for or encodes the BRCA1 polypeptide.
200 The gene that contains the mutation or polymorphism exists in nature. However, until it was isolated, it could not be used to identify the mutation or polymorphism. Once it was isolated, the presence of the mutation or polymorphism that indicates a likelihood of cancer could not be determined without comparison with the tables of the Patent. This reflects a difference between the gene in its natural state and after isolation.
201 Ms D’Arcy says that the exonic sequence of SEQ.ID No:1 does encode for the BRCA1 polypeptide when in the cell, but that sequence was obtained from an isolate which cannot be translated or transcribed into the polypeptide. It is an artificial sequence that does not exist in the cell; it is the sequence of a cDNA that was made artificially by the process leading up to the identification of the BRCA1 gene. Ms D’Arcy says that the cDNA ‘consists of the very sequence of exons that exists in the cell’ but that is misleading. The mRNA contains the exons but that is not the same as the cellular or genomic DNA. The cDNA is made artificially, by reverse transcription of the mRNA, to which it is complementary but not identical. So, the polynucleotide sequence of cDNA is different to that of genomic DNA.
202 The comparison of Claim 1 is between the sequence of the Table of the Patent (which is the result of invention, but not claimed) and the natural coding sequence containing natural mutations, but only when the naturally occurring sequence is isolated from the person’s genome and removed from its cellular environment.
203 Is that isolation, enabling the comparison to be made, sufficient to give patentability? Ms D’Arcy says that the sequences are, ‘subject to the question of isolation’, not the product of human endeavour but products of nature. She accepts that human endeavour and ingenuity found the naturally occurring BRCA1 sequence and that human endeavour and ingenuity found the naturally occurring mutations. She points out that the isolation of the sequence to be compared with the cDNA sequence of SEQ.ID No:1 is conventional.
CONCLUSION
204 This case is not about the wisdom of the patent system. It is about the application of Australian patent law, as set out in the Act and as developed by the courts since the Statute of Monopolies.
205 It is not about whether, for policy or moral or social reasons, patents for gene sequences should be excluded from patentability. That has been considered by the ALRC and by Parliament and has not occurred. It is not a matter for the court, but for Parliament to decide. Parliament has considered the question of the patentability of gene sequences and has chosen not to exclude them but to make amendments to the Act to address, in part, the balance between the benefits of the patent system and the incentive thereby created, and the restriction on, for example, subsequent research.
206 This case is about whether, under Australian law and the concept of patentable invention as discussed by the courts, in particular by the High Court in NRDC, the challenged claims of the patent are to patentable inventions, that is, whether they are properly the subject of letters patent. NRDC is not to be applied in a narrow sense. The principles of patentability as there discussed are principles which are apposite to the present case and with which, with respect, we fully agree. Questions of novelty and inventive step do not arise. Novelty has not been challenged and it is not in dispute that an inventive step was involved in the invention as claimed in the challenged claims.
207 In Australia, there is no statutory or jurisprudential limitation of patentability to exclude “products of nature”. To the contrary, the High Court has specifically rejected such an approach. A mere discovery is not patentable and an idea is not patentable, but a “manner of manufacture”, as that term has been developed, is.
208 In NRDC, the High Court upheld a patent for a herbicide, rejecting the argument that the claim was to a “mere” new use of a known material. For the High Court, what was required was ‘an inquiry not into the meaning of a word so much as into the breadth of the concept which the law has developed by its consideration of the text and purpose of the Statute of Monopolies’. The Court held that it is sufficient for a product to result in ‘an artificially created state of affairs’, leading to ‘an economically useful result’.
209 This was consistent with the High Court’s reasoning three months earlier in Commissioner of Patents v Microcell Ltd (1959) 102 CLR 232, where their Honours held that emphasis must be put on the phrase “nothing but” in Lord Buckmaster’s seminal caution of the patent ineligibility of ‘nothing but a claim for a new use of an old substance’ (at [8]).
210 The appeal centres on claim 1 of the patent; that is, to ‘an isolated nucleic acid coding for a mutant or polymorphic BRCA1 polypeptide, said nucleic acid containing in comparison to the BRCA1 polypeptide encoding sequence set forth in SEQ.ID No:1 one or more mutations or polymorphisms selected from the mutations set forth in Tables 12, 12A and 14 and the polymorphisms set forth in Tables 18 and 19’. There are a number of features of the subject matter of the claims:
It is to a compound; a nucleic acid. It is not a claim to information.
It is to the isolated nucleic acid; i.e. a nucleic acid taken out of the genome and removed from the cell. Isolated nucleic acid cannot be the subject of cellular processes like transcription and translation as can its naturally occurring counterpart; it has been removed from the cellular environment and thus from natural cellular processes (e.g. intron removal, dependent upon the spliceosome). It can only be transcribed and translated by artificial intervention. In the absence of transcription and translation, or following their malfunction, mutations may arise, resulting in disease or an increased risk thereof.
It contains the code for a particular polypeptide; a mutant or polymorphic protein.
It contains a sequence identified by comparison with tables created following extensive epidemiological research which describes the location of the mutations or polymorphisms as they exist in DNA. The DNA was constructed and these locations identified by the work of the inventors.
The nucleic acids have admitted valuable economic use.
211 In the decision of the US Court of Appeals for the Federal Circuit, Bryson J (dissenting) drew on a metaphor, likening an isolated nucleic acid and a branch being snapped off a tree. That is inapposite. The branch has not changed – it is simply divorced from the tree, whereas the chemical and physical makeup of the isolated nucleic acid renders it not only artificial but also different from its natural counterpart.
212 The claim is wider than for a “mere discovery”. The “magic microscope” theory relied upon by the parties in the Federal Circuit is that if an imaginary microscope could focus in on the claimed nucleic acid as it exists in the human body, the claim covers ineligible subject matter. This metaphor does not assist. What is being claimed is not the nucleic acid as it exists in the human body, but the nucleic acid as isolated from the cell. The claimed product is not the same as the naturally occurring product. There are structural differences but, more importantly, there are functional differences because of isolation. As Lourie J explains, ‘the ability to visualise a DNA molecule through a microscope, or by any other means, when it is bonded to other genetic material [and in a particular regulatory environment] is worlds apart from processing an isolated DNA molecule that is in hand and useable’.
213 To this extent we differ, with respect, to the primary judge. In our view the products the subject of claim 1 are different to the gene comprising the nucleic acid sequence as it exists in nature. It follows that the notice of contention based on this ground succeeds.
214 The isolation of the nucleic acid also leads to an economically useful result – in this case, the treatment of breast and ovarian cancers. This is surely what was contemplated by a manner of new manufacture in the Statute of Monopolies. As Moore J explained in the Federal Circuit, ‘it is not the chemical change alone, but that change combined with the different and beneficial utility which leads me to conclude that small isolated DNA fragments are patentable subject matter’.
215 The US Supreme Court rejected the claim over isolated nucleic acids for much the same reasons as those pressed by the appellant in this case. It is difficult to reconcile that Court’s endorsement of the reasoning in Chakrabarty, with its rejection of isolated nucleic acid as eligible for patentability. With respect, the Supreme Court’s emphasis on the similarity of ‘the location and order of the nucleotides’ existing within the nucleic acid in nature before Myriad found them is misplaced. It is the chemical changes in the isolated nucleic acid which are of critical importance, as this is what distinguishes the product as artificial and economically useful.
216 The fact that, hypothetically, if the isolated DNA sequence were replaced into the cell it would express the same proteins is irrelevant. Following Chakrabarty and NRDC, the isolated nucleic acid has ‘markedly different characteristics from any found in nature’; Myriad did not merely ‘separate that gene from its surrounding genetic material’. It should make no difference that in Chakrabarty there was an “addition” (of the plasmids) to the natural product (the bacterium); this is not the appropriate test. Myriad’s claim, properly considered is not, as the US Supreme Court considered, concerned ‘primarily with the information contained in the genetic sequence [rather than] with the specific chemical composition of a particular molecule’.
217 The reasoning of Lourie and Moore JJ of the Federal Circuit is persuasive. It accords with the High Court’s reasoning in NRDC and Microcell. The US Supreme Court accepted that cDNA is patentable. It rejected the isolated nucleic acid of claim 1 because it accepted wrongly, with respect, that the isolated nucleic acid is a “product of nature”. In any event, that exclusion is not in accordance with the principles of patent law in Australia and has been specifically rejected as a reason for exclusion in NRDC.
218 The isolated nucleic acid, including cDNA, has resulted in an artificially created state of affairs for economic benefit. The claimed product is properly the subject of letters patent. The claim is to an invention within the meaning of s 18(1) of the Act.
219 The appeal should be dismissed.
Associate:




